Curing cancer - How metastases arise
Part 3b-2 - How cells normally migrate
Part 1 of this series discussed the basics of metastatic disease.
Part 2 discussed features of human biology that are important in understanding cancer and metastases.
Part 3 discussed basic principles of how these features are altered during the development of cancer and metastases, with specifics to be discussed in parts 3a through 3d.
Part 3a discussed how the malignant properties of normal cells are switched on, focusing on rapid or otherwise inappropriate cell division.
Part 3b-1 discussed what cells normally migrate.
Part 3b-2 discusses normal triggers of migration and the mechanics of cell migration.
What triggers the initiation of migration?
Migration is initiated by random cell migration, dispersion, hitchhiking of cells and chemotaxis, often working together.
Random cell migration
Random cell migration (random motility) refers to the intrinsic ability of cells to migrate. This is demonstrated by fibroblasts plated onto media, which migrate on their own, based on adherence to the underlying matrix (Tschumperlin 2013).
Fibroblast migration video
Thus, fibroblasts and cells with similar properties inherently migrate, unless other factors in their microenvironment (i.e., other cells, the extracellular matrix and nearby biomolecules) prevent them from doing so. This means that inappropriate migration may be due not to new migration properties but to the lack of migration restraining properties.
Embryonic cells can activate random motility using genetic signals that prompt cells to colonize new territories within the embryo (Reig 2014) and to ensure that large populations of cells initiate their migration synchronously on a tight temporal cue (Kurosaka 2008). Often these cells are unable to sense chemotactic cues, which otherwise would cause these cells to migrate (Reig 2014).
Dispersion
Random cell migration has a dispersive effect that prompts cells to colonize new territories within the embryo (Reig 2014) due to contact inhibition of locomotion (CIL), in which colliding cells either migrate away from each other or cease migrating (Roycroft 2016). This dispersion appears to be based on cellular interactions with the underlying substrate that allows them to migrate (Hassan 2019). Whether a group of migrating cells disperses as individual cells or as a collective cell group (streams or sheets) depends on the balance between attractive and repulsive forces (Reig 2014).
The left panel shows contact inhibition of locomotion; when neural crest cells collide, they immediately move apart. In the middle and right panels, there is no dispersion because the enzymes that mediate this dispersion are blocked.
Note that contact inhibition of locomotion is different from contact inhibition, which is a density dependent inhibition of cell growth in which cells reduce their rate of proliferation when they become confluent (Roycroft 2016). Contact inhibition has little to do with migration.
Hitchhiking of cells
In some lower organisms, cells adhere to other endodermal cells and, as these move, go along for the ride (Reig 2014, Chihara 2012). In humans, primordial germ cells may hitchhike onto other cells in the mid-rear area of the embryo to reach their destination in the genital ridges (Freeman 2003 but see Grimaldi 2020).
Chemotaxis
Chemotaxis, the directional movement of cells based on a gradient of increasing or decreasing concentration of a particular substance, often initiates migration. Virtually all organisms move toward chemoattractants and away from repellents. Bacteria migrate based on a gradient of nutrients required for survival. In humans, oral bacteria secrete tyrosine phosphatase and other enzymes, which induce epithelial cell migration (Bai 2022). For inflammatory cells (neutrophils, B and T cells, monocytes, macrophages and dendritic cells), the gradients are cytokines and surface stiffness (George 2022). Other gradients include light, electric fields and temperature (Li 2020). In addition, cells can create their own gradients from random disturbances in systems of chemical reactions, as discovered by Alan Turing (Turing 1952), the founder of computer science.
In humans, chemotaxis is important beginning early in life. For fertilization to occur, the egg produces progesterone, which attracts sperm to move toward the egg (Teves 2009). During embryogenesis, chemotaxis directs the movement of cells during gastrulation and of neural cells at later times. In adults, chemotaxis directs immune system cells toward invading pathogens. For wounds, it directs skin keratinocytes and dermal fibroblasts toward the site of current healing. To maintain homeostasis in tissues whose cells have short lifespans, it directs stem cells of various organs to replenish tissue (Li 2020).
Flagella
Sperm cells use flagella to propel them towards the fallopian tubes. Maternal factors are also important in this step as well as allowing sperm to survive and be capable of fertilization (Fujihara 2018, Mahé 2021).
Cell migration acts as a complex system
Cell migration appears to behave as a complex system, in which the behavior of the whole is greater than the sum of the behavior of the parts. This means we cannot predict overall behavior from studying each interaction, although that is what we traditionally attempt to do.
Cell migration appears to be based on
- the cell's recent behavior
- the cell's microenvironment, including the physical features of neighboring cells (cell - cell contacts) and the intercellular matrix
- chemical signals from within the cell, from neighboring cells and systemically. This also includes resolution signals; initiating a process such as inflammation often also initiates the process to end it (Serhan 2005)
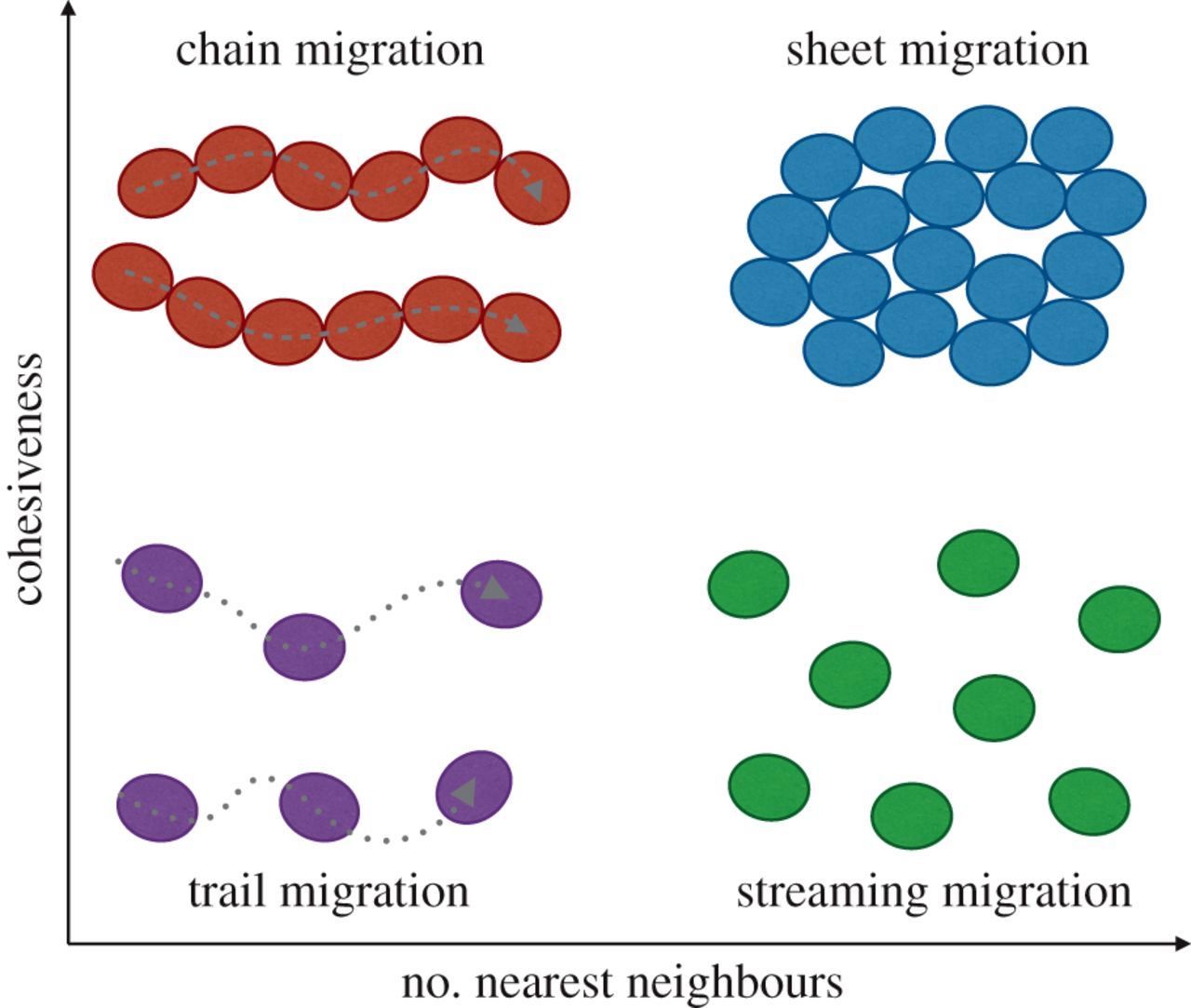
Individual cell migration versus collective cell migration
Cells migrate as individual cells or as clusters (collective cell migration). Collective cell migration is the prevalent mode of migration during development, wound healing and tissue regeneration and may be the dominant mode of migration during metastases (Trepat 2012). Motion results from forces produced by each cell and transmitted to the neighboring cells and to the substrate (Vazquez 2022). Collectively migrating cells use similar mechanisms as single cells but also (a) maintain tissue cohesiveness and organization, (b) create large gradients of soluble factors, (c) distribute tasks between specialized mobile and nonmobile cells and (d) propagate mechanical signals via cell - cell junctions. Types of collective cell migration are indicated below:
Mechanics of cell migration
Regardless of the initiating factor, all cells migrate by similar mechanisms using intricate molecular machines that sense the environment, respond to signals and modulate cell behavior (Kurosaka 2008).
To initiate migration in a developing organism, individual cells receive signals, which set in motion the complex and highly coordinated molecular machinery that drives these cells to move in the right direction with the appropriate speeds and to arrive at their destinations at exactly the right time. While on the organismal level migration is initiated and coordinated by global extracellular signaling, for each individual cell it is regulated from within, by forming transient structures that allow the cell to polarize, protrude and retract in response to its environment (Kurosaka 2008).
Migration of cells involves 4 steps: polarization, protrusion, adhesion and retraction.
In polarization, the migrating cells develop a protruding front (active leading edge) and a trailing edge that retracts as the cell moves forward.
Cell locomotion, cell motility, cell migration
Cells create protrusions in the direction of movement, adhere to the substances that surround them to provide traction, contract their cell body to pull themselves forward and then retract the rear of the cell (Reig 2014). The orientation of the cell may be influenced by contact guidance, in which cells sense and use inhomogeneities (e.g., microgrooves) in the substrate to adhere, polarize and orient their migration.
Cells migrate differently through three dimensional substances than they do on hard, two dimensional surfaces, which makes this more complicated to understand (Kurosaka 2008).
Epithelial - mesenchymal transition (EMT)
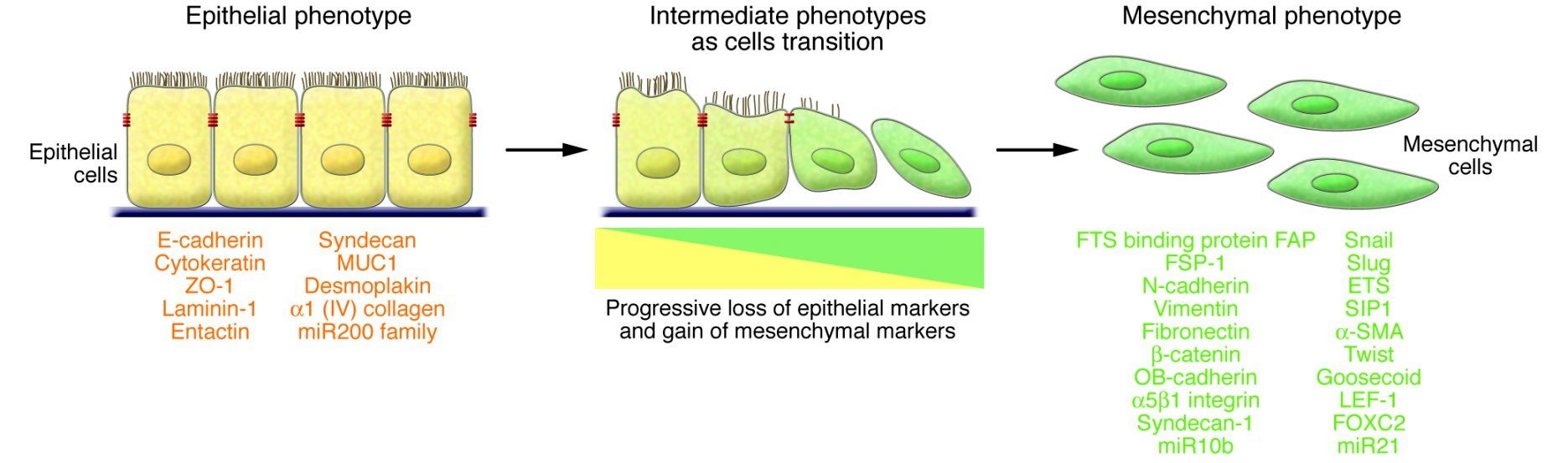
Much of our body is composed of epithelial cells, which line the inside and outside surfaces including skin, blood vessels and organs. These cells are relatively fixed and have a low migration ability. Mesenchymal cells are stem cells that can differentiate into different types of connective tissue such, as fibrous tissue, adipose (fat), muscle, bone and cartilage. Unlike epithelial cells, they have an inherent capacity to migrate.
Most migratory events occur by mesenchymal cells, which may originate through epithelial - mesenchymal transition (EMT) (Lu 2021), a process in which epithelial cells receive signals from their microenvironment and acquire mesenchymal phenotypes and behavior. Initially, the epithelial cells have stable epithelial cell - cell junctions, apical - basal polarity and interactions with the basement membrane that prevent their movement. During EMT, changes in gene expression and regulation mechanisms repress these epithelial characteristics and cause the acquisition of mesenchymal characteristics, including fibroblast-like features (flattening of cell shape, loss of apical - basolateral polarity and loss of cell contacts) and increased migratory capacity (Yang 2020). They may also have invasive properties (i.e., the ability to cross basement membranes that normally constrain the movement of cells and invade blood vessels) (Bai 2022).
Part 3b-3 will discuss what activates the migration functions of cells during malignant transformation.