Curing cancer - Curative cancer treatment based on complexity theory
Author:
Nat Pernick, M.D.
Revised 19 June 2022, originally posted at:
This essay summarizes my recommendations on how to substantially reduce cancer deaths. In general, we should view cancer as primarily a disorder of a complex biological system that is composed of interacting networks and not as a modular system with faulty parts (Pernick: What should our national cancer goals be, 2021). I also believe that substantially reducing cancer deaths is primarily a management problem to be solved by identifying and implementing a series of appropriate public policy, medical and scientific tasks (Pernick: Strategic plan to substantially reduce cancer deaths, 2021), and not by trying to discover a miracle treatment (Pernick: What will success look like in the war on cancer, 2021).
1. Network medicine. Adult cancer, which accounts for almost all cancer deaths, is a systemic disease. It arises from, and is maintained due to, dysfunctional cellular networks, not just mutated genes in a simple pathway (Pernick: New ideas about cancer, 2021).
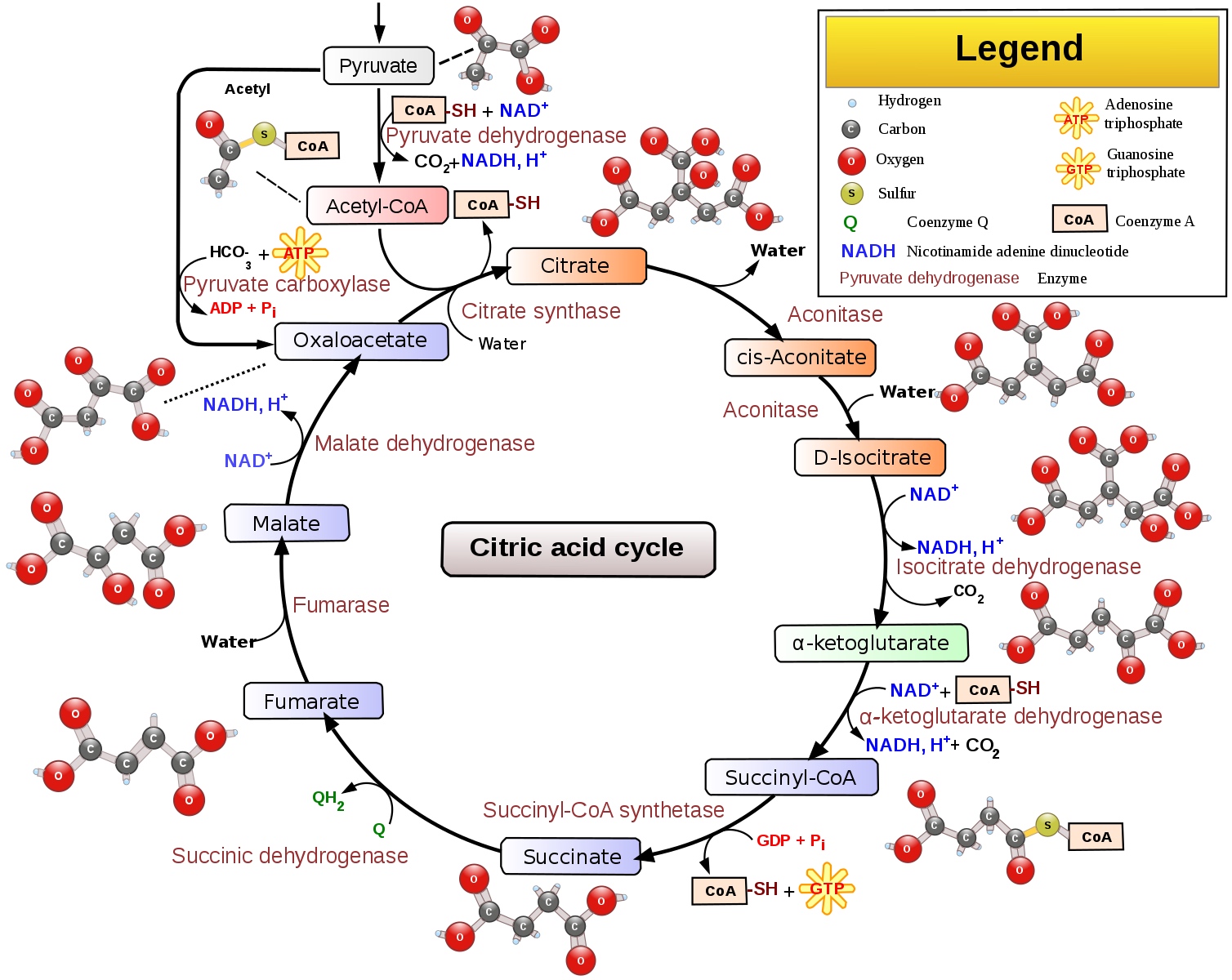
A network is a complex set of interactions, or relationships, between different entities. By contrast, a simple pathway is a linear process with changes that occur one step at a time (e.g., an assembly line). Scientists often think about biological pathways as a circular assembly line with small changes at each step until the pathway's function is completed, such as activating an enzyme; then the pathway begins again.
In contrast, complex biological pathways, such as those related to cell division, interact with each other at many steps, resembling sets of intersecting circles forming a three dimensional web of pathways that, when viewed as a whole, may perform a higher level function.

Advanced disease in adults may begin with simple changes but large cancers are sustained by years or decades of supportive network changes throughout the body, called an altered systems biology (Koutsogiannouli 2013, Paul 2021). These systemic changes typically will not revert to normal even if a substantial number of cancer cells are destroyed. Thus, a focus on "network medicine" is mandatory (Barabási 2011, Parini 2020).
2. Kill as many cancer cells as possible. High cancer cell kill is important because: (a) cancer cells directly damage normal cells, tissue and organ systems, interfering with their physiologic functions which maintain life; (b) cancer cells create an increased workload, both by producing biological substances that interfere with optimal physiology and by stimulating a response to destroy them; and (c) cancer cells have molecular heterogeneity so the death of any cancer cell may also destroy a different strategy of the cancer cell and its progeny to overcome the body's anticancer defenses.
3. Block multiple pathways and block each pathway at multiple points. We have cured some cancers in children and young adults, including childhood leukemia, Hodgkin lymphoma and testicular cancer. These cancers are caused by inherited or constitutional cancer predisposition or developmental mutations (Kentsis 2020) and exhibit a limited number of somatic (acquired) cancer mutations (Sweet-Cordero 2019). However, single therapies are not curative. In the 1940s, Dr. Sidney Farber, a Harvard pathologist, gave his childhood leukemia patients a new drug, aminopterin, which blocked the effect of folic acid, which is needed for cells to divide (Dana-Farber Cancer Institute, accessed 31May22). Amazingly, these children, who usually died within weeks of diagnosis, went into remission. But their cancer soon became resistant to treatment and the children relapsed (McNeer 2019, Benshang 2020).
We now know that it may take 3-5 drugs with different mechanisms of action to create enough blocks to completely disable these cancer cell networks in children (Mukherjee: Emperor of All Maladies, 2010). Disabling the activity of dysfunctional networks often requires drug combinations due to the weblike interaction of networks that can readily bypass a single block in a particular pathway. In addition, some treatments do not work for some patients (Palmer 2017, Plana 2022).
Curing adult cancers may require even more treatment diversity due to: (a) their complex and heterogeneous mutational landscape (de Sousa 2018, Blank 2018, Samuel 2011), (b) the field effects generated by cancer promoters / risk factors acting over decades of exposure and (c) associated systemic network changes that must also be addressed by treatment.
Although drug combinations are typically more effective than single agents (Mokhtari 2017, Palmer 2022), determining which combinations are most effective is time consuming. However, "deep learning," other computational approaches and modeling methods may help screen possible combinations for effectiveness (Kuenzi 2020, Sidorov 2019). Combining different types of therapy may also be effective; for example, regional hyperthermia may kill therapy resistant cancer stem cells (Oei 2017), be synergistic with immune checkpoint inhibitors (Li 2020) and improve survival (Fiorentini 2019).
4. Combinations of combinations of treatment. Since adult cancers are due to dysfunction in many key systemic networks (see below), with each often requiring a different set of combinatorial therapies, curative therapy may require combinations of combinations of treatment to adequately alter these networks. We speculate that for each cancer type, even the most aggressive, there exists a combination of 8-10 therapies that individually may be only partially effective but together can be substantially effective, although determining the optimal combinations will be difficult (Pernick: Combinations of therapy to substantially reduce cancer deaths, 2021).
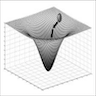
5. Move local cancer cell networks into less lethal states. Curative treatment, in addition to killing large numbers of cancer cells through multiple mechanisms, should "normalize" or reduce the malignant traits of cancer cells that survive (Heudobler 2019). Fifty years ago, Kauffman discovered that a complex network of thousands of mutually regulating genes in normal cells may produce a stable equilibrium state called an attractor that corresponds to gene expression profiles specific to each cell type (Kauffman 1969). Essentially, the environment of biological substances when together forces them to have a limited behavior even though they behave very differently when isolated. Attractors have been analogized to a low energy state or valley on a topographic diagram that pulls in cells with similar network configurations (Waddington 1957, Noble 2015), see diagrams at Vallacher 2013, Goldberg 2007.
Attractors maintain cellular network stability against common disruptions in both normal cells and cancer cells. In normal cells, this stability may be disturbed by cancer "super promoters" (risk factors), acting over long time periods, that push cell networks into malignant pathways (Pernick 2021a). In cancer cells, these "cancer attractors" create network stability that makes them resistant to anticancer treatment (Huang 2009).
For example, some cancer cells have an embryonic phenotype, which is associated with aggressive behavior. In the microenvironment of the fertilized egg, coordinated network activity ultimately moves embryonic related networks towards mature, differentiated phenotypes in the fetus and newborn. However, cancer risk factors stimulate these networks in a noncoordinated manner to trigger embryonic properties, such as rapid cell division (Kermi 2017), cell migration (Reig 2014, Kurosaka 2008) and changes to cell differentiation (Li 2014) that do not mature over time.
Curative treatment should include agents to promote this maturation, such as retinoids used in acute promyelocytic leukemia and childhood neuroblastoma (Madan 2020, Nowak 2009, Matthay 1999), progestins for endometrial hyperplasia, a premalignant condition (Gallos 2013), myeloid differentiation promoting cytokines (McClellan 2015) and other cancer cell reprogramming drugs (Gao 2019, Gong 2019). Constant disturbing of parts of the network may also be useful (Cho 2016, Kim 2017).
6. Disrupt the inflammatory process, which plays a central role in promoting and sustaining carcinogenesis. Tumors (i.e. solid collections of cancer cells) have been described as wounds that do not heal (Dvorak 1986, Dvorak 2015). Activation of the inflammatory system, which promotes wound healing and accompanies many malignancies (Coussens 2002, Pernick 2020), has been considered a major cause of cancer since 1863, when Virchow speculated that some irritants enhance cell proliferation through tissue injury and chronic inflammation (Schottenfeld 2006). Many cancer risk factors activate Inflammation, including excess weight, cigarette smoking, heavy alcohol consumption, aging and a Western diet (high fat, highly processed foods, low consumption of vegetables, fruits and whole grains) (Antwi 2016, Pernick 2020).
Inflammation may play a central role in promoting carcinogenesis because: (a) it is widely connected to other networks, (b) it rapidly initiates relatively unstable processes related to repair, antimicrobial and anticancer activities, which may propagate to local and systemic networks, promote their instability and ultimately lead to malignancy (Morgillo 2018, Bui 2022), (c) inflammation triggered by cancer risk factors differs from inflammation triggered by trauma or infection in that it has no physiologic mechanism to stop.
Physiologic inflammation, when triggered by trauma or infection, is coordinated with the simultaneous initiation of its resolution (Serhan 2005, Serhan 2020). As the trauma is repaired or the threat from foreign organisms subsides, the resolution pathways cause networks to revert towards their initial states to prevent bystander damage to tissue or propagation of instability to other systems (Sugimoto 2016). Cancer risk factors also trigger inflammation but through unconventional means that do not simultaneously initiate the resolution process (Fishbein 2021). This causes persistent inflammation, which may wear down stabilizing factors in inflammatory and adjacent networks, particularly when accompanied by other risk factors, which further drives the malignant process (Shimizu 2012, Huang 2009).
Curative cancer therapy needs to antagonize or diminish this persistent inflammatory process. Suggested options include: (a) triggering pro-resolution pathways (Fishbein 2021, Park 2020); (b) using anti-inflammatory agents to diminish inflammation in general (Zappavigna 2020, Bruserud 2020); (c) mimicking the halting mechanisms associated with wound healing (Shah 2018, Kareva 2016) and liver regeneration (Abu Rmilah 2019, Hadjittofi 2021); and (d) countering germline (inherited genetic) changes that promote instability in the inflammatory process.
7. Disrupt the cancer microenvironment (inflammation, vasculature, stroma and the extracellular matrix) that nurtures cancer cells at primary and metastatic sites. Cancer risk factors produce a microenvironment that nurtures mutated cells (Mbeunkui 2009), steers cellular networks towards malignant pathways, helps them escape immune surveillance (Labani-Motlagh 2020) and ultimately promotes invasion by activating cells to mimic physiologic "invasion" of wounded epithelium through the extracellular matrix (Bleaken 2016, Coussens 2002). Cancers require a fertile "soil" for the cancer "seeds" to grow (Fidler 2003, Tsai 2014). For example, Hodgkin Reed-Sternberg cells produce cytokines that assist the survival and proliferation of lymphoma cells (Wang 2019, Opinto 2021) and pancreatic cancer cells produce cytokine IL1β and proinflammatory factors that establish a supportive microenvironment (Das 2020, Huber 2020). From a network perspective, there is a complex crosstalk among cancer cells, other local cells and the extracellular matrix (Sounni 2013).
Curative treatment should disrupt or normalize the microenvironment by targeting inflammation, the vasculature, stroma (Hauge 2020) and extracellular matrix (Mpekris 2020). For example, anti-VEGF or anti-VEGF receptor treatment can normalize vasculature by reducing vascular permeability (Gkretsi 2015, Wu 2018). Normalizing the microenvironment may also enhance drug delivery and effectiveness (Polydorou 2017, Stylianopoulos 2018) or make existing cancers or premalignant states more susceptible to immune system attack (Ganss 2020).
It is also important to disrupt the microenvironment of possible metastatic sites. Typically, cancer cells die at secondary sites but the malignant process may precondition the otherwise hostile microenvironment of the secondary site so it can sustain their colonization (Houg 2018, Kaplan 2005).
8. Repair immune system dysfunction that coevolves with carcinogenesis. The immune system consists of a web of interacting networks whose effectiveness is systematically degraded with malignant progression. Immune dysfunction in cancer is typically not just the failure of one particular pathway (Stephen 2021). Curative treatment should attempt to improve immune system function with combinatorial therapy that targets multiple aspects of immune dysfunction (Sodergren 2020).
9. Antagonize hormonal expression that promotes cancer cell growth. Physiologic (i.e. normal) levels of estrogens and androgens and elevated levels of insulin are associated with breast (Dall 2017), endometrial / uterine (Rodriguez 2019), prostate (Liu 2020, Dai 2017) and pancreatic cancer (Andersen 2017, Li 2019, Perry 2020). The primary mechanism may involve promotion of cell growth at a stage when these cells are particularly vulnerable to instability.
Simple antagonism of hormonal pathways is possible using tamoxifen for estrogens, antiandrogens for testosterone and metformin for insulin (Wan 2018). One block in these hormonal networks is apparently adequate for normalization, in contrast to the 3-5 blocks required for other cancer cell networks. Behavioral changes, such as weight loss, exercise, a healthier diet and reducing alcohol and tobacco use may also be therapeutic by either altering hormone levels or changing their interaction with other risk factors.
10. Investigate attacking global changes of cancer cells:
(a) Promote the activation of gene networks that support stable, multicellular processes and suppress networks that promote unicellular processes that enable malignant type behavior. Multicellular organisms evolved from unicellular organisms by adding new genes and more intricate controls to existing networks for metabolism and replication (Trigos 2018, Trigos 2019). This enables greater communication and coordination between cells and makes possible higher level functions, such as cell differentiation and programmed cell death (Trigos 2018). The new control mechanisms keep cellular and systemic processes on track and shift the survival focus from individual cells towards the organism as a whole (Davies 2011). The operation of multicellular and unicellular programs appears to be somewhat mutually exclusive. Inflammation and DNA alterations may damage these multicellular controls, activating the existing genetic toolkit of preprogrammed, malignant behavior in unicellular networks based on what has been described as the atavism hypothesis of cancer (Davies 2011, Trigos 2017, Bussey 2017, Bussey 2021). Theoretically, it should be possible to shift the balance towards promotion of the multicellular networks and repression of the unicellular networks (Lineweaver 2014, Kasperski 2021).
(b) Repress epithelial-mesenchymal transition, a common mechanism of malignancy (Gaponova 2020, Hay 1995). Epithelial-mesenchymal transition (EMT) is a physiological process in which epithelial cells attain the properties of mesenchymal cells, both morphologically (in appearance) and physiologically (in function). Since EMT plays an important role in tumor progression through metastasis, apoptotic resistance and immune evasion, repressing EMT may be important for curative therapy.
(c) Target the weaknesses of cancer cells by applying a specific cellular stress that is readily dealt with by healthy cells using evolved capabilities or multicellular programming but not by cancer cells with predominantly unicellular programming. This includes "lethal challenges" of high dose methotrexate with leucovorin rescue (Howard 2016) or targeting other aspects of chaotic or unstable states, such as cell-extracellular matrix detachment (Crawford 2017).
11. Antagonize inherited genetic changes that promote malignant behavior. Genetic testing of noncancer cells (germline testing) is recommended for all patients with pancreatic cancer (Stoffel 2019) and select patients with other cancers or family histories of cancer (Daly 2020, Lincoln 2020). Results are currently used to determine anticancer therapy (Zhu 2020) as well as for cancer screenings, reproductive choices and genetic counseling. We suggest using these results to also provide treatment that moves premalignant or malignant cells into less harmful pathways as discussed above or to counter common germline changes in inflammatory, DNA repair, cell cycle stability, immune system function or other networks that promote malignancy.
12. Focus on reducing death and disability and not necessarily killing every cancer cell. Although killing every cancer cell is important for curing placental choriocarcinoma (Freireich 2002, Wikipedia-Min Chiu Li, accessed 9Jun22) and as discussed in #2 above, this may not be an appropriate treatment goal for all adult tumors; some patients may benefit from strategies of cancer growth containment rather than eradication (Bussey 2021, Ruscetti 2018, Liao 2021, Wang 2022).
It may also be important to achieve "marginal gains" at all steps of the disease process by optimizing specific aspects of care, even if minor, such as reducing perioperative morbidity, improving management of jaundice and addressing malnutrition. These small efforts may aggregate to produce substantial improvements, leading to additional treatment options (Powell-Brett 2021) and a reduction in the sense of futility that may promote death (Pernick 2021b).
We should also study whether guiding therapy based on patient preference, to the extent possible, reduces cancer death (Gärtner 2019, Seghers 2022).
13. Monitoring key networks. To optimize treatment, it may be important to monitor the status of key networks as treatment is given including the inflammatory process in general, the immune system's anticancer capabilities, different aspects of the cancer microenvironment, embryonic networks that promote lack of cell differentiation, hormonal expression that promotes cancer cell growth and inherited changes that promote malignant behavior (Pernick: The importance of systemic disease in cancer treatment, 2021). For each of these networks, we must determine which biological molecules to monitor, how best to do so, how changes in their expression affect treatment and how these values will impact long term survival rates.
14. Early cancer deaths. We should identify key physiologic networks disturbed in early cancer death and develop treatment strategies to rapidly normalize them and prevent death (Pernick: Curing Cancer Blog: Part 9 - How cancer kills 2021). Once these physiologic networks are stabilized, the underlying cancer can then be treated.
15. Clinical trials. Every cancer patient should be enrolled in a clinical trial, if possible. These trials are needed to determine: (a) the effectiveness of individual treatments, combinations of treatments and combinations of combinations of treatments, (b) long term survival rates (Pernick: Curing cancer - Adult versus childhood cancer 2022); (c) how to reduce side effects and (d) what adjustments to make for particular patients.
16. Public health and preventative programs. Government at all levels, the private sector and nonprofits should promote a culture of healthy living and low cancer risk that includes reducing tobacco use, excess weight and alcohol abuse, encouraging a healthy diet and exercise and obtaining appropriate medical care and vaccinations (American Code Against Cancer, accessed 10Jun22).
We should develop well run public health and medical care systems that promote risk factor reduction to prevent many cancers from arising. These systems can act as a societal or behavioral immune system (Schaller 2015) to reduce cancer risk factors, similar to our physiologic immune system, which prevents numerous cancers from being clinically evident, as demonstrated by the high cancer rate in immunosuppressed patients.
We should reduce medical misinformation and promote adequate and appropriate medical care for all patients (Pernick: Strategic plan to substantially reduce cancer deaths, 2021, Simcock 2020).
We should develop more effective programs to identify premalignant or malignant lesions in both high risk patients and current patients being monitored for relapse.
Together, these recommendations should play an important part in substantially reducing cancer deaths.
- 29 Dec 2020: Part 3 - Curative cancer treatment based on complexity theory
- 10 Jan 2021: Part 4 - Principles of curative treatment
- 17 Jan 2021: Part 5 - Key network issues that affect the primary tumor
- 21 Jan 2021: Part 6 - Key systemic network issue
This essay summarizes my recommendations on how to substantially reduce cancer deaths. In general, we should view cancer as primarily a disorder of a complex biological system that is composed of interacting networks and not as a modular system with faulty parts (Pernick: What should our national cancer goals be, 2021). I also believe that substantially reducing cancer deaths is primarily a management problem to be solved by identifying and implementing a series of appropriate public policy, medical and scientific tasks (Pernick: Strategic plan to substantially reduce cancer deaths, 2021), and not by trying to discover a miracle treatment (Pernick: What will success look like in the war on cancer, 2021).
1. Network medicine. Adult cancer, which accounts for almost all cancer deaths, is a systemic disease. It arises from, and is maintained due to, dysfunctional cellular networks, not just mutated genes in a simple pathway (Pernick: New ideas about cancer, 2021).
A network is a complex set of interactions, or relationships, between different entities. By contrast, a simple pathway is a linear process with changes that occur one step at a time (e.g., an assembly line). Scientists often think about biological pathways as a circular assembly line with small changes at each step until the pathway's function is completed, such as activating an enzyme; then the pathway begins again.

Example of a simple biologic pathway - citric acid cycle
In contrast, complex biological pathways, such as those related to cell division, interact with each other at many steps, resembling sets of intersecting circles forming a three dimensional web of pathways that, when viewed as a whole, may perform a higher level function.

Complex web of interactions for the MAP kinase pathway, related to cell division
Advanced disease in adults may begin with simple changes but large cancers are sustained by years or decades of supportive network changes throughout the body, called an altered systems biology (Koutsogiannouli 2013, Paul 2021). These systemic changes typically will not revert to normal even if a substantial number of cancer cells are destroyed. Thus, a focus on "network medicine" is mandatory (Barabási 2011, Parini 2020).
2. Kill as many cancer cells as possible. High cancer cell kill is important because: (a) cancer cells directly damage normal cells, tissue and organ systems, interfering with their physiologic functions which maintain life; (b) cancer cells create an increased workload, both by producing biological substances that interfere with optimal physiology and by stimulating a response to destroy them; and (c) cancer cells have molecular heterogeneity so the death of any cancer cell may also destroy a different strategy of the cancer cell and its progeny to overcome the body's anticancer defenses.
3. Block multiple pathways and block each pathway at multiple points. We have cured some cancers in children and young adults, including childhood leukemia, Hodgkin lymphoma and testicular cancer. These cancers are caused by inherited or constitutional cancer predisposition or developmental mutations (Kentsis 2020) and exhibit a limited number of somatic (acquired) cancer mutations (Sweet-Cordero 2019). However, single therapies are not curative. In the 1940s, Dr. Sidney Farber, a Harvard pathologist, gave his childhood leukemia patients a new drug, aminopterin, which blocked the effect of folic acid, which is needed for cells to divide (Dana-Farber Cancer Institute, accessed 31May22). Amazingly, these children, who usually died within weeks of diagnosis, went into remission. But their cancer soon became resistant to treatment and the children relapsed (McNeer 2019, Benshang 2020).
We now know that it may take 3-5 drugs with different mechanisms of action to create enough blocks to completely disable these cancer cell networks in children (Mukherjee: Emperor of All Maladies, 2010). Disabling the activity of dysfunctional networks often requires drug combinations due to the weblike interaction of networks that can readily bypass a single block in a particular pathway. In addition, some treatments do not work for some patients (Palmer 2017, Plana 2022).
Curing adult cancers may require even more treatment diversity due to: (a) their complex and heterogeneous mutational landscape (de Sousa 2018, Blank 2018, Samuel 2011), (b) the field effects generated by cancer promoters / risk factors acting over decades of exposure and (c) associated systemic network changes that must also be addressed by treatment.
Although drug combinations are typically more effective than single agents (Mokhtari 2017, Palmer 2022), determining which combinations are most effective is time consuming. However, "deep learning," other computational approaches and modeling methods may help screen possible combinations for effectiveness (Kuenzi 2020, Sidorov 2019). Combining different types of therapy may also be effective; for example, regional hyperthermia may kill therapy resistant cancer stem cells (Oei 2017), be synergistic with immune checkpoint inhibitors (Li 2020) and improve survival (Fiorentini 2019).
4. Combinations of combinations of treatment. Since adult cancers are due to dysfunction in many key systemic networks (see below), with each often requiring a different set of combinatorial therapies, curative therapy may require combinations of combinations of treatment to adequately alter these networks. We speculate that for each cancer type, even the most aggressive, there exists a combination of 8-10 therapies that individually may be only partially effective but together can be substantially effective, although determining the optimal combinations will be difficult (Pernick: Combinations of therapy to substantially reduce cancer deaths, 2021).
5. Move local cancer cell networks into less lethal states. Curative treatment, in addition to killing large numbers of cancer cells through multiple mechanisms, should "normalize" or reduce the malignant traits of cancer cells that survive (Heudobler 2019). Fifty years ago, Kauffman discovered that a complex network of thousands of mutually regulating genes in normal cells may produce a stable equilibrium state called an attractor that corresponds to gene expression profiles specific to each cell type (Kauffman 1969). Essentially, the environment of biological substances when together forces them to have a limited behavior even though they behave very differently when isolated. Attractors have been analogized to a low energy state or valley on a topographic diagram that pulls in cells with similar network configurations (Waddington 1957, Noble 2015), see diagrams at Vallacher 2013, Goldberg 2007.
Attractors maintain cellular network stability against common disruptions in both normal cells and cancer cells. In normal cells, this stability may be disturbed by cancer "super promoters" (risk factors), acting over long time periods, that push cell networks into malignant pathways (Pernick 2021a). In cancer cells, these "cancer attractors" create network stability that makes them resistant to anticancer treatment (Huang 2009).
For example, some cancer cells have an embryonic phenotype, which is associated with aggressive behavior. In the microenvironment of the fertilized egg, coordinated network activity ultimately moves embryonic related networks towards mature, differentiated phenotypes in the fetus and newborn. However, cancer risk factors stimulate these networks in a noncoordinated manner to trigger embryonic properties, such as rapid cell division (Kermi 2017), cell migration (Reig 2014, Kurosaka 2008) and changes to cell differentiation (Li 2014) that do not mature over time.
Curative treatment should include agents to promote this maturation, such as retinoids used in acute promyelocytic leukemia and childhood neuroblastoma (Madan 2020, Nowak 2009, Matthay 1999), progestins for endometrial hyperplasia, a premalignant condition (Gallos 2013), myeloid differentiation promoting cytokines (McClellan 2015) and other cancer cell reprogramming drugs (Gao 2019, Gong 2019). Constant disturbing of parts of the network may also be useful (Cho 2016, Kim 2017).
6. Disrupt the inflammatory process, which plays a central role in promoting and sustaining carcinogenesis. Tumors (i.e. solid collections of cancer cells) have been described as wounds that do not heal (Dvorak 1986, Dvorak 2015). Activation of the inflammatory system, which promotes wound healing and accompanies many malignancies (Coussens 2002, Pernick 2020), has been considered a major cause of cancer since 1863, when Virchow speculated that some irritants enhance cell proliferation through tissue injury and chronic inflammation (Schottenfeld 2006). Many cancer risk factors activate Inflammation, including excess weight, cigarette smoking, heavy alcohol consumption, aging and a Western diet (high fat, highly processed foods, low consumption of vegetables, fruits and whole grains) (Antwi 2016, Pernick 2020).
Inflammation may play a central role in promoting carcinogenesis because: (a) it is widely connected to other networks, (b) it rapidly initiates relatively unstable processes related to repair, antimicrobial and anticancer activities, which may propagate to local and systemic networks, promote their instability and ultimately lead to malignancy (Morgillo 2018, Bui 2022), (c) inflammation triggered by cancer risk factors differs from inflammation triggered by trauma or infection in that it has no physiologic mechanism to stop.
Physiologic inflammation, when triggered by trauma or infection, is coordinated with the simultaneous initiation of its resolution (Serhan 2005, Serhan 2020). As the trauma is repaired or the threat from foreign organisms subsides, the resolution pathways cause networks to revert towards their initial states to prevent bystander damage to tissue or propagation of instability to other systems (Sugimoto 2016). Cancer risk factors also trigger inflammation but through unconventional means that do not simultaneously initiate the resolution process (Fishbein 2021). This causes persistent inflammation, which may wear down stabilizing factors in inflammatory and adjacent networks, particularly when accompanied by other risk factors, which further drives the malignant process (Shimizu 2012, Huang 2009).
Curative cancer therapy needs to antagonize or diminish this persistent inflammatory process. Suggested options include: (a) triggering pro-resolution pathways (Fishbein 2021, Park 2020); (b) using anti-inflammatory agents to diminish inflammation in general (Zappavigna 2020, Bruserud 2020); (c) mimicking the halting mechanisms associated with wound healing (Shah 2018, Kareva 2016) and liver regeneration (Abu Rmilah 2019, Hadjittofi 2021); and (d) countering germline (inherited genetic) changes that promote instability in the inflammatory process.
7. Disrupt the cancer microenvironment (inflammation, vasculature, stroma and the extracellular matrix) that nurtures cancer cells at primary and metastatic sites. Cancer risk factors produce a microenvironment that nurtures mutated cells (Mbeunkui 2009), steers cellular networks towards malignant pathways, helps them escape immune surveillance (Labani-Motlagh 2020) and ultimately promotes invasion by activating cells to mimic physiologic "invasion" of wounded epithelium through the extracellular matrix (Bleaken 2016, Coussens 2002). Cancers require a fertile "soil" for the cancer "seeds" to grow (Fidler 2003, Tsai 2014). For example, Hodgkin Reed-Sternberg cells produce cytokines that assist the survival and proliferation of lymphoma cells (Wang 2019, Opinto 2021) and pancreatic cancer cells produce cytokine IL1β and proinflammatory factors that establish a supportive microenvironment (Das 2020, Huber 2020). From a network perspective, there is a complex crosstalk among cancer cells, other local cells and the extracellular matrix (Sounni 2013).
Curative treatment should disrupt or normalize the microenvironment by targeting inflammation, the vasculature, stroma (Hauge 2020) and extracellular matrix (Mpekris 2020). For example, anti-VEGF or anti-VEGF receptor treatment can normalize vasculature by reducing vascular permeability (Gkretsi 2015, Wu 2018). Normalizing the microenvironment may also enhance drug delivery and effectiveness (Polydorou 2017, Stylianopoulos 2018) or make existing cancers or premalignant states more susceptible to immune system attack (Ganss 2020).
It is also important to disrupt the microenvironment of possible metastatic sites. Typically, cancer cells die at secondary sites but the malignant process may precondition the otherwise hostile microenvironment of the secondary site so it can sustain their colonization (Houg 2018, Kaplan 2005).
8. Repair immune system dysfunction that coevolves with carcinogenesis. The immune system consists of a web of interacting networks whose effectiveness is systematically degraded with malignant progression. Immune dysfunction in cancer is typically not just the failure of one particular pathway (Stephen 2021). Curative treatment should attempt to improve immune system function with combinatorial therapy that targets multiple aspects of immune dysfunction (Sodergren 2020).
9. Antagonize hormonal expression that promotes cancer cell growth. Physiologic (i.e. normal) levels of estrogens and androgens and elevated levels of insulin are associated with breast (Dall 2017), endometrial / uterine (Rodriguez 2019), prostate (Liu 2020, Dai 2017) and pancreatic cancer (Andersen 2017, Li 2019, Perry 2020). The primary mechanism may involve promotion of cell growth at a stage when these cells are particularly vulnerable to instability.
Simple antagonism of hormonal pathways is possible using tamoxifen for estrogens, antiandrogens for testosterone and metformin for insulin (Wan 2018). One block in these hormonal networks is apparently adequate for normalization, in contrast to the 3-5 blocks required for other cancer cell networks. Behavioral changes, such as weight loss, exercise, a healthier diet and reducing alcohol and tobacco use may also be therapeutic by either altering hormone levels or changing their interaction with other risk factors.
10. Investigate attacking global changes of cancer cells:
(a) Promote the activation of gene networks that support stable, multicellular processes and suppress networks that promote unicellular processes that enable malignant type behavior. Multicellular organisms evolved from unicellular organisms by adding new genes and more intricate controls to existing networks for metabolism and replication (Trigos 2018, Trigos 2019). This enables greater communication and coordination between cells and makes possible higher level functions, such as cell differentiation and programmed cell death (Trigos 2018). The new control mechanisms keep cellular and systemic processes on track and shift the survival focus from individual cells towards the organism as a whole (Davies 2011). The operation of multicellular and unicellular programs appears to be somewhat mutually exclusive. Inflammation and DNA alterations may damage these multicellular controls, activating the existing genetic toolkit of preprogrammed, malignant behavior in unicellular networks based on what has been described as the atavism hypothesis of cancer (Davies 2011, Trigos 2017, Bussey 2017, Bussey 2021). Theoretically, it should be possible to shift the balance towards promotion of the multicellular networks and repression of the unicellular networks (Lineweaver 2014, Kasperski 2021).
(b) Repress epithelial-mesenchymal transition, a common mechanism of malignancy (Gaponova 2020, Hay 1995). Epithelial-mesenchymal transition (EMT) is a physiological process in which epithelial cells attain the properties of mesenchymal cells, both morphologically (in appearance) and physiologically (in function). Since EMT plays an important role in tumor progression through metastasis, apoptotic resistance and immune evasion, repressing EMT may be important for curative therapy.
(c) Target the weaknesses of cancer cells by applying a specific cellular stress that is readily dealt with by healthy cells using evolved capabilities or multicellular programming but not by cancer cells with predominantly unicellular programming. This includes "lethal challenges" of high dose methotrexate with leucovorin rescue (Howard 2016) or targeting other aspects of chaotic or unstable states, such as cell-extracellular matrix detachment (Crawford 2017).
11. Antagonize inherited genetic changes that promote malignant behavior. Genetic testing of noncancer cells (germline testing) is recommended for all patients with pancreatic cancer (Stoffel 2019) and select patients with other cancers or family histories of cancer (Daly 2020, Lincoln 2020). Results are currently used to determine anticancer therapy (Zhu 2020) as well as for cancer screenings, reproductive choices and genetic counseling. We suggest using these results to also provide treatment that moves premalignant or malignant cells into less harmful pathways as discussed above or to counter common germline changes in inflammatory, DNA repair, cell cycle stability, immune system function or other networks that promote malignancy.
12. Focus on reducing death and disability and not necessarily killing every cancer cell. Although killing every cancer cell is important for curing placental choriocarcinoma (Freireich 2002, Wikipedia-Min Chiu Li, accessed 9Jun22) and as discussed in #2 above, this may not be an appropriate treatment goal for all adult tumors; some patients may benefit from strategies of cancer growth containment rather than eradication (Bussey 2021, Ruscetti 2018, Liao 2021, Wang 2022).
It may also be important to achieve "marginal gains" at all steps of the disease process by optimizing specific aspects of care, even if minor, such as reducing perioperative morbidity, improving management of jaundice and addressing malnutrition. These small efforts may aggregate to produce substantial improvements, leading to additional treatment options (Powell-Brett 2021) and a reduction in the sense of futility that may promote death (Pernick 2021b).
We should also study whether guiding therapy based on patient preference, to the extent possible, reduces cancer death (Gärtner 2019, Seghers 2022).
13. Monitoring key networks. To optimize treatment, it may be important to monitor the status of key networks as treatment is given including the inflammatory process in general, the immune system's anticancer capabilities, different aspects of the cancer microenvironment, embryonic networks that promote lack of cell differentiation, hormonal expression that promotes cancer cell growth and inherited changes that promote malignant behavior (Pernick: The importance of systemic disease in cancer treatment, 2021). For each of these networks, we must determine which biological molecules to monitor, how best to do so, how changes in their expression affect treatment and how these values will impact long term survival rates.
14. Early cancer deaths. We should identify key physiologic networks disturbed in early cancer death and develop treatment strategies to rapidly normalize them and prevent death (Pernick: Curing Cancer Blog: Part 9 - How cancer kills 2021). Once these physiologic networks are stabilized, the underlying cancer can then be treated.
15. Clinical trials. Every cancer patient should be enrolled in a clinical trial, if possible. These trials are needed to determine: (a) the effectiveness of individual treatments, combinations of treatments and combinations of combinations of treatments, (b) long term survival rates (Pernick: Curing cancer - Adult versus childhood cancer 2022); (c) how to reduce side effects and (d) what adjustments to make for particular patients.
16. Public health and preventative programs. Government at all levels, the private sector and nonprofits should promote a culture of healthy living and low cancer risk that includes reducing tobacco use, excess weight and alcohol abuse, encouraging a healthy diet and exercise and obtaining appropriate medical care and vaccinations (American Code Against Cancer, accessed 10Jun22).
We should develop well run public health and medical care systems that promote risk factor reduction to prevent many cancers from arising. These systems can act as a societal or behavioral immune system (Schaller 2015) to reduce cancer risk factors, similar to our physiologic immune system, which prevents numerous cancers from being clinically evident, as demonstrated by the high cancer rate in immunosuppressed patients.
We should reduce medical misinformation and promote adequate and appropriate medical care for all patients (Pernick: Strategic plan to substantially reduce cancer deaths, 2021, Simcock 2020).
We should develop more effective programs to identify premalignant or malignant lesions in both high risk patients and current patients being monitored for relapse.
Together, these recommendations should play an important part in substantially reducing cancer deaths.