22 September 2022 - Case of the Month #519
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Drs. Mario Marques-Piubelli and Roberto Miranda, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA, for contributing this case and discussion and to Dr. Genevieve Crane, Cleveland Clinic, Cleveland, Ohio, USA, for reviewing the discussion.

Case of the Month #519
Clinical history:
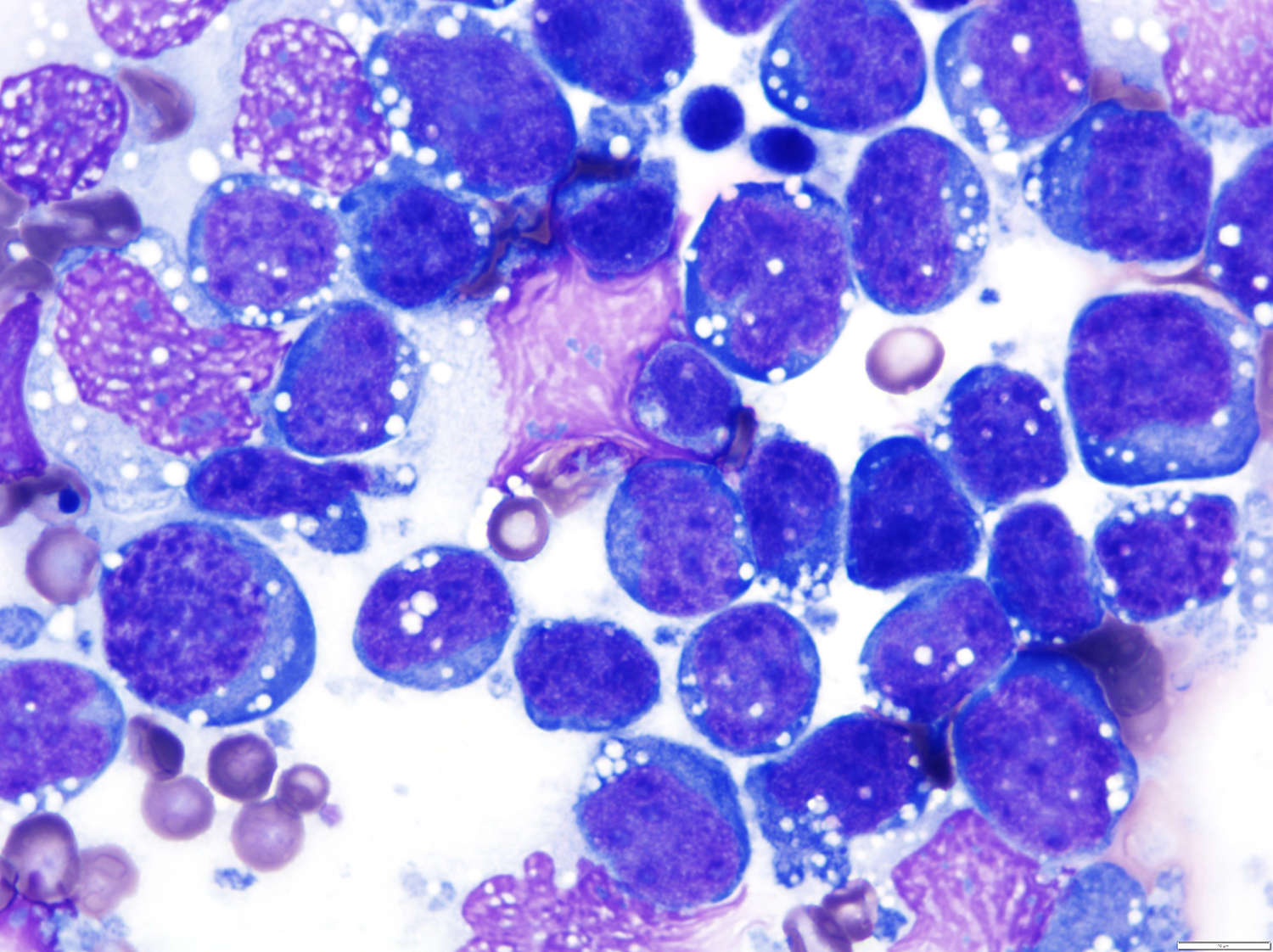
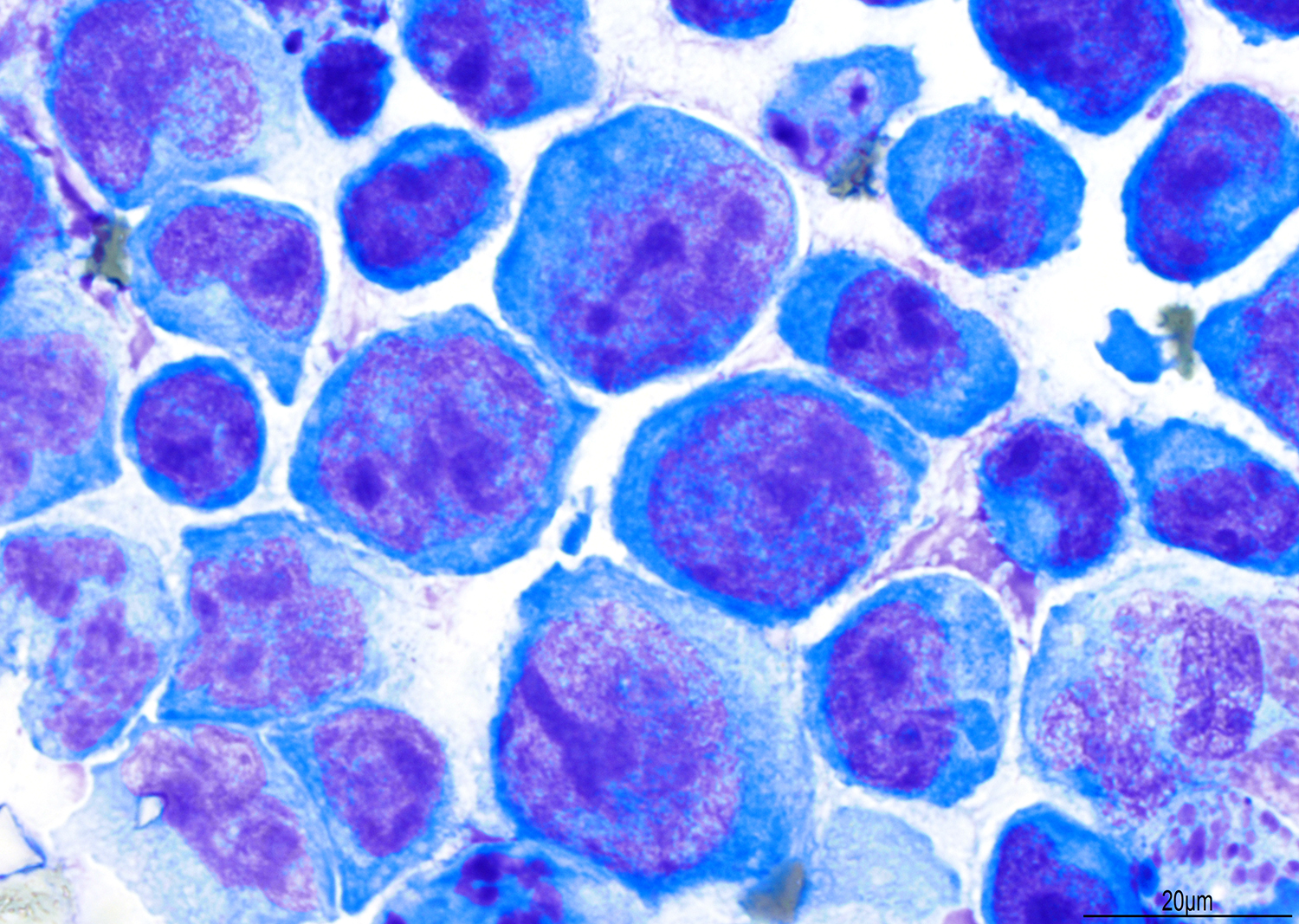
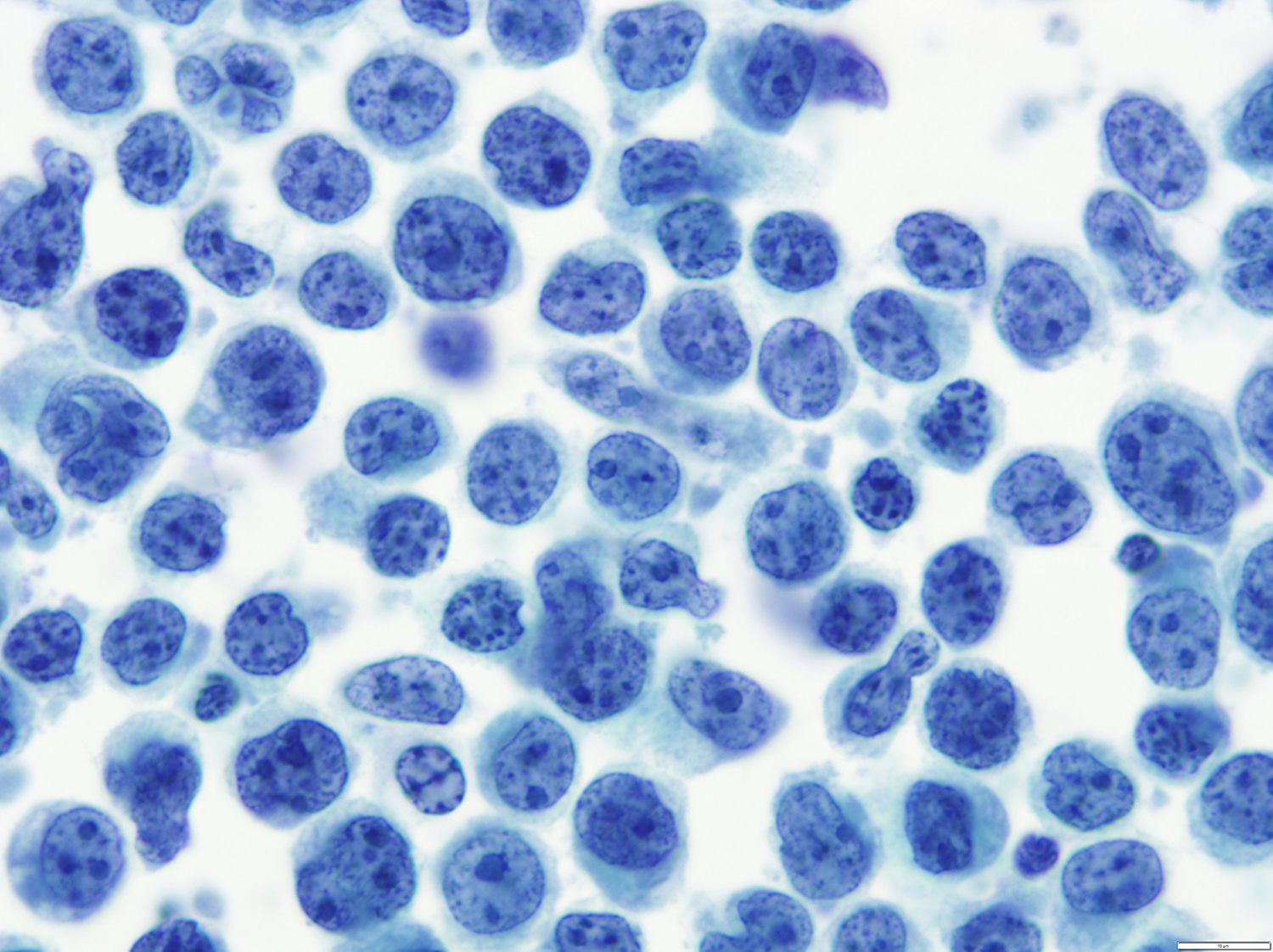
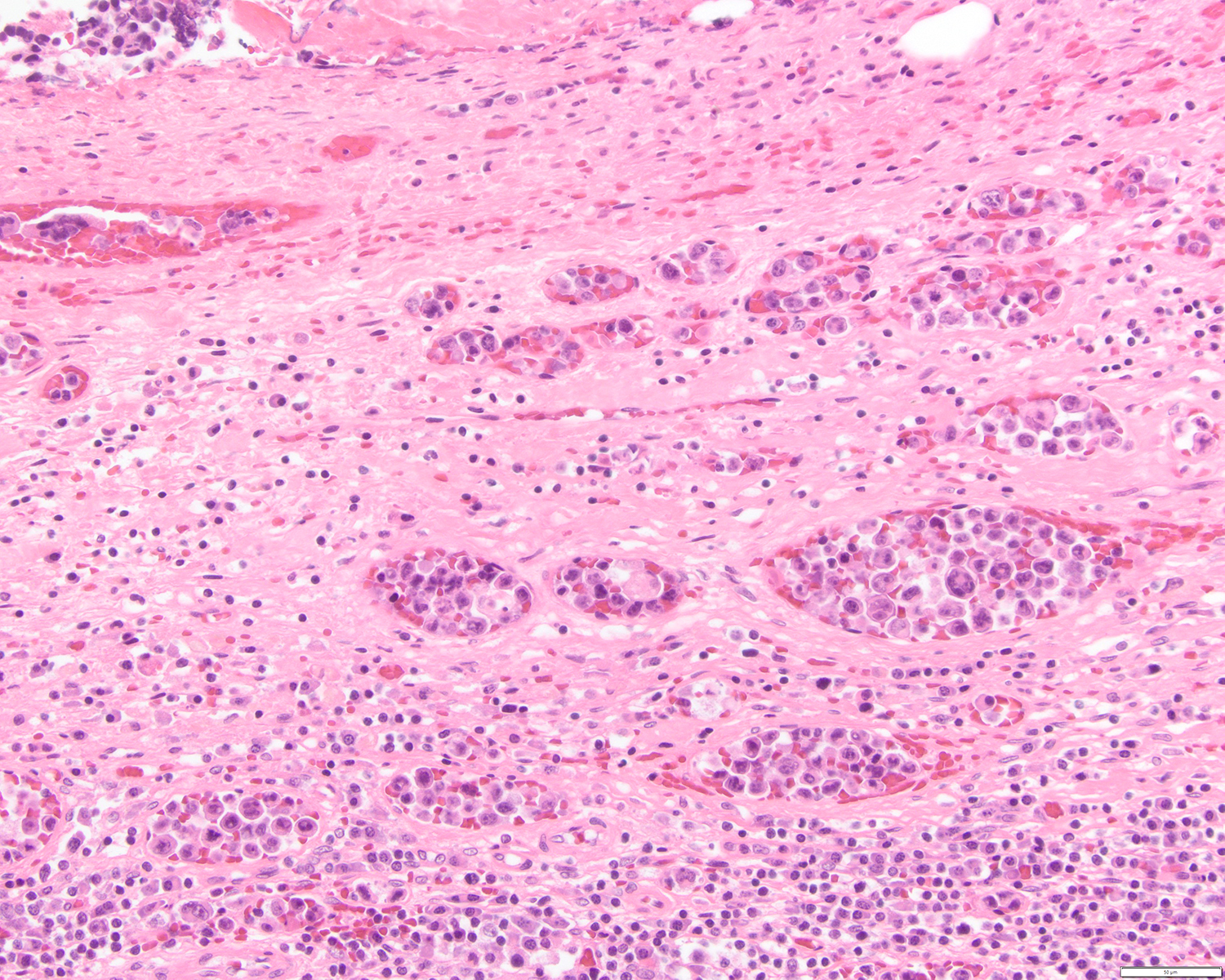
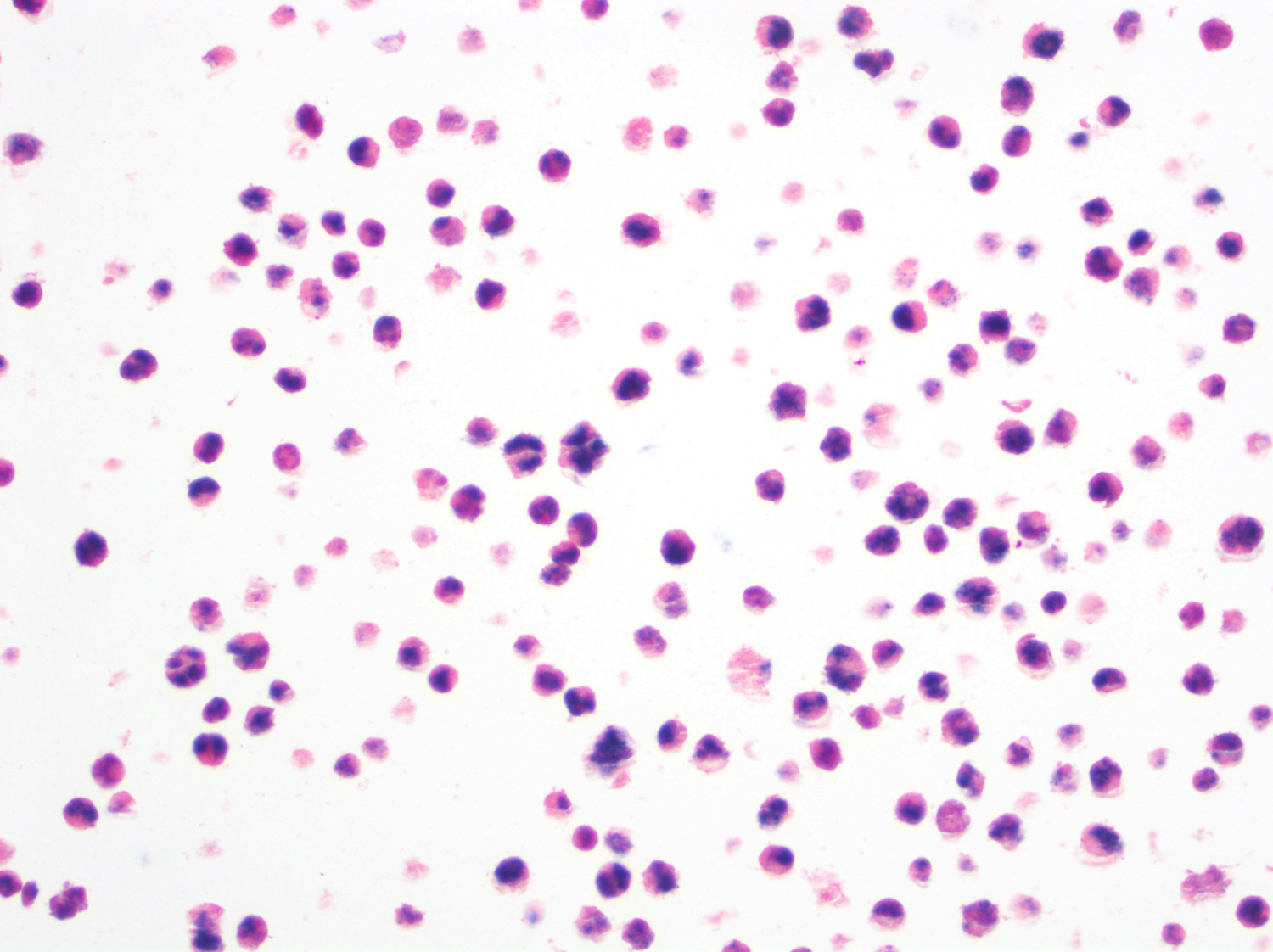
A 51 year old man with a previous medical history of human immunodeficiency virus (HIV) infection with poor follow up and irregular use of antiretroviral therapy was admitted to the emergency room with cough, dyspnea, chest pain and fever for the past 10 days. Peripheral blood work up revealed pancytopenia and a CT of the thorax showed notable pleural and pericardial effusion, slight pleural thickening and absence of parenchymal abnormalities or masses. A pleurocentesis was performed and a cytologic analysis showed the findings below.
Cytology and histopathology images:
What is your diagnosis?
Diagnosis: Primary effusion lymphoma (PEL)
Test questions (answer at the end):
1. Which of the following pair of viruses are associated with primary effusion lymphoma (PEL)?
A. EBV and HCV
B. EBV and HHV8
C. HHV8 and COVID-19
D. HHV8 and HPV
2. Which is the latency pattern of the Epstein-Barr virus found associated with primary effusion lymphoma (PEL)?
A. Latency Pattern 1: EBER(+), EBNA-1(+), LMP-1(-)
B. Latency Pattern 1: EBER(+), EBNA-1(+), LMP-1(+)
C. Latency Pattern 3: EBER(+), EBNA-2(-), LMP-1(+)
D. Latency Pattern 2: EBER(+), EBNA-2(-), LMP-1(+)
Stains
Discussion:
Primary Effusion Lymphoma (PEL) is a type of non-Hodgkin lymphoma that was first recognized as a distinct entity in the 2001 World Health Organization (WHO) classification of hematopoietic and lymphoid tissues, which predominantly presents with serous cavity effusions (Leuk Lymphoma 2013;54:1947, Leuk Lymphoma 2012;53:2378). It usually occurs in a setting of immunodeficiency and it is strongly associated with human herpesvirus 8 (HHV8) which is also called Kaposi sarcoma associated herpesvirus (KSHV) infection and is a frequent coinfection with Epstein-Barr virus (EBV) (Am J Hematol 2016;91:233, Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152, Am J Pathol 2014;184:618, Mod Pathol 2010;23:773).
The disease is rare and usually occurs in adult males (median age: 52 years, range: 27 to 86 years). In HIV+ patients, this type of lymphoma corresponds to < 5% of lymphomas, with a higher frequency of EBV coinfection when compared to other causes of immunodeficiency (Leuk Lymphoma 2012;53:2378, Am J Hematol 2016;91:233, Histopathology 2014;65:693, Mod Pathol 2010;23:773). Other diseases related to HHV8 infection, such as Kaposi sarcoma and multicentric Castleman’s disease, may be present at diagnosis (Mod Pathol 2017;30:745, Am J Surg Pathol 2012;36:1129).
After the HHV8 infection, several homologue viral genes are encoded, resulting in upregulation of anti-apoptotic pathways and, consequently, cellular proliferation (Arch Pathol Lab Med 2013;137:1152). The coinfection with EBV usually acts to block B cell differentiation due to the overexpression of activator B cell factor 1 (ABF1) and inhibitor of differentiation 2 (ID2) (Br J Haematol 2007;137:342). The clinical presentation of the disease is usually aggressive and includes two main forms: classical / cavitary and extracavitary. The cavitary form presents with effusion in pleura, pericardium, peritoneum and less likely in joint effusion, although mass lesions can be present (Am J Hematol 2016;91:233, Arch Pathol Lab Med 2013;137:1152). The extracavitary form, also known as solid variant, presents with only mass lesions and may occur in the gastrointestinal tract, spleen, central nervous system, liver, skin and lymph nodes. The clinical course is usually poor in both forms (Am J Hematol 2016;91:233, Am J Pathol 2014;184:618, Am J Surg Pathol 2012;36:1129).
The classic form is usually diagnosed in effusion preparations, which show a uniform proliferation of round to ovoid large cells with high mitotic activity and occasional necrosis (Histopathology 2014;65:693, Am J Surg Pathol 2012;36:1129). The neoplastic cells exhibit a reticular chromatin, round to irregular prominent nucleoli and abundant eosinophilic or basophilic cytoplasm with occasional vacuoles. A “jellyfish-like” nuclear morphology with prominent clear Golgi zone adjacent to nucleus is also noted. The morphology spectrum can include immunoblastic, plasmablastic and anaplastic variants (Am J Hematol 2016;91:233, Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152, Am J Surg Pathol 2012;36:1129, Blood 2003;101:4115).
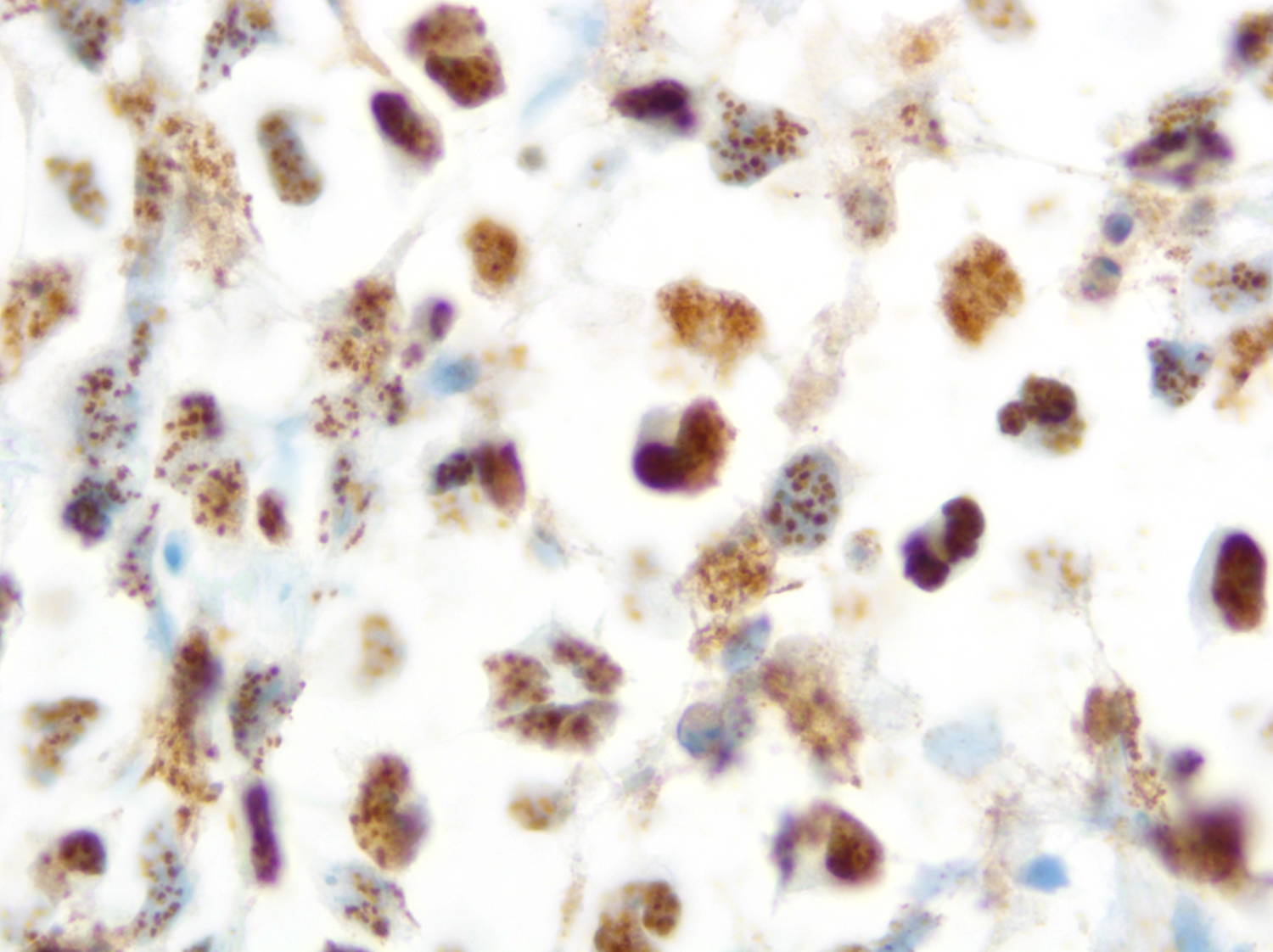
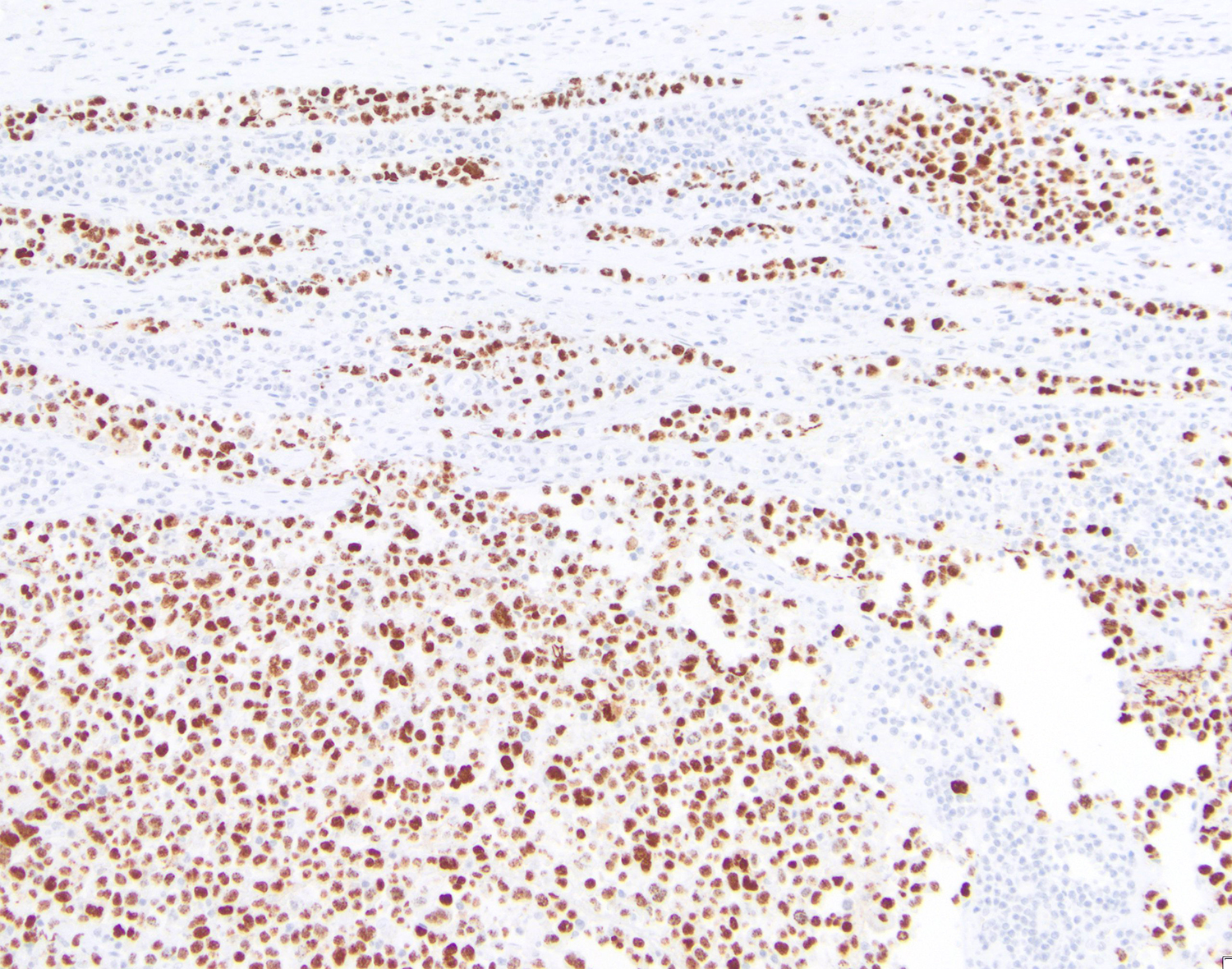
HHV8 is present in almost 100% of cases, playing a fundamental role in the diagnosis. Rare cases of HHV8 negative and EBV negative primary effusion lymphoma have been reported (Blood Adv 2020;4:4442, Am J Surg Pathol 2018;42:1607, Am J Surg Pathol 2013;37:241). These patients are usually HIV negative, elderly, with a previous medical history of HCV infection and have a better prognosis when compared with classic PEL. This led to the proposal of a new provisional entity named “HHV8 and EBV negative primary effusion based lymphoma” in “The International Consensus Classification of Mature Lymphoid Neoplasms” (Blood 2022 Jun 2 [Epub ahead of print]). The classic forms have plasmablastic differentiation and are positive for CD138, IRF4 / MUM1, CD38 and EMA. Pan B cell markers (e.g. CD20, CD79a, PAX5 and CD19), as well as CD10 and LMP1 are usually negative. A subset of cases can express CD30, BCL6, CD45 (LCA) and MUM1 [35,40,43,46]. EBER and EBNA1 are usually positive, demonstrating the latency pattern type 1 (Leuk Lymphoma 2013;54:1947, Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152, Am J Surg Pathol 2012;36:1129).
Complex karyotype is often present and a recurrent chromosomal abnormality has not been identified (Am J Hematol 2016;91:233). Monoclonal IGH gene rearrangement is a constant finding, while rearrangement of MYC, BCL2, BCL6 and CCND1 are absent. Somatic hypermutations of IGH variable regions are usually present (Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152). Secretome studies showed an increase of proteins involved in inflammation and immune response (HMGB1, GRAA and PCBP2), cell cycle (LEG1) and mRNA processing (ANM1) (Am J Pathol 2014;184:618).
Additional references
Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017, Swerdlow: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th Edition, 2017
Test question answers:
1. B. EBV and HHV8
2. A. Latency Pattern 1: EBER(+), EBNA-1(+), LMP-1(-)
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Drs. Mario Marques-Piubelli and Roberto Miranda, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA, for contributing this case and discussion and to Dr. Genevieve Crane, Cleveland Clinic, Cleveland, Ohio, USA, for reviewing the discussion.

Case of the Month #519
Clinical history:
A 51 year old man with a previous medical history of human immunodeficiency virus (HIV) infection with poor follow up and irregular use of antiretroviral therapy was admitted to the emergency room with cough, dyspnea, chest pain and fever for the past 10 days. Peripheral blood work up revealed pancytopenia and a CT of the thorax showed notable pleural and pericardial effusion, slight pleural thickening and absence of parenchymal abnormalities or masses. A pleurocentesis was performed and a cytologic analysis showed the findings below.
Cytology and histopathology images:
What is your diagnosis?
Click here for diagnosis, test question and discussion:
Diagnosis: Primary effusion lymphoma (PEL)
Test questions (answer at the end):
1. Which of the following pair of viruses are associated with primary effusion lymphoma (PEL)?
A. EBV and HCV
B. EBV and HHV8
C. HHV8 and COVID-19
D. HHV8 and HPV
2. Which is the latency pattern of the Epstein-Barr virus found associated with primary effusion lymphoma (PEL)?
A. Latency Pattern 1: EBER(+), EBNA-1(+), LMP-1(-)
B. Latency Pattern 1: EBER(+), EBNA-1(+), LMP-1(+)
C. Latency Pattern 3: EBER(+), EBNA-2(-), LMP-1(+)
D. Latency Pattern 2: EBER(+), EBNA-2(-), LMP-1(+)
Stains
Discussion:
Primary Effusion Lymphoma (PEL) is a type of non-Hodgkin lymphoma that was first recognized as a distinct entity in the 2001 World Health Organization (WHO) classification of hematopoietic and lymphoid tissues, which predominantly presents with serous cavity effusions (Leuk Lymphoma 2013;54:1947, Leuk Lymphoma 2012;53:2378). It usually occurs in a setting of immunodeficiency and it is strongly associated with human herpesvirus 8 (HHV8) which is also called Kaposi sarcoma associated herpesvirus (KSHV) infection and is a frequent coinfection with Epstein-Barr virus (EBV) (Am J Hematol 2016;91:233, Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152, Am J Pathol 2014;184:618, Mod Pathol 2010;23:773).
The disease is rare and usually occurs in adult males (median age: 52 years, range: 27 to 86 years). In HIV+ patients, this type of lymphoma corresponds to < 5% of lymphomas, with a higher frequency of EBV coinfection when compared to other causes of immunodeficiency (Leuk Lymphoma 2012;53:2378, Am J Hematol 2016;91:233, Histopathology 2014;65:693, Mod Pathol 2010;23:773). Other diseases related to HHV8 infection, such as Kaposi sarcoma and multicentric Castleman’s disease, may be present at diagnosis (Mod Pathol 2017;30:745, Am J Surg Pathol 2012;36:1129).
After the HHV8 infection, several homologue viral genes are encoded, resulting in upregulation of anti-apoptotic pathways and, consequently, cellular proliferation (Arch Pathol Lab Med 2013;137:1152). The coinfection with EBV usually acts to block B cell differentiation due to the overexpression of activator B cell factor 1 (ABF1) and inhibitor of differentiation 2 (ID2) (Br J Haematol 2007;137:342). The clinical presentation of the disease is usually aggressive and includes two main forms: classical / cavitary and extracavitary. The cavitary form presents with effusion in pleura, pericardium, peritoneum and less likely in joint effusion, although mass lesions can be present (Am J Hematol 2016;91:233, Arch Pathol Lab Med 2013;137:1152). The extracavitary form, also known as solid variant, presents with only mass lesions and may occur in the gastrointestinal tract, spleen, central nervous system, liver, skin and lymph nodes. The clinical course is usually poor in both forms (Am J Hematol 2016;91:233, Am J Pathol 2014;184:618, Am J Surg Pathol 2012;36:1129).
The classic form is usually diagnosed in effusion preparations, which show a uniform proliferation of round to ovoid large cells with high mitotic activity and occasional necrosis (Histopathology 2014;65:693, Am J Surg Pathol 2012;36:1129). The neoplastic cells exhibit a reticular chromatin, round to irregular prominent nucleoli and abundant eosinophilic or basophilic cytoplasm with occasional vacuoles. A “jellyfish-like” nuclear morphology with prominent clear Golgi zone adjacent to nucleus is also noted. The morphology spectrum can include immunoblastic, plasmablastic and anaplastic variants (Am J Hematol 2016;91:233, Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152, Am J Surg Pathol 2012;36:1129, Blood 2003;101:4115).
HHV8 is present in almost 100% of cases, playing a fundamental role in the diagnosis. Rare cases of HHV8 negative and EBV negative primary effusion lymphoma have been reported (Blood Adv 2020;4:4442, Am J Surg Pathol 2018;42:1607, Am J Surg Pathol 2013;37:241). These patients are usually HIV negative, elderly, with a previous medical history of HCV infection and have a better prognosis when compared with classic PEL. This led to the proposal of a new provisional entity named “HHV8 and EBV negative primary effusion based lymphoma” in “The International Consensus Classification of Mature Lymphoid Neoplasms” (Blood 2022 Jun 2 [Epub ahead of print]). The classic forms have plasmablastic differentiation and are positive for CD138, IRF4 / MUM1, CD38 and EMA. Pan B cell markers (e.g. CD20, CD79a, PAX5 and CD19), as well as CD10 and LMP1 are usually negative. A subset of cases can express CD30, BCL6, CD45 (LCA) and MUM1 [35,40,43,46]. EBER and EBNA1 are usually positive, demonstrating the latency pattern type 1 (Leuk Lymphoma 2013;54:1947, Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152, Am J Surg Pathol 2012;36:1129).
Complex karyotype is often present and a recurrent chromosomal abnormality has not been identified (Am J Hematol 2016;91:233). Monoclonal IGH gene rearrangement is a constant finding, while rearrangement of MYC, BCL2, BCL6 and CCND1 are absent. Somatic hypermutations of IGH variable regions are usually present (Histopathology 2014;65:693, Arch Pathol Lab Med 2013;137:1152). Secretome studies showed an increase of proteins involved in inflammation and immune response (HMGB1, GRAA and PCBP2), cell cycle (LEG1) and mRNA processing (ANM1) (Am J Pathol 2014;184:618).
Additional references
Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017, Swerdlow: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th Edition, 2017
Test question answers:
1. B. EBV and HHV8
2. A. Latency Pattern 1: EBER(+), EBNA-1(+), LMP-1(-)