15 July 2021 - Case of the Month #505
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Dr. Borislav Alexiev, Northwestern University Feinberg School of Medicine, Chicago, Illinois (USA), for contributing this case and writing the discussion and to Dr. Jose Mantilla, University of Washington, Seattle, Washington (USA), for reviewing the discussion.

Advertisement
Case of the Month #505
Clinical history:
A 68 year old woman presented with a 8.7 x 8.4 x 6.7 cm, hypervascular but centrally hypoenhancing, left retroperitoneal soft tissue mass anterior to the psoas, causing anterior medial displacement of the colon and small bowel.
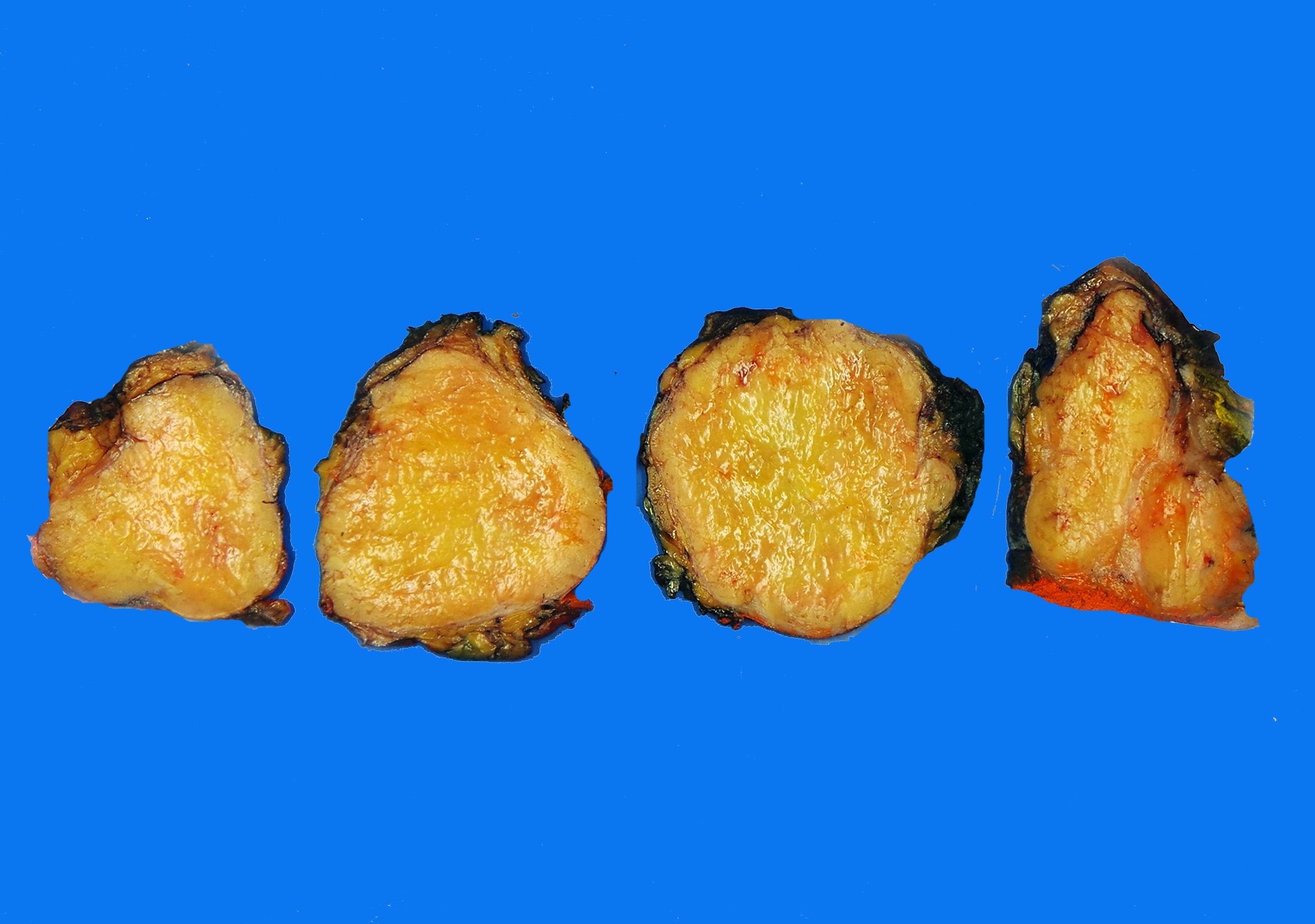
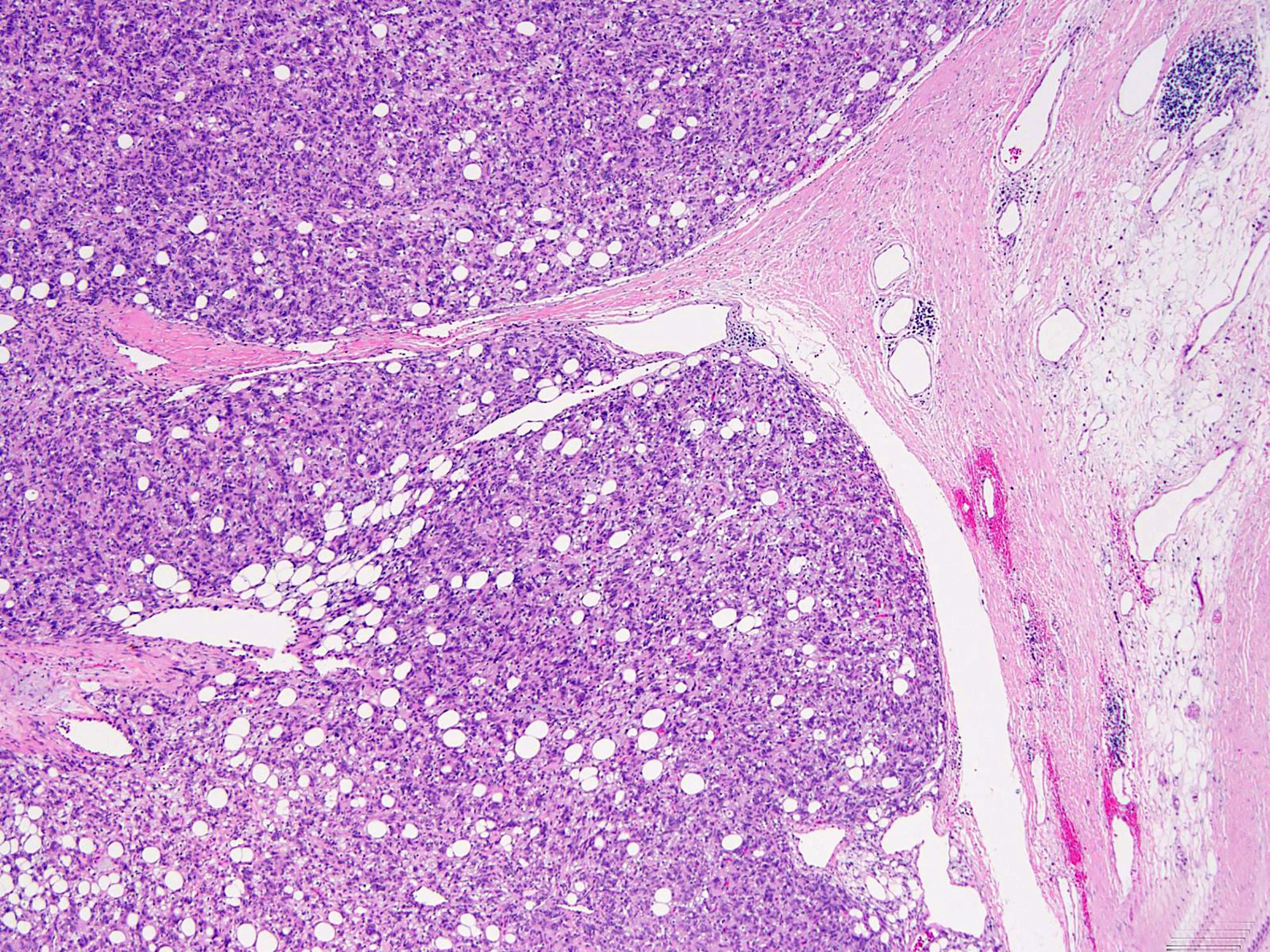
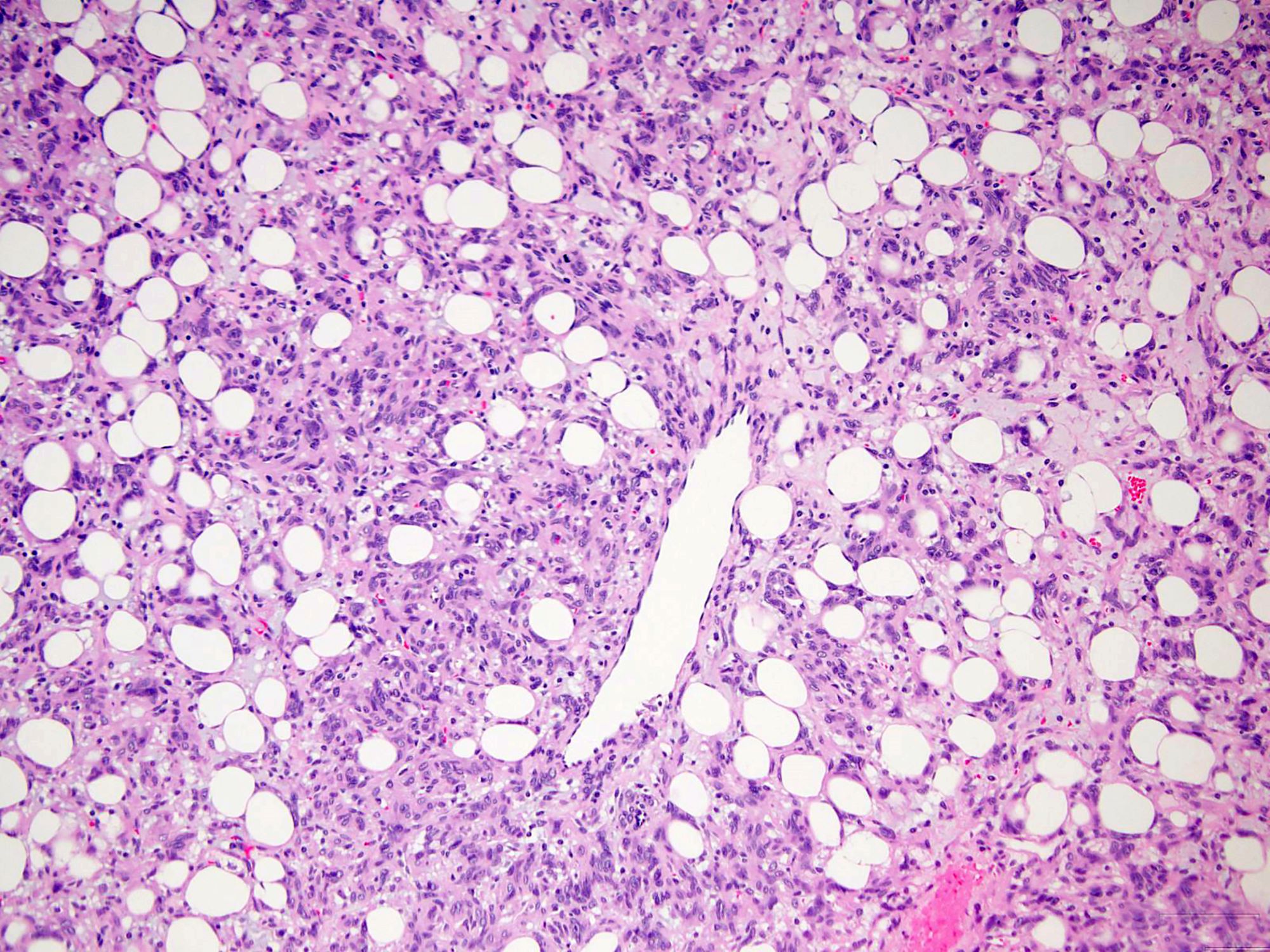
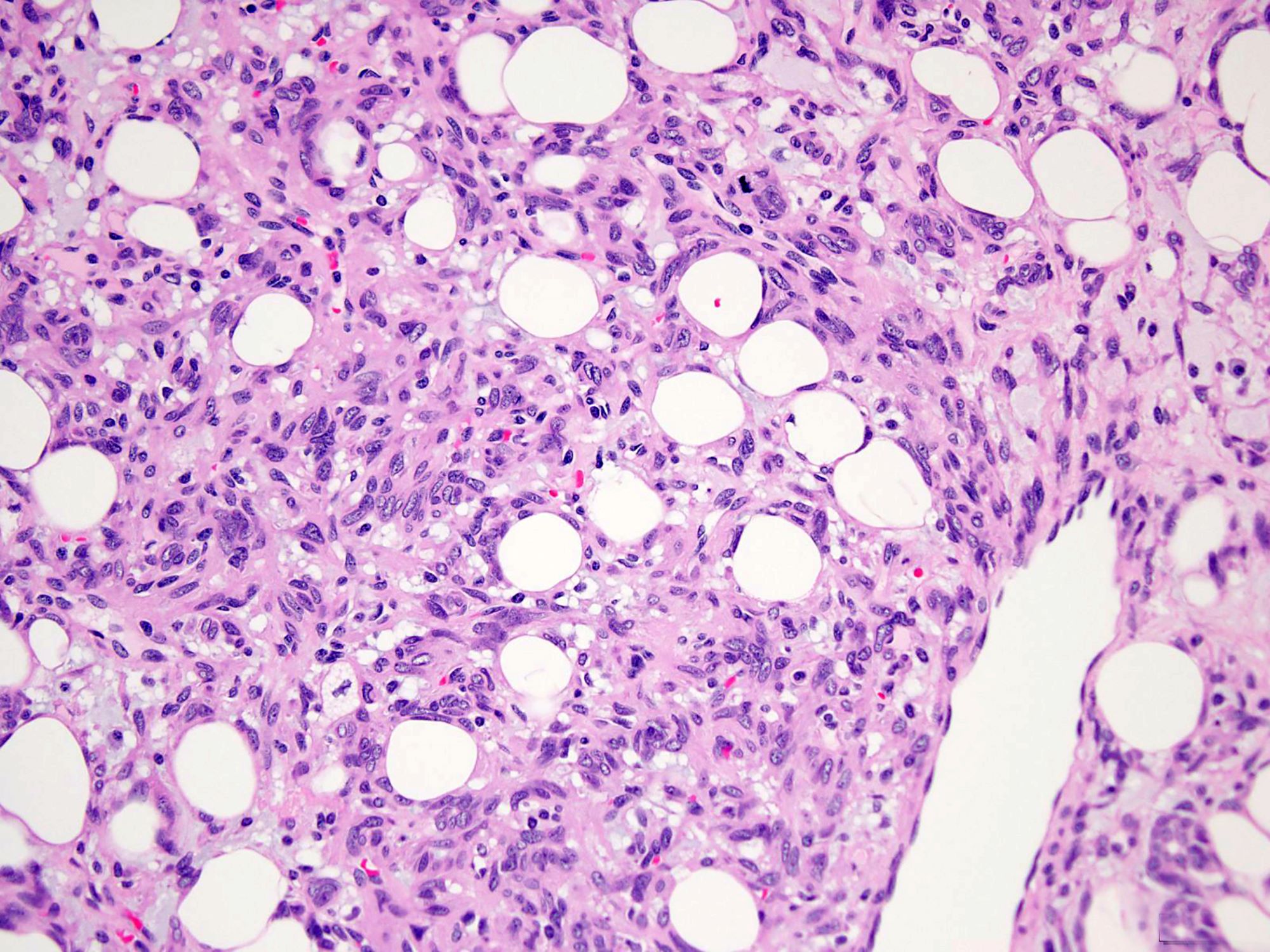
Gross pathology and histopathology images:
What is your diagnosis?
Diagnosis: Fat forming solitary fibrous tumor
Test question (answer at the end):
Which of the following is true about the majority of fat forming solitary fibrous tumor cases?
A. All tumors show reactivity for CD34
B. Fat forming solitary fibrous tumors usually occur in younger adults, predominantly in females
C. Multivacuolated lipoblasts are more common in the malignant subset
D. The imaging findings of fat forming solitary fibrous tumor are specific
E. The mediastinum is the most commonly affected site
Stains:
Discussion:
Fat forming solitary fibrous tumor, previously known as lipomatous hemangiopericytoma, is a rare variant of solitary fibrous tumor (SFT) (Korean J Pathol 2014;48:69). This entity was first described by Nielsen et al in 1995 (Am J Surg Pathol 1995;19:748).
Fat forming SFT usually arises in middle aged adult males (Am J Surg Pathol 1999;23:1201) and has a wide intra and extrathoracic anatomical distribution but the deep soft tissues of the retroperitoneum and thigh are the most commonly affected sites (Br J Radiol 2011;84:e203, In Vivo 2018;32:649). The patients usually present with a slowly enlarging mass of variable duration (Arch Pathol Lab Med 1999;123:941).
Fat forming SFT often presents as a heterogeneous lesion, with solid enhancing regions and scattered low attenuating adipose areas on CT examination. The adipose areas show high signal intensity on T1 weighted MRI that decreases in intensity with fat suppression sequences. Benign and malignant SFT cannot be differentiated based only on imaging findings (J Radiol Case Rep 2013;7:1).
Fat forming SFTs are grossly well delineated and solid. On cut sections, the tumors are tan-white and rubbery and have admixed, soft, yellow lipid rich foci (Arch Pathol Lab Med 1999;123:941, Am J Surg Pathol 1995;19:748).
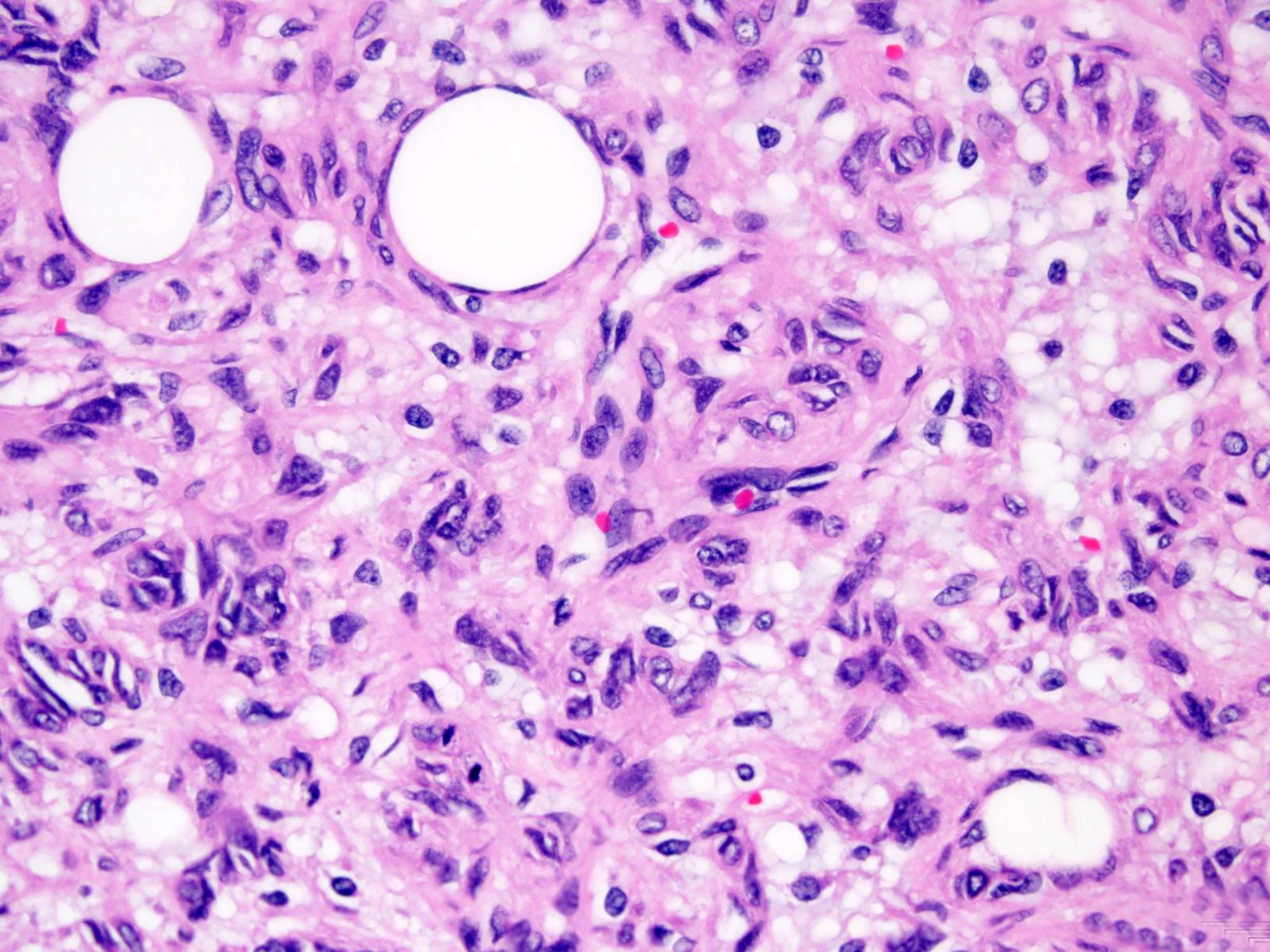
Histologically, the lesions exhibit a lobular growth pattern and are composed of cellular nodules with the classic appearance of SFT, patternless distribution of both oval and spindle shaped cells with uniform spindled nuclei and scant cytoplasm in a variably collagenous stroma and branched, often thick walled, hemangiopericytoma-like vessels (Ann Diagn Pathol 2018;34:142) admixed with adipocyte rich lobules (Arch Pathol Lab Med 1999;123:941). Mitotic figures are infrequent. Some cases may show moderate nuclear atypia. Mature fat varies in amount but usually occupies approximately one quarter to three quarters of the area of tumor (Am J Surg Pathol 1999;23:1201). The majority of adipocytes are uniform, round, univacuolar cells with peripherally located, compressed nuclei. Variation in adipocyte size and multivacuolated small cells with nuclear scalloping (lipoblast-like cells) may be evident focally (Arch Pathol Lab Med 1999;123:941).
Malignant fat forming SFTs exhibit at least focal hypercellularity, mitoses > 4/10 high power fields, at least moderate atypia and necrosis. Some tumors contain only mature adipose tissue, whereas others contain multivacuolated lipoblasts and may have areas resembling atypical lipomatous tumor (ALT) (Am J Surg Pathol 2011;35:1177). The presence of lipoblasts or ALT-like areas, although described in some benign examples of fat forming SFT, seems much more common in the malignant subset and may prompt a careful search for morphologic evidence of malignancy in any case of fat forming SFT (Am J Surg Pathol 2011;35:1177).
Dedifferentiated fat forming SFTs have areas of conventional SFT admixed with adipocyte rich lobules adjacent to areas resembling a high grade sarcoma (Arch Pathol Lab Med 2018;142:761). The dedifferentiated areas are typically diffusely hypercellular and lack the morphologic features of conventional SFT. The high grade component can undergo heterologous rhabdomyosarcomatous, osteosarcomatous and chondrosarcomatous differentiation (Arch Pathol Lab Med 2018;142:761, Am J Surg Pathol 2012;36:1202, Ann Diagn Pathol 2013;17:457, Virchows Arch 2014;465:615).
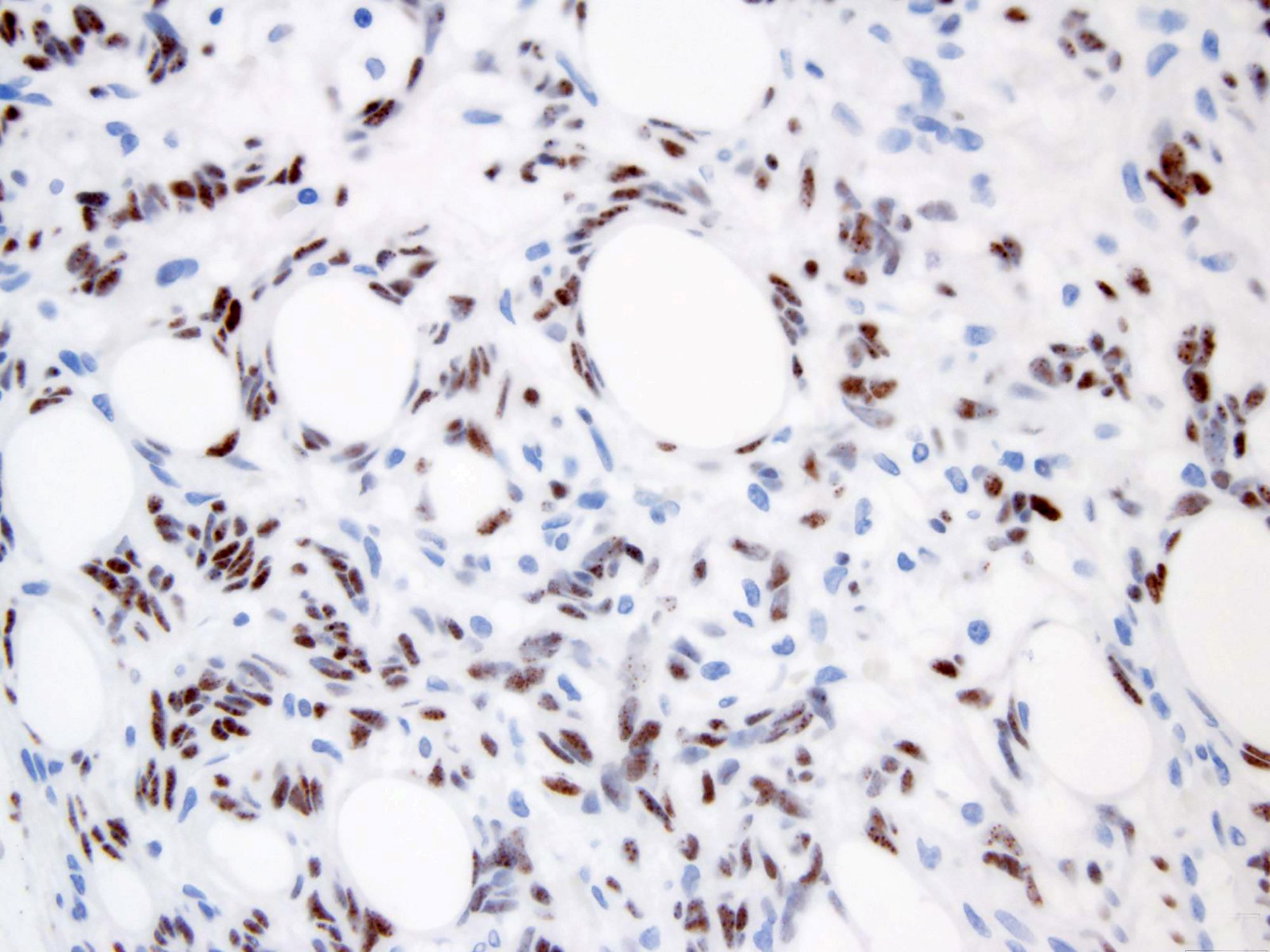
STAT6 is a useful diagnostic immunohistochemical marker for SFT (Pathol Res Pract 2017;213:1404). Mature adipocytes are positive for S100 protein (Int J Clin Exp Pathol 2015;8:8632).
Little is known about the underlying molecular changes in fat forming SFT. In conventional SFTs, many different breakpoints and fusions between NAB2 and STAT6 have been identified (Am J Pathol 2014;184:1209). The most common fusion type, NAB2ex4-STAT6ex2/3, correlates with an intrathoracic location and less aggressive phenotype. On the other hand, tumors with NAB2ex6-STAT6ex16/17 fusion type tend to be hypercellular, deep seated tumors with high mitotic activity and have frequent recurrences in younger patients (Hum Pathol 2015;46:347, Cancer Med 2016;5:159, Mod Pathol 2015;28:1324).
Differential diagnosis: The differential diagnosis includes spindle cell lipoma, myolipoma of soft tissue, angiomyolipoma (AML), atypical lipomatous tumor / well differentiated liposarcoma, dedifferentiated liposarcoma, synovial sarcoma and dermatofibrosarcoma protuberans (DFSP).
Spindle cell lipoma may show similar morphology. Differentiating features include loss of nuclear RB1 protein expression in spindle cell lipoma (Am J Surg Pathol 2012;36:1119, Virchows Arch 2021 Jan 3 [Epub ahead of print]) and STAT6 positivity in fat forming SFT.
Myolipoma of soft tissue is composed of an intimate admixture of mature fat and spindled cells, in variable proportions. Stains for desmin, h-caldesmon and smooth muscle actin confirm smooth muscle differentiation. HMGA2 is identified in 60% of cases and ER and PR are often positive (Am J Surg Pathol 1991;15:121, Am J Surg Pathol 2017;41:153, Histopathology 1996;29:184, Pathol Int 2004;54:460). STAT6 is negative.
Classic AML demonstrates admixture of fatty tissue, thick walled blood vessels and smooth muscle, while epithelioid AML is composed mainly of epithelioid cells (J Egypt Natl Canc Inst 2013;25:125). AMLs show consistent coexpression of melanocytic (HMB45, MelanA) and smooth muscle markers (muscle specific actin, calponin, smooth muscle actin) (Arch Pathol Lab Med 2007;131:122) and ER and PR are often positive. STAT6 is negative.
ALT / well differentiated liposarcoma and dedifferentiated liposarcoma may enter the differential diagnosis of fat forming SFT. The fat component in fat forming SFT is most often mature adipose tissue without scattered intervening atypical cells as seen in ALT / well differentiated liposarcoma (Am J Surg Pathol 2011;35:1177). Adipocyte size variation is not a helpful parameter in this context, as adipocytes with smaller cytoplasmic vacuoles, sometimes to the degree of mimicking lipoblasts, are not uncommonly seen at the interface between fatty and fibrous components in fat forming SFT (Am J Surg Pathol 2011;35:1177). Dedifferentiated liposarcoma can sometimes show SFT-like morphology and focal weak nuclear expression of STAT6, further complicating the differential diagnosis. However, demonstration of positivity for MDM2 and CDK4 by immunohistochemistry or MDM2 amplification by FISH can help confirm the diagnosis of ALT / well differentiated liposarcoma and dedifferentiated liposarcoma (Mod Pathol 2014;27:390).
Synovial sarcoma may show similar morphology, including hemangiopericytoma-like vessels and fat component (Asian J Androl 2015;17:343). Immunohistochemistry can distinguish the two entities: synovial sarcoma is often positive for keratin and negative for STAT6 (Appl Immunohistochem Mol Morphol 2020;28:311). The detection of SYT-SSX fusion has been established as a molecular diagnostic test for this tumor (Mod Pathol 2007;20:482).
DFSP is a dermal tumor composed mainly of spindle cells that are radially oriented in a storiform or cartwheel pattern. Microscopic extension into subcutaneous fat with honeycomb entrapment of fat is characteristic (Am J Surg Pathol 1998;22:576). Foci with prominent hemangiopericytoma-like branching vessels within the tumor may be identified (Am J Surg Pathol 1998;22:576). An underlying genetic alteration, t(17;22)(q22;q13), resulting in a COL1A1-PDGFB fusion, has been linked to this neoplasm. DFSP typically exhibits diffuse expression of CD34 (Appl Immunohistochem Mol Morphol 2017;25:586). No STAT6 expression is seen in DFSP (Appl Immunohistochem Mol Morphol 2020;28:311).
Because even benign appearing SFTs can be locally recurrent and metastatic, wide resection is recommended for both benign and malignant SFTs. Large tumor size (> 10 cm), inadequate resection margins, malignant histologic features, dedifferentiation and tumor location within the abdomen / pelvis are associated with adverse oncologic outcomes (Am J Clin Oncol 2018;41:687). Radiotherapy may be used for preoperative tumor shrinkage or as adjuvant therapy in patients with malignant disease or incomplete surgical margins. Chemotherapy with molecular targeted therapies has produced promising results (Am J Clin Oncol 2018;41:687).
Test question answer:
C. Multivacuolated lipoblasts are more common in the malignant subset
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Dr. Borislav Alexiev, Northwestern University Feinberg School of Medicine, Chicago, Illinois (USA), for contributing this case and writing the discussion and to Dr. Jose Mantilla, University of Washington, Seattle, Washington (USA), for reviewing the discussion.

Advertisement
Case of the Month #505
Clinical history:
A 68 year old woman presented with a 8.7 x 8.4 x 6.7 cm, hypervascular but centrally hypoenhancing, left retroperitoneal soft tissue mass anterior to the psoas, causing anterior medial displacement of the colon and small bowel.
Gross pathology and histopathology images:
What is your diagnosis?
Click here for diagnosis, test question and discussion:
Diagnosis: Fat forming solitary fibrous tumor
Test question (answer at the end):
Which of the following is true about the majority of fat forming solitary fibrous tumor cases?
A. All tumors show reactivity for CD34
B. Fat forming solitary fibrous tumors usually occur in younger adults, predominantly in females
C. Multivacuolated lipoblasts are more common in the malignant subset
D. The imaging findings of fat forming solitary fibrous tumor are specific
E. The mediastinum is the most commonly affected site
Stains:
Discussion:
Fat forming solitary fibrous tumor, previously known as lipomatous hemangiopericytoma, is a rare variant of solitary fibrous tumor (SFT) (Korean J Pathol 2014;48:69). This entity was first described by Nielsen et al in 1995 (Am J Surg Pathol 1995;19:748).
Fat forming SFT usually arises in middle aged adult males (Am J Surg Pathol 1999;23:1201) and has a wide intra and extrathoracic anatomical distribution but the deep soft tissues of the retroperitoneum and thigh are the most commonly affected sites (Br J Radiol 2011;84:e203, In Vivo 2018;32:649). The patients usually present with a slowly enlarging mass of variable duration (Arch Pathol Lab Med 1999;123:941).
Fat forming SFT often presents as a heterogeneous lesion, with solid enhancing regions and scattered low attenuating adipose areas on CT examination. The adipose areas show high signal intensity on T1 weighted MRI that decreases in intensity with fat suppression sequences. Benign and malignant SFT cannot be differentiated based only on imaging findings (J Radiol Case Rep 2013;7:1).
Fat forming SFTs are grossly well delineated and solid. On cut sections, the tumors are tan-white and rubbery and have admixed, soft, yellow lipid rich foci (Arch Pathol Lab Med 1999;123:941, Am J Surg Pathol 1995;19:748).
Histologically, the lesions exhibit a lobular growth pattern and are composed of cellular nodules with the classic appearance of SFT, patternless distribution of both oval and spindle shaped cells with uniform spindled nuclei and scant cytoplasm in a variably collagenous stroma and branched, often thick walled, hemangiopericytoma-like vessels (Ann Diagn Pathol 2018;34:142) admixed with adipocyte rich lobules (Arch Pathol Lab Med 1999;123:941). Mitotic figures are infrequent. Some cases may show moderate nuclear atypia. Mature fat varies in amount but usually occupies approximately one quarter to three quarters of the area of tumor (Am J Surg Pathol 1999;23:1201). The majority of adipocytes are uniform, round, univacuolar cells with peripherally located, compressed nuclei. Variation in adipocyte size and multivacuolated small cells with nuclear scalloping (lipoblast-like cells) may be evident focally (Arch Pathol Lab Med 1999;123:941).
Malignant fat forming SFTs exhibit at least focal hypercellularity, mitoses > 4/10 high power fields, at least moderate atypia and necrosis. Some tumors contain only mature adipose tissue, whereas others contain multivacuolated lipoblasts and may have areas resembling atypical lipomatous tumor (ALT) (Am J Surg Pathol 2011;35:1177). The presence of lipoblasts or ALT-like areas, although described in some benign examples of fat forming SFT, seems much more common in the malignant subset and may prompt a careful search for morphologic evidence of malignancy in any case of fat forming SFT (Am J Surg Pathol 2011;35:1177).
Dedifferentiated fat forming SFTs have areas of conventional SFT admixed with adipocyte rich lobules adjacent to areas resembling a high grade sarcoma (Arch Pathol Lab Med 2018;142:761). The dedifferentiated areas are typically diffusely hypercellular and lack the morphologic features of conventional SFT. The high grade component can undergo heterologous rhabdomyosarcomatous, osteosarcomatous and chondrosarcomatous differentiation (Arch Pathol Lab Med 2018;142:761, Am J Surg Pathol 2012;36:1202, Ann Diagn Pathol 2013;17:457, Virchows Arch 2014;465:615).
STAT6 is a useful diagnostic immunohistochemical marker for SFT (Pathol Res Pract 2017;213:1404). Mature adipocytes are positive for S100 protein (Int J Clin Exp Pathol 2015;8:8632).
Little is known about the underlying molecular changes in fat forming SFT. In conventional SFTs, many different breakpoints and fusions between NAB2 and STAT6 have been identified (Am J Pathol 2014;184:1209). The most common fusion type, NAB2ex4-STAT6ex2/3, correlates with an intrathoracic location and less aggressive phenotype. On the other hand, tumors with NAB2ex6-STAT6ex16/17 fusion type tend to be hypercellular, deep seated tumors with high mitotic activity and have frequent recurrences in younger patients (Hum Pathol 2015;46:347, Cancer Med 2016;5:159, Mod Pathol 2015;28:1324).
Differential diagnosis: The differential diagnosis includes spindle cell lipoma, myolipoma of soft tissue, angiomyolipoma (AML), atypical lipomatous tumor / well differentiated liposarcoma, dedifferentiated liposarcoma, synovial sarcoma and dermatofibrosarcoma protuberans (DFSP).
Spindle cell lipoma may show similar morphology. Differentiating features include loss of nuclear RB1 protein expression in spindle cell lipoma (Am J Surg Pathol 2012;36:1119, Virchows Arch 2021 Jan 3 [Epub ahead of print]) and STAT6 positivity in fat forming SFT.
Myolipoma of soft tissue is composed of an intimate admixture of mature fat and spindled cells, in variable proportions. Stains for desmin, h-caldesmon and smooth muscle actin confirm smooth muscle differentiation. HMGA2 is identified in 60% of cases and ER and PR are often positive (Am J Surg Pathol 1991;15:121, Am J Surg Pathol 2017;41:153, Histopathology 1996;29:184, Pathol Int 2004;54:460). STAT6 is negative.
Classic AML demonstrates admixture of fatty tissue, thick walled blood vessels and smooth muscle, while epithelioid AML is composed mainly of epithelioid cells (J Egypt Natl Canc Inst 2013;25:125). AMLs show consistent coexpression of melanocytic (HMB45, MelanA) and smooth muscle markers (muscle specific actin, calponin, smooth muscle actin) (Arch Pathol Lab Med 2007;131:122) and ER and PR are often positive. STAT6 is negative.
ALT / well differentiated liposarcoma and dedifferentiated liposarcoma may enter the differential diagnosis of fat forming SFT. The fat component in fat forming SFT is most often mature adipose tissue without scattered intervening atypical cells as seen in ALT / well differentiated liposarcoma (Am J Surg Pathol 2011;35:1177). Adipocyte size variation is not a helpful parameter in this context, as adipocytes with smaller cytoplasmic vacuoles, sometimes to the degree of mimicking lipoblasts, are not uncommonly seen at the interface between fatty and fibrous components in fat forming SFT (Am J Surg Pathol 2011;35:1177). Dedifferentiated liposarcoma can sometimes show SFT-like morphology and focal weak nuclear expression of STAT6, further complicating the differential diagnosis. However, demonstration of positivity for MDM2 and CDK4 by immunohistochemistry or MDM2 amplification by FISH can help confirm the diagnosis of ALT / well differentiated liposarcoma and dedifferentiated liposarcoma (Mod Pathol 2014;27:390).
Synovial sarcoma may show similar morphology, including hemangiopericytoma-like vessels and fat component (Asian J Androl 2015;17:343). Immunohistochemistry can distinguish the two entities: synovial sarcoma is often positive for keratin and negative for STAT6 (Appl Immunohistochem Mol Morphol 2020;28:311). The detection of SYT-SSX fusion has been established as a molecular diagnostic test for this tumor (Mod Pathol 2007;20:482).
DFSP is a dermal tumor composed mainly of spindle cells that are radially oriented in a storiform or cartwheel pattern. Microscopic extension into subcutaneous fat with honeycomb entrapment of fat is characteristic (Am J Surg Pathol 1998;22:576). Foci with prominent hemangiopericytoma-like branching vessels within the tumor may be identified (Am J Surg Pathol 1998;22:576). An underlying genetic alteration, t(17;22)(q22;q13), resulting in a COL1A1-PDGFB fusion, has been linked to this neoplasm. DFSP typically exhibits diffuse expression of CD34 (Appl Immunohistochem Mol Morphol 2017;25:586). No STAT6 expression is seen in DFSP (Appl Immunohistochem Mol Morphol 2020;28:311).
Because even benign appearing SFTs can be locally recurrent and metastatic, wide resection is recommended for both benign and malignant SFTs. Large tumor size (> 10 cm), inadequate resection margins, malignant histologic features, dedifferentiation and tumor location within the abdomen / pelvis are associated with adverse oncologic outcomes (Am J Clin Oncol 2018;41:687). Radiotherapy may be used for preoperative tumor shrinkage or as adjuvant therapy in patients with malignant disease or incomplete surgical margins. Chemotherapy with molecular targeted therapies has produced promising results (Am J Clin Oncol 2018;41:687).
Test question answer:
C. Multivacuolated lipoblasts are more common in the malignant subset