14 January 2021 - Case of the Month #499
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Dr. Patricia Tsang, Geisinger Commonwealth School of Medicine, Wilkes Barre, Pennsylvania (USA) for contributing this case and to Dr. Morgan Hrones, Yale New Haven Medical Center, New Haven, Connecticut (USA) for writing the discussion. The discussion was reviewed by Dr. Tsang.

Advertisement
Case of the Month #499
Clinical history:
A 74 year old man presented with weight loss, ascites, liver lesions and a 5 cm growing soft tissue mass in the abdominal wall.
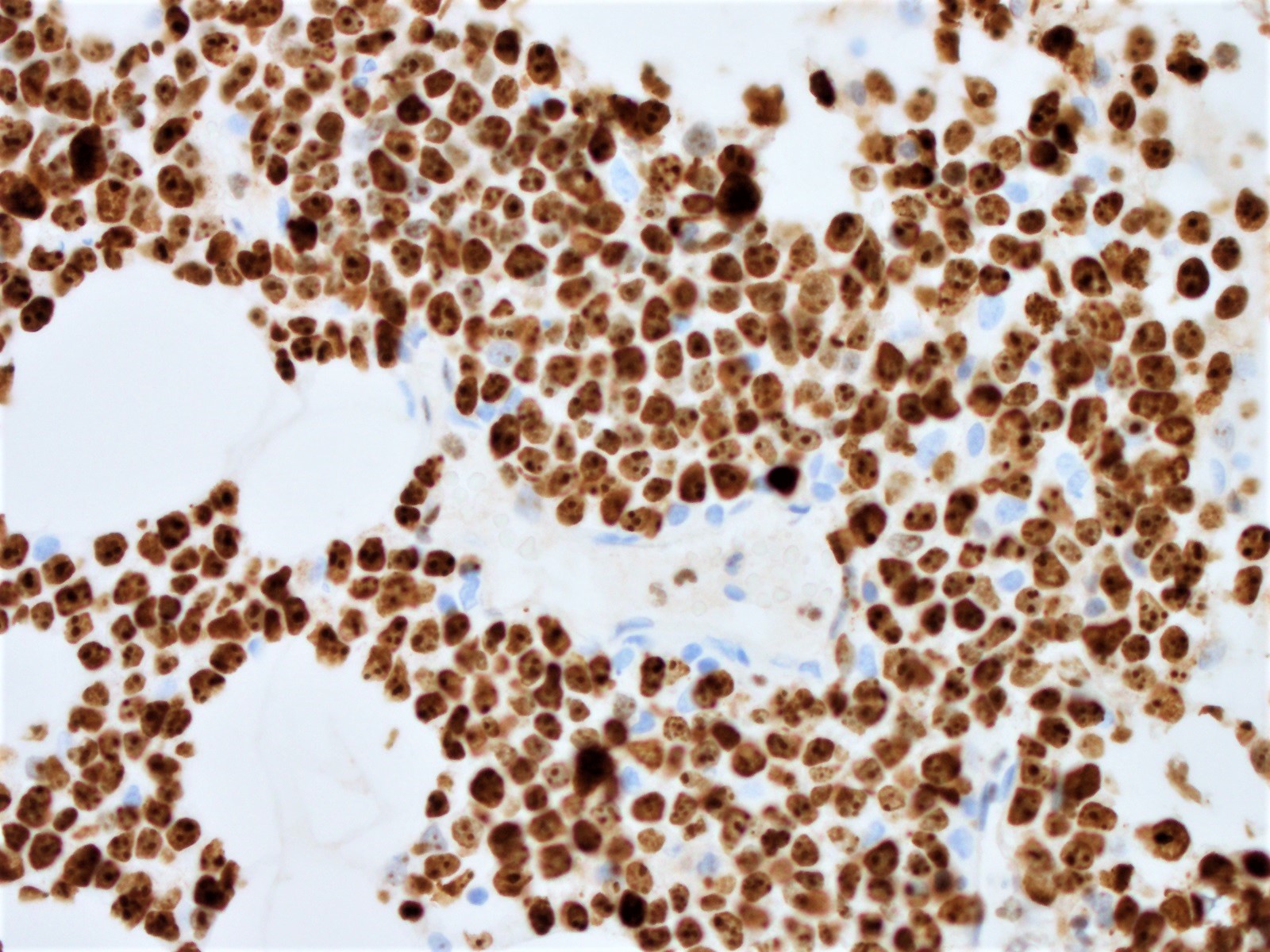
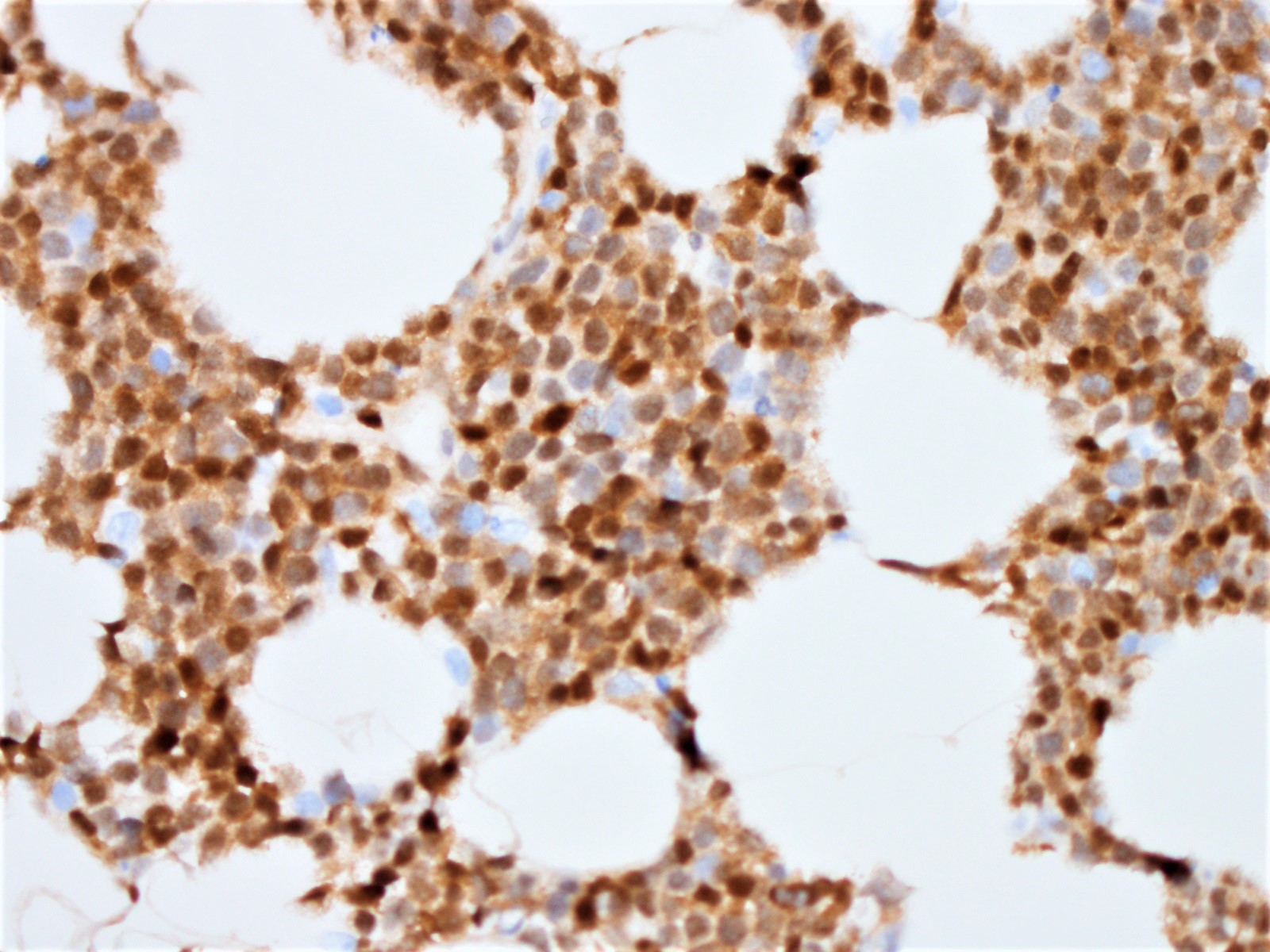
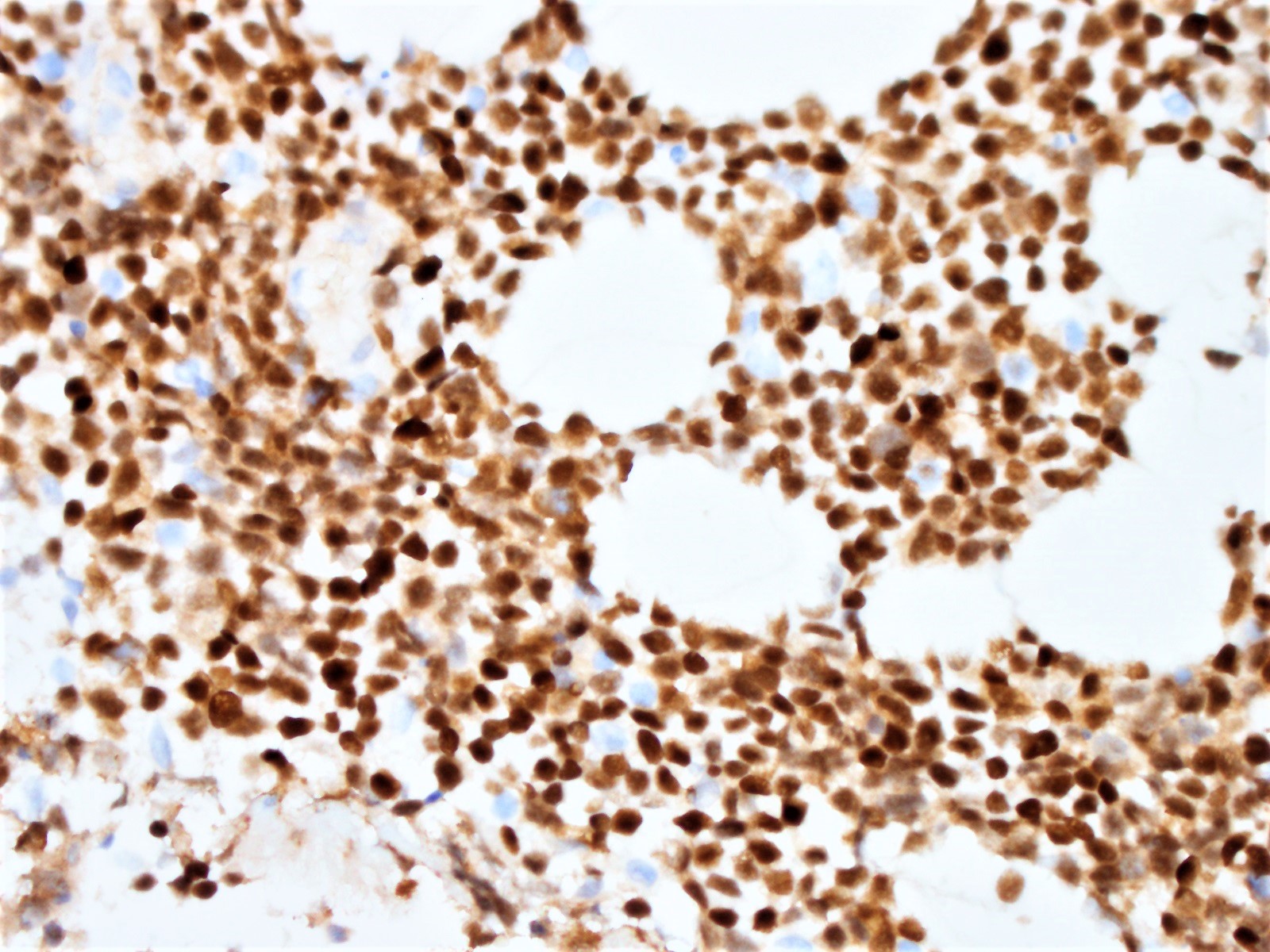
Histopathology images:
What is your diagnosis?
Diagnosis: Blastoid variant of mantle cell lymphoma (CD5 negative variant)
Test question (answer at the end):
Which of the following is true about the majority of blastoid mantle cell lymphoma cases?
A. Association with EBV
B. Association with t(8;14)
C. Clinical course is similar to that of leukemic non-nodal mantle cell leukemia subtype
D. Clonal rearrangement of T cell receptor gamma gene
E. Expression of cyclin D1 and SOX11
Discussion:
Mantle cell lymphoma (MCL) is classified as a distinct and generally aggressive type of B cell non-Hodgkin’s lymphoma (NHL) with much variability in its presentation and clinical course (Am J Hematol 2019;94:710). Most cases are associated with cyclin D1 (CCND1) overexpression, with evidence of CCND1 clonal rearrangement or the presence of t(11;14) confirmed by FISH or karyotypic analysis (Am J Surg Pathol 2019;43:1052). MCL may be classified into distinct morphologic subtypes: classic, pleomorphic or blastoid (Ocul Oncol Pathol 2019;5:245).
MCL comprises approximately 3 - 10% of all NHLs, with a higher preponderance among non-Hispanic white males (Am J Hematol 2019;94:710). The median age is 68 years at initial diagnosis (Am J Hematol 2019;94:710). Compared with the classic form, the blastoid variant of MCL is less common and generally has a poorer prognosis (Leuk Lymphoma 2016;57:1327).
Patients with MCL most often present at an advanced stage with a bulky tumor and widespread involvement. Typical complaints at presentation include systemic B symptoms or gastrointestinal (GI) symptoms (Leuk Lymphoma 2016;57:1327). Extranodal site involvement is common; however, nodal and leukemic forms can also be present at the time of presentation (Am J Hematol 2019;94:710). Since those presenting with GI complaints may have involvement in any region of the GI tract as lymphomatous polyposis, diagnosis may be made from nodal or extranodal tissue biopsy (ISRN Radiol 2013;2013:483069). Mesentery involvement may be present at diagnosis as well (ISRN Radiol 2013;2013:483069).
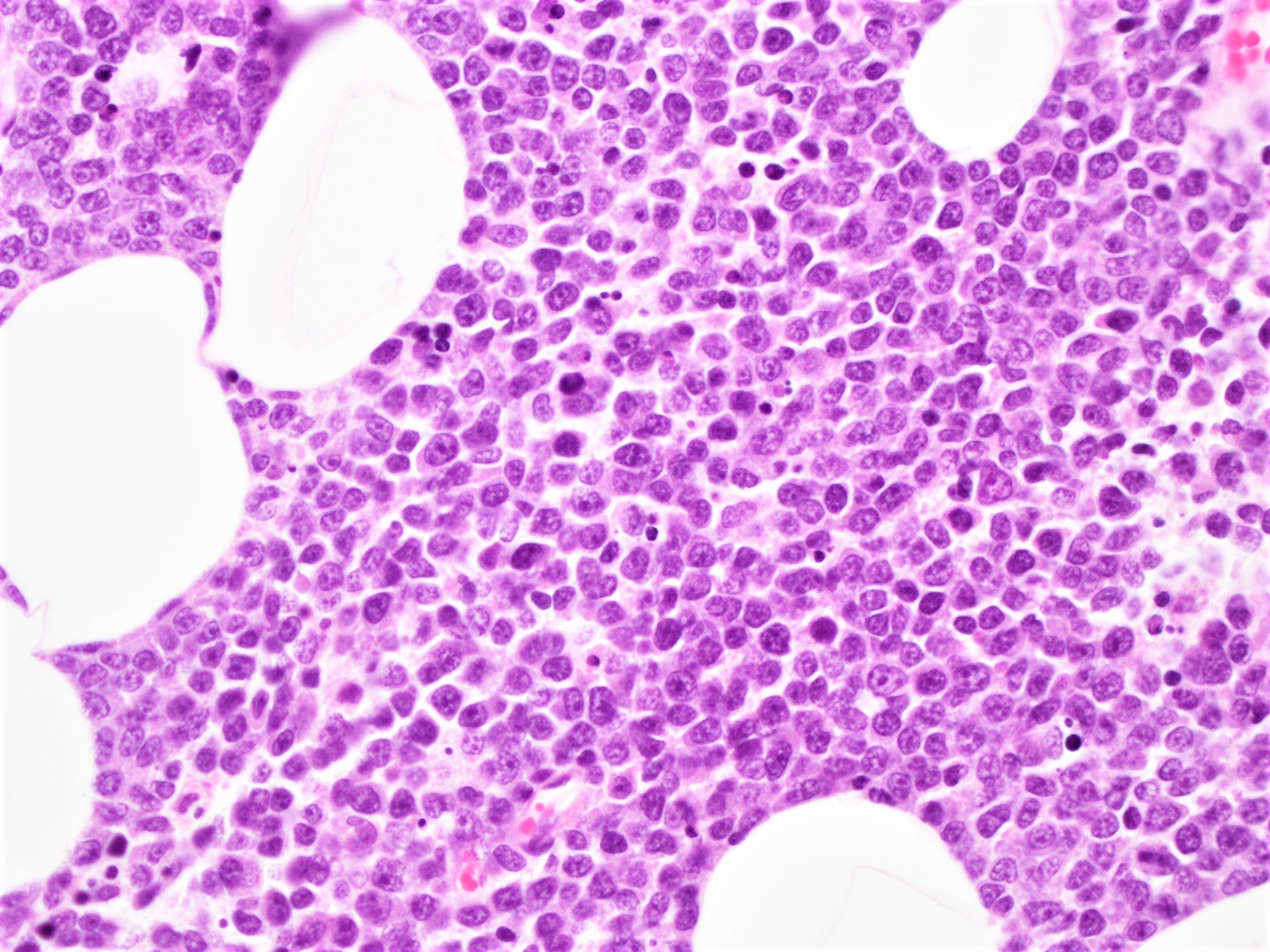
The pleomorphic and blastoid morphologic variants together account for about 10% of MCL and exhibit particularly aggressive behavior (Sci Rep 2019;9:12857). The growth pattern is most often diffuse and, less commonly, nodular. While the lymphoma cells in the classic variant are characteristically intermediate in size with slightly irregular nuclear contours, the pleomorphic variant typically has large cells which may resemble diffuse large B cell lymphoma.
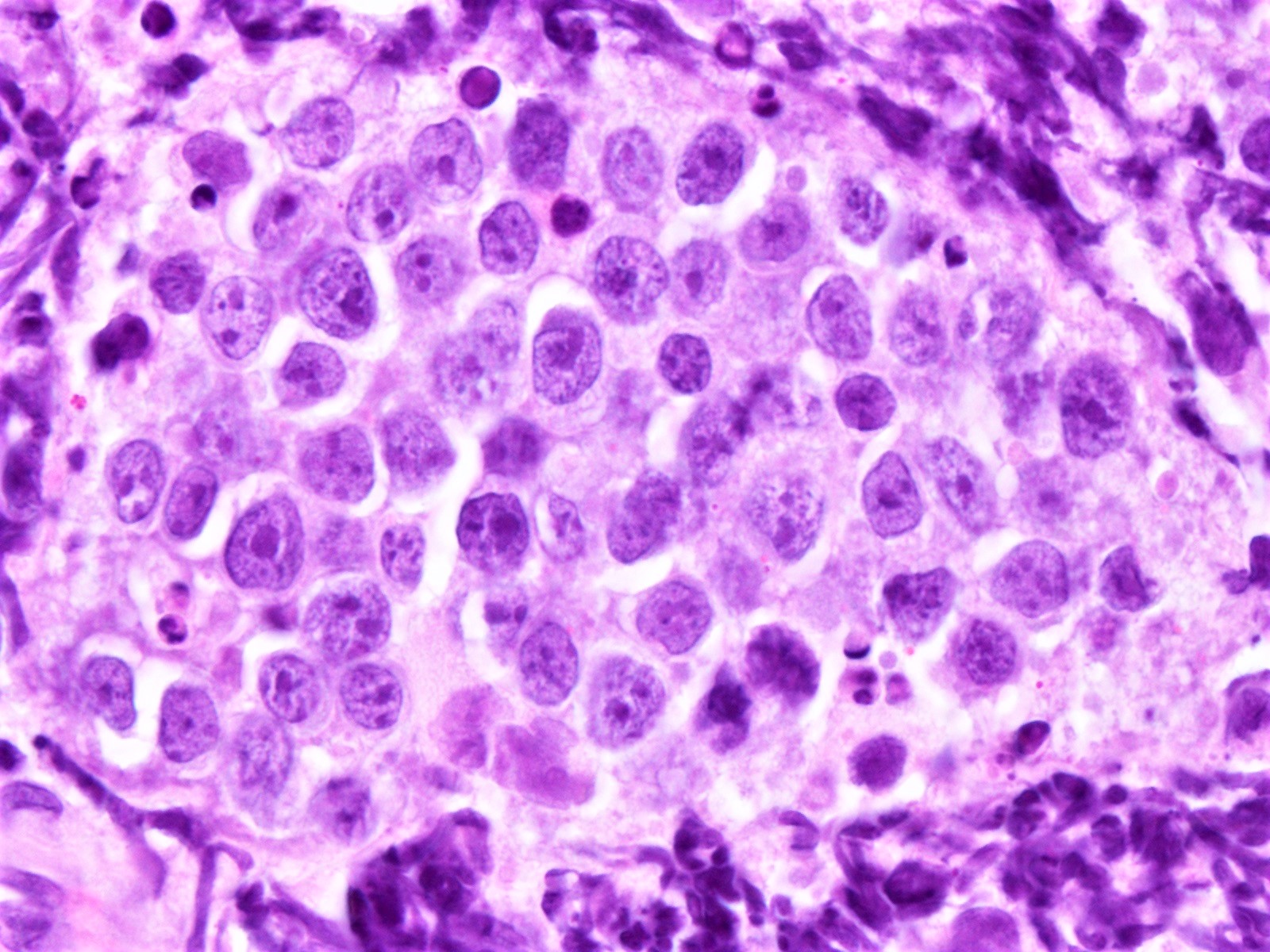
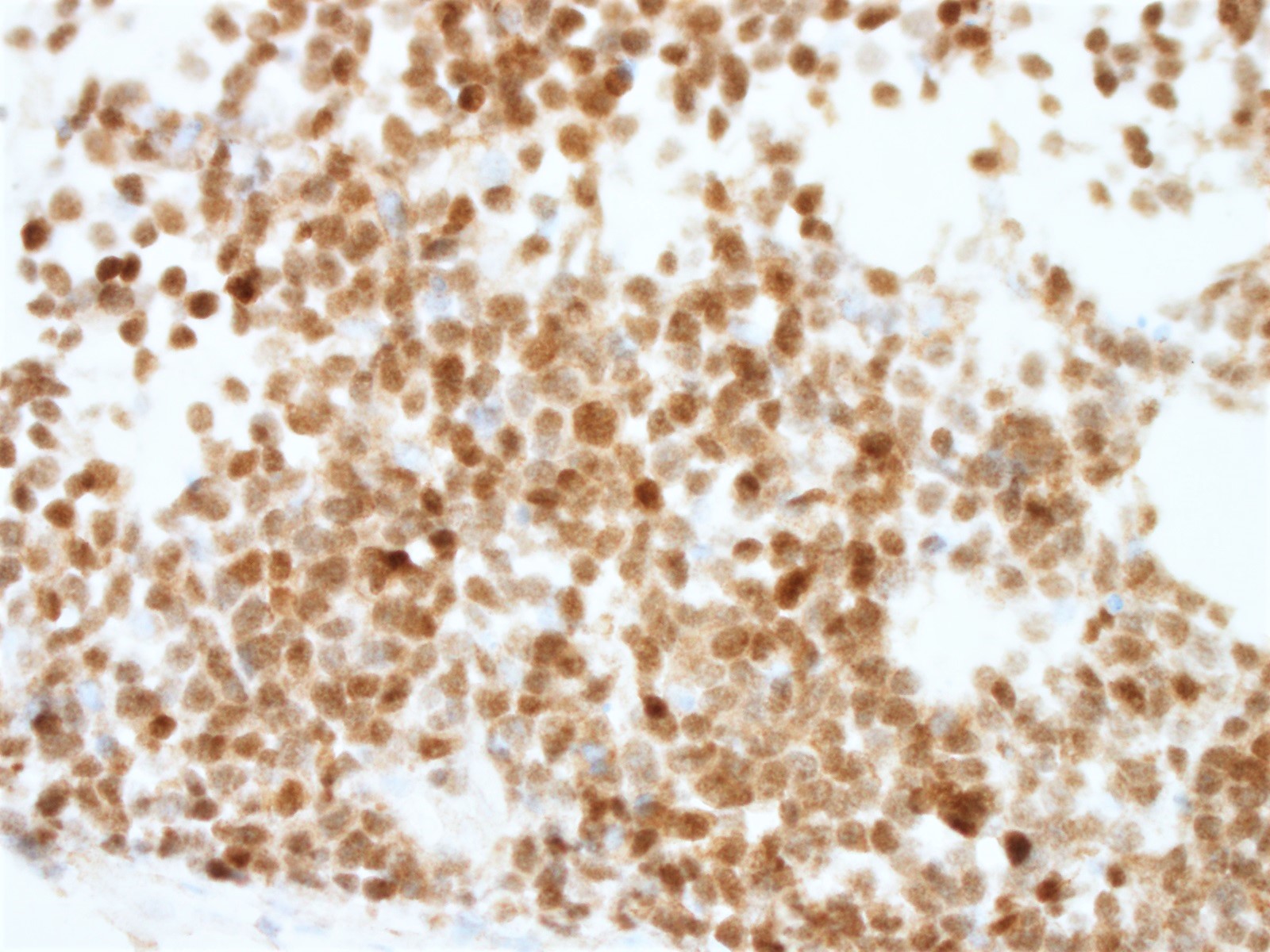
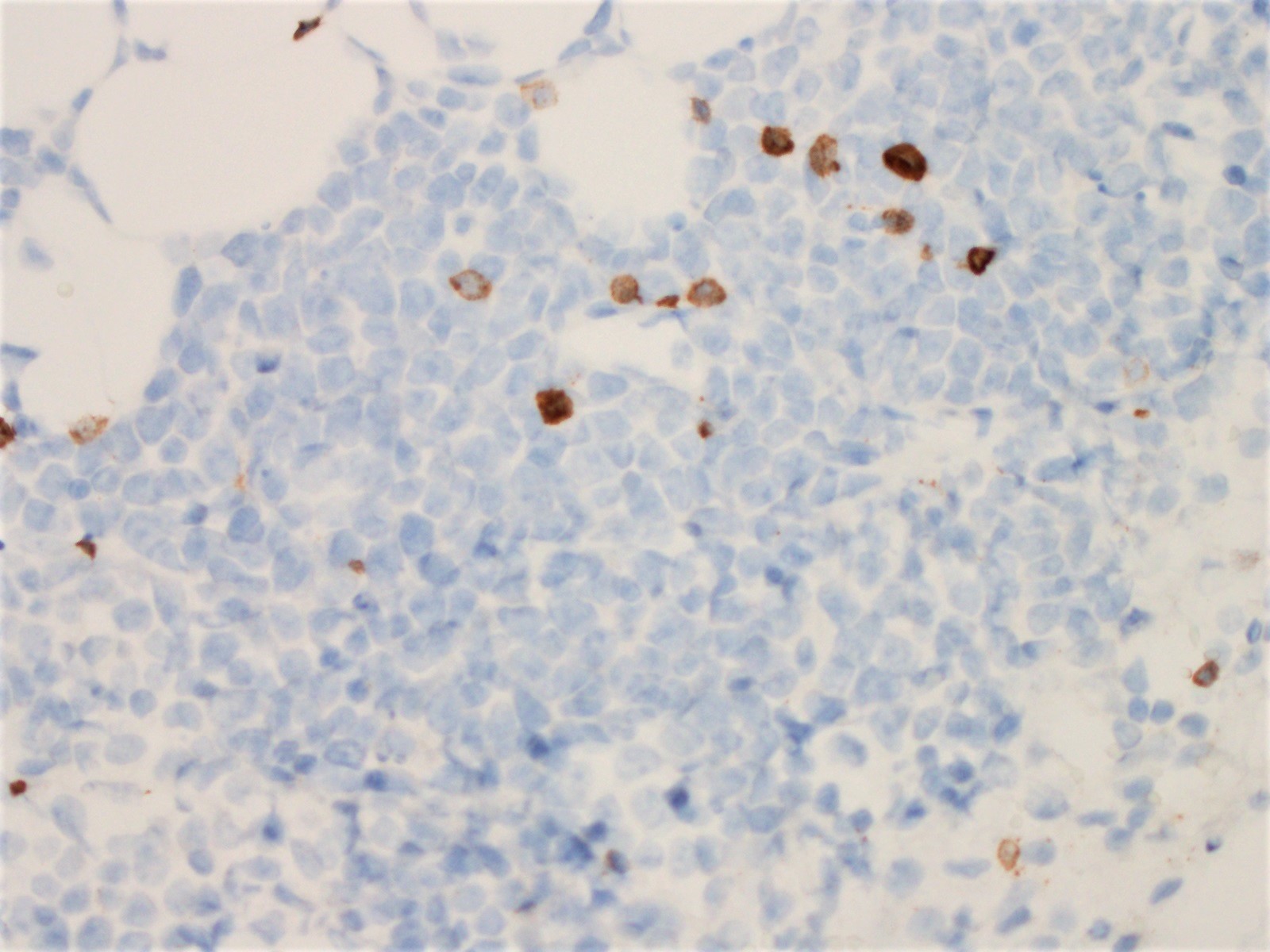
The neoplastic cells of the blastoid variant may be reminiscent of lymphoblasts, with a fine chromatin pattern and occasional nucleoli (Blood 2018;132:2722). Most cases of blastoid MCL show a high mitotic rate (> 20 to 30 per 10 high power fields) and high proliferative fraction based on Ki67/MIB1 labeling index (Sci Rep 2019;9:12857). Similar to the classic form of MCL, the blastoid variant typically shows CD5 positivity on both IHC and flow cytometry but CD5 negative cases have been reported (Am J Surg Pathol 2019;43:1052), as in this case. SOX11 immunoreactivity is demonstrable on most cases but negative cases can occur. Cytogenetic or FISH analysis reveals translocation t(11;14), resulting in CCND1 rearrangement with IGHV chain region on chromosome 14 (Ocul Oncol Pathol 2019;5:245).
MCL exhibits wide clinical heterogeneity. It can be stratified into 2 subtypes based on clinical presentation: conventional (or classic) MCL (cMCL) or leukemic non-nodal MCL (nnMCL) (Blood 2016;127:2375). cMCL is more often associated with SOX11+ expression and a more aggressive disease state (Blood 2016;127:2375). On the other hand, nnMCL is associated with greater genomic stability and a more indolent clinical course (Am J Hematol 2019;94:710). However, some cases of nnMCL have been shown to later acquire secondary mutations, notably TP53, enabling their cellular transformation to the more aggressive form of the disease (Ocul Oncol Pathol 2019;5:245).
The majority of blastoid variant cases of MCL studied have been thought to arise from the more aggressive cMCL subtype (Am J Hematol 2019;94:710). However, rare cases of the blastoid variant representing transformation of the more indolent nnMCL subtype have been reported (Blood 2016;127:2375).
Differential diagnoses: Burkitt lymphoma may show a similar morphology and exhibit a similarly high proliferative fraction. Differentiating features include CD10 positivity and subset EBV positivity in Burkitt lymphoma (Arch Pathol Lab Med 2008;132:1346). Definitive distinction may require FISH analysis, which confirms blastoid MCL when t(11;14) is identified or Burkitt lymphoma when t(8;14) is present (Arch Pathol Lab Med 2008;132:1346). Lymphoblastic lymphoma (LBL) may show a similar morphology and exhibit a similarly high proliferative fraction. IHC can distinguish the two entities; LBL is often positive for TdT and negative for CCND1 (Am J Clin Pathol 2014;141:305). Diffuse large B cell lymphoma (DLBCL) stains negatively for SOX11 and CCND1, except in rare cases (Am J Clin Pathol 2014;141:305). EBV may sometimes be seen in large B cell lymphoma but not in blastoid MCL (Arch Pathol Lab Med 2008;132:1346).
At follow up, the patient has responded to combination chemotherapy regimen for treatment of high risk, high stage B cell lymphoma.
Test question answer:
E. Expression of cyclin D1 and SOX11 by immunohistochemistry is seen in the majority of blastoid and classic variants of mantle cell lymphoma.
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Dr. Patricia Tsang, Geisinger Commonwealth School of Medicine, Wilkes Barre, Pennsylvania (USA) for contributing this case and to Dr. Morgan Hrones, Yale New Haven Medical Center, New Haven, Connecticut (USA) for writing the discussion. The discussion was reviewed by Dr. Tsang.

Advertisement
Case of the Month #499
Clinical history:
A 74 year old man presented with weight loss, ascites, liver lesions and a 5 cm growing soft tissue mass in the abdominal wall.
Histopathology images:
What is your diagnosis?
Click here for diagnosis, test question and discussion:
Diagnosis: Blastoid variant of mantle cell lymphoma (CD5 negative variant)
Test question (answer at the end):
Which of the following is true about the majority of blastoid mantle cell lymphoma cases?
A. Association with EBV
B. Association with t(8;14)
C. Clinical course is similar to that of leukemic non-nodal mantle cell leukemia subtype
D. Clonal rearrangement of T cell receptor gamma gene
E. Expression of cyclin D1 and SOX11
Discussion:
Mantle cell lymphoma (MCL) is classified as a distinct and generally aggressive type of B cell non-Hodgkin’s lymphoma (NHL) with much variability in its presentation and clinical course (Am J Hematol 2019;94:710). Most cases are associated with cyclin D1 (CCND1) overexpression, with evidence of CCND1 clonal rearrangement or the presence of t(11;14) confirmed by FISH or karyotypic analysis (Am J Surg Pathol 2019;43:1052). MCL may be classified into distinct morphologic subtypes: classic, pleomorphic or blastoid (Ocul Oncol Pathol 2019;5:245).
MCL comprises approximately 3 - 10% of all NHLs, with a higher preponderance among non-Hispanic white males (Am J Hematol 2019;94:710). The median age is 68 years at initial diagnosis (Am J Hematol 2019;94:710). Compared with the classic form, the blastoid variant of MCL is less common and generally has a poorer prognosis (Leuk Lymphoma 2016;57:1327).
Patients with MCL most often present at an advanced stage with a bulky tumor and widespread involvement. Typical complaints at presentation include systemic B symptoms or gastrointestinal (GI) symptoms (Leuk Lymphoma 2016;57:1327). Extranodal site involvement is common; however, nodal and leukemic forms can also be present at the time of presentation (Am J Hematol 2019;94:710). Since those presenting with GI complaints may have involvement in any region of the GI tract as lymphomatous polyposis, diagnosis may be made from nodal or extranodal tissue biopsy (ISRN Radiol 2013;2013:483069). Mesentery involvement may be present at diagnosis as well (ISRN Radiol 2013;2013:483069).
The pleomorphic and blastoid morphologic variants together account for about 10% of MCL and exhibit particularly aggressive behavior (Sci Rep 2019;9:12857). The growth pattern is most often diffuse and, less commonly, nodular. While the lymphoma cells in the classic variant are characteristically intermediate in size with slightly irregular nuclear contours, the pleomorphic variant typically has large cells which may resemble diffuse large B cell lymphoma.
The neoplastic cells of the blastoid variant may be reminiscent of lymphoblasts, with a fine chromatin pattern and occasional nucleoli (Blood 2018;132:2722). Most cases of blastoid MCL show a high mitotic rate (> 20 to 30 per 10 high power fields) and high proliferative fraction based on Ki67/MIB1 labeling index (Sci Rep 2019;9:12857). Similar to the classic form of MCL, the blastoid variant typically shows CD5 positivity on both IHC and flow cytometry but CD5 negative cases have been reported (Am J Surg Pathol 2019;43:1052), as in this case. SOX11 immunoreactivity is demonstrable on most cases but negative cases can occur. Cytogenetic or FISH analysis reveals translocation t(11;14), resulting in CCND1 rearrangement with IGHV chain region on chromosome 14 (Ocul Oncol Pathol 2019;5:245).
MCL exhibits wide clinical heterogeneity. It can be stratified into 2 subtypes based on clinical presentation: conventional (or classic) MCL (cMCL) or leukemic non-nodal MCL (nnMCL) (Blood 2016;127:2375). cMCL is more often associated with SOX11+ expression and a more aggressive disease state (Blood 2016;127:2375). On the other hand, nnMCL is associated with greater genomic stability and a more indolent clinical course (Am J Hematol 2019;94:710). However, some cases of nnMCL have been shown to later acquire secondary mutations, notably TP53, enabling their cellular transformation to the more aggressive form of the disease (Ocul Oncol Pathol 2019;5:245).
The majority of blastoid variant cases of MCL studied have been thought to arise from the more aggressive cMCL subtype (Am J Hematol 2019;94:710). However, rare cases of the blastoid variant representing transformation of the more indolent nnMCL subtype have been reported (Blood 2016;127:2375).
Differential diagnoses: Burkitt lymphoma may show a similar morphology and exhibit a similarly high proliferative fraction. Differentiating features include CD10 positivity and subset EBV positivity in Burkitt lymphoma (Arch Pathol Lab Med 2008;132:1346). Definitive distinction may require FISH analysis, which confirms blastoid MCL when t(11;14) is identified or Burkitt lymphoma when t(8;14) is present (Arch Pathol Lab Med 2008;132:1346). Lymphoblastic lymphoma (LBL) may show a similar morphology and exhibit a similarly high proliferative fraction. IHC can distinguish the two entities; LBL is often positive for TdT and negative for CCND1 (Am J Clin Pathol 2014;141:305). Diffuse large B cell lymphoma (DLBCL) stains negatively for SOX11 and CCND1, except in rare cases (Am J Clin Pathol 2014;141:305). EBV may sometimes be seen in large B cell lymphoma but not in blastoid MCL (Arch Pathol Lab Med 2008;132:1346).
At follow up, the patient has responded to combination chemotherapy regimen for treatment of high risk, high stage B cell lymphoma.
Test question answer:
E. Expression of cyclin D1 and SOX11 by immunohistochemistry is seen in the majority of blastoid and classic variants of mantle cell lymphoma.