All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
This case was contributed by Dr. Julia Braza, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Attend the
Annual Summer Update in Clinical Immunology,
Microbiology and Infectious Diseases
July 14-18, 2008
Snow King Resort
Jackson, Wyoming (USA)

This 25.75-hour review and update in clinical immunology, microbiology and infectious diseases is intended to improve knowledge about the pathogenesis and clinical manifestations of infectious diseases, immunological mechanisms of disease and disease prevention, appropriate approaches to the diagnosis of infections and immunologic disorders, and utilization of the clinical microbiology and immunology laboratory, including selection and interpretation of results.
This course will provide a forum for the exchange of ideas dealing with microbial infections as well as immunity to infectious diseases and immunologic disorders.
Case #121
Clinical history:
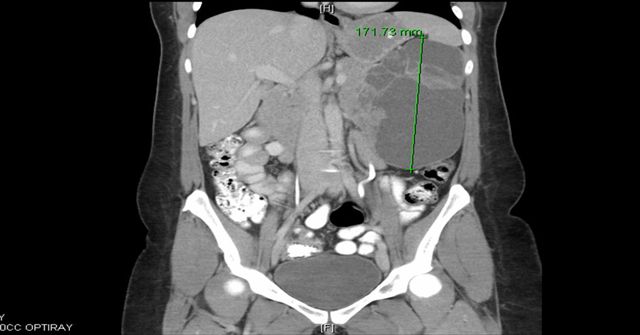
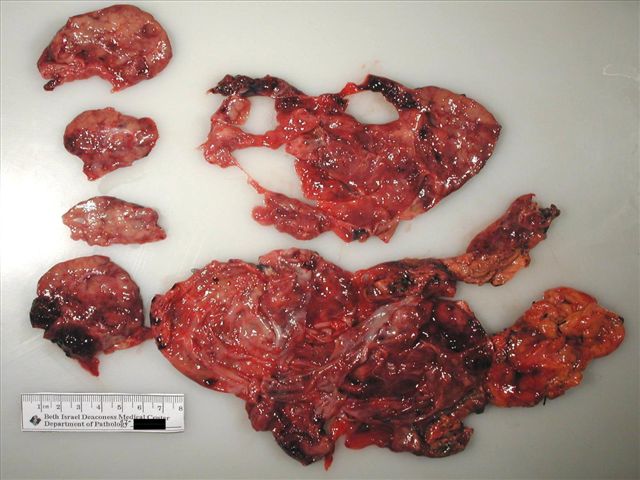
A 33 year old woman presented with progressively worsening left upper quadrant and back pain for one month, accompanied by anorexia due to early satiety. A CT scan showed a large, multilocular cystic mass arising from the pancreatic tail, displacing adjacent structures, with no invasion or distant disease. The specimen consisted of a distal pancreatectomy with omentum and gallbladder. The pancreas contained a 20 cm partially solid and cystic mass. The cystic portion had no excrescences or papillations and the solid portions had soft, friable and flesh-like cut surfaces, with areas of hemorrhage.
Radiology and gross images:
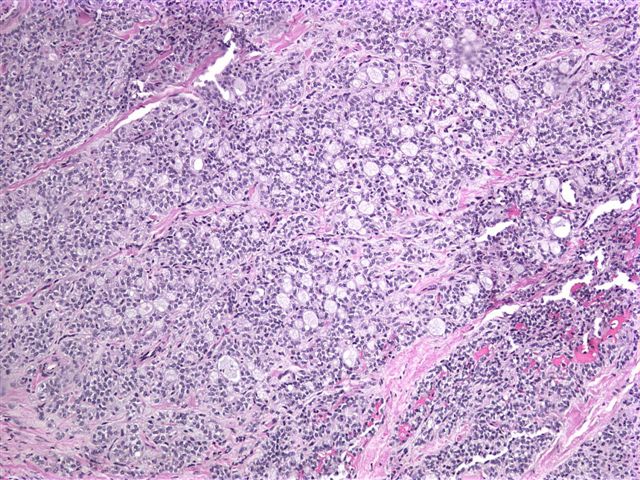
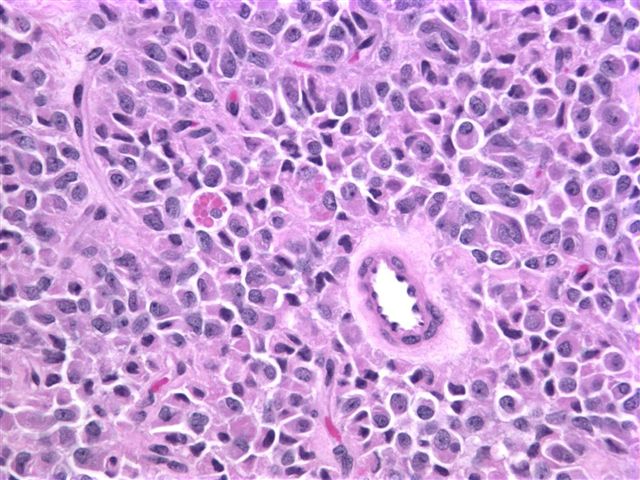
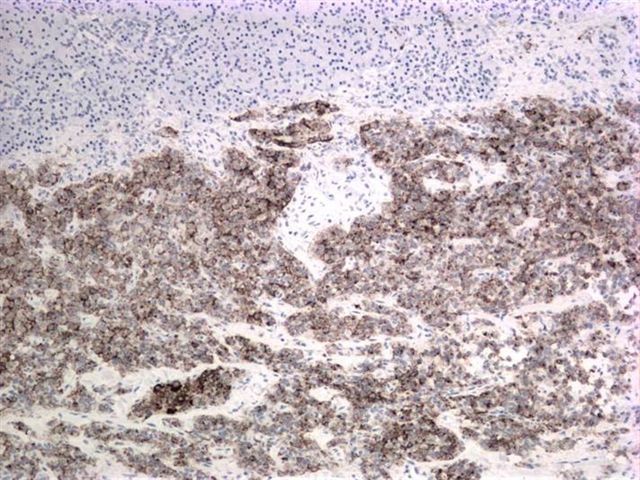
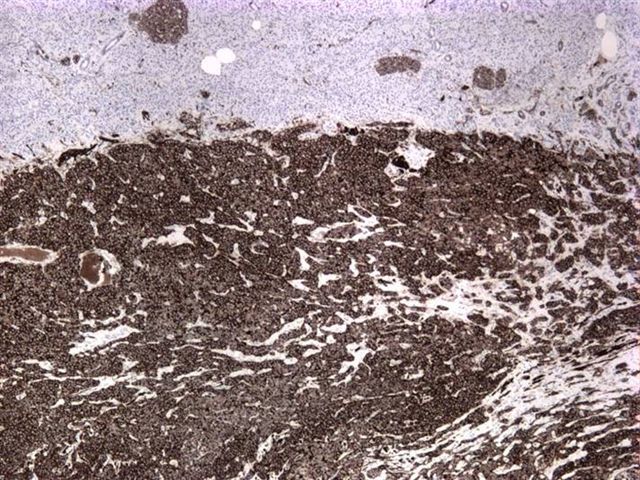
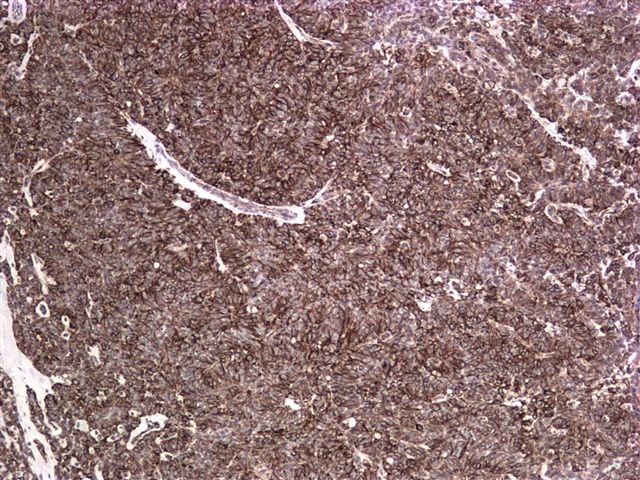
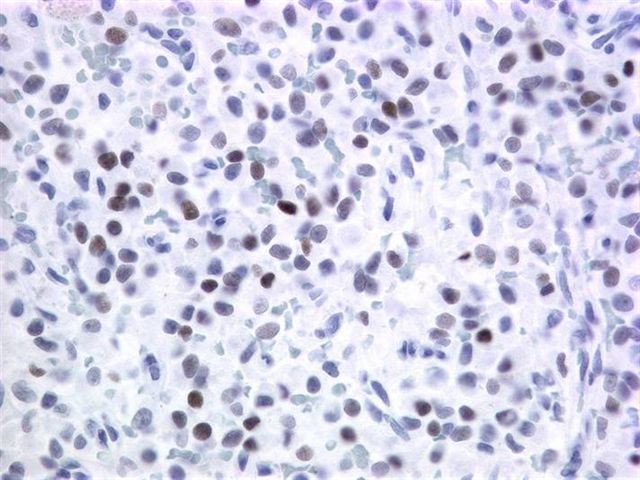
Microscopic images:
What is your diagnosis?
Diagnosis: Solid pseudopapillary neoplasm of pancreas
Stains:
Discussion:
Immunostain results were: CD10+, CD56+, vimentin+, PR+ and alpha-1 antitrypsin+.
Solid pseudopapillary neoplasm of the pancreas, also called papillary cystic neoplasm and Frantz tumor, accounts for 1 - 2% of nonendocrine pancreatic neoplasms. It typically affects young women, often in their twenties but is not associated with any clinical syndrome. This tumor appears to be unique to the pancreas but its origin is unclear, as it lacks clear evidence of ductal, acinar or endocrine differentiation (Mod Pathol 2007;20:S71).
Grossly, it is usually large (mean 9 cm) and encapsulated, with variable solid and cystic areas, as well as hemorrhagic and necrotic foci. Microscopically, the tumors contain pseudopapillae formed from solid nests, with loss of cells degenerating away from delicate small vessels. There is no stromal reaction to the tumor cells. The pseudopapillae have hyalinized fibrovascular cores and are composed of bland epithelial cells with clear to eosinophilic cytoplasm and variable PAS+ intracytoplasmic hyaline inclusions. The nuclei are round / oval with uniform, finely stippled chromatin and frequent longitudinal nuclear grooves but no distinct nucleoli. Mitotic figures are rare.
Almost all tumors have mutations in exon 3 of the beta-catenin gene, which causes abnormal immunostaining patterns for beta catenin (nuclear and cytoplasmic, compared to membranous staining in normal pancreas) and overexpression of Cyclin D1 (Am J Pathol 2002;160:1361). Recent studies have also shown nuclear localization of E-cadherin with absence of membranous and cytoplasmic localization, which may account for the dyscohesive nature of the tumor cells (Am J Surg Pathol 2008;32:1). Tumor cells are also immunoreactive for CD10, CD56, progesterone receptor, alpha-1 antitrypsin and vimentin and negative for chromogranin (Am J Surg Pathol 2000;24:1361).
The differential diagnosis includes pancreatic pseudocyst and pancreatic endocrine neoplasm. Pancreatic pseudocysts may be grossly similar but have no epithelial cells lining the cystic structures, even after careful search. In addition, patients are usually older and male and have a history of pancreatitis. Pancreatic endocrine neoplasms lack degenerative pseudopapillae, intracytoplasmic hyaline globules and longitudinal nuclear grooves. They are immunoreactive for chromogranin and negative for CD10 and nuclear beta catenin. Acinar cell carcinoma may be a consideration but typically has acinar formations, prominent nucleoli and mitotic activity, as well as immunostaining for trypsin, chymotrypsin and lipase (Mod Pathol 2007;20 Suppl 1:S94).
Solid pseudopapillary neoplasm has an excellent prognosis, with a high cure rate after resection (J Surg Oncol 2007;95:304). Metastases to the liver or peritoneum are seen in 10 - 15%, although patients usually survive even with metastases.
Additional reference: Hruban: Tumors of the Pancreas (AFIP Atlas of Tumor Pathology; 4th Series Fascicle 6)