Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Parra-Herran C. Progestin therapy related changes. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusprogestinrelated.html. Accessed April 2nd, 2025.
Definition / general
- Hysterectomy is the standard treatment for endometrioid intraepithelial neoplasia (atypical hyperplasia) and low grade endometrial endometrioid adenocarcinoma
- Hormonal therapy is a valid alternative for premenopausal women who desire to preserve fertility, as well as patients in which surgical treatment is not feasible (those with obesity or multiple other comorbidities)
Essential features
- Fertility sparing treatment is increasingly considered in the management of patients with endometrioid intraepithelial neoplasia (endometrioid intraepithelial neoplasia [EIN], atypical endometrial hyperplasia) and low grade endometrial endometrioid carcinoma
- Management recommendations include follow up samples, which determine whether the lesion is responding to progestin and to what degree
- Pathologists need to be aware of the changes seen in the neoplasm and background endometrium, as well as the terminology currently recommended to report such changes
ICD coding
Epidemiology
- Proportion of premenopausal women with these diagnoses is increasing: 15 - 25% are premenopausal, 10% are < 45 years, 4% are < 40 years (Int J Womens Health 2014;6:691)
- In these patients and those in which surgical management is not advisable, fertility sparing treatment is indicated
Diagrams / tables
Clinical features
- Indications for fertility sparing treatment
- Young woman with high desire to retain reproductive capabilities
- Diagnosis of endometrioid intraepithelial neoplasia / atypical hyperplasia or FIGO grade 1 endometrial endometrioid adenocarcinoma
- No myometrial invasion on imaging (MRI)
- The following patients are generally not eligible for fertility sparing treatment but can be considered on a case to case basis: patients with obesity, anovulation, grade 1 endometrioid carcinoma with superficial myoinvasion on imaging, nonmyoinvasive grade 2 endometrioid carcinoma
- Treatment modalities
- 2 main types of fertility sparing treatment are oral progestins and a levonorgestrel releasing intrauterine device
- Current recommendations for oral progestins include medroxyprogesterone acetate at 400 - 600 mg/day or megestrol acetate at 160 - 320 mg/day for at least 6 months, with follow up using dilation and curettage and imaging (Ann Oncol 2016;27:16, Int J Gynecol Cancer 2015;25:1258)
- Levonorgestrel releasing intrauterine device releases 52 mg of intrauterine progestin at a consistent rate for up to 5 years but declines thereafter (Obstet Gynecol Sci 2020;63:417)
- Limitations
- 10 - 30% of patients present at advanced stage (FIGO stage III - IV), usually with ovarian / adnexal metastases, which may be only detected on hysterectomy / bilateral salpingo-oopherectomy (BSO) but not with progestin treatment (Int J Womens Health 2014;6:691)
- Moreover, 5 - 29% of premenopausal women have a synchronous ovarian carcinoma, which may be only detected on hysterectomy / BSO but not with progestin treatment (Obstet Gynecol 2005;106:693)
Diagnosis
- Initial and follow up diagnosis in the setting of endometrioid intraepithelial neoplasia / endometrioid carcinoma treated with progestins require endometrial sampling (dilation and curettage preferred over pipelle biopsy)
- In principle, the histopathologic diagnosis of endometrioid intraepithelial neoplasia / atypical hyperplasia and endometrial endometrioid carcinoma in the setting of exogenous progestin therapy relies on the architectural and cytologic criteria used conventionally but with the caveat that the cytologic appearance is influenced / confounded by the progestin effect
- In this context, a lesion may appear cytologically bland but if it meets criteria for endometrioid intraepithelial neoplasia or endometrioid carcinoma, the diagnosis should be rendered as such
- If criteria are not met but there is gland crowding suggestive of residual neoplasia, descriptive terminology should be used (see Sample pathology report)
- Morphology of the background normal endometrium should always be reported, as the presence of progestin related changes indicates circulating progestins and adherence to the treatment; conversely, the absence of these changes (for instance, proliferative or inactive endometrium) may alert the treating clinician to the possibility of unsuccessful treatment or lack of patient adherence to the therapy
- Reference: Int J Gynecol Pathol 2022;41:142
Prognostic factors
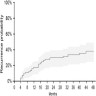
- A meta analysis of 24 studies involving 370 patients undergoing fertility sparing treatment showed that the remission probability ascended to 72% after 6 months of treatment and then plateaued with 78% and 81% remission chance after 12 months and 24 months of treatment, respectively (Fertil Steril 2014;101:785)
- Conversely, the recurrence probability was estimated at 10%, 17% and 29% at 6, 12 and 24 months, respectively, based on 100 patients in 10 studies; this data suggests that therapy beyond 6 months confers only marginal benefit compared to the steadily increasing risk of lesion recurrence or progression (Fertil Steril 2014;101:785)
- The following factors have been associated with higher likelihood of remission under progestin therapy: young age, previous pregnancy, infertility, index diagnosis of endometrioid intraepithelial neoplasia / atypical hyperplasia (versus endometrioid carcinoma) and treatment with megestrol acetate (versus other forms of treatment) (Fertil Steril 2014;101:785)
- In a meta analysis that included 351 patients from 22 studies, 32% of patients with fertility sparing treatment had at least one pregnancy, 54% of those using assisted reproductive technologies (Fertil Steril 2014;101:785)
- In previous literature, pregnancy was achieved in 35% of patients
- Rates are higher in those women actively attempting pregnancy: 73% pregnancy rate and 66% live birth rate (Int J Womens Health 2014;6:691, Obstet Gynecol 2013;121:136)
Gross description
- Progestin induced changes in endometrioid intraepithelial neoplasia / atypical hyperplasia or endometrioid carcinoma do not have macroscopic correlates
Frozen section description
- Intraoperative consultation with frozen section is not indicated in the setting of endometrioid intraepithelial neoplasia / atypical hyperplasia or endometrial endometrioid carcinoma treated with progestins
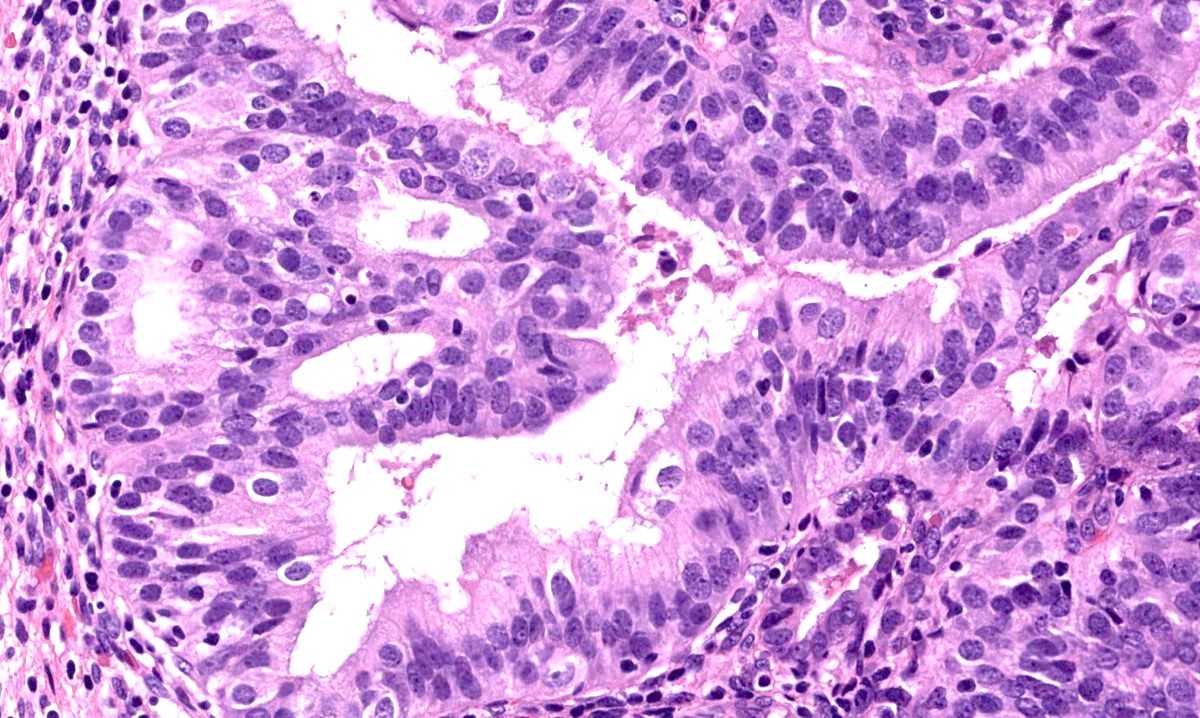
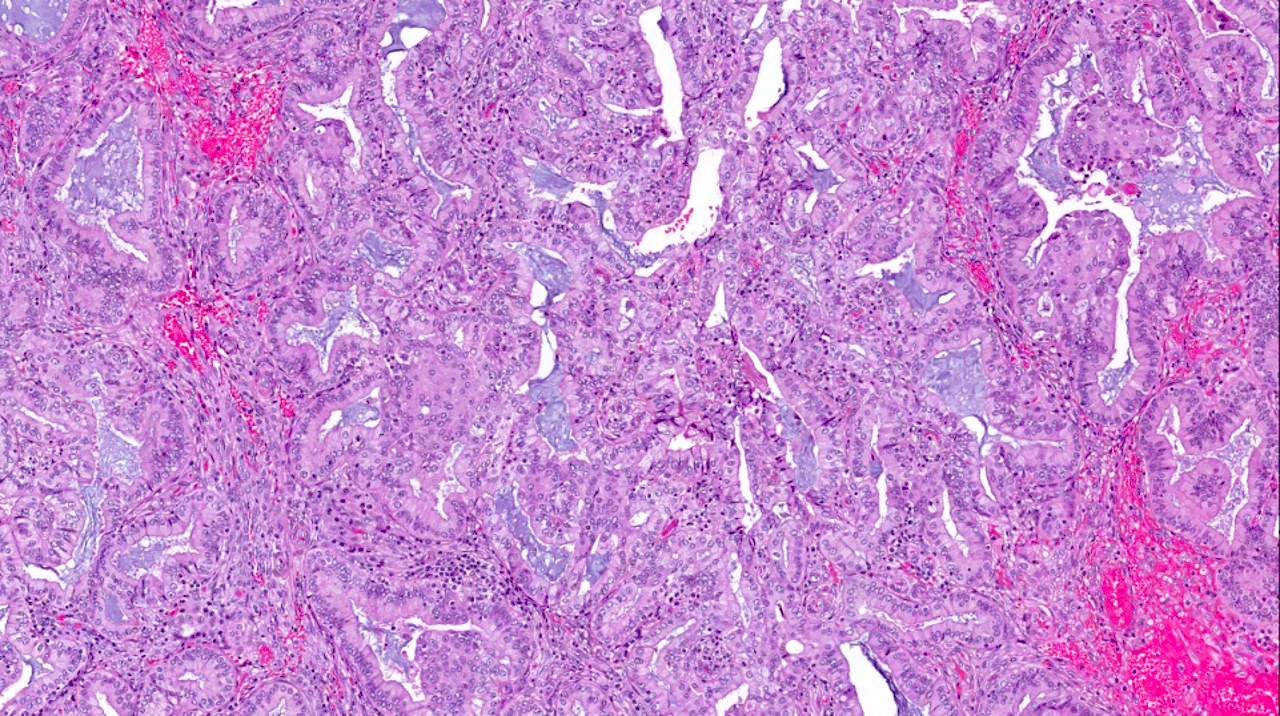
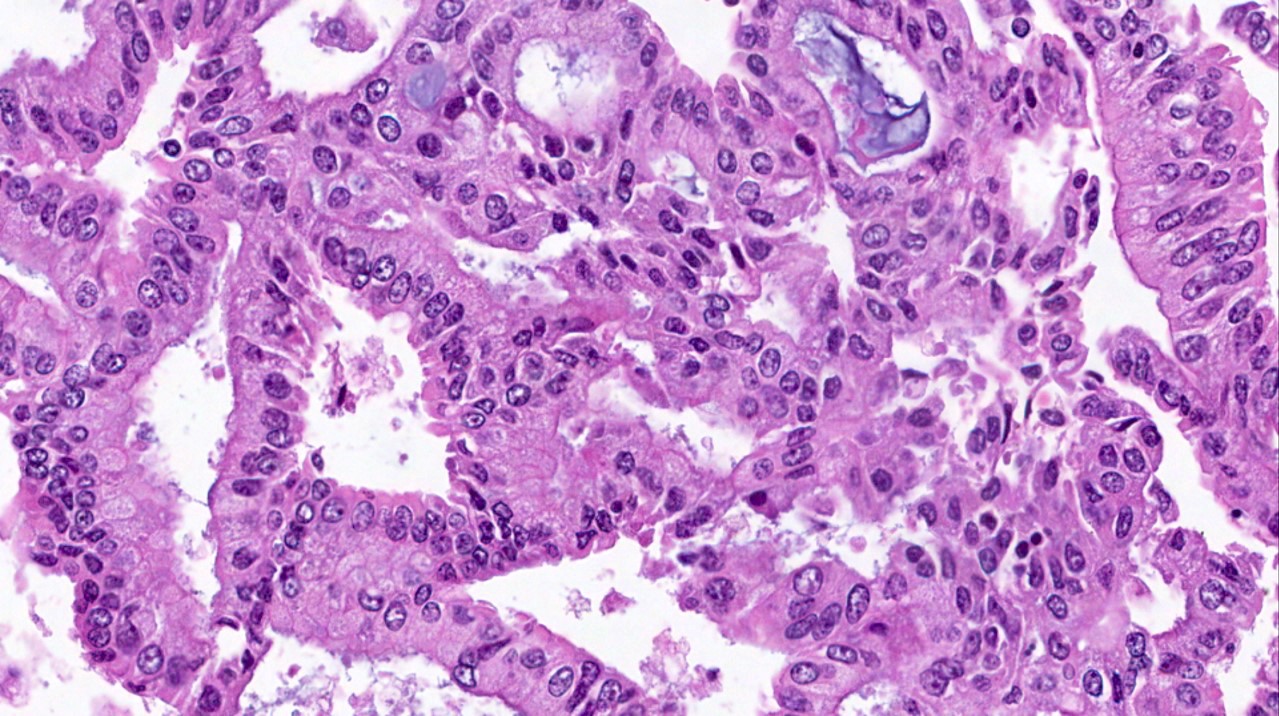
Microscopic (histologic) description
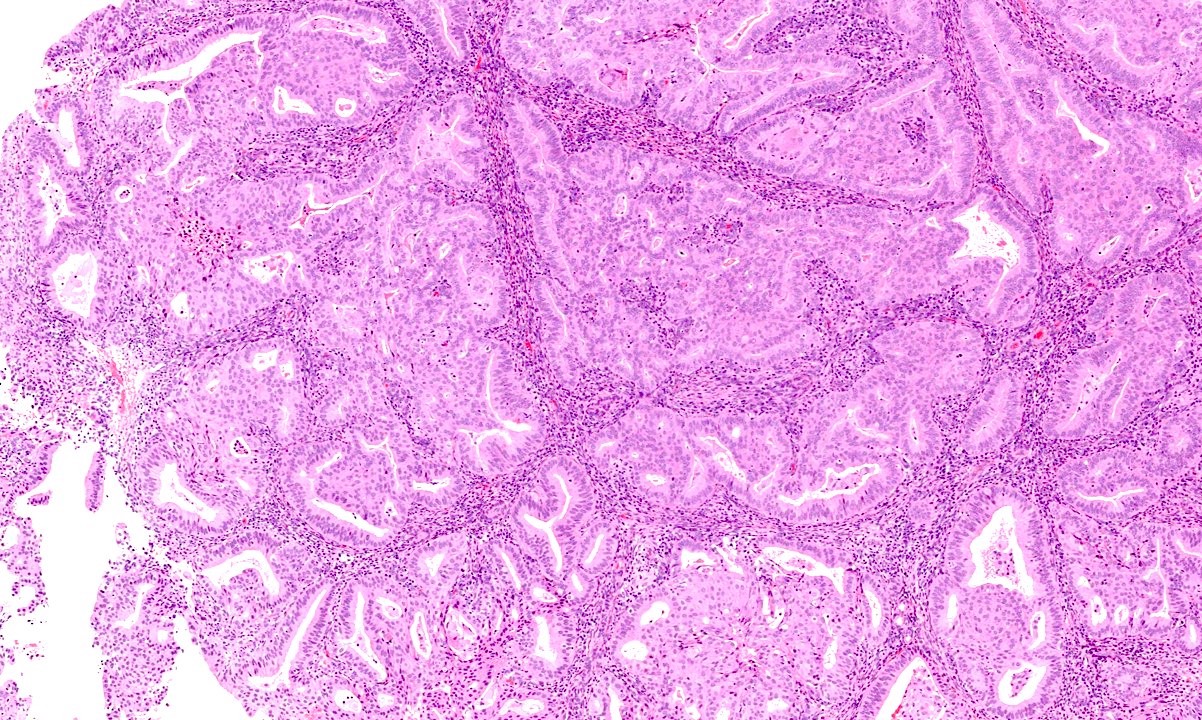
- Progestin therapy related changes in the neoplastic endometrium include (Am J Surg Pathol 2007;31:988, Am J Clin Pathol 2012;138:524, Gynecol Oncol 2014;132:33)
- Architectural changes
- Decreased volume of disease (% and number of involved fragments)
- Decreased glandular crowding
- Low to absent nuclear stratification
- Decreased cellularity (associated with complete response)
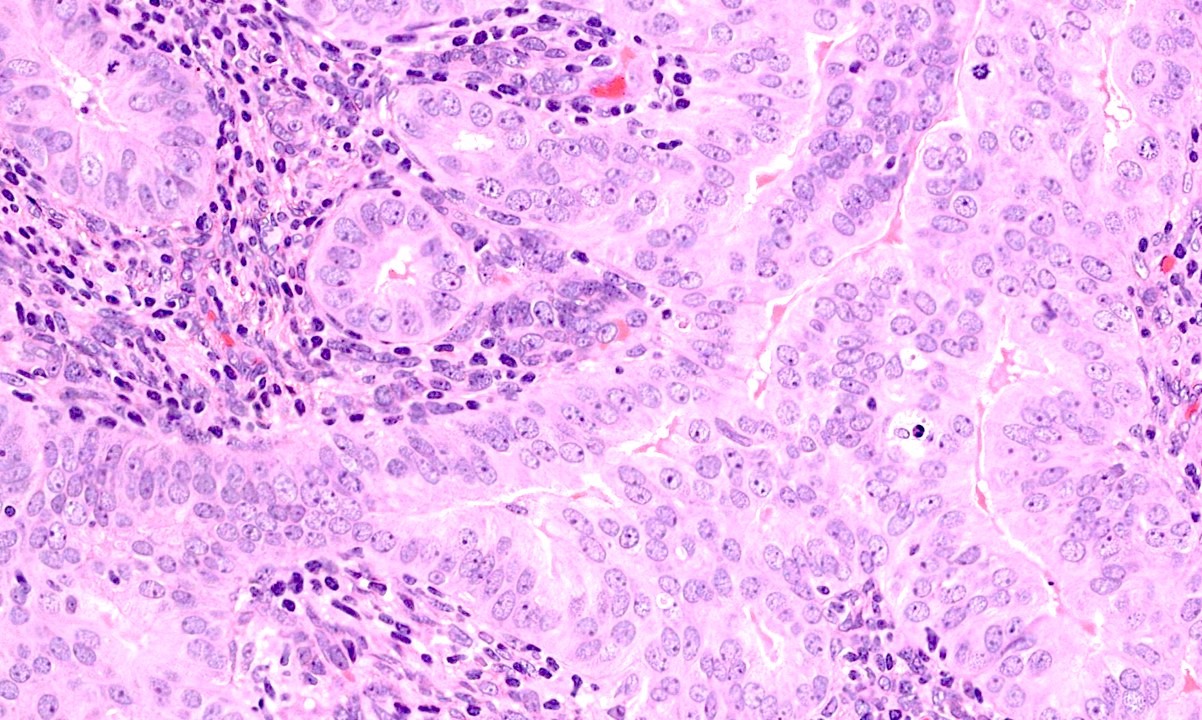
- Cytologic changes
- Decreased nuclear to cytoplasmic ratio
- Decreased nuclear size
- Cytoplasmic eosinophilia
- Nuclear rounding
- Metaplasia (secretory, squamous, mucinous)
- Architectural changes
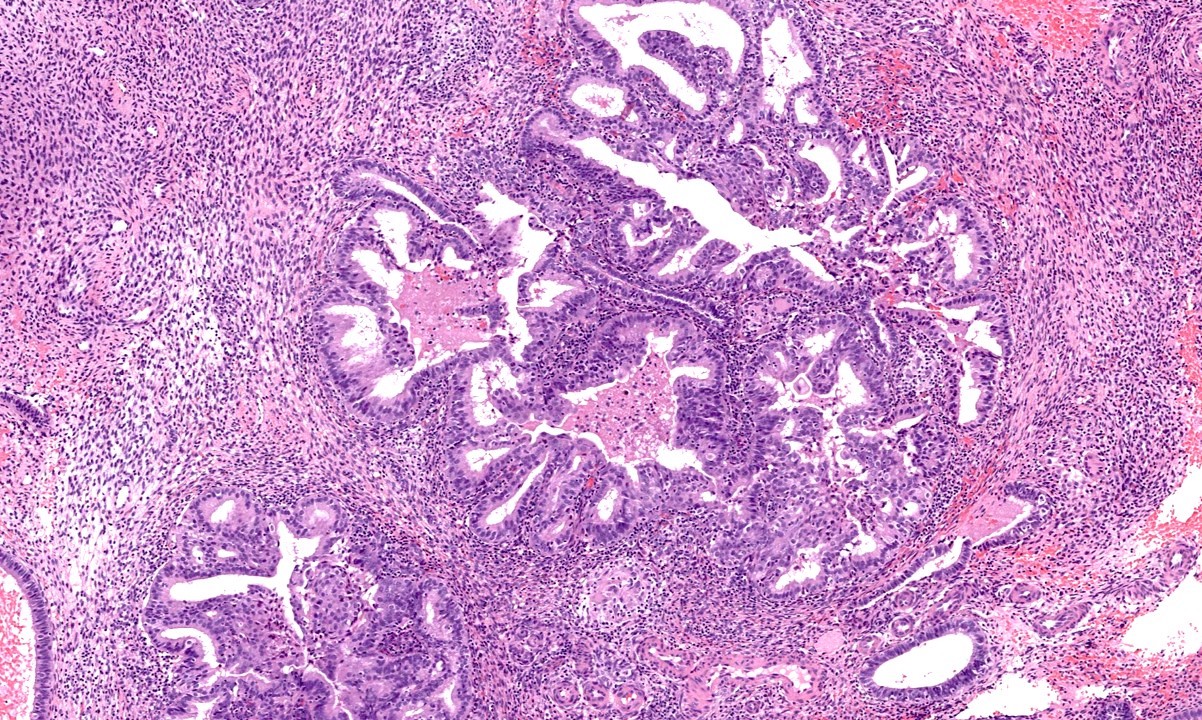
- Progestin related glandular and stromal changes in the background benign endometrium
- Their presence is an indicator of patient compliance with the treatment
- Conversely, their absence suggests a lack of patient adherence (in case of oral progestins) or malfunction (in case of intrauterine device)
- Diagnosis of the degree of response to progestin therapy
- A minimum of 6 months of therapy has been recommended based on the likelihood of persistence or progression of the neoplastic lesion while on progestin; after 6 months, therapy appears to confer only marginal benefit (Am J Surg Pathol 2007;31:988, Fertil Steril 2014;101:785)
- Within the first 6 months of progestin therapy, persistence of architectural complexity is allowed as it does not seem to adversely impact the outcome
- However, architectural complexity and cytologic atypia that was identified at the 6 month evaluation point or later correlated with adverse overall outcome (persistence or progression of endometrioid neoplasia)
- A minimum of 6 months of therapy has been recommended based on the likelihood of persistence or progression of the neoplastic lesion while on progestin; after 6 months, therapy appears to confer only marginal benefit (Am J Surg Pathol 2007;31:988, Fertil Steril 2014;101:785)
- Diagnostic workup of follow up endometrial samples in patients with fertility sparing treatment
- Document the time interval between initial diagnosis and follow up
- Compare initial and follow up samples (if possible)
- Determine the degree of response (see chart below)
- Determine the status of the background benign endometrium (with or without progestin therapy related changes)
- Endometrioid intraepithelial neoplasia is now equivalent to the term atypical endometrial hyperplasia
- Response to fertility sparing treatment can be framed in 4 categories: resolution, regression, persistence or progression
- Definition of each term depends on the index diagnosis (pretreatment)
Initial (pretreatment) diagnosis Endometrioid intraepithelial neoplasia / atypical hyperplasia FIGO grade 1 endometrioid carcinoma Resolution Negative for residual hyperplasia or carcinoma Negative for residual hyperplasia or carcinoma Regression Gland crowding or hyperplasia with progestin treatment effect Endometrial hyperplasia with or without progestin treatment effect Persistence Atypical endometrial hyperplasia with no progestin treatment effect FIGO 1 endometrioid carcinoma with or without progestin treatment effect Progression Endometrial endometrioid adenocarcinoma FIGO 2 or 3 endometrioid carcinoma
- Following a recent survey of practicing pathologists, the following diagnostic terminology and definitions have been proposed by Ganesan et al. (table adapted from Int J Gynecol Pathol 2022;41:142)
Category Glandular morphology Stromal morphology Treatment implications Negative for residual hyperplasia / carcinoma Well separated, inactive glands
Minor glandular irregularity or dilatation
Focal nuclear enlargement that appears reactive or degenerative
No cytologic atypiaStromal decidual change Treatment has been effective and can end
Follow up to ensure that there is no recurrenceResidual hyperplasia with treatment effects Foci of crowded glands or papillary architecture
Squamous metaplasia may be prominent
No cytologic atypiaStromal decidual change Treatment is working but further treatment is needed (at least 6 months in total based on most authors)* Residual atypical hyperplasia with treatment effects Foci of crowded glands or papillary architecture
Cytologic atypia presentStromal decidual change Treatment is working but further treatment is needed (at least 6 months in total based on most authors)* Atypical hyperplasia / endometrioid carcinoma without treatment effects Atypical hyperplasia / endometrioid carcinoma without stromal decidual change No stromal decidual change at the site of gland crowding The atypical hyperplasia / endometrioid carcinoma is not responding to progesterone treatment* None of the above (describe changes) *Treatment regimen should last at least 6 months; the likelihood of response after 6 months sharply decreases and at this point definitive treatment (e.g., hysterectomy) should be considered
Microscopic (histologic) images
Positive stains
- Immunohistochemistry is usually not necessary in the evaluation of progestin therapy in endometrial samples
- PTEN has been regarded as useful in the diagnosis of endometrioid intraepithelial neoplasia / atypical hyperplasia; in fact, PAX2 and PTEN have been used to determine success of progestin therapy in follow up samples; however, the use of these markers has not been validated or widely accepted by the pathology community (Am J Surg Pathol 2022;46:404, Anticancer Res 2015;35:6401)
- In this regard, PTEN can be downregulated under progesterone effect; therefore, PTEN expression can be weak or absent and should not be interpreted as a sign of endometrioid intraepithelial neoplasia / atypical hyperplasia; similar downregulation occurs with hormone receptors (J Clin Endocrinol Metab 2000;85:2334, Gynecol Oncol 2004;92:1008)
- In a 2016 study, changes in the expression of HE4 (H score) during progestin therapy correlated with therapy response but not with lesion relapse (Br J Cancer 2016;115:e15)
Negative stains
- See Positive stains
Molecular / cytogenetics description
- Identifying genes and molecular signatures predictive of response to hormonal treatment is the subject of active research
- A recent study reported differences in gene expression between responder and nonresponder lesions and predictive models using 9 genes (FOXO1, IRS2, PDGFC, DIO2, SOX9, BCL11A, APOE, FYN and KLF4) were shown to have > 90% predictive accuracy (Front Genet 2022;13:952083)
Videos
PathCast lecture on benign endometrial pathology (May 2020)
Sample pathology report
- Endometrium, biopsy / curettage:
- Endometrioid intraepithelial neoplasia / atypical hyperplasia / endometrioid carcinoma without treatment effects (see comment)
- Endometrium with changes consistent with exogenous progestin therapy
- Comment: Neoplastic glands do not show cytologic changes expected with exogenous progestin treatment.
- Endometrium, biopsy / curettage:
- Endometrioid intraepithelial neoplasia / atypical hyperplasia / endometrioid carcinoma without treatment effects (see comment)
- Background proliferative endometrium
- Comment: Neoplastic glands do not show cytologic changes expected with exogenous progestin treatment. Likewise, the background endometrium lacks morphology indicative of progestin effect. Correlation with clinical history and treatment adherence is recommended.
- Endometrium, biopsy / curettage:
- Residual endometrioid intraepithelial neoplasia / atypical hyperplasia / endometrioid carcinoma with treatment effects (see comment)
- Endometrium with changes consistent with exogenous progestin therapy
- Comment: Neoplastic glands and background endometrium show cytologic changes expected with exogenous progestin treatment. Changes are diagnostic of endometrioid intraepithelial neoplasia or carcinoma in context with progestin therapy related changes.
- Endometrium, biopsy / curettage:
- Glandular crowding consistent with residual endometrial neoplasia / hyperplasia with treatment effects (see comment)
- Endometrium with changes consistent with exogenous progestin therapy
- Comment: Changes are not diagnostic of endometrioid intraepithelial neoplasia / atypical hyperplasia or carcinoma but in context, likely represent residual endometrioid neoplasia with progestin therapy related changes.
- Endometrium, biopsy / curettage:
- Endometrium with changes consistent with exogenous progestin therapy (see comment)
- Comment: No residual endometrioid neoplasia or carcinoma identified.
Additional references
Board review style question #1
Board review style answer #1
D. Mucinous metaplasia. A range of metaplasias can be observed under progestin therapy, including secretory, squamous and mucinous metaplasia. Answers A - C are incorrect because progestin also is associated with decreases in the nuclear to cytoplasm ratio, cellularity and degree of nuclear stratification. Answer E is incorrect because nonmorular solid growth is a feature of endometrial endometrioid carcinoma used for grading purposes.
Comment Here
Reference: Progestin therapy related changes
Comment Here
Reference: Progestin therapy related changes