Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Laboratory | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Virk M. Massive transfusion. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedmassive.html. Accessed April 25th, 2024.
Definition / general
- Traumatic injury is the most common cause of hemorrhage requiring massive transfusion
- Other causes include gastrointestinal, obstetric and surgical bleeding
- Various definitions for massive transfusion (Indian J Anaesth 2014;58:590)
- Replacement of 1 entire blood volume within 24 hours
- Transfusion of > 10 units of red blood cells (RBCs) in 24 hours
- Transfusion of > 20 units of RBCs in 24 hours
- Transfusion of > 4 units of RBCs in 1 hour when ongoing need is foreseeable
- Replacement of 50% of total blood volume (TBV) within 3 hours
Essential features
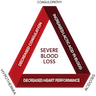
- Severe blood loss leading to the lethal triad of trauma (hypothermia, coagulopathy, acidosis)
- Transfuse RBCs, platelets and plasma in 1:1:1 ratio
- O negative RBCs reserved for females of childbearing age and pediatric patients with unknown blood type
- Type A plasma may safely be used in trauma patients with unknown blood type
- Early administration of tranexamic acid (TXA) reduces mortality from bleeding in trauma patients
Terminology
- Massive transfusion protocol (MTP)
- Massive transfusion guideline (MTG)
Pathophysiology
- Severe blood loss leading to the lethal triad of trauma (hypothermia, coagulopathy, acidosis)
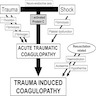
- Trauma induced coagulopathy resulting in a hypocoagulable state (Curr Opin Anaesthesiol 2016;29:212)
- Increased tissue factor expression and thrombin generation due to trauma
- Rapid consumption of coagulation factors (especially factors V and VIII)
- Increased thrombomodulin and activated protein C
- Increased plasmin activity leading to hyperfibrinolysis
- Thrombocytopenia, platelet dysfunction and decreased platelet margination
- Iatrogenic hemodilution with crystalloids can cause edema induced ischemia and exacerbate coagulopathy and hypothermia
Clinical features
- Progressive symptoms depending on severity of hemorrhagic shock (N Engl J Med 2018;378:370)
- Tachycardia is the earliest indicator
- Pale / cool skin and extremities due to shunting of blood to vital organs
- Oliguria
- Lightheadedness, confusion or loss of consciousness
- Chest pain or abdominal pain and swelling may indicate internal hemorrhage
- Vomiting blood or blood in stool may indicate GI bleed
Laboratory
- Laboratory values may be normal in early acute hemorrhage and progressively deteriorate as interstitial fluid enters the intravascular space and crystalloids are infused
- Anemia and thrombocytopenia
- Arterial blood gas demonstrates decreased pH (acidosis) and decreased oxygen saturation
- Acute renal failure during oliguric phase
- Decreased glomerular filtration rate (GFR), increased serum creatinine and blood urea nitrogen (BUN); increased urine specific gravity and osmolality
- Conventional coagulation testing
- Increased prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR)
- Decreased fibrinogen
- Viscoelastic testing / thromboelastography (TEG)
- May demonstrate hypercoagulable tracing in early hemorrhage or moderate trauma
- Hypocoagulable tracing and hyperfibrinolysis with increasing injury severity score
- Electrolyte & metabolic disturbances due to massive transfusion
- Decreased plasma ionized calcium (iCa < 1.15 mmol/L) due to chelation by citrate in transfused blood products
- Hypocalcemia can manifest as tingling in fingers and lips, muscle cramps, tetany, arrhythmias
- Potassium accumulation in RBC supernatant may cause hyperkalemia with the potential for cardiac arrest
- Massive transfusion of neonates can result in transient hyperglycemia followed by large insulin release and rebound hypoglycemia
- Reference: Curr Anesthesiol Rep 2014;4:189
Case reports
- 14 month old girl requiring massive transfusion during neurosurgery (Am J Case Rep 2016;17:211)
- 16 year old girl, 35 year old woman and 36 year old man in cases with use of warm fresh whole blood in massive hemorrhage (Ulus Travma Acil Cerrahi Derg 2016;22:195)
- 35 year old woman with massive transfusion in emergency cesarean section (Case Rep Womens Health 2019;23:e00130)
- 60 year old woman with fatal hyperkalemia after massive transfusion (J Nippon Med Sch 2009;76:258)
- Male soldier with use of TEG after massive transfusion (Anaesthesia 2011;66:52)
Treatment
- Transfuse RBCs, platelets and plasma in 1:1:1 ratio (JAMA 2015;313:471)
- Adult patients receive 5 - 6 RBCs and equivalent number of plasma units and whole blood derived platelets
- 1 apheresis platelet is equivalent to a 6 pack (pool) of whole blood derived platelets
- Intraoperative cell salvage may distort blood product order ratios with an increased need for plasma and platelets relative to RBCs
- Whole blood offered in military or remote settings and increasingly in civilian trauma
- Benefit includes longer shelf life of whole blood than that of platelets
- Blood product selection
- Patients with valid type and screen may receive ABO type specific or compatible RBCs and plasma for massive transfusions if institutional policy allows
- O negative RBCs reserved for females of childbearing age and pediatric patients with unknown blood type; O positive should be used for other patients
- Type A plasma may safely be used in trauma patients with unknown blood type (Transfusion 2017;57:1879)
- Any platelet can be selected regardless of patient blood type but institutional policy may require ABO compatibility or the use of low titer platelets depending on patient age or weight
- Cryoprecipitate not universally included in massive transfusion sets but may provide mortality benefit (Br J Anaesth 2015;115:76)
- Early administration of tranexamic acid (TXA) reduces mortality from bleeding in trauma patients and in postpartum hemorrhage (Lancet 2010;376:23, Lancet 2017;389:2105)
- TXA: a synthetic analog of lysine
- Calcium chloride infusion as needed to maintain iCa > 2 mEq/L (4 mg/dL)
- Laboratory guided transfusion
- Can be used after initial resuscitation with massive transfusion
- Plasma for INR > 2.0 or prolonged ACT/R time on TEG
- Cryoprecipitate or fibrinogen concentrate for fibrinogen < 100 mg/dL or prolonged K time/decreased α angle on TEG
- Platelets for count < 50,000 per microliter or decreased max amplitude on TEG
- Tranexamic acid for increased LY30 (amplitude at 30 minutes) on TEG, indicative of increased fibrinolysis
- Recombinant factor VIIa can be utilized in the setting of oozing with a normal TEG and abnormal prothrombin time
Board review style question #1
A young female is brought into the trauma center after a motor vehicle accident with several injuries and large volume bleeds from the abdomen and lower extremities. The patient was infused with crystalloid solution on the way to the hospital due to blood loss and vital sign changes indicating hypovolemic shock. An order is placed for a massive transfusion while the patient is taken to the operating room. What would be the most appropriate set of blood products to issue in this situation if the patient's blood type is still unknown?
- 6 units O positive RBCs, 6 units AB plasma and a 6 pack of whole blood derived platelets
- 1 unit O negative RBC, 1 unit A plasma and 1 apheresis platelet unit
- 6 units O negative RBCs, 6 units A plasma and 6 apheresis platelet units
- 1 unit O negative RBC, 1 unit AB plasma and 1 whole blood derived platelet unit
- 6 units O negative RBCs, 6 units AB plasma and 1 apheresis platelet unit
Board review style answer #1
E. 6 units O negative RBCs, 6 units AB plasma and 1 apheresis platelet unit. The patient is a young female with an unknown blood type and therefore should receive type O negative RBCs. Type O RBCs will prevent an acute hemolytic transfusion reaction due to ABO incompatibility. RhD negative units will prevent alloimmunization to the RhD antigen and the potential for hemolytic diseases of the fetus / newborn (HDFN) in a subsequent pregnancy. Although type A plasma could safely be used for massive transfusion in trauma patients with an unknown blood type, neither of the answer choices with this option (B and C) have the correct choice of RBCs or platelets. An adequate platelet dose for a massive transfusion in an adult would be 1 apheresis unit or a 6 pack pool of whole blood derived platelets. Answer choice A supplies the correct volume of platelets but the use of O positive RBCs would not be the first choice for a young female and answer choice B does not supply an adequate volume of RBCs or plasma.
Comment Here
Reference: Massive transfusion
Comment Here
Reference: Massive transfusion
Board review style question #2
A patient with a severe gastrointestinal bleed required a massive transfusion. The bleeding has been controlled but the patient is now complaining of perioral tingling, hand numbness and muscle cramping. Which of the following labs would be abnormal and associated with the patient's symptoms?
- Hemoglobin
- Ionized calcium
- Platelet count
- Potassium
- Prothrombin time (PT) / international normalized ratio (INR)
Board review style answer #2
B. Ionized calcium. Blood products contain citrate as part of the preservative solution to prevent coagulation during collection and storage. Citrate is also capable of chelating calcium and may cause hypocalcemia after a large volume transfusion. Hypocalcemia initially presents with perioral tingling and paresthesia but may progress to tetany, confusion and arrhythmias. It is important to note that the total calcium may be normal and the ionized calcium should specifically be monitored during massive transfusions.
Comment Here
Reference: Massive transfusion
Comment Here
Reference: Massive transfusion