Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Molecular / cytogenetics images | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Numbere N, Huber AR. HER2 stomach/GE junction. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsher2GI.html. Accessed May 11th, 2024.
Definition / general
- Cell surface receptor that functions in normal cell growth, encoded by ERBB2
- Gene amplification and protein overexpression lead to tumorigenesis
- HER2 testing identifies tumors that may respond to HER2 targeted therapy with trastuzumab
Essential features
- A cytoplasmic marker, overexpression of which bodes poor prognosis in untreated gastric and gastroesophageal junction (GEJ) adenocarcinomas
- Receptor overexpression or gene amplification identifies tumors that might respond to HER2 targeted therapy with trastuzumab
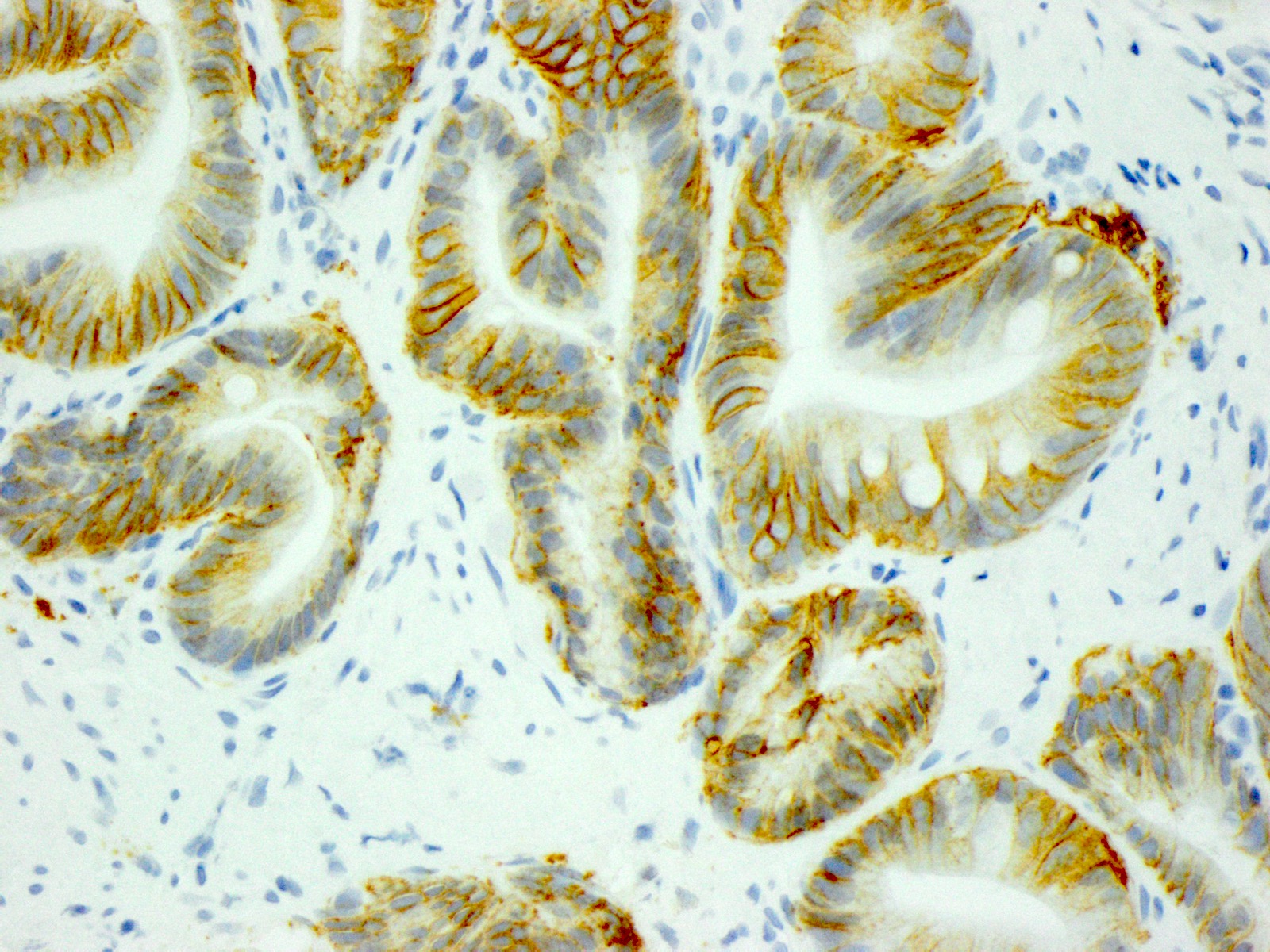
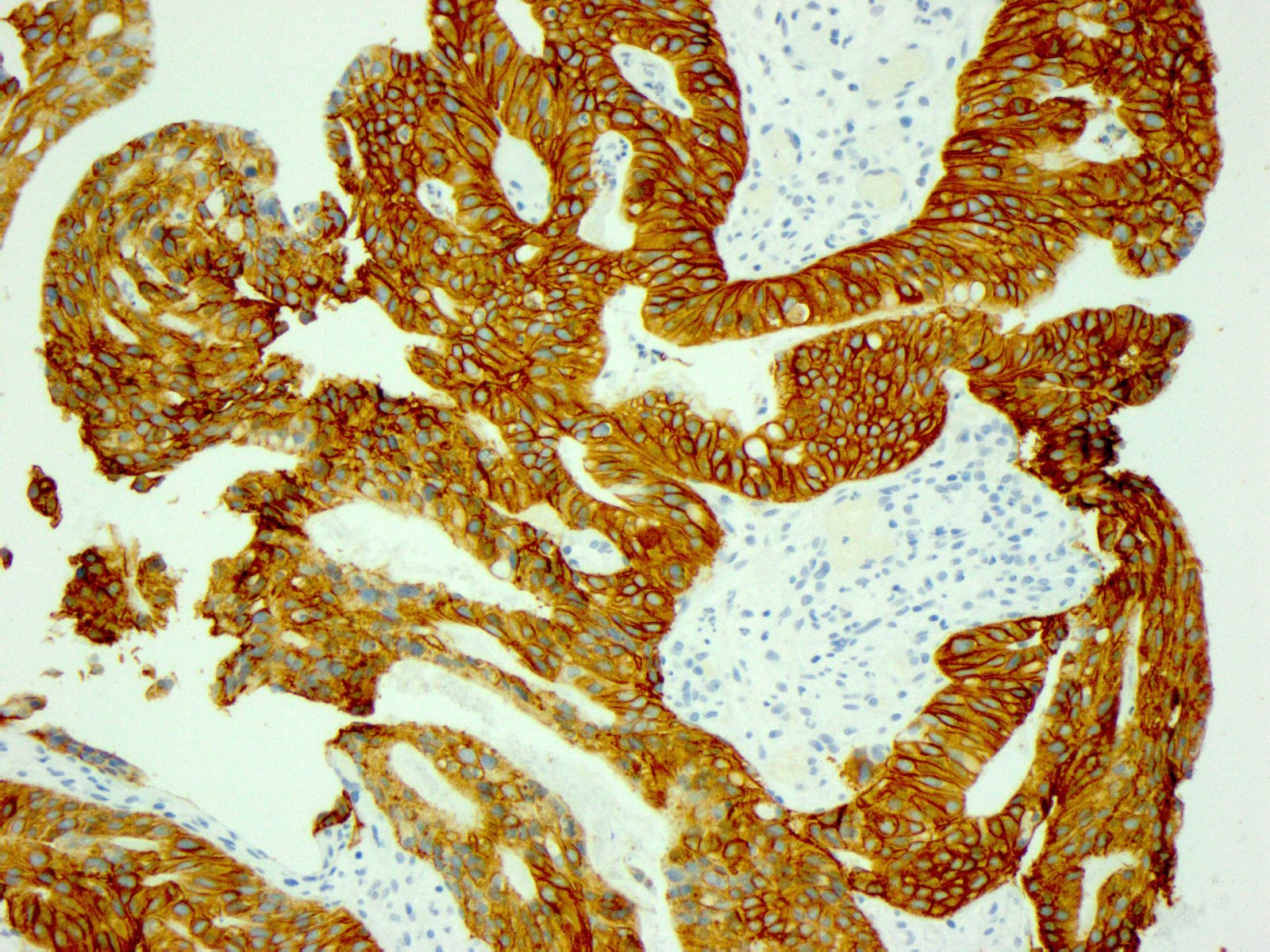
- Immunohistochemistry for HER2 is scored on a 4 tiered scale that ranges from 0 to 3+
- Strong lateral or complete basolateral membrane staining is adequate for positivity in gastric and GEJ adenocarcinomas, unlike in breast cancers, where circumferential membrane reactivity is required for positivity
- HER2 is assessed differently in resection and biopsy specimens, with the major difference being that only a cluster of 5 positive cells is required in a biopsy
Terminology
- Gene is ERBB2 (erythroblastosis oncogene B2)
- Protein has variably been termed CD340, HER-2, HER2 and HER2 / neu
Pathophysiology
- ERBB2 gene is a proto-oncogene located on chromosome 17q12
- HER2, the protein product of the gene, is a transmembrane receptor tyrosine kinase of the epidermal growth factor receptor (EGFR) family
- Other members of the EGFR family are HER1, HER3 and HER4
- HER2 has no ligand but forms dimeric complexes with other EGFR family receptors after binding of ligand to their extracellular domains
- Dimerization leads to phosphorylation of tyrosine residues in the cytoplasmic domain and activation of downstream signaling
- This leads to cell proliferation and suppression of apoptosis (Biochim Biophys Acta 1994;1198:165)
- In normal cells, receptor activation and dimerization are tightly regulated and short lived events
- In tumors, HER2 gene amplification results in increased number of receptors, constitutive receptor signaling in the absence of ligand binding, markedly increased gene expression, excessive uncontrolled growth and tumorigenesis
- HER2 also enhances invasiveness and metastasis independent of its effects on cell proliferation (Cancer Res 1997;57:1199)
Diagrams / tables
Clinical features
- Reported rates of HER2 positivity in gastric and GEJ adenocarcinomas range from 8.2 - 35% (Gastric Cancer 2014;17:1, J Gastric Cancer 2017;17:52)
- Higher rates of HER2 positivity are seen in intestinal type tumors, patients younger than 60 years of age, moderately differentiated tumors and GEJ tumors (versus tumors in more distal gastric sites) (Cancer 2000;88:238, World J Gastroenterol 2012;18:2402, Lancet 2010;376:687)
- Testing multiple samples from the same patient increases the rate of HER2 positivity due to tumor heterogeneity (Am J Clin Pathol 2019;151:461)
- HER2 testing is carried out by immunohistochemistry (IHC) or by in situ hybridization (ISH) on formalin fixed and paraffin embedded tissue
- Currently not enough evidence to recommend for or against genomic tests (Arch Pathol Lab Med 2016;140:1345)
- HER2 IHC assesses the level of expression of the membrane receptor
- Intensity of membrane staining is scored; in surgical specimens, the percentage of cancer cells with positive immunoreactivity is also factored into scoring
- Scores range from 0 to 3+ (Table 1) (Histopathology 2008;52:797)
- ISH assesses the status of gene amplification
- Most common use of ISH is for confirmation of IHC equivocal (2+) cases
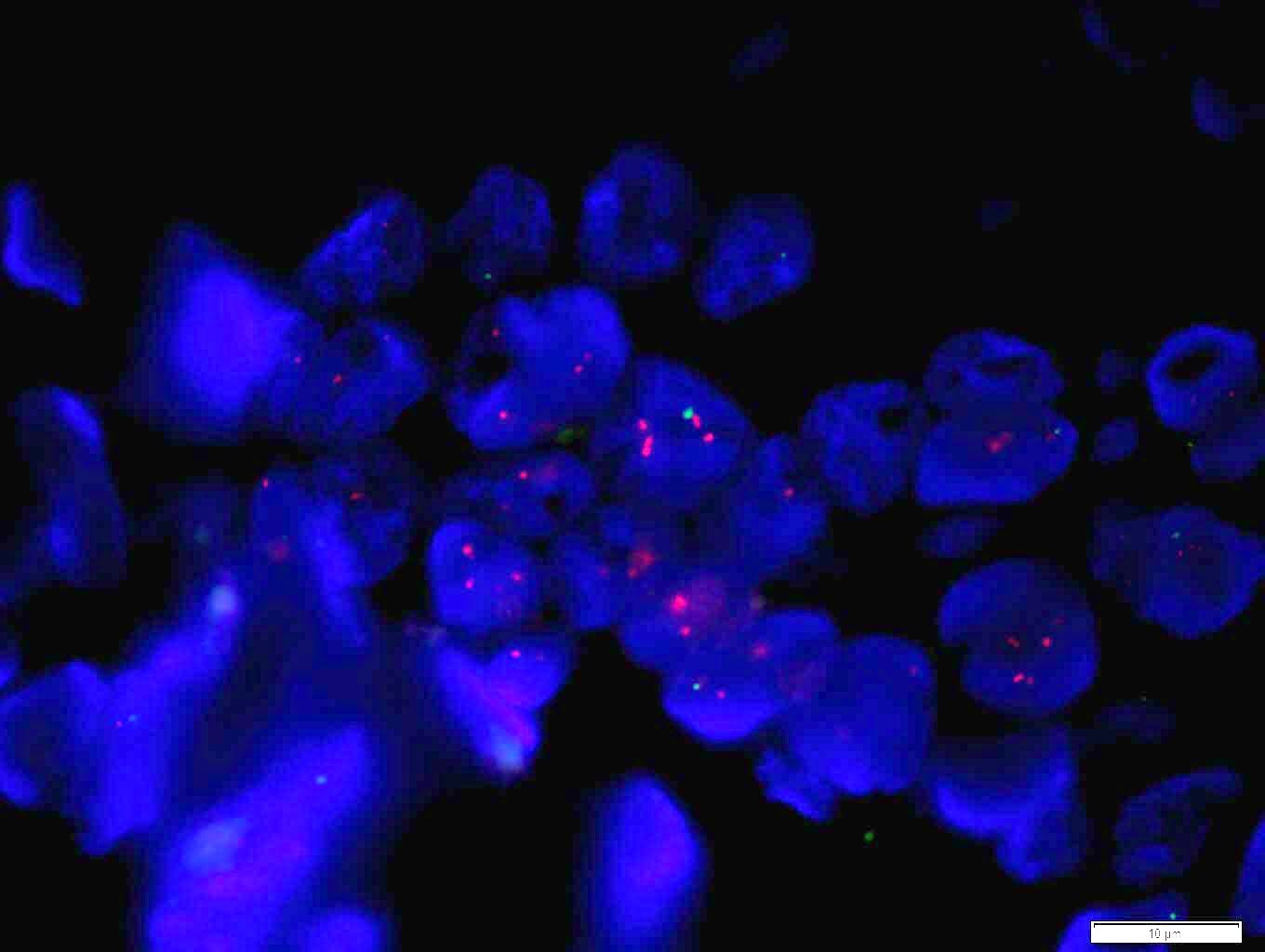
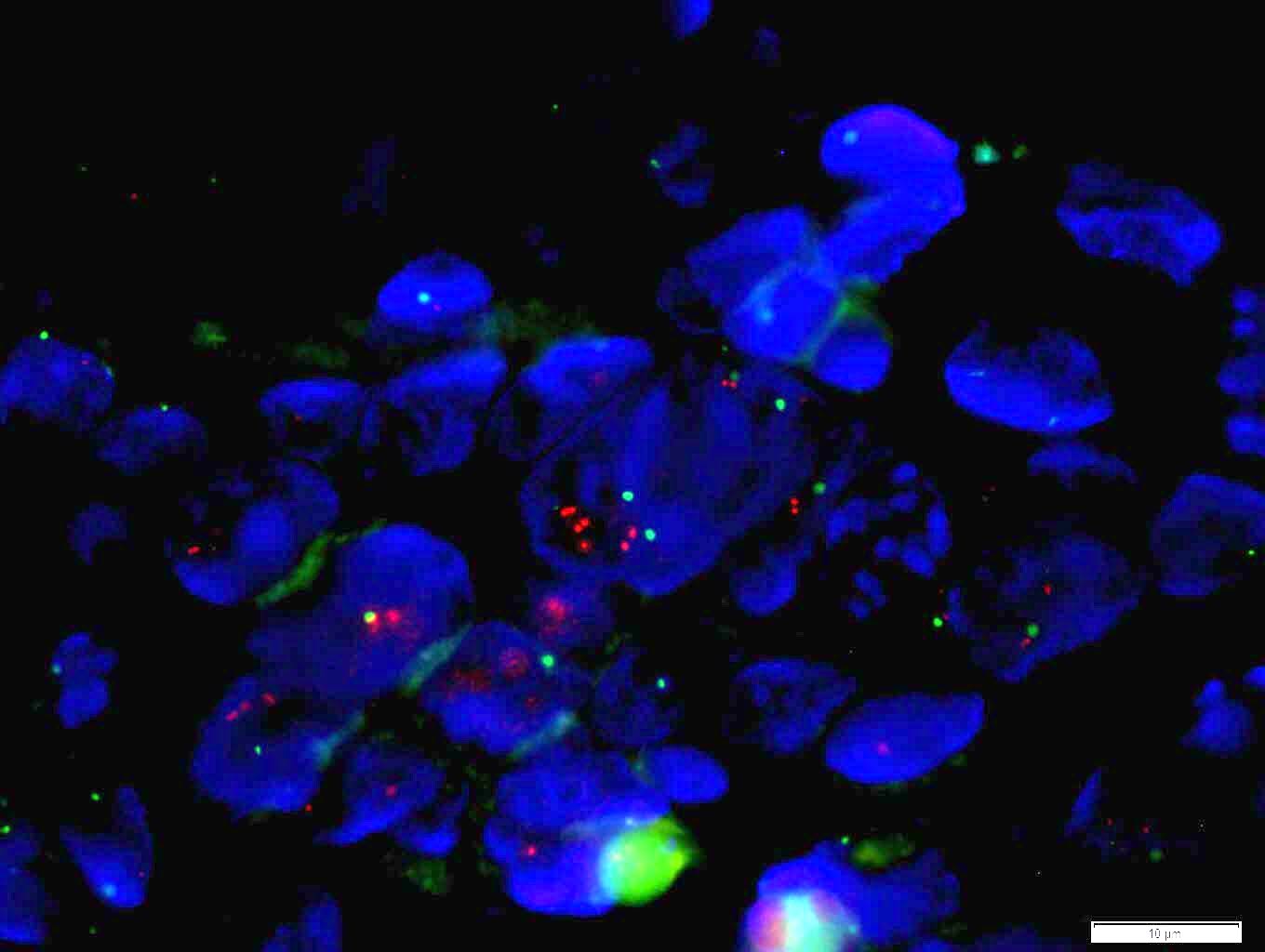
- ISH techniques include fluorescence in situ hybridization (FISH), dual in situ hybridization and bright field in situ hybridization methodologies like chromogenic in situ hybridization (CISH) and silver enhanced in situ hybridization
- ISH is reported as negative (nonamplified) or positive (amplified)
- The following are part of the guidelines for HER2 testing issued by the College of American Pathologists and the American Society of Clinical Oncology (Arch Pathol Lab Med 2016;140:1345)
- Recommended that testing be carried out before initiation of HER2 targeted therapy
- Biopsy and surgical specimens are preferred but FNA cell block specimens are acceptable as an alternative
- Areas of the tumor with the lowest grade morphology should be selected for testing
- If the tumor is morphologically heterogeneous, more than 1 tissue block may be selected for testing
- ISH scoring should be performed on areas of invasive adenocarcinoma with the strongest HER2 expression by IHC
Interpretation
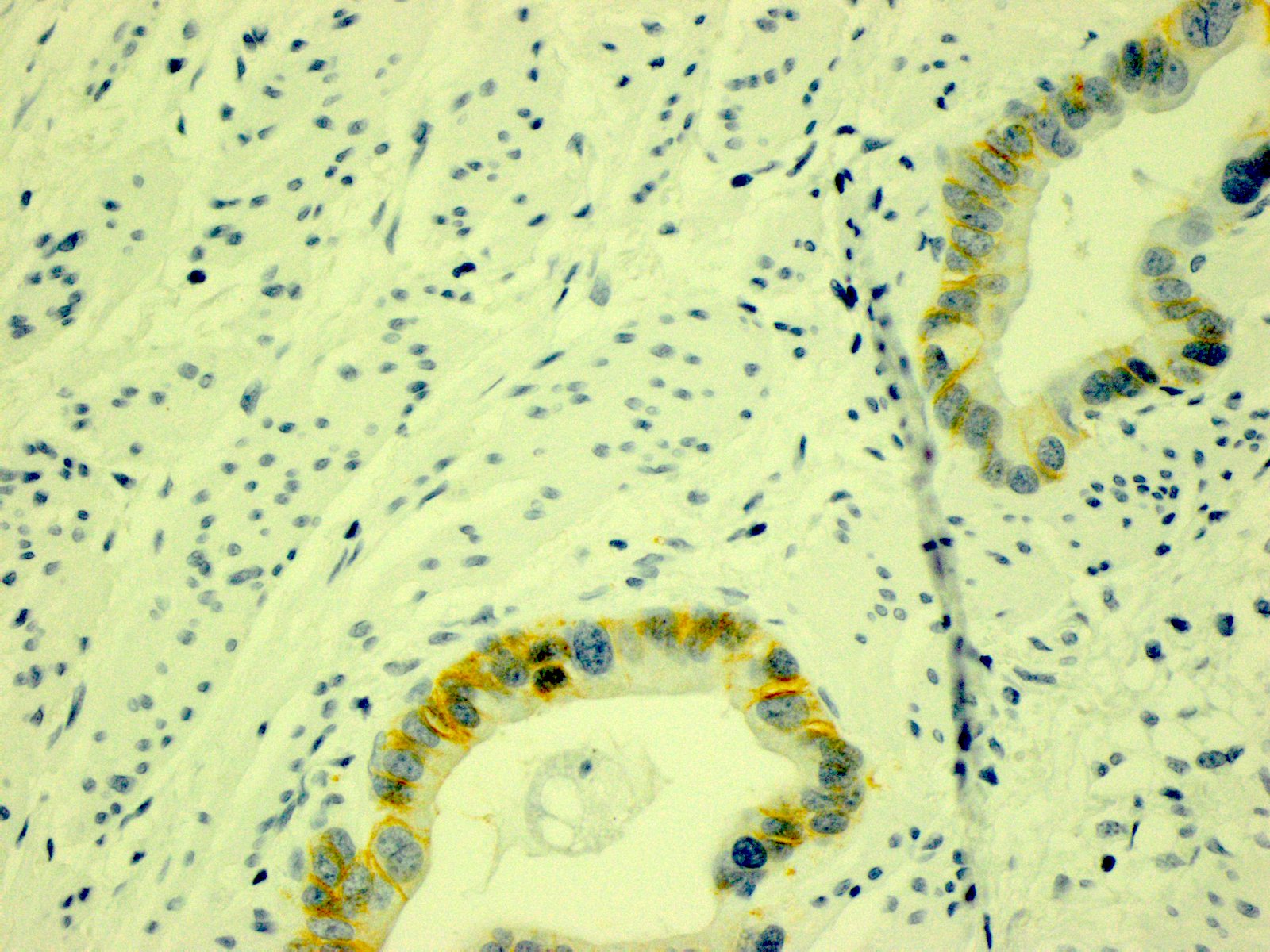
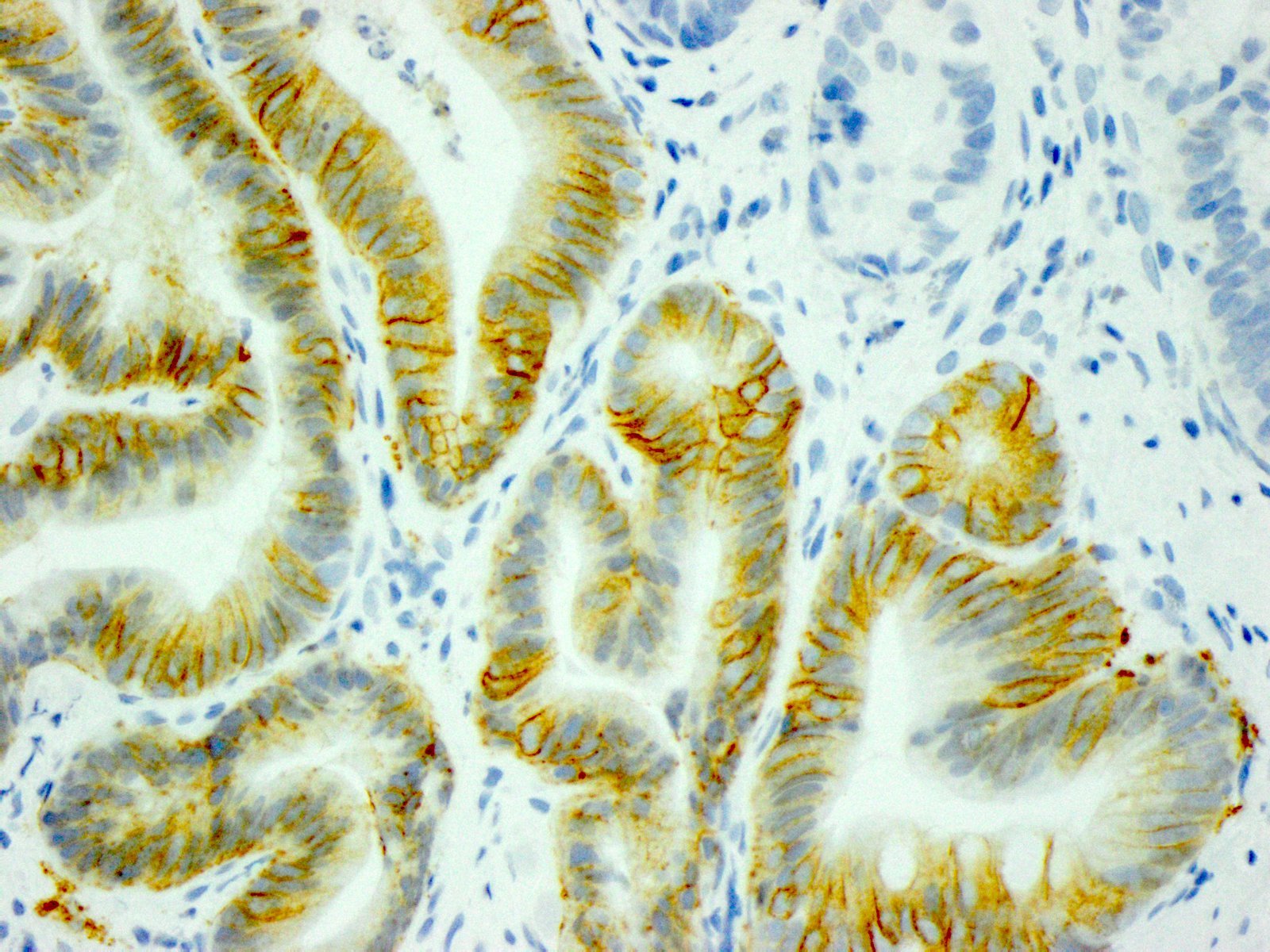
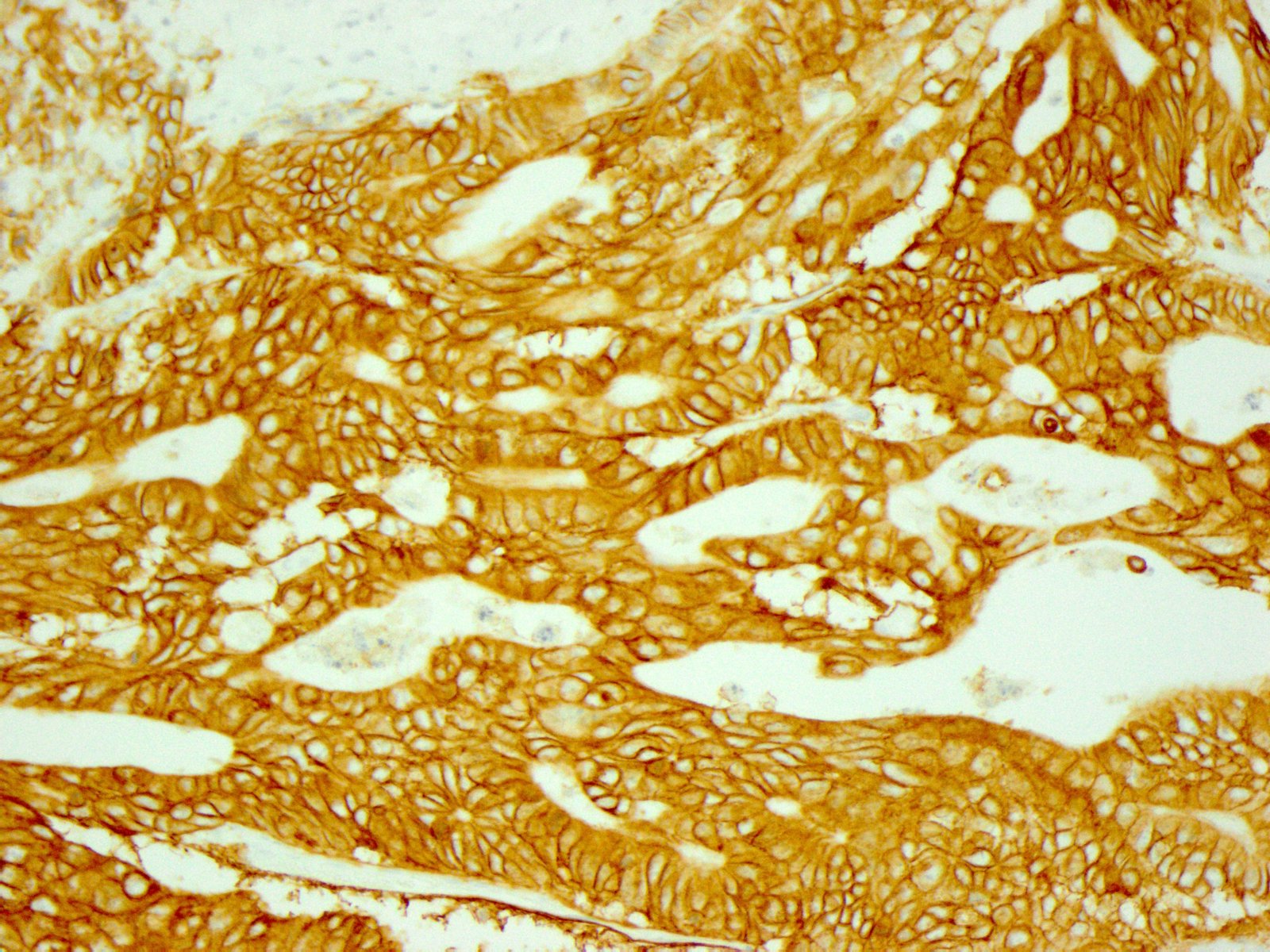
- In gastric adenocarcinomas, only lateral or basolateral membranous staining (not circumferential staining) by IHC is necessary for positivity
- Positive FISH is HER2:CEP17 ratio ≥ 2.0 (dual probe assays) or HER2 copy number > 6.0 (single probe assays) (Mod Pathol 2012;25:637)
- In the U.S., a positive HER2 testing result is IHC 3+ or positive ISH (Gastric Cancer 2014;17:1)
- In Europe, a positive result is IHC 3+ or IHC 2+ with positive ISH (Gastric Cancer 2014;17:1)
Table 1: HER2 testing for gastric and GEJ adenocarcinomas (Histopathology 2008;52:797, Arch Pathol Lab Med 2016;140:1345)
| Pattern of HER2 membrane reactivity by IHC in surgical specimen | Pattern of HER2 membrane reactivity by IHC in biopsy specimen | HER2 score by IHC | HER2 status by IHC | Recommendations for ISH testing and treatment |
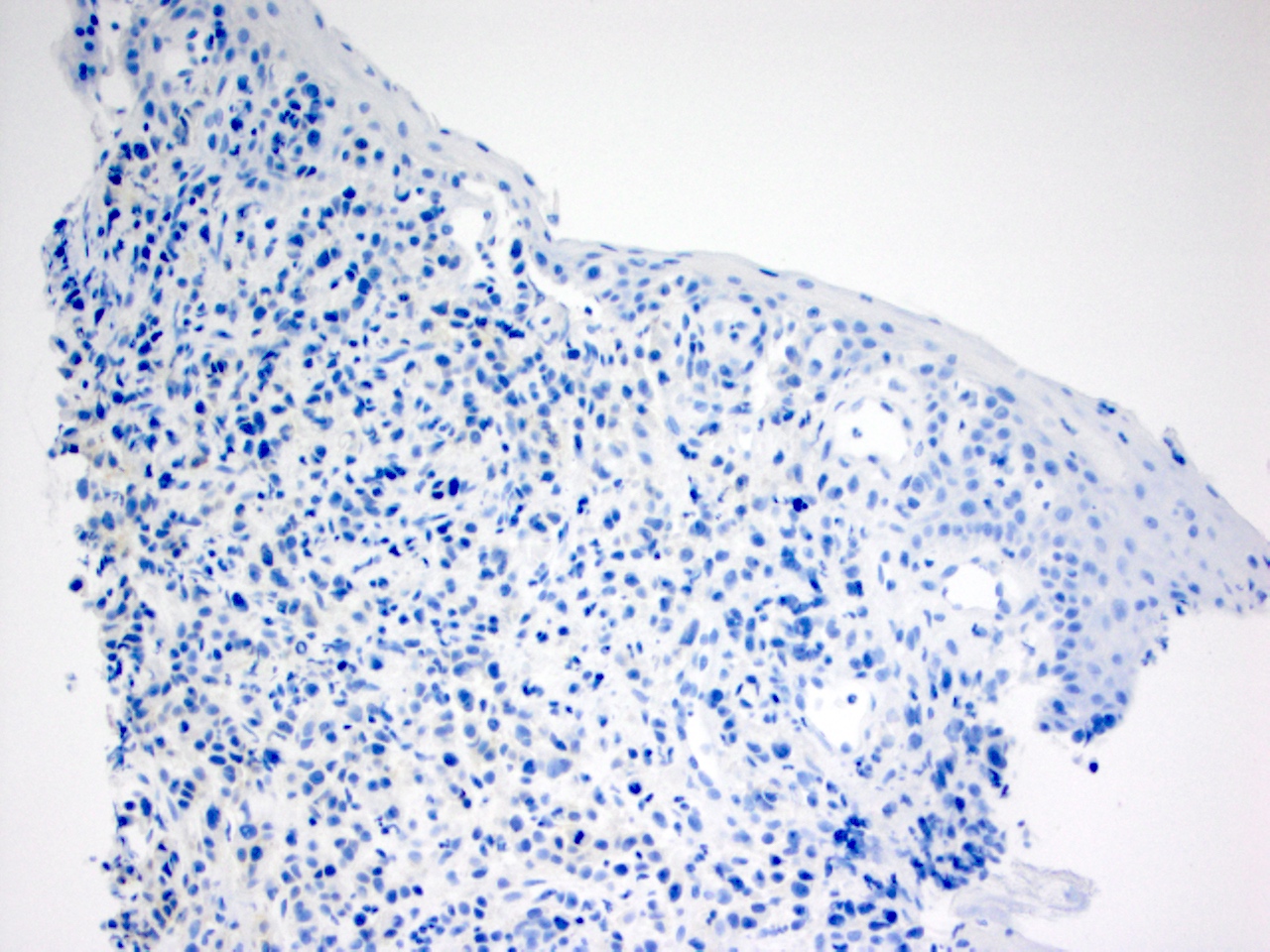
| No reactivity or membrane reactivity in < 10% of tumor cells | No membrane reactivity in cancer cells | 0 | Negative | No ISH testing HER2 targeted treatment not recommended |
| No more than faint partial membrane reactivity in 10% or more of tumor cells | A cluster of at least 5 cancer cells with no more than faint membrane reactivity regardless of the percentage of positive cancer cells | 1+ | Negative | No ISH testing HER2 targeted treatment not recommended |
| 10% or more of the tumor cells show weak to moderate lateral or complete basolateral membrane reactivity | A cluster of at least 5 cancer cells with weak to moderate lateral or complete basolateral membrane reactivity regardless of the percentage of positive cancer cells | 2+ | Equivocal | Reflex to ISH Administer HER2 targeted treatment only if ISH positive |
| 10% or more of the tumor cells show strong lateral or complete basolateral membrane reactivity | A cluster of at least 5 cancer cells with strong lateral or complete basolateral membrane reactivity regardless of the percentage of positive cancer cells | 3+ | Positive | No ISH testing Eligible for HER2 targeted treatment |
Uses by pathologists
- Testing for HER2 protein overexpression or gene amplification identifies patients who may derive benefit from HER2 targeted therapy with agents like trastuzumab (Lancet 2010;376:687)
- Trastuzumab is a humanized recombinant anti-HER2 targeting monoclonal antibody approved for combination treatment (with chemotherapy) of patients with inoperable locally advanced, recurrent or metastatic gastric or GEJ adenocarcinomas (Ther Adv Med Oncol 2013;5:143)
Prognostic factors
- In many studies, HER2 overexpression in untreated gastric and gastroesophageal cancers has been found to be associated with more aggressive biologic behavior, shorter survival, serosal invasion, higher stage and higher rates of lymph node and distant organ metastasis (J Cancer 2012;3:137, World J Gastroenterol 2012;18:2402)
- However, other studies have found HER2 overexpression in adenocarcinomas of the esophagus, GEJ or gastric cardia to be associated with better survival, lower tumor grade and stage and lower rates of lymph node metastasis (BMC Cancer 2019;19:38, Clin Cancer Res 2012;18:546)
- Intratumoral heterogeneity (nonuniform HER2 IHC scores in different areas of a tumor), is seen in up to 26% of metastatic or unresectable gastric adenocarcinoma and is associated with lower progression free and overall survival (World J Clin Cases 2019;7:1964)
- HER2 overexpression (by IHC or FISH) identifies patients who might derive benefit from HER2 targeted therapy
- Trastuzumab based chemotherapy in patients with HER2 overexpression results in prolongation in median survival, progression free survival, overall response rate, clinical benefit rate and duration of response (Lancet 2010;376:687)
Microscopic (histologic) images
Molecular / cytogenetics images
Sample pathology report
- Gastroesophageal junction, mass, biopsy:
- Adenocarcinoma
- Immunohistochemistry HER2 analysis:
- Results: Positive
- Interpretation: 3+
- Methodology:
- Antibody and assay methodology: Rabbit anti-human HER2, HerceptestTM (FDA-approved test kit), (Dako, Carpenteria, CA).
- Control slides examined: External kit slides provided by manufacturer (cell lines with high, low and negative HER2 protein expression) and in-house known HER2 amplified control tissue were evaluated along with the test tissue and showed appropriate staining.
- Interpretation: Interpretation based on recommendations put forth by Hofmann et al. in assessment of a HER2 scoring system for gastric cancer: results from a validation study in Histopathology 2008;52:797.
Interpretation criteria
| 3+ Positive | Surgical specimen with moderate to strong complete or basolateral membranous reactivity in more than 10% of cancer cells Biopsy sample with cohesive cluster with moderate to strong complete or basolateral membranous reactivity |
| 2+ Equivocal | Surgical specimen with weak to moderate complete or basolateral reactivity in more than 10% of cancer cells Biopsy sample with cohesive cluster with weak to moderate complete, basolateral or lateral membranous reactivity |
| 1+ Negative | Surgical specimen with faint barely perceptible membranous reactivity in more than 10% of cells Biopsy sample with cohesive cluster with faint or barely perceptible membranous reactivity |
| 0 Negative | Surgical specimen with no reactivity or reactivity in less than 10% of cells Biopsy sample with no membranous reactivity in any cancer cell |
- See also the HER2 biomarker reporting template of the College of American Pathologists at: CAP: Template for Reporting Results of HER2 (ERBB2) Biomarker Testing of Specimens from Patients with Adenocarcinoma of the Stomach or Gastroesophageal Junction [Accessed 5 August 2020]
Additional references
Board review style question #1
While reviewing the HER2 immunohistochemical stains of a biopsy specimen of gastroesophageal junction adenocarcinoma, the pathologist observes that multiple groups of more than 5 tumor cells that constitute 5% of the neoplasm show strong complete lateral and basolateral membranous reactivity. The correct interpretation of this finding is
- Equivocal because only strong complete basolateral (and not lateral) membrane reactivity is a positive result
- Negative because a positive result is strong circumferential membrane staining in > 10% of tumor cells
- Negative because a positive result is strong lateral or complete basolateral membrane reactivity in ≥ 10% of the cancer cells
- Positive because a positive result is moderate lateral or basolateral reactivity in at least 10% of neoplastic cells
- Positive because there is strong lateral or complete basolateral membrane positivity in a cluster of more than 5 cancer cells
Board review style answer #1
E. A positive HER2 result on immunohistochemistry (a 3+ score) in a gastric biopsy specimen is strong lateral or complete basolateral staining in a cluster of at least 5 cancer cells, regardless of the percentage of the tumor that the cluster comprises. The remaining answer choices are incorrect because they deviate from this criterion. It is noteworthy that HER2 is interpreted differently in surgical specimens than in biopsies as at least 10% of the neoplastic cells in surgical specimens must show strong complete lateral or basolateral staining to count as a positive result (answer choice C). Choice B is the definition of a positive result (3+ score) in breast cancers. HER2 is also scored using different criteria in gastric and breast cancers.
Comment Here
Reference: HER2 stomach/GE junction
Comment Here
Reference: HER2 stomach/GE junction