Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Interpretation | Uses by pathologists | Microscopic (histologic) images | Cytology images | Positive staining - disease | Molecular / cytogenetics description | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Zhou F. EGFR mutation specific antibodies. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsegfrmutationspecific.html. Accessed May 11th, 2024.
Definition / general
- ~90% of EGFR mutations in lung adenocarcinoma can be attributed to 2 targetable mutations detectable by immunohistochemistry (IHC) (J Thorac Oncol 2017;12:612)
- EGFR L858R point mutation in exon 21
- EGFR E746_A750 del (15 base pair deletion) in exon 19
Essential features
- EGFR gene mutations are present in 10 - 50% of lung adenocarcinoma patients
- Prevalence depends on population subgroup, including ethnicity
- ~90% of EGFR mutations in the lung can be attributed to 2 targetable mutations, which can be detected by immunohistochemistry (IHC) if the specimen is inadequate for molecular testing
- When using EGFR mutation specific IHC, use stringent interpretation criteria and close quality assurance (QA) to maintain high specificity (~99%) and inform clinicians of relatively low sensitivity (~70 - 80%)
- Beware of cross reactivity with HER2 (rare)
Terminology
- EGFR: epidermal growth factor receptor
- Synonyms: HER1, ERBB, ERBB1, others (NIH: EGFR Epidermal Growth Factor Receptor [Accessed 5 January 2024])
- Mutations detected by mutation specific EGFR IHC
- EGFR c.2573T>G (p.L858R): point mutation in exon 21
- Detectable using IHC EGFR antibody clone 43B2
- EGFR c.2185_2283del (p.E746_A750del): in frame 15 base pair deletion in exon 19
- Detectable using IHC EGFR antibody clone 6B6
- EGFR c.2573T>G (p.L858R): point mutation in exon 21
- Not all EGFR antibodies are appropriate for IHC staining of lung adenocarcinomas
- EGFR total protein IHC is not mutation specific and should not be used in lung adenocarcinoma cases, as there is no treatment for EGFR amplification
Pathophysiology
- EGFR encodes a 170 kDa transmembrane glycoprotein of the protein kinase superfamily
- EGFR protein is activated by binding of epidermal growth factor (EGF) and transforming growth factor alpha (TGFα) → dimerization → tyrosine kinase activity → cell growth
- Certain EGFR mutations = constitutive activity
- Tyrosine kinase inhibitor (TKI) drugs work on some of these EGFR mutations
Clinical features
- EGFR gene mutations are present in 10 - 50% of lung adenocarcinoma patients
- Prevalence depends on population subgroup, including ethnicity
- ~90% of EGFR mutations in the lung can be attributed to 2 targetable mutations, which can be detected by IHC
- EGFR L858R point mutation in exon 21
- EGFR E746_A750 del (15 base pair deletion) in exon 19
- References: Am J Cancer Res 2015;5:2892, J Thorac Oncol 2017;12:612
Interpretation
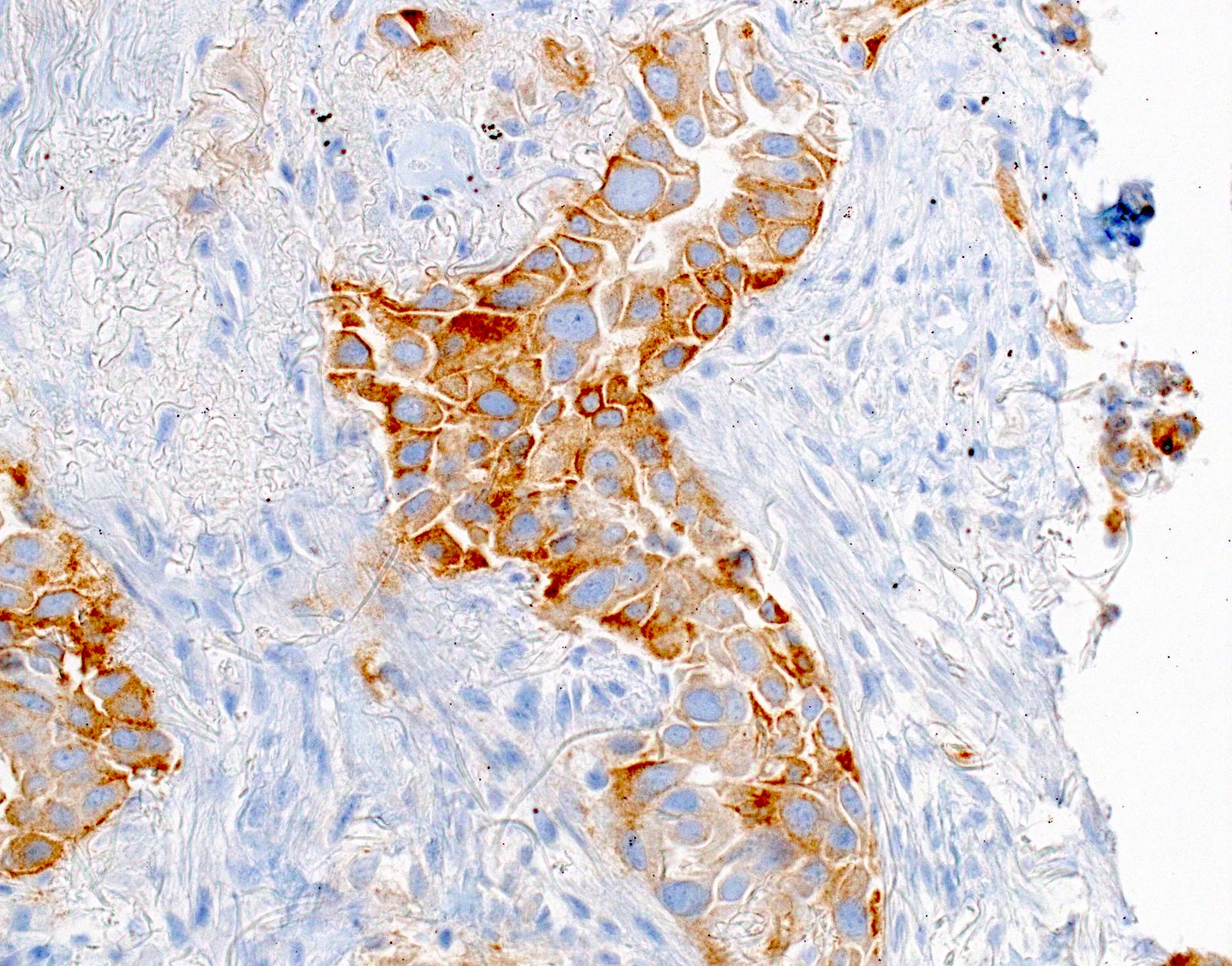
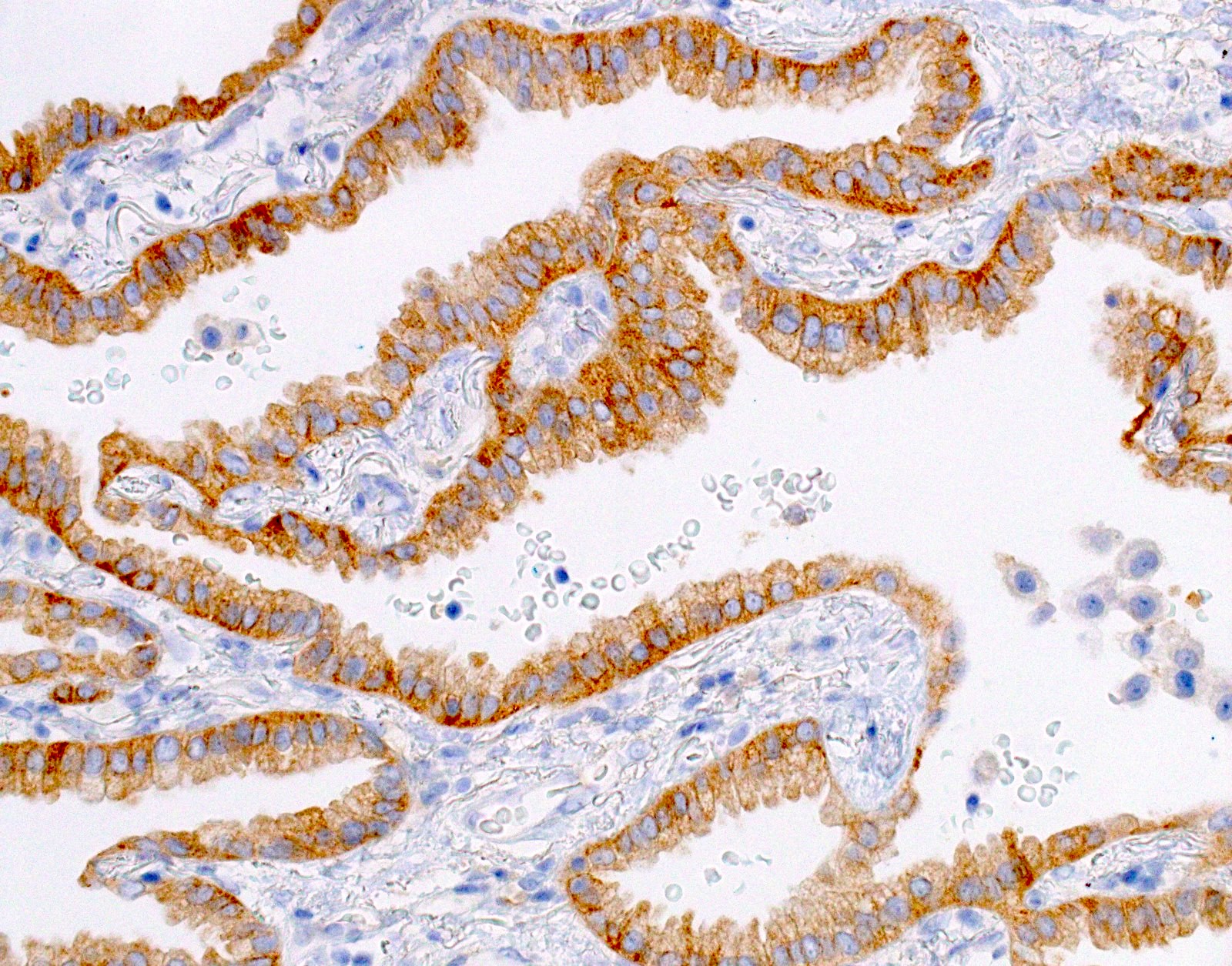
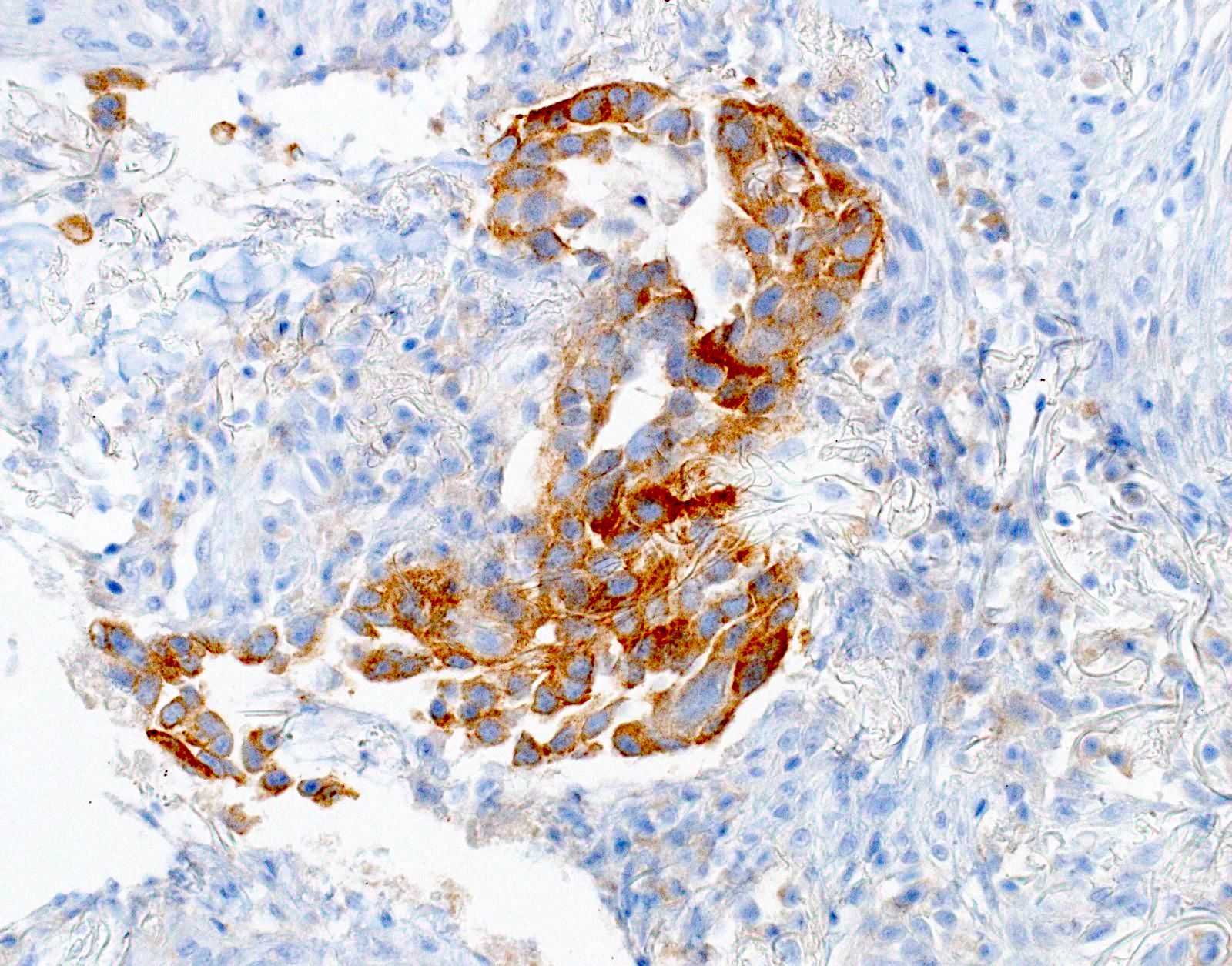
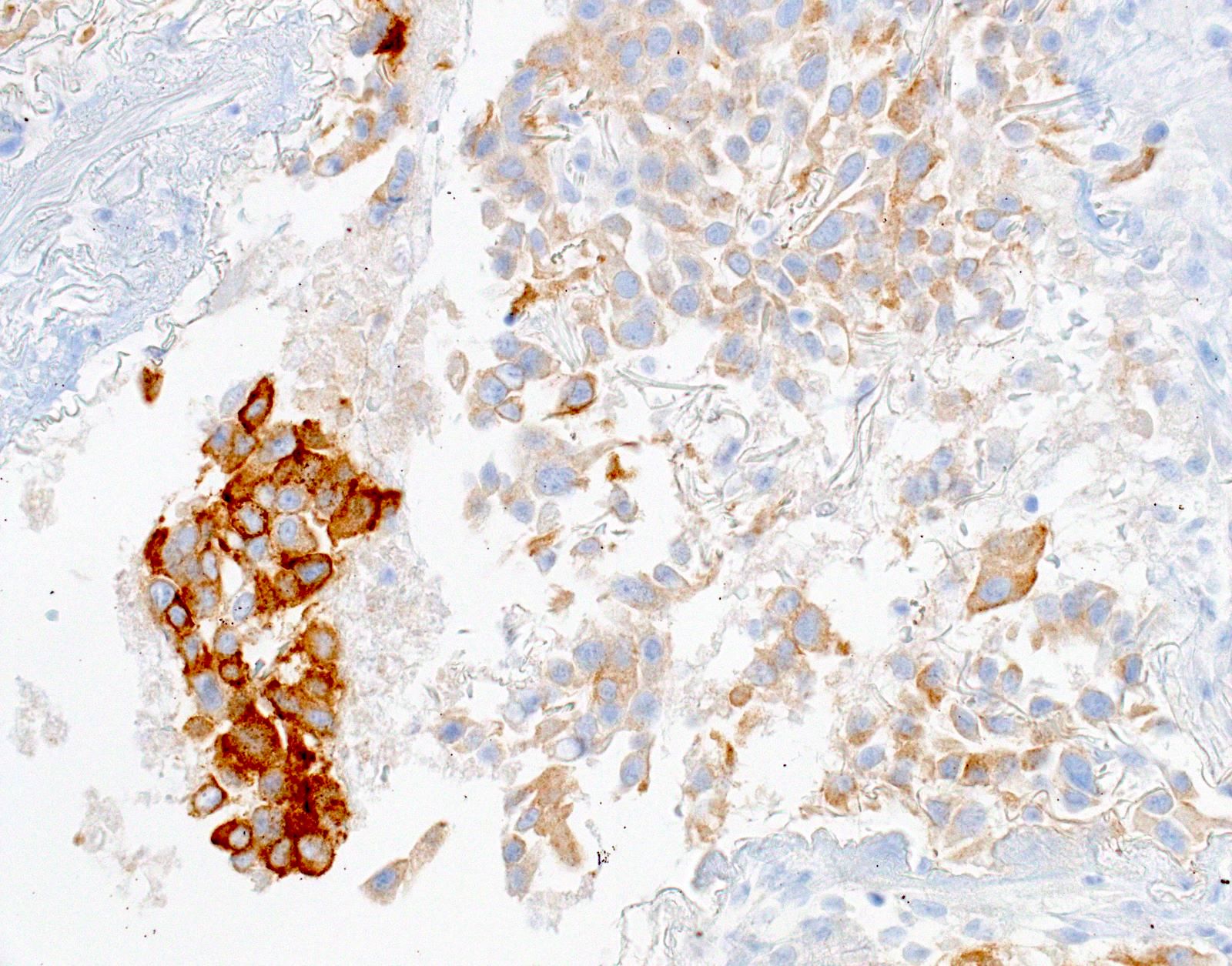
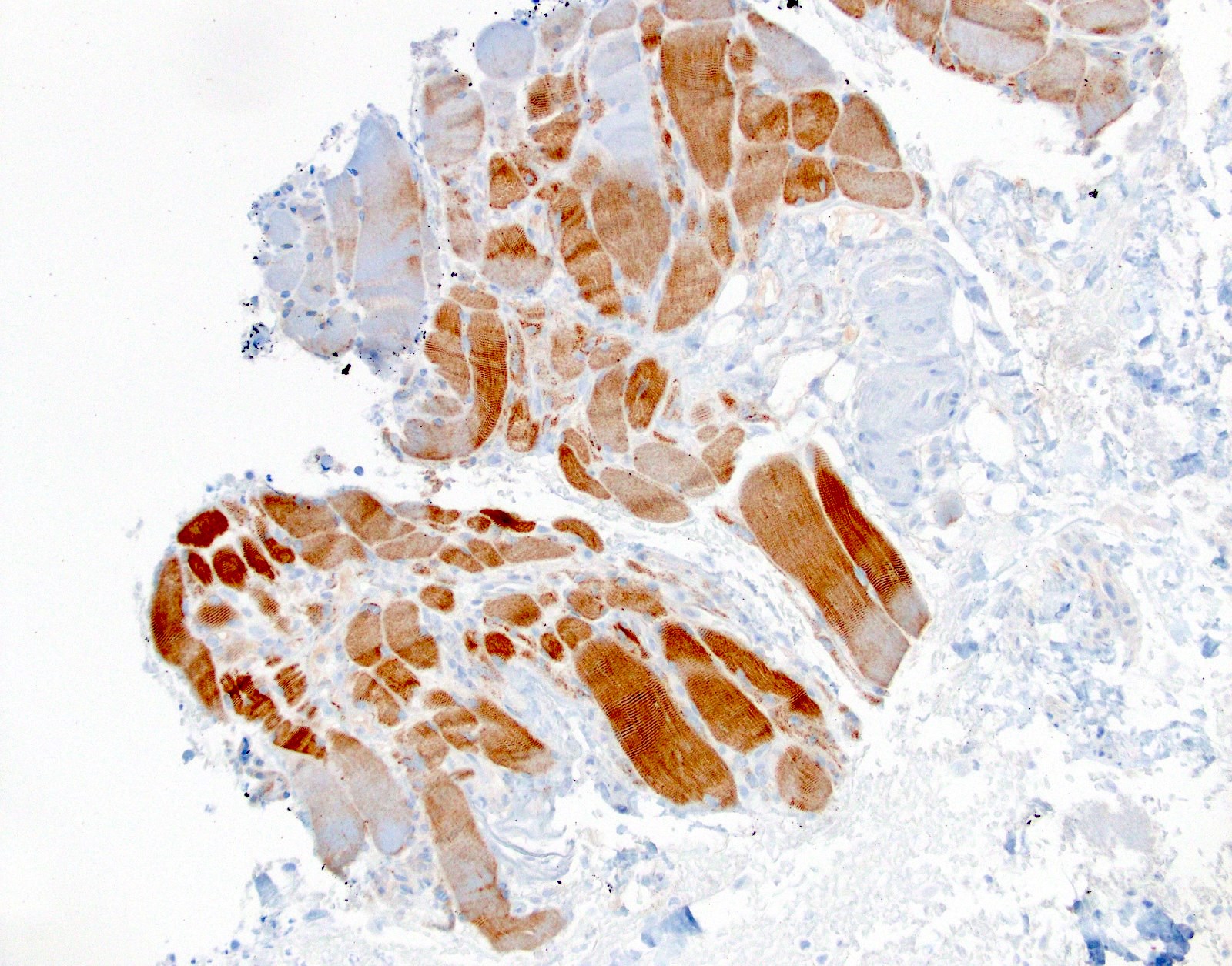
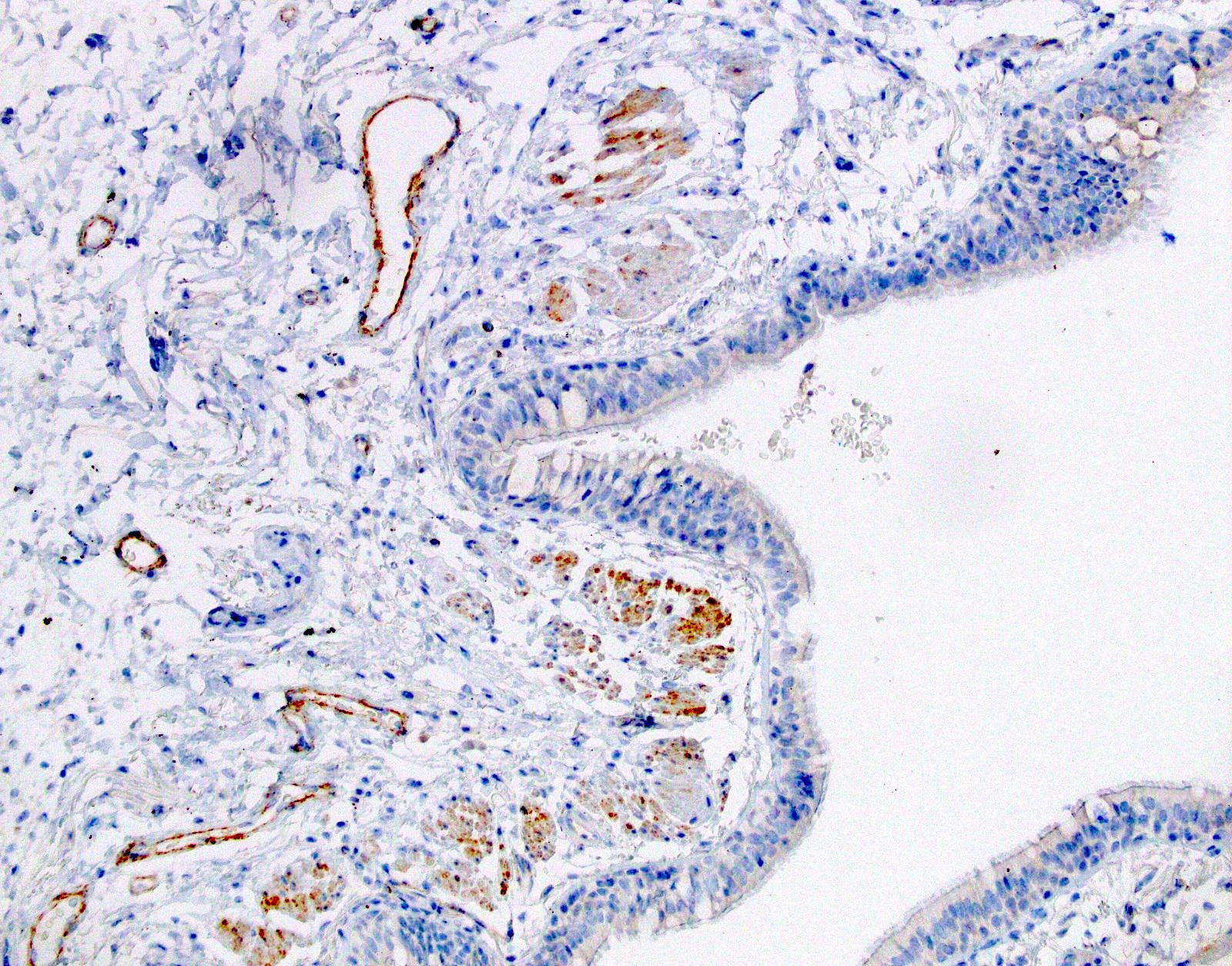
- Positive: strong, diffuse membranous staining, variable granular cytoplasmic staining
- Use stringent criteria to avoid false positive results; high specificity (~99%)
- Positive IHC result for EGFR clone 43B2 or 6B6 associated with response to EGFR TKI drugs
- ~1% false positives (cross reactivity with HER2 overexpression)
- Usually 2+ but rarely 3+ (Mod Pathol 2013;26:1197, see Microscopic images)
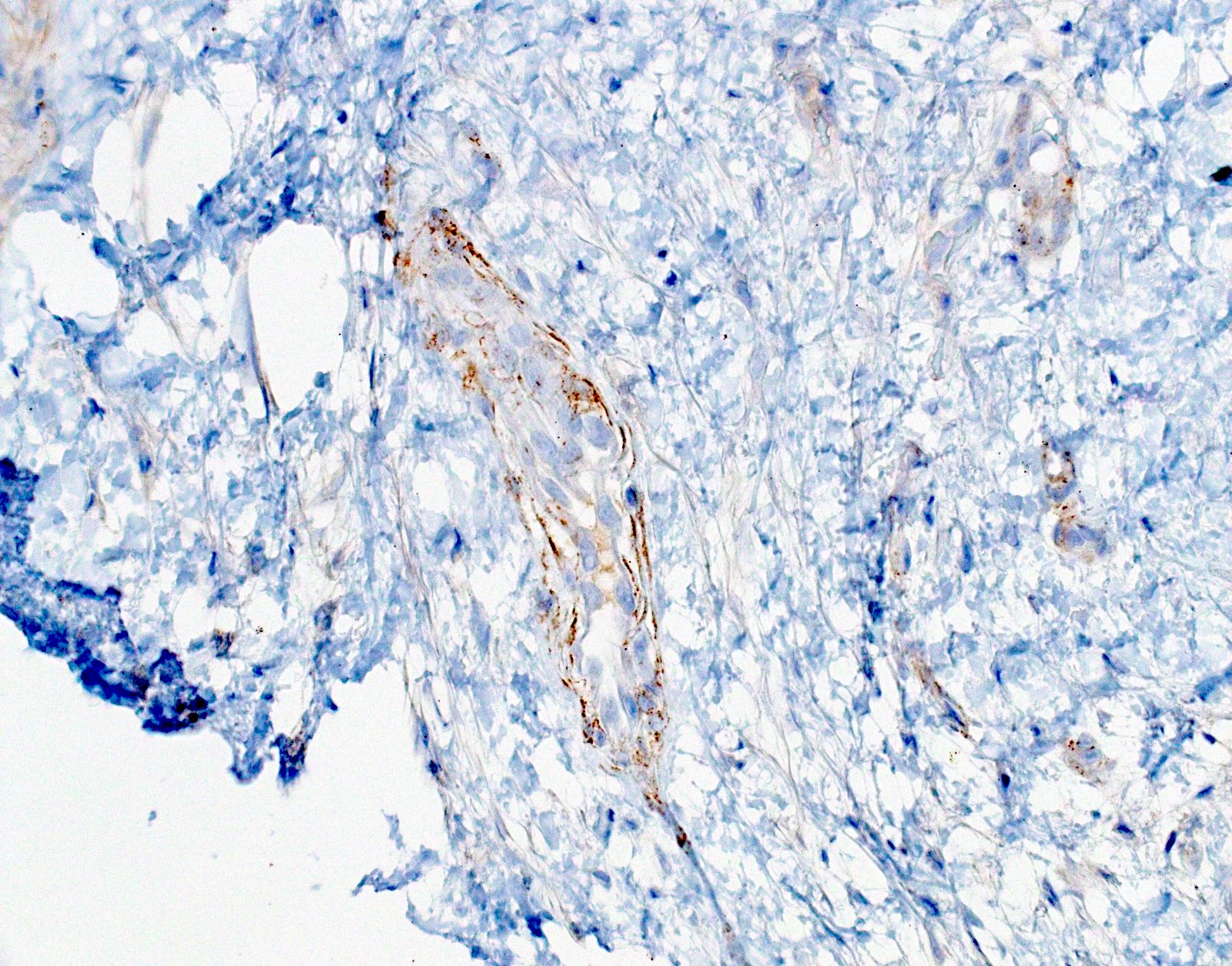
- Equivocal or negative: moderate / patchy membranous staining or cytoplasmic staining only
- Negative: no staining or weak membranous staining or weak cytoplasmic staining only
- Low sensitivity (~70 - 80%)
- All results (positive, equivocal and negative) should be confirmed by molecular testing, including molecular profiling of multiple genes whenever possible
- Suboptimal fixation and processing can cause false negatives and equivocal results
- References: Cytopathology 2022;33:14, Arch Pathol Lab Med 2016;140:1331
Uses by pathologists
- EGFR IHC can be used as a predictive marker to quickly identify patients who can benefit from oral tyrosine kinase inhibitors using minimal cellular material
- Results can be reported using CAP's synoptic template (CAP: Cancer Protocol Templates [Accessed 19 January 2024])
- Recent molecular society guidelines encourage molecular profiling of multiple genes whenever possible, rather than single gene / mutation testing
- EGFR mutation specific IHCs are useful when material is insufficient for molecular testing
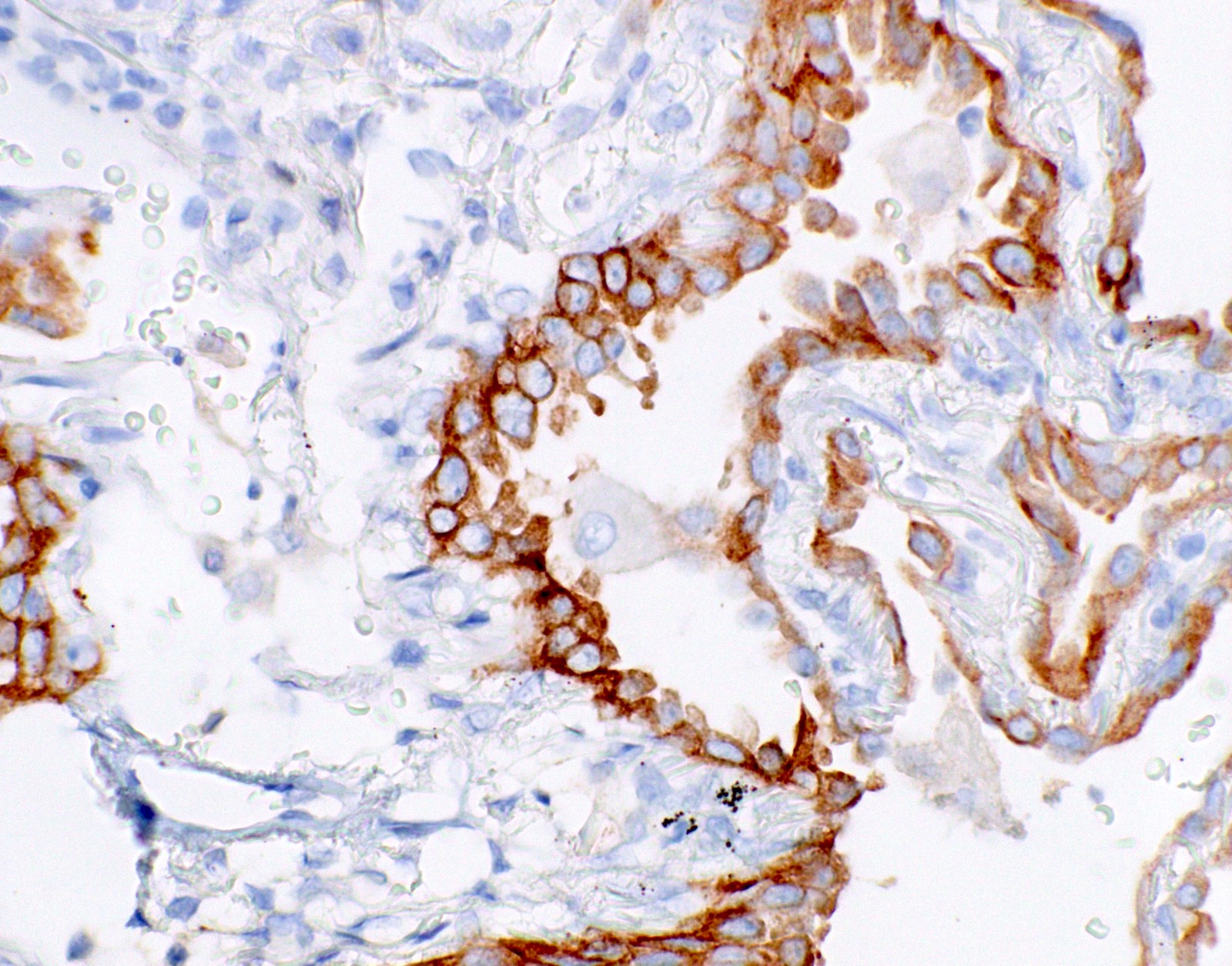
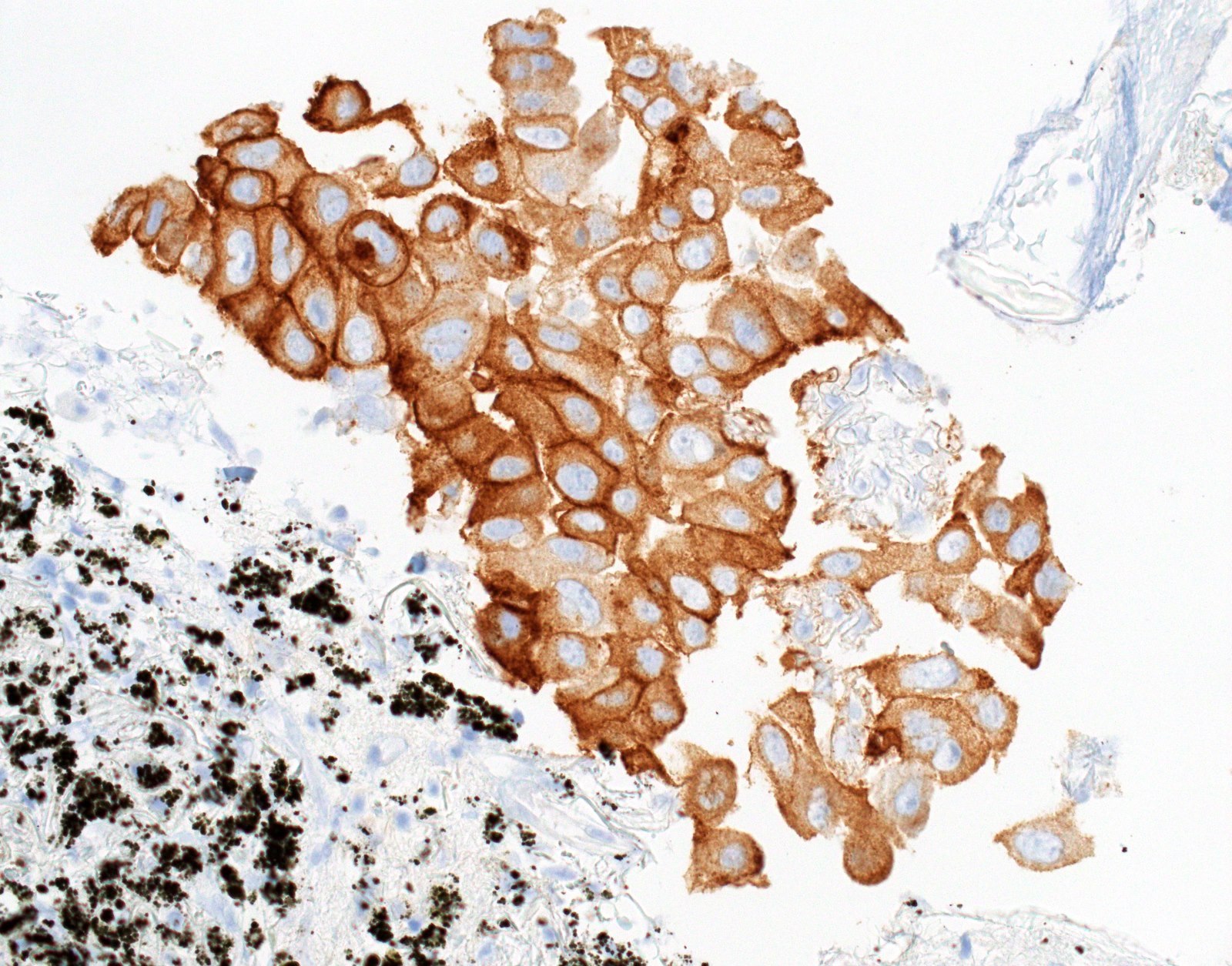
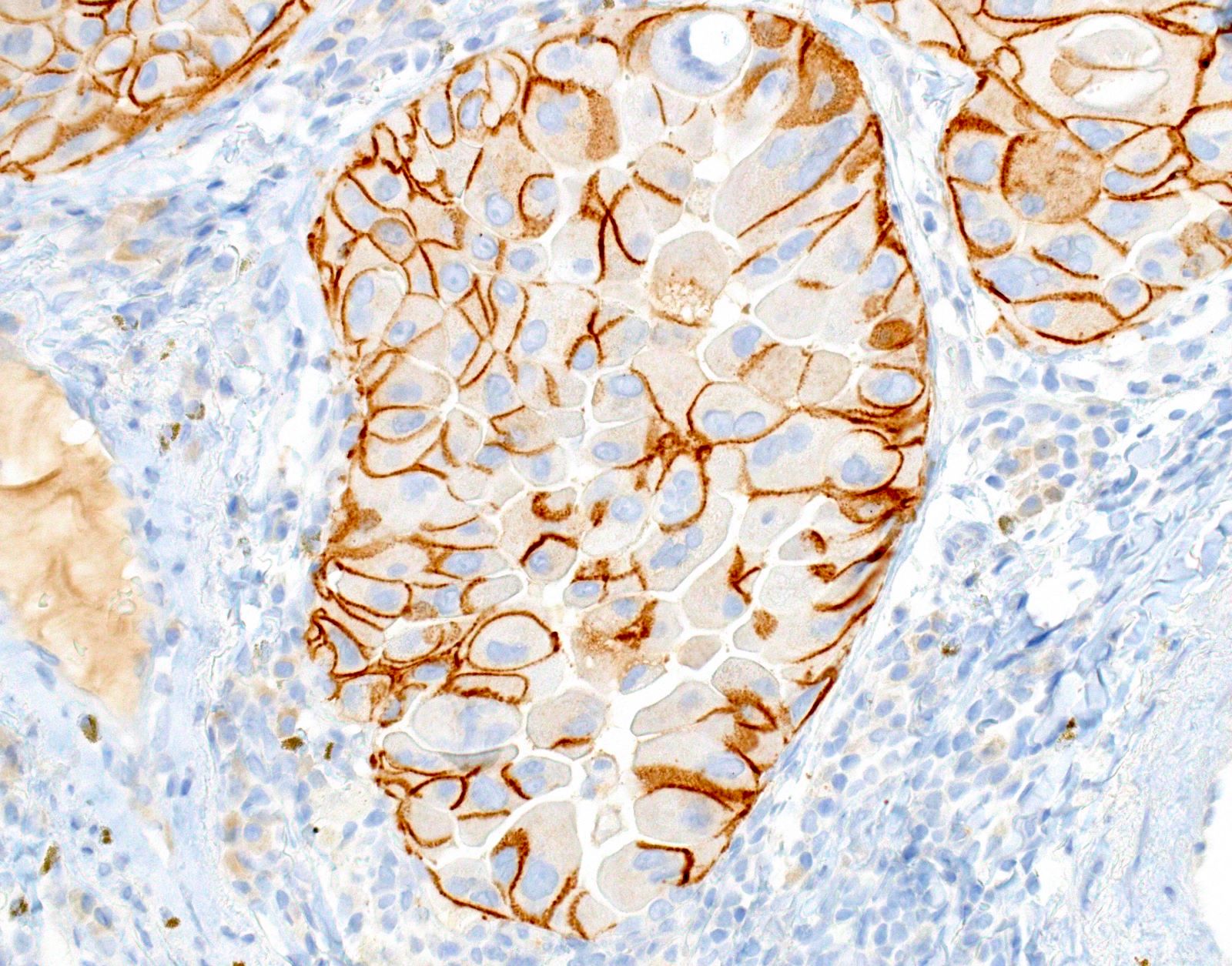
Microscopic (histologic) images
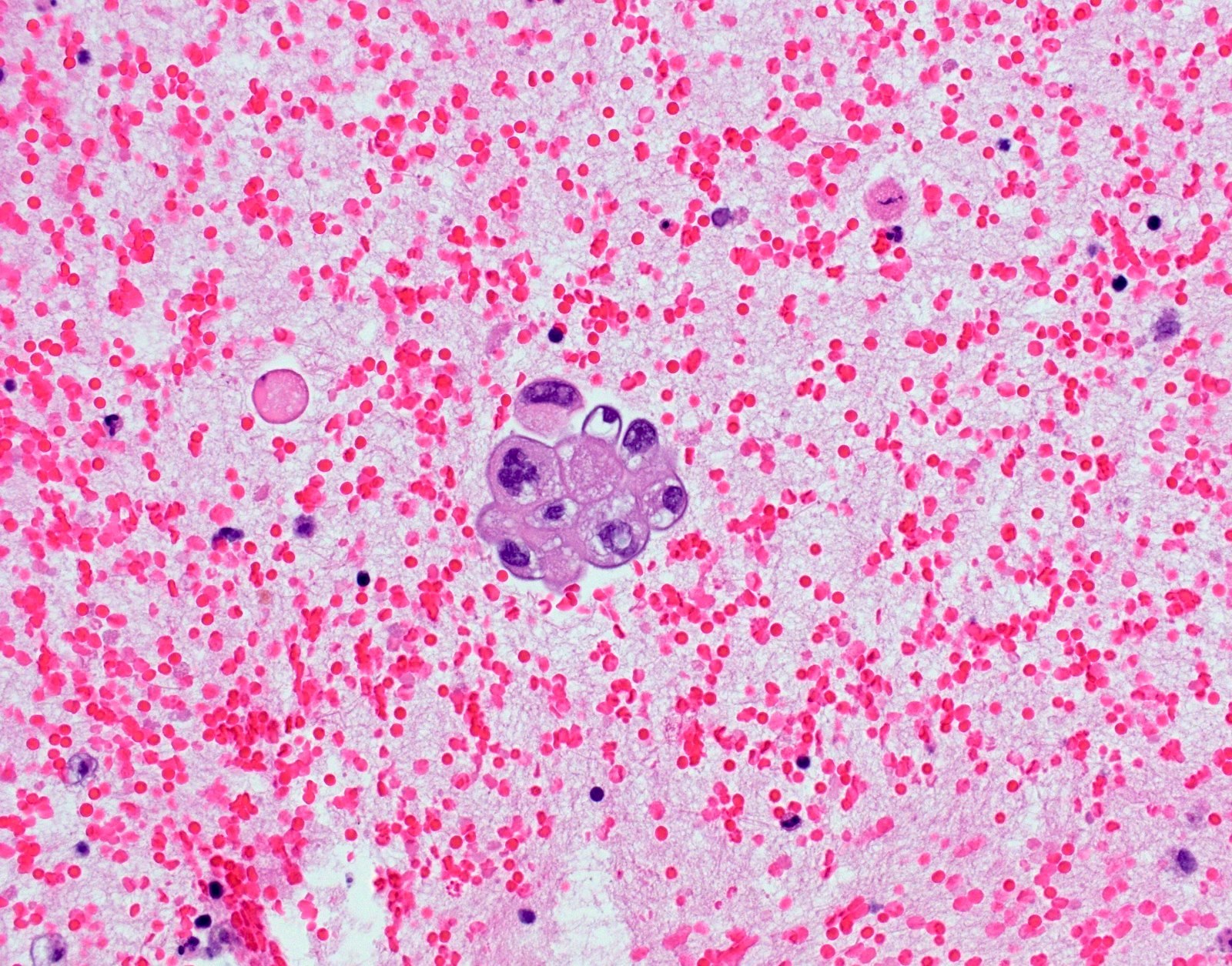
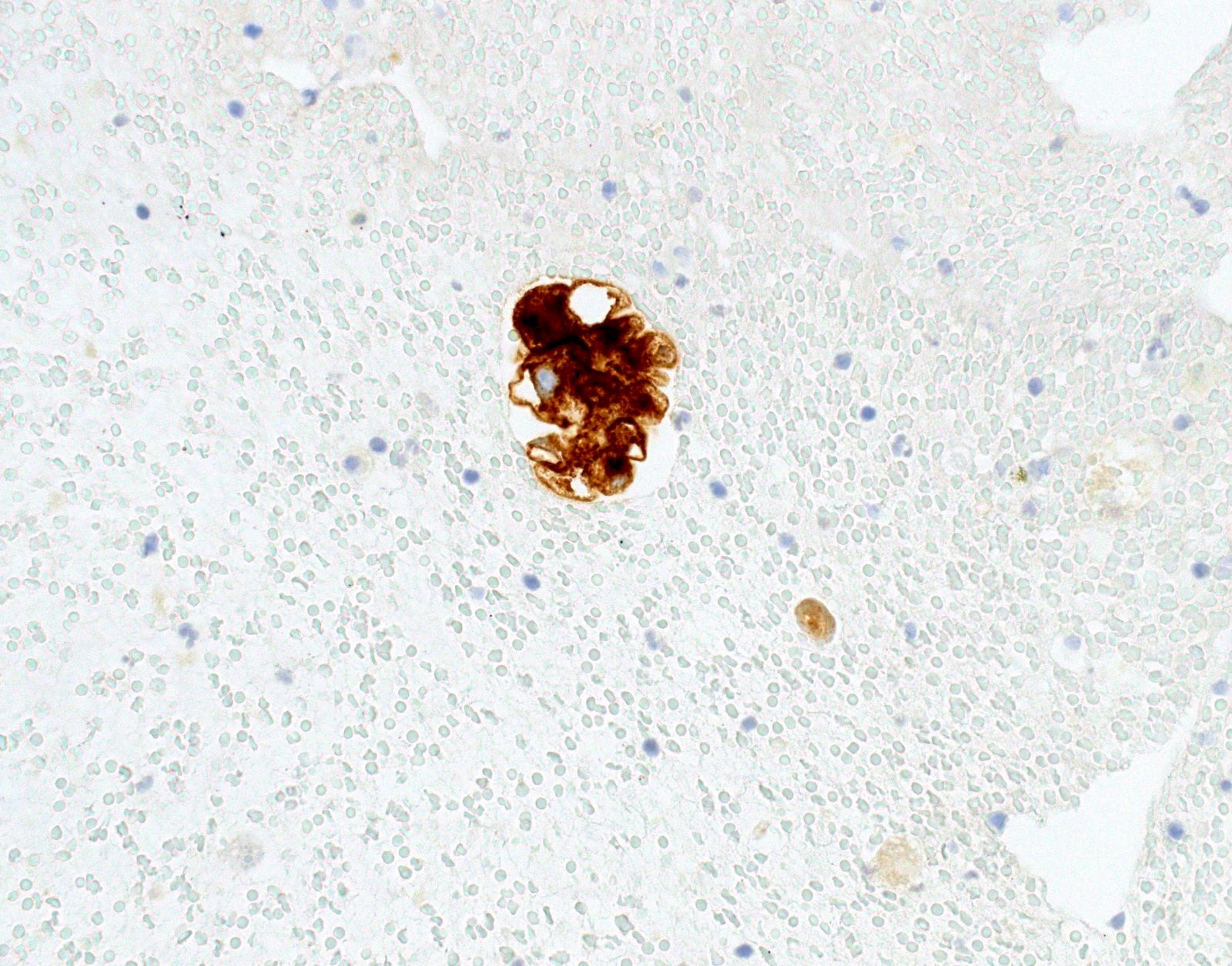
Cytology images
Positive staining - disease
- EGFR mutations in lung adenocarcinoma
- White patients: ~10%
- Asian patients: ~50%
- 2 targetable mutations comprise ~90% of all EGFR mutations in lung adenocarcinoma (J Thorac Oncol 2017;12:612)
- EGFR L858R point mutation in exon 21: IHC clone 43B2
- EGFR E746_A750del (15 base pair deletion) in exon 19: IHC clone 6B6
- Mutation specific IHC assays are available
- Notes on these 2 mutation specific IHC antibodies (Arch Pathol Lab Med 2016;140:1331)
- High specificity if using careful and conservative interpretation guidelines = positive results are associated with response to tyrosine kinase inhibitors (TKIs), specificity ~99%
- Positive cases should have strong, diffuse membranous staining, with or without cytoplasmic staining
- Cytoplasmic staining only = 80% do not have the mutation (PLoS One 2013;8:e59183)
- Antibodies cross react with HER2, causing false positive results in HER2+ cases (rare, ~1%)
- Low sensitivity for these 2 mutations (~80%)
- They also do not detect other targetable or TKI resistance associated EGFR mutations (~10%)
- They do not detect EGFR amplification (not targetable anyway)
- Generally not recommended if there is access to (and adequate material for) high sensitivity molecular assays that may detect more mutations in EGFR and other genes (Arch Pathol Lab Med 2018;142:321)
- Particularly useful in the following situations
- Specimen is inadequate for molecular assays (low tumor cellularity or purity or decalcified)
- There is simultaneous wild type allele amplification that obscures interpretation of EGFR molecular results (Mod Pathol 2021;34:2168)
- Quick turnaround time is needed (1 - 2 days)
- No access to molecular testing
- High specificity if using careful and conservative interpretation guidelines = positive results are associated with response to tyrosine kinase inhibitors (TKIs), specificity ~99%
Molecular / cytogenetics description
- Molecular sequencing detects the corresponding mutations detected by the IHC stains
Sample pathology report
- Lung, right lower lobe, biopsy:
- Adenocarcinoma (see comment)
- Comment: The tumor cells are positive for TTF1 while negative for p40, supporting the diagnosis. Please see synoptic report for the results of biomarker testing.
- CAP lung cancer biomarker reporting protocol (synoptic report)
Specimen Adequacy of sample for testing Adequate
Results EGFR EGFR L858R by immunohistochemistry (clone 43B2) Negative EGFR exon 19 deletion (E746_A750del) (clone 6B6) Positive Interpretation EGFR E746_A750del immunohistochemical staining is positive,
which is associated with response to EGFR tyrosine kinase inhibitorsALK ALK immunohistochemistry Negative PDL1 IHC PDL1 IHC interpretation Positive Percentage of tumor cells with staining (TPS) 40% Methods Antibody 22C3 Controls Internal control cells present; expected immunoreactivity External controls available; expected immunoreactivity Assay information Laboratory developed test
Board review style question #1
Board review style answer #1
C. EGFR L858R and E746_A750del. Among EGFR mutations in lung adenocarcinoma, these 2 mutations are the most common. Overall, KRAS is the most commonly mutated gene in lung adenocarcinoma (it is more commonly mutated than EGFR) and targeted therapies against specific KRAS mutations (such as KRAS p.G12C) are being developed. Answers A, B, D and E are incorrect because they include rare EGFR variants.
Comment Here
Reference: EGFR mutation specific antibodies
Comment Here
Reference: EGFR mutation specific antibodies
Board review style question #2
What is the current consensus recommendation for molecular testing in patients with lung adenocarcinoma?
- A broad diagnostic IHC panel should be used in the biopsy diagnosis of lung cancer
- ALK rearrangement should be tested by IHC only
- Cytology specimens are never considered adequate for molecular testing
- EGFR mutation specific IHC testing should take priority
- Molecular profiling of multiple genes should be performed whenever possible
Board review style answer #2
E. Molecular profiling of multiple genes should be performed whenever possible to detect targetable, rare and other mutations that may qualify patients for clinical trials. Answer D is incorrect because whenever possible, molecular profiling of multiple genes (including EGFR) is preferred over EGFR only testing. Answer C is incorrect because cytology cell blocks can be used successfully for molecular testing. Even smears may be used (alcohol is superior to formalin for nucleic acid preservation). Answer B is incorrect because ALK rearrangements are not only detectable by IHC but also by FISH, PCR and NGS methods. Answer A is incorrect because the current consensus recommendation is to preserve tissue for molecular detection of targetable mutations when possible. Most cases of non-small cell lung carcinoma can be classified as adenocarcinoma or squamous cell carcinoma using 2 IHC stains: TTF1 (clone 8G7G3/1) and p40. If both markers are negative or very focal, the need for additional diagnostic IHC is determined by clinical history and imaging findings (Arch Pathol Lab Med 2018;142:321, Curr Oncol 2022;29:4981, J Thorac Oncol 2019;14:377).
Comment Here
Reference: EGFR mutation specific antibodies
Comment Here
Reference: EGFR mutation specific antibodies