Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Aksionau A, Yeh YA. HGPIN with adjacent atypical glands. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostatePINATYP.html. Accessed April 25th, 2024.
Definition / general
- High grade prostatic intraepithelial neoplasia (HGPIN) with small atypical glands present in adjacent stroma (Epstein: Biopsy Interpretation of the Prostate, 6th Edition, 2021)
Essential features
- Single or a few small atypical glands located within 0.01 - 0.4 mm of adjacent HGPIN (Hum Pathol 2001;32:389)
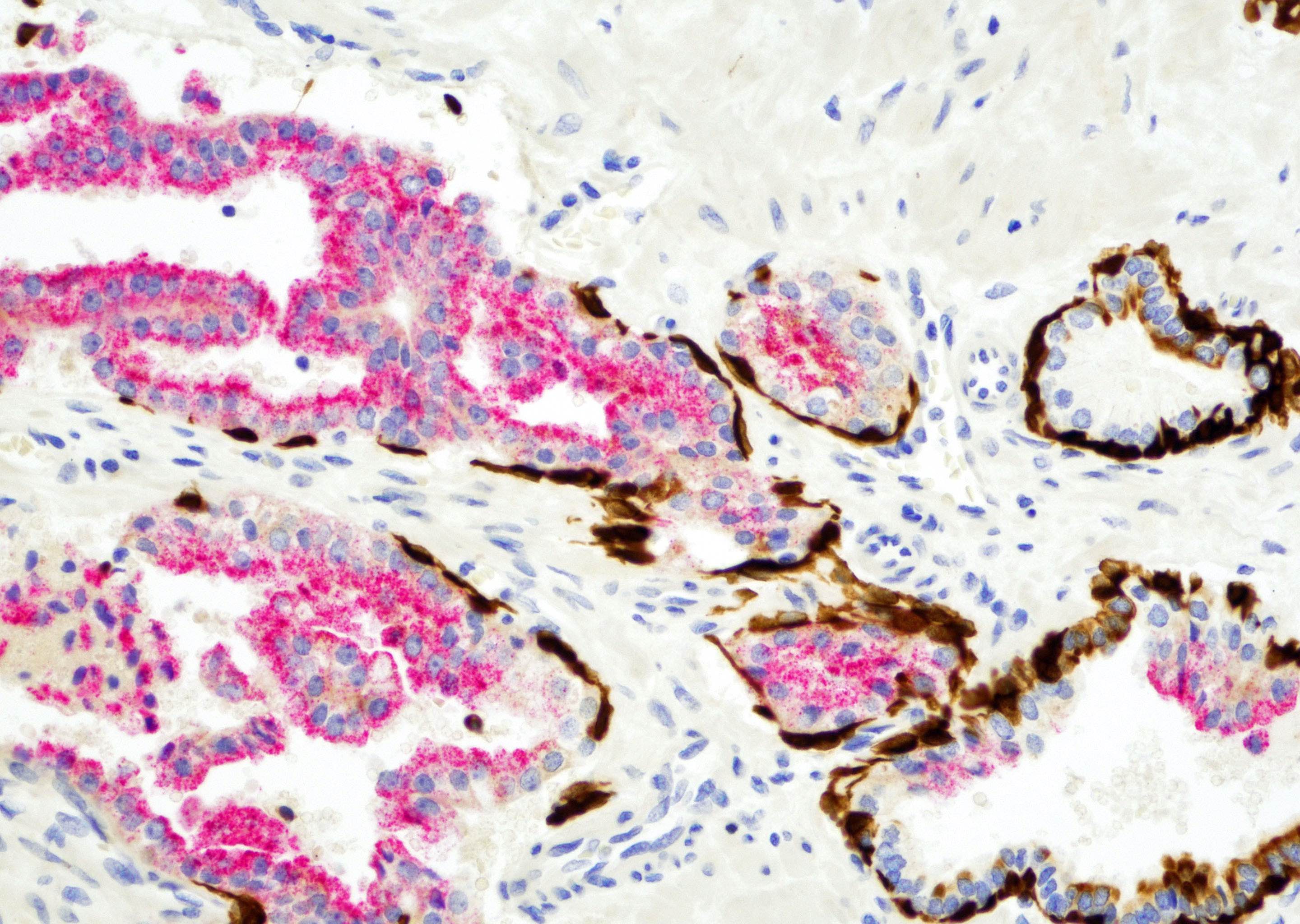
- Adjacent atypical glands stained positive (focal or patchy) on p63 or CK903 immunohistochemical stains
Terminology
- HGPIN with adjacent small atypical glands (Hum Pathol 2001;32:389)
- HGPIN with adjacent small atypical glands suspicious for infiltrating carcinoma (PINATYP) (Am J Surg Pathol 2006;30:1184)
- HGPIN with adjacent atypia (Urology 2001;57:296)
- HGPIN and ASAP (atypical small acinar proliferation) (BJU Int 2007;99:780)
ICD coding
Epidemiology
- More commonly arises in the peripheral zone
- Incidence of HGPIN with adjacent atypical glands (2.5%) is lower than that of HGPIN alone (4.3%) (Urology 2001;57:296)
Sites
- More commonly arises in the peripheral zone of the prostate (Urology 2001;57:296)
Pathophysiology
- Hypothesis of proliferative inflammatory atrophy as a precursor to HGPIN and prostate cancer (N Engl J Med 2003;349:366)
- Focal epithelial atrophy occurs in prostatic glands
- Chronic inflammatory cells infiltrate immediately adjacent to the areas of glandular atrophy
- HGPIN and localized prostate carcinoma develops as a result of DNA methylation and inactivation of DNA repair genes including GSTP1 and MGMT
- Reduced expression of PTEN, NKX3.1 and p27 is involved in prostate cancer progression (Nat Rev Urol 2018;15:222)
Etiology
- Gene mutation
- Germline mutations in familial prostate cancer: ELA2 (HPC2), MSR, RNASEL
- Somatic mutations: TP53, PTEN, AR, ZFHX3, RB1, APC, MLL2, OR5L1, CDK12, ATM, FOXA1, EZH2 (Nature 2012;487;239, Genes Dev 2018;32:1105)
- Gene fusion
- TMPRSS2::ERG gene fusion results in ETS overexpression (Cold Spring Harb Perspect Med 2019;9:a034942)
- 19% in HGPIN with cancer foci, 48.5% in localized prostate adenocarcinoma
- WNT signaling and beta catenin
- Increased level of CTNNB1 detected by immunohistochemical staining in prostate cancer
- DNA hypermethylation (Biochim Biophys Acta 2004;1704:87)
- Invasion: GSTP1, MGMT, RASSF1A, RARβ2, CDH1 (E-cadherin), CAV1 (caveolin-1), LAMA3, LAMB3, LAMC2
- Metastasis: APC, CDH1 (E-cadherin), CD44
- Histone modification: MLL2 mutation
- MicroRNA upregulation: overexpression of DICER, a key gene involved in biosynthesis of miRNA
Clinical features
- May be asymptomatic, present with dysuria or hematuria (JAMA 2017;317:2532)
Diagnosis
- May present with dysuria or hematuria if there is a coexisting prostatic nodule or urinary tract infection
- Solid, firm nodule on digital rectal examination (DRE) may be detected
- A few atypical glands immediately adjacent to HGPIN is present on microscopic examination of the prostatic samples obtained from biopsy, TURP or prostatectomy (Urology 2001;57:296, JAMA 2017;317:2532)
Laboratory
- Elevated PSA (ng/mL): HGPIN (mean 6.71), ASAP (mean 7.2), HGPIN and ASAP (mean 8.0) (Am J Surg Pathol 2005;29:1201)
Prognostic factors
- Higher cancer detection rates: HGPIN alone (23%), ASAP (37%), HGPIN and ASAP (33%) (Am J Surg Pathol 2005;29:1201)
- HGPIN or ASAP: 47% risk for cancer on repeat biopsy (J Urol 2001;166:866)
- Higher incidence of cancer on repeat biopsy: HGPIN alone (14%), HGPIN and ASAP (75%) (Urology 2001;57:296)
Treatment
- No definitive treatment for ASAP and HGPIN
- For ASAP and multifocal (> 2 sites) HGPIN, rebiopsy within 6 months; includes sampling more from the previous affected and adjacent areas
- For focal HGPIN, close follow up with PSA and DRE within 6 - 24 months; consider other testing, including free PSA, 4Kscore, PHI, PCA3 or ConfirmMDx (J Natl Compr Canc Netw 2016;14:509)
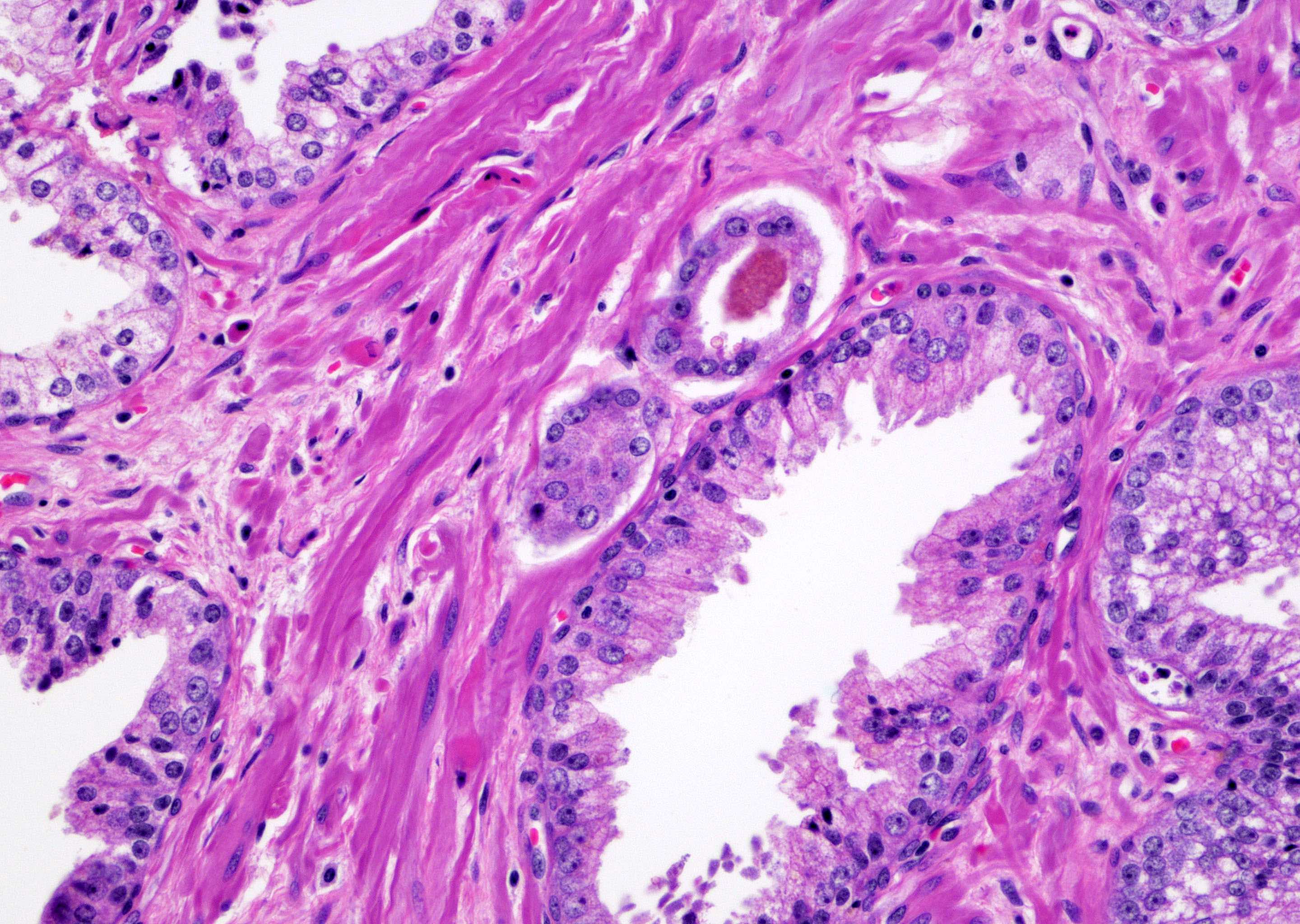
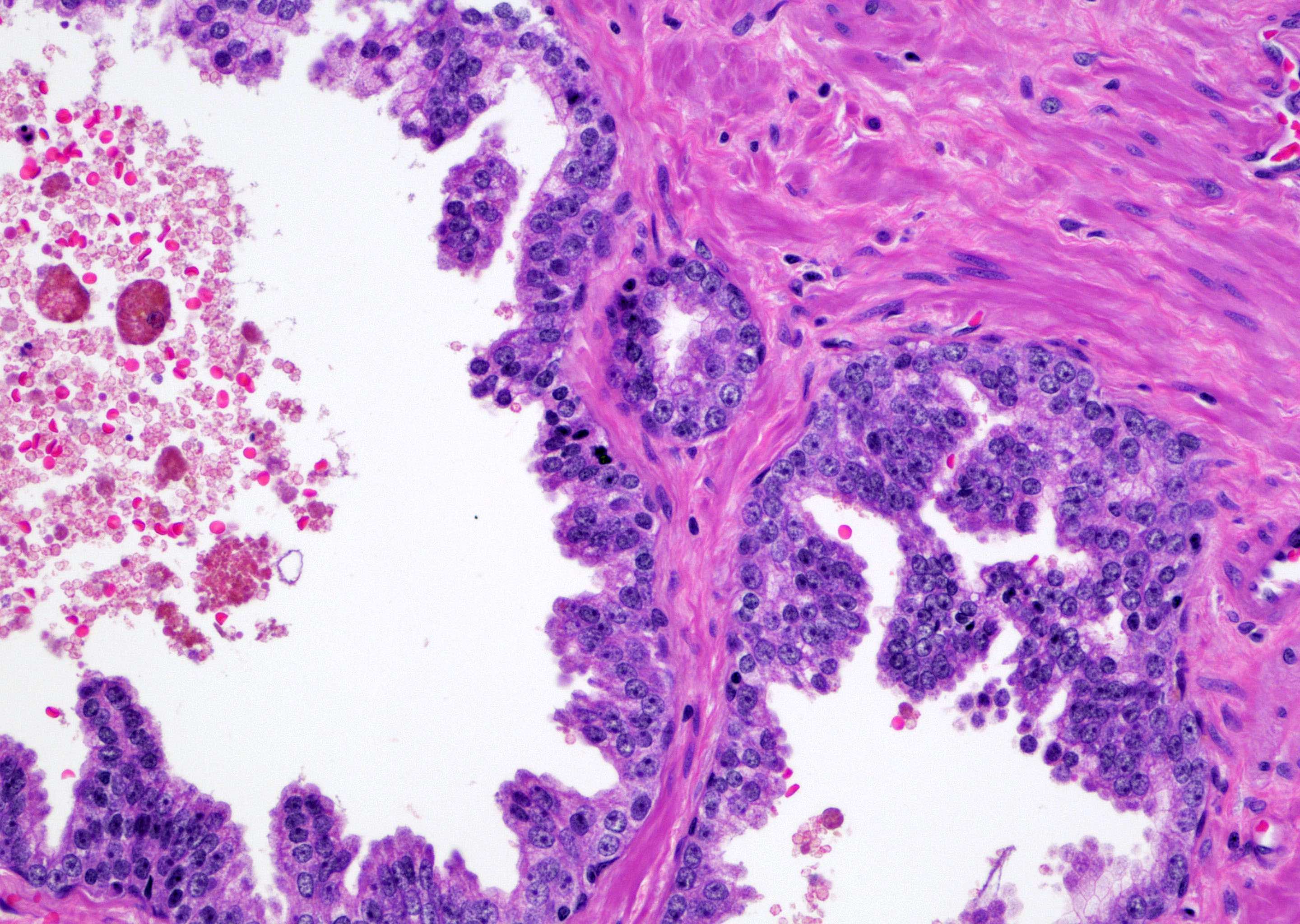
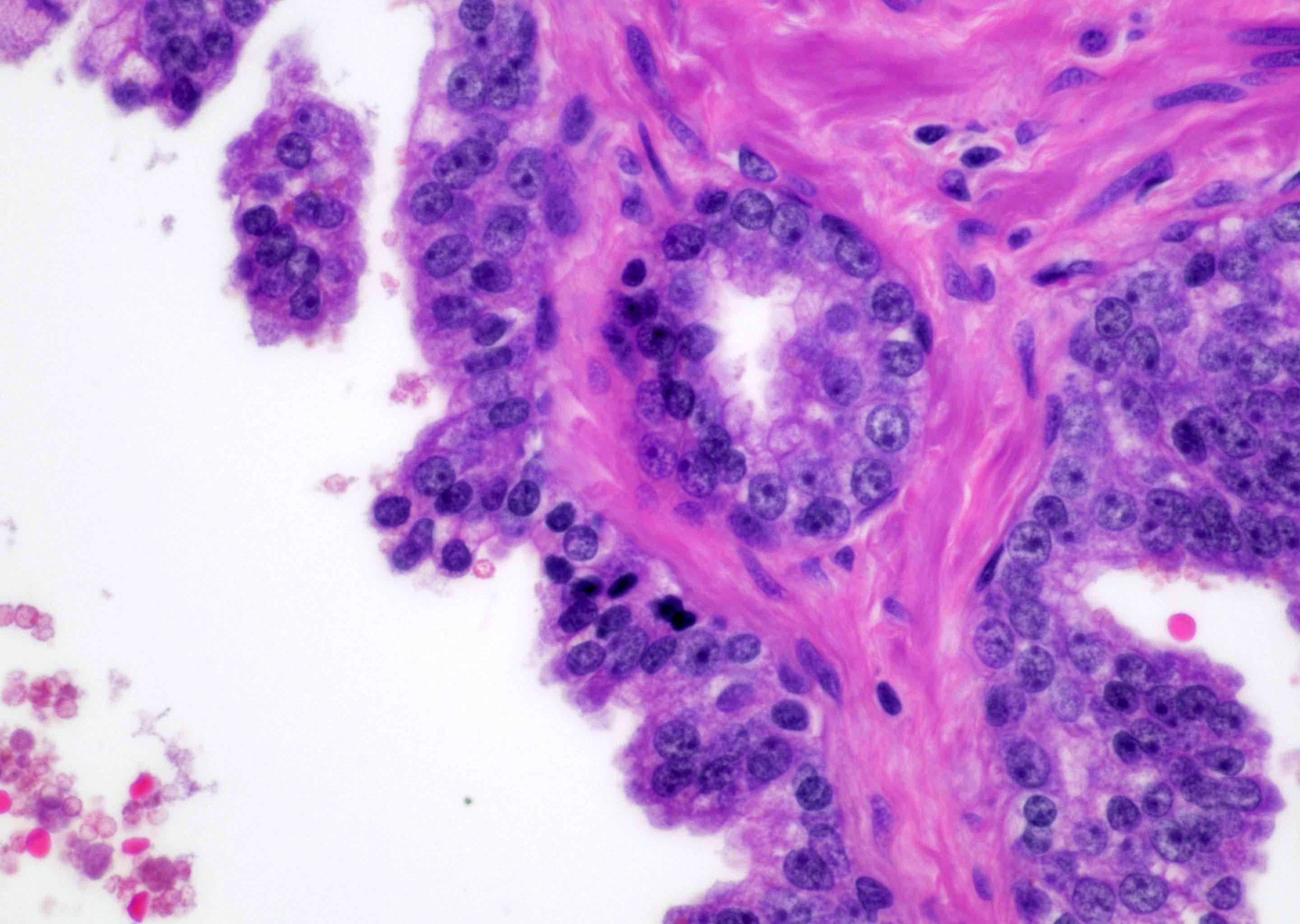
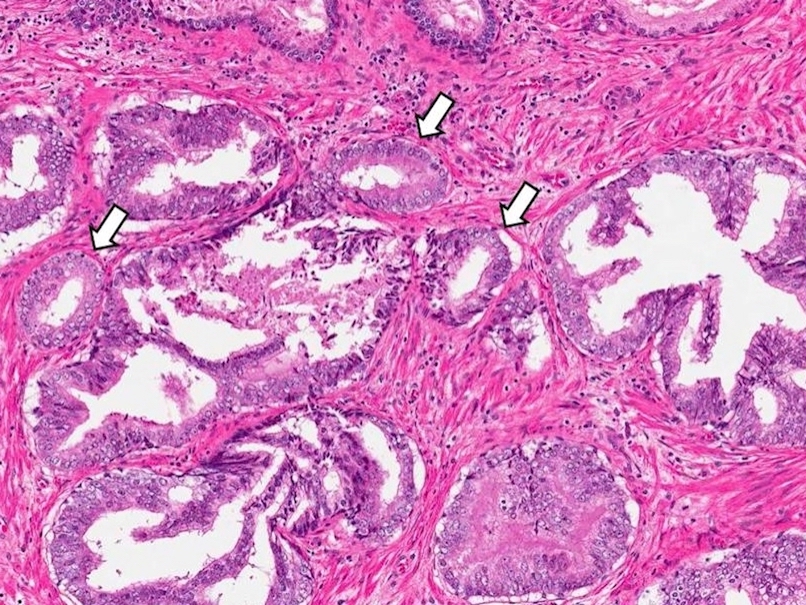
Microscopic (histologic) description
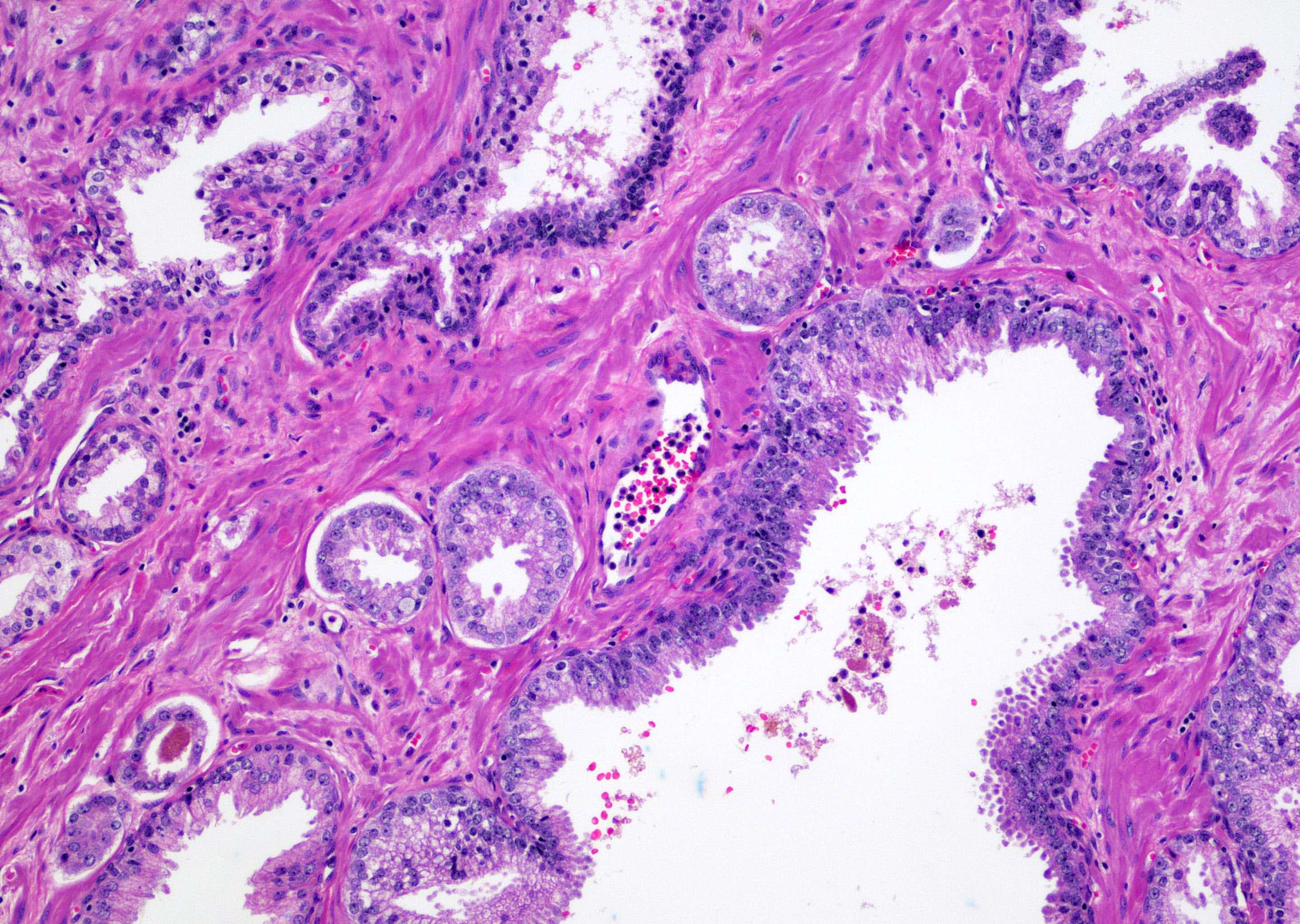
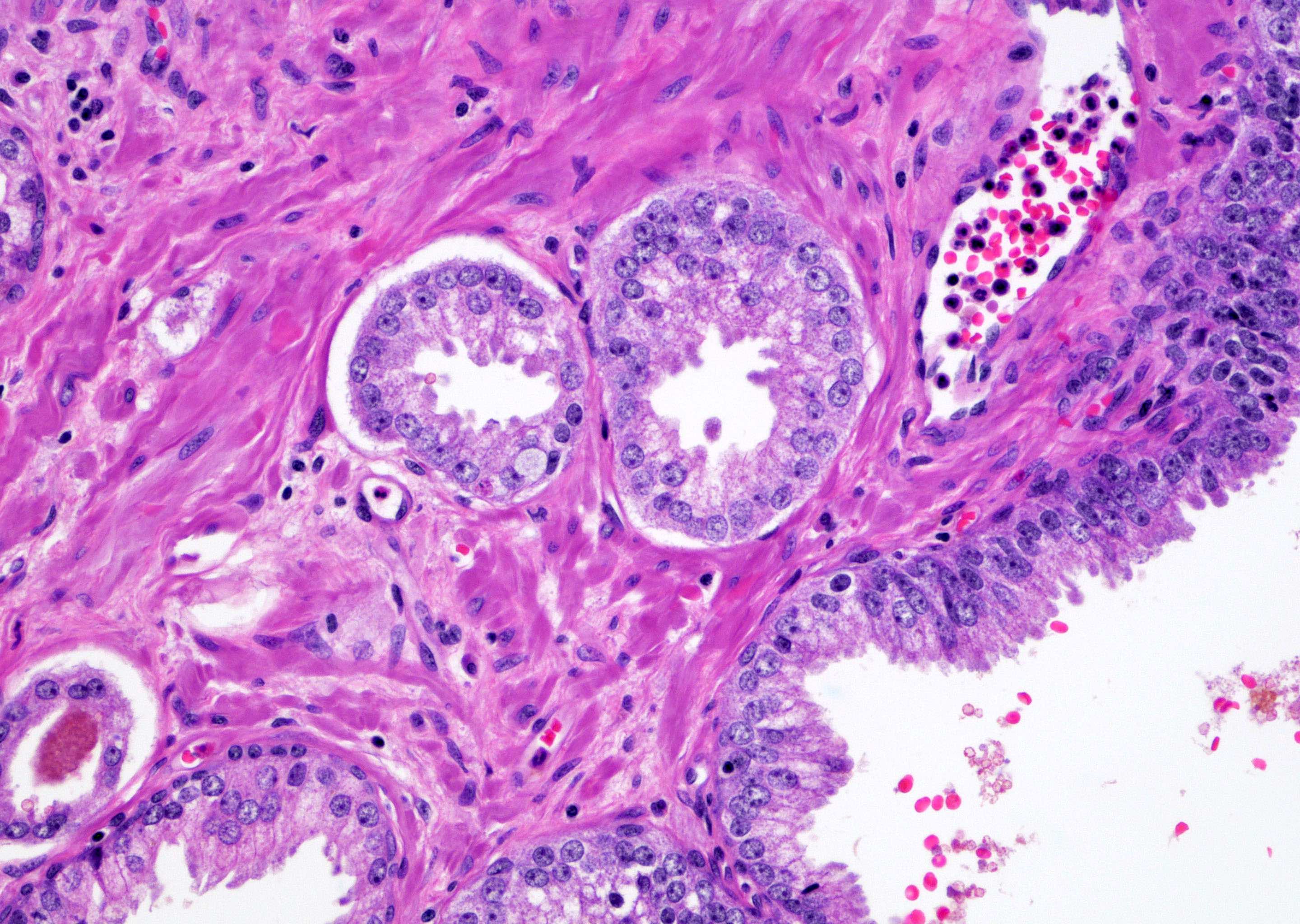
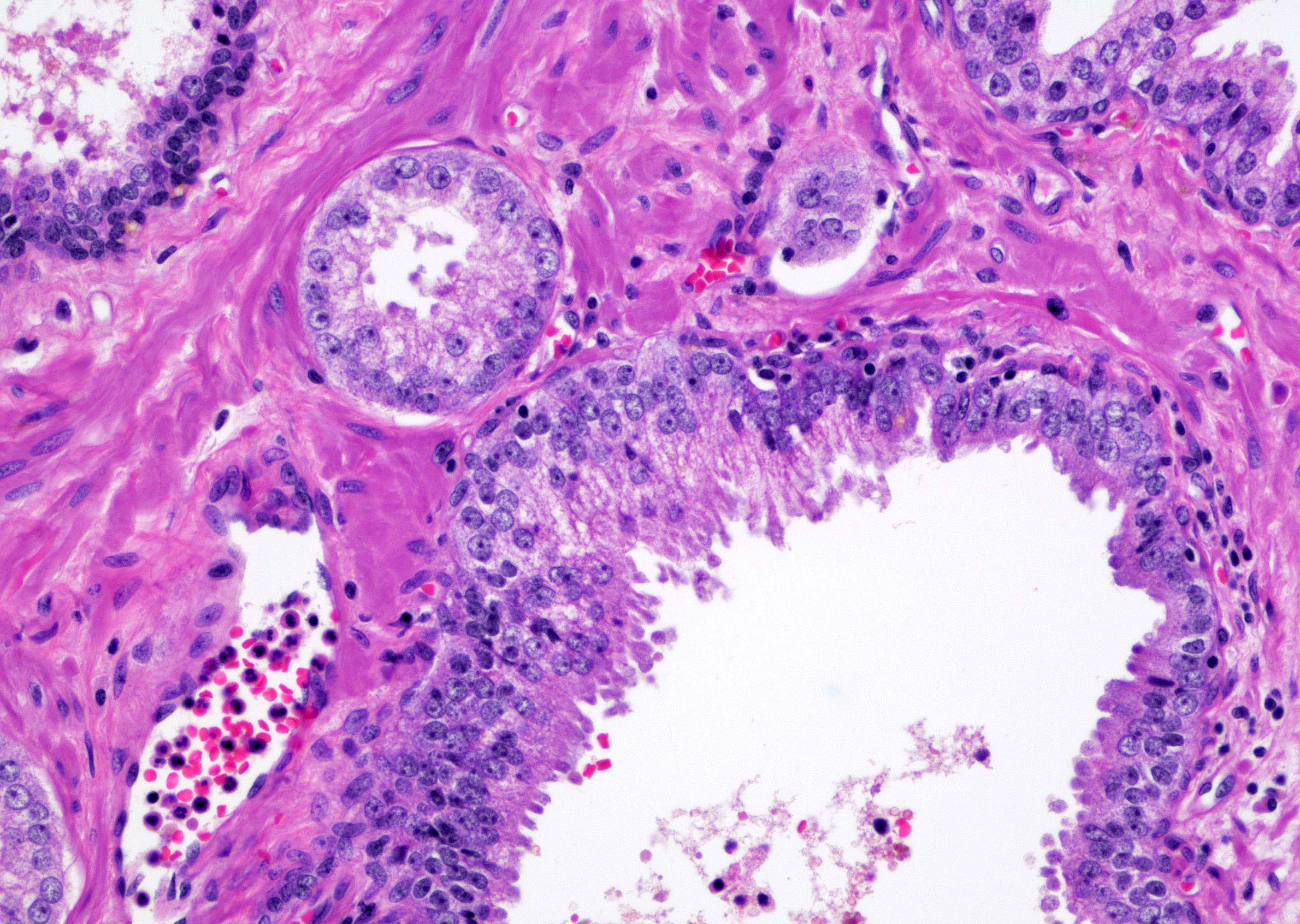
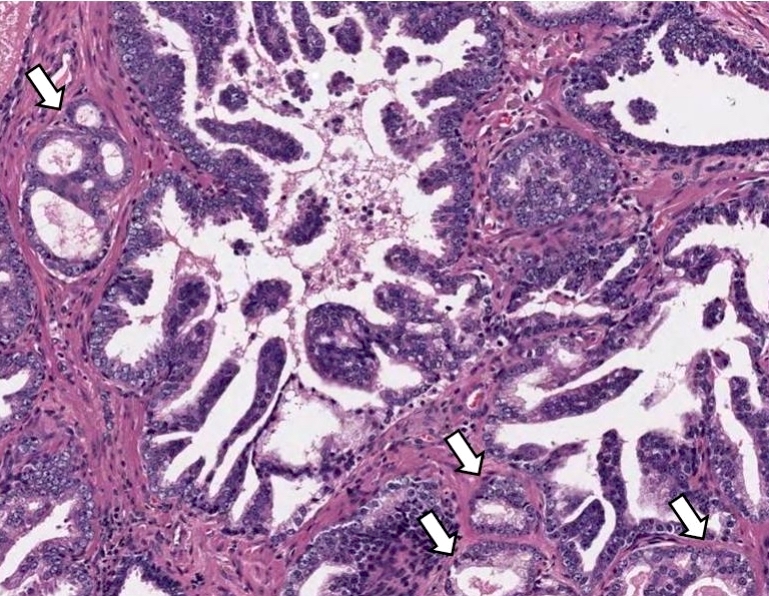
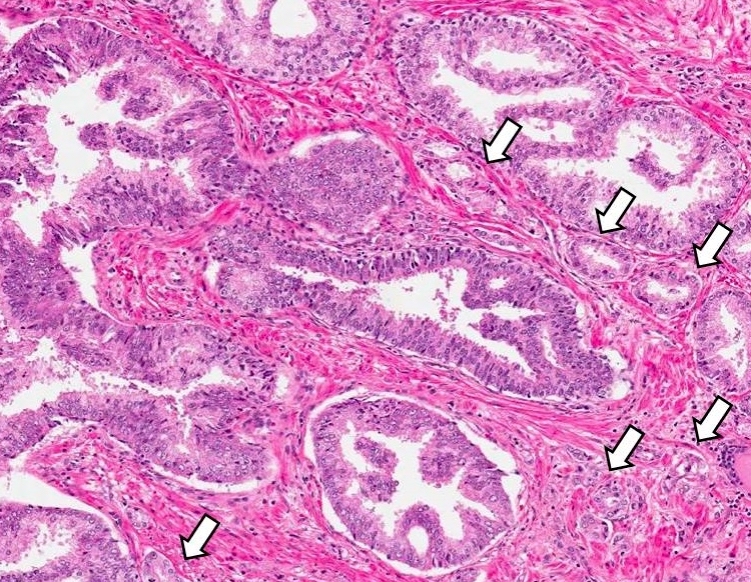
- Single or a few small atypical glands located within 0.01 - 0.4 mm of adjacent HGPIN (Hum Pathol 2001;32:389)
- The lining epithelial cells of HGPIN show high grade nuclei with nuclear enlargement, hyperchromasia and prominent nucleoli
- A layer of horizontally lined basal cells presents in the HGPIN
- Epithelial cells of the atypical glands show nuclear enlargement, prominent nucleoli and amphophilic cytoplasm, mimicking the cytologic features of HGPIN
Microscopic (histologic) images
Positive stains
- HGPIN (Epstein: Biopsy Interpretation of the Prostate, 6th Edition, 2021)
- p63 (continuous or patchy)
- High molecular weight cytokeratin CK903 (continuous or patchy)
- AMACR
- Adjacent atypical glands (Epstein: Biopsy Interpretation of the Prostate, 6th Edition, 2021)
- p63 (focal or patchy)
- High molecular weight cytokeratin CK903 (focal or patchy)
- AMACR
Negative stains
- Adjacent atypical glands
- Very few in total, could be negative for p63 or high molecular weight cytokeratin CK903 (Epstein: Biopsy Interpretation of the Prostate, 6th Edition, 2021)
Sample pathology report
- Prostate, needle biopsy:
- Atypical small acinar proliferation (ASAP)
- High grade prostatic intraepithelial neoplasia (see comment)
- Comment: The prostatic needle biopsy shows prostatic tissue with high grade intraepithelial neoplasia (HGPIN). Immediately adjacent to the HGPIN are 3 well formed small glands composed of cells with enlarged nuclei and prominent nucleoli, mimicking the cellular features of HGPIN. Immunohistochemical stains p63 and CK903 show focal positive staining in the small atypical glands and continuous positive staining in HGPIN. AMACR immunomarker staining is positive in both lesions. These features are consistent with HGPIN with adjacent atypical glands (HGPIN and ASAP). It has been shown that the cancer detection rate of HGPIN and ASAP is higher than that of HGPIN alone. Repeat biopsy within 6 months is advised (Am J Surg Pathol 2005;29:1201).
Differential diagnosis
- Acinar adenocarcinoma (Hum Pathol 2001;32:389):
- HGPIN with tangential sectioning or outpouching of glands (BJU Int 2007;99:780):
- Immediately adjacent to HGPIN
- Cellular features and immunostaining pattern mimicking HGPIN (continuous or patchy staining pattern with basal cell immunomarkers)
- May have connection with HGPIN on deeper levels of tissue sectioning
Additional references
Board review style question #1
A 68 year old man presented with dysuria. A firm nodule was detected on digital rectal examination. PSA level was 6.4 ng/mL. Prostatic needle biopsy followed by microscopic examination was performed. The photomicrograph is shown in the image above. Immunohistochemical stains are performed. The large and small glands show patchy positive staining with p63 and CK903. AMACR immunomarker is positive in the large and small glands. What is the best interpretation?
- High grade prostatic intraepithelial neoplasia
- High grade prostatic intraepithelial neoplasia with atypical glands
- Prostatic adenocarcinoma, Gleason 3+3=6
- Prostatic intraductal adenocarcinoma
Board review style answer #1
B. High grade prostatic intraepithelial neoplasia with atypical glands
Comment Here
Reference: HGPIN with adjacent atypical glands
Comment Here
Reference: HGPIN with adjacent atypical glands
Board review style question #2
What is the best clinical management of high grade prostatic intraepithelial neoplasia with adjacent atypical glands?
- Close follow up with PSA and digital rectal examination within 6 months
- Close follow up with PSA only within 6 months
- Rebiopsy including increased sampling from previous affected and adjacent areas within 6 months
- Rebiopsy within 6 to 12 months
Board review style answer #2
C. Rebiopsy including increased sampling from previous affected and adjacent areas within 6 months
Comment Here
Reference: HGPIN with adjacent atypical glands
Comment Here
Reference: HGPIN with adjacent atypical glands