Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Al Amri R, Liu YC. Myeloid sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/leukemiagranulocytic.html. Accessed December 4th, 2024.
Definition / general
- Tumor mass consisting of myeloblasts occurring at an anatomical site other than the bone marrow and distorts the normal tissue architecture
- Although commonly associated with concurrent acute myeloid leukemia, myeloid sarcoma can occur in isolation
- Diagnosis of myeloid sarcoma should be considered an equivalent to a diagnosis of acute myeloid leukemia (AML)
Essential features
- Based on the WHO classification, myeloid sarcoma must present as a tumor mass with tissue architectural effacement
- Important distinction
- Leukemia cutis (LC) is a somewhat nonspecific term defined as a cutaneous infiltration by neoplastic leukocytes (myeloid or lymphoid; mature or immature) (Am J Clin Pathol 2008;129:130)
- Leukemia cutis is not an equivalent of myeloid sarcoma without fulfilling the myeloid sarcoma diagnostic criteria by WHO classification
- Leukemia cutis could be seen in non-AML patients with myeloproliferative neoplasm (MPN), myelodysplastic syndrome (MDS) or myelodysplastic / myeloproliferative neoplasm (MDS / MPN), such as chronic myelomonocytic leukemia (CMML)
- Can present as de novo lesions, as therapy related myeloid neoplasms or as disease progression of MPN, MDS or MDS / MPN
Terminology
- Granulocytic sarcoma
- Chloroma
- Extramedullary myeloid tumor
ICD coding
- ICD-10: C92.3 - myeloid sarcoma
Epidemiology
- Can occur at any age
Sites
- Can involve any site; the most common sites of involvement are lymph nodes, skin, soft tissue, GI tract, bone and testicles
Pathophysiology
- Exact mechanism is not fully understood
- Chemokine / chemokine receptor interactions are implicated in the migration of leukemia cells migration to skin (Pediatr Blood Cancer 2010;55:344)
- Matrix metalloproteinases (MMPs) and integrin are implicated in the leukemia cells migration and the blood brain barrier disruption (Blood 2009;114:3008, PLoS One 2011;6:e20599)
- CXCR4, CXCL12 and CD56 have been previously reported to be associated with specific sites of myeloid sarcoma, which was not confirmed by a more recent study (Am J Surg Pathol 2016;40:1473)
Clinical features
- Variable clinical presentation depending on the location of tumor (Clin Lymphoma Myeloma Leuk 2017;17:263)
- Primary myeloid sarcoma (de novo): no pre-existing diagnosis of myeloid neoplasm or AML
- Secondary myeloid sarcoma: concurrent or pre-existing diagnosis of myeloid neoplasm or AML
- Isolated myeloid sarcoma: primary or secondary myeloid sarcoma with no bone marrow involvement
Diagnosis
- Broad panel of immunohistochemical stains required to avoid misdiagnosis (Am J Clin Pathol 2015;144:219)
- Cases without concurrent bone marrow involvement may have no fresh material for karyotyping and flow cytometric studies; fluorescence in situ hybridization (FISH) studies and mutation analysis should still be attempted on formalin fixed, paraffin embedded (FFPE) samples
Laboratory
- Findings vary, depending on the location of the tumor and the bone marrow involvement status
Prognostic factors
- Limited data on the prognostic significance of myeloid sarcoma
- Survival likely is similar to that for AML (Acta Haematol 2009;122:238, Leukemia 2003;17:1100)
- Tumor site may play a role in the prognosis
Case reports
- 47 year old woman with 2 large vulvar ulcers (Diagn Pathol 2019;14:126)
- 54 year old man with repeated symptoms of intestinal obstruction (Surg Case Rep 2020;6:2)
- 58 year old woman with right breast mass and history of essential thrombocythemia (ET) (Hematol Oncol Stem Cell Ther 2018;11:178)
- 84 year old man with superior mediastinal mass causing airway obstruction (Proc (Bayl Univ Med Cent) 2017;30:195)
Treatment
- No universal consensus on treatment (Leukemia 2021;35:1193)
- Treatment strategies may depend on the disease presentation (initial or relapse) and vary from institution to institution
- Treatment includes systemic chemotherapy, local therapy (surgery and radiation therapy), bone marrow transplant and target therapy
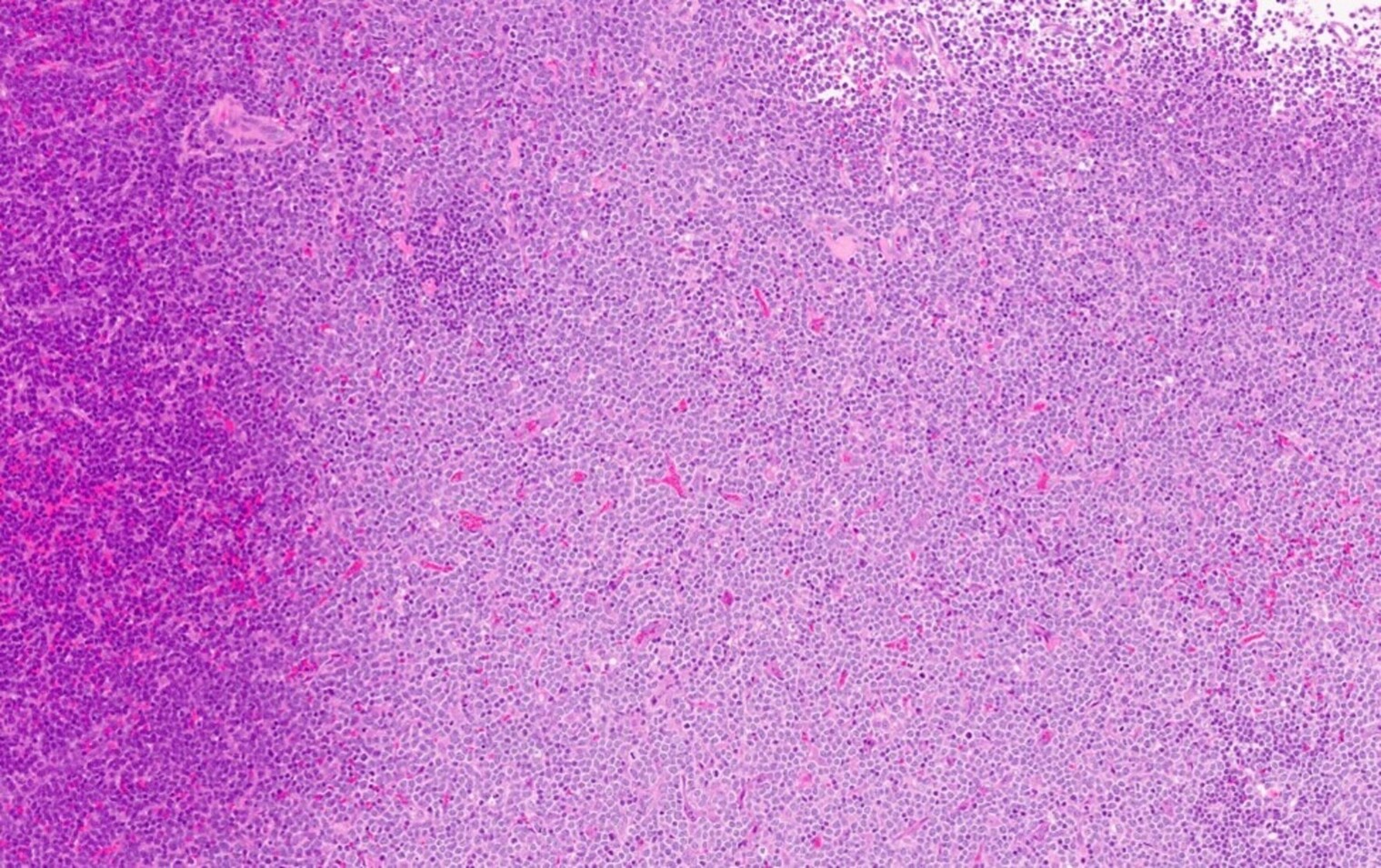
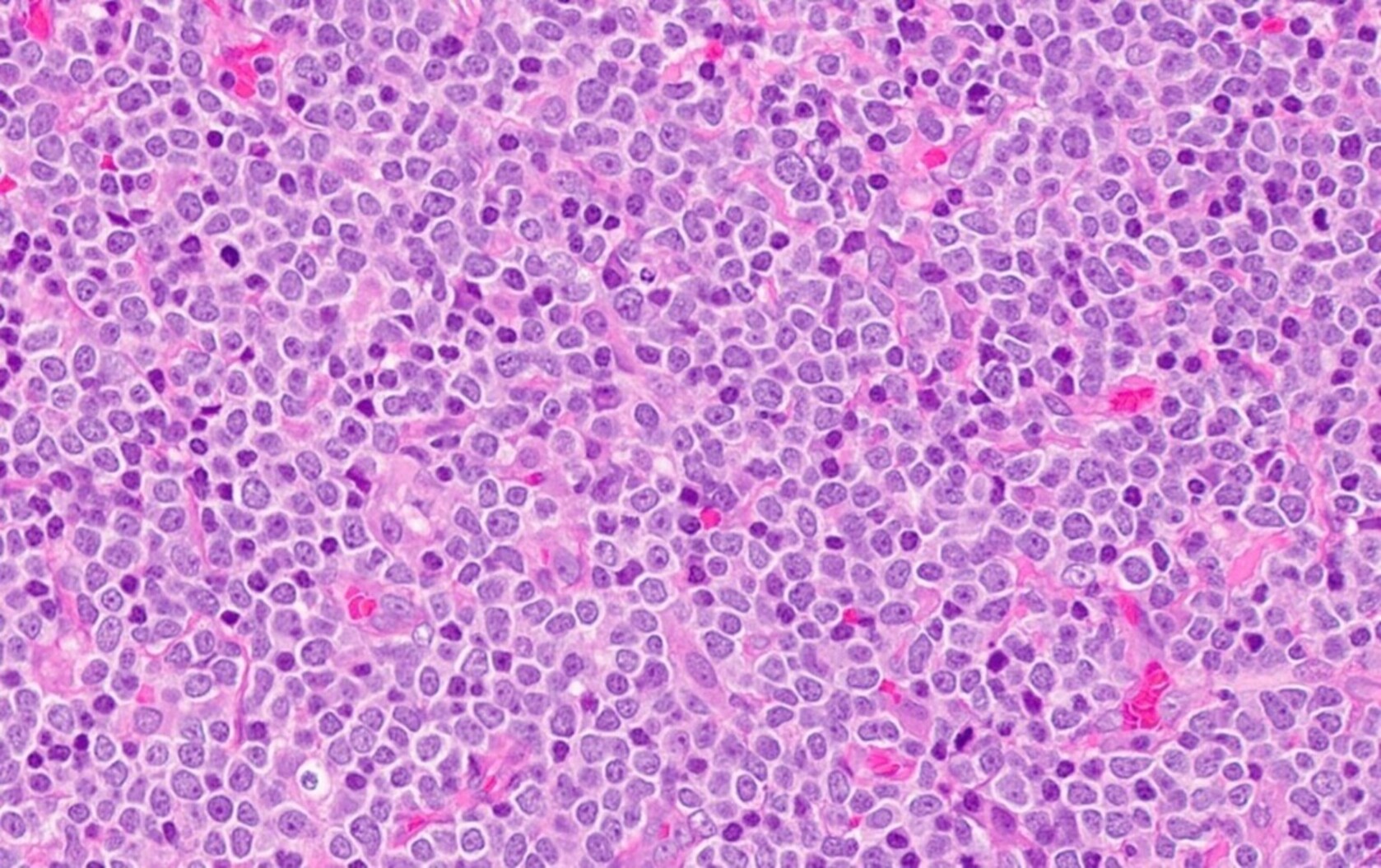
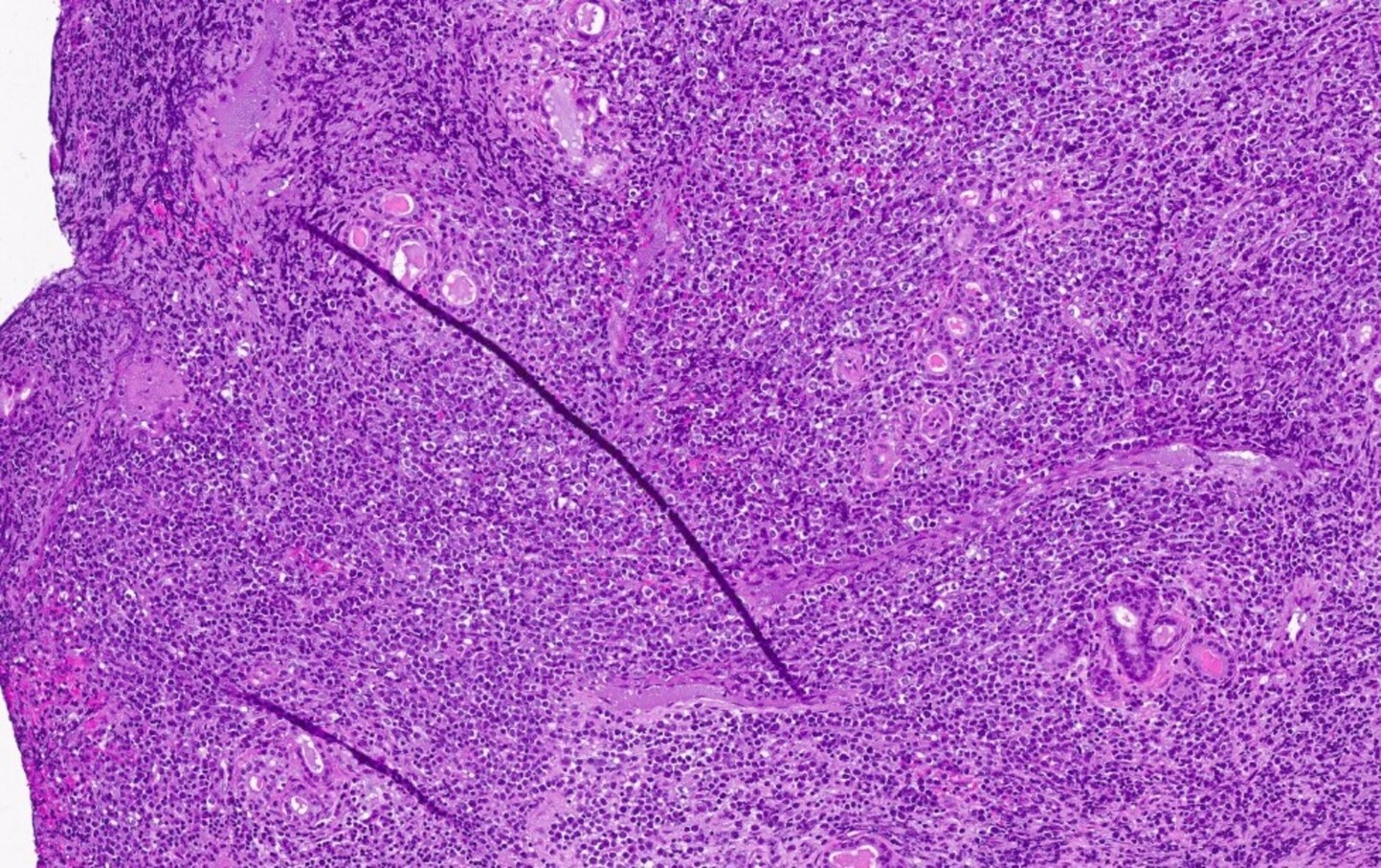
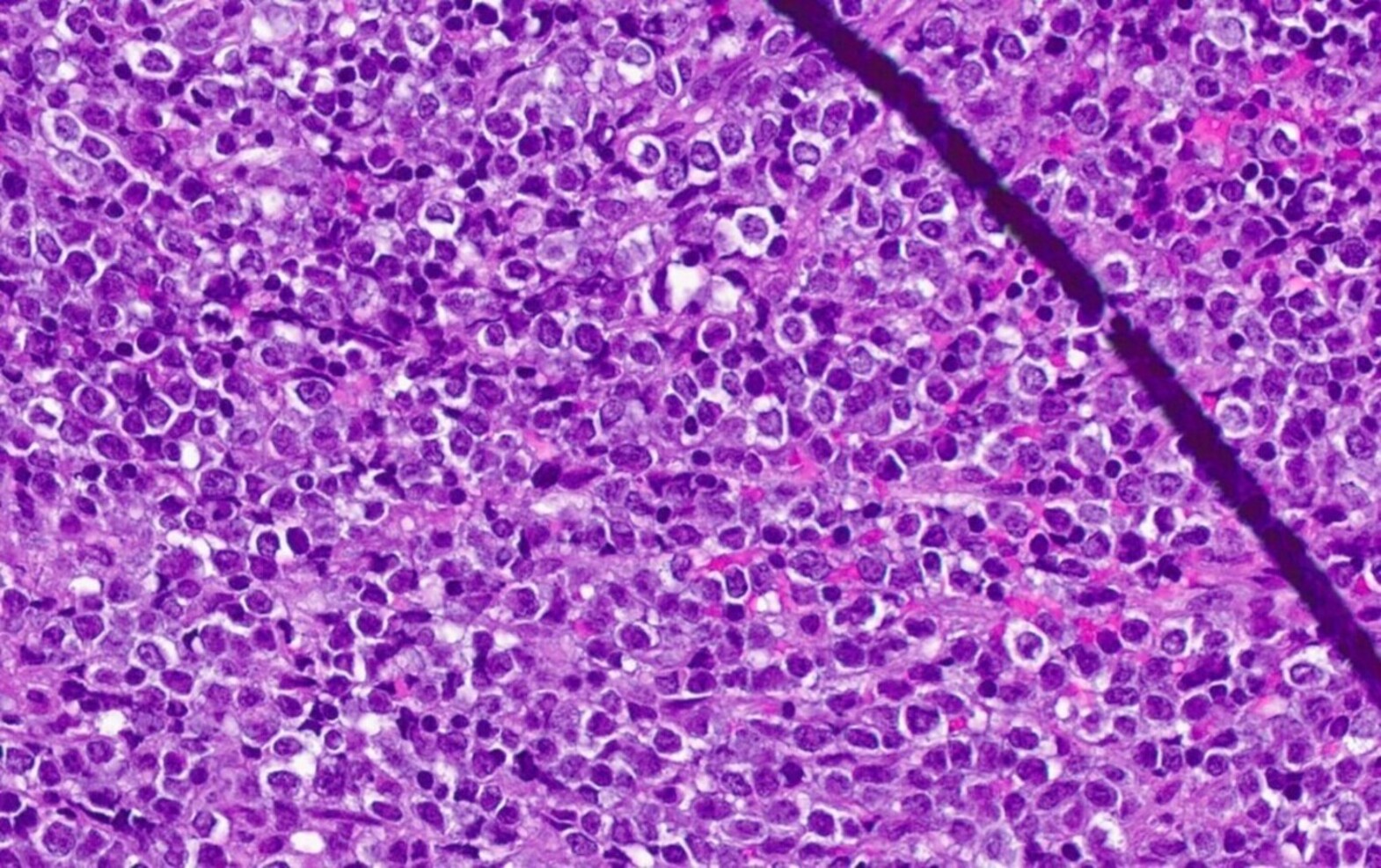
Microscopic (histologic) description
- At least partial effacement of the architecture
- Neoplastic cells can be seen forming cohesive nests, mimicking carcinoma or lymphoma or infiltrating as single files
- Granulocytic, monocytic or rarely, erythroblastic or megakaryoblastic differentiation can be seen
- Neoplastic cells usually demonstrate dispersed chromatin with high N/C ratio
- Mitotic figures and apoptosis are commonly identified indicating a high proliferation rate
Microscopic (histologic) images
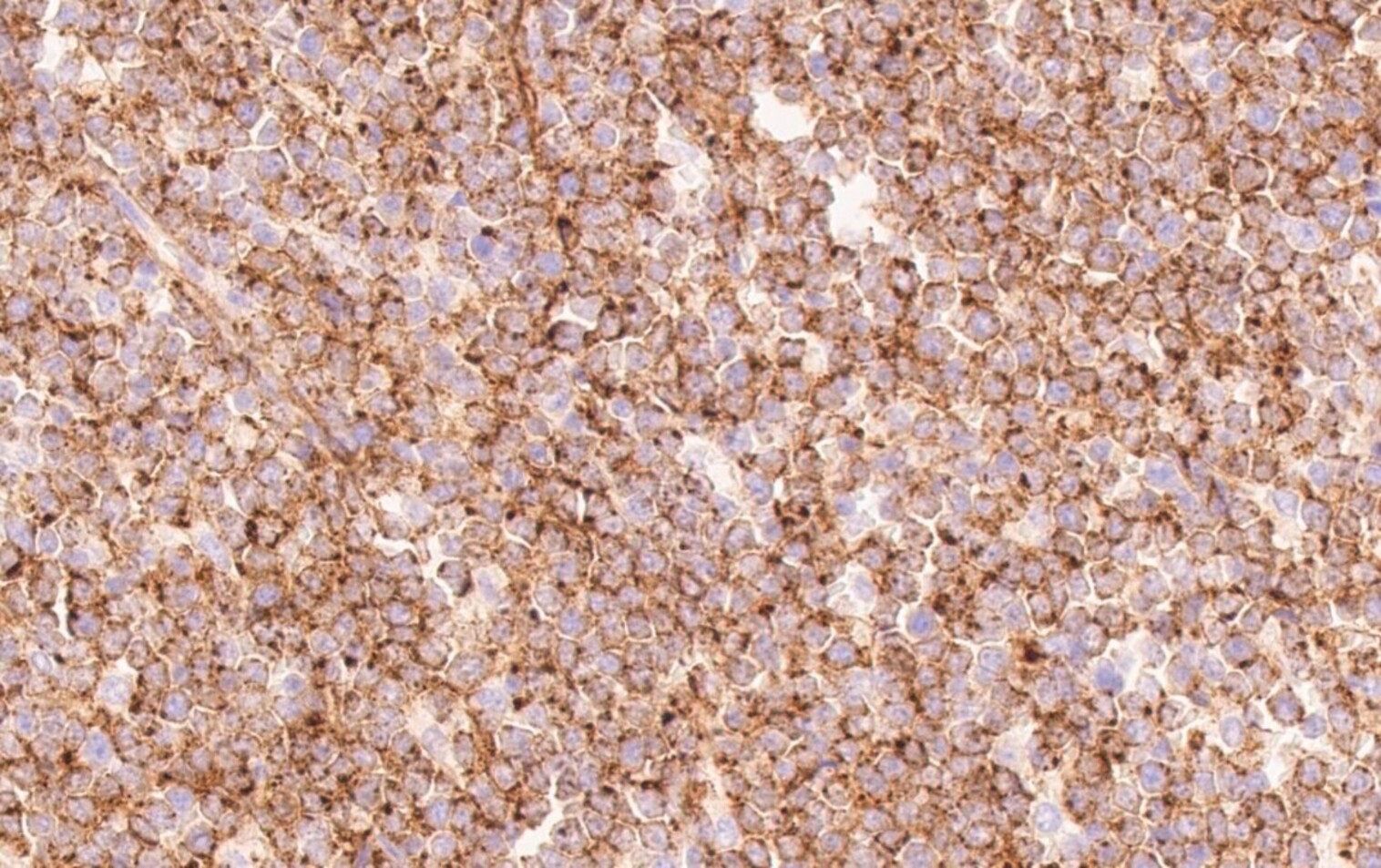
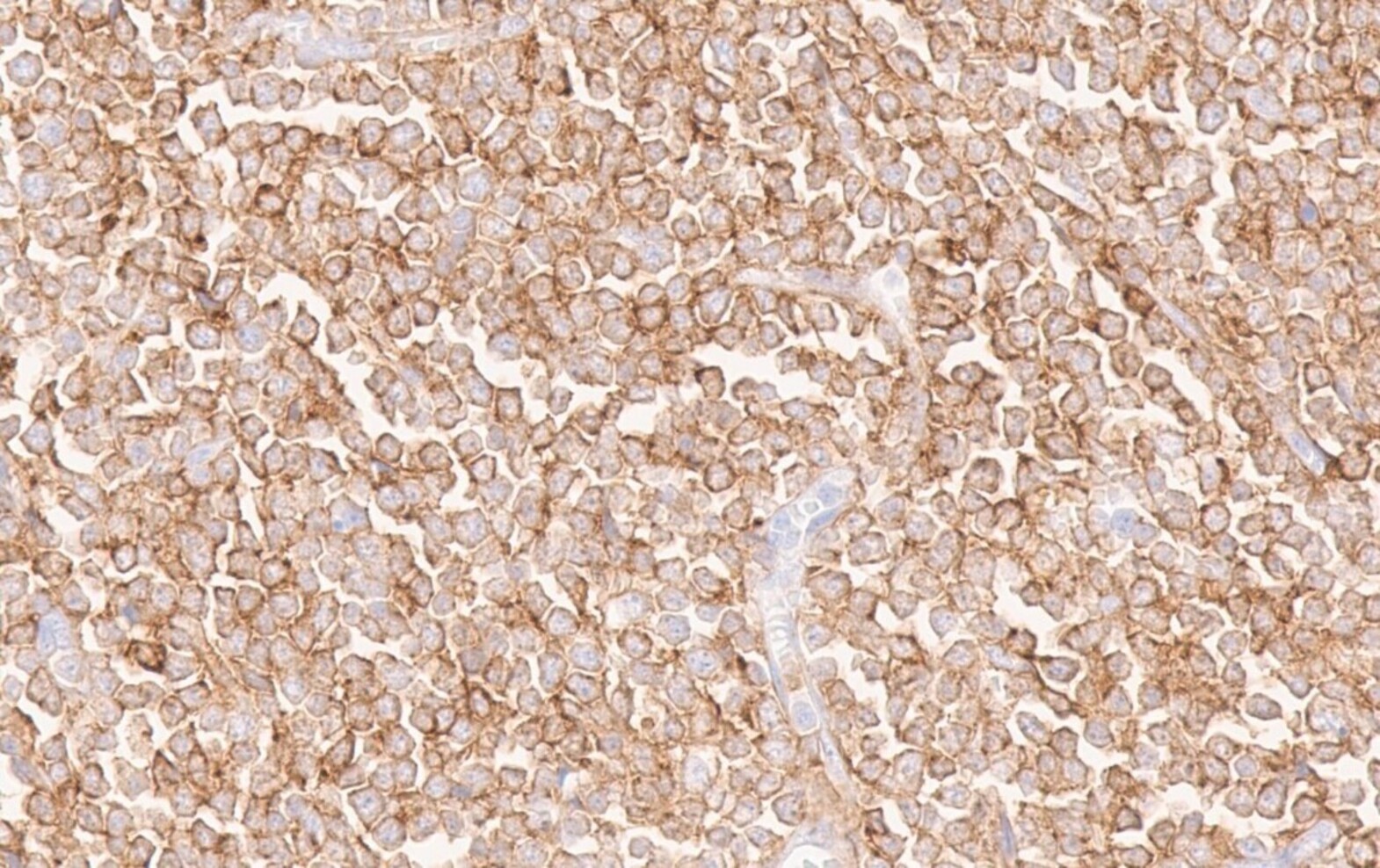
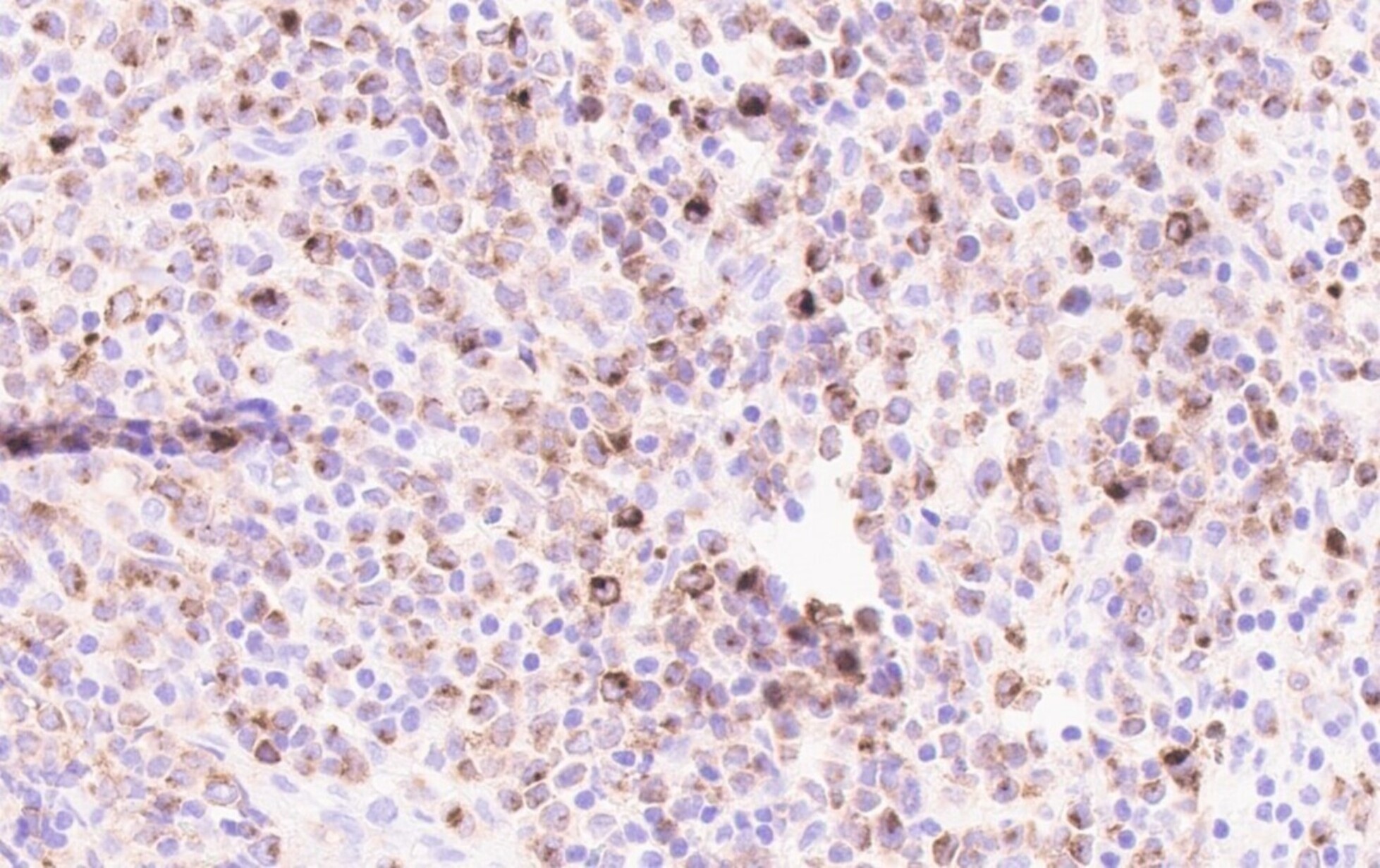
Positive stains
- Positivity may depend on the patterns of differentiation (Diagn Pathol 2007;2:42)
- CD68 / KP1, lysozyme, CD33, CD43: positive in high percentage of cases
- CD45, MPO and CD117 are also commonly seen but are not present in all cases (likely in > 50% of cases)
- CD56, CD34 and TdT: positive in only subset of cases (likely in < 50% of cases)
- CD71 and glycophorin for lesions with erythroblastic differentiation; CD42b and CD61 for lesions with megakaryoblastic differentiation
Negative stains
- Negative for lymphoid lineage markers, such as CD20 and CD3
- Usually negative for CD19 and PAX5 but association with t(8;21)(q22;q22.1) RUNX1-RUNX1T1 can show positive expression (Blood 1992;80:470)
Flow cytometry description
- Workup similar to that for acute leukemia but fresh material is usually lacking
Molecular / cytogenetics description
- Roughly half of cases show cytogenetic abnormalities
- Rare cases may show discrepancies from the karyotype obtained from the corresponding bone marrow, which may suggest clonal evolution
- Cytogenetic and molecular abnormalities seen in myeloid sarcoma are similar to those seen in AML
- Common translocations include t(8;21)(q22;q22) RUNX1-RUNX1T1 and inv(16)(p13.1q22) CBFB-MYH11
- Complex karyotype can be seen, usually in secondary myeloid sarcoma (Eur J Haematol 2018;100:603)
- Common mutations include NRAS, NPM1, FLT3-ITD, KRAS and IDH2 (Blood Cancer J 2018;8:43)
- JAK2 V617F mutation can be seen, especially in cases with pre-existing MPN
- BCR-ABL1 fusion can be seen and may represent disease progression of chronic myeloid leukemia (CML)
Sample pathology report
- Nasopharyngeal mass, biopsy:
- Myeloid sarcoma (see comment)
- Comment: The histologic sections demonstrate sheets of immature mononuclear cells. The immature cells are positive for CD34, CD33, CD117, MPO and negative for CD19 and CD3 by immunohistochemical stains. The morphologic and immunohistologic findings in the current sample support a diagnosis of myeloid sarcoma. A bone marrow examination to investigate for potential involvement by a myeloid neoplasm / acute myeloid leukemia will be of interest if clinically indicated. If no fresh diagnostic material can be retrieved from additional tissue / bone marrow examination, fluorescence in situ hybridization studies and molecular studies can be requested on the current sample for further characterization.
Differential diagnosis
- Lymphoblastic lymphoma:
- Can demonstrate similar blast-like morphology
- Relies on immunophenotyping to differentiate
- Positive for T cell or B cell lineage markers; commonly positive for TdT
- Burkitt lymphoma:
- Usually has a prominent starry sky appearance, though the starry sky appearance is not entirely specific
- Positive for B cell lineage markers, not myeloid markers
- MYC rearrangement present
- Diffuse large B cell lymphoma:
- Predominately large cells; cytologic features are less likely blastoid
- Positive for B cell lineage markers, not myeloid markers
- Histiocytic sarcoma:
- Can be a very challenging differential diagnosis due to significant overlapping immunophenotypic features
- Less likely to demonstrate bone marrow involvement
- Nonhematopoietic tumor:
- Relies on immunophenotyping in some scenarios
- Commonly used markers for screening include cytokeratin, CD45 and S100
- Extramedullary hematopoiesis:
- More likely occurs in patients with MPN
- Trilineage hematopoiesis with maturation is usually present
- Blastic plasmacytoid dendritic cell neoplasm (BPDCN):
Additional references
Board review style question #1
A 49 year old man with a history of MPN presented with small intestinal obstruction and was found to have a soft tissue mass on abdominal CT scan. Excisional biopsy showed sheets of immature mononuclear cells with fine chromatin. Tumor cells were positive for MPO, CD34, CD117 and CD33 and negative for TdT. A diagnosis of myeloid sarcoma was rendered. Which of the following statements is true about myeloid sarcoma?
- Always presents as a solitary mass
- Can occur with a concurrent myeloid disorder
- CD43 is not a good marker for the diagnosis
- Only occurs in the GI tract
Board review style answer #1
B. Can occur with a concurrent myeloid disorder (Clin Lymphoma Myeloma Leuk 2017;17:263)
Comment Here
Reference: Myeloid sarcoma
Comment Here
Reference: Myeloid sarcoma
Board review style question #2
Which of the following statements is true about myeloid sarcoma?
- Always shows megakaryoblastic differentiation
- Can only be identified with concurrent acute myeloid leukemia in bone marrow
- Never shows cytogenetic abnormalities
- NPM1 mutation can be found in myeloid sarcoma and lesions with NPM1 mutations tend to demonstrate myelomonocytic morphology
Board review style answer #2
D. NPM1 mutation can be found in myeloid sarcoma and lesions with NPM1 mutations tend to demonstrate myelomonocytic morphology (Clin Lymphoma Myeloma Leuk 2017;17:263, Blood 2016;127:2391)
Comment Here
Reference: Myeloid sarcoma
Comment Here
Reference: Myeloid sarcoma