Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Younes IE, Zhang L. PDGFRA rearrangement. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativePDGFRA.html. Accessed April 23rd, 2024.
Definition / general
- Myeloid, lymphoid or combined myeloid / lymphoid neoplasms often accompanied by eosinophilia and associated with FIP1L1-PDGFRA fusion, other PDGFRA gene rearrangements or an activating mutation of the PDGFRA gene
Essential features
- Myeloid or lymphoid neoplasm, often accompanied by prominent eosinophilia
- Involved PDGFRA gene, cytogenetic location 4q12 (N Engl J Med 2003;348:1201)
- Rearrangement of PDGFRA must be defined by FISH or reverse transcription PCR
- Multiple fusion partners are identified
- Can have different bone marrow pictures, most commonly chronic eosinophilic leukemia (CEL) or other diseases (e.g., acute transformation to acute myeloid leukemia or T cell acute lymphoblastic leukemia [T ALL])
- Very sensitive to imatinib therapy
- Associated mast cell hyperplasia
Terminology
- Myeloid and lymphoid neoplasms with PDGFRA rearrangement
- Myeloid and lymphoid neoplasms associated with PDGFRA rearrangement
ICD coding
- ICD-11: 2A50 - myeloid neoplasm associated with PDGFRA rearrangement
Epidemiology
- M:F = 17:1
- Age range 7 - 77; mostly between 25 - 55
- Reference: Immunol Allergy Clin North Am 2007;27:377
Sites
- Usually, peripheral blood and bone marrow
Pathophysiology
- Most common translocation partner is FIP1L1; FIP1L1-PDGFRA is generated from a submicroscopic, 800 kb interstitial deletion on chromosome 4, del(4)(q12q12), a cryptic deletion of CHIC2
- Other fusion partners have been identified:
- t(4;10)(q12;p11) KIF5B:PDGFRA (Leukemia 2006;20:827)
- ins(9;4)(q33;q12q25) CDK5RAP2:PDGFRA (Genes Chromosomes Cancer 2006;45:950)
- t(4;12)(q12;p13) PDGFRA:ETV6 (Br J Haematol 2007;138:77)
- t(4;22)(q12;q11) BCR:PDGFRA
- t(4;2) (q12;p24) STRN:PDGFRA
- t(4;10)(q12;q23) PDGFRA:TNKS2
- t(4;22)(q12;q11) BCR-PDGFRA translocation is a neoplasm with features intermediate between chronic myeloid leukemia and chronic eosinophilic leukemia (CEL) (Immunol Allergy Clin North Am 2007;27:377)
- Extramedullary myeloid tumor can harbor PDGFRA rearrangement despite the bone marrow not displaying the rearrangement (Cancer Genet 2018;220:13)
- Activating mutations of PDGFRA can play a role in tumorigenesis (e.g., Y288C mutation) (Nat Commun 2018;9:4583)
Etiology
- Unknown
- Some cases occurred after cytotoxic chemotherapy (Br J Haematol 2006;134:547)
Clinical features
- Always involves peripheral blood and bone marrow; however, other organs can be affected (e.g., lungs, CNS, skin and GI tract)
- Usually presents as chronic eosinophilic leukemia (CEL); can present as acute myeloid leukemia, T acute lymphoblastic leukemia or myeloid sarcoma (Leukemia 2007;21:1183, Head Neck Pathol 2021;15:1399)
- Symptoms range from asymptomatic to generalized fatigue, respiratory (dyspnea, cough) or GI symptoms (Leuk Lymphoma 2013;54:897)
- Can present with cardiac symptoms, such as endomyocardial fibrosis, Loeffler endocarditis and valvular regurgitation (Korean J Intern Med 2018;33:642)
- Splenomegaly is more common than hepatomegaly
- Can present as arterial or venous thrombosis or thrombotic thrombocytopenic purpura (TTP) (Case Rep Hematol 2019;2019:2820954)
- May present with anemia and thrombocytopenia
Diagnosis
- Peripheral blood and bone marrow examination both usually show eosinophilia (sometimes normal)
- FISH is the most used method for diagnosis (also known as CHIC2 deletion) and chromosomal microarray; however, reverse transcription PCR is more sensitive (Oncol Lett 2020;19:3587)
- Phosphoflow cytometry can be used for detection of PDGFRA rearrangement; however, it is not very sensitive in patients on treatment (Cytometry A 2021;99:784)
- Next generation sequencing can show additional mutations associated with this rearrangement (e.g., somatic KIT and CSF3R mutations) (Haematologica 2018;103:e348, Am J Hematol 2016;91:E10)
- Diagnostic criteria for myeloid / lymphoid neoplasms with eosinophilia associated with FIP1L1-PDGFRA or a variant fusion gene (A):
- Myeloid or lymphoid neoplasm, usually with prominent eosinophilia and presence of FIP1L1-PDGFRA fusion gene or a variant fusion gene with rearrangement of PDGFRA or an activating mutation of PDGFRA (B):
- A) Cases presenting as a myeloproliferative neoplasm, acute myeloid leukemia or lymphoblastic leukemia / lymphoma with eosinophilia and FIP1L1-PDGFRA gene fusion are assigned to this category
- B) If appropriate molecular analysis is not possible, this diagnosis should be suspected if there is a myeloproliferative neoplasm with no Philadelphia chromosome and with the hematological features of chronic eosinophilic leukemia associated with splenomegaly, marked elevation of serum vitamin, elevation of serum tryptase (> 12 ng/dL and < 20 ng/dL) and an increased number of bone marrow mast cells
- Myeloid or lymphoid neoplasm, usually with prominent eosinophilia and presence of FIP1L1-PDGFRA fusion gene or a variant fusion gene with rearrangement of PDGFRA or an activating mutation of PDGFRA (B):
Laboratory
- Complete blood count usually shows eosinophilia, usually > 1,500/uL; however, normal counts can be found
- Increased vitamin B12 levels > 1,900 ng/L in all cases with FIP1L1-PDFGRA due to increased levels of haptocorrins (Am J Hematol 2019;94:1149)
- Increased tryptase levels > 12 ng/dL and < 20 ng/dL (minor criteria for systemic mastocytosis)
Prognostic factors
- Good prognosis with absence of cardiac involvement
Case reports
- 51 year old woman with cutaneous T cell lymphoma with PDGFRA rearrangement and absence of eosinophilia (Am J Dermatopathol 2018;40:610)
- 60 year old man with chronic eosinophilic leukemia with PDGFRA rearrangement associated with tumor lysis syndrome as a complication of imatinib therapy (J Glob Oncol 2018;4:1)
- 70 year old man presenting with myeloid sarcoma in the retromolar area with PDGFRA rearrangement (Head Neck Pathol 2021;15:1399)
Treatment
- Usually sensitive to imatinib; alternative tyrosine kinase inhibitors (TKIs; e.g., midostaurin or sorafenib) may be effective
- Treatment with imatinib even in cases presenting with acute myeloid leukemia or T cell acute lymphoblastic leukemia
- However, T674I mutation within the adenosine triphosphate (ATP) binding domains of PDGFRA is resistant to imatinib and second generation tyrosine kinase inhibitors; a panresistant D842 variant was also identified (Hemasphere 2019;3:e182, Leukemia 2009;23:845)
- Third generation TKI ponatinib is active against both FIP1L1-PDGFRα p.T674I and p.D842 V (Hemasphere 2019;3:e182)
- Midostaurin and sorafenib are alternative regimens
- Some activating mutations of PDGFRA (e.g., Y288C mutations) are resistant to PDGFRA inhibitors; however, they are sensitive to PI3K / mTOR inhibitors (Nat Commun 2018;9:4583)
- Cases of acute myeloid leukemia with PDGFRA rearrangement and another mutation can achieve complete remission with tyrosine kinase inhibitors (Oncol Lett 2020;19:3587)
- End point of treatment is unclear; in general, imatinib can effectively suppress but not eliminate the FIP1L1-PDGFRA clone in most patients (Am J Hematol 2019;94:1149)
- Allogeneic hematopoietic stem cell transplantation would be the option for those resistant / refractory cases (Blood 2017;129:704)
Microscopic (histologic) description
- Monocytes and basophil counts are normal
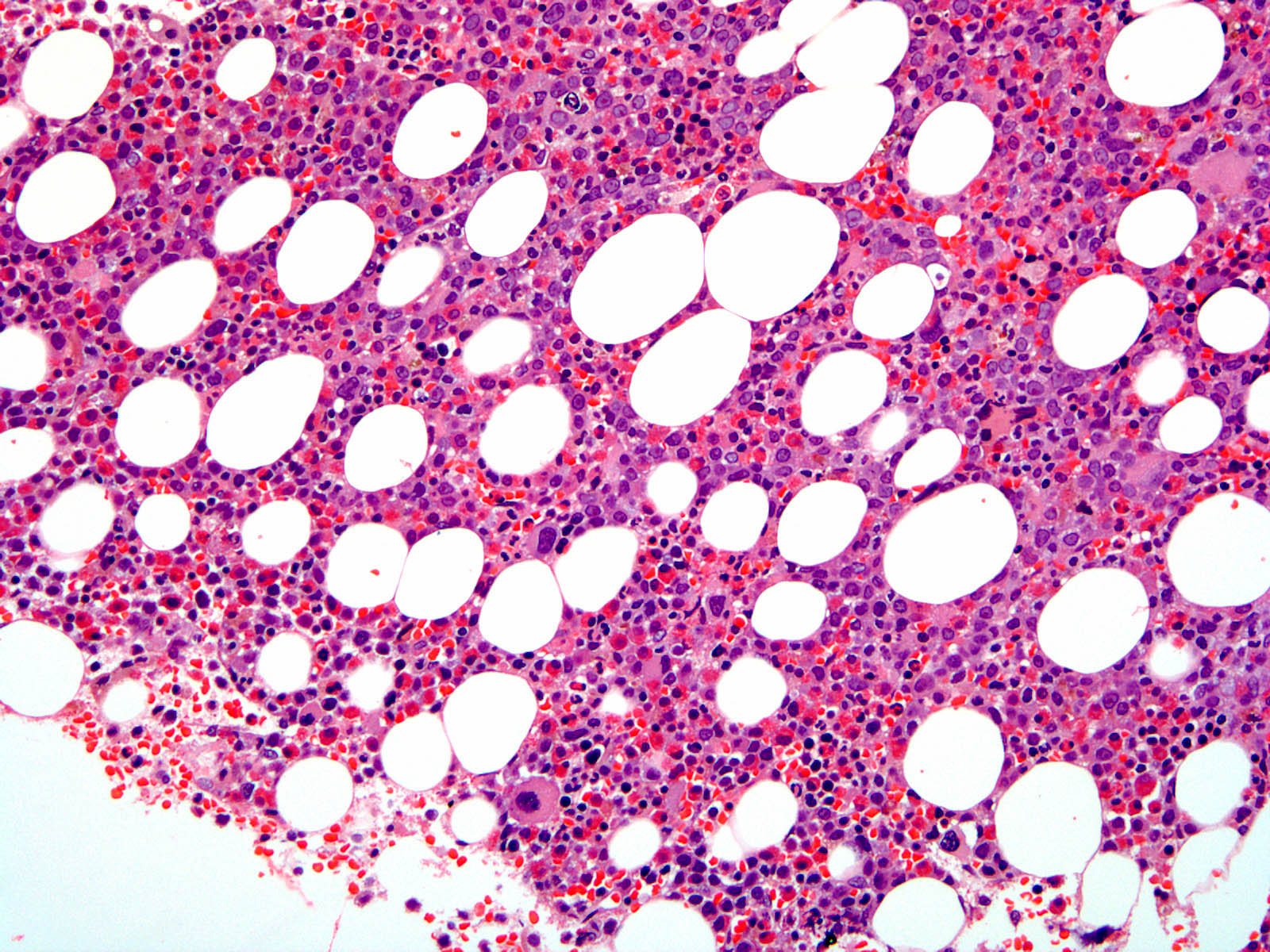
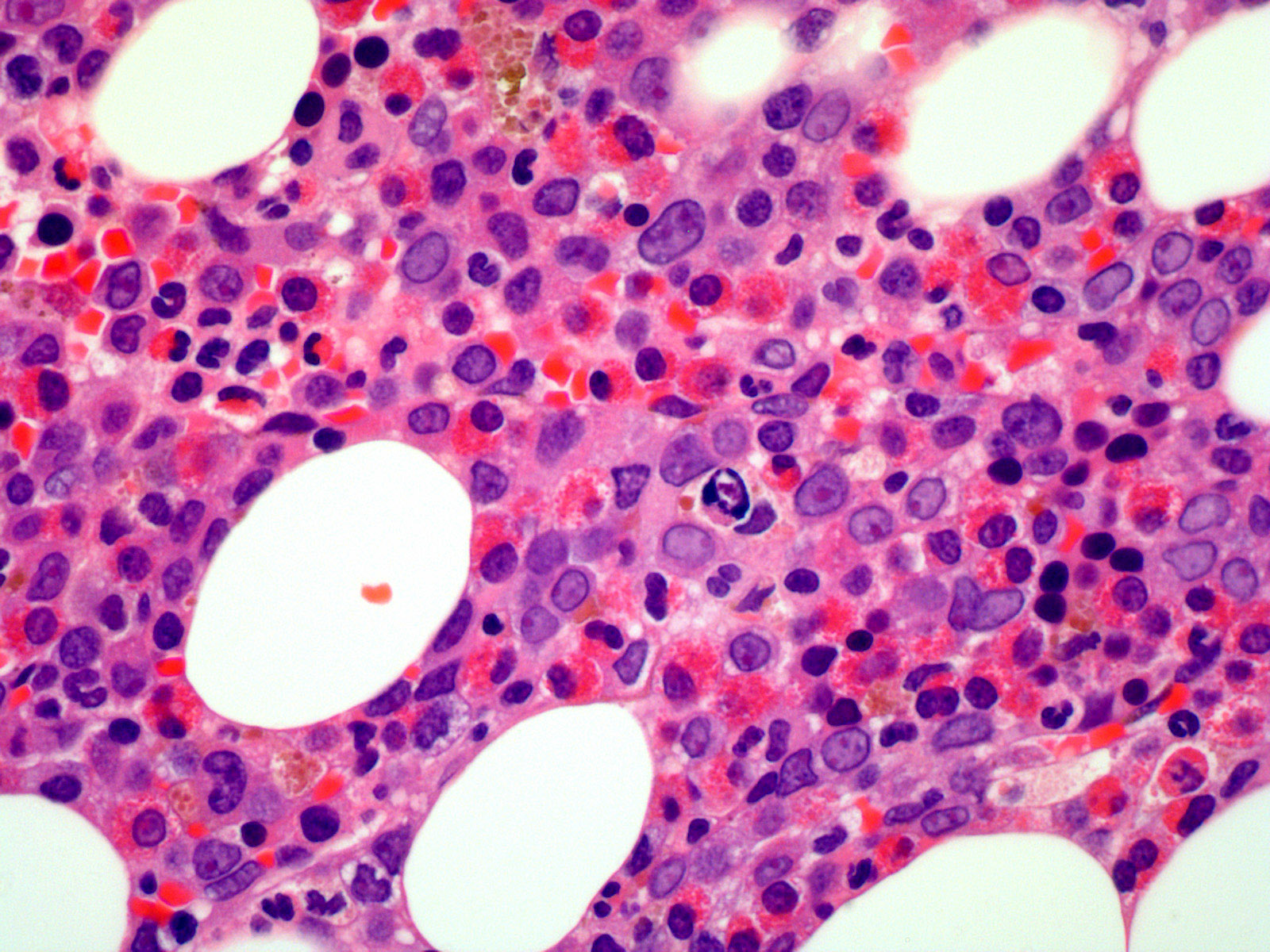
- Hypercellular marrow with increased eosinophils and its precursors (see Microscopic [histologic] images); clusters or scattered, increased spindle shaped CD25+ or CD2- mast cells present (Blood 2003;101:4660)
- Reticulin fibrosis is identified; sometimes can be normal (Blood 2004;103:473)
- Increased blasts identified in accelerated / blastic phase of myeloproliferative neoplasms or de novo acute myeloid leukemia or T cell acute lymphoblastic leukemia (see Microscopic [histologic] images)
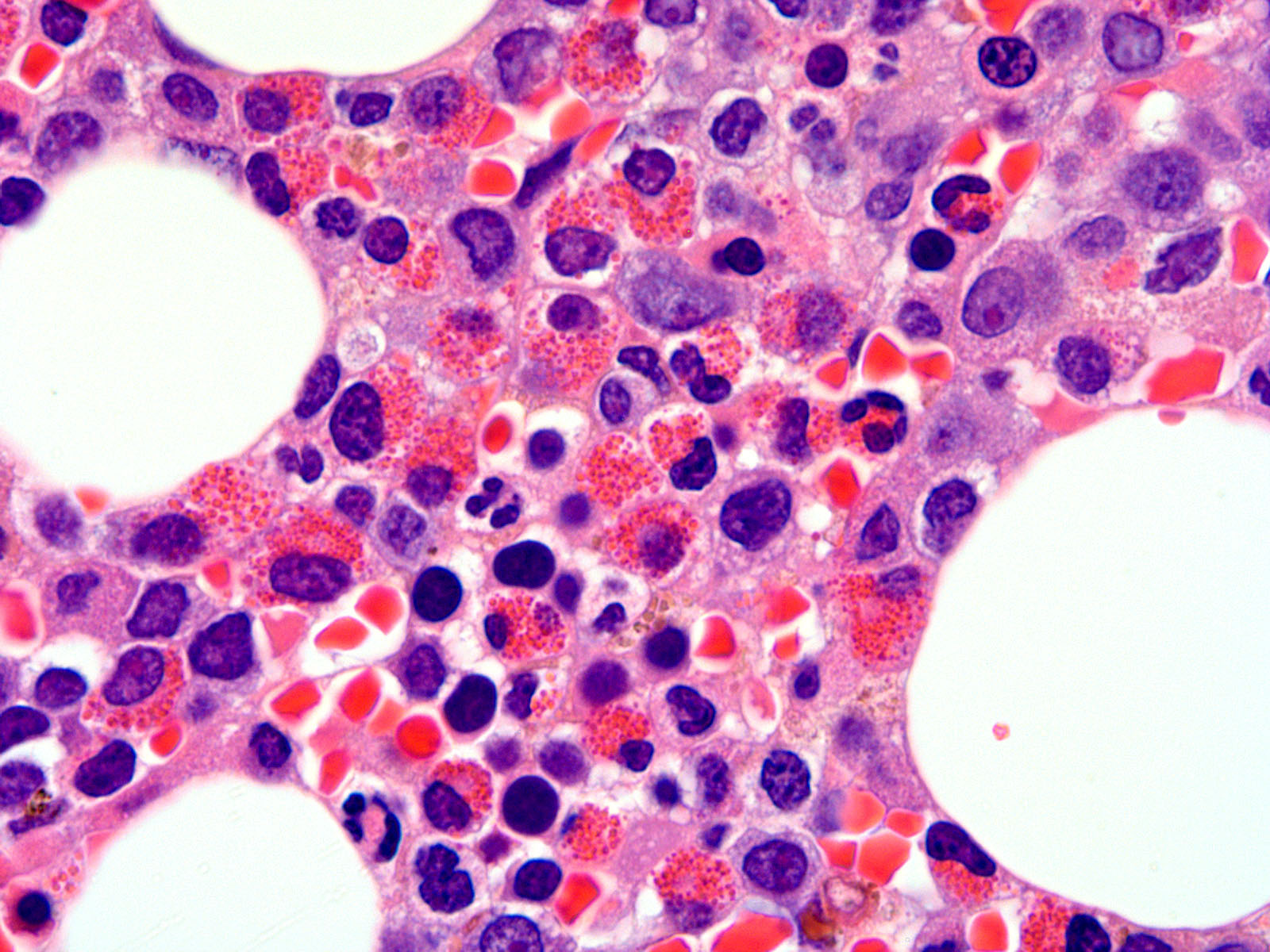
- Tissue eosinophilic infiltrate forming eosinophilic microabscesses or identifiable Charcot-Leyden crystals
Microscopic (histologic) images
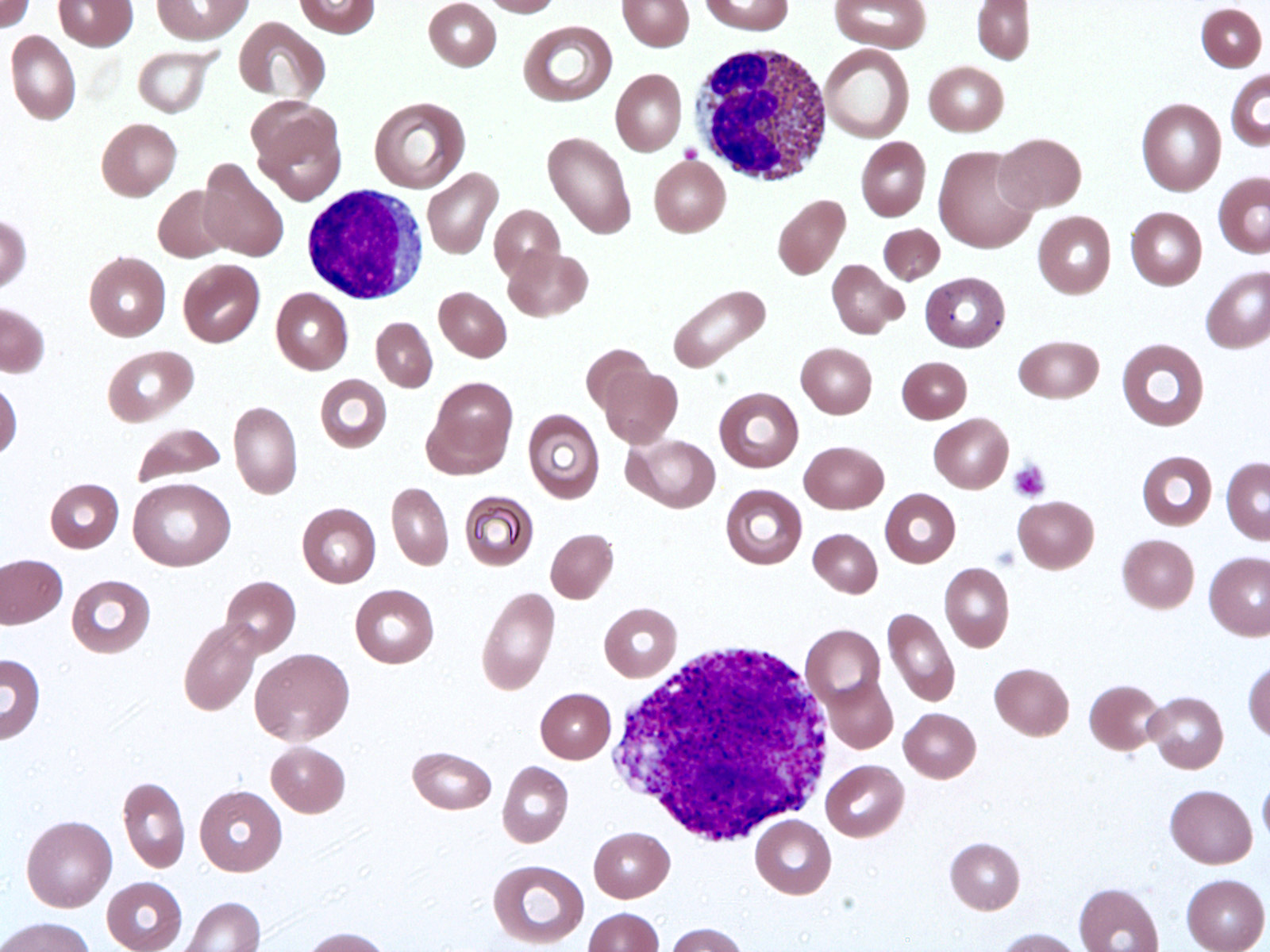
Peripheral smear description
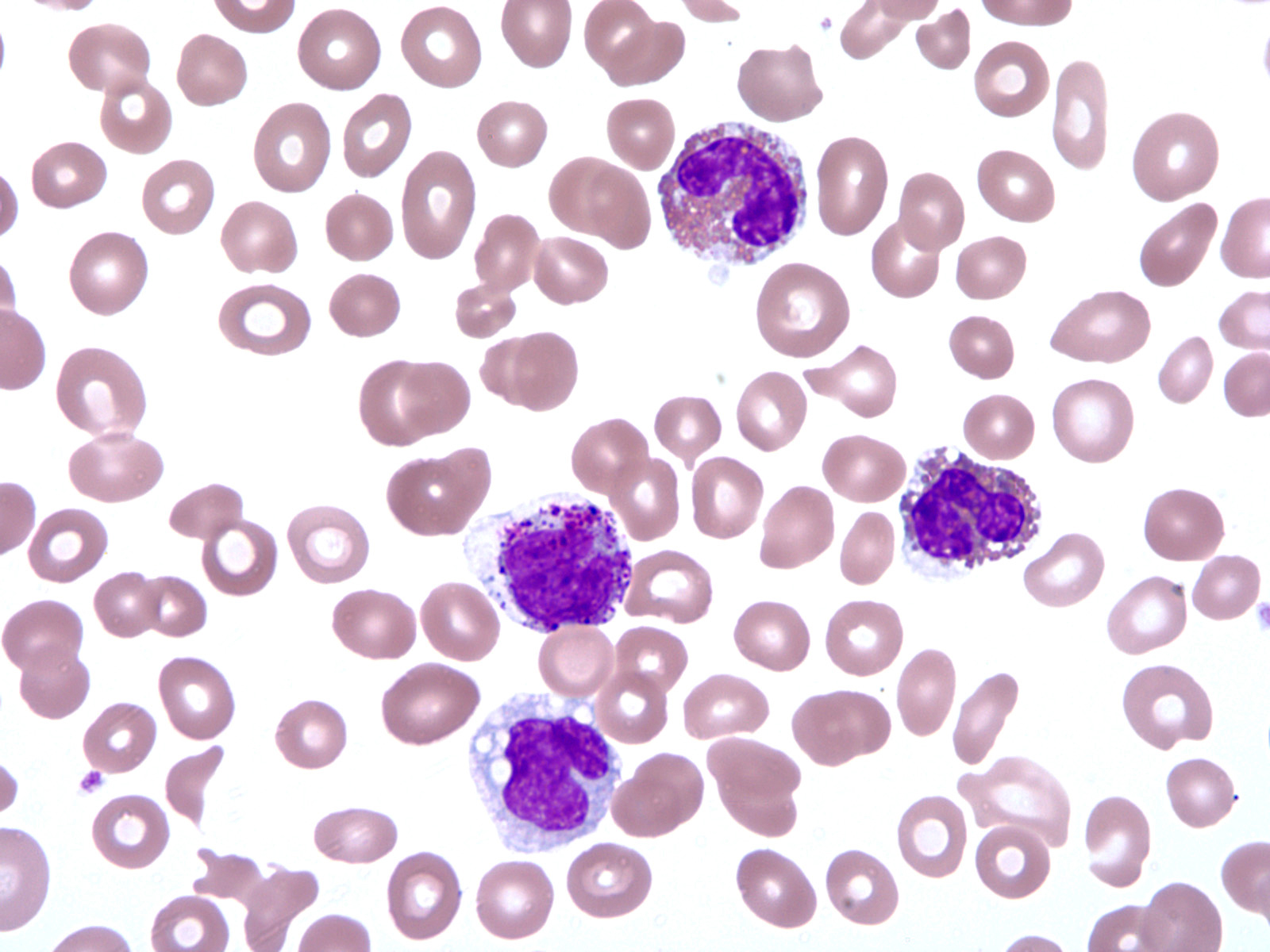
- Peripheral blood eosinophilia, occasionally neutrophilia; eosinophils are usually mature
- Variable morphologic abnormalities can be present in the eosinophils, including cytoplasmic vacuolation, nuclear hypersegmentation / hyposegmentation; morphologic changes are not unique as reactive conditions can have these changes as well (Foucar: Bone Marrow Pathology, 4th Edition, 2019)
- Some cases will present with increased blasts if the patient has acute lymphocytic leukemia or acute myeloid leukemia
Peripheral smear images
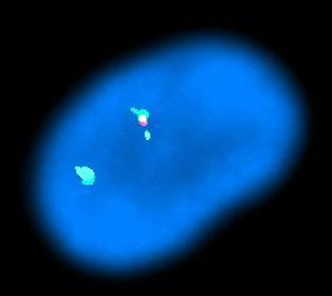
Molecular / cytogenetics description
- FISH shows loss of CHIC2 gene
Molecular / cytogenetics images
Sample pathology report
- Peripheral blood:
- Microcytosis with mild anisopoikilocytosis
- Moderate neutropenia with marked eosinophilia
- Moderate thrombocytopenia
- Bone marrow, left posterior iliac crest, aspirate and biopsy:
- Hypercellular marrow with marked hypereosinophilia and FIP1L1-PDGFRA rearrangement (see comment)
- Marked reticulin and diffuse collagen fibrosis
- Absent iron stores
- Comment: Cytogenetics confirms a normal male karyotype (46,XY). FISH identifies a microdeletion of the CHIC2 gene on chromosome 4q12, resulting in fusion of FIP1L1-PDGFRA. No abnormality of PDGFRB (5q33) is identified.
- Microscopic description:
- Peripheral smear: The hemoglobin is normal, with microcytic indices and mild anisopoikilocytosis. The reticulocyte count is decreased for the degree of anemia. The total white cell count is moderately increased, with a moderately severe neutropenia and a marked eosinophilia composed of relatively mature appearing eosinophils with normal granulation. Circulating blasts are not seen. The platelet count is moderately decreased, with relatively normal platelet morphology.
- Aspirate smear: The Wright stained aspirate smears are hypercellular with several poorly spread spicules, adequate for evaluation. Megakaryocytes are noted in moderately increased numbers, including many large hyperlobulated forms and few hypolobulated forms with increased cytoplasm. Myeloid and erythroid precursors are evenly distributed, with an M:E ratio calculated at 2.6. Myeloid precursors show adequate maturation and relatively normal morphology. There is no increase in blast cells. There is a marked eosinophilia, with predominantly mature segmented eosinophils showing normal granulation. Erythroid precursors are admixed, with adequate maturation and mild megaloblastic change. There is no significant increase in mast cells.
- Clot section and core biopsy: The clot section (A) and core biopsy (B) are fixed in B-Plus Fix (BBC) fixative. The core biopsy (B) is decalcified in Nitrical (5% nitric acid). 1 level of the clot section (A) and 1 level of the core biopsy (B) are stained with PAS to evaluate myeloid maturation and more accurately estimate an M:E ratio. 1 level of the clot section (A) and 1 level of the core biopsy (B) are stained with Prussian blue to evaluate iron stores and the average value reported. 1 level of the core biopsy (B) is stained with reticulin stain to evaluate marrow fibrosis.
- Thickened bony trabeculae show extensive evidence of bony remodeling and surround a marrow that is variably densely fibrotic and variably hypercellular, with prominent dilatation of the marrow sinuses. The overall cellularity approaches 100%. An occasional interstitial benign lymphoid aggregate is noted. Megakaryocytes showing the aforementioned abnormalities are present, forming distinct clusters and few small aggregates. Large erythroid islands are noted, with interspersed marrow parenchyma containing predominantly mature eosinophils. Admixed neutrophils are also seen. An M:E ratio is estimated at 10:1. Stainable iron is absent. There is a diffuse marked reticulin fibrosis associated with the aforementioned collagen fibrosis. Collagen accounts for approximately 20% of the total marrow space.
Differential diagnosis
- Chronic eosinophilic leukemia, NOS:
- Sustained eosinophilia (> 1.5 x 109/L) with other reactive processes excluded
- < 20% blasts in peripheral blood or bone marrow, no inv(16)(p13.1q22), t(16;16)(p13.1q22), t(8;21)(q22;q22.1)
- Bone marrow aspirate is hypercellular with abundant eosinophils and eosinophilic precursors with dysplastic features
- Clonal cytogenetic (e.g., +8, -20q) or molecular genetic abnormalities (e.g., TET2, ASXL1 and DNMT3A); if no clonal abnormalities are found, then blast count must be > 2% in peripheral blood or > 5% in bone marrow aspirate
- No PDGFRA, PDGFRB, FGFR rearrangement, BCR-ABL1, PCM1-JAK2, ETV6-JAK2 or BCR-JAK2 fusions
- No lymphoid or myeloid neoplasms (e.g., myelodysplastic syndrome, myeloproliferative neoplasms, systemic mastocytosis or acute myeloid leukemia) are identified
- Hypereosinophilic syndrome:
- Sustained eosinophilia (> 1.5 x 109/L) for at least 6 months with other reactive processes excluded
- Tissue damage must be present
- Exclude cytokine related lymphoid variant hypereosinophilia
- Bone marrow shows increased eosinophils with no dysplastic features and no increase in blast cells
- No cytogenetic or molecular alteration is identified
- No PDGFRA, PDGFRB, FGFR rearrangement, BCR-ABL1, PCM1-JAK2, ETV6-JAK2 or BCR-JAK2 fusions; no inv(16)(p13.1q22), t(16;16)(p13.1q22) or t(8;21)(q22;q22.1)
- No lymphoid or myeloid neoplasms (e.g., myelodysplastic syndrome, myeloproliferative neoplasms, systemic mastocytosis or acute myeloid leukemia) are identified
- Reactive eosinophilia:
- Can present with the increased eosinophils and can have abnormal eosinophils in the peripheral blood
- Secondary causes (infection, allergies, drugs, skin diseases, asthma, adrenal insufficiency, gastrointestinal eosinophilia) are present
- No evidence of PDGFRA rearrangement or active mutation or other cytogenetic / molecular abnormalities
- Idiopathic hypereosinophilia:
- Sustained eosinophilia (> 1.5 x 109/L) for at least 6 months
- No tissue damage
- No reactive causes, lymphoid or myeloid neoplasms are identified
- Bone marrow shows increased eosinophils with no dysplastic features and no increase in blast cells
- No molecular alteration is identified
- No PDGFRA, PDGFRB, FGFR rearrangement, BCR-ABL1, PCM1-JAK2, ETV6-JAK2 or BCR-JAK2 fusions
- Systemic mastocytosis with eosinophilia:
- Major diagnostic criteria: 4 minor criteria or multifocal infiltrates of mast cells (≥ 15 mast cells in aggregates) + 1 minor criterion
- Minor criteria:
- Bone marrow shows eosinophilia with variable dysplastic features
- No PDGFRA rearrangement
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
What is the most deleted gene in myeloid and lymphoid neoplasms with PDGFRA rearrangement?
- ABL
- BCR
- CHIC2
- FGFR
- TNK2
Board review style answer #2