Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Easwar A, Siddon AJ. Juvenile myelomonocytic leukemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativeJMML.html. Accessed April 24th, 2024.
Definition / general
- Clonal proliferation of (predominantly) granulocytic and monocytic precursors in children, with < 20% blasts in blood and bone marrow
- Mutations involving RAS pathway are characteristic
Essential features
- Myelodysplastic / myeloproliferative disorder in childhood, can occur in children from 1 month of age to adolescence
- Approximately 75% of cases occur in children less than 3 years old
- Greater than 75% of cases have mutations in the RAS pathways: PTPN11, KRAS, NRAS, CBL and NF1
- About 15% of cases are diagnosed in patients with Noonan syndrome (or Noonan-like syndrome) and ~10% occur in conjunction with neurofibromatosis type 1
- Patients present with hepatosplenomegaly and sustained increased monocyte counts
- Stem cell transplant improves survival
Terminology
- Juvenile chronic myelomonocytic leukemia
ICD coding
Epidemiology
- About 1.2 per million people per year
- 75% of cases occur in children < 3 years old, more frequent in males (~2:1)
- Median age of 2 years
- 10% of cases are associated with neurofibromatosis 1 and deletion of NF1 gene
- 15% of cases in infants with Noonan syndrome (CBL gene mutation)
- Rare association with EBV infection (Leuk Res 2008;32:181)
Sites
- Primarily involves peripheral blood and bone marrow
- Nearly all cases involve the liver and spleen
- Other common sites of involvement include lymph nodes, skin and respiratory tract
- Reference: Blood 1998;91:365
Pathophysiology
- Due to stem cell defect causing deranged hematopoiesis (Blood 2019;133:1060)
- In vitro hypersensitivity to GM-CSF, considered to be a hallmark of the disease (and previously used for diagnosis)
- Multiple genes affecting the RAS / MAPK signaling pathways have been implicated
- Gain of function mutations in more than 75% of patients
- Somatic mutations in NRAS or KRAS identified in ~30% of cases, which can lead to accumulation of activated protein
- ~30% of cases have PTPN11 mutations, which can be somatic or germline as seen in Noonan syndrome
- Loss of function mutation in NF1, which can be somatic or germline
- ~15 - 20% of cases can have NF1 mutation or deletion without neurofibromatosis
- CBL mutation, which can be somatic or germline (Noonan-like syndrome)
- ~10 - 15% of cases can have this loss of function mutation
- Gain of function mutations in more than 75% of patients
- Leads to reduced degradation of tyrosine kinase receptors and therefore increased RAS pathway signaling
Clinical features
- Varied clinical presentation may include failure to thrive, malaise, fever, bleeding, pallor, infection, with or without lymphadenopathy; rarely CNS involvement (J Pediatr Hematol Oncol 2007;29:770)
- Splenomegaly: found in all cases and prerequisite for diagnosis of JMML but may be initially absent at presentation
- Skin lesions: can be seen in patients with germline mutations (e.g., café au lait spots in NF1 and CBL mutations)
- Gastrointestinal and pulmonary symptoms can also be observed
- Most patients die of disease, although it may wax and wane
Diagnosis
- Multiple diagnostic criteria, according to WHO
- Genetic criteria (at least 1):
- Somatic mutations in PTNP11, KRAS or NRAS
- If present, germline mutations or transient abnormal myelopoiesis of Noonan syndrome have to be excluded
- NF1 mutation or clinical diagnosis of neurofibromatosis type 1
- CBL mutation (germline) or loss of heterozygosity
- Somatic mutations in PTNP11, KRAS or NRAS
- Clinical and hematologic criteria (must fulfill all 4):
- Sustained peripheral blood absolute monocyte count ≥ 1 x 109/L
- < 20% blasts + promonocytes in blood and bone marrow
- No Philadelphia chromosome or BCR::ABL1 fusion
- Splenomegaly
- If no genetic criteria are present, then the following criteria should be used (along with the clinical and hematologic criteria):
- Monosomy 7 or other chromosomal abnormality
- Or 2 or more of the following criteria:
- Increased hemoglobin F (for age)
- Myeloid / erythroid precursors on peripheral blood smear
- In vitro hypersensitivity to granulocyte macrophage colony stimulating factor (GM-CSF or CSF2)
- Hyperphosphorylation of STAT5
- Typically, WBC count is 20 - 30 x 109/L with granulocytes and monocytes and occasional dysplasia (which may not be prominent) (Blood 2019;133:1060)
Laboratory
- Peripheral blood monocytosis ≥ 1 x 109/L
- Abnormally increased hemoglobin F for patient's age
- Peripheral smear showing leukocytosis with myeloid left shift, in addition to thrombocytopenia and often anemia
- Nucleated RBCs are often seen
- Reference: Blood 1998;91:365
Prognostic factors
- According to WHO, low platelet counts, age at diagnosis greater than 2 years and high HgF levels are clinical predictors of shorter survival
- PTPN11 mutated JMML or in patients with NF1 is fatal without rapid treatment
- Aggressive JMML with increased risk of progression to AML seen in patients with more than 1 RAS activating mutation (Leukemia 2020;34:1658)
- BMP4, CALC4, CDKN2B, RARB, RASA4 isoform 2: other genes known to be hypermethylated in JMML
- Methylation pattern in JMML divided into 3 categories with different prognosis:
- Favorable prognosis: low methylation, underlying somatic mutations in NRAS and CBL
- Poor prognosis: high methylation, somatic PTPN11 mutations
- Intermediate category: somatic KRAS mutations and monosomy 7
- Reference: Blood Adv 2021;5:5507
Case reports
- 22 month old boy with concurrent neurofibromatosis type 1 and juvenile xanthogranuloma diagnosed with JMML (Pediatr Dermatol 2017;34:114)
- 2 year old boy with JMML with t(3;5), Auer rods and marked myelodysplasia (Pathol Res Pract 2018;214:919)
- 2 year old girl who presented with MDS / MPN driven by a cryptic NUP98::NSD1 fusion (J Pediatr Hematol Oncol 2021;43:e808)
Treatment
- Stem cell transplantation
- Recommended early in disease in patients with PTPN11, KRAS or NF1 mutations
- Deferred in patients with CBL or NRAS (germline) mutations (mainly due to spontaneous regression of JMML)
- Reference: Haematologica 2015;100:17
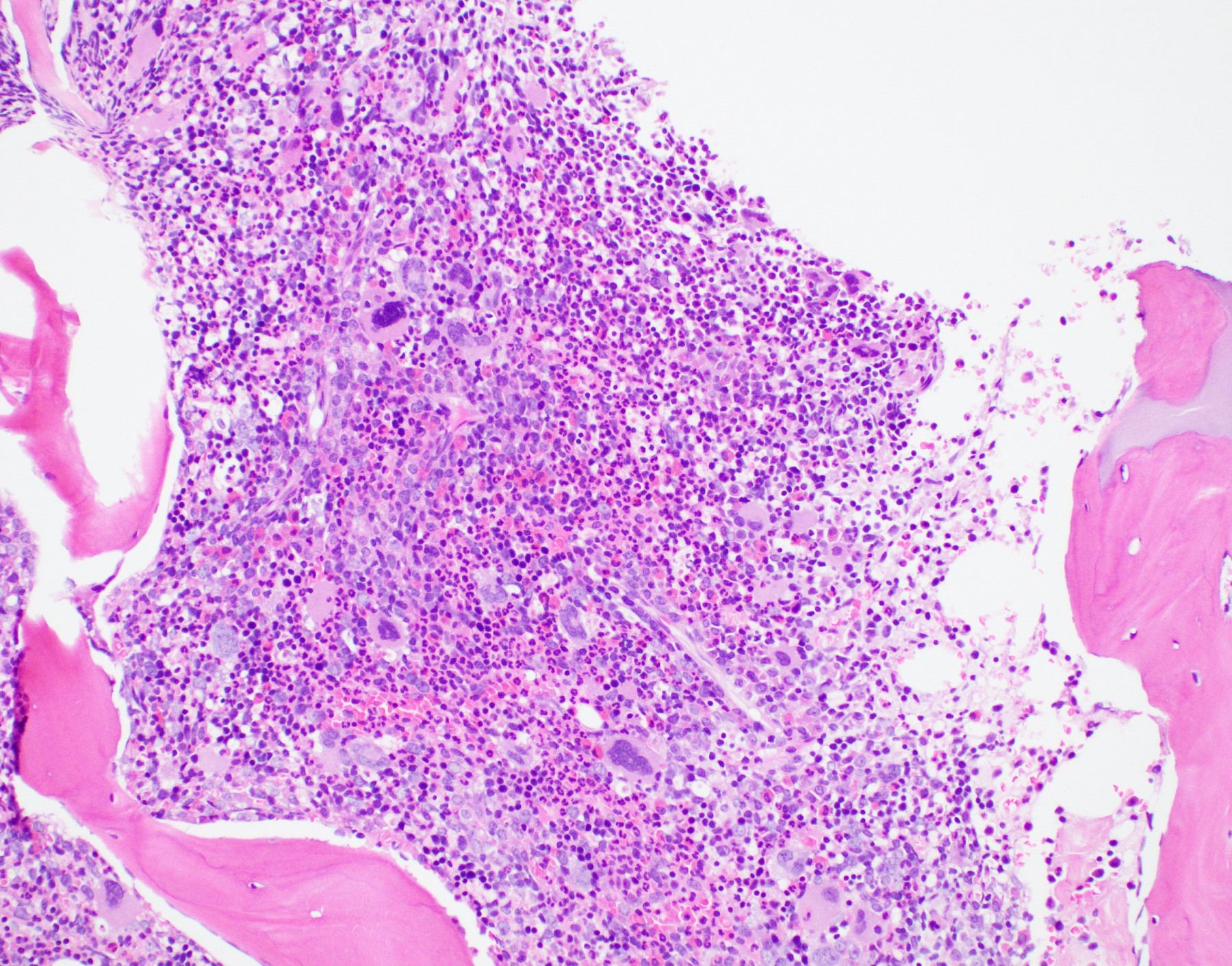
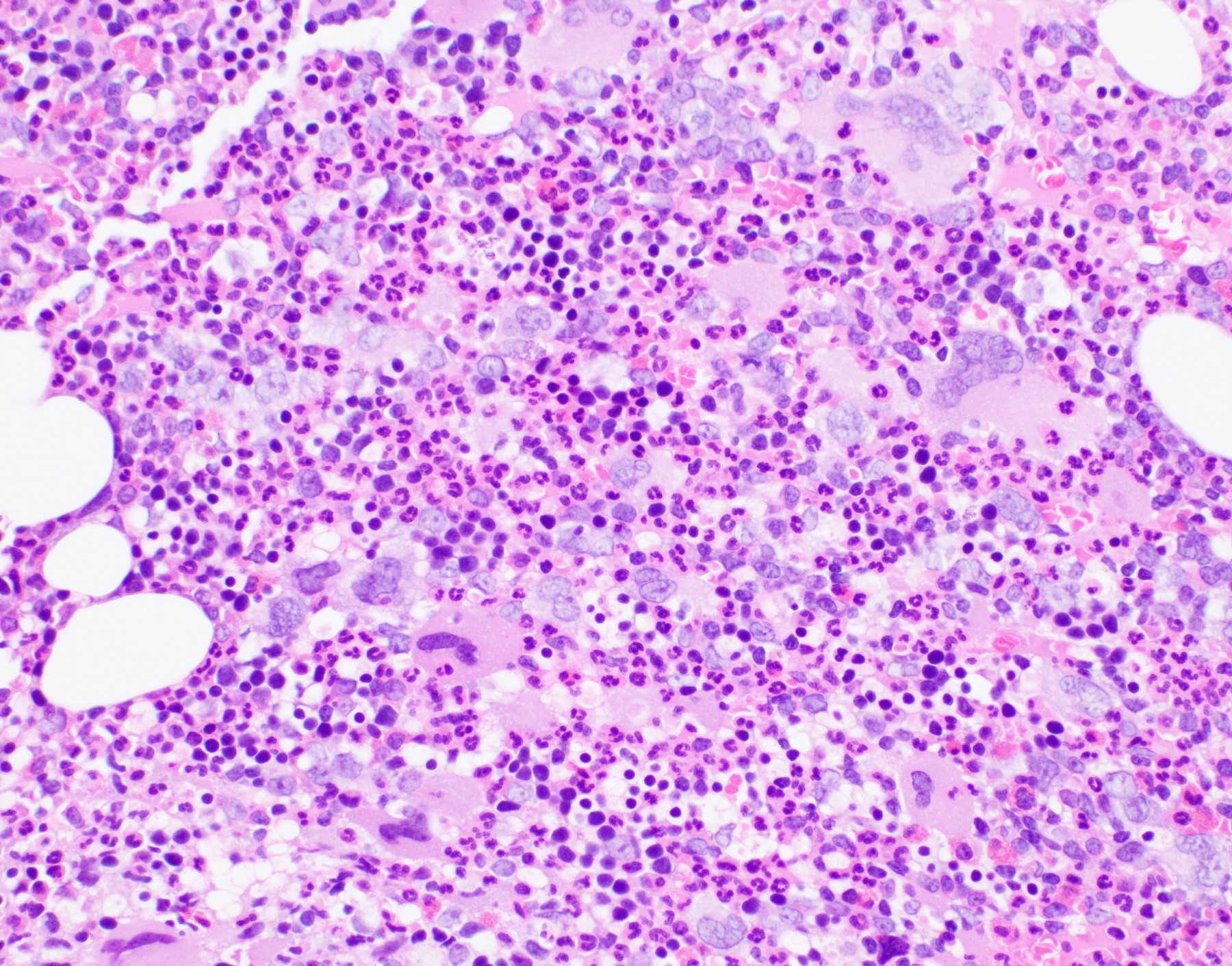
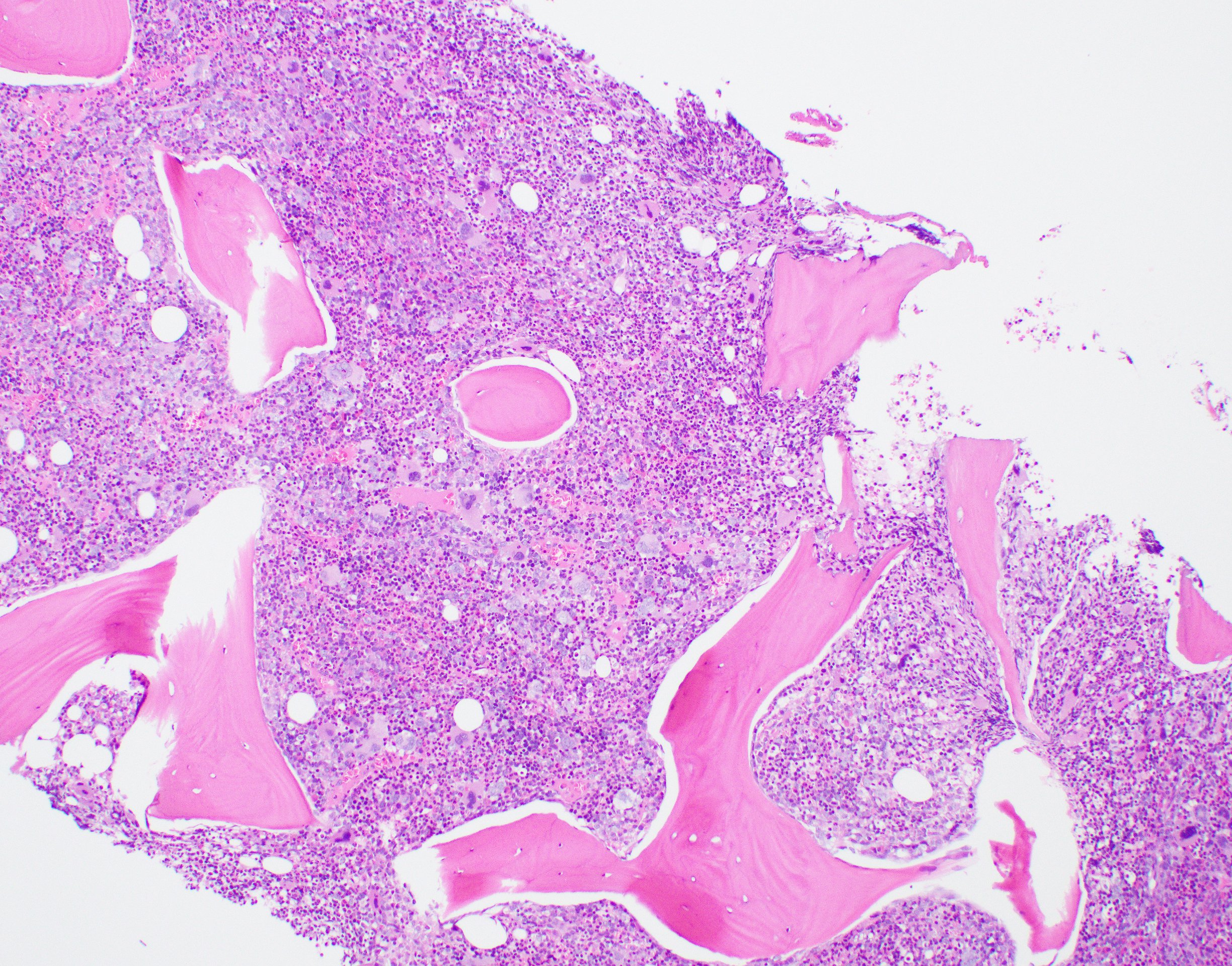
Microscopic (histologic) description
- Bone marrow: hypercellular with granulocytic hyperplasia, 5 - 30% monocytes, decreased megakaryocytes, blasts + promonocytes < 20%, dysplasia not prominent, reticulin fibrosis is rare (Blood 1997;89:3534)
- Skin: common presentation, with increased myelomonocytic infiltrates in the papillary and reticular dermis
- Liver: leukemic infiltrates in the portal regions and sinusoids
- Lung: leukemic infiltrates spread from the capillaries within the alveolar septa into alveoli
- Spleen: infiltrates in the red pulp with a preference for trabecular and central arteries
Microscopic (histologic) images
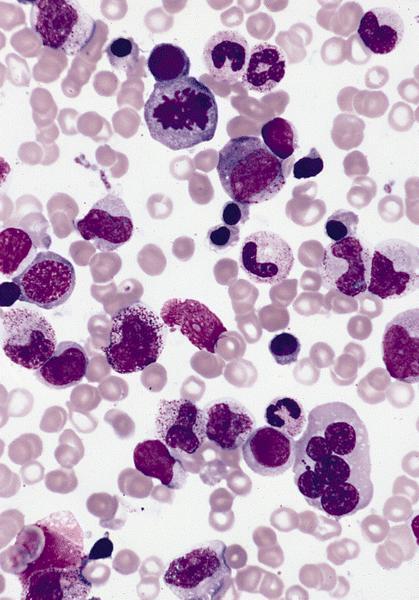
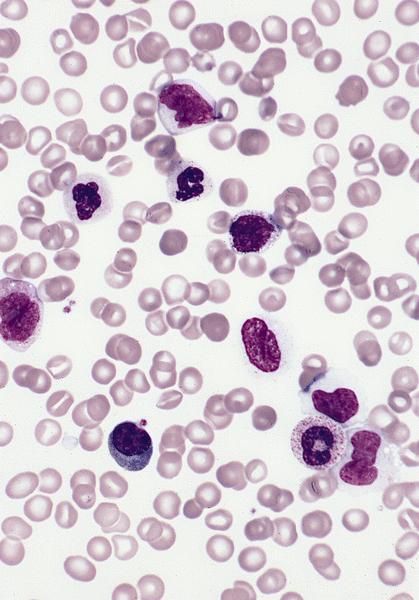
Peripheral smear description
- Important specimen for diagnosis
- Leukocytosis with neutrophilia with immature forms and monocytosis, WBC count is 20 - 30 x 109/L with granulocytes and monocytes and occasional dysplasia (which may not be prominent) (Blood 2019;133:1060)
- Anemia: most commonly normochromic and nucleated red blood cells are often identified
- Macrocytosis seen in cases with monosomy 7
- Thrombocytopenia
- Blasts and blast equivalents are usually less than 5% (no more than 20%)
Positive stains
- CD68 and lysozyme helpful to identify the monocytic component
- Cytochemical stains for alpha naphthyl acetate esterase / butyrase helpful to identify monocytic cells
- Blasts, if present: CD34 (should be < 20%)
Flow cytometry description
- Can see increased monocytes
Molecular / cytogenetics description
- PCR used for KRAS, NRAS, NF1, PTNT11 or CBL mutations
- RAS pathway mutations can occur in about 90% cases
- Next generation sequencing (NGS) has identified other mutations in patients with JMML
- SETBP1: found at disease initiation and progression, also considered a potential marker for how aggressive the disease is
- JAK3, ALK, ROS1
- EZH2, ASXL1 and DNMT3A: in about 15% of cases
- 35% of cases can have abnormal karyotype, most commonly monosomy 7
- References: Am J Blood Res 2021;11:1, Pediatr Int 2016;58:681
Sample pathology report
- Left posterior iliac crest, core biopsy and aspirate smear:
- Hypercellular marrow with granulocytic proliferation and (< 20%) blasts, consistent with juvenile myelomonocytic leukemia (see comment)
- Comment: The bone core biopsy demonstrates hypercellularity and myeloid predominance, with no increase in blasts. In conjunction with peripheral blood monocytosis, splenomegaly, negative BCR::ABL1 studies and a clinical diagnosis of neurofibromatosis type 1, the findings are consistent with juvenile myelomonocytic leukemia.
Differential diagnosis
- Childhood myelodysplastic syndrome:
- More likely to have karyotype abnormalities than gene mutations
- May be hypocellular for age
- Myeloproliferative neoplasms such as chronic myeloid leukemia:
- Must assess for BCR::ABL1 to exclude CML
- Viral infections:
- EBV, CMV or HHV6 also have increase in granulocytic and monocytic precursors, hypersensitivity to GM-CSF / CSF2 and NRAS mutation in proliferating B cells (J Pediatr Hematol Oncol 2002;24:136, Leuk Res 2008;32:181)
- Wiskott-Aldrich syndrome:
- Atopic dermatitis-like eczema, no intracellular WASP expression, WASP gene mutation (J Pediatr Hematol Oncol 2007;29:836)
- Infantile malignant osteopetrosis:
- Radiologic evidence of increased bone density
- Familial hemophagocytic lymphohistiocytosis (HLH):
- Splenomegaly and extensive specific clinical and genetic criteria
- Acute leukemia with KMT2A (MLL) rearrangement:
- Patients with massive hepatosplenomegaly and low blast count (Oman Med J 2019;34:553)
Additional references
Board review style question #1
A 9 year old boy with relevant prior history was noted to have enlarged tonsils and cough. His CBC showed leukocytosis with monocytosis. His physical exam was notable for lymphadenopathy and splenomegaly. Flow cytometry of blood showed no increase in blasts or evidence of acute leukemia. A bone marrow biopsy was pursued, which showed a myeloid predominant marrow with no increase in blasts and little granulocytic dysplasia. A pathogenic mutation in which gene is most supportive of a diagnosis of juvenile myelomonocytic leukemia?
- IDH2
- KRAS
- NPM1
- SF3B1
Board review style answer #1
B. KRAS. Greater than 75% of cases of JMML have RAS pathway mutation.
Comment Here
Reference: Juvenile myelomonocytic leukemia
Comment Here
Reference: Juvenile myelomonocytic leukemia
Board review style question #2
Which of the following is true of juvenile myelomonocytic leukemia?
- Average age of newly diagnosed patients is 50
- Commonly associated with neurofibromatosis type 2
- There is often increased hemoglobin F for age
- Usually driven by Epstein-Barr virus
Board review style answer #2
C. There is often increased hemoglobin F for age. The hemoglobin F can be a hint as it is often abnormally increased for the patient's age.
Comment Here
Reference: Juvenile myelomonocytic leukemia
Comment Here
Reference: Juvenile myelomonocytic leukemia