Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ewais M, Boland JM. Carcinoid tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/mediastinumcarcinoid.html. Accessed March 30th, 2025.
Definition / general
- Neuroendocrine epithelial neoplasm of thymic origin
- Low grade nuclear features
Essential features
- Histologic examination showing neuroendocrine morphology and low grade nuclear features is required for diagnosis
- Demonstration of strong expression of keratin and neuroendocrine markers is desirable for diagnosis
- Typical carcinoid tumor has < 2 mitoses/2 mm2 and no necrosis; atypical carcinoid tumor has 2 - 10 mitoses/2 mm2 or foci of necrosis
- In contrast to the lung, atypical carcinoids are much more common than typical carcinoids in the thymus
Terminology
- Typical carcinoid tumor (well differentiated neuroendocrine tumor of the thymus, grade 1)
- Atypical carcinoid tumor (well differentiated neuroendocrine tumor of the thymus, grade 2)
ICD coding
- ICD-O
- ICD-11
- 2C27.1 & XH9LV8 - carcinoid tumor or other neuroendocrine neoplasms of thymus & neuroendocrine tumor, grade 1
- 2C27.1 & XH51K1 - carcinoid tumor or other neuroendocrine neoplasms of thymus & neuroendocrine tumor, grade 2
Epidemiology
- Thymic carcinoid tumors are rare, representing 0.4% of all neuroendocrine tumors and < 5% of thymic neoplasms (J Thorac Oncol 2019;14:1472)
- Mean patient age for thymic typical carcinoids is 49 years, while atypical carcinoids occur at a slightly older age (48 - 55 years); both typical and atypical thymic carcinoids have a male predominance (Ann Thorac Cardiovasc Surg 1999;5:285, Mod Pathol 2001;14:985)
- Atypical carcinoids are much more common than typical carcinoids in the thymus (Mod Pathol 2001;14:985)
Sites
- Anterior mediastinum
Pathophysiology
- Unknown
Etiology
- Family history of MEN1 is reported in 25% of patients with thymic carcinoid (J Intern Med 1998;243:501)
- Smoking is a risk factor for thymic carcinoid only in men with MEN1 gene mutation (World J Surg 2009;33:1197)
Clinical features
- > 50% of patients present with clinical symptoms such as shortness of breath, cough, chest pain or superior vena cava syndrome (Mod Pathol 2001;14:985)
- Lymph node metastasis is common at presentation (40 - 50%) (Ann Thorac Cardiovasc Surg 1999;5:285)
- Paraneoplastic syndromes may occur due to associated hormone production
- Cushing syndrome due to ectopic secretion of adrenocorticotropin (ACTH) (Ann Thorac Cardiovasc Surg 1999;5:285)
- Hypercalcaemia / hypophosphatemia due to production of parathyroid hormone related protein (PTHrP) (Surg Today 1994;24:544)
- Inappropriate production of antidiuretic hormone or atrial natriuretic peptide (Ann Intern Med 1994;121:75)
- Acromegaly due to ectopic growth hormone releasing hormone (GHRH) (Clin Endocrinol (Oxf) 1998;48:243)
- Recurrence may occur late (> 5 years after diagnosis), including distant metastases to lung, brain, bone, liver, adrenals and soft tissue sites (Mod Pathol 2001;14:985, Am J Clin Pathol 2000;114:100)
Diagnosis
- Tissue diagnosis obtained from a biopsy or cytology samples can be used to evaluate mediastinal lesions; however, definitive tumor classification can be difficult in small samples and primary resection may be considered
Laboratory
- Increased plasma chromogranin A, although it is not specific (Hematol Oncol Clin North Am 2016;30:83)
Radiology description
- Nonspecific features on imaging
- Computed tomography (CT): large, heterogenous enhancing mass and with irregular contours, with or without surrounding capsule
- Magnetic resonance imaging (MRI): heterogeneous T2 hyperintense signal
- Thymic carcinoids are typical FDG avid on positron emission tomography (PET) / CT (Br J Radiol 2017;90:20150341)
Prognostic factors
- Data on the prognosis of thymic carcinoids is sparse but suggests that there is no statistically significant difference in survival rates between typical and atypical thymic carcinoid tumors (Ann Thorac Cardiovasc Surg 1999;5:285)
- Surgery and radiotherapy seem to improve outcome (Interact Cardiovasc Thorac Surg 2018;26:18)
- 5 year survival is 50 - 70% (Genes Chromosomes Cancer 2014;53:738, Am J Clin Pathol 2000;114:100)
- Study of 58 patients showed a median survival of 126 months for thymic typical thyroid carcinoids and 52 months for thymic atypical carcinoids
Case reports
- 25 year old man presented with abnormal mental behavior due to ectopic adrenocorticotrophic hormone syndrome caused by thymic carcinoid (Gland Surg 2022;11:767)
- 32 year old man presented with cyclic Cushing syndrome due to ACTH secreting atypical thymic carcinoid (Arch Endocrinol Metab 2021;65:512)
- 33 year old man with atypical thymic carcinoid presented with ACTH ectopic syndrome (Medicine (Baltimore) 2023;102:e33847)
- 53 year old man with thymic carcinoid presented with gastrointestinal symptoms and pericardial effusion (BMC Cardiovasc Disord 2021;21:54)
Treatment
- Surgical resection
- Postoperative radiotherapy may be considered (Interact Cardiovasc Thorac Surg 2018;26:18)
- Systemic therapy using somatostatin analog for patients with advanced disease (Cancer Med 2023;12:7893)
Gross description
- Variable size; average of 8 - 10 cm (Am J Clin Pathol 2000;114:100)
- May range from circumscribed to frankly invasive
- Firm, gray-white to tan cut surface; may be calcified
- Often lacks the prominent gross fibrous bands of thymoma
Frozen section description
- Monomorphic cells with fine chromatin arranged in organoid pattern with hyalinized stroma
Frozen section images
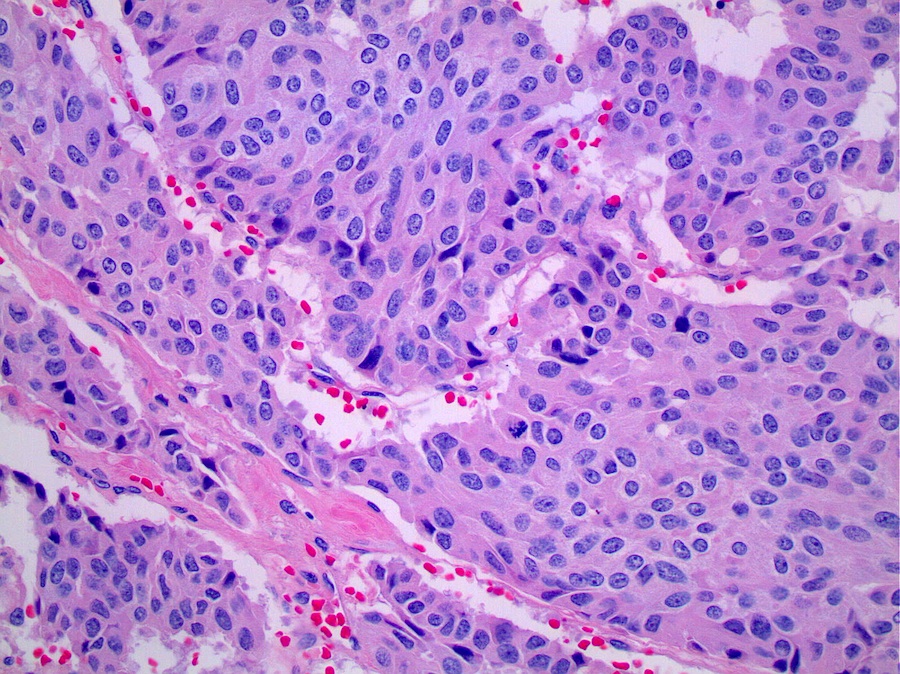
Microscopic (histologic) description
- Uniform, polygonal to spindle shaped tumor cells arranged in nests that commonly show classic neuroendocrine growth patterns including trabecular growth or rosettes
- Finely granular nuclear chromatin and scant to moderate pale eosinophilic cytoplasm
- Typical and atypical thymic carcinoid tumors are distinguished based on mitotic activity and necrosis, which are often patchy and can only be adequately assessed on resection specimens; therefore, definitive subclassification as typical or atypical usually cannot be made on small biopsy or cytology specimens
- Reference: Semin Diagn Pathol 1991;8:35
Microscopic (histologic) images
Cytology description
- Definitive tumor classification as typical or atypical carcinoid cannot be performed based on the cytologic features
- Small to medium sized, round, elongated or plasmacytoid tumor cells arranged in clusters or rosettes with scant to moderate cytoplasm and salt and pepper chromatin (Cibas: Cytology - Diagnostic Principles and Clinical Correlates, 4th Edition, 2014)
Positive stains
- Pankeratin
- Neuroendocrine markers (synaptophysin, chromogranin A, INSM1, CD56)
- PAX8, polyclonal (50%) (Am J Surg Pathol 2015;39:850)
- Ki67 is often < 2% in typical carcinoids and 2 - 10% in atypical carcinoids but can show overlap and is not currently used in classification; can be a very useful maker to differentiate carcinoids with low proliferative index from high grade neuroendocrine carcinomas with very high proliferation index (usually > 50%)
Negative stains
- TTF1 (may help differentiate thymic carcinoid from pulmonary carcinoid which are positive in 50%, although thymic carcinoid can occasionally be positive in up to 14% of cases)
- CDX2 (useful marker for neuroendocrine tumor of an intestinal origin) (Appl Immunohistochem Mol Morphol 2007;15:407)
Molecular / cytogenetics description
- Thymic carcinoids seem to demonstrate recurrent chromosomal gains and losses that appear different from those observed in pulmonary carcinoids (Mod Pathol 2005;18:358, Genes Chromosomes Cancer 2014;53:738)
Sample pathology report
- Thymus, mass, biopsy:
- Carcinoid tumor (see comment)
- Comment: In the current biopsy specimen, no increased mitotic activity or necrosis is identified; however, these features can be patchy and definitive classification as typical or atypical carcinoid tumor requires examination of a resection specimen.
Differential diagnosis
- Paraganglioma:
- Thymoma (Arch Pathol Lab Med 2006;130:1612):
- Variable proportions of epithelial cells and thymic lymphocytes (thymocytes)
- p63+ and p40+; may have TdT+ thymocytes
- Neuroendocrine markers (synaptophysin, chromogranin A) are negative
- Small cell carcinoma:
- High N:C ratio, scant cytoplasm, nuclear molding, inconspicuous nucleoli, marked necrosis and apoptotic bodies
- Increased mitosis > 10 per 2 mm2
- Ki67 proliferation index usually > 80%
- Large cell neuroendocrine carcinoma:
- Increased nuclear pleomorphism, prominent nucleoli, marked necrosis and apoptotic bodies
- Increased mitosis > 10 mitoses/2 mm2
- Ki67 proliferation index usually > 30%
- Thymic carcinoma with neuroendocrine differentiation (Am J Clin Pathol 2016;145:393):
Additional references
Board review style question #1
A thymic biopsy is performed and the tumor is shown above. Which of the following is true regarding this thymic neoplasm?
- Ki67 proliferation index is required for the diagnosis
- They are the most common thymic neoplasm
- Tumor cells are positive for pankeratin
- Tumor subclassification can reliably be made on cytologic evaluation
Board review style answer #1
C. Tumor cells are positive for pankeratin. This is a thymic carcinoid tumor, which would be expected to be positive for pankeratin. Answer B is incorrect because thymic carcinoids represent < 5% of thymic neoplasms. Answer D is incorrect because the diagnosis of carcinoid tumor can be established on small biopsy samples but definitive tumor classification as typical or atypical carcinoid should usually only be made on a resection specimen. Answer A is incorrect because Ki67 proliferation index is not currently one of the diagnostic criteria to differentiate typical and atypical thymic carcinoid tumors.
Comment Here
Reference: Mediastinum - Carcinoid tumor
Comment Here
Reference: Mediastinum - Carcinoid tumor
Board review style question #2
Which of the following criteria supports a diagnosis of thymic large cell neuroendocrine carcinoma over thymic carcinoid tumor?
- 20 mitoses per 2 mm2
- Ki67 proliferation index of 10%
- Negative keratin stain
- Tumor necrosis
Board review style answer #2
A. 20 mitoses per 2 mm2. Per WHO criteria, thymic carcinoid tumors cannot exceed a mitotic count of 10 per 2 mm2. Answers C and D are incorrect because both atypical thymic carcinoids and large cell neuroendocrine carcinoma can have tumor necrosis and should express keratin. Answer B is incorrect because Ki67 proliferation index is not currently one of the diagnostic criteria to differentiate thymic neuroendocrine tumors but a very high proliferation index can be helpful in supporting a diagnosis of large cell neuroendocrine carcinoma or small cell carcinoma over a carcinoid tumor.
Comment Here
Reference: Mediastinum - Carcinoid tumor
Comment Here
Reference: Mediastinum - Carcinoid tumor