Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy images | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Sathirareuangchai S, Bychkov A. Aspergillus. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumoraspergillosis.html. Accessed April 2nd, 2025.
Definition / general
- Infection of the lung by a hyaline mold in genus Aspergillus, with various manifestation based on host immune status
- Word origin: aspergillum, a ritual liturgical implement used in Roman Catholic ceremonies to sprinkle holy water (Arch Pathol Lab Med 2008;132:606)
Essential features
- Aspergillus histomorphology: acute angle, dichotomous branching, septate hyphae; however, fungal culture is required for definite identification
- Noninvasive form in immunocompetent host: cavitary lesion with fungal ball and surrounding chronic inflammation
- Invasive form in immunocompromised host: necrotizing pneumonia with angioinvasion and infarct
Terminology
- Allergic bronchopulmonary aspergillosis (ABPA)
- Chronic pulmonary aspergillosis (CPA)
- Simple / single aspergilloma
- Aspergillus nodule
- Chronic cavitary pulmonary aspergillosis (CCPA)
- Chronic fibrosing pulmonary aspergillosis (CFPA)
- Subacute invasive aspergillosis (formerly known as chronic necrotizing pulmonary aspergillosis)
- Invasive pulmonary aspergillosis (IPA)
ICD coding
- ICD-10:
- ICD-11:
Epidemiology
- Causes a wide spectrum of diseases in humans, depending on the underlying immune status of the host (Clin Microbiol Rev 2019;33:e00140)
- One of the most common causes of infectious death in severely immunocompromised patients
- Mortality rate = 50% in neutropenic patients, 90% in hematopoietic stem cell transplantation (HSCT) recipients (QJM 2007;100:317)
- Colonization in asymptomatic patient is common
- Aspergillus DNA has been found in 37% of lung biopsy specimens of healthy adults (Clin Infect Dis 2011;52:1123)
- Culture proven colonization occurs in up to 30% of chronic obstructive pulmonary disease (COPD) patients (Med Mycol 2012;50:433)
Sites
- Lung
- Upper lobes are more frequently affected in chronic pulmonary aspergillosis (Respir Med 2018;141:121)
- Other commonly affected organs: central nervous system, eye, paranasal sinus, middle ear, heart, bone and soft tissue, skin, gastrointestinal tract (Clin Infect Dis 2016;63:e1)
- Disseminated infection
Pathophysiology
- Acquired by inhalation of airborne conidia
- Virulence factors: endotoxins, heparin-like factors and oxalic acid (Arch Pathol Lab Med 2008;132:606)
Etiology
- Saprophytic fungi in genus Aspergillus, ubiquitous in environment, can be isolated from soil, household, hospital environment, food, etc.
- 300 species, only 8 species are responsible for infection in humans (Arch Pathol Lab Med 2008;132:606)
- Aspergillus fumigatus (most common), followed by A. niger
- Other: A. nidulans, A. terreus, A. clavatus, A. flavus, A. niveus and A. ustus
Diagrams / tables
Clinical features
- 3 broad categories of clinical manifestation in pulmonary aspergillosis (Respir Med 2018;141:121)
- Allergic bronchopulmonary aspergillosis (ABPA)
- Chronic pulmonary aspergillosis (CPA)
- Invasive pulmonary aspergillosis (IPA)
- Allergic bronchopulmonary aspergillosis (ABPA)
- Immunologic pulmonary disorder caused by hypersensitivity to Aspergillus fumigatus
- Pre-existing airway disease is present
- 1 - 2% of asthma patients (Arch Pathol Lab Med 2005;129:924)
- 10.5% of cystic fibrosis patients (Eur Ann Allergy Clin Immunol 2020;52:104)
- Other predisposing conditions: hyper IgE syndrome, chronic granulomatous disease (J Allergy Clin Immunol 1999;104:1265)
- Clinically: worsening asthma symptoms, chronic productive cough and wheezing (Respir Med 2018;141:121)
- Characteristic: golden brownish mucus plugs, found in 50% of patients (Chest 2009;135:805)
- Chronic pulmonary aspergillosis (CPA)
- A spectrum of diseases in immunocompetent patients with a pre-existing pulmonary condition, including tuberculous and nontuberculous mycobacterial infections (most common), ABPA, COPD, treated lung cancer, asthma, pneumonia and fibrocavitary sarcoidosis (Respir Med 2018;141:121)
- Aspergilloma (Respir Med 2018;141:121)
- Fungal ball consisting of Aspergillus hyphae, fibrin and other debris
- Present in almost all forms of CPA
- Simple / single aspergilloma
- Single pulmonary cavity containing a fungal ball (Eur Respir J 2016;47:45)
- Chronic cavitary pulmonary aspergillosis (CCPA)
- Most common form of CPA
- Can progress to chronic fibrosing pulmonary aspergillosis (CFPA) if untreated
- Aspergillus nodule
- Unusual form of CPA, mimicking malignancy, tuberculoma or coccidioidomycosis
- Frequent necrosis but no tissue invasion
- Subacute invasive aspergillosis
- Commonly grouped under CPA but diagnosed and treated similarly to IPA (Respir Med 2018;141:121)
- Formerly known as chronic necrotizing pulmonary aspergillosis
- Mildly immunocompromised or severely debilitated patients
- Similar clinical and radiological features to CCPA but progresses rapidly (Eur Respir J 2016;47:45)
- Most common symptom is cough (> 90%) (Medicine (Baltimore) 2017;96:e8315)
- Other symptoms: shortness of breath, chest pain, hemoptysis, fever, weight loss and night sweats
- Invasive pulmonary aspergillosis (IPA)
- Most serious form of pulmonary aspergillosis in immunocompromised patients (Respir Med 2018;141:121)
- Common in HSCT and solid organ transplant (SOT) recipients, hematologic malignancy, rare in HIV infection (Arch Pathol Lab Med 2008;132:606)
- Presence of tissue invasion by Aspergillus hyphae
- Prolonged neutropenia is the most significant risk factor (Clin Infect Dis 2016;63:e1)
- Aspergillus tracheobronchitis: unique form of IPA where infection is confined in the tracheobronchial tree (Medicine (Baltimore) 2012;91:261)
- Commonly found at the anastomotic site in lung transplant patient (Semin Diagn Pathol 2017;34:530)
- Most serious form of pulmonary aspergillosis in immunocompromised patients (Respir Med 2018;141:121)
Diagnosis
- Allergic bronchopulmonary aspergillosis (ABPA)
- Lung biopsy not routinely performed
- International Society for Human and Animal Mycology (ISHAM) diagnostic criteria (Clin Exp Allergy 2013;43:850)
- Predisposing conditions:
- Asthma
- Cystic fibrosis
- Obligatory criteria (both required):
- Type I aspergillus skin test positive or elevated IgE levels against A. fumigatus
- Elevated total IgE levels > 1,000 IU/mL (unless all other criteria are met, then total IgE levels can be < 1,000 IU/mL)
- Other criteria (at least 2 out of 3):
- Presence of IgG antibodies against A. fumigatus or precipitating antibodies
- Presence of fleeting or fixed pulmonary opacities on chest radiograph consistent with ABPA
- Eosinophils > 500/μL in steroid naive patient (may be a historical value)
- Chronic cavitary pulmonary aspergillosis (CCPA)
- Diagnostic criteria from Infectious Diseases Society of America (IDSA) guidelines (Clin Infect Dis 2016;63:e1)
- 3 months of chronic pulmonary symptoms or chronic illness or progressive radiographic abnormalities, with cavitation, pleural thickening, pericavitary infiltrates and sometimes a fungal ball
- Aspergillus IgG antibody elevated or other microbiological data
- No or minimal immunocompromise, usually with 1 or more underlying pulmonary disorders
- Diagnostic criteria from Infectious Diseases Society of America (IDSA) guidelines (Clin Infect Dis 2016;63:e1)
- Invasive pulmonary aspergillosis (IPA)
- Histopathological examination of lung tissue is the gold standard (Respir Med 2018;141:121)
- Flexible bronchoscopy with bronchoalveolar lavage (BAL) fluid for cytology and culture can be performed if biopsy is not advised (Clin Infect Dis 2016;63:e1)
- Clinical or radiological findings consistent with infection also required (Eur Respir Rev 2011;20:156)
Laboratory
- Standard fungal culture and identification
- Species differentiation by fruiting body (conidial heads) morphology
- Galactomannan (GM)
- Polysaccharide cell wall component of Aspergillus spp., which is released during growth (Clin Infect Dis 2006;42:1417)
- Can be measured in serum and bronchoalveolar lavage (BAL) fluid, using double sandwich ELISA technique
- Marker of invasive aspergillosis in certain subpopulation (e.g. hematologic malignancy and HSCT recipient) (Clin Infect Dis 2016;63:e1)
- Not recommended for screening in solid organ transplant (SOT) recipients (Scand J Infect Dis 2012;44:600)
- Serum 1,3-beta-D glucan
- Major cell wall components in various fungi (e.g., Aspergillus spp., Candida spp. and Pneumocystis jiroveccii) (J Clin Microbiol 2012;50:7)
- Protease zymogen based colorimetric assay, commercially available as Fungitell®, an FDA cleared in vitro diagnostic rapid screening test
- Marker of invasive fungal infection, not specific for genus Aspergillus
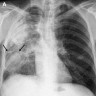
Radiology description
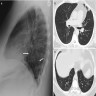
- Allergic bronchopulmonary aspergillosis (ABPA)
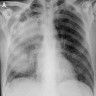
- Chest Xray (Indian J Radiol Imaging 2011;21:242)
- May be normal in early stage
- Tram line shadows, band-like (toothpaste) shadows, finger in glove opacities; represents mucoid impaction in dilated bronchi with occlusion of the distal end
- High resolution computed tomography (HRCT) (J Clin Diagn Res 2014;8:RC05)
- Centrilobular nodules, tree in bud pattern, mosaic attenuation and mucus impaction
- High attenuation mucus (HAM) (J Clin Diagn Res 2014;8:RC05)
- Pathognomonic for ABPA
- Mucus that appears denser than the skeletal muscles on HRCT (70 - 90 Hounsfield units)
- Chest Xray (Indian J Radiol Imaging 2011;21:242)
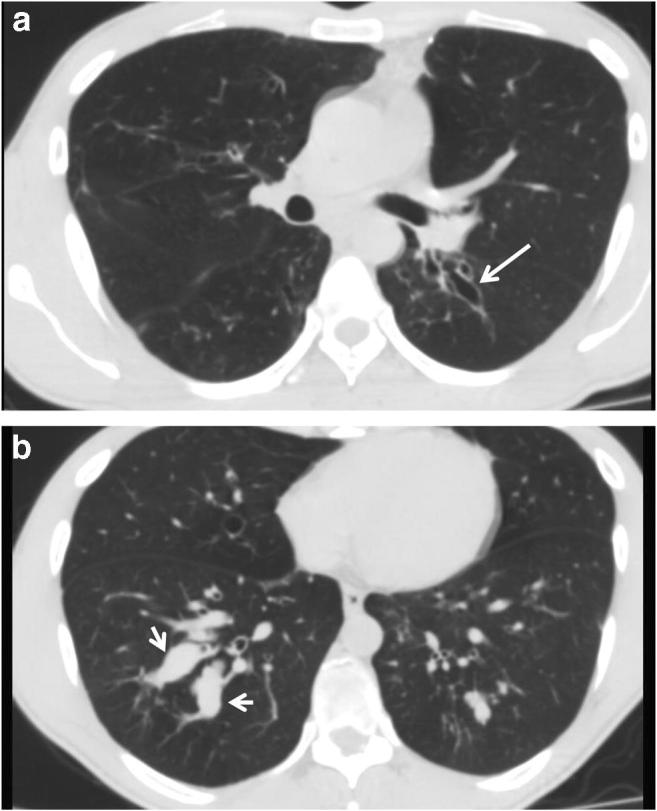
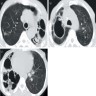
- Chronic cavitary pulmonary aspergillosis (CCPA)
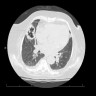
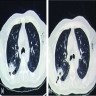
- New or expanding pre-existing cavities of variable wall thickness in the setting of chronic lung disease, with or without fungal ball, often with pleural thickening and marked parenchymal destruction or fibrosis (Eur Respir J 2016;47:45)
- Fungal ball: upper lobe, solid, round or oval, intracavitary mass, partially surrounded by a crescent of air (air crescent or Monad sign) (Eur Respir J 2016;47:45)
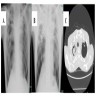
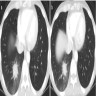
- Invasive pulmonary aspergillosis (IPA)
- HRCT is the imaging of choice when IPA is suspected (Clin Microbiol Infect 2018;24:e1)
- Classical HRCT findings: macronodules (> 1 cm), surrounded by a halo of ground glass attenuation (halo sign)
- Other findings: pleural based wedge shaped areas of consolidation, alveolar consolidations, mass-like lesion, ground glass opacities and pleural effusion
- Delayed findings: cavity or air crescent sign
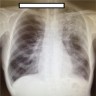
Radiology images
Prognostic factors
- Prognosis of ABPA is not well characterized (Mayo Clin Proc 2001;76:930)
- If ABPA is detected early and is treated promptly, the prognosis is good (Clin Dev Immunol 2011;2011:843763)
- Unfavorable prognosis can be seen in untreated patients with irreversible lung fibrosis, cor pulmonale and respiratory failure (Clin Pharm 1993;12:24)
- Poor prognostic factors in CPA (Eur Respir J 2017;49:1601062)
- History of a nontuberculous mycobacterial infection or COPD
- Low body mass index (BMI)
- Low serum albumin
- Old age
- Elevated inflammatory markers
- Lower activity
- Bilateral aspergillomas
- Risk factor for developing IPA (Eur Respir Rev 2011;20:156)
- Prolonged neutropenia (< 500 cells/mm3 for > 10 days)
- Solid organ transplantation (highest risk is with lung transplantation and HSCT)
- Prolonged (> 3 weeks) and high dose corticosteroid therapy
- Hematological malignancy (risk is higher with leukemia)
- Chemotherapy
- AIDS
- Chronic granulomatous disease
- Baseline factors that predict treatment outcome in patients with invasive aspergillosis (Mycoses 2019;62:651)
- Kidney and liver failure
- ICU admission
- Uncontrolled underlying disease
- Prolonged neutropenia
- Imaging results associated with negative outcome
- Multiple consolidations
- Bilateral pulmonary lesions
- Pleural effusion
Case reports
- 45 year old woman status post lung transplant with hemoptysis (Kobe J Med Sci 2020;65:E114)
- 61 year old man with hypertension, severe fever and thrombocytopenia (Viruses 2021;13:1086)
- 62 year old man with COPD and acute respiratory failure (Med Mycol Case Rep 2018;20:39)
- 64 year old man with chills and mild dyspnea on exertion after chemotherapy (Clin Case Rep 2018;6:2475)
- 68 year old man smoker with COPD and pseudomembranous lesion in the bronchus (Indian J Pathol Microbiol 2017;60:285)
- 69 year old man with fever and cavitary lung lesion after chemotherapy (Respirol Case Rep 2020;8:e00523)
- 75 year old with near drowning (Int Med Case Rep J 2020;13:77)
- 78 year old man with a recent diagnosis of nonspecific interstitial pneumonia (Intern Med 2018;57:3619)
- 79 year old man with COVID-19 and respiratory failure (J Fungi (Basel) 2021;7:230)
- 82 year old woman with wheezing, shortness of breath and productive cough (Cureus 2020;12:e11736)
Treatment
- Based on guidelines from the Infectious Diseases Society of America (IDSA), endorsed by the Pediatric Infectious Diseases Society (PIDS) (Clin Infect Dis 2016;63:e1)
- Allergic bronchopulmonary aspergillosis (ABPA)
- Itraconazole
- Alternative: oral voriconazole, posaconazole
- Corticosteroids for exacerbation
- Aspergilloma
- Observation
- Surgical resection in symptomatic patient (e.g., hemoptysis)
- Alternative: itraconazole, voriconazole
- Chronic cavitary pulmonary aspergillosis (CCPA), invasive pulmonary aspergillosis (IPA), tracheobronchial aspergillosis; same treatment
- Voriconazole
- Alternative: amphotericin B, isavuconazole, caspofungin, micafungin, posaconazole, itraconazole
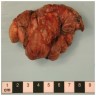
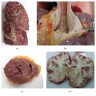
Gross description
- Allergic bronchopulmonary aspergillosis (ABPA)
- Bronchiectasis from mucus impaction
- Airway occlusion by mucus
- Chronic cavitary pulmonary aspergillosis (CCPA)
- Corresponds with the imaging finding of cavitary lesion, with or without fungal ball
- Invasive pulmonary aspergillosis (IPA) (Arch Pathol Lab Med 2008;132:606)
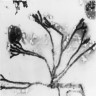
- Targetoid necrosis: central thrombosed vessels secondary to angioinvasion
- Confluent bronchopneumonia or dense lobar consolidation may be seen
- Foci of infarcted lung yields an infected pulmonary sequestrum
Gross images
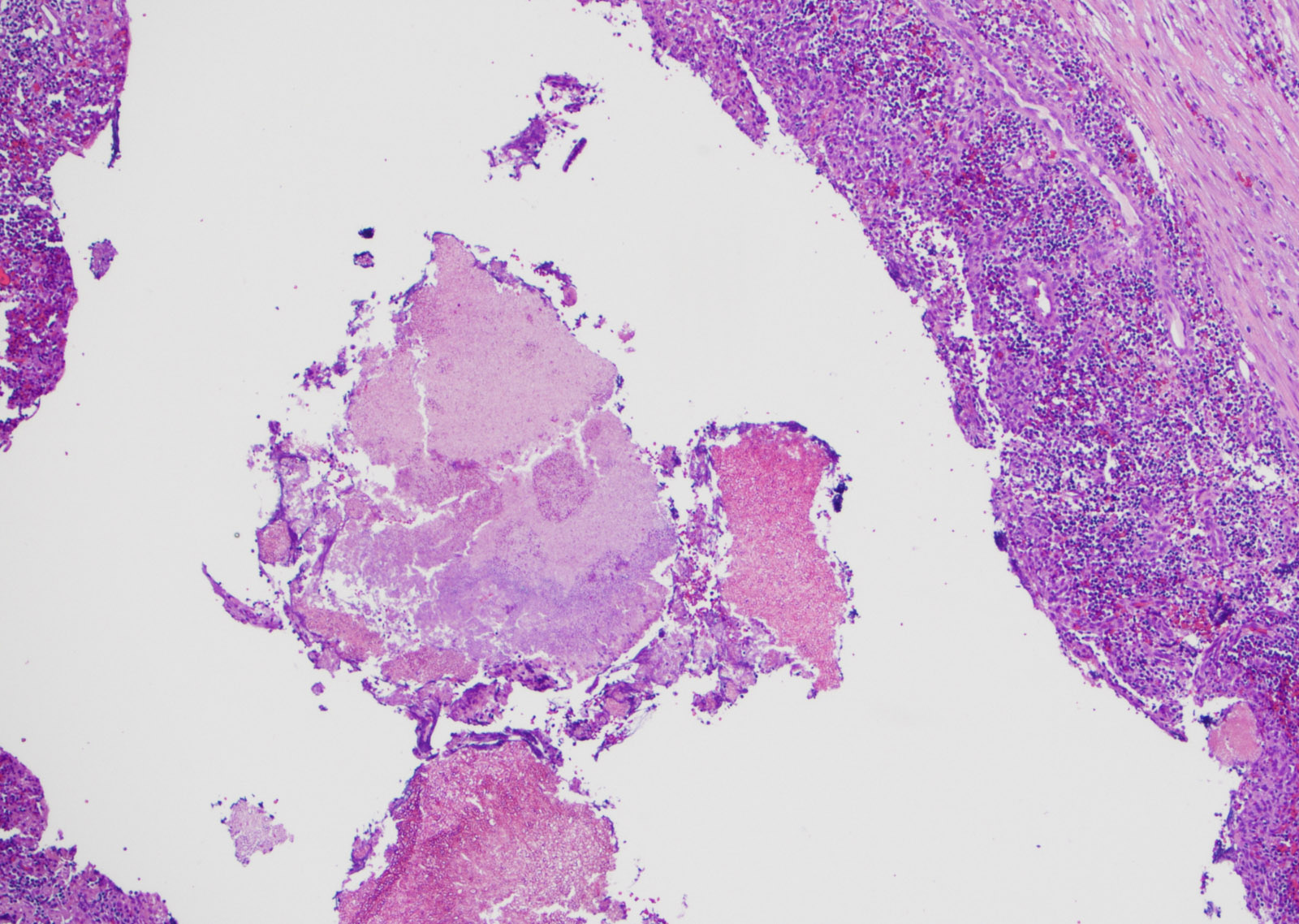
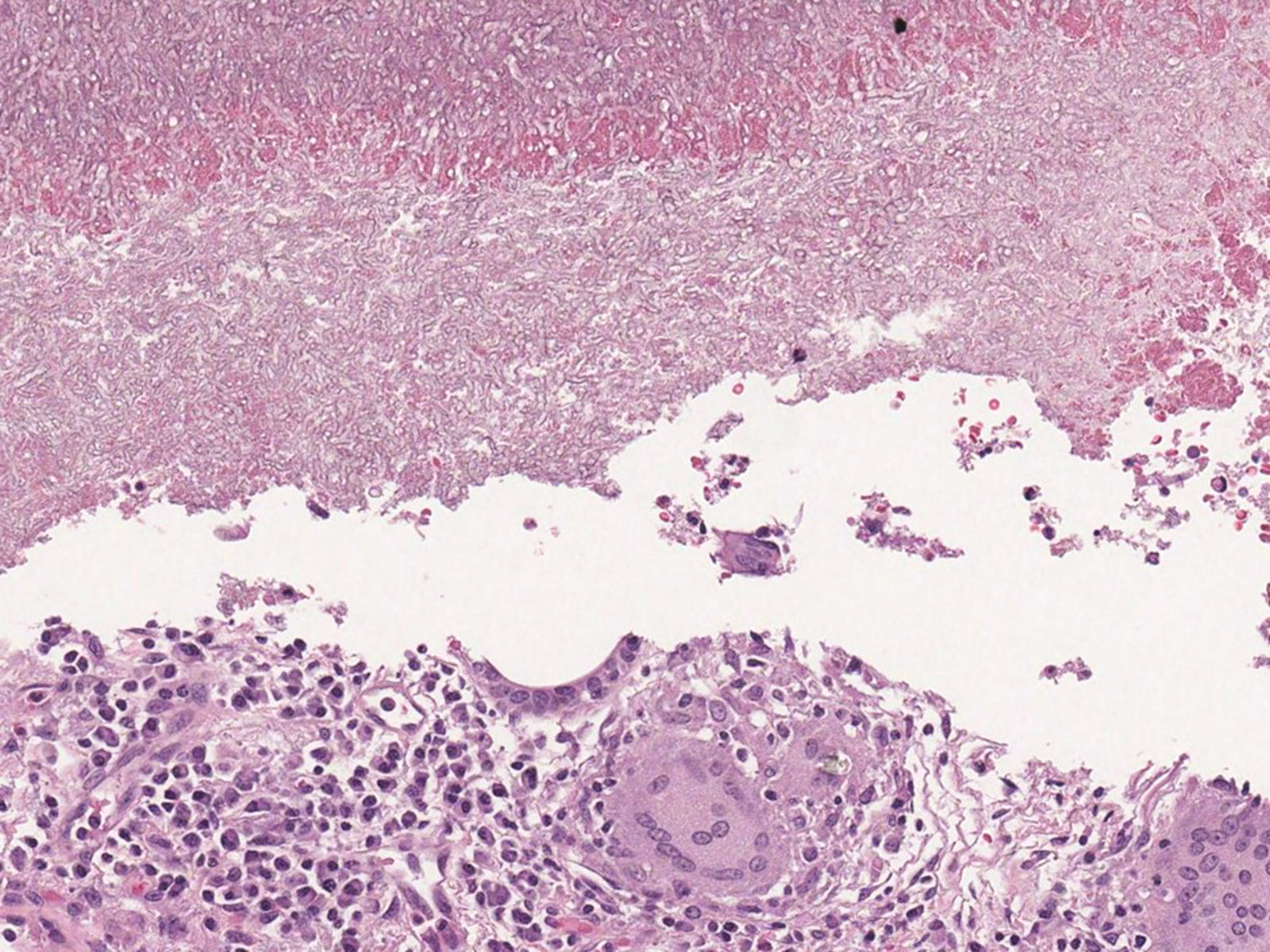
Microscopic (histologic) description
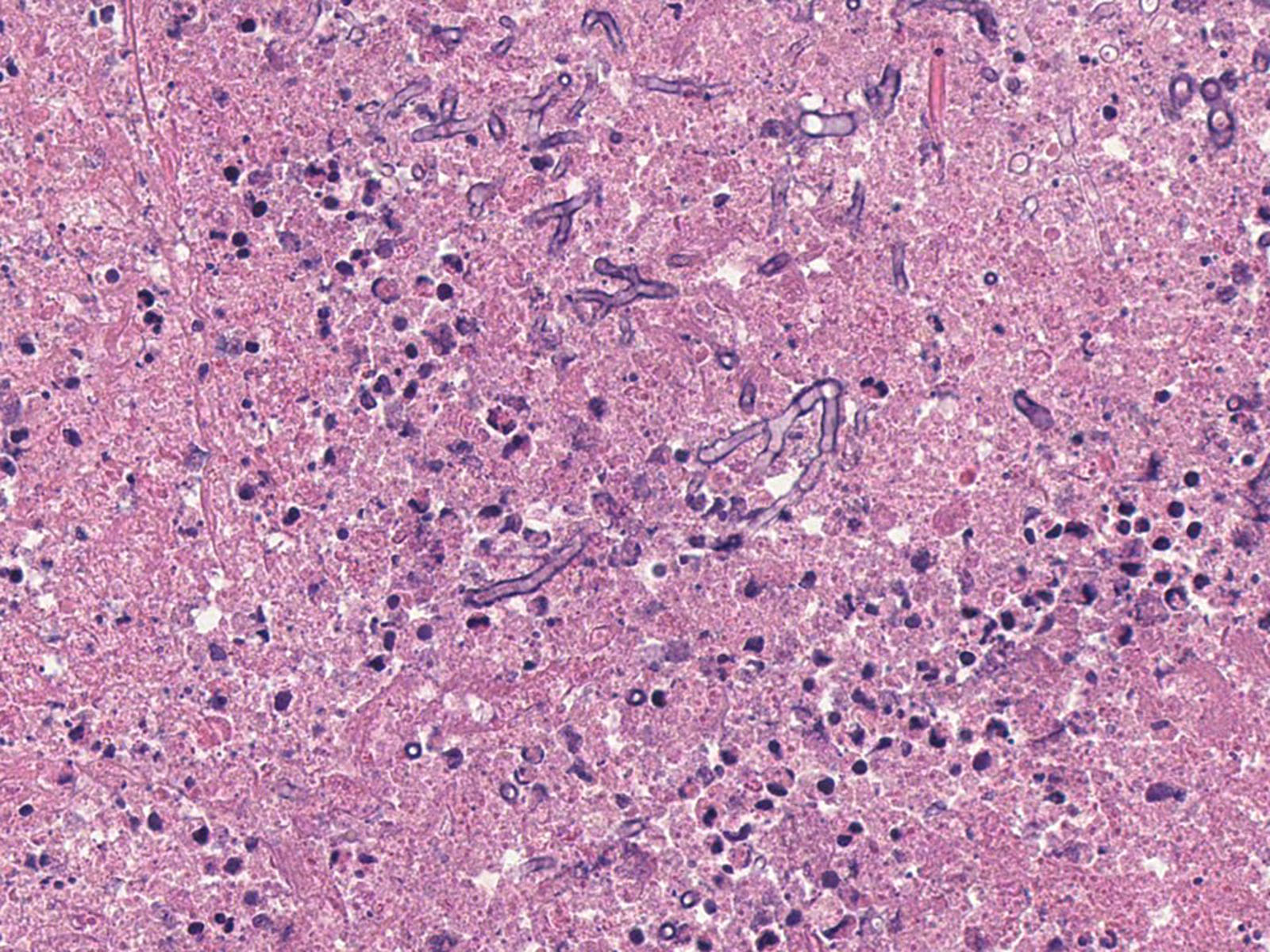
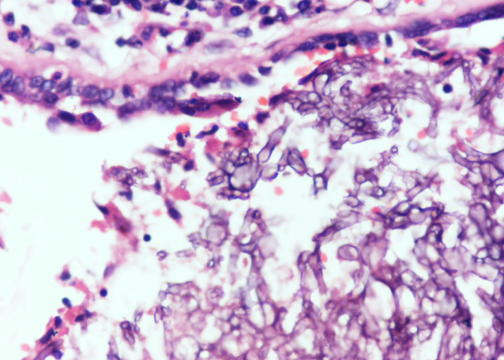
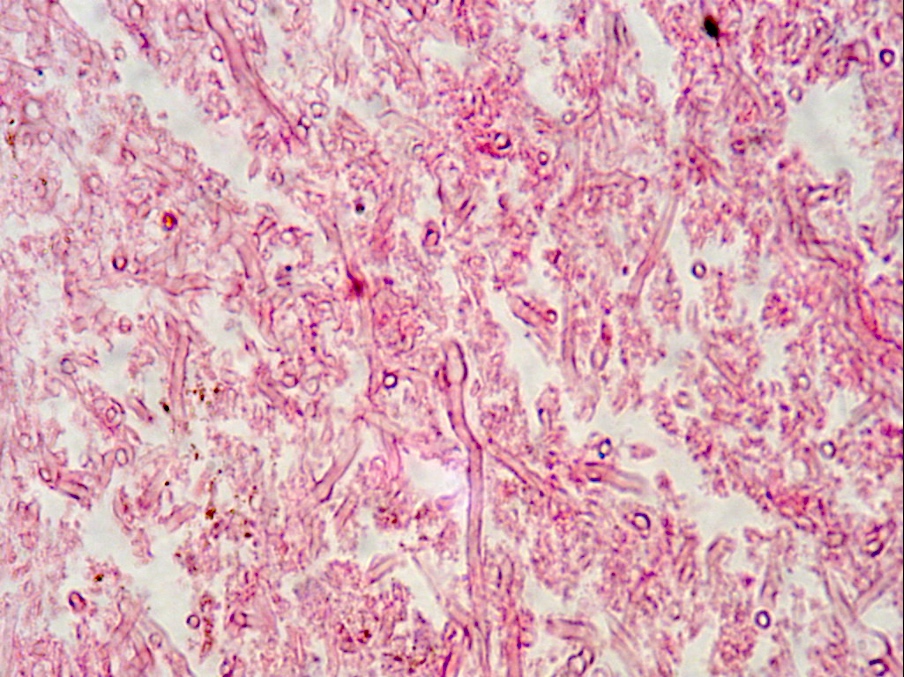
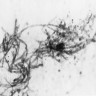
- Organism: acute angle (< 45°) or dichotomous branching, septate hyphae, 2.5 - 4.5 µm in diameter
- Aspergillus has a genus specific fruiting body (Arch Pathol Lab Med 2008;132:606)
- Develops from mycelia in areas of high oxygen tension (e.g. lung, sinus cavities)
- Does not develop in tissue
- Composed of a vesicle and either 1 or 2 layers of phialides that produce conidia
- Since histomorphology alone is not accurate in identification, definite classification should be based on microbiologic culture
- Aspergillus has a genus specific fruiting body (Arch Pathol Lab Med 2008;132:606)
- Allergic bronchopulmonary aspergillosis (ABPA) (Arch Pathol Lab Med 2005;129:924)
- Mucoid impaction of bronchi, composed of mucus and inflammatory cells (predominantly eosinophils), AKA allergic mucin
- Bronchocentric granulomatosis
- Airway centric necrotizing granulomatous inflammation with destruction of the airway wall
- Dense inflammatory infiltrate with prominent eosinophils
- May be seen in other conditions: nonfungal infection, malignancy, autoimmune disease
- Eosinophilic pneumonia
- Chronic or exudative bronchiolitis
- Fungal hyphae are rarely identified, usually fragmented
- Chronic pulmonary aspergillosis (CPA)
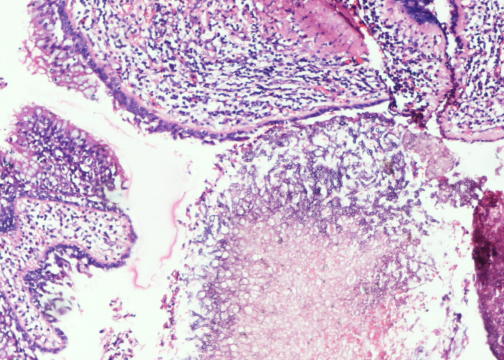
- Characteristic cavitary lesion with fungal ball (Arch Pathol Lab Med 2008;132:606)
- Cavity wall: superficial ulceration with granulation tissue, granulomatous inflammation or metaplastic squamous epithelium
- Wall must be carefully examined, to exclude subacute invasive aspergillosis (chronic necrotizing pulmonary aspergillosis) which shows invasion of lung parenchyma but no angioinvasion
- A. niger fungus ball can be associated with chronic pulmonary oxalosis (Arch Pathol Lab Med 2008;132:606)
- Oxalic acid is produced by A. niger
- Prothrombotic effect by oxalate leads to extensive ischemic necrosis
- Resection of the fungus ball is definitive treatment
- Splendore-Hoeppli phenomenon: deposition of antigen antibody complexes and debris from host inflammatory cells, resulting in amorphous eosinophilic material coating the mycelia (J Oral Maxillofac Pathol 2018;22:161)
- Characteristic cavitary lesion with fungal ball (Arch Pathol Lab Med 2008;132:606)
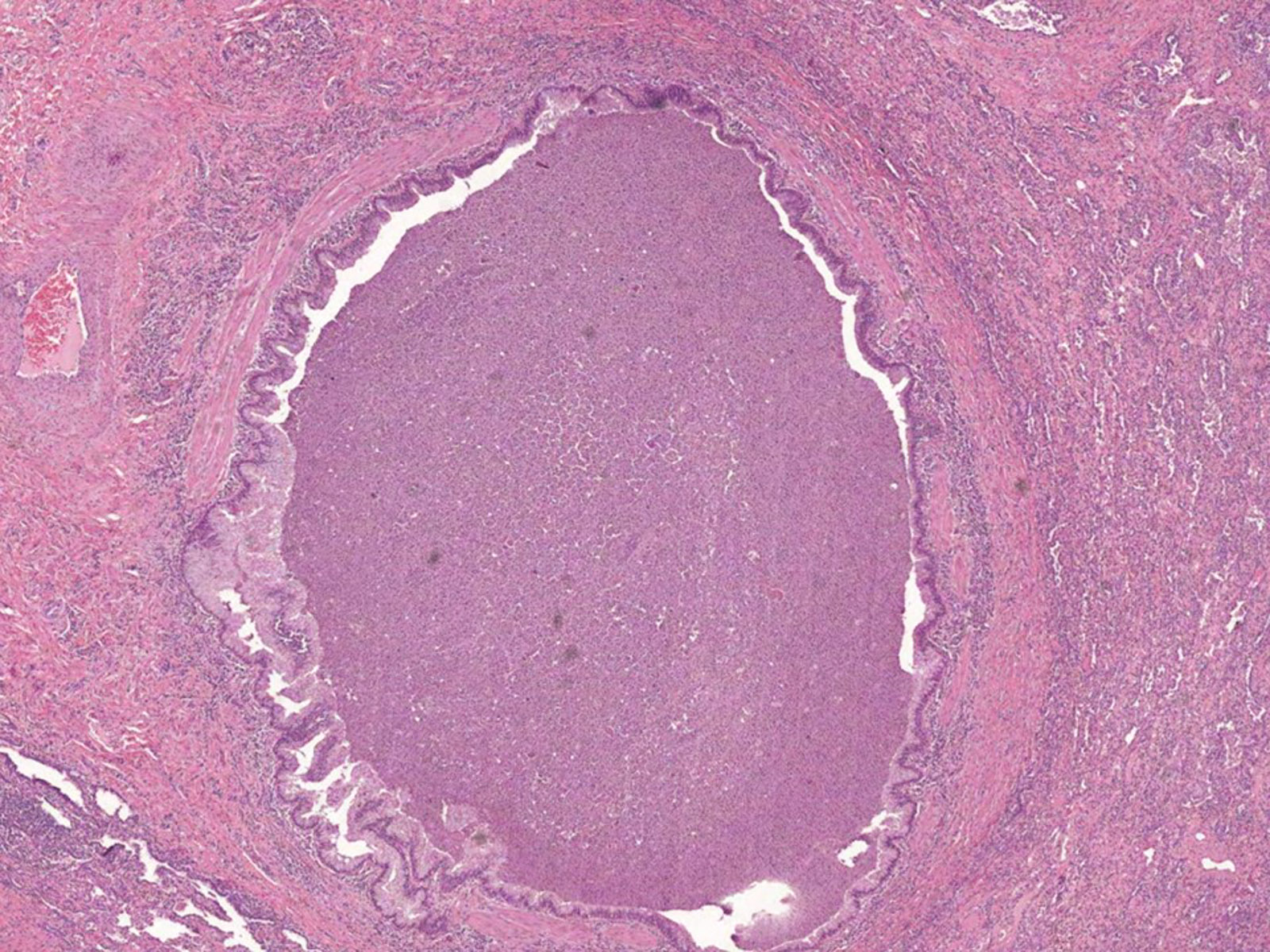
- Invasive pulmonary aspergillosis (IPA)
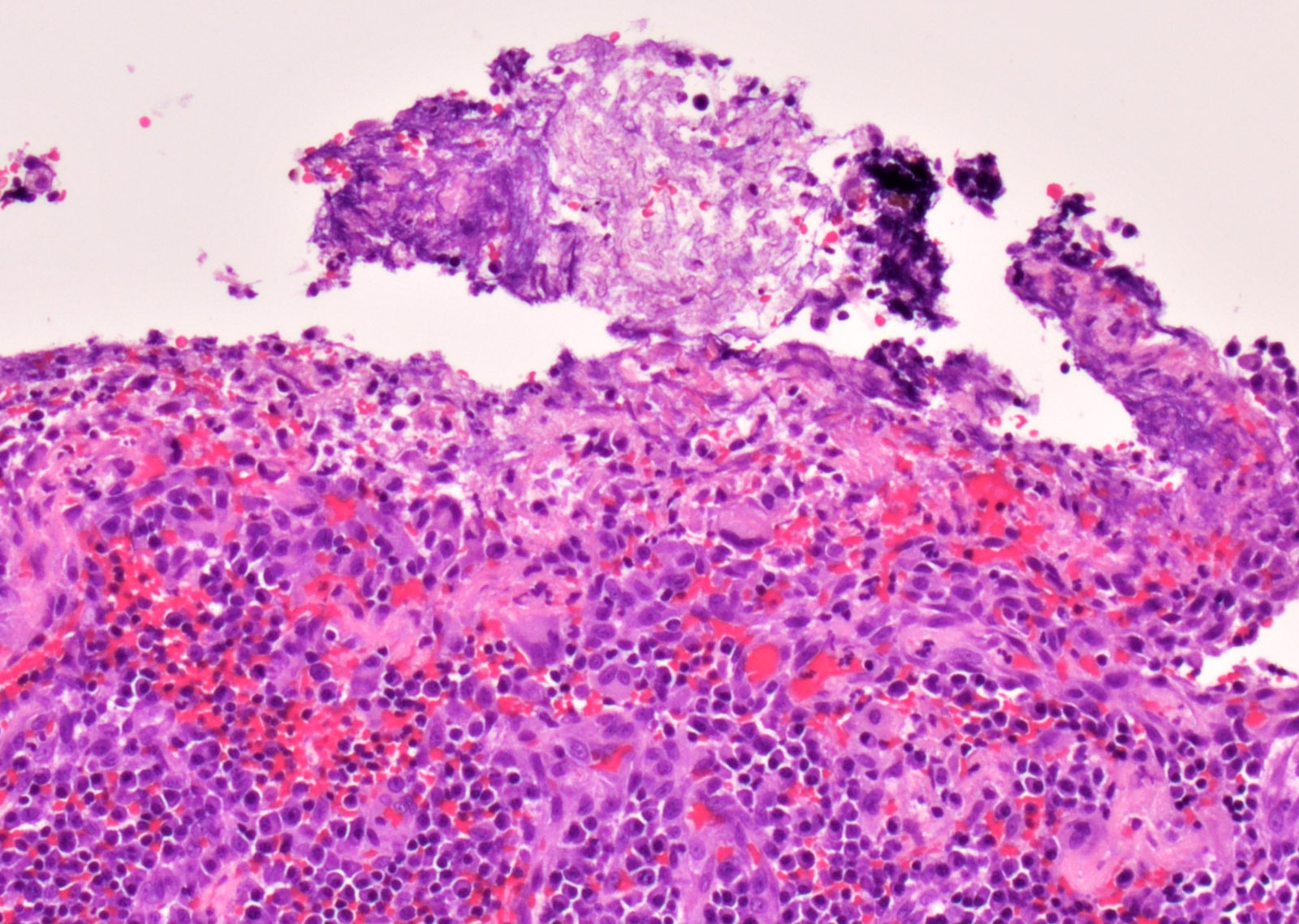
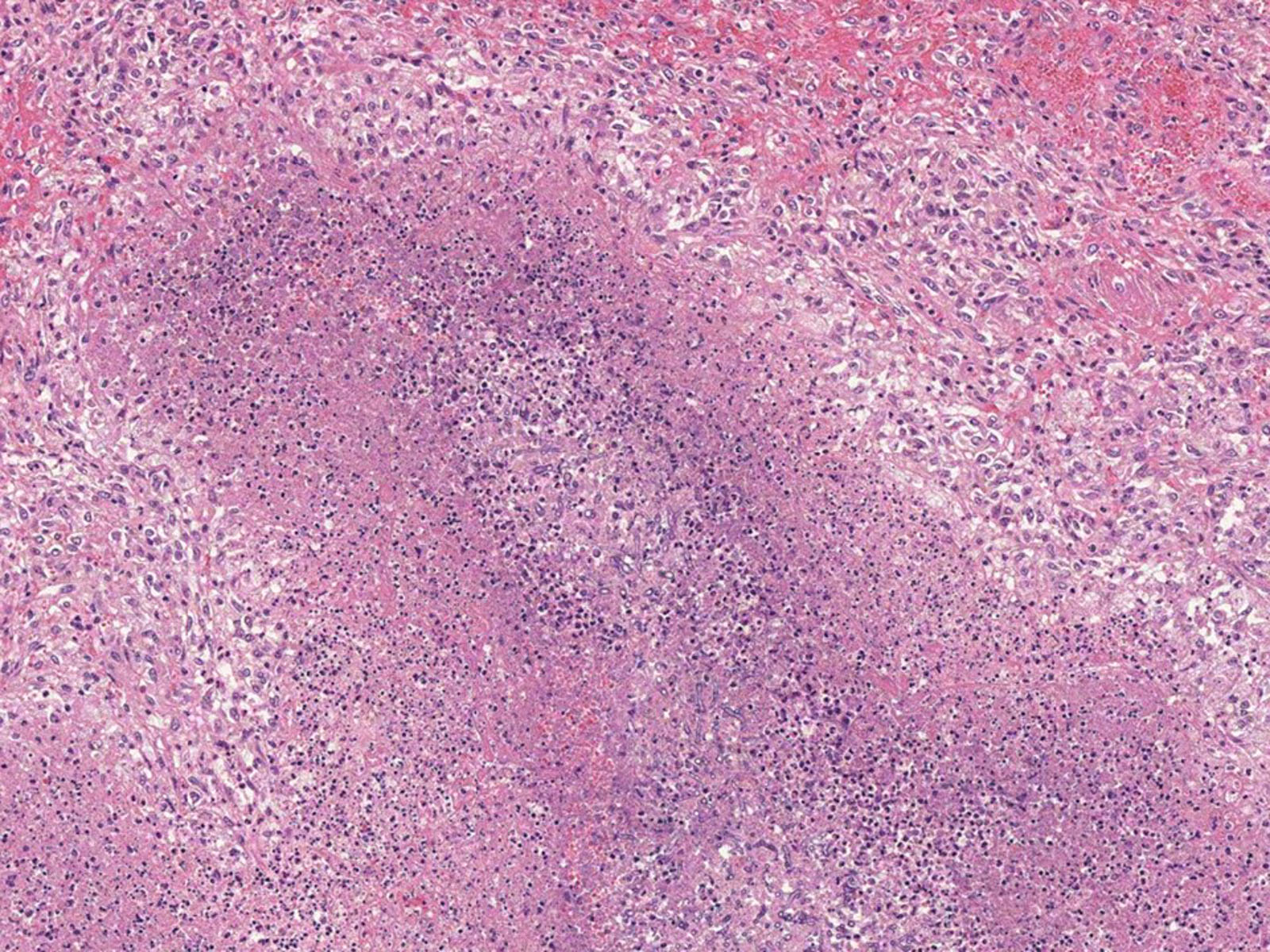
- Necrotizing pneumonia with areas of hemorrhage and acute and granulomatous inflammation (Arch Pathol Lab Med 2008;132:606)
- Fungal hyphae occlude the lumen of the pulmonary artery with associated infarcted area (Semin Diagn Pathol 2017;34:530)
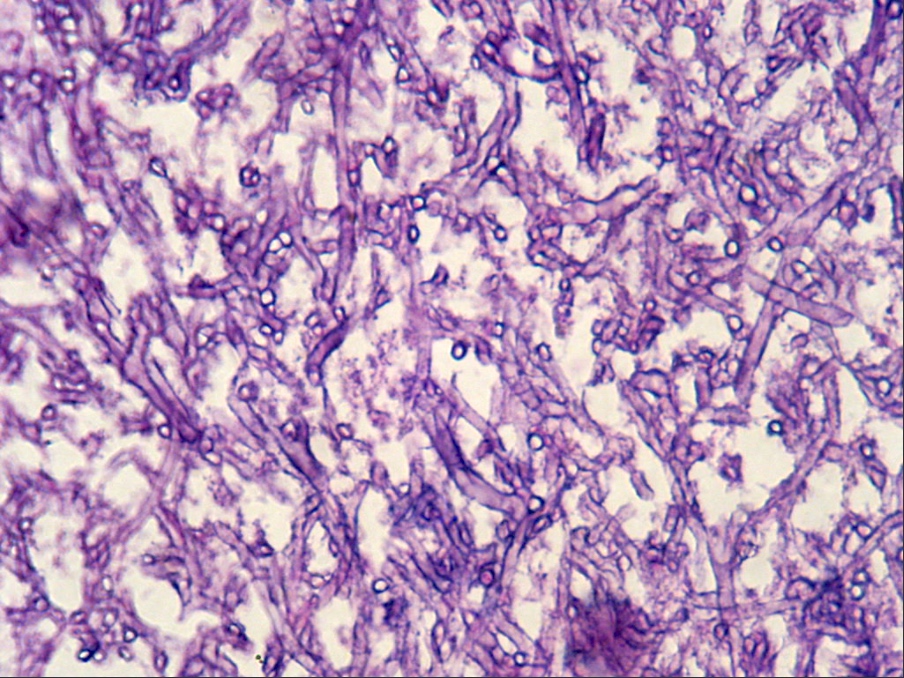
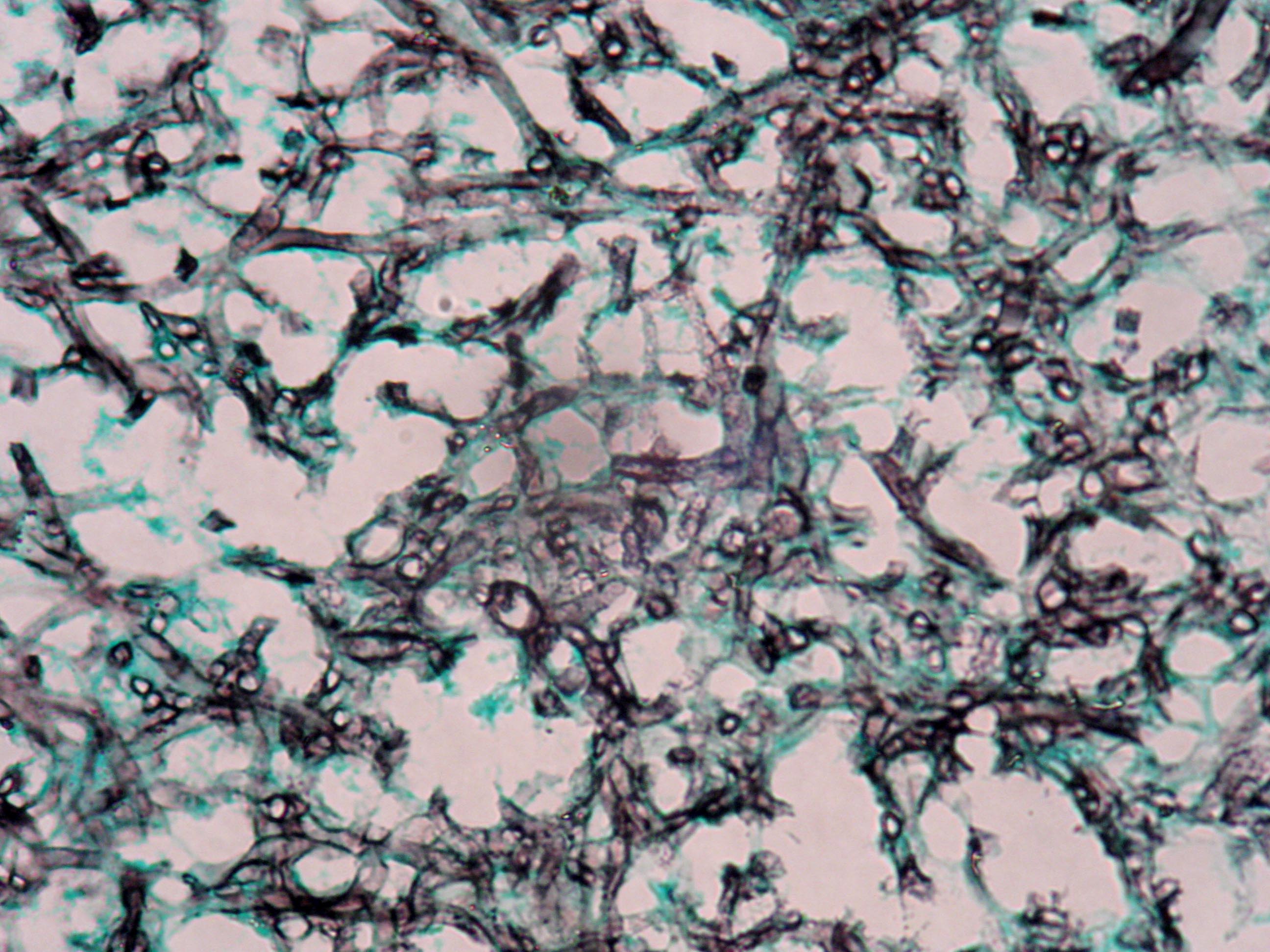
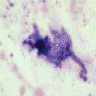
Microscopic (histologic) images
Contributed by Sakda Sathirareuangchai, M.D., Venna Maheshwar, M.D., Kiran Alam, M.D., Anshu Jain and Claudia Mendez, M.D.
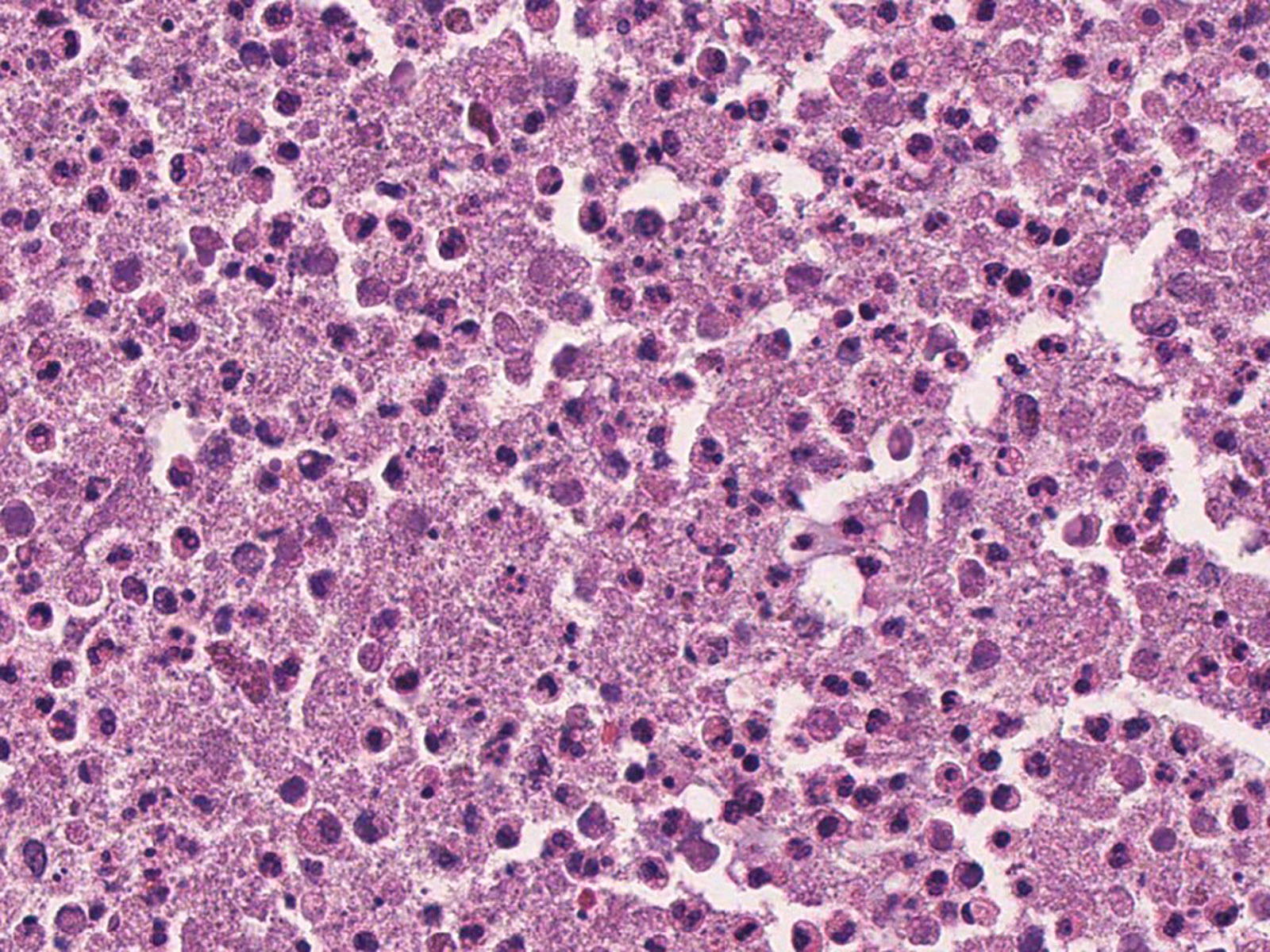
Cytology description
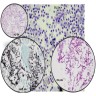
- Relatively large (3 - 6 μm), septate, fungal hyphae, with regular, progressive dichotomous branching at a 45° angle (Clin Microbiol Rev 1998;11:341)
- Fine needle aspiration (FNA) of the lung shows granulomatous process (Orell & Sterret's Fine Needle Aspiration Cytology, 5th Edition, 2011)
- Epithelioid histiocytes in cohesive clusters
- Few nonpigmented multinucleate histiocytes
- Granular, calcific, mucoid or acute inflammatory debris
- Lymphocytes
- Severely reactive pneumocytes may mimic carcinoma (Clin Microbiol Rev 1998;11:341)
- Sputum cytology might show calcium oxalate crystal (Arch Pathol Lab Med 1986;110:1176)
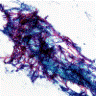
Cytology images
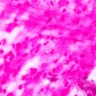
Positive stains
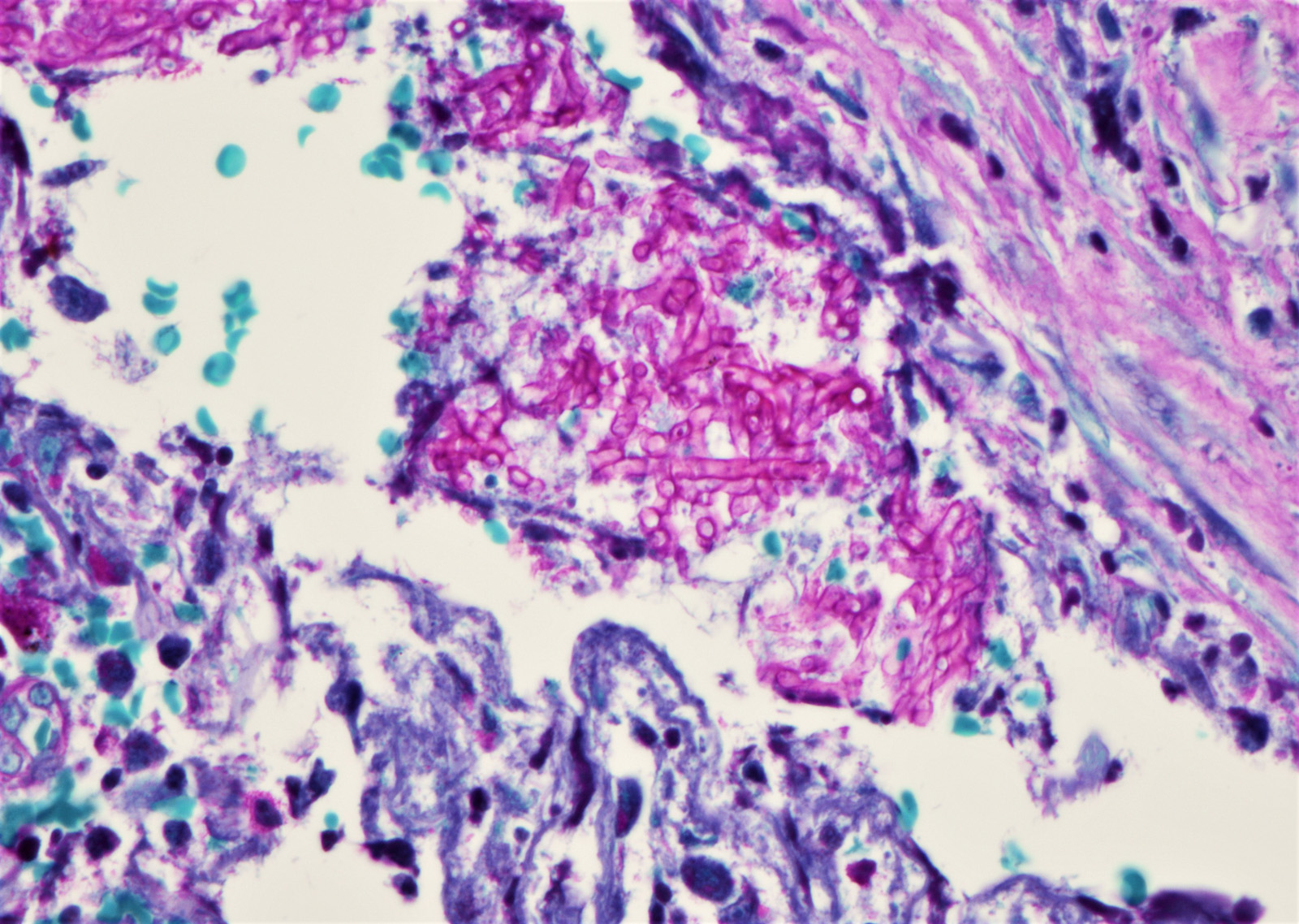
- Grocott and Gomori methenamine silver (GMS)
- Hyphae wall stains black or dark brown
- PAS (Periodic Acid-Schiff)
- Hyphae wall stains pink to purple
- Immunohistochemistry
- Not routinely used
- Sensitivity 85%, specificity 100% (Mycoses 2019;62:1006)
- Polyclonal antibody is preferred over monoclonal antibody (Appl Immunohistochem Mol Morphol 2009;17:524)
Negative stains
- Mucicarmine
- Stains the capsule of Cryptococcus (Semin Diagn Pathol 2017;34:530)
- Fontana-Masson (melanin stain)
- Stain black to dark brown color in Cryptococcus and the dematiaceous fungi (Hum Pathol 2012;43:898)
Videos
Pulmonary aspergillosis
Sample pathology report
- Lung, left upper lobe, wedge resection:
- Cavitary lesion with chronic inflammation
- Fungal hyphae identified (see comment)
- Comment: Fungal hyphae is septate dichotomous branching. GMS stain is positive. The differential diagnosis includes Aspergillus spp., Fusarium and spp.
Differential diagnosis
- Mucormycosis:
- Previously known as zygomycosis
- Nonseptate, broad hyphae with right angle (90°) branching, well stained by standard H&E
- Less common than aspergillosis
- Affects only immunocompromised host, especially diabetics
- Pseudallescheria boydii (anamorph Scedosporium apiospermum):
- Same histomorphology as Aspergillus
- Culture is required for definite identification (Respirology 2008;13:478)
- Fusarium spp.:
- An opportunistic mold with the same histomorphology as Aspergillus
- Culture is required for definite identification (J Clin Diagn Res 2017;11:ED04)
- Pulmonary tuberculosis:
- Also has necrotizing granulomatous inflammation
- Can coexist with chronic pulmonary aspergillosis
- AFB stain positive
- Non small cell lung carcinoma:
- Can present with cavitary and mass lesion
- Reactive pneumocytes from the surrounding tissue in aspergillosis may mimic carcinoma
Board review style question #1
A 65 year old man presented with a mass lesion in his left lung. He underwent a wedge biopsy, which showed benign lung parenchyma with chronic inflammation and an organism shown in the image. What is the most likely causative organism?
- Actinomyces israelii
- Aspergillus spp.
- Candida albicans
- Cryptococcus neoformans
- Mucor spp.
Board review style answer #1
Board review style question #2
A 50 year old man presented with an enlarging mass-like lesion in an existing lung cavity from prior pulmonary tuberculosis. His sputum AFB was negative. There was no other parenchymal lesion in the lung. Fine needle aspiration of the lesion showed acute angle branching septate hyphae with chronic inflammation. Fungal culture grew Aspergillus fumigatus. Which is the proper diagnosis in this patient?
- Allergic bronchopulmonary aspergillosis
- Aspergillus nodule
- Chronic cavitary pulmonary aspergillosis
- Invasive pulmonary aspergillosis
- Tracheobronchial aspergillosis
Board review style answer #2
Board review style question #3
Which of the following statement is true regrading allergic bronchopulmonary aspergillosis (ABPA)?
- Isolation of Aspergillus fumigatus is not required for diagnosis
- Lung biopsy is essential for diagnosis
- Mucous plug is rarely found in the airway
- Patients are immunocompromised host
- Serologic workup is not necessary
Board review style answer #3
A. Isolation of Aspergillus fumigatus is not required for diagnosis
Comment Here
Reference: Aspergillus
Comment Here
Reference: Aspergillus