Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Gadde R, Beck R. Acute myelomonocytic leukemia (AMML). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/leukemiaM4.html. Accessed April 1st, 2025.
Definition / general
- As per the current 5th edition of WHO classification (2022), acute myelomonocytic leukemia (AMML) is a distinct entity within acute myeloid leukemia (AML), defined by differentiation category (Leukemia 2022;36:1703)
- Acute myeloid leukemia, not otherwise specified (AML, NOS) according to the International Consensus Classification (ICC)
Essential features
- Acute myelomonocytic leukemia shows evidence of both granulocytic and monocytic differentiation
- Does not meet the criteria for inclusion in any of the other AML groups
- Blasts / blast equivalents include myeloblasts, monoblasts and promonocytes and must comprise at least 20% of blood or bone marrow cells
- Granulocytic differentiation is present but cells of monocytic lineage must be at least 20% of cells
Terminology
- AML M4 (FAB)
- AMMoL
- AMML
ICD coding
Epidemiology
- AMML represents 5 - 10% of all cases of AML (Blood 2013;121:2424)
- AMML is seen in all age groups but is more common in older individuals (> 60 years of age)
- Median age is ~50 years
- 3% of childhood leukemia (Orphanet: Acute Myelomonocytic Leukemia [Accessed 21 November 2022])
- M:F = 1.4:1
Sites
- Bone marrow, peripheral blood
- Extramedullary infiltration in the spleen, lymph nodes, tonsils, skin or sites like the brain, testis and ovaries has been rarely noted (Am J Clin Pathol 2006;125:783, Case Rep Oncol Med 2019;2019:4189275)
Pathophysiology
- Proliferation of a malignant hematopoietic stem cell which retains the capacity to differentiate into both granulocytic and monocytic cells
- Genetic drivers of AML include both chromosomal alterations and gene mutations
Etiology
- Exact causes are unknown
Clinical features
- Signs and symptoms relate to anemia, thrombocytopenia and increased number of infections
- Early symptoms include fever, fatigue, weakness, weight loss, shortness of breath
- Also can present as organomegaly, lymphadenopathy and other tissue infiltration (monocytic infiltrate)
- Reference: Curr Oncol 2022;29:6245
Diagnosis
- Diagnosis is based on integration of morphology, immunohistochemistry, cytochemistry, flow cytometry studies, cytogenetics and molecular findings
- Immunophenotyping is useful to confirm that both myeloid and monocytic populations are present
- Genetic testing excludes AML with recurrent genetic abnormalities which have myelomonocytic morphology
- Criteria for diagnosis:
- Blasts / blast equivalents comprise ≥ 20% of bone marrow cells
- Blasts / blast equivalents include myeloblasts, monoblasts and promonocytes
- Cells of monocytic lineage (monoblasts, promonocytes, monocytes) overall comprise at least 20% of cells
- AMML is similar to but distinct from acute monoblastic / monocytic leukemia, which requires ≥ 80% of blasts / blast equivalents to be of monocytic lineage
- Additional criteria: ≥ 3% of blasts should be positive for MPO by cytochemical stain (indicates myeloid lineage)
- Monocytic lineage may be ascertained by nonspecific esterase stain (not always present) or monocytic immunophenotype (e.g., expression of CD14, CD11c, CD64, CD163, lysozyme)
- No recurrent genetic abnormalities identified (Leukemia 2022;36:1703)
- AMML of AML, defined by differentiation, morphologically resembles AML with CBFB::MYH11 fusion (formerly known as FAB M4Eo), AML with KMT2A rearrangement and some cases of AML with NPM1 mutation
- Blasts / blast equivalents comprise ≥ 20% of bone marrow cells
Laboratory
- Leukocytosis is common
- Anemia, thrombocytopenia
Prognostic factors
- Poor prognostic factors:
- Adverse mutation profile, including wild type NPM1 and FLT3 ITDhigh, mutated RUNX1 and mutated TP53 (Am J Hematol 2018;93:1267)
- Complex karyotype
Case reports
- 54 year old woman with pure erythroid leukemia subsequent to acute myelomonocytic leukemia (Medicine (Baltimore) 2021;100:e25528)
- 65 year old woman presented with de novo acute myeloid leukemia with complex karyotype (AML M4) (Mol Cytogenet 2013;6:18)
- 82 year old woman with acute myelomonocytic leukemia with uncommon morphological features (Ann Hematol 2020;99:351)
Treatment
- Standard induction chemotherapy with cytarabine given twice daily for 10 days combined with an anthracycline drug for 3 days
- Consolidation therapy often with higher doses of same drugs used for induction or an allogenic stem cell transplant
- Targeted therapy if respective mutations are present (e.g., FLT3 or IDH1 / IDH2 inhibitors)
- Reference: Curr Oncol 2022;29:6245
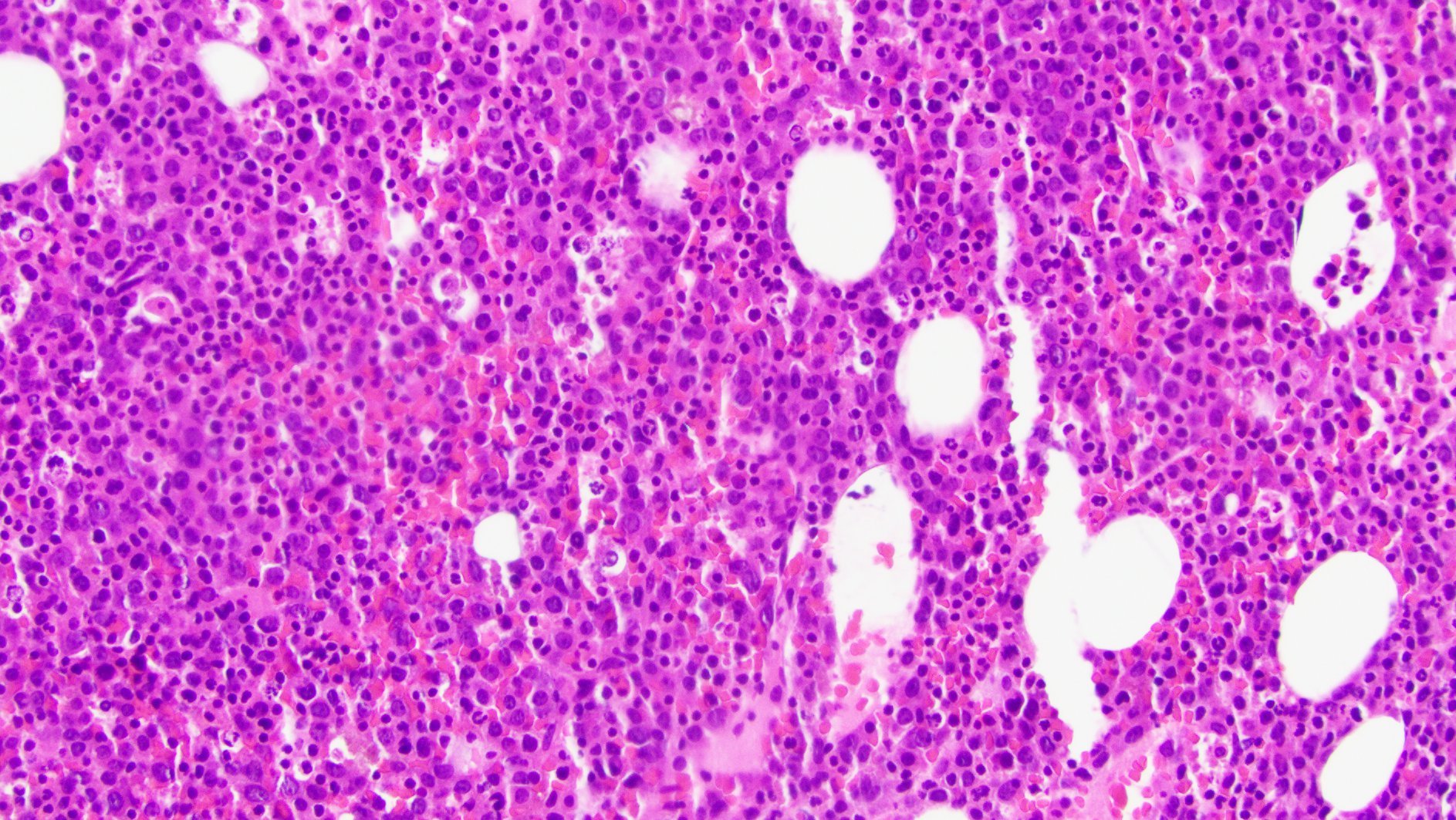
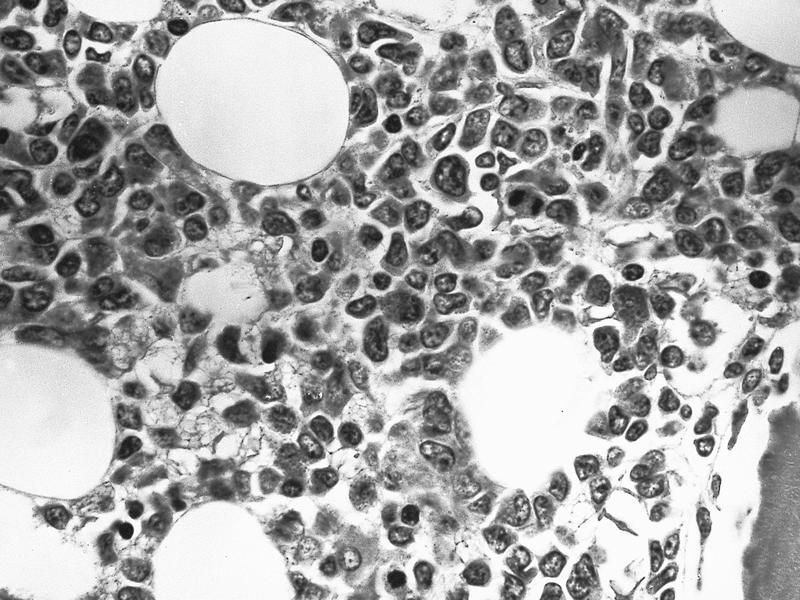
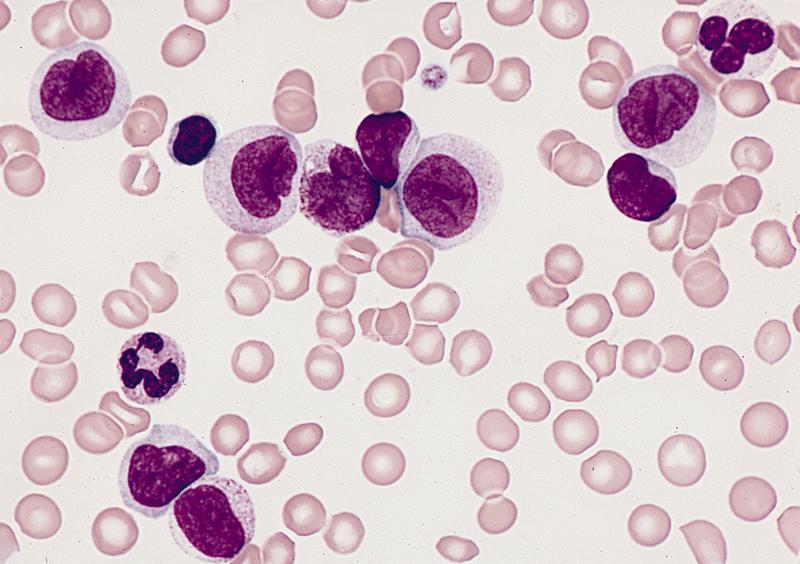
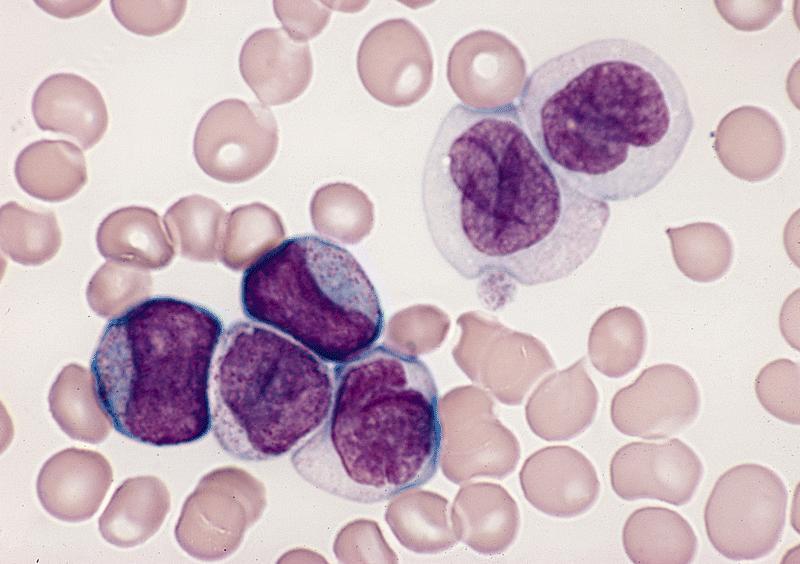
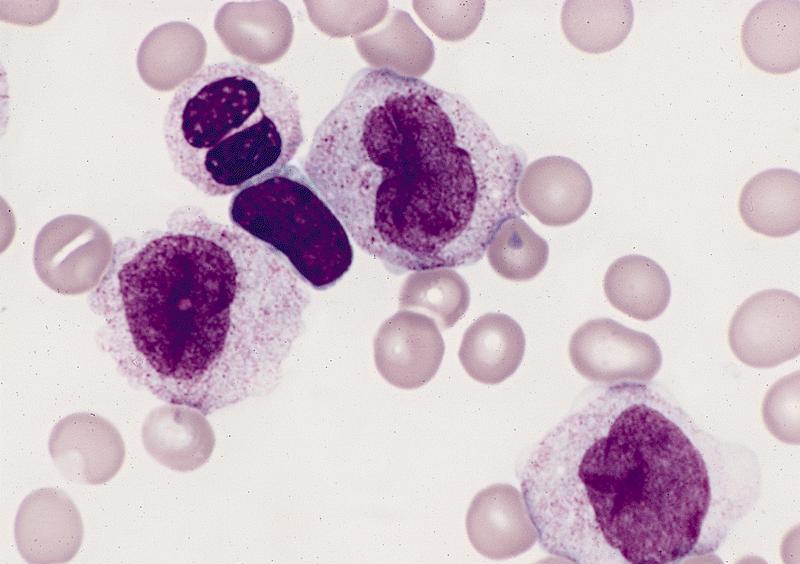
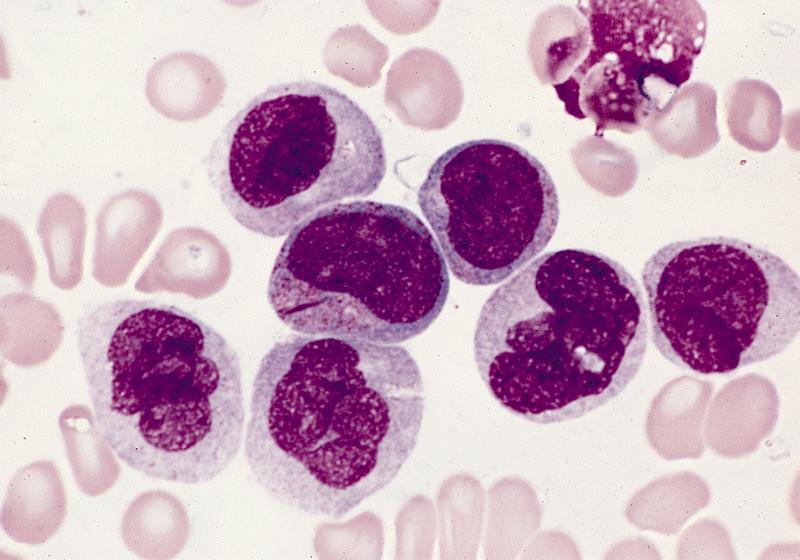
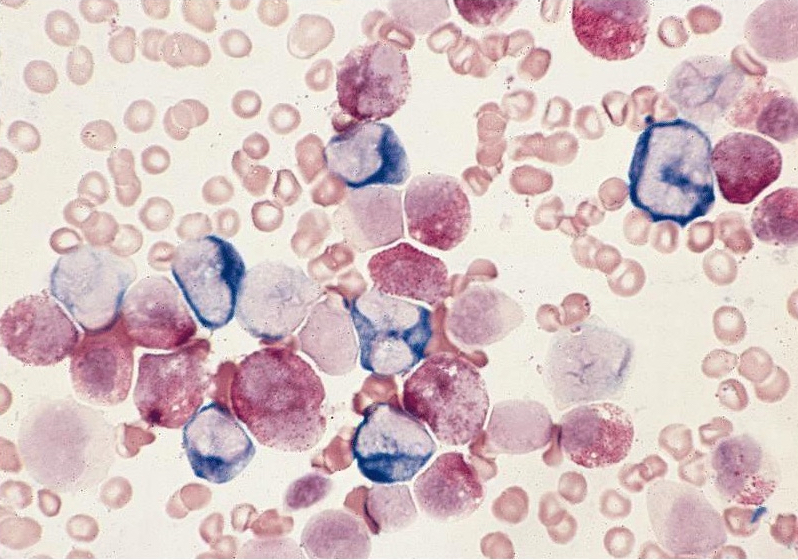
Microscopic (histologic) description
- By definition, blasts and blast equivalents comprise at least 20% of cells in marrow or blood, while cells of monocytic lineage (monoblasts, promonocytes and monocytes) are also at least 20% of cells
- Myeloblasts have high N:C ratios, round nuclei and variably granular cytoplasm; Auer rods occasionally seen
- Monoblasts are large cells with abundant, moderately to intensely basophilic cytoplasm
- May have pseudopod formation, scattered fine azurophilic granules and vacuoles
- Often with round nuclei with lacy chromatin and one or more large nucleoli
- Promonocytes have more irregular nuclei, typically with delicate folds and abundant, less basophilic cytoplasm which is more obviously granulated with occasional large azurophilic granules and vacuoles
- Reference: Haematologica 2009;94:994
Microscopic (histologic) images
Contributed by Ramya Gadde, M.D., Rose Beck, M.D., Ph.D. and AFIP
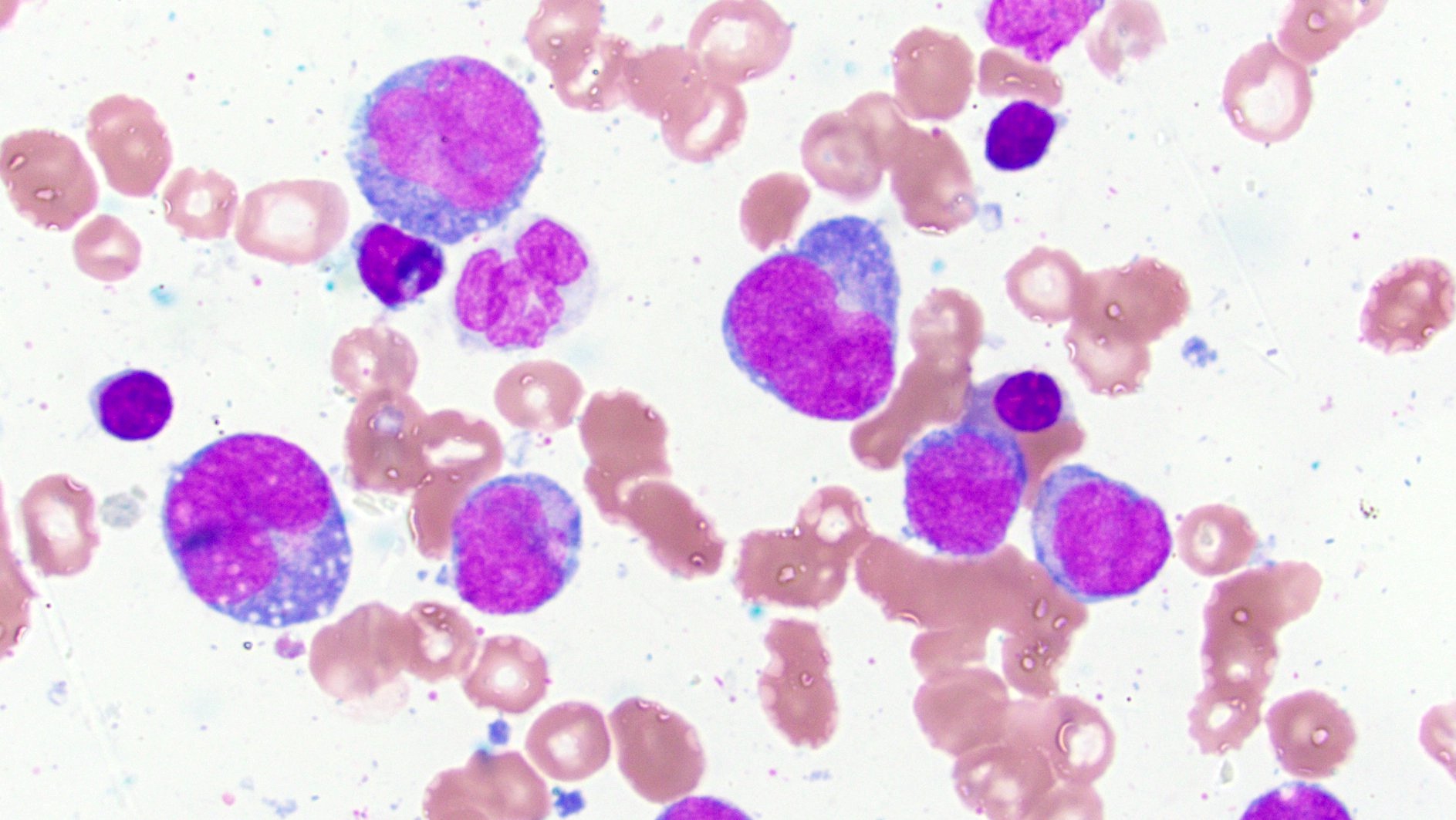
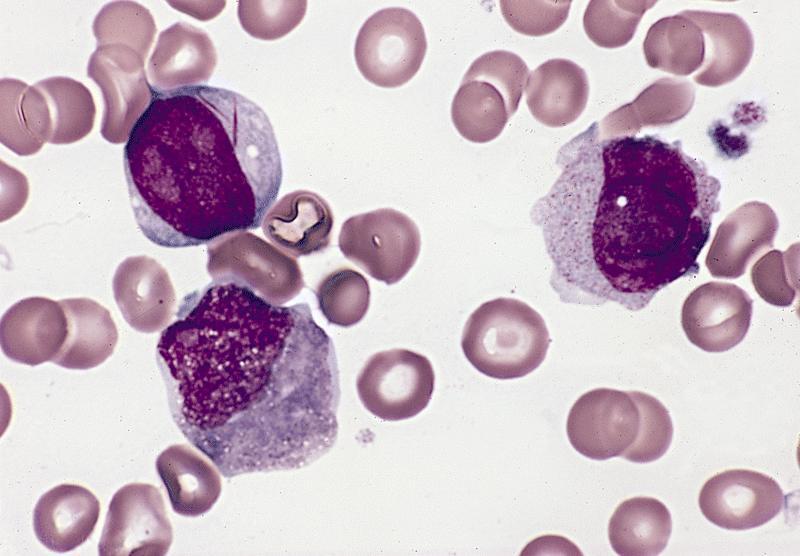
Peripheral smear description
- Peripheral blood often has leukocytosis with blasts, may show increased monocytes and is often more mature than the monocytic cells observed in bone marrow
Positive stains
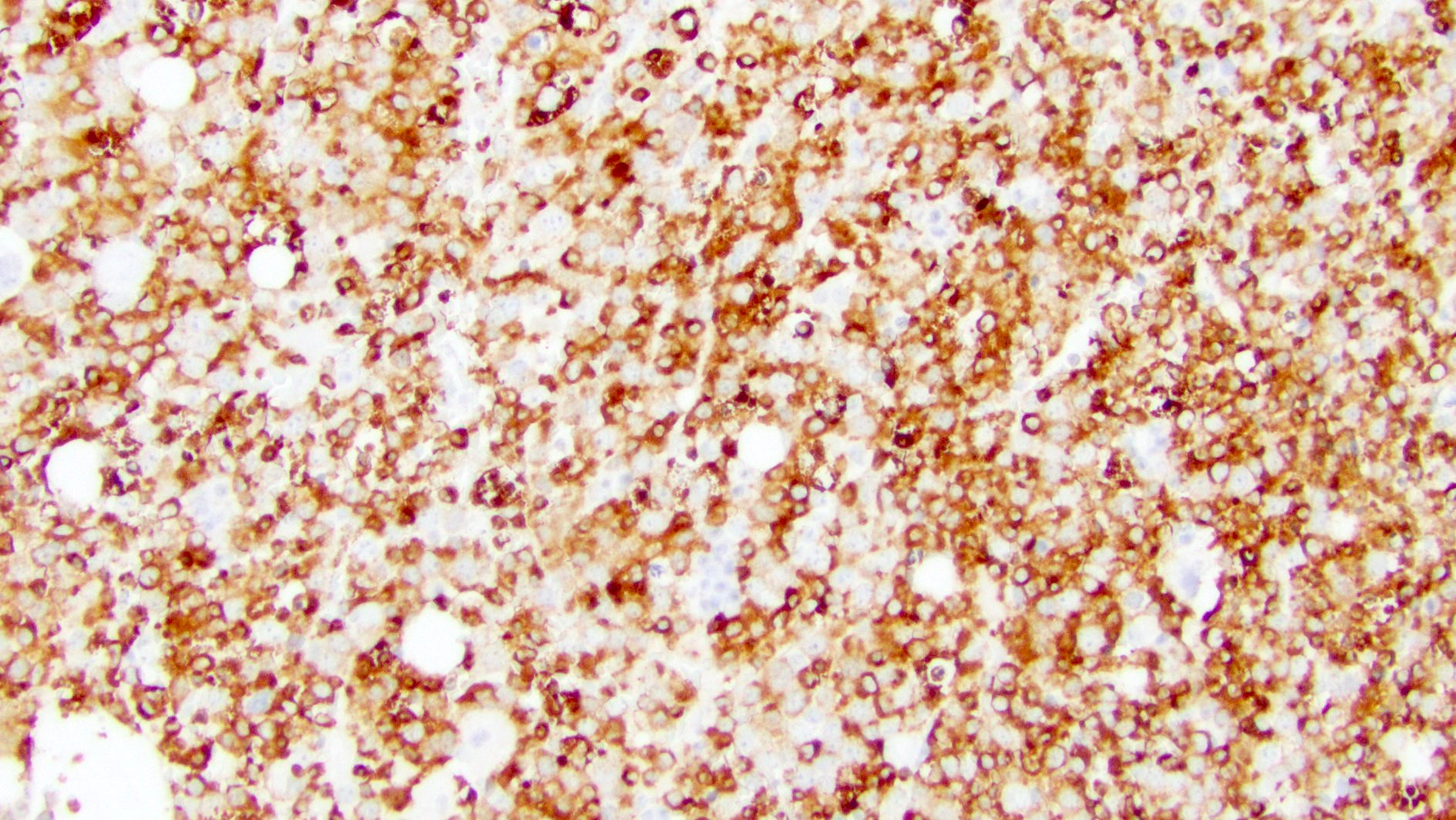
- Expression of markers may vary between monocytic and myeloid populations
- Variably express myeloid antigens CD13, CD33, CD65, CD15
- CD34 and CD117 typically present on the myeloblast population, which may be small
- Monocytic cells typically express CD14, CD64, CD11b, CD11c, CD4, CD36, CD68 (PGM1), CD163, lysozyme
- Most cases express HLA-DR
- Coexpression of CD15, CD36 and CD64 is characteristic of monocytic differentiation
- Reference: Am J Clin Pathol 2004;122:865
Negative stains
- CD41, CD61, glycophorin A
- Lymphoid markers (minority of cases may have aberrant expression of CD7)
Flow cytometry description
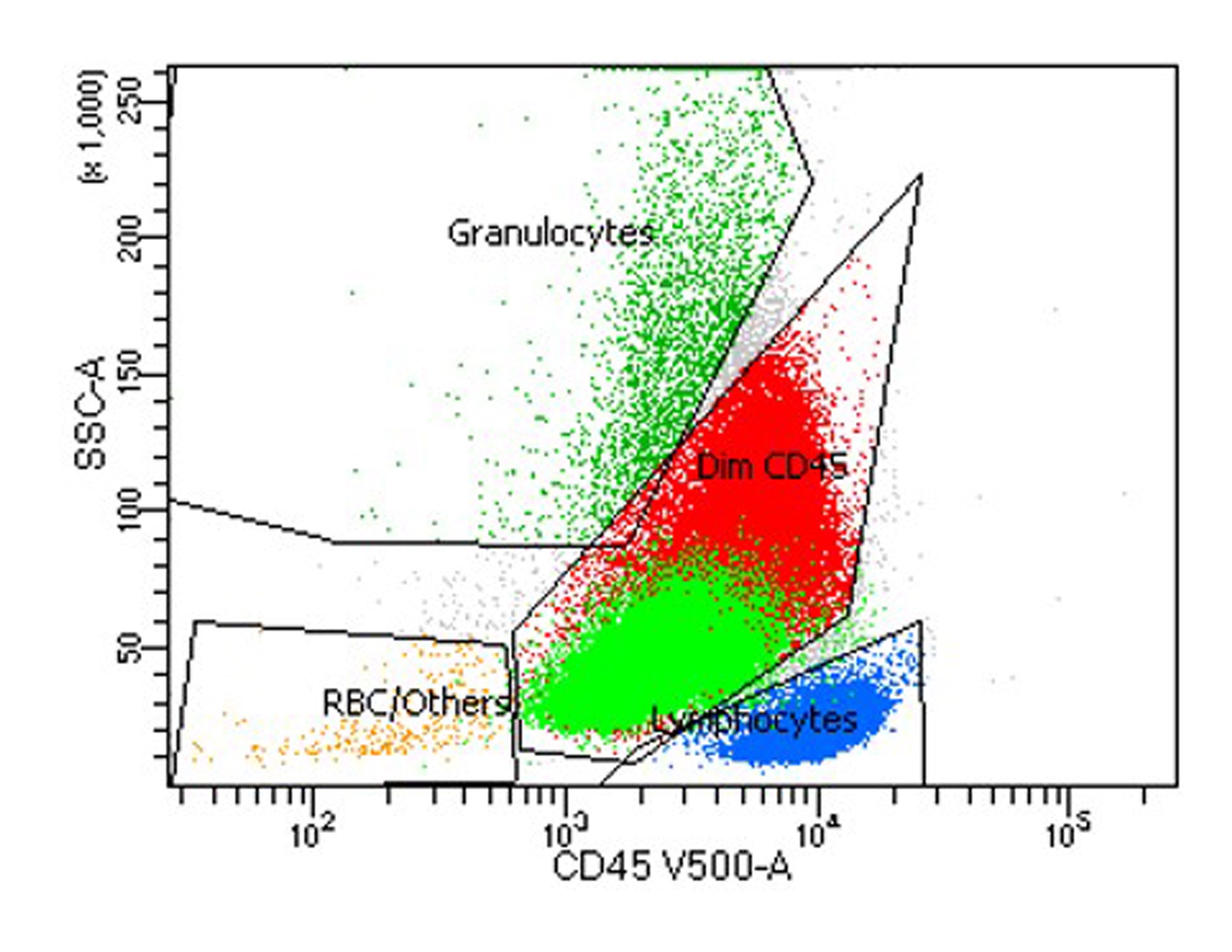
- Myeloid and monocytic cells share a common progenitor
- In the progression of myelomonocytic pathway, increase in the expression of CD15, CD33 and CD64 markers are noted
- In general, myeloblasts show dim to moderate CD45, low side scatter and express CD13, CD33, CD34, CD117 and HLA-DR
- Monoblasts and promonocytes are CD45 bright with side scatter slightly higher than myeloblasts
- References: Clin Lab Med 2017;37:753, Methods Mol Biol 2019;2032:281, Methods Cell Biol 2004;75:665, Cytometry B Clin Cytom 2017;92:218
Flow cytometry images
Molecular / cytogenetics description
- Nonspecific cytogenetic abnormalities in most cases, such as +8, monosomy 7 or del (7q), monosomy 5 or del(5q)
Sample pathology report
- Bone marrow, core biopsy with touch imprint, clot section, aspirate smears, left iliac crest:
- Acute myelomonocytic leukemia (see comment)
- Comment: Hypercellular bone marrow for age (80 - 90%) with involvement by acute myeloid leukemia, defined by differentiation. Immature myelomonocytic cells are increased. Erythroid precursors are decreased. Mature myeloid cells are decreased. Megakaryocytes are adequate and appear normal in morphology.
- Bone marrow aspirate smear is adequate. Blasts / blast equivalents including myeloblasts, monoblasts and promonocytes are increased (50%). Megakaryocytes are adequate in number and show normal morphology.
- Flow cytometry studies demonstrate an abnormal myeloid blast population with expression of dim CD45, CD34, CD15 (subset), CD33, CD13, HLA-DR and CD117. There is also an immature monocytic population with CD64, CD11b, CD11c, CD13, CD14, CD33 and CD64 expression.
Differential diagnosis
- AML with maturation:
- ≥ 3% blasts positive for MPO (by immunophenotyping or cytochemistry) or Sudan Black B by cytochemistry
- Maturing cells of the granulocytic lineage constitute ≥ 10% of the nucleated bone marrow cells
- Monocyte lineage cells constitute < 20% of bone marrow cells
- Expression of 2 or more myeloid associated antigens, such as MPO, CD13, CD33 and CD117 (Leukemia 2022;36:1703)
- AML with NPM1 mutation:
- Blast count < 20% is acceptable; presence of blasts with cup shaped nuclei and mutations in NPM1 gene is characteristic (Leukemia 2022;36:1703, Acta Haematol 2019;141:232)
- AML with KMT2A rearrangement:
- Blast count < 20% is acceptable; presence of KMT2A::MLL gene rearrangements is characteristic (Acta Haematol 2019;141:232)
- AML with CBFB::MYH11 fusion:
- Blast count < 20% is acceptable; presence of abnormal eosinophils, eosinophil precursors and CBFB::MYH11 fusion [inv(16)(p13.1;q22) or t(16;16)(p13.1;q22)] is characteristic (Acta Haematol 2019;141:232)
- Chronic myelomonocytic leukemia:
- Absolute and relative monocytosis; blasts are < 20% of the cells in blood and bone marrow
- Features of both a myeloproliferative neoplasm and a myelodysplastic syndrome are present
- G-CSF related transient atypical monocytosis (Clin Lab Haematol 2004;26:359):
- Recent administration of growth factor; lacks clonal cytogenetic and molecular alterations (Blood 2011;118:4283)
- Leukemoid reaction:
- Leukocytosis with neutrophilia and left shift; high leukocyte alkaline phosphatase (LAP) score
- Lacks clonal cytogenetic and molecular alterations
- Acute monocytic leukemia:
- ≥ 80% monocytes or their precursors (monoblasts or promonocytes)
- < 20% maturing granulocytic cells (Leukemia 2022;36:1703)
- Acute promyelocytic leukemia, microgranular variant:
- Presence of micro / hypogranular promyelocytes; PML::RARA translocation or a variant RARA translocation is characteristic
- Therapy related AML:
- Known history of cytotoxic therapy or radiation therapy administered for neoplastic and nonneoplastic conditions
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
In acute myelomonocytic leukemia (AMML), flow cytometry would most likely show a blast population that has which of the following expression profiles?
- CD34+, CD117+, CD33+, HLA-DR+
- CD34-, CD117+, CD33+, HLA-DR-
- CD34-, CD117-, CD14+, CD33+, HLA-DR+
- CD34+, CD117+, CD33+, HLA-DR+ (myeloblast population) and CD34-, CD117-, CD14+, CD33+, HLA-DR+ (monocytic population)
Board review style answer #2
D. CD34+, CD117+, CD33+, HLA-DR+ (myeloblast population) and CD34-, CD117-, CD14+, CD33+, HLA-DR+ (monocytic population)
Comment Here
Reference: AMML
Comment Here
Reference: AMML