Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Anderson D, Tretiakova M. Collecting duct carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignantcollectingduct.html. Accessed April 20th, 2024.
Definition / general
- Rare (< 1% of kidney cancers) aggressive carcinoma of renal medulla arising from the principal cells of the distal collecting ducts of Bellini
- Morphology: irregular, infiltrating tubules with high grade cells often containing mucin; marked stromal desmoplasia and inflammatory infiltrate
- Essentially a diagnosis of exclusion (Am J Surg Pathol 2020;44:e47)
Essential features
- Major diagnostic criteria
- Medullary involvement
- Predominantly tubular architecture
- Marked desmoplasia
- Cytologically high grade cuboidal or hobnail cells
- Infiltrative growth pattern
- Minor diagnostic criteria
- Central location (large tumors)
- Tubulopapillary architecture
- Inflammatory stroma with neutrophils
- Extensive renal, extrarenal and vascular infiltration
- Mucin positive
- Required diagnostic criteria
- Exclusion of other types of renal cell carcinoma (RCC), urothelial carcinoma and metastasis (Transl Androl Urol 2021;10:1506)
- See Differential diagnosis
Terminology
- Collecting duct carcinoma (preferred)
- Bellini duct carcinoma or carcinoma of the collecting ducts of Bellini (historic name, discouraged)
ICD coding
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
Epidemiology
- M:F = 2:1
- Mean age 55 years; median patient age in studies ranging from 43 to 63 (WHO 5th edition)
- Wide age range reported from 8 - 83 years (Pediatr Nephrol 1996;10:29, Can Urol Assoc J 2015;9:E589)
- Some debate whether it afflicts younger patients
- Questionable association with analgesic nephropathy (Am J Surg Pathol 1980;4:565)
Sites
- Arising centrally from renal medulla but often involving both the cortex and medulla because of large size
Clinical features
- Approximately 67% of patients present with pain, gross hematuria, weight loss, fatigue and flank mass
- Has the poorest prognosis of common renal cell carcinoma subtypes
- Often limited responses to immunotherapy and chemotherapy (J Urol 2007;177:1698)
Diagnosis
- Diagnosis is made primarily on H&E with a focus on the major, minor and required criteria; there should be adequate sampling, including of the adjacent renal pelvis
- Presence of any component of the tumor showing classic appearances of clear cell renal cell carcinoma, papillary renal cell carcinoma or mucinous tubular and spindle cell carcinoma likely supports a diagnosis of the corresponding entity with high grade features / transformation
- Clinical history, prior cytology or pathology, and radiologic correlation may sometimes be helpful in considering a metastasis or urothelial carcinoma in the differential
- Panel of immunostains such as PAX8, 34 beta E12, GATA3, SMARCB1, OCT 3/4 and fumarate hydratase may be useful in the diagnosis
Radiology description
- CT imaging shows good correlation with histopathologic findings
- Mass in the renal medulla and involving renal sinus; 50% with a cystic component
- Lymphadenopathy and metastases noted in 56% and 33% of cases, respectively
- Perinephric stranding and vascular invasion present in 56% and 28% cases, respectively
- Infiltrative growth (67%) and preservation of the renal contour (61%) more common than expansile growth (33%) and exophytic configuration (39%) (Eur J Radiol 2006;57:453)
- Features distinguishing cystic renal cell carcinoma from collecting duct carcinoma (Cancer Imaging 2021;21:52)
Prognostic factors
- Median survival: 7.6 - 11 months with 67% of patients dying within the first 2 years (Can Urol Assoc J 2015;9:E589, Curr Oncol 2013;20:e223, J Urol 2009;182:2595)
- Distant metastases in most patients either at presentation or after nephrectomy; metastases are usually to lymph nodes, liver, lungs, bones, adrenal gland or contralateral kidney (J Clin Oncol 2002;20:2376)
- Approximately 40% present with metastasis (WHO 5th edition)
- One study showed that absence of lymph node metastasis, distant metastasis, sarcomatoid differentiation; earlier stage could be used to predict a better survival
- Multivariable analysis showed that sarcomatoid differentiation and absence of renal surgery were predictors of mortality (Urology 2022;164:163)
- One study associated neutrophil to lymphocyte ratio ≥ 4 (median) with worse cancer specific survival in collecting duct carcinoma (Jpn J Clin Oncol 2018;48:692)
Case reports
- 39 year old man with possible BK virus associated de novo collecting duct carcinoma in renal allograft (Oncotarget 2018;9:15157)
- 54 year old man, a Caucasian engineer, referred with multifocal cardiac metastases (Urol Case Rep 2016;5:27)
- 59 year old man, a renal transplant recipient, with collecting duct carcinoma of the native kidney (Case Rep Transplant 2017;2017:4527104)
- 65 year old woman with collecting duct carcinoma masquerading as hydatid cyst (Indian J Pathol Microbiol 2018;61:410)
- 68 year old man with collecting duct carcinoma, sarcomatoid change and paraneoplastic syndrome (Urol Case Rep 2020;33:101322)
Treatment
- Nephrectomy in addition to cytotoxic chemotherapy, with or without radiation (Urol Oncol 2017;35:540.e13)
- In an open label phase II trial of 23 patients treated with gemcitabine plus cisplatin, objective responses were seen in 6 patients (26%) and median overall survival was approximately 11 months (J Urol 2007;177:1698)
- May respond to palbociclib in metastatic disease bearing a CDKN2A homozygous deletion (JCO Precis Oncol 2017;1:1)
- Use of tyrosine kinase and immune checkpoint inhibitors, antiprogrammed death 1 antibody and cytotoxic T lymphocyte associated antigen 4 antibodies are investigational (Int J Clin Oncol 2011;16:153, Clin Genitourin Cancer 2018;16:e521, Anticancer Res 2014;34:1027, Eur J Cancer 2018;100:1, Case Rep Urol 2021;2021:9936330, Int Cancer Conf J 2019;9:32, Front Oncol 2021;11:764352, J Med Case Rep 2022;16:193, Cancers (Basel) 2021;13:3807, Clin Genitourin Cancer 2016;14:e431, Medicine (Baltimore) 2018;97:e13173, Mol Clin Oncol 2017;7:988)
- Other immunotherapy treatments, including neoantigen based vaccination and neoantigen reactive T cells, are experimental (J Immunother Cancer 2020;8:e000217)
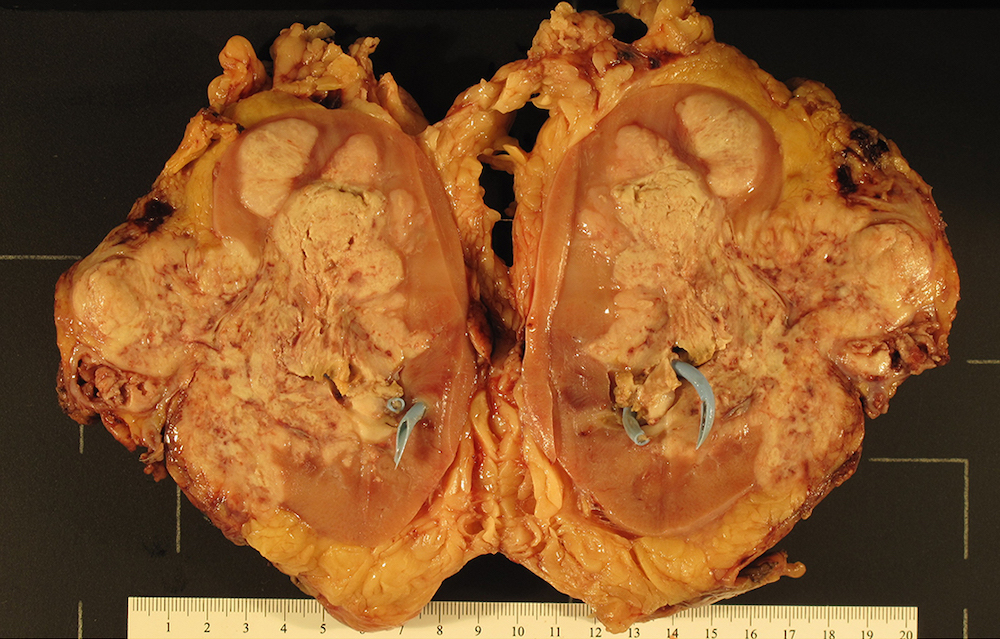
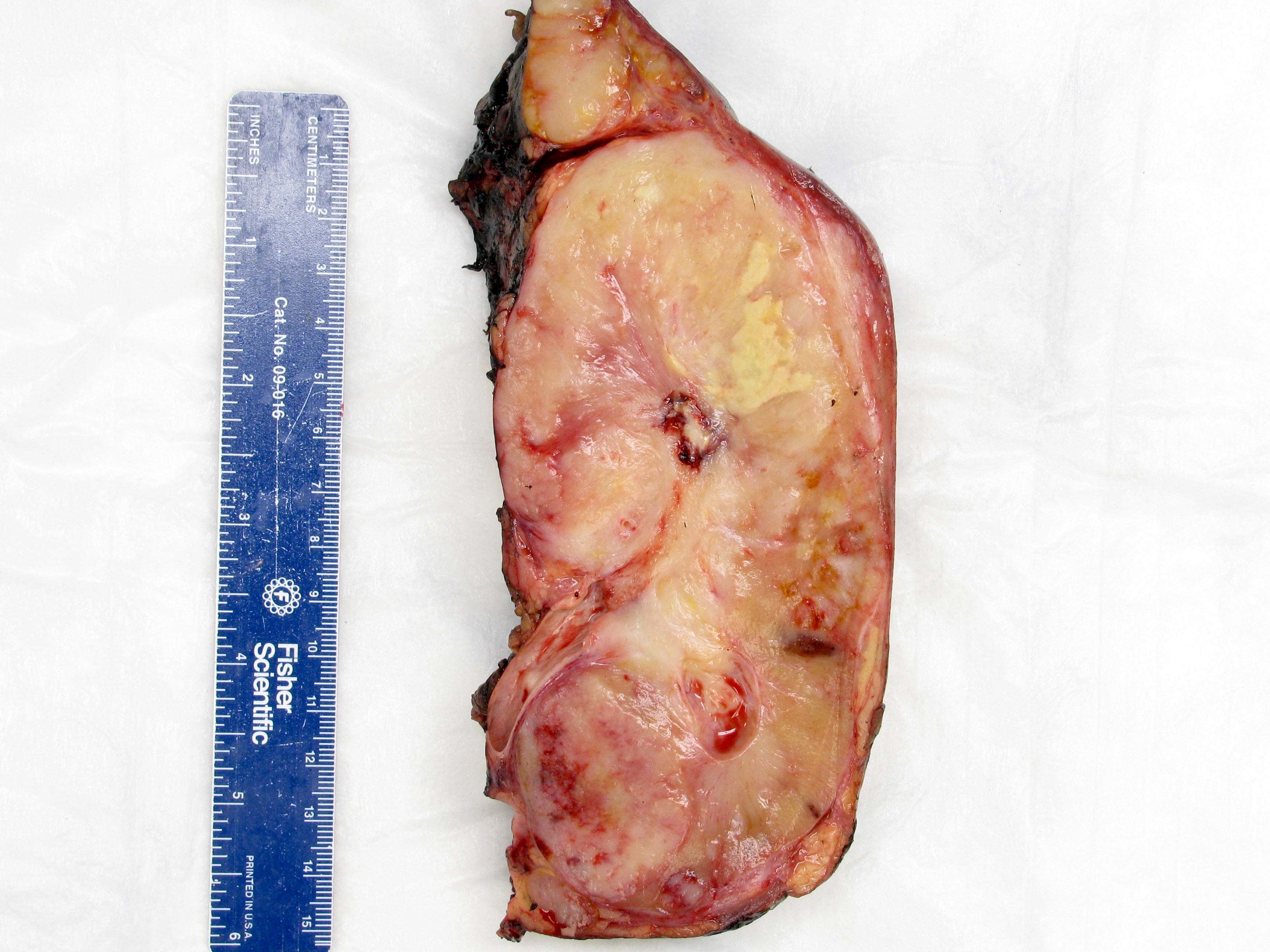
Gross description
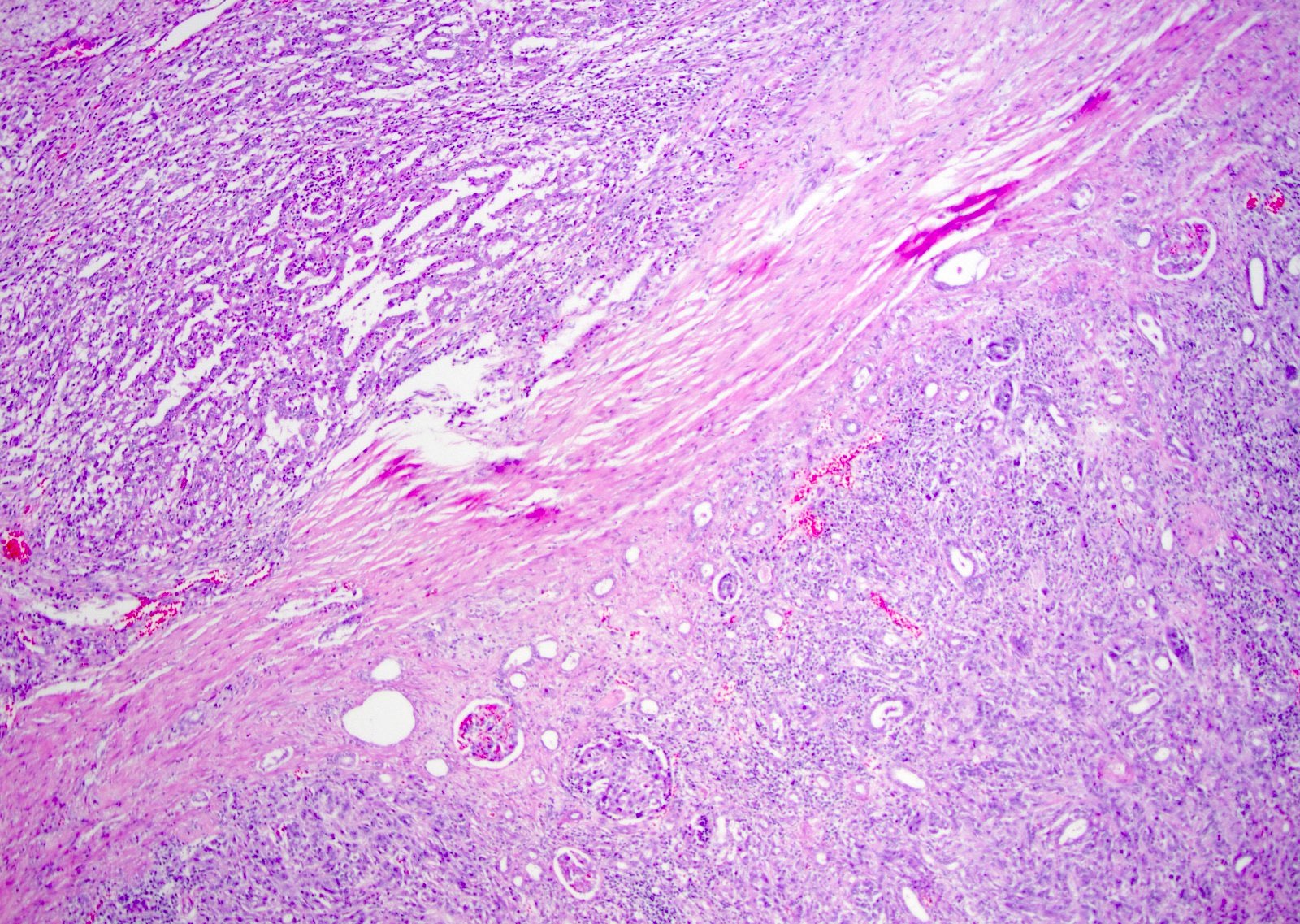
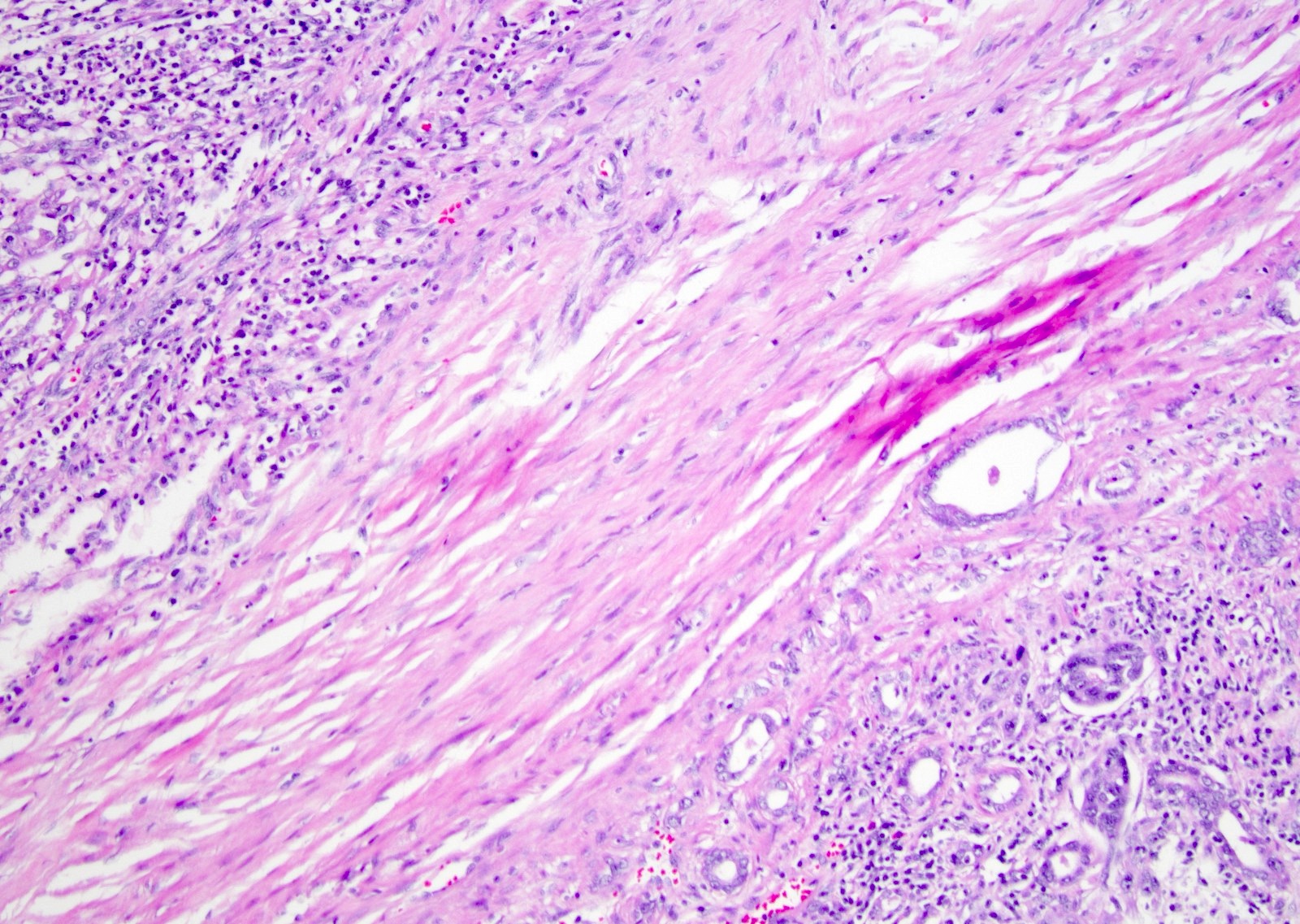
- Infiltrative, firm gray or white mass
- Centered in the medulla but often involving both cortex and medulla
- Tumor size range from 2.5 - 12 cm (mean 5 cm)
- Hemorrhage, necrosis and cystic changes are common
- May have satellite nodules and renal vein invasion
- Often infiltrates perirenal and renal sinus fat
Gross images
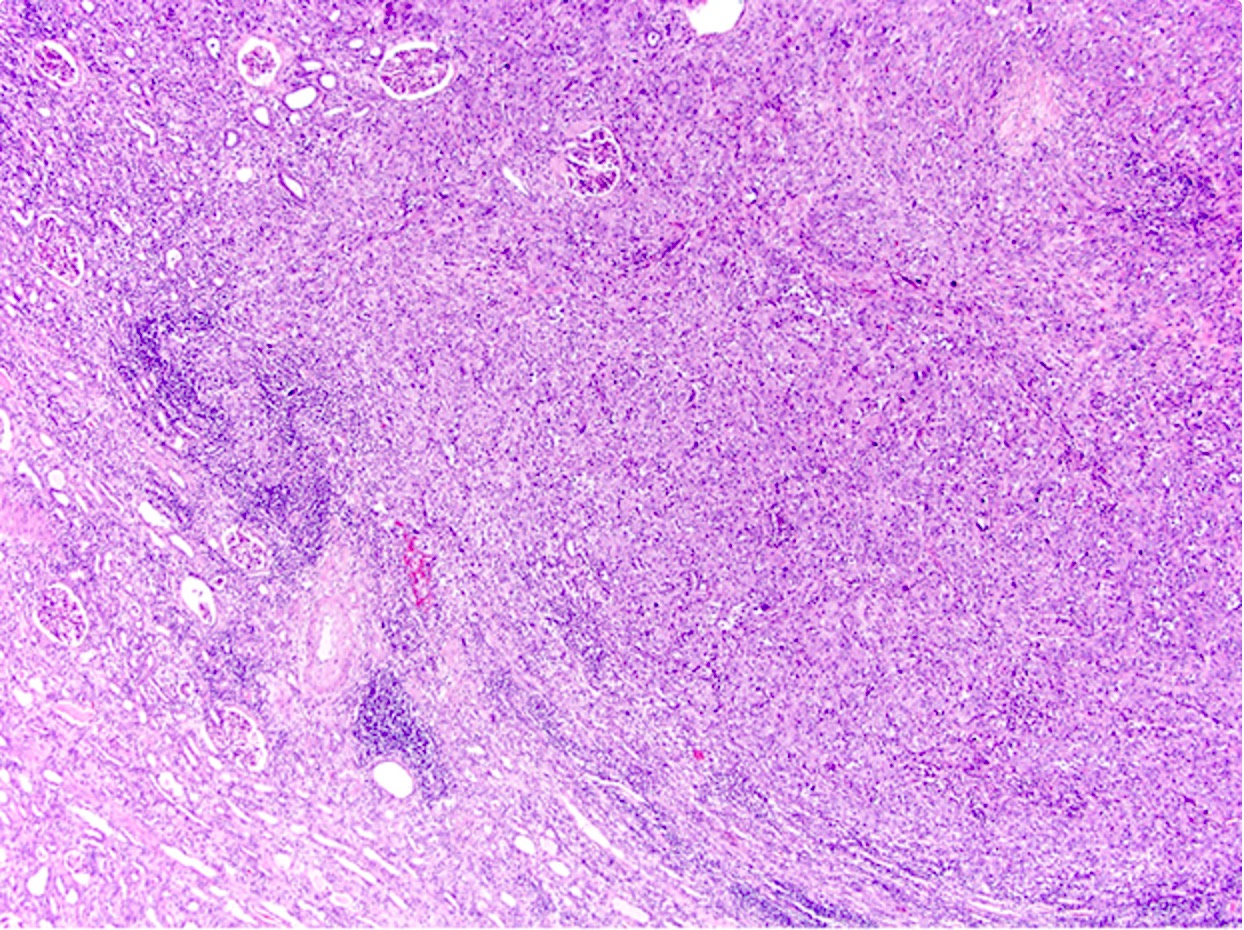
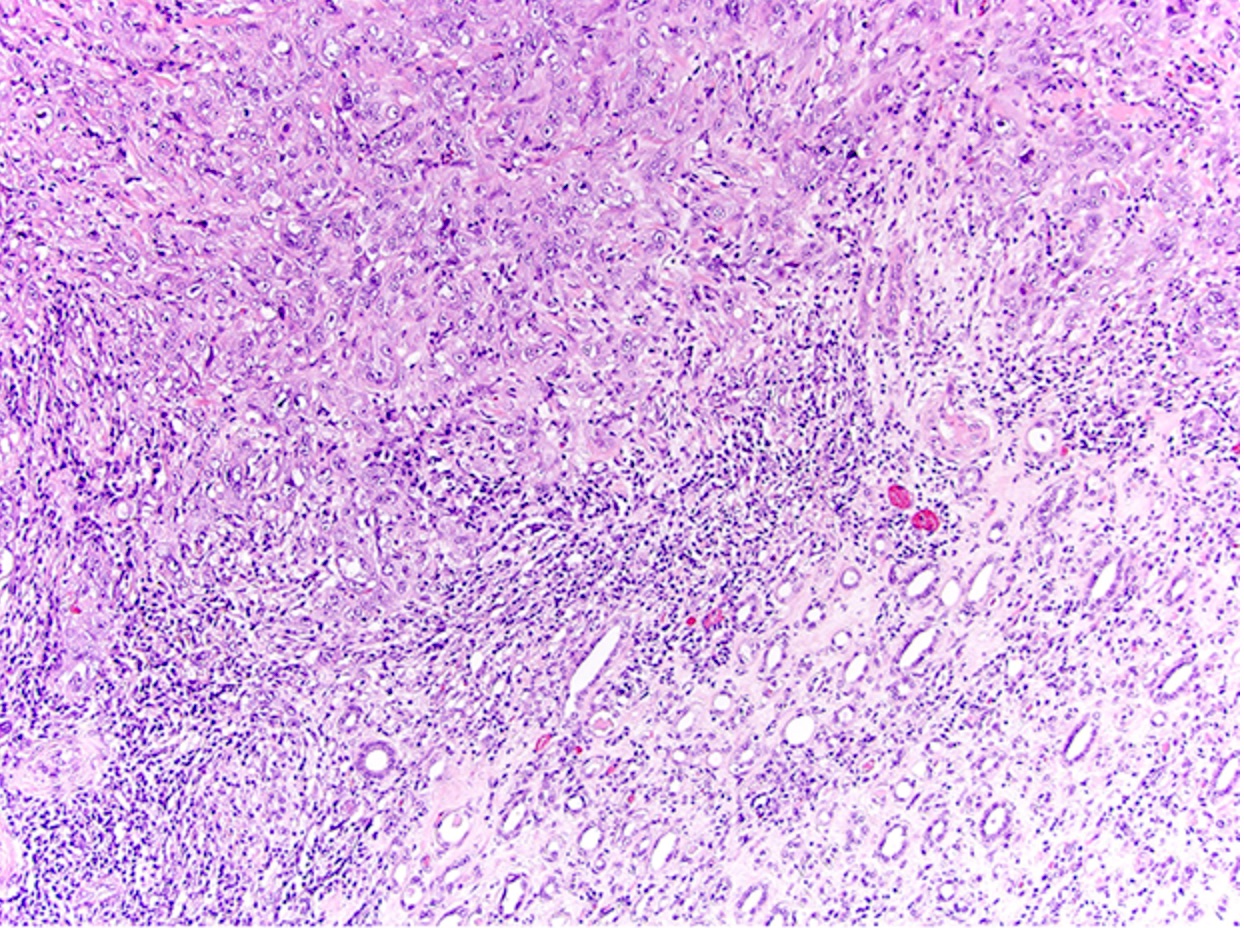
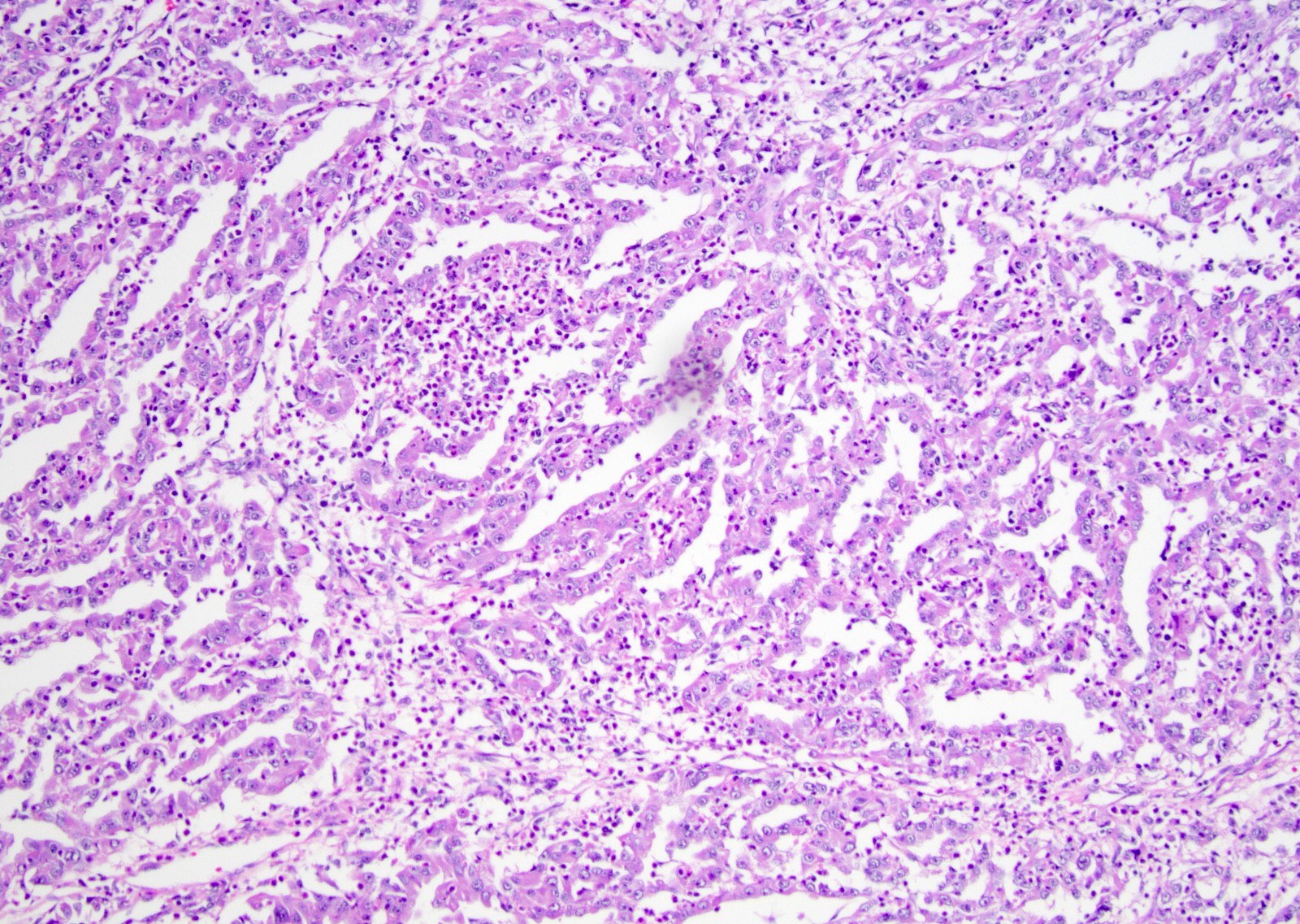
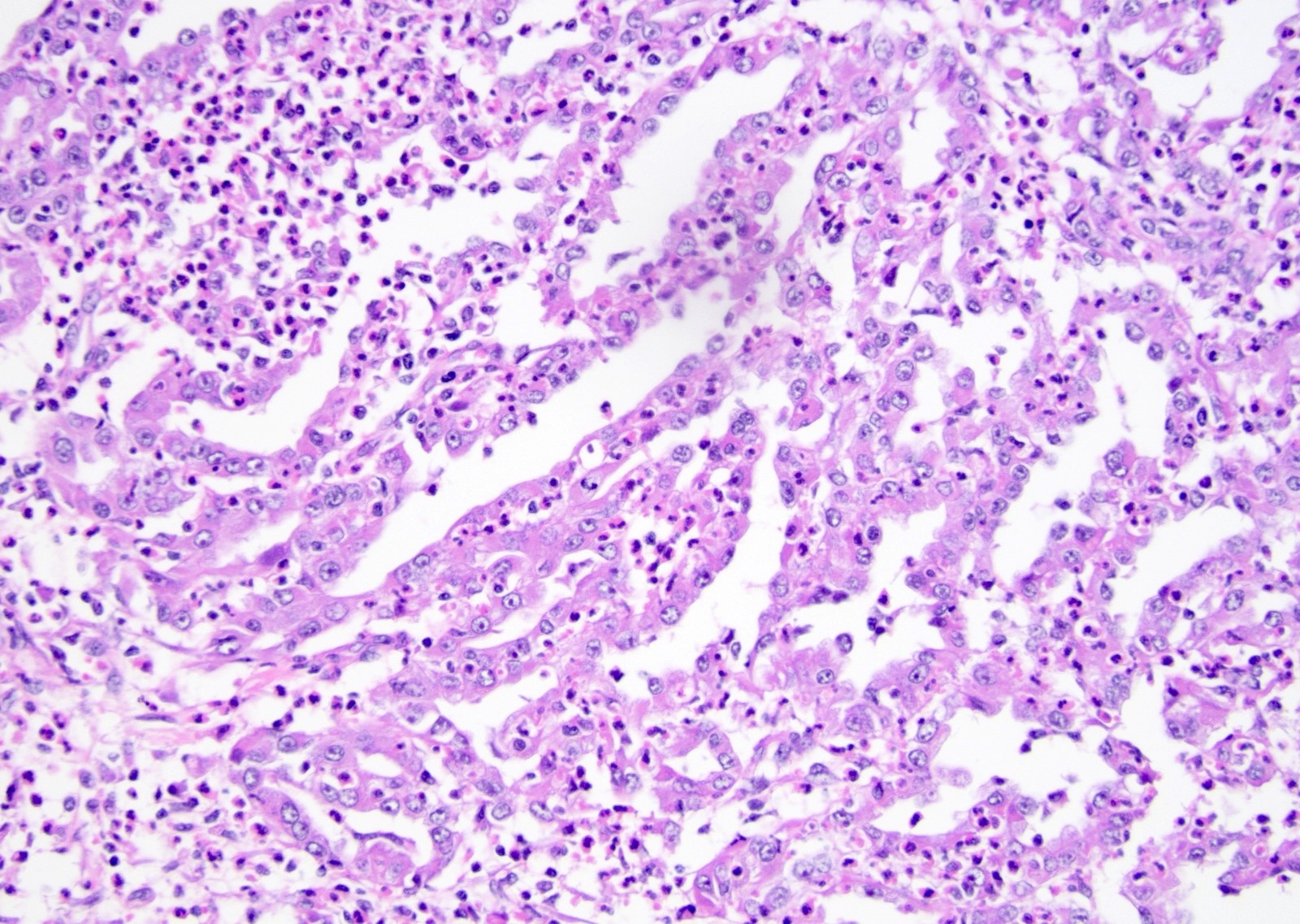
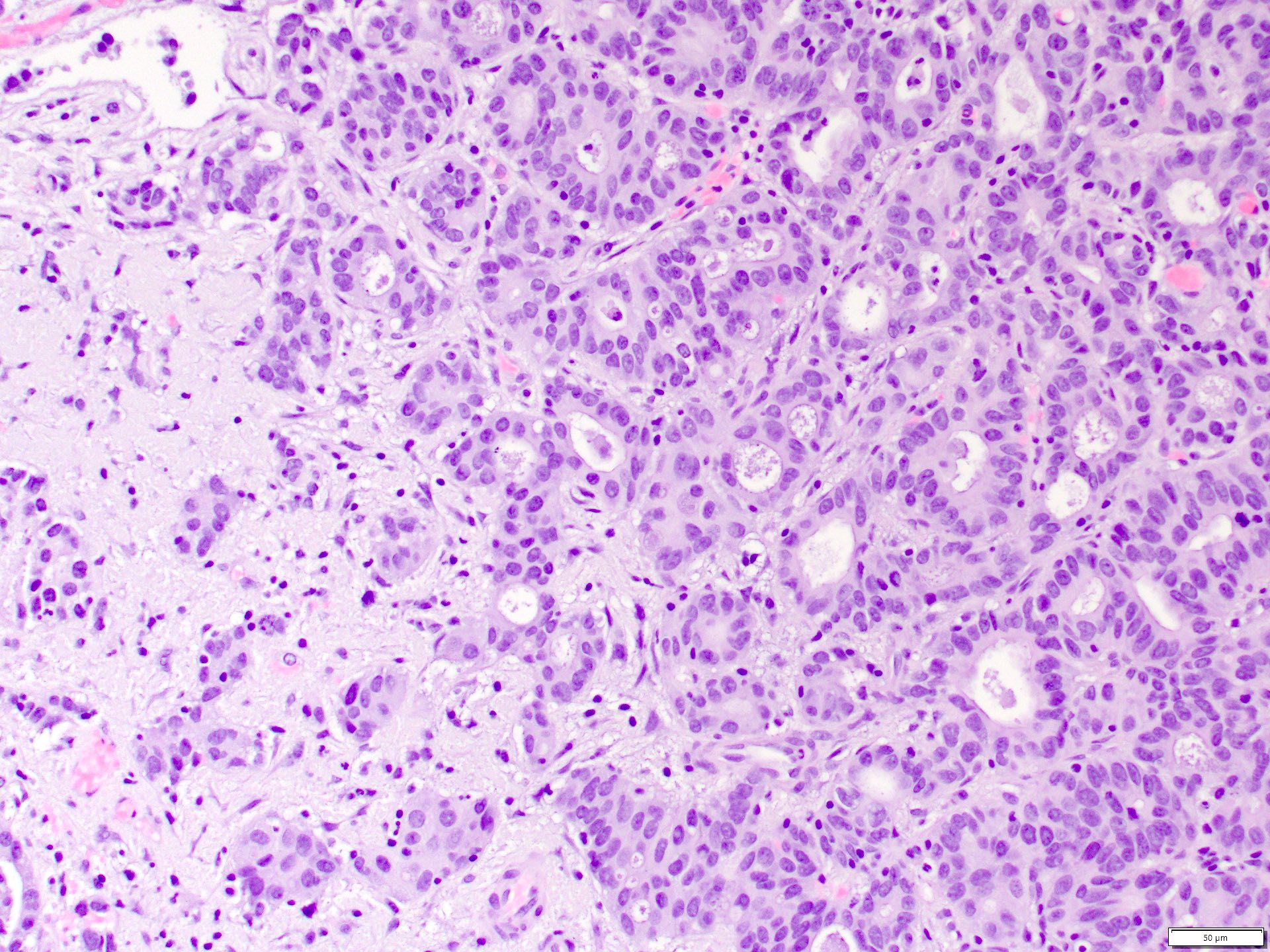
Microscopic (histologic) description
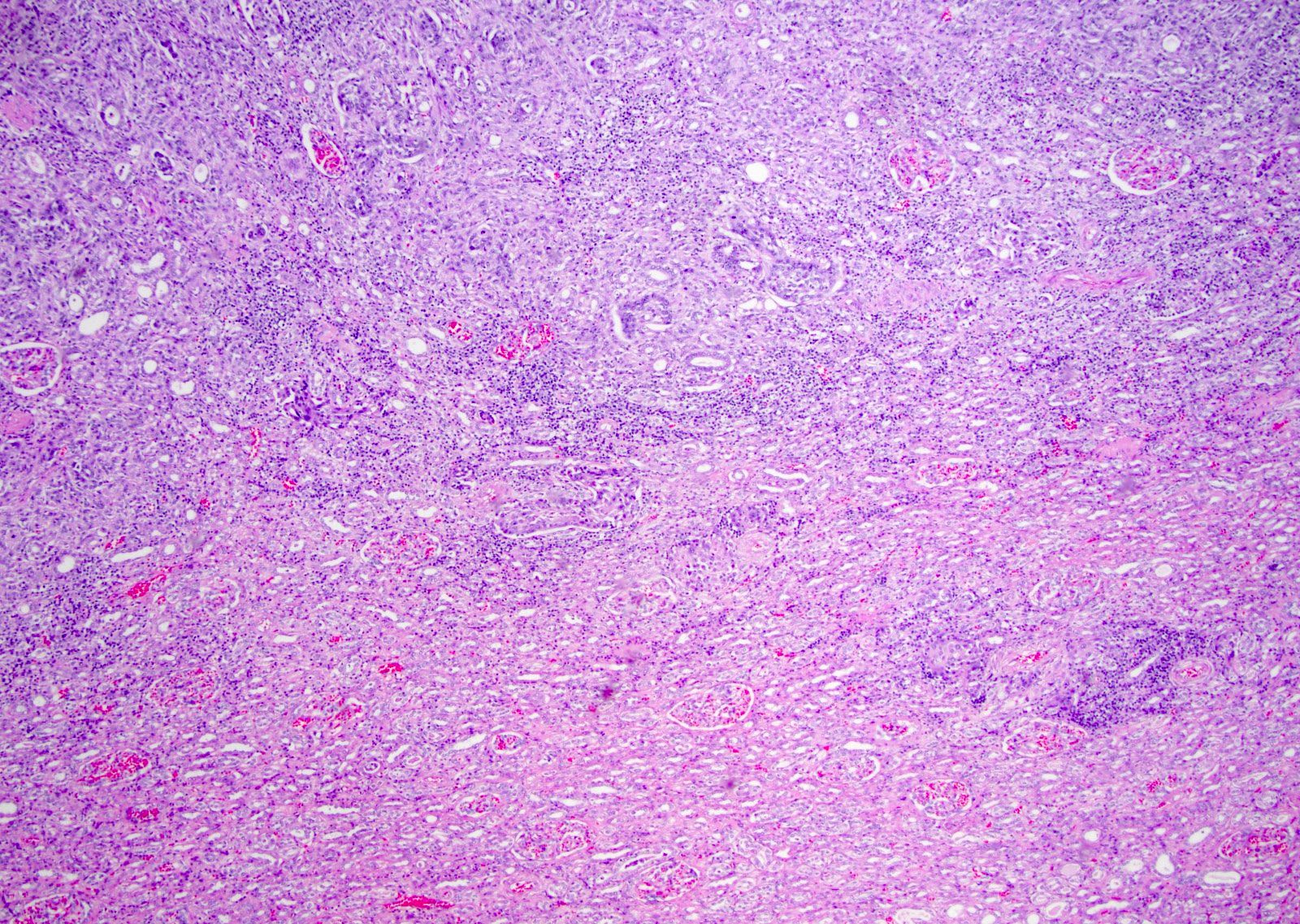
- Complex, infiltrative, poorly circumscribed, sometimes multinodular tumor
- Mainly interstitial growth patterns with preserved glomeruli
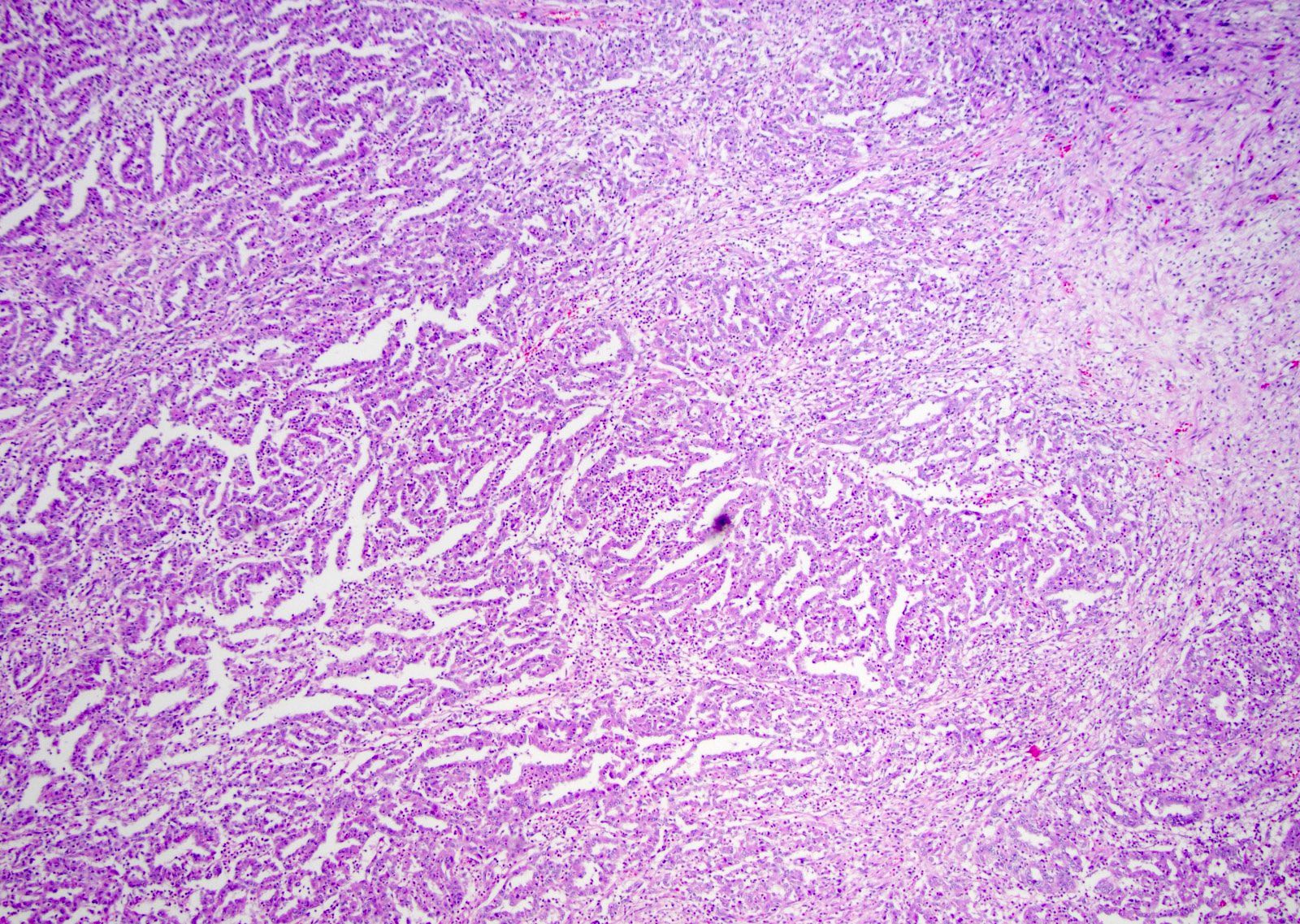
- Composed of cords, tubules or tubulopapillary structures with infiltrating glands embedded into inflamed desmoplastic stroma
- Solid, sheet-like growth, nested, cord-like, papillary, micropapillary growth or individual cells may be present
- Sieve-like / cribriform, intracystic papillary and reticular / yolk sac tumor-like patterns were identified as minor components
- Intracystic pattern demonstrated delicate fibrovascular cores and hyalinization present in 33% of cases in one study (Am J Surg Pathol 2018;42:279)
- Tubulocystic growth was not seen in any case in one study (Am J Surg Pathol 2018;42:279)
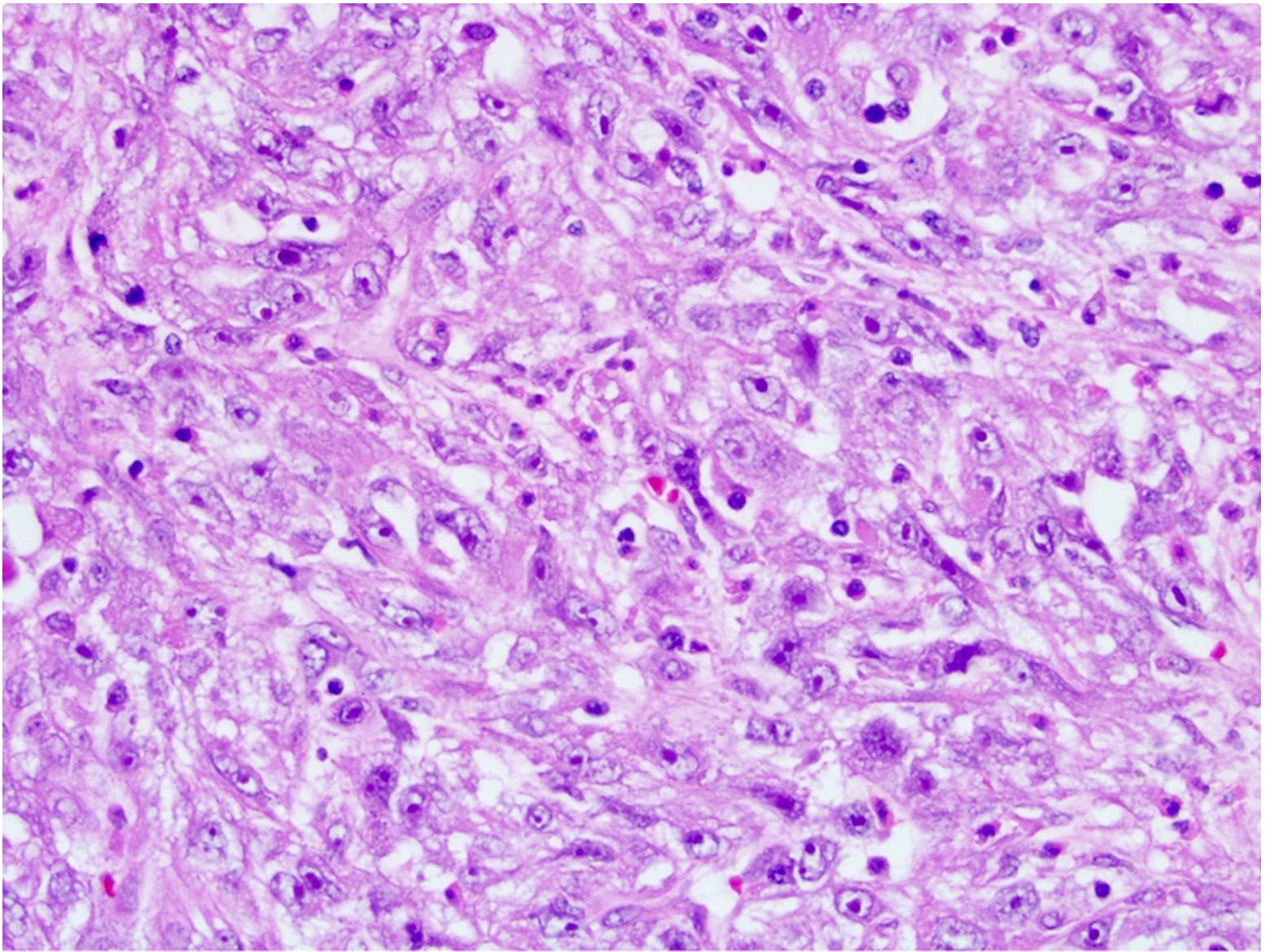
- Irregular channels are lined by high grade cuboidal to hobnail cells, usually with eosinophilic cytoplasm
- Nuclei are large, pleomorphic with prominent nucleoli and coarse chromatin
- Numerous mitotic figures; apoptotic cells and necrosis is common
- Intracytoplasmic and intraluminal mucin may be present
- May have microcystic changes from dilation of the tubular structures
- May have sarcomatoid differentiation
- Adjacent to the tumor, tubular epithelium lining collecting ducts may appear dysplastic
- Lymphovascular invasion and regional lymph node involvement are common
- Often prominent inflammatory reaction, including neutrophils, within and around the tumor
- Reference: Am J Surg Pathol 2018;42:279
Microscopic (histologic) images
Contributed by Daniel Anderson, M.D., M.B.A. and Garrison F. Pease, M.D.
Virtual slides
Cytology description
- Aspirate may contain cohesive nests of tumor cells with glandular features or individual cells
- Eosinophilic, vacuolated cells with intracytoplasmic mucin; nuclei are large irregular hyperchromatic with vesicular chromatin and large nucleoli
- Ductal / tubular differentiation with benign, dysplastic and malignant features, prominent desmoplastic stroma, neutrophils (Acta Cytol 2004;48:843)
- Sarcomatoid features may be seen (Diagn Cytopathol 2010;38:603)
- Cases of collecting duct carcinoma cells in urine cytology have been reported (Diagn Cytopathol 1997;16:446)
Positive stains
- PAX8 and PAX2 (Am J Surg Pathol 2010;34:965)
- High molecular weight cytokeratin (34 beta E12) and CK7
- Ulex europaeus lectins (Ulex1), peanut lectin agglutinin (PNA), mucin (strong)
- Vimentin, CK8/18, CK19, EMA, S100A1
- Usually SMARCB1 / INI1 / BAF47 retained (Urology 2011;78:474.e1)
- Positive (retained) fumarate hydratase
Negative stains
- p63, uroplakin II, GATA3 (Am J Surg Pathol 2010;34:965)
- Usually negative for CD10, AMACR, E-cadherin, CAIX, OCT 3/4, CD117
Electron microscopy description
- Features of adenocarcinoma, including intracellular and extracellular lumina
- Well formed cell junctions, prominent basal lamina and short apical microvilli
Molecular / cytogenetics description
- A defining set of molecular alterations is not currently recognized
- Not associated with loss of 3p
- Not associated with trisomies 7 and 17
- Complex chromosomal aberrations, especially copy number losses or loss of heterozygosity involving multiple chromosomes, such as 1p, 6, 8, 9, 14 and 22 (WHO 5th edition)
- HER2 / neu amplifications have been reported in 45% of cases (J Urol 1997;158:245)
- Genomic alterations in NF2 (29%), SETD2 (24%), SMARCB1 (18%) and CDKN2A (12%) (Eur Urol 2016;70:516)
- Whole exome sequencing and transcriptome sequencing (RNASeq) showed recurrent somatic single nucleotide variants (SNV) in MLL in 2 samples; somatically mutated SNVs identified in ATM, CREBBP, PRDM1, CBFB, FBXW7, IKZF1, KDR, KRAS, NACA, NF2, NUP98, SS18, TP53 and ZNF521
- Single nucleotide polymorphism (SNP) array identified a CDKN2A homozygous deletion and SNV analysis showed a nonsense mutation of the CDKN2A gene with unknown somatic status
- Dysregulation in solute carrier family genes, including overexpression in SLC7A11 (cystine transporter, xCT), a cisplatin resistance associated gene (Oncotarget 2016;7:29901)
- Other gene expression study showed downregulation in 4 solute carrier genes: SLC3A1, SLC9A3, SLC26A7 and SLC47A1; also upregulated keratin 17 and downregulation MARC2, cubulin (Cancers (Basel) 2019;12:64)
- RNA sequencing study found shift towards aerobic glycolysis and overexpression of immune genes (Sci Rep 2016;6:30988, Cancers (Basel) 2021;13:2903)
- Molecularly heterogeneous tumor with at least 2 subtypes distinguished by cell signaling and metabolic and immune related alterations (Cancers (Basel) 2021;13:2903, Cancers (Basel) 2021;13:3807)
- Whole genome sequencing study of a Chinese population showed 8 recurrently somatically mutated genes: BM14, MTUS1, GAK, DST, ASPM, CDC27, RNF213 and XIRP2
- TP53 / RB1 and CDKN2A alterations documented
- Copy number variant mutation spectrum appeared unique from urothelial carcinoma and other renal cell carcinoma subtypes
- CDKN2A altered patients displayed a worse overall survival (OS) (BMC Med Genomics 2022;15:1)
Videos
Collecting duct carcinoma
Sample pathology report
- Kidney, nephrectomy:
- Collecting duct carcinoma (X cm); tumor invades perirenal fat and renal sinus fat; margins are negative (see synoptic report and comment)
- Comment: Immunohistochemistry is performed. The carcinoma cells are positive for PAX8, 34 beta E12, fumarate hydratase (retained), SMARCB1 / INI1 (retained) and negative for GATA3 and OCT 3/4.
Differential diagnosis
- Metastatic carcinoma, including from GI or lung:
- Usually, well defined borders; multiple foci may be present
- Clinical history and radiologic review could be helpful
- Immunohistochemistry may be helpful in excluding a metastasis
- Urothelial carcinoma showing glandular differentiation:
- Usually associated with prior history of lower tract urothelial carcinoma
- Filling defect on imaging, positive urine cytology
- If located in renal calyces or pelvis it would favor urothelial carcinoma; however, involvement of cortex is found in higher stage disease
- Location of in situ urothelial carcinoma or residual papillary component is helpful
- Carcinoma in situ (CIS) may be present in renal pelvis
- CIS can colonize the collecting ducts with solid nests as opposed to dysplastic glandular cells in collected duct carcinoma
- Careful sampling of renal pelvis recommended
- Usually, lower stage disease at presentation
- Urothelial carcinoma typically p63+ / GATA3+ / uroplakin II+ and negative for PAX8 (Am J Surg Pathol 2010;34:965, Hum Pathol 2013;44:2651)
- Fumarate hydratase (FH) deficient renal cell carcinoma / hereditary leiomyomatosis RCC:
- Often viral inclusion-like (CMV-like) macronucleoli with perinucleolar halos are seen
- Tubulocystic and intracystic papillary with or without hyalinization patterns may favor FH deficient RCC (Am J Surg Pathol 2018;42:279)
- Loss of fumarate hydratase, nuclear and cytoplasmic expression of 2SC
- In one study, 25% of cases diagnosed previously as collecting duct carcinoma were reclassified as fumarate hydratase deficient renal cell carcinoma using fumarate hydratase and 2SC IHC (Am J Surg Pathol 2018;42:279)
- Papillary renal cell carcinoma:
- More well circumscribed, predominantly papillary, often psammoma bodies and foamy macrophages within papillae; usually no angiolymphatic invasion, no desmoplasia or inflammation, no dysplasia of collecting duct epithelium
- CEA-, HMWCK-, Ulex europaeus-, AMACR+, CD10+, trisomy 7 and 17 (Hum Pathol 2008;39:1350)
- SMARCB1 deficient renal medullary carcinoma (Am J Surg Pathol 2014;38:871):
- Occurs in those with sickle trait or related hemoglobinopathies; sickled RBCs (drepanocytes) may be found within tumor (Transl Androl Urol 2021;10:1506)
- Distinction from collecting duct not possible on morphologic grounds alone
- Sieve-like / cribriform and reticular / yolk sac tumor-like patterns favor renal medullary (Am J Surg Pathol 2018;42:279)
- Both positive for PAX8; p63 and GATA3 usually negative
- Inactivation of tumor suppressor gene INI1 on long arm of chromosome 22 is frequently detected
- INI1 negative (loss) and OCT 3/4 positive in 50% of cases (Urology 2011;78:474.e1, Histopathology 2012;61:428, Am J Surg Pathol 2018;42:279)
- Occurs in those with sickle trait or related hemoglobinopathies; sickled RBCs (drepanocytes) may be found within tumor (Transl Androl Urol 2021;10:1506)
- Mucinous tubular spindle cell carcinoma with high grade transformation
- Tubulocystic carcinoma:
- Often detected incidentally and usually without pain, hematuria, flank mass, etc.
- Cortical with involvement of medullary region at times
- Well circumscribed with multilocular sponge-like appearance without necrosis or hemorrhage (versus more solid appearance of collecting duct)
- Consists of tubules and variable sized cysts; should not display infiltrative glands, cords, solid sheets, necrosis, hemorrhage and desmoplastic stroma that is relatively common in collecting duct carcinoma
- Less mitotically active
- Will not have dysplastic medullary collecting duct structures or extension into perirenal adipose tissue
- If the size of the tumor obscures the site of origin, infiltrative growth that extends between normal structures, such as glomeruli and tubules, can be seen as a clue that the tumor is medullary based
- This pattern can be seen in collecting duct carcinoma, medullary carcinoma and invasive urothelial carcinoma; similar patterns can also be seen in metastatic tumors and lymphoma (Surg Pathol Clin 2015;8:587)
Additional references
Board review style question #1
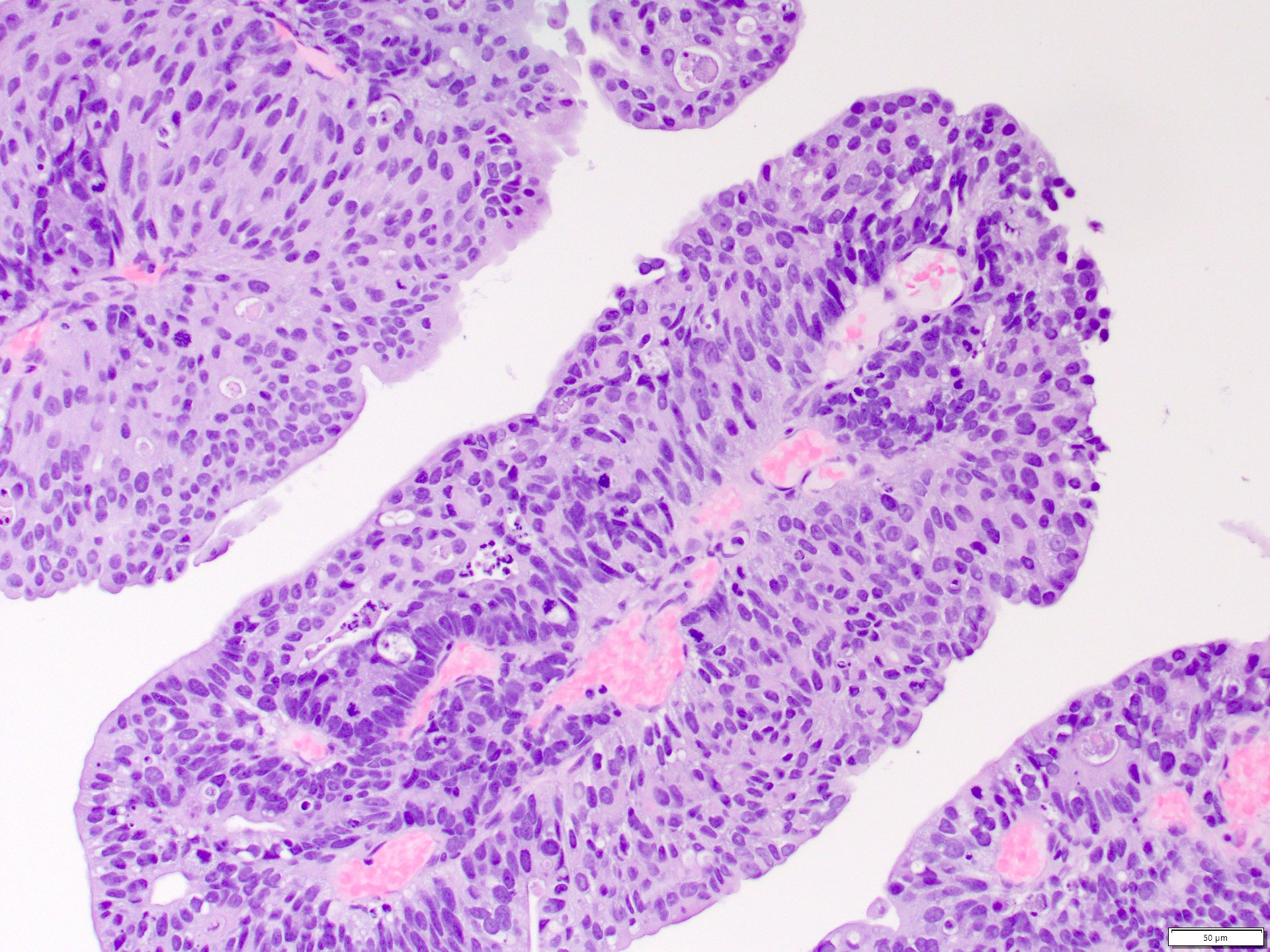
A 76 year old man with history of noninvasive low grade papillary urothelial carcinoma undergoes a nephrectomy for a 5 cm centrally located urothelial mass involving the renal pelvis, medulla and cortex. Photomicrographs are shown of the invasive component and results of renal pelvis sampling. Which of the following is true regarding this neoplasm?
- Has a multilocular, sponge-like cut surfaces on gross examination
- May show aneuploidy in chromosome 3, 7 or 17 or loss of 9p21 locus
- Will often demonstrate loss of fumarate hydratase by immunohistochemistry
- Will often show positive immunohistochemical expression for PAX8 and negative for p63 and GATA3
Board review style answer #1
B. May show aneuploidy in chromosome 3, 7 or 17 or loss of 9p21 locus. The findings support the diagnosis of invasive urothelial carcinoma with glandular differentiation. The clinical history and demonstration of a noninvasive high grade papillary component in the renal pelvis supports the diagnosis. Urothelial carcinoma often demonstrates aneuploidy in chromosomes 3, 7 or 17 or loss of 9p21 locus, which is the basis for the UroVysion FISH assay. The criteria for positivity on UroVysion is ≥ 4 of 25 morphologically abnormal cells showing a gain of ≥ 2 chromosomes in the same cell or ≥ 12 of the 25 cells having a loss of 9p21. Urothelial carcinomas will be positive for p63, GATA3 and negative for PAX8 (as opposed to renal cell carcinomas). They will demonstrate retained fumarate hydratase (as opposed to fumarate hydratase deficient RCC). A multilocular sponge-like cut surface on gross evaluation is characteristic of tubulocystic renal cell carcinoma.
Comment Here
Reference: Collecting duct carcinoma
Comment Here
Reference: Collecting duct carcinoma
Board review style question #2
A 59 year old man is diagnosed with collecting duct carcinoma. Which of the following is true regarding this entity?
- Has no morphologic overlap with fumarate hydratase deficient
- Has prominent stromal desmoplasia, infiltrative growth and inflammatory cell reaction
- Is correlated with sickle cell trait
- Will show papillary growth with foamy macrophages in papillae
Board review style answer #2
B. Has prominent stromal desmoplasia, infiltrative growth and inflammatory cell reaction. Collecting duct carcinoma (CDC) often presents with advanced disease over a wide age range and with male predominance. The carcinoma arises from the principal cells of the distal collecting ducts of Bellini and often shows a multinodular, infiltrative growth pattern of cords, tubules, tubulopapillary, nested, solid or sheet-like growth with high grade nuclei and associated inflammatory reaction. The presence of papillary growth in CDC can raise the differential of papillary renal cell carcinoma; however, the latter will show typical papillary morphology, including foamy macrophages within papillae. CDC has morphologic overlap with fumarate hydratase deficient renal cell carcinoma. In one study, 25% of cases of CDC were reclassified to fumarate hydratase deficient renal cell carcinoma using fumarate hydratase and 2SC IHC stains. CDC is morphologically similar to medullary carcinoma. Renal medullary carcinoma usually presents in younger patients with sickle cell trait or hemoglobin SC disease; few have sickle cell disease. Renal medullary carcinoma usually demonstrates loss of SMARCB1 (also known as INI1, SNF5 or BAF47).
Comment Here
Reference: Collecting duct carcinoma
Comment Here
Reference: Collecting duct carcinoma