Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Erem AS, Turashvili G. Melanoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/vulvamelanoma.html. Accessed April 18th, 2024.
Definition / general
- Primary malignant melanocytic tumor
Essential features
- Malignant melanocytic tumor arising from melanocytes in mucosal or cutaneous areas of the lower genital tract
- Tumor thickness is the most important prognostic factor
Terminology
- Malignant melanoma (MM)
- Primary malignant melanoma of vulva
- Primary malignant melanoma of vagina
- Primary malignant melanoma of lower genital tract
ICD coding
- ICD-O
- ICD-10
- ICD-11
- 2C70 - malignant neoplasm of the vulva
- Specific anatomy (use additional code, if desired)
- 2C71 - malignant neoplasms of vagina
- Specific anatomy (use additional code, if desired)
- 2C7Y - other specified malignant neoplasms of female genital organs
- 2C7Z - malignant neoplasms of female genital organs, unspecified
Epidemiology
- Accounts for < 5% of all vulvar and vaginal malignancies (Lancet Oncol 2008;9:973)
- 200 of 100,000 (0.2%) women per year will be diagnosed with vulvar melanoma (Obstet Gynecol 2007;110:296, Gynecol Oncol 2011;122:612)
- Difference in incidence of vulvar and vaginal melanomas among ethnic groups is significantly smaller than in cutaneous melanomas of other sites (Melanoma Res 2010;20:153)
- Vulvar melanoma
- Although vulvar melanomas are rare, accounting for 1 - 2% of all melanomas, they represent the most common primary genitourinary (GU) melanomas and primary gynecologic melanomas (J Natl Compr Canc Netw 2016;14:450, Case Rep Oncol 2021;14:1144, J Am Acad Dermatol 2014;71:1241, J Am Acad Dermatol 2016;75:144, Urol Oncol 2016;34:166.e7)
- Review of the Survival Epidemiology and End Results (SEER) database between 1973 - 2010 indicates that 75% of primary GU melanomas are vulvar and 25% are vaginal (Urol Oncol 2016;34:166.e7)
- Melanoma is the second most common vulvar malignancy following squamous cell carcinoma (Am J Surg Pathol 2002;26:1450, Lancet Oncol 2008;9:973, Obstet Gynecol 2007;110:296)
- Occurs predominantly in postmenopausal White patients in the fifth to eighth decades (Obstet Gynecol 2007;110:296, J Am Acad Dermatol 2014;71:1241)
- Association with lichen sclerosus has been reported in the pediatric population (Cancers (Basel) 2022;14:5217, Pediatr Dermatol 2016;33:e190, Pediatr Dermatol 2004;21:473, Am J Dermatopathol 1984:6:253, Arch Dermatol 1997;133:345)
- Although vulvar melanomas are rare, accounting for 1 - 2% of all melanomas, they represent the most common primary genitourinary (GU) melanomas and primary gynecologic melanomas (J Natl Compr Canc Netw 2016;14:450, Case Rep Oncol 2021;14:1144, J Am Acad Dermatol 2014;71:1241, J Am Acad Dermatol 2016;75:144, Urol Oncol 2016;34:166.e7)
- Vaginal melanoma: occurs in postmenopausal women in their fifth to seventh decades (J Gynecol Oncol 2013;24:330, Int J Gynecol Cancer 2014;24:149)
Sites
- Vulvar melanoma
- Labia majora and labia minora > periclitoral / clitoris > midline structures (e.g., periurethral, introitus and posterior fourchette) (Ann Surg Oncol 2015;22:1959)
- Most common: glabrous (hairless, mucosal) site in about 50% of cases, followed by hairy - glabrous skin junction (38%) and hairy skin (13%) (Acta Oncol 2004;43:421)
- Vaginal melanoma
- Most common: lower third of vagina, in the anterior wall (J Cancer Res Ther 2019;15:1392)
Pathophysiology
- Pathogenesis of all subtypes is poorly understood (Biomedicines 2021;9:758)
- Vulvar melanoma (sun protected cutaneous melanomas, majority originating in mucosal epithelium)
- Similar pathway to cutaneous melanomas arising in sun protected areas (e.g., acral and subungual melanomas)
- It has been hypothesized that a single melanocyte can act as a spontaneous promoter
- Vitamin D receptor (VDR) expression has been implicated (Am J Cancer Res 2020;10:4017)
- Molecular profile differs from cutaneous melanomas (J Low Genit Tract Dis 2023;27:40)
- Rate of KIT and NRAS mutations is higher than in cutaneous melanomas
- Both KIT and NRAS are upstream to BRAF and part of Ras / Raf / MEK / ERK pathway
- BRAF is relatively rare (< 10%) (J Low Genit Tract Dis 2015;19:350)
- Vaginal melanomas (J Low Genit Tract Dis 2023;27:40)
- Although the data on strictly vaginal melanomas are limited and typically grouped with vulvar melanomas, KIT mutations appear to play a role in pathogenesis (Melanoma Res 2014;24:360, Int J Gynecol Pathol 2020;39:587, Br J Dermatol 2017;177:1376)
Etiology
- Unlike cutaneous melanomas, ultraviolet (UV) radiation exposure does not appear to impact disease onset (J Am Acad Dermatol 2014;71:366, J Cutan Pathol 2005;32:191)
- HPV infection may play a role (Oncologist 2021;26:e473, Am J Dermatopathol 2007;29:13)
Diagrams / tables
Clinical features
- Clinical presentation is often delayed due to a variety of symptoms and rare self inspection (Gynecol Oncol 2021;161:202)
- Symptoms are nonspecific (Cancers (Basel) 2022;14:5217, Asian J Urol 2022;9:407, Gynecol Oncol 2021;161:202, Obstet Gynecol 2007;110:296)
- Early symptoms: pruritis, spontaneous bleeding or atypical discharge
- May present as a distinct mass, swelling or lump sensation
- Lymphadenopathy in advanced cases
- May be asymptomatic (J Low Genit Tract Dis 2016;20:e24, Melanoma Res 2008;18:127)
- On physical examination (Cancers (Basel) 2022;14:5217, Asian J Urol 2022;9:407, Gynecol Oncol 2021;161:202, Obstet Gynecol 2007;110:296)
- ABCD: asymmetry, border irregularity, color variation / discoloration, diameter (concerning, > 6 mm)
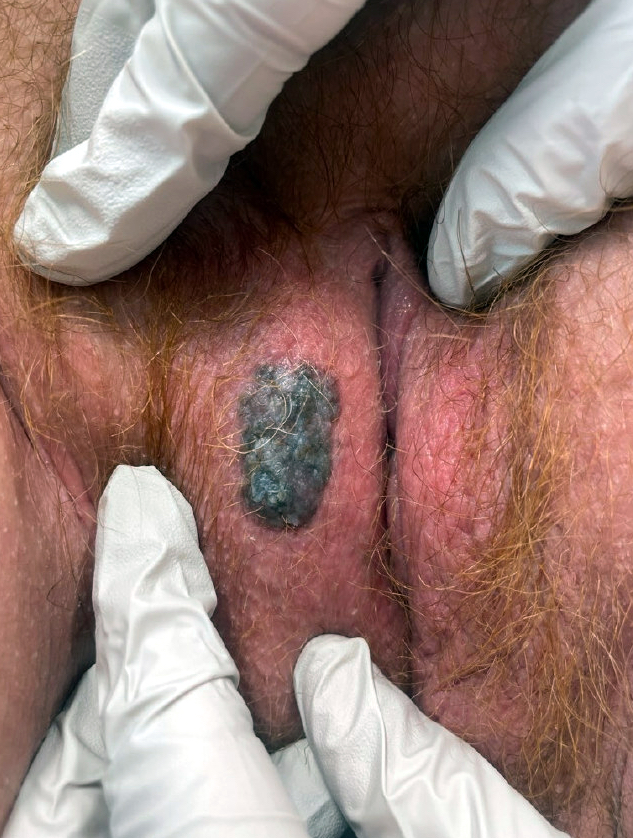
- 25 - 30% of cases are amelanotic, particularly on mucosal surfaces where melanoma may appear as a nonpigmented red polyp (J Am Acad Dermatol 2004;50:554, Dermatol Ther 2010;23:449, Melanoma Res 2008;18:127)
- May be multifocal (Dermatol Ther 2010;23:449)
- See table 1 and table 2
Table 1: Clinical and histologic differences between atypical genital nevus and vulvar melanoma (adapted from Hoang: Melanocytic Lesions - A Case Based Approach, 2014)
| Proposed diagnosis | Atypical genital nevus of special anatomic site | Vulvar melanoma |
| Age | Premenopausal, young adult | Postmenopausal |
| Size | < 1 cm | > 1 cm |
| Delineation | Well circumscribed | Infiltrative |
| Symmetry | Present | Absent |
| Lateral extension of junctional component | Focal | Present |
| Lentiginous junctional component | Focal | Present |
| Junctional nests | Discohesive | Confluent |
| Retraction artefact | Present | Absent |
| Ulceration | Absent or due to trauma | Often present |
| Pagetoid upward spread | Focal, central, inconspicuous | Prominent |
| Cytologic atypia | Superficial, mild to moderate | Deep, moderate to severe |
| Dermal mitoses | Rare and superficial | Conspicuous, atypical, deep |
| Dermal maturation | Present | Absent |
| Melanin pigmentation | Coarse, uniform | Fine, irregular |
| Dermal fibrosis | Broad zone of superficial coarse dermal fibrosis | Regression type |
Table 2: Clinical differences in mucosal melanoma of the vulva and vagina (adapted from Ann Surg Oncol 2015;22:1959 and Am J Cancer Res 2020;10:4017)
| Proposed diagnosis | Vulvar melanoma | Vaginal melanoma |
| Frequency | 3 - 10% of vulvar tumors | 4 - 5% of vaginal tumors |
| Age | Postmenopausal (50 - 70 years) | Postmenopausal (50 - 70 years) |
| Location | Labia majora and labia minora > periclitoral / clitoris > midline structures (e.g., periurethral, introitus and posterior fourchette)* | Lower vagina |
| Presentation | Bleeding, abnormal discharge, pruritis or visible exophytic mass to the patient | Similar to vulvar melanoma; polypoid mass may be only seen on closer gynecologic examination |
| Prognosis | Poor; 5 year survival of 25 - 60% | Very poor; 5 year survival of < 20% |
| Histologic growth patterns | Lentiginous (most common) Superficial spreading (less common) Nodular and pagetoid (rare) | Nodular (most common) |
| Differential diagnosis | Poorly differentiated carcinoma Metastatic melanoma Melanocytic nevus Lymphoma Soft tissue neoplasm | Poorly differentiated carcinoma Metastatic melanoma Lymphoma Soft tissue neoplasm |
Diagnosis
- Dermoscopy for nonmodified mucous membranes is useful
- Dermoscopic evaluation aids in avoiding unnecessary biopsies (Arch Dermatol 2011;147:1181, Dermatology 2011;222:157)
- Combination of blue, gray or white color with structureless zones and atypical vascularization is highly predictive of melanoma (Arch Dermatol 2011;147:1181, Dermatology 2011;222:157)
- In the absence of melanoma specific dermoscopic features, shiny white streaks (SWS), inverse pigment network and vascular network in a symmetrical pattern can be seen in atypical melanocytic nevi of genital type (AMNGT) (Dermatology 2011;222:157, Dermatology 2010;221:55)
- Experienced dermatologist will take a biopsy of clinically or dermoscopically concerning lesions
- See table 3
- Histologic diagnosis is confirmatory
Table 3: Differential diagnosis of vulvar melanocytic nevi, melanosis and melanoma (adapted from J Am Acad Dermatol 2014;71:1241)
| Reassuring features suggestive of a benign process | Concerning features for possible malignancy | |
| Clinical |
|
|
| Dermoscopy |
|
|
| Reflectance confocal microscopy |
|
|
Laboratory
- Lactate dehydrogenase (LDH) has been suggested as a potential predictive and prognostic biomarker (Cell Mol Biol Lett 2020:25:35, Cancer Immunol Immunother 2014;63:449)
- This has not been widely adopted and may lack sensitivity and specificity depending on LDH isoform utilized
Prognostic factors
- Recurrence rate
- Overall recurrence rate of 60% (Gynecol Oncol 2021;161:202, J Low Genit Tract Dis 2016;20:e24, Int J Gynecol Cancer 2016;26:1307)
- Independent prognostic factor for local recurrence: tumor size (Int J Gynecol Cancer 2016;26:1307)
- Independent prognostic factor for distant recurrence: American Joint Committee on Cancer (AJCC) stage and lymph node involvement (Int J Gynecol Cancer 2016;26:1307, N Engl J Med 2017;376:2211, Ann Surg Oncol 2000;7:738)
- Survival (Cancers (Basel) 2022;14:5217)
- Clinical presentation
- Central vulvar involvement is associated with worse overall survival (OS) and increased lymph node involvement (Clin Cancer Res 2017;23:2093, Urol Oncol 2016;34:166.e7)
- Multifocal involvement is associated with worse overall survival (Urol Oncol 2016;34:166.e7)
- Histopathologic factors
- 5 year overall survival (5Y OS) is associated with number of mitoses and lymph node status (J Low Genit Tract Dis 2016;20:e24, Ann Surg Oncol 2000;7:738)
- Factors independently associated with overall survival and disease specific survival (DSS): tumor thickness (≤ 2 mm2 or > 2 mm2) and dermal mitotic rate (< 2 mm2 or ≥ 2 mm2) (Clin Cancer Res 2017;23:2093)
- Independent prognostic factor: tumor infiltrating lymphocytes (Cancer Med 2018;7:583, J Am Acad Dermatol 2017;76:264)
- Ulceration is associated with worse survival (Cancer Med 2018;7:583, J Am Acad Dermatol 2017;76:264)
- Lymphovascular invasion (LVI) is associated with positive sentinel lymph nodes (Cancer Med 2018;7:583, J Am Acad Dermatol 2017;76:264)
- Stage by AJCC 2017 (Am J Clin Dermatol 2021;22:639)
- 5 year disease specific survival (5Y DSS) decreased from 32.3% at stage I to 4.9% at stage IV
- 5 year overall survival decreased from 73.6% to 3.9%, at stage I to stage IV, respectively
- Other prognosticators
- Age is a widely accepted independent prognostic factor for 5 year overall survival, disease free survival (DFS) and overall survival (Obstet Gynecol 2007;110:296, Int J Gynecol Cancer 2013;23:1118, Lancet Oncol 2016;17:757, Clin Cancer Res 2017;23:2093, Urol Oncol 2016;34:166.e7)
- See table 4 and table 5
- Clinical presentation
Table 4: Revised 2017 AJCC TNM staging for melanoma
| Primary tumor (T) | ||
| TX | Primary tumor thickness cannot be assessed (i.e., diagnosis by curettage) | |
| T0 | No evidence of primary tumor (i.e., axillary metastases without known primary tumor) | |
| Tis | Intraepithelial (i.e., melanoma in situ) | |
| T1 | Tumor ≤ 1.0 mm thick, without or with ulceration | |
| T1a | ≤ 0.8 mm thick and Clark level II or III, without ulceration | |
| T1b | < 0.8 mm thick and Clark level IV or V or with ulceration 0.8 - 1.0 mm thick and Clark level IV or V, with or without ulceration | |
| T2 | Tumor 1.01 - 2.0 mm thick, without (T2a) or with ulceration (T2b) | |
| T3 | Tumor 2.01 - 4.0 mm thick, without (T3a) or with ulceration (T3b) | |
| T4 | Tumor > 4.0 mm thick without (T4a) or with (T4b) ulceration | |
| Regional lymph node (N) | ||
| Nx | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis to 1 lymph node or in transit, satellite or microsatellite metastases with no tumor involved nodes | |
| N1a | Clinically occult (i.e., detected by SLNB) | |
| N1b | Clinically apparent (i.e., macroscopic) | |
| N1c | In transit, satellite or microsatellite metastases with no tumor involved nodes | |
| N2 | Metastasis to 2 or 3 regional lymph nodes or in transit, satellite or microsatellite metastases with no tumor involved nodes | |
| N2a | Clinically occult (i.e., detected by SLNB) | |
| N2b | Clinically apparent (i.e., macroscopic) | |
| N2c | In transit, satellite or microsatellite metastases combine with 1 clinically occult or apparent | |
| N3 | Metastasis to 4 or more regional lymph nodes, matted lymph nodes or combination of in transit metastasis or satellite(s) and metastatic regional lymph node(s) | |
| N3a | Clinically occult (i.e., detected by SLNB) | |
| N3b | Clinically apparent (i.e., macroscopic) or presence of matted nodes | |
| N3c | In transit, satellite or microsatellite metastases combine with 2 or more clinically occult or clinically detected or presence of matted nodes | |
| Distant metastasis (M) | ||
| Mx | Distant metastasis cannot be assessed | |
| M0 | No distant metastasis | |
| M1 | Distant metastasis, LDH status (designated as 0 for not elevated and I for elevated level; no suffix is used if LDH is not recorded or is unspecified) | |
| M1a | Distant skin, subcutaneous or lymph node | |
| M1b | Lung | |
| M1c | All other non-central nervous system (CNS) visceral sites | |
| M1d | CNS | |
Table 5: 2017 eighth AJCC prognostic stage group for cutaneous melanoma
| Stage | T | N | M |
| 0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 |
| IB | T1b | N0 | M0 |
| T2a | |||
| IIA | T2b | N0 | M0 |
| T3a | |||
| IIB | T3b | N0 | M0 |
| T4a | |||
| IIC | T4b | N0 | M0 |
| IIIA | T1a, T1b | N1, N2a | M0 |
| T2a | |||
| IIIB | T0 | N1b, N1c | M0 |
| T1a / b - T2a | N1b / c, N2b | ||
| T2b / T3a | N1a - N2b | ||
| IIIC | T0 | N2b / c, N3b / c | M0 |
| T1a - T3a | N2c, N3 | ||
| T3b / T4a | Any N ≥ N1 | ||
| T4b | N1a - N2c | ||
| IIID | T4b | N3 | M0 |
| IV | Any T, Tis | Any N | M1 |
Case reports
- 18 year old Black woman with widely metastatic melanoma originating from a primary vulvar lesion (Pediatr Dermatol 2023;40:749)
- 47 year old woman with metastatic vulvar melanoma, harboring an exon 17 KIT mutation (Ther Adv Med Oncol 2020:12:1758835920946158)
- 47 year old woman with vulvar melanoma and a family history of cutaneous melanoma (Cancer Genet 2022:262:102)
- 51 year old woman with recurrent vulvar melanoma with neurofibromatosis and postmenopausal bleeding (BMJ Case Rep 2019;12:e224744)
- 54 year old gravida 2 para 0 woman with recurrent metastatic vaginal melanoma (Gynecol Oncol Rep 2019:30:100508)
- 69 year old woman with melanoma of the vulva treated with topical imiquimod (Gynecol Oncol Rep 2021:38:100875)
- 73 year old woman with metastatic vaginal mucosal melanoma (Gynecol Oncol 2021;161:645)
- 84 year old woman with primary vulvar melanoma and melanocytic metastatic tissue (Curr Oncol 2022;29:3130)
- 89 year old woman with primary amelanotic vaginal melanoma (Diagn Cytopathol 2019;47:230)
Treatment
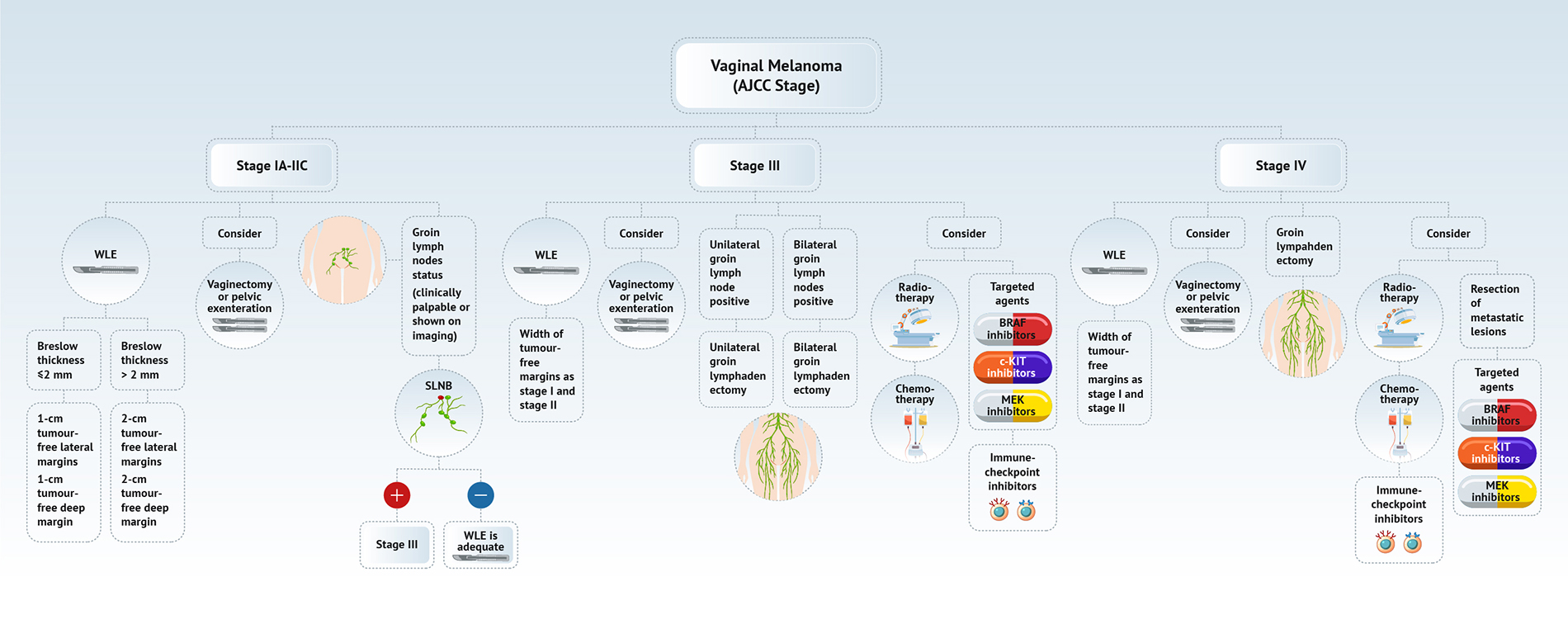
- Vulvar melanoma
- Clinical management is mainly based on cutaneous melanomas (Eur J Cancer 2017:81:36, Int J Gynecol Cancer 2014;24:S117, Cancers (Basel) 2022;14:5217)
- For localized disease: wide local excision (WLE) with adequate surgical margins
- Sentinel lymph node biopsy (SLNB) use is recommended (Cancers (Basel) 2022;14:5217)
- If there is clinical or radiologic evidence of positive lymph nodes
- If Breslow thickness is > 4 mm (J Obstet Gynaecol Can 2019;41:762, Eur J Cancer 2020:135:22, Int J Gynecol Cancer 2014;24:S117)
- Benefit of SLNB in intermediate thickness (1 - 4 mm) is unclear (Int J Gynecol Cancer 2019;29:1077)
- Bilateral SLNB is appropriate if the tumor is 2 cm from the midline (Int J Gynecol Cancer 2019;29:1077, Cancers (Basel) 2022;14:5217)
- If SLNB is positive, the benefit of complete groin dissection is currently unclear; it will be based on a multidisciplinary treatment plan and individual circumstances (Cancers (Basel) 2022;14:5217)
- DeCOG-SLT study suggested a complete groin dissection only for SLN metastases > 1 mm (Lancet Oncol 2016;17:757)
- In case of inguinofemoral metastases, an elective lymph node dissection (ELND) should be considered (Eur J Cancer 2020:135:22, Int J Gynecol Cancer 2014;24:S117)
- Data on adjuvant and neoadjuvant therapy in mucosal melanoma is based on retrospective case series, which makes the development of clinical guidelines challenging (Cancers (Basel) 2022;14:5217, Am J Cancer Res 2020;10:4017)
- Personalized therapy is based on the success of cutaneous melanoma treatment and is currently under investigation (Cancers (Basel) 2022;14:5217, Biomedicines 2021;9:758, Am J Cancer Res 2020;10:4017)
- Immunotherapy: programmed death 1 (PD-1), cytotoxic T lymphocyte antigen (CTLA4) checkpoint inhibitors show some success in locally advanced tumors and tumors with distant metastases; however, the clinical data is limited and the trials are ongoing (Immunotargets Ther 2018:7:35)
- Potential targeted therapy: BRAF, c-KIT, and MEK inhibitors with c-KIT show limited success in comparison to cutaneous melanoma (Ther Adv Med Oncol 2020:12:1758835920946158, N Engl J Med 2010;363:711, Br J Cancer 2010;102:1219)
- Chemotherapy: currently considered second line treatment (Cancers (Basel) 2022;14:5217, Am J Cancer Res 2020;10:4017)
- Radiation therapy plays a limited role and its utility remains to be investigated (Cancers (Basel) 2022;14:5217, Am J Cancer Res 2020;10:4017)
- See diagram 1 for a summary of vulvar melanoma management (Am J Cancer Res 2020;10:4017)
- Vaginal melanoma
- Complete local resection with adequate surgical margins (1 - 2 cm based on Breslow thickness) (Ann Surg Oncol 2004;11:34, Ann Surg Oncol 2002;9:840)
- Radical surgery alone or in combination with adjuvant radiotherapy can be considered in cases where complete resection is not feasible, the tumor is closely approaching resection margin, the tumor is large (> 3 cm) or the lymph nodes are positive (Ann Surg Oncol 2004;11:34, J Gynecol Oncol 2013;24:330)
- Groin or pelvic lymphadenectomy is recommended with evidence of positive lymph nodes on clinical or radiologic evaluation (Am J Cancer Res 2020;10:4017)
- SLNB utility is currently unclear (Am J Cancer Res 2020;10:4017)
- Role of chemotherapy remains to be determined (Am J Cancer Res 2020;10:4017)
- Personalized therapy may be considered with targetable mutations (Biomedicines 2021;9:758)
- Potential targeted therapy to be investigated: c-KIT, SF3B1, NRAS, BRAF
- PD-1 and CTLA4 checkpoint inhibitors have been utilized with mixed success and their utility in specifically vaginal melanoma remains to be determined (J Gynecol Oncol 2019;30:e94, J Low Genit Tract Dis 2021;25:146, Cancers (Basel) 2022;14:5217, J Eur Acad Dermatol Venereol 2018;32:e39)
- See diagram 2 for treatment strategies for vaginal melanoma (Am J Cancer Res 2020;10:4017)
- See table 6 for treatment strategies based on melanoma subtype
Table 6: Clinicopathologic correlates of melanoma in the lower genital tract (adapted from Am J Cancer Res 2020;10:4017)
| Subtype | Sun protected cutaneous vulvar melanoma | Mucosal melanoma (vulvar and vaginal) |
| Molecular alterations | BRAF, NRAS, TERT, CDKN2A, PTEN | KIT, NRAS, BRAF, NF1, CDKN2A, TERT, PTEN, SF3B1 |
| Metastatic pattern | Initially involving regional lymph nodes, distant metastases (lung, brain) at later stage | More likely to develop distant metastases (lung, liver, brain) |
| Surgical modality | Wide local excision with adequate margins | Complete excision |
| Lymph node assessment | Sentinel lymph node biopsy, regional lymphadenectomy if needed | Sentinel lymph node biopsy or routine regional lymphadenectomy not recommended |
| Systemic therapy | ||
| Chemotherapy | Interferon, dacarbazine, paclitaxel | Cisplatin, vinblastine, dacarbazine, interferon |
| Radiotherapy | Neoadjuvant or adjuvant | Palliative treatment of local or metastatic disease |
| Targeted therapy | BRAF and MEK inhibitors | BRAF and c-KIT inhibitors |
| Immunotherapy | Programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA4) checkpoint inhibitors | |
| Palliative therapy | Palliative surgery or radiotherapy | |
| Prognosis factors | AJCC staging, Breslow thickness, lymph node status, distant metastases | Breslow thickness, depth of invasion, lymph node status, distant metastases |
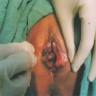
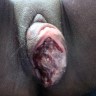
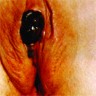
Clinical images
Gross description
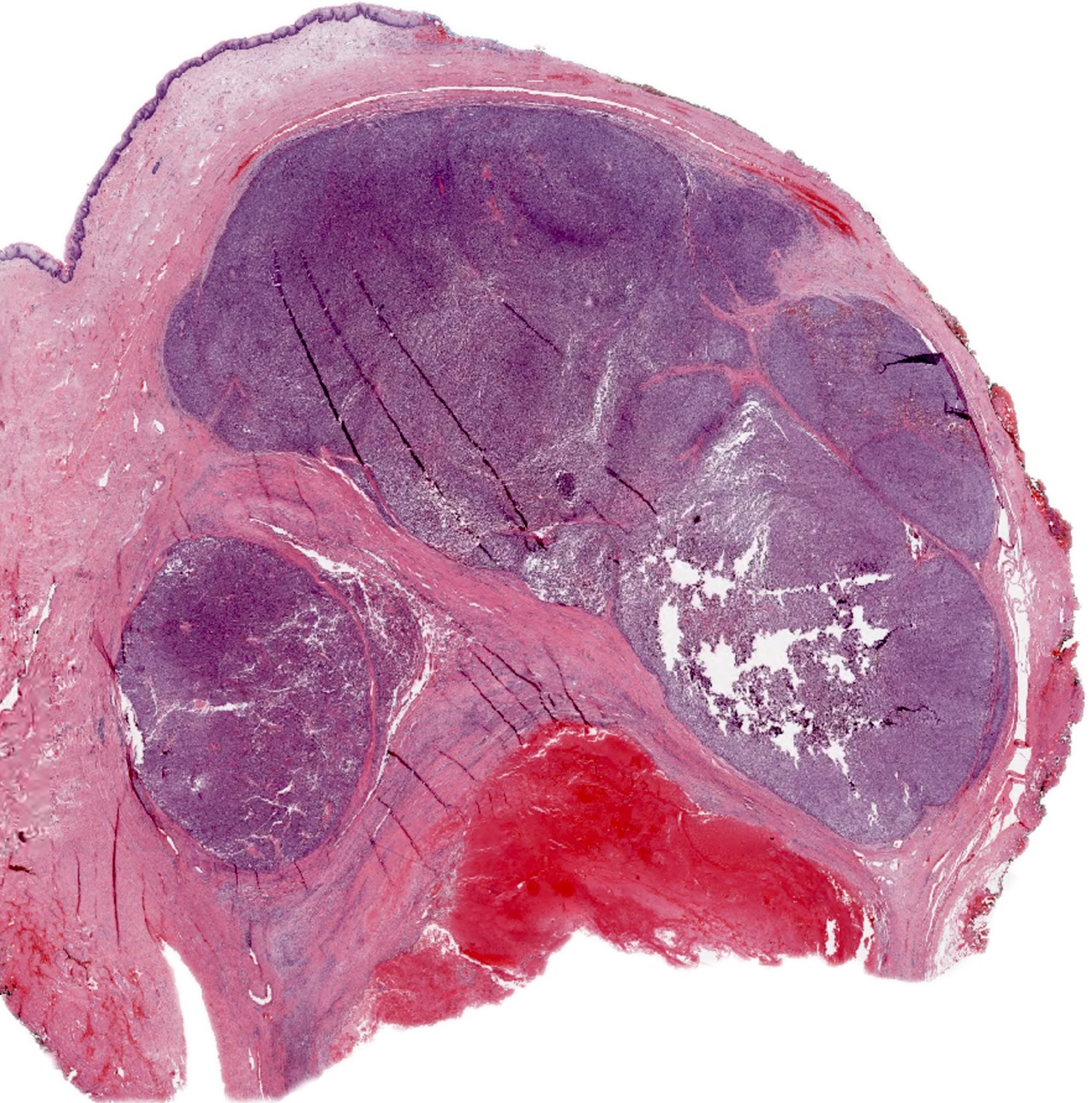
- Vulvar melanoma
- Usually presents as a pigmented plaque or amelanotic ulcerated nodule (Cancers (Basel) 2022;14:5217, Dermatol Ther 2010;23:449)
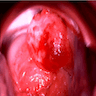
- Vaginal melanoma (Asian J Urol 2022;9:407)
- Usually presents as a large, ulcerated, nodular mass, often extending to the cervix
- Rarely, an associated nevus may be present
Gross images
Microscopic (histologic) description
- Vulvar melanoma
- Per AJCC and College of American Pathologists (CAP), vulvar melanoma are staged as primary cutaneous melanomas
- 25 - 30% of tumors are amelanotic (Cancer 1999;86:1273, J Am Acad Dermatol 2004;50:554, Dermatol Ther 2010;23:449, Melanoma Res 2008;18:127)
- Radial growth pattern (lentiginous / mucosal and superficial spreading) >> vertical growth pattern (nodular) > unclassified (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598)
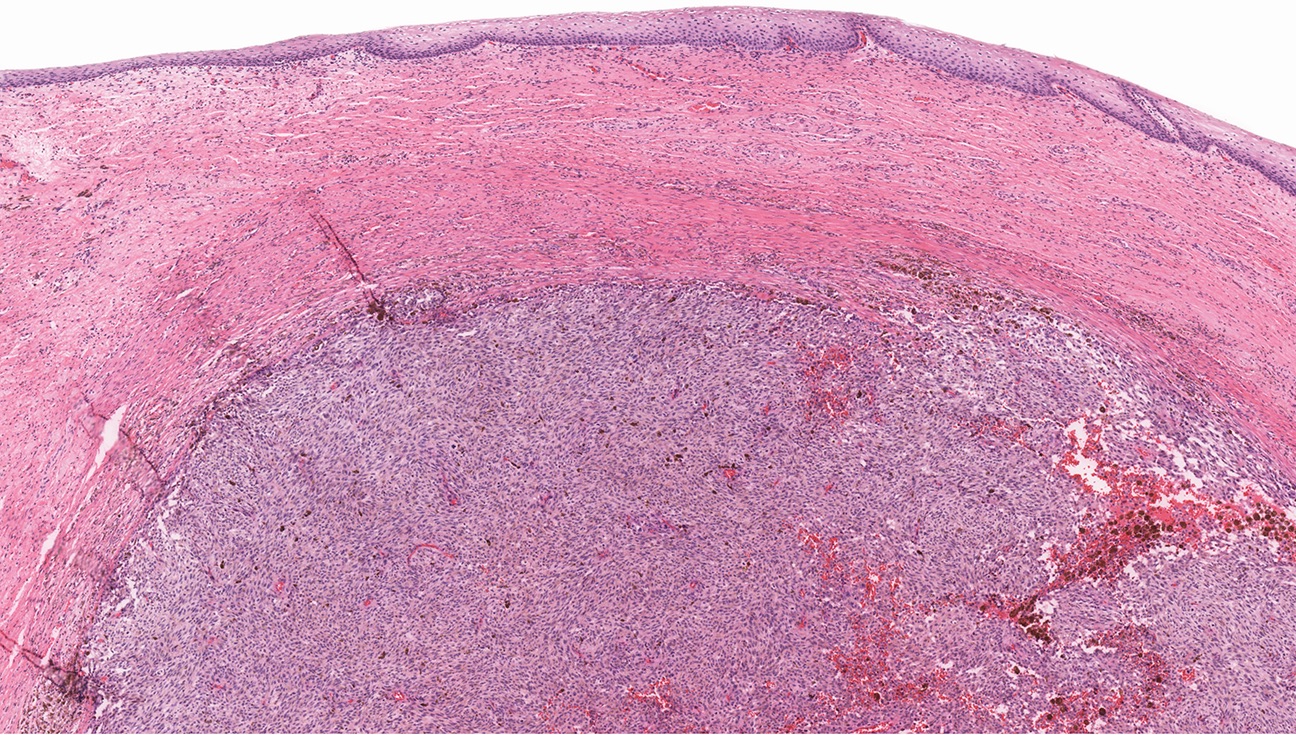
- Most tumors are asymmetric or ill defined lesions (Ann Diagn Pathol 2018:37:91)
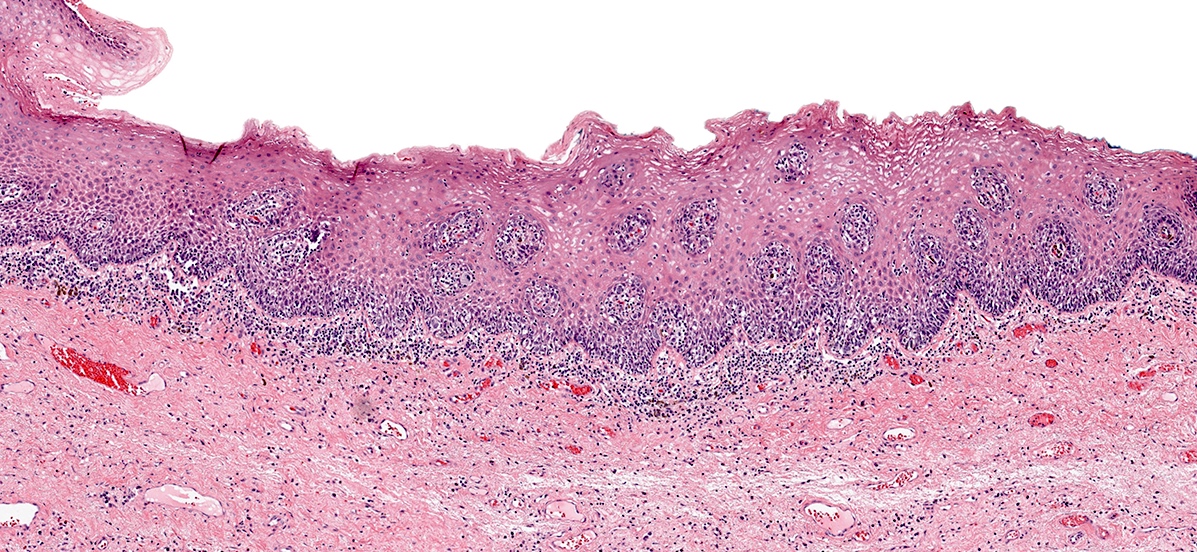
- Lentiginous pattern (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598, Ann Diagn Pathol 2018:37:91)
- Solitary units of melanocytes with nuclear atypia irregularly distributed along the dermoepidermal junction (DEJ)
- Occasional nests with skip areas and consumption of the rete ridges
- Adnexal involvement by isolated melanocytes or small nests is common
- Follicular extension along the epithelial stromal junction may be evident
- Overlying epidermis may resemble lichen planus with
- Hyperkeratosis and hypergranulosis
- Focal epidermal atrophy
- Dense lichenoid infiltrate
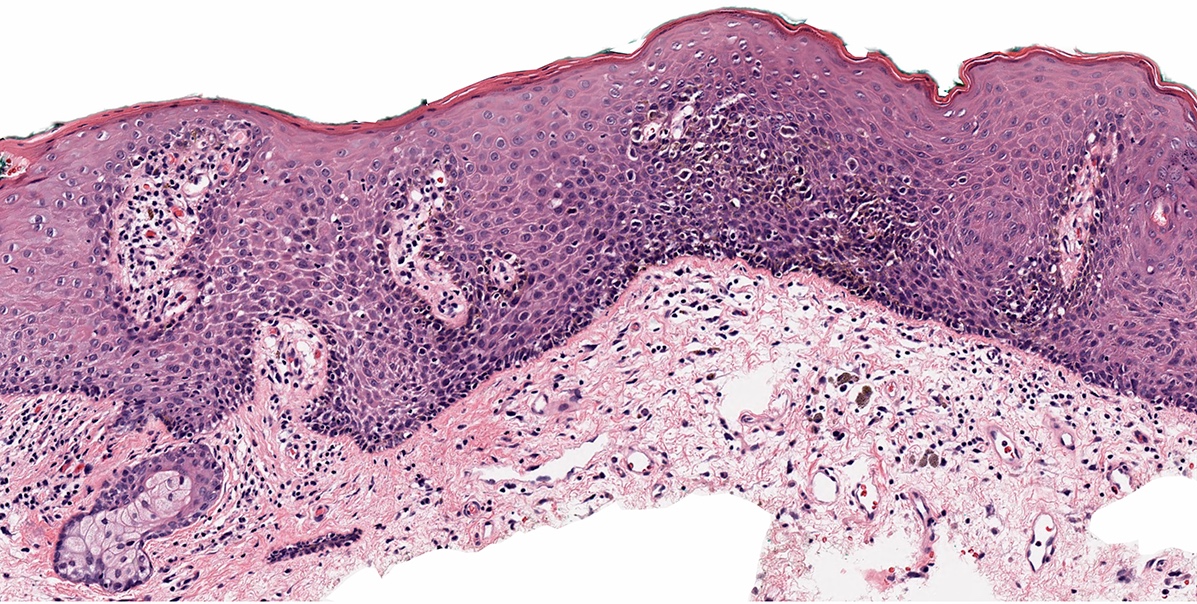
- Superficial spreading type (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598, Ann Diagn Pathol 2018:37:91)
- In situ component shows isolated cells and nests of atypical epithelioid melanocytes spreading into spinus layer in a pagetoid pattern
- Variable pagetoid patterns (J Am Acad Dermatol 2012;67:598)
- Amelanotic pagetoid melanoma in situ may closely resemble extramammary Paget disease and Bowen disease
- Immunohistochemical studies will support a definitive diagnosis
- Epithelioid morphology is most common, followed by mixture of epithelioid and spindle cells, although pure spindle cell melanomas have been described (Am J Surg Pathol 2004;28:1518)
- Effacement of dermal rete with moth eaten appearance
- Dermal component shows no maturation with severely atypical epithelioid or spindle cells with brisk mitoses forming sheets, nests, cords, single cells and rarely fascicles (Cancer 1999;86:1273, J Am Acad Dermatol 2012;67:598, Ann Diagn Pathol 2018:37:91)
- Nodular subtype (J Am Acad Dermatol 2013;68:568)
- In situ component should not extend beyond the periphery of the invasive tumor nodule; therefore, caution should be exercised in small biopsies
- Excisional specimens are the most helpful to examine the entire epidermis adjacent the nodular component
- May display a wide variation of architectural and cytologic features
- Usually polypoid / exophytic but can present with endophytic / infiltrative growth
- Nests with a mixture of fascicles and high cell density with solid growth pattern
- Ulceration, necrosis and LVI are common
- Desmoplastic (very rare but can be a diagnostic pitfall) (Pathology 1997;29:241, Gynecol Oncol 1997;64:180, Pathologica 2011;103:337)
- May induce an extensive desmoplastic reaction (Am J Surg Pathol 2004;28:1518, Ann Diagn Pathol 2018:37:91)
- Typically paucicellular with mild cytologic atypia and low mitotic activity
- Pure desmoplastic melanoma contains single spindle cells arranged in small nests or short fascicles embedded in the desmoplastic stroma
- Mixed / combined desmoplastic melanoma contains an additional epithelioid or spindled invasive dermal component (Ann Diagn Pathol 2018:37:91, Pathologica 2011;103:337)
- Peripheral lymphoid aggregates on low power are a soft sign of desmoplastic melanoma (Ann Diagn Pathol 2018:37:91)
- Perineural invasion is more common (Am J Surg Pathol 2004;28:1518, Ann Diagn Pathol 2018:37:91)
- Unless it is paucicellular (early onset) of lentiginous pattern, vulvar melanoma is usually associated with variable lymphocytic (typically lymphoplasmacytic) infiltrate
- In the absence of malignant cells, dermal fibrosis, increased vascularity and lymphocytic infiltrate are indicative of regression
- See table 7
Table 7: AJCC versus CAP features recommended for histopathologic assessment of primary vulvar melanomas (adapted from CAP: Protocol for the Examination of Specimens From Patients With Melanoma of the Skin [Accessed 30 November 2023])
| Histopathologic feature | AJCC recommended | CAP recommended |
| Histologic type | Yes | |
| Clarka / anatomicb level | ||
| Breslowa / tumorb thickness | Yes | Yes |
| Radial (nontumorigenic) growth phase | ||
| Vertical (tumorigenic) growth phase | ||
| Mitotic rate (number/mm2) | Yes | Yes |
| Ulceration | Yes | Yes |
| Regression | ||
| Lymphovascular invasion | Yes | |
| Perineural invasion | ||
| Microscopic satellitosis | Yes | Yes |
| Tumor infiltrating lymphocytes | ||
| Associated precursor melanocytic nevus | ||
| Predominant cytology | ||
| Margins | Yes |
b Used in evaluation of primary melanomas of mucosal origin
Table 8: Clark and Chung classifications comparative aspects (adapted from Biomedicines 2021;9:758, Ann Surg 1970;172:902, Cancer Res 1969;29:705, Obstet Gynecol 1975;45:638)
| Level of invasion | Clark classification Level of invasion of cutaneous melanoma | Chung classification Level of invasion of mucosal melanoma |
| I | Lesions involving only the epidermis (in situ melanoma) | Tumor confined to the epithelium (in situ melanoma) |
| II | Invasion of the papillary dermis, does not reach the papillary - reticular dermal interface | Invasion into the basement membrane to a depth of ≤ 1 mm |
| III | Invasion fills and expands the papillary dermis, does not extend to reticular dermis | Invasion to a depth of 1 - 2 mm |
| IV | Invasion into the reticular dermis, does not extend to the subcutaneous tissue | Invasion to a depth of > 2 mm, does not extend to the subcutaneous fat |
| V | Invasion through the reticular dermis into the subcutaneous tissue | Tumor penetrates the subcutaneous fat |
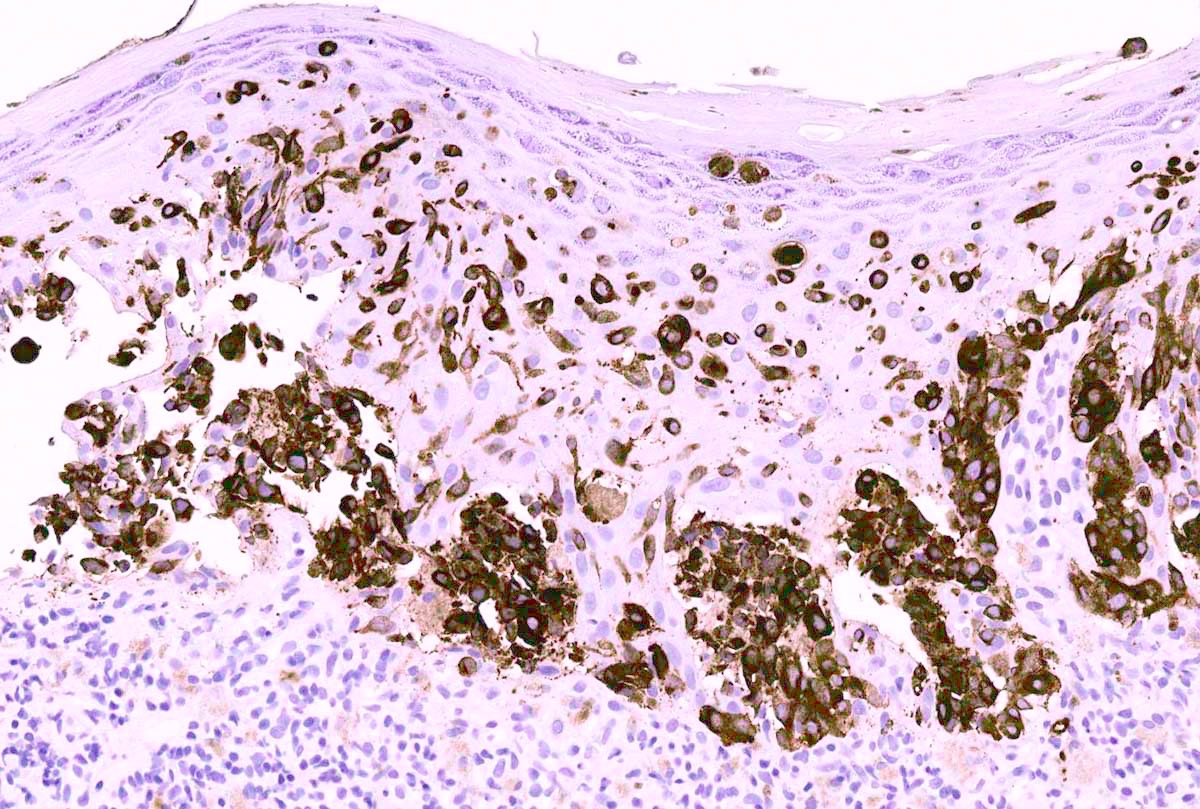
Microscopic (histologic) images
Contributed by Anna Sarah Erem, M.D., Gulisa Turashvili, M.D., Ph.D. and Priya Nagarajan, M.D., Ph.D.
Positive stains
- S100, SOX10 and nerve growth factor receptor (NGFR) are the most sensitive markers to visualize invasive melanoma
- Melanocytic markers
- MelanA (MART1) helps to highlight invasion and intraepidermal melanocytes (J Cutan Pathol 2020;47:446)
- Caution: staining of dendritic processes from melanocytes wrapped around keratinocytes may lead to overestimation and false positive interpretation
- HMB45 (Ann Diagn Pathol 2023:67:152211, Biomed Rep 2016;5:327, J Am Acad Dermatol 2012;67:446)
- Homogenous expression in primary conventional melanomas, especially epithelioid
- Absence of gradient or maturation pattern (does not diminish from top to bottom)
- May be weak or completely lost in spindle cell and desmoplastic melanomas
- MelanA (MART1) helps to highlight invasion and intraepidermal melanocytes (J Cutan Pathol 2020;47:446)
- SOX10 highlights junctional melanocytes to help differentiate melanoma in situ from benign counterparts (Appl Immunohistochem Mol Morphol 2014;22:142)
- PRAME (preferentially expressed antigen in melanoma) is typically diffusely and strongly expressed in melanomas (J Cutan Pathol 2020;47:1123)
- Melanomas of vulva usually lack BRAF mutation (Br J Dermatol 2010;162:677)
Negative stains
- p16: complete loss of expression is usually seen in melanomas
- Its utility in differentiation between benign and malignant melanocytic lesions has been questioned
Molecular / cytogenetics description
- Current studies have not consistently separated melanomas arising from glabrous / mucosal from hair bearing vulva; hence, their molecular distinction is challenging (J Low Genit Tract Dis 2023;27:40)
- KIT mutations are the most common alterations in mucosal melanomas (J Clin Oncol 2006;24:4340, Melanoma Res 2014;24:360, J Low Genit Tract Dis 2023;27:40)
- Vulvar melanoma > vaginal melanomas >> cutaneous melanomas
- NRAS, NF1, TP53, TERT, CDKN2A, PTEN have also been identified
- BRAF mutation in vulvovaginal melanomas (J Low Genit Tract Dis 2023;27:40)
- Rare and identified in < 10% of cases by next generation sequencing
- Fluorescence in situ hybridization (FISH) or comparative genomic hybridization to confirm or rule out melanoma
- Sensitivity of both tests may be lower than expected due to decreased cellularity in vulvar lesions
- For vulvar melanomas, pTERT and CDK4 can be considered but may not be part of each FISH panel (Nat Commun 2019;10:3163, Am J Cancer Res 2020;10:4017)
- See table 6 for clinicopathologic correlates
Videos
Vulvovaginal melanoma
by Dr. Lewis Hassell
Vaginal melanoma radiology
by Dr. Ayushi Gupta
Vaginal melanoma
Sample pathology report
- Vulva, right labium majus, excision:
- Melanoma, superficial spreading growth pattern, with features of regression (Breslow thickness = 1.7 mm, Clark level = IV; pT2a)
- Compound nevus
- Dermal scar
- All margins are free of melanoma (see comment and synoptic report)
- Comment: Immunohistochemical stains for MelanA performed on blocks A3 - A7 highlight the invasive melanoma. A BRAF V600E stain performed on block A6 is positive in the tumor cells.
- Vagina, radical hysterectomy and bilateral salpingo-oophorectomy:
- Vagina:
- Multifocal melanoma, nodular type (largest focus: 4.7 x 1.7 x 1.0 cm)
- Vaginal cuff margin involved by melanoma
- Cervix:
- Involved by melanoma (direct extension)
- Uterus:
- Endometrium: secretory
- Myometrium: adenomyosis
- Uterine serosa and cervix: benign
- Fallopian tubes: benign, fimbriated
- Ovaries: benign
- Vagina:
Differential diagnosis
- Atypical melanocytic nevus of genital type (AMNGT):
- May be most challenging to differentiate from superficial spreading subtype of melanoma
- Symmetrical lesion with dermal component maturation
- Rare or very few atypical melanocytes in the epidermis as single cells with rare pagetoid spread, predominantly in the center of the lesion
- S100, SOX10 and nerve growth factor receptor (NGFR) fail to demonstrate invasive growth
- HMB45 highlights a gradient pattern (diminishing or loss of expression from top to bottom)
- PRAME is lost or very weakly expressed (J Cutan Pathol 2020;47:1123)
- BRAF is the most common alteration in AMNGT (N Engl J Med 2015;373:1926, J Invest Dermatol 2016;136:1858)
- Pigmented epithelioid melanocytoma (PEM) of the vulva:
- PEM family consists of multiple, usually slow growing, distinct histologic melanocytic entities with potential to metastasize but with a better prognosis than melanoma (WHO 5th edition)
- Infiltrative deep dermal tumor that may involve subcutis
- Hypercellular tumor with cells ranging from medium sized epithelioid cells to large epithelioid cells and spindled cells
- Low mitotic activity
- PRKAR1A loss in 67% of PEMs (Am J Surg Pathol 2017;41:1333, Am J Surg Pathol 2019;43:480)
- Dysplastic nevus of vulva:
- Differentiation requires clinical - pathologic correlates
- Relative symmetry
- Presence of junctional shoulders: extension of junctional component at least 3 rete ridges beyond the dermal component
- Superficial nests are usually very similar and may show focal bridging or coalescence of the nests
- Elongation and bridging of the rete ridges with nests
- Melanocytes may scatter suprabasally (confined to the lower epidermal layer and centrally)
- 2 tier grading of cytologic atypia is recommended by World Health Organization (WHO) classification and is largely based on nuclear features (WHO 5th edition) (Hum Pathol 1999;30:500)
Additional references
- Am J Surg Pathol 1995;19:792, J Cutan Pathol 2008;35:889, J Cutan Pathol 2008:35:24, McKee: Pathology of the Skin - With Clinical Correlations, 3rd Edition, 2005, Busam: Pathology of Melanocytic Tumors, 1st Edition, 2018, Amin: AJCC Cancer Staging Manual, 8th Edition, 2017, WHO Classification of Tumours Editorial Board: Skin Tumours, 5th Edition, 2023, American Cancer Society: Key Statistics for Melanoma Skin Cancer [Accessed 5 December 2023], Nucci: Gynecologic Pathology - A Volume in Foundations in Diagnostic Pathology Series, 2nd Edition, 2020, Frumovitz: Diagnosis and Treatment of Rare Gynecologic Cancers, 1st Edition, 2022
Board review style question #1
A 67 year old woman was recently diagnosed with vulvar melanoma of the right labium majus. What is the most likely molecular alteration in this lesion?
- ALK fusion
- BRAF mutation
- KIT mutation
- NTRK 1/3
- PRKAR1A loss
Board review style answer #1
C. KIT mutation. KIT mutations are more common in vulvar melanomas compared with cutaneous melanomas. Answers A and D are incorrect as ALK fusions and NTRK rearrangements are common in Spitz nevi. Answer E is incorrect as PRKAR1A loss is characteristic of pigmented epithelioid melanocytoma. Answer B is incorrect as BRAF mutations are more common in cutaneous melanomas and are only rarely encountered (< 10%) in vulvovaginal melanomas; however, nevi in genital areas, including atypical melanocytic nevi of genital type, frequently have a driver mutation in BRAF.
Comment Here
Reference: Melanoma
Comment Here
Reference: Melanoma
Board review style question #2
A 69 year old woman presented to the OBGYN clinic with severe pruritis. Clinical exam showed a 12 x 15 mm asymmetric, dark brown patch on the right labia minora extending into the clitoral hood. Biopsy demonstrated a broad, asymmetric, junctional melanocytic proliferation with dense lichenoid infiltrate. What is most likely to be demonstrated by the immunohistochemical stains for this patient’s lesion?
- HMB45 showing diffuse nuclear expression
- Loss of PRAME expression
- MelanA highlighting invasive melanoma cells
- p16 demonstrating strong nuclear expression
- SOX10 highlighting increased number of melanocytes with focal pagetoid spread
Board review style answer #2
E. SOX10 highlighting increased number of melanocytes with focal pagetoid spread. The histologic features are consistent with melanoma in situ, lentiginous growth pattern. SOX10 and MelanA will highlight increased melanocytes and pagetoid growth in melanoma. Answer C is incorrect as there is no evidence of invasive melanoma on H&E section. Answers A, B and D are incorrect as HMB45 is a cytoplasmic stain, PRAME is usually diffusely positive in melanoma and p16 typically demonstrates loss of nuclear expression in melanoma.
Comment Here
Reference: Melanoma
Comment Here
Reference: Melanoma