Table of Contents
Definition / general | Essential features | Terminology | Embryology | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Mohamed N, Asirvatham JR. Embryology. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cervixembryology.html. Accessed April 1st, 2025.
Definition / general
- The upper third of the vagina, the cervix, the uterus and both fallopian tubes are derived from the paramesonephric / Müllerian ducts (StatPearls: Embryology, Uterus [Accessed 22 December 2022])
Essential features
- Cervix develops from mesoderm derived Müllerian (paramesonephric) ducts
- Deviations from normal development (e.g., abnormal formation, fusion or resorption of Müllerian ducts) result in anomalies
- Müllerian anomalies are classified into 7 classes; they can be asymptomatic or manifest as amenorrhea, infertility, dyspareunia and repeated miscarriage
Terminology
- Müllerian ducts: Paramesonephric ducts
- Wolffian ducts: Mesonephric ducts
- AMH: Anti-Müllerian hormone
- DES: Diethylstilbestrol
- UVC: Uterovaginal canal
Embryology
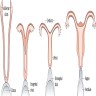
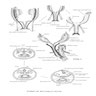
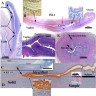
- At 6 weeks, 2 sets of paired genital ducts are present: the mesonephric (Wolffian) and the paramesonephric (Müllerian) ducts
- From ~8 - 12 weeks
- In the male fetus: anti-Müllerian hormone (AMH) and SRY gene (via testosterone production) cause regression of Müllerian ducts and differentiation of the Wolffian ducts into epididymides, vasa deferentia, seminal vesicles and ejaculatory ducts
- In the female fetus: in the absence of AMH and testosterone production, the Wolffian ducts degenerate and the Müllerian ducts continue to develop
- Müllerian ducts elongate and canalize forming 3 regions: cranial, horizontal and caudal
- Funnel shaped cranial regions open directly into the primitive peritoneal cavity forming the fimbria of the fallopian tubes
- Horizontal regions extend caudomedially and form the remainder of the fallopian tubes
- Caudal regions fuse together in the midline and form a single Y shaped tubular structure, the uterovaginal primordium / uterovaginal canal (UVC)
- UVC meets the posterior wall of the urogenital sinus caudally at the Müllerian tubercle (location of vaginal orifice at hymenal ring)
- 2 solid evaginations (the sinovaginal bulbs) grow from the urogenital sinus and encircle the caudal end of the UVC
- After fusion a septum remains, separating the 2 Müllerian ducts; the septum later is resorbed through apoptosis creating a patent uterine cavity, cervix and upper vagina
- UVC gives rise to the fibromuscular wall of vagina, epithelium and glands of corpus uteri and cervix; the endometrial stroma and myometrium develop from adjacent mesenchyme
- Epithelium of the vagina is largely derived from urogenital sinus by upgrowth of sinovaginal bulbs that fuse to form the vaginal plate; the plate subsequently breaks down centrally and leaves the periphery as vaginal epithelium
- Reference: StatPearls: Embryology, Mullerian Ducts (Paramesonephric Ducts) [Accessed 22 December 2022]
Pathophysiology
- The traditional hypothesis states that the Müllerian ducts are fused in a caudal cranial direction
- An alternative hypothesis indicates that fusion and resorption start at the isthmus and proceed bidirectionally at the same time or in a segmental pattern (J Hum Reprod Sci 2020;13:352)
- Müllerian anomalies result from failure of Müllerian duct formation, arrested development, failure of fusion of the Müllerian ducts or failure of resorption of the medial septum
- Müllerian anomalies can be associated with abnormalities in gastrointestinal and urinary system
- Gartner duct is the remnant of the distal Wolffian duct and can result in cystic structure in the lower vaginal wall called Gartner duct cyst
Etiology
- Diethylstilbestrol (DES) exposure in utero can lead to congenital Müllerian anomalies and adenosis (resulting in increased risk of clear cell adenocarcinoma of vagina and cervix)
- Congenital cervical anomalies associated with in utero DES exposure include hypoplasia, collar, hood, polyps and ectropion (presence of cervical glandular mucosa on the ectocervix)
- These are grossly evident in about 20% of patients with DES exposure in utero
- DES daughters have a 40 fold increased risk for cervical and vaginal clear cell adenocarcinoma
Diagrams / tables
Clinical features
- Müllerian anomalies can be asymptomatic or manifest as amenorrhea, infertility, dyspareunia, repeated miscarriage, complicated pregnancy or difficult labor
- These anomalies are classified into 7 categories (StatPearls: Embryology, Mullerian Ducts (Paramesonephric Ducts) [Accessed 22 December 2022]):
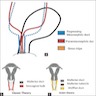
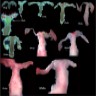
- Class I: hypoplasia / uterine hypoplasia (Mayer-Rokitansky-Küster-Hauser syndrome) (5 - 10%)
- Class II: unicornuate uterus (20%)
- Class III: uterus didelphys (5%)
- Class IV: bicornuate uterus (10%)
- Class V: septate uterus (55%)
- Class VI: arcuate uterus (limited data)
- Class VII: diethylstilbestrol in fetal life (T shaped uterus, cervical anomalies: hypoplasia, collar, hood, ectropion)
Diagnosis
- Müllerian anomalies present in ~17% of women with recurrent miscarriage
- Gynecological exam can reveal vaginal and some cervical anomalies
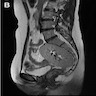
- Diagnosis depends on radiology, including 2 dimensional or 3 dimensional ultrasound, MRI, hysterosalpingo contrast sonography, Xray hysterosalpingography, video hysteroscopy and video laparoscopy
- Reference: Taiwan J Obstet Gynecol 2020;59:183
Radiology description
- 2 dimensional or 3 dimensional ultrasound imaging is the initial diagnostic modality in cases with high index of suspicion
- MRI is considered the gold standard imaging study for identifying Müllerian anomalies
- Hysterosalpingo contrast sonography provides good information about the cervix and uterine cavity (Taiwan J Obstet Gynecol 2020;59:183)
Radiology images
Case reports
- 24 year old woman with ruptured rudimentary horn pregnancy (Cureus 2021;13:e15873)
- 33 year old woman with unicornuate uterus diagnosed during labor (J Med Case Rep 2020;14:209)
- 39 year old woman has a double cervix with normal uterus and vagina (Int J Fertil Steril 2019;13:83)
Treatment
- Some Müllerian malformations can be treated by corrective surgery
Gross description
- Cervix develops from the middle two - fourths of the uterovaginal canal
- 8 - 10 weeks: the boundaries between the cervix and the uterine corpus or the vagina are unclear
- 18 - 20 weeks: the vaginal fornices are recognizable with the ectocervix projecting into the vagina
- Reference: Differentiation 2017;97:9
Microscopic (histologic) description
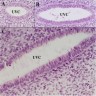
- Initially, a simple columnar Müllerian epithelium lines the uterovaginal canal throughout
- During development, the epithelium lining of the caudal portion of the uterovaginal canal becomes stratified
- Glands are well developed within the cervix at 20 weeks of gestation (Differentiation 2017;97:9)
Microscopic (histologic) images
Positive stains
Negative stains
- FOXA1 positive in urogenital sinus and its derivatives but not in the Müllerian duct derivatives
Molecular / cytogenetics description
- Many transcription factors and signaling molecules are necessary for the development of the Müllerian ducts
- PAX2, PAX8, EMX2, Lim1, PBX1 and HNF-1B are expressed in Müllerian epithelial progenitors
- Wnt4 and DMRT1 are expressed in the mesenchymal progenitor cells
- Disruption of any of these can result in anomalies throughout development and their presence at birth (Dis Model Mech 2021;14:dmm047977, Front Cell Dev Biol 2021;9:605301)
Videos
Female genital tract embryology
Additional references
Board review style question #1
Which of the following is the most common congenital Müllerian anomaly?
- Bicornuate uterus
- Septate uterus
- Unicorunate uterus
- Uterus didelphys
Board review style answer #1
B. Septate uterus comprises 55% of uterine anomalies. Bicornuate uterus (answer A) comprises 10% of uterine anomalies. Unicorunate uterus (answer C) comprises 20% of uterine anomalies. Uterus didelphys (answer D) comprises 5% of uterine anomalies (StatPearls: Embryology, Mullerian Ducts (Paramesonephric Ducts) [Accessed 22 December 2022]).
Comment Here
Reference: Cervix - Embryology
Comment Here
Reference: Cervix - Embryology
Board review style question #2
Diethylstilbestrol (DES) exposure in utero is known to be associated with congenital anomalies, subfertility and increased risk of precursor lesions and cancers of the reproductive tract, especially clear cell adenocarcinoma (CCA) of the cervicovaginal region. DES exposure is also associated with gross cervical anomalies. Compared to the normal population, what is the increase in risk for CCA after in utero DES exposure and frequency of gross cervical anomalies?
- 2x, 10%
- 10x, < 1%
- 20x, 20%
- 40x, 20%
Board review style answer #2
D. 40x, 20%. DES daughters have an increased risk for clear cell adenocarcinoma that is 40 fold. Congenital cervical anomalies associated with in utero DES exposure include hypoplasia, collar, hood, polyps and ectropion. These are grossly evident in ~20% of patients with DES exposure in utero
(NIH: Diethylstilbestrol (DES) Exposure and Cancer [Accessed 22 December 2022], Am J Obstet Gynecol 1986;154:1312).
Comment Here
Reference: Cervix - Embryology
Comment Here
Reference: Cervix - Embryology