Table of Contents
Definition / general | Essential features | Background | 2014 Bethesda system for reporting cervical cytology - report elements | Definitions of interpretation categories | Sites | Prognosis and management | Case reports | Cytology description | Cytology images | Molecular / cytogenetics description | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Jug R, Bean SM. Bethesda system. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cervixcytologybethesda.html. Accessed December 20th, 2024.
Definition / general
- The Bethesda system provides a framework for consistent interlaboratory terminology for the reporting of cervicovaginal cytology specimens
Essential features
- Report elements include specimen type, specimen adequacy, general categorization, interpretation / result and other optional elements such as ancillary testing, computer assisted interpretation, educational notes and comments
- Interpretations / results include the general categories of negative for intraepithelial lesion or malignancy (NILM), epithelial cell abnormalities and other malignancies
- Prognosis and management of cervical cytology screening diagnostic categories is based on the 2019 ASCCP Risk Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors (J Low Genit Tract Dis 2020;24:102)
Background
- The Bethesda system provides a framework for consistent interlaboratory terminology for the reporting of cervicovaginal cytology specimens
- First conceived in 1988 with updates in 1991, 2001 and 2014 (JAMA 2002;287:2114, Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
- By 2016, the 2014 update was implemented by most labs (67.2%) in the College of American Pathologists' PAP education program and 20.1% had planned to implement the updates (Arch Pathol Lab Med 2019;143:1196)
2014 Bethesda system for reporting cervical cytology - report elements
- Specimen type: conventional smear, liquid based preparation or other
- Specimen adequacy (mandatory)
- Satisfactory or unsatisfactory for evaluation
- Satisfactory
- Adequate number of well visualized or preserved squamous or squamous metaplastic cells
- Conventional smear: minimum of 8,000 to 12,000 cells
- Liquid based preparation: minimum of 5,000 cells
- Woman's postchemotherapy, radiotherapy, postmenopausal, atrophic changes or posthysterectomy may have < 5,000 cells and be deemed adequate at laboratory's discretion (if > 2,000 cells)
- Exception: adequate if any abnormal cells are present
- Unsatisfactory
- > 75% of cells obscured by inflammation, bacteria or interfering substances (lubricants and blood)
- Glacial acetic acid treatment may be applied to liquid based collections that are inadequate based on the Bethesda system to facilitate the removal of mucus, erythrocytes, inflammatory cells and debris (J Clin Microbiol 2012;50:2129)
- If 50 - 75% of cells are obscured, include a disclaimer describing how they are obscured and the percentage of cells obscured
- Report specific reason for unsatisfactory evaluation whether specimen is rejected or not processed or processed but unsatisfactory for evaluation
- > 75% of cells obscured by inflammation, bacteria or interfering substances (lubricants and blood)
- Quality indicators
- Presence or absence of endocervical or transformation zone component
- At least 10 well preserved endocervical or metaplastic cells
- Absence of transformation zone does not necessitate repeat testing (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
- General categorization (optional)
- Negative for intraepithelial lesion or malignancy
- Epithelial cell abnormality: specify squamous or glandular (see Interpretation / result)
- Other (e.g., endometrial cells in a woman ≥ 45 years of age) (see Interpretation / result) (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
- Interpretation / result (mandatory)
- Negative for intraepithelial lesion or malignancy (NILM)
- State NILM in General categorization or Interpretation / result sections of report and then specify nonneoplastic findings, including organisms, if present
- Nonneoplastic cellular findings
- Squamous metaplasia
- Keratotic changes
- Tubal metaplasia
- Atrophy
- Pregnancy associated changes
- Reactive cellular changes with association specified
- Inflammation (with or without repair)
- Lymphocytic (follicular) cervicitis
- Radiation
- Intrauterine contraceptive device changes
- Glandular cells posthysterectomy
- Organisms
- Trichomonas vaginalis
- Fungal organisms morphologically consistent with Candida species
- Shift in flora suggestive of bacterial vaginosis
- Bacteria morphologically consistent with Actinomyces species
- Cellular changes associated with herpes simplex virus
- Cellular changes associated with cytomegalovirus
- Other
- Endometrial cells (in a woman ≥ 45 years of age)
- Epithelial cell abnormalities
- Squamous cell
- Atypical squamous cells
- Of undetermined significance (ASCUS)
- Cannot exclude HSIL (ASC-H)
- Low grade squamous intraepithelial lesion (LSIL)
- High grade squamous intraepithelial lesion (HSIL)
- With features suspicious for invasion (if invasion suspected)
- Squamous cell carcinoma
- Atypical squamous cells
- Glandular cell
- Atypical
- Endocervical cells (NOS or specify in comment)
- Endometrial cells (NOS or specify in comment)
- Glandular cells (NOS or specify in comment)
- Endocervical cells, favor neoplastic
- Glandular cells, favor neoplastic
- Endocervical adenocarcinoma in situ
- Adenocarcinoma
- Endocervical
- Endometrial
- Extrauterine
- Not otherwise specified (NOS)
- Atypical
- Other malignant neoplasms (specify) (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
- Squamous cell
- Negative for intraepithelial lesion or malignancy (NILM)
- Other reporting considerations (optional)
- Ancillary testing: describe test method and report result (e.g., immunohistochemical stains performed on cell block material)
- Computer assisted interpretation of cervical cytology: specify automated instrument used, whether specimen was successfully processed by device, result and whether additional manual screening / review of specimen was performed
- Educational notes and comments: may be appended to cytology reports (e.g., references to relevant publications such as clinical guidelines published by professional organizations) (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
Definitions of interpretation categories
- Negative for intraepithelial lesion or malignancy (NILM)
- Adequate squamous cells in the absence of an intraepithelial lesion or malignancy with or without the presence of notable nonneoplastic cellular findings, reactive cellular changes, organisms and glandular cells (posthysterectomy or endometrial in origin ≥ 45 years old)
- Epithelial cell abnormalities
- Squamous cells
- Atypical squamous cells
- Cytologic changes in squamous cells suggestive of a squamous intraepithelial lesion (increased N:C ratio and minimal nuclear changes) but qualitatively or quantitatively insufficient for a definitive interpretation
- Atypical squamous cell: squamous intraepithelial lesion (ASC:SIL) ratio is used as a quality assurance measure and while the median ratio is 1.5:1, laboratories serving high risk populations should aim for an ASC:SIL ratio at or below 3:1 (Diagn Cytopathol 2010;38:180, Cibas: Cytology - Diagnostic Principles and Clinical Correlates, 4th Edition, 2014)
- Atypical squamous cells of undetermined significance (ASCUS)
- Changes suggestive of LSIL, including nuclei 2.5 - 3 times the size of a normal intermediate cell nucleus with or without minimal nuclear hyperchromasia and mildly irregular nuclear contours or poorly formed cytoplasmic halos or vacuoles, resembling koilocytes
- Includes atypical parakeratosis, atypical repair and atypia in postmenopausal women and in atrophy
- Atypical squamous cells cannot exclude HSIL (ASC-H)
- Scant atypical cells with changes suggestive of HSIL
- Includes atypical immature metaplastic cells, crowded sheets of cells, markedly atypical repair, severe atrophy and postradiation changes suspicious for recurrent or residual carcinoma
- Low grade squamous intraepithelial lesion (LSIL)
- Synonyms: mild dysplasia / cervical intraepithelial neoplasia (CIN) I; these terms are not recommended
- Large squamous cells with intermediate or superficial (mature) squamous cell type cytoplasm
- Nuclear enlargement (3 times the size of normal intermediate cell nuclei)
- Nuclear hyperchromasia, anisonucleosis, uniform chromatin, variable nuclear membranes, bi or multinucleation, inconspicuous nucleoli and koilocytosis or perinuclear cavitation
- High grade squamous intraepithelial lesion (HSIL)
- Synonyms: moderate dysplasia, severe dysplasia, CIN2, CIN3, carcinoma in situ (CIS); these terms are not recommended
- Smaller squamous cells than LSIL occurring singly, in sheets or in syncytial-like aggregates (hyperchromatic crowded groups) with a high N:C ratio, nuclear hyperchromasia, nuclear contour irregularities with indentations and grooves and generally absent nucleoli
- Specify presence of features suspicious for invasion (if invasion suspected), including highly pleomorphic HSIL cells without background features of invasion (necrosis or tumor diathesis) or background features of tumor diathesis without overtly malignant cells
- Includes HSIL involving endocervical glands
- Squamous cell carcinoma
- Single or (less commonly) cellular aggregates of variable cell sizes and shapes with nuclear membrane irregularities, dense opaque nuclei, coarsely granular chromatin, tumor diathesis (necrotic debris and broken down blood elements) and keratotic changes if keratinizing squamous cell carcinoma
- Encompasses invasive squamous cell tumors of varying degrees of differentiation, including nonkeratinizing and keratinizing carcinomas
- Nonkeratinizing squamous cell carcinoma may be differentiated from adenocarcinoma using immunohistochemistry on cell blocks made from residual liquid based material
- Atypical squamous cells
- Glandular cells
- Atypical NOS
- Atypical glandular cells should be categorized by site of origin whenever possible to guide management but can be labeled as not otherwise specified (NOS) when further classification is not possible
- Endocervical cells (NOS or specify in comment)
- Endocervical cell nuclear atypia (mild nuclear enlargement 3 - 5 times normal endocervical nuclei, overlap, pseudostratification, variation in size or shape, hyperchromasia, chromatin irregularities, rare nucleoli and mitoses) beyond reactive or reparative changes and without definite features of in situ or invasive endocervical adenocarcinoma
- Endometrial cells (NOS or specify in comment)
- Small groups of endometrial cells with enlarged nuclei, mild hyperchromasia, chromatin heterogeneity, occasional small nucleoli, scant with or without vacuolated cytoplasm and ill defined cell borders
- Glandular cells (NOS or specify in comment)
- Interpretive category used when unable to distinguish between mildly atypical endocervical and endometrial cells
- Atypical, favor neoplastic
- Endocervical cells, favor neoplastic
- Atypical endocervical cell morphology (nuclear atypia [above] plus nuclear elongation with rare cells forming rosettes or glands) quantitatively or qualitatively insufficient for a diagnosis of in situ or invasive endocervical adenocarcinoma
- Glandular cells, favor neoplastic
- Interpretive category used when unable to distinguish between atypical endocervical and endometrial cells quantitatively or qualitatively insufficient for a diagnosis of in situ or invasive adenocarcinoma
- Endocervical cells, favor neoplastic
- Endocervical adenocarcinoma in situ (AIS)
- Groups of cells in clusters, pseudostratified strips and rosettes with nuclear crowding, enlargement, hyperchromasia, coarsely granular chromatin, inconspicuous nucleoli, pseudostratification, apoptotic bodies and mitotic activity in a clean background
- Adenocarcinoma
- Endocervical
- Cytologic findings seen in endocervical AIS with the addition of more pleomorphism, macronucleoli, nuclear clearing with uneven distribution of chromatin and features indicative of invasion, including background tumor diathesis
- Endometrial
- Single or small clusters of cells with variation in nuclear size (dependent on grade), loss of nuclear polarity, moderate hyperchromasia, chromatin clearing (in higher grade tumors), increasing prominence of nucleoli with grade, scant vacuolated cytoplasm, intracytoplasmic neutrophils and finely granular, watery tumor diathesis
- Extrauterine
- Adenocarcinoma cells without tumor diathesis raise suspicion for tumors originating outside the uterus and cervix
- Some cytologic features may provide insight into the site of origin (such as psammoma bodies and papillary clusters suggestive of Müllerian origin), otherwise extra material can be made into a cell block on which immunohistochemical stains may be performed to help define a site of origin
- Not otherwise specified (NOS)
- Cells consistent with adenocarcinoma without features differentiating between site of origin (endocervical, endometrial or extrauterine)
- Endocervical
- Atypical NOS
- Squamous cells
- Other malignant neoplasms (apart from squamous cell carcinoma or adenocarcinoma; specify) (e.g., gynecologic primaries involving cervix or vagina by direct extension or secondary or metastatic tumors to the uterine cervix) (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
Sites
- Cervix, vagina, anus
Prognosis and management
- According to the 2019 ASCCP Risk Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors, the prognosis of cervical cytology screening results is expressed as their threshold of risk for having or developing CIN3+ and is based on current and previous screening results as well as history of previous precancer treatment
- Thresholds of risk are used to guide management recommendations
- Management options include return to routine screening, 1 year or 3 year surveillance, colposcopy or treatment corresponding to a risk stratum (J Low Genit Tract Dis 2020;24:102)
Case reports
- 34 year old woman, who was operated on for a left borderline ovarian tumor 2 years ago, was found to have glandular dysplasia in a Pap smear screening which supported a diagnosis of serous borderline tumor (Acta Cytol 2013;57:96)
- 42 year old woman with history of cutaneous melanoma 3 years prior was diagnosed by a routine cervical Pap smear with metastatic melanoma (Diagn Cytopathol 2018;46:1045)
- 52 year old woman underwent routine gynecologic and Pap smear examination that found a large number of hyphae and many fruiting bodies of Aspergillus species (Acta Cytol 2009;53:229)
Cytology description
- See Definitions of interpretation categories section
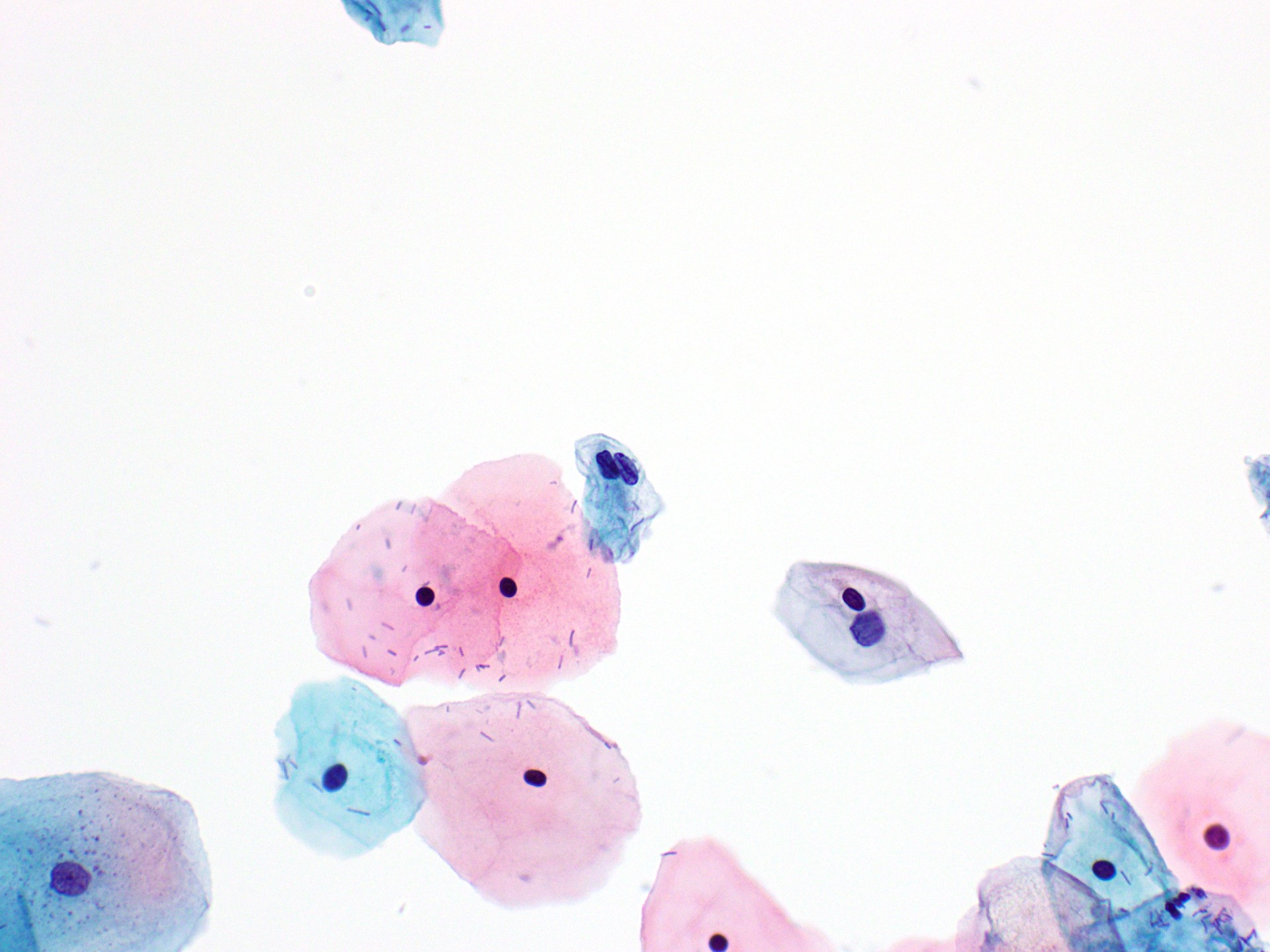
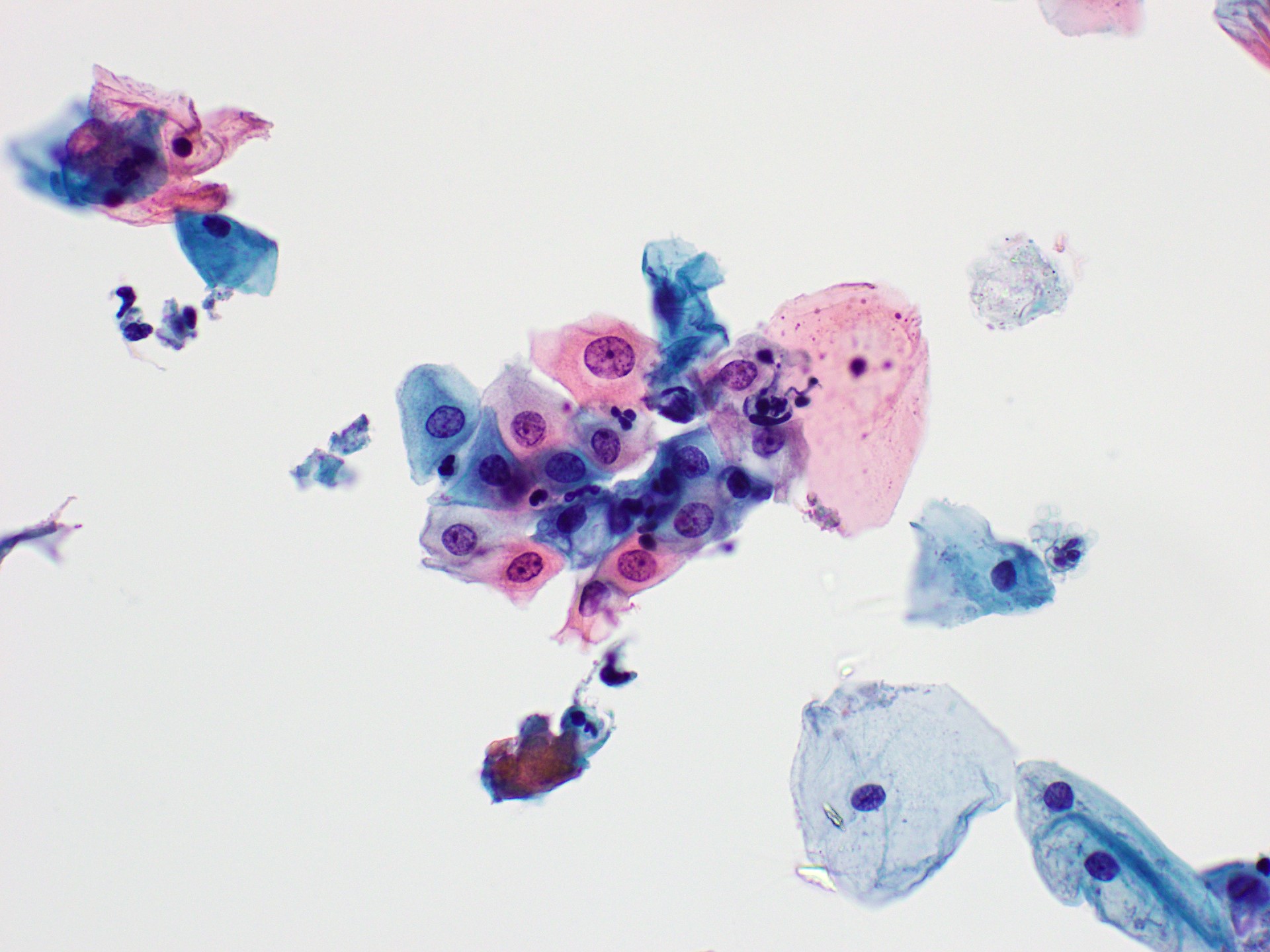
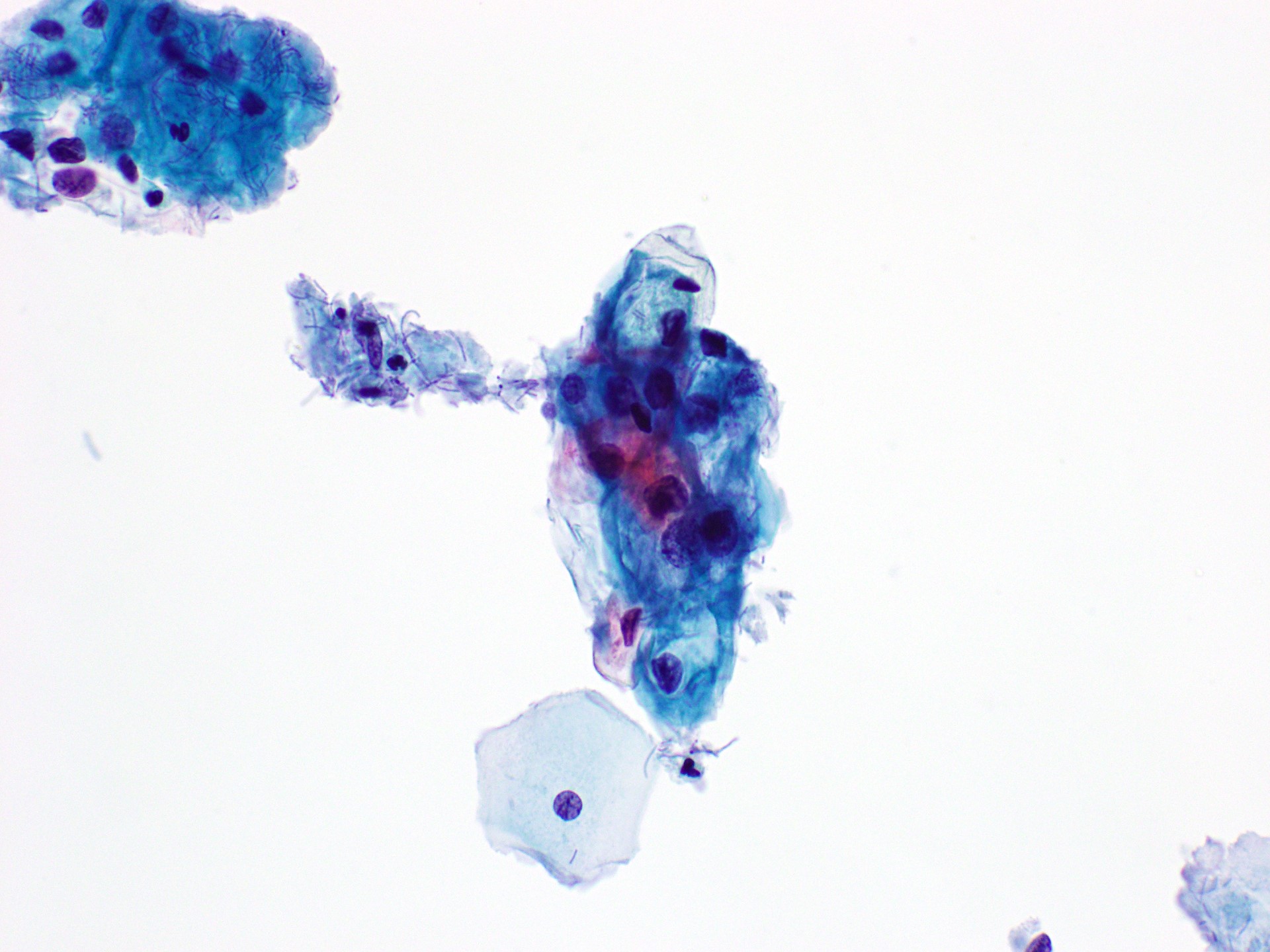
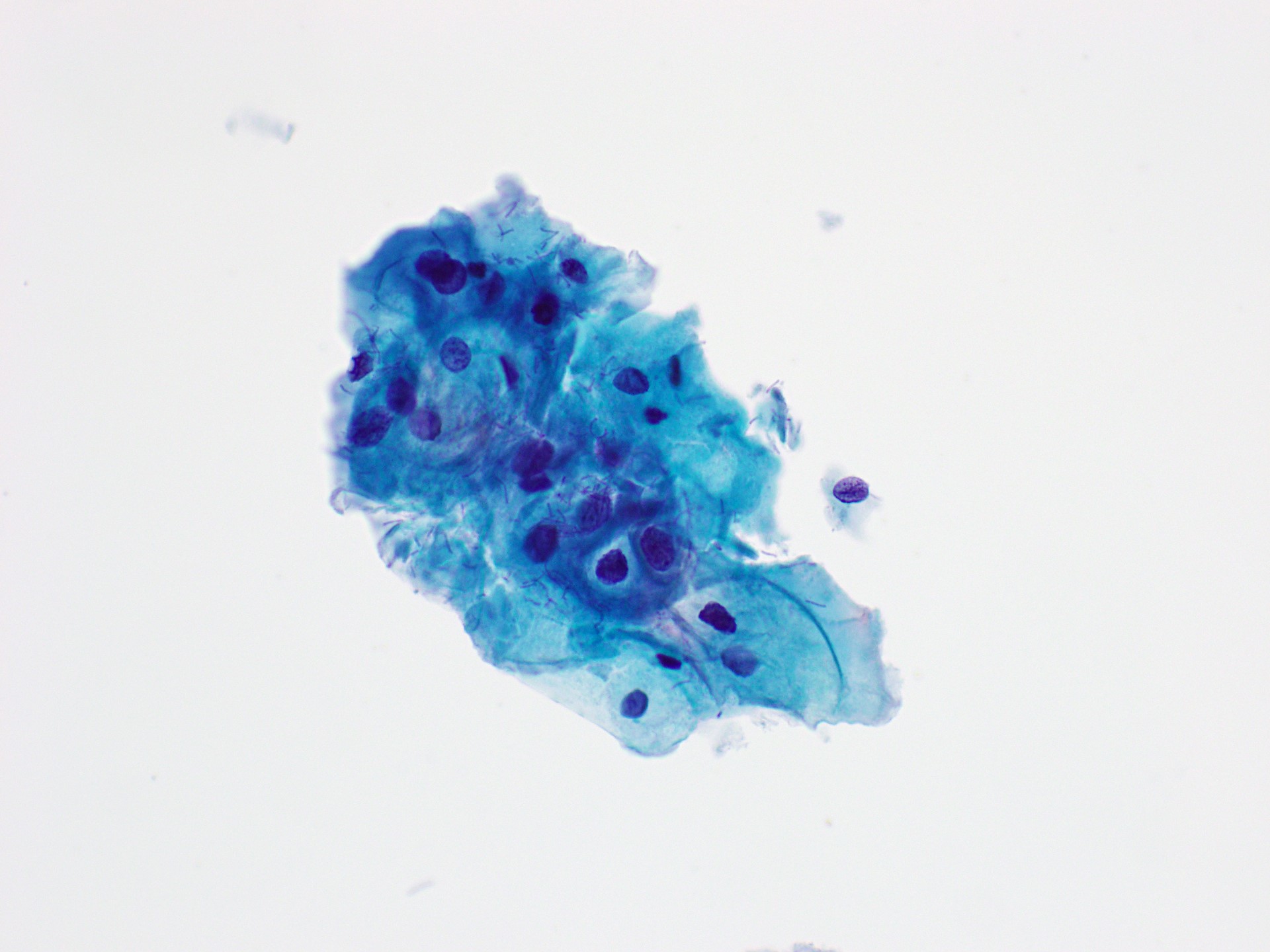
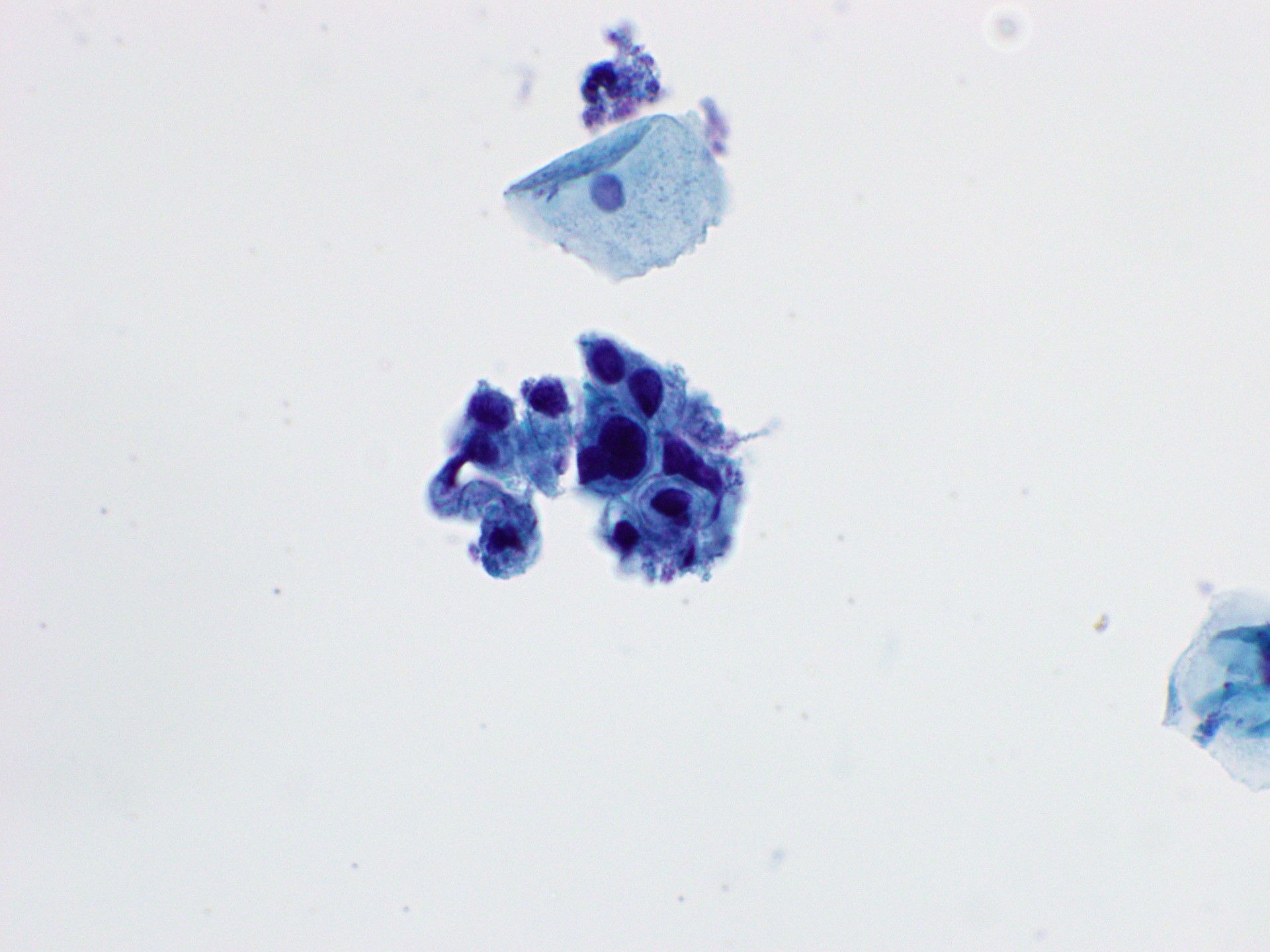
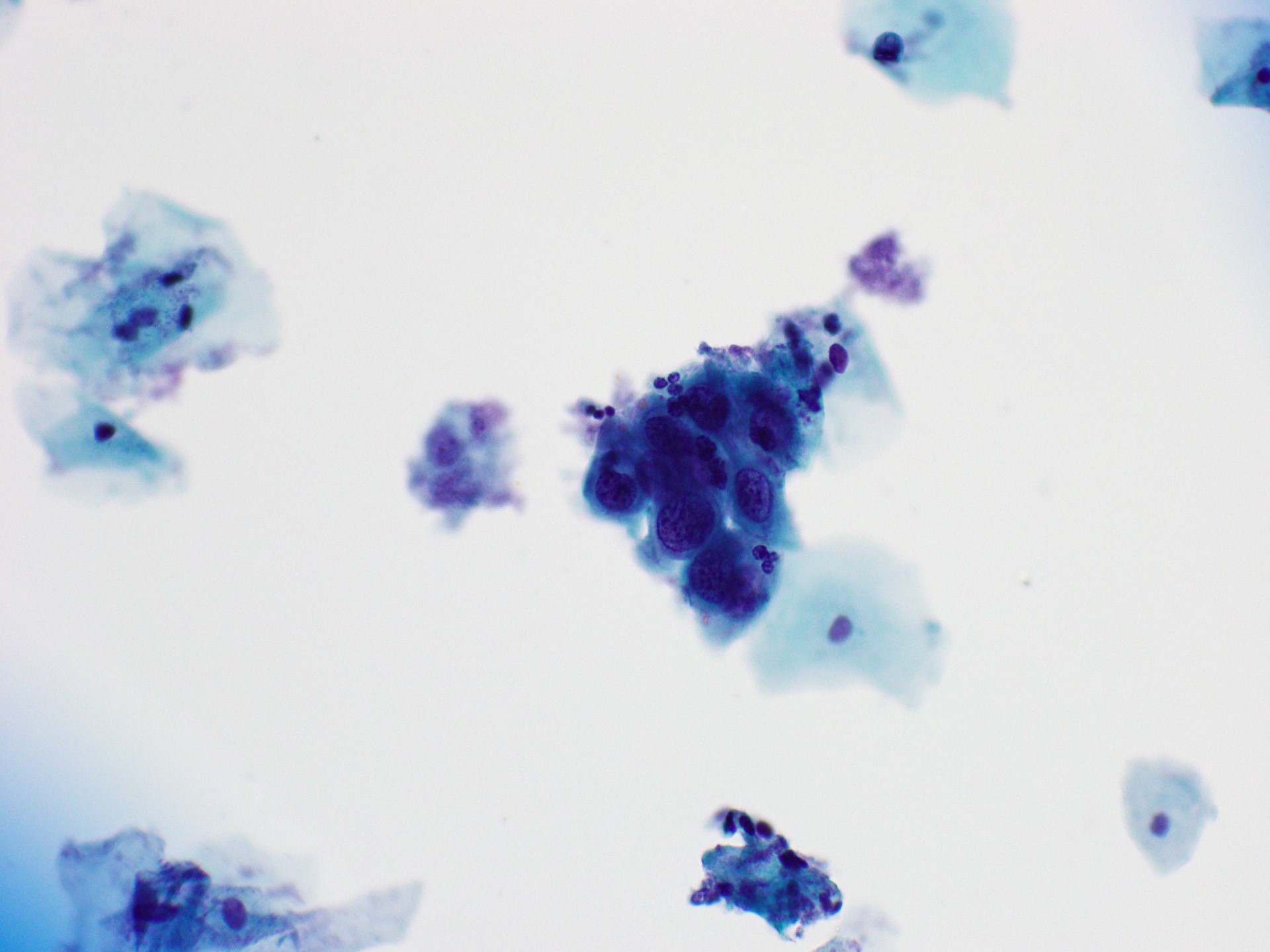
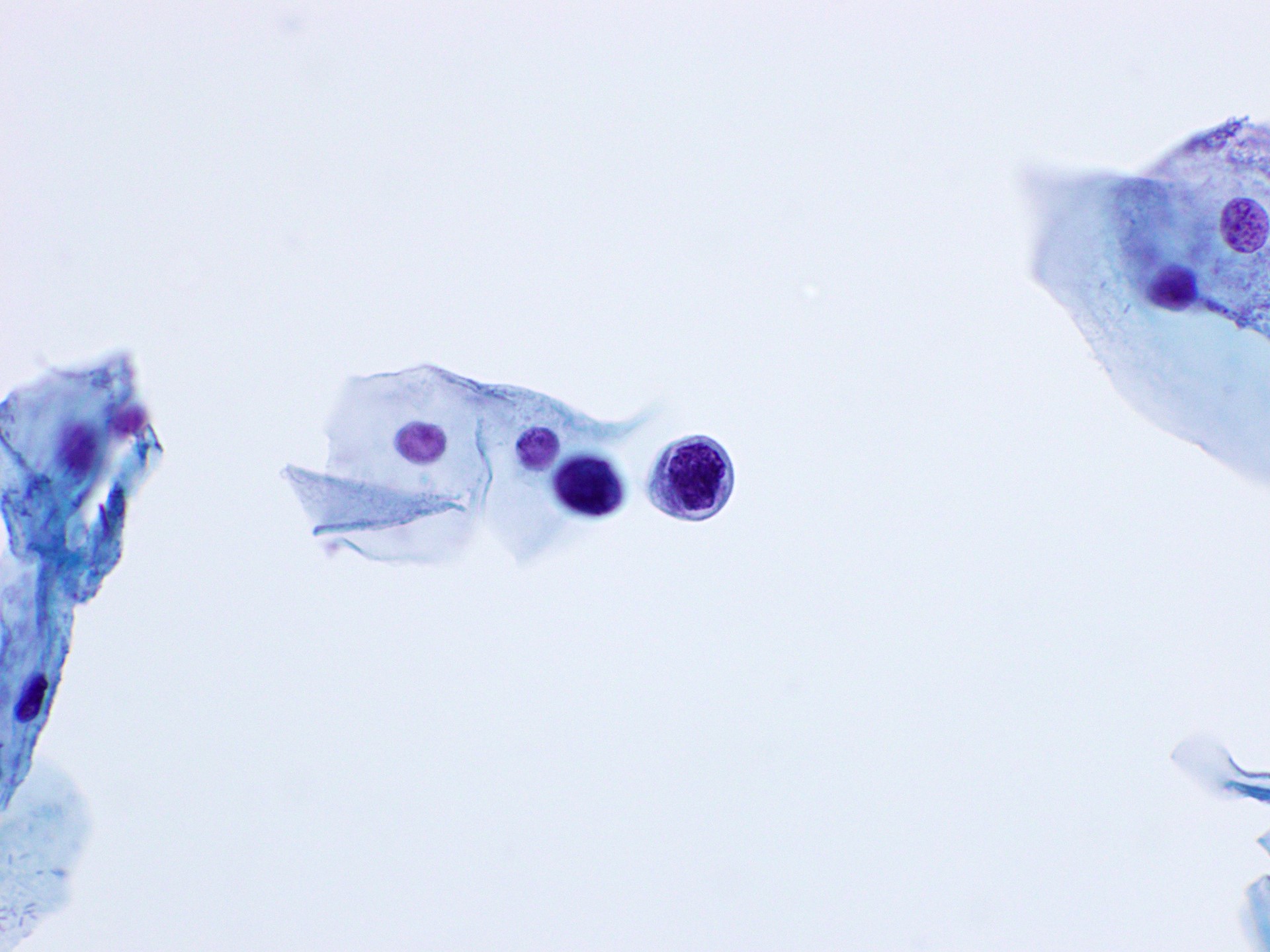
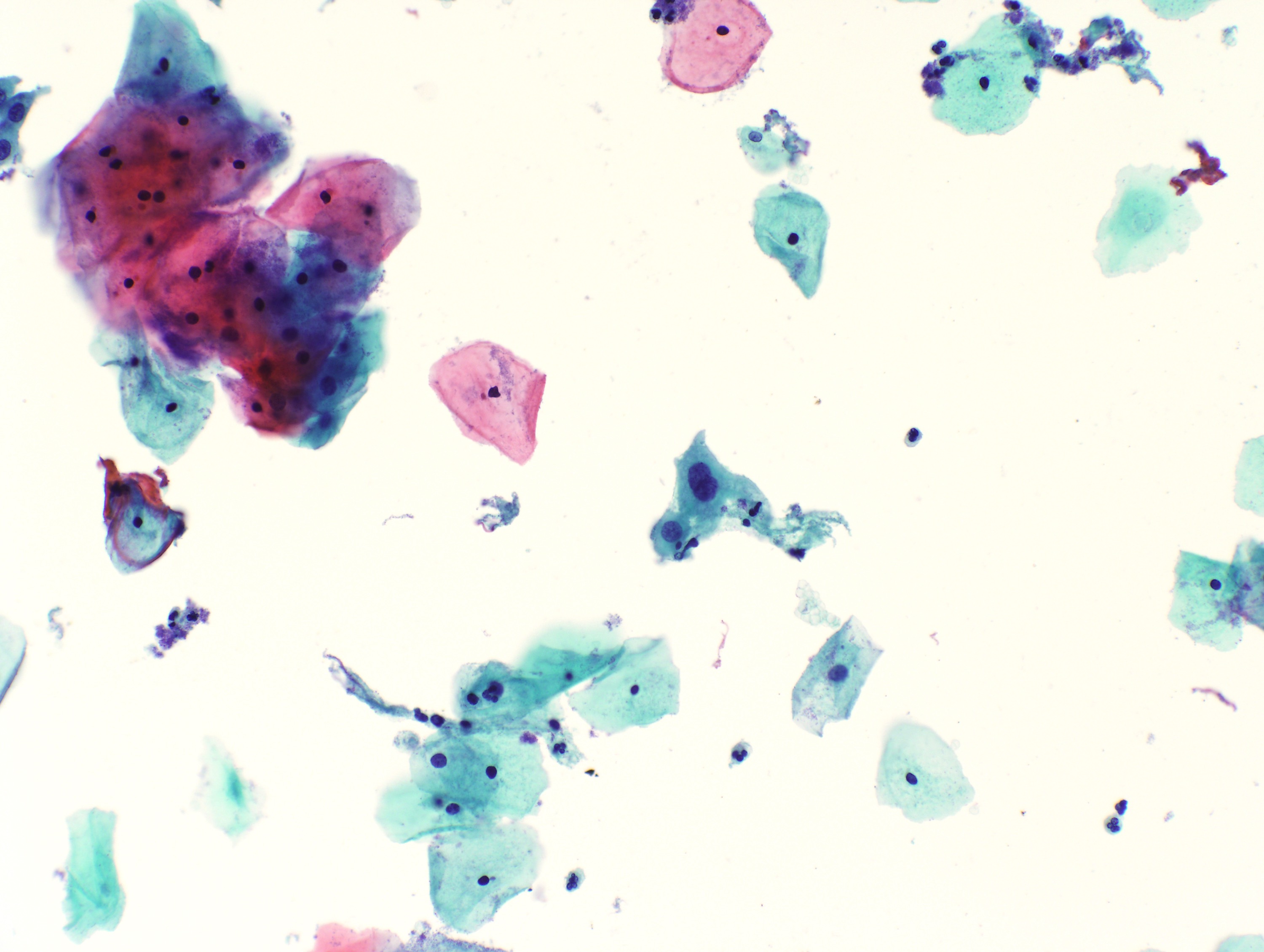
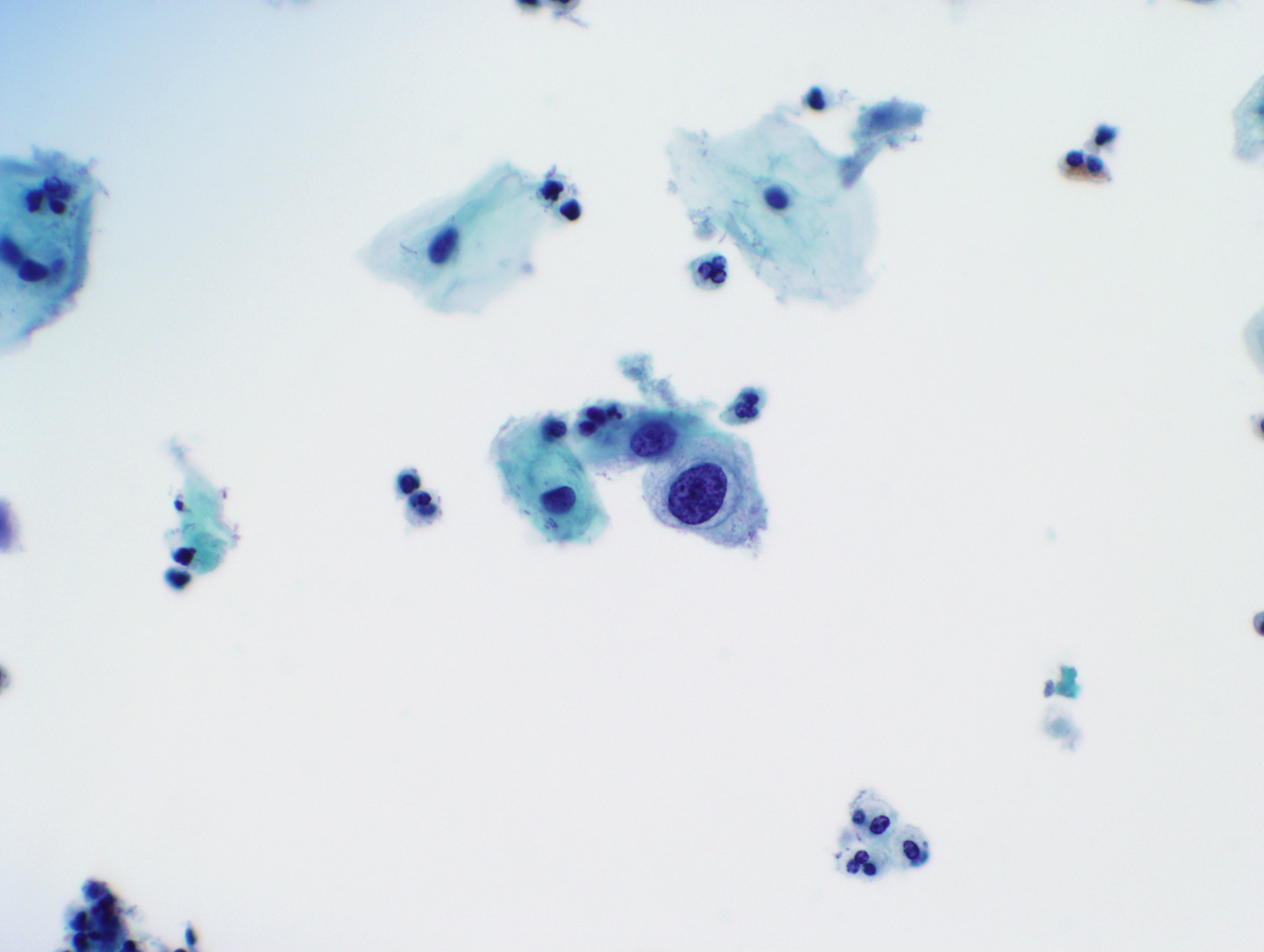
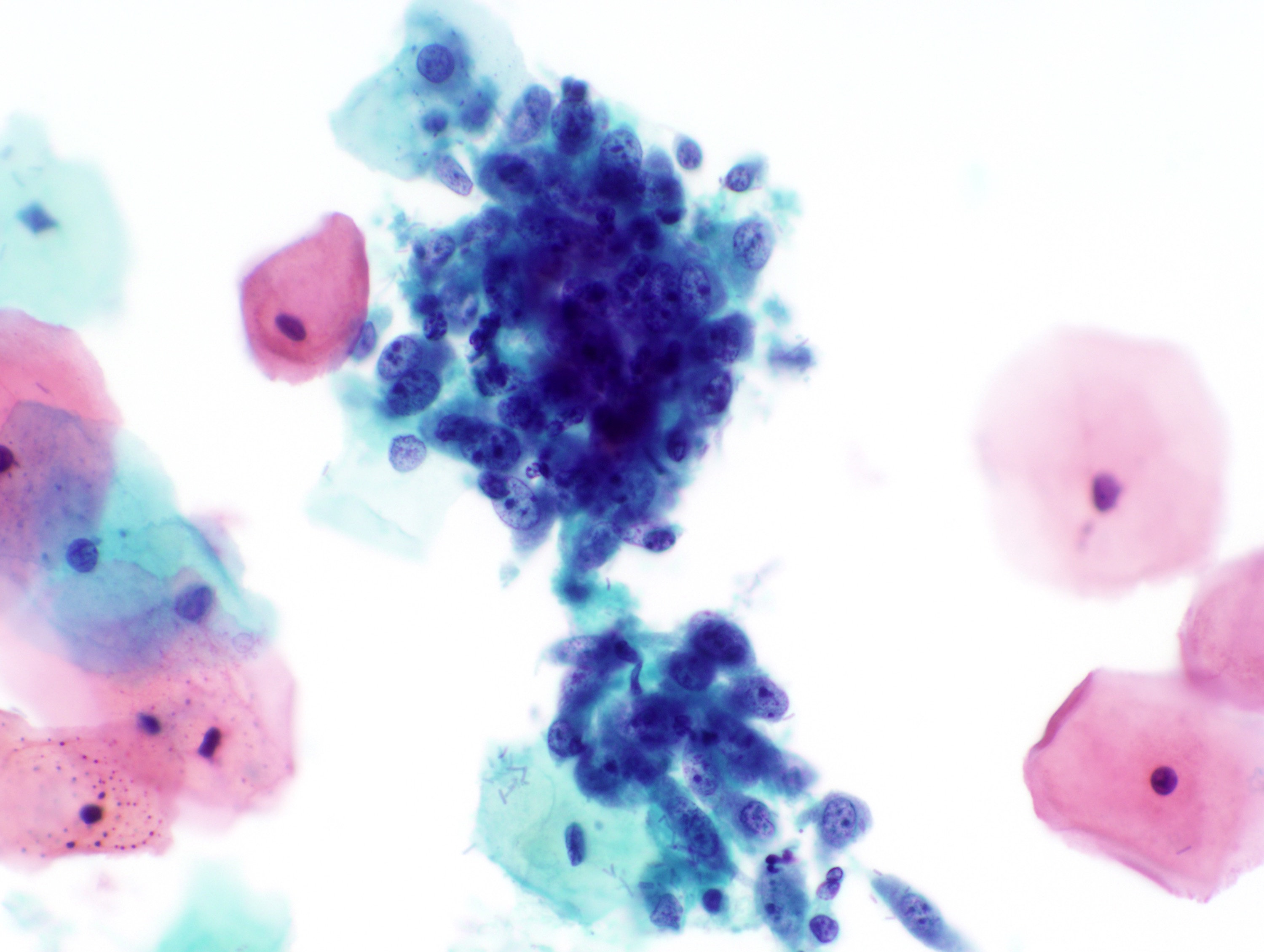
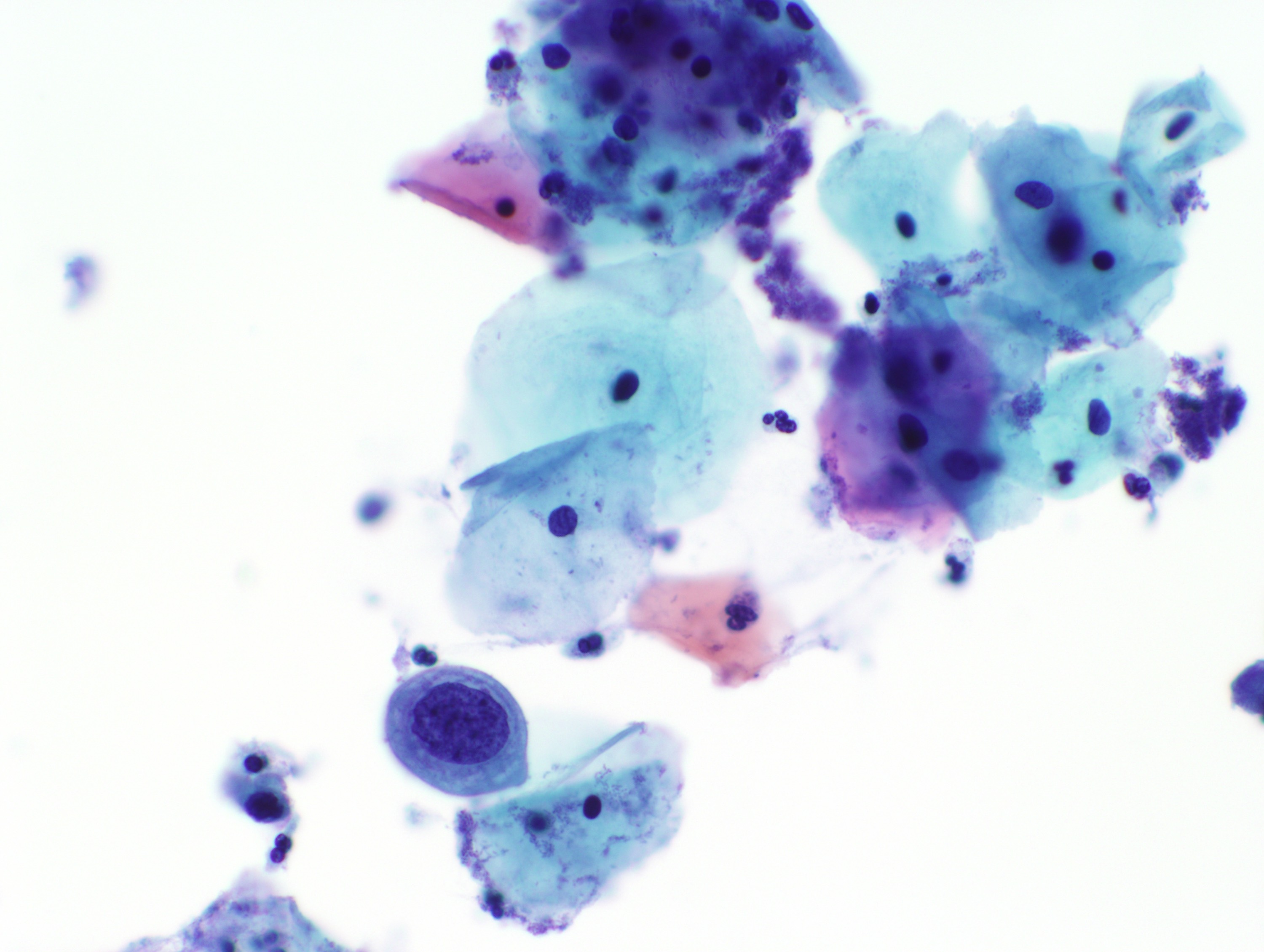
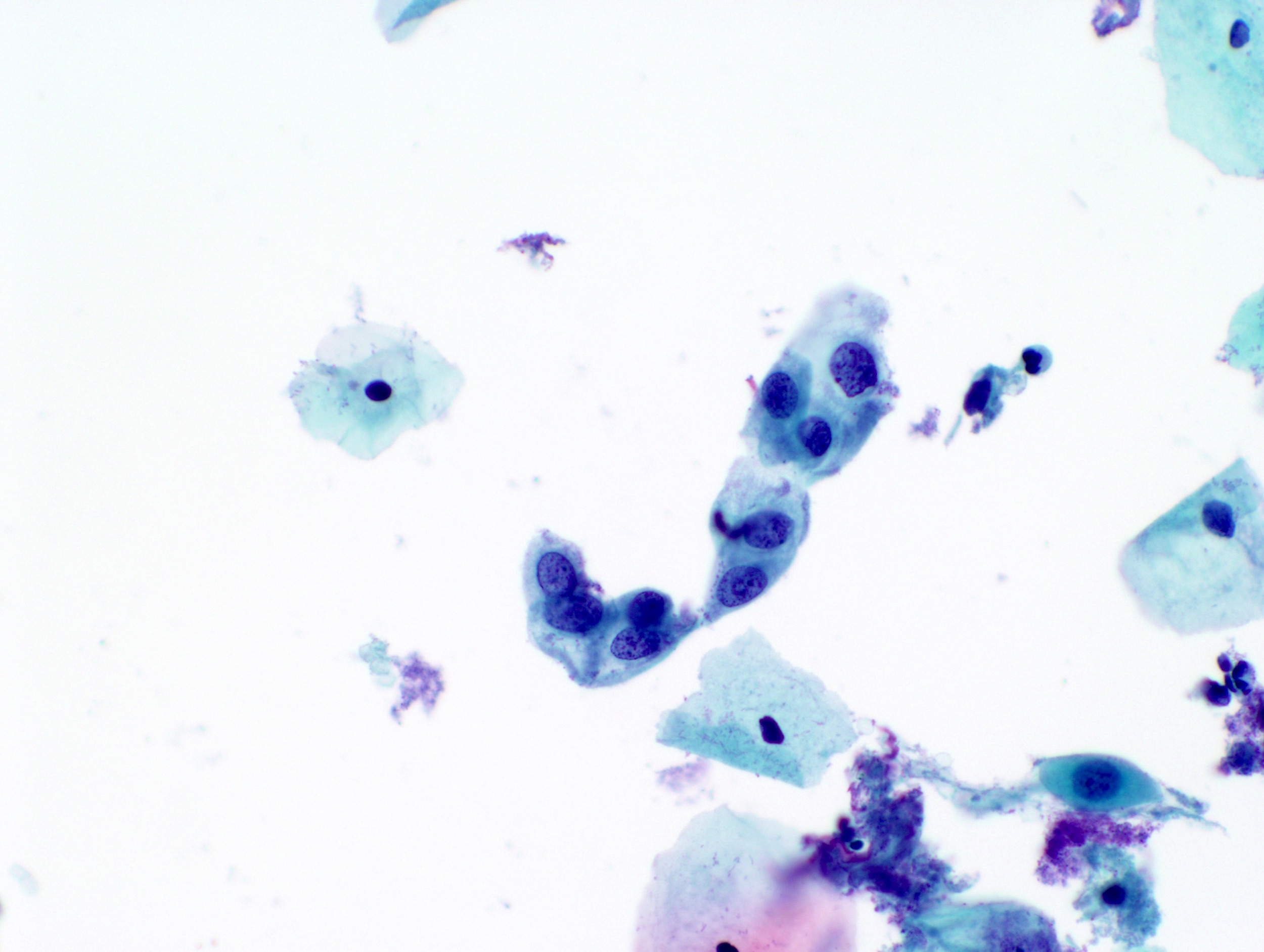
Cytology images
Contributed by Rachel Jug, M.B.B.Ch., B.A.O. and Sarah M. Bean, M.D.
Molecular / cytogenetics description
- HPV testing methods: HPV tests using a hybrid capture assay, invader chemistry technology, real time PCR and transcription mediated amplification (Cytojournal 2014;11:5)
- HPV testing indications
- Patients with atypical squamous cells (ASC)
- Normal cytology samples in women over the age of 30 years
- Samples with suspected glandular lesions (Cytojournal 2014;11:5)
Sample pathology report
- Cervix, Pap smear:
- Cytologic diagnosis:
- Specimen adequacy:
- Satisfactory for evaluation - endocervical component absent
- Specimen adequacy:
- Interpretation:
- Epithelial cell abnormality
- Low grade squamous intraepithelial lesion (LSIL)
- Fungal organisms morphologically consistent with Candida species
- Comment: This specimen has been analyzed by the ThinPrep Imaging System (Hologic Corp), along with an additional manual rescreening by a cytotechnologist or pathologist.
- Cytologic diagnosis:
- Human papillomavirus DNA, high risk:
- HPV 16: negative
- HPV 18: negative
- HPV OTH: positive
- Human papillomavirus 16, 18 or high risk pool (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) is detected by an FDA approved DNA amplification test. Positive for the high risk pool indicates that one or more of the genotypes was detected. A negative for any of the genotypes indicates that the genotype(s) was undetectable or below the threshold of the test.
Additional references
Board review style question #1
- What is the minimum number of well visualized or preserved squamous or squamous metaplastic cells in a liquid based preparation that must be present in order to be considered an adequate cervical cytology sample?
- 2,000
- 5,000
- 8,000
- 10,000
- 12,000
Board review style answer #1
B. 5,000. A minimum of 5,000 well visualized / well preserved squamous or squamous metaplastic cells is considered adequate for a liquid based preparation from a woman with a cervix. Certain circumstances based on the patient’s clinical history may allow for a lower adequacy threshold of 2,000 cells in the instance of postmenopausal atrophic changes, prior chemotherapy / radiation therapy or women posthysterectomy. The cellularity threshold for a conventional preparation is a minimum of 8,000 to 12,000 well preserved and well visualized squamous epithelial cells.
Comment Here
Reference: Bethesda system
Comment Here
Reference: Bethesda system
Board review style question #2
- Under the interpretation category of the Bethesda system, which epithelial cell abnormality is described by squamous cells with nuclear enlargement 3 times the size of the normal intermediate cell nucleus, hyperchromasia, anisonucleosis, uniform chromatin, bi or multinucleation and koilocytosis or perinuclear cavitation?
- Atypical glandular cells, not otherwise specified
- Atypical squamous cells of undetermined clinical significance (ASCUS)
- High grade squamous intraepithelial lesion (HSIL)
- Low grade squamous intraepithelial lesion (LSIL)
- Negative for intraepithelial lesion or malignancy (NILM)
Board review style answer #2
D. Low grade squamous intraepithelial lesion (LSIL). In contrast to normal squamous epithelial cells of the cervix (consistent with a diagnosis of NILM), squamous type epithelial cell abnormalities feature changes in nuclear size and features along a spectrum. ASCUS nuclei are enlarged by 2 to 3 times, have smooth to slightly irregular nuclear membranes, finely granular chromatin, inconspicuous nucleoli and questionable cytoplasmic cavitations. LSIL nuclei are enlarged by 3 to 4 times, have smooth to slightly irregular nuclear membranes, slightly more granular chromatin, absent nucleoli and diagnostic HPV cytoplasmic cavitations. HSIL nuclei are more variable than LSIL but the cytoplasmic area is decreased, resulting in a higher N:C ratio. HSIL nuclei have more irregular membranes, nuclear hyperchromasia with fine or coarse chromatin, generally absent nucleoli and can occur singly or in sheets or syncytial-like aggregates. Atypical glandular cells have features such as increased N:C ratio, mitotic activity, feathering and may show pseudostratification.
Comment Here
Reference: Bethesda system
Comment Here
Reference: Bethesda system
Board review style question #3
- Which interpretation category best describes single or small clusters of cells with variably sized nuclei, loss of nuclear polarity, nuclear hyperchromasia, prominent nucleoli, scant vacuolated cytoplasm, intracytoplasmic neutrophils and watery tumor diathesis?
- Adenocarcinoma, endocervical
- Adenocarcinoma, endometrial
- Adenocarcinoma, extrauterine
- Adenocarcinoma, not otherwise specified
- Endocervical adenocarcinoma in situ
Board review style answer #3
B. Adenocarcinoma, endometrial. Endometrial adenocarcinoma cells may be seen in Pap smears as single or small clusters of cells with variably sized nuclei, loss of nuclear polarity, nuclear hyperchromasia, prominent nucleoli, scant vacuolated cytoplasm, intracytoplasmic neutrophils and watery tumor diathesis. In contrast, endocervical adenocarcinoma cells may present as pseudostratified strips of cells with crowding, nuclear enlargement and peripheral feathering. Block positive p16 staining can help differentiate endocervical adenocarcinoma in situ (or invasive) from endometrial adenocarcinoma. Extrauterine adenocarcinoma will often present in a clean background as opposed to endometrial adenocarcinoma with tumor diathesis.
Comment Here
Reference: Bethesda system
Comment Here
Reference: Bethesda system