Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Tariq MU, Ud Din N. Osteofibrous dysplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/boneosteofibrousdysplasia.html. Accessed December 18th, 2024.
Definition / general
- Osteofibrous dysplasia (OFD) is a benign fibro-osseous tumor of the pediatric age group with strong predilection for the anterior tibial diaphysis
Essential features
- Usually occurs in first 2 decades of life, without gender predilection and most commonly involves anterior diaphysis of tibia
- Radiologically, tumor appears intracortical, multiloculated and radiolucent with well defined sclerotic margins
- Microscopically, woven bone trabeculae with osteoblastic rimming are seen against bland fibroblastic stroma with storiform pattern
- Maturation of bone trabeculae towards periphery (zonal architecture) and scattered keratin positive cells in the stroma are useful diagnostic features
- Has the potential to recur; tumors may regress after skeletal maturity
Terminology
- Ossifying fibroma of long bones (not recommended) (Arch Pathol 1966;82:218)
- Congenital fibrous defect of tibia (not recommended) (J Bone Joint Surg Am 1975;57:854)
ICD coding
- ICD-O: 9261/0 - osteofibrous dysplasia
- ICD-11: 2E83.5 & XH6M86 - benign osteogenic tumors of bone or articular cartilage of limbs and ossifying fibroma
Epidemiology
- Accounts for 0.2% of all primary bone tumors (Clin Radiol 2014;69:200)
- Age range: 3 days to 65 years, usually in first 2 decades of life; median: 10 years for males and 13.5 years for females (Hum Pathol 1993;24:1339, Radiol Case Rep 2021;17:825, Clin Radiol 2014;69:200, Yonsei Med J 2007;48:502)
- No gender predilection (Hum Pathol 1993;24:1339)
Sites
- Anterior tibial diaphysis is the most common location (> 95% of cases); synchronous involvement of fibula in 12% of cases (Clin Radiol 2014;69:200)
- Some tumors may be metaphyseal in location, multifocal and bilateral (Hum Pathol 1993;24:1339, Korean J Radiol 2014;15:114)
- Rarely, other bones can also be involved, such as the humerus, radius, ulna and clavicle (Rare Tumors 2018;10:2036361318808852, J Bone Joint Surg Br 2001;83:1178)
Pathophysiology
- Earlier studies had suggested some relationship between OFD and adamantinoma (it was unclear whether it was the precursor lesion or regressive form of adamantinoma); however, recent studies couldn't find any definitive evidence in favor of this hypothesis (J Bone Joint Surg Am 1994;76:1482, Mod Pathol 2012;25:56, Hum Pathol 1993;24:1339, Bone Joint J 2017;99-B:409)
- It has been hypothesized that the tumor arises as a result of misplacement of neural crest derived epithelial cells or as a result of traumatic implantation of epithelial cells into bone during fetal development (Surg Oncol 2021;38:101626)
- Some authors suggest that the mesenchymal to epithelial transformation occurs as a result of tumor regression (Mod Pathol 2012;25:56)
- No causative genetic abnormality has been identified; germline mutations of the MET gene have been observed in hereditary cases (Clin Oral Investig 2016;20:1709)
- Cutaneous / skeletal mosaic RASopathy cases may also develop OFD (Pediatr Dermatol 2020;37:890)
Etiology
- Etiology is unknown
- Familial cases have been rarely reported (Clin Oral Investig 2016;20:1709)
Clinical features
- Most lesions are incidentally found on imaging
- Can be associated with pain (31% of cases), pathologic fracture (19%), bowing deformity (13%) and localized swelling (Surg Oncol 2021;38:101626, Hum Pathol 1993;24:1339, Clin Radiol 2014;69:200)
- Tumor length ranges from 2 - 8.5 cm; mean: 6.1 cm (Skeletal Radiol 2008;37:1077)
- No causal connection or association
Diagnosis
- Xrays, MRI or CT scan are performed to assess tumor location, extent, pathological fracture and surgical staging
- Incisional / Tru-Cut biopsy is done to histologically confirm the diagnosis before undergoing tumor excision or curettage
- On small biopsy material, differentials should be given of OFD, OFD-like adamantinoma and classic adamantinoma because the latter 2 lesions also contain OFD-like areas (Surg Oncol 2021;38:101626, Orthopedics 2007;30:211, J Am Acad Orthop Surg 2010;18:358, Int J Surg Case Rep 2021;80:105599)
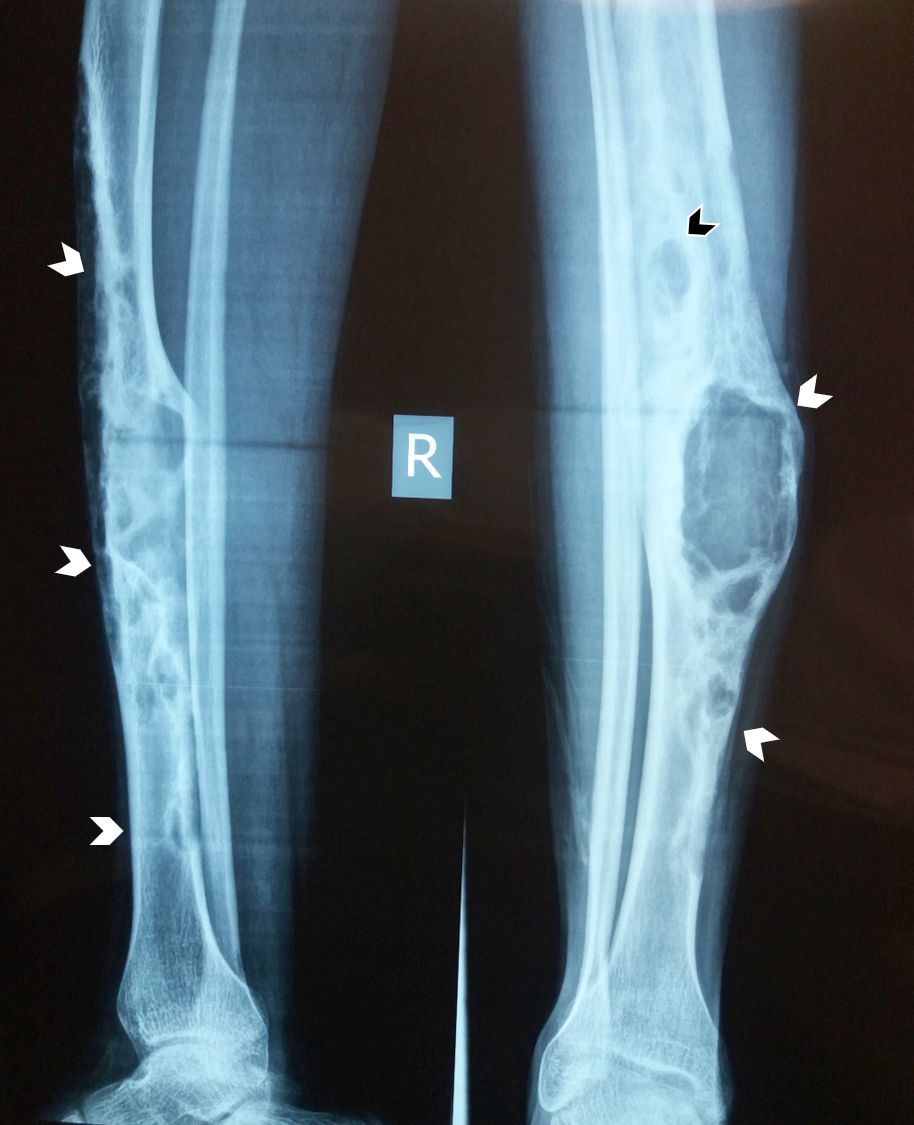
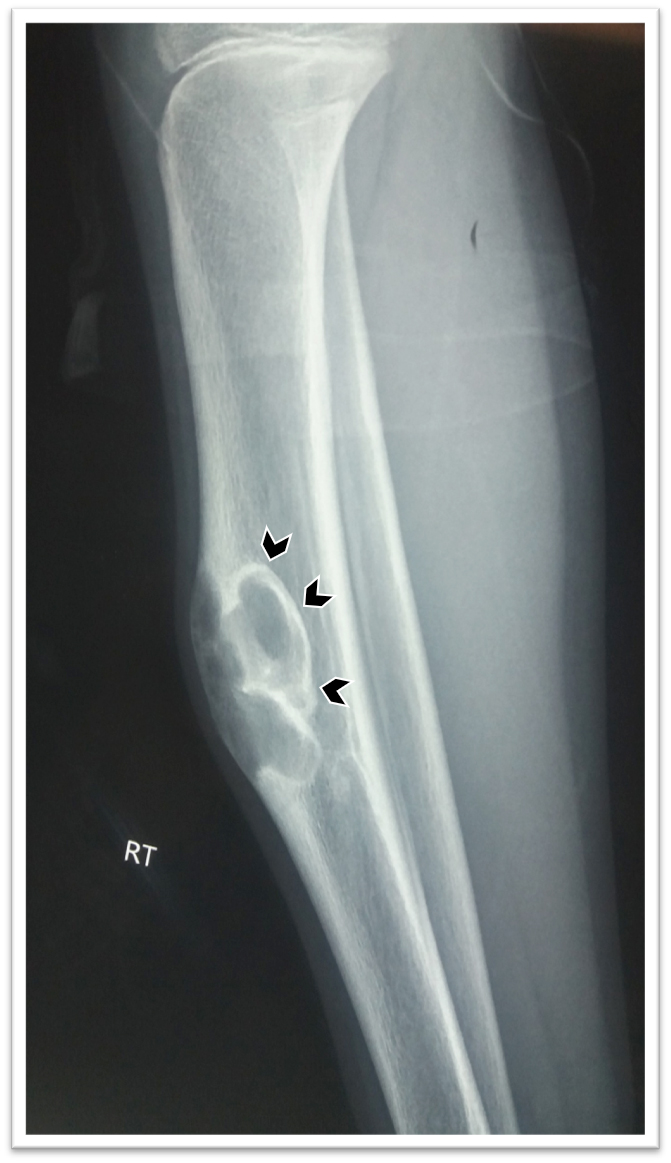
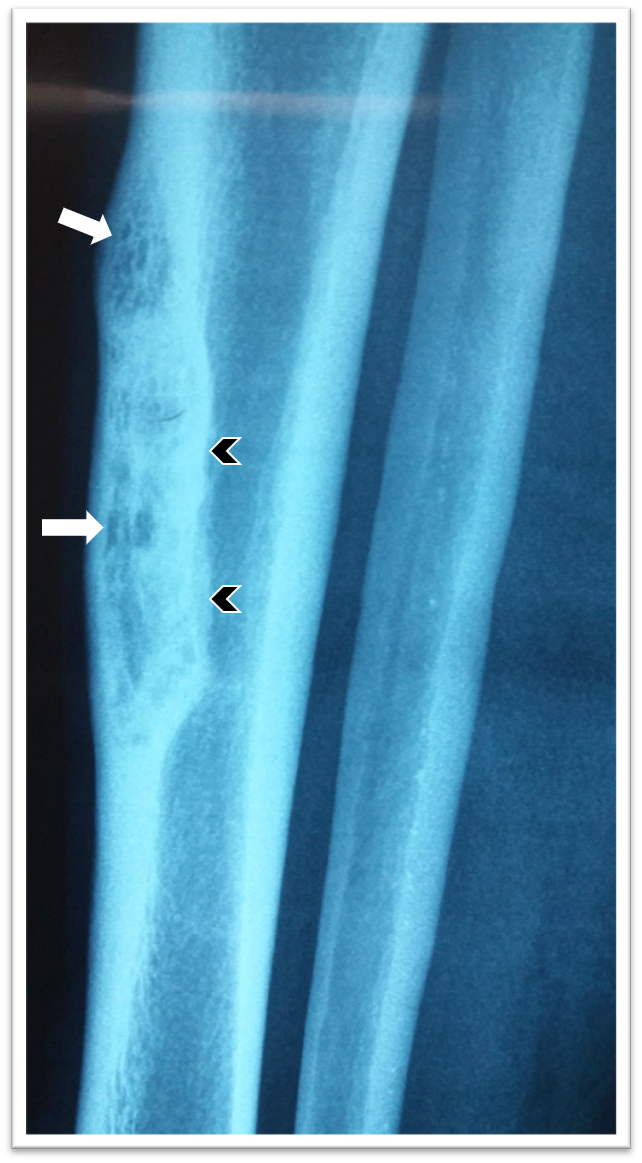
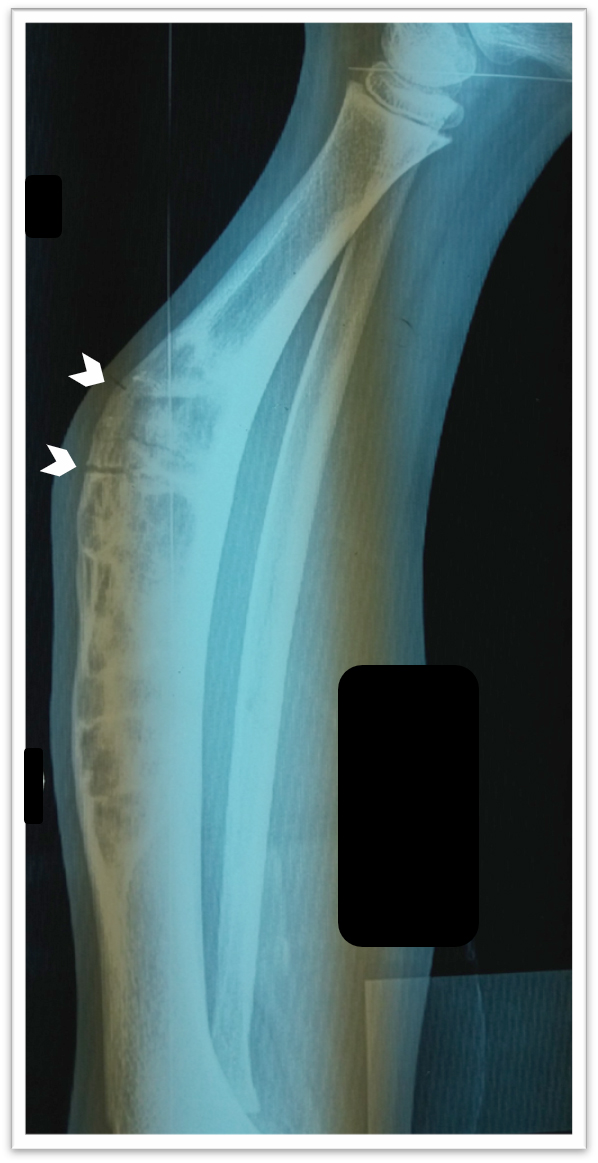
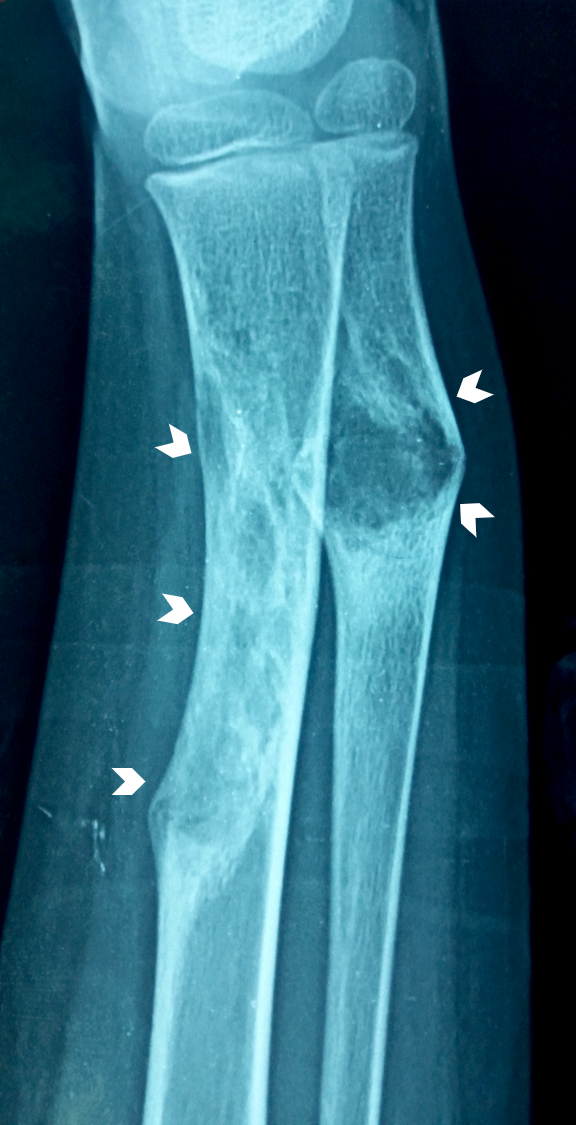
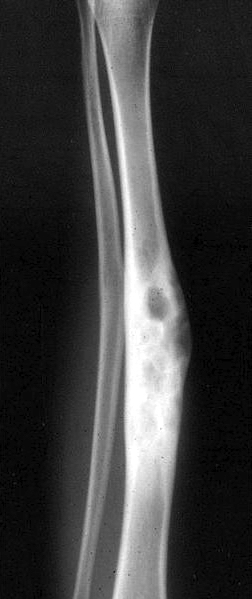
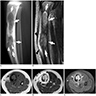
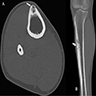
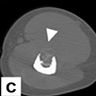
Radiology description
- Xray: typically intracortical, eccentric, multiloculated radiolucent / osteolytic lesion with internal septa and sharply defined sclerotic borders
- Some lesions may show ground glass appearance, marked sclerosis and ill defined borders
- Larger lesions cause cortical expansion, bowing deformity and fracture (Clin Radiol 2014;69:200, Surg Oncol 2021;38:101626)
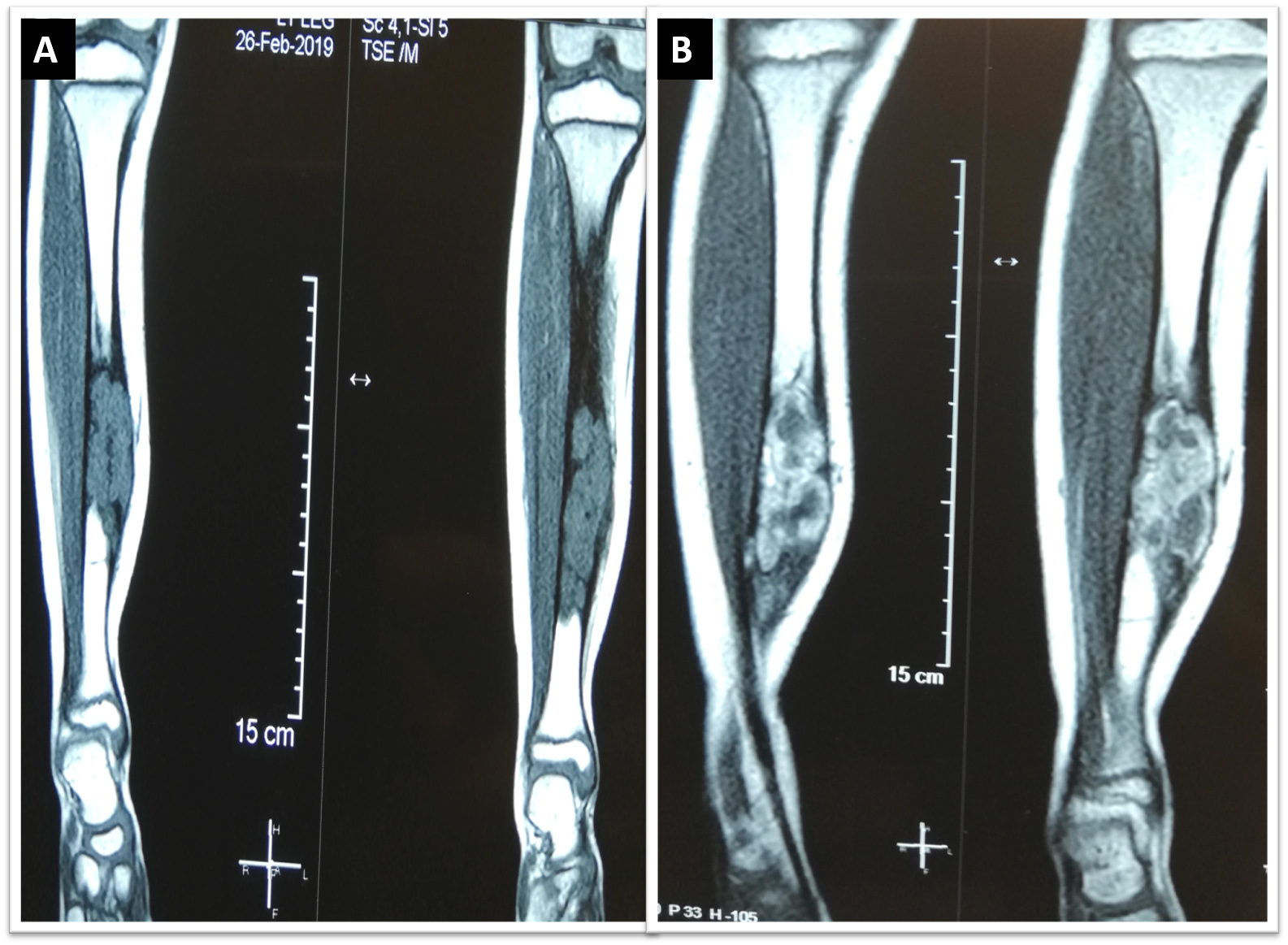
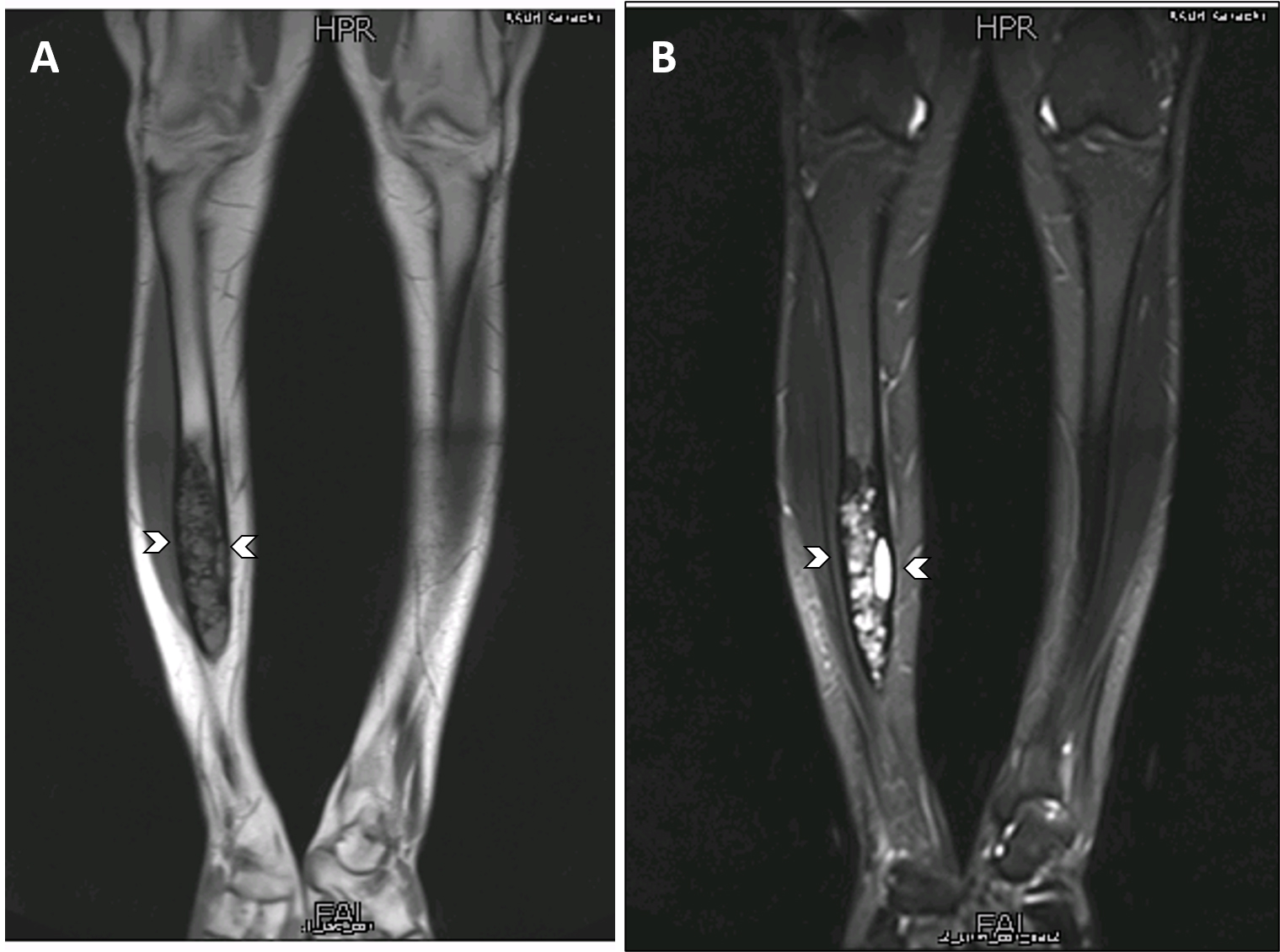
- On MRI scan, T1 weighted images show intermediate intensity signals while T2 weighted images show intermediate to hyperintense signals; with contrast, enhancement of homogenous or heterogenous patterns is observed (Korean J Radiol 2014;15:114)
- MRI scan is useful for assessing medullary involvement and surgical staging but not useful in discrimination from the differentials (Clin Radiol 2014;69:200)
- Involvement of the medullary cavity is uncommon; however, it has been reported in up to 40% of cases (Clin Radiol 2014;69:200)
- In a single study, partial or complete medullary involvement was observed on MRI scan in all 24 cases (Korean J Radiol 2014;15:114)
- CT scan is useful for assessing matrix mineralization, pathological fracture, lack of periosteal reaction and transitional zone; not superior to MRI scan (Clin Radiol 2014;69:200, Radiol Case Rep 2015;6:546)
Radiology images
Contributed by Nasir Ud Din, M.B.B.S., Mark R. Wick, M.D. and AFIP images
Images hosted on other servers:
Prognostic factors
- Favorable prognosis, tendency to recur, may regress after puberty (Surg Oncol 2021;38:101626)
- Recurrence rate after curettage and resection is 25% (Clin Radiol 2014;69:200)
Case reports
- 3 day old girl presenting with congenital osteofibrous dysplasia of the tibia (Radiol Case Rep 2021;17:825)
- 3 year old girl with a rapidly progressive osteofibrous dysplasia of the ulna (J Bone Joint Surg Br 2001;83:1178)
- 11 year old boy with a twice recurrent giant osteofibrous dysplasia of the tibia (Sarcoma 2004;8:51)
- 14 year old girl with osteofibrous dysplasia of the clavicle, clinically mimicking chronic osteomyelitis (Indian J Radiol Imaging 2016;26:290)
- 34 year old man with painful osteofibrous dysplasia of the humerus (Rare Tumors 2018;10:2036361318808852)
Treatment
- Surgical excision (with or without grafting) or curettage for larger tumors and cases with tibial bowing
- Some tumors are only observed, since the tumors regress after puberty (Surg Oncol 2021;38:101626)
- Some authors recommend extraperiosteal excision for all cases due to the low recurrence rate (J Bone Joint Surg Br 2006;88:658)
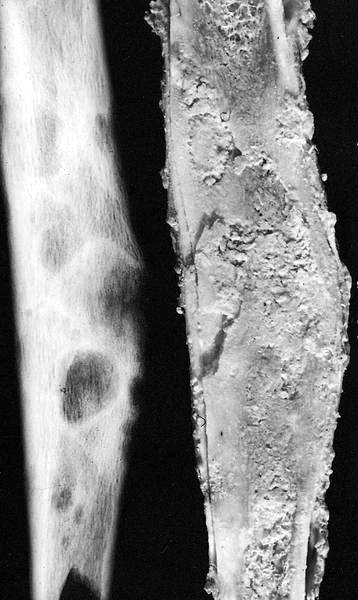
Gross description
- Usually confined to the cortex; yellow to white with gritty, fibrous consistency (Hum Pathol 1993;24:1339)
Gross images
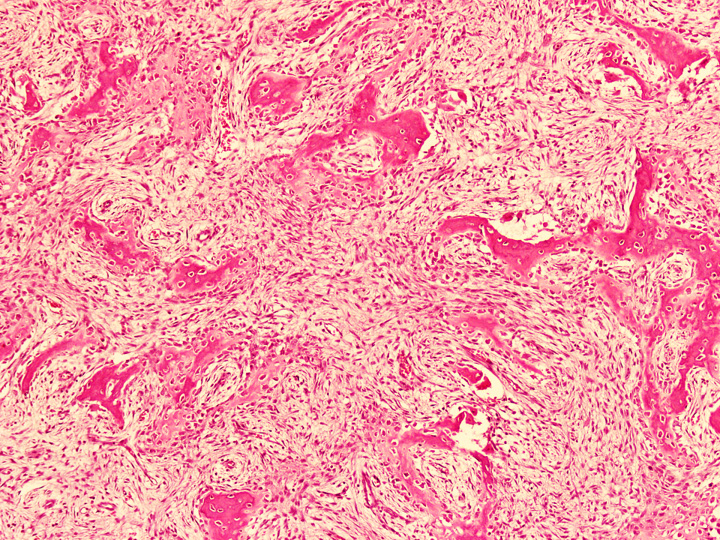
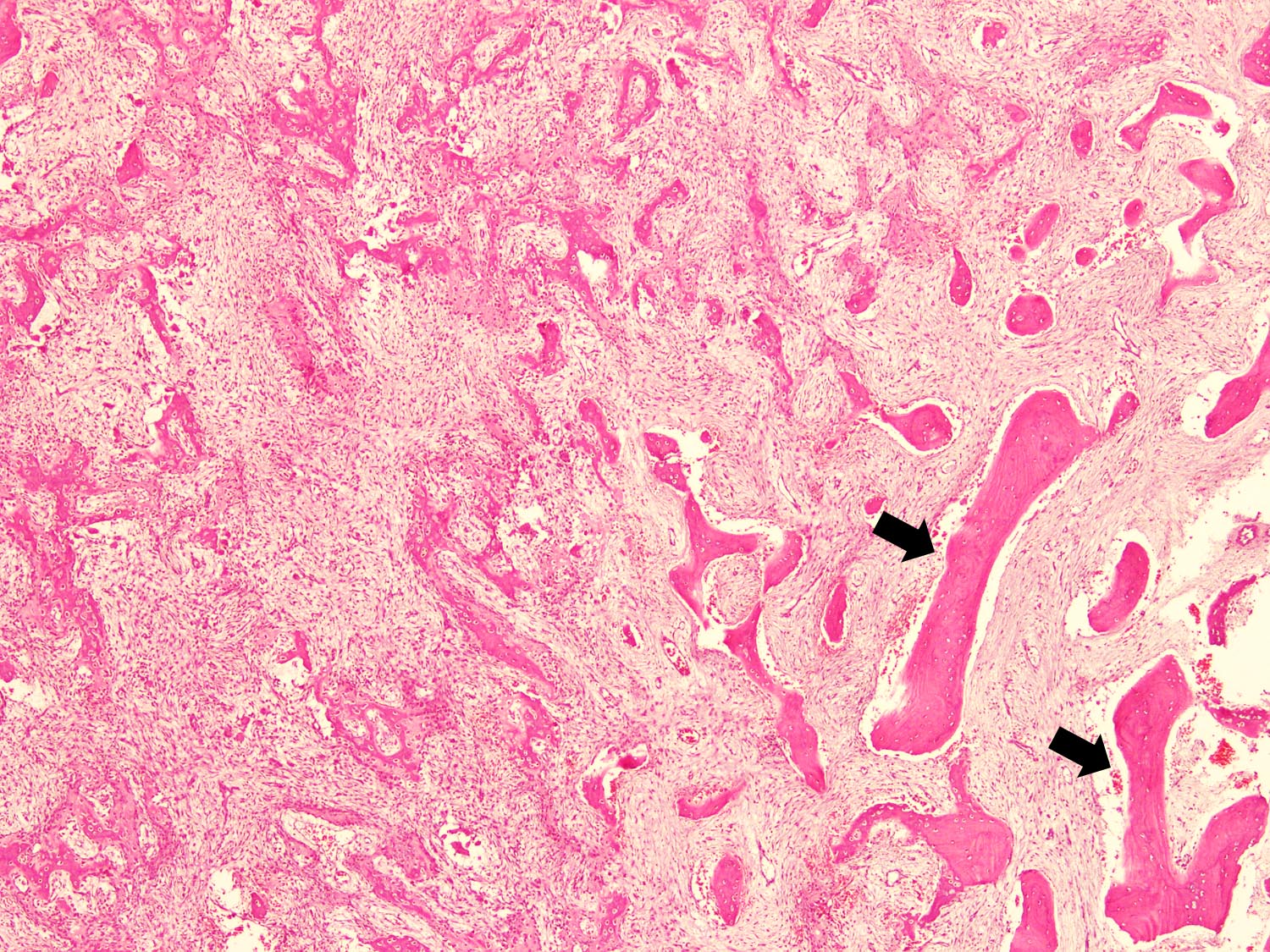
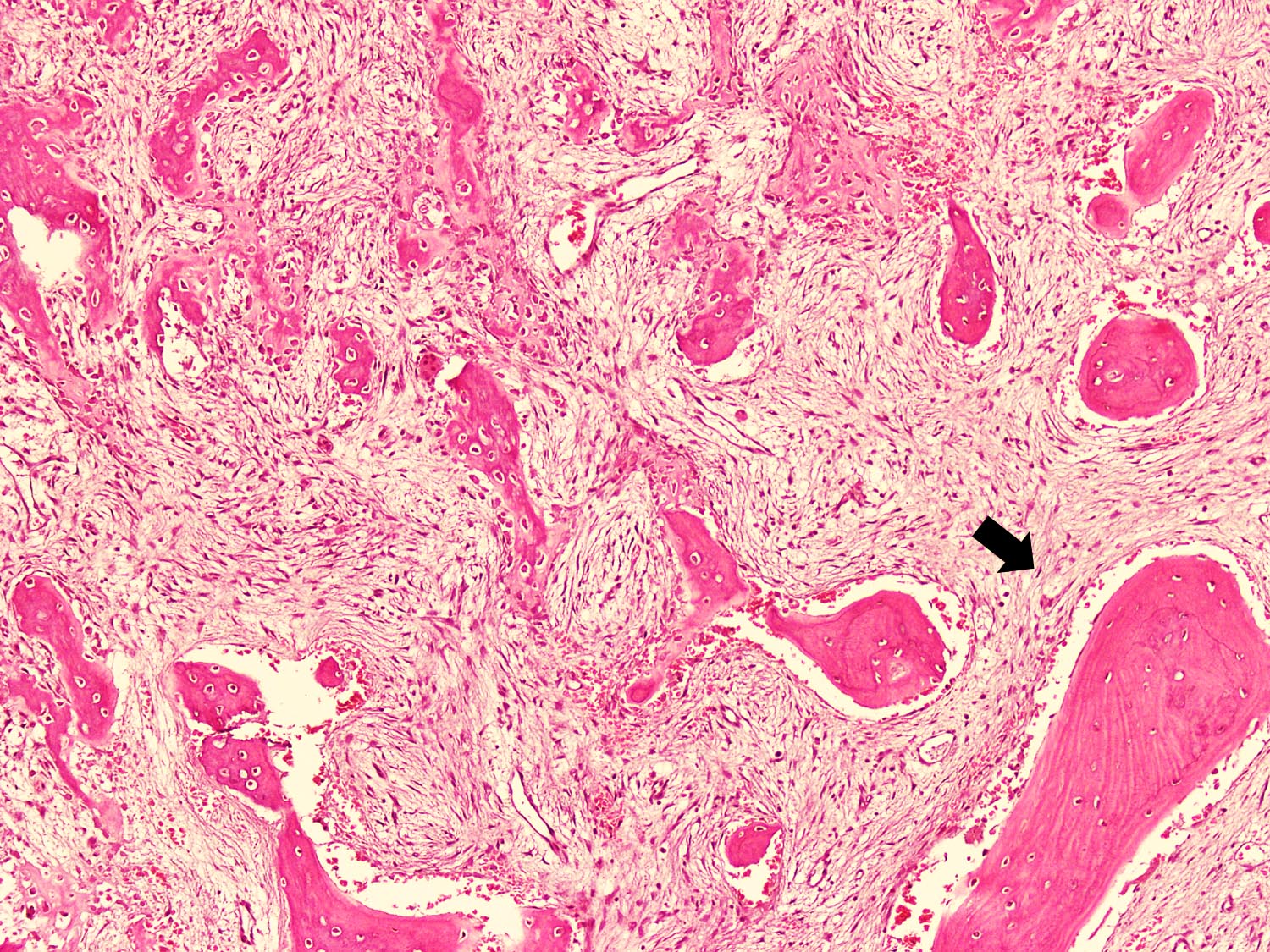
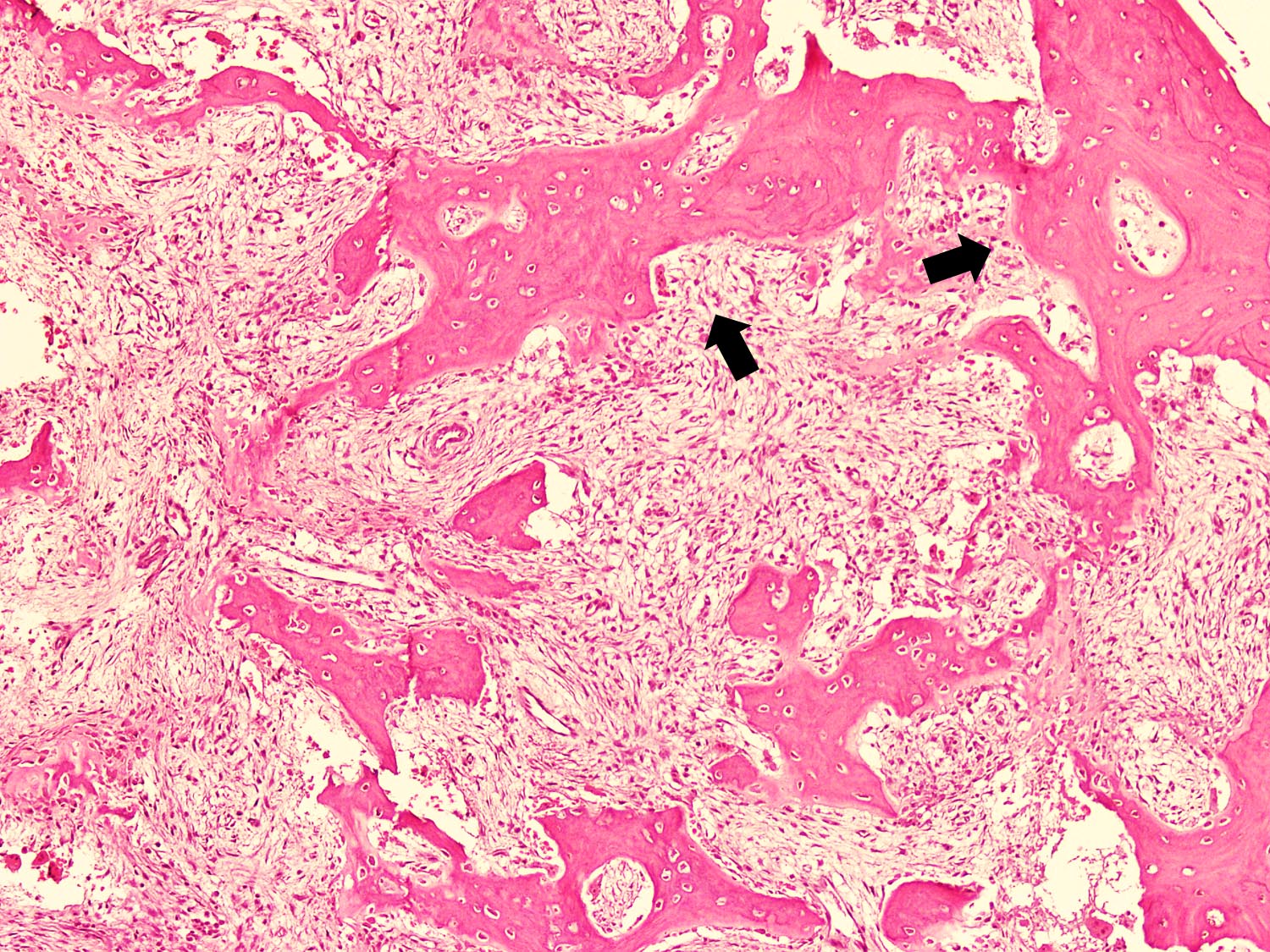
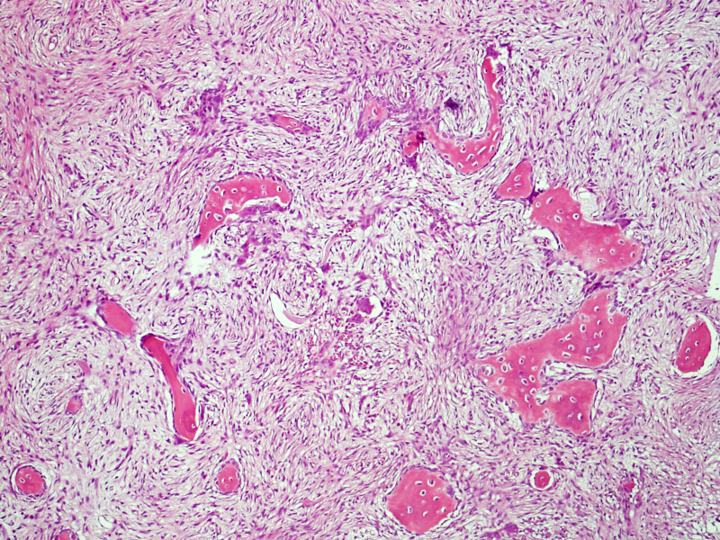
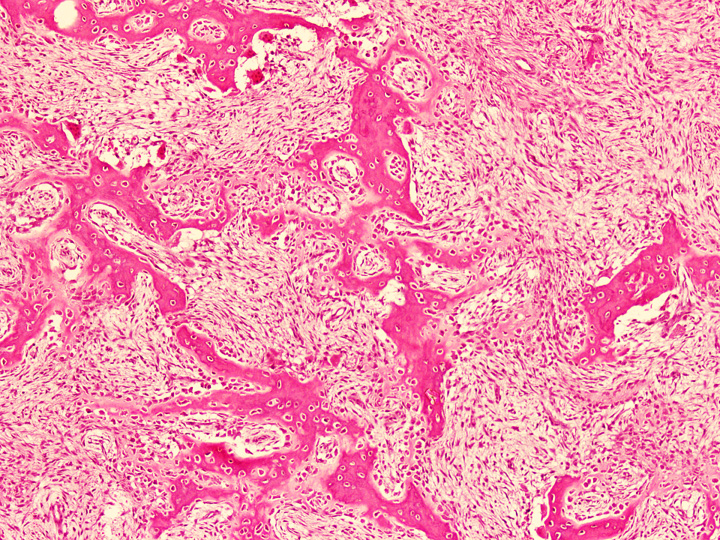
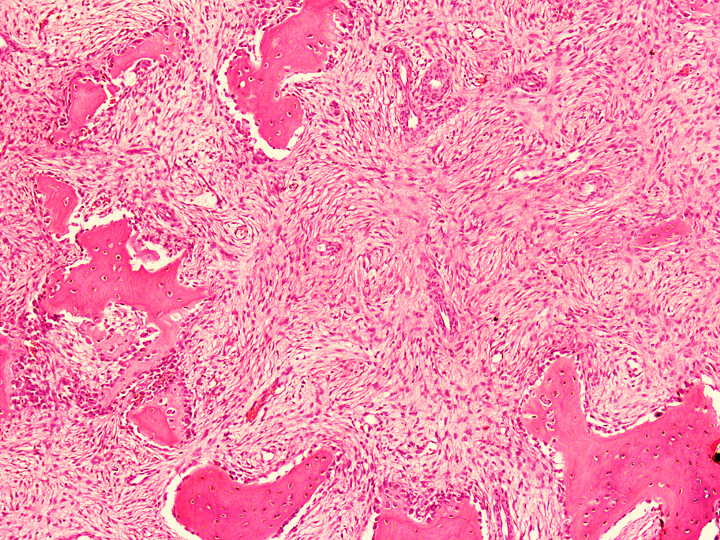
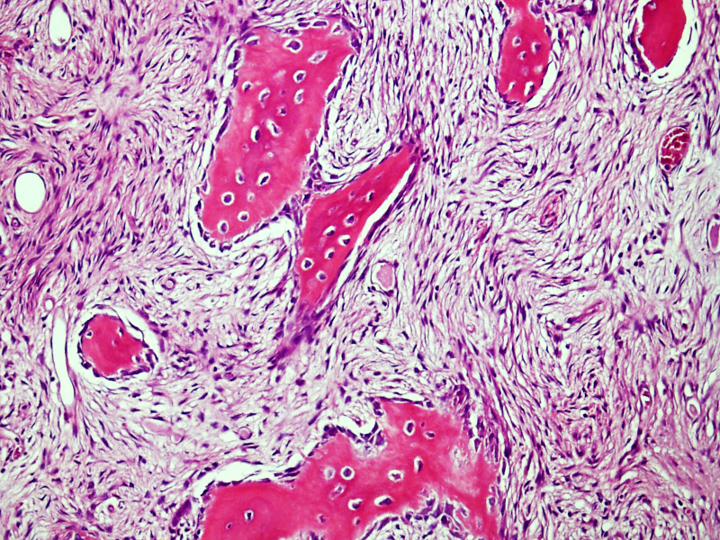
Microscopic (histologic) description
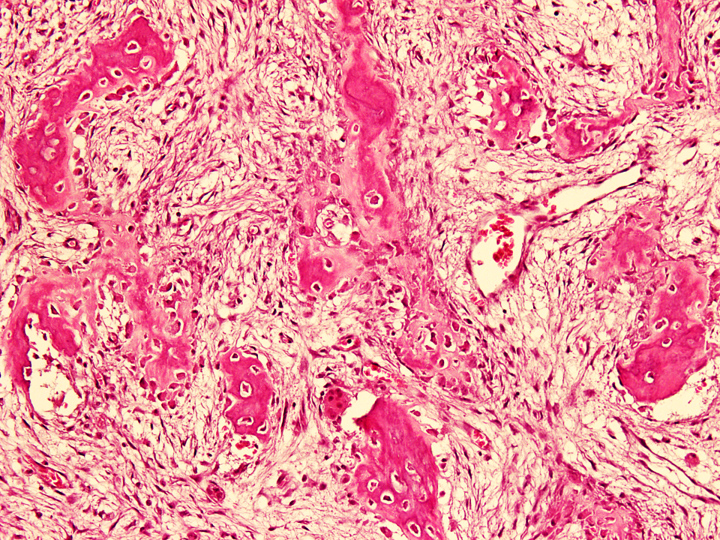
- Tumor has 2 basic components: fibrous stroma and bone trabeculae
- Zonation pattern is a characteristic feature
- In the center, the lesion is more fibrous and the newly formed woven bone trabeculae are thin
- Bone trabeculae become more numerous, thicker and mature (lamellar) and merge with the outer and inner cortices at the periphery
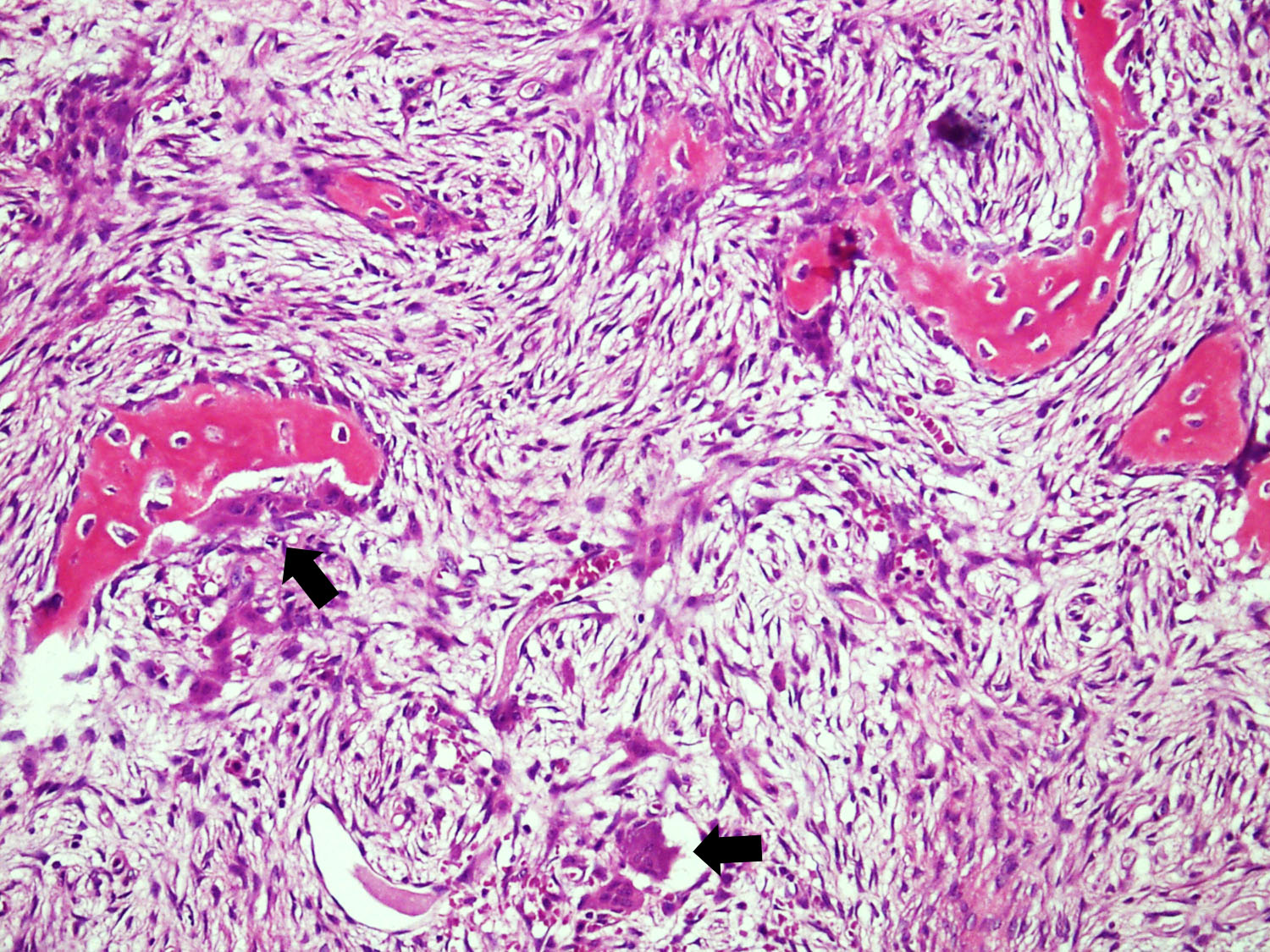
- Bone trabeculae are rimmed by epithelioid / active osteoblasts
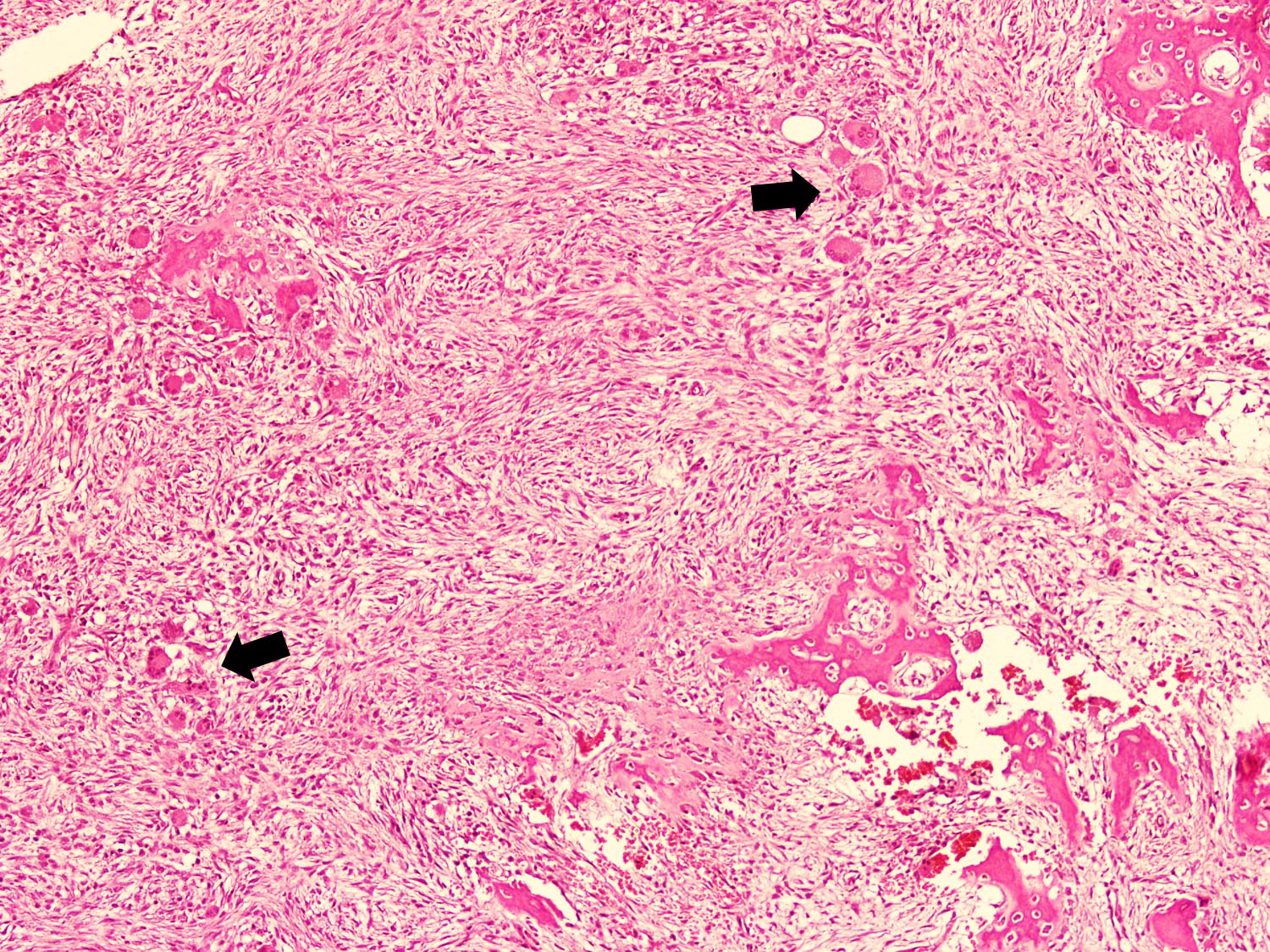
- Fibrous stromal component is composed of spindle to stellate cells arranged in short fascicles and vague storiform pattern; background is myxoid
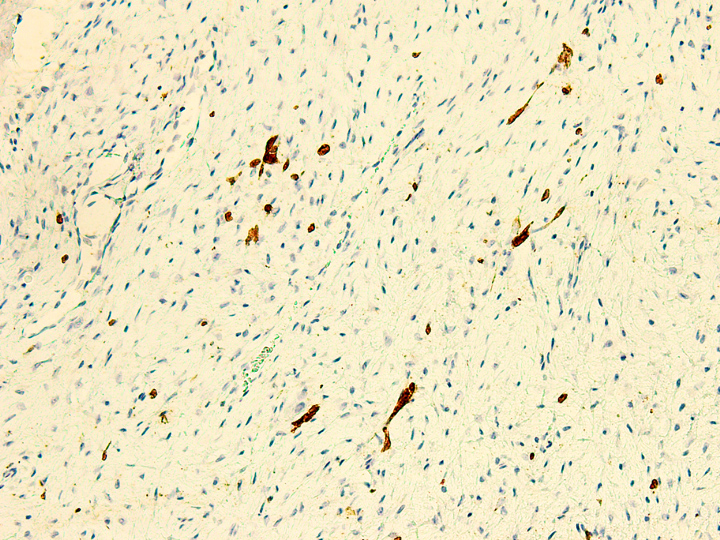
- Rare epithelial cells in the stroma may be highlighted on cytokeratin
- Scattered multinucleated giant cells, hemosiderin laden macrophages, hemosiderin and foamy macrophages may also be seen (Hum Pathol 1993;24:1339, Surg Oncol 2021;38:101626)
Microscopic (histologic) images
Contributed by Nasir Ud Din, M.B.B.S. and Mark R. Wick, M.D.
Positive stains
- Scattered epithelial cells in the stroma may show positivity for CK AE1 / AE3, CK1, CK5, CK14, CK17, CK19, EMA and p63 (Pathol Int 2000;50:801, Mod Pathol 2012;25:56, Virchows Arch 2011;459:109)
- TGFβ1 and TGFβ2 in fibroblasts and osteoblasts (Pathol Int 2000;50:801)
- Collagen IV, laminin, galectin3, osteonectin and fibronectin in stromal component (Hum Pathol 2004;35:69)
Electron microscopy description
- Stromal cells show fibroblast-like cells with prominent rough endoplasmic reticulum; epithelial differentiation was not seen
- Thickened woven bone trabeculae showed increased remodeling with osteoblastic and osteoclastic activity (Mod Pathol 2012;25:56)
Molecular / cytogenetics description
- Molecular / cytogenetic studies are not routinely performed
- In few cases, trisomies of chromosomes 7, 8, 12, 21 and 22 are detected by standard Giemsa banding and FISH techniques (Pediatr Dev Pathol 2004;7:148, Cancer 1994;73:1746)
- Trisomies have been identified only in neoplastic spindle stromal cells (Int J Surg Case Rep 2021;80:105599)
Videos
Osteofibrous dysplasia
Fibrous dysplasia and osteofibrous dysplasia: bone forming tumors,
part 4
Imaging osteofibrous dysplasia
Sample pathology report
- Tibia, Tru-Cut / incisional biopsy:
- Fibro-osseous tumor (see comment)
- Comment: Morphological features are those of osteofibrous dysplasia (OFD); however, the possibility of classic adamantinoma and OFD-like adamantinoma cannot be entirely ruled out because these lesions also contain areas with morphological features of OFD. Correlation with clinical and radiological findings is recommended. A more definitive diagnosis would be possible on excision biopsy specimen.
- Tibia, curettage / resection specimen:
- Fibro-osseous tumor, consistent with osteofibrous dysplasia (see comment)
- Comment: Histological examination showed a fibro-osseous tumor. The osseous component is composed of woven bone trabeculae rimmed by osteoblasts and fibroblastic stroma. CK AE1 / AE3 is positive in scattered stromal cells. No epithelial nests are seen. Correlation with clinical and radiological findings is recommended. OFD has the potential to recur locally; therefore, close follow up is advised.
Differential diagnosis
- Classic adamantinoma:
- Usually occurs in patients > 20 years of age (Mod Pathol 2012;25:56)
- Radiologically, adamantinomas are larger and more frequently and extensively involve the medullary cavity; may show ill defined (moth eaten) margins and soft tissue involvement (Clin Radiol 2014;69:200)
- Microscopically, islands and nests of epithelial cells with tubular, squamous, basaloid and spindle morphology are seen against osteofibrous background
- Low grade malignancy with metastatic potential (Clin Radiol 2014;69:200)
- Osteofibrous dysplasia-like adamantinoma:
- Presents with long history of dull pain
- Radiological distinction is difficult (Int J Surg Case Rep 2021;80:105599)
- Microscopically, similar to OFD on routine H&E stain, except for the presence of small nests and clusters of epithelial cells, which are highlighted on cytokeratin immunostains (Clin Radiol 2014;69:200)
- Osteoblasts and osteoclasts are less commonly seen than OFD (Int J Surg Case Rep 2021;80:105599)
- Fibrous dysplasia:
- Primarily a medullary lesion which may involve cortex (Clin Radiol 2014;69:200)
- Lacks zonation pattern, no maturation to lamellar bone, rare osteoblastic rimming, no keratin positive cells in stroma
- Presence of GNAS mutations (Hum Pathol 1993;24:1339)
- Osteoid osteoma and osteoblastoma:
- Radiologically, more extensive perilesional soft tissue and marrow edema, as compared to the size of nidus (Korean J Radiol 2014;15:114)
- Microscopically, osteoid deposition in the center and haphazardly arranged woven bone trabeculae with prominent osteoblastic rimming; loose fibrovascular stroma is present between the bone trabeculae (Am J Surg Pathol 2019;43:1661)
Additional references
Board review style question #1
A 10 year old boy complained of pain in his right leg. Xray of right leg showed a intracortical multiloculated, osteolytic lesion with a well defined sclerotic margin involving the diaphysis of tibia. Microscopically, the tumor showed trabeculae of woven bone with osteoblastic rimming. The background showed fibroblastic stroma with storiform pattern. On immunohistochemistry, only rare stromal cells showed positivity for cytokeratin. What is the most likely diagnosis?
- Fibrous dysplasia
- Nonossifying fibroma
- Osteofibrous dysplasia
- Osteofibrous dysplasia-like adamantinoma
- Osteoid osteoma
Board review style answer #1
C. Osteofibrous dysplasia. Clinical, radiological, histological and immunohistochemical features are characteristic of osteofibrous dysplasia. Fibrous dysplasia is a fibroosseous lesion that usually lacks osteoid rimming of bone trabeculae. Nonossifying fibroma lacks bone trabeculae. Osteofibrous dysplasia-like adamantinoma closely resembles osteofibrous dysplasia. On IHC, small clusters and cords of cytokeratin positive epithelial cells are seen in the stroma rather than scattered epithelial cells seen in osteofibrous dysplasia. Osteoid osteoma shows loose fibrovascular stroma between bone trabeculae.
Comment Here
Reference: Osteofibrous dysplasia
Comment Here
Reference: Osteofibrous dysplasia
Board review style question #2
Which of the following is the most helpful feature in differentiating osteofibrous dysplasia from osteofibrous dysplasia-like adamantinoma?
- Bone trabeculae without osteoblastic rimming
- Clusters and small nests of epithelial cells
- Patient's age
- Radiological findings
- Tumor location
Board review style answer #2
B. Clusters and small nests of epithelial cells. Osteofibrous dysplasia-like adamantinoma closely resembles osteofibrous dysplasia and the distinction is not possible clinically or radiologically. The differentiating feature on histology is the presence of cluster, cords and small nests of epithelial cells in the stroma of osteofibrous dysplasia-like adamantinoma, which are further highlighted on cytokeratin immunostains. Histologically, epithelial cells are not seen in the stroma of osteofibrous dysplasia and only scattered cytokeratin positive epithelial cells are highlighted on cytokeratin immunostains. Absence of osteoblastic rimming around bone trabeculae is a feature of fibrous dysplasia.
Comment Here
Reference: Osteofibrous dysplasia
Comment Here
Reference: Osteofibrous dysplasia






%20xray.jpg)
%20xray4.jpg)
%20gross.jpg)
%20keratin%20immunost.jpg)