Table of Contents
Acquired adrenocortical hyperplasia | Adrenal cytomegaly | Congenital adrenal hyperplasia | Macronodular hyperplasia | Macronodular hyperplasia with marked adrenal enlargement | Micronodular hyperplasia | Pigmented adrenal cortical hyperplasiaCite this page: Gellert L, Pernick N. Adrenal hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/adrenalacquiredhyper.html. Accessed April 1st, 2025.
Acquired adrenocortical hyperplasia
Definition / general
Case reports
Gross description
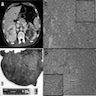
Gross images
Images hosted on other servers:
Microscopic (histologic) description
Molecular / cytogenetics description
Electron microscopy description
Differential diagnosis
- Defined as nonneoplastic increase in adrenal cortical cells
- Diffuse (62%) or nodular (20%) or hyperplasia with ectopic ACTH syndrome due to tumors (18%)
- Diffuse cases are usually bilateral, associated with elevated ACTH produced by pituitary gland, ACTH producing tumor or excess corticotropin releasing factor production and rarely due to ACTH receptor autoantibodies (as with Grave disease)
- Nodular cases usually are unrelated to ACTH production; may represent a later stage of diffuse hyperplasia, in which the lesion has evolved from ACTH dependent to adrenal gland dependent
- Associated with MEN1 (not neoplastic in one case); also thyroid neoplasms, leiomyomas, hepatic focal nodular hyperplasia and renal angiomyoliomas (Mod Pathol 1999;12:919)
Case reports
- 42 year old woman with lipomatous metaplasia (Arch Pathol Lab Med 1999;123:167)
Gross description
- Nodular variant: at least one nodule 0.5 cm in diameter, often diffusely nodular adrenal cortex which weighs ≥ 6 grams without fat; glands have rounded edges
Gross images
Images hosted on other servers:
Microscopic (histologic) description
- Increased thickness of zona reticularis and fasciculata
- Cells in fasciculata may appear lipid depleted
- Atypical cells with large hyperchromatic nuclei may be present in nodules (endocrine atypia)
- Small micronodules may be present near central vein, containing lipid laden clear cells similar to zona fasciculata
Molecular / cytogenetics description
- Cells may be aneuploid or polyploid
Electron microscopy description
- Abundant smooth endoplasmic reticulum, long microvilli
Differential diagnosis
- Adenoma:
- Single nodule, clonal
Adrenal cytomegaly
Definition / general
Case reports
Gross description
Microscopic (histologic) description
Differential diagnosis
- Relatively common finding within fetal cortex in newborns, particularly premature infants (3 - 7%) or infants with Rh incompatibility
- May be associated with Beckwith-Wiedemann syndrome
- Cells do not represent carcinoma in situ
- Pseudoinclusions (Arch Pathol Lab Med 1981;105:358)
Case reports
- Occurrence in 2, apparently normal adults (Arch Pathol Lab Med 1986;110:1072)
Gross description
- Hyperplastic adrenal glands
Microscopic (histologic) description
- Numerous, markedly enlarged (up to 150 microns) and bizarre polyhedral cells with eosinophilic, granular cytoplasm and large, hyperchromatic nuclei with pseudoinclusions in adrenal cortex
- No / rare mitotic figures
Differential diagnosis
- CMV infection:
- Usually infants, basophilic cytoplasm and large intranuclear inclusion surrounded by a clear halo
Congenital adrenal hyperplasia
Definition / general
Clinical features
Etiology
Treatment
Gross description
Microscopic (histologic) description
Differential diagnosis
- Various autosomal recessive syndromes due to enzyme deficiencies in biosynthesis of adrenal steroids, diverting production to other pathways and causing elevated ACTH levels and adrenocortical hyperplasia
Clinical features
- Usually children, rarely adults; no gender preference
- Symptoms depend on specific defect; include salt wasting, virilization, adrenogenital syndrome and hypertension
- Salt wasting syndrome: usually evident soon after birth (in utero, maternal kidneys maintain electrolytes and fluids); hypotension due to decreased serum sodium and increased serum potassium (from lack of aldosterone production), acidosis, cardiovascular collapse and death
- Simple virilizing syndrome: easier to detect in females (clitoral hypertrophy) than males
- Non-classic virilizing syndrome: more common than simple virilizing syndrome; asymptomatic or only hirsutism
- Adrenogenital syndrome: adrenal secretes excess androgens, causing changes towards adult masculinity in children or female adults; 50% occur before puberty, 80% are female; diagnose based on elevated dehydroepiandrosterone; rarely associated with male adult feminization due to increased 17-ketosteroids
- Congenital adrenal hyperplasia tumors: virilization in adult women is usually associated with carcinoma, particularly if Cushings syndrome also present; feminization in adult men is almost always associated with carcinoma; congenital adrenal hyperplasia is associated with testicular tumors that arise from ectopic adrenal cortical rests and rarely with similar ovarian tumors (Arch Pathol Lab Med 2000;124:785, Am J Surg Pathol 2001;25:1443)
- Congenital lipoid adrenal hyperplasia: very rare, low cortisol and aldosterone secretion, high levels of ACTH, FSH, LH and plasma rennin; present with severe adrenal insufficiency in neonatal period; usually die in infancy
Etiology
- Specified enzyme deficiencies
- 21-hydroxylase deficiency: causes 95% of cases; incidence of 1 per 5,000 to 14,500 births (1 in 60 in the normal population are heterozygotes); block in production of aldosterone and cortisol leads to accumulation of 17-hydroxypregnenolone and its catabolite pregnanetriol, also high plasma ACTH; causes virilizing syndrome, cortisol deficiency and variable salt wasting syndrome; also Leydig cell hyperplastic nodules without Reinke crystalloids
- Non-classic 21-hydroxylase deficiency: very common autosomal recessive disorder (1% incidence in parts of US), with mild cortisol deficiency, excessive adrenal androgens and no salt wasting; usually diagnosed by early adulthood
- 11-beta hydroxylase deficiency: second most common form (5%), incidence of 1 per 100,000 live births; associated with increased androgens and deoxycorticosterone; causes virilization and hypertension
- 17-alpha hydroxylase deficiency: causes 1% of cases, all patients have female external genitalia due to increased deoxycorticosterone; also hypertension
- 3 beta hydroxysteroid dehydrogenase deficiency: impaired synthesis of all steroid hormones, adrenal gland is similar to normal fetus; patients present in early infancy with adrenal insufficiency, variable virilization in females
Treatment
- Exogenous glucocorticoids and mineralocorticoids to provide cortisol and suppress ACTH levels
- Surgical correction of external genitalia
Gross description
- Marked adrenal enlargement (15g each gland) with cerebriform appearance, tan-brown
- Secondary to elevated ACTH (due to reduced cortisol secretion)
Microscopic (histologic) description
- Diffuse cortical hyperplasia, particularly of zona reticularis-like compact cells
Differential diagnosis
- Bilateral hyperplasia due to ectopic ACTH:
- Not grossly cerebriform, may have metastatic carcinoma and differentiate clinically
Macronodular hyperplasia
Definition / general
Laboratory
Treatment
Gross description
Microscopic (histologic) description
Differential diagnosis
- Large (≥ 0.5 cm), bilateral nodules of histologically unremarkable adrenocortical tissue in adrenal cortex or protruding into adjacent adipose tissue
- Rare (< 100 cases reported)
- Mean age 45 - 55 years
Laboratory
- Elevated plasma cortisol not suppressed by dexamethasone, low serum ACTH
Treatment
- Bilateral adrenalectomy, does not appear to recur
Gross description
- Enlarged adrenal glands, up to 200 grams
- May be massive
- Nodules from 0.2 to 4.0 cm, yellow-tan and not encapsulated
Microscopic (histologic) description
- Nodules composed of clear and compact cells with variable lipid
- Variable myelolipomatous change, osseous metaplasia or atrophic cortex between nodules
- No / rare mitotic figures or atypia
Differential diagnosis
Macronodular hyperplasia with marked adrenal enlargement
Definition / general
Essential features
Clinical features
Case reports
Treatment
Clinical images
Images hosted on other servers:
Gross description
Gross images
Images hosted on other servers:
Microscopic (histologic) description
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Differential diagnosis
- ACTH independent multinodular adrenocortical proliferation
- Usually bilateral
- Bimodal age distribution: newborns and age 40+
- Occasionally associated with McCune-Albright syndrome in children
- Occasionally associated with hereditary leiomyomatosis and renal cell cancer (HLRCC) in adults (J Clin Endocrinol Metab 2005;90:3773)
Essential features
- ACTH independent multinodular adrenocortical proliferation
- Usually bilateral
- Occasionally associated with hereditary leiomyomatosis and renal cell cancer (HLRCC) or McCune-Albright syndrome
Clinical features
- Usually present with either typical or atypical Cushing syndrome
Case reports
- 29 year old man with incidentally discovered bilateral adrenal enlargement (J Clin Endocrinol Metab 2011;96:E243)
- 31 year old woman with bilateral ovarian steroid cell tumors, massive macronodular adrenocortical disease and renal cell carcinoma (Pathology 2012;44:360)
- 46 year old man with bilateral nodular adrenal enlargement and Cushing syndrome (Internist Berl 2007;48:870)
- 65 year old woman with hereditary leiomyomatosis and massive macronodular adrenocortical disease (J Clin Endocrinol Metab 2005;90:3773)
- Four patients with adrenocorticotropic hormone (ACTH) independent bilateral massive adrenal gland enlargement (J Comput Assist Tomogr 1991;15:773)
Treatment
- Adrenalectomy
Clinical images
Images hosted on other servers:
Gross description
- Glands up to 180 g, nodules up to 4 cm and nonnodular cortex may be atrophic
Gross images
Images hosted on other servers:
Microscopic (histologic) description
- Predominantly diffuse and vaguely nodular proliferation of clear cortical cells
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Differential diagnosis
- Adrenalcortical adenoma or carcinoma
- Metastatic carcinoma:
- AE1 / AE3 positive
- Pigmented micronodular adrenocortical disease:
- Multiple small, pigmented nodules are characteristic
Micronodular hyperplasia
Definition / general
Gross description
Microscopic (histologic) description
Differential diagnosis
- Small (< 0.5 cm) nodules of histologically unremarkable adrenocortical tissue in adrenal cortex or protruding into adjacent adipose tissue
- Often multiple and bilateral; microscopic or grossly visible
- Associated with older age, hypertension and diabetes
- At autopsy, present in 3% of all ages, 20% with hypertension and 29% of women with mean age 81 years
- May represent localized overgrowth of adrenocortical cells
- Usually nonfunctional; no clinical significance
Gross description
- Unencapsulated, may protrude into capsule and may completely detach from capsule
Microscopic (histologic) description
- Cortical cells may stream into periadrenal fat
- Adrenal arteries often are hyalinized with intimal proliferation that causes luminal obliteration
- May have neuromelanin pigment; may undergo myelolipomatous or osseous metaplasia
Differential diagnosis
- Adrenocortical adenoma:
- Usually solitary, circumscribed
- Adrenocortical carcinoma:
- Mitotic activity, atypical mitotic figures and necrosis
Pigmented adrenal cortical hyperplasia
Definition / general
Laboratory
Treatment
Gross description
Microscopic (histologic) description
Electron microscopy description
Differential diagnosis
- Also called primary pigmented nodular adrenocortical disease
- Rare cause of ACTH independent Cushing syndrome (Mod Pathol 1992;5:23)
- May be familial, autosomal dominant and associated with Carney complex [cutaneous abnormalities in 80% (lentigenes, blue nevi, ephelides, myxomas), cardiac myxomas (72%, may be life threatening), large cell calcifying Sertoli tumors and Leydig cell tumors of testis (56%), primary pigmented nodular adrenocortical disease with Cushing syndrome (32 - 45%), myxoid fibroadenomas of breast in females (42%), growth hormone secreting pituitary adenomas (10%), uterine myxomas (8%), oral cavity myxomas (8%) and psammomatous melanotic schwannomas (5%); also neurofibromatosis, cerebral hemangioma]; associated with 2p16 abnormalities
- Sporadic and nonfamilial patients are usually infants or age < 30 years
- Note: Carney complex is also called LAMB syndrome (Lentigenes, Atrial myxomas, Mucocutaneous myxomas, Blue nevi), NAME syndrome (Nevi, Atrial Myxomas, Myxoid neurofibroma, Ephelides) or Swiss syndrome
- References: Am J Surg Pathol 1989;13:921, Am J Surg Pathol 1984;8:335
Laboratory
- Moderately elevated plasma cortisol but no diurnal rhythm, resistant to dexamethasone suppression and low / undetectable plasma ACTH
Treatment
- Bilateral adrenalectomy
Gross description
- Variably sized adrenal glands with multiple brown-black pigmented cortical nodules, 1 mm to 3 cm and atrophy of adjacent cortical tissue
- Nodules may extend into corticomedullary junction or periadrenal fat
Microscopic (histologic) description
- Sharply circumscribed but unencapsulated nodules composed of large eosinophilic lipid poor cells similar to zona reticularis but often with enlarged pleomorphic nuclei, prominent nucleoli and prominent lipofuscin deposits
- Also lipid rich fasciculata-like cells
- May have focal necrosis, mitotic activity, trabecular growth pattern, myelolipomatous change and lymphocytic infiltrates
Electron microscopy description
- Zona reticularis and fasciculata type cells
- Abundant lipofuscin type bodies
Differential diagnosis
- Melanoma