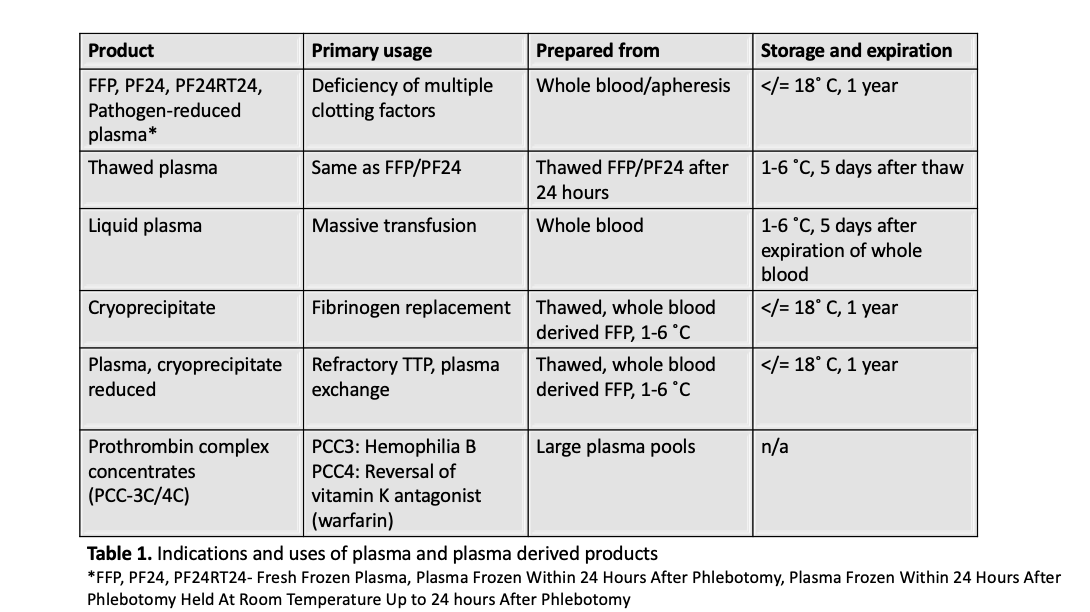
Superpage
Superpage Topics
ABO / H system
ABO discrepancies (pending)
ABO incompatible HSCT
Acute hemolytic transfusion reaction (AHTR)
Adsorption studies
Allergic / anaphylactic
Antibody identification & panel interpretation (pending)
ASFA guidelines overview
Automation
Bacteria
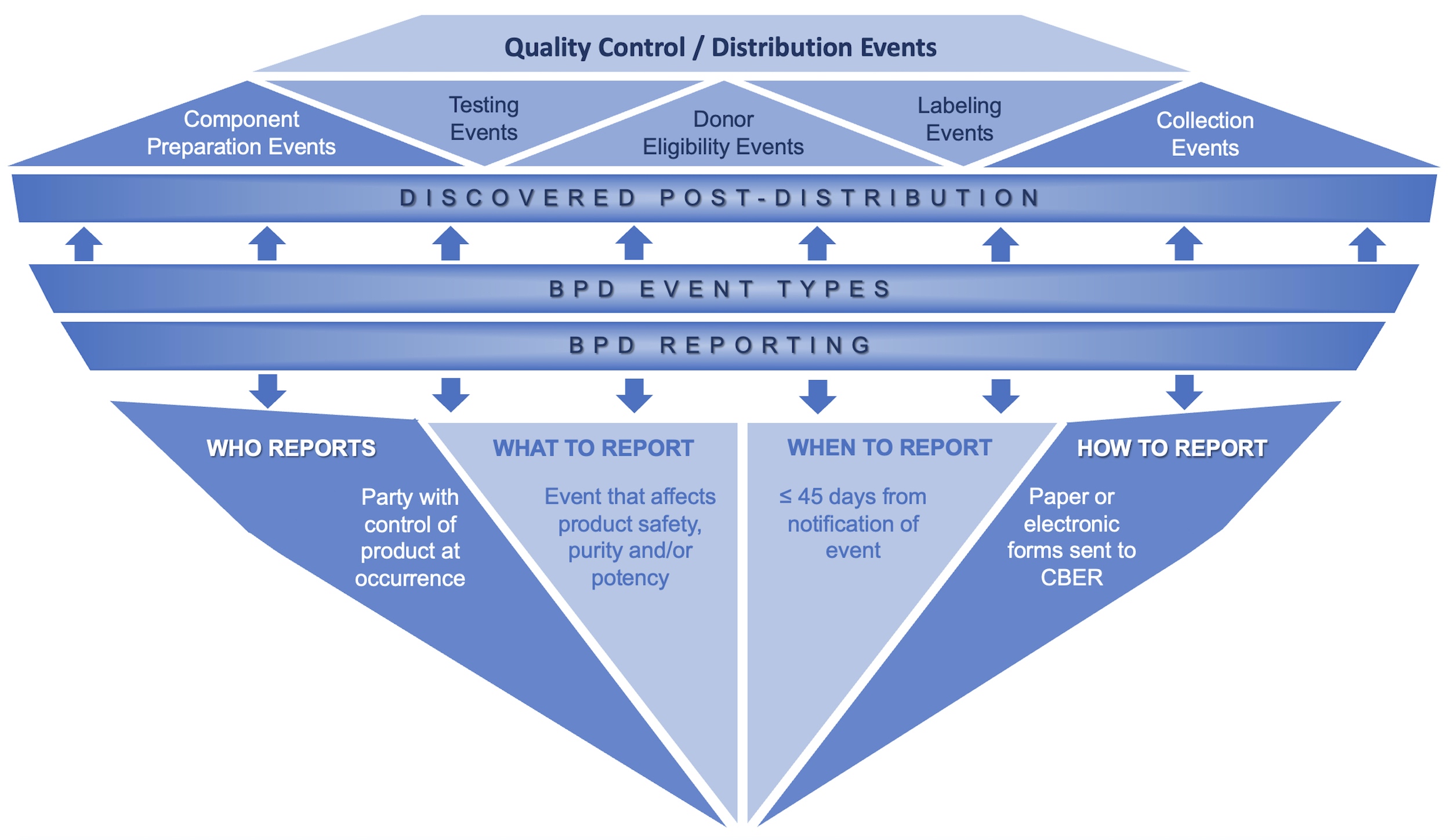
Biological product deviation
Blood alternatives (pending)
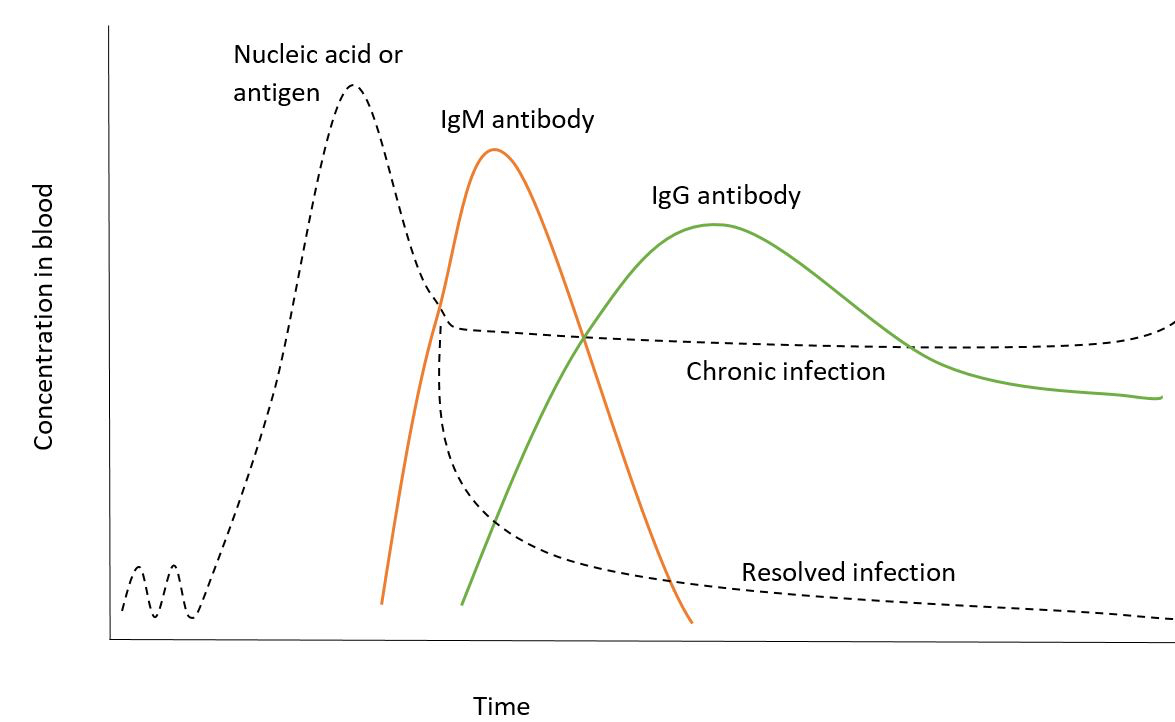
Blood donor testing
CAR T cell therapy
CAR T cell therapy
Cellular therapy reactions
Cold stored platelets
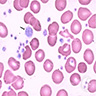
Coombs test / DAT
COVID-19 convalescent plasma
Cryoprecipitate use
DAT negative autoimmune hemolytic anemia
Delayed hemolytic and delayed serologic reactions
Donor collection
Donor eligibility
Donor reactions
DTT for monoclonal antibody (datatumumab) workup (pending)
Duffy system
Enzyme treatment (pending)
Ethics (pending)
FACT compliance (pending)
Febrile nonhemolytic
Fetal / neonatal alloimmune thrombocytopenia
Granulocyte use
Hemolytic disease of the fetus and newborn
Hemovigilance
Hemovigilance (pending)
Hypotension
Ii system
Iron overload
Irradiation
Kell system
Kidd system
LDL apheresis
Leukocytapheresis
Lewis system
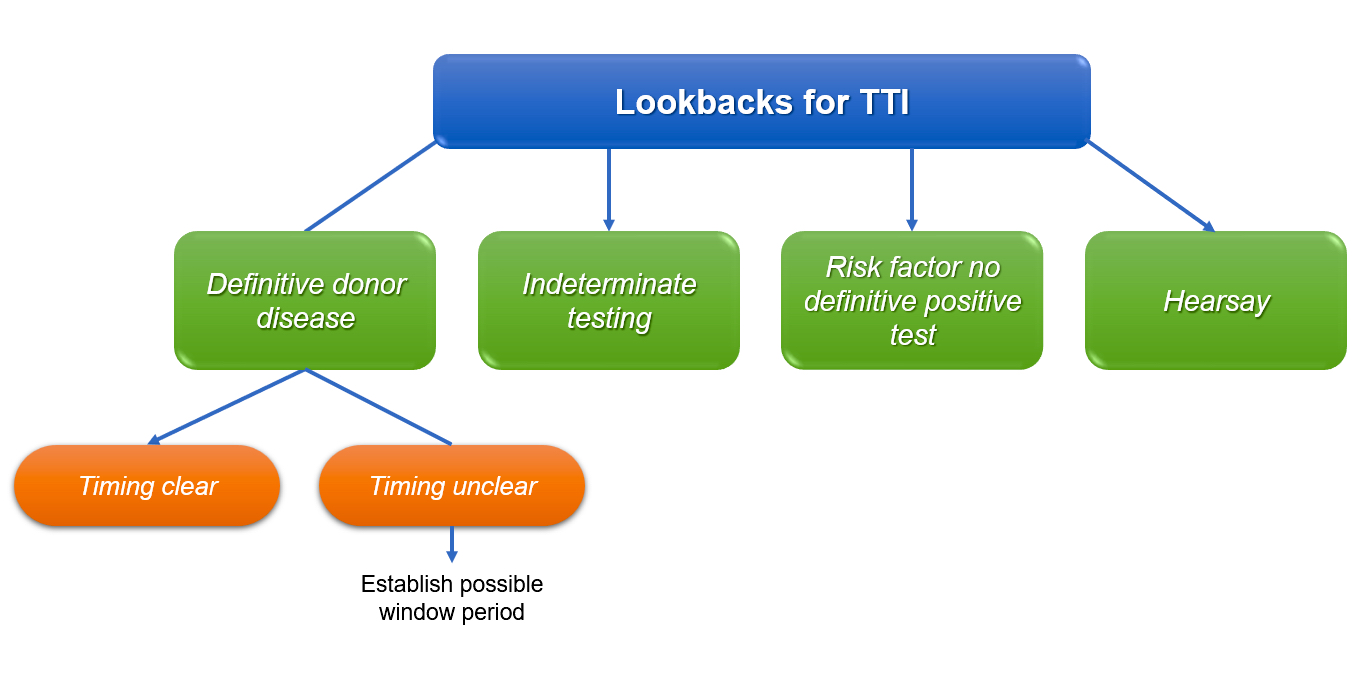
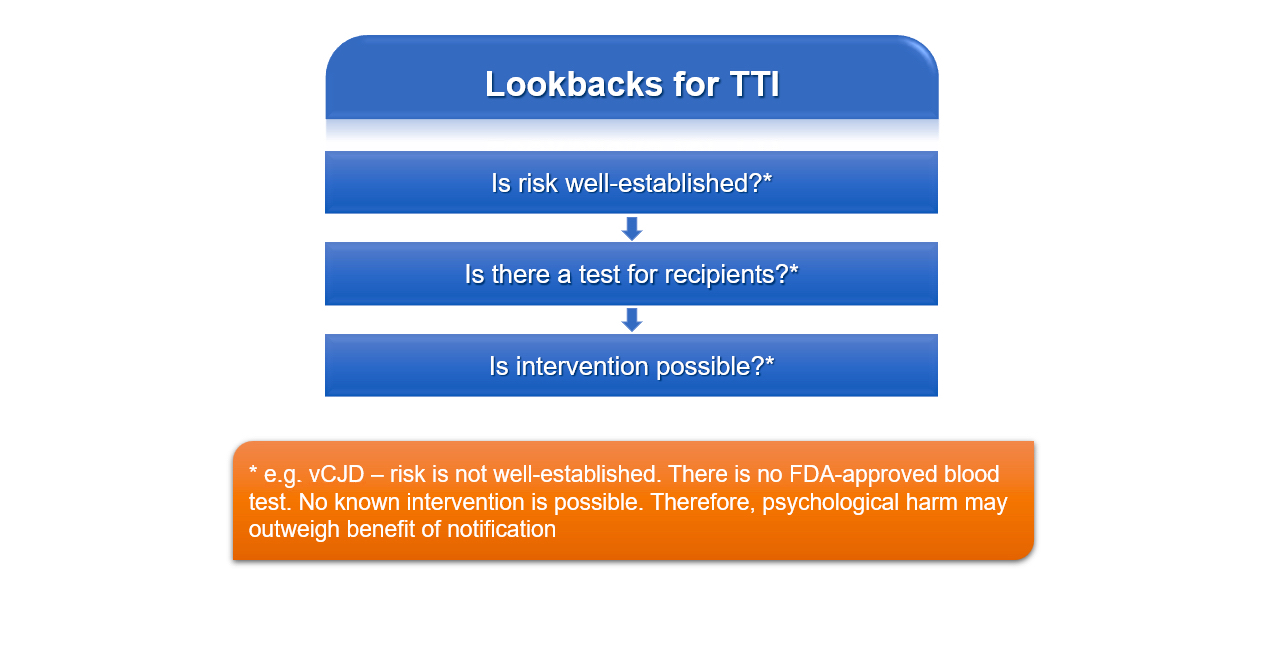
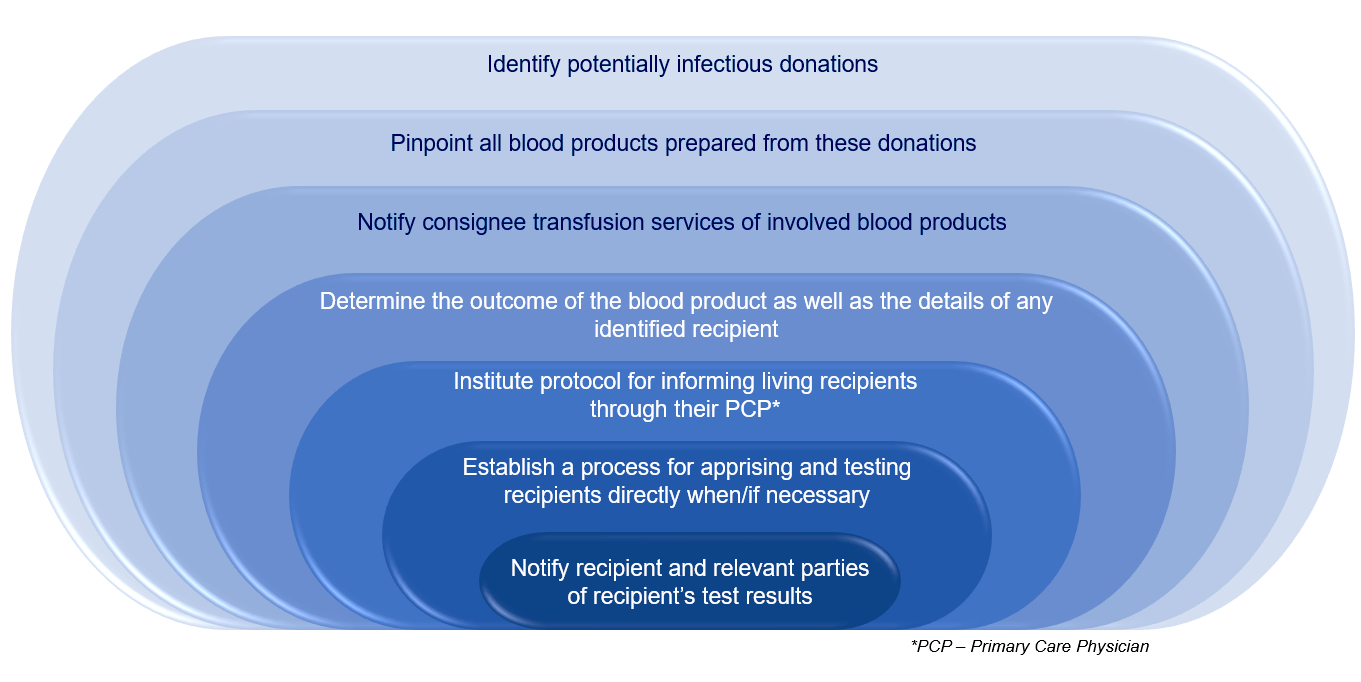
Lookbacks
Massive transfusion
MNS system
Monoclonal antibody therapy
Neutralization and lectins (pending)
P1PK GLOB system
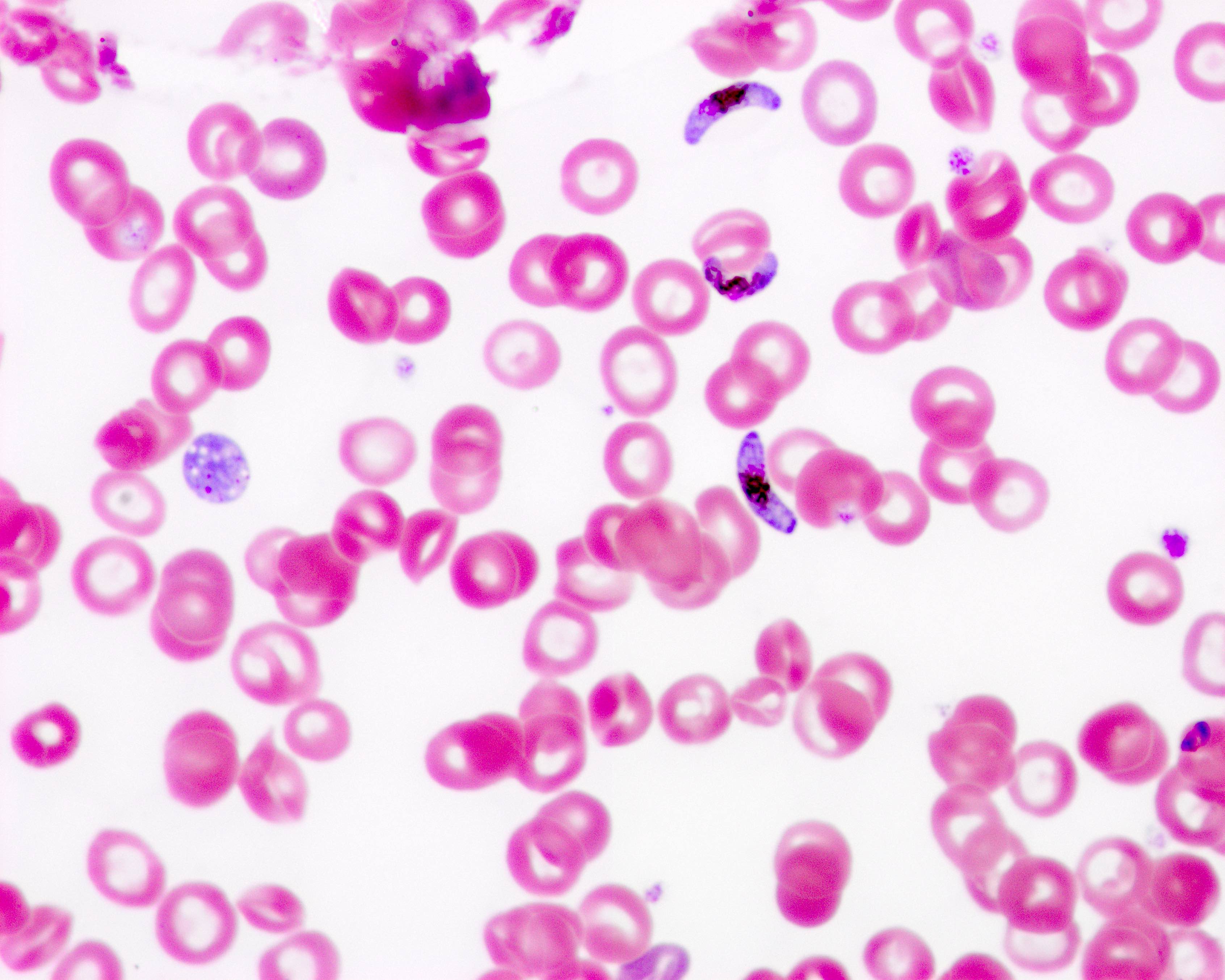
Parasites
Pathogen inactivation
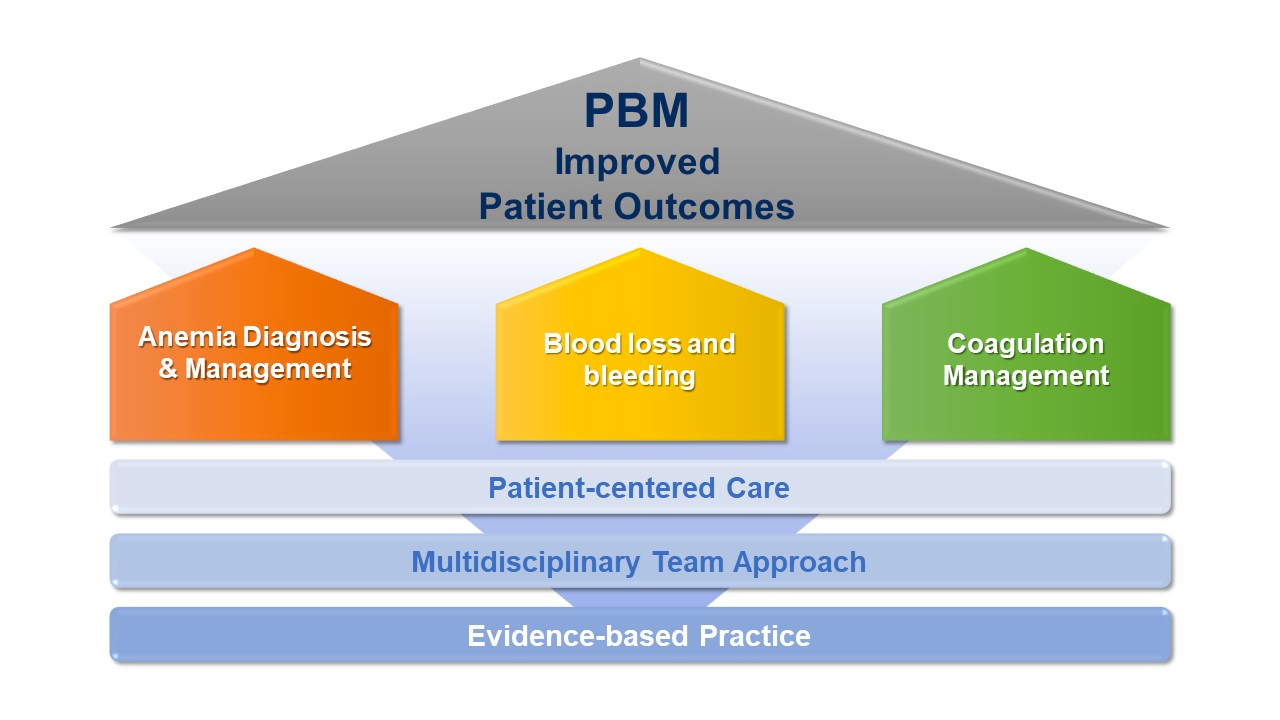
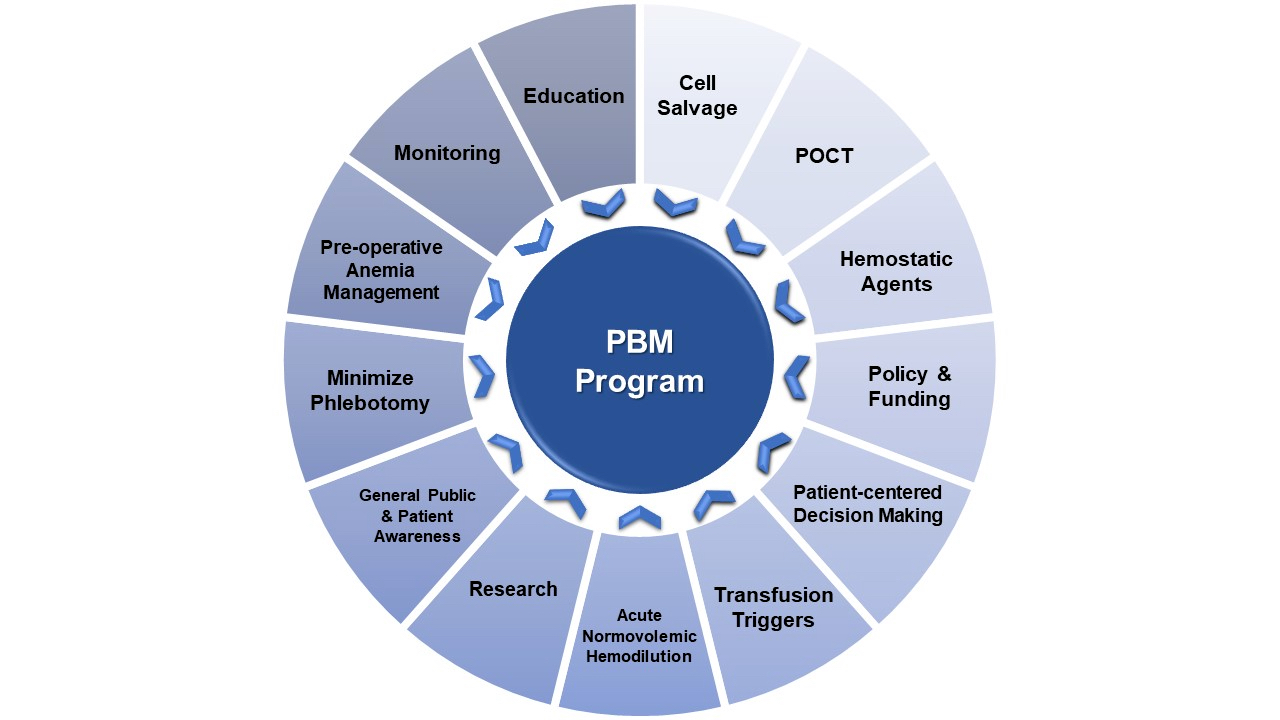
Patient blood management
Photopheresis
Plasma use
Platelet refractoriness
Platelet use
Plateletpheresis
Post-transfusion purpura
Pretransfusion testing
Red blood cell alloimmunization
Red blood cell exchange
Red blood cell genotyping (pending)
Red blood cell use
Rh immune globulin
Rh system
Shelf lives of products
Sickle cell disease
Stem cell collection
Therapeutic plasma exchange (TPE)
Tissue banking
Transfusion associated circulatory overload
Transfusion associated graft versus host disease
Transfusion related acute lung injury
Transfusion related immunomodulation (TRIM)
Variant Creutzfeldt-Jakob
Viruses
Warm autoantibody testing (pending)
Washing & volume reduction
Whole blood therapy
Zika virusABO / H system
Table of Contents
Definition / general | Essential features | Antigens | Antibodies | Terminology | Pathophysiology | Clinical features | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Most clinically important blood group
- Incompatible red blood cell transfusion causes intravascular, complement mediated hemolysis
- Originally described by Landsteiner (AABB: Technical Manual, 19th Edition, 2017)
- Antibodies are clinically significant
Essential features
- Patient’s blood type is based on their ABO phenotype
- Patients naturally form ABO antibodies or isohemagglutinins due to exposure to bacterial moieties mimicking ABO antigens
- Type O individuals have the H antigen which is unmodified and the blood group system may be referred to as ABH or ABO
- Anti-ABH antibodies are mostly IgM with a smaller component of IgG but can cause hemolysis and hemolytic disease of the fetus and newborn (HDFN)
- There are A and B subgroups, which may be recognized due to weaker expression of these antigens or unexpected reactivity (e.g., anti-A1 antibodies in A2 subgroup individuals) (AABB: Technical Manual, 19th Edition, 2017)
- Some individuals may lack H on red cells or in secretions and develop antibodies (i.e. Bombay or Parabombay patients) (AABB: Technical Manual, 19th Edition, 2017)
Antigens
- Type: carbohydrate
- H antigen produced by galactoside 2-alpha-L-fucosyltransferase 1 (FUT1) on red cells and alpha-1, 2-L- fucosyltransferase (FUT2) in secretions; the amount of H varies based on ABO subtype as follows: O > A2 > B > A2B > A1 > A1B (AABB: Technical Manual, 19th Edition, 2017)
- A antigen is a modification of the H antigen N-Acetylgalactosamine added to the terminal end
- There are various A subgroups based on the amount of A antigen present on red cells
- A1 subgroup is associated with the most A antigen on the surface of red cells with A2 being the second most common and having relatively less A antigen and more H antigen
- A2 patients and A2B patients may occasionally form anti-A1 antibodies which are rarely clinically significant
- Reference: AABB: Technical Manual, 19th Edition, 2017
- B antigen is a modification of the H antigen D-galactose added to the terminal end
- Individuals are type O due to an unmodified H antigen and have a higher proportion of H on the red cell surface (see above)
- Most common blood groups are as follows in descending order: O, A, B, AB
Race / Ethnicity O A B AB White non-Hispanic 45.2% 39.7% 10.9% 4.1% Hispanic 56.5% 31.1% 9.9% 2.5% Black non-Hispanic 50.2% 25.8% 19.7% 4.3% Asian 39.8% 27.8% 25.4% 7.1% Native American 54.6% 35% 7.9% 2.5% All donors 46.6% 37.1% 12.2% 4.1%
- Adapted from: Transfusion 2004;44:703
Antibodies
- Majority IgM, some IgG, anti-A,B, IgG in type O individuals (AABB: Technical Manual, 19th Edition, 2017)
- Can cause hemolytic reactions and hemolytic disease of the fetus and newborn
- This is the most common cause of hemolytic disease of the fetus and newborn; occurs most commonly in O mothers with A or B infants via anti-A,B antibodies
- Hemolytic disease of the fetus and newborn can occur in the first pregnancy, as no sensitizing pregnancy must occur; it develops with IgG antibodies crossing the placenta and generally causes mild anemia
- Naturally occurring at 3 - 6 months; also known as isohemagglutinins
- Newborns characteristically lack intrinsic ABO antibodies
Terminology
- Isohemagglutinins - ABO antibodies
- Alpha-1, 2-L- fucosyltransferase (FUT2) - also known as the secretor gene that produces H antigen present in secretions
Pathophysiology
- Bombay phenotype occurs when patients lack H antigen and naturally form anti-H antibodies
- This is an autosomal recessive condition where patients lack galactoside 2-alpha-L-fucosyltransferase 1 (FUT1)
- Patients will type as an O individual (denoted as Oh) but will characteristically have a panagglutinin on screening and panel cells
- Para-Bombay patients lack FUT1 but have a functional FUT2 gene that will produce H in secretions but can still develop anti-H antibodies and should functionally be treated like a Bombay patient
- Some proteins are posttranslationally modified by ABO antigens such as von Willebrand Factor, which is stabilized with the addition of A or B antigens; likewise, ABO antigens are occasionally implicated in the pathogenesis of certain infectious diseases
- However, the full scope of the ABO antigens' physiologic functions is not yet determined
Clinical features
- Acquired B antigen can occur due to enzymatic modification of the A antigen by enteric bacteria in patients with sepsis
- Reference: Dean: Blood Groups and Red Cell Antigens [Accessed 07 October 2020]
Laboratory
- ABO typing
- Forward type is based on reagents reacting with ABO antigens on patient red cells
- Back type is based on patient plasma reacting with ABO antigens on reagent red cells
- Typing discrepancies can occur and must be interrogated prior to transfusion
- Reference: Dean: Blood Groups and Red Cell Antigens [Accessed 07 October 2020]
Blood Group Antigens Antibodies Genotype A A Anti-B AA or AO B B Anti-A BB or BO AB A and B None AB O None Anti-A, Anti-B and Anti-A,B OO
Case reports
- Newborn boy born to a 28 year old woman at 35 weeks gestation developed hemolytic disease of the fetus and newborn due to ABO incompatibility (Indian J Hematol Blood Transfus 2018;34:183)
- 23 year old Indonesian man found to have a Parabombay phenotype (J Korean Med Sci 2019;34:e258)
- 40 year old man with a gunshot wound was given ABO incompatible blood and was successfully treated with red blood cell exchange transfusion (J Hematol 2019;8:141)
- 54 year old man with an A subgroup found to have a typing discrepancy (Asian J Transfus Sci 2019;13:129)
- 61 year old woman with blood group A developed acute intravascular hemolysis following transfusion of out of group (group O) single donor platelets (Am J Case Rep 2019;20:1075)
Additional references
Board review style question #1
A 27 year old mother of 3 delivers a baby boy at 39 weeks. The mother is O- and the son is A+. The mother received all routine shots and has been followed by OBGYN during all of her pregnancies. She has no history of transfusion. On day 2 of life the son is found to be listless with visible jaundice. Which of the following is the most likely cause of hemolytic disease of the fetus and newborn in this patient?
- Anti-D antibodies
- Anti-Jka antibodies
- Anti-K1 antibodies
- Anti-M antibodies
- Isohemagglutinins
Board review style answer #1
E. Isohemagglutinins. IgG ABO antibodies are the most common cause of hemolytic disease of the fetus and newborn. This generally occurs in type O mothers with type A babies.
Comment Here
Reference: ABO / H group
Comment Here
Reference: ABO / H group
Board review style question #2
A 36 year old man is rushed to the hospital by helicopter after a logging accident. While in flight, the patient receives a unit of O negative red blood cells. Upon arrival the patient is in disseminated intravascular coagulation and laboratory assessment is consistent with hemolysis. He expires and no autopsy is performed; however, a sample in the blood bank typed as O was shown to have anti-H antibodies. Which enzyme or type of enzyme was this patient missing?
- ADAMTS13
- Amylase
- Fucosyltransferase
- G6PD
Board review style answer #2
C. Fucosyltransferase. This patient lacks the ability to make the H antigen and has the so called Bombay phenotype.
Comment Here
Reference: ABO / H group
Comment Here
Reference: ABO / H group
ABO discrepancies (pending)
[Pending]
ABO incompatible HSCT
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Symptoms | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- ABO blood group matching is not a barrier to successful hematopoietic stem cell transplantation (HSCT)
- ABO blood group incompatibility has important implications for transfusion medicine support
- Reference: Hematology Am Soc Hematol Educ Program 2015;2015:378
Essential features
- ABO incompatible transplants are classified as major, minor or bidirectional
- In a major ABO incompatible transplant, the recipient has isohemagglutinins directed against the donor red cells
- In a minor ABO incompatible transplant, the donor graft has isohemagglutinins directed against the recipient red cells
- In a bidirectional ABO incompatible transplant, there are both major and minor ABO incompatibilities
- ABO incompatible transplants are associated with acute hemolysis immediately after infusion as well as delayed complications that may include pure red cell aplasia in major ABO incompatible transplants and passenger lymphocyte syndrome in minor ABO incompatible transplants
Terminology
- ABO mismatched HSCT
Pathophysiology
- ABO incompatible HSCT is possible because ABO blood group antigens are not expressed on pluripotent or early committed hematopoietic progenitor cells
- Complications associated with ABO incompatible HSCT are due to naturally occurring (anti-A or anti-B) antibodies present within the recipient or the donor graft
- Reference: Biol Blood Marrow Transplant 2013;19:1152
Diagrams / tables
Major / minor / bidirectional incompatibility
| O | A | B | AB | ||
| O | Compatible | Major | Major | Major | |
| A | Minor | Compatible | Bidirectional | Major | |
| B | Minor | Bidirectional | Compatible | Major | |
| AB | Minor | Minor | Minor | Compatible | |
Clinical features
- 40 - 50% of all HSCTs are ABO incompatible (Vox Sang 2010;98:455)
- ABO incompatible transplants are classified as major, minor or bidirectional
- All are associated with both immediate and delayed complications
- Major ABO incompatibility is the presence of naturally occurring ABO antibodies in the recipient directed against the donor's red cell antigens
- Acute hemolysis of infused donor red cells can occur immediately during graft infusion
- Acute hemolysis may be prevented via graft manipulation to reduce donor red cells
- Delayed red cell engraftment and pure red cell aplasia (PRCA) are both delayed complications that arise due to the continued production of antidonor ABO antibodies by the residual B cells and plasma cells in the recipient
- Delayed red cell engraftment and PRCA occur more frequently in reduced intensity conditioning (RIC) transplantation that uses less chemotherapy and radiation than the standard myeloablative regimen
- Minor ABO incompatibility is the presence of naturally occurring ABO antibodies in the donor graft directed against the recipient's red cell antigens
- Acute hemolysis of recipient red cells can occur immediately during graft infusion but is typically mild and self limited
- Acute hemolysis may be prevented via graft manipulation to reduce donor plasma
- Passenger lymphocyte syndrome (PLS) is a delayed complication that occurs 5 - 15 days after transplantation due to the production of ABO isohemagglutinins by donor lymphocytes
- Bidirectional incompatibility is the presence of both major and minor incompatibility and is associated with the complications of both
- Reference: Transfusion 2011;51:1143
Symptoms
- Acute hemolysis classically presents with fever, flank pain and red urine; however, the presentation depends on the type of incompatibility and the volume infused
- Delayed red cell engraftment and pure red cell aplasia may both present with the symptoms of anemia, such as pallor and exercise intolerance
- Passenger lymphocyte syndrome presents with the symptoms of delayed hemolytic anemia, such as pallor, tachycardia, tachypnea and hypotension (Transfus Med Rev 2020;34:178)
Blood donor screening
- Hematopoietic progenitor cell donor screening includes a health history questionnaire and physical exam (AABB: Circular of Information for the Use of Cellular Therapy Products [Accessed 17 February 2022])
Blood donor testing
- Infectious disease testing includes HIV, hepatitis B, hepatitis C, syphilis, West Nile virus, Chagas disease, human T cell lymphotropic virus and cytomegalovirus (AABB: Circular of Information for the Use of Cellular Therapy Products [Accessed 17 February 2022])
Donor deferral
- Some donors may not meet all the eligibility requirements but may be approved for donation due to a patient's clinical circumstance
- Products from a donor with abnormal screening or test results may be used if the recipient has been advised of the risk, the recipient's physician has authorized use of the product and the product is labeled appropriately
- Reference: AABB: Circular of Information for the Use of Cellular Therapy Products [Accessed 17 February 2022]
Laboratory
- Acute hemolysis:
- Positive direct antiglobulin test (DAT)
- Low haptoglobin
- Elevated bilirubin
- Elevated lactate dehydrogenase (LDH)
- Decreased hemoglobin
- Passenger lymphocyte syndrome:
- ABO antibody directed against recipient ABO group antigen on reverse type or antibody titers
- Positive direct antiglobulin test (DAT)
- Low haptoglobin
- Elevated bilirubin
- Elevated LDH
- Decreased hemoglobin
- Delayed red cell engraftment and pure red cell aplasia:
- Low reticulocyte count
- ABO antibody directed against donor ABO group antigen on reverse type or antibody titers
- ABO forward type shows no conversion to donor blood type
- Chimerism study may show full engraftment of all cell lines except red blood cells
- Reference: Hematology Am Soc Hematol Educ Program 2015;2015:378
Case reports
- 27 year old woman with pure red cell aplasia following major ABO incompatible HSCT for B cell acute lymphoblastic leukemia (J Zhejiang Univ Sci B 2021;22:695)
- 38 year old woman with passenger lymphocyte syndrome following a minor ABO incompatible HSCT for mixed phenotype acute leukemia (Hematology 2021;26:835)
- 48 year old man with pure red cell aplasia following a major ABO incompatible HSCT for high risk acute myeloid leukemia (Biol Blood Marrow Transplant 2018;24:1765)
Treatment
- Acute hemolysis is treated with hydration and transfusion
- Passenger lymphocyte syndrome is generally self limited; may require transfusion support and red blood cell exchange
- Pure red cell aplasia may be treated with erythropoietin, immunosuppression taper, donor lymphocyte infusion, rituximab and plasma exchange (American Society for Apheresis [ASFA] category III)
- References: J Clin Apher 2016;31:149, Hematology Am Soc Hematol Educ Program 2015;2015:378
Sample assessment & plan
- A 50 year old man with a history of myelodysplastic syndrome underwent a bidirectional ABO incompatible hematopoietic stem cell transplant (group B recipient, group A donor) 6 weeks prior and demonstrates 100% donor chimerism; however, the patient has yet to convert to the donor blood type. He remains transfusion dependent with a reticulocyte count of 0.4% and a hemoglobin of 6.9 g/dL. The findings may represent delayed red cell engraftment in the setting of ABO incompatible HSCT; however, disease relapse and parvovirus B19 should be excluded.
Differential diagnosis
- Transplant associated or drug induced thrombotic microangiopathic hemolytic anemia (TA-TMA, DIHA):
- Microangiopathic hemolytic anemia, including schistocytes and thrombocytopenia on peripheral blood smear
- DIHA is associated with cyclosporine, tacrolimus and sirolimus (Hematology Am Soc Hematol Educ Program 2015;2015:378)
- Autoimmune hemolytic anemia:
- Spherocytes on peripheral blood smear
- Positive direct antiglobulin test (DAT) with eluate demonstrating a panagglutinin (an agglutinin that agglutinates the red cells of all blood groups)
- Infection (e.g., parvovirus B19):
- Clinical signs of infection (fever, rash, fatigue, etc.)
- Positive serology or PCR test
- Graft failure:
- Loss of donor cell chimerism
- Disease relapse:
- Histologic or flow cytometry immunophenotypic evidence of recurrent disease
Board review style question #1
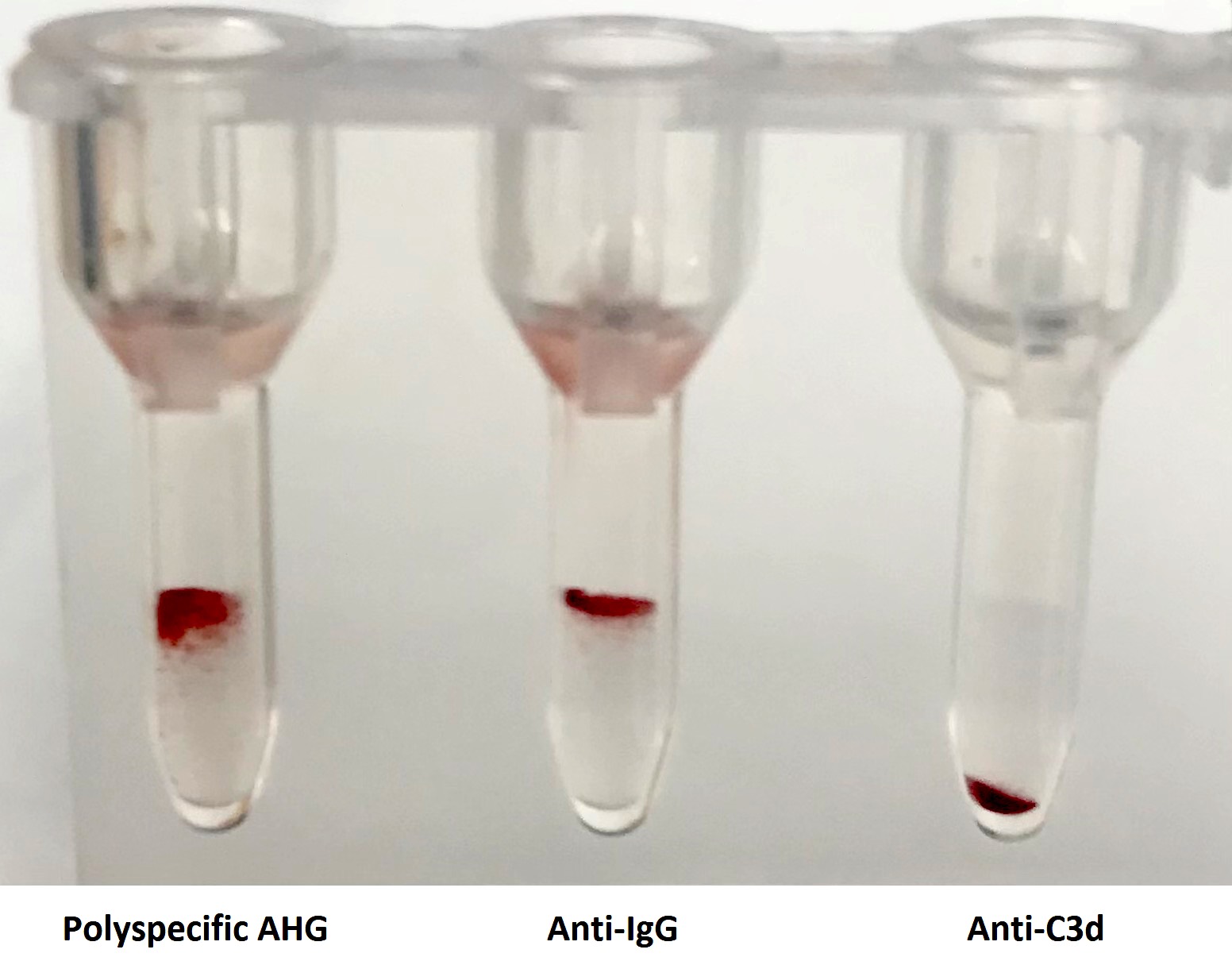
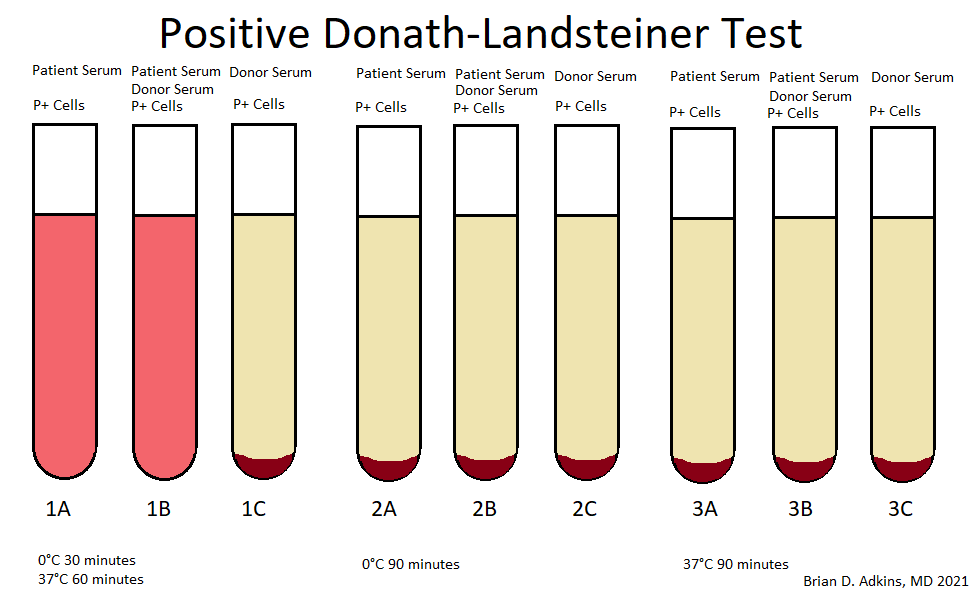
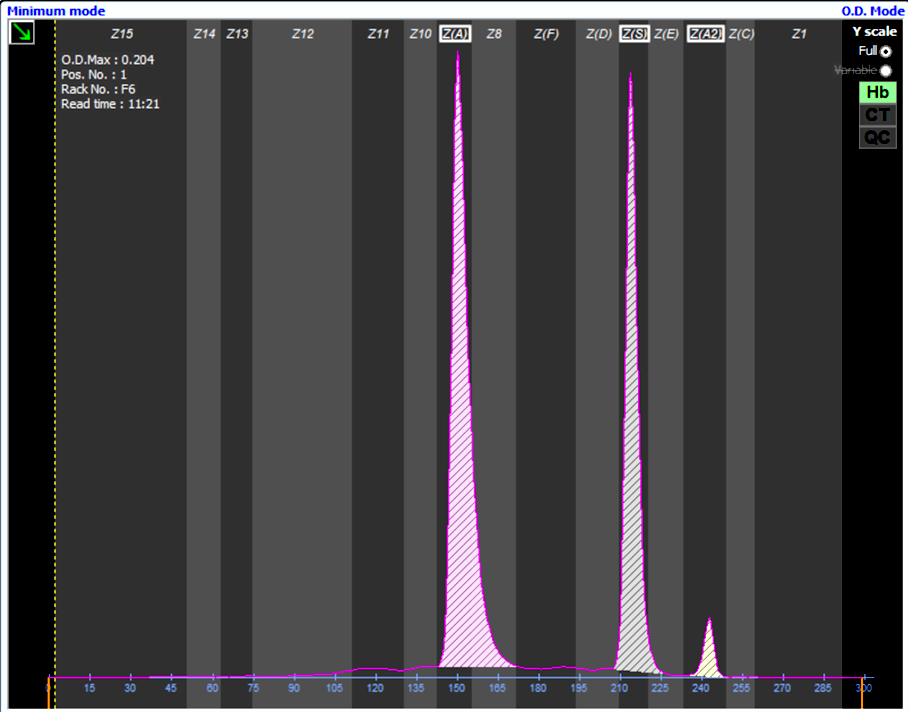
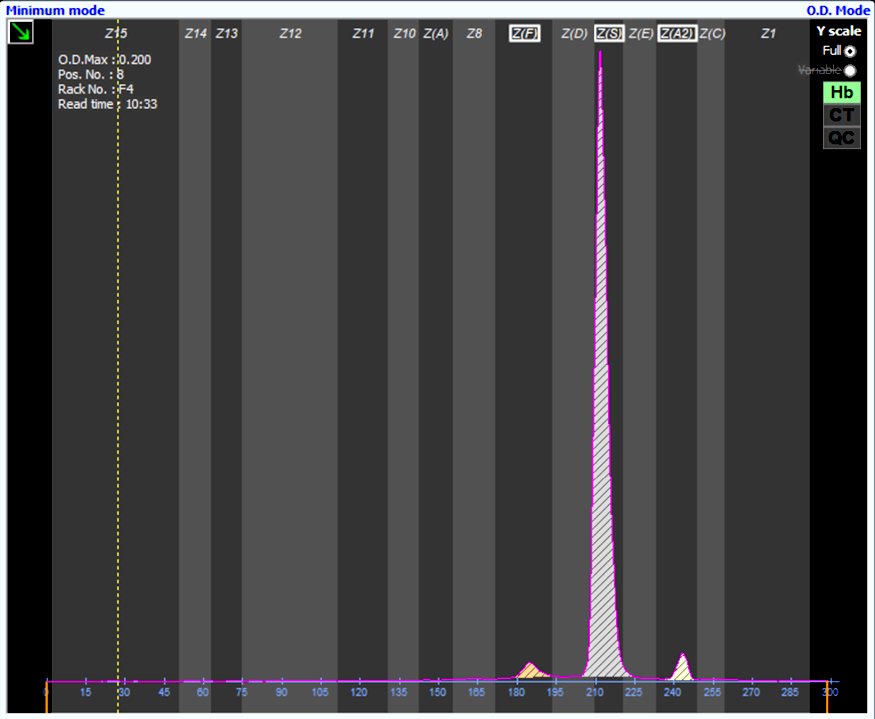
A 65 year old, A positive woman experiences a mild drop in hemoglobin following a bidirectional ABO incompatible hematopoietic stem cell transplant with a B positive donor. Her direct antiglobulin test is positive. Additional testing is performed shown in the image above. What is the most likely cause of these results?
- Acute hemolytic transfusion reaction
- Autoimmune hemolytic anemia
- Graft failure
- Passenger lymphocyte syndrome
- Pure red cell aplasia
Board review style answer #1
D. Passenger lymphocyte syndrome is due to the production of ABO isohemagglutinins by donor lymphocytes directed against recipient red cell antigens. The recipient is group A while the donor is group B and therefore produces anti-A isohemagglutinins against recipient red blood cells.
Comment Here
Reference: ABO incompatible HSCT
Comment Here
Reference: ABO incompatible HSCT
Board review style question #2
Major ABO incompatible hematopoietic stem cell transplantation is associated with a delayed engraftment of which of the following?
- Granulocytes
- Lymphocytes
- Platelets
- Red cells
Board review style answer #2
D. Red cells. A major ABO incompatible transplant is associated with a delayed red cell engraftment beyond the expected recovery period of 2 - 3 weeks due to recipient isohemagglutinins directed against engrafted red cell progenitors.
Comment Here
Reference: ABO incompatible HSCT
Comment Here
Reference: ABO incompatible HSCT
Acute hemolytic transfusion reaction (AHTR)
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission / incidence | Laboratory | Transfusion of significant incompatible plasma | Case reports | Treatment | Prevention | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Acute hemolysis due to incompatible RBC transfusion
- Most commonly ABO incompatible; it can also be caused by non-ABO antigens, such as Kell, Kidd and Duffy
- All of these antibodies strongly bind complement and cause intravascular hemolysis
Essential features
- Must occur within 24 hours of blood component administration and often during transfusion itself
- Mostly due to transfusion of incompatible RBC but may also result from incompatible plasma containing components such as FFP or platelets
- Usually due to mistransfusion of ABO incompatible RBC
- Transfusion service error
- Typing
- Labeling
- Crossmatching
- Clinical service error
- Wrong patient sample
- Wrong unit transfused at the bedside to a patient
- Transfusion of incompatible RBCs (could be ABO incompatible to patient antibodies)
- Transfusion service error
Terminology
- RBC: red blood cells
- FFP: fresh frozen plasma
- DAT: direct antiglobulin test
- AHTR: acute hemolytic transfusion reaction
- CBC: complete blood count
- LDH: lactate dehydrogenase
- PT: prothrombin time
- APTT: activated partial thromboplastin time
- DIC: disseminated intravascular coagulation
- IVH: intravascular hemolysis
- EVH: extravascular hemolysis
- TRALI: transfusion related acute lung injury
Pathophysiology
- Intravascular hemolysis:
- ABO incompatible: pre-existing naturally occurring anti A or anti B antibodies of IgM type → fixation of complement → formation of membrane attack complex → red cell lysis
- Some IgG antibodies can also fix complement → mild to fatal AHTR
- Usually occurs when mistransfusion of antigens in Kell, Duffy and Kidd systems
- DIC, shock and renal failure:
- Free hemoglobin from hemolysis → binds to nitric oxide (NO) → vasoconstriction
- RBC stroma → activates platelets and coagulation cascade → DIC
- RBC stroma → damages to renal tubules → renal failure
Clinical features
- Fever (important): early sign of AHTR – need to monitor vital signs frequently during the initial minutes of transfusion:
- Oozing from IV site
- Flank and back pain
- Hyper or hypotension
- Hemoglobinuria
- Anxiety, sense of doom
- Nausea, vomiting
- Pain at infusion site
- Diffuse bleeding
Transmission / incidence
- It is estimated that ABO incompatible transfusions occur in about 1 in 40,000 transfusions
- AHTR incidence estimated at about 1 in 76,000 transfusions (AABB: Technical Manual of the American Assoc of Blood Banks, 20th Edition, 2020)
- Up to 47% of cases of ABO incompatible mistransfusion had no adverse effect
- Less than 10% of ABO incompatible transfusions cause death
- Risk of death from ABO incompatible transfusion is estimated to be 1:1.8 million and correlates with the amount of incompatible blood transfused (Blood 2009;113:3406)
- Causes 10 - 30 deaths in US each year
Laboratory
- Contact the transfusion service immediately
- Return implicated blood bag, tags and attached administration set to blood bank
- Check for clerical error (i.e. patient identity and the patient identity on RBC unit)
- Centrifuge postreaction blood sample and examine serum / plasma for hemolysis; if noted, compare with pre-reaction specimen
- Perform DAT; if positive, compare with pre-reaction specimen DAT
- Repeat ABO type of the donor unit, pre- and posttransfusion patient sample
- Repeat antibody screen on the pre- and posttransfusion sample
- Repeat crossmatch with the pre- and posttransfusion sample
- If there is evidence of a hemolytic transfusion reaction, additional testing should be performed as needed:
- Laboratory studies for hemoglobinemia and hemoglobinuria (the free hemoglobin causes both plasma and urine to appear red), decreased serum haptoglobin (very sensitive marker of hemolysis) and increased lactate dehydrogenase
- Perform coagulation tests for DIC: PT, APTT, fibrinogen, platelet count
Transfusion of significant incompatible plasma
- Occurs less frequently
- Due to minor ABO mismatch - transferring of donor anti A, anti B or anti A,B in plasma containing products (platelets, FFP) → hemolysis of patient's own ABO incompatible RBCs
- Most often due to transfusion of out of group platelet (most commonly group O platelets to group A patient, Arch Pathol Lab Med 2007;131:909); also donor with high titer of anti A (> 1,000), transfusion of large volume of plasma into a smaller patients (such as neonate, infants or children)
- Clinical symptoms are identical to transfusion of incompatible pRBCs
Case reports
- Due to antibodies:
- Infant girl suffered massive hemolysis (J Clin Apher 2005;20:225)
- 18 year old woman with anti-IH in a patient with sickle cell disease (Transfusion 2000;40:828)
- 28 year old woman anti-Jka not detected by polybrene screen (Ann Clin Lab Sci 2006;36:101)
- 37 year old woman with anti-Bg HLA antibodies (Transfusion 2003;43:753)
- 58 year old man with anti-Coa (Immunohematol 2001;17:45)
- 69 year old Japanese man with anti-Jra (Transfusion 2004;44:197)
- 74 year old woman with anti-P1 (Transfusion 1998;38:373)
- 81 year old man with anti-Dombrock (a) (Transfusion 2006;46:244)
- 82 year old African American woman with anti-Fy3 in a non sickle cell disease patient (Immunohematol 2005;21:48)
- ABO incompatible platelet products (Am J Clin Pathol 1999;111:202)
- Due to nonantibodies:
- 61 year old man with chimeric red cells in a donor who was a twin (Transfusion 2003;43:1449)
- Two cases of severe HTR in sickle cell disease (Transfusion 2001;41:323)
Treatment
- Stop the transfusion
- Maintain IV access
- Provide good supportive care
- Maintain good urine output (> 1ml/kg per hour), can use diuretics (furosemide)
- Treat shock and support cardiac and respiratory function
- Administer low dose dopamine for hypotension (1 - 5 µg/kg per min)
- Manage DIC and hemorrhage as clinically indicated
Prevention
- Strictly adhere to the protocol to prevent mislabeled and miscollected samples
- Always perform bedside check before administering blood products
- Other innovations include barcoding of blood components and patient ID
- Educate physicians and nurses regarding transfusion practice
- Other innovations include barcoding of blood components and patient ID has been proven to reduce incorrect transfusion (Transfusion 2019;59:972)
Sample assessment & plan
- Monitor patient carefully during transfusion
- Stop the transfusion at first sign or symptom of an adverse reaction
- Recheck the identification of the patient and donor unit; verify that the unit is correct
- Notify the patient physician and the transfusion service
- Maintain IV fluids and urinary output; monitor and maintain vital signs
- Collect posttransfusion blood and urine samples
- Complete the transfusion investigation request, documenting patient symptoms, vital signs and the amount of blood transfused
- Send to the transfusion service:
- Postreaction blood and urine samples from the patient
- Transfusion reaction investigation request
- Blood bag with the attached administration set and IV solutions
Differential diagnosis
- Septic transfusion reaction:
- Contaminated blood product; bacteria (e.g. Pseudomonas, Escherichia coli, Yersinia enterocolitica) produce endotoxin
- Chills, fever, hypotension
- Broad spectrum antibiotics and resuscitative therapy
- TRALI:
- Caused by donor antibody attacking recipient HLA or WBC antigens
- Fever, chills, shortness of breath, respiratory failure, hypotension or non cardiogenic pulmonary edema
- Supportive care, administer oxygen, intubate (mechanical ventilation) if necessary
- Febrile nonhemolytic reaction:
- Recipient antibody to donor WBC leads to IL-1 production; accumulation of cytokines (IL-1) from donor WBC in blood product; underlying condition
- Temperature increase ≥ 1.8° F (1°C) with or without chills
- Premedicate with antipyretics; use leukoreduced products for future transfusions
- Premedication is not recommended under most recent literature, especially without evidence of repeat reactions (Hematol Educ Program 2020;2020:523)
- Nonimmune hemolytic transfusion reaction:
- Hemolysis of pRBCs from nonimmune causes, such as storage or inappropriate handling of blood products (microwave, using small needle for transfusion, transfusion of pRBCs with lactated Ringer solution, use of hyper or hypo-osmotic fluids, thermal injury, etc.)
- Always need to rule out immune causes of hemolysis
- Lysed red cells may cause hemodynamic, renal and pulmonary problems, possibly death
- Clinical features and treatment are similar to acute hemolytic transfusion reaction
- Stop transfusion and maintain IV access
- Contact transfusion service to rule out immune cause of hemolysis
- Monitor urine output
- Should also consider possibility of transfusion related infection (malaria, babesiosis)
- Hemoglobinemia, hemoglobinuria, possibly hyperkalemia (if renal failure)
Additional references
Board review style question #1
- 15 minutes after starting a transfusion of a group A Rh negative pRBC, a patient typed as group A Rh positive has a new onset fever, back pain, abdominal cramping, rigors and dyspnea. Hypotension is also noted. Red urine is evident in the Foley catheter bag, but urine output decreases in the next hour. The pretransfusion antibody screen was negative. What is the most likely cause of this patient's symptoms?
- Bacterial contamination of the unit
- Cytokines elaborated by donor white blood cells
- Laboratory errors in compatibility testing
- Error at the time of either patient or specimen identification
- Transfusion of Rh negative blood
Board review style answer #1
D. The patient is exhibiting acute hemolytic transfusion reaction. The dramatic symptoms and signs presenting early in a transfusion reaction represent an AHTR until proven otherwise. Immediate cessation of the transfusion and prompt evaluation are called for, often while normal saline is being given in an effort to maintain urine flow and prevent severe renal damage. The vast majority of these reactions are caused by incompatibilities involving the ABO system. These are overwhelmingly caused by improper identification of the recipient or through mislabeled samples. Laboratory error in ABO typing has become a rare event through the efforts of laboratory regulatory agencies which require maintenance of staff proficiency and competency as well as quality controls systems for sample processing and reporting results. Antibodies with specificity for the A and B antigen occur naturally and are expected to be present in antigen negative individuals. These antibodies are always IgM class and, although reactive at colder temperatures like most IgM antibodies, making their detection at room temperature possible, they bind to antigen positive red cells at 37°C. This leads to rapid onset of intravascular hemolysis and activation of complement and other inflammatory cascades, releasing vasoactive peptides. The symptoms experienced by this patient may overlap with other adverse reactions that can present early in transfusion but the red urine is a clue that IVH is present. Rh antibodies rarely fix complement and cause chiefly EVH, with paucity of most other symptoms. Besides, Rh negative blood lacks the RhD antigen and can be safely given to Rh positive individuals. Bacterial contamination is commonly associated with platelets products, which must be stored at 22°C, rather than in the refrigerator like red cell units. Storage under refrigerated conditions keeps the incidence of reactions to contaminated red cells extremely low. Although cytokine mediated reactions may present early in transfusion and cause some overlapping symptoms, especially fever and hypotension, they are not associated with hemolysis.
Comment Here
Reference: Acute hemolytic transfusion reaction (AHTR)
Comment Here
Reference: Acute hemolytic transfusion reaction (AHTR)
Board review style question #2
- A 35 year old man with group A Rh positive recently underwent a second peripheral blood stem cell transplantation from the same group A Rh positive matched sibling donor for relapsed acute myeloid leukemia and has persistent thrombocytopenia 1 month after transplantation. He is scheduled for platelet transfusion. 15 minutes and 100 ml into the transfusion of a blood group O single donor platelet component, he develops severe back and flank pain associated with hypertension and chest pain; vital signs are otherwise stable. The transfusion is discontinued; a posttransfusion specimen is grossly hemolyzed and a urine specimen is dark brown. The hemoglobin has decreased from 9.7 gm/dL before transfusion to 5.5 gm/dL. The DAT is positive for IgG and complement. Which of the following is the most appropriate next step in the management of this patient's symptoms?
- Initiate corticosteroids
- Transfuse a unit of pRBC, group A, crossmatch compatible
- Transfuse a unit of pRBC, group A, crossmatch incompatible
- Transfuse another bag of platelets, group A, washed
- Transfuse another bag of platelets, group O, washed
Board review style answer #2
B. In routine adult transfusion practice, platelets are transfused without regard to ABO compatibility (ABO incompatible hematopoietic stem cell transplantation recipients are the exception). Platelets are suspended in plasma. Not surprisingly, ABO antibodies in incompatible plasma occasionally cause a hemolytic transfusion reaction (with a positive DAT) after platelet transfusions. The patient is typically group A and the platelet component group O (minor incompatibility). This patient is symptomatic from the anemia so the priority should be to be transfuse RBC that are crossmatch compatible. A positive DAT is seen in autoimmune hemolytic anemia (AIHA), for which crossmatch incompatible units may need to be transfused (answer D) and corticosteroids initiated (answer E); this patient does not have AIHA. Future platelet transfusions should be restricted to ABO identical components; if group A platelets are not available for this patient, group O platelets should be washed (plasma depleted) prior to transfusion.
Comment Here
Reference: Acute hemolytic transfusion reaction (AHTR)
Comment Here
Reference: Acute hemolytic transfusion reaction (AHTR)
Adsorption studies
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Peripheral smear images | Screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Adsorption (or adsorption studies) is an advanced serologic testing method used to separate warm autoantibodies from serum in order to appropriately identify underlying alloantibodies
- Antibodies can be strategically removed from the serum by allowing them to adsorb onto the surface of red blood cells (RBCs) that express the target antigen
- Adsorbed serum can then be tested for remaining alloantibody reactivity
Essential features
- Warm autoantibodies may mask alloantibodies on routine serologic testing
- Adsorption allows for the identification of any underlying alloantibodies, an essential step in patient transfusion safety
Terminology
- Warm autoantibody (WAA): antibody directed against high incidence antigens on patient's own RBCs, optimally bound at or near body temperature (~37 °C)
- Cold autoantibody: antibody directed against high incidence antigens on patient's own RBCs, optimally bound at colder temperatures (room temperature or 4 °C)
- Drug related WAA: many medications have the potential to cause hemolysis in vivo; routine serologic testing of these samples look similar to WAA
- Elution: testing technique that dissociates bound IgG from RBCs
- Multiple methods available (e.g., acid elution kits, heat, freeze - thaw)
- Eluate can then be tested against reagent red cells to delineate WAA from drug related WAA
- Eluate with WAA is panreactive
- Eluate with drug related WAA, in the majority of cases, is negative (Cohn: Technical Manual, 20th Edition, 2020)
- Autoadsorption: adsorption technique used if the patient has no recent (within 3 months) transfusion history; patient RBCs can be used to adsorb the WAA while alloantibodies, if present, will remain in serum
- Alloadsorption: adsorption technique used if the patient has recent transfusion history (within 3 months), patient cells are not used in the adsorption testing as the sample would contain native and transfused cells, the latter which may adsorb alloantibodies (Immunohematology 2018;33:1)
- Adsorbed serum (or plasma): serum remaining after each adsorption process, may contain additional antibodies
- Cold adsorption: similar adsorption technique performed at cold temperatures, the temperature at which the antibody is optimally bound should be used for incubation
- Reference: Harmening: Modern Blood Banking & Transfusion Practices, 7th Edition, 2018
Pathophysiology
- Autoantibodies are directed against RBCs
- Can cause destruction (autoimmune hemolytic anemia [AIHA]) of circulating RBCs
- Phagocytosis of autoantibody coated RBCs occurs in the spleen
- WAA present challenges in pretransfusion compatibility testing
- Optimally reactive at 37 °C
- May mask the presence of clinically significant alloantibodies by agglutinating most or all RBCs tested
- Incidence of clinically significant alloantibodies is higher in patients with WAAs than in multiply transfused patients without AIHA
- Reference: Cohn: Technical Manual, 20th Edition, 2020
Clinical features
- WAAs may be associated with a variety of clinical conditions (e.g., autoimmune illnesses including systemic lupus erythematosus, lymphomas) or may be idiopathic
- Certain drugs may also elicit a warm autoantibody reaction (e.g., methyldopa, penicillin, quinolones)
- Elution is positive in WAAs but is normally negative in drug related WAA reactivity
- WAAs may be associated with decreases in hemoglobin and hematocrit and therefore related symptoms (noted below)
- ~7% of WAA cases suffer from Evans Syndrome, the combined entity of warm AIHA and immune thrombocytopenia (NIH: Evans Syndrome [Accessed 9 May 2023], J Clin Med 2020;9:3851)
Symptoms
- Patients receiving RBC transfusion without appropriately identifying and honoring their auto and alloantibodies have the potential for acute or delayed hemolytic transfusion reactions (Immunohematology 2014;30:153)
- Severity of hemolysis ranges from mild to severe
- Signs and symptoms of hemolysis include
- Anemia
- Fatigue
- Pallor
- Shortness of breath
- Back pain
- Dark urine
- Fevers / chills
- Hypotension
- Oliguria / anuria / renal failure (CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 9 May 2023])
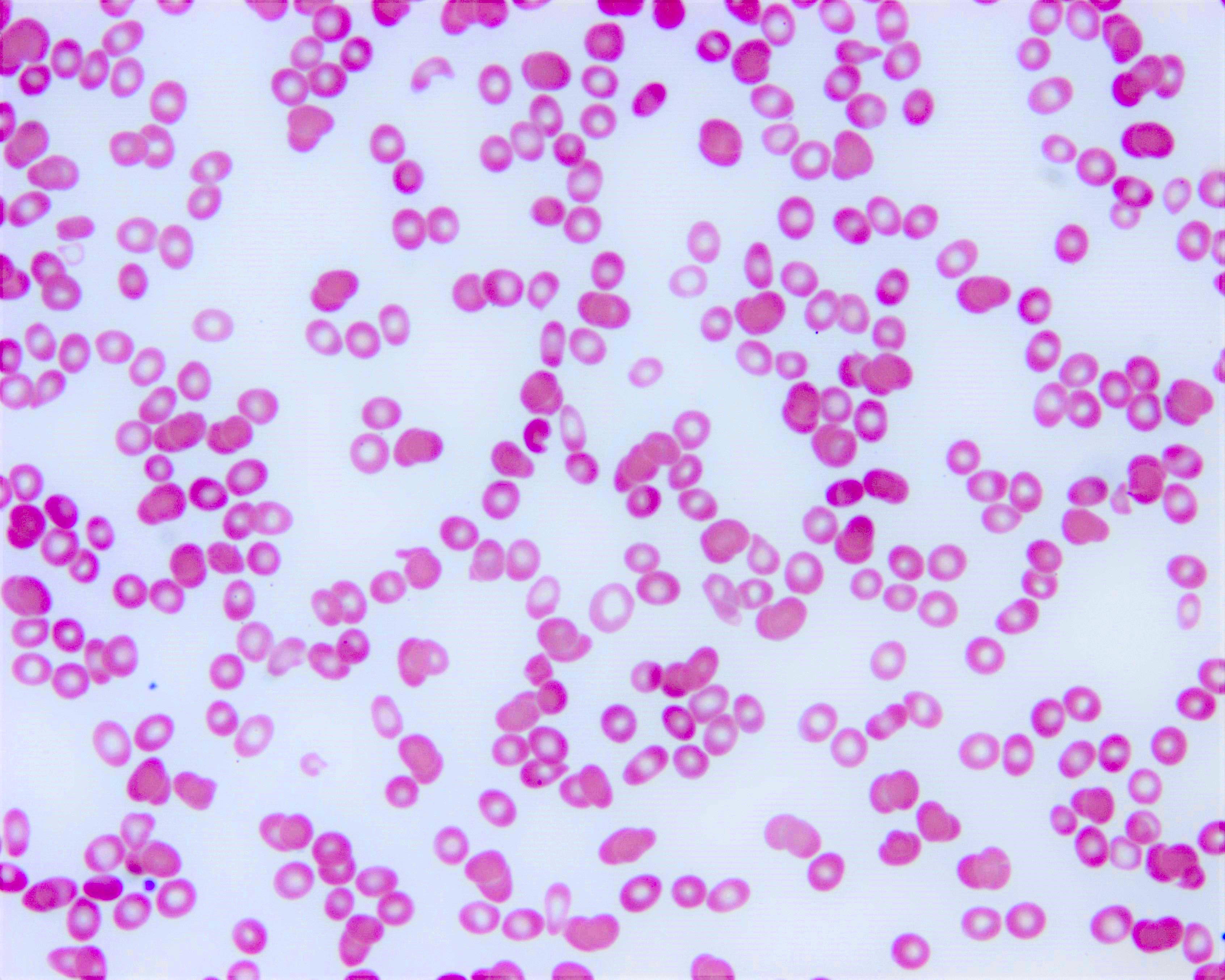
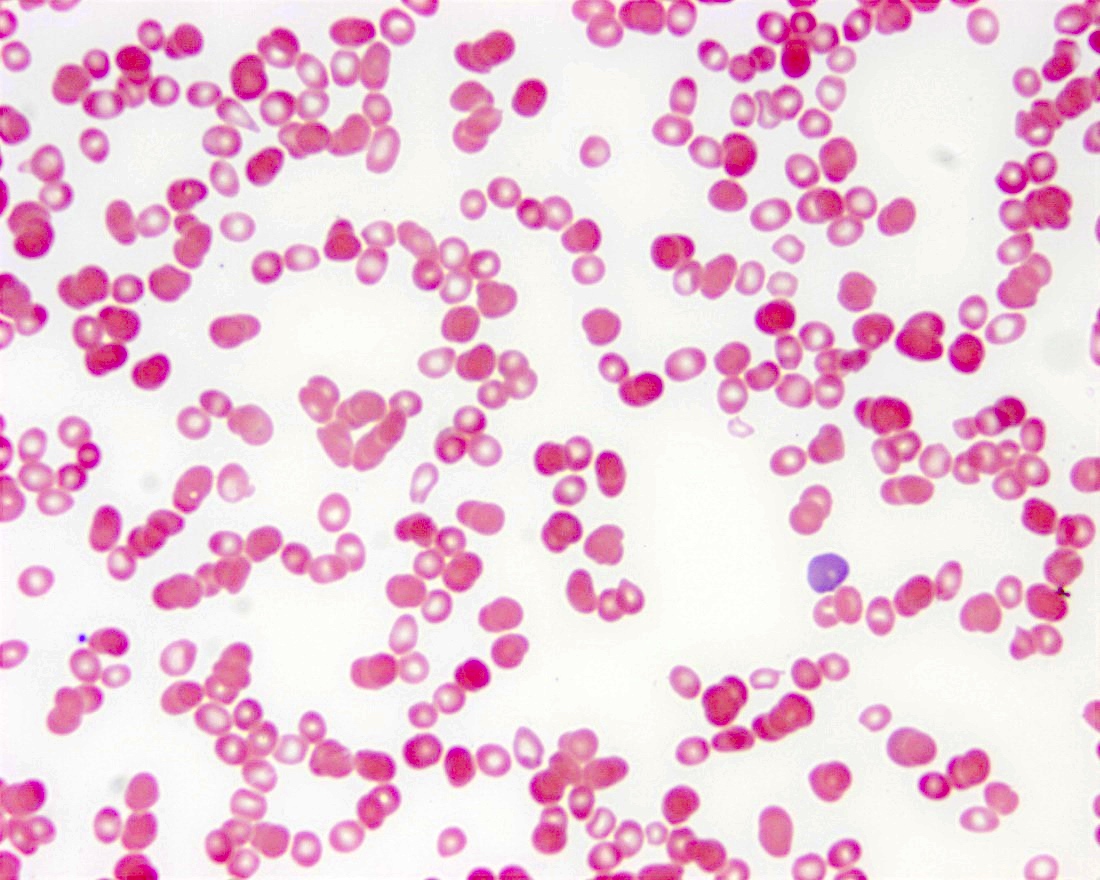
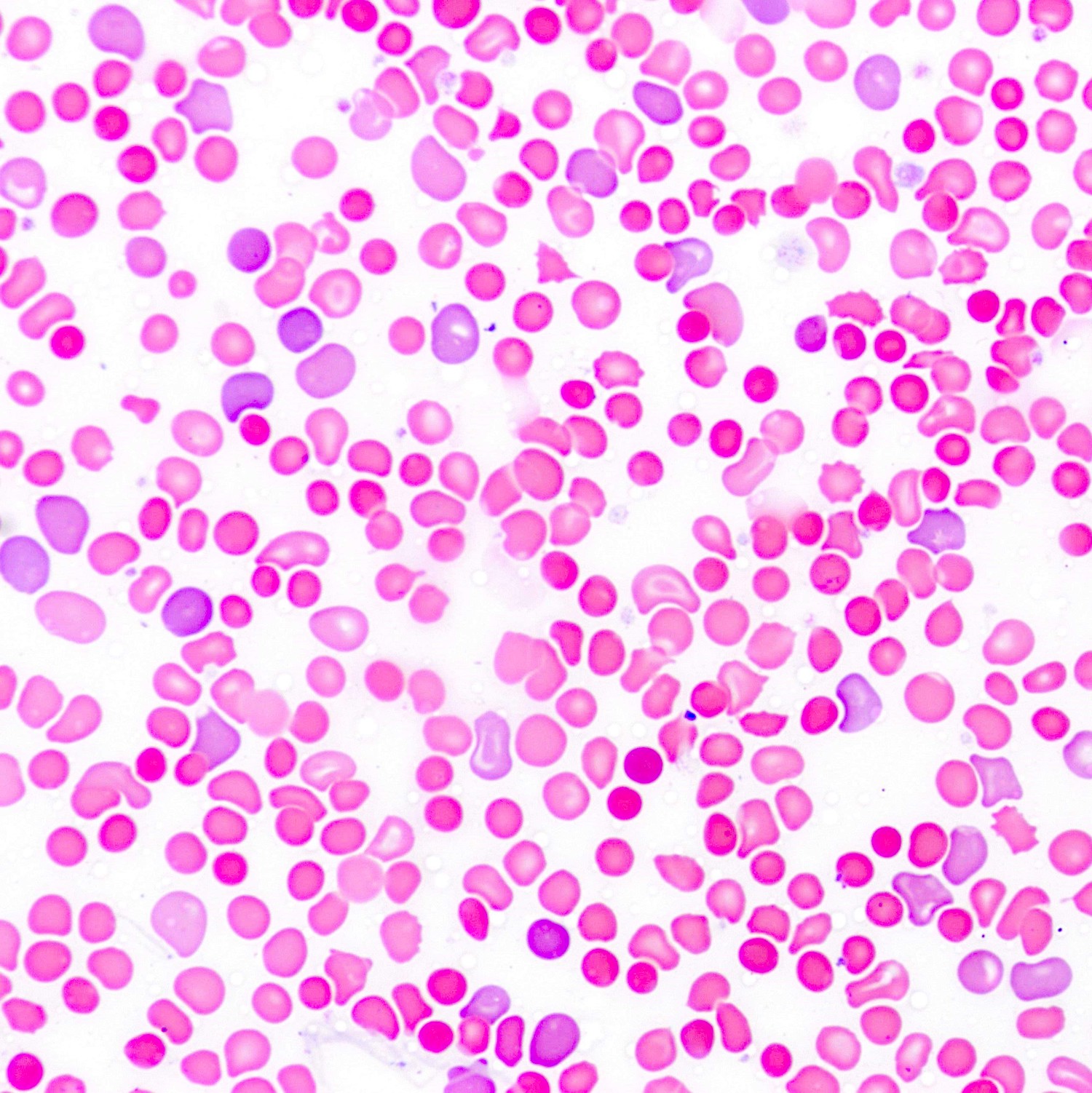
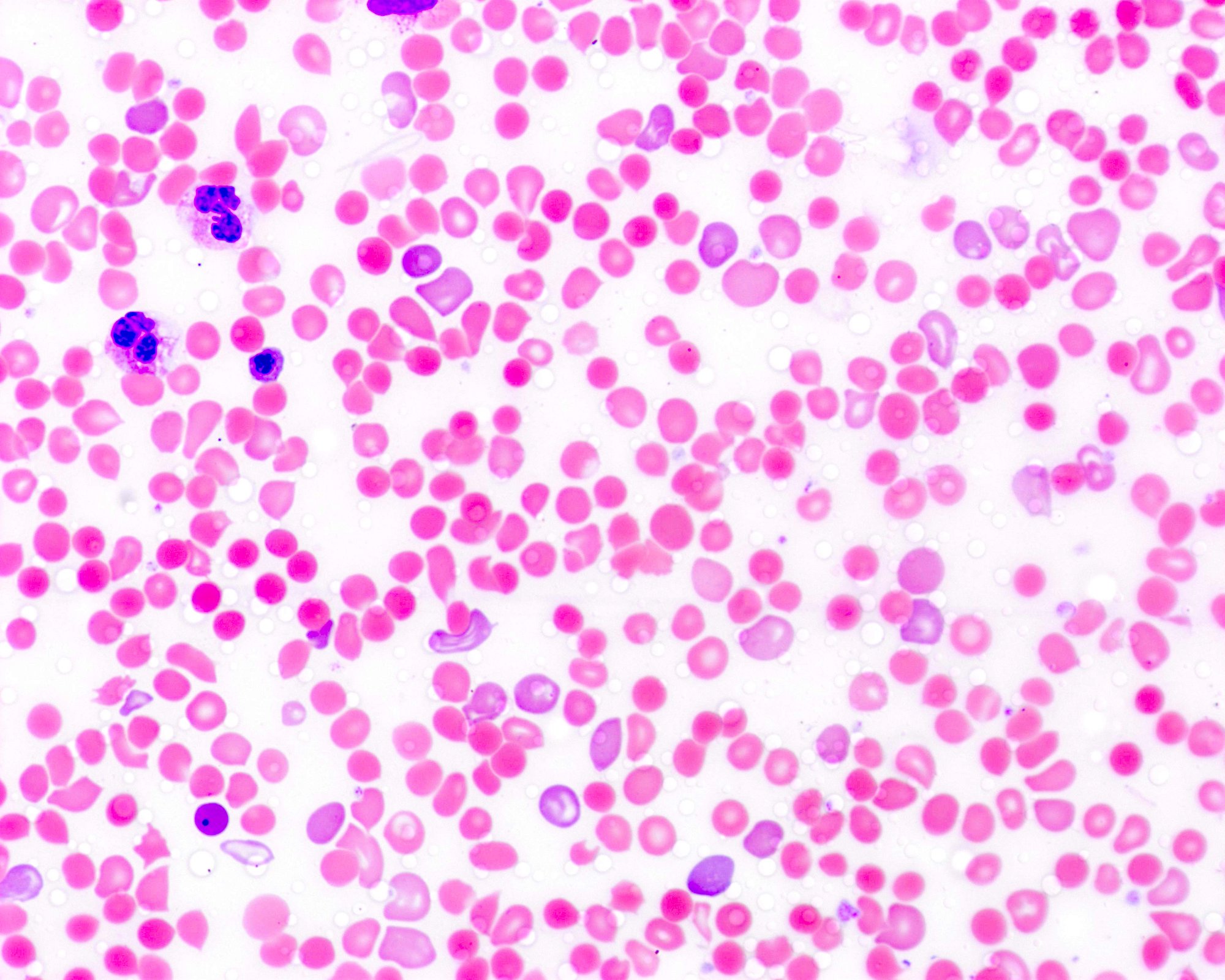
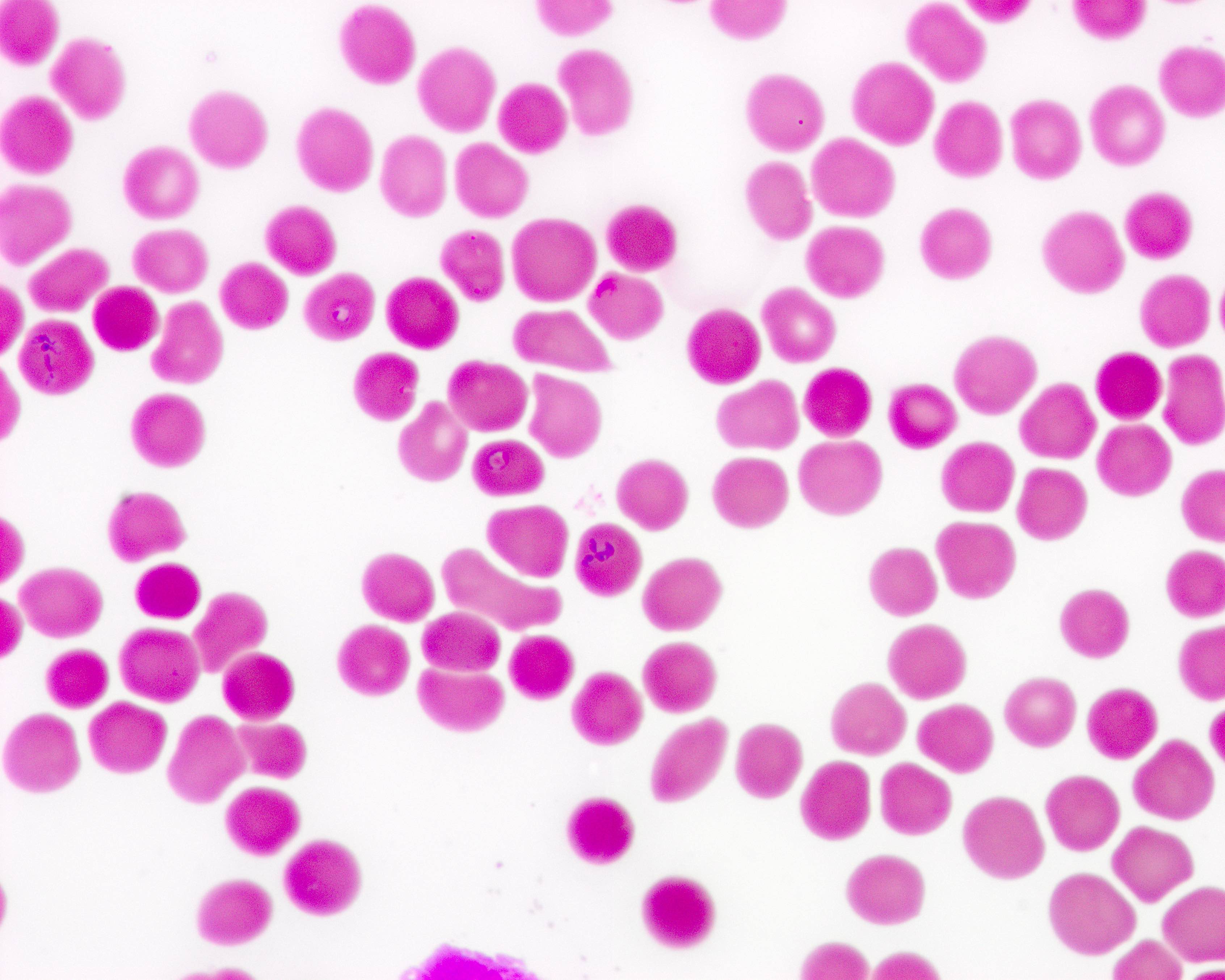
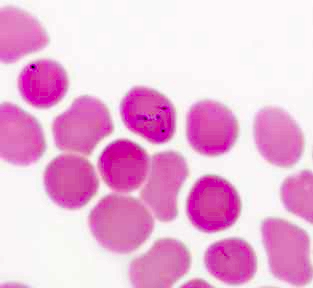
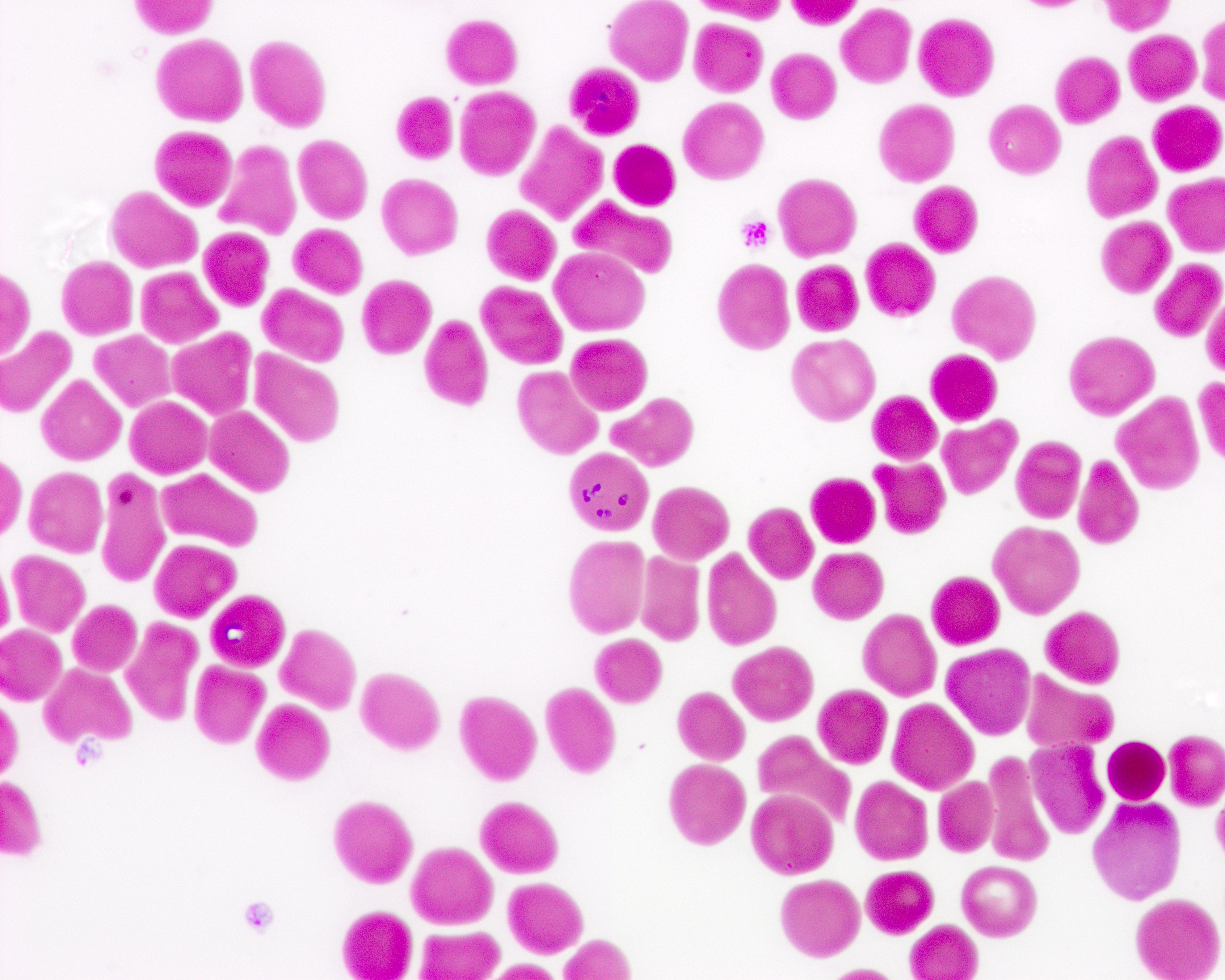
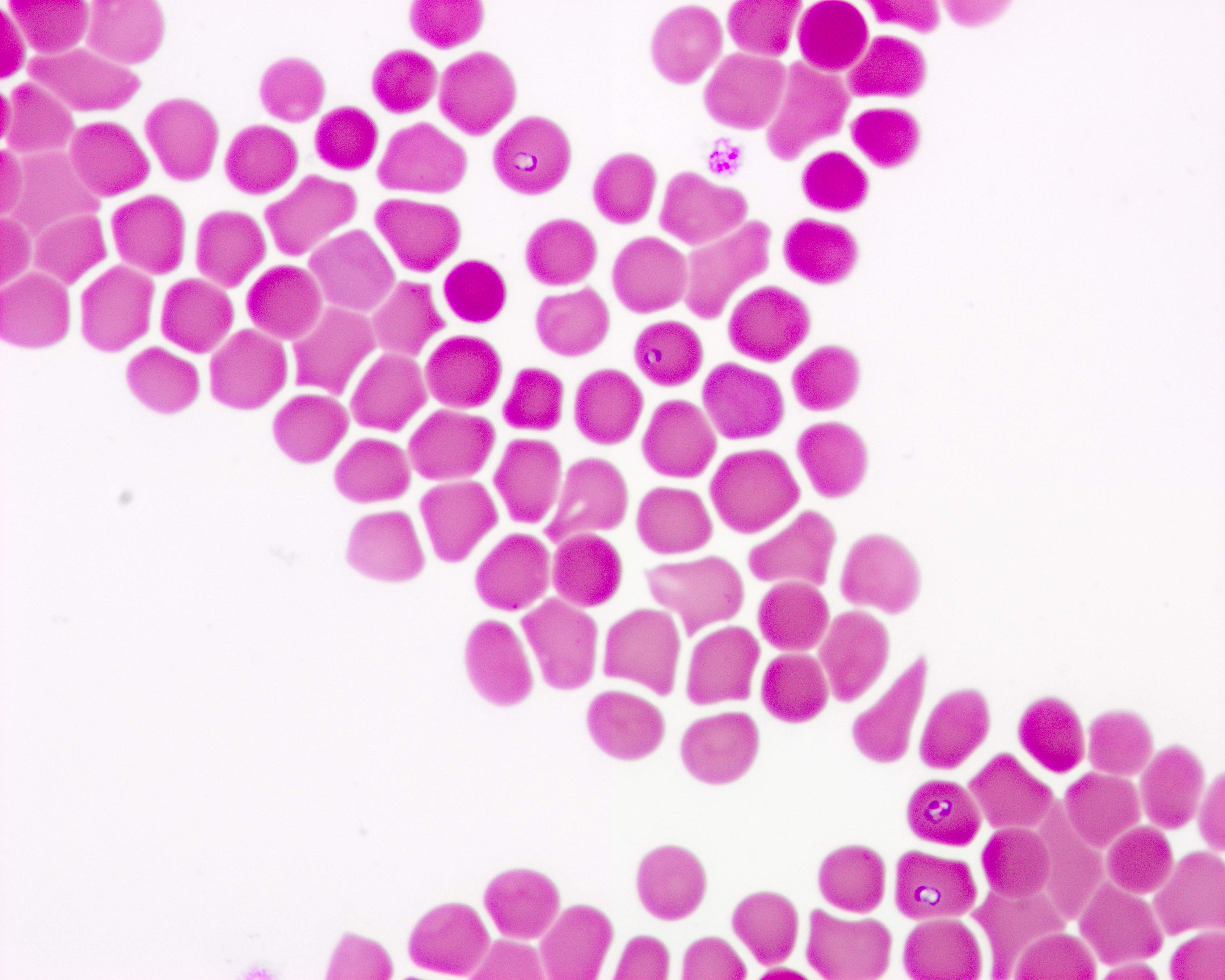
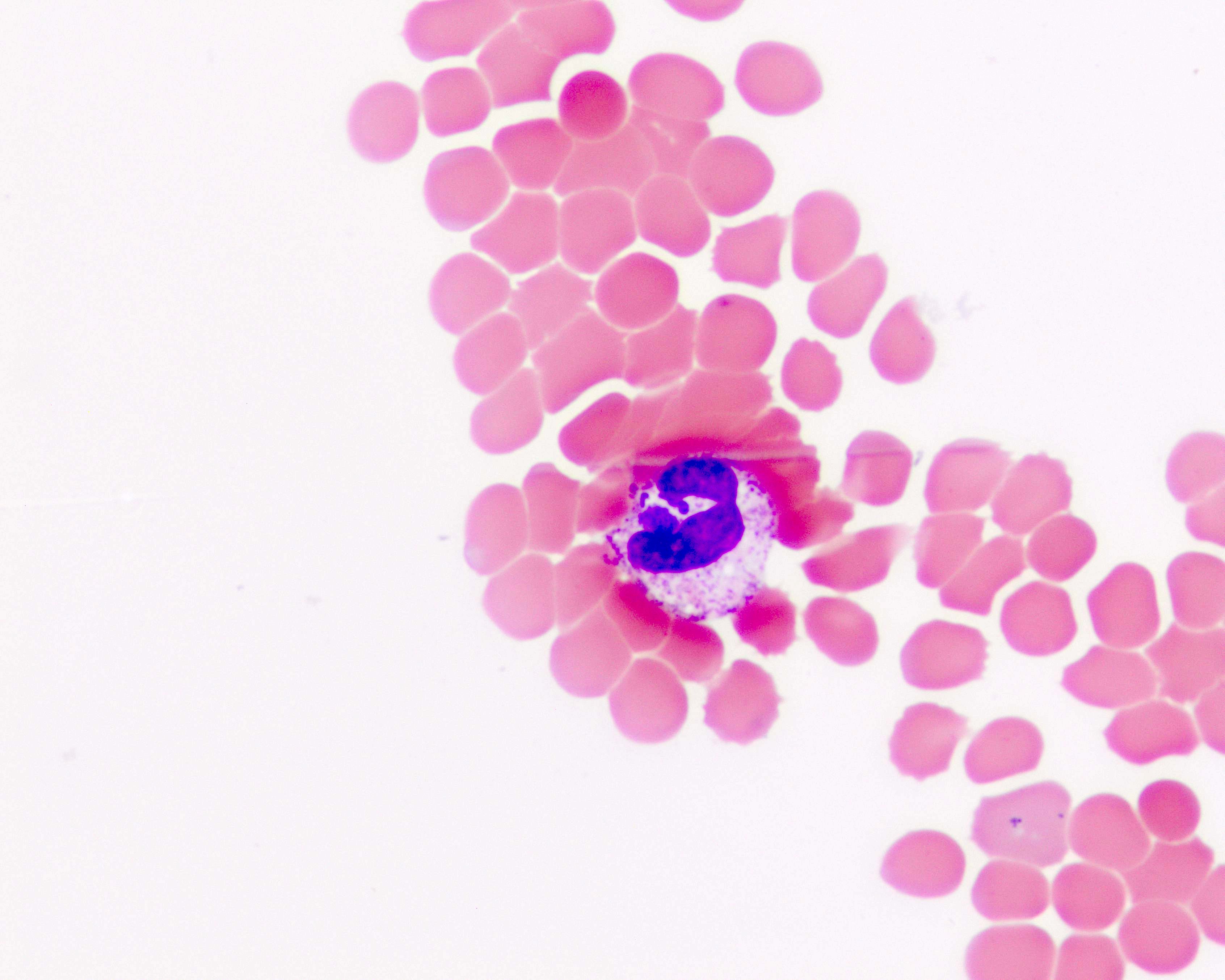
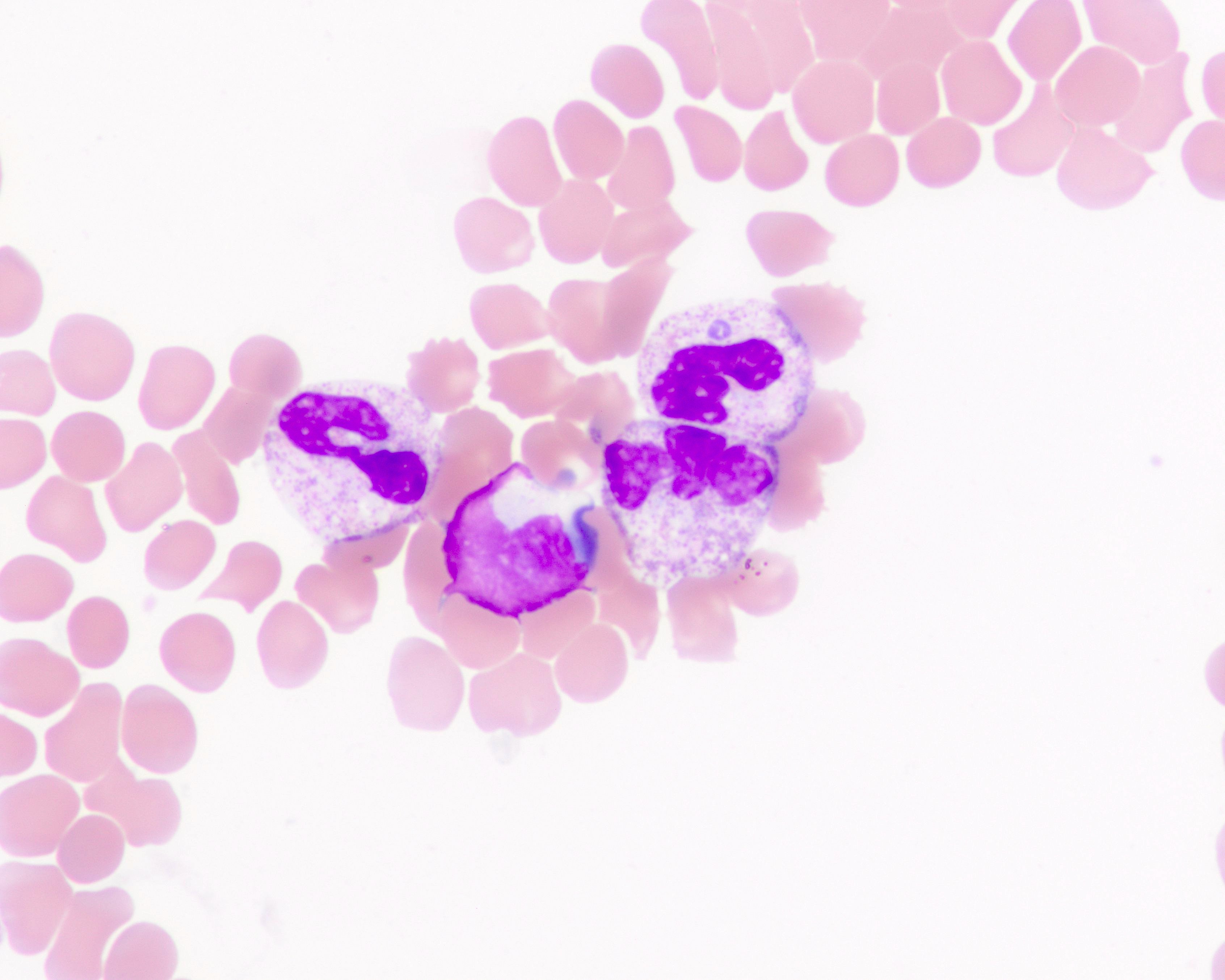
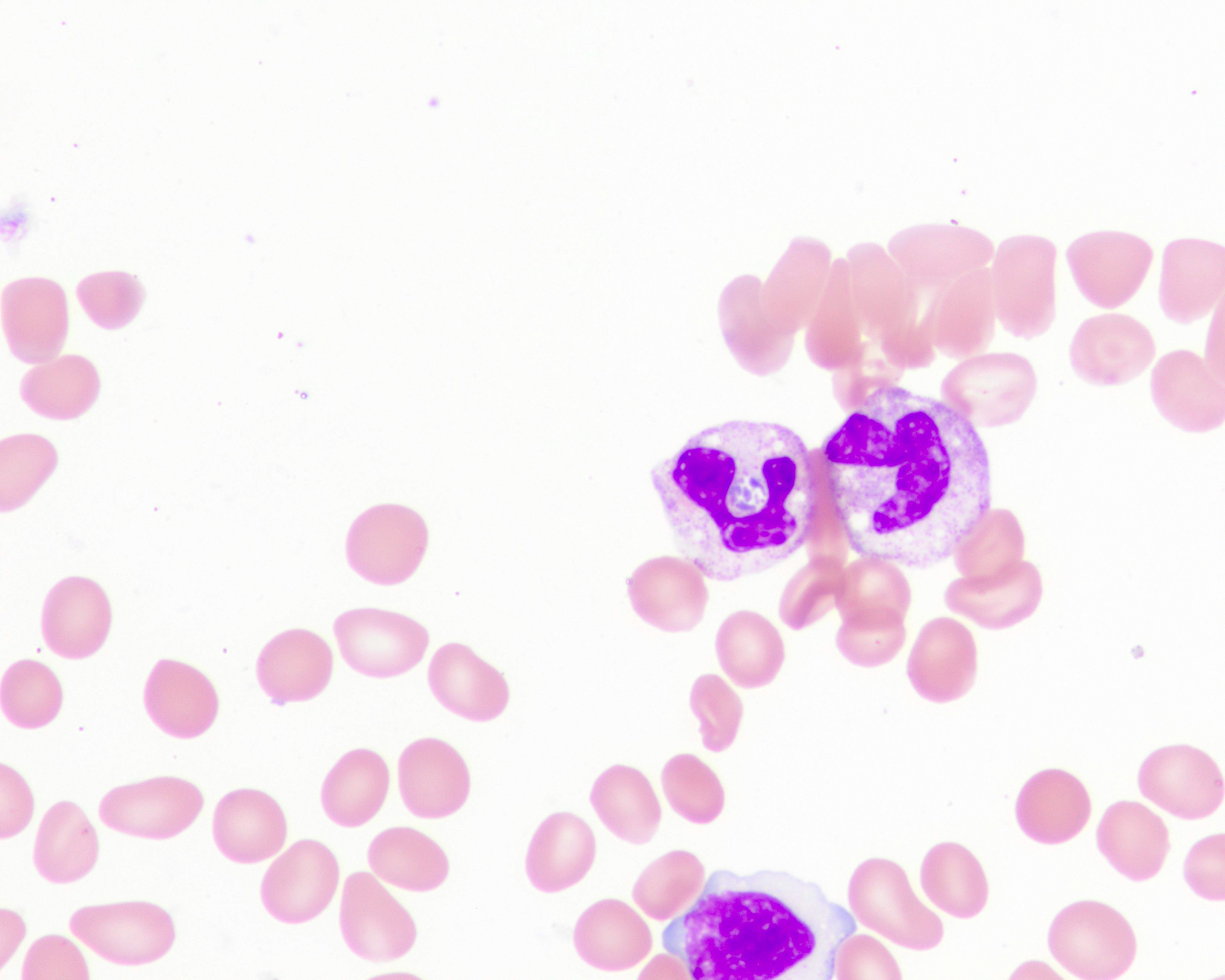
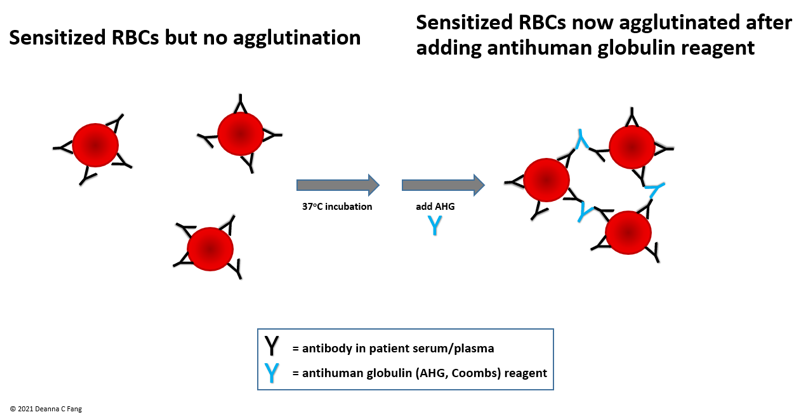
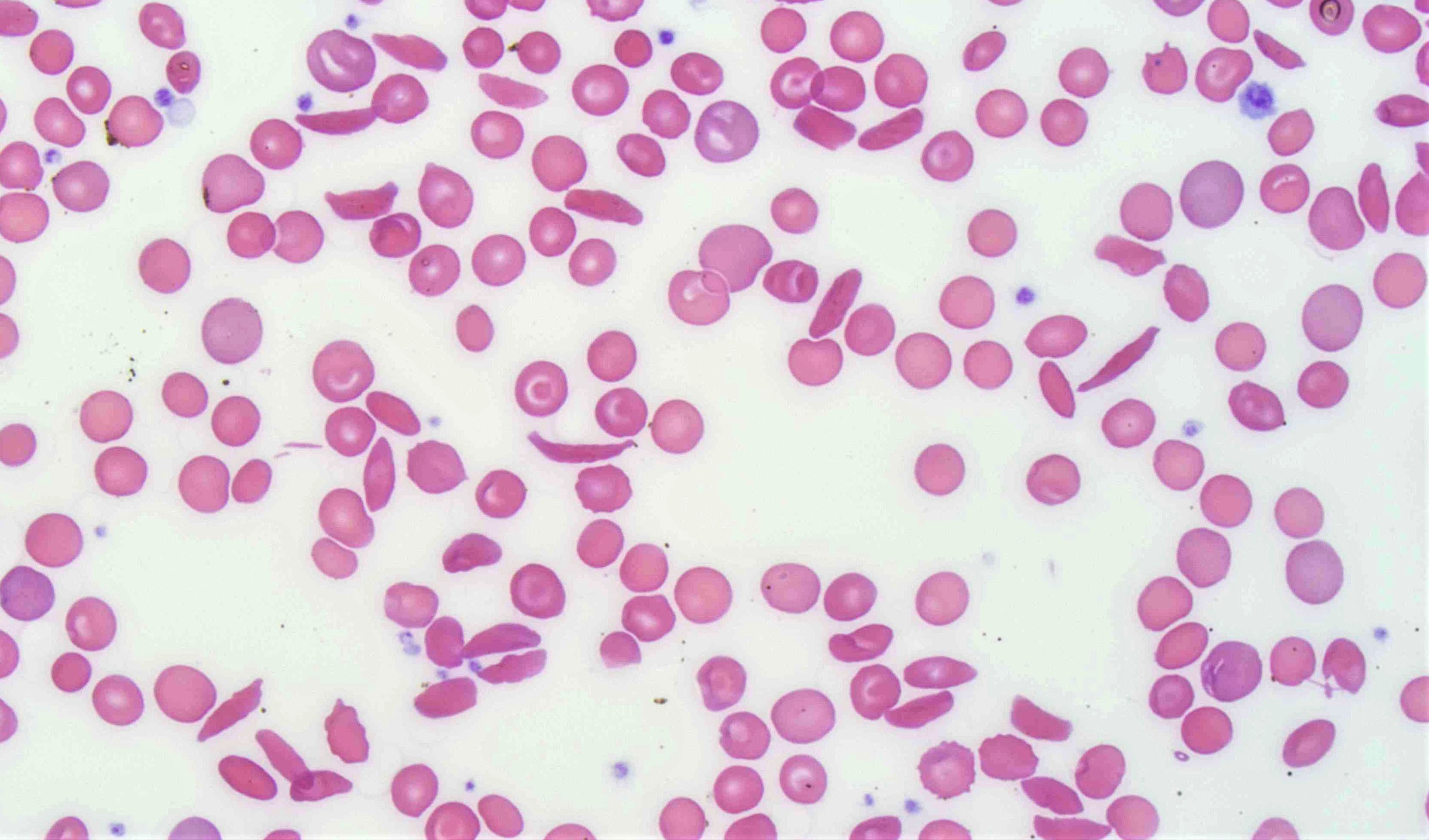
- Peripheral blood smear may show spherocytes (see Peripheral smear images below)
Peripheral smear images
Screening
- WAAs can be detected when
- Antibody screen / panel demonstrates panagglutination at 37 °C and antihuman globulin (AHG) phases
- Autocontrol is positive
- Direct antiglobulin test (DAT) is positive at IgG with or without complement C3
- Elution is panreactive (if elution is negative, a drug related antibody is suspected)
- Peripheral blood smear demonstrates increased spherocytes, polychromasia, anisopoikilocytosis
- pRBC units selected for transfusion may be incompatible
- Decisions about future adsorption studies can be made based upon changes in strength of DAT
- If DAT strength increases, suspect either new alloantibody or increase in strength of WAA
- If DAT reactivity has become stronger, consider adsorption to rule out alloantibody
- If DAT strength decreases, suspect WAA has become weaker and there is likely not an underlying alloantibody
- If DAT reactivity is the same or weaker, may be able to avoid adsorption and continue with least incompatible blood
- If DAT strength increases, suspect either new alloantibody or increase in strength of WAA
- References: Cohn: Technical Manual, 20th Edition, 2020, Hematology Am Soc Educ Program 2022;2022:96
Blood donor testing
- Donor testing includes antibody screening, auto or alloantibodies should be detected at this stage
Donor deferral
- Donors with positive antibody screens are generally deferred, except in particular circumstances
Laboratory
- Blood bank testing leading up to the need for adsorption studies often includes
- In the case of autoantibodies
- Panreactive antibody screen and panel
- Positive autocontrol
- Positive DAT
- Panreactive elution
- 2 types of adsorption
- Autoadsorption if patient has not been transfused recently (within past 90 days)
- Alloadsorption if patient has been transfused (within past 90 days)
- Autoadsorption involves using the patient's own RBCs to adsorb autoantibodies from serum
- Alloantibodies are left in plasma
- Adsorbed serum is tested against a panel to identify alloantibodies
- Traditional allogeneic adsorptions use a set of 3 adsorbing cells with known phenotypes: R1R1, R2R2 and rr
- At least 1 of the adsorbing cells should be negative for K and Jka or Jkb
- At least 1 of the reagent cells should also lack S or s and Fya or Fyb unless the cells are pretreated to denature the antigens
- A separate aliquot of patient serum is incubated with each adsorbing cell
- Once all autoantibody reactivity is removed, the 3 adsorbed serums are tested to eliminate or prove the presence of any underlying alloantibody
- Each aliquot of adsorbed serum contains alloantibody corresponding to those antigens for which the adsorbing cell was negative
- Once adsorption is complete, test the adsorbed serum against reagent RBCs to rule out the presence of underlying alloantibodies
- For instance, if the adsorbing cell is R1R1, S-, K-, Jka-, then the following alloantibodies can be ruled out in that aliquot of adsorbed serum: anti-E, -c, -S, -K and -Jka
- References: Immunohematology 2014;30:153, Hematology Am Soc Educ Program 2022;2022:96
- In the case of autoantibodies
Case reports
- 10 month old boy with AIHA, allogeneic adsorption and heat elution identified auto-anti-f, auto-anti-Jk(a) (Transfus Apher Sci 2020;59:102762)
- 30 year old man with hyperhemolysis syndrome and sickle cell disease, allogeneic adsorption identified possible anti-Fy3 / Fy5 (Transfusion 2022;62:1447)
- 78 year old man, adsorption elution studies identified anti-C, anti-D, anti-G (Ann Lab Med 2018;38:280)
Treatment
- In the case of strong WAAs, when underlying alloantibodies have been ruled out, least incompatible pRBC units may be the only option and transfusion should not be postponed if the patient requires blood components
- In the case of weaker WAAs, crossmatch compatible units may be identified using additional serologic techniques
- When 1 or more alloantibodies are identified, the patient should receive only antigen negative units corresponding to the alloantibodies present
- Infrequently, autoantibodies have specificity to particular antigens
- Occasional specificity to simple Rh antigens but also specificities in the LW, KEL, JK, FY and DI systems have been reported (Cohn: Technical Manual, 20th Edition, 2020)
- In this case, antigen avoidance may not be advantageous, particularly if this exposes the patient to an antithetical antigen they do not possess, creating the potential formation of an alloantibody to that antithetical antigen
- Antigen avoidance in these scenarios should be determined on a case by case basis
- Strategies
- Transfuse least incompatible nonphenotypically matched RBCs
- Transfuse least incompatible RBCs that are phenotypically matched for either the full phenotype or only for the common antigens in the Rh blood group system and for K
- Prevalence is low when indirect antiglobulin test is negative (< 1%)
- Significant portion of patients with WAA form new RBC alloantibody (~15%); however, the use of prophylactic antigen matched (PAM) approach for RBC selection does not appear to be protective against new alloimmunization (Vox Sang 2020;115:515)
Sample assessment & plan
- Assessment: John Doe is a 66 year old man with O, Rh(D) positive blood type and history of acute myelogenous leukemia who presented with neutropenic fever. A type and screen performed by the hospital transfusion service identified a warm autoantibody; panreactivity was identified at AHG phase with polyethylene glycol (PEG) enhancement. Testing was also positive using 1 hour saline technique. The autocontrol was positive and the DAT was positive for IgG. An elution was panreactive (1+ grade of reaction). No prior history of antibodies. His last transfusion was 1 year ago.
- Plan:
- If red cell transfusion is required, O, Rh(D) positive or negative, least incompatible units using saline technique will be issued.
- Recommend additional specimen (X # of red top and X # of lavender top tubes) be sent to the blood bank for autologous adsorption studies to exclude underlying alloantibodies.
- An addendum will be issued with results of the adsorption studies.
Differential diagnosis
- Antibody to a high prevalence antigen:
- Unless the patient had been recently transfused, the autocontrol would be negative (Harmening: Modern Blood Banking & Transfusion Practices, 7th Edition, 2018)
- Multiple alloantibodies:
- If not recently transfused, the autocontrol should be negative; additionally, the strengths of the reactions may vary more widely than an autoantibody without underlying alloantibodies
- If recently transfused, the autocontrol may be positive, as may the DAT
- In this case, an elution may demonstrate specificity
- Retrospective antigen typing of the transfused red cells should match the specificity seen in the eluate (Harmening: Modern Blood Banking & Transfusion Practices, 7th Edition, 2018)
- Drug induced autoimmune hemolytic anemia:
- Depending on the drug, the elution may be negative (most common)
- In rare instances (e.g., methyldopa), the eluate may appear panreactive similar to a WAA; in this case, review of the patient's medication list would be helpful (Transfusion 2007;47:697, Transfus Med Rev 1993;7:242)
- Monoclonal antibody interference (e.g., daratumumab):
- Autocontrol may or may not be positive
- DTT pretreated RBCs should remove the interfering monoclonal antibody (AABB: Association Bulletin #16-02 - Mitigating the Anti-CD38 Interference with Serologic Testing [Accessed 9 May 2023])
Board review style question #1
A 74 year old man with relapsed acute myeloid leukemia (AML) currently on chemotherapy had a complicated clinical course following COVID-19 infection, with subsequent fungal pneumonia and septic shock. He had known red blood cell antibodies against E and P1. During his hospital course, he developed new reactivity. Anti-Jka was identified and about a month later, a peripheral blood smear was reviewed on the patient. The slide signed out by the clinical pathologist on service noted: "moderate anisopoikilocytosis with spherocytes, occasional ovalocytes and teardrop cells... The findings are consistent with patient's history of chemotherapy for AML. The presence of spherocytes, positive autocontrol, positive DAT at IgG phase and panreactive elution, is suggestive of a warm autoantibody."
The patient was transfused several packed red blood cell and platelet units in the past 90 days. What would be the next best step in pretransfusion compatibility testing?
The patient was transfused several packed red blood cell and platelet units in the past 90 days. What would be the next best step in pretransfusion compatibility testing?
- Allogeneic adsorption
- Autologous adsorption
- Cold adsorption
- Dithiothreitol (DTT) treatment
Board review style answer #1
A. Allogeneic adsorption. In the setting of a warm autoantibody in a patient who already has alloantibodies and has been transfused within the past 90 days, allogeneic adsorption is the most appropriate next step. Answer B is incorrect because recent transfusion precludes autologous transfusion. Answer C is incorrect because the reactivity is seen at IgG phase and therefore at 37 °C, cold adsorption is not indicated. Answer D is incorrect because dithiothreitol (DTT) is useful for decreasing CD38 on red blood cells and therefore reducing / eliminating interference from daratumumab therapy often used in plasma cell myeloma and sometimes for pure red cell aplasia / delayed engraftment status post-bone marrow transplant. However, there is no indication from the clinical history that the patient is receiving this therapy.
Comment Here
Reference: Adsorption studies
Comment Here
Reference: Adsorption studies
Board review style question #2
A type and screen in a 56 year old woman with newly diagnosed Waldenström macroglobulinemia reveals panreactivity at immediate spin phase only. The autocontrol is positive at immediate spin phase and the direct antiglobulin test (DAT) is positive for complement. No reactivity is seen at antihuman globulin (AHG) phase using polyethylene glycol (PEG) enhancement; the autocontrol at AHG phase is also negative. The patient has no history of transfusions and no prior history of red cell antibodies. What is the most likely next step(s) for the technologist to exclude any underlying alloantibody?
- No additional testing is necessary
- Repeat the DAT; the results are erroneous given the absence of a DAT positive for IgG
- Send out for allogeneic adsorption studies
- Send out for autologous adsorption studies
Board review style answer #2
A. No additional testing should be necessary. The panel described above is most compatible with a cold autoantibody or cold agglutinin, likely secondary to the patient's diagnosis of Waldenström macroglobulinemia. Cold agglutinins that do not show reactivity at AHG phase are unlikely to be clinically significant (i.e., cause in vivo hemolysis). Even if there were an underlying cold alloantibody, this would be unlikely to be clinically significant in the absence of reactivity at AHG phase; therefore, no additional testing is needed. AHG crossmatch compatible units of RBCs can safely be issued to this patient. Answers B - D are incorrect because these tests are not indicated in this case.
Comment Here
Reference: Adsorption studies
Comment Here
Reference: Adsorption studies
Board review style question #3
A 60 year old woman was recently admitted to the hospital. Below are pertinent laboratory and blood bank results.
IS: immediate spin; AHG: antihuman globulin
Based on these results, which of the following is true?
| Parameter | Patient results | Normal range |
| Hemoglobin | 10.2 g/dL | 11.7 - 15.0 g/dL |
| Hematocrit | 30.5% | 35 - 44% |
| Lactate dehydrogenase (LDH) | 175 U/L | 135 - 250 U/L |
| Total bilirubin | 1.1 mg/dL | 0.0 - 1.2 mg/dL |
| Haptoglobin | 76 mg/dL | 30 - 200 mg/dL |
| Parameter | Patient results |
| Antibody screen | IS: negative; AHG: panreactivity (1+) |
| Antibody panel | IS: negative; AHG: panreactivity (1+) |
| Autocontrol | Positive |
| DAT | IgG: 1+; C3: 0 |
| Elution | Panreactivity (2+) |
Based on these results, which of the following is true?
- Alloantibody to a high prevalence antigen is present
- Cold autoantibody is present
- Warm autoantibody is present
- Without evidence of hemolysis, a warm autoantibody can be ruled out
Board review style answer #3
C. Warm autoantibody is present. These serologic results indicate a warm autoantibody and the diagnosis of a warm autoantibody can be made based on these results alone. While warm autoantibodies have the potential to cause in vivo hemolysis, not all do. The diagnosis of warm autoimmune hemolytic anemia (WAHA), however, requires the presence of a warm autoantibody and evidence of in vivo hemolysis (e.g., drop in hemoglobin / hematocrit, elevated bilirubin, elevated LDH, decreased haptoglobin, peripheral smear findings). Answer D is incorrect because a warm autoantibody cannot be ruled out in the absence of hemolysis. Answer B is incorrect because immediate spin results were negative, which is not consistent with a cold autoantibody. Answer A is incorrect because in the case of an alloantibody or even multiple alloantibodies, panreactivity is common but the autocontrol would likely be negative.
Comment Here
Reference: Adsorption studies
Comment Here
Reference: Adsorption studies
Allergic / anaphylactic
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Symptoms | Screening | Blood donor screening | Laboratory | Case reports | Treatment | Prevention | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Allergic reactions are the most common type of transfusion reaction
- Usually mild but can range from simple urticarial reactions to life threatening anaphylaxis
- Symptoms generally start within minutes of transfusion onset but can occur up to 4 hours following transfusion
- Incidence:
- Mild reactions: 0.03 - 0.61% RBC transfusions; 0.3 - 6% platelet transfusions; 1 - 3% plasma transfusions
- Anaphylaxis: 1/20,000 - 1/47,000 transfusions (Autoimmun Rev 2014;13:163)
- Most commonly occur following platelet or plasma transfusions but can occur following any blood component transfusion
- Leukoreduction does not reduce the incidence
Essential features
- Nonhemolytic in nature
- Allergic transfusion reactions are common and generally mild, presenting as urticaria (hives) and pruritus (itching)
- For a mild allergic reaction, a transfusion can be paused, the patient given appropriate medication (e.g. diphenhydramine) and if symptoms resolve completely, the transfusion may continue with observation; this is the only transfusion reaction that does not require a complete stop of the transfusion with a workup
- Anaphylaxis is rare; historically attributed to IgA deficiency
- IgA deficient patients should have an appropriate workup and do not require washed products unless a documented severe reaction has occurred in the past
Terminology
- BAT (basophil activation test)
- ELISA (enzyme linked immunosorbent assay)
- PAS (platelet additive solution)
- RBC (red blood cell)
- TACO (transfusion associated circulatory overload)
- TRALI (transfusion associated acute lung injury)
Pathophysiology
- Not well understood
- Type I / immediate hypersensitivity reaction:
- B cells produce IgE antibodies in response to allergens (plasma proteins); these antibodies bind causing basophil and mast cell activation and degranulation (Transfus Med Rev 2018;32:43)
- Plasma protein deficiencies:
- Congenital or acquired protein deficiencies (IgA, haptoglobin, C3 / C4): patients can form antibodies against absent plasma proteins
- IgA deficiency (IgA < 0.05 mg/dL):
- Anti-IgA IgG antibodies are formed in response to a sensitizing event (transfusion, pregnancy)
- Prevalence: IgA deficiency 1/300 - 1/1,200 in Caucasians (much lower in Asians); IgA deficiency with anti-IgA antibodies 1/994 - 1/1,560 (Autoimmun Rev 2014;13:163, Transfusion 2015;55:199)
- Anaphylactic transfusion reactions are rare and patients generally do not require modified blood products (Br J Haematol 2013;160:434, Transfusion 2015;55:199)
- Congenital haptoglobin deficiency
- Antibodies formed in response to sensitizing event similar to IgA deficiency
- Anhaptoglobineamia more common in East Asian populations (Blood 2000;95:1138)
- Reactions may by mild (Transfusion 2013;53:1361)
- IgA deficiency (IgA < 0.05 mg/dL):
- Passive sensitization:
- Passive transfer of donor allergens (e.g. peanut) or donor antibodies (e.g. allergen-specific IgE) is possible; transient allergic sensitivity to allergens (Allergy 2005;60:1192, Br J Haematol 2013;160:434, Transfusion 2015;55:199)
- Congenital or acquired protein deficiencies (IgA, haptoglobin, C3 / C4): patients can form antibodies against absent plasma proteins
Symptoms
- Fever is not a symptom of allergic or anaphylactic reactions
- The earlier in the transfusion the reaction starts, generally the more severe it is
- Mild allergic reactions:
- Urticaria, flushing, pruritus, mild / localized angioedema (eyes, lips, throat fullness)
- Severe / anaphylactic reactions:
- Allergic symptoms plus hypotension, dyspnea with or without signs of airway obstruction (wheezing, stridor), angioedema, abdominal pain, vomiting, loss of consciousness, shock
- References: Shaz: Transfusion Medicine and Hemostasis, 2nd Edition, 2013, Fung: AABB Technical Manual, 19th Edition, 2017
Screening
- There is no effective screening method for blood donors
Blood donor screening
- For patients with true IgA deficiency who require plasma transfusion, the American Rare Donor Program (a joint program of the AABB and American Red Cross) may be able to provide a source (Transfus Med Hemother 2014;41:342)
- IgA deficient plasma is very rare
Laboratory
- Basophil activation test (BAT):
- Basophil activation assessed using flow cytometry
- May be useful in the diagnosis of allergic transfusion reactions but not commonly performed
- IgA deficiency evaluation:
- Immunoglobulin serum concentrations (IgM, IgG, IgA)
- If severe IgA deficiency (< 0.05 mg/dL, typical adult normal range: 70 - 400 mg/dL depending on laboratory assay), perform anti-IgA antibody ELISA
- Evaluation in children should occur after 6 months of age; in children younger than 4 years, the diagnosis is considered preliminary as levels may normalize into adolescence (Autoimmun Rev 2014;13:163)
Case reports
- 88 year old woman, IgA deficient, with recurrent transfusion related anaphylactoid reactions (Transfusion 2018;58:2320)
- Peanut allergen passive transfer causing anaphylaxis (N Engl J Med 2011;364:1981)
Treatment
- Urticarial reaction (only):
- Stop the transfusion
- Administer antihistamine
- Symptom resolution: restart the transfusion, no laboratory work up required
- No symptom resolution: discontinue the transfusion, provide supportive care, report reaction to transfusion service
- All other reactions:
- Stop the transfusion
- Provide supportive care including but not limited to: epinephrine (intramuscular injection is first line for anaphylaxis), H1 and H2 receptor antagonists, corticosteroids, respiratory support
- Report reaction to transfusion service
- References: Shaz: Transfusion Medicine and Hemostasis, 2nd Edition, 2013, Fung: AABB Technical Manual, 19th Edition, 2017
Prevention
- Prophylactic premedication is not recommended in patients with no history of allergic reaction
- History of allergic reactions:
- Premedication with antihistamines, H2 receptor antagonists or corticosteroids may be helpful depending on previous reaction severity
- Patients with history of severe reactions (Blood 2019;133:1831):
- Washed products (pRBCs, platelets) to remove donor plasma may be indicated
- Platelets in platelet additive solution (PAS) may eliminate reactions with better posttransfusion platelet increases than washed platelets
- Solvent detergent treated plasma
- IgA deficient patients:
- Majority do not require washed or modified products; trial with unmodified products first
- If a history of anaphylactic reactions to transfusion, washed pRBCs and platelets, PAS platelets and IgA deficient plasma (rare donor program) may be indicated
Sample assessment & plan
- Example 1: IgA deficiency evaluation
- Assessment: Jane Smith is a 36 year old woman with a history of IgA deficiency. Laboratory review shows low IgA (< 10 mg/dL; normal adult range: 70 - 400 mg/dL) and anti-IgA is negative. The patient has no history of allergic or anaphylactic transfusion reactions; however, transfusion history is not available (patient required 2 units of pBRC for postpartum bleeding at an outside hospital in 2013). The patient is being evaluated for elective cholecystectomy.
- Plan: If required, we will issue unmodified blood products
- Although the patient has low IgA levels, the anti-IgA is negative
- No history of allergic reaction during prior transfusions in 2013
- Cholecystectomy has a low risk of bleeding complications
- Example 2: allergic transfusion reaction
- Assessment: Patient is a 17 year old boy with Hodgkin lymphoma and thrombocytopenia secondary to chemotherapy. The patient developed a rash across the chest and left upper arm with pruritus approximately 20 minutes into a transfusion of platelets which were irradiated and leukocyte reduced. The patient had received approximately 53 mL of platelets at the time of the reaction. The patient did not have any signs of respiratory distress and vital signs were stable. The transfusion was discontinued and the patient was given diphenhydramine, after which all symptoms resolved. Blood bank workup showed no clerical errors. There is no evidence of hemolysis and the DAT was negative on both pre and posttransfusion samples.
- Plan: No evidence of hemolysis. This is categorized as a mild transfusion reaction.
- Premedication is suggested only if the patient has additional allergic reactions; evidence has found that there is limited benefit to premedication for mild transfusion reactions
- Patient may receive additional transfusions as needed; if another mild reaction occurs, the transfusion may be paused and the patient can be given antihistamines; if all symptoms resolve within 20 minutes, the transfusion may be resumed
- For any transfusion reaction with fever, respiratory distress, hypertension or hypotension, the transfusion must be discontinued and reported to the blood bank immediately
Differential diagnosis
- Vasovagal or hypotensive reaction:
- Hypotension usually within first 15 minutes of transfusion
- Absence of other signs with resolution following discontinuation of transfusion
- Hemolytic transfusion reaction:
- Fever, dyspnea
- Frequently pain at infusion site
- Transfusion related acute lung injury (TRALI):
- Fever and pulmonary edema causing dyspnea
- Asthma exacerbation:
- History of asthma
- Absence of allergic symptoms (urticarial, pruritus)
- Allergens may provoke an asthma exacerbation
Additional references
Board review style question #1
A healthy, 65 year old woman is admitted to the hospital for total hip arthroplasty. During the procedure, her vital signs are within normal limits but she experiences significant bleeding. In the recovery room, she is found to have a hemoglobin of 6.2 g/dL (normal female reference
range: 12 - 16 g/dL) and complains of shortness of breath. A RBC transfusion is ordered. Halfway through transfusion, the patient begins to complain of itching with red raised wheals on her arms and chest. She remains short of breath, although her other vital signs are stable. Which of the following adverse reactions to transfusion has the patient most likely experienced?
- Allergic transfusion reaction
- Anaphylaxis
- Bacterial contamination of the donor unit
- Transfusion related acute lung injury (TRALI)
- Transfusion associated circulatory overload (TACO)
Board review style answer #1
A. Allergic transfusion reaction. While allergic transfusion reactions are most commonly seen with platelets, they can occur with any blood product. The patient displays a classic urticarial reaction, which is frequently accompanied by pruritus. She remains short of breath as her anemia has not yet been resolved. Her symptoms are not severe enough at this point to be considered an anaphylactic reaction. She is afebrile, making TRALI or bacterial contamination unlikely. She only received part of a unit of pRBCs following a surgery, making it unlikely that TACO is responsible. TACO also does not present with an urticarial reaction.
Comment Here
Reference: Allergic and anaphylactic transfusion reaction
Comment Here
Reference: Allergic and anaphylactic transfusion reaction
Board review style question #2
A 42 year old man, status post bone marrow transplant, arrives at the hospital infusion clinic for continued transfusion support. Following labs, he is found to be thrombocytopenic and a platelet unit is ordered. 10 minutes into the platelet transfusion, large red wheals appear across the patient's chest. His vital signs are stable and he reports no shortness of breath. The nurse stops the transfusion and administers 50 mg of diphenhydramine PO. His symptoms resolve. With regard to the remaining platelet volume, the nurse should
- Discard the remainder of the unit and notify the transfusion service so they may initiate a transfusion reaction workup
- Discontinue the transfusion and return the unit to the transfusion service for transfusion reaction workup
- Restart the transfusion, monitoring the patient for the return of symptoms
- Restart the transfusion without further patient monitoring
Board review style answer #2
C. Restart the transfusion, monitoring the patient for return of symptoms. A mild urticarial allergic reaction that resolves with antihistamines is the only scenario in which a transfusion can be restarted and in which the transfusion service does not need to be notified that a reaction has occurred. If the patient again develops urticaria following restarting of the unit, the transfusion must be permanently discontinued and the unit returned to the blood bank for further investigation.
Comment Here
Reference: Allergic and anaphylactic transfusion reaction
Comment Here
Reference: Allergic and anaphylactic transfusion reaction
Antibody identification & panel interpretation (pending)
[Pending]
ASFA guidelines overview
Table of Contents
Definition / general | Essential features | Terminology | Diagrams / tables | Case reports | Treatment | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- American Society for Apheresis (ASFA) was formed in 1982 with a mission to advance apheresis sciences and set a stage for physicians and allied health care workers to share their knowledge
- ASFA publishes guidelines to systematically review the available evidence and provides a categorical way to approach the request of apheresis procedures in different diseases
- ASFA guidelines are updated every few years; other than a few examples, this entry does not detail specifics of particular disease states
Essential features
- ASFA guidelines organize diseases and their therapies into category and grading systems
- Category describes the efficacy and priority of apheresis in treatment of a disease
- Grading describes the quality of evidence to support apheresis treatment of a disease
Terminology
- ASFA: American Society for Apheresis
- Apheresis: a modality in which the blood of a person is passed through a device that separates blood into different components, removes 1 constituent and returns the rest, with or without a replacement fluid (J Clin Apher 2013;28:3)
Diagrams / tables
Category definitions for therapeutic apheresis
| Category | Description |
| I | Apheresis is accepted as first line therapy, either as a primary standalone treatment or in conjunction with treatment modalities |
| II | Apheresis is accepted as second line treatment, either standalone or in conjunction with other treatment modalities |
| III | Apheresis decision should be individualized; the optimum role of apheresis is not established |
| IV | Disorders for which apheresis is ineffective or harmful based on the published data |
Grading recommendations and evidence for therapeutic apheresis
| Description | Methodological quality of supporting evidence | Implications | |
| Grade 1A | Strong recommendation, high quality evidence | Randomized controlled trials without important limitations or overwhelming evidence from observational studies | Strong recommendation; can apply to most patients in most circumstances without reservation |
| Grade 1B | Strong recommendation, moderate quality evidence | Randomized controlled trials with important limitations (inconsistent results, methodological flaws, indirect or imprecise) or exceptionally strong evidence from observational studies | Strong recommendation; can apply to most patients in most circumstances without reservation |
| Grade 1C | Strong recommendation, low quality or very low quality evidence | Observational studies or case series | Strong recommendation but may change when higher quality evidence becomes available |
| Grade 2A | Weak recommendation, high quality evidence | Randomized controlled trials without important limitations or overwhelming evidence from observational studies | Weak recommendation; best action may differ depending on circumstances or patients' or societal values |
| Grade 2B | Weak recommendation, moderate quality evidence | Randomized controlled trials with important limitations (inconsistent results, methodological flaws, indirect or imprecise) or exceptionally strong evidence from observational studies | Weak recommendation; best action may differ depending on circumstances or patients' or societal values |
| Grade 2C | Weak recommendation, low quality or very low quality evidence | Observational studies or case series | Very weak recommendation; other alternatives may be equally reasonable |
- Reference: Curr Opin Hematol 2019;26:461
Case reports
- 16 year old girl with systemic lupus erythematosus and thrombocytopenia was ultimately diagnosed with thrombotic thrombocytopenic purpura (Medicine (Baltimore) 2022;101:e28908)
- 33 year old man with chronic kidney disease, highly HLA sensitized, successfully treated with plasma exchange (Transpl Immunol 2022;74:101656)
- 66 year old woman with weight loss and loss of appetite was found to have white blood cell (WBC) count of 122K (Oncol Lett 2014;8:1825)
Treatment
- Plasma exchange (PLEX): plasma is removed from whole blood and replaced with albumin, fresh frozen plasma (FFP), cryo poor FFP or a combination of these fluids
- Red blood cell exchange (erythrocytapheresis): red cells are removed from whole blood and replaced with donor RBCs
- Leukocytapheresis: white blood cells are removed from whole blood and no replacement fluid is needed
- Thrombocytapheresis: platelets are removed from whole blood and no replacement fluid is needed
- Extracorporeal photopheresis: buffy coat is collected from whole blood, psoralen is added to the buffy coat and cells are photoactivated and returned to the patient (J Clin Apher 2019;34:171)
Sample assessment & plan
- Case 1: A 35 year old man presented to the emergency department with altered mental status and easy bruising. His significant other reports that he was in his usual state of health until last night when he started to have a headache and was talking incoherently. He was going to the bathroom when he fell and hit his arm, where he had a large bruise. He has no history of solid organ or stem cell transplant.
- On exam he seems pale, sclera is icteric, large bruise on his right arm. He is febrile and mildly tachycardic.
- Labs: significant for hemoglobin (Hgb) 7 g/dL, platelet (PLT) 20 x 109/L, lactate dehydrogenase (LDH) of 1,200 IU/L
- The findings may represent thrombotic thrombocytopenic purpura, since patient has acute unexplained thrombocytopenia, microangiopathic hemolytic anemia, fever and neurological findings. An emergent plasma exchange needs to be carried out.
- Daily plasmapheresis treatments should occur with fresh frozen plasma as replacement fluid until the platelet count is > 150 x 109/L for 2 - 3 days
- Each treatment generally exchanges 1 plasma volume
- Stopping treatment versus tapering is controversial
- Ancillary treatments, such as rituximab or eculizumab, may be considered after ceasing plasmapheresis
- Therapeutic monoclonal antibodies may be removed by plasmapheresis and consideration must be given to timing such treatments after rather than concurrent with apheresis
- Case 2: A 70 year old man with no significant history presented to the emergency with fatigue for the past 2 weeks and an inability to thrive. He has lost 10 pounds in the past 2 weeks. In the emergency department he suddenly becomes hypoxic, requiring high flow nasal cannula O2 support with 20 liters per minute (LPM). He also has sudden onset of altered mental status.
- His labs are notable for WBC: 200 x 109/L, Hgb of 6.6 gr/dL, PLT of 30 x 109/L
- These findings of hyperleukocytosis and end organ damage are worrisome for symptomatic hyperleukocytosis. Emergent leukocytapheresis needs to be carried out.
- The role of leukapheresis in improving long term outcomes in leukemia with hyperleukocytosis is controversial
- Leukapheresis is generally contraindicated in promyelocytic leukemia
- Leukapheresis should not delay more definitive chemotherapeutic interventions
- The primary team should be reminded of the potential for pseudohyperkalemia in hyperleukocytosis
- Whole blood potassium measurements are preferred over serum potassium for this reason
- 1.5 to 2 blood volumes are generally processed
- One procedure can reduce the WBC by 30 - 60%
- Case 3: A 22 year old man with HgbSS presented to the emergency department with right side facial droop and right side body weakness. Imaging demonstrates left middle cerebral artery stroke and multiple silent strokes.
- On exam, the patient has right side facial droop, he is not able speak in full sentences and he is not able to move the right side of his body.
- His labs are notable for Hgb of 10g/dL, hemoglobin S (HgbS) of 50%, WBC and PLT are within normal limits (WNL).
- Weight: 150 Ib; height: 5'3"
- The patient shows signs of acute stroke and his HgbS needs to rapidly be reduced to < 30%. He requires red blood cell exchange.
- The role of ongoing prophylactic red cell exchange in stroke prevention for patients with HbSS is controversial
- Patients receiving ongoing red cell exchange often experience venous access issues
- Exact HbS measurement might not be available off hours, so estimation might be necessary
- Target hematocrit should be 30 + 3% to avoid hyperviscosity
- End HbS should be < 30%
- The volume of packed red blood cell units to be exchanged can be calculated based upon starting and end hematocrit, fraction of patient's own cells remaining (FCR) and starting and end HbS levels
Additional references
Board review style question #1
A 25 year old man with no significant medical history presents to the emergency department, where his significant other describes that he was not himself for the past day and had a fever. He is also looking pale compared to before. Last night, he hit his arm while trying to get to the bathroom, which caused a large area of bluish discoloration.
A medicine resident inquires about the evidence for plasma exchange procedure. What does American Society for Apheresis (ASFA) recommend for this patient?
- On arrival vitals: temperature of 39.0 °C, heart rate of 110, blood pressure of 110/70, O2 of 95% on radial artery (RA), respiration rate (RR) of 20
- Labs significant for hemoglobin (7 g/dL), hematocrit (14.2%), platelet count (10 x 109/L), haptoglobin (< 30 mg/dL), lactate dehydrogenase (LDH; 1,885 U/L) and indirect bilirubin (3.2 mg/dL)
- Peripheral blood smear revealed an abnormally high reticulocyte count (18.7%) and presence of schistocytes
A medicine resident inquires about the evidence for plasma exchange procedure. What does American Society for Apheresis (ASFA) recommend for this patient?
- ASFA category I, grade 1A
- ASFA category I, grade 1B
- ASFA category II, grade 1A
- ASFA category II, grade 1B
- ASFA category III, grade 2C
Board review style answer #1
A. This indication for plasma exchange (PLEX) carries ASFA category I, grade 1A recommendation. This patient presents with profound thrombocytopenia, hemolytic anemia, fever and neurological changes, which are consistent with thrombotic thrombocytopenic purpura (TTP) until proven otherwise. The most recent 2019 ASFA guidelines recommend PLEX as first line therapy, which would be category I indication with grade 1A recommendation (strong recommendation, high quality of evidence). The categories and grading system are explained in tables 1 and 2.
Comment Here
Reference: ASFA guidelines overview
Comment Here
Reference: ASFA guidelines overview
Board review style question #2
A 65 year old woman with a history of diabetes and hypertension presents to her primary care office with fatigue and difficulty breathing for the past week. As part of workup, her primary care provider orders a CBC and results come back with WBC 150 x 109/L, hemoglobin (Hgb) 7.5 g/dL and platelet (PLT) count of 10 x 109/L. The primary care provider refers her to the emergency department immediately but she wants to stay home. After a couple of days, she becomes increasingly short of breath and is brought in by ambulance with O2 requirement of 10 L.
A hematology oncology fellow who has already looked at the American Society for Apheresis (ASFA) guidelines calls you and states that he found this indication carries a category II, grade 2C recommendation. What does this ASFA recommendation indicate?
- In the emergency department, her WBC went up to 190 x 109/L, Hgb 7.0 g/dL
- Peripheral smear shows 80% of blasts consistent with acute leukemia
- Coagulation profile is within normal limits
A hematology oncology fellow who has already looked at the American Society for Apheresis (ASFA) guidelines calls you and states that he found this indication carries a category II, grade 2C recommendation. What does this ASFA recommendation indicate?
- Apheresis should be considered as first line standalone therapy with strong recommendation and high quality evidence
- Apheresis should be considered as a second line treatment with strong recommendation and high quality evidence
- Apheresis should be considered as a second line treatment with strong recommendation and low quality evidence
- Apheresis should be considered as a second line treatment with weak recommendation and low quality evidence
- Apheresis should not be considered with strong recommendation and high quality evidence
Board review style answer #2
D. Apheresis should be considered as a second line treatment with weak recommendation and low quality evidence. Category II: apheresis procedure is considered as a second line treatment either standalone or in conjunction with other treatment modalities. Grade 2C: weak recommendation with low quality of evidence.
Comment Here
Reference: ASFA guidelines overview
Comment Here
Reference: ASFA guidelines overview
Automation
Table of Contents
Definition / general | Essential features | Terminology | Application of automation in transfusion medicine | Automated and semiautomated methodologies | Pros and cons of automation | Existing platforms of automated equipment | Electronic crossmatching | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Use of automatic operational equipment in the manufacturing or processing of whole blood or pretransfusion testing
- Semiautomated systems allow for partial manual labor with automated interpretation
Essential features
- Blood bank automation is an alternate method to manual tube testing and blood processing in transfusion medicine
- Promotes standardization of interpretation, increased transfusion safety, specimen batching and efficiency in turn around times
- 3 methodologies currently exist for automated pretransfusion testing and 1 for whole blood separation
- Implementation requires appropriate equipment verification
Terminology
- Automated blood bank system
- Automated pretransfusion compatibility testing
- Automated blood processing system (ABPS)
- Solid phase red cell adherence assay (SRCA)
- Column agglutination test (CAT)
- Erythrocyte magnetized technique (EMT)
Application of automation in transfusion medicine
- Blood component collection and processing
- Separation into plasma, red blood cell (RBC) and platelet components by apheresis
- Leukoreduction to prevent transmission of cytomegalovirus (CMV)
- Separation and processing of whole blood unit collections
- RBC washing for IgA deficient patients (Transfusion 2015;55:2415)
- Platelet agitation
- ABO typing, antibody screening, ABO titers, selected cell panels and compatibility testing in pretransfusion (immunohematology) testing
- Product safety and detection of growth of microorganisms in platelets through culture: BacT / ALERT (Transfus Med 2002;12:303)
- Supply of uncrossmatched or crossmatched blood via remote allocation in blood storage machines located away from the blood bank (Transfusion 2018;58:372)
- Possible use in manufacture of blood components (Transfusion 2021;61:568)
- Electronic crossmatch in patients with negative antibody screens
Automated and semiautomated methodologies
- Automated blood processing system (ABPS)
- Method: separates whole blood units into components through centrifugation and expression of components into individual product bags
- Degree of automation: full automation of manual whole blood separation steps
- Application: processing of whole blood collections
- Limitation: higher levels of platelets in plasma units (Transfus Med Hemother 2021;48:290)
- Solid phase red cell adherence assay (SRCA)
- Methods
- Standard: serum added for antigen antibody reaction in microplates containing solid medium with reagent RBCs showing a visible tight or effaced button as a negative or positive reaction, respectively
- Degree of automation: semiautomated and fully automated platforms
- Application: pretransfusion testing
- Limitation: does not detect IgM (method requires incubation at 37 °C), limiting application to the identification of non-ABO antibodies
- Additional features: capacity for platelet serology
- Methods
- Column agglutination test (CAT)
- Method: microtubes with gel or microbead matrix containing antisera or antihuman globulin
- Serum mixed with reagent RBC, agglutination of cells in the gel or microbead matrix constitutes test positivity
- Open system
- Degree of automation: semiautomated and fully automated platforms
- Application: pretransfusion testing
- Limitation: very sensitive; increased false positives due to lack of wash step
- Additional features: modified CAT, dilution factor based on degree of agglutination
- Method: microtubes with gel or microbead matrix containing antisera or antihuman globulin
- Erythrocyte magnetized technique (EMT)
- Method: serum added to magnetized RBC inside a microplate that agglutinates after a magnetic force is applied
- Degree of automation: fully automated platforms
- Limitation: does not detect IgM
- Additional features: eliminates the need for centrifugation and washing steps
- These methodologies have superior sensitivity to conventional tube testing (Asian J Transfus Sci 2012;6:140)
- Pretransfusion testing commonly uses agglutination (hemagglutination) as the standard to measure a possible reaction
- Agglutination techniques can be modified for various purposes of detection (e.g., latex agglutination, sample mixed with latex particles coated with an antigen or antibody)
- Increased sensitivity is achieved with low ionic strength saline or bromelin methyl cellulose resulting in increased potential for false positives
- Does not allow for unrestricted modifications of testing like dithiothreitol (DTT) treatment and proteolytic enzymes; laboratories with high complexity patients must retain tube agglutination methods to allow for modified testing protocols for specified situations
Pros and cons of automation
- Increases blood bank testing and unit processing capacity and throughput compared with manual technique (Transfus Med Hemother 2021;48:290, Vox Sang 2013;105:225, Transfus Apher Sci 2015;53:58)
- Limits human errors and reduces staffing requirements (Asian J Transfus Sci 2015;9:S6)
- Improves test reproducibility, traceability and patient identification with the use of specimen barcodes (Transfus Med 2022;32:299)
- Requires extensive validation (which falls under the blood bank quality management structure) and must meet Food and Drug Administration (FDA) compliance (FDA: Part 11, Electronic Records; Electronic Signatures - Scope and Application [Accessed 8 February 2023])
- All equipment must be certified for safety and verified in compliance with Clinical Laboratory Improvement Amendments (CLIA) quality standards (Electronic Code of Federal Regulations: Part 493 - Laboratory Requirements [Accessed 4 May 2023])
- Guidelines for validation are provided by the International Society of Blood Transfusion (ISBT) (Vox Sang 2010;98:1)
- Automated systems are more costly to acquire and maintain than manual methods and must be compatible with the institution's informatics
- Specific sample collection requirements and staff training are vital for adequate function
- A backup system is necessary in anticipation of down time
Existing platforms of automated equipment
- All platforms perform antibody screening, ABO group testing, crossmatching and direct antiglobulin test (DAT)
- Blood processing
- Reveos by Terumo Blood and Cell Technologies (Tokyo, Japan)
- ABPS
- 2 separation procedures
- RBC, plasma and residual leukocytes
- RBC, plasma, interim platelet units and residual leukocyte units
- Self contained
- System management for workflow management and reporting
- Reveos by Terumo Blood and Cell Technologies (Tokyo, Japan)
- Pretransfusion testing
- IH-1000 and IH-500 by Bio-Rad (USA)
- CAT
- Continuous reagents and sample loading
- Additional features: extended phenotyping
- Qwalys 3 by DIAGAST (France)
- EMT
- Continuous reagents and sample loading
- Additional features: weak D testing and extended phenotyping
- Studies have shown higher IgM titration levels when compared to tube and CAT methods (Vox Sang 2020;115:233)
- Galileo and NEO by IMMUCOR (USA)
- SRCA
- Continuous reagents and sample loading
- Additional features: weak D, Rh phenotyping, ABO titers and platelet antibody screening and crossmatch
- Wadiana and Erytra by Grifols (Singapore)
- CAT
- Batch testing for Wadiana and continuous loading or batch testing for Erytra
- Additional features: enzyme assays, weak D (Erytra) and extended phenotyping
- Autovue Innova and ORTHO VISION by Ortho Clinical Diagnostics (USA)
- CAT
- Continuous reagents and sample loading
- Additional features: indirect antiglobulin test and RH / Kell / Duffy phenotyping, selected cell panels, serial dilutions for titers (ORTHO VISION)
- PK7300 / 400 by Beckman Coulter (USA)
- SRCA
- Rh and Kell phenotyping
- Fully automated, batch testing of large volume samples (300/hour)
- SRCA
- TANGO by Bio-Rad (USA)
- 2 testing options
- Erytype S: SRCA; precoated microplates with dried antisera
- Rh and Kell phenotyping, Cord blood ABO and Rh
- Solid screen II: SRCA with protein A coated microwells
- Antibody screen and identification, DAT, weak D, crossmatch
- Erytype S: SRCA; precoated microplates with dried antisera
- Fully automated, batch testing
- 2 testing options
- IH-1000 and IH-500 by Bio-Rad (USA)
Electronic crossmatching
- Computer based crossmatch using recipient blood bank history to match with stored units
- Software matches compatible stored units at the blood bank based on recipient historical blood bank data (ABO / Rh and antibody testing)
- Substitutes for immediate spin compatibility testing (recipient's plasma / donor RBCs)
- Benefits
- Accurate assignment of compatible units and verification of historical data
- Faster release of units
- Automated verification of unit's compatibility through barcodes
- Promotes better use of storage by prioritizing units closer to expiration
- Limitations
- Accuracy depends on extensive validation with the laboratory information system (LIS)
- Typically not used for recipients with history of or newly formed clinically significant antibodies
- Modified or additional restrictions are placed per institution (e.g., exclusion of recipients of ABO incompatible hematopoietic stem cells) (Vox Sang 2013;104:350)
Additional references
Board review style question #1
- As a blood bank director, you are appointed to oversee the implementation of a new automated system for pretransfusion testing. Which of the following is a requirement before implementation for all equipment used in collection, processing, testing or storage of blood components?
- Acceptable performance on external proficiency testing
- Calibration
- Equipment verification
- Inspection from accreditation agencies (e.g., CAP)
Board review style answer #1
C. Equipment verification is required before implementation. Answer A is incorrect because performance on proficiency testing is a way of monitoring specific tests or measurements of individual laboratories and comparing it with other laboratories. Answer B is incorrect, as the calibration of an instrument is a process for verification of the accuracy of results after implementation. Answer D is incorrect because inspections by accreditation agencies (e.g., CAP) aim to assess the compliance of a laboratory with the agency requirements.
Comment Here
Reference: Automation
Comment Here
Reference: Automation
Board review style question #2
- Which of the following patients is the best candidate for an RBC transfusion using electronic crossmatching?
- 18 year old man with sickle cell disease and multiple RBC transfusions presenting with increased lactate dehydrogenase (LDH), haptoglobin of < 10 mg/dL and hemoglobin of 5.4 g/dL
- 35 year old woman with history of systemic lupus erythematosus (SLE) and history of warm autoantibody and mild thrombocytopenia of 100 K/cmm
- 45 year old woman with laboratories showing a mean corpuscular volume (MCV) of 84.3 fL, hemoglobin of 7 g/dL, serum iron of 12 microg/dL (normal: 40 - 160 microg/dL), who had a positive antibody panel, showing a nonspecific antibody 5 years ago; current direct antiglobulin test (DAT) and antibody screen is negative
- 87 year old man with anemia (Hgb 6.9 g/dL) and newly diagnosed multiple myeloma, no history of transfusions and a negative antibody screen
Board review style answer #2
D. 87 year old man with anemia (Hgb 6.9 g/dL) and newly diagnosed multiple myeloma, no history of transfusions and a negative antibody screen. This answer is correct because the patient has no prior history of transfusions as well as a negative type and screen. Answer A is incorrect, as the patient's laboratory findings are consistent with hemolytic anemia likely attributed to a hemolytic transfusion reaction due to the presence of ≥ 1 alloantibodies suggested by the history of multiple transfusions. The patient's alloantibody(ies) must be identified in order to provide antigen negative full crossmatch compatible RBCs. Answer B is incorrect because a warm autoantibody causes a positive antibody screen and panagglutinin in the cell panel. Other special testing techniques (e.g., adsorption studies) are needed to rule out any alloantibodies in order to use electronic crossmatch. Answer C is incorrect since electronic crossmatching should not be used on patients with positive antibody screens without a certain alloantibody specificity.
Comment Here
Reference: Automation
Comment Here
Reference: Automation
Bacteria
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Symptoms | Screening | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Transfusion transmitted bacterial infections (TTBI) present with fever, chills and hypotension immediately or up to 24 hours after a transfusion
- Platelets are by far the most commonly bacterially contaminated blood component due to room temperature storage and TTBI is one of the leading causes of death due to transfusion each year
- Bacterial risk control strategies for platelets include donor testing for bacteria and pathogen reduction technology (PRT)
Essential features
- TTBI presents with fever, chills and hypotension
- Source of bacteria is typically from donor's skin but also asymptomatic bacteremia can occur
- TTBI is one of the principal causes of death due to transfusion
- Donor testing strategies have some residual risk of contamination due to false negative results
- The contamination rate detected by primary culture is 1 in 4,348 for apheresis platelets and 1 in 2,632 for whole blood derived platelets collected by the platelet rich plasma method (Transfusion 2020;60:986)
- The September 2019 FDA Guidance on bacterial risk control strategies outlines permissible approaches that include bacterial testing or pathogen reduction technology (FDA-2014-D-1814) (FDA: Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion [Accessed 8 June 2020])
- If a transfusion reaction is considered, stop the transfusion immediately and report to Transfusion Services
Terminology
- Transfusion transmitted bacterial infection (TTBI)
- Intravenous drug use (IVDU)
- Transfusion related acute lung injury (TRALI)
- Whole blood derived (WBD)
- Disseminated intravascular coagulation (DIC)
- Pathogen reduction technology (PRT)
- Large volume delayed sampling (LVDS)
Pathophysiology
- Bacteria on donor skin or asymptomatic bacteremia that transfers to the collected blood component
- Bacteria proliferate at a rate that is dependent on lag time, doubling time and growth medium (the heterogeneous platelet component)
- Most common bacterial pathogens detected in blood components
- Staphylococcus epidermidis and Staphylococcus aureus
- Gram negative bacteria, such as Klebsiella species
- Anaerobic bacteria, such as Clostridium perfringens and Propionibacterium / Cutibacterium species (this latter category of organisms may have limited pathogenic potential)
- Bacterial risk control strategies (such as donor arm disinfection, diversion, testing and careful visual inspection of product prior to release) fail (Vox Sang 2014;106:23)
- Transfusion of bacteria above a certain threshold of harm (e.g. 103 - 105 colony forming units/ml) causes systemic inflammatory response in patient: fever, chills, hypotension (Clin Infect Dis 2008;46:1214, Transfusion 2017;57:2174)
- Platelet components most commonly implicated due to room temperature storage
- Rarely, bacterially contaminated red blood cell components have been reported to cause septic transfusion reactions
Clinical features
- TTBI rate likely underreported due to passive reporting of suspected transfusion reactions (Blood 2016;127:496)
- Surveillance data demonstrate nearly all septic transfusion reactions are associated with platelets issued on the last 2 days of shelf life (Transfusion 2020;60:220)
Transmission
- Bacteria are transferred from donor into collected blood component(s) and bacterial risk control strategies fail to result in interdiction of component(s)
- Contaminated blood component is transfused into the blood stream of a transfusion recipient
Symptoms
- Fever ≥ 38°C
- Chills
- Rigors
- Hypotension and shock
- Develop symptoms during or up to 24 hours after transfusion (Fung: Technical Manual, 19th Edition, 2017)
Screening
- Note that pathogen reduction technology does not require bacterial testing (screening)
- Permissible risk control strategies using FDA approved methods are outlined below with some useful naming codes in parentheses:
- Apheresis platelets or prestorage pools of whole blood derived platelets
- Single step methods:
- Primary culture no sooner than 24 hours, 3 day shelf life (24C-3)
- Large volume delayed sampling at 36 hours postcollection, 5 day shelf life (36C-5)
- Large volume delayed sampling at 48 hours, 7 day shelf life (48C-7)
- Pathogen reduction technology, 5 day shelf life (PRT)
- 2 step methods:
- Primary culture no sooner than 24 hours with secondary culture performed no sooner than day 3, 5 day shelf life (24C-D3C-5)
- Primary culture no sooner than 24 hours with secondary culture no sooner than day 4, 7 day shelf life (24C-D4C-7)
- Primary culture no sooner than 24 hours with rapid testing, 5 day shelf life (24C-R-5)
- Primary culture no sooner than 24 hours with rapid testing, 7 day shelf life (24C-R-7)
- Large volume delayed sampling no sooner than 36 hours with secondary culture no sooner than day 4, 7 day shelf life (36C-D4C-7)
- Large volume delayed sampling no sooner than 36 hours with secondary rapid testing, 7 day shelf life (36C-R-7)
- Single step methods:
- Single units and poststorage pools of whole blood derived platelets
- Rapid testing for 5 day shelf life of single units and 4 hours after pooling for poststorage pools of whole blood derived platelets
- Single culture with sampling performed no sooner than 36 hours after collection with a 5 day shelf life
- Single culture with sampling performed no sooner than 24 hours after collection for 5 day shelf life, if transfused after day 3 of storage, secondary rapid testing may be considered (FDA-2014-D-1814) (FDA: Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion [Accessed 8 June 2020])
- Apheresis platelets or prestorage pools of whole blood derived platelets
| List of permissible bacterial risk control testing policies for apheresis platelets and their characteristics | |||||||
| Policy code | Primary culture | Component expiration time (hours) | Secondary test | Availability (hours) | |||
| Sample time (hours) | Hold time (hours) | Test type | Sample time (hours) | Hold time (hours) | |||
| 24C-3 | 24 | 12 | 72 | N/A | N/A | N/A | 36 |
| 36C-5 | 36 | 12 | 120 | N/A | N/A | N/A | 72 |
| 48C-7 | 48 | 12 | 168 | N/A | N/A | N/A | 108 |
| 24C-D3C-5 | 24 | N/A | 120 | culture | 72 | * | 72 |
| 24C-D4C-7 | 24 | N/A | 168 | culture | 96 | 12 | 120 |
| 36C-D4C-7 | 36 | N/A | 168 | culture | 96 | 12 | 108 |
| 24C-R-5 | 24 | N/A | 120 | rapid | 12 hours prior to issue | N/A | 84 |
| 24C-R-7 | 24 | N/A | 168 | rapid | 12 hours prior to issue | N/A | 132 |
| 36C-R-7 | 36 | N/A | 168 | rapid | 12 hours prior to issue | N/A | 120 |
*Time period not specifically defined in FDA guidance; a hold period is required in the guidance but not specified for this testing policy (Walker BS et al. The comparative safety of bacterial risk control strategies for platelet components: A simulation study. Transfusion. Accepted.)
- False positive results are not uncommon
- If bacterial testing is positive, recommend sampling the platelet bag for a confirmation culture
- Organism should be identified and retrieval of any co-components is required
- Donor should be notified if bacteria not a likely skin contaminant
- Non-PRT strategies (i.e. those involving testing) have residual risk of contamination and septic transfusion reactions due to false negative results, which depends on strategy used and bacterial growth characteristics (Transfusion 2018;58:1647, Vox Sang 2012;103:1)
- Acinetobacter spp. and several other species have been reported as causing septic transfusion reactions in PRT and platelet additive solution products (FDA: Important Information for Blood Establishments and Transfusion Services Regarding Bacterial Contamination of Platelets for Transfusion [Accessed 11 March 2022])
Blood donor screening
- Questionnaire, specifically, to address illness within few days before / after donation
- Vitals within normal limits
- Antecubital skin examination: free of lesions and evidence of intravenous drug use (Fung: Technical Manual, 19th Edition, 2017)
Blood donor testing
- No specific bacterial testing of donors is performed directly, only on collected components
- Bacterial contamination is mitigated by improved donor skin disinfection and diversion of first 10 - 40 mL of donor blood
Donor deferral
- Medical director dependent, likely indefinite or permanent deferral
Laboratory
- When a septic transfusion reaction is suspected, bacterial culture of remaining transfused component and patient blood culture with same organism identified
- Gram stain of the residual transfusion component may be positive for bacteria
- Direct antiglobulin test (DAT) and visual inspection for hemolysis are usually negative
- Clerical check and repeat of patient ABO expected to be correct (Fung: Technical Manual, 19th Edition, 2017)
Case reports
- 21 year old woman with TTBI due to Streptococcus bovis in contaminated platelet unit (Am J Hematol 2004;77:282)
- 50 and 51 year old women received contaminated platelet unit (N Engl J Med 2002;347:1075)
- 56 year old woman with TTBI due to Pseudomonas poae in contaminated red blood cell unit (Emerg Infect Dis 2019;25:1445)
- 60 and 67 year old men received contaminated platelet unit (Clin Infect Dis 2004;39:e106)
- 2 fatal and 1 severe TTBI due to Klebsiella pneumoniae contaminated split platelet unit (Am J Clin Pathol 2018;149:293)
- 4 patients received bacterially contaminated platelets (MMWR Morb Mortal Wkly Rep 2019;68:519)
Treatment
- Stop the transfusion immediately
- Broad spectrum antibiotics until bacterial sensitivity / specificity known, then optimize coverage
- Supportive care
- Quarantine any co-components still in inventory as quickly as possible (Fung: Technical Manual, 19th Edition, 2017)
Sample assessment & plan
- Assessment: Patient X is a 50 year old male with acute myeloid leukemia undergoing hematopoietic stem cell transplant. He received a unit of apheresis platelets for bleeding prophylaxis (platelet count 5,000/uL). Within 1 hour of starting the transfusion, he developed tachycardia, hypotension and chills. The transfusion was stopped. A suspected transfusion reaction investigation was initiated with return of the component and associated tubing and a new blood sample to the Transfusion Service. Clerical check was correct. Visual hemolysis and DAT were negative. ABO was correct. Patient blood culture and residual platelet component culture were positive for Staphylococcus aureus.
- Plan: Supportive care and extended spectrum antibiotic coverage followed by more specific coverage when organism is identified and sensitivities performed. Quarantine any co-components. The patient may receive future transfusions as clinically indicated.
Differential diagnosis
- Consider underlying medical conditions, i.e. sepsis
- Acute transfusion reactions (< 24 hours):
- Acute hemolytic transfusion reaction:
- Chills, fever, hemoglobinuria, hypotension, renal failure with oliguria, hemorrhage (DIC), back pain, pain along infusion vein, anxiety
- Febrile nonhemolytic transfusion reaction:
- Fever, chills / rigors, headache, vomiting
- Anaphylactic transfusion reaction:
- Hypotension, urticarial, angioedema, bronchospasm, stridor, abdominal pain
- Transfusion related acute lung injury:
- Hypoxemia, respiratory failure, hypotension, fever, bilateral pulmonary edema
- Hypotensive transfusion reaction:
- Systolic blood pressure drops below 80 mm Hg and an overall decrease of at least 30 mm Hg from pretransfusion value (Fung: Technical Manual, 19th Edition, 2017)
- Acute hemolytic transfusion reaction:
Board review style question #1
A 65 year old man with a history of acute lymphoblastic leukemia is undergoing induction and has required multiple transfusions for anemia and thrombocytopenia. During a platelet transfusion, he develops fever, chills and hypotension. The transfusion is stopped and the product is returned to the Transfusion Service. Chest Xray shows no pulmonary edema or infiltrates. The Transfusion Service reports no hemolysis and the DAT is negative. What is the next most important step?
- The patient should be given an antihistamine
- The patient should be transferred to the ICU for close monitoring
- The patient should receive an antipyretic medication
- The patient's blood and the residual product should be cultured and the patient should be treated empirically with antibiotics
- The product and any other products created from the same donation should be thrown away
Board review style answer #1
D. The patient and the residual product should be cultured for aerobic and anaerobic bacteria. Additionally, a Gram stain should be performed to immediately provide clinicians a preliminary identification but are variably negative. If the cultures are positive, the organism should be the same. Additional testing, such as pulsed field gel electrophoresis of DNA macrorestriction, can lend information as to the similarities of the bacterial isolates. Since culture is a time intensive process, if there is clinical concern for a TTBI, the patient should be empirically treated with broad spectrum antibacterial coverage until identification with antibiotic sensitivities are available. While additional donor products should be identified and isolated, the products should be cultured, not thrown away. The ICU is likely necessary in a septic patient but further workup is still necessary for a diagnosis. The patient does not have symptoms solely suggestive of an allergic reaction, which would include urticarial, angioedema, bronchospasm, etc. An antipyrectic medication may be appropriate but the hypotension is concerning for a more serious transfusion reaction than a febrile, nonhemolytic reaction.
Comment Here
Reference: Bacterial contamination of blood components
Comment Here
Reference: Bacterial contamination of blood components
Board review style question #2
A donor presents to donate an apheresis platelet. The donor history questionnaire, vitals and hemoglobin / hematocrit are acceptable. The donor donates without incident. Viral testing returns negative. The product is cultured at 24 hours. According to the September 2019 FDA guidance, what is one permissible next step?
- After the appropriate amount of hold time (12 hours), the product is ready for transfusion with a 7 day shelf life
- After the appropriate hold period (12 hours), the product is ready for transfusion with a 4 day shelf life
- Rapid testing may be used according to the manufacturer's package insert with up to a 7 day shelf life of the platelet product
- Secondary culture cannot be used to extend shelf life
Board review style answer #2
C. Rapid testing may be used according to the manufacturer's package insert with up to a 7 day shelf life of the platelet product. The product is an apheresis platelet with a primary culture performed at 24 hours, which without a subsequent secondary test, has a shelf life of 3 days. A secondary test may be used to extend the shelf life. These may include rapid testing or secondary culture no sooner than day 4 for up to a 7 day shelf life. These strategies are based on the September 2019 FDA Guidance, which has several permissible strategies that are outlined above in this chapter.
Comment Here
Reference: Bacterial contamination of blood components
Comment Here
Reference: Bacterial contamination of blood components
Biological product deviation
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Diagrams / tables | Basis of BPDs / blood and biologic guidance | Responsibility (who must report) | Events to report | Events not required to report | Timing of report (when to report) | Modality for reporting (how and where to report) | Types of events | Implementation | Reporting fatalities associated with transfusion | Case reports | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Error or deviation from the standard operating procedure (nonconformance) related to the manufacture, storage or distribution of licensed biological products (e.g., blood products)
- Could potentially affect the safety, purity or potency of the product
- May include issues regarding the testing, processing, packing, labeling or storage of blood components
Essential features
- Biological product deviation (BPD) refers to unexpected events in manufacture, storage or distribution that deviate from the standard operating procedure
- BPD reporting is mandated by the FDA and governed by title 21 of the Code of Federal Regulations (CFR)
- Events occur after distribution and are categorized according to the phase of manufacture, storage or distribution in which they occur
Terminology
- Nonconformance
- Unexpected event
- Center for Biologics Evaluation and Research (CBER)
- Center for Drug Evaluation and Research (CDER)
- Code of Federal Regulations (CFR)
- U.S. Food and Drug Administration (FDA)
- Safety means "relative freedom from harmful effect to persons affected, directly or indirectly, by a product when prudently administered, taking into consideration the character of the product in relation to the condition of the recipient at the time" (eCFR: 21 CFR 600.3(p) [Accessed 11 August 2023])
- Purity is the "relative freedom from extraneous matter in the finished product, whether or not harmful to the recipient or deleterious to the product; purity includes but is not limited to relative freedom from residual moisture or other volatile substances and pyrogenic substances" (eCFR: 21 CFR 600.3(r) [Accessed 11 August 2023])
- Potency is defined as "specific ability or capacity of the product, as indicated by appropriate laboratory tests or by adequately controlled clinical data obtained through the administration of the product in the manner intended, to effect a given result" (eCFR: 21 CFR 600.3(s) [Accessed 11 August 2023])
Epidemiology
- Quality control and distribution events constituted 38.0% of the BPD events reported by licensed blood establishments to the FDA and 60.2% of BPD events from unlicensed registered blood establishments in fiscal year 2022
- 1,785 reports (29.1%) by licensed blood establishments involved blood collection deviations in fiscal year 2022
- 17.5% of reports from unlicensed registered blood establishments in fiscal year 2022 were due to routine testing deviations
- Quality control and distribution nonconformances constituted 54.3% of reports from transfusion services in fiscal year 2022
- Routine testing deviations constituted 28.5% of the reports from transfusion services to the FDA in fiscal year 2022 (FDA: Biological Product and HCT/P Deviation Reports - Annual Summary for Fiscal Year 2022 [Accessed 11 August 2023])
Diagrams / tables
Basis of BPDs / blood and biologic guidance
- Governed by title 21 of the CFR
- FDA mandates specific nonconformances in manufacturing be reported (eCFR: 21 CFR 600.14 [Accessed 11 August 2023], eCFR: 21 CFR 606.171 [Accessed 11 August 2023], eCFR: 21 CFR 1271.350(b) [Accessed 11 August 2023])
- Encompasses human tissue, cellular and tissue based products
- Investigations of nonconformances must
- Be timely
- Include preventative and corrective action plans
- Provide for processes for timely recall of nonconforming products
- Include proper disposal of all impacted products
Responsibility (who must report)
- Responsible party is the one that had control over the blood product at the time the nonconformance occurred
- Control is defined as responsibility for ensuring the safety, purity and potency of a product
- Control also covers responsibility for maintaining compliance with applicable standards and good manufacturing practices
- Manufacturing steps contracted out by a blood establishment still fall under the control of the primary blood establishment
- Manufacturing contractors are not mandated to report BPDs to the FDA
- Manufacturing performed by contractors must conform to current good manufacturing practice (CGMP) (United States Code: Title 21 - Food and Drugs [Accessed 11 August 2023])
- Examples of usual contracted manufacturing steps include blood collection, storage, distribution and irradiation
- Establishments who should report BPDs include
- Licensed manufacturers of blood products
- Unlicensed registered blood establishments
- Transfusion services
- Establishments that conduct compatibility testing for a transfusion service have control of this step and are in charge of reporting any nonconformance related to this aspect
- Reference: eCFR: 21 CFR 606.171(a) [Accessed 11 August 2023]
Events to report
- Any event related to manufacturing, inclusive of testing, processing, labeling, storage, distribution of blood or its components that deviates from current good manufacturing practices, relevant standards and regulations
- Any manufacturing events that impact the safety, purity or potency of a product
- Events discovered after product was distributed even if not transfused
- Usually unforeseen or unexpected events
- Happen within reporting facility or establishments in contract with the facility
- Events involving distributed blood or its components
- References: eCFR: 21 CFR 606.171(b) [Accessed 11 August 2023], eCFR: 21 CFR 600.14 [Accessed 11 August 2023]
Events not required to report
- When likely impacted products were not distributed
- Impact donor safety only but do not compromise the safety, purity or potency of the product
- Information received postdonation that may have prompted donor deferral if available at time of donation
- Identified before distribution and rectified
- Report of a delay in reporting a nonconformance
- Reference: eCFR: 21 CFR 606.171 [Accessed 11 August 2023]
Timing of report (when to report)
- Within 45 days of notification of event in question
- For contracting facilities, timing of report begins when the contractor becomes aware of the event
- Reference: eCFR: 21 CFR 606.171(c) [Accessed 11 August 2023]
Modality for reporting (how and where to report)
- Mandated use of Form FDA 3486
- Reporting by paper or electronic form
- Electronic reports submitted via CBER website (FDA: Electronic Submission of Biological Product Deviation Reports (eBPDR) [Accessed 11 August 2023])
- Paper reports are mailed to CBER document control center
- 1 report submitted per event
- 1 report submitted for single event involving multiple products
- References: eCFR: 21 CFR 606.171(d) [Accessed 11 August 2023], eCFR: 21 CFR 606.171(e) [Accessed 11 August 2023]
Types of events
- Donor eligibility
- Occurs during screening and deferral of donors
- Examples include
- Donor inappropriately deemed eligible when available information should result in deferral
- Unacceptable donor temperature
- Missing responses to high risk questions
- Inaccurate omission of a donor from deferral list
- Collection events
- Take place during collection and are only identified postdistribution
- Examples include
- Contaminated or possibly contaminated products
- Use of expired anticoagulant, collection set or bag
- Omission of arm preparation
- Clotted or hemolyzed product
- Component preparation
- Happens during preparation or processing
- Identified after product distribution
- Examples include
- Products contaminated during pooling
- Components prepared outside of stipulated interval following collection
- Products manufactured outside of specified procedures including
- Components prepared from whole blood stored at an unsuitable temperature
- Inaccurate irradiation dosage
- Wrong filter used in leukoreduction
- Testing events
- Arise during testing
- Identified after product distribution
- Examples include
- Testing performed outside of manufacturer's instructions
- Incomplete testing
- Omission of testing or the documentation of testing
- Testing conducted with unacceptable quality control
- Testing with out of date reagents
- Mislabeled specimens used in pretransfusion testing with subsequent product distribution based on the results
- Compatibility testing results are misread and products are distributed off of the interpretation
- Labeling events
- Occur during labeling
- Identified after distributing the products
- Encompass omitted, inaccurate or misleading information on product labeling, including tie tags, transfusion records, information circular and labels on product container
- Examples include
- Inaccurate ABO group, Rh or antigen type, volume, anticoagulant, expiration date
- Missing information on ABO blood group, Rh type or expiry date
- Labeling with wrong recipient name or identification
- Labeling with inaccurate or omitted donor identification
- Omitted or inaccurate testing information on labels
- Inaccurate or omitted product name
- Inaccurate extended expiry date despite product being transfused within the correct dating interval
- Quality control and distribution
- Arises during quality control procedures or in the quarantine and distribution of products distributed
- Examples include
- Products processed with instruments or reagents with unacceptable quality control
- Omission or nondocumentation of daily or weekly quality control
- Improper release from quarantine and distribution of a product not meeting established requirements
- Omission of quarantine and the distribution of units from an ineligible donor
- Improper distribution of a product before resolving a discrepancy with manufacturing or testing
- Distribution of an expired product
- Shipping or storage of a product at a wrong temperature prior to distribution
- Lack of documentation on appropriate storage temperature of a product
- Identification of a donor as a source of transmission transmitted infection
- Issuance of inaccurate products or units for a particular patient
- Omission of or nondocumentation of visual check of products before distribution
- Receipt, acceptance and subsequent identification of a hemolyzed product after distribution
- Reference: FDA: Biological Product Deviation Reporting for Blood and Plasma Establishments - Guidance for Industry [Accessed 11 August 2023]
Implementation
- Reporting a BPD involves analysis of the system(s) in which there was a failure that led to an unsuitable product being distributed
- Includes donor screening, quality control, distribution
Reporting fatalities associated with transfusion
- Any fatality confirmed to be a complication of blood transfusion or collection must be reported to the FDA
- Process is product centered and multistepped
- Multiple modalities for reporting include email, express mail, telephone and facsimile
- Initial reporting must occur as soon as possible after notification of fatality
- Written report must be submitted within 7 days
- Initial report includes
- Name of facility
- Time of notification
- Demographics of the patient
- Time of death
- Suspected cause of fatality
- Product unit number
- Blood supplier information
- Date of transfusion
- Circumstances around the patient's death
- References: FDA: Notifying FDA of Fatalities Related to Blood Collection or Transfusion [Accessed 11 August 2023], FDA: Transfusion / Donation Fatalities [Accessed 11 August 2023]
Case reports
- Single institution's retrospective review of management of blood product withdrawal (Ann Clin Lab Sci 2020;50:536)
Sample assessment & plan
- The director of a licensed blood establishment received a telephone communication from a client transfusion service regarding leukoreduced red blood cell (LRBC) products supplied to them. The client notified the director that the transfusion service received a batch of leukoreduced red blood cells whose bags were out of date. This nonconformance had been detected by the inventory officer at the transfusion service upon receipt of the supply.
- Assessment: The director determined this was a blood product deviation affecting quality control / distribution and involving multiple products. The quality assurance (QA) officer at the establishment had needed to go on an emergency family and medical leave, resulting in the role being filled in by other personnel on ad hoc basis each day. The usual automatic alerts for expiry dates and review of supplies were not recognized for 2 days during that period; therefore, multiple blood products and components bags were affected, including leukoreduced red blood cells, fresh frozen plasma and cryoprecipitate collection bags. He effected a recall on all the distributed products processed on those days.
- Plan: The licensed blood establishment outlined details in the report to the CBER using Form FDA 3486 to highlight
- Reporting facility information
- BPD information including date(s) event occurred, date discovered, date reported
- Description of contributing factors / root cause
- Details of affected products type, collection and expiration dates, product codes, disposition and notification
- Follow up and prevention plan
- Recall affected distributed products / components from all client facilities
- Implement a central automated alert system for the inventory software for any batch of component collection bags with expiration dates of 3 days or less
- Commence physical storage of collection bags according to months / years of expiry
- Implement mandatory inspection of expiry dates on 3 random product units in each dispatch
- Train and ensure competency of all personnel in QA and inventory / distribution divisions
Additional references
Board review style question #1
A 68 year old man has a transfusion attribute in the blood bank laboratory information system that all cellular products (packed red blood cells and platelets) for him should be irradiated. He carries a diagnosis of chronic lymphocytic leukemia / small lymphocytic lymphoma and blood bank policy indicates that blood products for patients with hematological malignancies should receive irradiated cellular products. During a particularly busy shift in the blood bank, a unit of packed red blood cells is issued to this patient without having been irradiated. An astute nurse in the infusion room notices that the unit does not have the usual irradiation indicator sticker on it and calls the blood bank. The blood bank requests the unit back, irradiates it and reissues it to the same patient. The temperature indicator on the unit is still acceptable. What are the next steps in handling a potential biological product deviation?
- Do not report it to the FDA but do report it to your institutional quality program since the unit was not transfused until after it was irradiated
- Do not report it to the institutional quality program or the FDA, since the unit was not transfused until after it was irradiated
- Report it to your institutional quality program and to the FDA since an error occurred that was caught after distribution, even though it was caught before transfusion
- Report it within your institutional quality program but do not report it to the FDA, since the unit was not transfused until after it was irradiated
Board review style answer #1
C. Report it to your institutional quality program and to the FDA since an error occurred that was caught after distribution, even though it was caught before transfusion. Since the error was not caught until after distribution, it is therefore reportable to both the institutional quality program as well as the FDA, even though there was no patient harm. Answers A, B and D are incorrect because despite a lack of patient harm, the error was not caught until after distribution. Therefore, it is reportable to internal and external quality monitors.
Comment Here
Reference: Biological product deviation
Comment Here
Reference: Biological product deviation
Board review style question #2
On an unusually busy evening shift, a medical laboratory scientist (MLS) in the blood bank is concerned because she witnessed one of the new staff members starting to deviate from the standard operating procedure (SOP) in making an aliquot of pRBC for an infant. The unit had 2 previous aliquots drawn for this same infant over the past 2 days and the new staff member was preparing to draw an aliquot off the source bag in an open fashion, without creating a sterile weld. The unit had 72 hours of shelf life left. The new staff member misremembered that the SOP specifies that it is acceptable to create an open system if the source bag is due to expire within 24 hours and instead thought it was okay for 72 hours. He had previously been covering the day shift where there were more staff members around to ask for help or clarify instructions. The observing medical laboratory scientist intervenes and demonstrates the correct way to access the main unit in a sterile fashion and draw off an aliquot. The new staff member admits that he got a little overconfident and notes that he should have kept the SOP open on the screen in front of him since he has only done this before during training. He takes the time to carefully review the SOP and carefully opens SOPs for all other tasks that evening. He also asks the other staff member to double check a visual inspection on a unit of platelets he is issuing. What is the next most appropriate step?
- Reinforce adherence to the standard operating protocol and do not report the error to the FDA, because it was caught before distribution and the product / patient was not harmed
- Reinforce adherence to the standard operating protocol and report the error to the FDA because it involved a blood component being prepared for an infant
- Report the new staff member to human resources, quarantine every product that he has accessed and report the error to the FDA
- Report this error to the internal quality program and to the FDA, as well as any other aliquots made by this staff member, as it is unclear if he ever made this error before
Board review style answer #2
A. Reinforce adherence to the standard operating protocol and do not report the error to the FDA, because it was caught before distribution and the product / patient was not harmed. This reflects a near miss that was caught before the product was distributed. New medical laboratory scientist staff members are more likely to make a few mistakes and this is an opportunity for professional growth. This error was caught before the product was compromised and there was no patient harm. Even if the aliquot had been drawn in an open system, it would have changed the expiration date and potentially wasted product but there would not have been harm to the intended recipient. Answer B is incorrect because there is no difference in how errors are handled / reported based on adult versus pediatric patients. Answers C and D are incorrect because there is no evidence of widespread errors from this particular staff member.
Comment Here
Reference: Biological product deviation
Comment Here
Reference: Biological product deviation
Blood alternatives (pending)
[Pending]
Blood donor testing
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Diagrams / tables | Laboratory | Case reports | Prevention | Special considerations | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Donor testing aims to:
- Detect transfusion transmissible disease in donors who have already been qualified through the donor history questionnaire
- Evaluate vital signs and hemoglobin
- Conduct a mini physical examination
Essential features
- Donor testing is skewed toward sensitivity over specificity
- Confirmatory testing (preferably FDA approved) often needed to diagnose infectious disease
Terminology
- Cytomegalovirus (CMV)
- Enzyme immunoassay (EIA)
- Enzyme linked immunosorbent assay (ELISA)
- Nucleic acid testing (NAT)
- Hepatitis B virus (HBV)
- Hepatitis B core antigen (HBc)
- Hepatitis B surface antigen (HBsAg)
- Hepatitis C virus (HCV)
- Human immunodeficiency virus (HIV)
- Human T cell lymphotropic virus (HTLV1 / HTLV2)
- Recombinant immunoblot assay (RIBA)
- West Nile virus (WNV)
- Zika virus (ZIKV)
- Chemiluminescence immunoassay (ChLIA)
Epidemiology
| Infectious disease | Frequency of detecting infection in donor (per screened donations) | Risk of transfusion transmission (per units screened) |
| Babesiosis (Babesia spp.) | ~1 in 250 in endemic state | ~1 in 100,000 in endemic* state, as high as 1 in 18,000 in endemic counties within endemic state |
| Chagas disease (Trypanosoma cruzi) | ~1 in 15,000 | Unknown |
| Hepatitis B (HBV) | ~1 in 12,000 | < 1 in 1 million |
| Hepatitis C (HCV) | ~1 in 5,000 | < 1 in 2 million |
| Human immunodeficiency virus (HIV) | HIV1: ~1 in 33,000 HIV2: ~1 in 57 million | < 1 in 2 million |
| HTLV1 / HTLV2 | ~1 in 27,000 | < 1 in 2 million |
| Treponema pallidum (syphilis) | Unknown | No cases recorded in > 50 years in the U.S. |
| West Nile virus (WNV) | Low: ~2,500 positives from 2003 - 2019 | 1 in 84 million; 1 in 35 million during summer (peak transmission season) |
| Zika virus** (ZIKV) | During the peak of the outbreak in 2016 - 2017, ~1 in 950,000 (all exposures were found to be outside the U.S. or in Florida) | Unknown |
- *14 states in the U.S. considered babesia endemic / contiguous to an endemic state: ME, VT, NH, CT, MA, NY, NJ, DE, RI, MD, PA, VA, MN, WI and the District of Columbia
- **Zika virus no longer considered threat to blood supply (FDA: Zika Virus Response Updates from FDA [Accessed 17 September 2021])
- Note: cytomegalovirus (CMV) not routinely tested
- Leukoreduced blood products = CMV safe
- Rare recipients may need serologically CMV negative units
- Residual risk of window period donation = length of window period x incidence of infections in repeat donors
- References: American Red Cross: Infectious Disease, HLA and ABO Donor Qualification Testing [Accessed 17 September 2021], Cohn: AABB Technical Manual, 20th Edition, 2020
Pathophysiology
- Pattern by which serologic markers become positive is the basis of blood donor testing
- Transfusion transmissible illness antigens and nucleic acids are the first detectable markers
- Antibody response
- IgM antibodies
- First to form in immune response
- Taper to moderate levels in chronic infection
- Decrease to low levels or become undetectable in resolved infection
- IgG antibodies
- Begin to rise at peak levels of IgM antibodies
- Taper to moderate levels as part of sustained immune response
- Relationship of infectious disease marker concentration to timing of immune response (see Diagrams)
- IgM antibodies
- References: Cohn: AABB Technical Manual, 20th Edition, 2020, AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2020
Diagrams / tables
Laboratory
- First transfusion transmissible disease to be screened (1940 - 1950): syphilis (Treponema pallidum)
- In the 1960s, > 30% of transfusion recipients developed posttransfusion hepatitis (PTH)
- Hepatitis B accounted for about 25% of PTH
- Development of sensitive hepatitis B surface antigen test (HBsAg)
- Non-A, non-B hepatitis (NANB) accounted for the remainder
- Occurred more frequently in paid donors than volunteer donors
- No specific antigen test available in 1960 - 1970
- Implementation of surrogate marker test to identify donors at increased risk of NANB PTH
- Presence of antibody to hepatitis B core antigen (anti-HBc) or presence of elevated alanine aminotransferase
- Concern about nonspecific nature of tests led to delay in implementation
- Hepatitis B accounted for about 25% of PTH
- 1980s: concerns over transmission of acquired immunodeficiency syndrome (AIDS) by transfusion reinvigorated interest in surrogate testing
- These realizations led to expansion of donor screening beyond looking for known agents
- Donor history evaluation for increased risk of exposure to:
- Blood borne infections
- Sexually transmitted infections
- Donor history evaluation for increased risk of exposure to:
- Window period
- Transmission of HIV continued after antibody testing was initiated
- Time between infection and detectability of infection by testing used
- Including HIV, p24 antigen test can further reduce the serological window period by 3 to 7 days before antibodies are detectable
- Antibody levels: seroconversion - IgM antibodies form first, followed by IgG
- Lessons learned to reduce the risk of transfusion transmitted infection:
- Donor education and screening questions to exclude donors with high risk behaviors and risk of transfusion transmitted illnesses for which there is no test currently available
- Reducing the window period of tests and improving the sensitivity
- Adopting current good manufacturing practice (cGMP) regulations
- Surveillance for known and emergent transfusion transmissible illness
- Donor screening tests
- Required by FDA (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021])
- Changes / updates provided in FDA guidance documents
- Guidance documents not technically legal requirement, however, are considered standard of care
- AABB (formerly American Association of Blood Bank) also issues recommendations via bulletins or the standards for blood bank and transfusion services (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2020)
- Required by FDA (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021])
- Sample
- Generally about 5 test tubes drawn
- Nucleic acid testing (NAT)
- ABO / Rh testing and testing for syphilis antibodies
- Viral antibody markers
- Antibodies against T. cruzi (Chagas)
- Tube kept for retention in case repeat testing is necessary
- Generally about 5 test tubes drawn
- Serologic screening tests: immunoassays for antigens or antibodies (host response to infection)
- Enzyme linked immunosorbent assay (EIA or ELISA)
- Can detect antibodies:
- Anti-HIV1 / HIV2
- Anti-HTLV1 / HTLV2
- Anti-HCV
- Anti-HBc (hepatitis B core)
- Anti-T. cruzi
- Can detect antigens:
- Hepatitis B surface antigen (HBsAg)
- Methodology:
- Indirect antibody detection
- Antigen is bound to a microtiter plate well
- Patient sample is added
- Incubation
- Wash step to remove unbound antibody
- Antihuman antibody conjugated to enzyme is added
- Example enzyme is horseradish peroxidase
- Helps produce a color change
- Bound antihuman antibody binds to patient antibody in sample attached to antigen fixed in microtiter plate well
- Chromogen (color changing compound) causes visible color change in response to antibody detection
- Antigen detection
- Generally sandwich technique
- Antibody against a target is bound to a microtiter plate well
- Donor sample is added
- Incubation
- Wash step to remove unbound antigen
- Antibody conjugated to enzyme targeting different portion of antigen added
- Conjugated antibody binds to antigen / antibody complex bound in well
- Chromogen allows detection via visible color change
- Indirect antibody detection
- Can detect antibodies:
- Chemiluminescent immunoassay (ChLIA)
- Can detect antibodies
- Can detect antigens
- Methodology
- Similar to EIA or ELISA
- Uses chemical reaction that generates light rather than color change
- Interpretations for immunoassays are as follows:
- Readout below established cutoff = negative
- Readout exceeds established cutoff = reactive
- Reactive samples retested in duplicate
- Repeat reactive = positive
- Both repeat results negative = donor is eligible
- Aims to decrease issues from nonspecific binding in these tests
- Repeatedly positive results require further evaluation by FDA approved supplemental assay if such assay is available
- Still subject to serological limits of detection (window period)
- Currently available FDA approved supplemental assays include:
- HBsAg neutralization
- HIV type 1 (HIV1) antibodies
- HCV antibodies
- HTLV1 / HTLV2 antibodies
- T. cruzi antibodies
- Confirmatory testing
- Specific antigen neutralization for HBsAg reactive confirmation
- HBsAg reactive samples that are HBV DNA reactive do not require further testing by neutralization
- FDA licensed enzyme strip immunoassay (ESA)
- Recombinant proteins containing antigens targeted by antibodies from those with T. cruzi infection
- Placed on lines on nitrocellulose membrane strips and incubated
- Antibodies, if present, bind the test strip
- Involves a conjugate antibody addition prior to second incubation and color intensity interpretation at each antigen site on the strip
- Used for T. cruzi supplemental testing (FDA: ABBOTT ESA Chagas [Accessed 17 September 2021])
- FDA licensed western blot
- Also known as a protein immunoblot
- First step is to separate proteins by the length of the polypeptide using gel electrophoresis
- Electrophoretic transfer onto a membrane, such as nitrocellulose
- Immunostaining to visualize a certain protein on the blot membrane
- Used for HTLV1 and HTLV2
- Specific antigen neutralization for HBsAg reactive confirmation
- Nucleic acid testing (NAT)
- Detects deoxyribonucleic acid (DNA) or ribonucleic acid (RNA)
- Extraction of nucleic acid from donor plasma or serum
- Nucleic acid amplification
- Detection of genetic sequences of viruses
- Techniques include:
- Polymerase chain reaction (PCR)
- Permits synthesis of large amounts of genetic material for analysis
- Components include target DNA, oligonucleotide primers, DNA polymerase, deoxyribonucleotide triphosphate base pairs, electrolyte solutions and buffers
- 3 steps:
- Denaturation: reaction mixture is heated to separate strands of DNA
- Annealing: cool to allow primers to bind specifically with a target DNA
- Extension: DNA polymerase initiates extension of primers (strands of DNA)
- Primary extension products dissociate from target DNA through heating
- Each extension fragments can serve as a template for additional rounds of kneeling and extension
- Many variants of this process possible
- Transcription mediated amplification (TMA)
- Isothermal nucleic acid amplification technique
- Begins with RNA target, usually single stranded and therefore no need for heat denaturation step
- Sequence specific DNA primer binds RNA target
- Reverse transcriptase extends primer creates DNA-RNA hetero duplex
- 5' end of sequence specific primer contains promoter for T7 bacteriophage polymerase
- Synthesizes DNA strand complementary to initial RNA target containing T7 promoter at 5' end
- Reverse transcriptase enzyme degrades initial RNA template while synthesizing complementary DNA
- Advantages include no need for denaturation step and use of isothermal processes that do not require thermocyclers
- Polymerase chain reaction (PCR)
- Reduces window period from weeks to days
- Performed in mini pools (MP) of 6 - 16 samples (cost and labor savings)
- If pool is reactive, component samples tested individually
- Fully automated NAT systems are available
- Followed by virus specific testing for donor notification / counseling
- Positive donor is deferred
- Used for:
- Babesia
- HBV
- HCV
- HIV
- West Nile virus (WNV)
- Zika virus (ZIKV; no longer considered a threat per May 12, 2021 FDA guidance) (FDA: Zika Virus Response Updates from FDA [Accessed 17 September 2021], McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2016, Cohn: AABB Technical Manual, 20th Edition, 2020)
- Detects deoxyribonucleic acid (DNA) or ribonucleic acid (RNA)
- Enzyme linked immunosorbent assay (EIA or ELISA)
- Basic screening tests (Cohn: AABB Technical Manual, 20th Edition, 2020):
- Current FDA licensed blood donor screening tests (U.S.)
- Babesia spp.
- DNA / RNA test using TMA or PCR for screening test; supplemental test via research antibody and PCR
- T. cruzi (Chagas)
- IgG antibody (once in lifetime), ChLIA or EIA; supplemental ESA
- HBV
- HBsAg by ChLIA or EIA; supplemental via neutralization assay, positive HBV DNA
- HBc antigen by ChLIA or EIA
- HBV DNA by TMA or PCR
- HCV
- IgG antibody via ChLIA or EIA; supplemental via positive HCV RNA
- HIV1 / HIV2
- IgM, IgG antibody via ChLIA or EIA; supplemental via positive HIV RNA, HIV1 IFA or western blot, HIV2 EIA
- HIV1 RNA by TMA or PCR
- HTLV I - II
- IgG antibody via ChLIA or EIA; supplemental via western blot
- T. pallidum (syphilis)
- IgG or IgG / IgM screening via microhemagglutination, particle agglutination, EIA or immunofluorescence; supplemental via T. pallidum screening test
- WNV
- RNA by TMA or PCR; supplemental repeat or alternate NAT test or antibody (IgG, IgM)
- ZIKV
- RNA TMA or PCR; supplemental repeat or alternate NAT and antibody (IgG, IgM)
- Babesia spp.
- False positives
- Nonspecific reactivity or crossreactivity to antigens and antibodies may occur
- False positive nucleic acid testing is possible as well
- Donors believed to demonstrate false positive results may be entered into a donor eligibility reentry algorithm
- Reentry algorithm is complex and features many decision points
- Reentry criteria is best accessed from current FDA guidance
- Current FDA licensed blood donor screening tests (U.S.)
- References: FDA: Requalification Method for Reentry of Blood Donors Deferred Because of Reactive Test Results for Antibody to Hepatitis B Core Antigen (Anti-HBc) [Accessed 17 September 2021], FDA: "Lookback" for Hepatitis C Virus (HCV) [Accessed 17 September 2021]
Case reports
- 62 year old asymptomatic man apheresis platelet donor with intermittent E. coli bacteremia (Transfusion 2015;55:2606)
- 69 year old man with West Nile disease following apheresis platelet transfusion (Transfusion 2020;60:424)
- Cases of transfusion transmitted hepatitis B virus prior to nucleic acid testing (Transfusion 2013;53:291)
- Cases of transfusion transmitted babesiosis prior to nucleic acid testing (Transfusion 2017;57:2348)
Prevention
| Transfusion transmissible illness (TTI) | Window period by serological testing in days | Window period by NAT in days |
| HBV HBsAg | 30 - 38 | 18.5 - 26.5 |
| HCV | 45 - 60 | 7.4 |
| HIV (CDC: Types of HIV Tests [Accessed 17 September 2021]) | Antigen test: 18 - 45 Antibody test: 18 - 90 | 9.1 |
| HTLV1 / HTLV2 | Unknown | No test available |
- Whole blood donation NAT for HIV and HCV costs millions of U.S. dollars per year to avert small numbers of HIV, HCV and HBV infections
- High cost to benefit ratio
- Ethical standards of protecting recipients outweigh pure material costs
- References: American Red Cross: Infectious Disease, HLA and ABO Donor Qualification Testing [Accessed 17 September 2021], Transfusion 2003;43:721
Special considerations
- Human cells, tissues and cellular and tissue based products (HCT / P)
- Requirements outlined in 21 Code of Federal Regulations 1271 and FDA guidance documents (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021], FDA: Eligibility Determination for Donors of Human Cells, Tissues and Cellular and Tissue Based Products [Accessed 17 September 2021])
- Autologous donations
- FDA requires infectious disease testing of autologous donations being shipped from one facility to another
- If receiving facility does not permit autologous donations to enter into general inventory if not used for donor, FDA requires testing of only the first donation each 30 day period (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021])
- Cytomegalovirus (CMV) testing for immunocompromised recipients (Cohn: AABB Technical Manual, 20th Edition, 2020):
- CMV is lipid enveloped Herpesviridae DNA virus
- Causes lifelong infection
- Latent phase and white blood cells
- Potential for reactivation
- Primary infection in immunocompetent host is usually minor
- Immunocompromised patients are in danger of severe or even fatal primary or reactivated disease
- Fetuses
- Low birth weight premature infants of CMV negative mothers
- CMV seronegative recipients of solid organ or hematopoietic stem cell transplants from seronegative donors pathogen reduced products
- Live, intact white blood cells in cellular blood products can transmit virus
- Frozen or thawed plasma products do not appear to transmit CMV
- Most blood donors have had exposure to CMV reduce risk options for vulnerable recipients include:
- Leukoreduction
- CMV seronegative donors
- Pathogen inactivation (platelets only in United States at present)
- Pathogen inactivation
- Donor screening cannot fully eliminate risk
- Not possible to test for every potentially transmissible illness
- Window periods
- Sensitivity / specificity of testing available
- Developing test takes time and resources
- As of yet, unknown pathogens cannot be tested for
- Reduces infectivity of residual pathogens and blood products
- Methods include:
- Solvent detergent (SD) treated pooled plasma products (Octaplas) (FDA: Octaplas [Accessed 17 September 2021])
- SD and methylene blue / visible light treatment works in plasma but damages cellular membranes and cannot be used for red blood cell or platelet products
- Plasma to be used in these products are prescreened for parvovirus B19, hepatitis E virus (HEV) and hepatitis A virus (HAV) (nonenveloped)
- Amotosalen / psoralen ultraviolet A (UVA) light treated products (Cerus: How INTERCEPT Works [Accessed 17 September 2021])
- Amotosalen / psoralen intercalates between nucleic acid base pairs
- UVA illumination activates amotosalen and causes permanent cross links between nucleic acid strands
- Prevents further replication and inactivates bacteria, viruses and leukocytes
- Available in U.S. for plasma and platelets
- Significant activity against HIV, HBV, HCV, HTLV, WNV, CMV, Zika virus, Babesia and parasites, syphilis and many bacteria sp.
- Solvent detergent (SD) treated pooled plasma products (Octaplas) (FDA: Octaplas [Accessed 17 September 2021])
- Donor screening cannot fully eliminate risk
Additional references
Board review style question #1
Which of the following disease markers are part of the panel of those required to be tested for during voluntary whole blood and apheresis donations in the United States?
- Cytomegalovirus nucleic acid test, hepatitis B virus surface antigen, hepatitis B virus nucleic acid test
- Hepatitis B virus surface antigen, HIV nucleic acid test, West Nile virus nucleic acid test
- HIV1, HIV2 antibody testing, syphilis, dengue nucleic acid test
- West Nile virus nucleic acid test, syphilis nucleic acid test, hepatitis C virus nucleic acid test
Board review style answer #1
B. Hepatitis B virus surface antigen, HIV nucleic acid test, West Nile virus nucleic acid test. Answer A is incorrect because CMV is not a required infectious disease marker and its residual risk is reduced by leukoreduction of cellular blood products or pathogen reduction technology. Answer C is incorrect because dengue is not currently one of the required infectious disease markers. Answer D is incorrect because syphilis (T. pallidum) is not screened for by nucleic acid testing.
Comment Here
Reference: Blood donor testing
Comment Here
Reference: Blood donor testing
Board review style question #2
A premature low birthweight infant needs red blood cell transfusion. What additional intervention might be recommended to lower the risk of transfusion transmissible infection?
- Irradiation
- Leukoreduction
- Volume reduction
- Washing of the unit / aliquot
Board review style answer #2
B. Leukoreduction is considered cytomegalovirus (CMV) safe because CMV tends to reside in white blood cells where it can cause latent infection or pose risk of new infection in a transfusion recipient. Answer A, irradiation, reduces the risk of transfusion associated graft versus host disease (TA-GVHD), however, it generally is not considered protective against infectious diseases. Answer C, volume reduction, may be helpful for reducing the amount of potentially incompatible plasma if no better alternative product is available, especially in the case of platelets whereby ABO identical products may not always be available. Answer D, washing of a unit or aliquot, is helpful to prevent potassium overload or recurrence allergic reaction in susceptible patients, however, it is not indicated for the prevention of any transfusion transmissible illness.
Comment Here
Reference: Blood donor testing
Comment Here
Reference: Blood donor testing
CAR T cell therapy
Table of Contents
Definition / general | Essential features | Terminology | History | Procedure to create CAR T product | Pathophysiology | Types of CAR T cells | Diagrams / tables | Challenges | Adverse effects | Clinical features | Laboratory | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Chimeric antigen receptor T cells (CAR T) are a form of cellular immunotherapy involving the genetic engineering of T cells to produce surface receptors targeted at specific cell surface receptors
- CAR T is primarily used to treat hematologic malignancies, with acute lymphoblastic leukemia (ALL) being the first disease targeted and diffuse large B cell lymphoma (DLBCL) being the second; its application to solid organ tumors is being studied
Essential features
- CAR T cell therapy was originally developed for B cell acute lymphoblastic leukemia
- It is also now approved for diffuse large B cell lymphoma (DLBCL)
- Process starts with collecting autologous T cells via leukocytapheresis, shipping the cells to be engineered / manufactured, expanding the cell population to achieve appropriate dose, cryopreserving CAR T cells and shipping to the treating facility
- Patient is given lymphocyte depleting chemotherapy prior to receiving the treatment
- Key steps of CAR T therapy are trafficking, recognition, control, microenvironment, proliferation / persistence
- Safety measures are built in to allow ablation of CAR T cells if severe toxicity develops
- Severe toxicity can include cytokine release syndrome and a CAR T encephalopathy
Terminology
- CART-19: CAR T directed against CD19 antigen on B cells
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Approved for relapsed acute lymphoblastic B cell leukemia / lymphomas
- Approved for patients with relapsed or refractory large B cell lymphoma including diffuse large B cell lymphoma, high grade B cell lymphoma and diffuse large B cell lymphoma arising from follicular lymphoma
- Axicabtagene ciloleucel, also known as Axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Approved for aggressive, relapsed or refractory diffuse large B cell lymphoma, primary mediastinal B cell lymphoma and transformed follicular lymphoma
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
History
- 1989: Discovery that it was possible to redirect T cell signaling to an antigen of choice, independent of major histocompatibility complex (MHC) restrictions
- 2006: First results published of human clinical trials using chimeric antigen receptor T cell technology (J Clin Oncol 2006;24:e20, Clin Cancer Res 2006;12:6106)
- 2010: First use of CAR T was in a 5 year old girl with relapsed B cell acute lymphoblastic leukemia (ALL); received CAR T directed against CD19 antigen on B lymphoblasts (CART-19)
- Developed complications, treated with toclilizumab, an anti-IL6 monoclonal antibody
- Patient survived and became global ambassador for CAR T therapy
- 2014: CTL019 was granted breakthrough therapy designation by the United States Food and Drug Administration (Immunol Rev 2015;263:68)
Procedure to create CAR T product
- Collect autologous T cells via leukocytapheresis
- Obtain approximately 109 T cells
- Unknowns:
- Is there an optimal time window for collection?
- How to predict an adequate collection?
- How to separate T cells from collected mononuclear cell apheresis product? (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
- Ship T cells to manufacturing site
- CAR structure is composed of an extracellular antigen recognition domain fused to intracellular TCR signaling domains (CD3z) and costimulatory domains such as CD28
- Manufacture CAR T cells by CD3 / CD28 bead stimulation and lentiviral transduction (Tisagenlecleucel)
- Manufacture CAR T cells by CD3 antibody / IL2 stimulation and retroviral transduction (Axicabtagene)
- Expand cell population over 6 - 10 days
- Achieve patient dose of 2 - 4 x 106 CAR expressing T cells / kg patient body weight
- Cryopreserve CAR T cells
- Ship to treating facility
- Patient is given lymphodepleting chemotherapy (usually fludarabine and cyclophosphamide)
- Deplete endogenous T cells that might reject the CAR T cells
- Increase likelihood of expanding CAR T population in recipient
- Enhance antigen presentation capabilities
Pathophysiology
- Structure:
- Receptor: combines facets of normal T cell activation into single protein
- Links extracellular antigen recognition domain to an intracellular signaling domain to activate the T cell when an antigen is bound
- 4 parts:
- Antigen recognition domain
- Exposed to the outside of the cell in the ectodomain portion of the receptor
- Allows the CAR T cell to attack any cell that expresses the matching molecule
- Derived from variable regions of a monoclonal antibody linked together as a single chain variable fragment (scFv)
- A scFv is a chimeric protein made up of the light (VL) and heavy (VH) chains of immunoglobulins connected with a short linker peptide
- VL and VH regions are selected to bind the target antigen like CD19
- Extracellular hinge region
- Structural domain between the antigen recognition region and the cell's outer membrane
- Optimizes flexibility of the scFv receptor head to promote antigen binding between CAR T and target antigen
- Transmembrane domain
- Anchors the CAR to the plasma membrane, links the extracellular hinge and antigen recognition domains with the intracellular signaling region
- Stabilizes the entire structure
- CD28 transmembrane domain often used and is very stable
- Should not use CD3-zeta transmembrane domain because it can cause incorporation of the artificial TCR into the native T cell receptor
- Intracellular T cell signaling domain
- Receives and perpetuates signal after antigen binds external recognition portion
- Cytoplasmic domain of CD3-zeta is often used to mimic the normal T cell activation dependent on phosphorylation of immunoreceptor tyrosine-based activation motifs
- Includes one or more chimeric domains from costimulatory proteins
- Antigen recognition domain
- Function of CAR T cells
- Trafficking
- Engineered T cell must be able to get to site of tumor cells
- Target tumor cells to be killed
- Possibility of introducing chemokine receptors into CAR T cells to improve trafficking to tumors that produce cognate chemokines
- Recognition
- Recognize target tumor cells
- Discriminate and ignore bystander tissues
- Control
- T cells are relatively autonomous once they are infused
- New work on regulatory processes to modulate the survival of T cells, timing, strength and location of their activity
- Microenvironment
- Resist immunosuppression
- Prime / mobilize endogenous immunity
- Proliferation / persistence
- Expand the population of CAR T cells to optimize activity (Cell 2017;168:724)
- Trafficking
- Safety measures: allow ablation of CAR T cells if severe toxicity develops
- Inclusion of suicide gene, iCaspase9
- Surface tag such as epidermal growth factor receptor
Types of CAR T cells
- First generation: contained only TCR complex CD3-ξ chain domain with additional costimulatory domains
- Second generation: incorporated costimulatory domains like CD28 or CD137 to boost survival, proliferation and antitumor activity
- Third generation: combined CD3-ξ chain domain with additional costimulatory domains (Cell 2016;164:780)
- Fourth generation: T cells redirected for universal cytokine killings (TRUCKS)
- Improve tumor microenvironment
- May have potential for solid tumors
- Newer generation: additional gene editing such as CRISPR-Cas9
- Armored CARs: CAR T cells modified to express cytokines, ligands or scFv that help turn suppressive tumor environment into pro-inflammatory to better fight tumor
- Tandem CARs: CAR molecule engineered to recognize multiple antigens via two binders on a single molecule
- Designer CARs: modified by CRISPR-Cas9 to edit CAR T genomes for advantageous features, such as being less susceptible to immunosuppressive effects
- Smart CARs: logic gated, regulated
- Have new receptors that function independently of CAR / TCR pathways but interfere with CAR activity in controlled way
- Use of modular receptors called synthetic Notch (synNotch) receptors
- Use extracellular domain like scFv to recognize target antigen, without triggering T cell activation
- Ligand engagement results in cleavage of receptor and subsequent release of transcriptional activator domain, which enters nucleus and drives expression of user specified target genes (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2016;164:780)
- Split, universal and programmable (SUPRA) CARs
- Universal receptor found on T cells and tumor targeting scFv adaptor molecule
- Two component receptor system with universal receptor (zipCAR) expressed on T cells and tumor targeting scFv adaptor (zipFv)
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- scFv of zipFv binds tumor antigen
- Leucine zipper binds and activates zipCAR on T cells
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- Regulate activity to limit over activation, decrease cytokine secretion and improve tumor targeting (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2018;173:1426)
Challenges
- Tumor specific antigens
- CD19 is a great target, since a loss of normal B cells can be compensated for by replacement antibody therapy (IVIG)
- How specific must they be?
- Tumor cells that do not express the target antigen may evade therapy
- Tumor cells with splice variants, lacking a specific epitope may also escape targeting (Blood 2018;131:2621)
- Can we pinpoint antigens expressed by tumors versus normal cells reliably?
- Do all tumor cells in a given tumor express the same antigens?
- Can a tumor be effectively treated even if only some of the cells are susceptible to targeting?
- How significant is bystander toxicity? (Blood 2018;131:2621)
- Rare chance of accidentally introducing CAR gene into a tumor cell during manufacture
- Proliferation of tumor cells
- Tumor cells escape detection by CAR T cells
- Response in non-Hodgkin lymphoma (NHL) worse than in ALL
- CD19 loss variants
- Microenvironment factors that limit proliferation and effect of CAR T cells
- Remission rates 70 - 80%
- Solid tumors
- Lack of suitable cell surface molecules for the CAR T cells to target
- Attempts to engineer T cells with T cell receptors capable of recognizing tumor specific antigens from intracellular proteins
- Modify tumor microenvironment to be more hospitable to CAR T cells
Adverse effects
- Toxicity
- On target effects: reversible when target cells are eliminated or CAR T cell engraftment is terminated
- B cell aplasia
- More severe than that caused by anti-CD20 monoclonal antibody rituximab
- Rapidly reversed after ablation of CAR T cells
- May require immunoglobulin therapy
- Cytokine release syndrome
- Initial flu-like presentation
- Fevers, hypotension, hypoxia, neurologic changes
- Can progress to capillary leak
- T cell activation and high levels of cytokines, IL6 and interferon-γ
- Neurotoxicity: CAR T related encephalopathy syndrome (Blood 2014;123:2625, Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
Clinical features
- Patient selection considerations may differ from autologous stem cell transplant (ASCT) and take into account previous therapies, upper age limit, severity of comorbidities and resistance to chemotherapy
- Some clinical trials may include transplant ineligible patients
- Other clinical trials are studying CAR T cell therapy as second-line treatment and for both transplant eligible and ineligible patients
- Other factors that may be considered in therapy include:
- Performance status
- Organ function
- T cell count
- B-acute lymphoblastic leukemia (ALL), target antigen is CD19
- CART-19: CAR T directed against CD19 antigen on B lymphoblasts
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as Axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Diffuse large B cell lymphoma, target antigen is CD19, no specific age limit
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as Axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Other diagnoses under investigation for possible CAR T therapies include:
- Hodgkin lymphoma (HL), target antigen is CD30
- Anaplastic large cell lymphoma, target antigen is CD30
- Myeloma, target antigens: SLAMF7, B cell maturation antigen (BCMA)
- Acute myeloid leukemia (AML), target antigens are CD123, CD33, Lewis Y and FOLR2
- Other potential costimulatory domains are PD1 / CD28 and CD200R / CD28
- T cell malignancies are a challenge since candidate target antigens are found on normal T cells
- CARs post transplant: maximize graft versus tumor effect while minimizing graft versus host disease (GVHD)
- CARs in donor leukocyte infusions (DLI): may help improve survival in relapses of hematologic malignancy
- CARS in virus specific T cells
- Chimeric autoantibody receptor T cells (CAARs): autoimmune disease such as pemphigus vulgaris (Immunol Rev 2015;263:68, Clin Cancer Res 2016;22:1875)
Laboratory
- Polymerase chain reaction (PCR) molecular assays are available for all CARs produced, however, such testing might not accurately reflect if the CAR is actually expressed on the cell surface and is generally performed retrospectively
- Flow cytometry may be a useful modality to monitor real time expansion and response
- CD19 CARs are known to expand rapidly, clear target tumor cells, then contract
- CARs have single chain variable fragments (scFv) for specificity against a target antigen and have little else on the cell surface
- There are three potential ways to detect the scFv using flow cytometry
- Develop an anti-idiotype antibody to the scFv
- Use Fc-conjugated soluble antigen such as Fc-CD22 that interacts with CD22 CAR T cells followed by a secondary detection antibody (anti-Fc)
- Use biotinylated protein L followed by a streptavidin-conjugated fluorophore
- There are benefits and deficits to each method and the process is labor intensive
- A well constructed assay provides meaningful information for individual patient care and a better understanding of CARs as a whole
- Additionally, there is need for standardized methods to profile memory phenotype of CAR Ts to evaluate quality and promote manufacturing improvements
- Use of a standardized memory T cell panel can help evaluate how T cell phenotype impact the efficacy and longevity of response in patients receiving CAR T therapies
- One attempt at this includes a dried memory T cell panel containing a pre-validated mixture of 7 antibodies for the identification of naïve, stem cell memory, central memory and effector memory CD4+ and CD8+ T cell subsets (BD Biosciences)
- One study used this pre-validated mix and additional drop in antibodies can complement the panel and enable more in depth evaluation of the T cell phenotype and monitor changes in expression of PD-1, TIM-3, LAG-3, HLA-DR, CD45RO and CXCR3 on T cells transduced to express a novel anti-CD37 CAR (Transfus Med Hemother 2019;46:15, Blood 2019;134:5626)
Case reports
- 18 month old girl and 52 year old woman treated with CAR T for B-ALL with mixed lineage leukemia gene (MLL) mutations developed AML clonally related to their B-ALL, suggesting CD19-negative immune escape (Blood 2016;127:2406)
- 20 year old man relapsing 9 months after CD19-targeted CAR T cell (CTL019) therapy (Nat Med 2018;24:1499)
- Mini review of control mechanisms for future CAR T products (Front Immunol 2020;11:326)
- Advancements in safety for new CAR T models (Mol Cancer 2019;18:125)
- Applications of CAR T for community oncology physicians (Oncologist 2016;21:608)
Treatment
- Cytokine release syndrome: treated with anti-IL6 monoclonal antibody, tocilizumab
Board review style question #1
- A 10 year old boy diagnosed with acute lymphoblastic leukemia with several relapses is on a clinical trial for CD19 CAR T therapy. He develops the rapid onset of flu-like symptoms, including fever, and progresses with mental status change and clinical concern for cytokine release syndrome. What would be an appropriate treatment for suspected cytokine release syndrome?
- Anti-CD20 (rituximab)
- Anti-CD38 (daratumumab)
- Anti-IL6 (tocilizumab)
- Azathioprine
- Interferon-γ
Board review style answer #1
C. Anti-IL6 (tocilizumab). Cytokine release syndrome is likely due to high levels of IL6 and Interferon-γ. Anti-IL6 (tocilizumab) has mainly been used in inflammatory conditions such as juvenile rheumatoid arthritis and has been effective in decreasing the IL6 levels involved in cytokine release syndrome.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy
Board review style question #2
- In which function of CAR T cells does the engineered T cell travel to the site of tumor cells targeted for destruction?
- Control
- Microenvironment
- Proliferation / persistence
- Recognition
- Trafficking
Board review style answer #2
E. Trafficking is the first function of a CAR T cell. The engineered cell must be able to travel to the site of the target tumor cells.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy
CAR T cell therapy
Table of Contents
Definition / general | Essential features | Terminology | History | Procedure to create CAR T product | Pathophysiology | Types of CAR T cells | Diagrams / tables | Challenges | Adverse effects | Clinical features | Transmission | Symptoms | Screening | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Chimeric antigen receptor T (CAR T) cells are a form of cellular immunotherapy involving the genetic engineering of T cells to produce surface receptors targeted at specific cell surface receptors
- CAR T cell therapy is primarily used to treat hematologic malignancies, with acute lymphoblastic leukemia (ALL) being the first disease targeted
Essential features
- CAR T cell therapy was originally developed for B cell acute lymphoblastic leukemia
- It is also now Food and Drug Administration (FDA) approved for B cell non-Hodgkin lymphoma (NHL), follicular lymphoma, mantle cell lymphoma and multiple myeloma; its application to solid organ tumors is being studied
- Process starts with collecting autologous T cells via leukocytapheresis, shipping the cells to be engineered / manufactured, expanding the cell population to achieve appropriate dose, cryopreserving CAR T cells and shipping to the treating facility
- Patient is given lymphocyte depleting chemotherapy prior to receiving the treatment
- Key steps of CAR T cell therapy are trafficking, recognition, control, microenvironment, proliferation / persistence
- Safety measures are built in to allow ablation of CAR T cells if severe toxicity develops
- Severe toxicity can include cytokine release syndrome and a CAR T encephalopathy
Terminology
- CART-19: CAR T directed against CD19 antigen on B cells
- BCMA: B cell maturation antigen found on plasma cells in multiple myeloma
FDA approved CAR T cell therapies (NIH: CAR T Cells - Engineering Patients' Immune Cells to Treat Their Cancers [Accessed 9 April 2024])
| Generic name | Brand name | Target antigen | Targeted disease | Patient population |
| Tisagenlecleucel | Kymriah® (Novartis, Basel, Switzerland) | CD19 | B cell acute lymphoblastic leukemia (ALL) | Children and young adults with refractory or relapsed B cell ALL |
| B cell non-Hodgkin lymphoma (NHL) | Adults with relapsed or refractory B cell NHL | |||
| Axicabtagene ciloleucel | Yescarta® (Kite Pharma / Gilead, Los Angeles, CA) | CD19 | B cell NHL | Adults with relapsed or refractory B cell NHL |
| Follicular lymphoma | Adults with relapsed or refractory follicular lymphoma | |||
| Brexucabtagene autoleucel | Tecartus | CD19 | Mantle cell lymphoma (MCL) | Adults with relapsed or refractory MCL |
| B cell ALL | Adults with relapsed or refractory B cell ALL | |||
| Lisocabtagene maraleucel | Breyanzi | CD19 | B cell NHL | Adults with relapsed or refractory B cell NHL |
| Idecabtagene vicleucel | Abecma® (Bristol‐MyersSquibb) | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
| Ciltacabtagene autoleucel | Carvykti® (Janssen Pharmaceutical Companies of Johnson & Johnson) | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
History
- 1989: discovery that it was possible to redirect T cell signaling to an antigen of choice, independent of major histocompatibility complex (MHC) restrictions
- 2006: first results published of human clinical trials using chimeric antigen receptor T cell technology (J Clin Oncol 2006;24:e20, Clin Cancer Res 2006;12:6106)
- 2010: first use of CAR T cell therapy was in a 5 year old girl with relapsed B cell acute lymphoblastic leukemia (ALL); received CAR T cell therapy directed against CD19 antigen on B lymphoblasts (CART-19)
- Developed complications, treated with tocilizumab, an anti-IL6 monoclonal antibody
- Patient survived and became global ambassador for CAR T cell therapy
- 2014: CTL019 was granted breakthrough therapy designation by the United States Food and Drug Administration (Immunol Rev 2015;263:68)
- 2021: ide-cel was FDA approved as a CAR T cell construct with murine BCMA targeting plasma cells in multiple myeloma (CA Cancer J Clin 2023;73:275)
- 2022: cilta-cel became second FDA approved anti-BCMA CAR T cell therapy (CA Cancer J Clin 2023;73:275)
Procedure to create CAR T product
- Collect autologous T cells via leukocytapheresis
- Obtain approximately 109 T cells
- Unknowns
- Is there an optimal time window for collection?
- How to predict an adequate collection?
- How to separate T cells from collected mononuclear cell apheresis product? (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
- Ship T cells to manufacturing site
- CAR structure is composed of an extracellular antigen recognition domain fused to intracellular TCR signaling domains (CD3z) and costimulatory domains such as CD28
- Manufacture CAR T cells by CD3 / CD28 bead stimulation and lentiviral transduction (Tisagenlecleucel)
- Manufacture CAR T cells by CD3 antibody / IL2 stimulation and retroviral transduction (Axicabtagene)
- Expand cell population over 6 - 10 days
- Achieve patient dose of 2 - 4 x 106 CAR expressing T cells/kg patient body weight
- Cryopreserve CAR T cells
- Ship to treating facility
- Patient is given lymphodepleting chemotherapy (usually fludarabine and cyclophosphamide)
- Deplete endogenous T cells that might reject the CAR T cells
- Increase likelihood of expanding CAR T population in recipient
- Enhance antigen presentation capabilities
Pathophysiology
- Targets (Front Immunol 2021;12:744823)
- CD19
- CD20, CD22 and BCMA
- Developing targets include CD70, CD7, CD5
- NKG2DL, GD2 and mesothelin for solid tumors potentially
- Structure
- Receptor: combines facets of normal T cell activation into single protein
- Links extracellular antigen recognition domain to an intracellular signaling domain to activate the T cell when an antigen is bound
- 4 parts
- Antigen recognition domain
- Exposed to the outside of the cell in the ectodomain portion of the receptor
- Allows the CAR T cell to attack any cell that expresses the matching molecule
- Derived from variable regions of a monoclonal antibody linked together as a single chain variable fragment (scFv)
- A scFv is a chimeric protein made up of the light (VL) and heavy (VH) chains of immunoglobulins connected with a short linker peptide
- VL and VH regions are selected to bind the target antigen like CD19
- Extracellular hinge region
- Structural domain between the antigen recognition region and the cell's outer membrane
- Optimizes flexibility of the scFv receptor head to promote antigen binding between CAR T and target antigen
- Transmembrane domain
- Anchors the CAR to the plasma membrane, links the extracellular hinge and antigen recognition domains with the intracellular signaling region
- Stabilizes the entire structure
- CD28 transmembrane domain is often used and is very stable
- Should not use CD3 zeta transmembrane domain because it can cause incorporation of the artificial TCR into the native T cell receptor
- Intracellular T cell signaling domain
- Receives and perpetuates signal after antigen binds external recognition portion
- Cytoplasmic domain of CD3 zeta is often used to mimic the normal T cell activation dependent on phosphorylation of immunoreceptor tyrosine based activation motifs
- Includes 1 or more chimeric domains from costimulatory proteins
- Antigen recognition domain
- Function of CAR T cells
- Trafficking
- Engineered T cell must be able to get to site of tumor cells
- Target tumor cells to be killed
- Possibility of introducing chemokine receptors into CAR T cells to improve trafficking to tumors that produce cognate chemokines
- Recognition
- Recognize target tumor cells
- Discriminate and ignore bystander tissues
- Control
- T cells are relatively autonomous once they are infused
- New work on regulatory processes to modulate the survival of T cells, timing, strength and location of their activity
- Microenvironment
- Resist immunosuppression
- Prime / mobilize endogenous immunity
- Proliferation / persistence
- Expand the population of CAR T cells to optimize activity (Cell 2017;168:724)
- Trafficking
- Safety measures: allow ablation of CAR T cells if severe toxicity develops
- Inclusion of suicide gene, iCaspase9
- Surface tag such as epidermal growth factor receptor
Types of CAR T cells
- First generation: contained only TCR complex CD3ξ chain domain with additional costimulatory domains
- Second generation: incorporated costimulatory domains like CD28 or CD137 to boost survival, proliferation and antitumor activity
- Third generation: combined CD3ξ chain domain with additional costimulatory domains (Cell 2016;164:780)
- Fourth generation: T cells redirected for universal cytokine killings (TRUCKS)
- Improve tumor microenvironment
- May have potential for solid tumors
- Newer generation: additional gene editing such as CRISPR-Cas9
- Armored CARs: CAR T cells modified to express cytokines, ligands or scFv that help turn suppressive tumor environment into proinflammatory to better fight tumor
- Tandem CARs: CAR molecule engineered to recognize multiple antigens via 2 binders on a single molecule
- Designer CARs: modified by CRISPR-Cas9 to edit CAR T genomes for advantageous features, such as being less susceptible to immunosuppressive effects
- Smart CARs: logic gated, regulated
- Have new receptors that function independently of CAR / TCR pathways but interfere with CAR activity in controlled way
- Use of modular receptors called synthetic Notch (synNotch) receptors
- Use extracellular domain like scFv to recognize target antigen, without triggering T cell activation
- Ligand engagement results in cleavage of receptor and subsequent release of transcriptional activator domain, which enters nucleus and drives expression of user specified target genes (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2016;164:780)
- Split, universal and programmable (SUPRA) CARs
- Universal receptor found on T cells and tumor targeting scFv adaptor molecule
- 2 component receptor system with universal receptor (zipCAR) expressed on T cells and tumor targeting scFv adaptor (zipFv)
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- scFv of zipFv binds tumor antigen
- Leucine zipper binds and activates zipCAR on T cells
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- Regulate activity to limit over activation, decrease cytokine secretion and improve tumor targeting (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2018;173:1426)
- Universal CAR T (UCAR T) (Front Immunol 2021;12:744823)
- Potential advantages
- Could be taken from healthy allogeneic donors
- Could have batched rather than customized manufacturing
- More immediate availability
- Lower cost
- Possible application in T cell malignancies
- Potential disadvantages
- Need for additional gene editing to avoid graft versus host disease and rejection
- Lower amplification and shorter persistence in vivo
- Potential advantages
Diagrams / tables
Challenges
- Tumor specific antigens
- CD19 is a great target, since a loss of normal B cells can be compensated for by replacement antibody therapy (IVIG)
- How specific must they be?
- Tumor cells that do not express the target antigen may evade therapy
- Tumor cells with splice variants, lacking a specific epitope may also escape targeting (Blood 2018;131:2621)
- Can we pinpoint antigens expressed by tumors versus normal cells reliably?
- Do all tumor cells in a given tumor express the same antigens?
- Can a tumor be effectively treated even if only some of the cells are susceptible to targeting?
- How significant is bystander toxicity? (Blood 2018;131:2621)
- Rare chance of accidentally introducing CAR gene into a tumor cell during manufacturing
- Proliferation of tumor cells
- Tumor cells escape detection by CAR T cells
- Response in non-Hodgkin lymphoma (NHL) is worse than in ALL
- CD19 loss variants
- Microenvironment factors that limit proliferation and the effect of CAR T cells
- Remission rates 70 - 80%
- Solid tumors
- Challenges identifying suitable cell surface molecules for the CAR T cells to target
- Attempts to engineer T cells with T cell receptors capable of recognizing tumor specific antigens from intracellular proteins
- Modify tumor microenvironment to be more hospitable to CAR T cells
Adverse effects
- Toxicity
- On target effects: reversible when target cells are eliminated or CAR T cell engraftment is terminated
- B cell aplasia
- More severe than that caused by anti-CD20 monoclonal antibody rituximab
- Rapidly reversed after ablation of CAR T cells
- May require immunoglobulin therapy
- Cytokine release syndrome
- Initial flu-like presentation
- Fevers, hypotension, hypoxia, neurologic changes
- Can progress to capillary leak
- T cell activation and high levels of cytokines, IL6 and interferon γ
- Neurotoxicity: CAR T related encephalopathy syndrome (Blood 2014;123:2625, Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
Clinical features
- Patient selection considerations may differ from autologous stem cell transplant (ASCT) and take into account previous therapies, upper age limit, severity of comorbidities and resistance to chemotherapy
- Some clinical trials may include transplant ineligible patients
- Other clinical trials are studying CAR T cell therapy as second line treatment and for both transplant eligible and ineligible patients
- Other factors that may be considered in therapy include
- Performance status
- Organ function
- T cell count
- B acute lymphoblastic leukemia (ALL), target antigen is CD19
- CART-19: CAR T directed against CD19 antigen on B lymphoblasts
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Diffuse large B cell lymphoma, target antigen is CD19, no specific age limit
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Other diagnoses under investigation for possible CAR T cell therapies include
- Hodgkin lymphoma (HL), target antigen is CD30
- Anaplastic large cell lymphoma, target antigen is CD30
- Myeloma, target antigens: SLAMF7, B cell maturation antigen (BCMA)
- Acute myeloid leukemia (AML), target antigens are CD123, CD33, Lewis Y and FOLR2
- Other potential costimulatory domains are PD1 / CD28 and CD200R / CD28
- T cell malignancies are a challenge since candidate target antigens are found on normal T cells
- CARs posttransplant: maximize graft versus tumor effect while minimizing graft versus host disease (GVHD)
- CARs in donor leukocyte infusions (DLI): may help improve survival in relapses of hematologic malignancy
- CARS in virus specific T cells
- Chimeric autoantibody receptor T cells (CAARs): autoimmune disease such as pemphigus vulgaris (Immunol Rev 2015;263:68, Clin Cancer Res 2016;22:1875)
Transmission
No information provided
Symptoms
No information provided
Screening
No information provided
Blood donor screening
No information provided
Blood donor testing
No information provided
Donor deferral
No information provided
Laboratory
- Polymerase chain reaction (PCR) molecular assays are available for all CARs produced, however, such testing might not accurately reflect if the CAR is actually expressed on the cell surface and is generally performed retrospectively
- Flow cytometry may be a useful modality to monitor real time expansion and response
- CD19 CARs are known to expand rapidly, clear target tumor cells, then contract
- CARs have single chain variable fragments (scFv) for specificity against a target antigen and have little else on the cell surface
- There are 3 potential ways to detect the scFv using flow cytometry
- Develop an anti-idiotype antibody to the scFv
- Use Fc conjugated soluble antigen such as Fc-CD22 that interacts with CD22 CAR T cells followed by a secondary detection antibody (anti-Fc)
- Use biotinylated protein L followed by a streptavidin conjugated fluorophore
- There are benefits and deficits to each method and the process is labor intensive
- Well constructed assay provides meaningful information for individual patient care and a better understanding of CARs as a whole
- Additionally, there is need for standardized methods to profile memory phenotype of CAR T cells to evaluate quality and promote manufacturing improvements
- Use of a standardized memory T cell panel can help evaluate how T cell phenotype impact the efficacy and longevity of response in patients receiving CAR T cell therapies
- One attempt at this includes a dried memory T cell panel containing a prevalidated mixture of 7 antibodies for the identification of naïve, stem cell memory, central memory and effector memory CD4+ and CD8+ T cell subsets (BD Biosciences)
- One study used this prevalidated mix and additional drop in antibodies can complement the panel and enable more in depth evaluation of the T cell phenotype and monitor changes in expression of PD-1, TIM3, LAG3, HLA-DR, CD45RO and CXCR3 on T cells transduced to express a novel anti-CD37 CAR (Transfus Med Hemother 2019;46:15, Blood 2019;134:5626)
Case reports
- 18 month old girl and 52 year old woman treated with CAR T cell therapy for B ALL with mixed lineage leukemia gene (MLL) mutations developed AML clonally related to their B ALL, suggesting CD19 negative immune escape (Blood 2016;127:2406)
- 20 year old man relapsing 9 months after CD19 targeted CAR T cell (CTL019) therapy (Nat Med 2018;24:1499)
- 38 year old woman with CD19 directed CAR T cell therapy combined with BTK inhibitor and PD-1 antibody against secondary central nervous system lymphoma (Front Immunol 2022;13:983934)
- Mini review of control mechanisms for future CAR T cell products (Front Immunol 2020;11:326)
- Advancements in safety for new CAR T cell therapy models (Mol Cancer 2019;18:125)
- Applications of CAR T cell therapy for community oncology physicians (Oncologist 2016;21:608)
Treatment
- Cytokine release syndrome: treated with anti-IL6 monoclonal antibody, tocilizumab
Sample assessment & plan
No information provided
Differential diagnosis
No information provided
Additional references
None
Board review style question #1
A 10 year old boy diagnosed with acute lymphoblastic leukemia with several relapses is participating in a clinical trial for CD19 CAR T cell therapy. He develops rapid onset of flu-like symptoms (including fever) and progresses with mental status change and clinical concern for cytokine release syndrome. What would be an appropriate treatment for suspected cytokine release syndrome?
- Anti-CD20 (rituximab)
- Anti-CD38 (daratumumab)
- Anti-IL6 (tocilizumab)
- Azathioprine
- Interferon γ
Board review style answer #1
C. Anti-IL6 (tocilizumab). Cytokine release syndrome is likely due to high levels of IL6 and interferon γ. Anti-IL6 (tocilizumab) has mainly been used in inflammatory conditions such as juvenile rheumatoid arthritis and has been effective in decreasing the IL6 levels involved in cytokine release syndrome. Answer A is incorrect because anti-CD20 (rituximab) is used in the treatment of B cell lymphomas. Answer B is incorrect because anti-CD38 (daratumumab) is used in the treatment of multiple myeloma. Answer D is incorrect because azathioprine can be used to treat immune disorders such as Crohn's disease, renal transplant rejection and rheumatoid arthritis. Answer E is incorrect because interferon γ is used to treat various autoimmune diseases.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy
Board review style question #2
Which of the following functions of CAR T cells refers to the movement of the engineered T cells to the site of tumor cells targeted for destruction?
- Control
- Microenvironment
- Proliferation / persistence
- Recognition
- Trafficking
Board review style answer #2
E. Trafficking is the first function of a CAR T cell. The engineered cell must be able to travel to the site of the target tumor cells. Answer A is incorrect because control refers to the relative autonomy of T cells once they are infused. Answer B is incorrect because microenvironment refers to background immunity that impacts the effect of CAR T cells. Answer C is incorrect because proliferation / persistence refers to the ability of the engineered cells to circulate. Answer D is incorrect because recognition is the ability of the cells to recognize their target.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy
Cellular therapy reactions
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Symptoms | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Cellular therapy reaction prevention strategies | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Infusion of hematopoietic progenitor cells (HPCs) can cause a range of reactions from transient and self limiting to severe, regardless of HPC origin (i.e., marrow versus peripheral blood versus cord blood)
- Therefore, it is important to acknowledge that these infusions are actually transfusions and all the components that make up the product have the potential to cause an adverse reaction in the recipient
- Reference: Kopko: Transfusion Reactions, 5th Edition, 2021
Essential features
- Infusion reactions related to cellular therapy infusion can occur with either cryopreserved or fresh HPCs
- Acute reactions can occur during or within 12 hours after infusion with HPCs; additional delayed reactions (days) are expected for haploidentical hematopoietic stem cell transplants (HSCT) and chimeric antigen T cell receptor (CAR T) cell therapies
- Reactions are more common with cryopreserved HPC due to both cryoprotectant, cell debris and passive infusion of inflammatory mediators; signs and symptoms attributed to cryopreserved HPC can overlap classic transfusion reactions
- Reactions with fresh HPC (e.g., bone marrow) are similar to other blood components including allergic reactions and volume overload
- Reference: Kopko: Transfusion Reactions, 5th Edition, 2021
Terminology
- Hematopoietic progenitor cells (HPCs), HPC(M) = marrow, HPC(C) = cord blood, HPC(A) = peripheral blood by apheresis
- Hematopoietic stem cell transplant (HSCT)
- Cytokine release syndrome (CRS)
- Reference: Kopko: Transfusion Reactions, 5th Edition, 2021
Pathophysiology
- Cryoprotectant
- Dimethyl sulfoxide (DMSO) is the most common cryoprotectant used for cellular therapy products, usually at a 10% final vol/vol concentration; HPC(C) and many CAR T may include dextran as well (Bone Marrow Transplant 1993;11:389)
- Autologous peripheral blood HPC (HPC[A]) and cord blood HPC (HPC[C]) are always cryopreserved; bone marrow HPC (HPC[M]) is not typically frozen due to high red blood cell (RBC) content
- DMSO
- Is oncotic, leading to hyperosmolality and volume expansion, hypertension, headache, bradycardia
- Can cause histamine release with flushing, cough, hypoxia, hypotension, arrhythmias
- Can cross the blood - brain barrier with sedation, dizziness, encephalopathy, seizures
- Is toxic and can cause nausea, vomiting, hepatic transaminitis, elevated creatinine kinase, intravascular hemolysis
- Infusion toxicity is proportional to the total volume of DMSO infused (Biol Blood Marrow Transplant 2012;18:220)
- Small pediatric patients, patients with renal insufficiency or amyloidosis are at increased risk for toxicity
- Total DMSO should not exceed 1 gm DMSO/kg patient weight per day
- DMSO is excreted as DMSO and dimethylsulfone (DMSO2) by kidney and as dimethylsulfide (DMSH2) by lungs; DMSH2 is responsible for the garlic aftertaste, halitosis, body odor and nausea in many patients
- Dextran used in HPC(C) and other non-HPC cellular therapy products can cause anaphylactic reactions
- Freezing related cell damage
- Unlike mononuclear cells (HPC, lymphocytes, monocytes), granulocytes and red cells do not survive standard DMSO cryopreservation, resulting in cell lysis
- Granulocytes
- Granulocyte colony stimulating factor (G CSF) mobilized HPC(A) have high levels of activated neutrophils; infusion toxicity has been linked to a high prefreeze granulocyte dose
- Lysis of G CSF activated granulocytes leads to passive infusion of WBC derived inflammatory cytokines and chemokines, apoptotic cells and cell debris
- Severe reactions can occur at doses > 5 x 109 total granulocytes (Biol Blood Marrow Transplant 2012;18:220)
- Red cells (Bone Marrow Transplant 1990;5:25, Bone Marrow Transplant 1999;23:533)
- HPC(C) and HPC(M) contain significant RBC quantities: HPC(A) contain < 5 mL RBC
- Toxicity attributed to infusion of free hemoglobin and cell debris
- HPC(C) are always cryopreserved and at risk for passive infusion of lysed RBC with high rates of cardiovascular toxicity
- HPC(C) are now RBC depleted before freezing; however, older cord units still contain significant RBC quantities
- Electrolyte disturbances (Nephrol Dial Transplant 2008;23:359)
- Cryopreserved HPC products have very low Ca++ and high K+ concentrations upon thawing
- Citrate anticoagulant binds free calcium
- Cell lysis with released intracellular K+
- Risk for localized cardiac electrolyte abnormalities with rapid intravenous (IV) push, central line infusion
- Rarely change in peripheral blood electrolytes
- Cryopreserved HPC products have very low Ca++ and high K+ concentrations upon thawing
- T cell activation and expansion (Biol Blood Marrow Transplant 2012;18:220, Nat Med 2019;25:1341, J Immunother Cancer 2018;6:56)
- Expected in haploidentical HSCT (1 - 2 days postinfusion) and CAR T cellular therapies (4 - 10 days)
- T cell activation causes the release of interferon (IFN) gamma, which results in a cascade of proinflammatory reactions resulting in a cytokine storm or cytokine release syndrome (CRS); interleukin 6 is a major driver in CRS and a target of therapy
- Presents as fever and can progress to hypoxia, hypotension, organ failure and death
- Donor plasma
- Allergic reactions, transfusion related acute lung injury (TRALI), ABO and other red cell alloantibodies
- Volume expansion
- Reference: Kopko: Transfusion Reactions, 5th Edition, 2021
Diagrams / tables
Table 1: Infusion toxicity by etiology
| DMSO | Cytokines | RBC / hemoglobin | Plasma | Volume | Citrate | |
| Nausea / emesis | X | |||||
| Fever / chills | X | X | X | |||
| Cough | X | X | ||||
| Flushing | X | X | ||||
| Shortness of breath, hypoxia | X | X | X | X | X | |
| Hypotension | X | X | X | |||
| Hypertension | X | X | X | |||
| Bradycardia | X | X | ||||
| Arrythmia | X | X | X | X | ||
| Neurologic | X | X | X | |||
| Gastrointestinal pain | X | X | X |
Clinical features
- Patient monitoring (Blood 1990;75:781, Anticancer Res 1998;18:4705)
- During and after transfusion
- Vital signs every 5 - 10 minutes during the first infusion, every 30 minutes for 2 - 4 hours after
- Electrocardiogram (ECG) before and after infusion to monitor cardiac toxicity
- Volume overload (Biol Blood Marrow Transplant 2013;19:1152)
- Pretransplant hydration, particularly with ABO incompatible HSCT to prevent renal injury
- HPC(M) infusion: small pediatric patients are at particular risk
- Infusion of multiple cryopreserved HPC(A) units due to saline hydration, total volume and DMSO infused with hyperosmolality and intravascular expansion
- Underlying cardiac and renal insufficiency
- Hemolysis (Bone Marrow Transplant 1990;5:25, Bone Marrow Transplant 1999;23:533)
- Immune hemolysis (see ABO incompatible HSCT)
- Hemolysis of donor red blood cells associated with ABO major incompatibility HPC(M)
- Hemolysis of recipient red blood cells associated with ABO minor incompatibility HPC(A)
- Passive infusion of lysed RBC in cryopreserved HPC(C) and HPC(M)
- Immune hemolysis (see ABO incompatible HSCT)
- Allergic reactions (Transfus Med Rev 2018 Jun 1 [Epub ahead of print])
- Typically mild, pruritis, rash
- Increased risk in patients with a history of allergy, allergic transfusion reactions
- Usually associated with allogeneic HPC(A) containing donor plasma
- Severe allergic reaction 1:1400 rate (Transfusion 1998;38:30S)
- Rare cases of anaphylaxis due to dextran in HPC(C)
- Pulmonary / respiratory symptoms (Bone Marrow Transplant 1990;5:25, Transfusion 2017;57:1522)
- Volume overload, especially with HPC(M) infusions
- DMSO induced
- Coughing, transient hypoxia
- Decrease in forced vital capacity (> 15%)
- Rare, respiratory depression
- Severe allergic / anaphylaxis (rare)
- Transfusion related acute lung injury (rare)
- Diffuse alveolar hemorrhage (rare)
- Cytokine release syndrome (see CRS below)
- Cardiac toxicity (Blood 1990;75:781, Transfusion 1993;33:578)
- Decreased pulse rate most common; atrioventricular (AV) block and arrhythmias also seen
- Onset 1 - 12 hours postinfusion (Bone Marrow Transplant 1992;10:435, Bone Marrow Transplant 1994;13:789)
- Increased risk for cardiac events with
- Cryopreserved HPC(M) and older, red cell replete HPC(C) units
- Amyloid patients at risk for severe cardiac events during and after infusion
- Cytokine release syndrome (CRS)
- Haploidentical HPC transplants (Hematol Oncol Stem Cell Ther 2017;10:79, Biol Blood Marrow Transplant 2016;22:141, Pediatr Transplant 2015;19:918, Transfusion 2015;55:2023)
- CAR T therapies
- Majority of patients (> 50%) require active monitoring and treatment
- Onset first 1 - 2 weeks, median of 4 days (Biol Blood Marrow Transplant 2019;25:625)
- Fever initial symptom
- Capillary leak, hypotension and hypoxia
- 10 - 45% of patients experience severe CRS (grade > 3) including death (Nat Med 2019;25:1341)
- High disease burden, leukemia risk factors
- CRS also seen with bispecific T cell engagement (BITE) monoclonal antibody therapy(s)
- Neurologic
- Cryopreserved, DMSO related (Cytotherapy 1999;1:311)
- Headache related to hyperosmolality, volume expansion, hypertension
- Less common: seizures, reversible severe encephalopathy, hemorrhagic stroke, global transient amnesia
- Immune effector cell associated neurotoxicity syndrome (ICANS) (Biol Blood Marrow Transplant 2019;25:625)
- CAR T cell therapies (0 - 67% of patients) (J Immunother Cancer 2018;6:56)
- Onset > 10 days postinfusion, duration of weeks
- Often but not always associated with a history of CRS
- Lethargy, tremor, difficulty writing, naming objects
- Severe ICANS seen in 10% of patients
- Global aphasia, depressed responsiveness, seizures, diffuse cerebral edema
- Cryopreserved, DMSO related (Cytotherapy 1999;1:311)
- Bacterial or fungal contamination
- HPC(M) has the highest rate of bacterial contamination (PLoS One 2015;10:e0141152, Braz J Infect Dis 2015;19:571)
- Multiple skin punctures during marrow harvest
- Normal skin flora (e.g., Cutibacterium, S. epidermitidis)
- HPC(C) are at risk for contamination by vaginal and enteric organisms, especially when collected ex vivo after vaginal delivery and delayed transport / processing of the delivered placenta (Transfusion 2012;52:1770)
- HPC(A) has a low rate of bacterial or fungal contamination
- Most are false positive cultures due to laboratory contamination during sample culturing
- Donor derived contamination is rare and usually associated with HPC collection in autologous HPC donors with colonized central lines
- HPC(M) has the highest rate of bacterial contamination (PLoS One 2015;10:e0141152, Braz J Infect Dis 2015;19:571)
- Reference: Kopko: Transfusion Reactions, 5th Edition, 2021
Symptoms
- Fresh, noncryopreserved HPC infusions (Bone Marrow Transplant 1990;5:25, Bone Marrow Transplant 1999;23:533, Transfus Med Rev 2018 Jun 1 [Epub ahead of print])
- Infused by gravity similar to other blood products
- Risk for transfusion reactions similar to other blood products
- Lower rate of infusion reactions (~10% overall) compared to cryopreserved HPC
- HPC(M): risk for hypertension, headache, volume overload due to product volume
- ABO major incompatible HPC(M) at risk for hemolytic transfusion reactions
- HPC(A): risk for allergic reactions, postinfusion fever (first 24 hours)
- Cryopreserved HPC infusions (Anticancer Res 1998;18:4705)
- Thawed at bedside, infused by IV push (10 mL/min)
- Significant infusion toxicity, reaching > 70% in some studies
- Common (> 10%)
- Nausea, vomiting, abdominal pain
- Hypertension, hypotension
- Bradycardia
- Coughing
- Less common (< 10%)
- Chest tightness, dyspnea
- Fever, chills
- Flushing
- Neurologic
Blood donor screening
- HPC donors are subjected to a more extensive donor history questionnaire than regular blood donors, including history of autoimmune disease and family history for potential inheritable diseases
- Marrow HPC(M) donors must be evaluated and cleared by anesthesia
- Reference: Abutalib: Best Practices of Apheresis in Hematopoietic Cell Transplantation, 1st Edition, 2020
Blood donor testing
- Donors undergo standard infectious disease testing at least 30 days prior to HPC collection
- Infectious disease testing is repeated on the day of donation
Donor deferral
- Unlike volunteer blood donors, autologous and allogeneic HPC donors may be accepted based on medical need regardless of donor history and infectious disease testing
- Donors may be deferred based on donor safety / health issues related to the risks associated with HPC donation
- Reference: Abutalib: Best Practices of Apheresis in Hematopoietic Cell Transplantation, 1st Edition, 2020
Laboratory
- Patients may undergo testing as appropriate for standard transfusion reactions
- ABO incompatible HSCT
- Hemolysis markers (haptoglobin, lactate dehydrogenase [LDH], direct antiglobulin test [DAT])
- Recipient ABO titer
- Peripheral smear
- Volume overload
- Brain natriuretic peptide (BNP)
- Reactions associated with cryopreserved HPC
- Units/volume HPC and DMSO infused (< 1 gm DMSO/kg recipient weight)
- Total prefreeze granulocyte dose infused
- ABO incompatible HSCT
- References: Kopko: Transfusion Reactions, 5th Edition, 2021, Abutalib: Best Practices of Apheresis in Hematopoietic Cell Transplantation, 1st Edition, 2020
Case reports
- 16 year old boy undergoes allogeneic hematopoietic cell transplantation (HCT) and experiences a severe infusion reaction due to dextran (Bone Marrow Transplant 2017;52:1051)
- 52 year old man who experienced tonic clonic seizure within minutes after the initiation of DMSO cryopreserved autologous peripheral blood progenitor cell (PBPC) infusion (Cell Tissue Bank 2018;19:831)
- 63 year old woman with multiple myeloma presented for autologous peripheral blood HSC collection (J Clin Apher 2022;37:316)
Cellular therapy reaction prevention strategies
- Premedication options (Blood Adv 2020;4:3041, Transfusion 1991;31:521, Biol Blood Marrow Transplant 2008;14:1425)
- Antihistamines (diphenhydramine, 50 mg or chlorphenamine, 10 mg)
- Steroids (hydrocortisone, 250 mg or methylprednisolone, 20 mg)
- Antipyretic, due to the frequency of fever and chills
- Antiemetics
- Strawberry lollipops, lemon drops and orange slices were reported to reduce nausea and vomiting by counteracting noxious taste due to DMSO
- ABO incompatible, saline hydration to prevent acute renal failure (maintain urine output > 100 mL/hour)
- Prophylactic antibiotic coverage
- Culture positive products may need additional antibiotic coverage
- Contamination / transfusion transmitted infection (Arch Pathol Lab Med 2003;127:e19)
- Donors are screened for transfusion transmitted disease prior to and on day of donation
- Donors are screened for active infection, possible bacteremia prior to HPC collection
- HPC(M): some centers infuse clindamycin prophylactically to mitigate bacterial contamination associated with bone marrow harvest
- HPC products are processed in monitored clean rooms following good tissue practices (GTP) and good manufacturing practices (GMP)
- All HPC products are cultured for contamination (aerobic, anaerobic, fungal); products are cultured after collection as well as during and after final processing as required
- During thawing of cryopreserved HPC, products are placed in sterile overwraps and thawed in sterile water bath
- Most patients receive prophylactic antibiotic coverage; patients receiving known heavily contaminated HPCs should begin appropriate antibiotic prophylaxis 2 days before infusion
- Change the HPC infusion rate (Bone Marrow Transplant 1993;11:389)
- Cryopreserved HPCs are infused at 10 mL/minute
- HPCs in 10% DMSO are viable up to 1 hour postthaw, permitting a slower infusion rate if necessary
- Fresh, noncryopreserved HPC are infused by gravity drip at 3 - 4 mL/min with less infusion toxicity
- Cryopreserved HPCs are infused at 10 mL/minute
- Red cell, plasma volume reduction (Blood Transfus 2014;12:150)
- RBC depletion required in ABO major incompatible HPC(M) HSCT to prevent hemolytic transfusion reactions
- RBC depletion now required in HPC(C) products to prevent severe infusion toxicity
- Plasma reduction can be used with
- Can be used with ABO minor incompatibilities
- For prevention of volume overload (i.e., large grafts, children)
- Can result in fourfold decrease in allergic reactions
- Reducing DMSO concentration (Bone Marrow Transplant 1993;11:389)
- Divided infusion over 2 days
- Freeze at higher cell concentrations
- Reduce the number of units/DMSO volume infused
- Average concentration is 1 x 108 cells/mL: increase to 5 - 7 x 108 cells/mL without affecting CD34 viability
- Product wash postthaw
- Pros: can remove DMSO, red cells and cellular debris
- Cons: postthaw cells are fragile and osmotically challenged, loss of CD34+ cells
- Dilution
- Common method for thawing HPC(C)
- Add albumin - dextran 40 solution to postthaw cells to a ratio of 1:2 to 1:3
- Pros: increase in postthaw cell viability
- Cons: reports have implicated dextran 40 with severe cardiopulmonary reactions
- Reducing granulocyte toxicity (Biol Blood Marrow Transplant 2012;18:220)
- Limit infused cell dose to < 1.63 x 109 cells/kg/day
- Pros: reduces severe reactions by 85%
- Cons: increases the number of required days for infusion
- Optimize HPC collection
- Minimize the number of apheresis procedures
- Addition of plerixafor for poor mobilizers
- Limit infused cell dose to < 1.63 x 109 cells/kg/day
- Alternative freezing solutions (Transfusion 2004;44:245)
- Freezing with reduced DMSO concentration at 5% with or without 6% hetastarch and 4% albumin at -80°C
- Non-DMSO cryoprotectants (not yet commercially available)
- CD34+ cell selection (Vox Sang 2008;95:70)
- Pros: absence of any measurable toxicity
- Cons: high costs, cell loss (up to 50%) and limited institutional availability
- Reference: Kopko: Transfusion Reactions, 5th Edition, 2021
Differential diagnosis
- Many signs and symptoms can overlap with the patient's underlying clinical condition(s)
- Medication adverse effects
- Nonblood product related infection
Board review style question #1
A 49 year old, 82.7 kg man with a medical history of multiple myeloma is admitted for an autologous hematopoietic stem cell transplant (HSCT) with cryopreserved, plasma reduced HPC(A). He was premedicated with 650 mg of Tylenol and 50 mg of intravenous (IV) Benadryl. He was infused with a total of 200 mL HPC(A) containing 9.1 x 106 CD34+ cells/kg, 6.9 x 108 MNC/kg and 20 mL dimethyl sulfoxide (DMSO). Shortly after the infusion, the patient began to experience headaches, facial flushing, coughing and difficulty taking deep breaths. The nurse slowed the infusion and the patient's symptoms improved. Vital signs before, during and after infusion are shown below. What was the most likely cause of the patient's reaction?
| Before | During | After | |
| Time | 12:03 | 12:44 | 16:33 |
| Temperature (Celsius) | 36.5 | 36.5 | 37.1 |
| Heart rate | 92 | 97 | 86 |
| Resp rate | 16 | 20 | 16 |
| Blood pressure | 148/83 | 132/78 | 120/66 |
| SpO2% | 96 | 95 | 99 |
| O2 device | None (room air) | None (room air) | None (room air) |
- Anxiety: psychological stress and anxiety surrounding the infusion
- DMSO toxicity from cryopreservation
- Severe allergic reaction without anaphylaxis due to plasma
- Volume overload: due to rapidly increasing intravascular volume
Board review style answer #1
B. DMSO toxicity from cryopreservation. The patient began to have difficulty with deep breaths, flushing, coughing and headaches shortly after his infusion began. This patient was experiencing a classic reaction to DMSO. DMSO can cause a wide array of reactions including headache, nausea, dizziness, halitosis, hypoxia, chest tightness, coughing and hypotension. DMSO is hyperosmolar with expansion of intravascular volume and capable of inducing histamine release with flushing, coughing, chest tightness or dyspnea. Answer A is incorrect because the question stem does not mention any signs or symptoms specifically related to anxiety. In addition, he was premedicated with 50 mg IV Benadryl, which is very sedating; however, it is paramount to keep a broad differential diagnosis when assessing patients with possible reactions to infusion of any products. Answer C is incorrect because a severe allergic reaction is unlikely since this was plasma reduced and autologous stem cells. Answer D is incorrect because a volume overload is unlikely since the infusion was only (2 units - 200 mL). One strategy to reduce reactions due to infusion includes slowing the rate (Kopko: Transfusion Reactions, 5th Edition, 2021).
Comment Here
Reference: Cellular therapy reactions
Comment Here
Reference: Cellular therapy reactions
Board review style question #2
A 19 year old, O+ blood type, 52.1 kg woman with acute myeloid leukemia (AML) was admitted for a matched (8/8), unrelated, allogeneic ABO incompatible HPC(M) bone marrow transplant from a group A+ donor. The original marrow volume was exceptionally large (1,694 mL) and bloody (Hct = 30%). After red cell and plasma depletion, the processed marrow was 390 mL with 164.6 mL residual donor red blood cells (RBC) and a total cell loss of 8% for a final yield of 3.1 x 106 CD34 cells/kg. The patient was premedicated with Tylenol 500 mg PO, intravenous (IV) Solu-Cortef 100 mg and IV diphenhydramine 50 mg. HPC(M) was infused by gravity flow at 3 - 4 mL/minute. During infusion, the patient developed headaches that did not resolve with Tylenol. Toward the end of the transplant, her blood pressure rose from 123/74 to 152/92. Hydralazine and magnesium boluses were ordered and blood pressure began to downtrend. Hemoglobinuria was noted after the completion of the transplant. What is the reaction this patient is experiencing and what is the most likely cause?
- Acute dimethyl sulfoxide (DMSO) toxicity: due to rate of infusion
- Adverse medication reaction: premedication with Solu-Cortef
- Dehydration: patient has not consumed any fluids since the procedure due to an upset stomach
- Immune hemolysis: due to major ABO incompatibility (A donor / O recipient)
Board review style answer #2
D. Immune hemolysis: due to major ABO incompatibility (A donor / O recipient). This patient is experiencing an acute hemolytic transfusion reaction due to infusion of ABO incompatible HPC(M) marrow transplant. HPC(M) are quite bloody and still contain significant red cells even after RBC depletion because of the need to balance depletion against CD34 cell losses. Her pretransplant anti-A IgG titer was 256 and anti-A IgM titer was 32. Her headache was caused by a dramatic increase in systolic blood pressure (123 → 154) because of excess volume from transplant and aggressive hydration to prevent acute kidney injury and nitric oxide scavenging with heme mediated vasoconstriction. Answer A is incorrect because she was not exposed to DMSO; the patient received an allogeneic marrow transplant, which is not cryopreserved and infused fresh. Answer B is incorrect because this patient would not have hemoglobinuria due to an adverse medication reaction to Solu-Cortef . Answer C is incorrect given hypertension, not hypotension (Kopko: Transfusion Reactions, 5th Edition, 2021).
Comment Here
Reference: Cellular therapy reactions
Comment Here
Reference: Cellular therapy reactions
Cold stored platelets
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Treatment | Board review style question #1 | Board review style answer #1Definition / general
- Platelets may be stored at 1 - 6°C without agitation for up to 3 days for usage in actively bleeding trauma patients (21 CFR 640.24 and 640.25)
- A 2019 variance by the FDA allows for apheresis platelets to be stored at 1 - 6°C for 14 days without agitation for use in military settings including actively bleeding patients or in the absence of available room temperature platelets
Essential features
- Cold platelets undergo cytoskeletal rearrangements that allow for priming of the platelet and provide a hemostatic advantage over room temperature platelets in the actively bleeding patient
- Circulation of cold stored platelets is reduced
- Room temperature autologously transfused platelets have a survival of 6 - 7 days while the survival of allogeneic transfused platelets is 3 - 4 days
- Autologously transfused cold stored platelets have a survival of 2 - 3 days and allogeneically transfused cold stored platelets circulate for one day or less (Transfusion 2017;57:2836)
- Currently approved to be stored at 1 - 6°C without agitation for up to 3 days for use in actively bleeding trauma patients
Terminology
- COLD-PLT(S): cold stored platelets
- RT-PLT(S): room temperature platelets
- CCI: corrected count increment
Pathophysiology
- Cold stored platelets are activated during storage, offering the advantage of improved clotting and hemostatic effects
- Ultrastructurally, refrigeration results in cytoskeletal rearrangements causing increased actin filaments and loss of microtubule banding
- Shortly after starting refrigeration (< 16°C), membrane flipping of the platelet results in the release and exposure of P selectin from the alpha granules, which facilitates platelet-platelet binding as well as increasing platelet-leukocyte-endothelial interactions (Curr Opin Hematol 2018;25:500)
- Increased intracellular calcium and exposure of negatively charged phosphatidylserine contribute to initiation of coagulation cascade
- Other procoagulatory changes that occur in cold stored platelets include microparticle formation, increased degranulation, pseudopod formation and CD40L ligand expression (Transfusion 2019;59:1467)
- In vitro analysis shows enhanced clot formation in cold stored platelets
- At approximately 24 hours, the effects of refrigeration become irreversible and platelets will show permanent disc to sphere configuration and rapid clearance by the liver after transfusion
- Increased removal of cold stored platelets from circulation is multifactorial and involves the following mechanisms (see Diagrams / tables) (Curr Opin Hematol 2018;25:500, Transfusion 2019;59:1467):
- Glycoprotein Ibα clustering, which induces 14-3-3 protein associated apoptosis
- Cold induced removal of sialic acid, causing exposure of galactose, which in turn leads to increased removal by hepatic macrophages via the Ashwell-Morell receptor
- Exposure of phosphatidylserine resulting in macrophage induced phagocytosis
Diagrams / tables
Clinical features
- Compared with cold stored platelets, room temperature platelets offer the advantage of providing increased platelet counts with predictable recovery and improved corrected count increment, being the preferred method of storage for platelets used for prophylaxis of nonbleeding patients with hyporegenerative thrombocytopenia or thrombocytopathies (Transfusion 2019;59:1467)
- Corrected count increment = posttransfusion - pretransfusion platelet count (x1011) x body surface area (m2)
number of platelets transfused (x1011) - Cold stored platelets have increased aggregation and decreased risk of bacterial contamination when compared with room temperature platelets
- Bacterial risk in room temperature platelets has reduced the shelf life to 5 days
- Bacterial contamination can be detected in approximately 1 in every 5,000 platelet products, resulting in sepsis in 1 in every 100,000 units transfused and in death in 1 in every 500,000 - 1,000,000 platelet products transfused
- In U.S., 2 - 4 deaths per year are attributed to transfusion of bacterially contaminated platelet products (FDA: Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion [Accessed 10 March 2020])
- Recent FDA guidance requires further bacterial detection by either large volume delayed sampling or additional cultures / rapid testing assays at the point of release or pathogen reduced platelets (FDA: Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion [Accessed 10 March 2020])
- To increase the sensitivity of detection methods of bacterial contamination (large volume delayed sampling or cultures / rapid testing assays at the point of release), the maximum expiration date for room temperature platelets has been extended to 7 days
- Cold platelets mitigate the risk of bacterial contamination allowing for not only a product that offers enhanced hemostatic potential but also an improved microbial safety profile and potentially an extended lifespan to 14 - 21 days (Transfusion 2019;59:2652, FDA: Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion [Accessed 10 March 2020])
- Cold platelets have the potential to alleviate much of the burden of platelet inventory issues
Treatment
- Administered in patients with trauma induced coagulopathy
Board review style question #1
Cold stored platelets are currently approved by the FDA. Which of the following is one of the current properties or criteria for transfusion of cold stored platelets?
- In vivo circulation of 6 - 7 days
- Prophylactic platelet transfusion
- Require continual agitation
- Stored up to 5 days at 1 - 6°C
- Trauma induced coagulopathy
Board review style answer #1
E. Trauma induced coagulopathy. Due to activation of platelets during refrigeration, they are rapidly removed from circulation within 48 hours by macrophages and hepatocytes and thus currently are not approved for patients requiring prophylactic platelet transfusion. While room temperature platelets still offer better recovery beyond 48 hours, cold stored platelets offer the benefit of shortened bleeding times and faster clot formation, making this product desirable for the bleeding patient. Cold stored platelets are only approved for up to 3 days when stored at 1 - 6°C without agitation for trauma patients; however, a recent variance from the FDA has permitted the military to allow usage of cold stored platelets for up to 14 days without agitation.
Comment Here
Reference: Cold stored platelets
Comment Here
Reference: Cold stored platelets
Coombs test / DAT
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Screening | Blood donor screening | Blood donor testing | Donor deferral | Case reports | Treatment | Clinical images | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Used to determine whether a patient’s red blood cells (RBCs) are coated with immunoglobulins or complement in vivo
- Originally described using Coombs reagent: polyclonal rabbit antihuman globulin serum
- Modern Coombs reagents include anti-immunoglobulin G (IgG) and anticomplement antibodies either mixed together into one reagent (polyspecific) or used separately (monospecific)
Essential features
- A positive Coombs test suggests that antibodies or activated complement proteins from the patient are binding to RBCs in the patient’s blood
Terminology
- Direct antiglobulin test (DAT or Coombs test): tests whether patient RBCs are coated with antibodies present in vivo
- Indirect antiglobulin test (IAT or indirect Coombs test): tests whether antibodies from a patient bind to reagent RBCs in vitro
- Autoantibody: antibody that binds patient’s own RBCs and can bind reagent / transfused RBCs as well
- Panagglutinin: a molecule (can be an IgG) that binds all RBCs tested, causing agglutination of the RBCs in tube testing
- Alloantibody: antibody that does not bind a patient’s RBCs but does bind reagent RBCs or RBCs from a donor
Pathophysiology
- Indicates whether antibodies or complement are bound to RBCs within the patient
- Polyspecific (anti-immunoglobulin and anticomplement) reagent is used in a single step screen
- If polyspecific reagent is positive, then monospecific reagents are used to determine whether the RBCs are coated with IgG or complement
- IgM antibodies often deposit complement proteins more efficiently than IgG antibodies
- IgG antibodies may or may not cause detectable complement deposition on RBCs
- Positive DAT could be due to alloantibody (alloantibody binding transfused RBCs in circulation) or autoantibody (warm or cold autoimmune hemolytic anemia as well as paroxysmal cold hemoglobinuria)
- Passenger lymphocyte syndrome: posttransplant patients can develop a positive DAT due to antibody production from donor derived lymphocytes (Blood Transfus 2015;13:423)
- Positive DAT can also be due to passively transferred antibodies, e.g. hemolytic disease of the fetus and newborn (HDFN), passive transfer of antibodies due to recent transfusion of plasma or administration of concentrated immunoglobulins (e.g. intravenous immunoglobulin (IVIG) or RhoGAM) (Transfusion 1987;27:28)
Clinical features
- Hemolysis may or may not be present in patients with a positive Coombs test
- Patients recently transfused with allogeneic RBCs who develop a positive Coombs test may have formed a RBC alloantibody
- In these cases, an eluate can be performed to determine the antigenic specificity of the antibody
- Positive Coombs test with negative eluate (i.e. no alloantibody identified in the eluate) can occur in cases of drug induced RBC antibodies (Asian J Transfus Sci 2008;2:20)
- Recently transfused patients who have antibodies against the transfused RBCs may have a negative DAT despite clear signs of hemolysis, since the transfused RBCs may have been entirely cleared from circulation prior to performing the DAT (Transfus Med Hemother 2008;35:346)
- DAT may be negative in rare cases of autoimmune hemolytic anemia (Immunohematology 1997;13:115)
- Since IgG antibody crosses the placenta, the presence of a positive DAT in cord blood suggests a maternal derived IgG that binds fetal RBCs
- This can be due to maternal alloantibodies such as anti-D but most commonly is due to maternal derived anti-A, anti-B or anti-A,B (Laeknabladid 2016;102:326)
- Maternal fetal ABO incompatibility exists in 15 - 25% of pregnancies with a 1% chance of positive cord blood DAT in these pregnancies but only ~ 23% of infants with a positive DAT will have enough hemolysis to cause clinically significant jaundice (Arch Pediatr 2011;18:279)
Symptoms
- Signs and symptoms of hemolysis may or may not be present in a patient with a positive DAT
Screening
- DAT is often performed as part of blood bank type and screen testing when the results are abnormal or when a DAT is specifically ordered by a clinician
- Prevalence of DAT positivity in hospitalized patients is high (J Clin Pathol 1979;32:1014)
- If a DAT is negative, a control reaction with check cells (abbreviated as “cc”) is performed to confirm that the Coombs reagent is working; the check cells should be positive and serve as a positive control
Blood donor screening
- Blood donors are not routinely tested with DAT
- Studies have shown approximately 0.008% of donors have a positive DAT (Asian J Transfus Sci 2019;13:70)
- Presence of a positive DAT in a blood donor may correlate with an increased risk for future diagnosis of malignancy, especially hematologic malignancies; however, larger studies are needed to confirm this finding (Transfusion 2009;49:838)
Blood donor testing
- Not routinely performed
Donor deferral
- Although there is currently no universal guideline to defer a donor who has a positive DAT, a blood donor center medical director may elect to defer the donor out of caution
Case reports
- 37 year old liver transplant recipient who developed hemolytic anemia and positive DAT in the setting of posttransplant passenger lymphocyte syndrome (Transfusion 2017;57:1262)
- 70 year old patient incompatible with a donor RBC unit in AHG phase due to the donor having a positive DAT (Transfus Apher Sci 2014;50:239)
- 72 year old man with CLL presenting with panagglutinin and positive DAT (CMAJ 2006;174:305)
Treatment
- Positive DAT without signs of hemolysis is not an indication for treatment
- In recently transfused patients, a positive DAT (especially a newly positive DAT) is an indication for performing an eluate to evaluate for the development of an alloantibody
- Alloantibodies can cause a positive DAT and positive eluate in recently transfused patients even if the serum antibody screen is negative
- If an alloantibody is identified on an eluate, then antigen negative crossmatch compatible RBCs are indicated for future transfusions
- Reference: Blood 2019;133:1821
Sample assessment & plan
- Positive DAT, negative eluate and negative antibody screen:
- Assessment: The patient is a 75 year old woman with a history of recent Bactrim use. The blood group antibody screen is negative for common clinically significant alloantibodies. The direct antiglobulin test (DAT) is positive with anti-IgG. An eluate was performed, which did not react with any of the control cells tested. Potential causes of a positive DAT with negative eluate include drug-induced RBC antibodies and hypergammaglobulinemia (Arch Pathol Lab Med 2017;141:305).
- Plan: Drug dependent RBC antibody testing is available upon request. Patients with a positive DAT should be monitored for signs and symptoms of hemolysis.
- Positive DAT due to autoantibody:
- Assessment: The patient is an 80 year old man with a history of lymphoma. The blood group antibody screen is positive at 37 °C for a panagglutinin and the direct antiglobulin test is positive using anti-IgG and anti-C3d reagents. An eluate was performed which showed reactivity against all controls cells tested. The patient has not been transfused in the past, therefore an autoabsorption was performed. Autoabsorped plasma showed no reactivity against the control cells tested, ruling out an underlying alloantibody against common clinically significant antigens. These findings are consistent with a warm autoantibody.
- Plan: The patient should be monitored for signs of hemolysis.
Differential diagnosis
- Nonantibody mediated RBC agglutination due to Wharton jelly contamination of cord blood or rouleaux
- Nonspecific antibody binding due to elevated immunoglobulin levels, secondary to antiphospholipid antibodies or infection
- Incomplete washing of RBCs, overcentrifugation or other lab error (Arch Pathol Lab Med 2017;141:305)
Additional references
Board review style question #1
A 45 year old woman with no prior history of RBC transfusion presented with anemia. The results of a patient’s direct antiglobulin (DAT) / Coombs test are shown above. An eluate was performed, which reacted with 11 of 11 control RBCs tested by indirect antiglobulin test (IAT) in gel. Which of the following is the most likely diagnosis?
- Cold agglutinin disease
- Warm autoimmune hemolytic anemia
- Hemolytic transfusion reaction
- Drug dependent RBC antibody
Board review style answer #1
Board review style question #2
Plasma from patient A was crossmatched with four randomly selected RBC units using antihuman globulin (AHG) reagent in gel: 1 of the 4 crossmatches were positive. However, patient A’s plasma did not react with any reagent screening cells or any other RBC units tested. The RBC unit that yielded the positive crossmatch with patient A’s plasma also resulted in a positive AHG crossmatch when tested with plasma from five other patients who all had negative antibody screens.
What is the most likely explanation?
What is the most likely explanation?
- Patient A has an antibody against a low frequency RBC antigen
- Patient A has an autoantibody
- The donor of the reactive RBC unit has an alloantibody
- The donor of the reactive RBC unit has a warm autoantibody
Board review style answer #2
D. The donor of the reactive RBC unit has a warm autoantibody
Comment Here
Reference: Coombs test / DAT
Comment Here
Reference: Coombs test / DAT
COVID-19 convalescent plasma
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Symptoms | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Sample assessment & plan | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Convalescent plasma is obtained from a blood donor who has recently recovered from the relevant infection
- Considered experimental or investigational and must be approved by the FDA for each disease entity
- Currently approved under an Emergency Use Authorization (EUA) (see Essential features for eligibility criteria)
- Originally permitted as an investigational new drug (IND) by the FDA on March 27, 2020; patients who have recovered from COVID-19 are eligible to donate convalescent plasma after they have been symptom free for 14 days, if they 1) have proof of infection (positive PCR by nasopharyngeal swab or positive antibody titer), and 2) are eligible for regular blood transfusion
- Effectiveness of COVID-19 convalescent plasma (CCP) in patients has not been definitively proven
- Benefit has been suggested by studies where CCP with a high titer of neutralizing antibodies is transfused to patients who do not have detectable SARS-CoV-2 antibodies in < 3 days after admission
- Benefit has been suggested where CCP is administered to inpatients with COVID-19 and concurrent immunosuppression
- Benefit has been suggested where CCP is administered to outpatients with COVID-19 who are at high risk for disease progression (Ann Intern Med 2022;175:1310)
- For disease specific information, see Lung - COVID-19 topic
Essential features
- COVID-19 convalescent plasma is an investigational blood product used to treat certain patients who are infected with the novel coronavirus
- FDA EUA has been modified multiple times, most recently December, 28, 2021; transfusion of CCP in hospitalized immunocompetent patients is not authorized due to lack of proven clinical benefit (FDA: Convalescent Plasma COVID-19 Letter of Authorization [Accessed 16 February 2023])
- EUA currently authorizes use of CCP with high titers of anti-SARS-CoV-2 antibodies for the treatment of COVID-19 patients with immunosuppressive disease or receiving immunosuppressive treatment either in the outpatient or inpatient setting (FDA: Investigational COVID-19 Convalescent Plasma [Accessed 16 February 2023])
- Donors must have evidence of COVID-19 infection, either with symptoms and a positive test by an FDA approved, cleared or authorized method or donors who have not had symptoms but test positive on 2 different tests approved, cleared or authorized by the FDA to test for SARS-CoV-2 antibodies
- Donors must be eligible for blood donation, have been COVID symptom free for 10 days and be male, never pregnant female or female who has tested negative for HLA antibodies since her last pregnancy
- Donors who have only had the vaccine are not eligible; donors who are vaccinated and have had SARS-CoV-2 are eligible if they have symptoms and test positive for SARS-CoV-2 and are within 6 months of infection
- Plasma donation must be tested for high titers of anti-SARS-CoV-2 antibodies using a test methodology approved, cleared or authorized by the FDA
- Recipients are eligible at the discretion of their healthcare provider, with documentation of consent in the patient's chart
- CCP carries the same risks as FFP or FP24: allergic transfusion reaction, transfusion related acute lung injury, transfusion associated circulatory overload, hemolytic transfusion reaction and infectious disease transmission; no risks specific to COVID-19 have been identified (J Clin Invest 2020;130:4791)
Terminology
- Convalescent plasma: plasma (FFP or FP24) that has been collected from a recently recovered patient for the purpose of transfusion to a currently infected or recently exposed patient
- COVID-19: the disease caused by the SARS-CoV-2 coronavirus, first reported in Wuhan, Hubei Province, China in 2019
- SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
- SARS-CoV-2 variants: variants of the SARS-CoV-2 virus with novel spike protein mutations that are able to evade immune response
- Variants of concern include Alpha, Beta, Gamma, Delta and Omicron
- Delta and Omicron variants have driven increases in COVID-19 cases worldwide
- CCP: COVID-19 convalescent plasma
- FFP: plasma frozen < 8 hours from collection
- FP24: plasma frozen < 24 hours from collection
- PCR: polymerase chain reaction
- DHQ: donor health questionnaire
- EUA: emergency use authorization
- EAP: expanded access protocol
- eIND: emergency investigational new drug authorization
- FDA: Food and Drug Administration
- TACO: transfusion associated circulatory overload
- TRALI: transfusion related acute lung injury
Pathophysiology
- See Lung - COVID-19 topic
Clinical features
- See Lung - COVID-19 topic
Transmission
- COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an enveloped, positive stranded RNA virus that is adapted to infect many animals, including humans
- Transmission is primarily airborne, person to person via respiratory droplets; evidence is most suggestive of short range exposure (< 6 feet), via contact with an infected person (e.g., handshake) or touching a contaminated surface (fomite transmission, e.g., door knob)
- Risk factors for airborne transmission include prolonged time in an enclosed space with an infected person (especially with shouting or singing) and inadequate air ventilation (Emerg Infect Dis 2020;26:1343)
- Physical distancing (> 1 m), hand washing, face mask use (specifically N95 masks) and eye protection are associated with decreased risk of SARS-CoV-2 infection (Lancet 2020;395:1973, BMJ 2021;375:e068302)
- Reference: CDC: Scientific Brief - SARS-CoV-2 Transmission [Accessed 16 February 2023]
Symptoms
- Fever
- Chills
- Cough
- Shortness of breath or difficulty breathing
- Headache
- Fatigue
- Muscle aches
- Loss of taste or smell
- Sore throat
- Congestion or runny nose
- Cough
- Nausea or vomiting
- Diarrhea
- Asymptomatic infection is common and re-infections are becoming more common with the rise of SARS-CoV-2 variants (Clin Infect Dis 2021;73:e2946, Lancet Infect Dis 2021;21:52, J Investig Med 2021;69:1253)
Blood donor screening
- CCP donors must:
- Have previous symptoms consistent with COVID-19
- Have either a positive COVID-19 PCR nasopharyngeal swab or positive antibody test (antibody testing can be confirmed on a sample collected with the plasma)
- Be symptom free for 10 days
- Be eligible for blood donation via all standard criteria required by the FDA (FDA: Blood & Blood Products [Accessed 16 February 2023])
- Asymptomatic individuals can be eligible to donate if they have positive antibody tests on 2 different platforms approved / cleared by the FDA (i.e., incidentally discovered during routine blood donation)
- Regular blood donors are eligible to donate (whole blood, platelets, etc.) after 28 days without symptoms, regardless of testing status
- Vaccination against SARS-CoV-2 alone does not qualify a donor for convalescent plasma donation
- Reference: FDA: Convalescent Plasma COVID-19 Letter of Authorization [Accessed 16 February 2023]
Blood donor testing
- CCP donors must pass all infectious disease testing required by the FDA
- Emergency investigational new drug (March 27 - August 23, 2020) recommended titers of ≥ 1:160 with a minimum recommendation of ≥ 1:80 (FDA: Recommendations for Investigational COVID-19 Convalescent Plasma [Accessed 16 February 2023])
- Emergency use authorization (December 28, 2021) requires the use of the following approved tests (table modified from Appendix A in FDA: Convalescent Plasma COVID-19 Letter of Authorization [Accessed 16 February 2023])
Tests acceptable for use in the manufacture of COVID-19 convalescent plasma with high titers of anti-SARS-CoV-2 antibodies Manufacturer Assay Qualifying result Date of listing under the EUA Abbott AdviseDx SARSCoV-2 IgG II (ARCHITECT and Alinity i) ≥ 1,280 AU/mL December 28, 2021 Diasorin LIAISON SARS-CoV-2 TrimericS IgG ≥ 87 AU/mL December 28, 2021 EUROIMMUN Anti-SARS-CoV-2 S1 Curve ELISA (IgG) > 55 RU/mL February 9, 2022 GenScript cPass SARS-CoV-2 Neutralization Antibody Detection Kit Inhibition ≥ 80% December 28, 2021 Kantaro COVID-SeroKlir, Kantaro Semi-Quantitative SARS-CoV-2 IgG Antibody Kit Spike ELISA > 69 AU/mL December 28, 2021 Ortho VITROS Anti-SARS-CoV-2 IgG Quantitative Reagent Pack > 200 BAU/mL December 28, 2021 Roche Elecsys Anti-SARS-CoV-2 S > 210 U/mL December 28, 2021
Donor deferral
- Potential donors who have received convalescent plasma as part of their treatment are deferred for 3 months, consistent with blood transfusion deferral guidelines recently updated by the FDA (FDA: Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products [Accessed 16 February 2023])
- Donors who received a monoclonal antibody therapy (investigational or approved) for COVID-19 are deferred for 3 months after receipt of therapy
- Individual blood collection centers and in-hospital research protocols (investigational new drug) may have additional restrictions at their discretion
Laboratory
- Convalescent plasma must be stored at ≤ 18 °C and expires in 1 year from the time of collection
- Convalescent plasma must be labeled as an investigational drug under the FDA
- Patients should receive type compatible plasma; if type compatible is not available, incompatible plasma with a low titer of anti-A or anti-B (≤ 1:128) may be considered, although the impact of this has not been studied
- Reference: FDA: Investigational COVID-19 Convalescent Plasma [Accessed 16 February 2023]
Case reports
- 5 cases of COVID-19 treated with convalescent plasma in Wuhan, China (JAMA 2020;323:1582)
- 6 cases of COVID-19 treated with convalescent plasma in Wuhan, China (J Med Virol 2020;92:1890)
- 10 cases of COVID-19 treated with convalescent plasma (Proc Natl Acad Sci U S A 2020;117:9490)
- First 25 patients treated with convalescent plasma in Houston, Texas, United States (Am J Pathol 2020;190:1680)
Treatment
- Convalescent plasma has been shown to be most effective when given early (< 3 days) and with a high titer IgG dose (≥ 1:1,350) (Am J Pathol 2020;190:2290, medRxiv 2020 Aug 12 [Preprint])
- Mayo expanded access protocol enrolled over 30,000 patients at multiple sites who received at least 1 dose of CCP; the study was closed in conjunction with the FDA's emergency use authorization
- Standard dose is 200 mL (1 plasma unit), although this may be adjusted based on the size of the patient, when only low titer products are available and if the patient is part of a clinical trial with alternative or repeat dosing algorithms
Sample assessment & plan
- Assessment: 72 year old man with history of acute myeloid leukemia, recently started on induction chemotherapy, was found to have a fever up to 39.1 °C, respiratory distress with oxygen saturations 90 - 92% on room air (RA), requiring supplemental oxygen, cough and loss of taste and smell x 3 days. COVID-19 PCR positive by nasopharyngeal swab. Due to the patient's immunosuppressive therapy, convalescent plasma is warranted as treatment. I discussed this plan with the patient, including the risks of receiving a plasma blood product (transfusion related acute lung injury, transfusion associated circulatory overload, hemolysis, infectious disease, allergic reaction, which may be severe) and also discussed that this is still an investigational product under the FDA and thus the treatment may be ineffective or there may be risks that we have not yet identified. The patient expressed understanding of the risks and wishes to proceed with treatment.
- Plan:
- Type and screen, CBC with diff, CMP, PT / PTT, fibrinogen
- COVID-19 convalescent plasma 200 mL, ABO type compatible
- Isolation precautions
- Dexamethasone 6 mg daily for 10 days
- Oxygen 2 L nasal cannula (NC), titrate to maintain O2 saturation > 95%
Board review style question #1
A potential candidate for convalescent plasma donation presents to the donor center. He has a history of a confirmed positive PCR for COVID-19 performed by nasopharyngeal swab. He had mild symptoms of cough, fever and fatigue, and states his last symptoms were 9 days ago. Is he eligible to proceed with donation?
- No, he is not eligible because he also needs a COVID-19 antibody titer
- No, he is not eligible because he needs a repeat PCR to prove he is negative for COVID-19
- No, he is not eligible because he needs to be symptom free for 10 days
- No, he is not eligible to donate until he has been symptom free for 28 days
- Yes, he is eligible to donate
Board review style answer #1
C. No, he is not eligible until he has been symptom free for 10 days. Eligibility for donation for COVID-19 convalescent plasma (CCP) requires 1) confirmation of disease by either a positive PCR by nasopharyngeal swab or positive antibody test, 2) donor has been symptom free for 10 days and 3) must pass all other blood donor criteria established by the FDA. Blood donors who want to proceed with regular blood donation are eligible after 28 days of being symptom free, regardless of testing status.
Comment Here
Reference: COVID-19 convalescent plasma
Comment Here
Reference: COVID-19 convalescent plasma
Board review style question #2
A 27 year old man, who recently received a hematopoietic stem cell transplant (HSCT) for B cell acute lymphoblastic lymphoma (B ALL), blood type A negative, positive for COVID-19 by viral PCR, has been in the ICU for 3 days with bilevel positive airway pressure (BIPAP) for severe respiratory distress. His family recently heard about convalescent plasma for COVID-19 (CCP) on the news and wants him to receive this therapy. The blood bank has 1 high titer (1:1,350) CCP unit available that is blood type O positive. The clinical team wants your thoughts on how to respond to the family.
- The patient can receive the unit because it is type compatible and he qualifies based on the severity of the disease and his preexisting conditions
- The patient can receive the unit but only if the anti-A titers are ≤ 1:128 due to type incompatibility; he qualifies based on the severity of the disease and his preexisting conditions
- The patient should not receive the unit, because of the risk of anti-A in a plasma unit
- The patient should not receive the unit, because he is past 72 hours of disease, so the CCP would not be effective
- The patient should not receive the unit, because his age and preexisting conditions do not put him in a high risk category
Board review style answer #2
B. The patient can receive the unit but only if the anti-A titers are ≤ 1:128 due to type incompatibility; he qualifies based on the severity of the disease and his preexisting conditions. This patient is very ill with COVID-19, with significant risk factors (B ALL, status post-HSCT). He would likely still benefit from convalescent plasma (based on current available data). The problem is that the only available unit is not compatible with his blood type. Recall that a blood type O unit will have anti-A and anti-B in the plasma (see table). This unit is associated with a risk of causing hemolytic transfusion reaction. Although it has not been studied, it is at the discretion of the provider to allow incompatible units for the patient. In this scenario, an anti-A titer of ≤ 1:128 is recommended but may vary by institution.
Comment Here
Reference: COVID-19 convalescent plasma
| Blood type | Antigen on red cell | Antibody in plasma |
| O | None | Anti-A and anti-B |
| A | A | Anti-B |
| B | B | Anti-A |
| AB | A and B | None |
Comment Here
Reference: COVID-19 convalescent plasma
Cryoprecipitate use
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Treatment | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cryoprecipitate is the insoluble byproduct of fresh frozen plasma (FFP) when thawed at 1 - 6 °C and contains concentrated amounts of fibrinogen (factor I), factor VIII, factor XIII, von Willebrand factor and fibronectin
- Primary usage is treatment of bleeding patients with acquired fibrinogen deficiency
Essential features
- Cryoprecipitate production (see figure 1)
- Cryoprecipitate preparation:
- Whole blood derived fresh frozen plasma is thawed at 1 - 6 °C
- Centrifugation of the thawed fresh frozen plasma produces an insoluble aggregate; the insoluble protein is resuspended in approximately 15 mL of plasma, representing the cryoprecipitate (Br J Anaesth 2014;113:922)
- Remaining supernatant plasma can be transferred to a satellite bag and relabeled as "plasma, cryoprecipitate reduced"
- Primary indication of "plasma, cryoprecipitate reduced" is plasma exchange in thrombotic thrombocytopenic purpura (J Blood Transfus 2016;2016:4860284)
- Cryoprecipitate must be frozen within an hour of production at 18 °C or colder and typically is pooled before freezing for ease of use post thaw, with most pools consisting of 5 or 10 units
- Shelf life = 12 months
- Cryoprecipitate thawing:
- Frozen cryoprecipitate is thawed at 37 °C, then stored at 20 - 24 °C; the expected turnaround time in the blood bank to thaw cryo is approximately 25 - 30 minutes (Methods Mol Biol 2011;728:259)
- In adults, units of cryo are pooled and will expire at either 4 hours in an open system or 6 hours in a closed system
- Cryoprecipitate preparation:
- FDA quality assurance (QA) mandates that each unit tested contains (Br J Anaesth 2014;113:922):
- 150 mg fibrinogen
- 80 IU factor VIII
- Each unit also contains: factor XIII (~170 units/bag), von Willebrand factor (vWF) (~60 units/bag) and fibronectin
- Primary use of cryoprecipitate is treatment of patients with acquired fibrinogen deficiency, with a goal of at least 100 - 150 mg/dL in the bleeding patient
Terminology
- Cryoprecipitated antihaemophilic factor; cryoprecipitated (AHF); cryoprecipitate (cryo)
- Plasma, cryoprecipitate reduced; cryosupernatant; cryo reduced plasma (CRP)
- Fresh frozen plasma (FFP)
- von Willebrand factor (vWF)
- Plasma frozen within 24 hours after phlebotomy (PF24)
- Plasma frozen within 24 hours after phlebotomy held at room temperature up to 24 hours after phlebotomy (PF24RT24)
Pathophysiology
- Fibrinogen is a heterodimeric molecule produced in the liver and the normal concentration in plasma ranges from 2 - 4 mg/mL (Blood Rev 2015;29:17)
- Upon tissue injury, fibrinogen is acted upon by thrombin to produce fibrin monomers
- Individual monomers of fibrin polymerize and are crosslinked to other strands by FXIIIa, forming a stable, mature clot
- Low levels of fibrinogen may be found in various conditions and may be congenital or acquired (trauma induced injury, obstetric patients and certain invasive procedures)
- Fibrinogen defects may be qualitative (dysfibrinogenemia) or quantitative (hypofibrinogenemia, afibrinogenemia)
Diagrams / tables
Clinical features
- Primary indication for cryoprecipitate is hypofibrinogenemia, either acquired or congenital (Br J Anaesth 2014;113:922)
- Currently, there are 2 FDA approved human fibrinogen concentrates (RiaSTAP, Fibryga) for the treatment of congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia (Haemophilia 2020;26:25)
- Cryo may also be administered in the following circumstances as second line therapy for uremic bleeding after DDAVP failure:
- Factor VIII, von Willebrand disease, factor XIII deficiency (congenital or acquired) as a second line therapy when factor concentrates are unavailable
- Patients with disseminated intravascular coagulation (DIC) and low fibrinogen are probably best treated with a combination of fresh frozen plasma and cryoprecipitate, to minimize the risk of inducing thrombosis with transfusion of cryoprecipitate alone (Br J Anaesth 2014;113:922)
- See table 1 for primary uses of cryoprecipitate compared with fresh frozen plasma and other plasma derived products (Blood Transfus 2010;8:149, J Blood Transfus 2016;2016:4860284)
- Adverse events:
- Similar to fresh frozen plasma including transmission of blood borne pathogens and rare reported cases of cryoprecipitate associated transfusion related acute lung injury (Br J Anaesth 2014;113:922)
- Infrequently, cryoprecipitate may cause a positive direct antiglobulin test or mild hemolysis when ABO incompatible cryo is transfused in large volumes or to pediatric patients (Transfusion 2012;52:635, Vox Sang 2011;101:55)
- Sample assessment & plan
Treatment
- Fibrinogen levels of more than 50 mg/dL are considered sufficient to support physiologic hemostasis; adequate transfusion should be given to maintain the fibrinogen level above 100 mg/dL
- Each unit of cryoprecipitate must contain a minimum of 150 mg fibrinogen and 80 IU of factor VIII, representing 40 - 70% of the original amount of these factors in the original plasma (AABB: Standards for Blood Banks and Transfusion Services, 31st Edition, 2018)
- Cryoprecipitates result in a combination of high molecular weight factors, microparticles containing clotting factors and complexed lipids and lipoproteins, which result in a favorable hemostatic effect higher than the addition of the isolated factors in acquired bleeding coagulopathies
- Currently, there is not enough data to identify whether cryoprecipitates are superior or inferior to fibrinogen concentrates in the treatment of acquired hypofibrinogenemia (Br J Anaesth 2014;113:922, Blood 1986;68:307)
- Cryoprecipitate contains only 10 - 15 mL of plasma and pools of cryoprecipitates have reduced levels of anti-A and anti-B antibody and may be used universally for adult recipients (Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 2nd Edition, 2013)
- Standard adult dosing
- Cryoprecipitate, pools of 10
- Each unit will raise fibrinogen by 5 to 10 mg/dL
- Cryoprecipitate dosing for fibrinogen replacement = (fibrinogendesired (mg/dL ) - fibrinogencurrent (mg/dL )) × plasma volume × (1.0 dL/100 mL) × (1 unit cryoprecipitate/150 mg)
- Plasma volume = weight (kg) × 70 mL/kg × (1.0 - hematocrit) (Br J Anaesth 2014;113:922)
- Pediatric dosing
- 1 - 2 units/10 kg, ~100 mg/dL rise in fibrinogen
- Fibrinogen levels should be reassessed after cryoprecipitate administration to ensure a high enough dose was administered
- In adults, ABO compatible cryoprecipitate is not required
- Cryoprecipitate, pools of 10
Additional references
Board review style question #1
A 76 kg male patient is admitted to the intensive care unit for workup of a consumptive coagulopathy. Laboratory evaluation reveals a hematocrit of 35% and a fibrinogen level of 35 mg/dL (reference range: 200 - 400 mg/dL). How many units of cryoprecipitate would be necessary to raise fibrinogen to 100 mg/dL?
- 5 units
- 15 units
- 24 units
- 32 units
Board review style answer #1
B. 15 units. Calculation of cryoprecipitate dosing requires knowledge of the formula:
Comment Here
Reference: Cryoprecipitate use
- Cryoprecipitate dosing for fibrinogen replacement = (fibrinogendesired (mg/dL ) - fibrinogencurrent (mg/dL )) × plasma volume × (1.0 dL/100 mL) × (1 unit cryoprecipitate/150 mg)
- Plasma volume = weight (kg) × 70 mL/kg × (1.0 - hematocrit)
- Cryoprecipitate dosing for fibrinogen replacement = (100 mg/dL - 35 mg/dL) × (76 kg × 70 mL/kg × (1.0 - .35)) × (1.0 dL/100 mL) × (1 unit cryoprecipitate / 150 mg) = 15 units of cryoprecipitate
Comment Here
Reference: Cryoprecipitate use
Board review style question #2
Which of the following is true?
- ABO compatible cryoprecipitate is required in adult patients
- Cryoprecipitate contains concentrated amounts of factors VIII and XIII
- Cryoprecipitate when thawed is stored at 1 - 6 °C with a 24 hour shelf life
- "Plasma, cryoprecipitate reduced" contains high levels of von Willebrand factor
- There are currently no FDA approved human fibrinogen concentrates in the United States
Board review style answer #2
B. Cryoprecipitate contains concentrated amounts of factors VIII and XIII. Cryoprecipitate is produced when whole blood derived fresh frozen plasma is thawed at 1 - 6 °C, followed by centrifugation. The resulting insoluble protein is collected with ~15 mL of plasma. Cryo contains concentrated levels of fibrinogen, factor VIII, FXIII, von Willebrand factor and fibronectin. The remaining supernatant is collected and labeled as "plasma, cryoprecipitate reduced," which is deficient in von Willebrand factor as well as the other proteins present when the insoluble protein is collected from the thawed fresh frozen plasma. When cryoprecipitate is thawed, it is stored at 20 - 24 °C and expires at 4 hours or 6 hours if it was pooled in an open or closed system respectively. ABO compatibility is typically not necessary in adult patients as the amount of plasma present is minimal. There are currently 2 approved human fibrinogen concentrates in the United States.
Comment Here
Reference: Cryoprecipitate use
Comment Here
Reference: Cryoprecipitate use
DAT negative autoimmune hemolytic anemia
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Screening | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- The vast majority of cases of autoimmune hemolytic anemia (AIHA) can be separated into warm reacting, cold reacting, mixed type and paroxysmal cold hemoglobinuria (PCH), each with a classic pattern of direct antiglobulin test (DAT) positivity; however, some patients with clinical and laboratory evidence of AIHA have a negative DAT (Cohn: AABB Technical Manual, 20th Edition, 2020)
Essential features
- Between 3% and 11% of hemolytic anemia cases clinically consistent with AIHA have a negative DAT (Blood Cells Mol Dis 2014;52:152)
- High clinical suspicion of autoimmune hemolysis can guide additional testing to confirm the diagnosis and guide treatment
- Clinical course of DAT negative AIHA is similar to DAT positive cases and similar therapies are utilized
- Appropriately categorizing AIHA contributes to transfusion safety
Terminology
- Coombs negative autoimmune hemolytic anemia (AIHA)
- AIHA associated with a negative DAT
- DAT negative hemolysis
Pathophysiology
- AIHA is the process in which shortened red blood cell (RBC) survival is caused by RBC destruction via an immune response, specifically by humoral autoantibody
- RBC autoantibodies are directed toward the individual's own RBC antigens but given that these are usually high incidence antigens, the antibodies can bind not only to the individual's own cells but also those from a transfused blood component
- Presence of autoantibody alone does not necessarily cause decreased RBC survival
- It is not completely understood who will be clinically affected by autoantibodies (i.e., develop hemolytic anemia); the following may be involved (Harmening: Modern Blood Banking & Transfusion Practices, 7th Edition, 2018)
- Thermal amplitude of antibody
- IgG subclass of antibody
- Amount of antibody bound to RBCs
- Ability of antibody to fix complement
- Individual's macrophage activity
- Quantitative or qualitative change in band 3 and proteins 4.1 and 4.2 in the RBC membrane
- If hemolysis does occur and the rate of RBC destruction outpaces that of RBC production, anemia may result
- Intravascular and extravascular RBC destruction may be present
- In the vast majority of cases, autoimmune hemolysis can be confirmed with a positive DAT
- However, there are a minority of cases of AIHA with a negative DAT; these can often be explained by the following
- IgG antibody concentration below the limit of detection of standard DATs
- Low affinity IgG antibodies
- Sensitization via IgA antibodies (not routinely demonstrated using commercial reagents)
- Sensitization via warm reacting and monomeric IgM antibodies (not routinely demonstrated using commercial reagents, unless complement is also bound) (Blood 2017;129:2971)
Clinical features
- Similar to DAT positive AIHA
- Symptoms of anemia
- Fatigue
- Dyspnea
- Pallor
- Hemoglobin at presentation is often between 7 - 10 g/dL
- 33% of cases may feature hemoglobin < 7 g/dL
- Depending on coexisting conditions, may include
- Leukocytosis (chronic lymphocytic leukemia)
- Thrombocytopenia (Evan syndrome)
- Absolute reticulocyte count is normally elevated
- Coexisting conditions that limit reticulocyte response
- Iron deficiency
- Primary bone marrow disorder
- Drug effect
- Antibodies or viral effect against RBC precursors
- Haptoglobin may be low
- Lactate dehydrogenase (LDH) is high
- Indirect bilirubin is high
- Aspartate transaminase (AST) may be high
- Mean corpuscular hemoglobin is high, reflecting increased reticulocytosis
- Hemoglobin A1C may be unexpectedly low due to hemolyzed RBCs having limited time for glycation
- Peripheral blood smear may show spherocytes
- Pertinent negatives include absence of schistocytes (evidence against microangiopathic hemolytic anemias) and absence of sickle cells
- Coexisting conditions that limit reticulocyte response
- References: Hematol Oncol Clin North Am 2022;36:315, Hematol Oncol Clin North Am 2022;36:307, Blood 2021;137:1283
Symptoms
- Fatigue
- Weakness
- Dyspnea
- Pallor
- Reference: Hematol Oncol Clin North Am 2022;36:315
Screening
- Antibody screen is likely to be negative
- DAT will be negative
- There may be clinical suspicion by the patient's physician of AIHA despite a negative DAT, possibly due to the presence of anemia and reticulocytosis with no other obvious etiology
- Peripheral blood smear may demonstrate spherocytes or microspherocytes
- Hemolytic labs may suggest in vivo hemolysis; these include
- Low haptoglobin
- Elevated indirect bilirubin
- Elevated LDH
- Hemoglobinuria
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016
Laboratory
- DAT negative AIHA workup is not 1 test but a battery of tests used to evaluate potential etiologies
- This workup typically starts with more sensitive tests to identify lower concentration or low affinity IgG and if negative, expands to additional studies
- Each immunohematology reference lab (IRL) will have its own testing algorithm, often including
- Repeat DAT with more sensitive reagents (depending on reagent, fewer than 300 - 500 IgG molecules per red cell may not be detected) (Blood 2017;129:2971)
- Repeat DAT with an alternate method (e.g., gel card) and performed under differing temperatures
- Repeat DAT testing with monospecific reagents (separate reagents for anti-IgG, anti-IgA, anti-IgM, anti-C3) (Eur J Haematol 2003;70:60)
- Testing with enhancement media (e.g., polyethylene glycol (PEG) and polybrene [hexadimethrine bromide])
- Elution with concentrated plasma / serum sample
- Antibody detection with and without enzymes
- Each immunohematology reference lab (IRL) will have its own testing algorithm, often including
- Based on the suspected etiology of the negative DAT, many advanced testing options are available (Blood Cells Mol Dis 2014;52:152)
- Identifying IgG that is present but not detectable necessitates more sensitive testing methods
- Flow cytometry
- Enzyme linked anti-IgG assay
- Testing with enhancement media
- Direct polybrene test
- Direct polyethylene glycol test
- Povidone (polyvinylpyrrolidone) augmented antiglobulin test
- Eluate with papain treated red cells
- Concentrated eluate assay
- Radiolabeled anti-IgG
- Column agglutination or gel test
- Solid phase test
- Complement fixation antiglobulin consumption test
- Monocyte monolayer assay
- Identifying low affinity IgG antibodies
- DAT after 4 °C or low ionic strength saline red cell wash, to avoid the loss of antibody that can be seen with room temperature or 37 °C washing
- Flow cytometry may also identify these antibodies
- Isolated IgA or monomeric IgM warm reacting antibodies
- Test with anti-IgA antisera
- Test with anti-IgM antisera or IgM radioimmune antiglobulin test
- This testing should help to identify the rare IgM antibodies that do not bind complement
- Flow cytometry
- Natural killer (NK) cell mediated hemolysis
- 51Cr release assay (identifying NK cell destruction of autologous but not ABH matched allogeneic red cells)
- Identifying IgG that is present but not detectable necessitates more sensitive testing methods
Case reports
- 28 year old man with hypothyroidism and DAT negative, steroid refractory AIHA (Case Rep Hematol 2022;2022:6423143)
- Woman in early 30s with DAT negative AIHA secondary to mature cystic teratoma (BMJ Case Rep 2022;15:e243017)
- 53 year old woman with SARS-CoV-2 triggered, IgA mediated AIHA (Leuk Lymphoma 2021;62:2037)
- 55 year old man with pneumonia, anemia and DAT negative AIHA (J Clin Med 2020;9:3858)
Treatment
- Similar to DAT positive AIHA
- Corticosteroids (first line treatment)
- Consideration of using rituximab as first line therapy in addition to corticosteroids
- When refractory
- Splenectomy
- Rituximab
- Other immunosuppressive drugs (azathioprine, cyclophosphamide, cyclosporine, mycophenolate mofetil)
- Other therapies
- Intravenous immunoglobulins
- Danazol
- Plasma exchange
- Alemtuzumab
- High dose cyclophosphamide
- Novel therapeutics under further investigation include
- Inhibitors of neonatal Fc receptor with FcRn targeting monoclonal antibodies
- Nipocalimab
- Rozanolixizumab
- Orilanolimab
- Inhibitors of cellular mediators of phagocytosis
- Fostamatinib (splenic tyrosine kinase inhibitor)
- Inhibitors of signal transducers
- Rilzabrutinib (a reversible, covalent Bruton tyrosine kinase inhibitor)
- Inhibitors of neonatal Fc receptor with FcRn targeting monoclonal antibodies
- Reference: Semin Hematol 2015;52:304
Sample assessment & plan
- Assessment: John Doe is a 56 year old man, blood type A positive, who presented with lightheadedness and fatigue and was found to have anemia and clinical evidence of hemolysis (elevated LDH, decreased haptoglobin, indirect hyperbilirubinemia). An antibody screen was negative. Routine DAT was also negative. Peripheral blood smear demonstrated increased spherocytes.
- Plan:
- Due to clinical suspicion for autoimmune hemolytic anemia, specimen was sent out to our immunohematology reference laboratory, which demonstrated a warm reacting (IgG mediated) autoantibody following enhanced DAT testing
- If red blood cell transfusion is required, A or O, Rh(D) positive or negative, full crossmatch compatible blood will be issued
- Consider referral to hematology / oncology for management of this autoimmune hemolytic anemia
Differential diagnosis
- Congenital and acquired nonimmune hemolytic anemias:
- Hemoglobinopathies:
- Consider hemoglobin electrophoresis
- Red blood cell membranopathies:
- Consider ektacytometry or osmotic fragility or flow cytometry
- Red blood cell enzymatic deficiencies:
- Consider gene panel
- Microangiopathic hemolytic anemia:
- May see schistocytes on peripheral blood smear
- Thrombocytopenia likely present
- Paroxysmal nocturnal hemoglobinuria (PNH):
- Peripheral blood flow cytometry may help with diagnosis
- Autoimmune lymphoproliferative syndrome (American Society of Hematology: DAT-Negative Autoimmune Hemolytic Anemia - There Shall Be ALPS [Accessed 26 July 2023]):
- Physical exam may reveal splenomegaly, lymphadenopathy, a skin rash or clinical history may reveal symptoms of lymphoma such as fever or night sweats
- Elevated levels of double negative T cells
- Genetic testing for the FAS gene mutation
- May have elevated B12 levels
- Hemolytic disease of the newborn:
- Consider clinical context, such as blood type O mother and A or B blood type newborn
- Hemoglobinopathies:
- Autoimmune hemolytic anemia in someone recently transfused:
- If heavily transfused, the DAT could be falsely negative due to the predominance of donor red cells present (Hematol Oncol Clin North Am 2022;36:307)
- Reference: Hematology Am Soc Hematol Educ Program 2021;2021:331
Board review style question #1
A 7 year old boy is referred to pediatric hematology from a general pediatrician for new onset anemia with normal white blood cell (WBC) and platelet count after an upper respiratory infection. Iron studies are normal and there is no family history to suggest hemoglobinopathy, membranopathy or enzyme defect. He is found to be anemic with no other complete blood count (CBC) abnormalities. The pediatric hematologist is concerned about autoimmune hemolytic anemia and orders a direct antiglobulin test (DAT), which is negative and requests a peripheral blood smear, which shows increased spherocytes and polychromasia in the image above.
The pediatric hematologist then orders an enhanced DAT or DAT negative autoimmune hemolytic anemia workup. Which of the following is the most likely cause of the patient's apparent hemolysis and explanation for the negative DAT?
- Hereditary spherocytosis
- IgG antibody concentration below the limit of detection of standard DATs
- Lab error in the original DAT
- Microangiopathic hemolytic anemia
Board review style answer #1
B. IgG antibody concentration below the limit of detection of standard DATs. This likely represents a case of low level or low affinity IgG autoantibodies below the level of detection of a standard DAT. Answer A is incorrect because the child was previously healthy and there is no familial history of a membranopathy. Answer C, while possible, is unlikely because of the use of check cells for negative reactions at antihuman globulin phase in blood bank as a check and balance on negative results. Additionally, most DAT panels include 3 reagents: polyspecific IgG plus C3, monospecific IgG and monospecific C3. Even if there had been a problem with one of the reagents, the built in redundancy of the polyspecific reagent increases the likelihood of detecting any sort of reactivity in the DAT. Answer D is incorrect because there is no significant evidence to suggest microangiopathic hemolytic anemia; namely, there is no major increase in schistocytes, as compared to spherocytes.
Comment Here
Reference: DAT negative autoimmune hemolytic anemia
Comment Here
Reference: DAT negative autoimmune hemolytic anemia
Board review style question #2
A 55 year old woman with chronic lymphocytic lymphoma (CLL) presented with anemia and reticulocytosis, a negative routine direct antiglobulin test (DAT) for both complement and IgG, a negative red cell antibody screen, elevated lactate dehydrogenase (LDH), severely decreased haptoglobin and indirect hyperbilirubinemia. At this point, which of the following is the recommendation the blood bank medical director will most likely give to the consulting physician when contacted for assistance with evaluation for possible autoimmune hemolytic anemia (AIHA)?
- Peripheral blood smear
- Send out testing to immunohematology reference lab (IRL) for DAT negative AIHA
- Send out testing to IRL for Donath-Landsteiner testing
- Urinalysis
Board review style answer #2
B. Send out testing to the immunohematology reference lab (IRL) for DAT negative AIHA evaluation. Answers A and D are incorrect because at this point, finding spherocytes on a peripheral blood smear or evidence of hemoglobinuria on urinalysis are unlikely to provide any further information to confirm autoimmune hemolytic anemia, as there is already evidence of in vivo hemolysis. The consulting physician is likely to already have started corticosteroids for the treatment of presumed AIHA and confirmation of a DAT negative AIHA would support staying the course, as would clinical improvement with steroids. Answer C is incorrect because send out testing for a Donath-Landsteiner test would be of lesser value in this patient demographic. Unless the patient had known syphilis, paroxysmal cold hemoglobinuria (PCH) is unlikely in an elderly patient. It is most common in pediatric patients after a viral illness. It is confirmed with a positive Donath-Landsteiner test.
Comment Here
Reference: DAT negative autoimmune hemolytic anemia
Comment Here
Reference: DAT negative autoimmune hemolytic anemia
Delayed hemolytic and delayed serologic reactions
Table of Contents
Definition / general | Essential features | Pathophysiology | Clinical features | Signs and symptoms | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- A delayed hemolytic transfusion reaction is a potentially significant adverse event caused by antibody mediated removal of recently transfused red cells due to an amnestic immune response from antibodies against an incompatible minor red blood cell antigen
Essential features
- This transfusion reaction can cause an unexpected drop in hemoglobin 3 - 28 days after a transfusion and can cause clinically observed symptoms, including fever
- Caused by an amnestic immune response, where red cell alloantibodies increase in titer and trigger removal of incompatible red cells via splenic macrophages
- Diagnosis is supported by a new positive direct antiglobulin test (+IgG or +IgG and C3) and the detection of an alloantibody by antibody screen / panel or elution
- Future transfusions should involve red cells that are antigen negative for the detected alloantibody
Pathophysiology
- Prior red cell exposure through pregnancy, transfusion or transplantation → sensitization to minor red blood cell antigens → primary immune response → alloantibody formation
- Commonly implicated minor antigens: Rh (D, C, c, E, e), Kidd (Jka, Jkb), Duffy (Fya, Fyb), Kell (K, k), MNS (M, N, S, s); antibodies are usually IgG reactive at 37°C and may or may not fix complement
- Commonly implicated minor antigens: Kidd antibodies (Jk) are most common and most severe
- Alloantibody titer decreases over time with no reexposure (transfusion / pregnancy) → pretransfusion testing becomes negative (Blood 2019;133:1821)
- Reexposure to antigens by offending transfusion / pregnancy → amnestic response → production of IgG → extravascular hemolysis
- Removal of sensitized donor red cells by splenic macrophages leads to typical laboratory or clinical findings
- Reaction severity depends on the thermal range (a wider thermal range can be more severe than a narrow thermal range), antibody specificity (antibodies to some antigens, such as anti-Jk or anti-K, tend to more commonly result in hemolysis than others), and IgG subclass (the subclasses that bind well to complement and Fc receptors on phagocytic cells, such as IgG3 and IgG1, can result in more severe reactions)
- Note: this reaction can rarely be due to a primary (nonamnestic) immune response (Immunohematology 2004;20:184)
Clinical features
- Clinical symptoms and laboratory findings (see below) typically occur 3 - 10 days (up to 28 days) after a transfusion of red blood cells that originally appeared compatible by testing
- Patients have an unexpected drop in hemoglobin or less than expected rise in hemoglobin post transfusion
- Delayed hemolytic transfusion reaction (DHTR) is typically mild but may necessitate additional transfusions
- When amnestic antibody production does NOT cause detectable hemolysis = delayed serologic transfusion reaction (DSTR)
- In the U.S., DHTR incidence ranges approximately from 1:1,200 - 7,000 transfused patients (Transfusion 2010;50:1444, Transfusion 2017;57:2903)
- In one single institution study, DHTR incidence has fallen between 1999 and 2007, and was associated with a transition from polyethylene glycol (PEG) to gel technology
- DSTR is twice as likely as DHTRs (Transfusion 1995;35:26)
- Incidence of DHTR is significantly increased in sickle cell patients
- One French study found the incidence to be 4.2% per transfusion episode and 6.8% per patient (Am J Hematol 2017;92:1340)
- DHTR can mimic vasoocclusive crisis (VOC) in patients with sickle cell disease and new alloantibodies are not always detectable, hindering recognition of this reaction in these patients (Transfusion 2002;42:37)
- Patients with sickle cell disease and DHTR can also develop hyperhemolysis: hemolysis of bystander red blood cells in addition to the transfused red cells (Pediatrics 2003;111:e661)
Signs and symptoms
- Continued anemia (from unexpected drop in hemoglobin or less than expected rise in hemoglobin)
- Fever
- Chills / rigors
- Back pain
- Jaundice
- Malaise
- Renal failure (rare)
- Disseminated intravascular coagulation (rare)
- Hemoglobinuria (rare)
- Generalized pain (sickle cell disease) (Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019)
Laboratory
- Positive direct antiglobulin test with IgG or IgG / C3
- Positive eluate with the causative alloantibody
- Positive antibody screen with a newly identified alloantibody
- Positive reticulocytosis
- Positive leukocytosis
- Positive unconjugated hyperbilirubinemia
- Positive urine urobilinogen
- Positive spherocytes on peripheral smear (Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019)
Case reports
- 28 year old woman with a history of sickle cell disease and DHTR from anti-Doa and anti-N (Transfusion 2019;59:1907)
- 32 year old African American woman with a history of sickle cell disease and DHTR from anti-Joa (Immunohematology 2017;33:73)
- 44 year old woman with a history of hereditary hemorrhagic telangiectasia and DHTR from anti-e (Transfusion 2019;59:3570)
- 64 year old woman with a history of liver failure and liver transplant with DHTR from anti-e (J Clin Apher 2017;32:59)
- 83 year old woman with a history of 13 previous pregnancies and DHTR from anti-K (BMJ Case Rep 2017;2017)
Treatment
- Specific intervention usually not indicated but symptomatic or supportive management can safely be used (i.e. antipyretics for fever)
- Close communication between transfusion medicine physician and clinical team is critical to explain current and future risks
- If patient requires red blood cells before the transfusion reaction workup is completed, then benefit of transfusion must be weighed against risk of additional hemolysis
- Hydration and close monitoring are key for such cases
- After workup is completed, if clinically indicated, patient should receive antigen negative, crossmatch compatible, red blood cells that avoid the newly detected alloantibody
- Prevention is key:
- Accurate transfusion histories are critical to avoid delayed hemolytic transfusion reactions; contact other hospitals or transfusion medicine services for records
- Once a clinically significant alloantibody is identified, patient will receive red blood cell units that lack that antigen regardless of whether the antibody is detectable
- Both AABB and College of American Pathology standards mandate permanent preservation of all records of potentially clinically significant alloantibodies, as well as a review of previous records prior to red blood cell transfusion
- Patients receiving chronic therapeutic red cell transfusions should have a red blood cell phenotype completed (molecular methods are now preferred) for at least Rh, Kell, Duffy, Kidd and MNS antigens before initiation of transfusion therapy
- Patients with sickle cell disease or thalassemia should prophylactically receive red blood cells matched for C, E and K at a minimum (Blood Adv 2020;4:327)
Sample assessment & plan
- Assessment:
- This patient is a 55 year old woman with a recent history of acute gastrointestinal bleeding who received a single red cell transfusion on X/XX/20XX due to symptomatic anemia (hemoglobin: 6.2 g/dL). The transfusion workup at that time revealed no unexpected alloantibodies, a negative direct antiglobulin test and the unit prepared for transfusion was crossmatch compatible. Her hemoglobin responded appropriately to the transfusion and no adverse events were noted. On X/XX/20XX, the patient developed a fever up to 101.3°F. This was accompanied by an unexpected drop in hemoglobin (6.4 g/dL), a rise in bilirubin and a new positive direct antiglobulin test (+IgG). A new anti-K was detected by antibody screen and red cell elution. Evidence for infection or a new GI bleed has been negative to date. The red cell unit provided on X/XX/20XX was tested positive for the K antigen.
- Diagnosis:
- Delayed hemolytic transfusion reaction due to anti-K
- Plan:
- All future red cell transfusions should be negative for the K-antigen. Please be aware that increased time may be needed to procure antigen matched blood and such red cell units may not be available quickly in an emergency. Symptoms associated with a delayed reaction, such as fever, will be self limited, and can be managed symptomatically. Please contact the medical director on service if you have additional questions about this case.
Differential diagnosis
- Warm autoimmune hemolytic anemia:
- Can present with similar signs and symptoms as a DHTR
- Antibody screen and panel results will most often show panreactivity rather than antigen specificity
- Elution from a positive direct antiglobulin test will also most often show panreactivity rather than antigen specificity
- Vaso-occlusive pain crisis:
- In patients with sickle cell disease, DHTR can mimic pain crises
- Antibody screen and panel results will show no new alloantibody
- Direct antiglobulin test will likely be negative (or at least not newly positive)
- Passenger lymphocyte syndrome:
- Patient will have had a recent minor ABO incompatible organ transplant (i.e. group O liver into a group A patient)
- Anti-A or B or A, B will be found on red cells
- Malaria or Babesia infected red blood cell product:
- Patient will have symptoms typical of malaria or Babesia
- Organism will be identified within infected red cells
- Antibody screen and panel results will show no new alloantibody
- Direct antiglobulin test will be negative (or at least not newly positive)
Additional references
Board review style question #1
- A 50 year old woman received a single red cell transfusion 1 week ago due to postsurgery anemia. A second red cell transfusion is now requested from the blood bank for this patient as her anemia has returned to pretransfusion levels. She has no prior transfusion history but has had 4 previous pregnancies. Her blood bank workup 1 week ago was negative. Today, the pretransfusion blood sample is positive for a newly identified anti-C and anti-Fya. The patient's direct antiglobulin test is 3+ positive for IgG and negative for C3d. The elution demonstrates the presence of anti-C and anti-Fya. The patient's phenotype, using a sample from one week ago, is C negative and Fya negative. The unit provided a week ago is now 3+ crossmatch incompatible against the patient’s current blood sample. What is the best explanation for these findings?
- Best explanation cannot be determined because the patient has no history of prior transfusions
- Patient experienced a delayed hemolytic transfusion reaction
- Patient has a clinically significant cold agglutinin disease
- Patient has a new diagnosis of a warm autoimmune hemolytic anemia
- Patient has evidence of osmotic hemolysis secondary to G6PD deficiency
Board review style answer #1
B. Patient experienced a delayed hemolytic transfusion reaction
Comment Here
Reference: Delayed hemolytic transfusion reaction (DHTR)
Comment Here
Reference: Delayed hemolytic transfusion reaction (DHTR)
Board review style question #2
- A 75 year old man received a crossmatch compatible red cell transfusion 3 days ago. The patient had no detectible alloantibodies and received a crossmatch compatible, ABO compatible unit. The transfusion service was contacted to evaluate this patient for a possible transfusion reaction as the patient has now developed an unexpected fever, hyperbilirubinemia and jaundice 72 hours after the transfusion event. The patient's phenotype on file reveals that patient is positive for the following antigens: D, C, e, K, k, Jkb, Fya, S, M and N. If the patient’s direct antiglobulin test is now positive for IgG, which of the following alloantibodies would we most likely find in this patient's plasma?
- Anti-c
- Anti-E
- Anti-Fyb
- Anti-Jka
- Anti-s
Board review style answer #2
Donor collection
Table of Contents
Definition / general | Essential features | Terminology | Symptoms | Blood donor screening | Whole blood collection | Apheresis collection | Blood donor testing | Donor deferral | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Therapeutic blood products may be collected as whole blood and processed into components or collected by apheresis for the targeted component(s)
Essential features
- Donor collection is the process by which whole blood or individual components are collected from an eligible blood donor via phlebotomy or by apheresis, respectively
- Whole blood collection may proceed to postcollection manufacture of individual components through centrifugation, the addition of preservatives / storage solutions and appropriate labeling
- Apheresis collection targets a specific component from the outset and can be labeled with minimal postcollection manufacturing
Terminology
- Donor collection
- Whole blood collection
- Apheresis collection
- Donor history questionnaire (DHQ)
Symptoms
- Complications to apheresis or whole blood components; see Donor reactions
Blood donor screening
- Donor screened to identify whether blood donation will be safe for the individual and to ensure safety of the blood product for transfusion recipients
- Screening includes self assessment of donor following review of educational material at time of donation
- Donor given health questionnaire (DHQ) assessing current health status, travel history, behavioral risks, exposure risks, medications, pregnancy status
- Interview by screener with potential donor conducted to clarify responses to the DHQ
- If deemed eligible to donate that day, a mini physical exam is conducted that includes:
- Assessment of vital signs
- Point of care hemoglobin
- Visual inspection of phlebotomy site
- At time of collection, blood sample taken for infectious disease testing and blood typing (see Blood donor testing)
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016
Whole blood collection
- Roughly 45 - 60 minute process for above screening, collection and observance for postdonation adverse events
- Performed at fixed donor site or mobile unit
- Aseptic technique used prior to venipuncture, per establishment's protocol
- Donor is either seated upright, semirecumbent in chair or lying on cot or table
- Following venipuncture, blood specimen collected for ABO / Rh typing and infectious disease testing
- Whole blood collection of approximately 500 mL (or ~10% of the donor's blood volume) takes on average < 10 minutes
- Collections exceeding 15 - 20 minutes may not be eligible for processing into plasma containing products (Cohn: AABB Technical Manual, 20th Edition, 2020)
- Collections taking only a few minutes to complete indicate possible arterial puncture
- May be associated with pulsating needle, bright red blood entering collection bag
- Cutoff times for collection established by medical director via standard operating procedures (SOPs)
- Following collection, the collection bag is clamped, needle removed and donor is instructed to elevate arm slightly or hold direct pressure over venipuncture site with gauze
- Donor is encouraged to slowly sit up and eventually walk to canteen area for food / drink
- Staff uses this time to observe donor for any reactions (see Donor reactions)
- U.S. donors must wait 56 days prior to attempting subsequent donations (whole blood or apheresis)
- This can allow iron levels to improve
- Roughly 238 - 265 mg iron removed with each donation
- May also avoid subsequent deferral due to low hemoglobin
- May mitigate fatigue or other symptoms of iron deficiency
- U.K. donors can donate every 12 weeks and 16 weeks for males and females, respectively (Transfusion 2016;56:2005)
- In Germany and France, whole blood donations may occur every 8 and 12 weeks for males and females, respectively
- In the interval study conducted in Europe, more frequent donations were associated with increased frequency of certain symptoms (such as fatigue and dizziness) but did not have major effects on quality of life or physical and mental performance (Lancet 2017;390:2360)
- Iron supplementation has been shown to increase iron levels and avoid iron deficiency (Transfusion 2016;56:2005)
- Following collection, the whole blood collection undergoes component processing
- Blood products made from a given donation are linked to unique donor identification number (DIN)
- DIN is a federally required label to allow hospitals / blood banks to trace origin of donation
- 13 digit code unique to collection facility and individual donor
- Needed in event of transfusion transmissible infection or other biological product deviation investigation
- Whole blood may be separated into up to 4 different components, potentially:
- Red blood cells
- Plasma
- Platelets
- Cryoprecipitate
- Whole blood in primary bag is centrifuged to separate red cells, which sediment to the bottom of the bag due to their high density relative to the other components
- Platelet rich plasma (typically straw colored) settles on layer above (U.S. method of processing / centrifugation settings)
- Thin white-gray layer of white blood cells may be visible at the interface between the red cells and plasma; granulocytes cannot be donated from whole blood processing but by apheresis
- Plasma expressor (automated or manual) expresses off the plasma into a separate bag / container
- This can be frozen for fresh frozen plasma or to be further processed into cryoprecipitate (Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016)
- If platelets will be made from whole blood collection, a second spin in the centrifuge is performed to better separate the platelets from the plasma
- Platelets can later be leukocyte reduced (leukoreduced)
- Platelets combined from several donors to constitute one transfusable unit
- AABB Standards requires < 8.3 x 105 residual lymphocytes for whole blood derived platelets to be considered leukoreduced
- Following separation from the plasma, the red cells may be hung on equipment and attached to a leukoreduction filter
- Leukoreduction decreases the amount of residual lymphocytes from the red cells
- Per AABB Standards < 5 x 106 for red blood cells to be considered leukoreduced
- Reference: AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021
Apheresis collection
- Collection via automated apheresis, of which several FDA approved platforms exist
- Includes collection of platelets, red blood cells, plasma, granulocytes
- Similar requirements for whole blood donors
- Donors must be monitored for red cell loss, which cannot exceed 200 mL in 56 days
- Plasma and platelet donors must be monitored for plasma loss as well
- Collections centers must maintain ongoing records documenting the above for those donating more frequently than every 56 days and still meeting standard eligibility criteria
- Plateletpheresis:
- Collection from single donor for a platelet unit
- Per AABB Standards, 90% of sampled apheresis platelet units must have a minimum of 3 x 1011 platelets
- If a unit is below that threshold, the unit must be labeled with the platelet count (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021)
- May collect single units, double units and triple units, depending on the technology, donor weight and platelet count
- Collections may be for conventionally manufactured platelets or for pathogen reduced platelets (Cerus: How INTERCEPT Works [Accessed 27 May 2022])
- May process into platelet additive solution, limiting the residual plasma content to 33% of the original collection
- Donors may donate as frequently as every 2 days, not to exceed more than 2 days in a rolling week and cannot exceed more than 24 times in a rolling year
- Unless a directed donor or donor is a good match for a recipient with platelet refractoriness secondary to HLA or HPA antibodies, routine donors are likely to donate much less frequently
- Platelet counts may be monitored per blood center policy
- Platelet counts only required prior to donation for triple unit platelet collections
- Platelets may be collected concurrently with plasma (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021)
- Plasmapheresis:
- Infrequent donors (donate no more frequently than once a month) require same collection criteria as whole blood donors
- Cannot exceed more than 800 mL of plasma, dependent upon donor weight (McCullough: Transfusion Medicine, 5th Edition, 2021)
- Frequent donors (> monthly collections) require serum protein monitoring, which is used for source plasma donations
- Source (recovered) plasma is not transfused therapeutically at hospitals or infusion centers but manufactured into derivatives that are concentrates of specific plasma proteins (e.g., factor VIII concentrate) (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021)
- Generally, source plasma is plasma from women who have been identified as having HLA antibodies from prior pregnancy (TRALI risk, see Transfusion related acute lung injury) or because the donor is on a medication that cannot be transfused or from donors with certain disorders that do not impact the red cells. (e.g., von Willebrand disease)
- Red cells:
- Double red cells donation refers to equivalent of 2 units of packed red blood cells but from single apheresis donor
- Donor must wait 112 days before next donation (McCullough: Transfusion Medicine, 5th Edition, 2021)
- May collect equivalent of single red cell unit along with platelets or plasma by automated means
- Double red cells donation refers to equivalent of 2 units of packed red blood cells but from single apheresis donor
- Granulocytes:
- Leukocytapheresis (Transfusion 2018;58:598, Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016):
- Donors called upon for specific patients with:
- Severely low absolute neutrophil count
- Life threatening bacterial or fungal infection unresponsive to antimicrobial therapy
- Unique among cellular collections in that donor is stimulated with a corticosteroid (dexamethasone or hydrocortisone) or granulocyte colony stimulating factor (G-CSF) or both
- Repeat dexamethasone use in donors may have negative health consequences, such as cataracts (Transfusion 2011;51:921)
- G-CSF use appears to be safe (Bone Marrow Transplant 2015;50:334)
- Overall safety appears to be acceptable (J Clin Apher 2015;30:265)
- Apheresis collection occurs the next day
- Collected granulocyte product has higher hematocrit (~7.5%) compared with mononuclear cell collection (MNC) for hematopoietic progenitor cell (HPC) transplant
- Hydroxyethyl starch (HES) may be used as a sedimenting agent to aid separation of cellular layers
- Safety concerns regarding use of HES as a volume expander for treating hypovolemia
- Possible acute kidney injury, fluid retention, coagulopathy and allergic reactions (FDA: Labeling Changes on Mortality, Kidney Injury, and Excess Bleeding with Hydroxyethyl Starch Products [Accessed 27 May 2022])
- Donors called upon for specific patients with:
- Buffy coat preparation method (Vox Sang 2017:112:173):
- Used in Canada and some European countries
- Platelet concentrates produced from room temperature whole blood stored overnight, saline is added, then product is centrifuged to further separate out granulocytes
- 5 donor samples processed, purified and pooled
- Potentially better Candida albicans killing capacity
- Less risk to donors, no steroid, G-CSF or HES exposure
- Leukocytapheresis (Transfusion 2018;58:598, Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016):
Blood donor testing
Donor deferral
Board review style question #1
A 35 year old male blood donor takes 22 minutes to collect during a whole blood donation. Which of the following would be most likely, assuming the blood establishment has a conservative protocol regarding the whole blood collection time limit?
- His collection may be manufactured into all components: red cells, platelets and plasma
- His collection may be manufactured into plasma only
- His collection may be manufactured into platelets only
- His collection may be manufactured into red cells only
- His whole blood collection must be discarded
Board review style answer #1
D. His collection may be manufactured into red cells only. Whole blood collections exceeding 10 minutes may not be eligible for processing into plasma containing products, per blood center protocol, which affects both plasma and platelets (which are suspended in plasma).
Comment Here
Reference: Donor collection
Comment Here
Reference: Donor collection
Board review style question #2
A 50 year old woman attempts to donate whole blood. Her last whole blood donation was 40 days ago. Which of the following is she eligible to donate today?
- Double red cells by apheresis
- Plasma by plasmapheresis
- Platelets by plateletpheresis
- She is ineligible for any whole blood or apheresis donation today
- Whole blood donation
Board review style answer #2
D. Due to the red cell loss, she is ineligible for any whole blood or apheresis donation until 56 days from the date of her last whole blood donation.
Comment Here
Reference: Donor collection
Comment Here
Reference: Donor collection
Donor eligibility
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Donor requirements | Transmission | Blood donor screening | Donor deferral (not all inclusive) | Donor re-entry | Laboratory | Sample blood donor deferral letter | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Donor eligibility is established by donor history questionnaire, evaluation of vital signs and hemoglobin, brief physical examination and laboratory testing for transfusion transmissible infectious disease
Essential features
- Donor eligibility is determined by a combination of:
- Donor health / history questionnaire
- Brief physical examination
- Laboratory screening testing
- Overarching principles are to protect safety of the donor and recipient
- Screening is sensitive but not as specific
- Confirmatory testing (preferably FDA approved) needed to diagnose infectious disease
Terminology
- DHQ: donor history questionnaire
- ADHQ: abbreviated donor history questionnaire
- TTI: transfusion transmitted infection
Pathophysiology
- Donor screening is performed through donor history questionnaire, limited physical examination and laboratory testing of donor blood for transfusion transmissible infectious disease
- 2 basic principles:
- Protect the donor
- Blood donors must be:
- Feeling well on the day of donation to avoid a donor reaction
- Healthy enough to tolerate blood donation
- Adequate venous access
- Tolerance of volume loss
- Tolerance of red blood cell (RBC) oxygen carrying capacity loss
- Blood donors must be:
- Protect the recipient
- Blood donors must be (AABB: DHQ/aDHQ Medication Deferral List v2.1 [Accessed 20 July 2021]):
- Feeling well on day of donation (decreases risk of transmitting virus or subclinical bacterial sepsis)
- Free of transfusion transmitted infectious disease
- Not taking teratogenic medications
- Not taking medications that alter platelet function (if donating platelets) or clotting factors (if donating plasma containing products)
- Not currently on human immunodeficiency virus (HIV) preventive medication or HIV antiretroviral therapy
- Blood donors must be (AABB: DHQ/aDHQ Medication Deferral List v2.1 [Accessed 20 July 2021]):
- Protect the donor
Donor requirements
- Requirements for blood donation are outlined by:
- AABB (formerly American Association of Blood Banks)
- Food and Drug Administration (FDA)
- Other blood collectors
- Requirements include:
- Age
- At least 17 years old in most states
- Some states: 16 years old with parental consent
- Weight
- At least 110 lbs
- May have additional height and weight requirements for donors < 18 years old
- Vital signs
- Blood pressure
- Minimum: 90/50
- Maximum: usually 180/100
- Pulse
- Minimum: 50
- Maximum: 100
- Must be regular
- Medical director discretion if pulse is < 50 and donor is an athlete with high cardiovascular endurance
- Blood pressure
- Laboratory (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021)
- Hemoglobin (Hb) / hematocrit (Hct)
- Men: Hb > 13.0 g/dL, Hct > 39%
- Women: Hb > 12.5 g/dL, Hct > 38%
- Upper limit may be at medical director's discretion
- Iron deficiency sometimes an issue in repeat donors, especially women of childbearing age
- Education for donors on iron rich foods
- Possible iron supplementation
- Hemoglobin (Hb) / hematocrit (Hct)
- Cognitive ability to consent to donate
- Donor history questionnaire
- Absence of cancer (some medical director discretion)
- > 12 months remission / completion of treatment
- No deferral for lower risk in situ cancers like squamous cell or basal cell
- Hematologic malignancy is generally indefinite deferral
- Precancerous conditions (e.g. dysplasia of uterine cervix) if treated successfully, might not be deferral; medical director discretion
- Absence of blood disorders that could harm donor or render blood product less effective
- Coagulopathy
- Sickle cell disease
- Sickle cell trait is acceptable
- Other heterozygous alternate hemoglobin traits (e.g. hemoglobin C or E trait)
- Absence of history of transfusion transmissible infectious disease:
- HIV: indefinite deferral
- Hepatitis B virus (HBV): indefinite deferral
- Hepatitis C virus (HCV): indefinite deferral
- Human T lymphotropic virus (HTLV) I / II: indefinite deferral
- Zika virus: deferral 120 days after symptoms resolve
- West Nile virus: deferral 120 days after diagnosis
- Malaria (Centers for Disease Control and Prevention: Malaria - Blood Donor Screening [Accessed 20 July 2021])
- Travelers to endemic area: 1 year deferral
- Former residents of endemic area: 3 year deferral
- Malaria diagnosis: 3 year deferral after treatment, symptom free
- Babesiosis: deferral for at least 2 years after last positive test (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021)
- Lyme disease: deferral for 28 days after diagnosis and after completion of antibiotic therapy, whichever is longer (World Health Organization: Guidelines on Assessing Donor Suitability for Blood Donation [Accessed 20 July 2021])
- Absence of high risk behaviors for transfusion transmissible infectious disease (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021):
- High risk sexual practices (U.S. Food and Drug Administration: Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products [Accessed 20 July 2021])
- Household contact with individual with hepatitis B or C
- Absence of travel to / residence in regions with risk for (Transfusion 2017;57:1875, Vox Sang 2016;110:310, Transfusion 2020;60:694):
- Malaria (Centers for Disease Control and Prevention: Malaria - Blood Donor Screening [Accessed 20 July 2021])
- Chagas disease (Trypanosoma cruzi)
- Variant Creutzfeld-Jakob (vCJD): prior to August 2020 (U.S. Food and Drug Administration: Recommendations to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Components [Accessed 20 July 2021])
- Revising / removing prior recommendations to screen blood donors for:
- Travel risk: possible exposure to bovine spongiform encephalopathy (e.g. areas of United Kingdom and United States military bases in Europe)
- Receipt of a blood transfusion in certain vCJD risk countries
- Risk factors for iatrogenic CJD (cadaveric pituitary human growth hormone [hGH])
- Blood relatives with CJD
- Bovine insulin
- Not all emerging risks are screened for
- Absence of cancer (some medical director discretion)
- Age
Transmission
- Blood borne viruses, bacteria, parasites and, theoretically, prions may be transmitted by transfusion
- Screening and laboratory testing aims to exclude:
- Viruses: a risk with all blood products
- Bacteria: room temperature platelet products are highest risk (Centers for Disease Control and Prevention: Handling and Bacteriologic Work-up of Blood Components [Accessed 20 July 2021])
- Common bacteria include skin flora (e.g. Staphylococcus epidermidis, Staphylococcus aureus)
- Psychrophilic (cold tolerant) bacteria pose risk in refrigerated products (e.g. Yersinia enterocolitica)
- Unlikely to survive in frozen, acellular products, such as plasma and cryoprecipitate
- Parasites
- Plasmodium spp. (malaria)
- Babesia microti
- Prions (U.S. Food and Drug Administration: Recommendations to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Components [Accessed 20 July 2021], Transfusion 2017;57:1875, Vox Sang 2016;110:310, Transfusion 2020;60:694)
- Recent evidence that nonvariant Creutzfeld-Jakob Disease is not transfusion transmitted
Blood donor screening
- Sample
- Generally, about 5 test tubes drawn
- Nucleic acid testing (NAT) for infectious disease
- ABO / Rh testing and testing for syphilis antibodies
- Viral antibody markers
- Antibodies against T. cruzi (Chagas)
- Tube retained in case repeat testing is necessary
- Generally, about 5 test tubes drawn
- Screening has high sensitivity but lower specificity
- False positives are possible
- Confirmatory testing may be necessary for final disposition of donor eligibility
- See separate entry on Blood donor testing for more detail
Donor deferral (not all inclusive)
- General:
- Deferrals for safety of recipient:
- Definitive TTIs = indefinite deferral
- Self limited, high risk for TTI (e.g. one time events) used to be 1 year deferral, now usually 3 months
- Medications based on risk of teratogenicity if donated blood were to be transfused to a pregnant woman, depends on drug clearance
- Vaccine deferrals are quite variable
- Deferrals for safety of donor:
- Time needed to replenish red blood cells, plasma and platelets
- Blood collectors still have some discretion in creating policies potentially stricter than FDA
- Deferrals for safety of recipient:
- Indefinite:
- Confirmed TTI (HBV, HCV, HIV, etc.)
- Etretinate (also known as Tegison) use: psoriasis drug with teratogenic effects
- 3 years:
- Acitretin (Soriatane) use: psoriasis drug with teratogenic effects, high adipose distribution
- Immigration from malaria endemic area
- History of malaria (after becoming symptom free)
- 1 year:
- Incarceration for > 72 hours
- Residing with or sexual contact with person with hepatitis
- 6 months:
- Dutasteride (Avodart) use: drug for treating benign prostatic hyperplasia with teratogenic effects
- 3 months (most used to be a 1 year deferral) (U.S. Food and Drug Administration: Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products [Accessed 20 July 2021]):
- Exchanging sex for money or drugs
- Nonprescription injection drug use
- Sex with any of the following individuals:
- Person with any history of a positive test for HIV
- Person with a history in the past 3 months of exchanging sex for money or drugs
- Person with a history in the past 3 months of nonprescription injection drug use
- Receipt of blood product transfusion
- Mucous membrane exposure to blood / needlestick
- Tattoo (in states without health department oversight of tattoo parlors)
- History in the past 3 months of syphilis or gonorrhea or treatment for syphilis or gonorrhea
- For male donors: sex with another man
- For female donors: a history in the past 3 months of sex with a man who has had sex with another man in the past 3 months
- 4 months / 16 weeks:
- Double unit red cell collection by apheresis
- 2 months / 8 weeks:
- Whole blood donation
- 1 month / 4 weeks:
- Isotretinoin (Accutane): acne drug
- Finasteride (Proscar, Propecia)
- Live attenuated viral and bacterial vaccines
- German measles
- Varicella (chicken pox)
- 2 weeks:
- Live attenuated viral and bacterial vaccines
- Measles
- Polio (Sabin oral)
- Mumps
- Typhoid (oral)
- Yellow fever
- Live attenuated viral and bacterial vaccines
- Variable:
- Smallpox vaccine: if no complications 21 days or until scab falls off; if complications, 14 days after resolution
- 72 hours:
- Autologous whole blood donation
- > 48 hours:
- Between apheresis plasma, platelet or granulocyte donations
- 36 hours:
- Aspirin use, if platelets from donation are to be used
- No deferral:
- Toxoids
- COVID-19 vaccine
- Synthetic or killed (inactivated) vaccines, including:
- Anthrax
- Cholera
- Diphtheria
- Hepatitis A
- Hepatitis B
- Lyme
- Paratyphoid
- Pertussis
- Plague
- Pneumococcal polysaccharide
- Polio (Salk injection)
- Rabies
- Rocky Mountain Spotted Fever
- Tetanus
- Typhoid (injection)
- Medical director discretion
- Some donor eligibility issues fall outside usual categories
- Judged on case by case basis
- Assess potential risk to donor and recipient
- Reference: AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2021
Donor re-entry
- Guidances are complex
- Most up to date resources are:
- U.S. Food and Drug Administration: Blood Guidances [Accessed 20 July 2021]
- U.S. Food and Drug Administration: Nucleic Acid Testing (NAT) for Human Immunodeficiency Virus Type 1(HIV-1) and Hepatitis C Virus (HCV) [Accessed 20 July 2021]
- U.S. Food and Drug Administration: Use of Serological Tests to Reduce the Risk of Transfusion-Transmitted Human T-Lymphotropic Virus Types I and II (HTLV-I/II) [Accessed 20 July 2021]
Laboratory
- Typical findings
- Donors must be negative for screening tests
- Confirmatory testing may clarify nonspecific or false positive results
- Even false positives are deferred until negative screening testing is achieved
Sample blood donor deferral letter
Dear ### (Blood Donor Name),
In order to safeguard the blood supply, we perform a number of laboratory tests to make sure that potential donors do not have transfusion transmissible illnesses (TTI). This includes screening for various viruses and bacteria. Such testing is very sensitive but false positives may also occur. One of your tests warrants further investigation.
Please call the Blood Donor Center at (###) ###-#### between the hours of 8 a.m. to 4:30 p.m., Monday through Friday for more information.
Sincerely,
Medical Director of Transfusion Medicine
Reference: U.S. Food and Drug Administration: Guidance for Industry Donors of Blood and Blood Components: Notification of Donor Deferral Small Entity Compliance Guide [Accessed 20 July 2021]
In order to safeguard the blood supply, we perform a number of laboratory tests to make sure that potential donors do not have transfusion transmissible illnesses (TTI). This includes screening for various viruses and bacteria. Such testing is very sensitive but false positives may also occur. One of your tests warrants further investigation.
Please call the Blood Donor Center at (###) ###-#### between the hours of 8 a.m. to 4:30 p.m., Monday through Friday for more information.
Sincerely,
Medical Director of Transfusion Medicine
Reference: U.S. Food and Drug Administration: Guidance for Industry Donors of Blood and Blood Components: Notification of Donor Deferral Small Entity Compliance Guide [Accessed 20 July 2021]
Additional references
Board review style question #1
A 20 year old man schedules an appointment to donate blood at a blood drive held on his university campus. He previously donated blood while he was a high school student. On his donor questionnaire, he notes that he recently started taking isotretinoin (Accutane) for acne. What is the blood donation deferral period for taking this medication?
- 1 month after the last dose
- 1 year after the last dose
- 6 months after the last dose
- Indefinite
- No deferral
Board review style answer #1
A. Isotretinoin (Accutane) use is a 1 month deferral after the last dose due to the potentially teratogenic effects, should the donated blood be transfused to a pregnant woman.
Comment here
Reference: Donor eligibility
Comment here
Reference: Donor eligibility
Board review style question #2
A 35 year old woman presents to donate blood. Upon screening, she reveals that she was treated for Plasmodium vivax malaria after spending 3 months on a medical missions trip to Africa. Her treatment ended 6 months ago. How long should she be deferred from donating blood?
- 3 months after treatment; she has been eligible to donate for 3 months
- 3 years after treatment has ended and donor is symptom free
- 6 months after treatment; she is eligible to donate now
- 6 more months for a total of 1 year deferral after treatment
- Indefinite deferral
Board review style answer #2
B. Donors who have been diagnosed with malaria are deferred for a 3 year period after treatment and with the qualification that they are symptom free. Some forms of malaria may remain dormant in the liver and reactivate at a later time. Proper treatment is critical for preventing reactivation. A 3 year deferral takes the potential of reactivation into account. Individuals who have been successfully treated for the potential latent, liver phase of malaria are deferred for 3 years after completion of treatment. After this deferral period, provided there is no additional travel history or evidence of reinfection / reactivation of infection, these individuals are eligible for donation.
Comment here
Reference: Donor eligibility
Comment here
Reference: Donor eligibility
Donor reactions
Table of Contents
Definition / general | Essential features | Terminology | Clinical features | Symptoms | Blood donor screening | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Adverse events related to whole blood or apheresis donation
Essential features
- Complications may be localized due to phlebotomy or systemic
- Apheresis donations carry additional risk of hypocalcemia
Terminology
- Donor reactions
- Donor adverse events
- Donor adverse reactions
- Donor safety
- Donor hemovigilance
Clinical features
- Systemic / syncopal type:
- Presyncopal, prefaint
- Loss of consciousness
- Additional bodily injury from syncope related fall
- Lacerations, abrasions, head injury, fracture
- Most often vasovagal
- Decrease in blood pressure and pulse
- More common in younger donors (JAMA 2008;299:2279)
- May be caused by hypovolemia
- Decrease in blood pressure with increased pulse rate
- Phlebotomy related:
- Hematoma
- Bruising
- Arterial puncture
- Suspect if rapidly pulsating needle, bright red blood
- Infection
- Warmth, pain, erythema near phlebotomy site
- Thrombophlebitis
- Redness, swelling tracks along vein
- Upper extremity deep vein thrombosis
- Very rare; swelling, warmth, pain, possible palpable cord
- Nerve injury
- Radiating pain down arm, paresthesia
- Arm pain
- Arteriovenous fistula
- Pulsating mass, bruit on auscultation
- Hematoma
- Systemic other:
- Citrate toxicity (apheresis collections) causing hypocalcemia from anticoagulant binding calcium
- Perioral tingling, distal extremity paresthesia
- Allergic
- Localized urticaria to rare, severe anaphylactic
- Other
- Anxiousness, hyperventilation, headache
- Citrate toxicity (apheresis collections) causing hypocalcemia from anticoagulant binding calcium
- No universally accepted severity grading system at this time, although proposal in development (Transfusion 2020;60:1231)
- References: Transfusion 2008;48:1809, Blood Rev 2012;26:33, ISBT: Standard for Surveillance of Complications Related to Blood Donation [Accessed 7 May 2021]
Symptoms
- Lightheadedness
- Dizziness
- Seizure
- Loss of consciousness
- Pain at phlebotomy site
- Ecchymosis at phlebotomy site
- Palpable mass at phlebotomy site postdonation
- Extremity paresthesia
- Arm pain
- Arm weakness
- Pulsating needle during phlebotomy
- Reference: ISBT: Standard for Surveillance of Complications Related to Blood Donation [Accessed 7 May 2021]
Blood donor screening
- Blood donors are not screened specifically for risk factors for donor reactions
- Must be 16 or 17 years old, as determined by state regulations in the U.S.
- Maximum donation frequency, e.g. every 56 days for whole blood
- Provided information on donation process, infectious disease testing, potential reactions
- Screened for overall blood safety using the Donor History Questionnaire (DHQ) as published by the AABB and accepted by the FDA:
- General state of health
- High risk behavior for infectious disease
- Needle sticks / recent tattoos
- Travel history
- Medical conditions
- Recent vaccinations
- Medications
- Donors are also screened by a mini physical exam:
- Point of care hemoglobin
- Blood pressure
- Pulse
- Temperature
- Skin integrity around anticipated phlebotomy site (typically antecubital fossa)
- Interview with screener for clarification of any "yes" responses or concerns
- Reference: Fung: Technical Manual, 19th Edition, 2017
Case reports
- 20 year old woman with upper extremity deep venous thrombosis following whole blood donation (Transfusion 2004;44:586)
- 23 year old man with laceration / fracture following whole blood donation (Asian J Transfus Sci 2019;13:60)
- 26 year old man with mechanical hemolysis from apheresis donation (Transfus Apher Sci 2018;57:277)
- 40 year old woman with severe citrate toxicity from apheresis donation (J Clin Apher 2007;22:15)
- 49 year old woman with pseudoaneurysm following whole blood donation (Transfusion 1994;34:253)
Treatment
- Presyncopal / syncopal events are generally managed by:
- Placing individual in supine position with feet elevated (Trendelenburg position), if possible or having seated patient cross and flex legs (Prehosp Emerg Care 2020;24:64)
- Mitigation strategies include ingesting water or hypertonic fluids to increase predonation blood pressure (Transfusion 2011;51:2727)
- > 6 hours sleep predonation and increasing muscle tension near the end of the donation have also been associated with fewer vasovagal reactions (Transfus Med Hemother 2014;41:284)
- If hypovolemic, oral fluids may be encouraged once the patient is stable
- Allergic reactions typically treated with antihistamines if mild; potentially epinephrine if severe (Am Fam Physician 2017;95:717)
- Phlebotomy related events are typically managed with pressure, compression, elevation of the arm or ice
- Hematoma is fairly common in both whole blood and apheresis donors
- Up to 23% if donor follow up occurs weeks after donation (Transfus Med Rev 2013;27:44)
- Treat with compression, including compression dressing if necessary and ice (Wyatt: Oxford Handbook of Emergency Medicine, 5th Edition, 2020)
- Nerve injury rarely leads to long term sequelae but occasionally requires recovery times of several months
- In one study, 30% of donors with nerve injury required at least a month for symptom resolution
- Such extenuating cases may require physician consultation (Transfusion 1996;36:213)
- Hematoma is fairly common in both whole blood and apheresis donors
- Citrate toxicity or hypocalcemia is usually addressed with oral calcium (Popovsky: Transfusion Reactions, 4th Edition, 2012)
- More severe reactions may be evaluated by the medical director if on site, urgent care or emergency medical system
Board review style question #1
A 22 year old female whole blood donor complained of lightheadedness at the end of her donation, while still recumbent in the collection chair. Her 510 mL blood collection took approximately 11 minutes. Her predonation blood pressure was 116/74 and pulse was 74. Following the incident, her blood pressure was found to be 80/50 and her pulse was 85. Which of the following is the most likely donor reaction and recommended treatment?
- Allergic reaction, antihistamines
- Arterial puncture, direct pressure over the phlebotomy site
- Citrate toxicity, oral calcium carbonate
- Vasovagal reaction, Trendelenburg positioning
Board review style answer #1
D. Vasovagal reaction, Trendelenburg positioning. This is consistent with a mild vasovagal reaction and often responds to repositioning the patient with feet above the level of the heart. Alternatively, bearing down to increase muscle tension to increase blood return to the heart may also help, especially if the patient is sitting upright in a chair that does not recline. Allergic reactions (answer A) would most often manifest with pruritus / itching or hives. An arterial puncture (answer B) would cause the needle to rapidly pulsate with bright red blood during collection and the collection bag would fill within a few minutes. Hypocalcemia from citrate toxicity (answer C) occurs during automated collections (such as apheresis component collections or apheresis source plasma collections) and will usually respond to oral calcium supplementation.
Comment Here
Reference: Donor reactions
Comment Here
Reference: Donor reactions
Board review style question #2
During a plateletpheresis collection, a 30 year old man complains of numbness and tingling around his mouth and cramping of his muscles in his hands and feet. He has no significant change in vital signs predonation versus immediately after symptom onset. What is the most likely explanation for this type of donor reaction?
- Hyperventilation leading to lowered bicarbonate levels
- Hypocalcemia from citrate anticoagulant binding calcium
- Hypovolemia from dehydration
- Sensory nerve injury from phlebotomy
Board review style answer #2
B. Hypocalcemia from citrate anticoagulant binding calcium. The symptoms are most compatible with citrate toxicity. The anticoagulant necessary to prevent extracorporeal blood clotting in the apheresis tubing binds calcium, leading to these symptoms of hypocalcemia. Hyperventilation (answer A) may also cause numbness and tingling in the extremities. However, citrate toxicity is more common during an automated apheresis collection. Hypovolemia (answer C) would most likely lead to the sensation of lightheadedness and a decrease in blood pressure with increase in heart rate. Nerve injury (answer D) would cause paresthesia in the ipsilateral arm where phlebotomy was performed.
Comment Here
Reference: Donor reactions
Comment Here
Reference: Donor reactions
DTT for monoclonal antibody (datatumumab) workup (pending)
[Pending]
Duffy system
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Duffy blood group antigens are found on a glycoprotein on the surface of red blood cells and other cells in the body
- Antibodies clinically significant
Essential features
- Duffy antigens are common in whites but uncommon in the black population
- Antibodies can cause hemolysis and hemolytic disease of fetus and newborn (HDFN)
- Duffy acts as a receptor for malaria species, specifically Plasmodium vivax
- Mutations in the GATA1 promoter gene block red cell localization of Fyb
Terminology
- Duffy a, Fya or Fy1
- Duffy b, Fyb or Fy2
Pathophysiology
- GATA1 acts as a promoter for transcription of the Fyb antigen
- Point mutations in GATA1 disrupt binding of an erythroid transcription factor, which leads to a block in expression of Fyb in red cells (Fyb silent allele)
- As Fyb is expressed on other tissues throughout the body, these individuals should not produce Fyb antibodies
- Reference: Fung: Technical Manual, 19th Edition, 2017
Clinical features
- Duffy acts a receptor for P. vivax and P. knowlesi
- Antigens (type: peptide)
- 2 common antigens: Fya and Fyb, which are prevalent in the white population but less commonly expressed on red blood cells in individuals of African descent as a result of evolutionary pressures caused by malaria (Fung: Technical Manual, 19th Edition, 2017)
- Duffy glycoprotein is encoded by the ACKR1 (atypical chemokine receptor 1) gene on chromosome 1q21-q22 and acts as a transmembrane chemokine receptor (Fung: Technical Manual, 19th Edition, 2017)
- Duffy protein acts as a receptor for P. vivax and P. knowlesi; emerging data demonstrates that Duffy negative individuals can still be infected by P. vivax (Hematology 2006;11:389, Malar J 2020;19:229)
- Individuals of African descent commonly have mutations in the GATA1 gene, which normally acts as a promoter for erythroid transcription of the Fyb gene
- Inactivating GATA1 mutations turns off Fyb antigen expression on red cells only while other tissues continue to express it
- These individuals do not form Fyb antibodies and are protected from alloimmunization (Fung: Technical Manual, 19th Edition, 2017)
- True Duffy null individuals can form antibodies to Fy3, Fy4, Fy5 and Fy6, with Fy3 and Fy5 being clinically significant (Fung: Technical Manual, 19th Edition, 2017)
- The genes for Duffy may play a role in maintaining adequate neutrophil counts and there is an association between Duffy null individuals and benign ethnic neutropenia (BEN) (Blood Rev 2019;37:100586)
Adapted from Fung: Technical Manual, 19th Edition, 2017Race / Ethnicity Fy(a+b-) Fy(a+b+) Fy(a-b+) Fy(a-b-) White 20 48 32 Rare Black (U.S.) 10 3 20 67
- Many individuals of African descent have the GATA1 mutation and an Fy(a-b+) genotype without phenotypic Fyb expression
- Antibodies
- Majority IgG1 (Fung: Technical Manual, 19th Edition, 2017)
- Occur with exposure to products containing incompatible blood or pregnancy; rarely naturally occurring (Fung: Technical Manual, 19th Edition, 2017)
- Anti-Fya antibodies are most common (Fung: Technical Manual, 19th Edition, 2017)
- Fy3, Fy4, Fy5 and Fy6 antibodies can be formed in Duffy null individuals; Fy3 and Fy5 are clinically significant
Transmission
- Exposure to Duffy antigens secondary to pregnancy or transfusion
Laboratory
- Serologic phenotyping must be taken to the antihuman globulin phase and cannot be performed in the presence of a positive direct antiglobulin test (DAT); there has been interest in developing antibodies as a workaround (Br J Haematol 2004;126:277, Immunohematology 2006;22:161)
Case reports
- 24 year old woman with HDFN due to multiple alloantibodies, including Fya (Asian J Transfus Sci 2020;14:83)
- 69 year old woman with Fya antibodies who expired due to TRALI (Transfusion 2021;61:1336)
- 77 year old man with delayed hemolytic transfusion reaction with Fya antibodies successfully treated with automated red blood cell exchange (Arch Pathol Lab Med 2013;137:861)
- 10 cases of P. vivax in Duffy negative individuals (Am J Trop Med Hyg 2018;99:1128)
- Blood donor found to have novel Duffy antigen (Transfusion 2019;59:2158)
Additional references
Board review style question #1
A patient is found to have a Fya antibody. Blood from what group of donors would most likely be compatible?
- Asian
- Black
- Native American
- White
Board review style answer #1
B. Black. Negativity for Duffy antigens is most common in African individuals due to environmental pressures exerted by malaria. Fya antigen positivity is common in White, Asian and Native American populations.
Comment Here
Reference: Duffy system
Comment Here
Reference: Duffy system
Board review style question #2
Duffy acts as a receptor for which species of Plasmodium (malaria)?
- P. falciparum / P. ovale
- P. malariae
- P. ovale
- P. vivax
Board review style answer #2
Enzyme treatment (pending)
[Pending]
Ethics (pending)
[Pending]
FACT compliance (pending)
[Pending]
Febrile nonhemolytic
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Laboratory | Case reports | Treatment | Prevention | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Acute reaction that occurs during or within 4 hours of cessation of blood product transfusion
- Otherwise unexplained fever ≥ 38 °C (100.4 °F) and a change of at least 1 °C (1.8 °F) from pretransfusion value or chills / rigors
Essential features
- Common acute transfusion reaction, roughly 0.62% of transfusions (Transfusion 2016;56:2587)
- Important to treat as possible serious transfusion reaction (hemolytic, TRALI, etc.) until workup is complete, as these symptoms are identical (fever, chills / rigors, vital signs changes)
- Most common complication of platelet transfusion (115.3 FNHTRs per 100,000 components transfused)
- Incidence depends on
- Extent of reporting (active versus passive)
- Type of product transfused (platelet, red blood cell)
- Blood product modifications (leukocyte reduced, platelet additive solution, washed)
- Patient risk factors (higher risk in patients with hematologic disease and HLA alloimmunization from prior transfusions or pregnancy)
- Pretreatment of recipients with antipyretics
- Signs and symptoms must occur within 4 hours of transfusion
- Reaction due to cytokine release most commonly by activated donor leukocytes
- Diagnosis of exclusion
- Unrelated to underlying medical conditions
- Other transfusion reactions (hemolysis, sepsis, transfusion related acute lung injury, etc.) ruled out
Terminology
- Direct antiglobulin test (DAT) / Coombs test
- Febrile type reaction
- Febrile nonhemolytic transfusion reaction (FNHTR)
- Human leukocyte antigen (HLA)
- Human platelet antigen (HPA)
- Leukocyte reduced / leukoreduction (LR)
- Platelet additive solution (PAS)
- Platelet (PLT)
- Red blood cell (RBC)
- Transfusion related acute lung injury (TRALI)
Pathophysiology
- Pyrogenic / inflammatory cytokines (e.g. IL1β, IL6, TNFα) released from activated leukocytes, commonly from donor during product storage
- Cytokines lead to production of prostaglandin E2, causing the hypothalamus to increase the body temperature
- Nonimmune mediated usually associated with platelet transfusions
- Passive transfusion of accumulated inflammatory cytokines in the plasma of the transfused unit
- Immune mediated usually associated with red blood cell transfusions
- 2 general mechanisms:
- Recipient antibodies (mainly anti-HLA) activates donor leukocytes to release cytokines
- Recipient leukocytes are activated to release cytokines by immune complexes formed from recipient antibodies and donor leukocytes
- Other non-HLA antibodies have been implicated, including those directed against human platelet antigens (Clin Chim Acta 2017;474:120)
- 2 general mechanisms:
- Nonimmune mediated usually associated with platelet transfusions
Clinical features
- Not a life threatening condition
- See symptoms below
Symptoms
- Fever
- Chills / rigors
- Respiratory changes
- General discomfort
- Nausea / vomiting
- Headache
- Reference: CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 16 June 2020]
Laboratory
- No specific tests available
- Reaction workup negative (clerical check, direct antiglobulin test, visual hemolysis inspection, ABO confirmation)
- Hemolysis laboratory markers unremarkable
- Bacterial contamination ruled out
Case reports
- 19 year old woman with thalassemia and asymptomatic malaria has an unusual manifestation of delayed febrile posttransfusion reaction mimicking FNHTR (Transfusion 2011;51:469)
- 64 year old man with history of allogeneic hematopoietic stem cell transplantation with complication after receiving 1 red blood cell unit (Transfus Med Hemother 2019;46:384)
Treatment
- Stop transfusion immediately
- Notify blood bank / transfusion medicine service for reaction workup (direct antiglobulin test, visual inspection for hemolysis, etc.)
- Close observation with frequent vital signs
- Fever usually self limiting
- Antipyretics (e.g. acetaminophen) for symptom relief
- Meperidine for severe reactions with rigors
- Reference: Lancet 2016;388:2825
Prevention
- Prestorage leukoreduction (Transfusion 2004;44:25, Ann Med Health Sci Res 2015;5:185)
- May consider acetaminophen if persistent fever due to underlying disease or past history of FNHTR (Transfusion 2008;48:2285)
- Routine pretreatment with acetaminophen has limited preventative benefits and may obscure more serious transfusion complications
- Use of platelet additive solution (Transfusion 2014;54:1927)
- Plasma reduced or washed products if severe reactions (Transfusion 2002;42:556)
- Restrictive transfusion approach in high risk patients (Transfusion 2017;57:1674)
Sample assessment & plan
- Assessment: Jane Doe is a 26 year old female with a history of menorrhagia, anemia and mild fatigue. She received 1 unit of red blood cells that was well tolerated with stable vital signs throughout. 30 minutes after completion, she spiked a temperature of 101.3 °F with no other complaints. She was given acetaminophen and blood bank was notified for a transfusion reaction workup. The clerical check revealed no incompatibility. Direct antiglobulin test and antibody screening were both negative with an unremarkable visual inspection for hemolysis. Her temperature returned to baseline (98.9 °F) within 1 hour. The reaction is most appropriately classified as a febrile nonhemolytic reaction with no clinical sequelae.
- Plan: Ms. Doe is cleared for additional blood product transfusions if clinically indicated. May consider pretreatment with acetaminophen prior to future transfusions. Please continue to report any suspected transfusion reactions to the blood bank.
Differential diagnosis
- Hemolytic transfusion:
- Hemoglobinemia, hemoglobinuria, hypotension, flank pain, pain at needle site, unexpected bleeding
- Lab tests consistent with hemolysis
- Septic transfusion reaction:
- High and persistent temperature (temperature increase ≥ 2 °C)
- Signs of bacterial infection
- Unit culture positive
- Transfusion related acute lung injury:
- Hypoxemia
- Radiographic evidence of bilateral lung infiltration
Additional references
Board review style question #1
A 61 year man with end stage renal failure who received kidney transplantation 1 year ago was transfused 1 unit of red blood cells. About 1 hour into the transfusion, he complained of chills. The transfusion was stopped immediately. Upon examination, he appears uncomfortable. His vital signs are as follows: heart rate 78 beats/min, blood pressure 140/80 mmHg, respiratory rate 17/min, temperature 100.9 °F, SaO2 98%. His pretransfusion vital signs are heart rate 76 beats/min, blood pressure 145/80 mmHg, respiratory rate 18/min, temperature 98.9 °F, SaO2 99%. Transfusion workup confirms that he was transfused an ABO identical RBC unit, serum was pale yellow, DAT negative. Which of the following is the most likely cause of his symptoms?
- Febrile nonhemolytic transfusion reaction
- Hemolytic transfusion reaction
- Septic transfusion reaction
- Transfusion related acute lung injury
Board review style answer #1
A. Febrile nonhemolytic transfusion reaction
Comment Here
Reference: Febrile nonhemolytic transfusion reaction (FNHTR)
Comment Here
Reference: Febrile nonhemolytic transfusion reaction (FNHTR)
Board review style question #2
The previous patient was given a blanket to keep warm. His symptoms resolved an hour later. Which of the following blood product modifications would be the most effective way to decrease the possibility of the same type of transfusion reaction in the future?
- Leukocyte reduction before storage
- Leukocyte reduction before the transfusion
- Radiating the product
- Washed product
Board review style answer #2
A. Leukocyte reduction before storage
Comment Here
Reference: Febrile nonhemolytic transfusion reaction (FNHTR)
Comment Here
Reference: Febrile nonhemolytic transfusion reaction (FNHTR)
Fetal / neonatal alloimmune thrombocytopenia
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Screening | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, potentially life threatening bleeding disorder affecting the fetus / neonate
- Caused by maternal IgG antibodies targeting fetal / neonatal platelet antigens inherited from the father
Essential features
- Thrombocytopenia in neonate with or without associated fetal / neonatal bleeding
- Confirmation of maternal platelet antibody(ies) directed at paternally inherited platelet antigen(s)
- May affect fetus / neonate in first pregnancy
- May see increasing severity in subsequent pregnancies
- References: Cohn: AABB Technical Manual, 20th Edition, 2020, Br J Haematol 2013;161:3
Terminology
- Fetal neonatal alloimmune thrombocytopenia (FNAIT)
- Neonatal alloimmune thrombocytopenia (NAIT)
- Fetomaternal alloimmune thrombocytopenia (FMAIT)
- Neonatal alloimmune thrombocytopenic purpura (NATP)
Pathophysiology
- Platelet antigen nomenclature defined by the International Society for Blood Transfusion, Platelet Immunobiology Working Group (ISBT: Platelet Immunobiology [Accessed 22 March 2021])
- Human platelet antigens (HPA) named in chronological order of discovery with numerical nomenclature
- Human platelet antigen systems are biallelic
- High incidence allele designated as "a"
- Low incidence allele or polymorphism designated as "b"
- Molecular testing (e.g. PCR) can distinguish the antigens
- Human platelet antigens are located on platelet membrane glycoproteins or glycoprotein complexes
- Fetal neonatal alloimmune thrombocytopenia results from maternal alloimmunization during pregnancy or transfusion
- IgG antibodies cross the placenta and destroy corresponding platelets
- Antibodies against the antigens below are most commonly implicated (Transfusion 2004;44:1220):
- HPA1a ≈ 80% (Caucasian population) (i.e. mother homozygous for HPA1b develops anti-HPA1a antibodies against the paternally derived HPA1a allele on her offspring’s platelets)
- HPA5b ≈ 10% (Caucasian population)
- HPA4a more common in Asians (Transfus Med Rev 2008;22:255)
- Antibodies against human leukocyte antigens not known to cause fetal neonatal alloimmune thrombocytopenia
- Affected infants are thrombocytopenic and at risk of major bleeding complications, including intracranial hemorrhage
- References: Cohn: AABB Technical Manual, 20th Edition, 2020, Br J Haematol 2013;161:3
Clinical features
- Severe fetal / neonatal thrombocytopenia is most commonly caused by fetal neonatal alloimmune thrombocytopenia
- Affected infant may present with petechial hemorrhages, purpura and an extremely low platelet count
- Pregnant women not routinely screened for platelet antibodies
- Might not be known until birth of affected neonate
- References: Cohn: AABB Technical Manual, 20th Edition, 2020, Br J Haematol 2013;161:3
Symptoms
- Asymptomatic fetus
- Symptomatic fetus with:
- Intracranial hemorrhage, identified by ultrasound
- Asymptomatic neonate
- Neonate with:
- Bleeding
- Genitourinary hemorrhage
- Ocular hemorrhage
- Intraabdominal hemorrhage
- Pulmonary hemorrhage
- Spinal cord hemorrhage
- Intracranial hemorrhage
- Petechiae
- Purpura
- Reference: Transfusion 2016;56:1230
Screening
- Consider if neonate born with low platelet count
- When fetal neonatal alloimmune thrombocytopenia is considered in the differential diagnosis, testing may include:
- Maternal serum HPA antibody testing
- Maternal platelet genotyping
- Paternal platelet genotyping
- May monitor pregnancy if sibling with:
- History of fetal neonatal alloimmune thrombocytopenia
- Antenatal intracranial hemorrhage
- Unclear if screening more effective than simply treating mother and neonate when symptoms develop (BJOG 2019;126:e173)
Laboratory
- Diagnosis made when:
- HPA antibodies detected in maternal serum
- Corresponding antigen detected from paternal testing via HPA genotyping
- Less often, corresponding antigen detected by HPA genotyping of fetus
- Via chorionic villus sampling after 11 weeks gestational age
- Via amniocentesis after 16 weeks gestational age (BJOG 2019;126:e173)
- Maternal platelet count is normal, as opposed to immune thrombocytopenic purpura, where maternal count is also low
Case reports
- Neonates with fetal neonatal alloimmune thrombocytopenia with severe bleeding complications other than intracerebral hemorrhage (Transfusion 2016;56:1230)
- Newborn twin girls with fetal neonatal alloimmune thrombocytopenia, presumably from passive human leukocyte antigen antibodies (BMJ Case Rep 2017;2017:bcr2016218269)
- Term infant with fetal neonatal alloimmune thrombocytopenia secondary to maternal antibodies to HPA15 (Pediatrics 2018;141:S506)
- 27 year old pregnant woman with antenatal diagnosis of intracranial hemorrhage from fetal neonatal alloimmune thrombocytopenia (BMJ Case Rep 2015;2015:bcr2014209130)
- 43 year old woman with first pregnancy complicated by fetal neonatal alloimmune thrombocytopenia due to HPA5a antibodies (Transfus Apher Sci 2020;59:102880)
Treatment
- Prevention not possible at present
- Antenatal management:
- Intravenous immune globulin (IVIG) offered to at risk women
- Some offer to mother starting at 18 weeks gestation
- Others risk stratify mother based on prior pregnancy history:
- Very high risk: intracranial hemorrhage or platelets < 20 x 109/L in prior pregnancy
- Extremely high risk: thrombocytopenia and intracranial hemorrhage prior to 28 weeks gestation in prior pregnancy
- High risk: thrombocytopenia only in prior pregnancy
- No difference if IVIG alone or in combination with corticosteroids (Obstet Gynecol 2006;107:91)
- Intravenous immune globulin (IVIG) offered to at risk women
- Postnatal treatment of symptomatic neonates
- Thrombocytopenic neonates with or without bleeding
- Platelet threshold for transfusion varies by study:
- < 35 x 109/L or < 50 x 109/L
- Majority of intracranial hemorrhage occurs < 30 x 109/L
- Platelet threshold for transfusion varies by study:
- Platelet transfusions
- If the HPA antigen is known, HPA antigen negative platelets
- Both washed, irradiated platelets or HPA selected platelets effective (led to higher posttransfusion platelet counts)
- HPA selected more effective than unselected
- Random platelets
- Safe option; should not delay transfusion to infant trying to obtain HPA selected or washed maternal platelets (Br J Haematol 2019;185:549)
- Washed, irradiated maternal platelets
- May be difficult to obtain due to timing, ability to perform plateletpheresis collection on the mother
- Platelet transfusion plus IVIG but no clear benefit of adding IVIG (J Perinatol 2019;39:1329)
- If the HPA antigen is known, HPA antigen negative platelets
- Washed, irradiated platelets or HPA selected platelets led to higher posttransfusion platelet counts
- However, platelet transfusion of any kind resulted in clinical hemostasis
- More studies required to assess benefit of platelet transfusion with intravenous immune globulin in neonate
- Thrombocytopenic neonates with or without bleeding
- References: Transfus Apher Sci 2020;59:102704, BJOG 2019;126:e173
Sample assessment & plan
- Assessment:
- Patient is a 25 year old gravida 2, para 1 (G2P1) who previously delivered a neonate affected by fetal neonatal alloimmune thrombocytopenia without intracerebral hemorrhage
- Father in the previous pregnancy is heterozygous for the incompatible platelet antigen and is the father of the current pregnancy
- Mother is currently at 10 weeks gestation and given her history is being evaluated for a second pregnancy potentially affected by fetal neonatal alloimmune thrombocytopenia
- Plan:
- Close monitoring and management by maternal fetal medicine
- Start intravenous immunoglobulin at approximately 12 - 16 weeks gestation
- Fetal blood sampling may occur during the pregnancy but should not be automatic in all cases due to the risks associated with this invasive procedure
- Fetal HPA status can be determined by typing fetal DNA for platelet antigens, amniocentesis at 15 - 16 weeks of gestation, fetal blood is not necessary
- Ultrasound examinations to look for fetal intracranial hemorrhage beginning at 16 to 20 weeks and occurring every 4 - 6 weeks until delivery
- In viable pregnancies, early delivery or C section may be necessary to allow treatment of fetal / neonatal thrombocytopenia
Differential diagnosis
- Maternal immune thrombocytopenia (ITP):
- Check maternal platelet count
- Immune thrombocytopenic purpura may be considered when maternal thrombocytopenia present
- Infection:
- Newborn likely ill with fever
- Increased white blood cell count
- Blood cultures, serology or PCR likely positive for infectious organism
- Disseminated intravascular coagulopathy:
- Newborn likely ill with multiorgan failure
- Complete blood count may show increased white blood cell count
- Elevated coagulation studies
- Decreased fibrinogen
- Positive D-dimer
- Some congenital / inherited syndromes, such as:
- Thrombocytopenia absent radii (TAR) syndrome
- Suspect if skeletal abnormalities of thumb, radii
- Wiskott-Aldrich:
- X linked recessive primary immunodeficiency disorder
- Suspect if male with thrombocytopenia, decreased mean platelet volume, eczema
- Thrombocytopenia absent radii (TAR) syndrome
- Congenital leukemias:
- Suspect if increased blasts identified on peripheral blood smear
- Flow cytometry, bone marrow biopsy, cytogenetic studies to confirm
- Hemangiomas:
- Suspected based on skin lesion(s)
- Pseudothrombocytopenia from platelet clumping:
- If identified on capillary sample, repeat platelet count from venous sample
- Can review peripheral blood smear for platelet clumping
- References: Gomella's Neonatology, 8th Edition, 2020, Neonatology: A Practical Approach to Neonatal Diseases, 2nd Edition, 2018
Board review style question #1
26 year old gravida 1, para 1 (G1P1) patient gives birth to a neonate born with petechiae and a platelet count of 15 K/uL. Fetal neonatal alloimmune thrombocytopenia is suspected and paternal platelet antigen genotyping is performed, as is maternal HPA antibody testing and maternal platelet antigen genotyping. The 2 most common HPA antigens associated with this diagnosis are
- HPA1a and HPA1b
- HPA1a and HPA3b
- HPA1a and HPA5b
- HPA3a and HPA5b
- HPA5a and HPA5b
Board review style answer #1
C. HPA1a and HPA5b are the 2 most common antigens to which maternal antibody is directed. However, fetal neonatal alloimmune thrombocytopenia can be linked to any HPA antigen.
Comment Here
Reference: Fetal / neonatal alloimmune thrombocytopenia
Comment Here
Reference: Fetal / neonatal alloimmune thrombocytopenia
Board review style question #2
35 year old gravida 3, para 2 (G3P2) patient has a history of a severely fetal neonatal alloimmune thrombocytopenia affected neonate born with intracranial hemorrhage, followed by a subsequent pregnancy managed with intrauterine fetal transfusion. She is currently at 10 weeks gestation. What would be the most appropriate management for this pregnancy?
- Automatic intrauterine fetal transfusion of washed maternal platelets at 28 weeks, regardless of fetal platelet count
- High dose maternal corticosteroids, starting now until delivery
- Intrauterine fetal transfusion of washed maternal platelets to keep fetal platelet count > 100 x 109/L; delivery by Cesarian section only
- IVIG starting between 12 - 16 weeks gestation, with fetal monitoring / blood sampling and delivery near term at about 36 weeks
- Routine prenatal care, no additional interventions
Board review style answer #2
D. Recent articles show that IVIG started at about 12 - 16 weeks gestation is appropriate management for women with a history of fetal neonatal alloimmune thrombocytopenia affected pregnancy. Close monitoring is necessary. Most studies have not demonstrated definitive benefit for maternal corticosteroids and may show adverse outcomes (maternal gestational diabetes, etc). Fetal blood sampling may occur at intervals and governs the need for fetal intrauterine transfusion. It should not be considered automatic during the course of pregnancies. Fetal platelet transfusion thresholds are suggested to be between 25 - 50 x 109/L. Keeping fetal platelet count > 100 x 109/L would be an unnecessarily high threshold and may pose risk as percutaneous umbilical vein blood sampling and transfusions are not without risk.
Comment Here
Reference: Fetal / neonatal alloimmune thrombocytopenia
Comment Here
Reference: Fetal / neonatal alloimmune thrombocytopenia
Granulocyte use
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Blood donor screening | Blood donor testing | Donor selection / deferral | Apheresis granulocyte collection | Laboratory | Administration | Case reports | Treatment | Videos | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Granulocytes are white blood cells with visible granules, the most abundant of which are neutrophils, with important roles in protecting against bacterial and fungal infections
- Granulocyte transfusions are used for treatment of antimicrobial unresponsive bacterial and fungal infections in patients with severe neutropenia or defective granulocyte function (e.g. chronic granulomatous disease) when granulocyte recovery is anticipated
Essential features
- Clinical indications for granulocyte transfusions (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 9 June 2021]):
- Severe neutropenia with absolute neutrophil count (ANC) < 500/μL or impaired granulocyte function, such as chronic granulomatous disease
- Documented evidence of fungal or bacterial infection that is unresponsive to appropriate antimicrobial therapy for at least 24 to 48 hours
- To be used as a bridge with expectation of marrow recovery
- Granulocyte product characteristics:
- Each dose must contain at least 1.0 x 1010 granulocytes in at least 75% of units tested
- Stored at 20 - 24 °C without agitation
- Infused as soon as possible, with shelf life of 24 hours due to deterioration of function in storage
- Irradiated to prevent transfusion associated graft versus host disease (TA-GVHD)
- Crossmatch compatible with recipient's plasma due to 10 - 30 mL of red blood cells (RBCs) in the apheresis product
- Emergency release: results of donor infectious disease testing are generally unavailable within 24 hours of collection
- Cytomegalovirus (CMV) seronegative granulocytes must be provided for CMV seronegative recipients
- Must not be leukoreduced but administered with a standard blood infusion set with a 170 to 260 micron filter
- Granulocytes are not licensed blood products by the U.S. Food and Drug Administration (FDA)
Terminology
- Apheresis granulocytes: prepared from stimulated, single donor leukapheresis collection, contain ≥ 1.0 x 1010 granulocytes, 10 - 30 mL of RBCs, ~1.0 x 1011 platelets and up to 200 mL of plasma; total volume of product approximately 300 mL (Cytotherapy 2017;19:1256)
- Hydroxyethyl starch (HES): RBC sedimenting agent added during the leukapheresis collection to further separate RBCs from granulocytes
- Whole blood derived buffy coat granulocytes: the source of granulocytes in the U.K., each component is 50 mL, with 1 - 2 x 109 leukocytes, hematocrit of 45%, 90 x 109 platelets and 9.5 g of hemoglobin; individual units must be pooled to provide an adequate patient dose (NHS Blood and Transplant: Clinical Guidelines for the Use of Granulocyte Transfusions [Accessed 9 June 2021])
Pathophysiology
- Granulocytes migrate to sites of inflammation and infection to eliminate pathogens via phagocytosis and intracellular killing by generation of reactive oxygen species or by secretion of antimicrobial proteins to combat targets (Trends Immunol 2010;31:318)
- Neutrophils can also kill microbes by releasing web-like chromatin structures called neutrophil extracellular traps (NETs) (Nat Rev Immunol 2018;18:134)
- Infused granulocytes have been shown to migrate towards sites of superficial bacterial infections (Transfusion 2007;47:2185)
- Infused granulocyte half life in the peripheral blood is short: 6 - 8 hours (Trends Immunol 2010;31:318)
- Granulocytes are not recommended for prophylaxis during neutropenia in patients following chemotherapy or hematopoietic stem cell transplantation (Cytotherapy 2017;19:1256)
- Evidence for survival benefit of granulocyte transfusions has not been firmly established but some studies, including a recent trial (Resolving Infection in Neutropenia with Granulocytes [RING] trial), suggest that larger doses of granulocytes may offer clinical benefit (Cytotherapy 2017;19:1256)
Blood donor screening
- Community donors and directed (family) donors can be collected
- Granulocyte donors must be healthy, meeting the same standards for donor screening (donor history questionnaire and physical exam) as blood donors
- Additional considerations for blood donor screening are secondary to the adverse effects associated with the use of granulocyte colony stimulating factor (G-CSF), dexamethasone, HES and risks of apheresis procedures (see Apheresis granulocyte collection)
- Granulocyte donor suitability criteria vary across different institutions but may include:
- Adequate veins for peripheral vascular access
- No history of hypersensitivity reactions to HES
- No contraindications to steroid administration (hypertension, diabetes, gastrointestinal ulcers, cataract, active infections or other conditions adversely affected by steroids)
- Assessment of renal function (creatinine, cystatin C, estimated glomerular filtration rate [eGFR]) due to use of HES
- Eye exam: to screen for cataracts, especially for repeatedly stimulated donors
- Assessment of pregnancy status
- ABO / Rhesus (Rh) type
- Human leukocyte antigen (HLA) type: if HLA matching is performed by the blood center
Blood donor testing
- Similar to donor screening, donor infectious disease testing must abide by requirements outlined in Title 21 of the Code of Federal Regulations section 610.40 and 630.3(h), FDA Blood Guidances, standards of accreditation organizations such as American Association of Blood Banks (AABB) and state and local regulatory agencies (FDA: Code of Federal Regulations Title 21 [Accessed 21 June 2021], FDA: Blood Guidances [Accessed 21 June 2021])
- Required allogeneic donor testing include HBV DNA, HBsAg, anti-HBc, anti-HCV, HCV RNA, anti-HIV 1 / 2, HIV 1 RNA, anti-HTLV I / II, WNV RNA and syphilis by a serologic test
- Babesia spp. nucleic acid testing (NAT) is indicated in donations from states specified by the current FDA guidance (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 21 June 2021])
- Antibodies against Trypanosoma cruzi (T. cruzi) are tested at least once for each donor
- CMV testing: CMV seronegative recipients must be administered granulocytes from CMV seronegative donors
- HLA compatibility: further decreases the available donor pool
- Patients with HLA antibodies should have HLA matched granulocytes or HLA cognate antigen type compatible with the patient's anti-HLA
- Recipients planned to undergo hematopoietic stem cell transplantation should be provided with granulocytes lacking the prospective donor HLA antigens to avoid HLA alloimmunization and development of donor specific antibodies
- Emergency release: granulocytes are transfused as soon as possible under emergency release or authorized exception because results of donor infectious disease testing are not available until 24 - 48 hours after sample collection (Acad Pathol 2020;7:2374289520909500)
- Granulocyte donors may be selected from an established platelet donor pool whose donor infectious disease testing within the last 30 days is available
Donor selection / deferral
- Donor suitability assessment: stimulation with G-CSF or dexamethasone, as well as addition of HES in the apheresis collection, require assessment for donor safety (Transfusion 2012;52:2646)
- G-CSF adverse effects: bone pain, headache and fatigue; mild but treatable (J Clin Apher 2004;19:115)
- Corticosteroid adverse effects: fluid accumulation, weight gain, insomnia, posterior subcapsular cataracts
- Association of repeated corticosteroid stimulation with posterior subcapsular cataracts has been proposed but has not been established (Transfusion 2011;51:921)
- HES adverse effects: minimal adverse effects; rare hypersensitivity and pruritic reactions (Transfusion 2015;55:911)
- HES may be stored and accumulate in reticuloendothelial system over time (Intensive Care Med 2014;40:160)
- Long term followup of granulocyte donors repeatedly stimulated with G-CSF / dexamethasone appears to be safe (Transfusion 2009;49:513)
- Logistics of donor selection, stimulation, testing and collection are cumbersome and may be inconvenient for the donor, adding to constraints in granulocyte availability
- 2 - 3 donor visits / days are required for a collection procedure:
- Infectious disease testing
- Stimulation with steroid or G-CSF
- Day of granulocyte apheresis collection
- 2 - 3 donor visits / days are required for a collection procedure:
Apheresis granulocyte collection
- Collection of adequate numbers of granulocytes has historically been the limiting factor for the use of granulocytes because of both low yields and donor availability
- Stimulation with corticosteroids with or without G-CSF has greatly increased the yield of granulocyte collections but with ensuing adverse effects on donors (Transfusion 2000;40:642, Transfusion 2001;41:1037)
- Most blood centers in the U.S. provide steroid stimulated granulocytes
- Corticosteroid only stimulation: 2 - 3 times absolute neutrophil (ANC) count increase with peak at 24 hours; yield: ~1 x 1010 granulocytes
- G-CSF only stimulation: 7 - 10 times ANC increase with peak at 12 hours; yield: 2 - 6 x 1010 granulocytes
- G-CSF + corticosteroid stimulation: 10 - 13.5 times ANC increase with peak at 12 hours; yield: 4.5 - 9 x 1010 granulocytes
- Granulocyte donors can be community or family related (directed) donors
- Apheresis collection utilizes HES to increase collection efficiency by separating the RBC from the granulocyte layer during centrifuge separation apheresis
- Blood volume processing 7 - 10 L (procedure lasts for 2 - 3 hours)
- Anticipated granulocyte increment in the neutropenic patient after receipt of G-CSF + corticosteroid stimulated apheresis granulocyte has been reported to be a median peak ANC increment of 1,000/μL measured at 12 - 24 hours postinfusion after an average granulocyte dose of 4 x 1010/L granulocytes (Bone Marrow Transplant 2015;50:846)
Laboratory
- Storage condition: granulocytes are stored in 20 - 24 °C without agitation for a maximum of 24 hours
- Must not be refrigerated
- Irradiation: granulocytes must be irradiated to prevent transfusion associated graft versus host disease (TA-GVHD)
- Compatibility testing: must be crossmatch compatible because of 10 - 30 mL RBCs in the granulocyte product
- HLA compatibility: granulocyte transfusion should be avoided in patients with HLA antibodies; otherwise, the recipient should be HLA typed and the donor should be HLA matched or compatible with the patient's HLA antibodies, which may further decrease the available donor pool
- Repeat screening for HLA, HPA (human platelet antigen) and neutrophil antibodies if refractoriness to platelet or granulocyte transfusions develop
- Red cell incompatibility may be addressed by additional time on the bench for in vitro starch sedimentation, which removes 80 - 90% of RBC harvested during a granulocyte apheresis procedure; this may be necessary when (1) ABO major incompatibility exists and (2) recipient has clinically significant antibodies to RBC antigen(s) and an antigen negative donor cannot be recruited (Transfusion 2010;50:1203)
- See video below of RBC sedimentation in granulocyte product (Transfusion 2017;57:2551)
Administration
- Must not be infused through a leukoreduction filter (only a standard blood administration set: 170 - 260 microns)
- Potential adverse effects of granulocyte transfusions are similar to other blood products but more important associations include (Cytotherapy 2017;19:1256):
- Fever and chills (5 - 10%)
- Transfusion transmitted infections: especially CMV and leukocyte associated agents because leukoreduction cannot be performed
- Pulmonary complications (10 - 18%): transfusion associated acute lung injury (TRALI), hypoxemia, hemoptysis
- Alloimmunization to HLA and human neutrophil antigens (HNA): leading to reduced ANC increments to subsequent granulocyte transfusions and refractoriness to platelet transfusions
- Contrary to previous reports, pulmonary complications due to the infusion of granulocytes with amphotericin B infusions have not been proven or replicated (Haematologica 1997;82:71)
- Granulocyte transfusions are not widely utilized because of (Cytotherapy 2017;19:1256):f
- Inconclusive clinical efficacy based on controlled clinical trials; most evidence is from case reports and case series
- Challenges with collecting granulocytes by apheresis due to:
- Limited donor availability
- Low collection yield for an adequate dose
- Logistical difficulties with coordinating a multiday course of granulocyte transfusions
- Improved treatment of neutropenic patients with antimicrobial agents and hematopoietic growth factors (G-CSF)
Case reports
- 9 year old girl with leukocyte adhesion deficiency I (LAD I) who developed ecthyma gangrenosum treated with granulocyte transfusions (BMC Dermatol 2010;10:10)
- 16 year old girl with severe aplastic anemia and invasive aspergillosis awaiting stem cell transplantation (Blood 2012;119:1353)
- 35 year old woman with acute myeloid leukemia who developed TRALI after granulocyte transfusion (Transfusion 2003;43:1683)
Treatment
- Infusion of allogeneic granulocytes in neutropenic patients follows the same approach as RBC and platelet transfusions but granulocytes are underused because of the challenges of collecting an adequate dose and the conflicting evidence for their clinical efficacy
- Most published studies regarding efficacy of granulocyte transfusions are from case reports and case series
- RING trial was designed to investigate the efficacy of granulocyte transfusions and it showed that there was no difference between the survival of patients receiving antimicrobial therapy and patients receiving antimicrobials and granulocyte transfusions; however, the study was underpowered due to low enrollment and a definitive conclusion could not be drawn
- Post hoc secondary analysis suggested that patients who received higher doses have better outcomes than those who received a lower dose (Cytotherapy 2017;19:1256)
Sample assessment & plan
- Assessment: 7 year old boy with severe aplastic anemia status post hematopoietic stem cell transplant (HSCT) with subsequent graft rejection / failure. He is now planned for second HSCT from a new donor and currently has febrile neutropenia with an ANC of < 200. He is diagnosed with fungal pneumonia based on radiology and identification of Aspergillus fumigatus in bronchoalveolar lavage. Despite broad spectrum antifungal treatment regimens, he remains febrile with rapid radiologic progression of peripheral right lower lung infiltrates on CT scan.
- Plan: The patient is expected to undergo marrow reconstitution after a second HSCT as definitive therapy for severe aplastic anemia. Given the persistent febrile neutropenia and Aspergillus fungal pneumonia despite ongoing antifungal therapy, the use of granulocyte transfusions is warranted. The first granulocyte infusion will be available after approximately 4 - 7 days after donor recruitment with considerations given to HLA matching, CMV status and donor availability. Frequency and duration of granulocyte therapy will be determined by frequent reevaluation of the patient's clinical condition and will begin with 2 - 3 granulocyte transfusions/week during the neutropenic period
Additional references
Board review style question #1
An 8 year old boy with acute lymphoblastic leukemia is admitted for induction chemotherapy. During his admission, he developed high grade fever (103.5 °F), hypoxia and hypotension. Workup showed blood culture positive for Pseudomonas aeruginosa susceptible to cefepime. After 3 days of antibiotic therapy, he continued to experience high grade fevers, positive serial blood cultures and acute kidney injury. His absolute neutrophil count is 0.2 x 103/μL. Other pertinent lab results are: blood type A positive with a negative antibody screen and anticytomegalovirus (anti-CMV) IgG and IgM are negative. The clinical care team consulted the transfusion medicine service to initiate a course of granulocyte transfusions. Which of the following products should be provided to the patient?
- ABO crossmatch compatible, irradiated, cytomegalovirus (CMV) seronegative granulocytes because the patient is CMV seronegative
- ABO crossmatch compatible, leukoreduced, irradiated, CMV seronegative granulocytes because the patient is CMV seronegative
- Irradiated granulocytes; no donor CMV testing is required because granulocytes are CMV safe
- Leukoreduced, irradiated, CMV seronegative granulocytes because the patient is CMV seronegative
- Leukoreduced, irradiated granulocytes; no donor CMV testing is required because granulocytes are CMV safe
Board review style answer #1
A. ABO crossmatch compatible, irradiated, cytomegalovirus (CMV) seronegative granulocytes because the patient is CMV seronegative. Granulocytes collected by leukapheresis contain 10 - 30 mL of red blood cells and must be crossmatch compatible to avoid hemolytic transfusion reactions. In instances when major ABO incompatibility exists, additional starch sedimentation can be performed to further decrease incompatible red blood cells. Irradiation must be performed because of lymphocytes in the product and the risk of transfusion associated graft versus host disease. It is apparent that leukoreduction cannot be performed in granulocyte products. The granulocyte product must be CMV matched with the recipient; therefore, a CMV seronegative product must be provided.
Comment Here
Reference: Granulocyte use
Comment Here
Reference: Granulocyte use
Board review style question #2
What is true of granulocytes as a transfusion product?
- Can be stored after collection until infectious disease testing is reported
- Crossmatch is not necessary because it is released under emergent conditions
- Cytomegalovirus (CMV) status of the donor is not relevant because the product is irradiated
- Must be stored in a refrigerator to preserve phagocytic activity
- Must be stored without agitation with a maximum shelf life of 24 hours
Board review style answer #2
E. Must be stored without agitation with a maximum shelf life of 24 hours. Granulocytes are stored at room temperature (20 - 24 °C) without agitation. Infused granulocytes have a half life of 6 - 8 hours, must be infused under authorized medical exception and emergency release because infectious disease testing results are not available until 24 - 48 hours after collection. Because of the RBCs in granulocytes, the product must be crossmatched with the recipient's plasma. The only indication for irradiation is prevention of transfusion associated graft versus host disease (TA-GVHD), not CMV transmission. The granulocyte product must be CMV negative if the recipient is CMV negative due to the presence of lymphocytes in the product.
Comment Here
Reference: Granulocyte use
Comment Here
Reference: Granulocyte use
Hemolytic disease of the fetus and newborn
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission / incidence | Symptoms | Screening | Laboratory | Case reports | Treatment | Peripheral smear images | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hemolytic disease of the fetus and newborn (HDFN) is a complication of maternal alloantibodies directed at fetal / neonatal red blood cell antigens
- Symptoms can range from mild to severe or fatal for the fetus / neonate
Essential features
- Caused by maternal alloantibodies against fetal red blood cells
- May lead to fetal anemia and potentially hydrops fetalis and death in utero
- May lead to neonatal anemia, hyperbilirubinemia and kernicterus in the neonate
- Requires prenatal screening
- If clinically significant maternal antibodies detected, requires additional testing or monitoring prenatally / perinatally
Terminology
- Hemolytic disease of the fetus (HDF)
- Hemolytic disease of the newborn (HDN)
Pathophysiology
- Maternal IgG alloantibody(ies) cross the placenta, destroy fetal red blood cells
- May lead to extramedullary hematopoiesis
- Can manifest as splenomegaly, hepatomegaly
- May lead to portal hypertension, decreased albumin, ascites and edema
- Culminating in hydrops fetalis
- Potentially fatal from cardiac failure
- If not severe and fetus survives to delivery, neonatal anemia may be present
- Neonatal hyperbilirubinemia results from inability to adequately conjugate elevated unconjugated bilirubin levels
- Unaddressed, may result in kernicterus (bilirubin deposition in brain stem)
- In utero, bilirubin is cleared from fetal bloodstream via placenta / maternal circulation, protecting against kernicterus
- After delivery, maternal protection is lost and high bilirubin can accumulate in newborn's bloodstream and brain
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016, AABB: Technical Manual, 19th Edition, 2017
Clinical features
- Hemolytic disease of the fetus
- Fetal anemia
- Extramedullary hematopoiesis
- Splenomegaly, hepatomegaly
- Portal hypertension
- Cardiac failure
- Hydrops fetalis
- Hemolytic disease of the newborn
- Neonatal anemia
- Hyperbilirubinemia
- Kernicterus
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016
Transmission / incidence
- Maternal IgG mediated alloantibodies can cross the placenta and enter fetal circulation
- IgM antibodies cannot cross placenta
- Includes IgG anti-A, B (in blood type O mothers) attacking fetal red cells that are blood type A or B
- 1 - 4% incidence based on ethnicity (AABB: Technical Manual, 19th Edition, 2017)
- Includes IgG anti-D
- Without Rh immune globulin (RhIG) prophylaxis, roughly 16% incidence
- With RhIG prophylaxis, decreases risk to ≤ 2% (AABB: Technical Manual, 19th Edition, 2017)
- IgG antibodies also occur against non-ABO antigens and non-D antigens, such as other Rh blood group antigens, Duffy, Kidd and Kell antigens, etc.
- Severity depends on how well developed target antigen is on fetal red blood cells
- More than one antibody may occur (e.g. anti D and anti C)
Symptoms
- Discussed under Clinical features
Screening
- Type and screen of mother at prenatal examination
- Obtain serial titers of an alloantibody in the mother associated with HDFN
- Invasive cordocentesis / amniocentesis may be considered to assess fetal anemia via amniotic bilirubin levels
- Carries risk of fetal maternal hemorrhage
- Carries risk of spontaneous abortion
- Consider paternal zygosity testing to determine likelihood the fetus inherited the antigen from the father
- Cell free fetal DNA testing of maternal plasma may be used in some circumstances to determine antigen status of fetal cells
- Ultrasound Doppler studies of the middle cerebral artery (MCA) of the fetus
- MCA Doppler studies may be used to surveil for fetal anemia noninvasively
- Enhanced peak systolic velocity > 1.50 multiples of median (MoM) identifies moderate to severe fetal anemia
- Reference: Cunningham: Williams Obstetrics, 25th Edition, 2018
- To prevent HDFN from anti-D alloimmunization in Rh(D) negative of partial D phenotype women
- Give Rh immune globulin (RhIG) typically at 28 weeks gestational age (U.S. practice)
- Give RhIG within 72 hours of delivery if the neonate is Rh(D) positive
- Perform rosette test (or other fetal blood screening test) to determine if significant fetal maternal hemorrhage may have occurred
- Demonstrates presence of D positive cells in a D negative suspension using anti-D reagent
- Anti-D binds to D positive fetal RBCs → indicator D positive RBCs are added → rosettes are formed (see Peripheral smear images)
- If positive, perform quantification test to dose additional vials of RhIG
- Quantify the percent of fetal RBCs in maternal blood by flow cytometry or Kleihauer-Betke testing (see details in Laboratory)
- Perinatal fetomaternal hemorrhage (FMH)
- RhIG can be administered for any event associated with FMH during the perinatal period
- Events associated with perinatal FMH: abortion, ectopic pregnancy, amniocentesis, chorionic villus sampling, external cephalic version, abdominal trauma
- Reference: AABB: Technical Manual, 19th Edition, 2017
Laboratory
- ABO mediated hemolytic disease of the fetus and newborn
- Antibody screen may be negative
- Mother's blood group is generally type O
- Fetus / neonate's blood group is A or B
- Non-ABO mediated hemolytic disease of the fetus and newborn
- Prenatally, the mother's type and screen may reveal a known or new antibody
- Serial titers are often performed during pregnancy
- Clinically significant antibodies other than anti-K are critical ≥ 16
- Anti-K is significant at lower titers; 8 or even lower
- K is present on red cell and platelet precursors
- Anti-K causes suppression of erythropoiesis and even thrombopoiesis in addition to hemolysis
- Once a critical titer is reached, ultrasound Doppler studies are performed on the fetus' middle cerebral artery
- > 1.5 MoM (multiples of the median) may require intrauterine transfusion or delivery
- Positive direct antiglobulin test (DAT) on newborn blood may indicate HDFN
- DAT usually positive for IgG if the mother has non-ABO antibody
- Elution would be consistent with the mother's known antibody
- DAT may be positive for complement (C3d or C3b) if ABO related HDFN
- DAT usually positive for IgG if the mother has non-ABO antibody
- A peripheral blood smear may show findings of increased spherocytes, polychromasia, and sometimes normoblastemia (nucleated RBCs)
- For a peripheral blood smear from a 38 week gestation group A positive baby with mild HDFN born to a mother who was group O positive (see Peripheral smear images)
- Rosette screening test
- Screening test for FMH that detects fetal RhD positive red cells in maternal RhD negative blood (Transfusion 1991;31:303)
- Anti-D IgG added to maternal RBC suspension, which will bind to any fetal RhD positive RBCs, if present
- Indicator cells added that will bind to antibody coated fetal RBCs and form rosettes visible on microscopy
- A manufacturer specified number of rosettes (e.g., 3 or more in 10 fields) designates a positive result and suggests a significant FMH (> 30 mL), requiring quantification
- Acid elution quantification
- Commonly referred to as the Kleihauer-Betke test
- Based on the principle that red cells containing fetal hemoglobin (HbF) are less susceptible to acid elution than cells containing adult hemoglobin (HbA)
- Maternal peripheral smear is acid treated, washed and stained
- Pink stained fetal RBCs counted as a percentage of maternal RBCs, which will appear as ghost cells due to elution of hemoglobin
- Fetal RBC % used to calculate volume of hemorrhage and calculate dose of RhIG
- Reference: Cunningham: Williams Obstetrics, 25th Edition, 2018
Case reports
- 32 year old woman with HDFN from anti-D, anti-G with passive anti-D in breast milk (Transfusion 2017;57:2121)
- 41 year old woman with HDFN due to partial D (Transfusion 2018;58:2260)
- 45 year old type O woman with ABO HDFN in type AB twins (Transfusion 2010;50:2102)
- 4 women with HDFN due to Jk3 alloantibodies (Transfusion 2018;58:1157)
- 5 women with HDFN requiring intrauterine transfusion, therapeutic apheresis and IVIG (Transfusion 2018;58:677)
Treatment
- Prenatal treatments for hemolytic disease of the fetus include:
- Intrauterine transfusion
- Therapeutic plasma exchange
- Intravenous immune globulin (IVIG)
- Postnatal treatments for hemolytic disease of the neonate include:
- Phototherapy (ultraviolet light to convert serum unconjugated bilirubin to the water soluble form)
- Double volume exchange transfusion
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016
Peripheral smear images
Sample assessment & plan
- Assessment: Jane Doe is a 26 year old G2P1 woman with O positive blood type who presented for prenatal intake examination. A type and screen identified an anti-E alloantibody. The current titer is 2. Anti-E is capable of causing hemolytic disease of the fetus / newborn and hemolytic transfusion reactions.
- Plan:
- If red cell transfusion is required, O, Rh(D) positive or negative, E antigen negative, full crossmatch compatible units that are irradiated and leukocyte reduced will be issued.
- Recommend maternal-fetal medicine consultation.
- Zygosity testing of the father can be obtained by antigen typing the father for E, e antigens to determine inheritance risk for fetus.
- If father carries E antigen, consider monitoring the antibody via serial titrations.
- A fourfold increase is a clinically significant rise in titer. An absolute titer of ≥ 16 is considered a critical titer. If reached, recommend following with ultrasound middle cerebral artery Doppler flow velocity studies.
Differential diagnosis
- Infection / sepsis:
- White blood cell count may be elevated
- Pathogen specific testing may confirm
- Red blood cell enzymopathies:
- May be identified during newborn screening
- Peripheral blood smear may reveal red cell morphologic abnormalities
- Glucose-6-phosphate-dehydrogenase (G6PD) deficiency most common
- Thalassemia:
- Hemoglobin electrophoresis may identify
- Iron studies may be needed to exclude iron deficiency anemia
- Genetic studies can confirm
- Hemoglobinopathy:
- Hemoglobin electrophoresis to confirm
- Physiologic jaundice:
- Maternal antibody screen negative
- Direct antiglobulin test (DAT) negative
- Neonatal antibody screen (if performed) negative
- More likely if above are true and ABO related HDFN unlikely (i.e., maternal blood type not O and neonate blood type not A or B)
- Breast milk jaundice:
- Exact etiology unknown
- Consider when:
- Breastfeeding exclusively
- Nursing not going well or newborn weight loss > 8 - 10%
- Largely diagnosis of exclusion
- References: Am Fam Physician 2018;98:354, Semin Fetal Neonatal Med 2010;15:129
Board review style question #1
A 25 year old G2P1 woman has an anti-E alloantibody detected on her prenatal examination. During a serial titration with the current pregnancy, the titer rose from 8 to 64 one month later. Fetal middle cerebral artery (MCA) Doppler flow studies were initiated. What is true about these studies?
- Decreased flow rates may be indicative of fetal hyperbilirubinemia
- Increased flow rates may be indicative of fetal anemia
- It is an invasive technique that increases alloimmunization risk to the mother
- It is most useful for monitoring ABO mediated hemolytic disease of the fetus
Board review style answer #1
B. Increased flow rates suggest fetal anemia. Hemolytic disease of the newborn (but not fetus) is associated with hyperbilirubinemia, since the fetus has assistance of the mother to conjugate and excrete any indirect bilirubin. It is a noninvasive technique; therefore, it does not further increase risk of bleeding and alloimmunization for the mother. It is most useful for non-ABO alloantibodies, as the ABO type of the fetus is generally unknown until birth and ABO mediated hemolytic disease of the newborn is usually mild (full expression of ABO antigens occurs at 2 - 4 years old).
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Board review style question #2
A 30 year old woman with A negative blood type gives birth to a healthy male neonate who is blood type O positive. The mother received Rh immune globulin (RhIG) at 28 weeks estimated gestational age. What is proper management regarding RhIG administration and prevention of anti-D alloimmunization to the mother?
- 1 vial of 300 μg RhIG should be administered and a fetal blood screen should be performed on the mother
- 1 vial of 300 μg RhIG should be administered; no additional testing required
- RhIG is not indicated because it was given at 28 weeks
- RhIG is not indicated because the infant is Rh positive
Board review style answer #2
A. 1 vial of 300 μg RhIG should be given and a fetal blood screen / rosette test should be performed. If positive, a quantitative method for fetal to maternal hemorrhage, such as flow cytometry or Kleihauer-Betke (semiquantitative) study should be done to determine whether more than 1 vial of RhIG is required. The half life of RhIG is approximately 3 weeks, so dosing after delivery (when the infant is Rh positive) would be required even if given at 28 weeks estimated gestational age. RhIG is required following delivery when the mother is either Rh negative or a partial D phenotype and capable of forming an anti-D alloantibody and the neonate is Rh positive.
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Hemovigilance
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Diagrams / tables | Transmission | Symptoms | Screening | Blood donor screening | Blood donor symptoms | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Clinical images | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hemovigilance is the surveillance for complications resulting from blood donation or blood product transfusion, the evaluation of the complication data and the resulting quality improvement practices that are instituted as a result of the collected and analyzed data
Essential features
- Hemovigilance encompasses monitoring blood donor and blood product recipients for adverse events, collecting and compiling that data, then using that data to improve blood donation and blood product transfusion processes and procedures to decrease risk
- Internationally, hemovigilance systems can be comprised of nationally implemented and run programs with mandatory or voluntary reporting compared with hemovigilance systems that are made up of networks of public and private entities with voluntary reporting of adverse events
- In the United States, hemovigilance definitions are standardized through the Center for Disease Control's National Healthcare Safety Network Hemovigilance Module; 12 transfusion reaction entities are defined (case definition) and then are further classified based on severity and imputability
Terminology
- Hemovigilance: surveillance of the whole transfusion chain, including blood donation to blood transfusion, to minimize adverse events or reactions in donors and recipients and promote safe and effective use of blood components
- Biovigilance: encompasses principles of hemovigilance (blood recipients and blood donor) but also includes tissues, organs and cellular therapy (CT) components
- Imputability: the degree to which the adverse reaction was caused by transfusion
Pathophysiology
Not relevant to this topic
Clinical features
- Historical perspective
- Many international hemovigilance systems were established to monitor and mitigate transfusion transmitted viral infections, including transfusion transmitted HIV, hepatitis B and hepatitis C
- International hemovigilance
- Examples of early hemovigilance systems
- Japan: in 1993, the Japanese Red Cross Society was the first hemovigilance system that began collecting adverse event and infectious disease transmission data at a national level (Japanese Red Cross Society: Haemovigilance Information [Accessed 21 June 2023])
- United Kingdom: the United Kingdom established the Serious Hazards of Transfusion (SHOT) in 1996 (Br J Haematol 2013;163:303)
- Data collected through SHOT showed that there was an association between transfusion related acute lung injury (TRALI) and plasma and platelet units donated by females (Transfusion 2015;55:164, Serious Hazards of Transfusion: TRALI Tables [Accessed 21 June 2023])
- Current hemovigilance systems are comprised of nationally organized systems or hemovigilance systems that are a network of public and private organizations working together
- In 2014, the Association for the Advancement of Blood & Biotherapies (AABB) and International Society of Blood Transfusion (ISBT) published the first internationally harmonized standard definitions related to blood transfusions (Vox Sang 2016;110:185)
- Examples of early hemovigilance systems
- Hemovigilance in U.S.
- Hemovigilance in the United States is coordinated by the collaboration of public and private entities; there is no formal national hemovigilance program with required adverse event reporting
- In 2010, the CDC's National Healthcare Safety Network (NHSN) hemovigilance module began national hemovigilance data collection (Transfusion 2015;55:703)
- NHSN hemovigilance module relies on voluntary passive reporting of transfusion associated adverse events
- Passive reporting of transfusion reactions underestimated the rate of transfusion associated circulatory overload in a single institution (Vox Sang 2015;108:387)
- Elements of effective hemovigilance systems
- Timely reporting of adverse events
- Reporting of demographic and blood product utilization data
- Standardized definitions of donation related and transfusion related adverse event data reporting
- The CDC National Healthcare Safety Network has defined 12 transfusion reaction categories (CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 21 June 2023])
- 12 defined transfusion reaction types are
- Transfusion associated circulatory overload (TACO)
- Transfusion related acute lung injury (TRALI) type I
- Transfusion associated dyspnea (TAD)
- Allergic reaction
- Hypotensive transfusion reaction
- Febrile nonhemolytic transfusion reaction (FNHTR)
- Acute hemolytic transfusion reaction (AHTR)
- Delayed hemolytic transfusion reaction (DHTR)
- Delayed serologic transfusion reaction (DSTR)
- Transfusion associated graft versus host disease (TAGVHD)
- Posttransfusion purpura (PTP)
- Transfusion transmitted infection (TTI)
- Other or unknown
- Future directions of hemovigilance systems
- Digitally enabled hemovigilance
- Using artificial intelligence or algorithms built into electronic medical records (EHR) may help identify signs and symptoms of transfusion related adverse events in real time
- Baseline of passively reported suspected transfusion reactions was 0.34% and increased to 1.3% at a single institution that implemented a digitally enabled hemovigilance system with a hemovigilance unit (a 524% increase) (Transfusion 2022;62:1010)
- Active surveillance
- Actively identifying suspected adverse reactions through vital sign checks, chart reviews and patient evaluations is time and resource intensive but may capture more suspected adverse reactions than passive surveillance
- 12% of patients with Coronavirus disease 2019 (COVID-19) who received COVID-19 convalescent plasma (CCP) were found to meet diagnostic criteria for transfusion associated circulatory overload (TACO) at a single institution that implemented an active surveillance protocol to monitor for CCP related adverse events (Transfusion 2022;62:28)
- Digitally enabled hemovigilance
Diagrams / tables
NHSN hemovigilance module adverse reaction codes, severity codes and imputability
| Case definition | Severity | Imputability |
| Definitive: the adverse reaction fulfills all of the case definition criteria | Nonsevere: medical intervention (e.g., symptomatic treatment) is required but there is minimal risk of permanent damage to the transfusion recipient | Definite: there is conclusive evidence that the reaction can be attributed to the transfusion |
| Probable: the adverse reaction meets some of the clinical signs and symptoms or radiologic, laboratory evidence or available information but does not meet all definitive case definition criteria | Severe: inpatient hospitalization or prolonged hospitalization is directly attributable to the transfusion reaction, persistent or significant disability or incapacity of the patient as a result of the reaction or a medical or surgical intervention is necessary to preclude permanent damage or impairment of a body function | Probable: there are other potential causes present that could explain the recipient's symptoms but transfusion is the most likely cause of the reaction |
| Possible: the reported clinical signs or symptoms, radiologic or laboratory evidence and available information are not sufficient to meet definitive or probable case definition criteria | Life threatening: major intervention was required after the transfusion reaction (e.g., vasopressors, intubation, transfer to intensive care) to prevent death | Possible: there are other potential causes that are most likely; however, transfusion cannot be ruled out |
| Death: the recipient died as a result of the transfusion reaction | Doubtful: there is evidence clearing in favor of a cause other than the transfusion but transfusion cannot be excluded | |
| Not determined: the severity of the adverse reaction is unknown or not stated | Ruled out: there is conclusive evidence beyond reasonable doubt of a cause other than the transfusion | |
| Not determined: the relationship between the reaction and transfusion is unknown or not stated |
Table modified from: Transfusion 2015;55:703
Transmission
Not relevant to this topic
Symptoms
- In blood product recipients (CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 21 June 2023])
- Information capture includes blood product recipient vital signs at discrete intervals (pretransfusion, 15 minutes into the transfusion and posttransfusion) as well as signs and symptoms of an adverse event
- The most common adverse events are allergic reactions and febrile nonhemolytic transfusion reactions (Transfusion 2021;61:1424)
- The most common causes of transfusion related death are transfusion related acute lung injury and transfusion associated circulatory overload (Transfusion 2021;61:1424)
- Signs and symptoms of transfusion associated adverse events
- Fever, ≥ 1 °C / 1.8 °F rise in temperature to ≥ 38 °C / 100.4 °F
- Chills or rigors
- Headache
- Loss of consciousness
- Respiratory distress (wheezing, coughing, hypoxia, dyspnea, cyanosis, decreased SpO2 saturation < 90% on room air, tachypnea)
- Bronchospasm or stridor
- Pulmonary edema
- Evidence of cardiovascular system changes (elevated central venous pressure, evidence of left heart failure, widened pulse pressure, jugular venous distention, peripheral edema, enlarged cardiac silhouette)
- Evidence of fluid overload
- Hypertension
- Hypotension (drop in systolic BP of ≥ 30 mmHg and systolic BP ≤ 80 mmHg)
- Tachycardia
- Abdominal, chest, flank or back pain
- Pain at infusion site
- Skin manifestations such as rash, flushing, urticaria, pruritus
- Edema of conjunctiva, periorbital region, lips, tongue, uvula or angioedema
- Jaundice or hemoglobinuria
- Nausea / vomiting
- Abnormal bleeding, epistaxis
- Disseminated intravascular coagulation
- Oliguria / anuria
- Marrow aplasia or pancytopenia
- Thrombocytopenia
- Other
Screening
Not relevant to this topic
Blood donor screening
No information provided
Blood donor symptoms
- In blood donors (AABB: Donor Hemovigilance [Accessed 21 June 2023])
- Information capture includes blood donor vital signs at discrete intervals (predonation and postdonation) as well as signs and symptoms of an adverse event
- The most common donor adverse events are presyncopal reactions and small hematomas
- Signs and symptoms of donor related adverse events
- Vasovagal
- Prefaint (presyncopal), no loss of consciousness
- Loss of consciousness
- Local injury related to needle
- Hematoma / bruise
- Nerve irritation
- Arterial puncture
- Painful arm
- Delayed bleeding
- Infection
- Major blood vessel injury
- Injury
- Major injury
- Minor injury
- Apheresis related
- Citrate
- Hemolysis
- Air embolus
- Infiltration
- Allergic
- Local
- Systemic
- Anaphylactic
- Major cardiovascular event
- Angina pectoris within 24 hours
- Cardiac arrest
- Cerebrovascular accident
- Myocardial infarction within 24 hours
- Transient ischemic attack (TIA) within 24 hours
- Other
- Vasovagal
Blood donor testing
Not relevant to this topic
Donor deferral
Not relevant to this topic
Laboratory
- Transfusion reaction workups require the implicated blood product unit as well as a posttransfusion sample to be returned to the blood bank (Cohn: Technical Manual, 20th Edition, 2020)
- Blood bank performs the following
- Clerical check of the component bag, label, paperwork, and patient sample
- Repeat ABO testing of the posttransfusion sample
- Evaluate the pretransfusion and posttransfusion specimens for visual hemolysis
- Perform direct antiglobulin test (DAT) on posttransfusion sample
- Clerical check of the component bag, label, paperwork, and patient sample
- Blood bank performs the following
Case reports
- 23 year old splenectomized woman with transfusion transmitted malaria (Plasmodium knowlesi) (Malar J 2016;15:357)
- 40 year old woman with transfusion transmitted CMV as complication of liposuction in the Dominican Republic (BMJ Case Rep 2021;14:e236892)
- 3 female solid organ transplant recipients in their 40s and 50s with eastern equine encephalitis virus (EEEV) infection from a single organ donor (Clin Infect Dis 2019;69:450)
- 59 year old man with COVID-19 infection developed transfusion related acute lung injury (TRALI) after COVID-19 convalescent plasma transfusion (J Int Med Res 2021;49:3000605211032814)
Treatment
Not relevant to this topic
Sample assessment & plan
- Assessment: Patient is a 48 year old woman, A RhD positive, antibody screen negative, with a history of type II diabetes, end stage renal disease on dialysis and idiopathic cardiomyopathy with a left ventricular ejection fraction of 17%. She presents with a hemoglobin of 5.8 g/dL, fatigue and shortness of breath. She is transfused 2 units of A RhD positive red blood cells. During transfusion of the second unit she develops hypertension to 210/90, tachycardia to 112 bpm and desaturates to 89% O2 saturation on room air. A chest Xray is performed, which shows bilateral pulmonary edema. The transfusion reaction workup shows clerical check correct, no evidence of hemolysis and polyspecific direct antiglobulin test (DAT) is negative (see clinical image). The patient is placed on supplemental oxygen via nasal cannula and respiratory status improves after dialysis.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of transfusion associated circulatory overload (TACO), severity is nonsevere, imputability is probable.
- Recommend judicious transfusion and we suggest transfusing at a slow rate in the future if the clinical situation allows, e.g., 1 mL/kg/hr).
Differential diagnosis
Not relevant to this topic
Additional references
Board review style question #1
A 56 year old woman with a refractory / recurrent plasma cell neoplasm and multiple allergies to medications has received multiple transfusions of red blood cell and platelet units over years. She has had multiple mild allergic transfusion reactions, which the clinical team treated with Benadryl and steroids. She presents to the outpatient infusion clinic for a platelet transfusion (see image above). Premedication is not administered. 15 minutes into the platelet transfusion, she develops hives, itching, chest tightness, difficulty breathing, throat swelling, oxygen desaturation to 78% on room air and hypotension with blood pressure of 78/52. A code is called, epinephrine is administered, the patient is intubated and admitted to the medical intensive care unit. What is the case definition, severity and imputability of this transfusion reaction?
- Case definition: definitive; severity: life threatening; imputability: definite
- Case definition: definitive; severity: nonsevere; imputability: definite
- Case definition: possible; severity: nonsevere; imputability: definite
- Case definition: possible; severity: severe; imputability: probable
- Case definition: probable; severity: severe; imputability: probable
Board review style answer #1
A. Case definition: definitive; severity: life threatening; imputability: definite. The case definition is definitive as the patient presented with 2 or more of the diagnostic criteria during the transfusion. The patient had hypotension, respiratory distress, itching and hives. The severity is life threatening as the patient required intubation and admission to the intensive care unit. The imputability is definite as the reaction occurred during the transfusion and no other risk factors were noted. Answers B through E did not list the correct case definition, severity or imputability.
Comment Here
Reference: Hemovigilance
Comment Here
Reference: Hemovigilance
Board review style question #2
Which transfusion adverse event was mitigated after collection of hemovigilance data through the United Kingdom's Serious Hazards of Transfusion (SHOT)?
- Allergic reaction
- Febrile nonhemolytic transfusion reaction (FNHTR)
- Transfusion associated circulatory overload (TACO)
- Transfusion associated graft versus host disease (TA-GVHD)
- Transfusion related acute lung injury (TRALI)
Board review style answer #2
E. Transfusion related acute lung injury (TRALI). Hemovigilance data collected through the United Kingdom's Serious Hazards of Transfusion (SHOT) found an association between transfusion related acute lung injury (TRALI) and plasma and platelet products donated by females. Low risk TRALI donor strategies, including collecting plasma from males and never pregnant females, were instituted in the early 2000s. In a meta analysis, patient populations prone to develop TRALI were found to have a significant reduction in TRALI risk (OR, 0.51; 95% CI, 0.29 - 0.90). Answer A is incorrect because premedication is not supported for the prevention of an allergic reaction. If allergic reactions are severe and recurrent, washing of red blood cell units may be considered. Answer B is incorrect because febrile nonhemolytic transfusion reaction incidence has decreased with universal leukoreduction. Answer C is incorrect because transfusion associated circulatory overload can possibly be mitigated by slow transfusion rate (1 mL/kg/hr) but data is lacking. Answer D is incorrect because transfusion associated graft versus host disease can be mitigated with irradiation and pathogen inactivation technologies (Transfusion 2015;55:164, J Hosp Med 2020 Nov;15:684, Transfusion 2004;44:25, Transfusion 2004;44:16, Transfusion 2018 ;58:1506).
Comment Here
Reference: Hemovigilance
Comment Here
Reference: Hemovigilance
Hemovigilance (pending)
[Pending]
Hypotension
Table of Contents
Definition / general | Essential features | Pathophysiology | Clinical features | Symptoms | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Decrease in blood pressure during or within 1 hour after cessation of a transfusion
- In adults, hypotension is defined as a drop in systolic blood pressure of ≥ 30 mmHg and a systolic blood pressure of ≤ 80 mmHg
- All other adverse transfusion reactions which may present with hypotension, such as septic reactions, acute hemolytic reactions, transfusion related lung injury and anaphylaxis, must be excluded
- Reference: CDC: NHSN Biovigilance Component Surveillance Protocol [Accessed 28 May 2021]
Essential features
- Hypotensive transfusion reaction classically presents as a sudden, profound drop in blood pressure within 15 minutes of starting a transfusion and responds rapidly to stopping the transfusion and supportive care
- Thought to be mediated by bradykinin and is strongly associated with angiotensin converting enzyme (ACE) inhibitors, which prevent the breakdown of bradykinin
- In patients with a history of severe hypotensive transfusion reaction and who are on an ACE inhibitor or angiotensin receptor blocker (ARB), consider temporarily withholding the medication prior to transfusion
Pathophysiology
- Hypothesized to be related to the accumulation of bradykinin, a peptide and inflammatory mediator that causes vasodilation and increases vascular permeability
- Bradykinin accumulates in the presence of an angiotensin converting enzyme (ACE) inhibitor, which prevents bradykinin degradation (Transfusion 2004;44:1361)
- Bedside leukoreduction filters, which can generate bradykinin via activation of the contact system due to their negatively charged surface, have been implicated in the past but are no longer commonly used; prestorage leukoreduction is now a standard practice (Anesth Analg 2016;123:268)
Clinical features
- Isolated hypotensive transfusion reactions typically occur < 15 minutes after the start of the transfusion and respond rapidly to cessation of transfusion and supportive care
- Rare, accounting for 2.8% of reported transfusion reactions, however, the true incidence may be higher due to the difficulty in recognizing these reactions (Anesth Analg 2016;123:268)
- National Healthcare Safety Network (NHSN) Hemovigilance Module criteria defines hypotension as:
- In adults 18 years and older, a drop in systolic blood pressure of ≥ 30 mmHg and systolic blood pressure ≤ 80 mmHg
- In children and adolescents 1 year to < 18 years old, a greater than 25% drop in systolic blood pressure from baseline
- In neonates and small infants (< 1 year old or < 12 kg), a greater than 25% drop in baseline value using whichever measurement is being recorded (CDC: NHSN Biovigilance Component Surveillance Protocol [Accessed 28 May 2021])
Symptoms
- Uncommon and usually nonspecific (i.e. dizziness, nausea, headache, syncope) (Transfusion 2015;55:1668)
Laboratory
- No visual evidence of hemolysis
- Negative direct antiglobulin test (DAT)
Case reports
- 61 year old man with a history of hypertension on ramipril underwent prostatectomy and developed severe drop in blood pressure following intraoperatively autologous blood transfusion (Transfusion 2004;44:1361)
- 68 year old woman underwent lumbar fusion surgery and developed profound intraoperative hypotension immediately following transfusion (Am J Case Rep 2018;19:1283)
- 78 year old woman with a history of hypertension on lisinopril underwent laminectomy and fusion surgery and developed severe hypotension immediately following transfusion (A A Case Rep 2017;9:4)
Treatment
- Stop transfusion
- Maintain IV access
- Supportive care and if necessary, fluid bolus or vasopressors (Transfusion 2015;55:1668)
Sample assessment & plan
- Assessment: The patient had a severe drop in blood pressure within minutes of receiving a blood transfusion. Cessation of the transfusion resulted in normalization of the blood pressure and no further treatment was necessary. The patient exhibited no other symptoms. Posttransfusion testing of the patient and donor samples ruled out an acute hemolytic transfusion reaction.
- Plan: These findings are consistent with a hypotensive transfusion reaction.
- If the reaction is severe and the patient is on an ACE inhibitor or an ARB, consider temporarily withholding the medication prior to transfusion, as they are associated with hypotensive transfusion reactions and block the breakdown of bradykinins.
Differential diagnosis
- Acute hemolytic transfusion reaction (AHTR):
- Typically presents with hypotension but with additional symptoms, such as fever, rigors and back pain
- Should also show laboratory evidence of hemolysis with a positive direct antiglobulin test
- Transfusion related acute lung injury (TRALI):
- Presents with hypotension
- Also with hypoxemia and radiographic evidence of pulmonary edema
- Anaphylaxis:
- Presents with hypotension
- Also with a rash or airway symptoms, such as dyspnea and mucocutaneous edema
Board review style question #1
A 65 year old man with a history of hypertension, hyperlipidemia, coronary artery disease and diabetes is 1 day status postcoronary bypass surgery. He is transfused a unit of red blood cells for a hemoglobin of 7 g/dL. Within 10 minutes of the transfusion, his blood pressure decreases from 102/57 to 45/30. All other vital signs remain stable. He has no other symptoms. The transfusion is stopped, a bolus of normal saline is given and the patient quickly recovers. Which of the following is the most likely diagnosis?
- Acute hemolytic transfusion reaction
- Anaphylaxis
- Hypotensive transfusion reaction
- Transfusion associated circulatory overload (TACO)
- Transfusion associated lung injury (TRALI)
Board review style answer #1
C. Hypotensive transfusion reaction. Hypotensive transfusion reactions present as a sudden decrease in blood pressure of at least 30 mmHg typically within the first 15 minutes of a transfusion and respond rapidly to the cessation of transfusion and supportive care. Other transfusion reactions may present with hypotension; however, they often present with additional signs and symptoms. An acute hemolytic transfusion reaction may present with fever, rigors and back pain and will have laboratory evidence of hemolysis with a positive direct antiglobulin test. Transfusion associated lung injury (TRALI) presents with hypoxemia and radiographic evidence of pulmonary edema. Transfusion associated circulatory overload (TACO) presents with hypertension (not hypotension) due to volume overload. Anaphylaxis presents with airway symptoms, such as dyspnea and mucocutaneous edema, in addition to hypotension.
Comment Here
Reference: Hypotension
Comment Here
Reference: Hypotension
Board review style question #2
Which of the following statements is true regarding hypotensive transfusion reactions?
- Associated with the use of angiotensin converting enzyme (ACE) inhibitors
- Associated with the use of prestorage leukoreduced blood products
- Caused by the infusion of cytokines
- Caused by the infusion of leukocytes
- Prevented by premedicating patients with diphenhydramine
Board review style answer #2
A. Associated with the use of angiotensin converting enzyme (ACE) inhibitors. Hypotensive transfusion reactions are associated with the use of bedside leukocyte reduced blood products. They are thought to occur due to the accumulation of bradykinin and are strongly associated with ACE inhibitor use. ACE inhibitors block the degradation of bradykinin, leading to its accumulation. Hypotensive transfusion reactions have also occurred in patients receiving blood products that are filtered through bedside leukoreduction filters which are negatively charged and can generate kinins through the contact system, however, bedside leukoreduction filters are no longer used and have been replaced by prestorage leukoreduction. Infusion of cytokines is associated with febrile nonhemolytic transfusion reactions. Infusion of leukocytes is associated with febrile nonhemolytic transfusion reactions, HLA alloimmunization and transfusion associated graft versus host disease. Diphenhydramine may be used to treat allergic transfusion reactions.
Comment Here
Reference: Hypotension
Comment Here
Reference: Hypotension
Ii system
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ii antigens are carbohydrate antigens formed from multiple ABO moieties
- Cold reacting antibodies
Essential features
- Cold autoantibodies associated with infection
- Generally, not clinically significant
- Can cause cold agglutinin disease
Terminology
- I: antigen in I blood group system
- i: antigen in Ii collection
Pathophysiology
- Antigens are formed by repeating lactosamines associated with ABH antigens on red cell surfaces (Fung: Technical Manual, 19th Edition, 2017)
- i antigen is linear and less complex
- I is larger and more complex with branching
- I gene is GCNT2 and has multiple tissue specific isoforms (Immunohematology 2019;35:85)
Clinical features
- i is present in younger patients, with branching and complexity being added with age, and is converted to I antigens by 13 - 20 months of age
- Antibodies common with infection:
- Transient anti-i seen in infectious mononucleosis (EBV)
- Transient anti-I seen in Mycoplasma infection
- Antigens (type: carbohydrate) (Fung: Technical Manual, 19th Edition, 2017)
- I: larger and more complex, found in the RBC cell membrane of all adults
- i: small and linear without branching, found in fetus and infants
- Cord cells characteristically lack I
- Autoantibodies:
- Generally naturally occurring and not clinically significant
- Cold agglutinin disease associated with antibodies (usually IgM against I antigen) that have high thermal amplitude and can react near body temperature, a common cause of acquired hemolytic anemia
- Disease associations (Immunohematology 2019;35:85):
- GCNT2 mutations can cause adult i phenotype and are associated with congenital cataracts
- Decreased GCNT2 expression associated with melanoma progression
- Increased GCNT2 expression in leukemia / lymphoma cells associated with increased clearance by NK cells
Transmission
- Antibodies are naturally occurring
Laboratory
- May be present at immediate spin, leading to ABO mismatch and challenges in pretransfusion testing
- Antibodies common when testing at 4 °C
- Thermal amplitude study assesses the reactivity of serum or plasma with RBCs at different temperatures, typically 40 °C, 220 °C, 300 °C and 370 °C
- Reactivity at > 30 °C is clinically significant; the closer to body temperature clumping occurs, the more harmful the patient’s cold agglutinins are
- In patients undergoing cardioplegia where the body temperature is lowered, it is important to know the exact temperature of reactivity
- Direct antiglobulin test (DAT) may be utilized to screen for cold agglutinin disease
- Reference: Fung: Technical Manual, 19th Edition, 2017
Case reports
- 8 year old girl with EBV presenting with hemolytic anemia and anti-i antibodies (J Pediatr Hematol Oncol 2019;41:324)
- 56 year old woman and 73 year old man presenting with ABO backtyping issues associated with cold antibodies (J Clin Lab Anal 2021;35:e23894)
- 70 year old man with cold agglutinin disease and COVID-19 treated with therapeutic plasma exchange (BMJ Case Rep 2021;14:e244227)
- 78 year old man with cold agglutinin disease presenting with dry gangrene (Rheumatology (Oxford) 2021 Aug 13 [Epub ahead of print])
Additional references
Board review style question #1
A patient is found to have an i antibody. The patient is a 14 year old boy presenting to his primary care physician for fatigue and was found to be anemic. What infectious process may be causing this finding?
- Babesiosis
- EBV
- Mycoplasma
- Parvovirus
Board review style answer #1
Board review style question #2
A patient is preparing for cardiothoracic surgery and will be receiving cardioplegia. What lab test may help prevent hemolysis in this patient?
- Donath Landsteiner
- Ham test
- Thermal amplitude
- Type and screen
Board review style answer #2
Iron overload
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Screening and monitoring | Laboratory | Case reports | Treatment | Sample note / clinical documentation in the patient’s medical record | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Iron accumulates within various body organs after multiple transfusions
- Patients at risk are those receiving multiple transfusions, such as patients with sickle cell disease (SCD), thalassemia and myelodysplastic syndrome (MDS)
Essential features
- Occurs after transfusion of 10 - 20 units of red blood cells, affecting transfusion dependent patients
- Resulting cardiac and liver dysfunction are major causes of morbidity and mortality
- Patients on regular transfusions should be screened for iron loading after 10 units of transfusions, then regularly thereafter
- Iron chelation therapy is the primary mode of treatment
Terminology
- Hemochromatosis (secondary)
- Hemosiderosis
Pathophysiology
- Each unit of red blood cells transfused contains 200 - 250 mg of iron
- Human body lacks a mechanism for elimination of excess iron
- Iron released from transfused red blood cells is transported by transferrin
- After transferrin is saturated, nontransferrin bound iron (NTBI) and labile plasma iron (LPI) exert tissue damage, partly through reactive oxygen species (Hemasphere 2020;4:e357)
- Affected organs include heart, liver, pancreas, endocrine glands, bone marrow and skin
Clinical features
- Heart failure, liver cirrhosis, diabetes, hypothyroidism, hypoparathyroidism, hypogonadism and skin hyperpigmentation
- Cardiac iron loading was the leading cause of death in thalassemia before effective treatments were available
- References: Hematol Am Soc 2017;2017:265, Acta Biomed 2018;88:435, Mediterr J Hematol Infect Dis 2016;8:e2016058, PLoS One 2019;14:e0214148
Symptoms
- Asymptomatic at early stages
- Symptoms occur later after organ damage
- Growth retardation
- Jaundice, ascites
- Shortness of breath on exertion, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, lower limb edema
- Polyuria, polydipsia
- Skin discoloration
Screening and monitoring
- Patients who receive multiple transfusions must be screened for iron overload using serum ferritin measurement and organ assessment
- Should take place after transfusion of 10 units of red blood cells
- Liver iron concentration (LIC) as assessed by liver biopsy and direct absorption spectrophotometry provide a precise method for iron loading
- Reference range for LIC is 0.2 - 2 mg Fe/g dry weight (3.6 - 36 μg Fe/kg dry weight); measurements exceeding the upper limit are diagnostic for iron overload
- MRI T2 and T2*w of the liver also used for screening and monitoring and reported as LIC
- References: Magn Reson Imaging Clin N Am 2010;18:359, Br J Haematol 2017;177:703, Blood Transfus 2013;11:128
Laboratory
- High serum ferritin
- Serum ferritin above 1,000 ng/ml is usually considered an indication for initiation of treatment
- Typical normal range (μg/L): 30 - 300 (male); 10 - 200 (female) (N Engl J Med 2004;351:1548)
- However, serum ferritin may not be accurate for prediction of iron loading in all organs (Hematology Am Soc Hematol Educ Program 2019;2019:337)
- Since ferritin is an acute phase reactant, trends, rather than single values are usually used
- Serum ferritin above 1,000 ng/ml is usually considered an indication for initiation of treatment
- Elevated transferrin saturation
- Liver biopsy used to be the gold standard method for evaluation of liver iron concentration
- MRI T2 and T2* considered a reliable, noninvasive method for assessment of liver and myocardial iron loading; access to an MRI machine and special software required (Blood 2005;105:855, European Heart Journal 2001;22:2171)
- Features of organ dysfunction:
- Diabetes (high fasting and random blood glucose and high hemoglobin A1c)
- Hypoparathyroidism (low parathyroid hormone, low calcium)
- Hypothyroidism (high thyroid stimulating hormone, low T3 and T4)
Case reports
- 14 year old boy with hypoparathyroidism secondary to transfusional iron overload (J ASEAN Fed Endocr Soc 2020;35:129)
- 35 year old woman and 42 year old man with sickle cell disease and cardiomyopathy secondary to iron overload (Transfusion 2017;57:700)
- 56 year old woman with transfusional iron overload and end stage renal disease (Case Rep Oncol 2020;13:760)
Treatment
- Treatment is through iron chelators, including (Transfus Clin Biol 2017;24:223):
- Desferrioxamine, also known as deferoxamine (subcutaneously through a pump or intravenous infusion)
- Deferiprone (oral)
- Deferasirox (oral)
- Combinations can also be used specially for those with severe iron loading or iron loading in specific organs (liver or heart)
- Phlebotomy (venesection) is a primary treatment for hereditary hemochromatosis and can be used in patients with hemoglobinopathies or MDS after stem cell transplantation
- Automated red cell exchange has a therapeutic effect on iron loading in patients with SCD, in comparison with manual exchange transfusion
Sample note / clinical documentation in the patient’s medical record
- Note for venesection (therapeutic phlebotomy) procedure in a patient post SCT
- Mr. X underwent therapeutic phlebotomy for iron overload secondary to (diagnosis). Whole blood volume removed: 400 ml. No replacement fluids were required. He tolerated the procedure well with no complications. The next procedure is set for [date].
Differential diagnosis
- Hereditary hemochromatosis:
- Genetic (autosomal recessive with variable penetrance)
- Related to hepcidin deficiency
- Most commonly due to HFE gene mutation but could also result from mutations in the following genes: HJV, HAMP, TFR2, SLC40A1
- Ferroportin disease:
- Genetic (autosomal dominant)
- Related to defects of cellular iron export
- Hepatic Kupffer cells show marked iron accumulation; low transferrin saturation
- Due to loss of function mutations of the ferroportin gene (SLC40A1)
Additional references
Board review style question #1
In addition to liver biopsy, the severity of liver iron content can be accurately evaluated by
- Computerized tomography (CT)
- Liver enzymes (AST, ALT, GGT)
- Magnetic resonance imaging (MRI)
- Serum ferritin levels
Board review style answer #1
Board review style question #2
Which of the following is a common complication of transfusional iron overload?
- Diabetes insipidus
- Hyperparathyroidism
- Hypogonadism
- Lung fibrosis
Board review style answer #2
Irradiation
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Screening | Laboratory | Case reports | Treatment | Clinical images | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Irradiation of cellular blood components is required for certain transfusion recipients to prevent transfusion associated graft versus host disease (TA-GVHD), a rare but usually fatal transfusion related complication
- Populations at risk for TA-GVHD include those with severe impairment in cellular immunity, as well as recipients of donations from blood relatives due to shared human leukocyte antigen (HLA) haplotypes that render the recipient unable to recognize donor T lymphocytes as foreign
Essential features
- Transfusion associated graft versus host disease (TA-GVHD) is a rare but usually fatal complication of transfusion, with an estimated mortality rate that exceeds 80%
- There are limited viable treatment options for TA-GVHD and therefore, prevention is required for recipients identified to be at risk for this complication
- Irradiation of cellular blood components is currently the cornerstone of prevention of TA-GVHD
- There are minimum dosage requirements for blood product irradiation in the U.S. (see Clinical features) and Europe
- Irradiation can cause lipid peroxidation of red cell membranes and an increase in extracellular potassium levels, which may lead to clinical hyperkalemia in at risk recipients, such as neonates
Terminology
- Human leukocyte antigen (HLA)
- Transfusion associated graft versus host disease (TA-GVHD)
Pathophysiology
- TA-GVHD
- Due to engraftment and proliferation of viable T lymphocytes from the donor in the transfusion recipient
- Transfused donor T lymphocytes in cellular blood components recognize the recipient's tissues as foreign
- Recipient's immune system is unable to recognize the donor T lymphocytes as foreign, thereby allowing the donor mediated immune attack on the recipient's tissues, especially the skin, bone marrow and gastrointestinal tract, to continue unchecked
- Risk of TA-GVHD increases with:
- Number of viable lymphocytes in a transfused component
- Severity of impairment of cellular immunity of the recipient
- Number of HLA alleles shared by donor and recipient
- Signs and symptoms typically appear 10 - 14 days after the transfusion or even later in the case of neonates; this may result in delayed recognition
- Organ system manifestations are generally similar to GVHD associated with allogeneic stem cell transplant, including fever, skin rash, hepatitis or hepatomegaly and enterocolitis with diarrhea
- However, TA-GVHD is uniquely associated with pancytopenia, due to marrow aplasia, leading to its mortality rate > 90% from neutropenic sepsis (Transfus Med 2013;23:416, Transfusion 2007;47:1405)
- Transfusion recipients at risk for TA-GVHD include:
- Patients with severe congenital or acquired immunodeficiency states, e.g. severe combined immune deficiency (SCID), DiGeorge syndrome, hematopoietic stem cell transplant (HSCT) recipients
- Patients being treated with purine analogues (fludarabine, clofarabine, bendamustine), antithymocyte globulin or alemtuzumab (anti-CD52 therapy)
- Fetuses and neonates: includes fetuses receiving intrauterine transfusion, premature infants and neonates requiring red cell exchange, due to their underdeveloped cellular immunity and the risk of as yet unrecognized congenital immunodeficiency (Transfusion 2011;51:916)
- Partially HLA matched blood products, where the recipient and the donor share some but not all HLA antigens (haplotypes)
- Patients receiving transfusions from blood relatives (directed donations) in geographical areas with genetically homogeneous populations, e.g. Japan (AABB: Standards for Blood Banks and Transfusion Services, 30th Edition, 2016)
- Universal irradiation may be considered, as in one study, 50% of TA-GVHD cases occurred in patients who would not be predicted to be at risk for TA-GVHD by current guidelines for blood irradiation (Blood 2015;126:406)
- Lack of donor recipient HLA disparity can lead to TA-GVHD among immunocompetent individuals in the following situation: the donor is homozygous for an HLA haplotype but the recipient is heterozygous for that haplotype, leading to a one way haplotype match in which the recipient is unable to recognize the donor as foreign but the donor reacts to the recipient as foreign (Blood 2015;126:406)
- Universal irradiation may be considered, as in one systemic review of TA-GVHD cases, 50% of cases occurred in patients who would not be predicted to be at risk for TA-GVHD by current guidelines for blood irradiation; although in the cases for which HLA data were available, the vast majority were found to involve donor antigens not recognized as foreign by the recipient (Blood 2015;126:406)
- Japan is one nation that uses universal irradiation of cellular blood components due to previously high rates of TA-GVHD among immunocompetent patients who happened to share HLA haplotypes (Transfus Med 2000;10:315)
Clinical features
- Due to the high mortality rate and limited viable treatment options, risk mitigation strategies for prevention of TA-GVHD are required
- Irradiation
- Irradiation leads to DNA damage and cell cycle arrest
- Dosage studies have established the radiation dosages necessary to inactivate T cells in a cellular blood component without impairing granulocyte function (Blood 1994;83:1683, Transfus Med 1996;6:261)
- Other potential preventive measures:
- In vitro and animal models have shown that pathogen inactivation methods involving photoactive compounds (e.g. synthetic psoralens or riboflavin) that damage DNA when exposed to UV light, also inactivate T cells with an efficacy comparable to irradiation (Transfusion 2018;58:1506, Bone Marrow Transplant 2009;44:205)
- However, to date, irradiation and pathogen inactivation have not been directly compared in a clinical trial
- Leukoreduction is not considered an effective preventive measure, due to incomplete removal of viable T lymphocytes; however, there is some evidence that leukoreduction may reduce the risk of TA-GVHD compared with no preventive measure
- Blood products that have been subjected to a freeze thaw cycle confer a significantly lower risk of TA-GVHD, due to inactivation of donor lymphocytes
- These products include fresh frozen plasma (FFP), thawed plasma, plasma frozen within 24 hours after collection (PF24), cryoprecipitate or frozen and deglycerolized RBC components
- In contrast, liquid plasma contains potentially viable T lymphocytes that may cause TA-GVHD in at risk recipients, since it has never been subjected to a freeze thaw cycle
- Irradiation
- Red cell and whole blood units have most commonly been implicated in TA-GVHD, although there have been instances of TA-GVHD from platelets (Blood 2015;126:406)
- Adverse effects of radiation include damage to cell membranes, affecting overall product recovery and potassium leak, particularly in the case of red cells (Vox Sang 2014;106:379)
- Due to concerns about hyperkalemia in neonates, many institutions perform additional processing of irradiated units intended for pediatric patients, including washing or transfusion of fresher red cell units (Transfusion 2008;48:2302)
- Expiration date on irradiated red cell components is 28 days or its original expiration date, whichever is earlier
- Extracellular potassium concentration in the blood component increases with longer storage of the component postirradiation (Vox Sang 2015;108:141)
Symptoms
- TA-GVHD typically presents 10 - 14 days after transfusion and is associated with fever, a maculopapular rash, diarrhea, hepatitis and occasionally jaundice
Screening
- There is no specific investigation that detects whether a patient is at risk of TA-GVHD
Laboratory
- Diagnosis of TA-GVHD is based primarily on characteristic clinical findings, although laboratory studies and tissue or bone marrow biopsy may be useful
- Complete blood count (CBC) and differential will show pancytopenia
- Peripheral blood smear will show decreases in all cell lineages with normal cellular morphology and should be reviewed to exclude other causes of acute onset pancytopenia, such as a thrombotic microangiopathy (red cell schistocytes), acute hematologic malignancy (blasts), Evan syndrome (red cell spherocytes, with or without larger platelets)
- Skin or liver biopsy will show characteristic histological findings of GVHD:
- Satellite dyskeratosis, vacuolization of the basal layer, histiocyte infiltration in skin (Arch Dermatol 1975;111:1597)
- Bile duct changes, including cytoplasmic vacuolization and lymphocytic infiltrate in liver (J Gastrointest Oncol 2016;7:S21)
- Molecular studies showing leukocyte chimerism (dual populations of donor and recipient lymphocytes) establish imputability of the diagnosis based on NHSN criteria (Transfus Med 2013;23:416, CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 18 January 2021])
- Bone marrow biopsy is not required for diagnosis but will show severe hypoplasia or aplasia; the presence of increased blasts, increased fibrosis or dysplasia may suggest alternative etiologies of the pancytopenia
- Blood bank / transfusion services considerations
- Sources of radiation include gamma rays from either a cesium-137 or cobalt-60 source or Xrays
- In the U.S., the requirements for the minimum radiation dose are
- At least 25 gray (Gy) or 2500 centigray (cGy) and no more than 50 Gy or 5000 cGy to the midplane of the container
- At least 15 Gy or 1500 cGy to all other parts of the container (AABB: Standards for Blood Banks and Transfusion Services, 30th Edition, 2016)
- A radiation sensitive label is applied to the blood product prior to irradiation, with the words NOT IRRADIATED visible (see Clinical images)
- After an appropriate dose has been administered to the container, only the word IRRADIATED remains visible (see Clinical images)
- Irradiators are regulated by the FDA as part of their oversight of biological products, including blood and blood components
- Facilities that perform irradiation must register with the FDA and are subject to inspections, including prelicense / preapproval inspections, routine inspections and "for cause" inspections (Fung: AABB Technical Manual, 19th Edition, 2017)
- In the U.S., increased security measures are required around gamma irradiators to protect against unauthorized access to radioactive materials; this does not apply to Xray irradiators (U.S. NRC: Radioactive Material Security [Accessed 18 January 2021])
- FDA has released recommendations regarding the gamma irradiation of blood products available through their website, including recommendations regarding maintenance of standard operating procedures, dosage delivery validation and labeling (FDA: Gamma Irradiation of Blood Products [Accessed 25 May 2021])
- Verification of dose delivery to a fully loaded canister should be performed annually for cesium-137 as a radiation source and semiannually for cobalt-60 as a radiation source; the frequency of dose delivery verification for alternate radiation sources is per the manufacturer
- In addition, dose delivery verification should be performed if the irradiator is moved or undergoes major repairs (AABB: Standards for Blood Banks and Transfusion Services, 31st Edition, 2018)
- National Nuclear Security Administration has incentivized voluntary replacement of cesium irradiators with FDA approved nonradioactive Xray irradiators through the Cesium Irradiator Replacement Project (National Nuclear Security Administration: Office of Radiological Security [Accessed 25 May 2021])
Case reports
- 13 day old boy with familial hemophagocytic lymphohistiocytosis and development of TA-GVHD, with supportive skin biopsy after receiving a nonirradiated red cell unit (J Pediatr Hematol Oncol 2017;39:e309)
- 7 month old boy developed pneumococcal sepsis and subsequent TA-GVHD, manifested by skin rash and supported by chimerism studies and was later found to have combined immunodeficiency (Transfusion 2010;50:2484)
- 42 year old woman with refractory lupus nephritis who developed TA-GVHD from treatment with fludarabine and cyclophosphamide, resulting in profound myelosuppression (Transfusion 2003;43:1667)
- 59 year old man who received multiple units of nonirradiated red blood cells for a gastrointestinal bleed, then subsequently developed TA-GVHD in the setting of multifactorial immune suppression, with chimerism showing shared HLA haplotype with a blood donor (Transfusion 2013;53:174)
Treatment
- Treatment for TA-GVHD is largely palliative; prevention is essential
- A systematic review that included 348 cases of TA-GVHD showed a marginal survival benefit with hematopoietic stem cell transplant (HSCT) and immunosuppression (Blood 2015;126:406):
- HSCT is usually not considered a viable option, due to time limitations in identifying a potential donor
- Immunosuppressive agents that have been considered as therapeutic options include corticosteroids, cyclosporine, intravenous immune globulin (IVIG) and antithymocyte globulin (ATG) and antilymphocyte globulin (ALG)
Clinical images
Sample assessment & plan
- Assessment: A 75 year old man presented to a local emergency department in an obtunded state after sustaining major bleeding in a motor vehicle accident. He received 5 units of emergency released, uncrossmatched packed red cells. It was subsequently discovered that the patient had received a kidney transplant at another institution, which was complicated by acute rejection requiring antithymocyte globulin (ATG) treatment. 14 days into his hospitalization for the motor vehicle accident, he developed a fever and a diffuse, erythematous, bullous skin rash. Complete blood count (CBC) revealed pancytopenia, including an absolute neutrophil count less than 100 cells/mm³ and a review of prior records showed that his CBCs had previously been normal. Bone marrow biopsy revealed a severely hypocellular marrow (10% cellularity). The patient then developed septic shock with multiorgan failure and blood cultures were positive for Candida albicans.
- Plan: This patient's presentation is concerning for transfusion associated graft versus host disease (TA-GVHD). His treatment with antithymocyte globulin as well as his advanced age put him at risk for impaired cellular immunity. To cement the diagnosis, the patient's skin rash should be biopsied. In addition, chimerism studies of the patient's CD3+ cells can be performed. If his CD3+ cells are homozygous for a certain HLA haplotype and the patient is heterozygous for that haplotype, this supports a one way HLA match that would further increase his risk for TA-GVHD.
Differential diagnosis
- Acute infection:
- Can lead to transient myelosuppression with rash, especially in the case of a viral infection; however, severe neutropenia is generally not observed
- Drug reaction:
- Fever, rash and other systemic symptoms may be seen in drug reaction with eosinophilia and systemic symptoms (DRESS)
- Most patients recover in weeks to months after drug withdrawal
- Aplastic anemia:
- Presentation tends to be subacute / chronic and review of prior CBCs shows progressive worsening of cytopenias
- Hypocellularity of bone marrow, which may show features of dysplasia in MDS-AA overlap
- Peripheral blood flow cytometry may show a paroxysmal nocturnal hemoglobinuria (PNH) clone
- Acute leukemia:
- Can lead to pancytopenia, although skin involvement is rare (localized skin eruptions can be seen in leukemia cutis)
- Bone marrow biopsy shows infiltration by malignant blasts
Board review style question #1
What is the best way to mitigate the risk of transfusion associated graft versus host disease (TA-GVHD) in a 12 year old boy receiving granulocytes for a disseminated fungal infection during induction therapy for acute leukemia?
- Leukoreduction
- Freeze thaw
- Gamma irradiation with no more than 20 gray (Gy) delivered to the midline of the blood container
- Gamma irradiation with at least 25 gray (Gy) and no more than 50 Gy delivered to the midline of the blood container
- Risk mitigation is not required since this patient is not at risk for TA-GVHD
Board review style answer #1
D. Gamma irradiation with at least 25 gray (Gy) and no more than 50 Gy delivered to the midline of the blood container
Comment Here
Reference: Irradiation
Comment Here
Reference: Irradiation
Board review style question #2
A 1 day old baby born at 36 weeks gestation requires a red cell exchange for hyperbilirubinemia due to hemolytic disease of the fetus and newborn. To maximize safety, which of the following packed red blood cell products will be ideal for this patient?
- Leukoreduced pRBC with a negative screen for sickle cells
- Leukoreduced pRBC with a negative screen for sickle cells that was irradiated 15 days ago
- Leukoreduced pRBC with a negative screen for sickle cells that is irradiated just prior to issuing from the blood bank
- No special processing is needed since this patient is not at risk for TA-GVHD
Board review style answer #2
C. Leukoreduced pRBC with a negative screen for sickle cells that is irradiated just prior to issuing from the blood bank
Comment Here
Reference: Irradiation
Comment Here
Reference: Irradiation
Kell system
Table of Contents
Definition / general | Essential features | Antigens | Antibodies | Terminology | Clinical features | Transmission | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Highly immunogenic
- Antibodies clinically significant
Essential features
- Most Kell antigens are widely expressed on the surface of red blood cells in the donor population
- Antibodies can cause hemolysis and hemolytic disease of the fetus and newborn (HDFN)
- Kell is expressed in early erythroid precursors, which can lead to suppression of erythropoiesis in the fetus
Antigens
- Type: peptides found within the Kell protein (CD238); the Kell blood group antigens are expressed on a large transmembrane protein with extensive disulfide bonds; the function of this protein is unknown (AABB: Technical Manual, 19th Edition, 2017)
- 36 antigens total (AABB: Technical Manual, 19th Edition, 2017)
- K or KEL1, often referred to erroneously as Kell antigen, is highly immunogenic but has low prevalence; it is encoded by the KEL gene on chromosome 7 (AABB: Technical Manual, 19th Edition, 2017)
- k or K2 (archaic Cellano), is a high prevalence antigen; it is a codominant allele and can be expressed alongside KEL1 (AABB: Technical Manual, 19th Edition, 2017)
- Many Kell antigens are expressed in high frequency amongst the donor population, specifically k, Jsb, Kpb and Ku; K (KEL1), is expressed less frequently and often matched in sickle cell disease patients due to its high immunogenicity
- Kell antigens are destroyed by dithiothreitol treatment, which is commonly used when evaluating panels for patients on daratumumab; as such, patients on daratumumab must undergo phenotyping prior to drug administration or genotyping in order to provide Kell matched red blood cell products, as antibodies cannot be identified by routine methods
- Kx is a protein associated with Kell antigens required for normal expression on red cells; absence of Kx, which is encoded by the XK gene, leads to decreased expression of Kell antigens, acanthocytosis and the clinical presentation of McLeod phenotype (AABB: Technical Manual, 19th Edition, 2017)
- Kell null phenotype arises in individuals inheriting defective KEL gene (K0) and may form Ku antibodies; Kell null red cells have no expression of Kell antigens but increased levels of Kx
Table 1: prevalence of KEL phenotypes
| White | 91 | 8.8 | 0.2 | 97.7 | 2.3 | Rare | 100 | Rare | 0 |
| African American (US) | 98 | 2 | Rare | 100 | Rare | 0 | 80 | 19 | 1 |
Antibodies
- Majority IgG, fewer IgM (AABB: Technical Manual, 19th Edition, 2017)
- Occurs with transfusion, pregnancy or activities such as intravenous drug use
- Can cause hemolytic reactions and hemolytic disease of the fetus and newborn
- Kell antigens are expressed in early erythropoiesis so there is immune clearance of red cell precursors; this leads to severe anemia without significant fetal hemolysis
- Kell null individuals can develop antibodies against Ku, a high frequency Kell antigen and subsequently require blood from other K0 (Kell null) individuals
- Not generally affected by dosage and unaffected by enzyme treatment
Terminology
- K or KEL1
- k or K2 (archaic Cellano)
Clinical features
- McLeod syndrome is an X-linked disease characterized by neuromuscular symptoms, such as cognitive abnormalities, movement disorders and psychiatric symptomology and ongoing hemolytic anemia; caused by an absence of the Xk protein (which expresses the Kx antigen) (GeneReviews: McLeod Neuroacanthocytosis Syndrome [Accessed 25 November 2020])
- The gene responsible for X-linked Chronic Granulomatous Disease is near the XK gene on the X chromosome and may be also be lost in patients presenting with McLeod phenotype (Blood: Chronic Granulomatous Disease and Mcleod Syndrome: Single Center Report of Four Cases [Accessed 11 February 2021])
Transmission
- Exposure to Kell antigens secondary to pregnancy or transfusion
Case reports
- 59 year old woman with a history of lymphoma developed a Kpa alloantibody (Asian J Transfus Sci 2018;12:81)
- 66 year old man with McLeod syndrome (BMC Neurol 2019;19:301)
- 66 year old man with the first description of an anti-Ku antibody in Korea (Korean J Lab Med 2009;29:238)
- Atypical presentation of hemolytic disease of the fetus and newborn in a Kell null mother (Transfus Med Hemother 2017;44:53)
- Anti-K1 antibody present in breast milk (Case Rep Pediatr 2017;2017:6927813)
Additional references
Board review style question #1
Which group of antigens is expressed early in erythropoiesis, leading to hemolytic disease of the fetus and newborn without significant hemolysis?
- Duffy
- Kell
- Lutheran
- Rh
Board review style answer #1
B. Kell. Erythropoiesis is inhibited in the fetus by binding of Kell antigens, which are expressed on early erythroid precursors. (N Engl J Med 1998;338:798)
Comment Here
Reference: Kell group
Comment Here
Reference: Kell group
Board review style question #2
Kell antigens have a high prevalence in the donor population. Which Kell antigen is expressed by almost all donors?
- K1
- K2
- Kpa
- Ku
Board review style answer #2
D. Ku. Ku or universal is expressed by the majority of the donor population. Of note, many Kell antigens are high frequency antigens such as k, Jsb and Kpb. K (KEL1) is less frequently expressed (~9%) but highly immunogenic.
Comment Here
Reference: Kell group
Comment Here
Reference: Kell group
Kidd system
Table of Contents
Definition / general | Essential features | Terminology | Antigens | Antibodies | Pathophysiology | Clinical features | Transmission | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Antigenic
- Antibodies clinically significant
Essential features
- Most Kidd antigens are widely expressed in the donor population
- Antibodies can cause hemolysis and hemolytic disease of the fetus and newborn (HDFN)
- Kidd antibodies often drop below the level of detection and are commonly implicated in delayed hemolytic transfusion reactions
- Kidd antibodies are often capable of activating complement and can cause intravascular hemolysis
Terminology
- Kidd a or Jka
- Kidd b or Jkb
Antigens
- Type: peptide
- 3 significant antigens: Jka, Jkb and Jk3 (AABB: Technical Manual, 20th Edition, 2020)
- Jka and Jkb are frequently expressed in most ethnic populations (AABB: Technical Manual, 20th Edition, 2020)
- Individuals negative for both antigens Jk(a-b-) are infrequent except in Polynesian and Finnish populations but can form antibodies against Jk3, a high incidence antigen in all individuals with any Jk positivity (Transfus Med Rev 2017;31:165)
- Kidd glycoprotein is encoded by the HUT11 / UT-B / JK (SLC14A1) gene encoded at 18q12-q21 (Transfus Med Rev 2017;31:165)
- Kidd glycopeptide functions as a transmembrane urea transporter on red cells, which helps to maintain cellular osmotic stability; also helps the kidney concentrate urine (AABB: Technical Manual, 20th Edition, 2020)
| Jk(a+b-) | Jk(a+b+) | Jk(a-b+) | Jk(a-b-) | |
| White | ||||
| Black (U.S.) | ||||
| Asian |
Antibodies
- Majority IgG, fewer IgM (AABB: Technical Manual, 20th Edition, 2020)
- Occur with exposure to products containing incompatible blood or pregnancy
- Antibodies tend to decrease over time; may become undetectable by blood bank methodology, posing a risk of transfusion reaction
- Can cause hemolytic reactions and hemolytic disease of the fetus and newborn
- Majority IgG1 or IgG3 and can fix complement (AABB: Technical Manual, 20th Edition, 2020)
- Antibodies are enhanced when using enzyme treated red cells
Pathophysiology
- Antibodies tend to decrease in level over time and then rapidly increase with subsequent exposure
- Plasma cell memory is called an anamnestic response
- Decrease in antibody level over time to a potentially undetectable level is known as evanescence
- Antibodies formed tend to be IgG1 or IgG2, which are better at fixing complement; this causes intravascular hemolysis clinically
- Reference: AABB: Technical Manual, 20th Edition, 2020
Clinical features
- Kidd antibodies often drop below the level of detection and are commonly implicated in delayed hemolytic transfusion reactions
- Kidd antibodies are often capable of activating complement and can cause intravascular hemolysis
- Reference: AABB: Technical Manual, 20th Edition, 2020
Transmission
- Exposure to Kidd antigens secondary to pregnancy or transfusion
Laboratory
- 2M urea testing can be used to distinguish Kidd positive versus Kidd null individuals; Kidd positive cells will lyse with a rapid influx of urea while Kidd null cells will remain intact
- Conversely, 5M urea is the concentration used for determining factor XIII deficiency
- Resistant to proteolytic enzymes, such as papain and ficin
- Reference: AABB: Technical Manual, 20th Edition, 2020
Case reports
- Newborn girl with hemolytic disease caused by anti-Jkb antibodies (Indian J Hematol Blood Transfus 2014;30:135)
- 32 year old woman with sickle cell disease developed hyperhemolysis secondary to multiple antibodies including Jkb (Indian J Hematol Blood Transfus 2016;32:251)
- 73 year old man with Jk3 sensitization after cardiac surgery (Kyobu Geka 2017;70:457)
- Case series of Jk3 antibody positive mothers (Transfusion 2018;58:1157)
- Novel Jk allele associated with Jk3 antibody production (Transfusion 2018;58:1078)
Additional references
Board review style question #1
A patient has a negative antibody screen and receives a red blood cell transfusion for symptomatic anemia. 2 days later, the patient develops hematuria and is found to have a positive direct antiglobulin test (DAT [IgG and C3]). A screen demonstrates a previously unidentified Jkb antibody. What antibody characteristic likely contributed to this delayed hemolytic transfusion reaction?
- Dosage
- Dithiothreitol treated red cells
- Enzyme treated red cells
- Evanescence
Board review style answer #1
Board review style question #2
What molarity of urea is used to determine if individuals are Jk(a-b-) and to evaluate for factor XIII deficiency, respectively?
- 1M / 2M
- 2M / 2M
- 2M / 5M
- 5M / 5M
Board review style answer #2
LDL apheresis
Table of Contents
Definition / general | Instrumentation | Epidemiology | Vascular access | Indications | Volume exchanged and technical details | Adverse events | Laboratory | Treatment | Additional referencesDefinition / general
- LDL apheresis: removal of LDL ("bad" cholesterol) by separating whole blood into its components and then selectively removing LDL from plasma by filtration / absorption; see guidelines at Circulation 2014;129:S1
Instrumentation
- Liposorber (Kaneka Pharma America):
- Whole blood is anticoagulated with heparin and then plasma is separated and run over a dextran sulfate column (U.S. Food and Drug Administration: Liposorber® LA-15 System [Accessed 28 January 2021])
- Typically processes 3 blood volumes, resulting in 56 - 65% reduction in LDL
- Plasmat Secura (B. Braun Medical):
- Can also precipitate the LDL heparin complex (B.Braun Medical: LDL Apheresis Therapy [Accessed 1 November 2017])
- LDL typically decreases by 67% after each treatment
- Both instruments are U.S. FDA approved to reduce LDL cholesterol
- There is minimal impact on HDL ("good") cholesterol
Epidemiology
- Familial hypercholesterolemia:
- High LDL levels from birth secondary to genetic mutation in ApoB / E (LDL) receptor
- Autosomal dominant
- Associated with early LDL deposits in tendons forming xanthomata; also early onset coronary artery disease
- Patients with 2 mutations have more severe disease (OMIM: Hypercholesterolemia, Familial [Accessed 1 November 2017])
- Lipoprotein(a) hyperlipoproteinemia:
- Lipoprotein(a) is a modified LDL unit that is coupled to apolipoprotein A via a disulfide bond
- Excess lipoprotein(a) can lead to accelerated atherosclerosis (OMIM: Apolipoprotein(a); LPA [Accessed 1 November 2017])
- Refsum disease:
- Autosomal recessive disorder that results in tetrad of abnormalities:
- Retinitis pigmentosa, peripheral neuropathy, cerebellar ataxia and elevated protein levels in cerebrospinal fluid, without an increase in the number of cells
- Most common presenting symptom is night blindness (OMIM: Refsum Disease, Classic [Accessed 1 November 2017])
- Treat by removing excess phytanic acid plus dietary modification (avoid dairy)
- Autosomal recessive disorder that results in tetrad of abnormalities:
- Sudden sensorneurial hearing loss:
- Multiple etiologies, including elevated cholesterol or fibrinogen, which compromises the terminal blood supply to inner ear (cochlea or cochlear nerve), causing unilateral or bilateral hearing loss (National Institutes of Health: Sudden Deafness [Accessed 1 November 2017])
Vascular access
- LDL apheresis can be performed either with peripheral IV access or with a central venous catheter (CVC)
- However, chronic treatment and CVC is nearly always required; may begin at ages 6 - 7 years for homozygous familial hypercholesterolemia cases
Indications
- For therapeutic procedure, the American Society for Apheresis (ASFA) delineates LDL apheresis based on the acuity of the clinical presentation
- Familial hypercholesterolemia (FH) LDL apheresis homozygotes: Category I
- Familial hypercholesterolemia LDL apheresis heterozygotes: Category II
- Liprotein(a) hyperlipoproteinemia LDL apheresis: Category II
- Peripheral vascular diseases LDL apheresis: Category III
- Phytanic acid storage disease (Refsum disease) LDL apheresis: Category II
- Sudden sensorineural hearing loss LDL apheresis: Category III
Volume exchanged and technical details
- All LDL apheresis systems use heparin alone or in combination with citrate as its anticoagulant so must screen patients for history of heparin induced thrombocytopenia (HIT)
- The Liposorber instrument only uses heparin; citrate interferes with the interaction between the column and apoB lipoproteins
Adverse events
- Overall adverse event rate is approximately 11% with no difference between instruments
- Most are minor: post procedure bleeding, vomiting, hypotention, hypoglycemia
- Discontinue ACE inhibitor medication prior to adsorption LDL apheresis; it causes excess bradykinin accumulation, leading to hypotensive reactions; can use angiotensin receptor blockers (ARBs) as an alternative
- Longstanding LDL apheresis can lower vitamin B12, ferritin and transferrin, contributing to anemia and possible need for dietary supplements
Laboratory
- Direct measurement of LDL levels is infrequently performed
- LDL levels are usually calculated using the Friedewald calculation: LDL = Total Cholesterol - HDL - Triglycerides/5.0 (mg/dL) (MDCalc: LDL Calculated [Accessed 1 November 2017], Clin Chem 1972;18:499, Clin Chem 2009;55:888)
Treatment
- For FH, treatment can be lifelong to stabilize or improve coronary artery atherosclerosis
- Frequency of procedure is adjusted to maintain target LDL level
- Multiple LDL procedures are needed to reduce the time averaged LDL levels
- LDL level can decrease dramatically after single procedure but long term goal is > 60% reduction from baseline
Additional references
Leukocytapheresis
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Vascular access | Indications | Volume exchanged and technical details | Adverse events | Laboratory | Case reports | Treatment | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Leukocytapheresis is the removal of white blood cells via apheresis
Essential features
- Therapeutic leukocytapheresis is performed to acutely lower a patient's white blood cell (WBC) count due to symptoms associated with hyperleukocytosis or increased viscosity
Terminology
- Hyperleukocytosis (J Clin Apher 2019;34:171)
- Generally defined as WBC count or blast count > 100,000/μL
- Can be symptomatic with clinical manifestations or asymptomatic
- Can lead to hyperviscosity / leukostasis syndrome
- Hyperviscosity / leukostasis symptoms (J Clin Apher 2019;34:171)
- Cerebrovascular insufficiency (altered mentation, transient ischemic attack, stroke)
- Pulmonary complications (dyspnea, hypoxemia)
- Disseminated intravascular coagulopathy (DIC)
- More common in leukemia patients with monocytic or myeloid differentiation
Pathophysiology
- Usually associated with (J Clin Apher 2019;34:171)
- Acute myeloid leukemia (AML) with WBC > 100,000/μL
- Incidence of AML with WBC > 100,000/μL is 12 - 18% in children and 5 - 18% in adults
- Can occur with blast count > 50,000/μL especially with monocytic differentiation
- Cells release cytokines which can lead to cellular adhesion (Blood 2015;125:3246)
- Lymphoblastic leukemia / lymphoma (ALL) with WBC > 400,000/μL (> 3% of ALL patients)
- Acute myeloid leukemia (AML) with WBC > 100,000/μL
Clinical features
- Patients may present with DIC, tumor lysis syndrome or hypoxic ischemic type injury
- Symptoms include vision changes, headache and shortness of breath
- Neurologic symptoms may progress in the setting of intracranial hemorrhage
Symptoms
- Hyperleukocytic symptoms manifest as hypoxic ischemic type injury associated with poor perfusion
- A grading schema for hyperleukoctyic leukemia was described by Novotny, et al. (Eur J Haematol 2005;74:501)
Grading schema (adapted from Eur J Haematol 2005;74:501)
| Group | Probability of leukostasis syndrome | Severity of symptoms | Pulmonary symptoms | Neurologic symptoms | Other organ systems |
| 0 | Not present | No limitations | No symptoms and no limitations in ordinary activities | No neurologic symptoms | No symptoms |
| 1 | Possible | Slight limitations | Mild symptoms and slight limitation during ordinary activity, comfortable at rest | Mild tinnitus, headache, dizziness | Moderate fatigue |
| 2 | Probable | Marked limitations | Marked limitation in activity because of symptoms, even during less than ordinary activity, comfortable only at rest | Slight visual disturbances, severe tinnitus, headache, dizziness | Severe fatigue |
| 3 | Highly probable | Severe limitations | Dyspnea at rest, oxygen or respirator required | Severe visual disturbances (acute inability to read), confusion, delirium, somnolence, intracranial hemorrhage | Myocardial infarction, priapism, ischemic necrosis |
Vascular access
- Usually performed emergently using femoral apheresis or a dialysis catheter, which does not require radiographic confirmation
- Central venous catheter, which requires radiographic confirmation, can be used in nonemergent situations
Indications
- For therapeutic procedure, the American Society for Apheresis (ASFA) delineates leukapheresis based on the severity of the clinical presentation (J Clin Apher 2019;34:171)
- Symptomatic leukostasis is ASFA category II
- Prophylaxis is ASFA category III
- ALL: WBC or blast count > 400,000/μL
- AML: WBC or blast count > 100,000/μL
- May be considered in monocytic subtypes of AML with WBC as low as 50,000/μL
- Acute promyelocytic leukemia (APL) is a relative contraindication as studies have shown no improvement and one group showed increased mortality (J Clin Apher 2019;34:171)
- APL has a strong association with DIC, and leukapheresis can induce a cytokine storm
- All trans retinoic acid (ATRA) therapy is first line treatment for APL
- Leukocytosis is now a category III indication regardless of symptoms (J Clin Apher 2023;38:77)
Volume exchanged and technical details
- Generally limited to 1 procedure but number of treatments depends on WBC count decrease
- 1.5 - 2 blood volume is usually performed for therapeutic leukapheresis procedure
- This usually reduces the WBC count by 30 - 60%, although predicting the postprocedural WBC count is difficult due to mobilization of leukocytes from extramedullary sites
- 6% hydroxyethyl starch (HES) is not necessary in leukemic patients
- Red blood cell (RBC) units may be used with caution to prime the apheresis machine in patients with severe anemia but undiluted RBCs can increase the blood viscosity
- Replacement fluid is recommended
- Reference: J Clin Apher 2019;34:171
Adverse events
- Leukapheresis is an apheresis procedure, with its typical complications
- Also complications from the underlying leukemia, hypotension, citrate related toxicity, bleeding and infection
Laboratory
- Elevated WBC
- Elevated lactate dehydrogenase (LDH)
Case reports
- 16 year old boy with hyperleukocytosis found to have T lymphoblastic leukemia / lymphoma (T ALL) was treated with leukocytapheresis and developed intracranial hemorrhage (J Pediatr Hematol Oncol 2021;43:e812)
- 25 year old woman with chronic myeloid leukemia (CML) treated with leukocytapheresis (Cureus 2020;12:e12375)
- 77 year old woman with chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL / SLL) was managed with leukocytapheresis (Am J Case Rep 2020;21:e924798)
Treatment
- Chemotherapy should be initiated as quickly as possible and should supersede leukocytapheresis if patients are asymptomatic
- Therapeutic leukapheresis is not curative; WBC reduction is short lived and only a bridging therapy to definitive treatments such as chemotherapy
- Studies show no improvement in early mortality with leukapheresis, though patients with symptomatic leukostasis are more likely to undergo the procedure (Transfusion 2020;60:2360)
- Most studies are observational given the critically ill population investigated (Hematology 2022;27:141)
- Symptomatic leukostasis treatment (J Clin Apher 2019;34:171)
- Can be performed daily or as needed to treat leukemic patients with high WBC count with hyperviscosity syndrome
- WBC count has a poor correlation with clinical symptoms; thus defining a WBC or blast goal is not optimal
- Treatment should be continued until symptoms are resolved and WBC or blast count < 400,000/μL in ALL patients or < 50,000 - 100,000/μL in AML patients
- Chemotherapy should be given concurrently with leukapheresis to prevent the rapid reaccumulation of WBC and blasts
- Leukapheresis might decrease early death in leukemic patients with hyperviscosity but does not appear to increase overall survival
- Can be performed daily or as needed to treat leukemic patients with high WBC count with hyperviscosity syndrome
- For prophylaxis (J Clin Apher 2019;34:171)
- Leukapheresis is not superior to aggressive chemotherapy and supportive care
- Might be useful in children with ALL and WBC > 400,000/μL (50% of these patients develop pulmonary complication from leukostasis)
- Goal
- ALL: WBC or blast count > 400,000/μL
- AML: WBC or blast count > 100,000/μL
- AML with monocytic differentiation: > 50,000/μL
Sample assessment & plan
- Assessment: A 17 year old boy with T ALL underwent leukocytapheresis for hyperviscosity. 1.5 blood volumes were processed. Postprocedure WBC count is pending. The patient tolerated the procedure well.
- Plan: Additional procedures will be performed as necessary to reduce WBC count > 400,000/μL but should not prevent initiation of chemotherapy.
Additional references
Board review style question #1
A patient presents with the microgranular variant of acute promyelocytic leukemia (APL) with a white blood cell (WBC) count of 125,000/μL. What is the appropriate first step when a diagnosis is suspected?
- All trans retinoic acid (ATRA) therapy
- Induction chemotherapy
- Leukocytapheresis
- Stat PML::RARA t(15;17) fluorescence in situ hybridization (FISH)
Board review style answer #1
A. All trans retinoic acid (ATRA) therapy. Before a diagnosis is established, ATRA should be administered if APL is suspected or presumed as this can rapidly improve the risk of mortality. Newly diagnosed APL is a contraindication for leukocytapheresis due to risk of increased mortality. FISH analysis to confirm the diagnosis would be a good next step.
Comment Here
Reference: Leukocytapheresis
Comment Here
Reference: Leukocytapheresis
Board review style question #2
What is the white blood cell (WBC) count suggested for prophylactic leukocytapheresis in B lymphoblastic leukemia / lymphoma (B ALL)?
- 20,000/μL
- 50,000/μL
- > 100,000/μL
- > 400,000/μL
Board review style answer #2
D. > 400,000/μL. For lymphoblastic leukemia / lymphoma, leukocytapheresis triggers are WBC or blast count > 400,000/μL. For acute myeloid leukemia (AML), WBC or blast count > 100,000/μL. For AML with monocytic differentiation, > 50,000/μL.
Comment Here
Reference: Leukocytapheresis
Comment Here
Reference: Leukocytapheresis
Lewis system
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Lewis antigens are carbohydrates that are present in the plasma and adsorbed to the red cell surface
- Antibodies are generally not clinically significant
Essential features
- Lewis antigens are created by enzymatic modification of type 1 chains
- Lewis antibodies are generally clinically insignificant
- Levels drop with increase in plasma volume, specifically common in pregnancy
- Lewis acts as a receptor for H. pylori
Terminology
- Lea (LE1)
- Leb (LE2)
Pathophysiology
- Lewis antigens are carbohydrates synthesized from type 1 chains with the fucosyltransferase FUT3
- Unmodified type 1 chains are converted to Lea, while H chains are converted to Leb
- FUT2 (secretor) is more efficacious than FUT3 and therefore most type 1 chains are converted to H and Leb rather than Lea
- Nonsecretors cannot produce Leb and only make Lea
- Absence of FUT2 and FUT3 leads to the Le(a-b-) phenotype
- Rarely patients have weak secretor activity and produce a Le(a+b+)
- This phenotype is more common in Asian populations
- Individuals with Leb typically have some Lea present, which may not be detected with serologic phenotyping
- Lewis antigens are present in the plasma and secretions (e.g., saliva and milk) and passively adhere to the RBC surface
- In order for individuals to elaborate Lewis A, unmodified type I chains must be modified by FUT3
- To produce Lewis B, H antigens produced by individuals with the secretor gene (FUT2) are subsequently modified to form Lewis B antigens
- FUT2 acts on type I chains more avidly and therefore most of the Lewis antigens produced in secretors with FUT3 are Lewis B, though a small proportion of Lewis A is likely present based on the low rate of anti-Lewis A in this population
- Reference: Fung: Technical Manual, 19th Edition, 2017
Clinical features
- Lewis antigens modify chains with FUT3 to form Lea and H to form Leb
- Levels can decrease during pregnancy and patient’s cells may phenotype as Lea-Leb-
- At this time, patients may develop Lewis antibodies, which are clinically insignificant
- Not associated with hemolytic disease of the fetus and newborn (HDFN)
- Most antibodies are IgM, which does not cross into fetal circulation; Lewis antigens are not expressed extensively on fetal red cells
- Single report of HDFN with Lea (Reid: The Blood Group Antigen FactsBook, 3rd Edition, 2012)
- Leb acts as a receptor for H. pylori
- Antigens (type: carbohydrate) (Fung: Technical Manual, 19th Edition, 2017)
- 2 common antigens: Lea and Leb
- Leb antigens can be modified by A and B enzymes leading to ALeb and BLeb
Adapted from Fung: Technical Manual, 19th Edition, 2017Race / ethnicity Le(a+b+) Le(a+b-) Le(a-b+) Le(a-b-) White Rare 22 72 6 Black (U.S.) Rare 23 55 22
- Antibodies
- Majority are IgM but some are IgG (Lea > Leb) and not clinically significant
- Lewis substance circulates freely in the plasma and can readily bind antibodies without associating with RBCs
- Lewis antigens are not strongly expressed on cord blood cells, effectively preventing HDFN, even in the presence of an IgG component (Reid: The Blood Group Antigen FactsBook, 3rd Edition, 2012)
- Occur with exposure to products containing incompatible blood; naturally occur during pregnancy (Fung: Technical Manual, 19th Edition, 2017)
- Rare reports of hemolytic transfusion reactions; a single report of HDFN with Lea (Reid: The Blood Group Antigen FactsBook, 3rd Edition, 2012)
- Majority are IgM but some are IgG (Lea > Leb) and not clinically significant
- Disease associations (Wiley Interdiscip Rev Syst Biol Med 2016;8:517):
- Norovirus found to bind to Lewis antigens as well as A and H antigens
- Lewis nonsecretors Le(a+,b-) may have more severe cholera infections and Lewis null individuals have more prolonged diarrheal illness
- H. pylori lipopolysaccharide demonstrates mimicry of Lewis antigens leading to antibody formations; H. pylori infection is associated with extranodal marginal zone lymphoma of mucosa associated lymphoid tissue (MALT lymphoma) (Gut 2000;47:10)
Transmission
- Exposure to Lewis antigens secondary to pregnancy or transfusion
Laboratory
- Levels can decrease during pregnancy or with increased plasma volume; patient's cells may phenotype as Lea-Leb-
- Lewis antigen levels decrease with red cell storage and therefore phenotyping is most reliable on fresh units
- Lewis antibodies can sometimes cause in vitro hemolysis (Lea > Leb)
- References: Fung: Technical Manual, 19th Edition, 2017, Kreuter: Transfusion Medicine Self-Assessment and Review, 2017
Case reports
- 19 year old man, a blood donor, was found to have clinically significant anti-Leb causing transfusion reaction (Asian J Transfus Sci 2020;14:198)
- 21 - 65 year old patients with Lewis system antibodies (Asian J Transfus Sci 2020;14:54)
- 30 year old African American woman with acute hemolytic transfusion reaction due to anti-Le(b) (Transfusion 2015;55:2486)
- 31 year old woman with Le(a-b+) phenotype and novel para-Bombay phenotype (Transfus Med Hemother 2021;48:254)
Additional references
Board review style question #1
A pregnant woman is found to have a Leb antibody. The OB / GYN resident consults the blood bank for advice. What do you recommend?
- Counsel the patient that these antibodies do not cause hemolytic disease of the fetus and newborn (HDFN)
- Percutaneous umbilical cord blood sampling
- Serial titers
- Transcranial Doppler ultrasound
Board review style answer #1
A. Counsel the patient that these antibodies do not cause HDFN. Lewis antibodies tend to be IgM, which do not cross the placenta; likewise, fetal red cells do not express Lewis antigens. Pregnant patients may transiently develop Lewis antibodies later in their pregnancy.
Comment Here
Reference: Lewis
Comment Here
Reference: Lewis
Board review style question #2
Lewis antigen b (Leb) acts as a receptor for which microorganism?
- Brachyspira aalborgi
- Giardia lamblia
- H. pylori
- P. ovale
Board review style answer #2
Lookbacks
Table of Contents
Definition / general | Essential features | Terminology | Applications | Implementation | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Traces the source of a relevant transfusion transmissible infection (RTTI) or transfusion transmitted disease (TTD) by a recipient of blood product(s) or notifies a recipient who was transfused with blood product(s) from a donor who is currently confirmed positive for a RTTI, in accordance with Food and Drug Administration (FDA) regulations
- Additionally, recalls / market withdrawals may be issued in situations such as improper donor testing or postdonation information
Essential features
- Performed to find previously distributed units from donors who are considered positive / reactive for a given infectious disease
- Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) require a lookback according to the U.S. FDA
- If blood or a blood product / component implicated in a lookback has been transfused, consignees of the product must have a process to notify a recipient, their physician of record, their legal representative or in the case of HIV, next of kin
Terminology
- Nonconformance
Applications
- Receipt of consignee notification for a product disposition from a collection facility
- Provider notifies transfusion services that a patient has tested positive for a possible TTD after receiving a blood component transfusion at their facility
- TTD or RTTI is identified during a transfusion reaction investigation, RTTIs are defined by the FDA (eCFR: Code of Federal Regulation Title 21 [Accessed 9 November 2023])
Implementation
- Blood establishments must
- Quarantine and destroy any products within their control and notify consignees (those in receipt of the blood or blood component, such as a hospital transfusion service)
- Note: for the purpose of this outline, transfusion service and consignee will be used interchangeably
- Test the donor per FDA's code of federal regulations or relevant guidance documents, depending on the infectious disease agent
- Notify transfusion services so that they can notify recipients, if required by FDA or per medical director discretion or transfusion service policy when no FDA regulation exists
- Quarantine and destroy any products within their control and notify consignees (those in receipt of the blood or blood component, such as a hospital transfusion service)
- When a lookback notification process by the blood establishment to the transfusion service is required, the transfusion service must disposition the unit and report the status back to the donor center
- If not transfused
- Quarantine recalled products in inventory
- Destroy or return to the collection facility as requested
- Return documentation of product's final disposition to the blood collection facility which issued the withdrawal / lookback
- If the product has been shipped, request final disposition from the receiving facility
- Discarded
- Expired, discarded
- Report back to supplier
- No further action necessary
- If transfused
- Review FDA Code of Federal Regulations
- FDA guidance for industry
- American Association of Blood Banks (AABB) standards / bulletins
- In situations where notification is recommended, it is advisable to contact the recipient's physician first
- If that physician is unavailable or declines to notify the recipient, the medical director of the transfusion medicine service or designee assumes responsibility for notifying the recipient
- In general, the only clearly defined lookbacks by the FDA mandating notification of the recipient or the recipient's physician or a legal representative involve blood components considered positive with HIV or HCV
- Lookbacks require notification of transfusion recipients in receipt of blood or blood components collected during the 12 months before the date of reactive testing
- HIV also requires notification of next of kin, should the recipient be deceased
- For HIV, this would occur when either the nucleic acid test (NAT) for HIV is positive / reactive or HIV antibody testing and confirmatory testing is also positive or indeterminate
- For HCV, this would occur when either nucleic acid testing for HCV is positive / reactive, anti-HCV is repeatedly reactive or another reliable test indicates HCV infection
- Notification of the recipient, their physician, legal representative or next of kin when it applies, should be provided within 12 weeks of receiving the test results (FDA: Code of Federal Regulations Title 21 - Sec. 610.46 [Accessed 9 November 2023], FDA: Code of Federal Regulations Title 21 - Sec. 610.47 [Accessed 9 November 2023], FDA: Lookback for Hepatitis C Virus [Accessed 9 November 2023])
- Blood establishments issue market withdrawals and recalls for nonlegally binding situations involving positive testing or when postdonation information is disclosed to the blood establishment, leading to deviation from protocol and where there is potential for harm to a transfusion recipient, such as
- Hepatitis B virus (HBV), typically due to positive HBV nucleic acid test or hepatitis B surface antigen (HBsAg) with positive neutralization assay results
- Babesia reactivity in cellular blood products (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 9 November 2023])
- Malaria, in order to monitor recipient for 3 months posttransfusion of cellular blood product, except source plasma (FDA: Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria [Accessed 9 November 2023])
- Trypanasoma cruzi (Chagas disease), to encourage consignees to notify the recipient's physician (FDA: Use of Serological Tests to Reduce the Risk of Transmission of Trypanosoma cruzi Infection in Blood and Blood Components [Accessed 9 November 2023])
- West Nile virus (FDA: Assessing Donor Suitability and Blood and Blood Product Safety in Cases of Known or Suspected West Nile Virus Infection [Accessed 9 November 2023])
- Ebola virus, where the FDA recommends consignees notify the recipient's physician for notification and monitoring (FDA: Recommendations for Assessment of Blood Donor Eligibility, Donor Deferral and Blood Product Management in Response to Ebola Virus [Accessed 9 November 2023])
- Variant Creutzfeldt-Jakob disease (vCJD), so that the consignee medical director can consider notifying the recipient's physician of record for counseling purposes (FDA: Recommendations to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Components [Accessed 9 November 2023])
- Postdonation information involving certain medications that compromise coagulation or are teratogenic, disclosure of high risk behavior, travel, illness, etc. (Ann Clin Lab Sci 2020;50:538)
Diagrams / tables
Additional references
Board review style question #1
Which of the following includes 3 relevant considerations for the risks / benefits of notification of a recipient for a TTI?
- Existence of a test for donors, moderate risk and existence of a possible intervention
- Existence of a test for recipients, existence of a test for donors and presence of a probable risk
- Presence of small theoretical risk, existence of a test for donors and existence of a possible intervention
- Presence of well established risk, existence of a test for recipients and existence of a possible intervention
Board review style answer #1
D. Presence of well established risk, existence of a test for recipients and existence of a possible intervention. In recalls without mandatory notification of recipients of a transfused blood component, relevant considerations that may guide the decision to notify include the presence of a well established risk, the existence of a test for recipients and existence of a possible intervention in the case of a positive test result. When these 3 are not present, the risk of the psychological harm associated with notifying a recipient or their relative may outweigh the benefit of such notification. Answers A and C are incorrect as the risk to the donor must be well established. Answer B is incorrect since both the possibility for intervention and the presence of a well established risk to the donor must be present in addition to an available test.
Comment Here
Reference: Lookbacks
Board review style question #2
What transfusion transmitted infections listed in the FDA's code of federal regulations require lookbacks?
- Babesia and HIV
- Ebola and HCV
- HIV and HCV
- Trypanosoma cruzi
Board review style answer #2
C. HIV and HCV. Only HIV and HCV are listed in the FDA's code of federal regulations as having required lookbacks. Answer A is incorrect because Babesia is not addressed in the FDA's code of federal regulations as a lookback. Answer B is incorrect because FDA's code of federal regulations does not require a lookback for Ebola; however the recommendation is to inform the recipient's physician. Answer D is incorrect because a lookback is also not required for Trypanasoma cruzi according to the FDA's code of federal regulations; however it is recommended that consignees of transfused products notify the recipient's physician.
Comment Here
Reference: Lookbacks
Comment Here
Reference: Lookbacks
Board review style question #3
What is the time limit within which notification to the recipient, their physician, legal representative or next of kin should be done when a blood component has been implicated in a lookback mandated by the FDA?
- 72 hours
- 2 weeks
- 12 weeks
- 12 months
Board review style answer #3
C. 12 weeks. Notification to the recipient, their physician, legal representative or next of kin when it applies should be done within 12 weeks. Lookbacks require notification of transfusion recipients in receipt of blood or blood components collected during the 12 months before the date of reactive testing. Answers A and B are incorrect because 12 weeks allows for further testing to confirm the presence of HCV or HIV if screening results are reactive. Answer D is incorrect because 12 months may increase the risk of viral transfer to others.
Comment Here
Reference: Lookbacks
Comment Here
Reference: Lookbacks
Massive transfusion
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Laboratory | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Traumatic injury is the most common cause of hemorrhage requiring massive transfusion
- Other causes include gastrointestinal, obstetric and surgical bleeding
- Various definitions for massive transfusion (Indian J Anaesth 2014;58:590)
- Replacement of 1 entire blood volume within 24 hours
- Transfusion of > 10 units of red blood cells (RBCs) in 24 hours
- Transfusion of > 20 units of RBCs in 24 hours
- Transfusion of > 4 units of RBCs in 1 hour when ongoing need is foreseeable
- Replacement of 50% of total blood volume (TBV) within 3 hours
Essential features
- Severe blood loss leading to the lethal triad of trauma (hypothermia, coagulopathy, acidosis)
- Transfuse RBCs, platelets and plasma in 1:1:1 ratio
- O negative RBCs reserved for females of childbearing age and pediatric patients with unknown blood type
- Type A plasma may safely be used in trauma patients with unknown blood type
- Early administration of tranexamic acid (TXA) reduces mortality from bleeding in trauma patients
Terminology
- Massive transfusion protocol (MTP)
- Massive transfusion guideline (MTG)
Pathophysiology
- Severe blood loss leading to the lethal triad of trauma (hypothermia, coagulopathy, acidosis)
- Trauma induced coagulopathy resulting in a hypocoagulable state (Curr Opin Anaesthesiol 2016;29:212)
- Increased tissue factor expression and thrombin generation due to trauma
- Rapid consumption of coagulation factors (especially factors V and VIII)
- Increased thrombomodulin and activated protein C
- Increased plasmin activity leading to hyperfibrinolysis
- Thrombocytopenia, platelet dysfunction and decreased platelet margination
- Iatrogenic hemodilution with crystalloids can cause edema induced ischemia and exacerbate coagulopathy and hypothermia
Clinical features
- Progressive symptoms depending on severity of hemorrhagic shock (N Engl J Med 2018;378:370)
- Tachycardia is the earliest indicator
- Pale / cool skin and extremities due to shunting of blood to vital organs
- Oliguria
- Lightheadedness, confusion or loss of consciousness
- Chest pain or abdominal pain and swelling may indicate internal hemorrhage
- Vomiting blood or blood in stool may indicate GI bleed
Laboratory
- Laboratory values may be normal in early acute hemorrhage and progressively deteriorate as interstitial fluid enters the intravascular space and crystalloids are infused
- Anemia and thrombocytopenia
- Arterial blood gas demonstrates decreased pH (acidosis) and decreased oxygen saturation
- Acute renal failure during oliguric phase
- Decreased glomerular filtration rate (GFR), increased serum creatinine and blood urea nitrogen (BUN); increased urine specific gravity and osmolality
- Conventional coagulation testing
- Increased prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR)
- Decreased fibrinogen
- Viscoelastic testing / thromboelastography (TEG)
- May demonstrate hypercoagulable tracing in early hemorrhage or moderate trauma
- Hypocoagulable tracing and hyperfibrinolysis with increasing injury severity score
- Electrolyte & metabolic disturbances due to massive transfusion
- Decreased plasma ionized calcium (iCa < 1.15 mmol/L) due to chelation by citrate in transfused blood products
- Hypocalcemia can manifest as tingling in fingers and lips, muscle cramps, tetany, arrhythmias
- Potassium accumulation in RBC supernatant may cause hyperkalemia with the potential for cardiac arrest
- Massive transfusion of neonates can result in transient hyperglycemia followed by large insulin release and rebound hypoglycemia
- Reference: Curr Anesthesiol Rep 2014;4:189
Case reports
- 14 month old girl requiring massive transfusion during neurosurgery (Am J Case Rep 2016;17:211)
- 16 year old girl, 35 year old woman and 36 year old man in cases with use of warm fresh whole blood in massive hemorrhage (Ulus Travma Acil Cerrahi Derg 2016;22:195)
- 35 year old woman with massive transfusion in emergency cesarean section (Case Rep Womens Health 2019;23:e00130)
- 60 year old woman with fatal hyperkalemia after massive transfusion (J Nippon Med Sch 2009;76:258)
- Male soldier with use of TEG after massive transfusion (Anaesthesia 2011;66:52)
Treatment
- Transfuse RBCs, platelets and plasma in 1:1:1 ratio (JAMA 2015;313:471)
- Adult patients receive 5 - 6 RBCs and equivalent number of plasma units and whole blood derived platelets
- 1 apheresis platelet is equivalent to a 6 pack (pool) of whole blood derived platelets
- Intraoperative cell salvage may distort blood product order ratios with an increased need for plasma and platelets relative to RBCs
- Whole blood offered in military or remote settings and increasingly in civilian trauma
- Benefit includes longer shelf life of whole blood than that of platelets
- Blood product selection
- Patients with valid type and screen may receive ABO type specific or compatible RBCs and plasma for massive transfusions if institutional policy allows
- O negative RBCs reserved for females of childbearing age and pediatric patients with unknown blood type; O positive should be used for other patients
- Type A plasma may safely be used in trauma patients with unknown blood type (Transfusion 2017;57:1879)
- Any platelet can be selected regardless of patient blood type but institutional policy may require ABO compatibility or the use of low titer platelets depending on patient age or weight
- Cryoprecipitate not universally included in massive transfusion sets but may provide mortality benefit (Br J Anaesth 2015;115:76)
- Early administration of tranexamic acid (TXA) reduces mortality from bleeding in trauma patients and in postpartum hemorrhage (Lancet 2010;376:23, Lancet 2017;389:2105)
- TXA: a synthetic analog of lysine
- Calcium chloride infusion as needed to maintain iCa > 2 mEq/L (4 mg/dL)
- Laboratory guided transfusion
- Can be used after initial resuscitation with massive transfusion
- Plasma for INR > 2.0 or prolonged ACT/R time on TEG
- Cryoprecipitate or fibrinogen concentrate for fibrinogen < 100 mg/dL or prolonged K time/decreased α angle on TEG
- Platelets for count < 50,000 per microliter or decreased max amplitude on TEG
- Tranexamic acid for increased LY30 (amplitude at 30 minutes) on TEG, indicative of increased fibrinolysis
- Recombinant factor VIIa can be utilized in the setting of oozing with a normal TEG and abnormal prothrombin time
Board review style question #1
A young female is brought into the trauma center after a motor vehicle accident with several injuries and large volume bleeds from the abdomen and lower extremities. The patient was infused with crystalloid solution on the way to the hospital due to blood loss and vital sign changes indicating hypovolemic shock. An order is placed for a massive transfusion while the patient is taken to the operating room. What would be the most appropriate set of blood products to issue in this situation if the patient's blood type is still unknown?
- 6 units O positive RBCs, 6 units AB plasma and a 6 pack of whole blood derived platelets
- 1 unit O negative RBC, 1 unit A plasma and 1 apheresis platelet unit
- 6 units O negative RBCs, 6 units A plasma and 6 apheresis platelet units
- 1 unit O negative RBC, 1 unit AB plasma and 1 whole blood derived platelet unit
- 6 units O negative RBCs, 6 units AB plasma and 1 apheresis platelet unit
Board review style answer #1
E. 6 units O negative RBCs, 6 units AB plasma and 1 apheresis platelet unit. The patient is a young female with an unknown blood type and therefore should receive type O negative RBCs. Type O RBCs will prevent an acute hemolytic transfusion reaction due to ABO incompatibility. RhD negative units will prevent alloimmunization to the RhD antigen and the potential for hemolytic diseases of the fetus / newborn (HDFN) in a subsequent pregnancy. Although type A plasma could safely be used for massive transfusion in trauma patients with an unknown blood type, neither of the answer choices with this option (B and C) have the correct choice of RBCs or platelets. An adequate platelet dose for a massive transfusion in an adult would be 1 apheresis unit or a 6 pack pool of whole blood derived platelets. Answer choice A supplies the correct volume of platelets but the use of O positive RBCs would not be the first choice for a young female and answer choice B does not supply an adequate volume of RBCs or plasma.
Comment Here
Reference: Massive transfusion
Comment Here
Reference: Massive transfusion
Board review style question #2
A patient with a severe gastrointestinal bleed required a massive transfusion. The bleeding has been controlled but the patient is now complaining of perioral tingling, hand numbness and muscle cramping. Which of the following labs would be abnormal and associated with the patient's symptoms?
- Hemoglobin
- Ionized calcium
- Platelet count
- Potassium
- Prothrombin time (PT) / international normalized ratio (INR)
Board review style answer #2
B. Ionized calcium. Blood products contain citrate as part of the preservative solution to prevent coagulation during collection and storage. Citrate is also capable of chelating calcium and may cause hypocalcemia after a large volume transfusion. Hypocalcemia initially presents with perioral tingling and paresthesia but may progress to tetany, confusion and arrhythmias. It is important to note that the total calcium may be normal and the ionized calcium should specifically be monitored during massive transfusions.
Comment Here
Reference: Massive transfusion
Comment Here
Reference: Massive transfusion
MNS system
Table of Contents
Definition / general | Essential features | Terminology | Antigens | Antibodies | Pathophysiology | Clinical features | Transmission | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- MNSU blood group system is antigenic on the surface of red blood cells (RBC)
- Antibodies to M and N are generally clinically insignificant
- Antibodies to other antigens, such as S, s and U, are clinically significant
Essential features
- Antibodies to M and N are generally clinically insignificant
- Antibodies to other antigens, such as S, s and U, are clinically significant
- There are numerous high frequency and low frequency antigens, some of which are clinically significant
Terminology
- M (MNS1), N (MNS2), S (MNS3), s (MNS4)
- U (MNS5) or universal
- Glycophorin A (GPA, CD235A)
- Glycophorin B (GPB, CD235B)
Antigens
- Type: peptide
- M and N are present on glycophorin A protein, while S, s and U are present on glycophorin B protein
- Glycophorins A and B are encoded by 2 homologous genes, GYPA and GYPB, respectively, both of which are located on chromosome 4
- ~50 antigens in total (Fung: Technical Manual, 19th Edition, 2017)
- Glycophorin molecules contribute to RBC membrane structure with an association to band 3 and likewise act as ligands for Plasmodium falciparum (Fung: Technical Manual, 19th Edition, 2017)
- U is a high frequency antigen found on glycophorin B (Fung: Technical Manual, 19th Edition, 2017)
- Mur antigen is present in ~10% of Chinese and Thai individuals (Fung: Technical Manual, 19th Edition, 2017)
| Race / ethnicity | M+N- | M+N+ | M-N+ | M-N- | S+s- | S+s+ | S-s+ | S-s- |
| White | 30% | 49% | 21% | Rare | 10% | 42% | 48% | Rare |
| Black (U.S.) | 25% | 49% | 26% | Rare | 6% | 24% | 68% | 2% |
- Adapted from Fung: Technical Manual, 19th Edition, 2017
Antibodies
- Antibodies to M and N are usually cold reactive, not clinically significant and naturally occurring, while S, s and U antibodies are clinically significant
- M antibodies tend to be IgM and are generally not associated with hemolytic disease of the fetus and newborn (HDFN) (rarely reported), though assessment for IgG conversion should be considered (AJP Rep 2017;7:e205)
- N antibodies are generally IgM
- S, s, U and other antibodies tend to be IgG and can cause hemolytic transfusion reactions and HDFN (Fung: Technical Manual, 19th Edition, 2017)
- N antigen exists on the N terminus of the glycophorin A peptide, similar to the N terminus of all glycophorin B peptides; hence antibodies against N are less common (Fung: Technical Manual, 19th Edition, 2017)
- Mur antibodies are common in Southeast Asia (Fung: Technical Manual, 19th Edition, 2017)
Pathophysiology
- Antibodies to M can form naturally without exposure, with auto anti-M commonly encountered
- S, s and U antibodies are clinically significant
- Exposure to antigens can lead to sensitization
- Reference: Fung: Technical Manual, 19th Edition, 2017
Clinical features
- African Americans have a deletion in the coding region of GYPB and can form antibodies against S, s and U (a high prevalence antigen)
- High prevalence of U antigen and scarcity of U negative donors render management of patients with anti-U challenging
- Presence of an N antibody suggests a lack of glycophorin B and may indicate patients can form anti-U antibodies
- Reference: Fung: Technical Manual, 19th Edition, 2017
Transmission
- Exposure to MNS antigens secondary to pregnancy or transfusion
Laboratory
- M, N, S and s are generally destroyed by enzyme treatment (Fung: Technical Manual, 19th Edition, 2017)
- M and N are trypsin sensitive but only slightly sensitive to alpha chemotrypsin (Fung: Technical Manual, 19th Edition, 2017)
- S and s are trypsin resistant but are sensitive to alpha chemotrypsin (Fung: Technical Manual, 19th Edition, 2017)
- IgM antibodies can be destroyed using dithiothreitol (DTT), which is useful for determining if there is an IgG component in pregnant patients
Case reports
- Fetal GP*Mur causing HDFN (Transfusion 2020;60:870)
- 1 year old girl, a hematology / oncology patient, presenting with anti-M antibody (J Clin Diagn Res 2017;11:ED20)
- 24 year old woman with pregnancy complicated by anti-S antibody and malaria infection (Transfus Apher Sci 2014;50:281)
- 28 year old woman with an Mk antibody with recurrent pregnancy loss (Transfusion 2017;57:376)
- 71 year old woman with anti-N antibody presenting as ABO discrepancy (Asian J Transfus Sci 2019;13:140)
Additional references
Board review style question #1
A gravida 1 para 0 mother is found to have an anti-M antibody. The clinical team wants to determine if the antibody is IgG or IgM. Treatment of the patient serum with which reagent destroys IgM antibodies and helps to determine if this is an IgG antibody?
- Addition of antihuman globulin
- Dithiothreitol (DTT)
- Enzyme treatment
- Polyethylene glycol (PEG) enhancement
Board review style answer #1
Board review style question #2
A Jehovah's Witness patient agrees to receive plasma products only. A type and screen is found to be positive. The patient is disturbed when he sees this result in the patient portal, as he has no transfusion history. What is the best explanation to provide to the patient?
- He has likely been transfused with RBCs at some point and this is evidence
- These antibodies can be naturally occurring
- This is likely passive transfer from his recent FFP transfusion
- This patient needs a RBC transfusion
Board review style answer #2
Monoclonal antibody therapy
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Laboratory | Case reports | Treatment | Sample assessment & plan | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Monoclonal antibodies (mAbs) are novel therapeutics that are used in the management of hematologic malignancies, solid tumors, immune disorders, infections and several other conditions
- These antibodies may occasionally interfere with serologic testing in the blood bank and require protocolized testing methods to ensure transfusion safety
Essential features
- Monoclonal antibodies (mAbs) are used in the management of malignancies and immune disorders and may interfere with serologic testing in the blood bank
- Anti-CD38 and anti-CD47 therapies are currently the most widely used mAbs that cause serology interference and blood banks should have protocols established to manage these patients
- Anti-CD38 causes interference on antibody screens and requires reagent red cell treatment with diothiothreitol (DTT)
- Anti-CD47 therapy can result in ABO discrepancies, positive direct antiglobulin test (DAT) and interference on antibody screens
Terminology
- Naming conventions (Lancet 2022;399:24):
- Prefixes are unique to distinguish each drug
- Infixes designate the target (tu for tumor), antibody source (u for human) and modifications (zu for humanized)
- Suffix was historically mab to designate monoclonal antibody; however, recent additions have been made to provide information about immunoglobulin structure
Pathophysiology
- Mechanism of action (Oncogene 2003;22:9097):
- mAbs generally target cell surface antigens or plasma soluble molecules such as proteins or drugs
- Target and affinity for antigen is determined by variable region / complementarity determining region (CDR)
- Fc (fragment crystallizable) portion supports the recruitment of other immune cells and complement factors to act on target
- Monoclonal antibodies most relevant to transfusion medicine:
- Anti-CD20 (rituximab) (Blood 2017;129:2971):
- CD20 present on B cell lineage
- Therapy results in downregulation of B cell receptor activity, decreased serum immunoglobulin production and increased apoptosis
- Used to treat various lymphomas, leukemias and autoimmune diseases
- Frequently used in conjunction with plasmapheresis for autoimmune neurologic disorders
- May be considered in certain cases of autoimmune hemolytic anemia, pure red cell aplasia, thrombotic thrombocytopenic purpura (TTP), idiopathic thrombocytopenic purpura (ITP) and Evans syndrome
- Anti-CD38 (daratumumab and isatuximab) (Pathology 2021;53:427):
- CD38 is overexpressed on multiple myeloma cells
- Daratumamab causes indirect apoptosis through antibody dependent cellular cytoxicity (ADCC) via natural killer cells and complement dependent cytotoxicity (CDC)
- Isatuximab induces direct cell apoptosis along with indirect apoptosis via ADCC and CDC
- CD38 is expressed at low levels on red blood cells (RBCs)
- See Laboratory for blood bank serologic testing issues related to CD38 expression on RBCs
- Anti-CD47 (magrolimab - Hu5F9-G4) (Pathology 2021;53:427):
- CD47 is widely expressed in the human body and acts as an antiphagocytic "do not eat me" marker on healthy cells
- Therapy is primarily used to treat acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS)
- CD47 expression on RBCs can result in transient hemolysis upon initiation of therapy
- See Laboratory for blood bank serologic testing issues related to CD47 expression on RBCs
- Anti-C5 (eculizumab) (Transfus Med Rev 2019;33:256):
- Inhibits the cleavage of C5 into C5a, an anaphylatoxin and proinflammatory molecule, and C5b, which forms the C5b-9 membrane attack complex that creates cytotoxic pores on cell surfaces
- FDA approved for paroxysmal nocturnal hemoglobinuria (PNH), complement mediated thrombotic microangiopathy (referred to as atypical hemolytic uremic syndrome [aHUS]), refractory generalized myasthenia gravis and antiaquaporin 4+ neuromyelitis optica
- Frequent off label use for other forms of thrombotic microangiopathy and other hematologic and autoimmune disorders
- Anti-CD20 (rituximab) (Blood 2017;129:2971):
Clinical features
- Adverse effects (Physiol Res 2016;65:S455):
- Mild allergic infusion reactions are common and can be managed by pausing infusion, slowing infusion and with diphenhydramine
- Acute hypersensitivity reactions and severe allergic reactions occur less frequently
- Cytokine release syndrome (CRS) is a severe immune reaction that occurs in response to immunotherapy for certain cancers and leads to elevation in inflammatory cytokines
- There is potential for infections and autoimmune reactions with the use of mAbs that reduce immune function
Laboratory
- CD38 and CD47 expression on RBCs can cause serology interference and require protocols for testing and managing patients on therapies targeting these antigens (Pathology 2021;53:427)
- Anti-CD38:
- Therapy can result in interference with indirect antiglobulin testing (IAT)
- Results in panreactive antibody screen and positive serologic crossmatch
- Baseline ABO / Rh typing and antibody screen should be performed prior to initiating therapy
- Baseline RBC phenotype / genotype to assess Rh, Kell, Jk, Fy and MNS antigen expression also recommended
- Antibody screening should be performed with dithiothreitol (DTT) treatment of reagent RBCs to eliminate anti-CD38 binding and interference
- Provide Kk compatible units due to DTT destruction of Kell antigens on reagent RBCs and inability to detect Kell alloantibodies
- Soluble CD38 protein may also be used to neutralize anti-CD38 in patient plasma but it is not licensed for routine testing and difficult to acquire
- Novel F(ab')2 fragments have been shown to directly block CD38 antigens on RBCs and overcome daratumumab interference but are not yet licensed or widely available (N Engl J Med 2018;379:90)
- Anti-CD47:
- Therapy can result in ABO discrepancies, positive DATs and positive antibody screens
- Baseline ABO / Rh typing and antibody screen should be performed prior to initiating therapy
- Baseline RBC phenotype / genotype to assess Rh, Kell, Jk, Fy and MNS antigen expression also recommended
- Use type O RBCs if baseline ABO not determined or anomalous results encountered after initiating therapy
- Adsorption studies with papain treated RBCs can be utilized for satisfactory antibody screening in cases with interference (Transfusion 2019;59:730)
- Use of antihuman globulin (AHG) reagents that do not bind to IgG4 molecules has also demonstrated reduced interference as magrolimab is an IgG4 antibody (Transfusion 2019;59:730)
Case reports
- 2 year old boy treated with daratumumab for post-hematopoietic stem cell transplantation refractory hemolytic anemia (Pediatr Blood Cancer 2020;67:e28010)
- 19 year old woman treated with daratumumab in life threatening autoimmune hemolytic anemia following hematopoietic stem cell transplantation (Blood Adv 2018;2:2550)
- 35 year old woman with posttransplant Evans syndrome treated with daratumumab (Br J Haematol 2019;187:e48)
- 60 year old man treated with daratumumab for thrombocytopenia posttransplant (Blood Adv 2020;4:815)
- 72 year old man treated with daratumumab for delayed red cell engraftment posttransplant (N Engl J Med 2018;379:1846)
Treatment
- Monoclonal antibodies are usually administered parenterally but can also be administered intravenously, subcutaneously or intramuscularly, depending on the exact therapy and formulation
- Use during plasmapheresis:
- mAbs are present in the plasma and may be removed during plasmapheresis
- Therapy plans that include apheresis should carefully consider dose timing to limit removal and allow for distribution to extravascular fluid compartments
Sample assessment & plan
- Transfusion service serology report:
- 70 year old man with multiple myeloma being treated with daratumumab (anti-CD38). Blood bank was alerted of the patient's medications and followed protocol for type and screen for patients on anti-CD38 therapy. Baseline testing on this patient prior to initiation of therapy demonstrated a blood type of A positive and a negative antibody screen. Repeat testing at this time confirms the A positive blood type. Antibody screening on the current sample was performed with DTT treatment of reagent red cells and also demonstrates a negative result.
- Due to the use of DTT for antibody screening, we are unable to detect antibodies directed against antigens in the Kell blood group system and will provide the patient with K-k+ RBCs based on the patient's phenotype.
Board review style question #1
Your blood bank receives a type and screen sample for a 73 year old female patient that is new to your hospital and laboratory. The ABO / Rh typing is completed without issue and the patient demonstrates a blood type of O positive. The antibody screen is performed and shows that the patient's plasma causes 4+ agglutination with all 3 reagent red cells and the autocontrol is also positive. The blood bank technologist obtains additional clinical information and finds that this patient has multiple myeloma and is being treated with daratumumab. What serology method can be performed to eliminate the panreactivity on the antibody screen and identify underlying alloantibodies?
- Alloadsorption of patient's plasma
- Autoadsorption of patient's plasma
- Dithiothreitol treatment of patient's plasma
- Dithiothreitol treatment of reagent red cells
- Enzyme treatment of reagent red cells
Board review style answer #1
D. Dithiothreitol treatment of reagent red cells
Comment Here
Reference: Monoclonal antibody therapy
Comment Here
Reference: Monoclonal antibody therapy
Board review style question #2
Your blood bank receives a type and screen sample for a 75 year old male patient that is new to your hospital and laboratory. The ABO / Rh typing is completed without issue and the patient demonstrates a blood type of O positive. The antibody screen is performed and shows that the patient’s plasma causes 4+ agglutination with all 3 reagent red cells and the auto control is also positive. The blood bank technologist obtains additional clinical information and finds that this patient has multiple myeloma and is being treated with daratumumab. The blood bank technologist successfully uses DTT treatment to perform the antibody screen and finds that the screen is now negative. Which of the following blood products should be issued to the patient if an RBC is ordered?
- A positive irradiated RBCs because daratumumab reduces ABO antigen detection on forward typing
- O negative irradiated RBCs because daratumumab causes false positive RhD agglutination on forward type
- O positive irradiated RBCs with no additional special attributes
- O positive irradiated RBCs phenotypically matched for D, Cc and Ee antigens
- O positive irradiated RBCs phenotypically matched for K and k antigens
Board review style answer #2
E. O positive irradiated RBCs phenotypically matched for K and k antigens
Comment Here
Reference: Monoclonal antibody therapy
Comment Here
Reference: Monoclonal antibody therapy
Neutralization and lectins (pending)
[Pending]
P1PK GLOB system
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Laboratory | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Antibodies to P and PP1Pk are clinically significant
- Typically, cold reacting antibodies
Essential features
- Antibodies to P and PP1Pk are clinically significant
- Autoanti-P antibodies seen in paroxysmal cold hemoglobinuria and are associated with infection
- P acts as a receptor for parvovirus B19
Terminology
- P, GLOB (globoside) system antigen
- P1, first P1PK antigen described
- Pk, high prevalence P1PK antigen
- Anti-PP1Pk (arachaic Tja), antibody formed by p individuals
- Reference: Fung: Technical Manual, 19th Edition, 2017
Pathophysiology
- P1, Pk and NOR are synthesized by enzymatic modification of glycolipids
- P is derived from enzymatic modification of Pk by β1,3-N-acetylgalactosaminyltransferase
- Individuals lacking these antigens due to recessive phenotypes can produce naturally occurring P1PK and GLOB system antibodies such as anti-PP1Pk in p (null) phenotype individuals
- Reference: Fung: Technical Manual, 19th Edition, 2017
Clinical features
- P acts as a receptor for parvovirus B19
- Individuals can develop autoanti-P with syphilis or upper respiratory infection
- Antibodies are IgG, bind at cold temperatures and cause complement mediated hemolysis upon warming
- Anti-PP1Pk is occasionally associated with hemolytic disease of the fetus and newborn but can lead to early abortion as P1PK and GLOB system antigens are expressed at high levels on placental surfaces
- Antigens (type: carbohydrate) (Fung: Technical Manual, 19th Edition, 2017)
- Most high prevalence with expression on several tissue types
- P1 predominately expressed on red cells
Incidence among Caucasians
Adapted from Reid: The Blood Group Antigen FactsBook, 3rd Edition, 2012Antigen Incidence (%) P 99.9 P1 79 Pk High prevalence
- Antibodies:
- Antibodies to P and PP1Pk are clinically significant
- Anti-P autoantibodies associated with paroxysmal cold hemoglobinuria
- Disease associations (Immunohematology 2020;36:99):
- P1PK antigens may be bound by Shiga toxins
- Some cancers such as breast and ovarian may express P1PK and GLOB system antigens
- P acts as a receptor for parvovirus B19
- Pk may bind HIV enabling entry
- P1 motif antigens are produced in hydatid (echinococcal) cyst fluid leading to antibodies, likewise, hydatid cyst fluid can be used as a neutralizing substance for anti-P1
Transmission
- Antibodies are generally naturally forming in null individuals but sensitization can occur due to pregnancy or transfusion
- P1 antibodies can occur in pigeon fanciers
- Reference: Clin Allergy 1980;10:643
Laboratory
- Autoanti-P antibodies are characterized with the Donath Landsteiner test (Arch Dis Child Educ Pract Ed 2021 Aug 25 [Epub ahead of print])
- Hemolysis with incubation at 0 °C followed by incubation at 37 °C in tubes with patient serum and patient serum / donor serum and P+ cells (see below)
Contributed by Brian D. Adkins, M.D.
- Anti-P1 antibodies can be neutralized with pigeon egg whites or echinococcal cyst fluid
- The P1 phenotype is the most common phenotype (~80% of white individuals), with P2 representing the second most common phenotype (~20% of white individuals); variable phenotypes are seen amongst differing races and serum reactivity is reviewed below:
Reactivity with reagent anti-sera
| Reactions with reagent serum | |||||
| Anti-P1 | | Anti-Pk | Anti-PP1Pk (Tja) | Antibodies in patient serum | Patient RBC phenotype |
| + | + | 0 / + | + | None | P1 |
| 0 | + | 0 / + | + | Anti-P1 (occasionally) | P2 |
| 0 | 0 (occasional weak positivity due to crossreactivity) | 0 | 0 | Anti-PP1Pk (Tja) | p (rare) |
| + | 0 | + | + | Anti-P,-PX2 | P1k (rare) |
| 0 | 0 | + | + | Anti-P,-P1, -PX2 | P2k (rare) |
Case reports
- 4 year old girl with hemolytic transfusion reaction with anti-PP1Pk (Transfus Med Hemother 2021;48:240)
- 5 year old boy with recurrent paroxysmal nocturnal hemoglobinuria (Transfusion 2017;57:1401)
- Case series of anti-PP1Pk in pregnant patients (Vox Sang 2021;116:591)
- 2 sisters with Pk phenotype presenting with recurrent miscarriage (Transfus Med 2019;29:202)
Additional references
Board review style question #1
Board review style answer #1
B. Echinococcal cyst fluid and pigeon egg white can be used to neutralize P1 antibodies, which commonly occur in individuals caring for pigeons.
Comment Here
Reference: P1PK GLOB
Comment Here
Reference: P1PK GLOB
Board review style question #2
P red cell antigen of the GLOB system acts as a receptor for which microorganism?
- Babesia microti
- Francisella tularensis
- Parvovirus B19
- Plasmodium vivax
Board review style answer #2
Parasites
Table of Contents
Babesiosis | Malaria | Trypanosoma cruzi | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3 | Board review style question #4 | Board review style answer #4 | Board review style question #5 | Board review style answer #5 | Board review style question #6 | Board review style answer #6Babesiosis
Definition / general
Essential features
Terminology
Pathophysiology
Clinical features
Transmission
Symptoms
Screening
Blood donor screening
Blood donor testing
Donor deferral
Diagnosis
Laboratory
Case reports
Treatment
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
Differential diagnosis
- Babesiosis is a disease caused by parasites that infect red blood cells (RBCs)
- Most human cases of Babesia infection in the United States are caused by the parasite Babesia microti, which is spread by ticks, primarily Ixodes scapularis ticks (MMWR Surveill Summ 2019;68:1)
Essential features
- The vast majority of babesiosis cases in the United States are caused by Babesia microti, the species that is prevalent in the Northeast and upper Midwest of the U.S.
- Tickborne transmission usually peaks during warm months (N Engl J Med 2012;366:2397)
- Babesiosis is the most frequently reported tickborne pathogen disease transmitted by blood transfusion in the U.S. (Transfus Med Rev 2002;16:131)
- Babesia infection can range from asymptomatic to life threatening
- Risk factors for severe babesiosis include asplenia, advanced age (age > 50) and impaired immune function
- In 2019, the FDA licensed tests utilized for donor screening for detection of Babesia species and recommended year round regional testing for blood donations in areas endemic for babesiosis (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 22 December 2022])
Terminology
- See also: Babesia
Pathophysiology
- Nymphal stage of the Ixodes is the primary vector and requires attachment to a host for at least 36 to 72 hours to complete transmission of sporozoite forms
- These sporozoites attach and enter erythrocytes where they mature and divide to form merozoites; merozoites then rupture the host erythrocyte and continue infecting other erythrocytes, repeating the same cycle
- The spleen is essential in the host's ability to control this infection
- Erythrocytes infected with Babesia are recognized as abnormal as they pass through the spleen and are targeted for destruction by macrophages
- People with a history of splenectomy are at high risk for severe infection with high level parasitemia (Front Microbiol 2021;12:697669)
Clinical features
- Incubation period: 1 - 4 weeks following tick bite; 1 - 9 weeks after contaminated blood transfusion (up to 24 weeks)
- Asymptomatic infection has been reported in up to 20% of adults and 50% of children (StatPearls: Babesiosis [Accessed 22 December 2022])
- Symptomatic patients present with fever, general malaise, headache, hemolysis and rarely, renal failure and coagulopathy
Transmission
- Tickborne transmission babesiosis: 1 - 4 weeks following tick bite
- Transfusion transmitted babesiosis: 1 - 9 weeks after contaminated blood transfusion
- High risk patients include immunocompromised, elderly and asplenic patients (Transfusion 2000;40:285)
Symptoms
- Fever, chills, sweats
- Malaise, fatigue
- Myalgia, arthralgia, headache
- Gastrointestinal (GI) symptoms, such as anorexia and nausea (less common: abdominal pain, vomiting)
- Dark urine
- Less common: dry cough, sore throat, photophobia, conjunctival injection
- Mild splenomegaly, mild hepatomegaly or jaundice may occur in some patients
- Severe cases can be associated with marked thrombocytopenia, disseminated intravascular coagulation, hemodynamic instability, acute respiratory distress, renal failure, hepatic compromise, altered mental status and death
- Reference: MMWR Morb Mortal Wkly Rep 2012;61:505
Screening
- Screening for babesiosis is performed by donor questionnaire or by nucleic acid testing
- Babesia microti parasitemia (DNA) has been detected for > 1 year in immunocompetent patients treated with a standard course of antibiotic therapy and > 2 years in untreated individuals (N Engl J Med 2016;375:2236)
- Immunocompromised patients can experience persistent B. microti parasitemia and relapsing symptoms for > 2 years despite antimicrobial therapy
Blood donor screening
- Donor history questionnaire asking prospective donors if they have ever had a positive test result for Babesia, obtained from either a medical diagnosis or a reactive donor screening test
Blood donor testing
- Blood donor centers must test each donation for evidence of Babesia using a licensed nucleic acid testing (NAT) when collected in Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, Wisconsin and Washington, D.C.
- Testing must be performed year round and in accordance with the instructions for use of the device (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 22 December 2022])
Donor deferral
- Blood donor centers must defer donors with a reactive nucleic acid test (NAT) result for Babesia for at least 2 years from the date of the reactive donation
- Blood donor centers must defer donors who report a history of a positive test result for Babesia, obtained from either a medical diagnosis or a reactive donor screening test result for 2 years
- Reference: FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 22 December 2022]
Diagnosis
- Identification of intraerythrocytic Babesia parasites by light microscopic examination of a peripheral blood smear
- Ring forms are most commonly seen and can have multiple rings per cell
- Tetrad formations, also known as Maltese cross, are occasionally seen
- Positive Babesia (or B. microti) polymerase chain reaction (PCR) analysis
- Demonstration of a Babesia specific antibody titer by indirect fluorescent antibody (IFA) testing for total immunoglobulin (Ig) or IgG
- Reference: Clin Infect Dis 2021;72:e49
Laboratory
- Hemolytic anemia with decreased haptoglobin, elevated lactate dehydrogenase (LDH) values and reticulocytosis
- Thrombocytopenia
- Elevated creatinine and blood urea nitrogen (BUN) values
- Mildly elevated hepatic transaminase values
- Proteinuria
- Reference: CDC: Surveillance for Babesiosis [Accessed 25 January 2023]
Case reports
- Premature 1 month old infant with WA1 type Babesia (Transfusion 2002;42:1482)
- 40 year old man with fever, jaundice, greenish brown urine, loss of appetite and general malaise (Transfus Apher Sci 2020;59:102902)
- 45 year old man asymptomatic donor with multiple recipients (Transfusion 2002;42:1154)
- 46 year old healthy man with fever, myalgias and left sided abdominal pain radiating to the shoulder (J Gen Intern Med 2021;36:3869)
- 54 year old spleen intact man with transfusion associated Babesia microti infection after a heart transplant (Emerg Infect Dis 2003;9:116)
Treatment
- Treatment is indicated in symptomatic cases or in asymptomatic patients who have a positive blood smear or PCR for > 3 months
- Patients are usually treated for at least 7 - 10 days with a combination of medications, typically either clindamycin plus quinine or atovaquone plus azithromycin (Clin Infect Dis 2021;72:e49)
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
- Assessment: Jane Doe is a 68 year old woman with a history of splenectomy who presented 14 days after packed red blood cell transfusion with fever, fatigue and abdominal pain. The patient's labs show anemia and impaired kidney function. Peripheral smear shows ring forms and some tetrad formations.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of transfusion transmitted babesiosis.
- Recommend starting clindamycin and quinine for 7 - 10 days
- If patient's clinical status deteriorates, red blood cell exchange may be considered
- Partial or complete red blood cell exchange transfusion is indicated in patients presenting with a parasitemia of at least 10% and anemia with hemoglobin of < 10 g/dL
Differential diagnosis
- Lyme disease:
- Fever, headache, fatigue and a characteristic skin rash called erythema migrans
- Malaria:
- Fever and flu-like illness, including shaking chills, headache, muscle aches and fatigue
- Colorado tick fever:
- Biphasic fever, chills, headache, body aches and fatigue
- Anaplasmosis:
- Fever, chills, severe headache, muscle aches, nausea, vomiting, diarrhea, loss of appetite
Malaria
Definition / general
Essential features
Terminology
Pathophysiology
Clinical features
Transmission
Symptoms
Screening
Blood donor screening
Blood donor testing
Donor deferral
Laboratory
Case reports
Treatment
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
Differential diagnosis
Additional references
- Malaria is a mosquito borne disease caused by a parasite
- There are 5 Plasmodium species that cause malaria in humans: P. falciparum, P. malariae, P. ovale, P. vivax, P. knowlesi (Malar J 2018;17:36)
- Common transfusion transmitted infection worldwide
Essential features
- In the U.S., there are 1,300 new cases of malaria each year but only 1 - 2 cases are due to transfusion transmission
- Risk of transfusion transmitted disease is estimated to be 0.25 cases per million transfusions and usually results from transmission of P. falciparum
- Symptoms can occur a week to several months after transfusion
- Rarely fatal
- P. vivax and P. ovale have a dormant stage that can persist in the liver (if untreated) and cause relapses by invading the bloodstream weeks or even years later
Terminology
- See also: Plasmodium falciparum
Pathophysiology
- Natural history of malaria involves cyclical infection of humans and female Anopheles mosquitoes
- In humans, the parasites grow and multiply first in the liver cells and then in the RBCs
- In the blood, successive broods of parasites grow inside the red cells and destroy them, releasing daughter parasites (merozoites) that continue the cycle by invading other RBCs (MMWR Surveill Summ 2019;68:1)
Clinical features
- Fever, chills, sweats, headaches, muscle pains, nausea and vomiting (Baron: Medical Microbiology, 4th Edition, 1996)
Transmission
- Mosquito borne malaria
- Airport malaria: malaria caused by infected mosquitoes that are transported rapidly by aircraft from a malaria endemic country to a nonendemic country
- Congenital malaria: infected mothers transmit parasites to their child during pregnancy before or during delivery
- Transfusion transmitted malaria
- Reference: WHO: Malaria [Accessed 25 January 2023]
Symptoms
- People with malaria often experience nonspecific symptoms such as fever, chills, muscle ache and flu-like illness
- In severe malaria (primarily caused by Plasmodium falciparum), clinical findings (confusion, coma, neurologic focal signs, severe anemia, respiratory difficulties) are more striking and may increase the index of suspicion for malaria
Screening
- Incubation period in most cases varies from 7 to 30 days
- Shorter incubation periods are observed most frequently with P. falciparum and the longer ones with P. malariae
- Prevalence in donors (in endemic regions):
- Brazil: 1 - 3% (Rev Inst Med Trop Sao Paulo 2007;49:1)
- Kenya: 9% (East Afr Med J 2005;82:565)
- Nigeria: 10% (Trop Doct 2007;37:32)
- Countries with high donor infection rates may process donor blood with quinine or sulfadoxine pyrimethamine to kill parasites (Saudi Med J 2006;27:986, Am J Trop Med Hyg 2005;73:1119)
Blood donor screening
- Donor history questionnaire asking prospective donors if they have ever had malaria or have been in malaria endemic areas
Blood donor testing
- In the U.S., there is no FDA licensed test to detect the presence of malaria parasites in blood donors
- FDA strategy for reducing the risk of transfusion transmitted malaria (TTM) is to defer potential blood donors based on their travel or residence history in malaria endemic countries
Donor deferral
- A resident of a country nonendemic for malaria who has traveled to a malaria endemic area may be accepted as a donor 3 months after their return to the nonendemic country (irrespective of the use of chemoprophylaxis) if they have been free from malaria symptoms
- An immigrant or visitor from a malaria endemic country may be accepted as a donor 3 years after departure from the malaria endemic area if they have been free from malaria
- Former residents of malaria endemic countries who have lived in the United States for < 3 consecutive years and who return to visit a malaria endemic area may be accepted as donors 3 years after their most recent visit if they have been free from malaria
- Former residents of malaria endemic countries who have lived in the United States for ≥ 3 consecutive years and who return to visit a malaria endemic area may be accepted as donors 3 months after their most recent visit if they have been free from malaria
- Persons who have had a diagnosis of malaria should be deferred for 3 years after becoming asymptomatic
- References: CDC: Malaria - Blood Donor Screening [Accessed 25 January 2023], FDA: Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria [Accessed 25 January 2023]
Laboratory
- Blood smear remains the gold standard for laboratory confirmation of malaria with parasites seen within RBCs
- Antigen detection test was approved by FDA in 2007 to detect antigens derived from malaria parasites
- PCR: parasite nucleic acids may be detected; this is most useful for confirming the species of malarial parasite after the diagnosis has been established by either smear microscopy or antigen detection test
- Serology does not detect current infection but rather measures past exposure
- Reference: CDC: Malaria - Disease [Accessed 25 January 2023]
Case reports
- Patients between 2 days and 85 years old with transfusion transmitted malaria (N Engl J Med 2001;344:1973)
- 47 year old woman with striking fever (Mikrobiyol Bul 2005;39:101)
- 69 year old man in Texas with blood donor from Ghana (MMWR Morb Mortal Wkly Rep 2003;52:1075)
- 70 year old man with donor from Cameroon (Swiss Med Wkly 2001;131:320)
Treatment
- Depends on many factors including disease severity, the species of malaria parasite causing the infection and the part of the world in which the infection was acquired
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
- Assessment: Joe Doe is a 60 year old man who presented with fatigue, pallor and headaches. Patient has a history of traveling all around the world in the last 6 months. Lab peripheral blood smear shows a parasite inside a red blood cell. Malaria is confirmed with an antigen detection test.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of malaria.
- Recommend starting antimalarial therapy
- Patient will be deferred from blood donation for 3 years
Differential diagnosis
- Typhoid fever:
- Weakness, stomach pain, headache, diarrhea or constipation, cough and loss of appetite
- Influenza:
- Fever, cough, sore throat, congestion, headaches, body aches and fatigue
- Dengue fever:
- Fever, rash, eye pain, muscle pain, headache, nausea or vomiting
- Babesiosis:
- Fever, chills, dark urine, arthralgia, general malaise, loss of appetite
Additional references
Trypanosoma cruzi
Definition / general
Essential features
Terminology
Pathophysiology
Clinical features
Transmission
Symptoms
Screening
Blood donor screening
Blood donor testing
Donor deferral
Laboratory
Case reports
Treatment
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
Differential diagnosis
Additional references
- ~6 - 7 million people worldwide, mostly in Latin America, are estimated to be infected with Trypanosoma cruzi (T. cruzi), the parasite that causes Chagas disease
- Trypanosoma cruzi infection is curable if treatment is initiated soon after infection
- Blood screening is vital to prevent infection through transfusion and organ transplantation all over the world (WHO: Chagas Disease (American Trypanosomiasis) [Accessed 23 December 2022])
Essential features
- Triatoma infestans, Rhodnius prolixus and Triatoma dimidiata are the 3 most important insect vector in the transmission of T. cruzi to humans (WHO: Control of Chagas Disease - Second Report of the WHO Expert Committee [Accessed 23 December 2022])
- A large proportion of patients exhibit an acute phase characterized by flu-like symptoms followed by an asymptomatic phase
- Infected individuals may remain asymptomatic for years but they may have circulating parasites that are potentially transferable to an eventual recipient of a transfusion (Hematol Transfus Cell Ther 2019;41:262)
- FDA licensed a screening test for antibodies to T. cruzi in blood donors in 2006, which blood collection centers are currently using in the U.S.
- Photoinactivation may be useful for red cell suspensions, platelets or plasma (Transfus Apher Sci 2007;37:23, Transfusion 2007;47:434, Vox Sang 2006;91:285)
Terminology
- See also: Chagas disease (trypanosomiasis)
Pathophysiology
- In Latin America, T. cruzi parasites are mainly transmitted by contact with feces / urine of infected blood sucking triatomine bugs
- After the parasite enters through an open wound or mucous membrane, the infectious trypomastigote is found in the plasma
- There is a predilection for the myocardium or myenteric plexus of the GI tract, where the parasite replicates by binary fission (StatPearls: Chagas Disease [Accessed 23 December 2022])
Clinical features
- Acute phase: flu-like symptoms, unilateral palpebral edema (Romana sign) or local swelling at the inoculation site (chagoma)
- Chronic phase: megacolon, megaesophagus and cardiomyopathy
- 30 - 40% of infected patients will develop cardiomyopathy or digestive mega syndromes such as megacolon or megaesophagus
- Reference: MMWR Morb Mortal Wkly Rep 2002;51:210
Transmission
- T. cruzi is transmitted by the triatomine bug (vector borne), as well as orally (food borne), through blood / blood products, mother to child (congenital) transmission, via organ transplantation and by laboratory accidents
Symptoms
- Unilateral palpebral edema (Romana sign)
- Fever
- Lymphadenopathy
- Hepatosplenomegaly
- Myocarditis
- Megacolon / megaesophagus
- Meningoencephalitis
- References: MMWR Morb Mortal Wkly Rep 2002;51:210, Circulation 2018;138:e169
Screening
- Primary infection is asymptomatic in ~70% of people and occurs most frequently in children
- Incubation period is ~14 days and the clinical manifestations, when present, can last for 6 - 8 weeks
- Donor / control positive rates:
- Brazil: 2% (Rev Soc Bras Med Trop 2006;39:530)
- Columbia: 0.4% (Biomedica 2005;25:527)
- Mexico: 0.5% (Salud Publica Mex 2006;48:13)
- U.S. high risk regions: 1 per 5,000 (MMWR Morb Mortal Wkly Rep 2007;56:141)
Blood donor screening
- No specific questions on donor history questionnaire regarding T. cruzi or Chagas disease
Blood donor testing
- Donor centers must test donations for evidence of T. cruzi using a licensed screening test for antibodies to T. cruzi
- FDA recommends one time testing of each donor using a licensed test for antibodies to T. cruzi
Donor deferral
- Donors who test repeatedly reactive on a licensed screening test for T. cruzi antibody must be deferred
- Donors whose blood tests positive or indeterminate on the licensed supplemental test should be deferred permanently and informed of the likelihood and medical significance of infection with T. cruzi
- Reference: MMWR Morb Mortal Wkly Rep 2007;56:141
Laboratory
- Diagnosis of T. cruzi infection relies on serological or parasitological techniques
- Available serological tests include hemagglutination, indirect immunofluorescence or enzyme linked immunosorbent assay (ELISA)
- One of the most significant problems in the serological diagnosis of T. cruzi infection is the absence of a gold standard test
- Reference: Adv Parasitol 2011;75:19
Case reports
- 9 month old boy infant born to a seropositive mother to chronic Chagas in Mexico (Iran J Parasitol 2021;16:697)
- 3.5 year old girl with stage 4 neuroblastoma received multiple blood components and had transfusion acquired T. cruzi infection from Bolivian blood donor in the U.S. (Transfusion 2007;47:540)
- 86 year old woman with autoimmune rheumatic disease presents with reactivation of Chagas disease (Open Forum Infect Dis 2021;8:ofaa642)
Treatment
- Chagas disease can be treated with benznidazole or nifurtimox; both medicines are nearly 100% effective in curing the disease if given soon after infection, at the onset of the acute phase
- During the chronic phase of Chagas disease, most people are asymptomatic and unaware of their infection
- References: WHO: Chagas Disease (American Trypanosomiasis) [Accessed 23 December 2022], J Pediatric Infect Dis Soc 2019;8:461
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
- Assessment: Jane Doe is a 50 year old woman who moved from Mexico 5 years ago. Patient was recently diagnosed with rheumatoid arthritis and is currently on immunosuppression therapy. She presented with obstructive megacolon. Due to high suspicion of parasitemia, serological test was done and confirmed Chagas disease.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of Chagas disease.
- Recommend starting antiparasitic treatment with benznidazole or nifurtimox
Differential diagnosis
- Malaria:
- Fever and flu-like illness, including shaking chills, headache, muscle aches and fatigue
- Toxic megacolon:
- Pain, distention of the abdomen, fever, rapid heart rate and dehydration
- Myocarditis:
- Chest pain, fatigue, rapid or irregular heartbeat, shortness of breath, flu-like symptoms, light headedness
- Achalasia:
- Dysphagia, regurgitating food or saliva, heartburn, belching, chest pain, coughing at night, vomiting
Additional references
Board review style question #1
A 40 year old man with no significant medical history presented to the emergency room due to 1 week of generalized weakness, fatigue, myalgia, lightheadedness, achiness and sporadic fevers. Patient also has a history of a tick bite on the anterior abdomen 4 months prior to presentation. What pathognomonic presentation on peripheral smear would confirm a diagnosis of babesiosis?
- Crescent shaped gametocytes
- Ring forms
- Schistocytes
- Tetrad formations
Board review style answer #1
D. Tetrad formations. The pathognomonic presentation of babesiosis on peripheral smear is a tetrad formation within the red blood cell, also called a Maltese cross. Ring forms can be seen in babesiosis but are not pathognomonic for it as they are commonly seen in malaria. Crescent shaped gametocytes are also present in malaria disease. Gametocytes of Plasmodium falciparum are crescent or sausage shaped, and are usually ~1.5 times the diameter of a red blood cell in length. Schistocytes are red blood cell fragments indicative of intravascular hemolysis.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #2
A 40 year old man with no significant medical history presented to the emergency room due to 1 week of generalized weakness, fatigue, myalgia, lightheadedness, achiness and sporadic fevers. Patient also has a history of a tick bite on the anterior abdomen 4 months prior to presentation. If babesiosis is confirmed after a positive nucleic acid test, how long should this patient be deferred for blood donation?
- 1 year
- 2 years
- 3 years
- 4 years
Board review style answer #2
B. 2 years. A positive nucleic acid test for babesiosis requires a blood donation deferral for 2 years.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #3
A 25 year old woman presents with symptoms of fatigue, nausea, vomiting, headache and general malaise. Patient returned 1 month ago from studying abroad in Kenya. Laboratory testing shows anemia and a blood smear shows a parasite inside a red blood cell. What is the most common infectious pathogen to cause these symptoms and test results?
- P. falciparum
- P. malaria
- P. ovale
- P. vivax
Board review style answer #3
A. P. falciparum. The most common infectious pathogen of malaria is P. falciparum. P. vivax and P. ovale generally cause less serious illnesses but the parasites can remain dormant in the liver for many months, causing a reappearance of symptoms months or even years later.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #4
A 25 year old woman presents with symptoms of fatigue, nausea, vomiting, headache and general malaise. Patient returned 1 month ago from studying abroad in Kenya. Laboratory testing shows anemia and a blood smear shows a parasite inside a red blood cell. For how long should this patient be deferred from donating blood?
- 3 months
- 1 year
- 3 years
- 5 years
Board review style answer #4
C. 3 years. A patient diagnosed with malaria is deferred for 3 years after becoming asymptomatic.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #5
An 8 year old boy who recently moved from South America (Brazil) presents to the ER with left eyelid swelling associated with a history of general malaise and flu-like symptoms. His family states they have a farm and the boy has been in contact with several animals. The physician is concerned about a parasite induced disease. Considering the presentation, what would be the most likely area of entry of the parasite?
- Blood
- Ear
- Eye
- Mouth
Board review style answer #5
C. Eye. The most common area of entry when a patient presents with eyelid swelling (Romina sign) is the eye, which usually presents during the acute phase in Chagas disease.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #6
An 8 year old boy who recently moved from South America (Brazil) presents to the ER with left eyelid swelling associated with a history of general malaise and flu-like symptoms. His family states they have a farm and the boy has been in contact with several animals. The physician is concerned about a parasite induced disease. What is a common complication in chronic carriers of this disease?
- Anemia
- Diarrhea
- Fatigue
- Megacolon
Board review style answer #6
D. Megacolon. The most common complication of chronic phase of Chagas disease is megacolon. Most infected people develop a prolonged asymptomatic form of disease called chronic indeterminate, during which few or no parasites are found in the blood. ~20 - 30% of infected individuals will later develop severe and sometimes life threatening medical problems over the course of their lives, such as megacolon, cardiomyopathy or arrhythmias.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Pathogen inactivation
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Treatment of blood products that inactivate or reduce the communicability of infectious agents to reduce the risk of transfusion transmitted infections
- Different technologies are currently used for plasma and platelets with varying usage and approval in different countries
- Pathogen reduction of platelets on the rise in the U.S.
Essential features
- Treatment of blood products that inactivate or reduce the communicability of infectious agents
- Effective against a broad range of pathogens, including bacteria, viruses and parasites but not completely effective against all pathogens, based on factors such as pathogen concentration, species and spore formation
- Inactivates leukocytes
- Currently, used only on platelets and plasma products
- Pathogen reduction technologies currently approved in the U.S.:
- INTERCEPT for platelets and plasma
- Solvent / detergent treated pooled plasma
Terminology
- Leukoreduction is also known as leukofiltration
- Pathogen inactivation is used synonymously with pathogen reduction
- No current technology is able to completely eliminate the risk of pathogen contamination
- Effectiveness of pathogen reduction depends on factors such as pathogen concentration and species / strain (Transfus Apher Sci 2018;57:683)
Pathophysiology
- Leukoreduction
- Removal of leukocytes from blood products
- Filtration based method
- Filters remove leukocytes by pore size and charge exclusion
- Filtration can be performed either in line as part of a blood collection kit or after collection with gravity based filters
- Leukoreduction can be performed prestorage, in the transfusion service or at the beside of the transfusion recipient (poststorage)
- Prestorage leukoreduction preferred as this prevents donor leukocytes from releasing cytokines during storage (Oncotarget 2017;9:4385)
- Patients on ACE inhibitors have increased risk of hypotension with poststorage (beside) leukoreduction (J Heart Lung Transplant 2001;20:759)
- Most apheresis collection devices have built in leukoreduction mechanisms
- Red blood cells, whole blood and apheresis derived platelets must contain < 5 x 106 leukocytes to be considered leukoreduced per FDA requirements
- Whole blood derived platelets must contain < 8.3 x 105 leukocytes to be considered leukoreduced
- Red blood cell loss from filtration must not exceed 15% (at least 85% recovery of original red blood cells)
- Platelet loss in whole blood derived platelets must not exceed 15% and 75% of units must have a minimum of 5.5 x 1010 platelets
- Leukoreduction reduces the risk of transmission of leukocyte associated viruses
- Leukoreduced products are considered cytomegalovirus safe, which is considered equivalent to cytomegalovirus seronegative (Transfusion 2016;56:1581)
- Sickle cell trait (hemoglobin AS) can cause leukoreduction failure due to filter obstruction (Transfus Med Rev 2004;18:168)
- Granulocytes should never be leukoreduced
- Solvent / detergent plasma
- Pooled plasma is treated with solvent / detergent that disrupts lipid bilayers of cell membranes and lipid envelopes
- Plasma is filtered both before and after solvent / detergent treatment to remove cells, cell fragments, bacteria and debris
- Effective against bacteria, protozoa and enveloped viruses (including hepatitis B virus, hepatitis C virus and HIV) (Vox Sang 1998;74:207)
- Not used in cellular products including platelets since solvent / detergent directly damages cell membranes
- Reduces the risk of allergic transfusion reactions
- Likely reduces the risk of transfusion related acute lung injury (TRALI) as the use of pooled plasma dilutes antibodies implicated in TRALI pathogenesis compared to a single donor derived unit of plasma (Transfusion 2005;45:1628)
- Octoplas, a commercially available frozen solution of solvent / detergent treated pooled plasma, is FDA approved in the U.S.
- INTERCEPT Blood System
- Amotosalen, a synthetic psoralen compound, is added to the blood component followed by illumination with UVA light
- Photoactivated amotosalen forms covalent bonds with nucleic acids and intercalates between nucleotide bases, preventing nucleic acid replication
- Residual amotosalen and photoproducts are then absorbed and removed
- Effective against a broad spectrum of bacteria, viruses, protozoa and leukocytes
- Varying levels of effectiveness on nonenveloped viruses with no inactivation of hepatitis A virus or parvovirus
- Not effective against bacterial spores
- Licensed in the U.S. and Canada for use with plasma and platelets
- Mirasol Pathogen Reduction Technology
- Riboflavin (vitamin B2) is added to the blood component and exposed to UVA and UVB light
- Riboflavin associates with nucleic acids and generates reactive oxygen intermediates, leading to irreversible modification and damage of nucleic acids
- No need for removal of riboflavin after illumination
- Effective against both enveloped and nonenveloped viruses, protozoa, a broad range of bacteria and leukocytes (Transfus Med Hemother 2011;38:8)
- Has shown reduced efficacy against certain bacterial species such as Staphylococcus aureus (Vox Sang 2014;107:254)
- Has not been evaluated for inactivation of bacterial spores
- Licensed in Europe for use with plasma and platelets
- Theraflex methylene blue (Theraflex MB)
- Methylene blue, a phenothiazine dye, is added to plasma
- Methylene blue directly binds to nucleic acids
- Subsequent illumination with visible light leads to oxidative damage of nucleic acids, preventing nucleic acid replication
- After treatment, methylene blue and photoproducts are removed via filtration
- Effective against a broad range of viruses; however, no effect on hepatitis A virus (Transfus Med Hemother 2011;38:55)
- Primarily used to inactivate viruses but has shown some efficacy against bacteria, including spore forming bacteria (Vox Sang 2015;109:129)
- Licensed in Europe for use with plasma
Clinical features
- Benefits of pathogen inactivation
- Reduces risk of transfusion transmitted infections
- Potential to decrease transmission of emerging infectious agents
- Leukoreduction also decreases the risk of
- Febrile nonhemolytic transfusion reactions (Transfusion 2004;44:25)
- Alloimmunization to donor human leukocyte antigens (Transfusion 2014;54:672)
- INTERCEPT can replace cytomegalovirus testing or irradiation for transfusion associated graft versus host disease prevention
- Mirasol can replace irradiation for transfusion associated graft versus host disease prevention
- Currently, technologies are used for platelets and plasma with more rapid implementation for platelets in the U.S. given the increased risk of sepsis with platelets due to room temperature storage
- Reduces risk of transfusion transmitted infections
- Cons of pathogen inactivation
- Possible decreased effectiveness of blood product
- Leukoreduction can lead to red blood cell and platelet loss, which may not exceed 15% per FDA requirements
- Low to moderate loss of platelet function in vitro (Vox Sang 2015;108:328)
- Not effective against spore forming bacteria
- Variable effectiveness against nonenveloped viruses, depending on the virus strain and technology, with only Mirasol showing effectiveness against hepatitis A virus (Transfus Med Hemother 2011;38:8)
- Not effective against prions
- No current technologies approved for use on red blood cells or whole blood, with various clinical trials underway
- Issues with UV light penetration of red blood cells
- Leads to increased costs of blood products with lack of well established reimbursement policies (Transfusion 2019;59:3002)
- Possible decreased effectiveness of blood product
Case reports
- 74 year old woman with parvovirus B19 detected in her plasma after transfusion with INTERCEPT treated platelets (Transfus Med Hemother 2016;43:198)
- 81 year old woman who developed disseminated intravascular coagulation and multiorgan failure after transfusion of a leukoreduced red blood cell unit contaminated with Anaplasma phagocytophilum (Am J Clin Pathol 2012;137:562)
- Adult man who developed sepsis from an INTERCEPT treated platelet contaminated with Acinetobacter calcoaceticus / baumannii complex and Staphylococcus saprophyticus (Transfusion 2020;60:1960)
Additional references
Board review style question #1
A 26 year old African American woman presents to a blood donor center and attempts to donate a unit of whole blood; however, the leukoreduction filter clogs and the filtration attempt fails. What is the most likely cause of the leukoreduction failure?
- Bacterial sepsis
- Congenital immunodeficiency syndrome
- Low red blood cell count / low hematocrit
- Sickle cell trait
- Thrombocytopenia
Board review style answer #1
Board review style question #2
Which of the following is true regarding pathogen inactivation of blood products?
- Can inactivate all strains of bacteria
- Currently approved for use in the U.S. for plasma, platelets and red blood cells
- Is able to inactivate prions
- Reduces but does not eliminate the risk of transfusion transmitted infections
- Uses filtration as the principal method to eliminate pathogens
Board review style answer #2
D. Reduces but does not eliminate the risk of transfusion transmitted infections
Comment Here
Reference: Pathogen inactivation
Comment Here
Reference: Pathogen inactivation
Patient blood management
Table of Contents
Definition / general | Essential features | Terminology | Diagrams / tables | History / evolution | ABC toolbox | Drivers | Approaches related to surgery | Overarching approaches | Ongoing interventions | Case reports | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Multidisciplinary approach targeted toward optimization of patient care while employing the best available evidence in patients requiring blood transfusion (AABB: Technical Manual of the American Association of Blood Banks, 20th Edition, 2020)
- Peer developed definition by representatives of various patient blood management (PBM) organizations: "a patient centered, systematic, evidence based approach to improve patient outcomes by managing and preserving a patient's own blood, while promoting patient safety and empowerment" (Anesth Analg 2022;135:476)
Essential features
- Revolves around quality of care improvement, patient safety and transfusion economics
- Involves a transdisciplinary and multistrategic approach
- Blood conservation
- Optimization of red cell mass
- Incorporating patient oriented decision making
- Evidence based practice to achieve improved patient outcomes
- Applied to both surgical and nonsurgical patients
- Surgical patient blood management addresses perioperative and intraoperative care
- A strong PBM program requires
- Adequate funding and resources
- Education and strategic involvement of healthcare providers
- Ongoing research
- Continuous monitoring and feedback
- Use of appropriate quality indicators
- Public and patient awareness
Terminology
- Patient blood management (PBM)
- Blood management (no longer recommended)
- Association for the Advancement of Blood and Biotherapies (formerly the American Association of Blood Banks) (AABB)
- Bloodless medicine and surgery (BMS)
- Society for the Advancement of Patient Blood Management (SABM)
- International Foundation of Patient Blood Management (IFPBM)
History / evolution
- Advent of allogeneic transfusion in the early twentieth century decreased emphasis on anemia and bleeding prevention
- Bloodless surgery evolved in the latter half of the twentieth century, starting with intricate cardiovascular surgeries with minimization of allogeneic transfusion based on patient and available evidence (Ann Thorac Surg 1998;65:125)
- Terminologies adopted over time include
- Bloodless medicine and surgery
- Blood conservation
- Blood management
- Patient blood management was coined in 2005 (Anesth Analg 2022;135:476, Blood Transfus 2019;17:191, Transfus Apher Sci 2002;27:29, J Australas Ass Blood Conserv 2005;2:3)
ABC toolbox
- ABC toolbox of PBM from the IFPBM SABM workgroup summarizes practical principles of PBM to target 3 areas (Anesth Analg 2020;131:74)
- Anemia and iron deficiency
- Blood loss and bleeding
- Coagulopathy management
Drivers
- Increasing demand for quality improvement in healthcare
- Transfusion associated risks
- Economic benefits of decreased transfusion
- Advancement of evidence based practice
- Patient centered care, autonomy and satisfaction
- Excessive use of blood and blood components
- Increasing frequency of blood shortage (AABB: Technical Manual of the American Association of Blood Banks, 20th Edition, 2020)
Overarching approaches
- PBM program oversees staff education on
- Minimizing phlebotomy blood loss
- Increased use of POCT and microvolume sampling
- Use of noninvasive laboratory measurements such as hemoglobin when appropriate
- PBM program liaises with the laboratory to review test ordering patterns and the frequency of inadequate specimens to decrease irrelevant tests and the need for sample recollections (SABM: Administrative and Clinical Standards for Patient Blood Management Programs, 5th Edition, 2019)
- Transfusion triggers
- Packed red blood cells (pRBCs) (Choosing Wisely: A Watershed Moment in Health Care [Accessed 21 June 2023])
- Do not transfuse more units of RBCs or other components than absolutely necessary
- Restrictive threshold (7.0 - 8.0 g/dL) should be used for most stable patients without evidence of impaired tissue oxygenation
- Threshold of 8.0 g/dL is appropriate for patients with pre-existing cardiovascular disease
- Transfusion decisions should be influenced by clinical symptoms and hemoglobin
- Single unit red cell transfusions should be the starting point for nonbleeding, hospitalized patients
- Additional units should only be given after reassessment of clinical symptoms and lab values
- Do not transfuse RBCs for iron deficiency without hemodynamic instability
- Better options include oral or intravenous iron supplementation
- Do not perform serial blood counts on clinically stable patients
- Multiple blood draws can cause iatrogenic anemia and promote unnecessary transfusions
- Do not transfuse O negative blood except to O negative patients in emergencies for women of childbearing potential with unknown blood group
- Do not transfuse more units of RBCs or other components than absolutely necessary
- Plasma (FFP, thawed plasma, PF24, etc.) (Society of Hospital Medicine: Anemia Prevention and Management Program Implementation Guide, 2015)
- Do not transfuse plasma to correct coagulopathy in nonbleeding patients
- Do not routinely use blood products to reverse vitamin K antagonists (VKA); e.g., warfarin
- Reversal of VKA should be based on the international normalized ratio (INR) plus bleeding risks and urgency of reversal
- INR < 4.5 without bleeding
- Hold warfarin if not emergent
- Oral or IV vitamin K (5 - 10 mg)
- 4 factor prothrombin complex concentrate (4F PCC), a hemostatic concentrate of at least 4 coagulation factors (factors II, VII, IX and X), for reversal < 2 hours
- Plasma (15 mL/kg) if 4F PCC is not available
- INR 4.5 - 10 without bleeding
- Hold warfarin
- IV vitamin K (5 - 10 mg) for more rapid reversal
- 4F PCC for reversal < 2 hours
- Plasma (15 - 30 mL/kg) if 4F PCC is not available
- INR > 10 without bleeding
- Hold warfarin
- IV vitamin K (5 - 10 mg)
- 4F PCC for reversal < 2 hours
- Plasma (20 - 30 mL/kg) if 4F PCC is not available
- Serious life threatening bleeding at any INR
- IV vitamin K 10 mg
- 4F PCC for reversal < 2 hours
- Plasma (20 - 30 mL/kg) if 4F PCC is not available
- Platelets (Choosing Wisely: A Watershed Moment in Health Care [Accessed 21 June 2023], Ann Intern Med 2015;162:205)
- Do not transfuse platelets without laboratory guidance outside of fixed ratio massive transfusions
- Prophylactic transfusion for a platelet count of < 10 x 109 cells/L to reduce risk of spontaneous bleeding
- Single apheresis platelet unit or equivalent
- Half doses may be equally effective, especially in times of inventory shortage
- Prophylactic transfusion for a platelet count of < 20 x 109 cells/L for elective placement of central venous catheter
- Prophylactic transfusion for elective diagnostic lumbar puncture with a platelet count < 50 x 109 cells/L
- Prophylactic transfusion for major elective nonneuraxial surgery with a platelet count < 50 x 109 cells/L
- No indication for prophylactic transfusion for patients who are nonthrombocytopenic undergoing cardiac surgery with cardiac bypass
- Not enough evidence for or against transfusion for patients on antiplatelet therapy with intracranial hemorrhage
- Packed red blood cells (pRBCs) (Choosing Wisely: A Watershed Moment in Health Care [Accessed 21 June 2023])
Ongoing interventions
- Education
- Identify key stakeholders (physicians, nurses, administrators, laboratorians, phlebotomists)
- Chief medical officer: administrative / resource support
- Chief quality / safety officer: administrative / resource support
- Chief executive / financial officer: resource allocation
- Anesthesiology: optimization of pre / peri / postoperative hemostasis and hemoglobin
- Surgery: surgical techniques to minimize/manage bleeding
- Hematology oncology: optimize hemostasis in patients with cancer and hematologic disorders
- Clinical laboratory: appropriate laboratory utilization
- Blood bank: blood product stewardship
- Pharmacy: role in appropriate use of pharmacologic interventions that decrease blood usage (vitamin K, parental iron, tranexamic acid)
- Information technology: make changes in electronic medical record (EMR) such as computerized provider order entry (CPOE) and best practice alerts
- Identify key stakeholders (physicians, nurses, administrators, laboratorians, phlebotomists)
- Monitoring
- Determine what data is available from the EMR versus the blood bank computer system
- Pharmacy is a source of data on iron use and adjuvant medications to optimize hemostasis
- Determine if information obtained electronically is reliable for if additional chart search is necessary
- EMR interventions
- Clinical decision support (CDS)
- Reduction of errors and adverse events
- Promotion of best practices
- Quality and safety
- Appropriate utilization
- Cost reduction
- Cost profile improvement
- Use evidence based recommendations (J Am Med Inform Assoc 2018;25:1556)
- Computerized provider order entry (CPOE): embed best practices into EMR
- Alerts: automatic message to communicate essential information to provider at time of order
- May feature best practice guidelines or prompting questioning of order
- Passive alert: information is presented but does not interrupt the user workflow, no user interaction required
- May lead to alert fatigue and providers ignoring the messaging
- Overrides may pose dangers
- Hard stop: user is either prevented from taking an action altogether or allowed to proceed only with the external override of a third party
- Soft stop: user is allowed to proceed against the recommendations presented in the alert as long as an active acknowledgement reason is entered
- Clinical decision support (CDS)
Case reports
- 101,794 patients ages 18 years and older involved in a patient blood management monitoring and feedback program (Transfusion 2015;55;2807)
- Impact of a patient blood management monitoring and feedback program on allogeneic blood transfusions and related costs with 213,882 adult patients (Anaesthesia 2019;74:1534)
Sample assessment & plan
- Assessment: A 68 year old woman who observes a vegan diet has a past medical history significant for obesity and osteoarthritis. She is referred by her primary care physician to the orthopedic surgery clinic for a 3 year history of worsening right knee pain. On assessment for a right total knee replacement, her complete blood count showed a hemoglobin of 9.8 mg/dL (12 - 16 g/dL), mean corpuscular volume (MCV) of 72 fL (79 - 98 fL), mean corpuscular hemoglobin concentration (MCHC) of 28 g/dL (32 - 36 g/dL) and red cell distribution width (RDW) of 17.1% (11.1 - 14.9%). Iron studies showed a low serum iron and ferritin with an increased transferrin iron binding capacity. Vitamin B12 and folate levels were within normal limits.
- Right knee osteoarthritis
- Obesity
- Iron deficiency anemia
- Plan:
- Schedule for right total knee replacement in 8 weeks
- Stool for occult blood, ova and parasites (negative results)
- Commence trial of oral iron therapy for 4 weeks
- Repeat hemoglobin and iron studies in 4 weeks
- Repeat tests at the 4 week follow up visit showed a hemoglobin of 11.2 g/dL, the surgeon deemed this adequate response with no plan to use adjuvant erythropoietic agents
- Follow up assessment and plan:
- Resolve anemia
- Continue oral iron therapy
- Repeat hemoglobin 1 day prior to planned surgery: 12.5 g/dL
- Proceed with surgery as planned; administer intraoperative fibrinolytic / hemostatic agents
- Patient underwent knee replacement surgery without administration of intraoperative tranexamic acid and use of topical hemostatic agents. She had a moderate amount of intraoperative blood loss with a postoperative hemoglobin of 10.3 mg/dL. She was, however, stable and was discharged to a rehabilitation facility.
- Postoperative plan:
- Continue oral iron therapy and rehabilitation therapy
- Follow up in 4 weeks at anemia clinic
Additional references
Board review style question #1
You are the Medical Director of Transfusion Medicine at your hospital. You are currently working with Information Technology to implement some updates to the clinical decision support in your electronic medical records (EMR) for the ordering of blood products. Which best practice recommendations would you embed in the orders for packed red blood cells (pRBCs)?
- 1 unit pRBCs are not normally recommended; 2 should always be given
- Additional units of pRBCs should be given without checking hemoglobin (Hb) in stable patients to avoid unnecessary blood draws and iatrogenic anemia
- pRBC transfusion is not recommended in hemodynamically unstable cardiac patients with evidence of impaired tissue oxygenation until Hb < 7.0 g/dL
- pRBC transfusion is recommended in hemodynamically stable patients with evidence of impaired tissue oxygenation and Hb < 7.0 g/dL
Board review style answer #1
D. pRBC transfusion is recommended in hemodynamically stable patients with evidence of impaired tissue oxygenation and Hb < 7.0 g/dL. Answers A and B are incorrect because 1 unit pRBC transfusion is recommended for most indications and stable patients, followed by rechecking of hemoglobin for transfusion response. Answer C is incorrect because for patients who are hemodynamically unstable and have pre-existing cardiovascular disease, the hemoglobin threshold for transfusion is often higher at 8.0 g/dL rather than the more restrictive < 7.0 g/dL.
Comment Here
Reference: Patient blood management
Comment Here
Reference: Patient blood management
Board review style question #2
A patient is scheduled to undergo an elective central venous catheter insertion. The patient is not taking anticoagulants and has a reasonable hemoglobin level of 12.2 g/dL. What is the recommended platelet count transfusion threshold in this scenario?
- < 10 x 109 cells/L
- < 20 x 109 cells/L
- < 50 x 109 cells/L
- < 100 x 109 cells/L
Board review style answer #2
B. < 20 x 109 cells/L. Insertion of a central venous catheter and otherwise stable patient carries a recommendation for a platelet transfusion threshold of at least 20 x 109 cells/L. Answers A and C are incorrect because prophylactic platelet transfusion is recommended to avoid spontaneous bleeding in patients with a platelet count of < 10 x 109 cells/L and for patients who are to undergo lumbar puncture with platelet count of < 50 x 109 cells/L, respectively. Additionally, patients about to undergo neuroaxial surgical procedures should be transfused for platelet count < 50 x 109 cells/L. Answer D is incorrect because it refers to the older recommendation of transfusing for a platelet count of < 100 x 109 cells/L for patients undergoing any sort of neurosurgery; however, there is not much high quality evidence to support that practice.
Comment Here
Reference: Patient blood management
Comment Here
Reference: Patient blood management
Board review style question #3
A 40 year old woman with a history of heavy menstrual bleeding due to uterine fibroids is scheduled for elective hysterectomy. Her hemoglobin is 9.7 g/dL and her iron studies are consistent with iron deficiency anemia. She is otherwise healthy. Which of the following interventions is most likely to decrease the likelihood of allogeneic transfusion during her surgery?
- Acute normovolemic hemodilution during the surgery
- Preoperative anemia clinic management with correction of iron deficiency scheduled well in advance of her date of surgery
- Provider education with best practice guideline alerts in the EMR regarding appropriate hemoglobin thresholds for pRBC transfusion
- Use of point of care devices such as thromboelastography during surgery to assess clot formation in real time
Board review style answer #3
B. Preoperative anemia clinic management with correction of iron deficiency scheduled well in advance of her date of surgery. In advance of elective hysterectomy, optimization of hemoglobin with iron therapy would be the most appropriate PBM intervention and is very achievable if the surgery is scheduled several weeks in advance. Answer A is incorrect because acute normovolemic hemodilution is not normally used during procedures such as hysterectomy, which are not usually associated with multiple unit red blood cell loss. Answer C is incorrect since best practice alerts may be helpful in the stable in patient setting but are less likely to be helpful to aid decision making during surgeries. Answer D is incorrect because point of care devices such as thromboelastography are typically employed during high blood loss cases such as cardiothoracic surgery, trauma or liver transplant. They are not typically employed during routine hysterectomy cases.
Comment Here
Reference: Patient blood management
Comment Here
Reference: Patient blood management
Photopheresis
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Vascular access | Indications | Adverse events | Diagnosis | Laboratory | Case reports | Treatment | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Apheresis procedure wherein the buffy coat is removed and treated with psoralen and UV light, which leads to apoptosis and immune modulation of treated cells (J Clin Apher 2019;34:171)
Essential features
- Process of removing mononuclear cells (MNC) (monocytes and lymphocytes) from the patient and treating these mononuclear cells with 8-methoxypsoralen (8-MOP) and ultraviolet light (UVA) (320 - 400 nm wavelength)
- Following UVA photoactivation, the mononuclear cells are returned to the patient
- This process can be performed on a discontinuous device (UVAR XTS) or a continuous apheresis device (CellEx), both currently licensed in the U.S.
- This process of mononuclear cell collection, treatment and photoactivation takes place outside of the patient (ex vivo); thus, the process can also be called extracorporeal photopheresis (ECP)
- Initially approved by the FDA to treat cutaneous T cell lymphoma (CTCL) that is unresponsive to other treatments, photopheresis is the only FDA approved indication in the U.S. to date (Therakos CellEx Plus: Achieving Progress By Design [Accessed 22 February 2023])
- Also used to treat graft versus host disease (GVDH), heart and lung transplant rejection (Nat Clin Pract Oncol 2006;3:302)
Terminology
- Therakos UVAR XTS
- Operates on discontinuous cycle
- Single needle access
- Uses either 125 mL (small bowl) or 225 mL (Latham bowl) to collect mononuclear cells (3 - 6 cycles)
- Small bowl is used for pediatric patients, patients with anemia (HCT < 36%), lower body weight (< 45 kg) or hemodynamic instability
- Extracorporeal volume (ECV) ranges from 220 to 620 mL
- Typically takes ~4 hours to finish
- Therakos CellEx (Br J Dermatol 2009;161:167)
- Operates on continuous cycle
- Single or double venous access
- ECV is 266 mL for single needle and 216 mL for double needle procedures
- Can be used for patients who weigh as little as 22 kg
- Typically takes ~1.5 hours to finish
- 8-methoxypsoralen (8-MOP)
- Psoralen based medication used in ECP; this medication is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant (North Carolina Extension Gardener: Ammi Majus [Accessed 22 February 2023])
- Functions by binding to the DNA backbone when photoactivated with UVA, thus preventing future cell division / replication
Pathophysiology
- Exact mechanism of action for ECP is unclear
- Overall, this procedure helps to change the regulatory balance of the immune system with an emphasis on clearance of cytotoxic T cells
- Multiple theories exist as to how this is undertaken, including
- Apoptosis of cells exposed to treatment, with greatest effect in cytotoxic T cells
- Human leukocyte antigen (HLA) modulation of cytotoxic CD8+ cells
- Increasing activity of regulatory T cells
- Differentiation of monocytes into dendritic cells
- References: Blood Rev 2001;15:103, Transfus Med Hemother 2020;47:226, Transpl Immunol 2009;21:117
Clinical features
- Most common type of CTCL is mycosis fungoides (MF), which makes up ~50% of primary cutaneous lymphomas
- Sézary syndrome (SS) is an advanced, leukemic form of CTCL
- There are ~3,000 new cases of mycosis fungoides each year in the U.S.; ~15% are diagnosed as Sézary syndrome (Cutaneous Lymphoma Foundation: Sézary Syndrome [Accessed 22 February 2023], Blood 2005;105:3768, Blood 2009;113:5064)
- Acute graft versus host disease (GVHD; grade II - IV) occurs within 3 months after hematopoietic stem cell transplantation and affects 10 - 60% of these patients; chronic GVHD affects 6 - 80% of cases
- Lung transplant incidence is 4.8 per 1 million of the population in the U.S.
- Heart transplants: ~2,300 annually in U.S. (Clin Transpl 2008:35)
Vascular access
- Therakos UVAR XTS machine operates using a discontinuous flow; thus, it only requires single needle venous access (J Clin Apher 2017;32:462)
- Therakos CellEx machine operates using a continuous flow; thus, either single or double venous access can be used (J Clin Apher 2017;32:462)
- ECP can be performed either with peripheral IV access or with a central venous catheter (CVC)
- For patients undergoing chronic ECP, a CVC is often used to ensure adequate venous access
- Emergent procedures are not performed since numerous ECP procedures are often needed before there is evidence of clinical benefit
Indications
- ECP is a chronic treatment and not generally used as an acute intervention
- American Society for Apheresis (ASFA) has expanded their indications for ECP in recent years (J Clin Apher 2023;38:77)
Current indications for ECP according to ASFA 2023 guidelines (J Clin Apher 2023;38:77)
| Disease | Indication | Category | Grade |
| Atopic (neuro) dermatitis (atopic eczema), recalcitrant | III | 2B | |
| Cutaneous T cell lymphoma (CTCL); mycosis fungoides; Sézary syndrome | Erythrodermic | I | 1B |
| Nonerythrodermic | III | 2B | |
| Graft versus host disease (GVHD) | Acute | II | 1B |
| Chronic | II | 1B | |
| Inflammatory bowel disease | Crohn's disease | III | 2C |
| Nephrogenic systemic fibrosis | III | 2C | |
| Pemphigus vulgaris | Severe | III | 2C |
| Psoriasis | Disseminated pustular | III | 2B |
| Systemic sclerosis | III | 2A | |
| Transplantation, cardiac | Cellular / recurrent rejection | II | 1B |
| Rejection prophylaxis | II | 2A | |
| Transplantation, liver | Antibody mediated rejection / immune suppression withdrawal | III | 2B |
| Desensitization, ABOi | III | 2C | |
| Transplantation, lung | Chronic lung allograft dysfunction / bronchiolitis obliterans syndrome | II | 1C |
Adverse events
- ECP is a safe and well tolerated procedure
- ECP does not require exposure to blood / blood products
- Patients may require blood transfusion prior to ECP to maintain a safe extracorporeal blood volume
- Minor adverse events
- Low grade fevers may occur within 2 to 12 hours post infusion of mononuclear cells (Front Immunol 2023;14:1086006)
- Temporary increase in pruritis or erythema may be seen in patients with CTCL
- Psoralen medications can remain in the peripheral circulation following ECP therapy; patients may be photosensitive and should avoid UV exposure
- Recommended to avoid a high fat meal at least 7 hours prior to ECP, as plasma opacity from triglycerides could interfere with the separation as well as the photoactivation process
- Similar difficulty in the interface detection can occur in patients with high bilirubin levels
- Some patients may experience a foul taste with psoralen infusion
- Patients with limited or compromised cardiopulmonary reserve or impaired kidney function should be closely monitored for any significant changes in fluid shifts
- Contraindications
- Psoralen compounds are contraindicated in patients with aphakia or with a photosensitive disease (e.g., porphyria cutanea tarda)
- Patients with psoralen allergies should avoid ECP; additionally, patients allergic to common sources of psoralens like figs or celery should be evaluated by an allergist prior to initiation of therapy
- Heparin is often used as an anticoagulant in ECP; thus, it is contraindicated in patients with a history of heparin induced thrombocytopenia
Diagnosis
- Diagnosis of CTCL is based on history, physical, flow cytometry and biopsy assessment (Clin Lab Med 2017;37:527)
- Diagnosis of cardiac rejection can be made by biopsy assessment as well as clinical status changes
- Diagnosis of lung transplant rejection can be made by biopsy as well as followed by pulmonary lung function testing (PFT)
- Graft versus host disease can be assessed clinically (skin involvement, GI involvement [diarrhea], lung [PFT]) and by biopsy)
Laboratory
- Prior to initiating ECP, both the patient and laboratory values should be assessed
- Try to limit the amount of blood outside of a patient to < 15%
- For patients with low HCT, preprocedure transfusion may be required
- Additionally, there can be small platelet losses from ECP; therefore, a patient must be assessed for bleeding risk prior to ECP initiation
- Hemoglobin value of 10 g/dL and a platelet count of 20 x 109/L is recommended (Asian J Transfus Sci 2017;11:81)
Case reports
- 29 year old man with a history of metastatic melanoma who was managed with checkpoint inhibitor therapy and subsequently developed colitis (N Engl J Med 2020;382:294)
- 49 year old woman with checkpoint inhibitor associated systemic sclerosis who was managed with ECP (Clin Exp Rheumatol 2022;40:2004)
- 63 year old man with hepatitis B cirrhosis who developed GVHD after liver transplant and was successfully treated with ECP (Transfusion 2022;62:2409)
Treatment
- Combination therapy is often required for solid organ and stem cell transplant GVHD
- Use of ECP can help to reduce the overall steroid dose that many treatment refractory patients experience (Transfus Med Hemother 2020;47:214)
- For CTCL
- Mycosis fungoides is 1 cycle (2 consecutive days) for every 2 - 4 weeks for at least 6 months
- Sézary syndrome is 1 cycle for every 2 weeks for at least 6 months
- Maintenance therapy is 1 cycle every 6 - 12 weeks with the goal of discontinuation if no relapses occur
- If CTCL recurs, then the plan is 1 cycle for every 2 - 4 weeks
- For cardiac allograft rejection
- No consensus on the treatment protocol
- Example of a treatment plan reported in the literature is 1 cycle weekly initially, then every 2 - 8 weeks for several months
- No definite data exists regarding duration, interval or tapering protocol for ECP
- For lung allograft rejection
- No definite data exists regarding duration, interval or tapering protocol for ECP
- Largest case series:
- 24 procedures over 6 months
- 1 cycle is 2 treatments on consecutive days
- 5 cycles over the first month = 10 treatments
- 1 cycle biweekly x 2 months = 4 cycles = 8 treatments
- 1 cycle monthly x 3 months = 3 cycles = 6 treatments
- 24 procedures over 6 months
- For GVHD
- No definite data exists regarding duration, interval or tapering protocol for using ECP to treat acute or chronic GVHD
- Acute GVHD
- No consensus on the treatment protocol
- Example of a treatment plan reported in the literature is 1 cycle weekly until disease response (~4 weeks) and then tapering to biweekly before discontinuing (Transfus Med Hemother 2020;47:214)
- Chronic GVHD
- No consensus on the treatment protocol
- Example of a treatment plan reported in the literature is 1 cycle weekly until response or for 8 - 12 weeks, following by tapering to every 2 - 4 weeks until maximal response (Transfus Med Hemother 2020;47:214)
Sample assessment & plan
- Assessment: Patient is a 19 year old man with GVHD. The patient is currently undergoing extracorporeal photopheresis for GVHD. The patient does not report any significant interval change since the last procedure.
- Procedure: The patient tolerated the procedure well. 1.5 liters of the patient's whole blood was processed. Heparin was utilized as anticoagulation. The buffy coat was collected and treated with 8-MOP. Next treatment tomorrow.
- Plan: Next treatment is tomorrow. Will continue with 1 cycle weekly until a response or for 8 - 12 weeks. This will be followed by tapering to every 2 - 4 weeks until maximal response.
Additional references
Board review style question #1
Which of the following is an ASFA category I indication for extracorporeal photopheresis (ECP)?
- Erythrodermic cutaneous T cell lymphoma
- Graft versus host disease (GVHD)
- Inflammatory bowel disease (Crohn's disease)
- Pemphigus vulgaris
Board review style answer #1
A. Erythrodermic cutaneous T cell lymphoma. All other indications are noncategory I indications. Answers B - D are incorrect because these answers are category II or III indications.
Comment Here
Reference: Photopheresis
Comment Here
Reference: Photopheresis
Board review style question #2
Which of the following is a side effect of extracorporeal photopheresis (ECP)?
- Allergy to psoralen
- Dysgeusia
- Feeling of impending doom
- Slight increase in platelet count
Board review style answer #2
B. Dysgeusia. Though ECP is well tolerated, dysgeusia (or abnormal taste) has been reported with psoralen infusion. Answer A is incorrect because patients allergic to psoralens should avoid ECP, thus this is a contraindication not a side effect. Answer C is incorrect because a feeling of impending doom may be a sign of a hemolytic transfusion reaction but has not been described with ECP. Answer D is incorrect because there is a slight loss of platelets within the machine during the procedure so a slight decrease in platelet count would be observed.
Comment Here
Reference: Photopheresis
Comment Here
Reference: Photopheresis
Plasma use
Table of Contents
Definition / general | Essential features | Terminology | Characteristics | Pathophysiology | Clinical features | Blood donor screening | Blood donor testing | Treatment | Sample assessment & plan | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Plasma is the aqueous part of blood and contains albumin, coagulation factors including fibrinogen, immunoglobulins and other proteins (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020)
- Used directly for transfusion purposes or indirectly for preparation of specific plasma protein products through different fractionation processes (Cohn: Technical Manual, 20th Edition, 2020, ISBT Science Series 2008;3:148)
- Derived from whole blood or apheresis collection (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020, ISBT Science Series 2008;3:148)
Essential features
- Plasma used for transfusion purposes is either frozen (< -18 °C), refrigerated (1 - 6 °C), kept at room temperature (20 - 24 °C) or thawed after freezing (30 - 37 °C) (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020)
- Different plasma types (see below) are considered equivalent for transfusion purposes, although minor differences in some labile coagulation factors do exist
- Plasma transfusions are generally indicated in coagulopathic patients with active bleeding; all units should contain coagulation factors, natural anticoagulants and ADAMTS13 at acceptable levels but lower levels of heat labile Factors V and VIII can result if freezing of fresh plasma is delayed (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Volume: whole blood derived units contain 200 - 250 mL on average but apheresis derived units may contain 400 - 600 mL (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
Terminology
- Fresh frozen plasma (FFP): plasma frozen within 8 hours of collection (Transfusion Medicine and Hemostasis 2009:1;161)
- Plasma frozen within 24 hours after phlebotomy (PF24): plasma refrigerated within 8 hours of collection and frozen within 24 hours of collection (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Plasma frozen within 24 hours after phlebotomy held at room temperature up to 24 hours after phlebotomy (PF24RT24): plasma stored at room temperature for up to 24 hours after collection and frozen within 24 hours of collection (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Plasma, cryoprecipitate reduced: plasma prepared from thawed whole blood derived FFP with cryoprecipitate removed by centrifugation (FFP - cryoprecipitate) and refrozen within 24 hours of thawing (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Thawed plasma: frozen plasma (FFP, PF24 or PF24RT24) that has been thawed and refrigerated for up to 4 days after the initial 24 hour post thaw period (maintained in a closed system) (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Thawed plasma, cryoprecipitate reduced: frozen plasma (plasma, cryoprecipitate reduced) that has been thawed and refrigerated for up to 4 days after the initial 24 hour post thaw period (maintained in a closed system) (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Liquid plasma: plasma that has been prepared from whole blood and only refrigerated (never frozen) and can be transfused up to 5 days after the expiration date of the whole blood (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
| Product | Postcollection temporary hold | Frozen postcollection |
| FFP | Within 8 hours | |
| PF24 | Refrigerated within 8 hours | Within 24 hours |
| PF24RT24 | Room temperature for up to 24 hours | Within 24 hours |
| Post thaw 0 - 24 hours | Post thaw 24 - 120 hours |
| FFP | Thawed plasma |
| PF24 | Thawed plasma |
| PF24RT24 | Thawed plasma |
| Plasma, cryoprecipitate reduced | Thawed plasma, cryoprecipitate reduced |
Characteristics
- All types of plasma should contain coagulation factors, natural anticoagulants and ADAMTS13 at acceptable levels but lower levels of heat labile Factors V and VIII can result if freezing of fresh plasma is delayed
- All frozen plasma products should be infused immediately after thawing or refrigerated for up to 24 hours
- After 24 hours, the product must be relabeled as thawed plasma and can be stored in the refrigerator for an additional 4 days
- Thawed plasma has reduced levels of mainly Factors V, VII and VIII
- PF24 and PF24RT24 are prepared when whole blood or plasma cannot be transported, processed and frozen in time after phlebotomy due to geographic or logistical constraints
- Both have reduced levels of Factor VIII compared with FFP
- PF24 also has reduced levels of protein C while PF24RT24 furthermore has reduced levels of Factor V and protein S
- Plasma, cryoprecipitate reduced is deficient in fibrinogen, Factor VIII, Factor XIII and von Willebrand factor (vWF) due to removal of the cryoprecipitate
- Liquid plasma has variable levels of coagulation factors and other proteins
- Reference: AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021]
| Product | Compared with FFP, reduced levels of |
| PF24 | Factor VIII and protein C |
| PF24RT24 | Factors V and VIII and protein S |
| Thawed plasma | Factors V, VII and VIII |
| Liquid plasma | Variable; depends upon storage condition |
| Product | Shelf life |
| FFP | 12 months frozen 7 years at -65 °C (with FDA approval) |
| Thawed plasma | 4 days after the initial 24 hour post thaw period |
| Liquid plasma | 26 days (from anticoagulant citrate dextrose (ACD) / citrate phosphate dextrose (CPD) or citrate phosphate double dextrose (CP2D) whole blood units) 40 days (from citrate phosphate dextrose adenine (CPDA-1) whole blood units) |
Pathophysiology
- Transfusion reactions that are strongly associated with plasma transfusion include allergic / anaphylactic reactions, transfusion related acute lung injury (TRALI) and transfusion associated circulatory overload (TACO); although less common, other types of transfusion reaction may occur (Transfusion 2012;52:65S, Blood 2019;133:1840)
- TRALI is one of the leading causes of transfusion related mortality
- Characterized by acute hypoxemia and noncardiogenic pulmonary edema occurring within 6 hours of transfusion
- Most patients recover within 96 hours with respiratory support
- Higher risk from plasma containing blood products due to the presence of antibodies against human leukocyte antigens (HLA) or human neutrophil antigens (HNA)
- Current mitigation strategies to minimize the exposure risk to HLA alloantibodies include collection from men, never pregnant women or parous women who test negative for HLA antibodies
- Transfusion associated circulatory overload (TACO) has a similar presentation and should be included in the differential diagnosis for TRALI
- TACO is typically involved with large volume plasma transfusions in susceptible individuals with evidence of cardiogenic pulmonary edema and volume overload (increased brain natriuretic peptide [BNP] or N terminal pro B type natriuretic peptide [NT proBNP])
- References: Cohn: Technical Manual, 20th Edition, 2020, Hematology Am Soc Hematol Educ Program 2018;2018:585
Clinical features
- Plasma contains antibodies, including antibodies to red blood cell antigens (Transfusion Medicine and Hemostasis 2009:1;161)
- All units undergo antibody screen for non ABO antibodies; if the screen is positive, plasma will not be made from that unit (Transfusion Medicine and Hemostasis 2009:1;161)
- If a unit of plasma is prepared for transfusion, the only red cell alloantibodies (if present) are ABO antibodies (Transfusion Medicine and Hemostasis 2009:1;161)
- AB blood group is the universal plasma donor because it lacks anti-A and anti-B (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021])
- Group A plasma is readily available compared with AB; therefore, it has potential to relieve current pressure on the AB plasma inventory, especially in the trauma or other emergency settings (Transfusion 2014;54:1751)
- In comparison with restricting transfusions to ABO compatible plasma, evidence has shown that group B and AB patients transfused with group A plasma benefited without greater incidence in morbidity or mortality (Transfusion 2014;54:1751)
- Liquid plasma is increasingly used in emergencies and early trauma management to avoid delays in thawing with comparable results to frozen plasma products (Transfusion Medicine and Hemostasis 2009:1;161)
- Thawed plasma is also used in emergencies due to availability and can help reduce the wastage from frozen plasma products when not transfused within the first 24 hours after thawing (Transfusion Medicine and Hemostasis 2009:1;161, Transfusion 2009;49:2625)
- Plasma, cryoprecipitate reduced is almost exclusively used in patients with thrombotic thrombocytopenic purpura (TTP) as regular infusions or exchange transfusions (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020)
- It should never be used for repletion of coagulation factors (fibrinogen, Factor VIII, Factor XIII and vWF) or as a substitute for FFP, PF24 or thawed plasma (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020)
- Solvent / detergent treated plasma (SD plasma): pooled from many donor units, the major advantage is an extra safety measure and lower likelihood of allergic reactions, TRALI and transfusion transmitted infections (Shock 2016;46:468)
- Pathogen reduced / inactivated plasma (PRT plasma): new technology utilizing various methods (e.g amotosalen, UV light, etc.), as an additional safety measure against transfusion transmitted infections (Hematol Oncol Clin North Am 2019;33:749)
- Dried plasma: in military / trauma settings where conventional plasma storage is logistically difficult or unavailable; since 2018, FDA emergency authorization for pathogen reduced leukocyte depleted freeze dried plasma has been granted to the U.S. Department of Defense (U.S. FDA: FDA News Release [Accessed 14 July 2021], Transfusion 2016;56:S128)
Blood donor screening
- Per FDA requirements, all donors must be screened via donor history questionnaire (DHQ) for risk factors of transfusion transmitted infections and other conditions that may adversely affect the health of the donor or the safety, purity or potency of the blood or blood component (U.S. FDA: Implementation of Acceptable Full-Length and Abbreviated Donor History Questionnaires and Accompanying Materials for Use in Screening Donors of Source Plasma [Accessed 14 July 2021])
Blood donor testing
- Per FDA requirements, all donors must be tested for infectious disease markers (IDM) using FDA licensed donor screening tests (U.S. FDA: Testing Human Cells, Tissues, and Cellular and Tissue Based Product (HCT/P) Donors for Relevant Communicable Disease Agents and Diseases [Accessed 14 July 2021])
Treatment
- Actively bleeding patients with multiple coagulation factor deficiencies / defects
- Rapid vitamin K antagonist reversal in cases of Coumadin (warfarin) overdose, that results in bleeding or in patient undergoing an invasive procedure requiring prompt coagulation factor replacement when factor concentrates, such as 4 factor prothrombin complex concentrates, are not available or contraindicated
- During massive transfusions associated with significant coagulation factor deficiencies / defects
- Specific plasma protein factor deficiencies / defects, either congenital or acquired, when licensed factor concentrate is not available
- Transfusion or replacement fluid for therapeutic plasma exchange (TPE) in patients with TTP (first line treatment) or in conjunction with albumin as replacement fluid in patients with underlying coagulopathy, requiring therapeutic plasma exchange for other indications
- TPE is the mainstay treatment for TTP (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020)
- Patients with clinical and laboratory evidence of TTP (low levels or reduced ADAMTS13 activity) are treated with TPE to remove antibodies to ADAMTS13 and replete ADAMTS13 until their platelet counts recover (AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020)
- Perioperative management of coagulopathy (see note below)
- Dosing: typical dose of plasma is around 10 - 15 mL/kg
- Hemostasis is usually achieved when the coagulation factor activity is at least 25 - 30% of normal
- Infusion: infusion rates depend on the volume load that can be tolerated by the patient
- Suggested rates:
- Healthy individual: 2 - 4 mL/kg per hour
- Individual with volume overload or heart failure: 1 mL/kg per hour or less
- Note: other approaches should be explored whenever possible before transfusing with plasma to avoid the risks of an adverse event, which includes various types of blood product related transfusion reaction, transfusion transmitted infections, etc.
- References: AABB: Circular of Information for the Use of Human Blood and Blood Components [Accessed 2 June 2021], Cohn: Technical Manual, 20th Edition, 2020
Sample assessment & plan
- Assessment: 37 year old female with prior history of chronic relapsing thrombotic thrombocytopenic purpura (TTP) treated with multiple FFP transfusions / plasma exchanges. She presented to the emergency room directly from hematology clinic due to an acute drop in platelet count (28 x 10³/mcL), with undetectable ADAMTS 13 activity and elevated inhibitor level indicating relapsed TTP. The patient had history of bruises with prior episodes but currently denies bleeding, fever or neurological symptoms.
- Plan: The patient will start daily plasma exchange sessions using plasma as replacement fluid (1 - 1.5 plasma volume) until platelet counts recover (> 150 x 10³/mcL) and lactate dehydrogenase (LDH) is near normal for 2 - 3 consecutive days. An order for stat central venous catheter placement is placed. The patient will be premedicated with diphenhydramine before starting the procedure. Please report any transfusion reaction to blood bank.
- Reference: J Clin Apher 2019;34:171
Board review style question #1
Which of the following is a correct statement?
- Fresh frozen plasma (FFP) has a shelf life of 5 days
- Liquid plasma has a shelf life of 26 days if derived from citrate phosphate double dextrose (CP2D) whole blood
- Plasma frozen within 24 hours after phlebotomy (PF24) has a shelf life of 24 hours
- Thawed plasma has a shelf life of 3 days
Board review style answer #1
B. Liquid plasma has a shelf life of 26 days if derived from citrate phosphate double dextrose (CP2D) whole blood
Comment Here
Reference: Plasma use
Comment Here
Reference: Plasma use
Board review style question #2
Which of the following is a correct statement?
- Group A plasma may be given to any patients during a bleeding emergency
- Group A plasma should never be given to a group AB patient
- Group O plasma is the universal plasma
- Liquid plasma should not be used for bleeding emergency, as it has decreased levels of Factor V and VIII
Board review style answer #2
A. Group A plasma may be given to any patients during a bleeding emergency
Comment Here
Reference: Plasma use
Comment Here
Reference: Plasma use
Platelet refractoriness
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features and adverse effects | Screening / evaluation | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Defined as a suboptimal platelet count response after multiple platelet transfusions
- Often clinically assessed using the corrected count increment (CCI) calculation, which adjusts for the transfused platelet dose and the recipient's body size
- Majority of cases are due to nonimmune factors (e.g., fever, infection, bleeding) compared to immune mediated factors (e.g., anti-HLA antibodies)
- Poor platelet count response at 10 - 60 minutes may indicate immune mediated refractoriness and laboratory based testing is usually indicated (see Laboratory section), while poor response at 24 hours usually indicates nonimmune causes
Essential features
- Suboptimal response of platelet counts following 2 or more transfusions with CCI of < 5,000/μL (or < 7,500/μL) when assessed within 1 hour after transfusion
- Management and treatment vary based on etiology
- Nonimmune: treat underlying condition
- Immune: HLA, HPA and ABO matched platelets
- Increased HLA alloimmunization prevalence in
- Patients with hematologic malignancy or hematopoietic stem cell transplantation (HSCT)
- Females with history of pregnancy (compared to males and females who have never been pregnant)
- Leukoreduction of blood products has been shown to lower frequency of HLA alloimmunization and platelet refractoriness
Terminology
- PI: platelet increment
- CCI: corrected count increment
- PPR: percentage platelet recovery
- HSCT: hematopoietic stem cell transplant
- HLA: human leukocyte antigen
- HPA: human platelet specific antigen
- BSA: body surface area
- PD: platelet dose
- PRA: panel reactive antibody
- TXA: tranexamic acid
Pathophysiology
- Nonimmune mediated (≤ 80% of cases): usually from rapid consumption or sequestration (Blood 2005;105:4106)
- Sepsis / infection, fever (temperature > 38.4 °C), bleeding, hypersplenism, disseminated intravascular coagulation (DIC)
- Medications can have both nonimmune and immune mechanisms (e.g., vancomycin, amphotericin B and heparin)
- Other possible causes include hepatic sinusoidal obstruction syndrome and graft versus host disease (GVHD) in HSCT patients
- Immune mediated (≤ 20% of cases)
- Prior antigenic exposure via transfusion, pregnancy or transplantation leads to alloimmunization against
- Epitopes of class I HLA (A and B)
- Human platelet specific antigens (HPA), although rarely implicated
- ABO antigens are weakly expressed on platelets; therefore, incompatible platelet transfusion can reduce platelet survival (Br J Haematol 2015;171:297)
- Drug dependent antibodies
- Other immunogenic antigens implicated include CD36 (glycoprotein IV) due to anti-CD36 antibodies in CD36 deficiency recipients (Transfusion 2021;61:1932)
- Prior antigenic exposure via transfusion, pregnancy or transplantation leads to alloimmunization against
Clinical features and adverse effects
- Longer hospital stays and higher inpatient hospital costs (Am J Hematol 2000;64:251)
- Increased risk of bleeding (Bone Marrow Transplant 2000;26:315, Transfusion 2008;48:1959)
- Decreased survival (Transfusion 2008;48:1959)
Screening / evaluation
- Obtain platelet count within 10 - 60 minutes and at 24 hours posttransfusion on 2 separate occasions
- A single apheresis platelet transfusion should increase the platelet count by ~30K - 50K/μL in an average sized recipient
- Calculations used to assess platelet count response:
- Posttransfusion increment (PI):
- Simplest calculation, commonly used in routine clinical practice
- PI = posttransfusion platelet count - pretransfusion platelet count (K/μL)
- PI > 10,000/μL is considered a good response
- Simplest calculation, commonly used in routine clinical practice
- Corrected count increment (CCI):
- Calculated using the PI, the patient's body surface area (BSA) and the transfused platelet dose (PD)
- CCI = PI x BSA (m2)
PD (x 1011) - CCI > 5,000 (or > 7,500/μL) at 1 hour is considered an adequate response (Transfus Med Rev 2000;14:180)
- TRAP study defined platelet refractoriness as a 1 hour CCI of < 5,000/μL on 2 sequential occasions, when transfusing ABO identical fresh platelets (N Engl J Med 1997;337:1861)
- CCI =
- Calculated using the PI, the patient's body surface area (BSA) and the transfused platelet dose (PD)
- Percentage platelet recovery (PPR):
- Calculated using the PI, the patient's total blood volume (TBV) and the PD
- PPR = TBV x PIx 100
PD (x 1011) - PPR > 30% at 1 hour posttransfusion and > 20% at 20 - 24 h is considered a good response (Transfus Med 1993;3:91)
- PPR =
- Calculated using the PI, the patient's total blood volume (TBV) and the PD
- Posttransfusion increment (PI):
- In nonimmune platelet refractoriness, the posttransfusion platelet count may be adequate; however, return to pretransfusion levels on repeat 24 hour testing
Laboratory
- HLA testing is highly complex and is usually performed at reference labs
- HLA genotyping (PCR)
- HLA antibody analysis
- Panel reactive antibody (PRA)
- Historical method using a lymphocytotoxicity assay in which serum is reacted with a panel of HLA typed lymphocytes
- Antibody binding to specific HLA antigens induces complement mediated cell death and dye uptake which is visible on fluoroscopy as red cells indicating a positive reaction
- With negative reactions, the cells survive and dye is excluded, appearing green on fluoroscopy
- PRA > 20% suggests probable HLA alloimmunization
- Historical method using a lymphocytotoxicity assay in which serum is reacted with a panel of HLA typed lymphocytes
- Multiplex flow cytometric bead based assay
- Beads coated with individual HLA antigens
- Binding detected by staining with fluorescently labeled antihuman globulin
- Antibody level determined by flow cytometry, flow microarrays or enzyme linked immunosorbent assay (ELISA)
- Panel reactive antibody (PRA)
- HPA testing can be done concurrently with HLA testing or subsequently if HLA antibody testing is negative and patient continues to be refractory
- Reference: Virchows Arch 2019;474:139
Case reports
- 31 year old man with acute myeloid leukemia who developed platelet refractoriness (Asian J Transfus Sci 2021;15:90)
- 64 year old woman with resolution of platelet refractoriness with secondary acute myeloid leukemia after allogenic bone marrow transplantation (Haematologica 2019;104:e121)
- 69 year old woman who developed platelet refractoriness following atorvastatin use (Cureus 2021;13:e12502)
- 74 year old man who developed refractory thrombocytopenia within 1 day of receiving the Moderna SARS-CoV-2 vaccine (J Blood Med 2021;12:221)
Treatment
- Nonimmune mediated platelet refractoriness:
- Treat underlying condition (e.g., discontinue medication, treat infection, treat cause of DIC)
- Immune mediated platelet refractoriness:
- ABO identical platelets, stored < 48 hours
- Crossmatch compatible platelets
- Can detect incompatibility due to HLA or HPA antibodies
- Further alloimmunization possible
- Can be more convenient and less expensive than HLA based strategies depending on institution
- HLA matched platelets
- Molecular HLA typing and single antigen testing have supplanted the use of the antigen match grade system (e.g., A, BU, B2U, BX, C matches, etc.)
- Large HLA typed donor population required to find a match
- Potentially compatible platelets may be excluded even though not an HLA match
- Computer based matching algorithms help predict compatibility based on defined epitopes (Hum Immunol 2007;68:12)
- HLA antigen exclusion platelet units
- Lack the antigen for which the patient has specific antibodies
- Not indicated when the PRA is low (< 20%)
- Further alloimmunization possible
- HPA matched platelets if anti-HPA antibodies are detected
- Return to random platelets if patient continues to have poor posttransfusion increments with the strategies listed above
- Immunosuppressive therapy (e.g., IVIG, rituximab) with or without therapeutic plasma exchange may be considered in extenuating circumstances (limited data)
- For management of bleeding, consider
- Antifibrinolytic agents (lysine derivatives), e.g. tranexamic acid (TXA) and minocaproic acid
- Continued platelet transfusions (may provide some hemostatic benefit) (Br J Haematol 2015;171:297)
- Prevention:
- Leukoreduction reduces the exposure to multiple HLA class I and HPA antigens
- ABO matched platelets (limited data)
Sample assessment & plan
- Assessment: Jane Doe is a 55 year old woman with a recent diagnosis of acute myeloid leukemia (AML) admitted for induction chemotherapy. Her course was significant for severe thrombocytopenia (platelet count of 15,000/μL), diffuse petechiae and minor nose bleeds. Despite multiple platelet transfusions, the patient continues to have low counts and lower than expected platelet increments. The CCIs for the next 2 successive platelet transfusions were both < 5,000/μL. Transfusion medicine service was consulted for assistance in transfusion management.
- Plan: The patient's clinical history, current presentation and preliminary screening workup are all suggestive of immune mediated platelet refractoriness.
- Order reference lab workup for HLA / HPA antibody screen
- In the meantime, provide crossmatch compatible platelets (continue if adequate platelet response and compatible platelets are available)
- Depending on lab screening results, consider use of either HLA / HPA antigen matched and HLA antigen exclusion in order to optimize platelet availability
- If the strategies listed above fail, return to continued transfusion of random platelets to offer some hemostatic benefit
- Consider use of antifibrinolytics if bleeding continues
Differential diagnosis
- Immune thrombocytopenia (ITP):
- Thrombocytopenia without apparent etiology
- Thrombotic thrombocytopenic purpura (TTP):
- Pentad (fever, renal dysfunction, microangiopathic hemolytic anemia, thrombocytopenia, neurological dysfunction)
- Schistocytes on peripheral blood smear
- Heparin induced thrombocytopenia (HIT):
- Thrombocytopenia and thrombosis
- Neonatal alloimmune thrombocytopenia (NAIT):
- Due to maternal antibodies
- Nonimmune factors (e.g., fever, infection, hypersplenism, bleeding, etc.)
Additional references
Board review style question #1
A 56 year old woman with myelodysplastic syndrome (MDS) requiring weekly transfusions, presented to the Emergency Department with pancytopenia and bright red blood per rectum. Platelet count at presentation was 15K/uL. She received two units of RBCs and one apheresis platelet. A CBC obtained within an hour of platelet transfusion revealed a platelet count of 18K/uL and her hemoglobin increased from 5.6 g/dl to 8.0 g/dl. Despite repeated platelet transfusions, the CCI never went above 3K/uL when assessed within an hour after transfusion. What is the most probable cause of this patient’s refractoriness and the best initial step in management?
- Alloimmunization and stop platelet transfusions until HLA typing and antibody screen is resulted
- Alloimmunization and transfuse ABO matched platelets
- Immune thrombocytopenia (ITP) and start steroids
- Progression of MDS and start chemotherapy
Board review style answer #1
B. Alloimmunization and transfuse ABO matched platelets
This patient is currently bleeding, so it is important to continue providing therapeutic hemostatic support with platelet transfusion. The patient is in platelet refractory state as demonstrated by the failure to achieve an expected platelet count increment within an hour of transfusion on multiple occasions. As such, transfusion of ABO matched platelets as a first step is indicated, while evaluating for other causes of platelet refractories including the presence of anti-HLA antibodies. Patient’s MDS associated pancytopenia requires significant blood product transfusions, predisposing patient to multiple antigens and hence alloimmunization. Progression of her MDS would not necessarily have a suboptimal CCI, thus chemotherapy would not be the best initial step in management. ITP is a diagnosis of exclusion characterized by an acquired thrombocytopenia due to autoantibodies directed against platelet antigens. ITP does not affect multiple hematopoietic cell lineages; therefore, pancytopenia is not observed.
Comment Here
Reference: Platelet refractoriness
This patient is currently bleeding, so it is important to continue providing therapeutic hemostatic support with platelet transfusion. The patient is in platelet refractory state as demonstrated by the failure to achieve an expected platelet count increment within an hour of transfusion on multiple occasions. As such, transfusion of ABO matched platelets as a first step is indicated, while evaluating for other causes of platelet refractories including the presence of anti-HLA antibodies. Patient’s MDS associated pancytopenia requires significant blood product transfusions, predisposing patient to multiple antigens and hence alloimmunization. Progression of her MDS would not necessarily have a suboptimal CCI, thus chemotherapy would not be the best initial step in management. ITP is a diagnosis of exclusion characterized by an acquired thrombocytopenia due to autoantibodies directed against platelet antigens. ITP does not affect multiple hematopoietic cell lineages; therefore, pancytopenia is not observed.
Comment Here
Reference: Platelet refractoriness
Board review style question #2
A 39 year old woman with AML has become refractory to platelet transfusions, evidenced by multiple suboptimal platelet count increments assessed within 1 hour of transfusion. She has recently developed minor bleeding from her nasal mucosa as well as petechiae on her lower extremities. Which of the following HLA antigen typings would be the most useful to optimize her platelet transfusion response?
- HLA-A and HLA-B antigens
- HLA-A and HLA-C antigens
- HLA-A and HLA-DR antigens
- HLA-B and HLA-C antigens
Board review style answer #2
A. HLA-A and HLA-B antigens
Platelets express a variety of antigens on their surface, including HLA Class I, ABO and HPAs. Antibodies directed against HLA Class I (HLA-A and HLA-B) are associated with platelet refractoriness but rarely HLA-C antibodies. ABO identical / compatible platelets may help improve the platelet response in certain cases. Antibodies directed towards HPAs can be considered in cases where there is persistent high suspicion for immune mediated refractoriness while the HLA antibody screen is negative. HLA Class II antigens (HLA-DR, HLA-DQ, and HLA-DP) are not found on platelets.
Comment Here
Reference: Platelet refractoriness
Platelets express a variety of antigens on their surface, including HLA Class I, ABO and HPAs. Antibodies directed against HLA Class I (HLA-A and HLA-B) are associated with platelet refractoriness but rarely HLA-C antibodies. ABO identical / compatible platelets may help improve the platelet response in certain cases. Antibodies directed towards HPAs can be considered in cases where there is persistent high suspicion for immune mediated refractoriness while the HLA antibody screen is negative. HLA Class II antigens (HLA-DR, HLA-DQ, and HLA-DP) are not found on platelets.
Comment Here
Reference: Platelet refractoriness
Platelet use
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Symptoms and signs of thrombocytopenia | Screening | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Platelets are derived from single donor apheresis or pooled whole blood (from 4 - 6 donors) collections
- Platelet transfusions may be indicated prophylactically in thrombocytopenic patients or therapeutically in bleeding patients
Essential features
- Apheresis and whole blood derived platelets are collected from volunteer donors who meet the donor health questionnaire and other screening requirements
- Platelet units are tested for infectious diseases (viral and bacterial)
- Clinical practice recommendations for platelet use in adult inpatients include the following indications (with recommended platelet count threshold):
- Nonimmune thrombocytopenia, including prophylactic transfusion of hypoproliferative thrombocytopenic patients (≤ 10 × 10⁹/L)
- Prior to procedures not associated with significant blood loss (including some percutaneous procedures) (< 20 × 10⁹/L)
- Prior to epidural anesthesia or lumbar puncture, procedures with expected blood loss > 500 mL, major nonneuraxial surgery (< 20 - 50 × 10⁹/L)
- Significant bleeding (< 50 × 10⁹/L)
- Guideline references: Transfus Med Rev 2015;29:3, Ann Intern Med 2015;162:205, J Clin Oncol 2018;36:283
- Transfuse 1 dose (1 pool of whole blood derived platelets or 1 apheresis platelets unit) and reassess patient; 1 dose raises the platelet count by approximately 15 - 25 × 10⁹/L
- Platelet life span in vivo is 8 - 10 days (J Biol Chem 2013;288:6826)
- Platelet transfusions are associated with potential adverse events, the most common of which include febrile reaction, allergic reaction and bacterial sepsis
Terminology
- Apheresis platelets: collected from single donors using automated cell separator devices (contains ≥ 3 × 1011 platelets per bag in 100 - 500 mL of plasma or plasma with platelet additive solution) (Transfusion 2016;56:2173)
- Whole blood derived platelets: 4 - 6 random donor platelets are pooled to make an adult dose (contains ≥ 5.5 × 1010 platelets per bag in 40 - 70 mL of plasma) (Transfusion 2016;56:2173)
- Pediatric platelet dosing: 5 - 10 ml/kg up to 300 ml (adult dose)
- Neonates: 10 - 15 ml/kg
Pathophysiology
- Platelets are activated upon exposure to extracellular matrix of injured endothelium to adhere and aggregate, which forms a hemostatic plug
- This self amplifying mechanism maintains normal hemostasis and works in primary hemostasis to address injuries in bleeding (Blood Rev 2011;25:155)
- Platelet membrane antigens include glycoconjugates of the ABO system, class I human leukocyte antigens (HLA) and human platelet antigens (HPA)
- HLA matched platelets are indicated if the patient has anti-HLA antibodies reacting with antigens present on at least 20% of the donor population (i.e. PRA > 20%)
- If the patient has poor increments to HLA matched platelets, an investigation for anti-HPA antibodies should be performed to determine if HPA matched platelets are indicated
- HLA and HPA matched platelets are genotype matched products, HLA and HPA selected platelets lack HLA or HPA antigens to which the patient has formed HLA or HPA antibodies
- Antibody formation against platelet antigens is responsible for alloimmune thrombocytopenia, platelet transfusion refractoriness, passive alloimmune thrombocytopenia, neonatal alloimmune thrombocytopenia and posttransfusion purpura (Wien Klin Wochenschr 2001;113:806)
- Antiplatelet alloantibody - most prevalent in the obstetrical setting and in patients with history of multiple transfusions (see platelet antibodies)
- Reduced posttransfusion platelet count increments can be seen with major ABO incompatible platelet transfusions, compared with minor ABO incompatible or ABO compatible platelet transfusions (Asian J Transfus Sci 2015;9:117)
- While platelets themselves do not express RhD antigens, platelets from RhD positive donors are contaminated by RhD positive red blood cells that pose a risk of RhD alloimmunization to RhD negative recipients; prevention measures may be considered in women of childbearing age (Asian J Transfus Sci 2015;9:117)
Clinical features
- Causes of thrombocytopenia include:
- Reduced production by congenital and acquired causes of abnormal megakaryocytic development (drugs, hematologic malignancy, May-Hegglin syndrome, alcohol excess, infections, etc.)
- Immune and nonimmune platelet consumption
- Abnormal distribution of platelets (splenic sequestration)
- Dilutional (in massive blood transfusion) (Medicine 2017;45:221)
Transmission
- Platelet transfusions are associated with risks to recipients including the following adverse events (with approximate risk per platelet transfusion in the U.S.):
- Febrile reaction (1/14)
- Allergic reaction (1/50)
- Bacterial sepsis (1/75,000)
- Transfusion related acute lung injury (1/138,000)
- Hepatitis B virus infection (1/2,652,580)
- Hepatitis C virus infection (1/3,315,729)
- HIV infection (0 - 1/1,461,888) (Ann Intern Med 2015;162:205)
- Transmissible disease risk varies between jurisdictions based on donor population disease prevalence, donor screening criteria and donor unit testing methods
Symptoms and signs of thrombocytopenia
- Range from asymptomatic to petechiae, bruises, mucosal bleeding (from nose, GU or GI tract) and rarely intracranial hemorrhage (Medicine 2017;45:221)
Screening
- Platelet count should be measured pre and posttransfusion to help assess need for transfusion and the adequacy of response to a platelet transfusion, respectively
Blood donor screening
- Donors must meet specific eligibility criteria outlined by regulators such as the Food and Drug Administration, laboratory accrediting organizations such as AABB and individual donation centers, including:
- Age (16+), feeling well and healthy on day of donation, weight and hemoglobin requirements and deemed acceptable upon completing the donor health history questionnaire (AABB: Blood Donor Screening and Testing [Accessed 9 November 2020])
Blood donor testing
- In addition to standard infectious disease testing as per whole blood donations (including testing for [some regional and seasonal] hepatitis B and C, HIV, West Nile virus, Chagas disease, Zika, Babesia, HTLV1), platelets undergo additional testing:
- HLA antibody screening of donors with a history of transfusion or pregnancy to reduce the risk of transfusion related acute lung injury in recipients (Transfusion 2014;54:3036)
- Strategies to reduce bacterial contamination of platelets for transfusion include skin decontamination, blood (and skin plug) diversion, bacterial culture testing, storage duration limitations and pathogen reduction / inactivation technologies (Crit Care 2018;22:271)
Donor deferral
- In addition to standard deferrals for blood donation based on the donor health history questionnaire, patients with HLA antibodies are deferred from donating platelets since they pose a risk of transfusion related acute lung injury to recipients (Vox Sang 2012;103:10)
Laboratory
- Platelets are stored at room temperature under constant agitation to prevent cold platelet storage lesion and aggregation, respectively
- Cold platelet storage lesion describes a decrease in posttransfusion survival and hemostatic function (Curr Opin Hematol 2018;25:500)
- Room temperature storage conditions increase risk of bacterial contamination, hence the short 5 - 7 day shelf life of platelets, which challenges blood bank inventory management due to rapid outdating (Blood Transfus 2019;17:321)
- Measuring the pretransfusion count and posttransfusion platelet count approximately 1 hour after platelet transfusion can help distinguish between immune and nonimmune causes of platelet refractoriness, which is a complication most commonly encountered in the management of hypoproliferative thrombocytopenic patients
- At 1 hour posttransfusion, if the corrected count increment is less than 7.5 x 10⁹/L or the percentage platelet recovery is < 30%, this suggests alloimmune platelet refractoriness
- At 1 hour posttransfusion, if the corrected count increment is greater than 7.5 x 10⁹/L then falls below 7.5 x 10⁹/L at 24 hours, this suggests nonimmune platelet destruction (Canadian Blood Services: Platelet Transfusion, Alloimmunization and Management of Platelet Refractoriness [Accessed 10 December 2020])
- Various calculations can be used to define platelet refractoriness:
- Platelet increment (PI) = P2 - P1, where P1 is the pretransfusion platelet count and P2 is the posttransfusion platelet count
- Corrected count increment (CCI) = [(PI × BSA)/n] × 100, where BSA represents the body surface area in meters² and n is the number of platelets transfused
- Percentage platelet recovery (PPR) = [(PI × body weight (kg) × 0.075 (l/kg))/n] × 100; body weight (kg) × 0.075 (l/kg) is an estimate of blood volume (Transfus Med 1992;2:35)
Case reports
- 2 newborns with neonatal alloimmune thrombocytopenia (Kobe J Med Sci 2019;64:E197)
- 61 year old woman with acute intravascular hemolysis due to plasma in non-ABO identical platelets (Am J Case Rep 2019;20:1075)
- 61 year old woman with drug induced thrombocytopenia (Kobe J Med Sci 2016;62:E9)
Treatment
- Clinical practice recommendations for platelet use in adult inpatients by diagnosis / indication (with recommended platelet count threshold):
- Nonimmune thrombocytopenia, including prophylactic transfusion of hypoproliferative thrombocytopenic patients (< 10 × 10⁹/L)
- Prior to procedures not associated with significant blood loss (including some percutaneous procedures) (< 20 × 10⁹/L)
- Therapeutic anticoagulation that cannot be stopped (< 30 × 10⁹/L)
- Prior to epidural anesthesia or lumbar puncture, procedures with expected blood loss > 500 mL, major nonneuraxial surgery (< 50 × 10⁹/L)
- Patients with significant bleeding (< 50 × 10⁹/L)
- Prior to neuraxial surgery (< 100 × 10⁹/L)
- Head trauma, central nervous system hemorrhage, life threatening hemorrhage (< 100 × 10⁹/L)
- Platelet dysfunction (secondary to aspirin or clopidogrel) and significant bleeding (with or without cardiopulmonary bypass) or prophylactic invasive procedure (any platelet count)
- AABB does not recommend for or against platelet transfusion in the context of platelet dysfunction (secondary to aspirin or clopidogrel) in the context of intracranial hemorrhage due to low quality and conflicting evidence
- Immune thrombocytopenia (case specific; only for life threatening bleeding in consultation with a hematologist)
- Guideline references: Transfus Med Rev 2015;29:3, Ann Intern Med 2015;162:205, J Clin Oncol 2018;36:283
- Transfuse 1 dose (1 pool of platelets or 1 apheresis platelets unit) and reassess patient; 1 dose raises the platelet count by approximately 15 - 25 × 10⁹/L
- In patients with hypoproliferative thrombocytopenia, HLA matched platelets lead to better 1 hour posttransfusion count increments; however, the effect at 24 hours is inconsistent (Transfusion 2013;53:2230)
- Contraindications to platelet transfusion - only use platelet transfusions to treat life threatening bleeding in patients with:
- Thrombotic microangiopathies (e.g. thrombotic thrombocytopenic purpura) - increased risk of further thrombotic events
- Congenital platelet functional disorders (e.g. Glanzmann thrombasthenia) - increased risk of alloimmunization leading to refractoriness, consider alternatives (recombinant factor VIIa, tranexamic acid, desmopressin) (Br J Haematol 2017;176:365)
- Modifications to platelet products based on availability and indications:
- Gamma irradiation: to prevent transfusion associated graft versus host disease (TA-GVHD) in patients by inactivating donor T lymphocytes that are present in platelet units in small numbers despite leukocyte reduction (Blood 2015;126:406)
- Pathogen reduction technologies (such as amotosalen-ultraviolet A pathogen reduction) reduce the risk of transfusion-transmitted infection and TA-GVHD (Transfusion 2019;59:1953)
- Disadvantages include reduced platelet activatability and increased / accelerated platelet apoptosis and clearance (Haematologica 2017;102:1650)
Sample assessment & plan
- Assessment: John Doe is a 75 year old man undergoing induction chemotherapy for acute myeloid leukemia. He is pancytopenic with a platelet count of 2 × 10⁹/L and has bruising on his forearms.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of hypoproliferative thrombocytopenia.
- Recommend: prophylactic transfusion of 1 dose of platelets
- Perform platelet count 1 hour posttransfusion to assess response to platelet transfusion
Differential diagnosis
- Hypoproliferative thrombocytopenia:
- Decreased platelet production secondary to bone marrow dysfunction (hematological malignancy) or destruction (by metastatic infiltration)
- Immune thrombocytopenia:
- Most cases are autoimmune; some may be triggered by viral or bacterial infections
- Drug induced thrombocytopenia:
- Reported with drugs such as quinidine, trimethoprim-sulfamethoxazole, gold
- Idiopathic thrombocytopenia:
- Other causes of thrombocytopenia excluded
- Platelet dysfunction:
- Postcardiopulmonary bypass (via removal of GPIb from platelets and activated platelets from circulation)
- Antiplatelet drugs (clopidogrel, aspirin)
- Congenital (Bernard-Soulier, Glanzmann thrombasthenia, platelet storage pool disorders)
Additional references
Board review style question #1
In patients with hypoproliferative thrombocytopenia and who have a platelet count increment less than 5 - 10 × 10⁹/L 1 hour posttransfusion on 2 occasions, what is the next best step in their transfusion management?
- Irradiated platelets
- Random donor whole blood derived platelets
- Apheresis platelets
- HLA or HPA selected platelets
- Fresh as possible platelets
Board review style answer #1
D. In HLA or HPA alloimmunized patients who are refractory to platelet transfusions with apheresis or whole blood derived platelets, HLA or HPA selected platelets are recommended. If these are unavailable, use ABO identical or crossmatched apheresis platelets. Never delay lifesaving platelet transfusions while waiting for HLA or HPA matched platelets.
Comment Here
Reference: Platelet use
Comment Here
Reference: Platelet use
Board review style question #2
A 65 year old woman presents to the emergency department with a lower GI bleed and she is on dual antiplatelet therapy (DAPT). About 15 minutes into transfusion of 1 dose of platelets, she spikes a fever (temperature 38.8 °C = 101.8 °F) and experiences rigors. What adverse reaction do you suspect from her platelet transfusion?
- Mild allergic reaction
- Septic reaction
- Transfusion related acute lung injury
- Transfusion associated circulatory overload
- Hepatitis B virus infection
Board review style answer #2
B. Septic transfusion reactions occur at an approximate frequency of 1/100,000 platelet transfusions and present acutely with fever, chills, rigors, nausea, vomiting, diarrhea, abdominal and muscle pain or hypotension. Platelets are stored at room temperature, which makes them an ideal growth media for bacteria. Despite efforts to reduce bacterial contamination of platelets, including skin disinfection, volume diversion and microbiological culture of units, bacterial contamination leading to septic transfusion reactions still occurs. Pathogen reduction / inactivation technologies may help further prevent this adverse reaction.
Comment Here
Reference: Platelet use
Comment Here
Reference: Platelet use
Plateletpheresis
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Vascular access | Indications | Volume exchanged & technical details | Adverse events | Laboratory | Case reports | Treatment | Peripheral smear images | Sample assessment & plan | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Thrombocytapheresis (or plateletpheresis) is the removal of platelet cells via apheresis
Essential features
- Therapeutic thrombocytapheresis is performed to acutely lower a patient's platelet (PLT) count due to symptoms associated with thrombocytosis, such as hemorrhagic or thromboembolic complications
Terminology
- Thrombocytosis (J Clin Apher 2023;38:77, Blood 2014;124:1997)
- Usually defined as a platelet count > 450,000 - 500,000/µL
- Can be caused by an underlying hematologic malignancy (primary thrombocytosis) or by a reactive process (secondary thrombocytosis) (Leuk Res 2017;58:14)
- Primary thrombocytosis
- Due to a myeloproliferative disorder (MPD), including polycythemia vera, essential thrombocythemia and chronic myelogenous leukemia (CML) (see Peripheral smear images)
- Shorter platelet survival; normal circulating lifespan of a platelet is 8 - 10 days
- Increase in markers of platelet activation, such as platelet factor 4, thrombomodulin and beta thromboglobulin
- May experience hemorrhage or thrombosis
- Hemorrhage risk due to acquired von Willebrand syndrome
- Thrombosis usually occurs when platelet count is > 600,000/µL but risk does not correlate well with platelet count
- Secondary thrombocytosis
- More common, accounting for 80 - 90% of cases (Intern Med 2022;61:3323)
- Platelet function is relatively normal
- Carries significantly smaller risk of hemorrhage or thrombosis
Pathophysiology
- Thrombosis can occur as a result of congestion and increased viscosity secondary to high platelet counts
- This tends to be more common in clonal thrombocytosis unless patients have underlying predisposition to clot formation (N Engl J Med 2004;350:1211)
- Likewise, bleeding tends to occur as a result of qualitative platelet defects seen in myeloproliferative disorders
(N Engl J Med 2004;350:1211)
- Extremely high platelet counts tend to associate with an acquired von Willebrand syndrome with clearance of high molecular weight von Willebrand factor (vWF) and bleeding (Haematologica 2002;87:264)
Clinical features
- Hemorrhage or thromboembolism associated with platelet counts > 450,000/µL (Semin Thromb Hemost 2013;39:101)
Symptoms
- Patients may present with platelet type bleeding at mucous membranes or gastrointestinal tract, digital ischemia or massive thromboembolic events such as pulmonary embolism or hepatic vein thrombosis (N Engl J Med 2004;350:1211)
- Platelet count does not predict symptoms (J Clin Apher 2023;38:77)
Vascular access
- Usually performed emergently using femoral apheresis or a dialysis catheter, which do not require radiographic confirmation
- Background on femoral vein vascular access (StatPearls: Femoral Vein Central Venous Access [Accessed 31 January 2024])
- Central venous catheter, which requires radiographic confirmation, can be used in nonemergent situations
Indications
- The only indications for thrombocytapheresis according to the American Society for Apheresis (ASFA) are symptomatic and prophylactic or secondary thrombocytosis (J Clin Apher 2023;38:77)
- Symptomatic thrombocytosis - ASFA category II
- Bring platelet count to < 350 - 450 x 109/L in patients with an acute thrombohemorrhagic event
- Prophylaxis - ASFA category III
- Pregnant patients, surgical patients, etc.
- < 450 - 600 x 109/L may be an appropriate count for prophylaxis per ASFA
- Symptomatic thrombocytosis - ASFA category II
Volume exchanged & technical details
- 1 procedure or until resolution of symptoms
- 1.5 - 2 blood volumes
- This usually reduces the platelet count by 30 - 60%
- Patients with spleens will recover counts more quickly than asplenic patients due to splenic platelet content (Leuk Res 2017;58:14)
Adverse events
- This is an apheresis procedure, with its typical complications
- Also complications from the underlying myeloproliferative neoplasm, hypotension, citrate related toxicity, bleeding and infection
Laboratory
- Elevated platelet count
- Reduced vWF and factor VIII levels
Case reports
- 24 year old woman with essential thrombocythemia was treated prophylactically with thrombocytapheresis (Case Rep Oncol 2020;13:675)
- 25 year old man with triple negative essential thrombocythemia presented with thrombosis and acquired von Willebrand syndrome and was treated with thrombocytapheresis (Am J Case Rep 2020;21:e924560)
- 51 year old woman with essential thrombocythemia was treated for postoperative bleeding with 2 thrombocytapheresis procedures (Transfusion 2021;61:3277)
Treatment
- Treatment of underlying condition should be initiated for primary thrombocytosis with cytoreductive agents (hydroxyurea) (J Clin Apher 2023;38:77)
- Interferon alpha should be used in pregnancy
- In symptomatic or prophylactic thrombocytapheresis, the platelet goal should be < 450 x 109/L
Sample assessment & plan
- Assessment: A 63 year old man with JAK2+ myeloproliferative neoplasm underwent thrombocytapheresis due to acute thromboembolism.
- Plan: 1.5 blood volumes were processed. If counts remain high or patient experiences symptoms related to thrombocytosis, further intervention may be considered.
Board review style question #1
A patient presents with essential thrombocythemia and has ongoing mucocutaneous and gastrointestinal bleeding. A platelet count is found to be > 2,000 x 109. Unfortunately, 2 days later the patient remains symptomatic. What is the goal platelet count?
- < 10 - 20 x 109/L
- < 350 - 450 x 109/L
- < 750 - 1,000 x 109/L
- < 1,500 - 1,750 x 109/L
Board review style answer #1
B. < 350 - 450 x 109/L. ASFA guidelines recommend a count of 350 - 450 x 109/L for symptomatic thrombocytosis. The remaining answers fall outside of ASFA recommendations. Answer A is incorrect because it is too low and could lead to bleeding complications, requiring platelet transfusion. Answers C and D are incorrect because < 750 - 1,000 x 109/L and < 1,500 - 1,750 x 109/L are both still too high and could potentially contribute to depletion of von Willebrand factor, furthering the symptomatic thrombocytosis associated bleeds.
Comment Here
Reference: Plateletpheresis
Comment Here
Reference: Plateletpheresis
Board review style question #2
Why do asplenic patients with thrombocytosis recover their platelet counts more slowly than patients with spleens after thrombocytapheresis?
- Absence of extramedullary hematopoiesis
- Asplenia impacts fluid balance leading to thrombocytopenia
- Lack of splenic macrophages
- Spleen helps maintain platelet count with sequestered platelets
Board review style answer #2
D. Spleen helps maintain platelet count with sequestered platelets. The spleen contains ~25 - 30% of the body's platelets and some of these will peripheralize after thrombocytapheresis or in times of stress in patients with a spleen (Leuk Res 2017;58:14). Asplenic patients do not have this residual pool of platelets (StatPearls: Physiology, Spleen [Accessed 31 January 2024]). Answer A is incorrect because extramedullary hematopoiesis occurs mainly in very young patients or those with a bone marrow filling lesion, such as a hematologic malignancy, metastasis or marrow fibrosis; nevertheless, it does not act as a platelet reserve but rather a producer of platelets. Answer B is incorrect because fluid balance is maintained predominantly by the kidneys as opposed to the spleen. Answer C is incorrect because splenic macrophages help clear aging platelets and red cells and do not release or store platelets.
Comment Here
Reference: Plateletpheresis
Comment Here
Reference: Plateletpheresis
Post-transfusion purpura
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Post-transfusion purpura is a rare but serious complication of a blood component transfusion in which severe thrombocytopenia occurs 5 - 10 days after the transfusion event
- Bleeding may be severe
- Risk of significant morbidity from bleeding complications with a > 10% mortality rate
- Self limiting but due to the severity of complications treatment is warranted
- IVIG is the mainstay of treatment
Essential features
- Immune condition of severe thrombocytopenia occurring 5 - 10 days after a transfusion
- Classically occurs after an RBC transfusion but any blood component can trigger the immune process (RBCs, platelets, plasma)
- Transfusion recipient has antibodies against human platelet specific antigens (HPA) acquired through prior transfusion, transplant or pregnancy
- F:M = 26:1, but incidence in men increases in age > 65 (Am J Hematol 1996;52:205, Transfusion 2015;55:284)
Terminology
- PTP (post-transfusion purpura)
- RBC (red blood cell)
- HPA (human platelet antigen)
- HLA (human lymphocyte antigen)
- ELISA (enzyme linked immunosorbent assay)
- IVIG (intravenous immune globulin)
Pathophysiology
- Antiplatelet alloantibodies to human platelet antigens (HPA), usually due to prior transfusion, transplant or pregnancy
- Anti-HPA-1a is the most common culprit
- Other HPA antibodies have been implicated
- Anti-HPA antibody destroys autologous platelets; mechanism is not fully understood
- Passive transfer of antibody is possible (Br J Haematol 1987;66:113)
- Possible autoimmune platelet antibody masked by alloantibody (Transfus Med 2005;15:243)
- Antibody to autologous GPIIb/IIIa platelet receptor (Am J Hematol 2016;91:848)
- Immune complex binding to platelets (Vox Sang 1991;60:40)
- Adhesion of transfused alloantigen to autologous platelets (Br J Haematol 1989;72:539)
Clinical features
- Symptoms:
- Severe, sudden thrombocytopenia (< 10K/uL)
- Bleeding (mucosal or gastrointestinal)
- Petechiae or purpura
Laboratory
- Human platelet antigen (HPA) testing is a highly specialized methodology and is performed at reference labs:
- Detection of HPA antibodies by immunofluorescence
- Enzyme linked immunoassay (ELISA)
- Platelet genotyping (PCR)
- Monoclonal antibody specific immobilization of platelets assay (MAIPA) (Ann Hematol 2014;93:309)
Case reports
- 35 year old woman and 44 year old woman with post-transfusion purpura (Am J Hematol 1996;52:205)
- 49 year old man with severe thrombocytopenia after passive transfer of platelet (PLT) antibody (Transfusion 2008;48:1981)
- 56 year old woman who developed acute thrombocytopenia (Cureus 2017;9:e1207)
- 66 year old woman with unexpected development after surgery (Am J Hematol 2016;91:848)
- 68 year old man with post-transfusion purpura due to human platelet antigen-5b alloantibody (J Med Case Rep 2012;6:420)
- Women with post-transfusion purpura (Ann Hematol 2001;80:488)
Treatment
- Self limiting: untreated will persist for 7 - 28 days
- Platelet transfusion is generally not helpful in the acute phase, even HPA matched platelets are destroyed (Am J Hematol 1979;6:71)
- IVIG, high dose 400 - 500 mg/Kg per day for 5 days
- 1 g/Kg for two days in severe cases
- Glucocorticoids
- Therapeutic plasma exchange (Acta Med Scand 1978;203:539)
- Prevention:
- Patient with known history of post-transfusion purpura should receive washed RBCs
- Platelet transfusions should be crossmatched or HPA matched products
Sample assessment & plan
- Assessment: Jane Doe is a 63 year old woman who presented 11 days after PRBC transfusion with severe thrombocytopenia, altered mental status and diffuse petechiae. Send out testing identified the patient has both HLA class I antibodies and is homozygous for HPA-1b platelet antigen, with an antibody to HPA-1a. Further review of history reveals the patient has had 3 pregnancies, with one child affected by neonatal alloimmune thrombocytopenia at birth.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of post-transfusion purpura (PTP).
- Recommend starting IVIG at 1g/kg/day for three days.
- Platelets negative for the HPA-1a antigen have been requested. In the acute phase of the disease, platelet transfusion is not usually beneficial, however as this patient has an intracranial hemorrhage from the low platelets, we will issue platelet products as needed. Platelets usually have poor survival after transfusion due to existing patient antibodies.
- If the patient needs an RBC transfusion, we will issue washed products. Please allow extra time to prepare this product (2 hours).
Differential diagnosis
- Thrombotic thrombocytopenic purpura:
- Severe thrombocytopenia and microangiopathic hemolytic anemia
- Schistocytes on peripheral smear
- Fever, renal dysfunction and neurological dysfunction may be present
- Drug induced thrombocytopenia:
- History of medication or beverage (quinine water) proximate to thrombocytopenia
- Only occurs in presence of drug; complete recovery with removal of drug
- Heparin induced thrombocytopenia (HIT):
- Venous or arterial thrombosis
- Platelet count rarely drops < 20k/uL
- Idiopathic thrombocytopenia (ITP):
- Isolated thrombocytopenia without clinically apparent cause
- Accelerated platelet destruction, often splenic sequestration
- Evans Syndrome (pediatrics):
- Immune thrombocytopenia (ITP) with autoimmune hemolytic anemia
- Positive direct antiglobulin test (DAT)
- HLA platelet refractoriness:
- Poor increment increase post platelet transfusion
- Does not cause thrombocytopenia but will impair transfusion efforts
- Neonatal alloimmune thrombocytopenia (NAIT) (infants):
- Severe thrombocytopenia in infant present at birth, no thrombocytopenia in mother
- Anti-HPA-a1 most common cause
Additional references
Board review style question #1
A 75 year old man with type II diabetes and hypertension is admitted for emergent cardiac catheterization due to an episode of chest pain with elevated cardiac enzymes. During the catheterization he requires 2 units of RBC and 1 unit of platelets for unexpected bleeding. He is stabilized and discharged after 48 hours. Six days later, he returns to his PCP with diffuse bruising and a platelet count of 9K/uL. Which item of clinical history is most pertinent to the diagnosis?
- The patient was taking clopidogrel
- The patient was in a motor vehicle accident 15 years ago, requiring multiple blood products
- The patient has an allergy to trimethoprim-sulfamethoxazole
- The patient has a sister with a history of heavy menses and easy bruising
- The patient’s diabetes is poorly controlled with an A1C of 12.2%
Board review style answer #1
B. The patient is exhibiting post-transfusion purpura. Although this is rare and less common in males, it can occur, particularly as a patient’s comorbidities increased. Prior exposure to foreign platelet antigens, through transfusion, transplant or pregnancy can result in antihuman platelet antigen antibodies. This patient’s prior history of blood transfusion and the sudden severe thrombocytopenia (< 20K/uL) occurring 5 - 10 days after a blood transfusion is consistent with PTP. Clopidogrel is an antiplatelet medication used in patients with increased risk of cardiac events or other hypercoagulable states, it does not cause thrombocytopenia but rather a iatrogenic thrombocytopathy. Trimethoprim-sulfamethoxazole is a common culprit in drug induced thrombocytopenia. There is no evidence that this patient would have received this medication. A sister with heavy menses and easy bruising suggests von Willebrand’s Disease (vWD). Although this is an autosomal dominant condition, even if the patient had vWD, it would not cause thrombocytopenia 5 - 10 days after a surgery. Poorly controlled diabetes can contribute to peripheral neuropathy, which can result in increased bruising or other injuries since patients do not feel the event. It does not affect the platelet count.
Comment Here
Reference: Post-transfusion purpura (PTP)
Comment Here
Reference: Post-transfusion purpura (PTP)
Board review style question #2
A 62 year old woman is admitted for urosepsis and anemia. The clinical team calls you because they are preparing to give an RBC transfusion but the patient’s daughter is refusing the transfusion. The daughter says during a previous transfusion at a different hospital her mom had low platelets and severe bruising. You tell the clinical team that you recommend which of the following?
- The patient should receive leukocyte-reduced red cells and the team should provide reassurance to the daughter
- The patient should receive crossmatched platelets due to presumed HLA antibodies
- The patient should receive IgA deficient plasma
- The patient should receive washed RBCs to prevent post-transfusion purpura
- The patient may have an antibody and should receive phenotypically matched RBCs
Board review style answer #2
D. The history the daughter has provided suggests a prior history of post-transfusion purpura in the patient. Post-transfusion purpura carries a high risk of morbidity and mortality. Even though recurrence is exceedingly rare, washed products (to remove the offending HPA antibody) or matched products (it the HPA type is known) is recommended. Although HLA antibodies could be a concern in a multiparous woman, one would typically give an unmodified (other than leukocyte reduction) platelet and monitor the post-transfusion platelet count before assuming that crossmatching is needed. IgA deficient plasma is a rare, special order product that is given for patients with proven severe allergic reaction to IgA. If the patient has an antibody to a red cell antigen, that should be identified during routine type and screen, although it would be prudent to contact the prior hospital for history of any antibody that have become undetectable.
Comment Here
Reference: Post-transfusion purpura (PTP)
Comment Here
Reference: Post-transfusion purpura (PTP)
Pretransfusion testing
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Blood donor testing | Laboratory | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Testing performed by the transfusion service before a transfusion
- Current scope excludes donor testing performed by collection facility
Essential features
- Pretransfusion testing is based on serologic testing methods (molecular testing alone is not sufficient)
- Follow up is required for typing discrepancies; the blood bank must adhere to certain restrictions for donor units and patient transfusions until discrepancies are resolved
- Pretransfusion testing criteria are different for neonates compared with nonneonates
- Computer or electronic crossmatches are allowed as an alternative to serologic crossmatch only when particular criteria are met
Terminology
- Type and screen:
- In this context, type refers to ABO typing
- Forward typing for ABO uses anti-A and anti-B reagent to detect A and B antigens on patient red blood cells (RBCs)
- Reverse typing for ABO uses A1 and B reagent red blood cells to detect anti-A and anti-B in patient serum / plasma
- Typing for Rh (D) uses anti-D reagent to detect Rh (D) antigen on RBCs: reverse typing is not performed because anti-D is not naturally occurring (not expected, such as Anti-A or Anti-B)
- In this context, screen (or antibody screen) refers to screening the patient’s serum / plasma for unexpected antibodies to RBC antigens
- Unexpected antibodies: antibodies that we would not expect to see in a nonsensitized patient (e.g. patient with no transfusion / exposure history)
- This is in contrast to naturally occurring antibodies; the best example of these are anti-A and anti-B, which we expect to see in O, A or B patients (but should not expect to see in anti-A or Anti-B in AB patients)
Pathophysiology
- Clinically significant antibodies:
- Frequently associated with adverse clinical consequences, such as:
- Hemolytic transfusion reactions (acute or delayed)
- Hemolytic disease of the fetus and newborn (HDFN)
- Decreased RBC survival
- Clinically significant antibodies are typically:
- IgG antibodies
- Reactive at 37 °C (body temperature) (Harmening: Modern Blood Banking & Transfusion Practices, 5th Edition, 2005, American Association of Blood Banks: Technical Manual, 20th Edition, 2020)
- Most antibodies are IgG; ABO are IgM but clinically significant
- Frequently associated with adverse clinical consequences, such as:
- Crossmatch: assessment of compatibility between patient’s serum / plasma and donor RBCs (in this context); options include:
- Serologic (physical) crossmatch - physically testing patient serum / plasma against donor RBCs
- Immediate spin (IS) crossmatch:
- Primary objective of the IS crossmatch is to detect ABO incompatible RBC unit before it is transfused
- Patient’s serum / plasma is mixed with donor cells (suspended in saline) in a tube which is then immediately spun in a centrifuge; the tube is then assessed for any donor RBC hemolysis or agglutination that would indicate incompatibility between the patient’s serum / plasma and donor RBCs
- Patient’s anti-A and anti-B are typically IgM (pentameric and hence larger) antibodies that can bridge the gap between donor RBCs and thereby cause agglutination
- Other clinically significant antibodies may or may not be caught by the IS crossmatch, depending on the circumstance (Harmening: Modern Blood Banking & Transfusion Practices, 5th Edition, 2005, American Association of Blood Banks: Technical Manual, 20th Edition, 2020)
- Antihuman globulin (AHG) crossmatch
- Primary objective of the AHG crossmatch is to detect non-ABO incompatibility (due to clinically significant antibodies) before an RBC unit is transfused
- Antihuman globulin (AHG) is a reagent (historically referred to as Coombs reagent) that increases the sensitivity of the crossmatch
- Because clinically significant antibodies are typically IgG (monomeric and hence smaller) antibodies, even if they bind to donor RBCs (i.e. even if the donor RBCs are sensitized), agglutination may not occur without assistance
- AHG bridges the gap between sensitized RBCs, allowing donor RBCs to more easily agglutinate (see Figure 1)
- AHG reagents can be categorized into the following:
- Polyspecific: AHG reagent contains anti-IgG and anti-complement (C3d or C3b)
- Monospecific: AHG reagent contains either anti-IgG or anti-complement (C3d or C3b)
- Polyclonal
- Monoclonal
- Procedure for the AHG crossmatch typically continues from where the IS crossmatch left off (note: AHG crossmatch is unnecessary if the IS crossmatch is already positive)
- Patient’s serum / plasma is mixed with donor cells (suspended in saline) and incubated at 37 °C (incubation times vary depending on the laboratory procedure used); after washing the RBCs to remove excess (unbound) antibodies, AHG reagent is added and the tube / well is inspected for agglutination
- Positive AHG crossmatch can be caused by:
- Clinically significant antibodies
- Clinically insignificant antibodies (Harmening: Modern Blood Banking & Transfusion Practices, 5th Edition, 2005, American Association of Blood Banks: Technical Manual, 20th Edition, 2020)
- Immediate spin (IS) crossmatch:
- Computer (electronic) crossmatch: where a validated computer software system assesses ABO compatibility based on patient and donor ABO typing (donor sample and intended unit are not physically mixed)
- Serologic (physical) crossmatch - physically testing patient serum / plasma against donor RBCs
Blood donor testing
- See Laboratory section below
Laboratory
- Blood tube requirements for patient testing:
- Most laboratories use tubes with ethylenediaminetetraacetic acid (EDTA) (pink or lavender top) but tubes with acid citrate dextrose (ACD) (yellow top) or without anticoagulant (red top) are also acceptable if they have been validated by the laboratory
- It is the laboratory’s responsibility to validate the acceptable blood tube types for specimen collection for testing
Table A.
Regarding pretransfusion testing of donor: for allogeneic or autologous transfusions
| |||
| |
|||
| Red blood cells or whole blood or granulocytes |
ABO typing | Integrally attached segment |
Any discrepancy must:
|
Rh(D) negative typing
|
|||
| Non-ABO / Rh(D) red blood cell antigens | Not required if already labeled as negative for that antigen | ||
Table B.
| Testing of nonneonatal patient: for allogeneic transfusions | ||||
Any unit |
ABO typing |
Reagents:
|
Must be current sample |
|
| Rh typing | Reagents:
|
Weak D typing optional | ||
Red blood cells or Whole blood or Granulocytes |
Antibody screen |
Procedure must include a 37 °C incubation prior to adding AHG Reagent:
|
Patient sample must be obtained within 3 days of the scheduled transfusion if the patient has:
|
Day 0 = date of sample draw A positive antibody screen requires additional testing If the patient has a prior history of clinically significant antibody(s), the lab must use testing methods that identify additional clinically significant antibodies |
Table C.
| Testing on nonneonatal patient: for autologous transfusions |
| ABO typing |
| Rh typing |
| Crossmatch (electronic or serologic) |
Table D.
| Pretransfusion testing of neonatal patient: for allogeneic transfusions | ||||
| |
||||
| ABO typing | Reagents:
|
Not required until:
|
ABO reverse typing not required | |
| Rh typing | Reagent:
|
Weak D typing optional | ||
Antibody screen |
Procedure must include a 37 °C incubation prior to adding AHG Reagent:
|
If no history of discharge, use neonatal serum / plasma or maternal serum / plasma If the neonate has been already been discharged and is being readmitted neonate, use neonatal serum / plasma |
Positive screen requires additional antibody testing |
|
Crossmatch |
|
If initial antibody screen is negative:
If initial antibody screen is positive:
|
||
Anti-A, anti-B screening |
|
Serum / plasma of neonate |
Testing is required if:
|
|
Table E. Crossmatch: for autologous or allogeneic transfusions
| Serologic crossmatch | |||
| |
|||
Must:
|
Red blood cells | Integrally attached segment | Serum / plasma |
| Whole blood | |||
| Apheresis granulocytes with > 2 ml red blood cells | Can use donor red blood cells from sample on date of donation |
||
| Apheresis platelet with > 2 ml red blood cells | |||
| Computer crossmatch | |
| |
|
Patient eligibility requires all of the following:
|
|
Board review style question #1
A 51 year old man is admitted for an active gastrointestinal bleed. A single patient sample is sent to the blood bank with orders for ABO and Rh typing and it results as group O Rh+. Review of records shows that the patient received a red blood cell transfusion 2 months ago, at which time the ABO / Rh typing was O Rh+ and screen for unexpected antibodies was negative.
2 STAT red blood cell units are requested. Why, in the interest of time, is the patient currently not eligible for computer crossmatch?
2 STAT red blood cell units are requested. Why, in the interest of time, is the patient currently not eligible for computer crossmatch?
- ABO typing was not confirmed using second ABO testing from the same admission
- Clinical team did not yet order an antibody screen in this admission
- Only patients who type as AB are eligible for computer crossmatch
- This is an irrelevant question; the patient is currently eligible for computer crossmatch
Board review style answer #1
B. The patient is not currently eligible for computer crossmatch. Even though the patient had a prior negative antibody screen 2 months ago, because he was transfused within the past 3 months, he needs a current antibody screen result to confirm his antibody status and this test was not ordered yet by the team (and thus, not yet done by the transfusion service). Historical ABO / Rh typing from a previous admission is one of the allowed methods of confirming the patient's ABO / Rh typing. Blood type is not a factor in computer crossmatch eligibility.
Comment Here
Reference: Pretransfusion testing
Comment Here
Reference: Pretransfusion testing
Board review style question #2
A 48 year old woman has a scheduled cardiac surgery and arranged for 1 autologous red blood cell unit to be collected and held for her use. After testing, the collection facility labels the unit as group A Rh+ and sends it to the blood bank. Which of the following statements is correct regarding which pretesting is required by the transfusion service before issuing and transfusing the autologous red blood cell unit?
- None; no pretransfusion testing is required for issuing autologous units
- Confirmatory ABO and Rh typing of the donor unit only
- ABO and Rh typing of the patient and confirmatory ABO and Rh typing of the donor unit only
- ABO and Rh typing of the patient and confirmatory ABO typing of the donor unit only
- ABO and Rh typing of the patient, confirmatory ABO typing of the donor unit and crossmatch testing only
Board review style answer #2
E. Autologous transfusions require that the patient has ABO typing, Rh typing and crossmatch testing prior to transfusion. Any red blood cell unit (autologous or allogeneic) requires ABO confirmatory typing by the transfusion service before it is issued. Confirmatory Rh typing is not required if the unit is already labeled as Rh+.
Comment Here
Reference: Pretransfusion testing
Comment Here
Reference: Pretransfusion testing
Board review style question #3
Select the correct statement regarding the cutoff requirements for performing a serologic crossmatch:
Unless the patient is eligible for computer crossmatch, a serologic crossmatch is required for a platelet unit that contains
Unless the patient is eligible for computer crossmatch, a serologic crossmatch is required for a platelet unit that contains
- Greater than 0.1 ml red blood cells
- Greater than 0.2 ml red blood cells
- Greater than 0.5 ml red blood cells
- Greater than 1 ml red blood cells
- Greater than 2 ml red blood cells
Board review style answer #3
E. Serologic crossmatch is required if a platelet unit contains greater than 2 ml red blood cells, which rarely happens given that many platelet units were collected by apheresis procedures.
Comment Here
Reference: Pretransfusion testing
Comment Here
Reference: Pretransfusion testing
Red blood cell alloimmunization
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Screening | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Red blood cell (RBC) alloimmunization is the formation of antibodies to non-self RBC antigens (excluding naturally occurring anti-A and anti-B)
- Only occurs after exposure through transfusion, pregnancy or transplantation
- Incidence varies among different patient populations and is estimated to occur in 0.5 - 3% of the general population
Essential features
- If a patient has an identified RBC alloantibody, an RBC unit negative for the implicated antigen should be selected for transfusion
- RBC alloimmunization can result in acute hemolytic or, more commonly, delayed hemolytic transfusion reactions, which can be life threatening
Terminology
- Red blood cell (RBC)
Pathophysiology
- 1 - 1.6% of RBC transfusions are associated with antibody formation; primary alloimmunization occurs days to months after transfusion of antigen positive RBCs (Cohn: Technical Manual, 20th Edition, 2020)
- RBC alloimmunization involves multiple steps, including RBC antigen recognition and presentation by HLA class II, activation of CD4+ T cells and B cell differentiation into plasma cells (Blood 2012;120:528)
- Certain individuals seem to be genetically predisposed to RBC alloimmunization (responders) and have a 30% alloimmunization risk (Blood 2008;112:2546)
Clinical features
- Elevated rates of RBC alloimmunization in patients with sickle cell disease (19 - 43%), thalassemia major (5 - 45%) and myelodysplastic syndromes (15%) due to the high frequency of transfusion (Hematology Am Soc Hematol Educ Program 2016;2016:446, Blood 2018;132:1826)
- Prophylactic Rh and Kell matching is performed in certain populations, including patients with sickle cell disease or thalassemia major or those with warm autoantibody mediated hemolytic anemia, to reduce alloimmunization (Transfusion 2021;61:3027)
- RBC alloimmunization poses a unique challenge for people of childbearing potential (Transfus Med Rev 2018;32:213)
- Clinically significant RBC alloantibodies are associated with hemolytic transfusion reactions, hemolytic disease of the fetus / newborn (HDFN) and decreased survival of transfused RBCs
- Clinically significant antibodies more commonly cause delayed hemolytic transfusion reactions (via extravascular hemolysis) rather than acute hemolytic reactions
- Common clinically significant red blood antigen groups include Rh, Kell, Duffy, Kidd and Ss
- D antigen is the most immunogenic red cell antigen (other than ABO)
Screening
- RBC alloantibodies are detected by antibody screen and identification panels as part of pretransfusion testing
- RBC antibodies may become undetectable over time (as many as 30 - 60%) and missed on the antibody screen during pretransfusion testing so it is important to know if patient has a history of RBC alloantibodies (Cohn: Technical Manual, 20th Edition, 2020)
- Common cause of delayed hemolytic transfusion reactions
Laboratory
- Presence of RBC alloantibodies can complicate pretransfusion testing and delay blood product availability
- In non-alloimmunized patients, antigens other than ABO and RhD are not routinely considered in blood product selection for transfusion
- To prevent alloimmunization, RhD negative recipients (especially individuals of childbearing potential) should receive RhD negative red blood cells
- Monoclonal antibody therapies such as daratumumab (anti-CD38) interfere with pretransfusion antibody testing by causing panreactivity in indirect antiglobulin test and masking alloantibodies (AABB: Mitigating the Anti-CD38 Interference with Serologic Testing [Accessed 16 December 2021])
Case reports
- 38 year old woman with hemolytic disease of the fetus / newborn due to anti-D and anti-Jka in Nigeria (Hum Antibodies 2020;28:245)
- 83 year old woman with fatal delayed hemolytic transfusion reaction (BMJ Case Rep 2017;2017:bcr2017222343)
- 5 patients with sickle cell disease and RBC alloimmunization who experienced delayed hemolytic transfusion reactions or did not receive needed RBC transfusions and died (Transfusion 2016;56:107)
- Hemolytic disease of the fetus / newborn due to anti-E and anti-Jka in India (Immunohematology 2020;36:60)
- 5 women with severe HDFN and successful management of severe red blood cell alloimmunization in pregnancy (Transfusion 2018;58:677)
Treatment
- No specific treatment for RBC alloimmunization
- If a patient has an identified RBC alloantibody or multiple alloantibodies, an RBC unit negative for the implicated antigen(s) should be selected for transfusion
- Therapeutic plasma exchange has been used to reduce or prevent the red cell alloantibodies, particularly for RHD negative females who have received RH positive transfusions or as a method of antibody reduction for HDFN (J Clin Apher 2019;34:171)
Sample assessment & plan
- Assessment: Routine antibody testing demonstrated an unexpected antibody in 1/3 of the cells on the antibody screen and a full antibody panel demonstrated positivity in 4/12 cells with a negative auto control. An anti-E was identified while all other common clinically significant alloantibodies were ruled out. Anti-E is part of the Rh blood group system and can cause hemolytic transfusion reactions and hemolytic disease of the fetus and newborn (HDFN).
- Plan: E negative RBC units will be issued for all future transfusions. Please allow extra time for antihuman globulin (AHG) or Coombs crossmatch testing (1 hour).
Differential diagnosis
- Alloantibodies must be distinguished from autoantibodies, which develop to self antigens
- Autoantibodies may mask underlying alloantibodies
Board review style question #1
Which patient scheduled for surgery is most likely to have a red blood cell antibody?
- 8 year old child with congenital heart disease
- 26 year old woman with fibroids
- 60 year old woman who had a previous spinal surgery and has 2 children
- 65 year old man with 4 children and coronary artery disease
Board review style answer #1
C. 60 year old woman who had a previous spinal surgery and has 2 children. The patient who has a history of pregnancy and previous surgery with likely transfusion is most likely to have an RBC antibody. RBC alloimmunization occurs after exposure to non-self antigens through pregnancy, transfusion or transplantation.
Comment Here
Reference: Red blood cell alloimmunization
Comment Here
Reference: Red blood cell alloimmunization
Board review style question #2
Patients with which disease have the highest red blood cell alloimmunization rates?
- Acute leukemia
- Iron deficiency anemia
- Sickle cell disease
- Solid tumor malignancy
Board review style answer #2
C. Sickle cell disease. Patients with sickle cell disease have higher rates of RBC alloimmunization (19 - 43%) compared with 1 - 3% of the general population. Higher rates of RBC alloimmunization are also seen in patients with thalassemia major (5 - 45%) and myeloproliferative syndromes (15 - 59%) as well as patients with warm autoantibody mediated hemolytic anemia.
Comment Here
Reference: Red blood cell alloimmunization
Comment Here
Reference: Red blood cell alloimmunization
Red blood cell exchange
Table of Contents
Definition / general | Essential features | Terminology | American Society for Apheresis (ASFA) guidelines | Indications for red blood cell exchange | Indications for erythrocytapheresis | Replacement fluid | Exchange volume | Simple exchange, depletion and depletion / exchange | Vascular access | Anticoagulation | Circuit priming | Adverse events | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Automated technique that separates red blood cells (RBCs) from whole blood and replaces with replacement fluid
- When replacement fluid is donor packed red blood cells (pRBCs), the procedure is referred to as red blood cell exchange (RBCX)
- When replacement fluid is crystalloid or colloid solution, the technique is referred to as erythrocytapheresis (Greek: aphaíresis, "taking away")
Essential features
- Therapeutic technique directed at correcting disease driven by congenital or acquired abnormality of red blood cells
- Sickle cell disease (SCD) is the most common indication; American Society for Apheresis (ASFA) category I indications for RBCX in SCD include treatment of acute stroke and chronic prevention of stroke
- Other indications include parasitemias, such as babesiosis and malaria
- Red blood cell exchange requires donor pRBCs; having valid antibody screen testing on the patient is crucial and blood should be antigenically matched in sickle cell patients
- Hereditary hemochromatosis (HH) and polycythemia vera (PV) are ASFA category I indications for erythrocytapheresis, with modest efficacy advantages over simple therapeutic phlebotomy
Terminology
- Total blood volume (TBV):
- Estimated from patient height, weight and sex
- Preferred method is Nadler formula
- Red blood cell volume (mass) (RBCV):
- Calculated by multiplying total blood volume and hematocrit (Hct)
- Hemoglobin S as a percentage of blood hemoglobin (HbS%):
- Marker of disease severity in sickle cell disease best measured by high performance liquid chromatography (HPLC)
- Fraction of cells remaining (FCR):
- Fraction of residual patient red blood cells following exchange, a marker of procedure performance
- 1.0 would indicate no replacement of patient cells, 0.0 would reflect complete replacement
- Whole blood to anticoagulant ratio (WB:AC):
- Inversely reflects degree of anticoagulation in extracorporeal circuit
- Ratios of 10:1 to 15:1 are commonly used for un-anticoagulated patients
- Inlet rate:
- Refers to volume of whole blood drawn per unit time from patient
- Directly affects procedure runtime
American Society for Apheresis (ASFA) guidelines
- Published triennially (every 3 years)
- Categories published based on best evidence; organized into 4:
- Category I: disorders for which apheresis is accepted as first line therapy
- Category II: disorders for which apheresis is accepted as second line therapy
- Category III: optimum role of apheresis therapy is not established; decision making should be individualized
- Category IV: disorders in which published evidence demonstrates or suggests apheresis to be ineffective or harmful
- Reference: J Clin Apher 2019;34:171
Indications for red blood cell exchange
- Sickle cell disease:
- Congenital hemoglobinopathy caused by beta globin mutation
- Disease can be caused by homozygous HbS or compound heterozygotes (HbSC, HbS beta thalassemia)
- In HbSS patients with acute exacerbation, goal of RBCX is generally to reduce HbS to 30% or lower
- Homozygotic patients with no HbS% data available can generally be assumed to have 90 - 100% HbS unless transfused within past 100 days
- In patients with HbSC disease or other symptomatic compound heterozygous sickling disorder, goal of RBCX is generally to increase HbA to 70% or higher (i.e. the combined fraction of [HbS + HbX] is 30% or less)
- Acute indications
- Acute stroke: category I
- Acute chest syndrome, severe: category II
- Other complications (vaso-occlusive crisis, dactylitis, priapism, sequestration): category III
- In patients with prior stroke or other severe sequelae of SCD or to prevent complication of pregnancy / surgery, goal of RBCX is to maintain HbS < 30 - 50%; HbS% data is generally necessary for procedure planning
- Note: simple transfusion of pRBCs may be indicated as a temporizing measure while vascular access is obtained and blood is procured
- Chronic indications
- Chronic stroke prophylaxis (primary or secondary): category I
- Pregnancy: category II
- Recurrent veno-occlusive crises: category II
- Preoperative management: category III
- Parasitemias:
- Severe infection by intraerythrocytic parasites, typically Plasmodium or Babesia
- Diagnosis requires laboratory evidence of hemolysis and blood smear examination demonstrating organisms
- RBCX is generally adjunctive to antiparasitic therapy when > 10% parasitemia with severe symptoms
- 2 times RBC volume exchange is standard
- Babesiosis: category II
- Malaria: category III
- ABO incompatibility:
- Prevention / amelioration of hemolysis in major ABO incompatibility may be achievable through RBCX
- Major ABO incompatible red cell transfusion:
- Rationale is to remove incompatible transfused cells before total hemolysis occurs
- In practice, cells hemolyze rapidly and practice is not recommended
- Note: plasma exchange could be considered for removal of excess free hemoglobin to treat moderate to severe nephrotoxicity caused by hemolysis of incompatible RBCs
- Red cell alloimmunization:
- Usually to prevent immunization to RhD by RhD negative women in setting of accidental or emergency exposure to RhD positive cells or massive hemorrhage by RhD positive pregnancy
- Category III
- Minor ABO incompatible hematopoietic stem cell transplantation:
- To prevent hemolysis of recipient RBCs by donor isohemagglutinins
- Effectively accelerates patient ABO conversion
- Category III
- Reference: J Clin Apher 2019;34:171
Indications for erythrocytapheresis
- Hereditary hemochromatosis:
- Treat iron overload by removal of RBC mass, goal ferritin < 50 ng/mL
- Erythrocytapheresis can more rapidly correct hyperferritinemia than therapeutic phlebotomy but incurs a higher procedure cost
- Category I
- Polycythemia vera:
- Treat polycythemia by removal of RBC mass, goal hematocrit ≤ 45%
- Erythrocytapheresis can more rapidly correct polycythemia than therapeutic phlebotomy and may be preferred in hemodynamically unstable patients
- Category I
- Reference: J Clin Apher 2019;34:171
Replacement fluid
- For RBCX, obtain type and screen and procure donor pRBCs for replacement fluid, if needed
- Patients with sickle cell disease require prophylactic antigen matching per National Heart, Lung and Blood Institute (NHLBI) guidelines
- Procuring antigen negative compatible blood may cause delay
- ABO, RH group and K recommended for sickle cell patients which no prior history of antibody formation
- Extended matching (ABO, RH group, K, Duffy, Kidd and Ss systems) recommended for patients with a history of antibody formation
- Reference: JAMA 2014;312:1033
Exchange volume
- Removal of pathogenic substances in apheresis follows decay equation: Y/Yo = e-X
- Y = residual pathogenic substance (note: fraction of cells remaining = Y/Yo)
- Yo = initial pathogenic substance
- X = number of volumes exchanged (in RBCX, red blood cell volume equivalents)
- Determine the required number of volumes to exchange and convert into donor pRBC volume
- Recall that most pRBC units are stored with additive solution and have a hematocrit of approximately 60%
- Prediction of RBCX efficiency is optimized by accurate knowledge of pRBC unit hematocrit
- Example:
- Patient with 5 L total blood volume and 30% hematocrit has 90% HbS
- RBCX is requested with an end goal of 30%
- Goal fraction of cells remaining is (30/90) = 33%
- Solving for X indicates that ~1.1 times the RBC volume should be exchanged
- Patient's RBC volume is (5,000 mL x 0.3) = 1,500 mL
- 1,650 mL of red cell mass should be exchanged or (1,650/0.60) = 2,750 mL of pRBCs
- Exchange should be performed with 9 - 10 units of pRBCs
- Automated calculator mobile app is available for free from TerumoBCT
- Note that exchange becomes less efficient with higher volumes; most acute procedures tend to exchange about 1.0 times RBC volume
- Note: end procedure patient hematocrit can be programmed; increasing patient hematocrit in this fashion requires further pRBCS but may improve symptoms and reduce reticulocytosis
- Keep in mind that increasing end hematocrit will increase patient iron load and serum ferritin
- End hematocrit should generally be ≤ 33% (and never higher than 36%) to avoid hyperviscosity in SCD; end hematocrit of 30% is typical
- Reference: Transfusion 2018;58:1965
Simple exchange, depletion and depletion / exchange
- In simple exchange, donor pRBCs are transfused to patient in an isovolemic fashion to offset removal of diseased RBCs
- In depletion, crystalloid or colloid replacement is infused instead of donor pRBCs; this process is standard in erythrocytapheresis for polycythemia vera or hereditary hemochromatosis
- Hybrid depletion / exchange can be performed, wherein a brief depletion phase is followed by exchange with donor pRBCs
- This marginally improves procedure efficiency and may have implications on iron homeostasis (contested)
- Reference: Transfusion 2018;58:1965
Vascular access
- In adults, choice of vascular access should be able to support inlet rate of 30 mL/min or ideally up to and above 60 mL/min
- Generally, procedure is performed continuously with 2 points of intravenous access
- Peripheral IVs:
- Least invasive
- Should be considered in patients with adequate veins for peripheral access before resorting to central lines
- Central venous catheters:
- Femoral access confers higher infection rate but fewer placement complications (e.g. pneumothorax) than internal jugular or subclavian access and does not require imaging to confirm placement
- Nontunneled lines are appropriate for short term inpatient exchange in patients who fail peripheral access
- Tunneled lines (Permcath) can be used for intermediate duration of procedures on outpatient basis
- Peripherally inserted central catheters (PICCs) generally cannot sustain required inlet rates and are inappropriate for apheresis procedures
- Ports:
- Appropriate for patients requiring chronic exchange on outpatient basis
- Note required inlet rates; standard infusion ports are not adequate
- Vortex (AngioDynamics) or PowerFlow (Becton-Dickinson) ports are recommended
- Reference: Transfusion 2018;58:569
Anticoagulation
- Majority of apheresis procedures use citrate to anticoagulate extracorporeal circuit
- Patients who are systemically anticoagulated (e.g. high dose heparin therapy) may require less or even no anticoagulation
- Citrate prevents clotting by binding calcium that is required for secondary hemostasis
- Default whole blood to anticoagulant ratio is 12:1; increasing the ratio decreases the anticoagulation dose to patient and slightly increases speed of procedure at risk of extracorporeal clot formation
- Reference: Transfus Apher Sci 2019;58:132
Circuit priming
- Extracorporeal circuit must be purged of air; saline is used for initial purge as part of standard kit operation
- When exchanging patients with low total blood volume (infants or small children) or at risk of severe intraprocedural anemia, circuit may then be primed with pRBCs (1 unit is sufficient)
- pRBC prime if extracorporeal volume (ECV) exceeds 15% of patient total blood volume
- Spectra Optia therapeutic kit ECV is 185 mL plus blood warmer tubing volume or other circuit dead volume
- Rinseback refers to end of procedure purging of circuit back to patient
- If pRBCs were used for prime, rinseback may be modified, as rinseback would result in net transfusion of the unit used for priming
Adverse events
- Use of a blood warmer is recommended for prevention of hypothermia
- Citrate toxicity:
- Most frequent adverse effect, related to hypocalcemia
- Often limited to paresthesias or numbness
- Severe complications include nausea / vomiting, muscle spasms or cardiac arrhythmias
- Severe citrate toxicity in red blood cell exchange is rare; oral calcium repletion is generally sufficient to resolve mild symptoms
- Transfusion reactions:
- Apheresis provider should evaluate for acute reactions to donor pRBCs, including allergic reaction, transfusion related acute lung injury (TRALI) and acute hemolysis
- Apheresis and other patient providers should be alert to delayed transfusion reactions that occur 24 hours to 28 days later, such as delayed hemolysis or transfusion transmitted infection
- Line and procedural related complications:
- Improper line placement or pneumothorax following internal jugular or subclavian line placement must be ruled out with plain film chest Xray
- Hypotension during apheresis is common due to fluid shifts but is a known complication in setting of ACE inhibitor use due to contact activation of bradykinin in extracorporeal circuit
- Line infection and bacteremia may present as fever during procedure and should be prevented by proper line care
- Reference: Transfusion 1999;39:282
Sample assessment & plan
- Assessment and plan: John Doe is a 25 year old man with history of sickle cell disease (HbSS) who is admitted for leg and back pain consistent with veno-occlusive crisis. He is normally treated with Hydrea as an outpatient. His baseline hemoglobin is 10 g/dL and his current hemoglobin is also 10 g/dL. He has not been transfused this admission. 2 days into admission, he developed shortness of breath and an oxygen requirement (saturating 86% on room air). Chest plain film imaging shows bilateral opacities concerning for acute chest syndrome. Apheresis service is consulted for urgent red cell exchange by the inpatient Heme / Onc consulting team. A central line is currently being placed.
- Severe acute chest syndrome is a category II (grade 1C) indication for red cell exchange according to 2019 ASFA guidelines (J Clin Apher 2019;34:171). Emergent exchange is indicated for acute chest syndrome with severe features (SaO2 < 90%). The apheresis service will perform inpatient red cell exchange to reduce his HbS to < 30% and to effect an acute change in his respiratory condition with the following parameters:
- Height: 69 inches / 175 centimeters
- Weight: 165 pounds / 75 kilograms
- Hematocrit: 30% per most recent CBC
- TBV: ~5,000 mL
- RBCV: ~1,500 mL
- Current HbS%: currently unknown (no Hb eval sent), assume 90%
- Goal HbS%: < 30%
- Goal FCR: 33.3%
- Type & screen drawn on admission is negative. All past antibody screens are negative. Patient is group A positive. STAT order placed with blood supplier for 10 units group A pRBC as below.
- Primary team to verify appropriate line placement and vascular access.
- Nurse to perform simple red cell exchange with 10 units of antigen negative, crossmatch compatible pRBCs, negative for C, E, K antigens and negative for HbS (SickleDex).
- Set start and end hematocrit both to 30%. Set WB:AC ratio to 12.
- Nurse to monitor for signs and symptoms of hypocalcemia. 2 g PO calcium carbonate ordered PRN. Page blood bank physician on call for any issues or patient reactions.
- Postprocedure:
- Patient tolerated red cell exchange well. During the visit, he was comfortable and did not complain of itching, numbness or paresthesias. No adverse reactions were noted by nursing. Per Optia instrument, the final FCR was 29%; therefore, residual HbS% is expected to be approximately 26%.
- Primary team: Please draw hemoglobin electrophoresis to assess HbS%.
- This patient has been seen by and discussed with the attending blood bank physician. Their written attestation is to follow.
Additional references
Board review style question #1
Which of the following conditions is considered an American Society for Apheresis (ASFA) category I indication for red cell exchange?
- Acute chest syndrome in sickle cell disease
- Babesiosis
- Prevention of hemolysis in ABO major incompatible packed red blood cell transfusion
- Stroke in sickle cell disease
- Thrombotic thrombocytopenic purpura
Board review style answer #1
D. Stroke in sickle cell disease. In sickle cell disease, acute treatment or prevention of stroke are both category I ASFA indications for red blood cell exchange. Thrombotic thrombocytopenic purpura is a category I indication for therapeutic plasma exchange (TPE). Severe acute chest syndrome is a category II indication for red blood cell exchange. Prevention of hemolysis in major incompatible transfusion is unlisted in the 2019 ASFA guidelines but is not recommended. Babesiosis is a category II indication for red blood cell exchange.
Comment Here
Reference: Red blood cell exchange
Comment Here
Reference: Red blood cell exchange
Board review style question #2
A red cell exchange is requested for a 50 kg, 165 cm man with sickle cell disease with acute stroke. His hematocrit is 27% and his HbS is 90%. Because of antigen matching requirements, only 4 units of packed red blood cells (300 mL volume each, at 60% hematocrit) are immediately available. What is the expected postprocedural HbS if all 4 units are used and end hematocrit is left at 27%?
- 27%
- 30%
- 45%
- 50%
- 70%
Board review style answer #2
C. 45%. The available donor red cell mass to exchange is (300 mL x 0.60 x 4) = 720 mL. The patient's total blood volume is ~3,900 mL (via Nadler formula). At a hematocrit of 27%, his red blood cell volume is ~1,050 mL. Using all 4 units would amount to a (720/1,050) = 0.69 red blood cell volume exchange, with a resulting fraction of cells remaining of e-0.69 = 50%. If the patient's starting HbS is 90%, the expected postexchange HbS should be 45%.
Comment Here
Reference: Red blood cell exchange
Comment Here
Reference: Red blood cell exchange
Board review style question #3
Which of the following vascular access methods is inappropriate for red cell exchange?
- Arteriovenous fistula
- Existing extracorporeal circuits (e.g. extracorporeal membrane oxygenation)
- Mahurkar catheter
- Nonpower single lumen port
- Quinton catheter
Board review style answer #3
D. Nonpower single lumen port. Most single lumen nonpower injectable ports, such as most standard chemo infusion ports, cannot support the pressure and inlet rates needed for apheresis procedures. Additionally, 2 access sites are necessary for continuous apheresis. Quinton and Mahurkar catheters are both central venous catheters (CVCs) generally used for hemodialysis and are adequate for apheresis. Special attention should be given to the catheter diameter and length to ensure flow rates of up to 60 mL/min or greater can be supported. For reference, typical inlet rates in hemodialysis may exceed 120 mL/min. Arteriovenous fistulas are acceptable for access but require training for safe access. Extracorporeal circuits are also acceptable for apheresis procedures but the additional extracorporeal volume should be factored into consideration and coordination with extracorporeal membrane oxygenation team is necessary.
Comment Here
Reference: Red blood cell exchange
Comment Here
Reference: Red blood cell exchange
Red blood cell genotyping (pending)
[Pending]
Red blood cell use
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Symptoms | Blood donor screening | Blood donor testing (per FDA) | Donor deferral | Laboratory | Treatment | Special conditions | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Red blood cell (RBC) transfusion is used to improve oxygenation of the tissues to correct symptoms and prevent complications from anemia
Essential features
- RBC transfusions are used to improve tissue oxygenation in anemia
- For hemodynamically stable recipients, evidence favors restrictive over liberal RBC transfusion
- Blood donors are screened by history, observations and blood tests to maintain donor and recipient safety
- Manufacturing such as irradiation may be used to optimize RBC products for certain recipients
Terminology
- Packed RBCs (or packed cells): red cells that have been separated from whole blood and have a higher hematocrit
- Whole blood: blood stored as collected from donors in anticoagulant with all blood constituents
- Patient blood management refers to systems designed to maximize hemoglobin, prevent blood loss and allow tolerance of anemia and includes restrictive approaches to transfusion
Pathophysiology
- Main function of RBC transfusion is to improve oxygenation by increasing the oxygen carrying capacity of blood through an increased hemoglobin level
- RBC transfusion may also increase microvascular flow by increasing blood viscosity
- RBC transfusion, particularly exchange transfusion, may reduce sickling in sickle cell disease (Blood Adv 2020;4:327)
- In thalassemia major, adequate RBC transfusion suppresses extramedullary hematopoiesis and allows normal growth and development
Symptoms
- Side effects of RBC transfusion include:
- Transfusion reactions
- Iron overload
- Transfusion associated acute lung injury (TRALI)
- Post transfusion purpura
- Graft versus host disease
- Allergic and anaphylactic reactions and immune modulation
Blood donor screening
- Donor screening is designed to assure donor and recipient safety and adequacy of the collected product
- Donor questionnaire
- Assess for behaviors associated with increased infection transmission risk (travel to endemic areas, tattoos, intravenous drug use, male to male sex, sex with an at risk person, percutaneous or mucosal occupational blood exposure) (FDA: Implementation of Acceptable Full-Length and Abbreviated Donor History Questionnaires and Accompanying Materials for Use in Screening Donors of Blood and Blood Components [Accessed 26 October 2020])
- Pregnancy within the previous 6 weeks
- Adequate donation interval
- Symptoms of recent infection or other illness that may affect donor or recipient safety
- Medications
- Donor examination (FDA: Implementation of Acceptable Full-Length and Abbreviated Donor History Questionnaires and Accompanying Materials for Use in Screening Donors of Blood and Blood Components [Accessed 26 October 2020])
- Temperature: < 37.5 °C (99.5 °F)
- Weight: > 50 kg (110 pounds)
- Pulse: 50 - 100 bpm unless responsible physician certifies donor safety, as in trained athletes
- Blood pressure: 90 - 180/50 - 100 mmHg unless responsible physician certifies donor safety
- No inflammation or infection at phlebotomy site or recent intravenous drug injection marks
Blood donor testing (per FDA)
- Hemoglobin: > 12.5 g/dL (F) or > 13 g/dL (M); > 11 g/dL (autologous)
- Hepatitis B nucleic acid test, surface antigen and core antibody
- Hepatitis C antibody and nucleic acid test
- HIV1 and 2 antibodies and nucleic acid test
- HTLV1 and 2 antibodies
- Syphilis (Treponema pallidum) antibodies
- Trypanosoma cruzi antibodies
- West Nile virus antibodies seasonally from June 1 to October 31
- Babesia nucleic acid testing in affected states
- Zika virus nucleic acid testing is license but inadequate as a screen test; deferral based on contact or travel history
- CMV seronegative testing is only required if the product is to be CMV negative
Donor deferral
- Donor deferral may be based on results of screening or donor testing
- Indefinite - can't donate in the foreseeable future; requalification to donate possible with changes in regulation or guidance (e.g. intravenous drug use has been previously indefinitely deferred but may now requalify providing they meet all current donation criteria)
- Infections (e.g. with HIV, HBV, HCV, Ebola)
- Residence or blood transfusion within countries and timeframes at risk of bovine spongiform encephalopathy
- Temporary - deferred for known periods based on time or risk factor event
- Recent infection
- Recent travel to areas endemic for blood borne infections, such as malaria, Ebola
- Deferral for 3 months for:
- Male to male sex, sex with a prostitute or someone with (or at risk of) HIV
- Needlestick injury
- Transfusion or allogeneic transplant
- Tattoo or body piercing
- Travel to malaria endemic areas
- Deferral for 6 months following Zika virus infection or travel to areas of increased risk of Zika
- Deferral for 12 months following imprisonment
- Deferral for 3 years for prior residents (> 5 years) of malaria endemic regions or history of malaria
- Medications (AABB: Medication Deferral List [Accessed 02 October 2020])
Laboratory
- Stored at 2 - 6 °C for up to 42 days depending on anticoagulant (AABB: Circular of Information - For the Use of Human Blood and Blood Components [Accessed 26 October 2020])
- Progressive loss of ATP leading to crenation, loss of deformability, decreased 2,3-DPG and loss of potassium into supernatant
- Despite red cell storage lesion, older RBCs have been shown to be equivalent to younger RBCs in mortality and adverse events based on randomized trials (Blood 2016;127:40026626995)
Treatment
- Typical RBC unit increases hemoglobin by about 1 g/dL in adults
- For children, 10 mL/kg RBC increases hemoglobin by 2 g/dL(Transfusion 2007;47:212)
- Restrictive hemoglobin thresholds for RBC transfusion have been proven to be as safe or safer than liberal and provide noninferior outcomes in:
- Intensive care units (7 versus 9 g/dL) (N Engl J Med 1999;340:409)
- Pediatric intensive care (7 versus 9 g/dL) (N Engl J Med 2007;356:1609)
- Upper gastrointestinal hemorrhage (7 versus 9 g/dL) (N Engl J Med 2013;368:11)
- Cardiac surgery (7.5 versus 9.5 g/dL) (N Engl J Med 2018;379:1224)
- Recovery after hip fracture surgery (8 versus 10 g/dL) (N Engl J Med 2011;365:2453)
- Bone marrow transplantation (7 versus 9 g/dL) (J Clin Oncol 2020;38:1463)
- Acute myocardial infarction (7 versus 10 g/dL) (Blood Adv 2020;4:327)
- Premature neonates (variable thresholds depending on age and the need for respiratory support) (N Engl J Med . 2020;383:2639)
- No particular transfusion trigger based on hemoglobin or any other parameter has been supported by controlled trials in chronic hypoproliferative anemia; transfusion thresholds are based on levels where there is a demonstrated improvement in symptoms for the individual
- Many people with hemoglobin levels below 7 g/dL are able to tolerate anemia well and transfusion is not always required, especially when there is a reversible cause, such as iron deficiency
- Where there is a foreseeable potential need for transfusion, such as elective surgery, optimization of preoperative hemoglobin (consider iron or erythropoiesis stimulating agents as appropriate) is encouraged to reduce transfusion needs
- Where transfusion is indicated for anemia, best practice is to titrate the transfusion volume to needs by giving a single unit and reassessing clinically
- RBC may be transfused in sickle cell disease to prevent sickling in acute chest syndrome, during pregnancy or preoperatively
- Red cell exchange is preferred over simple red cell transfusion for chronic transfusion therapy or where RBC transfusion is otherwise indicated but there is high hemoglobin (Blood Adv 2020;4:327)
Special conditions
- Leukocyte reduced
- < 5 x 10⁶ leukocytes per unit
- Reduce HLA alloimmunization, febrile nonhemolytic transfusion reactions and CMV transmission
- CMV negative
- Serologically negative; doesn't exclude donors within window periods
- Leukocyte depleted have very low to negligible CMV risk
- Recommended during pregnancy or low birth weight premature neonates
- May be used in CMV negative transplant recipients or during intensive chemotherapy
- Irradiated
- To 25Gy to prevent transfusion associated graft versus host disease (by preventing the proliferation of immunocompetent donor lymphocytes, which can damage host tissue)
- Indicated for HLA matched or related donors, intrauterine transfusions or neonates who previously received intrauterine transfusions, stem cell transplant recipients or certain immunocompromised groups (Hodgkin lymphoma, after purine analogue therapy, aplastic anemia treated with antithymocyte globulin and some severe T cell immunodeficiency syndromes)
- GVHD risk is low but potentially fatal; emergency transfusion need not be delayed if irradiated blood is not immediately available
- Washed
- Indicated for the removal of plasma proteins associated with severe reactions (e.g. prior anaphylaxis to IgA)
- Deglycerolized
- Frozen red cell units stored in glycerol have this removed before transfusion, usually from rare donor phenotypes
- Red cell loss during processing means units are up to 20% smaller
- Whole blood
- Increasing use in trauma / critical bleeding setting to replace all blood components
- Rarely used outside this setting
- Reference: AABB: Circular of Information - For the Use of Human Blood and Blood Components [Accessed 26 October 2020]
Sample assessment & plan
- Assessment: A 27 year old primipara has a post partum hemorrhage following a term vaginal delivery managed with oxytocin and ergometrine. The following day she reports being tired. She has a blood pressure of 115/70 and a pulse rate of 84 bpm. Her hemoglobin is measured at 6.8 g/dL, which is 3 g/dL less than in first trimester when she was noted to be iron deficient.
- Plan: While there may be a more rapid improvement in symptoms with transfusion, this is very small when compared with intravenous iron therapy and insignificant when compared with allowing time to recover from a traumatic delivery. Iron therapy is more likely to lead to sustained increase in hemoglobin and replenishment of iron stores. Transfusion may be safely withheld; however, if transfusion is prescribed for symptoms, a single unit should be given followed by clinical reassessment (BMC Pregnancy Childbirth 2010;10:83).
Additional references
Board review style question #1
A 64 year old Caucasian woman has hemoglobin of 8.6 g/dL (12 - 15 g/dL), with an MCV of 72 fL (80 - 100 fL) one day after acute myocardial infarction, treated with left anterior descending stent insertion. She is A RhD negative and has an anti-D and an anti-C on red cell antibody screen. She is asymptomatic, has a blood pressure of 120/75 mmHg, a pulse of 68 bpm and no signs of congestive cardiac failure. You are asked to consult on transfusion management. The best advice is to
- Advise that alternatives to transfusion should be considered as the multiple antibodies will make it hard to source blood for transfusion
- Indicate that transfusion is unlikely to be required based on the clinical features and that further investigation for iron deficiency should be considered
- Advise that transfusion should be undertaken with leukocyte depleted blood to prevent further antibody formation
- Advise to transfuse 2 units of red cells to bring the hemoglobin up to 10 g/dL and consider the cause for anemia
- Supply washed red cells to prevent antibodies reacting with red cells
Board review style answer #1
B. Indicate that transfusion is unlikely to be required based on the clinical features and that further investigation for iron deficiency should be considered. Evidence indicates that transfusion with a Hb > 8 g/dL does not improve outcomes, even after AMI. The red cell indices indicate possible iron deficiency anemia, especially in a population with lower thalassemia prevalence. Best practice would indicate treatment of the underlying iron deficiency rather than transfusion and a search for the cause.
Comment Here
Reference: Red blood cell use
Comment Here
Reference: Red blood cell use
Board review style question #2
Washed red cells are indicated
- To prevent HLA alloimmunization in a 16 year old with acute myeloid leukemia
- To prevent a febrile nonhemolytic transfusion reaction in a patient undergoing partial hepatectomy with a history of a similar reaction 2 years prior following their last transfusion
- In all people having regular transfusions
- In a 57 year old having a total hip replacement with IgA deficiency and a history of anaphylaxis to fresh frozen plasma
- In a 36 year old with severe postpartum anemia who had a red cell transfusion stopped due to urticaria and has an IgA of 30 mg/dL (60 - 400)
Board review style answer #2
D. In a 57 year old having a total hip replacement with IgA deficiency and a history of anaphylaxis to fresh frozen plasma. People with IgA deficiency may develop anti-IgA and anaphylaxis to IgA present in all fresh blood products. Washing aims to remove the small amounts of IgA remaining in RBCs.
Comment Here
Reference: Red blood cell use
Comment Here
Reference: Red blood cell use
Rh immune globulin
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Laboratory | RhIG dosing | Case reports | Treatment | Peripheral smear images | Sample assessment & plan | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rh (Rhesus) immune globulin (RhIG) is a derivative of pooled human plasma manufactured by cold ethanol fractionation to prevent alloimmunization (antibody formation) to RhD antigen following exposure, such as through a blood transfusion or pregnancy
- Treatment option for immune thrombocytopenia (ITP)
- Available via intramuscular (IM) or intravenous (IV) preparations
Essential features
- RhIG is used to prevent RhD alloimmunization (antibody formation) and as treatment option for ITP
- RhIG is typically administered in 300 μg IM vials as prophylaxis during pregnancy for RhD negative females and at time of delivery if neonate is RhD positive
- In cases of fetal maternal hemorrhage, a fetal screen is indicated and if positive, flow cytometry or Kleihauer-Betke testing is performed to estimate appropriate number of additional vials of RhIG
Terminology
- Rh immune globulin, Rh immunoglobulin, Rh0(D) immunoglobulin
- Trade name preparations of Rh immune globulin include Rhophylac®, RhoGAM®, MICRhoGAM®, WinRho SDF®, HyperRHO S/D®
Pathophysiology
- An RhD negative person exposed to RhD positive red blood cells, either through a blood transfusion or fetal maternal hemorrhage during a pregnancy, is at risk of developing antibodies to RhD
- RhD negative phenotype occurs in roughly 15% of whites
- Predominantly due to RHD gene deletion
- RhD negative phenotype in blacks is ~7 - 8%
- Most due to mutant RHD gene coding for premature stop codon
- RhD negative phenotype in Asians is < 1%
- RhD negative phenotype occurs in roughly 15% of whites
- RhIG can suppress the formation of these antibodies, preventing their development
- Exposure to Rh(D) positive red blood cells via massive transfusion protocol or whole blood exists when
- There is no policy to avoid Rh(D) positive red blood cells or whole blood to trauma patients of child bearing potential
- Or there is a lack of Rh(D) negative red blood cells due to inventory shortfalls
- Or via exposure through Rh(D) positive platelets administered emergently during a massive transfusion
- In the first two scenarios, there is the potential for exposure to large quantities of red blood cells such that the risk of hemolysis from RhIG may outweigh the risk of anti-D sensitization
- There is currently no consensus on management of Rh(D) positive red cell exposure from 1 or more units of red blood cells or whole blood
- Case reports exist of success of dosing RhIG following 1 - 2 units of red blood cells, with or without coinciding red blood cell exchange procedures
- Regarding exposure from residual Rh(D) positive red cells in platelet units, dosing is far easier
- If it is an apheresis platelet unit, there is likely ≤ 1 mL of residual red blood cells
- If a 300 µg vial of RhIG covers 30 mL of whole blood or 15 mL of packed red cells, this would adequately cover roughly 10 units of Rh(D) positive platelets
- Prepooled random donor platelets contain < 2 mL of residual red blood cells, thus a single vial of 300 µg is also sufficient
- References: Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019, Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016, J Clin Apher 2010;25:70, Transfusion 2008;48:1990
Clinical features
- Consequences of anti-D alloimmunization causing hemolytic disease of the fetus may include:
- Fetal anemia
- Organomegaly from extramedullary hematopoiesis
- Portal hypertension
- Fetal hydrops
- Death from high output cardiac failure
- Consequences of anti-D alloimmunization causing hemolytic disease of the newborn may include:
- Neonatal anemia
- Jaundice
- Kernicterus
Symptoms
- Potential adverse effects following RhIG administration prevention of anti-D formation:
- Nausea, dizziness, headache, pain at injection site, malaise
- Potential adverse effects following RhIG administration for ITP:
- Chills / rigors, fever, headache, hemolysis (rare)
- Reference: PDR: RhoD Immune Globulin Human - Drug Summary [Accessed 11 August 2020]
Laboratory
- Following delivery, an RhD negative female bearing an RhD positive neonate should undergo a fetal blood screen
- Negative fetal screen means only 1 vial RhIG required
- Indicates either no or minimal fetal maternal hemorrhage (< 30 mL)
- Positive fetal screen requires a quantitative test, to dose additional vials of RhIG, such as:
- Flow cytometry
- Kleihauer-Betke test
- Detects fetal red cells in maternal circulation by their pink color
- EDTA (pink / lavender top) specimen required, drawn from the mother following delivery
- Hemoglobin in fetal red cells is resistant to acid treatment (bright pink / red)
- Hemoglobin in maternal red cells is destroyed by acid treatment (clear / light pink “ghost” cells)
- Administration of RhIG will look like an anti-D on antibody identification and is generally called passive anti-D
- Pattern of reactivity is indistinguishable from true alloimmunization; blood bank interpretations may include cautionary statement
- Following administration of RhIG, serologic detection of passive anti-D may occur up to 3 - 4 months by tube testing or gel methodology
- Up to 5 - 6 months by solid phase red cell adherence assay (SPRCA)
- Patients injected with IM RhIG should not develop a positive antibody screen until 7 to 8 hours after administration
- Earlier detection raises concern for true alloimmunization
- Different manufactured brands of RhIG are comparable in strength and duration of reactivity across test methods (Transfusion 2015;55:1444)
- Special circumstance: detection of possible anti-D and anti-C in woman of childbearing age, as this may represent anti-G
- Anti-G is an antibody directed against epitopes on both the C and D antigens
- Antibody titering: anti-G is suspected when the anti-C titer is consistently higher than the anti-D titer (Transfusion 1997;37:493)
- Important to investigate via adsorption studies to determine eligibility for RhIG
- Various combinations of antibodies against C, D and G may be present
- If anti-D is not detected, patient (if woman of childbearing age) is eligible for RhIG
- If anti-D is detected, patient is not eligible to receive RhIG
RhIG dosing
- Calculate volume of fetal maternal hemorrhage (FMH) based on Kleihauer-Betke result:
- [# pink / red (fetal) cells ÷ 2,000 cells counted) x maternal blood volume (5,000 mL is average)
- A 300 µg vial of RhIG will cover 30 mL of whole blood
- Therefore, volume of FMH ÷ 30 = vials of RhIG
- If the number of calculated vials is < 0.5, round down to the nearest whole number and add 1 vial
- If the number of calculated vials is ≥ 0.5, round up to nearest whole number and add 1 vial
- For example, if 8 fetal red cells are detected of 2000 cells counted
- Volume of FMH = (8/2000 * 5000 mL) / 30 mL = 0.67 vials
- Round up to 1 and add 1 vial
- Answer: 2 vials should be administered
Case reports
- RhIG for variant RHD:
- 2 cases of variant RHD in pregnant women, misclassified as RhD positive, who did not receive RhIG when indicated and formed anti-D (Immunohematology 2017;33:60)
- RhIG after fetal maternal hemorrhage (FMH):
- 31 year old woman with large RhIG dose requirement following FMH (Am J Clin Pathol 2016;145:744)
- RhIG after RhD positive red cell transfusion following trauma:
- 25 year old woman involved in car accident (J Clin Apher 2010;25:70)
- RhIG for anti-G when clinically indicated - generally, female children and women with childbearing potential:
- 78 year old man with anti-G (Ann Lab Med 2018;38:280)
- RhIG for immune thrombocytopenia (ITP):
- 5 year old boy with platelet count of 2 x 109/L, a theoretical case for consultation of RhIG for ITP (Hematology Am Soc Hematol Educ Program 2013;2013:283)
Treatment
- Rh immune globulin is given to RhD negative women:
- At approximately 28 weeks estimated gestational age (EGA)
- Again within 72 hours of delivery if the neonate is RhD positive
- Following trauma
- After invasive procedures (amniocentesis, cordocentesis)
- Spontaneous or elective abortions
- Pregnancy inversions
- Pregnant women who are considered D variants (weak or partial D) may be capable of forming an anti-D
- RHD genotyping can distinguish weak from partial D and determine if RhD negative blood or RhIG is indicated
- PCR based test requiring specimen collected in EDTA tube
- Weak D types 1, 2, 3 can be managed as RhD positive
- Recent data suggests types 4.0 and 4.1 can also be managed as RhD positive (Transfusion 2020;60:855)
- Immune thrombocytopenia (ITP)
- Only used when patient is RhD positive
- Anti-D acts like an immune decoy to decrease body's formation of antiplatelet antibodies
- References: Fung: Technical Manual, 19th Edition, 2017, Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019
Sample assessment & plan
- History: 35 year old woman presenting to hospital after motor vehicle accident
- Blood type: O negative
- Laboratory testing:
- Type and screen performed in the blood bank, followed by antibody identification which identifies reactivity consistent with anti-D plus anti-C
- Adsorption studies were performed at an immunohematology reference laboratory, identifying anti-G and anti-C; anti-D not identified
- Clinical significance:
- Anti-G is an antibody formed in almost all cases by Rh negative (D negative), G antigen negative patients. Anti-G reacts with epitopes on both the C antigen and the D antigen. Anti-G typically is seen in a D negative patient who has never knowingly been exposed to Rh positive blood, yet presents with an antibody that looks like a combination of both anti-D and anti-C.
- This antibody can form through previous exposure via pregnancy or transfusion. A patient with anti-G would be transfused in exactly the same way as a patient who has the combination of anti-D and anti-C: with Rh negative (D antigen negative) and C antigen negative blood.
- However, women of childbearing age with anti-G should receive Rh immune globulin during pregnancy to protect against the formation of a true alloimmunization against the D antigen, which could cause severe hemolytic disease of the newborn.
- Difficulty in finding compatible blood:
- This patient can receive O, RhD negative, C antigen negative packed red blood cells, which accounts for approximately 2% of the blood inventory
Board review style question #1
A 31 year old G2P1 woman presents to a new medical center after having moved from out of state. She is 16 weeks pregnant and was referred to maternal fetal medicine for a positive antibody screen. Antibody identification reveals a pattern consistent with anti-D and anti-C. Additional blood samples are requested by the blood bank for adsorption testing at an immunohematology reference laboratory, as anti-G is suspected. In which scenario is RhIG indicated for this patient?
- Anti-G, anti-C and anti-D are identified
- Anti-D and anti-C are identified but anti-G is not identified
- Anti-G and anti-C are identified but anti-D is not identified
- Anti-G and anti-D are identified but anti-C is not identified
Board review style answer #1
C. Anti-G and anti-C are identified but anti-D is not identified. Anti-G is an important consideration in the workup of a woman of childbearing age who appears to have anti-D and anti-C. Adsorption studies to discern the presence or absence of true anti-D should be undertaken to determine eligibility for RhIG in the interest of protecting against severe anti-D hemolytic disease of the newborn. Women of childbearing age who have not formed anti-D are eligible for RhIG. The presence or absence of of anti-G or anti-C does not influence RhIG administration.
Comment Here
Reference: Rh immune globulin
Comment Here
Reference: Rh immune globulin
Board review style question #2
A 27 year old G1P0 receives 300 μg RhIG at approximately 28 weeks gestational age because her blood type is A, RhD negative. She later delivers a full term healthy boy at 39 weeks gestational age. Cord blood testing of the infant reveals he is O, RhD positive. Additionally, a fetal screen performed on a postpartum sample from the mother is positive, indicating fetal maternal hemorrhage, and a Kleihauer-Betke is reflexively performed in order to properly dose RhIG. How many total vials of 300 μg RhIG are required if 2,000 total cells are counted and 8 fetal cells are identified (and a maternal blood volume of 5,000 mL is presumed)?
- 1 vial RhIG
- 2 vials RhIG
- 3 vials RhIG
- 4 vials RhIG
Board review style answer #2
B. 2 vials. Volume FMH identified by Kleihauer-Betke = (8 fetal cells / 2,000 total cells counted) x 5,000 mL maternal blood volume = 20 mL. 20 mL/30mL = 0.67 vial. In this case, 0.67 vial is rounded up to 1 vial and 1 additional vial is added, making the appropriate RhIG dose 2 vials.
Comment Here
Reference: Rh immune globulin
Comment Here
Reference: Rh immune globulin
Rh system
Table of Contents
Definition / general | Essential features | Antigens | Antibodies | Terminology | Pathophysiology | Transmission | Laboratory | Case reports | Treatment | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Most antigenic blood group outside of ABO
- Antibodies are clinically significant
Essential features
- Rh (Rhesus) antigens are widely expressed in the donor population
- Antibodies can cause hemolysis and hemolytic disease of the fetus and newborn (HDFN)
Antigens
- Type: peptide
- RhD is an antigen formed by RHD on chromosome 1
- D antigen has high frequency among all racial groups and products are designated as positive or negative based on its presence
- D variants include individuals who express less D antigen (weak D) and those who express structurally different D antigen (partial D)
- Partial D individuals may form antibodies against a normal D antigen
- Reference: AABB: Technical Manual, 19th Edition, 2017
- RHCcEe are antigens generally coinherited with RhD on chromosome 1 encoded by the RHCE gene
- The D antigen is most immunogenic, though other Rh antigens are very immunogenic and patients who alloimmunize at higher rates, such as individuals with sickle cell disease, are often matched for RhCc and RhEe antigens
- RHAG is a glycoprotein that helps localize Rh antigens to the red cell membrane; absence of this protein leads to RhNull disease
- RhNull disease is characterized by stomatocytosis, enhanced osmotic fragility and mild chronic hemolysis
- Rh antigens form structural epitopes, which may be separately antigenic
- G antigen is formed by four shared amino acids between RhD and RhC
- f antigen is a compound antigen found on red cells with Rh antigens ce
- Dce / CE in trans configuration leads to decreased RhD expression due to the Ceppellini effect; the decrease in D expression is most pronounced in the trans configuration but is present to a lesser extent in cis individuals as well (AABB: Technical Manual, 19th Edition, 2017)
Adapted from Transfusion 2004;44:703, AABB: Technical Manual, 19th Edition, 2017Race / Ethnicity RhD+ RhD- C c E e White non-Hispanic 82.7% 17.3% 68% 80% 29% Hispanic 92.7% 7.3% Black non-Hispanic 92.9% 7.1% 27% 96% 22% Asian 98.3% 1.7% Native American 90.3% 9.7% All donors 85.4% 14.6% 98% - RhD is an antigen formed by RHD on chromosome 1
Antibodies
- Majority IgG, fewer IgM (AABB: Technical Manual, 19th Edition, 2017)
- Can cause hemolytic reactions and hemolytic disease of the fetus and newborn
- Occur with exposure to products containing Rh incompatible blood or pregnancy
- Show dosage and improve with enzyme treatment
Terminology
| DCe | R1 |
| DcE | R2 |
| Dce | R0 |
| DCE | Rz |
| ce | r |
| Ce | r' |
| cE | r" |
| CE | ry |
Pathophysiology
- RhD sensitization occurs during the first pregnancy due to maternal exposure to fetal hemorrhage during gestation or delivery; this leads to an anti-D IgG antibody which can cross the placenta in subsequent pregnancies, leading to hydrops fetalis if not identified by obstetrics teams (AABB: Technical Manual, 19th Edition, 2017)
- Risk of sensitization in an RhD negative mother with an RhD positive fetus is 16%, though this is reduced to < 0.1% with appropriate Rh immunoglobulin administration (AABB: Technical Manual, 19th Edition, 2017)
- 1 vial of RHIg binds 30 mL whole blood or 15 mL RBC
- Alloimmunization in healthy individuals can be high; however, rates in hospitalized patients and trauma patients tend to be lower
- RhD remains the most immunogenic antigen due to its density on the red blood cell surface and amino acid differences from RhCc / Ee
Transmission
- Exposure to Rh antigens secondary to pregnancy or transfusion
Laboratory
- RhD positivity is determined
- O negative blood is the preferred universal donor blood type in emergency situations; many hospitals will provide RhD positive units in massive transfusion protocols, as RhD alloimmunization is of lower risk in this population; however, women of childbearing age (<50 years) should always receive RhD negative products
Case reports
- Newborn with D variant implicated in hemolytic disease of the fetus and newborn (Asian J Transfus Sci 2018;12:75)
- 34 year old woman with a case of hydrops fetalis due to Rh alloimmunization (Case Rep Obstet Gynecol 2018;2018:8412139)
- 39 year old Iranian woman with a rare Rh phenotype (Indian J Hematol Blood Transfus 2019;35:402)
- 43 year old Iranian woman was found to be RhNull (Int J Hematol Oncol Stem Cell Res 2018;12:181)
- 58 year old man with passenger lymphocyte syndrome with an anti-D alloantibody (Transfus Med Hemother 2014;41:153)
Treatment
- RhIg dosage may be administered in pregnant patients and in patients after exposure to RhD positive products
- RhD negative mothers with RhD positive partners receive a vial at 28 weeks gestation and after delivery
- In the situation of maternal fetal hemorrhage or other RhD exposure, a Kleihauer-Betke test may be performed in order to calculate RhIg dosing
- 1 vial contains 300 μg and theoretically should bind 30 mL of RhD positive or fetal blood
- Volume in ml of fetal blood = percentage of fetal cells x 50
- Volume of fetal blood/30 ml = number of vials of 300 mcg RhIg
- Number of vials = percentage of fetal cells x 50 / 30
- Round based on the tenth position after the decimal
- < 0.5, round down and add one vial
- ≥ 0.5, round up and add one vial
- Labs may also determine RhIg dosing using flow cytometry
- Reference: Krywko: Kleihauer Betke Test [Accessed 12 October 2020]
Additional references
Board review style question #1
A 27 year old mother of 2 presents at the OB/GYN. The mother is O- and has never received prenatal care. No heart rate is detected on Doppler and an ultrasound is performed. A hydropic fetus is identified. If no significant fetal maternal hemorrhage occurred in her prior pregnancies, when should this mother have received RhIg to prevent this outcome?
- At 8 weeks and 35 weeks
- At 28 weeks and after delivery
- At 28 weeks and 35 weeks
- Only after delivery
- Only at 28 weeks
Board review style answer #1
B. At 28 weeks and after delivery. Mothers are dosed at 28 weeks and at delivery to prevent RhD alloimmunization. The rate of RhIg failure is < 0.01%.
Comment Here
Reference: Rh group
Comment Here
Reference: Rh group
Board review style question #2
A 36 year old man is seen for a viral illness by his primary care physician. A complete blood count is performed and the patient is found to have mild anemia. The physician is concerned and orders a peripheral smear review at a follow up appointment, which is read as mild anemia with anisopoikilocytosis including frequent stomatocytes. What Rh abnormality may this patient have?
- DCe / Ce in trans configuration
- Partial D
- Partial e
- Weak D
- RhNull phenotype
Board review style answer #2
E. RhNull phenotype. This lack of all Rh antigens leads to structural abnormalities (stomatocytes) and chronic mild anemia, which is generally not clinically significant.
Comment Here
Reference: Rh group
Comment Here
Reference: Rh group
Shelf lives of products
Table of Contents
Definition / general | Essential features | Terminology | Blood product storage conditions and shelf life | Case reports | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Shelf life of a blood product refers to the period during which the product is expected to retain its intended characteristics, including appearance, functionality and safety
- This duration is influenced by factors such as the specific type of blood product, its intended use and the conditions under which it is stored
Essential features
- Red blood cell components are stored in plastic bags with anticoagulants and preservatives and are susceptible to storage related metabolic, morphologic and functional changes
- Whole blood components are stored in plastic bags and share susceptibility to storage related alterations found in red cell components
- Platelet components are stored in semipermeable plastic bags and have a shelf life defined by metabolic, morphologic and functional changes
- Granulocyte components achieve optimal efficacy by transfusing promptly upon receipt from the blood center
- Plasma derived component storage conditions and shelf life can affect coagulation factor activity levels
Terminology
- Anticoagulant / preservative solutions: acid citrate dextrose (ACD), citrate phosphate dextrose (CPD), citrate phosphate double dextrose (CP2D), citrate phosphate dextrose adenine 1 (CPDA-1); these are crucial anticoagulant solutions for preserving blood samples, preventing clotting during storage or processing
- Additive solutions: AS-1 (Adsol), AS-3 (Nutricel), AS-5 (Optisol) and AS-7 (SOLX); these contain adenine with or without mannitol to further extend the shelf life of blood products beyond anticoagulant / preservative solutions
- Whole blood derived (WBD)
- Large volume, delayed sampling (LVDS): a strategic product sampling approach used to mitigate the risk of bacterial contamination
Blood product storage conditions and shelf life
- Information and tables are modified from Cohn: Technical Manual, 21st Edition, 2023
Red blood cell components (whole blood derived or apheresis derived) (FDA: CFR - Code of Federal Regulations Title 21 [Accessed 22 February 2024])
- Stored in plastic bags that contain anticoagulants, preservatives, with or without additive solutions
- U.S. Food and Drug Administration (FDA) requires in vivo labeling studies demonstrate that ≥ 75% of the transfused red cells remain in circulation after 24 hours with < 1% hemolysis
- Storage lesions are an accumulation of biochemical and morphological changes that occur during blood product storage
- Morphological changes
- Alterations in red cell membrane shape and deformability
- Membrane vesiculation
- Biochemical changes
- Decreased pH, adenosine triphosphate and 2,3 diphosphoglycerate
- Increased lysophospholipids, potassium and free hemoglobin
- Morphological changes
| Blood product | Storage conditions | Transport conditions | Shelf life / expiration |
| Red blood cells (RBCs) | 1 - 6 °C | 1 - 10 °C | ACD / CPD / CP2D: 21 days CPDA-1: 35 days Additive solution: 42 days Open system: 24 hours |
| Deglycerolized RBCs | 1 - 6 °C | 1 - 10 °C | Open system: 24 hours Closed system: 14 days or as FDA approved |
| Frozen RBCs 40% glycerol | -65 °C or colder if 40% glycerol or as FDA approved | Maintain frozen state | 10 years (a policy shall be developed if rare frozen units are to be retained beyond this time) Freeze within 6 days of collection unless rejuvenated Freeze before expiration if rare unit |
| RBCs irradiated | 1 - 6 °C | 1 - 10 °C | Original expiration or 28 days from date of irradiation, whichever is sooner |
| RBCs leukocytes reduced | 1 - 6 °C | 1 - 10 °C | ACD / CPD / CP2D: 21 days CPDA-1: 35 days Additive solution: 42 days Open system: 24 hours |
| Rejuvenated RBCs | 1 - 6 °C | 1 - 10 °C | CPD, CPDA-1: 24 hours AS-1: freeze after rejuvenation |
| Deglycerolized rejuvenated RBCs | 1 - 6 °C | 1 - 10 °C | 24 hours or as approved by FDA |
| Frozen rejuvenated RBCs | -65 °C or colder | Maintain frozen state | CPD, CPDA-1: 10 years AS-1: 3 years (a policy shall be developed if rare frozen units are to be retained beyond this time) |
| Washed RBCs | 1 - 6 °C | 1 - 10 °C | 24 hours |
Whole blood components
- Used in situations requiring a combination of components, such as during massive blood loss or for certain surgical procedures
- Contain red cells, plasma and platelets
- Stored in plastic collection bags
- Subject to same storage lesions found in red cell components
| Blood product | Storage conditions | Transport conditions | Shelf life / expiration |
| Whole blood | 1 - 6 °C | 1 - 10 °C | CPD / CP2D: 21 days CPDA-1: 35 days |
| Whole blood irradiated | 1 - 6 °C | 1 - 10 °C | Original expiration or 28 days from date of irradiation (whichever is sooner) |
| Whole blood leukocytes reduced | 1 - 6 °C | 1 - 10 °C | CPD / CP2D: 21 days CPDA-1: 35 days Open system: 24 hours |
Granulocyte components
- Transfuse as soon as possible after receipt from blood center
- Always irradiate
- Should not be agitated
| Granulocyte components | Storage conditions | Transport conditions | Shelf life / expiration |
| Apheresis granulocytes | 20 - 24 °C | As close as possible to 20 - 24 °C | 24 hours |
| Apheresis granulocytes irradiated | 20 - 24 °C | As close as possible to 20 - 24 °C | No change from original expiration date |
Plasma components
- Coagulation factor activity is affected by storage conditions and shelf life
- Plasma derivatives include products like immunoglobulins and albumin, used for immune deficiency treatment, volume expansion and various therapeutic purposes
- Cryoprecipitate antihemophilic factor (cryo)
- Cryo is rich in clotting factors and is used to treat bleeding disorders, particularly hemophilia and fibrinogen deficiencies
| Plasma components | Storage conditions | Transport conditions | Shelf life / expiration |
| Cryoprecipitated antihemophilic factor (AHF) | -18 °C or colder | Maintain frozen state | 12 months from original collection |
| Cryoprecipitated AHF (after thawing) | 20 - 24 °C | As close as possible to 20 - 24 °C | Single unit: 6 hours |
| Pooled cryoprecipitated AHF (pooled before freezing) | -18 °C or colder | Maintain frozen state | 12 months from earliest date of collection of products in pool |
| Pooled cryoprecipitated AHF (after thawing) | 20 - 24 °C | As close as possible to 20 - 24 °C | Pooled in an open system: 4 hours Pooled using a sterile connection device: 6 hours |
| Pathogen reduced cryoprecipitated fibrinogen complex | -18 °C or colder | Maintain frozen state | -18 °C or colder: 12 months from collection Post-thaw, 20 - 24 °C: 5 days |
| Fresh frozen plasma (FFP) | -18 °C or colder or -65 °C or colder | Maintain frozen state | -18 °C or colder: 12 months from collection -65 °C or colder: 7 years from collection (FDA approval required if product is stored for longer than 12 months) |
| FFP (after thawing) | 1 - 6 °C | 1 - 10 °C | If issued as FFP: 24 hours |
| Plasma frozen within 24 hours after phlebotomy (PF24) | -18 °C or colder | Maintain frozen state | 12 months from collection |
| Plasma frozen within 24 hours after phlebotomy (after thawing) | 1 - 6 °C | 1 - 10 °C | If issued as PF24: 24 hours |
| Plasma frozen within 24 hours after phlebotomy held at room temperature up to 24 hours after phlebotomy (PF24RT24) | -18 °C or colder | Maintain frozen state | 12 months from collection |
| Plasma frozen within 24 hours after phlebotomy held at room temperature up to 24 hours after phlebotomy (after thawing) | 1 - 6 °C | 1 - 10 °C | If issued as PF24RT24: 24 hours |
| Thawed plasma | 1 - 6 °C | 1 - 10 °C | 5 days from date product was thawed or original expiration, whichever is sooner |
| Plasma cryoprecipitate reduced | -18 °C or colder | Maintain frozen state | 12 months from collection |
| Plasma cryoprecipitate reduced (after thawing) | 1 - 6 °C | 1 - 10 °C | If issued as plasma cryoprecipitate reduced: 24 hours |
| Thawed plasma cryoprecipitate reduced | 1 - 6 °C | 1 - 10 °C | If issued as thawed plasma cryoprecipitate reduced: 5 days from date product was thawed or original expiration, whichever is sooner |
| Liquid plasma | 1 - 6 °C | 1 - 10 °C | 5 days after expiration of whole blood |
| Recovered plasma (liquid or frozen) | Refer to short supply agreement | Refer to short supply agreement | Refer to short supply agreement (required) |
| Plasma pathogen reduced | -18 °C or colder | Maintain frozen state | 12 months from original collection |
Platelet components
- Used to treat bleeding disorders such as thrombocytopenia and to support patients undergoing chemotherapy or transplant procedures
- Storage bag must be semipermeable and allow diffusion of oxygen and carbon dioxide to maintain a pH > 6.2 (FDA: CFR - Code of Federal Regulations Title 21 [Accessed 22 February 2024])
- Metabolic, morphologic, functional changes define shelf life and storage conditions
- Shelf life limited by increased risk of bacterial growth
- Shelf life can be extended from 5 to 7 days using an FDA approved strategy (FDA: Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion [Accessed 22 February 2024])
- Large volume, delayed sampling (LVDS) no sooner than 48 hours after collection
- Use of a secondary culture of apheresis platelets no sooner than day 4 of storage in FDA cleared containers approved for 7 day storage
- Secondary rapid testing of apheresis or prestorage pooled WBD platelets
| Platelet components | Storage conditions | Transport conditions | Shelf life / expiration |
| Platelets | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | 24 hours to 5 days, depending on collection system |
| Platelets cold stored | 1 - 6 °C (agitation optional) | 1 - 10 °C | 14 days |
| Platelets irradiated | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | No change from original expiration date |
| Platelets leukocytes reduced | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | Open system: 4 hours Closed system: no change in expiration |
| Pooled platelets leukocytes reduced | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | 4 hours after pooling or 5 days following collection of the oldest unit in the pool |
| Pooled platelets (in open system) | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | Open system: 4 hours |
| Apheresis platelets | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | 24 hours or 5 days, depending on collection system |
| Apheresis platelets irradiated | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | No change from original expiration date |
| Apheresis platelets leukocytes reduced | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | Open system: within 4 hours of opening the system Closed system 5 days or 7 days |
| Apheresis platelets platelet additive solution added leukocytes reduced | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | 5 days |
| Apheresis platelets pathogen reduced | 20 - 24 °C with continuous gentle agitation | As close as possible to 20 - 24 °C Maximum time without agitation: 30 hours | 5 days |
Case reports
- 25 year old man presented to his primary care physician with 3 weeks of fatigue and intermittent low grade fevers (Acad Pathol 2020;7:2374289520909500)
- 63 year old man with chronic kidney and liver disease who received a pathogen reduced platelet transfusion (Transfusion 2021;61:641)
- 82 year old Caucasian man underwent weekly platelet and blood transfusion due to sustained severe thrombocytopenia (New Microbiol 2023;46:219)
Sample assessment & plan
- Assessment:
- A 65 year old man presented to a local hospital with shortness of breath and fatigue. According to his wife, he had been feeling more tired than usual over the last couple of weeks, particularly the past 3 days. During this time, he also noted occasional blood streaked stools.
- CBC revealed a Hgb of 5.9 g/dL. Sigmoidoscopy revealed 2 bleeding rectal polyps, which were removed.
- He is well known to the transfusion service, where they determined the presence of an anti-Jk3 as part of serological workup on a past admission when blood transfusion was also required. At the request of the treating physician, 2 cryopreserved (frozen 3 years ago) Jk3 antigen negative units in inventory were thawed and deglycerolized for transfusion. After the first unit was transfused, the clinical team decided to hold off on additional transfusion since the patient's symptoms appeared to resolve.
- Plan:
- The patient's response to treatment, including initial transfusion, does not warrant additional blood transfusion at this time.
- Due to the extreme scarcity of Jk3 antigen negative RBCs, the blood bank medical director will have the laboratory technologist refreeze the second Jk3 antigen negative RBC unit, as per local hospital transfusion policy.
Additional references
Board review style question #1
Which of the following statements is true regarding the shelf life and storage conditions of red blood cells (RBCs)?
- RBCs have a shelf life of 21 days when stored in citrate phosphate dextrose (CPD) at 1 - 6 °C
- RBCs have a shelf life of 35 days when stored in citrate phosphate double dextrose (CP2D) at 1 - 6 °C
- RBCs have a shelf life of 35 days when stored in citrate phosphate dextrose adenine 1 (CPDA-1) at 1 - 10 °C
- RBCs have a shelf life of 42 days when stored in CPD and additive solution at 1 - 10 °C
Board review style answer #1
A. RBCs have a shelf life of 21 days when stored in CPD at 1 - 6 °C. Answer C is incorrect because RBCs must be stored between 1 and 6 °C. Answer B is incorrect because storing RBCs collecting in CP2D has a shelf life of 21 days. Answer D is incorrect because storing RBCs at 1 - 10 °C is not a recommended storage temperature.
Comment Here
Reference: Shelf lives of products
Comment Here
Reference: Shelf lives of products
Board review style question #2
Which blood product has the shortest shelf life?
- Apheresis platelets stored at room temperature
- Cryoprecipitate stored at -18 °C
- Fresh frozen plasma (FFP) stored at -18 °C
- Red blood cells (RBCs) stored at 1 - 6 °C
Board review style answer #2
A. Apheresis platelets stored at room temperature.
Among the options listed, platelets have the shortest shelf life. They are most often stored at room temperature and have a shelf life of only 5 days. This shelf life can be extended to 7 days in certain circumstances outlined by the FDA bacterial contamination mitigation strategies. Cold stored platelets can have a longer time to expiration based on manufacturer's instruction. Answers B, C and D are incorrect because these blood products have longer shelf lives compared to platelets. FFP is stored at -18 °C or colder and can be stored for up to 1 year. Cryoprecipitate is derived from FFP and has a shelf life of 1 year when stored at -18 °C or colder. RBCs in liquid form have a shelf life ranging from 21 to 42 days depending on the anticoagulant, preservative and additive solution used.
Comment Here
Reference: Shelf lives of products
Comment Here
Reference: Shelf lives of products
Board review style question #3
What is the maximum allowable storage time for cryoprecipitate when stored at -18 °C or colder?
- 1 year
- 2 years
- 6 months
- 18 months
Board review style answer #3
A. 1 year.
Cryoprecipitate, when stored at -18 °C or colder, has a maximum allowable storage time of 1 year. This ensures the preservation of its clotting factor content. Answers B, C and D are incorrect because they either do not meet or exceed the recommended storage time for cryoprecipitate.
Comment Here
Reference: Shelf lives of products
Comment Here
Reference: Shelf lives of products
Sickle cell disease
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Symptoms | Screening | Blood donor screening | Laboratory | Laboratory images | Case reports | Treatment | Microscopic (histologic) description | Peripheral smear images | Positive stains | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sickle cell disease is a hemoglobinopathy caused by a point mutation in the β globin gene that leads to the production of hemoglobin S, which polymerizes under deoxygenated conditions and causes red blood cells (RBCs) to form a sickle shape
- Sickle RBCs result in hemolysis, vaso-occlusive pain crises and endothelial damage
Essential features
- Sickle cell disease is caused by a single point mutation in the β globin gene that results in the production of hemoglobin S, which polymerizes under deoxygenated conditions and causes RBCs to sickle (Lancet 2010;376:2018)
- Sickle RBCs are rigid and less deformable, which causes vaso-occlusive pain crises, hemolytic anemia, endothelial injury and inflammation (Lancet 2010;376:2018)
- Complications of sickle cell disease include stroke, acute chest syndrome, priapism, multiorgan failure, splenic / hepatic sequestration, intrahepatic cholestasis, aplastic crisis, pulmonary hypertension and avascular necrosis
- Current treatment options include simple and exchange RBC transfusions, disease modifying drug therapies and hematopoietic stem cell transplantation (Lancet 2017;390:311)
- Sickle cell trait is protective against the parasite Plasmodium falciparum
Terminology
- Sickle cell anemia
Pathophysiology
- Single point mutation (A to T substitution) in the first exon of the β globin gene, converting glutamic acid into valine
- Defective hemoglobin tetramers are formed (Hemoglobin S, Hgb S) that polymerize under deoxygenated conditions
- Polymerized Hgb S causes RBCs to form a sickle shape
- Sickle RBCs are rigid, less deformable and have a shortened lifespan of 10 - 20 days
- Rigid sickle RBCs lead to hemolysis, vascular occlusion, endothelial injury and inflammation
- Patients have hemolytic anemia and experience vaso-occlusive pain crises
- Sickle RBCs and leukocytes adhere to the vascular endothelium, causing vascular obstruction and tissue ischemia
- Chronic inflammation with an increased risk of thrombosis especially during vasooclussive event (J Thromb Thrombolysis 2013;35:352)
- Immunocompromised due to autoinfarction of spleen or surgical splenectomy (Birth Defects Orig Artic Ser 1975;11:322)
- Have hypercoagulable state based on thermoelastographic profiles (Arch Pathol Lab Med 2005;129:760, J Clin Pathol 1980;33:622)
Clinical features
- Stroke
- Acute chest syndrome
- Priapism
- Multiorgan failure
- Splenic / hepatic sequestration
- Intrahepatic cholestasis
- Aplastic crisis
- Pulmonary hypertension
- Avascular necrosis
- Reference: J Clin Med 2021;10:4232
Transmission
- Autosomal recessive gene inheritance pattern
- Variable genotypes: SS, SC, S/β0 thalassemia, S/β+ thalassemia, SD, SE are common ones
- Reference: Lancet 2010;376:2018
Symptoms
- Jaundice
- Fatigue
- Dyspnea
- Infection
- Pain and swelling
- Organ damage
- Reference: Lancet 2010;376:2018
Screening
- Newborn screening is performed as early as 24 - 48 hours after birth
- Methods used to detect the presence of Hgb S include gel electrophoresis and high performance liquid chromatography (HPLC) (Syst Rev 2020;9:250)
- Confirmatory testing is performed for positive screen results
Blood donor screening
- Donors with sickle cell disease are not eligible to donate blood; donors with sickle cell trait may be eligible
- Blood with sickle cell trait often causes failure of leukocyte reduction filters and may be deferred due to this
Laboratory
- Positive for anemia: low hemoglobin, low hematocrit, low RBC, high reticulocyte
- Positive for hemolysis: low haptoglobin, high lactate dehydrogenase, high total bilirubin, high indirect bilirubin
- Positive for Hgb S: high hemoglobin S
- Positive for inflammation: high C reactive protein, high erythrocyte sedimentation rate
- Positive for iron studies if chronically transfused and iron overloaded: high serum ferritin, high serum iron, high serum transferrin saturation, low total iron binding capacity
- Associated with increases in thrombin generation, fibrinolytic activation, platelet activation, increased antiphospholipid antibodies, decreased levels of circulating anticoagulants and contact factors
- Also increased circulating levels of tissue factor and endothelial cells expressing a tissue factor phenotype
- Reference: Ann Intern Med 2021;174:ITC1
Laboratory images
Case reports
- 8 year old boy with a rare hemoglobinopathy (hemoglobin S / hemoglobin Quebec-Chori) who presented with complications of sickle cell disease characterized by pain crises, osteomyelitis and priapism (J Pediatr Hematol Oncol 2020;42:e775)
- 16 year old girl with concurrent sickle cell anemia and ulcerative colitis (Paediatr Int Child Health 2021 Sep 3 [Epub ahead of print])
- 20 year old woman with sickle cell anemia (Hgb SS) and no history of alloantibodies, who developed a severe delayed hemolytic transfusion reaction 5 days after an elective preoperative packed RBC transfusion that rapidly progressed to multiorgan failure and death (J Pediatr Hematol Oncol 2019;41:624)
- 10 patients with sickle cell disease who developed COVID-19 infections in a developing country (Acta Haematol 2021 Sep 17 [Epub ahead of print])
Treatment
- RBC transfusions: simple or exchange (N Engl J Med 1998;339:5)
- Monitor for iron overload as a potential side effect of chronic transfusion therapy
- Prophylactic red cell antigen matching for Rh (C, E, K) recommended by American Society of Hematology (ASH) (Blood Adv 2020;4:327)
- Alloimmunization rate as high as 50% without prophylactic red cell antigen matching, 5 - 24% with limited prophylactic matching and ~7% with extended prophylactic matching (Pediatr Blood Cancer 2011;57:294, Transfusion 2001;41:1086, Pediatr Blood Cancer 2013;60:1487, Transfus Med Rev 2019;33:12, Transfusion 2011;51:1732)
- Extended red cell antigen matching (Jka / Jkb, Fya / Fyb, S / s) may further prevent alloimmunization (Blood Adv 2020;4:327)
- Disease modifying drug therapies: hydroxyurea, L glutamine, crizanlizumab, voxelotor (N Engl J Med 2017;376:429, Blood 2019;133:1865)
- Hematopoietic stem cell transplantation: allogeneic donor, autologous donor with gene therapy (clinical trials)
- Sickle cell patients should avoid handling reptiles and perhaps should avoid pet chickens due to an increased risk of salmonella infection (Pediatr Hematol Oncol 2002;24:75, CDC: Salmonella Outbreaks Linked to Backyard Poultry [Accessed 6 January 2022])
Microscopic (histologic) description
- May have increased normoblasts and megaloblastic changes due to folate deficiency
- Increased perivascular fibrosis in small and medium sized vessels (Arch Pathol Lab Med 2004;128:634)
- Occasional aplastic crisis
Positive stains
- CD1a, CD1b and CD1c in monocytes (Hum Immunol 2004;65:1370)
Sample assessment & plan
- Patient with sickle cell disease (Hgb SS) and a history of stroke, priapism and vaso-occlusive pain crisis presented with shortness of breath and was subsequently diagnosed with acute chest syndrome. Hemoglobin S level found to be 80%. Patient to undergo red blood cell exchange with 8 units of ABO compatible and crossmatch compatible packed RBCs that are Rh and K antigen matched, leukocyte reduced, irradiated and Hgb S negative. Post procedure target Hgb S is less than 30% and target hematocrit is 27%.
Additional references
Board review style question #1
A 12 year old girl with sickle cell disease presents to the emergency department with fever (102 °F), difficulty breathing, cough and chest pain. Her mother reported that the patient had a cold 2 weeks ago. On physical exam, her vitals were T 102 °F, BP 100/60, P 80, RR 25 and SaO2 80%. She was coughing and appeared to be in pain. No other abnormal findings were noted. A chest Xray was performed and showed a new pulmonary opacity. Her hematocrit was 15% and Hgb S level was 75%.
What is the most likely diagnosis for the patient?
What is the most likely diagnosis for the patient?
- Acute chest syndrome
- Asthma
- Influenza
- Pneumonia
Board review style answer #1
Board review style question #2
A 12 year old girl with sickle cell disease presents to the emergency department with fever (102 °F), difficulty breathing, cough and chest pain. Her mother reported that the patient had a cold 2 weeks ago. On physical exam, her vitals were T 102 °F, BP 100/60, P 80, RR 25 and SaO2 80%. She was coughing and appeared to be in pain. No other abnormal findings were noted. A chest Xray was performed and showed a new pulmonary opacity. Her hematocrit was 15% and Hgb S level was 75%.
The patient is being managed with red blood cell exchange. What should the target hematocrit (Hct) and Hgb S levels be?
The patient is being managed with red blood cell exchange. What should the target hematocrit (Hct) and Hgb S levels be?
- Hct > 30%, Hgb S < 30%
- Hct > 30%, Hgb S > 30%
- Hct < 30%, Hgb S < 30%
- Hct < 30%, Hgb S > 30%
Board review style answer #2
Stem cell collection
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Screening | Blood donor screening | Blood donor testing | Donor deferral | Laboratory | Case reports | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Hematopoietic stem cell transplantation, also known as bone marrow transplantation, is a procedure whereby stem cells are infused into a recipient after a conditioning regimen in order to repopulate the bone marrow that is depleted or dysfunctional (Biol Res 2012;45:307)
- Stem cells may be obtained from the bone marrow, peripheral blood or cord blood
- Stem cells may be from the recipient of the transplant (autologous) or from a healthy donor (allogeneic) (Biol Res 2012;45:307)
Essential features
- Minimum of 2 x 106 CD34+ cells/kg recipient weight must be collected for successful neutrophil and platelet engraftment (Pharmacotherapy 2010;30:485)
- Bone marrow, peripheral blood and cord blood are 3 sources of stem cells that each have pros and cons
- Bone marrow derived stem cells are associated with less graft versus host disease but lead to slower engraftment when compared with peripheral blood derived stem cells
- Human leukocyte antigen (HLA) matching for HLA-A, B, Cw, DR, DQ (10/10 match) ideal but transplantation may occur with fewer matches
- Donors are evaluated for suitability and eligibility prior to stem cell collection
Terminology
- Hematopoietic stem cell transplantation
- Stem cell transplantation
- Hematopoietic progenitor cell transplantation
Pathophysiology
- Stem cells are self renewing
- Stem cells differentiate into all blood cell lineages (i.e. white blood cells, red blood cells, platelets)
- Stem cells adhere to niche spaces in the bone marrow via adhesion molecule interactions
- CD34 antigen is a marker of stem cells
- At least 2 x 106 CD34+ cells/kg recipient weight is necessary for engraftment after transplantation
- Reference: Curr Opin Hematol 2019;26:258
Clinical features
- Bone marrow derived stem cells
- Operating room procedure
- Patient placed under anesthesia (i.e. general, epidural, spinal)
- Bone marrow harvested using syringe and needle
- Typical harvest sites are posterior iliac crest and anterior iliac crest
- Typical bone marrow product collected approximately 1 liter
- Collection takes several hours
- Large number of stem cells may be collected with fewer mature T cells
- Lower risk of chronic graft versus host disease compared with peripheral blood derived stem cells
- Slower engraftment compared with peripheral blood derived stem cells
- Peripheral blood derived stem cells
- Apheresis procedure
- Vascular access (e.g. peripheral venous, central venous catheter) required for apheresis collection
- 2 - 5 total blood volumes typically processed
- Collection takes several hours
- Mobilization agent (e.g. granulocyte colony stimulating factor [G-CSF], plerixafor) required to dissociate stem cells from the bone marrow into the peripheral blood (Pharmacotherapy 2010;30:485)
- Multiple days of collection possible to reach target CD34+ cell yield
- Fastest rate of engraftment compared with bone marrow derived stem cells and cord blood derived stem cells
- Highest rate of chronic graft versus host disease compared with bone marrow derived stem cells and umbilical cord blood derived stem cells
- Umbilical cord blood derived stem cells
- Collected from umbilical vein before or after placenta is delivered
- Low CD34+ cell content (approximately 3 - 4 x 106 cells per collection)
- Collection takes several minutes
- Lowest rate of acute and chronic graft versus host disease compared with bone marrow derived stem cells and peripheral blood derived stem cells
- Higher probability of finding a match if recipient has rare human leukocyte antigen type
- Rapidly available for procurement (approximately 2 weeks)
- No donor attrition
- Lower incidence of viral contamination
- Single collection only
- Common clinical indications (Br J Haematol 2016;174:515):
- Stroke or neurological event or deficit
- Recurrent acute chest syndrome
- Recurrent severe pain crises despite supportive care
- Recurrent vaso-occlusive painful episodes or recurrent priapism
- End organ damage (lung, heart, kidneys)
- Multiple red blood cell transfusions per year to prevent vaso-occlusive complications
- Clinical indications for HSCT (Center for International Blood & Marrow Transplant Research: The US Summary Slides - HCT Trends and Survival Data [Accessed 23 August 2021]):
- Autologous transplants:
- Multiple myeloma
- Lymphoma (Non-Hodgkin lymphoma and Hodgkin disease)
- Other malignancy
- Multiple myeloma and Lymphoma (Non-Hodgkin lymphoma and Hodgkin disease) accounted for 37% of all hematopoietic stem cell transplants in the U.S. in 2019
- Allogenenic transplants:
- Acute leukemias (acute myeloid leukemia, acute lymphoblastic leukemia)
- Myelodysplastic syndrome
- Nonmalignant disease
- Aplastic anemia
- Chronic myelogenous leukemia
- Other leukemia
- Acute leukemias (acute myeloid leukemia, acute lymphoblastic leukemia) and myelodysplastic syndrome accounted for 76% of all hematopoietic stem cell transplants in the U.S. in 2019
- Autologous transplants:
Screening
- Optimal adult donor matched at high resolution to HLA class I: A, B, C; HLA class II: DRB1 to prevent graft versus host disease, graft rejection and engraftment failure (N Engl J Med 2014;371:339)
- ABO antigen mismatch between donor and recipient is not a barrier to successful transplantation (Biol Blood Marrow Transplant 2013;19:1152)
Blood donor screening
- Donor suitability:
- Focuses on health of donor (i.e. is the donor healthy enough to donate stem cells?)
- Evaluated through medical history, physical exam, laboratory studies
- Donor eligibility:
- Focuses on health of recipient (i.e. are the stem cells from the donor safe for the recipient?)
- Evaluation of risk factors for infectious diseases
- Evaluated through donor history questionnaire, medical records review, physical exam, infectious disease marker testing from a blood sample
- References: U.S. Food & Drug Administration: CFR - Code of Federal Regulations Title 21, Subpart C, Sec. 1271 [Accessed 21 June 2021], U.S. Food & Drug Administration: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products [Accessed 21 June 2021], Foundation for the Accreditation of Cellular Therapy: Establishing Global Standards in Cellular Therapies [Accessed 21 June 2021]
Blood donor testing
- Infectious disease marker testing
- Human immunodeficiency virus (HIV), types 1 and 2
- Hepatitis B virus (HBV)
- Hepatitis C virus (HCV)
- Human T lymphotropic virus (HTLV), types 1 and 2
- Treponema pallidum (syphilis)
- Trypanosoma cruzi (Chagas)
- West Nile virus (WNV)
- Zika virus
- References: U.S. Food & Drug Administration: CFR - Code of Federal Regulations Title 21, Subpart C, Sec. 1271 [Accessed 21 June 2021], U.S. Food & Drug Administration: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products [Accessed 21 June 2021], Foundation for the Accreditation of Cellular Therapy: Establishing Global Standards in Cellular Therapies [Accessed 21 June 2021]
Donor deferral
- Donors with medical conditions that make stem cell collection unsafe are deemed unsuitable and are deferred from donation
- Donors with risk factors for infectious diseases or who have positive infectious disease markers are deemed ineligible and are deferred from donation; ineligible donors (i.e. nonconforming donors) may be used if there is documentation of urgent medical need
- Any potential donor who exhibits 1 or more of the following conditions or behaviors is determined to be ineligible (adapted from FDA: Guidance for Industry - Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) [Accessed 11 June 2021], FDA: Tissue Guidances [Accessed 11 June 2021]):
- Men who have had sex with another man in the preceding 5 years
- Persons who have injected drugs for a nonmedical reason in the preceding 5 years
- Persons with hemophilia or other related clotting disorders who have received human derived clotting factor concentrates in the preceding 5 years
- Persons who have engaged in sex in exchange for money or drugs in the preceding 5 years
- Persons who have had sex in the preceding 12 months with any person in the previous 4 bullet points or with any person who has HIV infection, HBV infection or a clinically active HCV infection
- Persons who have been exposed in the preceding 12 months to known or suspected HIV, HBV or HCV infected blood through a needlestick or through contact with an open wound, nonintact skin or mucous membrane
- Children born to mothers with or at risk for HIV infection if 18 months of age or younger or if breastfed within the preceding 12 months
- Persons who have been in juvenile detention, lockup, jail or prison for more than 72 consecutive hours in the preceding 12 months
- Persons who have lived with another person who has HBV or clinically active HCV infection in the preceding 12 months
- Persons who have undergone tattooing, ear piercing or body piercing in the preceding 12 months without using sterile procedures, supplies or equipment
- Persons who have had a past diagnosis of clinical, symptomatic viral hepatitis after their eleventh birthday, unless there is documented evidence that hepatitis A virus, Epstein-Barr virus or cytomegalovirus was the cause
- Persons who are deceased and have a documented medical diagnosis of sepsis or have documented clinical evidence consistent with a diagnosis of sepsis that is not explained by other clinical conditions at the time of death
- Persons who have had a smallpox vaccination in the preceding 8 weeks
- Persons who acquired a clinically recognizable vaccinia virus infection by contact with someone who received the smallpox vaccine
- Persons who have had a medical diagnosis or suspicion of WNV infection should be deferred for 120 days following diagnosis or onset of illness, whichever is later
- Persons who have tested positive or reactive for WNV infection using an FDA licensed or investigational WNV NAT (nucleic acid test) donor screening test in the preceding 120 days
- Persons who have been treated for or had syphilis within the preceding 12 months
- Persons who have been diagnosed with variant Creutzfeldt-Jakob disease (vCJD) or any other form of Creutzfeldt-Jakob disease (CJD)
- Persons who have been diagnosed with dementia or any degenerative or demyelinating disease of the central nervous system or another neurological disease of unknown etiology
- Persons who are at increased risk for CJD
- Persons who have a history of CJD in a blood relative
- Persons who spent 3 months or more cumulatively in the United Kingdom from 1980 - 1996
- Persons who are current or former U.S. military members, civilian military employees or dependents of a military member civilian employee who resided at U.S. military bases in Northern Europe for 6 months or more cumulatively from 1980 - 1990 or elsewhere in Europe for 6 months or more cumulatively from 1980 - 1996
- Persons who spent 5 years or more cumulatively in Europe from 1980 to the present
- Persons who received any blood or blood component transfusions in the United Kingdom or France between 1980 and the present
- Persons or their sexual partners who were born or lived in certain countries in Africa after 1977
- Persons who have received a blood transfusion or any medical treatment that involved blood in certain countries in Africa after 1977
- Persons who are recipients of xenotransplantation products or intimate contacts of a xenotransplantation product recipient
- Note: if a donor is ineligible due to 1 or more of the above criteria but there are no acceptable alternative matches / potential donors, the donor can still be used via an urgent medical need pathway, which requires additional disclosure to and consent by the recipient
Laboratory
- Complete blood count
- Complete metabolic panel
- Prothrombin time
- Partial thromboplastin time
- Fibrinogen
- Ionized calcium
- CD34+ cell count
- Note: acceptable values for stem cell collection vary by service / institution
- Reference: Foundation for the Accreditation of Cellular Therapy: Establishing Global Standards in Cellular Therapies [Accessed 21 June 2021]
Case reports
- 25 year old healthy man with a low peripheral blood stem cell collection yield after G-CSF administration (J Clin Apher 2009;24:262)
- 38 year old healthy man with severe hypersensitivity anaphylactoid reaction after G-CSF administration (J Oncol Pharm Pract 2019;25:2056)
- 58 year old man with end stage renal disease who donated peripheral blood stem cells by apheresis (Bone Marrow Transplant 2012;47:157)
- Case series of 5 patients with AL amyloidosis mobilized with plerixafor and G-CSF for peripheral blood stem cell collection (Amyloid 2014;21:149)
Sample assessment & plan
- Assessment:
- 40 year old woman with multiple myeloma who presented for peripheral blood stem cell collection. The patient was mobilized with G-CSF and plerixafor prior to the start of collection. The patient was given 2 g intravenous calcium gluconate in 100 mL normal saline piggyback. The patient did not complain of any symptoms. The procedure was completed without any adverse events. The total amount of CD34+ cells collected was 3 x 106 CD34+ cells/kg recipient weight.
- Plan:
- The patient’s collection goal of at least 5 x 106 CD34+ cells/kg has not been met. The patient will return for a second day of collection tomorrow. The patient will receive another dose of G-CSF tonight and tomorrow morning and plerixafor tonight.
Additional references
Board review style question #1
Which of the following antigens is a marker of stem cells?
- CD20
- CD34
- CD38
- CD45
Board review style answer #1
Board review style question #2
Which stem cell source is associated with the fastest engraftment?
- Bone marrow
- Cord blood
- Liver
- Peripheral blood
Board review style answer #2
Board review style question #3
Which blood cell type is responsible for graft versus host disease?
- B cells
- Neutrophils
- Plasma cells
- T cells
Board review style answer #3
Therapeutic plasma exchange (TPE)
Table of Contents
Definition / general | Indications | ASFA guidelines | Examples of TPE category I indications per 2019 ASFA guidelines (not comprehensive) | Vascular access | Anticoagulation | Replacement fluid | TPE removes plasma | Potential adverse events | Clinical documentation in the patient’s medical record | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Automated instrument removes whole blood from patient, separates and removes plasma, returns remainder of blood along with replacement fluid
- Instrument separation method: centrifugation (most common method in U.S.) or membrane filtration
- Volume treated: most commonly 1 - 1.5 plasma volumes
Indications
- In general, goal is to remove pathogenic substances present in plasma to mitigate disease processes
- American Society for Apheresis (ASFA) guidelines were most recently updated in 2019 (J Clin Apher 2019;34:171)
ASFA guidelines
- Published every 3 years
- For each indication, ASFA provides
- Category: I - IV (description of the role of apheresis in treatment of the indication)
- I: “Disorders for which apheresis is accepted as first-line therapy, either as a primary standalone treatment or in conjunction with other modes of treatment.”
- II: “Disorders for which apheresis is accepted as second-line therapy, either as a standalone treatment or in conjunction with other modes of treatment”
- III: “Optimum role of apheresis therapy is not established. Decision making should be individualized.”
- IV: “Disorders in which published evidence demonstrates or suggests apheresis to be ineffective or harmful. IRB approval is desirable if apheresis treatment is undertaken in these circumstances.”
- Grade: 1A - 2C (a reflection of the evidence used to assign the category)
- Background information about disease
- Practical guidance: replacement fluid, number of procedures, frequency of procedures, volume to treat
- Category: I - IV (description of the role of apheresis in treatment of the indication)
- Reference: J Clin Apher 2019;34:171
Examples of TPE category I indications per 2019 ASFA guidelines (not comprehensive)
- Catastrophic antiphospholipid syndrome
- Chronic inflammatory demyelinating polyradiculoneuropathy
- Focal segmental glomerulosclerosis recurrent in kidney transplant
- Myasthenia gravis acute, short term treatment (long term treatment is category II)
- Antibody mediated rejection following ABO compatible renal transplant (antibody mediated rejection following ABO incompatible renal transplant is category II)
- Thrombotic thrombocytopenic purpura
- Wilson disease, fulminant
- Reference (includes all indications): J Clin Apher 2019;34:171
Vascular access
- Access must accommodate high flow rates
- Peripheral veins, central venous catheter, ports, fistula, AV graft are options; choice will depend on urgency and frequency of plasma exchange needed
Anticoagulation
- Citrate or heparin
- Use of supplemental calcium with citrate anticoagulation varies by institution (some administer calcium to every patient, some according to patient ionized calcium values or patient symptoms of hypocalcemia)
Replacement fluid
- Typically, albumin or donor plasma (or a combination of these, for example, half albumin followed by half plasma)
- See ASFA guidelines for indication specific recommendations (J Clin Apher 2019;34:171)
- Plasma with coagulopathy, bleeding, peri-invasive procedure; always use plasma for TTP
- Typically, 1 - 1.5 plasma volumes are exchanged per TPE procedure; the Optia instrument can calculate this volume based on patient specific data
- One plasma volume = total blood volume x (1 - hematocrit)
TPE removes plasma
- Includes not only potential pathogenic substances but also normal plasma contents such as coagulation factors
- For a 1 plasma volume exchange using albumin as replacement fluid, coagulation factors may decrease by 25 - 50% and are expected to recover 80 - 100% by 48 hours; fibrinogen is expected to decrease by 63% and recover 65% by 48 hours; fibrinogen may need to be supplemented in some patients
- Can remove medications (especially those that are highly protein bound, small volume of distribution); for example, IVIG and monoclonal antibody medications will be extensively removed by plasma exchange; administration should be postponed until after the procedure
- For an extensive discussion of plasma content changes resulting from TPE, please see Winters: Apheresis: Principles and Practice, Volume 1: Therapeutic Apheresis, 4th Edition, 2020
Potential adverse events
- Associated with anticoagulant: e.g. symptoms of hypocalcemia secondary to citrate
- Associated with concurrent ACE inhibitor use: hypotension
- Associated with extracorporeal volume: e.g. hypotension
- Associated with replacement fluid: e.g. allergic reaction to albumin or plasma
- Associated with vascular access: e.g. infection
- Very rare, death
Clinical documentation in the patient’s medical record
- Note styles and content vary among institutions and among providers, but an example of a brief daily progress note follows:
- Ms. X underwent plasma exchange today for myasthenia gravis (MG) exacerbation. She tolerated the procedure without procedure related side effects. Ms. X reports that her MG symptoms continue to improve.
- Vital signs: temperature 37 C, pulse 74 bpm, BP 137/64 mmHg, RR 20 br/min
- General: appeared comfortable, no acute distress
- Laboratory values: hemoglobin: 11.5 g/dL, PLT: 268x10e9/L, fibrinogen: 194 mg/dL
- Ms. X is a 50 year old female who presented for plasma exchange for myasthenia gravis exacerbation. This was plasma exchange number X out of Y requested. The next procedure is planned for [date]. Further management per neurology.
- Ms. X underwent plasma exchange today for myasthenia gravis (MG) exacerbation. She tolerated the procedure without procedure related side effects. Ms. X reports that her MG symptoms continue to improve.
- Basic information to consider including in notes:
- Initial consult note: indication, ASFA category (if in ASFA guidelines), procedure type, number of planned procedures, anticipated schedule, replacement fluid to be used, volume to be exchanged, referring physician, access, vitals / brief physical, pertinent laboratory values, allergies, medications, past medical history
- Daily procedure notes: indication, procedure type, vitals / brief physical, pertinent laboratory values, interventions and outcome if procedure related side effects occurred, replacement fluid
Additional references
- Balogun: Principles of Apheresis Technology, 7th Edition, 2020
- Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019
- Schwartz: Therapeutic Apheresis - A Handbook, 6th Edition, 2019
- Cohn: Technical Manual, 20th Edition, 2020
- Wu: Apheresis Standard Operating Procedures Manual, 1st Edition, 2019
Board review style question #1
Which of the following entities is considered a category I indication for therapeutic plasma exchange?
- Amyloidosis, systemic
- Autoimmune hemolytic anemia, severe
- Catastrophic antiphospholipid syndrome (CAPS)
- Thyroid storm
Board review style answer #1
C. Catastrophic antiphospholipid syndrome (CAPS)
Comment Here
Reference: Therapeutic plasma exchange
Comment Here
Reference: Therapeutic plasma exchange
Board review style question #2
Which of the following entities is considered a category I indication for therapeutic plasma exchange?
- IgA nephropathy (Berger disease)
- Myasthenia gravis (acute, short term treatment)
- Myasthenia gravis (long term treatment)
- PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections) exacerbation
Board review style answer #2
B. Myasthenia gravis (acute, short term treatment)
Comment Here
Reference: Therapeutic plasma exchange
Comment Here
Reference: Therapeutic plasma exchange
Tissue banking
Table of Contents
Definition / general | Essential features | Terminology | Donor screening | Tissue recovery | Laboratory | Processing | Storage | Records | Case reports | Sample assessment and plan | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Retrieval, storage and distribution of human tissues for medical use (AATB: Standards of Tissue Banking [Accessed 16 October 2020])
- Tissue can be retrieved from both living and deceased donors for autologous or allogeneic use depending on tissue type
- 58,000 donors recovered annually, providing over 3.3 million tissue grafts from 120 accredited tissue banks in the United States (AATB: Who We Are [Accessed 16 October 2020])
- Most common transplants
- Allografts: bone, tendons / ligaments, corneas
- Autografts: skull flaps recovered from craniotomies
Essential features
- Includes bone, cardiac (e.g. valves) and vascular tissue, corneas and retinas, dura mater, reproductive tissue (including gestational tissue), skin and cellular tissues
- Does not include organ transplant (liver, kidney, heart)
- Most allogeneic tissue does not require ABO or human leukocyte antigen (HLA) compatibility
- Processing removes the majority of cellular material (Clin Lab Med 2005;25:473, Clin Lab Med 2005;25:499)
- Pooling of multiple donor tissues is not permitted (FDA: Electronic Code of Federal Regulations Title 21.1271.220(b) [Accessed 20 November 2020], Clin Lab Med 2005;25:487)
Terminology
- AATB: American Association of Tissue Banks
- Allograft: tissue transplanted into a genetically different individual
- Autograft: tissue implanted into the person from whom it was recovered
- DRAI: donor risk assessment interview
- FDA CBER: Food and Drug Administration Center for Biologics Evaluation and Research
- FDA CFR: Food and Drug Administration Code of Federal Regulations
- Xenograft: tissue transplant from one species to another
- Consent: informed consent obtained from donor or authorized person
Donor screening
- Tissue donor screening:
- Donor risk assessment interview (DRAI) conducted with donor or designee
- Physical assessment performed on all living and deceased allogeneic donors
- Relevant medical records review
- Autopsy report (if available)
- Donor exclusion criteria similar to whole blood donation
- If patient was transfused blood or colloids within 48 hours of asystole or crystalloids within 1 hour of asystole, plasma dilution must be assessed
Tissue recovery
- All tissue is collected aseptically using standard surgical preparations
- Skin prep must occur within 15 hours of asystole if donor’s body not cooled / refrigerated
- If donor body cooled / refrigerated within 12 hours, skin prep for recovery must occur within 24 hours of asystole
- If removed from cooling, cumulative noncooled time may not exceed 15 hours (AATB: Standards of Tissue Banking [Accessed 16 October 2020])
Laboratory
- Donor testing:
- FDA regulations require donors be screened to prevent infectious, malignant or structurally defective tissues from use
- AATB also requires donor screening as part of its accreditation standards (FDA: Code of Federal Regulations Title 21 Part 1270 [Accessed 19 October 2020], Clin Lab Med 2005;25:473)
- Infectious disease testing: performed within 7 days prior to or after donation for allogeneic tissue (exception: oocyte donors within 3 days prior to or 7 days post donation)
- Includes: HIV 1 / 2, hepatitis B, hepatitis C, syphilis, West Nile virus
- If tissue leukocyte rich (e.g. semen), test HTLV I / II
- FDA regulations require donors be screened to prevent infectious, malignant or structurally defective tissues from use
- Plasma dilution:
- Pretransfusion blood specimens should be used for donor infectious disease testing when the donor is known / suspected to have received large volume transfusion prior to death defined as:
- > 2,000 mL of whole blood, RBCs, colloids within 48 hrs prior to asystole
- > 2,000 mL of crystalloids within 1 hr prior to asystole
- Tissue banks can define more specific algorithms to assess plasma dilution if justified
- Pretransfusion blood specimens should be used for donor infectious disease testing when the donor is known / suspected to have received large volume transfusion prior to death defined as:
- Tissue testing:
- Detection of certain virulent organisms can result in discard
- Includes: Clostridium, fungi, Streptococcus pyogenes (Group A streptococcus) and additional exclusionary organisms for skin (Enterococci, Staphylococcus aureus, gram negative bacilli)
- Detection of certain virulent organisms can result in discard
- Reference: AATB: Standards of Tissue Banking [Accessed 16 October 2020]
Processing
- Depends on tissue type and expected use (Clin Lab Med 2005;25:499):
- Bone demineralization
- Chemical disinfection / sterilization: detergents, antibiotics, ethanol
- Chemical preservation
- Dehydration / desiccation
- Fresh / refrigerated (0 - 10°C): birth tissues, musculoskeletal tissue and osteoarticular grafts, skin, cellular tissue
- Freezing / cryopreservation: birth tissues, cardiac / vascular tissue (-100°C), musculoskeletal tissue and osteoarticular grafts ([-20] - [-40]°C), skin (-40°C), cellular tissue, reproductive tissue (liquid nitrogen)
- Lyophilization: skin (ambient temperature storage, temperature monitoring not required)
- Fresh allografts are highly antigenic; HLA should be considered (Clin Lab Med 2005;25:473)
Storage
| Tissue Type | Storage Temperature / Time | Uses |
| Amniotic / chorionic membrane | Validated by tissue bank | Wound care, neurosurgery and spinal surgery, burns |
| Cardiac / vascular tissue | Cryopreserved (≥ -100°C) | Congenital cardiac repairs, valvular insufficiency, bypass grafting, tissue revascularization |
| Corneas - for keratoplasty | 2 - 8°C (14 days) | Keratoconus correction |
| Musculoskeletal tissues - fresh | 1 - 10°C (5 days) | Skeletal reconstruction, bony defect fillers, dental implants, spinal fusion |
| Musculoskeletal tissues - frozen | (-20) - (-39)°C (< 6 months) ≥ -40°C (≤ 5 years) | |
| Reproductive tissue - semen and ova | ≥ -40°C (liquid / vapor phase nitrogen) | Infertility |
| Reproductive tissue - other than semen and ova | Validated by tissue bank | |
| Skin - refrigerated | 0 - 10°C* | Burn treatment, wound care |
| Skin - frozen | ≥ -40°C | |
| Skin - lyophilized | Ambient** |
**Does not require temperature monitoring
References: AATB: Standards of Tissue Banking [Accessed 16 October 2020], Clin Lab Med 2005;25:499, Clin Lab Med 2005;25:587
Records
- Tissue records (donor consent, eligibility, processing, storage, distribution) maintained for at least 10 years after date of tissue distribution, transplant, disposition or expiration (whichever is longest)
- Reference: AATB: Standards of Tissue Banking [Accessed 16 October 2020]
Case reports
- 4 year old girl with osteoarticular allograft reconstruction of posttraumatic skeletal defect (Injury 2016;47:2473)
- 23 year old man with autologous strut grafting repair in femoral nonunion (Chin J Traumatol 2011;14:241)
- 28 year old woman who developed Creutzfeldt-Jakob disease following dural graft (J Neurosurg 1988;69:766)
Sample assessment and plan
- Procurement eligibility: A 16 year old boy is pronounced deceased at the scene of a motor vehicle accident in a remote town following a heavy snowfall and temperatures below freezing. Time of death is unknown. Deceased was last seen alive by his parents approximately 6 hours earlier. Family has expressed an interest in organ and tissue donation.
- Assessment: As time of death is unknown, time last seen alive is used as the surrogate for asystole in tissue donation. Based on environmental conditions, the deceased can be considered cooled within 12 hours of death and is therefore a candidate for tissue recovery for 24 hours since last seen alive.
- Plan: Parents would be immediate next of kin with legal authority in this case.
- Gain informed consent from parents regarding donation process.
- Inform local tissue procurement agency of potential patient:
- Agency will perform DRAI with family, access all necessary medical records and perform procurement once acceptability determined.
Additional references
Board review style question #1
A 42 year old woman with no known medical history arrives in critical condition to the emergency room by ambulance following a pedestrian versus automobile accident. The patient undergoes multiple transfusions in an attempt to resuscitate but ultimately succumbs to her injuries. The patient's family is at the bedside and expresses their wish to have the patient be an organ and tissue donor. Is this patient a candidate for tissue donation based on the information provided below?
Height: 163 cm
Weight: 55.3 kg
Hemoglobin (pretransfusion sample): 6.5 g/dL
Hematocrit (pretransfusion sample): 20%
Volume of red blood cells transfused: 728 mL
Volume of crystalloids transfused: 2,250 mL
Height: 163 cm
Weight: 55.3 kg
Hemoglobin (pretransfusion sample): 6.5 g/dL
Hematocrit (pretransfusion sample): 20%
Volume of red blood cells transfused: 728 mL
Volume of crystalloids transfused: 2,250 mL
- No, a posttransfusion sample is not available
- No, she did not sign an organ donation card before death
- Yes, as long as a pretransfusion sample is available due to posttransfusion hemodilution
- Yes, based on her age, no additional information is needed
Board review style answer #1
C. Yes, as long as a pretransfusion sample is available due to posttransfusion hemodilution. Based on the volume of crystalloids and red blood cells received, this patient would be hemodilute following resuscitation and accurate infectious disease testing would not be able to be performed unless a pretransfusion sample is available. For tissue banking, the total colloid / crystalloid volume received must be less than 2,000 mL. In this case, the patient received 2,250 mL of crystalloid, which is greater than 2,000 mL (and greater than her plasma volume of 2,212 mL). As long as no high risk behaviors are identified during the DRAI interview and her physical exam is unremarkable for high risk behaviors, she would be a candidate to donate.
Comment Here
Reference: Tissue banking
Comment Here
Reference: Tissue banking
Transfusion associated circulatory overload
Table of Contents
Definition / general | Essential features | Pathophysiology | Clinical features | Symptoms | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Circulatory volume overload following transfusion
- Fluid accumulates in the lungs if the heart or kidneys are unable to compensate for the volume of the product transfused
Essential features
- Transfusion associated circulatory overload (TACO) is a form of cardiopulmonary edema due to the inability to tolerate the volume or rate of transfusion
- In patients with a history of heart failure, renal failure or evidence of positive fluid balance, carefully consider the need for transfusion
- Slowing the rate of transfusion or concurrent diuresis may prevent TACO in at risk patients
- TACO is the leading cause of death from transfusion in the US
Pathophysiology
- Transfusion increases intravascular volume
- If the heart is unable to increase cardiac output or the kidneys are unable to compensate for the increased volume, venous pressure will increase
- Increased pressure can force fluid from vasculature into the lungs
- Reference: Transfusion 2019;59:3617
Clinical features
- New onset or exacerbation of 3 or more of the following within 6 hours of the end of transfusion (CDC: National Healthcare Safety Network - Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 12 April 2021]):
- Acute respiratory distress (dyspnea, orthopnea, cough)
- Elevated B type natriuretic peptide (BNP)
- Elevated central venous pressure (CVP)
- Evidence of left heart failure
- Evidence of positive fluid balance
- Radiographic evidence of pulmonary edema
- Risk factors include (Am J Med 2013;126:357.e29):
- Positive fluid balance
- History of heart failure
- History of chronic renal failure
- Hemorrhagic shock
- Multiple blood products transfused
- TACO is a common transfusion reaction and is estimated to occur in 1 - 8% of transfused patients
- TACO caused the highest number of transfusion related fatalities (32%) in data reported to the FDA from 2014 to 2018 (FDA: Fatalities Reported to FDA Following Blood Collection and Transfusion [Accessed 12 April 2021])
- TACO is associated with increased morbidity measured by significantly increased hospital and ICU length of stay (Am J Med 2013;126:357.e29)
- Although TACO is the most frequent serious adverse event associated with blood transfusions, it is likely underrecognized and the incidence underestimated, especially in ICU patients and pediatrics (Crit Care Med 2019;47:849, Transfusion 2018;58:1037)
Symptoms
- Dyspnea, orthopnea, cough, headache, chest tightness, hypertension, tachycardia, hypoxia, widened pulse pressure, jugular venous distension
Laboratory
- Elevated circulating B type natriuretic peptide (BNP, formerly brain natriuretic peptide) or N-terminal-pro-BNP (NT-pro-BNP), a marker for congestive heart failure
- Post / pretransfusion NT‐proBNP ratio > 1.5 can aid in the diagnosis of TACO; posttransfusion levels of BNP < 300 or NT‐proBNP < 2000 pg/mL, drawn within 24 hours of the reaction, make TACO unlikely (Transfusion 2019;59:795)
Case reports
- 5 year old boy with neuroblastoma develops acute hypoxemia, tachypnea and tachycardia after red blood cell transfusion (Transfus Apher Sci 2017;56:445)
- 46 year old man develops acute dyspnea with hypoxemia following red blood cell transfusion (Indian J Crit Care Med 2014;18:396)
- 63 year old woman with diabetes develops acute respiratory distress following red blood cell transfusion (BMJ Case Rep 2020;13:e230426)
Treatment
- Stop transfusion
- Diuresis
- Supplemental oxygen
- Prevention:
- Avoid unnecessary transfusion (Transfusion 2014;54:2344)
- Reduce transfusion rate
- Diurese high risk patients before or during transfusion
Sample assessment & plan
- Assessment
- Given the patient’s history of heart failure, the presence of dyspnea and hypertension after transfusion, there is an increased concern for TACO. The chest Xray results after the transfusion show evidence of worsening pulmonary edema compared with a recent prior Xray. The patient's pre-transfusion BNP was 250 pg/ml and post-transfusion BNP is 400 pg/ml, further supporting the diagnosis of TACO. Clerical check of the transfused unit is correct and there is no visible evidence of hemolysis.
- Plan
- Carefully consider the need for transfusion, weighing it against the potential risks of transfusion and avoid unnecessary transfusions by adhering to restrictive thresholds for hemodynamically stable patients. Slowing the rate of transfusion or concurrent diuretic treatment may alleviate future incidents of TACO.
Differential diagnosis
- Transfusion related acute lung injury (TRALI) (Blood 2019;133:1840):
- Noncardiogenic pulmonary edema characterized by dyspnea, often accompanied by fever and hypotension
- Patients with TRALI do not have evidence of volume overload and do not respond to dieresis
- Transfusion associated dyspnea (TAD):
- Patients with TAD do not have evidence of volume overload and do not respond to diuresis
Board review style question #1
A 75 year old man with a history of heart failure presents to the hospital with a lower GI bleed. He requires a red blood cell transfusion for a hemoglobin of 7 g/dL. During the transfusion of the second red blood cell unit, he develops a cough, chest tightness, headache and hypertension. The transfusion is stopped, the patient is given supplemental oxygen with improvement of symptoms and a chest Xray is ordered (shown above). Which of the following is the most likely cause of his signs and symptoms?
- Acute hemolytic transfusion reaction
- Anaphylaxis
- Transfusion associated circulatory overload (TACO)
- Transfusion related acute lung injury (TRALI)
Board review style answer #1
C. Transfusion associated circulatory overload (TACO)
Transfusion associate circulatory overload is defined as the new onset or exacerbation of 3 or more of the following within 6 hours of cessation of transfusion:
Comment Here
Reference: Circulatory overload
Transfusion associate circulatory overload is defined as the new onset or exacerbation of 3 or more of the following within 6 hours of cessation of transfusion:
- Acute respiratory distress (dyspnea, orthopnea, cough)
- Elevated B type natriuretic peptide (BNP)
- Elevated central venous pressure (CVP)
- Evidence of left heart failure
- Evidence of positive fluid balance
- Radiographic evidence of pulmonary edema
Comment Here
Reference: Circulatory overload
Board review style question #2
Transfusion associated circulatory overload (TACO) may demonstrate which of the following features?
- Development of flank pain and dark colored urine
- Development of hypotension, dyspnea and angioedema
- Elevated B type natriuretic peptide (BNP) or NT-pro-BNP
- Presence of HLA antibodies in the transfused blood component
Board review style answer #2
C. Elevated B type natriuretic peptide (BNP) or NT-pro-BNP
Elevated circulating B type natriuretic peptide (BNP, formerly brain natriuretic peptide) or N-terminal-pro-BNP (NT-pro-BNP), markers for congestive heart failure, can indicate circulatory overload. Demonstration of anti-human leukocyte antigens or anti-human neutrophil antibodies in a donor provides support for transfusion related acute lung injury (TRALI). Acute hemolytic reactions classically present with flank pain and hematuria. Allergic reactions are associated with hypotension, respiratory distress (bronchospasm), angioedema, urticaria and rash.
Comment Here
Reference: Circulatory overload
Elevated circulating B type natriuretic peptide (BNP, formerly brain natriuretic peptide) or N-terminal-pro-BNP (NT-pro-BNP), markers for congestive heart failure, can indicate circulatory overload. Demonstration of anti-human leukocyte antigens or anti-human neutrophil antibodies in a donor provides support for transfusion related acute lung injury (TRALI). Acute hemolytic reactions classically present with flank pain and hematuria. Allergic reactions are associated with hypotension, respiratory distress (bronchospasm), angioedema, urticaria and rash.
Comment Here
Reference: Circulatory overload
Transfusion associated graft versus host disease
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Laboratory | Prevention | Risk factors / indications for irradiation | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Transfusion associated graft versus host disease (TA-GVHD) is a rare, fatal, delayed reaction to blood transfusion (Indian J Hematol Blood Transfus 2010;26:92)
- Life threatening, multisystemic syndrome observed in immunocompromised and immunocompetent individuals (Hematol Rep 2021;13:9280)
Essential features
- Highly preventable
- Most common clinical manifestations include fever (7 - 10 days after transfusion), pancytopenia, maculopapular rash on the face and trunk that spreads to extremities, diarrhea, abdominal pain, mucositis, hepatitis, jaundice and marrow aplasia (Arch Pathol Lab Med 2018;142:662)
Terminology
- Transfusion associated graft versus host disease (TA-GVHD)
Pathophysiology
- Active T lymphocytes (CD4, CD8 and NK cells) are transfused from a nonidentical donor (the graft) with human leukocyte antigen (HLA) incompatibility and recognize the transfused recipient (the host) as foreign, initiating an immune attack that causes tissue damage in the transfused recipient (Arch Pathol Lab Med 2018;142:662)
- Lymphocytes from the recipient cannot counterattack (immunosuppressed recipient) or do not recognize the graft (donor) as foreign (immunocompetent HLA heterozygous recipient from an HLA homozygous donor, i.e., HLA one way compatibility)
- Recipient fails to respond to neutralize the donor; viable, transfused T lymphocytes attack and lead to GVHD
Clinical features
- National Health and Safety Network (NHSN) established the criteria diagnostic of TA-GVHD based on a combination of clinical and histologic findings that occur between 2 days and 6 weeks after a transfusion (Blood 2015;126:406)
- All cellular blood products and fresh plasma are implicated; products that have been frozen do not pose a risk due to the destruction of donor lymphocytes by freezing
- Incidence of TA-GVHD is lower than that of bone marrow transplant related GVHD
- However, TA-GVHD is a more lethal condition; mortality rate is 87 - 100%, typically related to bone marrow failure (Indian J Hematol Blood Transfus 2010;26:92)
Symptoms
- Clinical manifestations affect the skin, GI tract, liver and bone marrow; severe neutropenia leading to untreatable infection is the primary cause of death in TA-GVHD (Am J Clin Pathol 2000;113:732)
- Constitutional: fever
- Skin: can range from erythematous maculopapular rash to toxic epidermal necrolysis
- Liver: hepatomegaly, jaundice
- GI: abdominal pain, diarrhea, vomiting, anorexia
Laboratory
- Complete blood count (CBC) shows pancytopenia
- Peripheral blood smear confirms pancytopenia with a low reticulocyte response
- Metabolic panel will show abnormal liver and kidney function
- Bone marrow: aplasia, pancytopenia, lymphoid and histiocytic infiltrates
- Liver or skin biopsy (rare) (Hematol Rep 2018;10:7724)
- Donor lymphocyte DNA (polymerase chain reaction [PCR] based HLA typing)
Prevention
- Blood products, such as whole blood, red blood cells, platelets, granulocytes and plasma that have not been frozen (e.g., liquid plasma), can contain viable T lymphocytes and cause TA-GVHD
- Gamma irradiation of cellular blood products
- T cells are sensitive to high doses of radiation
- Cellular blood products must be irradiated with a minimum of 25 Gy to the center of the unit and 15 Gy to the periphery
- Pathogen reduction technology: process uses a psoralen compound and UV light to crosslink DNA and RNA in the target pathogens (viruses / bacteria) but also crosslinks the DNA of any T lymphocytes in the product, which provides comparable results as irradiation (Vox Sang 2017;112:607)
- Leukocyte reduction does not prevent TA-GVHD but does reduce the risk, due to an overall lower dose of residual T lymphocytes in the product
Risk factors / indications for irradiation
- Selected immunosuppressed patients (Bone Marrow Transplant 2004;33:1, Hematol Rep 2018;10:7724):
- Congenital T cell deficiencies
- Intrauterine transfusion or neonatal exchange transfusion recipients
- Marrow transplant recipient or candidate
- Taking purine analogs such as fludarabine, anti-CD52 medications (alemtuzumab) or other drugs that affect T cells
- Hodgkin disease, leukemia or lymphoma patients
- Requiring granulocyte transfusions
- Irradiation is not required for patients with HIV / AIDS
- Selected immunocompetent patients:
- Those receiving blood transfusion from a first degree relative (in practice, products from any relative should be irradiated)
- Partial HLA matched units
- Neonates who have received intrauterine transfusions
- Healthy newborns do not need irradiation, however, some facilities may choose to irradiate up to a certain age to avoid the possibility of an immunodeficiency that has not manifested (e.g., DiGeorge syndrome, congenital neutropenia syndromes, such as all patients < 1 year of age)
Case reports
- 50 year old immunocompetent woman who received whole blood transfusion from an unrelated donor (Indian J Hematol Blood Transfus 2010;26:92)
- 53 year old man with TA-GVHD following hepatectomy for hepatocellular carcinoma (Kurume Med J 1993;40:193)
- 61 year old woman with chronic lymphocytic leukemia (CLL) was treated with fludarabine followed by combination chemotherapy / radioimmunotherapy and peripheral blood progenitor cell (PBPC) rescue (Transfusion 2002;42:1567)
Treatment
- Palliative care
- Immunosuppression: glucocorticoids, cyclosporine, intravenous immune globulin (IVIG) and antithymocyte globulin (ATG) or antilymphocyte globulin (ALG)
- Bone marrow transplant (HSC) might play an important role in the future (Pagano: Complications of Transfusion, 1st Edition, 2014, Blood 2015;126:406)
Sample assessment & plan
- Assessment: Patient X is a 63 year old man with a 60 pack year tobacco history, diabetes mellitus and hypertension. He was admitted to the hospital with acute, severe left chest pain and was found to have coronary artery disease. He underwent coronary artery bypass graft surgery, during which he received 4 units of nonirradiated packed red blood cells. 2 weeks after the procedure, he presented in the emergency department with a fever (104 °F), nausea, vomiting, erythematous rash on his trunk / extremities and abdominal pain. Laboratory tests revealed abnormal liver and kidney function, pancytopenia, bone marrow aplasia and increased levels of lactate dehydrogenase (LDH), amylase and lipase. HLA typing was performed on the patient and on the red blood cell transfusion donors, revealing that the donor shared an HLA haplotype. The skin biopsy showed vacuolar dermatitis with a lymphocytic infiltrate.
- Impression: Transfusion associated graft versus host disease
- Plan:
- Supportive care and steroids.
- Evaluation by hematology / oncology for bone marrow transplant may be considered, however, this disease process has an extremely poor outcome.
- Should have a discussion regarding prognosis with the patient.
- Palliative care consultation may be appropriate based on the patient's wishes.
Differential diagnosis
- Viral infections (such as hepatitis B, hepatitis C, HIV, cytomegalovirus, Epstein-Barr virus):
- Fever, skin rash, bone marrow failure and abnormal liver function tests
- Serologic testing and polymerase chain reaction (PCR) will identify the virus
- Adverse drug reaction:
- Skin rash / eruption, myalgia, pancytopenia, fever and eosinophilia
- Skin biopsy, complete blood cell (CBC) and liver function tests
- Liver failure:
- Etiology: infections, autoimmune reactions, medications, metabolic disorders (Wilson disease)
- Signs and symptoms of encephalopathy: altered mental state, progressive loss of memory and cognitive ability, myoclonus, nystagmus and asterixis
- Elevated coagulation tests: prothrombin time / international normalized ratio (INR)
- Allogeneic hematopoietic cell transplantation (HCT):
- Liver failure, rash, fever, diarrhea, nausea and abdominal pain
- Previous bone marrow transplant
Board review style question #1
A 68 year old man with lymphoma presents to the hospital to undergo cardiac surgery with low levels of platelets. The blood bank director recommended transfusion of irradiated platelets before surgery. Which is the correct explanation regarding the blood bank director's recommendation?
- Irradiation of platelets and red blood cells prevents acute hemolytic transfusion reactions (AHTR)
- Irradiation of platelets and red blood cells prevents febrile nonhemolytic transfusion reactions (FNHTR)
- Irradiation of platelets and red blood cells prevents transfusion associated graft versus host disease (TA-GVHD)
- Irradiation of platelets and red blood cells prevents transfusion related acute lung injury (TRALI)
- Irradiation of platelets and red blood cells prevents transfusion associated circulatory overload (TACO)
Board review style answer #1
C. Irradiation of platelets and red blood cells prevents transfusion associated graft versus host disease (TA-GVHD), especially in a patient with hematological malignancy such as this one.
Comment Here
Reference: Transfusion associated graft versus host disease
Comment Here
Reference: Transfusion associated graft versus host disease
Board review style question #2
A 4 month old boy was brought to the emergency department by his mother for fever, diarrhea, vomiting, abdominal pain and anorexia that started 3 days ago. On physical examination, there is a diffuse maculopapular skin rash. His significant previous medical history includes a preterm birth and having received a blood transfusion 8 days ago. Laboratory findings include pancytopenia and elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP). Which of the following is the most likely diagnosis in this patient?
- Cytomegalovirus colitis
- Drug reaction
- Henoch-Schönlein purpura
- Hepatitis B
- Transfusion associated graft host disease (TA-GVHD)
Board review style answer #2
E. Transfusion associated graft host disease (TA-GVHD)
Comment Here
Reference: Transfusion associated graft versus host disease
Comment Here
Reference: Transfusion associated graft versus host disease
Transfusion related acute lung injury
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Clinical features | Screening | Donor deferral | Prevention | Laboratory | Radiology images | Case reports | Treatment | Microscopic (histologic) description | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Transfusion related acute lung injury (TRALI) is an adverse outcome of transfusion in which acute respiratory distress occurs within 6 hours of a transfusion
- This is typically an antibody mediated process in which antibodies in the transfused product or less commonly in the recipient attract neutrophils to the pulmonary vasculature
Essential features
- Respiratory distress / acute lung injury developing within 6 hours of cessation of transfusion
- Radiographic evidence of new bilateral infiltrates (generally without cardiomegaly)
- Hypoxemia
- No previous evidence of acute lung injury (ALI), such as circulatory overload or acute respiratory distress syndrome (ARDS)
Terminology
- Transfusion related acute lung injury (TRALI)
- Acronym TRALI coined in 1985 by Popovsky and Moore (Transfusion 1985;25:573)
Epidemiology
- Difficult to pinpoint exact incidence due to likelihood of underreporting
- Likely decreasing with increased use of leukoreduced products leading to decreased alloimmunization and also reverse TRALI
- 1 in 5,000 transfusions (Transfusion 2003;43:1053, Transfusion 1985;25:573)
- TRALI has been reported following transfusion of all blood products
- Most common with plasma rich blood products (> 60 mL of plasma)
- Plasma from female donors (discussed later under Prevention)
- Directed blood donation from mother to child
Pathophysiology
- A two hit hypothesis has been proposed
- Hit 1: patient's underlying condition
- Hit 2: transfused blood component activates the neutrophils within the pulmonary vasculature, which in turn causes plugging and endothelial damage
- Antibody mediated
- Passive transfer of human leukocyte antigen (HLA) or human neutrophil antigen (HNA) antibodies from donor blood to a transfusion recipient in majority (80%) of cases (Vox Sang 2008;94:277)
- HLA class I molecules: A, B and C antigens carried on nucleated cells (white cells and endothelial cells) and platelets
- HLA class I antibodies can bind to neutrophils and prime them, which can alter their biological processes leading to mechanical plugging of capillary beds and damage to endothelium due to cytokine release
- Antibodies against HLA-A2, a frequent antigen, have been reported (Lancet 1984;1:244, Vox Sang 1986;51:102)
- Antibodies against class II antigens may bind to monocytes → release of cytokines → neutrophil activation (alternate TRALI pathway) (Transfusion 2003;43:177)
- HNA: structures predominantly expressed on neutrophils but are also found on monocytes, lymphocytes and platelets, not expressed on endothelium
- Antibodies against HNA-3a have been identified in numerous TRALI models (Am J Respir Crit Care Med 2001;164:896, Blood 1990;76:1438)
- Exposure to foreign HLA or HNA molecules through transfusion, transplantation or pregnancy can cause alloimmunization against foreign antigens
- Antibodies can cause immediate destruction of cells bearing cognate antigens → cascade of inflammatory events leading to TRALI (Vox Sang 2008;94:324)
- Antibodies are not always found in suspected TRALI cases (JAMA 2002;287:1968, Vox Sang 2007;93:70)
- Mechanism of action of lung damage
- Neutrophils normally tether to endothelium under shear stress
- Neutrophils are normally spherical with a diameter of 6 - 8 μm
- Pulmonary capillaries, which range in diameter from 2 - 15 μm, force neutrophils to flatten out to pass through and make them unable to roll (J Appl Physiol 1995;79:493)
- Priming of neutrophils (caused by HLA and HNA antibodies) disrupts neutrophil shape change and leads to mechanical plugging of pulmonary vessels
- Pulmonary inflammation → emigration of neutrophils from capillary beds into the alveolar spaces (J Appl Physiol 1995;79:493)
- Primed neutrophils release cytokines → damage endothelium → protein rich fluid leak through endothelial gaps → exudate into alveolar spaces → respiratory distress = TRALI (N Engl J Med 2005;353:2788)
- Inverse TRALI (induced by transfusion of neutrophils to patient with preformed antibodies against HLA / HNA)
- HLA or HNA alloimmunized patients receiving blood components that contain neutrophils, e.g. granulocytes
- Leukoreduction should decrease incidence
Clinical features
- Criteria for TRALI
- Respiratory distress / acute lung injury developing within 6 hours of cessation of transfusion
- Radiographic evidence of new bilateral infiltrates (generally without cardiomegaly)
- Hypoxemia defined by ≥ 1 of the following
- PaO2/FiO2 ≤ 300 mg Hg
- O2 saturation < 90% on room air
- Other clinical evidence of hypoxemia
- No previous evidence of ALI, such as circulatory overload or acute respiratory distress syndrome
- Findings that may be helpful but are not required for diagnosis include
- Frothy pink secretions in ventilator tubing
- No response to diuretics, as opposed to transfusion associated circulatory overload, which does respond
- Short lived, sudden drop in white blood cell count, particularly with neutropenia, resulting from neutrophil sequestration in small alveolar blood vessels
- While presence of antibodies against HLA or HNA are helpful for diagnosis, they are not absolutely required
- References: Transfusion 1992;32:589, Transfusion 1985;25:573, CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 16 January 2020]
Screening
- Women who have been pregnant have a higher likelihood of developing HLA and HNA antibodies due to alloimmunization from the fetus
- Most significant in plasma rich products such as fresh frozen plasma and apheresis platelets
- Initial efforts to limit products from female donors → use of male only plasma in U.K. and U.S. → reduced rate of TRALI dramatically
- Screening donors for anti-HLA
- References: Transfus Med 2008;18:348, Vox Sang 2008;95:313, Hematology Am Soc Hematol Educ Program 2018;2018:585
Donor deferral
- Donor lookback and deferral is critical to protect future patients
- Very important to identify cocomponents
- Hospital should notify blood center following a potential case of TRALI
- Investigate pregnancy, transfusion or transplantation history of the donor
- Determine if donor has anti-HLA or HNA antibodies; if yes, then patient is also tested to see whether an antigen - antibody interaction could be implicated
- Donors with anti-HLA or HNA antibodies matched to recipient antigens in a possible case of TRALI are deferred indefinitely (AABB: TRALI Risk Mitigation for Plasma and Whole Blood for Allogeneic Transfusion [Accessed 8 January 2020])
Prevention
- Defer donors confirmed to be implicated in TRALI
- Use only male plasma to avoid risk of anti-HLA antibodies formed in women during pregnancy
- Screen female donors of apheresis platelets for HLA antibodies, indefinitely defer those with antibodies
- References: Hematology Am Soc Hematol Educ Program 2018;2018:585, Vox Sang 2017;112:694, AABB: TRALI Risk Mitigation for Plasma and Whole Blood for Allogeneic Transfusion [Accessed 8 January 2020], Transfusion 2010;50:1312, AABB: Standards for Blood Banks and Transfusion Services, 31st Edition, 2018, Fung: Technical Manual, 19th Edition, 2017
Laboratory
- Short lived, sudden drop in white blood cell count, particularly with neutropenia, resulting from neutrophil sequestration in small alveolar blood vessels
- No elevation in brain natriuretic peptide (BNP) as in transfusion associated circulatory overload
- Pleural fluid is exudate rather than transudate (as in transfusion associated circulatory overload) with a protein content of > 2.9 g/dL and fluid protein to serum protein ratio of > 0.5, and cholesterol content of > 45 mg/dL
- References: Hematology Am Soc Hematol Educ Program 2018;2018:585, Fung: Technical Manual, 19th Edition, 2017, Transfusion 2004;44:1689, Transfusion 2005;45:1056, Transfusion 2008;48:2167, Vox Sang 2008;94:277, Curr Opin Hematol 2011;18:452, Transfusion 2004;44:1683, Vox Sang 2007;93:70
Case reports
- 4 month old girl and 78 year old woman, transfusions between mothers and daughters (Am J Clin Pathol 2004;121:590)
- 2 cases in children (Transfus Med 2006;16:343)
- 28 and 31 year old men with thalassemia (Mediterr J Hematol Infect Dis 2017;9:e2017060)
- 67 year old man with transfusion for iron deficiency anemia (CMAJ 2007;177:149), recurrence 2 years later (Transfus Med 2007;17:192)
- 77 year old woman with common variable immune deficiency (Transfusion 2014;54:3088)
- Patient with hematologic malignancy and neutropenia associated pneumonitis (Transfusion 2003;43:1683)
Treatment
- Stop the transfusion
- Report the transfusion to transfusion medicine service to start the investigation
- Supportive management only
- Mechanical ventilation may be needed in severe cases
- Most patients improve within 48 - 96 hours
- Mortality is 5 - 10%
- Indefinite deferral of blood donor to prevent future cases
- Deaths from TRALI: during fiscal years 2012 - 2016, the combined TRALI and possible TRALI cases caused the highest number of reported fatalities, 64 out of 186 total fatalities (34%) (FDA: Transfusion Related Acute Lung Injury (TRALI) [Accessed 8 January 2020])
Microscopic (histologic) description
- Acute respiratory distress syndrome with extensive intra-alveolar edema and presence of neutrophils
- Hyaline membranes and distortion of pulmonary architecture
- Capillary leukostasis
- Electron microscopy shows degranulation of neutrophils in contact with injured endothelium (Transfusion 1986;26:278, Chest 2001;119:219)
Sample assessment & plan
- Assessment: John Doe is a 75 year old man who developed respiratory distress 2 hours after a packed red blood cell (pRBC) transfusion. He had normal ejection fraction, no elevation in brain natriuretic peptide (BNP) and treatment with diuretics did not resolve his respiratory distress. The blood supplier was notified and performed testing of donor sample. Testing of the donor sample identified anti-HLA DR11, DR13 and DR52. Patient had matching cognate antigens - HLA type DR11, DR13 and DR52.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of transfusion related acute lung injury (TRALI).
Differential diagnosis
- Transfusion associated circulatory overload:
- Fluid overload → increased pulmonary capillary pressure → cardiogenic edema = congestive heart failure
- Patient often has predisposition to congestive heart failure
- Hypertension rather than the hypotension typically seen in TRALI
- Elevated brain natriuretic peptide (BNP), e.g. post to pretransfusion ratio of BNP ≥ 1.5 (indicates stretched ventricular muscle due to volume overload)
- Transudative rather than exudative alveolar edema
- All blood components have been implicated, volume issue, no particular product is more commonly associated
- Transfusion associated dyspnea:
- Diagnosis of exclusion that does not meet criteria for TRALI or transfusion associated circulatory overload
- No specific underlying pathophysiology has been identified
Additional references
Board review style question #1
One key feature seen in transfusion related acute lung injury but not in transfusion associated circulatory overload is
- Decreased oxygen saturation
- Hypotension
- More likely to have underlying cardiac dysfunction
- New pulmonary infiltrates
- Response to diuretics
Board review style answer #1
B. Hypotension is often seen in transfusion related acute lung injury, as opposed to hypertension seen in transfusion associated circulatory overload, as a result of expansion of intravascular fluid volume, making choice B the correct response. The answer choices of new pulmonary infiltrates and decreased oxygen saturation are seen in both. Response to diuretics and pre-existing, underlying cardiac dysfunction are more commonly seen in transfusion associated circulatory overload.
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Board review style question #2
A 55 year old woman with acute myeloid leukemia, status postallogeneic bone marrow transplant with severe neutropenia and unresponsive fungal infection is receiving granulocytes. She develops severe respiratory distress shortly after the transfusion is completed, necessitating mechanical ventilation. Her fluid balance is neutral and she has no pre-existing cardiac dysfunction. What laboratory testing would help confirm a diagnosis of inverse transfusion related acute lung injury?
- Anti-HLA / HNA antibodies in the donor that match antigens in the recipient
- Anti-HLA / HNA antibodies in the recipient that match antigens in the donor
- Elevated brain natriuretic peptide (BNP)
- Pleural fluid with protein content < 2.5 g/dL and fluid protein to serum protein ratio of < 0.5 and cholesterol content of < 45 mg/dL
- Short lived, sudden increase in neutrophils in complete blood count
Board review style answer #2
B. Anti-HLA / HNA antibodies in the recipient that match antigens in the donor. Inverse transfusion related acute lung injury refers to the recipient having antibodies against antigens in the donor, so choice B. Elevated brain natriuretic peptide and transudative pulmonary fluid are seen in transfusion associated circulatory overload. Choice A describes traditional transfusion related acute lung injury occurring when antibodies in the donor match cognate antigens in the recipient. Choice E is incorrect because transfusion related acute lung injury may feature a sudden, short lived decrease rather than increase in neutrophils in a complete blood count.
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Transfusion related immunomodulation (TRIM)
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Symptoms | Laboratory | Case reports | Treatment | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Transfusion related immunomodulation (TRIM) refers to the capability of transfused allogeneic blood products to interact with and impact recipient immune cell function
- Immunosuppressive or proinflammatory effects of transfusion have the potential to negatively impact patient outcomes
Essential features
- Existence and extent of the impact of TRIM is somewhat controversial
- Contributions of transfusion to an already immune dysregulated patient are difficult to isolate (Curr Opin Hematol 2017;24:551)
- Leukoreduction is a key strategy to mitigate TRIM effects
- However, TRIM has also been documented despite leukoreduction and may occur in a leukocyte independent manner
- Potential adverse effects related to TRIM include increased risk for infection, organ dysfunction and recurrence or progression of cancer
- Positive impacts, such as lower rejection rates of transplanted organs, have also been described
Terminology
- Immunomodulation by blood transfusions
- Immunomodulatory effects of blood transfusions
- Transfusion associated immunomodulation
Pathophysiology
- There are many proposed mechanisms by which donor blood products may interact with the recipient's immune system (Transfusion 2018;58:804)
- White blood cell mediated
- Donor antigen presenting cells expressing major histocompatibility complex (MHC) II molecules interact with recipient lymphocytes
- Engagement of recipient T cells without costimulatory signals leads to antigen specific anergy and shifts to immunosuppressive T helper type 2 (Th2) type response
- Donor white blood cells (WBCs) undergoing apoptosis during collection or storage
- Exposes phosphatidylserine on dying cell membranes, which interacts with recipient immune cells
- Recipient immune cells generate immunosuppressive signals (i.e., increased IL10 and TGFβ)
- Exposes phosphatidylserine on dying cell membranes, which interacts with recipient immune cells
- Donor WBCs producing soluble mediators including cytokines, degranulation products, soluble human leukocyte antigen (HLA) molecules
- May induce recipient immune cell apoptosis, impair chemotaxis, decrease natural killer (NK) cell activity
- Donor antigen presenting cells expressing major histocompatibility complex (MHC) II molecules interact with recipient lymphocytes
- Red blood cell mediated
- Hemolysis during storage and transfusion leading to release of metabolic wastes, microparticles, free iron and free heme
- Upregulation of proinflammatory cytokines
- Bias of macrophage phenotype from inflammatory M1 to immunosuppressive M2
- While it has been proposed that longer storage duration of red blood cell units may increase these effects, studies to date have not supported the clinical impact of longer red blood cell storage in the general population (N Engl J Med 2016;375:1937)
- Hemolysis during storage and transfusion leading to release of metabolic wastes, microparticles, free iron and free heme
- Platelet mediated
- In vitro work has suggested platelet derived microparticles are capable of immune system modulation
- In vivo contribution is unknown
- Bioactive lipid mediated
- Polyunsaturated fatty acids (arachidonic acid, linoleic acid, etc.) remain in blood products after leukoreduction
- In vitro studies have demonstrated a capability to prime neutrophils
- Rat models transfused with these lipids have shown transfusion related lung injury-like effects
- In vivo contribution is unknown
- Polyunsaturated fatty acids (arachidonic acid, linoleic acid, etc.) remain in blood products after leukoreduction
- Extracellular vesicle mediated
- In vitro studies have demonstrated a variety of proinflammatory and immunosuppressive interactions with monocytes, macrophages and neutrophils
- In vivo contribution is unknown
Clinical features
- Critically ill patients and patients with cancer may be at increased risk for complications of TRIM due to already dysregulated immune signaling (Sci Rep 2018;8:10186)
- Studies reporting seemingly paradoxical proinflammatory and immunosuppressive impacts from transfusion could be explained by recipient specific and contextual influences at the time of transfusion
- Immune response to critical illness is dynamic
Transmission
- Mononuclear cells and soluble mediators from white blood cells in nonleukoreduced allogeneic blood products
- Leukoreduction reduces the quantities of these but some residual white blood cells remain
- Soluble mediators also may persist despite removal of white blood cells
- Effect is still demonstrable in leukoreduced units and may be due to contributions from red blood cells, platelets, bioactive lipids and extracellular vesicles
- Reference: Best Pract Res Clin Anaesthesiol 2023;37:495
Symptoms
- Increased risk of
- Cancer recurrence (Transfus Apher Sci 2017;56:287)
- Opportunistic infections (Surg Infect (Larchmt) 2008;9:415)
- Postoperative complications (Circulation 1998;97:562)
- Improved outcomes in kidney transplantations (JAMA Netw Open 2023;6:e2332821)
Laboratory
- No specific findings
- In vitro features of an inappropriately paralyzed immune response to critical illness may be present (Transfusion 2017;57:195)
- Downregulated monocyte HLA-DR expression
- Reduced ex vivo production of TNFα in response to lipopolysaccharide (LPS) challenge
- Lymphocyte hyporeactivity with decreased IFNγ production in response to phytohemagglutinin or anti-CD3
- Can see changes related to complications of TRIM, such as infection or organ dysfunction
Case reports
- 9 year old boy with acute lymphoblastic leukemia (ALL), frequent blood transfusions and endogenous fungal endophthalmitis (BMC Ophthalmol 2011;11:30)
Treatment
- Leukoreduction of blood components is a key mitigation strategy
- Treatment of complications, such as infection or cancer progression, as otherwise clinically indicated
Sample assessment & plan
- Assessment:
- The patient is a 16 year old girl with osteosarcoma undergoing chemotherapy admitted with symptomatic anemia. She received 2 units of nonleukoreduced packed red blood cells. Within 72 hours of the transfusion, she developed a fever (39 °C), lactic acidosis and hypotension requiring vasopressor support. Despite aggressive antibiotic therapy, she developed acute kidney injury and hypoxic respiratory failure requiring ventilator support. Blood cultures are positive for fungal organisms.
- Impression:
- Multiple organ dysfunction from sepsis induced systemic inflammation and mitochondrial energy failure. Contributing factors including transfusion related immunomodulation from recent blood transfusion.
- Plan:
- Restrictive transfusion strategy with leukoreduced units
- Antibiotics and supportive care
Differential diagnosis
- Contributions solely from underlying disease
Board review style question #1
Which of the following modifications to blood products can help to reduce the risk of nosocomial infection development due to immunosuppression after allogeneic blood transfusion in the critically ill?
- Concentration of units
- Human leukocyte antigen (HLA) matching
- Leukoreduction
- Washing
Board review style answer #1
C. Leukoreduction. Leukoreduction is a key strategy to mitigate transfusion related immunomodulation (TRIM) effects. Answers D and A are incorrect because while washing and concentration will remove residual plasma from units and may decrease residual soluble mediators found in the plasma, leukoreduction has been associated with a multifold reduction in the quantity of immunosuppressive interleukins posttransfusion. Answer B is incorrect because HLA matching will not reduce the risk of TRIM.
Comment Here
Reference: Transfusion related immunomodulation (TRIM)
Comment Here
Reference: Transfusion related immunomodulation (TRIM)
Board review style question #2
Which of the following has been implicated as a mechanism of transfusion related immunomodulation (TRIM)?
- Downregulation of cytokines
- Intracellular vesicles
- Neutrophil priming via bioactivated lipids
- Upregulation of serotonin
Board review style answer #2
C. Neutrophil priming via bioactivated lipids. Neutrophil priming via bioactivated lipids is one of several mechanisms thought to lead to TRIM. Answer A is incorrect because upregulation of cytokines has been implicated in TRIM. Answer B is incorrect because extracellular vesicles, not intracellular, have been implicated in TRIM. Answer D is incorrect because upregulation of serotonin has not been associated with TRIM.
Comment Here
Reference: Transfusion related immunomodulation (TRIM)
Comment Here
Reference: Transfusion related immunomodulation (TRIM)
Variant Creutzfeldt-Jakob
Definition / general
- Prion disease results from the benign form of a prion protein changing to an insoluble protease resistant form → formation of plaques in brain
- Caused by prion protein that assumes an abnormal configuration, which promotes change to abnormal configuration of other proteins
Three forms of classic CJD:
- Sporadic (85%)
- Hereditary
- Acquired (through corneal transplants, pituitary gland derived growth hormone, dura mater transplants, inadequately sterilized brain electrodes)
- vCJD is human equivalent of "mad cow disease", due to epidemic of bovine spongiform encephalopathy, possibly due to prion in cattle entering human food supply
- In 1995, vCJD was documented in U.K.; transmission was through food chain
vCJD symptoms:
- Behavioral change, cerebellar ataxia and dementia; death in 7 - 38 months
- vCJD is rarely transmitted by transfusions of blood components but not plasma products
- As of 2007, in U.K., 66 individuals received transfusion products from donors who later developed vCJD; 3 have developed symptoms of vCJD, 1 had no symptoms of vCJD but evidence of abnormal prion proteins at autopsy for unrelated death
- In U.S., donors who have resided in U.K. or Europe are deferred
- Excluding transfusion recipients from donating blood is unlikely to change epidemiology of disease (Emerg Infect Dis 2007;13:89)
- References: epidemiologic study (Vox Sang 2006;91:221), Medscape: Variant Creutzfeldt-Jakob Disease and Bovine Spongiform Encephalopathy [Accessed 27 October 2017], Centers for Disease Control and Prevention: Variant Creutzfeldt-Jakob Disease (vCJD) [Accessed 27 October 2017]
Case reports
Variant Creutzfeldt-Jakob
- United Kingdom - confirmed (Lancet 2006;368:2061)
- Possible transmission (Lancet 2004;363:417)
- Preclinical disease in patient with unrelated death (Lancet 2004;364:527)
Viruses
CMV
- 50 - 85% of U.S. population is CMV+; 50% of U.S. donors are CMV seropositive, compared with 93% in Ghana (Ghana Med J 2006;40:99)
- Seroconversion rate is 1% per year
- CMV is almost always latent in immunocompetent adults; CMV virus is latent in leukocytes
- Leukoreduction reduces risk of CMV transmission
- Leukoreduced blood products are generally considered equivalent to CMV negative products
- CMV can cause pneumonitis, hepatitis, retinitis or organ failure in immunocompromised recipients, who should get CMV negative or leukoreduced blood components (Bone Marrow Transplant 2005;36:499, Transfus Med Rev 2005;19:181)
Hepatitis B
- Hepatitis B is a DNA virus, which is transmitted parenterally, sexually, perinatally
- Vaccination of infants and high risk adults led to a decrease in U.S. incidence from 260,000 to 60,000 new infections/year
- Vaccinated children may be susceptible in endemic areas (J Hepatol 2006;44:39)
- Hepatitis B infection can result in acute infection with subsequent clearance of virus and immunity OR chronic infection with persistent viremia
- Previously, hepatitis B transmission was most serious transfusion transmitted disease risk but due to serologic testing, risk of transfusion transmitted HBV is now 1:205,000 among repeat donors and 1:144,000 among all donors in the U.S., 1 per 1.3 million units in Australia (Intern Med J 2005;35:592), 1 per 640,000 in France (Transfus Clin Biol 2005;12:239), 1 per 150,000 in Canada (Transfusion 2007;47:316), 1 per 17,500 in Shenzhen, China (Transfusion 2007;47:529), 1 per 1,500 in Mexico (Rev Invest Clin 2006;58:101)
- Transfusion transmitted hepatitis B may be over reported or under reported
- To reduce rates even further, vigilance for errors and donor selection may be as important as further testing (Euro Surveill 2005;10:17)
- Value of nucleic acid testing (NAT) is controversial (helpful in high prevalence area - Vox Sang 2006;91:1, Transfusion 2005;45:1247; not helpful in low endemic areas, Mol Diagn Ther 2006;10:77)
- In U.S., minipool NAT testing does not decrease the window period significantly
- In U.S., blood banks use sensitive HBsAg and anti-HBc tests
- In most of the world, prevalence of anti-HBc is > 10% and use of HBc antibody test may exclude many otherwise healthy donors (J Clin Virol 2006;36:S33)
- Pathogen inactivation has eliminated transmission in U.S. licensed plasma derivatives since 1985; however cannot be used for cellular components (Arch Pathol Lab Med 2007;131:719)
- Only one confirmed transfusion related case in U.S. in 2003; false positive seroconversions may occur due to administration of immunoglobulin (Dtsch Med Wochenschr 2006;131:1325)
- Microarray multiplex assay may be useful to detect Hepatitis B, C and HIV (Biochem Biophys Res Commun 2007;356:1017)
Hepatitis C
- Hepatitis C virus is an RNA virus
- Transmitted parenterally, especially through blood transfusions (before testing) and intravenous drug use
- Historical risk for "non-A, non-B hepatitis" was 7% from volunteer donated blood and 28% from commercial blood
- 9% of Chinese patients with HCV due to unscreened blood have developed cirrhosis after a mean of 13 years (Zhonghua Gan Zang Bing Za Zhi 2006;14:199)
- Recommended to offer screening to patients (particularly children) who received blood products in 1992 (U.S.) or previously, when blood was not screened for HCV (Acta Paediatr 2007;96:1050, Transfusion 2007;47:615, Transfusion 2005;45:1020)
- Serologic testing and nucleic acid based testing have reduced risk, although developing countries may not screen (J Hepatol 2006;45:607)
In the U.S.:
- In 1970s, 10% of blood transfusion recipients had evidence of hepatitis C
- With current NAT testing, the risk of transfusion transmitted hepatitis C infection is 1:1.4 million products
- Documented breakthrough cases from nonreactive nucleic acid testing components are rare (Lancet 2000;355:41)
- Yield of HCV look back in Canada is likely zero when prior donations were tested by second or third generation EIA (Transfusion 2006;46:690)
- Identified in 1 per 250,000 donors in U.S. (Arch Pathol Lab Med 2007;131:702)
- Donors are usually asymptomatic
- Risk factors are injection drug use, hemodialysis, incarceration, receipt of blood products prior to HCV testing
- Also ozone enriched transfusion of autologous blood in Italy (Infect Control Hosp Epidemiol 2005;26:762)
- Antigen positive donors may not seroconvert (Transfusion 2000;40:1280)
- Pathogen inactivation has eliminated transmission in U.S. licensed plasma derivatives since 1985; however technique cannot be used for cellular components (Arch Pathol Lab Med 2007;131:719)
- References: Medscape: Pediatric Hepatitis C [Accessed 30 October 2017], Albert and Mary Lasker Foundation: Hepatitis C Virus and Its Detection in Blood for Transfusions [Accessed 30 October 2017]
HIV
- HIV is a lentivirus, a subgroup of the retrovirus family
- HIV is transmitted through sexual contact, childbirth, breast feeding and parenteral exposure to blood
- HIV transmission by blood products is efficient: infectivity is 90 - 100% for contaminated blood versus 0 - 2% for needlestick injuries (AIDS 2006;20:805)
- Transfusion associated HIV cases usually have an acute viral syndrome; if untreated, progress to AIDS in 10 years
- HIV1 and HIV2 both can cause AIDS; HIV2 is rare in U.S., with NO reported cases of transfusion transmission in U.S.
- Possibility of transfusion associated HIV is frightening to many patients but actual risk is only 1 per 2 million products tested for HIV1 and about 1 per 5 million - 8 million products transfused in U.S.
- Pathogen inactivation has eliminated transmission in U.S. licensed plasma derivatives since 1985; however technique cannot be used for cellular components (Arch Pathol Lab Med 2007;131:719)
- Identification of recipients of products from HIV+ donors is mandated by FDA
- Risk in France is 1 per 3 million (Euro Surveill 2005;10:5); in Ivory Coast, the risk is 1 per 6,000 (Transfus Clin Biol 2006;13:242)
- Current standard is serologic antibody testing (1 per 33,000 positive) plus nucleic acid testing (reduces window of seronegativity between time of infection and development of antibodies, Transfus Med 2007;17:200)
- Risk exists for blood donated through window of seronegativity, and 4 "breakthrough" cases have been identified (nonreactive by nucleic acid testing - Vox Sang 2004;86:171, Transfusion 2004;44:929)
- Most transfusion medicine litigation focuses on transfusion acquired HIV (Arch Pathol Lab Med 2007;131:615)
HTLV
- HTLV is transmitted by vertical transmission from mother to child, breast feeding, sexual relations, parenteral exposure
- HTLV1 infects mostly CD4+ lymphocytes while HTLV2 infects preferentially CD8+ lymphocytes
- HIV was originally called HTLV-III but no longer; HTLV3 describes another virus
- In U.S., seroprevalence is 10 - 20 per 100,000 donors
Prevalence in donors:
- Brazil HIV clinic - 2% (Sex Transm Dis 2006;33:302)
- California (U.S.) - 1 per 900,000 (Transfusion 2006;46:703)
- India - 0.2% (Indian J Pathol Microbiol 2006;49:532)
- Peruvian pregnant women - 2% (J Acquir Immune Defic Syndr 2006;42:604)
- Saudi Arabia - < 0.01% (Saudi Med J 2004;25:1419)
- Senegal - 0.2% (J Clin Microbiol 2006;44:1550)
- HTLV1 is associated with adult T cell leukemia / lymphoma; endemic in Caribbean and parts of Africa, Japan, South America
- Transfusion of RBCs, platelets and whole blood but not FFP, have led to seroconversion of recipients
- In U.S., seroconversion rates are 14 - 30% in recipients of seropositive cellular components
- Risk of transfusion transmission is 1 per 3 million products transfused
- Most positive donors are asymptomatic
- HTLVI is rare in U.S.; HTLV2 is common in IV drug users in U.S.
- Donors in U.S. are tested for HTLV1 and 2
- Leukoreduction may be effective in removing HTLV (Transfusion 2004;44:42 but see Blood 2002;100:677)
West Nile virus
- West Nile virus is a flavivirus primarily transmitted through mosquitoes, with birds as immediate hosts (Wikipedia: West Nile fever [Accessed 31 October 2017])
- Entered North America in 1999; still epidemic in North America due to mosquito and bird vectors
- First appeared in New York in 1999 and rapidly expanded its geographical area within 3 years
- Symptoms: headache, new rash, generalized weakness (Transfusion 2006;46:272)
- May cause meningoencephalitis (Am J Clin Pathol 2003;119:749)
- Transfusion transmission resulted in 23 infections in 2002 during an outbreak
- Detected by nucleic acid based testing although virus adheres to human red blood cells in whole blood (Clin Infect Dis 2007;45:181)
- NAT testing was implemented in 2003
- Use of only minipool NAT testing resulted in 7 transfusion transmission cases in 2003 - 2004
Cost effectiveness models:
- In areas with high levels of transmission, seasonal screening of individual samples and restricting screening to blood donations designated for immunocompromised recipients is cost effective
- In areas with low levels of infection, using a standard questionnaire is cost effective (PLoS Med 2006;3:e21, PLoS Med 2006;3:e99; see also Ann Intern Med 2005;143:486 and Ann Intern Med 2005;143:537)
- In 2003 - 2005, 1,425 infected blood donors were identified in U.S. (Transfusion 2006;46:2038)
- In 2003, only 6 cases of transfusion transmitted WNV were documented in U.S. from 12.6 million blood units donated and 23 million blood transfusions; fewer since strategy changed from minipool to individual nucleic acid testing method in epidemic areas (Dev Biol [Basel] 2007;127:43, N Engl J Med 2005;353:460)
- Viral inactivation systems may be useful (Vox Sang 2006;91:345, Antiviral Res 2005;68:84)
- Donor prevalence rates: Mexico - 0.03% (Transfusion 2006;46:111), Netherlands - 0% (Vox Sang 2006;90:166)
- Epidemiologic reports from Centers for Disease Control (2002 - 2007): MMWR 2002;51:790, MMWR 2002;51:823, MMWR 2002;51:884, MMWR 2002;51:973, MMWR 2003;52:769, MMWR 2003;52:916, MMWR 2004;53:281, MMWR 2004;53:842, Emerg Infect Dis 2005;11:1167, MMWR 2007;56:76
Warm autoantibody testing (pending)
[Pending]
Washing & volume reduction
Table of Contents
Definition / general | Essential features | Terminology | Clinical features | Case reports | Clinical images | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Washing of blood products replaces plasma with saline and reduces proinflammatory cells, molecules, additives and minerals
- Washing creates an open system and the unit expiration date is decreased
Essential features
- Blood product modification on red blood cell and platelet units to decrease proinflammatory cells, molecules, additives (mannitol) and minerals by replacing plasma with 0.9% sodium chloride (Transfusion 2011;51:955)
- Creates an open system; therefore, the unit expiration date is decreased to 24 hours or 4 hours, depending on how the unit is stored
- Indications for washing red blood cell (RBC) units include
- Hyperkalemia
- Removal of plasma proteins and inflammatory cells in patients who have had allergic transfusion reactions, including
- IgA deficient patients
- Patients with haptoglobinemia
- Red blood cell units for intrauterine transfusions
- Cryopreserved red blood cell units to remove glycerol
- Indications for washing platelet units
- Patients with allergic transfusion reaction history, unmitigated by antihistamines or steroids
- Decreases the volume of red blood cells and platelets
- Cell salvage - procedure performed in the operating room where shed blood is collected, washed or filtered and returned to the patient to reduce allogeneic transfusion
Terminology
- Washing: an automated or manual blood product modification process by which plasma is removed from platelet and red blood cell units and is replaced with saline solution (Transfusion 2011;51:955)
- Cell salvage: a process by which autologous blood is shed by a patient during a surgical procedure, collected, washed and then returned to the patient (Medicine (Baltimore) 2016;95:e4490)
Clinical features
- Washing of RBC can be performed either by manual or automated methods
- In a survey of 15 children's hospitals in the United States, 88% of hospitals used the COBE 2991 cell washer (see image below) (Transfusion 2011;51:955)
- In a small study of 8 RBC units, units washed on the COBE 2991 cell washer had the same or higher K+ concentrations at 24 hours after washing compared to prewash values (Transfusion 2011;51:955)
- Creates an open system and increases the risk of bacterial contamination
- Subsequently, the unit expiration date is decreased to
- 24 hours for products stored at 1 - 6 °C
- 4 hours for products stored at 20 - 24 °C
- Subsequently, the unit expiration date is decreased to
- Decreases the volume of a RBC unit by ~20 - 25% and a platelet unit by up to 33%
- Activates platelets and reduces platelet count recovery and survival (Anesth Analg 2004;99:17, Transfusion 2012;52:1221)
- Indications for RBC washing
- Hyperkalemia
- Defined as potassium (K+) concentration > 5.5 mmol/L
- Decreases the resting membrane potential of cardiac myocytes, leading to life threatening arrhythmias and cardiac arrest as K+ levels rise (Circ Arrhythm Electrophysiol 2017;10:e004667)
- Inhibition of the RBC membrane Na-K-ATPase pump in CPD-A or additive solution cold stored red blood cells results in leakage of K+ into the supernatant of red blood cell units
- Irradiation (gamma or Xray) further increases the K+ leak by damaging the red blood cell membrane (Transfus Med Rev 2022;36:133)
- Large volume transfusions in pediatric and adult patients may result in transfusion associated hyperkalemia (Transfus Med Rev 2022;36:133, Transfusion 2021;61:1093)
- Removes the supernatant and replaces it with sodium chloride solution
- Decreases the K+ volume
- Randomized controlled trials show conflicting results (Turk J Med Sci 2017;47:407, Eur J Cardiothorac Surg 2007;31:659)
- History of allergic transfusion reaction (ATR)
- Allergic transfusion reactions: type I hypersensitivity reactions where IgE antibodies interact with an allergen and cause activation of mast cells and basophils
- Biogenic amines, including histamine and adenosine, eosinophil and neutrophil chemotactic factors, bradykinin and proteoglycans mediate ATRs (Transfusion 2011;51:1676)
- Washing RBC and apheresis platelet units reduced the incidence of allergic transfusion reactions from 2.7% to 0.3% and 5.5% to 0.5%, respectively (Transfusion 2011;51:1676)
- In patients with IgA deficiency and haptoglobinemia, prior exposure results in antibodies to IgA or haptoglobin
- Future transfusions, with exposure to IgA or haptoglobin, result in allergic transfusion reactions
- Washing of RBC units and apheresis platelet units can decrease incidence of transfusion reactions (Transfusion 2018;58:2320, Ann Lab Med 2012;32:304, Transfusion 2015;55:2415)
- Allergic transfusion reactions: type I hypersensitivity reactions where IgE antibodies interact with an allergen and cause activation of mast cells and basophils
- Intrauterine transfusion (IUT)
- RBC units used for IUT should be group O, crossmatch compatible with maternal plasma, irradiated to prevent transfusion associated graft versus host disease, cytomegalovirus (CMV) safe (either leukoreduced or from a CMV seronegative donor), lacking Hgb S, collected within 5 - 7 days, washed or concentrated to a hematocrit of 70 - 85%
- In a survey of IUT practices in the U.S., 56% of 32 institutions always washed RBC units (Transfusion 2022;62:2449)
- Part of the deglycerolizing process
- RBC cryopreserved in glycerol (40% concentration) are thawed in a 37 °C water bath and then the glycerol needs to be removed via washing
- Glycerol is replaced with a 0.9% sodium chloride and 2% dextrose solution
- Hyperkalemia
- Indications for platelet unit washing
- Reducing exposure to antibodies against recipient antigens
- In neonatal alloimmune thrombocytopenia (NAIT), maternal antibodies against fetal platelet antigens, most commonly against HPA-1a, can cause severe thrombocytopenia
- Maternal platelets can be used for transfusion in the fetus or neonate and washing of the platelets can remove the maternal anti-HPA-1a antibodies (Transfusion 1982;22:125)
- History of allergic transfusion reaction (in particular, related to IgA deficiency / haptoglobinemia)
- In one study, washing apheresis platelet units reduced the incidence of allergic transfusion reactions from 5.5% to 0.5% (Transfusion 2011;51:1676)
- Reducing exposure to antibodies against recipient antigens
- Intraoperative cell salvage
- Blood shed during operative procedures is collected and either washed or filtered and then returned to the patient
- Cell salvage procedures have been shown to decrease the need for allogeneic RBC transfusions in cardiac, vascular and orthopedic surgery, among others (Medicine (Baltimore) 2016;95:e4490)
Case reports
- Neonate from a B r’r’ mother with anti-c and anti-S antibodies underwent RBC exchange for hyperbilirubinemia using 2 frozen antigen negative RBC units that were thawed and washed (Transfus Med 2019;29:128)
- 44 year old man who underwent emergent multivessel coronary artery bypass grafting requiring extracorporeal membrane oxygenation (ECMO), complicated by heparin induced thrombocytopenia (HIT) and disseminated intravascular coagulopathy (DIC), required massive transfusion as well as 19.7 L washed RBCs from cell salvage (Perfusion 2019;34:337)
- 50 year old woman, with relapsed acute promyelocytic leukemia and anhaptoglobinemia (deletion of Hp gene cluster) and haptoglobin antibodies, received washed apheresis platelet units (Ann Lab Med 2012;32:304)
- 88 year old woman with IgA deficiency, anti-IgA class IgG antibodies and monocytopenia and neutropenia after red blood cell transfusion, managed by red blood cell washing (Transfusion 2018;58:2320)
Sample assessment & plan
- Assessment: Jane Doe is a 78 year old woman with a history of residual / recurrent acute myeloid leukemia and a history of red blood cell transfusions. She developed an anaphylactic transfusion reaction 15 minutes into a red blood cell transfusion, requiring treatment with epinephrine, ICU transfer and ventilator support. Workup found IgA deficiency with anti-IgA class IgG antibodies.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of IgA deficiency with anti-IgA antibodies.
- The patient will be supported with washed red blood cell products. Please allow extra time to prepare this product (~2 hours).
- Limiting transfusions of plasma containing products is recommended.
Additional references
Board review style question #1
Board review style answer #1
A. 24 hours. A washed red blood cell unit expires 24 hours after it has been washed. Answers B - E are incorrect because the 24 hour expiration supersedes all other expiration time points. The shelf life for a unit collected in CPD-A is 35 days. An irradiated unit will expire 28 days after irradiation in Europe and 28 days from the irradiation date or the original expiration date in the U.S., whichever comes earlier. Red blood cell units collected in additive solutions have 42 day shelf lives.
Comment Here
Reference: Transfusion medicine - Washing
Comment Here
Reference: Transfusion medicine - Washing
Board review style question #2
For which patient would red blood cell washing be recommended?
- 26 year old man, O positive, with sickle cell disease with anti-C, anti-e, anti-Fyb antibodies who presents for his monthly red blood cell exchange of 6 red blood cell units and the blood bank has 6 antigen negative liquid units available
- 54 year old man with aplastic anemia requiring transfusion support with history of multiple previous mild allergic transfusion reactions
- 78 year old woman on dialysis with recent myocardial infarction and peripheral edema
- Intrauterine transfusion for a fetus with hematocrit of 25% in a mother with anti-D titer of 1:256
- Neonate being placed on extracorporeal membrane oxygenation (ECMO) and fresh red blood cell units are available
Board review style answer #2
D. Intrauterine transfusion for a fetus with hematocrit of 25% in a mother with anti-D titer of 1:256. RBCs used for intrauterine transfusion should be group O and crossmatch compatible with maternal plasma, be irradiated to prevent transfusion associated graft versus host disease, be CMV risk reduced (leukoreduced or from a CMV negative donor) and lack hemoglobin S. Units can be washed or concentrated to a hematocrit of 70 - 85%.
Answer A is incorrect because patients with sickle cell disease who have developed multiple alloantibodies may need rare cryopreserved red blood cell units. In this current case, the patient's red blood cell needs can be supported by available liquid units but if he is further alloimmunized and requires rare cryopreserved red blood cell units, cryopreserved units need to undergo washing to remove the glycerol preservative prior to being transfused.
Answer B is incorrect because a 54 year old man with history of mild allergic transfusion reactions does not need washed products at this time. If he has an anaphylactic transfusion reaction, that would be an indication for washed RBC units.
Answer C is incorrect because this patient is exhibiting risk factors for transfusion associated circulatory overload (TACO). Washing of RBCs can mitigate transfusion related acute lung injury (TRALI), possibly by removing proinflammatory cells and molecules stored in RBCs. Washing does not mitigate TACO. Recommended methods for TACO mitigation include judicious transfusion, slow transfusion rate (no greater than 4 hours) and diuresis.
Answer E is incorrect because a neonate on ECMO can be supported with fresh red blood cell units. As red blood cell units age, they leak potassium, which can cause cardiac arrhythmias in neonates. If no fresh red blood cell units are available, washed RBC units can be used.
Comment Here
Reference: Transfusion medicine - Washing
Answer A is incorrect because patients with sickle cell disease who have developed multiple alloantibodies may need rare cryopreserved red blood cell units. In this current case, the patient's red blood cell needs can be supported by available liquid units but if he is further alloimmunized and requires rare cryopreserved red blood cell units, cryopreserved units need to undergo washing to remove the glycerol preservative prior to being transfused.
Answer B is incorrect because a 54 year old man with history of mild allergic transfusion reactions does not need washed products at this time. If he has an anaphylactic transfusion reaction, that would be an indication for washed RBC units.
Answer C is incorrect because this patient is exhibiting risk factors for transfusion associated circulatory overload (TACO). Washing of RBCs can mitigate transfusion related acute lung injury (TRALI), possibly by removing proinflammatory cells and molecules stored in RBCs. Washing does not mitigate TACO. Recommended methods for TACO mitigation include judicious transfusion, slow transfusion rate (no greater than 4 hours) and diuresis.
Answer E is incorrect because a neonate on ECMO can be supported with fresh red blood cell units. As red blood cell units age, they leak potassium, which can cause cardiac arrhythmias in neonates. If no fresh red blood cell units are available, washed RBC units can be used.
Comment Here
Reference: Transfusion medicine - Washing
Whole blood therapy
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission | Screening | Blood donor screening | Blood donor selection | Laboratory | Treatment | Sample assessment & plan | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Cold, stored whole blood (WB) may be stored at 1 - 6°C for up to 35 days (should be distinguished from fresh, warm whole blood)
- Group O whole blood is used primarily for the resuscitation of trauma patients with massive bleeding
Essential features
- Used primarily in the treatment of trauma patients with massive bleeding
- Group O units can be used emergently across patient ABO types, making it practical as the initial resuscitation product
- Risk of hemolysis can be mitigated by using units that have low anti-A and anti-B titers
- May be effective tool for prehospital transfusion
Terminology
- CSWB: cold, stored whole blood
- LTOWB: low titer group O whole blood
- WFWB: warm, fresh whole blood
Pathophysiology
- Stored for 21 days at 1 - 6°C in CPD and CP2D anticoagulant or for 35 days at 1 - 6°C in CPDA-1 (AABB: Circular of Information for the use of human blood and blood components [Accessed 9 November 2020])
- Cold storage may improve the hemostatic function of platelets due to increased in vivo aggregation and superior correction of bleeding time of cold-stored relative to warm-stored platelets (J Trauma Acute Care Surg 2014;77:S114)
- More concentrated product contains less anticoagulant and additive solutions when compared with equivalent doses of component therapy with red blood cells (RBCs) stored in additive solutions (J Trauma 2009;66:S69)
- American Association of Blood Banks (AABB) standards allow the use of whole blood for non group O patients or patients with unknown ABO group (American Association of Blood Banks: Standards for blood banks and transfusion services, 31st Edition, 2018)
- WB from group O donors contains RBCs that are compatible with all recipients but the plasma in group O whole blood contains anti-A and anti-B antibodies that could potentially cause hemolysis in a non group O recipient (minor mismatch)
Clinical features
- Delivers all components in a single product, providing early inclusion of plasma in trauma patients to address the coagulopathy of trauma (JAMA 2015;313:471)
- Group O units can be used emergently across patient ABO blood types, making it practical as the initial resuscitation product in appropriate patients
- May be effective tool for prehospital transfusion (JAMA Surg 2016;151:15)
- Patients should be monitored for hemolysis after transfusion
- Risk of hemolysis can be mitigated by using units that have low anti-A and anti-B titers (low titer O whole blood)
Transmission
- WB may reduce the number of donor exposures and may reduce risk of transfusion transmitted infections
Screening
- All blood for transfusion is tested for the presence of certain infectious disease pathogens
- Titers are typically performed with each collection
- Low-titer group O whole blood (LTOWB) units are titered for both Anti-A and Anti-B antibodies; each institution is responsible for determining its acceptable titer but most are below 256 (Transfusion 2020;60:S45)
Blood donor screening
- Armed Services Blood Program collects whole blood from male and never pregnant female donors or from female donors testing negative for anti-human leukocyte antigens antibodies (this mitigates risk of transfusion associated acute lung injury, transfusion related acute injury, and is an AABB / FDA requirement)
- WB is collected from both Rh positive and negative donors (Mil Med 2018;183:44)
Blood donor selection
- Donors are TRALI risk mitigated
- Males and females who have never been pregnant
- Rh positive and negative
Laboratory
- Cold, stored whole blood may be stored at 1 - 6°C for up to 35 days (should be distinguished from fresh, warm whole blood)
Treatment
- Used primarily in the treatment of trauma patients with massive bleeding
- Protocols that use whole blood beyond day 21 should consider the need for supplemental platelet transfusion
Sample assessment & plan
- A: 27 year old man presents to a level I trauma center with several injuries after motor vehicle collision. SBP is < 90mmHg and EBL > 150 mL per minute. No labs including CBC and type and screen have been resulted.
- P: Initiate massive transfusion protocol with low titer group O whole blood. Switch to component based goal directed therapy when sufficient hemostasis has been achieved and allows for proper use of visoelastic and traditional coagulation testing. Send labs to monitor for the presence of hemolysis.
Additional references
Board review style question #1
How can the risk of hemolysis associated with the transfusion of group O whole blood to non group O recipients be mitigated?
- Agitating units in storage
- Leukoreduction
- Selecting units with low anti-A and anti-B titers
- Using units less than 7 days old
- Washing
Board review style answer #1
C. Selecting units with low anti-A and anti-B titers. The risk of hemolysis is due to the presence of anti-A and anti-B antibodies binding to recipient red cells. Selecting for low titers should minimize the risk. Washing would remove the plasma that is needed to provide coagulation factors that treat the trauma induced coagulopathy. Whole blood units can be leukoreduced but this does not impact hemolysis. Age of the units does not impact hemolysis. Whole blood is stored without agitation.
Comment Here
Reference: Whole blood therapy
Comment Here
Reference: Whole blood therapy
Zika virus
Table of Contents
Definition / general | Essential features | Transmission | Symptoms | Screening | Blood donor screening | Blood donor testing | Donor deferral | Case reports | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Zika virus (ZIKV) is a mosquito borne virus that is transmissible via blood transfusion (U.S. Food and Drug Administration: Information for Blood Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Transfusion-Transmitted Infection [Accessed 12 August 2021])
- Although most patients are asymptomatic, the disease causes severe fetal malformations (microcephaly)
- Testing and deferral periods are required by the Food and Drug Administration
Essential features
- Zika virus is a mosquito borne infectious disease
- It is also transmissible by transfusion, blood exposure and sexual intercourse
- Most infected are asymptomatic; symptoms are a flu-like illness with conjunctivitis and maculopapular rash
- Associated with severe birth defects such as microcephaly
- Donors are tested via nucleic acid testing and if positive, are deferred for 120 days
Transmission
- Primarily mosquito borne (Aedes aegypti > Aedes albopictus) (Centers for Disease Control and Prevention: Zika Virus: Prevention and Transmission [Accessed 1 July 2019])
- Other routes: perinatal, intrauterine, sexual, breast milk, laboratory acquired, transfusion transmission
- Not transmitted through saliva or urine
- First mosquito borne transmission in the United States: Puerto Rico, December 2015
- Also reported in Florida and Texas in 2016
Symptoms
- Most infected (80%) are asymptomatic or have mild symptoms lasting up to 1 week (Bull World Health Organ 2018;96:402)
- Most common symptoms are fever, conjunctivitis, maculopapular rash, arthralgia, myalgia, headache
- Usually self limiting
- Neurologic manifestations, including Guillain-Barré syndrome, have been reported
- Association of Zika infection during pregnancy and fetal malformations, microcephaly
Screening
- ZIKV can be detected in serum or plasma for 1 - 2 weeks after infection (N Engl J Med 2017;379:1234)
- Median value for ZIKV RNA persistence:
- Serum / plasma: 11 - 17 days
- Semen: 28 - 41 days
- Urine: 6 - 10 days
- Whole blood and red blood cells: up to 3 months
Blood donor screening
- If a donor volunteers a recent infection with Zika, blood or blood components must not be collected and the donor is deferred for 120 days (U.S. Food and Drug Administration: Information for Blood Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Transfusion-Transmitted Infection [Accessed 12 August 2021])
- Blood donor screening questions have not been determined to be an effective method of preventing infected asymptomatic individuals from donating
Blood donor testing
- Only approved method is the individual donor nucleic acid test for Zika virus (Roche Molecular Systems)
- Minipool nucleic acid testing (N Engl J Med 2018;378:1778)
- A positive minipool nucleic acid test must be resolved using individual donor nucleic acid testing or pathogen reduction of blood components (only plasma and platelets are Food and Drug Administration approved at this time)
- Individual donor nucleic acid testing is required for defined geographic collection areas at times of higher risk (i.e. suspected or documented Zika infection) as defined by positive ZIKV testing in a nearby blood center, Centers for Disease Control and Prevention or other surveillance notification
- Converting to individual donor nucleic acid testing is required within 24 hours of notice
Donor deferral
- Donor with confirmed positive ZIKV individual donor nucleic acid test is deferred for 120 days (U.S. Food and Drug Administration: Information for Blood Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Transfusion-Transmitted Infection [Accessed 12 August 2021])
- Donor must be notified and counseled regarding the medical significance of the results
- All in date blood products (including those of a prior donation) must be retrieved and quarantined
- Physicians who transfused any components from the infected donor collected during a prior donation (120 days prior) should be encouraged to notify the transfusion recipient
- Females of childbearing age should be advised that becoming pregnant is not recommended for 8 weeks from exposure
- Males should be advised that Zika is transmitted in semen; unprotected sex should be avoided for 6 months from infection
Case reports
- 25 year old woman who contracted Zika in first trimester had fetus with intrauterine growth retardation and severe microcephaly (N Engl J Med 2016;374:951)
- 44 year old man with potential sexual transmission of Zika virus (Emerg Infect Dis 2015;21:359)
- Probable transfusion transmitted Zika virus in Brazil (Hematol Transfus Cell Ther 2018;40:250)
Additional references
Board review style question #1
Postinfectious disease screening is positive for a 42 year old male blood donor who donated 2 days ago. The testing is positive for Zika virus by individual donor nucleic acid testing. You call the donor to inform him that he is now deferred. Because the donor is male, it is important to inform him of which of the following?
- Zika virus can be sexually transmitted for 6 months in semen
- Zika virus can be transmitted through urine, so he should not use a public restroom for 10 days
- Zika virus is latent and he will never clear the infection
- Zika virus is not contagious outside of blood transfusion and mosquito bites
Board review style answer #1
A. Zika virus is sexually transmitted and can cause fetal malformations in pregnancy. It is detectable in semen for 6 months.
Comment Here
Reference: Zika virus
Comment Here
Reference: Zika virus
Board review style question #2
You receive a letter from your blood provider, stating that a donor who last donated 8 weeks ago returned and the donor is now positive for Zika virus by individual donor nucleic acid testing. The donor was negative at the time of the transfusion. The blood donor is asymptomatic. A review of records reveals this unit was transfused to a 22 year old female postdelivery of a full term male infant. When you call the recipient to inform her of the exposure, she asks you what main risk of the disease is. You tell her
- She is at an increased risk of developing Guillain-Barré disease
- She may have a flu-like illness with fever, conjunctivitis, rash and headache
- There is no risk to her since the donor was negative for Zika at the time of donation
- This virus causes birth defects, so she should avoid pregnancy for at least 8 weeks
- This virus is transmissible through breast milk, so she should talk to her pediatrician
Board review style answer #2
E. This virus is transmissible through breast milk, so she should talk to her pediatrician. The mother already delivered at the time of transfusion, so it should not have been transmitted to her infant perinatally. It is transmissible through breast milk, though in general, the benefits of breastfeeding are thought to outweigh the risk of Zika transmission in an otherwise healthy infant. She should discuss this with her pediatrician.
Comment Here
Reference: Zika virus
Comment Here
Reference: Zika virus
Recent Transfusion medicine Pathology books
Find related Pathology books: hematopathology, lab medicine, other, transfusion, management