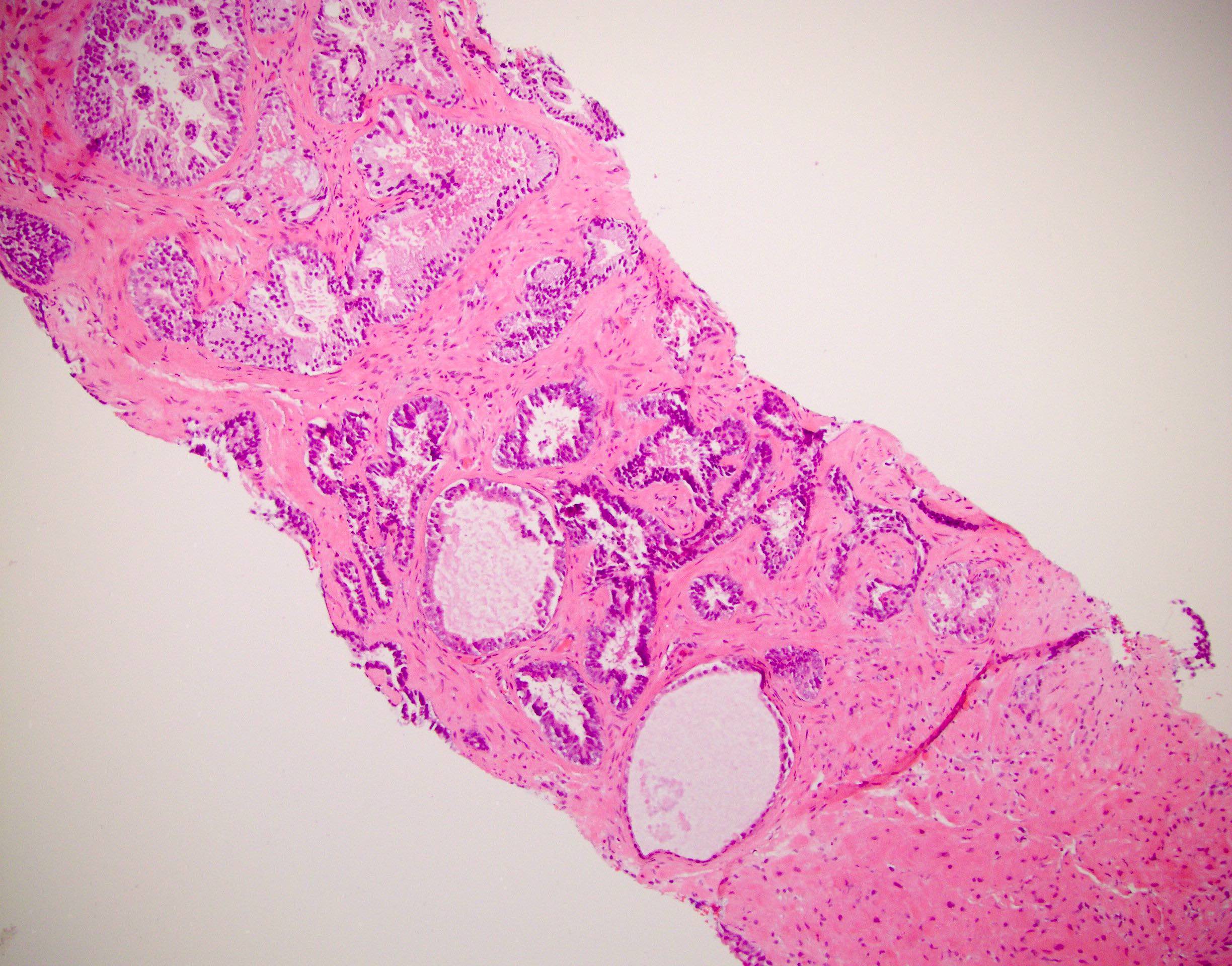
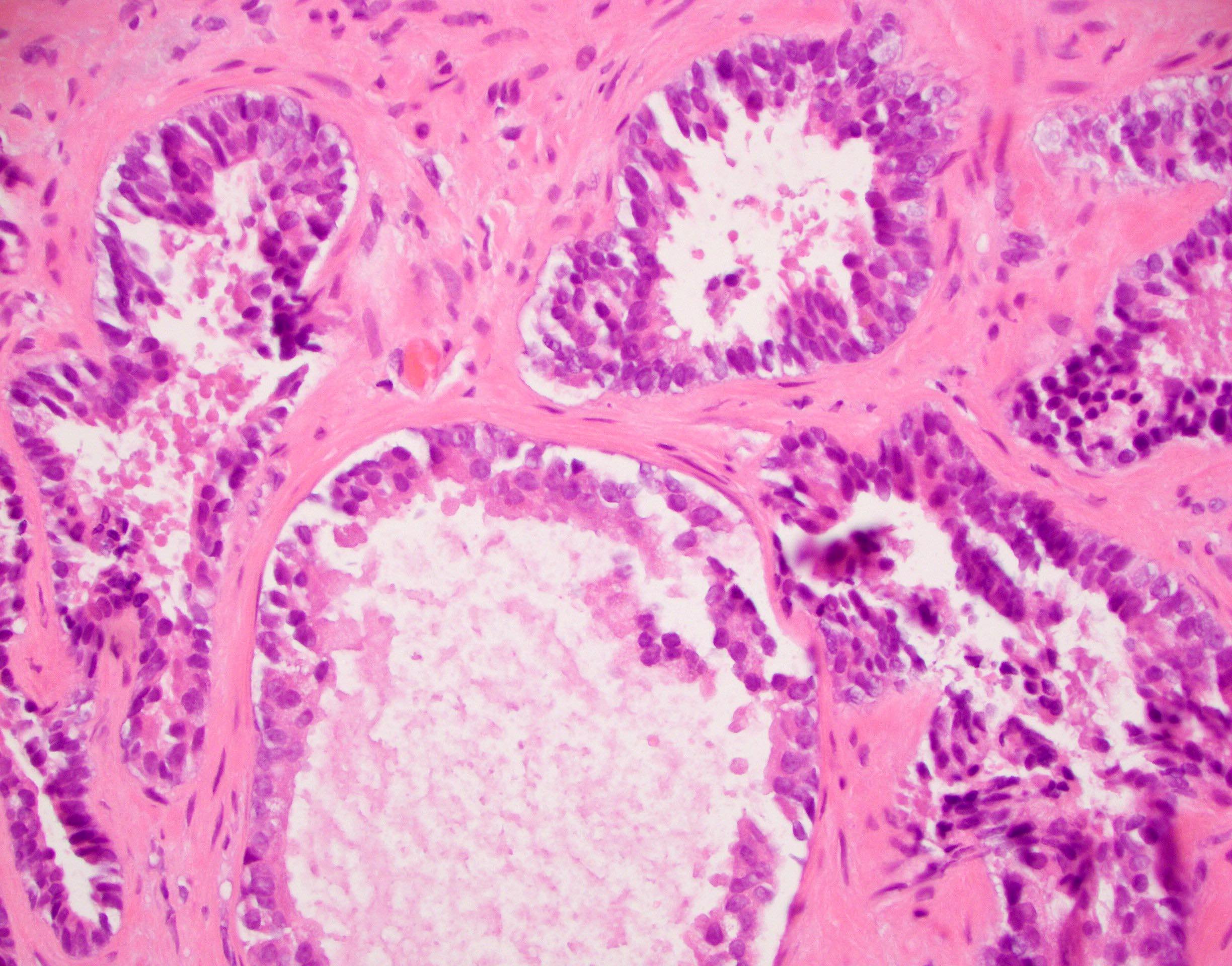
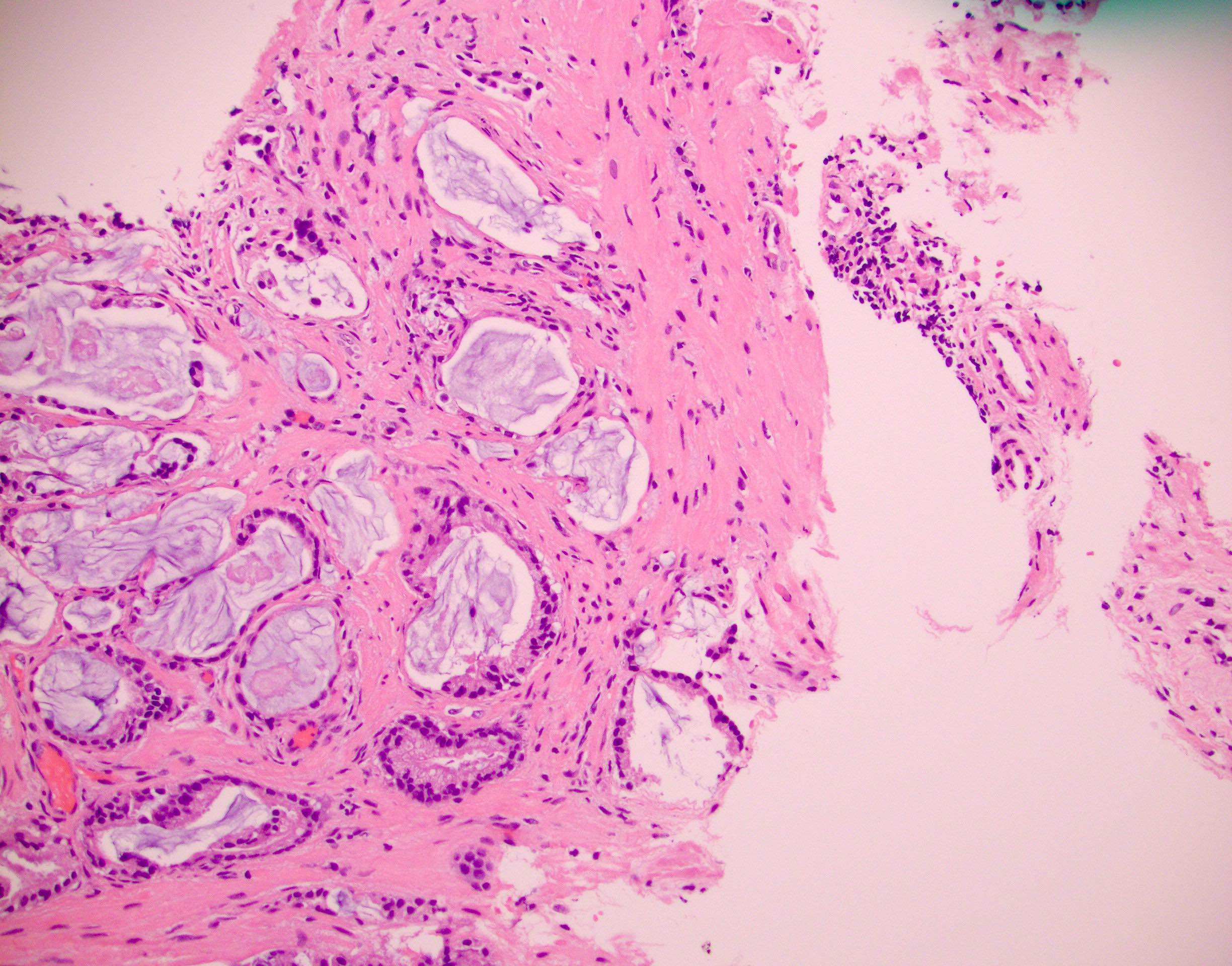
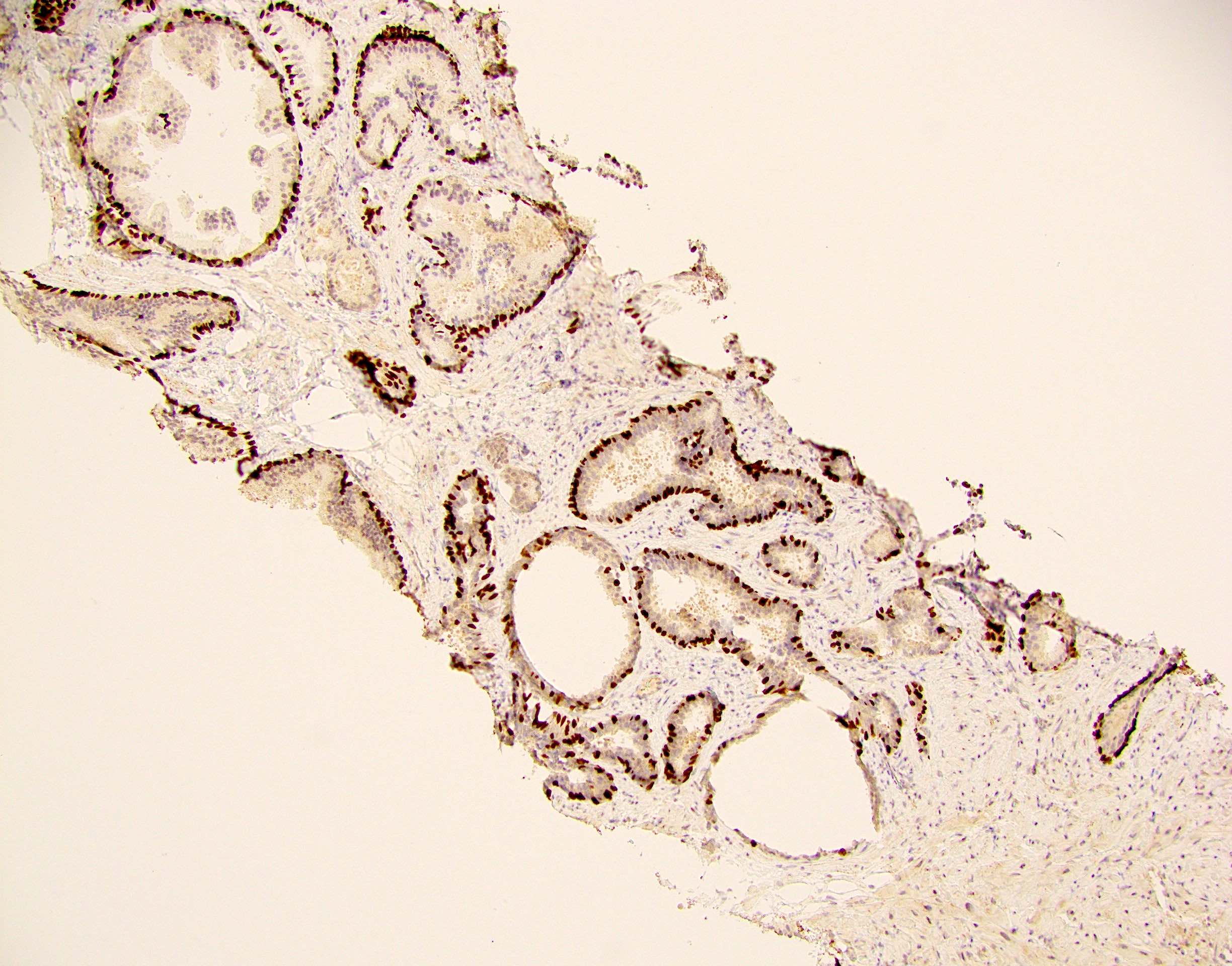
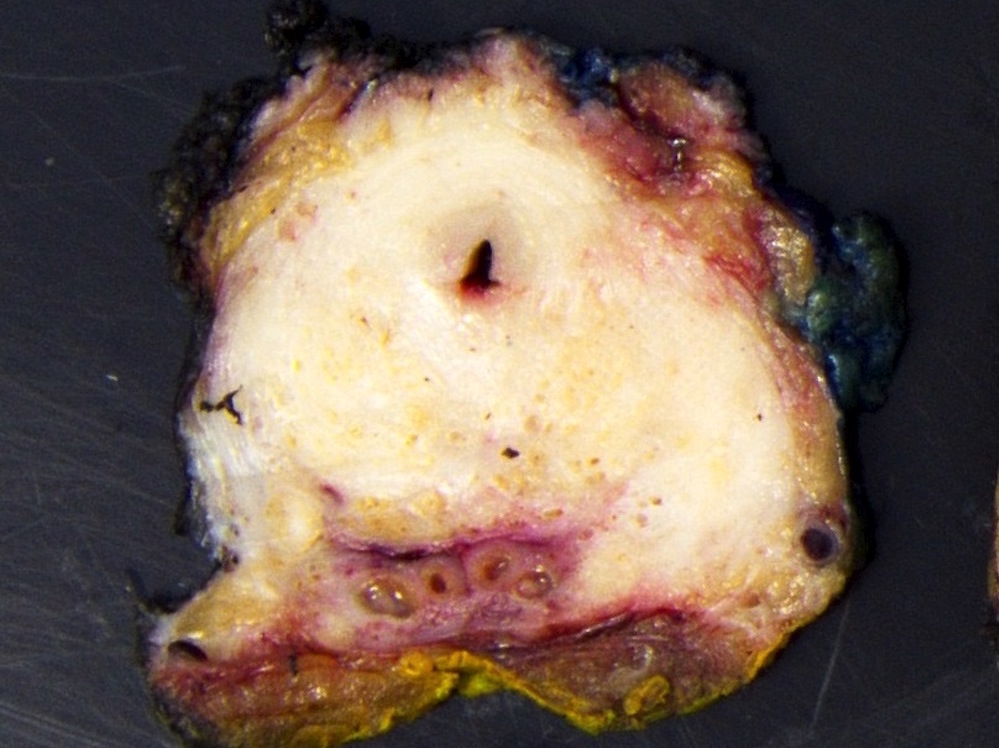
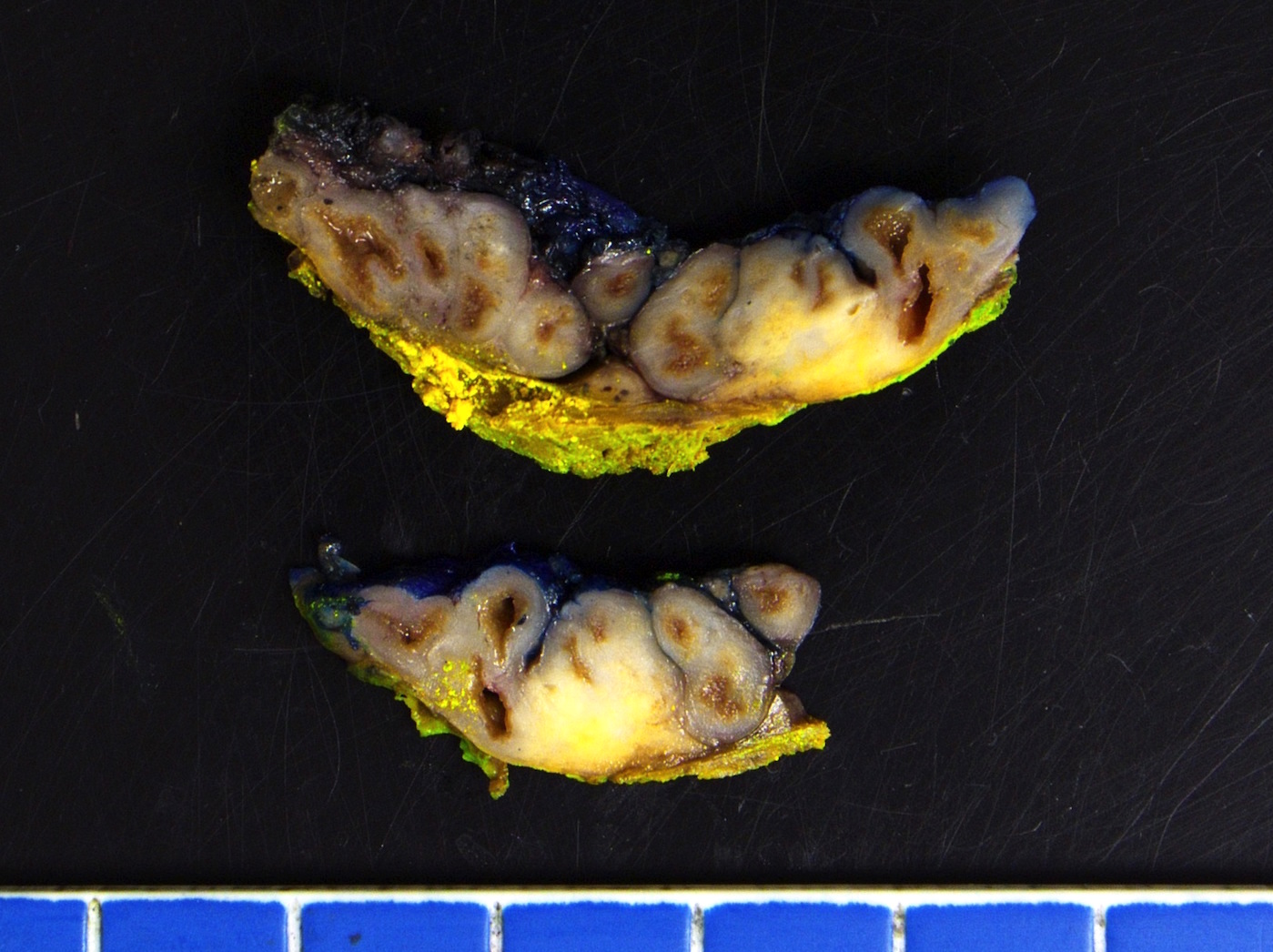
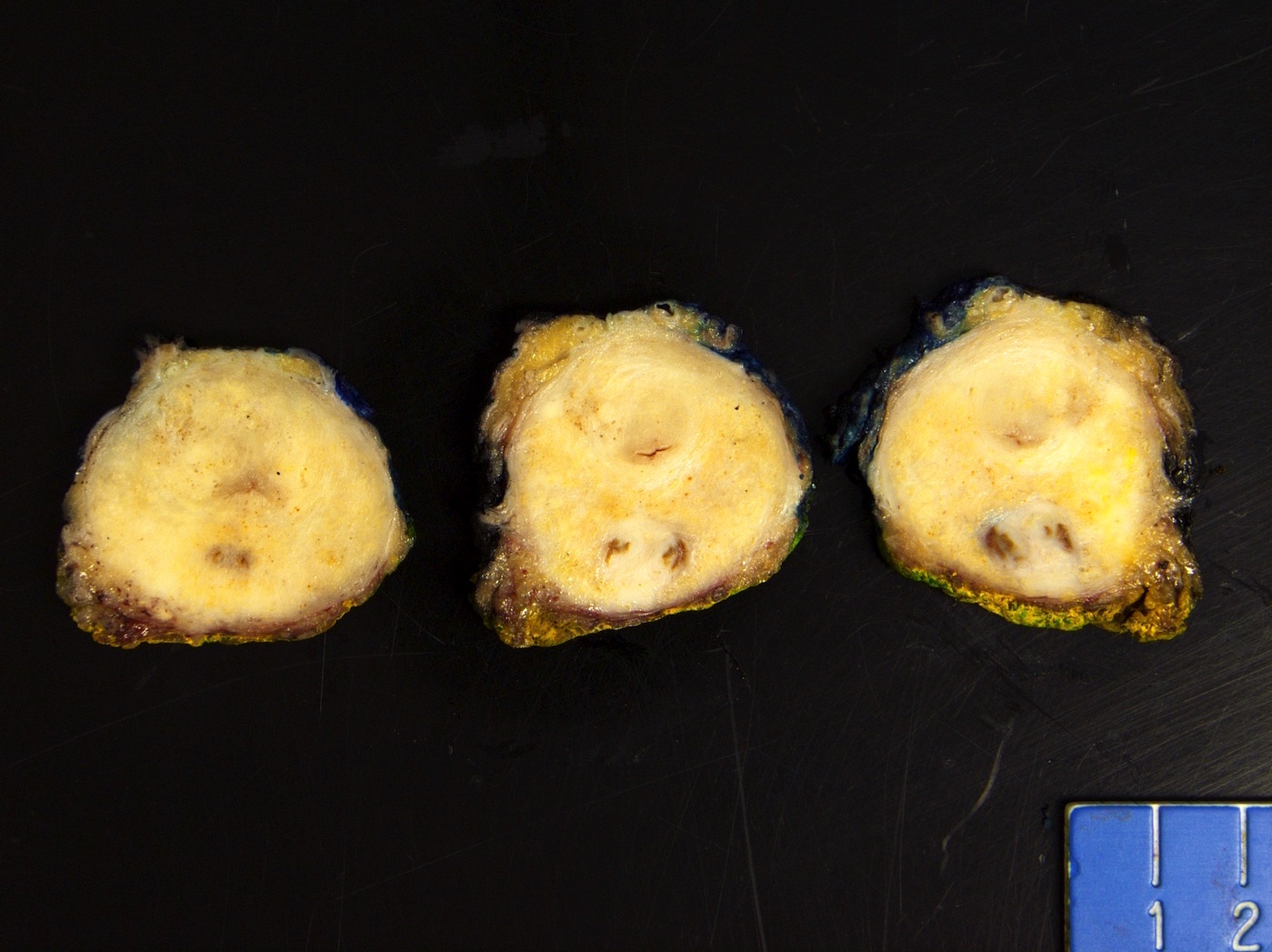
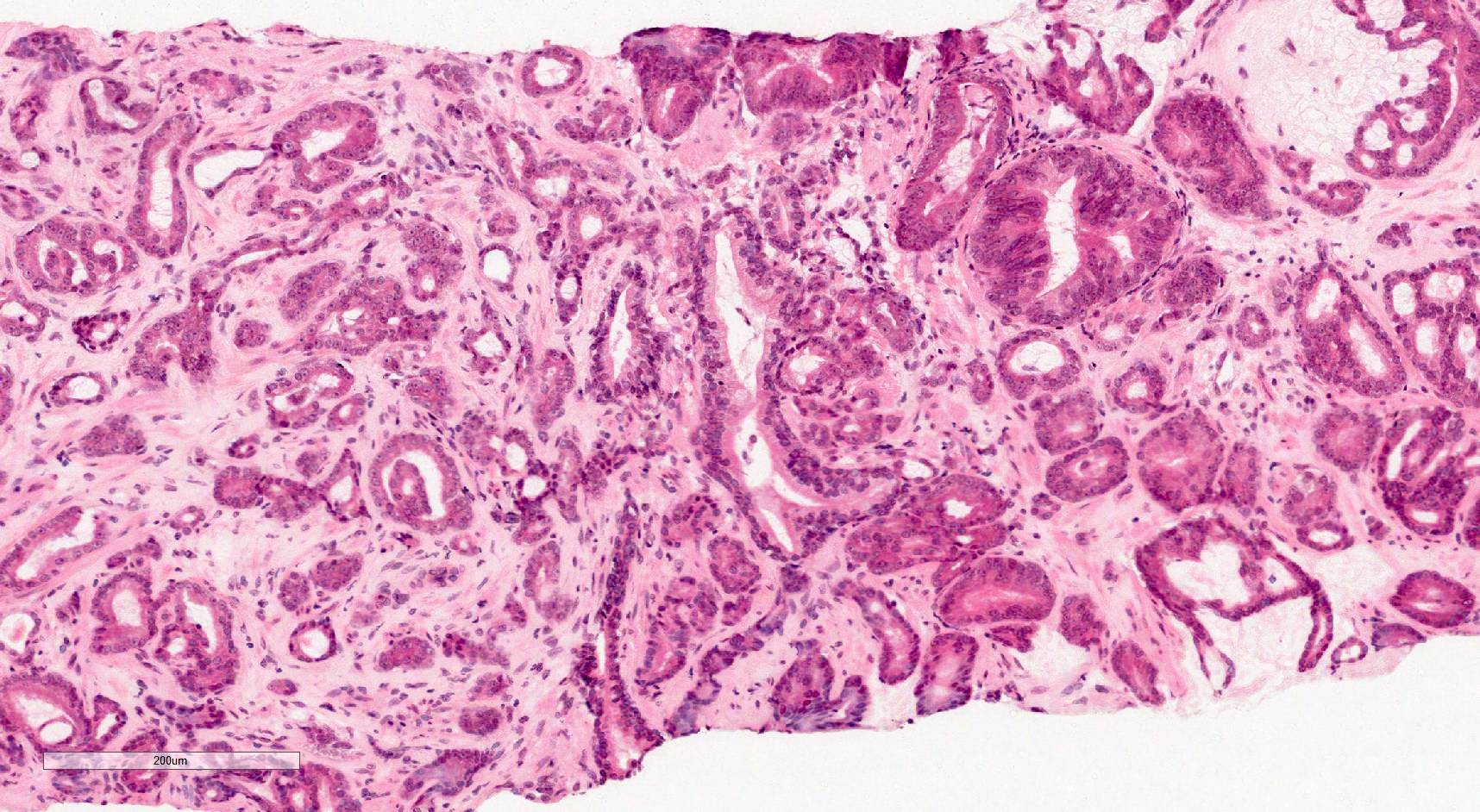
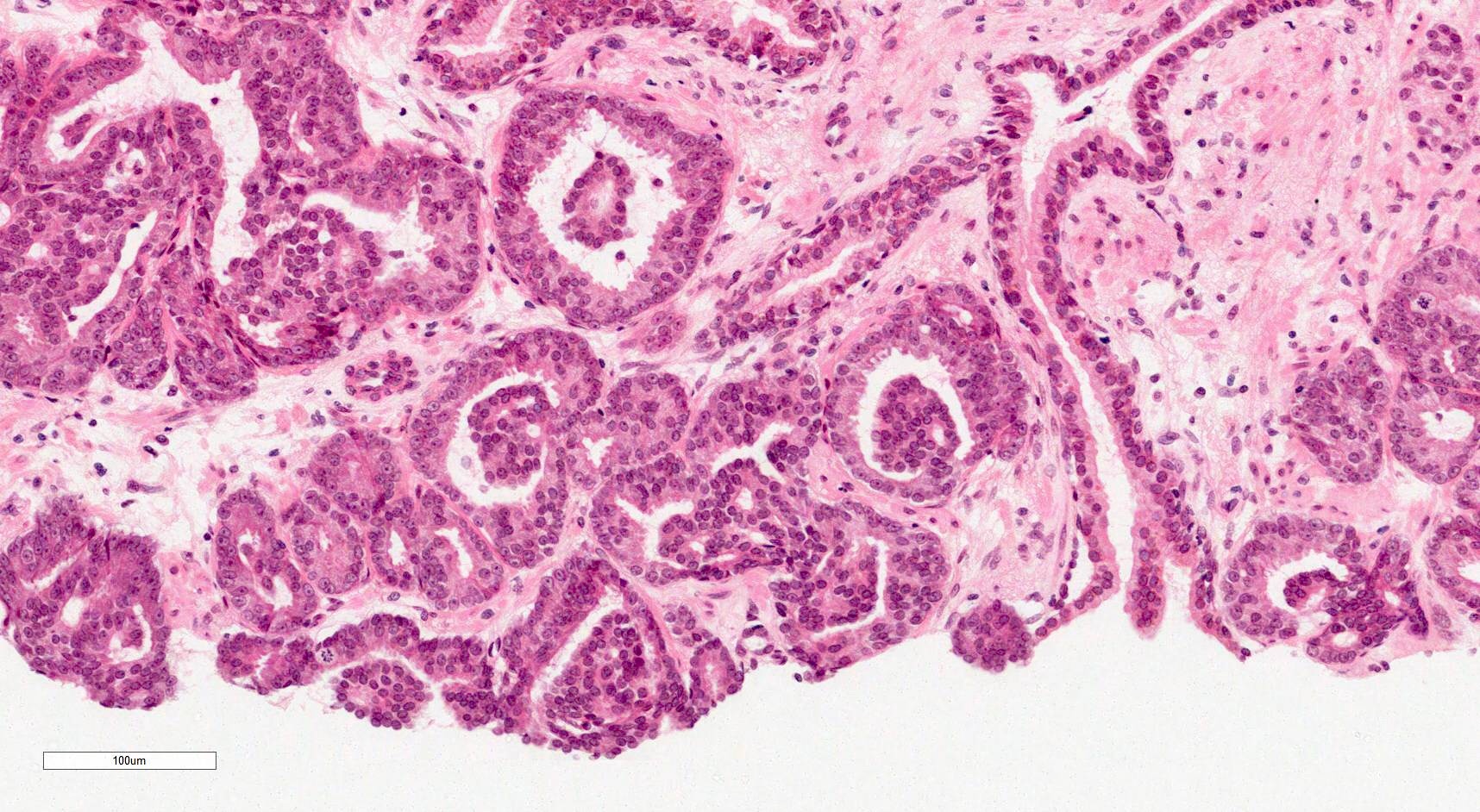
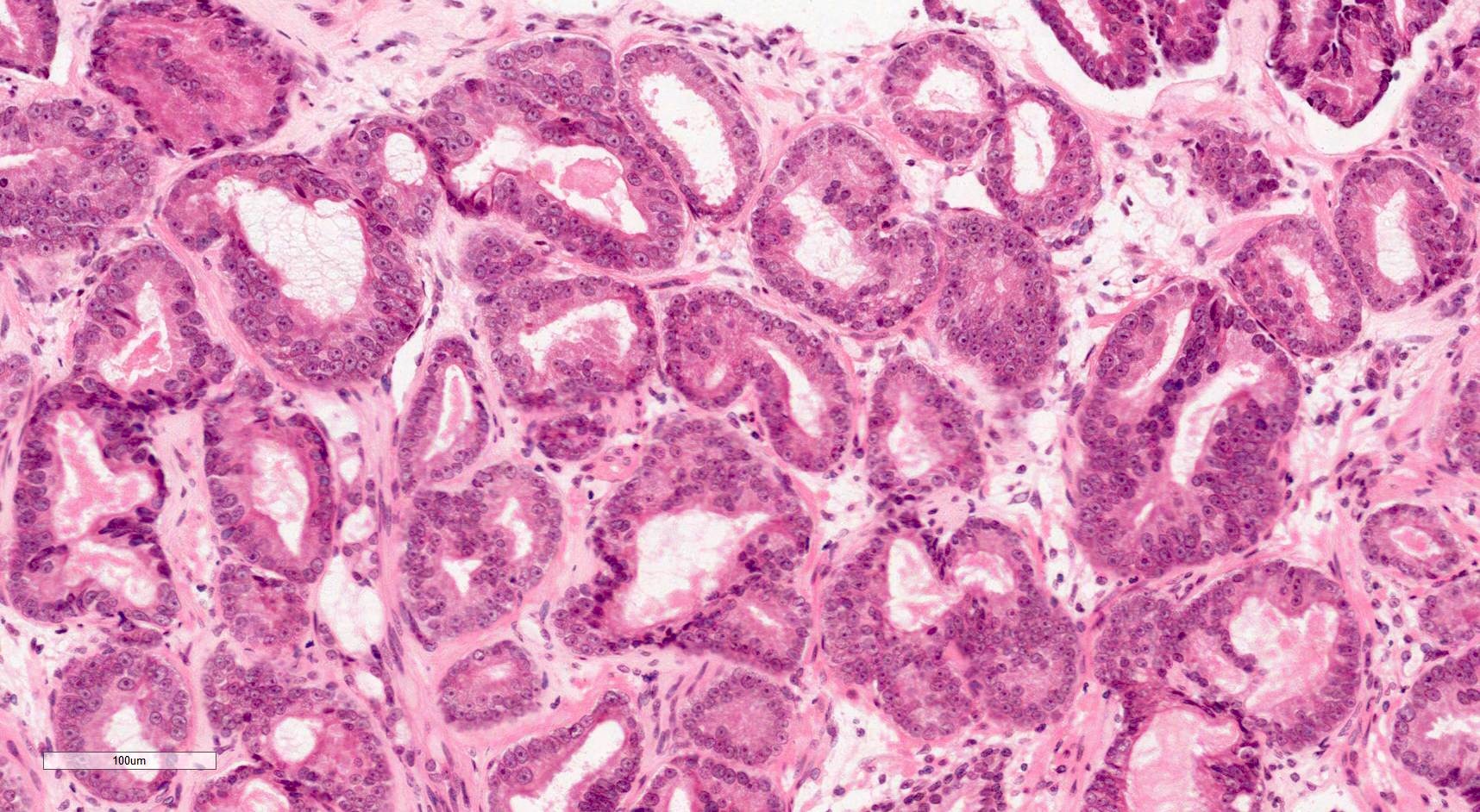
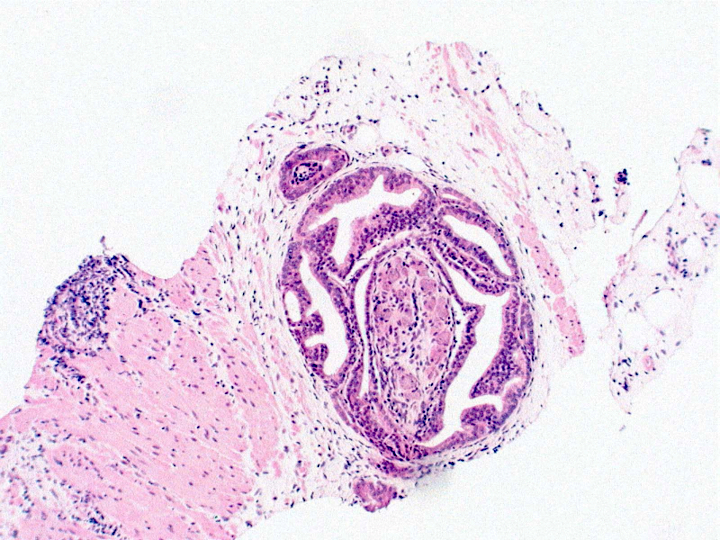
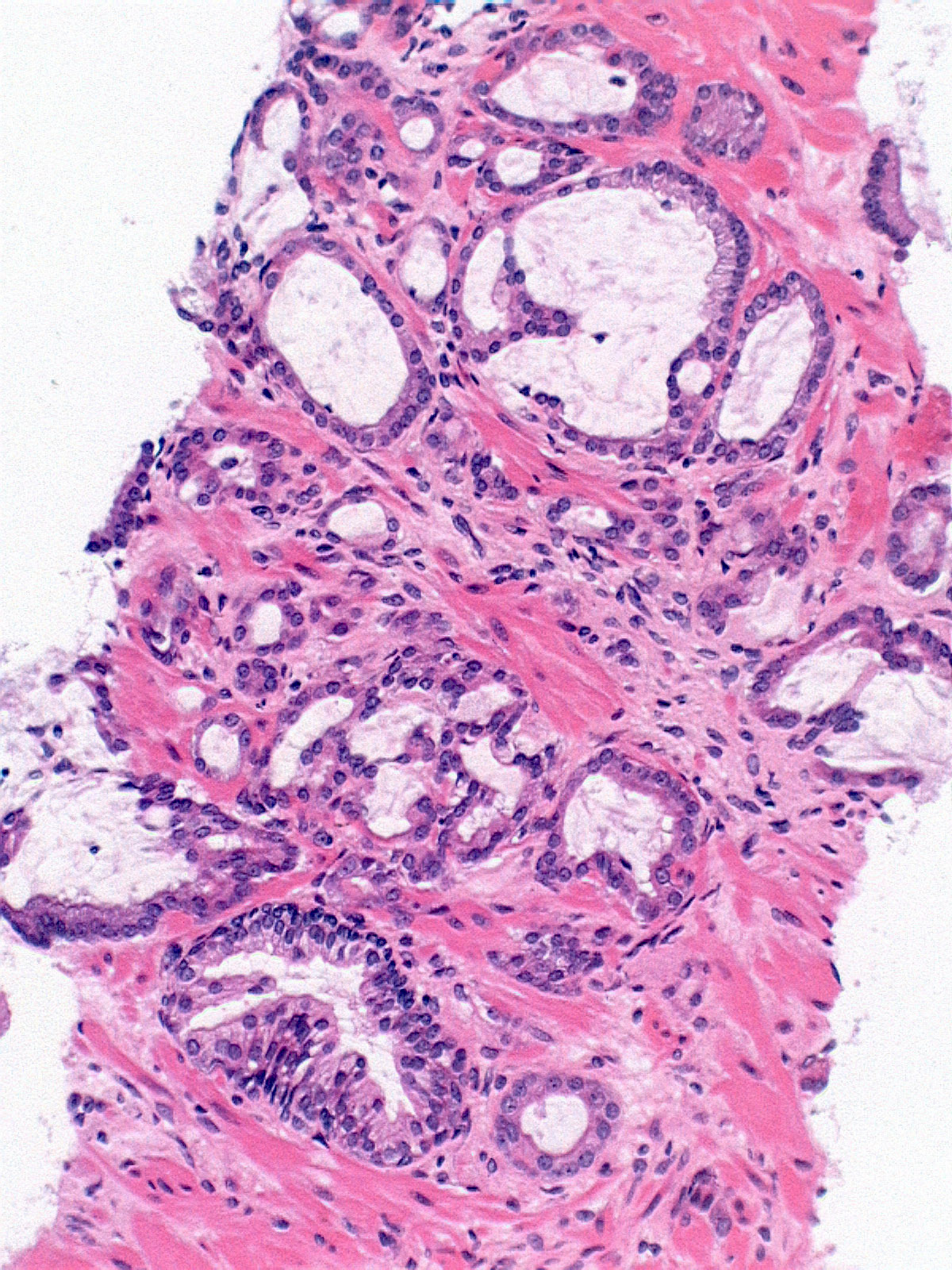
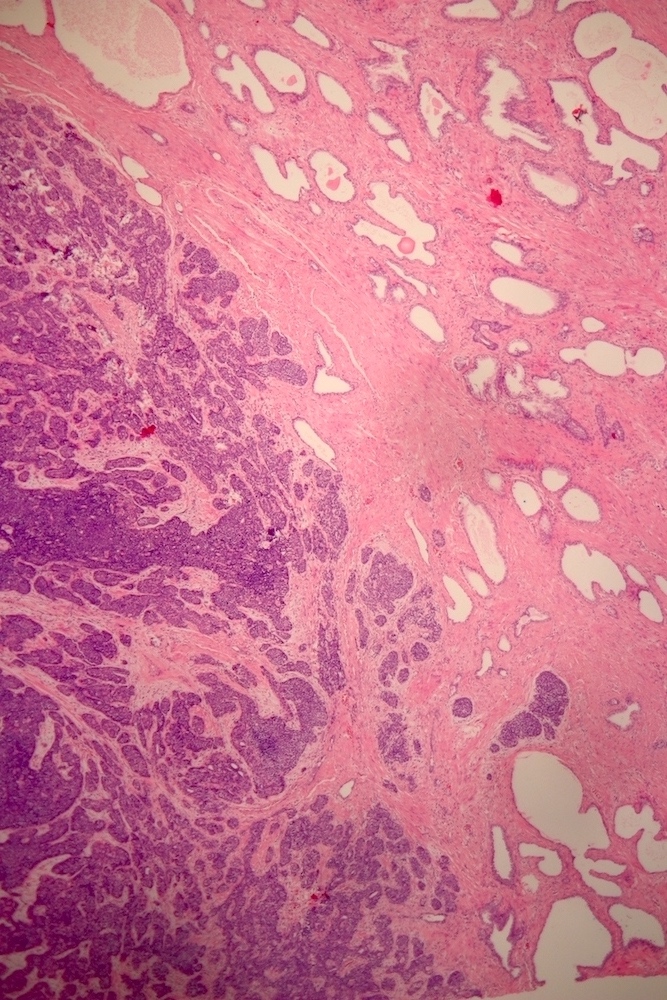
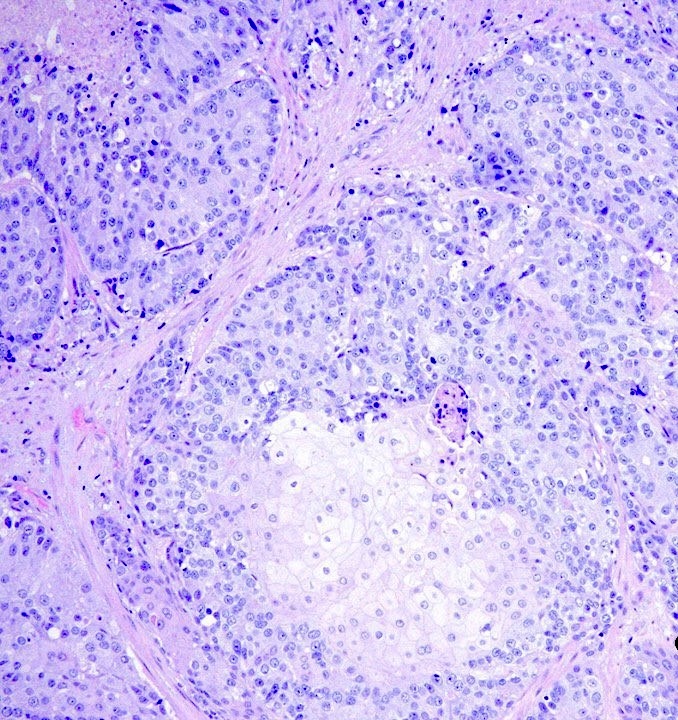
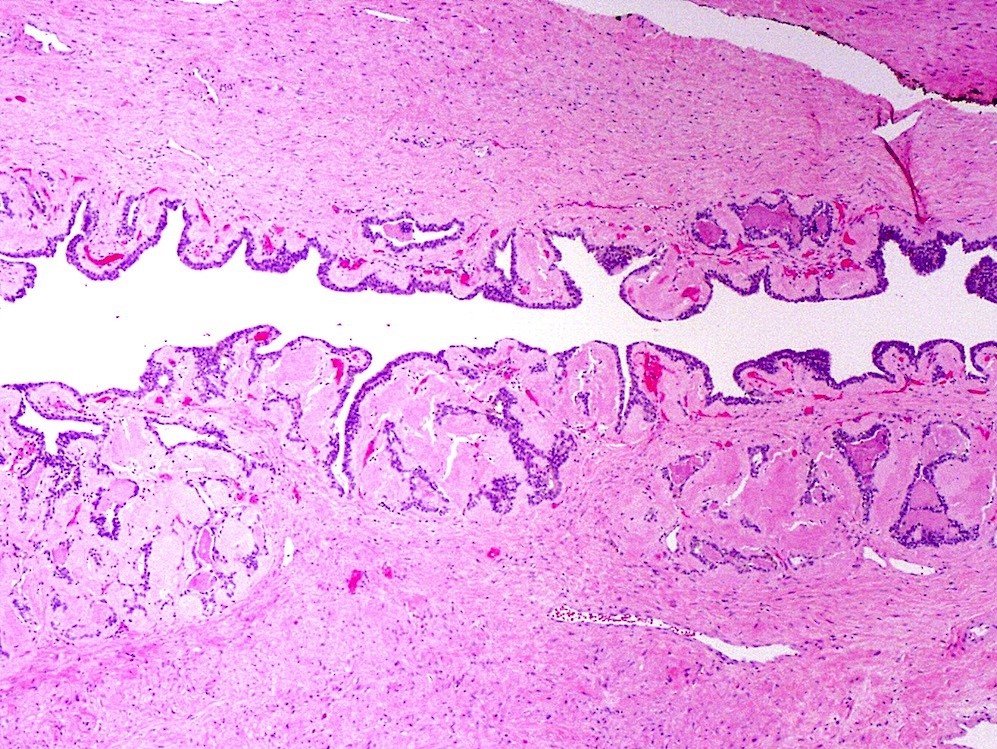
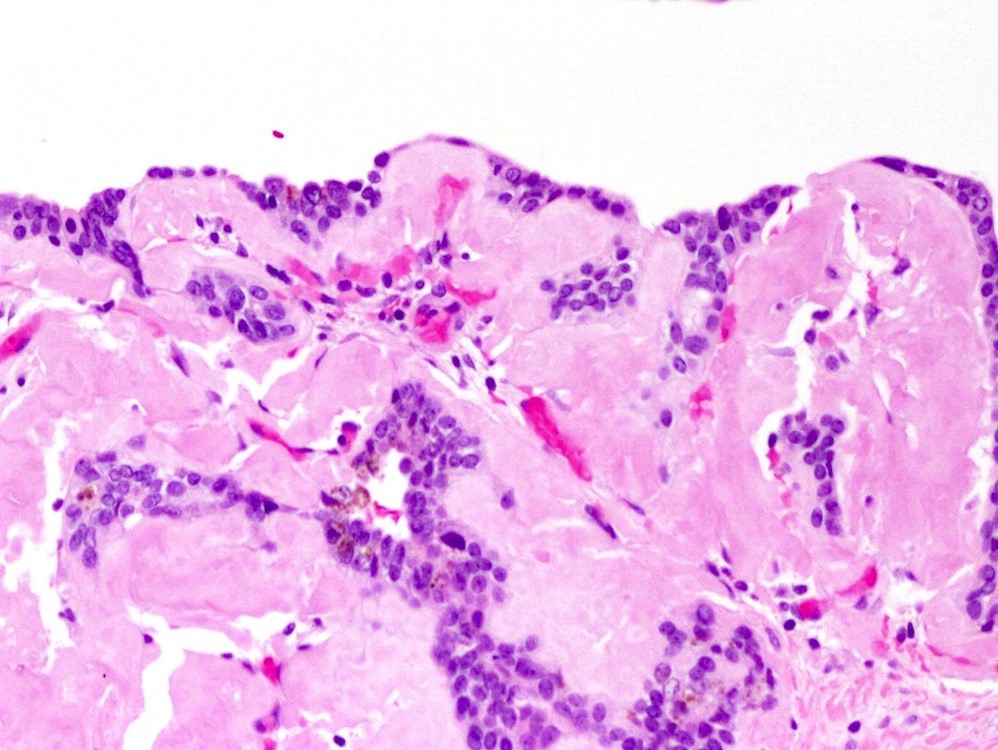
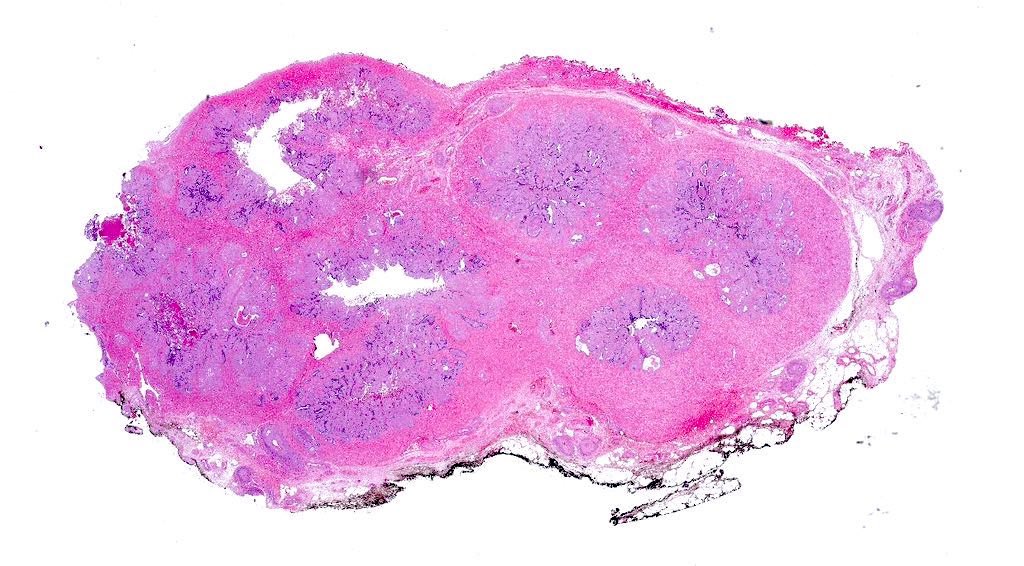
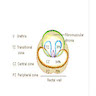
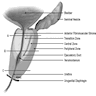
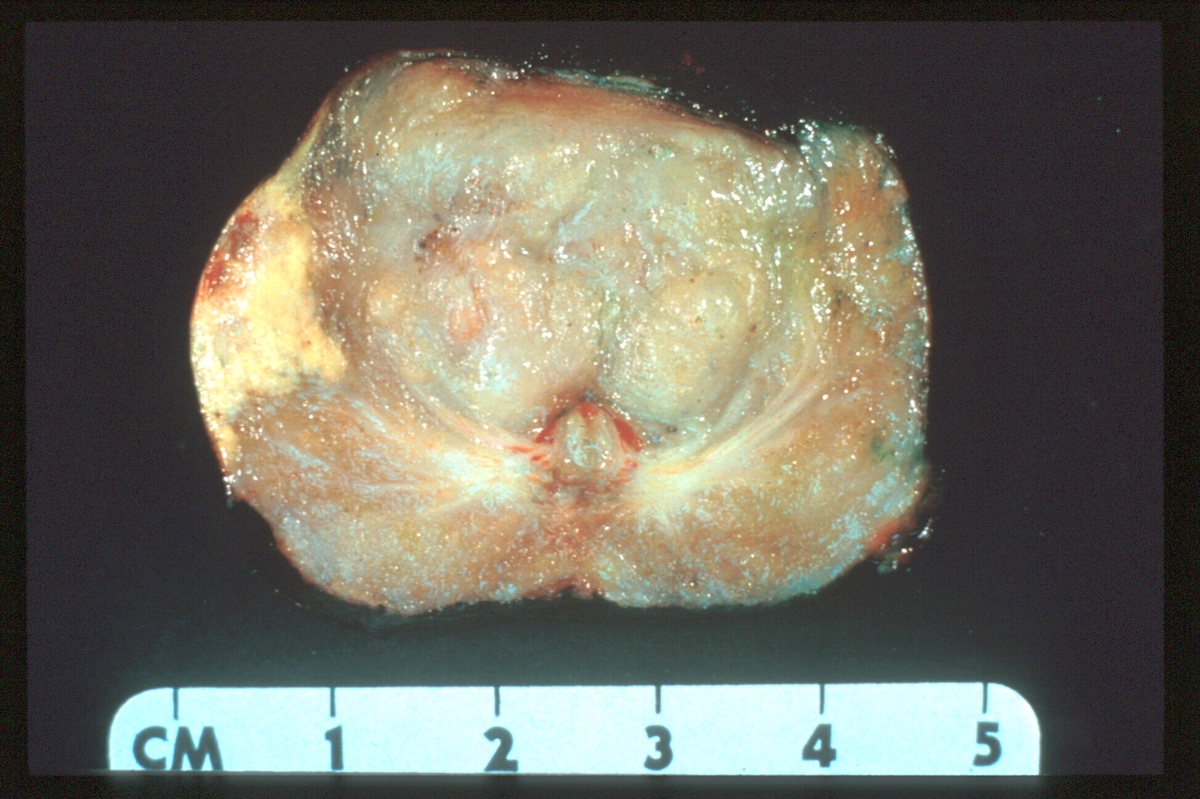
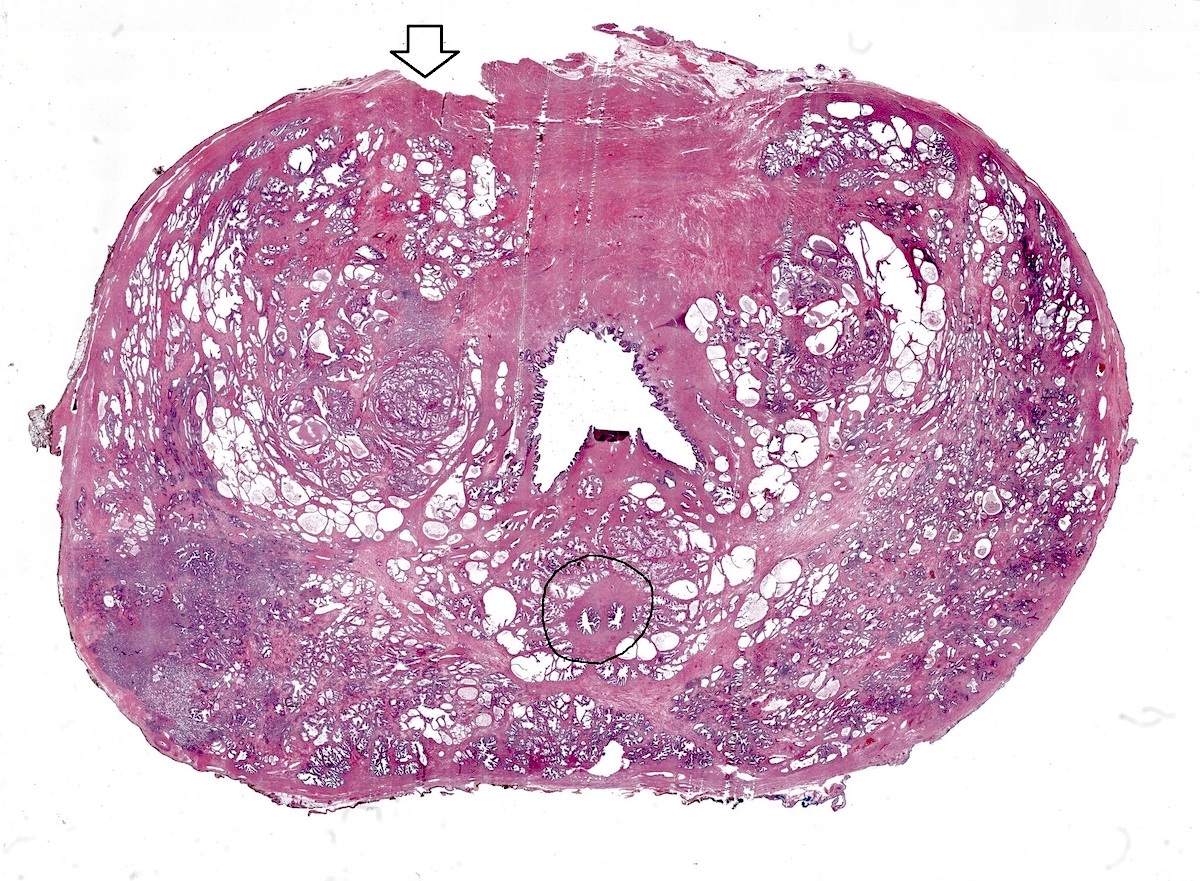
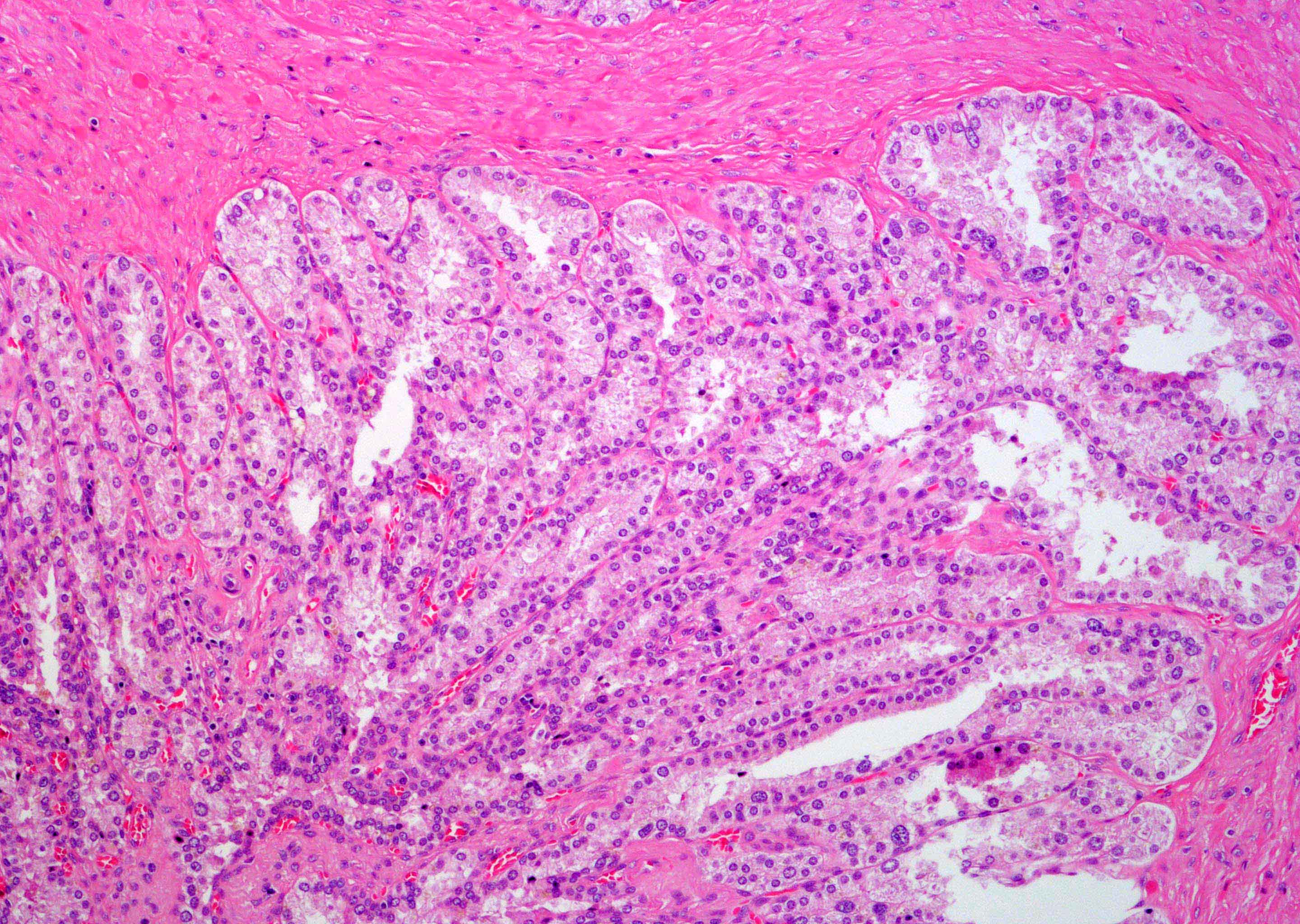
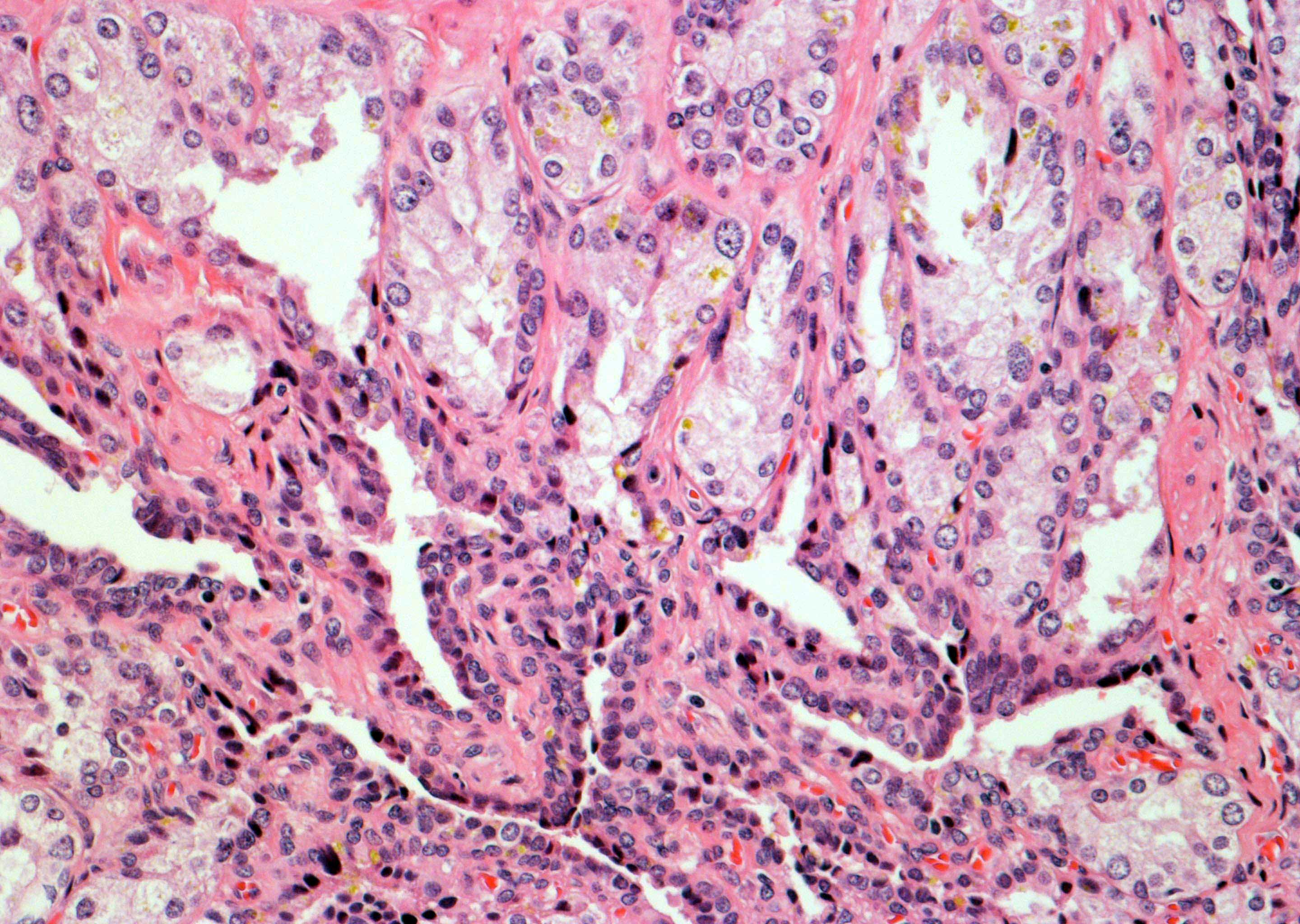
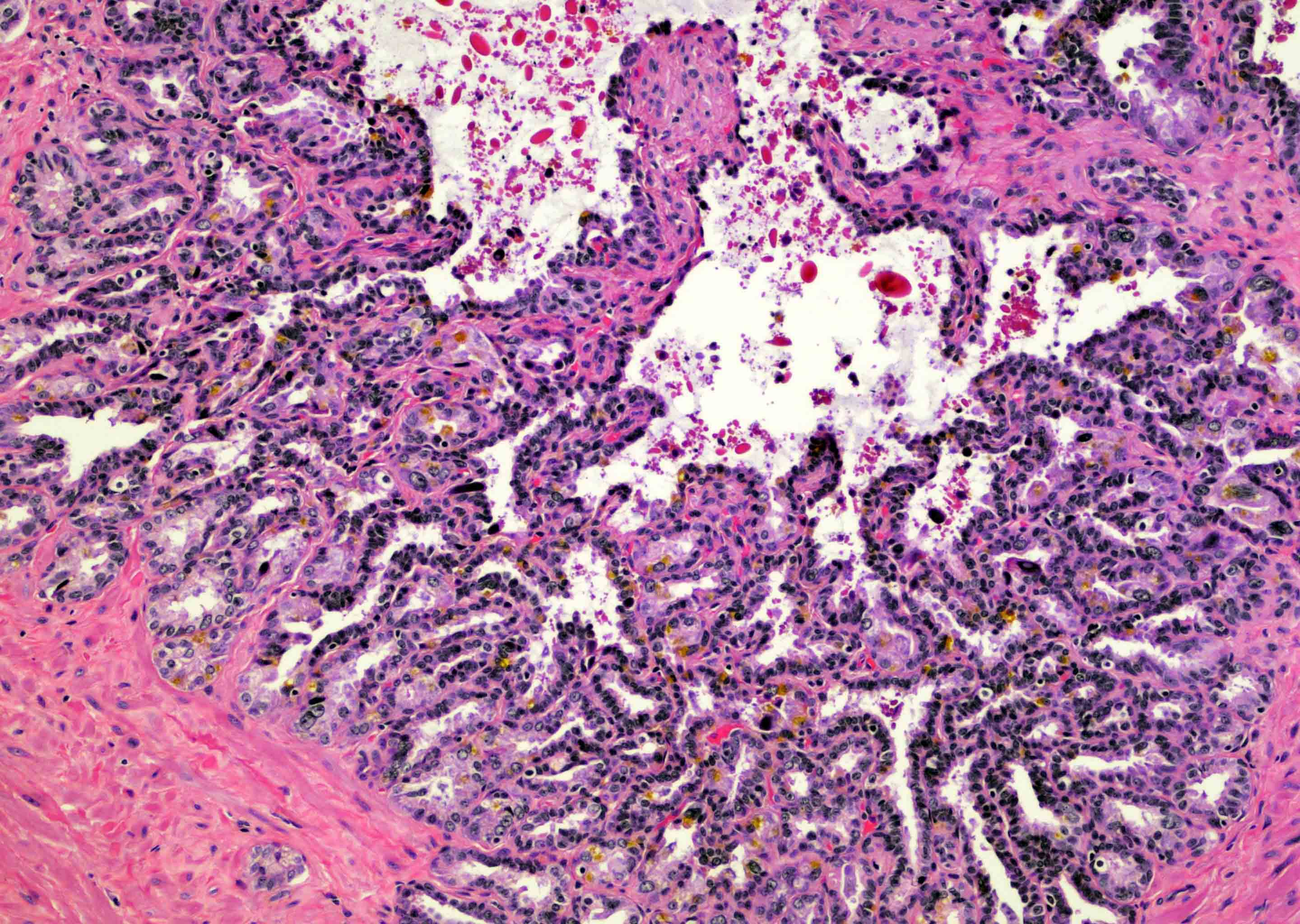
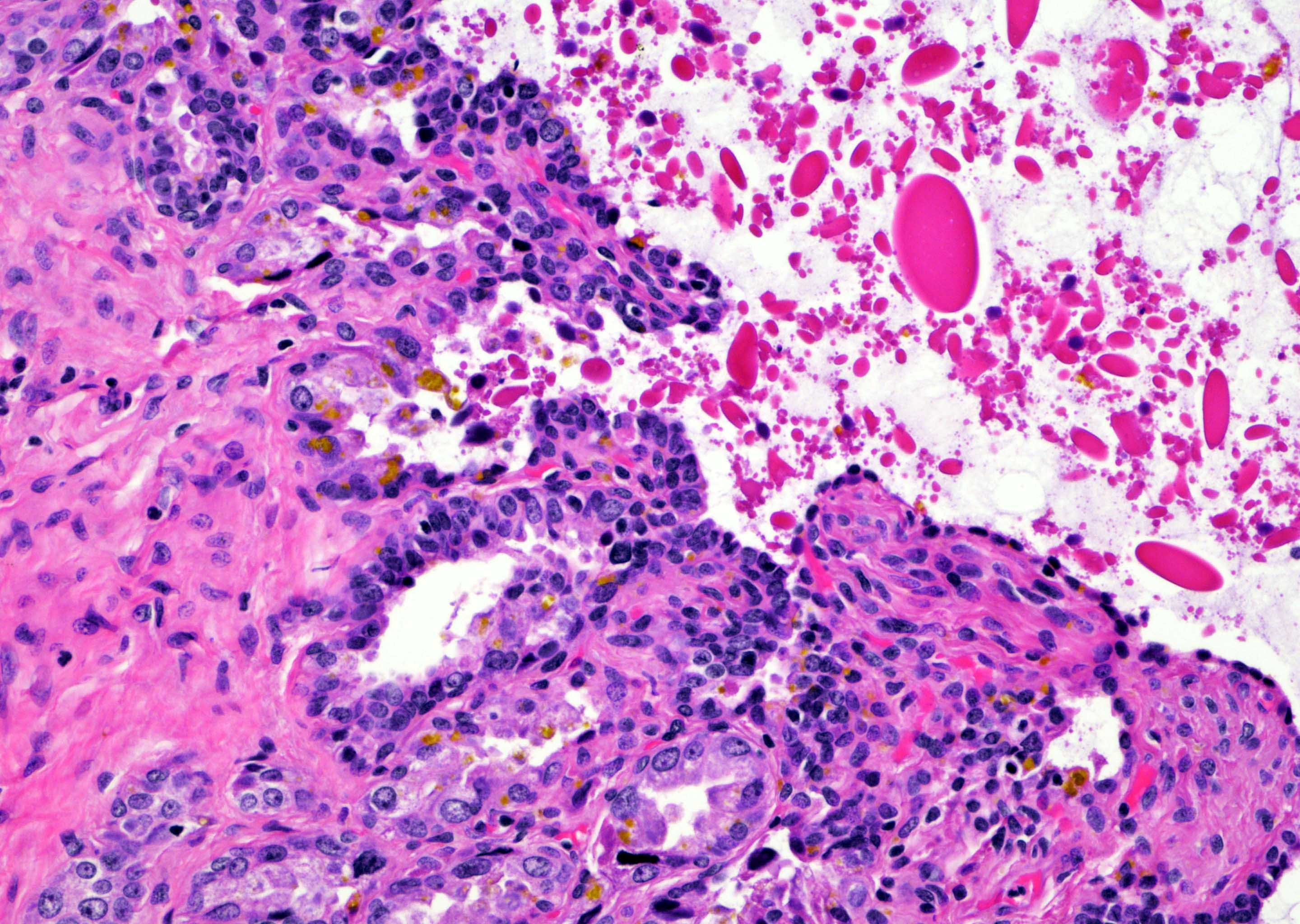
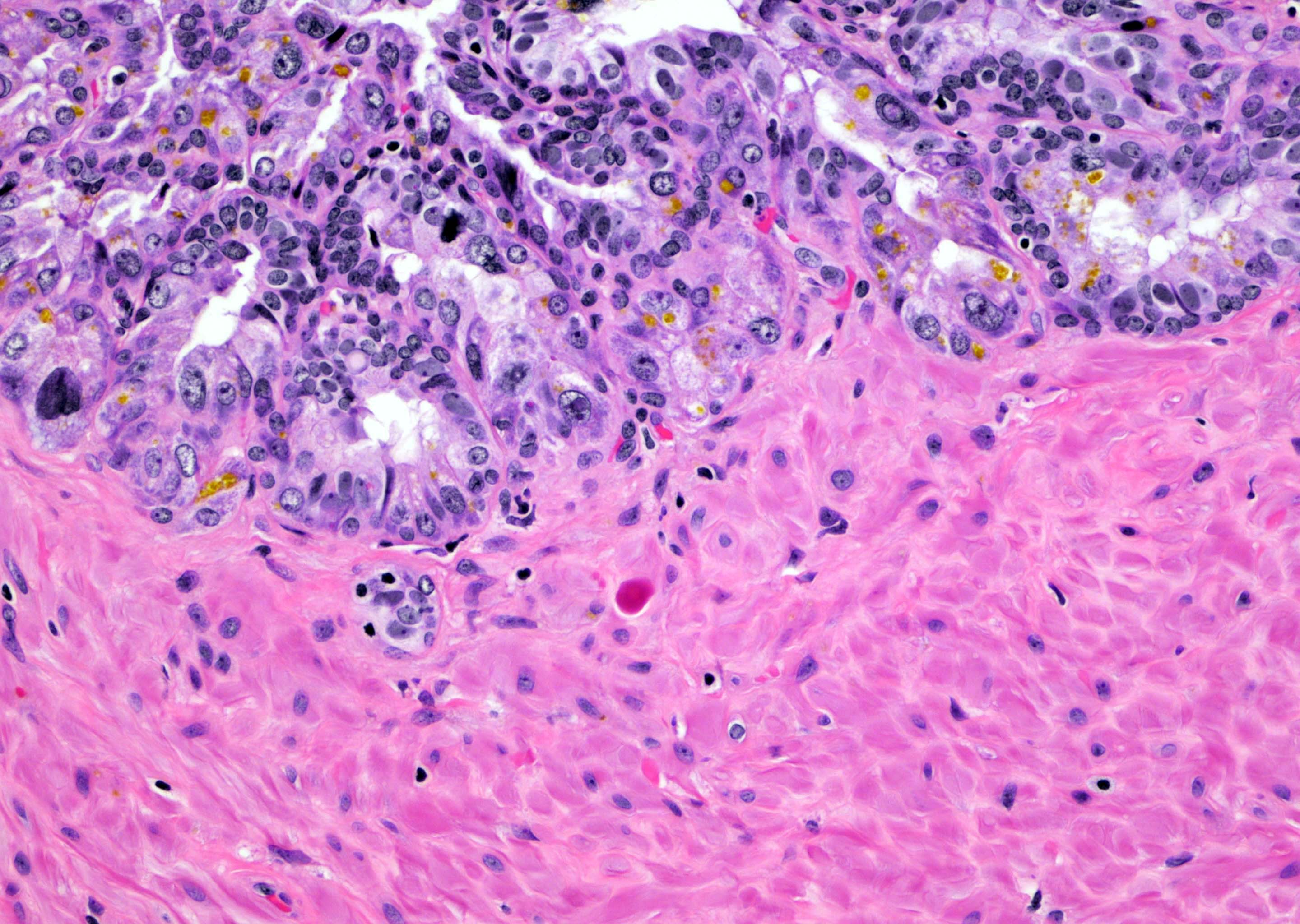
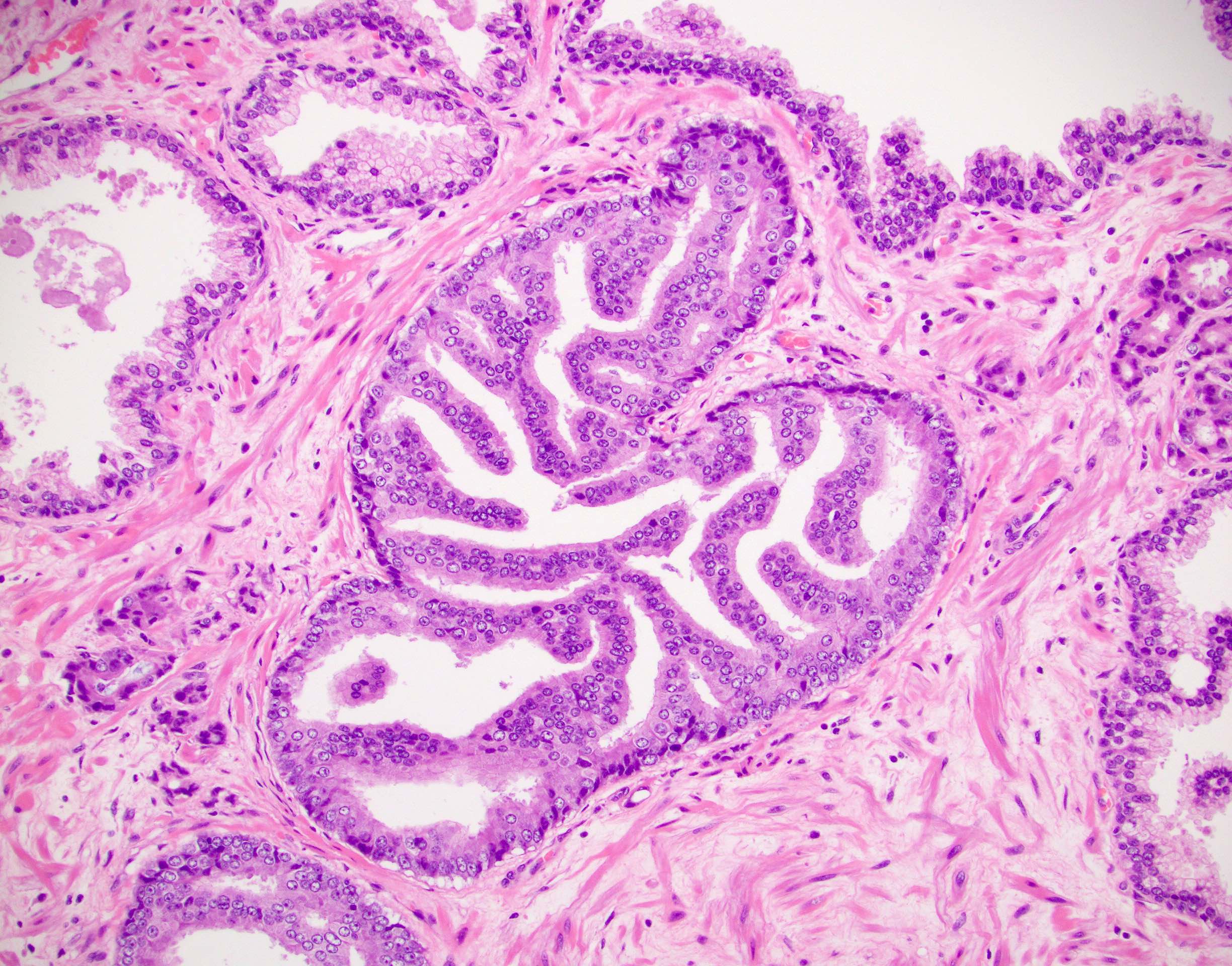
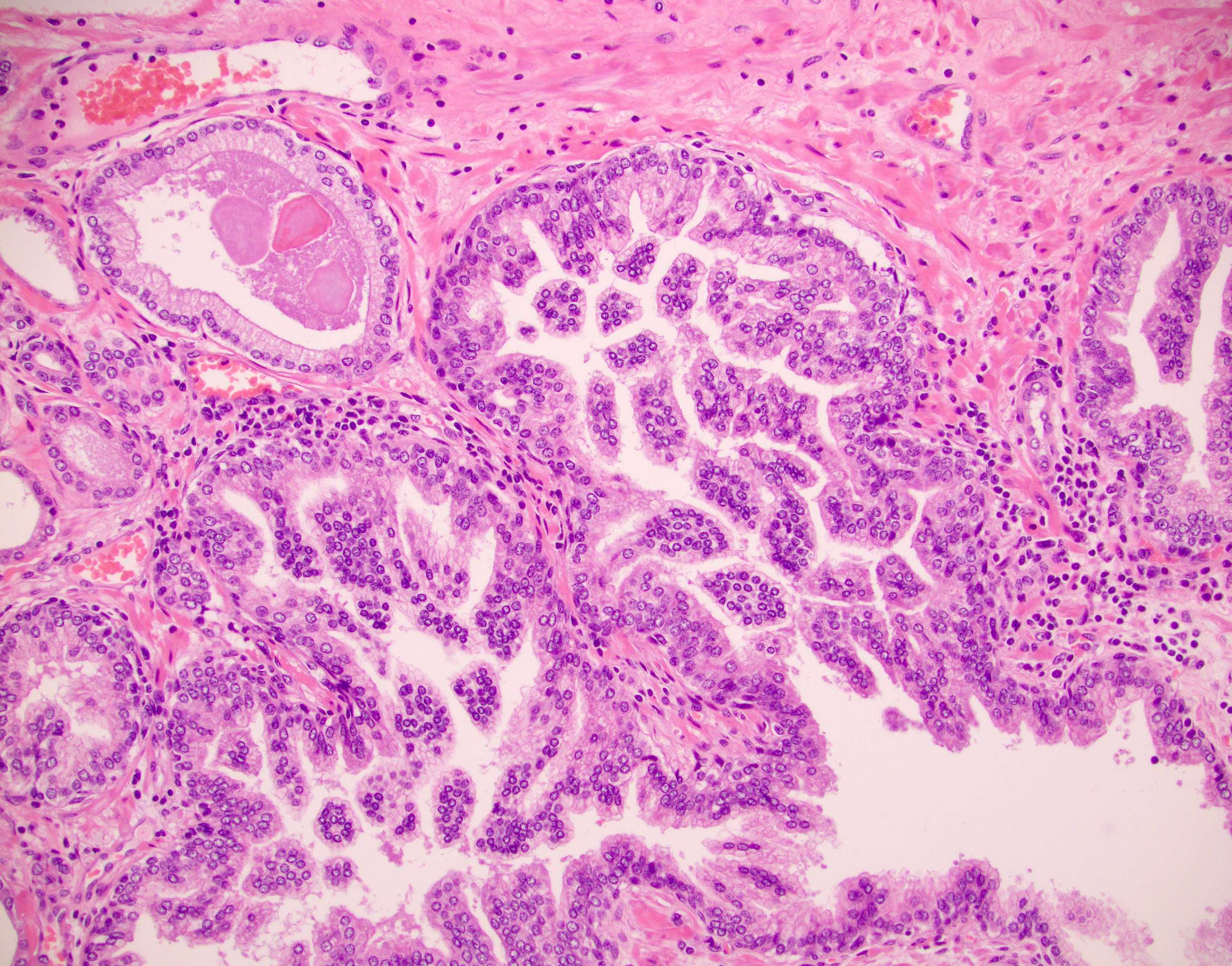
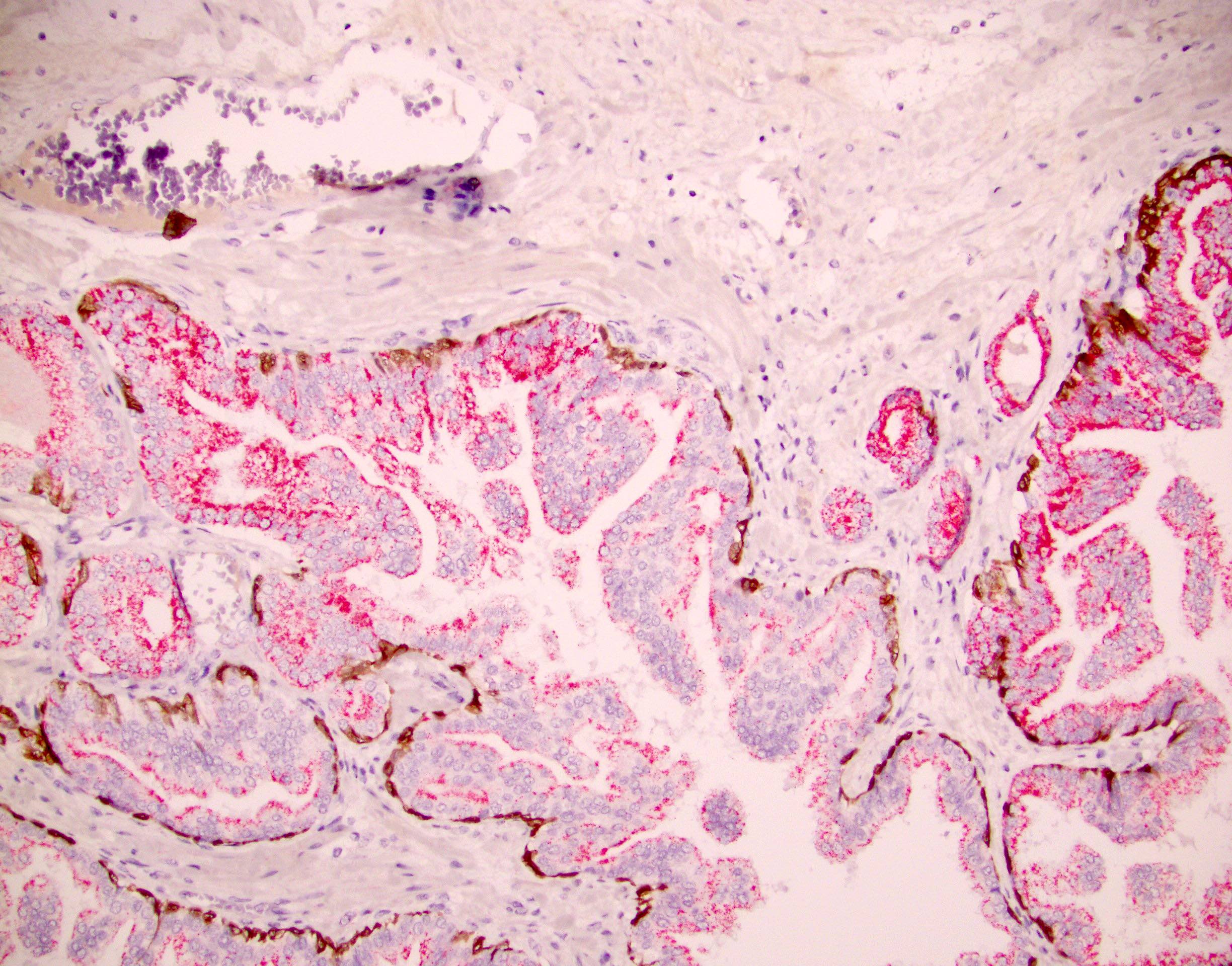
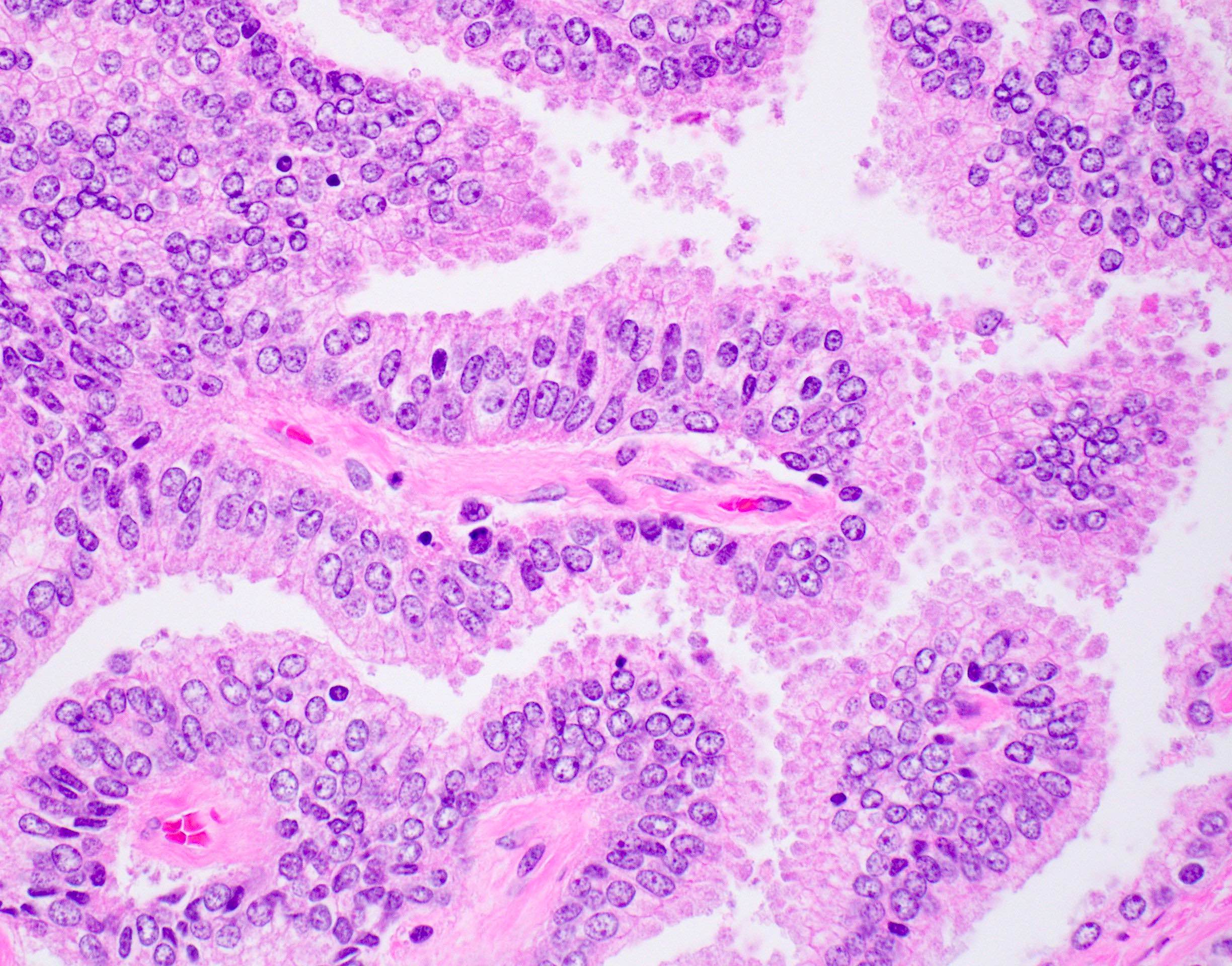
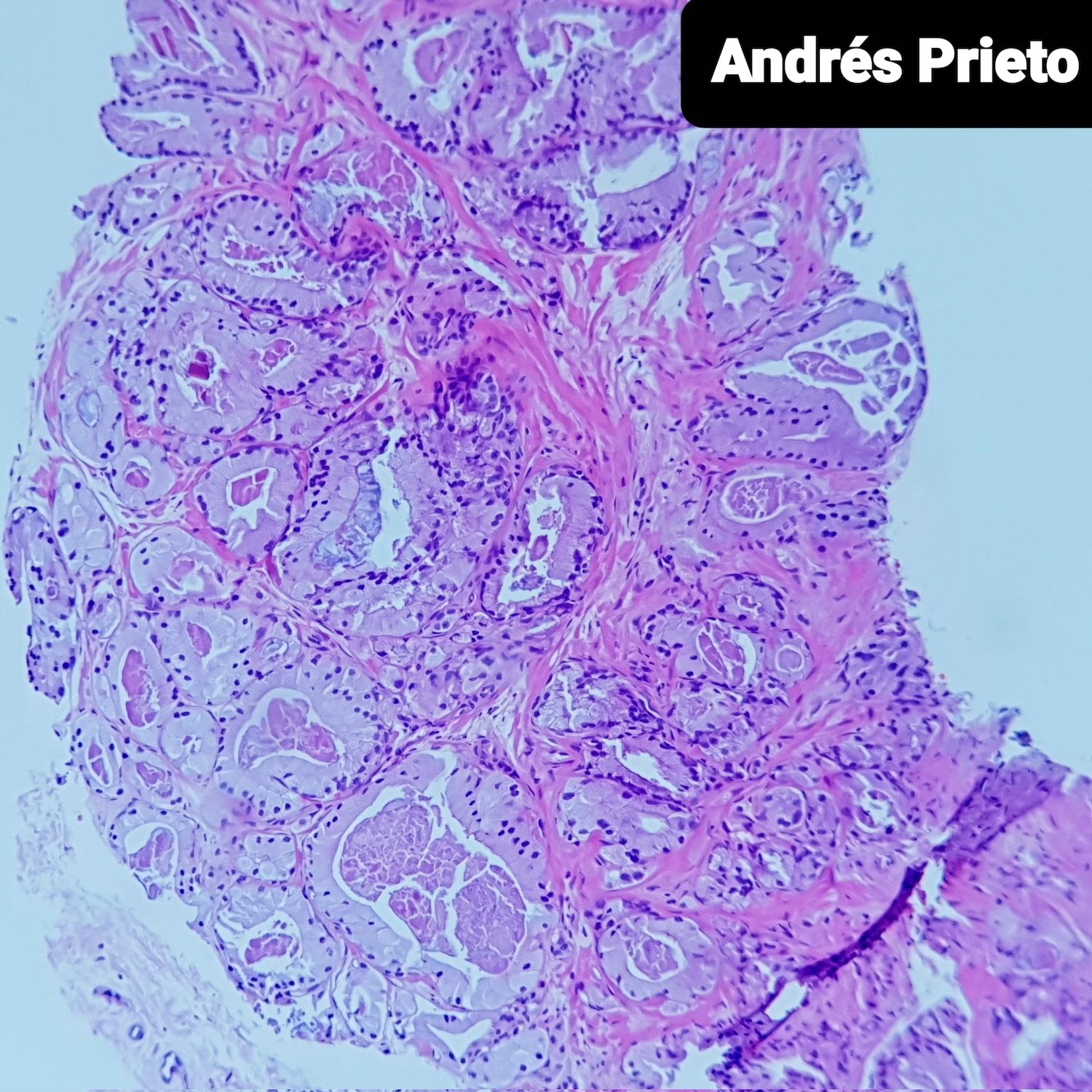
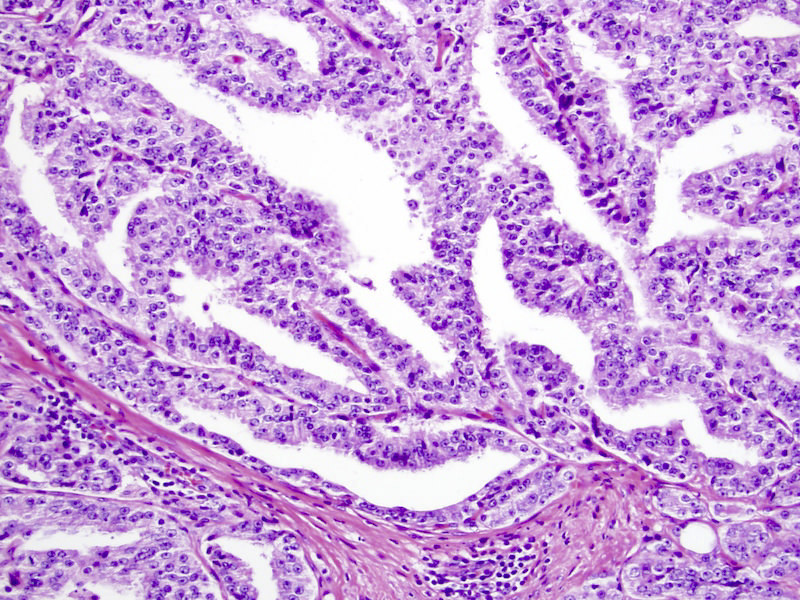
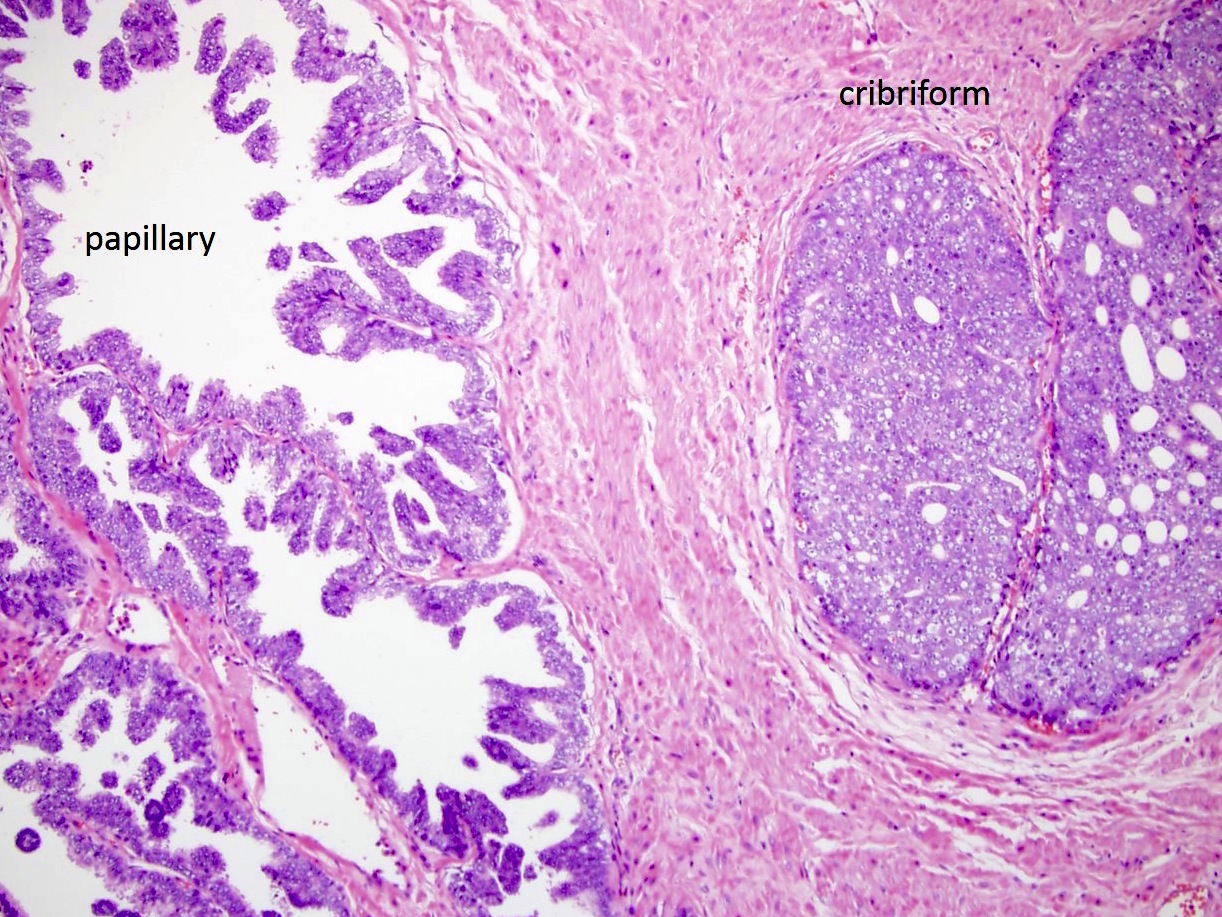
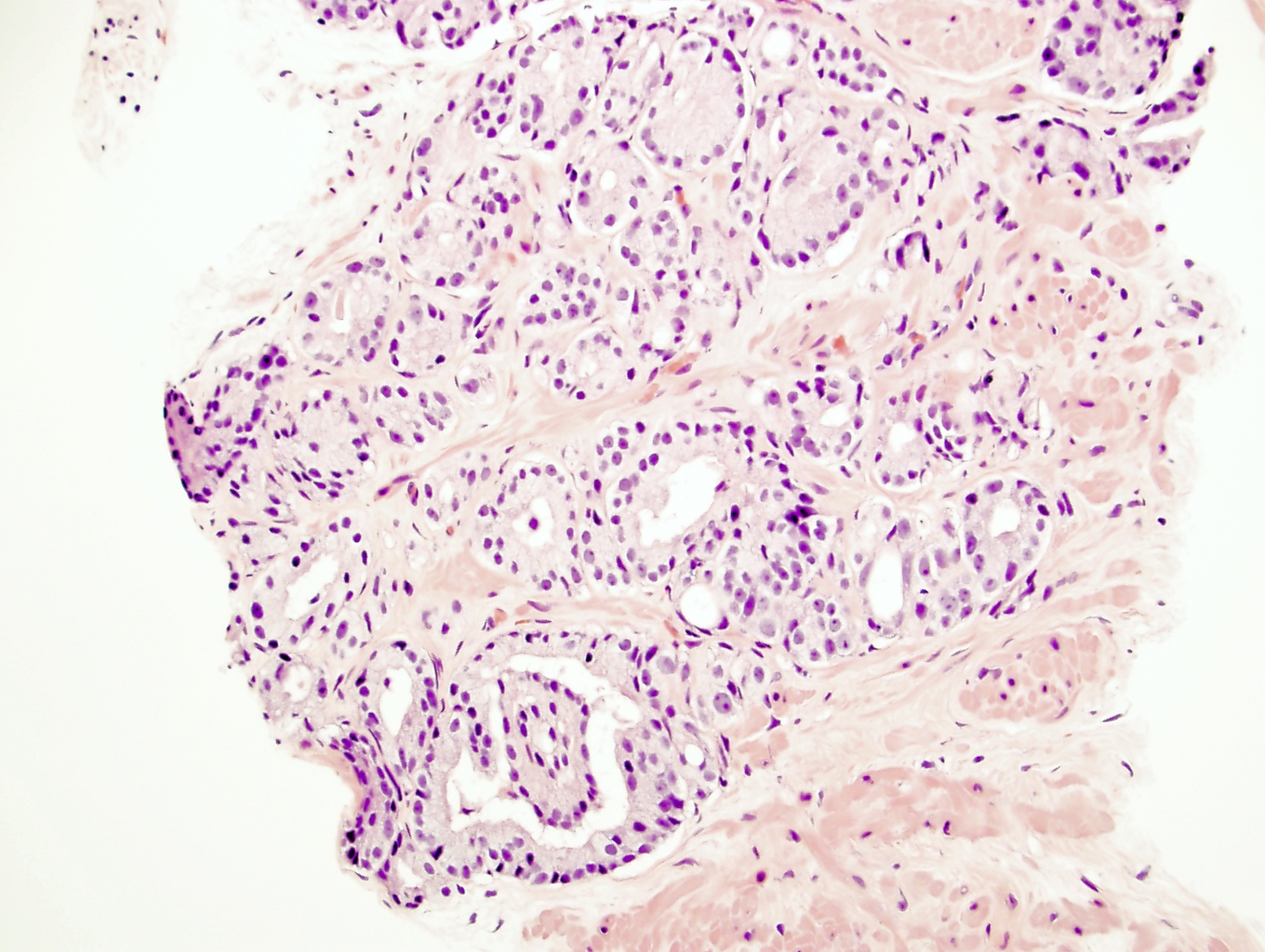
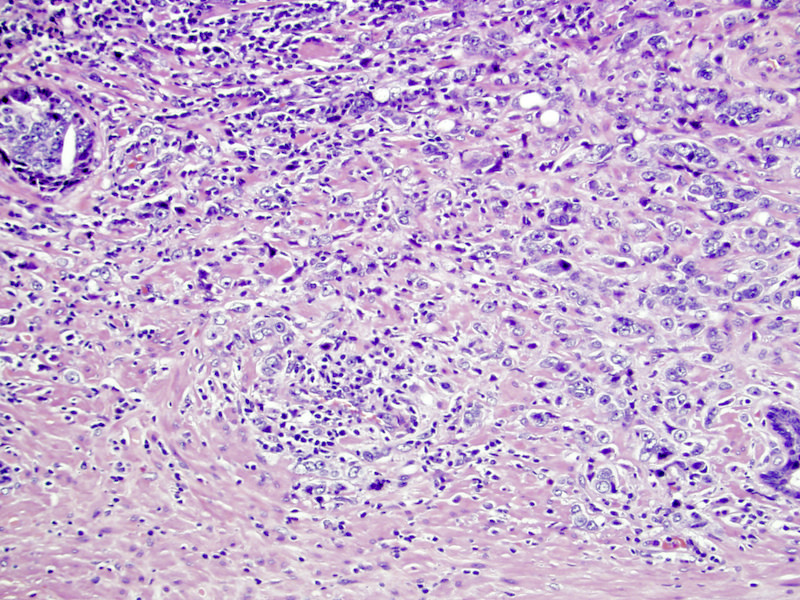
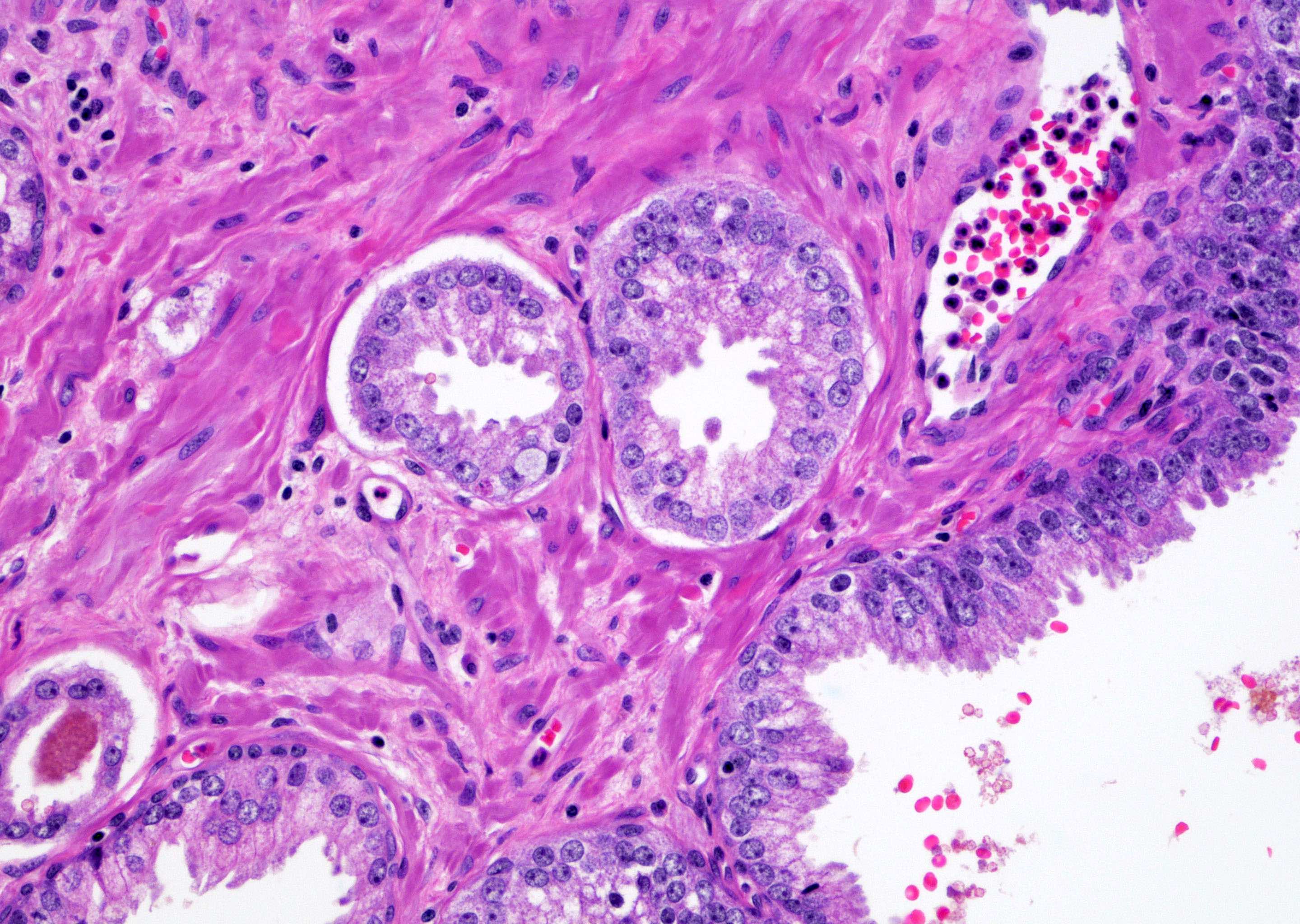
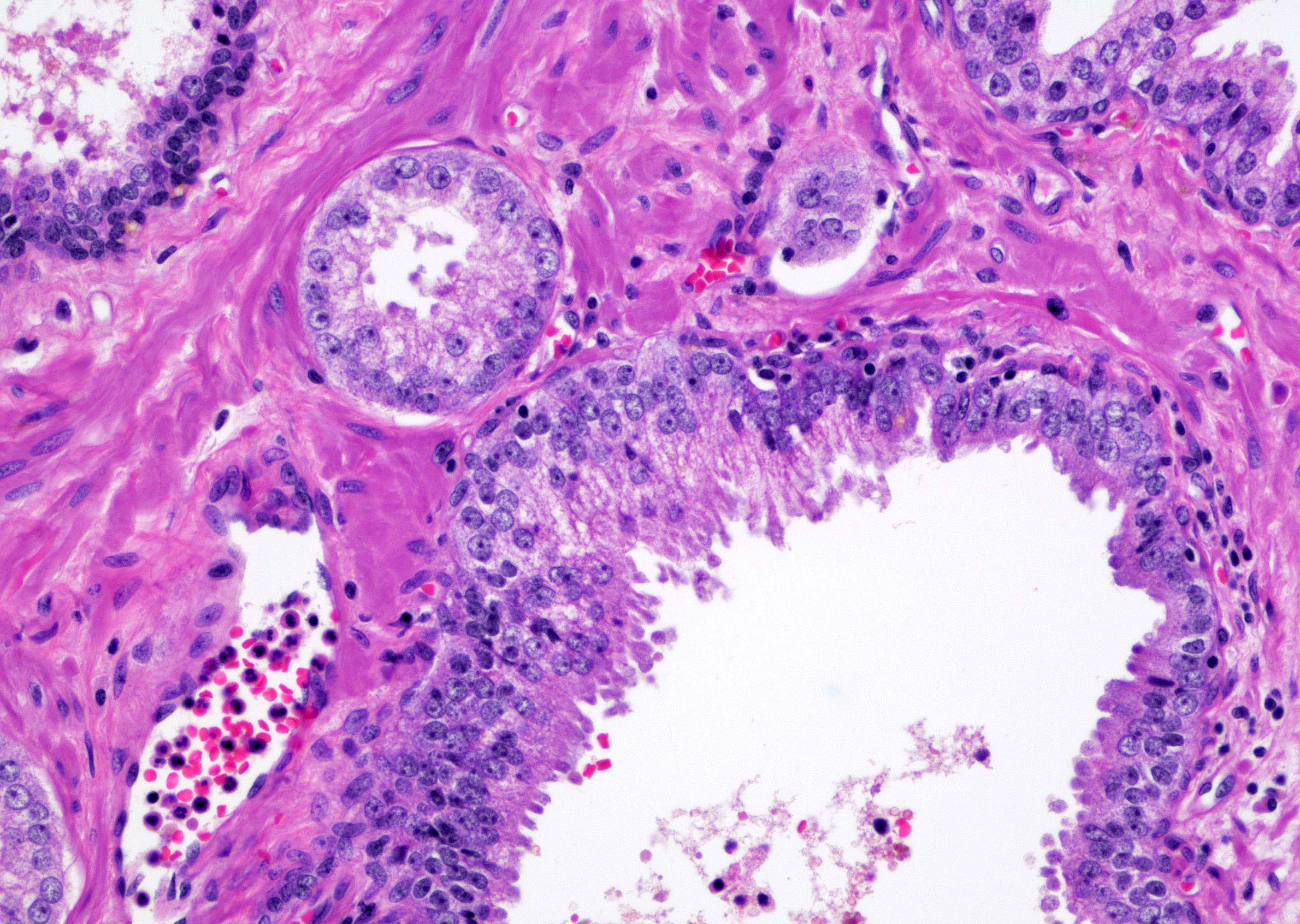
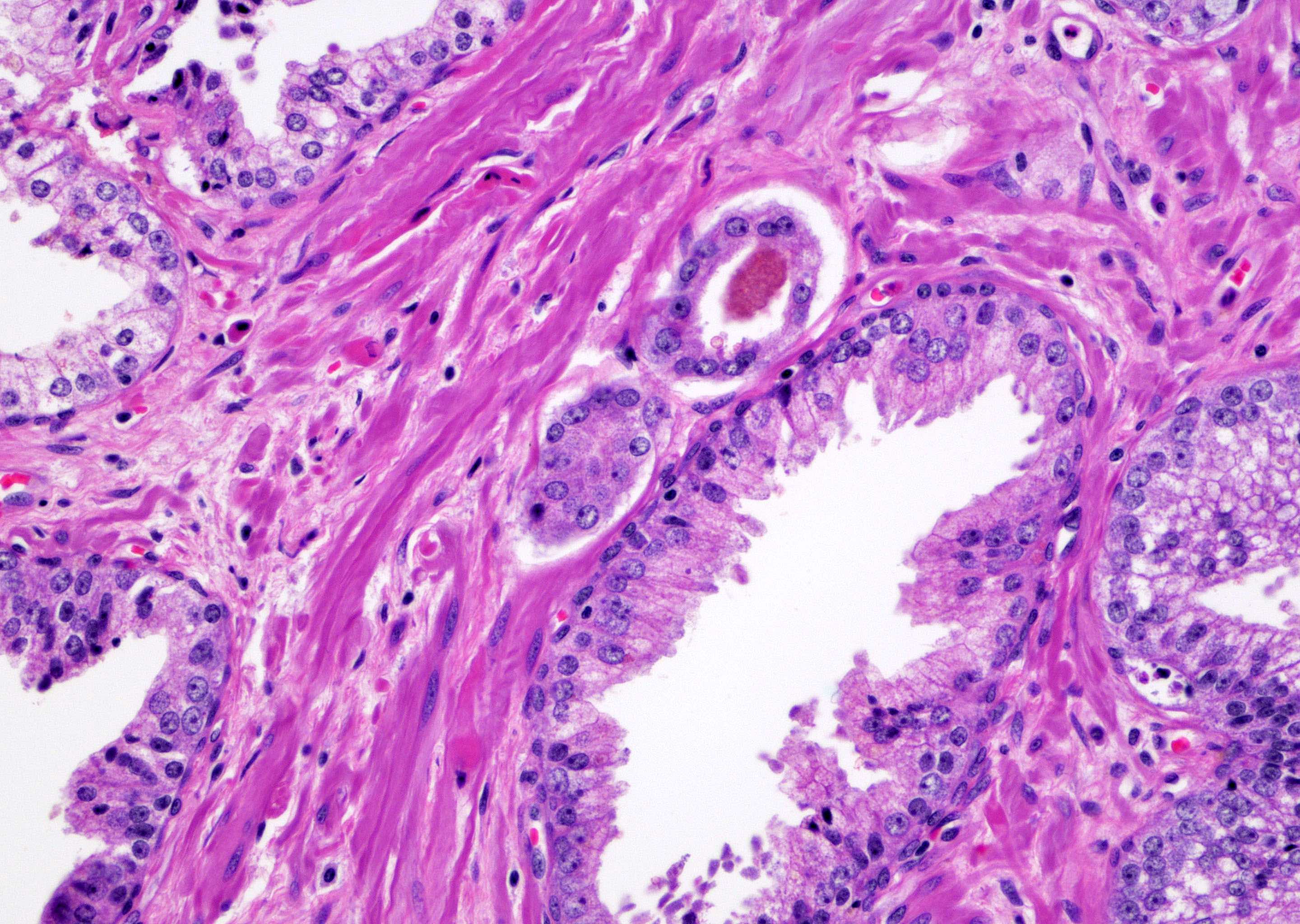
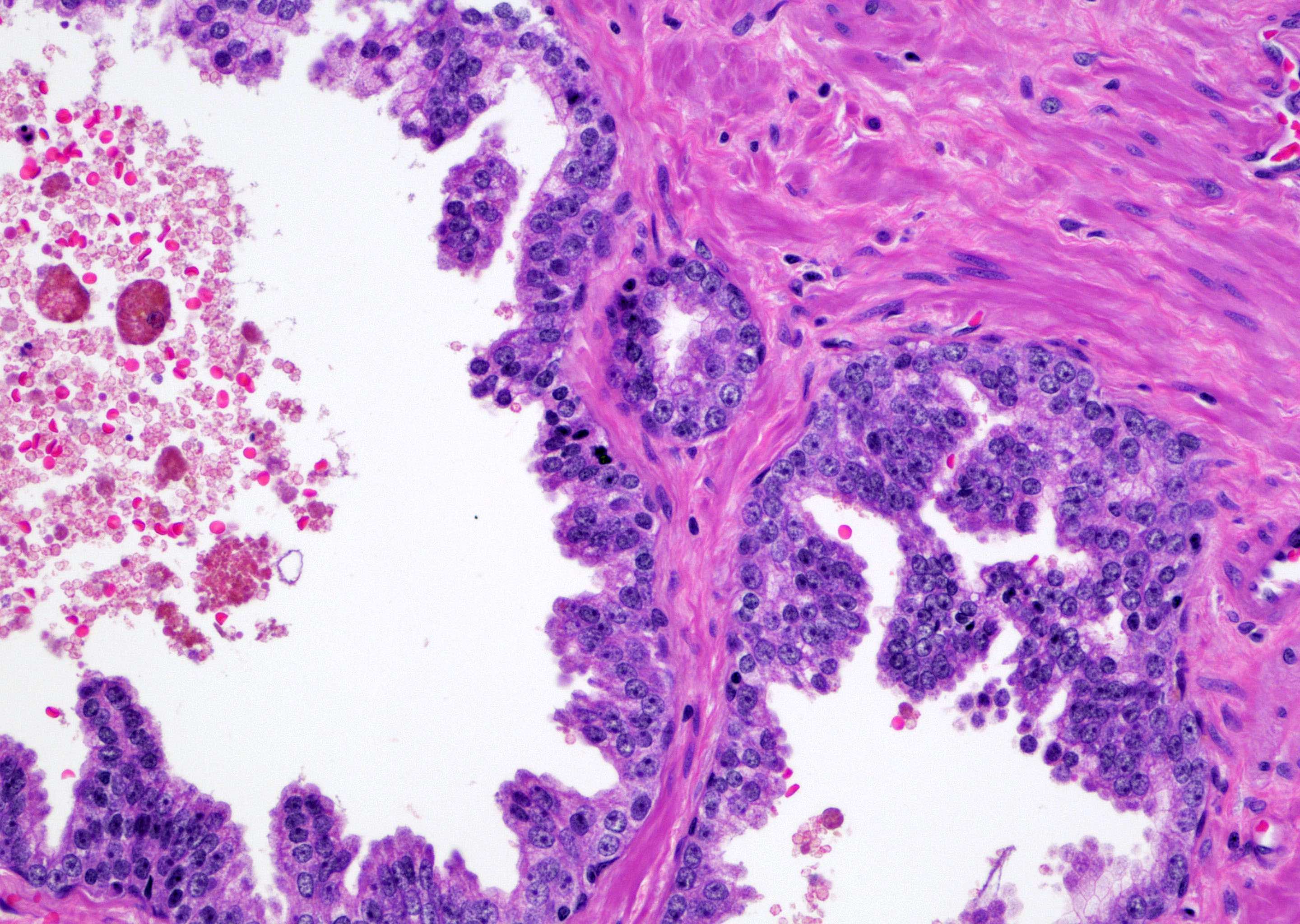
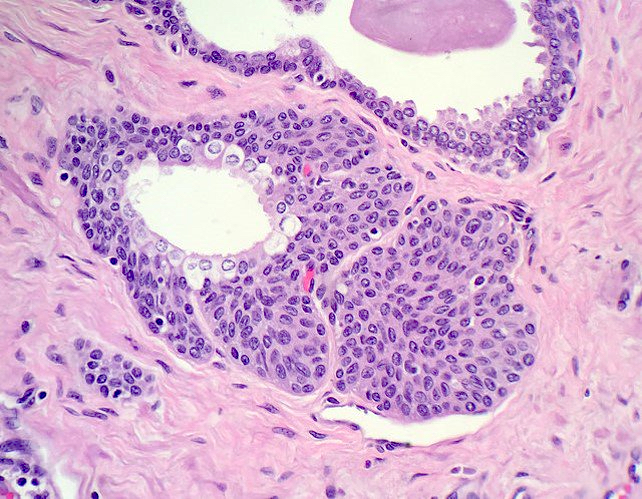
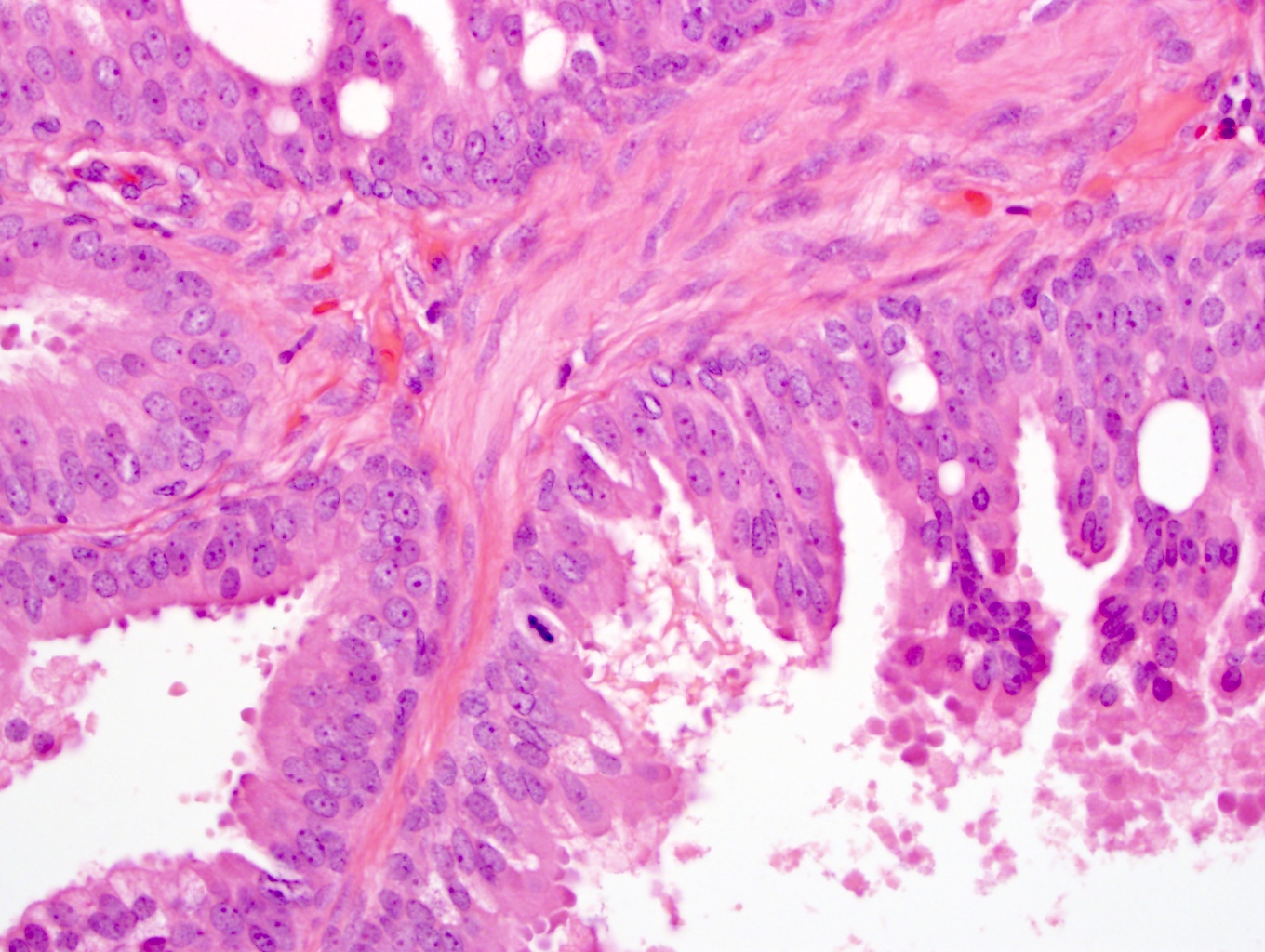
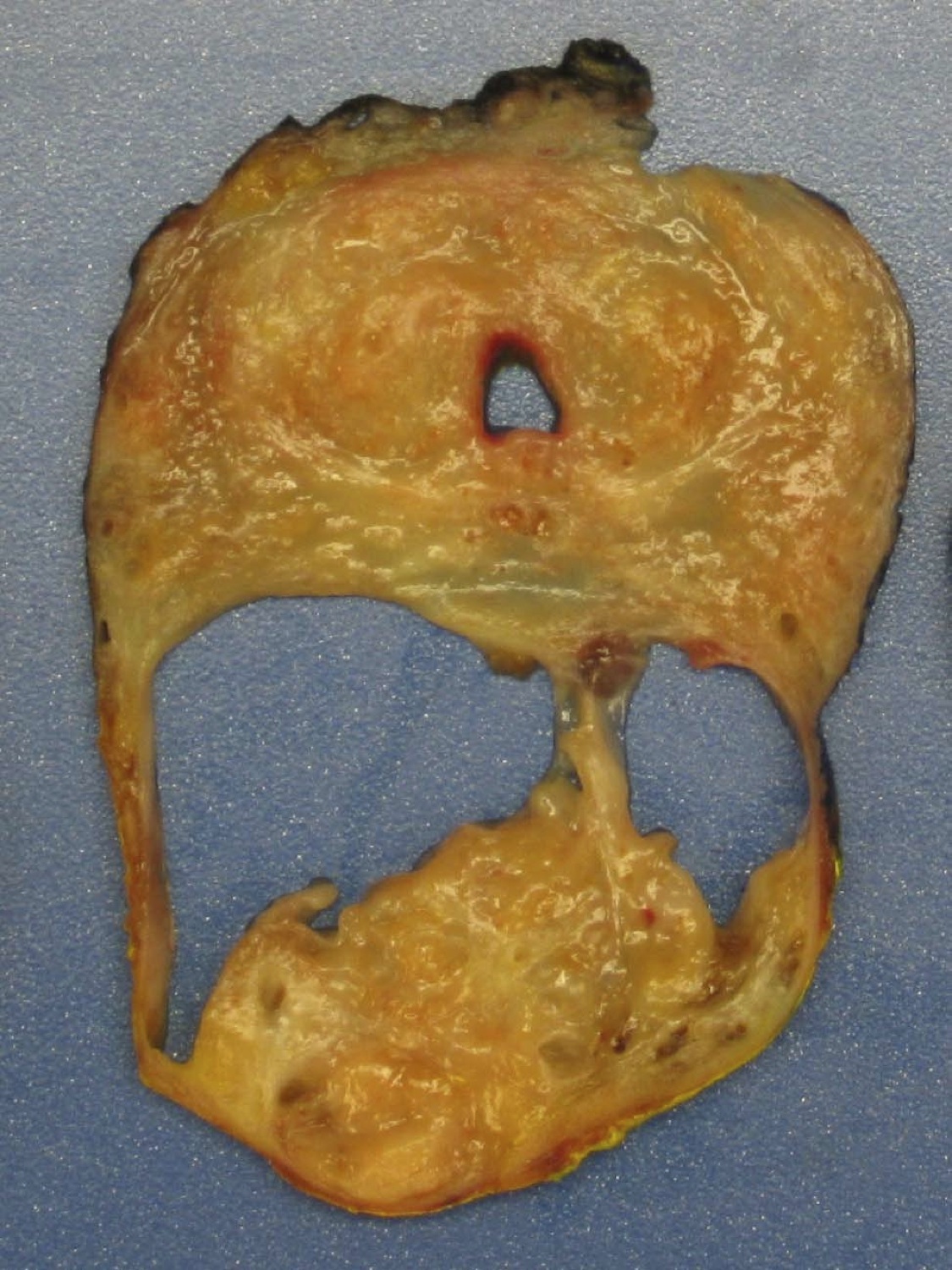
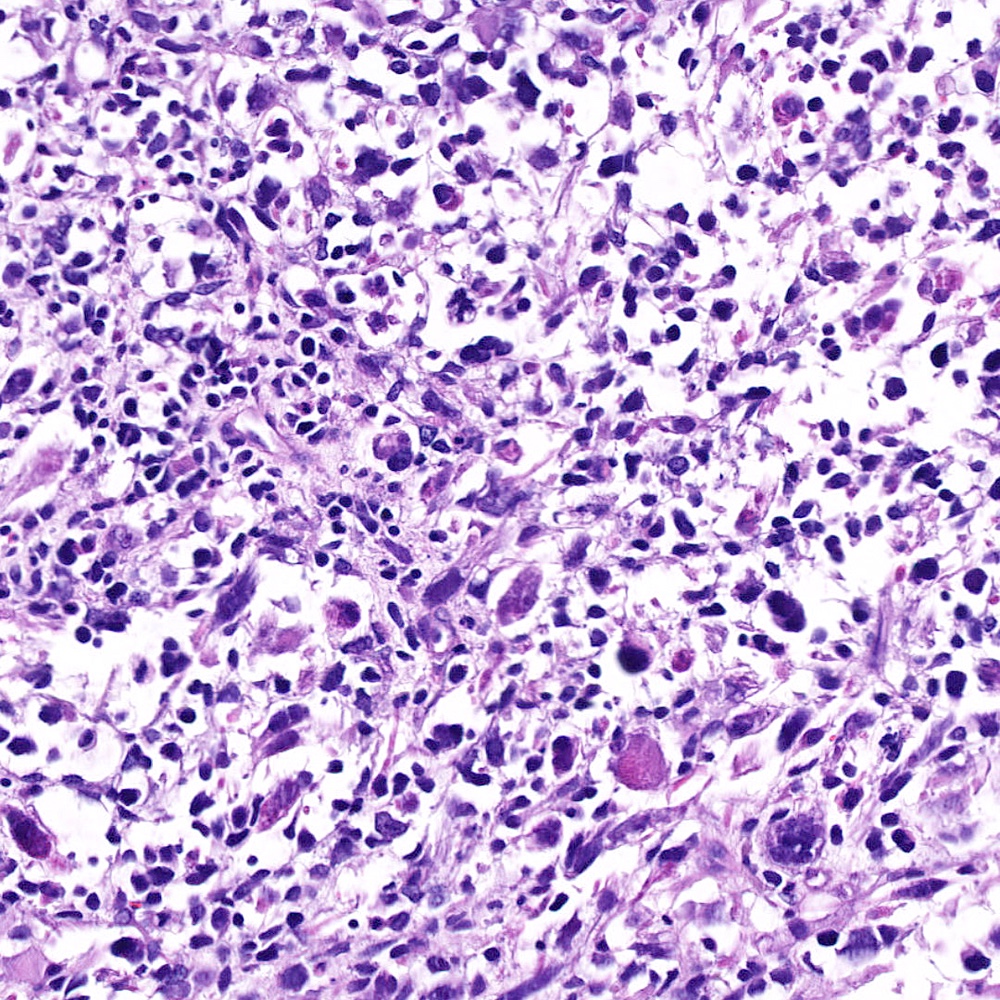
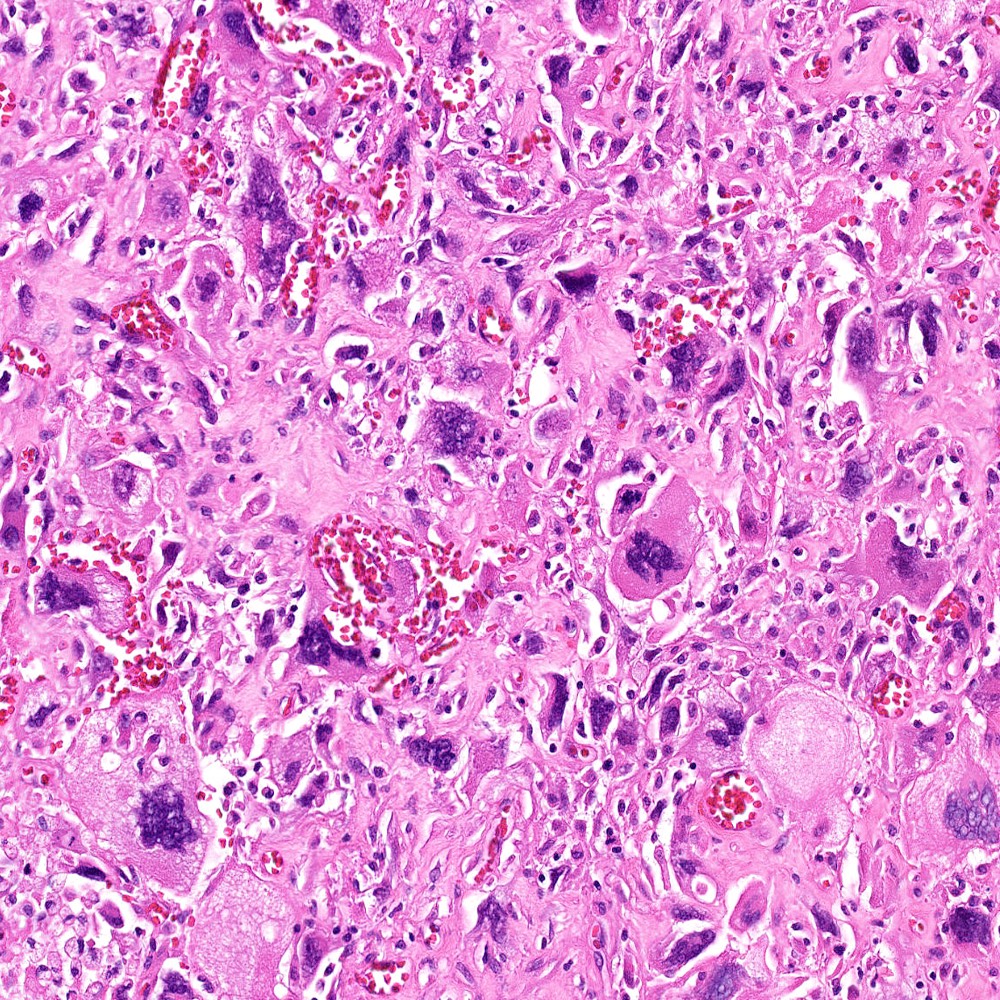
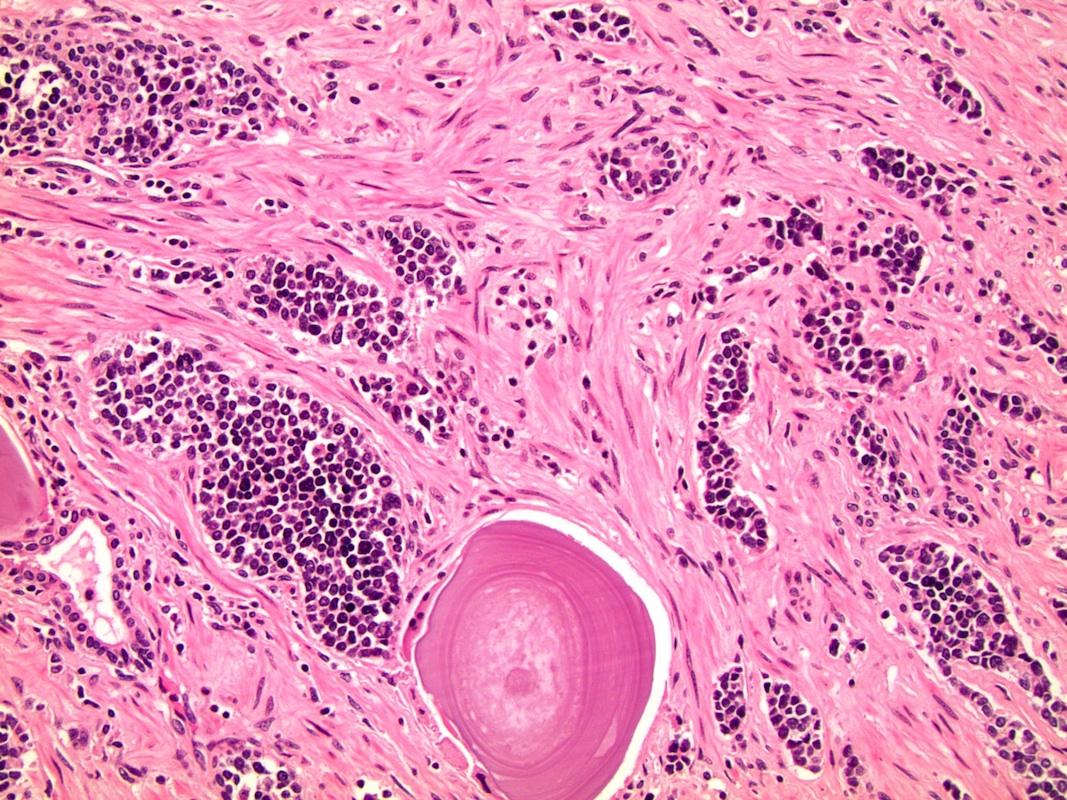
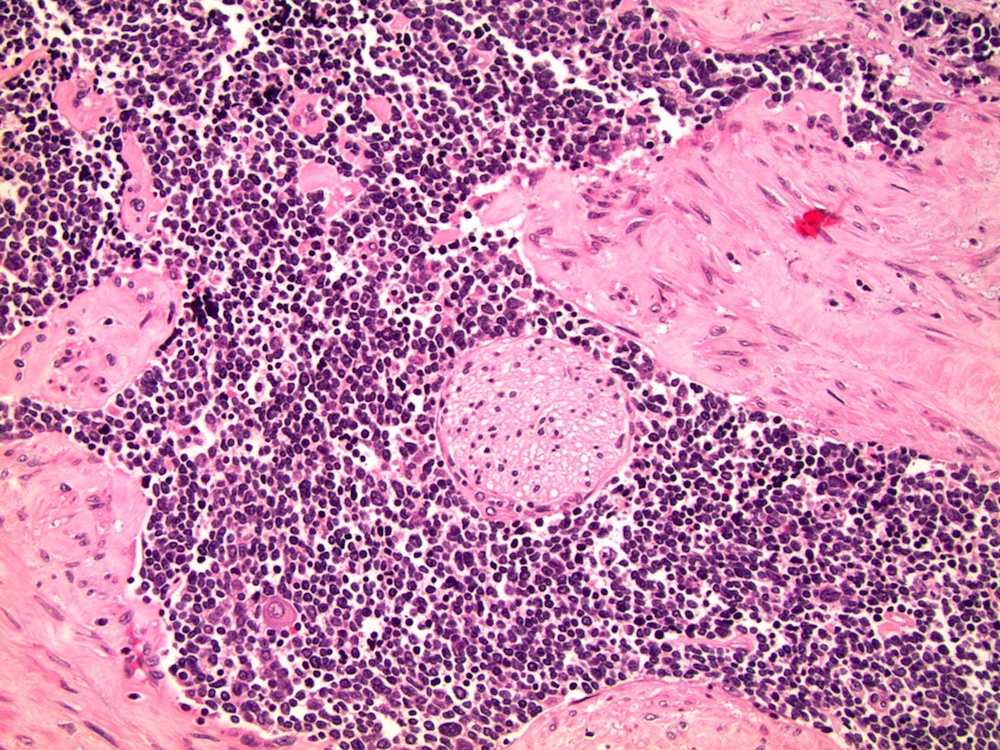
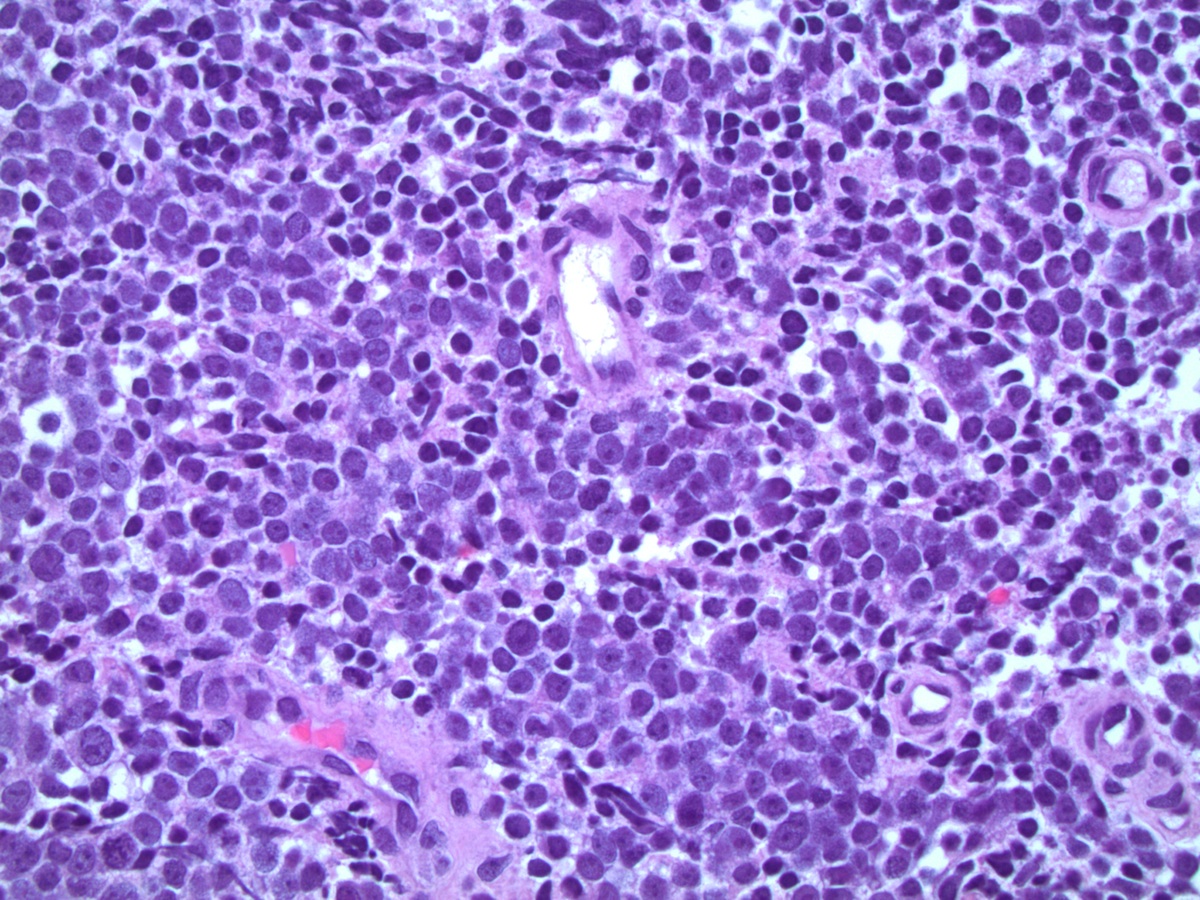
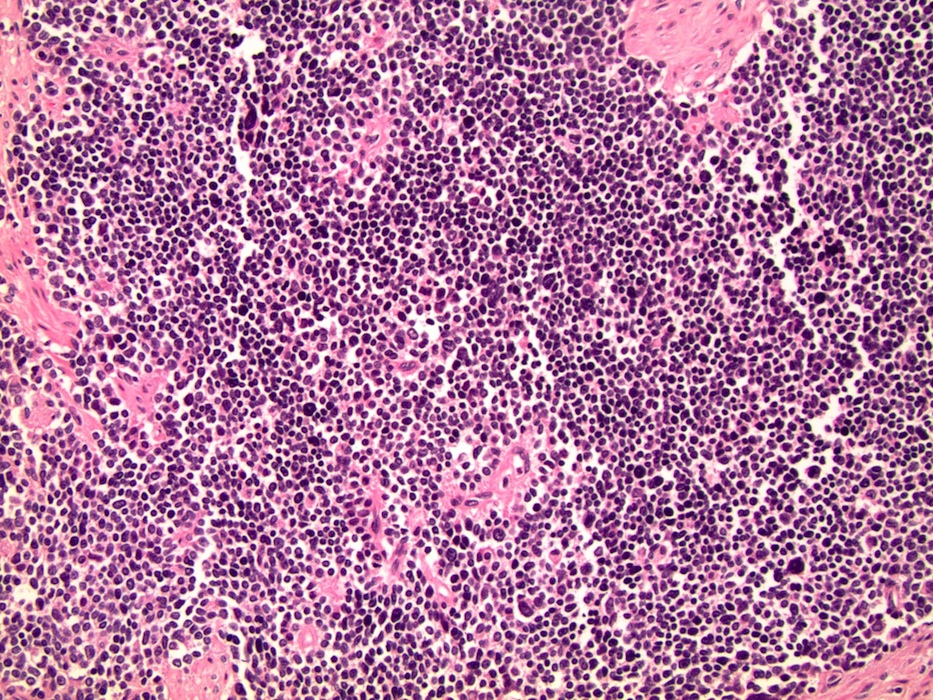
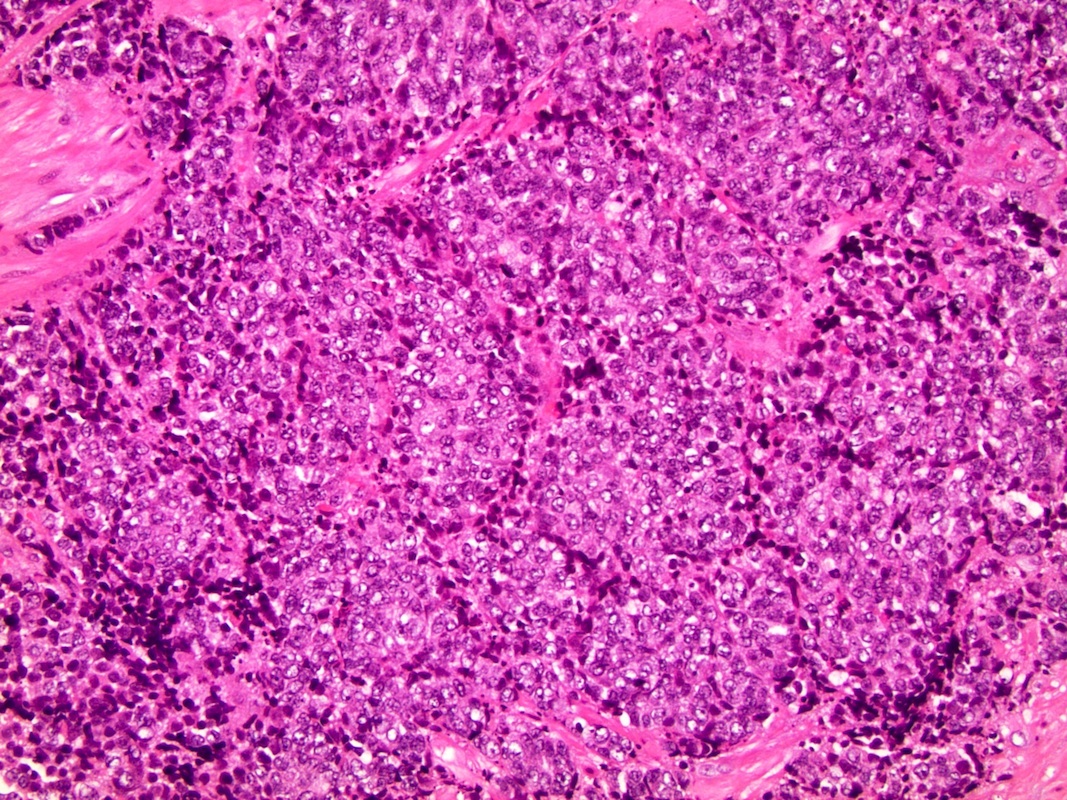
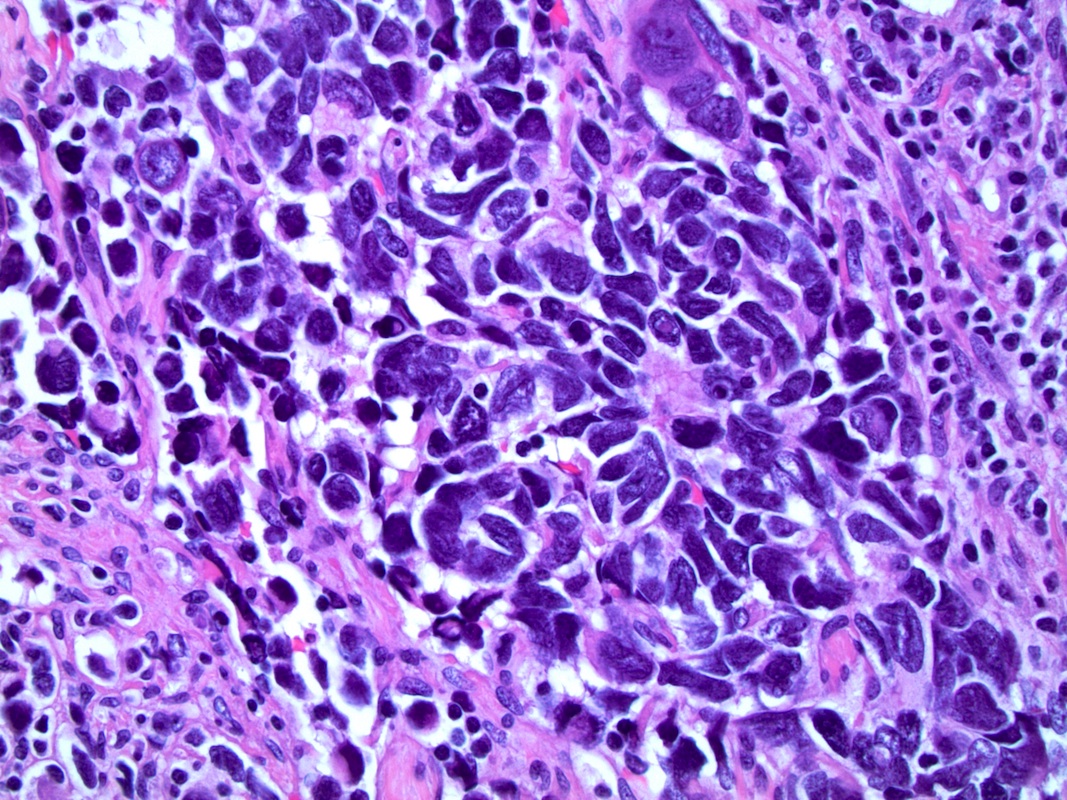
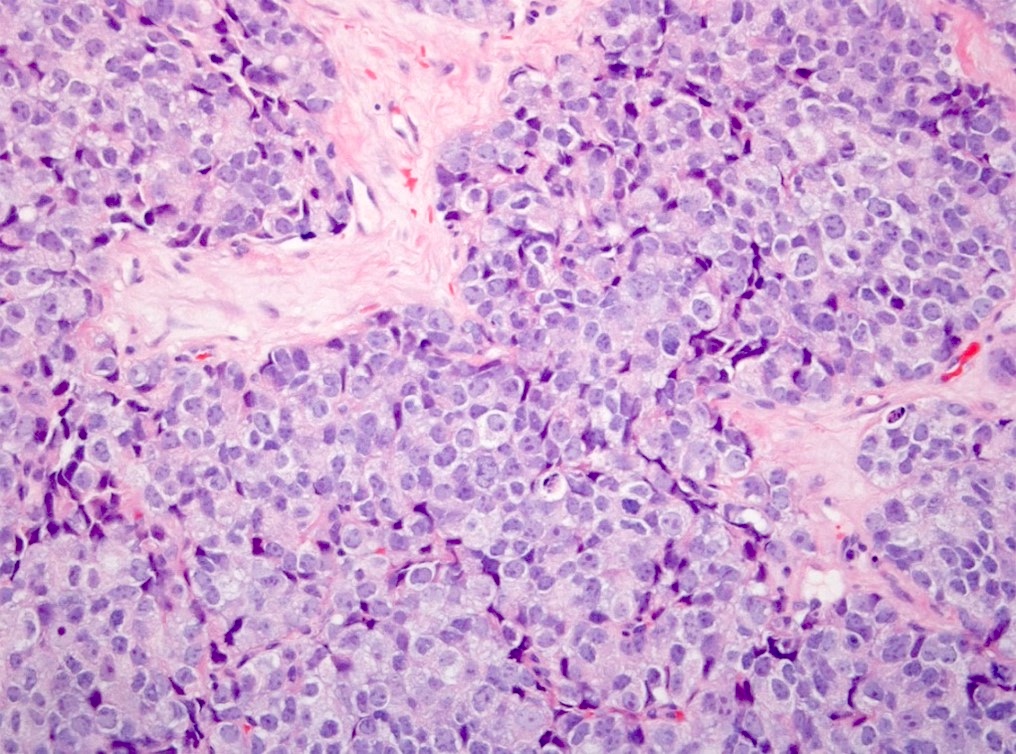
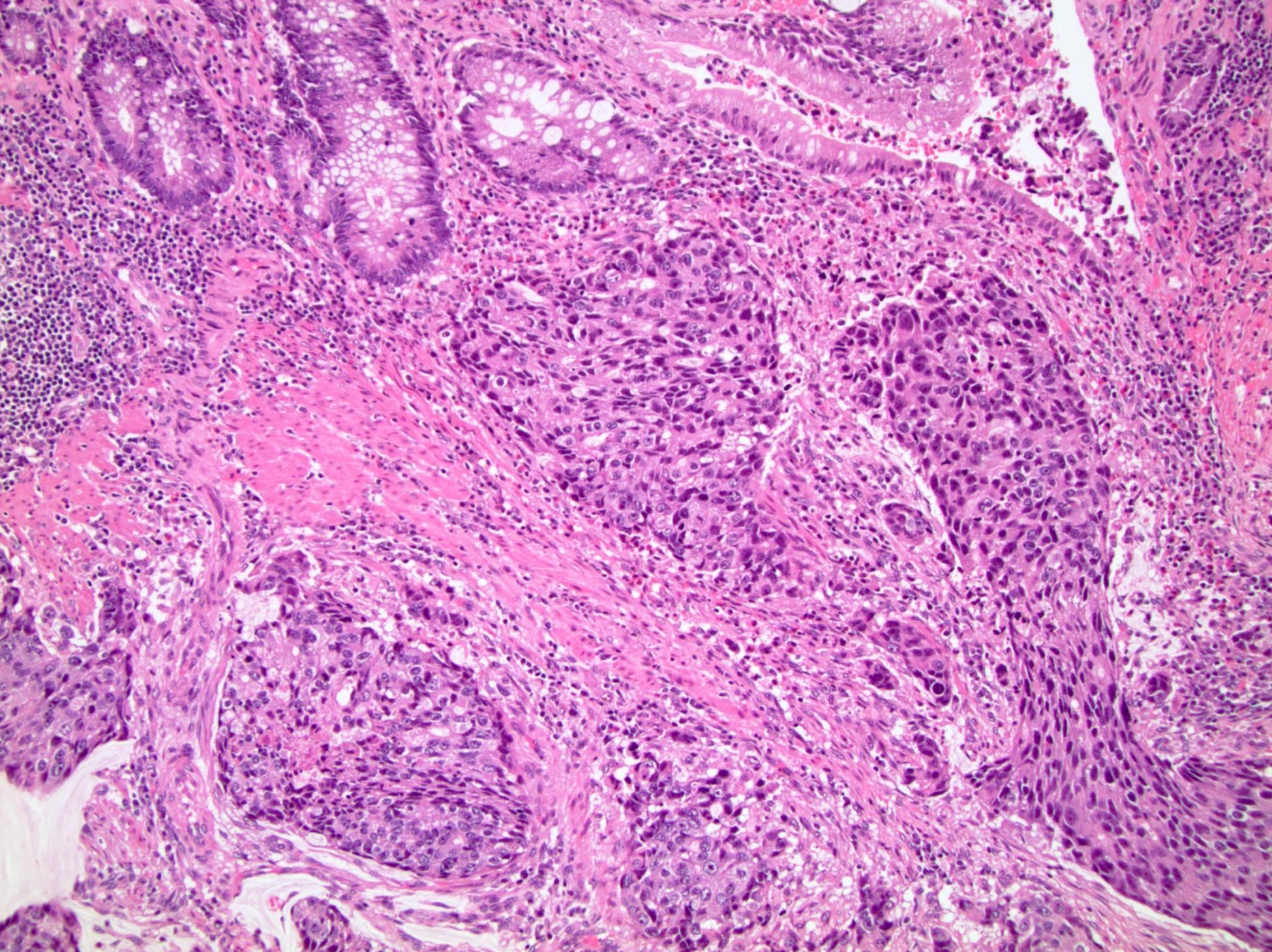
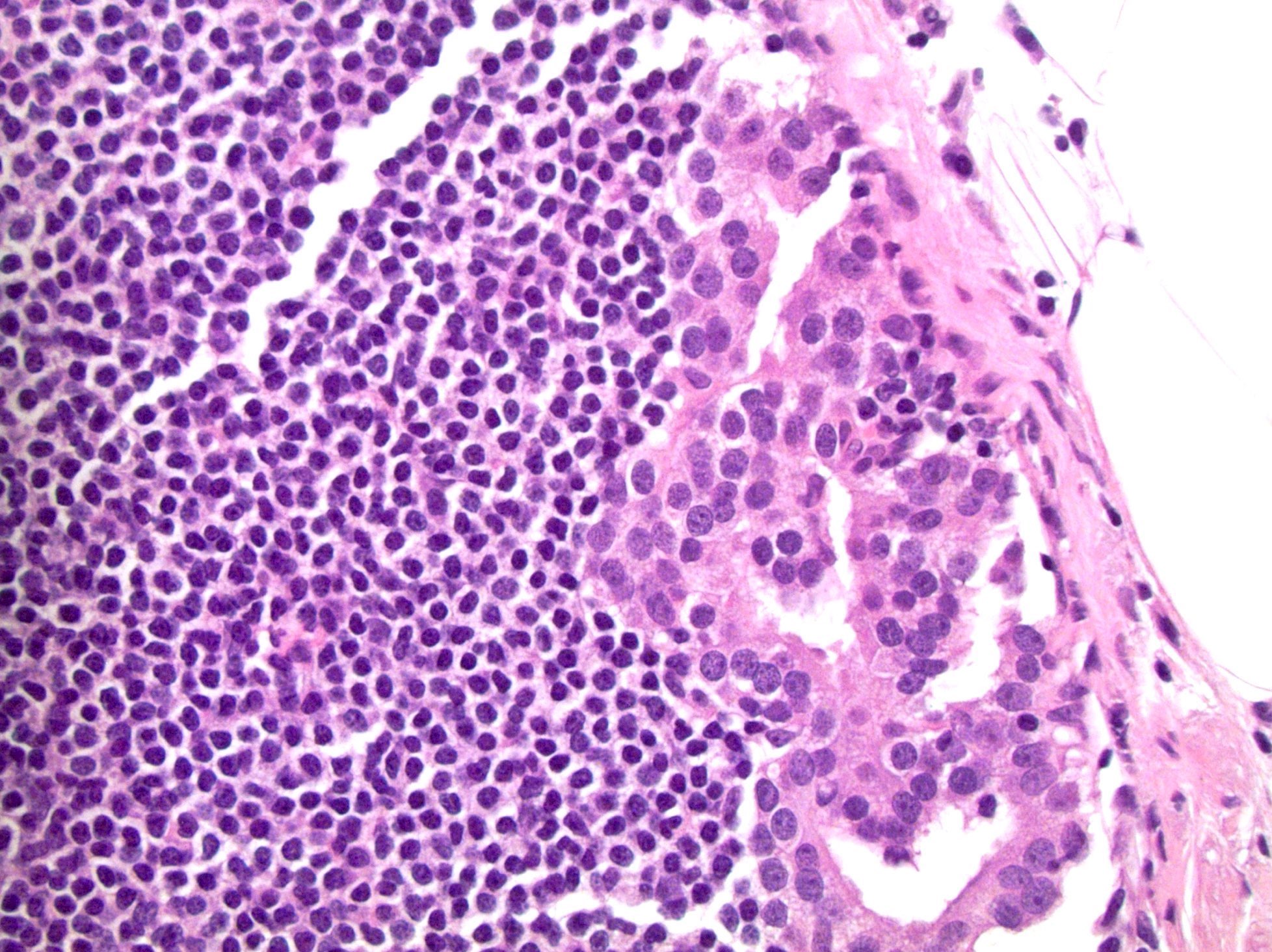
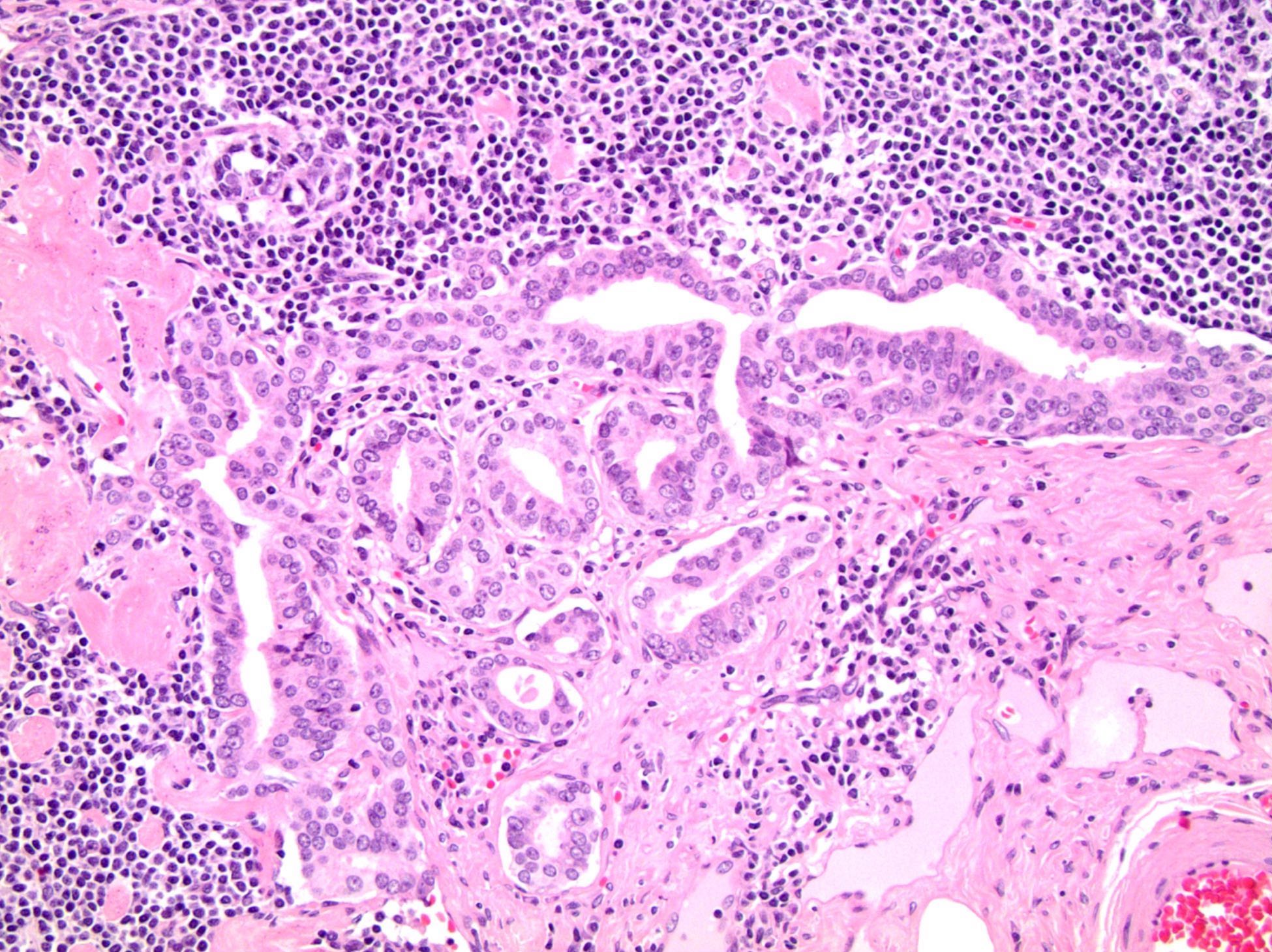
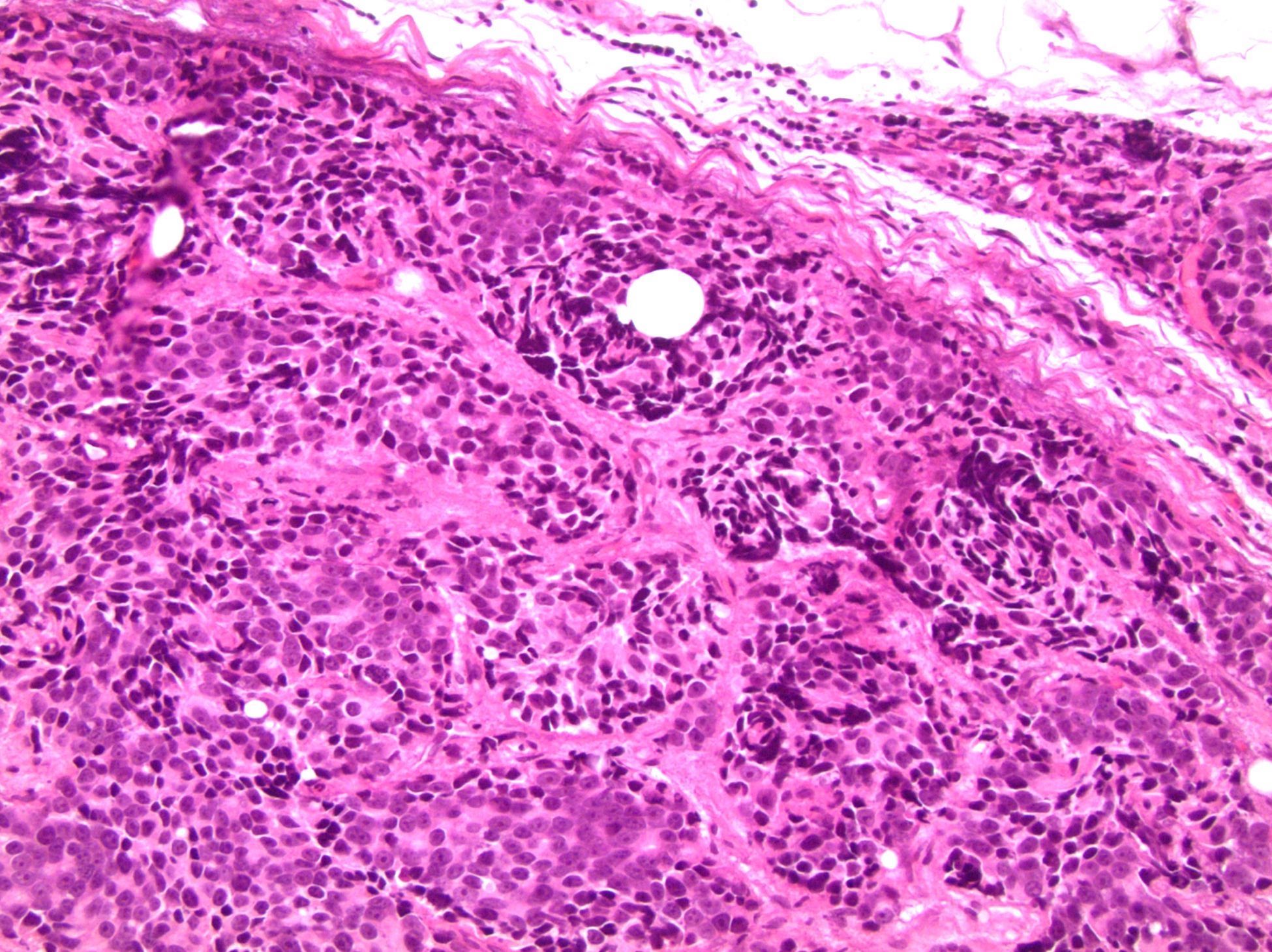
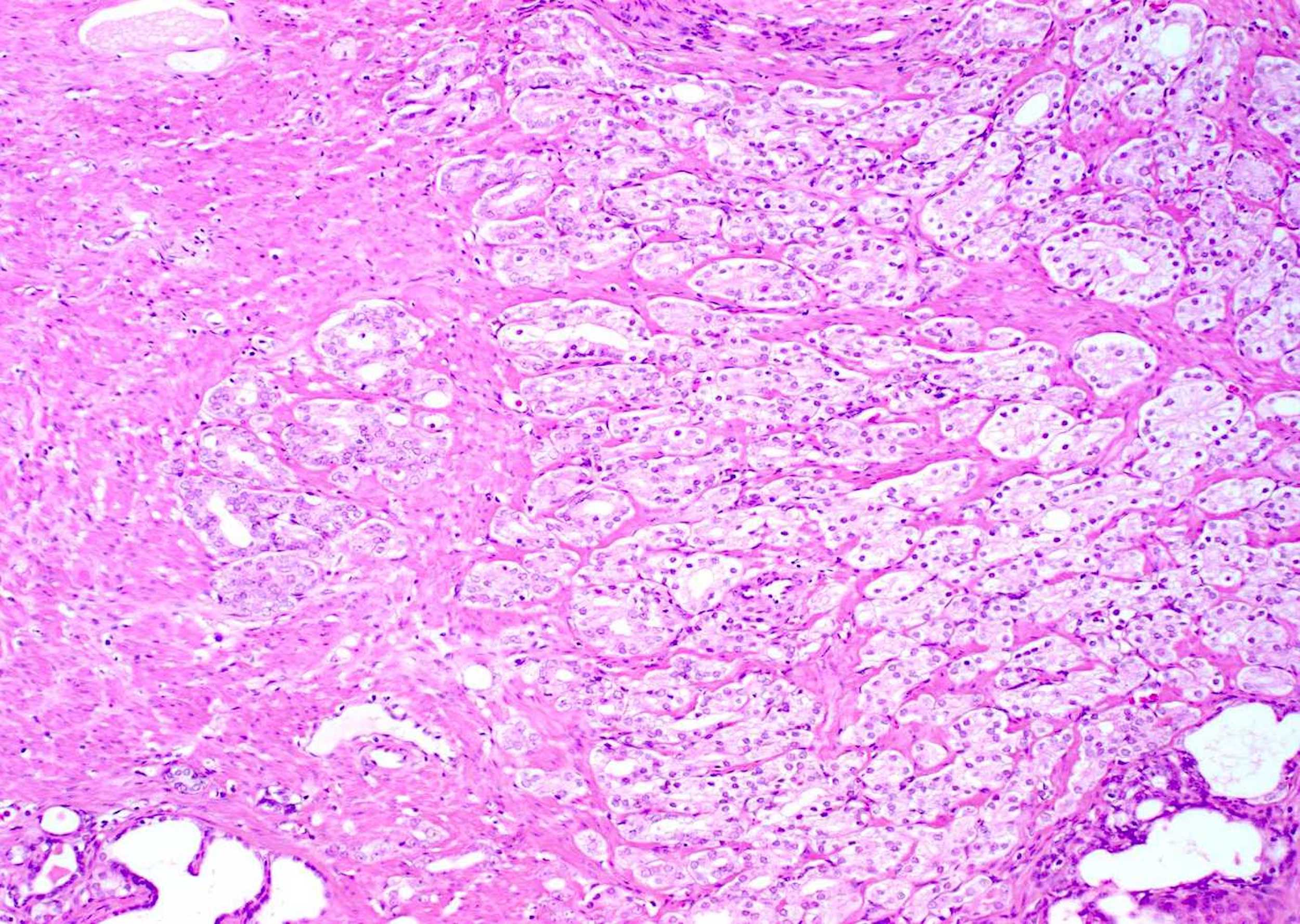
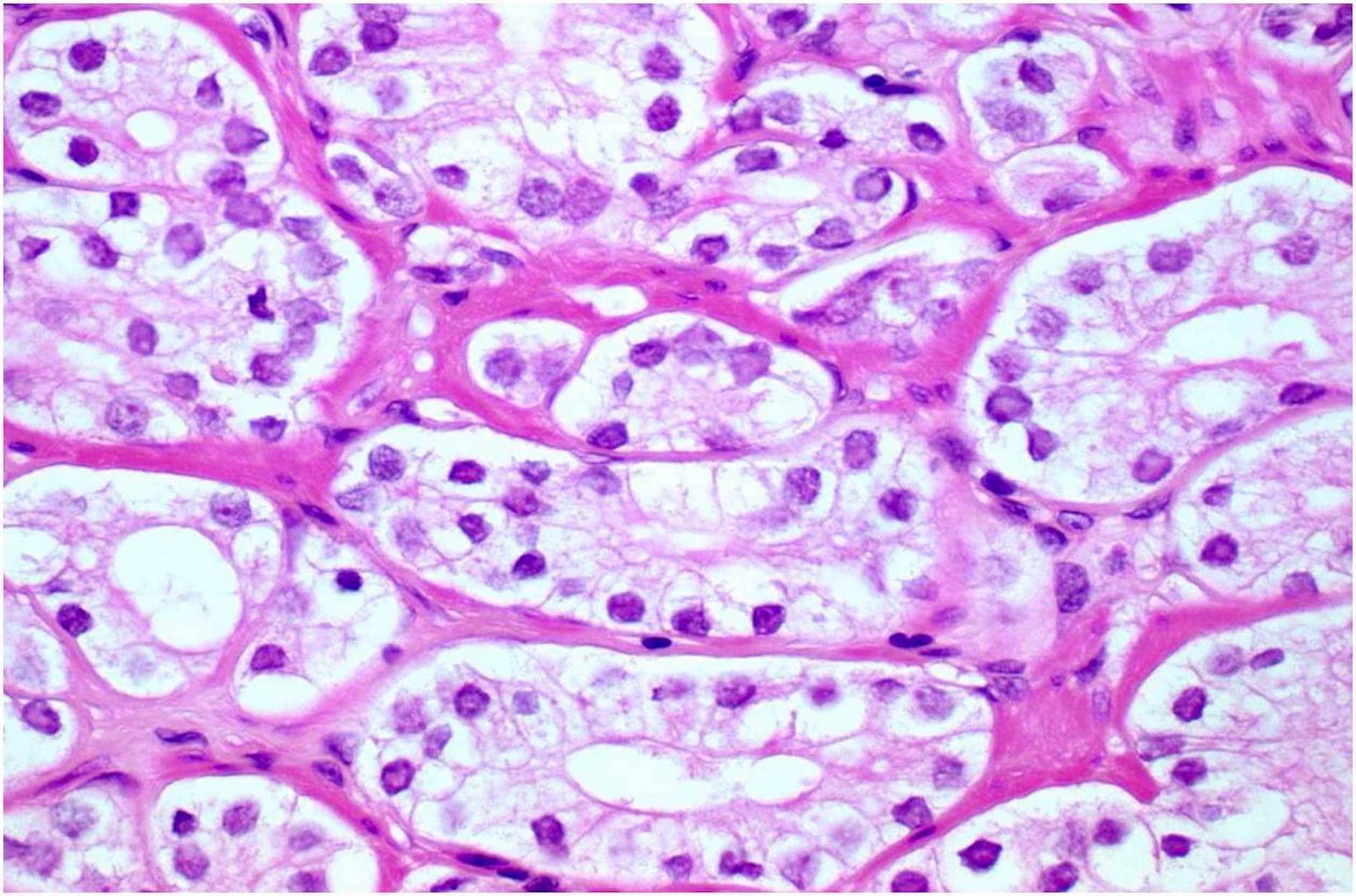
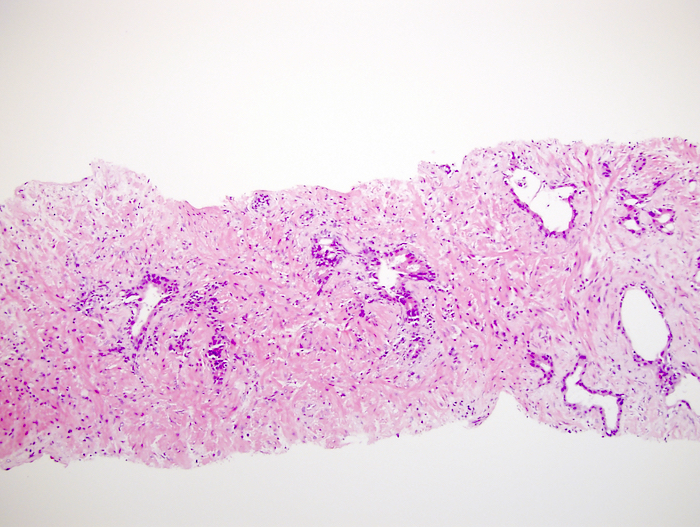
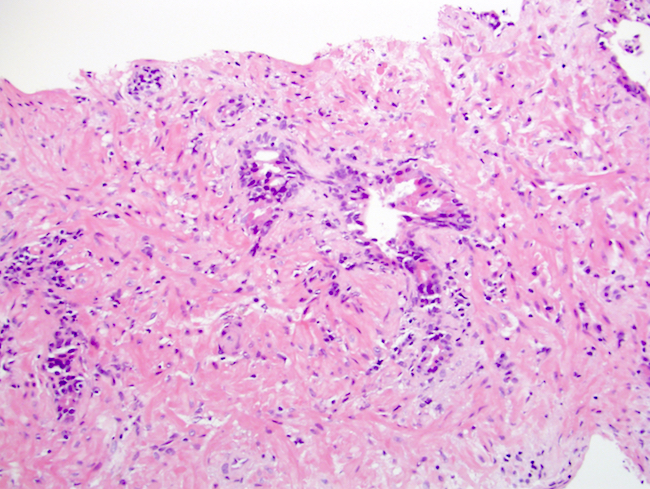
- pT2: organ confined
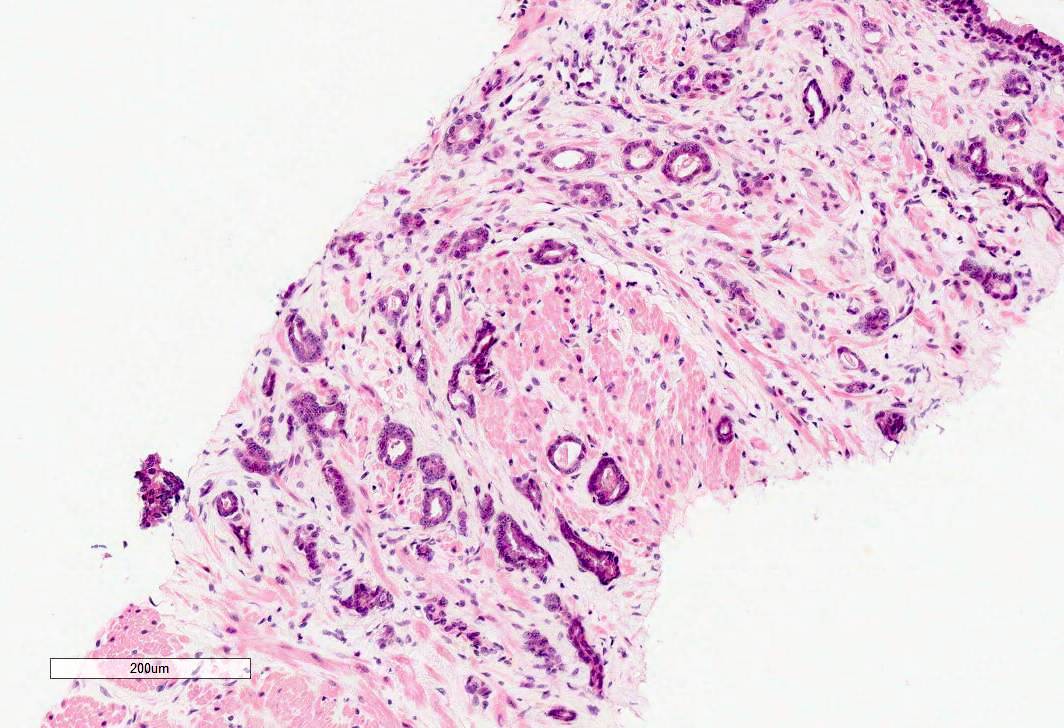
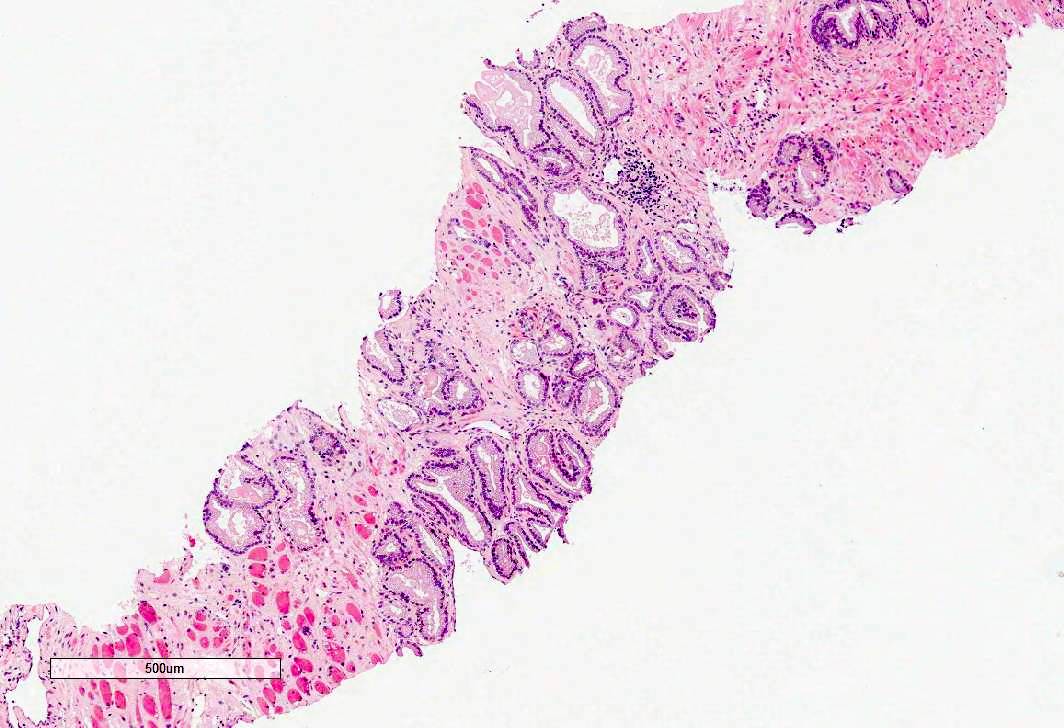
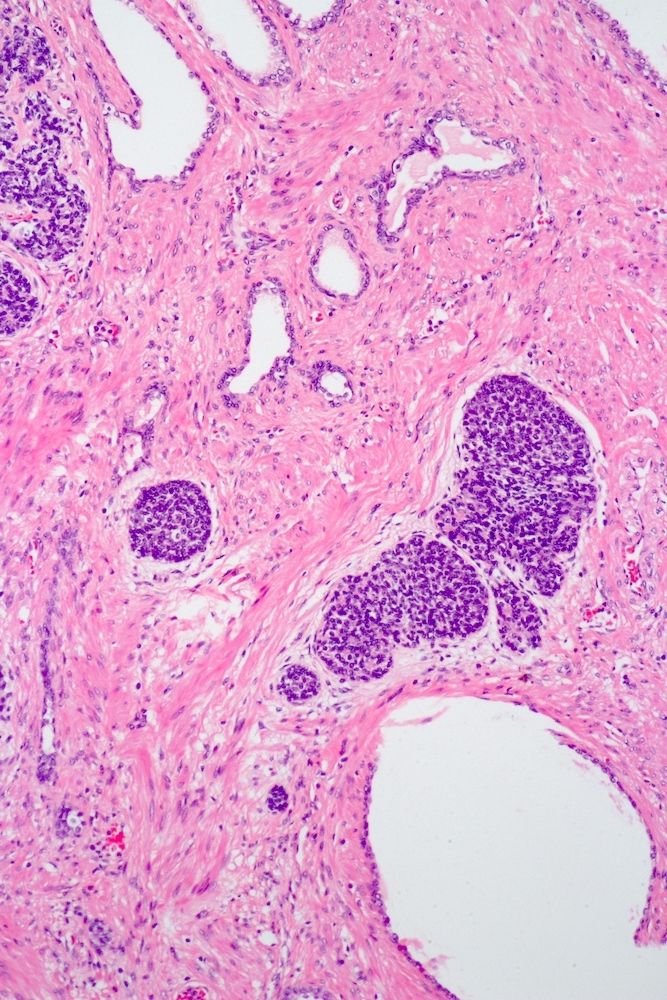
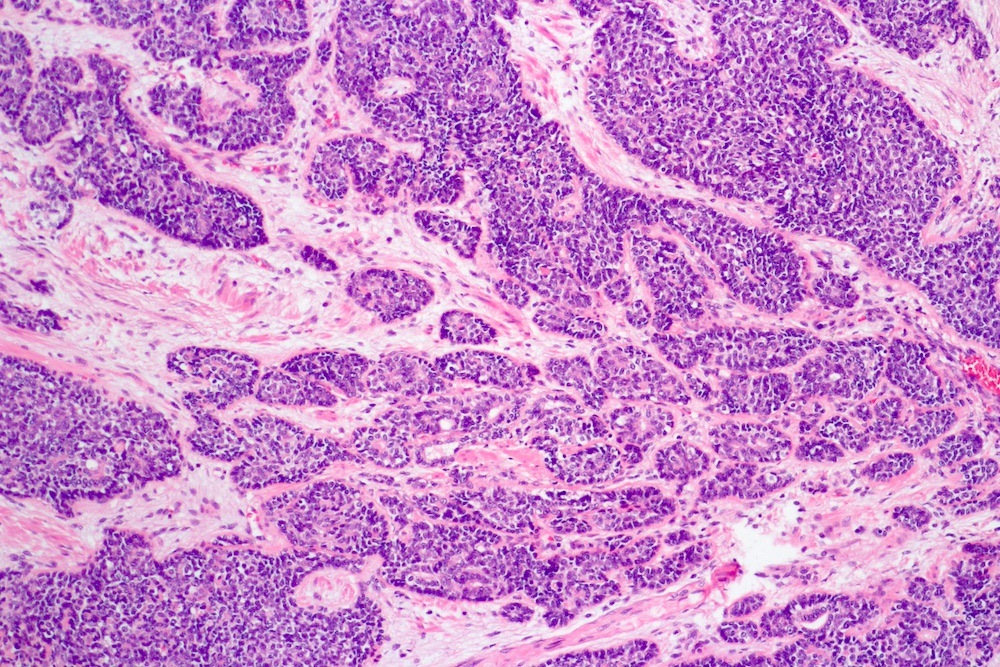
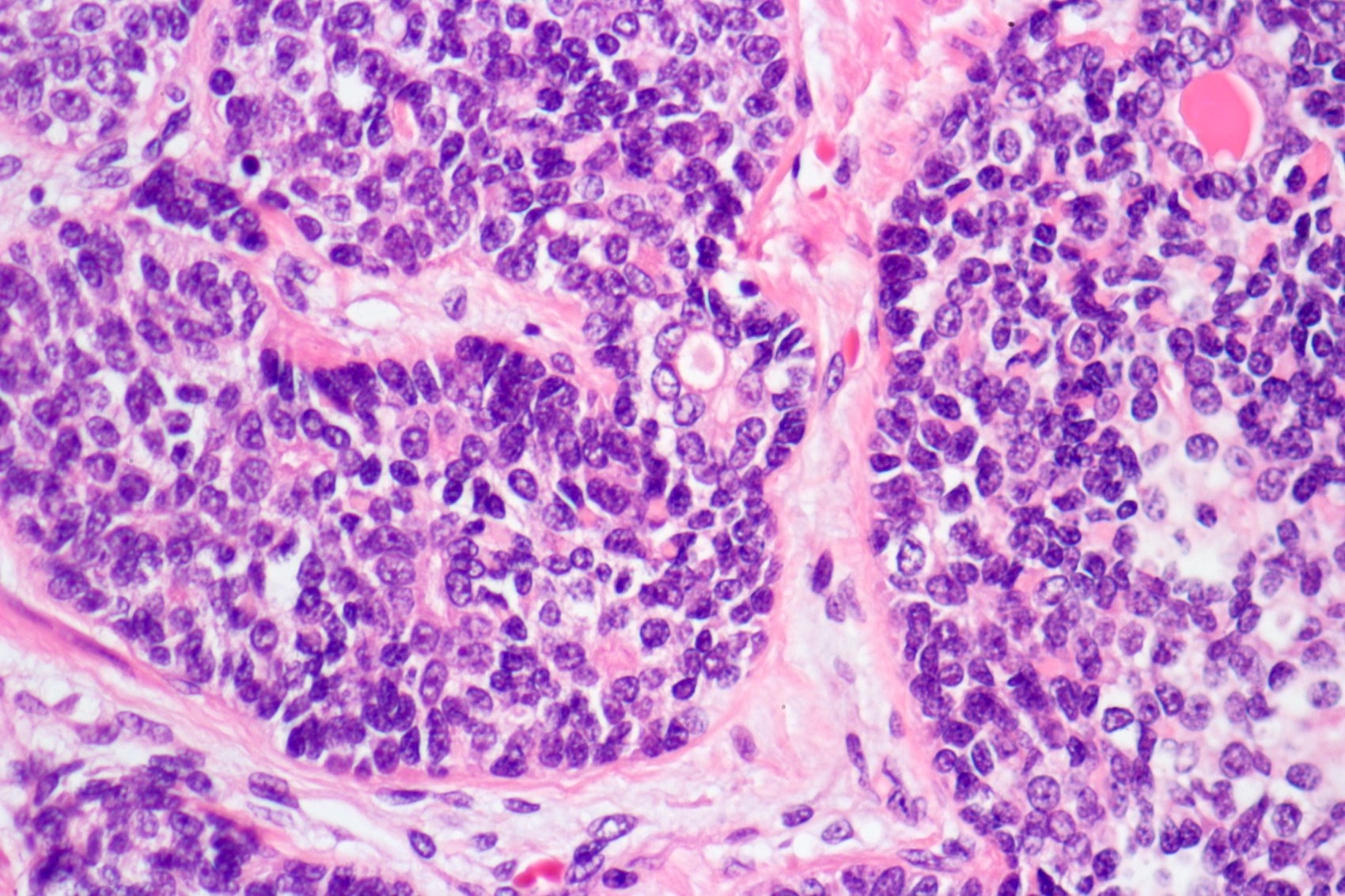
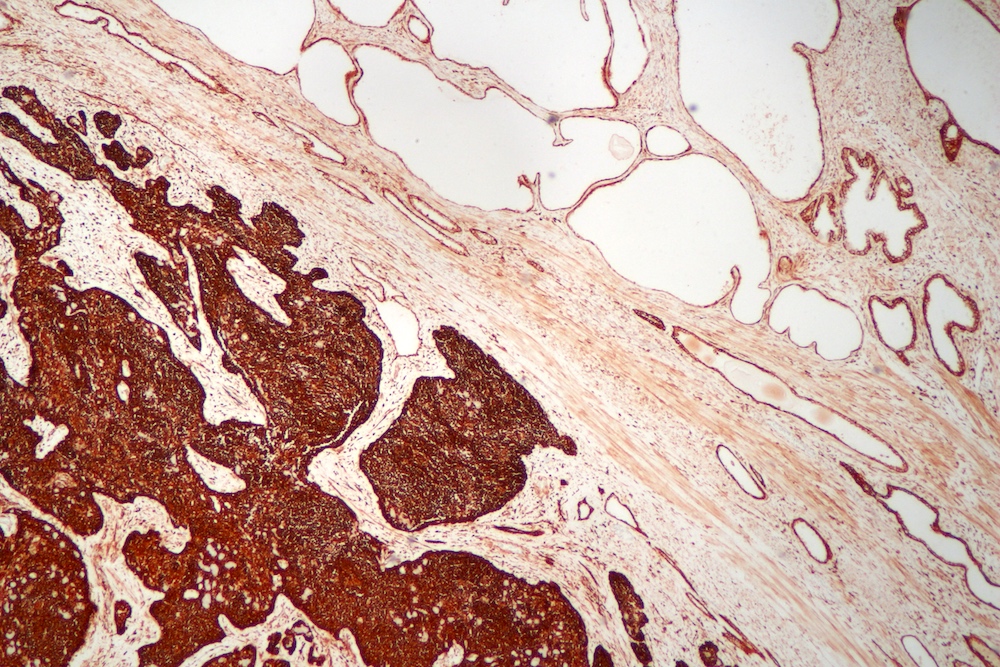
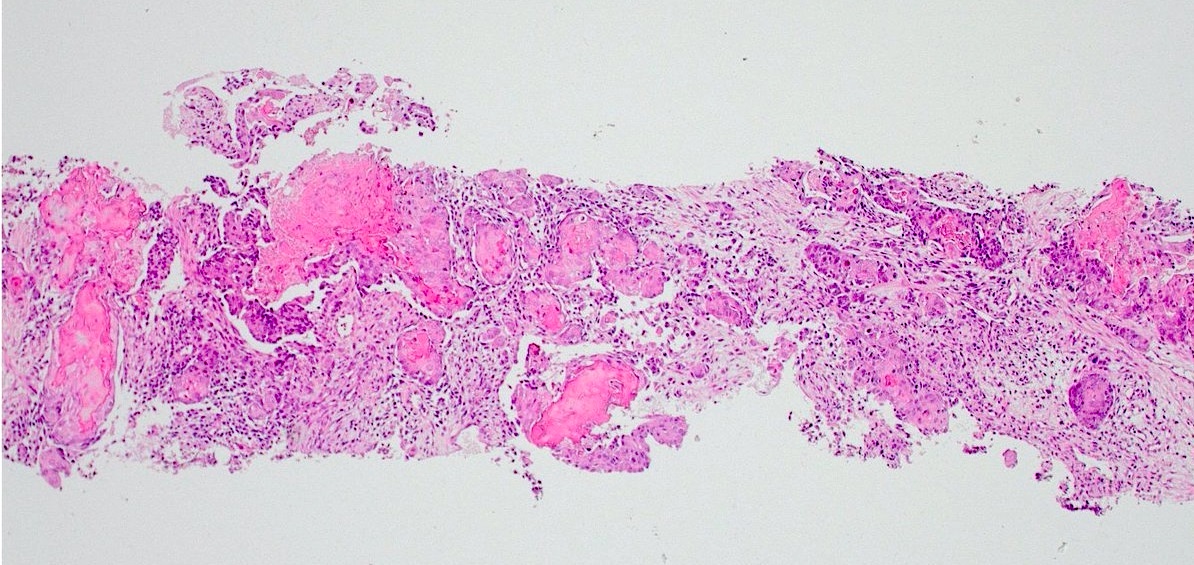
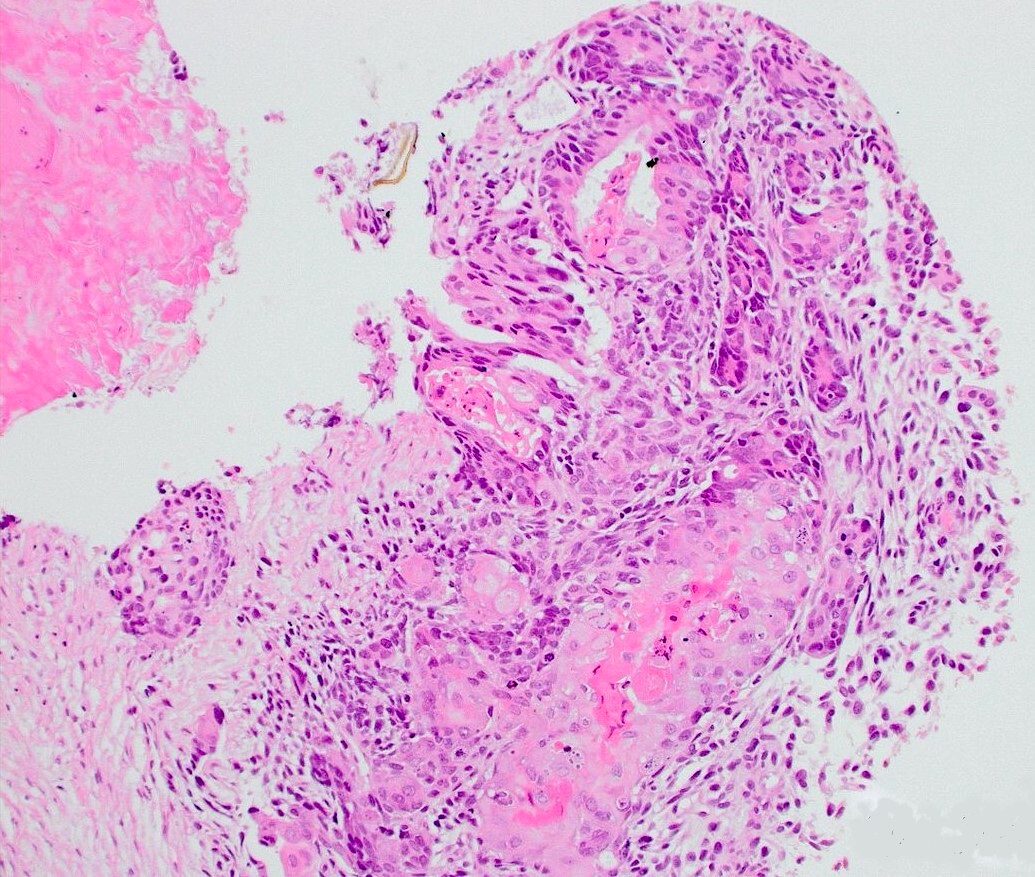
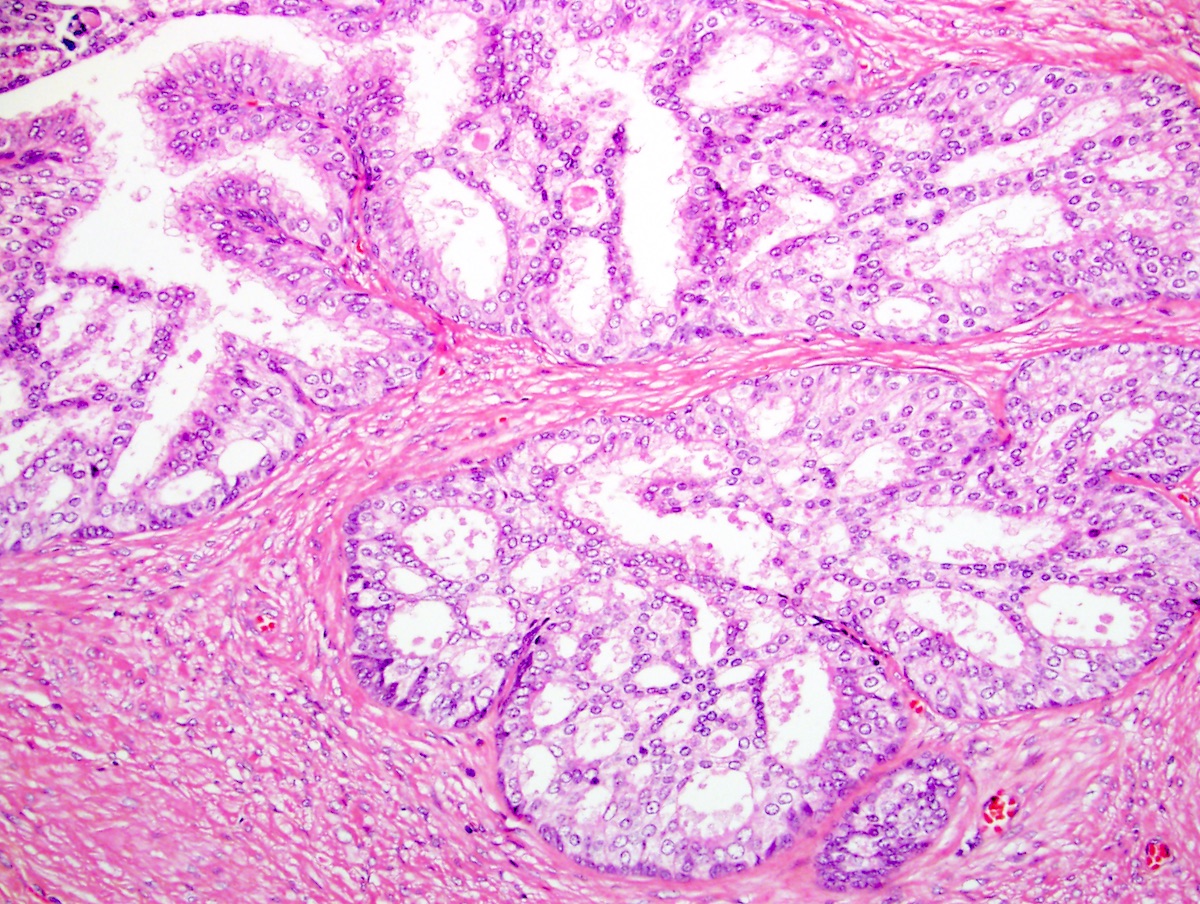
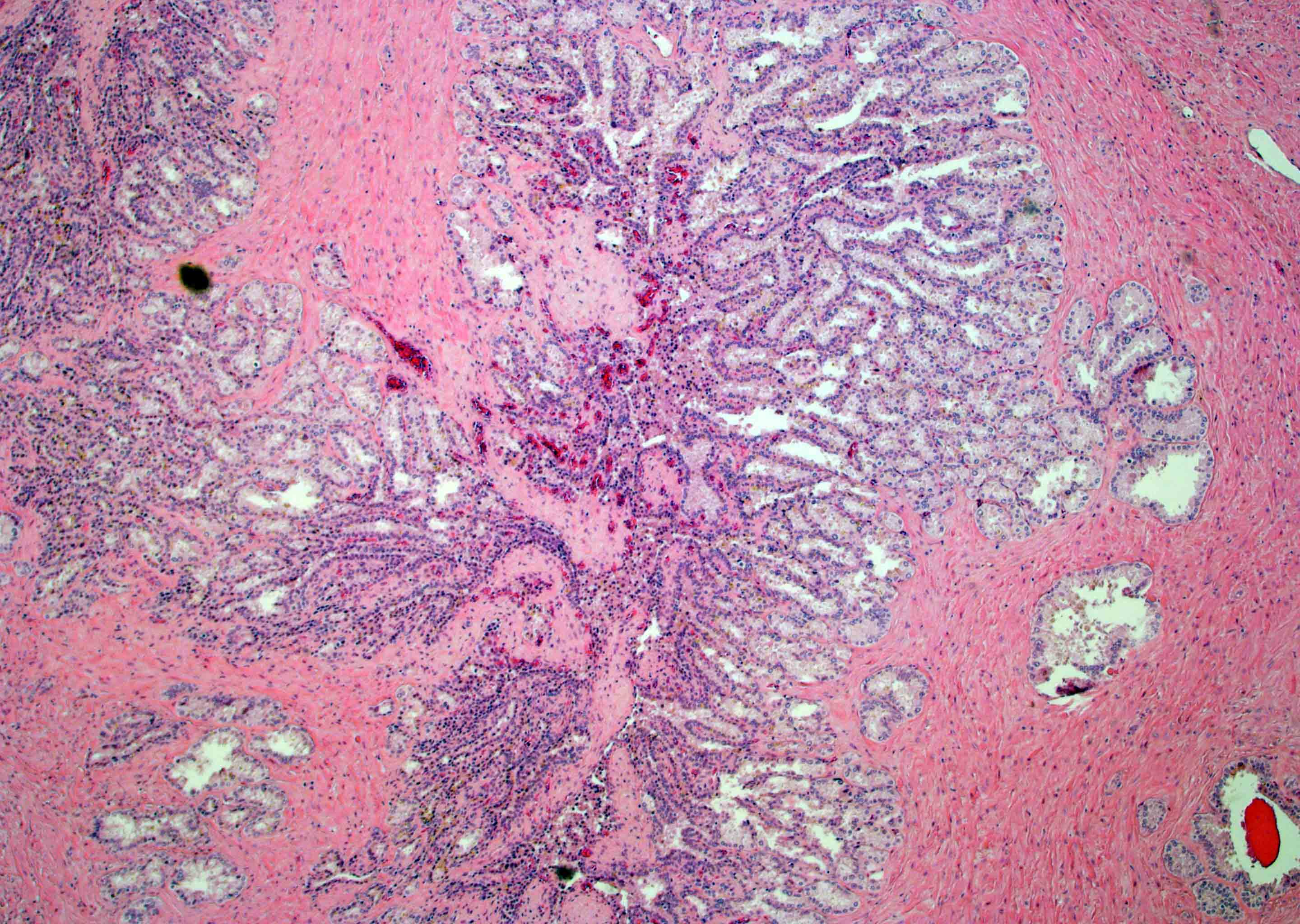
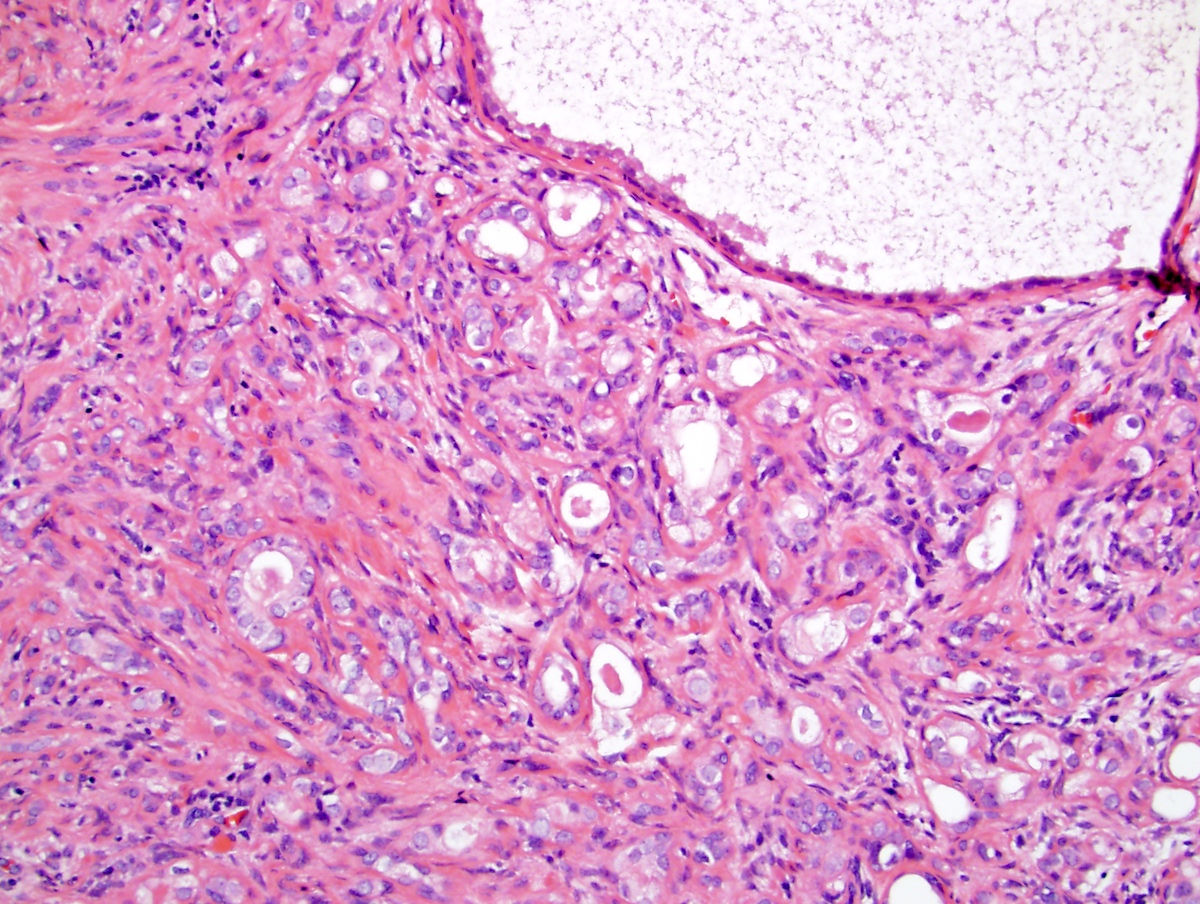
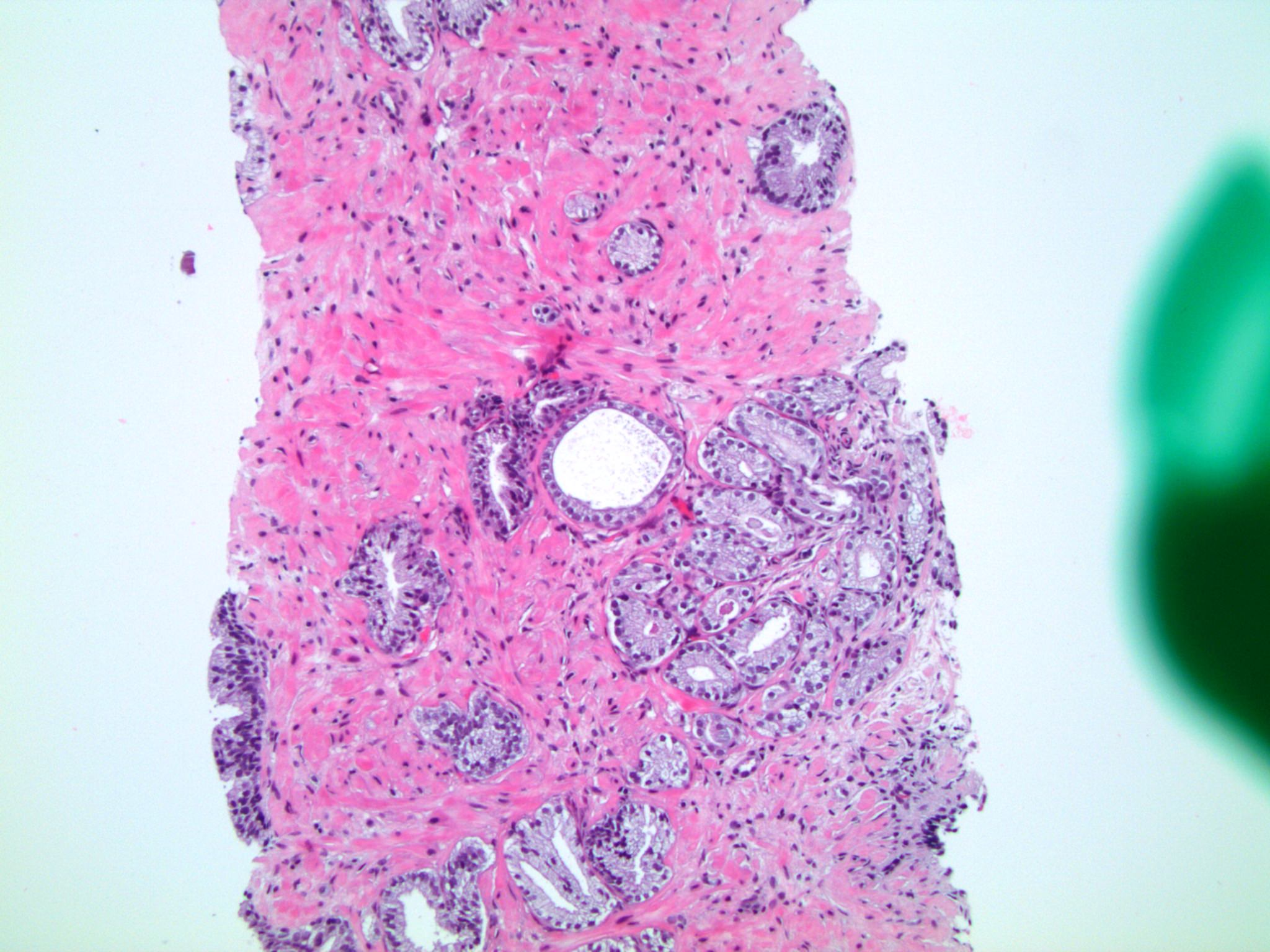
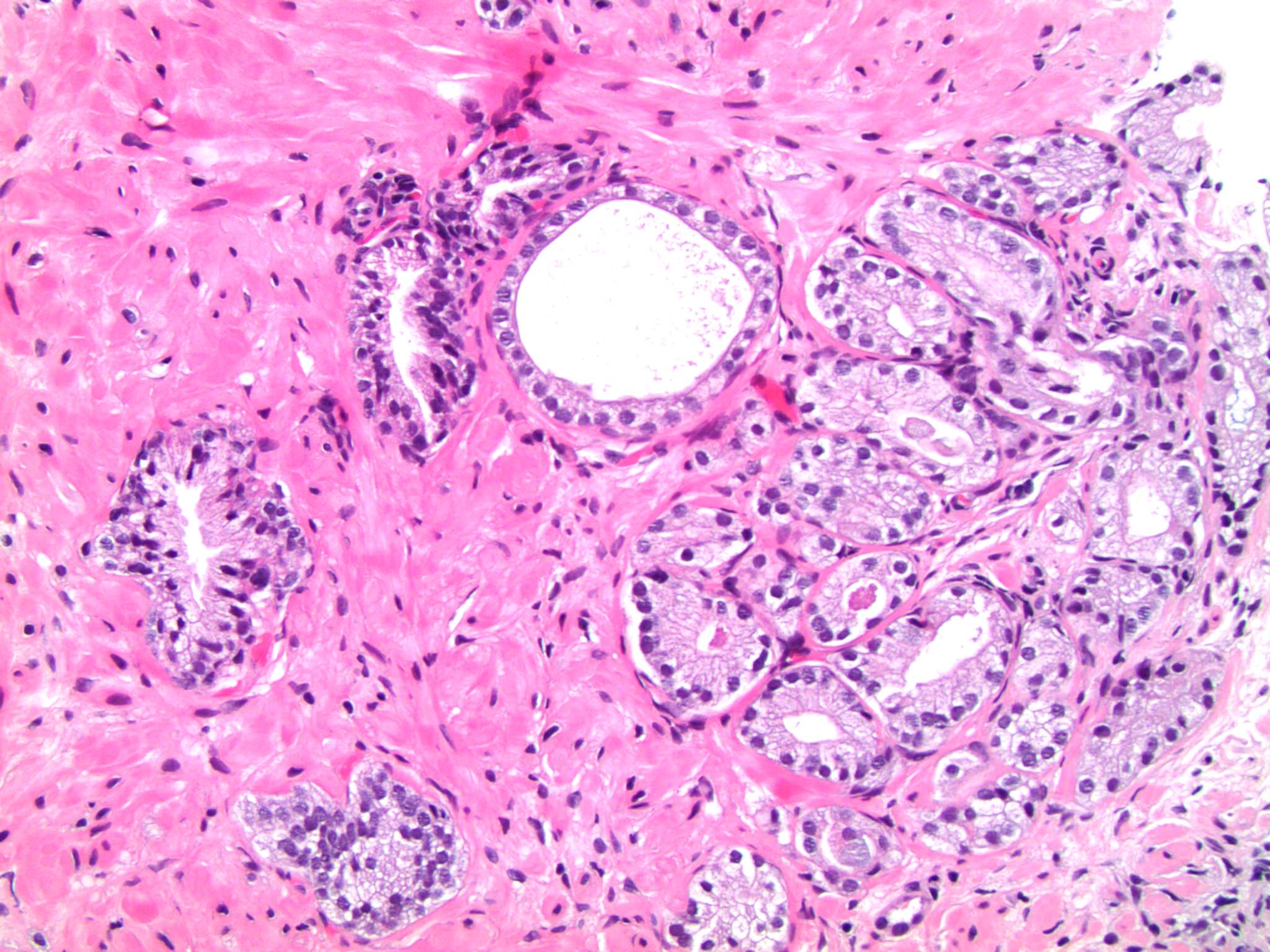
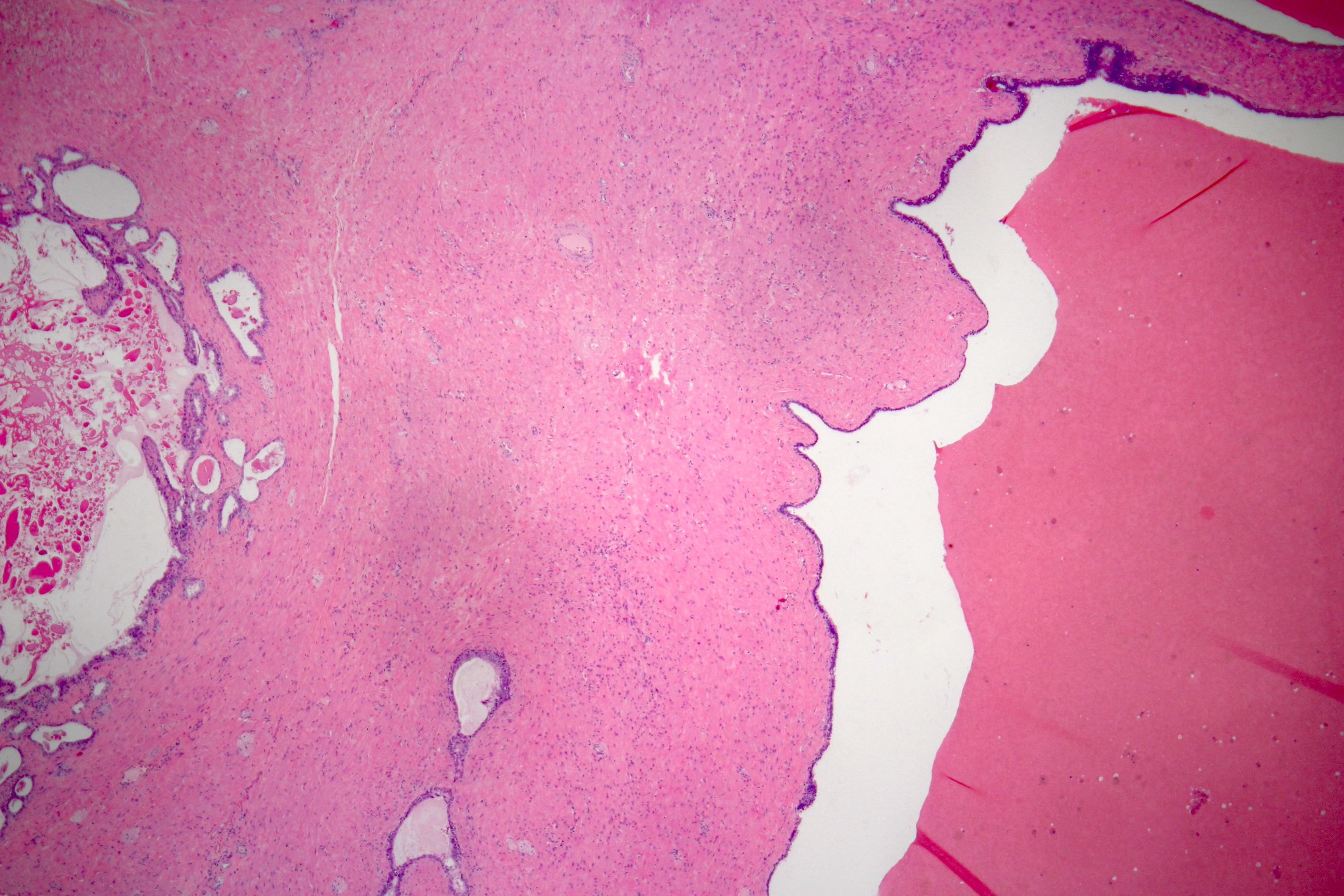
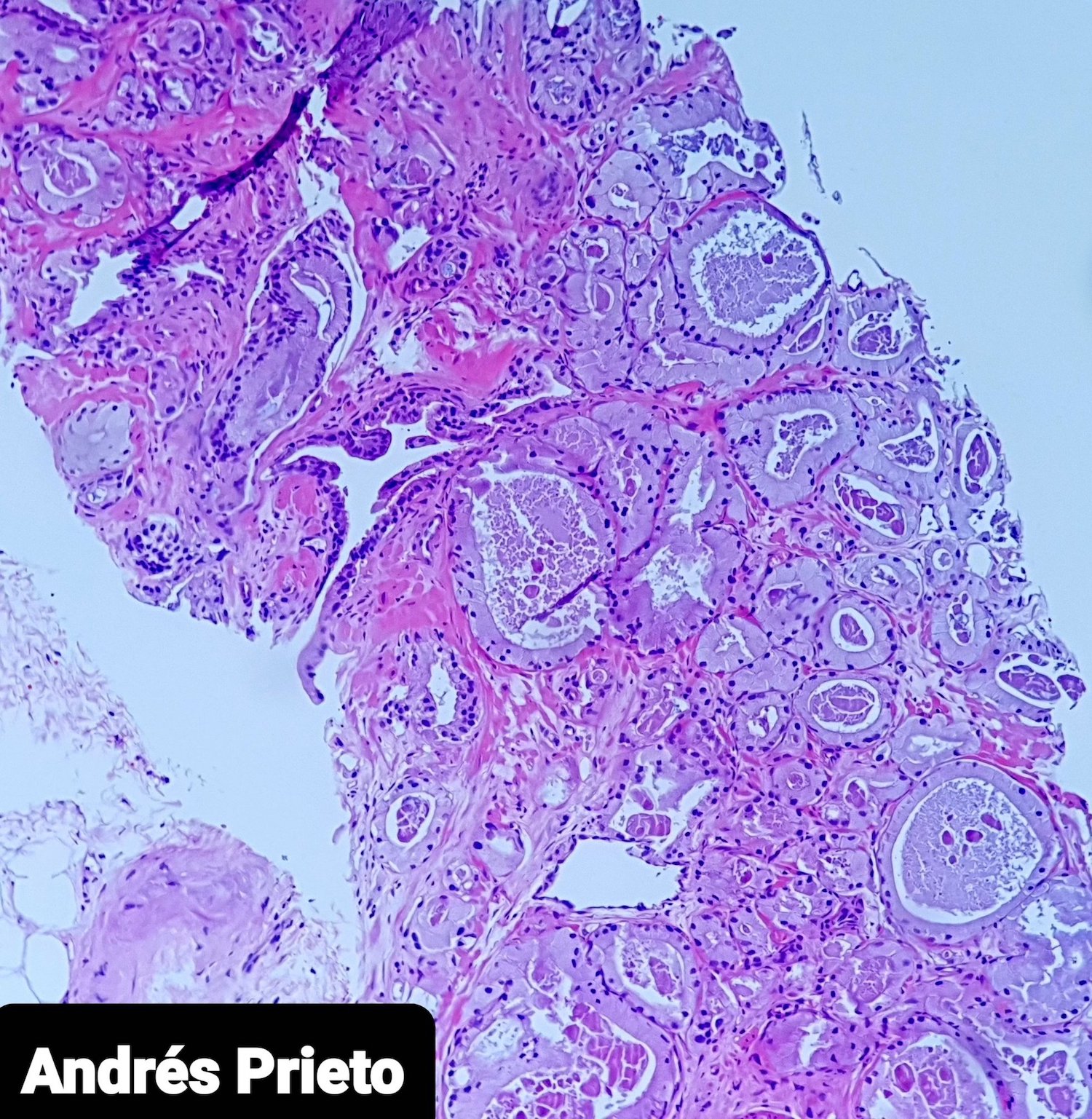
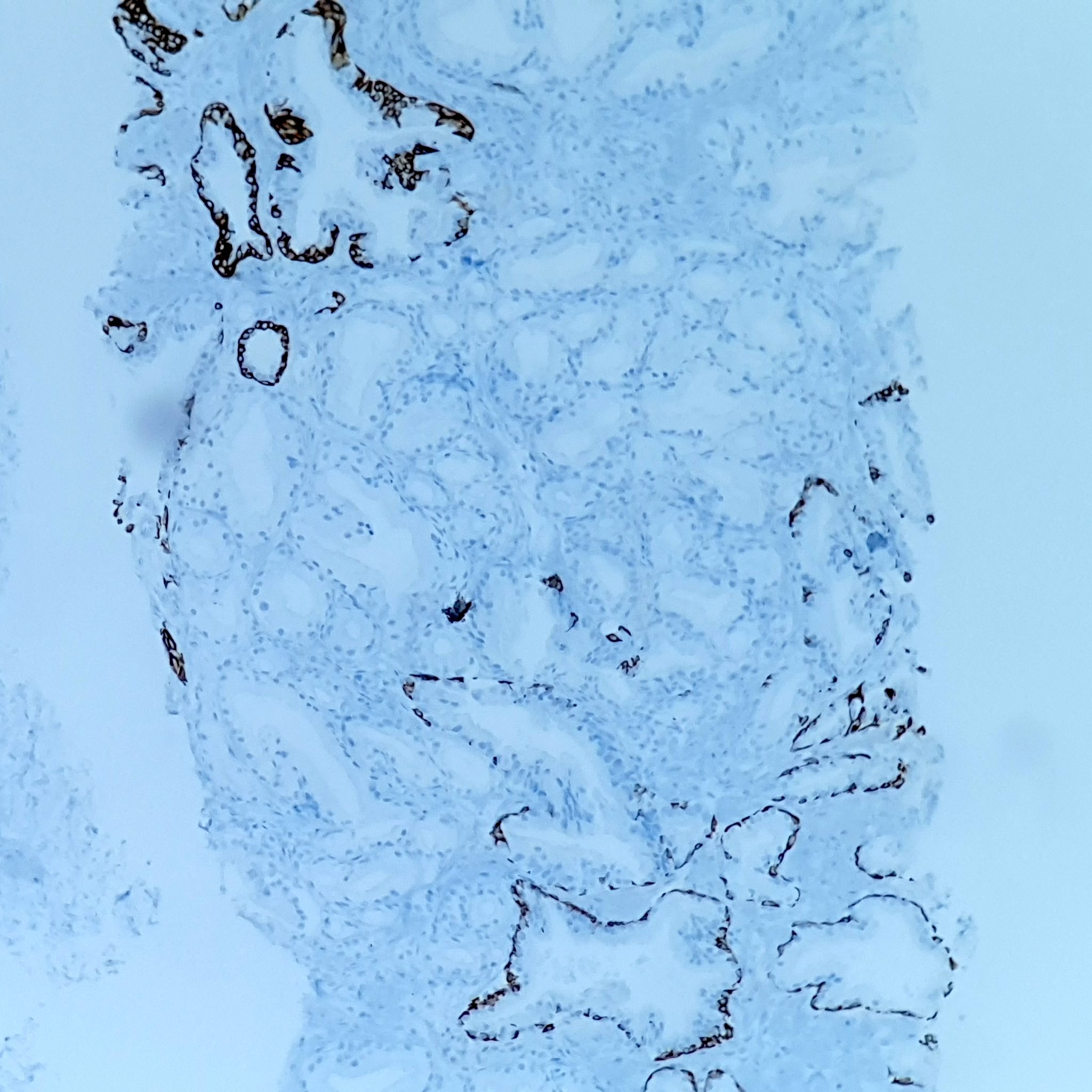
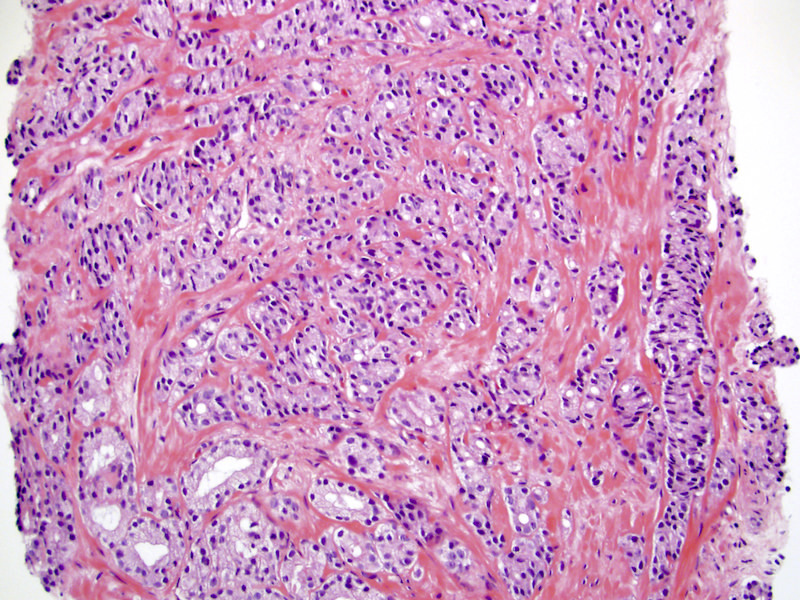
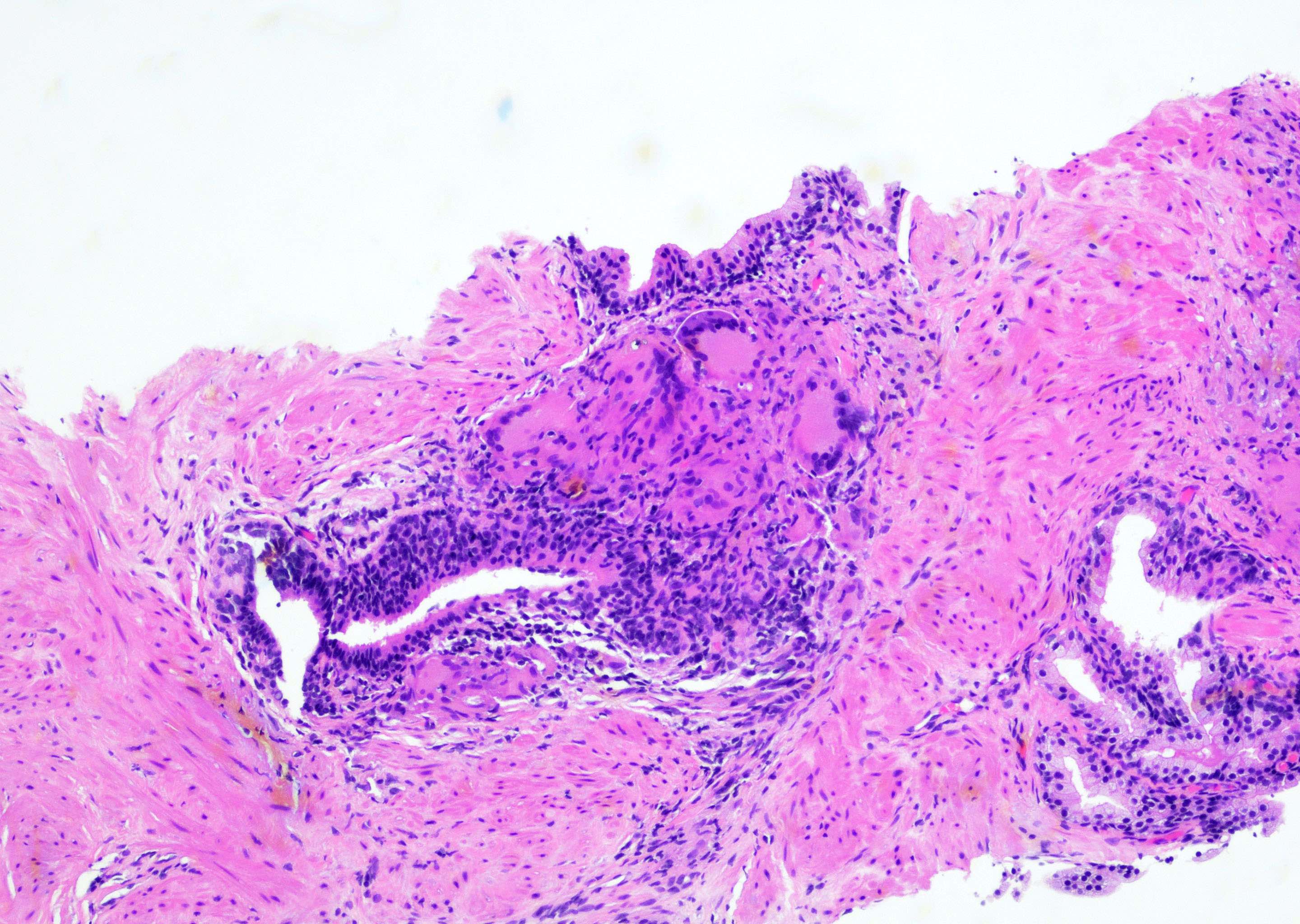
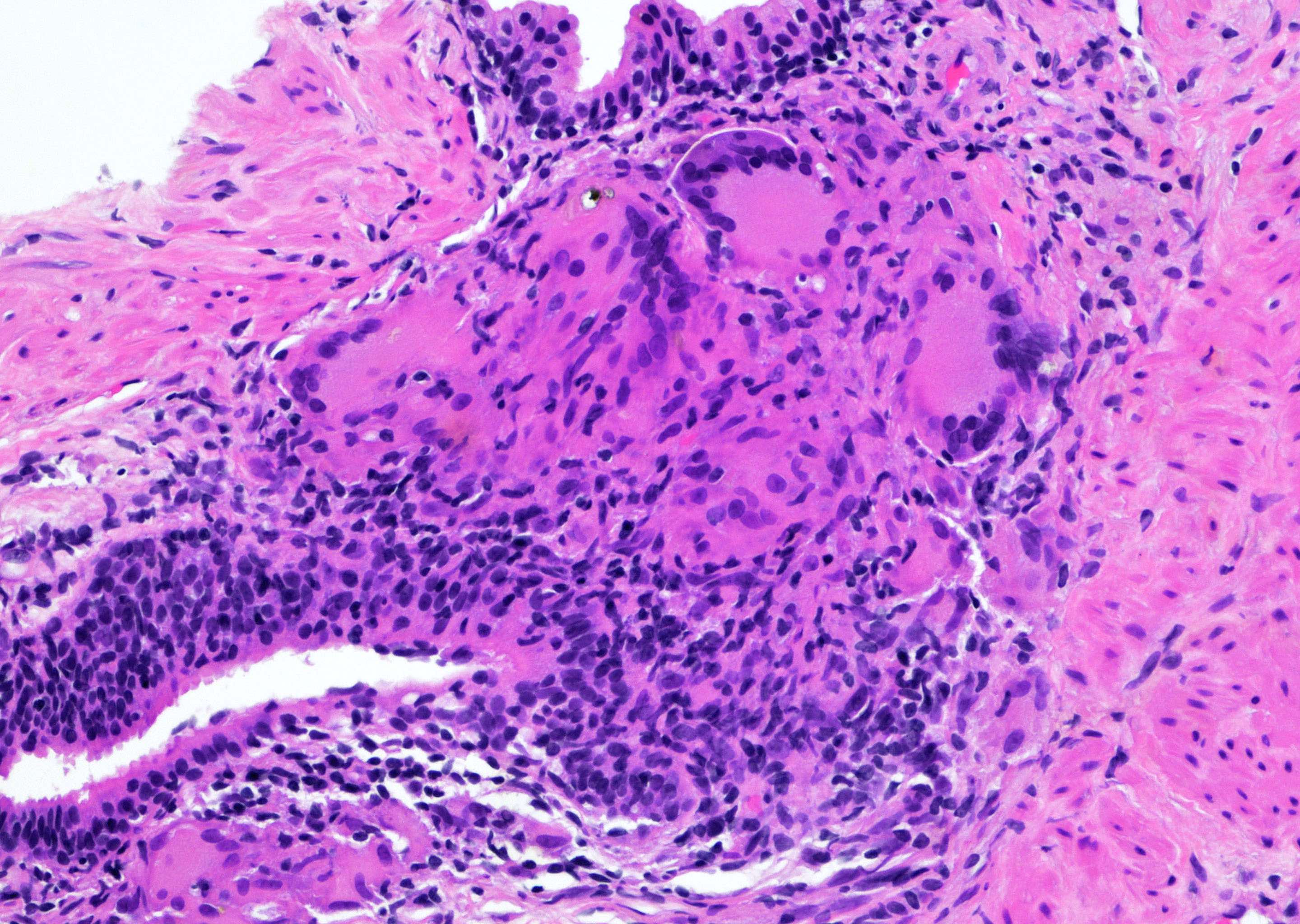
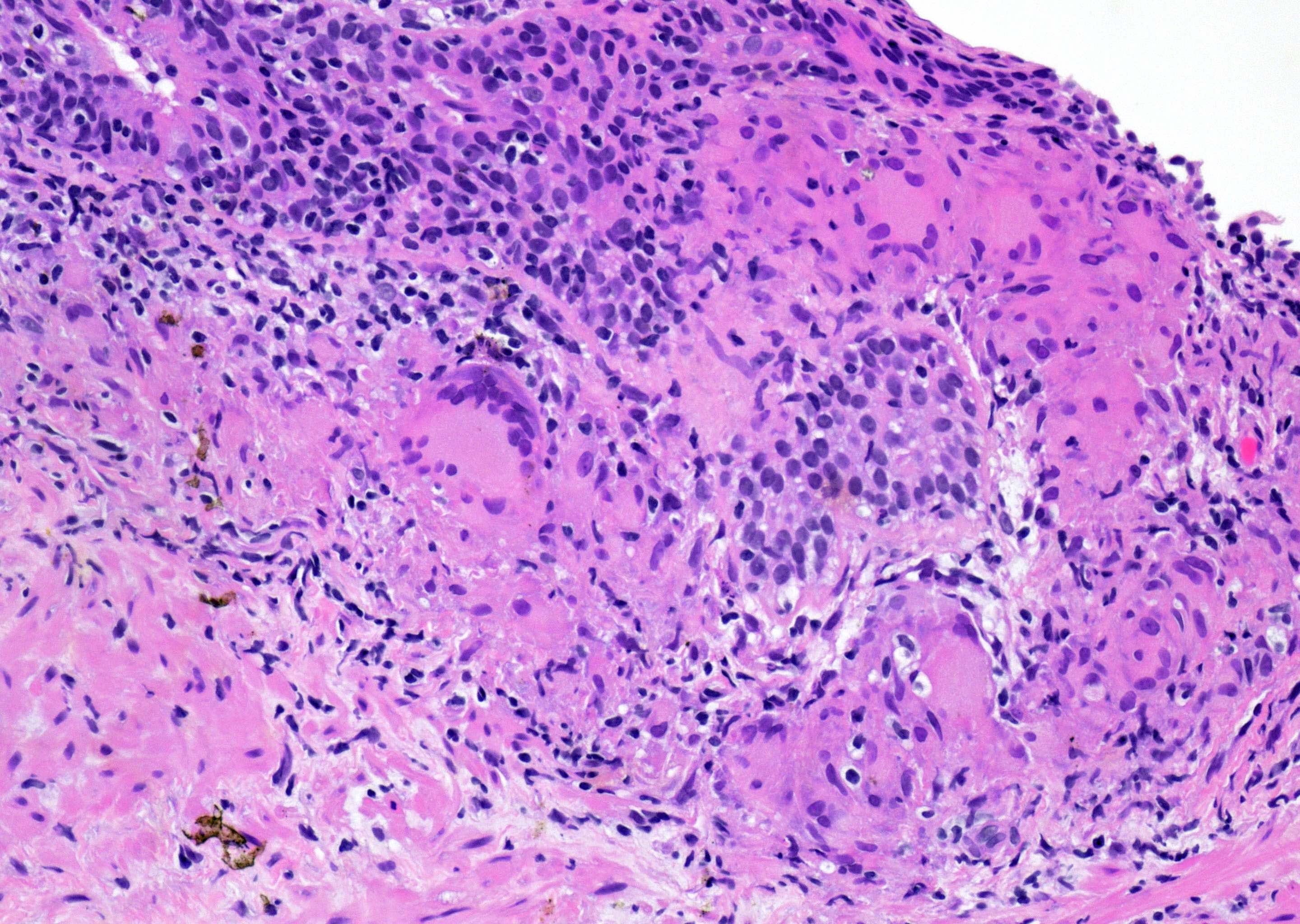
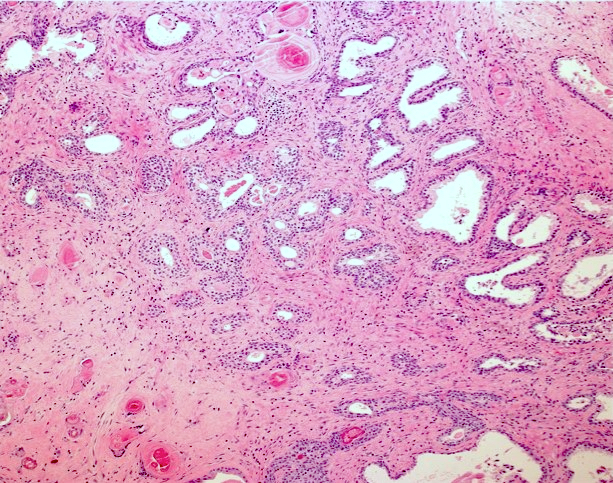
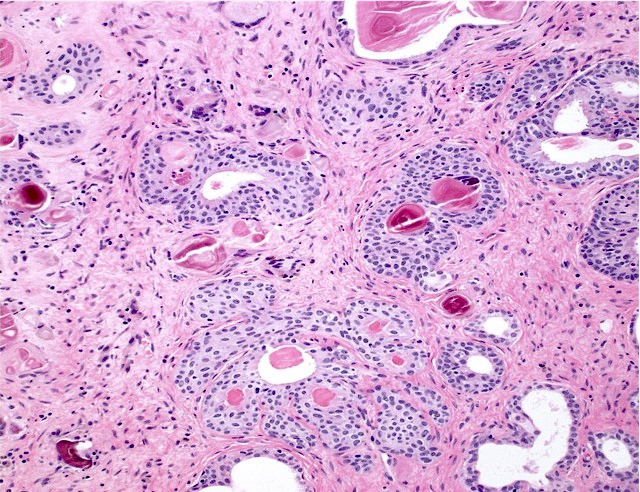
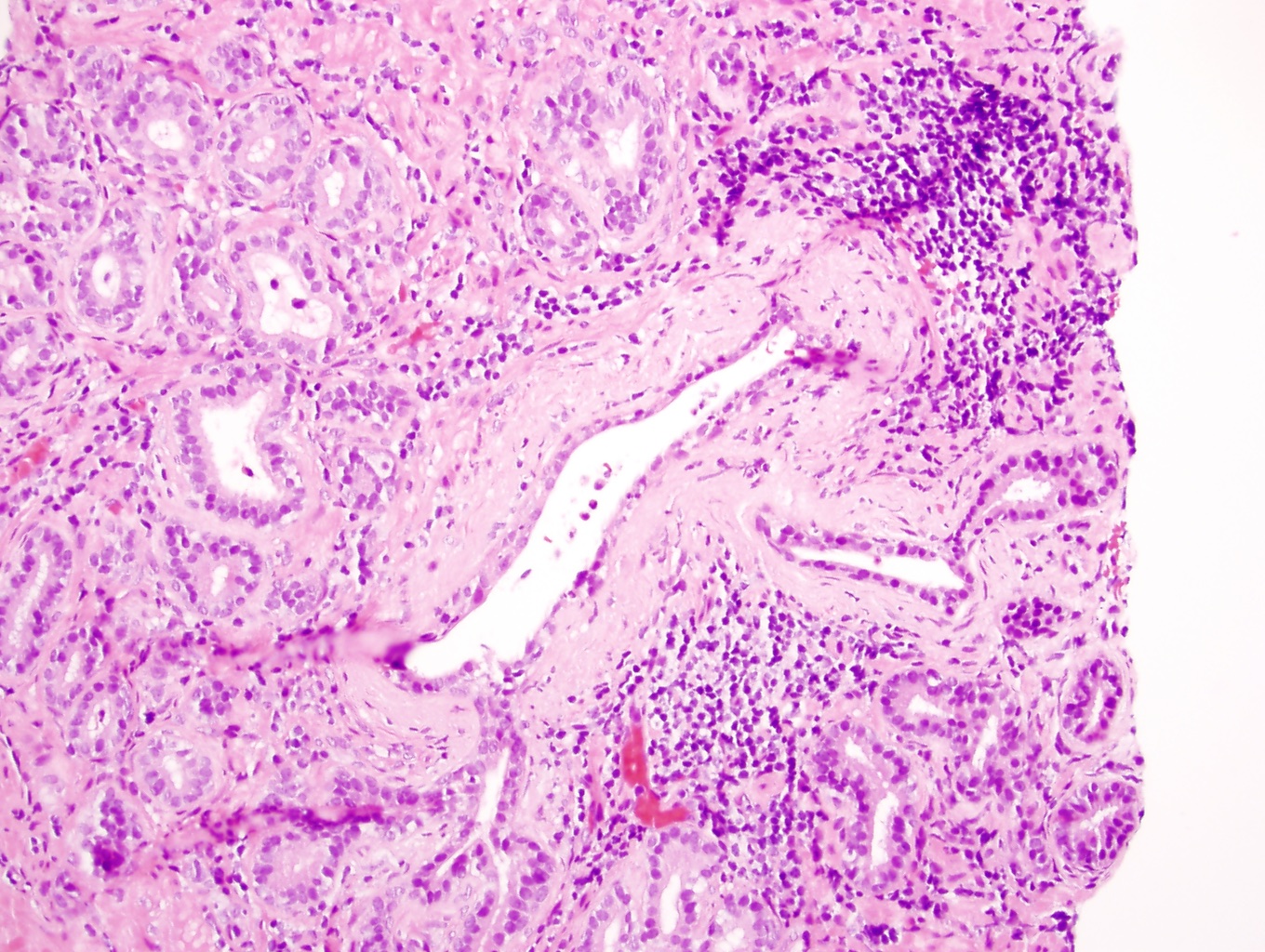
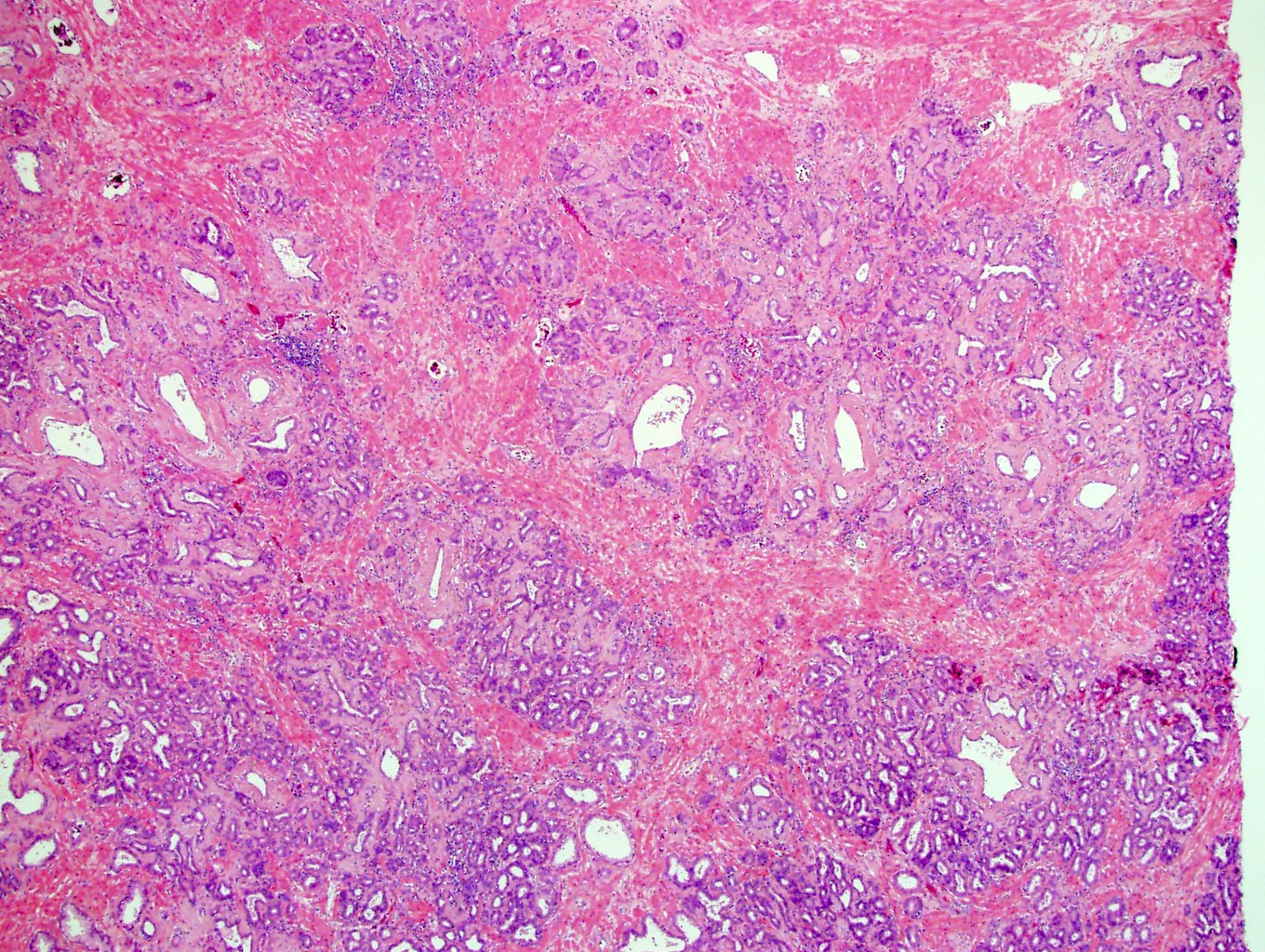
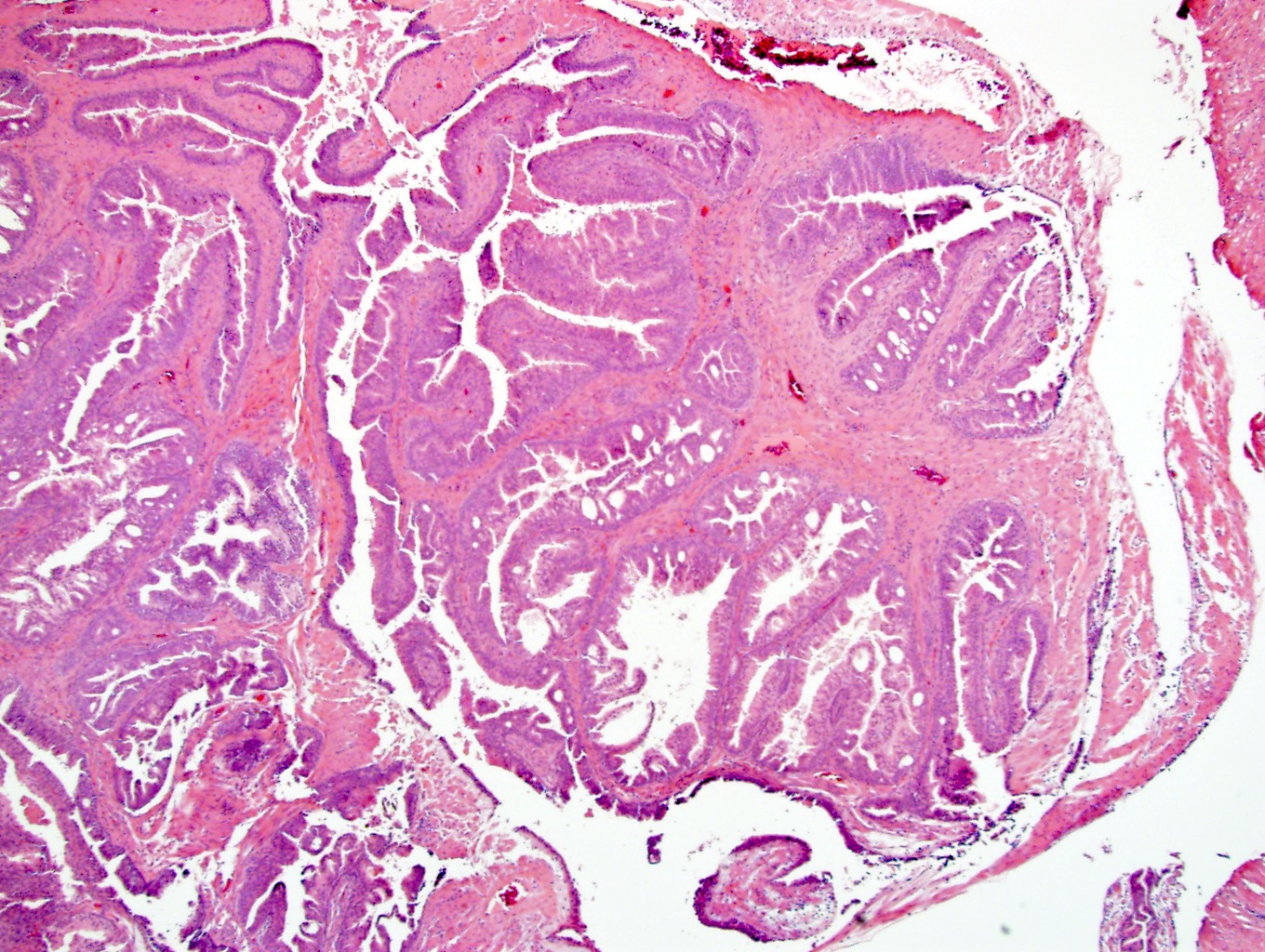
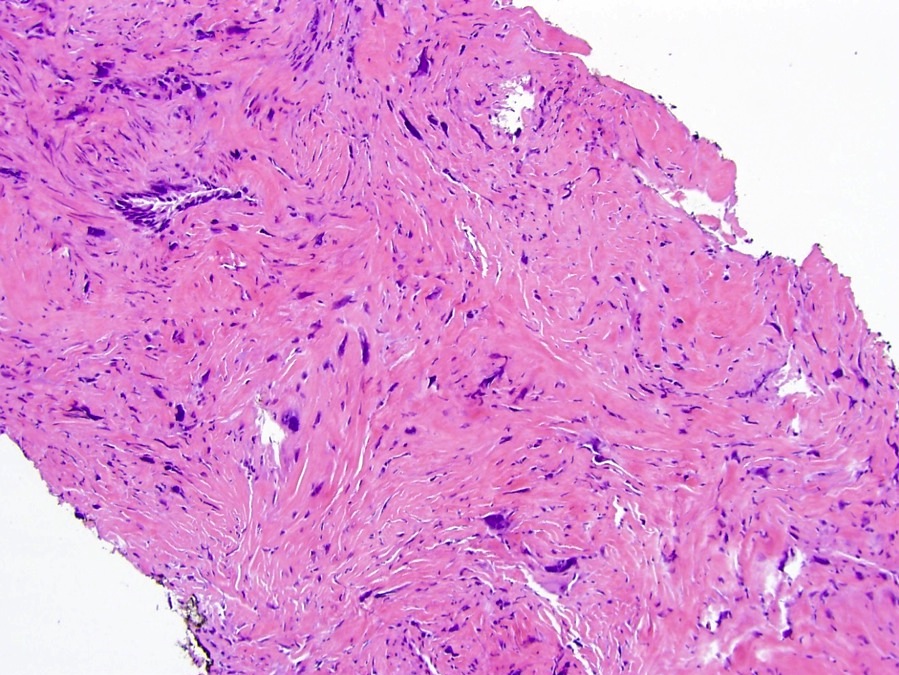
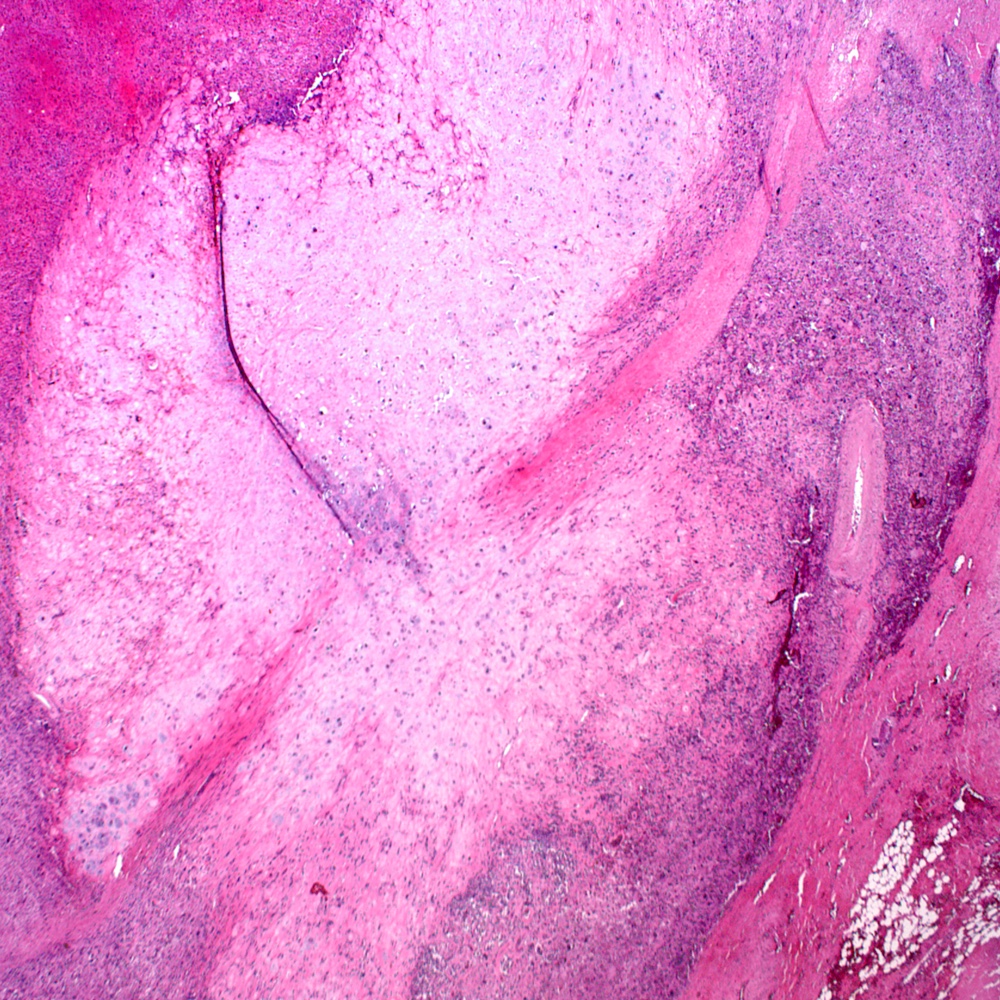
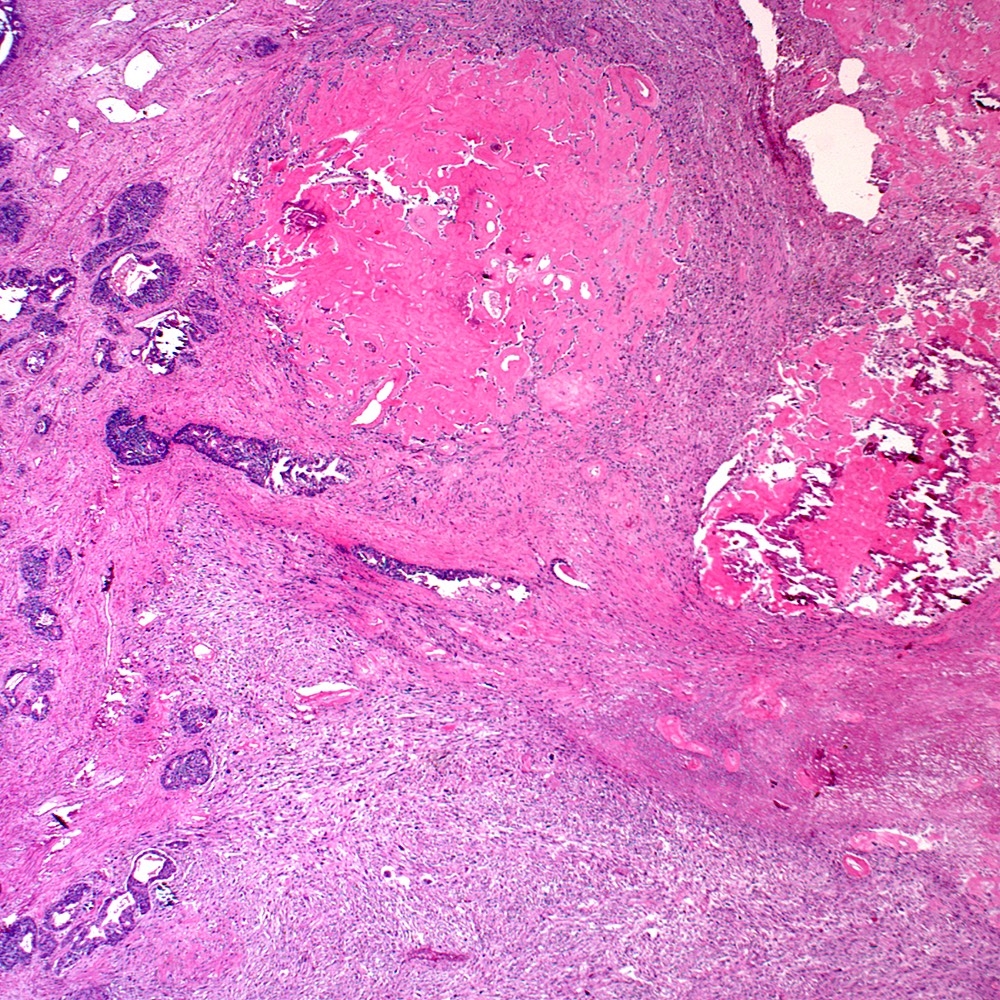
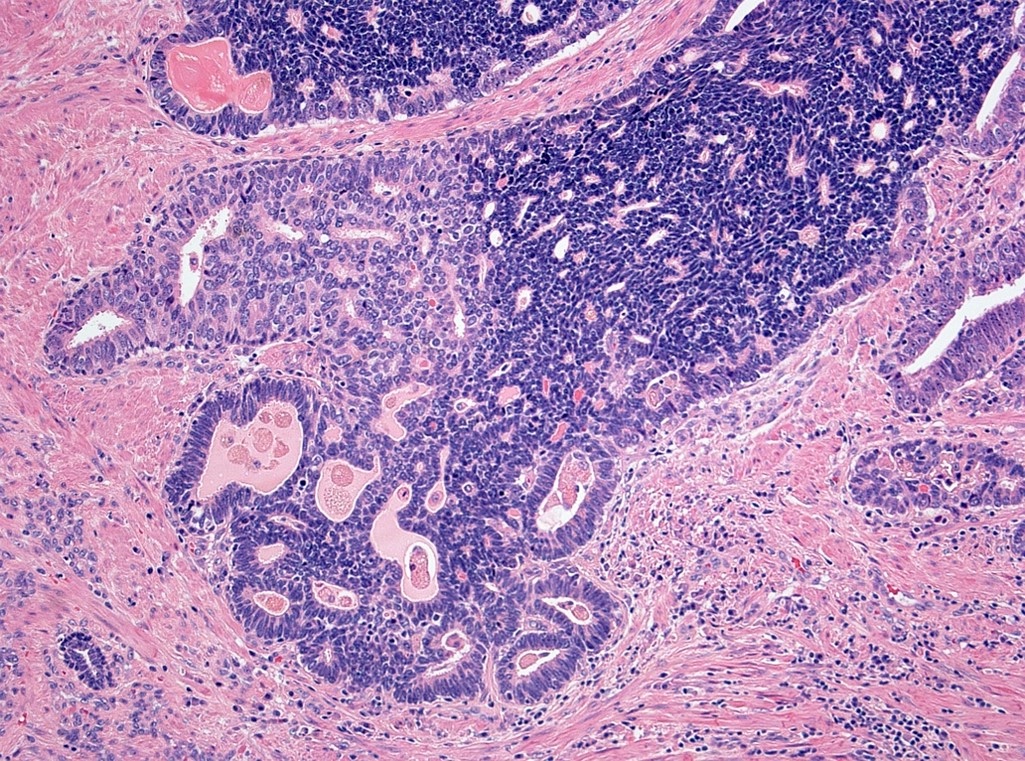
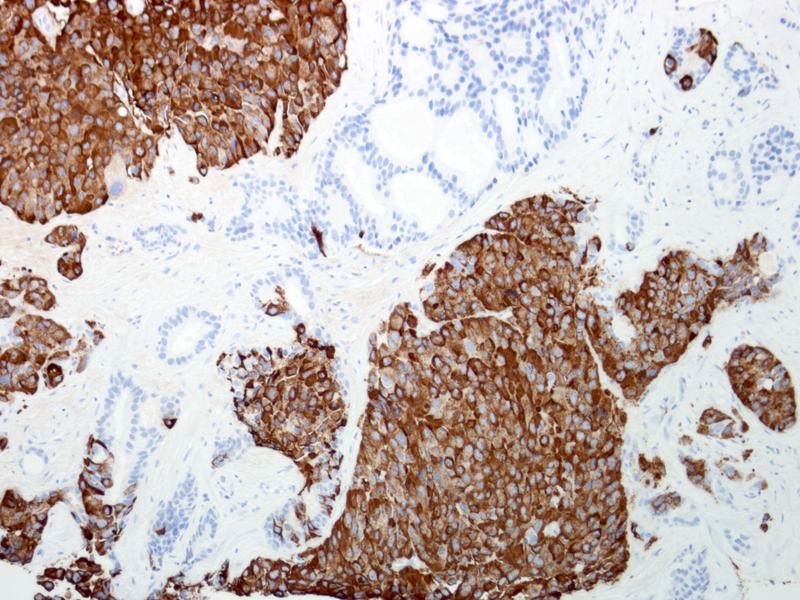
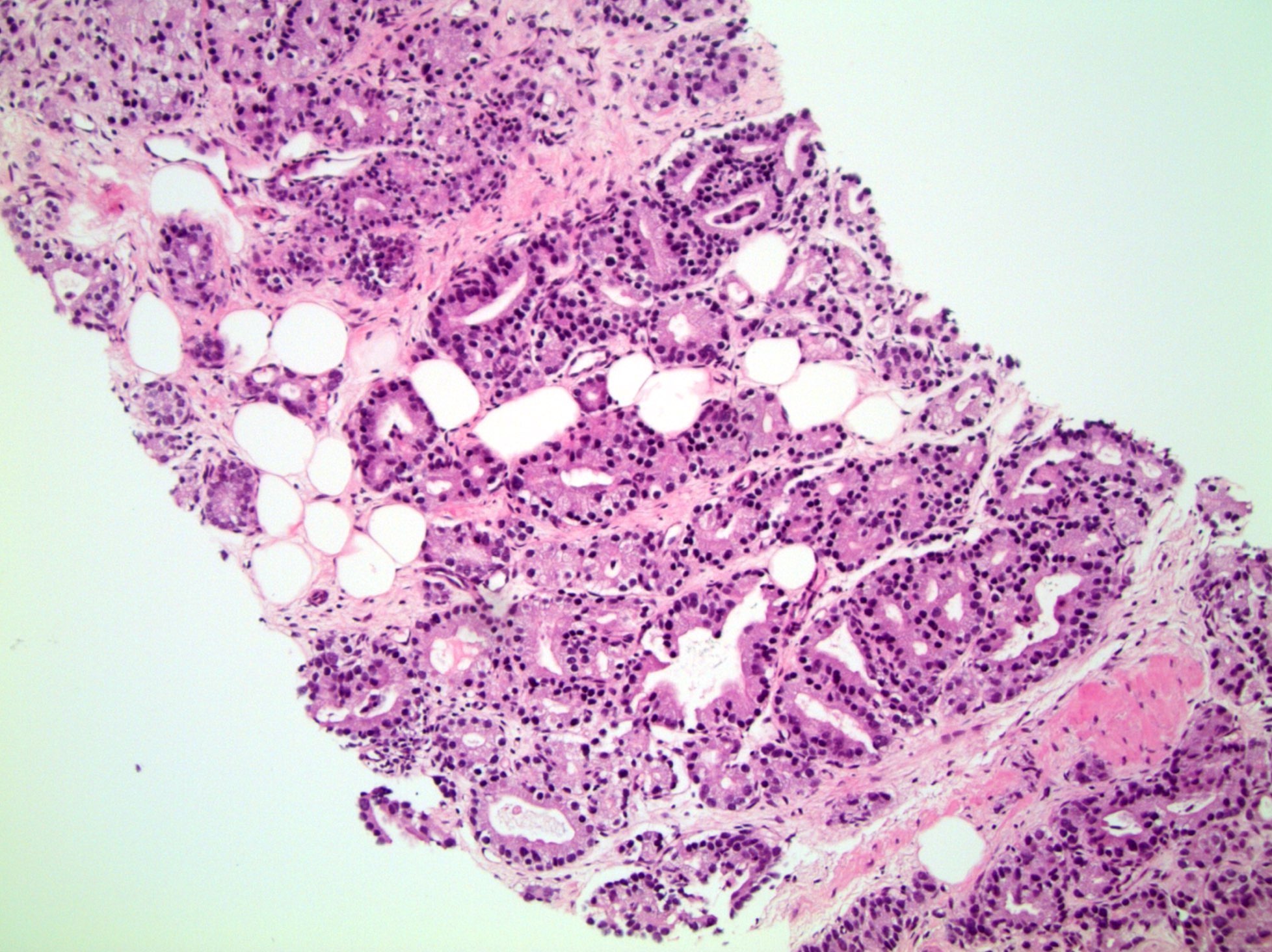
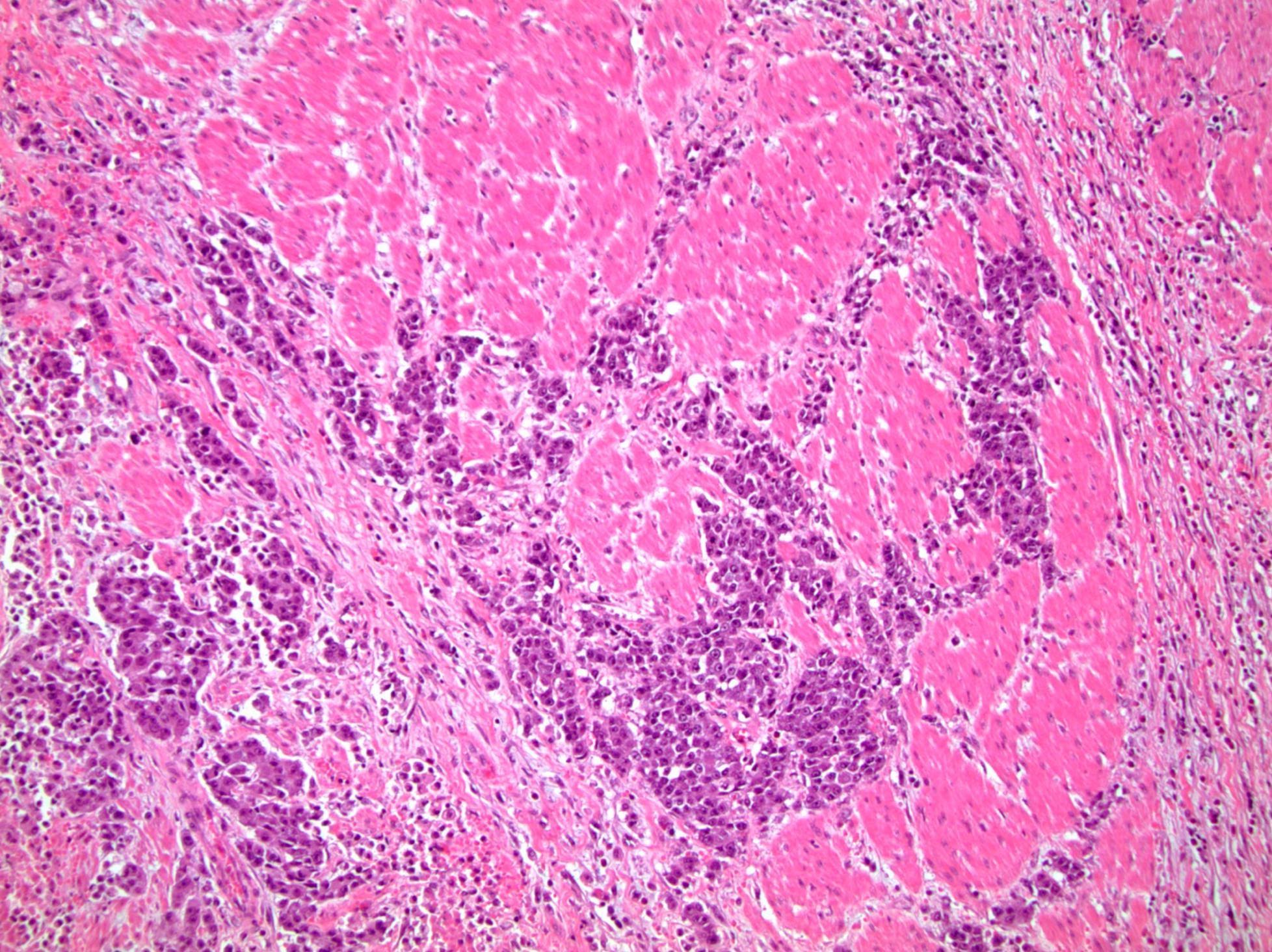
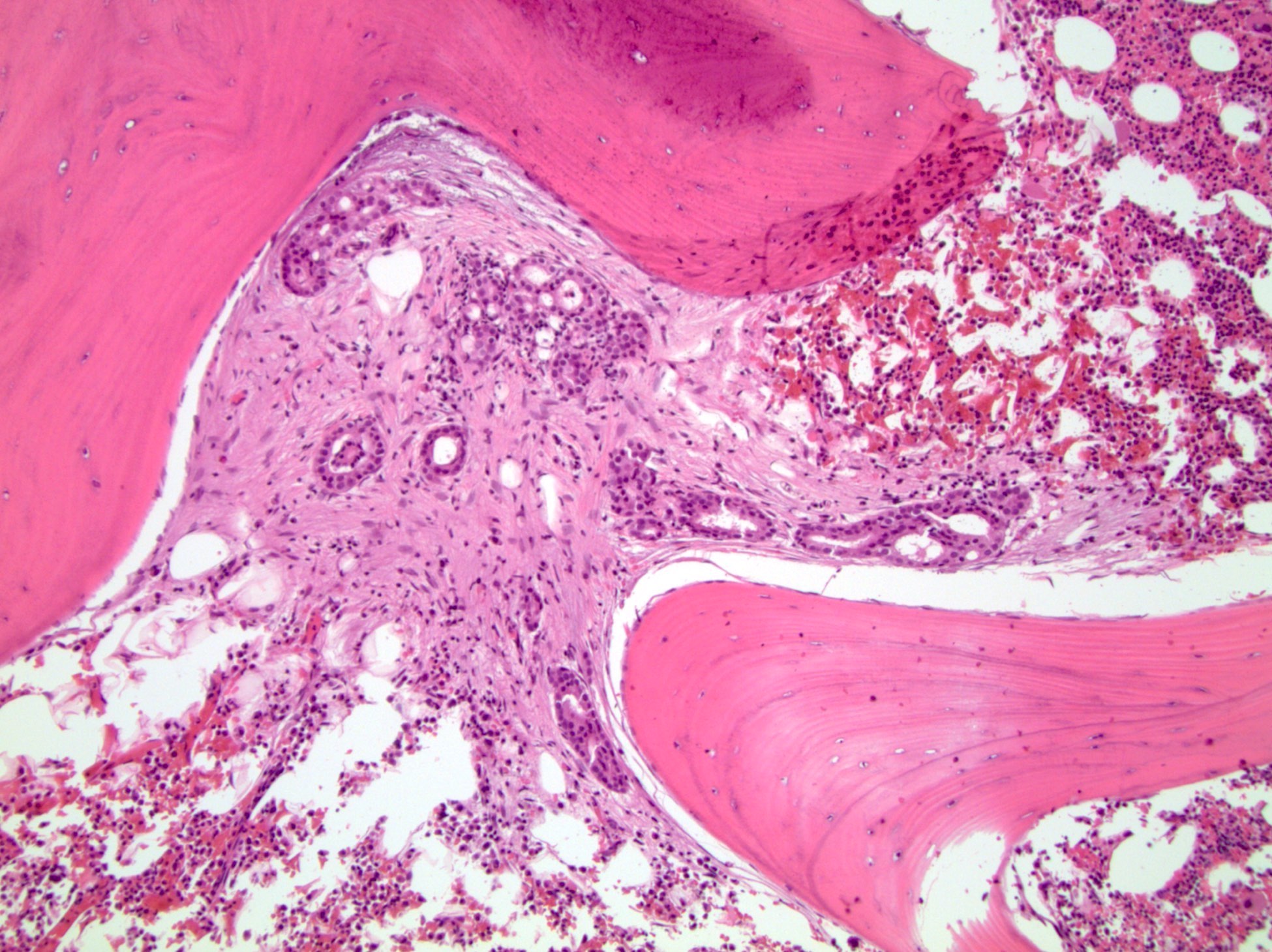
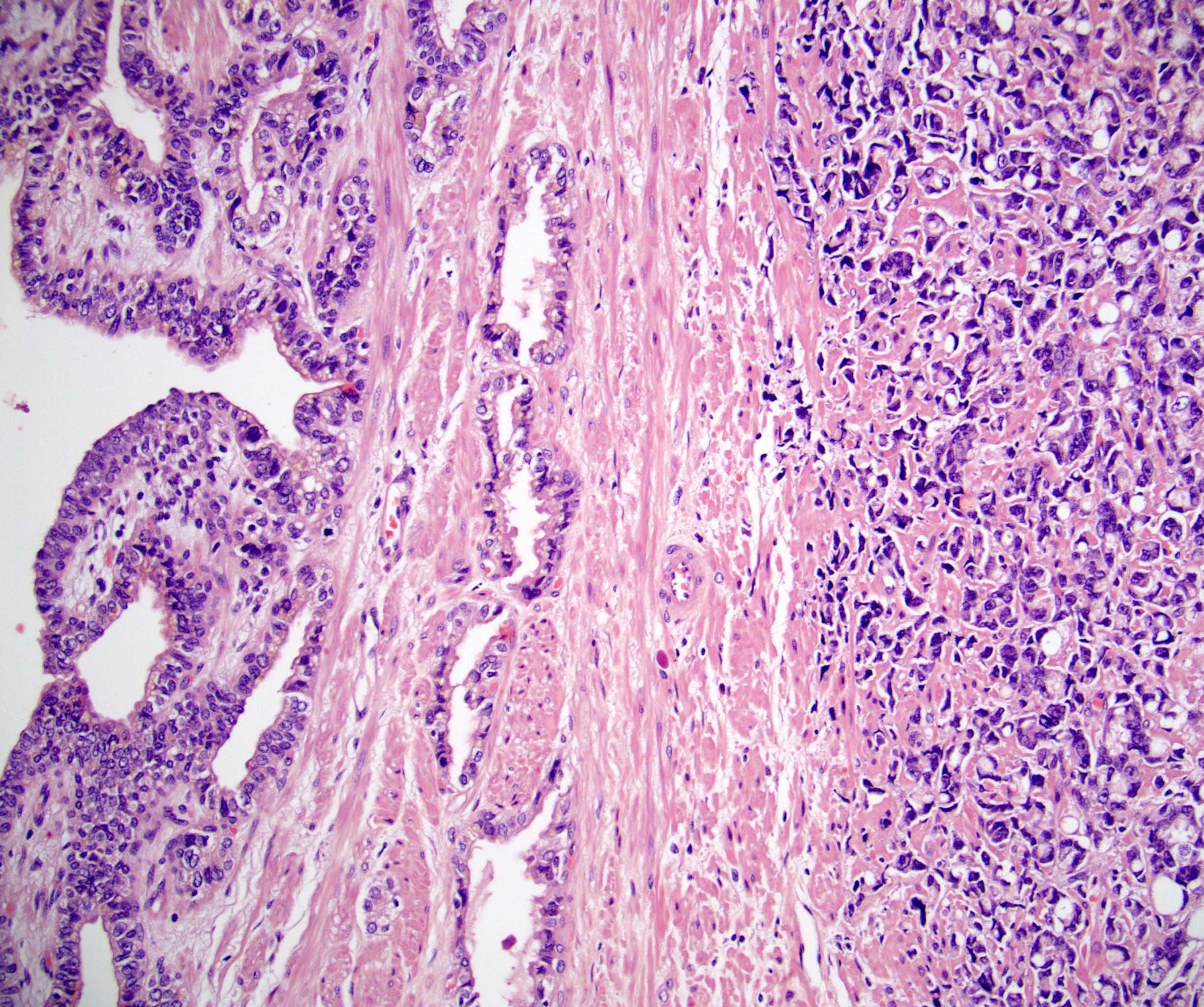
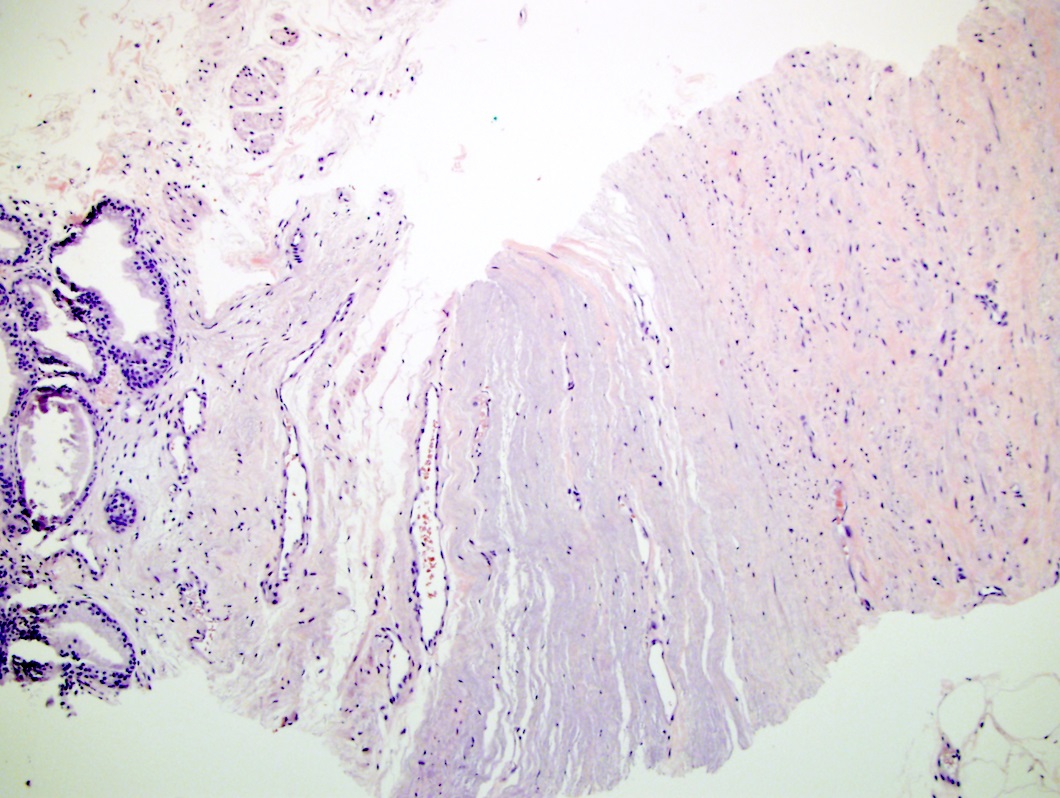
- pT3a: extraprostatic extension or microscopic invasion of bladder neck
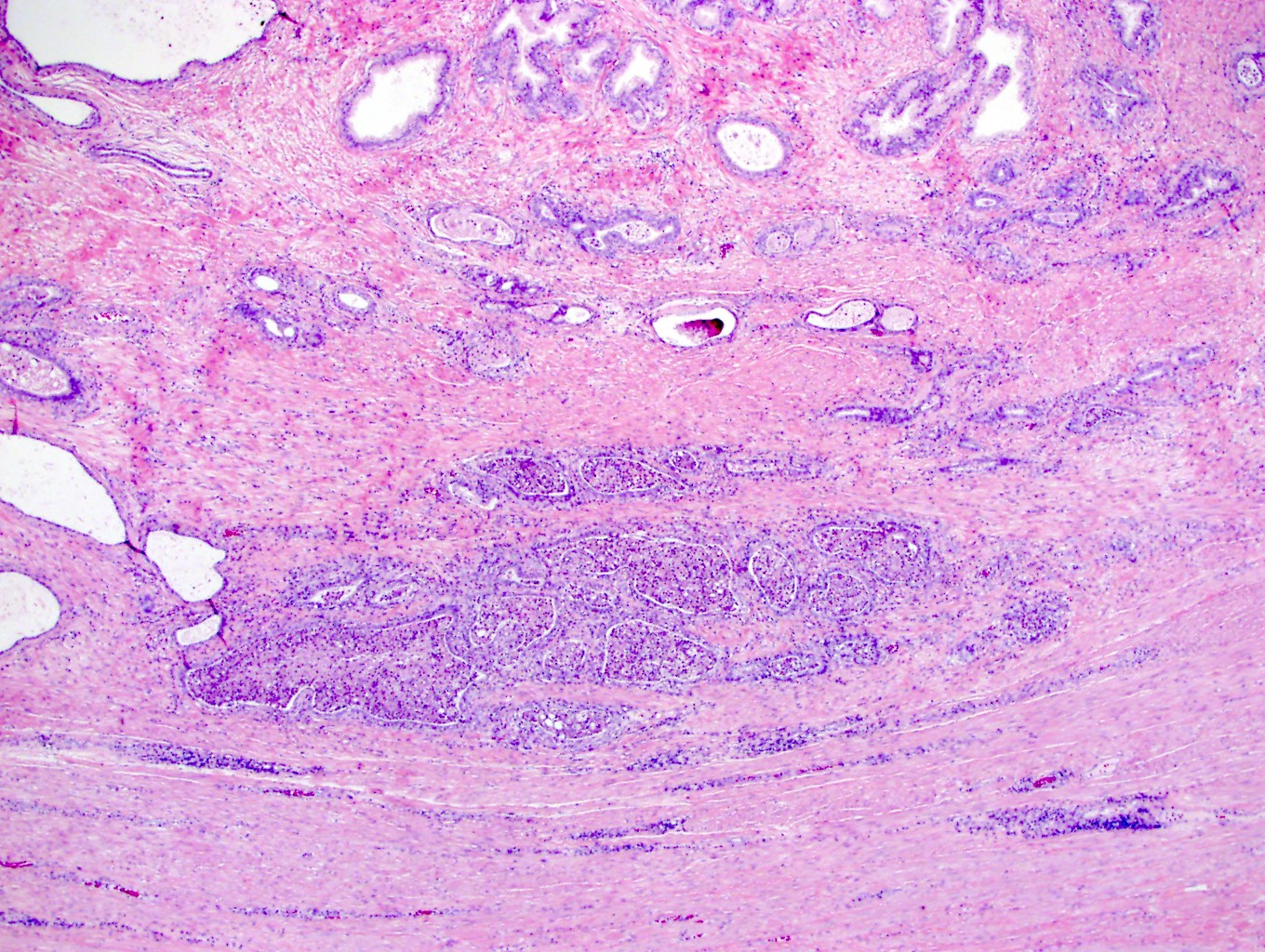
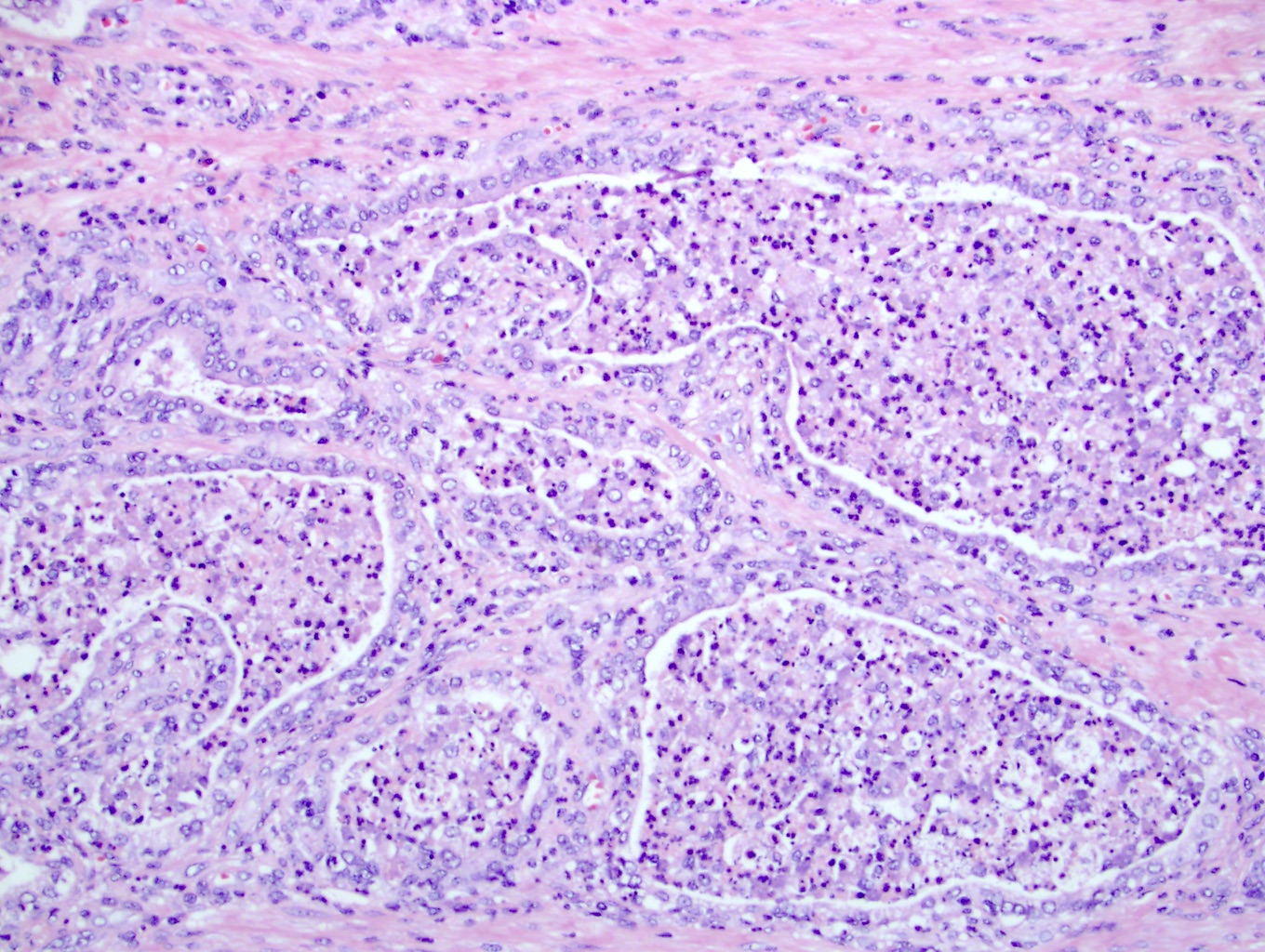
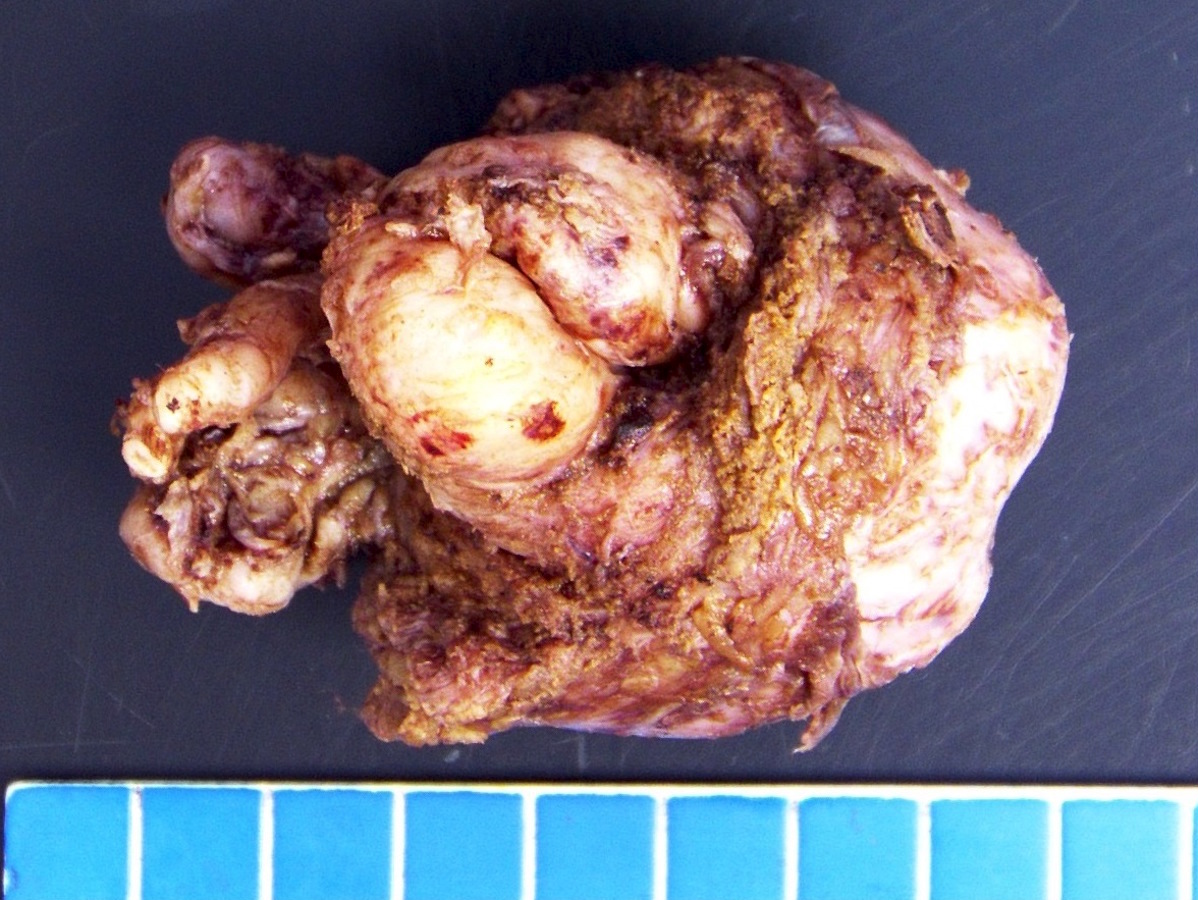
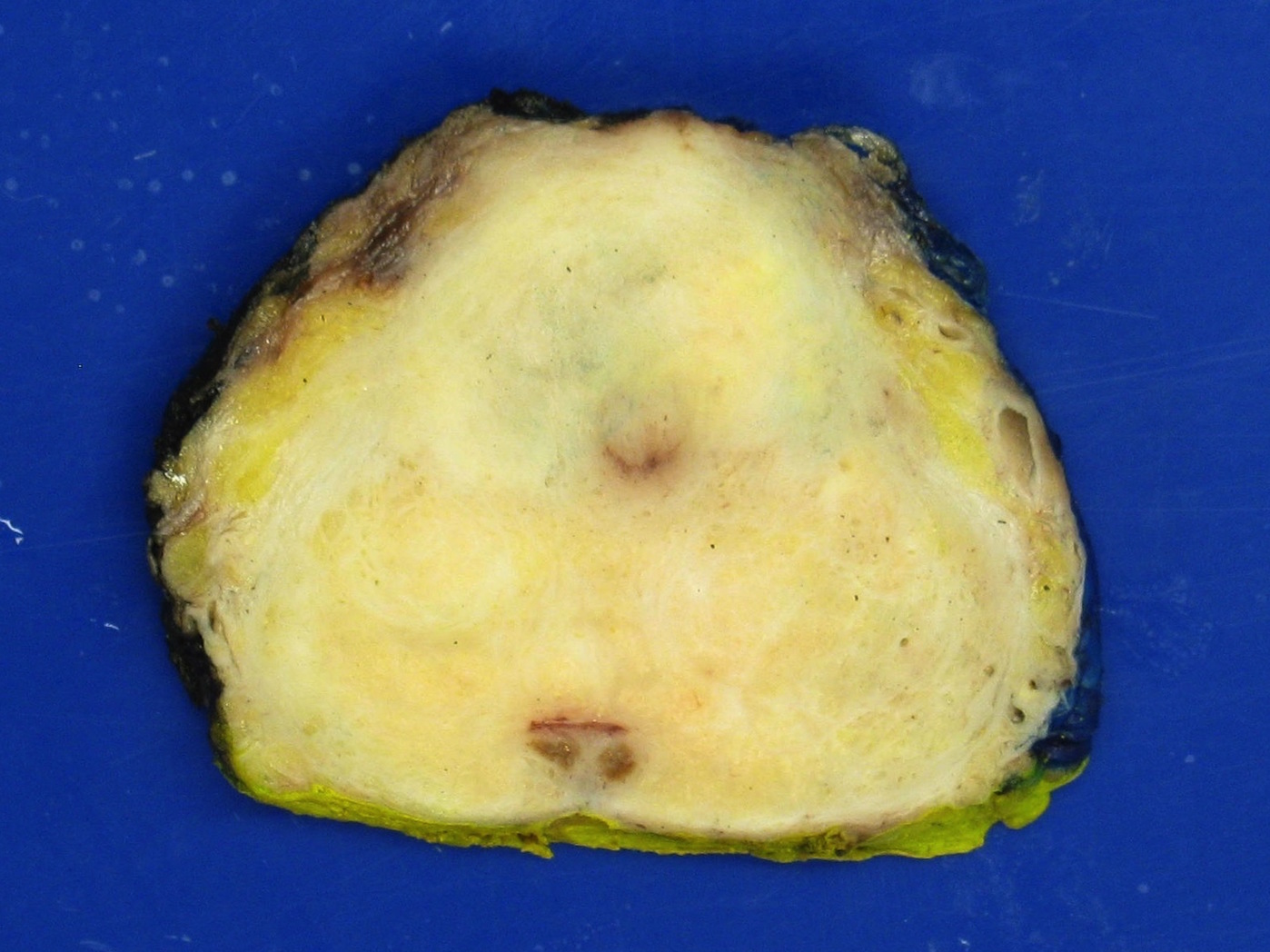
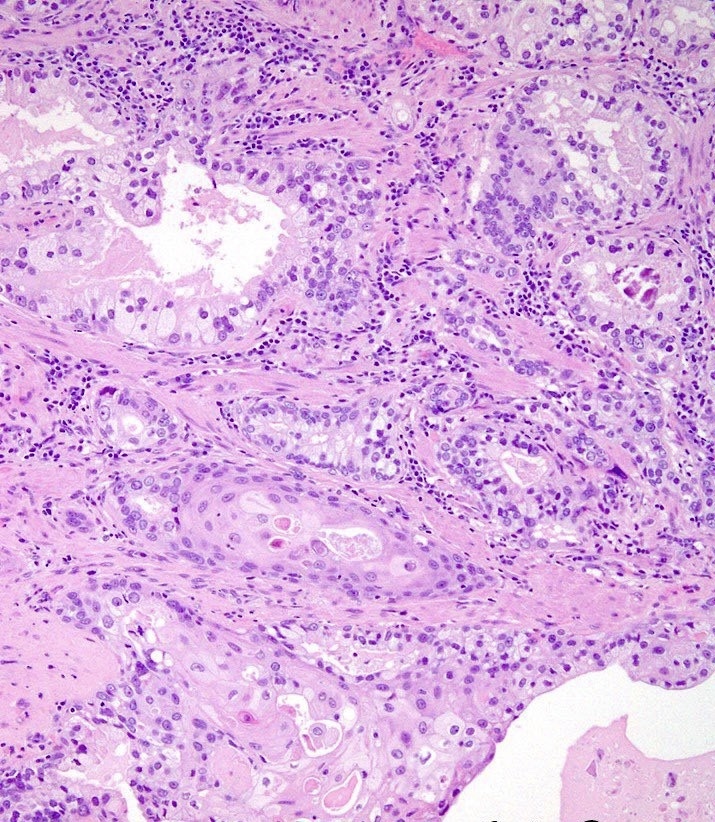
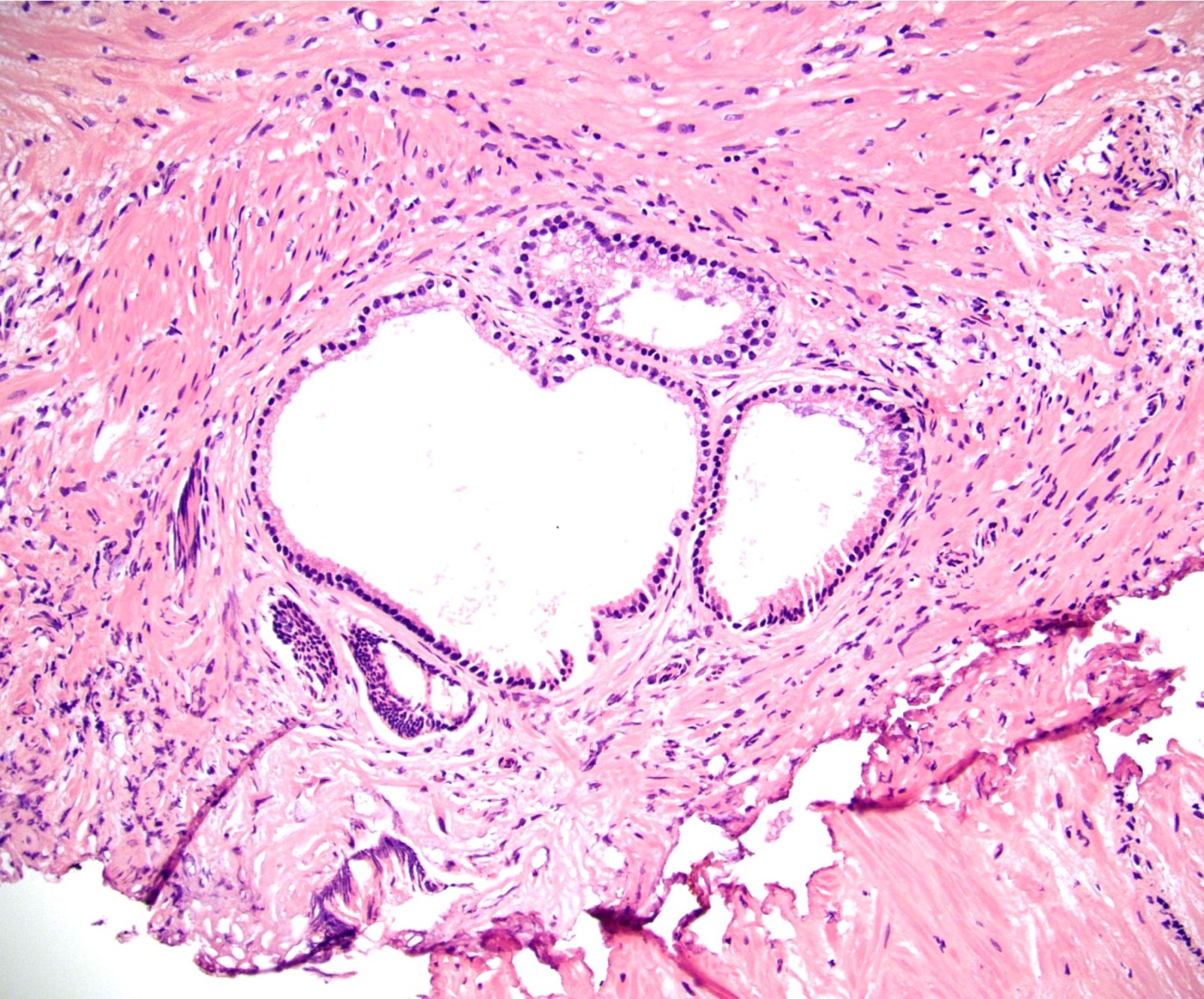
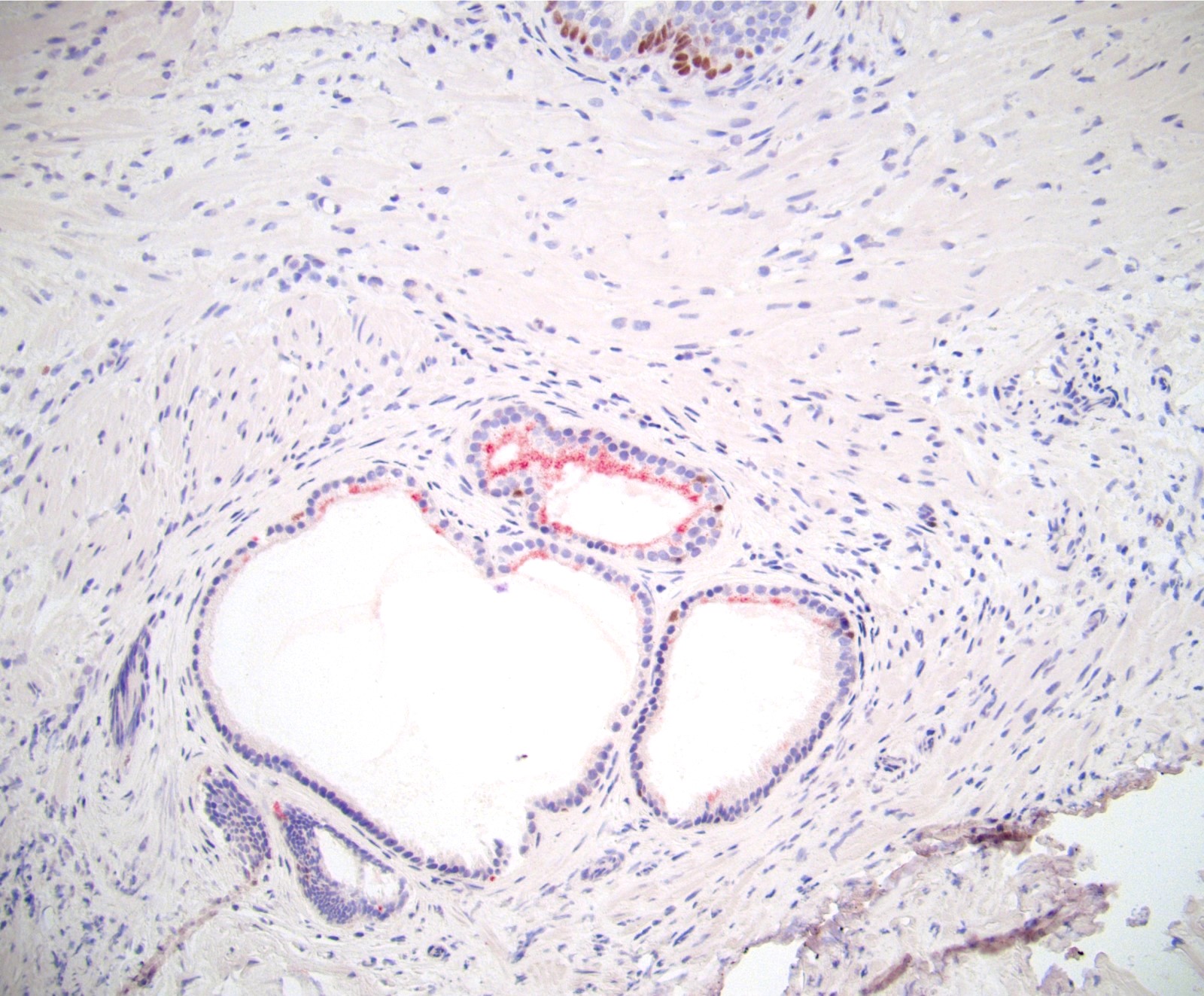
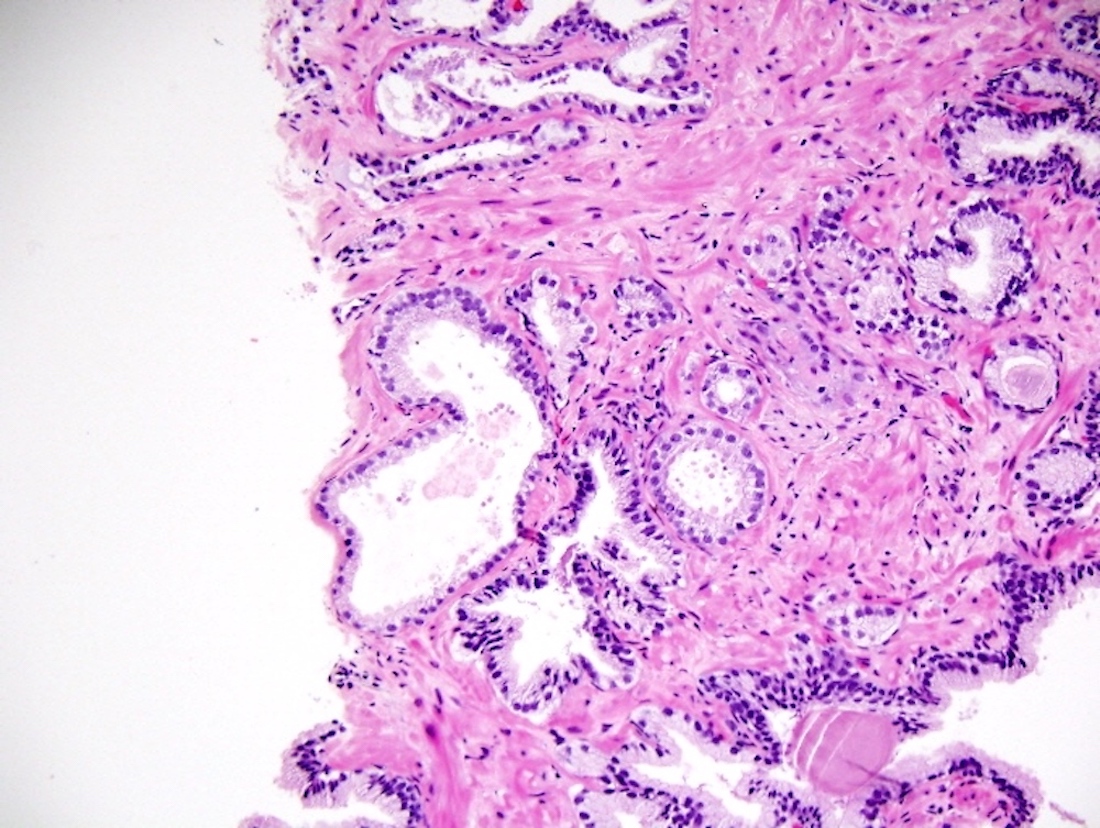
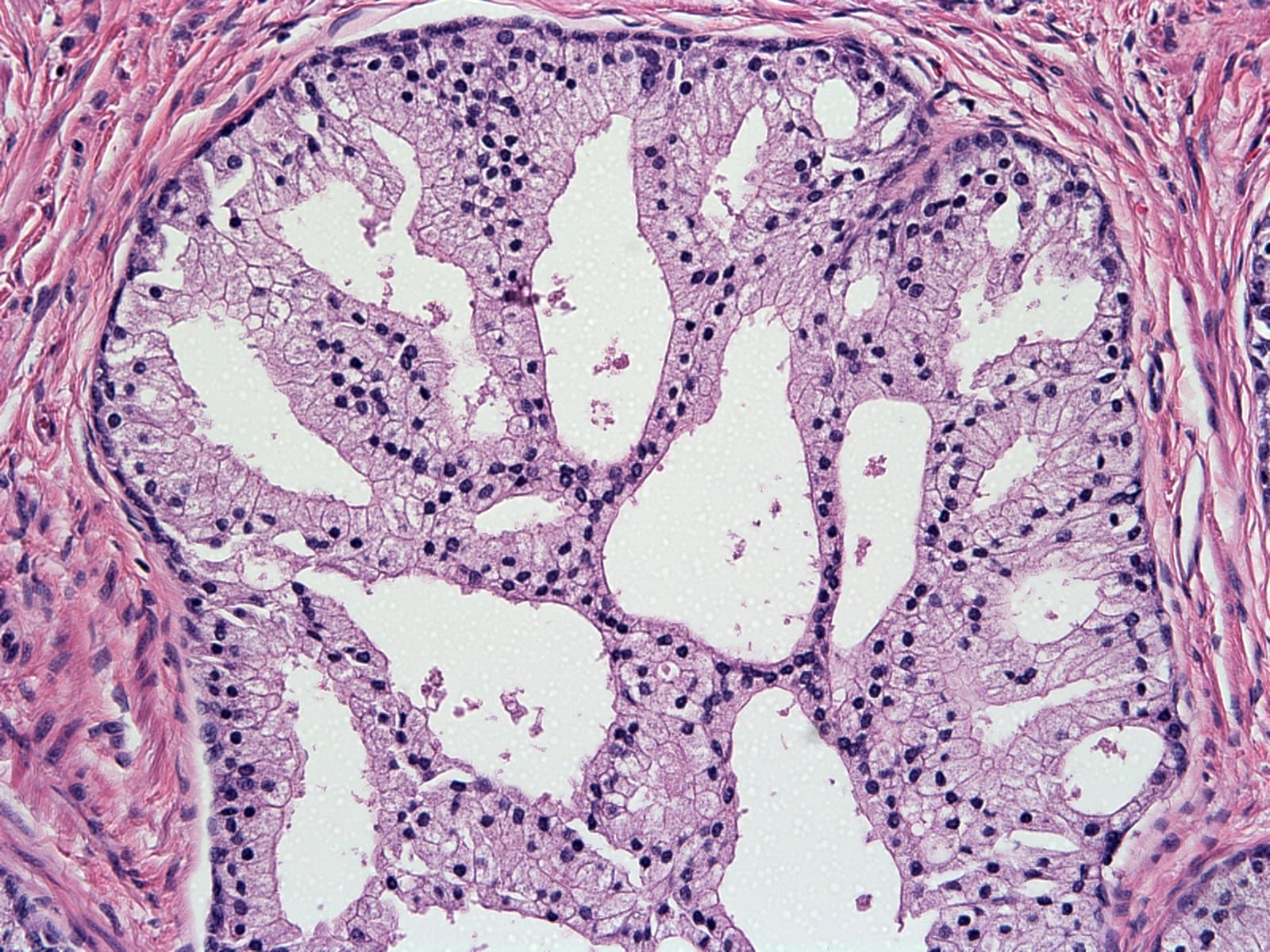
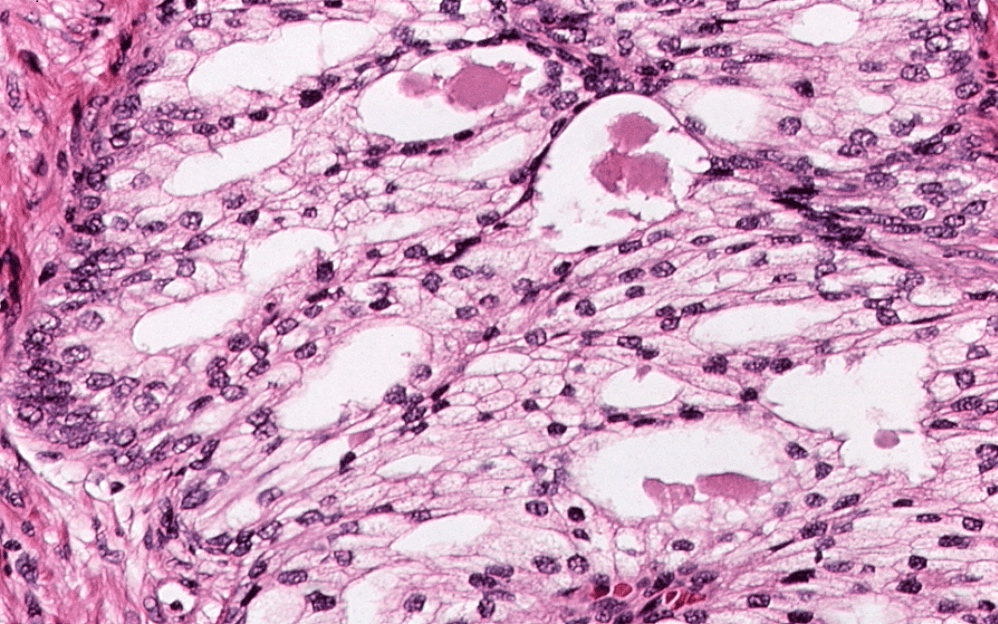
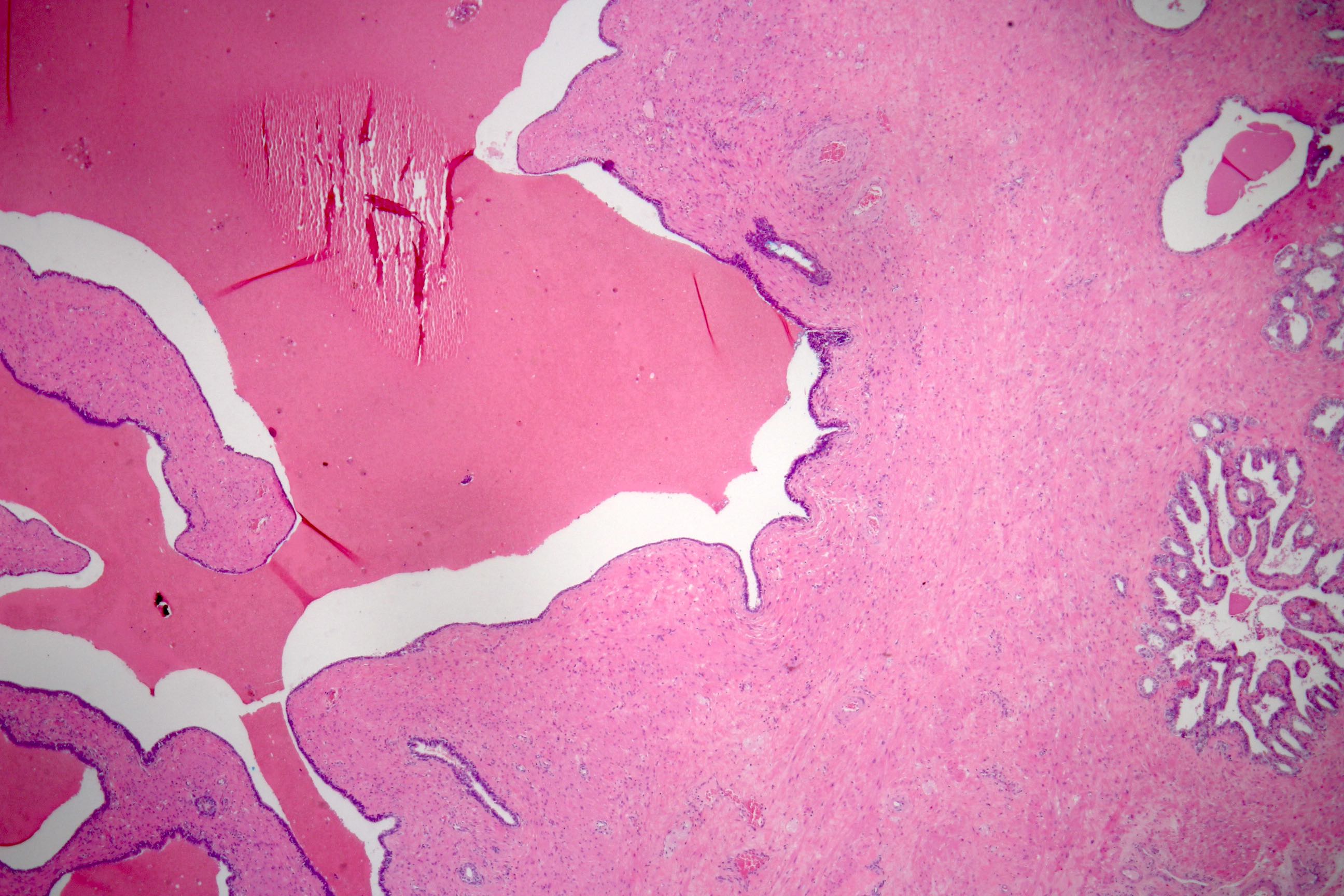
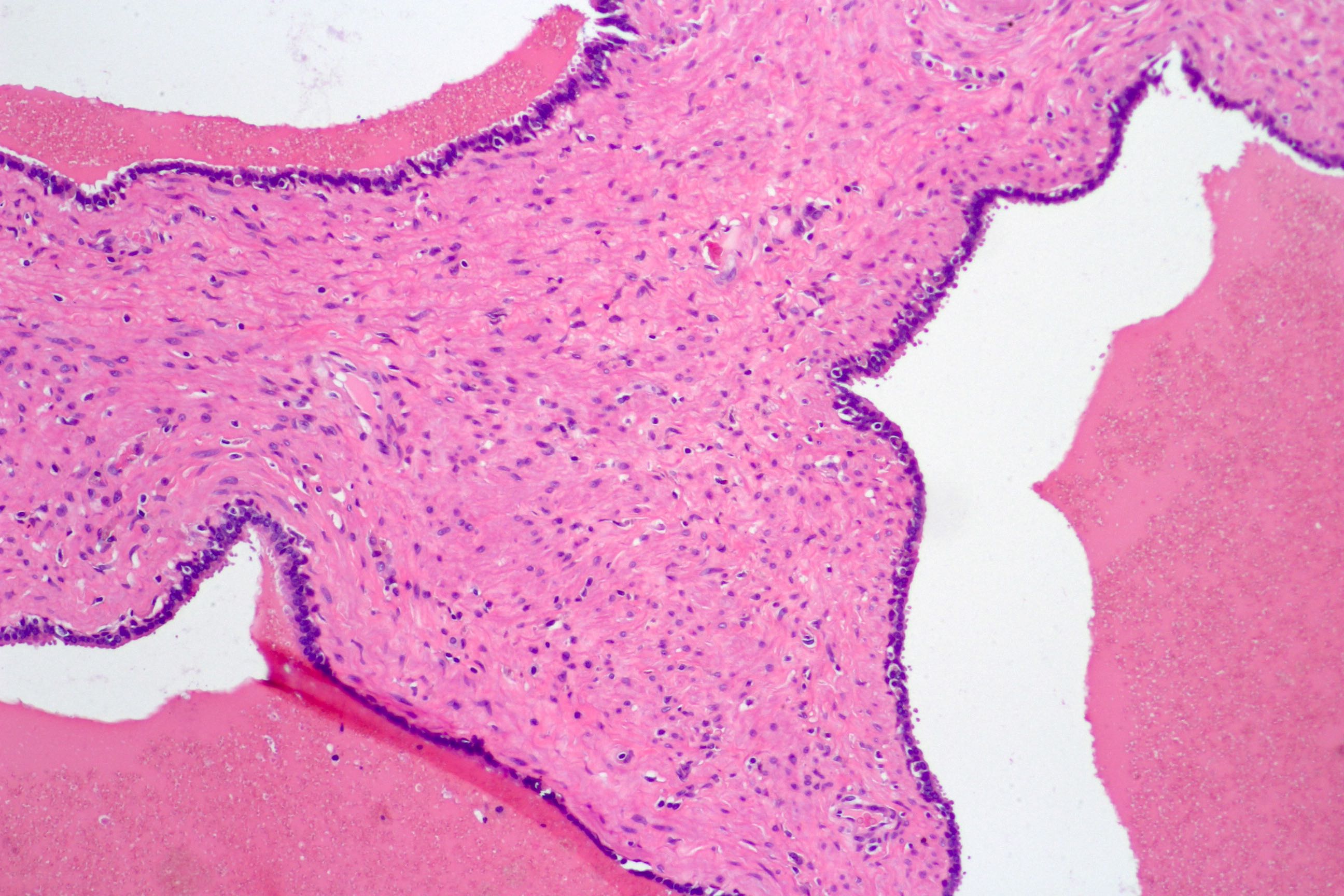
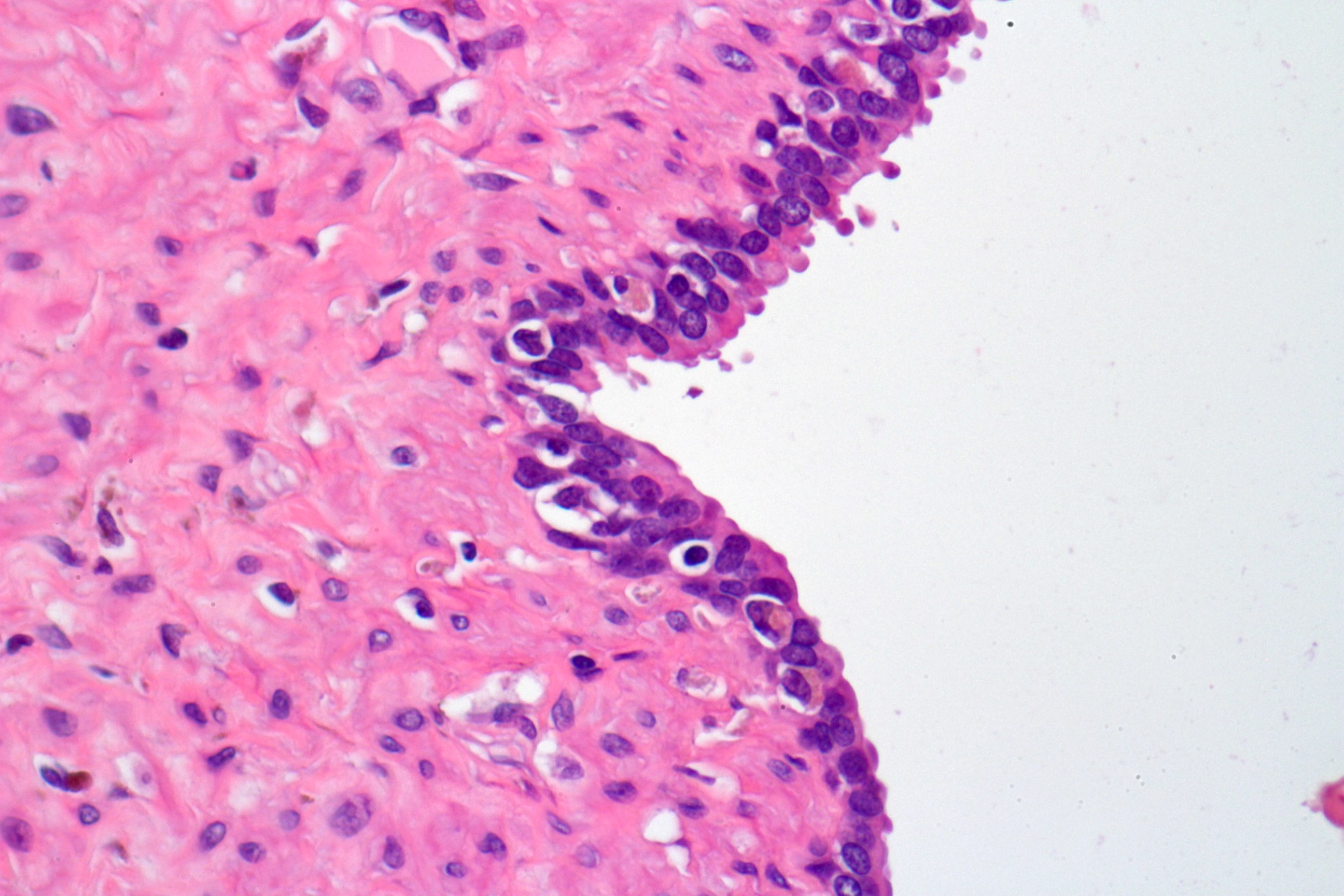
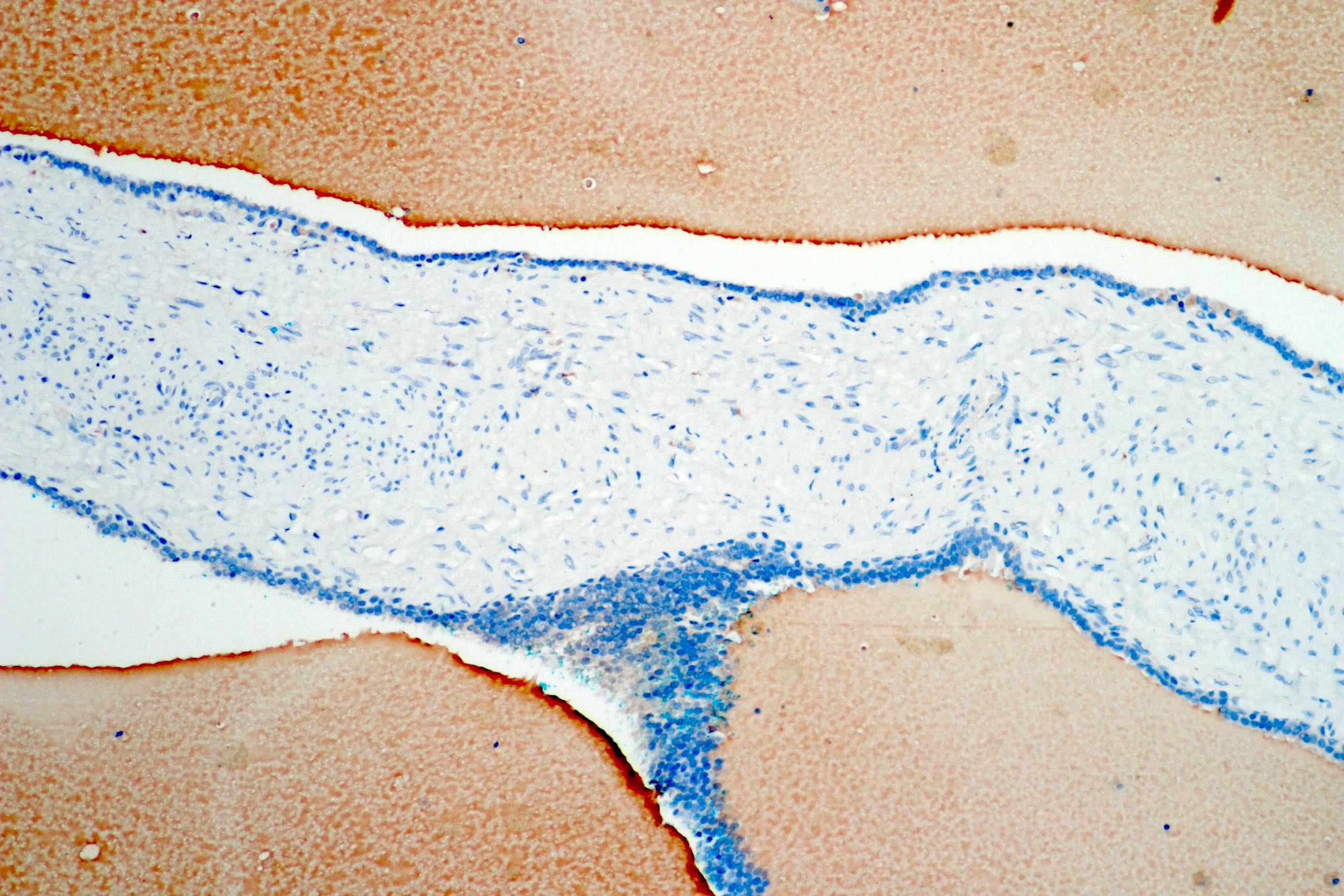
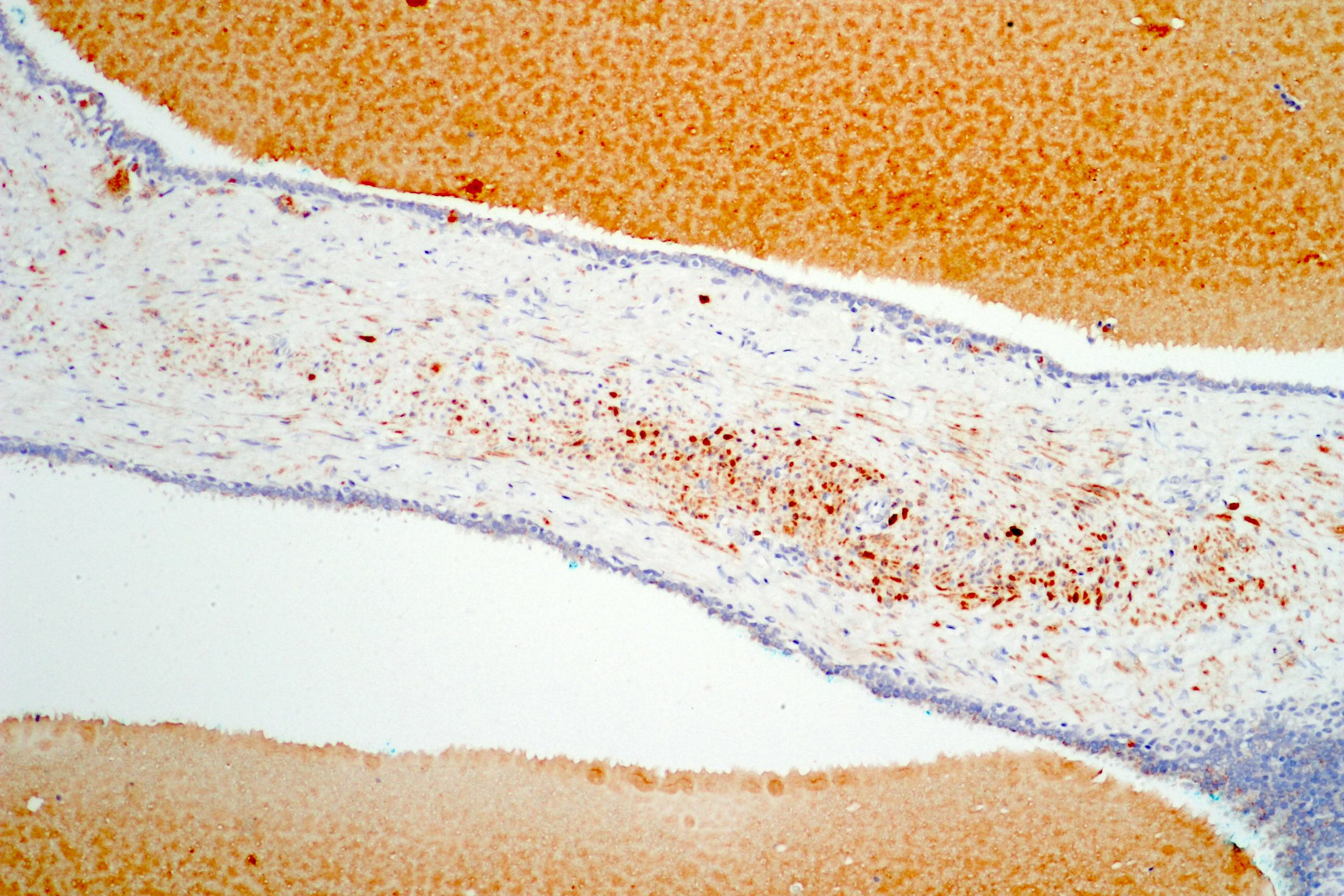
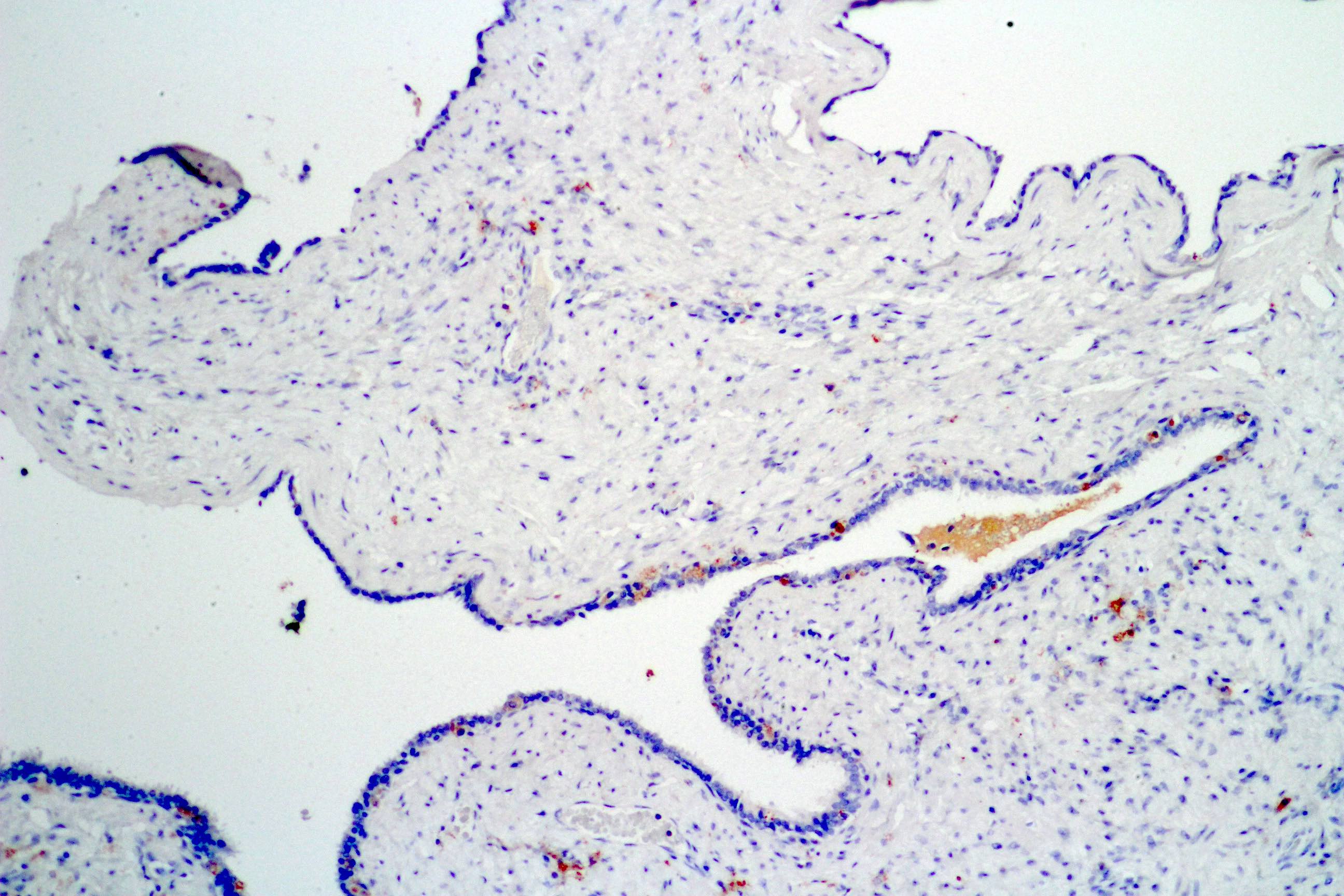
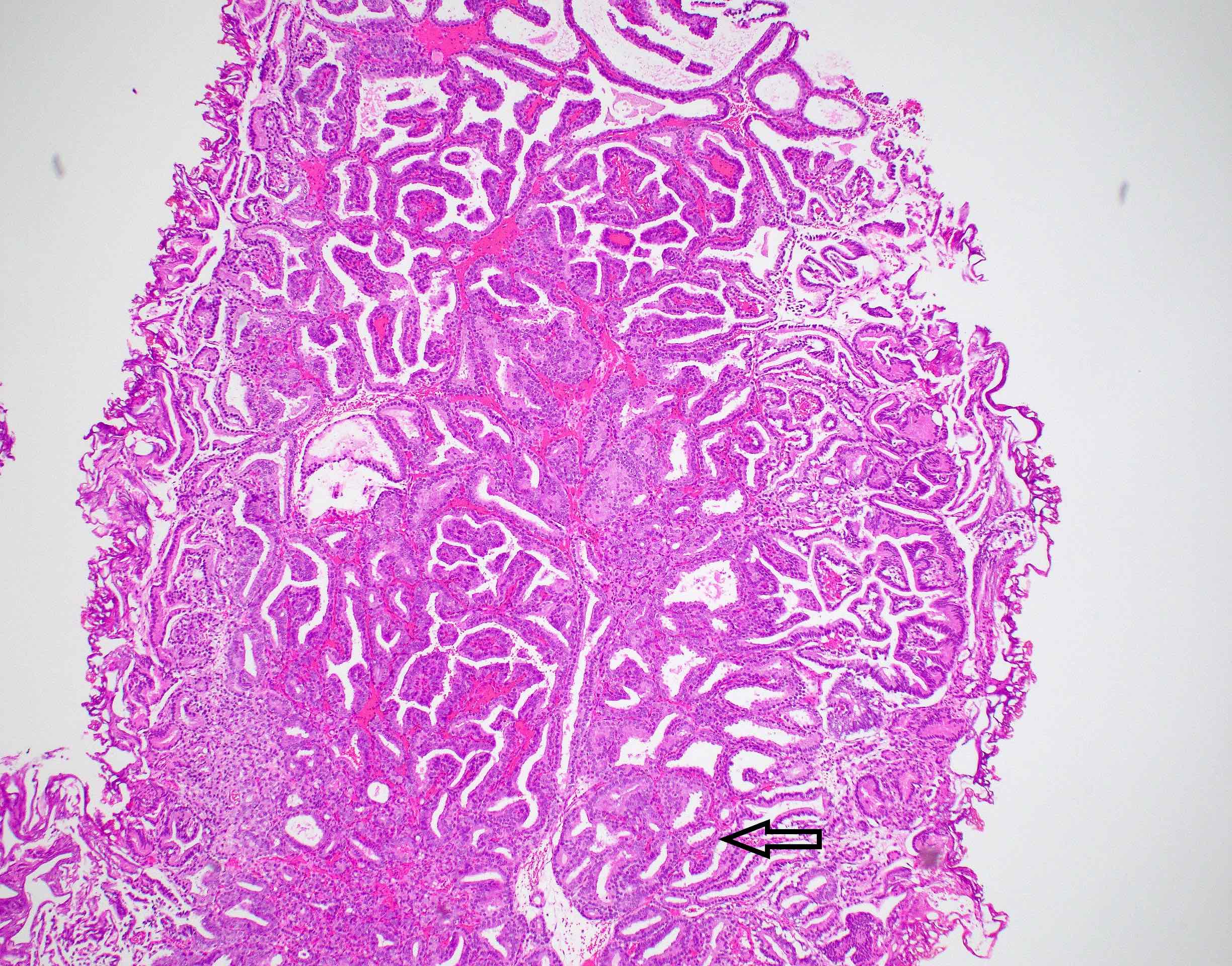
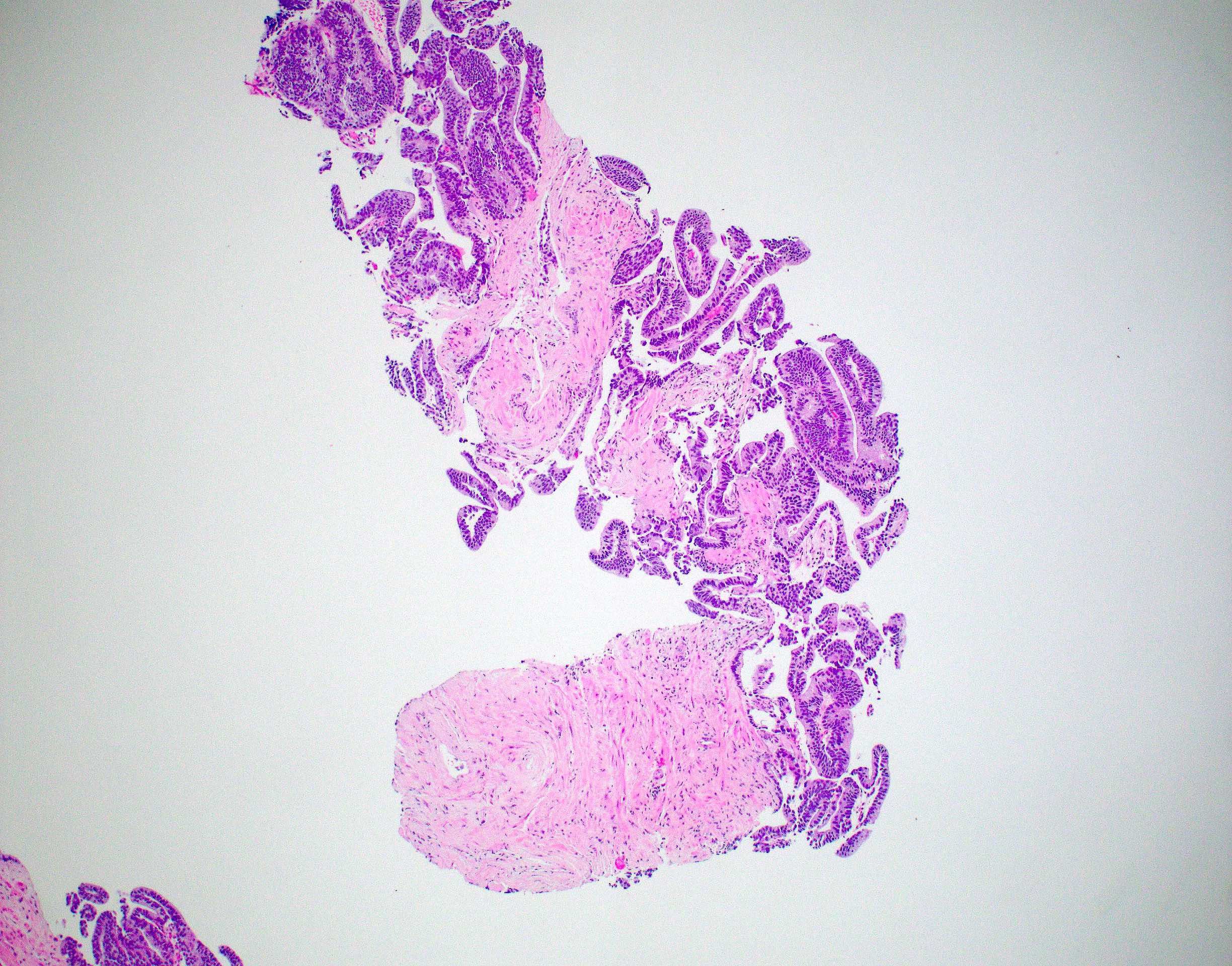
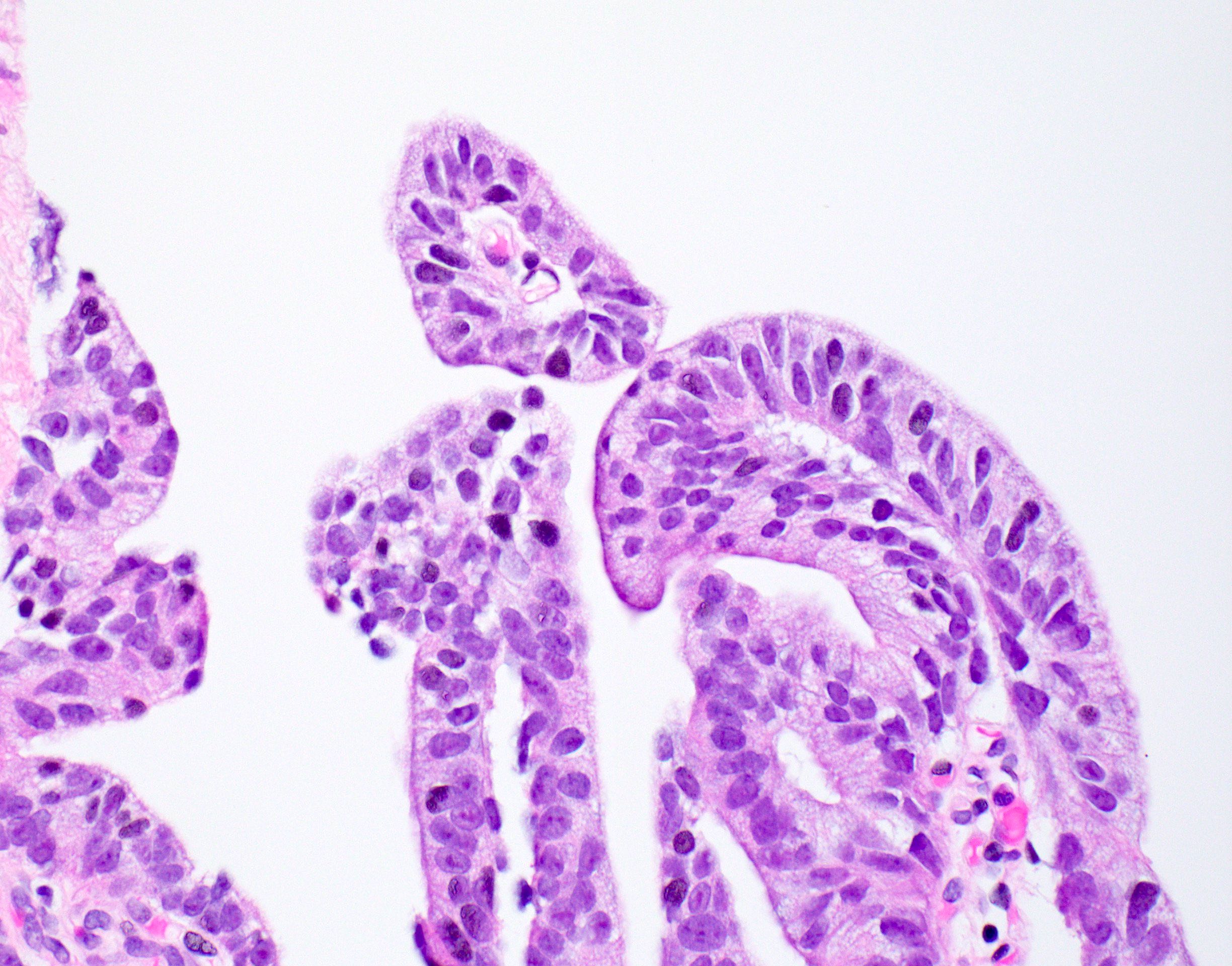
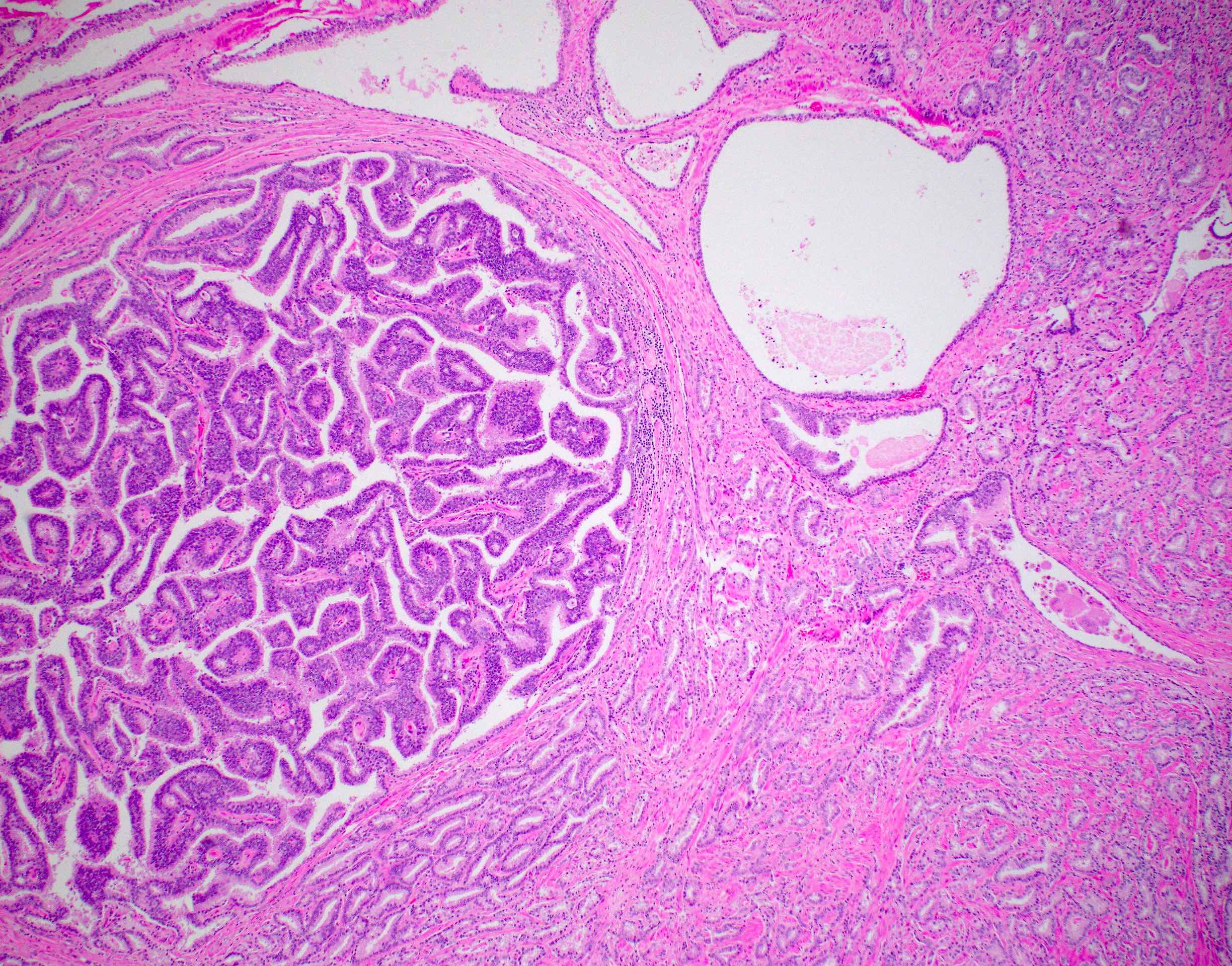
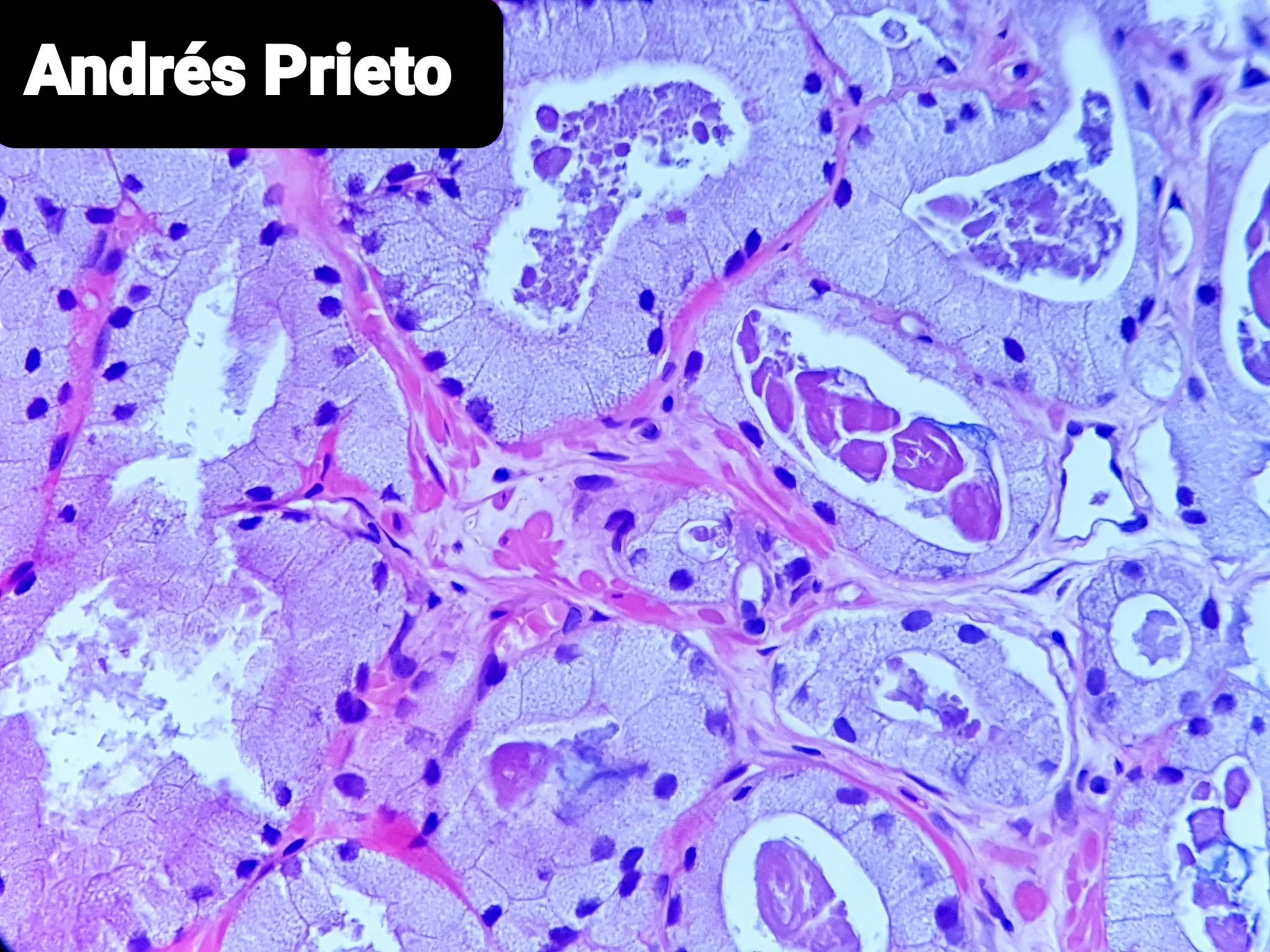
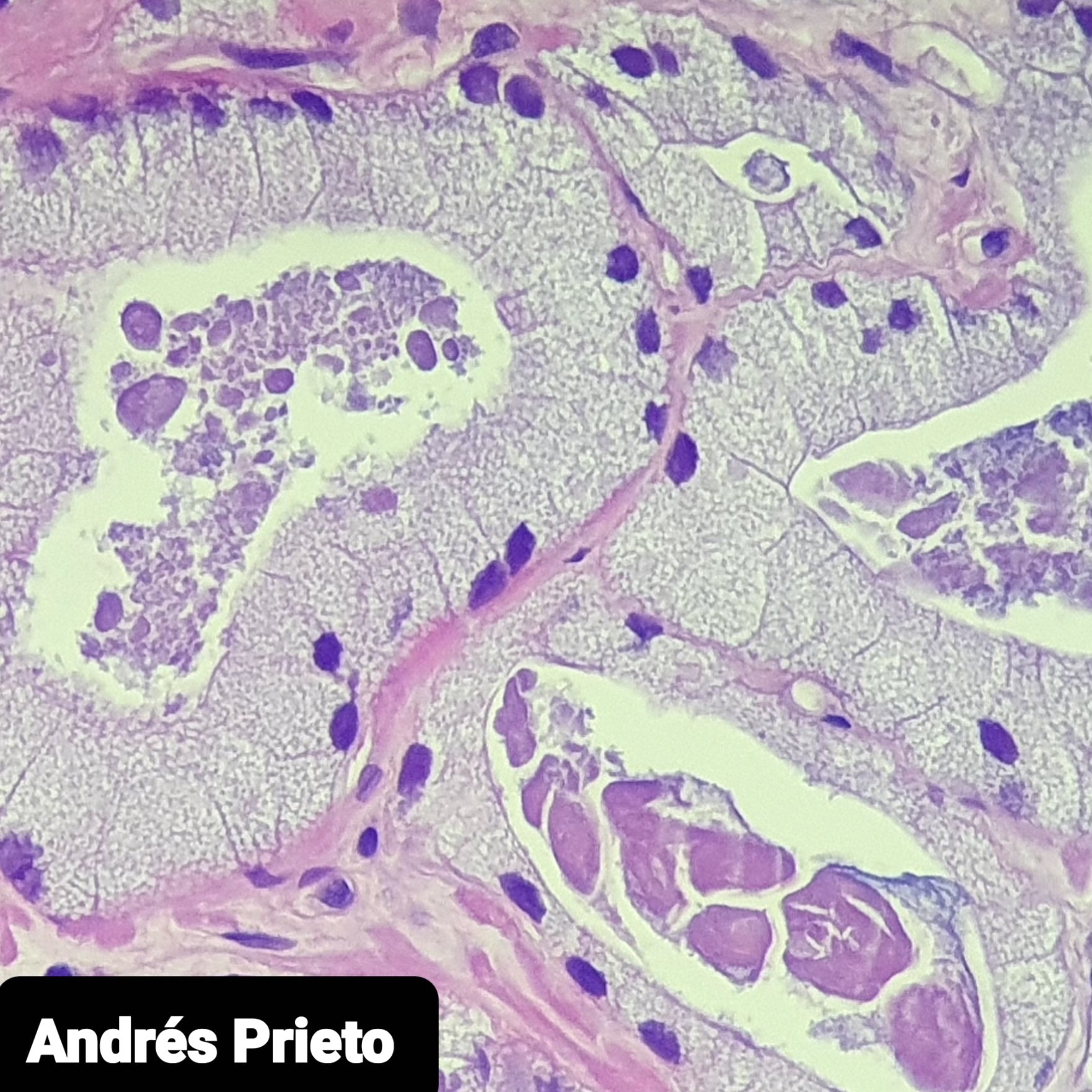
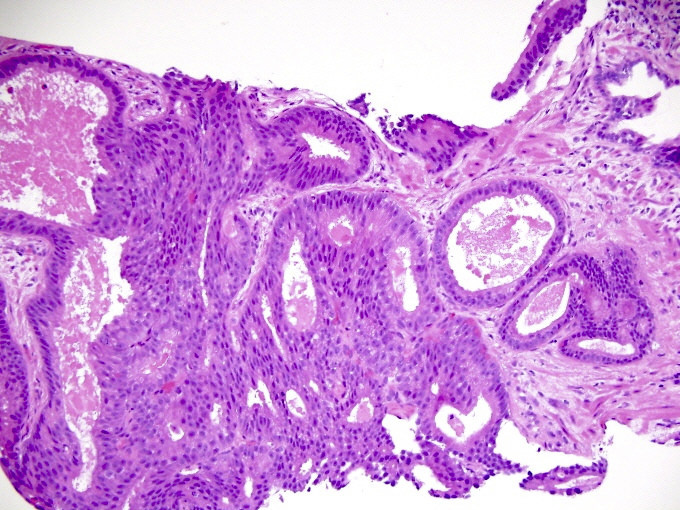
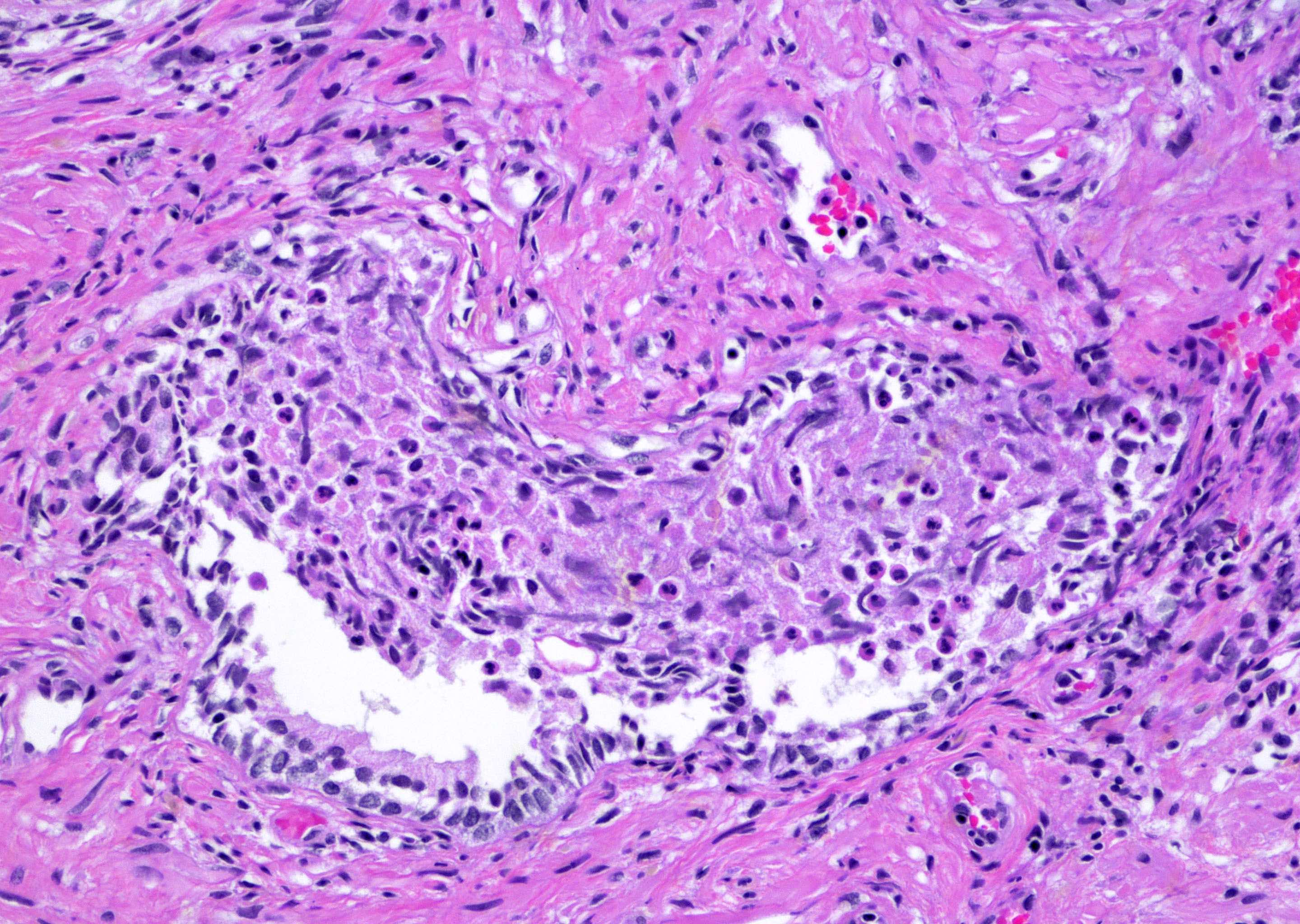
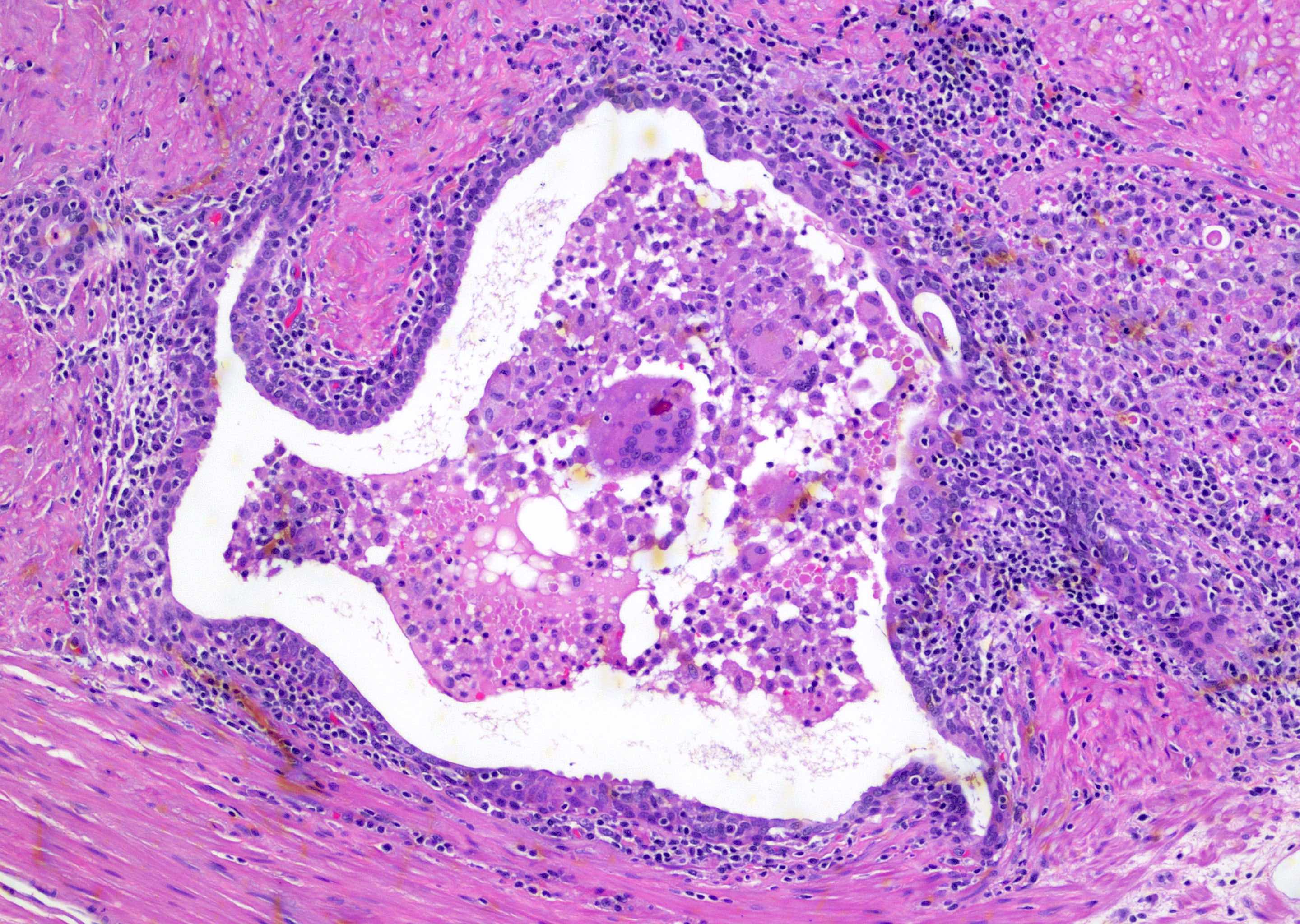
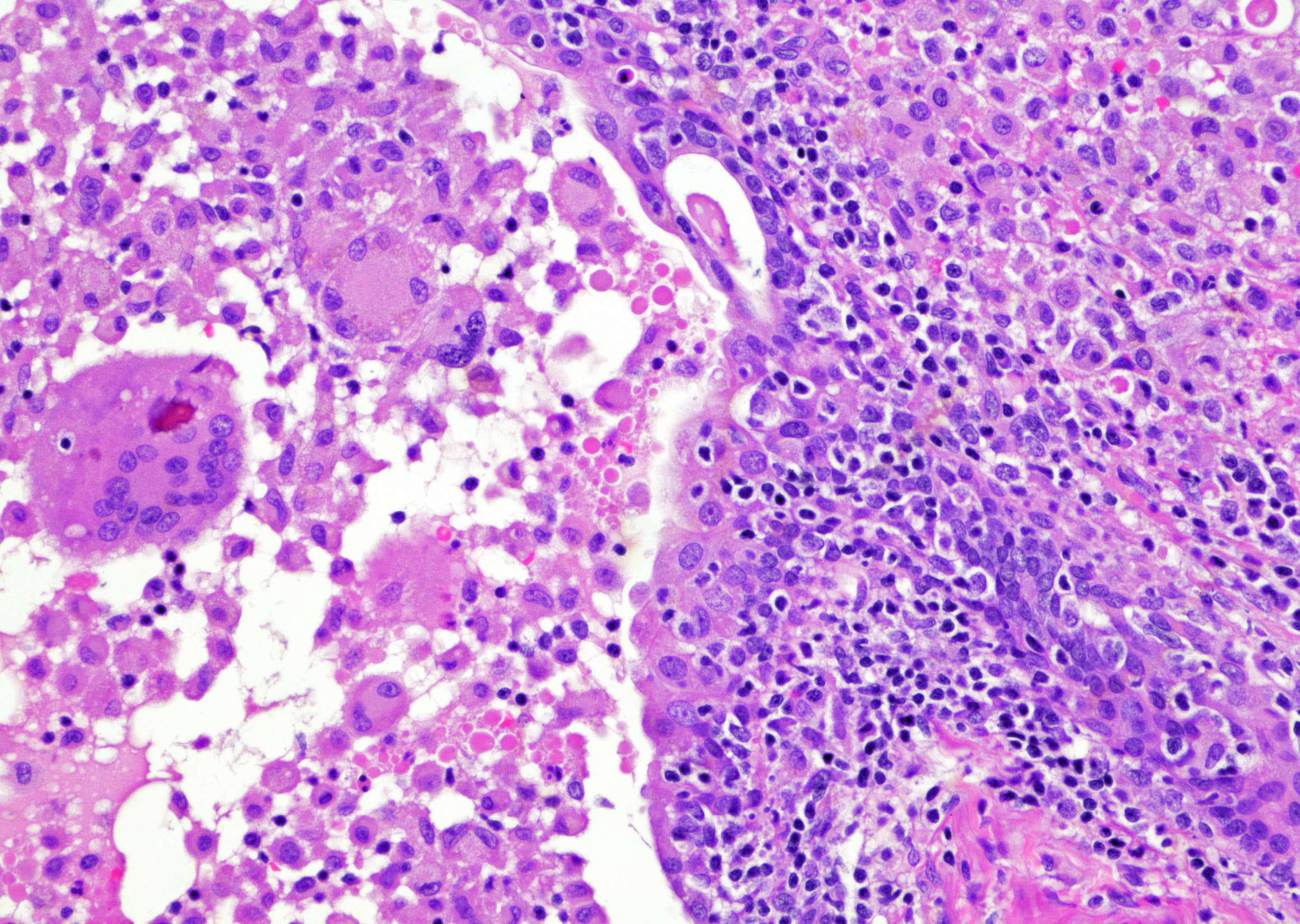
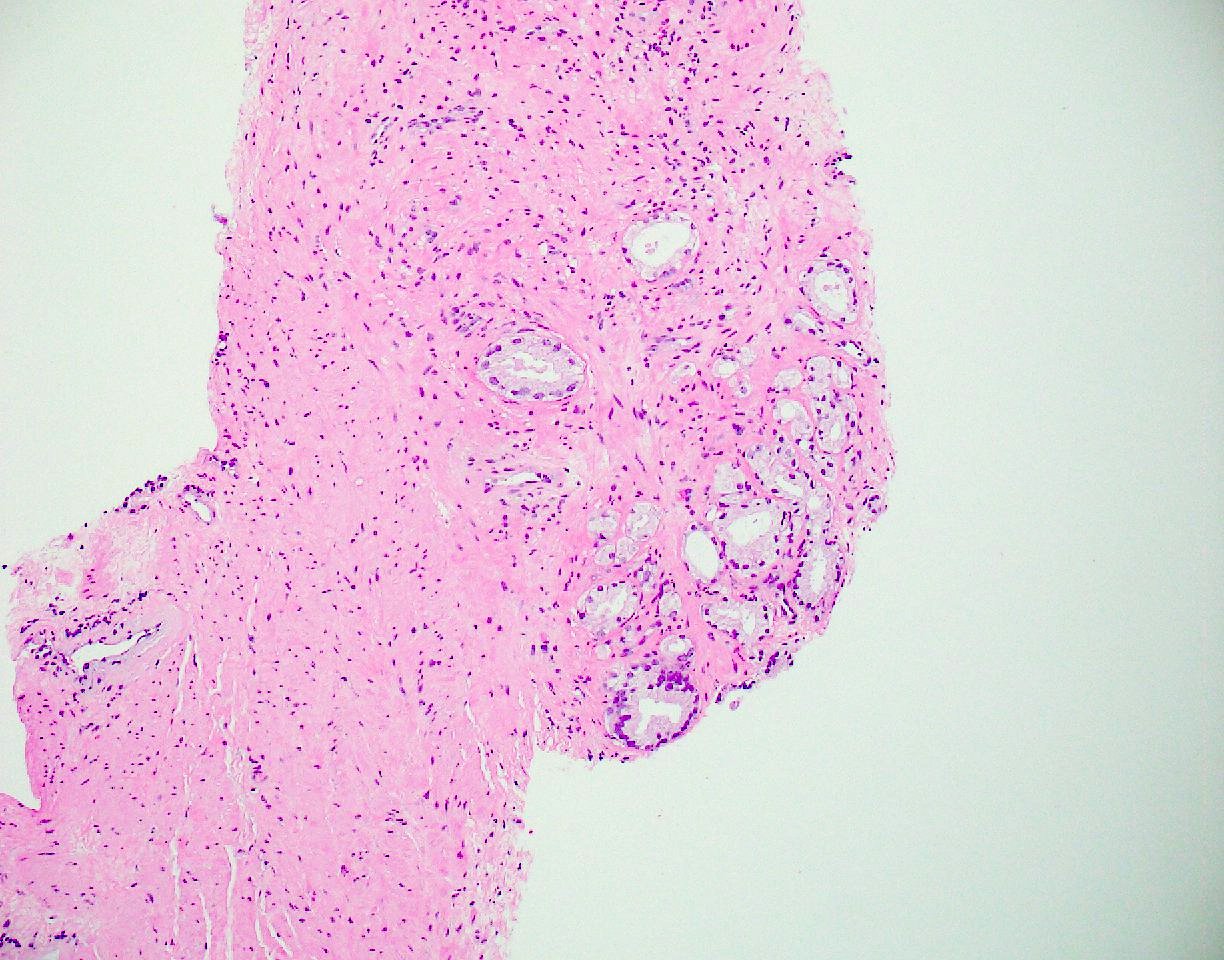
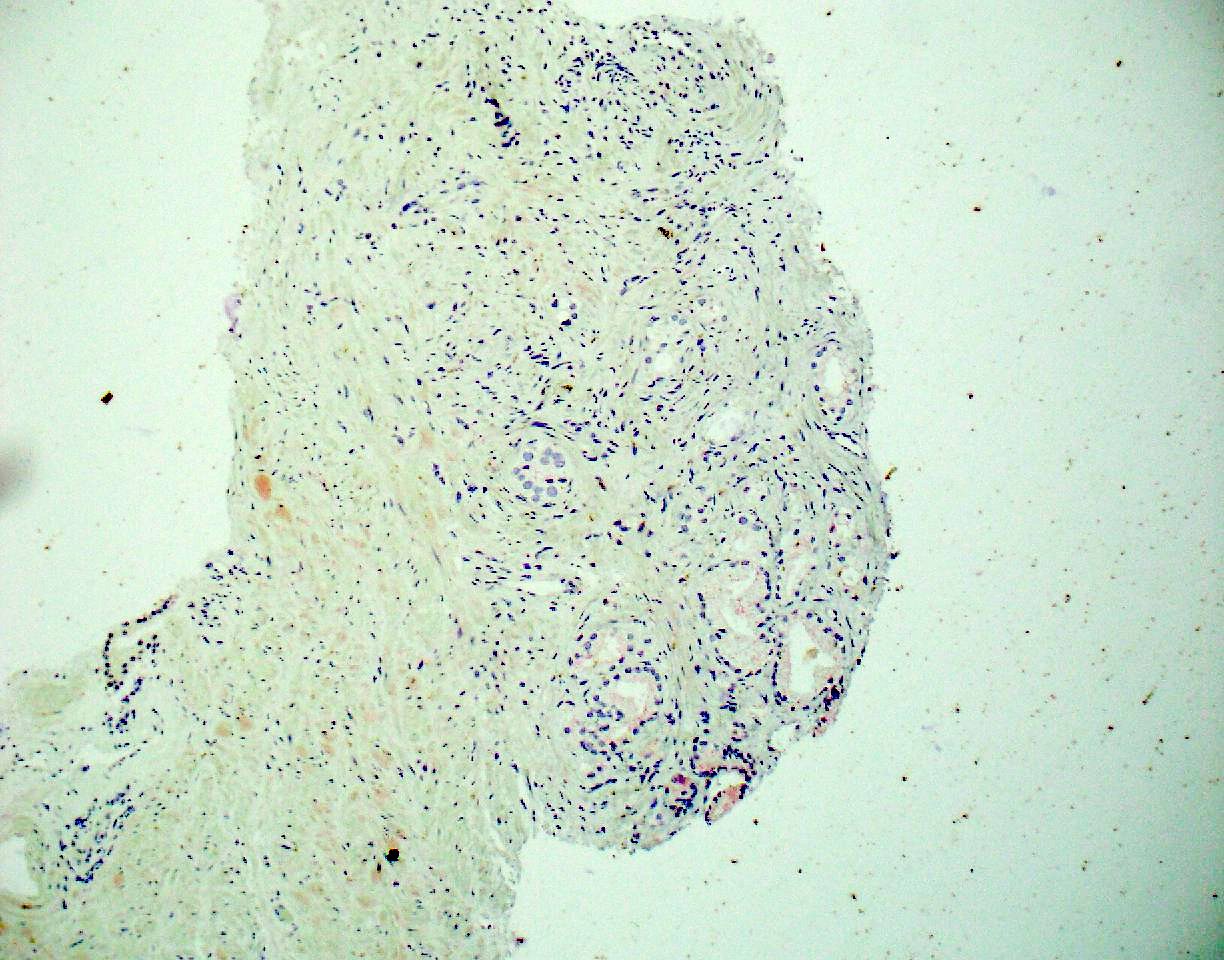
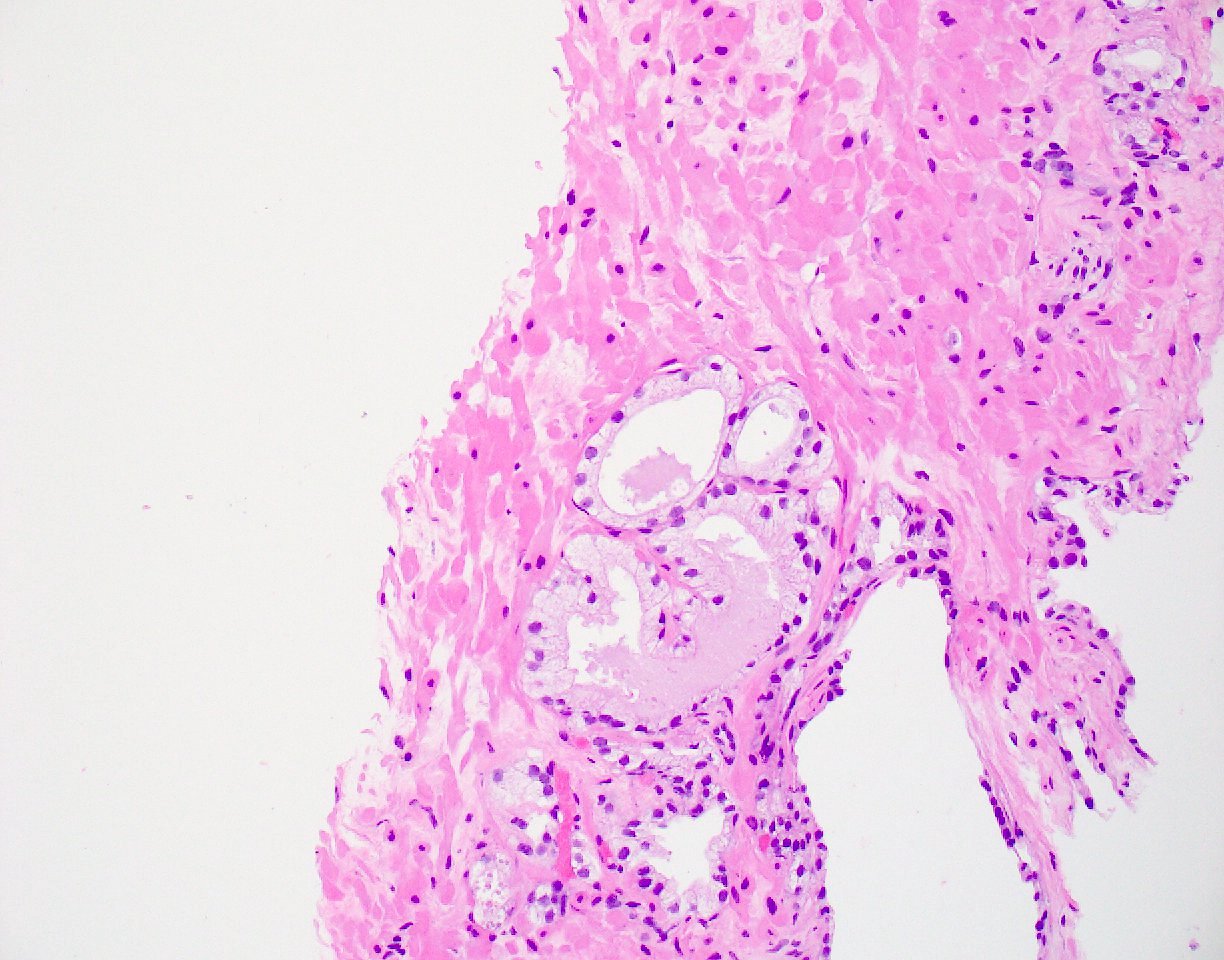
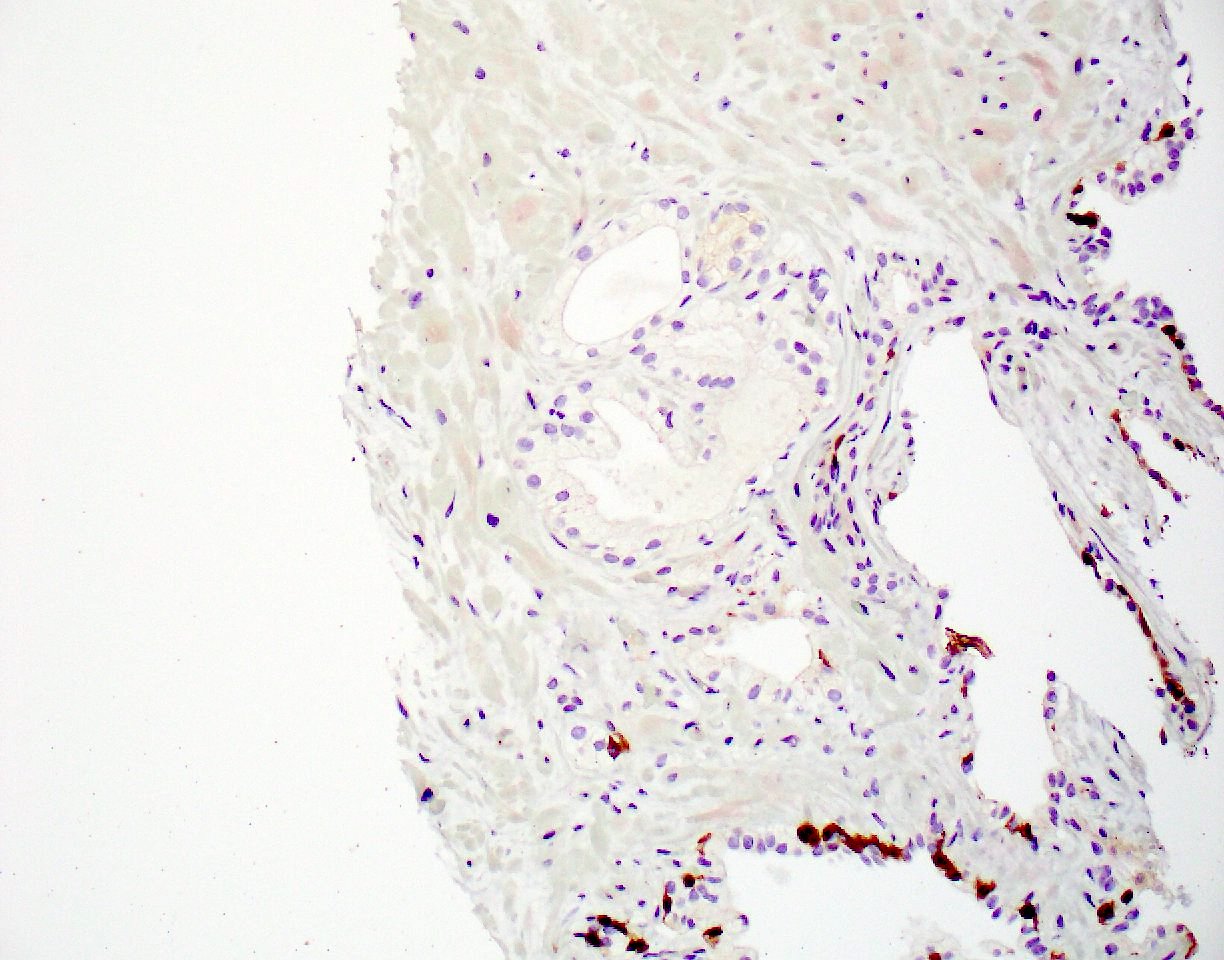
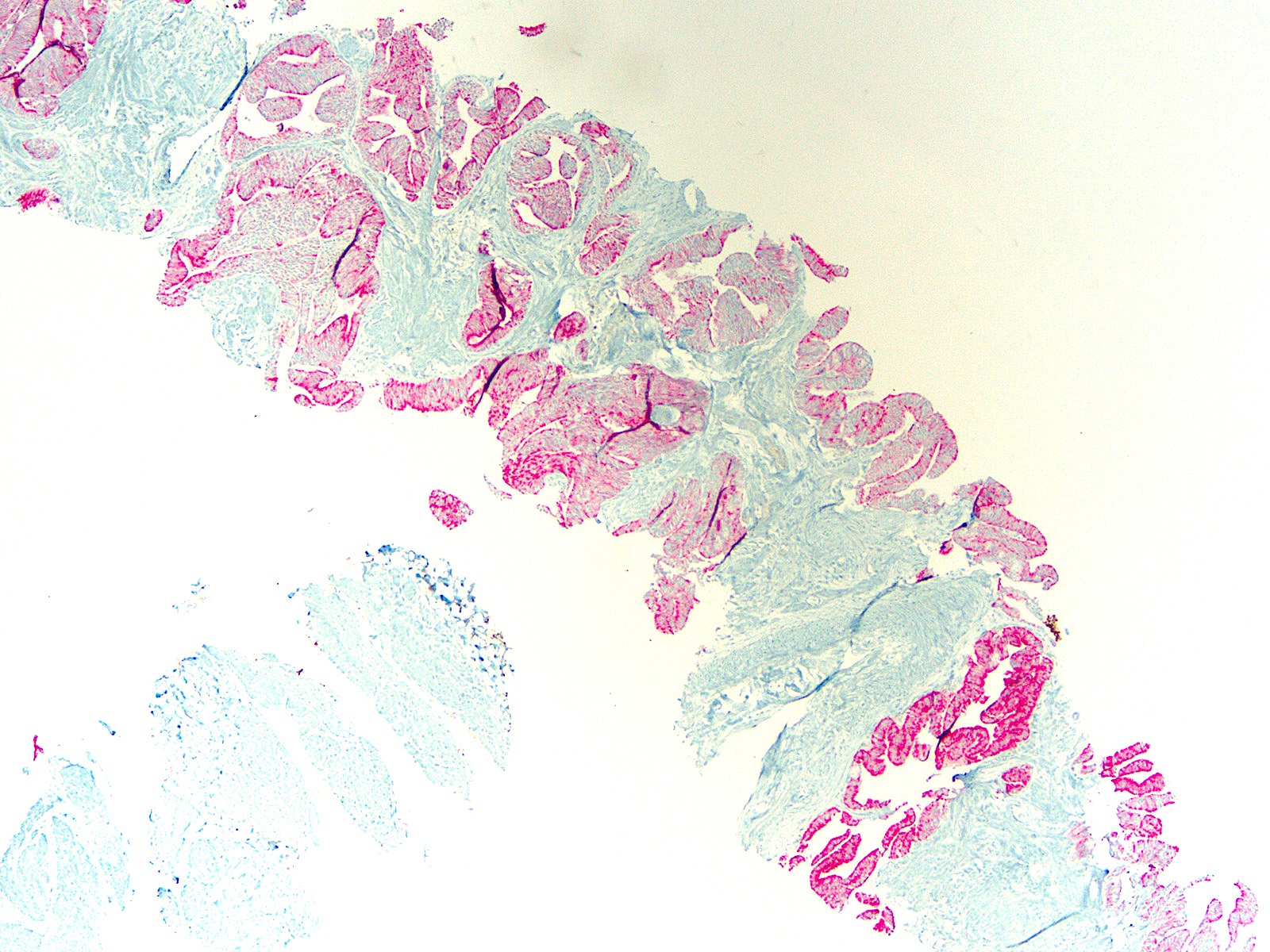
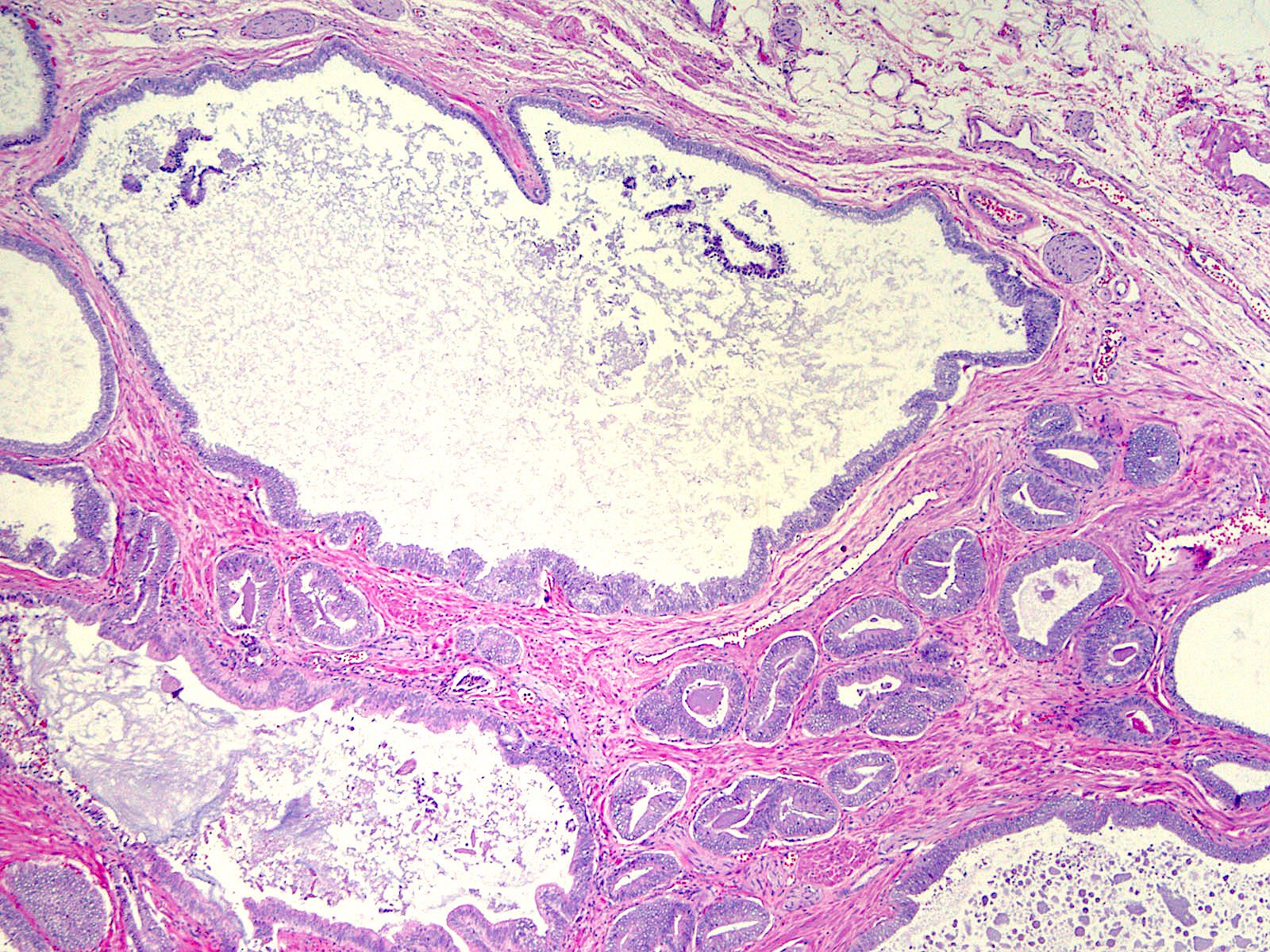
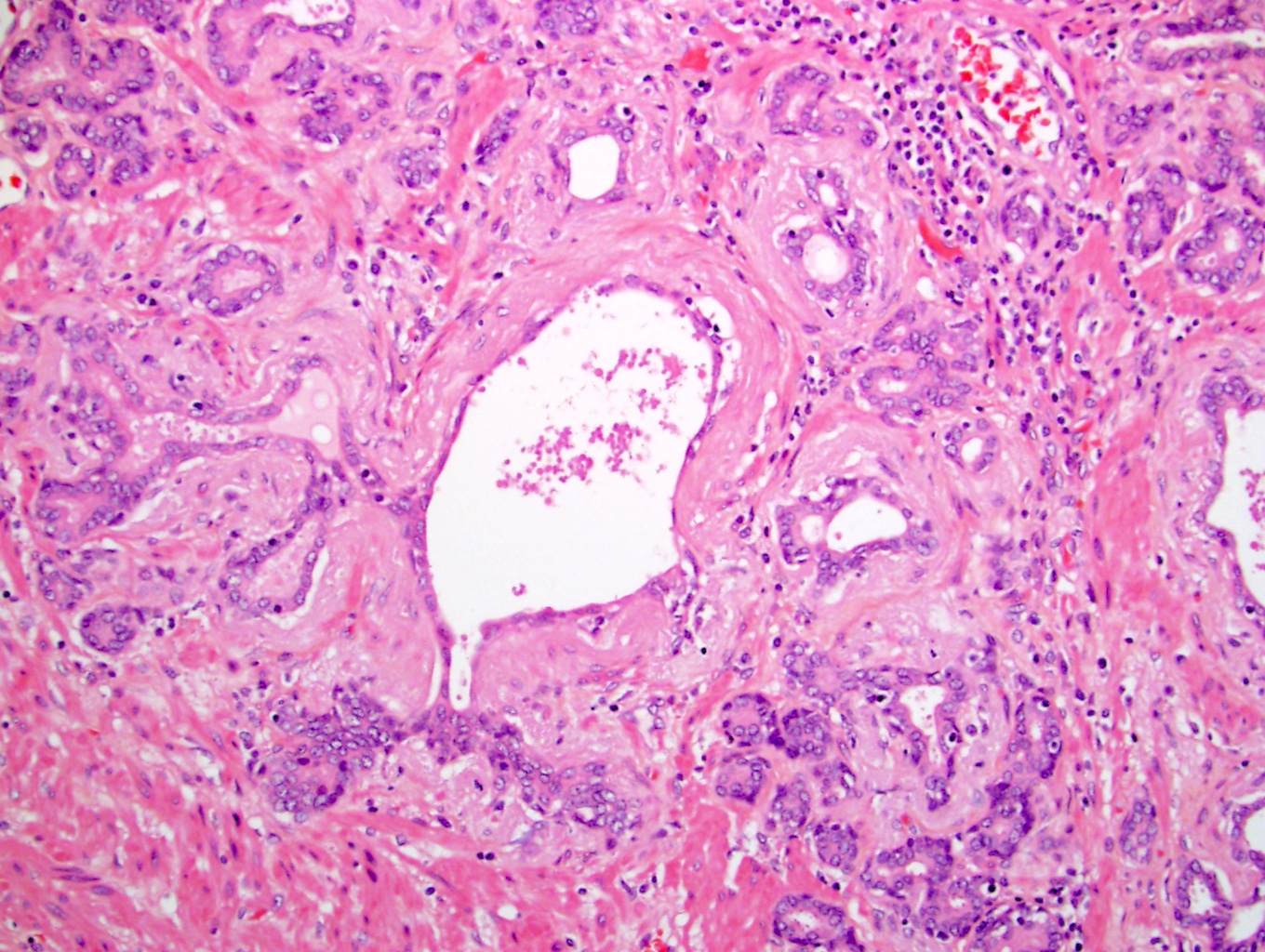
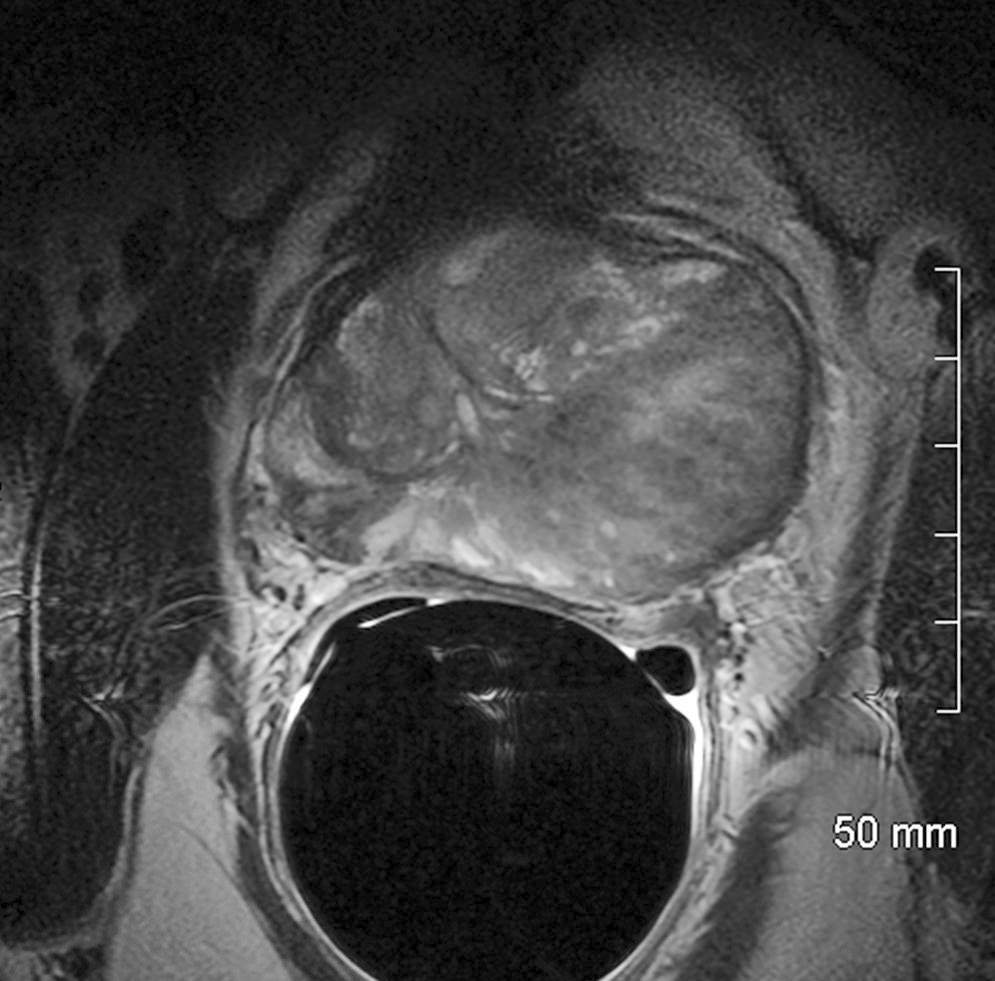
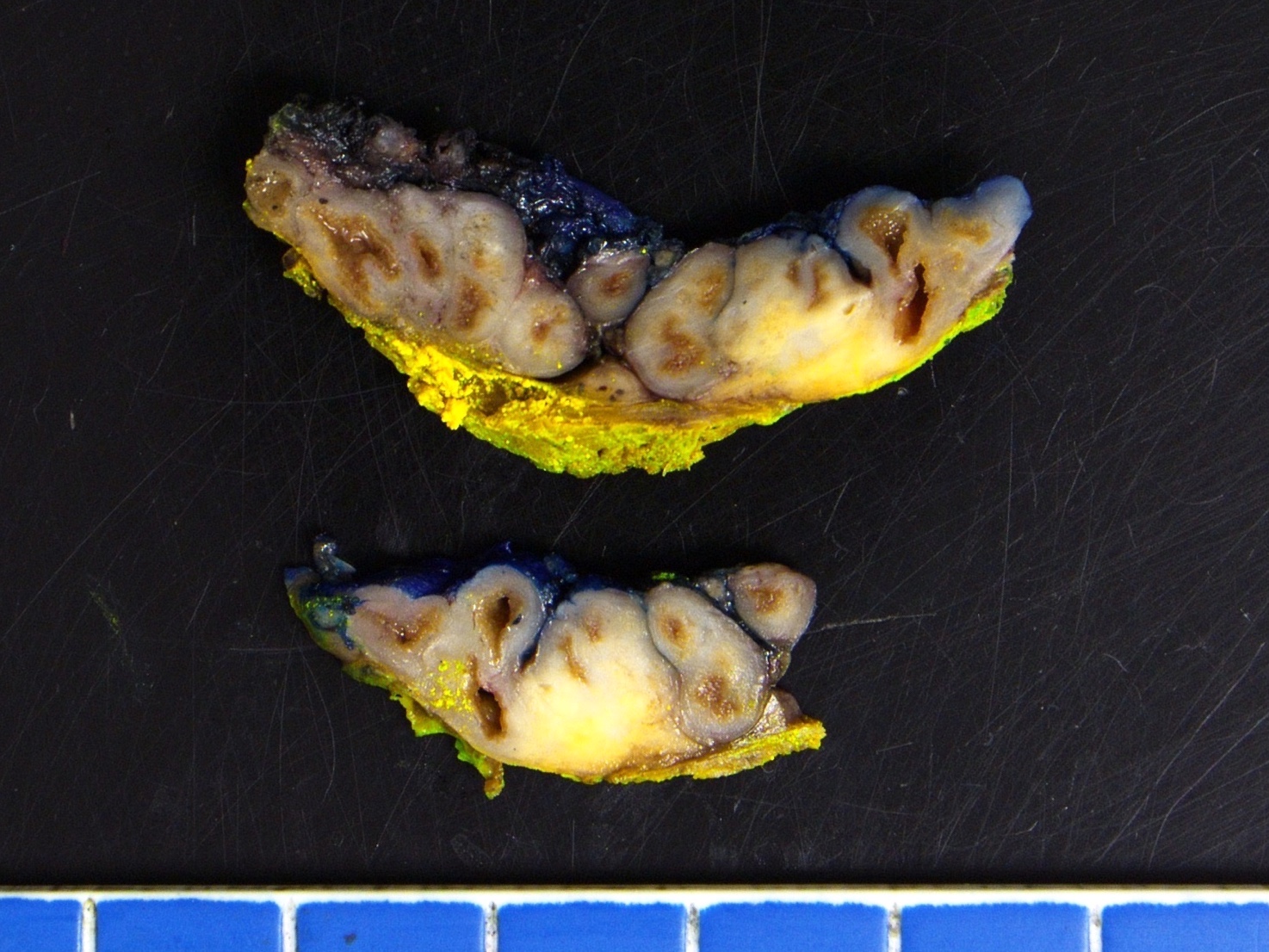
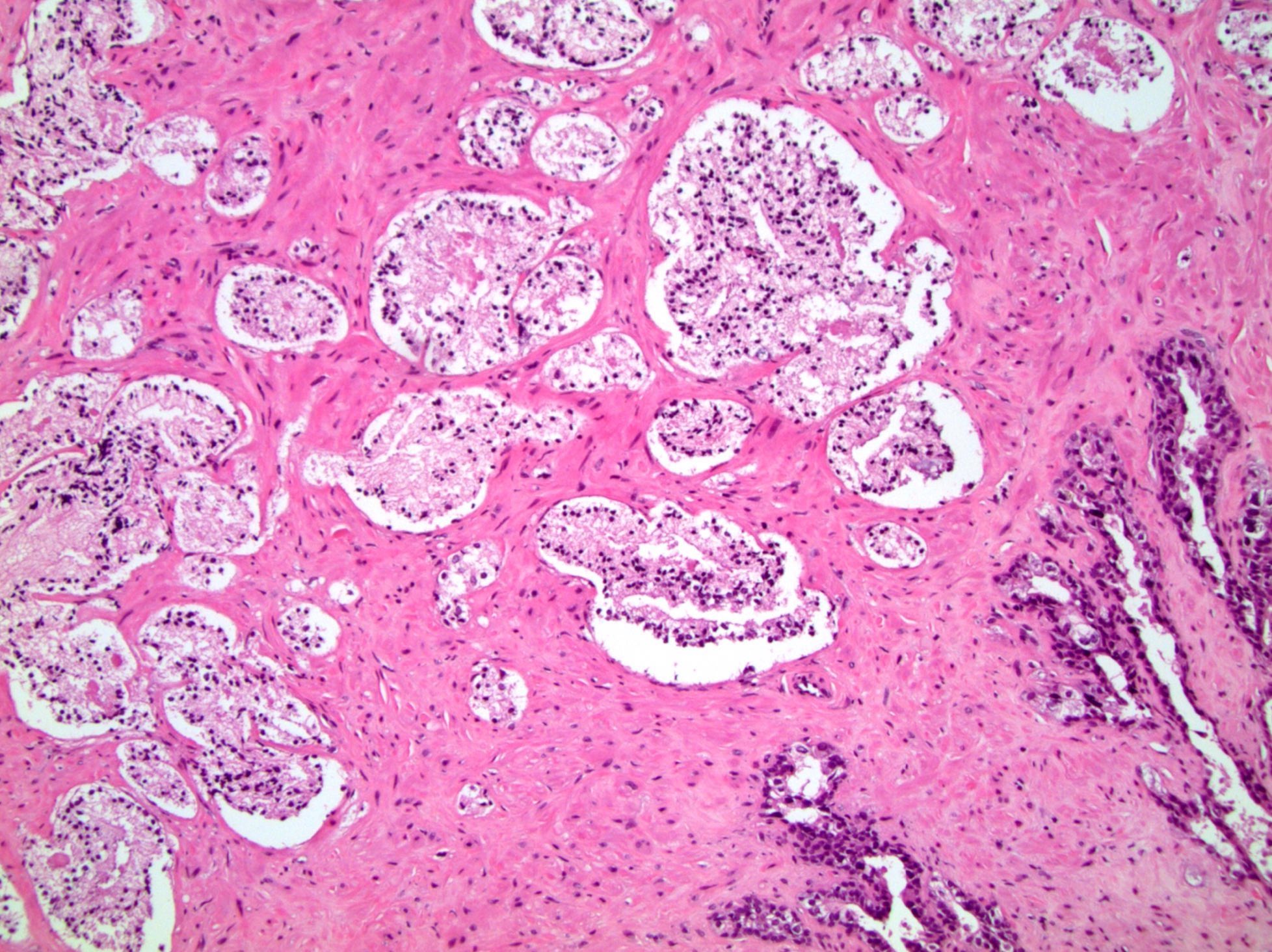
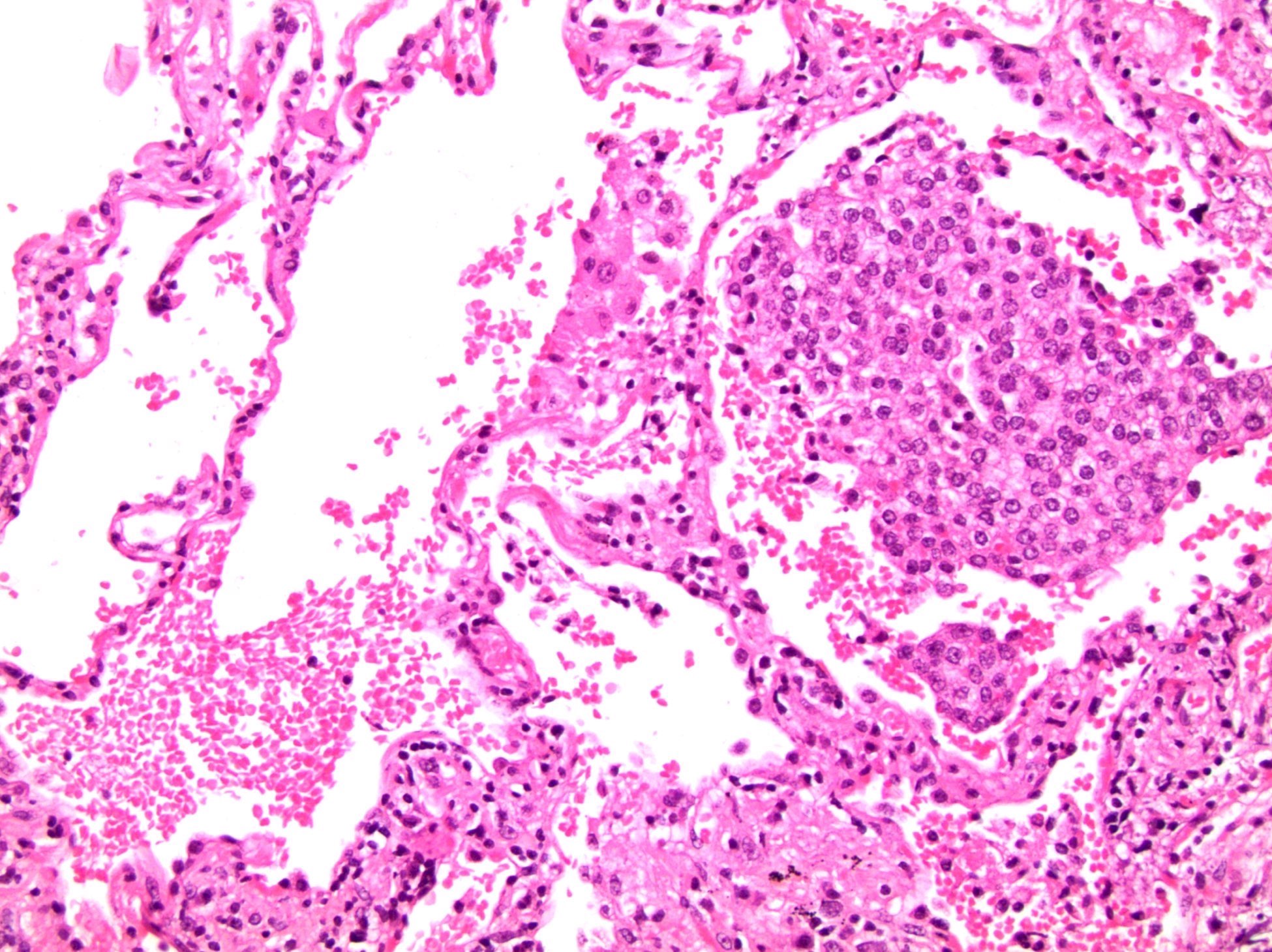
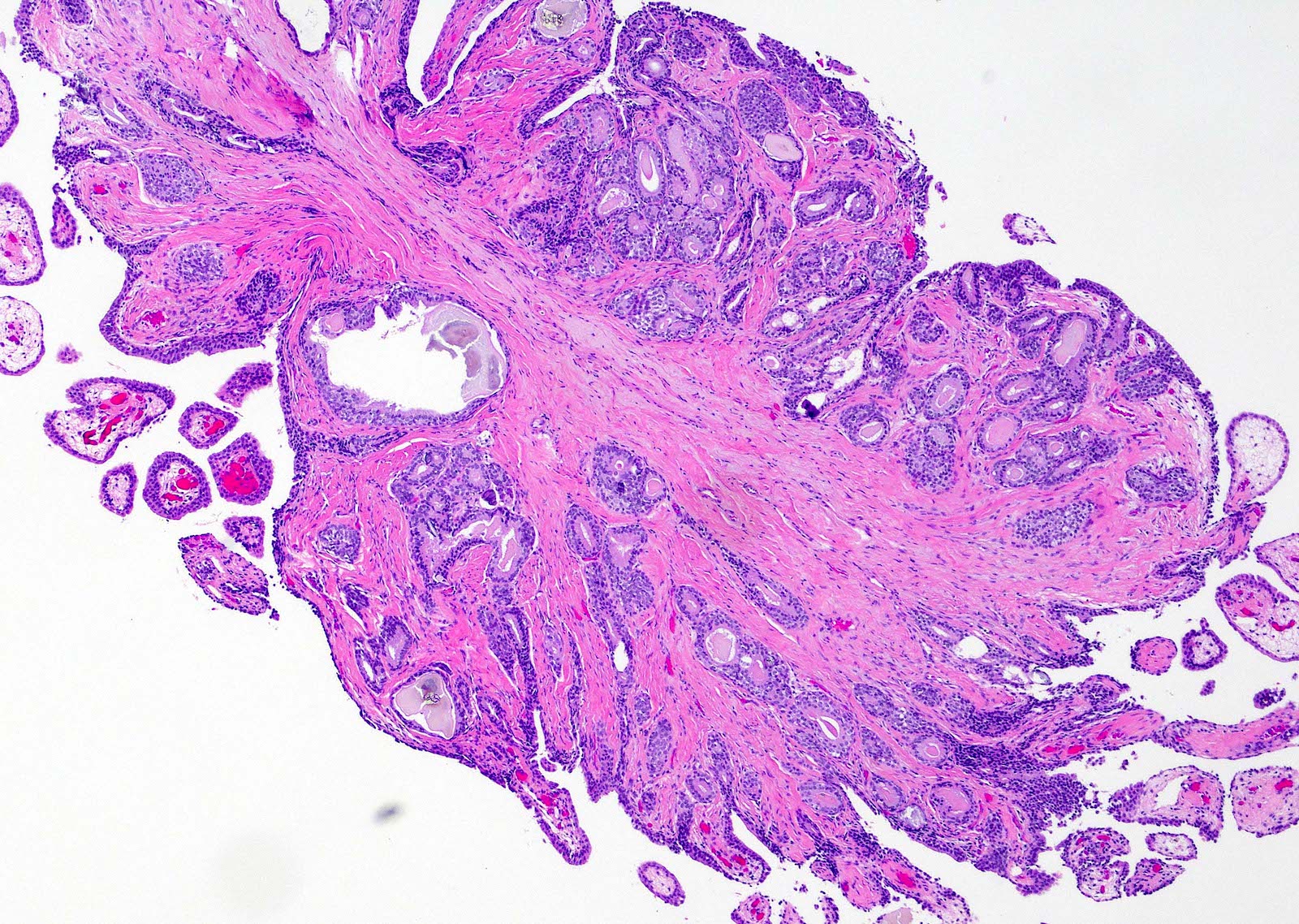
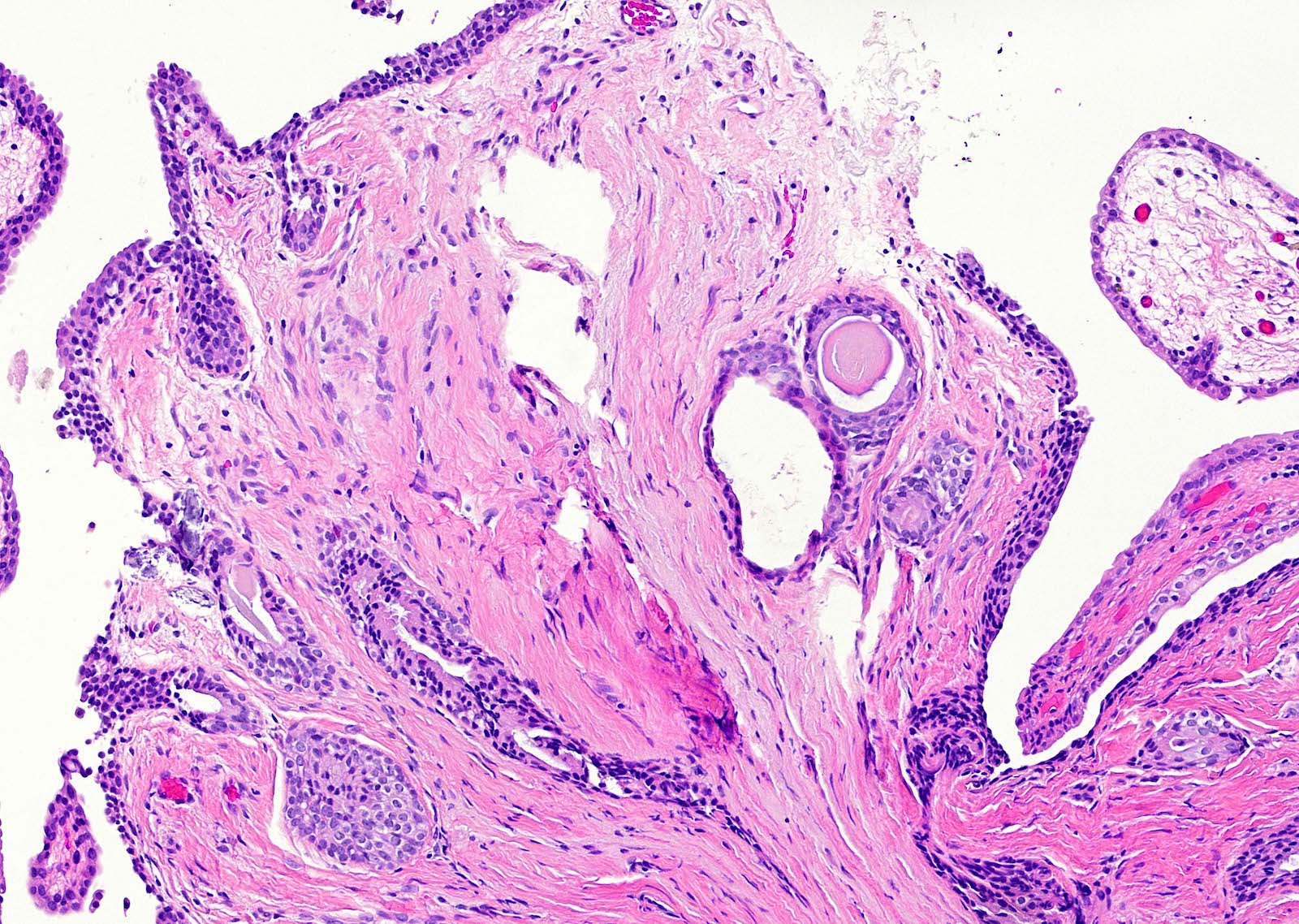
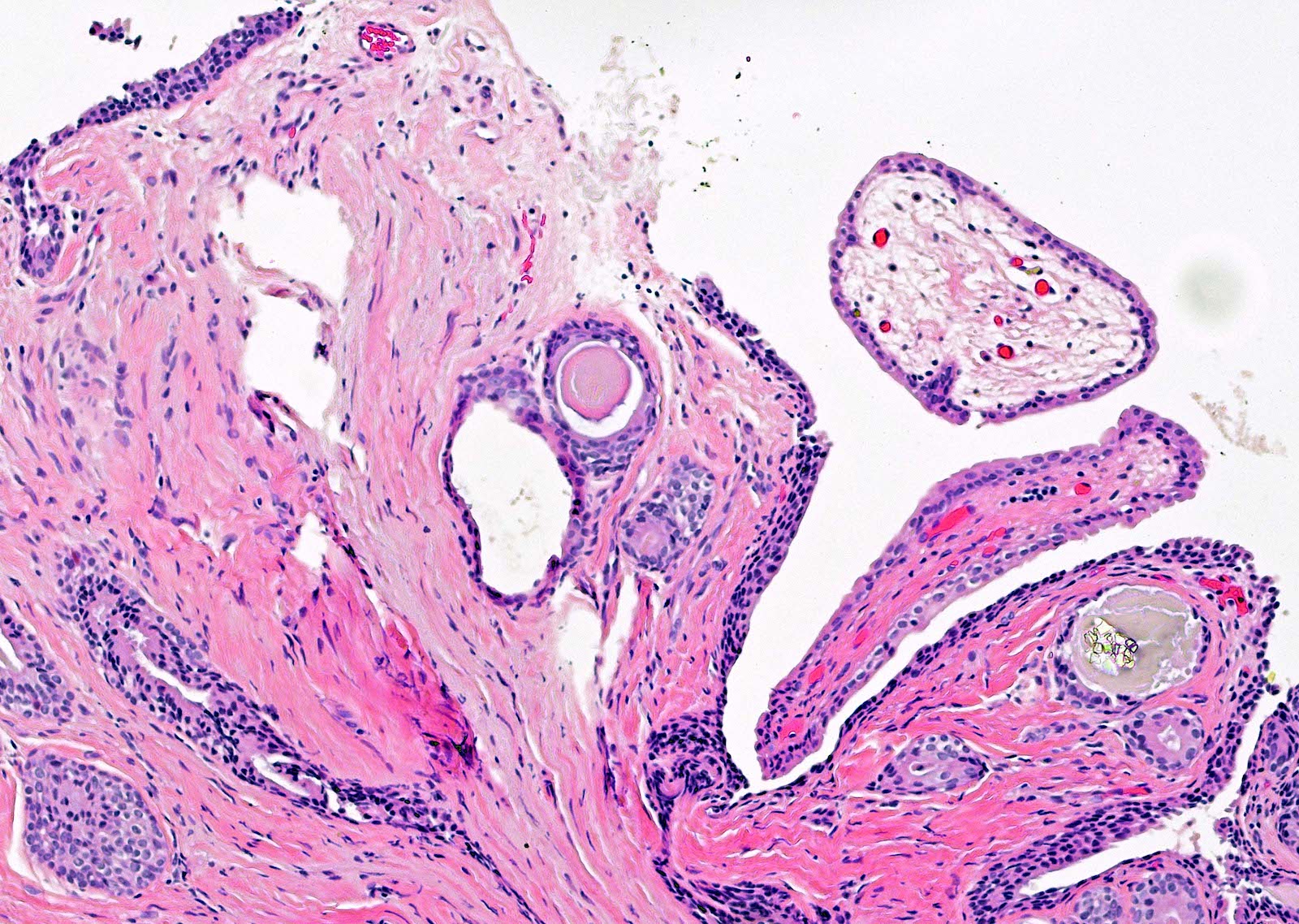
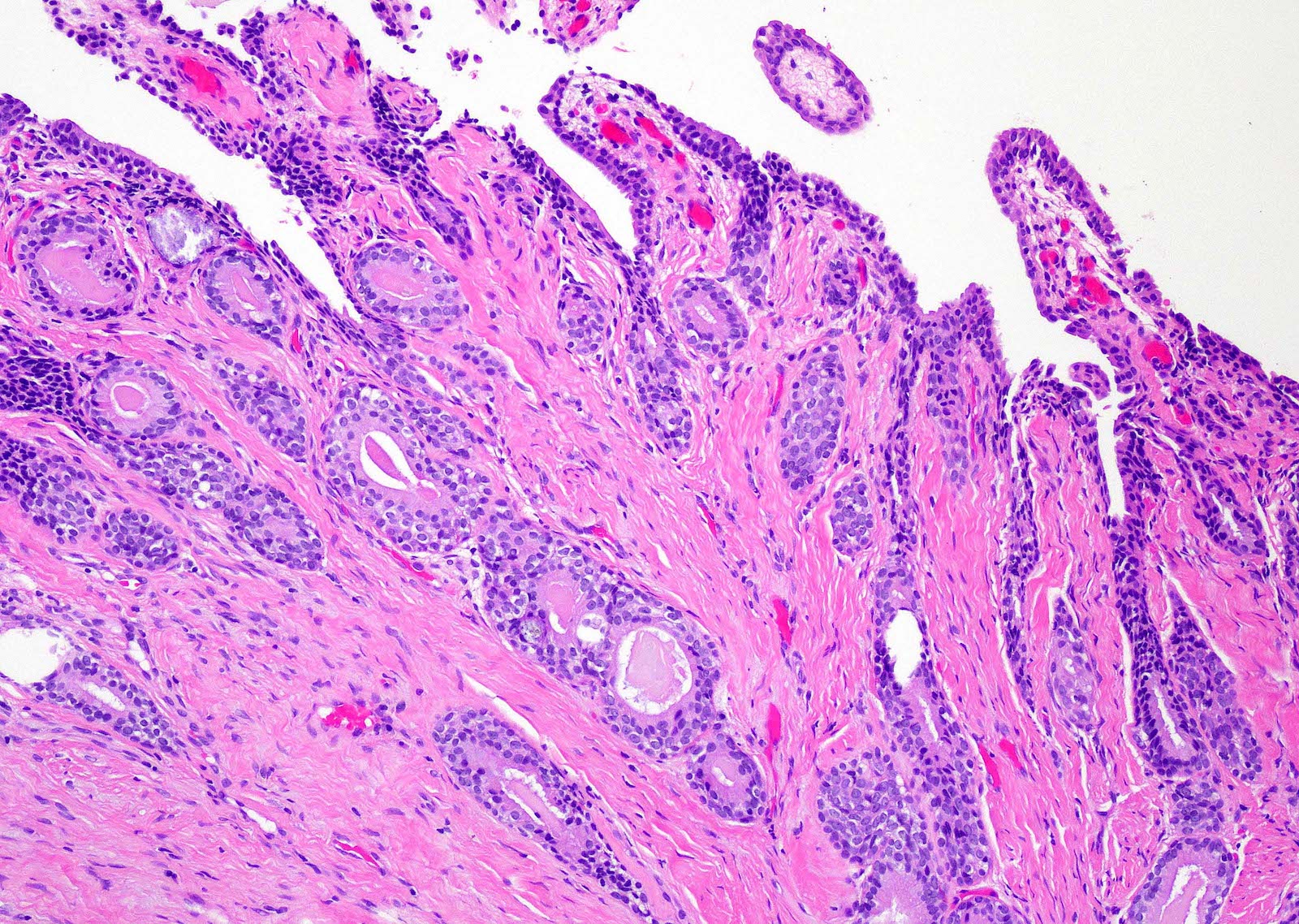
- pT3b: seminal vesicle muscle invasion
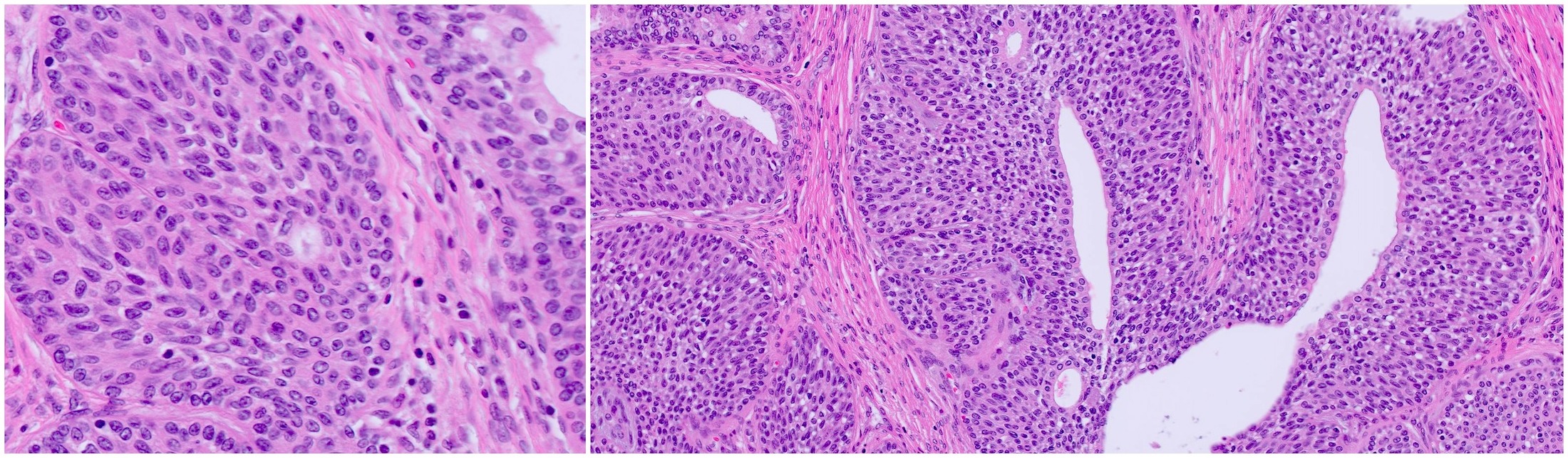
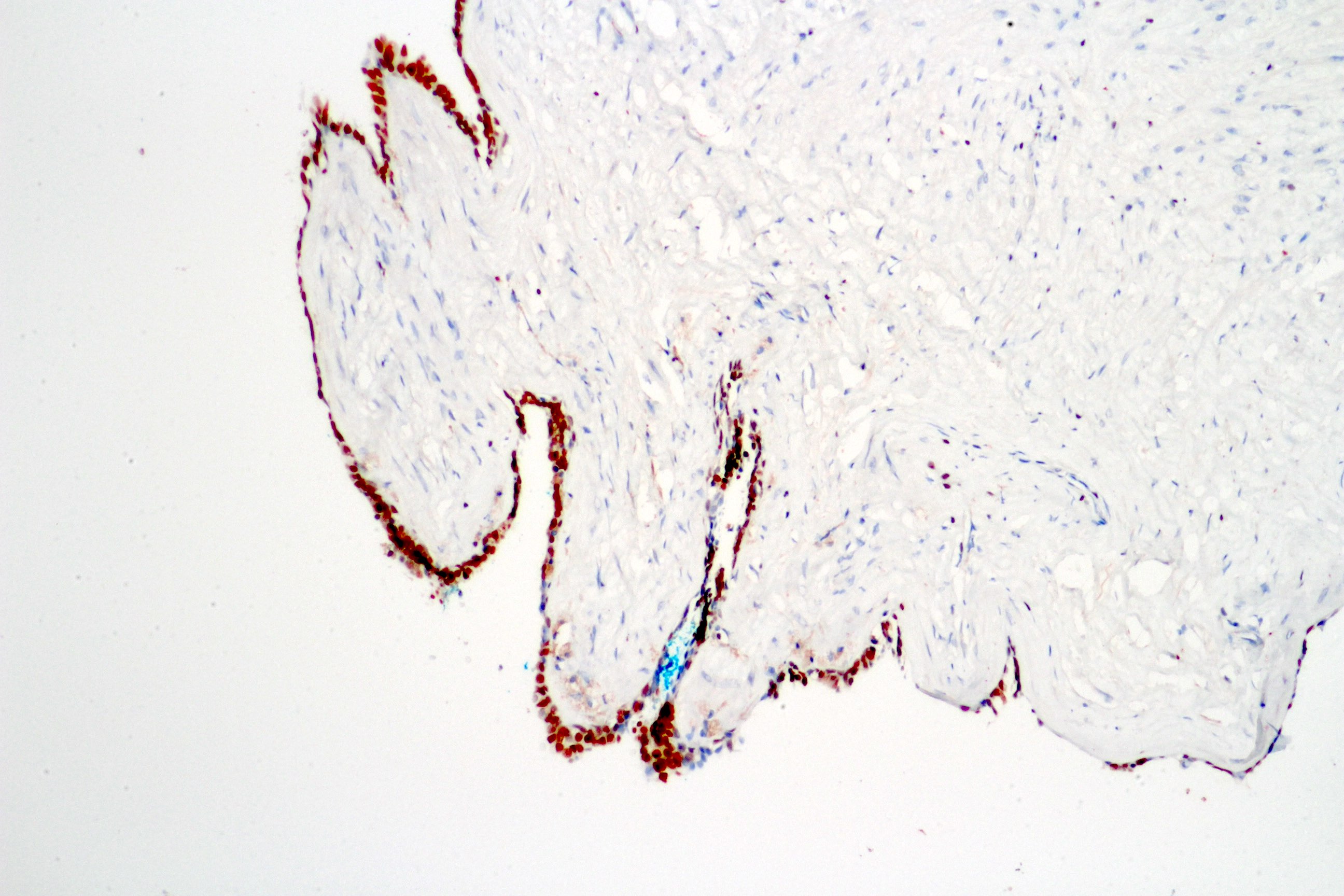
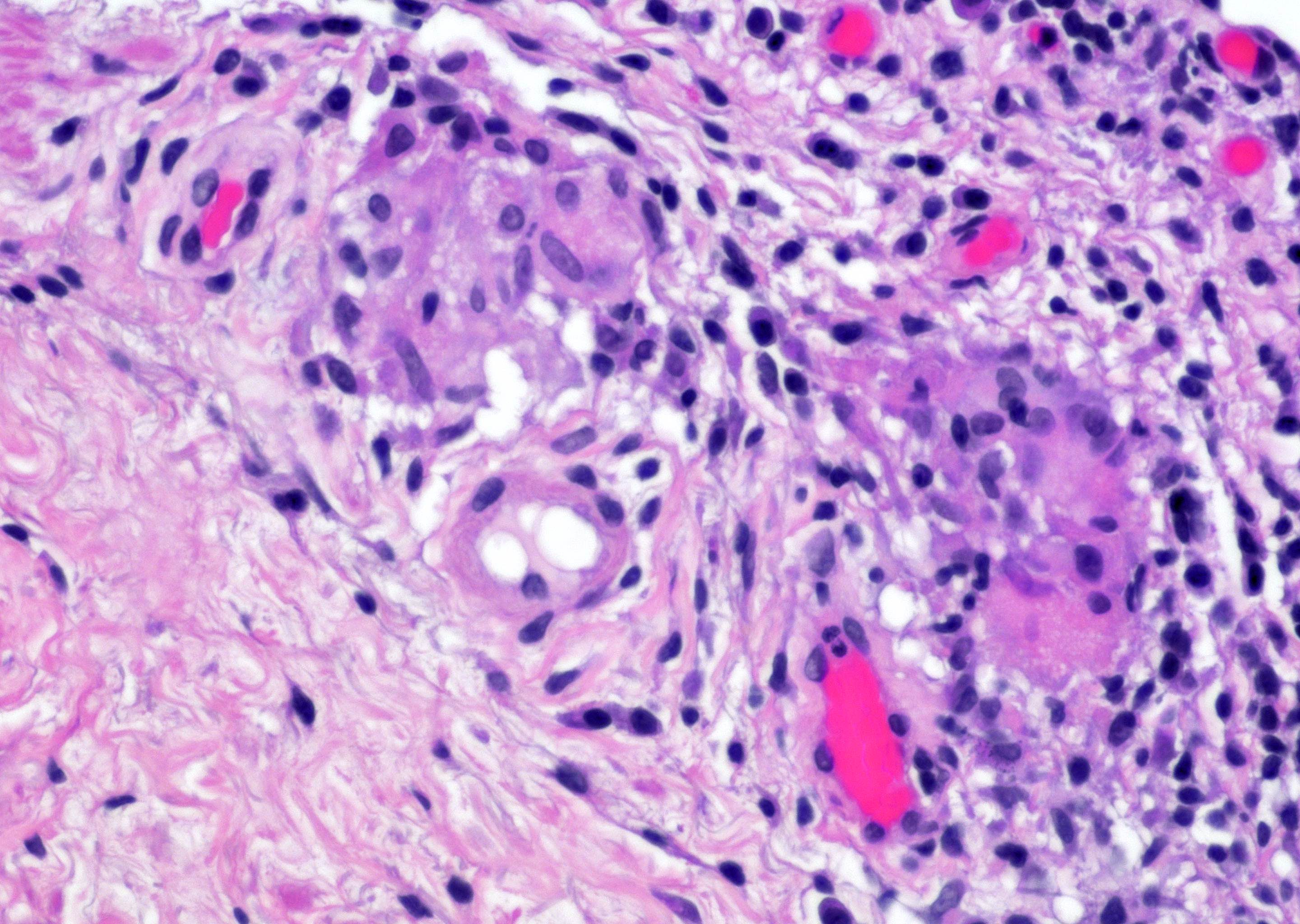
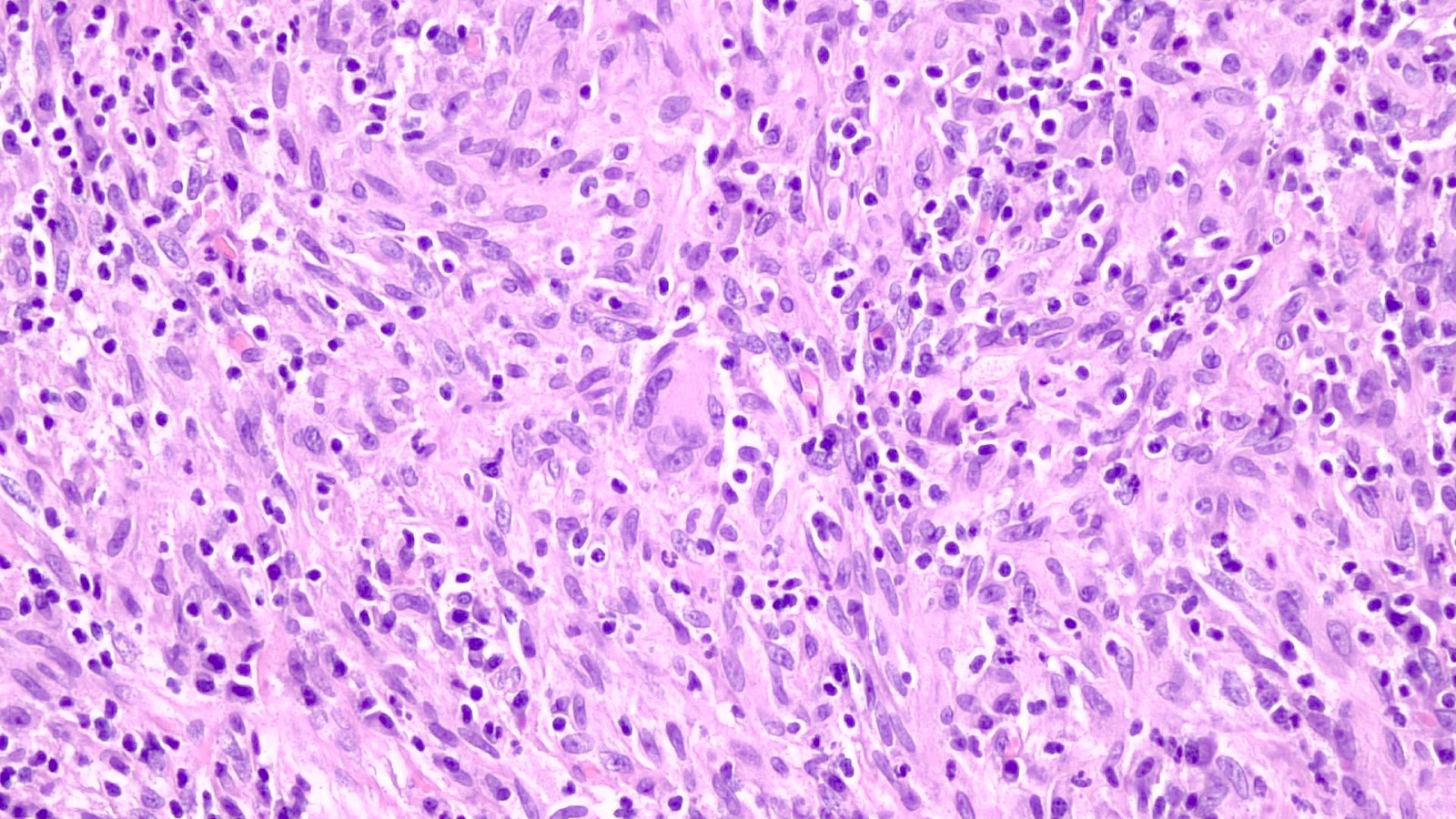
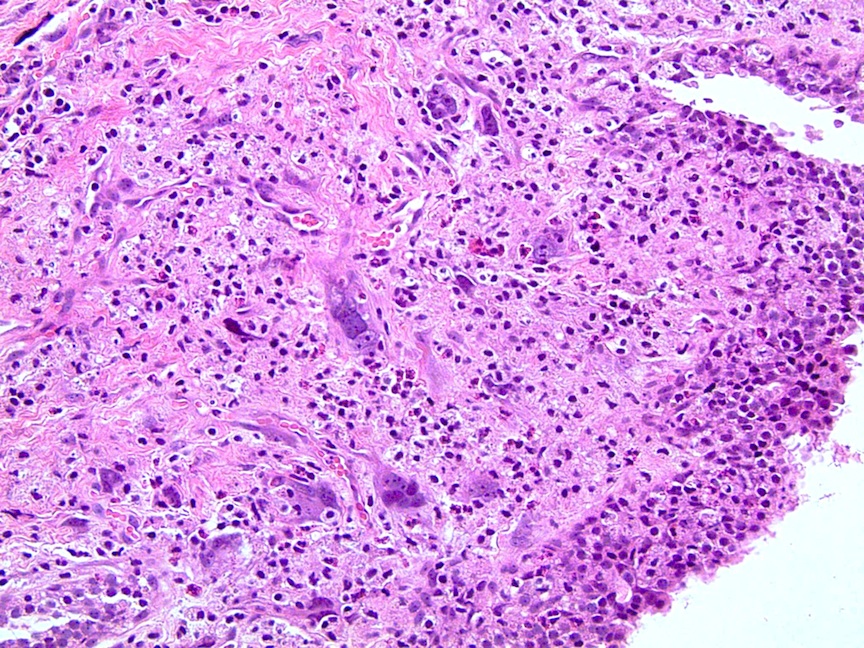
- pT4: fixed tumor or invasion of structures such as external sphincter, rectum, bladder, levator muscles or pelvic wall
Notes:
- There is no pT1 classification
- Note that cT1 is a part of clinical classification for clinically inapparent, nonpalpable tumor detected in specimens other than a prostatectomy such as a TURP or prostate biopsy with clinically inapparent, nonpalpable, incidental tumor in 5% or less of tissue defined as cT1a, clinically inapparent, nonpalpable, incidental tumor in > 5% defined as cT1b and clinically inapparent, nonpalpable tumor in a prostate needle biopsy defined as cT1c
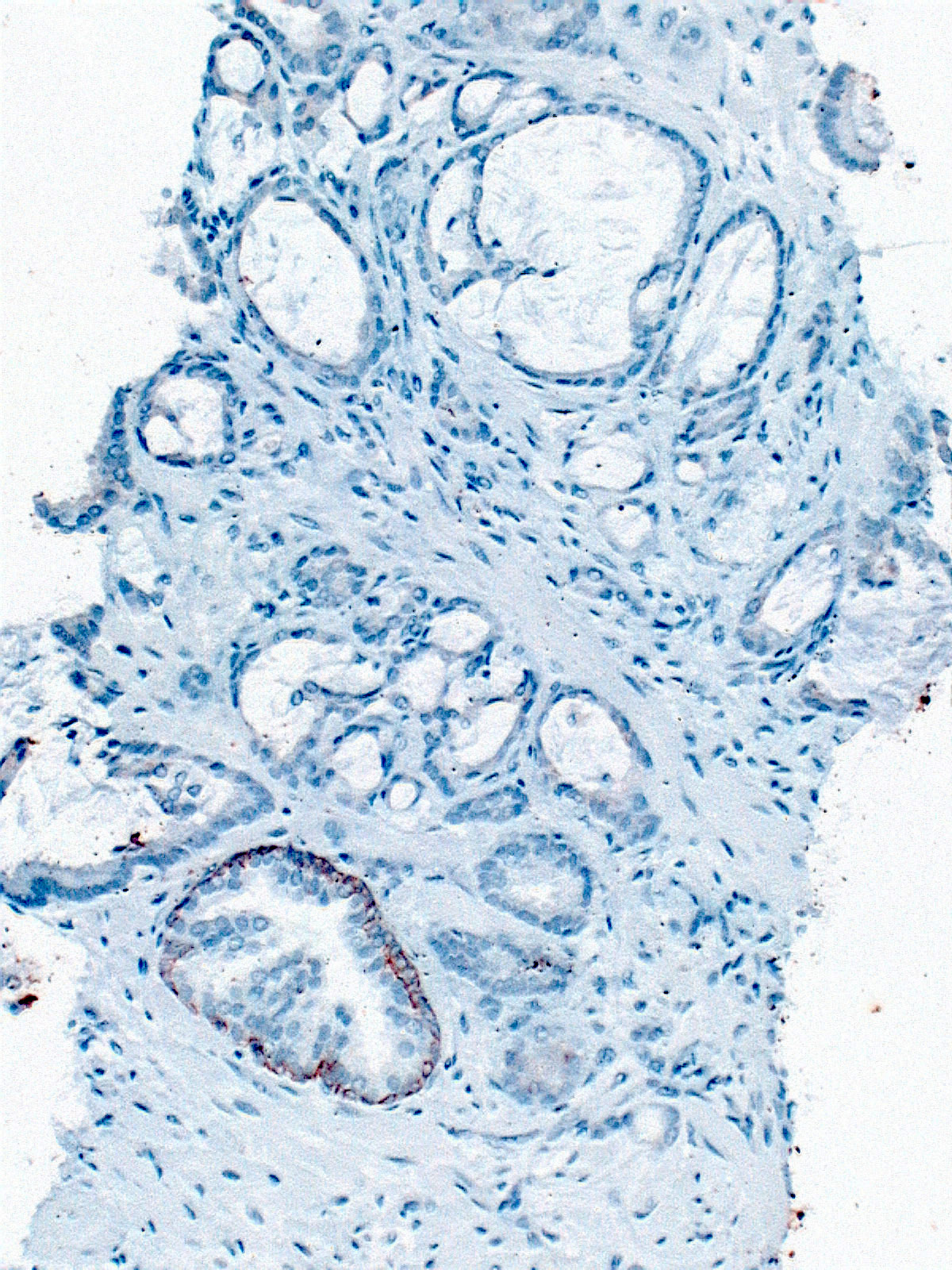
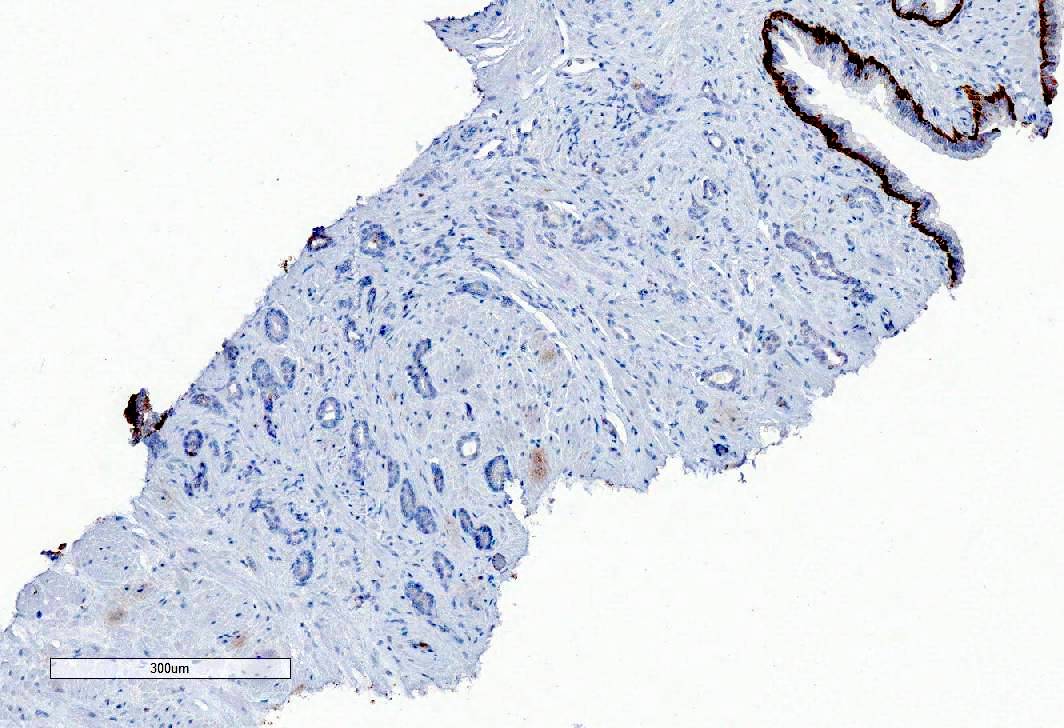
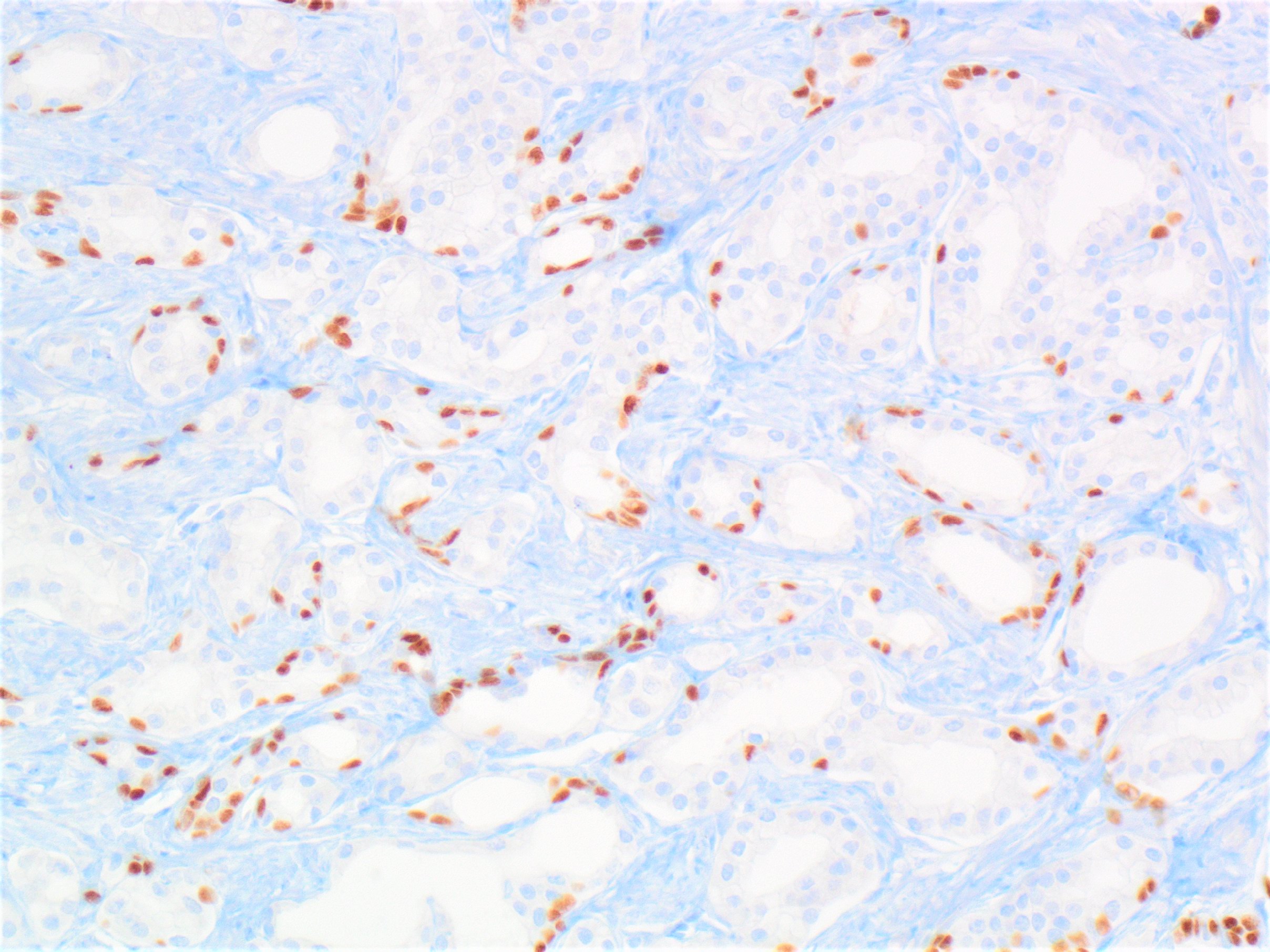
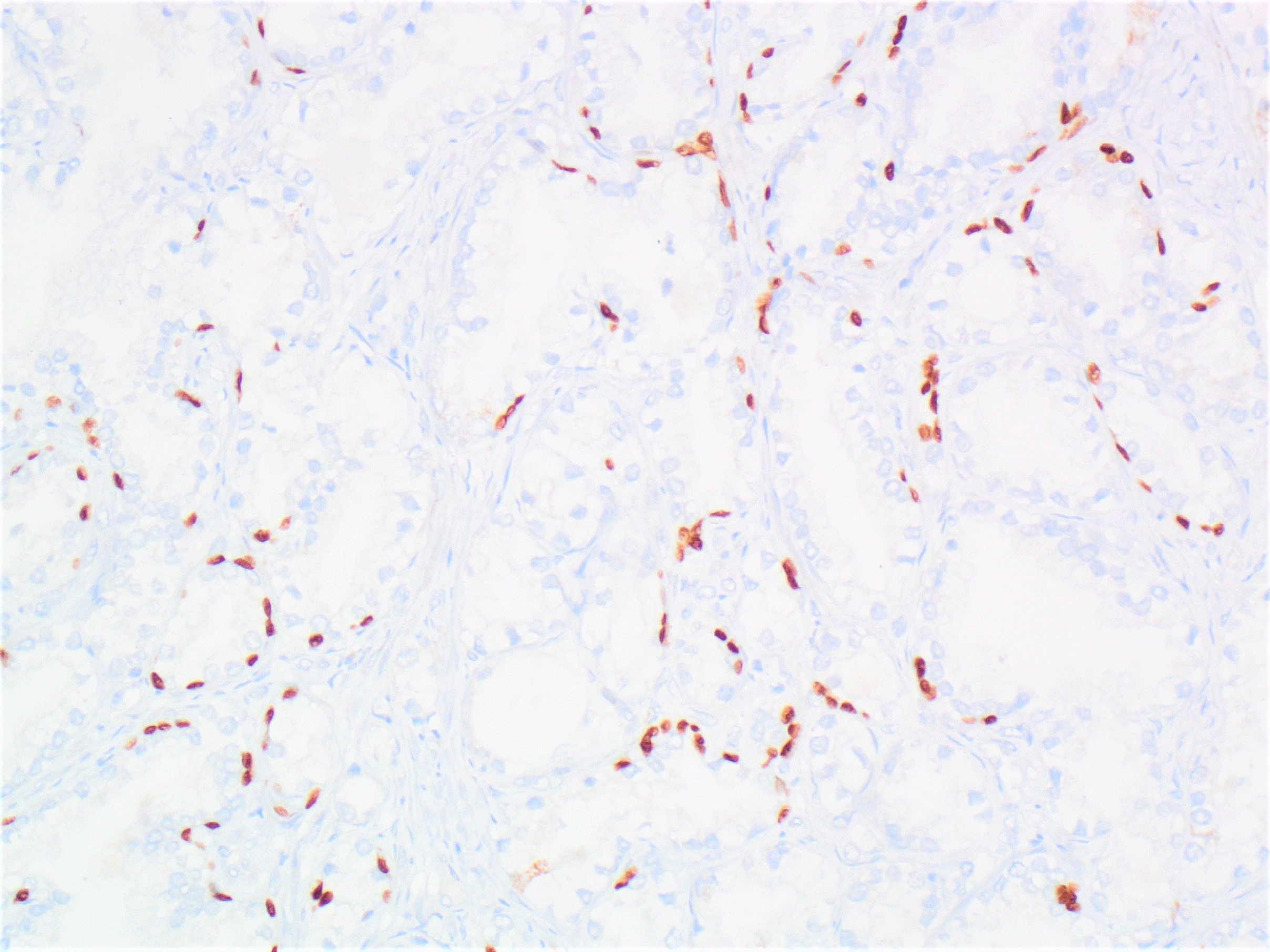
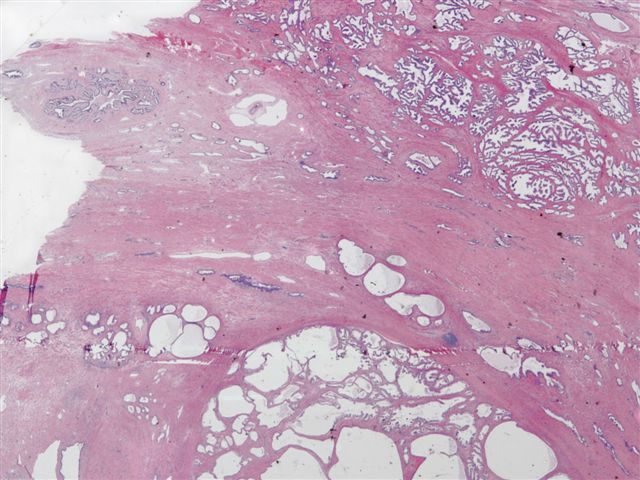
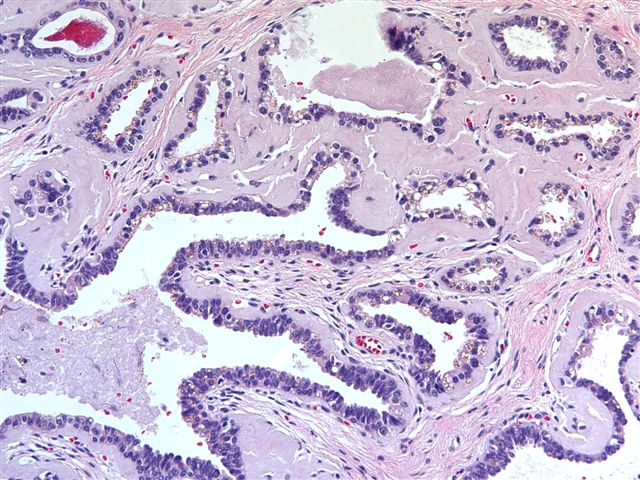
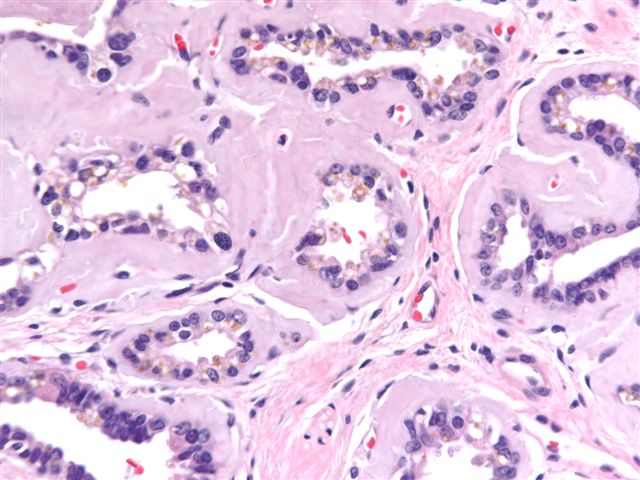
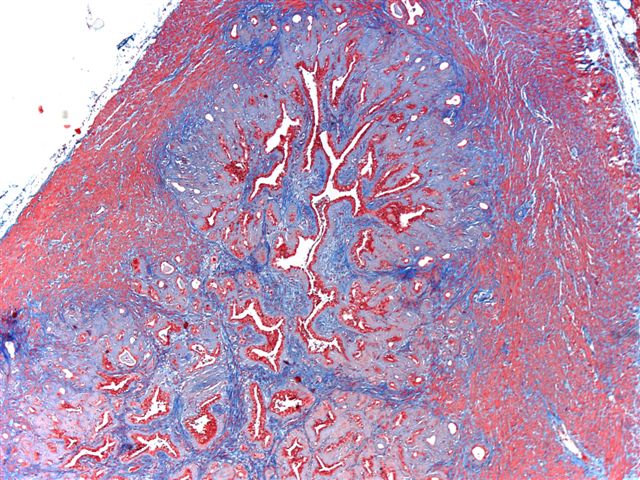
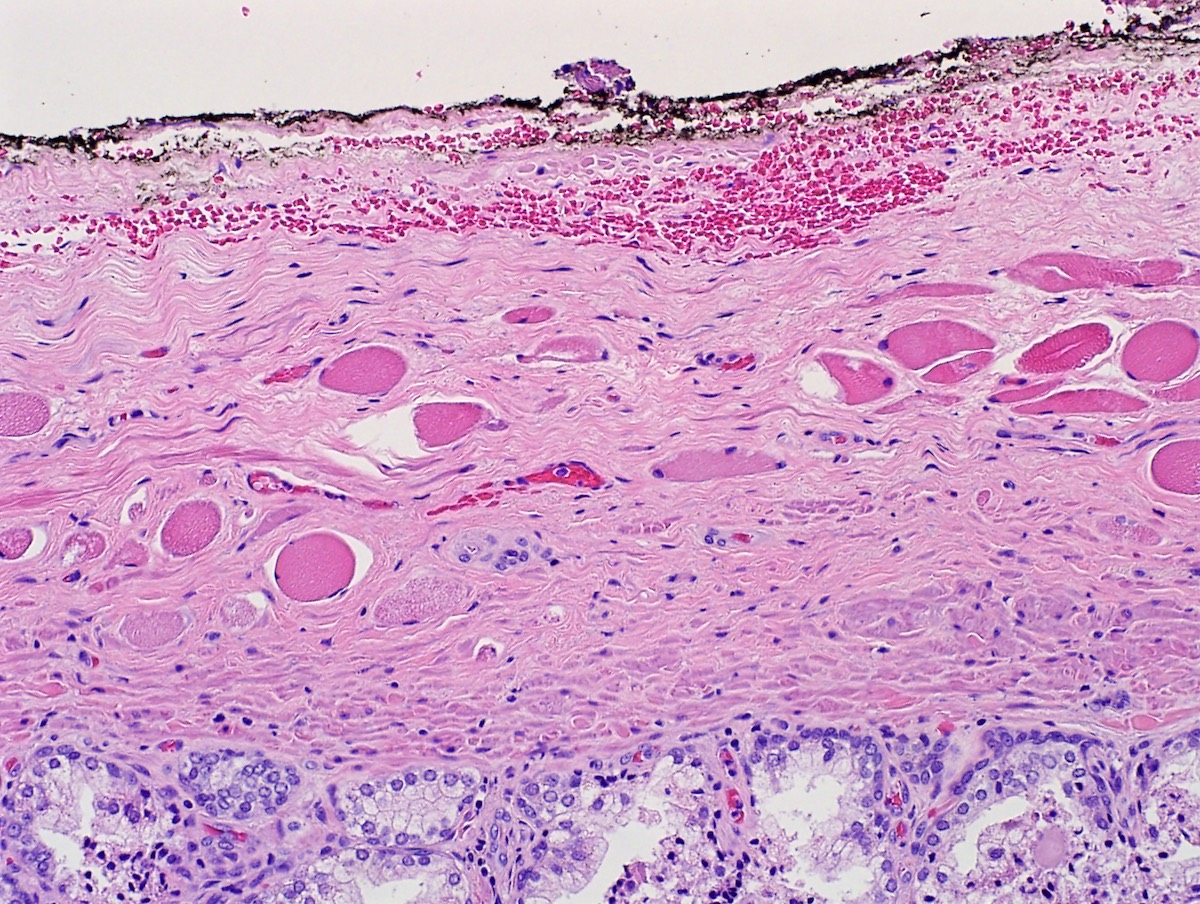
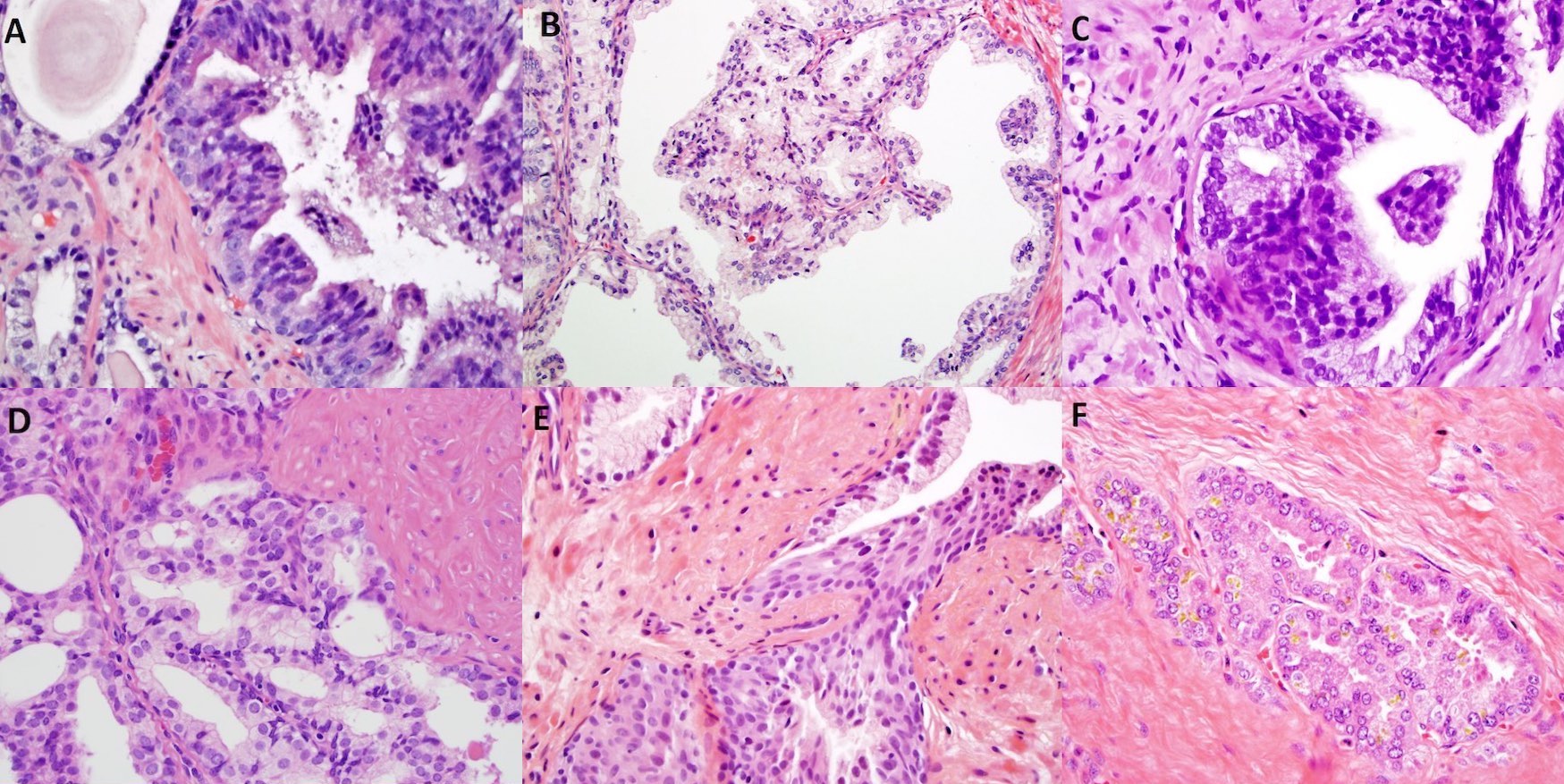
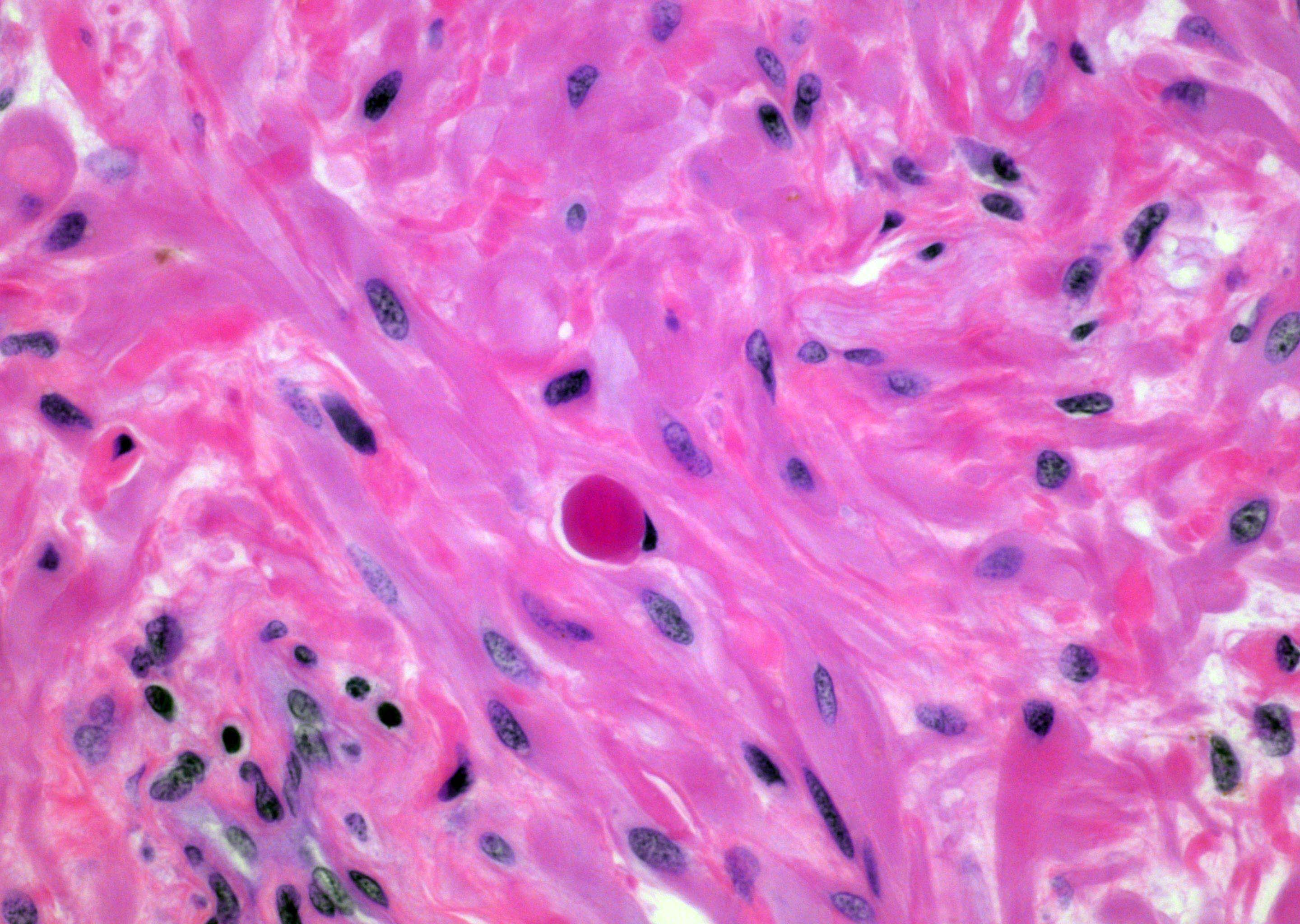
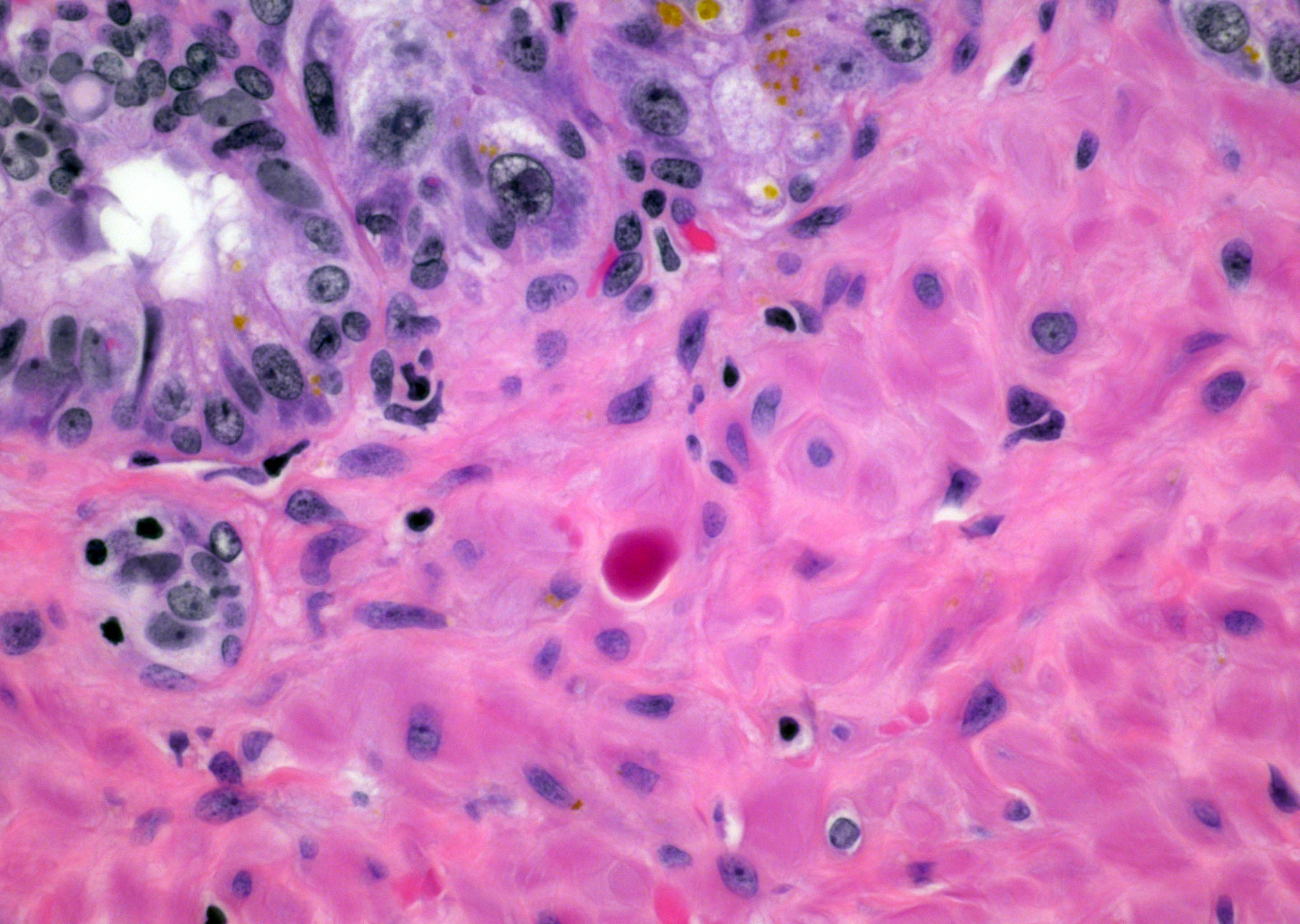
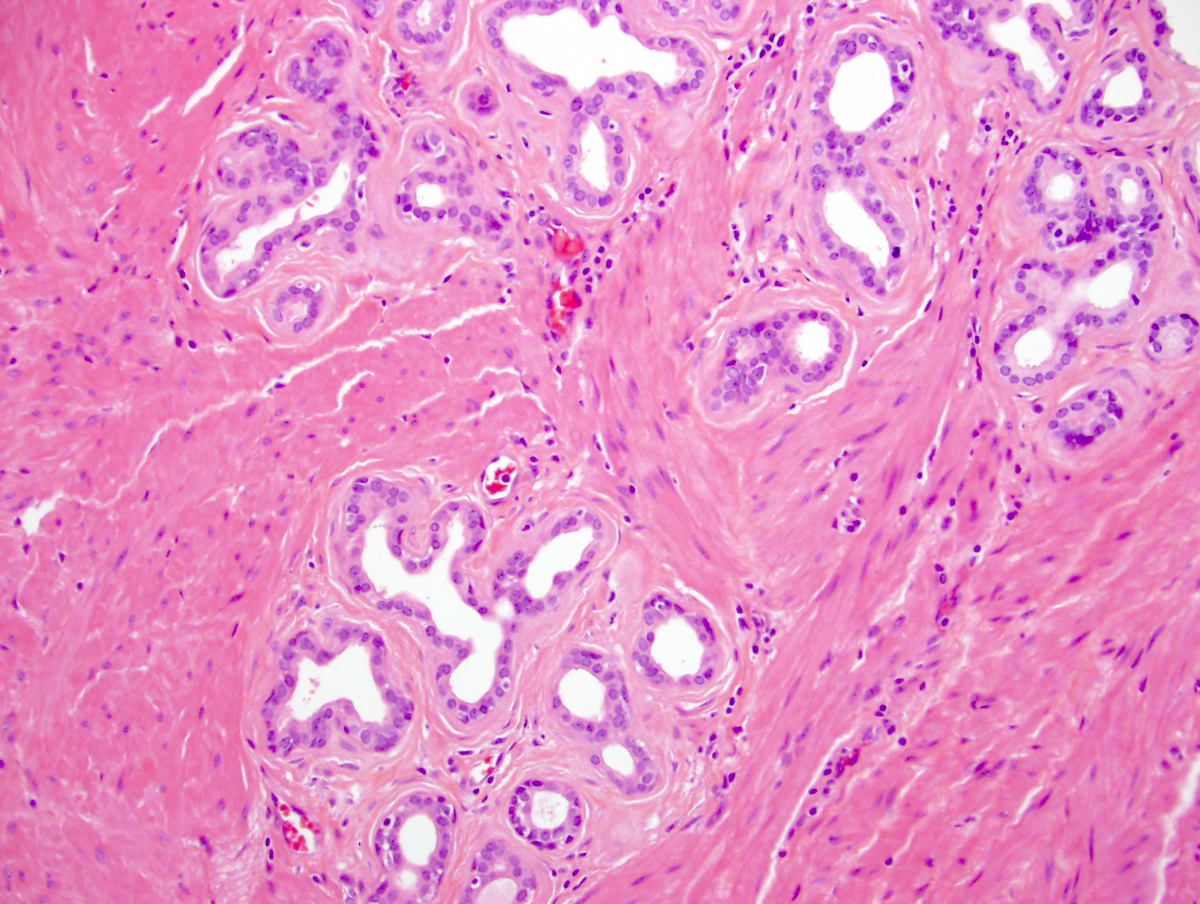
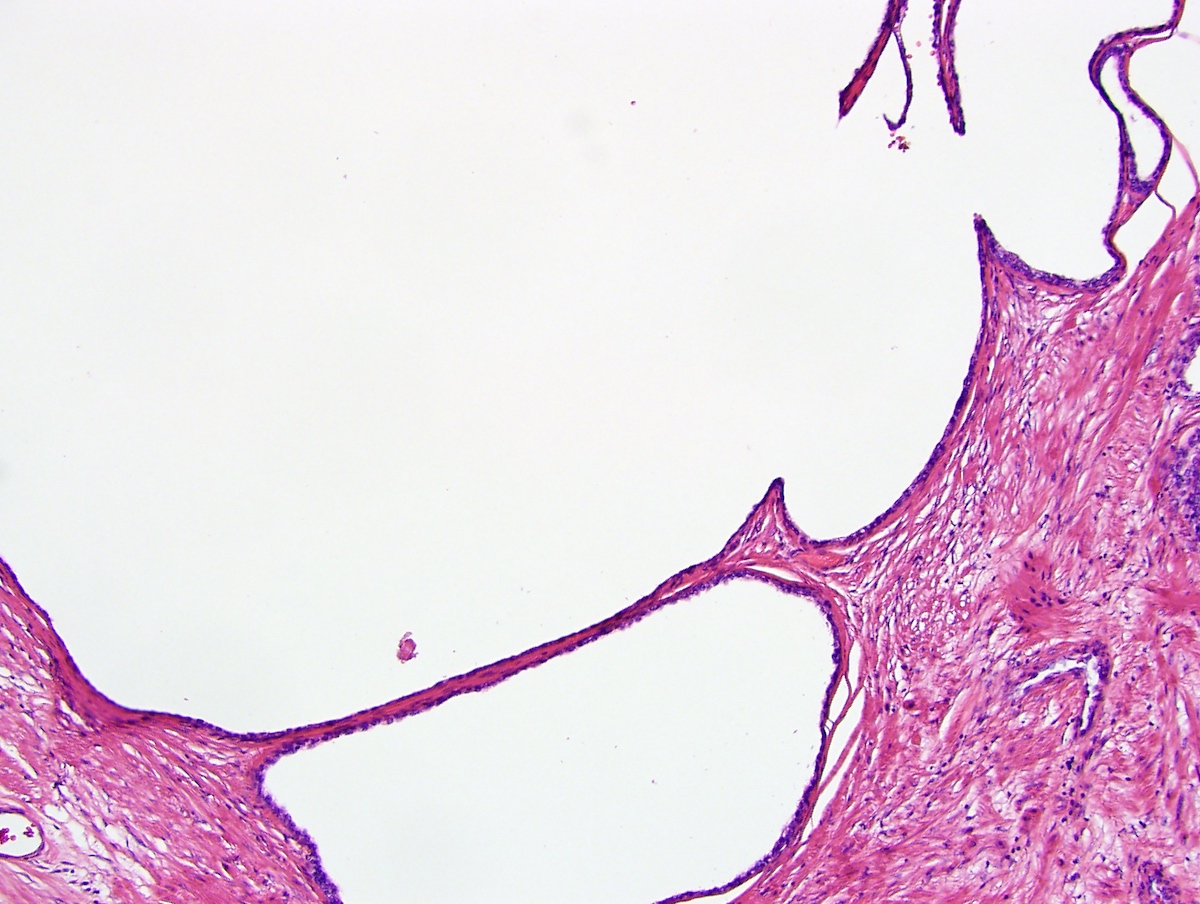
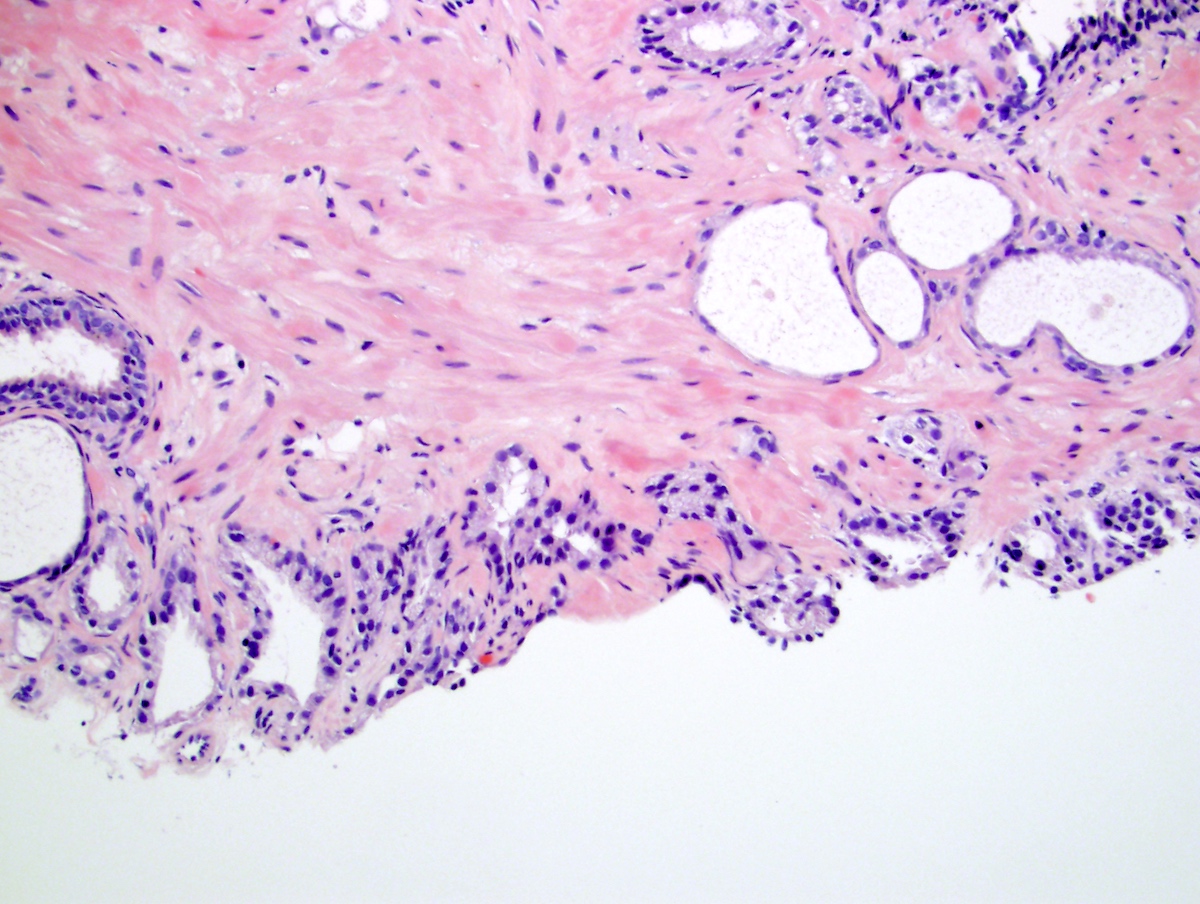
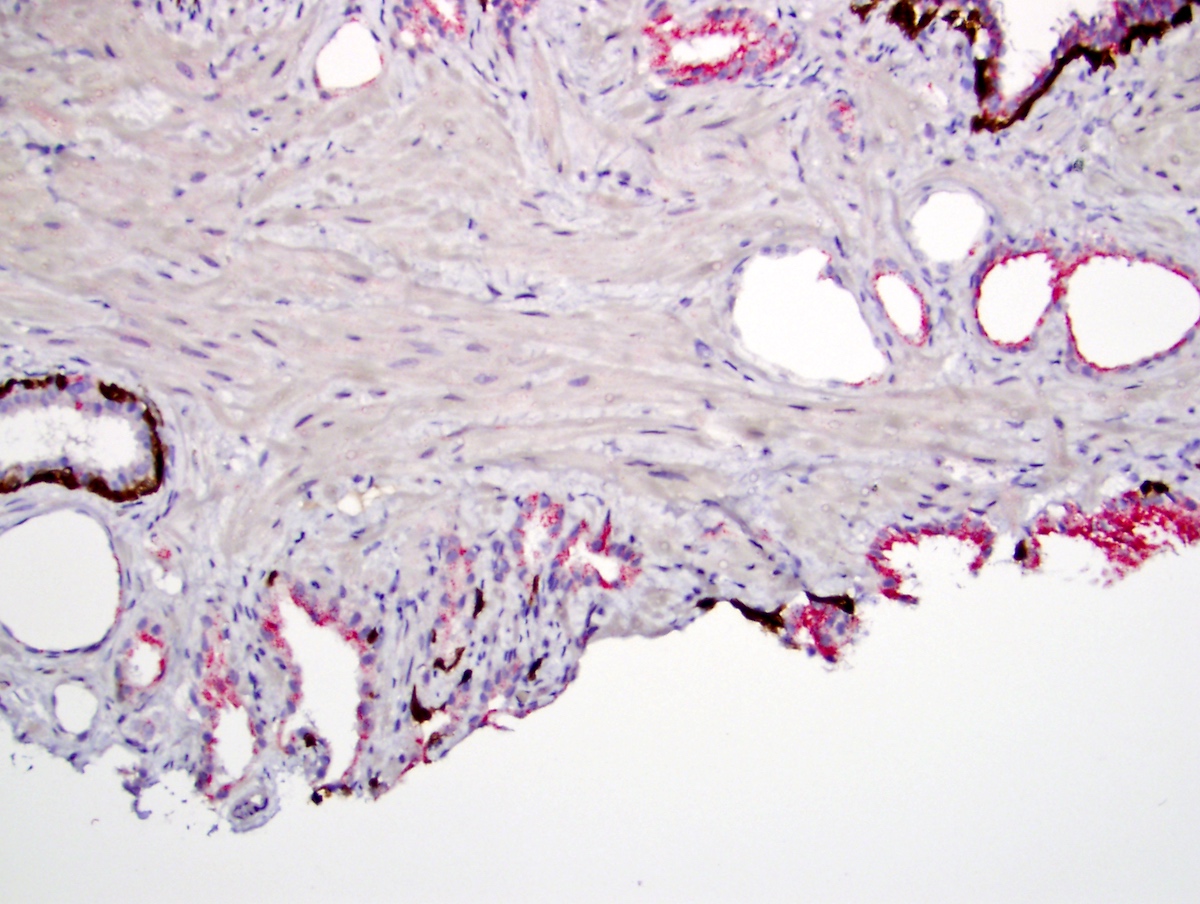
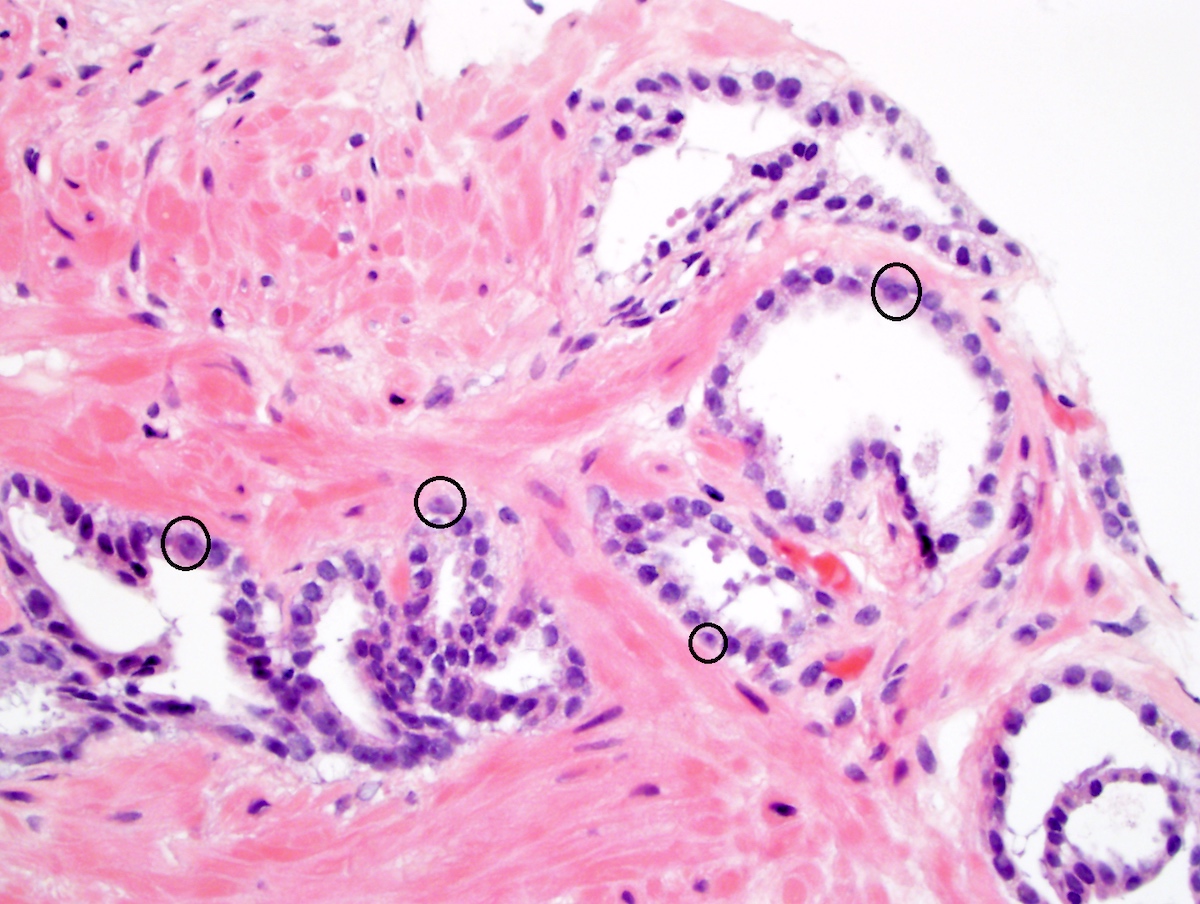
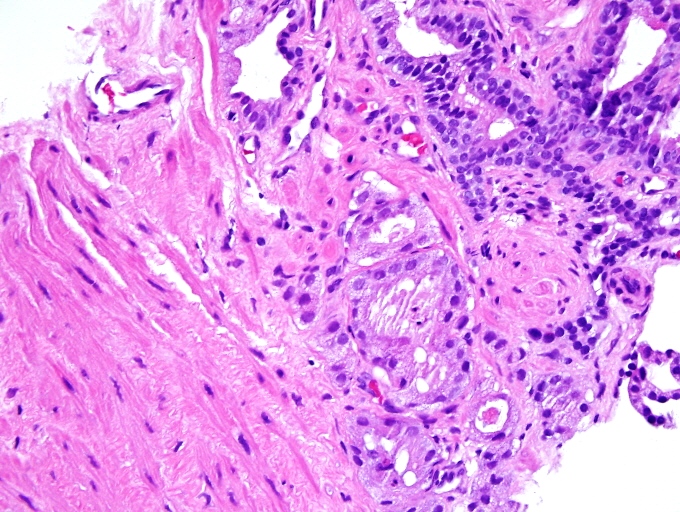
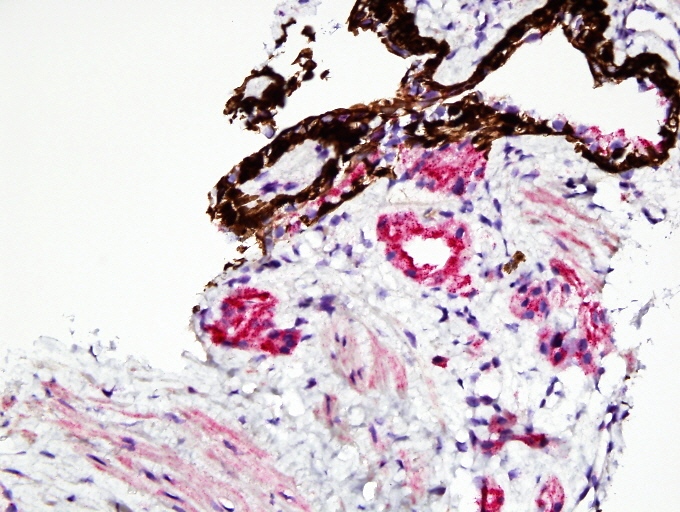
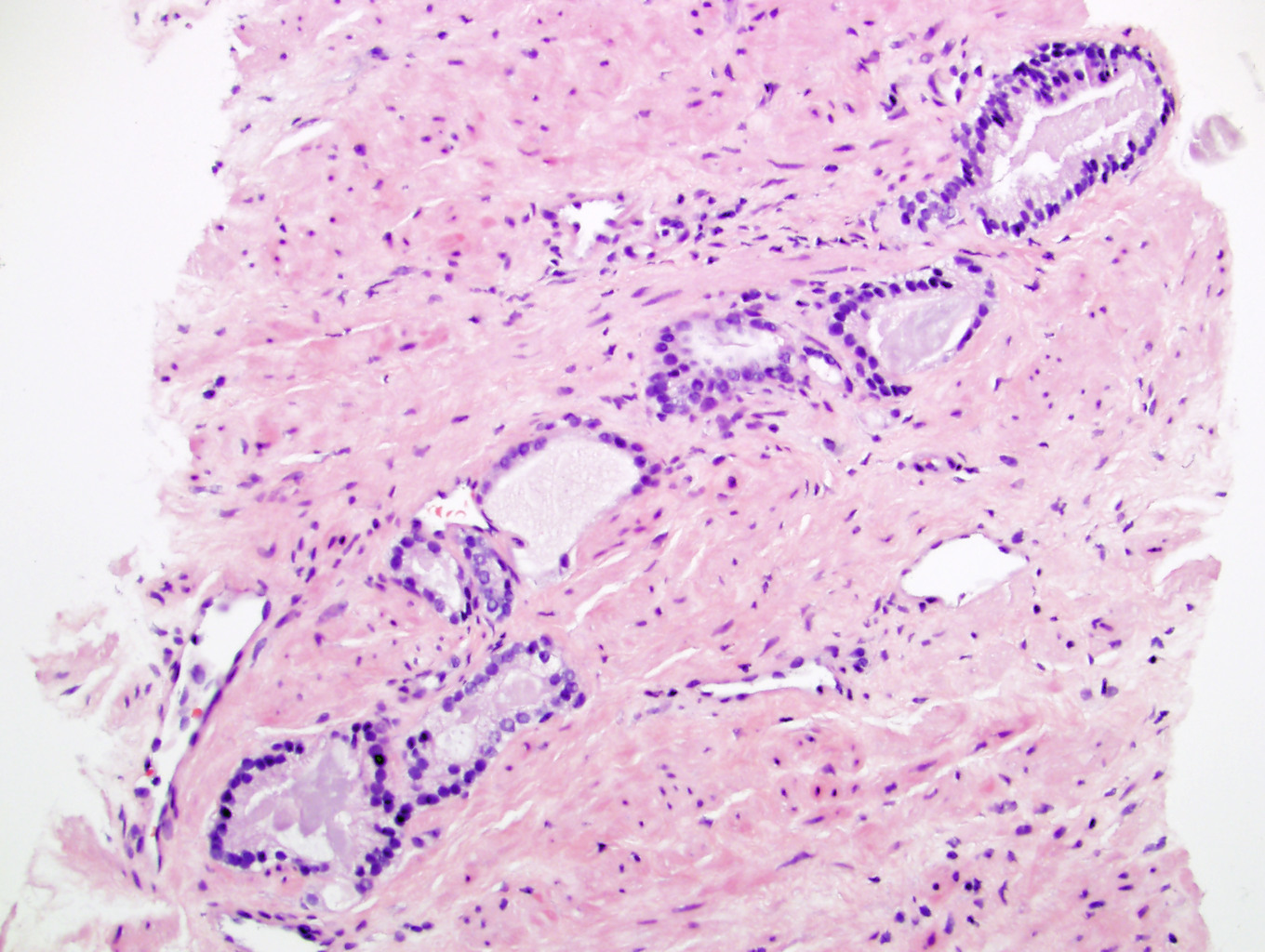
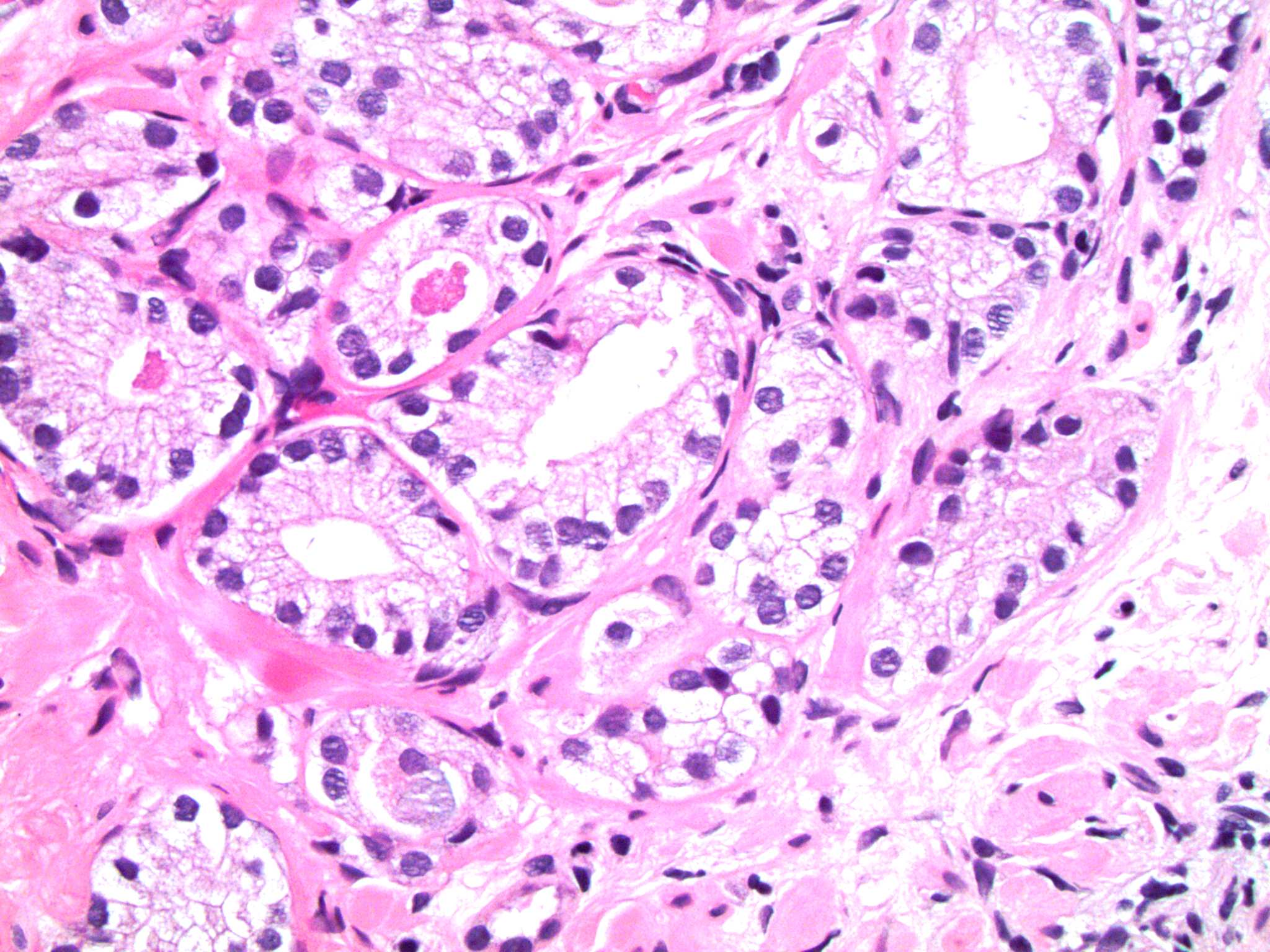
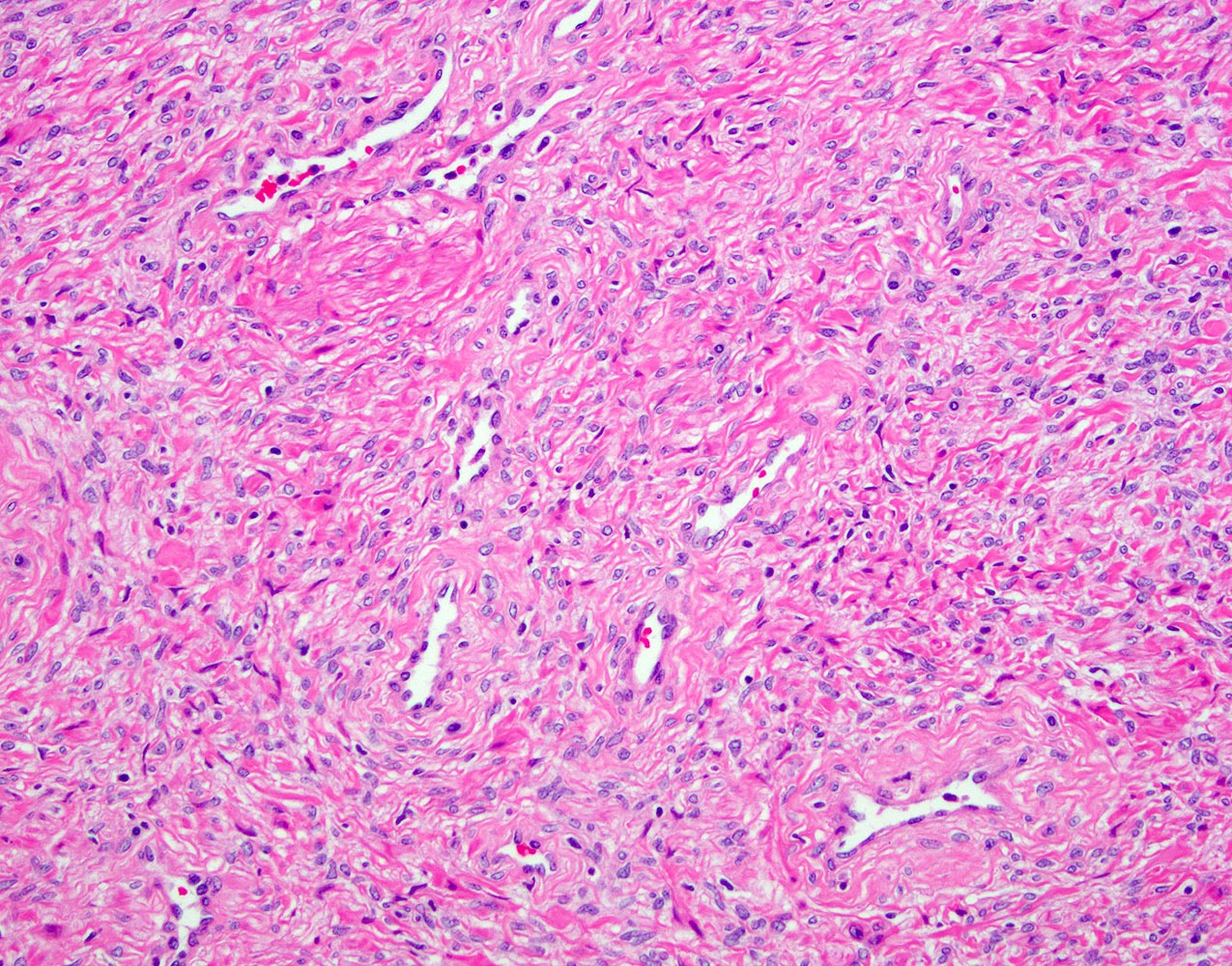
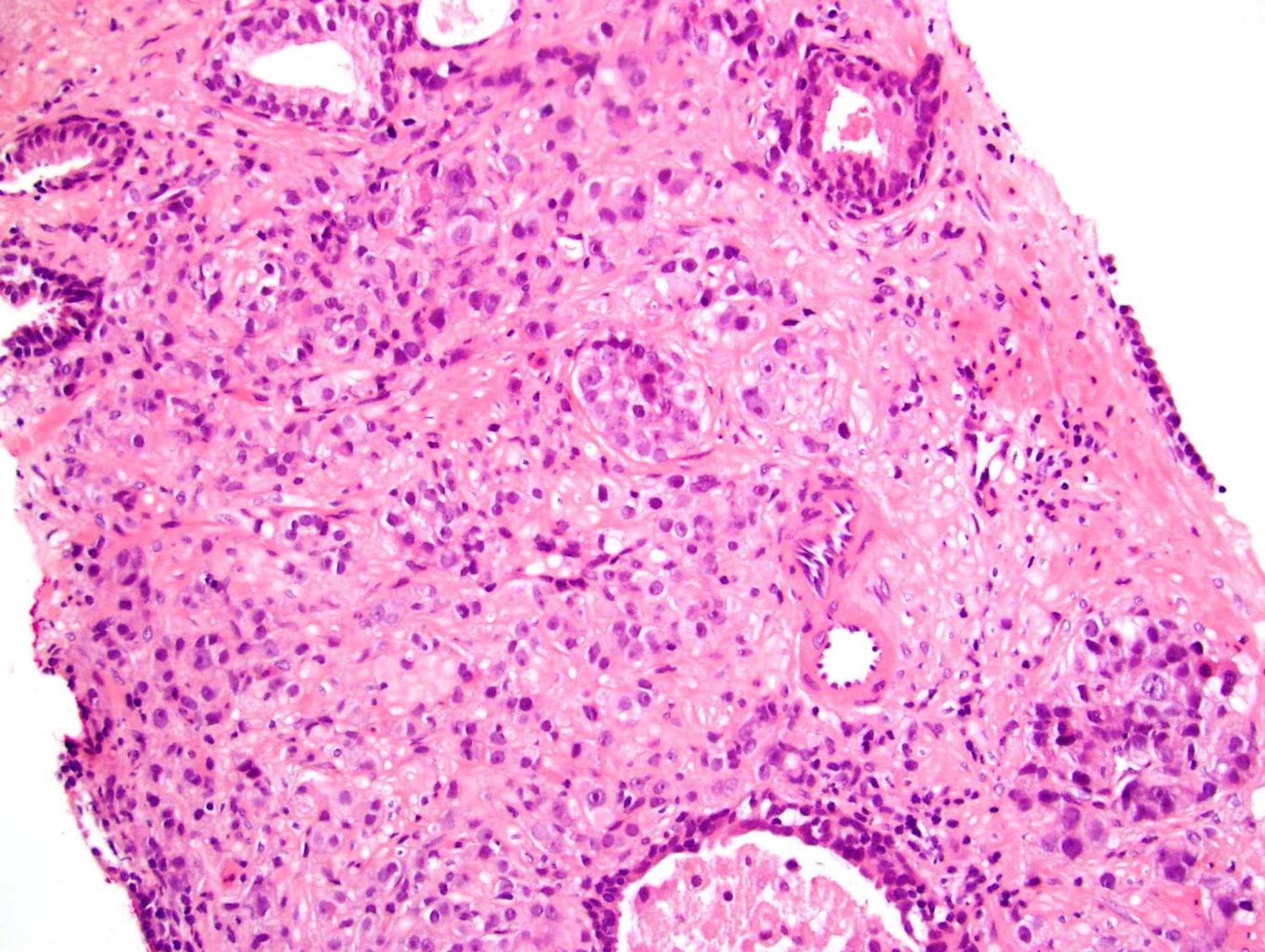
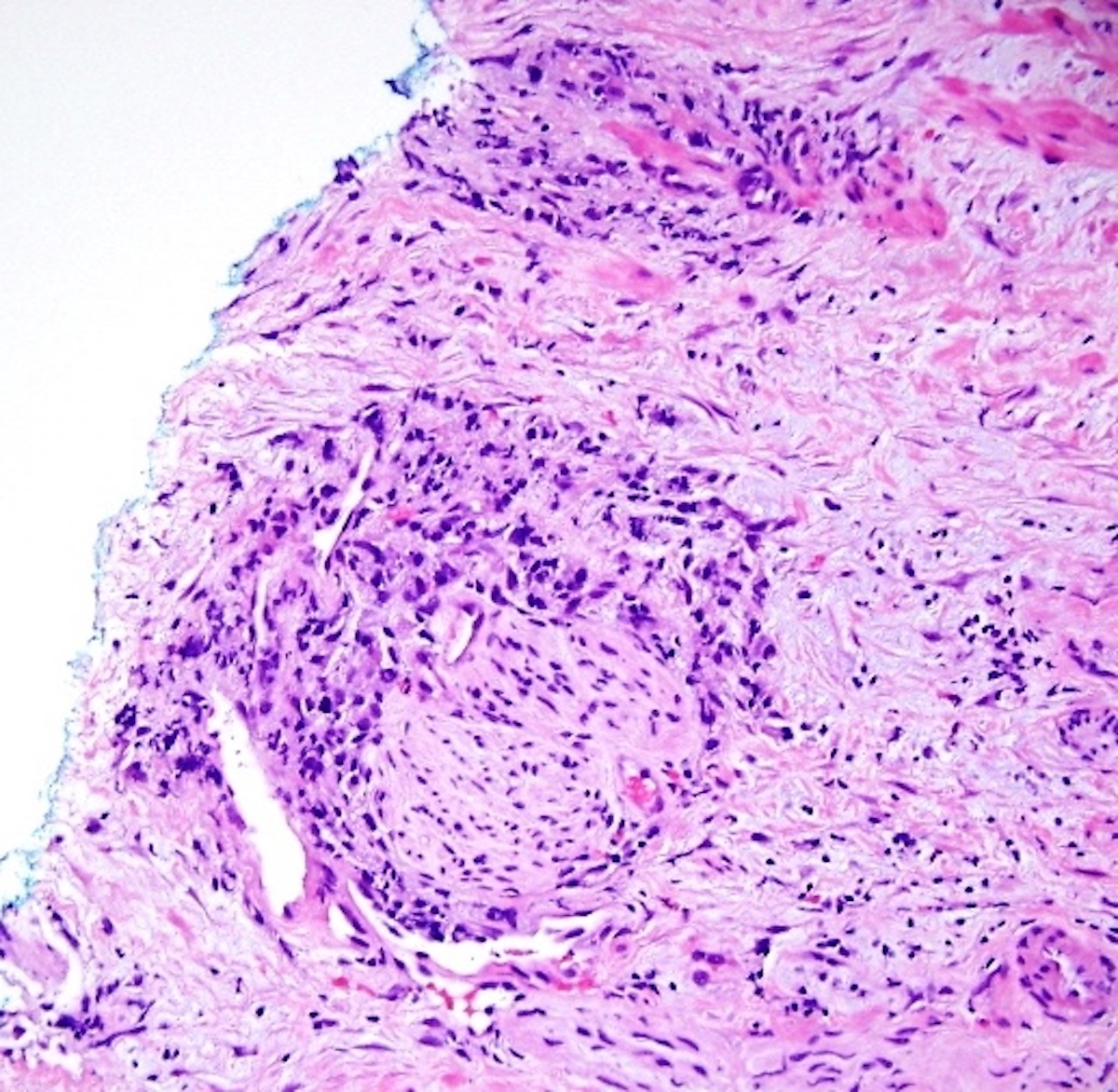
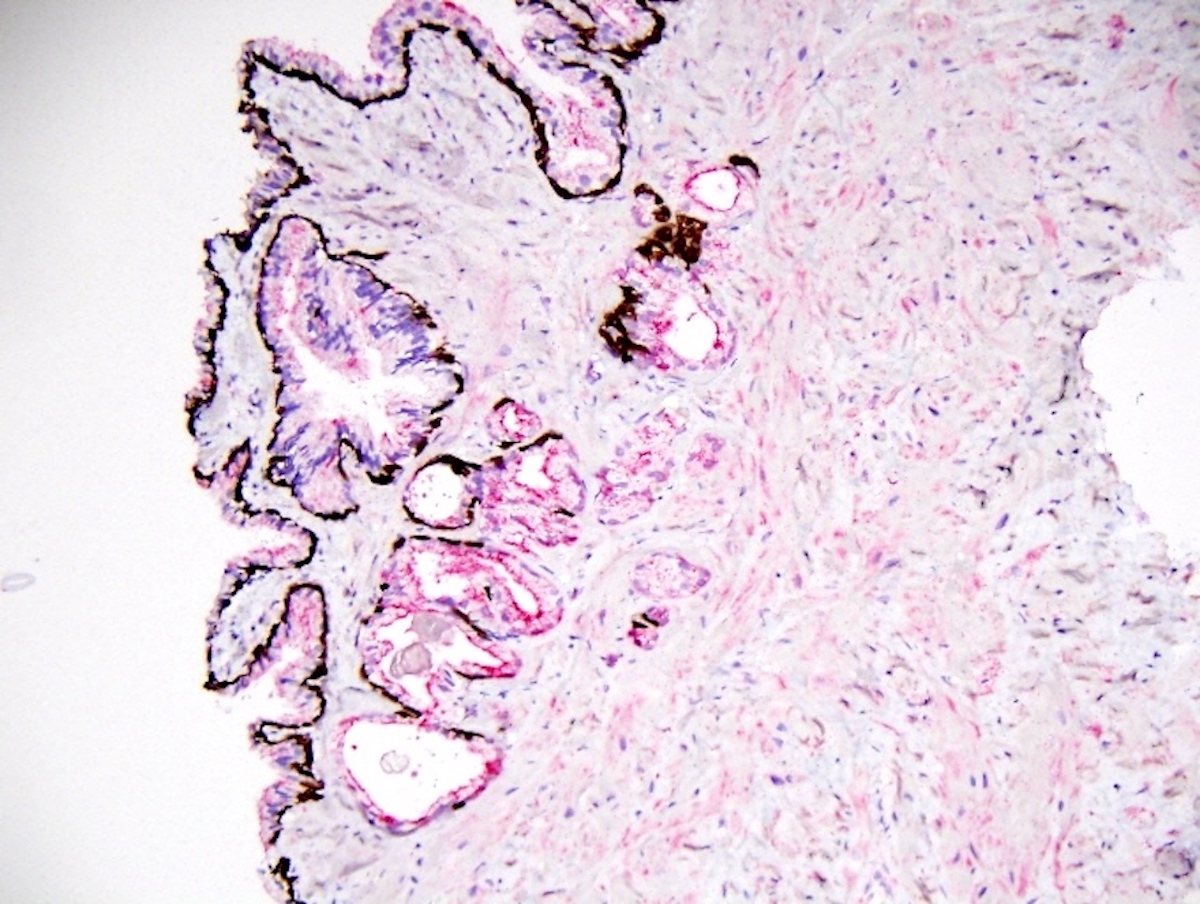
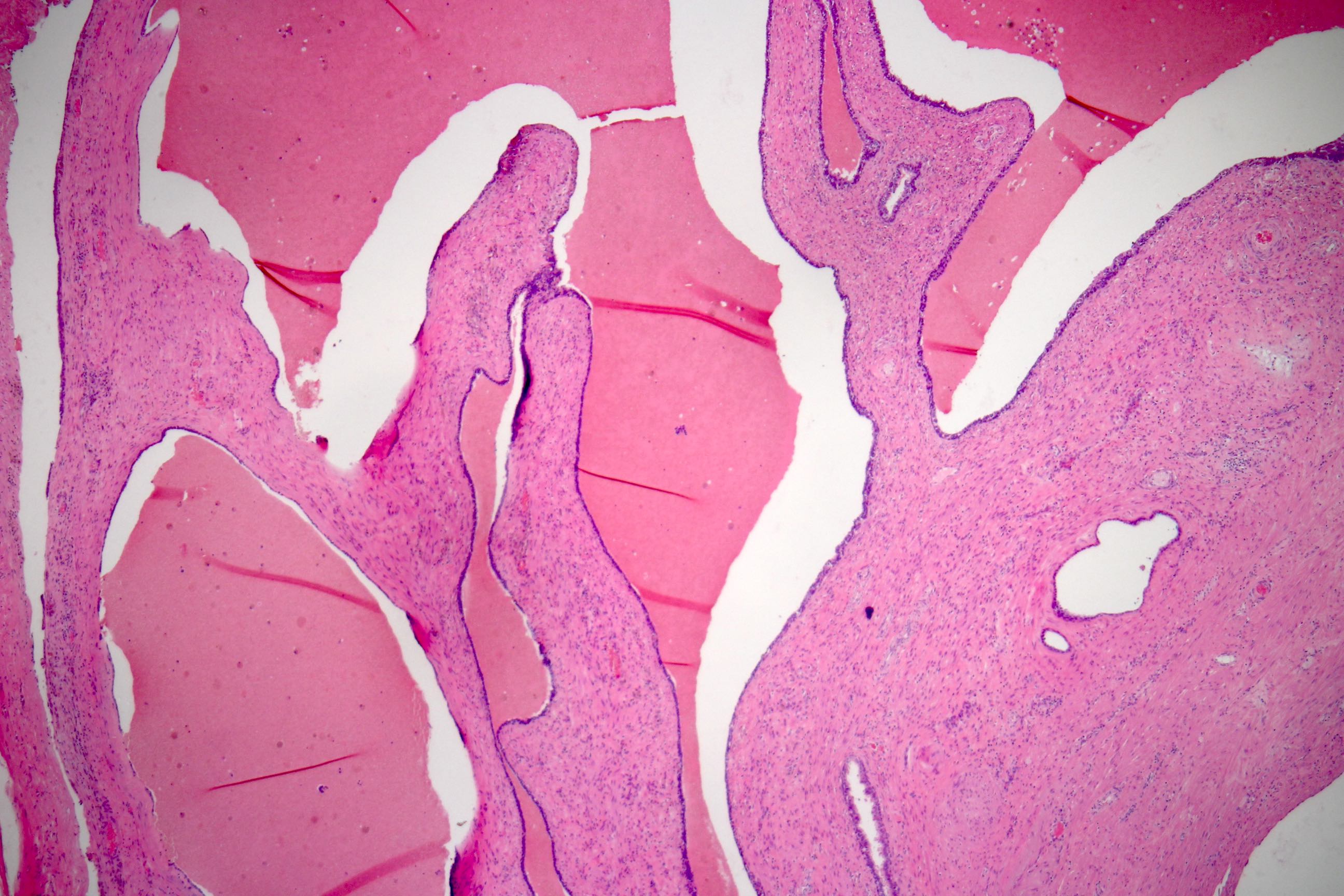
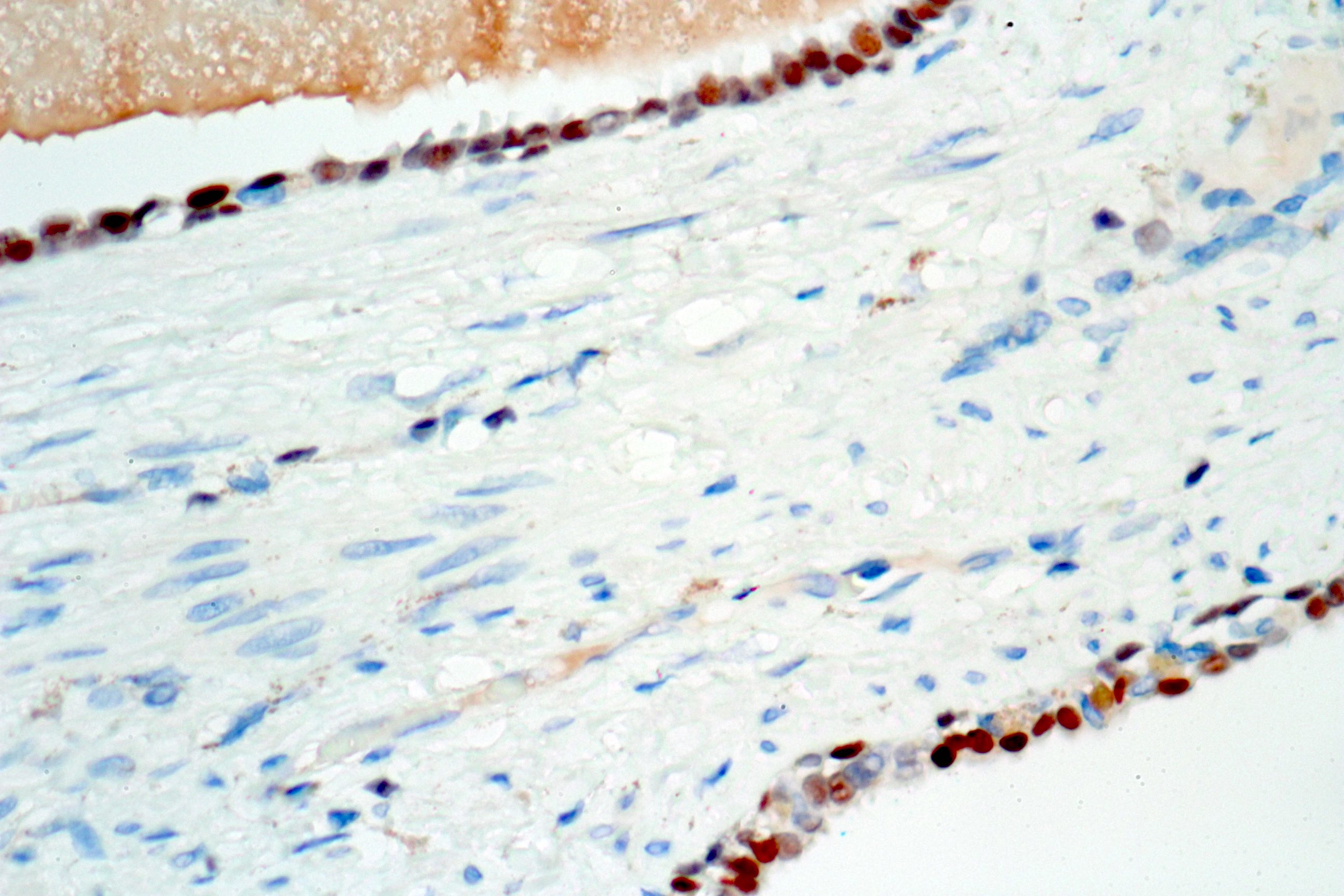
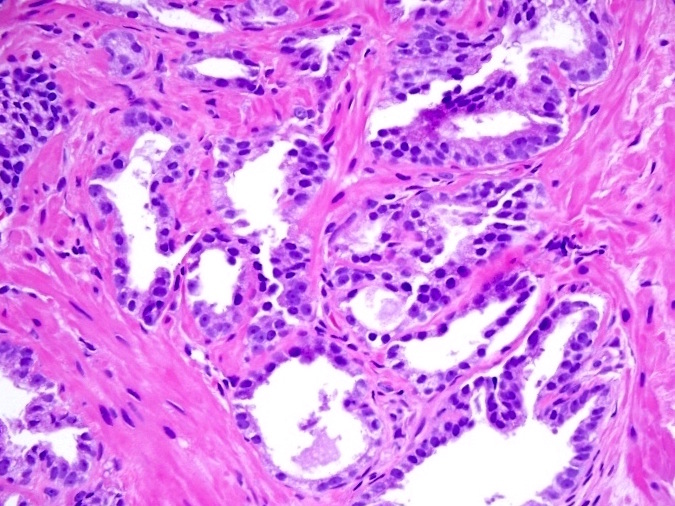
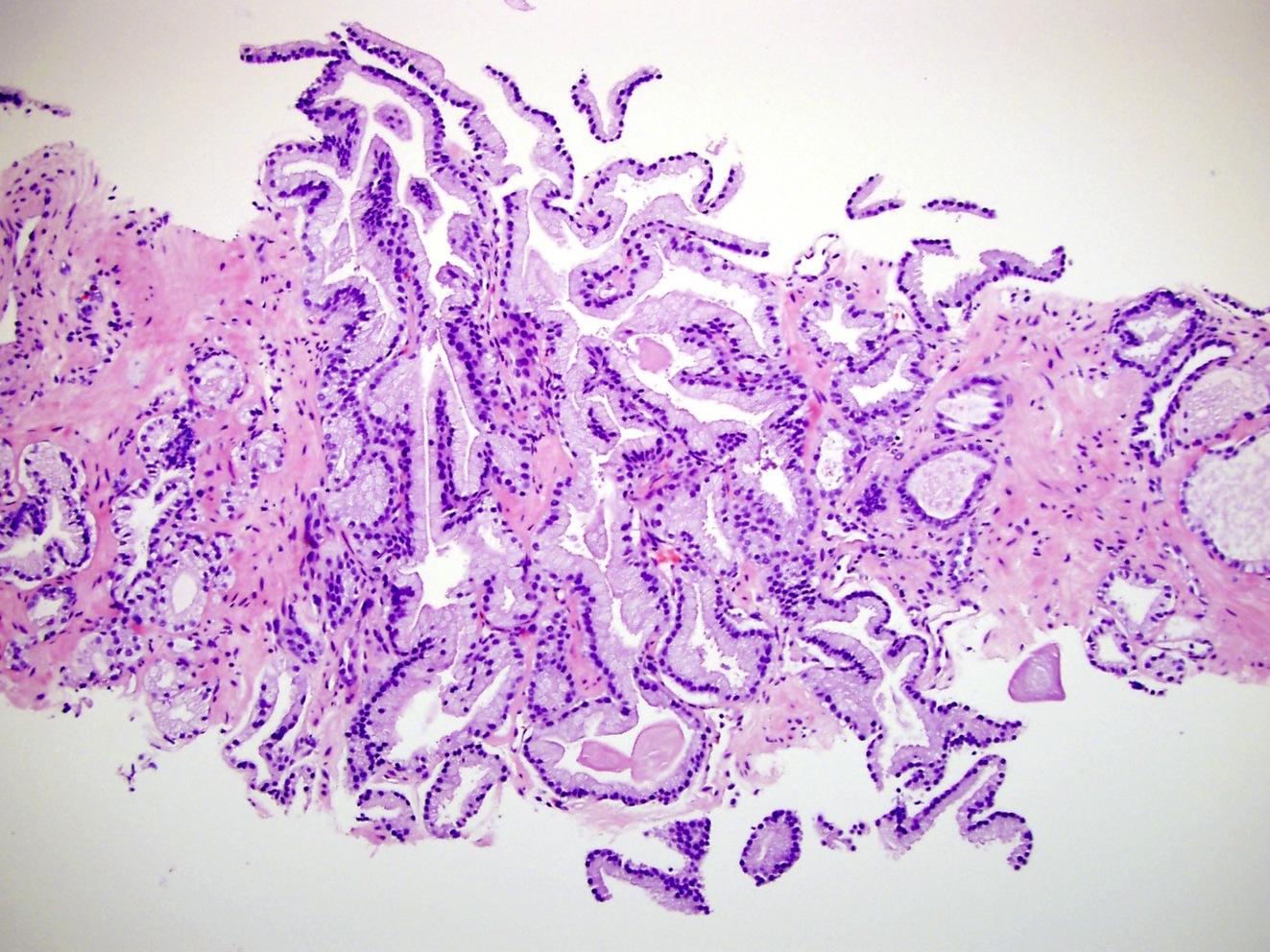
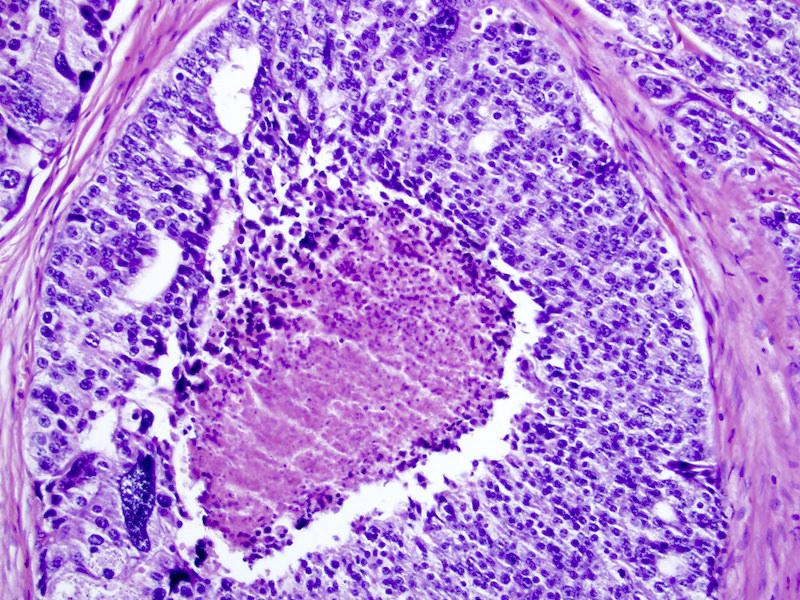
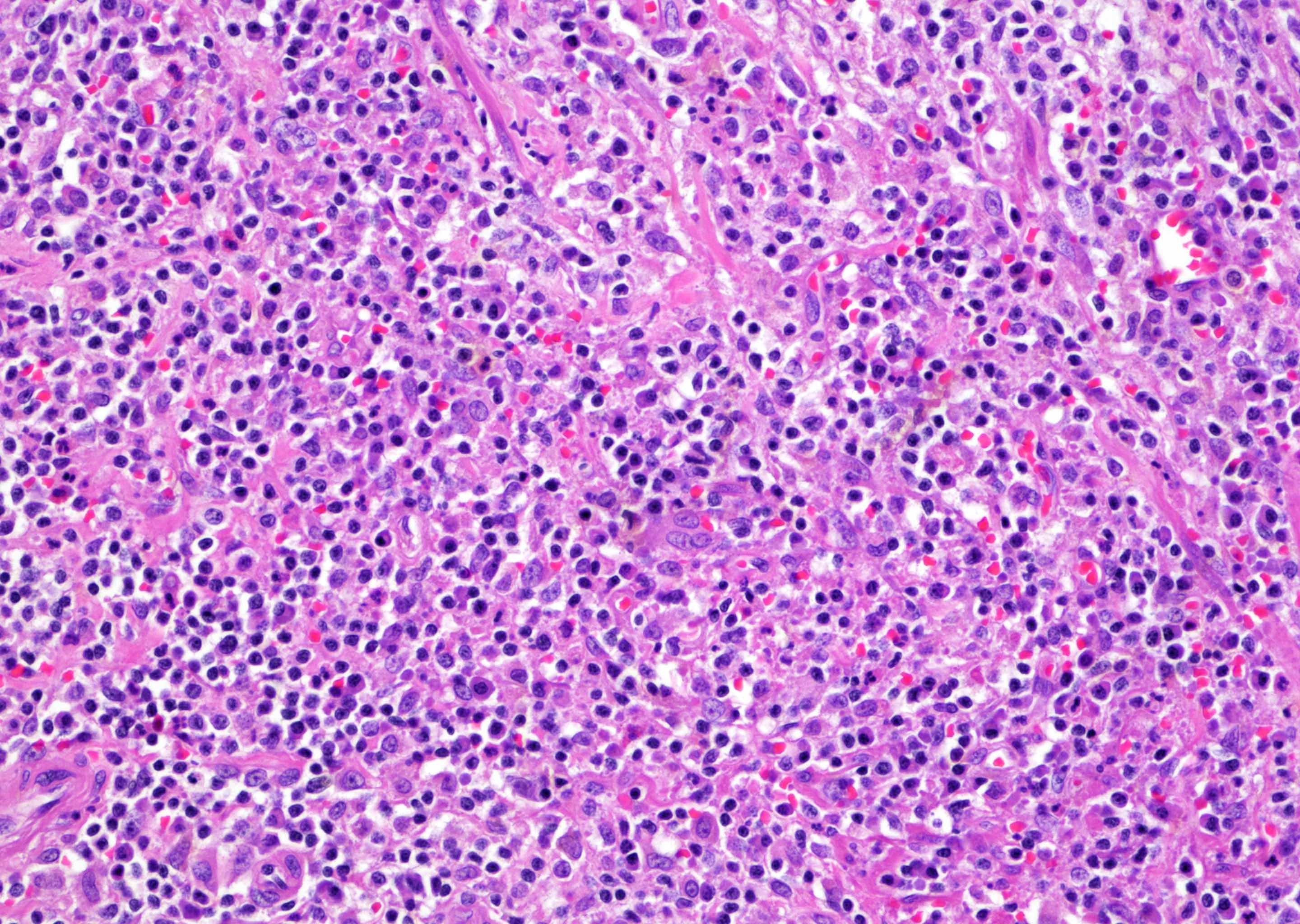
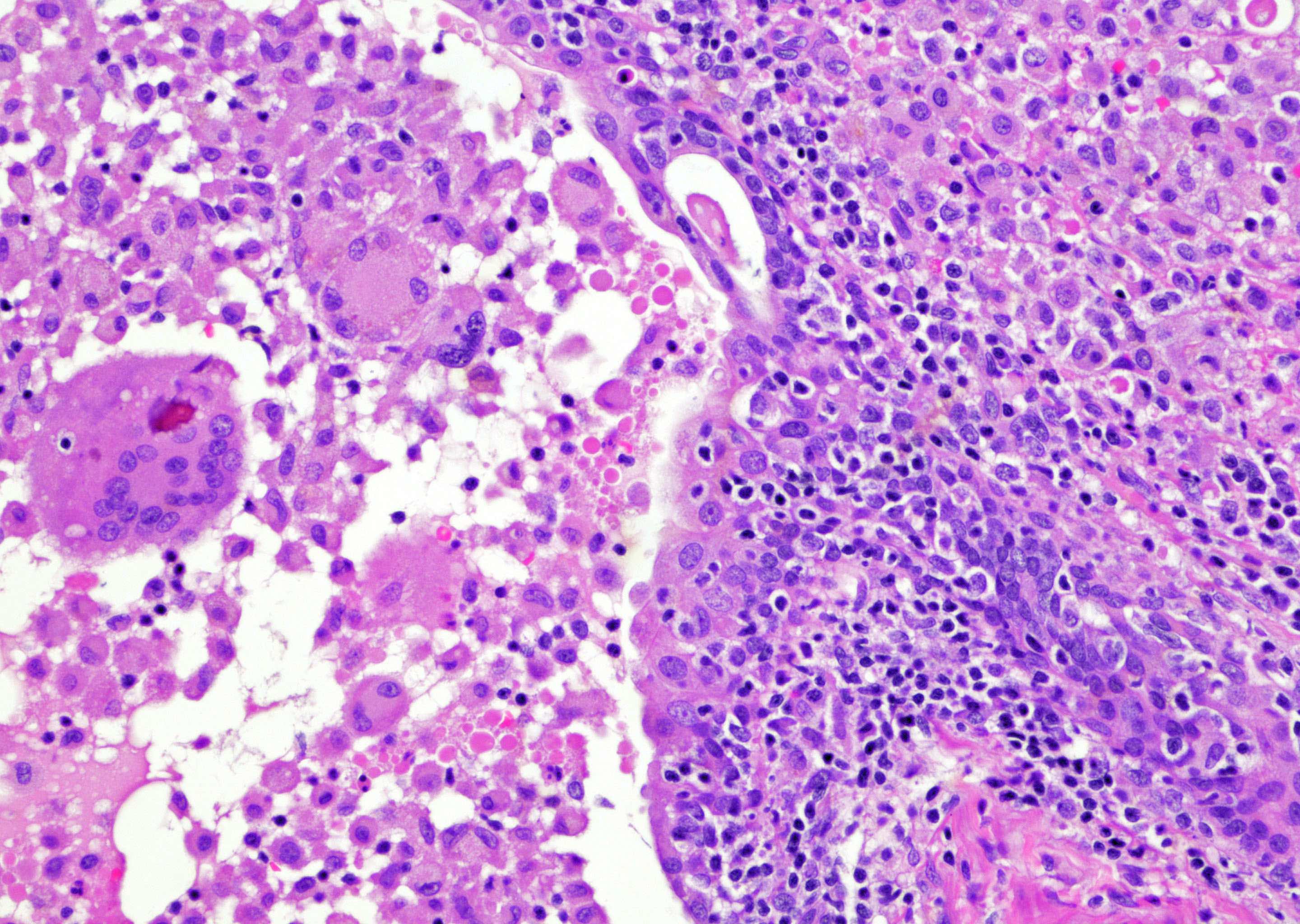
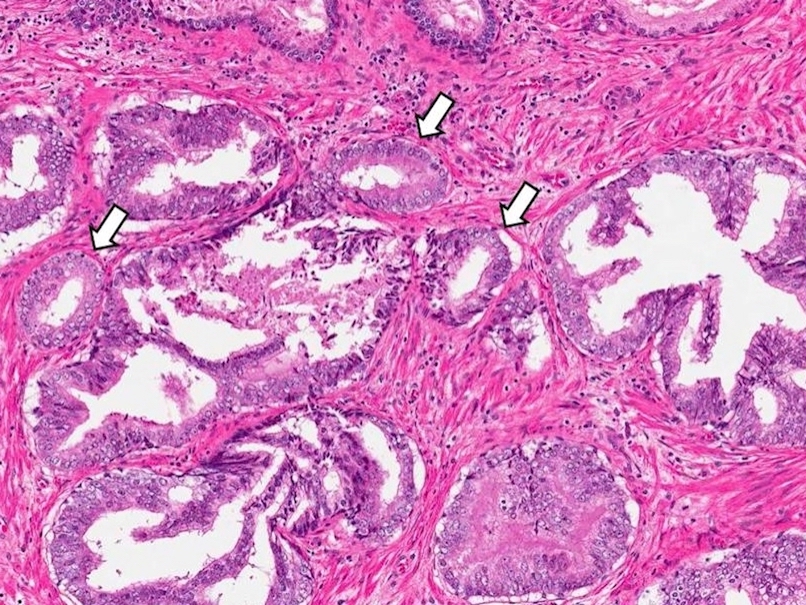
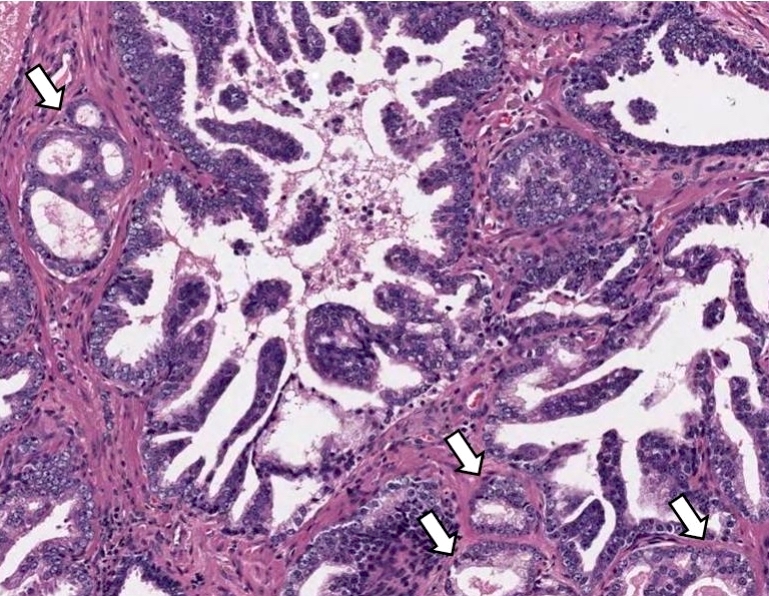
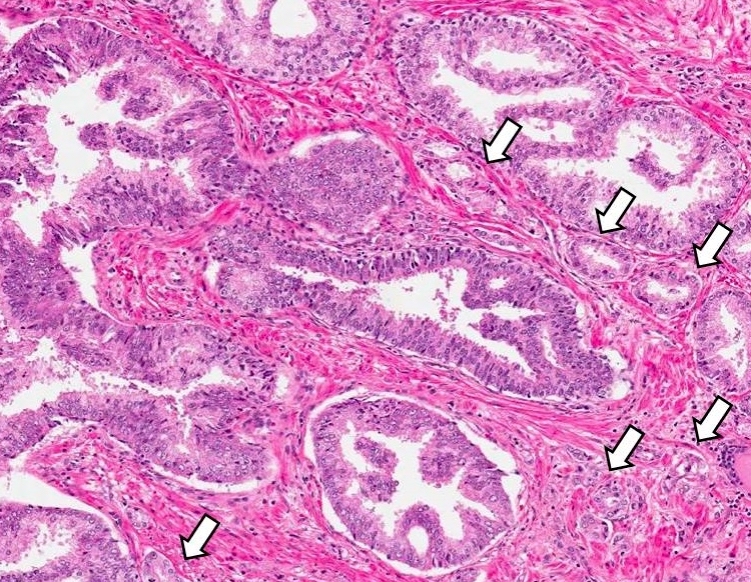
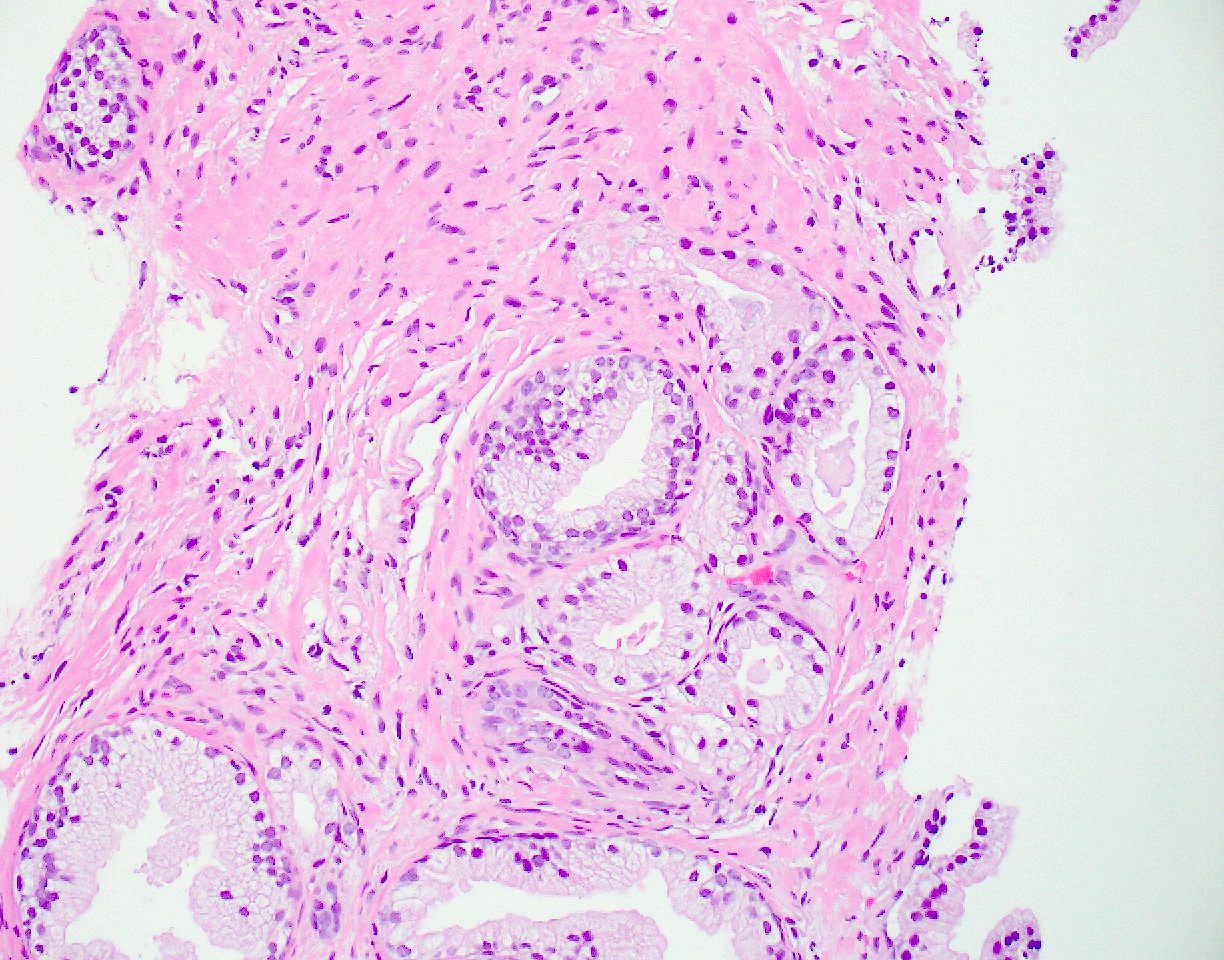
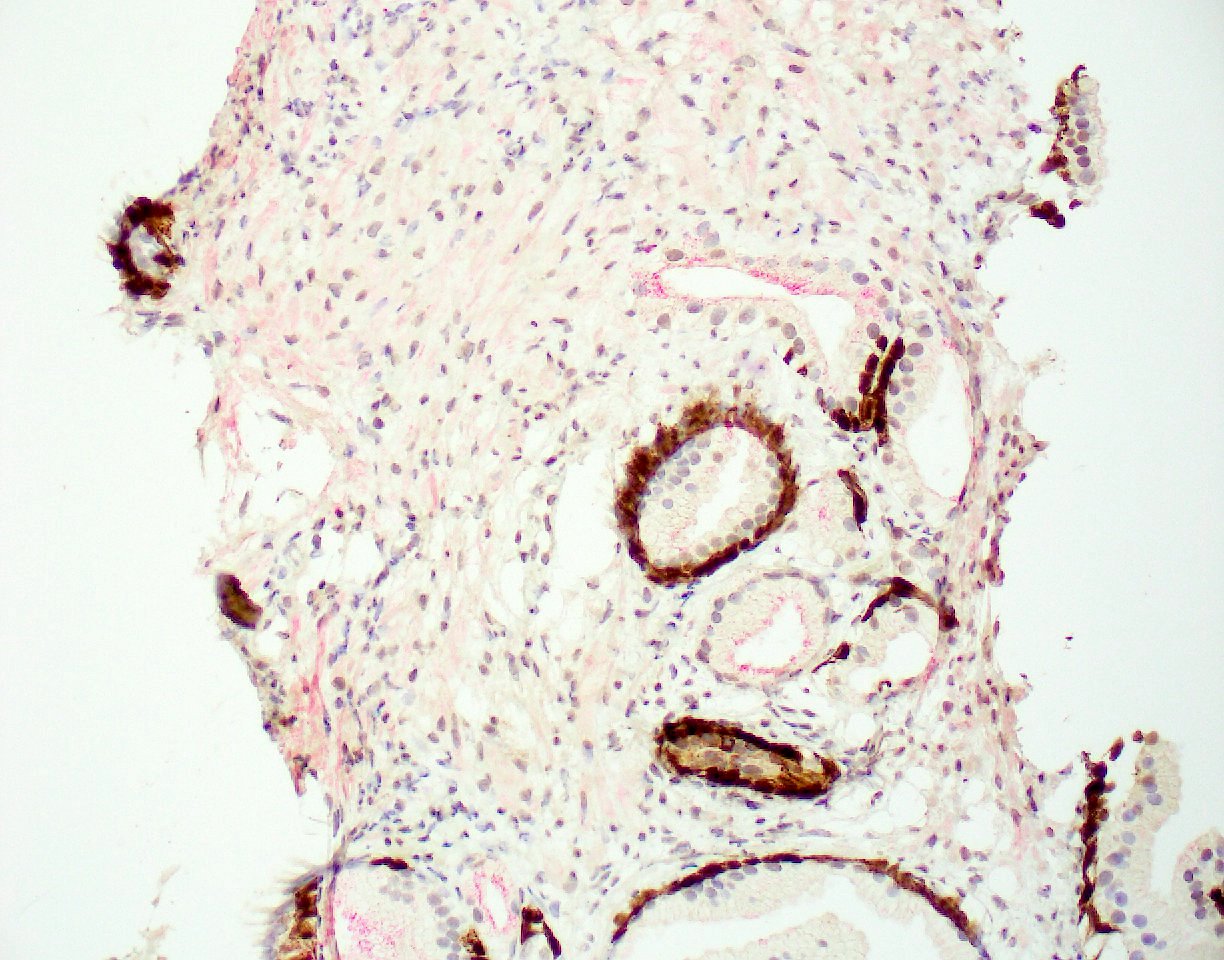
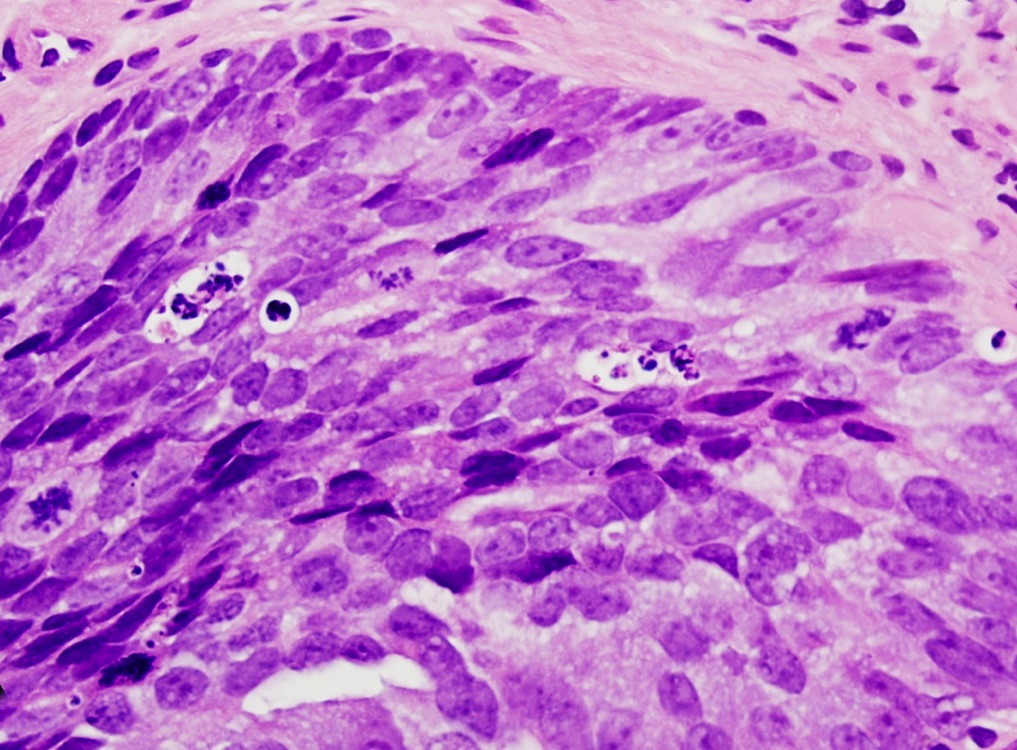
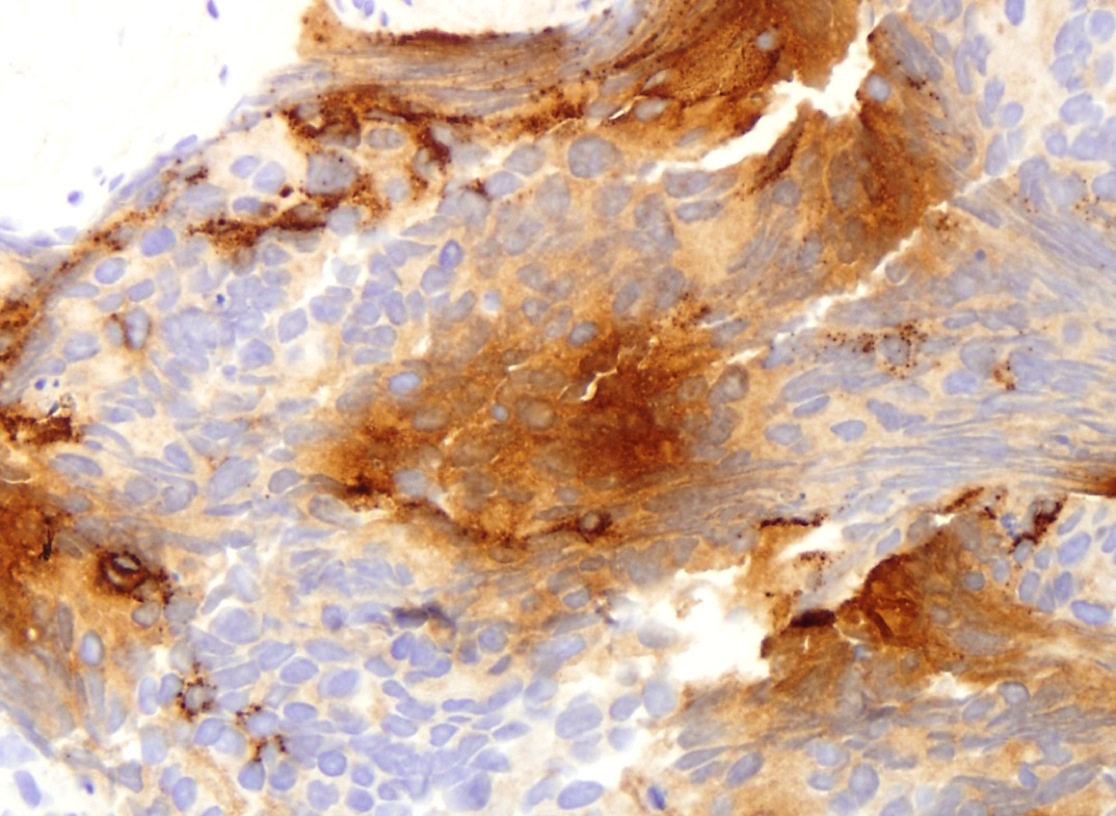
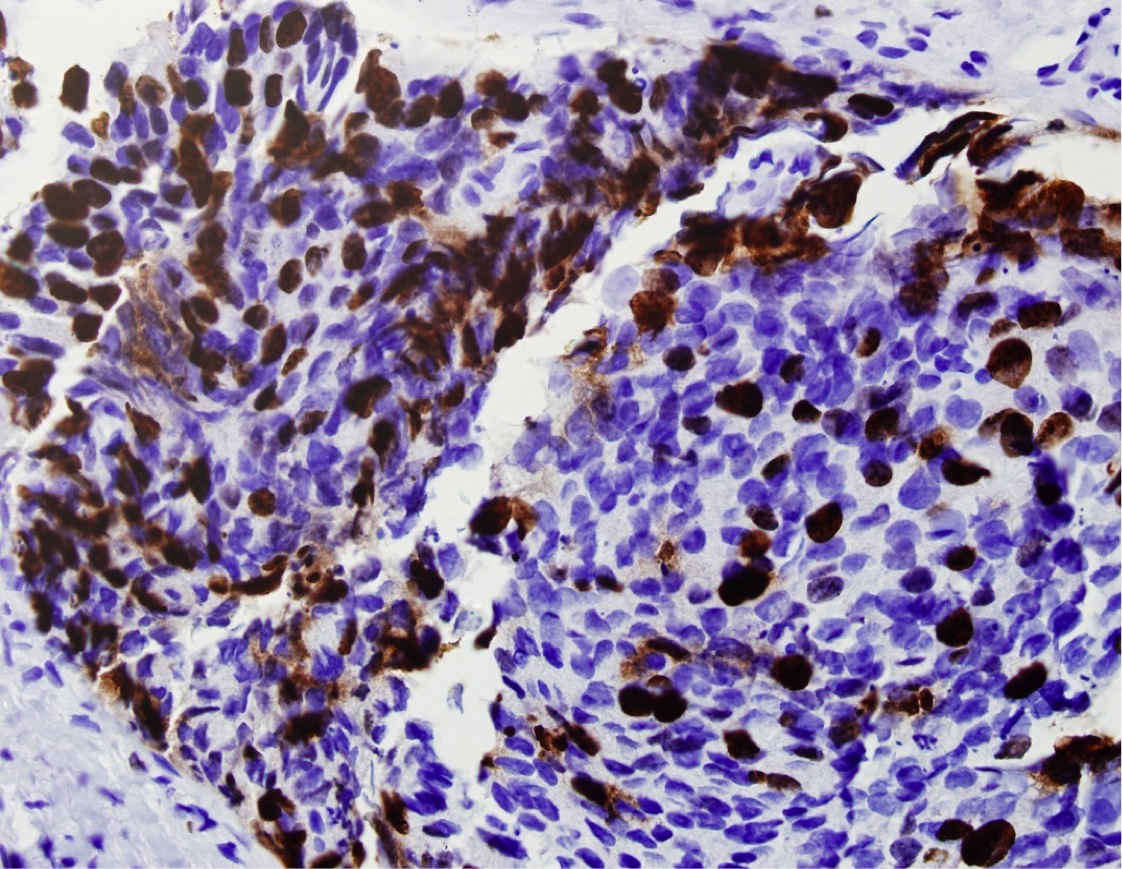
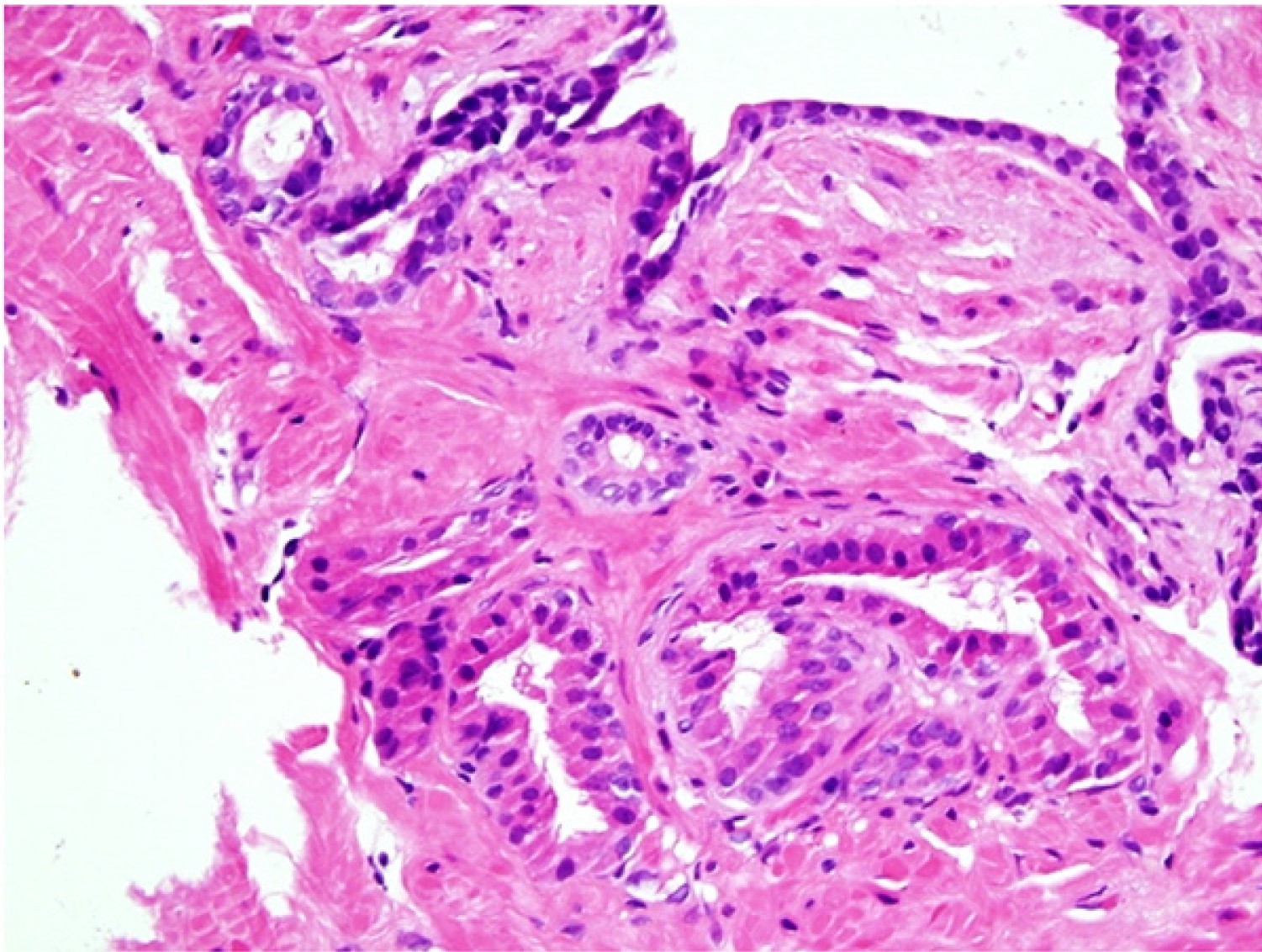
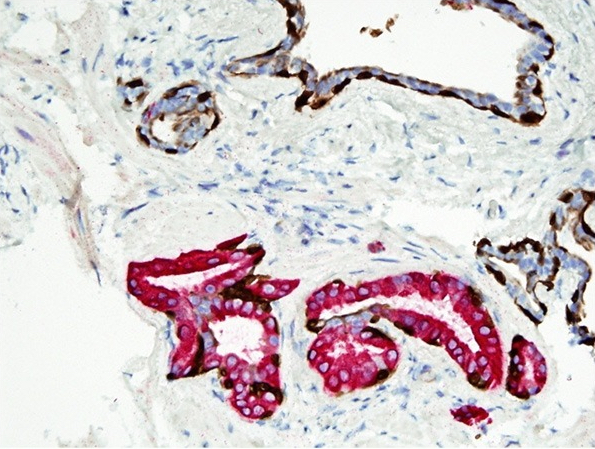
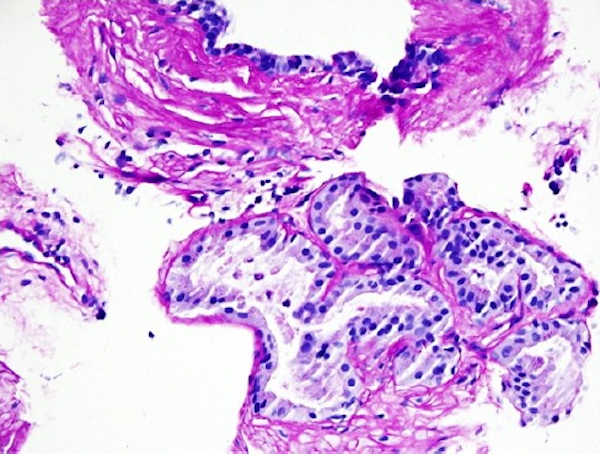
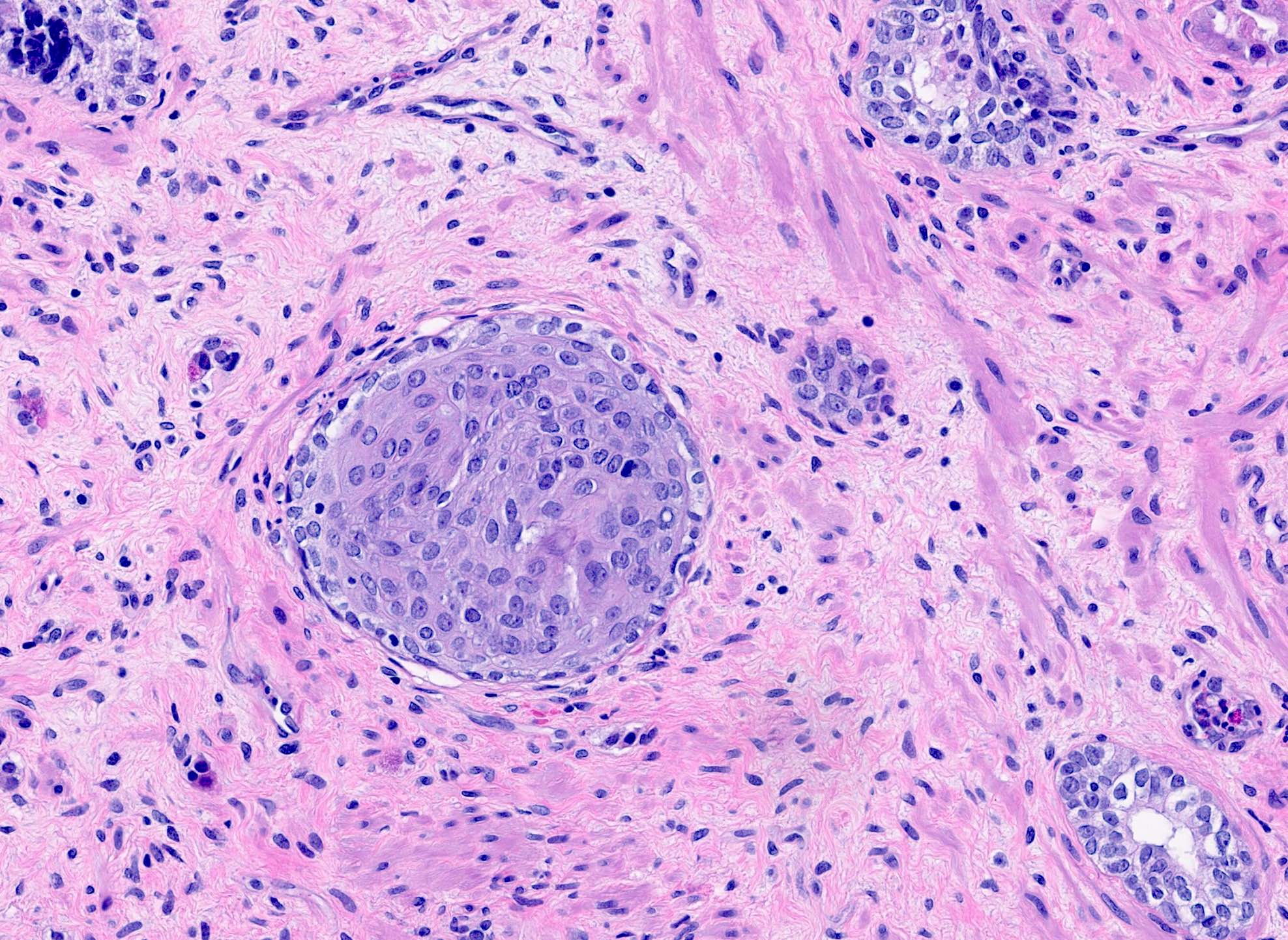
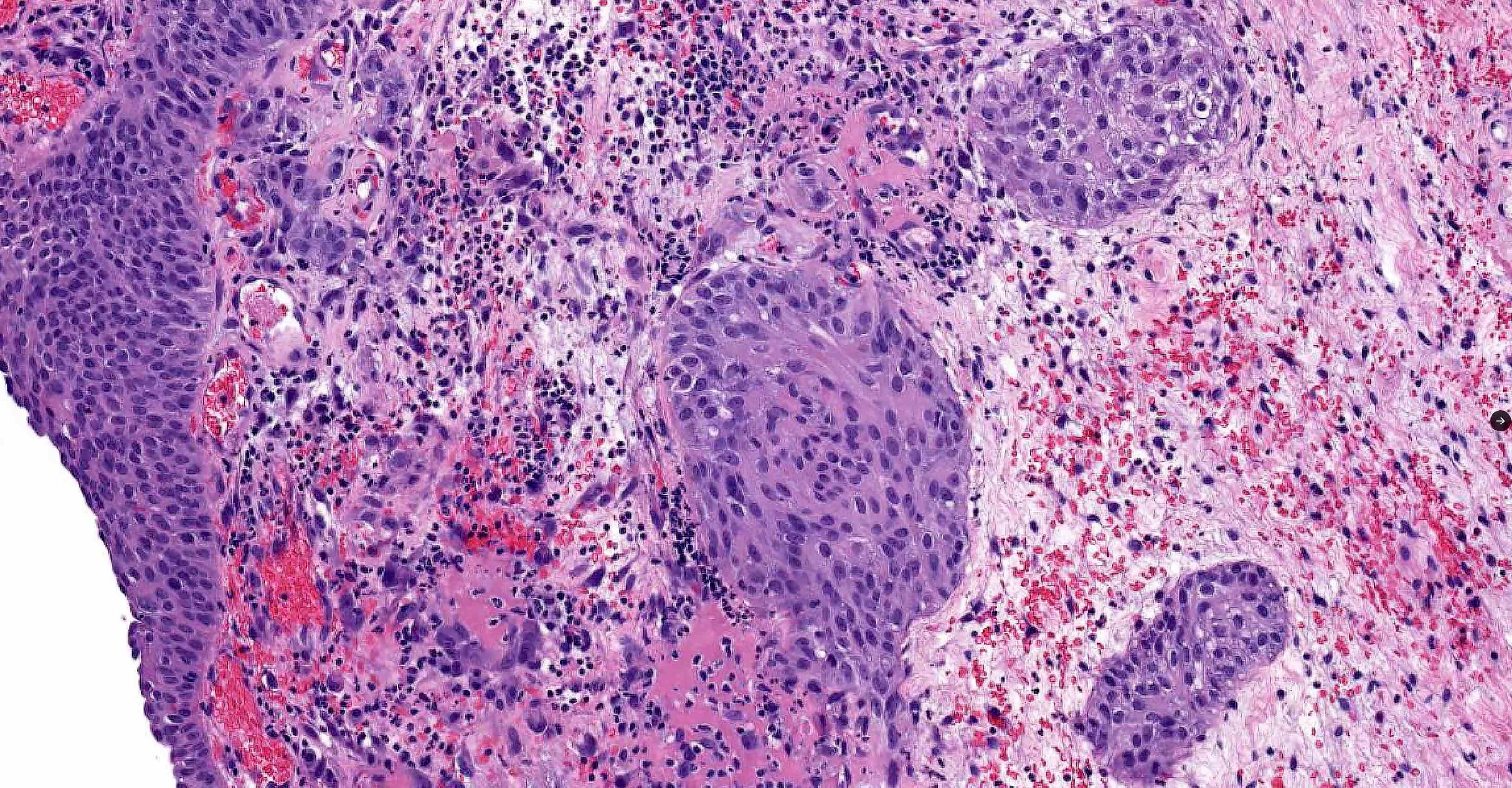
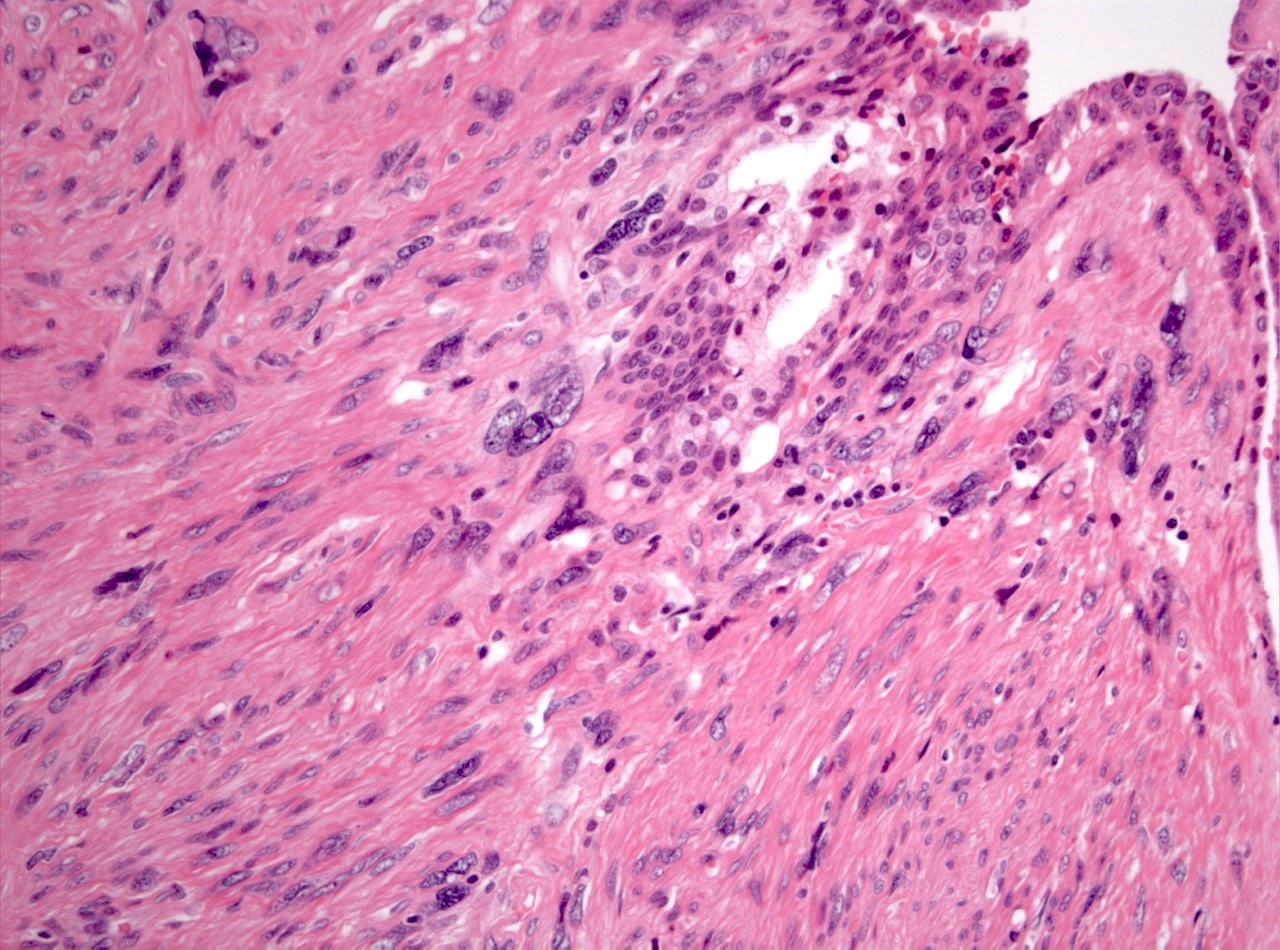
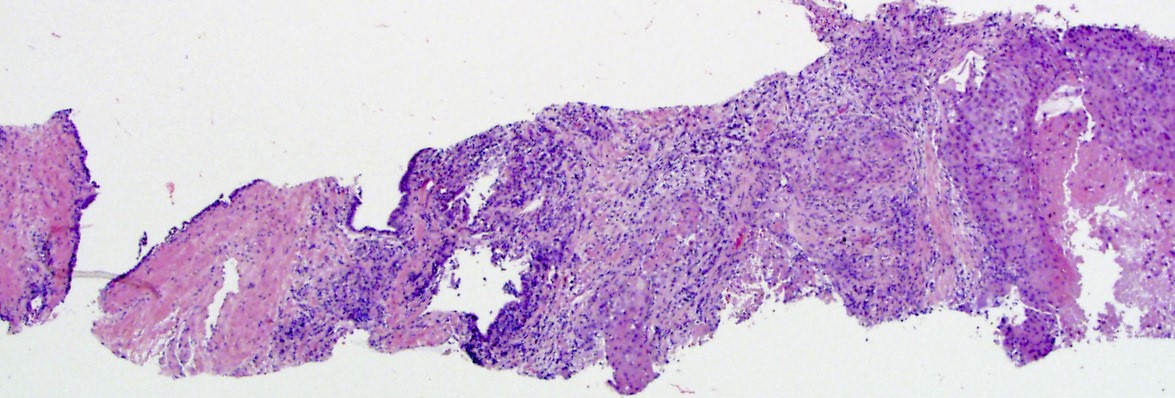
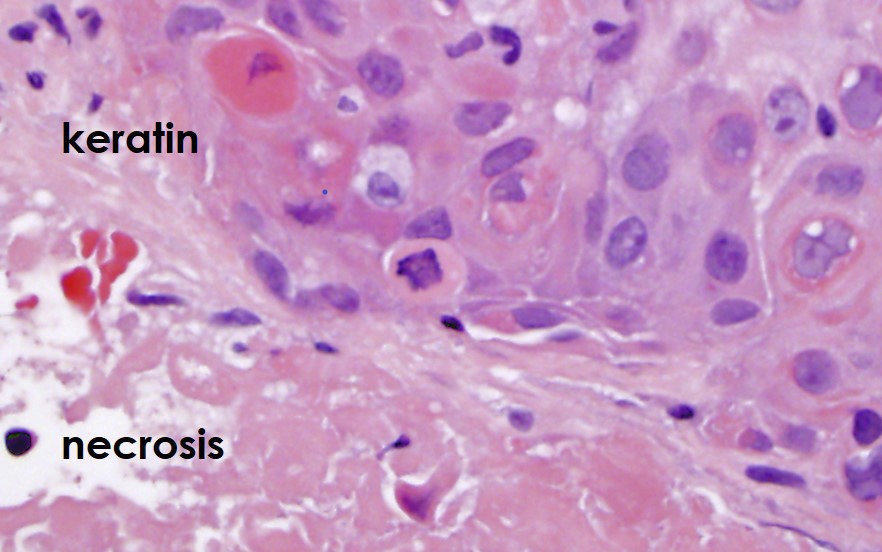
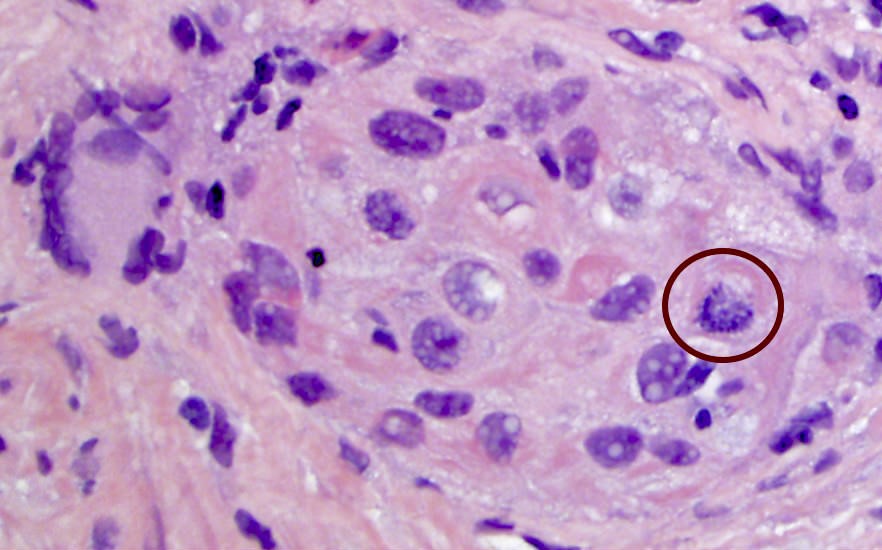
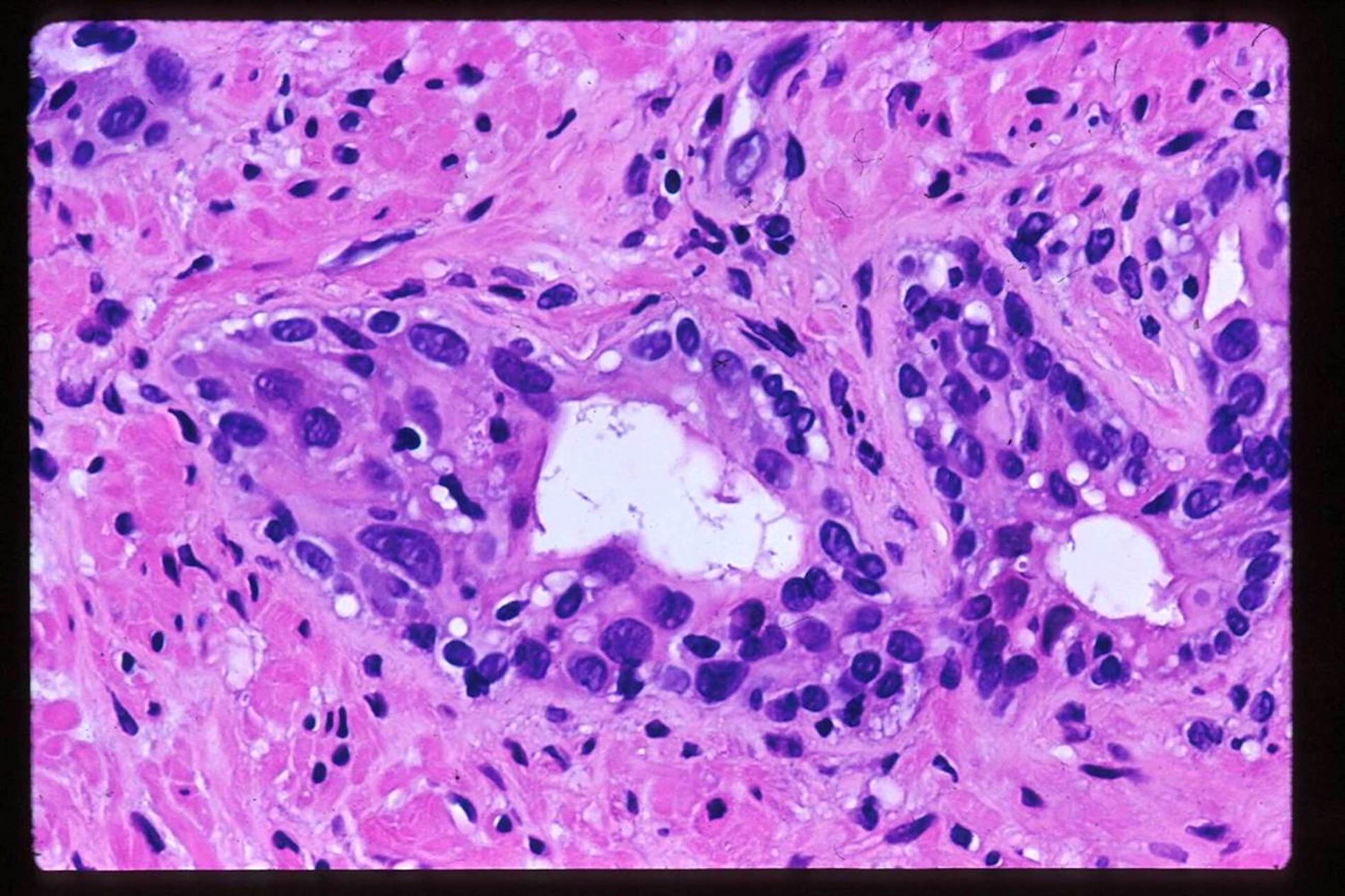
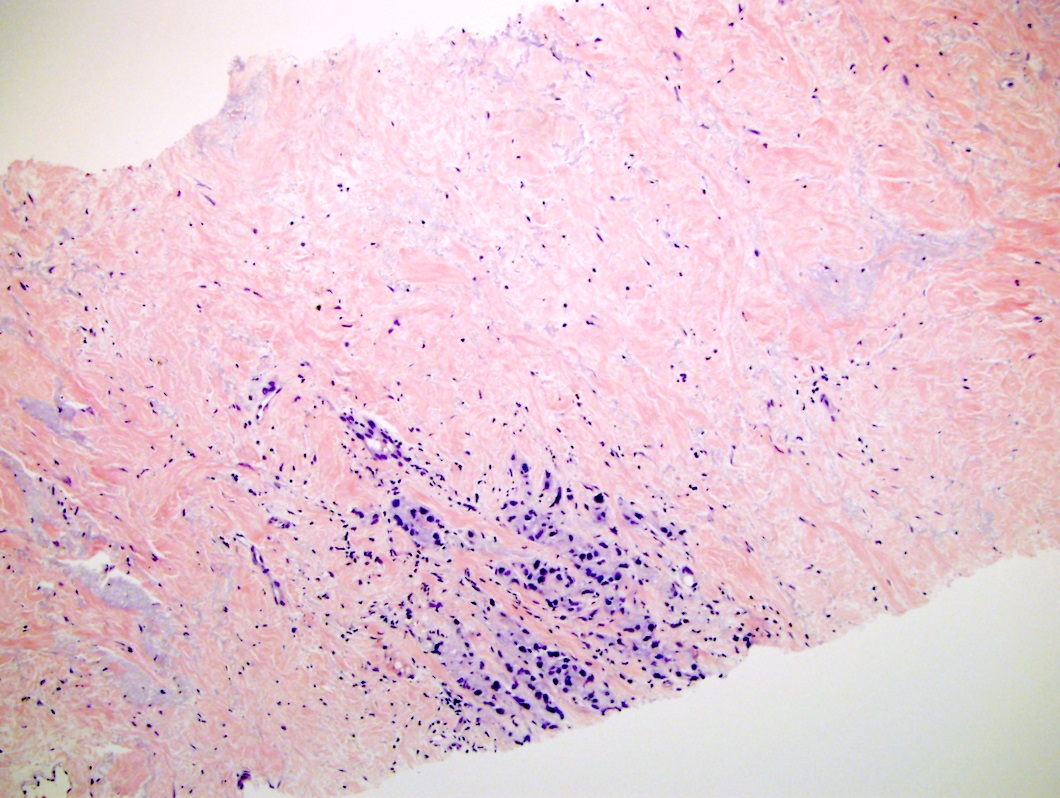
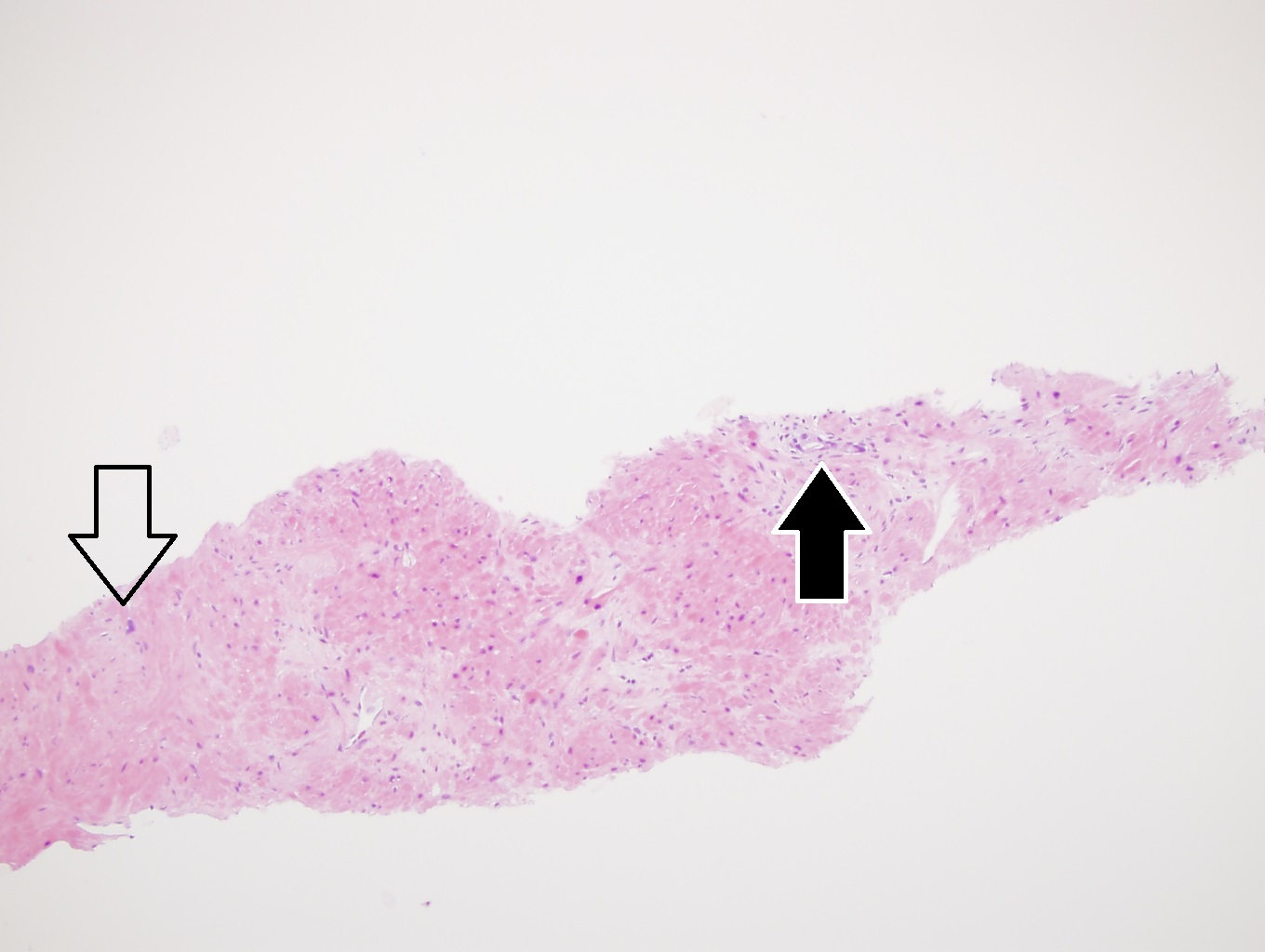
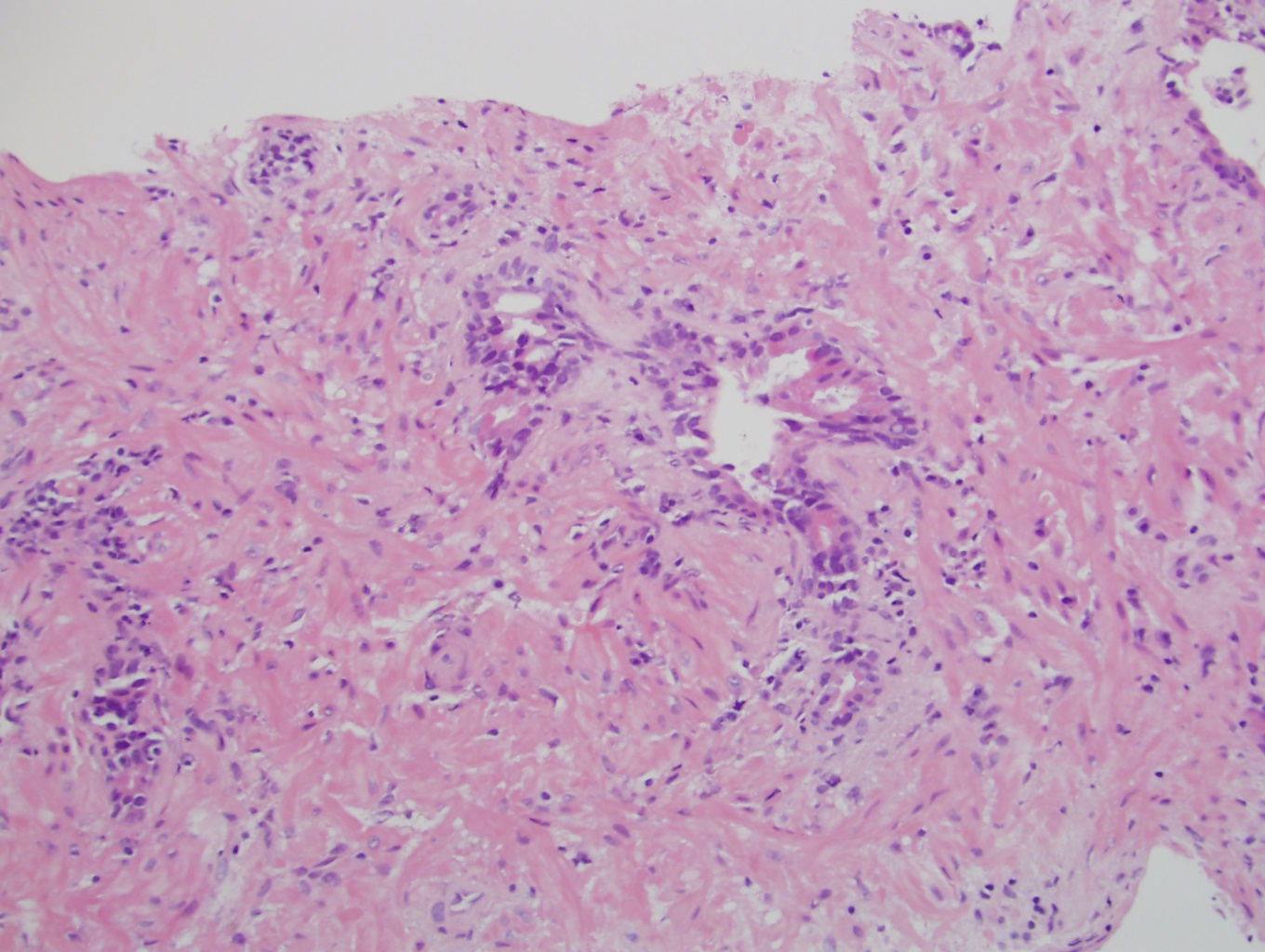
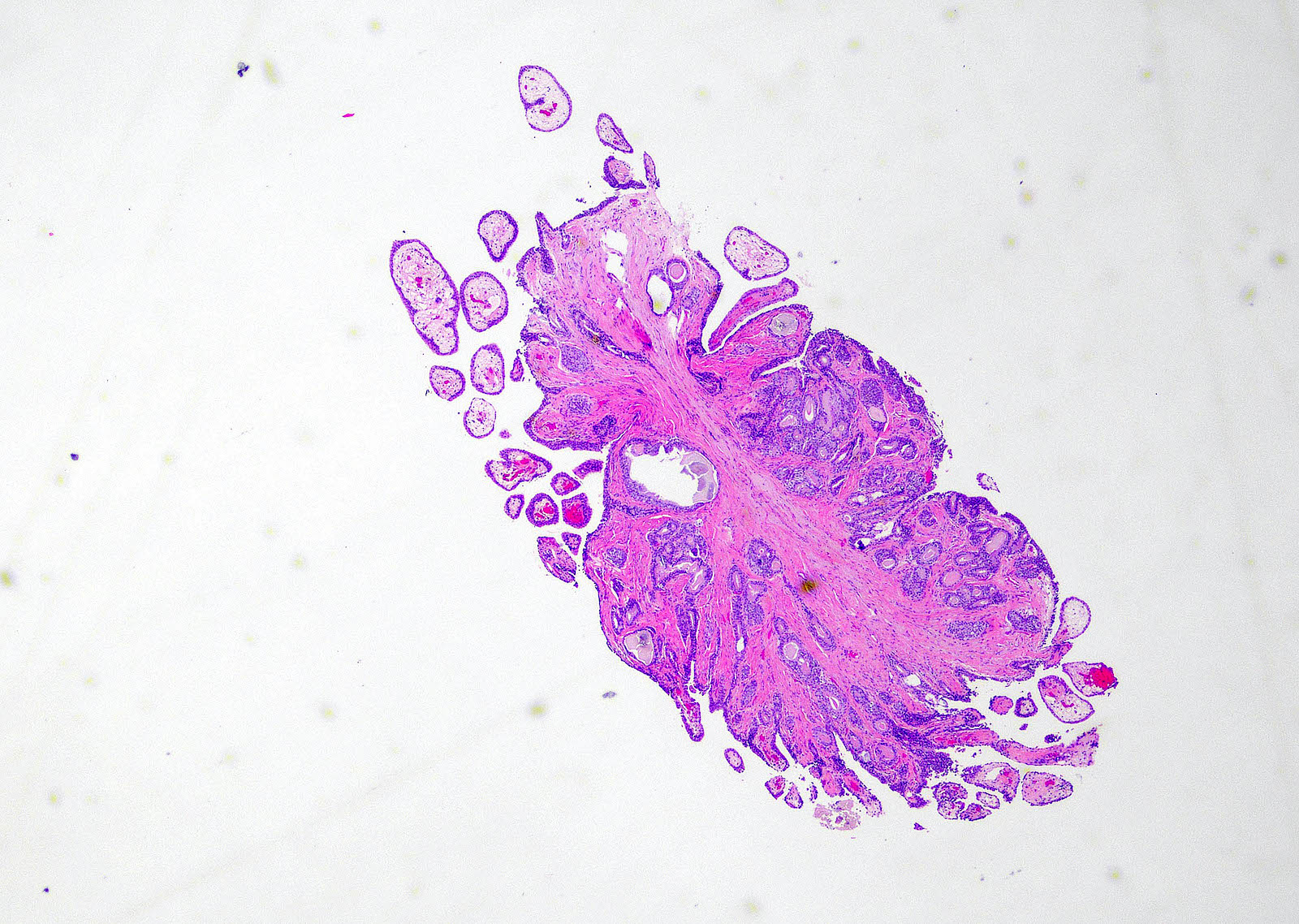
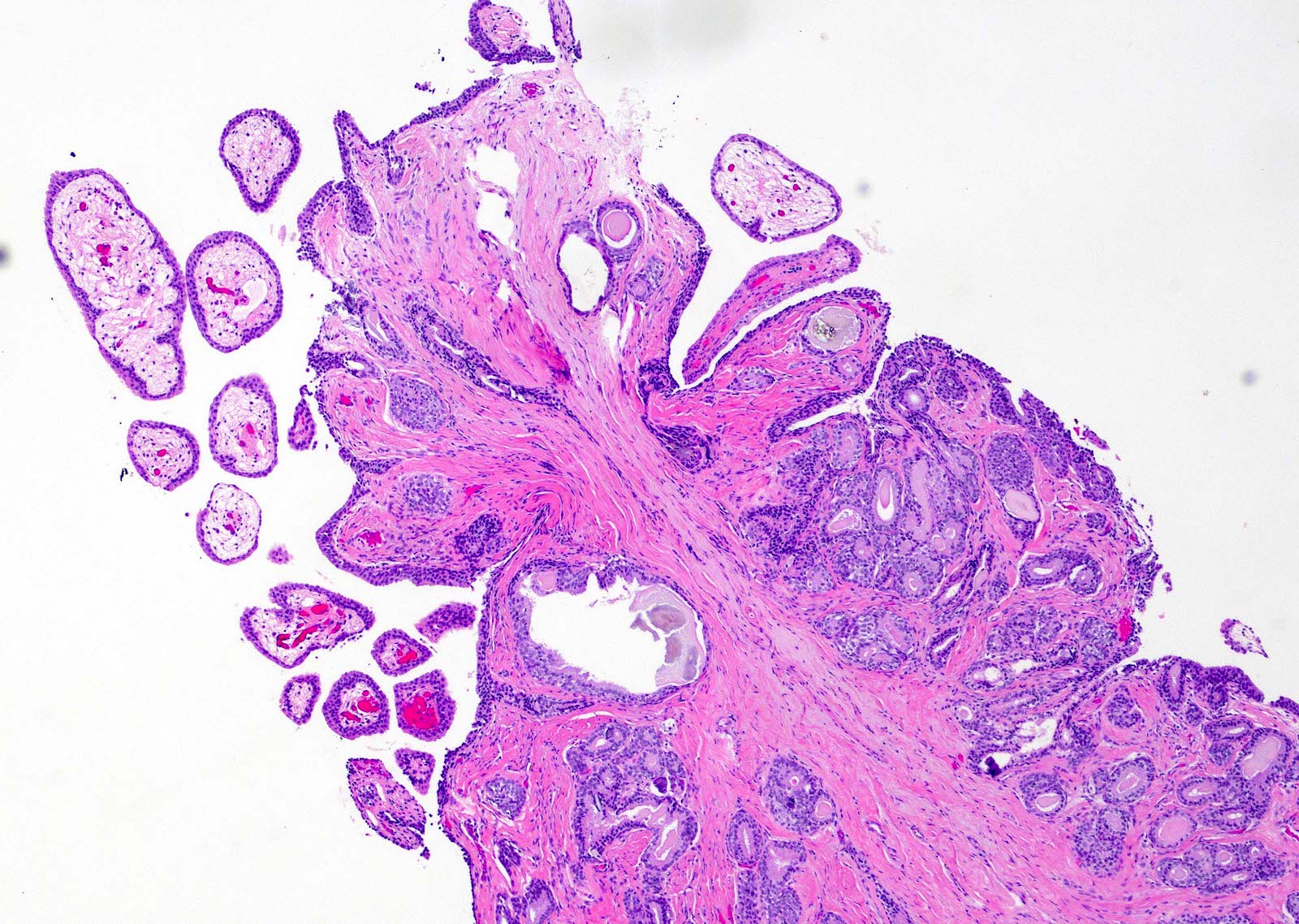
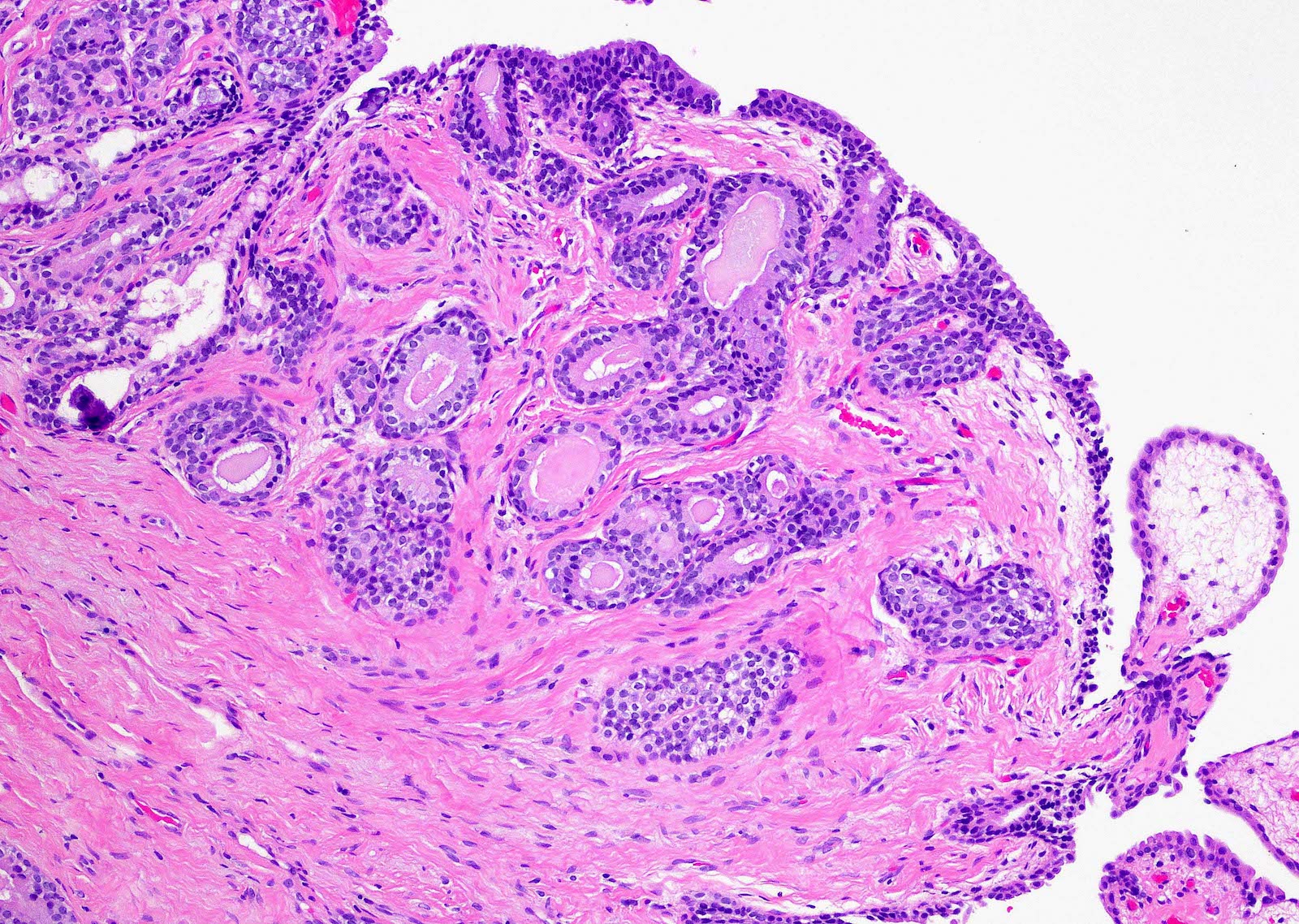
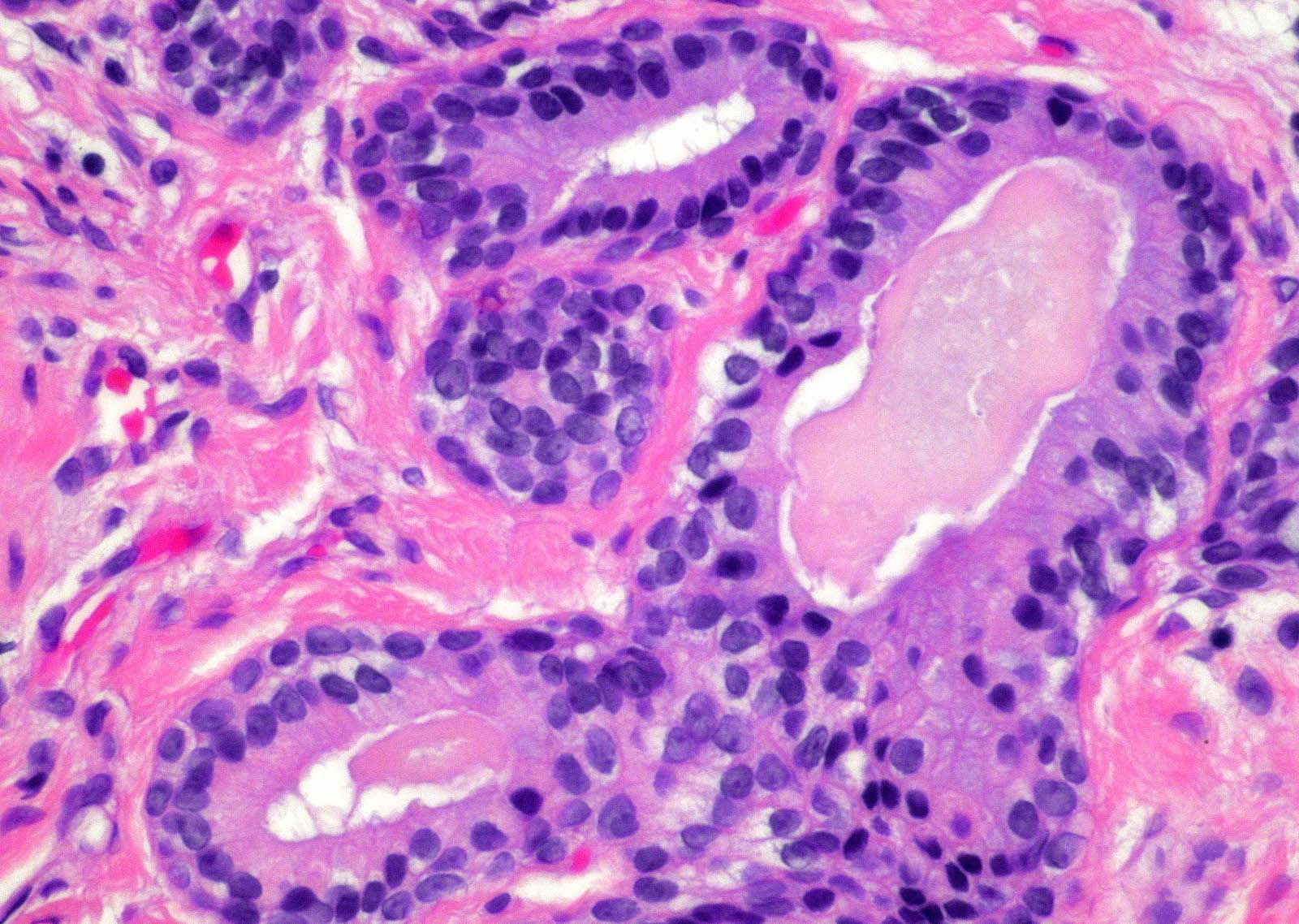
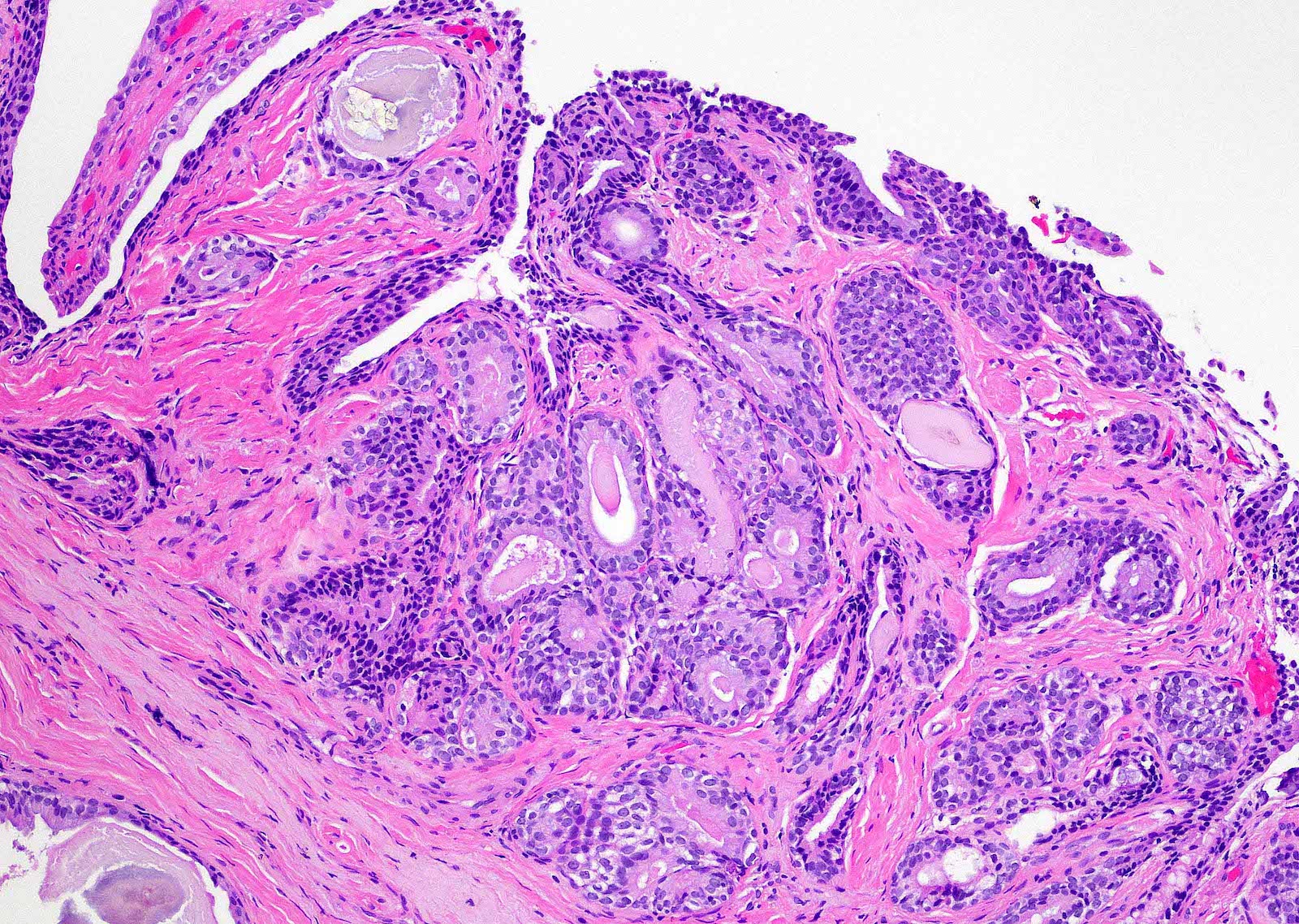
- Extraprostatic extension: usually this is dichotomized as focal (< 1 high power field in 1 - 2 slides) versus nonfocal / established (> 1 high power field in 1 - 2 slides)
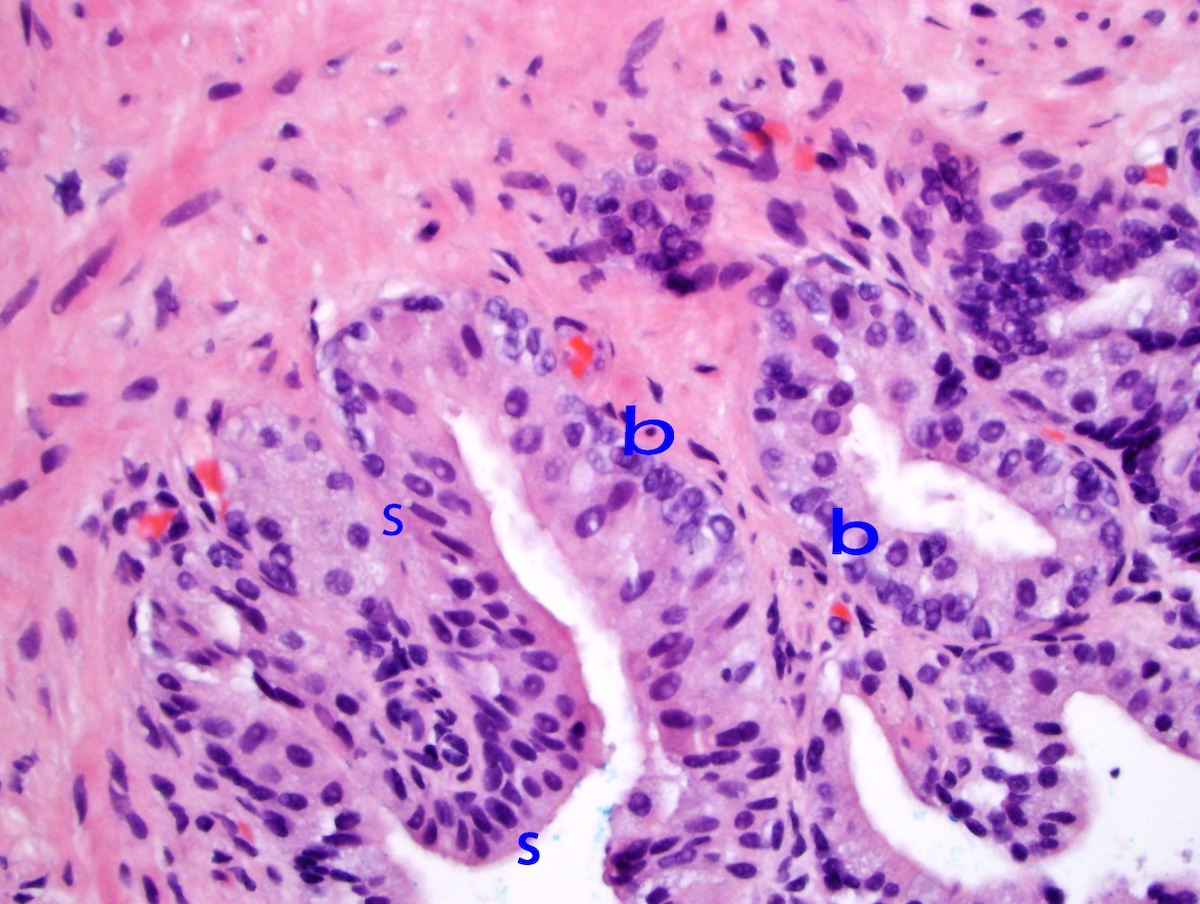
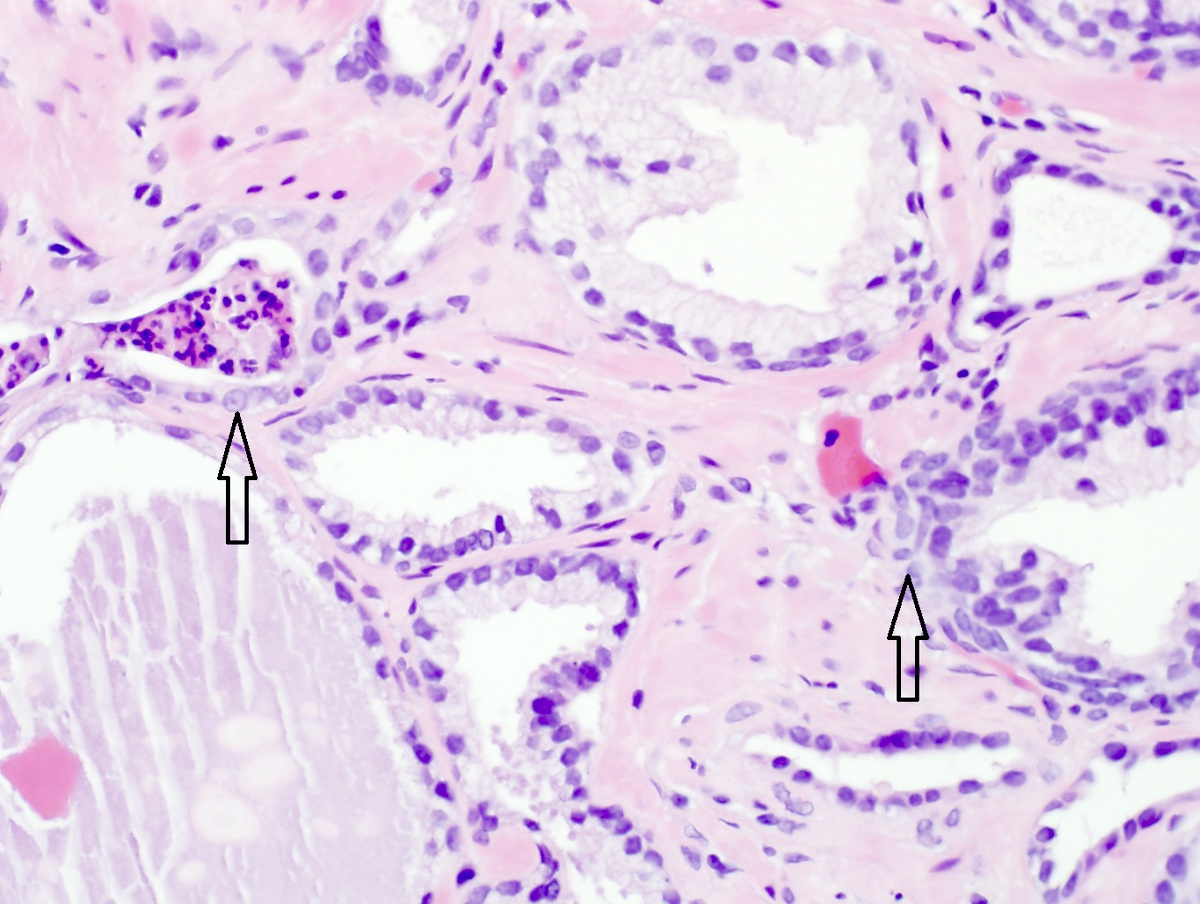
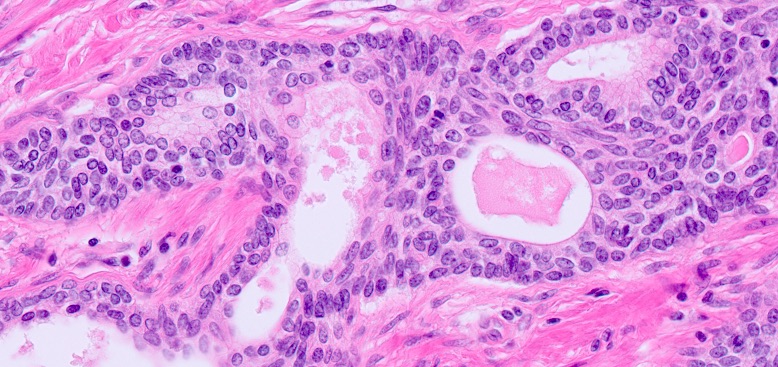
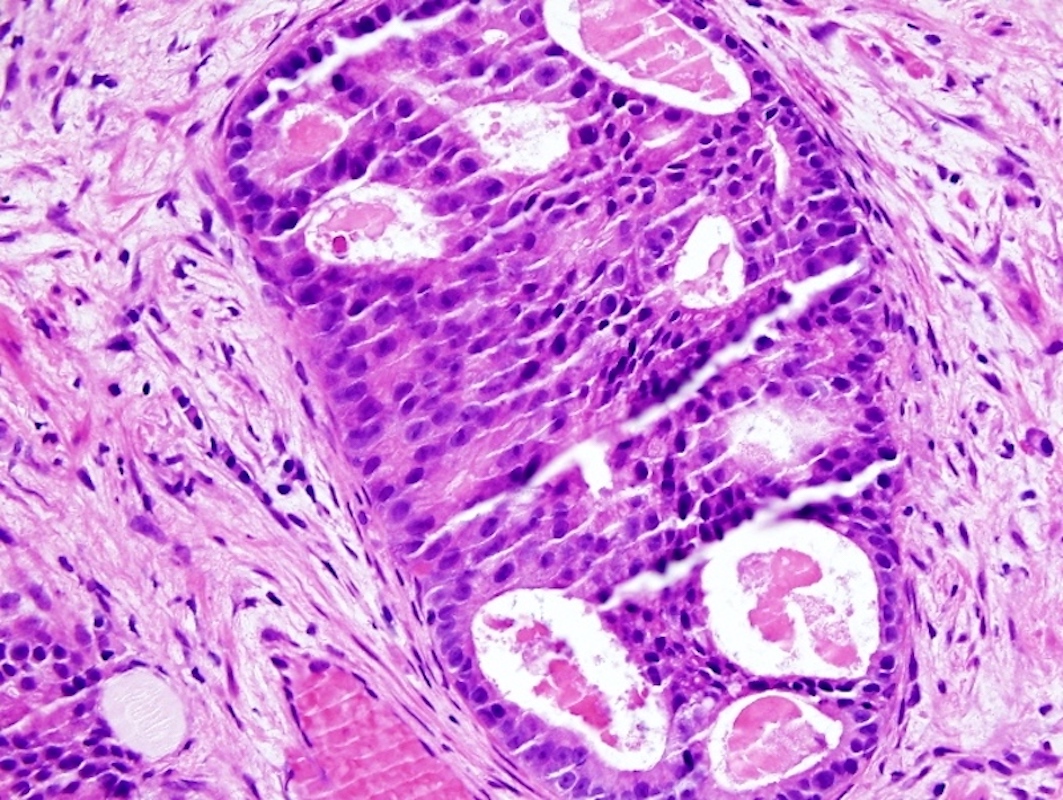
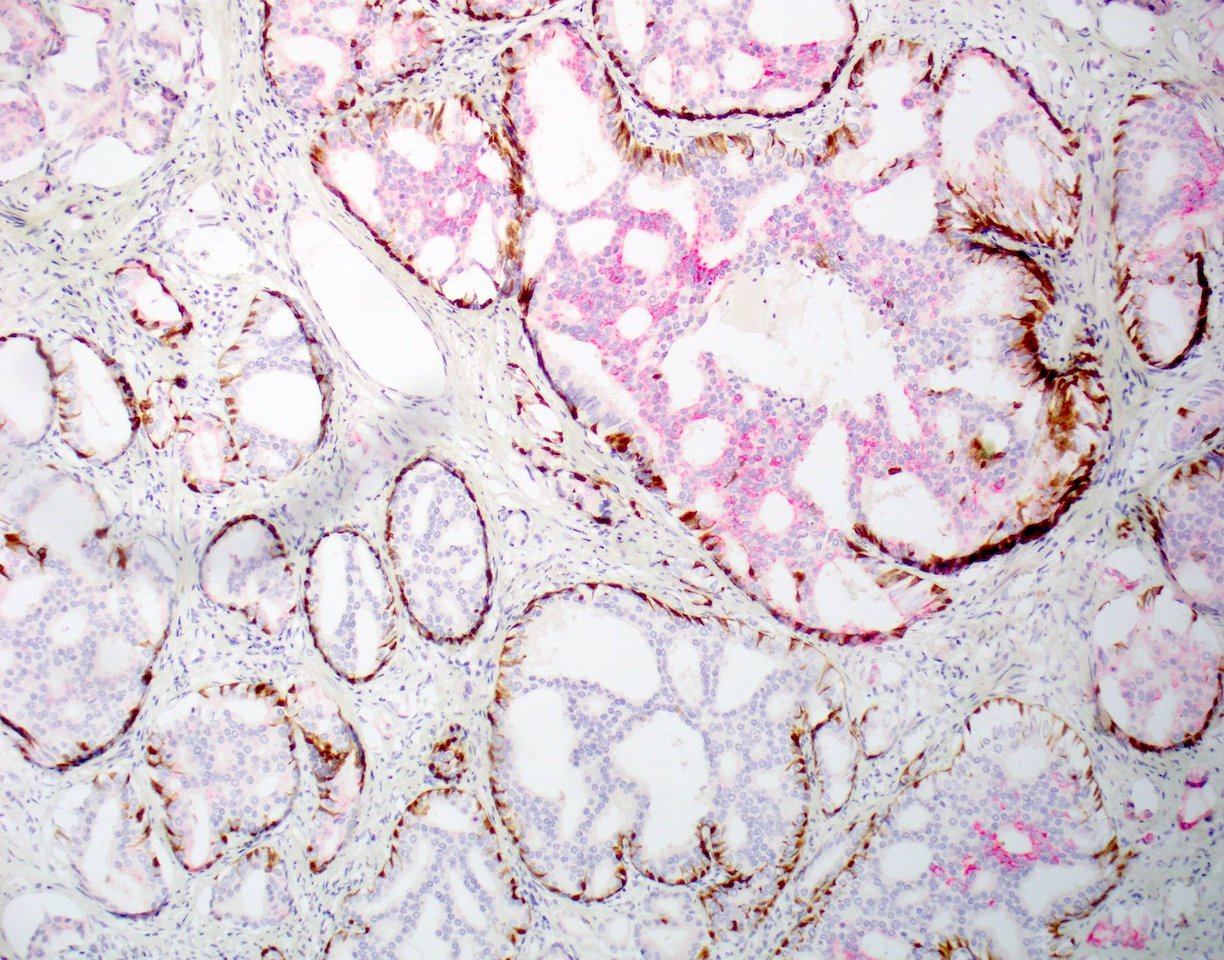
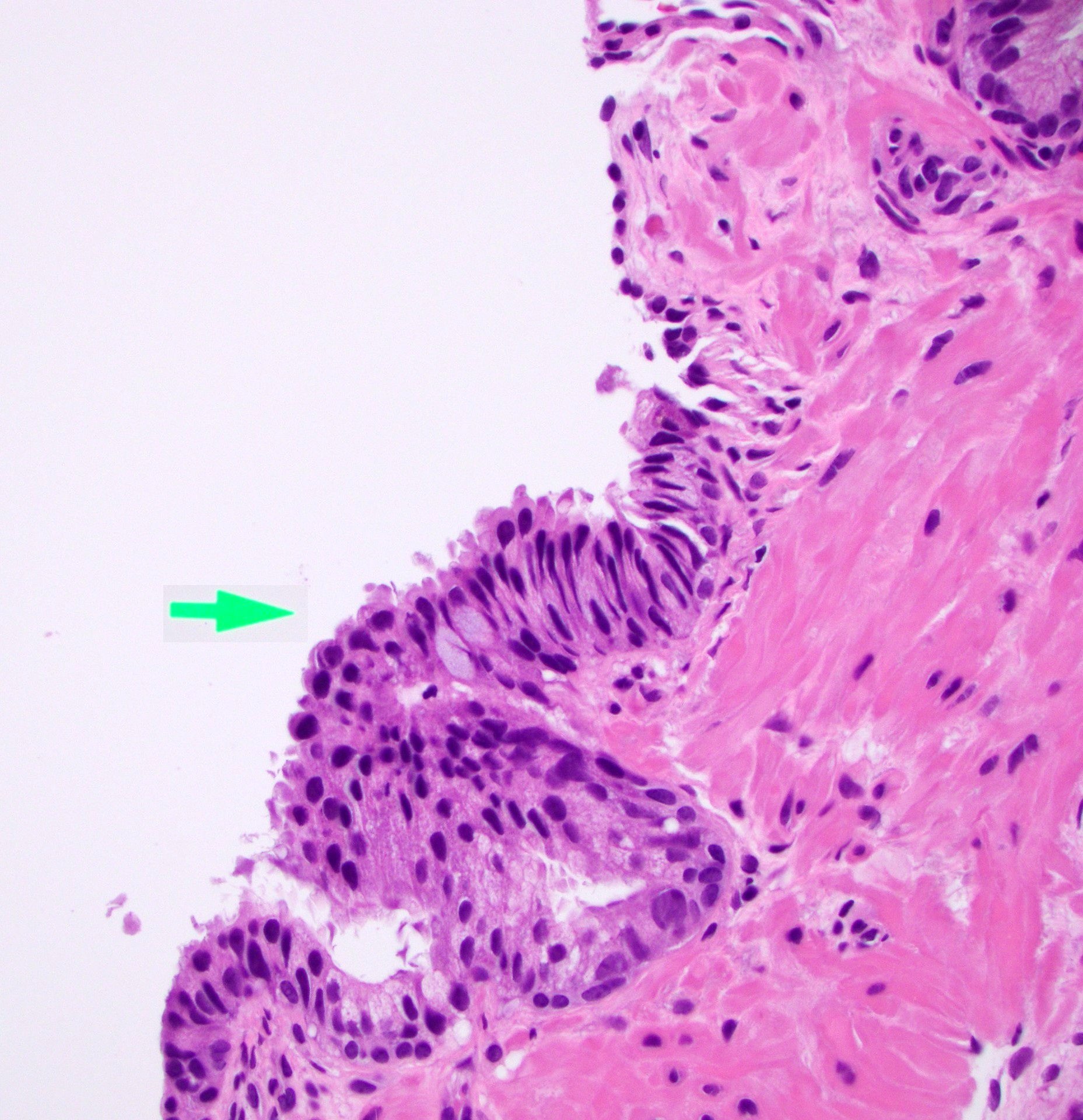
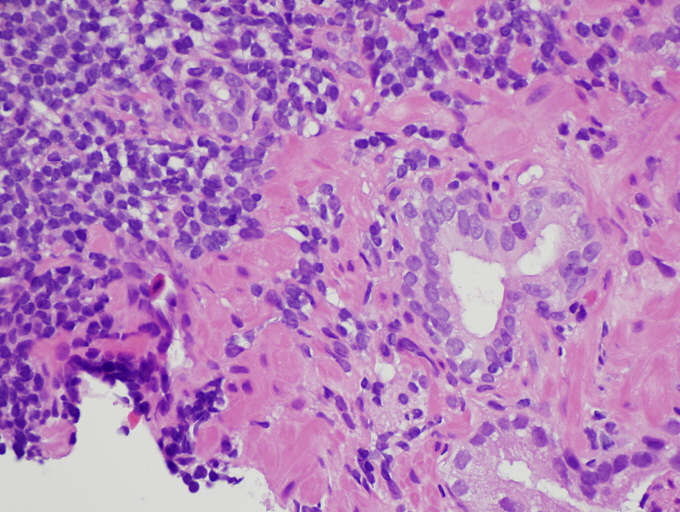
- Microscopic invasion of bladder neck: presence of tumor in thick muscle usually with no adjacent nonneoplastic glands
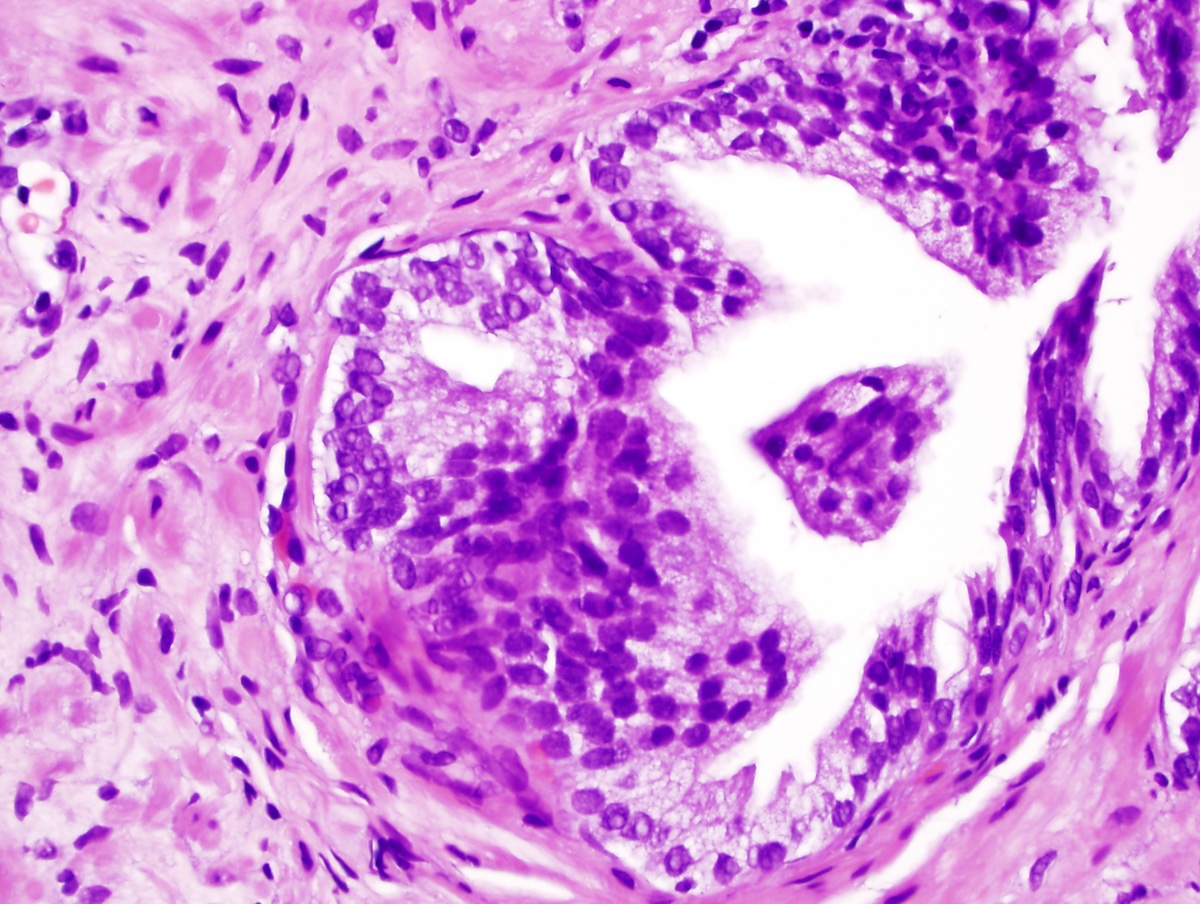
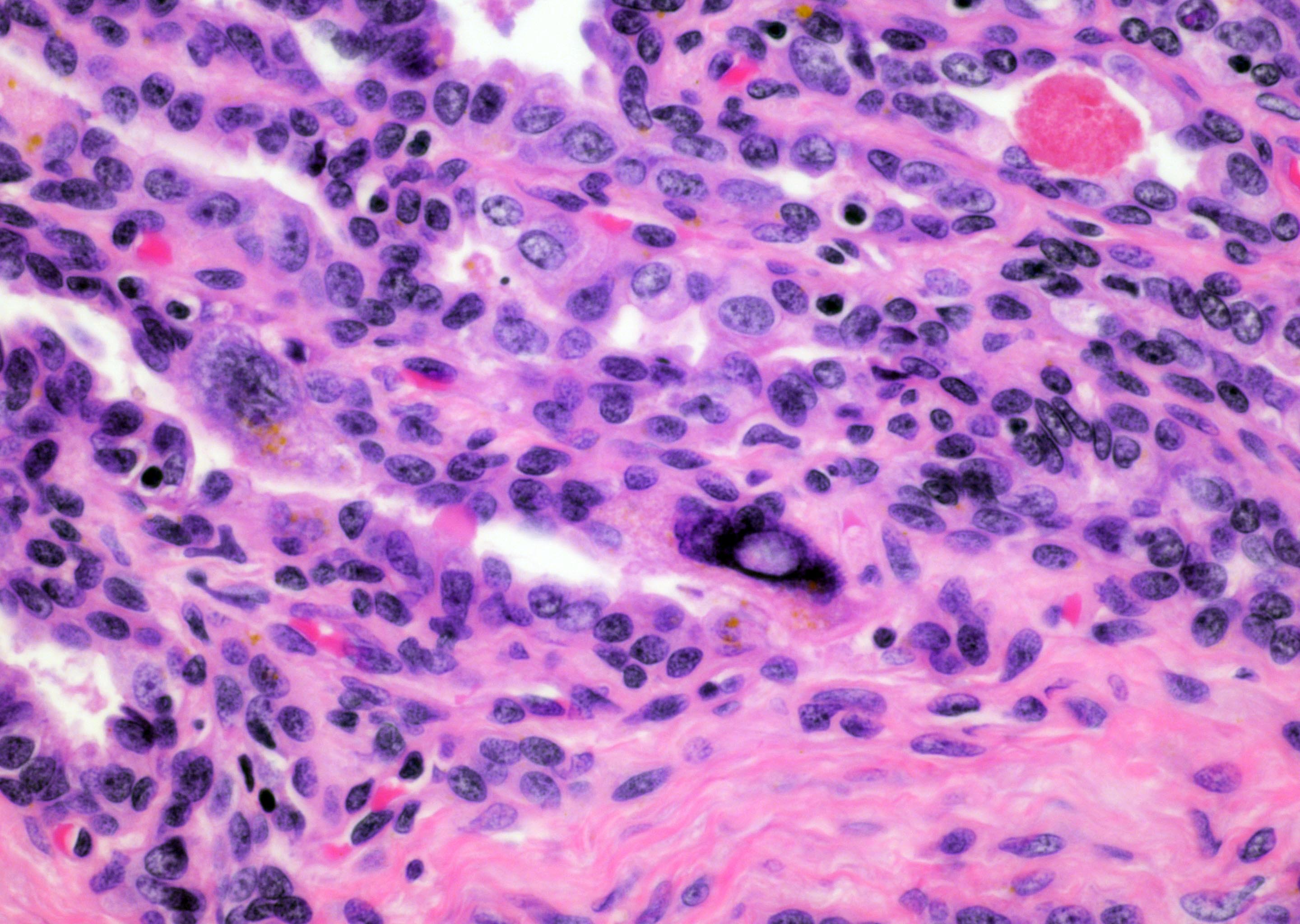
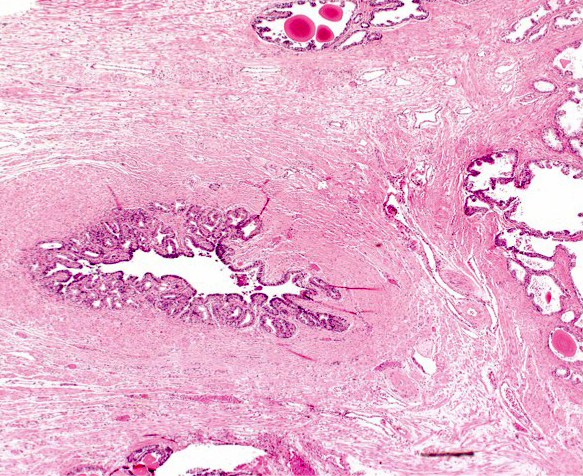
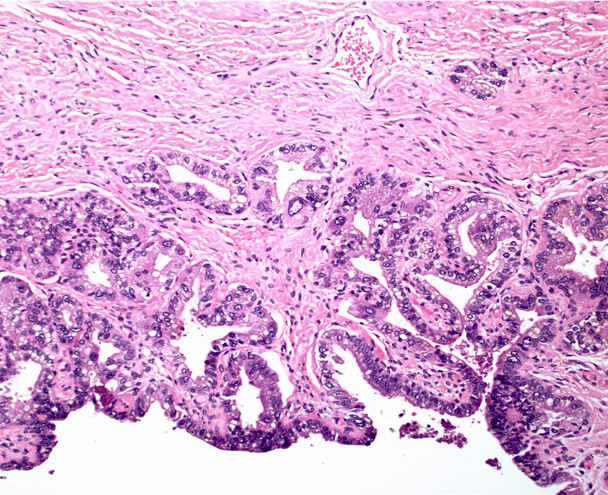
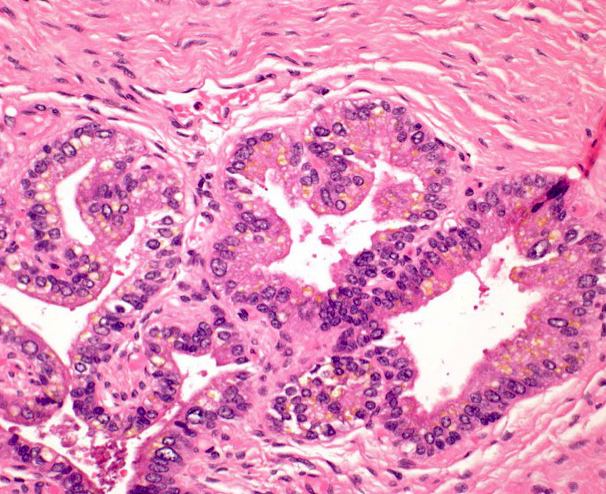
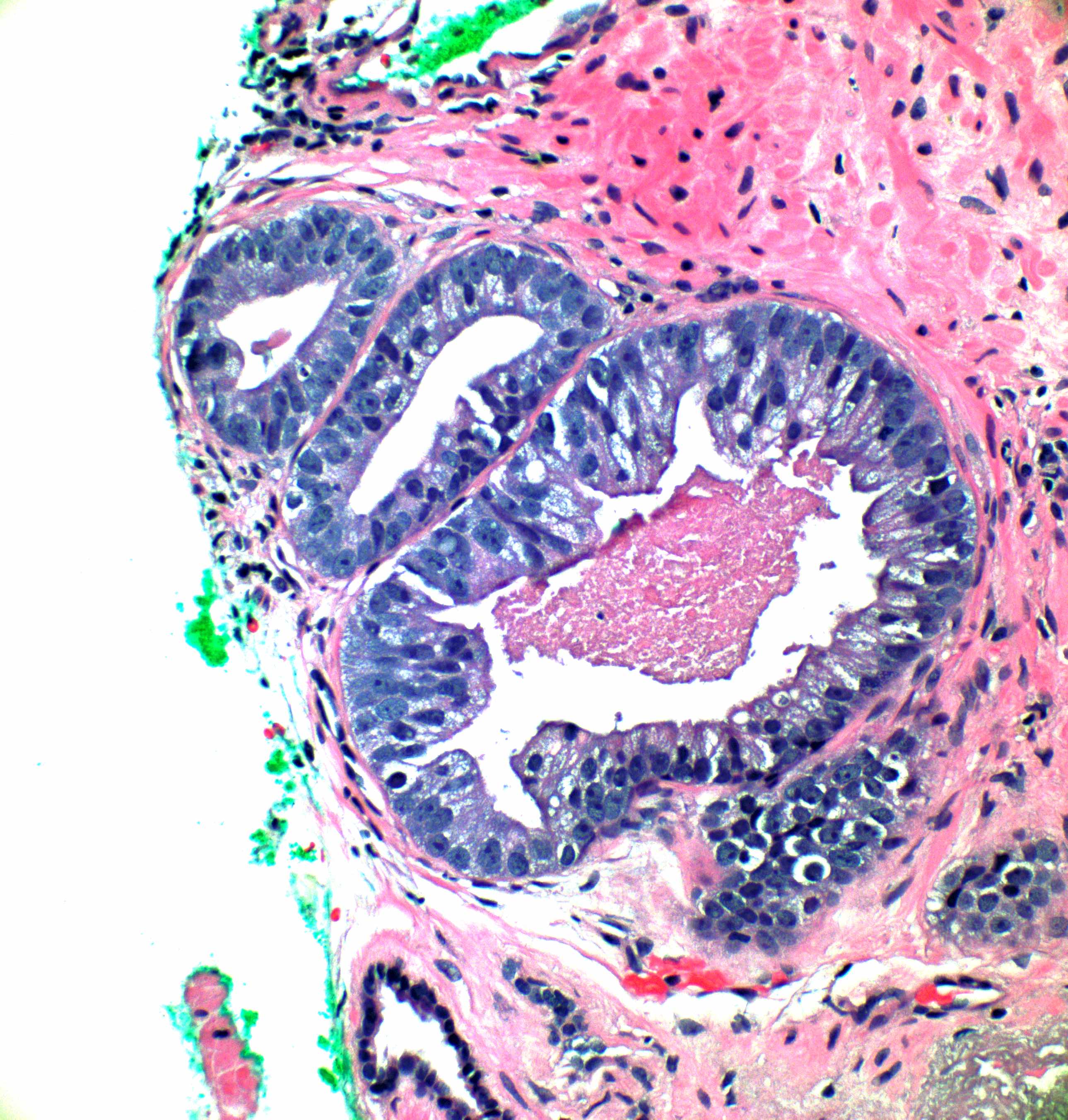
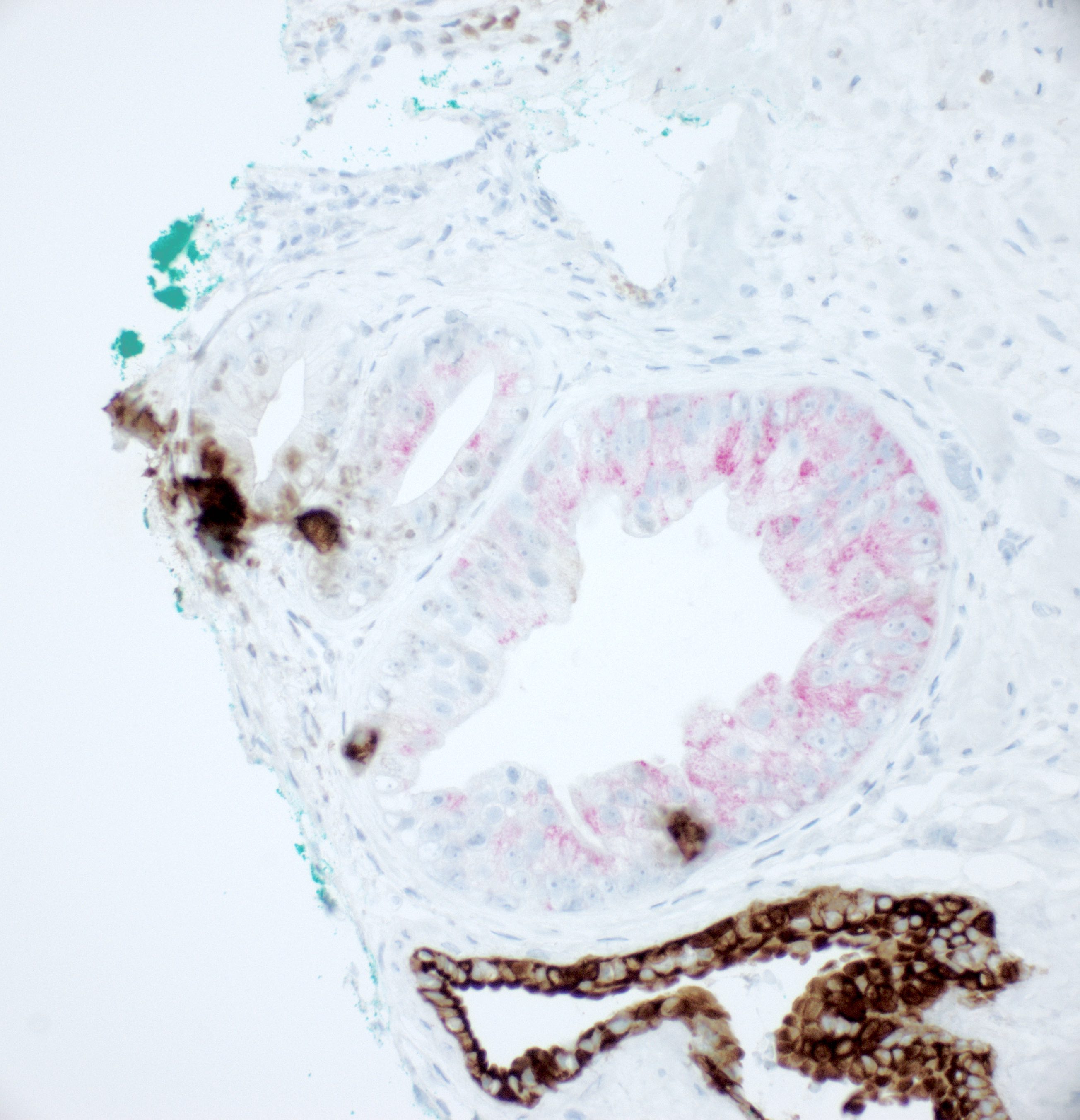
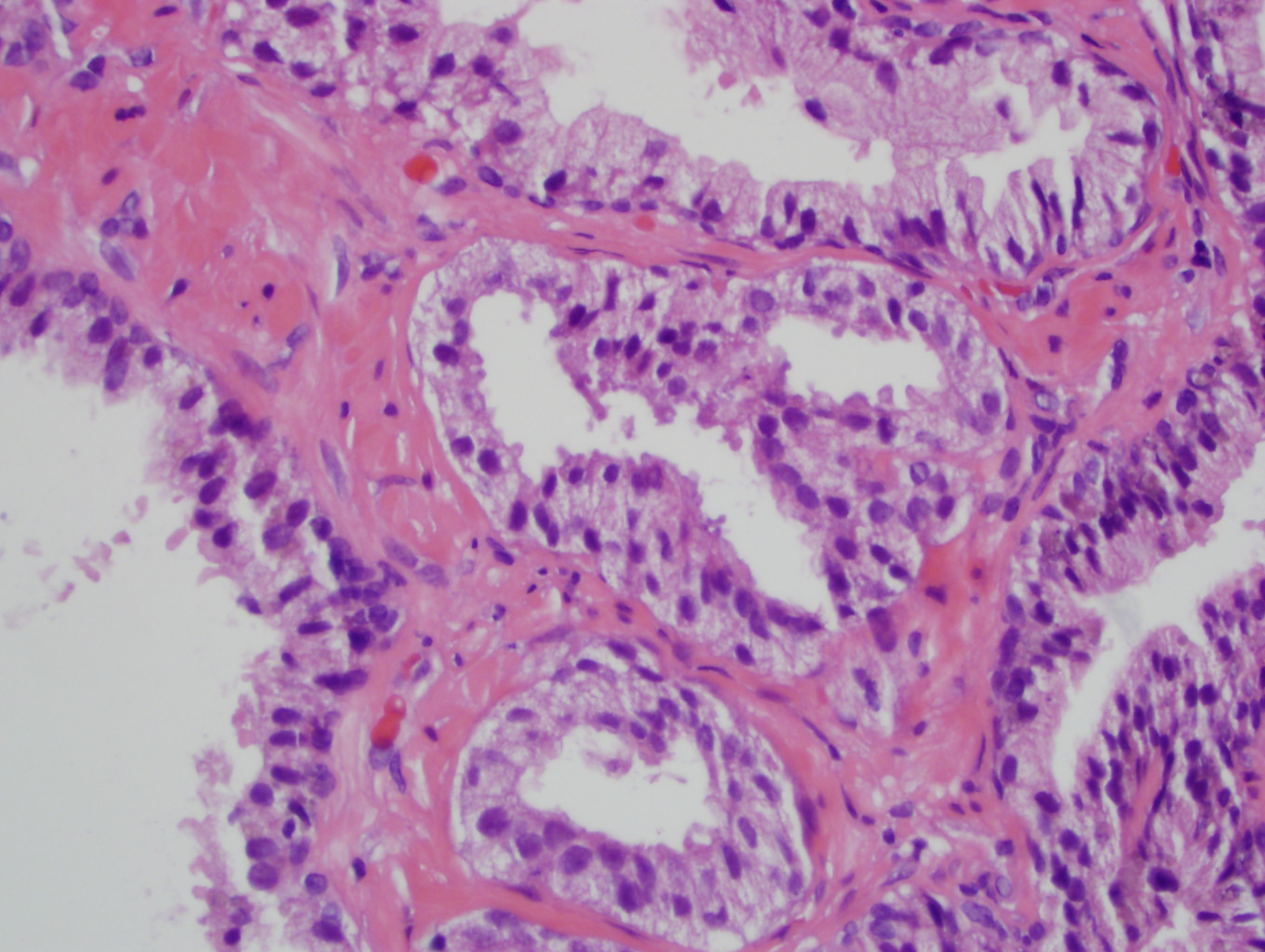
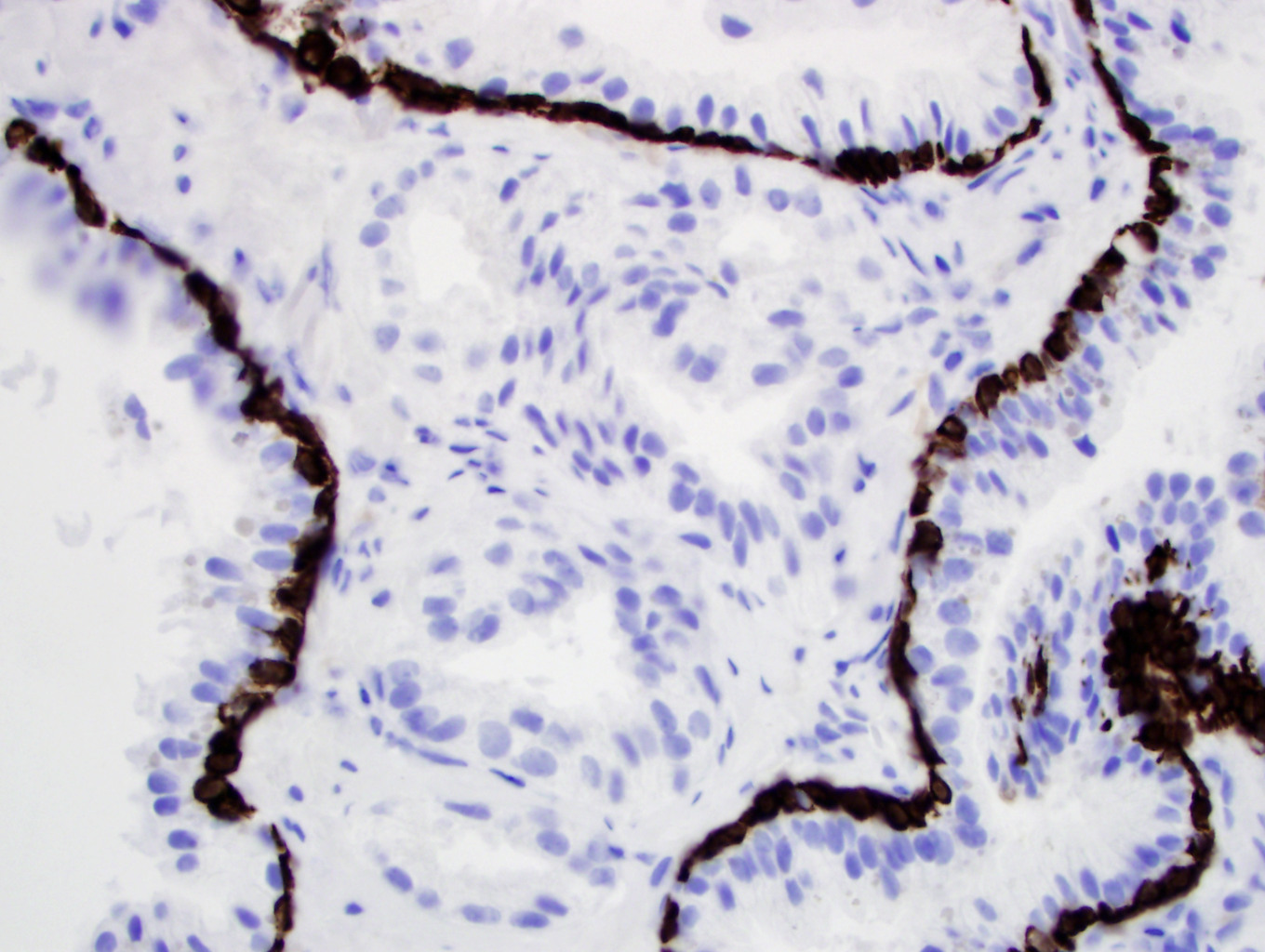
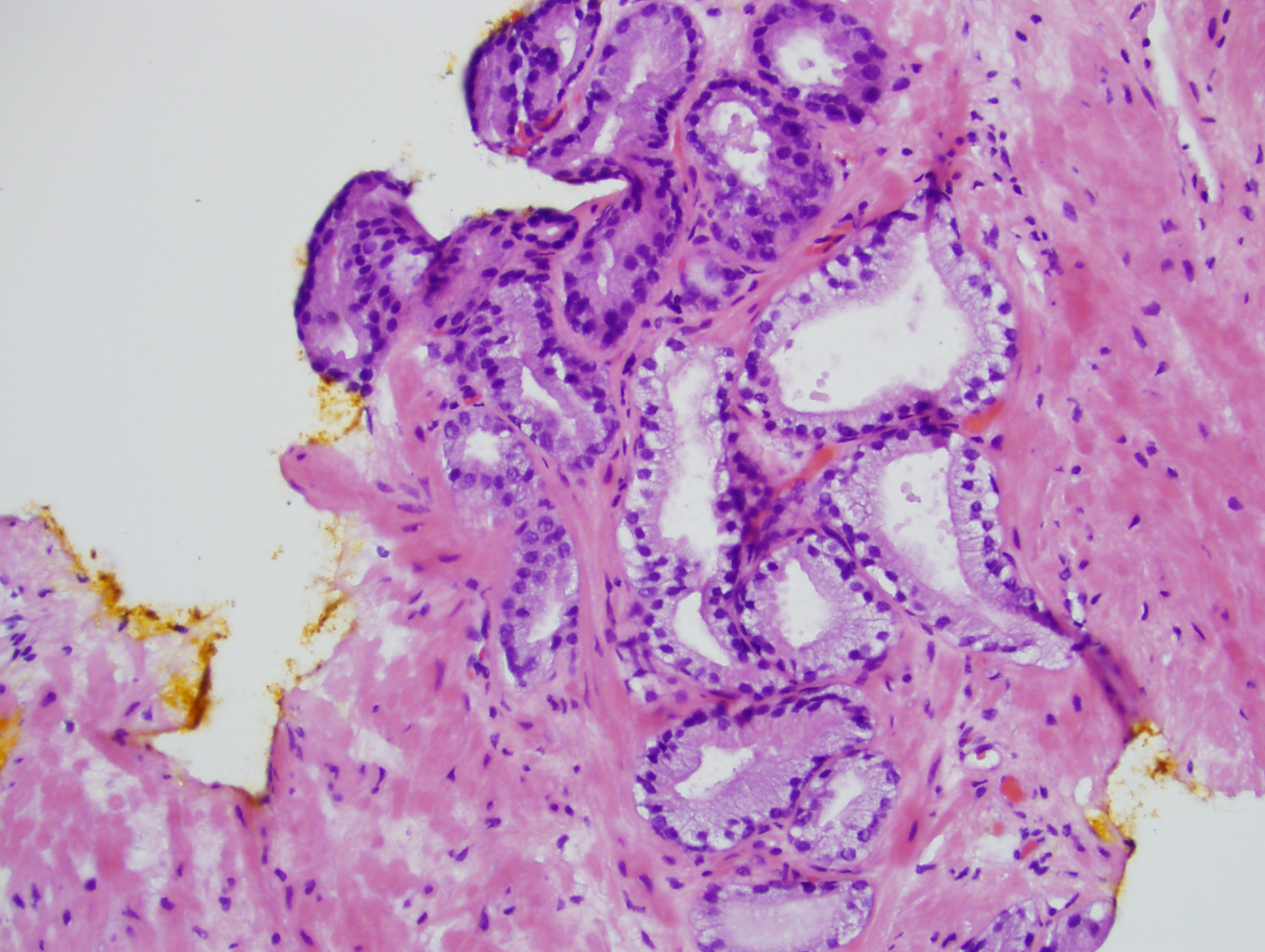
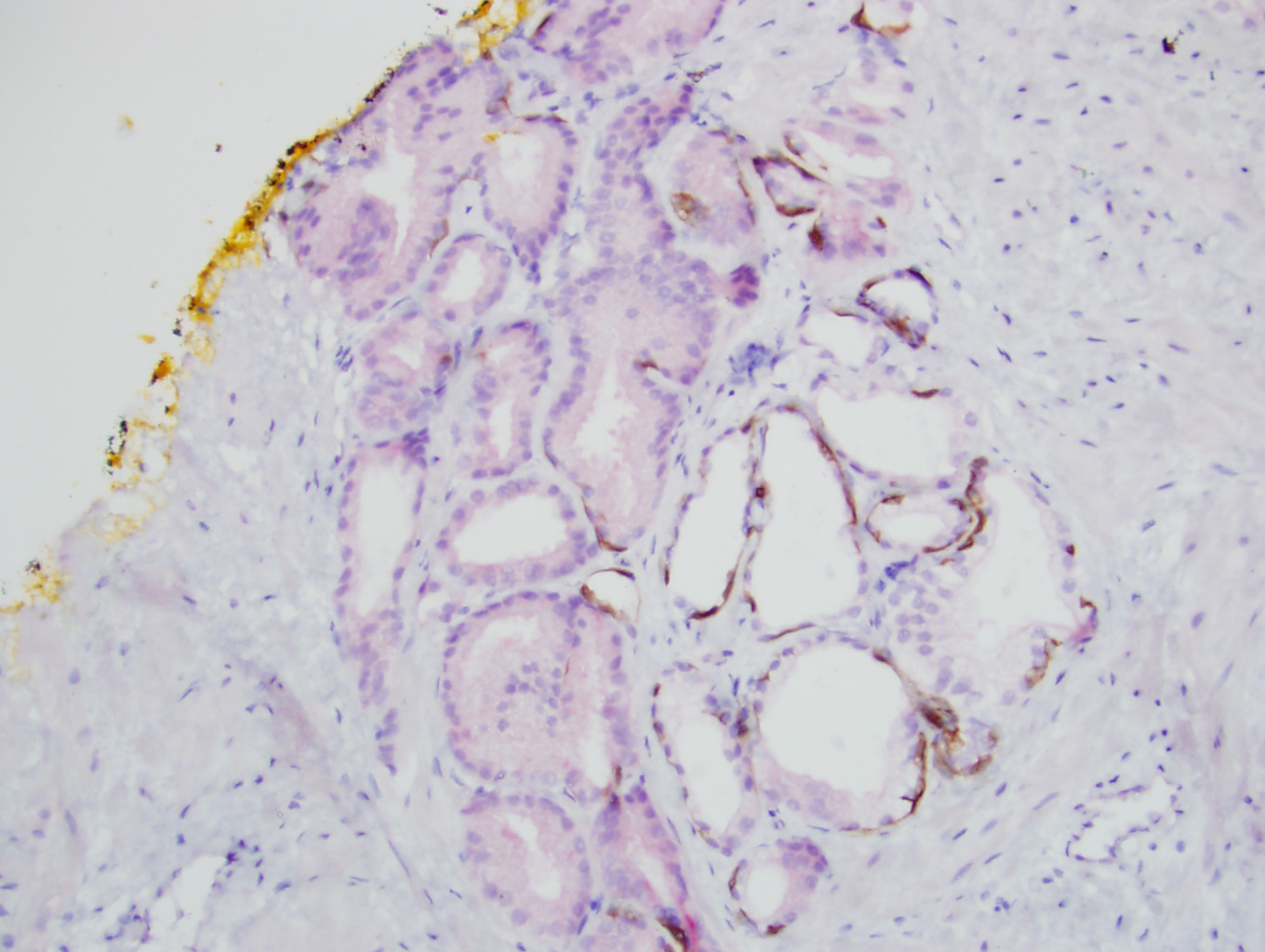
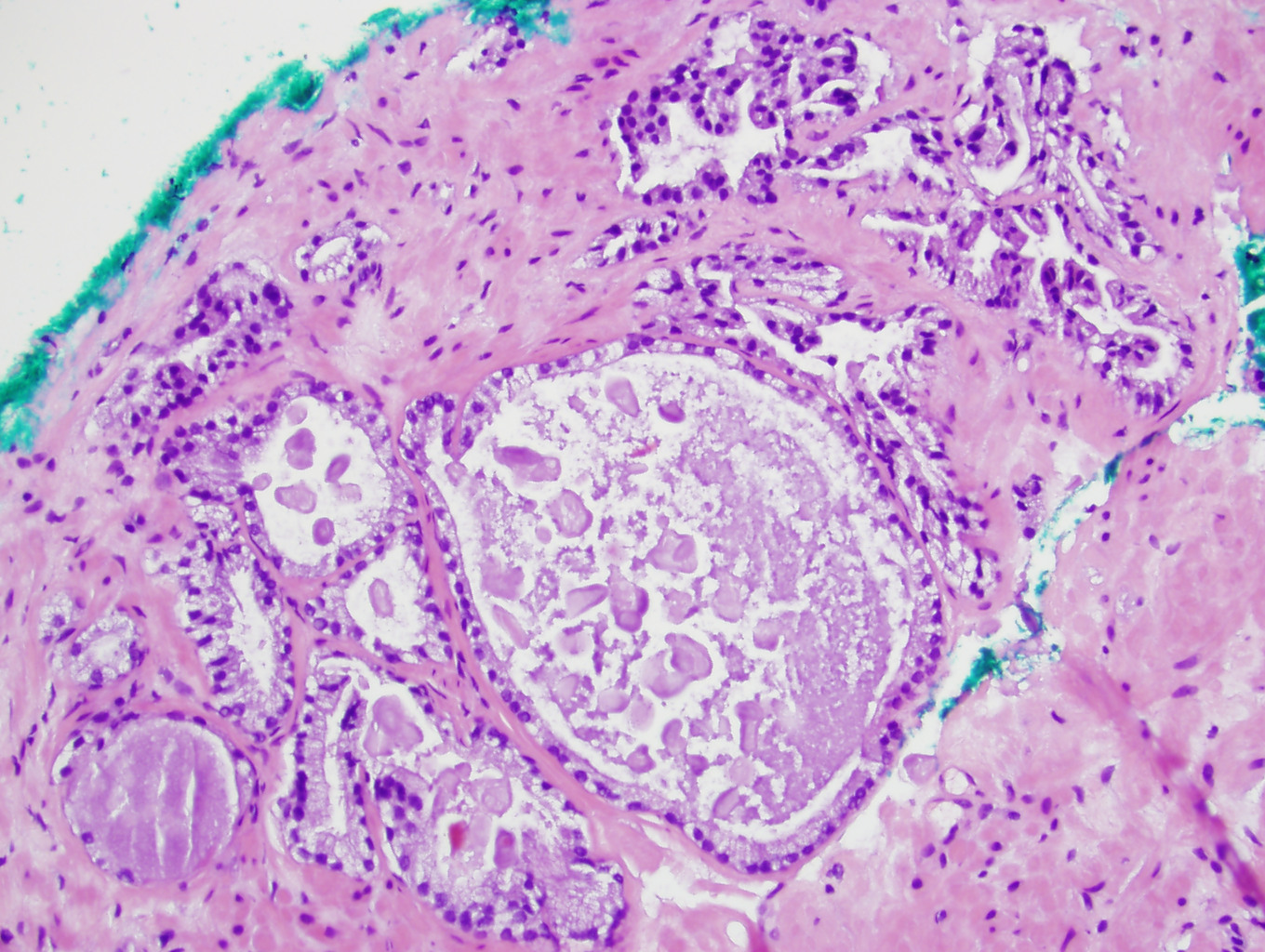
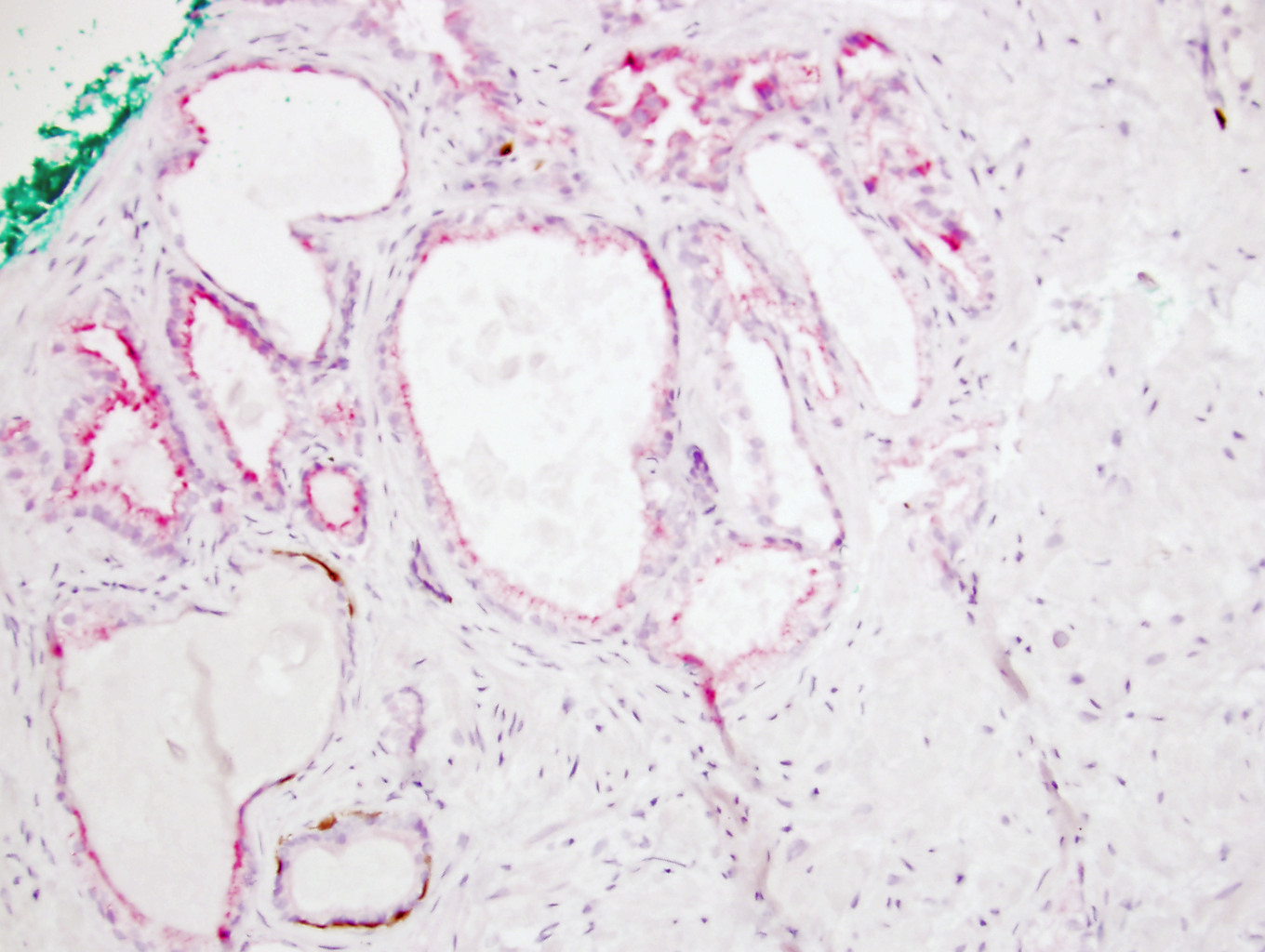
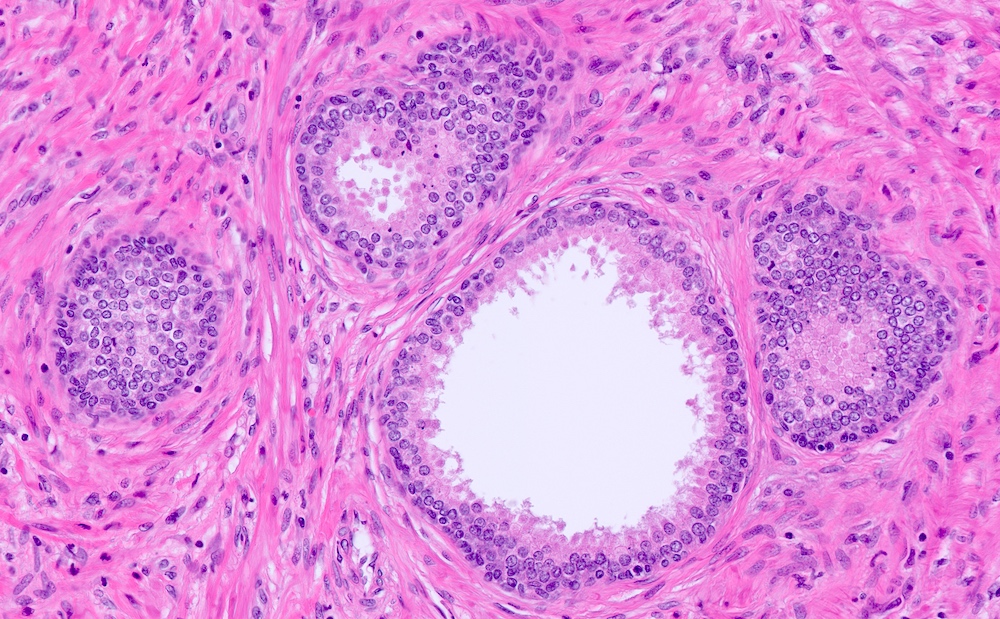
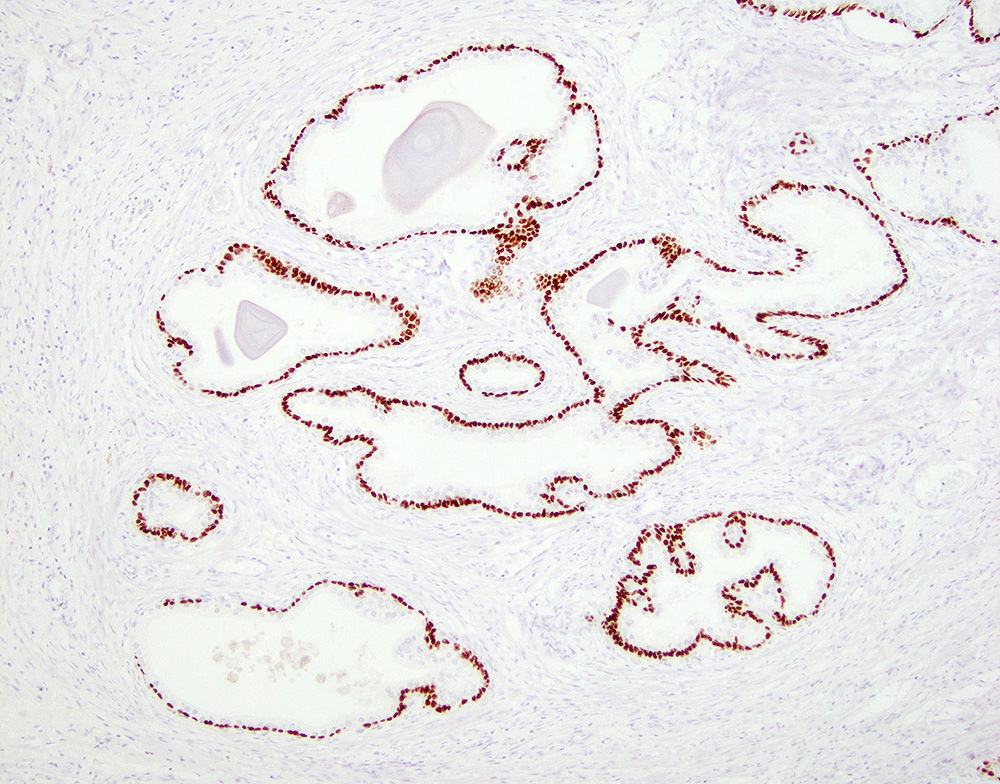
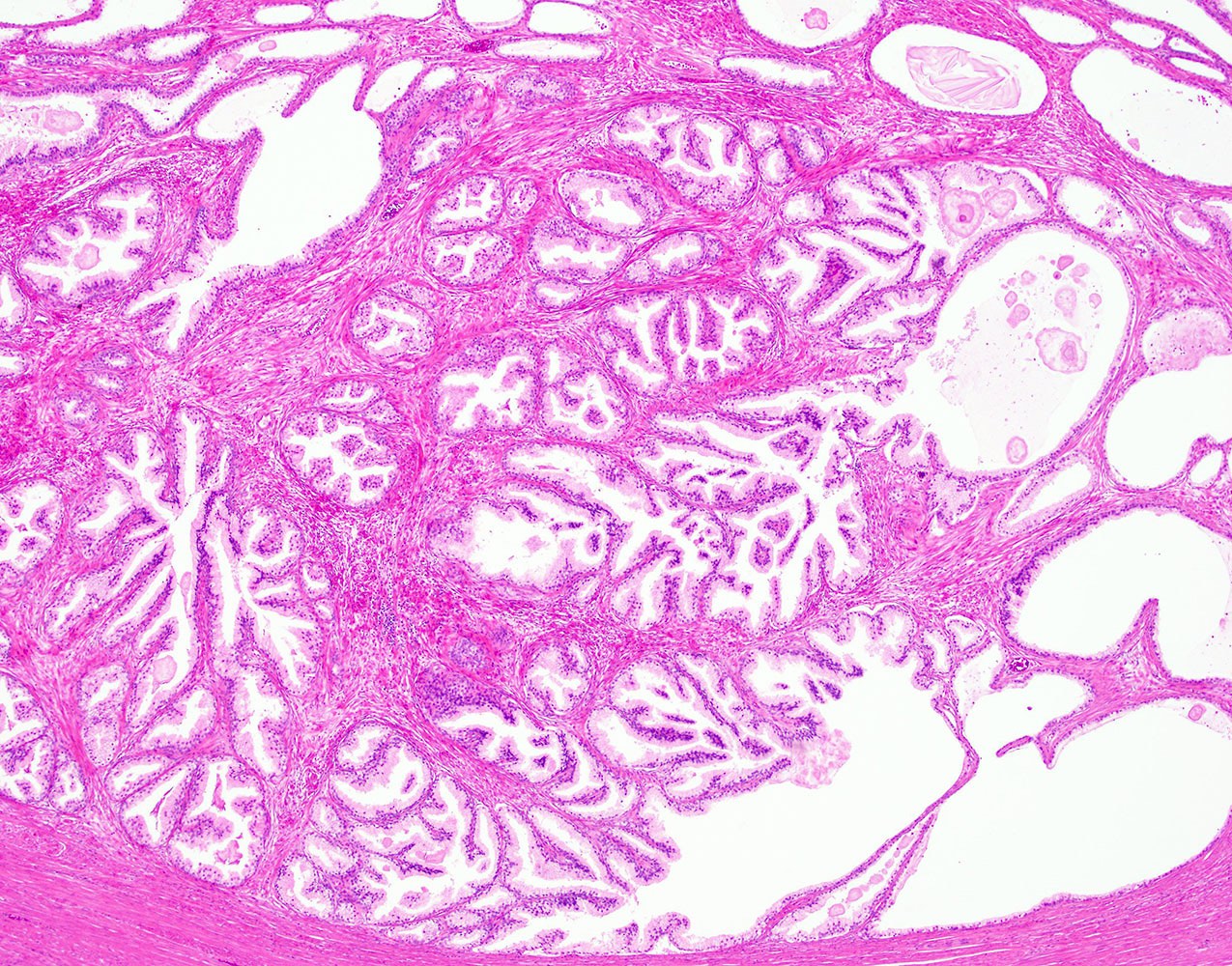
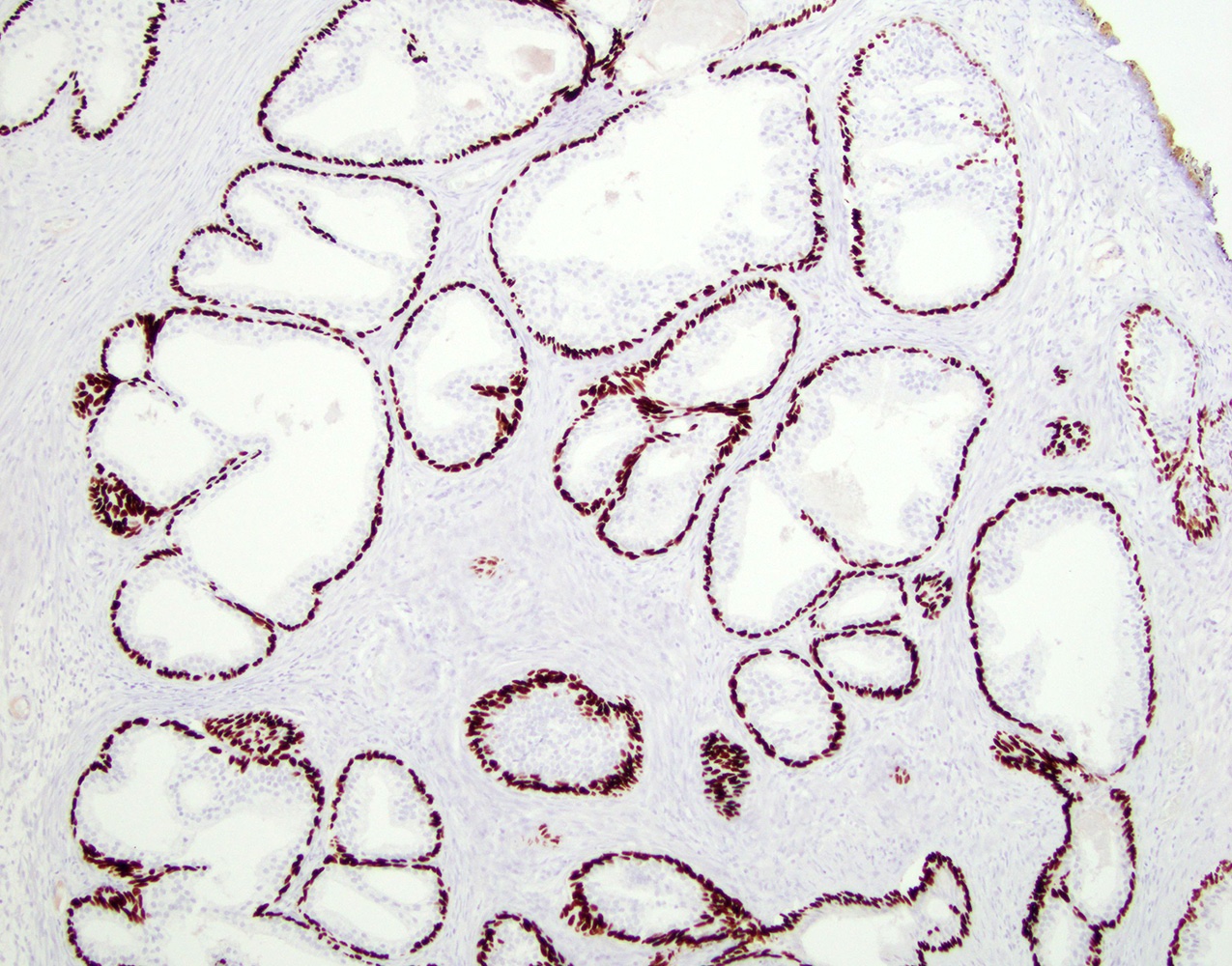
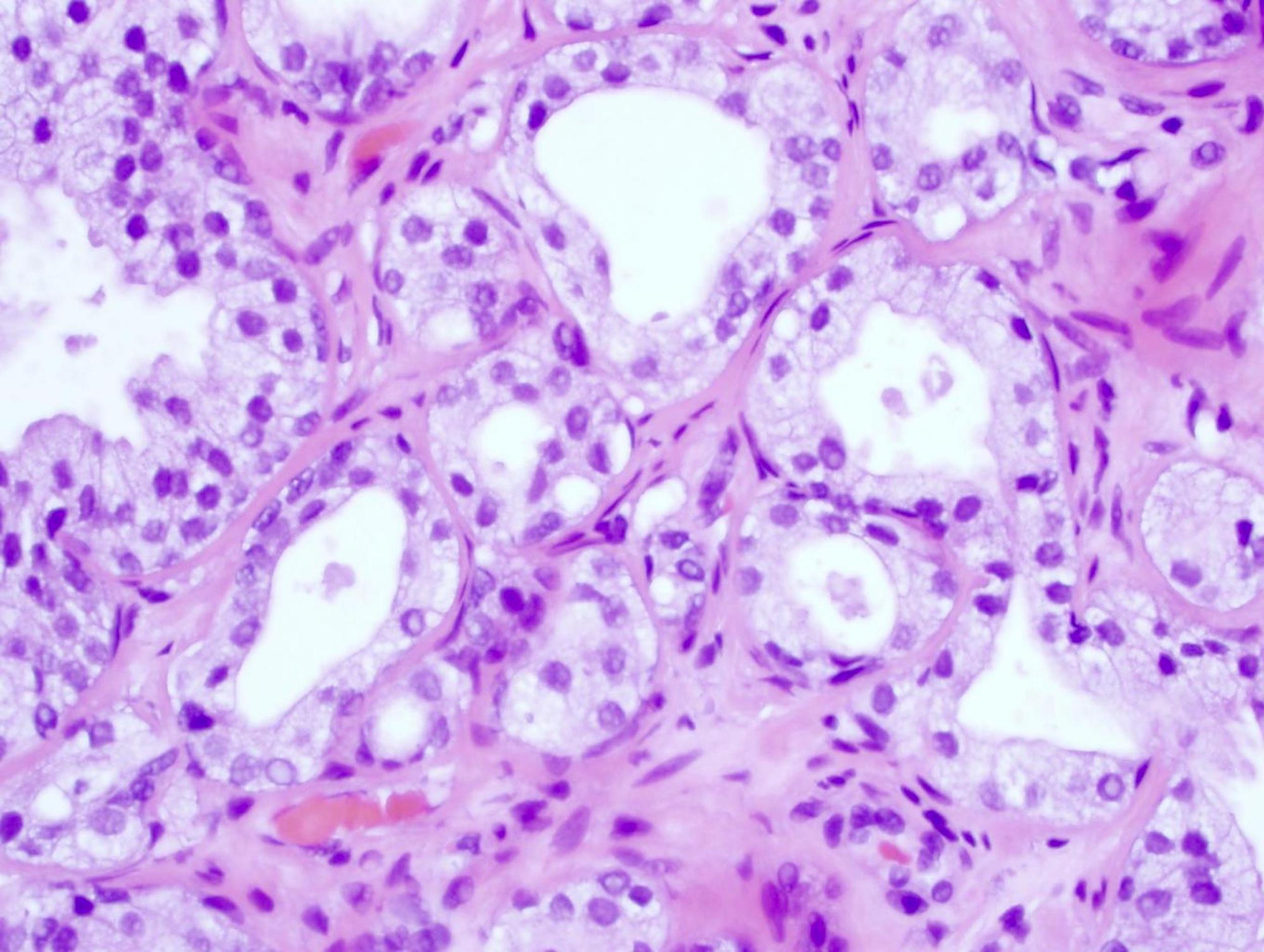
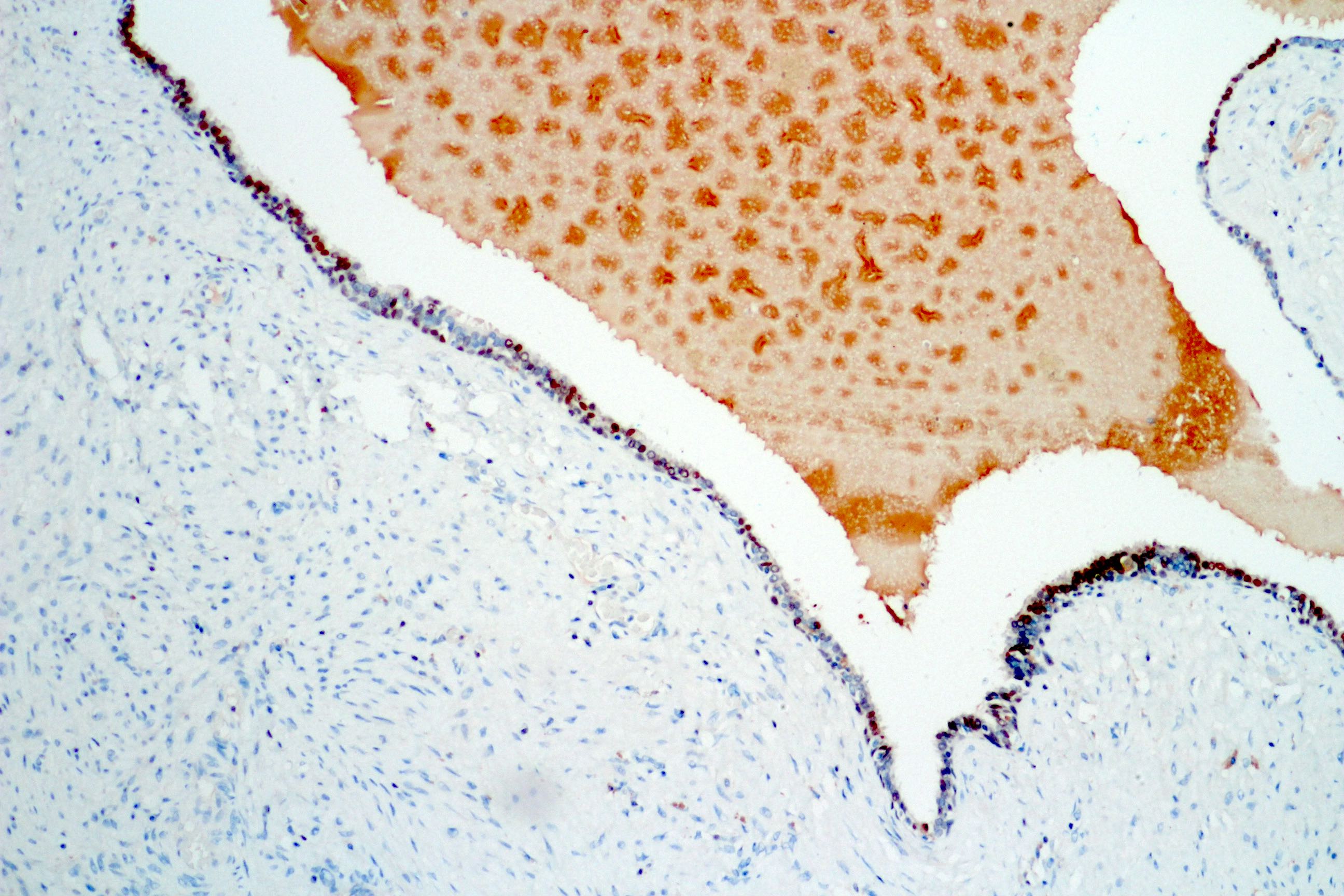
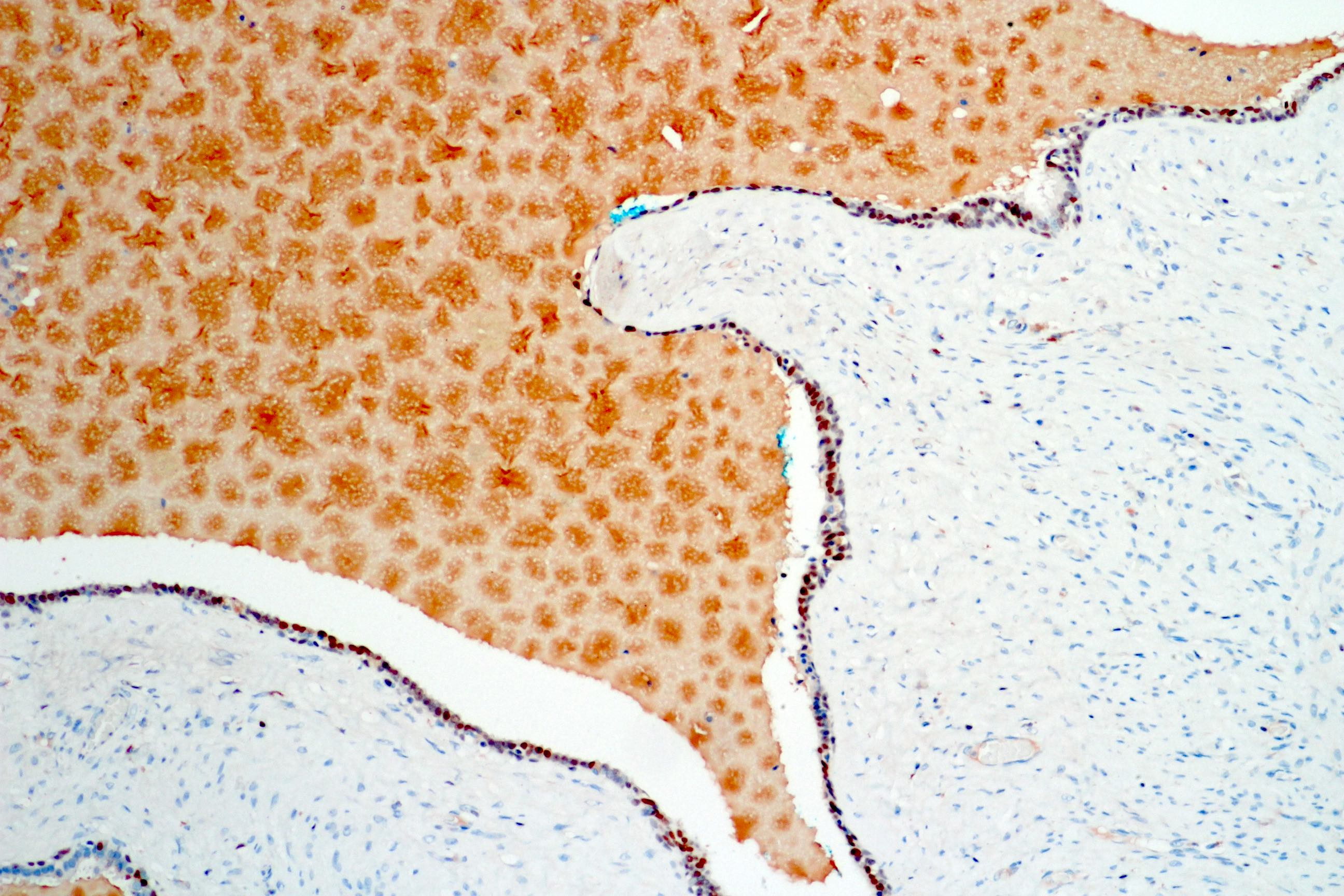
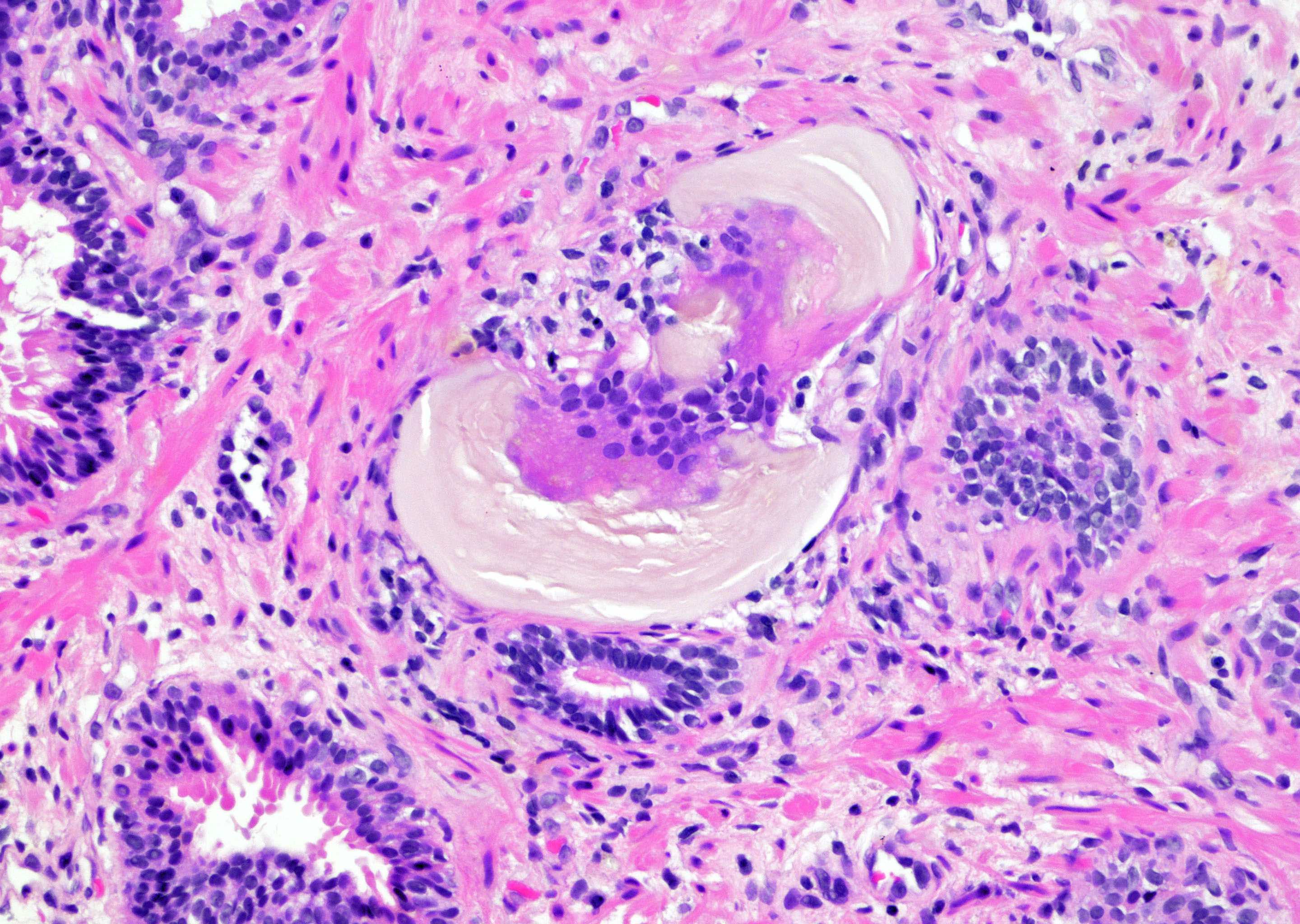
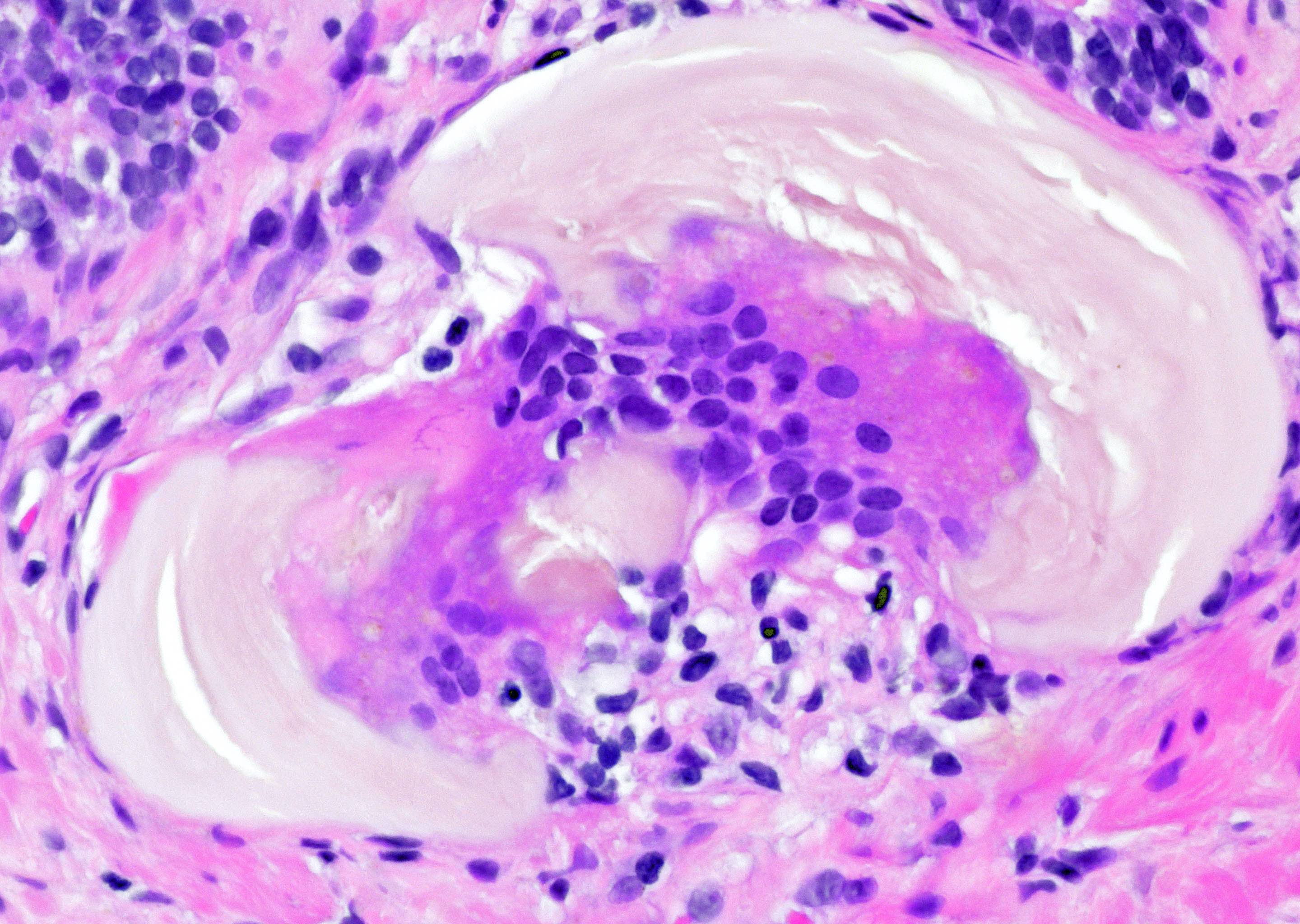
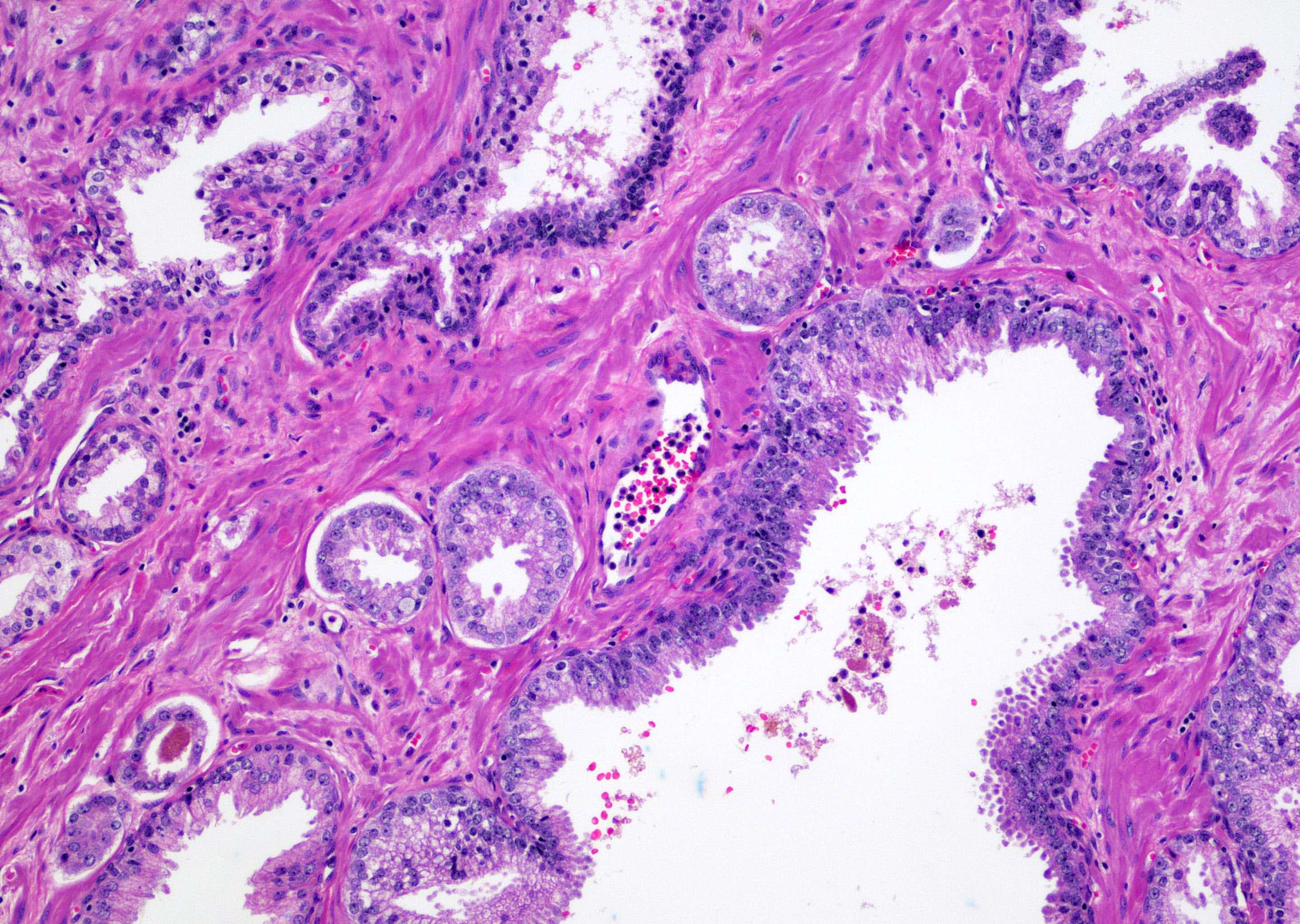
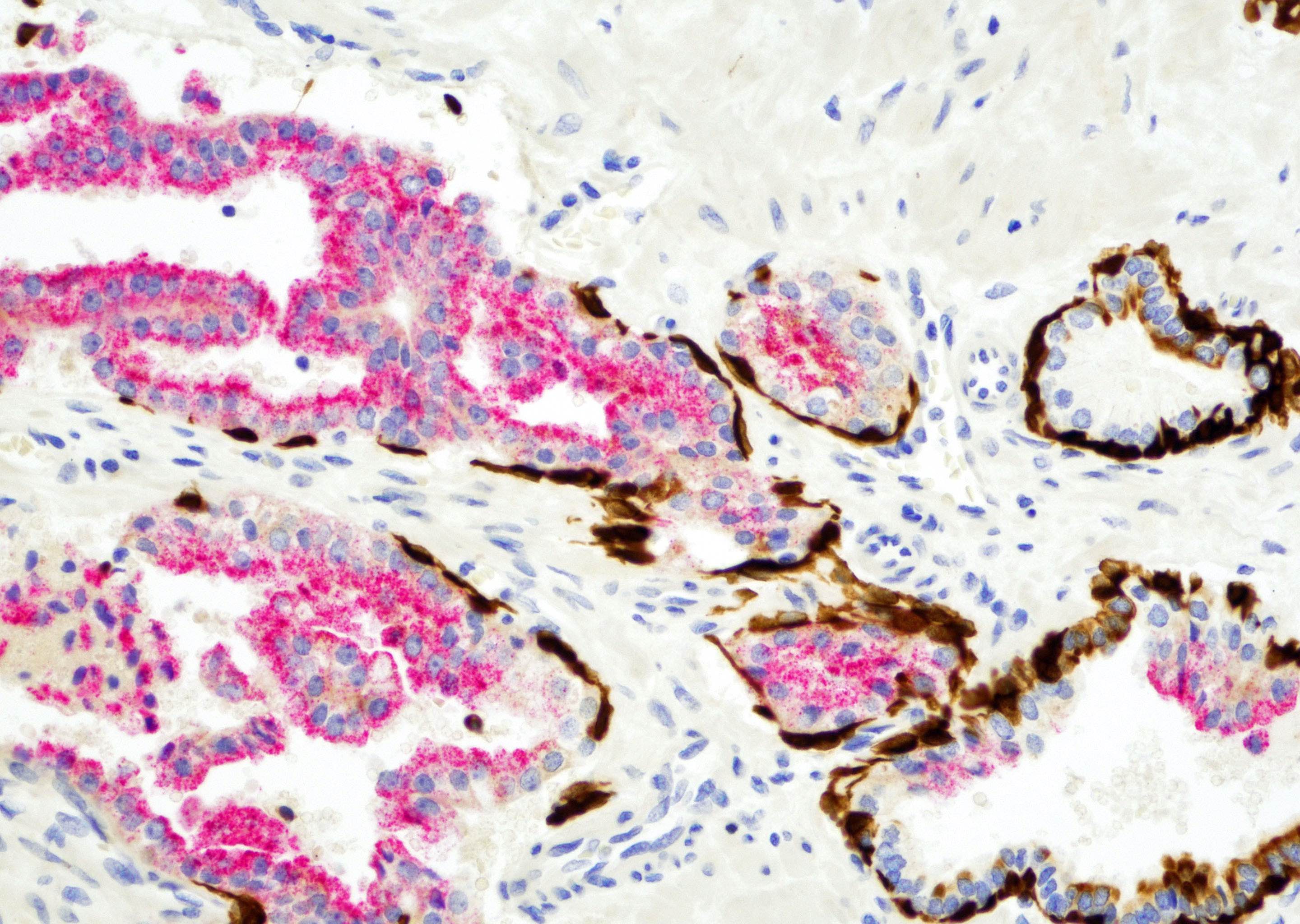
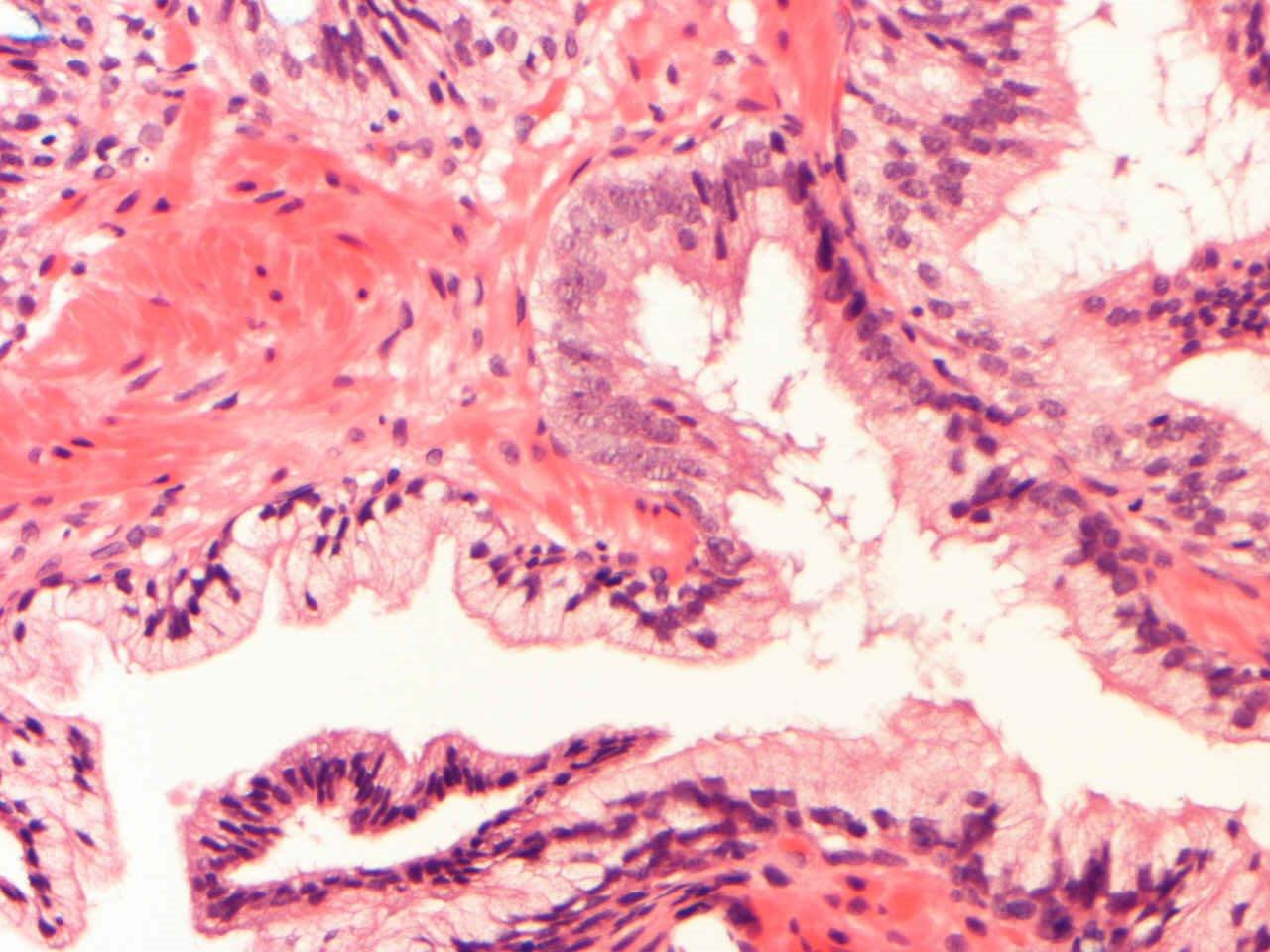
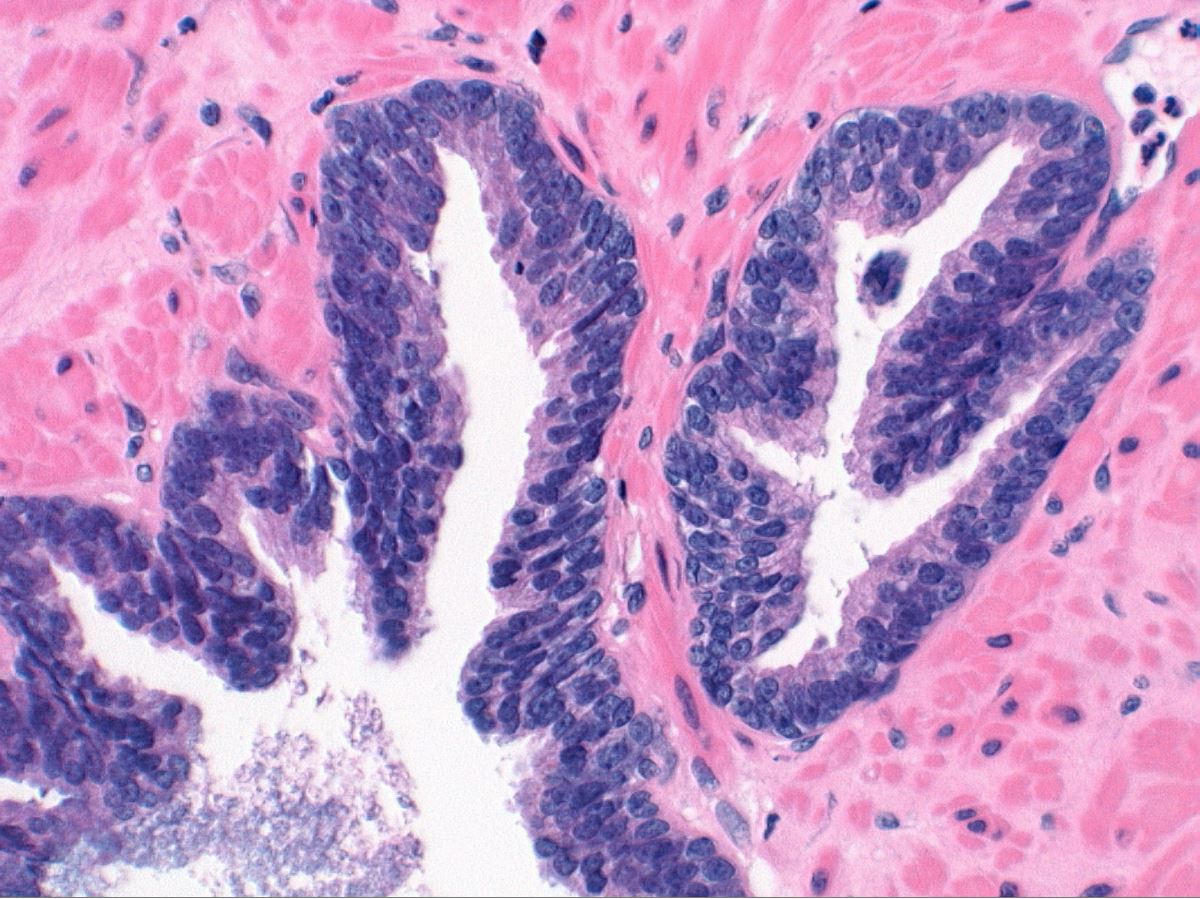
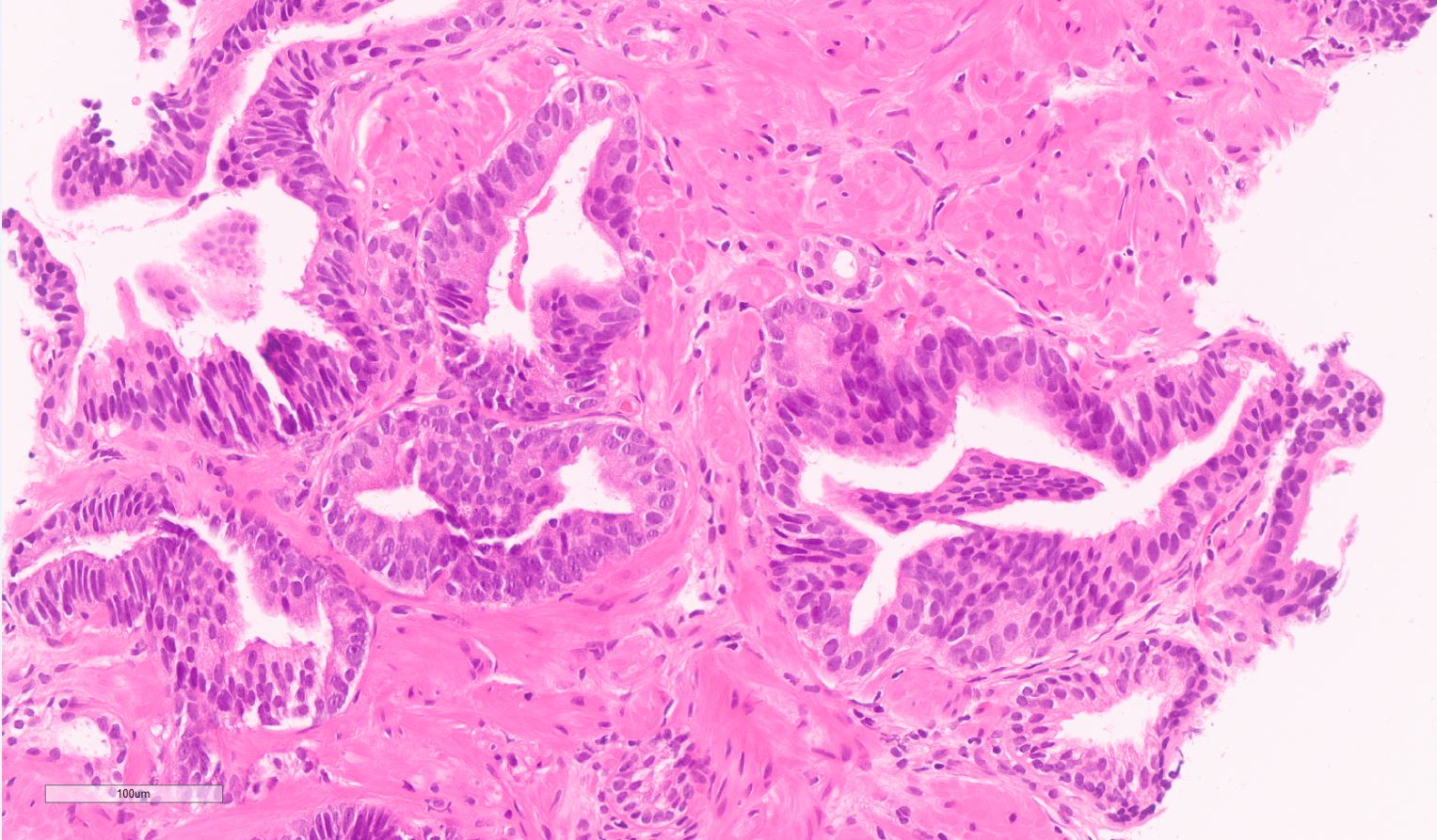
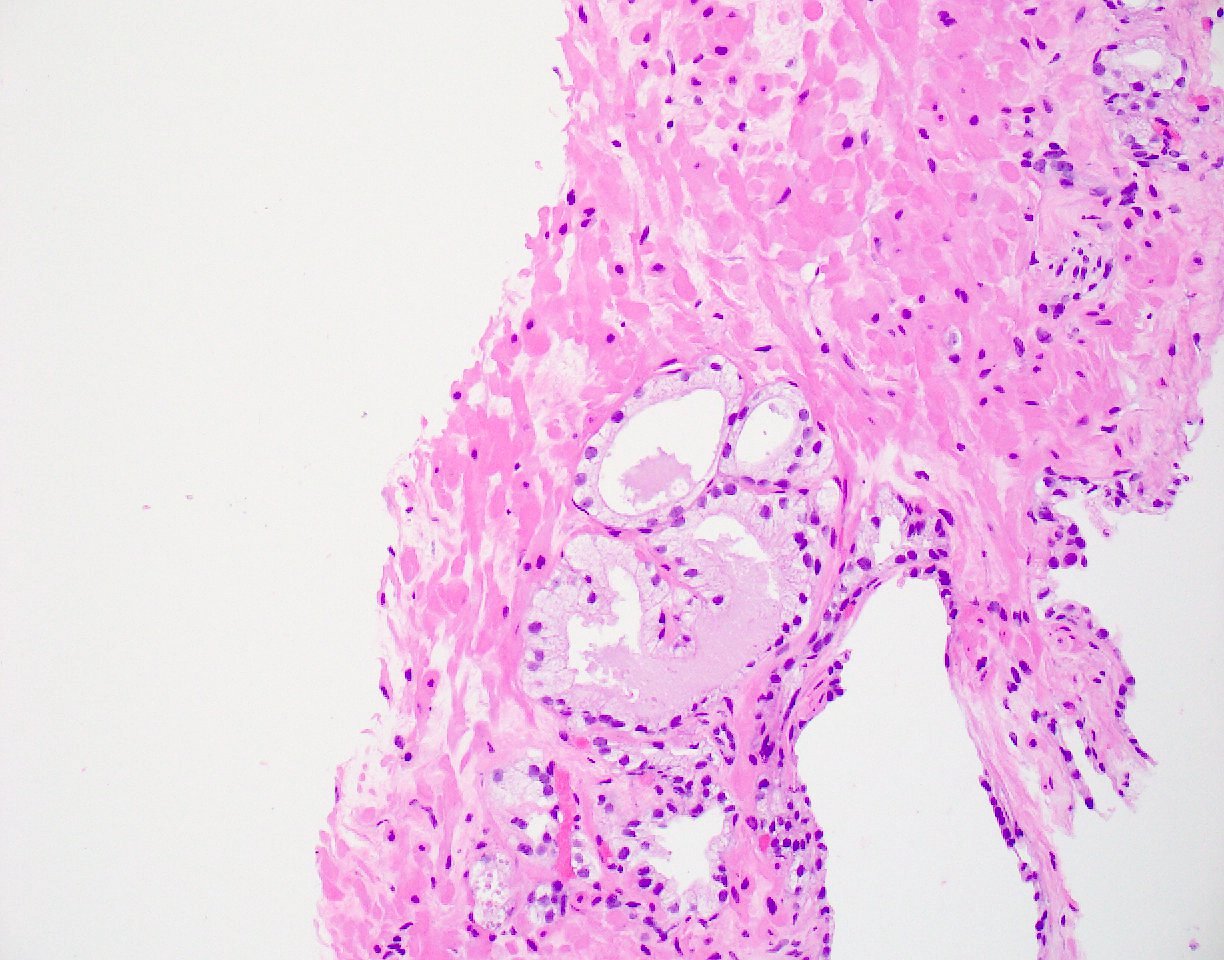
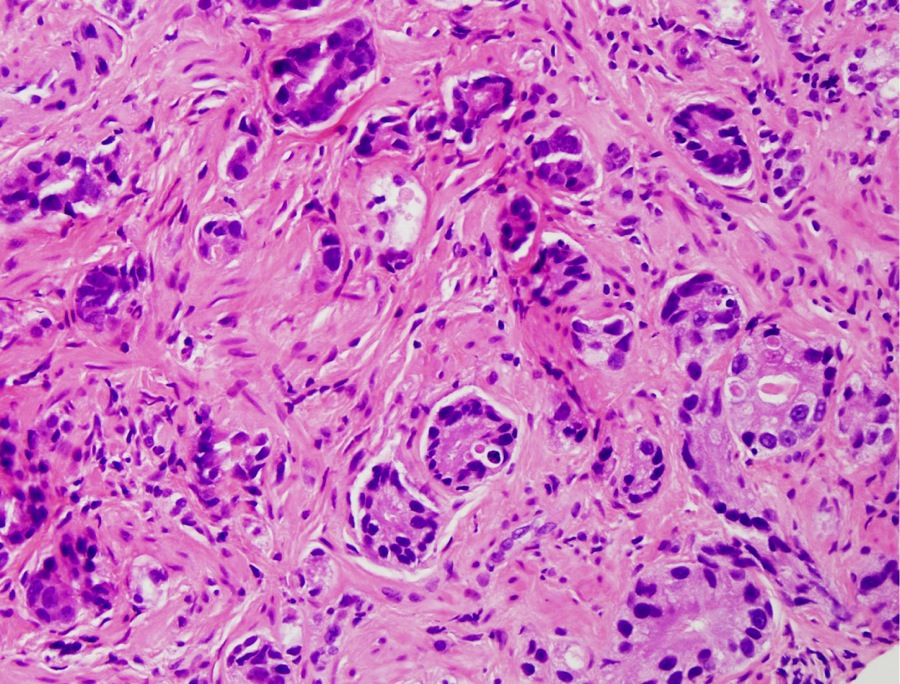
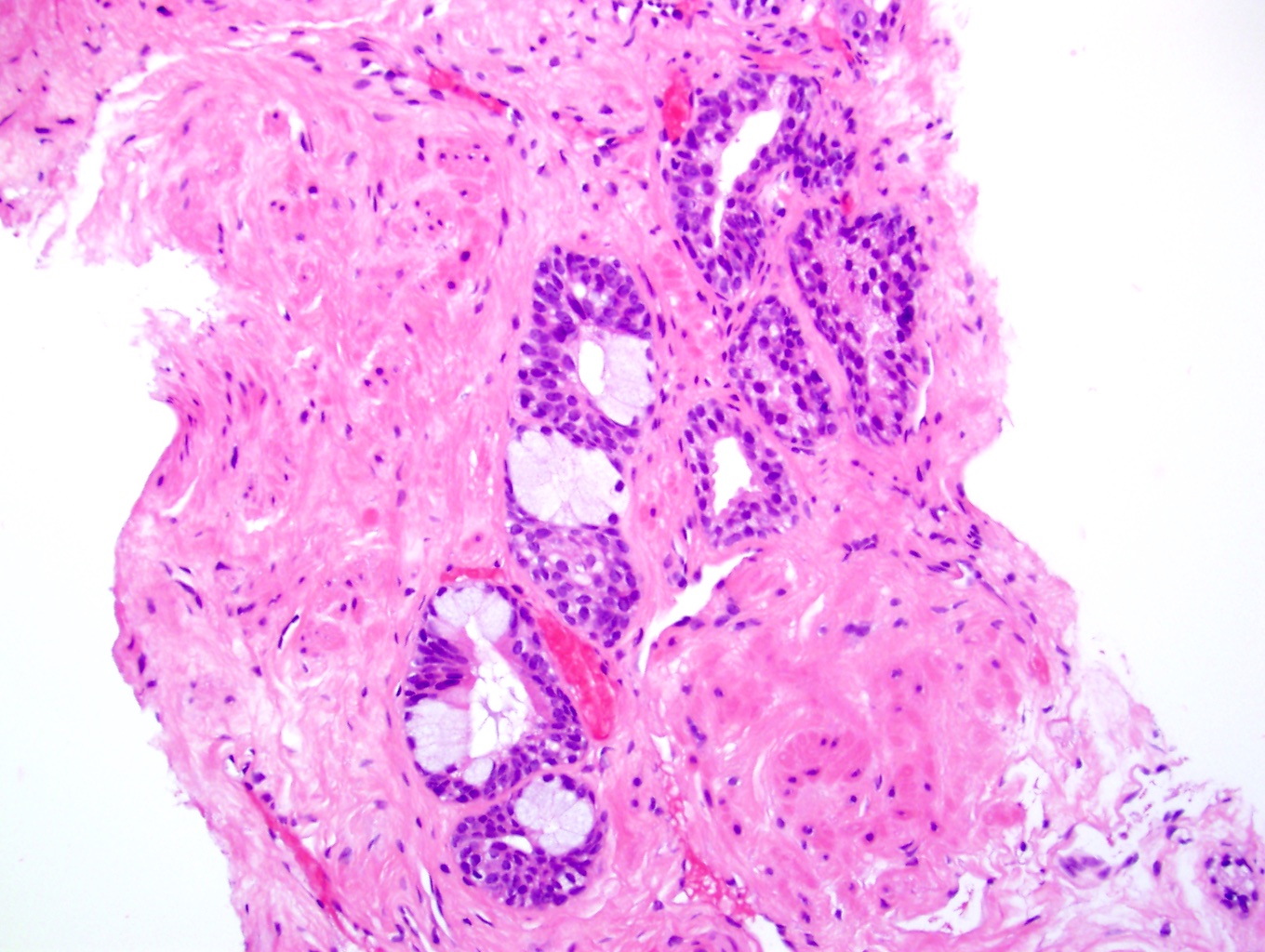
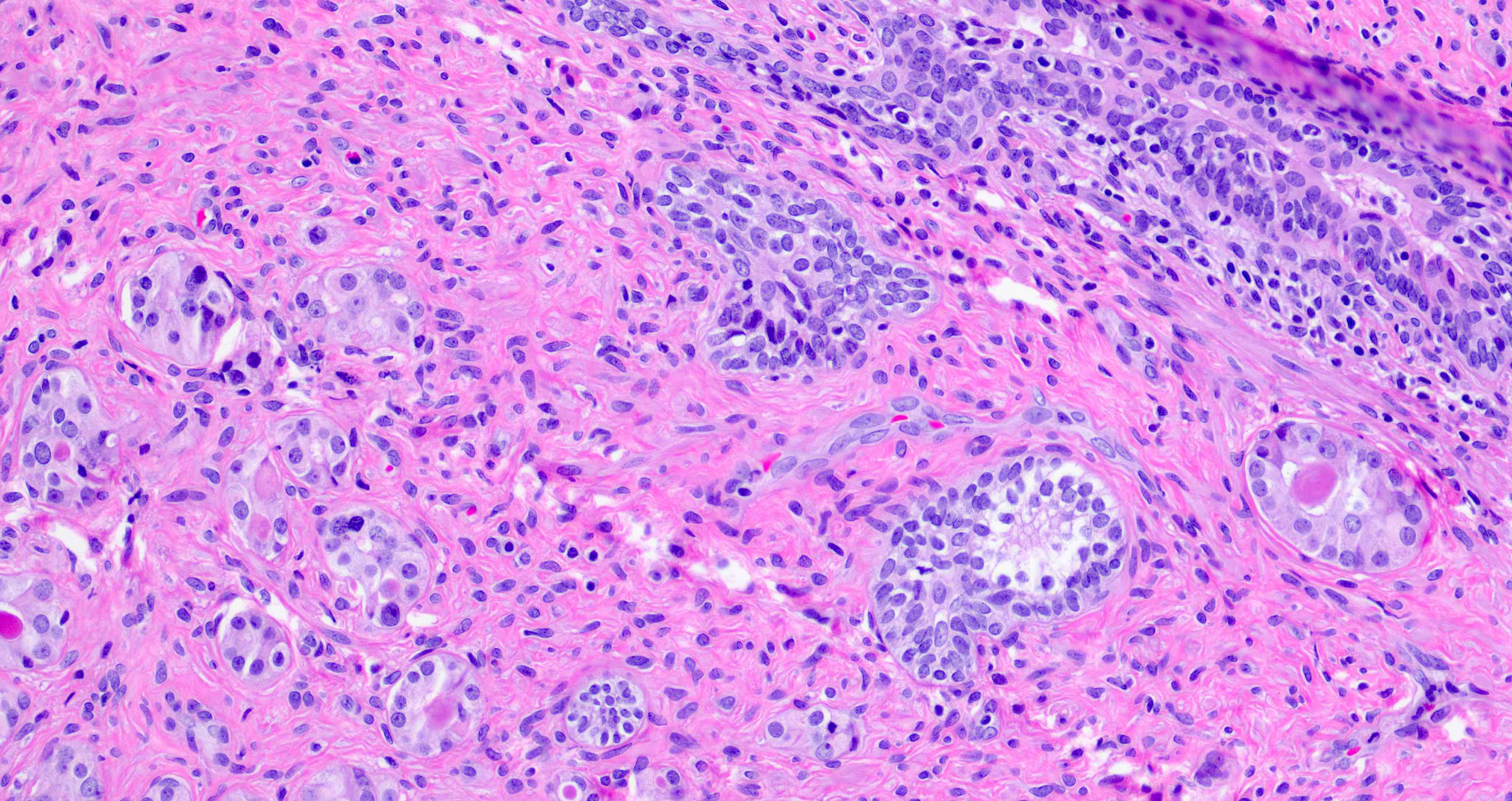
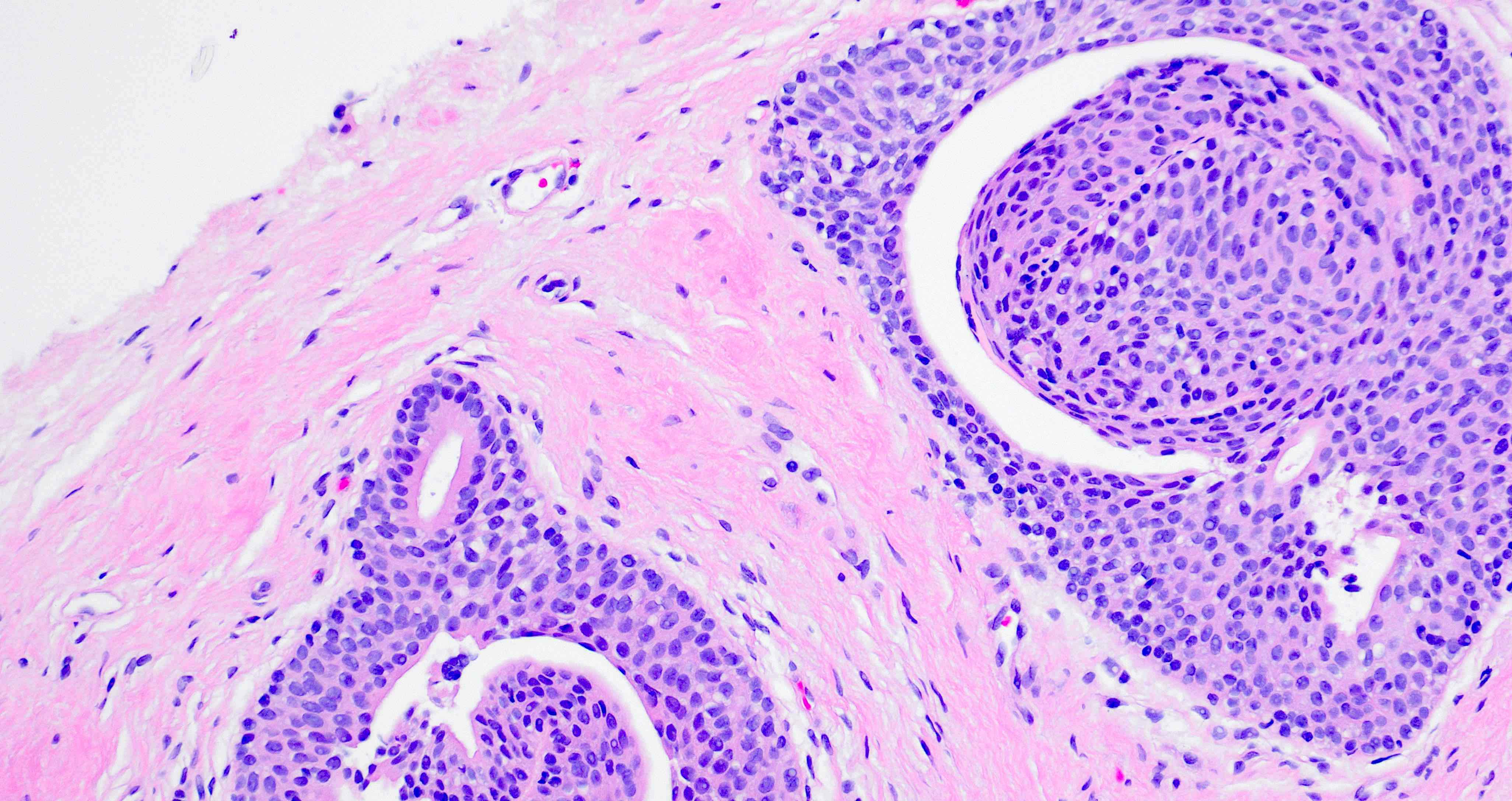
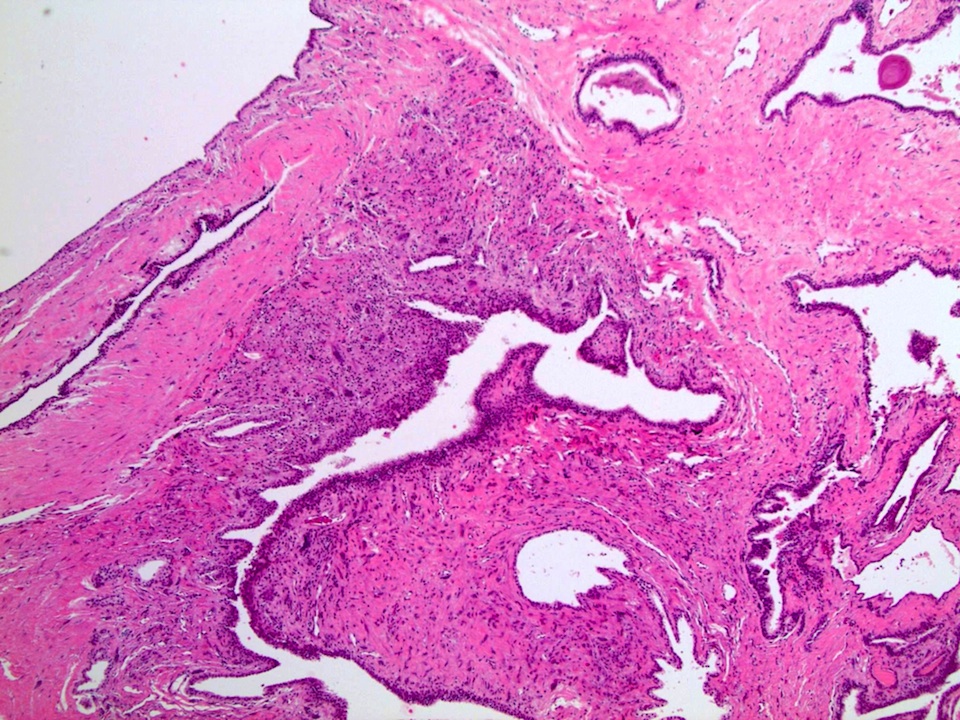
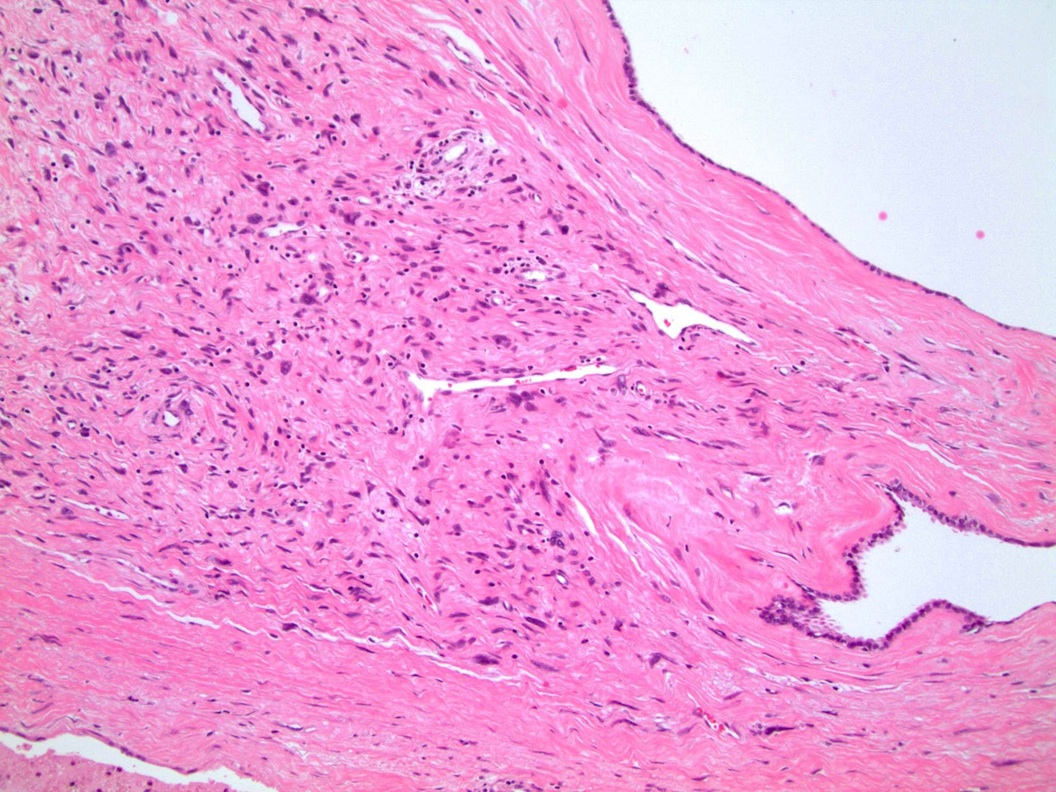
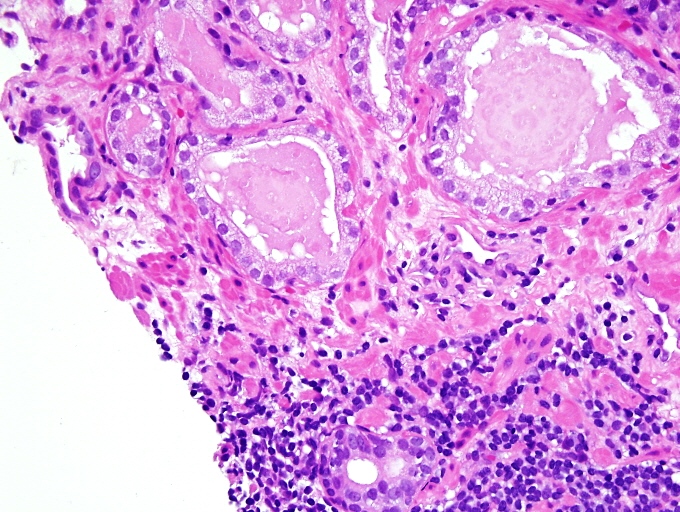
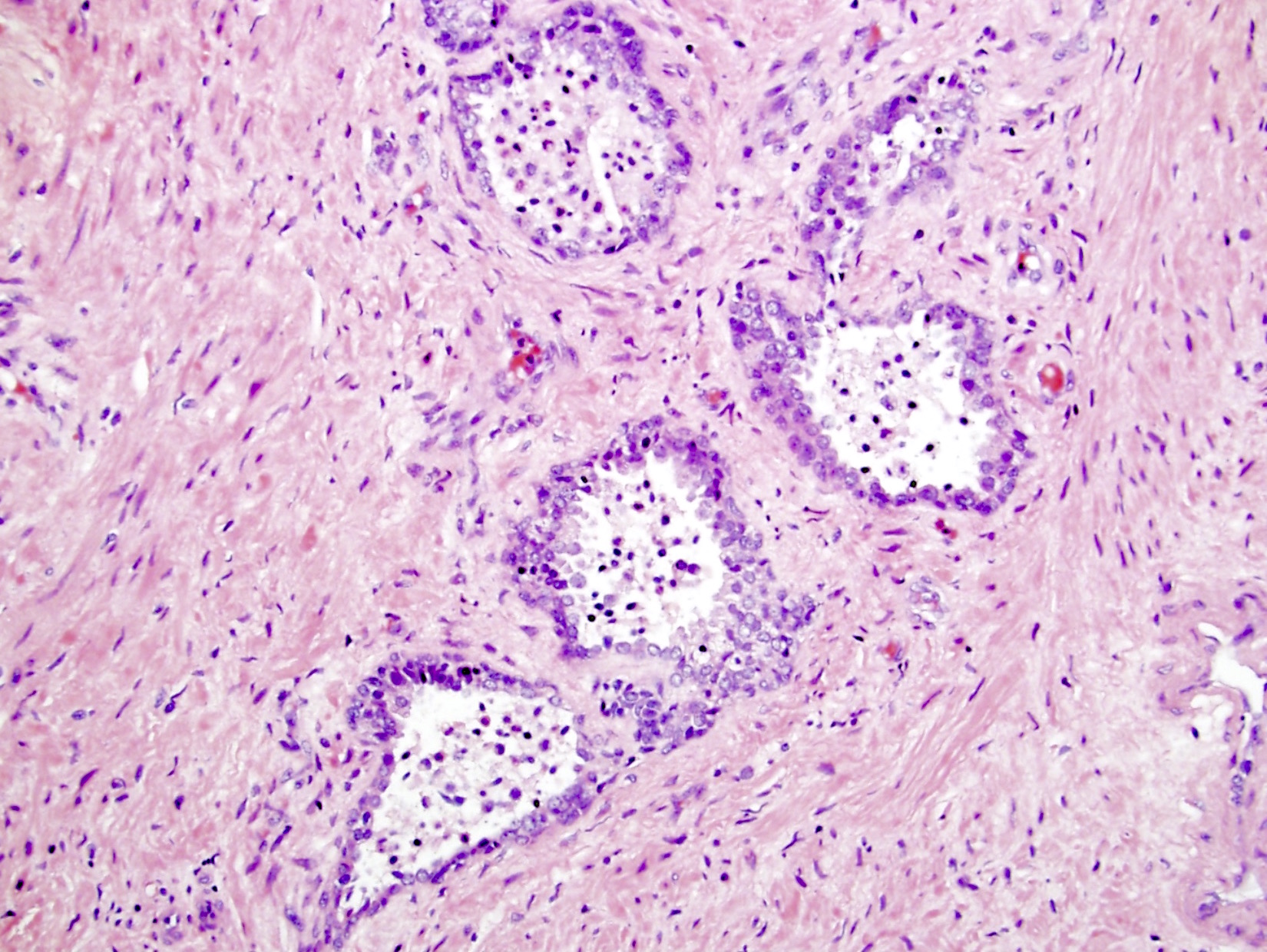
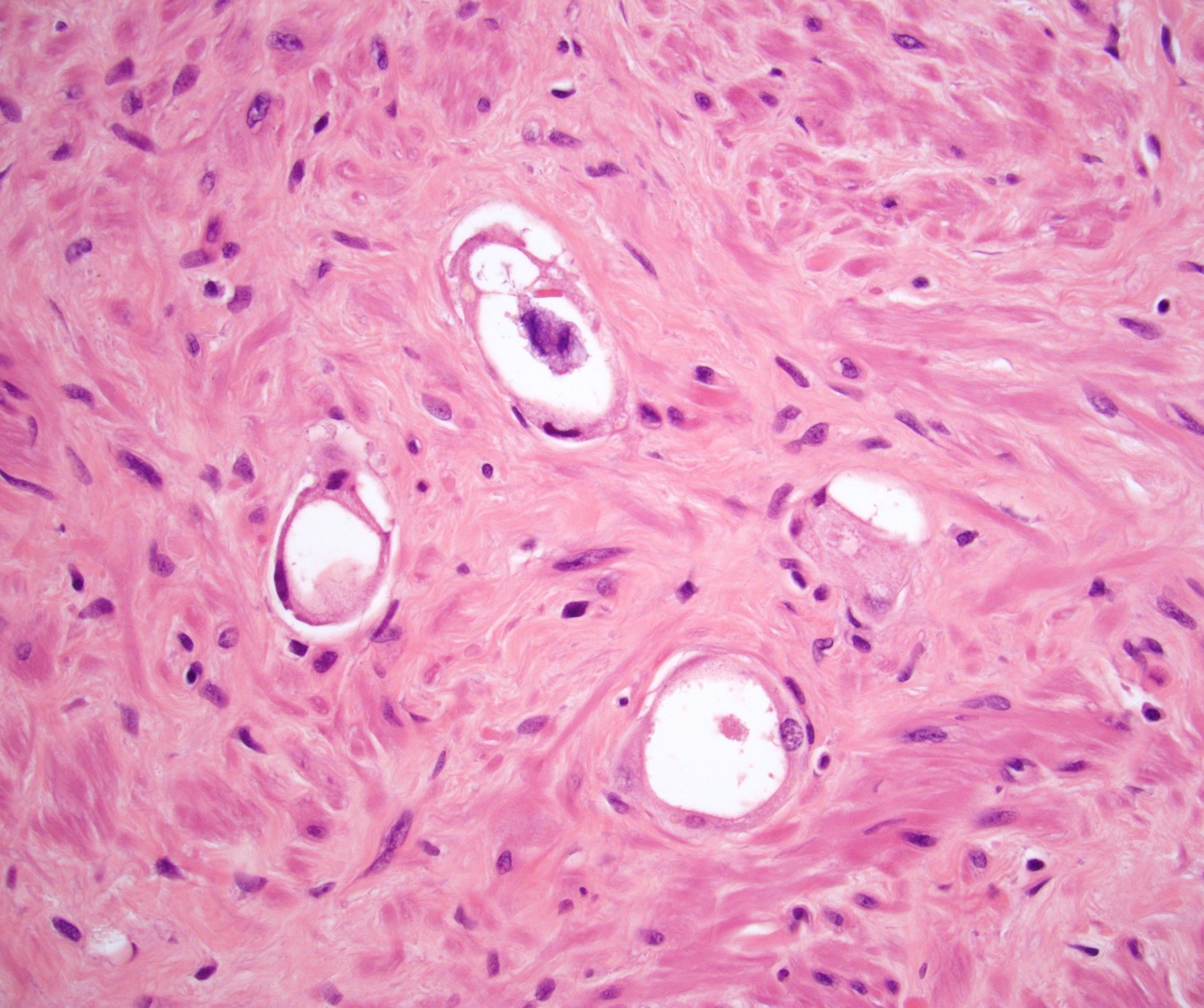
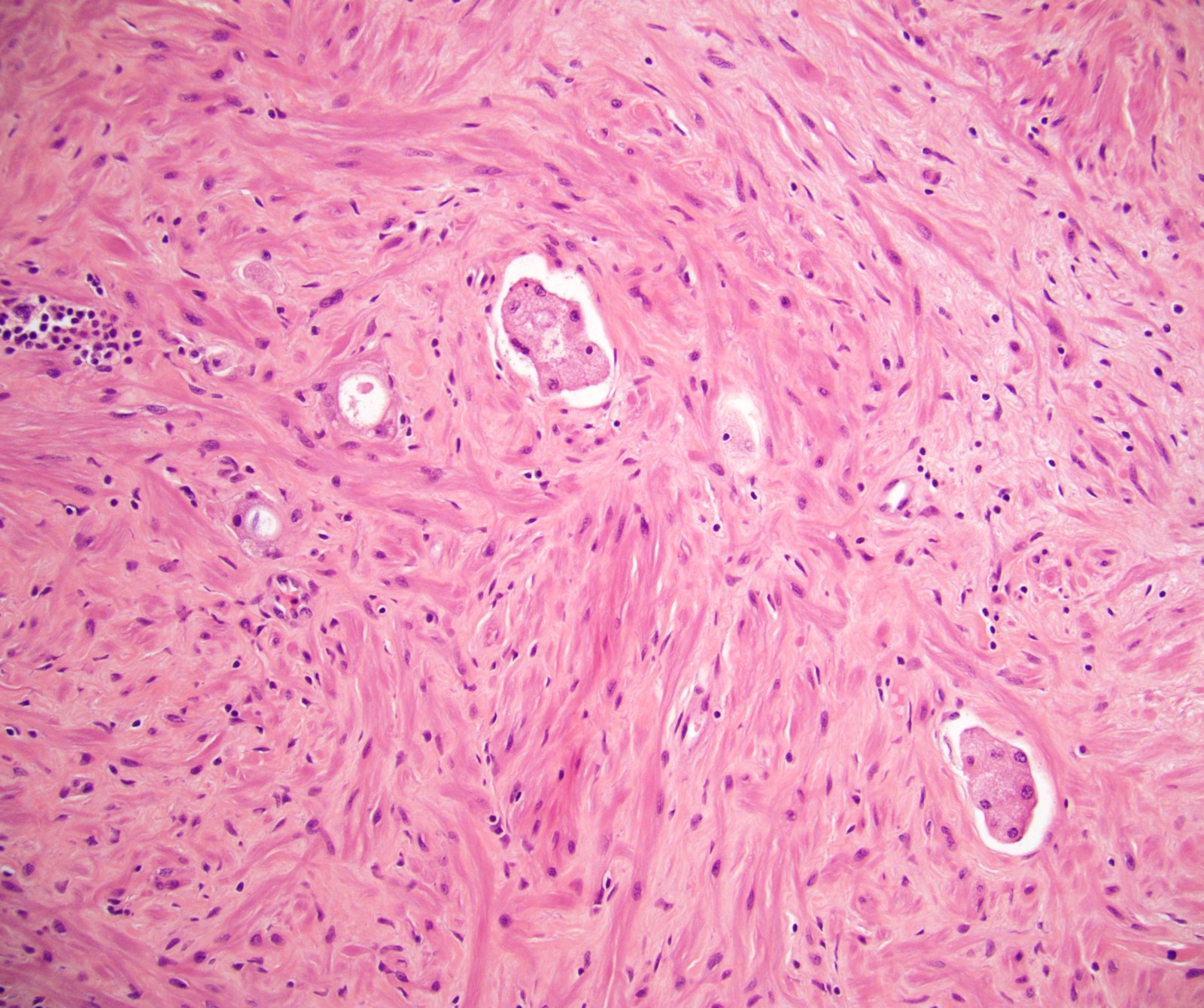
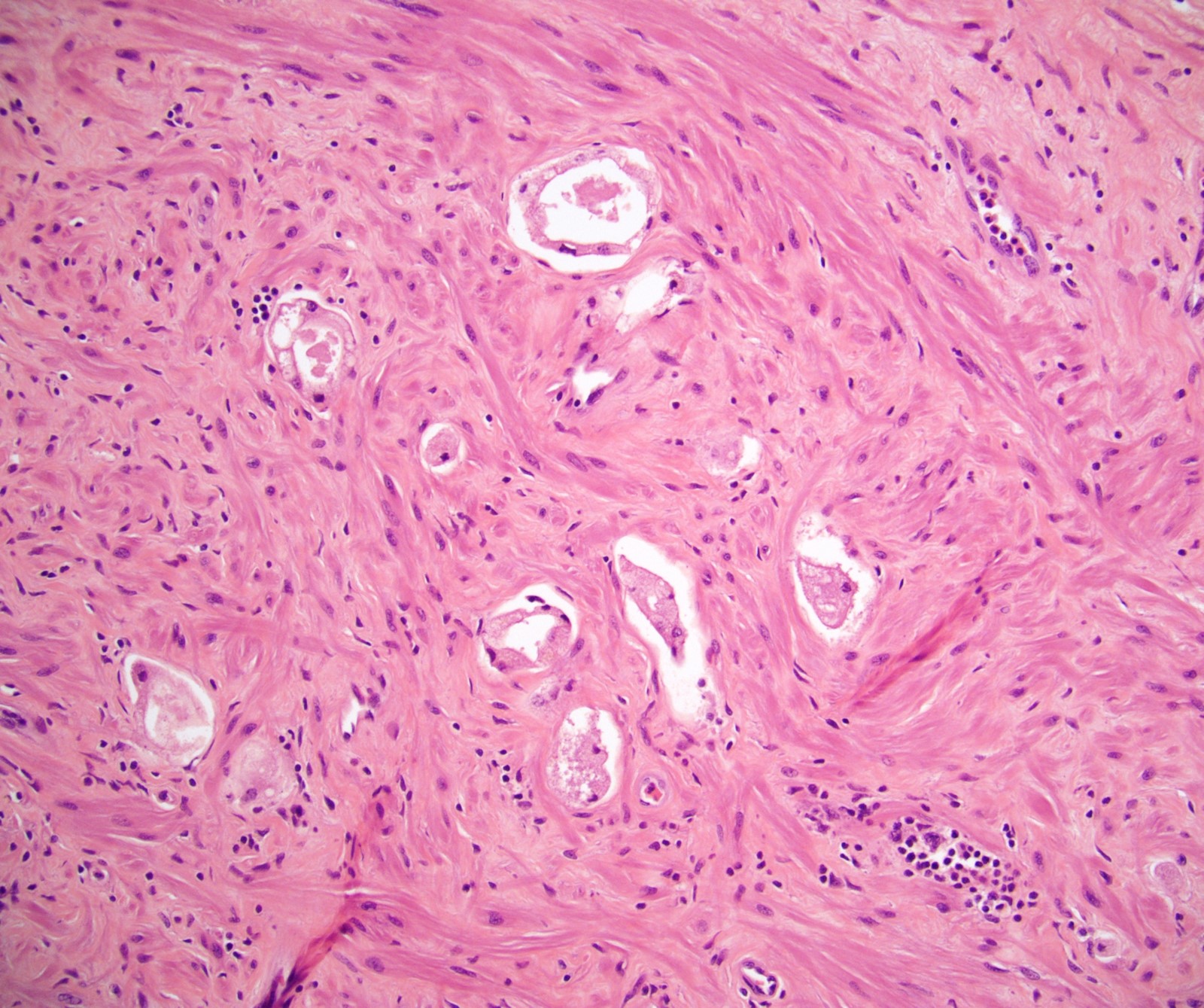
- Seminal vesicle invasion: invasion of the extraprostatic seminal vesicle muscular wall