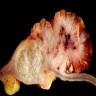
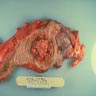
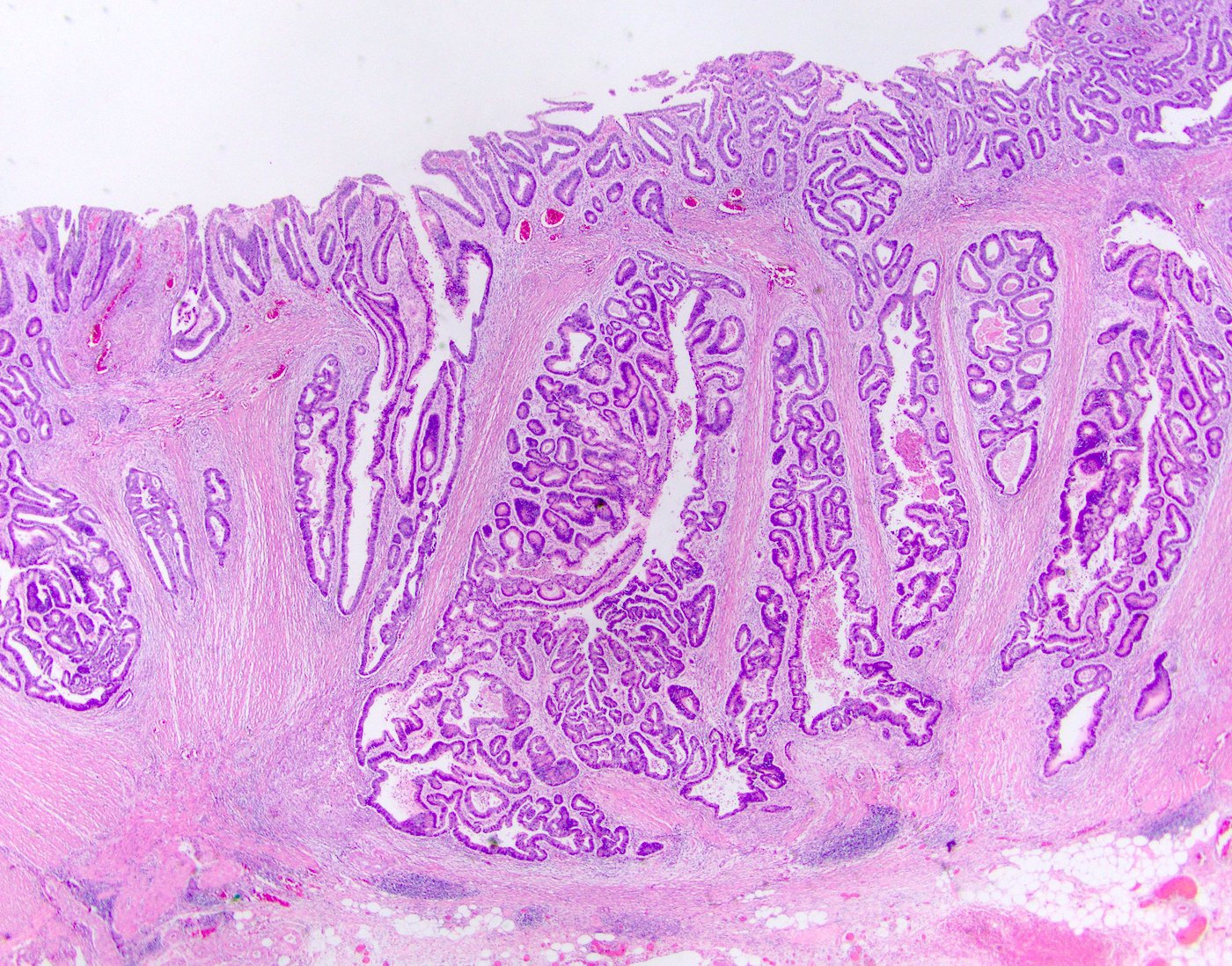
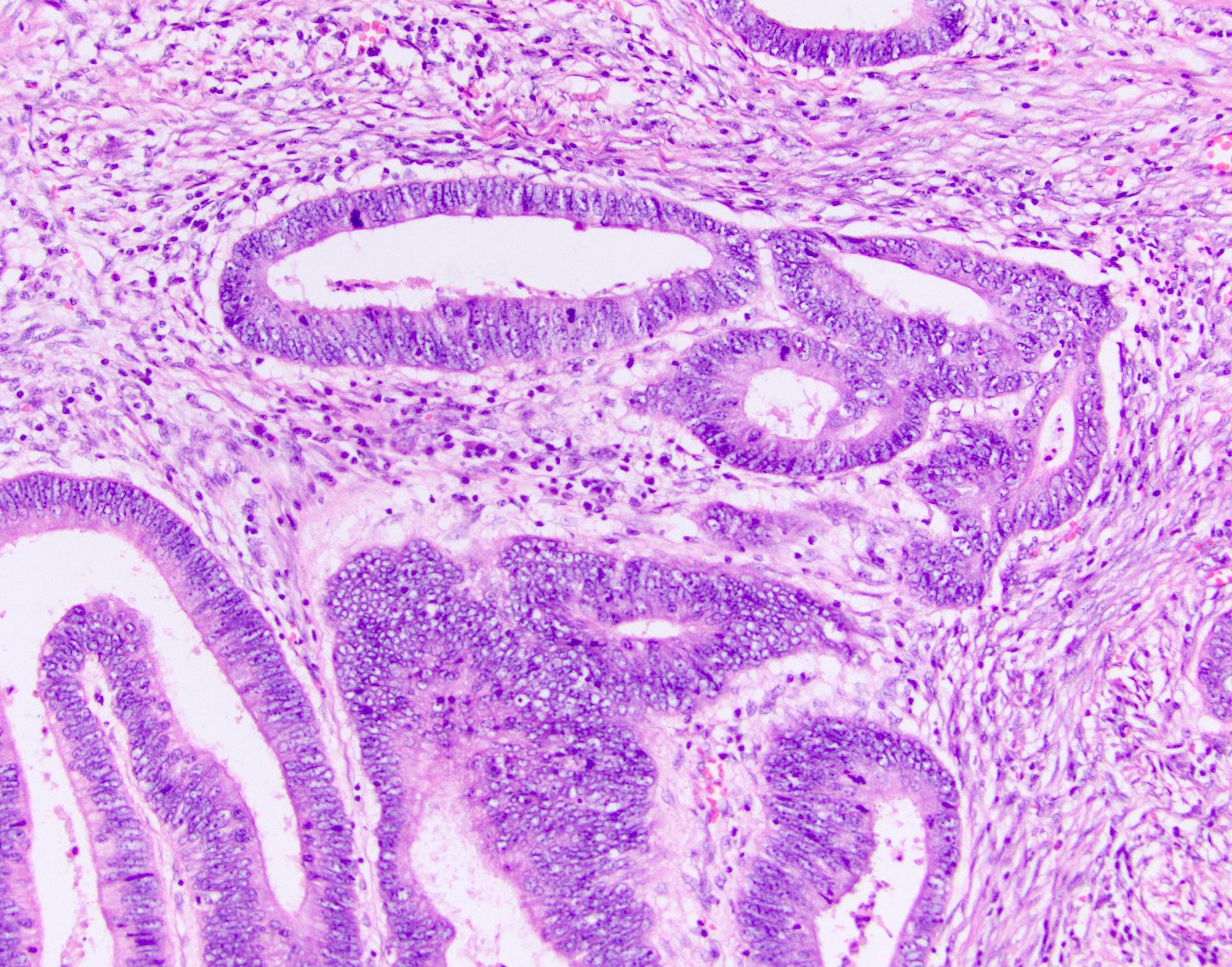
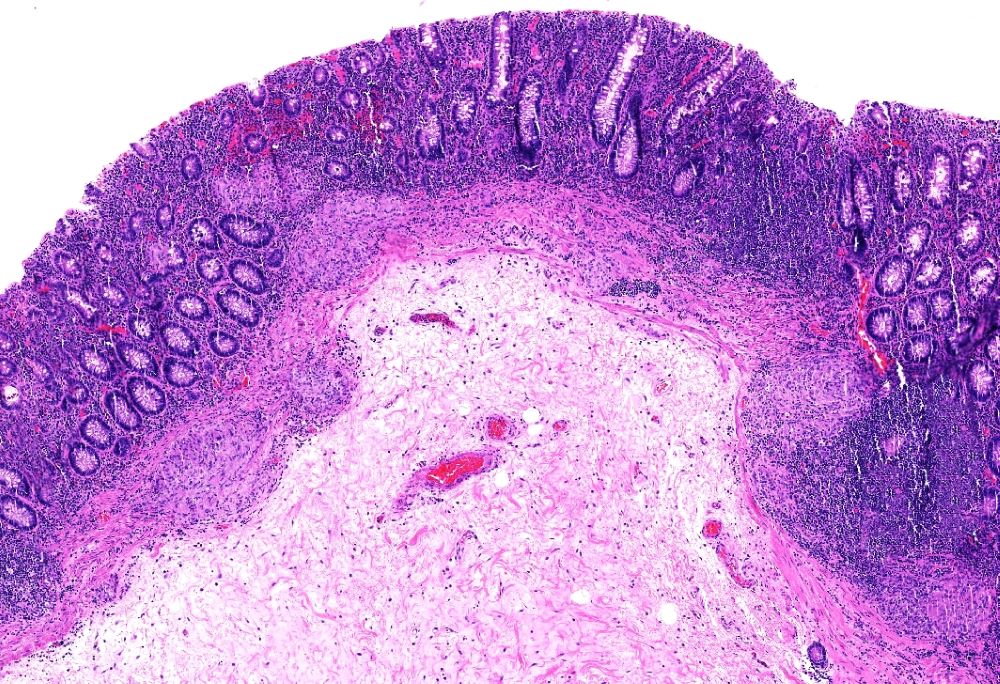
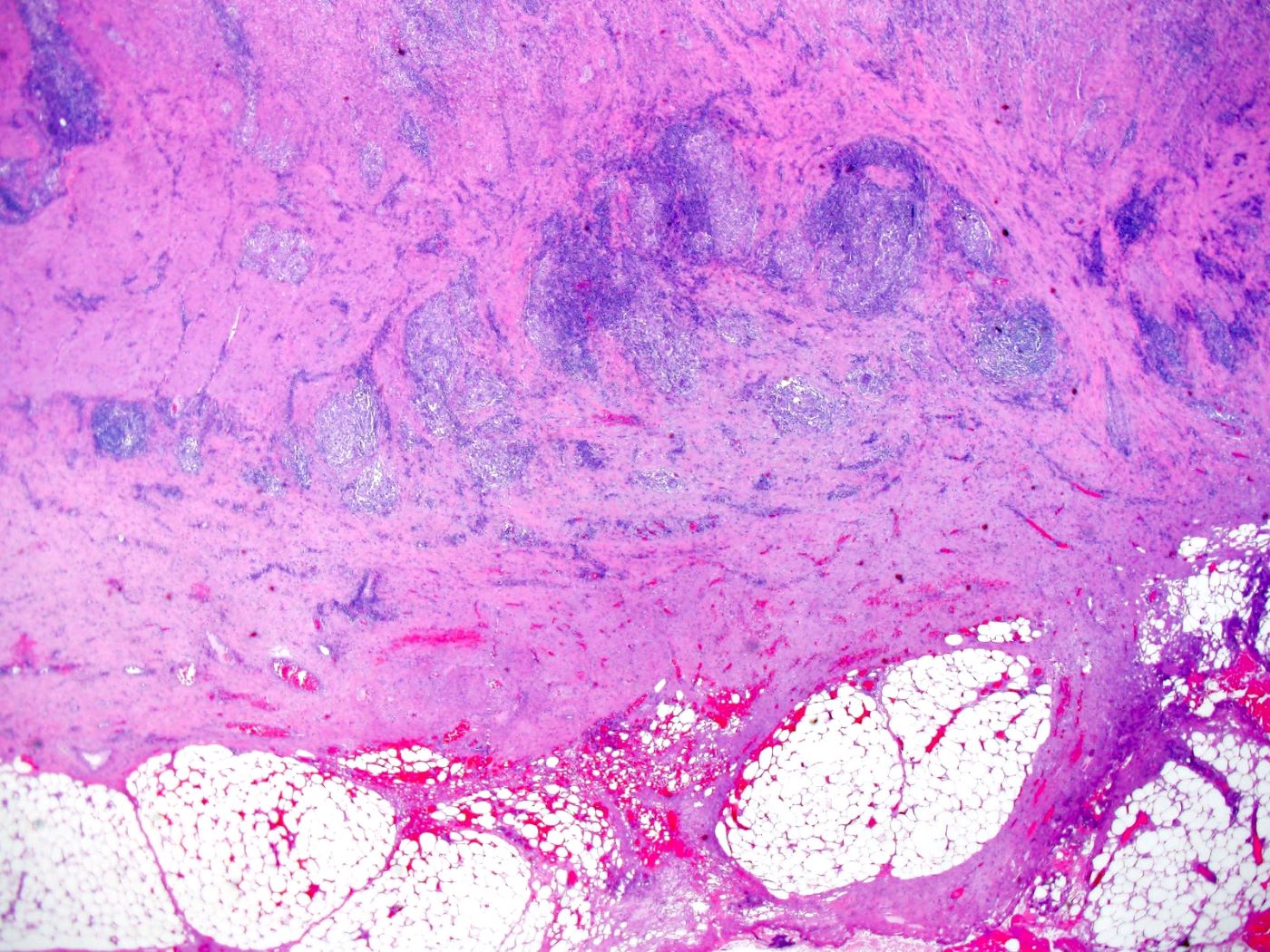
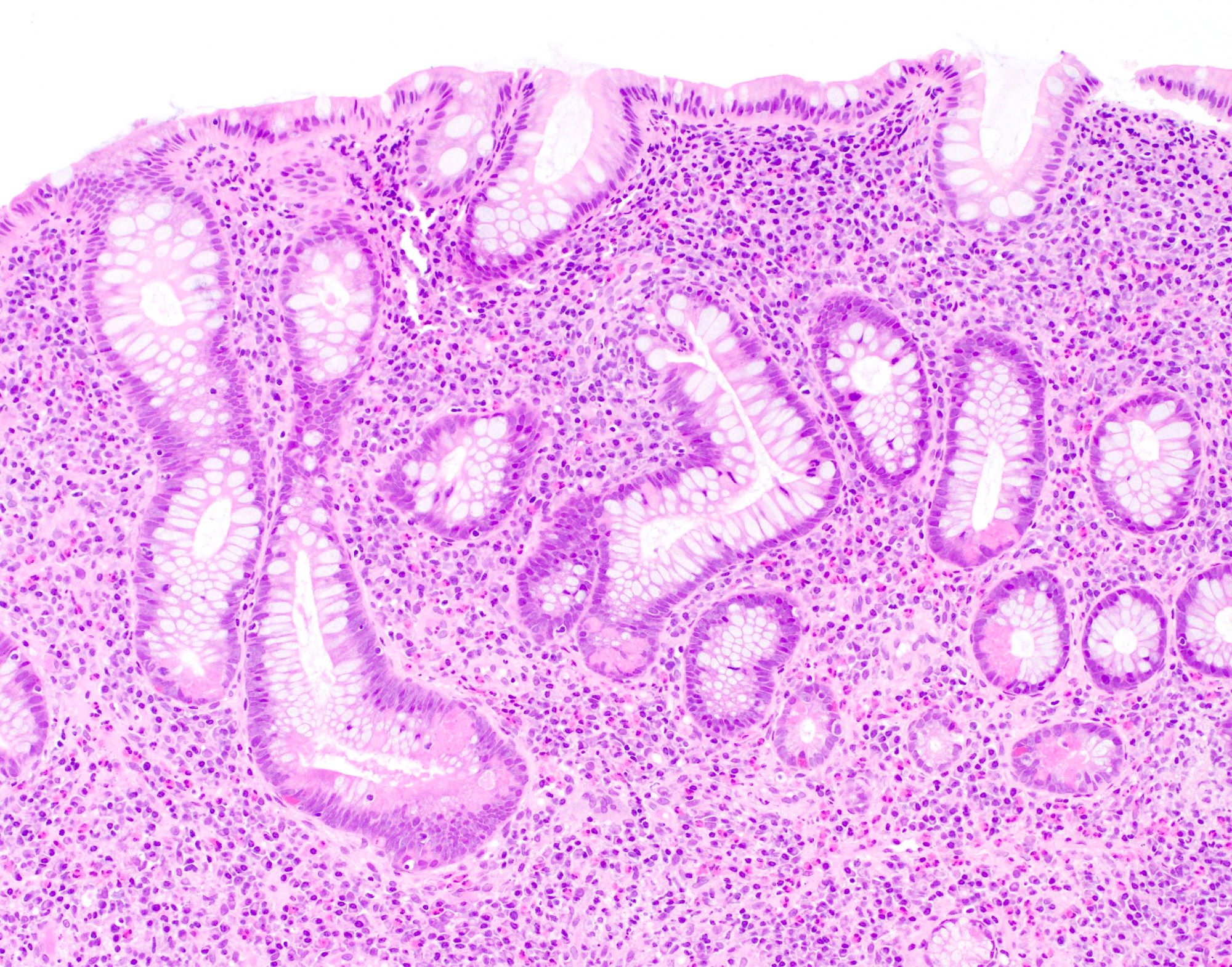
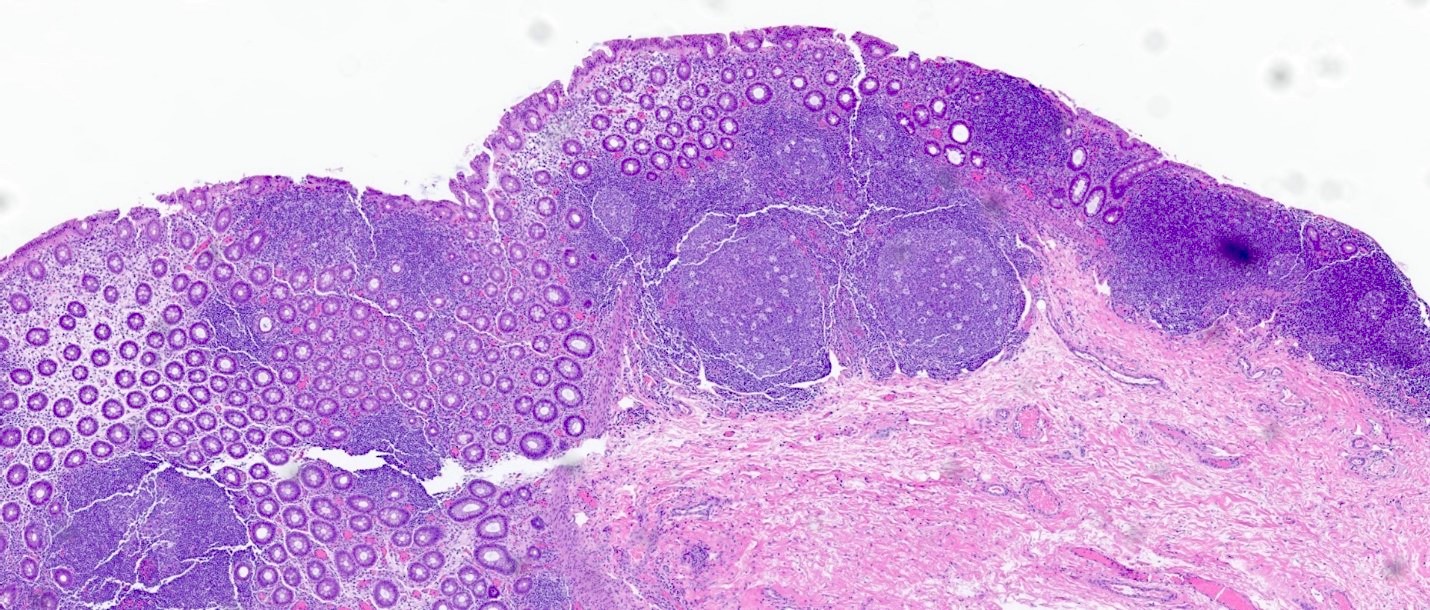
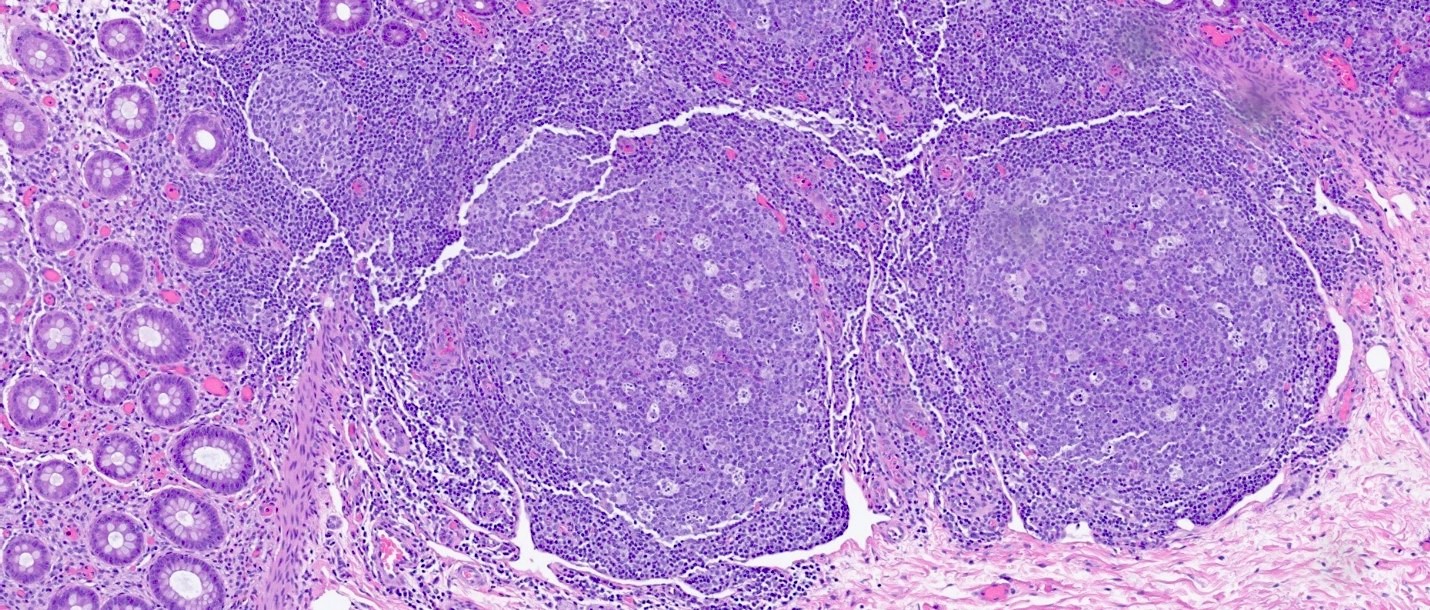
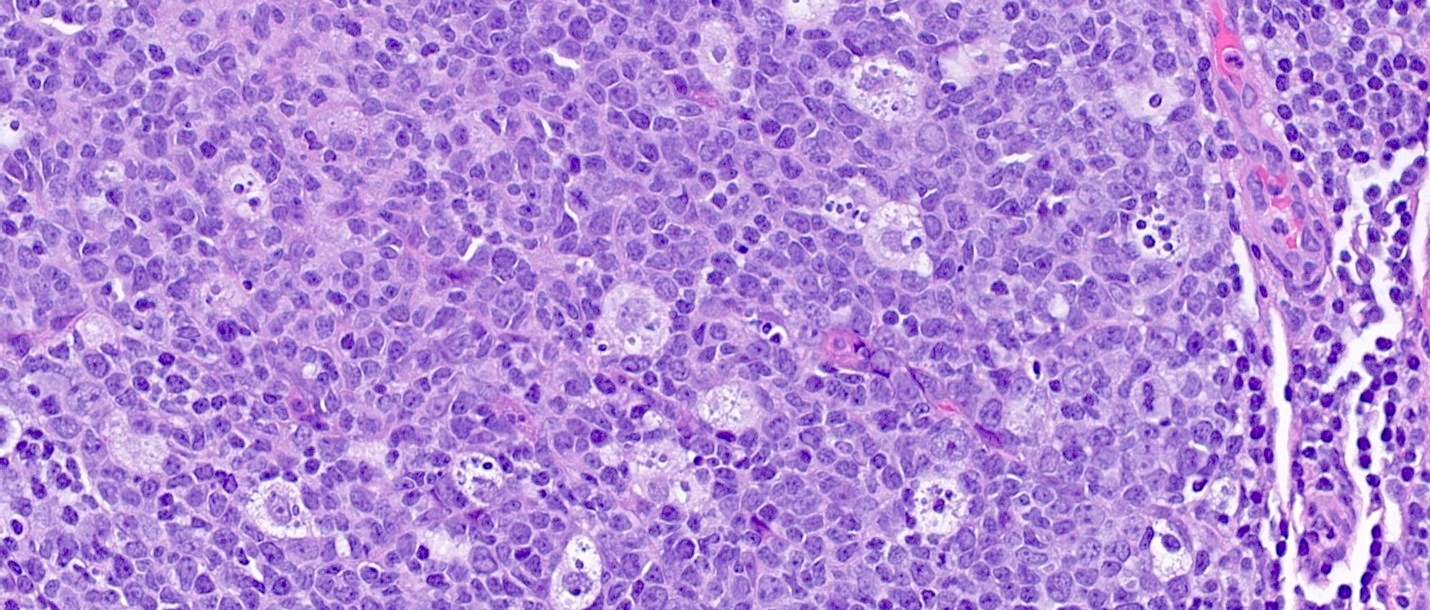
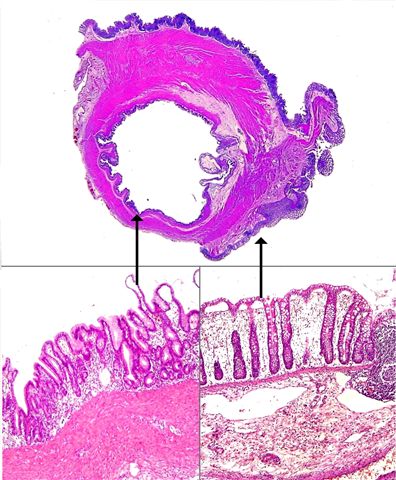
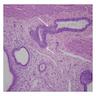
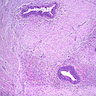
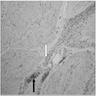
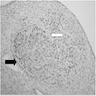
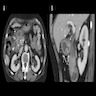
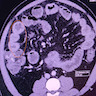
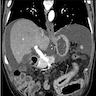
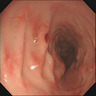
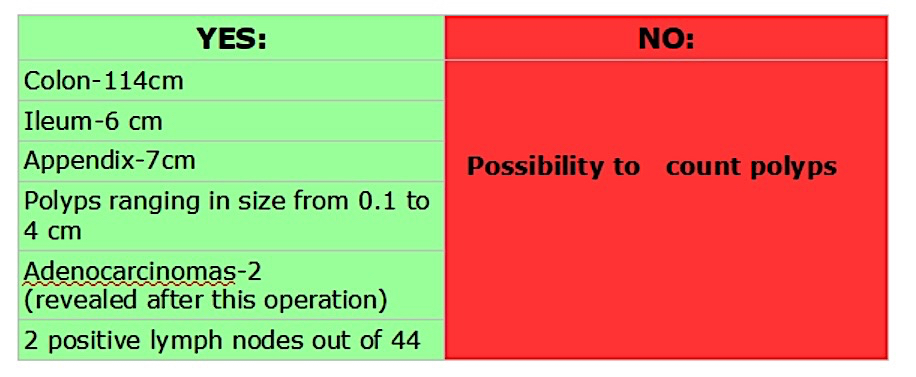
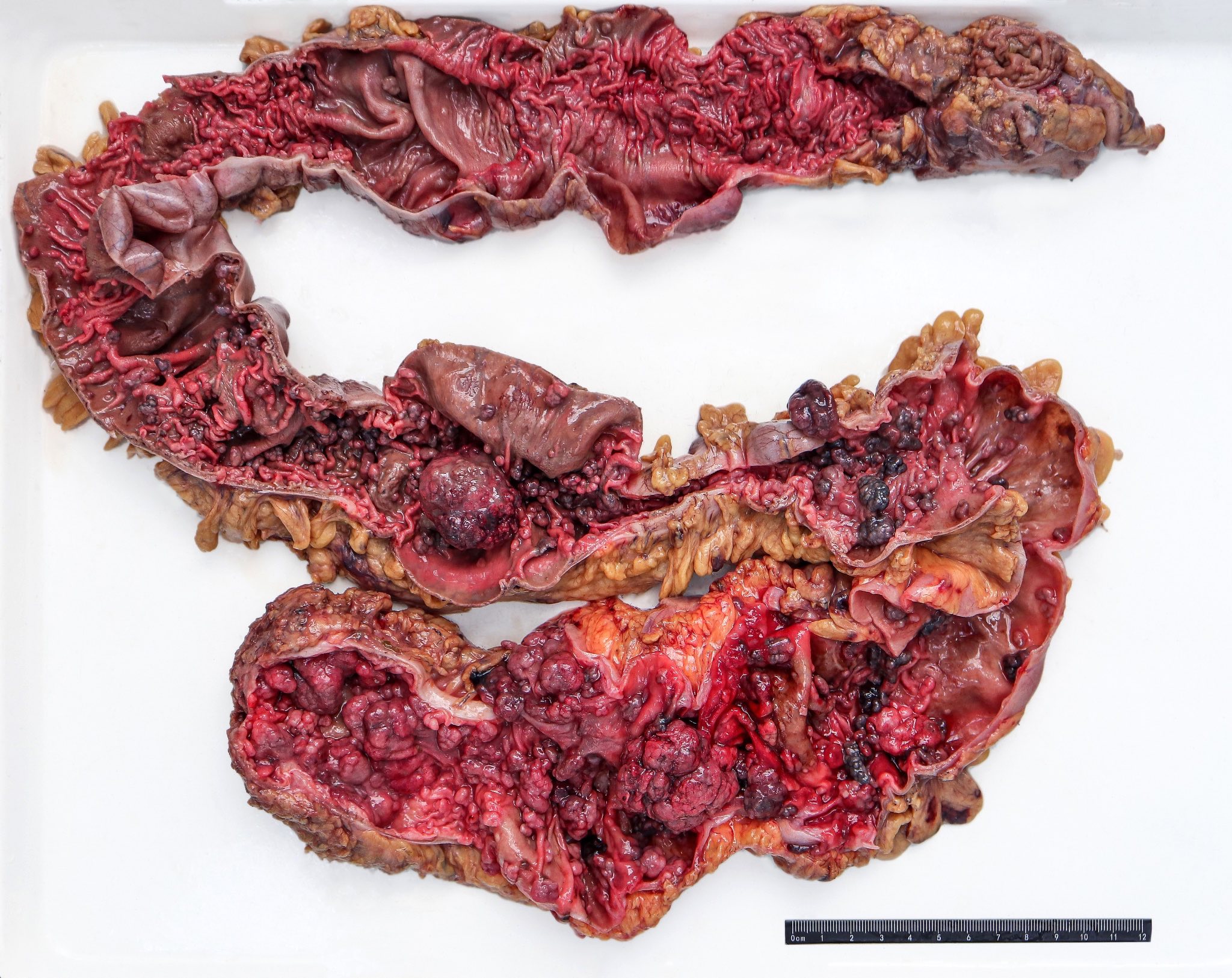
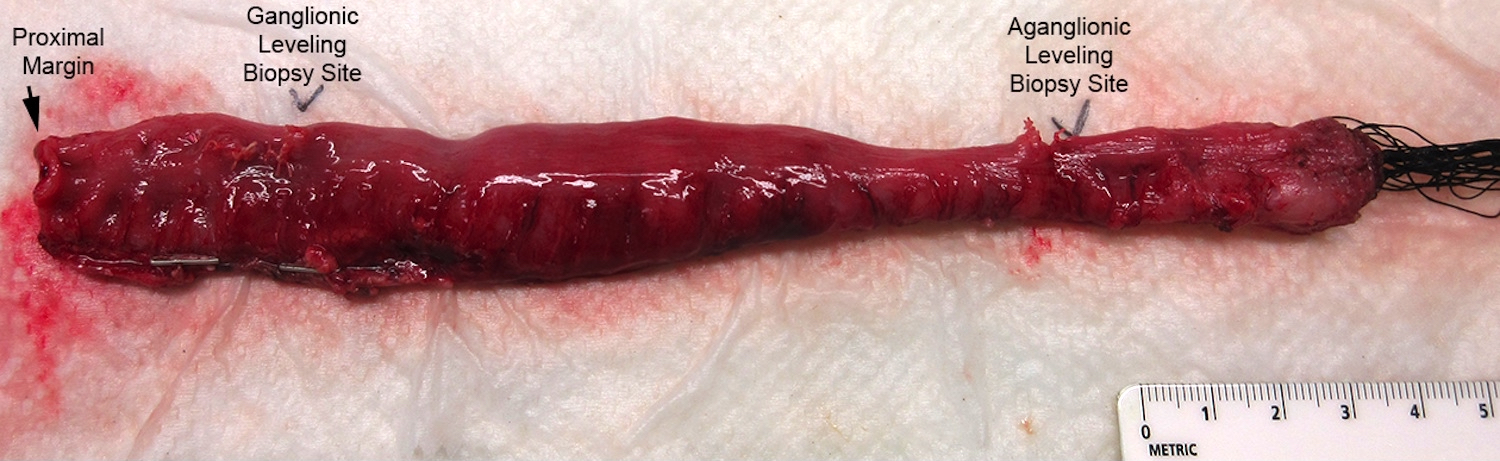
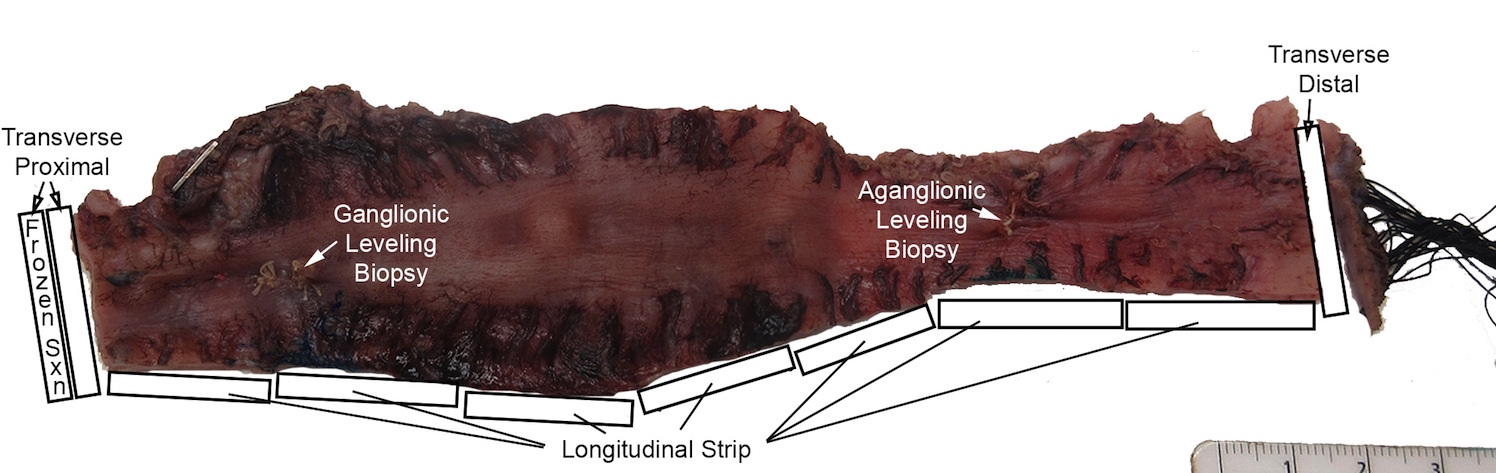
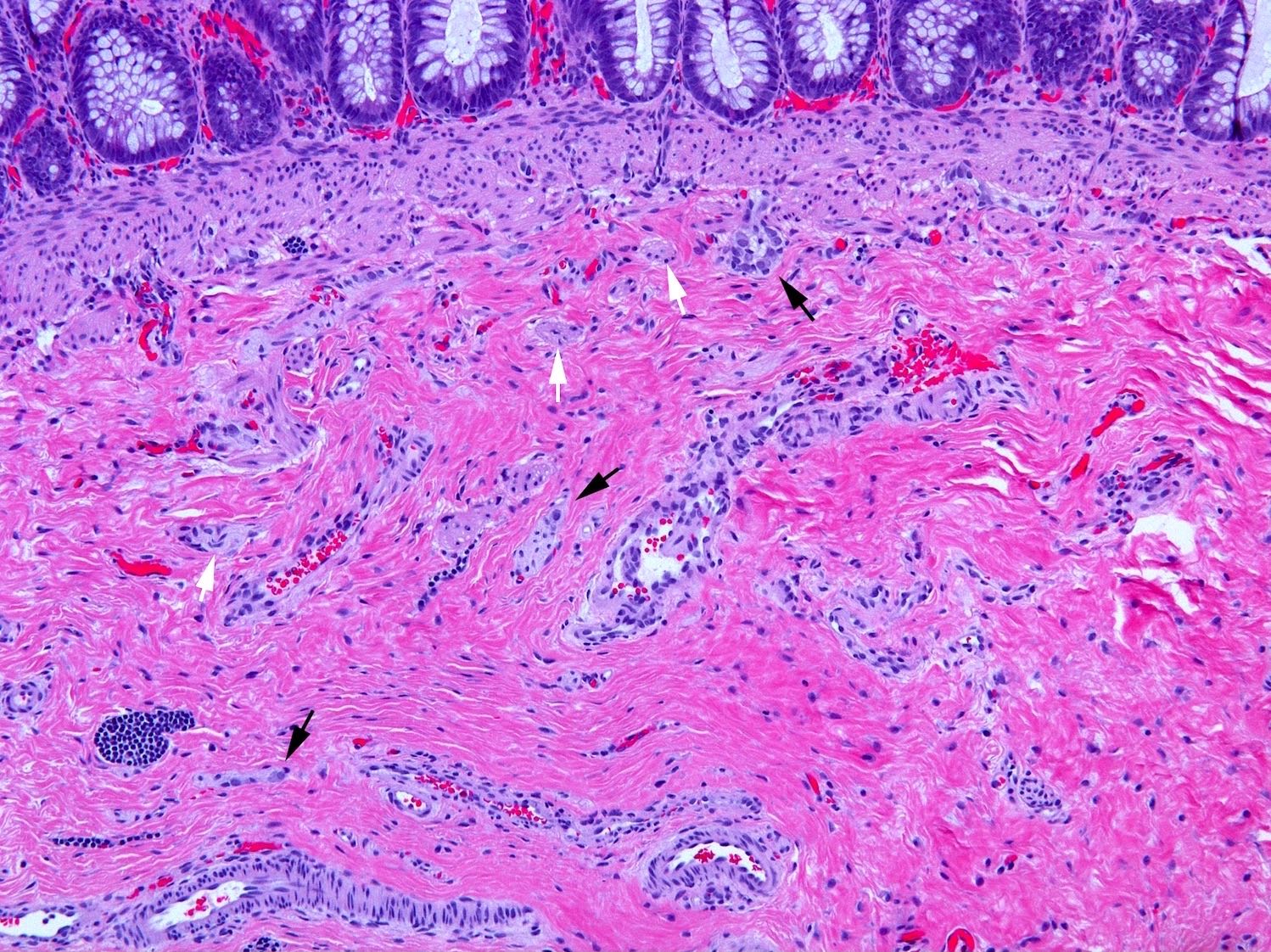
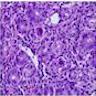
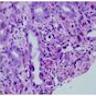
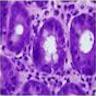
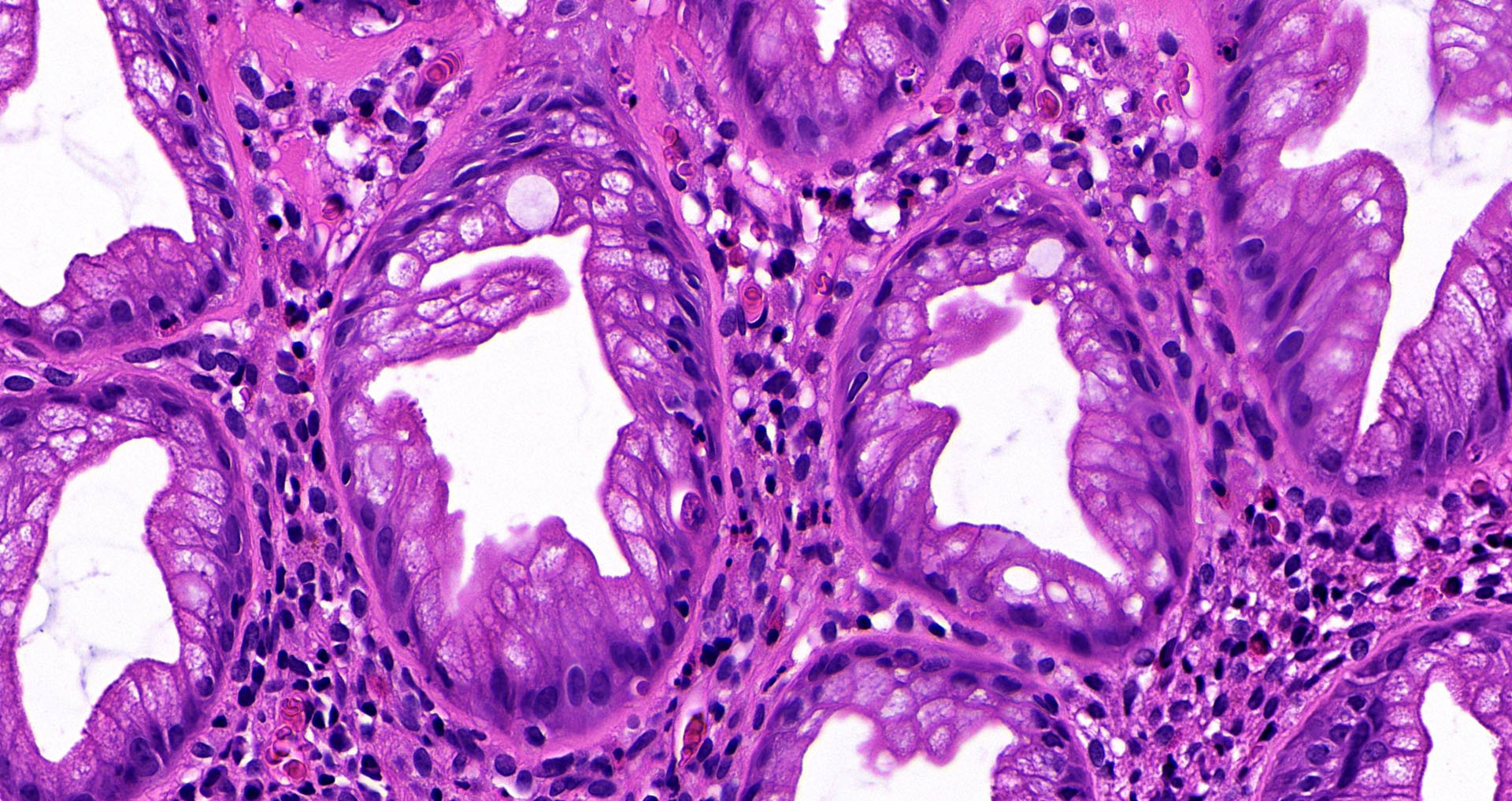
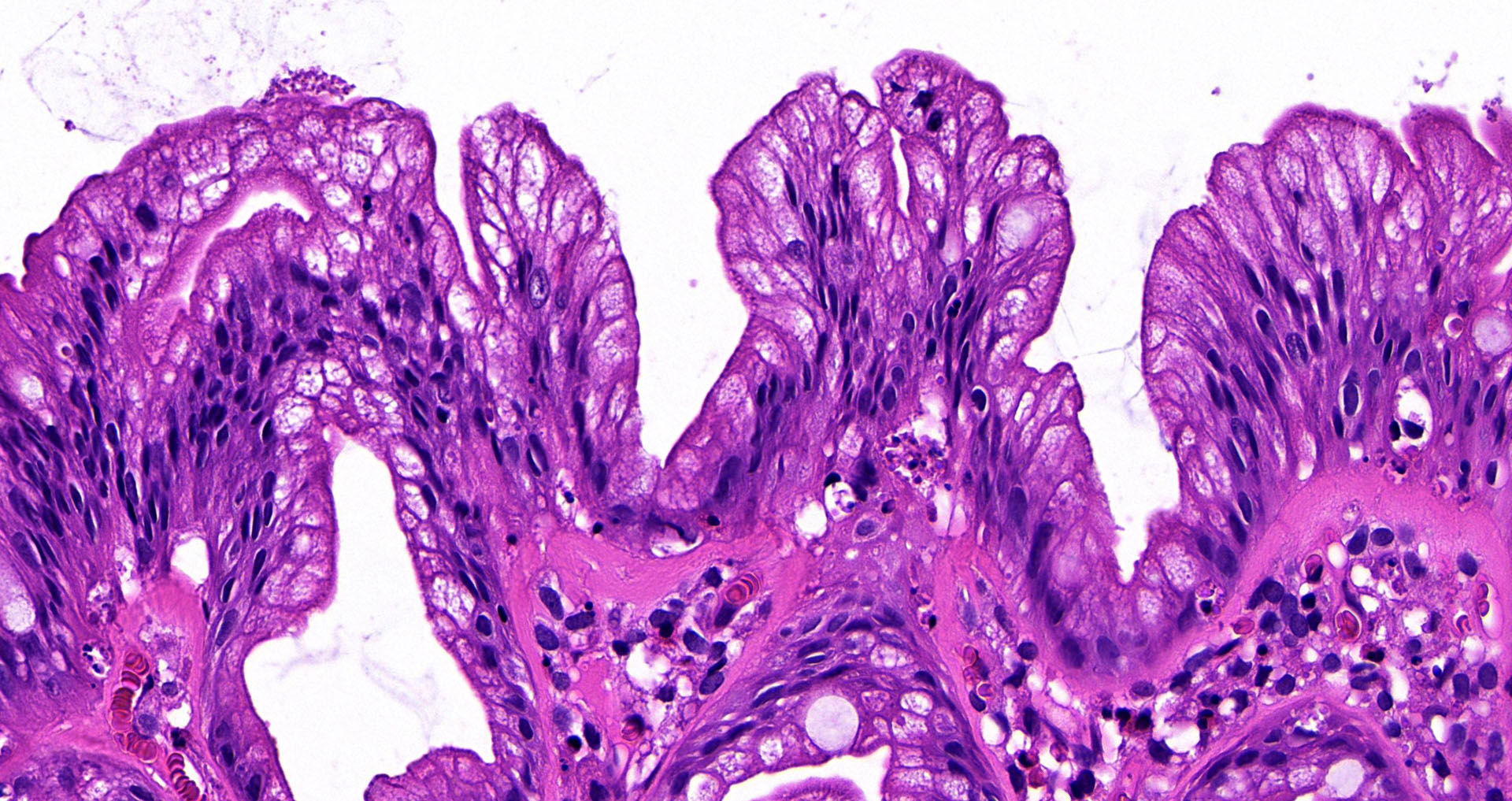
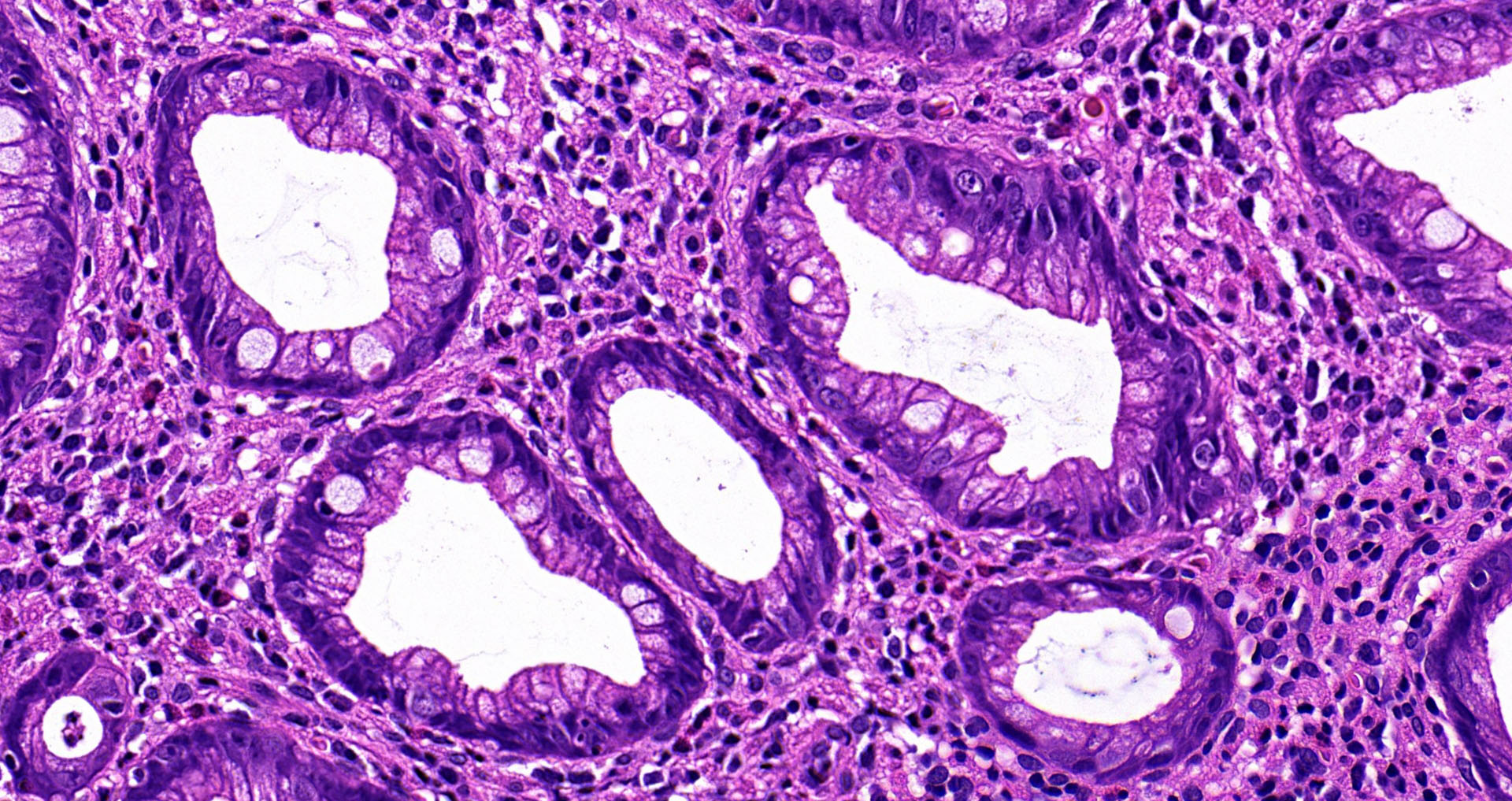
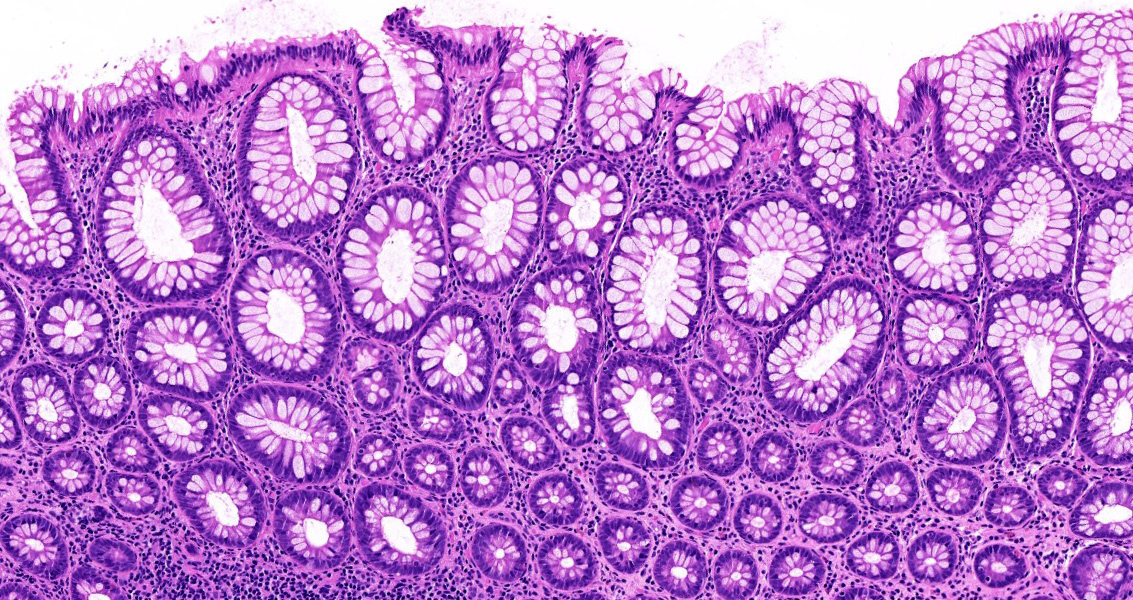
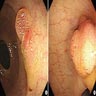
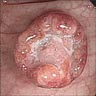
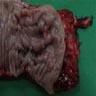
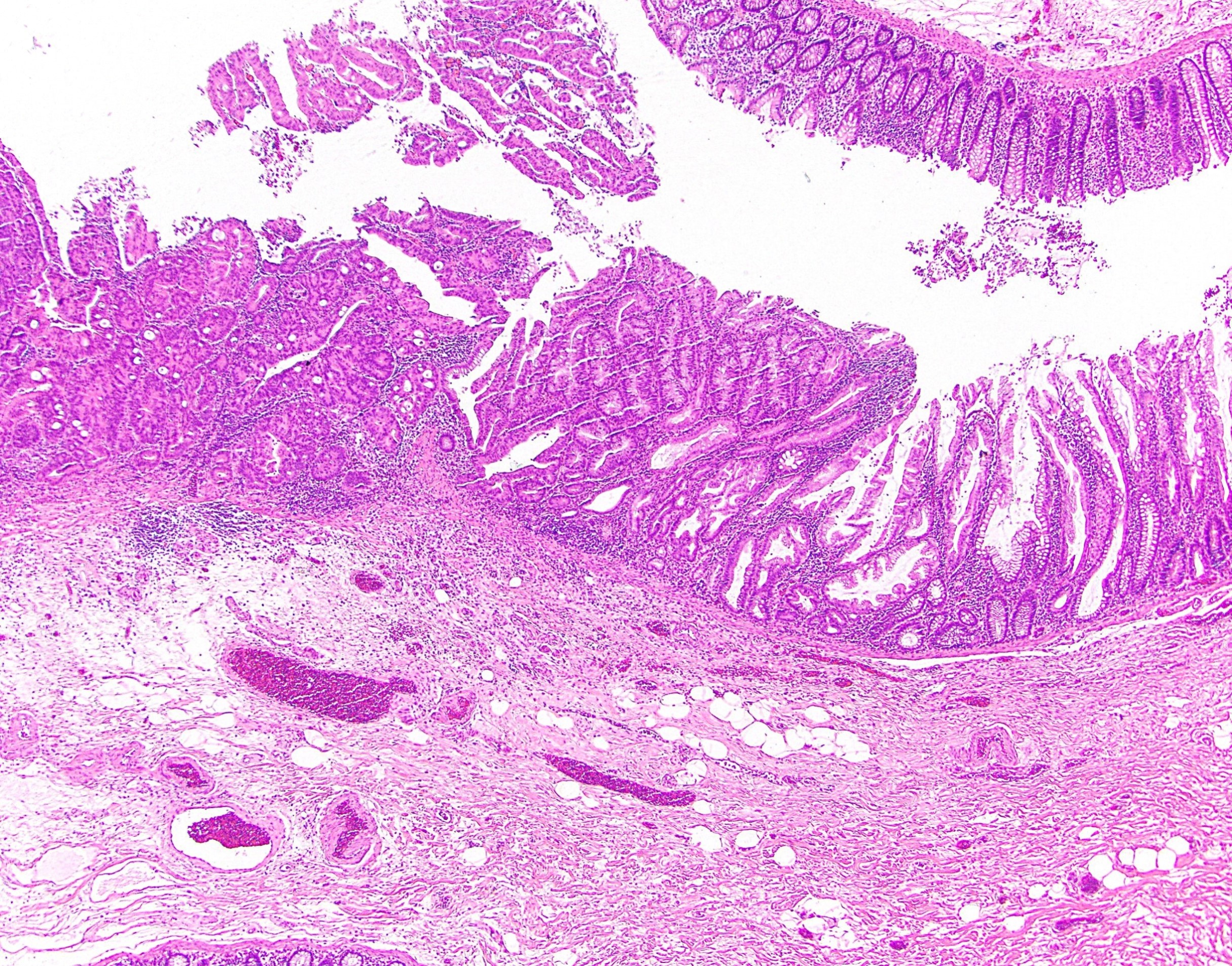
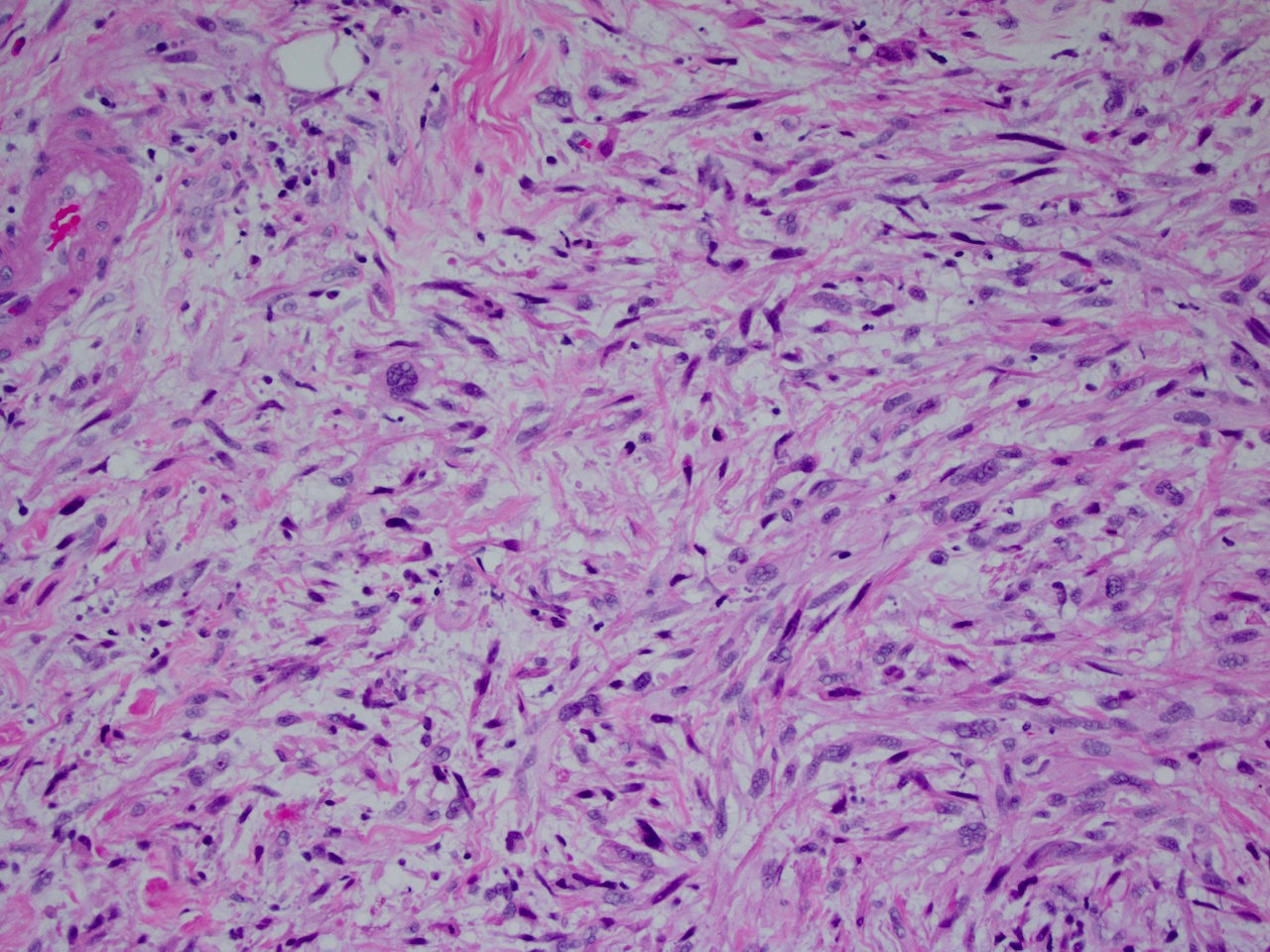
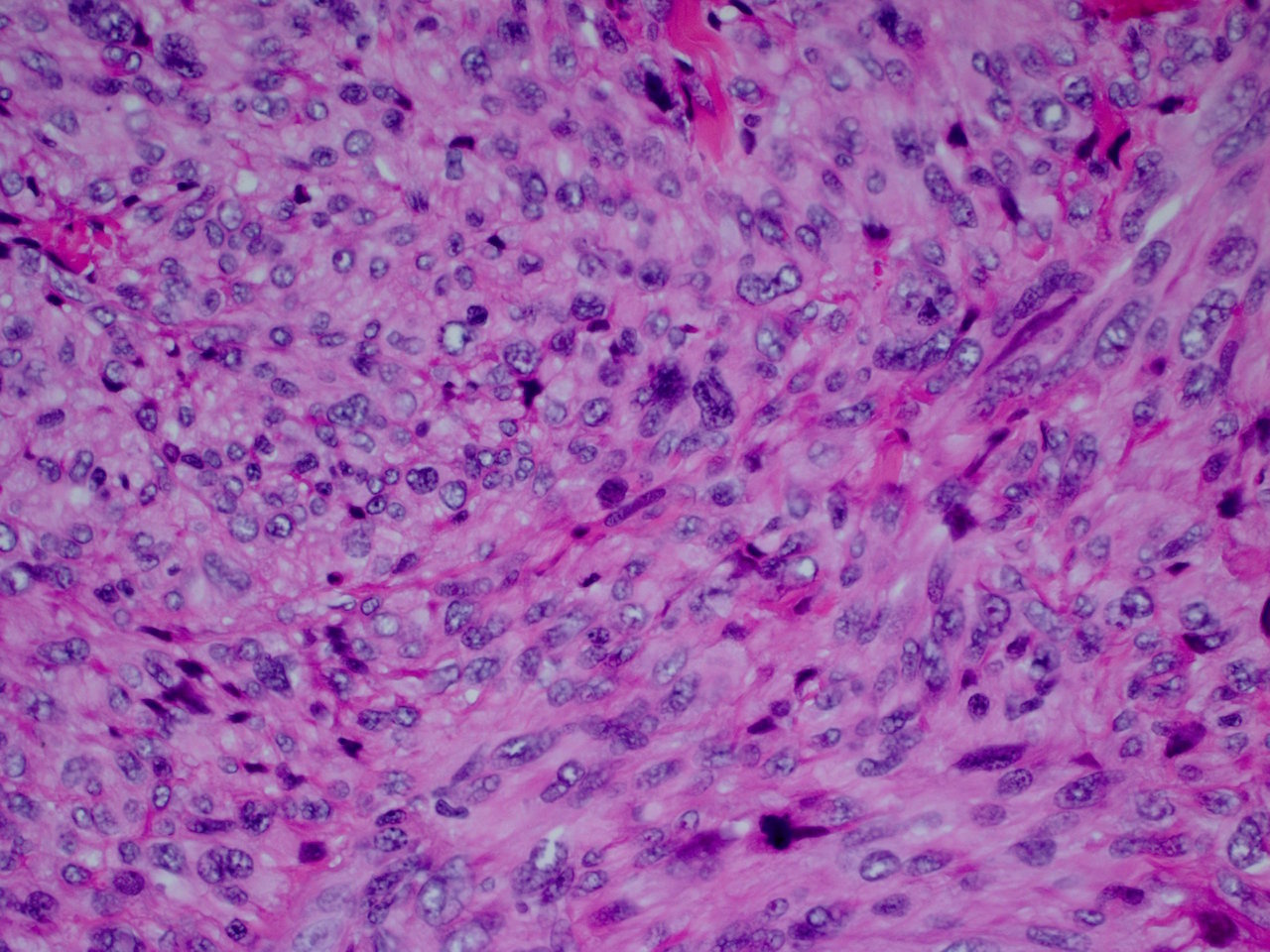
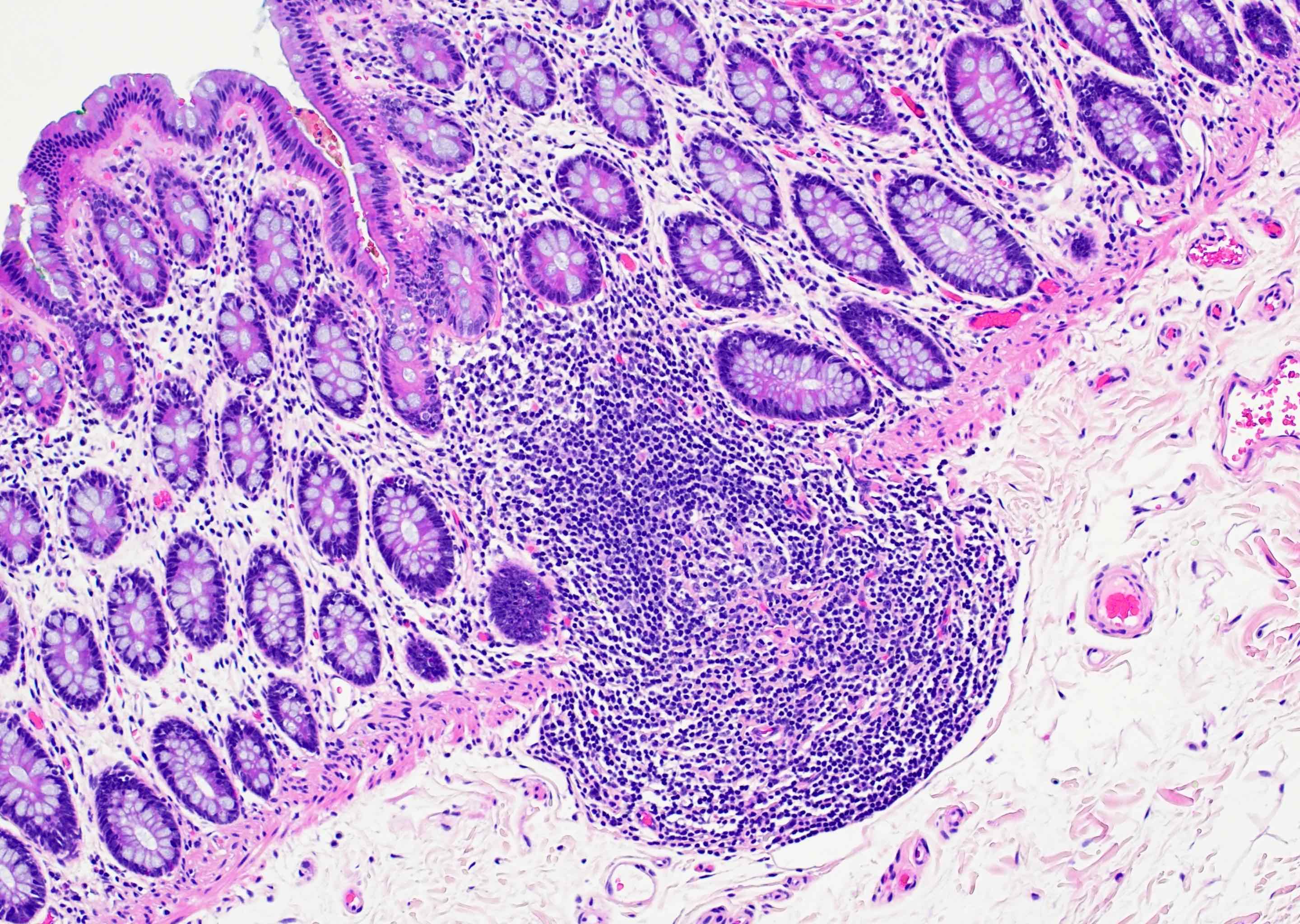
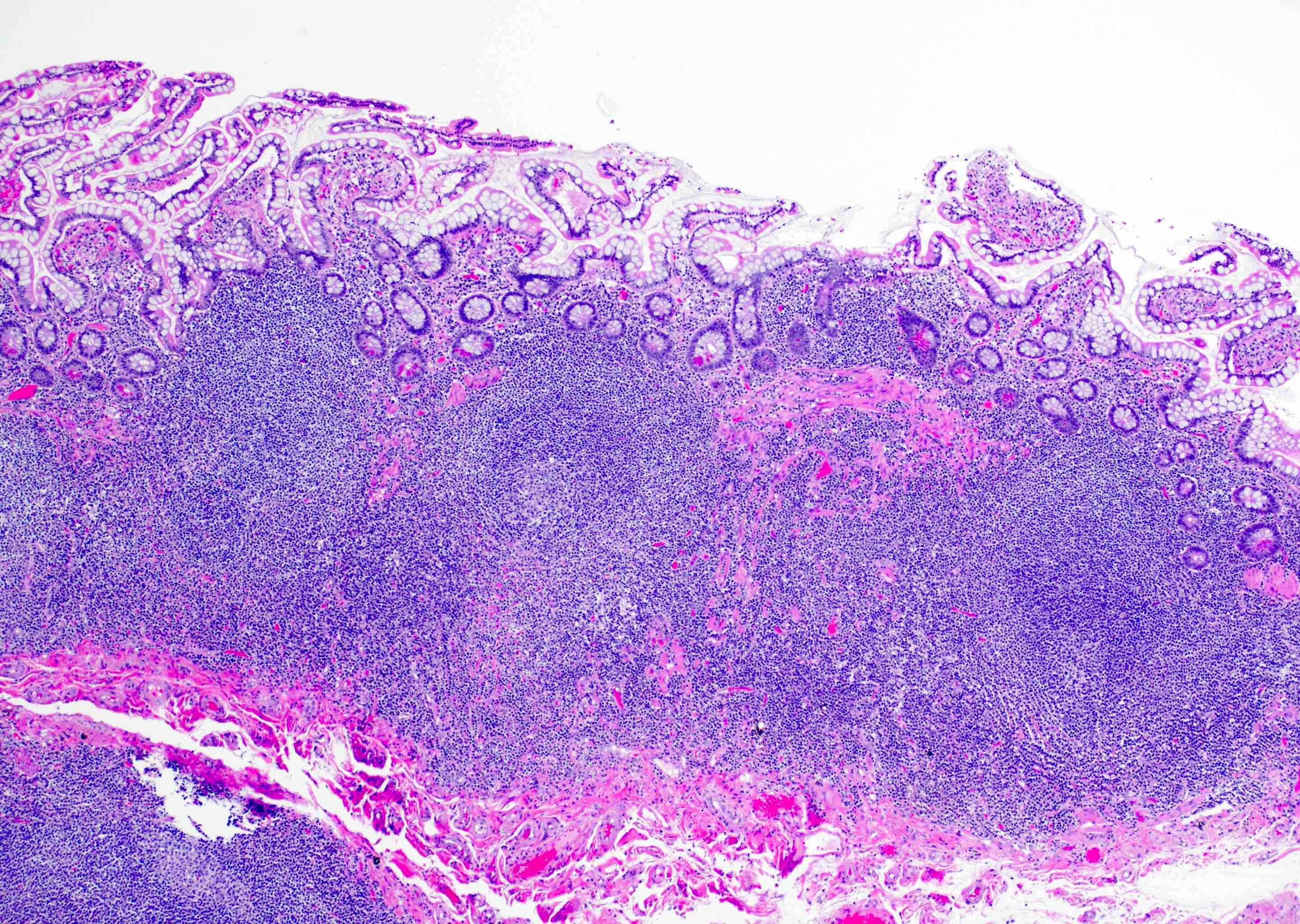
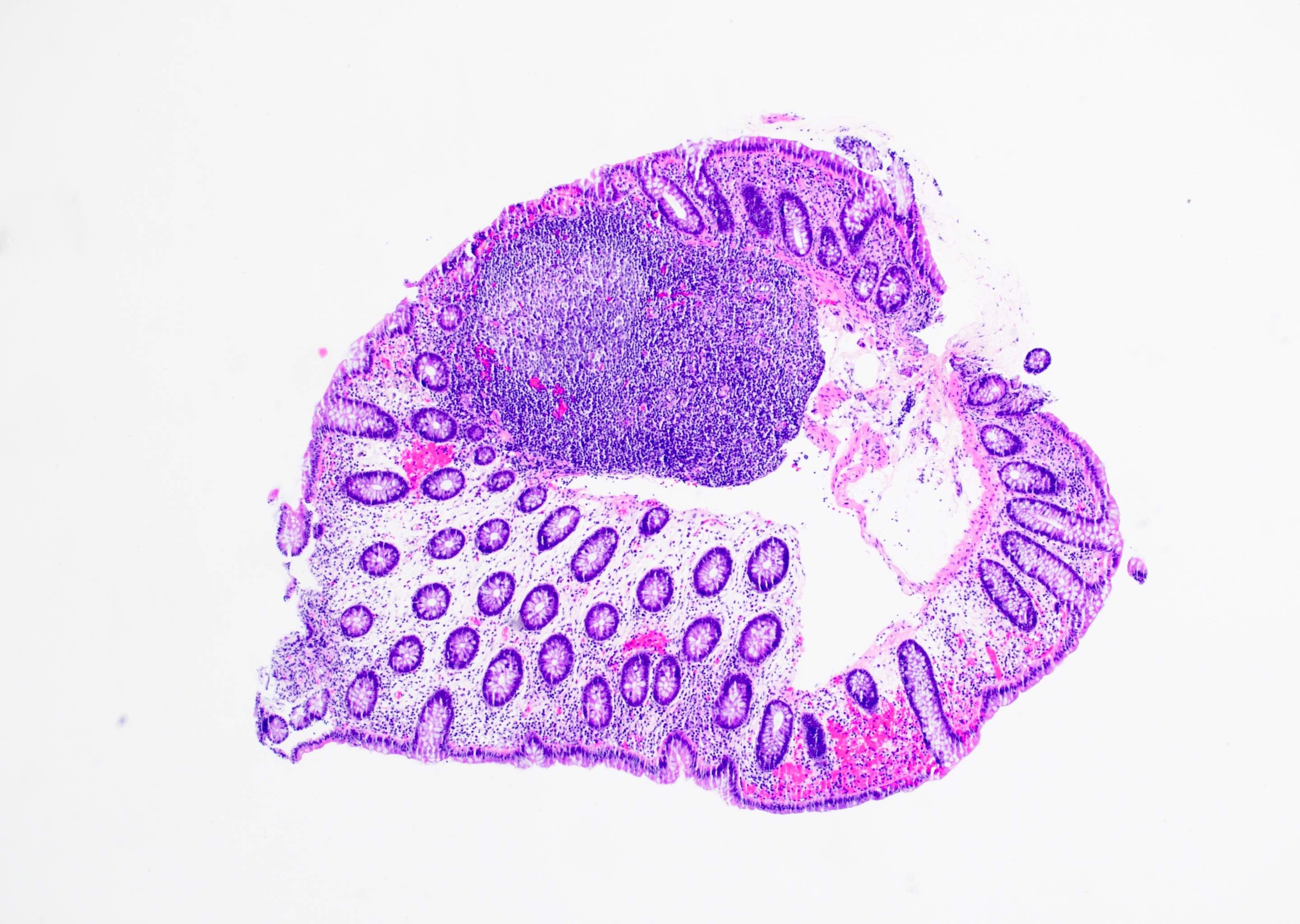
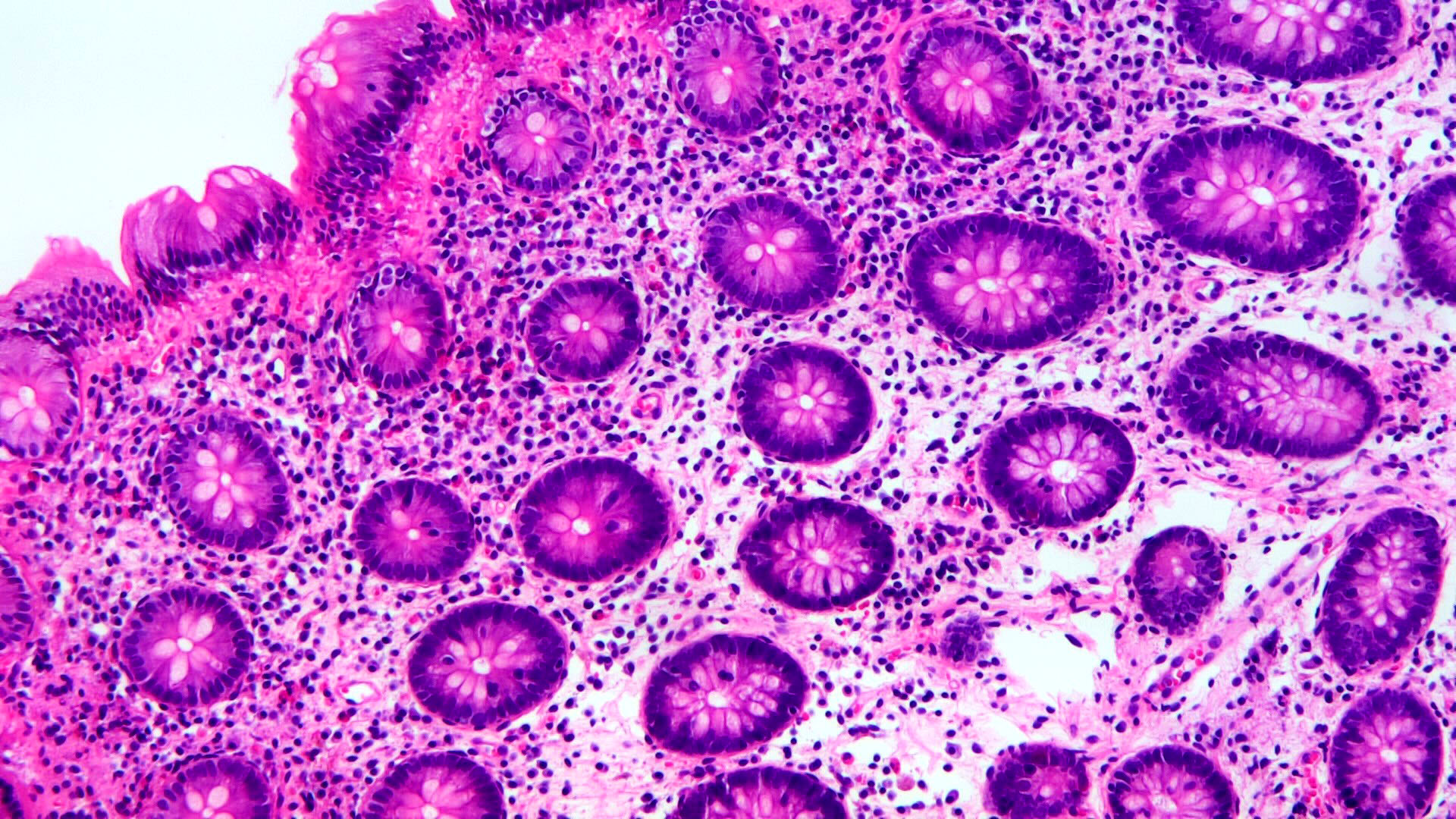
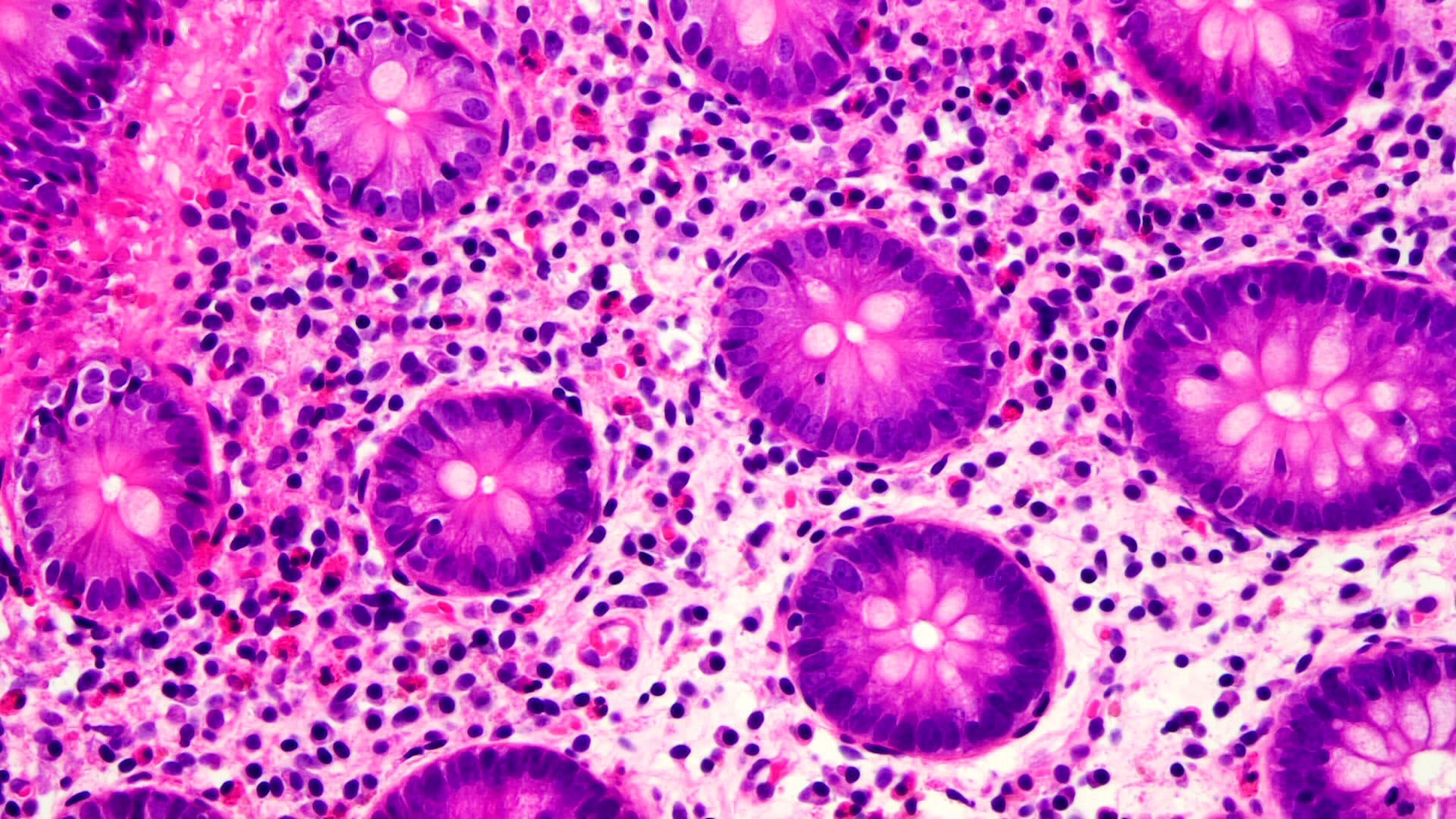
Superpage
Superpage Topics
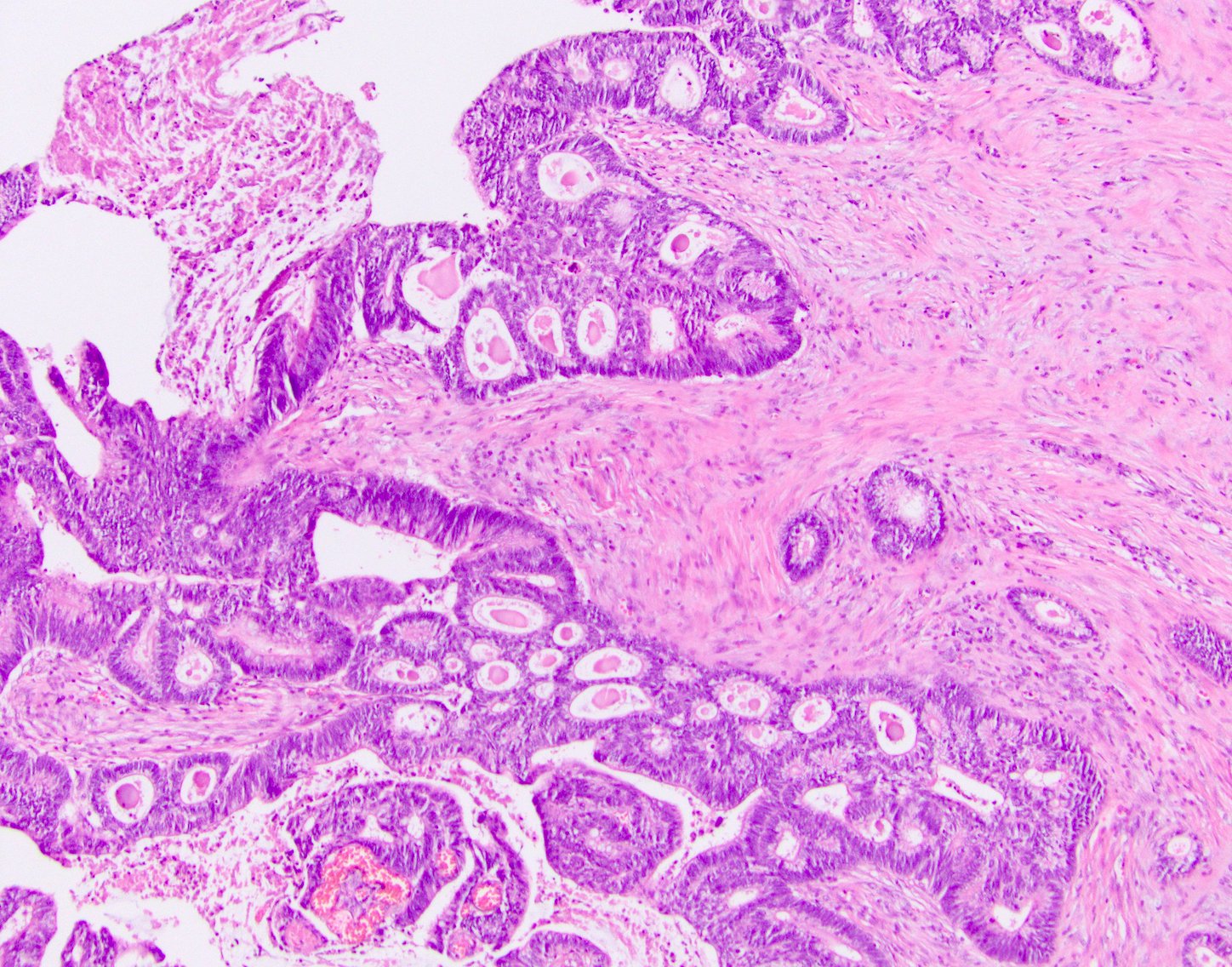
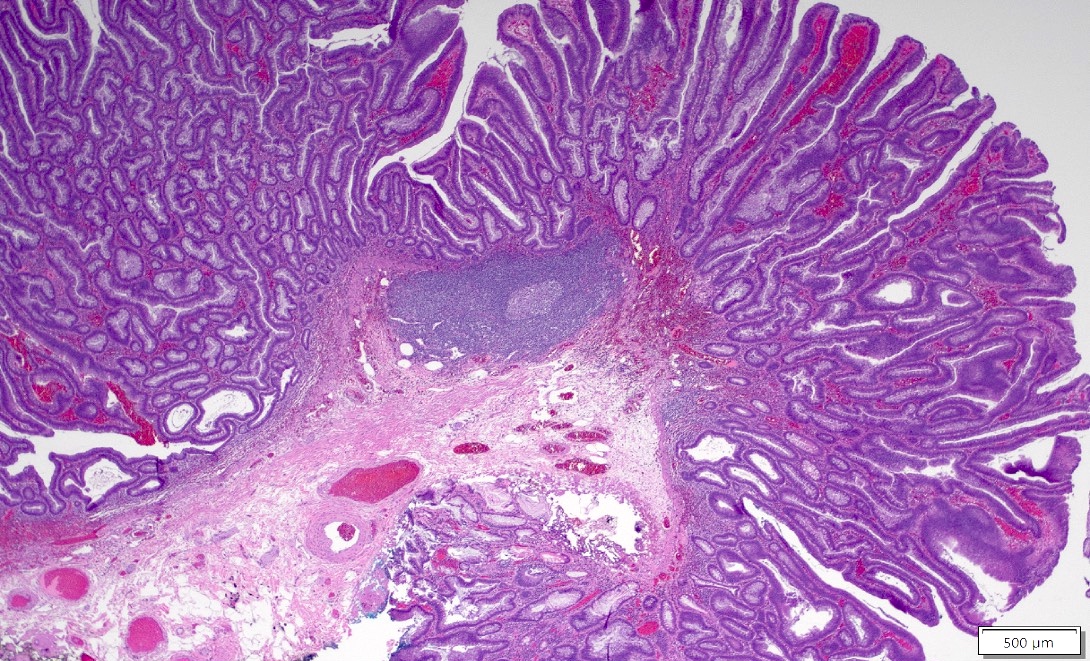
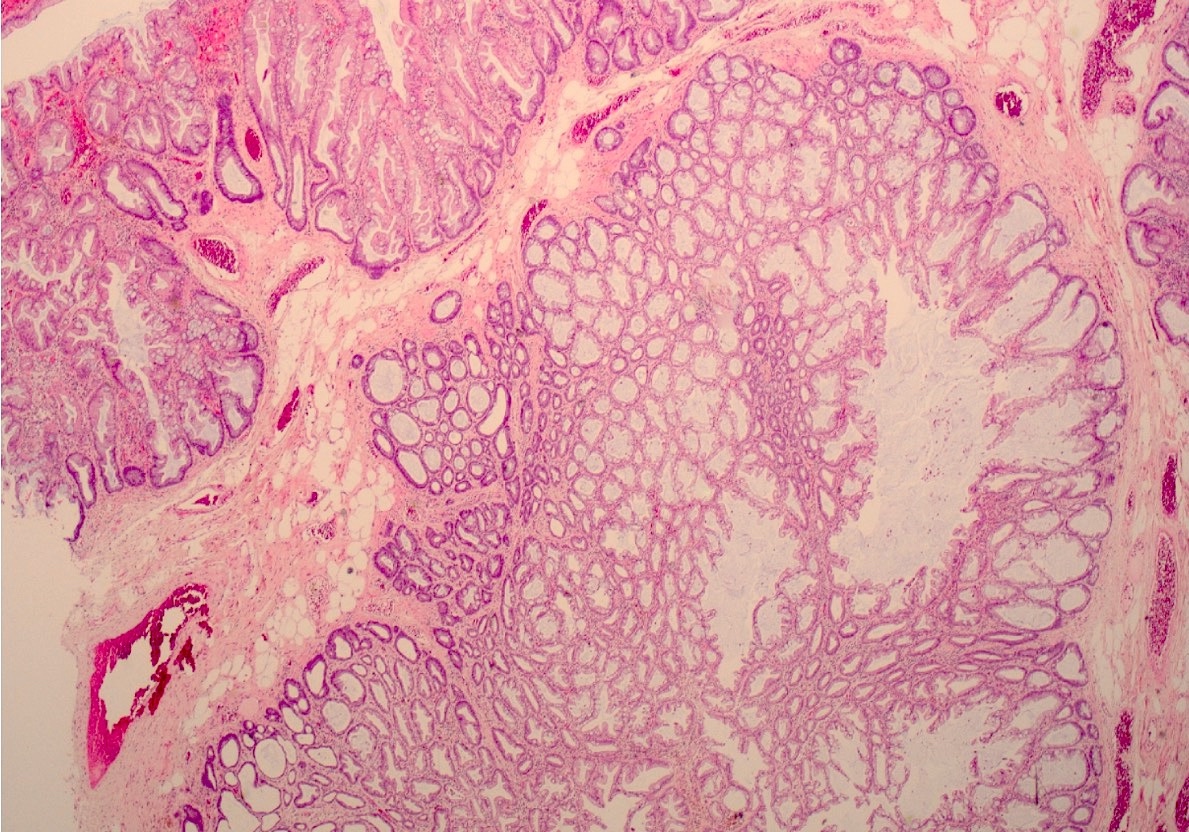
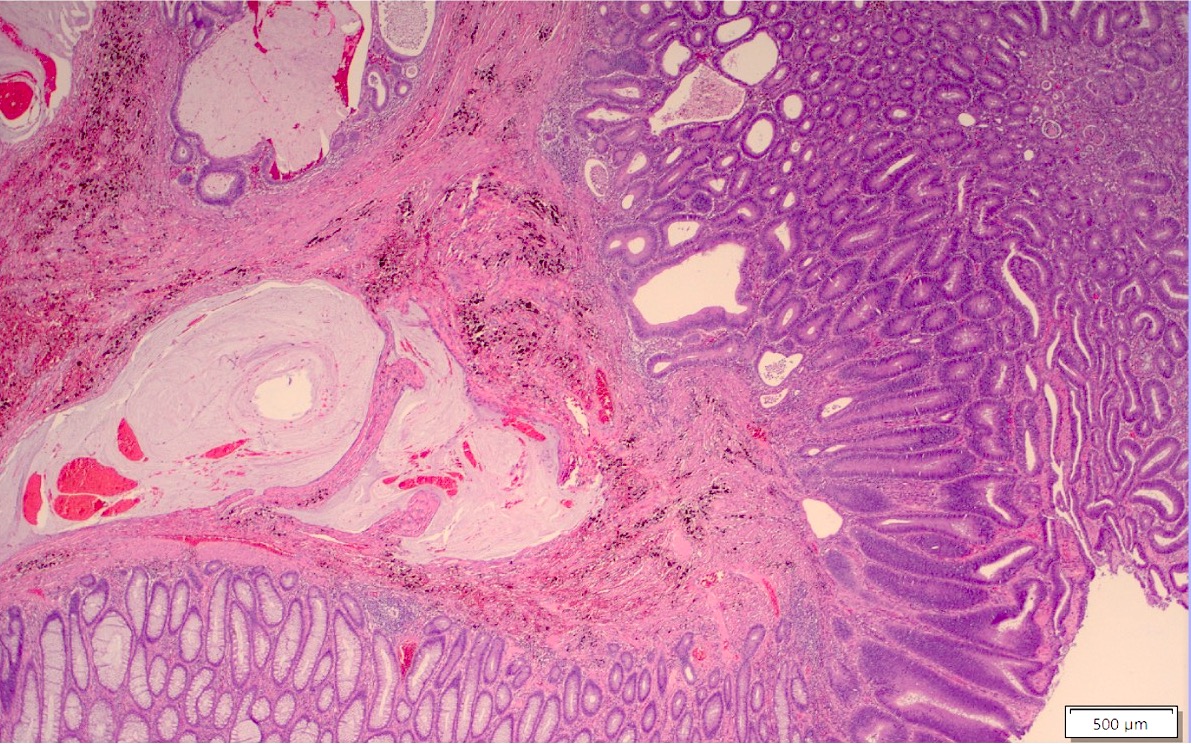
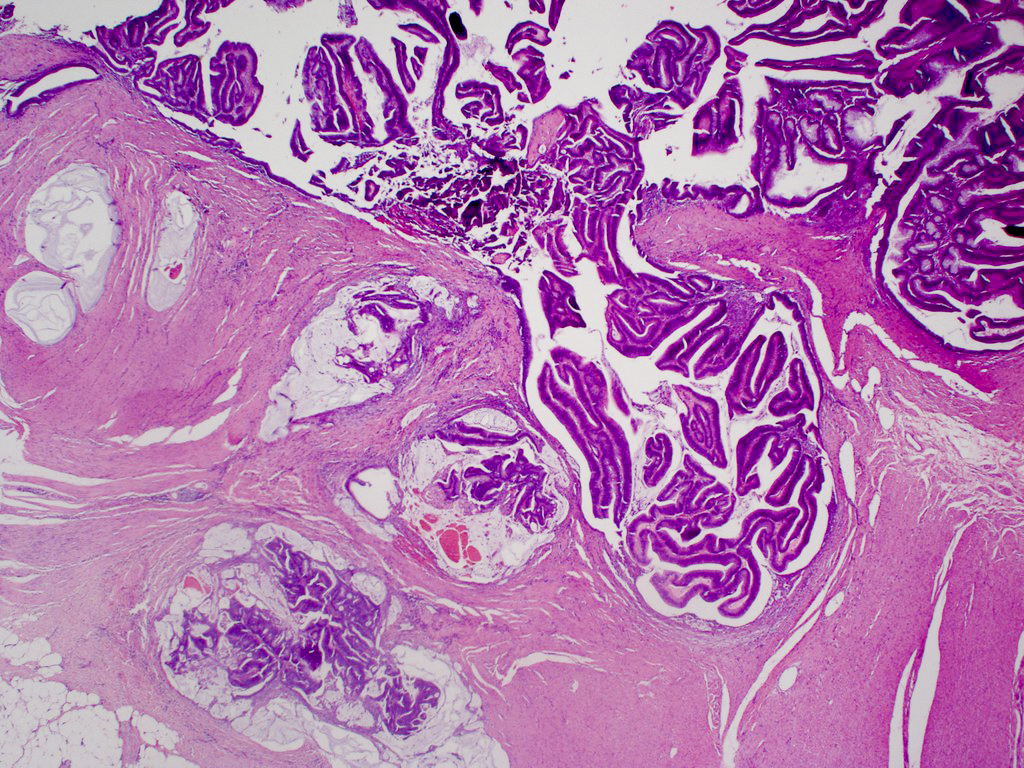
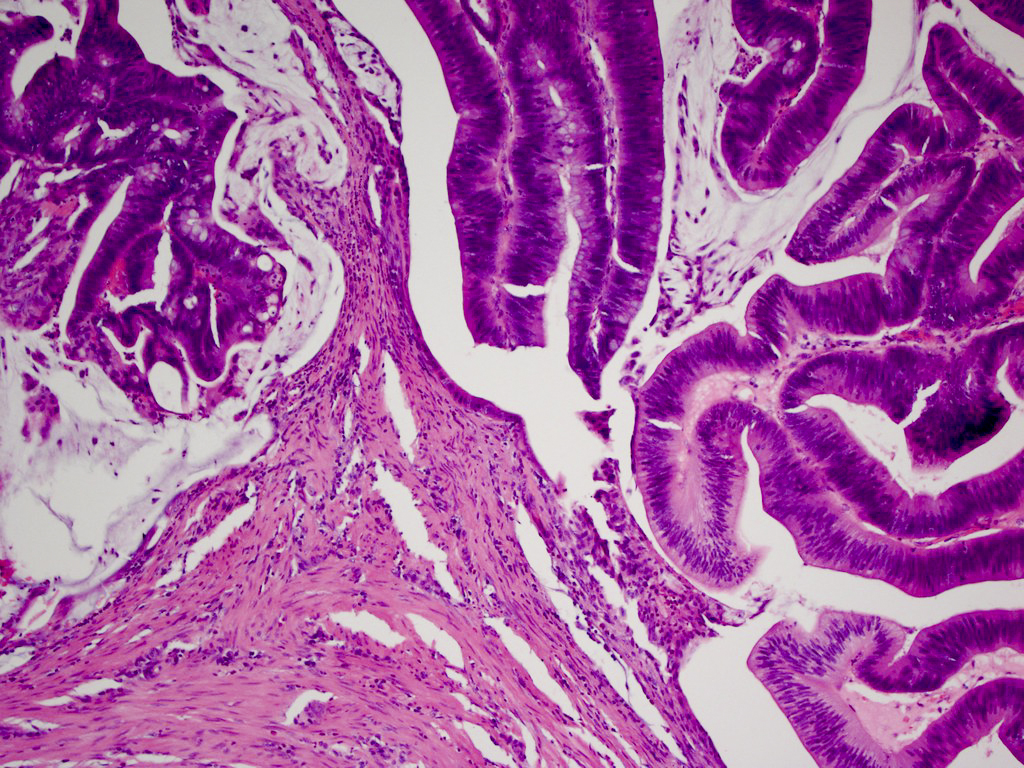
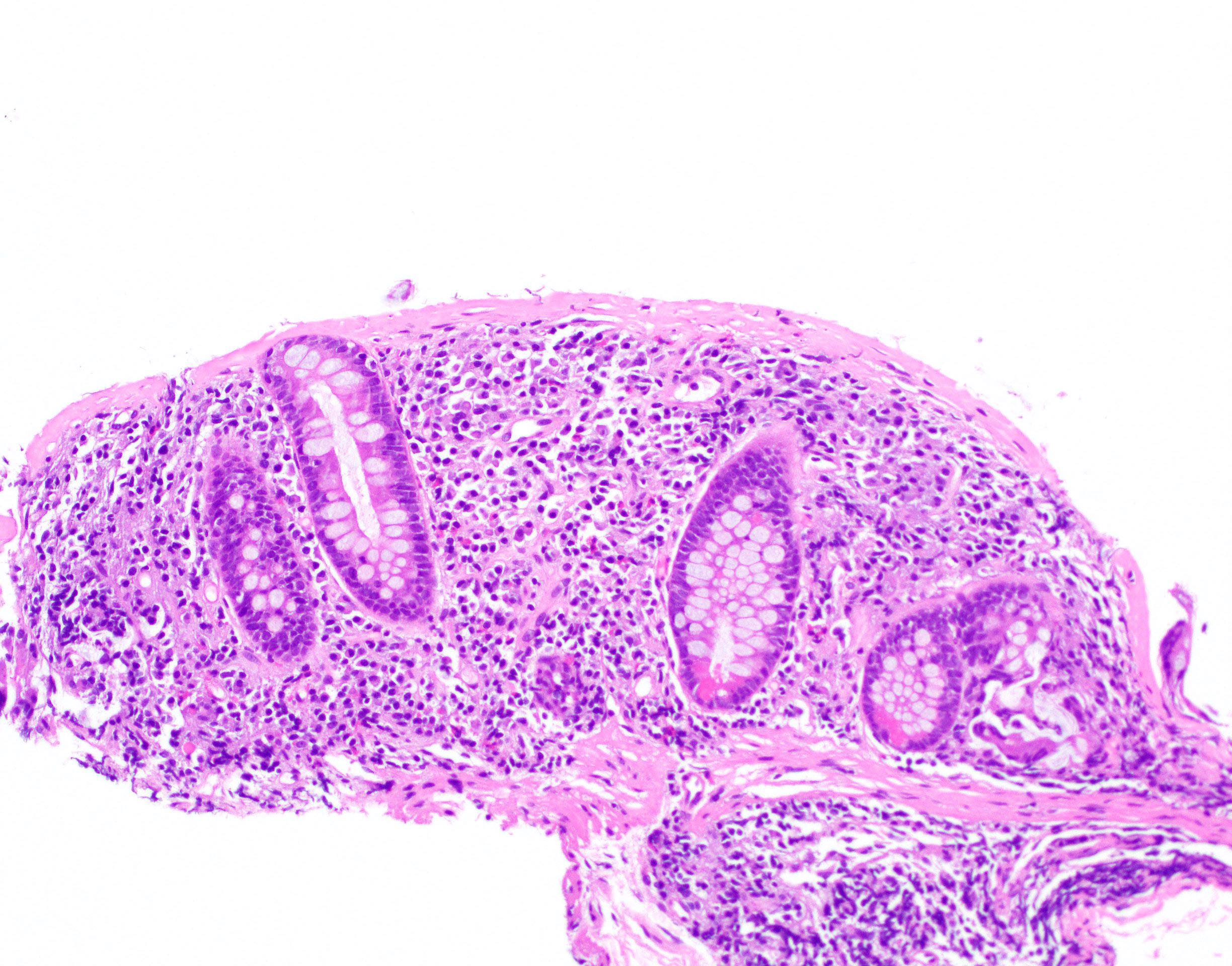
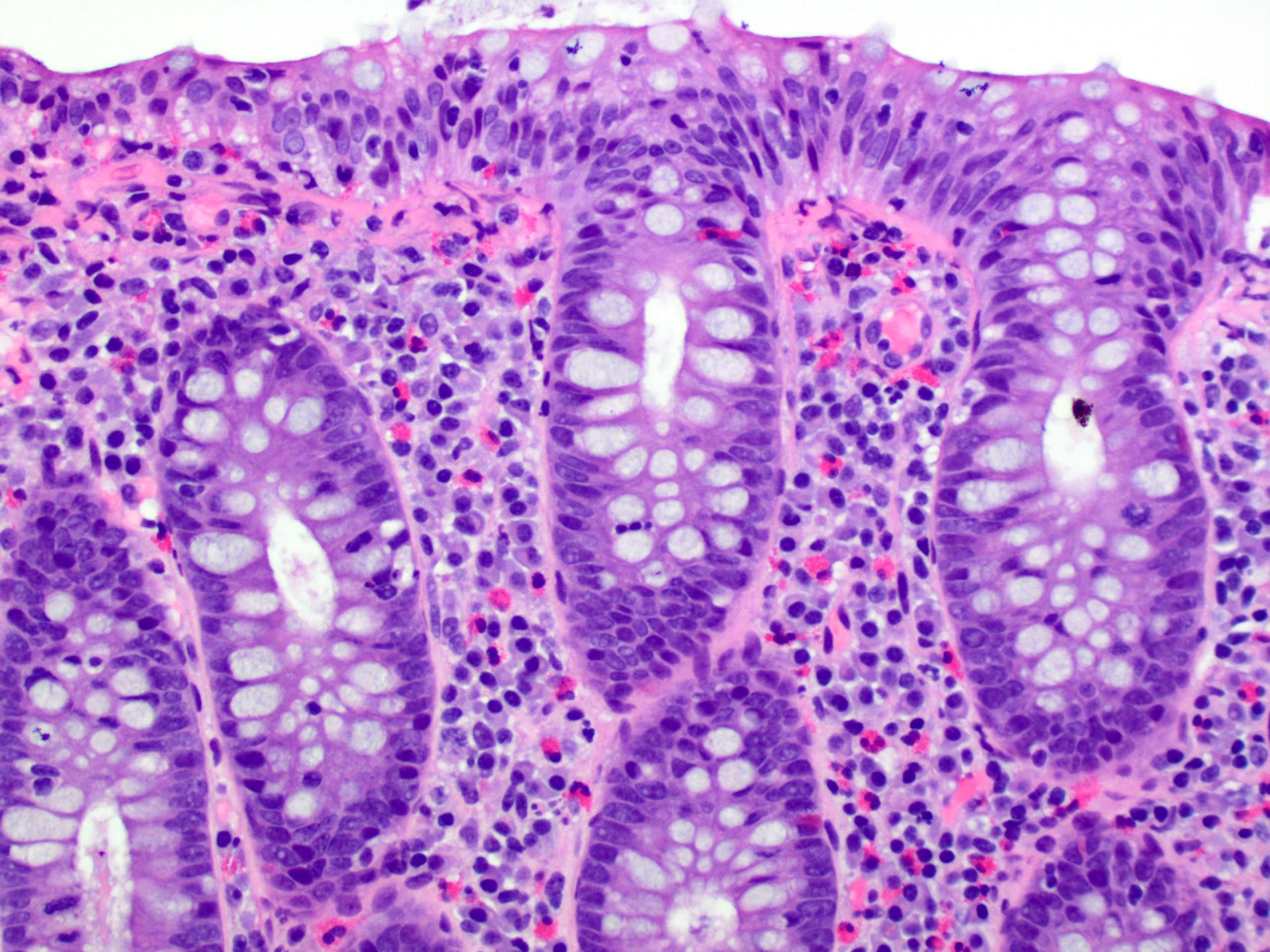
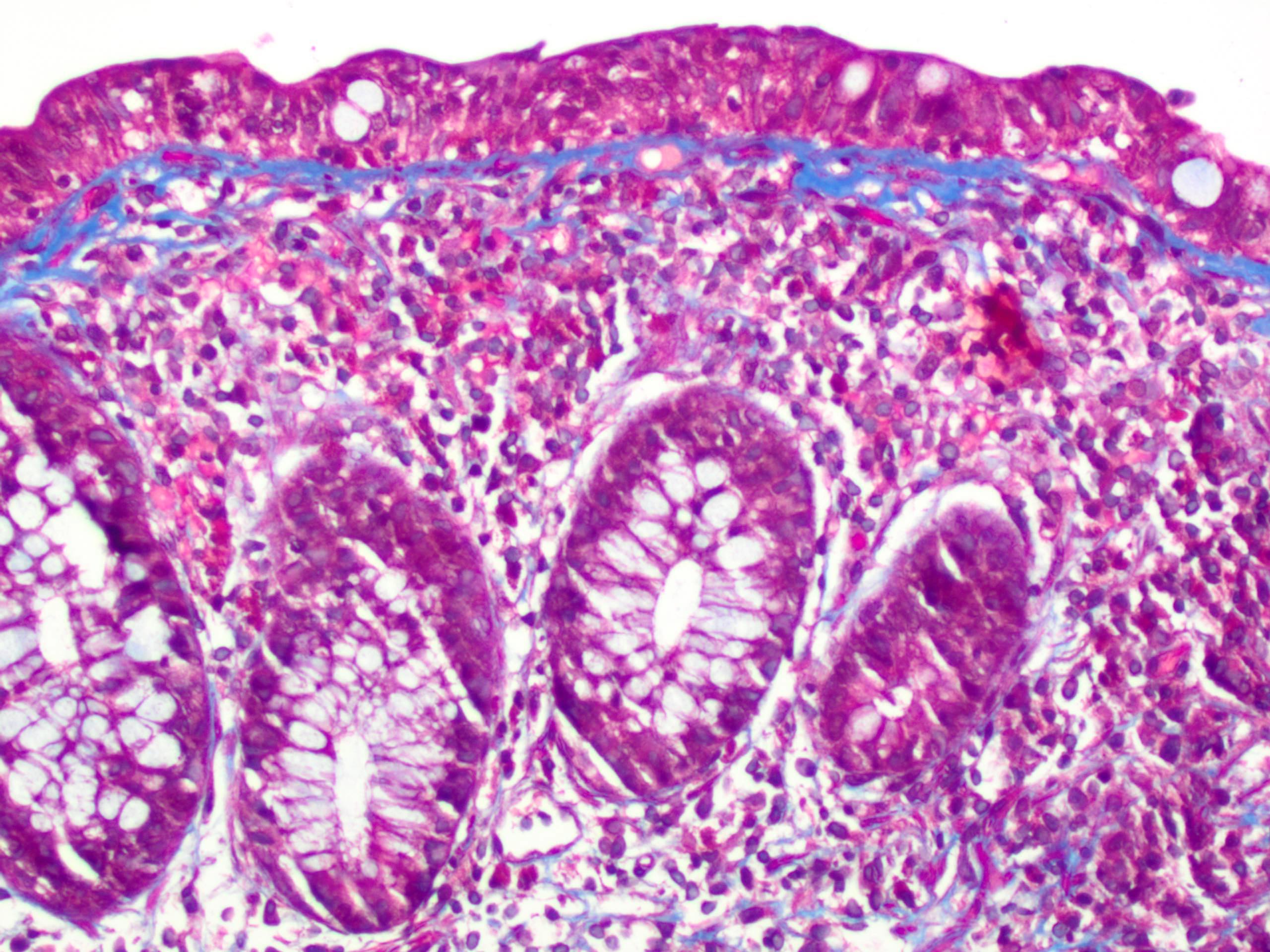
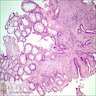
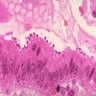
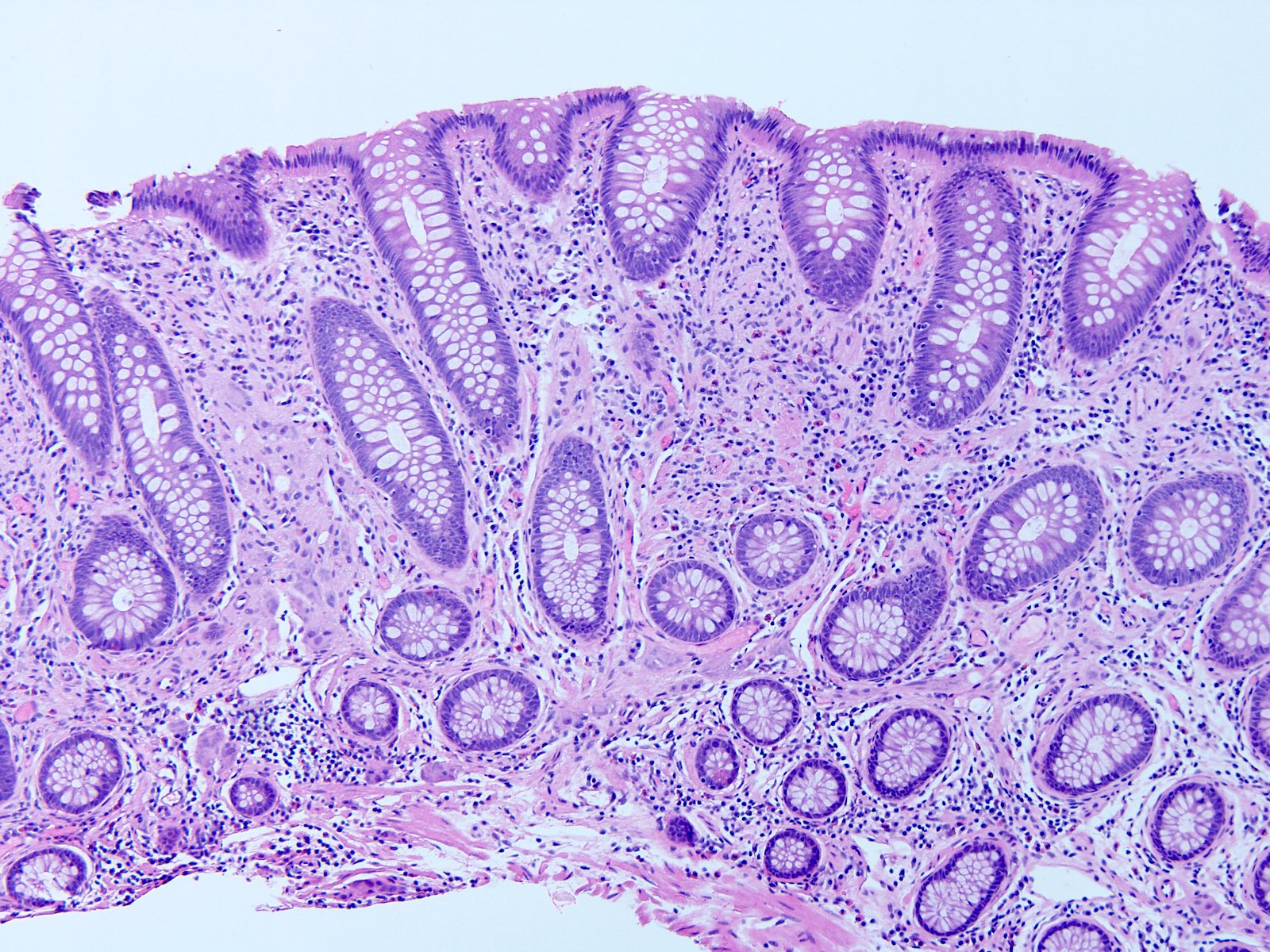
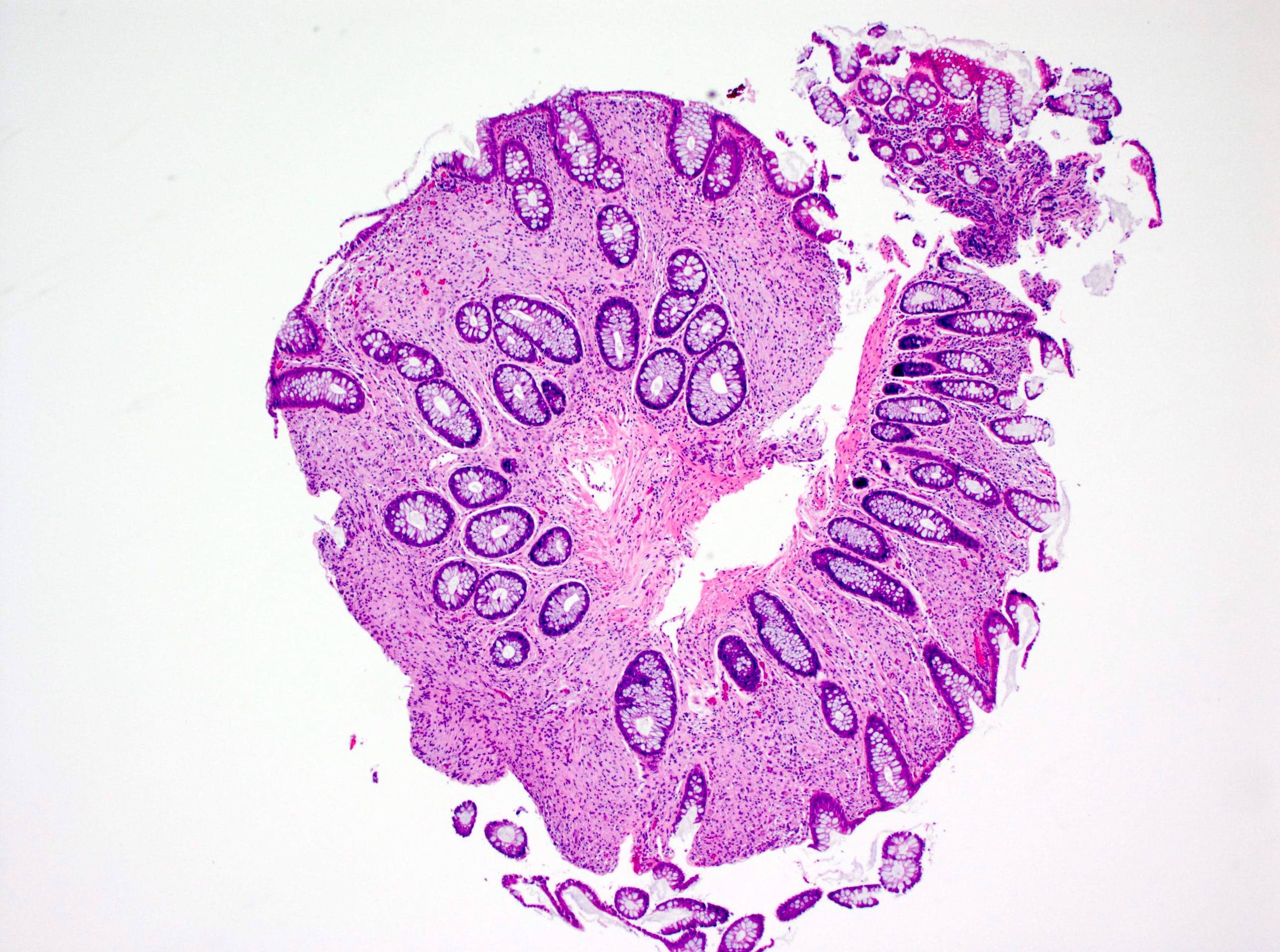
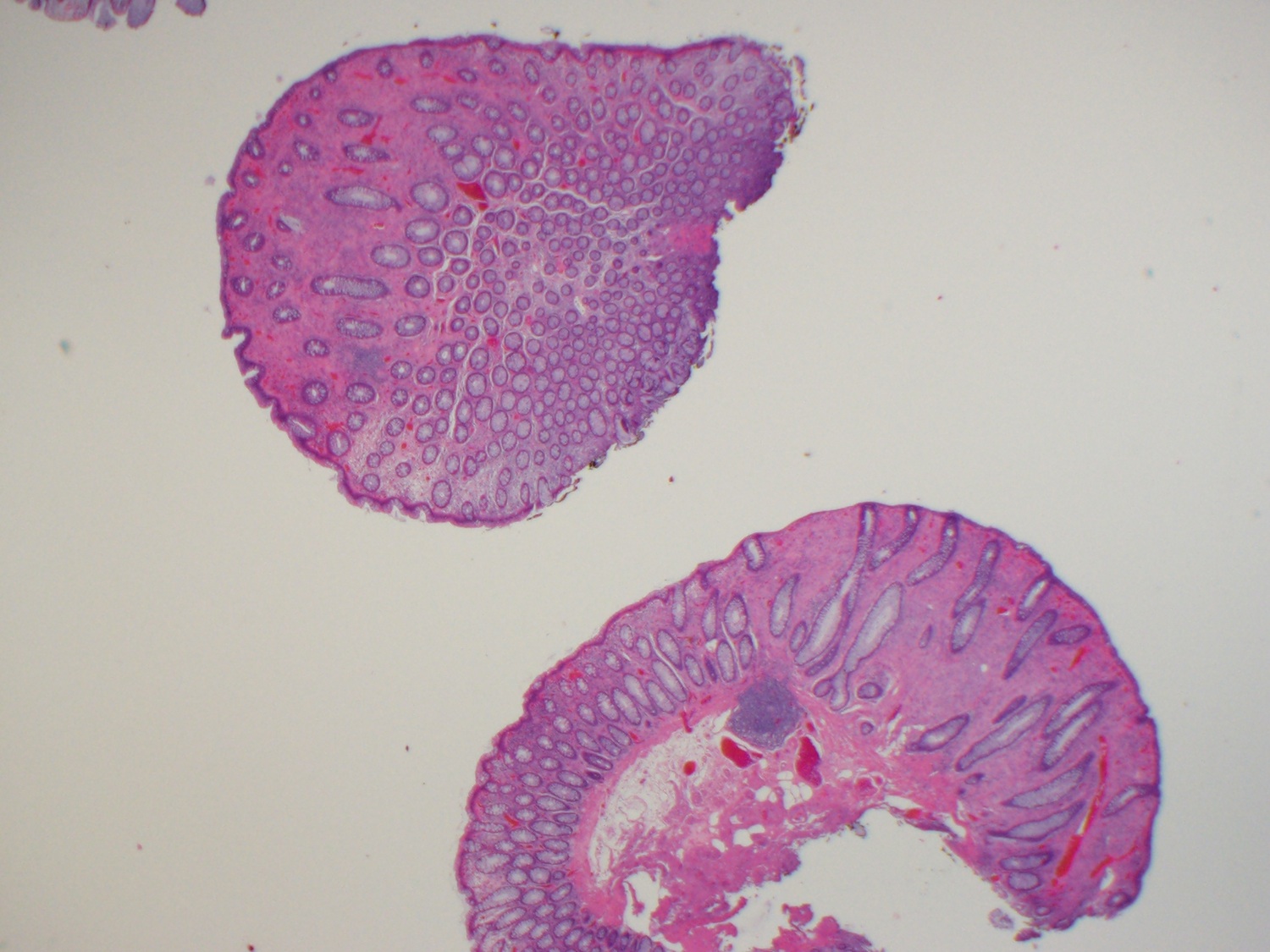
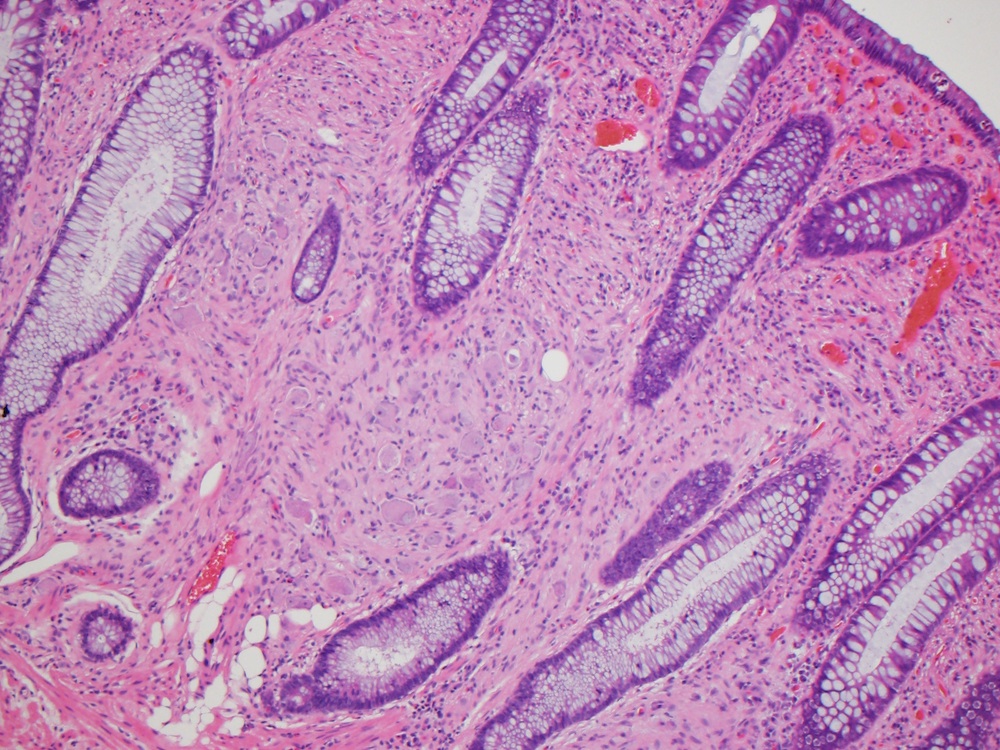
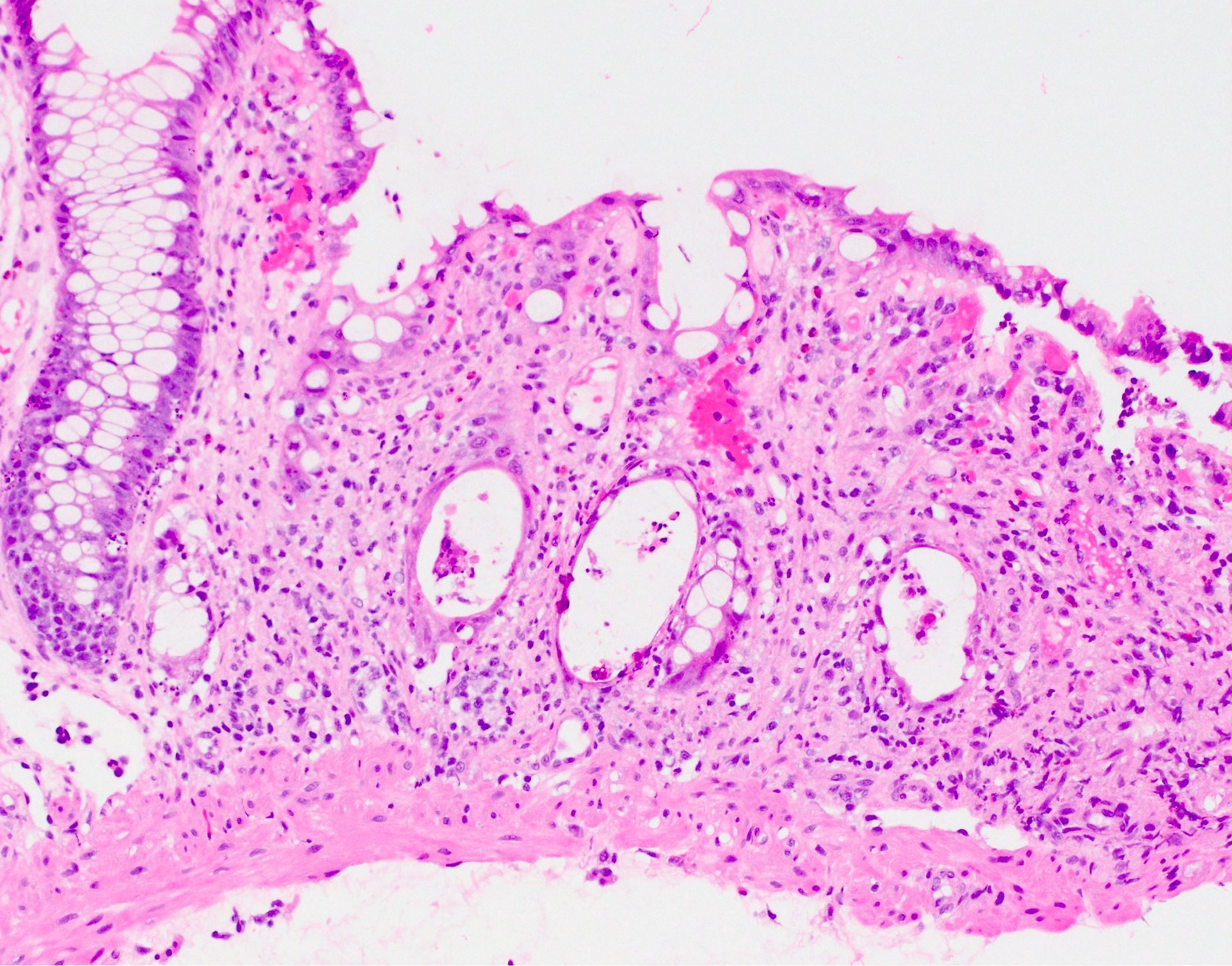
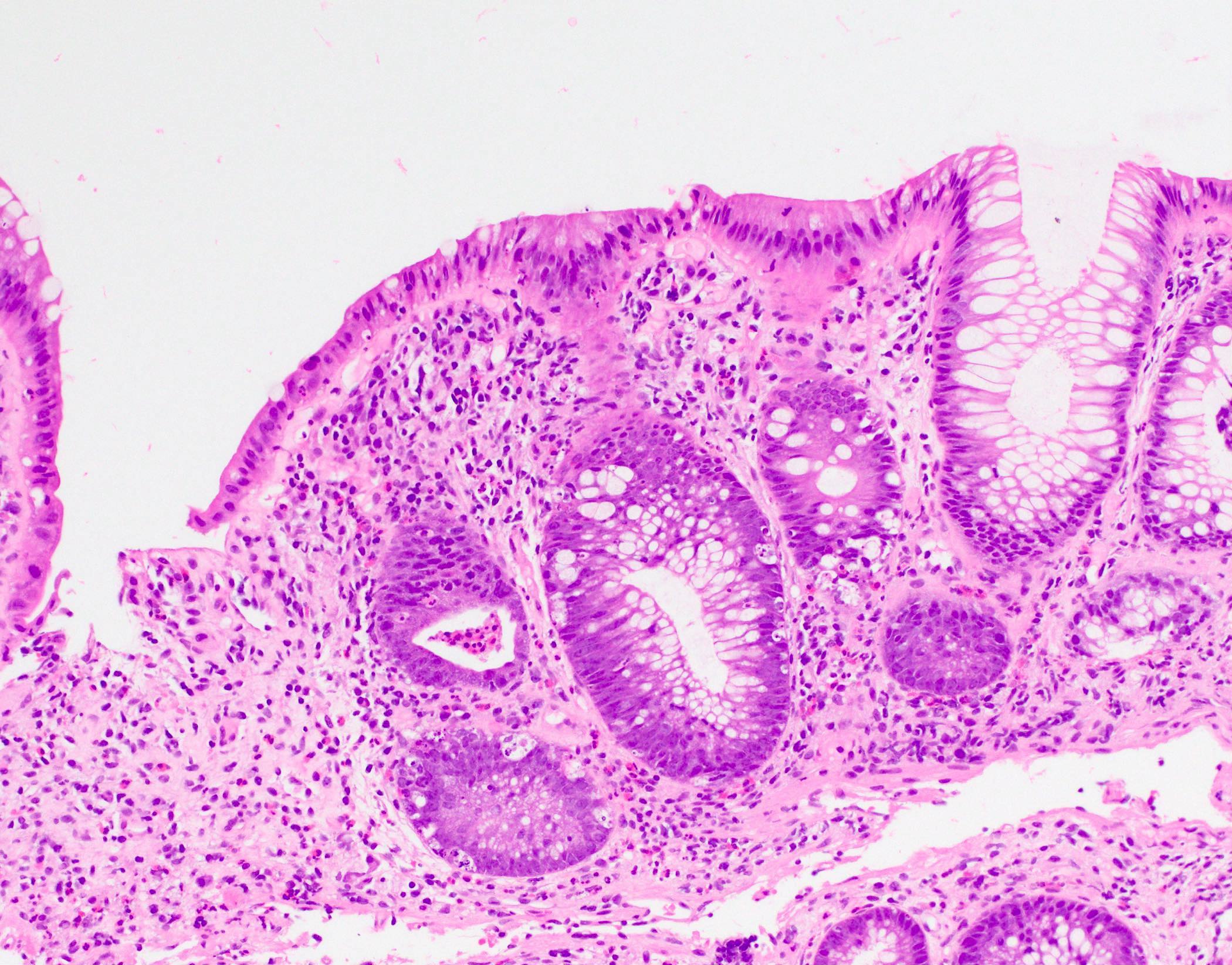
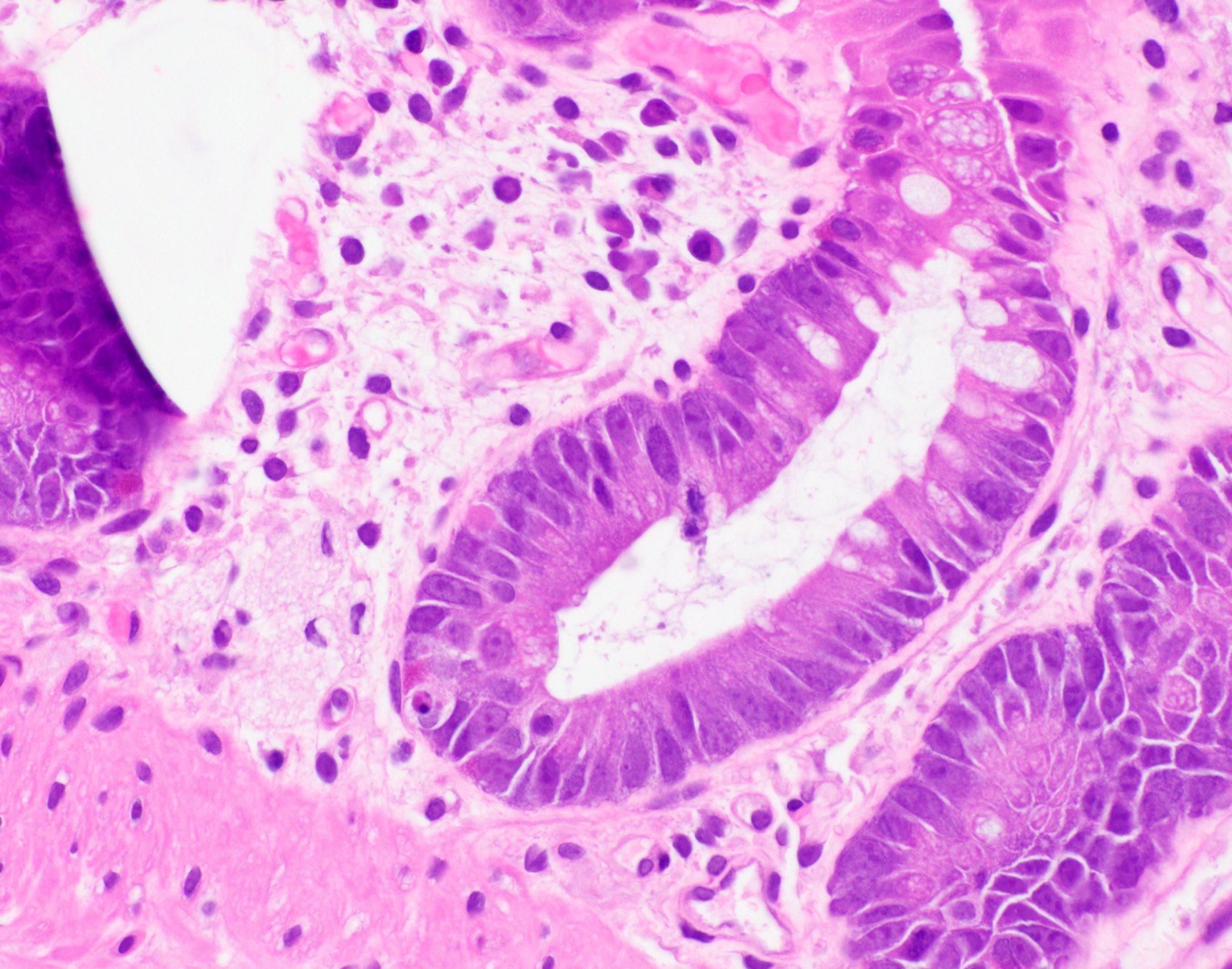
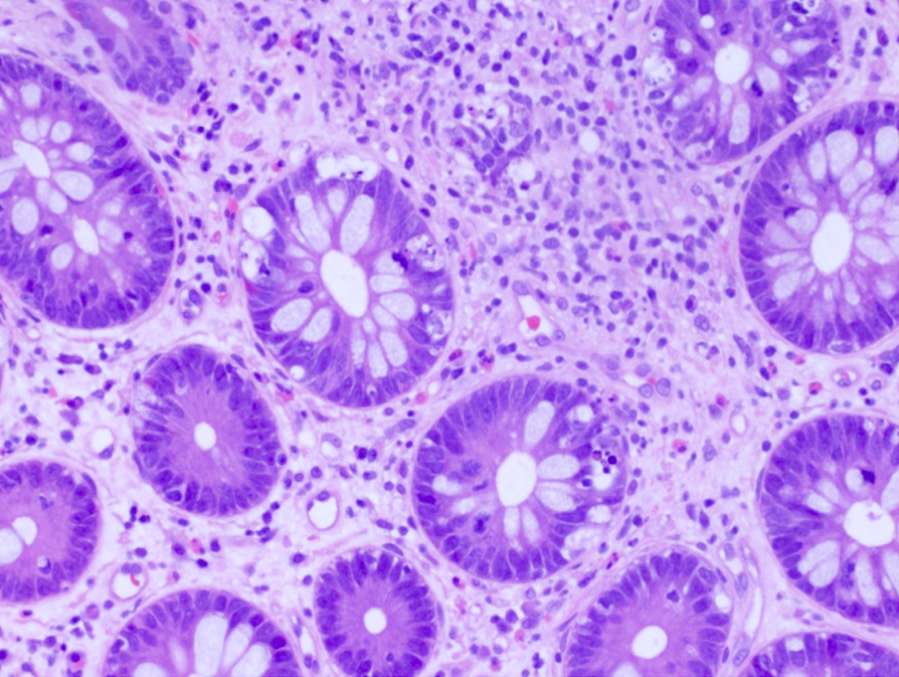
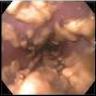
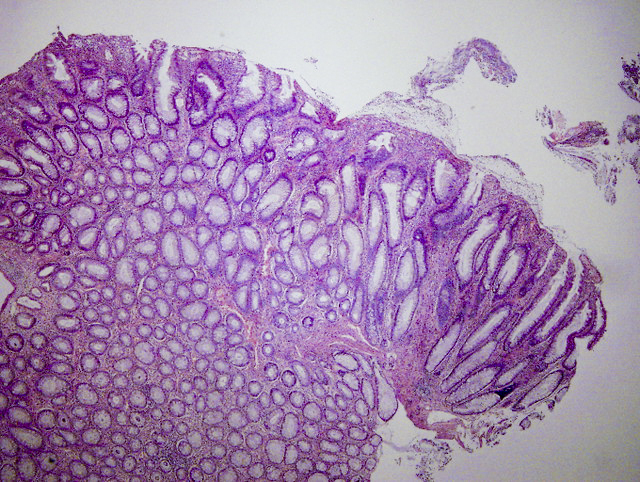
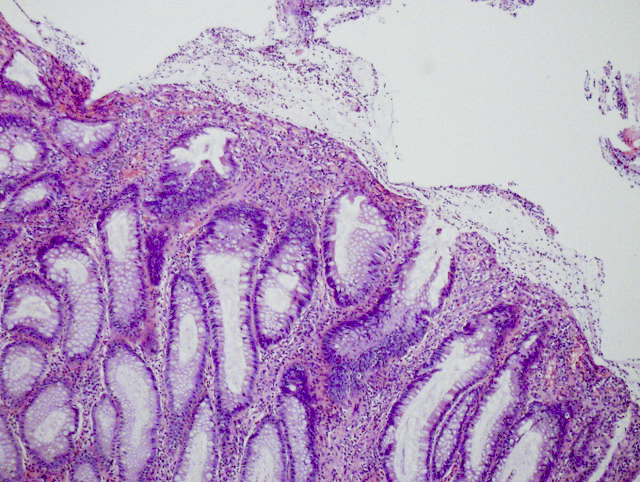
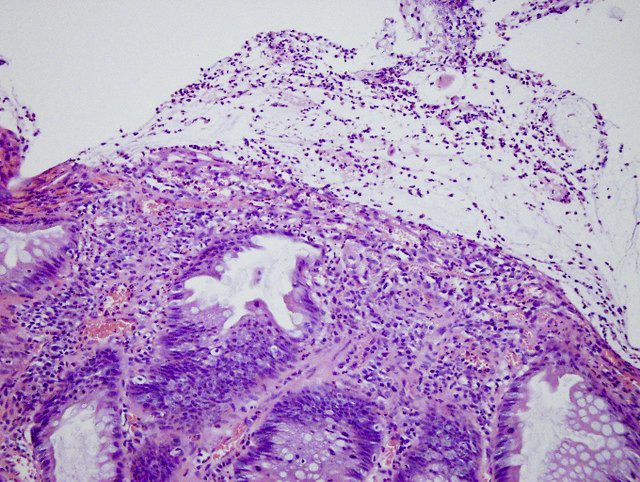
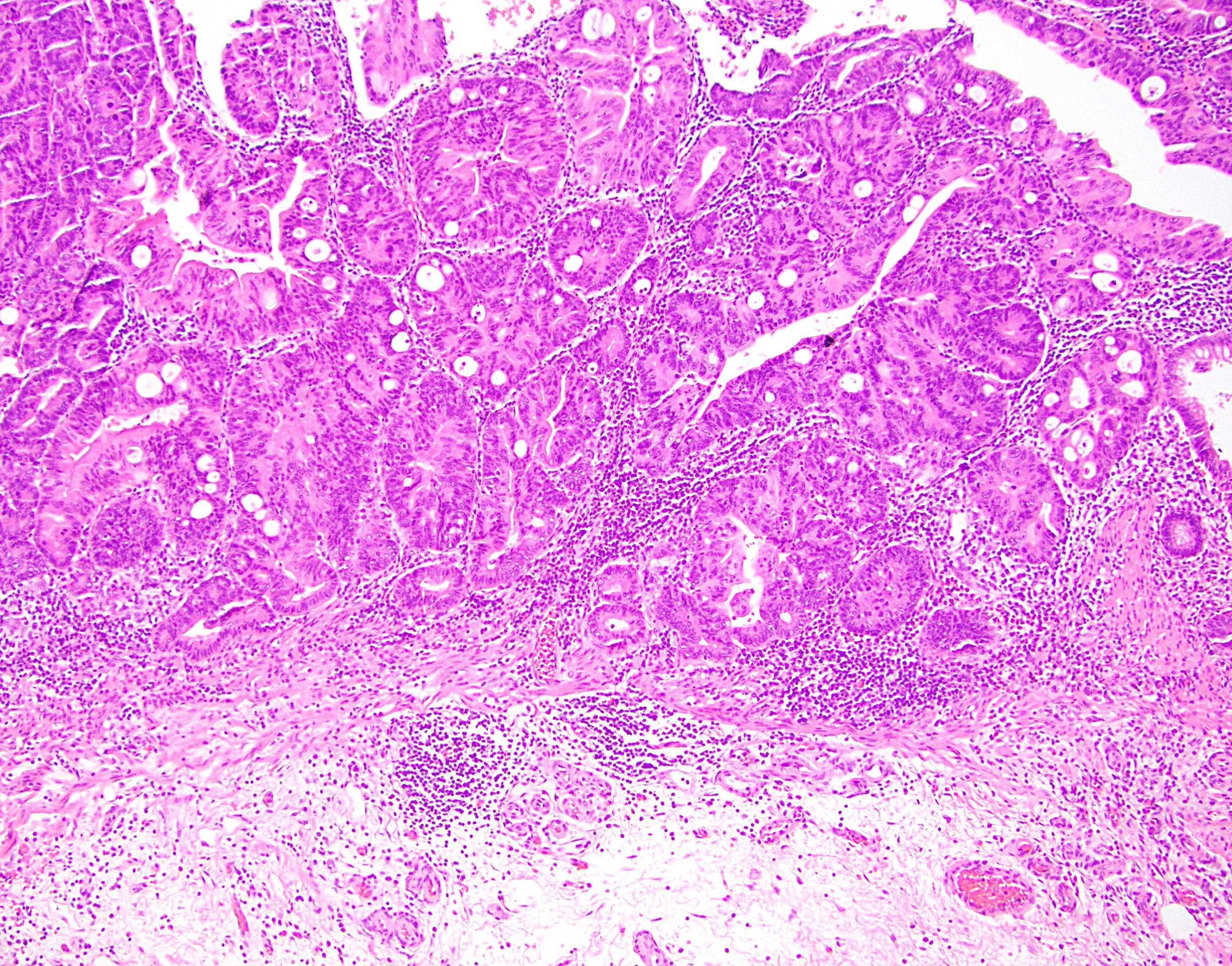
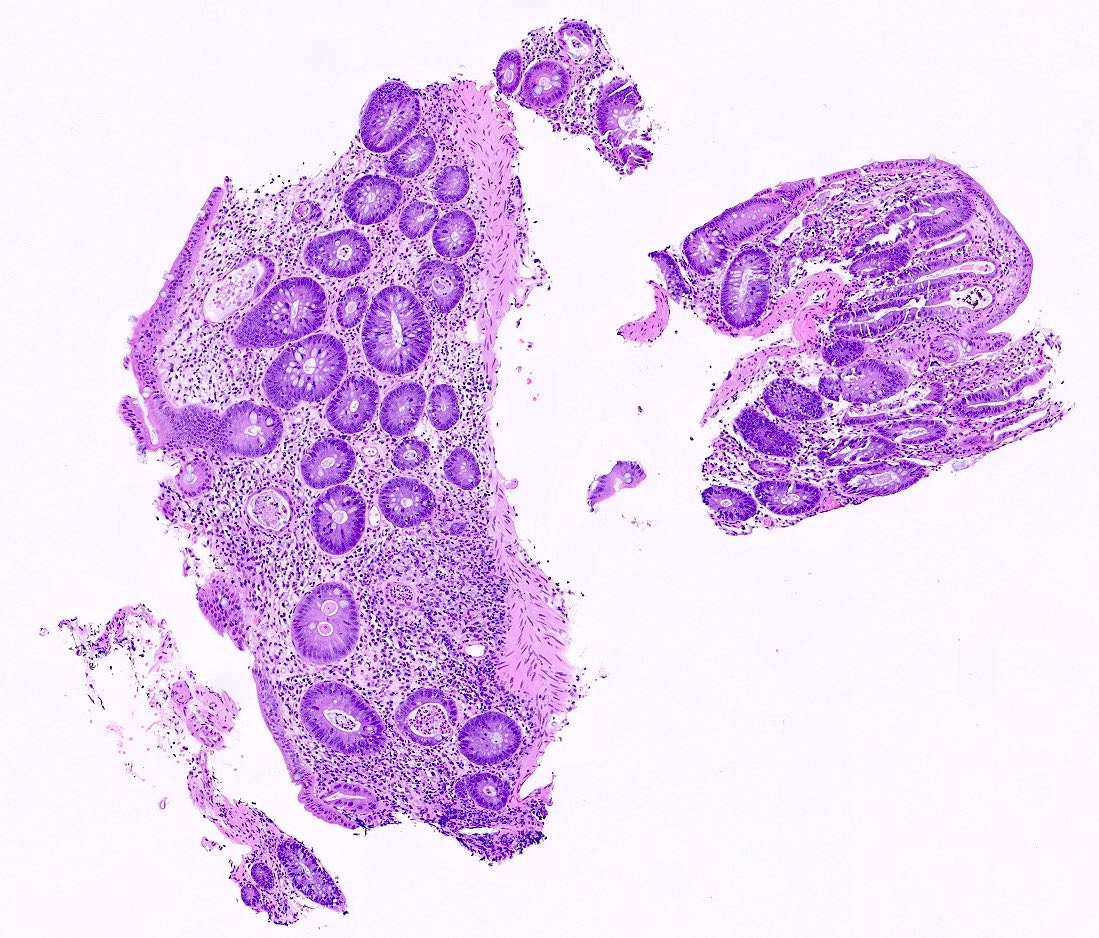
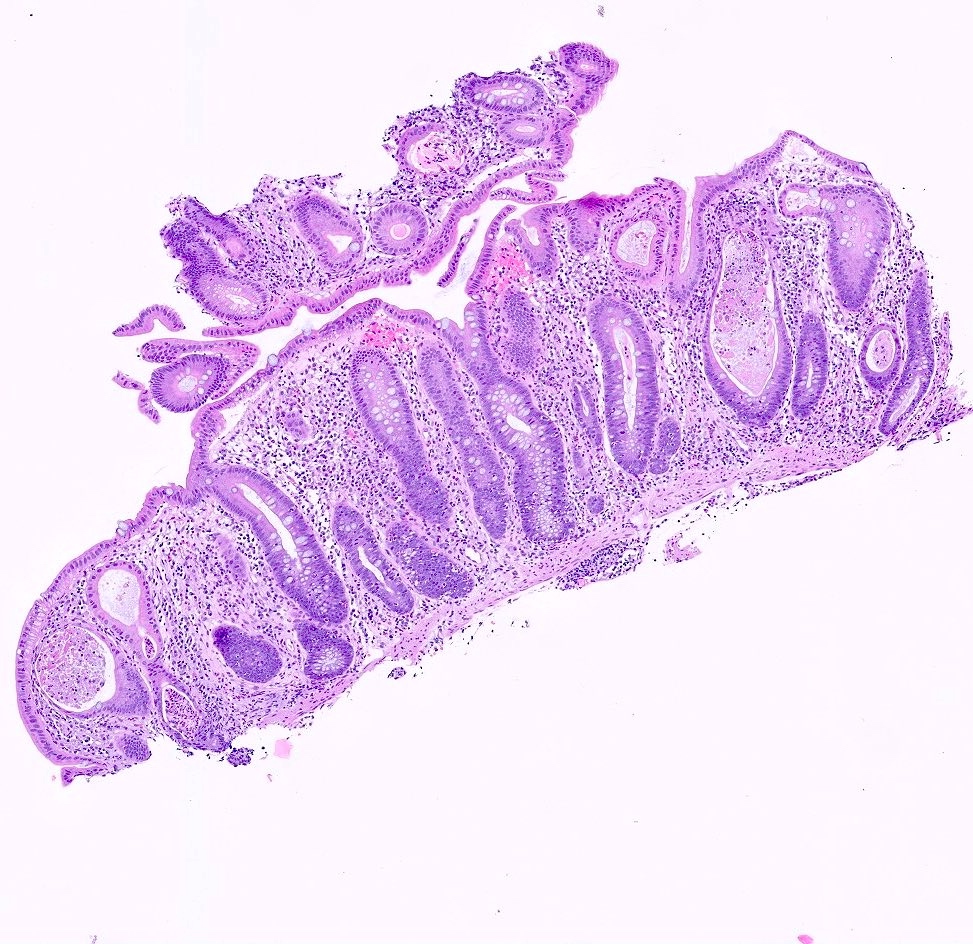
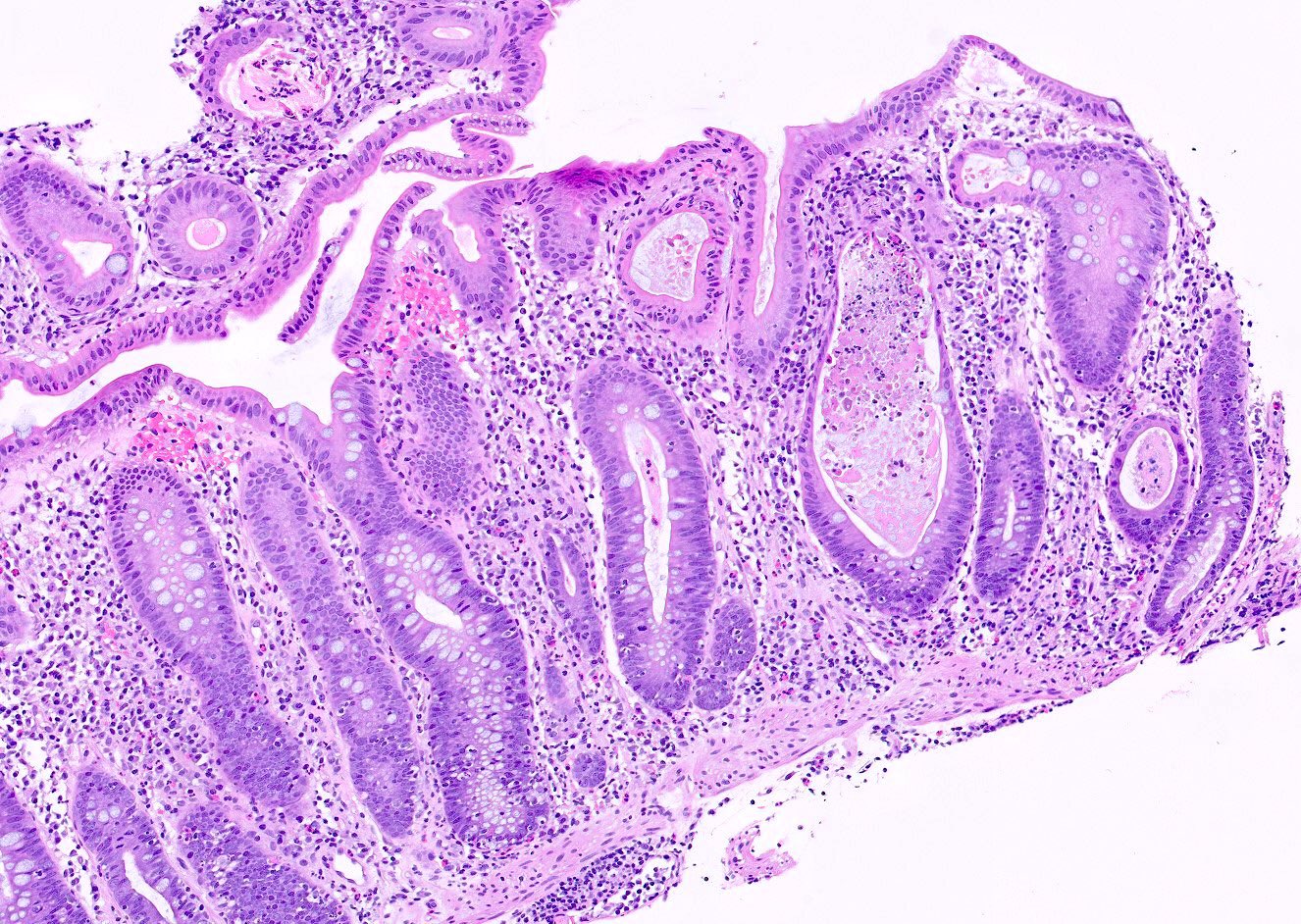
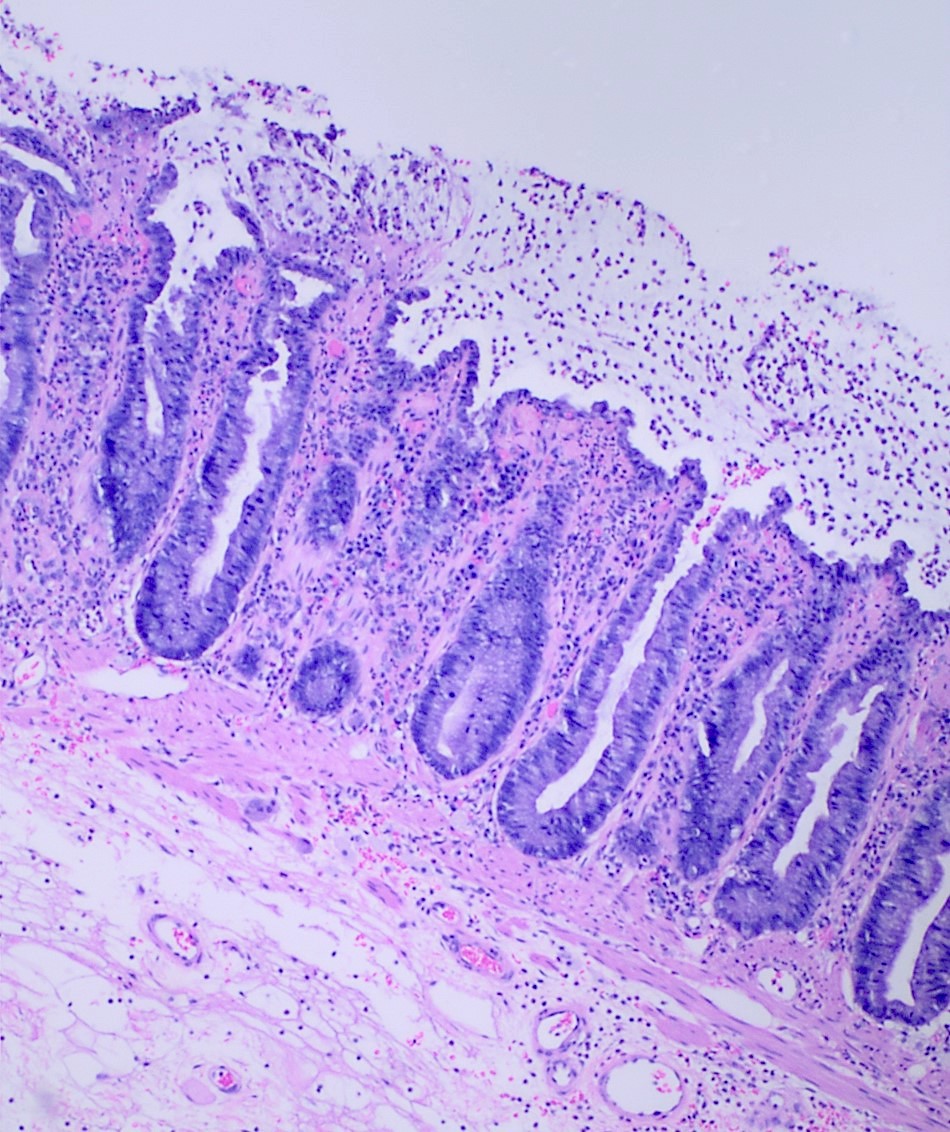
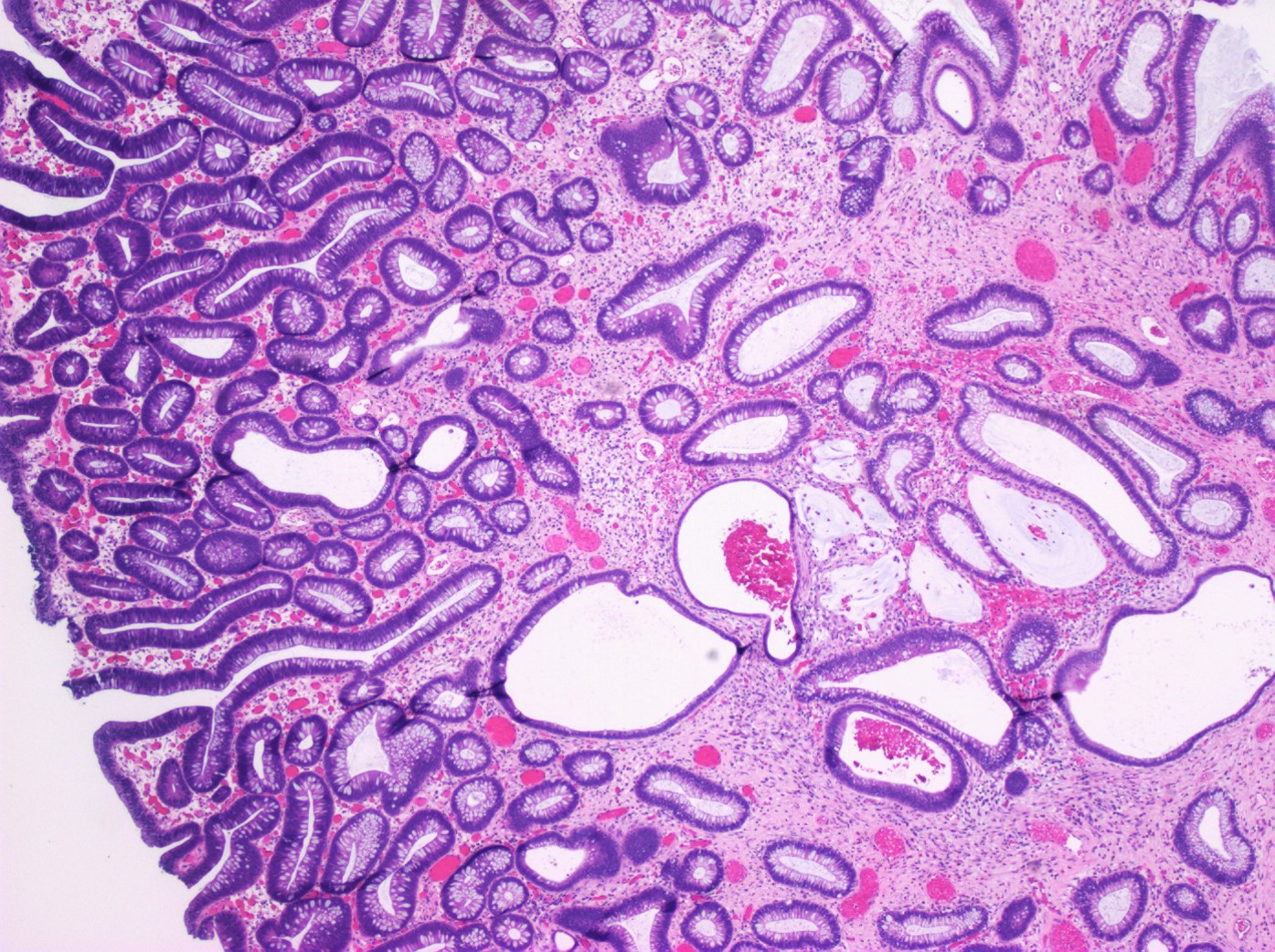
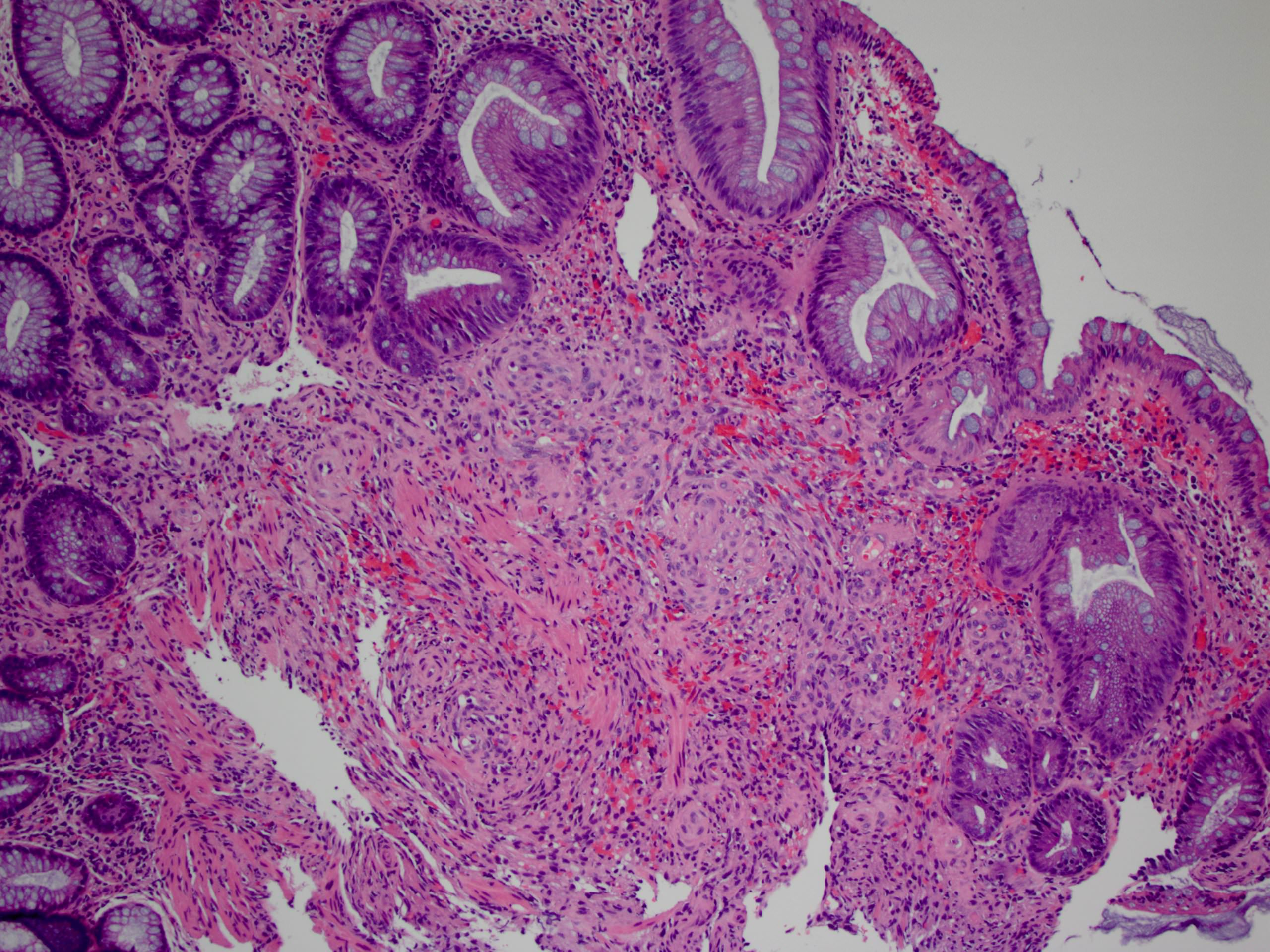
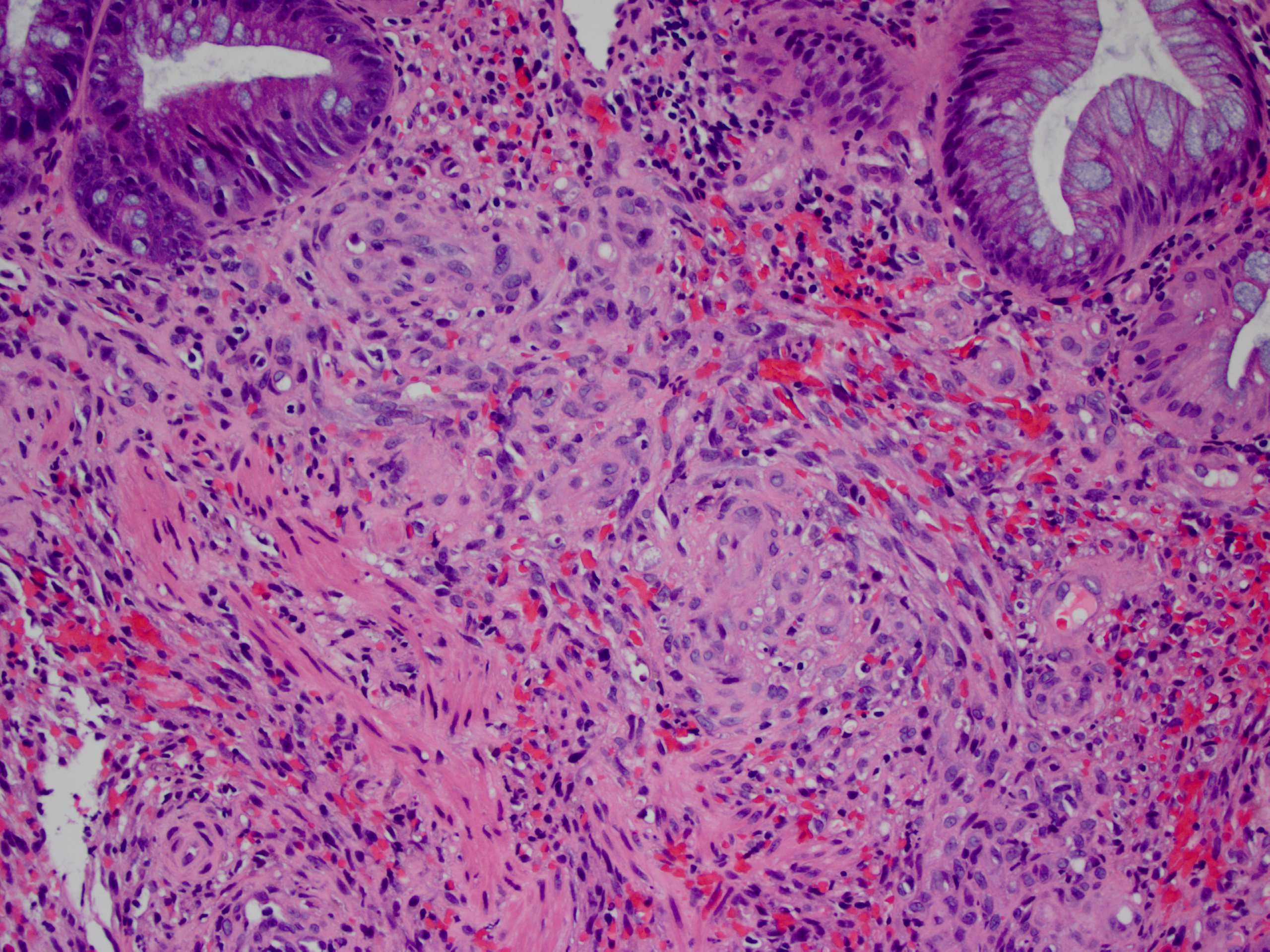
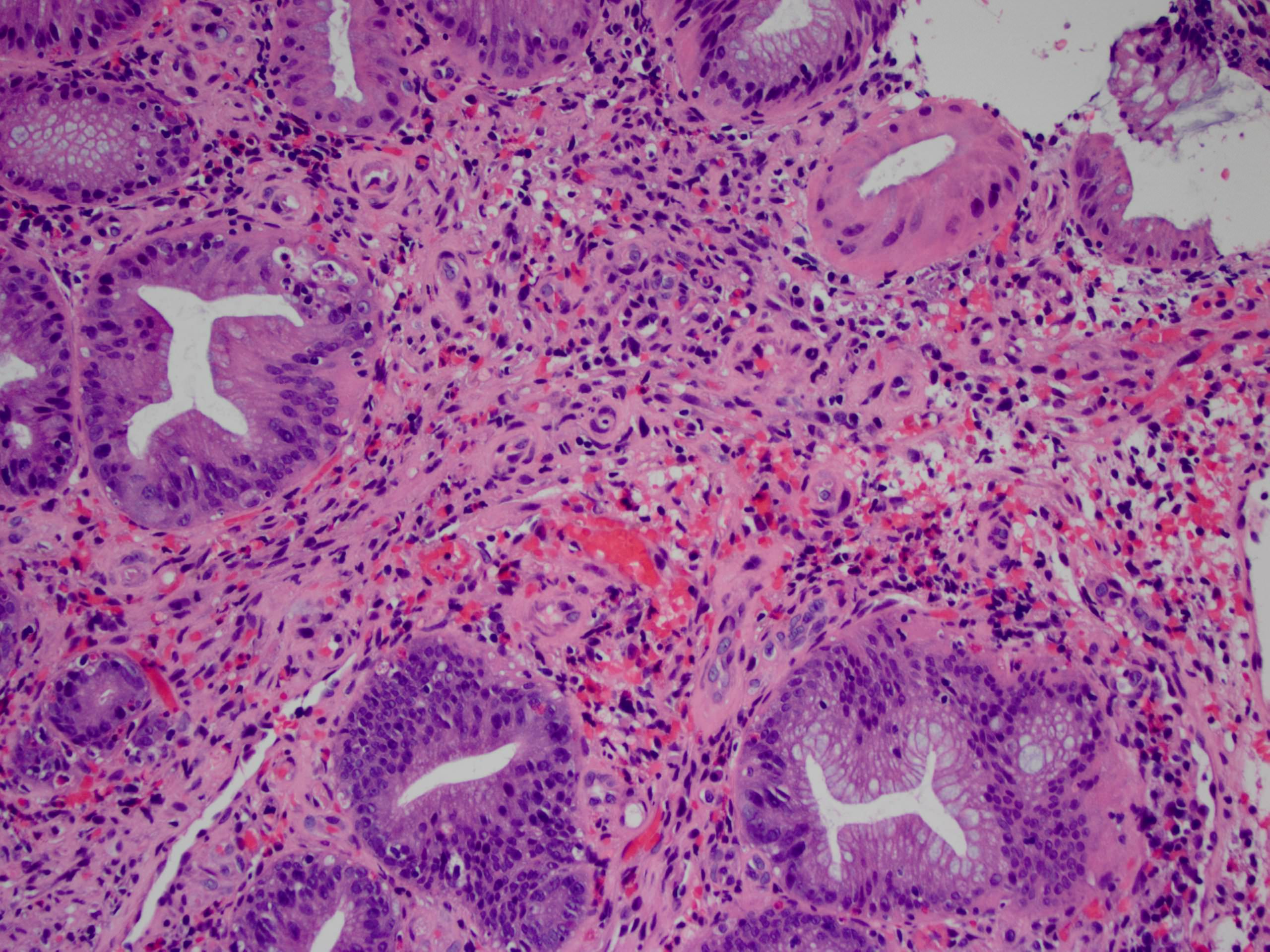
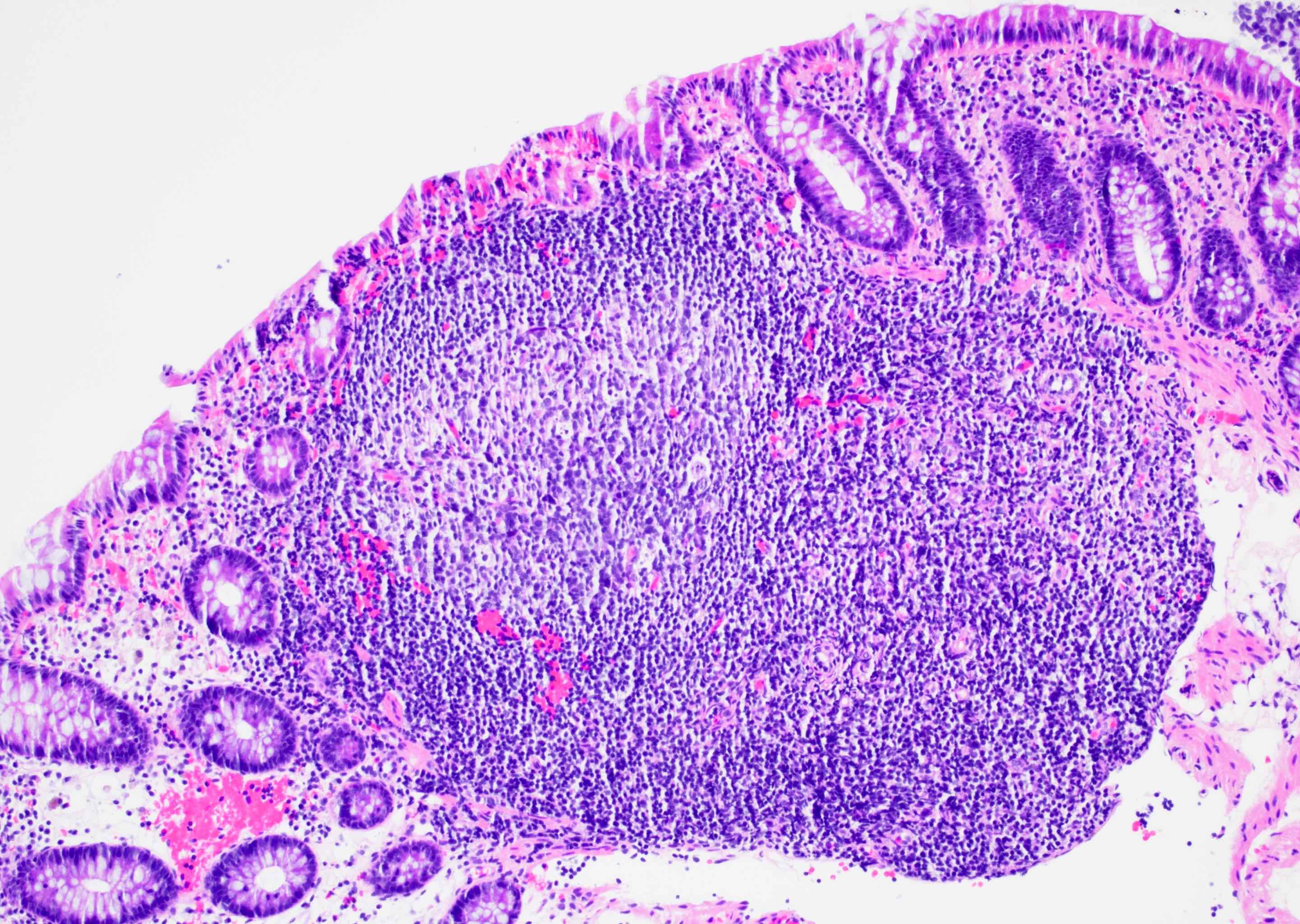
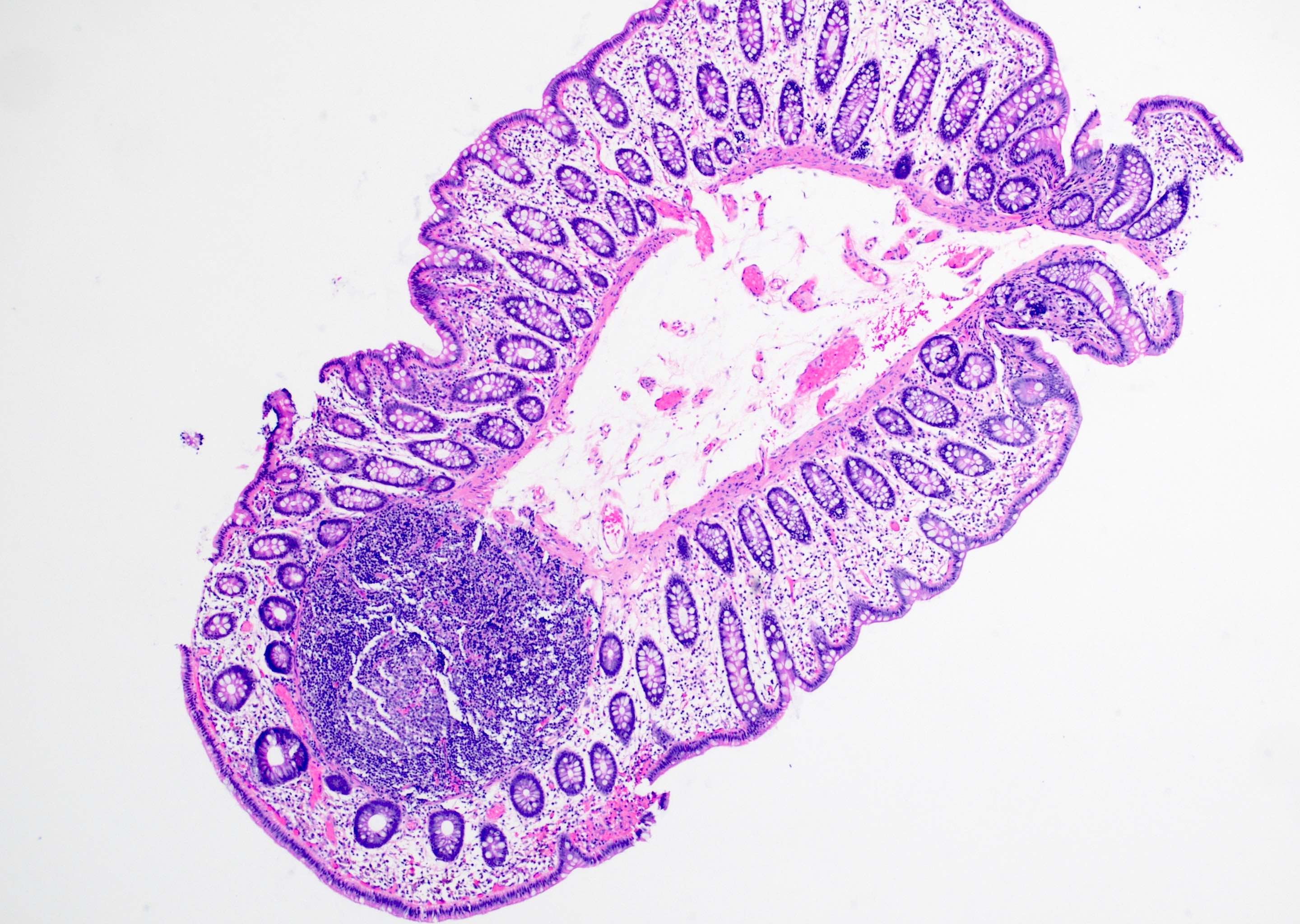
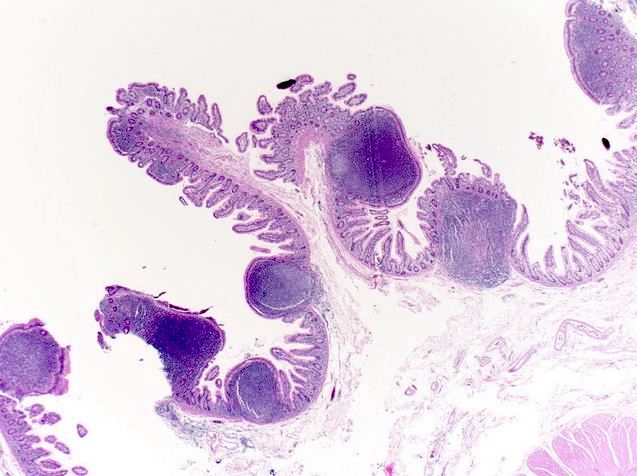
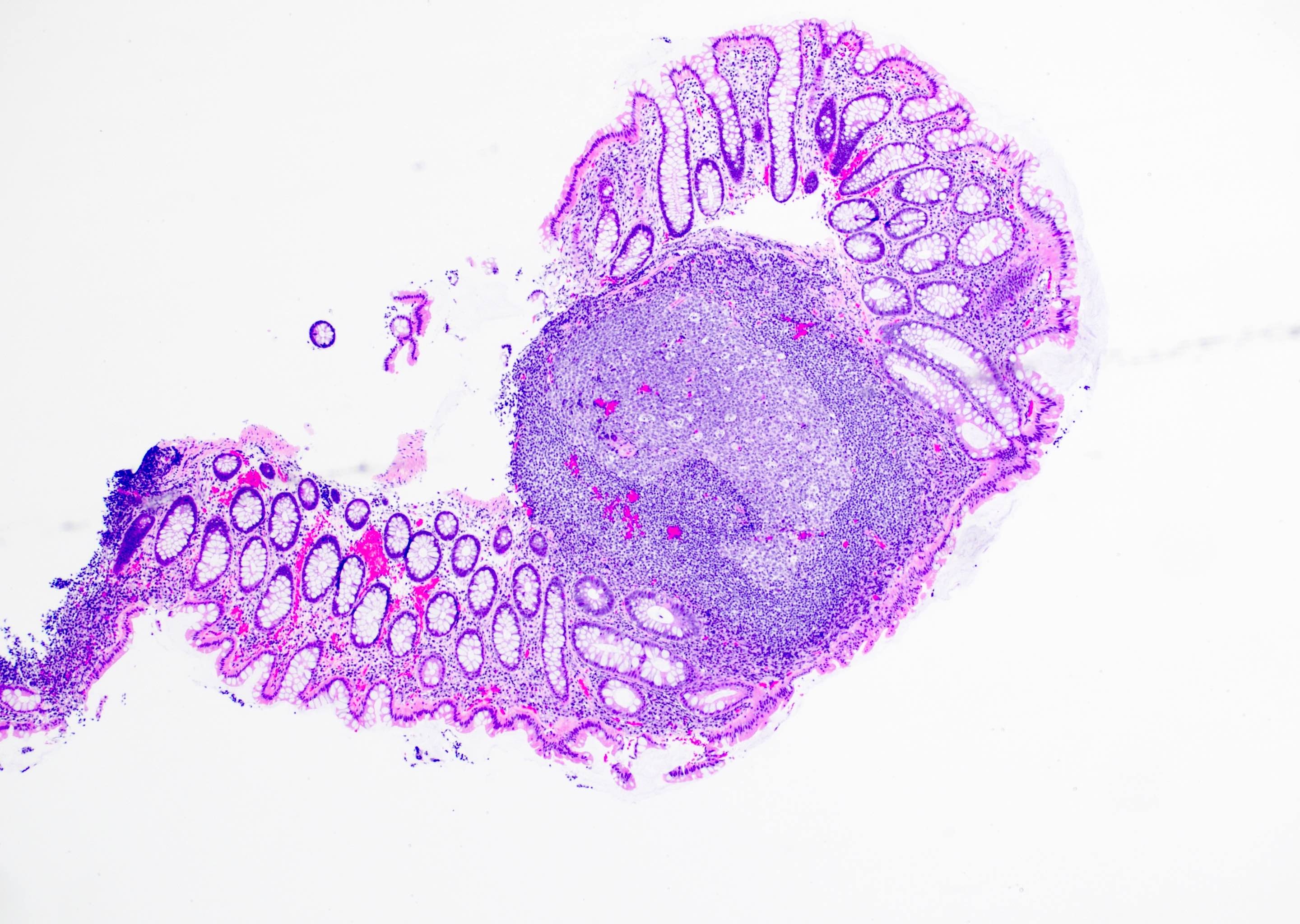
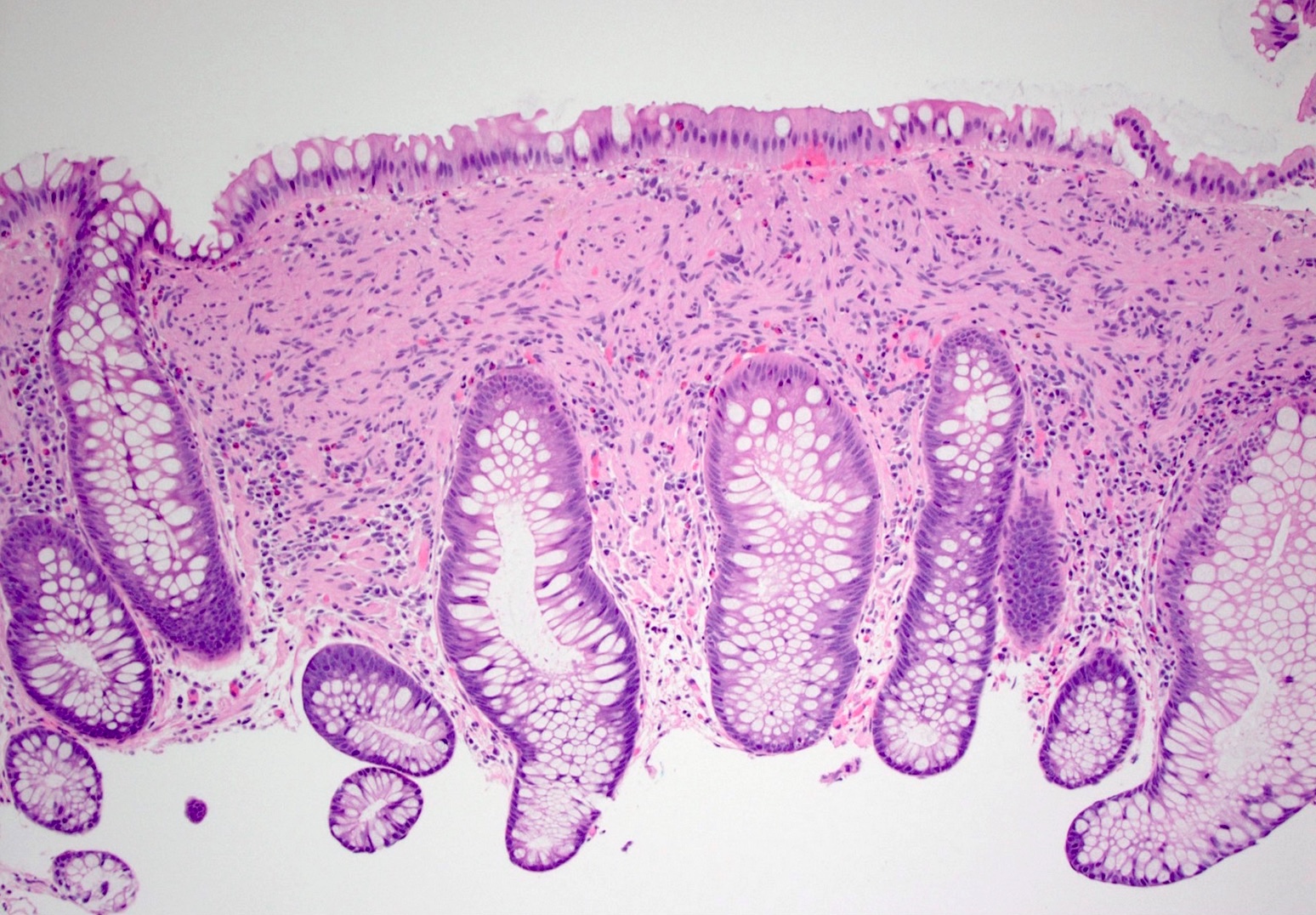
Aberrant crypt foci
Actinomycosis
Acute self limited colitis
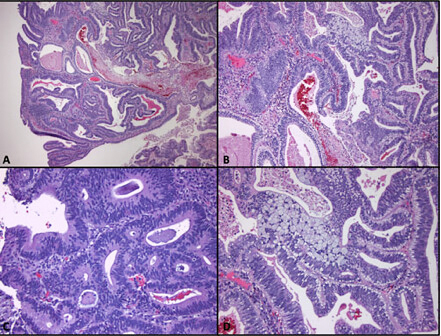
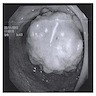
Adenocarcinoma
Adenoma overview
Adenoma with invasive carcinoma
Adenoma-like adenocarcinoma
Adenosquamous carcinoma
Adenovirus
Adhesions
Allergic colitis
Amebic colitis
Amyloidosis
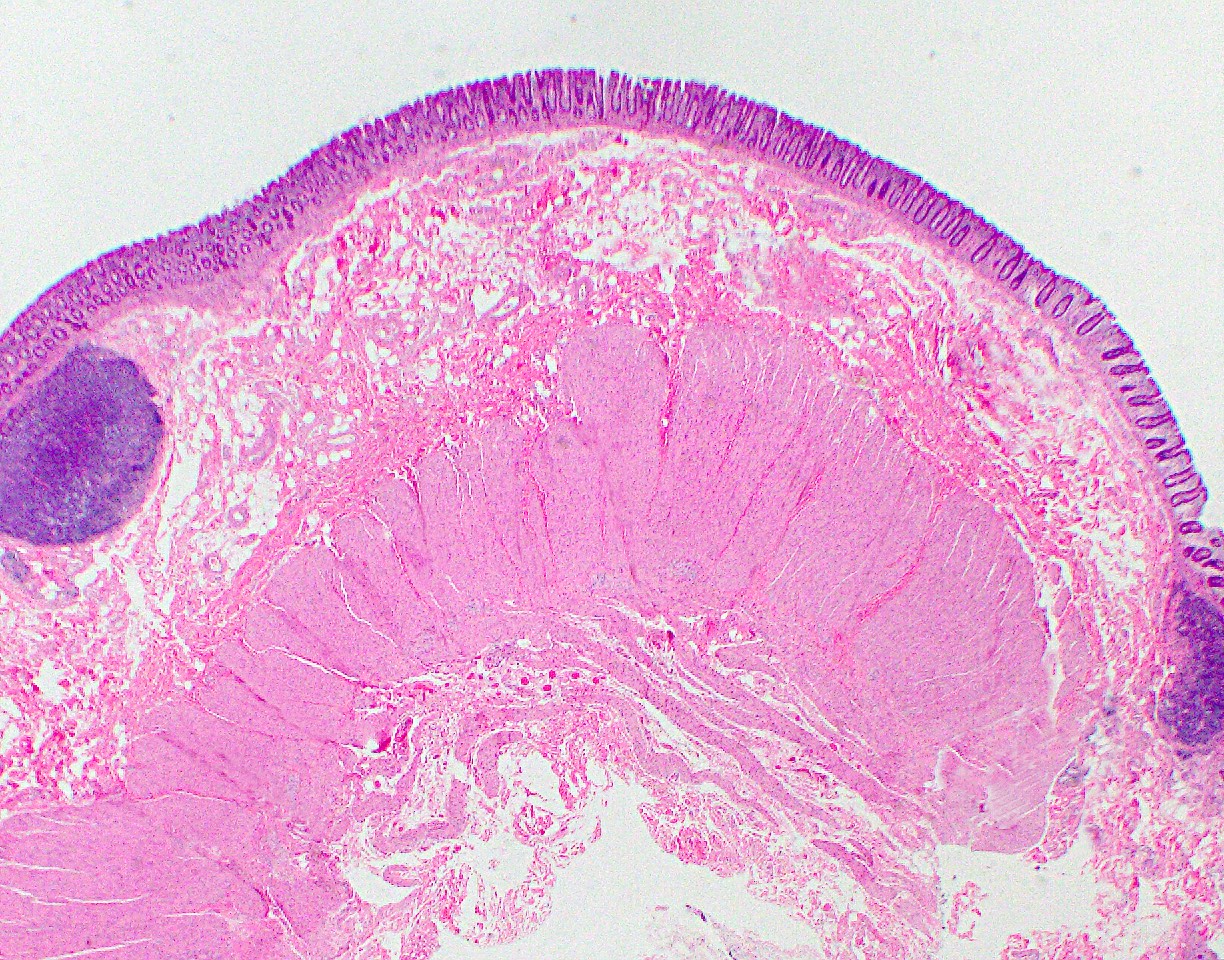
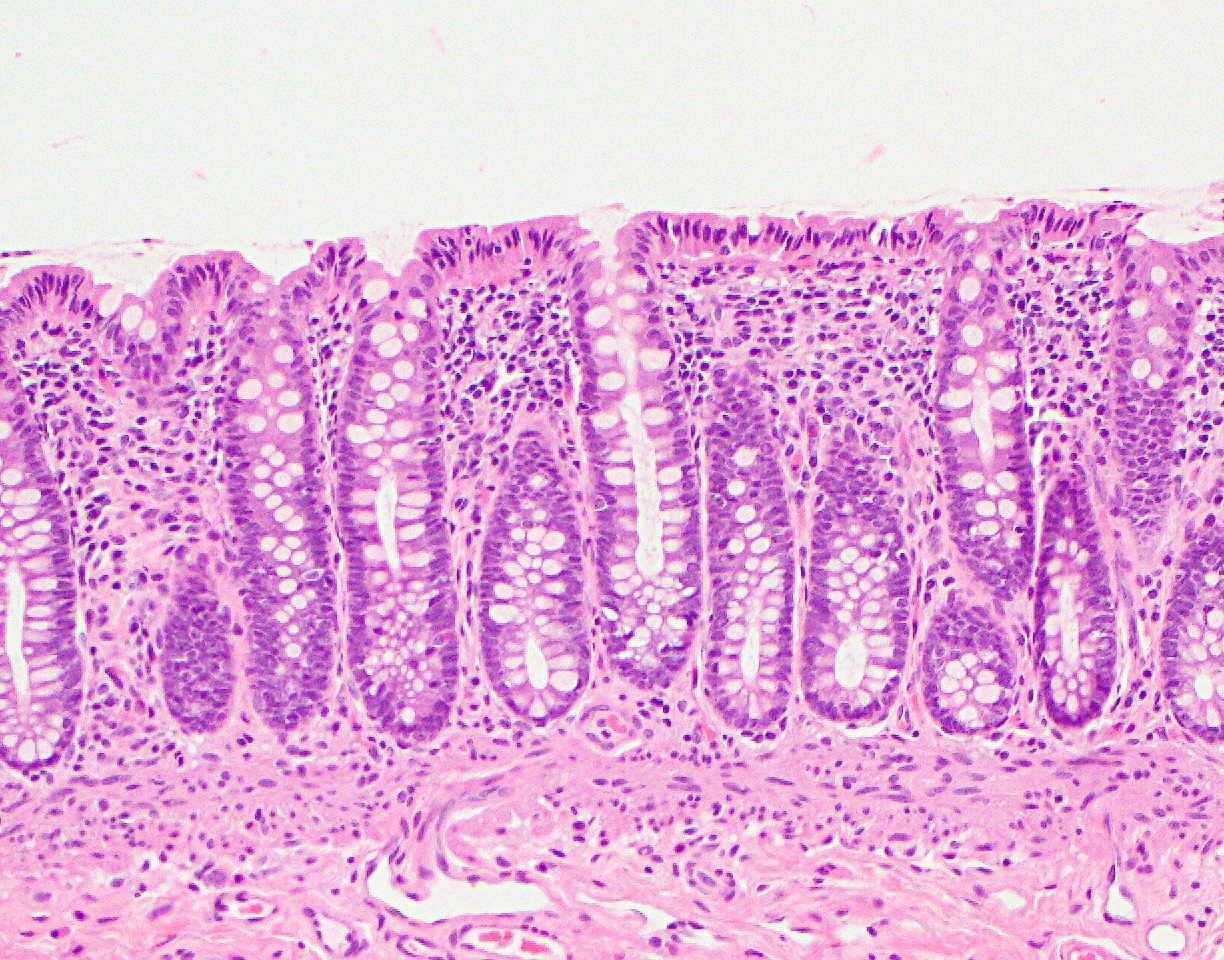
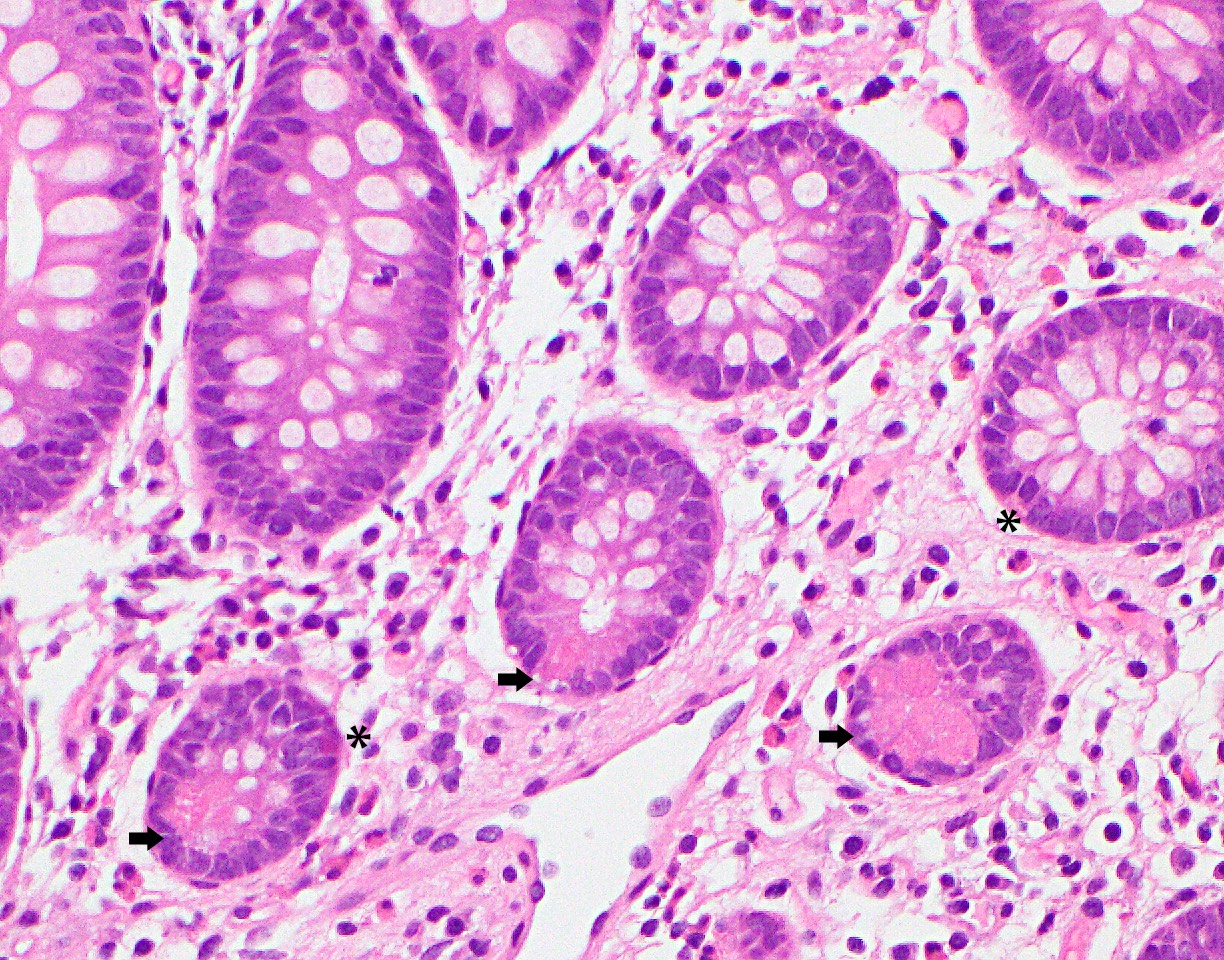
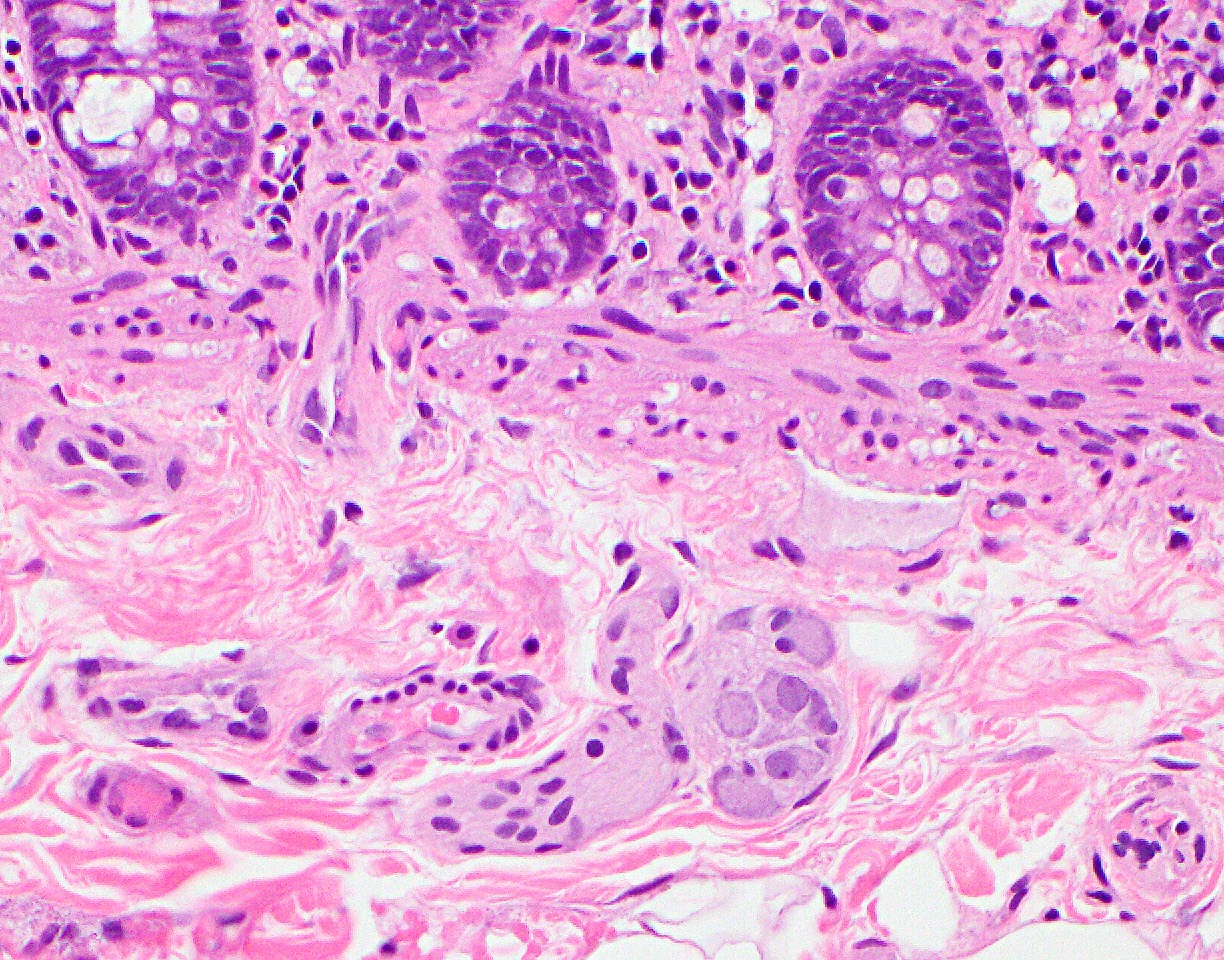
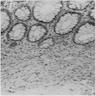
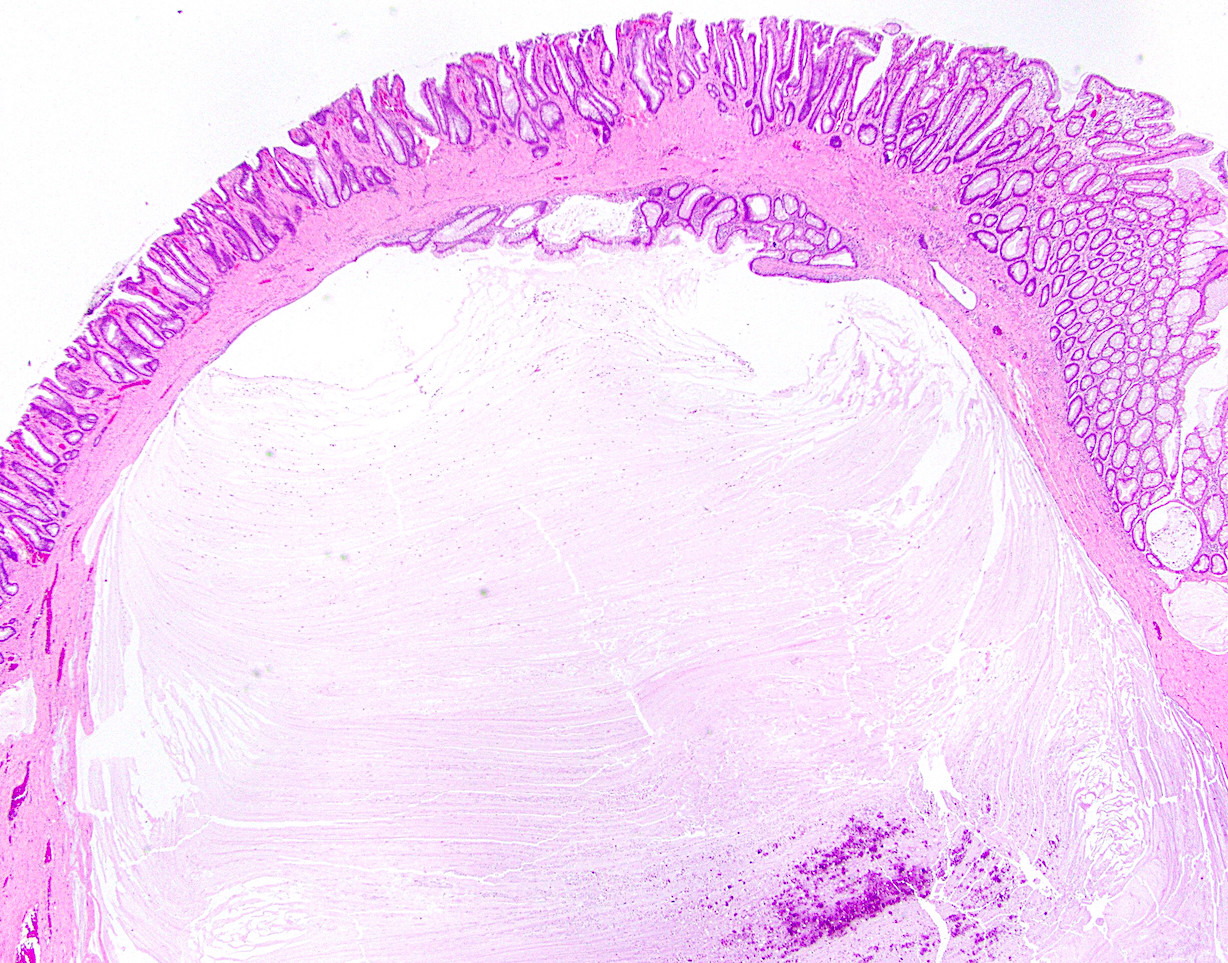
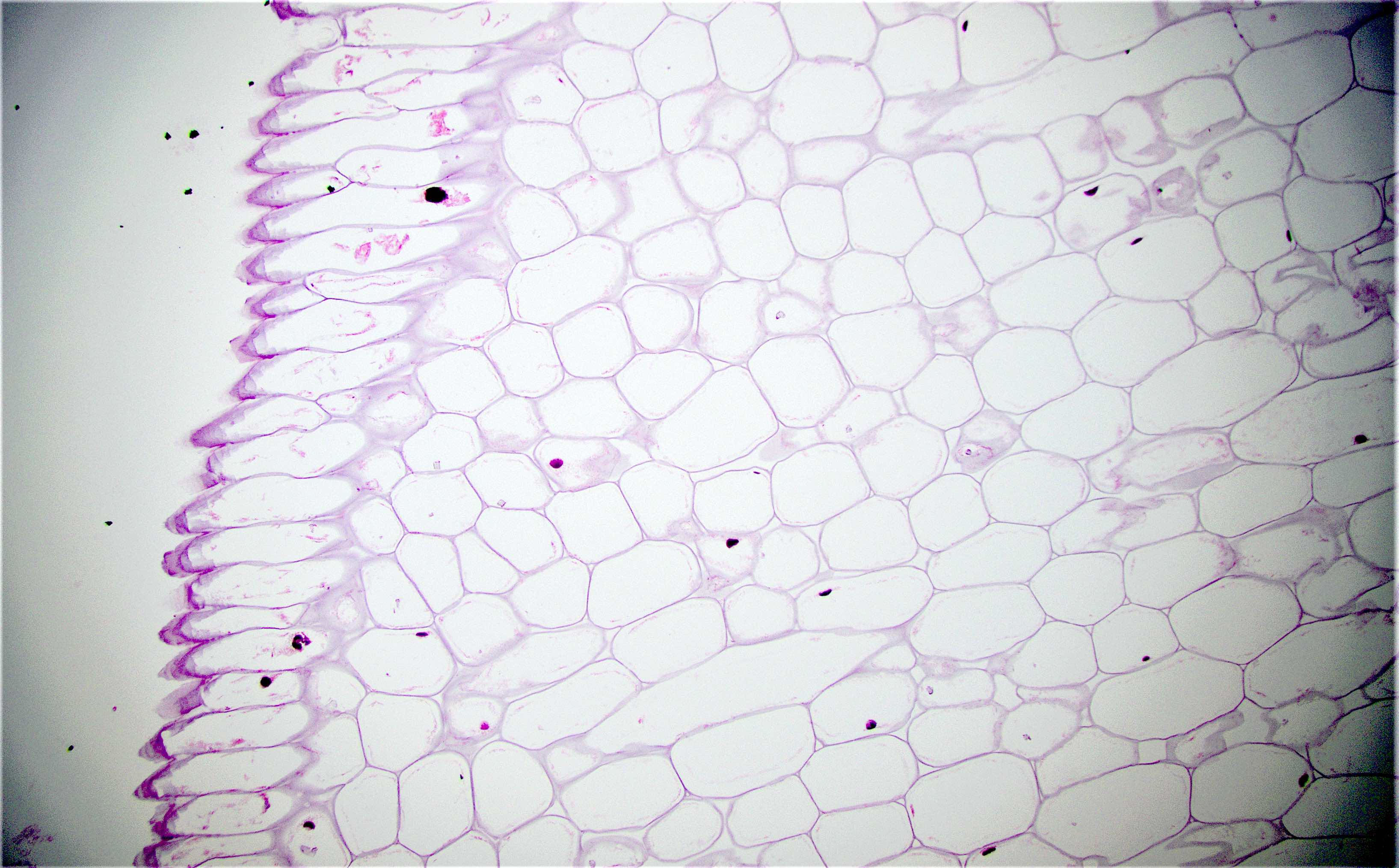
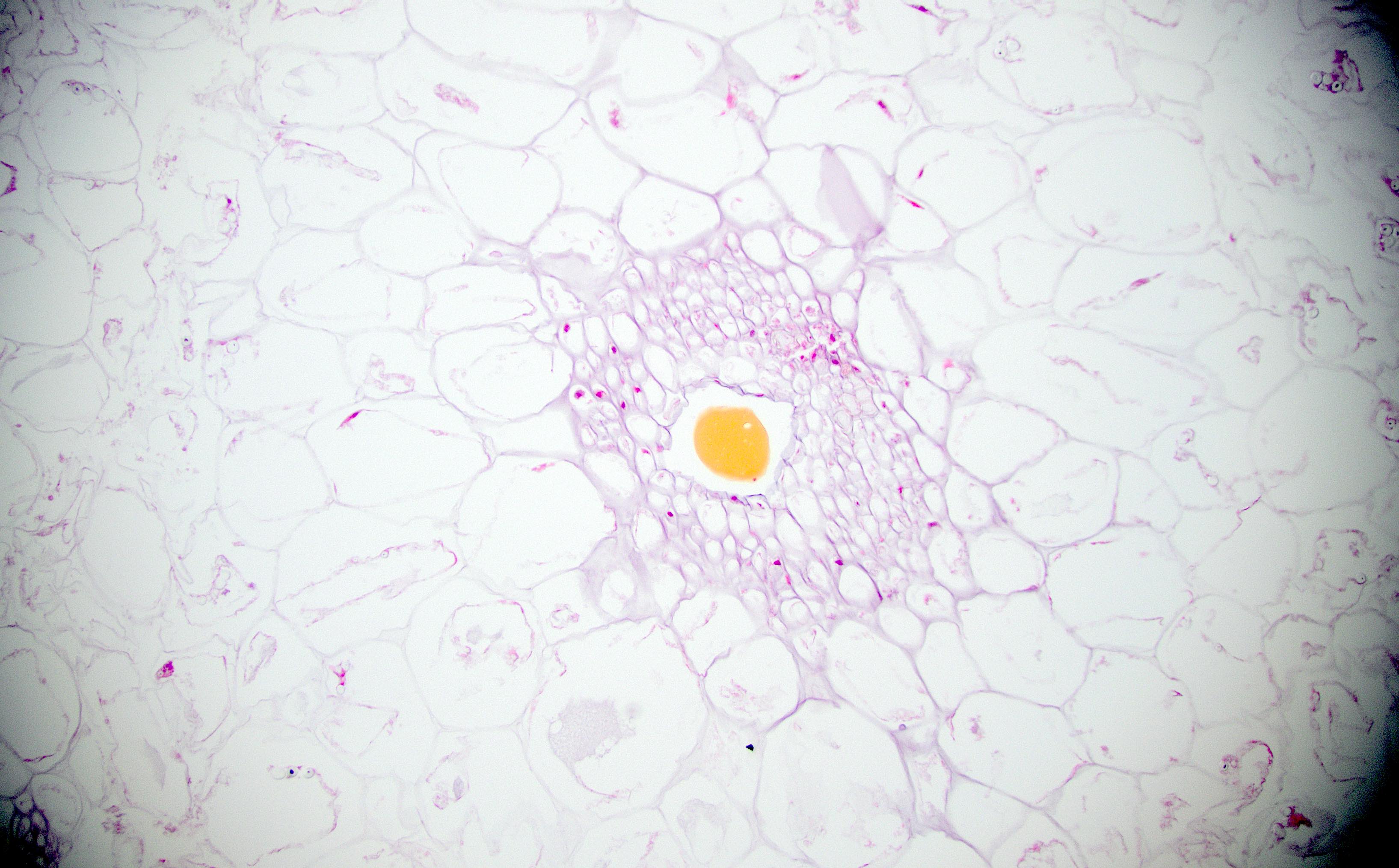
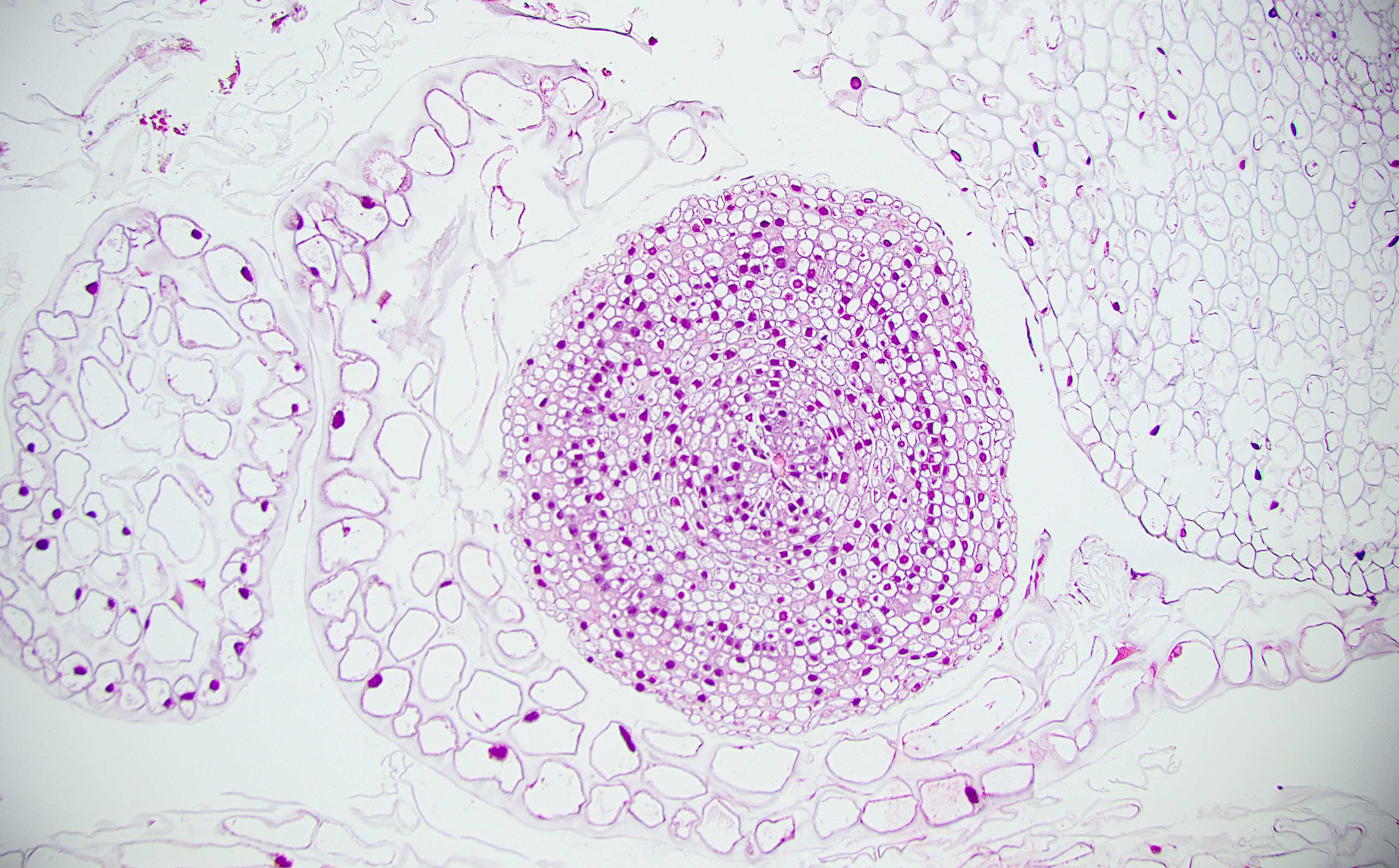
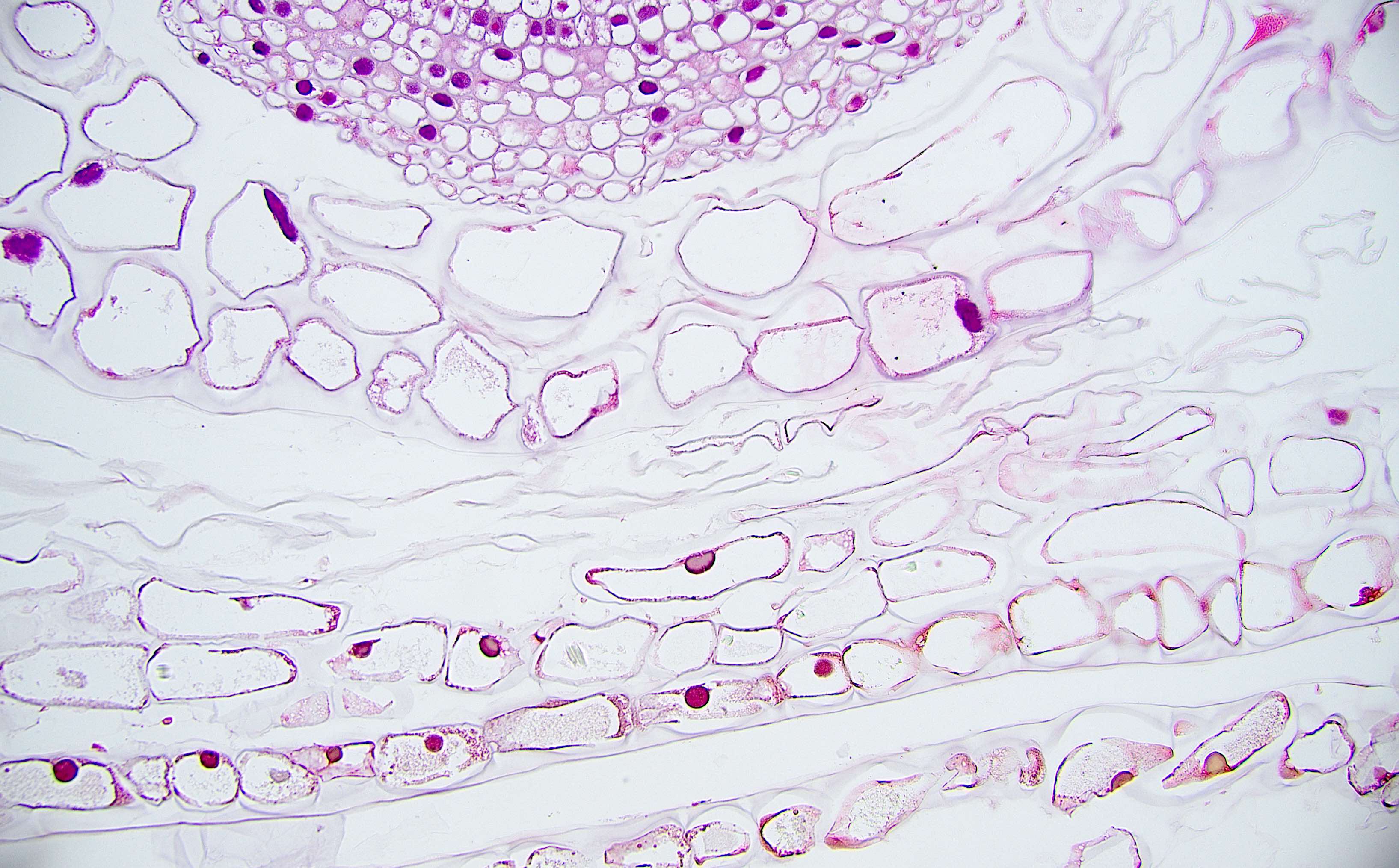
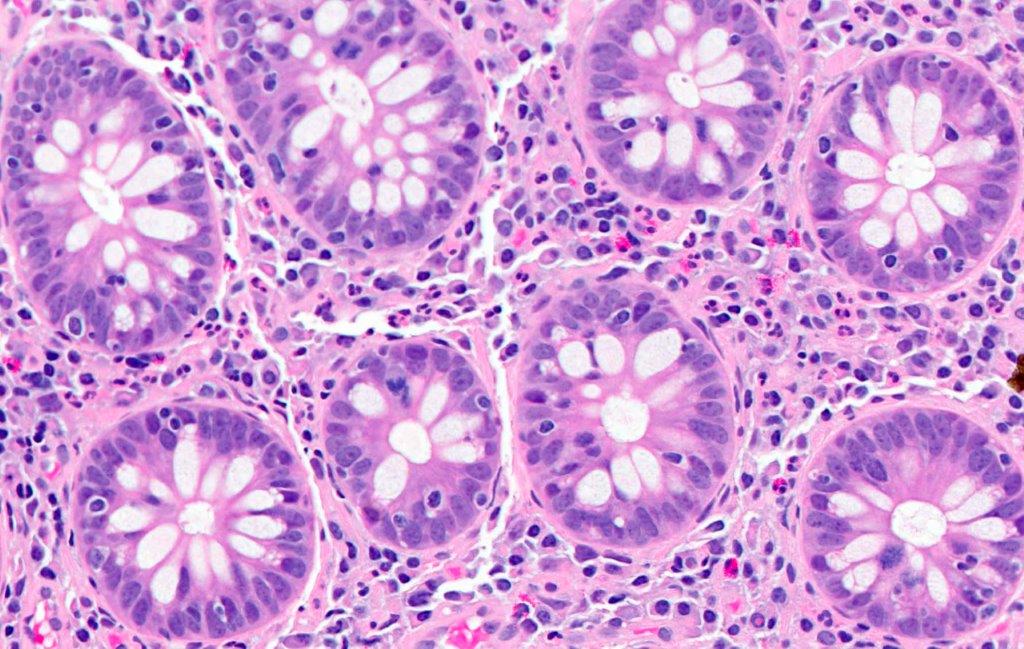
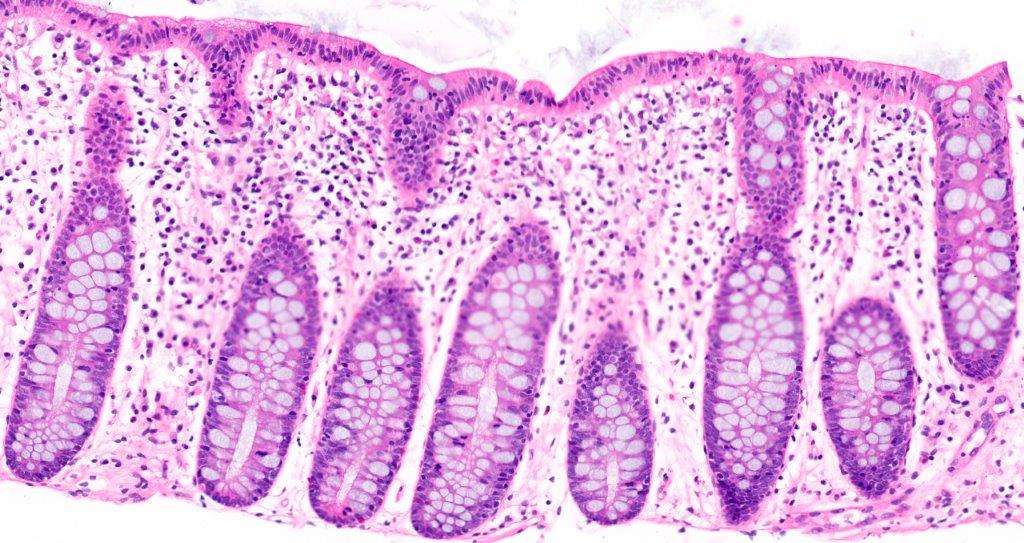
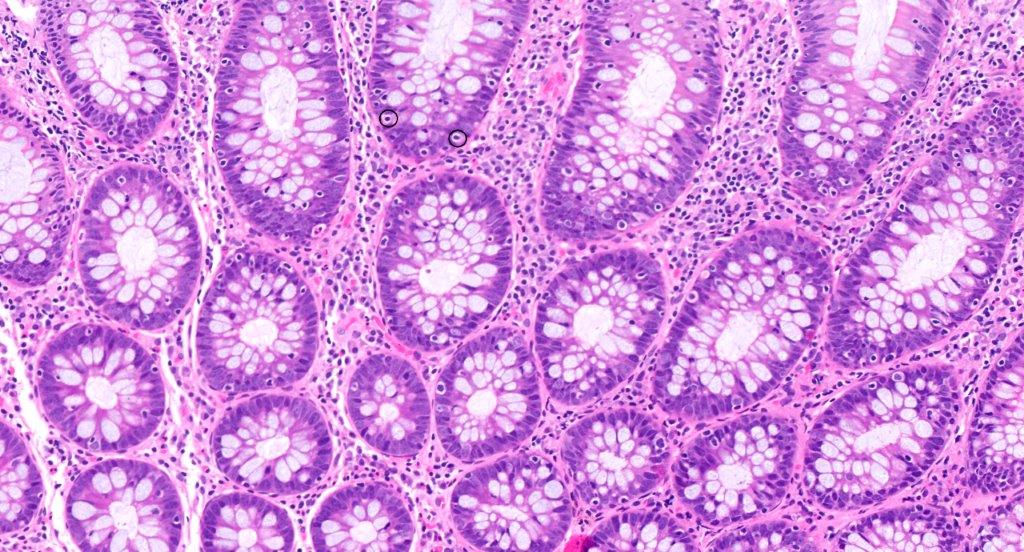
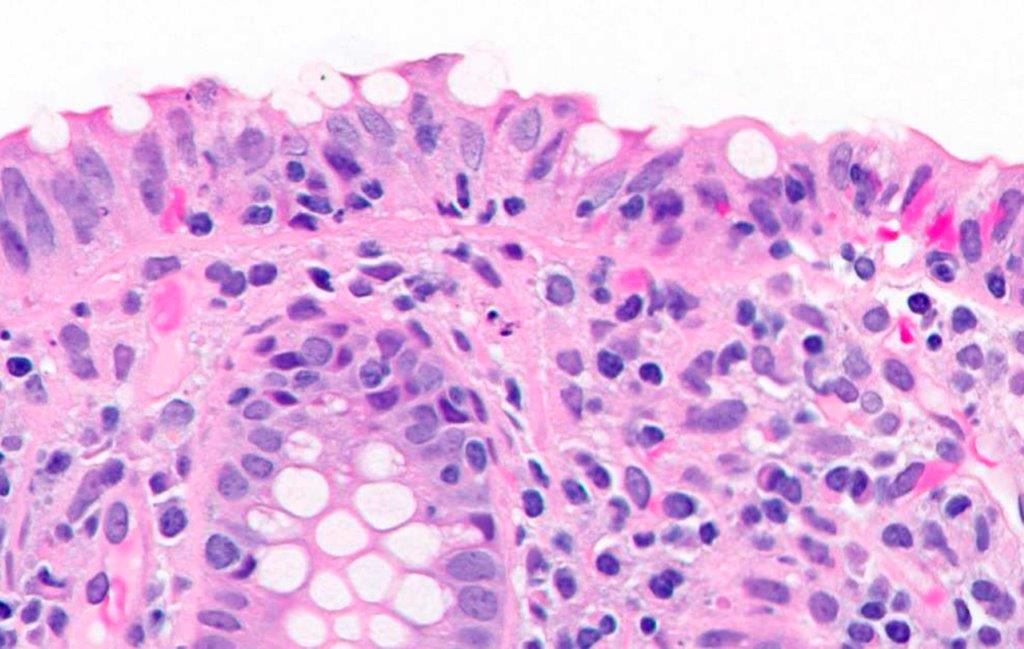
Anatomy & histology
Angiosarcoma (pending)
Anti-PD1 associated colitis
APC gene
Apoptotic colopathy
Atresia
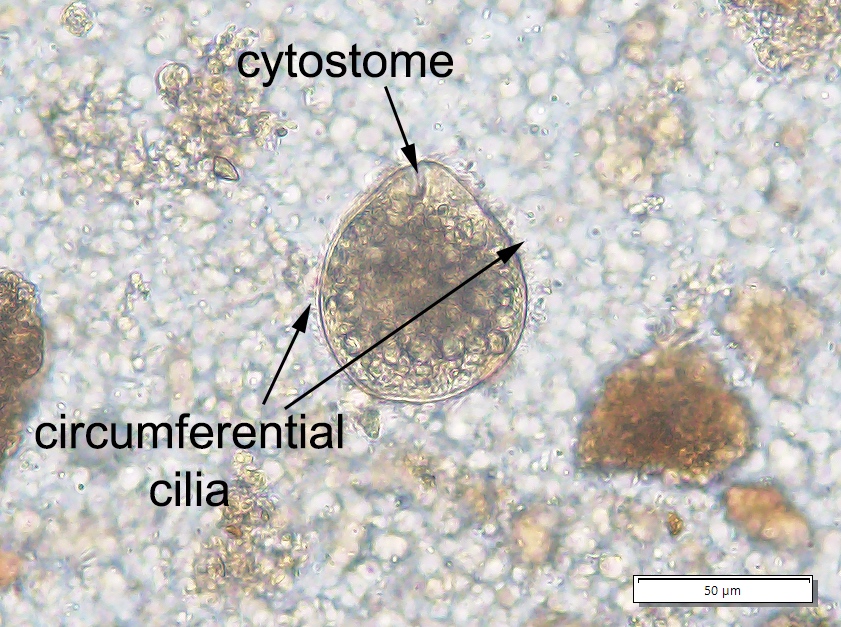
Balantidiasis
Basidiobolomycosis
Behcet's syndrome
Brainerd diarrhea
Brown bowel syndrome (pending)
Campylobacter jejuni
Candida
Chagas disease (trypanosomiasis)
Chemotherapy induced colitis (pending)
Chromosomal instability & MUTYH pathways
Chronic granulomatous disease (pending)
Chronic intestinal pseudo-obstruction
Clostridium botulinum
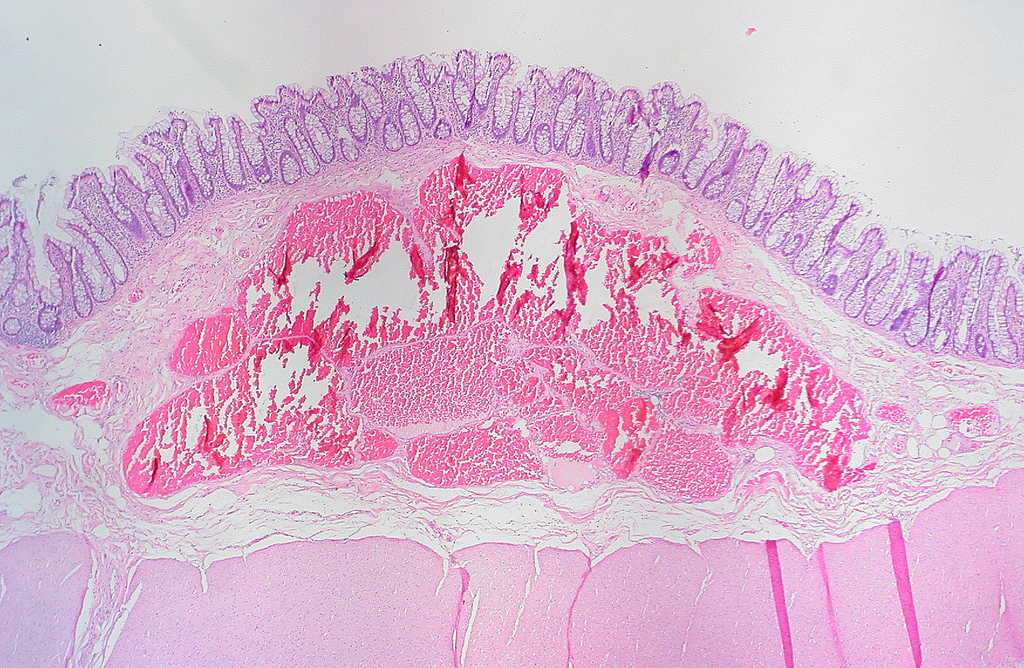
Colitis cystica profunda
Collagenous colitis
Colon carcinoma overview
Common variable immunodeficiency (CVID)
Congenital absence of muscularis propria (pending)
COVID-19 associated colitis
Cowden syndrome
Crohn's disease
Cronkhite-Canada syndrome
Cryptosporidium parvum
Cyst of retrorectal space
Cytomegalovirus (CMV)
Diarrhea / dysentery
Diversion colitis
Diverticular colitis
Diverticulosis
Drug induced colitis - overview (pending)
Duplication
Dysplasia
Ehlers Danlos syndrome
Elastofibromatous change
Endometriosis
Eosinophilic gastroenterocolitis
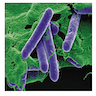
Escherichia coli
Familial adenomatous polyposis, attenuated
Familial adenomatous polyposis, classic
Focal active colitis
Foreign materials / food
Foreign materials / food (pending)
Ganglioneuroma
Ganglioneuromatosis
Gardner syndrome
Gastric heterotopia
Gastrointestinal stromal tumor
Graft versus host disease
Granulomatous colitis
Grossing & features to report
Hemangioma
Hepatoid carcinoma
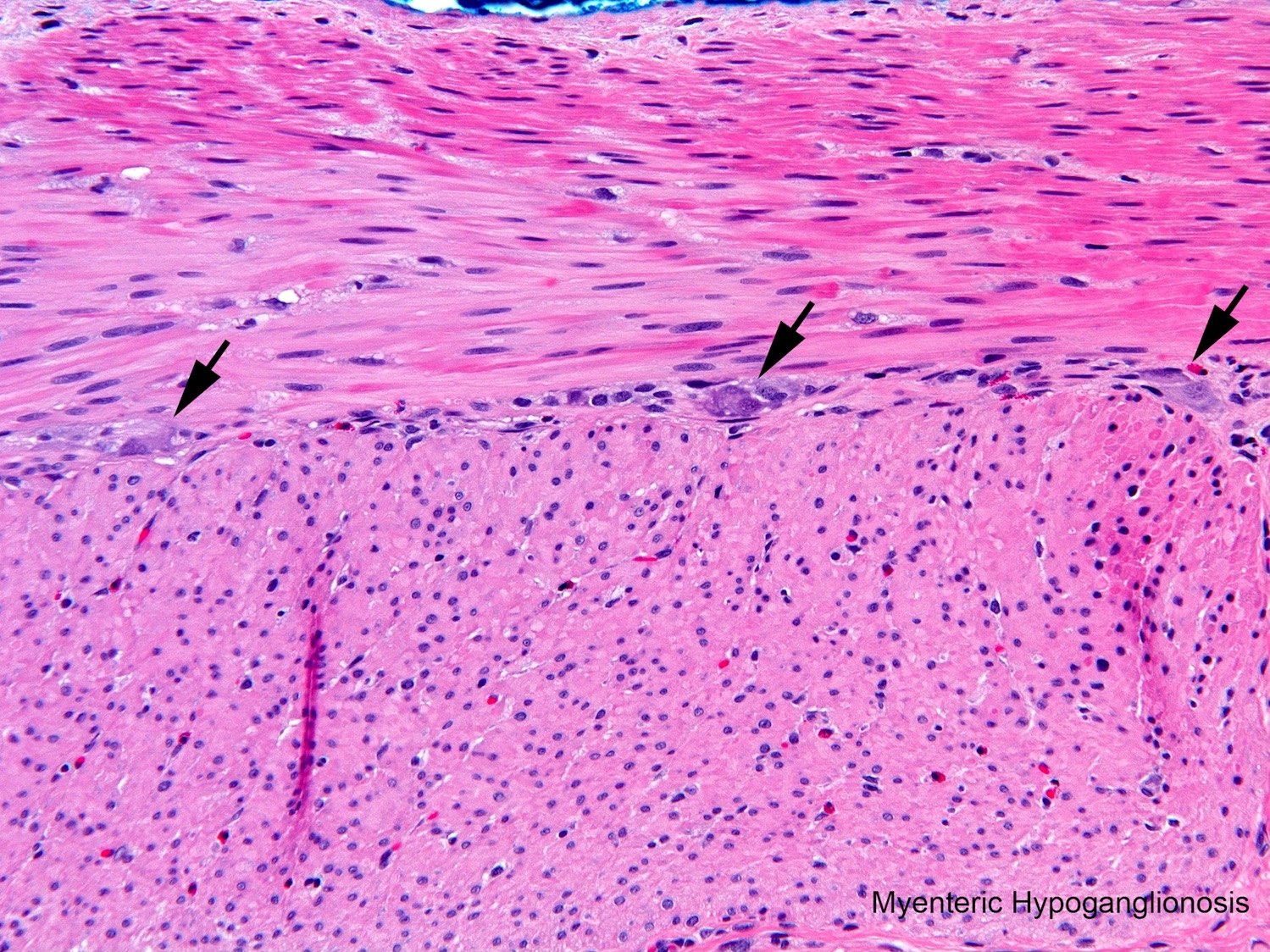
Hirschsprung disease
Histoplasmosis
HIV/AIDS associated
HSV
HSV
Hyperplastic polyp
IBD associated
Idelalisib associated
Idiopathic myointimal hyperplasia of mesenteric veins
Idiopathic retroperitoneal fibrosis
Infarct
Infarcted epiploic appendages
Inflammatory bowel disease, indeterminate type
Inflammatory cap polyp
Inflammatory fibroid polyp
Inflammatory myofibroblastic tumor
Inflammatory polyp
Intestinal neuronal dysplasia
Intestinal spirochetosis
Intramucosal carcinoma
Ipilimumab associated colitis
Ischemic colitis
Juvenile (retention) polyp
Juvenile polyposis syndrome
Kaposi sarcoma
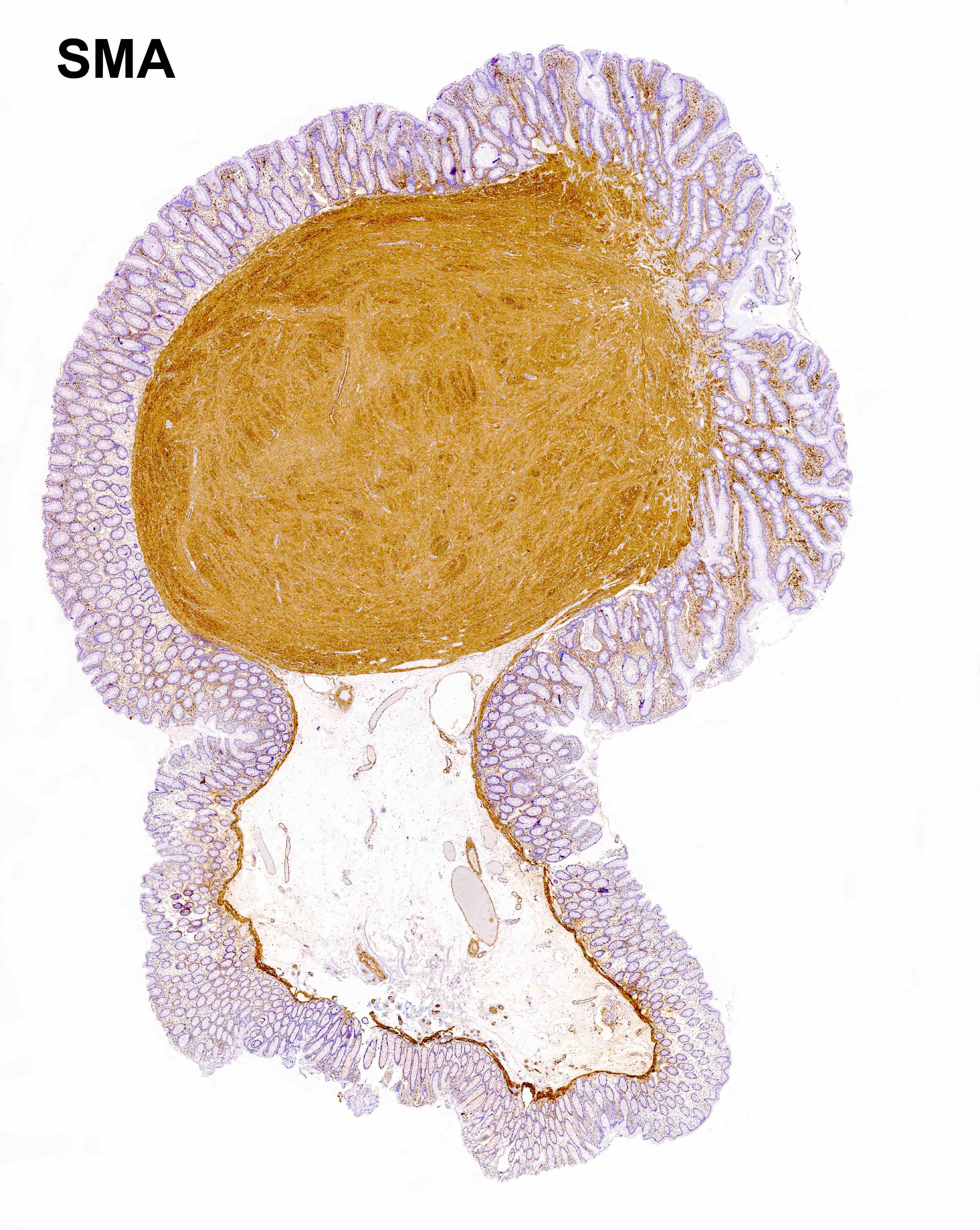
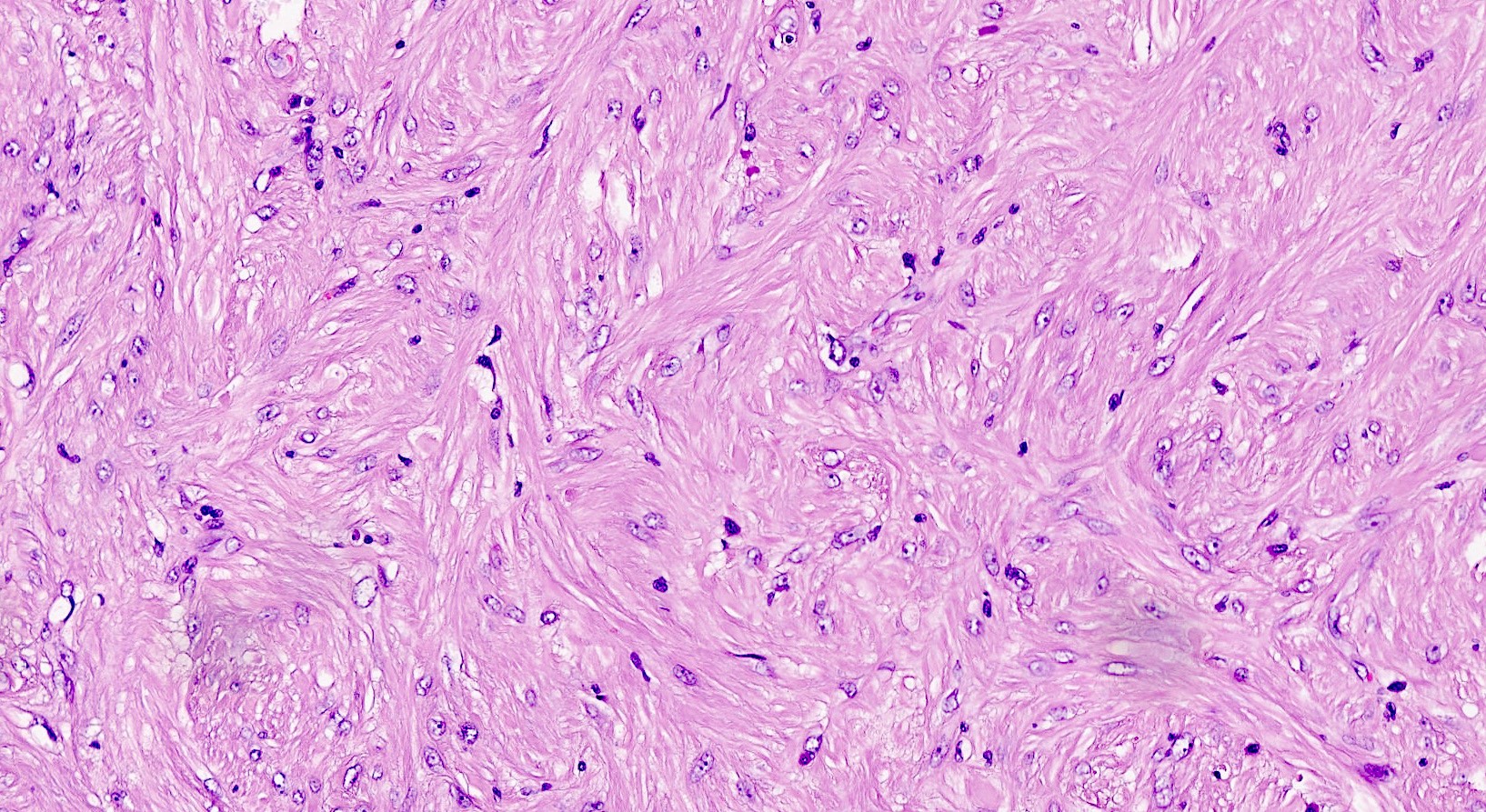
Leiomyoma
Leiomyosarcoma
Lifting agent granuloma
Lipoma
Low grade tubuloglandular adenocarcinoma
Lymphocytic colitis
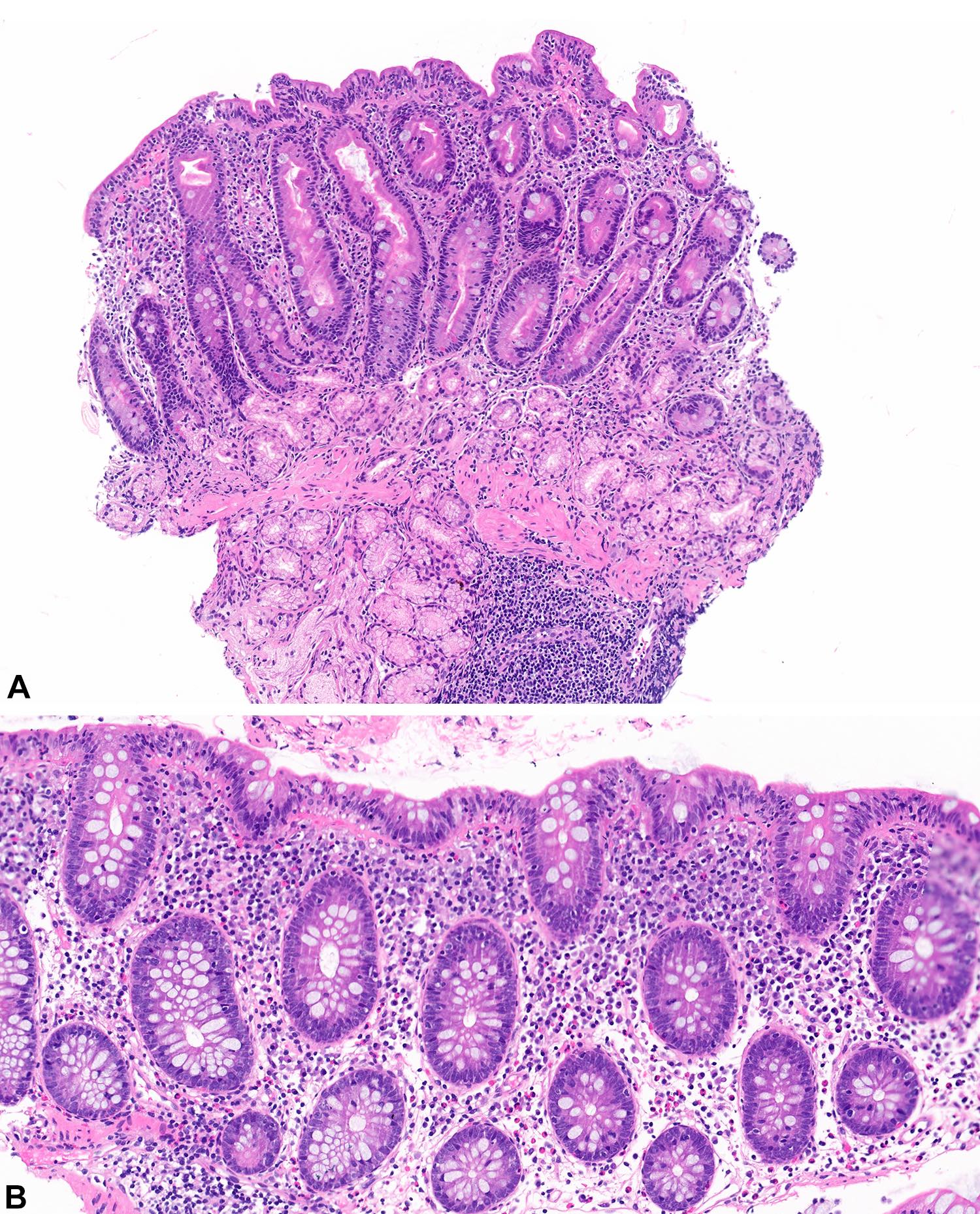
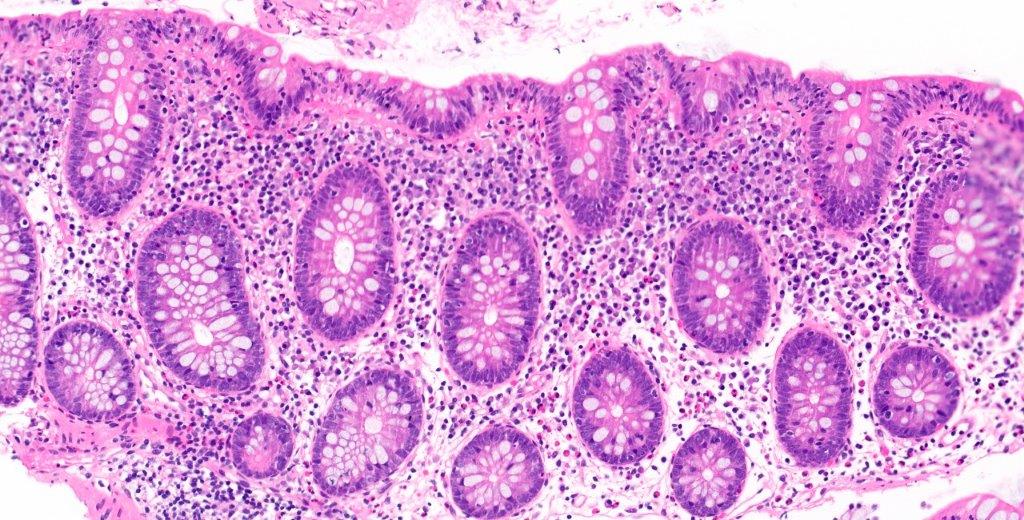
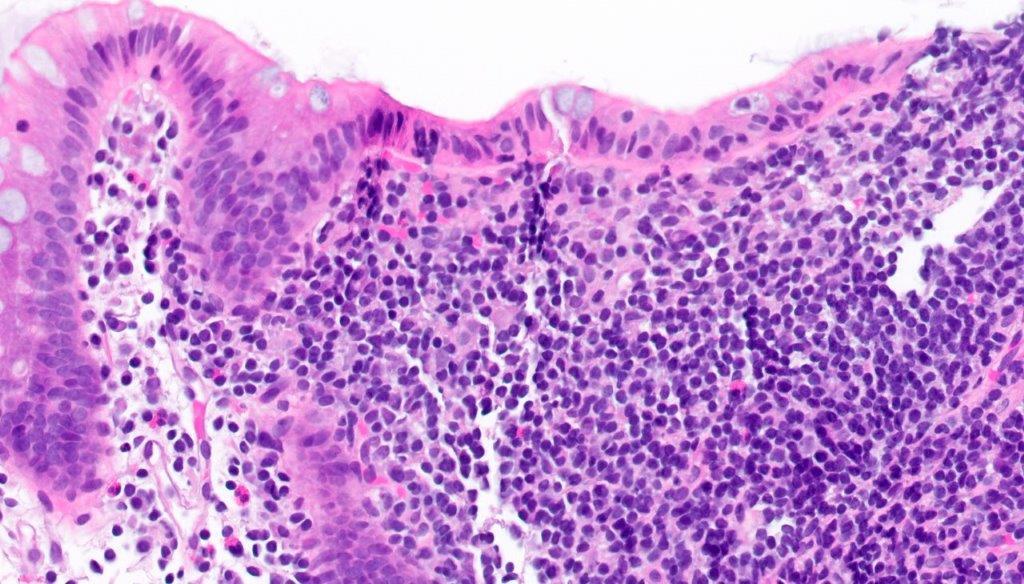
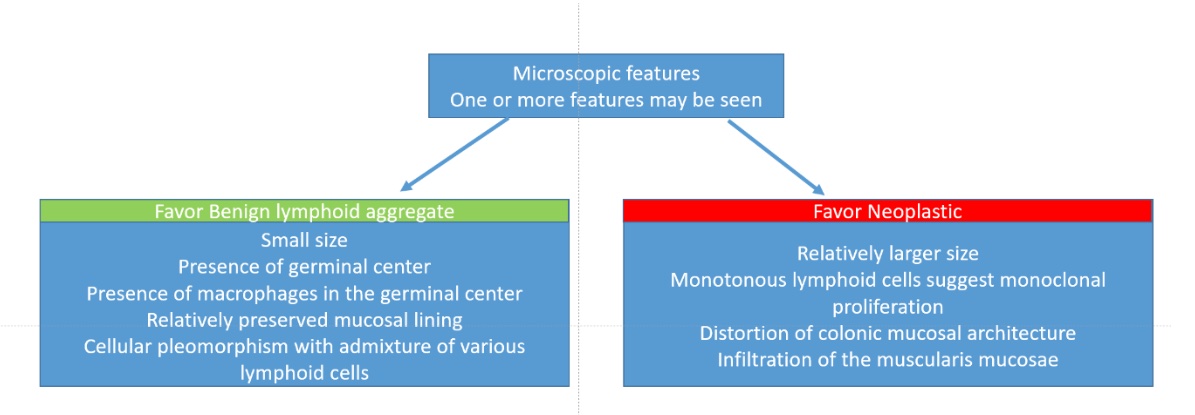
Lymphoid polyp
Lynch syndrome
Malakoplakia
Mantle cell lymphoma
Mast cell disorders
Medullary carcinoma
Melanosis coli
Metastases
Micropapillary carcinoma
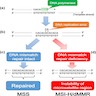
Microsatellite instability pathway
Mixed neuroendocrine nonneuroendocrine neoplasm
Mucinous adenocarcinoma
Mucosal prolapse polyp (pending)
Mucosal Schwann cell hamartoma
Muir-Torre syndrome
MUTYH associated polyposis
Mycobacterium avium-intercellulare (pending)
Mycophenolate mofetil associated colitis
Necrotizing enterocolitis
Neuroendocrine carcinoma
Neuroendocrine tumor
NSAID associated colitis
Omphalocele
Perineurioma
Peutz-Jeghers syndrome
Pill fragment associated colitis
Pneumatosis cystoides intestinalis
POLD1 / POLE
Polyp overview
Portal hypertensive colopathy (pending)
Posttreatment changes
Pseudomembranous colitis
Pulse granuloma
Pyogenic granuloma
Radiation enterocolitis
Reactive nodular fibrous pseudotumor
Salmonella (typhoid and nontyphoidal)
Sapovirus
Schistosomiasis
Schwannoma
Scleroderma (pending)
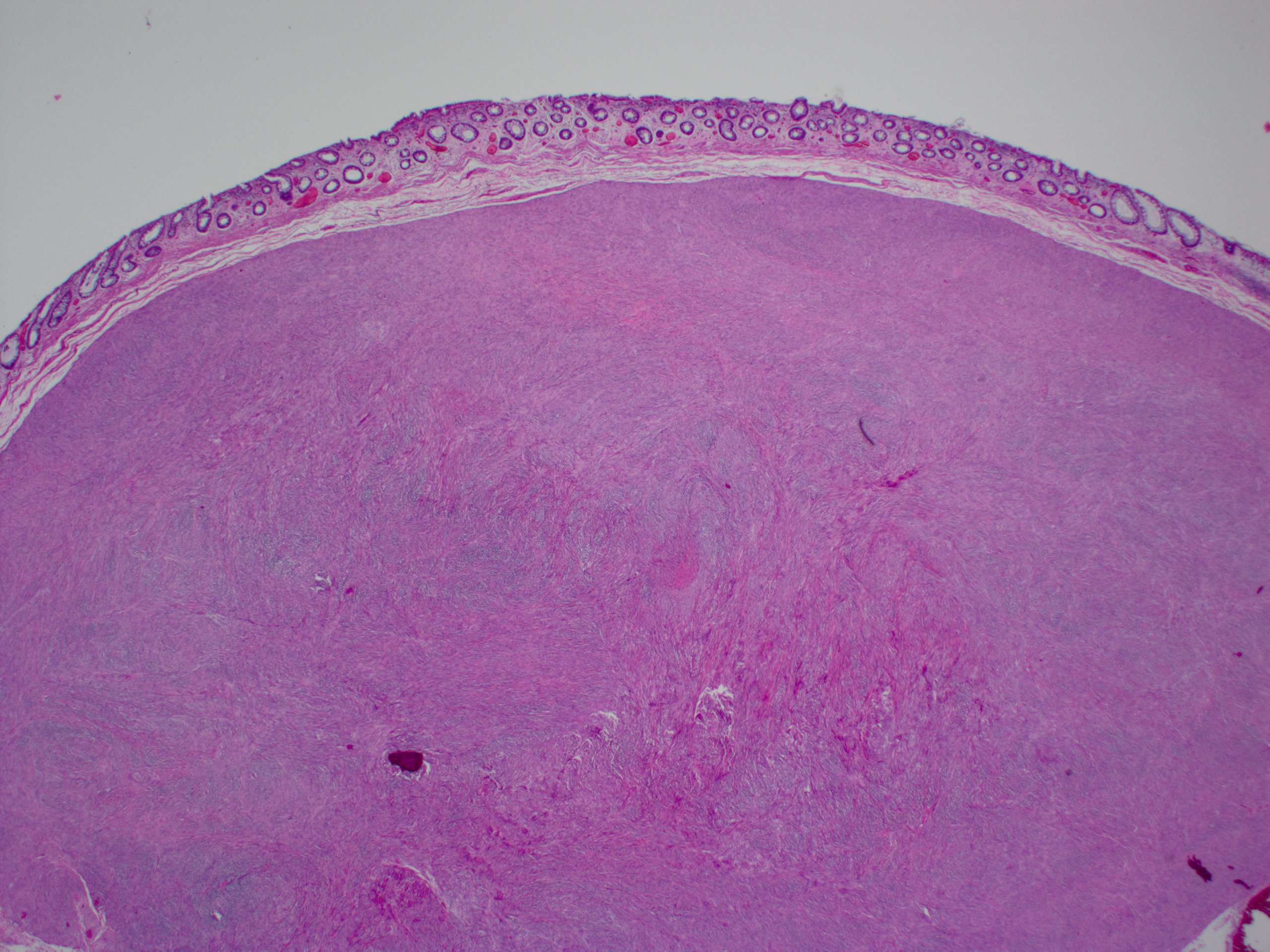
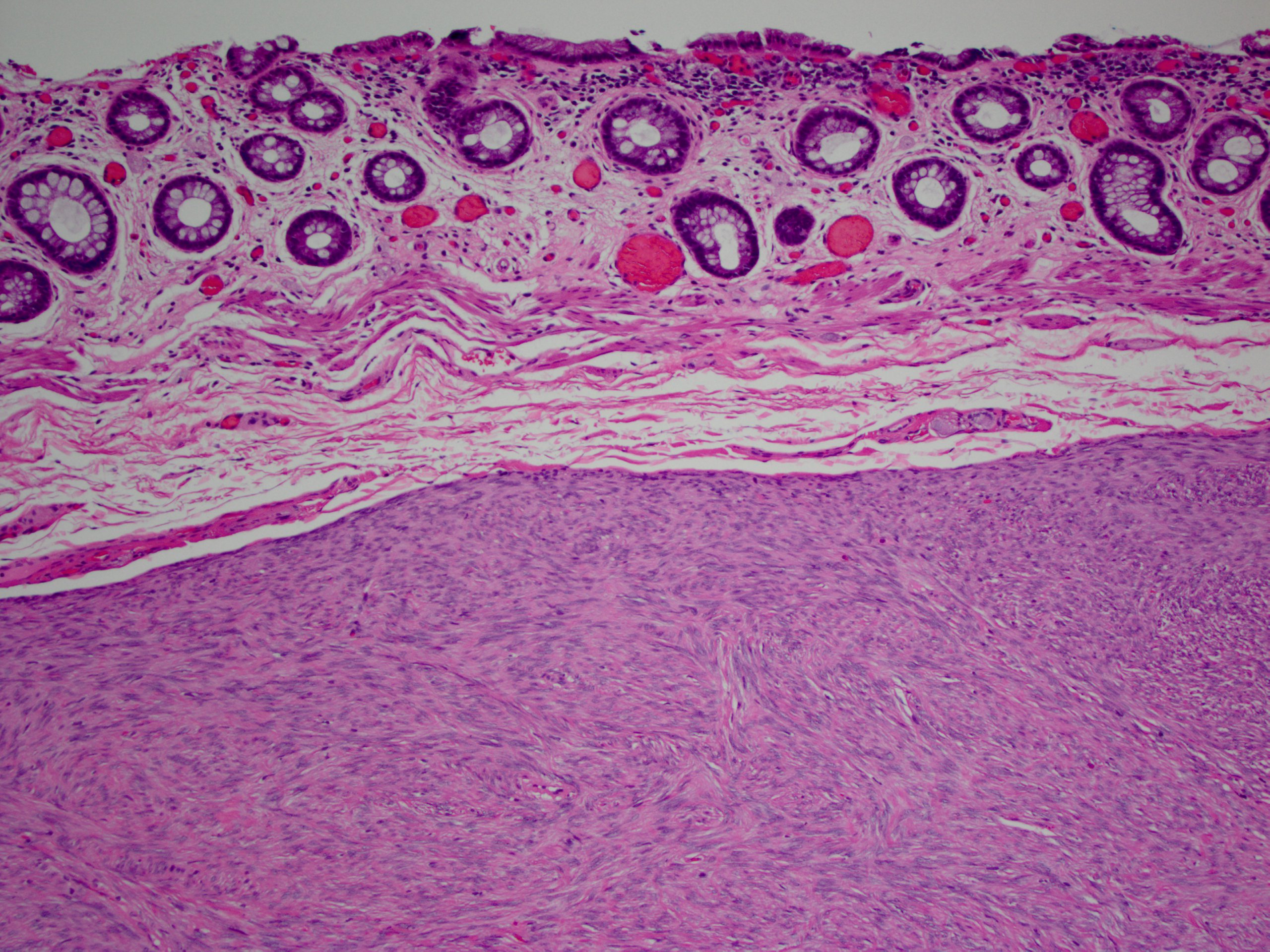
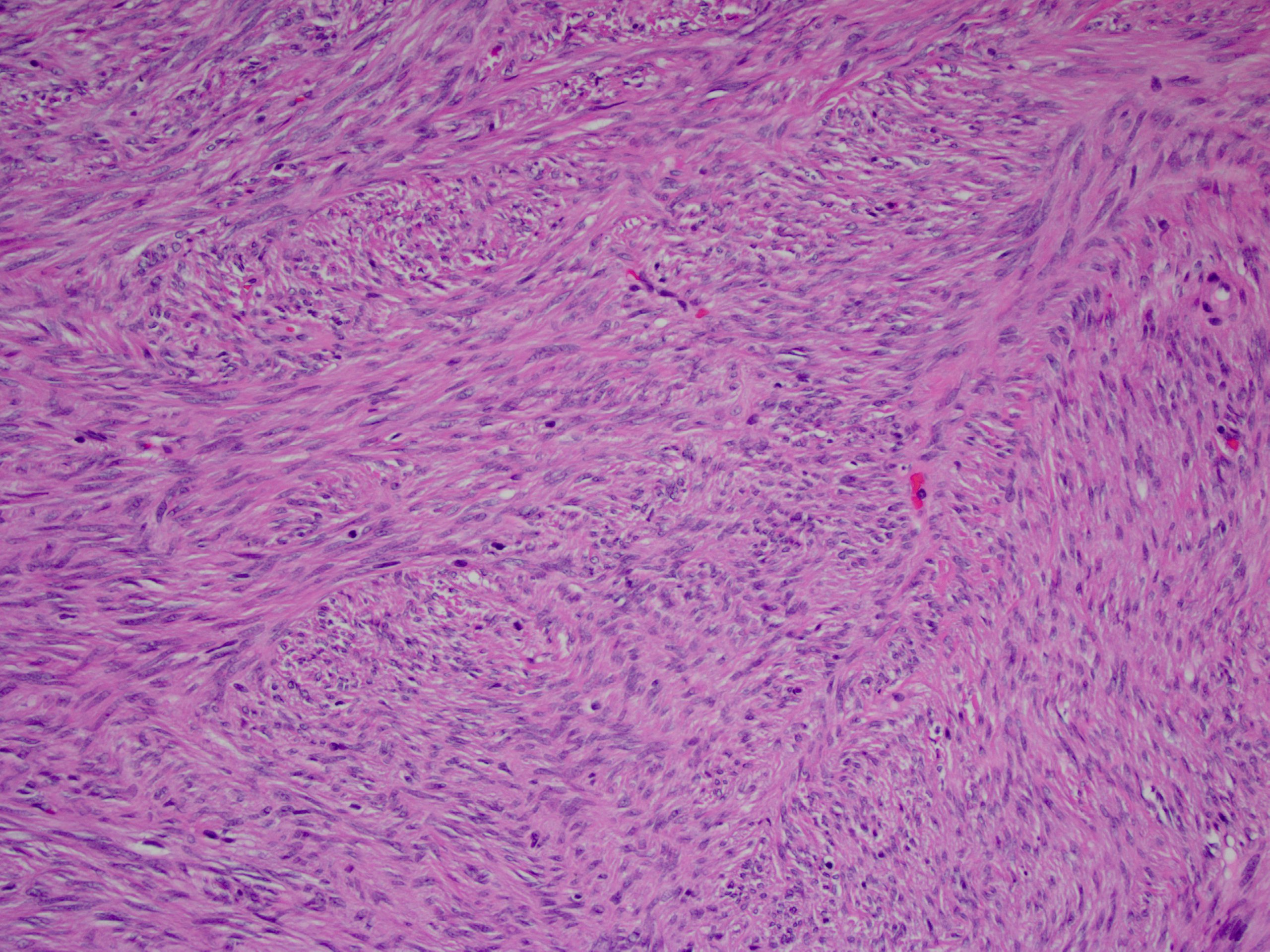
Sclerosing mesenteritis
Secukinumab induced colitis (pending)
Serrated adenocarcinoma (SAC)
Serrated lesions
Serrated pathway
Serrated polyposis
Sessile serrated adenoma
Sexually transmitted infectious colitis / proctitis
Shigella
Signet ring cell carcinoma
Solitary rectal ulcer syndrome
Squamous cell carcinoma
Staging-carcinoma
Staging-neuroendocrine
Stercoral ulcer
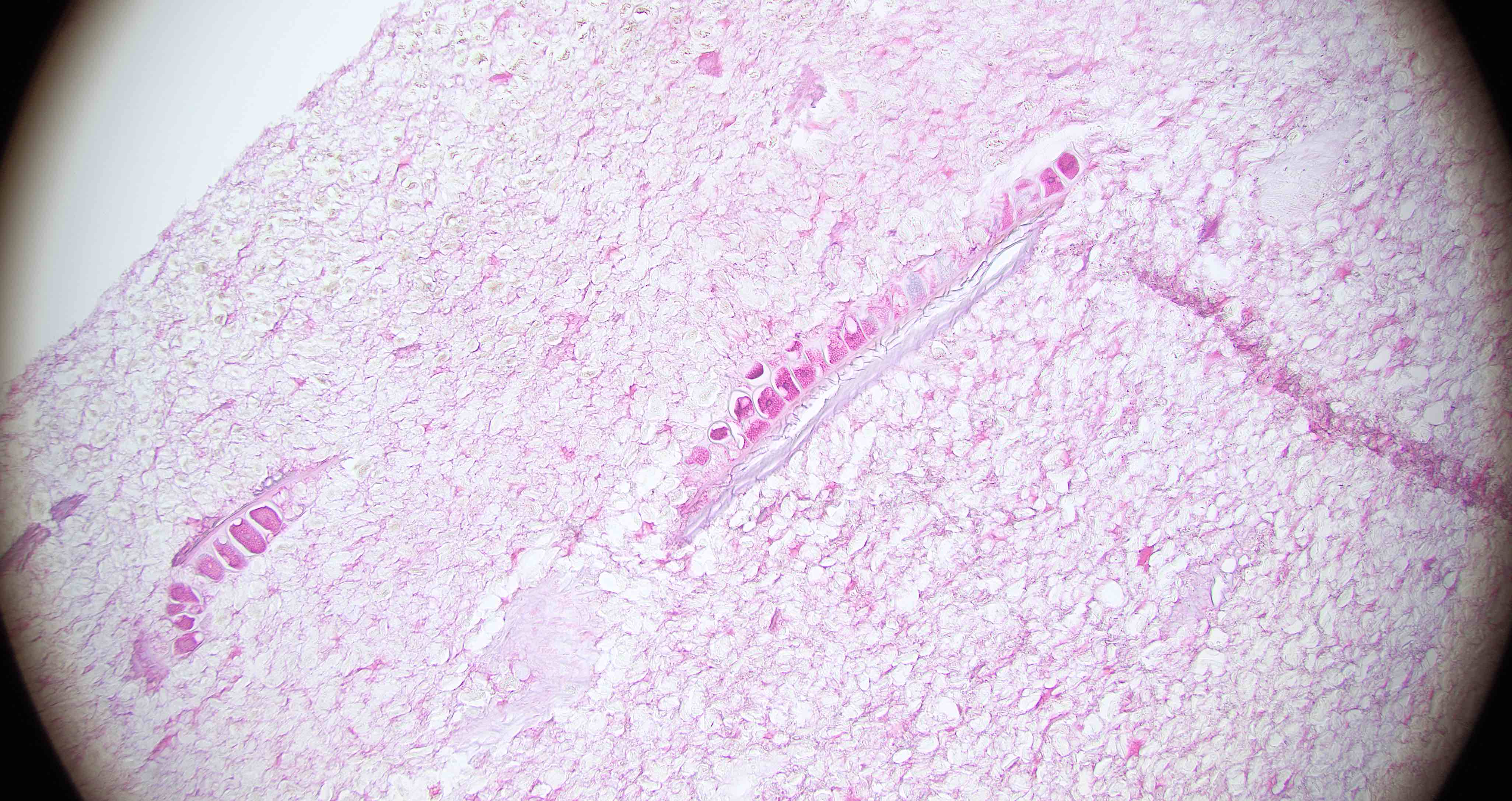
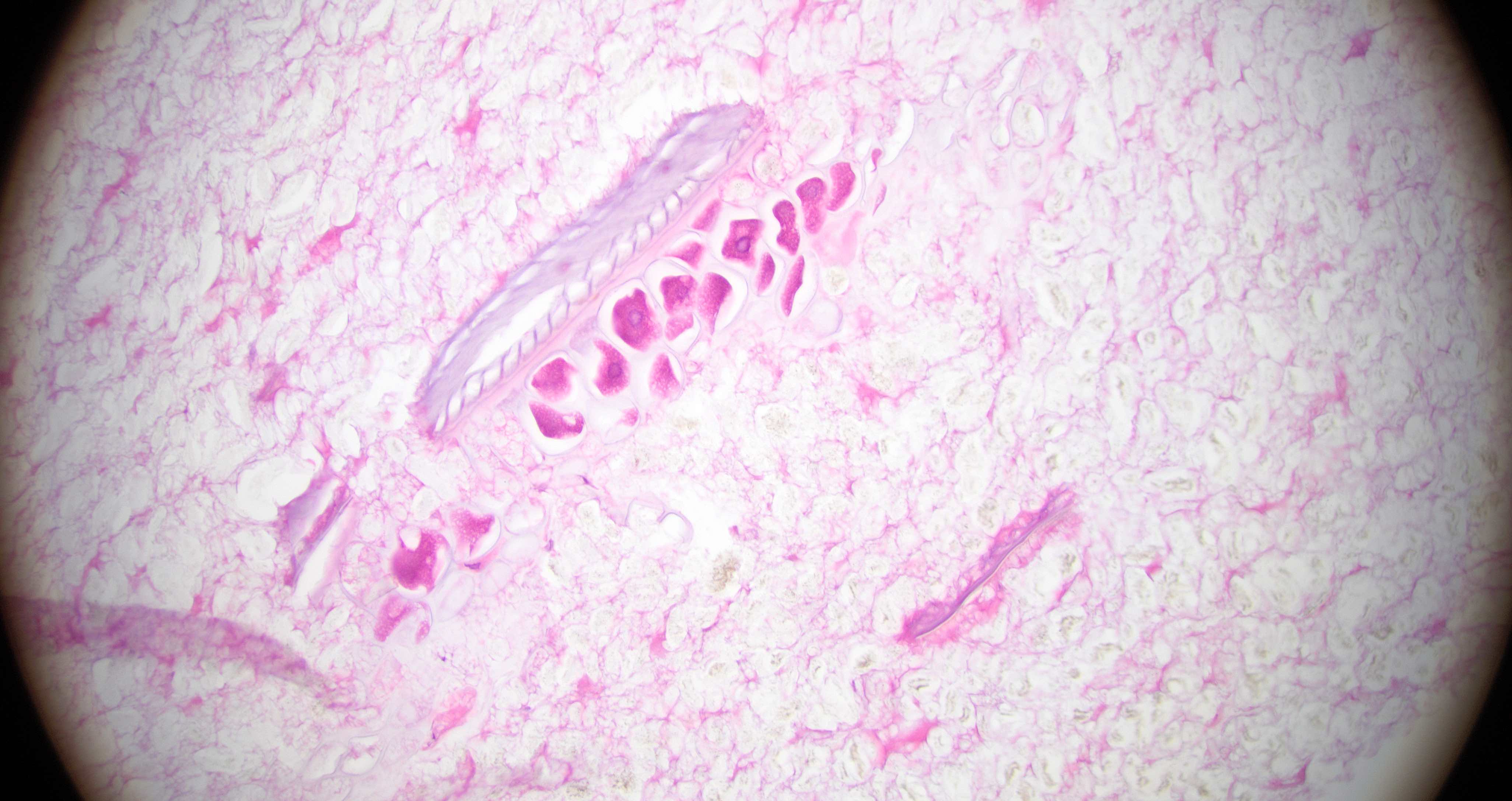
Strongyloides stercoralis
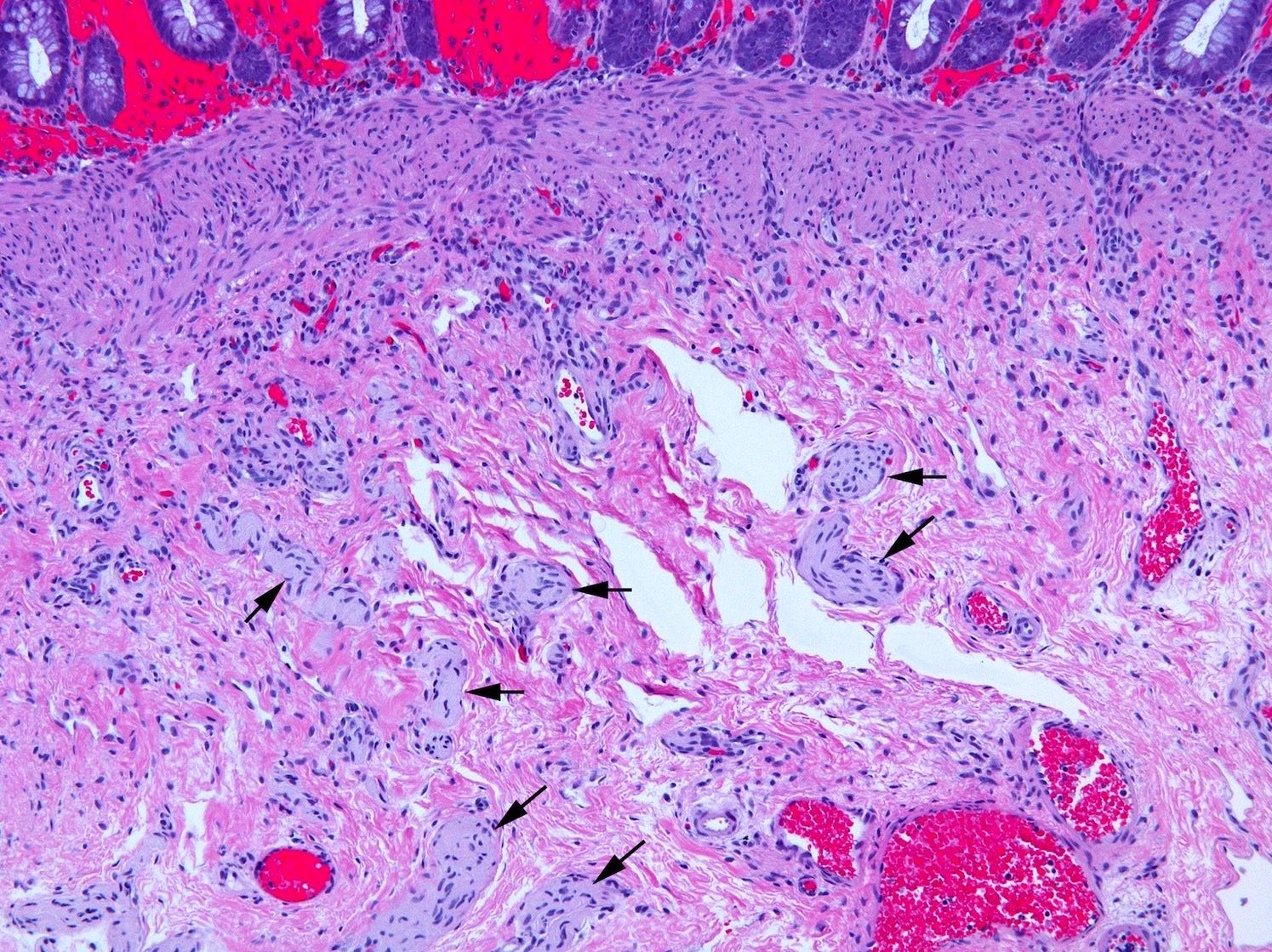
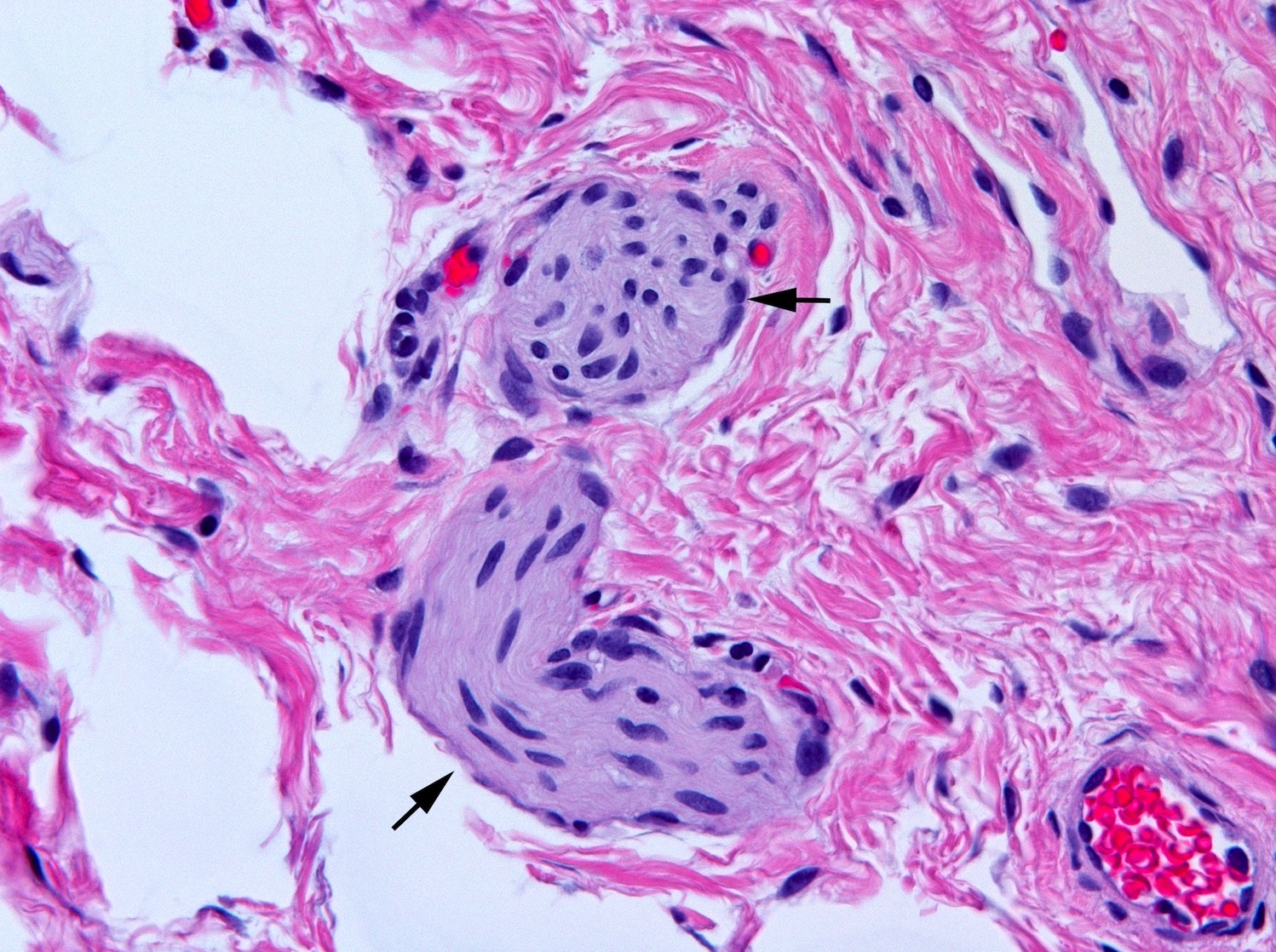
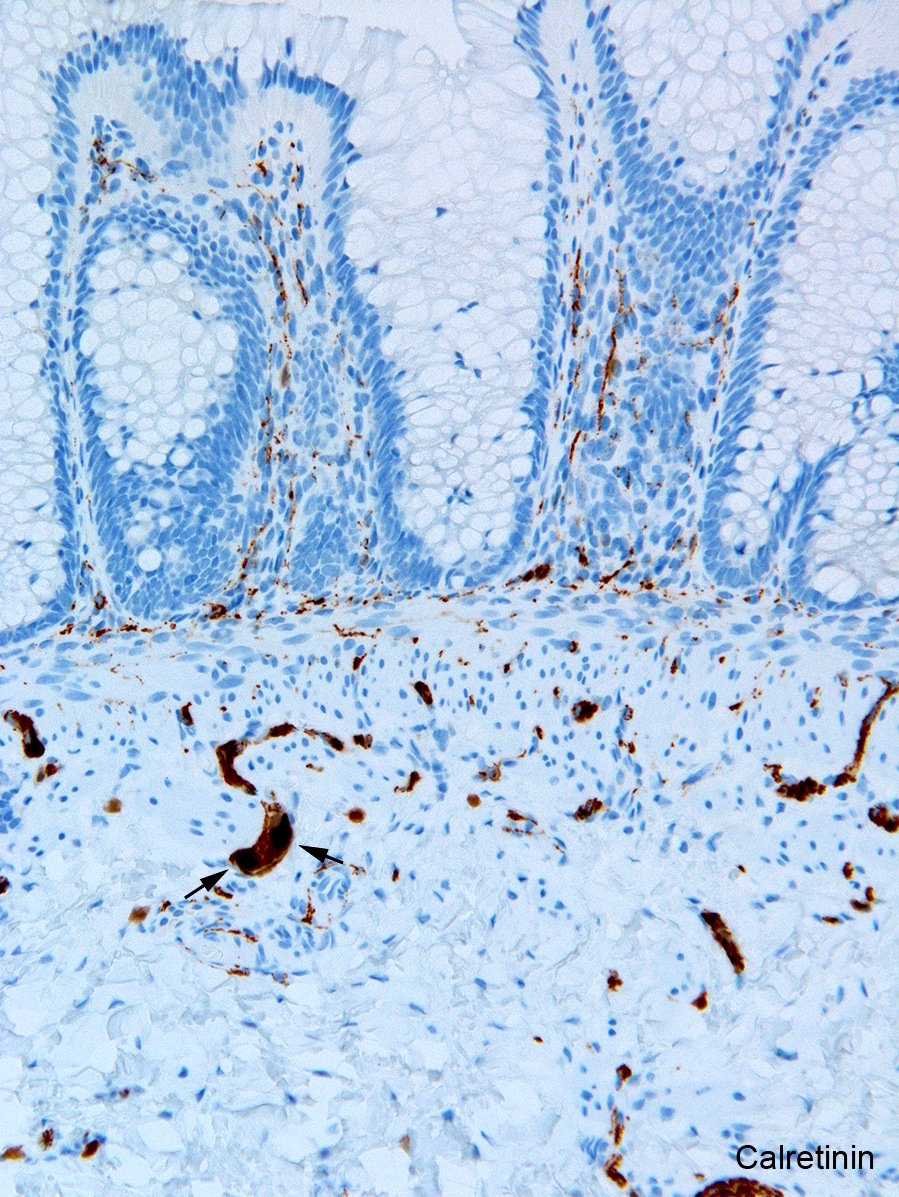
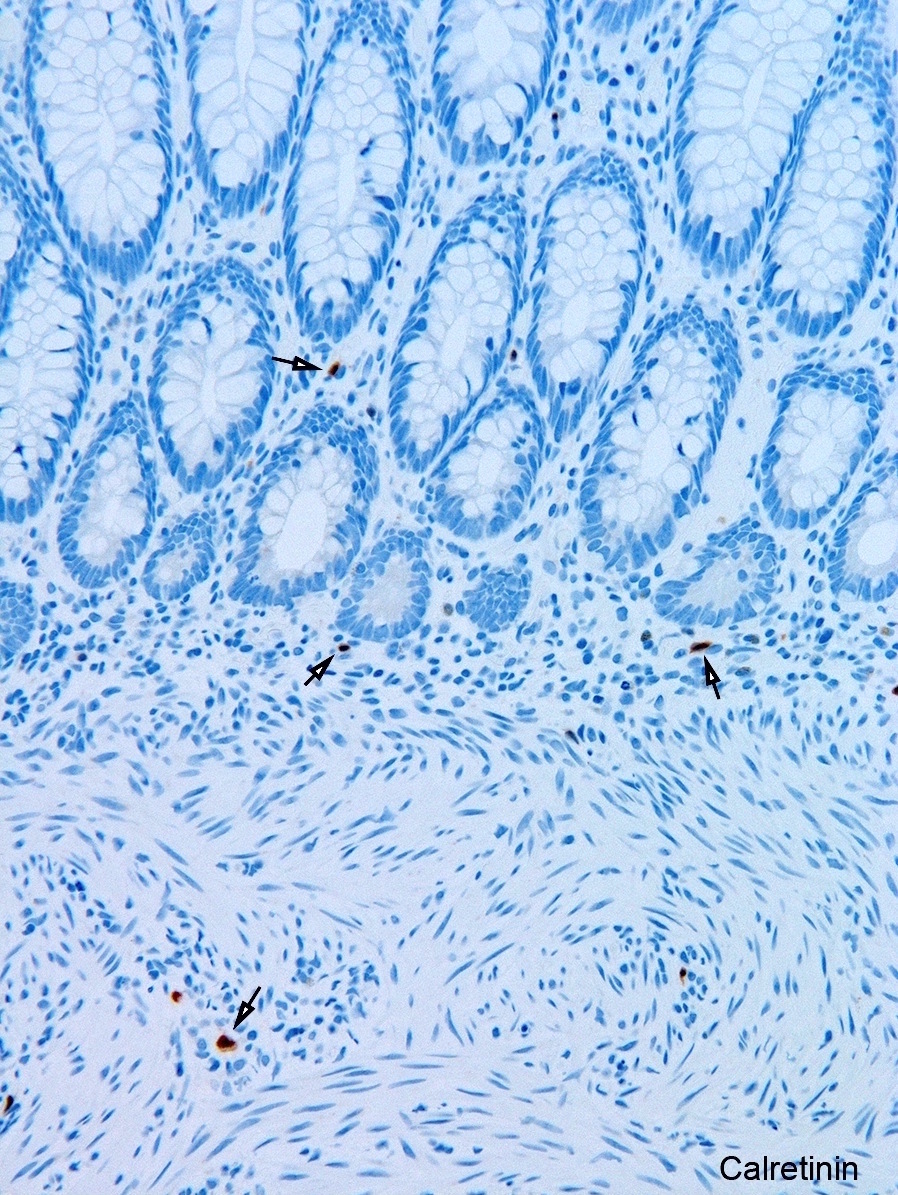
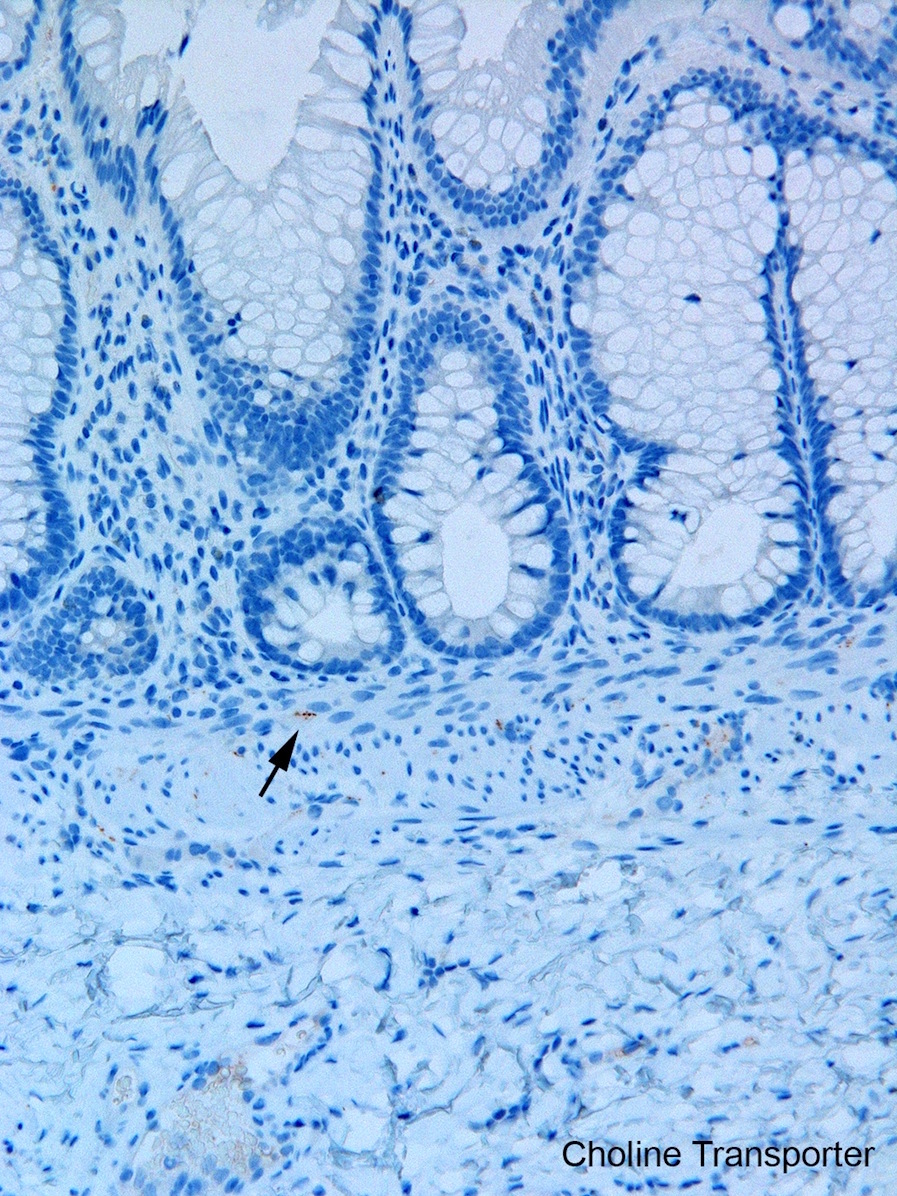
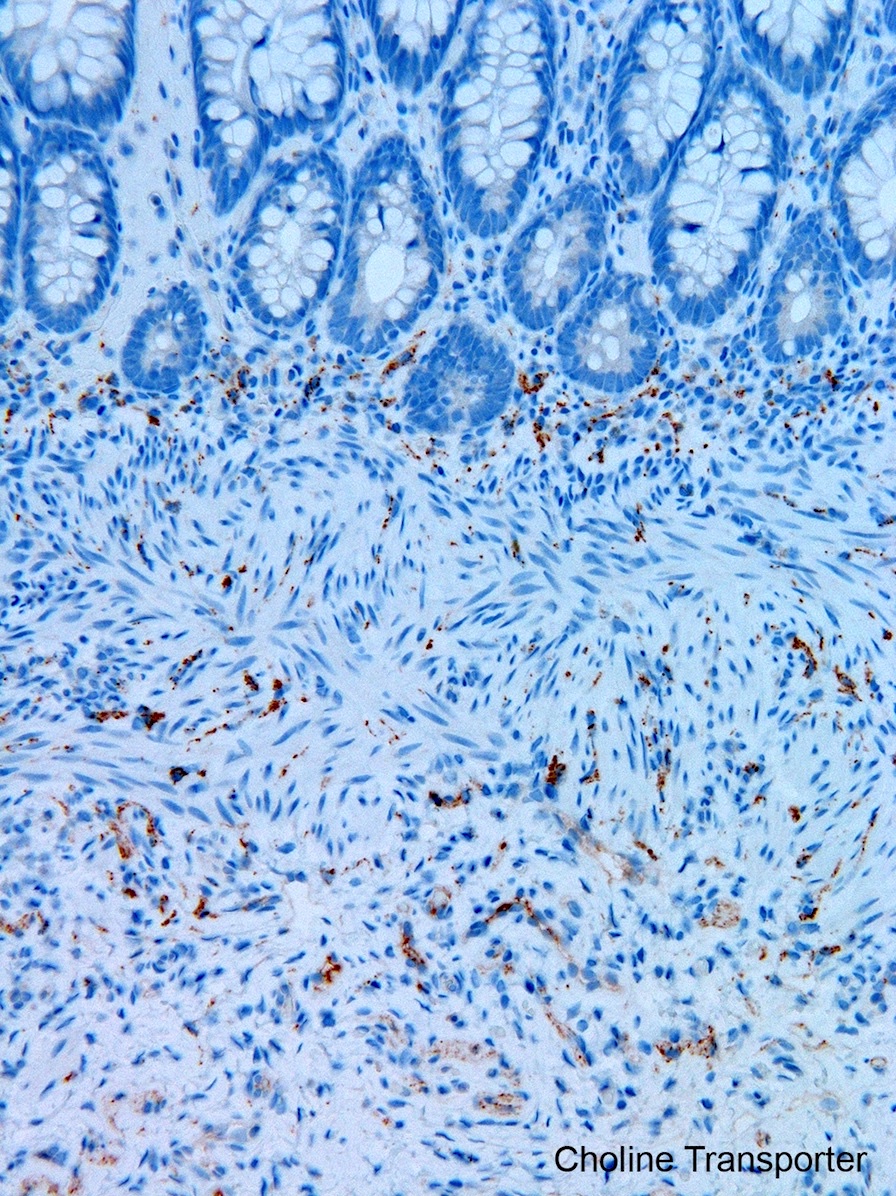
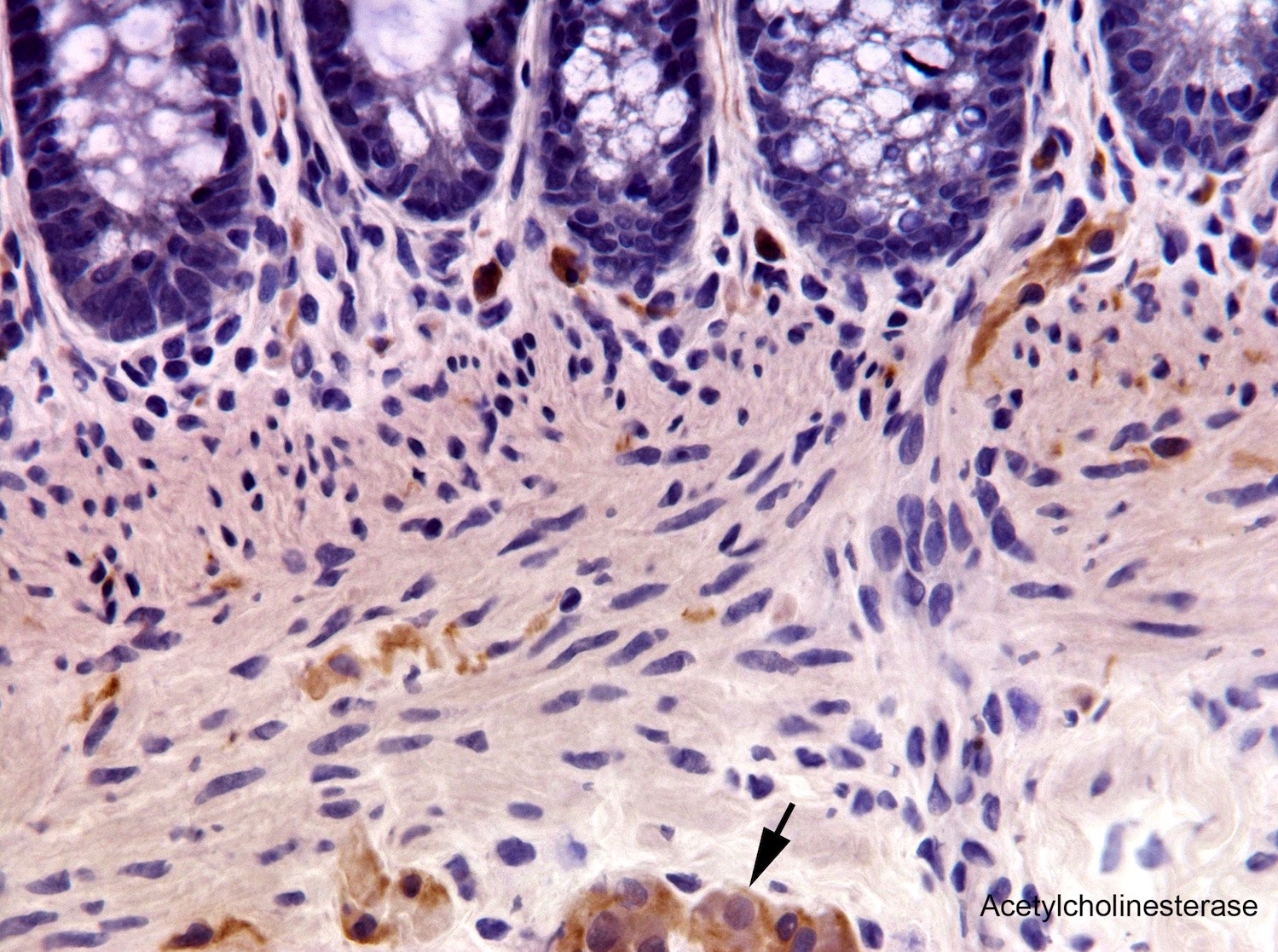
Tactile corpuscle-like bodies
Traditional serrated adenoma
Trichuriasis
Tuberculosis
Tubular adenoma
Tubulovillous / villous adenoma
Turcot syndrome
Typhlitis
Ulcerative colitis
Ulcerative proctitis
Vascular ectasia
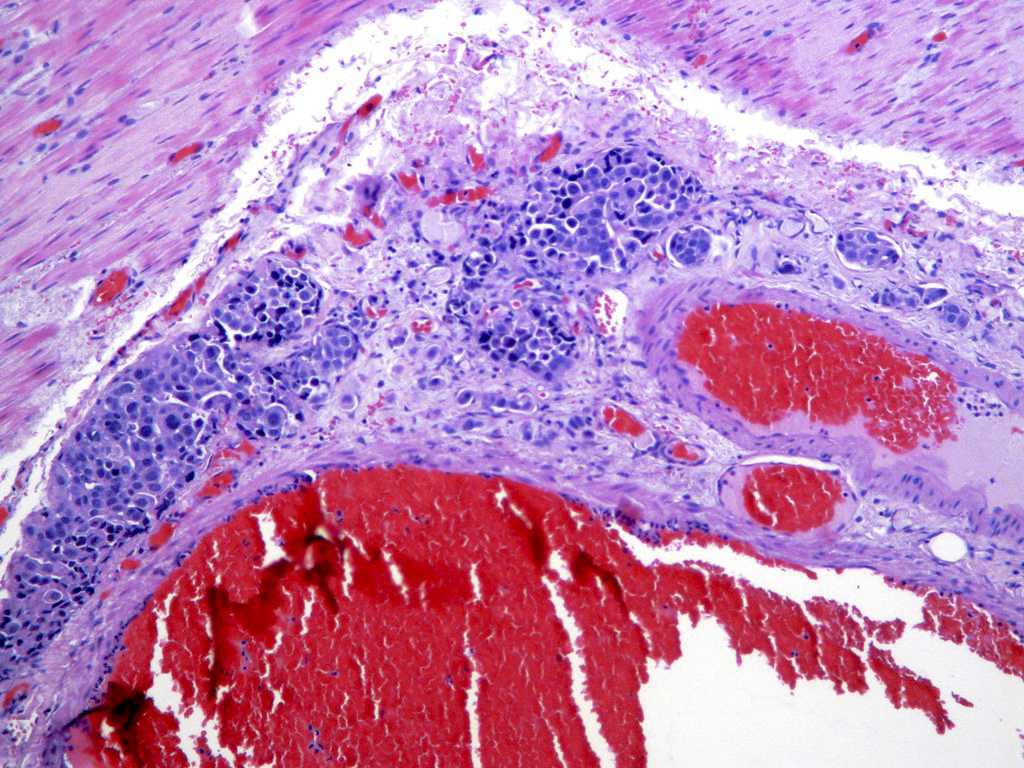
Vasculitis
Vibrio cholerae
Visceral myopathy (pending)
Volvulus
WHO classification
Xanthoma
YersiniosisAberrant crypt foci
Table of Contents
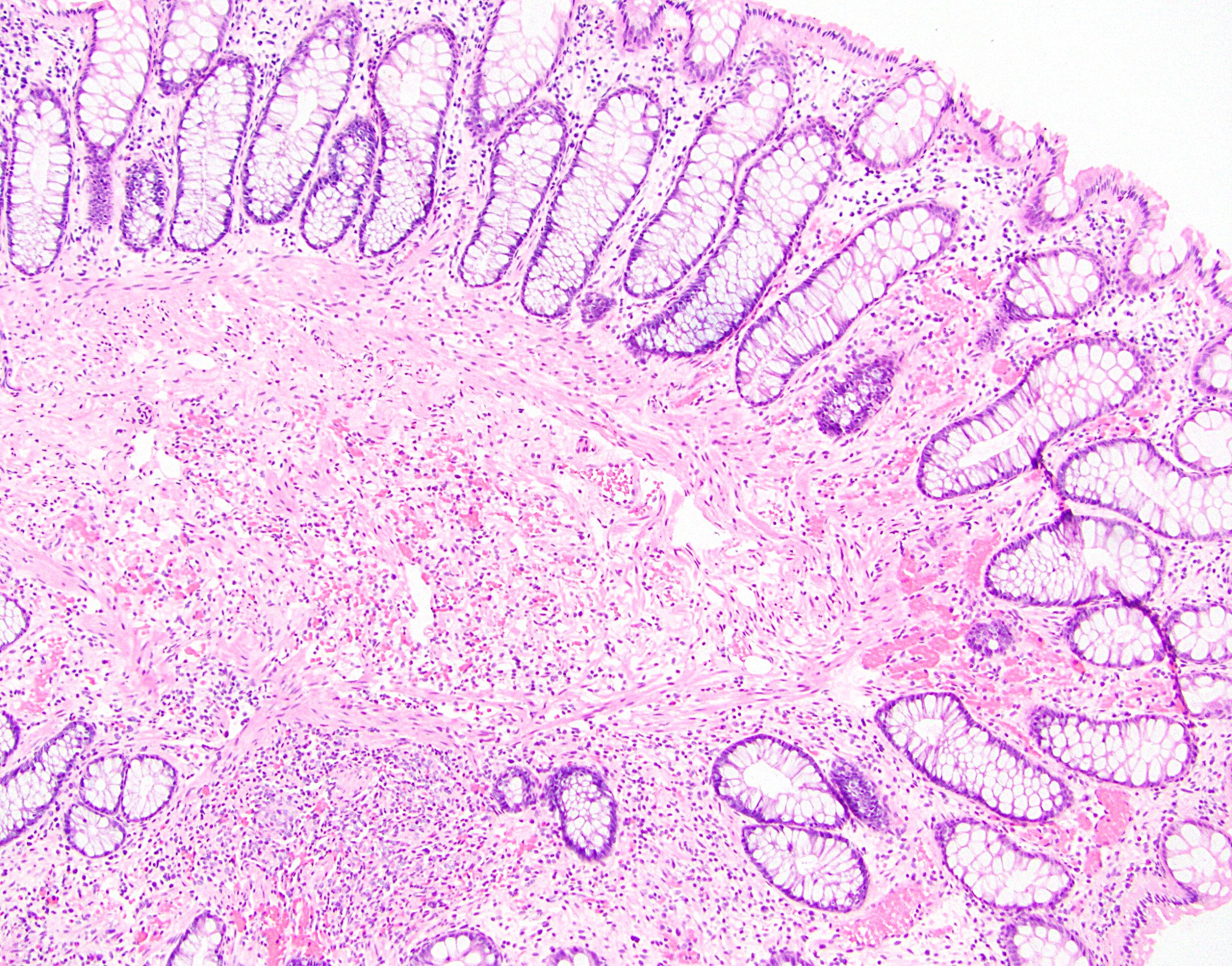
Definition / general | Prognosis and treatment | Microscopic (histologic) description | Molecular / cytogenetics description | Additional referencesDefinition / general
- Earliest neoplastic lesion of colon
- May predict future adenoma or carcinoma (Am J Gastroenterol 2006;101:1362, Am J Gastroenterol 2005;100:1283)
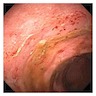
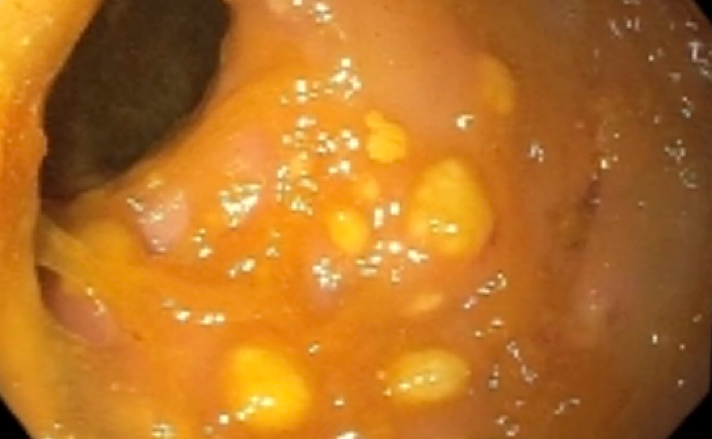
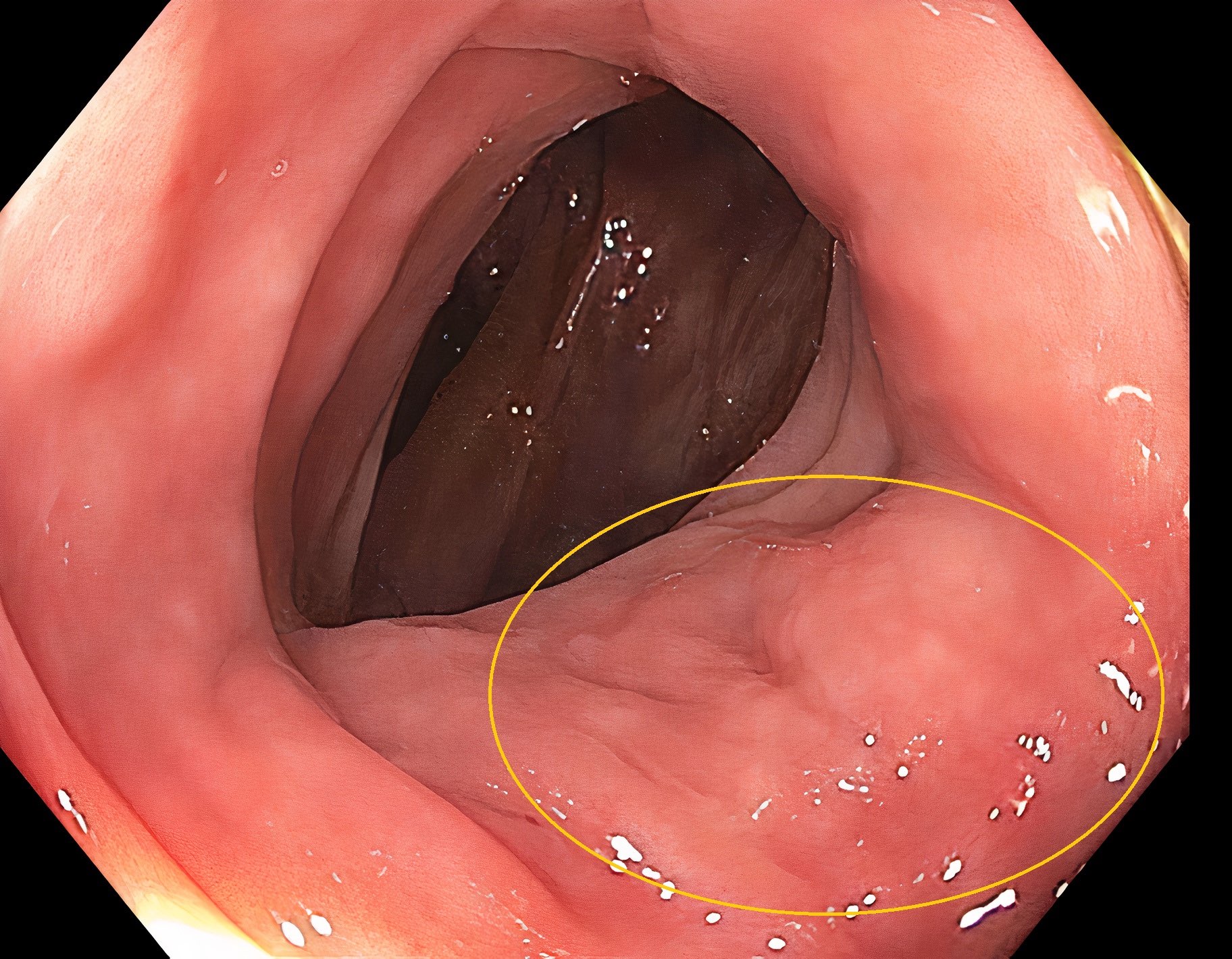
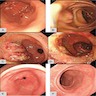
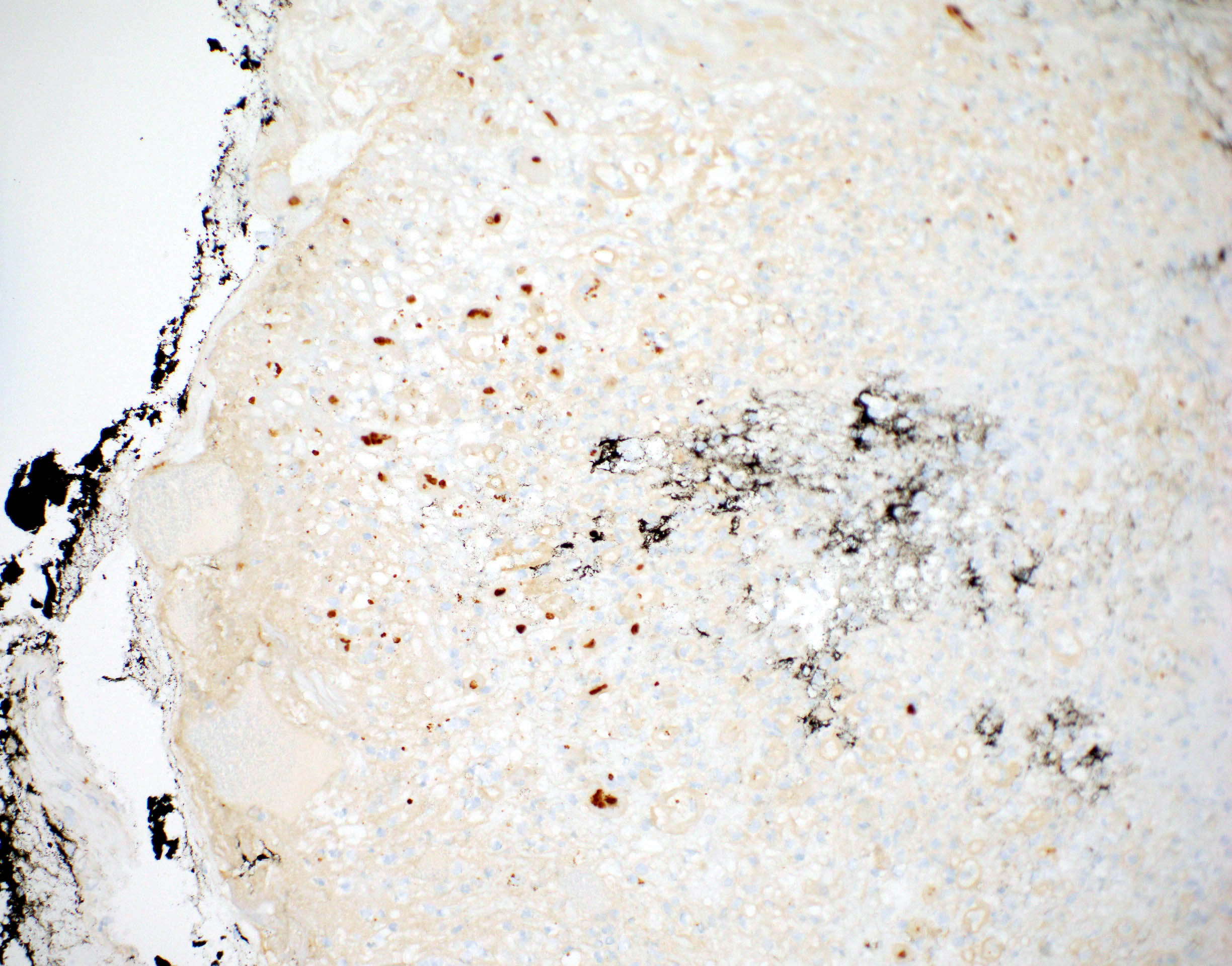
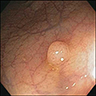
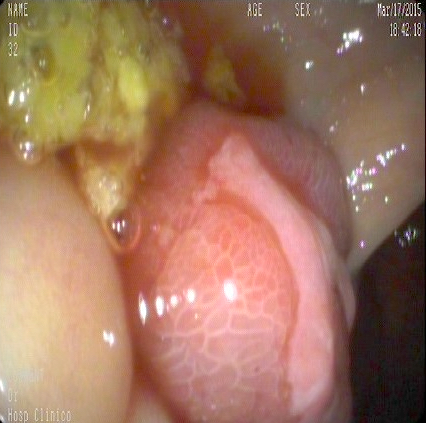
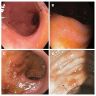
- Endoscopy: aberrent cryptic foci are stained darker than normal crypts with Methylene blue using high magnification chromoendoscopy, which helps in obtaining a targeted biopsy
Prognosis and treatment
- ACF are markers of increased colorectal cancer risk, particularly those with dysplastic features (J Surg Oncol 2008;98:207)
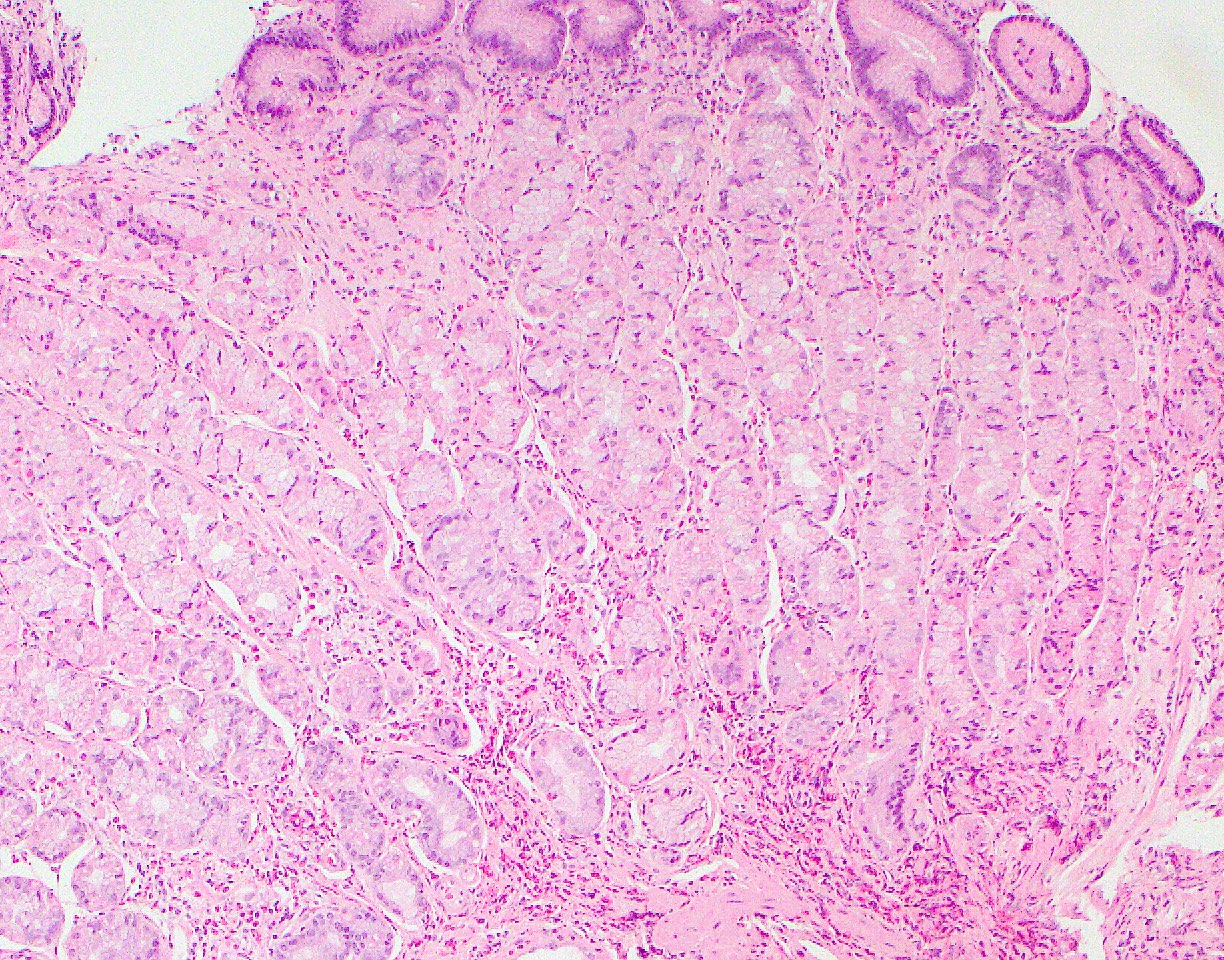
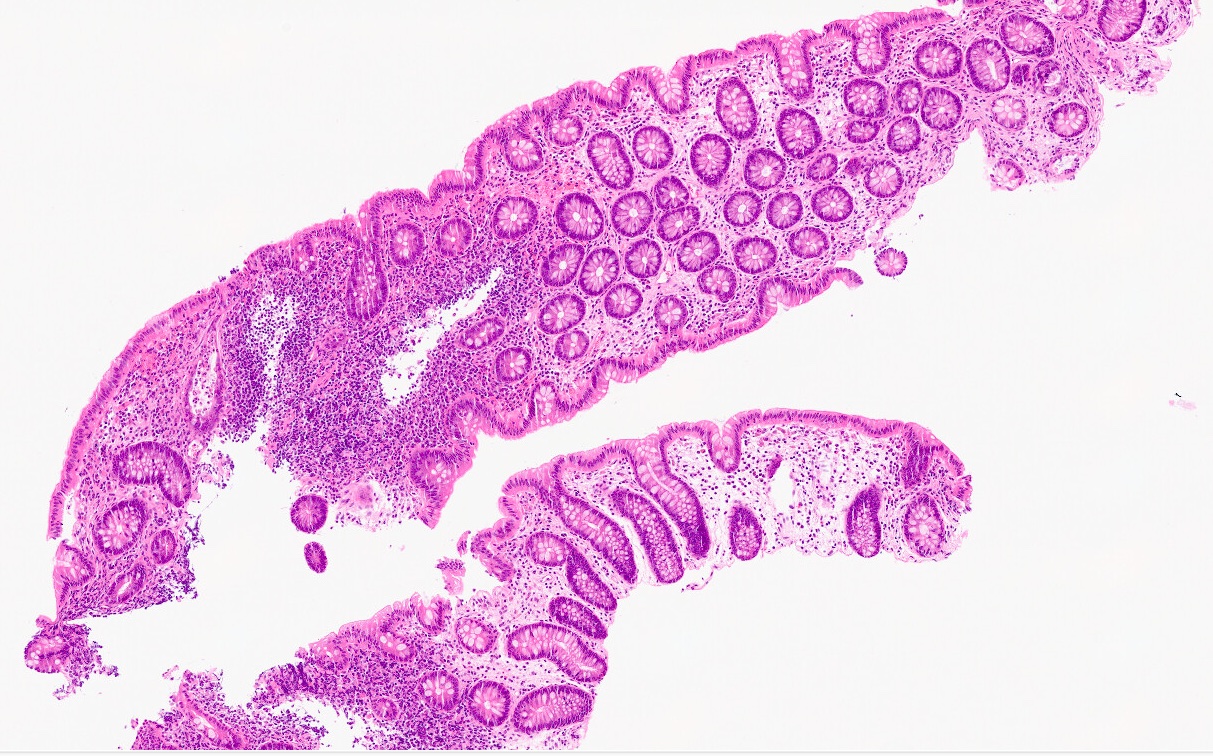
Microscopic (histologic) description
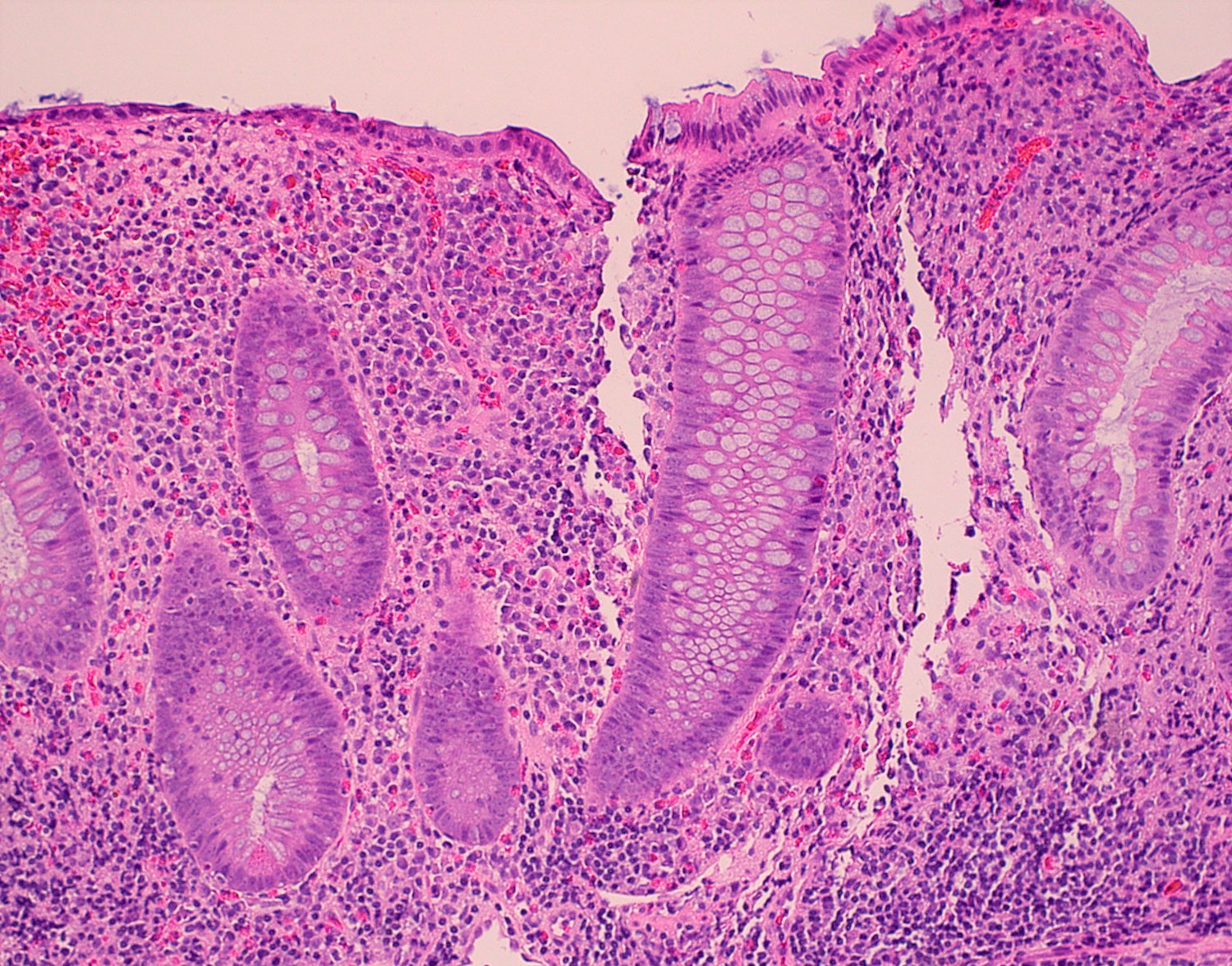
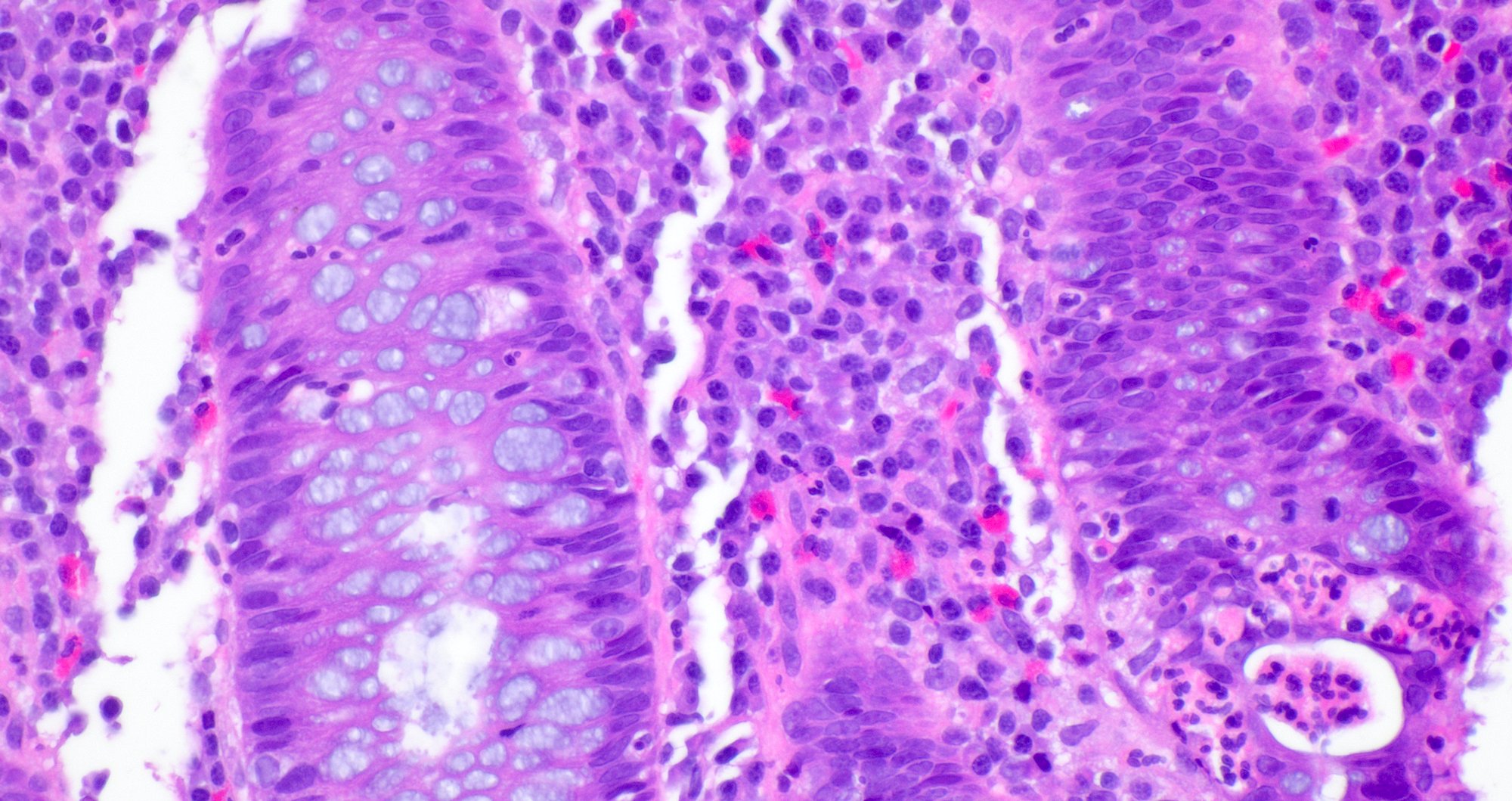
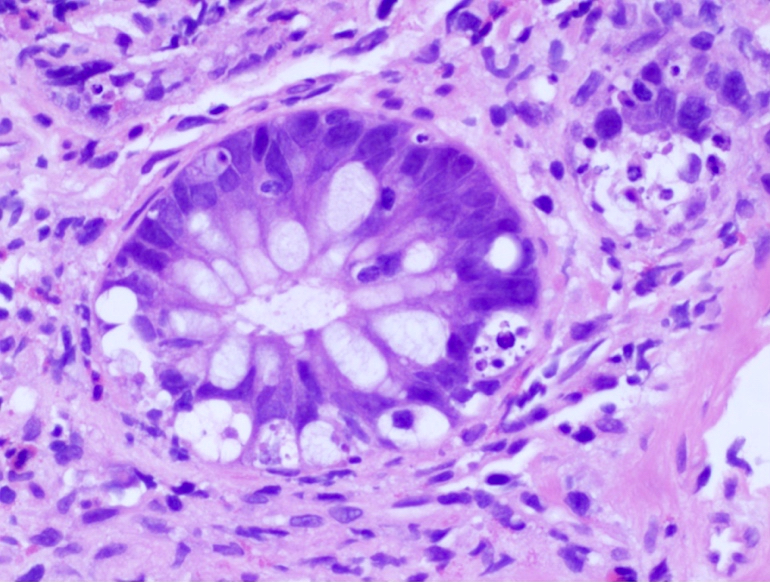
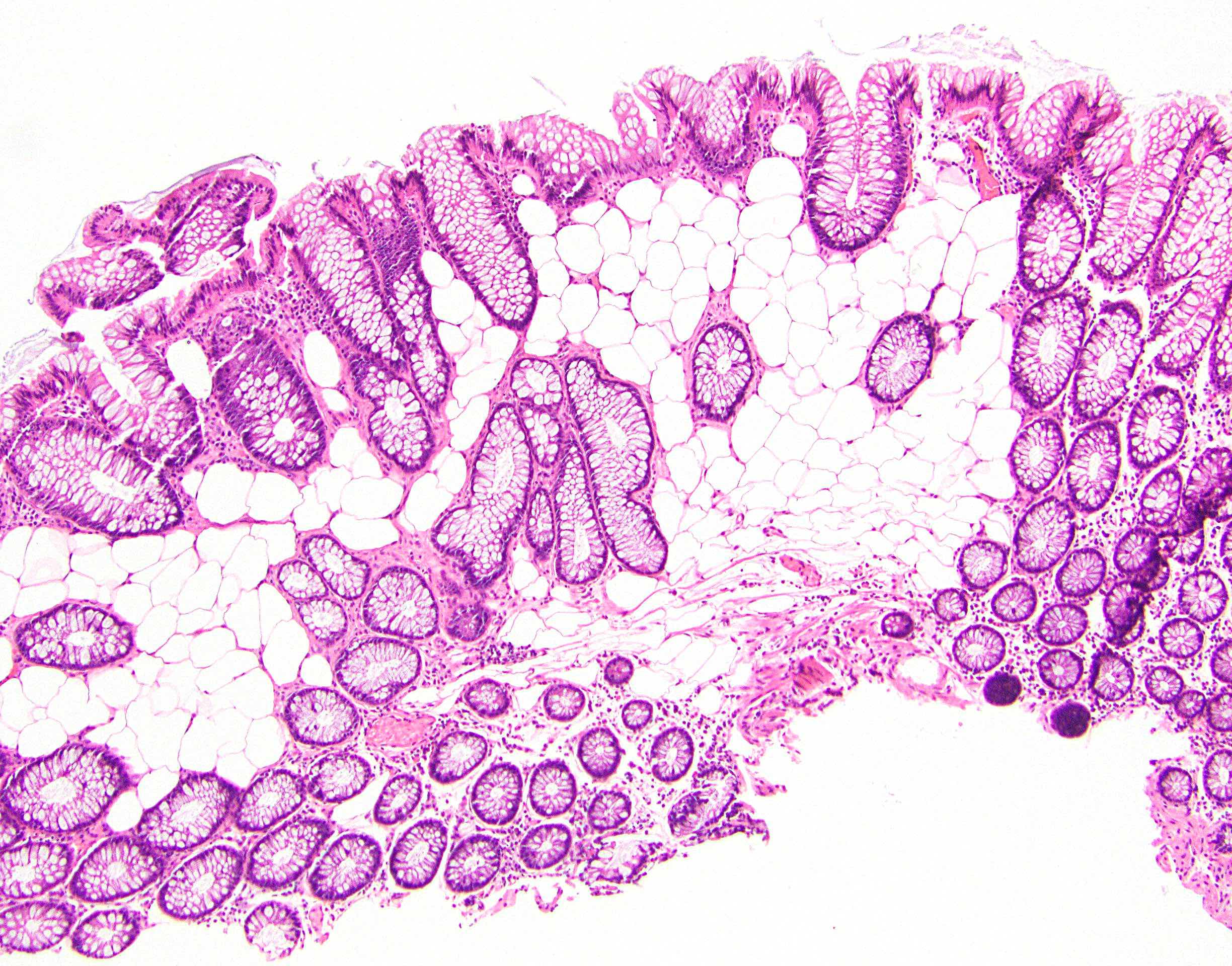
- Crypts are 2 - 3x larger and more dilated than normal crypts, may have a foci of dysplasia
- ACF can be subclassified as dysplastic, nondysplastic or mixed type
- Dysplastic ACF resemble adenomas
Molecular / cytogenetics description
- Dysplastic ACF are present both in familial adenomatous polyposis (FAP), which is the result of a germline mutation of the APC gene; and in sporadic colorectal carcinomas, due to mutations of KRAS (Am J Pathol 2005;166:1069)
Additional references
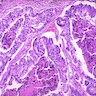
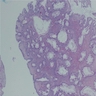
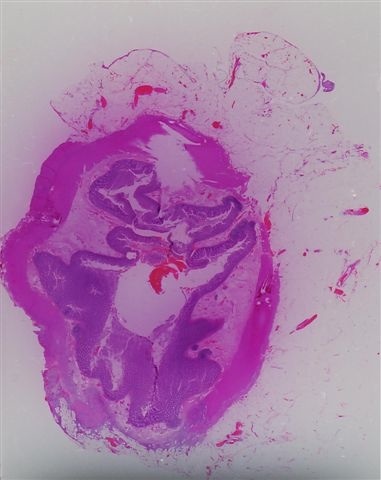
Actinomycosis
Table of Contents
Definition / general | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosis | Additional referencesDefinition / general
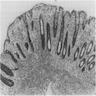
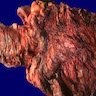
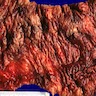
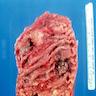
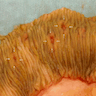
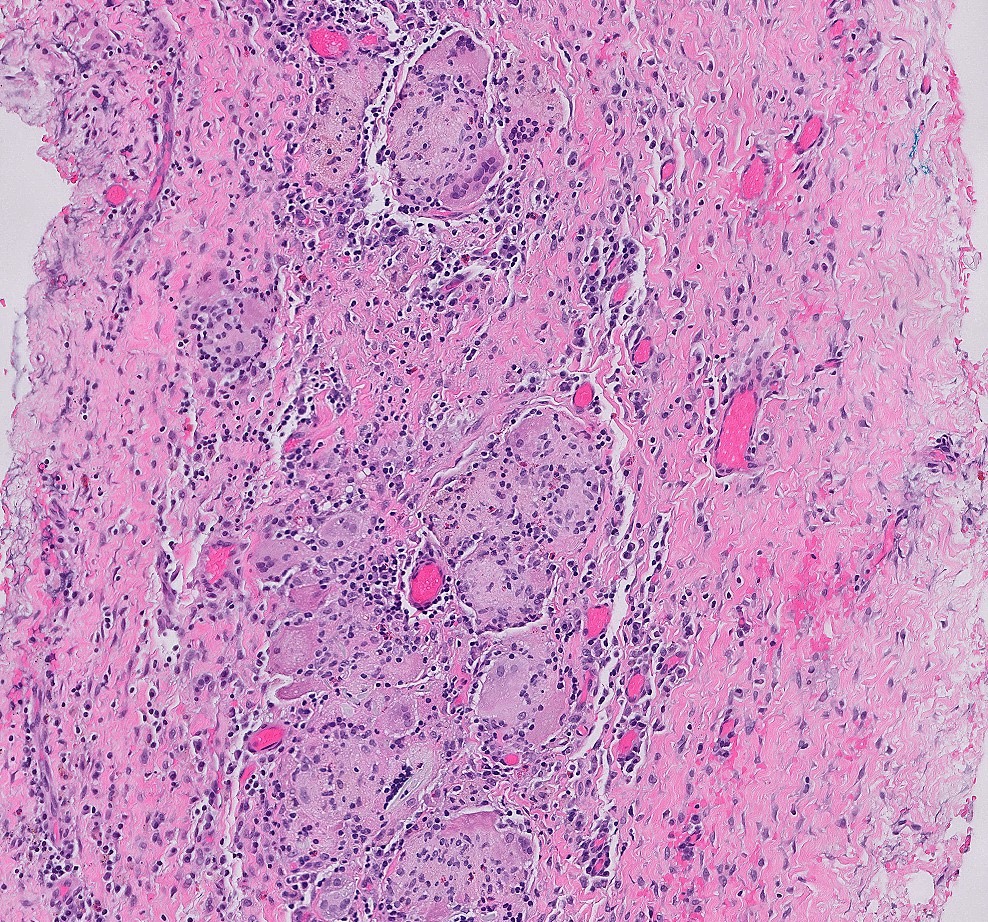
- Infection of large intestine by actinomycotic microorganisms
- Colonic actinomycosis is a subset of abdominal actinomycosis, but some sources do not discriminate between actinomycosis of abdomen and pelvis
- Other common sites are oral cervicofacial actinomycosis ("lumpy jaw", see secondary chronic osteomyelitis), skin, thoracic and pelvic (cervix, kidney, ovary)
Epidemiology
- Rare, but abdominal actinomycosis comprises 15 - 20% of actinomycosis in humans
- Males are more commonly affected
Sites
- Most common site is cecum, most often with concurrent ileal disease
- Some cases are secondary to disease in appendix or female genital tract
Pathophysiology
- Due to infection by anaerobic or microaerophilic, saprophytic bacteria of genus Actinomyces, a normal commensal of mouth, large intestine, vagina
- Disruption of mucosa is necessary for disease - causes are trauma, surgery, diverticular disease, ingestion of foreign body, or less commonly other inflammatory processes
- In surgery related cases, disease may appear months after perforation leading to appendectomy or bowel resection, but without evidence of actinomycosis at the time
- In some cases, no inciting event is identified; rare cases may originate through hematogenous spread
- While actinomycotic colonies may dominate the histologic picture, it is likely that companion, or "co-pathogenic" bacteria may also be necessary for disease to occur, possibly by lowering oxygen tension
Etiology
- Actinomyces israelii is the most common etiologic agent; however, at least five other species are implicated in human disease
- Using comparative 16s ribosomal RNA, some bacteria traditionally associated with actinomycotic disease have been reclassified as Aracanobacterium, Actinobaculum or Cellulomonas
Clinical features
- Usually indolent disease, but more severe in immunocompromised patients
- Patients usually present with non-specific symptoms including abdominal pain, fever, change in bowel habits, sensation of a mass
- Imaging studies reveal a mass-like lesion, cystic lesion or an abscess, often suggestive of malignancy
- May coexist with other inflammatory lesions of colon with fistula or sinuses
- Clumps of yellow bacteria known as sulfur granules are characteristically present
- No cases of human to human transmission have been reported and Actinomyces has not been cultured from nature
Diagnosis
- Usually by tissue biopsy or resection where the characteristic microorganisms are seen
- Abdominal actinomycosis is rarely suspected clinically and most patients are diagnosed after major surgery
- Diagnosis by microbiologic culture is uncommon as strict anaerobic conditions are necessary and antibiotics interfere with growth in culture
Prognostic factors
- Most patients respond well to therapy
Case reports
- 17 year old boy with mass over sigmoid colon (APSP J Case Rep 2011;2:4)
- 38 year old woman with severe large bowel obstruction (Scand J Infect Dis 2006;38:231)
- 41 year old man with renal, colonic and retroperitoneal actinomycosis (West Afr J Med 2005;24:343)
- 50 year old woman with combined intra- and extraabdominal actinomycosis (Rom J Gastroenterol 2004;13:337)
- 53 year old man with abdominal actinomycosis containing a fish bone (Surg Today 2006;36:187)
- 57 year old liver transplant recipient with abdominal actinomycosis (Transpl Infect Dis 2012;14:86)
- 58 year old man with actinomycosis simulating malignant large bowel obstruction (Braz J Infect Dis 2004;8:186)
- 63 year old man with abdominal actinomycosis and multiple myeloma (Oncol Lett 2014;8:1876)
- 67 year old woman with actinomycosis of the sigmoid colon (World J Gastrointest Surg 2009;1:62)
- 72 year old woman with ascending colon actinomycosis mimicking cancer (BMC Gastroenterol 2005 Jan 4;5:1)
- 74 year old woman with abdominal actinomycosis (Acta Clin Belg 2014;69:152)
- 79 year old man with colonic actinomycosis invading abdominal wall (Int J Surg Case Rep 2010;1:9)
Treatment
- Prolonged high dose antibiotics
- Even with advanced clinical disease, most patients can be effectively treated with medical management alone; however, this is very uncommon with abdominal disease
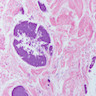
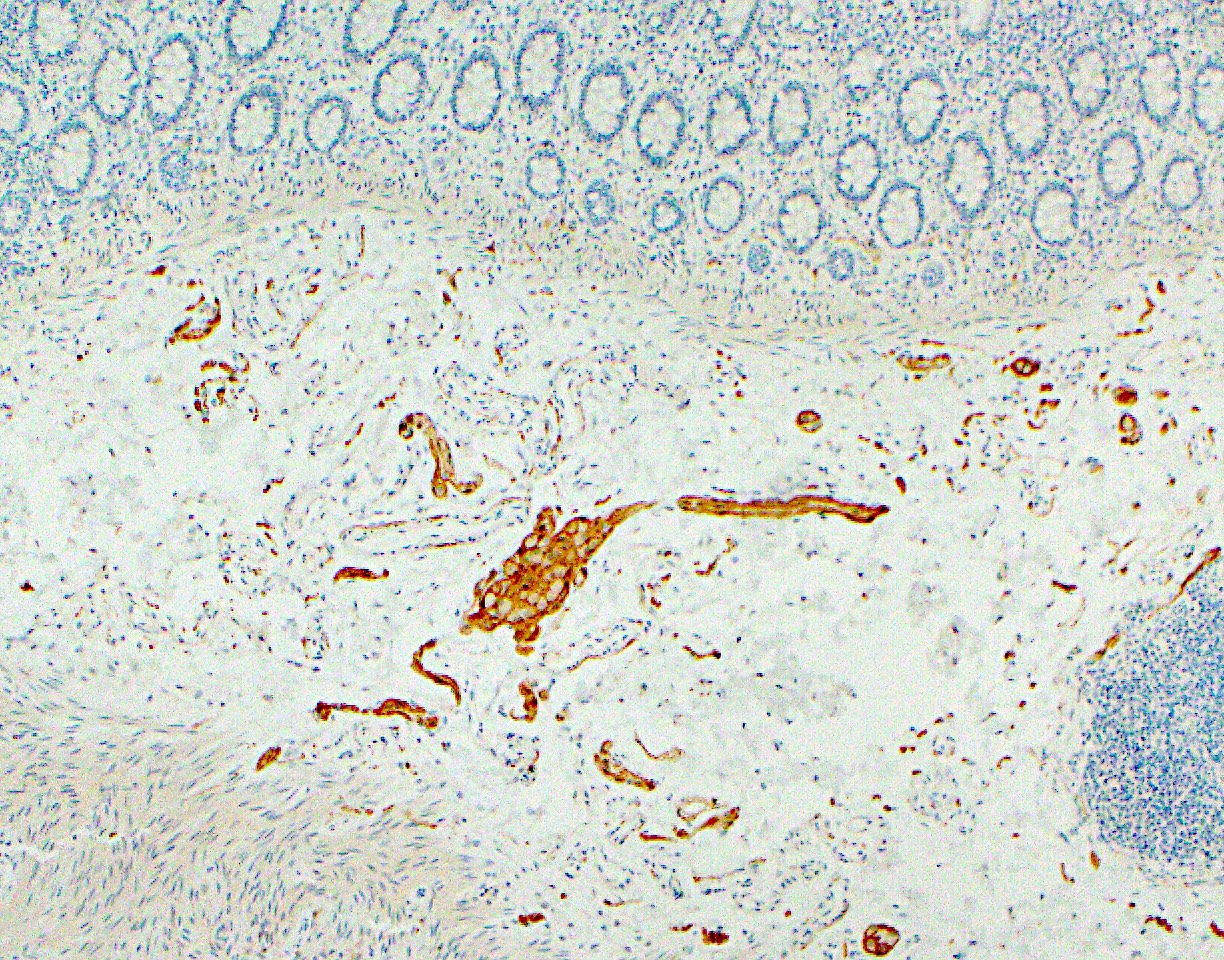
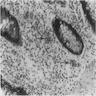
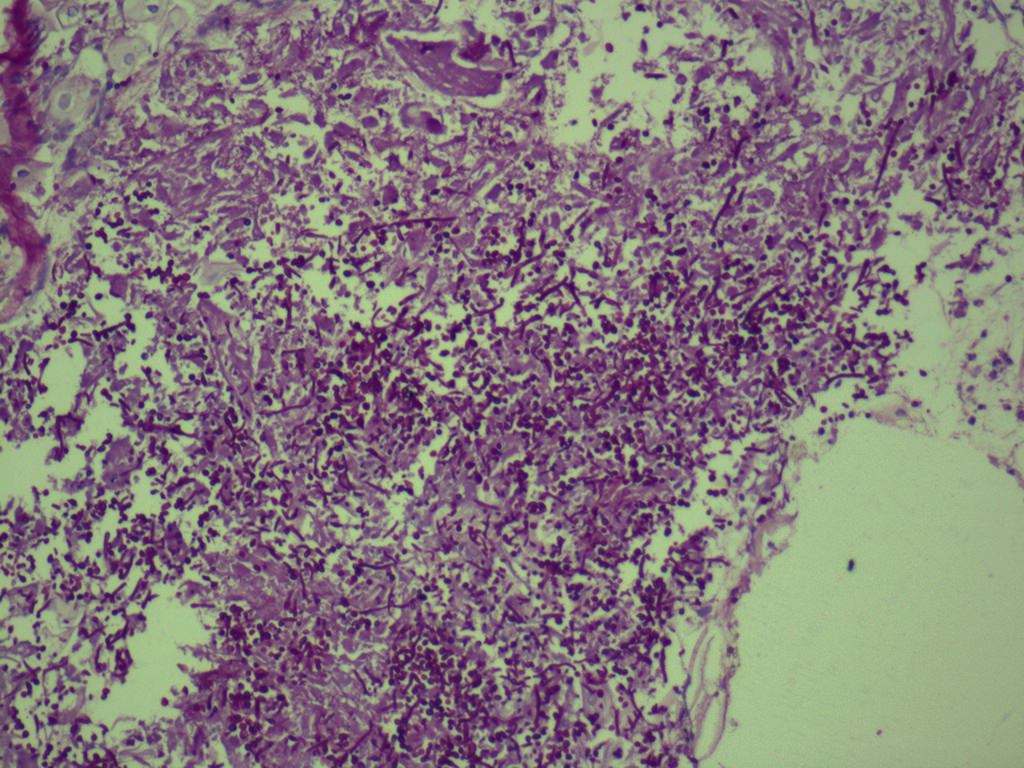
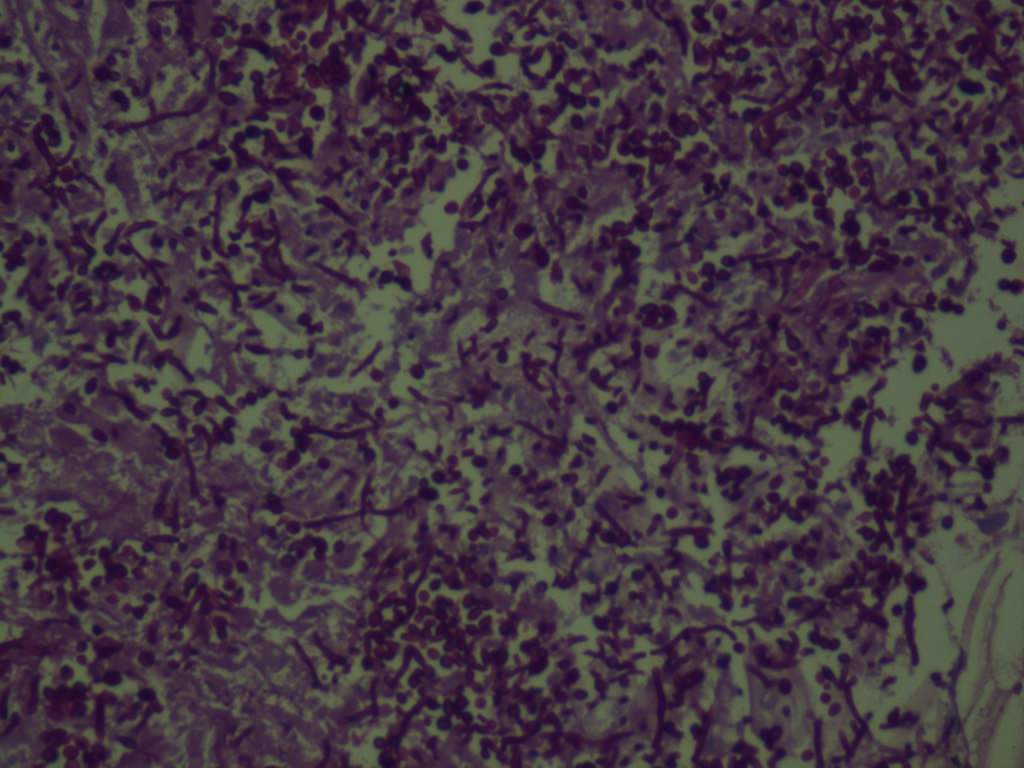
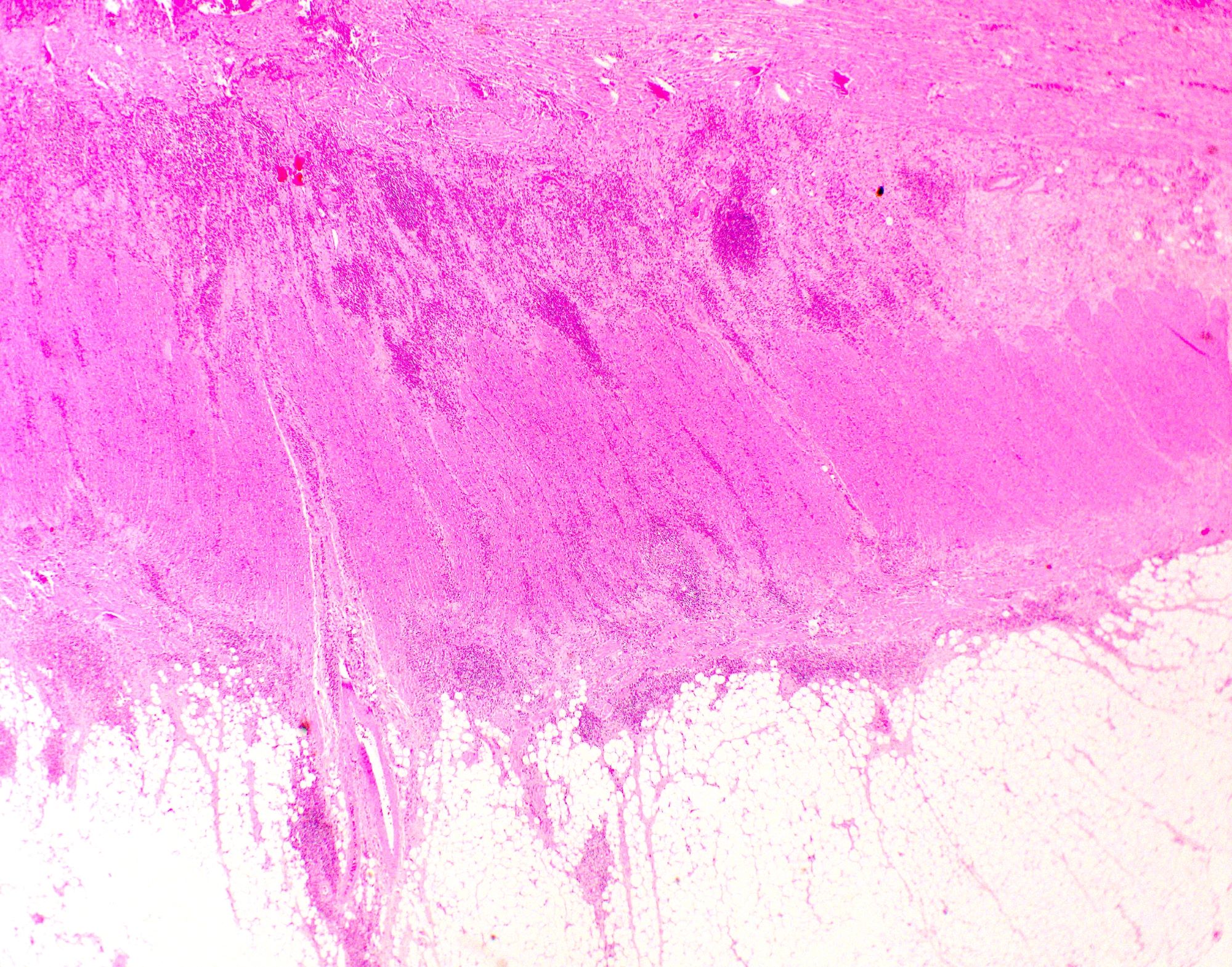
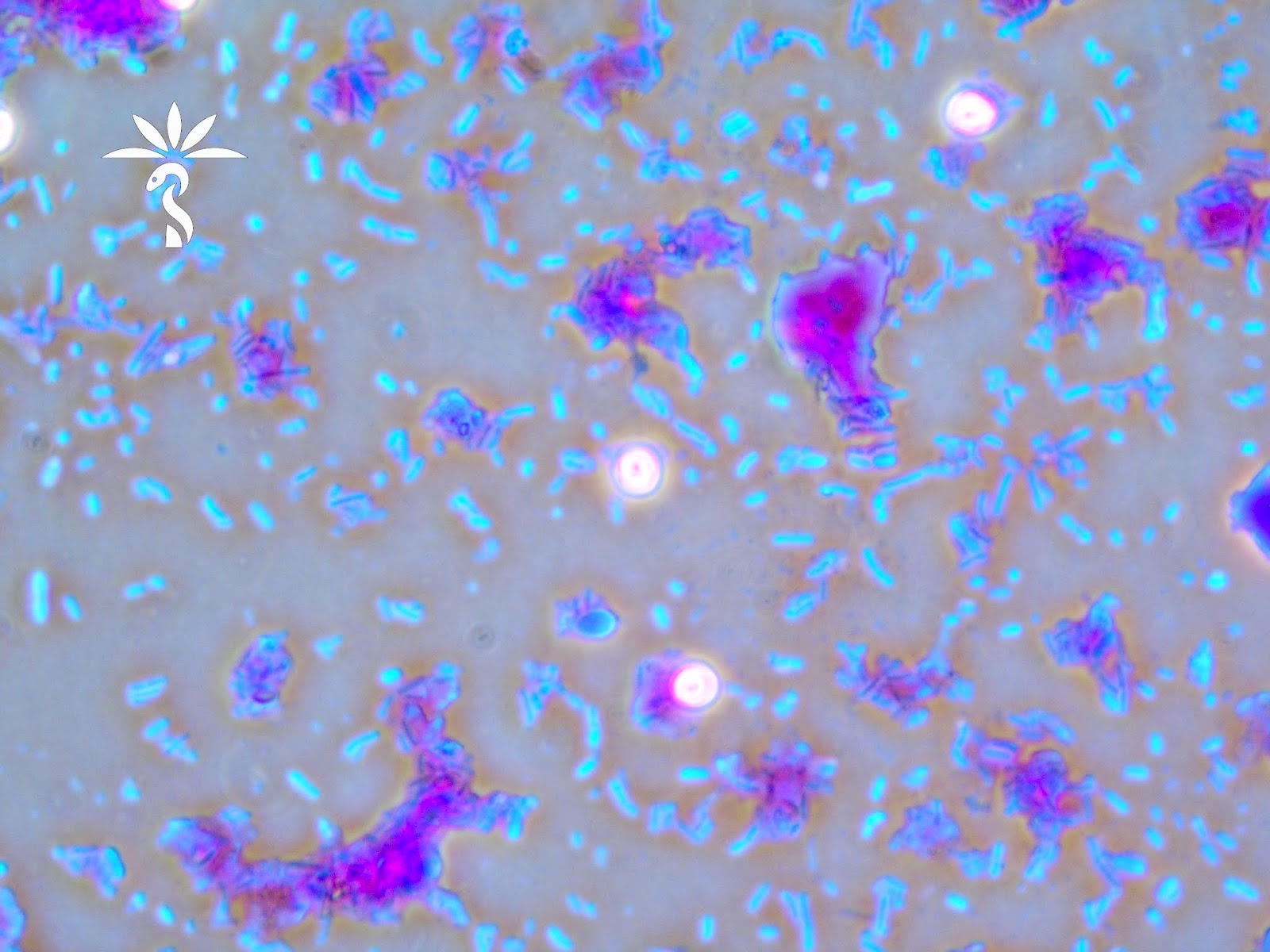
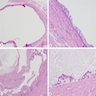
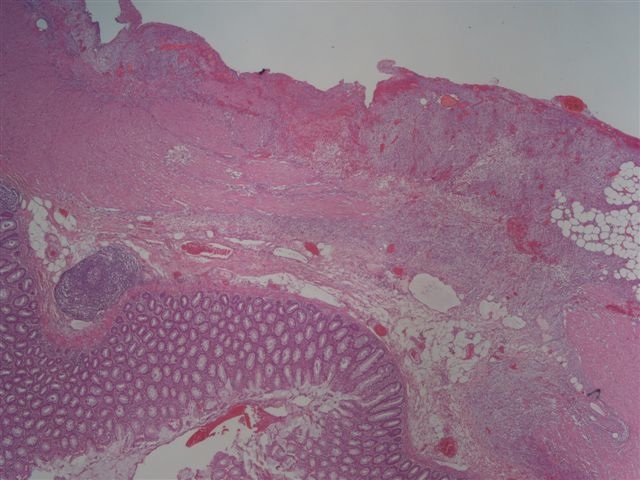
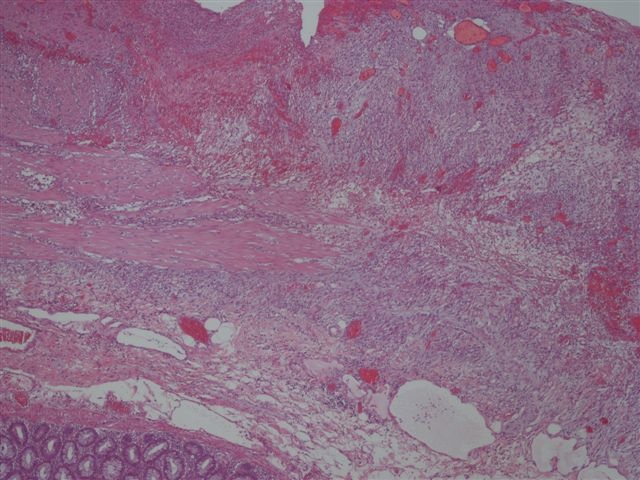
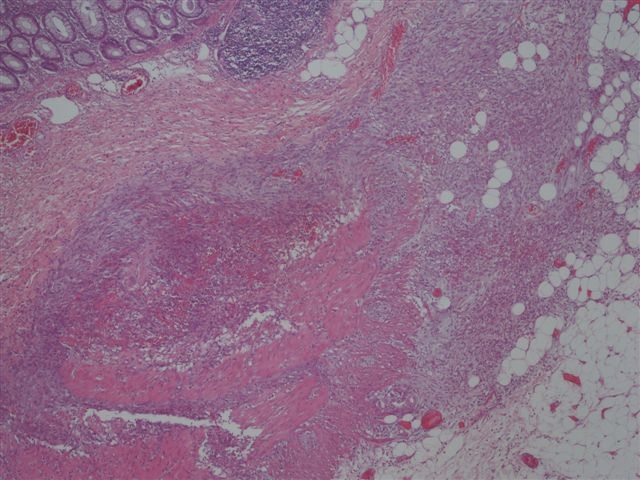
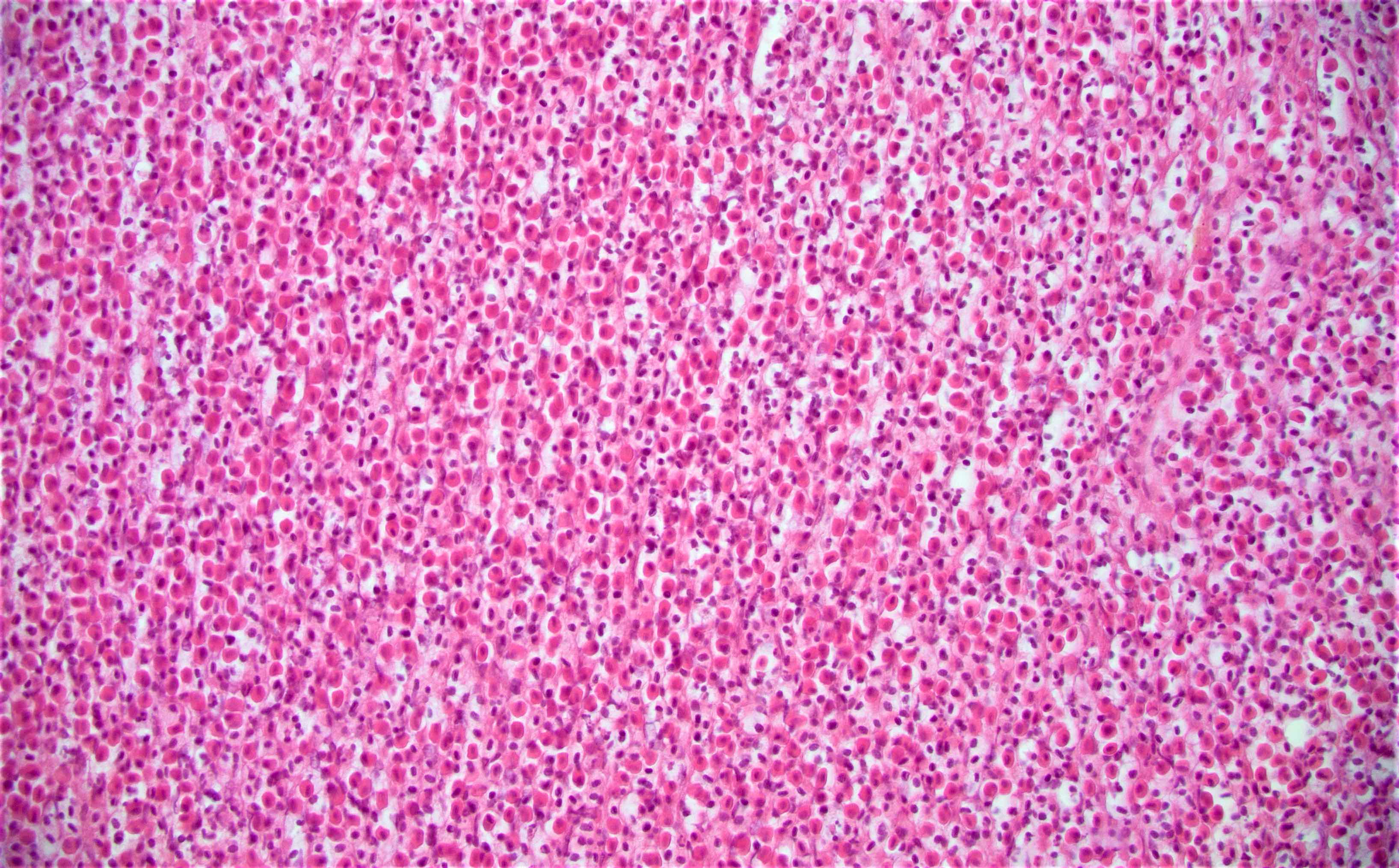
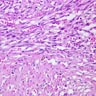
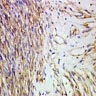
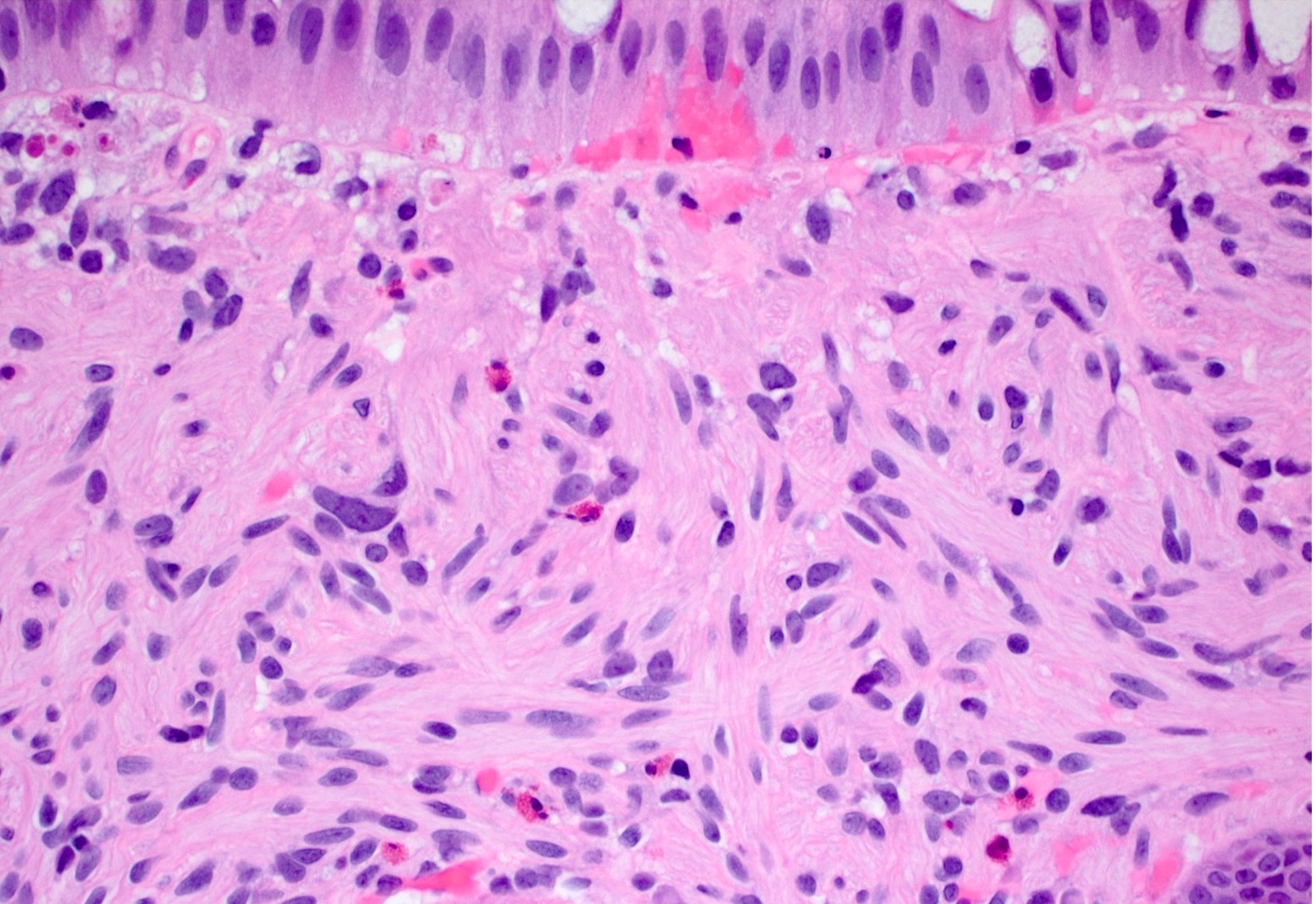
Microscopic (histologic) description
- Characteristic clumps of basophilic filamentous bacteria in a vaguely rosette-like configuration surrounded by acute inflammatory cells are characteristic
- Acute inflammation is accompanied by dense fibrosis described as "woody"
- Eosinophilic clubs may be found at periphery (Splendore-Hoeppli phenomena)
- Granulomatous inflammation may be present
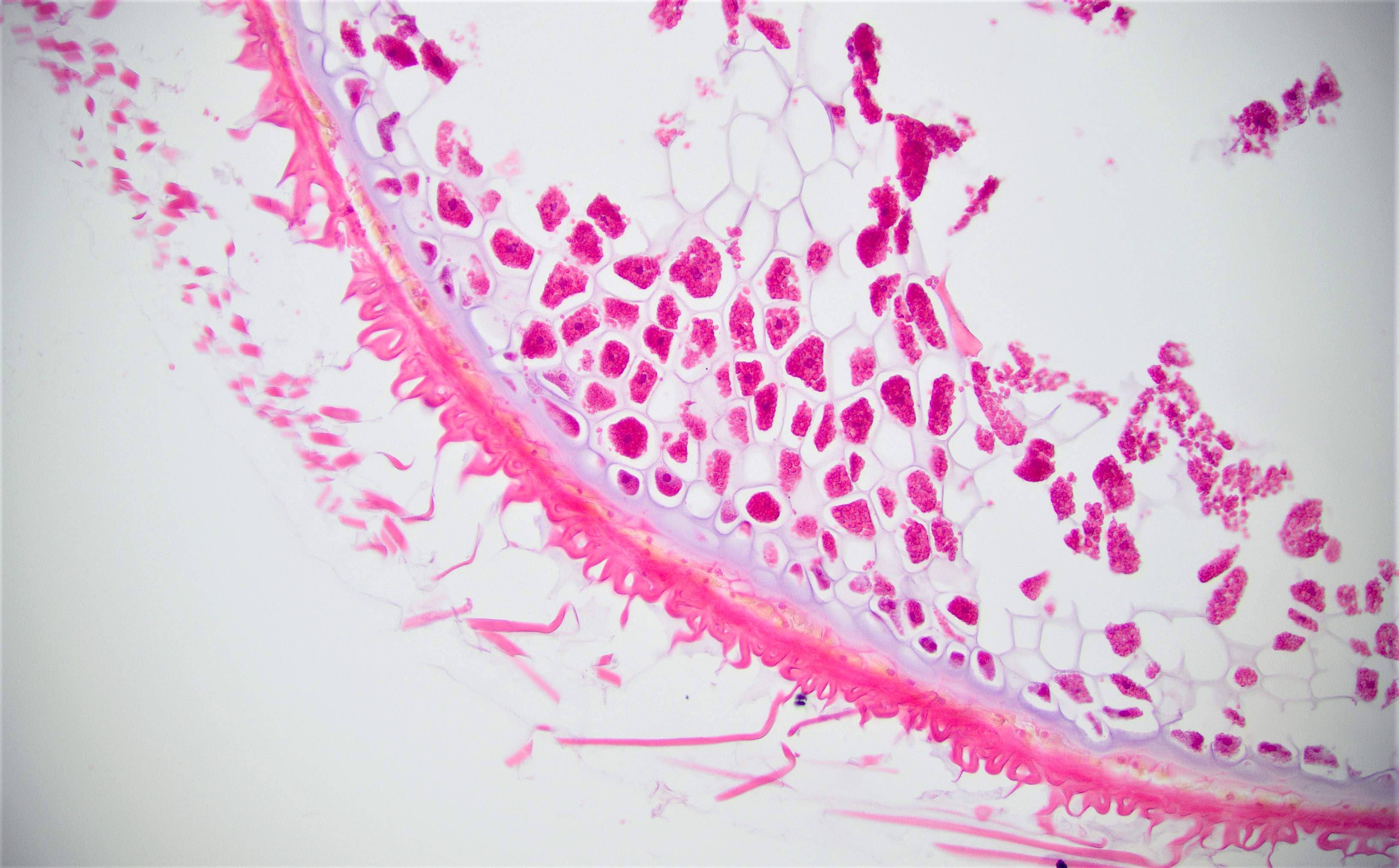
Microscopic (histologic) images
Negative stains
- Regular and modified acid fast stains
Differential diagnosis
- Chronic granulomatous infection
- Malignancy (especially by imaging)
- Nocardiosis
Additional references
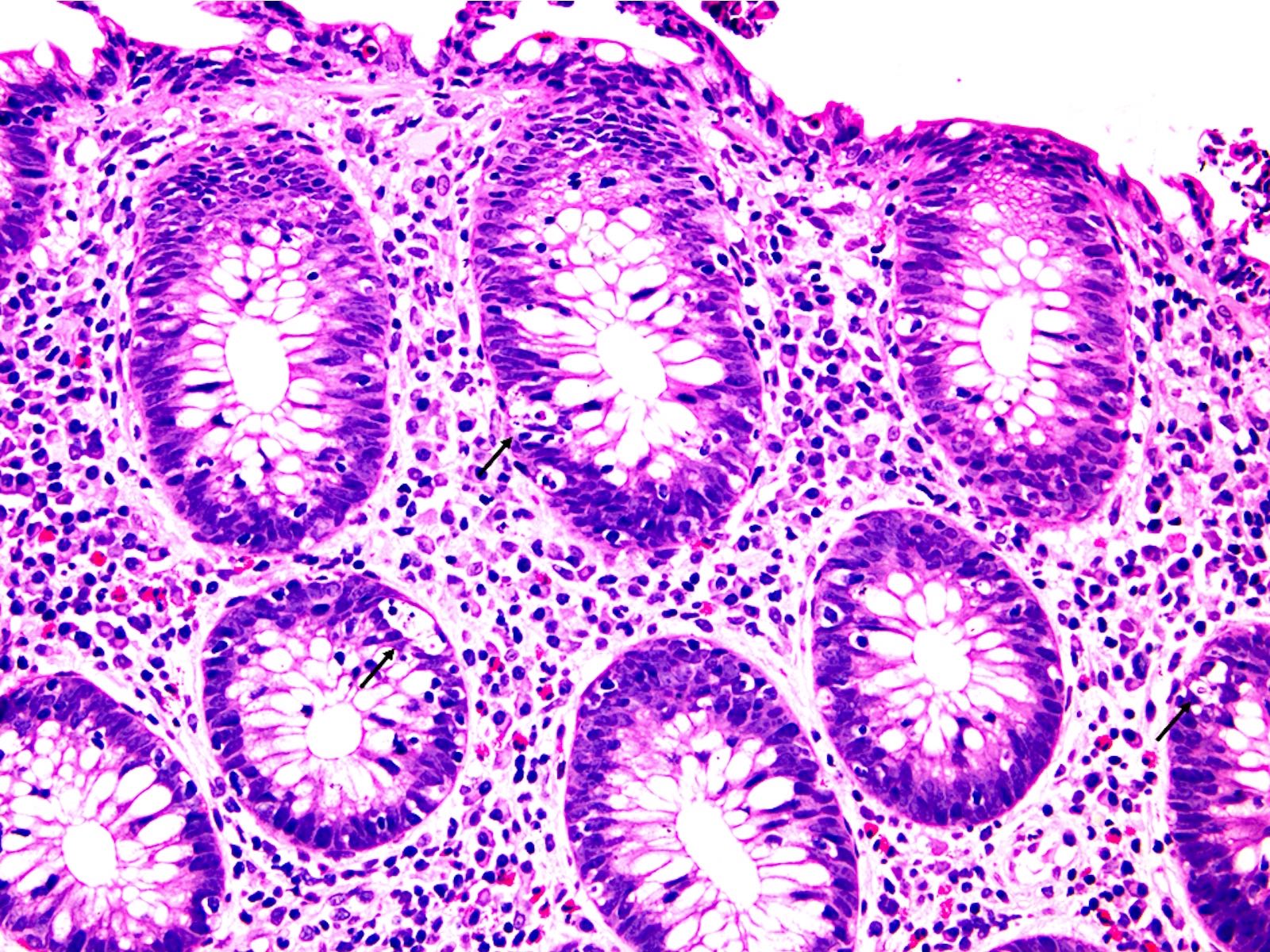
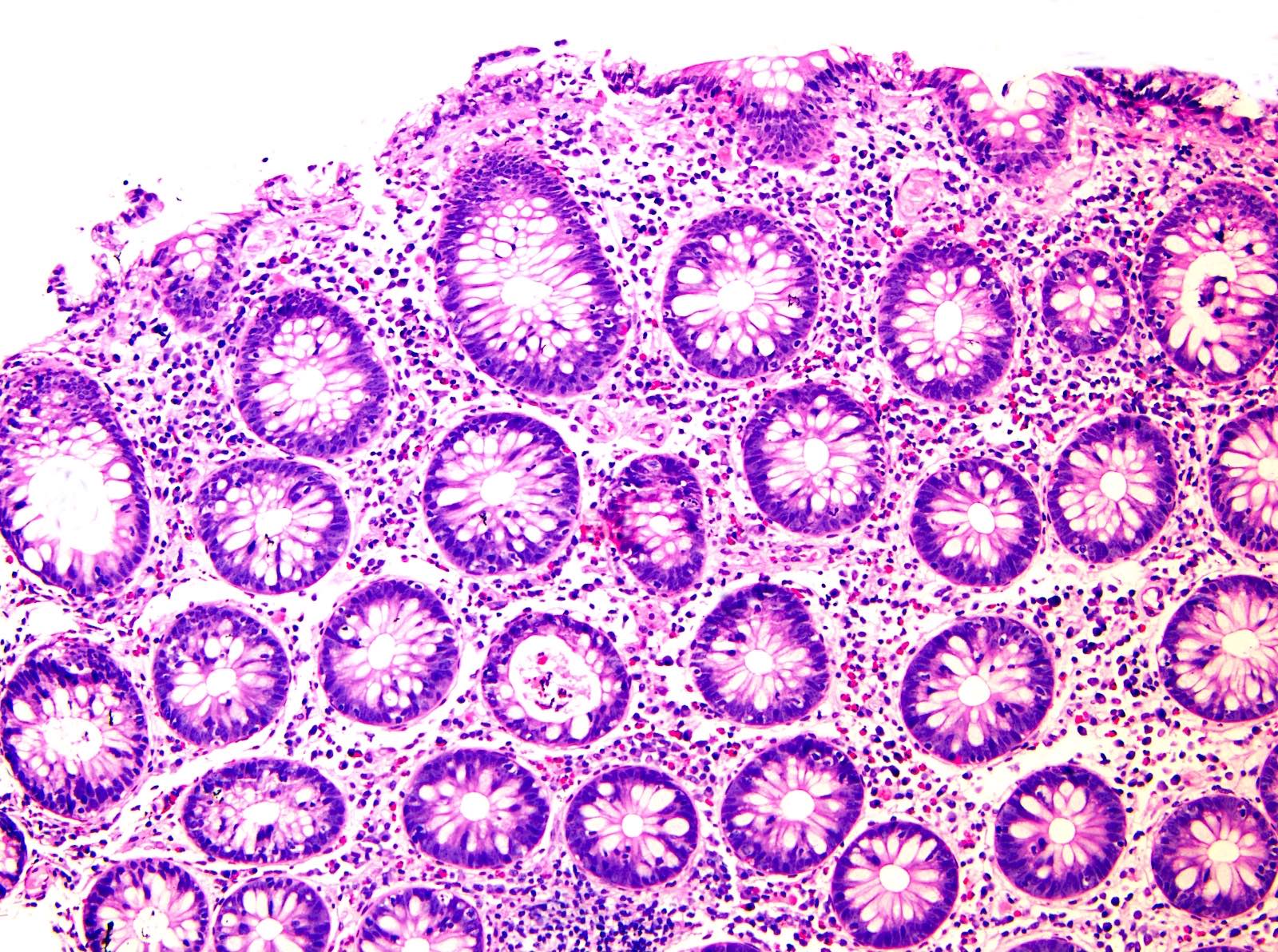
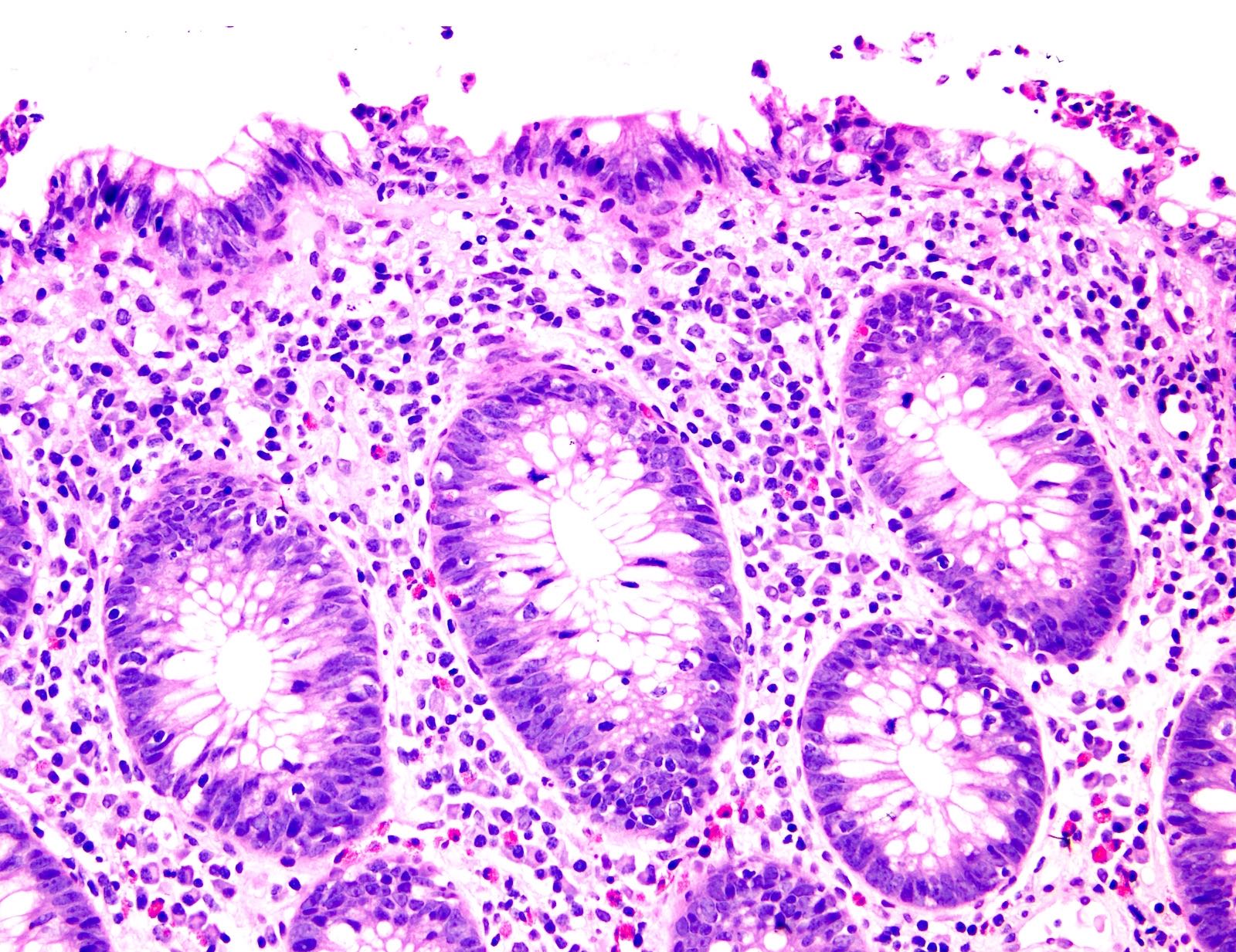
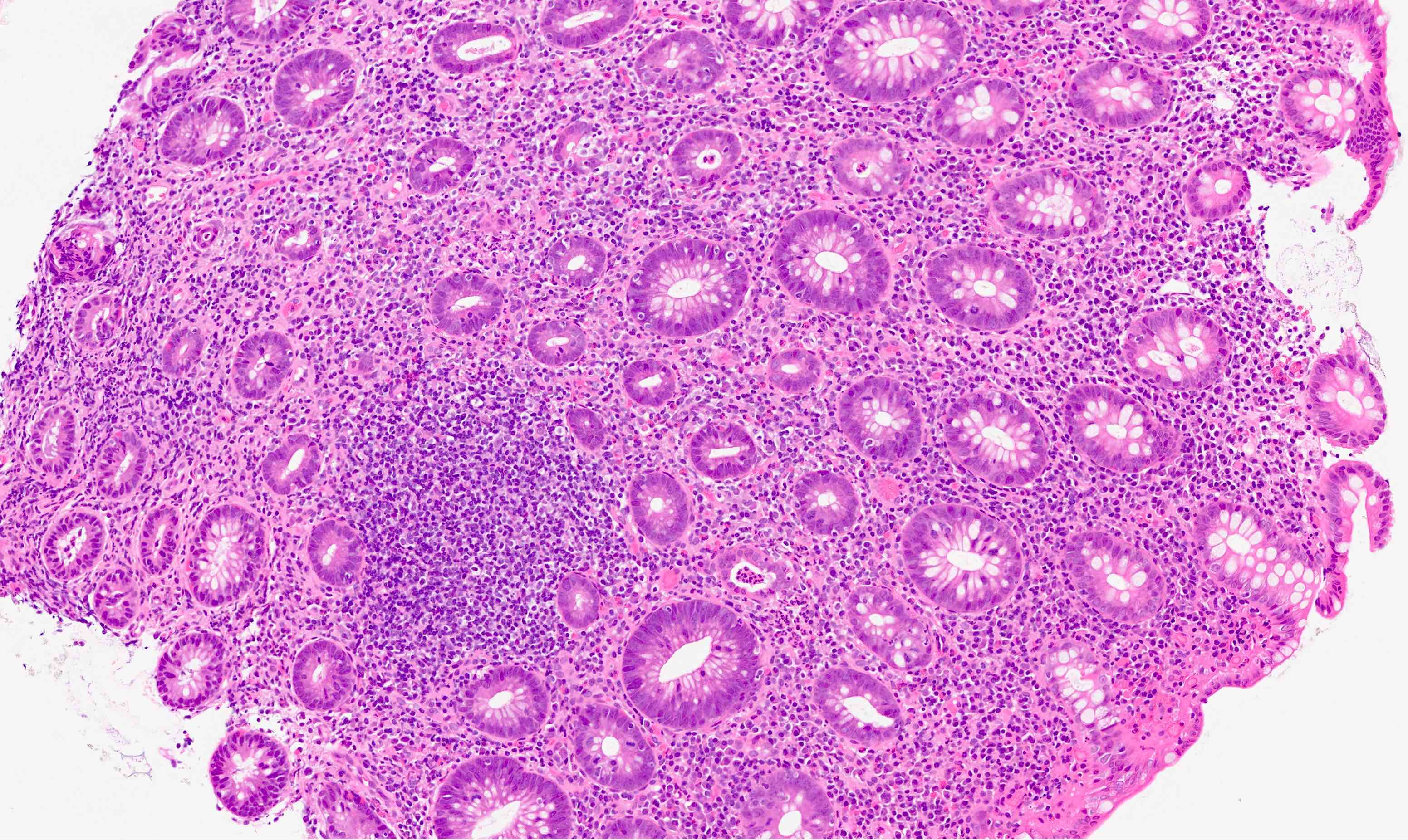
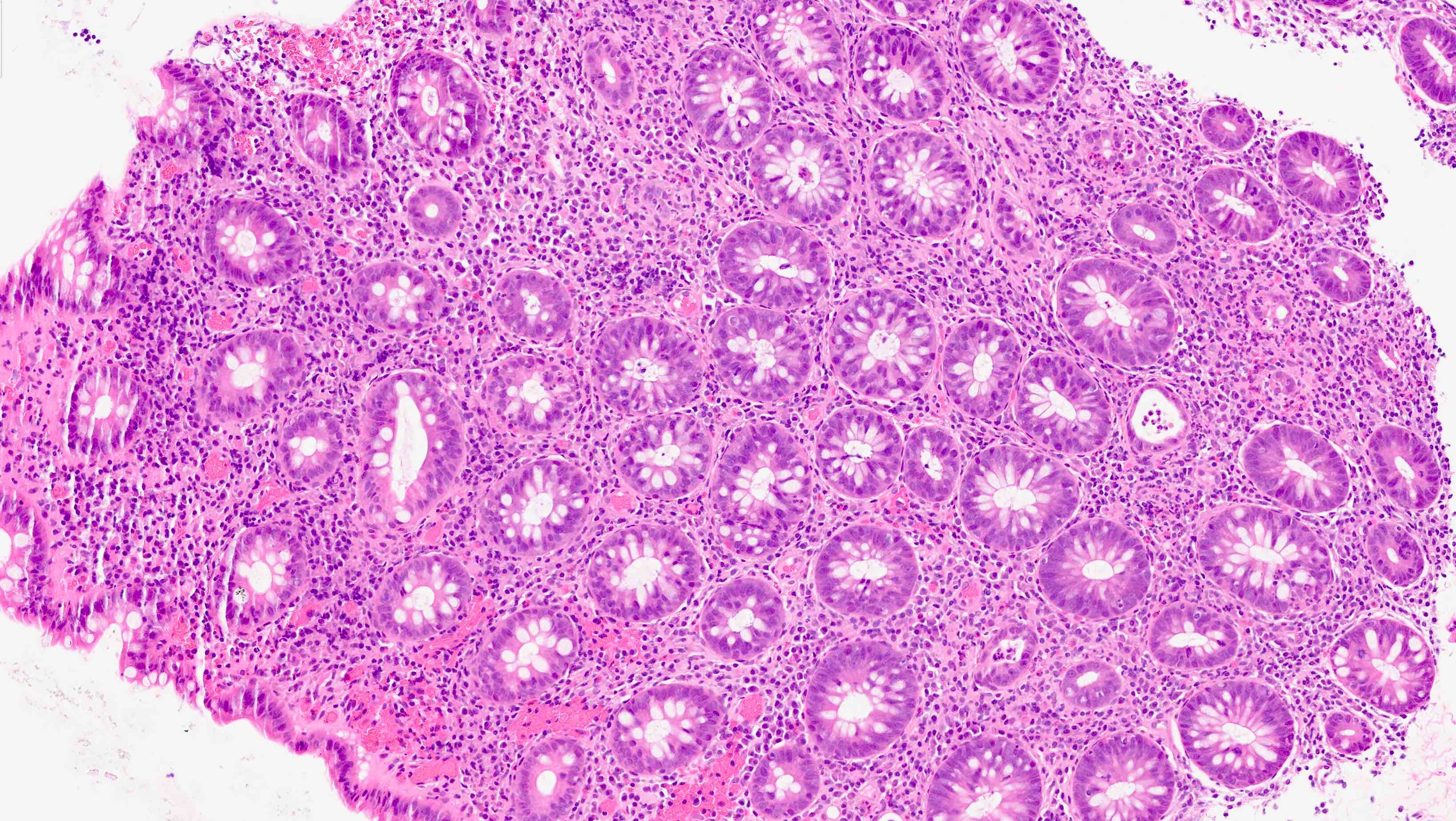
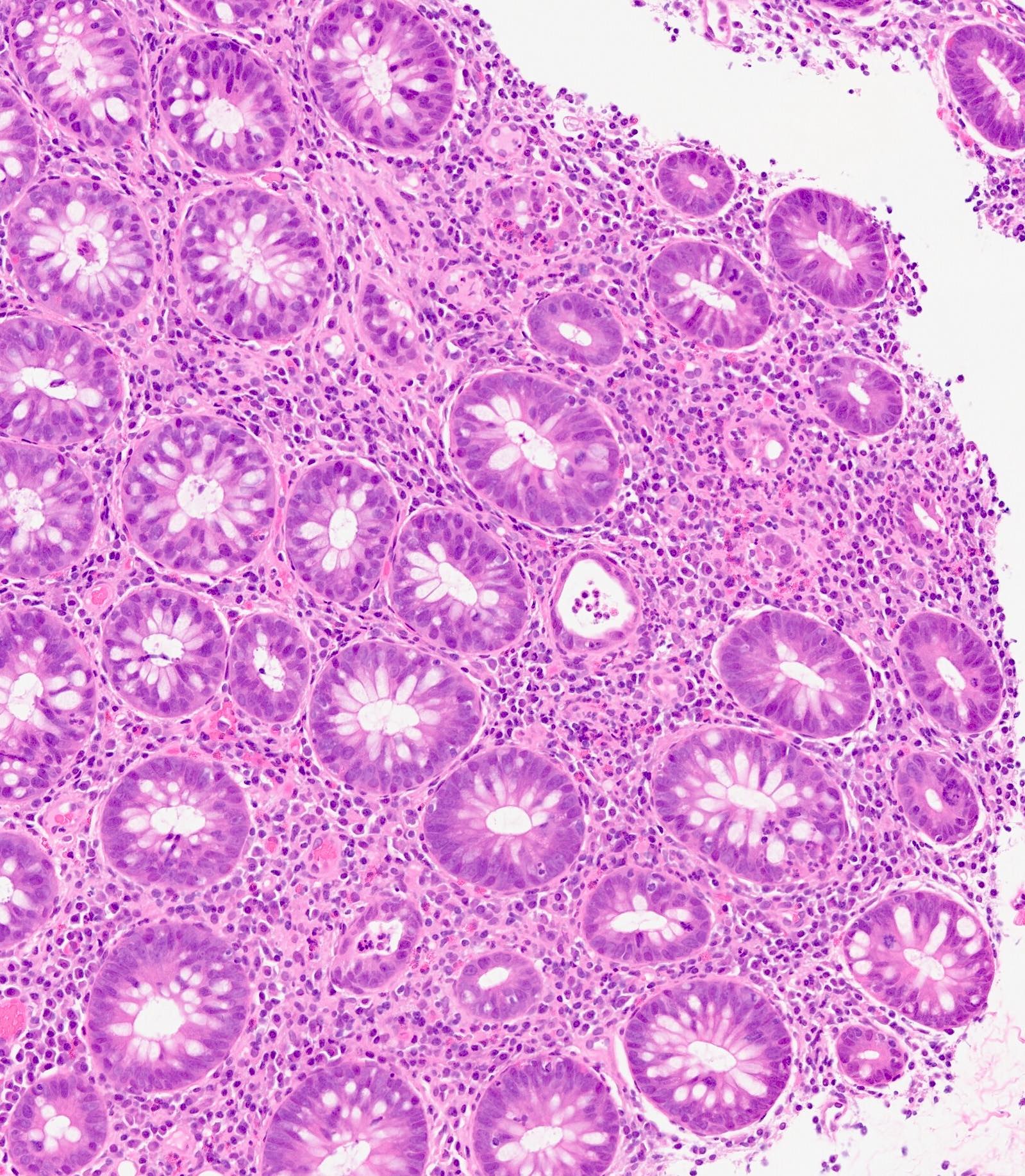
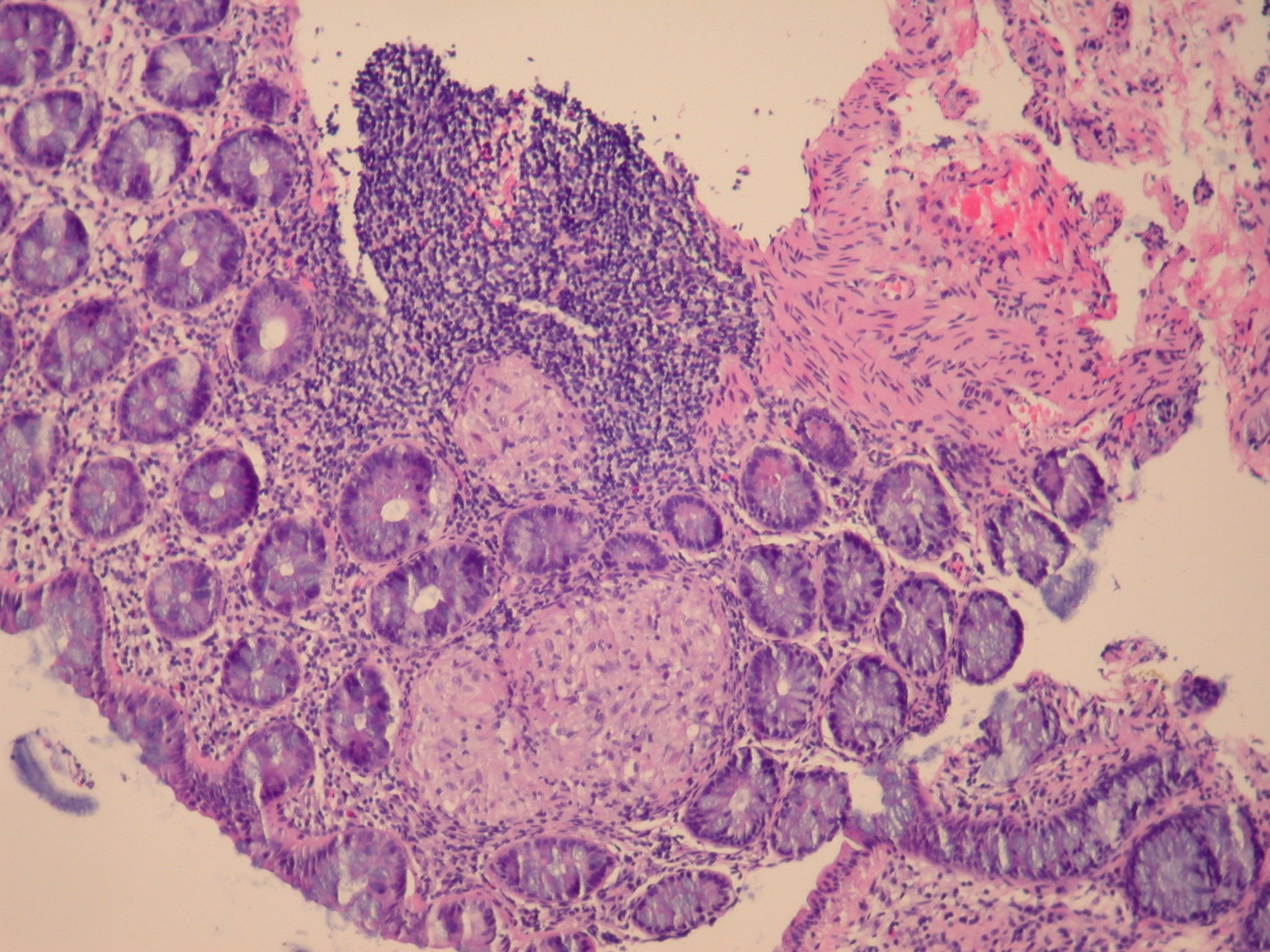
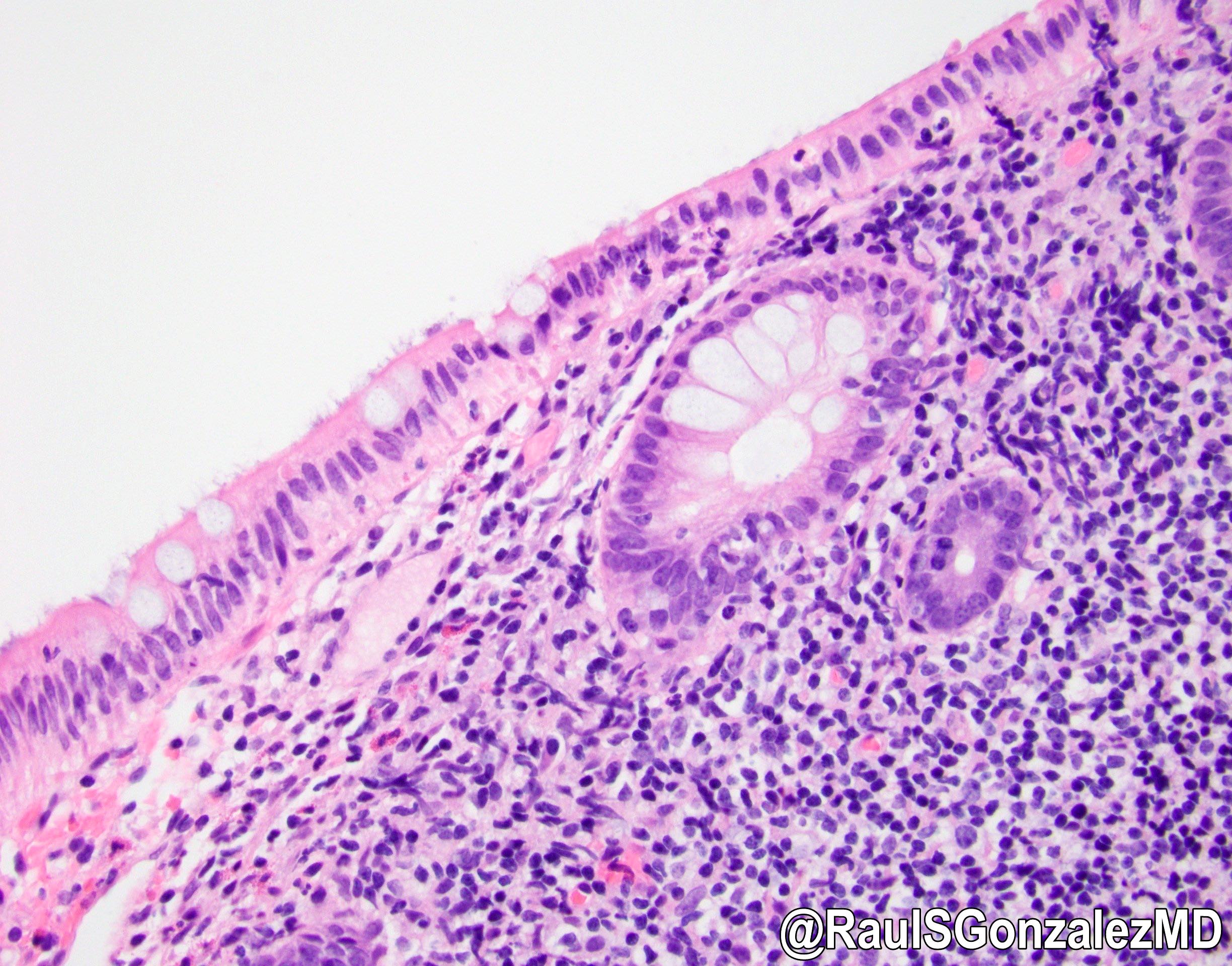
Acute self limited colitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Defined as a transient, most likely infectious, disorder of the colon, which usually resolves completely within 2 - 4 weeks (Odze: Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 3rd Edition, 2014)
- Due to infections, nonsteroidal anti-inflammatory drugs (NSAIDs) or other drugs, bowel preparation or procedure associated injury (e.g., gluteraldehyde disinfection of endoscope) (Arnold: Atlas of Gastrointestinal Pathology - A Pattern Based Approach to Neoplastic Biopsies, 1st Edition, 2019)
- Not always acute or self limited
Essential features
- Due to infections, NSAIDs or other drugs, bowel preparation or procedure associated injury (e.g., gluteraldehyde disinfection of endoscope) (Arnold: Atlas of Gastrointestinal Pathology - A Pattern Based Approach to Neoplastic Biopsies, 1st Edition, 2019)
- Wide variety of pathogens, most commonly bacterial organisms such as Campylobacter jejuni, Salmonella, Shigella species, E. coli and Yersinia enterocolitica (Odze: Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 3rd Edition, 2014)
- Sudden onset, early fever, often with numerous (> 6) bowel movements daily
Terminology
- Acute self limited colitis
- Infectious colitis
ICD coding
- ICD-10: K52.9 - noninfective gastroenteritis and colitis, unspecified
Sites
- Large intestine
Pathophysiology
- Most commonly associated with bacterial enterocolitis (Int J Mol Sci 2020;21:4748)
- Bacterial virulence factors include:
- Adherence to epithelial cells
- Enterotoxins
- Invasion factors
- Cytotoxicity
- Adherence:
- Via fimbriae or pili
- Process of adherence destroys the microvilli brush border
- Enterotoxins:
- Toxins bind to cell membrane, enter cells, activate massive electrolyte secretion (cholera toxin, E. coli heat labile and heat stable toxins produce traveler's diarrhea)
- No white blood cells in stool
- Invasion factors:
- C. jejuni, Salmonella and Shigella species, E. coli and Y. enterocolitica are organisms that invade the tissue and cause epithelial injury and death
- These organisms invade via microbe simulated endocytosis, then cause cell lysis and cell to cell spread
- Tissue invasion also results in the production of inflammatory cytokines that trigger an acute inflammatory reaction
- Cytotoxins are polypeptides that cause tissue injury by inhibiting protein synthesis, disrupting tight junctions and depleting adenosine triphosphate (ATP) within cells
- Endothelial injury results in the activation of the coagulation cascade that ultimately leads to an ischemic colitis pattern of tissue injury (Odze: Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 3rd Edition, 2014)
- Cytotoxicity:
- Shiga toxin, enterohemorrhagic E. coli
Etiology
- Wide variety of pathogens, most commonly bacterial organisms such as Campylobacter jejuni, Salmonella, Shigella species, E. coli and Yersinia enterocolitica (Odze: Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 3rd Edition, 2014)
- Clinical manifestation of acute self limited colitis is related to the ability of microorganisms to invade mucosa or produce enterotoxins
- Ingestion of preformed toxins (Staphylococcus aureus, Vibrio cholera, Clostridium perfringens)
- Infection by enteroinvasive organisms which invade and destroy mucosal epithelium cells
- Infection by viral organisms (cytomegalovirus [CMV], herpes simplex virus [HSV], HIV, etc.)
Clinical features
- Abdominal pain, watery or bloody diarrhea (Int J Mol Sci 2020;21:4748)
- Causes symptoms within hours (including explosive diarrhea)
- Sudden onset, early fever, often with numerous (> 6) bowel movements daily
- Complications of dehydration, sepsis, perforation can occur secondary to potential massive fluid loss and loss of mucosal barrier
- Inflammatory process usually resolves completely within 2 - 4 weeks
Diagnosis
- Stool cultures (Gastroenterology 1994;107:755)
- Colonoscopy with mucosal biopsy
- Imaging studies can show nonspecific thickening of the bowel wall; however, they are seldom performed
Laboratory
- Peripheral blood and fecal leukocyte count
Radiology description
- Limited role since inflammatory abnormalities are nonspecific (e.g., colonic wall thickening)
Prognostic factors
- Depends on specific underlying infectious agent (Gastroenterology 1994;107:755)
- Immune status of host
- Generally good with appropriate therapy
- Commonly self limited disease (2 - 4 weeks)
Case reports
- 8 week old and 10 month old immunocompetent boys with CMV colitis, causing self limited colitis (Pediatr Infect Dis J 2016;35:573)
- 34 year old woman with Yersinia enterocolitica colitis mimicking acute appendicitis (Emerg Radiol 2008;15:123)
- 72 year old immunocompromised man with multidrug resistant Campylobacter colitis (BMC Infect Dis 2016;16:409)
Treatment
- Supportive therapy with rehydration
- Rarely may require antibiotics or steroids
Gross description
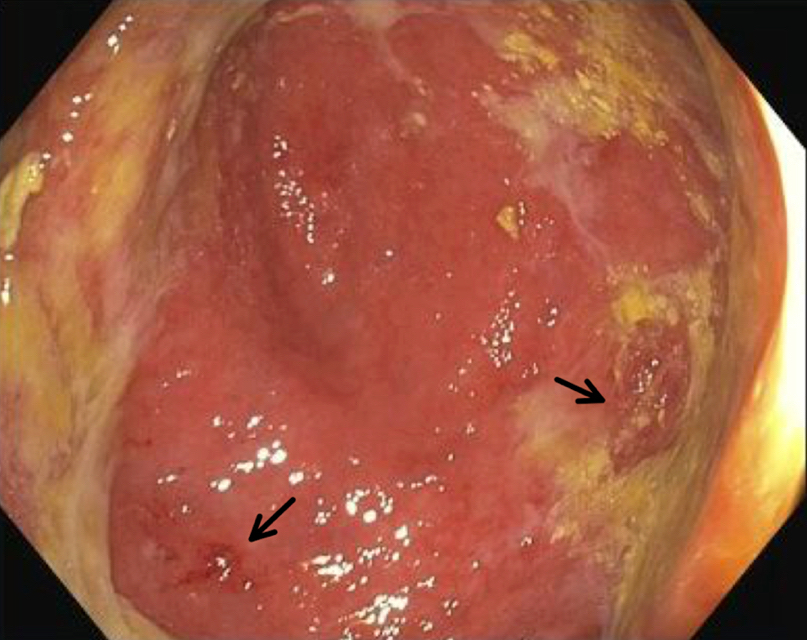
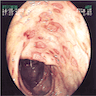
- Mucosal erythema, erosion, friability (Case Rep Infect Dis 2012;2012:810943)
- Ulceration, erosion, pseudopolyps, hyperemia
- Exudates may be present (pseudomembranes)
- Mass lesions are unlikely
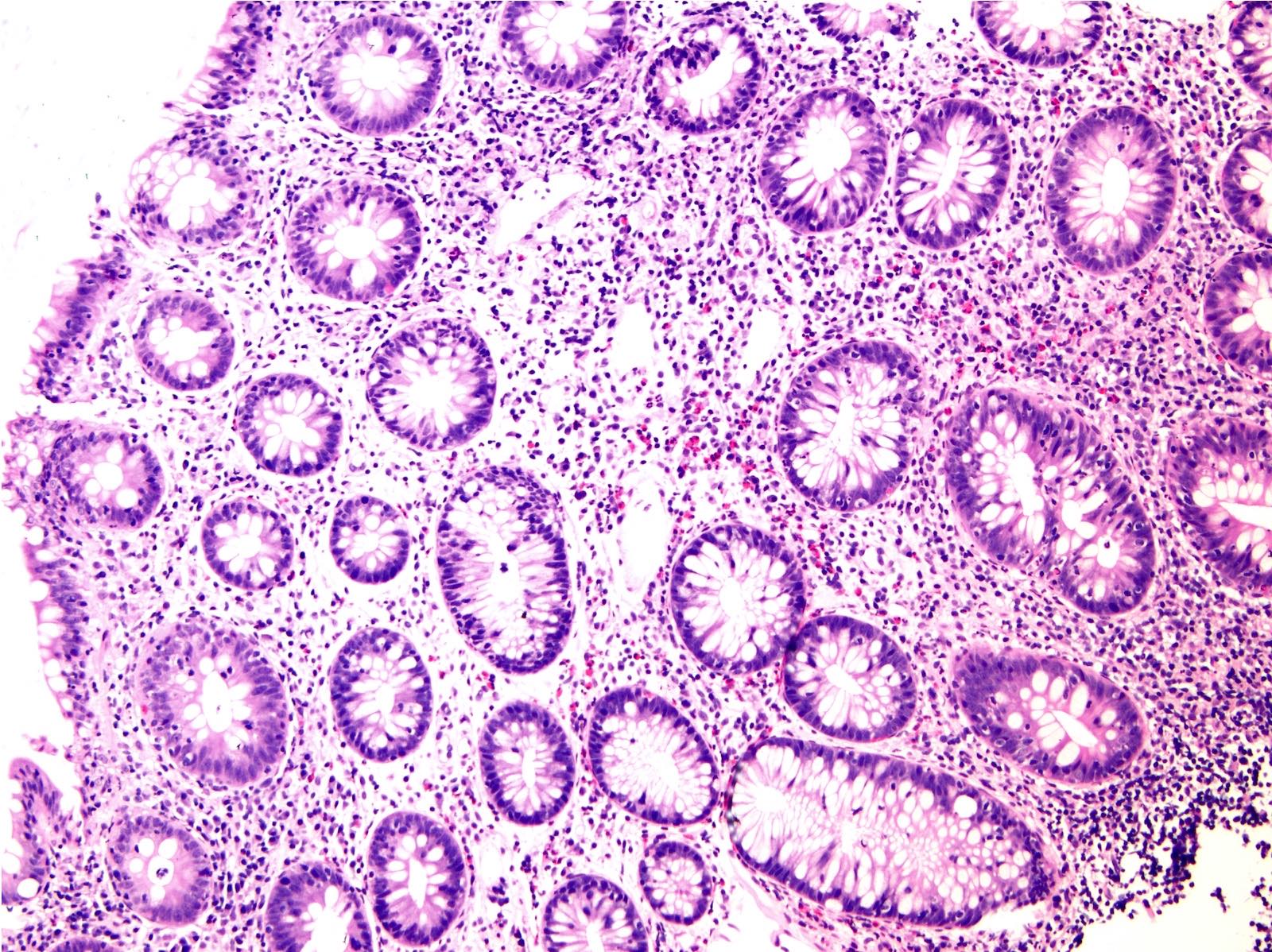
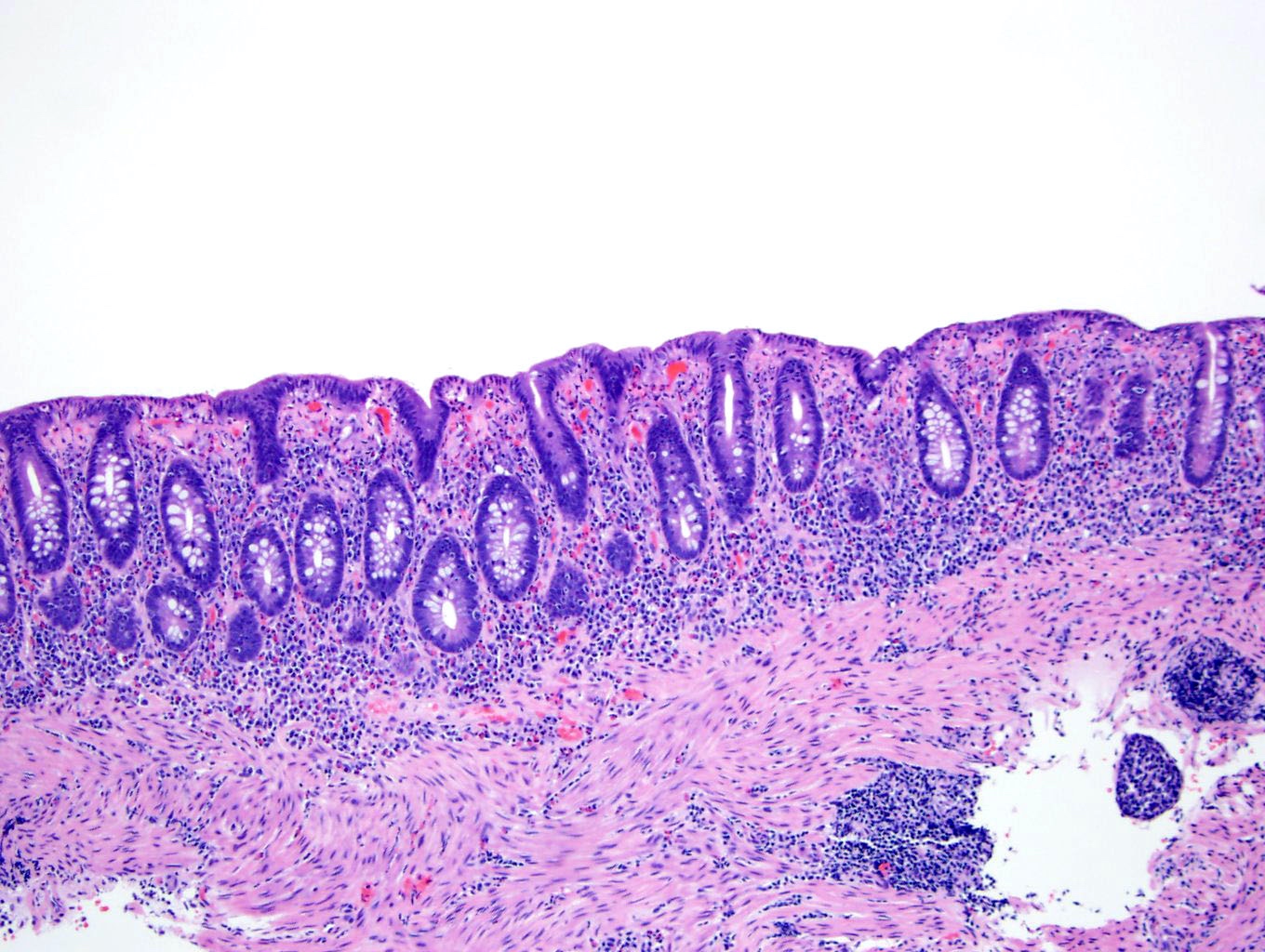
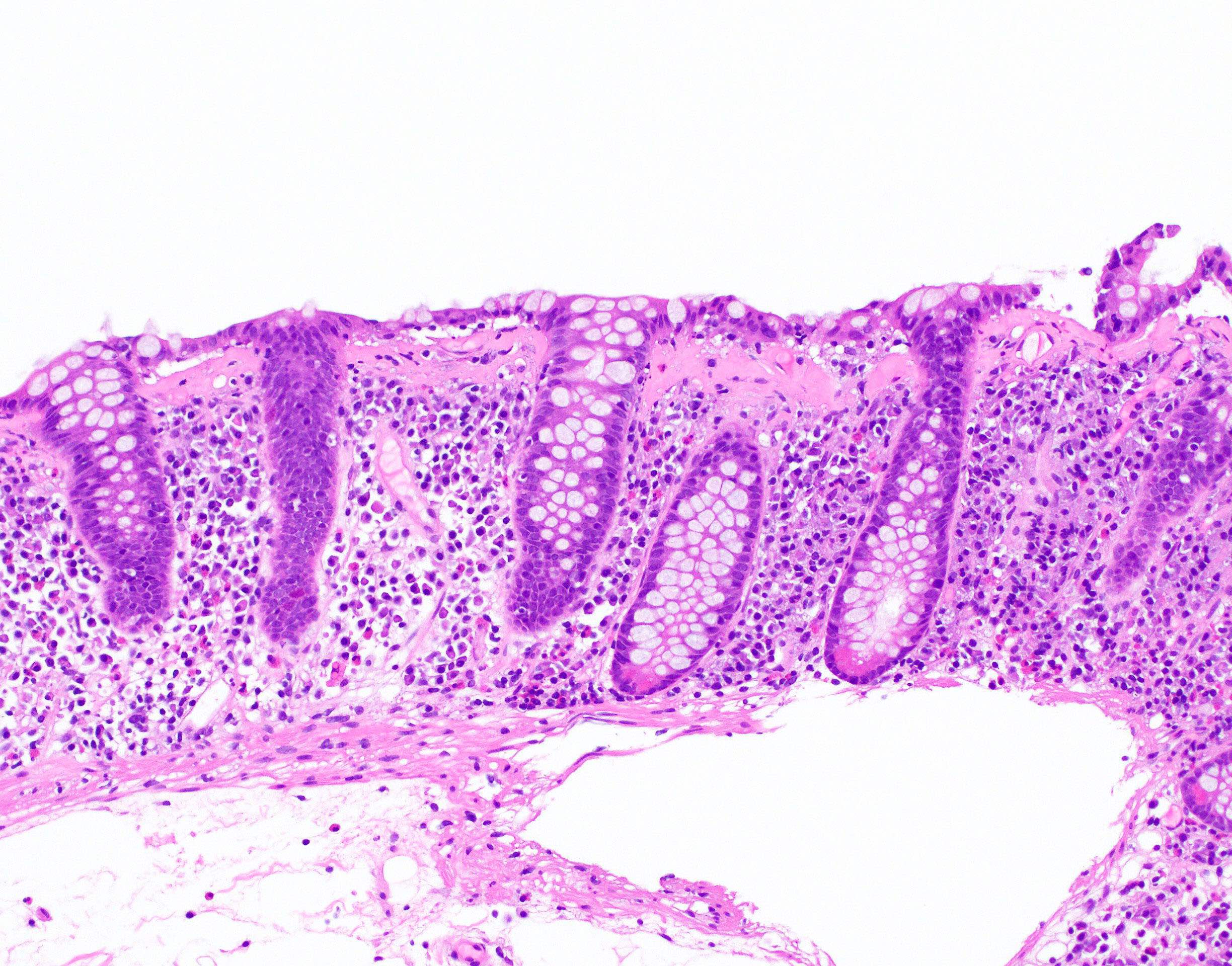
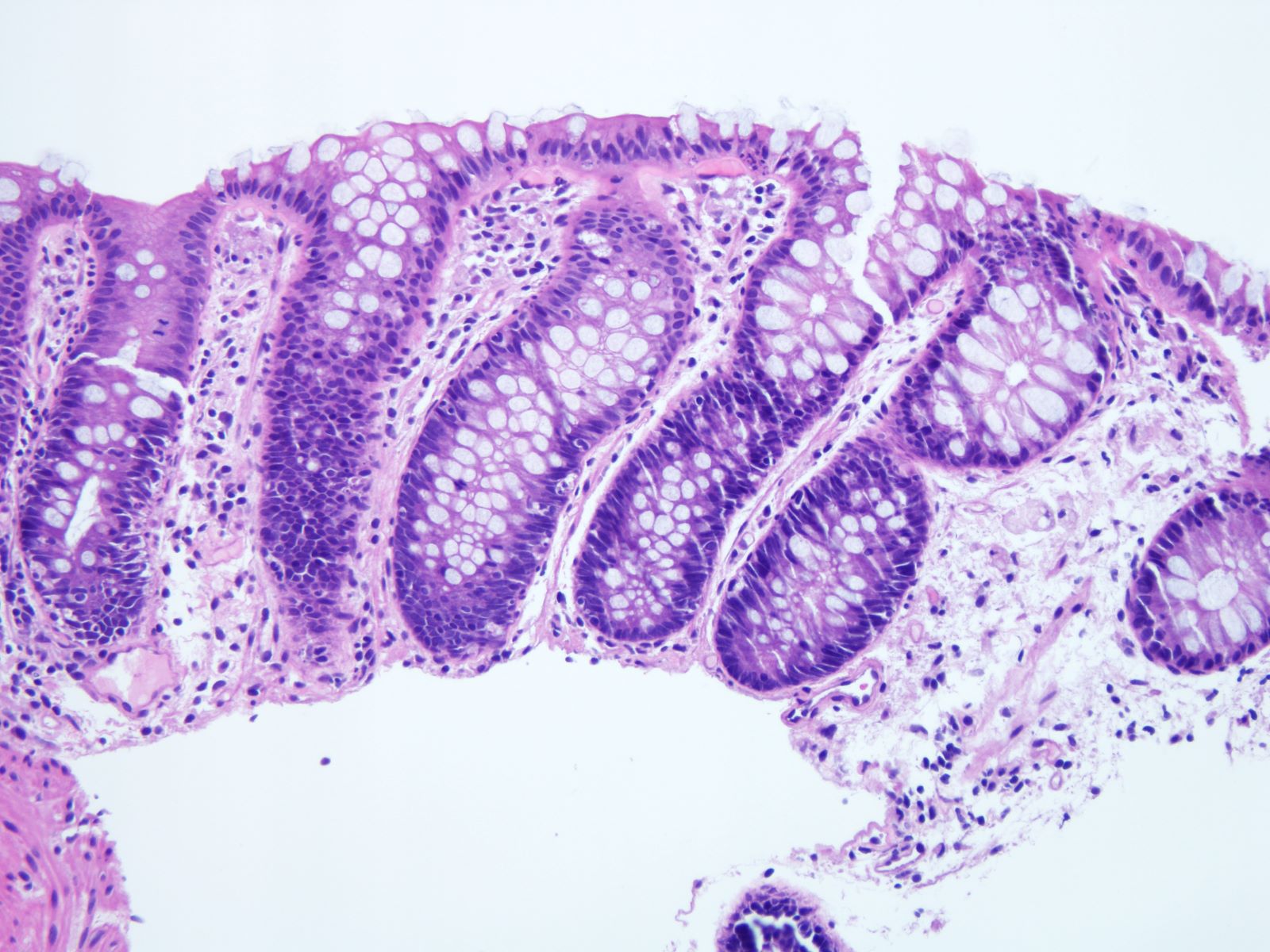
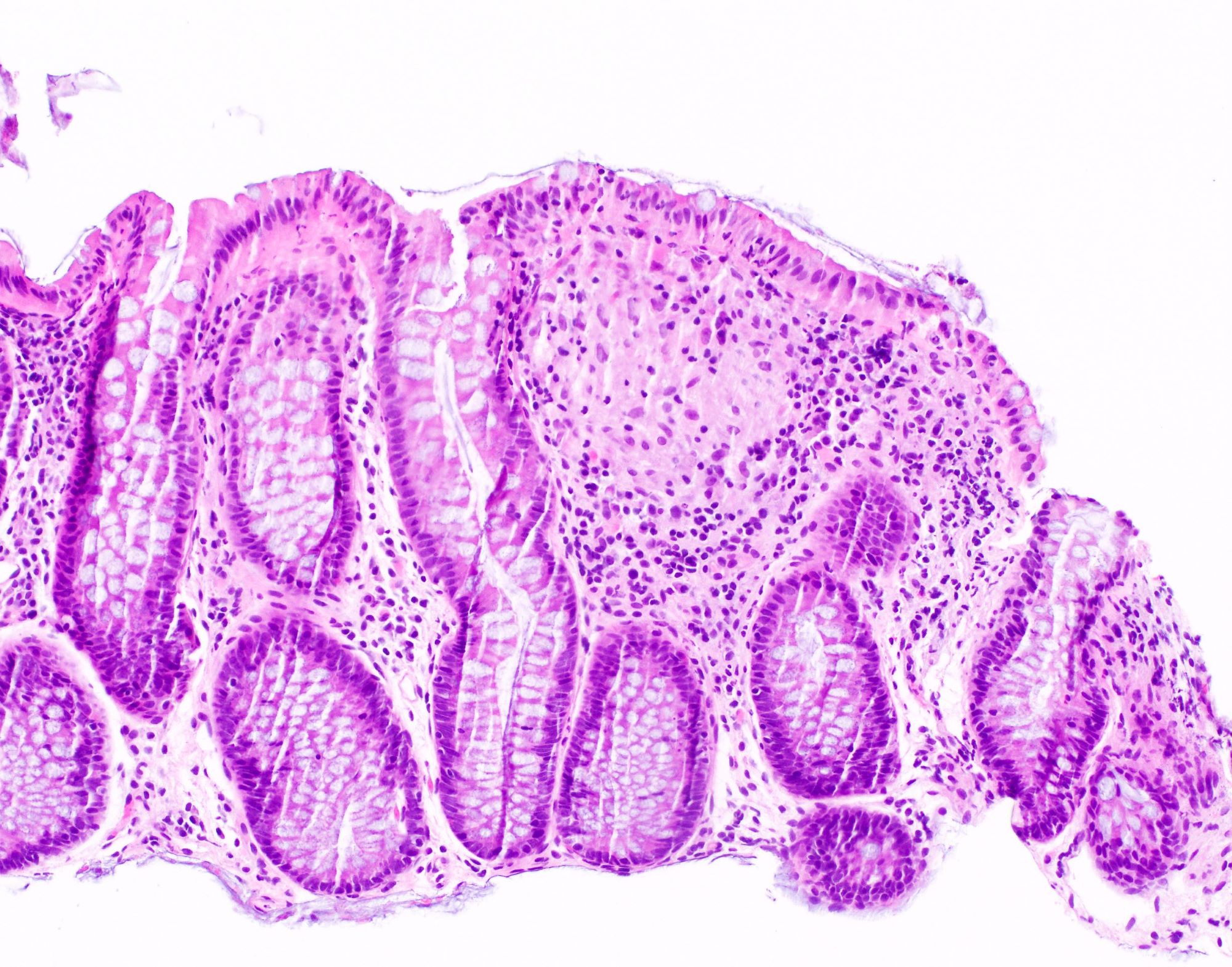
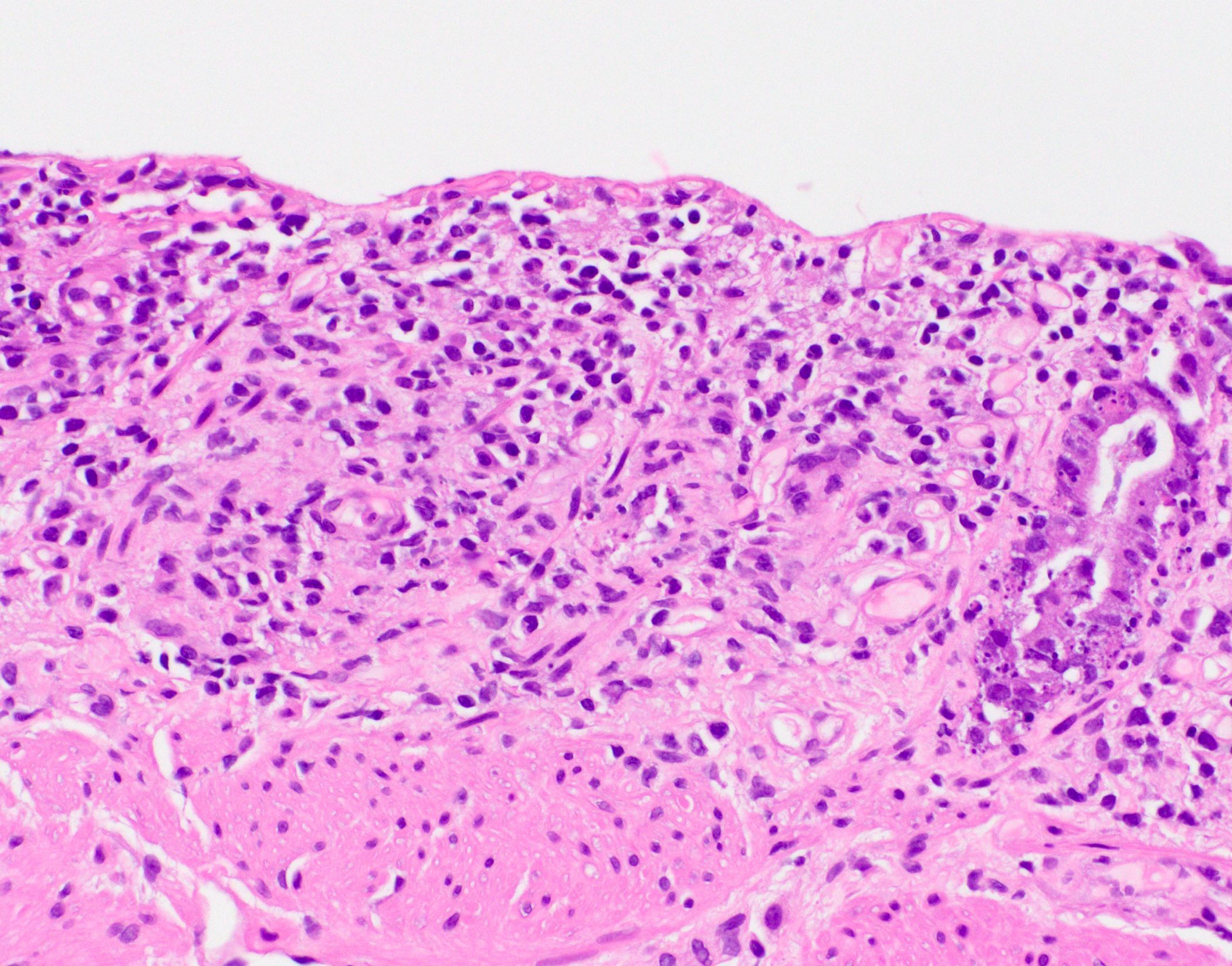
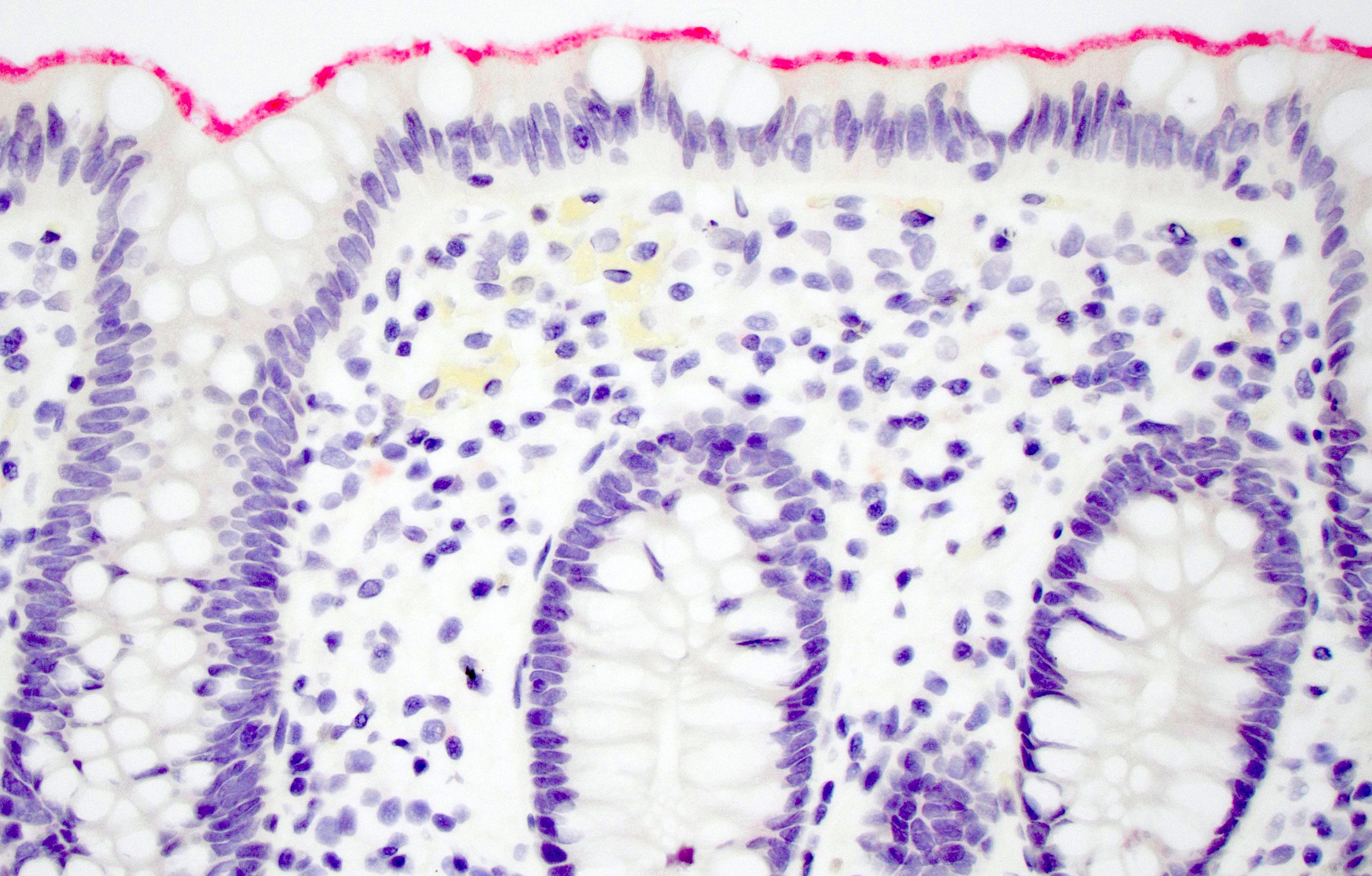
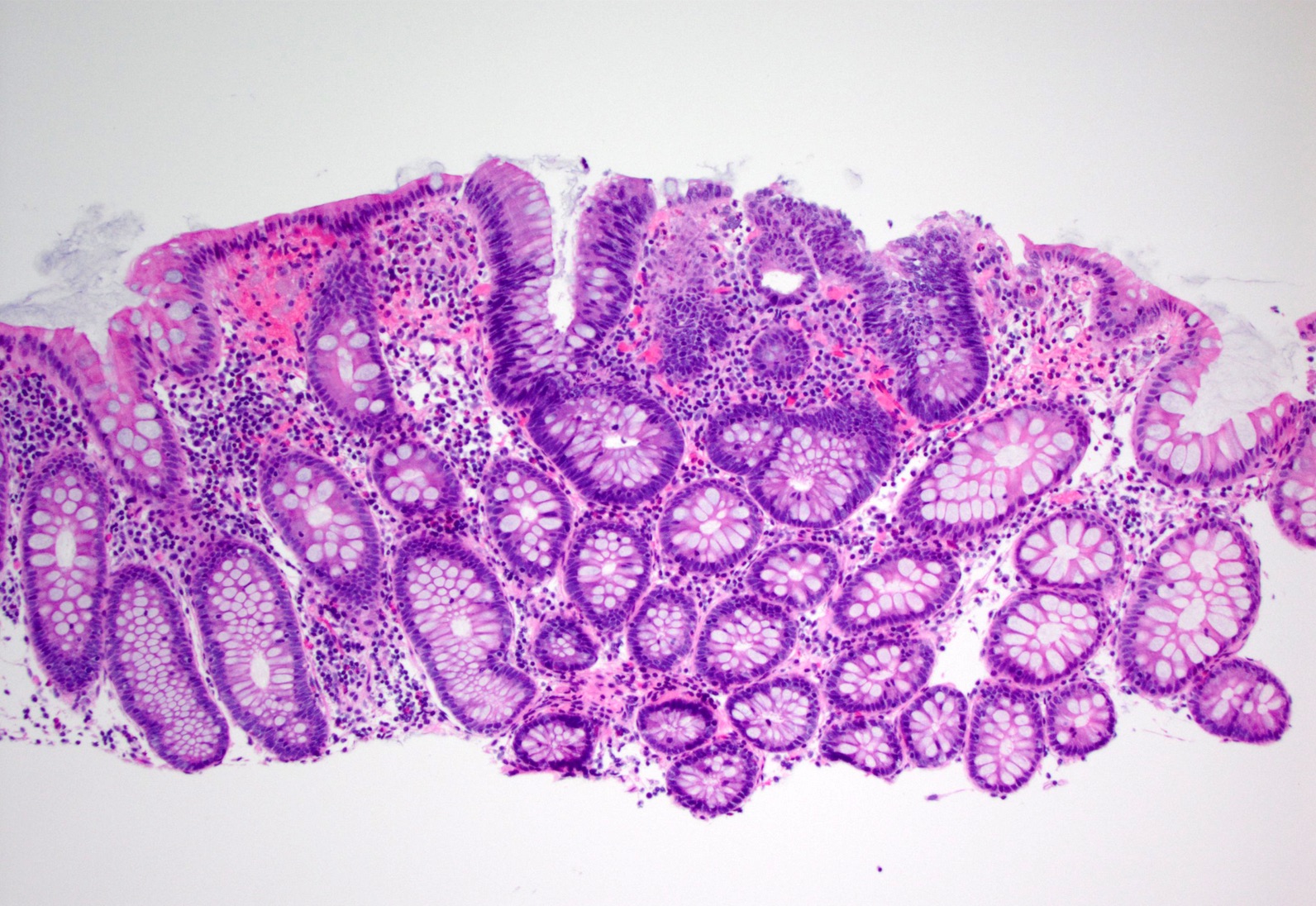
Microscopic (histologic) description
- Inflammation of lamina propria (active much more than chronic), edema, hemorrhage (Br Med J (Clin Res Ed) 1984;289:270)
- Usually lacks features of chronicity:
- Crypt architectural distortion
- Basal lymphoplasmacytosis
- Pyloric gland metaplasia
- Paneth cell metaplasia in the left colon
- Neutrophil induced epithelial injury (cryptitis or crypt abscess)
- Severe cases have crypt abscesses, extensive necrosis, hemorrhage and microthrombi
- Over time, neutrophils disappear (within second / third weeks) with persistent monocytic infiltration
- Colonic lymphocytosis can be seen in resolving phase
- Epithelial vacuolization, nuclear disarray, lymphocytosis, intranuclear inclusion can be seen in viral etiologies
Sample pathology report
- Colon, cecum, endoscopic biopsy:
- Active colitis (see comment)
- Comment: The cecal biopsy shows colonic mucosa with a variable degree of crypt architectural distortion and neutrophil mediated epithelial injury, diagnostic of active colitis. No granulomas or evidence of dysplasia is identified. The findings are nonspecific and may be seen in the setting of intestinal infection, medication / drug associated injury and idiopathic inflammatory bowel disease. Definitive diagnosis will require synthesis of all available clinical, endoscopic, radiologic and pathologic evidence.
Differential diagnosis
- Inflammatory bowel disease:
- Chronic mucosal injury, crypt distortion, basal lymphoplasmacytosis, pyloric gland metaplasia (Cureus 2017;9:e1817)
- Irritable bowel syndrome:
- Type of functional gastrointestinal disorder
- These problems cause the digestive tract to be very sensitive and change how the bowel muscles contract; the result is abdominal pain, diarrhea and constipation (Inflamm Bowel Dis 2018;24:2479)
- Adverse drug reaction:
- Apoptosis and intraepithelial lymphocytosis could be observed
Additional references
Board review style question #1
A 35 year old, previously healthy female complained of right lower abdominal pain and intermittent diarrhea. The clinical impression was acute appendicitis. An appendectomy was performed. Grossly, the appendix appeared normal. Histologically, there was patchy chronic and acute inflammation as shown in the image above. Which of the following statements about this infection is correct?
- A patient with this infection is likely to die within 6 months
- Long term antibiotics are the treatment of choice in this patient
- The patient most likely has a colitis and requires supportive therapy
- This infection may be disseminated and tests to discover additional foci are mandatory
Board review style answer #1
C. The patient most likely has a colitis and requires supportive therapy. This patient most likely has CMV colitis demonstrated by nuclear and cytoplasmic inclusions, commonly referred to as owl eye inclusions, which are Cowdry type A inclusions. CMV colitis can mimic chronic idiopathic inflammatory bowel disease, graft versus host disease and acute appendicitis.
Comment Here
Reference: Acute self limited colitis
Comment Here
Reference: Acute self limited colitis
Board review style question #2
Paneth cell metaplasia is an indicator for chronic mucosal injury in which part of the colon?
- Ascending colon
- Cecum
- Sigmoid colon
- Terminal ileum
- Transverse colon
Board review style answer #2
C. Sigmoid colon. Paneth cells are normally absent in the left colon. When present, they serve as an indicator for chronic mucosal injury. However, Paneth cells are normally present in the right colon; hence, sigmoid colon is the correct choice. If Paneth cell metaplasia is seen in the sigmoid colon, this is an indicator for chronic mucosal injury.
Comment Here
Reference: Acute self limited colitis
Comment Here
Reference: Acute self limited colitis
Adenocarcinoma
Table of Contents
Definition / general | Essential features | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
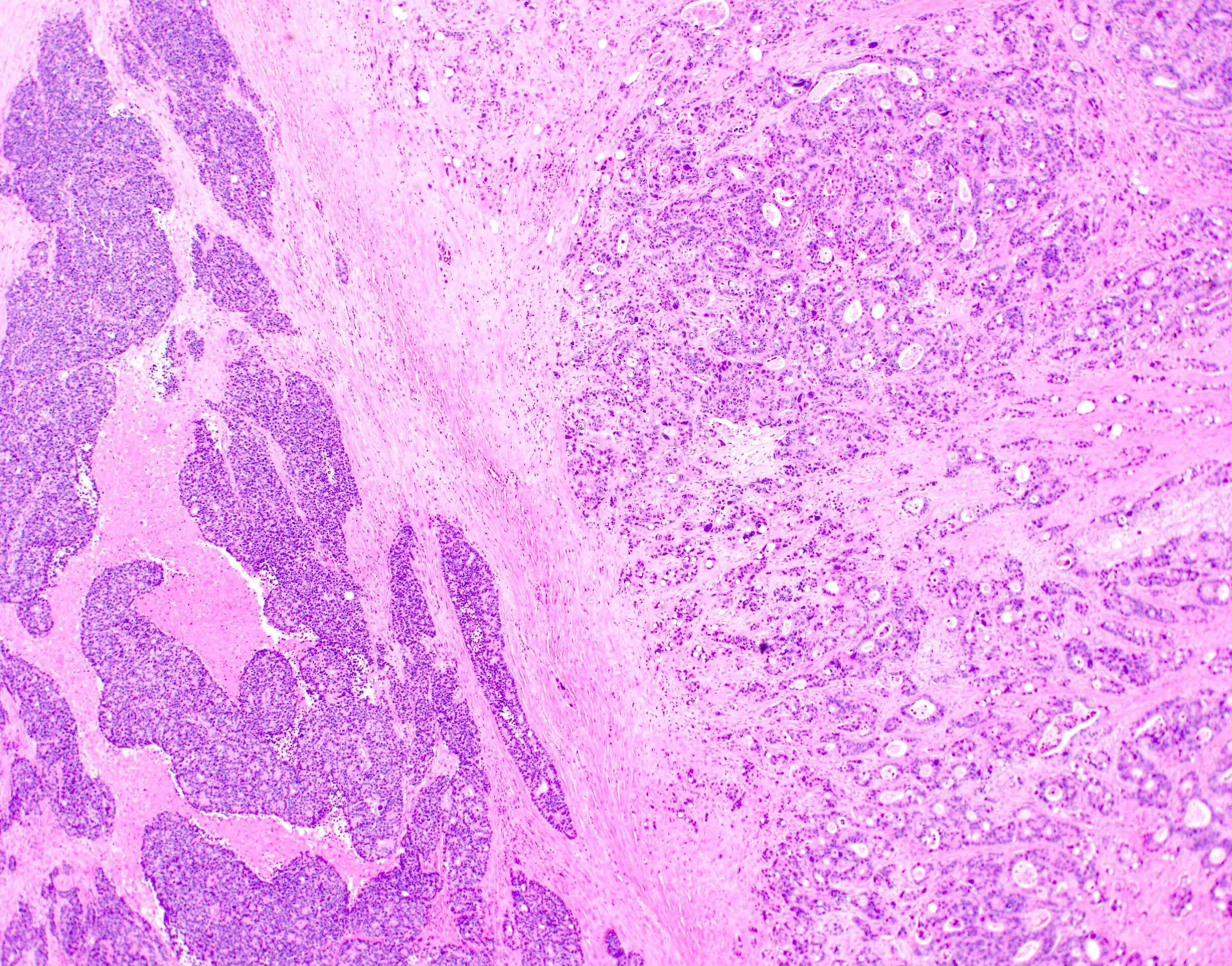
- Glandular neoplasm of the colorectum, representing 98% of colonic cancers (therefore, most details in the general colon carcinoma section pertain to adenocarcinomas)
- 9 WHO recognized subtypes: adenoma-like, adenosquamous, carcinoma with sarcomatoid components, medullary, micropapillary, mucinous, serrated, signet ring cell, undifferentiated
- Uncommon subtypes are clear cell adenocarcinoma, low grade tubuloglandular adenocarcinoma and villous / adenoma-like adenocarcinoma (Am J Surg Pathol 2006;30:1022, Histopathology 2016;68:183)
Essential features
- Most common primary colon carcinoma
- Typically arises through chromosomal instability pathway (70 - 80%) or microsatellite instability pathway (10 - 15%)
- Stage is most important prognostic factor
Clinical features
- Increased carcinoma risk in patients with polyposis syndromes, Lynch syndrome and inflammatory bowel disease
- Right sided tumors cause anemia, weakness and fatigue
- Left sided tumors cause change in bowel habits (diarrhea or constipation)
- Superficial tumors only rarely cause lymph node metastases due to distribution of lymphatics in colon
Diagnosis
- Generally discovered on colonoscopy and confirmed on biopsy
Prognostic factors
- Good prognostic factors:
- Microsatellite instability (Medicine (Baltimore) 2018;97:e0019)
- Increased tumor infiltrating lymphocytes (Am J Surg Pathol 2020;44:536)
- Poor prognostic factors:
- Advanced stage, higher grade, lymphovascular and perineural invasion (Surg Pathol Clin 2020;13:503)
- Positive margins
- High tumor budding (Mod Pathol 2012;25:1315)
- CDX2 loss (World J Gastroenterol 2015;21:1457)
- High stromal content (Histopathology 2018;73:197)
Case reports
- 29 year old man with colonic adenocarcinoma metastasizing as a germ cell neoplasm (Arch Pathol Lab Med 2001;125:558)
Treatment
- Surgical resection is generally required unless tumor is small and confined to a polyp
- Adjuvant therapy given for patients with lymph node metastases
- Neoadjuvant therapy often given for rectal carcinomas
Gross description
- Usually single, polypoid or ulcerated mass
- May cause serosal puckering if muscularis propria is involved
- Right colon tumors tend to be polypoid and exophytic, while left colon tumors tend to be annular, encircling lesions
Gross images
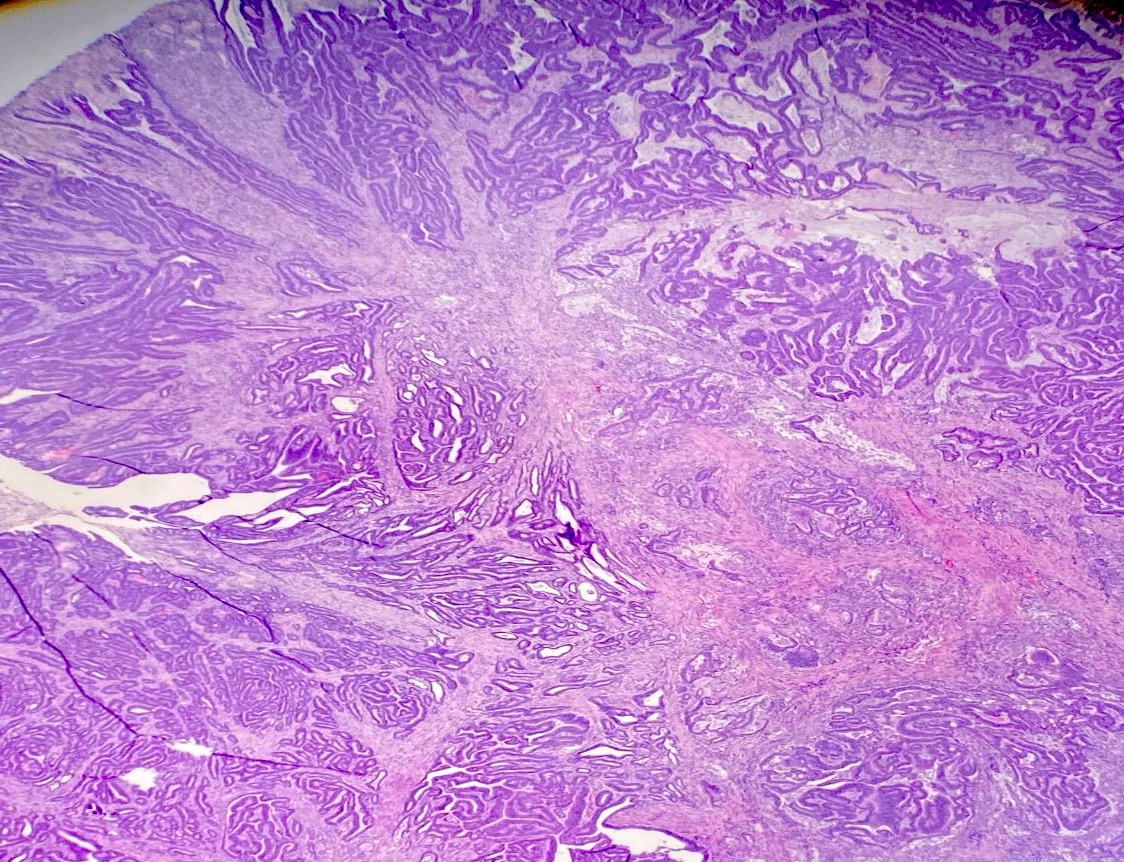
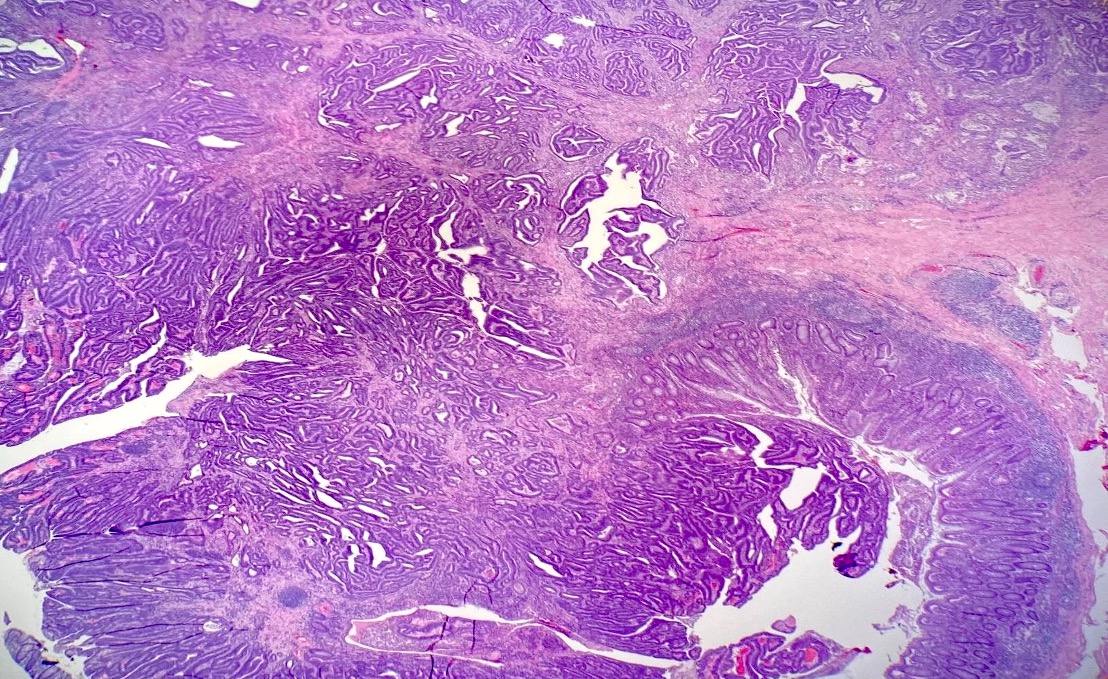
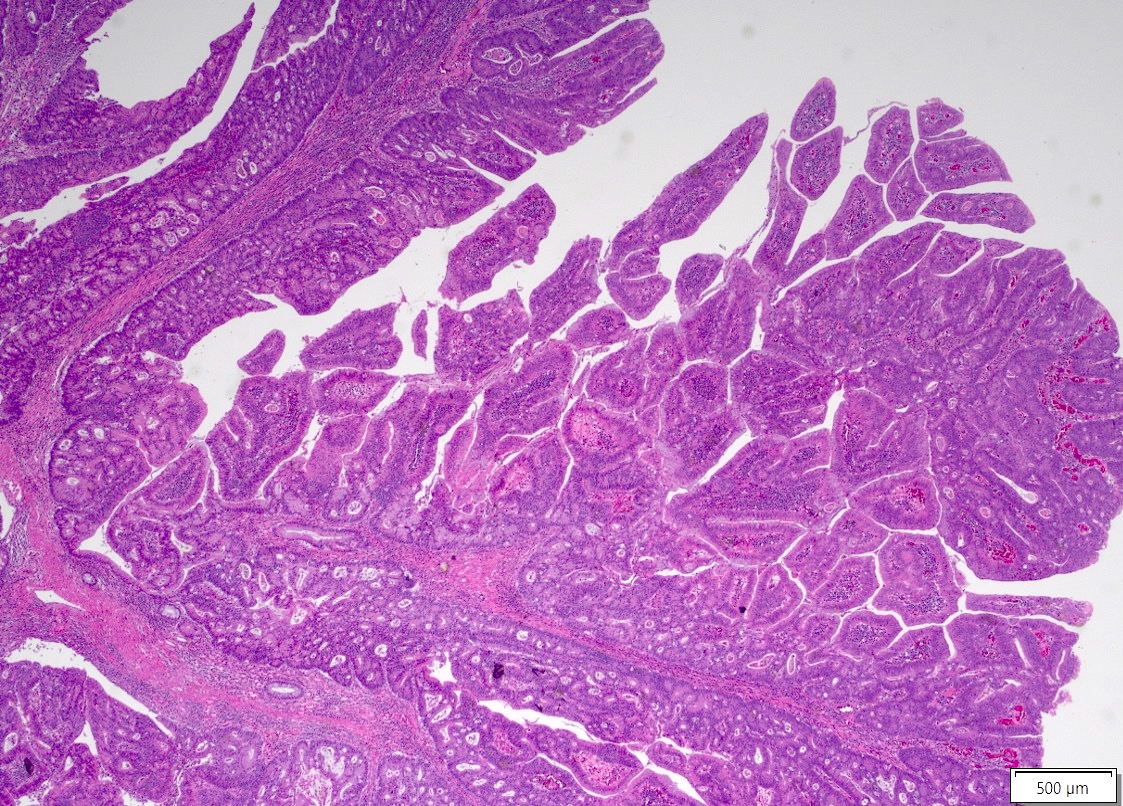
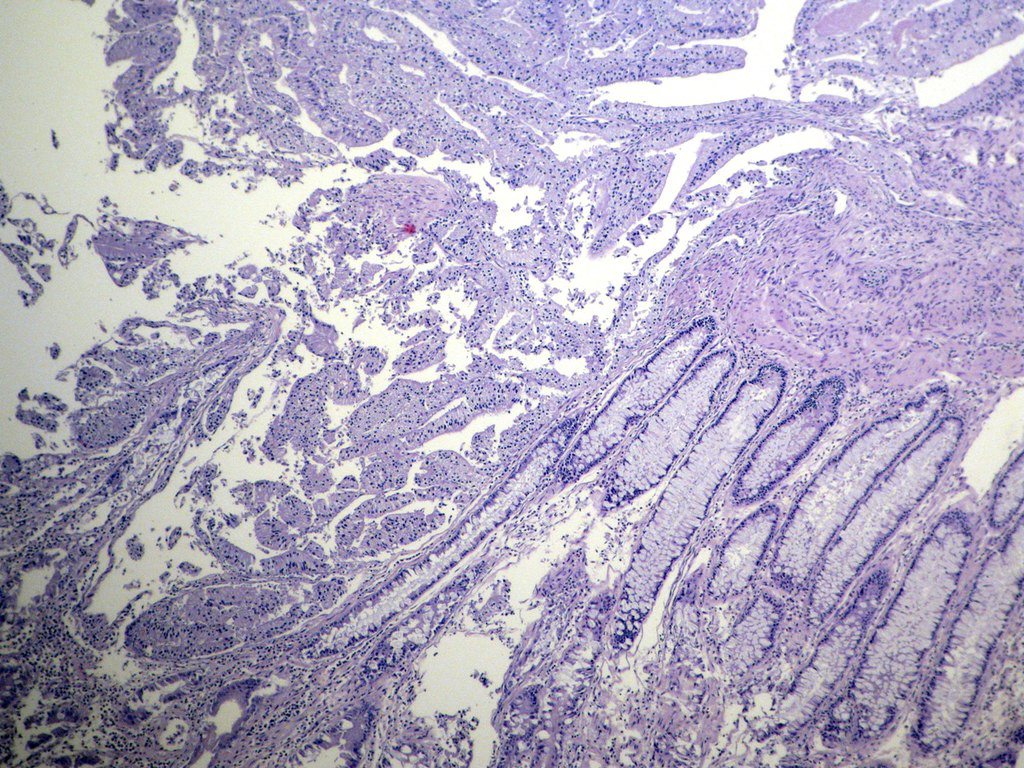
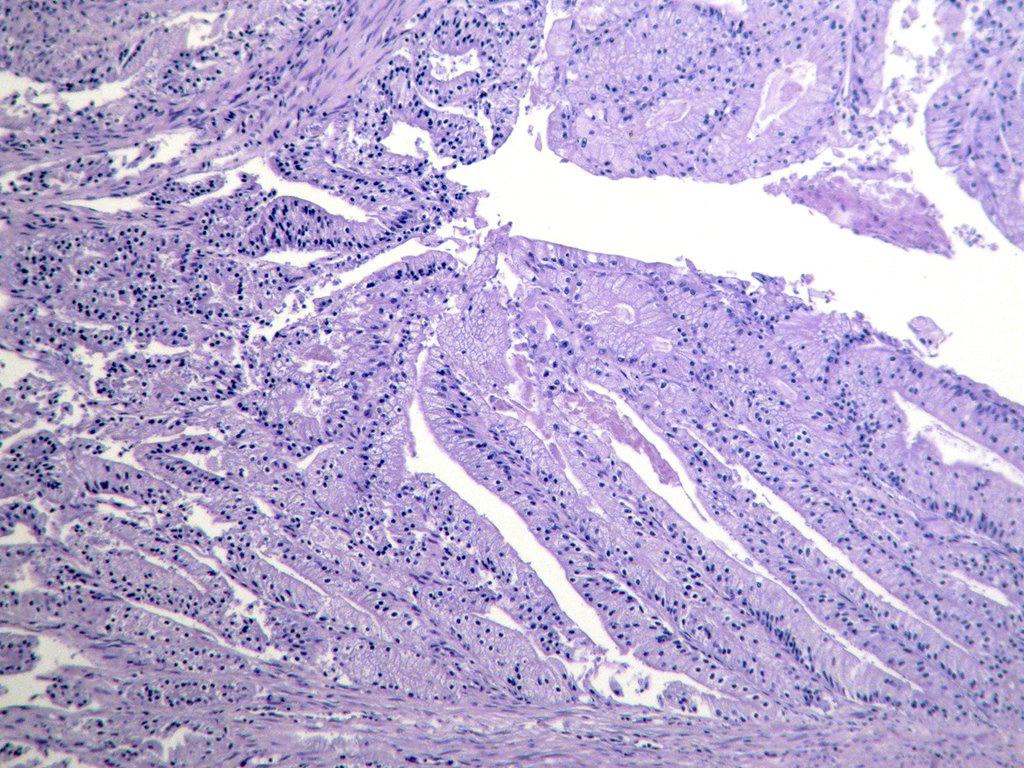
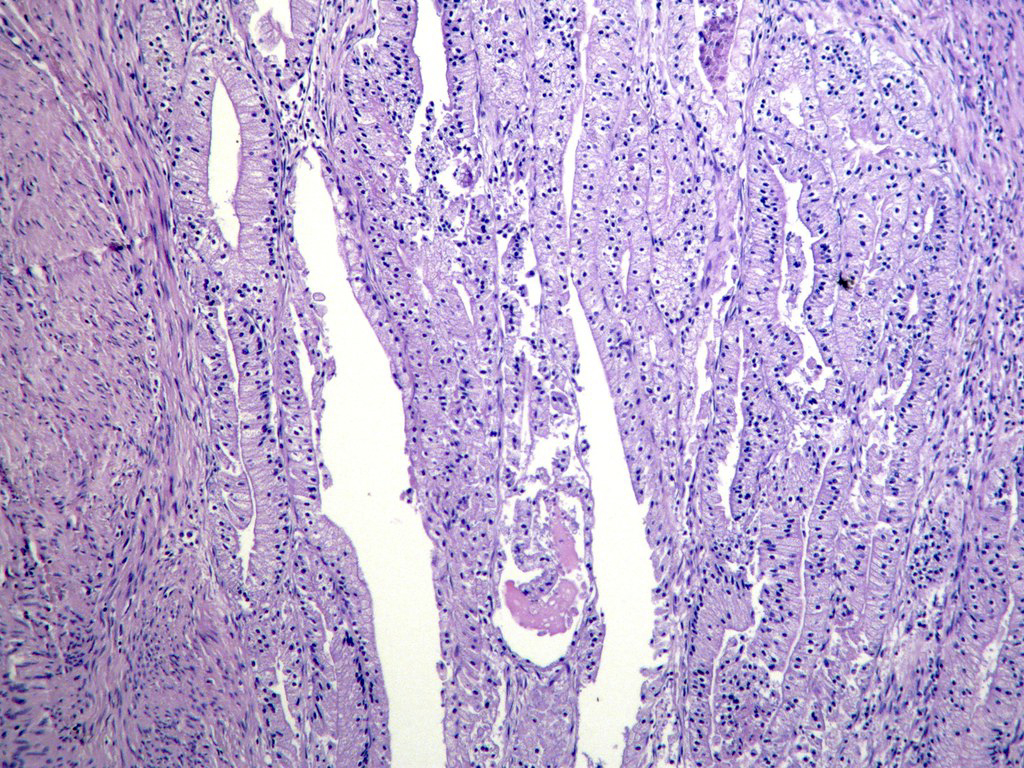
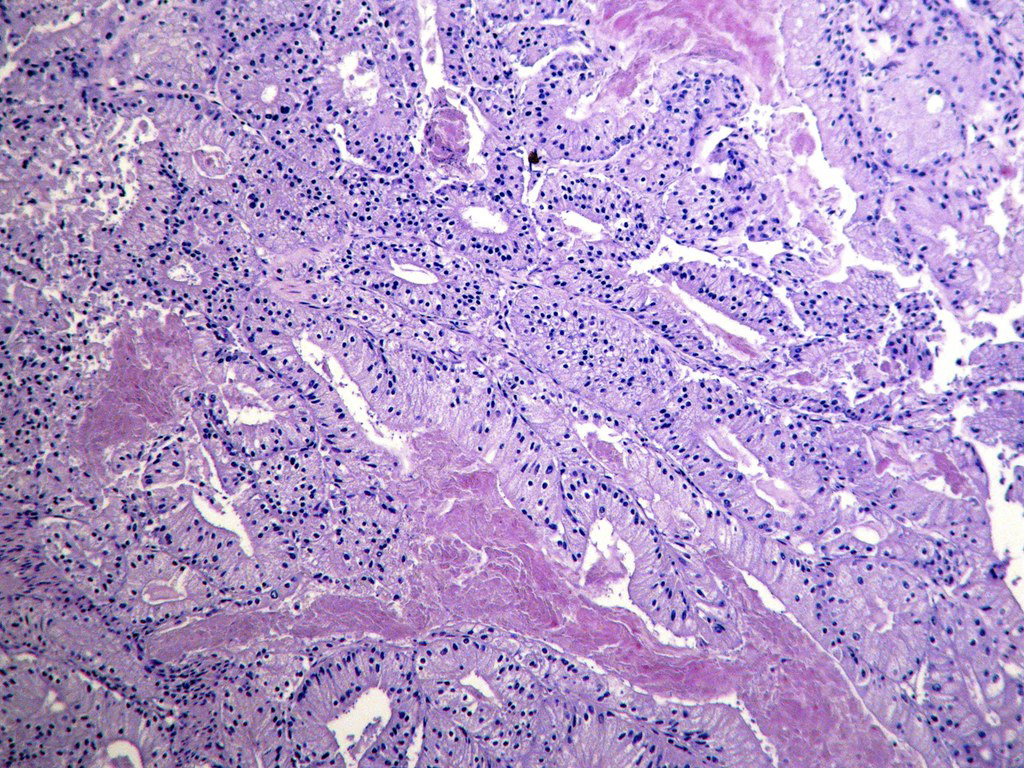
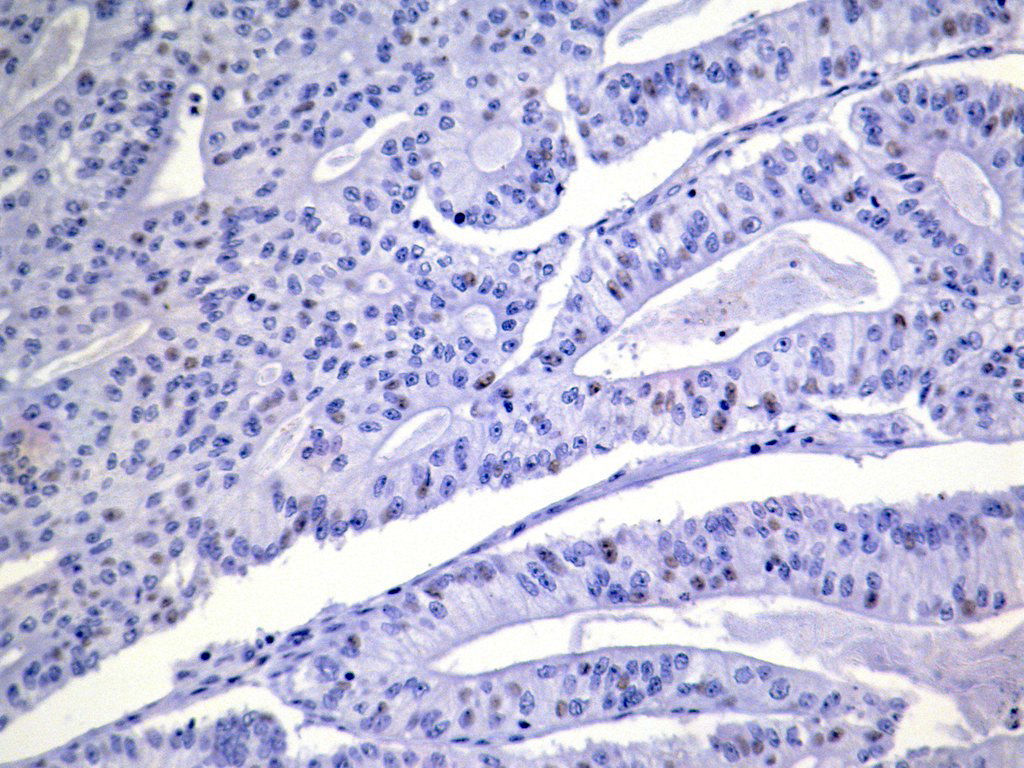
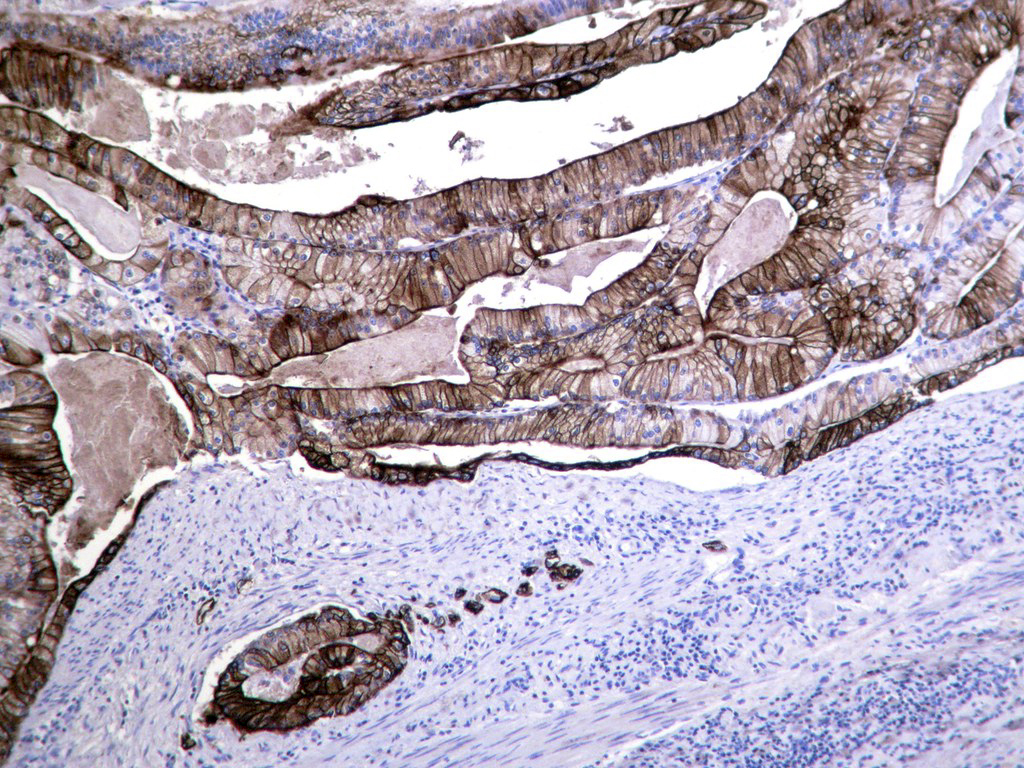
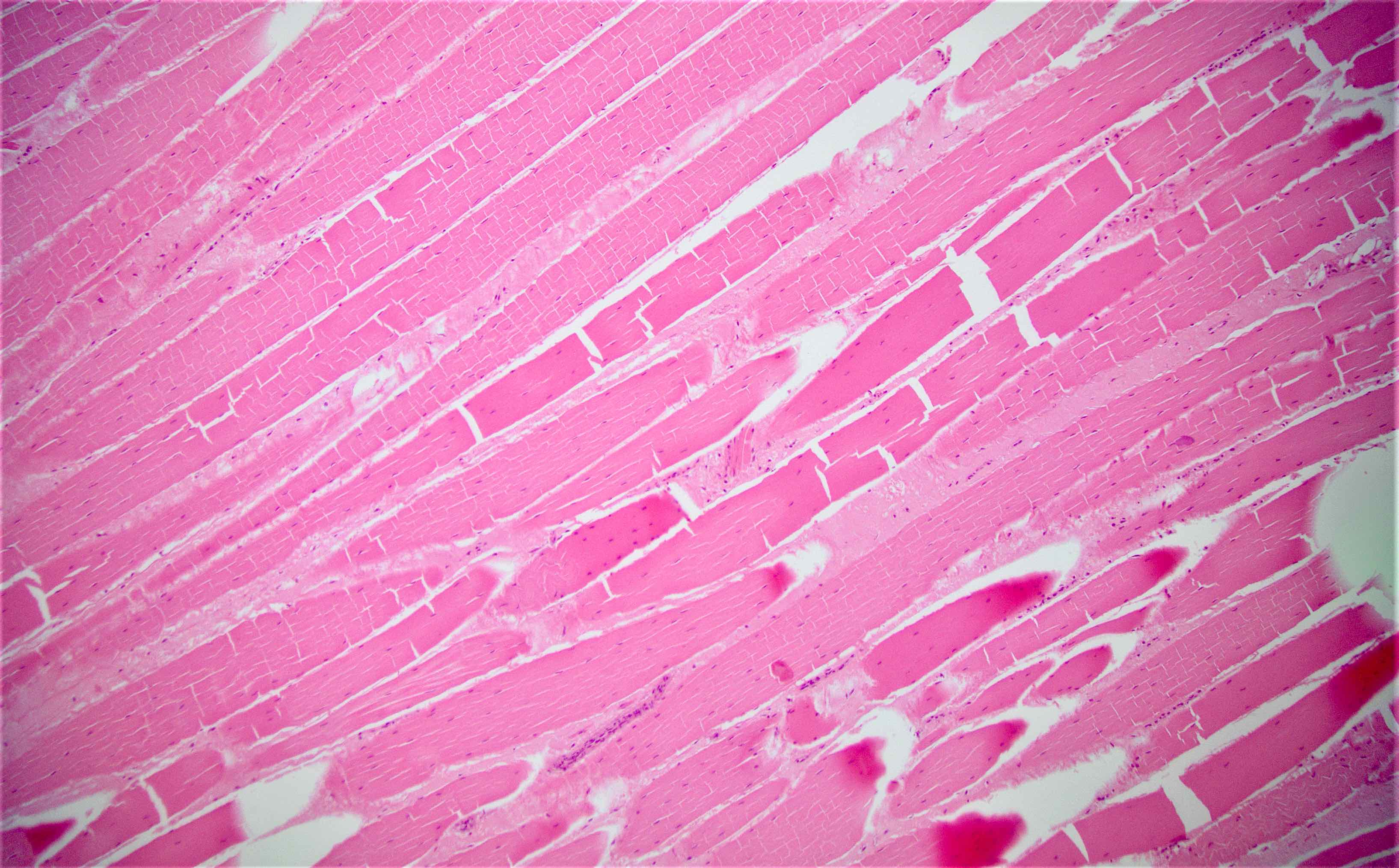
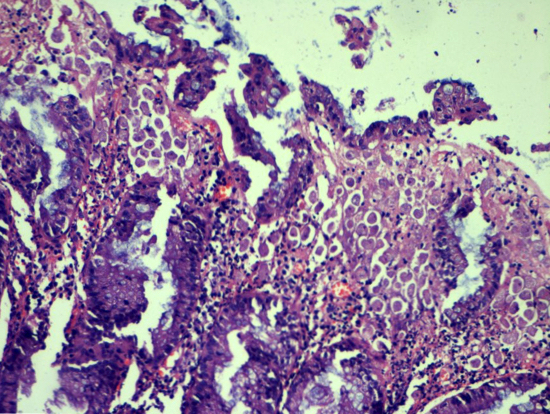
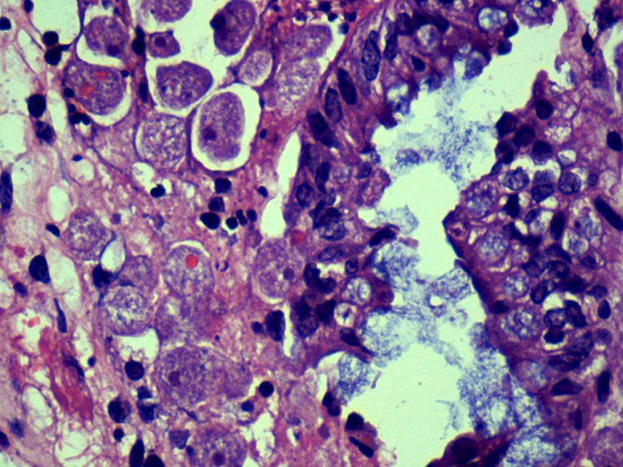
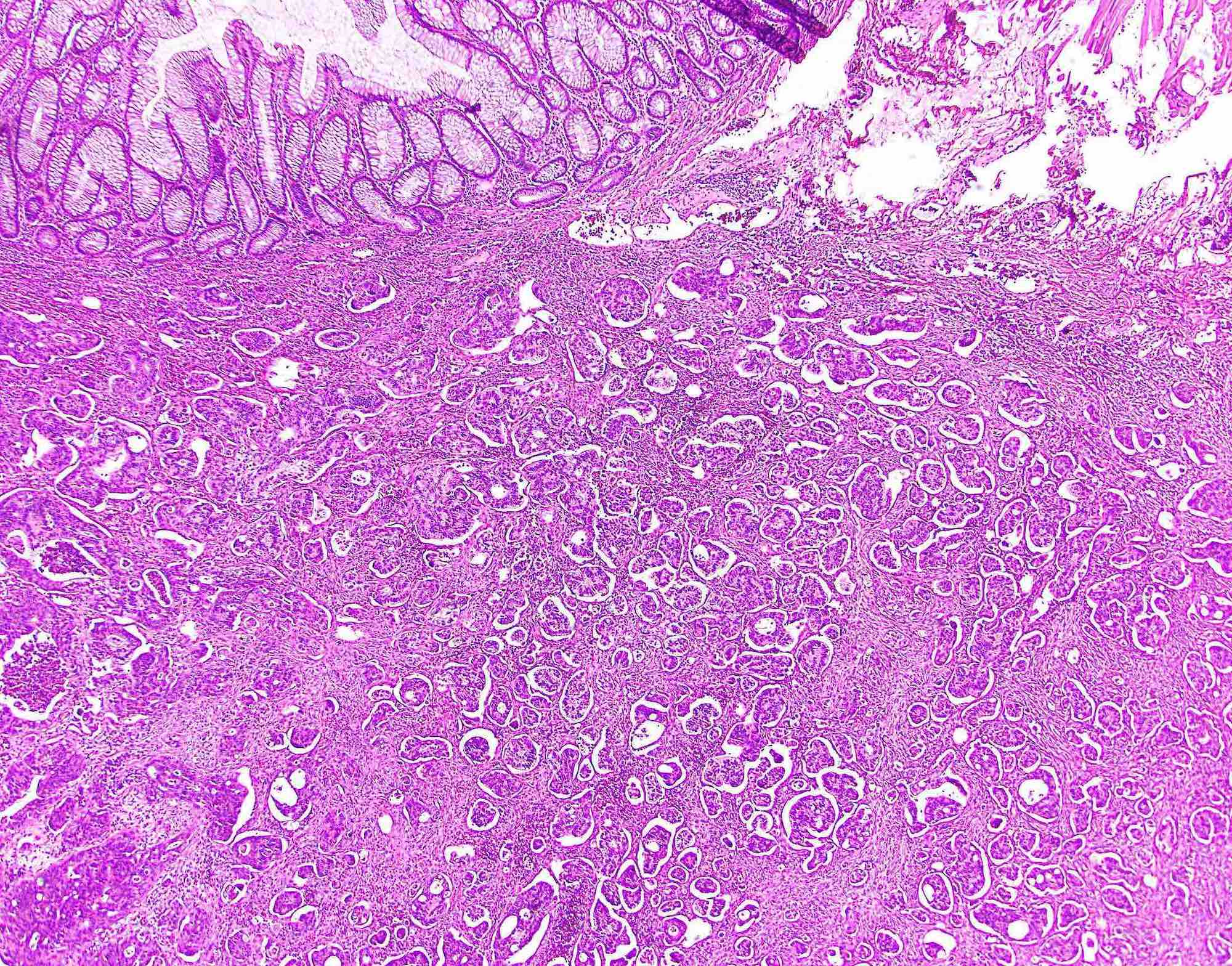
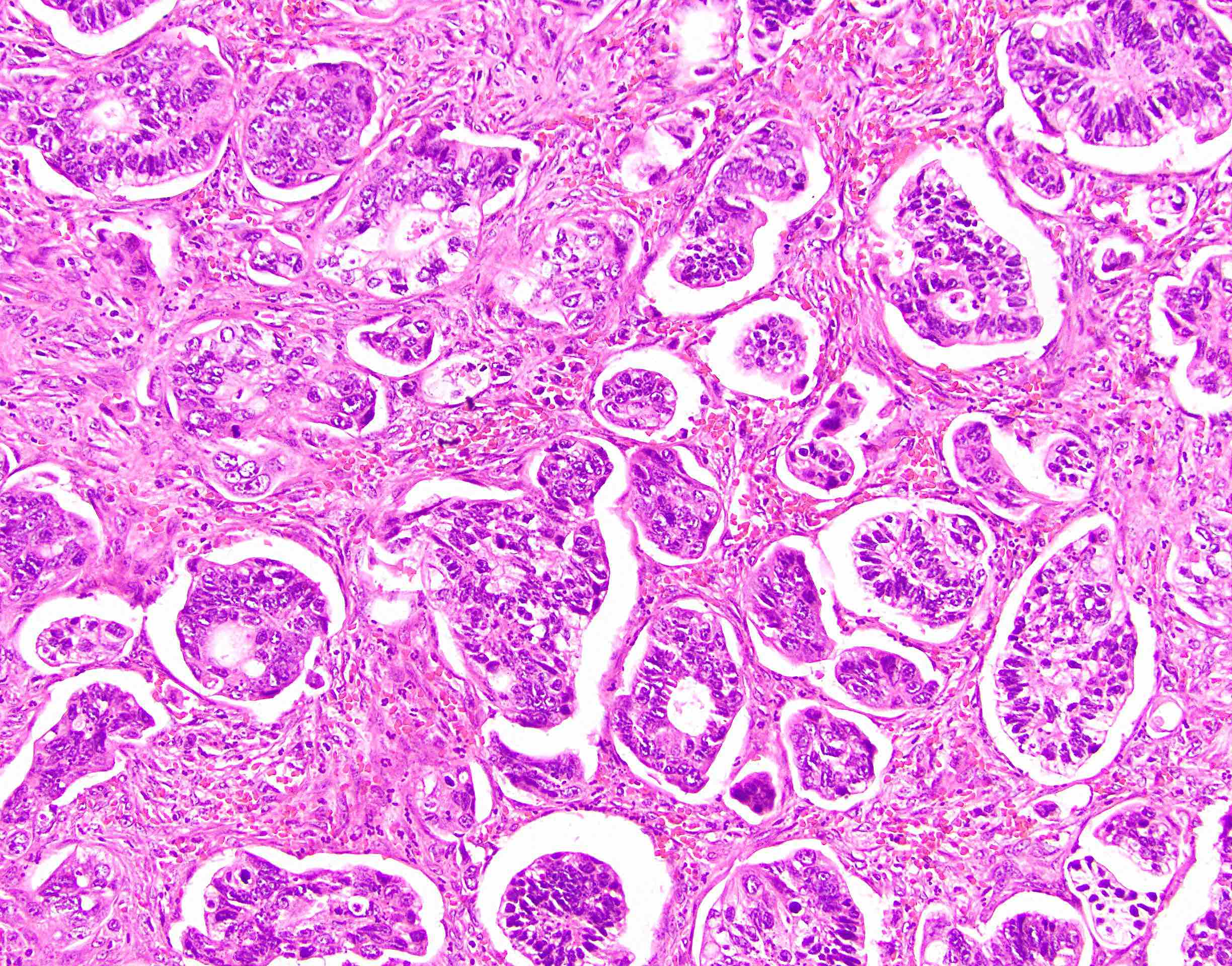
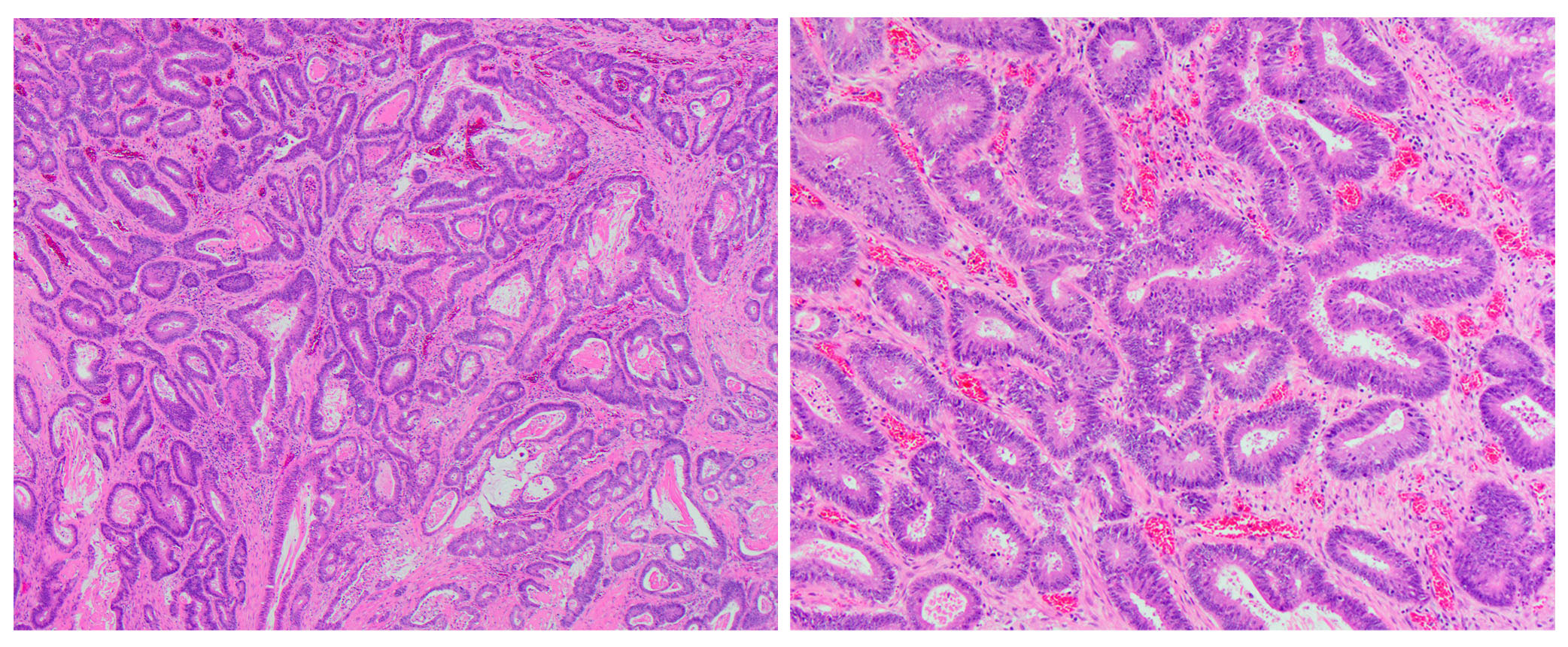
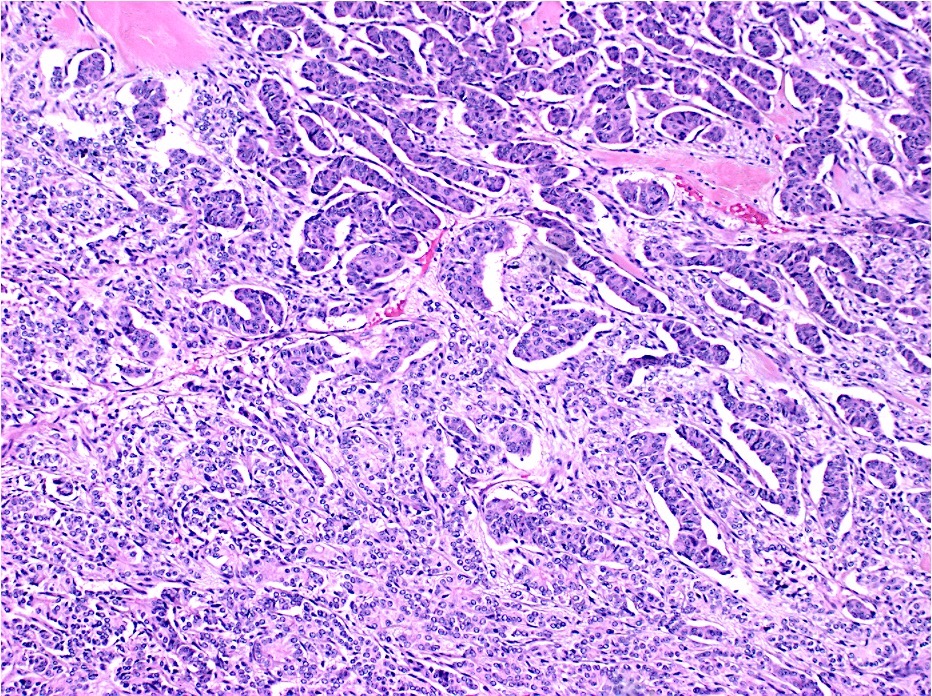
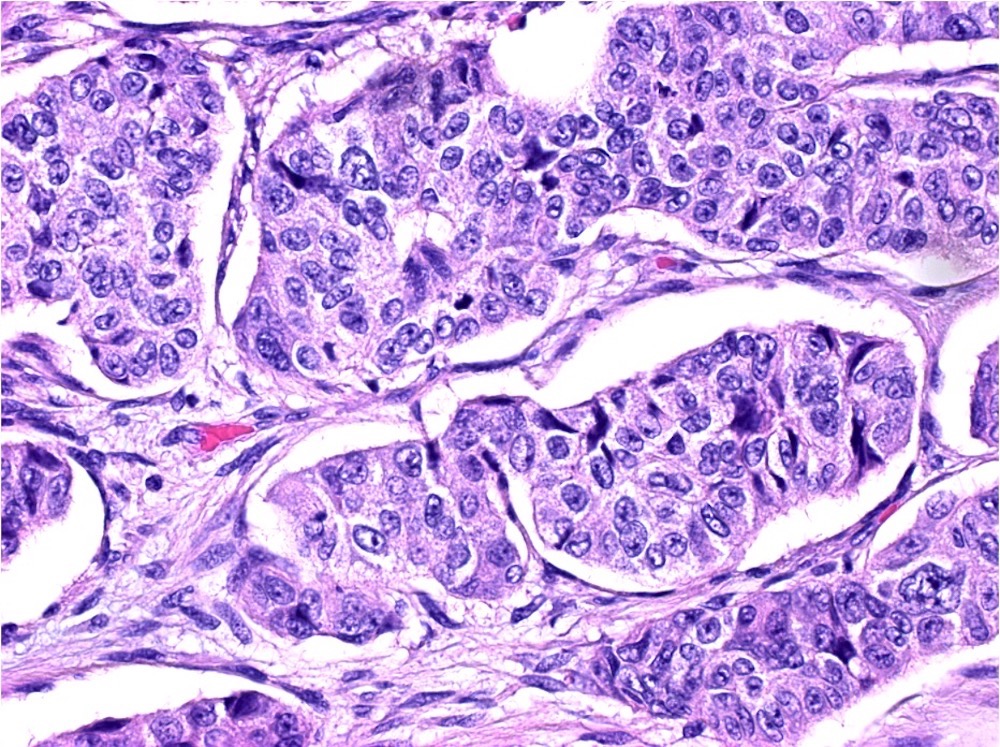
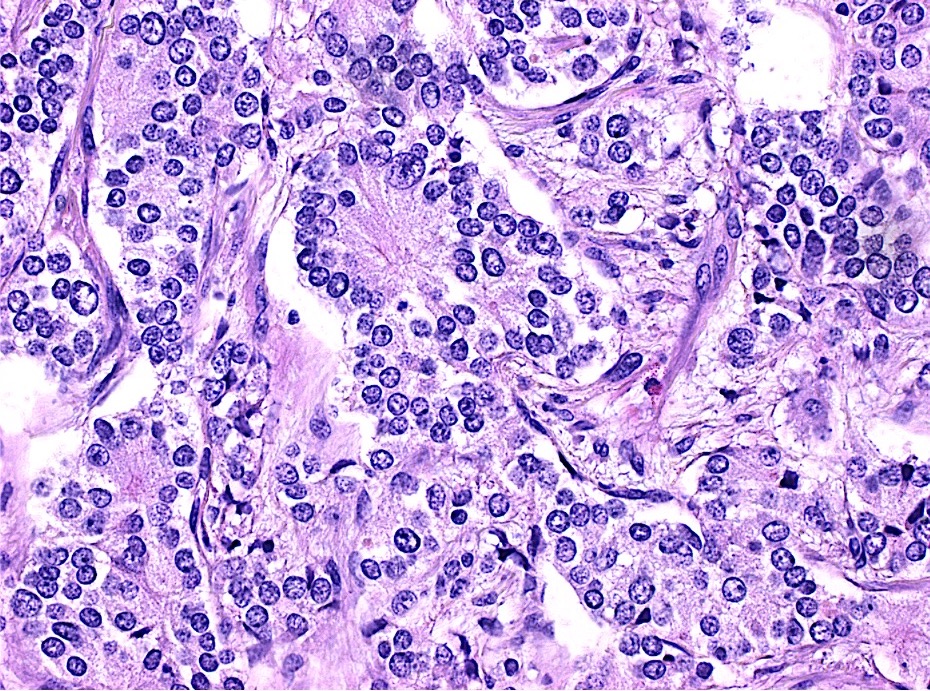
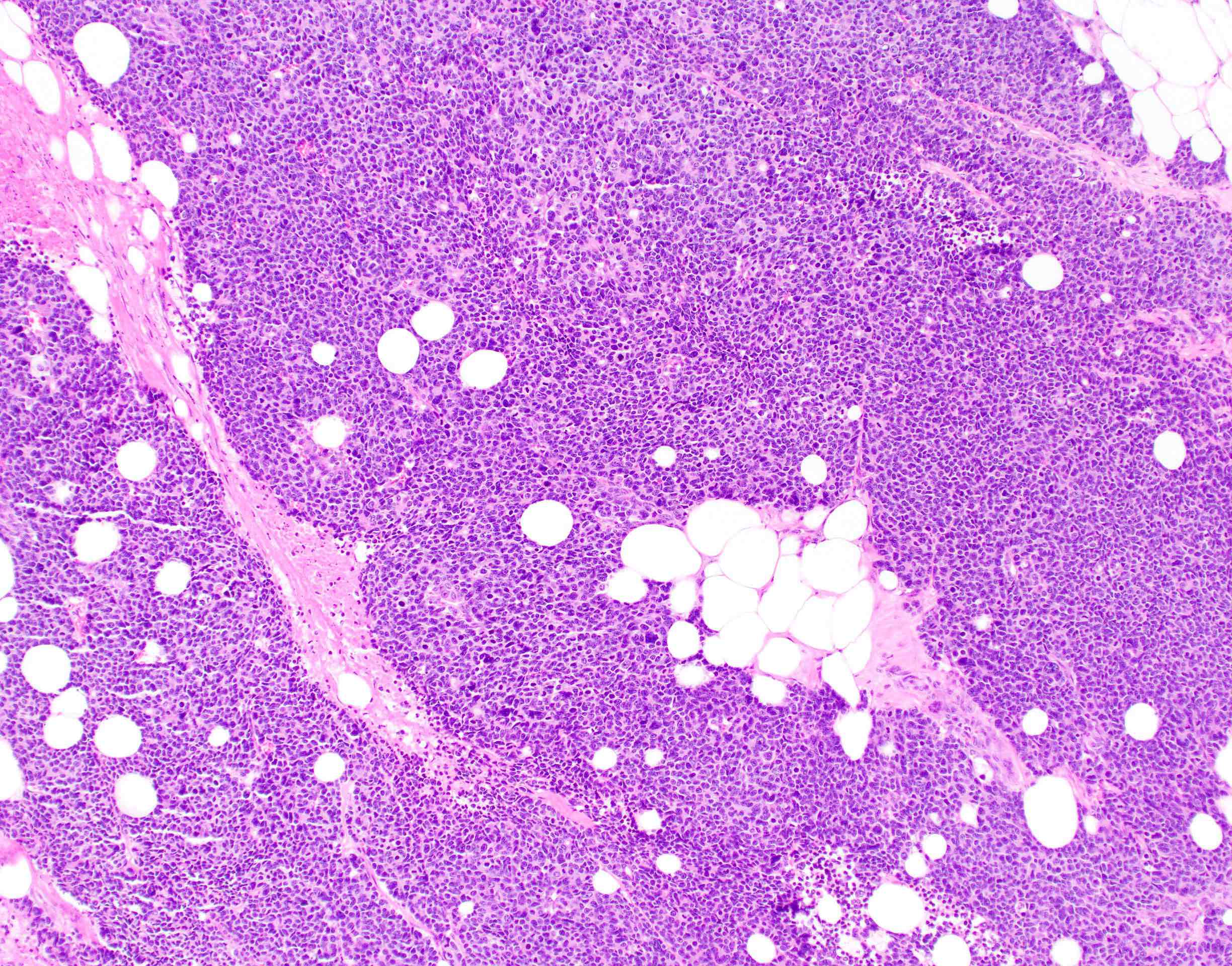
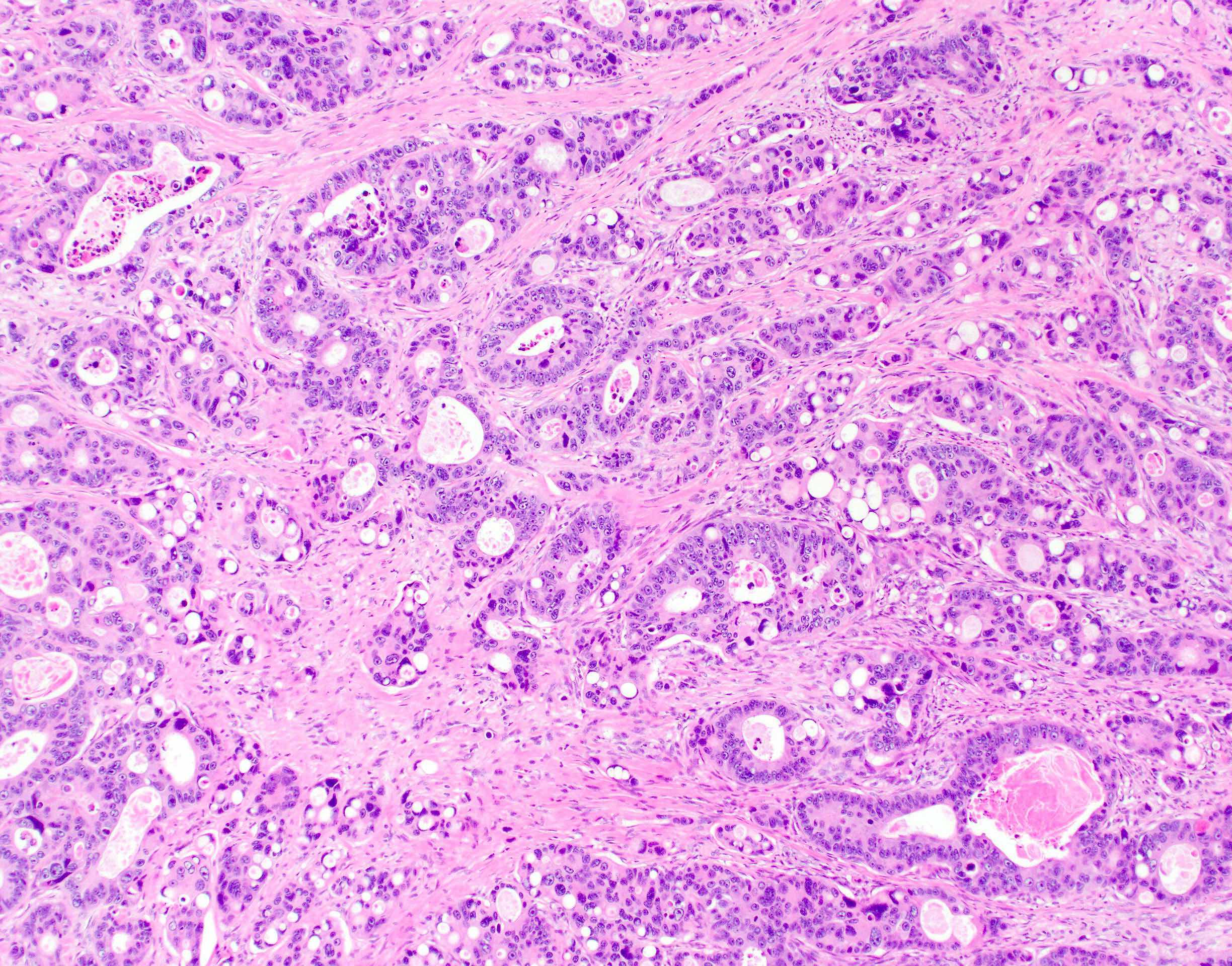
Microscopic (histologic) description
- Usually well or moderately differentiated gland forming carcinoma with marked desmoplasia, particularly at edge of tumor
- Glands often cribriform and filled with necrotic debris (dirty necrosis), in both primary and metastatic sites
- Inflammatory cells and scattered neuroendocrine cells are common (Pol J Pathol 2005;56:89)
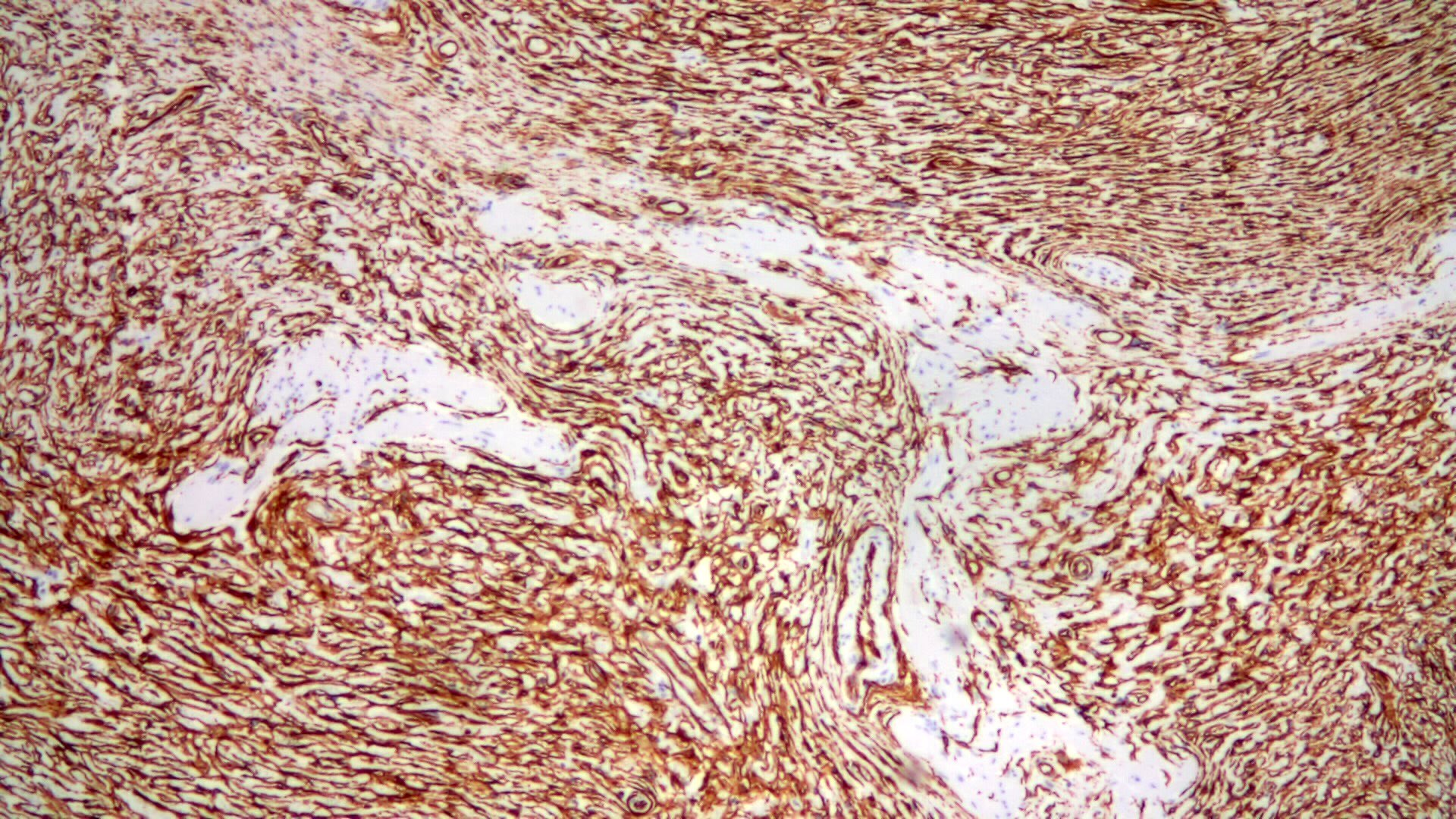
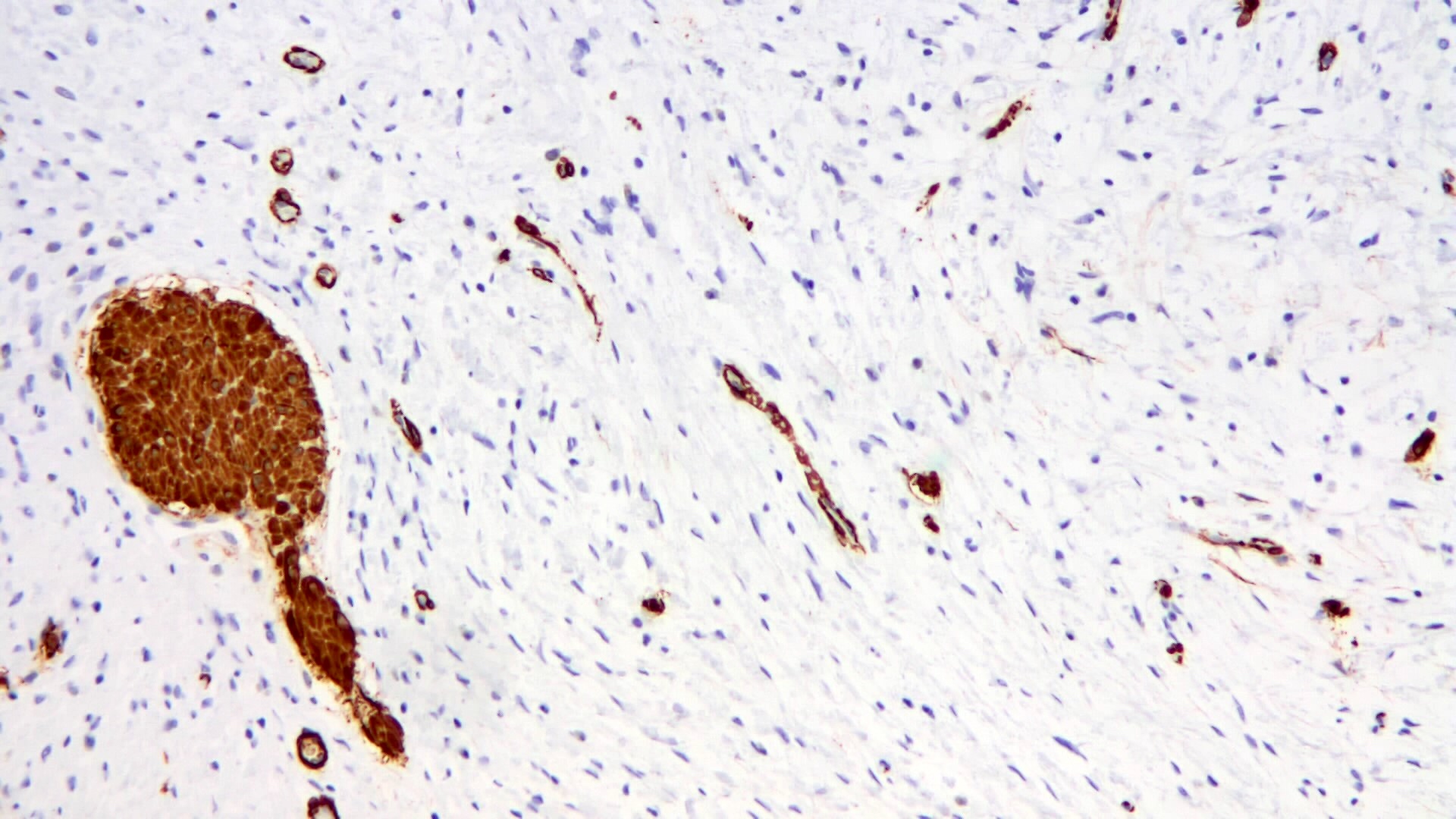
- Intramural venous invasion may be easier to identify using an elastin stain (J Clin Pathol 2002;55:17)
- Well differentiated:
- 15 - 20% of all carcinomas
- Well formed glands or simple tubules with uniform, basally oriented nuclei
- Somewhat resembles adenomatous epithelium
- Moderately differentiated:
- 60 - 70% of all carcinomas
- Tubules may be simple, complex or slightly irregular
- Nuclear polarity lost
- Poorly differentiated:
- 15 - 20% all of carcinomas
- Less than 50% gland formation
- Majority of tumor (excluding advancing edge) consists of sheets of cells without gland formation
- Usually right sided (Hepatogastroenterology 2004;51:1698)
- Note: preoperative histologic grading is not accurate (J Med Assoc Thai 2005;88:1535)
Microscopic (histologic) images
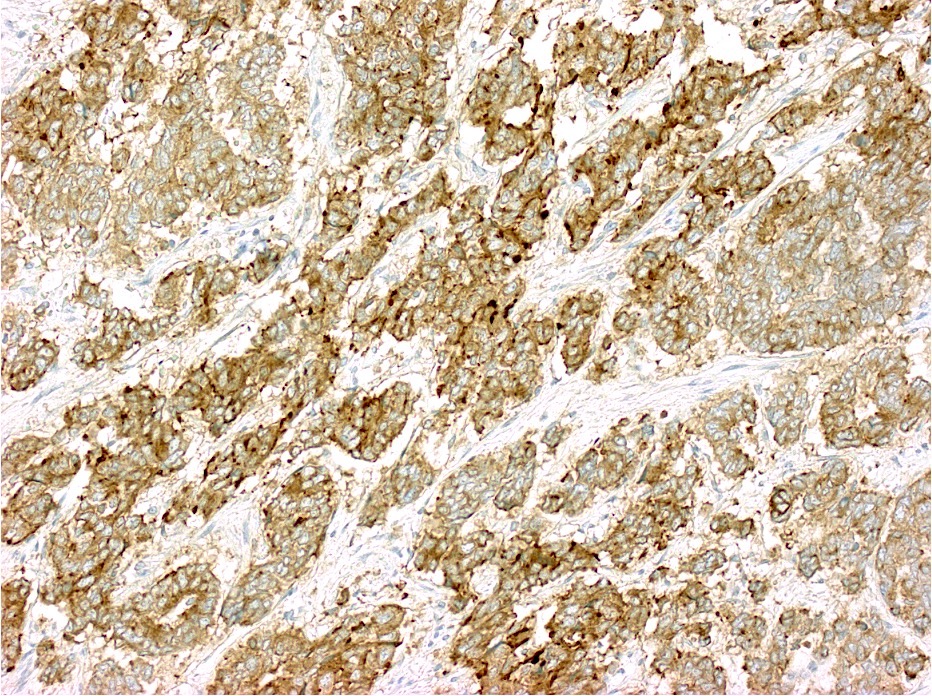
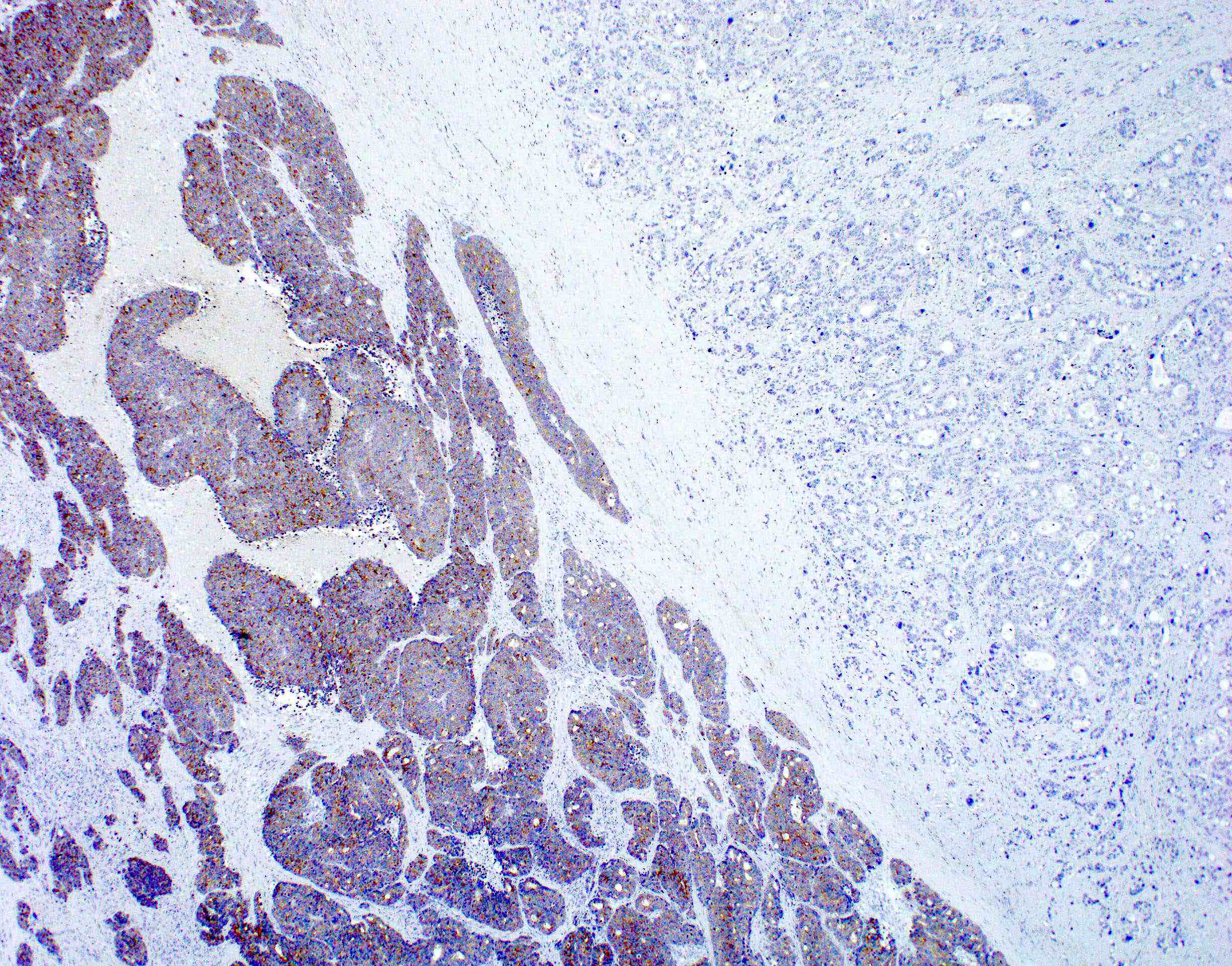
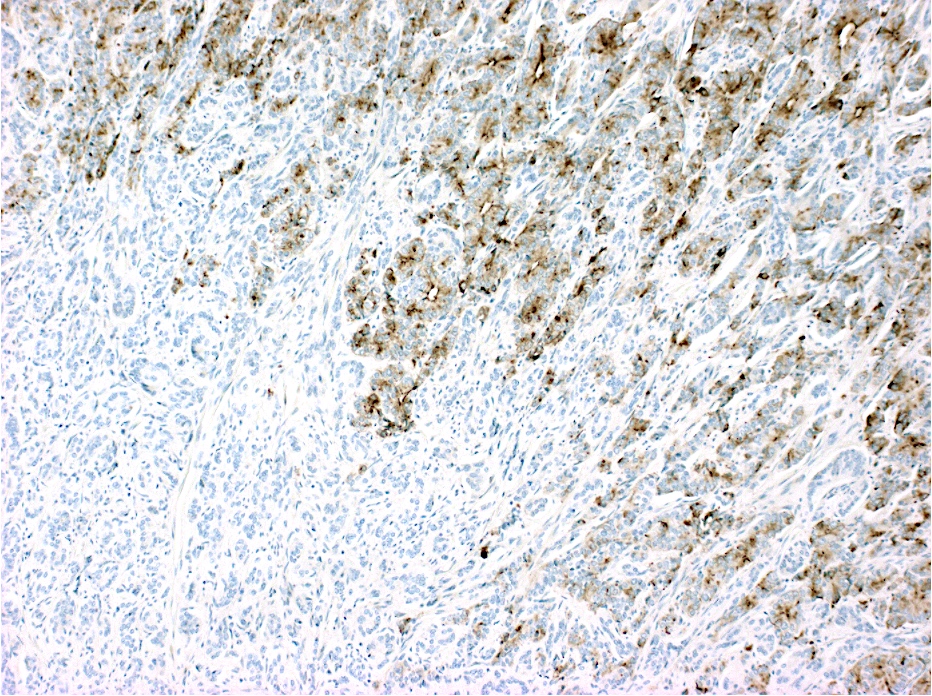
Contributed by Raul S. Gonzalez, M.D.
Contributed by Semir Vranic, M.D., Ph.D. and Beverly Wang, M.D.
Images hosted on other servers:
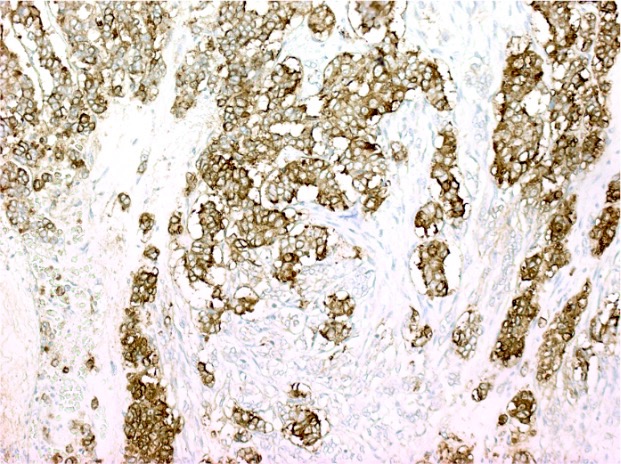
Positive stains
- CK20 (Mod Pathol 2000;13:962)
- CDX2 (superior to villin: Am J Surg Pathol 2003;27:303)
- Also: AMACR (Am J Surg Pathol 2002;26:926)
- Sometimes estrogen receptor (Hum Pathol 2001;32:940)
- CD10 in stromal cells (Hum Pathol 2002;33:806)
- SATB2
Negative stains
- CK7, except in rectal adenocarcinomas (Appl Immunohistochem Mol Morphol 2009;17:196)
Molecular / cytogenetics description
- Most commonly mutated genes include APC, TP53 and KRAS
- Molecular classification of carcinomas has been proposed (Histopathology 2007;50:113)
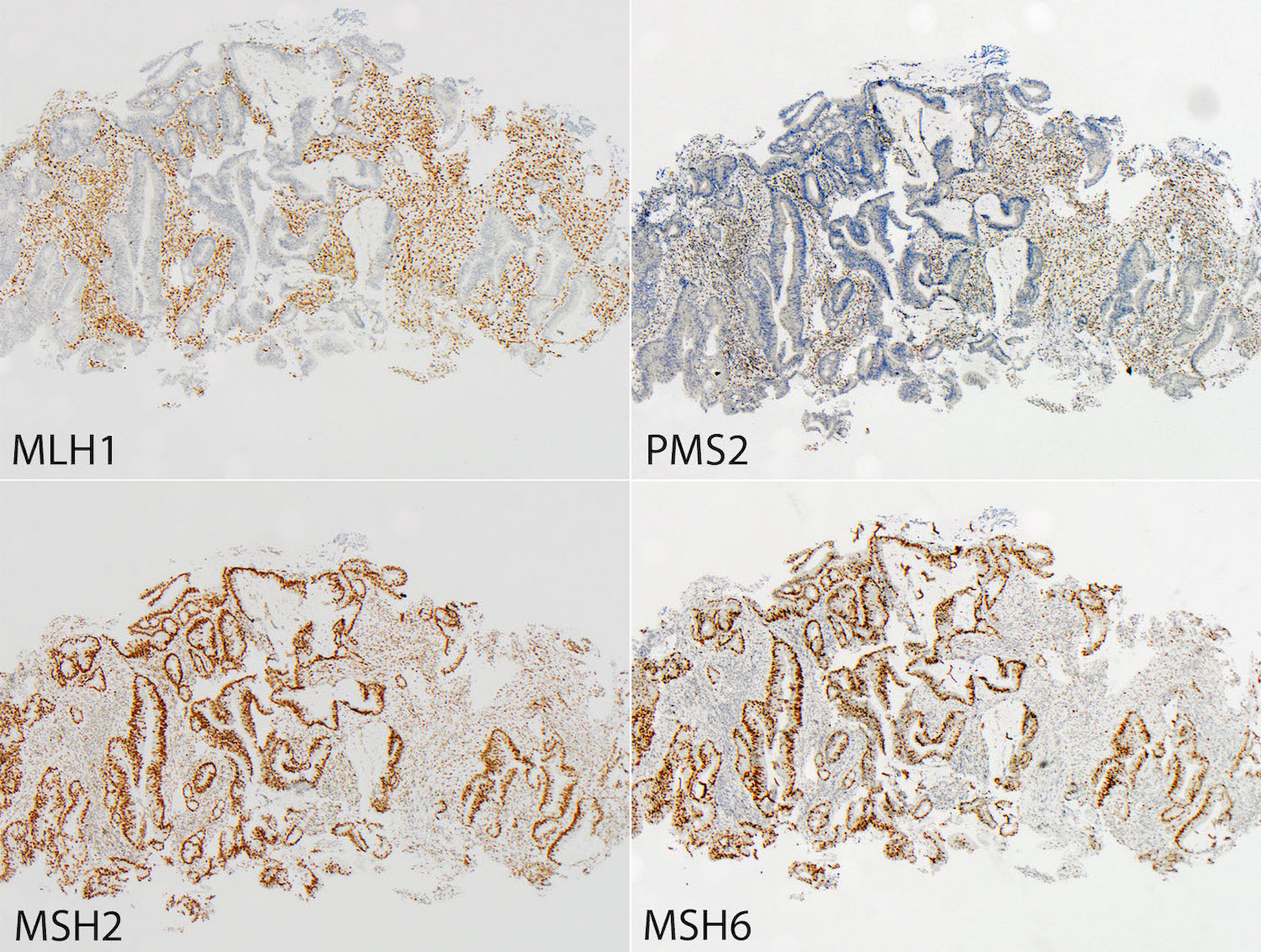
- Tumors can be screened for microsatellite instability via immunohistochemistry for MLH1, MSH2, MSH6 and PMS2
Videos
Histopathology colon adenocarcinoma
Sample pathology report
- Sigmoid colon, resection:
- Adenocarcinoma, moderately differentiated (see synoptic report)
Differential diagnosis
Additional references
Board review style question #1
- Which of the following is an official WHO recognized subtype of colorectal adenocarcinoma (per the 2019 classification)?
- Adenosquamous carcinoma
- Clear cell carcinoma
- Cribriform comedo carcinoma
- Low grade tubuloglandular adenocarcinoma
Board review style answer #1
Board review style question #2
- Which of the following is true about colon cancer?
- Commonly mutated genes include APC, TP53 and KRAS
- Most cases are poorly differentiated
- Most cases are positive for CK7 and negative for CK20 and CDX2
- Superficial / early tumors metastasize often
Board review style answer #2
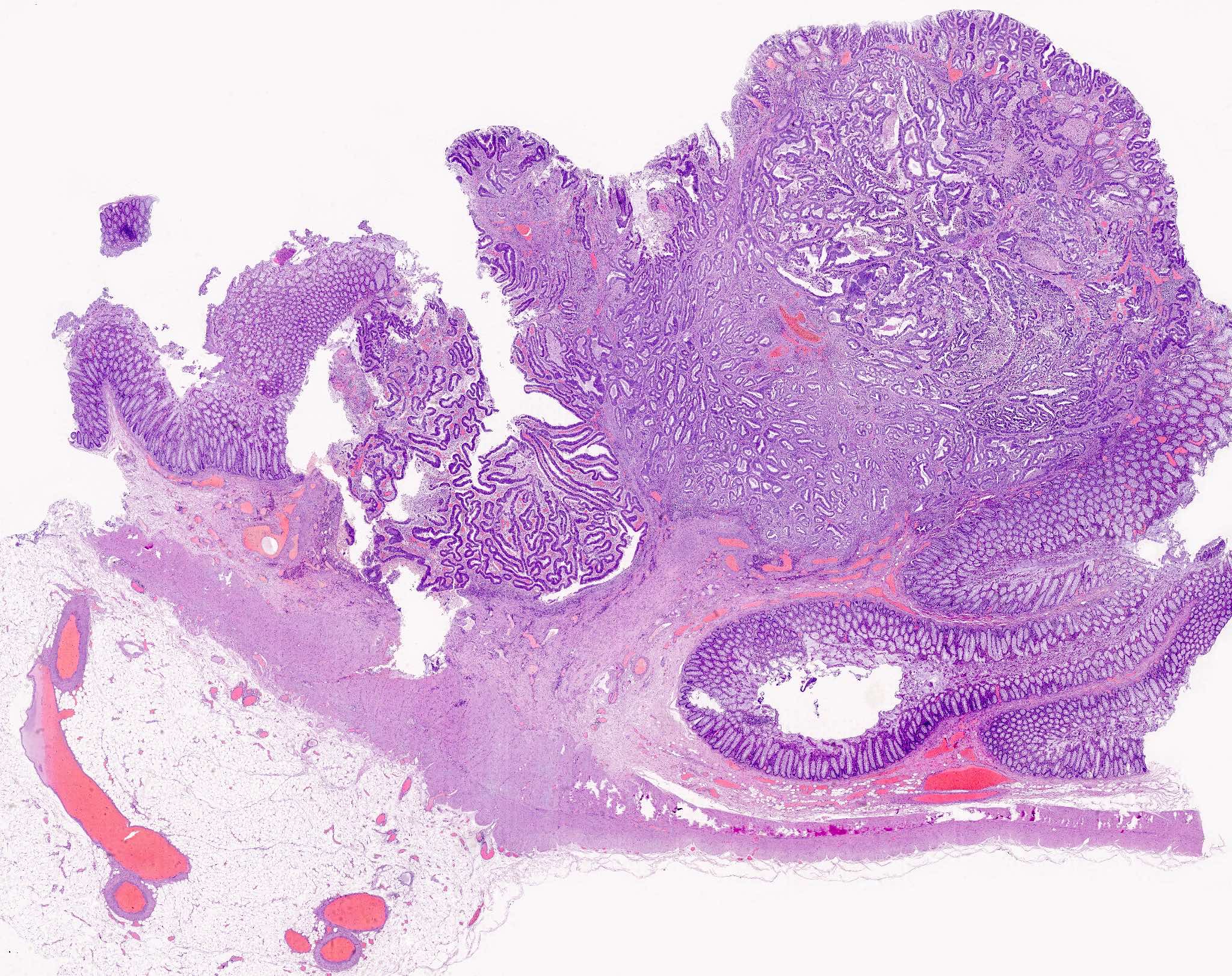
Adenoma overview
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sometimes referred to as conventional adenoma to be distinguished from serrated lesions
- Serrated lesions are of different pathway and are not included in this topic
- Benign, premalignant neoplasm composed of dysplastic colorectal epithelium
Essential features
- Premalignant lesions
- At least low grade dysplasia; absence of true invasion
- Subtypes include tubular adenoma (most common), villous adenoma, tubulovillous adenoma and advanced adenoma
Terminology
- Conventional colorectal adenoma
ICD coding
- ICD-O:
- ICD-11: 2E92.4Y & XH7SY6 - other specified benign neoplasm of the large intestine & tubular adenoma, NOS
Epidemiology
- High incidence in populations with diets typical of high income countries with a sedentary lifestyle
- High risk population overlap with those of colorectal adenocarcinoma (see Etiology)
Sites
- Colon
Pathophysiology
- Adenoma - carcinoma sequence: genetic changes that occur before morphologically identifiable tumor formation, including a small set of driver genes (APC, CNNTB1, KRAS, SMAD4 and TP53) (Proc Natl Acad Sci U S A 2013;110:1999)
- APC genetic alteration results in reduced degradation of beta catenin and dysregulated WNT signaling (Science 1997;278:120)
- Inherited (constitutional) APC alterations lead to familial adenomatous polyposis
- Activating KRAS mutations leads to growth dysregulation through MAPK pathway (Br J Cancer 1997;75:341)
- SMAD deletion leads to disruption of TGFb growth inhibitory pathway
- Alteration of PTEN or activation of PIK3CA disrupts PI3K pathway, inhibits apoptosis and promotes neoplastic cell survival (Nat Commun 2016;7:11971, Nat Commun 2014;5:4961)
- Alteration of TP53 allows the cells to survive DNA damage and other cellular stresses (Int J Cancer 1995;64:47)
- APC genetic alteration results in reduced degradation of beta catenin and dysregulated WNT signaling (Science 1997;278:120)
- Small subset of adenoma acquires defect in DNA mismatch repair genes (predominantly hypermethylation of the MLH1 promoter) (Gastroenterol Hepatol Bed Bench 2017;10:S117)
Etiology
- Increased risk associated with consumption of processed and red meat, alcohol, excess body fat (Lancet Oncol 2015;16:1599, Ann Oncol 2017;28:1788, N Engl J Med 2016;375:794)
- Decreased risk associated with consumption of dietary fiber and dairy products, increased levels of physical activity (Ann Oncol 2017;28:1788, BMJ 2016;354:i3857)
Clinical features
- See Diagnosis
Diagnosis
Prognostic factors
- Most adenomas do not progress through the adenoma carcinoma sequence
- Risk is associated with:
- Higher number of lesions
- Larger size
- Higher proportion of villous architecture
- Extent of high grade dysplasia
Case reports
- 61 year old man with rectal bleeding for 1 week (Medicine (Baltimore) 2020;99:e20985)
- 66 year old woman with homogeneous segmental bowel wall thickening (BJR Case Rep 2020;6:20200016)
- 76 year old man with positive fecal occult blood test (Tokai J Exp Clin Med 2016;41:22)
Treatment
- Major treatment: endoscopic biopsy or resection
- According to NCCN Guidelines for Colorectal Cancer Screening (version 2.2021), if pathology identified:
- Low risk adenoma: < 2 polyps and < 1 cm
- Repeat colonoscopy in 7 - 10 years
- High risk polyp:
- High grade dysplasia present
- Villous / tubulovillous histology
- 3 - 10 adenomatous polyps (serrated lesions are discussed in different section)
- Repeat colonoscopy in 3 years; if negative, repeat colonoscopy in 5 years
- If positive, treat according to the pathology finding
- Large colorectal polyps (> 1 cm in size) without invasion:
- If pedunculated, colonoscopy in 3 years
- Sessile morphology with no high risk endoscopic features for invasive cancer:
- If complete resection and no unfavorable risk factors, colonoscopy in 1 - 3 years
- If incomplete resection, referral for surgery evaluation or expertise in management of large colorectal polyps
- Sessile morphology with high risk endoscopic features, even if no invasive cancer identified by pathology - surgical evaluation or expertise in management of large colorectal polyps
- Low risk adenoma: < 2 polyps and < 1 cm
- Reference: National Comprehensive Cancer Network: NCCN Guidelines - Colorectal Cancer Screening [Accessed 18 October 2021]
Gross description
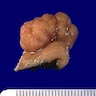
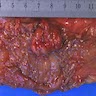
- Specimen is received in formalin, additionally labeled transverse colon polyps and consists of 2 soft, irregular, red-tan tissue fragments (0.5 x 0.3 x 0.2 cm in aggregate)
- Specimen is received in formalin, additionally labeled right colon and consists of multiple tan to white, soft, irregular mucosal tissue fragments (0.8 x 0.2 x 0.2 cm in aggregate)
Gross images
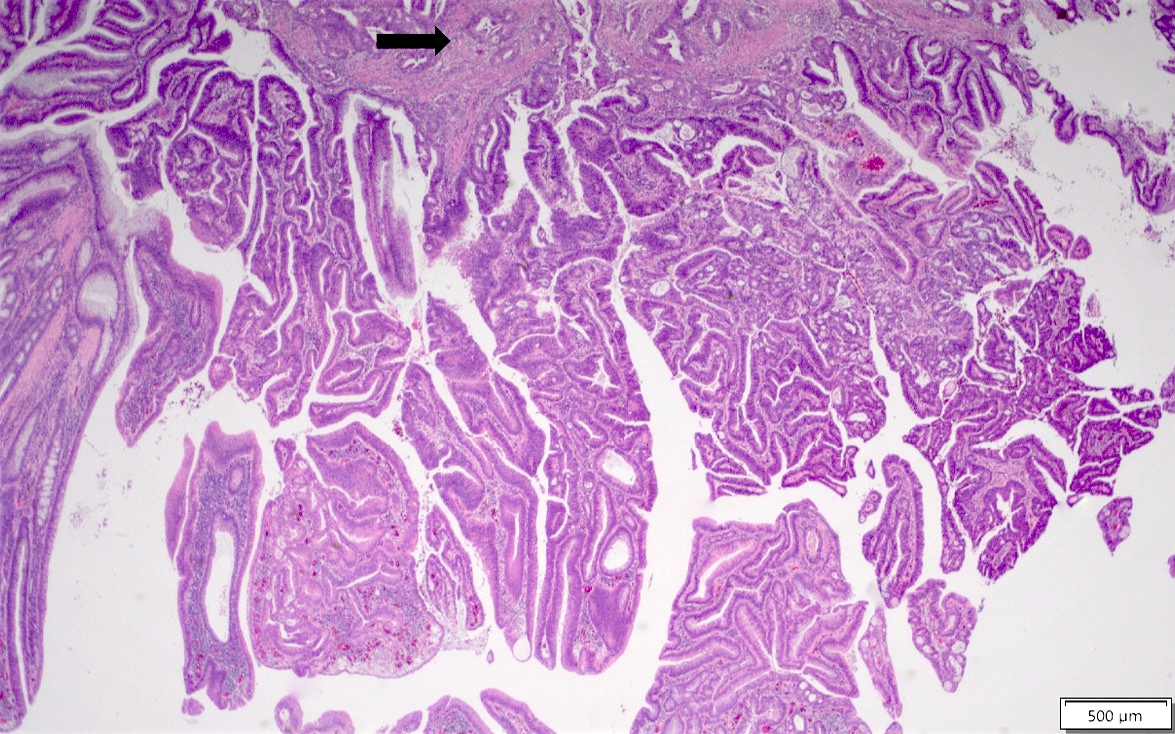
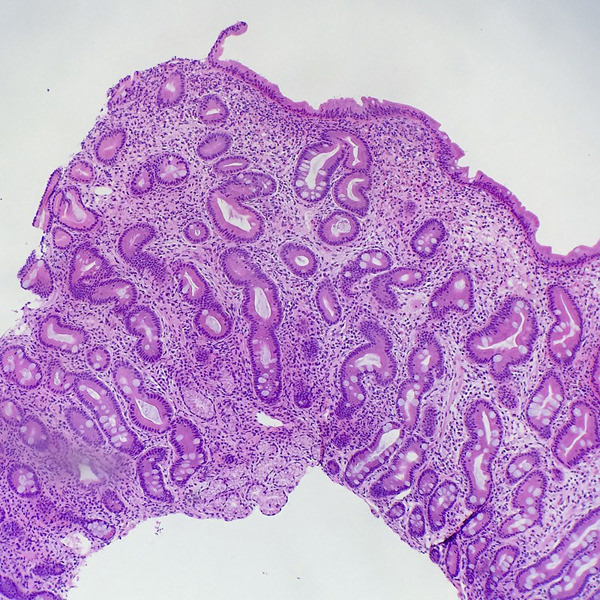
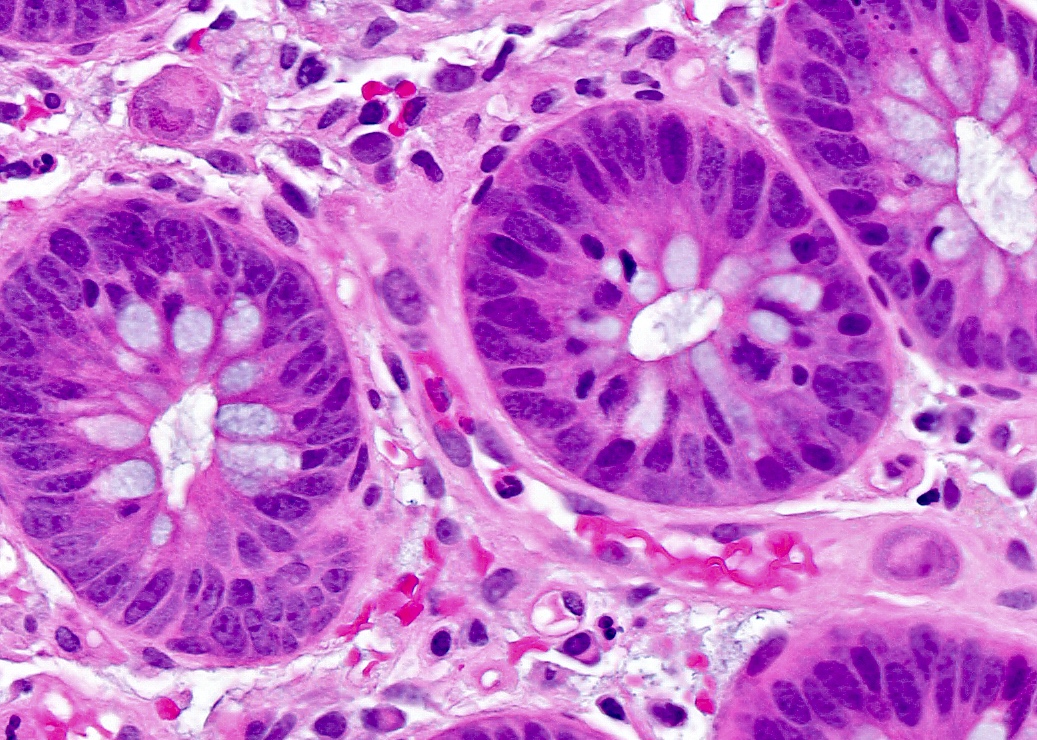
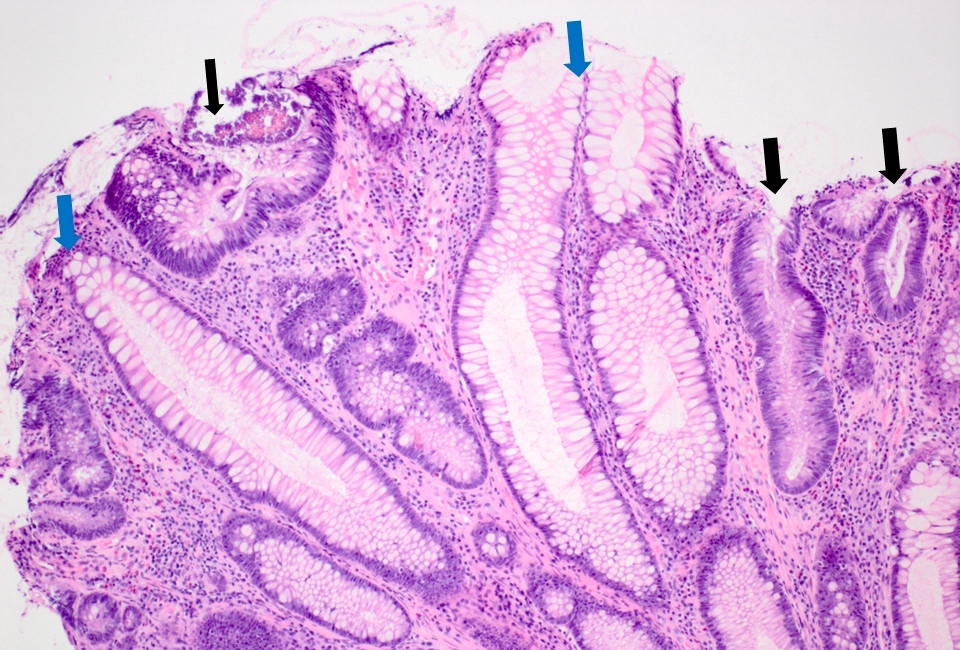
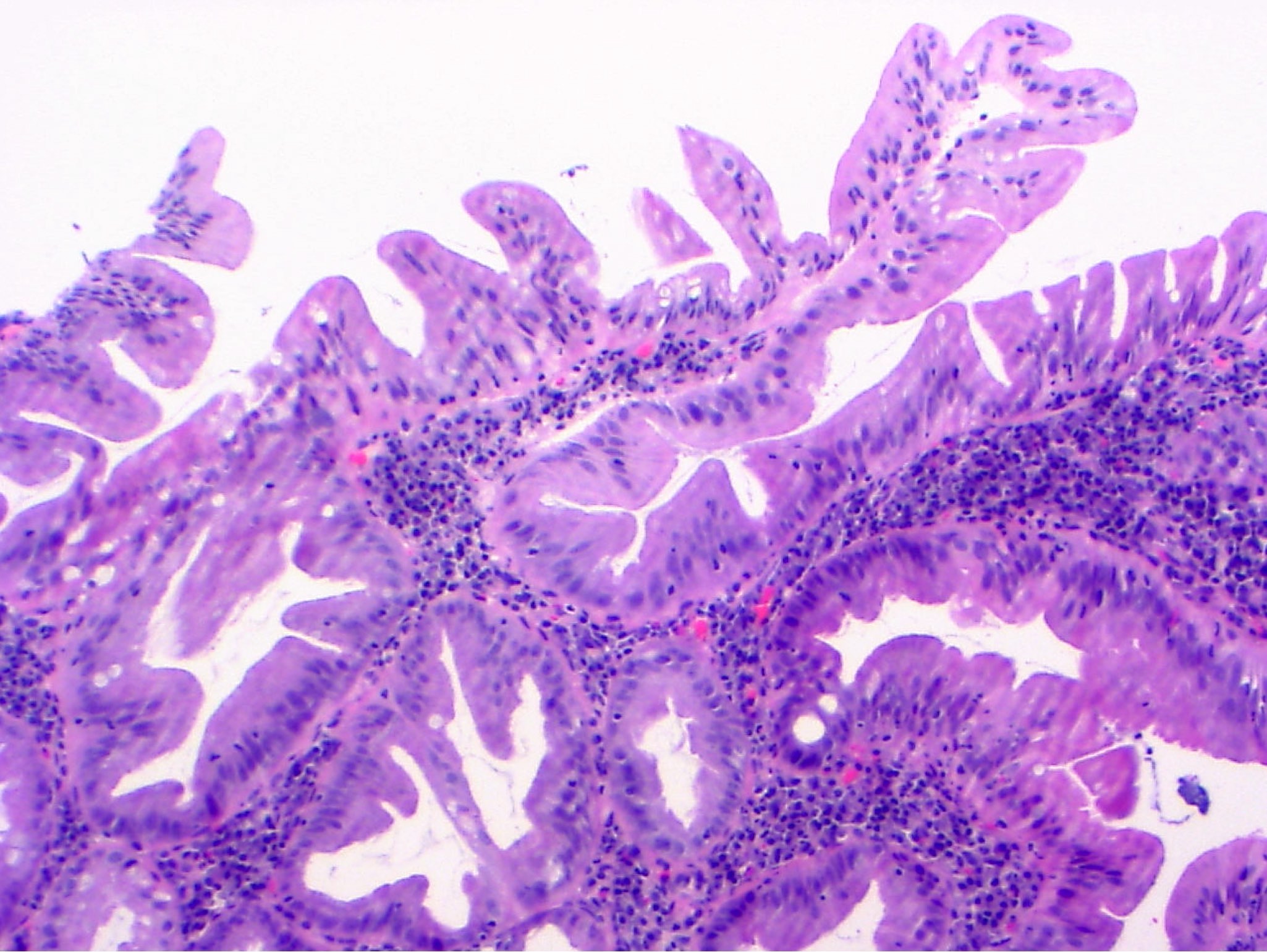
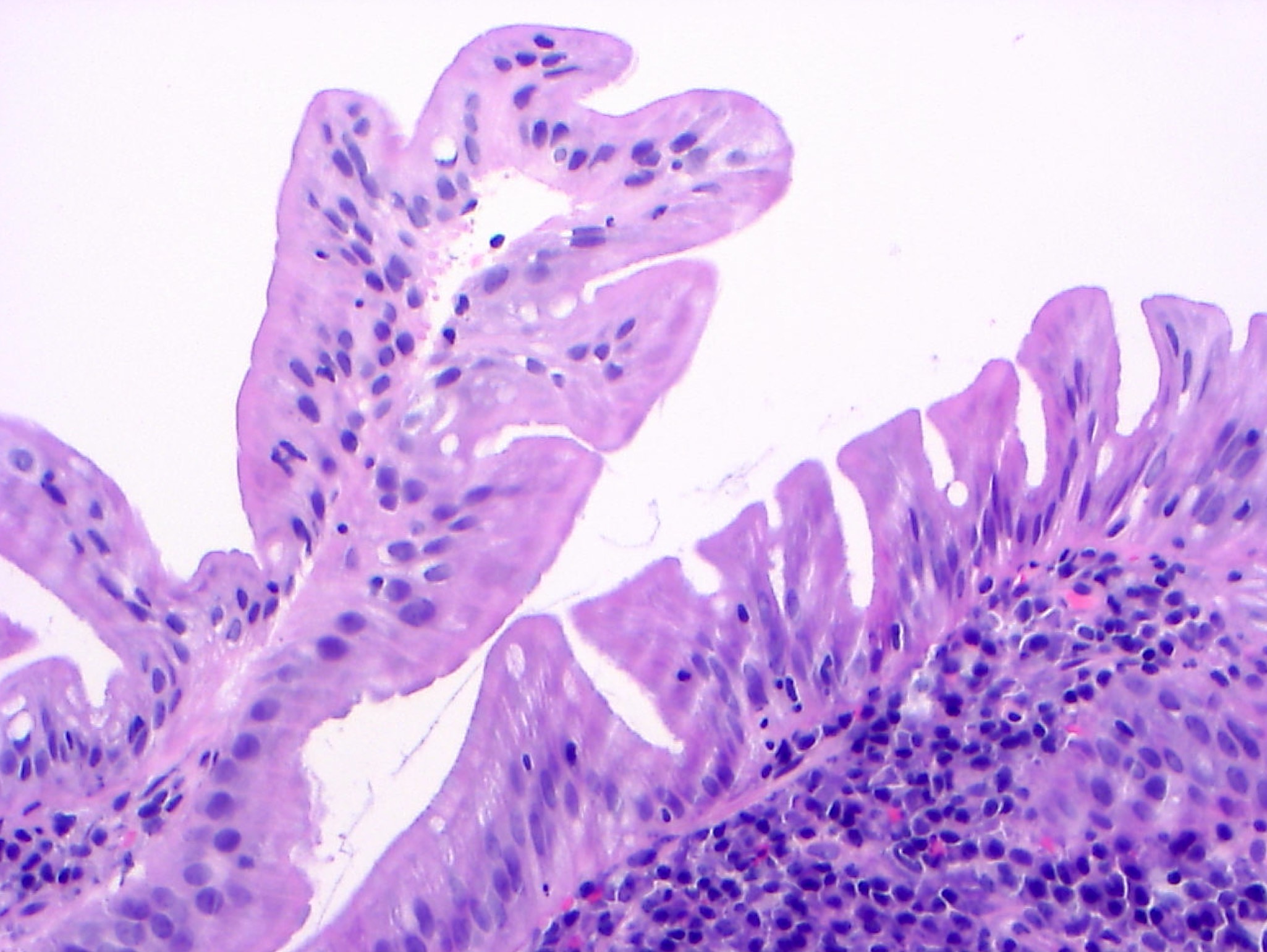
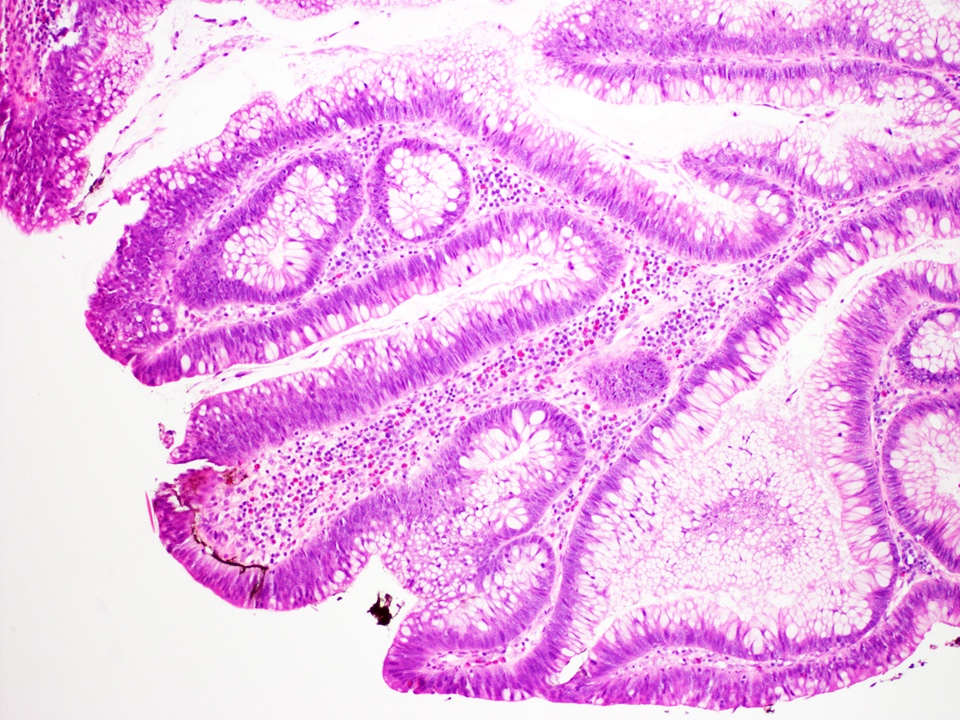
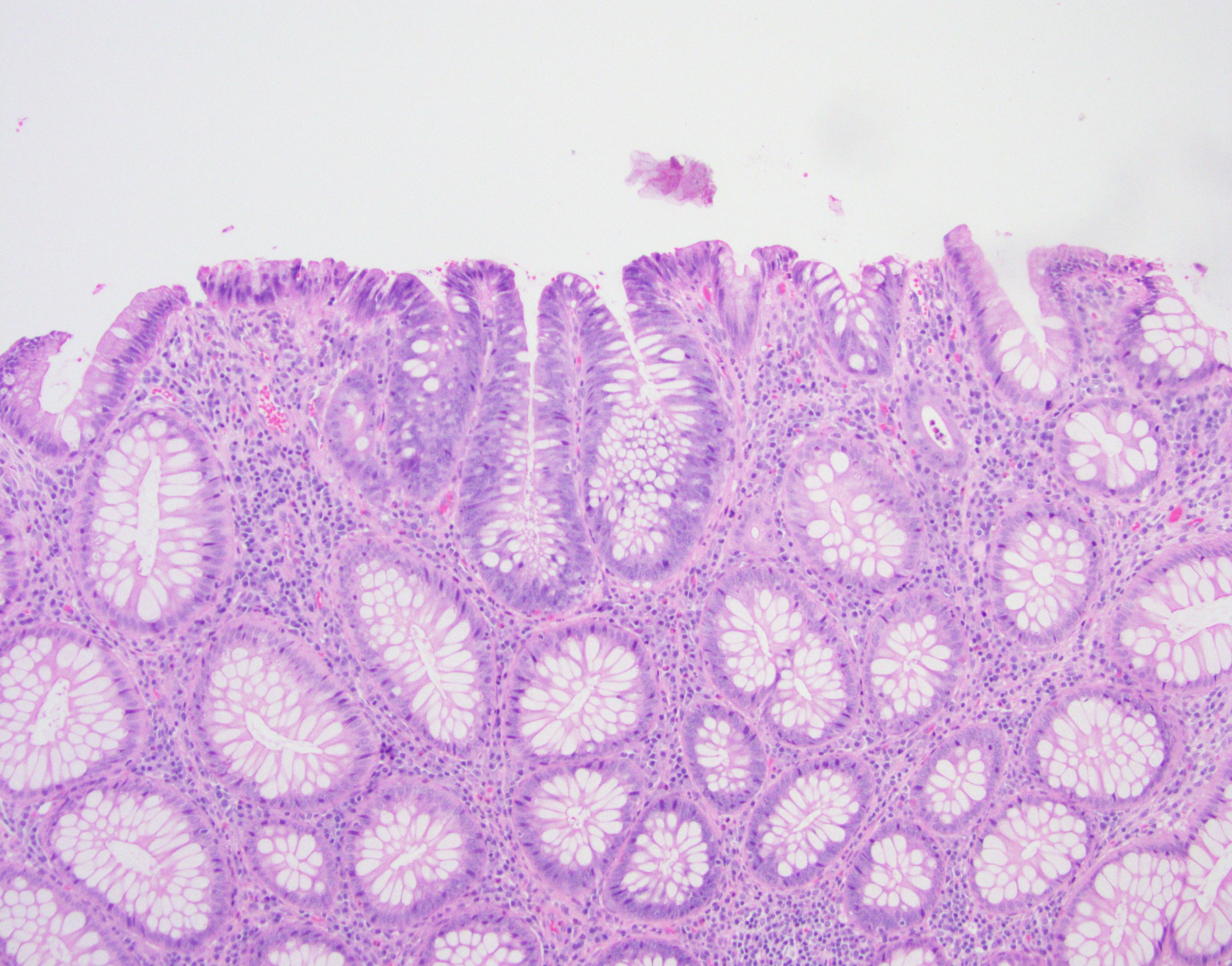
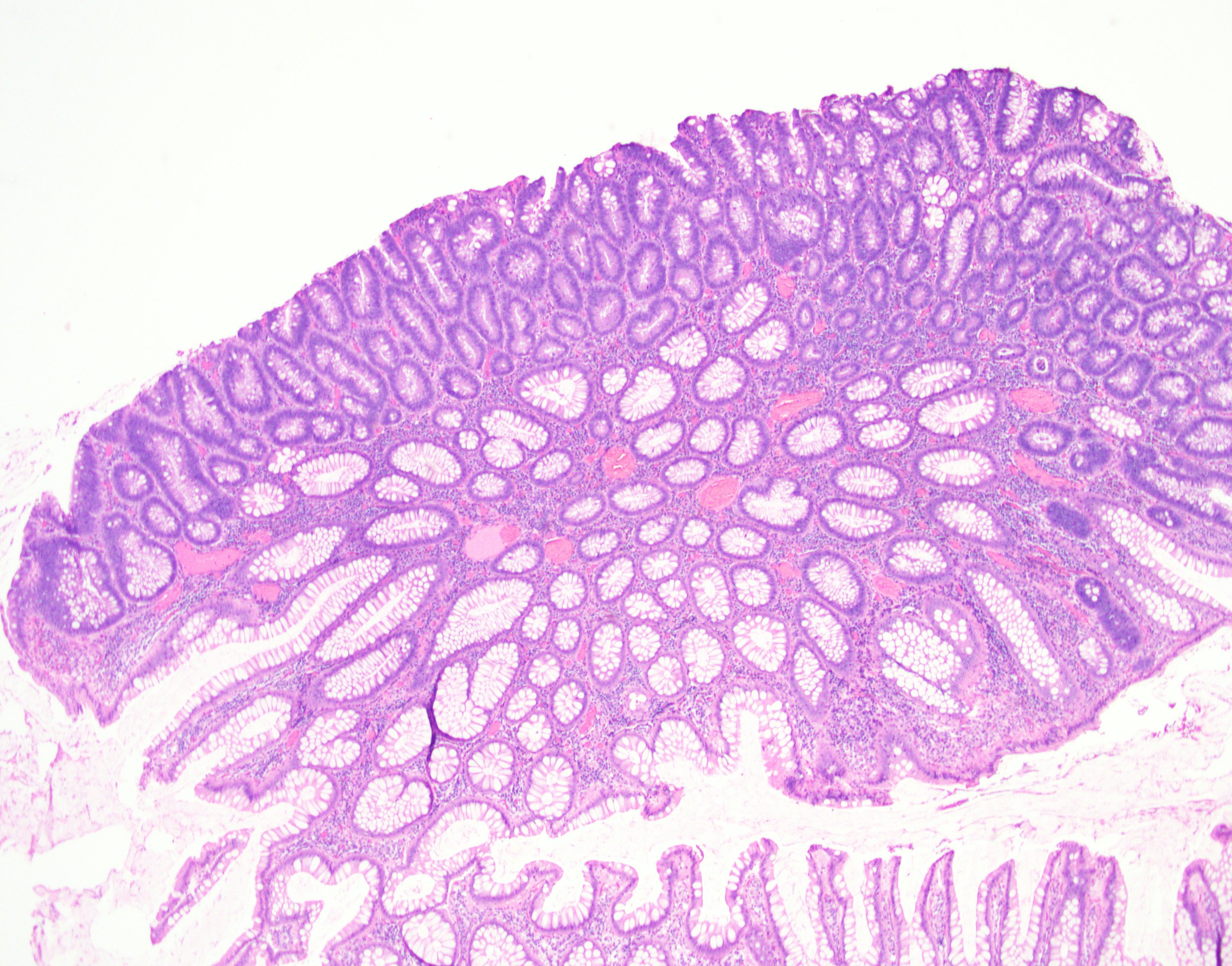
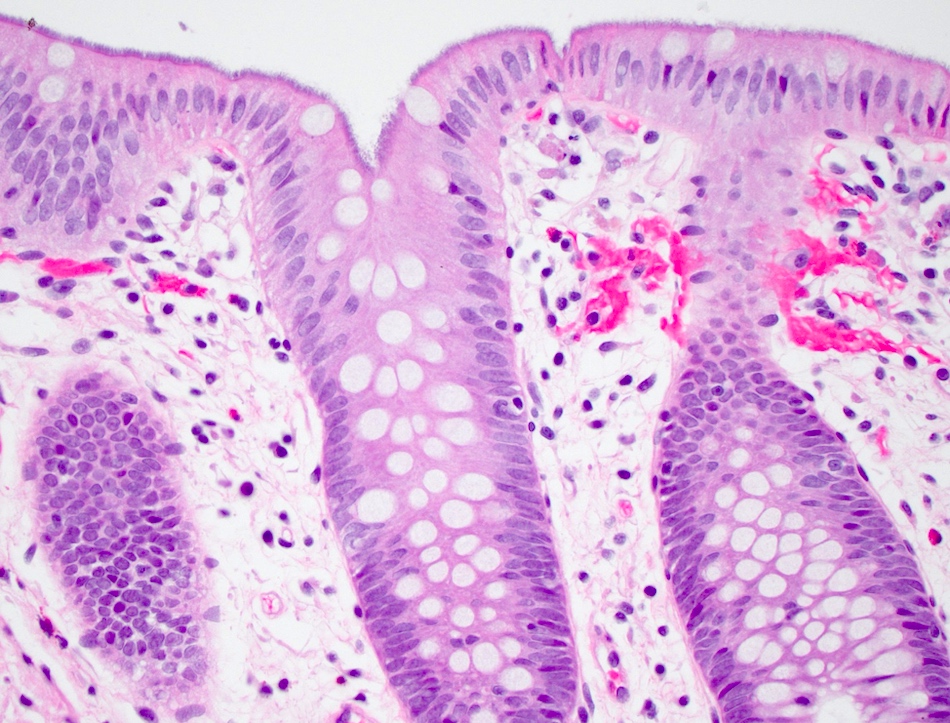
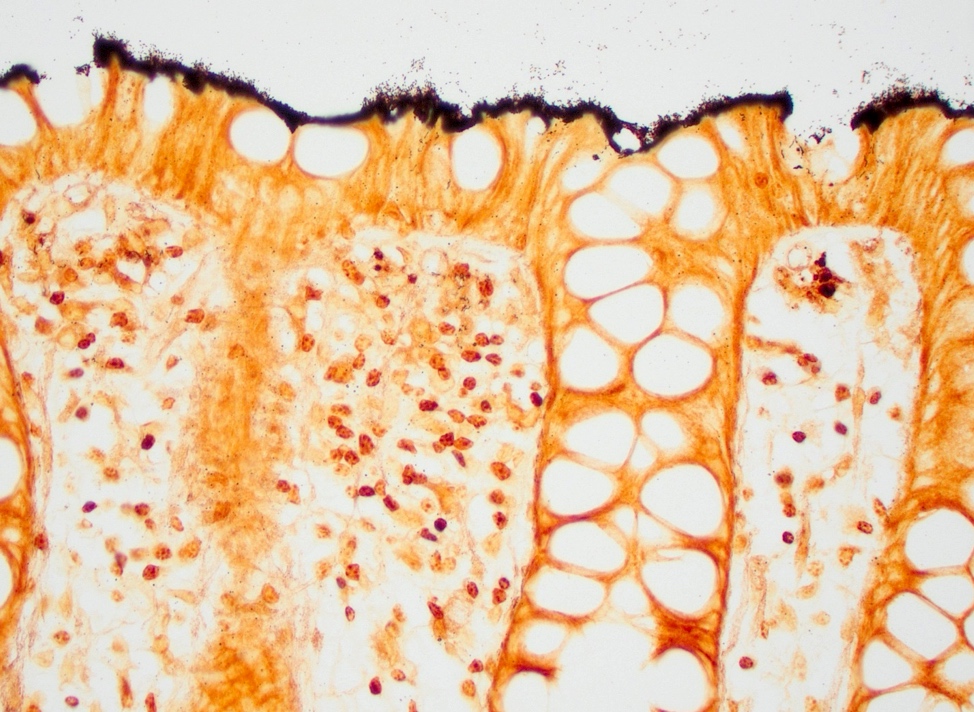
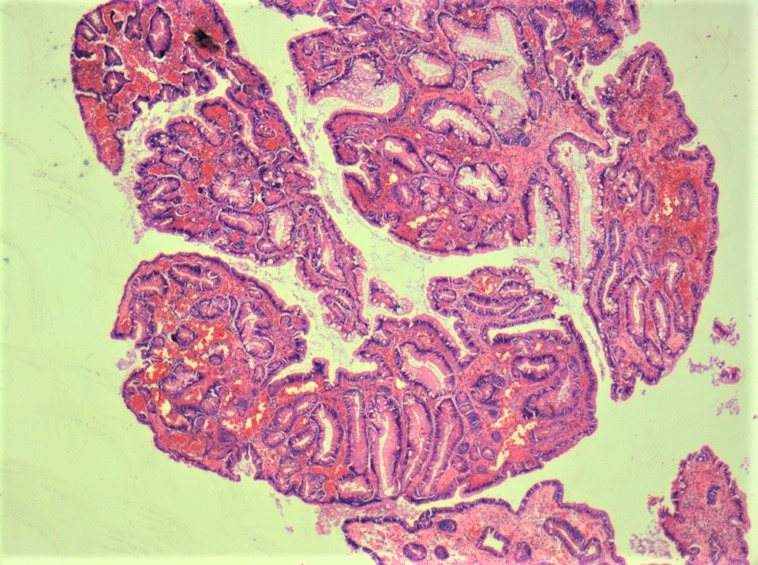
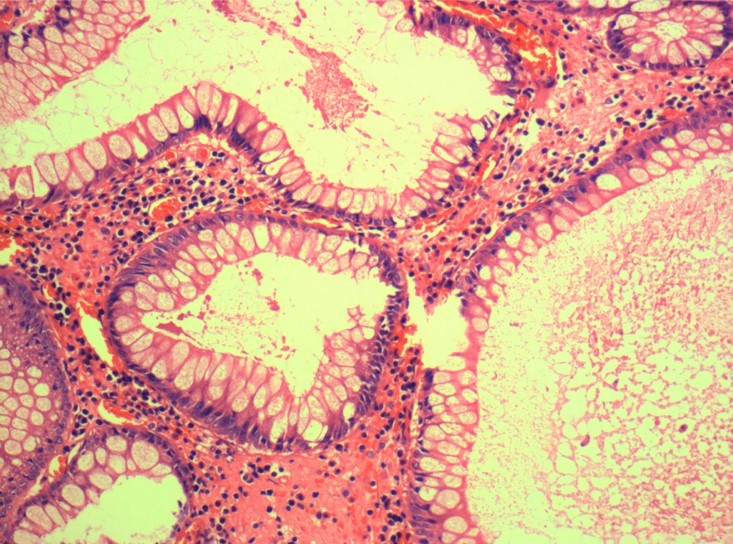
Microscopic (histologic) description
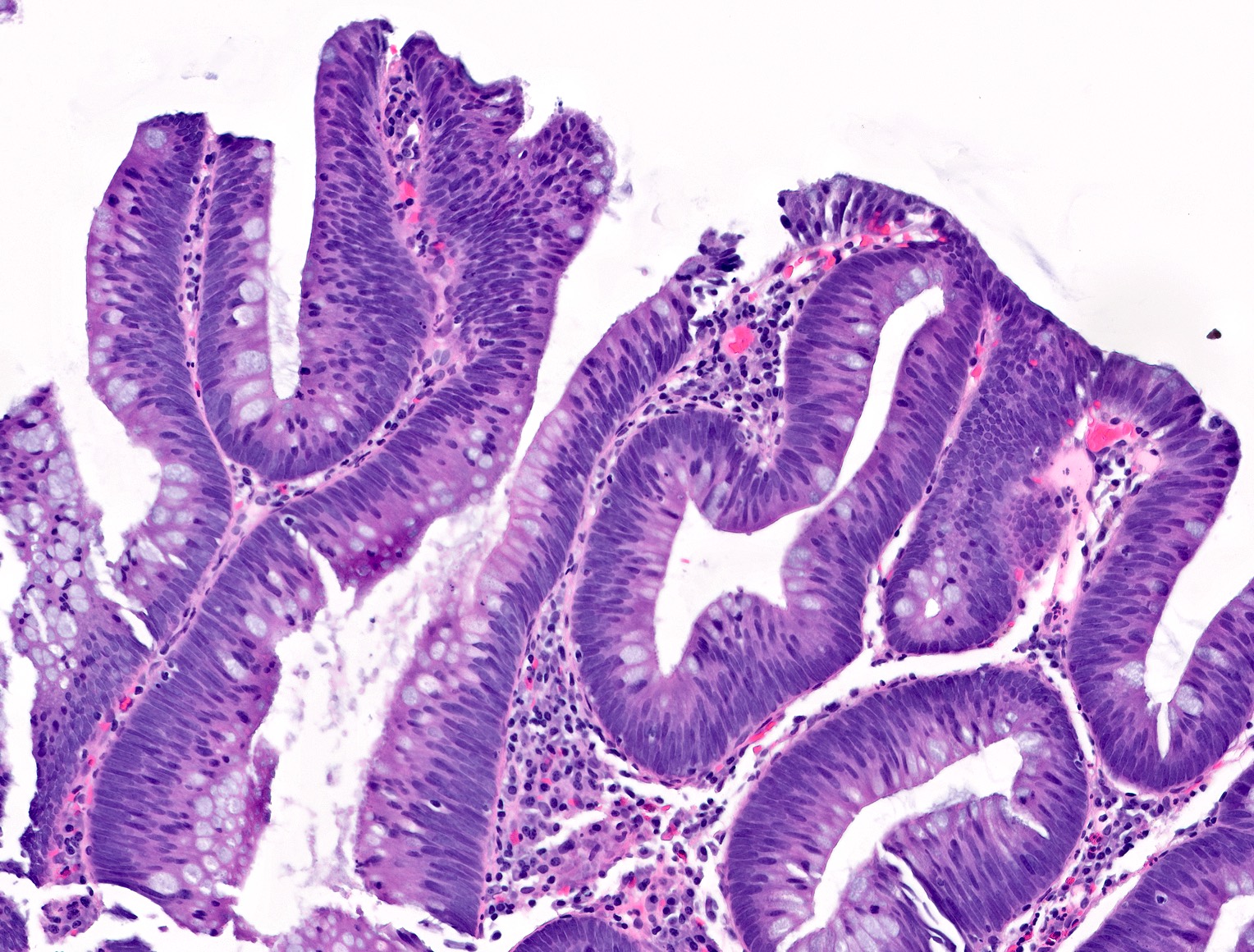
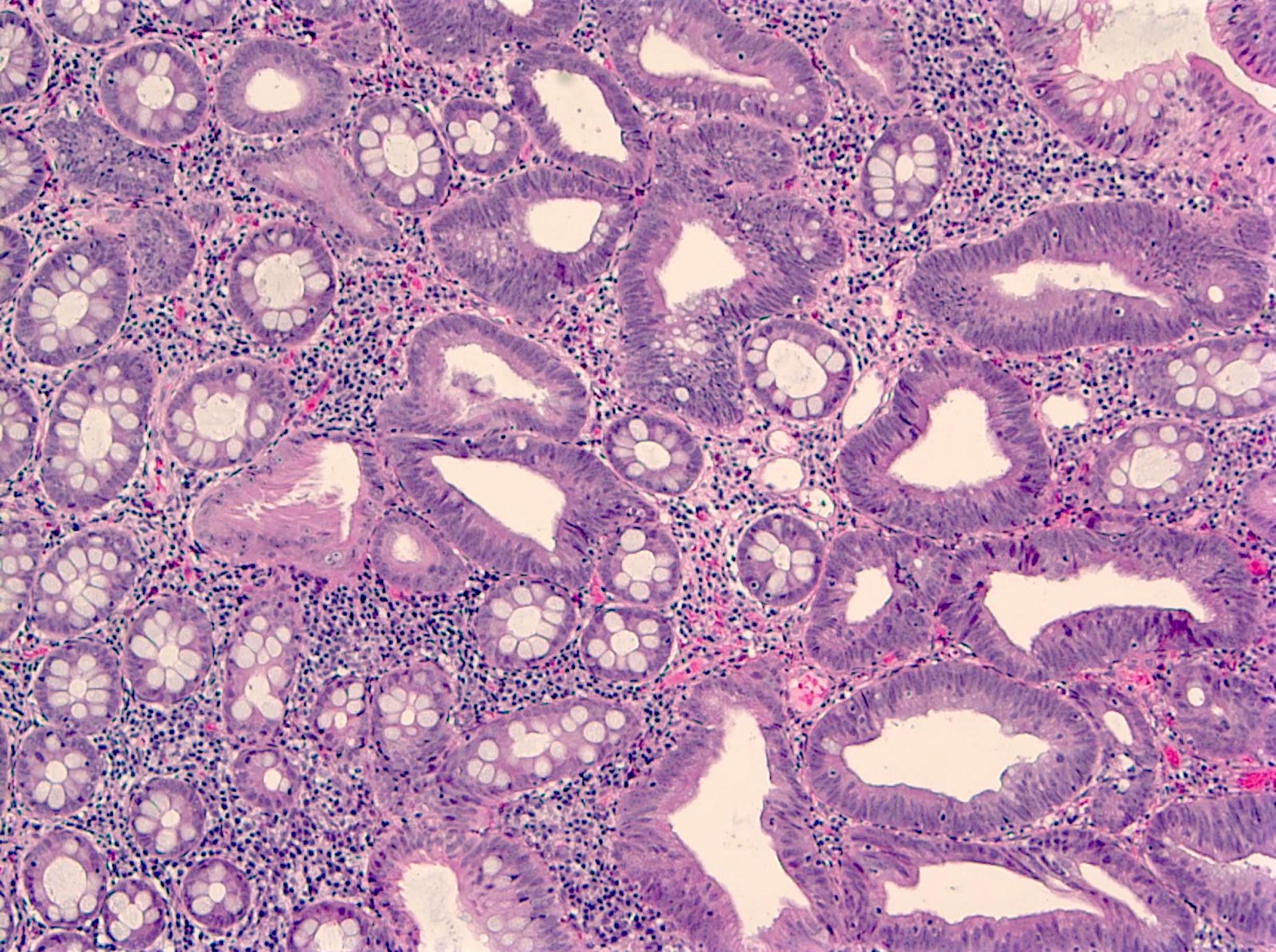
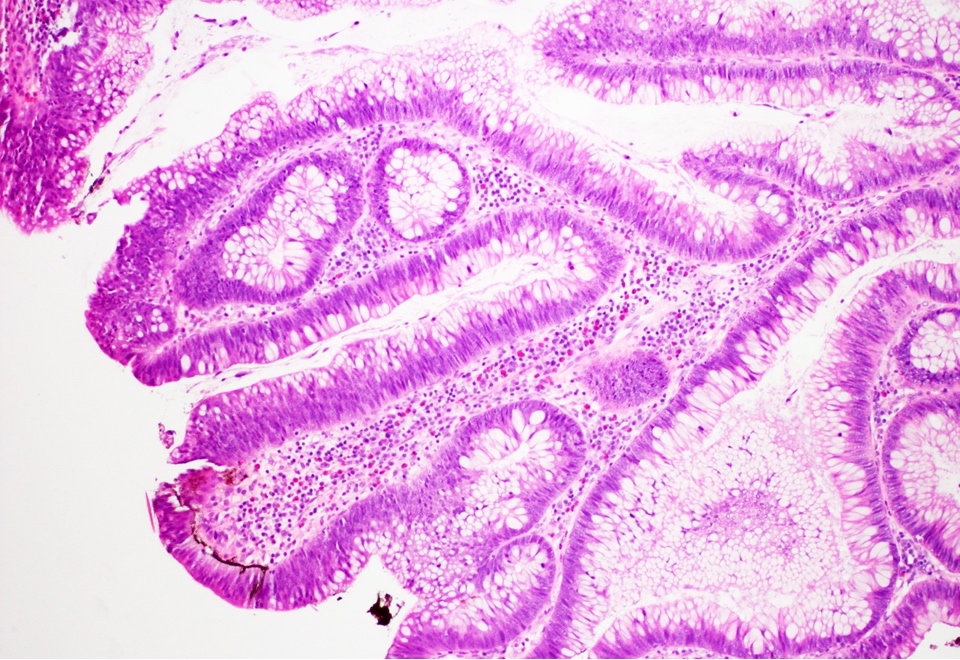
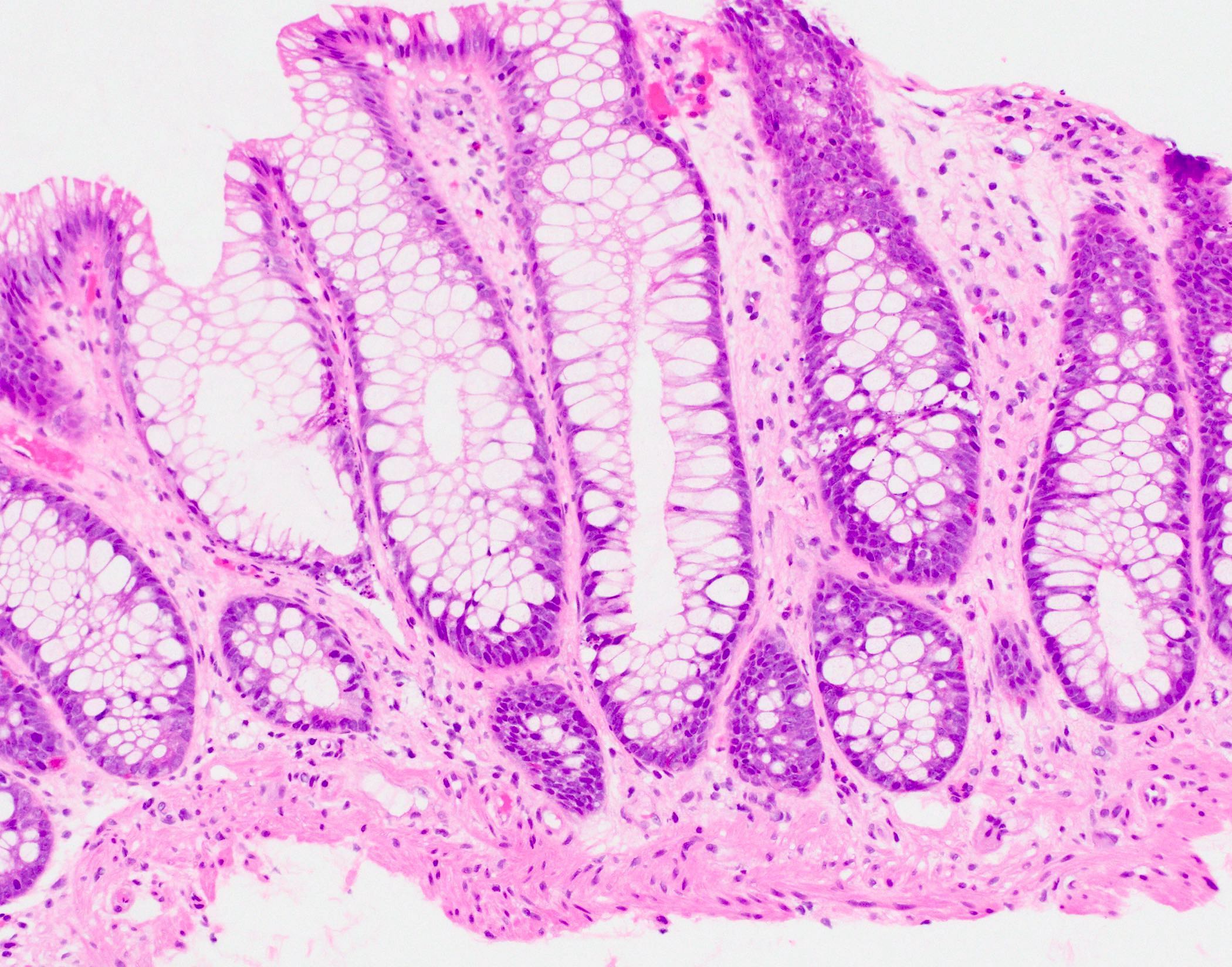
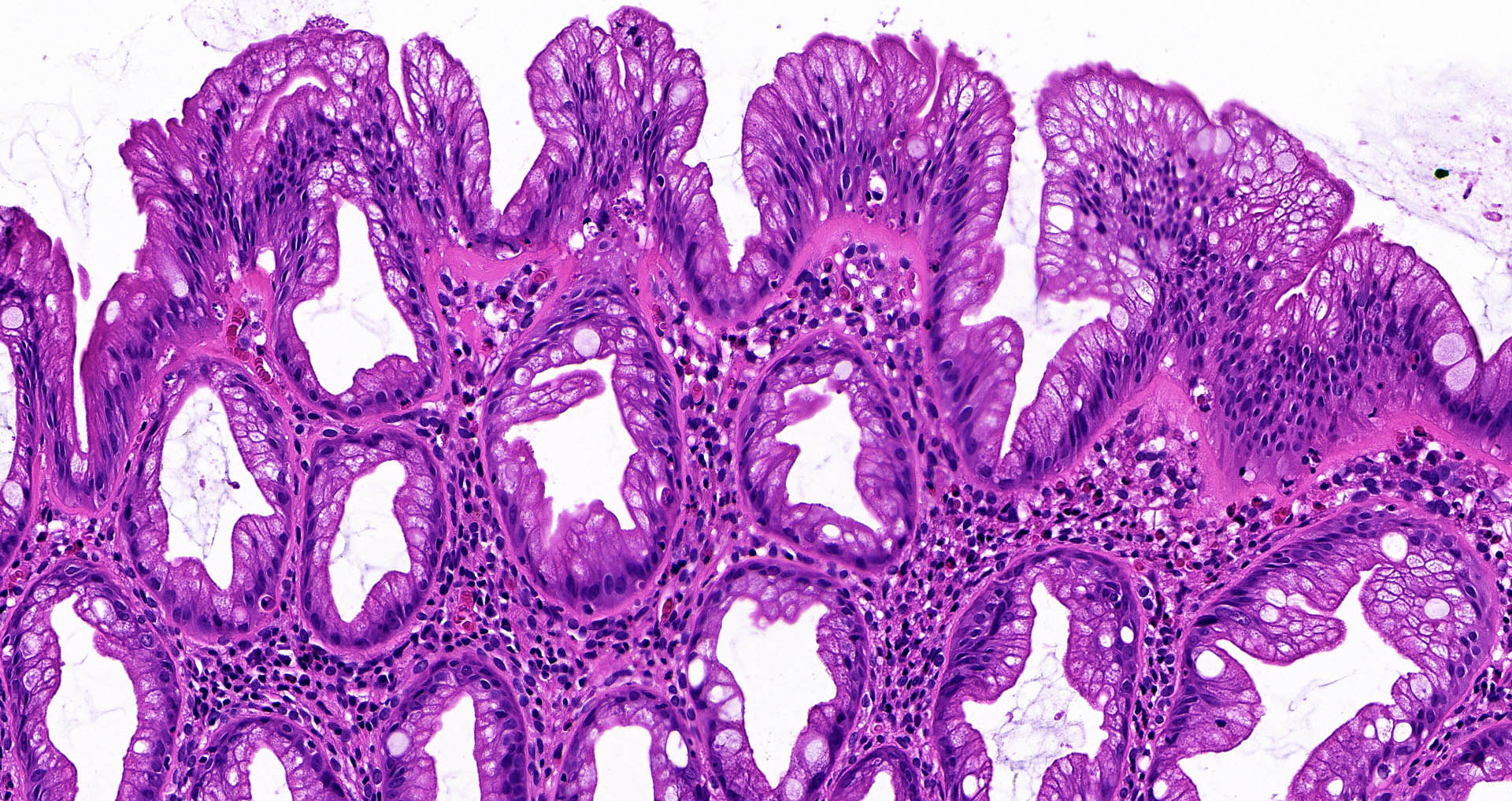
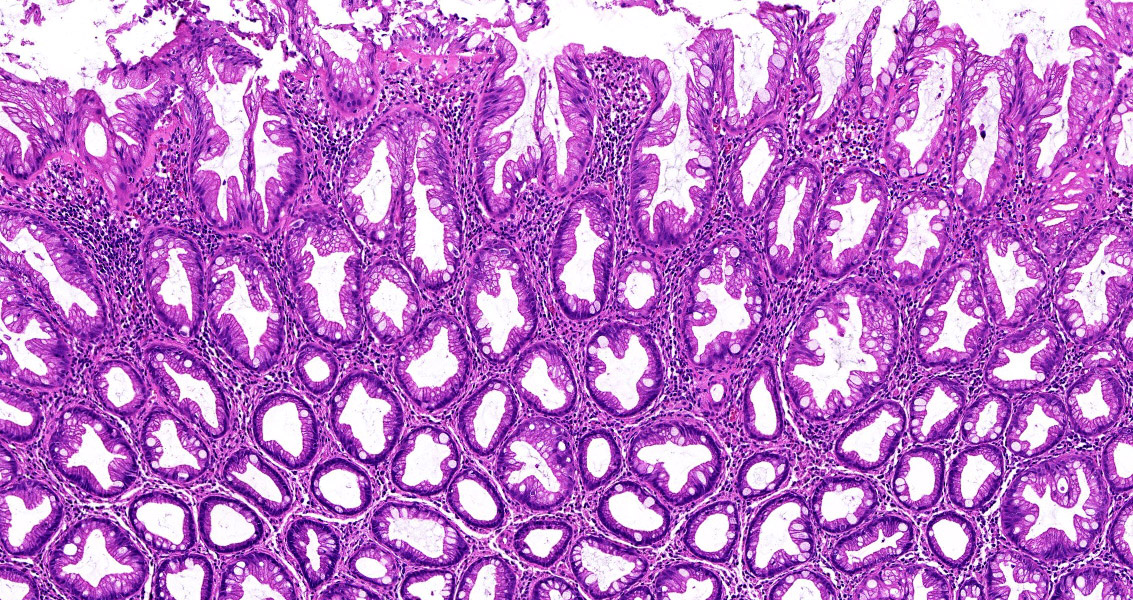
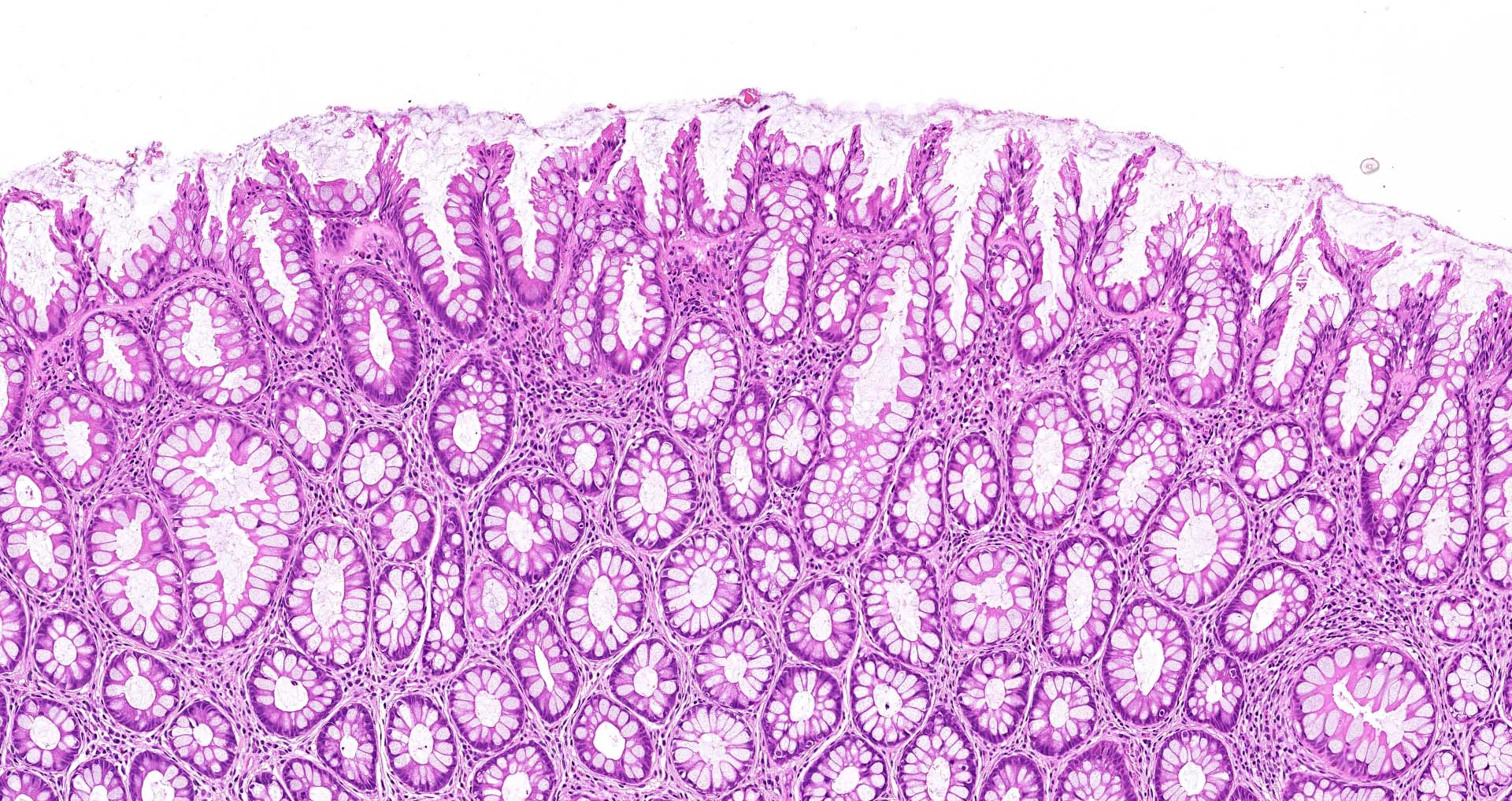
- Tubular adenomas:
- Conserved crypt architecture with variable elongation of the crypts and an increased number of glands
- At least low grade dysplasia: hyperchromatic nuclei, nuclear spindling and stratification, loss of cell polarity
- Decreased numbers of goblet cells and absorptive cells
- Small (< 25%) villous component is acceptable
- Tubulovillous adenoma:
- Similar to tubular adenoma but with 25 - 75% of villous component
- Villous component: architecture that resemble small intestinal villi
- Villous adenoma:
- > 75% of villous component
- Advanced adenoma:
- All adenomas with a size > 10 mm
- With tubulovillous or villous architecture
- With or without high grade dysplasia
- Highest risk of synchronous of metachronous adenomas
- Rare subtypes:
- Paneth cell rich subtype (more common in proximal colon or in younger patients) (Hum Pathol 2009;40:872, Sci Rep 2016;6:26129)
- Squamous components might be present as morules or squamous metaplasia (Pathobiology 2005;72:269)
- Other rare morphological findings (Histopathology 2021;78:348):
- Clear cell metaplasia or clear cell change
- Note the clear or vacuolated cytoplasm are not mucin
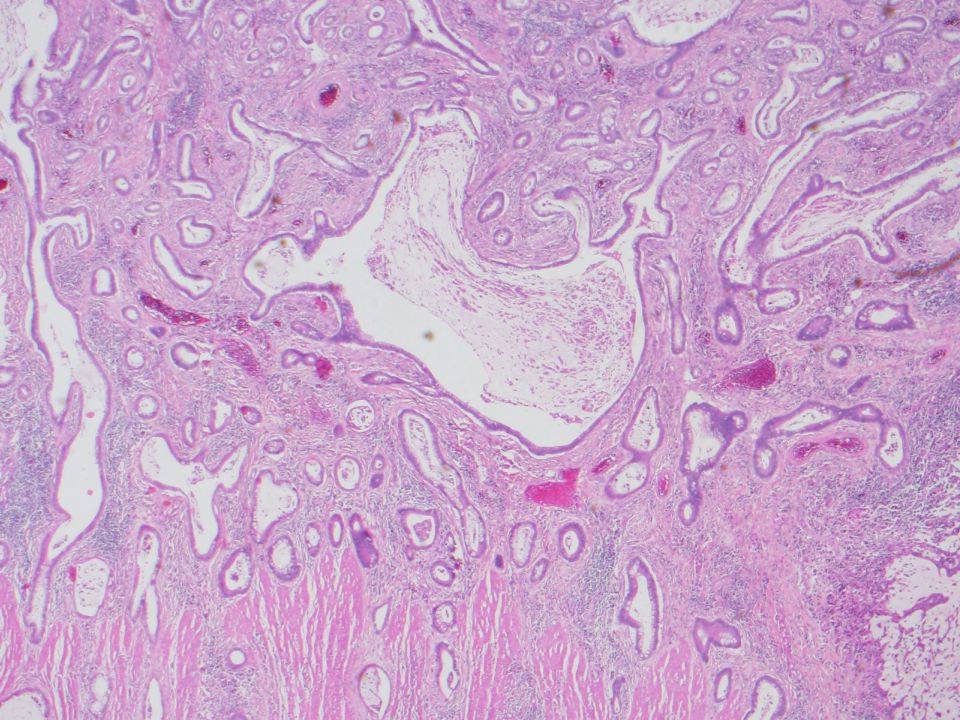
- Osseous metaplasia or heterotopic ossification
- Neuroendocrine differentiation
- Neuroendocrine hyperplasia
- Neuroendocrine metaplasia
- Neuroendocrine cell proliferation
- Composite intestinal adenoma microcarcinoid
- Mixed neuroendocrine - nonneuroendocrine neoplasm (MiNEN)
- Mixed adenoma - neuroendocrine tumor (MANET)
- Signet ring cell-like lesion
- Clear cell metaplasia or clear cell change
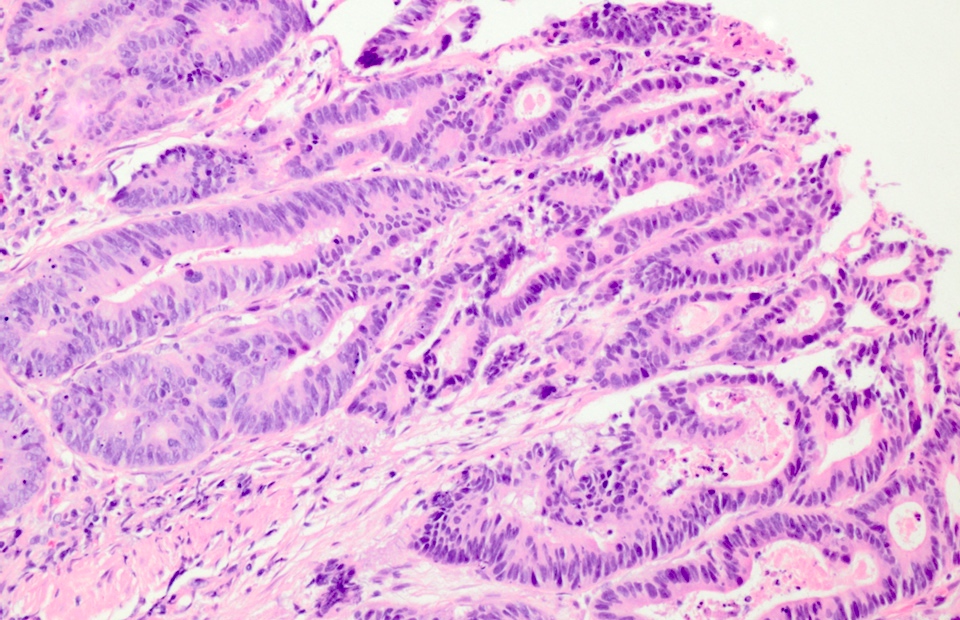
- Histology grading:
- 2 tiered system: low grade versus high grade
- Criteria for high grade dysplasia:
- Architecture: marked complex glandular crowding with glandular irregularity; cribriform architecture; intraluminal necrosis; can be observed at low power
- Cytology: substantial loss of cell polarity, marked enlarged nuclei with prominent nucleoli, dispersed chromatin pattern, atypical mitotic figures (Eur J Gastroenterol Hepatol 2002;14:183, Colorectal Dis 2015;17:682)
- Should be no evidence for invasion, however, pseudoinvasion (epithelial misplacement) could sometimes be seen due to prolapse (Mod Pathol 2015;28:S88)
- Features favoring pseudoinvasion / epithelial misplacement:
- Signs suggestive of previous epithelial trauma (extracellular mucin, hemorrhage or hemosiderin)
- Focus appears to be continuous with the surface epithelium with similar cytology
- Signs of mucosal prolapse such as muscular proliferation
- Acute necrosis of the surface
- Features favoring adenocarcinoma:
- Isolated glands without accompanying lamina propria
- Budding
- Vascular invasion
- Poor differentiation in morphology
- Concurrent review by more than 1 GI pathologist is suggested if high grade dysplasia is present
- Features favoring pseudoinvasion / epithelial misplacement:
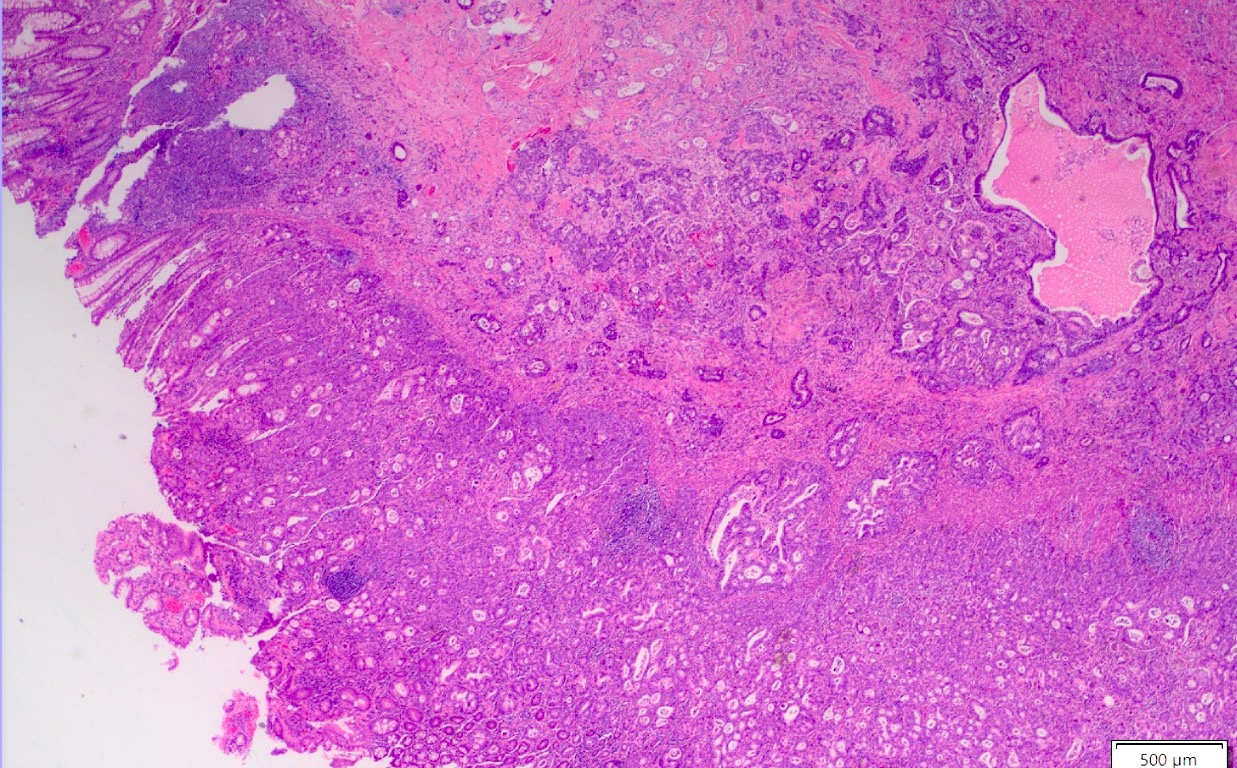
Microscopic (histologic) images
Molecular / cytogenetics description
- Not routinely performed, although majority of cases will have chromosomal instability; a subset (~25%) of cases will show TP53 mutations
Videos
Tubular adenoma
Colon dysplasia
Villous adenoma
Sample pathology report
- Colon, hepatic flexure polyp, biopsy:
- Tubular adenoma with focal high grade dysplasia
- Ascending colon, polyp, endoscopic mucosal resection:
- Fragments of tubulovillous adenoma (see comment)
- Comment: No high grade dysplasia is identified. Specimen fragmentation precludes assessment of specimen margins.
Differential diagnosis
- Reactive colonic mucosa:
- Smaller nuclei with basal orientation
- No or less significant hyperchromasia or pseudostratification
- More abundant cytoplasm and mucin
- Invasive adenocarcinoma:
- At least invasion through muscularis mucosa into the submucosa
- Desmoplasia, single cells
- Traditional serrated adenoma:
- Sawtooth luminal / surface contour
- Ectopic crypts characterized by aberrant budding crypts
Board review style question #1
Board review style answer #1
D. TP53. Alteration of TP53 allows cells to survive DNA damage and other cellular changes. It is found in ~25% of adenomas.
Comment Here
Reference: Adenoma overview
Comment Here
Reference: Adenoma overview
Board review style question #2
Board review style answer #2
A. The number of adenomatous polyps considered to be a low risk feature is < 3.
Comment Here
Reference: Adenoma overview
Comment Here
Reference: Adenoma overview
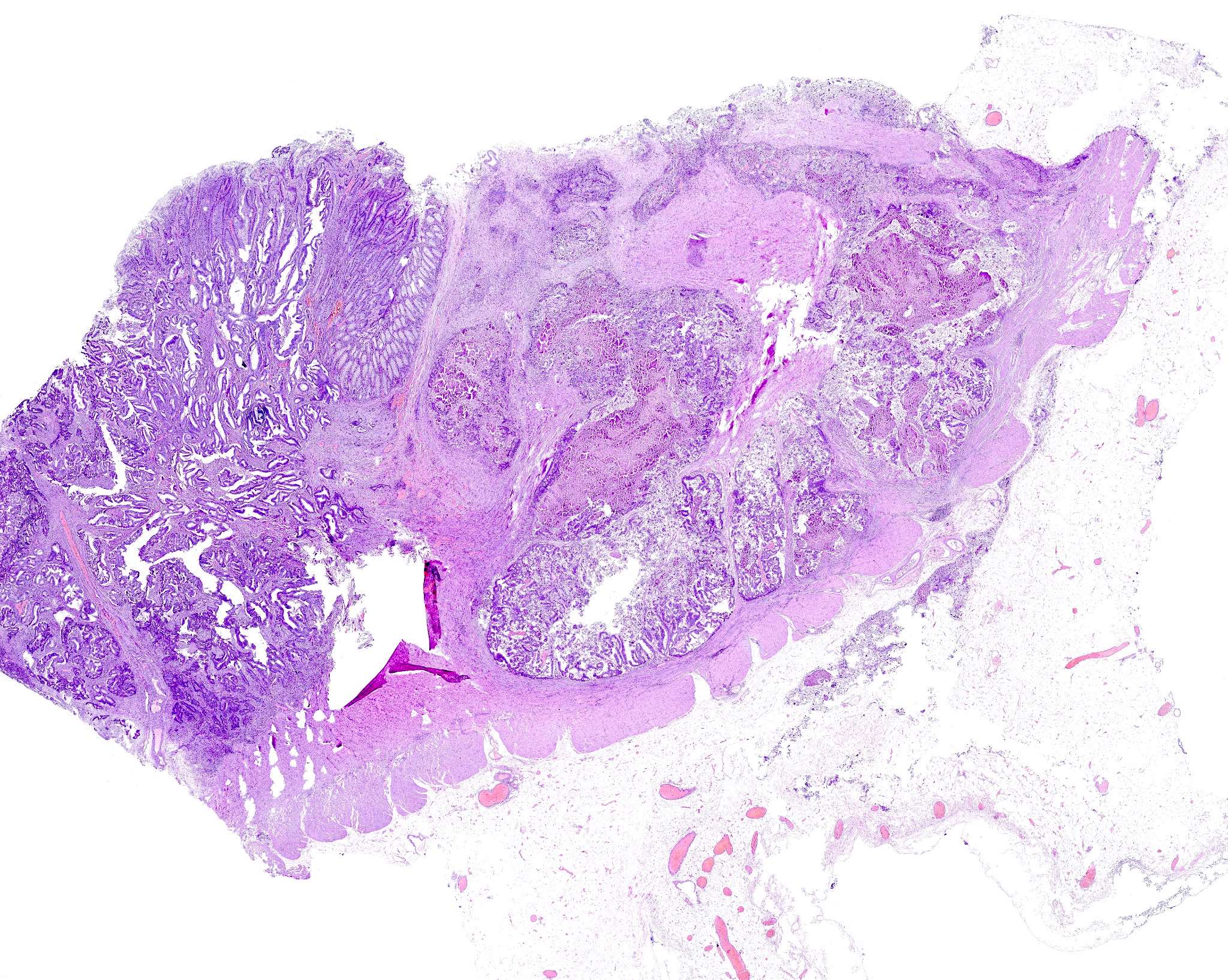
Adenoma with invasive carcinoma
Table of Contents
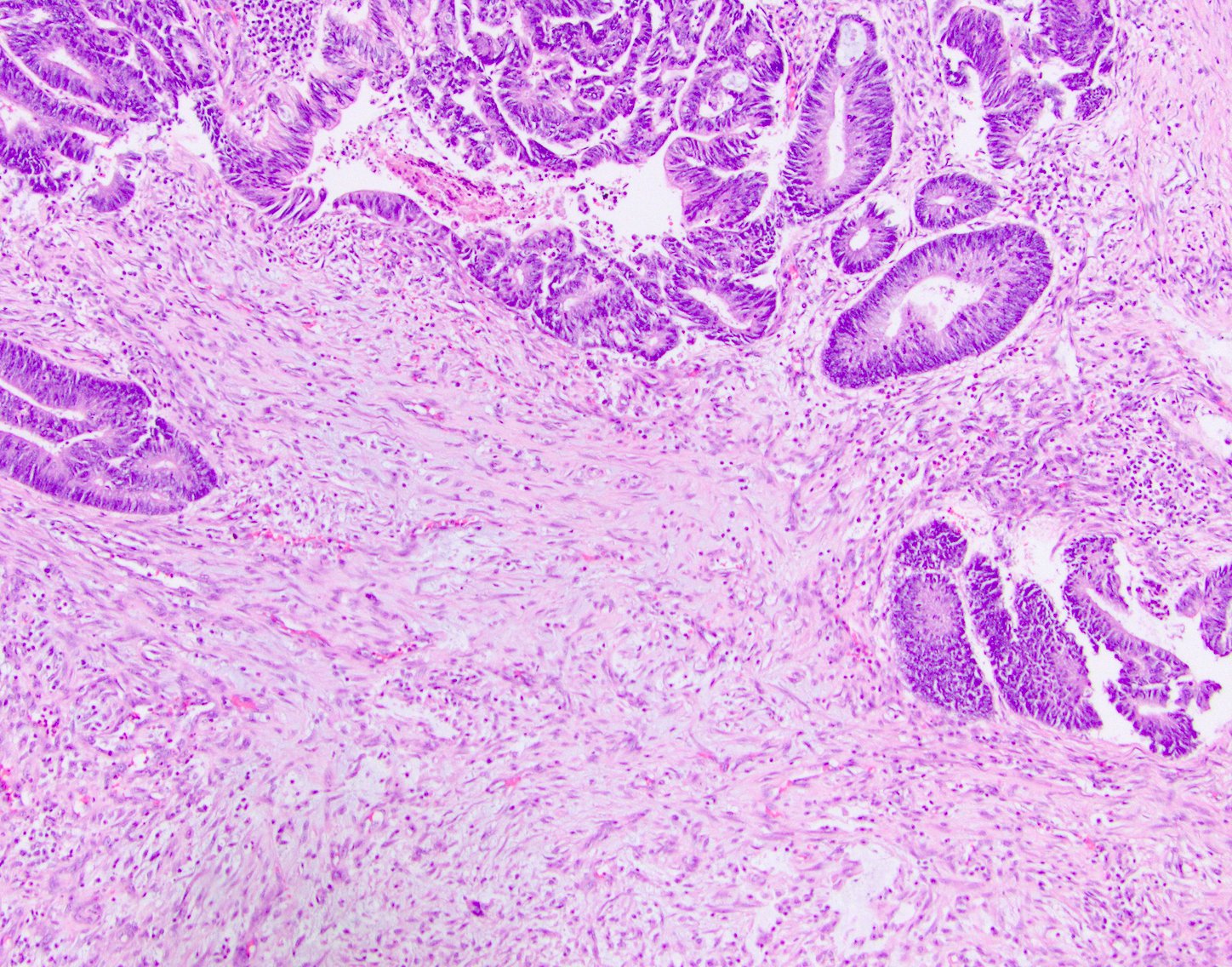
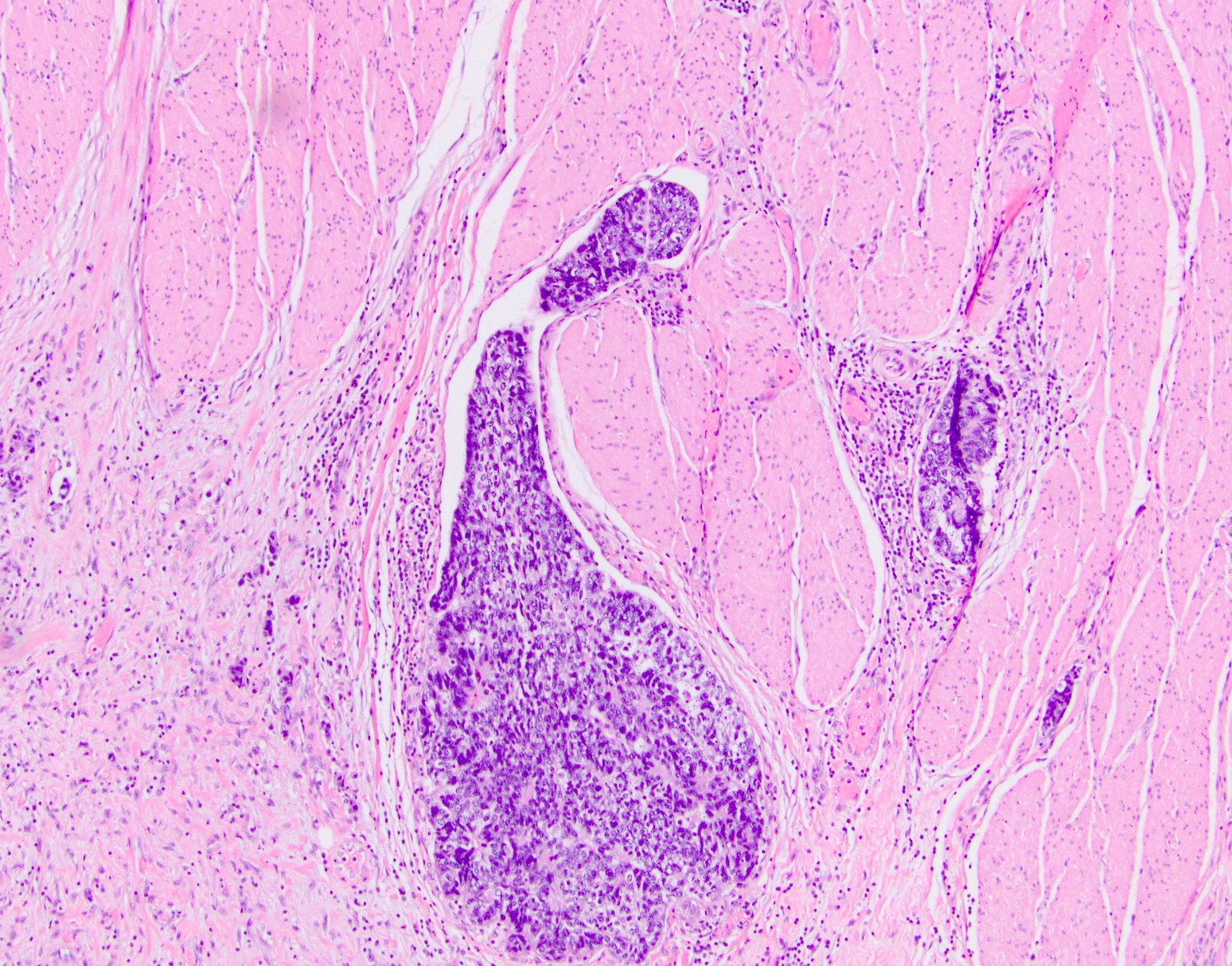
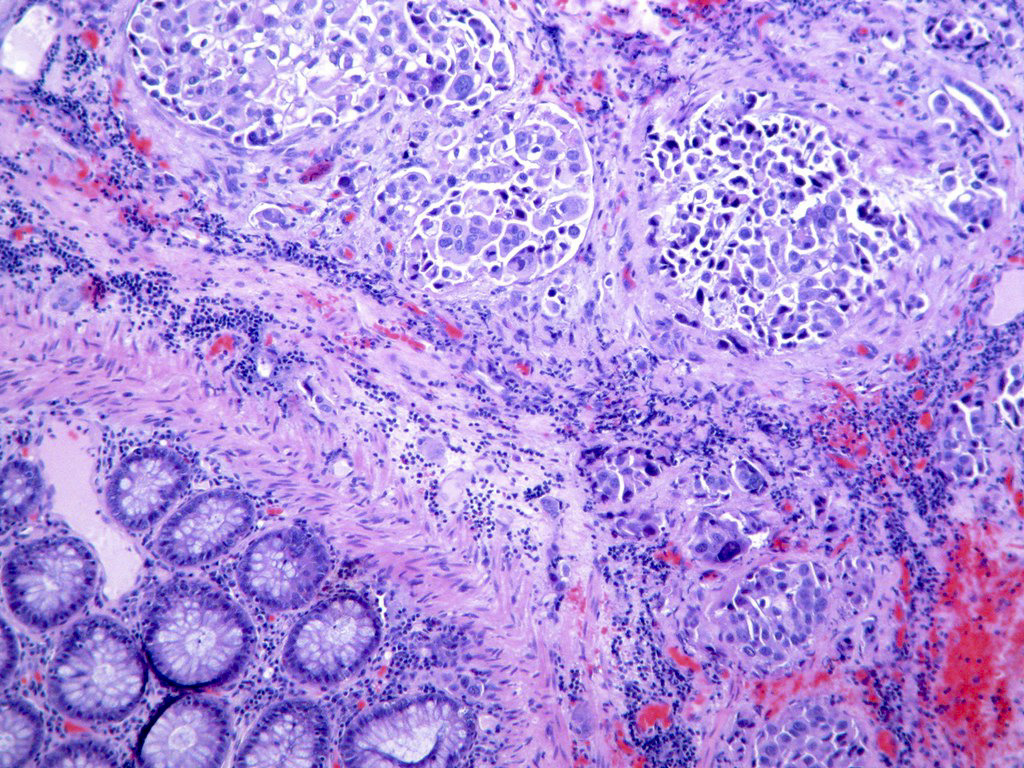
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
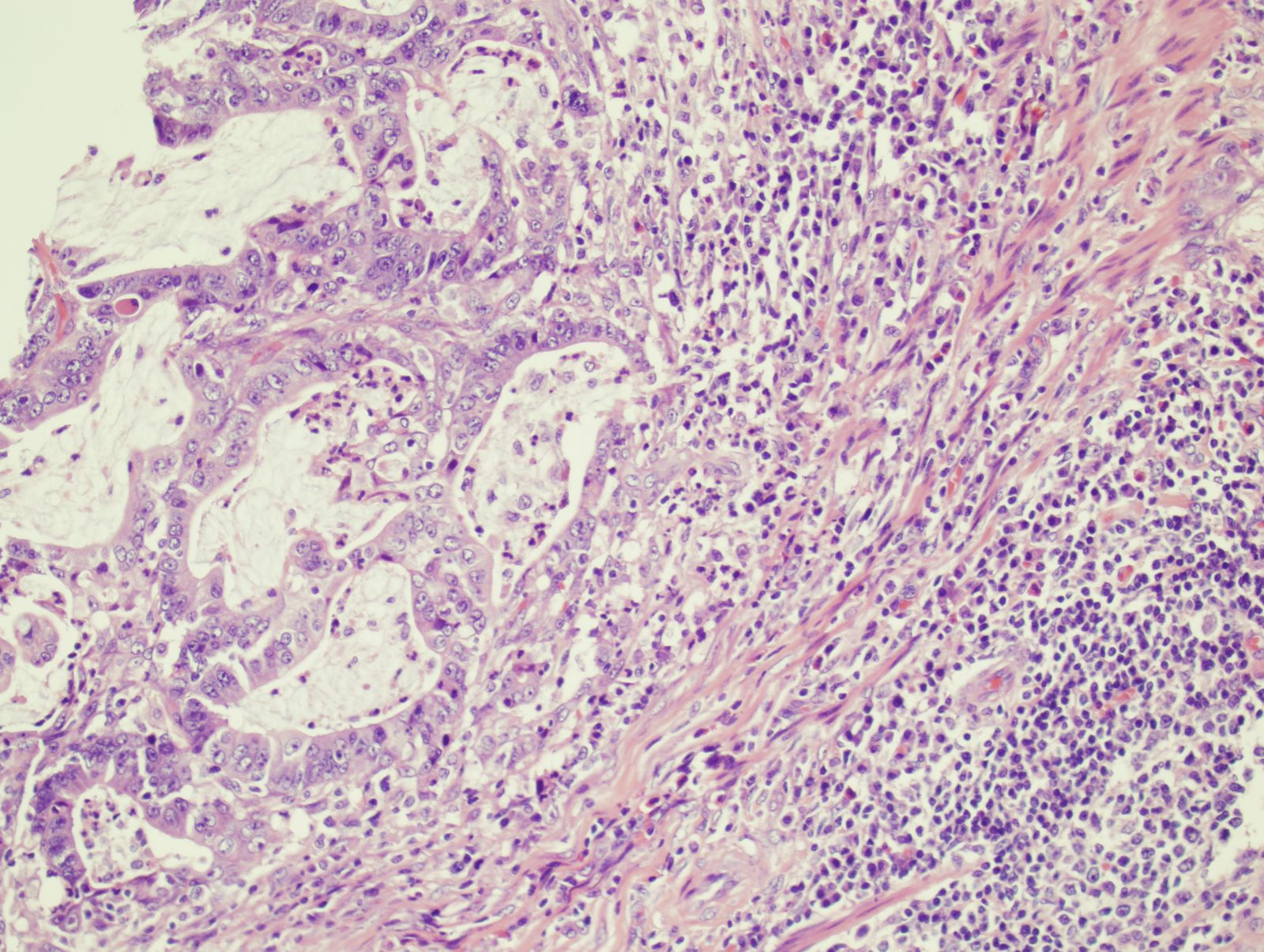
- Malignant colorectal polyps are colorectal adenomas containing invasive adenocarcinoma that extend through the muscularis mucosae into the submucosa and do not extend into the muscularis propria (Am J Gastroenterol 2020;115:1751)
- They are classified as pT1 in the current TNM classification system in the 8th edition of AJCC Cancer Staging Manual (CA Cancer J Clin 2017;67:93)
Essential features
- Colorectal adenoma with invasive carcinoma represents the earliest form of carcinoma because submucosal invasion leads to further risk for lymphatic and vascular metastasis even if the polyp / tumor has been completely resected (Gastroenterology 2004;127:385)
- Colorectal adenomas with intramucosal carcinomas limited to the surface epithelium, lamina propria or muscularis mucosae are staged as carcinoma in situ (Tis) and should be excluded from this category (Am J Gastroenterol 2020;115:1751)
- On a limited biopsy specimen when a clinical mass lesion is identified, we can diagnose invasive carcinoma in a colorectal adenoma if we see marked desmoplasia, infiltrative pattern, poorly differentiated morphology or lymphovascular space invasion (LVI) (Gastroenterology 1995;108:1657)
Terminology
- Also called malignant epithelial / colorectal polyp, cancerous polyps
ICD coding
- ICD-10: C18.9 - malignant neoplasm of colon, unspecified
Epidemiology
- Prevalence of malignant polyps is between 0.2% and 12.0% (average: ~5.0%) in endoscopic polypectomies (Endoscopy 1995;27:153)
- Incidence is increasing due to more efficacious colonoscopy screening programs, which are fundamental in the prevention of colorectal cancer and the treatment of some advanced polyps (World J Gastroenterol 2010;16:3103)
- Malignant polyps are also classified based on the depth of invasion, which is the most important feature; in 1985, Haggitt et al. put forward a classification system for pedunculated and sessile polyps based on the depth of invasion of adenocarcinoma (Gastroenterology 1985;89:328)
- Risk factors for finding invasive carcinoma in colonic adenomas depends on
- Size of adenoma
- 1% risk if adenoma is < 1 cm; 10% risk if adenoma is 1 - 2 cm adenoma; 46% risk if adenoma is > 2 cm (Cancer Epidemiol Biomarkers Prev 2002;11:622)
- But infrequently, > 20 cm sessile adenomas can be benign
- Villous component in adenomatous polyp
- High grade dysplasia has a 35% risk of having carcinoma (versus low grade dysplasia, which has only a 6% risk)
- Age of the patient (older patients are at higher risk of having malignant transformation of their colorectal polyps)
- Size of adenoma
Sites
- Any part of the colon
Pathophysiology
- 95% of colorectal cancers arise from adenomatous polyps and follow adenoma - carcinoma sequence, which is an indolent process taking many years to progress after a stepwise collection of genetic alterations
- Sessile serrated adenoma is presumed to be the precursor of right sided adenocarcinomas with high levels of microsatellite instability (MSI-H) (Am J Gastroenterol 2012;107:1315)
Clinical features
- Asymptomatic to rectal bleeding
Diagnosis
- Colonoscopy with polypectomy and histopathological evaluation
Radiology description
- Polyps are divided endoscopically by their size into (Am J Gastroenterol 2018;113:303)
- Diminutive: < 5 mm
- Small: 6 - 9 mm (account for > 80% of polyps encountered during colonoscopy and have little overall risk for advanced histology [0.8 - 1.6%] and malignancy [0 - 0.1%])
- Large: ≥ 10 mm
- Colon polyps > 10 mm have a 22.9% likelihood of advanced pathology, while those lesions that are 30 mm carry a 60% risk of high risk pathology (Gastrointest Endosc 2012;75:1022)
Prognostic factors
- Risk factors leading to lymph node metastasis or local recurrence from residual malignancy following polypectomies are (Endoscopy 2013;45:827)
- Higher histologic grade (poorly differentiated or undifferentiated carcinoma, signet ring cell carcinoma)
- Tumor ≤ 1 mm from the resection margin
- Lymphatic / venous vessel involvement
- Histologic factors that have adverse prognostic factors for distant metastasis are as follows (Mod Pathol 2017;30:1299)
- Quantity of tumor budding (Hum Pathol 2016;47:4)
- Depth or area of submucosal invasion (submucosal invasion > 1 mm) (World J Surg 2018;42:2635)
- In en block resections of pedunculated or nonpedunculated colorectal lesions with submucosal invasion, the pathologists need to measure and report the depth of invasion, distance of the tumor from the deep and lateral surgical resection margins, in addition to prognostic histologic features, such as degree of differentiation, presence or absence of lymphovascular invasion and tumor budding
Case reports
- 43 year old woman with a 2 cm pedunculated polyp in the descending colon showing poorly differentiated adenocarcinoma, invading into the submucosa and demonstrating a lymphatic invasion (World J Surg Oncol 2021;19:269)
- 60 year old woman with rectal bleeding and a smooth 0.8 cm polyp in the cecum, with a pathology revealing an infiltrating adenocarcinoma arising within the hyperplastic polyp (Am J Gastroenterol 2005;100:S211)
- 88 year old man presented with ischemic colitis and incidental 8 mm ascending colon polyp with invasive adenocarcinoma was found in a single section from the ascending colon (Cureus 2021;13:e13928)
Treatment
- Polypectomy with complete removal of the malignant colorectal polyp; prevention of colorectal cancer progression is the primary goal
- Endoscopic resection can provide complete resection and obviate the higher morbidity, mortality and cost associated with alternative surgical treatment
- Complete excision with conventional endoscopic snare polypectomy for < 1 cm adenomas, which accounts for 80 - 90% of colorectal polyps (Am J Surg Pathol 2018;42:1083)
- Cold or hot snare polypectomy (with or without submucosal injection) to remove 10 - 19 mm nonpedunculated lesions
- Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) are used for > 20 mm nonpedunculated colorectal polyps but are usually only available in specialized centers
- Benefit of EMR and ESD is the ability to obtain 1 large piece of tissue, avoiding fragmented excision with clear margins and making it easier to orient, section and evaluate margins
- Colectomy with lymph node dissection is recommended for pedunculated polyps with any unfavorable histology, invasion into submucosa of bowel wall (Haggitt level 4) and any sessile / flat adenomas with invasion (Haggitt level 1 - 4)
- First follow up surveillance colonoscopy is 6 months for larger colorectal polyps ≥ 20 mm and the interval to the next colonoscopy is at 1 year and then 3 years (Am J Surg Pathol 2018;42:1083)
Gross description
- Grossly the polyps are described as polypoid (pedunculated or sessile) and nonpolypoid (flat or ulcerated) subtypes according to the Paris classification (Gastrointest Endosc 2003;58:S3)
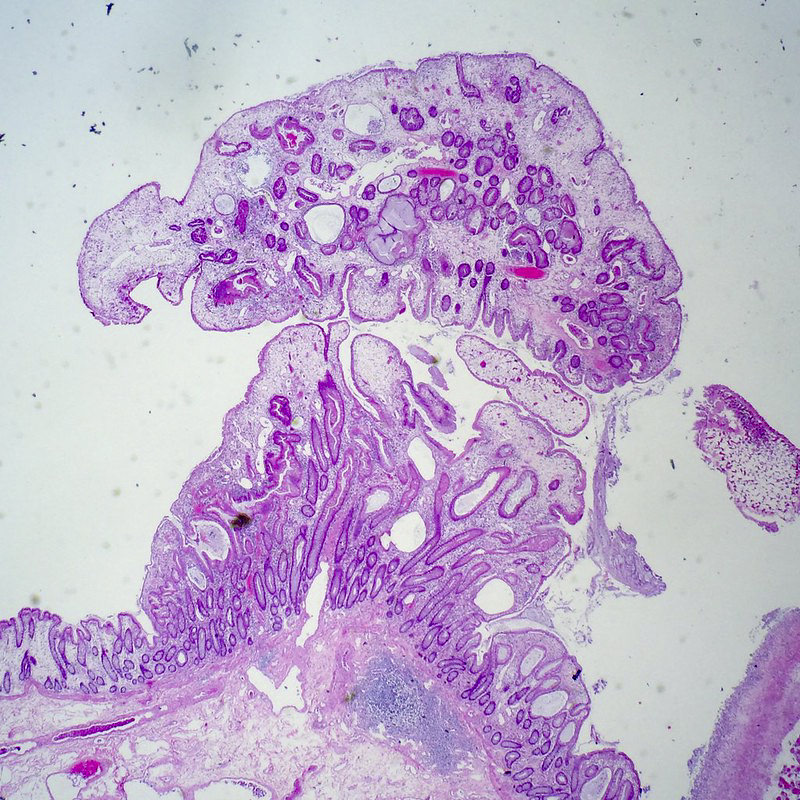
Microscopic (histologic) description
- Adenocarcinoma can arise in adenomatous (tubular, tubulovillous or villous), serrated (sessile serrated adenoma / polyp or traditional serrated adenoma) or hamartomatous polyps
- For malignant pedunculated polyps, submucosal involvement by carcinoma has been divided into 4 Haggit levels (head, neck, stalk and beyond stalk in the submucosa) (Gastroenterology 1985;89:328)
- Level 1: invasion limited to head of pedunculated polyp
- Level 2: invasion extends to neck of pedunculated polyp
- Level 3: invasion extends to stalk of pedunculated polyp
- Note: levels 1 - 3 have the lowest risk of metastasis (< 1%)
- Level 4: invasion of submucosa in bowel wall proper (beyond the stalk of pedunculated polyp)
- Note: level 4 has the highest risk of lymph node metastasis, up to 27%
- For malignant sessile polyps, submucosal involvement by carcinoma has been divided into superficial, mid and deep levels (Kikuchi levels SM1, SM2 and SM3) (Am J Gastroenterol 2020;115:1751)
- SM1: invasion into upper third of submucosa
- SM2: invasion into middle third of submucosa
- Note: SM1 / SM2 are associated with low risk of metastasis; reported to be 0% for SM1 and ~10% for SM2
- SM3: invasion into lower third of submucosa; the greatest risk of lymphatic spread, up to 25%
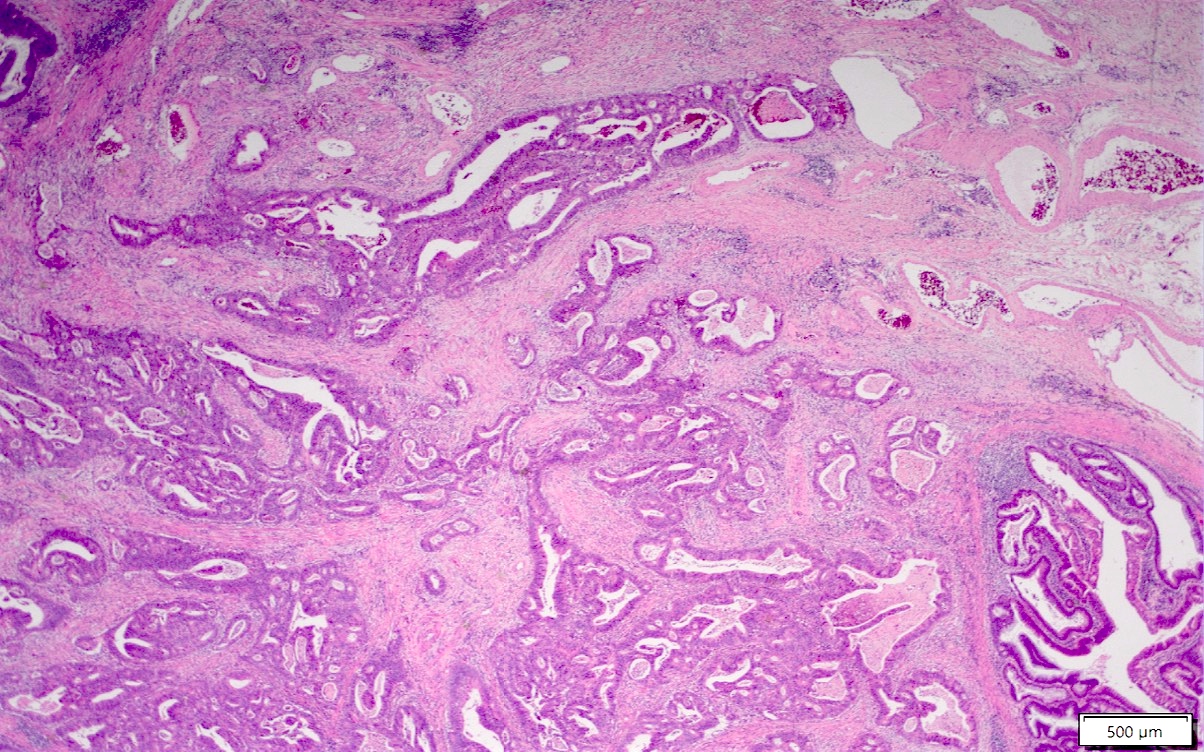
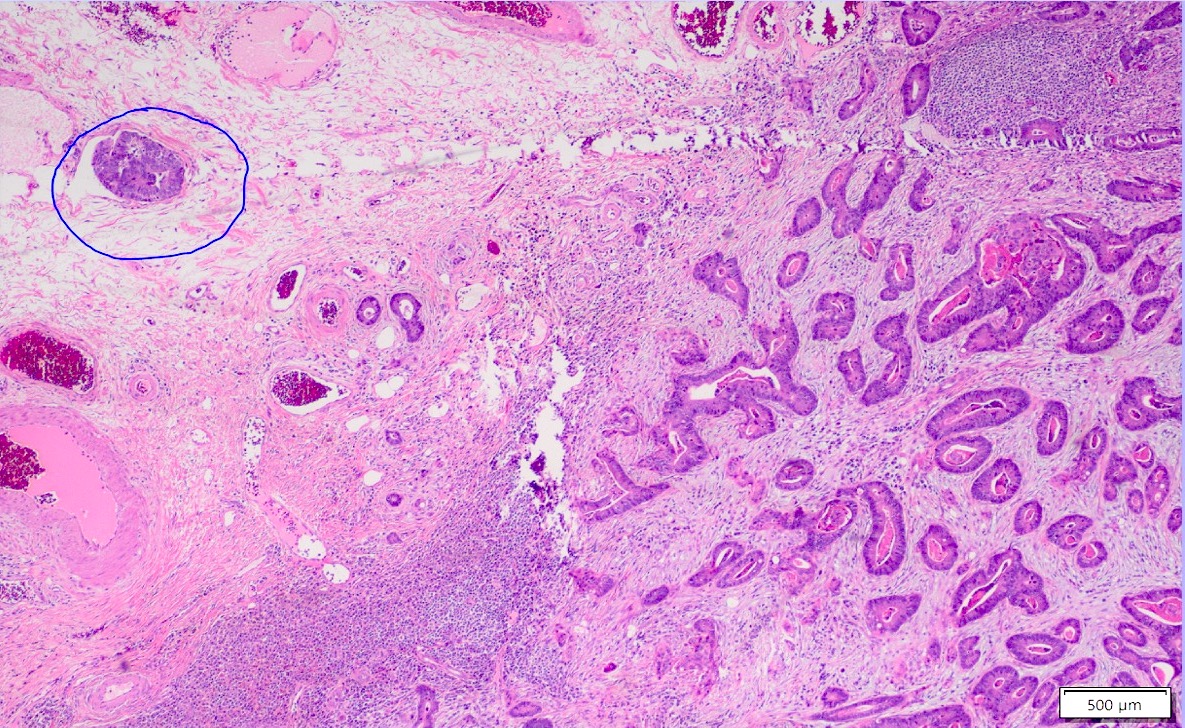
Microscopic (histologic) images
Contributed by Albina Joldoshova, M.D. and Naziheh Assarzadegan, M.D.
Molecular / cytogenetics description
- Sessile serrated adenoma more often shows high levels of microsatellite instability and MLH1 hypermethylation in sporadic cases (Am J Gastroenterol 2012;107:1315)
Sample pathology report
- Colon, ascending polyp, hot snare polypectomy:
- Invasive adenocarcinoma, moderately differentiated, arising in a tubular adenoma (see comment)
- Negative for lymphovascular space invasion
- Low tumor budding score
- Cauterized stalk margins negative for dysplasia or carcinoma
- Immunohistochemistry for mismatch repair proteins will be reported in an addendum
- Comment: Tumor invades into submucosa at the head of the polyp (depth on invasion is 0.5 mm). The distance to the deep margin is 2 mm. Complete excision of this lesion is considered adequate treatment; therefore, completeness of excision should be ensured clinically, if not already achieved.
Differential diagnosis
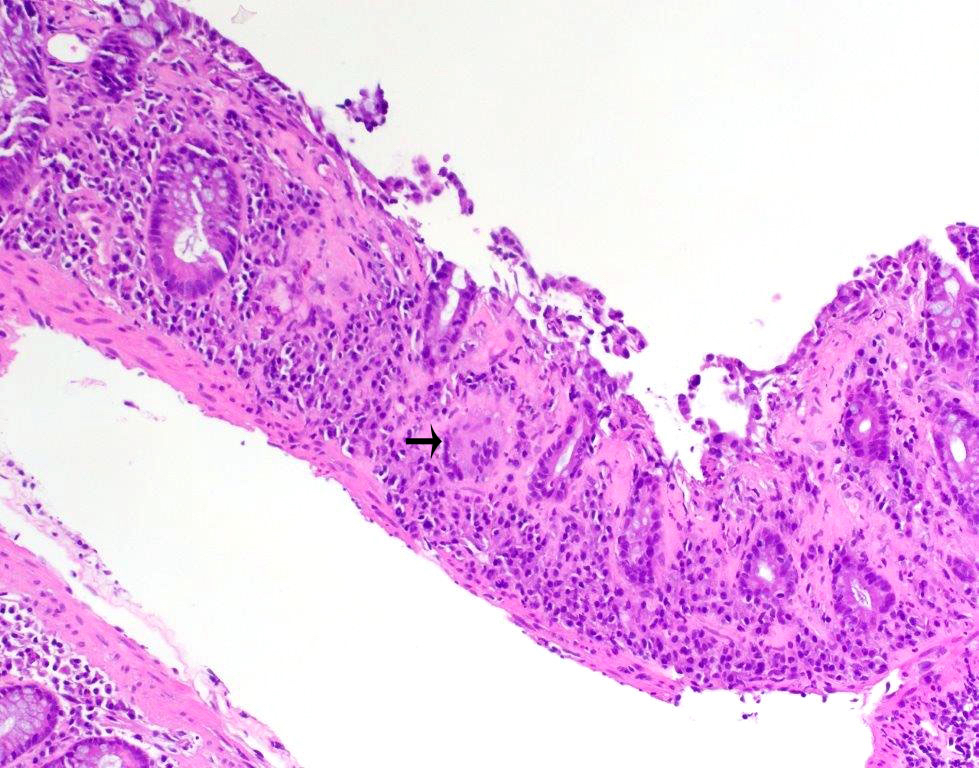
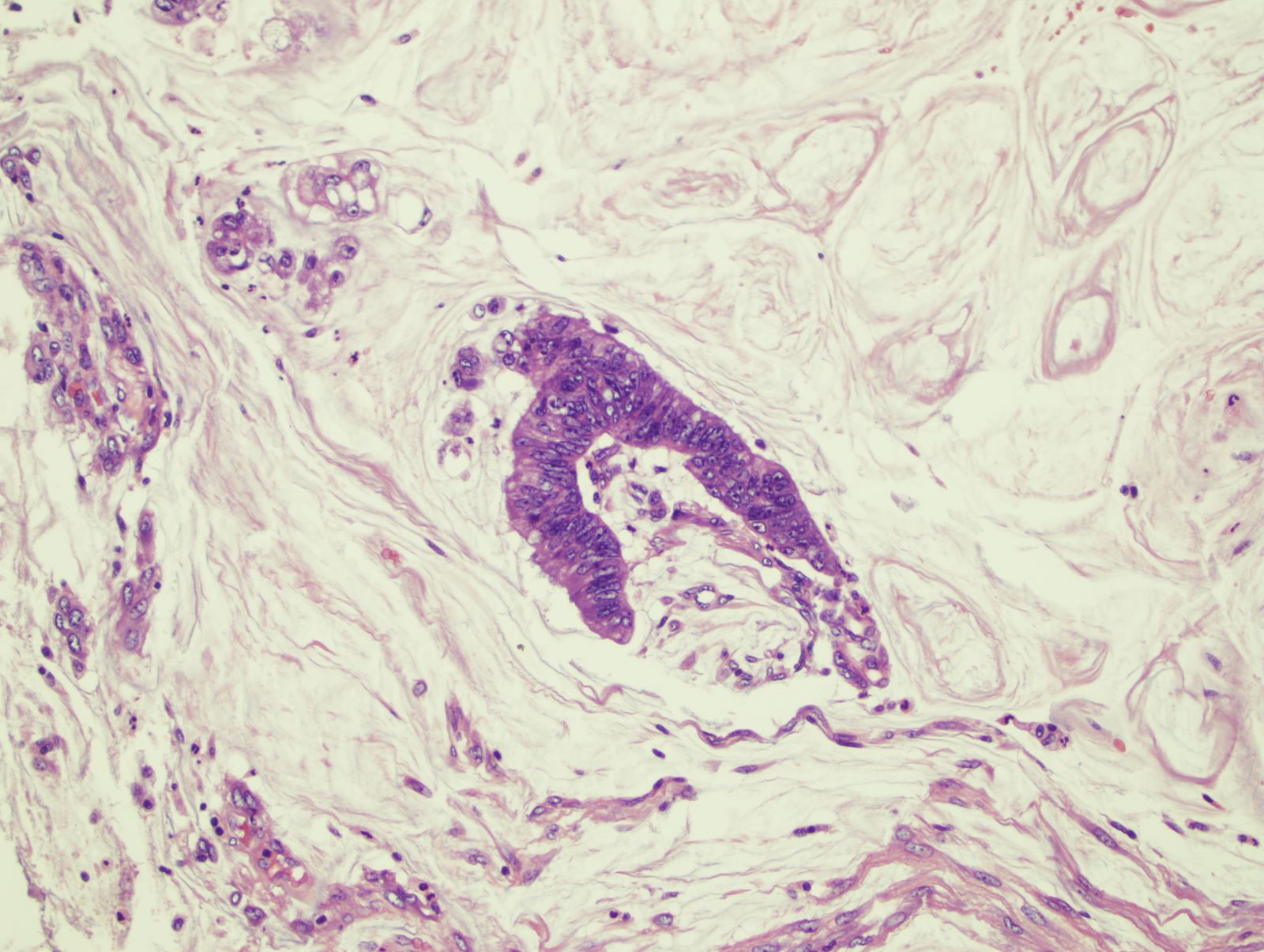
- Adenoma with pseudoinvasion (misplacement of benign or dysplastic glands) (Cancer 1974;33:206):
- Presence of dysplastic glandular epithelium of the mucosa beneath the muscularis mucosae in colonic polyps mimicking a submucosal invasion
- Usually occurs in large polyps (> 1 cm) (especially those with long stalks) and is mostly found in polyps of the sigmoid colon
- Overall, it has lobular architecture and crypts with smooth and rounded edges
- Nondysplastic or same grade of dysplasia as adenoma at the surface
- Usually surrounded by rim of lamina propria
- Absent desmoplastic stromal response
- Hemorrhage or hemosiderin deposition
- Sometimes acellular extracellular mucin associated with ruptured dilated mucinous cysts and inflammatory response may be noted; need to differentiate from an invasive mucinous carcinoma (colloid), which will have mucin pools with malignant cells, a feature lacking in pseudoinvasion
- Colonic adenomatous polyps involving submucosal lymphoglandular complexes (Am J Surg Pathol 2018;42:1083):
- Lymphoglandular complexes (LGCs) are lymphoid follicles, present in close apposition to lamina propria or muscularis mucosae or submucosa; rarely, colorectal adenomas involve submucosal lymphoglandular complexes, simulating invasive adenocarcinoma with associated extensive lymphoid response and presenting a diagnostic pitfall
- Tumor is contained within the lymphoid tissue and lack infiltrating single cells / small clusters, poorly formed, fused and irregular glands, solid tumor nests, desmoplastic reaction and lymphovascular invasion
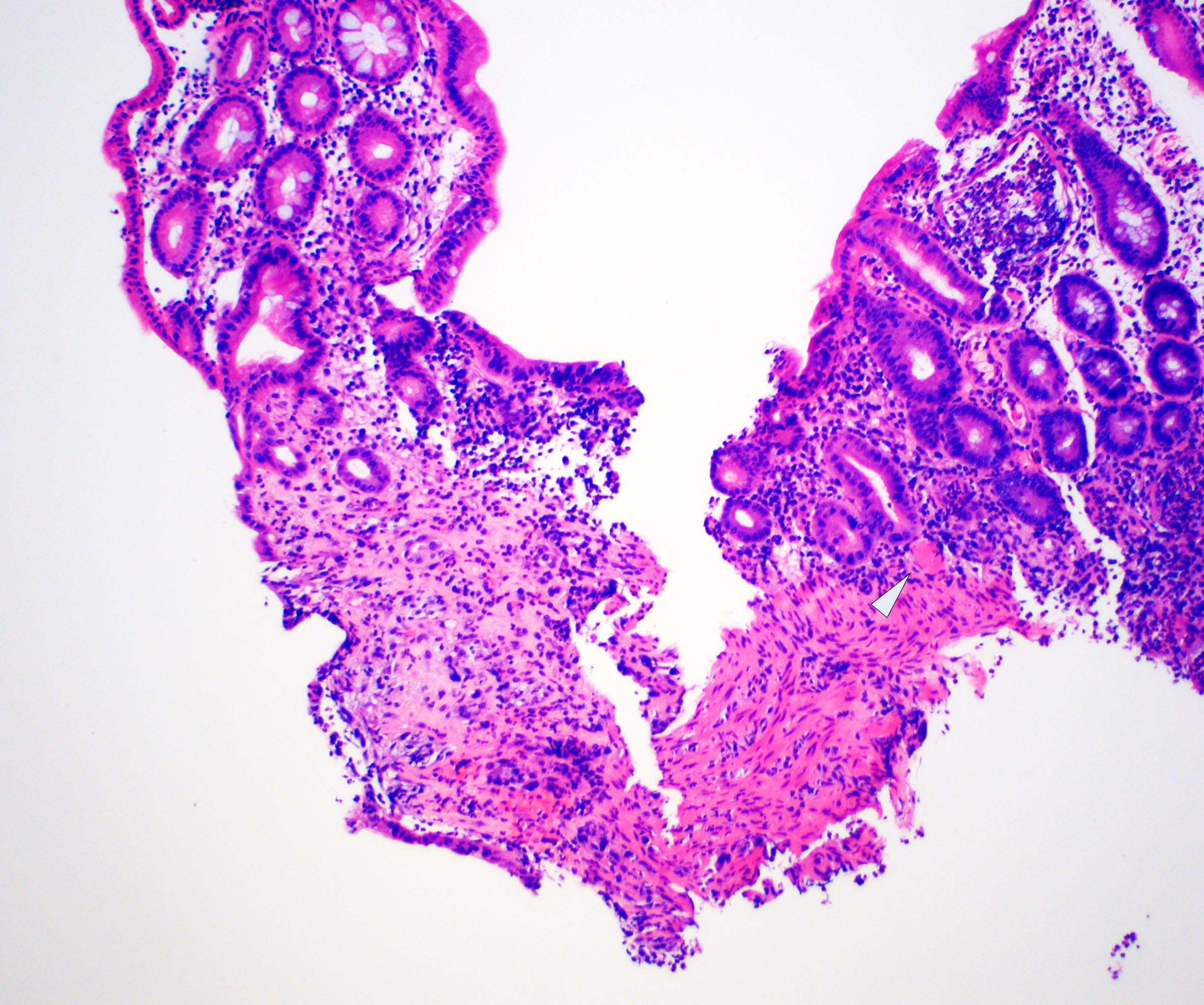
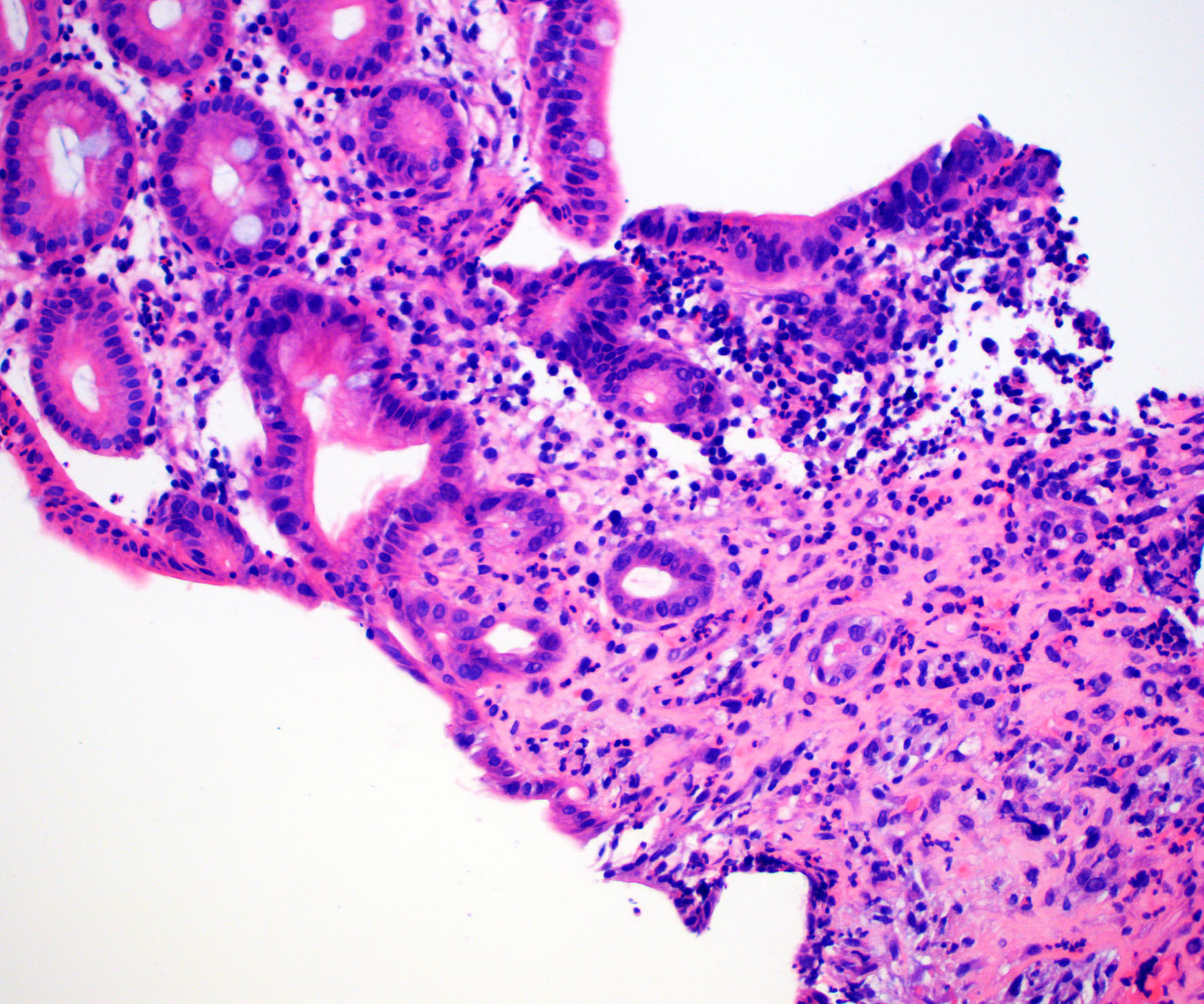
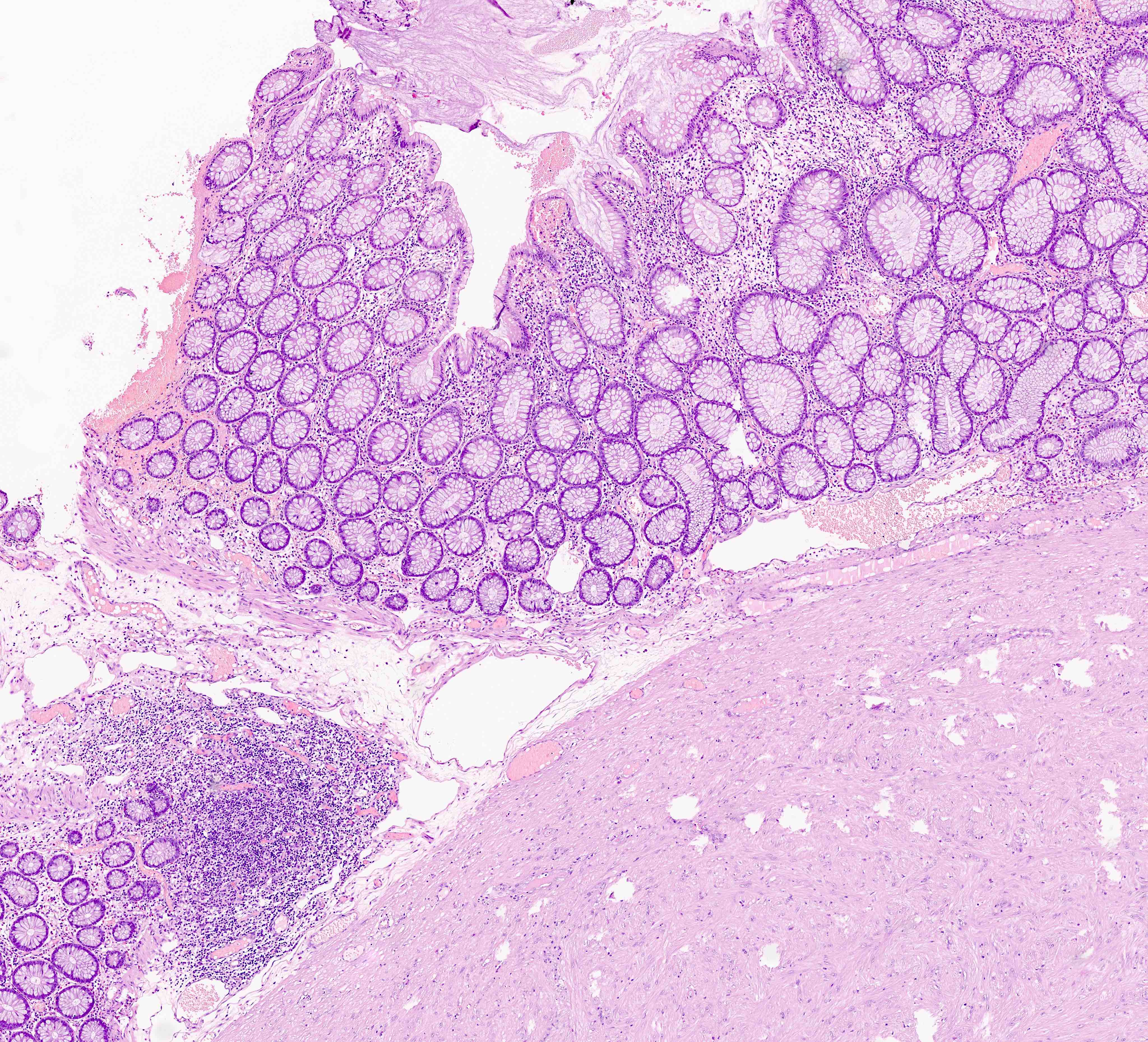
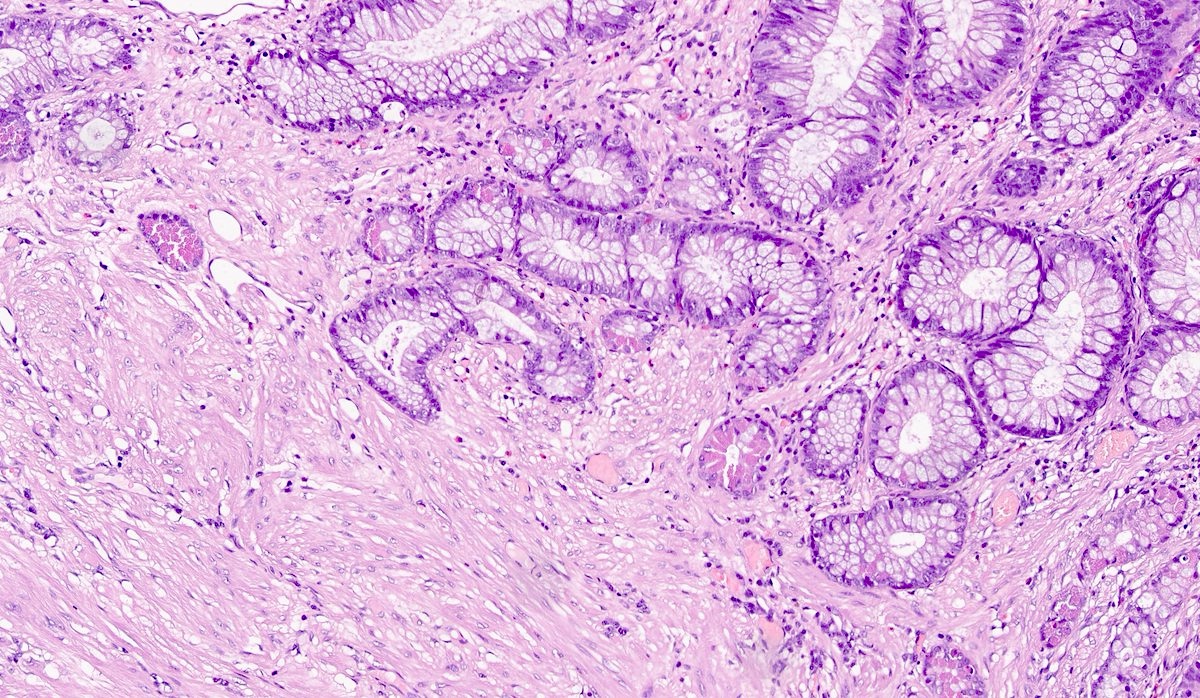
- Localized colitis cystica profunda:
- Glandular, nondysplastic epithelium in submucosa
- Overlying mucosa: usually ulcerated or hyperplastic
- Usually rectal; seen with prolapse or after irradiation
Board review style question #1
Board review style answer #1
D. Tubular adenoma with pseudoinvasion. This represents a tubular adenoma with pseudoinvasion, which shows misplacement of dysplastic glandular epithelium into submucosa mimicking a submucosal invasion. Pseudoinvasion will show associated stromal hemorrhage, hemosiderin deposition and acellular extracellular mucin with ruptured dilated mucinous cysts. Answer A is incorrect because of the additional features of pseudoinvasion. Answer B is incorrect because tubular adenomas with invasive adenocarcinoma will show true invasion of neoplastic glands beyond the muscularis mucosa into the submucosa. Answer C is incorrect because abundant expression of extracellular mucin within the tumor is not shown.
Comment Here
Reference: Adenoma with invasive carcinoma
Comment Here
Reference: Adenoma with invasive carcinoma
Board review style question #2
Which of the following are poor prognostic factors for malignant colorectal polyp?
- Large polyp size
- High grade dysplasia
- High tumor budding, lymphovascular invasion, higher histologic grade, positive margin and submucosal invasion > 1 mm
- Villous component in adenomatous polyp
Board review style answer #2
C. High tumor budding, lymphovascular invasion, higher histologic grade, positive margin and submucosal invasion > 1 mm are the adverse prognostic factors for distant metastasis. Answer A is incorrect because while a risk factor for finding an invasive carcinoma is higher (46%) in larger colorectal polyps (> 2 cm), this doesn’t have an adverse prognostic factor for distant metastasis or local recurrence. Answer D is incorrect because a risk factor for finding an invasive carcinoma is higher in colorectal polyps with a more villous component. Answer B is incorrect because while high grade dysplasia has a 35% risk of having carcinoma (versus low grade dysplasia, which has only a 6% risk), it is not a poor prognostic indicator.
Comment Here
Reference: Adenoma with invasive carcinoma
Comment Here
Reference: Adenoma with invasive carcinoma
Adenoma-like adenocarcinoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Clinical features | Diagnosis | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Well differentiated subtype of colorectal carcinoma with good prognosis that resembles villous adenoma on the surface (Anticancer Res 1998;18:2649)
- Formally recognized by the WHO
Essential features
- Very well differentiated subtype of colorectal carcinoma
- Can mimic tubulovillous adenoma on biopsy, making diagnosis challenging
- Very good prognosis, with metastasis uncommon
Terminology
- WHO uses term adenoma-like adenocarcinoma (Histopathology 2016;68:183)
- Also known as villous adenocarcinoma
Epidemiology
- Approximately 3 - 5% of colorectal carcinomas have villous / adenoma-like features
Clinical features
- Low rate of metastasis to lymph nodes and other organs
Diagnosis
- Biopsy may resemble villous adenoma, so clinical / endoscopic correlation is essential
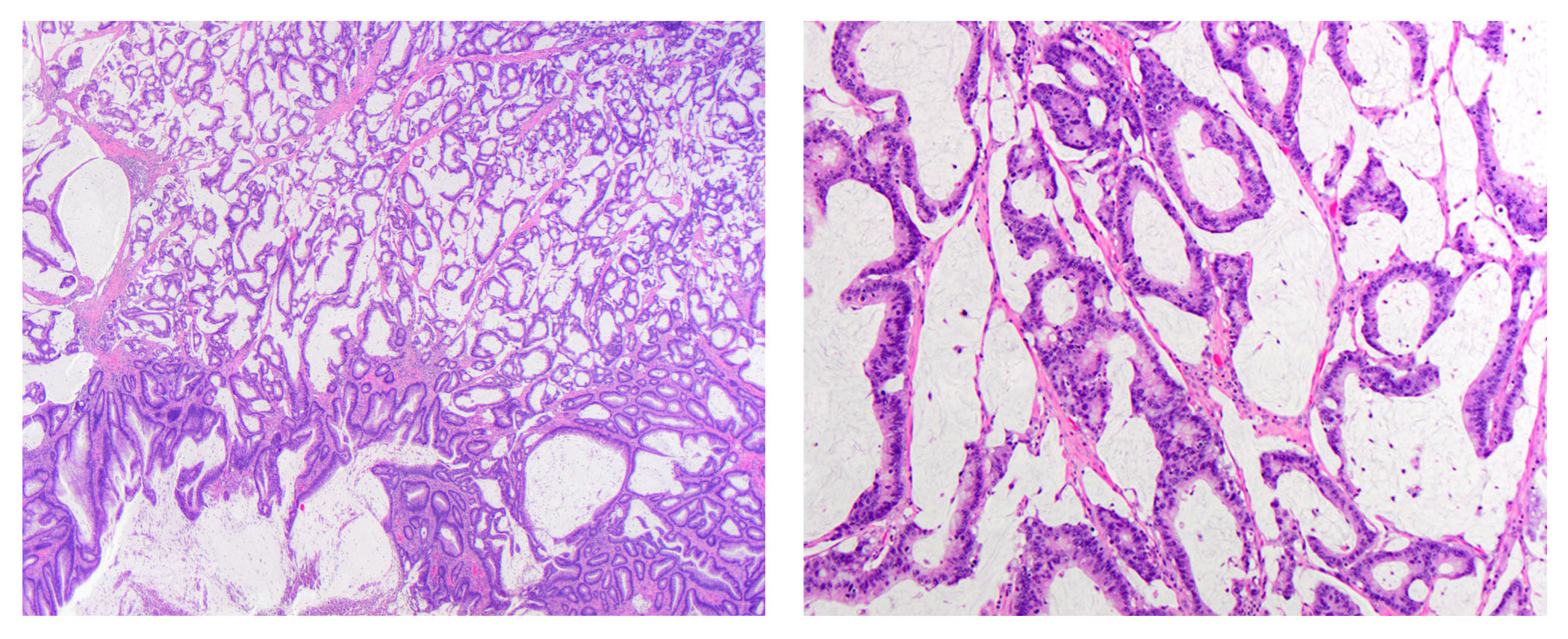
Microscopic (histologic) description
- Invasive carcinoma with architectural and cytologic features resembling villous adenoma
- May have traditional invasive component or invade by pushing
- Mucinous features often present
- Epithelial islands in desmoplastic stroma is a helpful finding (Am J Surg Pathol 2004;28:1460)
- May also have a component of conventional adenocarcinoma (Hum Pathol 2021;107:9)
Microscopic (histologic) images
Molecular / cytogenetics description
- 24% of cases have microsatellite instability
Sample pathology report
- Transverse colon, resection:
- Adenoma-like adenocarcinoma of the colon, well differentiated (see synoptic report)
Differential diagnosis
- Villous adenoma:
- Can be hard to distinguish on biopsy (subtle clues include gland distortion and desmoplasia)
Board review style question #1
Board review style answer #1
Board review style question #2
Which of the following is true about adenoma-like adenocarcinoma of the colon?
- It always demonstrates microsatellite instability
- It can be difficult to diagnose on biopsy
- It has a high rate of lymph node and liver metastasis
- It is always pure, without any component of conventional adenocarcinoma
Board review style answer #2
Adenosquamous carcinoma
Table of Contents
Definition / general | Essential features | Sites | Pathophysiology | Clinical features | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Rare (0.1% of colonic carcinomas) WHO recognized epithelial malignancy of colon with glandular and squamous elements (Am J Surg Pathol 1978;2:47)
Essential features
- Rare primary subtype of colorectal carcinoma
- Has higher metastatic rate and worse prognosis than conventional colorectal adenocarcinoma (Dis Colon Rectum 2012;55:509, Dis Colon Rectum 1999;42:258)
Sites
- Typically arises in right colon (Dis Colon Rectum 2001;44:341)
Pathophysiology
- HPV infection does not appear to play a role (Surg Today 2009;39:619, Eur J Surg Oncol 2002;28:657)
Clinical features
- Tumor stage is often advanced at presentation (Dis Colon Rectum 1996;39:1265)
- May cause paraneoplastic hypercalcemia (Int J Clin Oncol 2005;10:144, Am Surg 2001;67:585)
- Can occur in patients with ulcerative colitis (Dis Colon Rectum 1988;31:323)
Case reports
- 71 year old man with adenosquamous carcinoma of sigmoid colon (Int J Clin Exp Med 2013;6:390)
- 78 year old woman with associated paraneoplastic hypercalcemia (Int J Clin Oncol 2005;10:144)
- Adenosquamous carcinoma of colon (Am Surg 2006;72:754)
- Adenosquamous carcinoma of colorectum (J Exp Clin Cancer Res 2001;20:293)
Treatment
- Surgery with adjuvant treatment
Clinical images
Microscopic (histologic) description
- Resembles conventional colorectal adenocarcinoma but with areas of squamous differentiation, either admixed or distinct
Microscopic (histologic) images
Positive stains
Sample pathology report
- Ascending colon, biopsy:
- Adenosquamous carcinoma (see comment)
- Comment: The malignancy demonstrates both glandular elements and squamous elements. The former is positive for CDX2 by immunohistochemistry and the latter is positive for p40 by immunohistochemistry.
Differential diagnosis
- Squamous cell carcinoma:
- Also rare
- No glandular elements
Additional references
Board review style question #1
Board review style answer #1
Adenovirus
Table of Contents
Definition / general | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy images | Additional referencesDefinition / general
- Disease by adenovirus is most common in the upper respiratory tract, but also causes pneumonia and conjunctivitis, and may affect the genitourinary tract, liver, central nervous system or other sites
- Adenovirus is a common cause of pediatric diarrhea, although at least half of infections are subclinical
- The colon may be involved as part of systemic infection
- Most cases are community acquired but nosocomial outbreaks occur
- Infection is associated with immunodeficiency including HIV infection, solid organ and hematopoietic stem cell transplantation, congenital immunodeficiency (Hum Pathol 2010;41:1777)
- There are over 50 serotypes with seven species that affect humans
- In intestinal disease, serotypes 31, 40, and 41 are mostly reported in infants; 2, 3, and 5 mostly in children
- It is not well understood why specific serotypes are associated with specific syndromes; however, differences in mode of transmission and viral tropism likely play a role
Pathophysiology
- Adenovirus is ubiquitous; transmission is by respiratory droplets, fomites, fecal-oral route
- It is a hardy virus and prolonged survival in the environment is possible
- Adenovirus is a nonenveloped, lytic double stranded DNA virus (CDC - Adenoviruses)
- Adenovirus enters the cytoplasm after binding to a receptor and then is transported to the nucleus where replication occurs
- Cell rupture leads to dispersion of viral particles, cytokine production and an inflammatory response
- Chronic or latent infection, usually involving lymphoid tissue, may occur
- Virus may be shed for months to years after infection
Clinical features
- With only very rare exceptions, diarrheal disease is mild and self-limited in immunocompetent individuals
- Infants may develop watery diarrhea, fever and vomiting that lasts 8-12 days (Gastroenterol Clin North Am 2001;30:779)
- Subclinical disease is frequent
- Significant morbidity or mortality can occur in immunocompromised patients, with symptoms of fever, weight loss, abdominal pain
- Other infections, especially CMV, may coexist with adenovirus infection in immunocompromised patients
- Nosocomial outbreaks may occur; appropriate hand hygiene and isolation procedures effectively prevent this
- Infection may cause ileal or cecal intussusception in children
Diagnosis
- Characteristic inclusions in biopsy material are highly consistent with infection; immunohistochemical stains provide confirmation
- PCR, viral culture and stool electron microscopy may be used for diagnosis
- Serologic studies may also be obtained
Prognostic factors
- In immunocompromised patients, high viral load by PCR is associated with a poorer prognosis
Case reports
- Infant with necrotizing enterocolitis associated with adenovirus infection (J Pediatr Surg 2008;43:e5)
- 3 year and 17 year old boys with adenovirus enterocolitis following bone marrow transplantation (Arch Pathol Lab Med 2003;127:1615)
Treatment
- Generally only supportive care
- Cidofovir has been used in immunocompromised patients, but significant toxicity may occur (Biol Blood Marrow Transplant 2007;13:74)
- Immune reconstitution, if possible, is usually curative
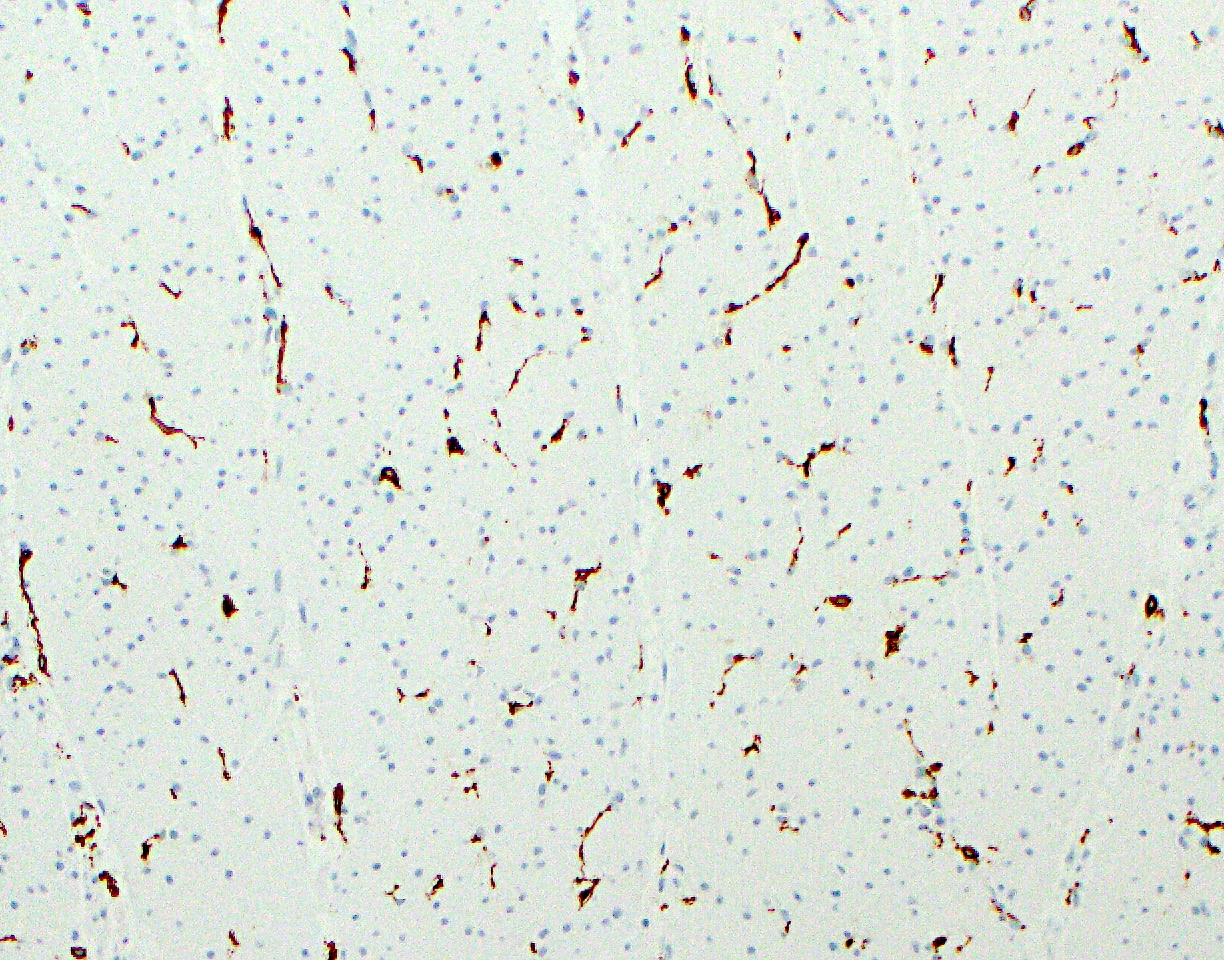
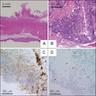
Microscopic (histologic) description
- Surface epithelial cells, especially goblet cells, are infected
- Cowdry type B nuclear inclusions with enlarged, homogeneous, smudgy basophilic nuclei (smudge cells) are more common than Cowdry type A inclusions, which are eosinophilic to amphophilic with nuclear halos
- Usually present are necrotic cells, apoptotic bodies and cellular debris with a mononuclear cell infiltrate and generally mild architectural distortion (Histopathology 2015;66:467)
Microscopic (histologic) images
Positive stains
- Adenovirus immunostain
Electron microscopy images
Additional references
Adhesions
Table of Contents
Definition / general | Essential features | Sites | Etiology | Clinical features | Diagnosis | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Board review style question #1 | Board review style answer #1Definition / general
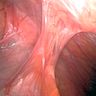
- Serosa based fibrous bands of scar tissue that cause colon to connect and adhere to nearby structures, typically other viscera
Essential features
- Fibrovascular scar tissue of the colonic serosa, usually secondary to injury or prior surgery
- Can distort anatomy and lead to complications such as obstruction, herniation or ischemia
Sites
- Can occur anywhere in GI tract, commonly between bowel segments or abdominal wall and operative site
Etiology
- Typically due to injury, such as prior surgical procedures, infection (i.e. peritonitis) or radiation damage
- Also Crohn's disease or serosal endometriosis
- Rarely congenital
Clinical features
- Common cause of abdominal obstruction (Colorectal Dis 2007;9:39)
- Cause hospital admissions in up to 33% within 10 years of colorectal surgery (Dis Colon Rectum 2001;44:822)
- Can complicate hospital stay after colorectal surgery (Am J Surg 2013;206:166)
- May create internal herniations (closed loops through which viscera slide)
- May result in segmental intestinal ischemia, often due to arterial occlusion
- Can necessitate conversion from laparoscopic to open surgery (Surg Technol Int 2011;21:147)
- Rarely cause colonic obstruction (Am Surg 1984;50:479)
Diagnosis
- Typically observed radiologically or during surgery; can also be seen microscopically
Treatment
- When severely symptomatic, consider partial or total colectomy (Int J Colorectal Dis 2013;28:1407)
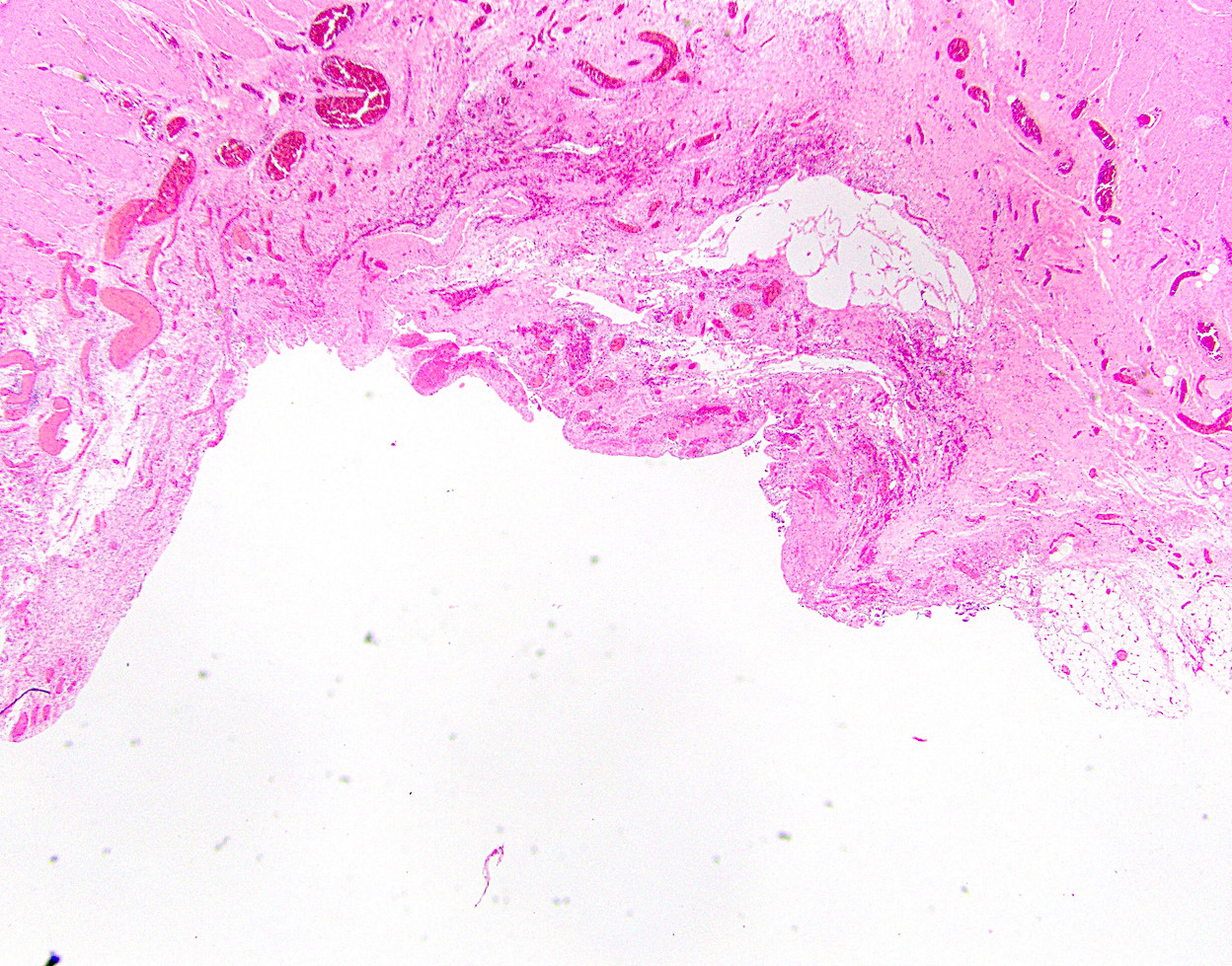
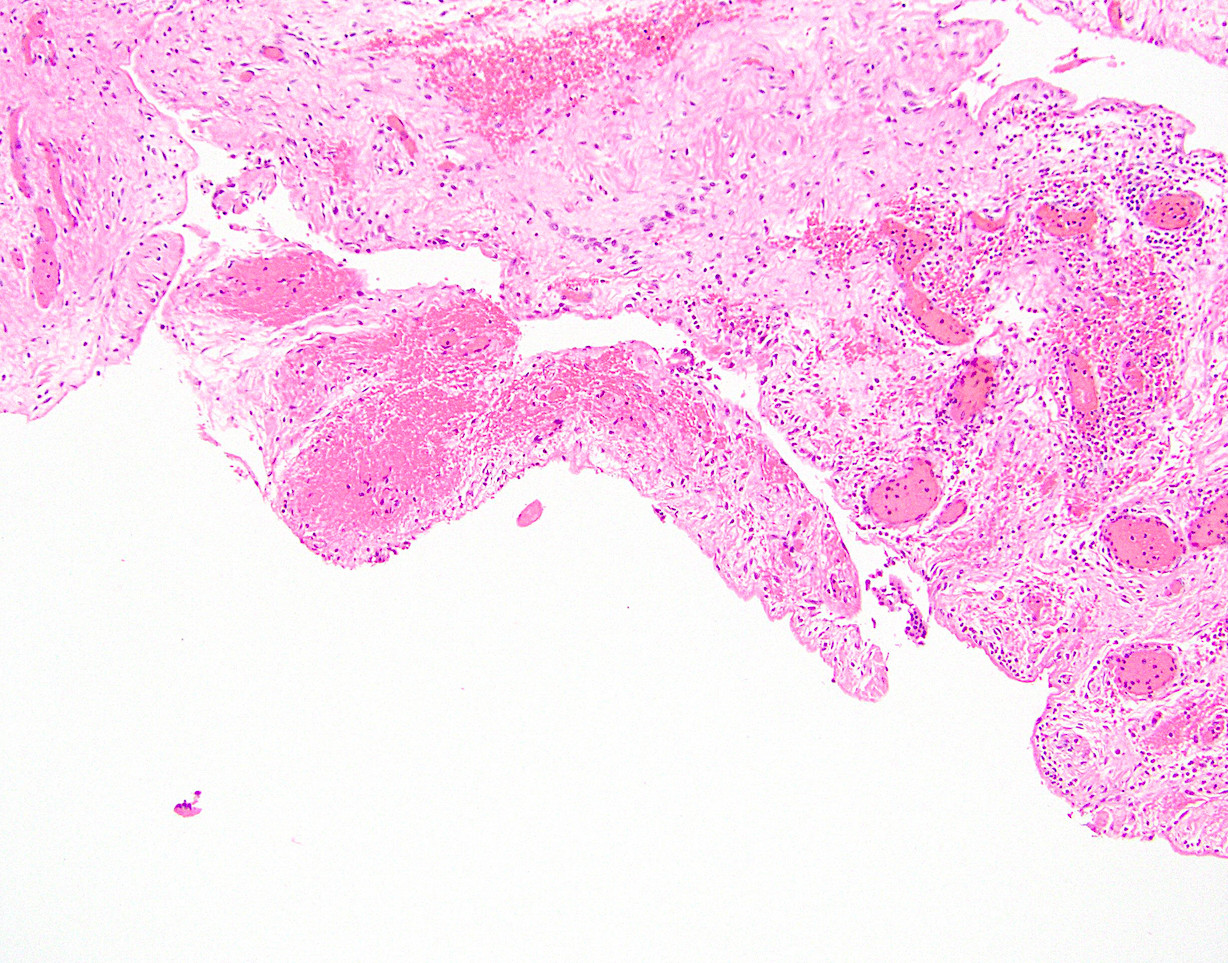
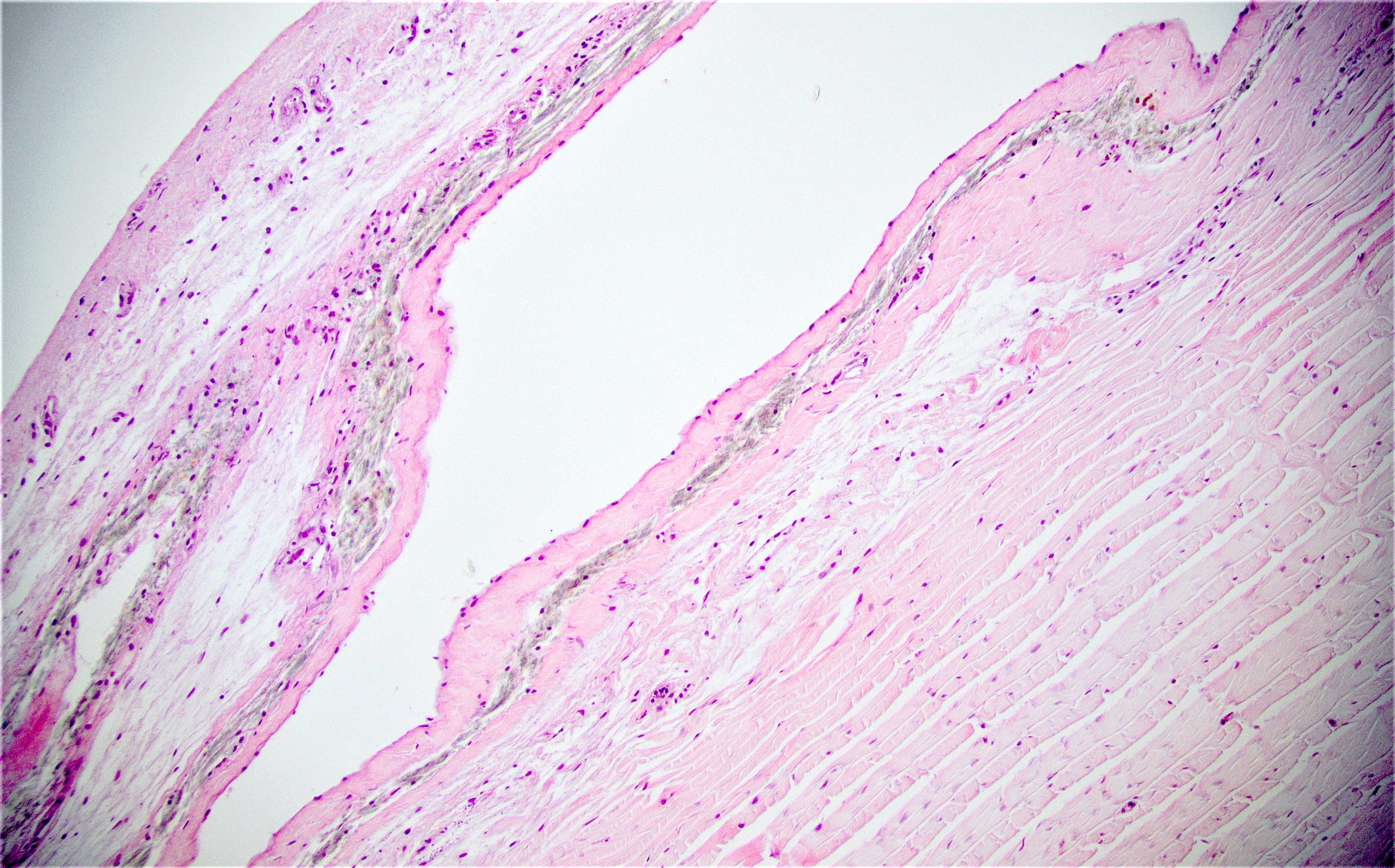
Microscopic (histologic) description
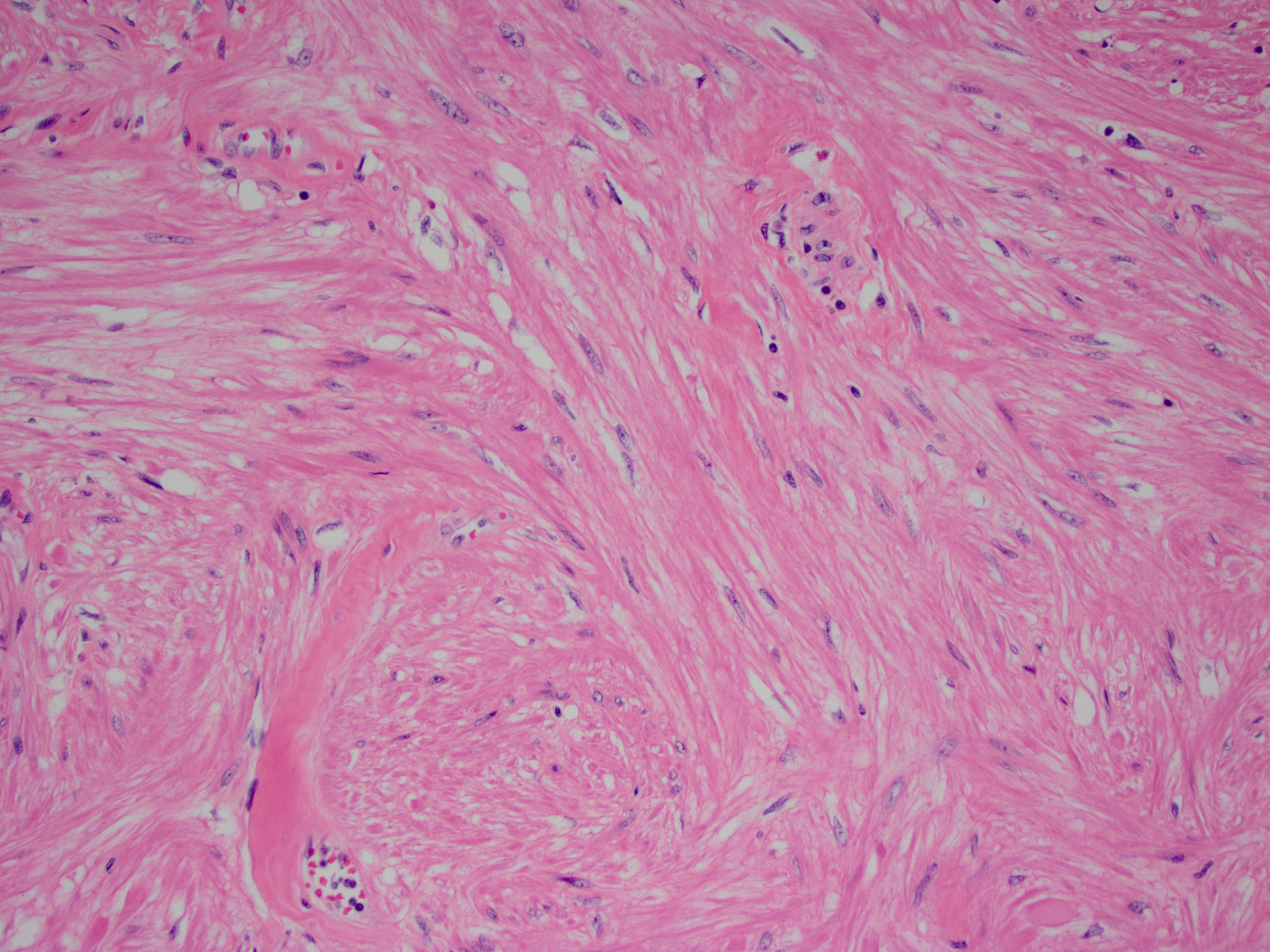
- Cellular fibrous connective tissue containing vessels and nerves; may contain fat and smooth muscle clusters (J Pathol 2000;192:67)
Microscopic (histologic) images
Sample pathology report
- Ascending colon, resection:
- Segment of colon with reactive change and prominent serosal adhesions
- Margins of resection unremarkable.
- Four benign lymph nodes.
Board review style question #1
Board review style answer #1
Allergic colitis
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Allergic proctocolitis: benign disorder of blood streaked stools in otherwise healthy-appearing infants
- Occurs in infants and children related to food, particularly cow's milk in infants and soy or eggs in older children
- Both IgE and non-IgE related mechanisms (Allergol Immunopathol (Madr) 2009;37:36)
- Some clinical cases of food allergy in young children are eventually diagnosed as inflammatory bowel disease (Med Wieku Rozwoj 2006;10:475)
- Italian study of allergic proctocolitis: atopy patch tests positive in 100% infants, multiple positivity in 50%; sensitization for breast milk in 100%, cow's milk in 50%; also soy (28%), egg (21%), rice (14%), wheat (7%) (BMC Gastroenterol 2011;11:82)
Clinical features
- Symptoms: rectal bleeding (although nonspecific, J Pediatr Gastroenterol Nutr 2005;41:16), variable diarrhea; may have peripheral eosinophilia
Case reports
- Premature infant with rectal bleeding after first formula feeding (Acta Paediatr 2005;94:1514)
Treatment
- Removal of cow's milk from infant or (if breast fed) mother’s diet
- Elemental diet based on amino acid formula typically resolves symptoms in 2-3 days, GI eosinophilic inflammation within 6 weeks (Pediatr Allergy Immunol 2007;18:360)
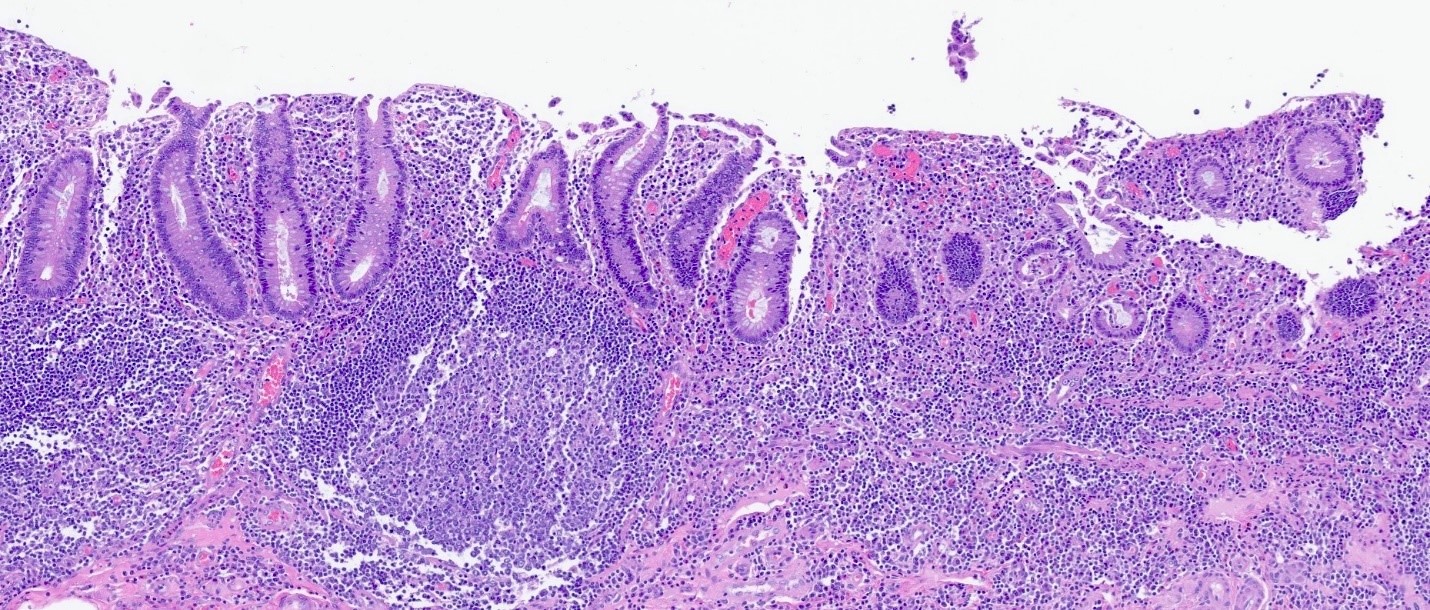
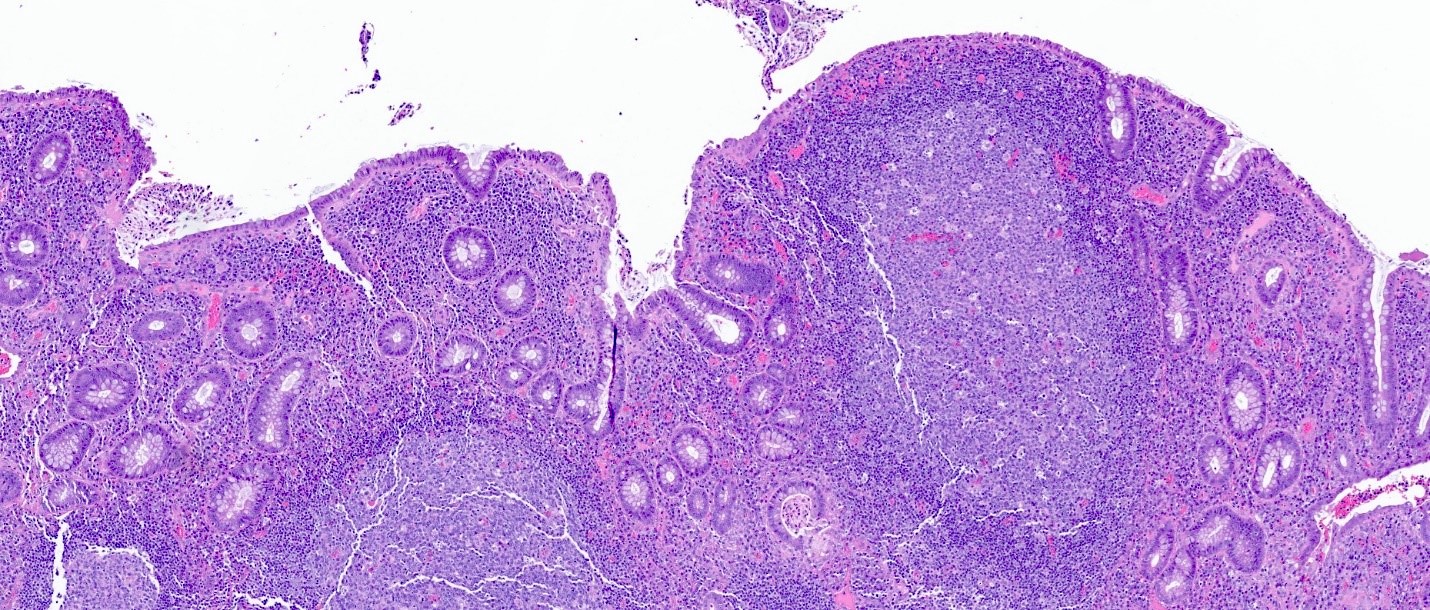
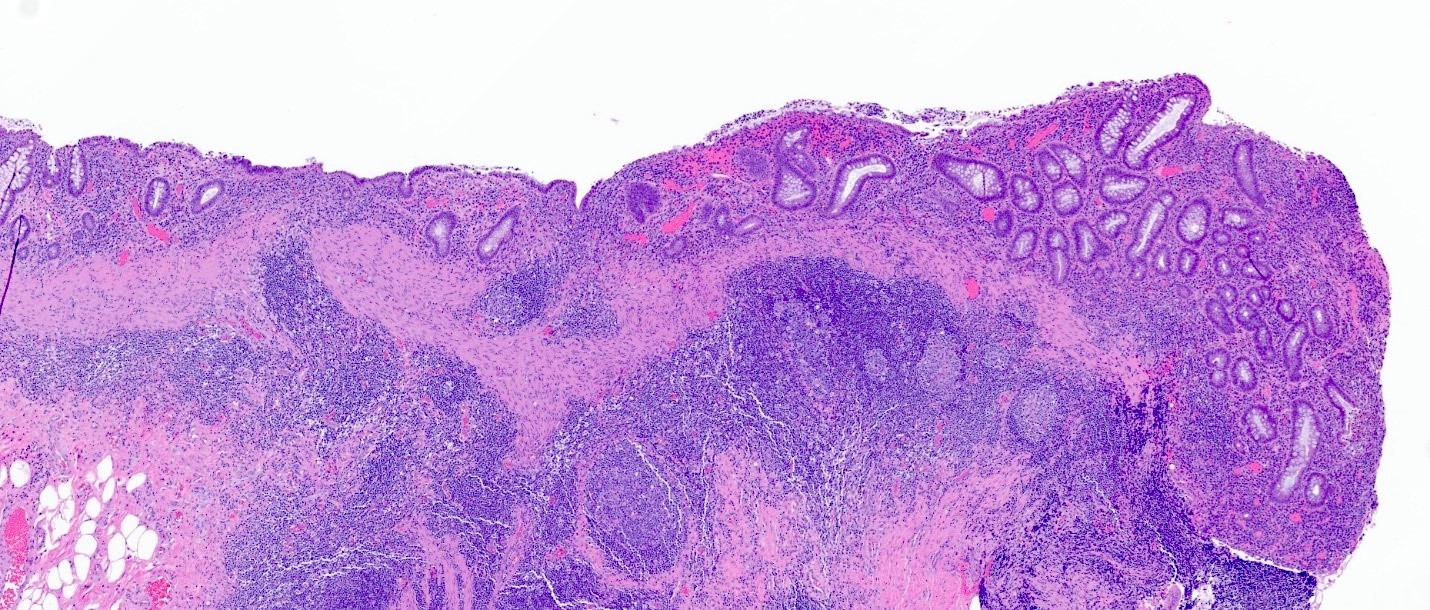
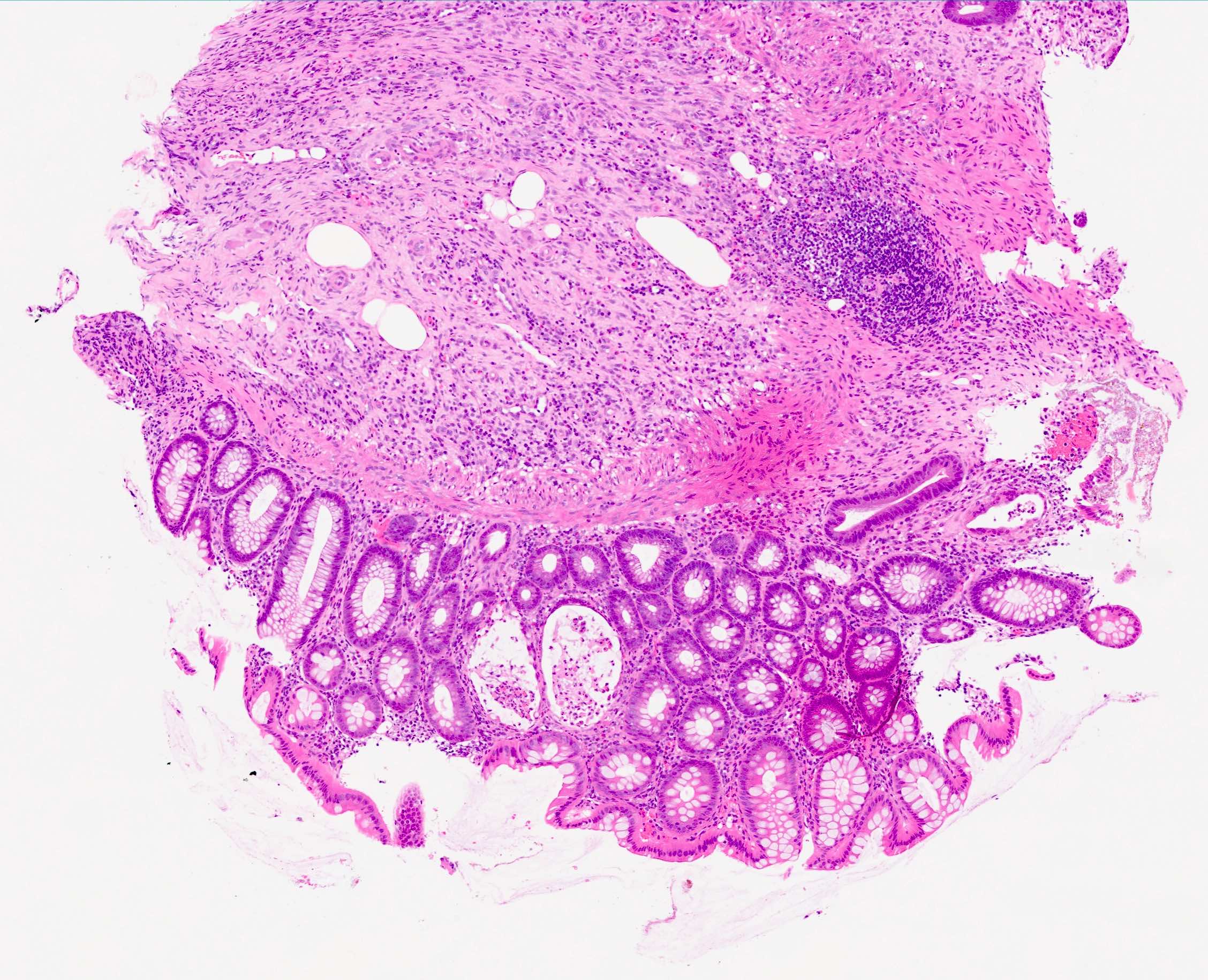
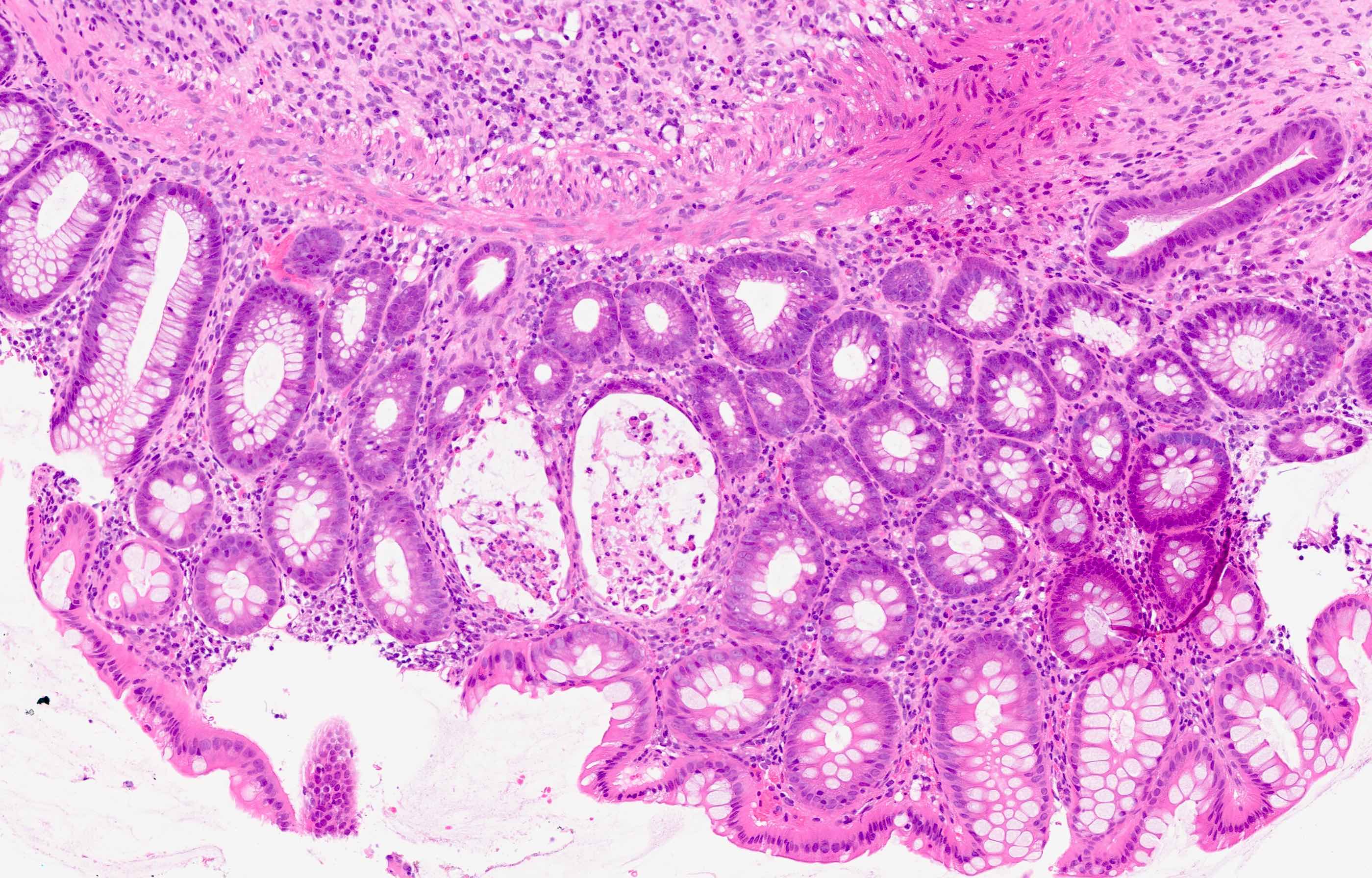
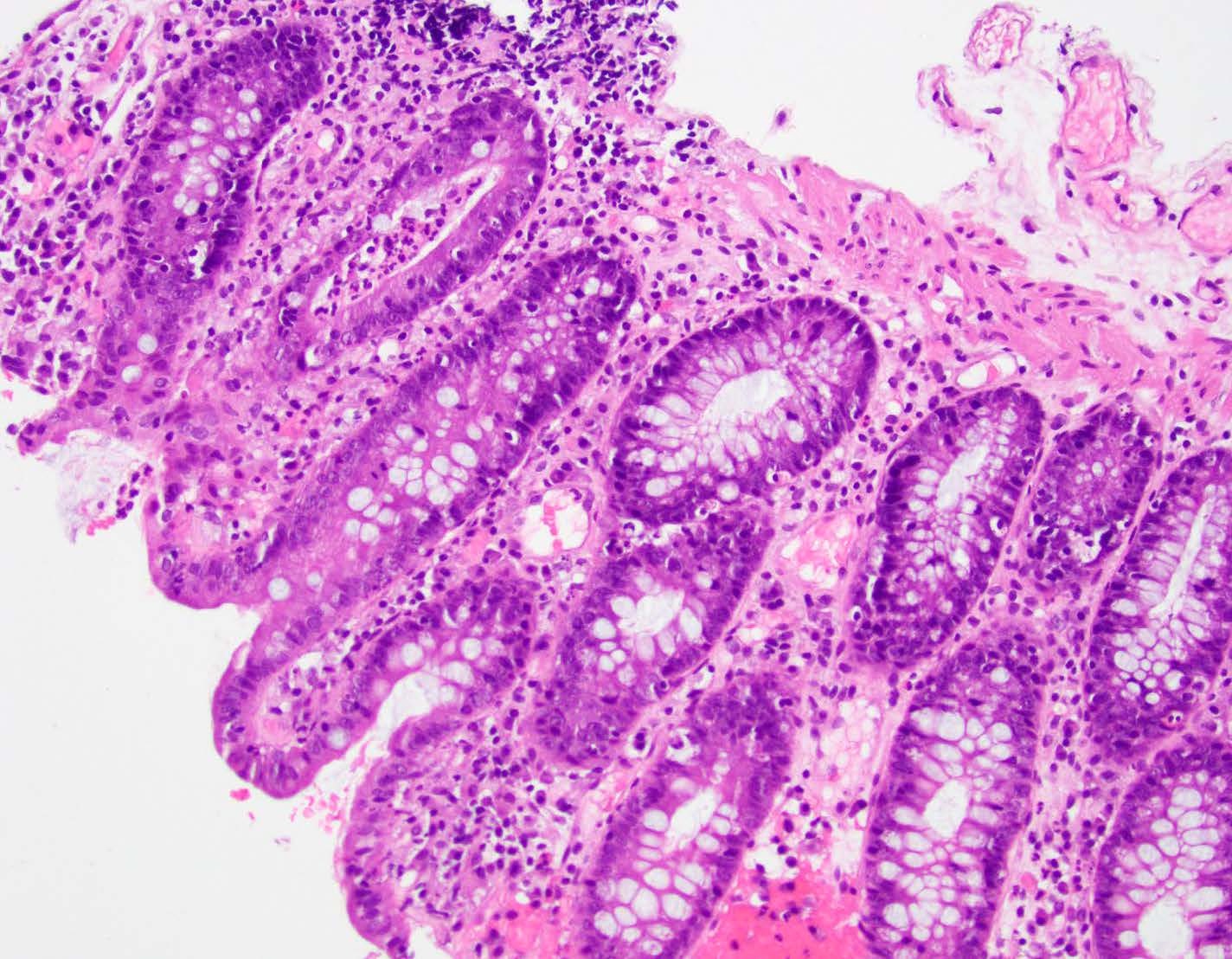
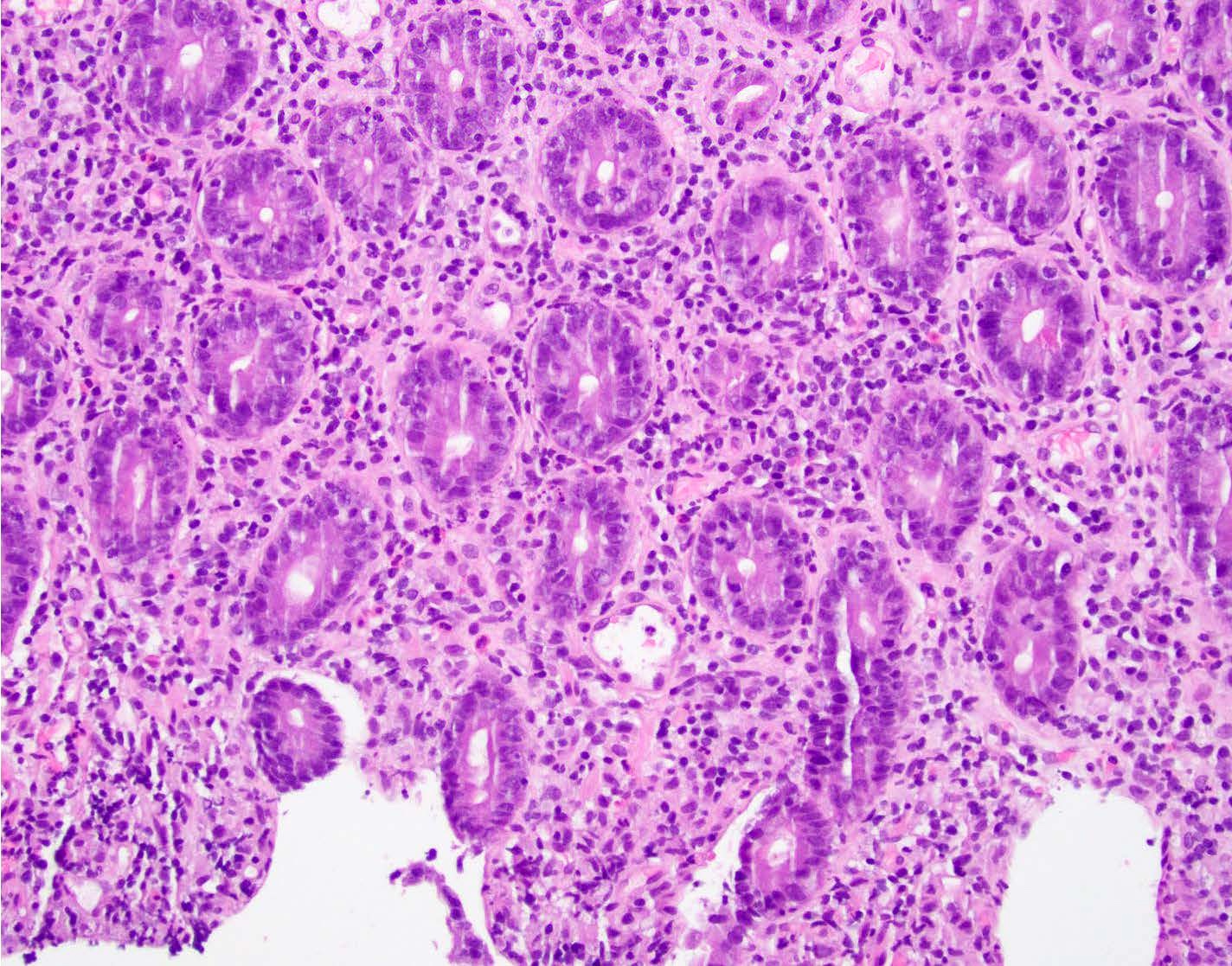
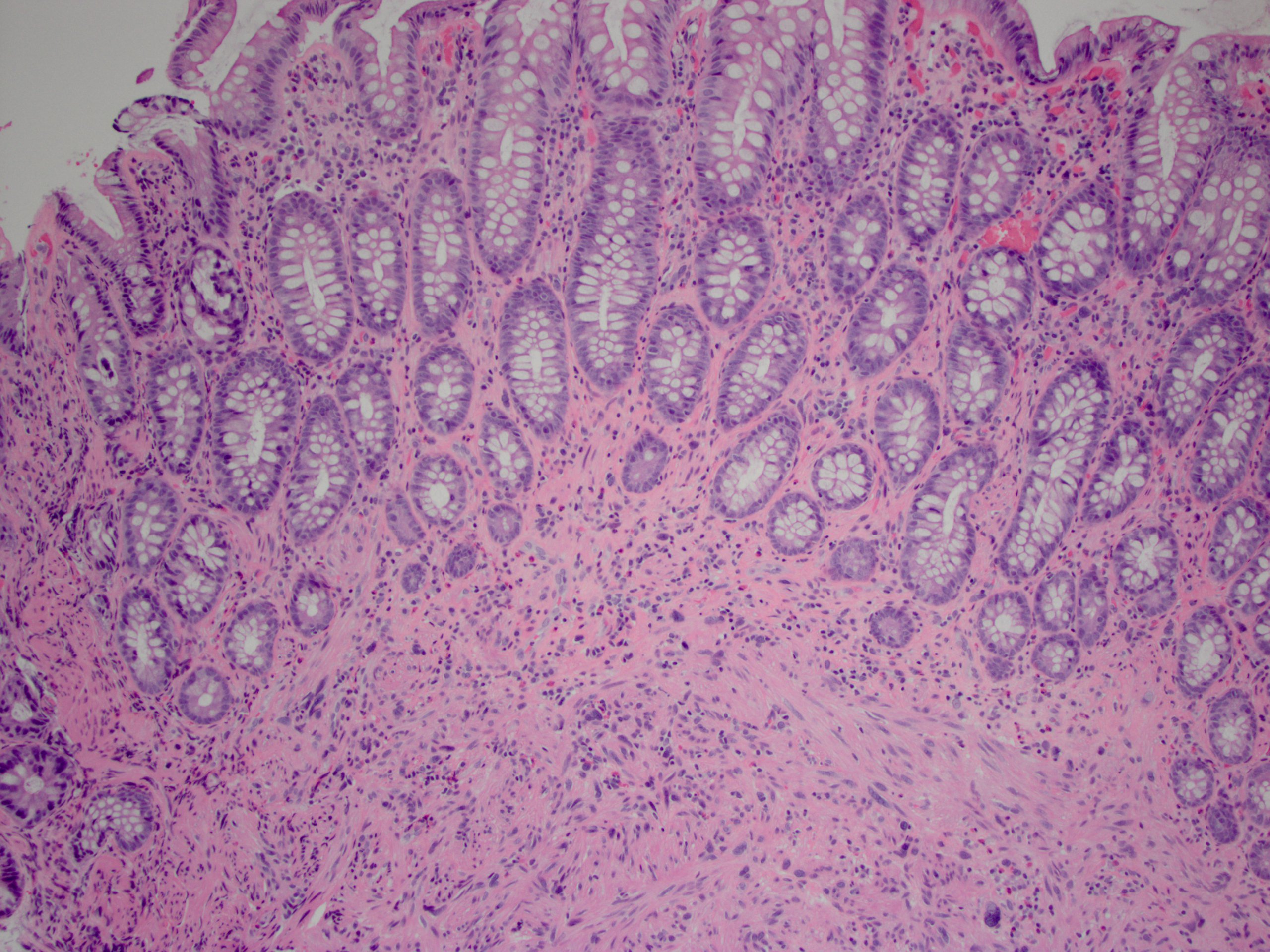
Microscopic (histologic) description
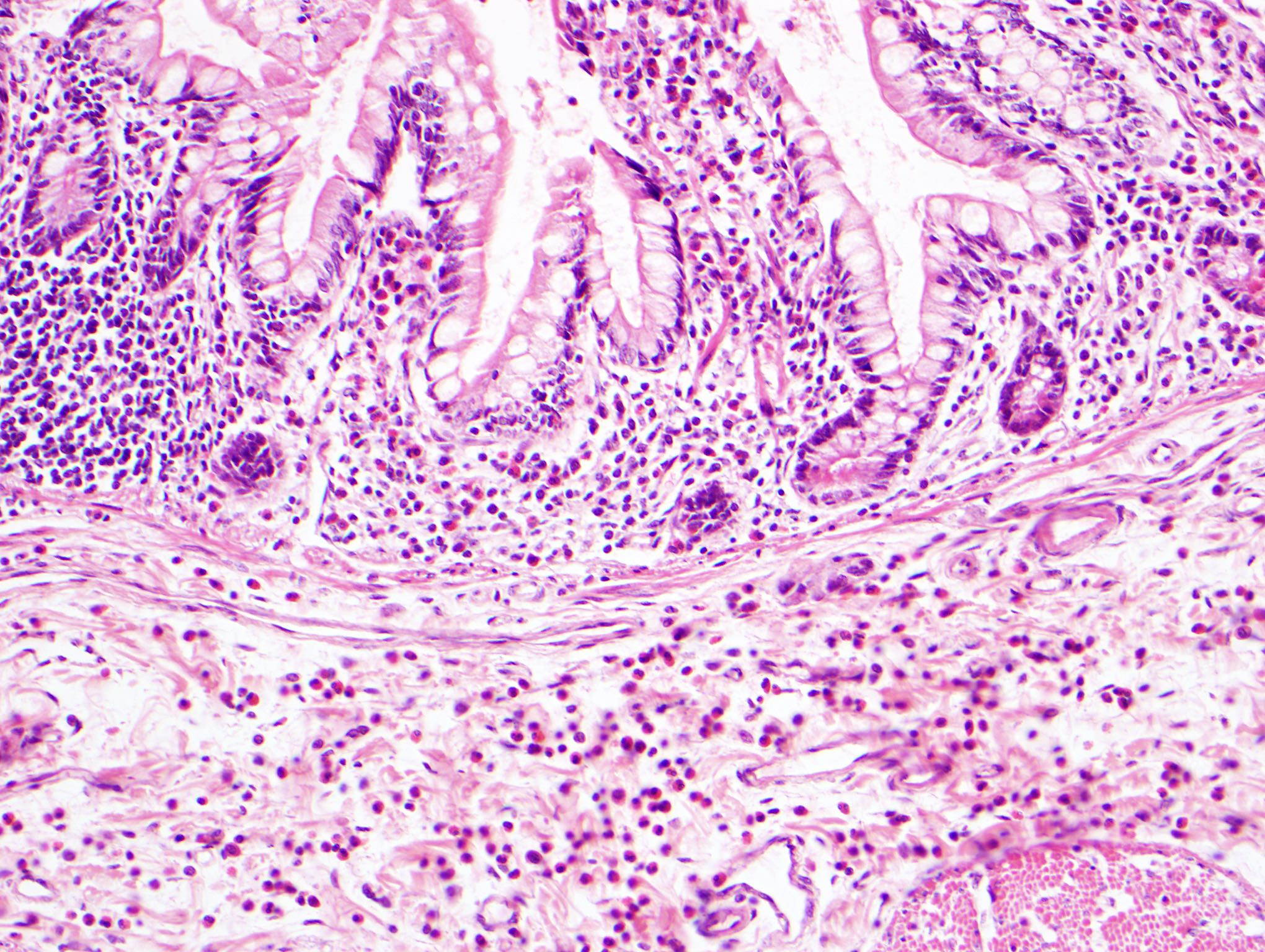
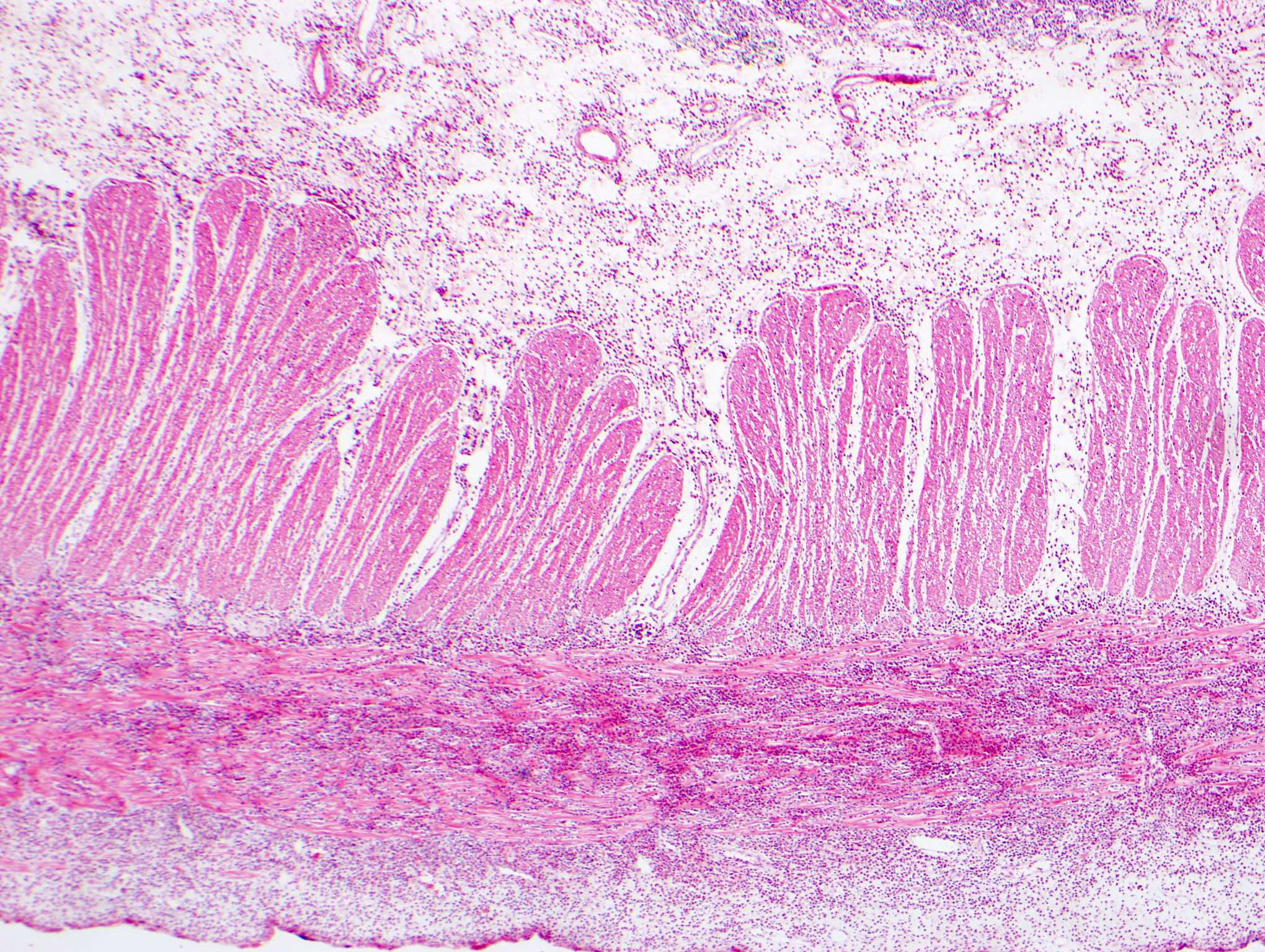
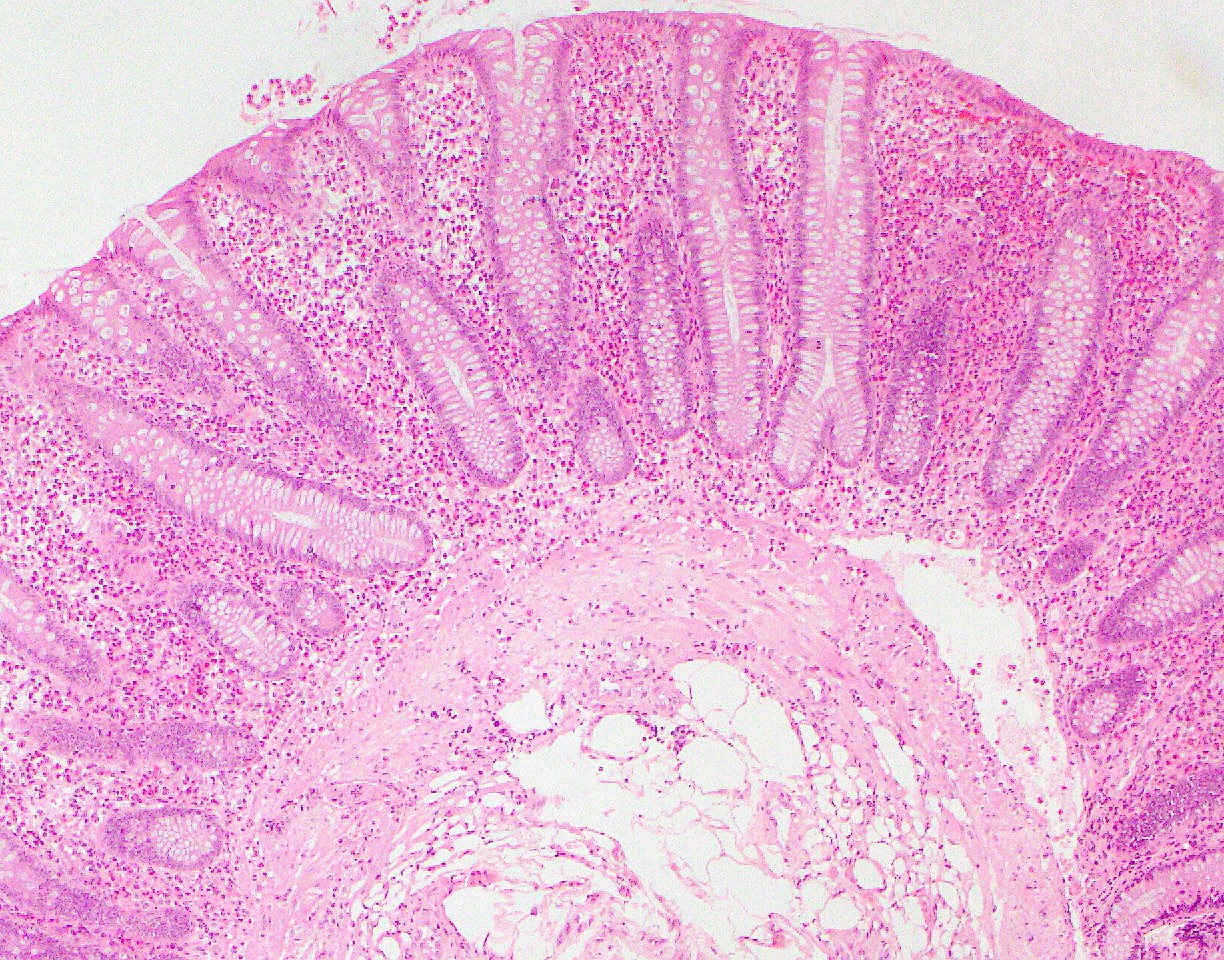
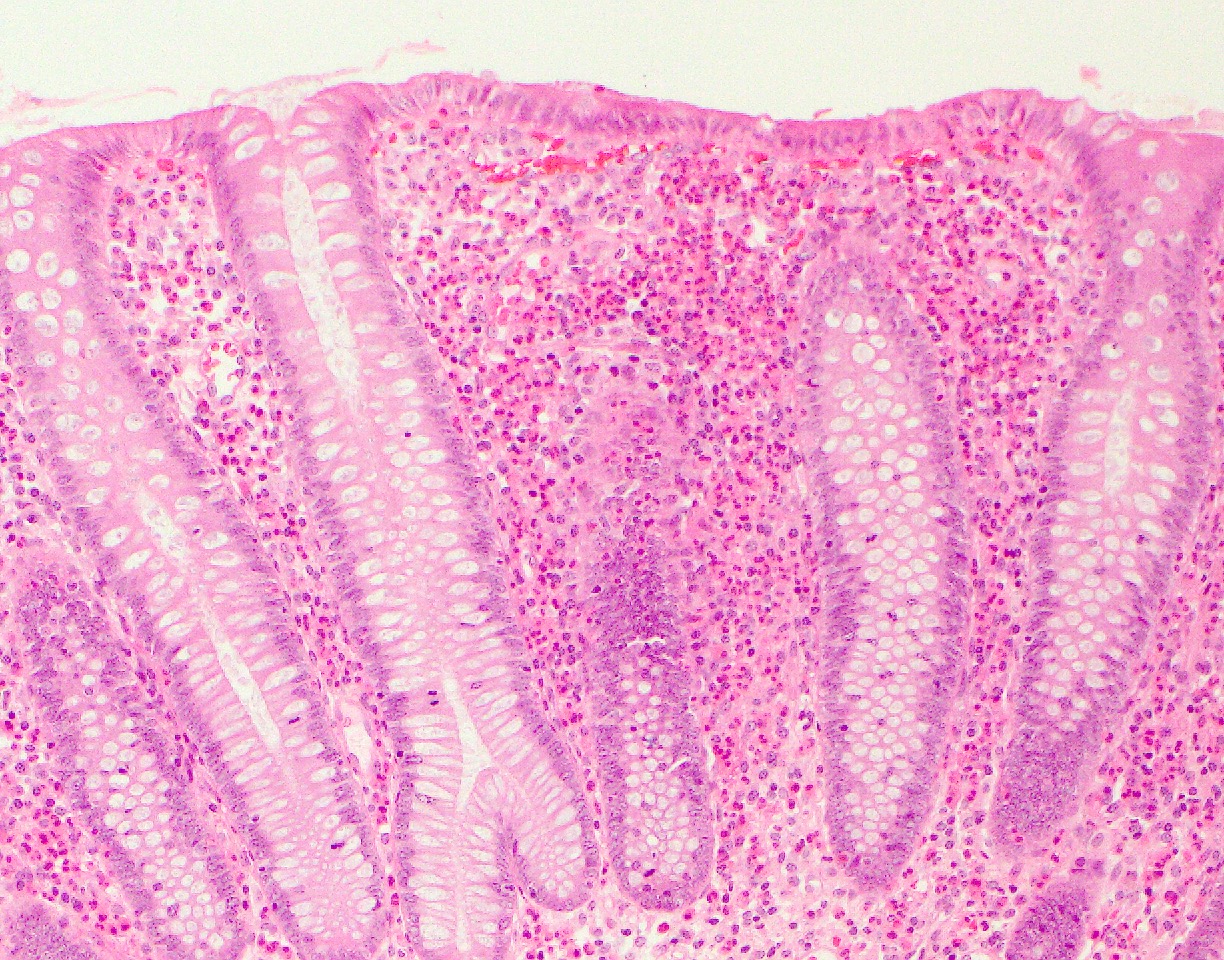
- Rectal biopsy shows mucosal edema, prominent eosinophils (>60 per 10 HPF) aggregating around lymphoid nodules, in crypt abscesses and around muscularis mucosa; may have mild focal active colitis (J Pediatr Gastroenterol Nutr 1994;19:22)
- May have granulomatous features (Histopathology 2009;55:758)
Differential diagnosis
- Dientamoeba fragilis infestation (J Pediatr Gastroenterol Nutr 1998;26:16)
- Eosinophilic colitis: different clinical history (Adv Anat Pathol 2011;18:335)
Amebic colitis
Table of Contents
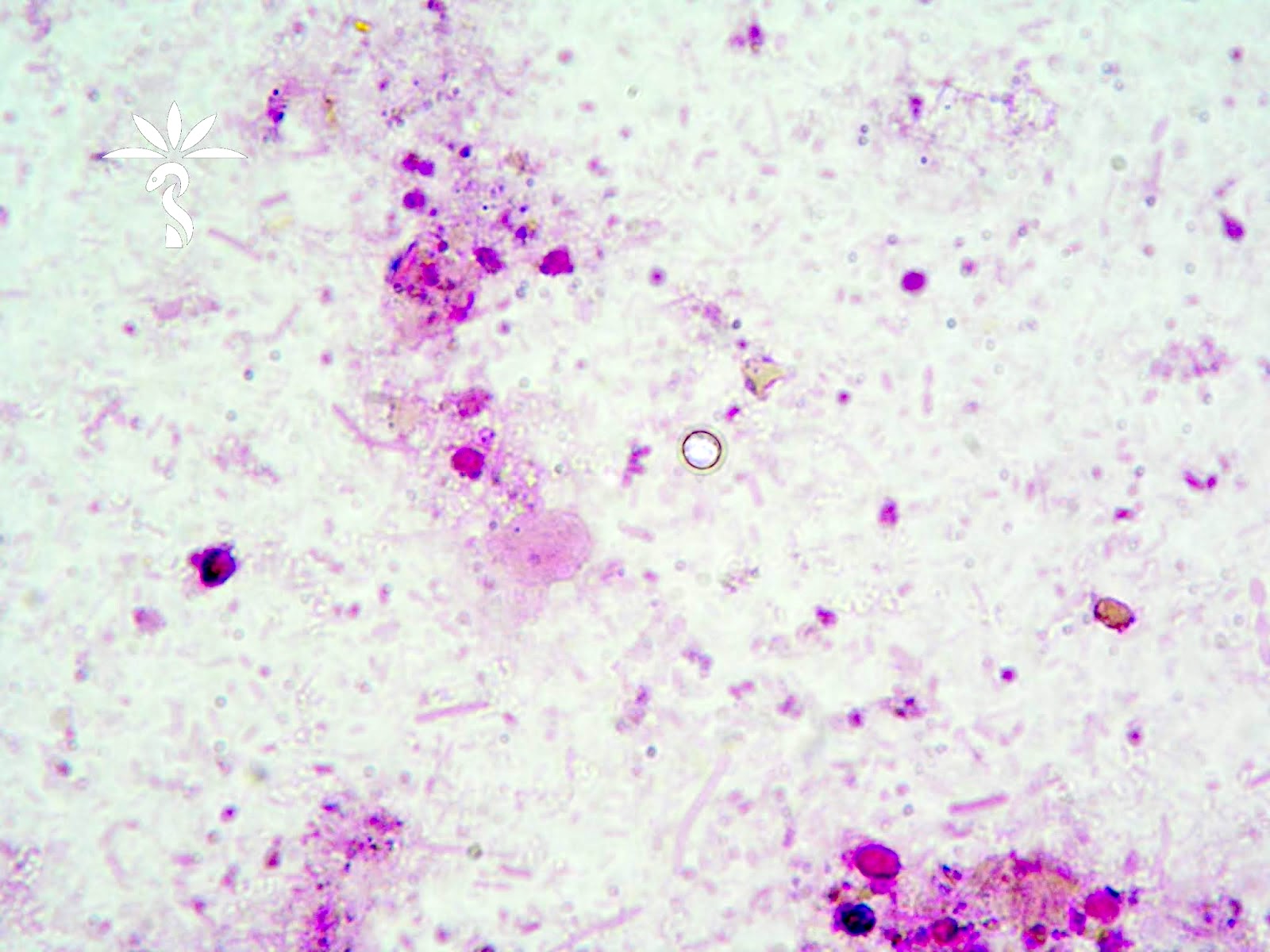
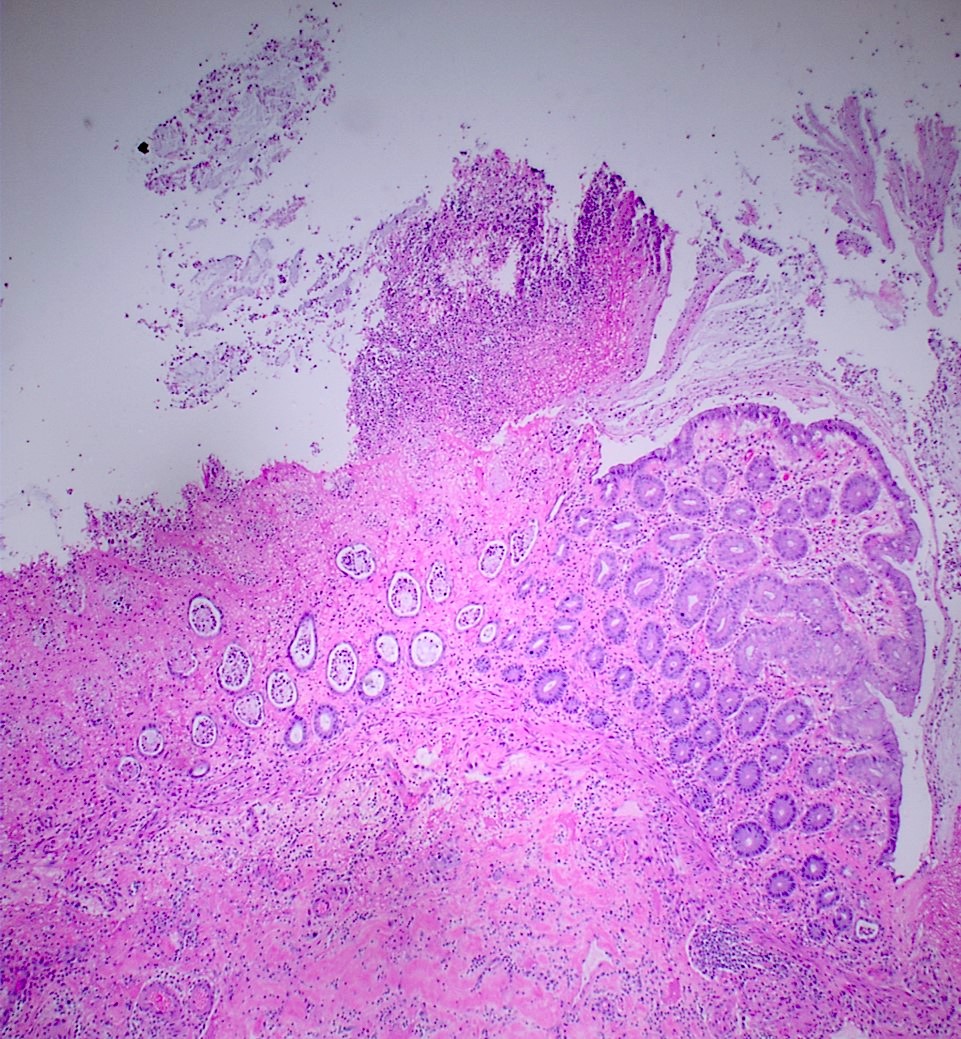
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Disease caused by infection of the large intestine with the protozoan parasite, Entamoeba histolytica
- Several protozoan species in the genus Entamoeba colonize humans but not all of them are associated with amebiasis (Centers for Disease Control and Prevention: Amebiasis [Accessed 13 October 2023])
Essential features
- Due to invasive infection with Entamoeba histolytica
- Transmission primarily by ingestion of E. histolytica cysts in fecally contaminated food or water (fecal - oral); also by sexual contact (oral - anal)
- Associated with fever, abdominal pain, tenesmus, diarrhea (with or without blood), dysentery
- Amebae may disseminate to the liver and other organs (see Entamoeba histolytica abscess)
- See flask shaped ulcers; rarely inflammatory mass (ameboma), perforation
- Amebic trophozoites invade the submucosa, undermining the overlying mucosa; no amebic cysts in tissue
- Trophozoites with pseudopod projections, ingested RBCs in cytoplasm, small round nucleus with dot-like karyosome and peripheral rim of condensed chromatin
- Trophozoites are CD68 negative, strongly PAS positive
- Treat invasive disease with metronidazole or tinidazole; also use paromomycin to eradicate luminal cysts
Terminology
- Amebiasis, amoebiasis
ICD coding
- ICD-10:
- ICD-11:
Epidemiology
- Occurs worldwide but more common in tropical and subtropical regions (Open Forum Infect Dis 2018;5:ofy161)
- In developed countries, affected patients are usually immigrants, travelers, men who have sex with men (MSM) or institutionalized individuals
- Microscopy based detection methods overestimate the prevalence of E. histolytica due to the presence of the morphologically identical amebae, E. dispar, E. moshkovskii, E. bangladeshi and E. nuttalli (CDC: Amebiasis [Accessed 22 April 2022])
- 1 of the top 15 causes of diarrhea in children; diarrhea is a leading cause of death in children < 5 years of age (Lancet Infect Dis 2017;17:909)
- Millions of cases are estimated to occur worldwide each year; estimated 67,900 deaths in 2015 with 15,500 deaths in children < 5 years of age (Lancet 2016;388:1459)
- < 5 deaths per year reported in the United States from 1990 - 2007 (Am J Trop Med Hyg 2011;85:1038)
Sites
- Colon; cecum is the most common, followed by right colon, rectum, sigmoid and appendix (BMC Gastroenterol 2021;21:367)
- Terminal ileum is usually spared
- Skip lesions are common
- Hematogenous dissemination may occur, usually to the liver (see Entamoeba histolytica abscess); also lungs, brain
- Rectovesical fistula and fistulous involvement of the skin may also occur
Pathophysiology
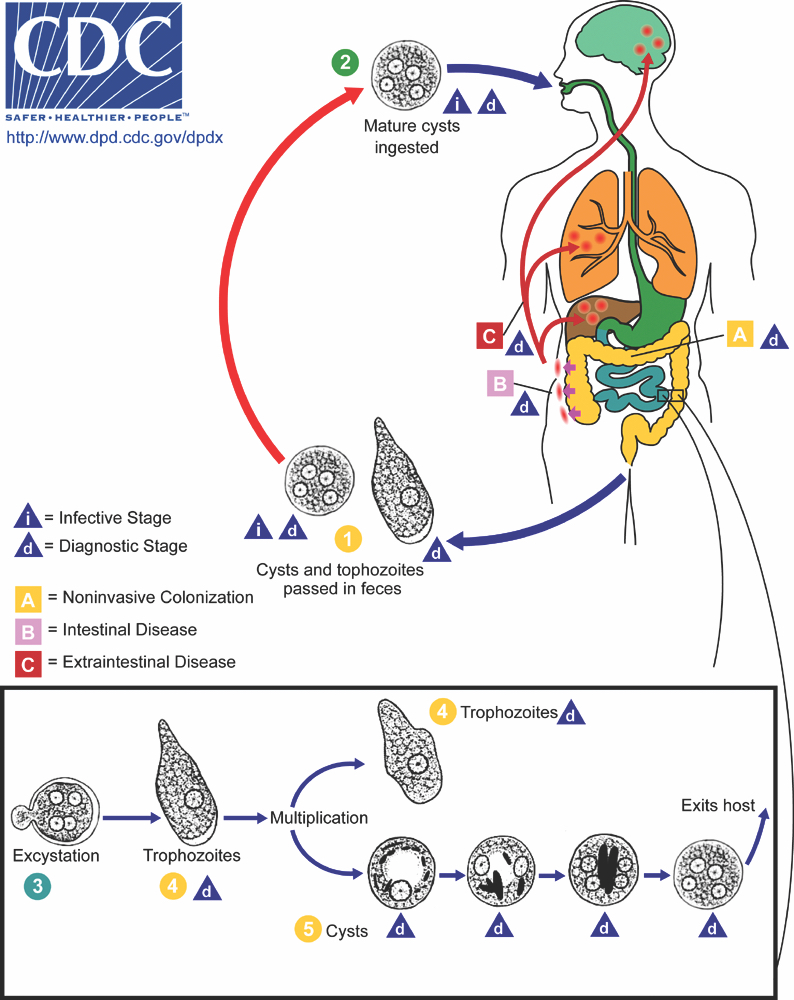
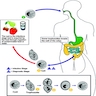
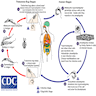
- Entamoeba histolytica cysts are ingested from fecally contaminated food or water; sexual transmission also occurs (see Diagrams / tables)
- Cysts are resistant to gastric acid (and chlorine in water supplies)
- Excystation occurs in the small intestine to release trophozoites
- Trophozoites are invasive and multiply by binary fission
- Estimated that 20% of infections are associated with intestinal wall invasion
- Susceptibility to invasive infection appears to be host dependent (Trends Parasitol 2011;27:254):
- Polymorphisms in leptin receptor affect susceptibility
- Risk factors for severe disease: malnutrition, malignancy, alcoholism, immunocompromised state, pregnancy, young age
- Cell adhesion and killing by E. histolytica trophozoites (Trends Parasitol 2011;27:254):
- Gal / GalNAc lectin on E. histolytica's surface binds galactose and N-acetyle-D-galactosamine residues found on O linked sugar side chains of host colonic mucin; degrades intestinal protective mucous barrier and allows penetration of epithelium
- Parasite secretion of proteinases, contact dependent cell lysis and apoptosis and the formation of amebapores result in host cell death
- E. histolytica ingests remnant erythrocytes (hemophagocytosis)
- Some trophozoites undergo encystation through signaling pathways, completing the cycle
Etiology
- Due to E. histolytica infection
Diagrams / tables
Clinical features
- 80 - 90% of individuals with E. histolytica infection are asymptomatic (Can J Gastroenterol Hepatol 2018;2018:4601420)
- Symptoms range from mild diarrhea (most common) to severe dysentery
- Subacute onset; develops over 3 - 4 weeks with worsening diarrhea and abdominal pain
- Symptoms may also develop acutely and mimic acute abdomen
- Cases may occur where symptoms develop months after infection
- Young children may develop intussusception or necrotizing colitis, that may lead to perforation
- Rare complications are toxic megacolon, fulminant necrotizing colitis, colonic amebomas, perianal fistulas
- 50% mortality rate with fulminant necrotizing colitis (Pathog Glob Health 2012;106:245)
- Disseminated disease is more common in men (see Entamoeba histolytica abscess)
- Morphologically similar species E. dispar is nonpathogenic (does not invade); the pathogenic potential of E. bangladeshi, E. moshkovskii and E. nuttalli is not fully understood
Diagnosis
- May be suspected based on epidemiologic factors, patient symptoms, radiologic or colonoscopic findings (Open Forum Infect Dis 2018;5:ofy161)
- Definitive diagnosis of amebic colitis is made by finding trophozoites that have invaded the intestinal mucosa
- Patients with amebic liver abscesses usually have antiamebic antibodies (see Entamoeba histolytica abscess)
- Cyst aspiration is sometimes performed; although it is unusual to see parasites, the absence of other microorganisms supports evidence of amebic liver abscess
Laboratory
- E. histolytica infection is usually detected via stool microscopy (ova and parasite examination), stool antigen or stool nucleic acid testing (Open Forum Infect Dis 2018;5:ofy161)
- Positive stool exam is not definitive for amebic colitis, since 80 - 90% of infections do not cause symptoms
- Stool microscopy detects cysts or trophozoites that are morphologically consistent with E. histolytica and other morphologically identical species, but cannot usually differentiate them
- Some antigen detection or nucleic acid amplification tests (NAATs) can distinguish E. histolytica from similar appearing amebae
- NAATs are the most sensitive detection methods performed on stool
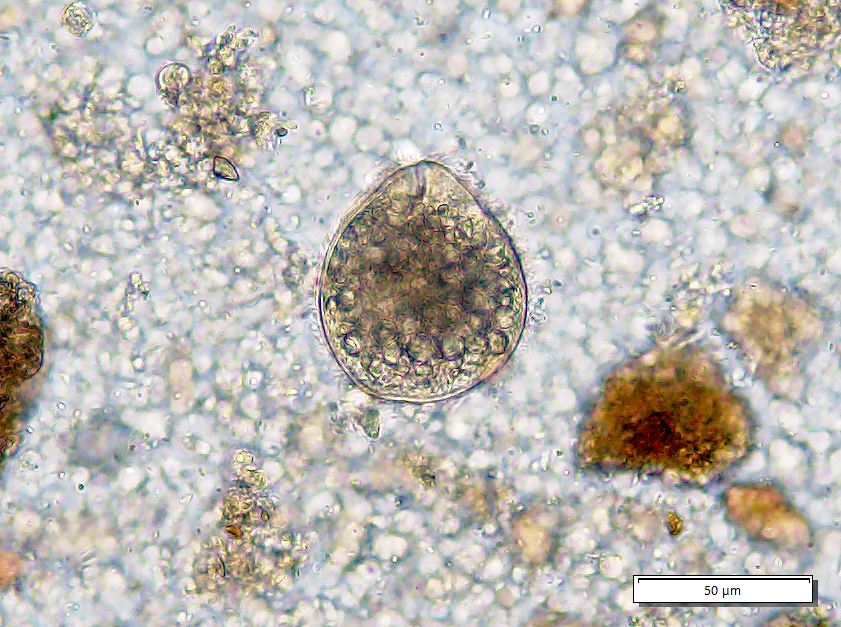
- Stool microscopy findings:
- Trophozoites measure 10 - 60 micrometers and possess a single nucleus with even peripheral chromatin and a pinpoint, often centrally located karyosome
- Ingested erythrocytes pathognomonic for E. histolytica infection; otherwise, needs to be distinguished from nonpathogenic species (e.g., E. dispar)
- Cysts are spherical, 10 - 20 micrometers in diameter; mature cysts have 4 nuclei and chromatoid bodies with bluntly rounded ends
- Serologic testing for E. histolytica antibodies supports the diagnosis of amebiasis but cannot differentiate current from past infection
- Most useful for diagnosing disseminated disease
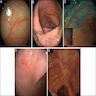
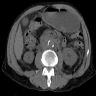
Radiology description
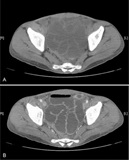
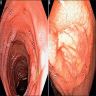
- Evidence of colitis may be seen on CT (Jpn J Radiol 2021;39:558):
- Acute colitis: nonspecific diffuse wall thickening, submucosal edema with stratified or target patterns (contrast enhanced CT)
- Chronic colitis: prominent thickened wall; may mimic malignancy
- Most common pattern: involvement of cecum and rectum with sparing of the terminal ileum
- Plain film radiographs using barium may reveal ulcerative changes
- Ameboma: localized wall thickening with minimal contrast enhancement on CT
- Fulminant necrotizing colitis:
- Prominent thickened wall on contrast enhanced CT with a thin enhancing rim
- Contiguous involvement or skip lesions
- Ultrasonography and CT can be used for detection of liver abscess
Case reports
- 40 year old man with fulminant necrotizing amebic colitis following receipt of corticosteroids for severe COVID-19 (BMJ Case Rep 2021;14:e246110)
- 43 year old married couple with amebic colitis treated with paromomycin monotherapy (PLoS Negl Trop Dis 2020;14:e0008013)
- 54 year old man with cecal amebiasis mimicking inflammatory bowel disease (J Int Med Res 2020;48:300060520922379)
- 56 year old man with advanced HIV infection requiring emergent subtotal colectomy of invasive intestinal amebiasis (J Clin Microbiol 2018;56:e01703)
- 67 year old man with sexually acquired amebiasis (BMJ Case Rep 2019;12:e228942)
- Patient with 2 different types of objects seen in a trichrome stained stool specimen (Pritt: Creepy Dreadful Wonderful Parasites Blog - Case of the Week 546 [Accessed 13 October 2023])
Treatment
- Amebic colitis is treated with both an amebicidal and luminal agent (Open Forum Infect Dis 2018;5:ofy161)
- Metronidazole or tinidazole amebicides, to target invading trophozoites
- Paromomycin to eradicate luminal cysts and prevent relapse / transmission to others
- Complicated disease may also require surgical resection of perforated / necrotic bowel, fluid resuscitation, broad spectrum antimicrobials for peritonitis
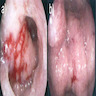
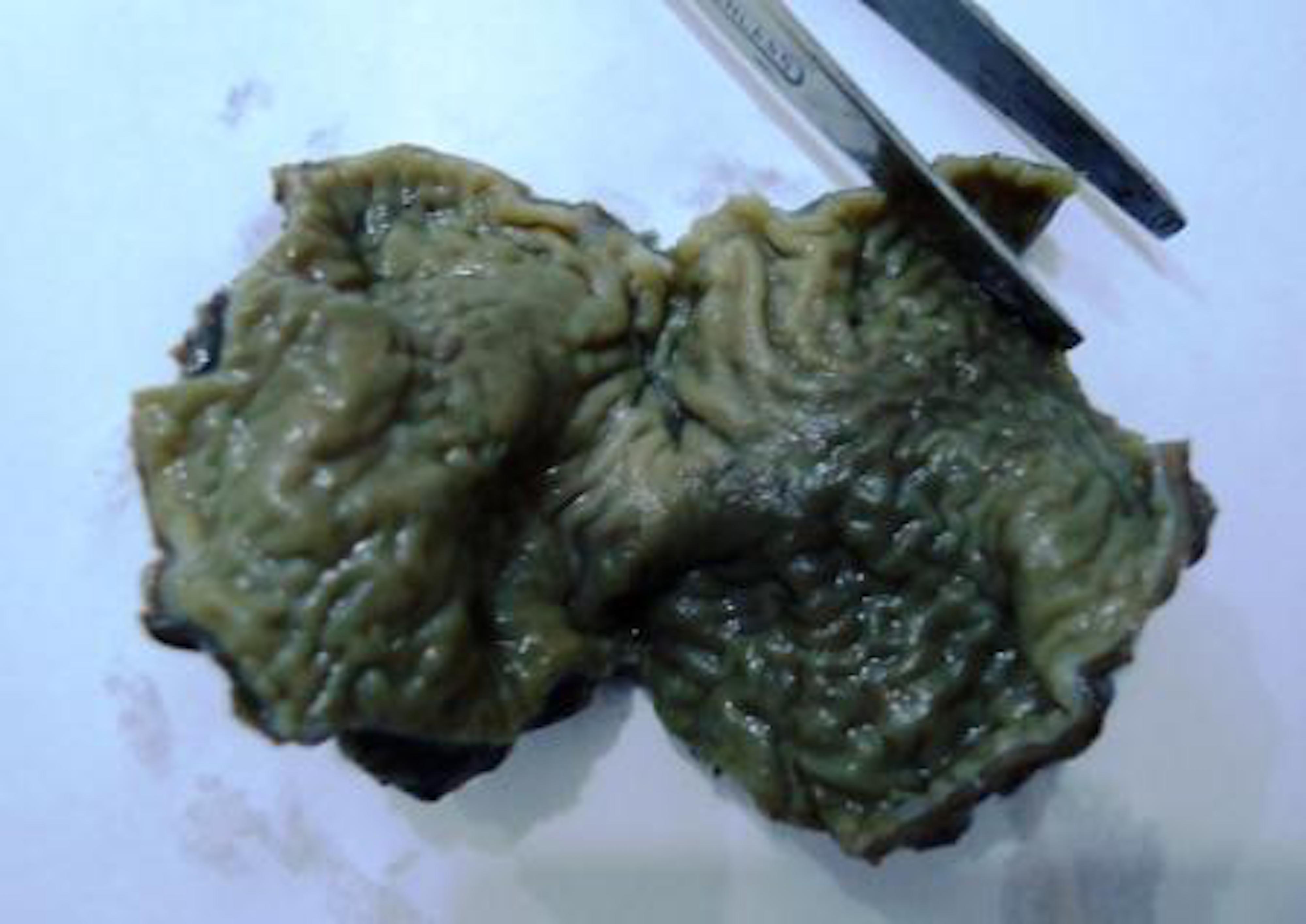
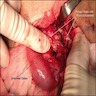
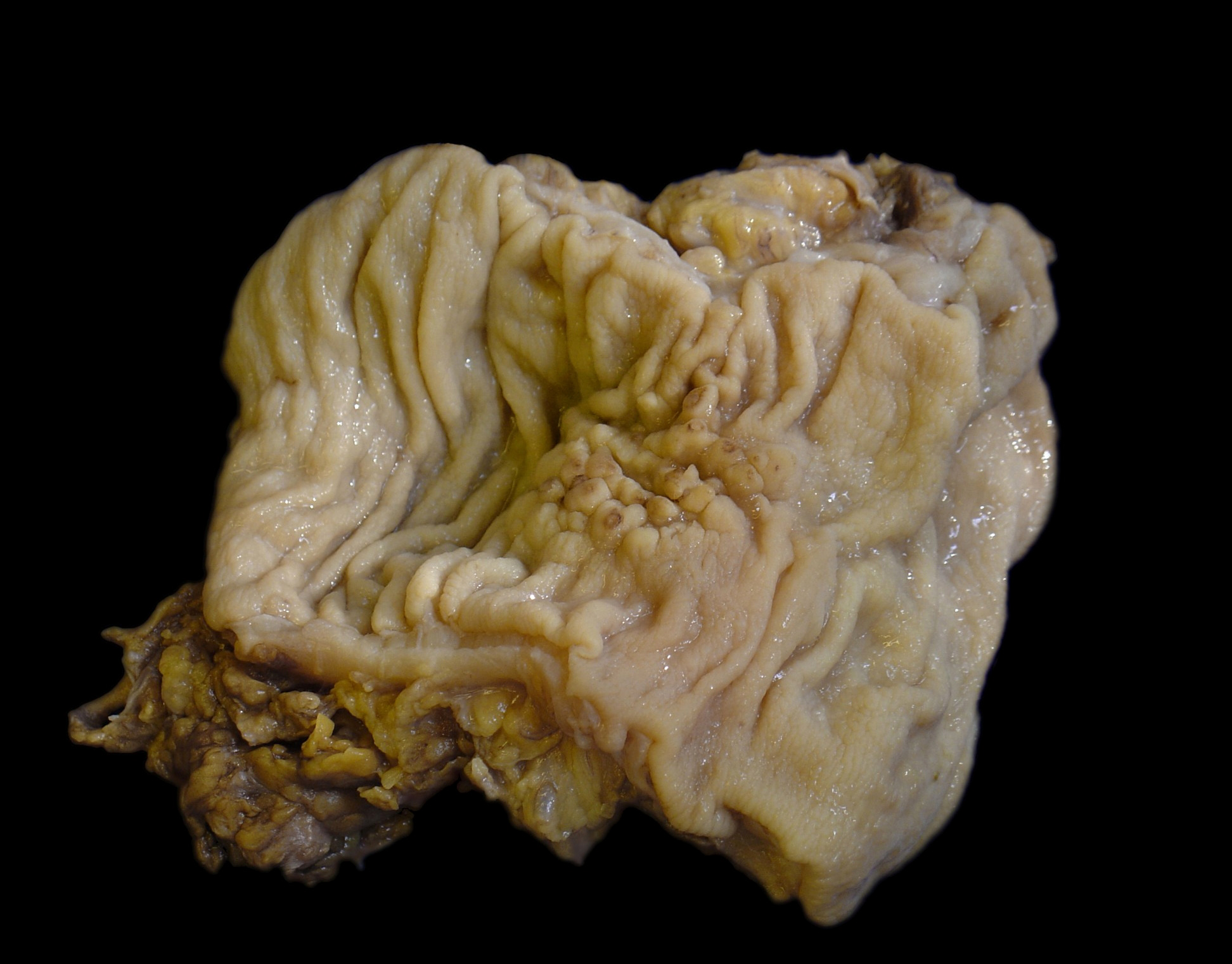
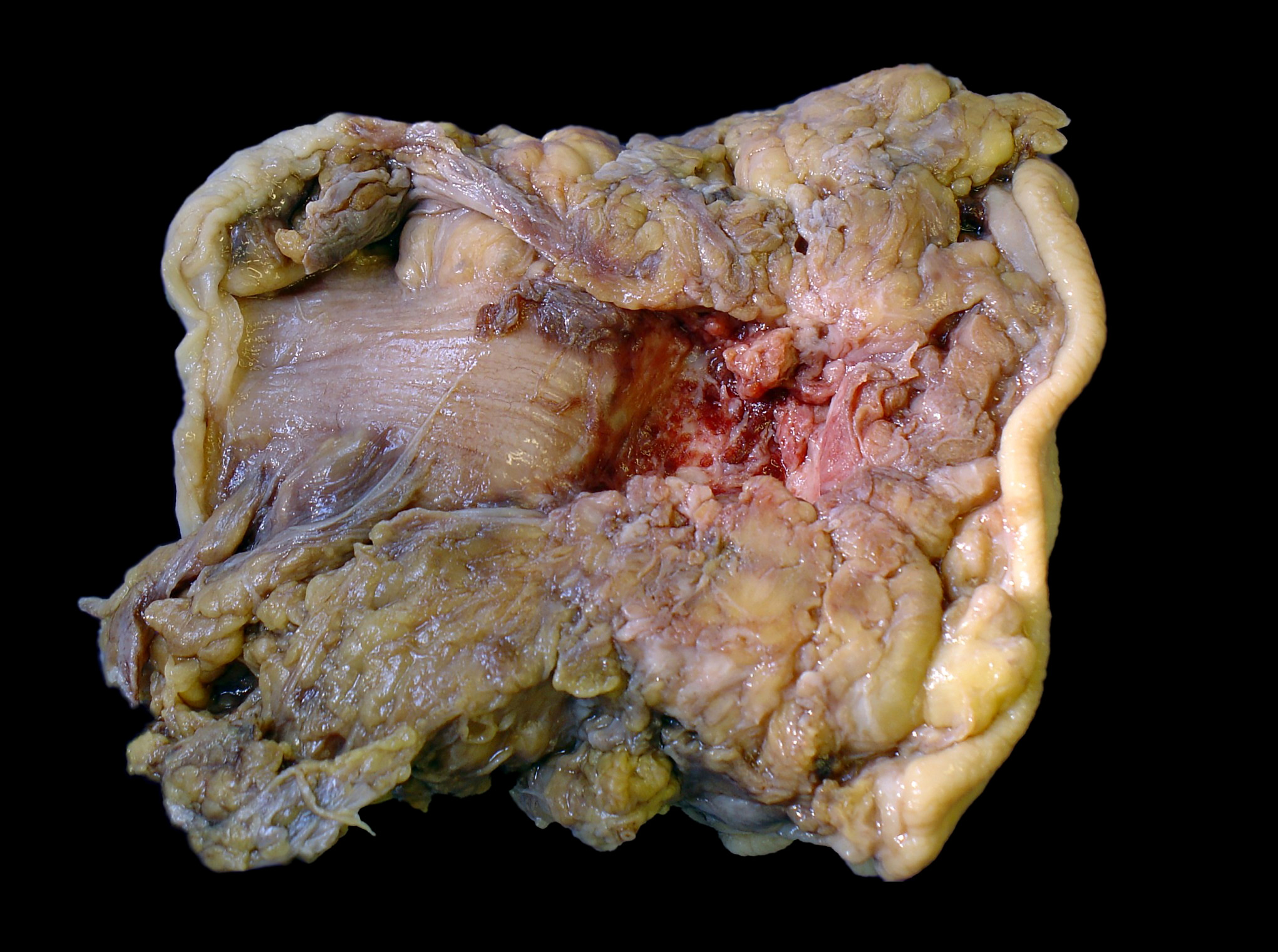
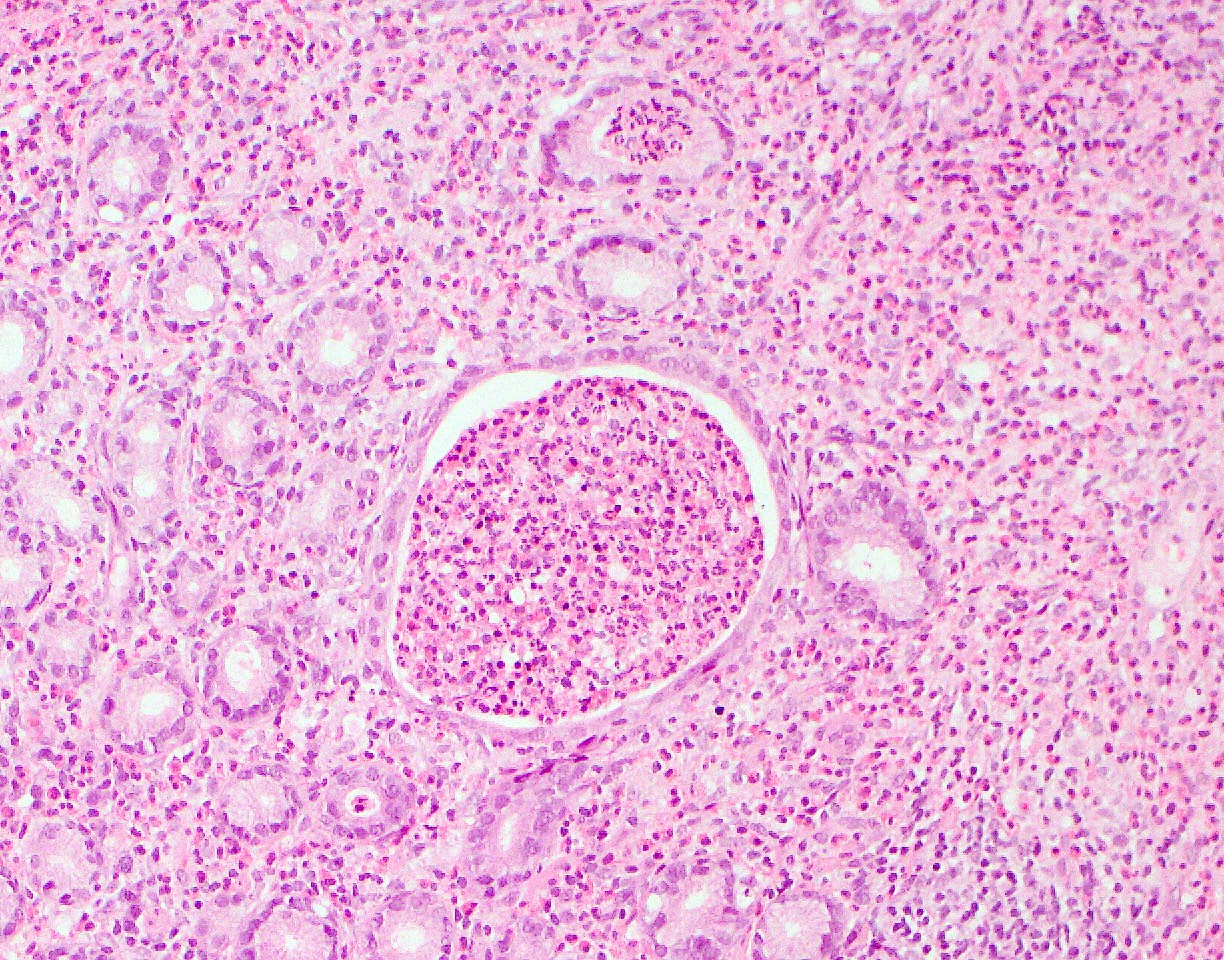
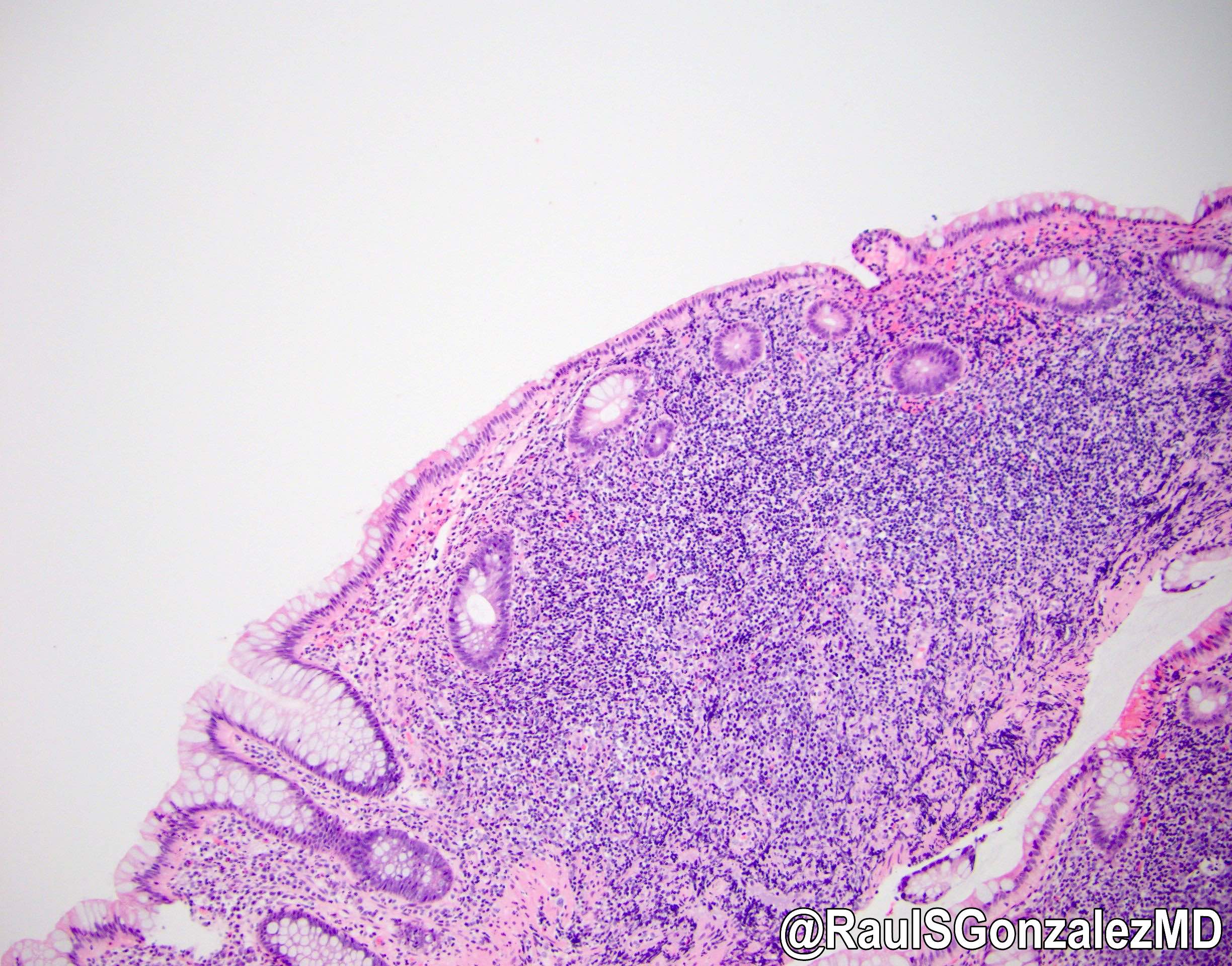
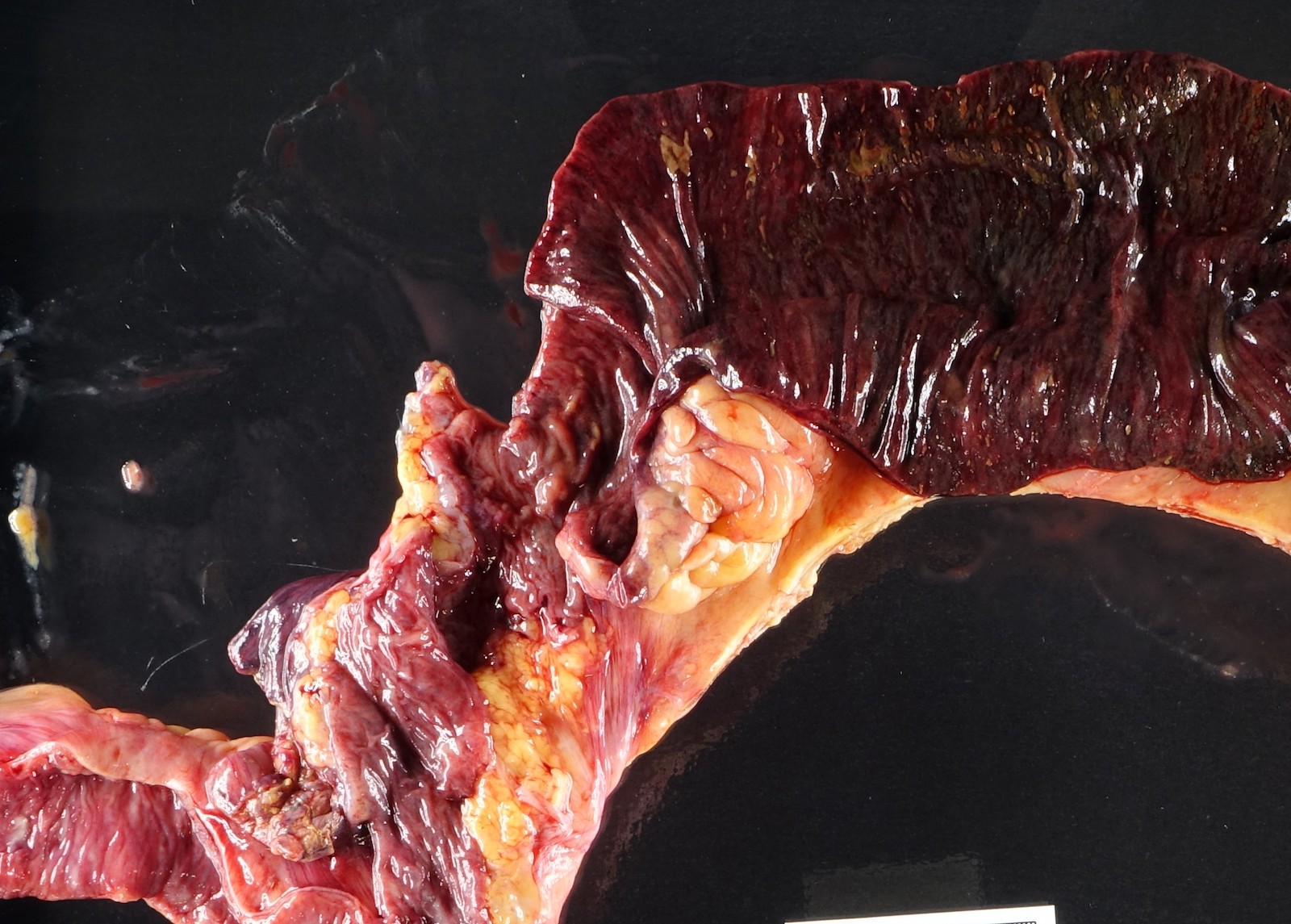
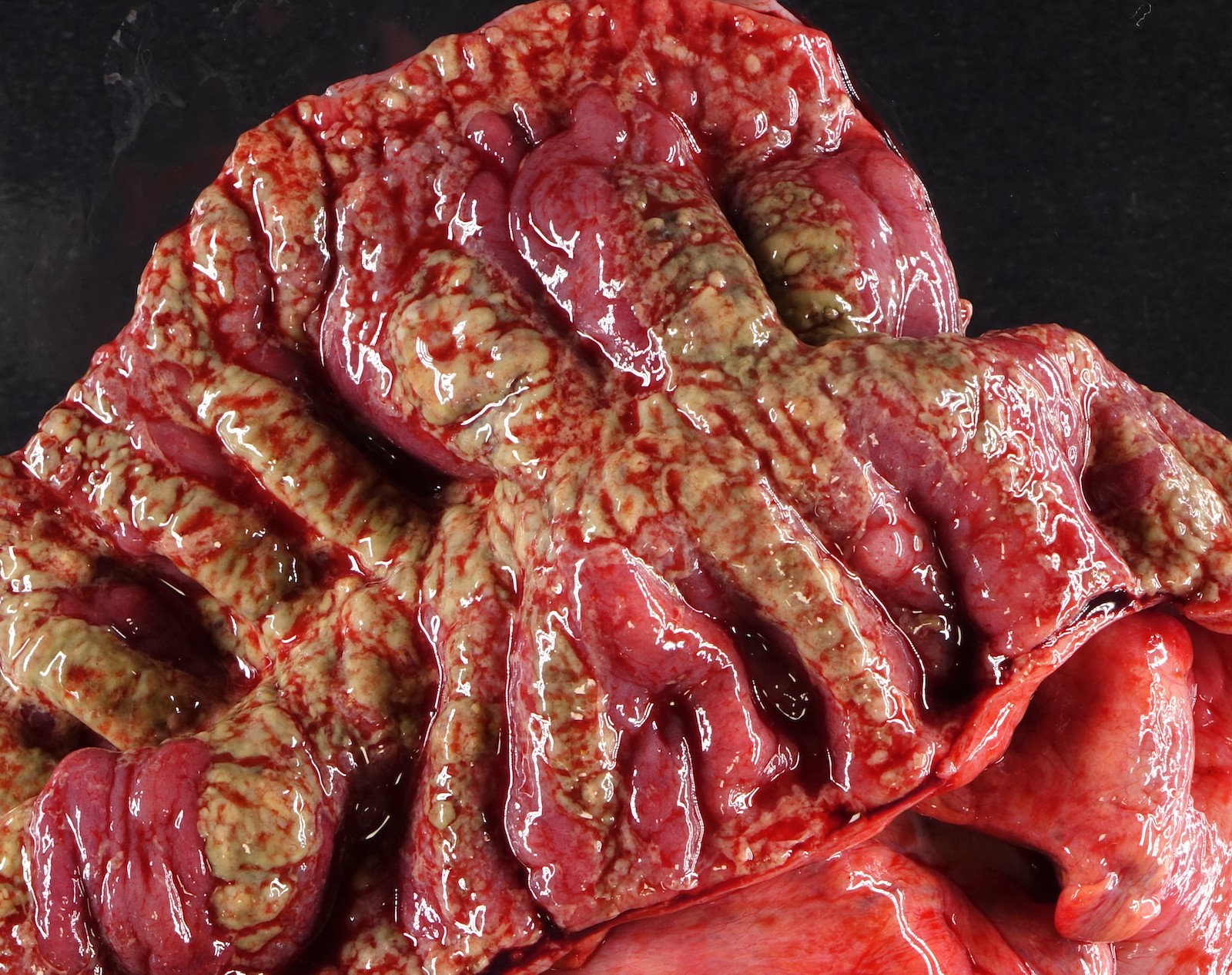
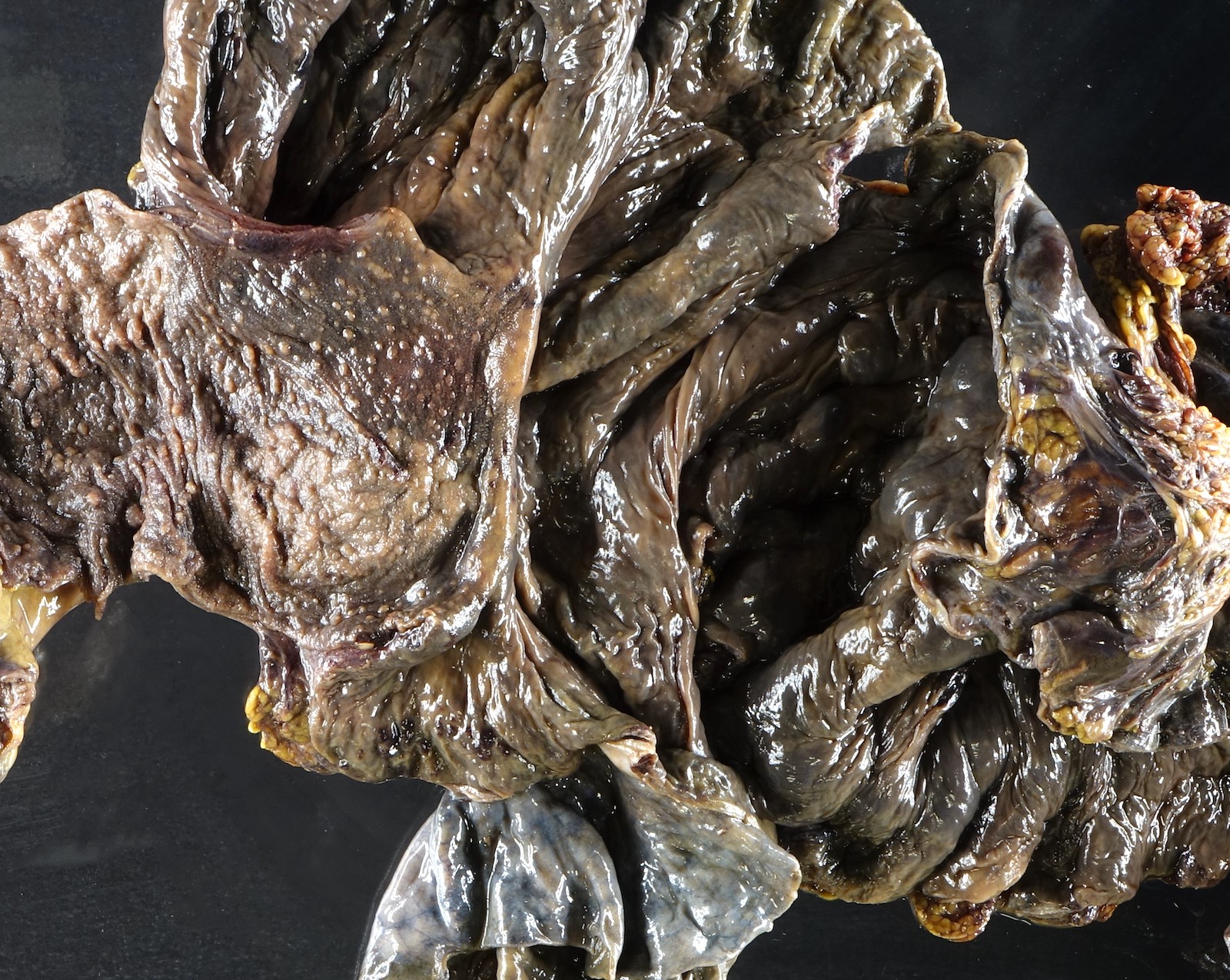
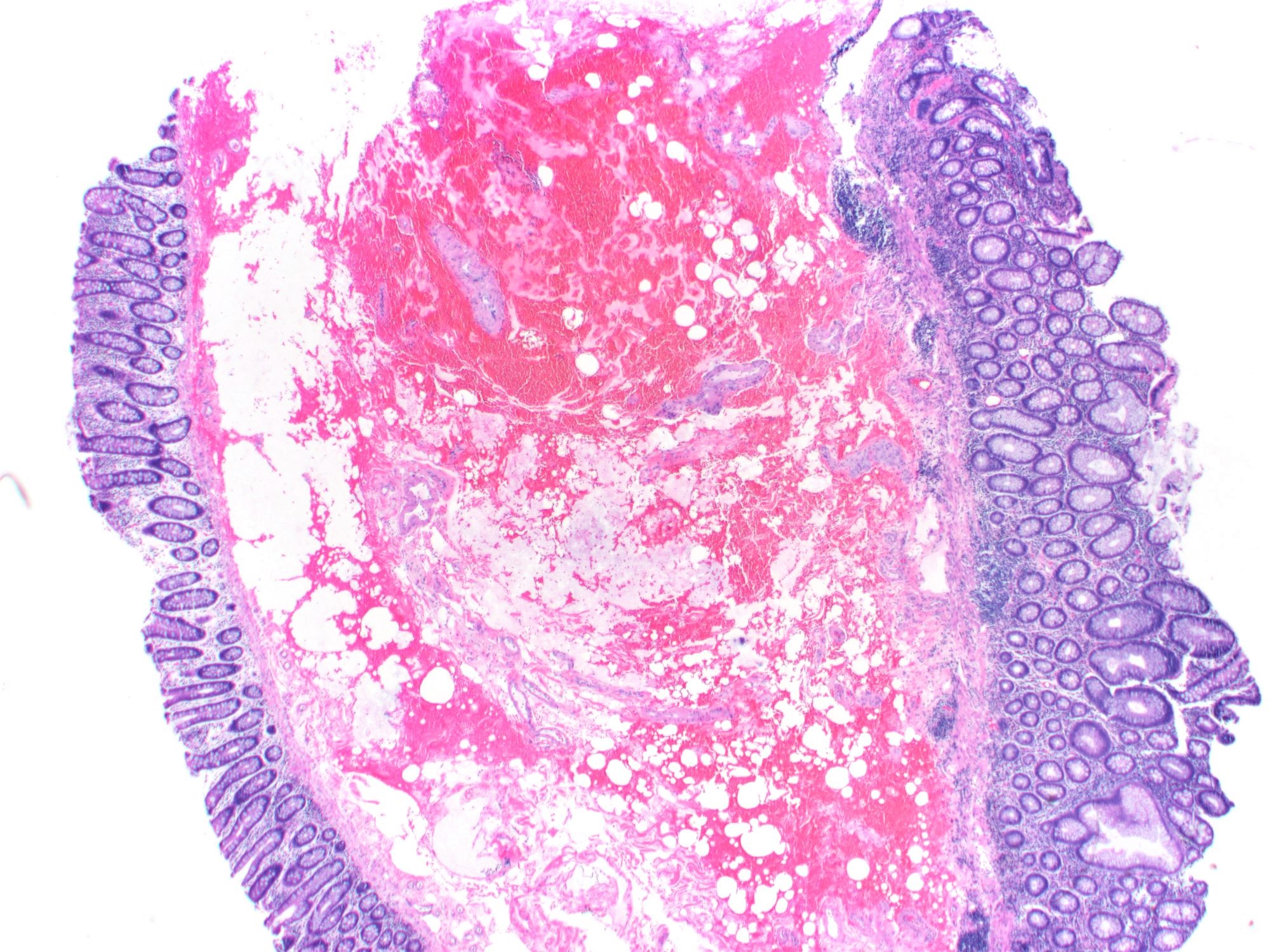
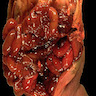
Gross description
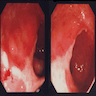
- Discrete, sharply defined ulcers and erosions with smooth, irregularly shaped borders (BMC Gastroenterol 2021;21:367, AFIP: Topics on the Pathology of Protozoan and Invasive Arthropod Diseases [Accessed 22 April 2022])
- Ulcers with white or yellow exudates, bloody exudates
- Normal intervening mucosa
- Ulcers may coalesce, resulting in larger lesions that may be necrotic in the center
- May show areas of colitis or inflammatory polyps
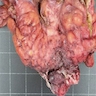
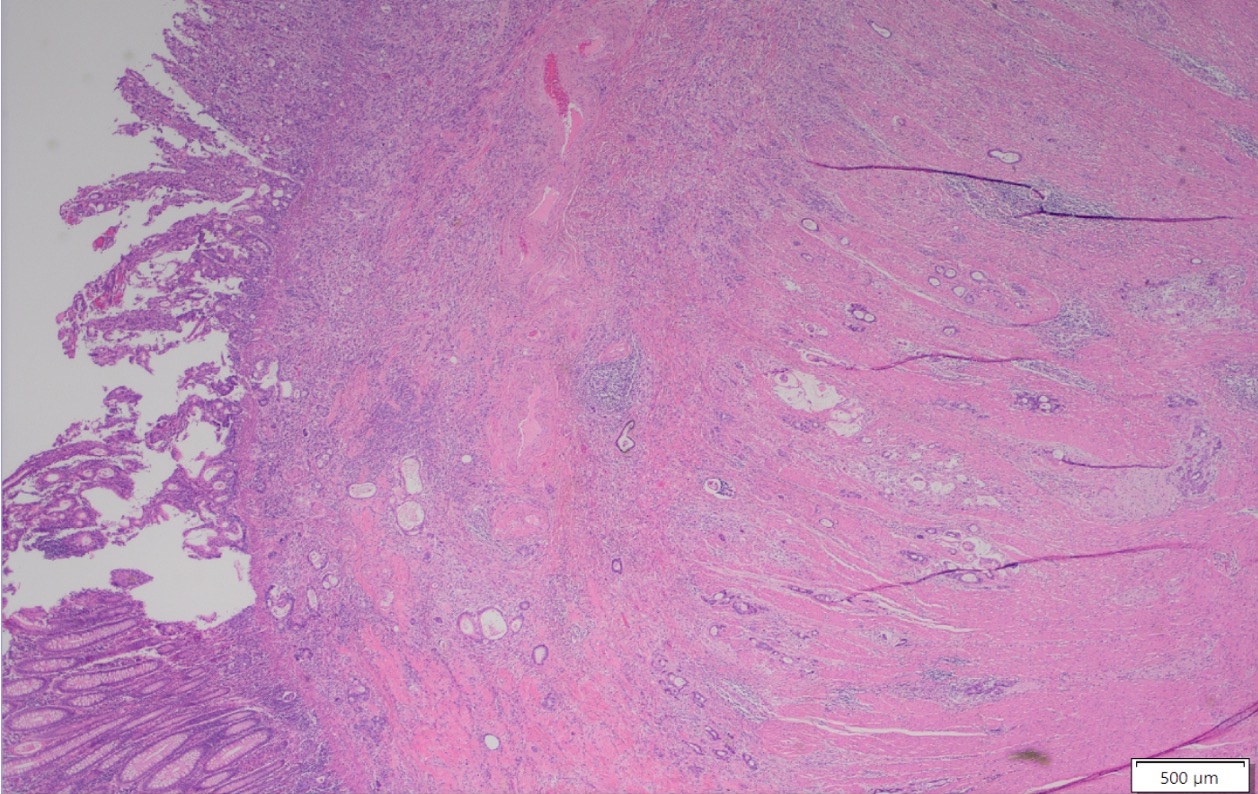
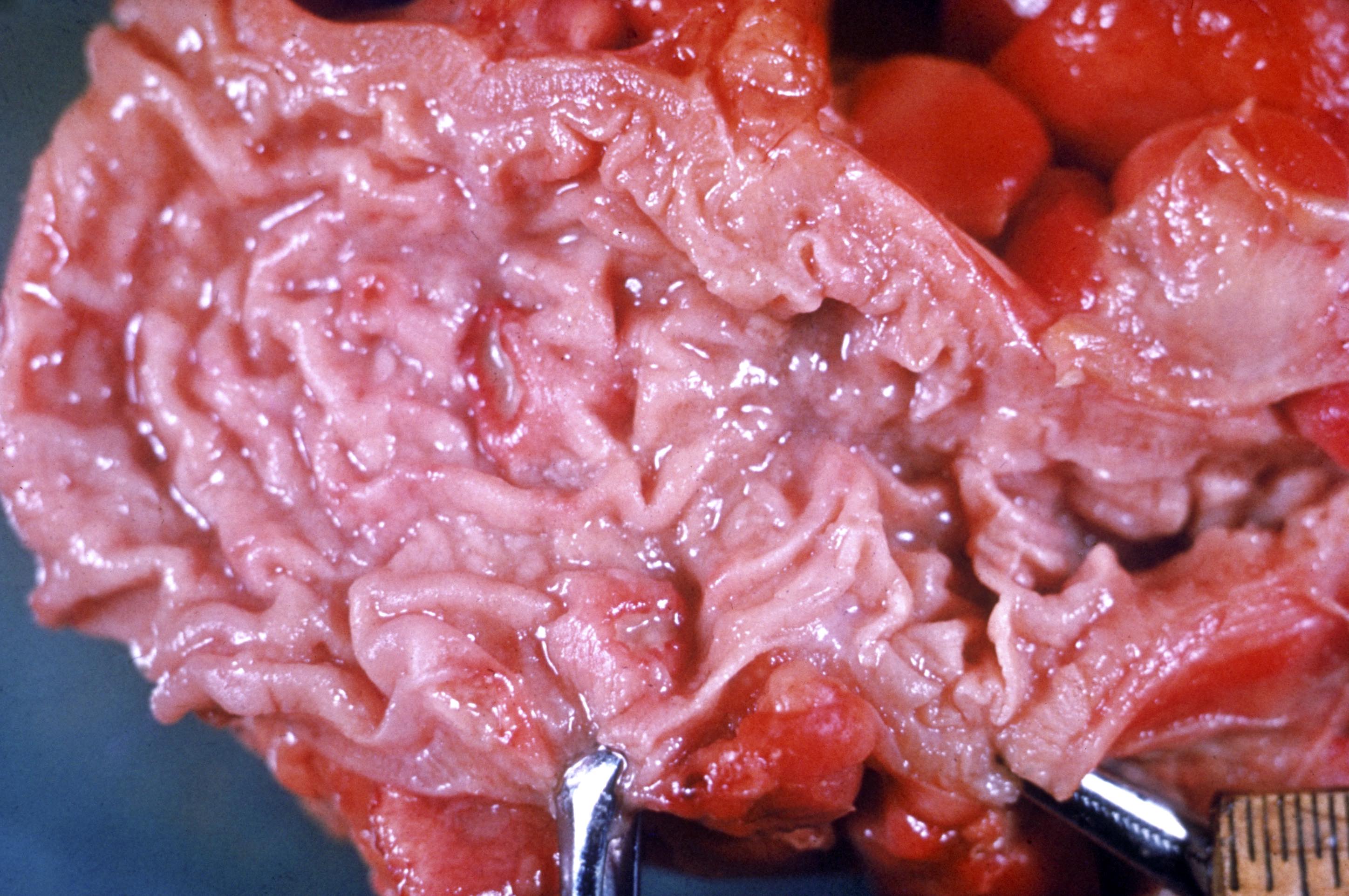
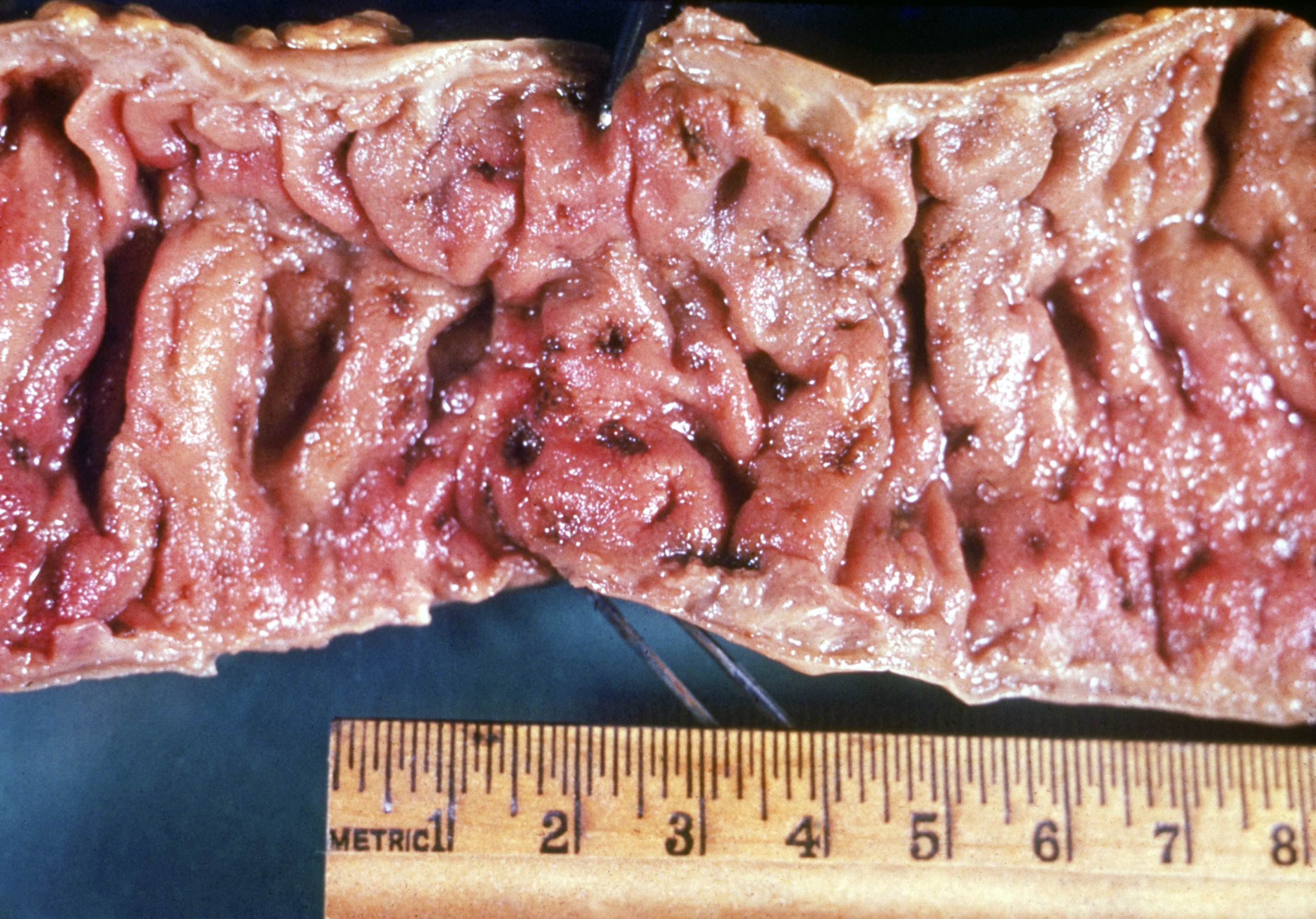
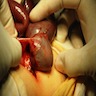
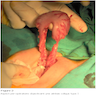
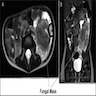
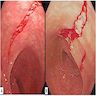
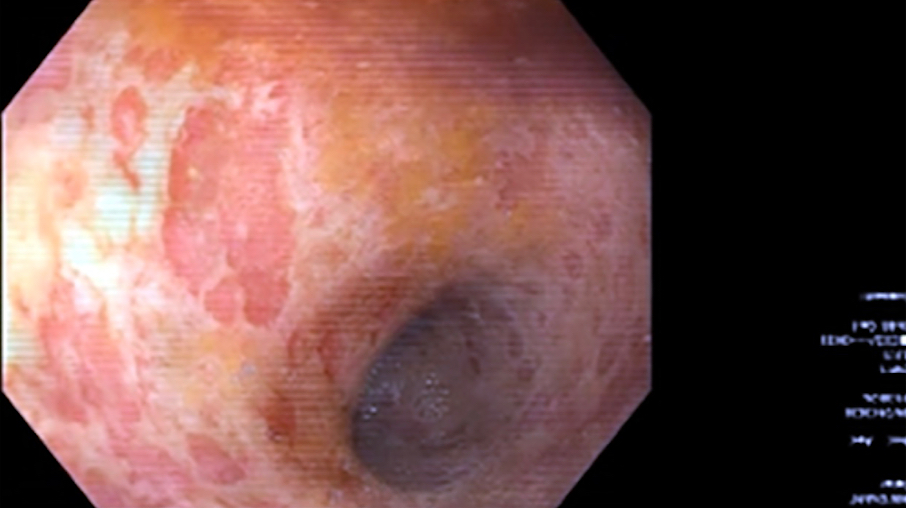
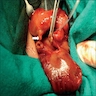
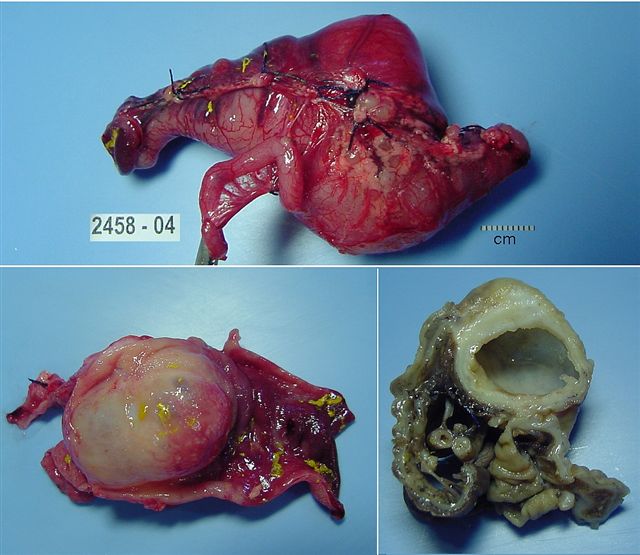
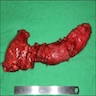
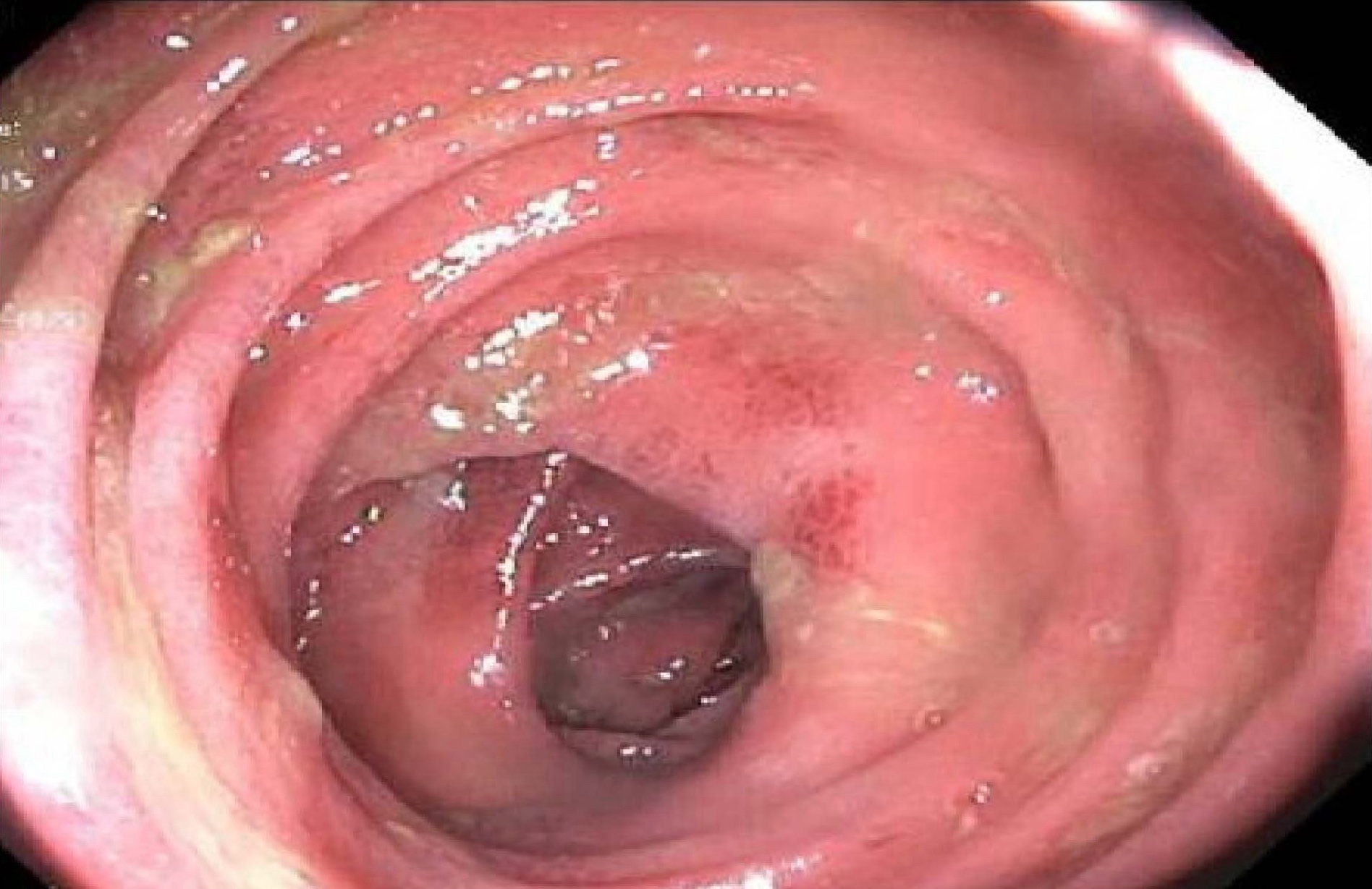
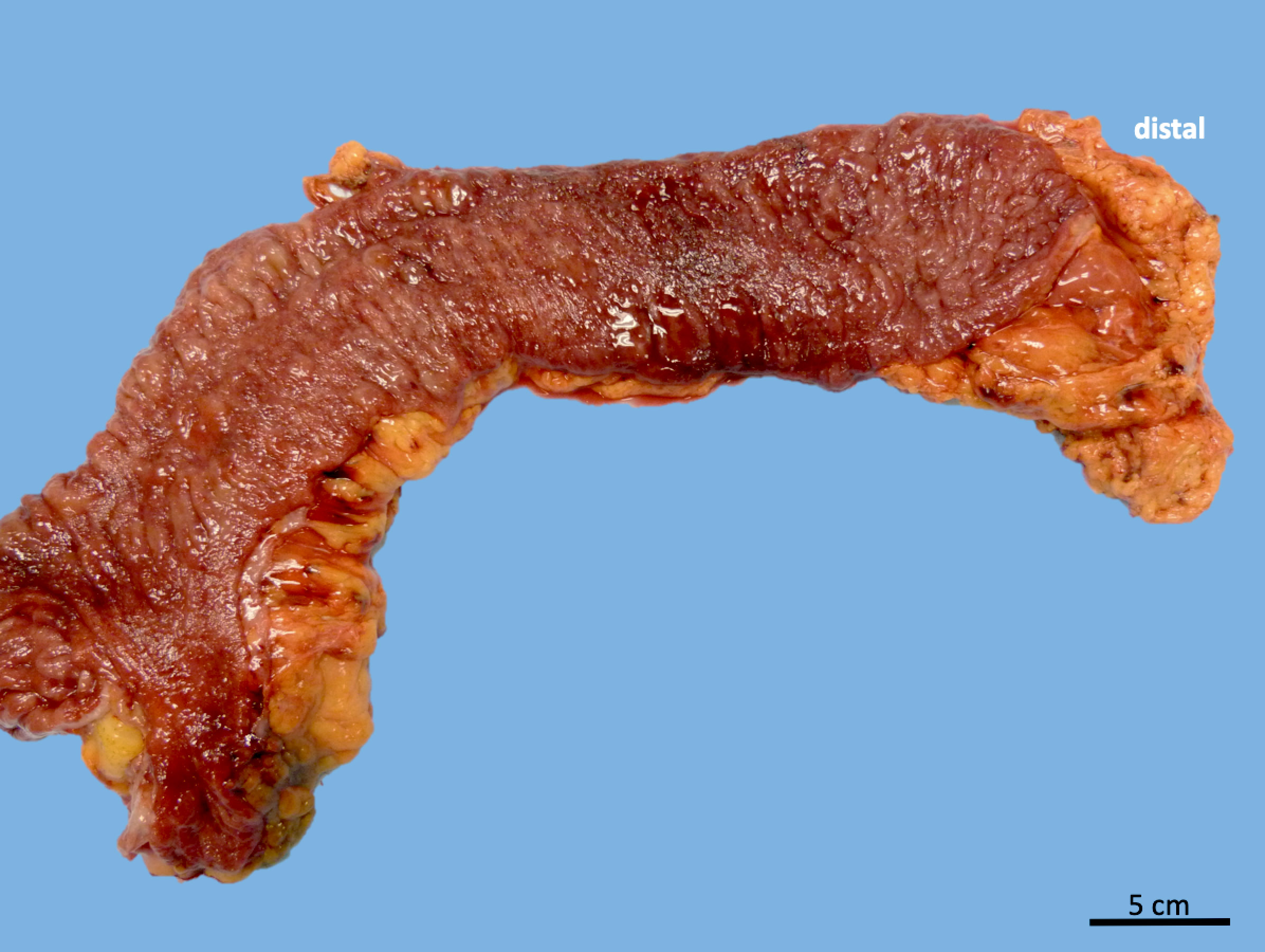
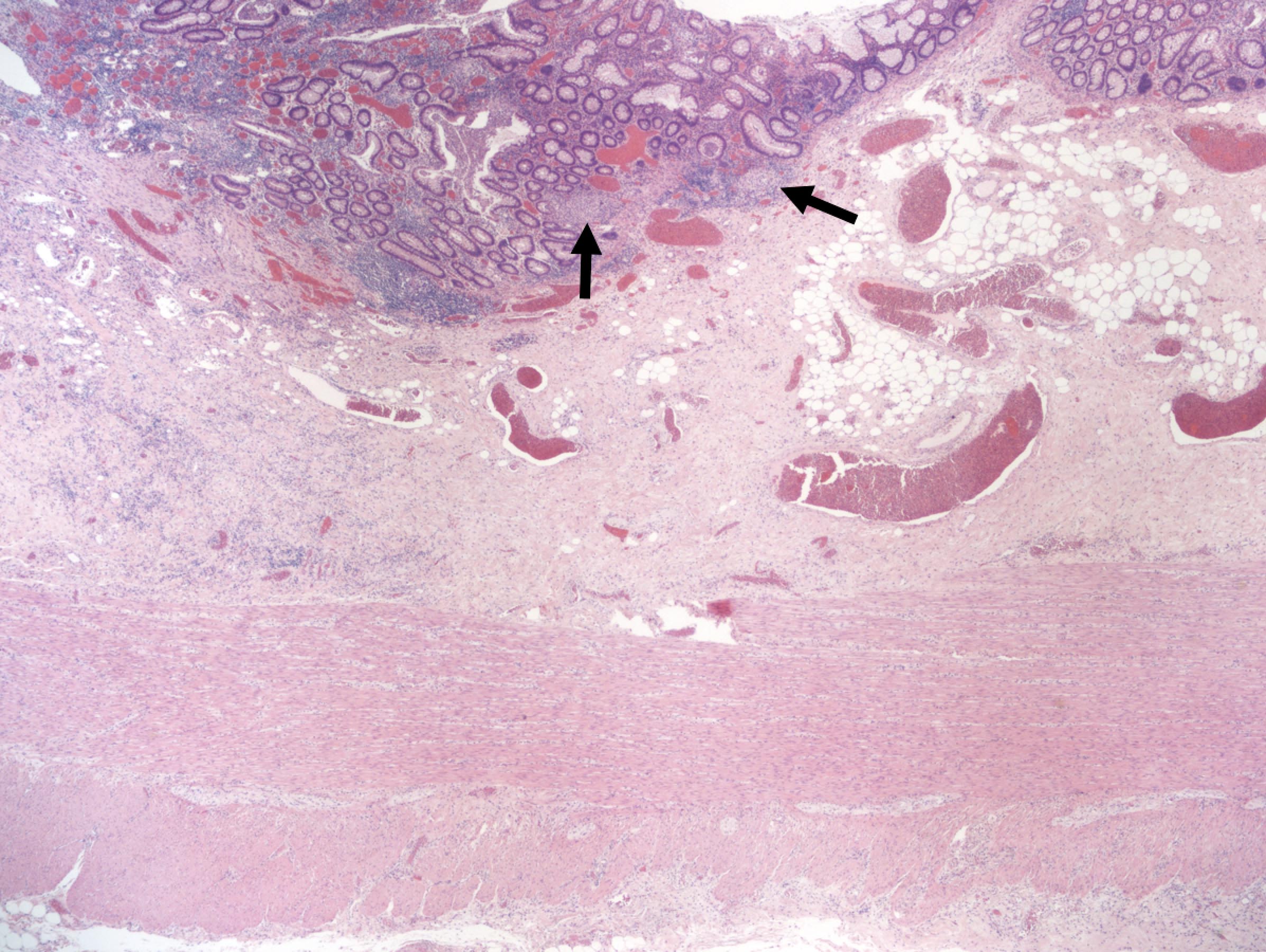
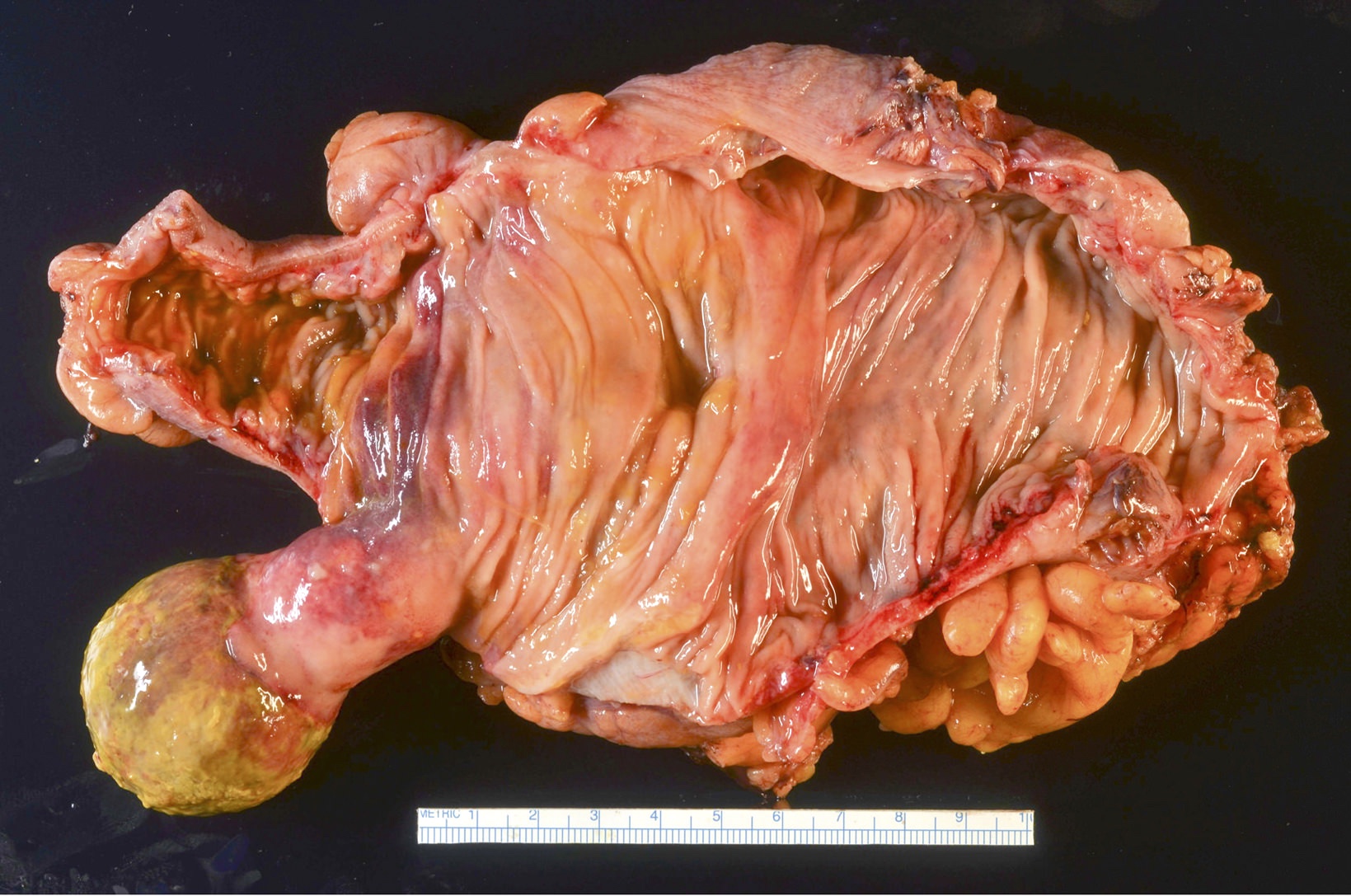
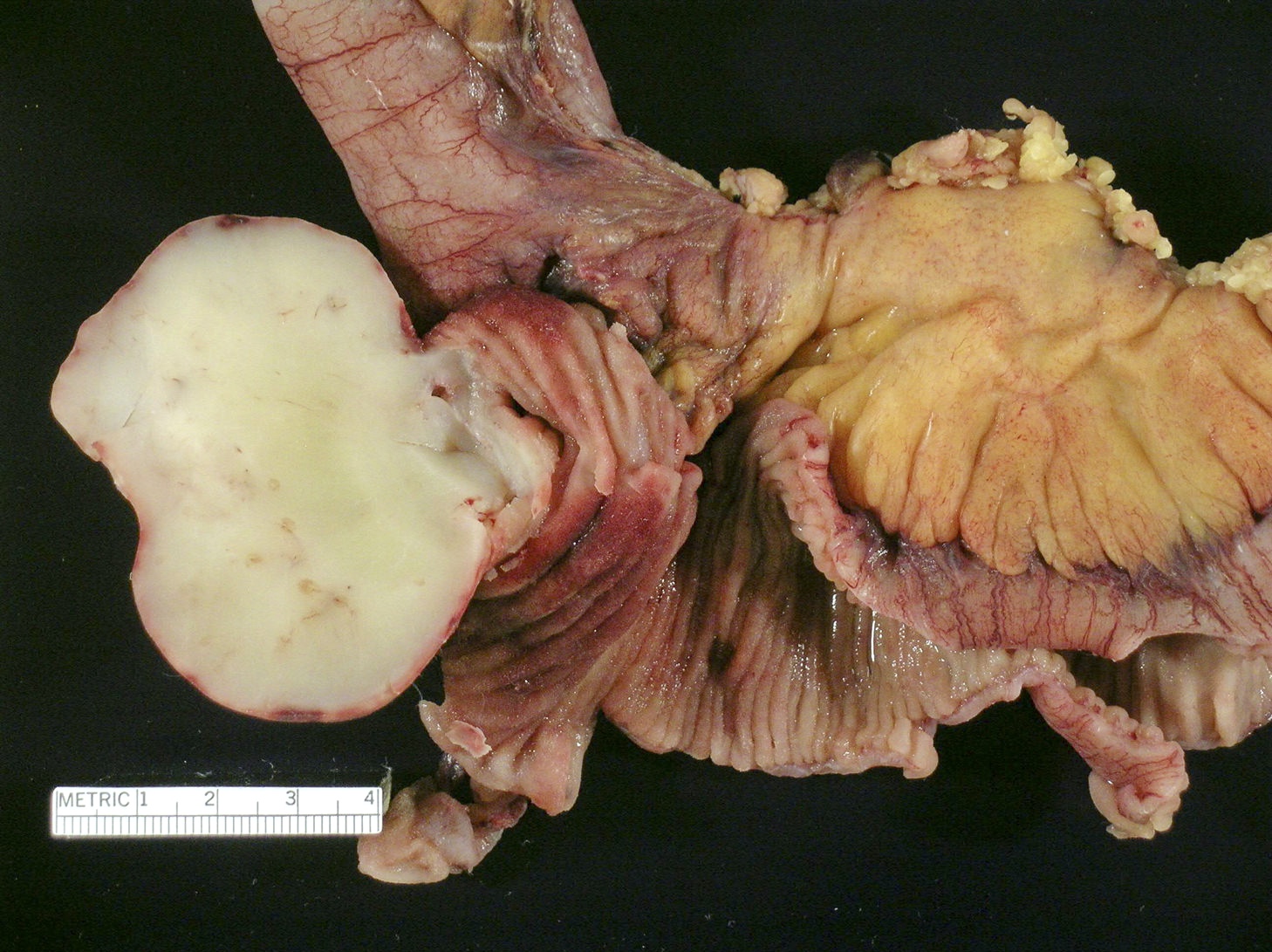
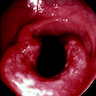
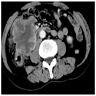
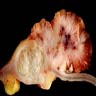
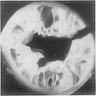
- Ameboma (amebic pseudotumor) presents as firm, well defined, annular inflammatory thickening of the colon wall, usually in the cecum or ascending colon; commonly causes a napkin ring deformity
Gross images
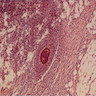
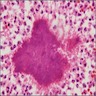
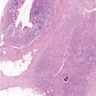
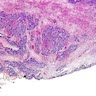
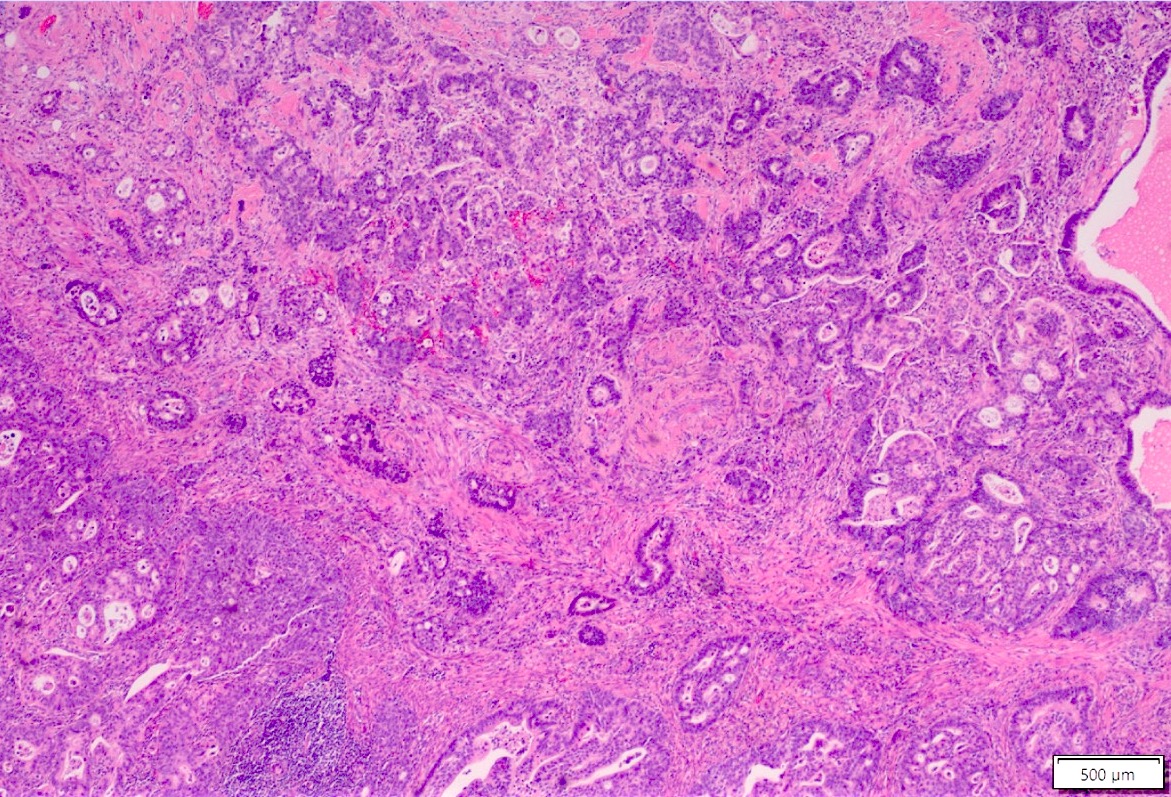
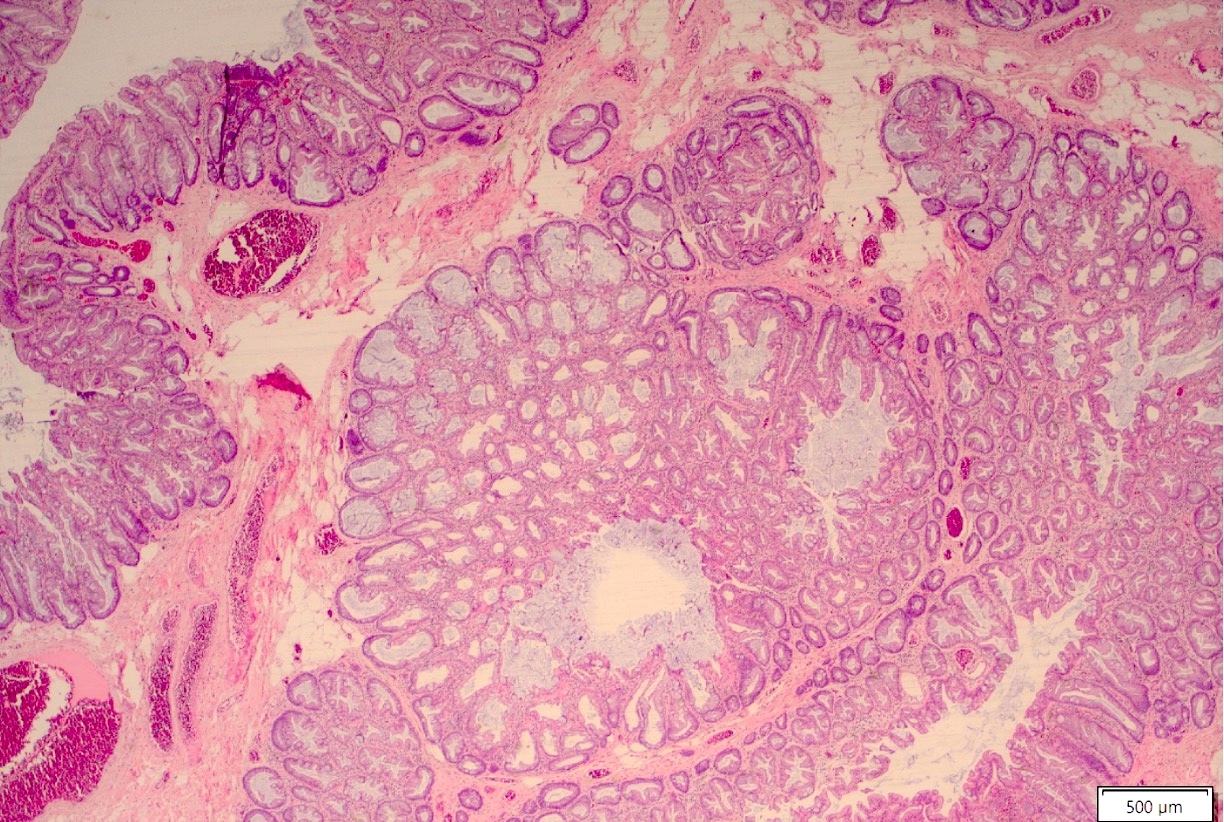
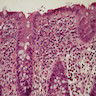
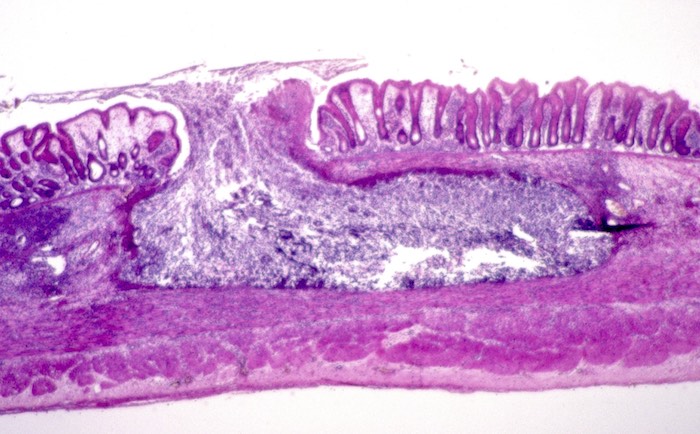
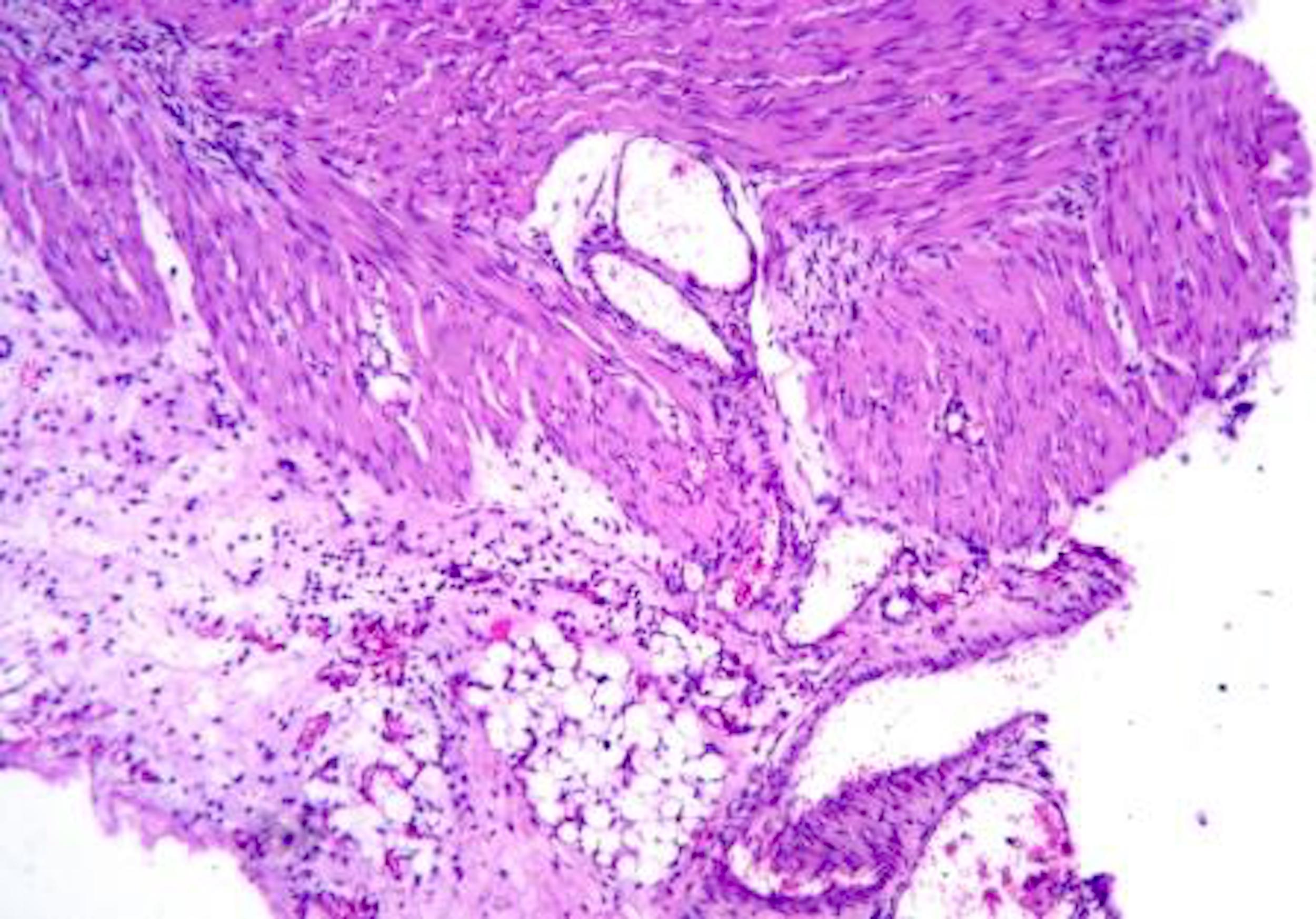
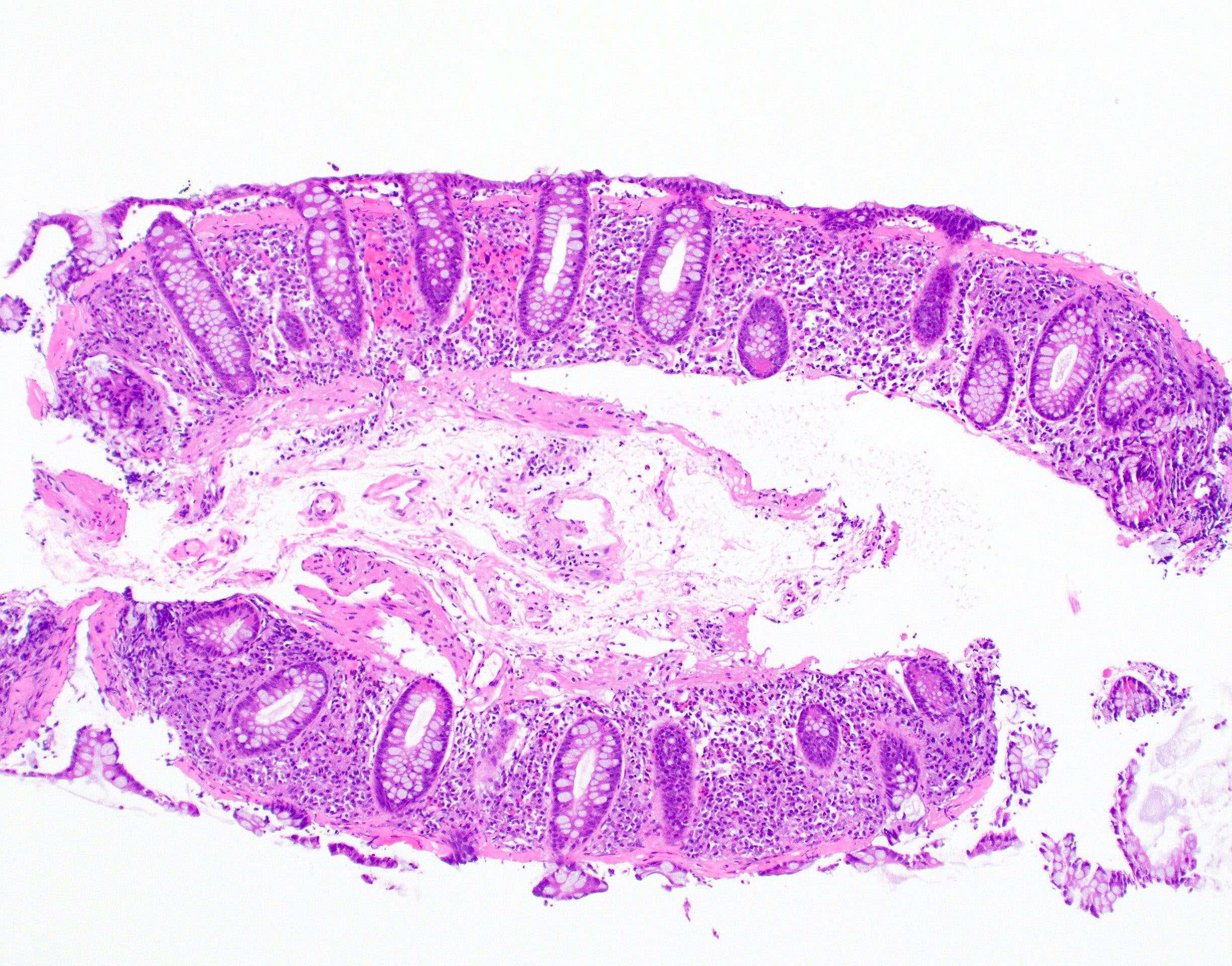
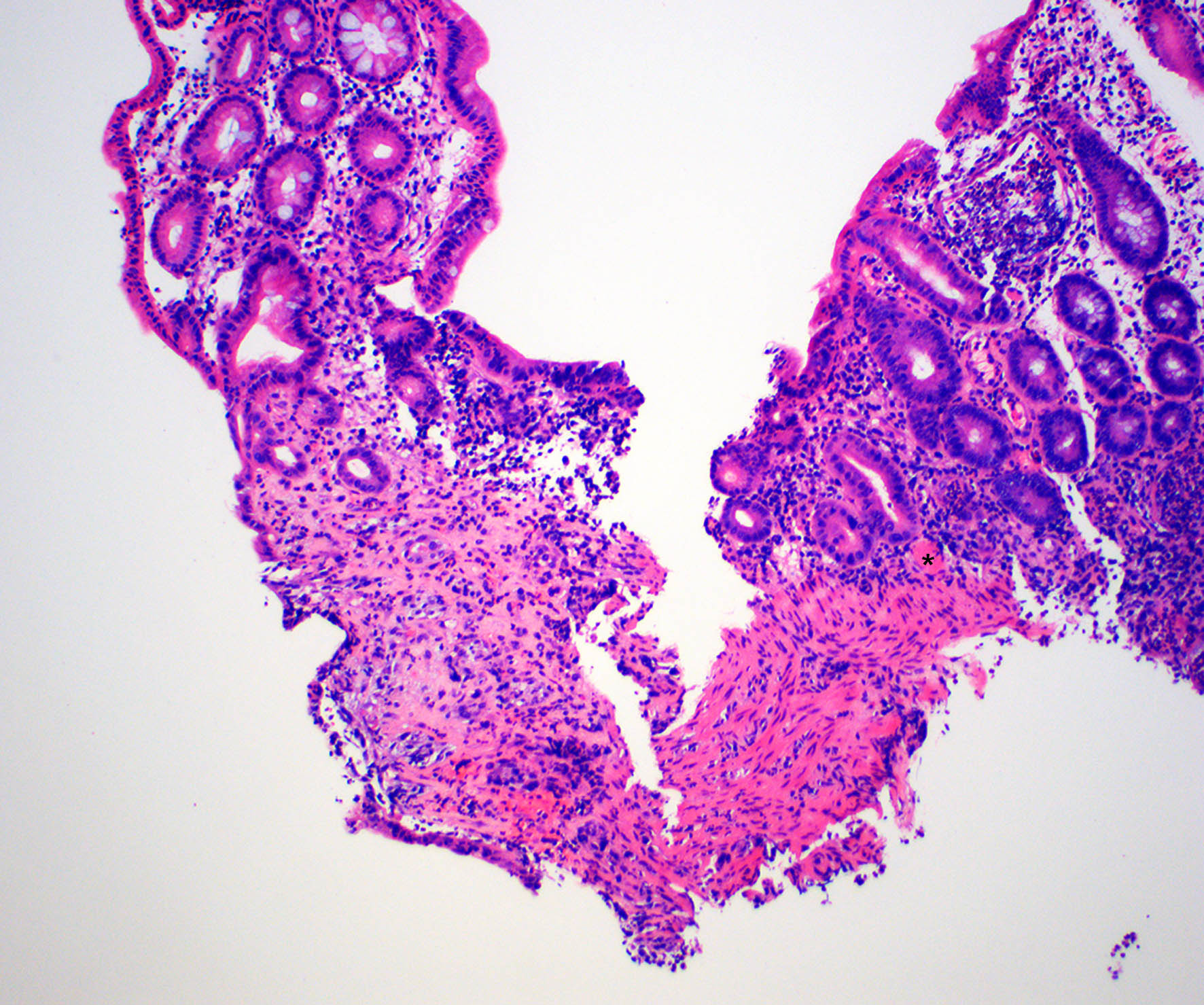
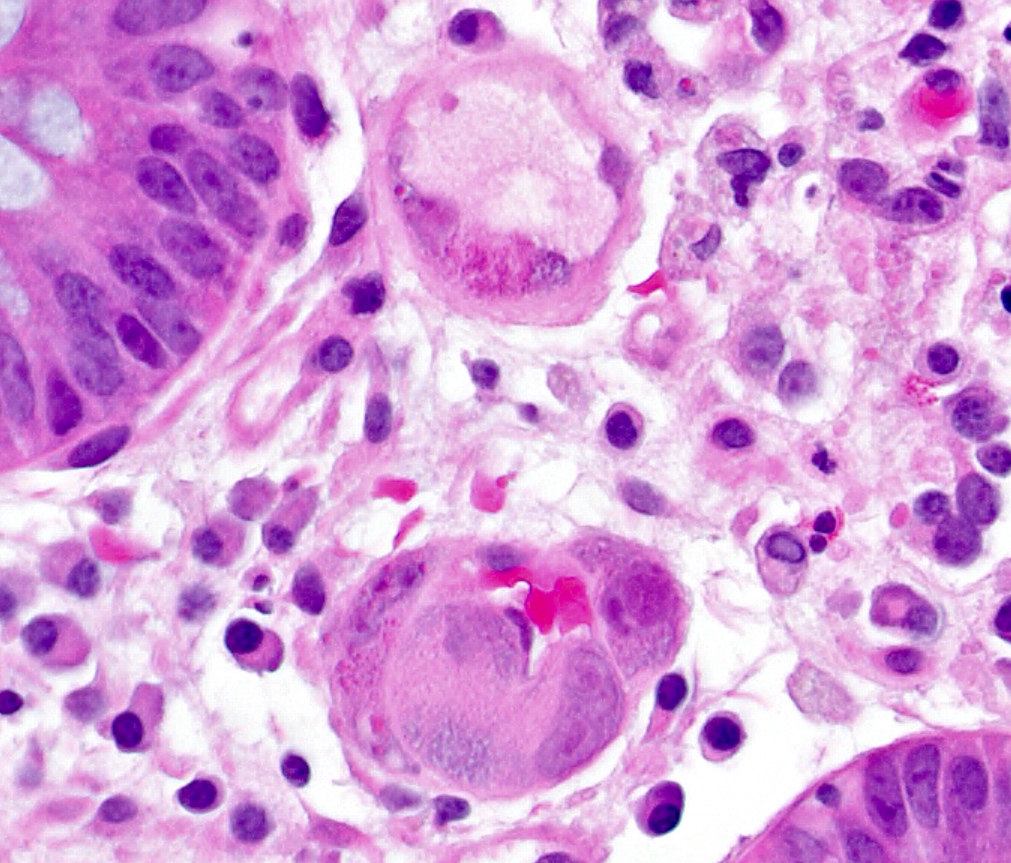
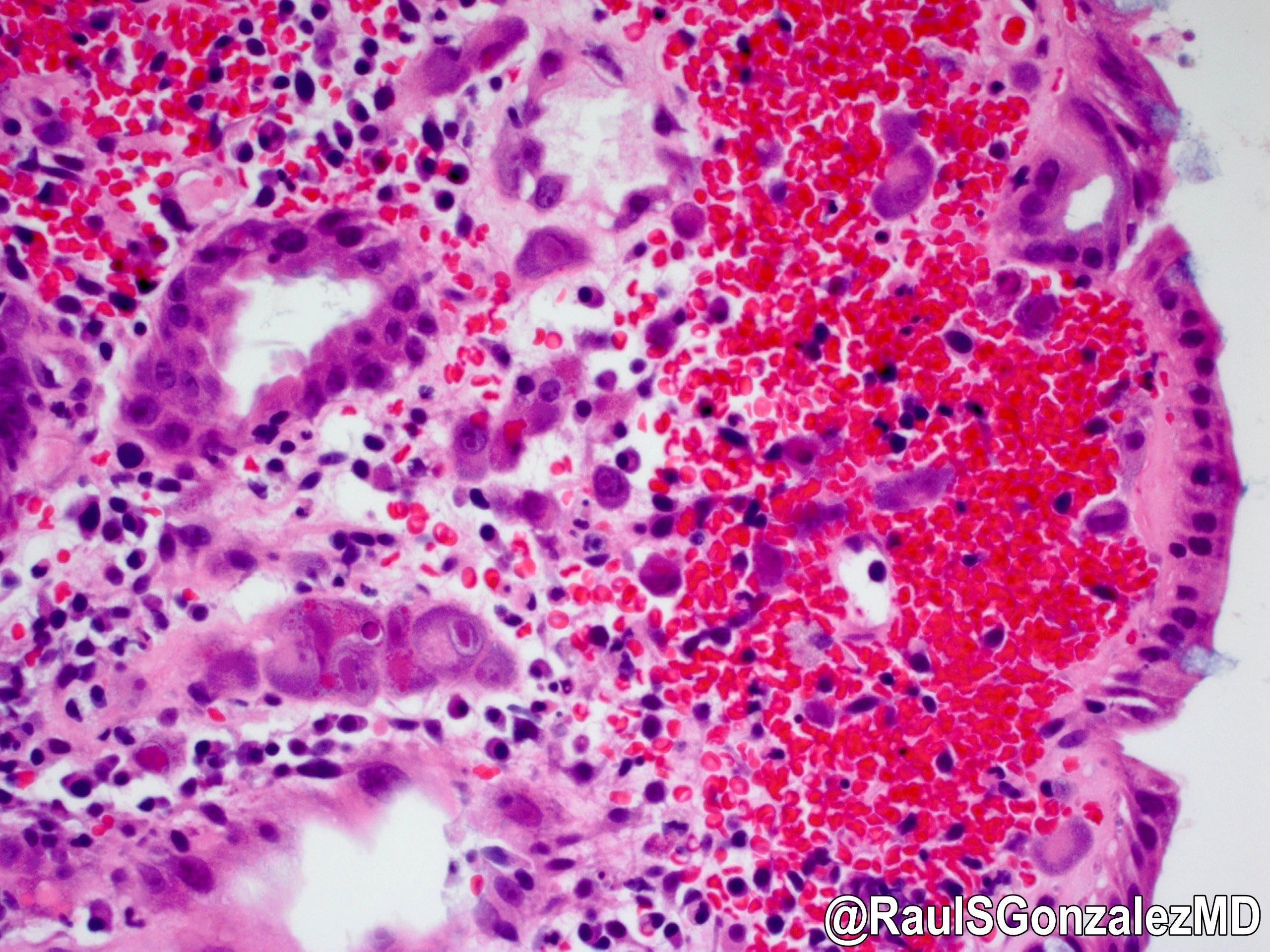
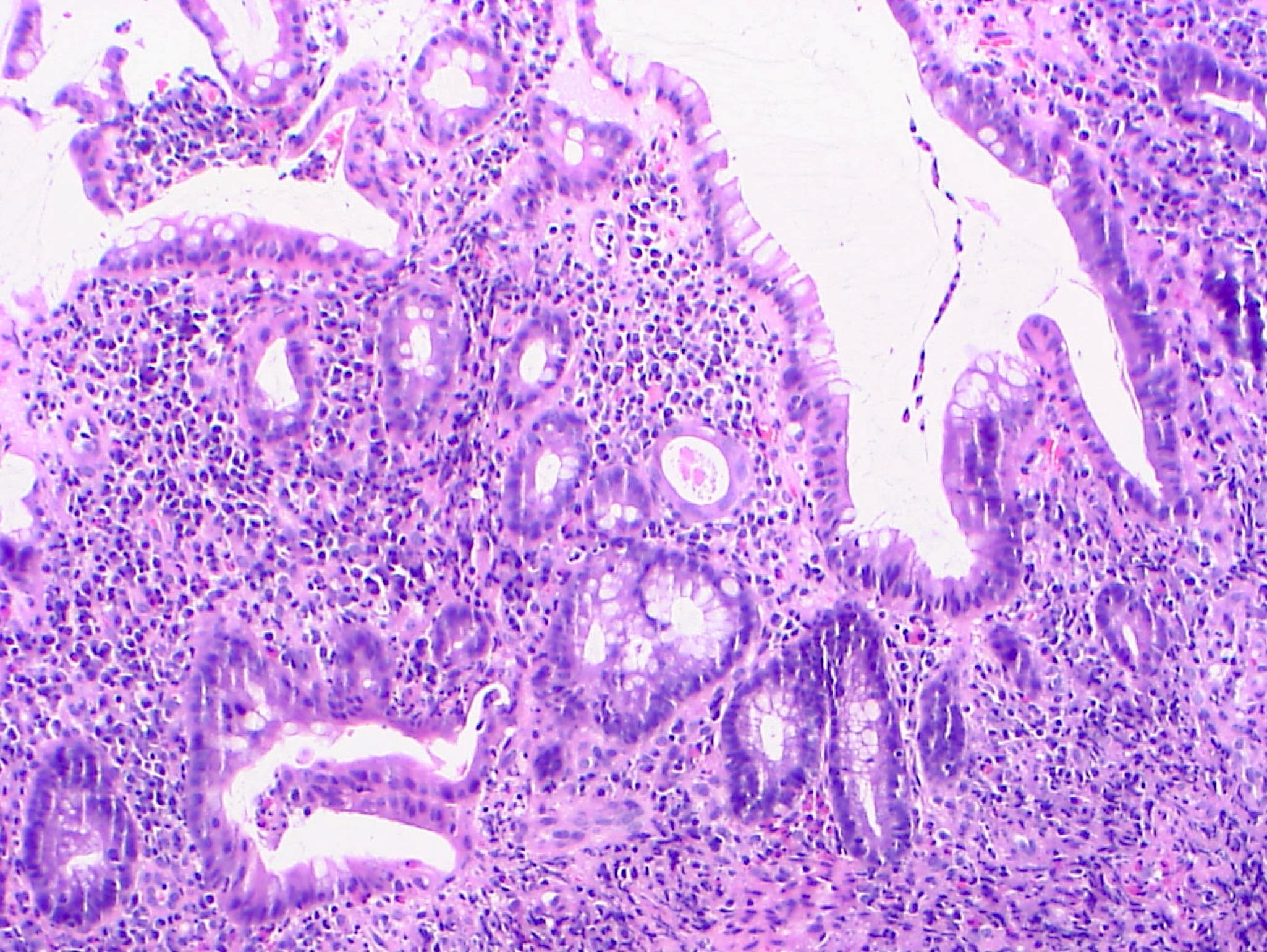
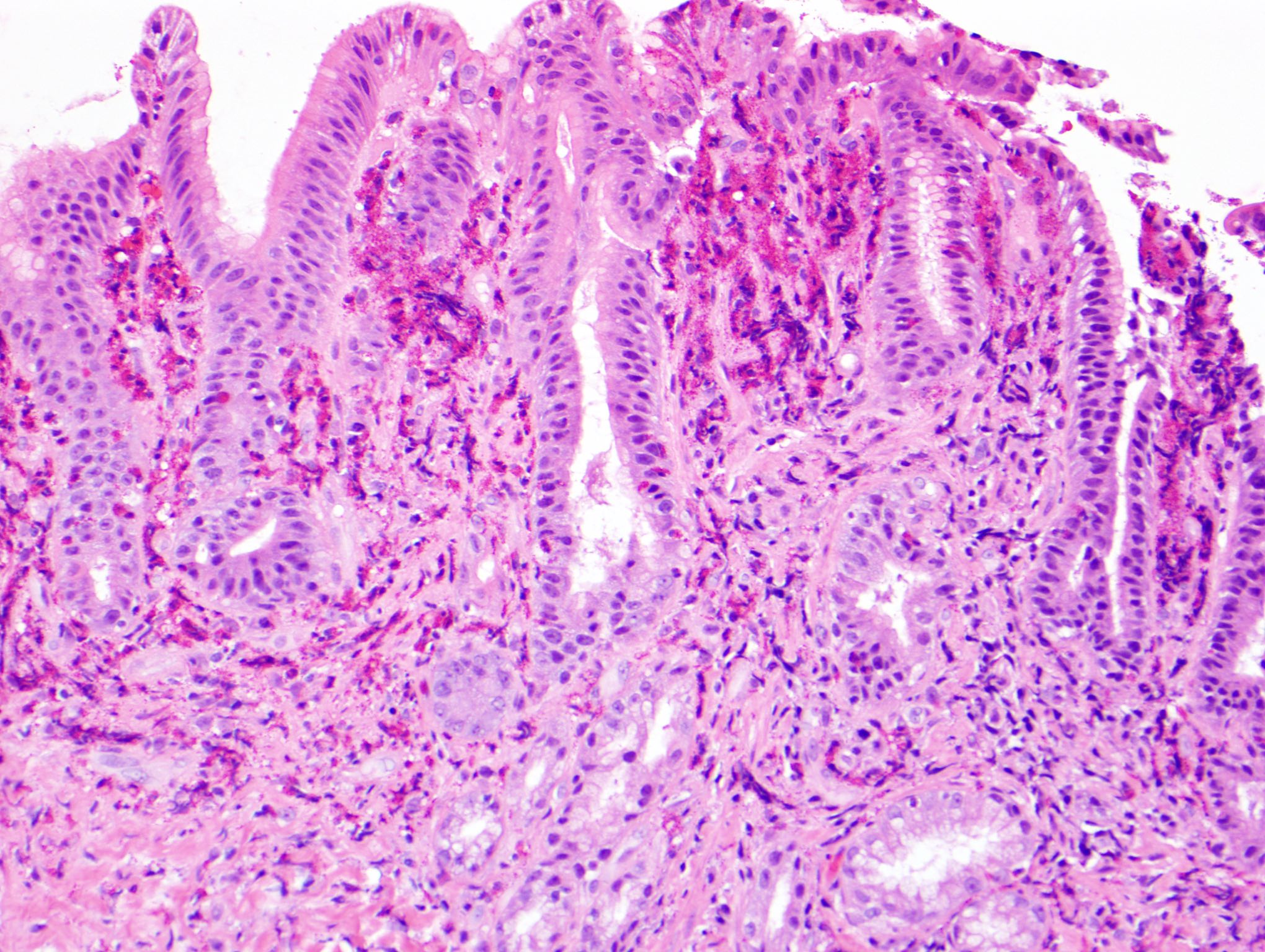
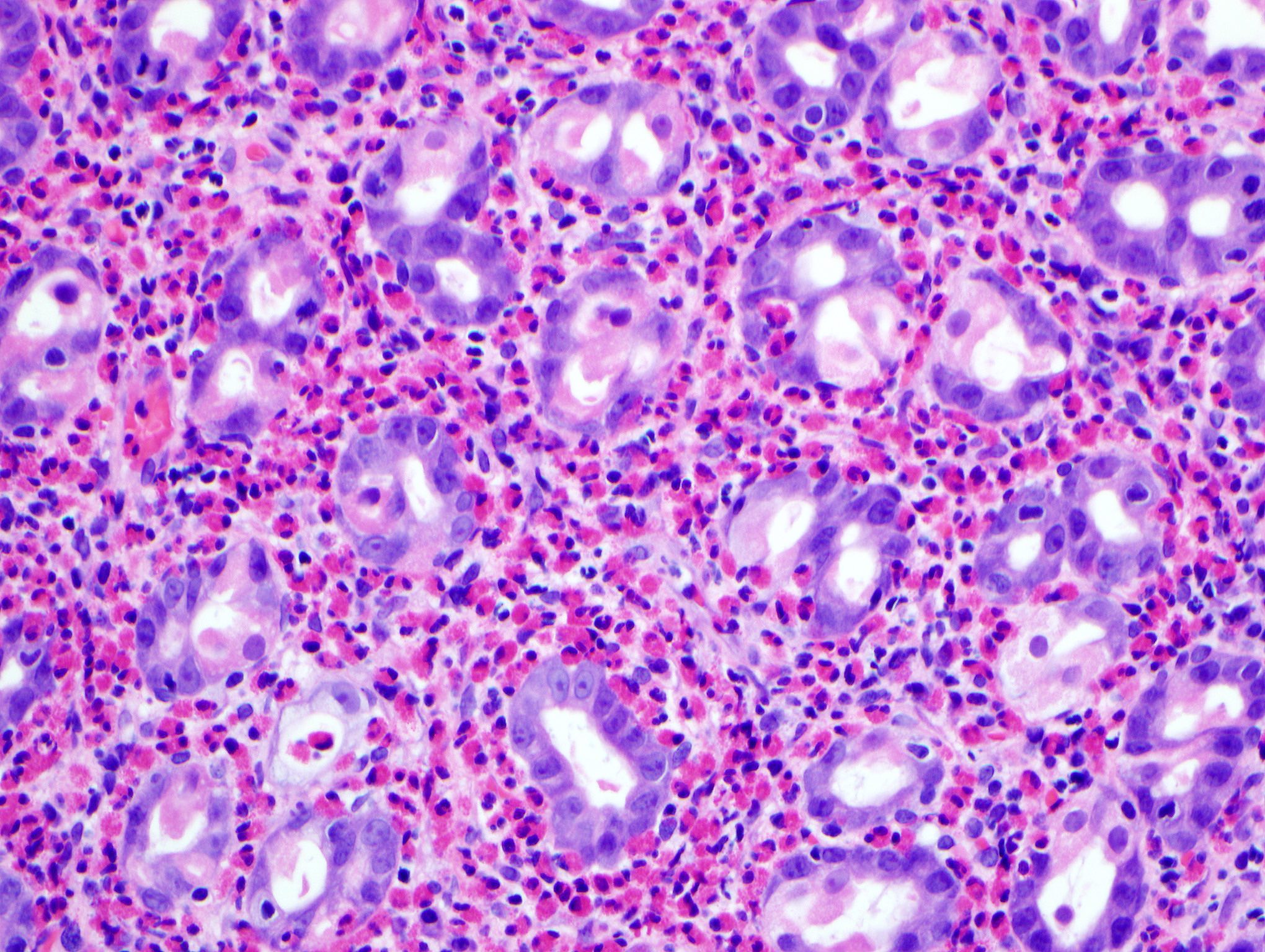
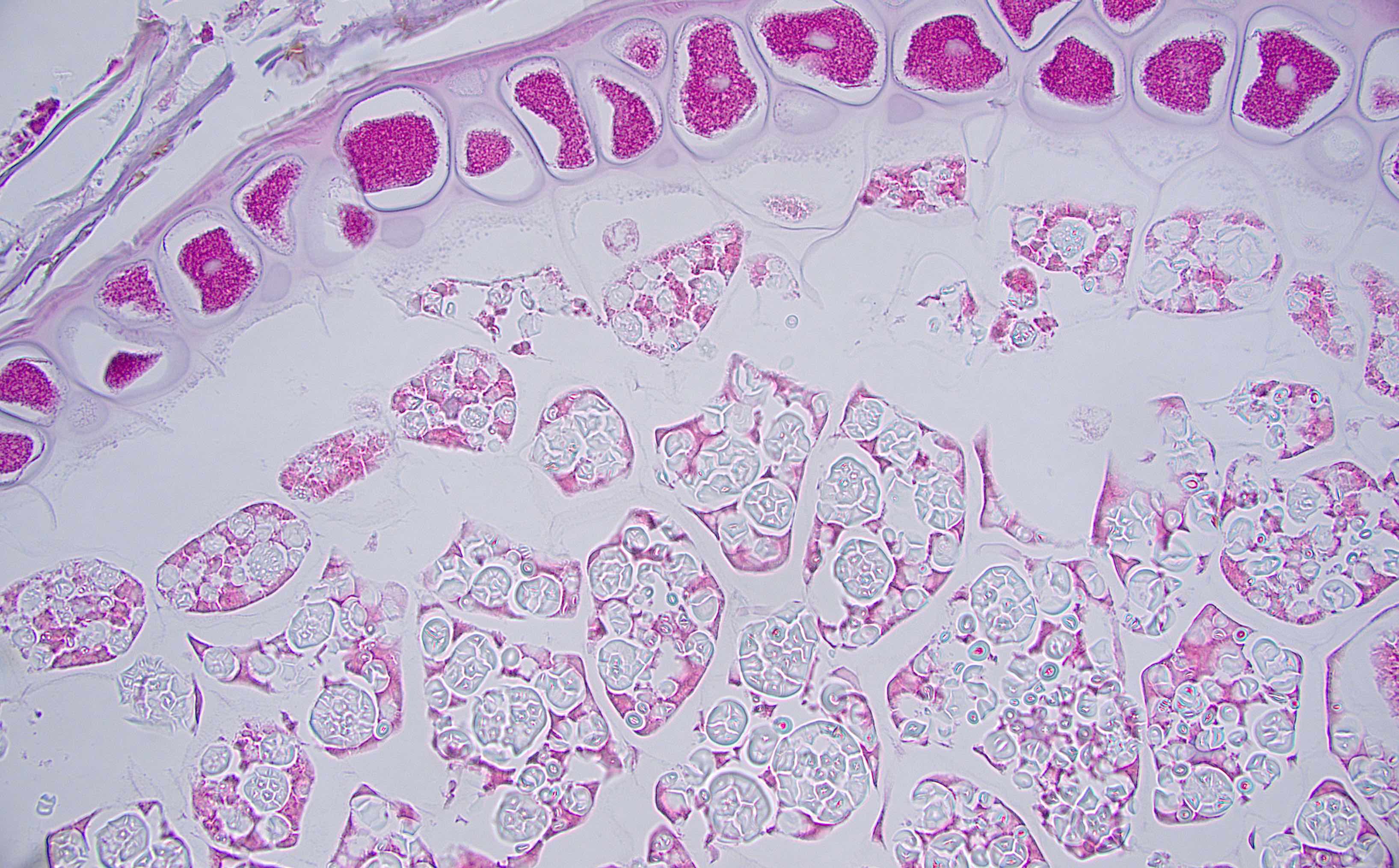
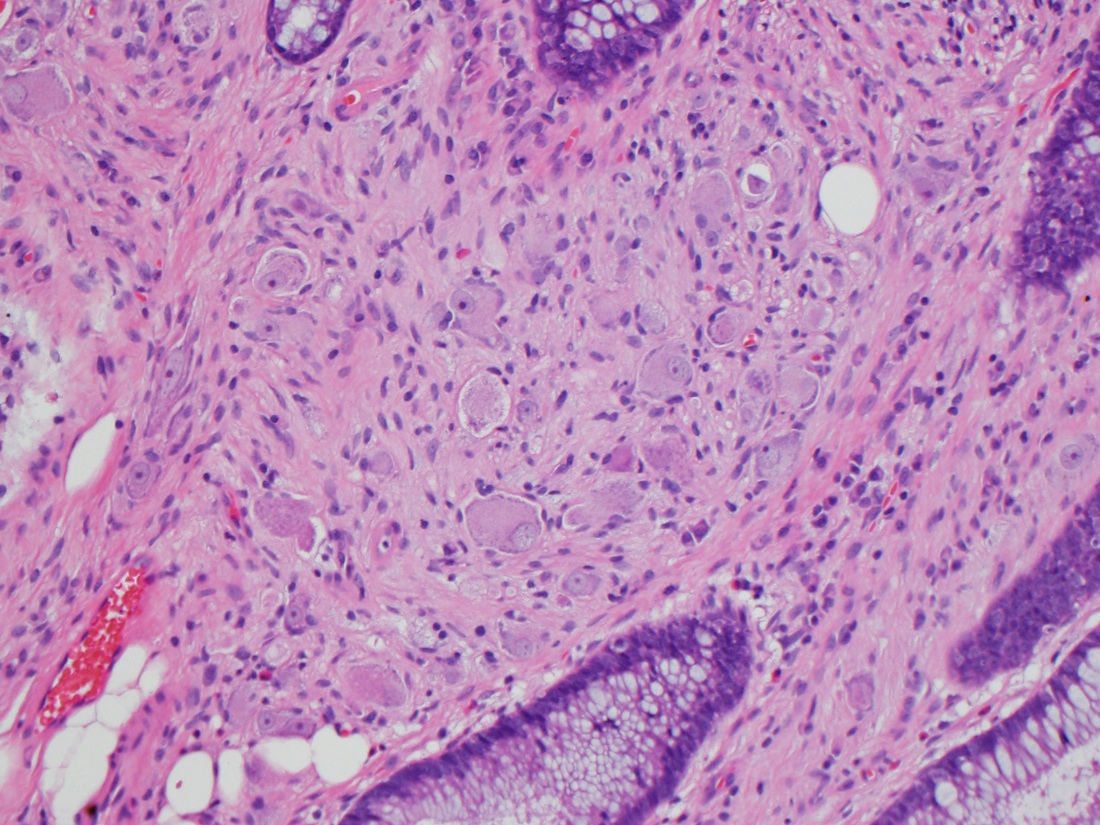
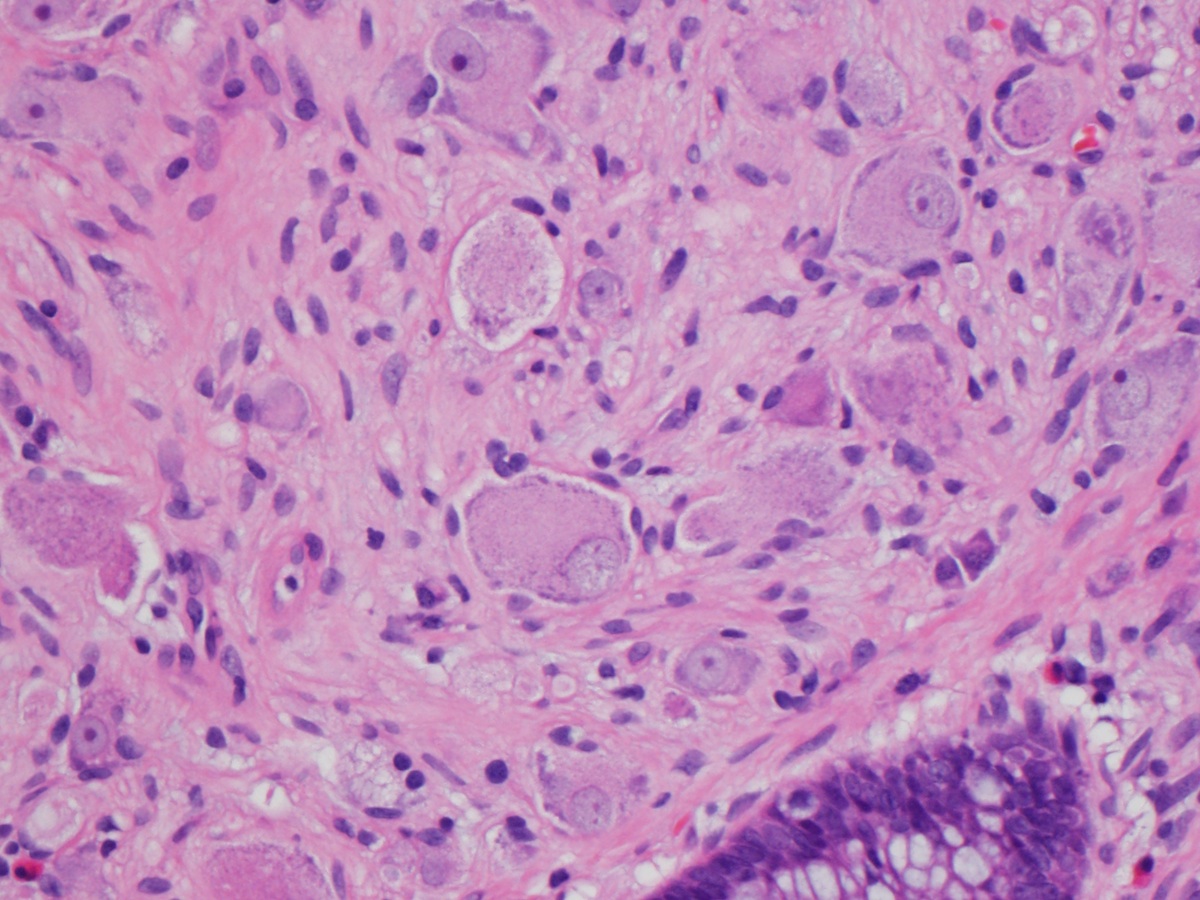
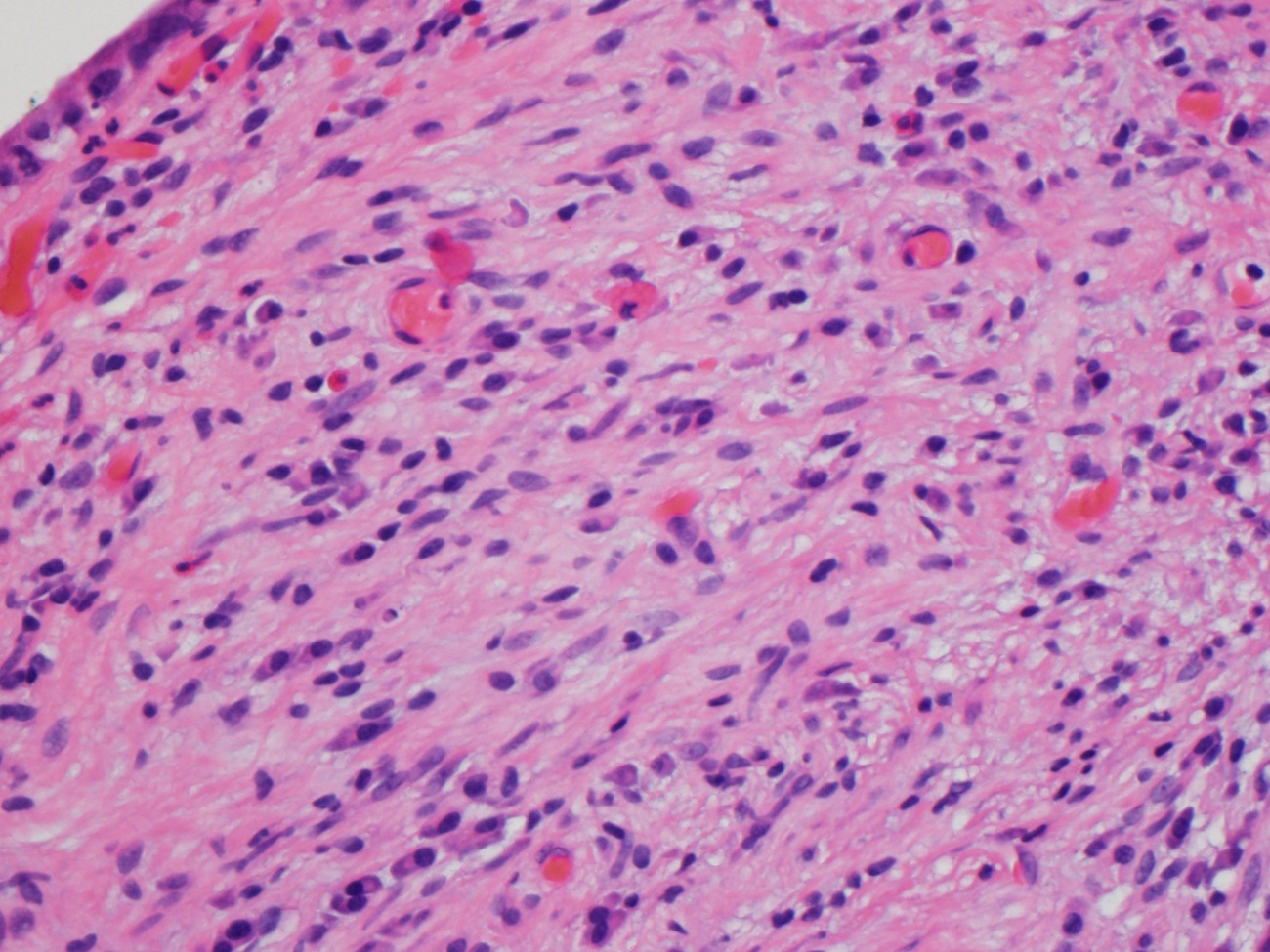
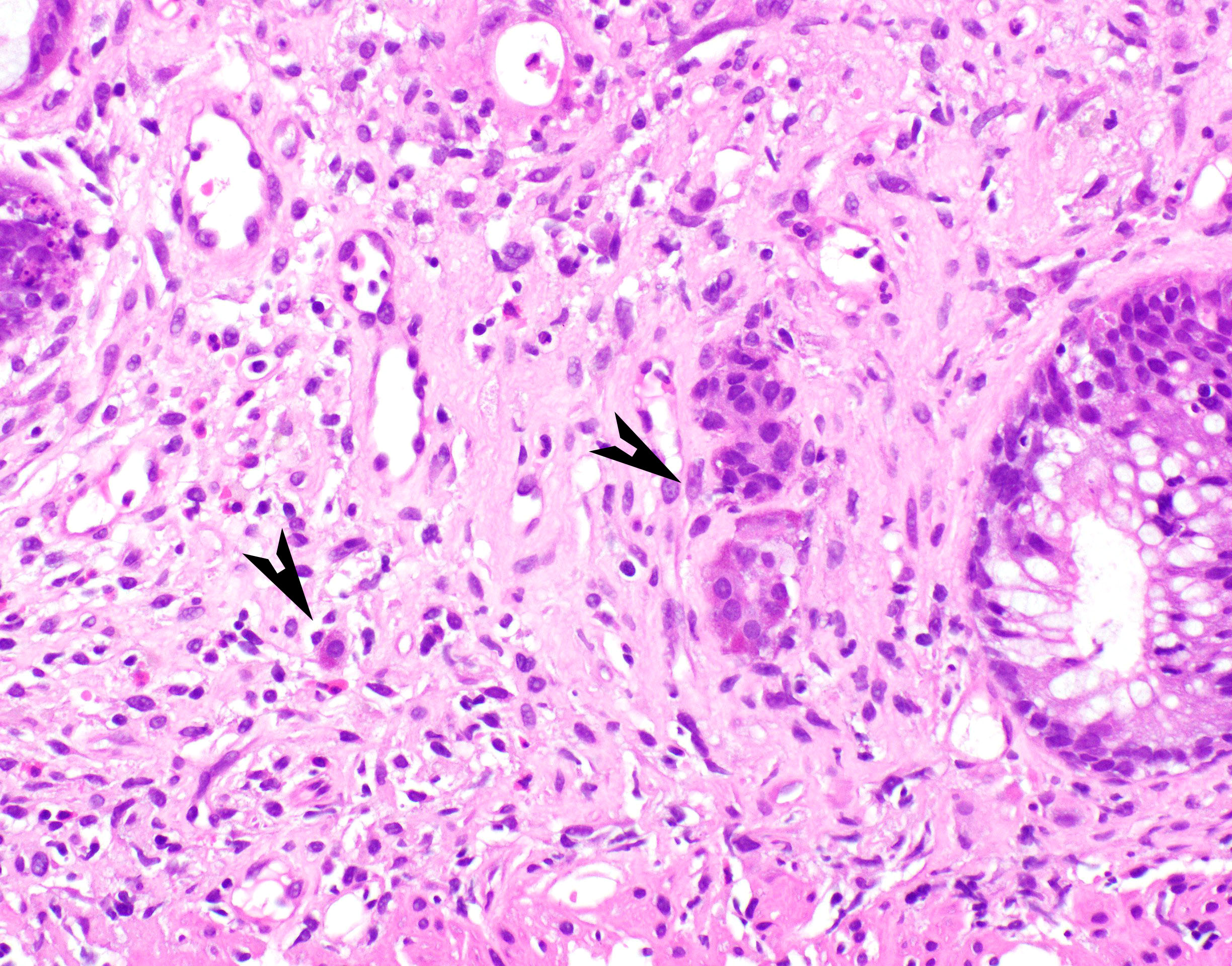
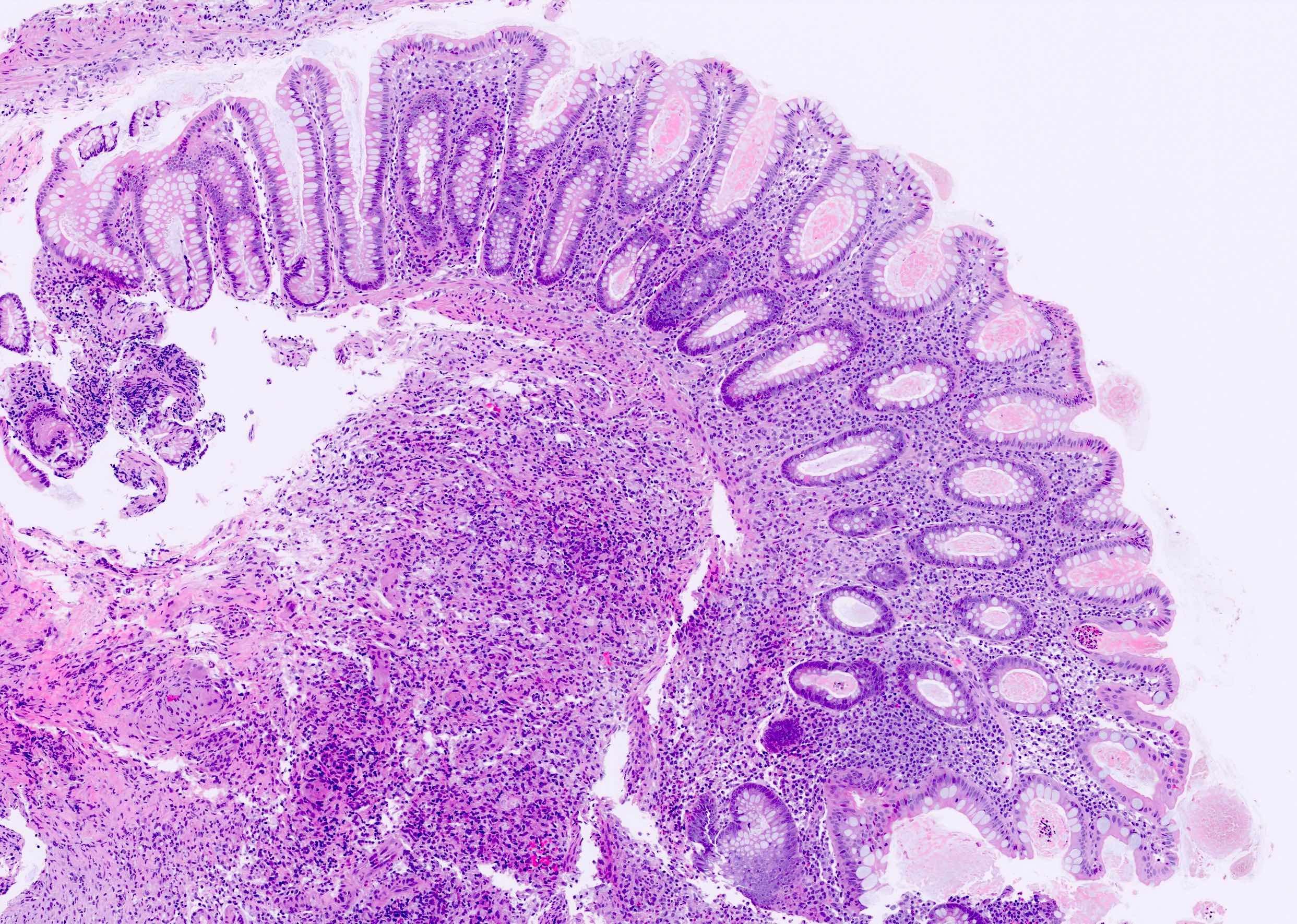
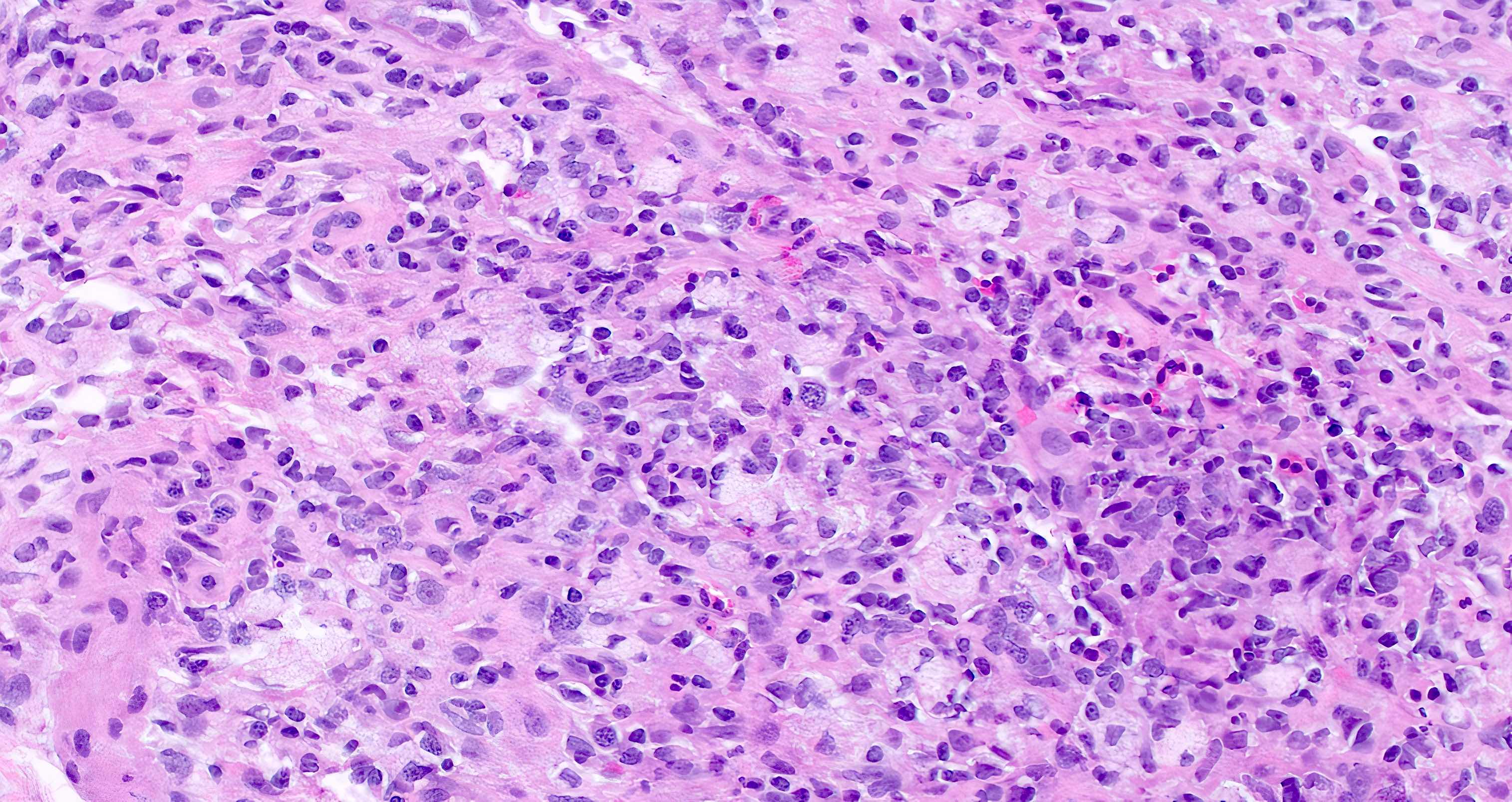
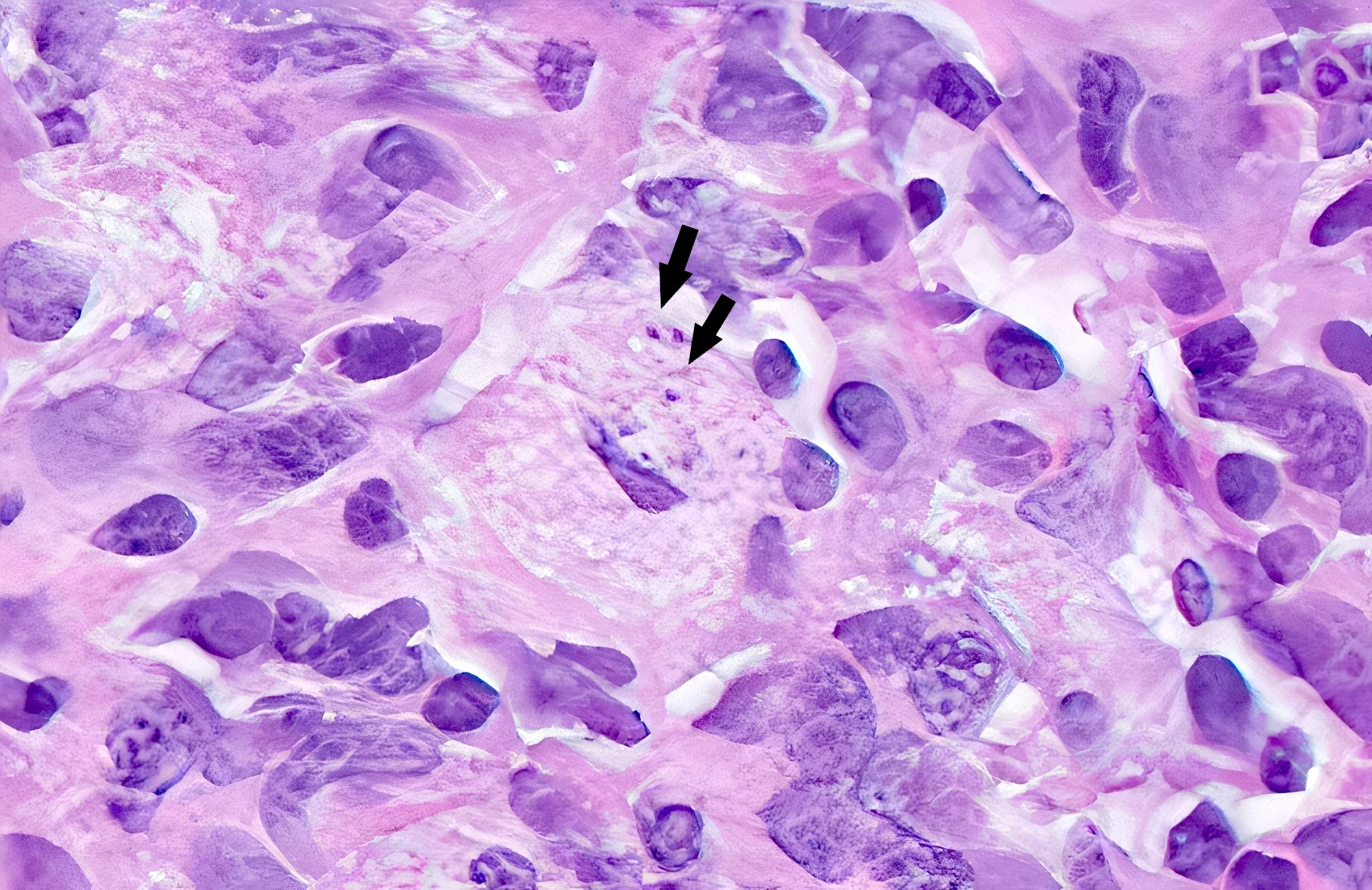
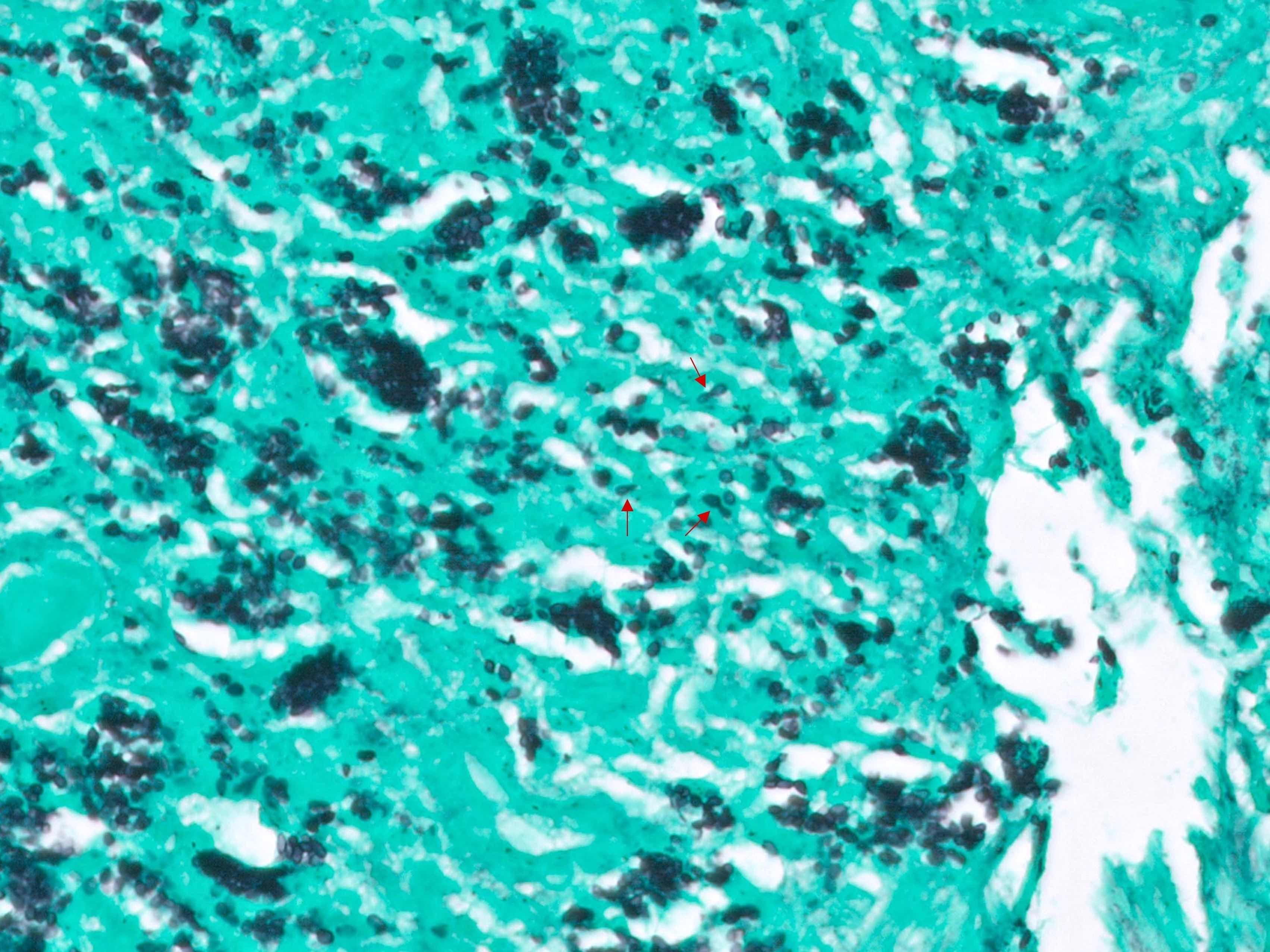
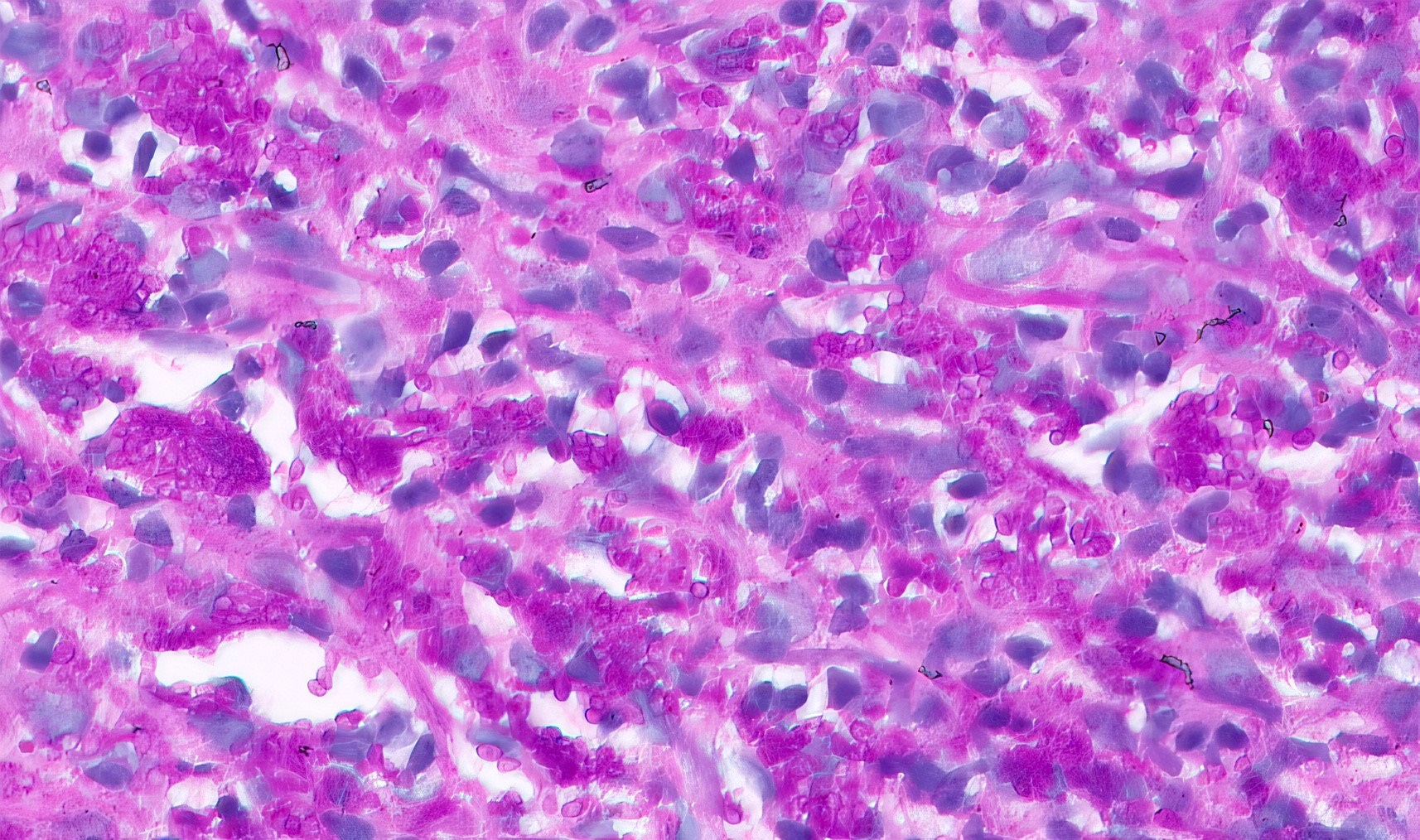
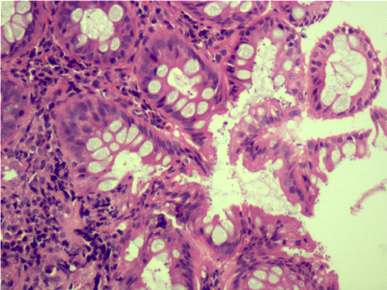
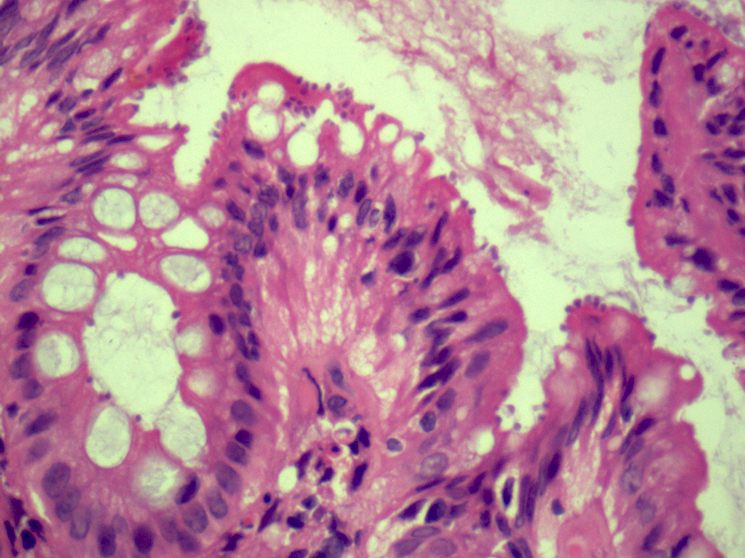
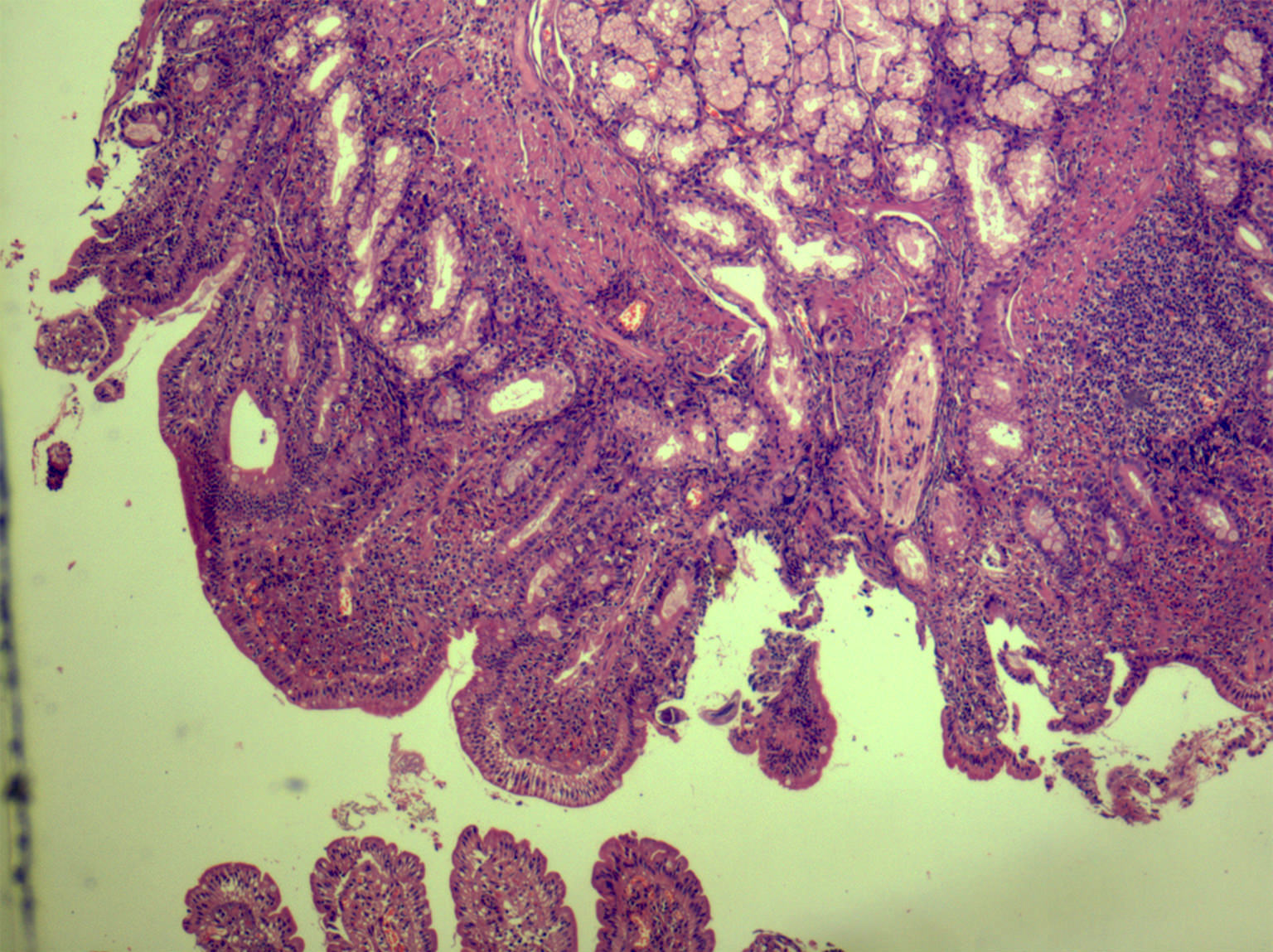
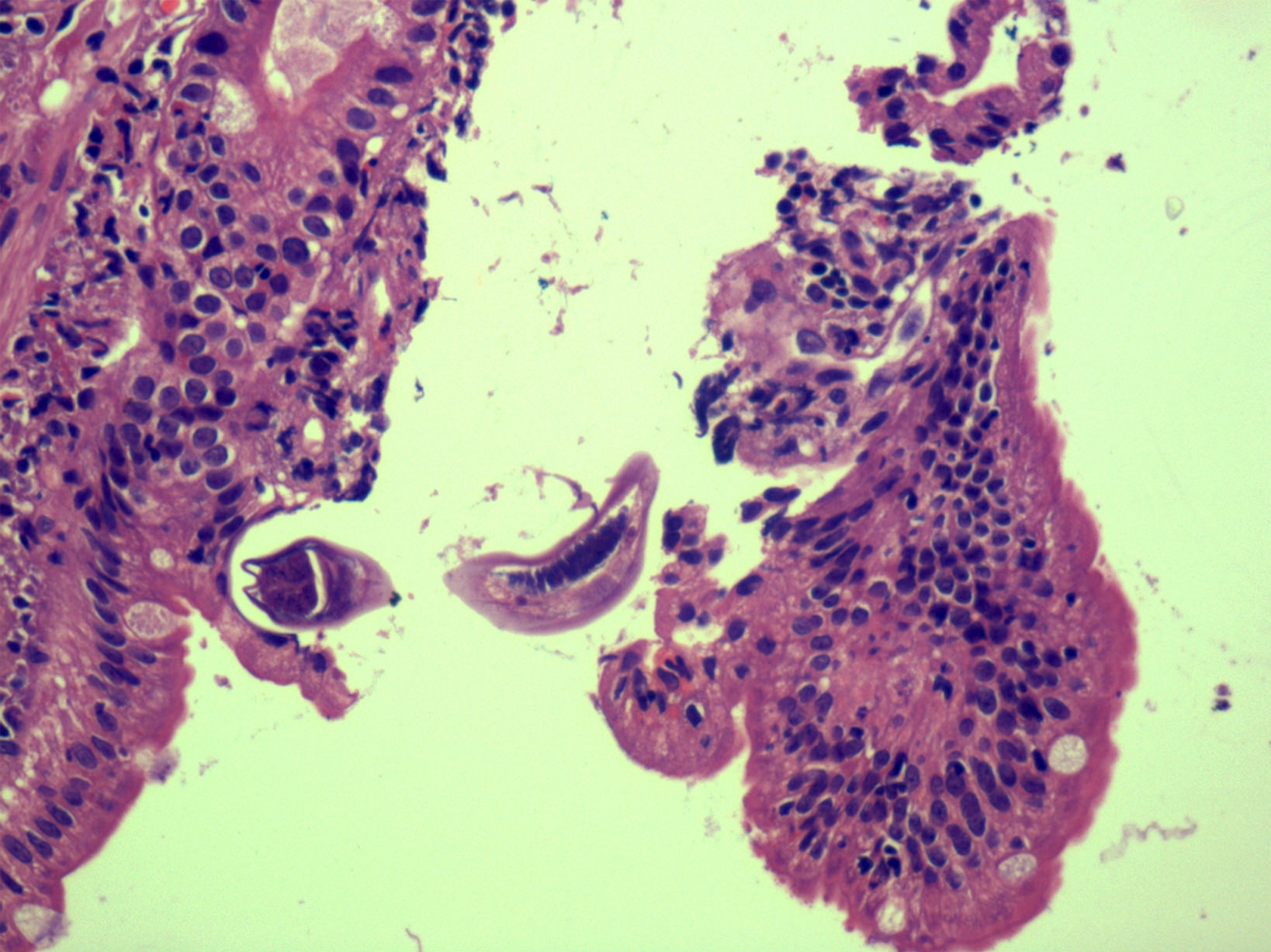
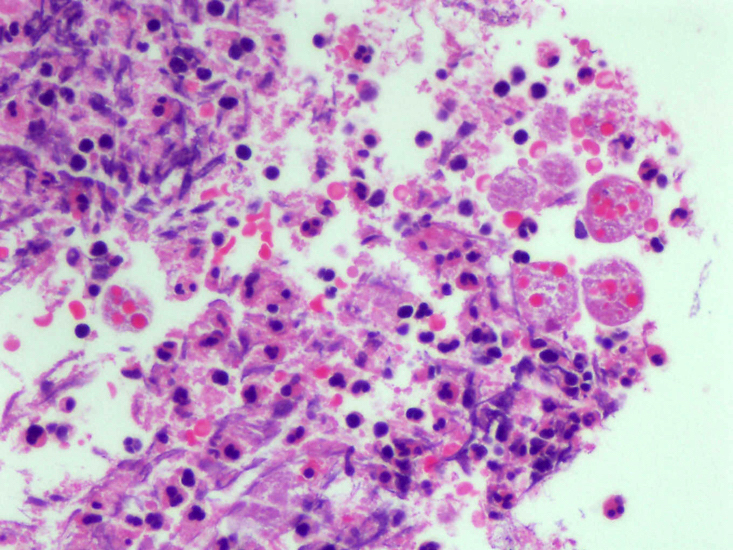
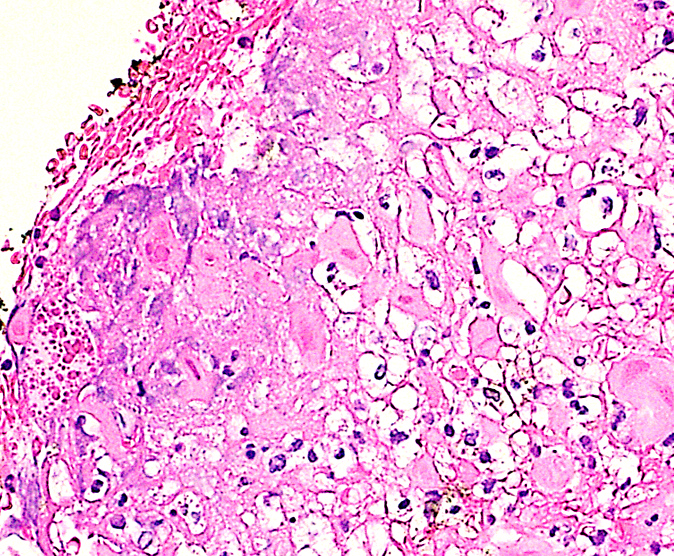
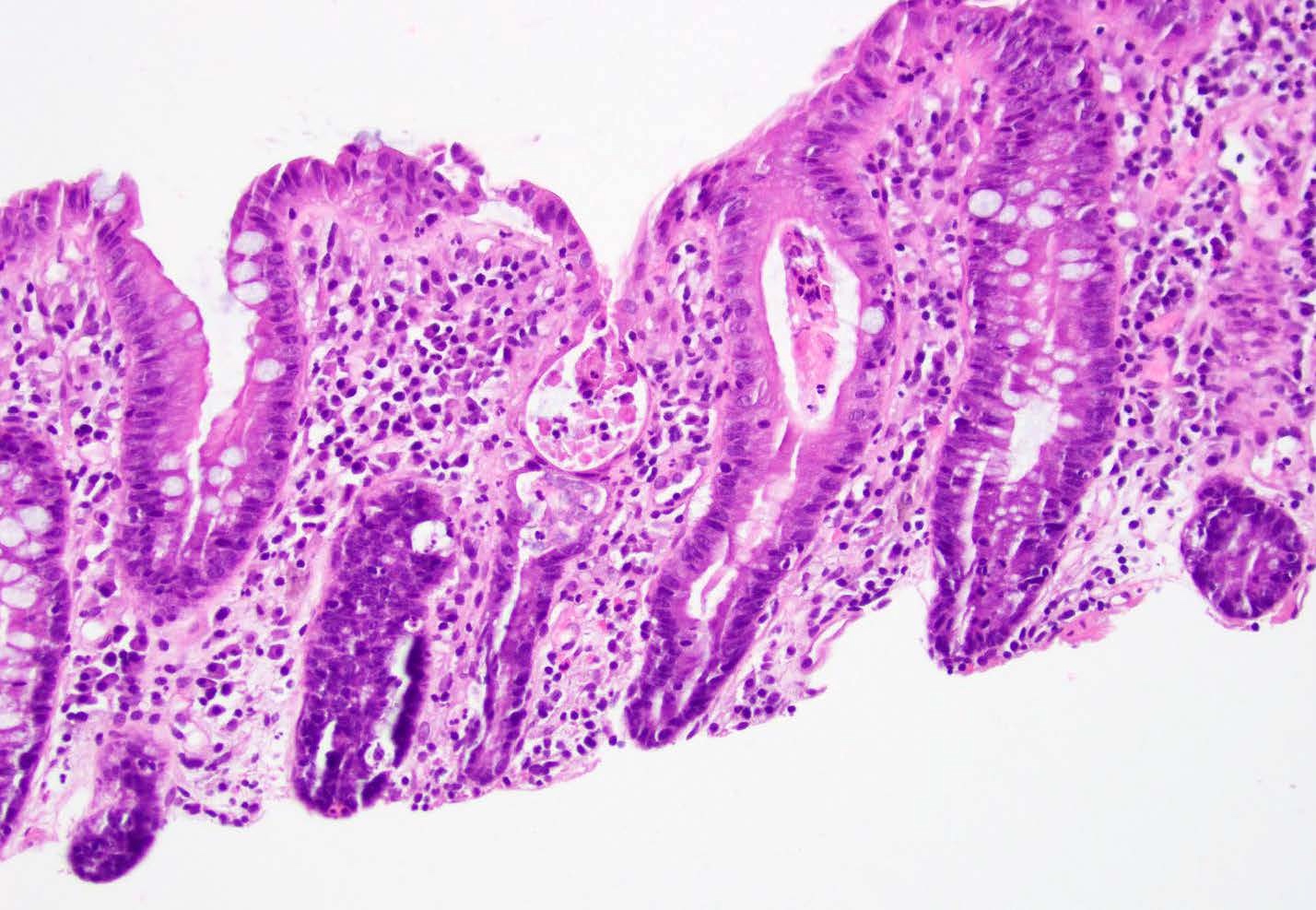
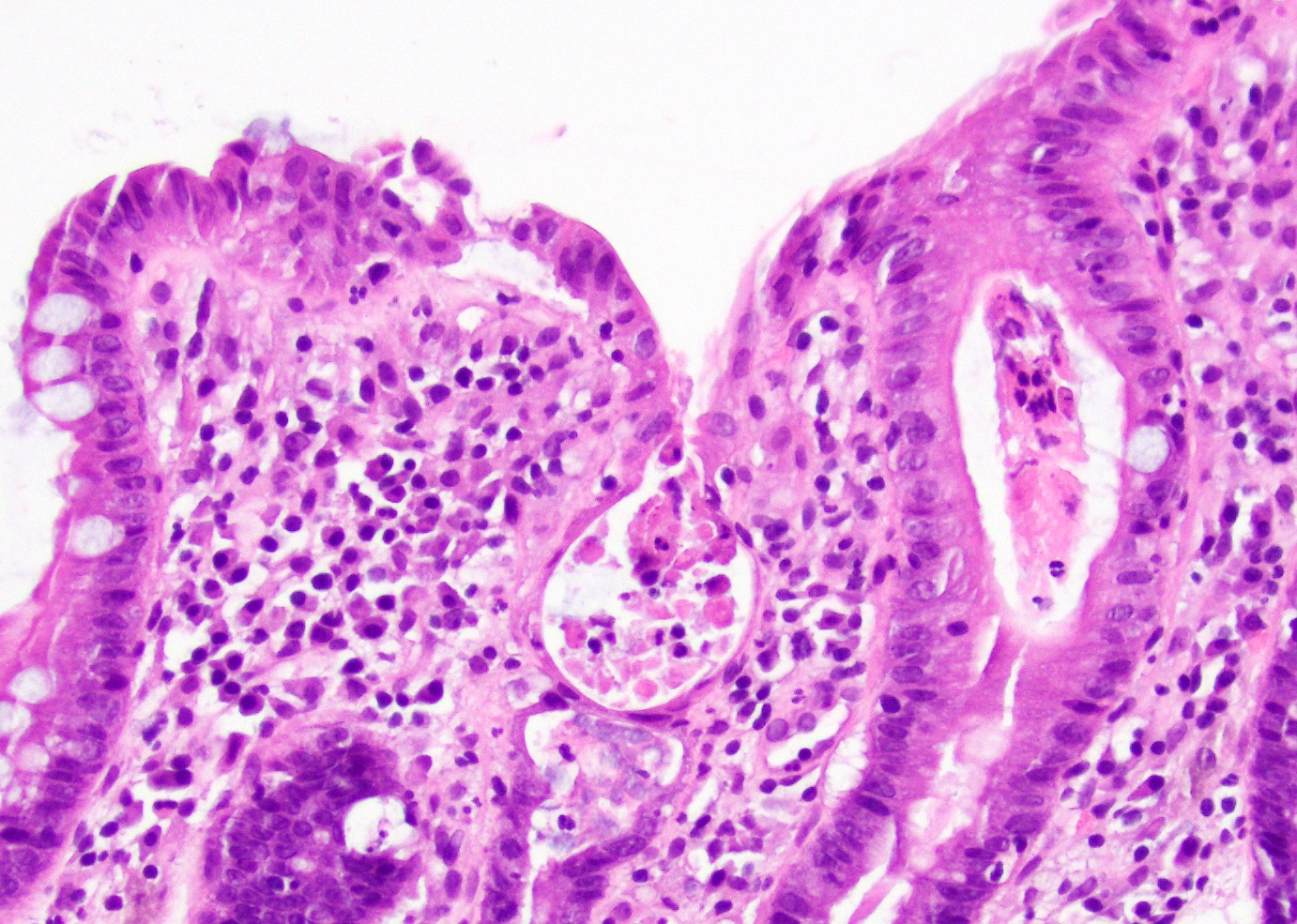
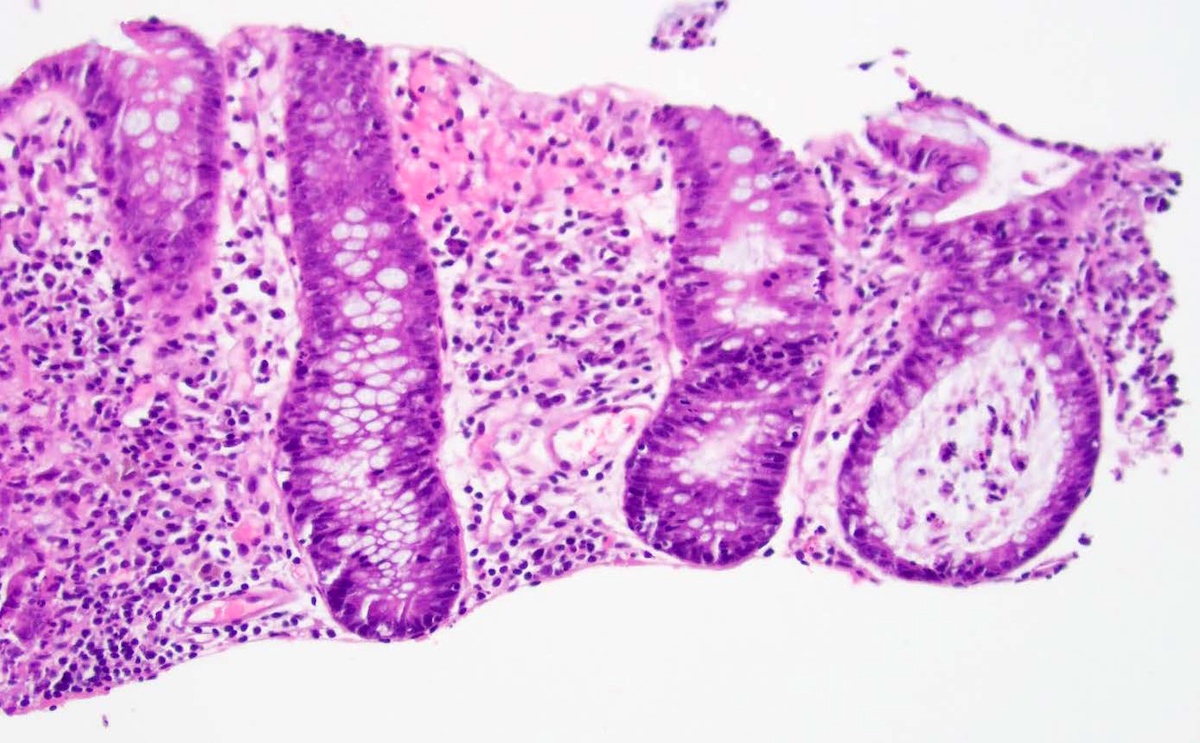
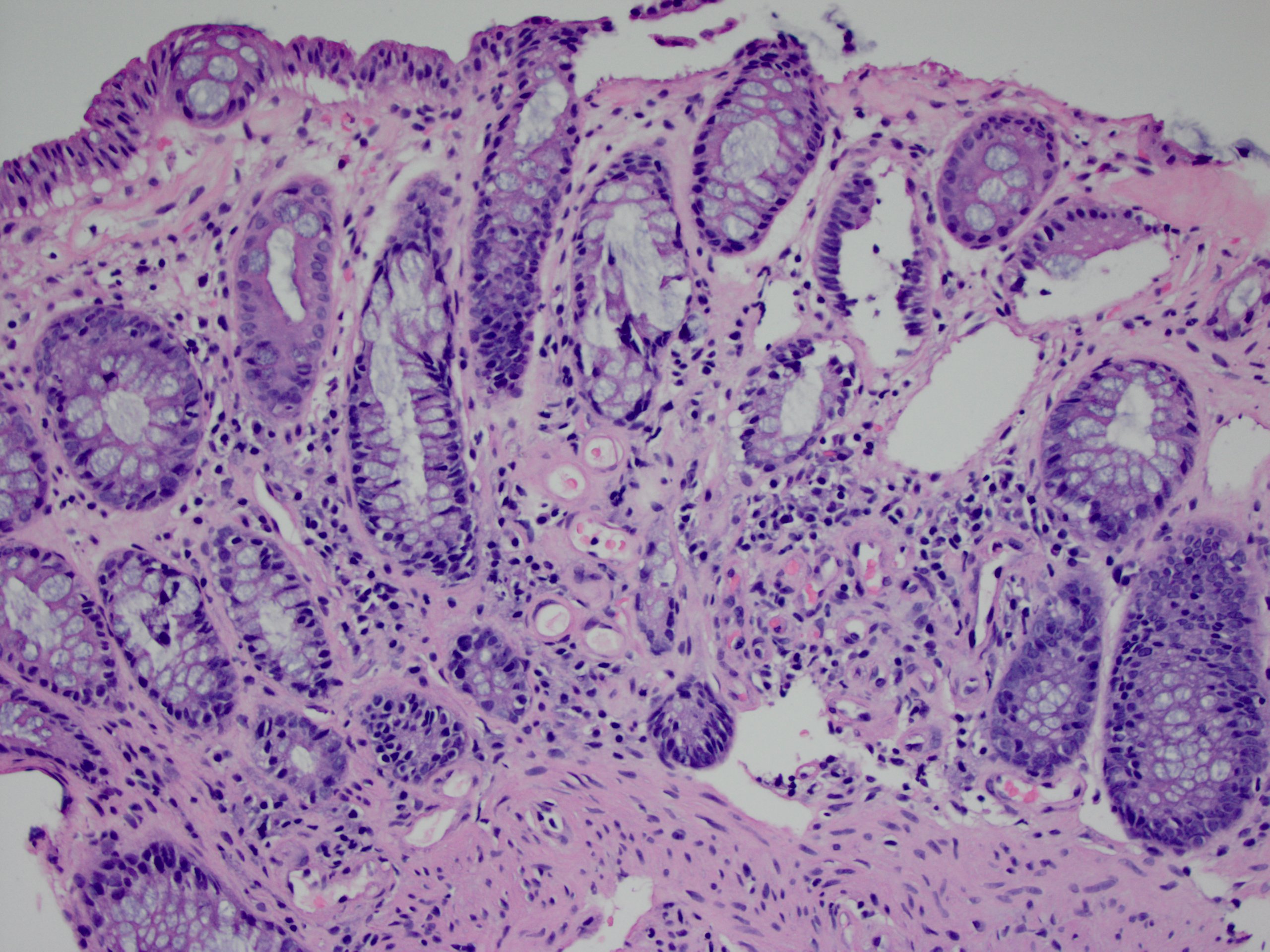
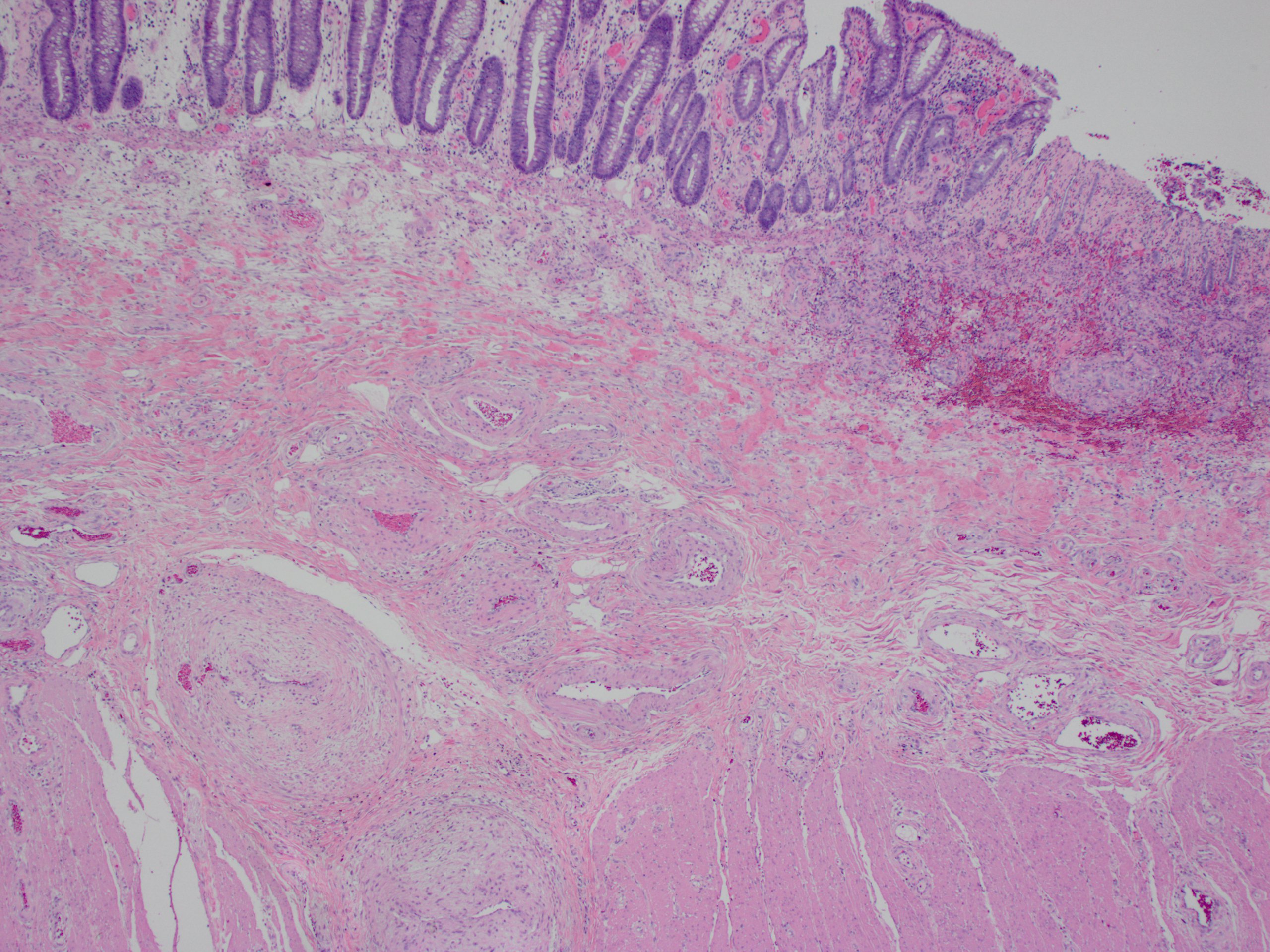
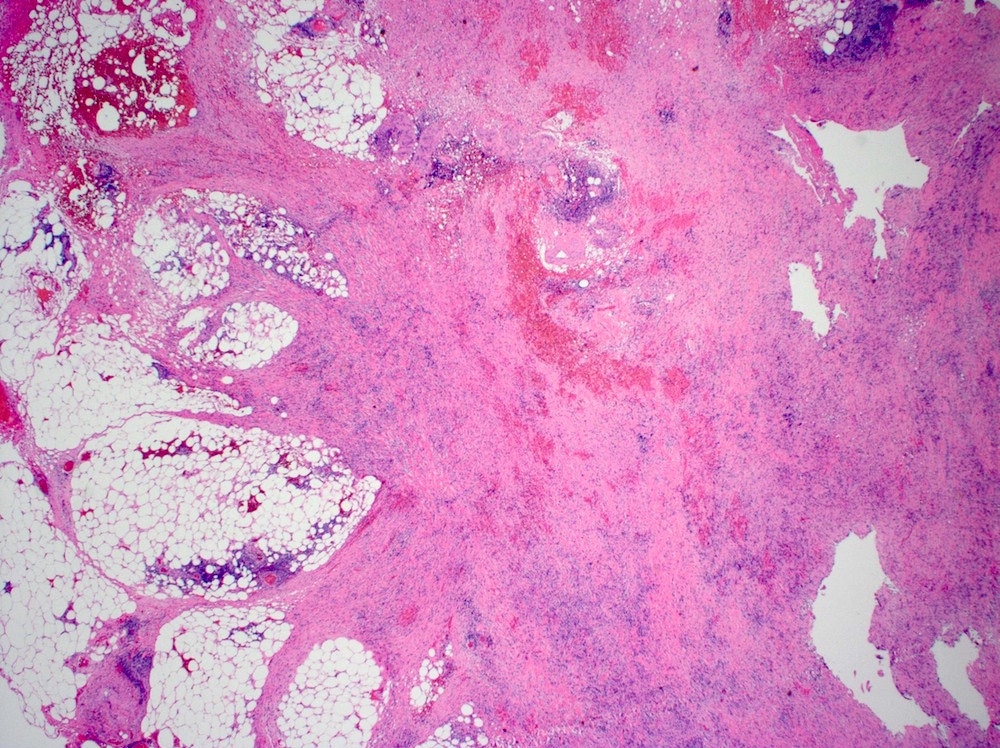
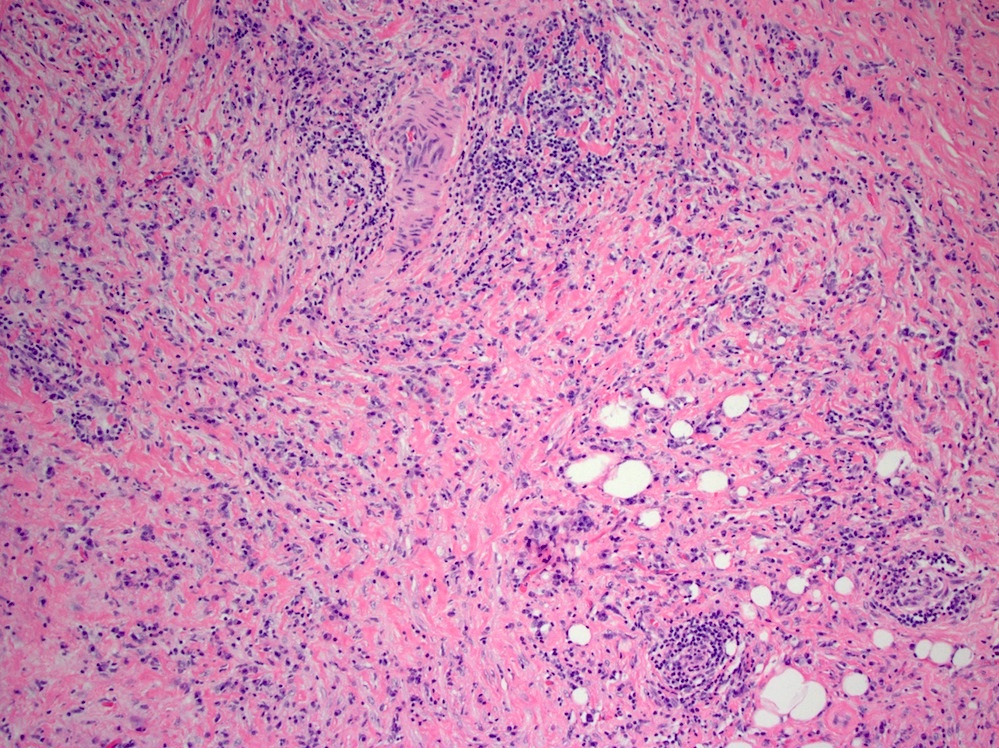
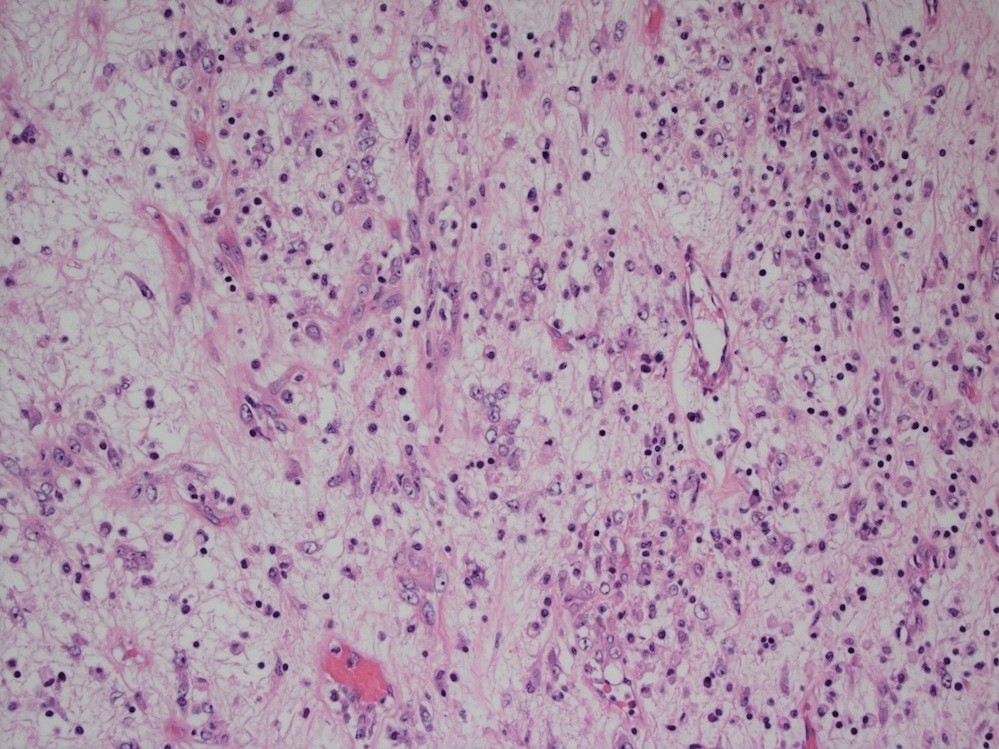
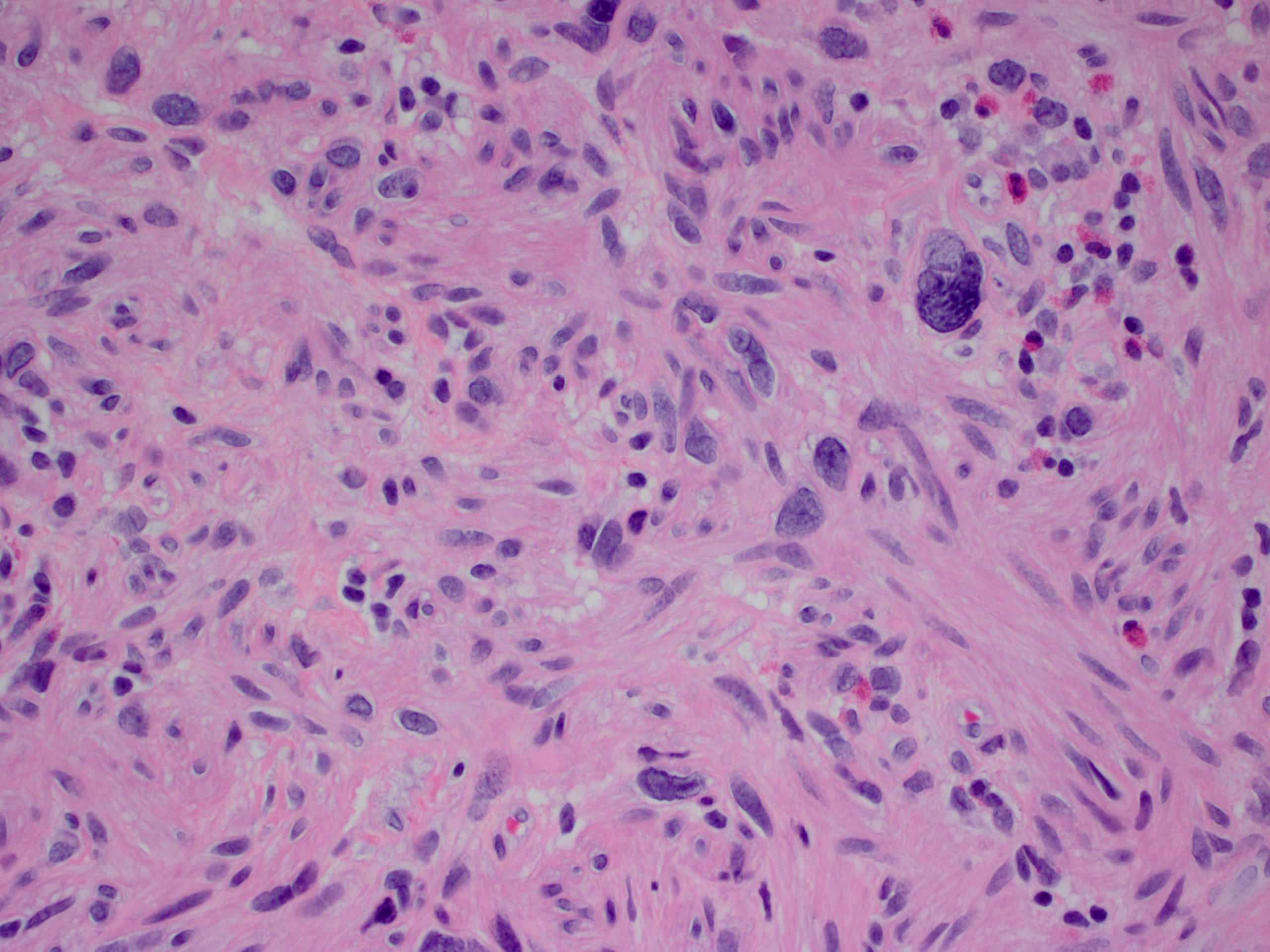
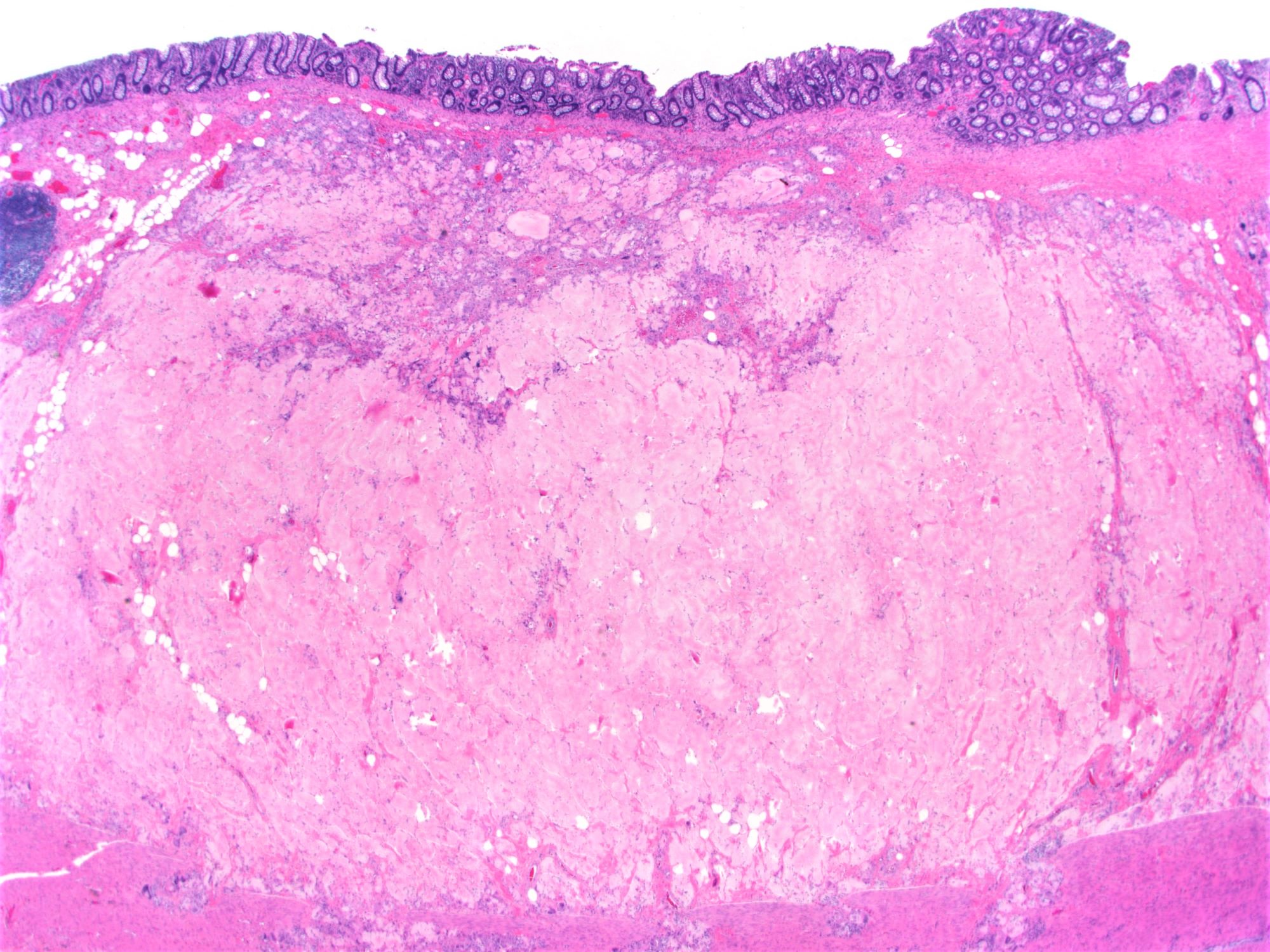
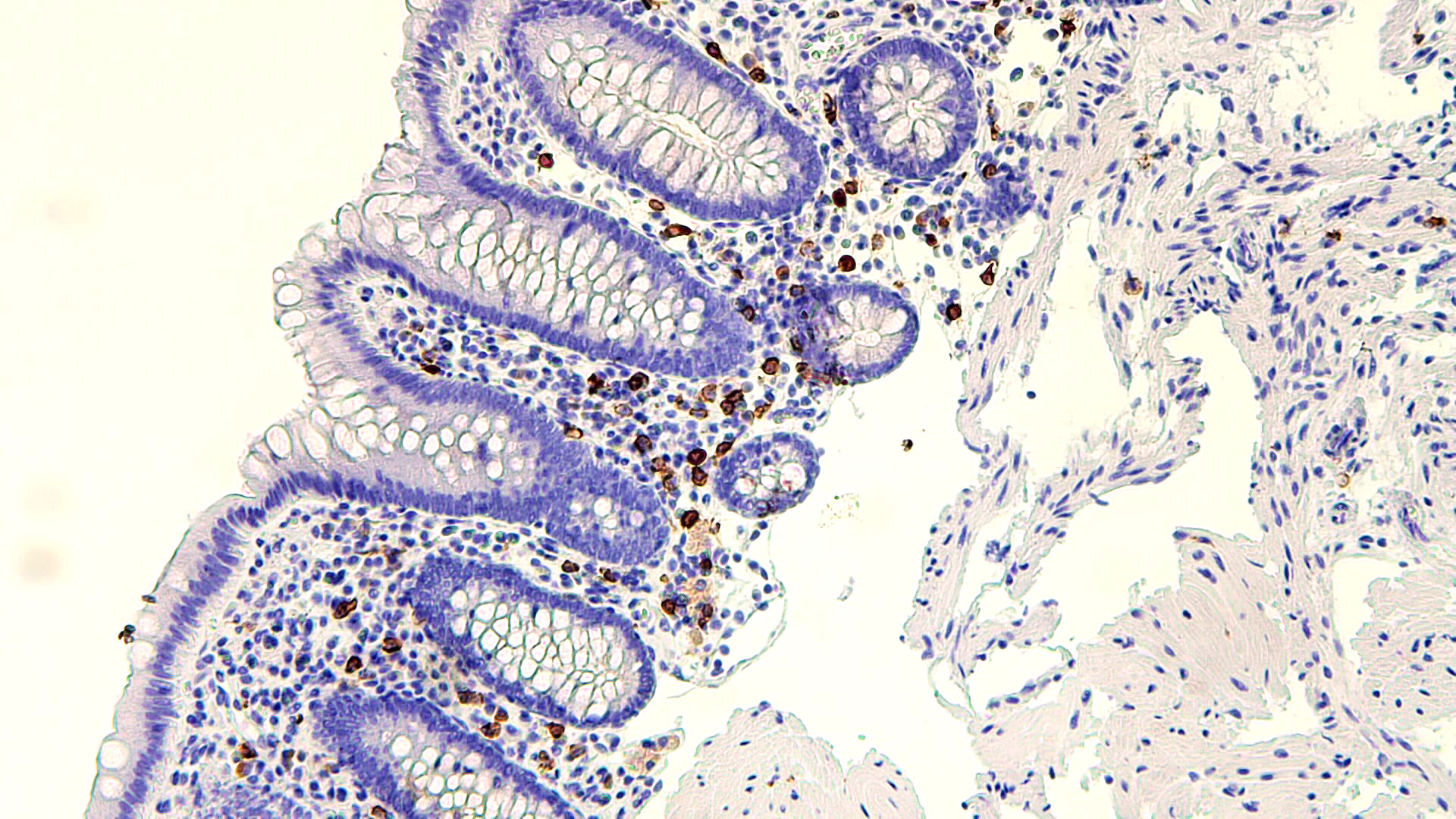
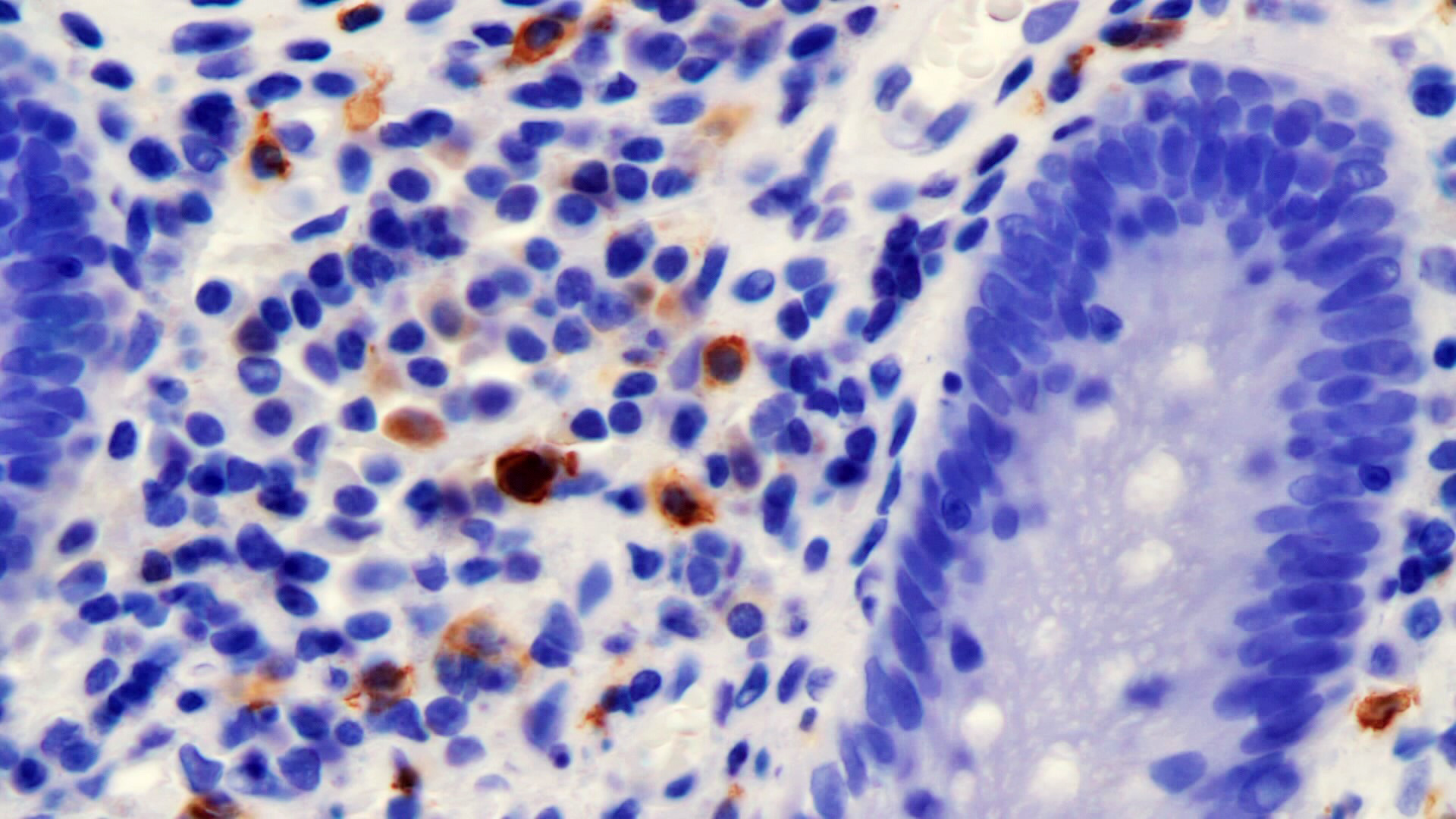
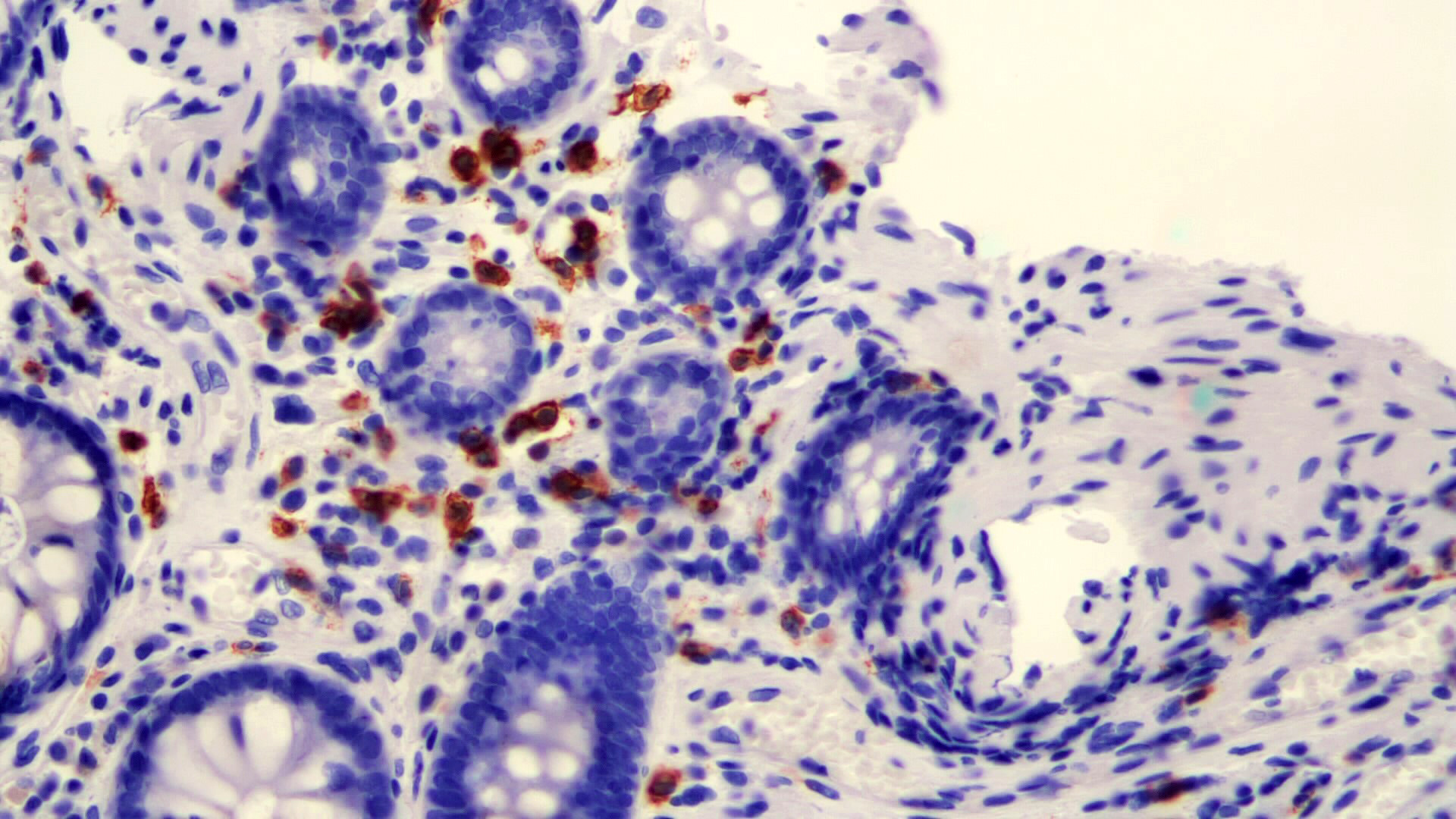
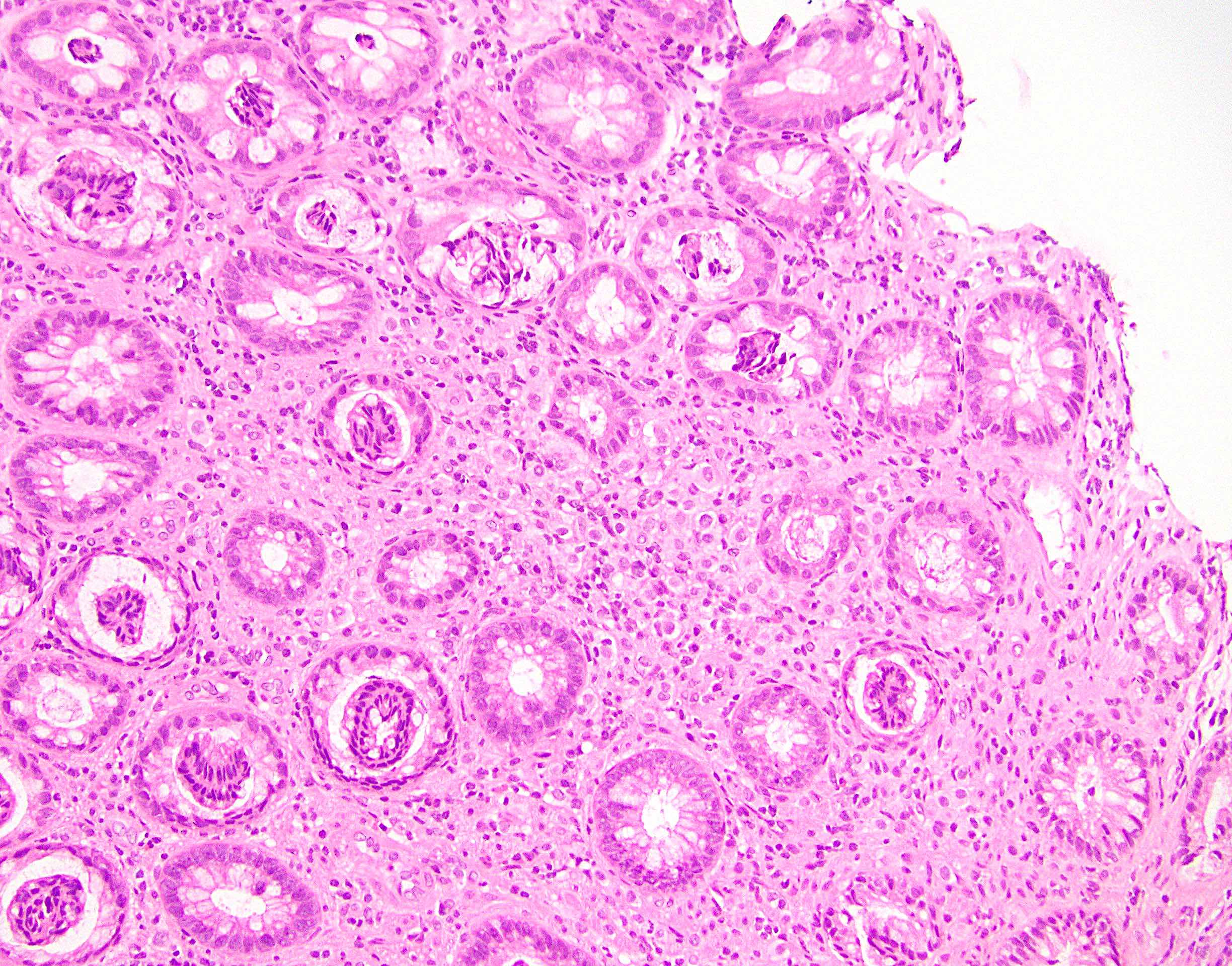
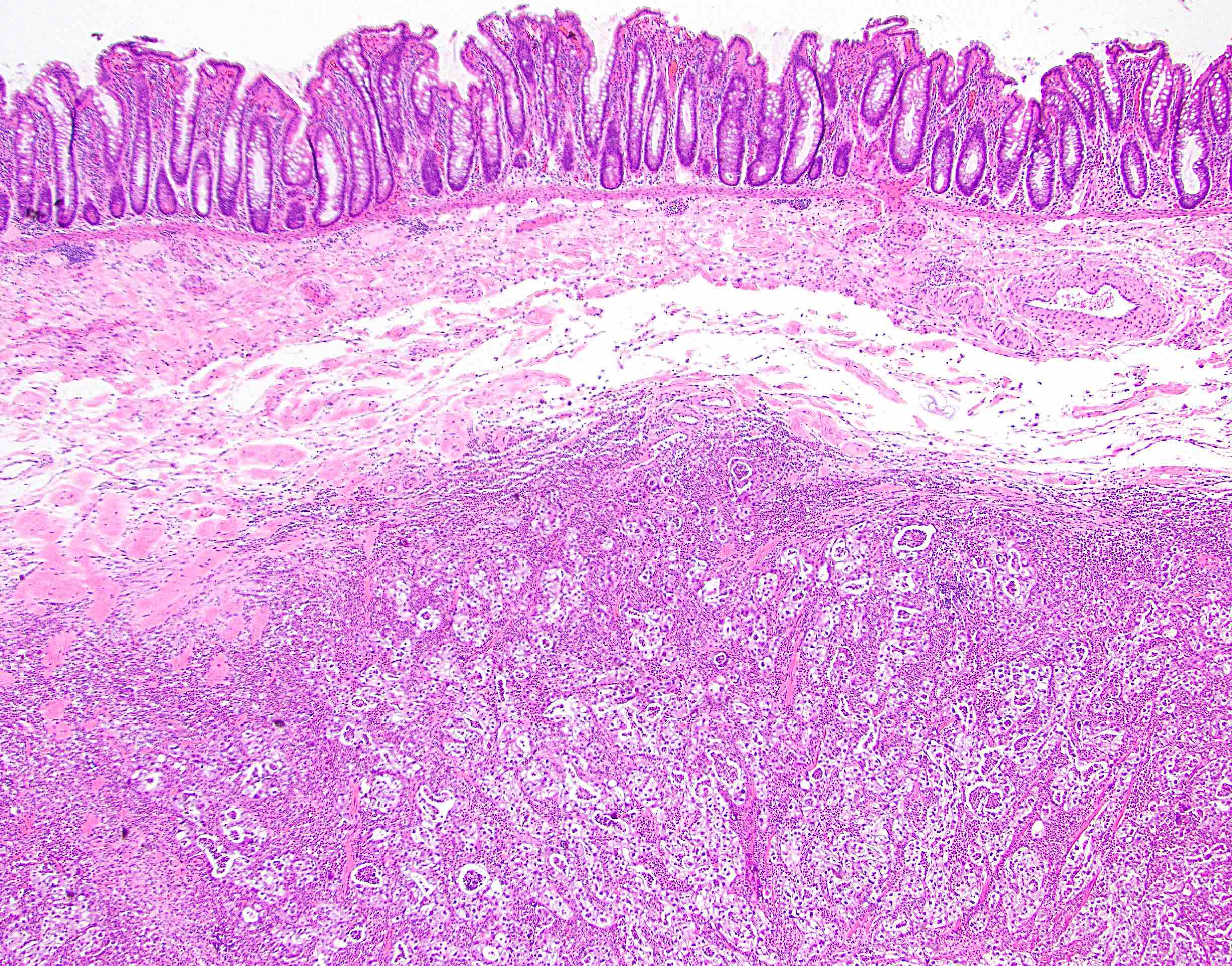
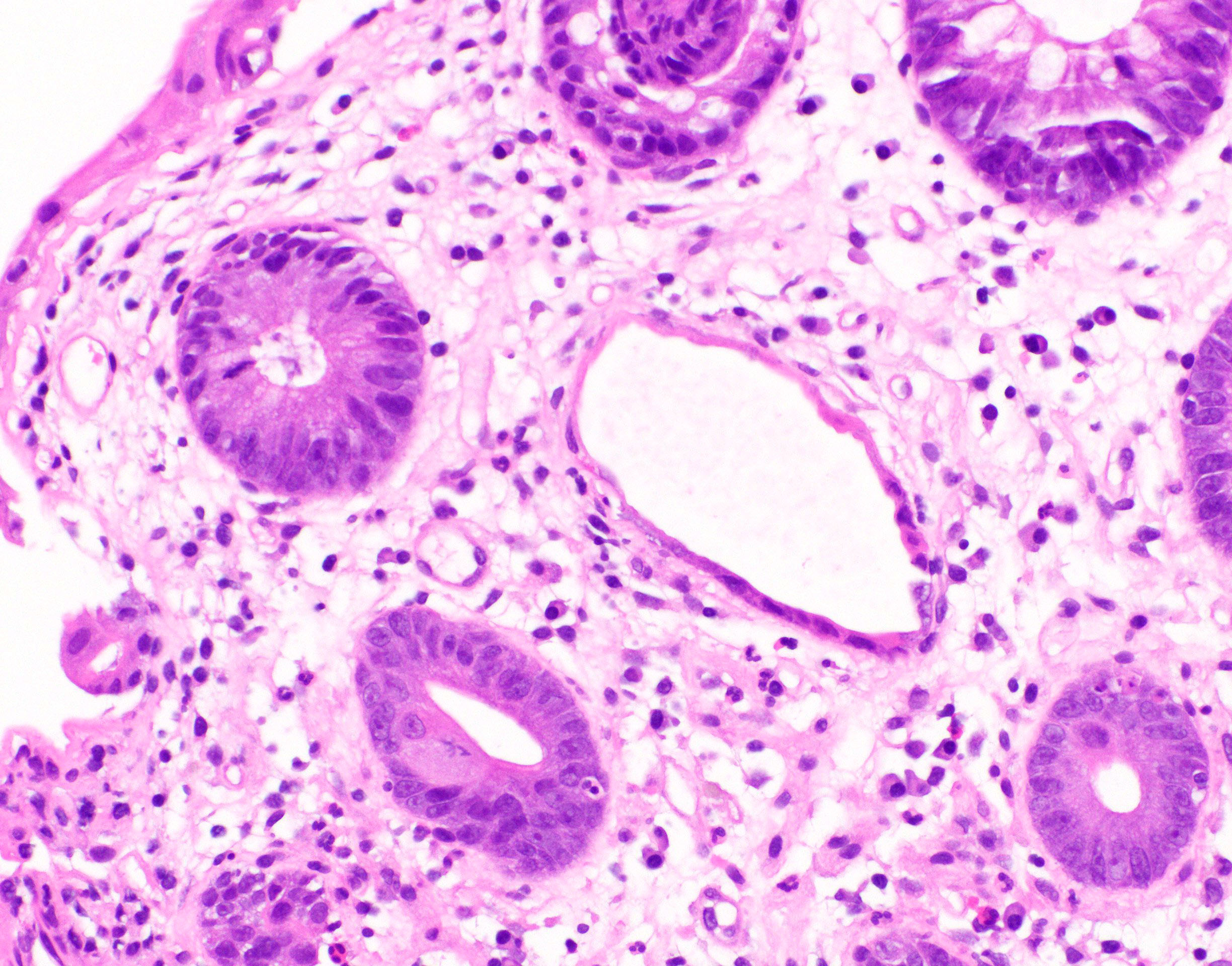
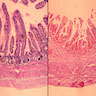
Microscopic (histologic) description
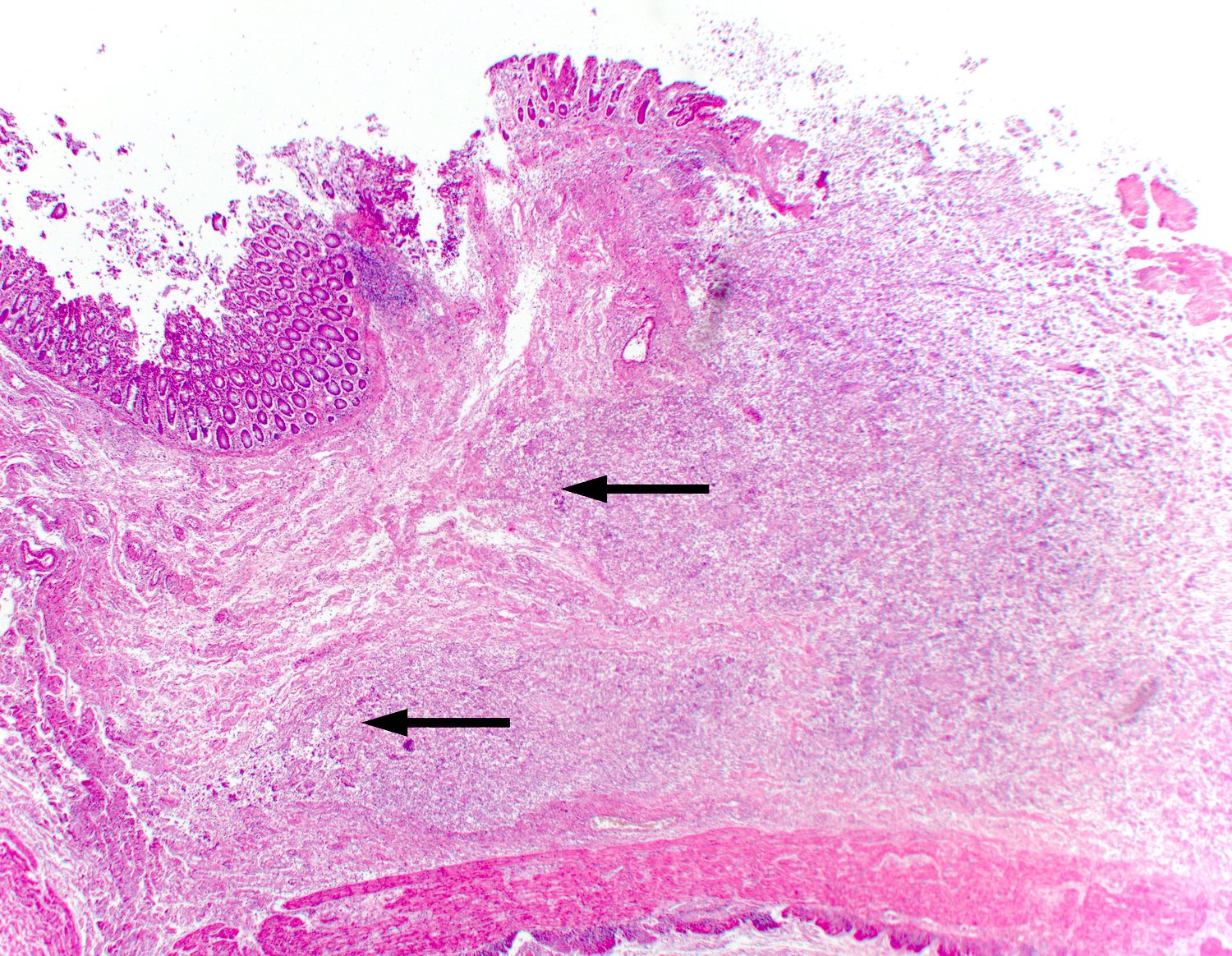
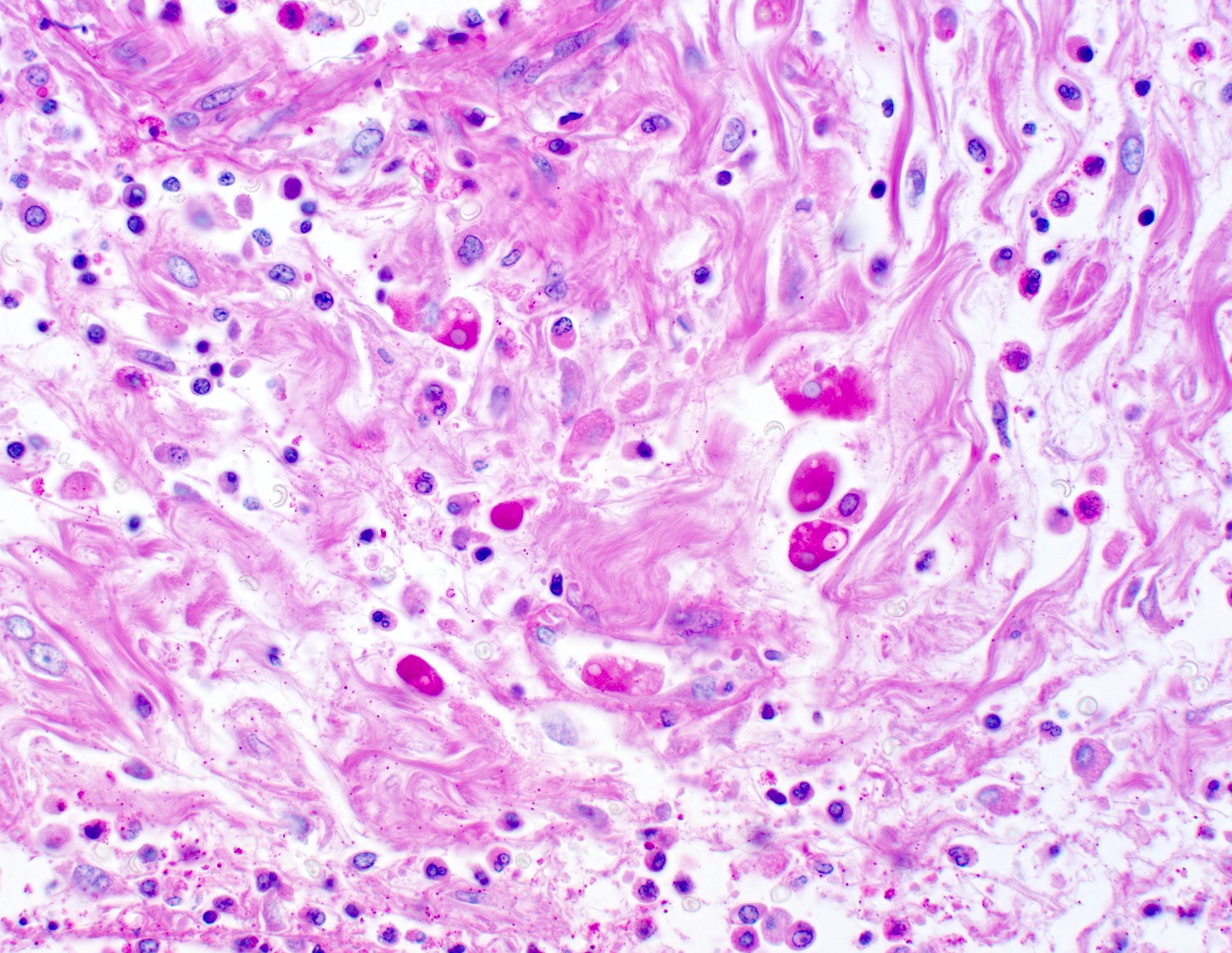
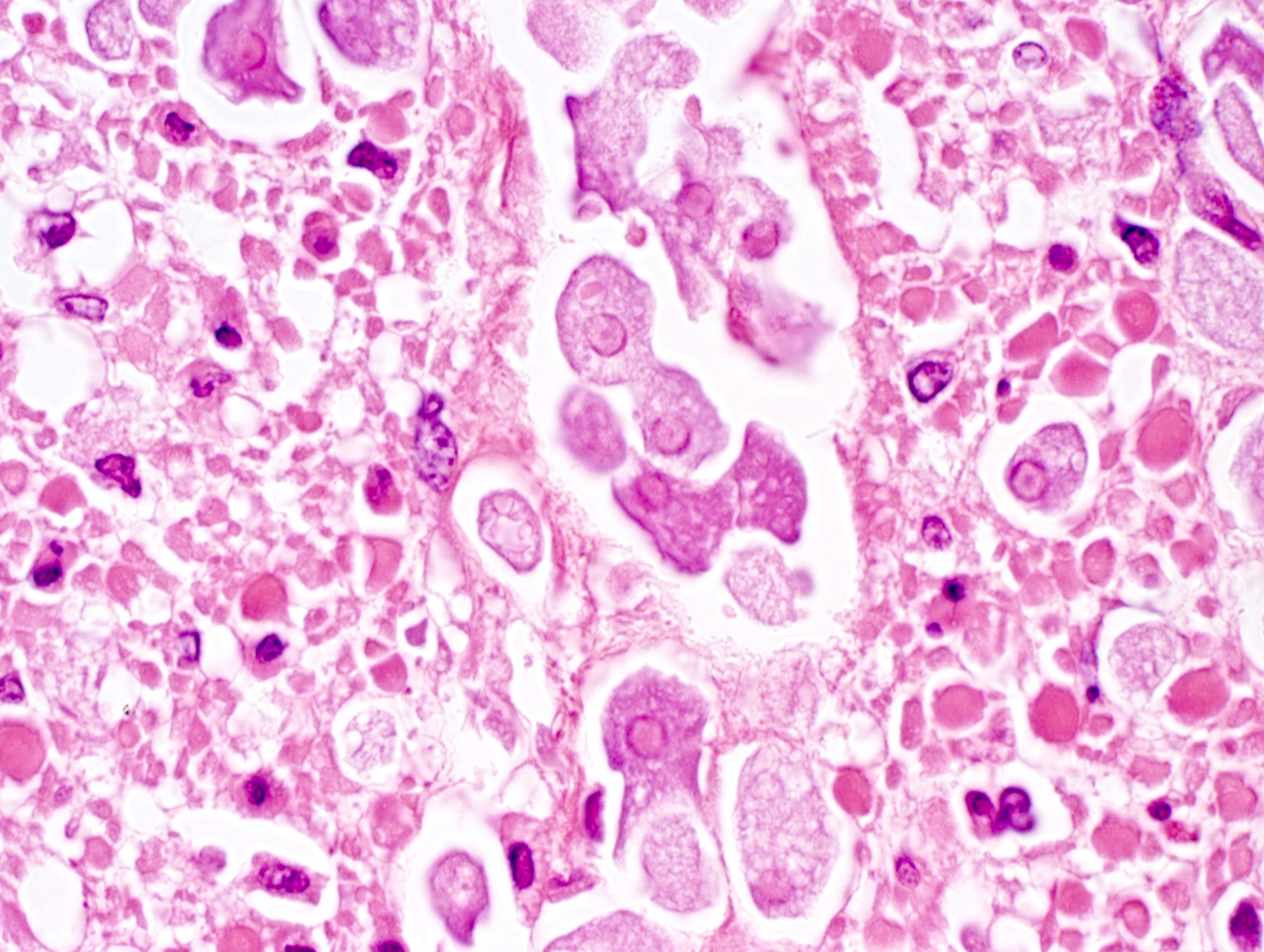
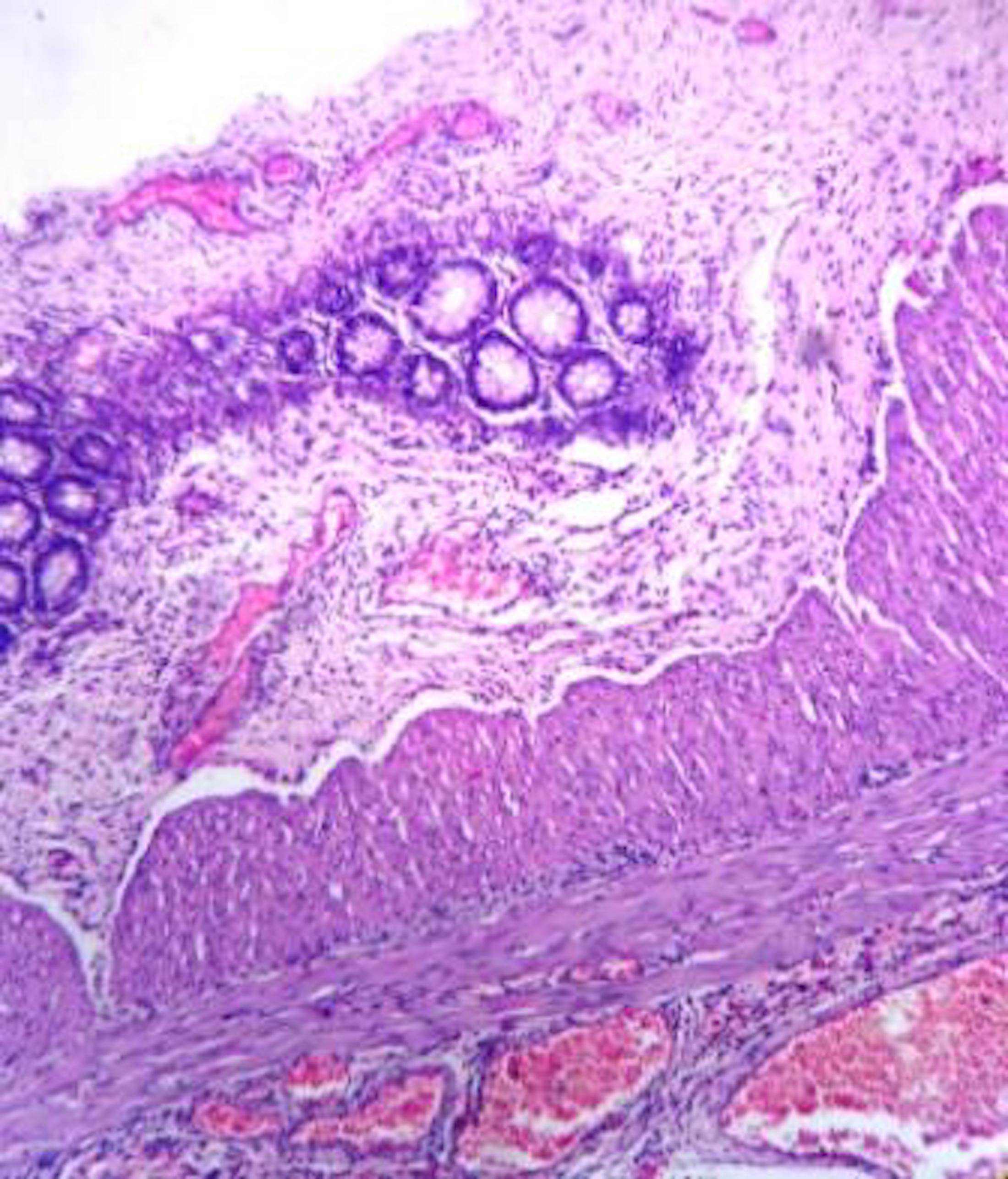
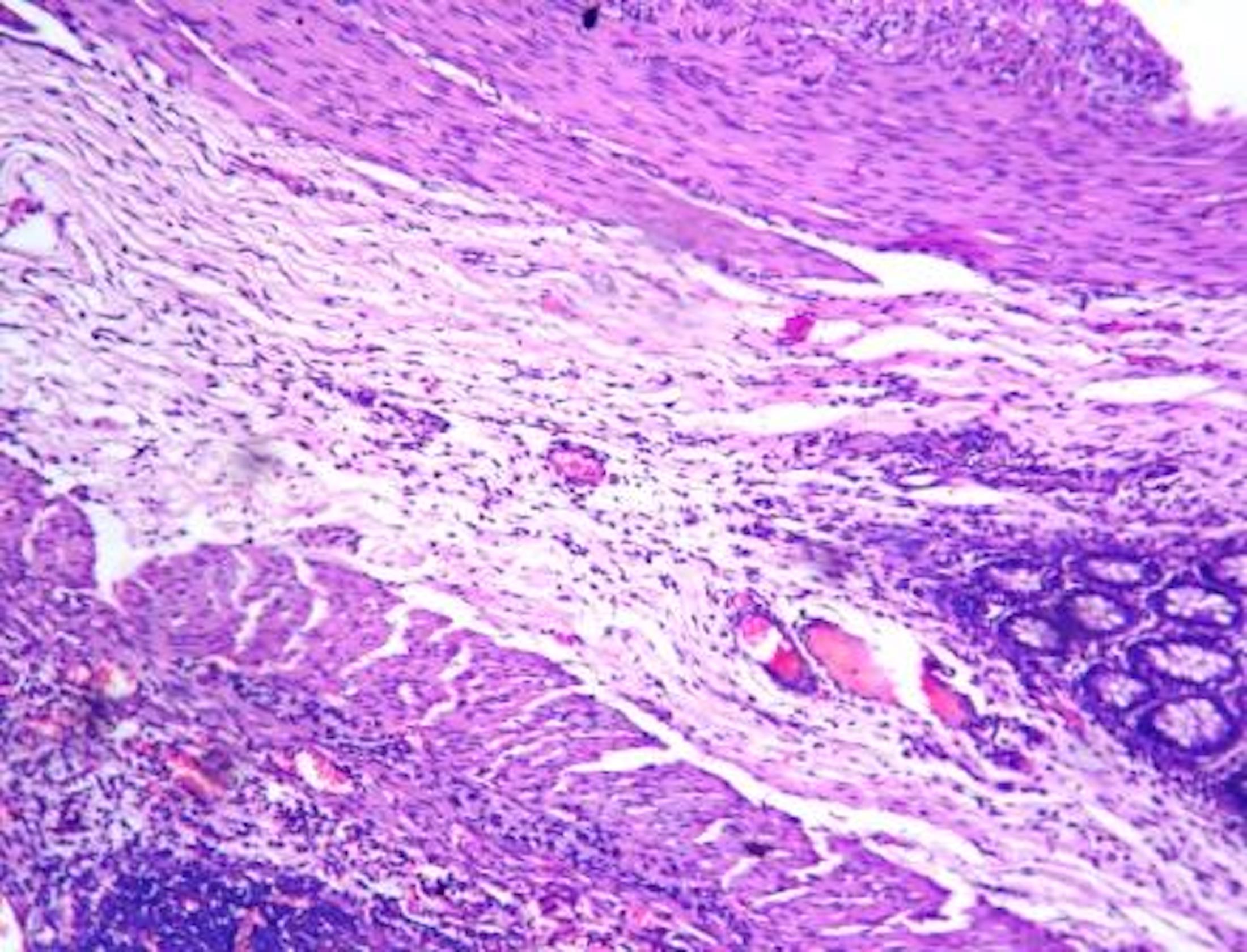
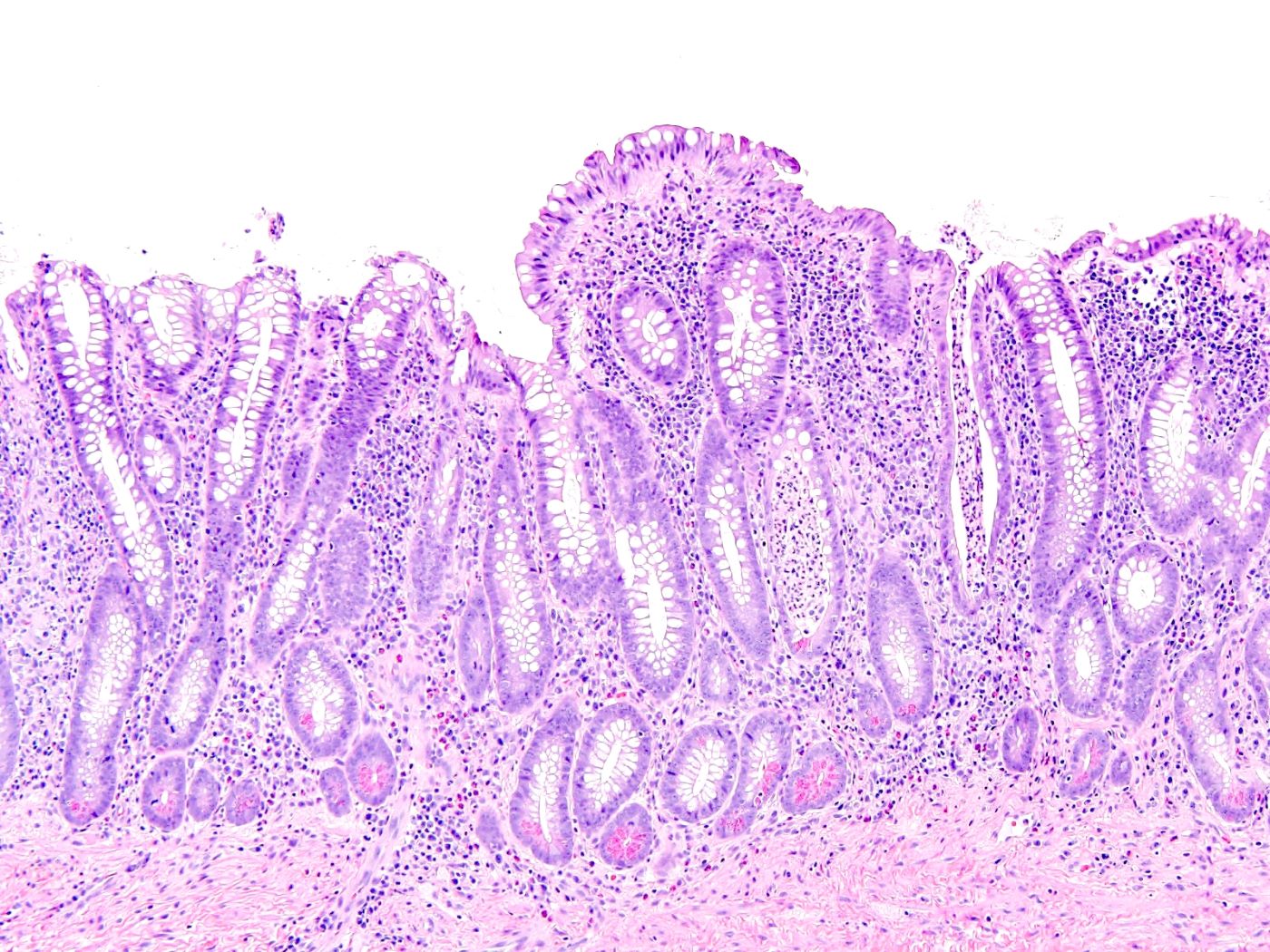
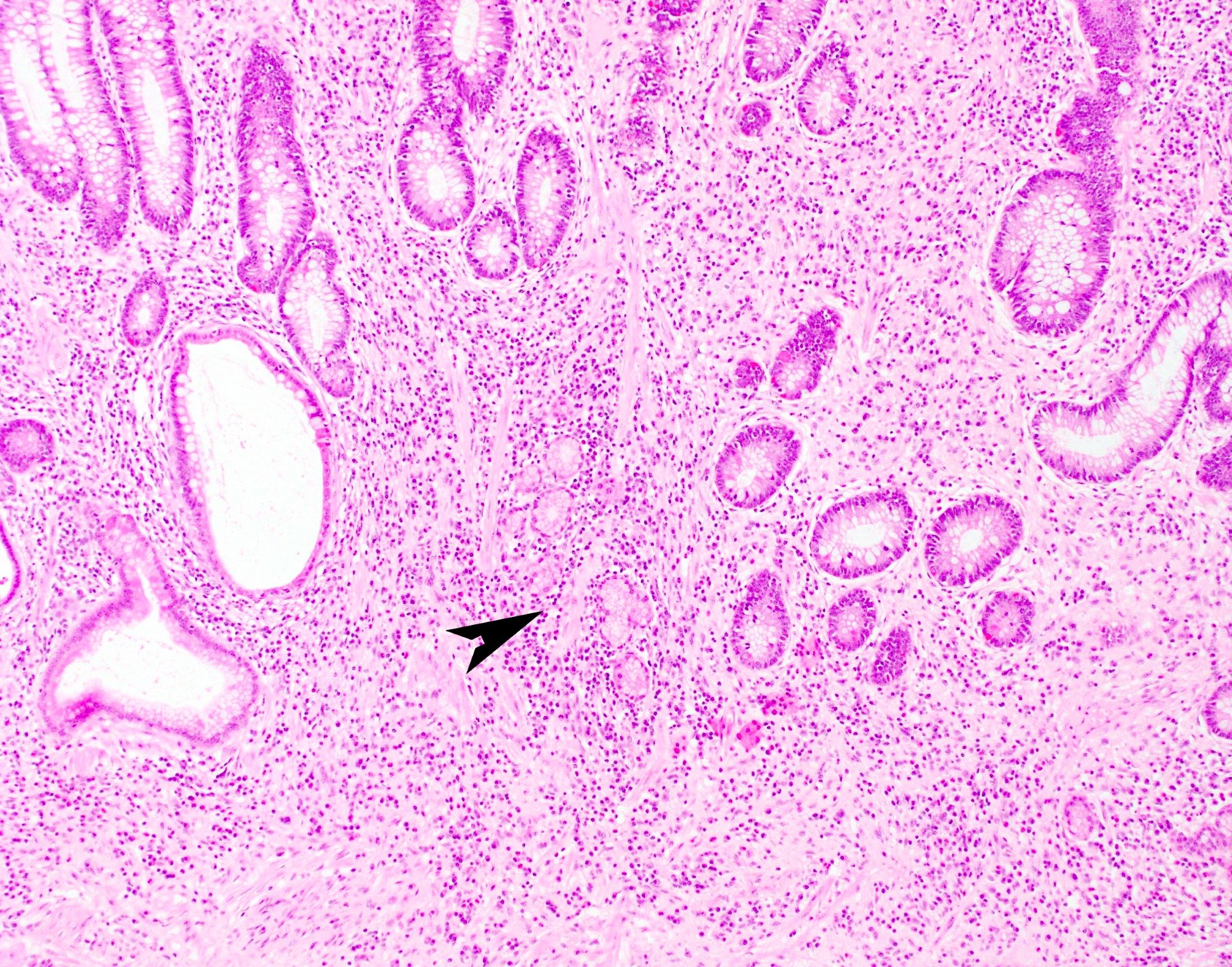
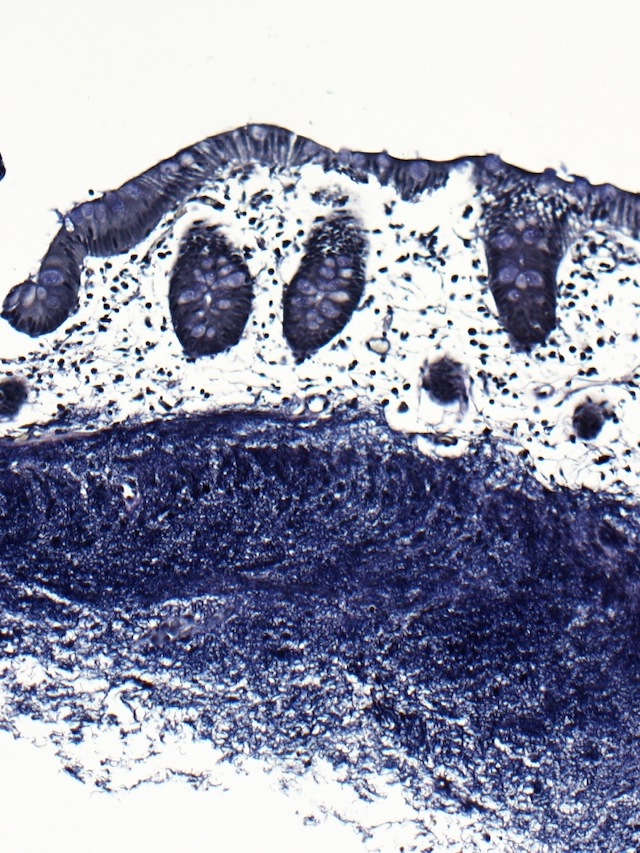
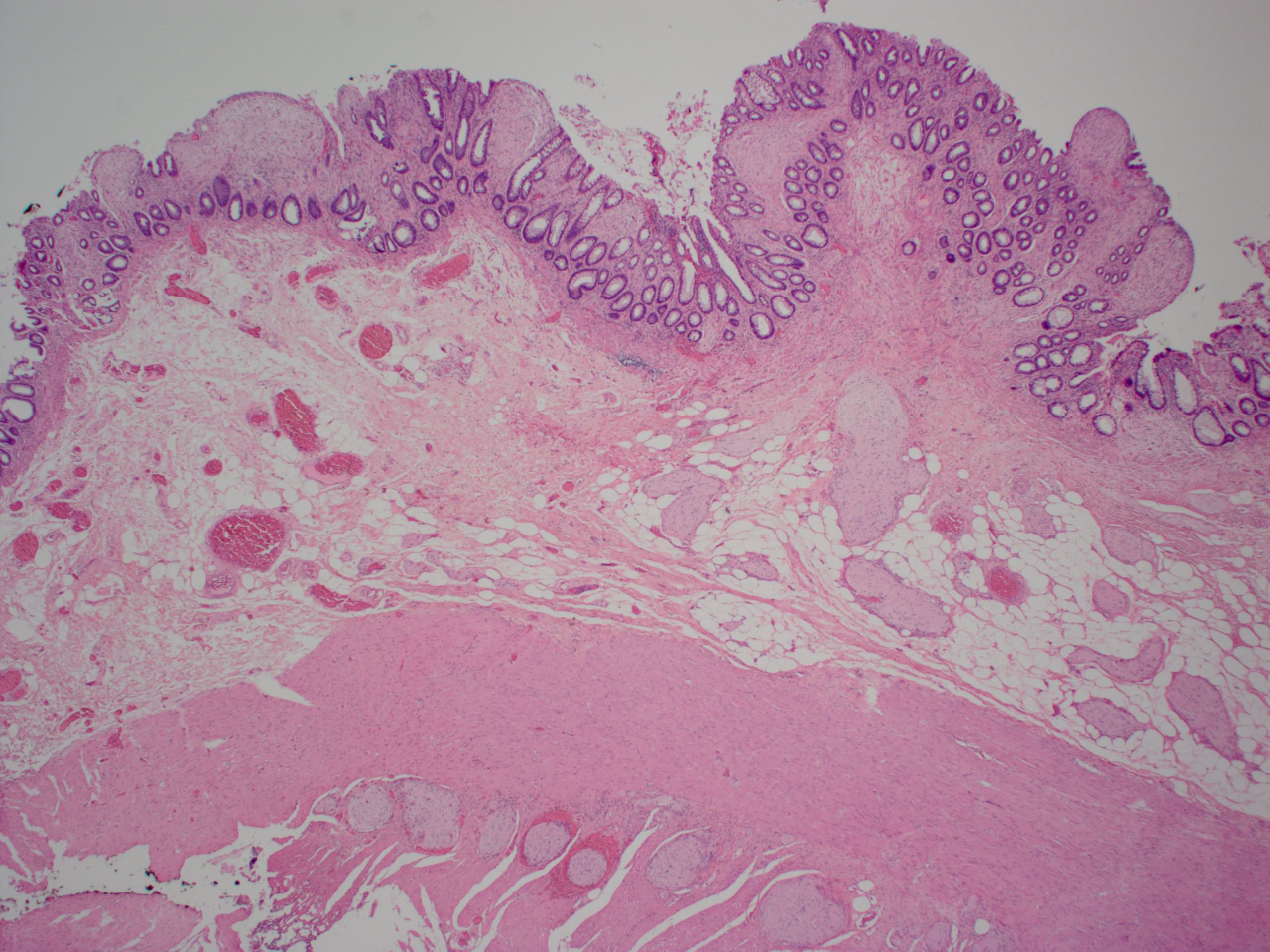
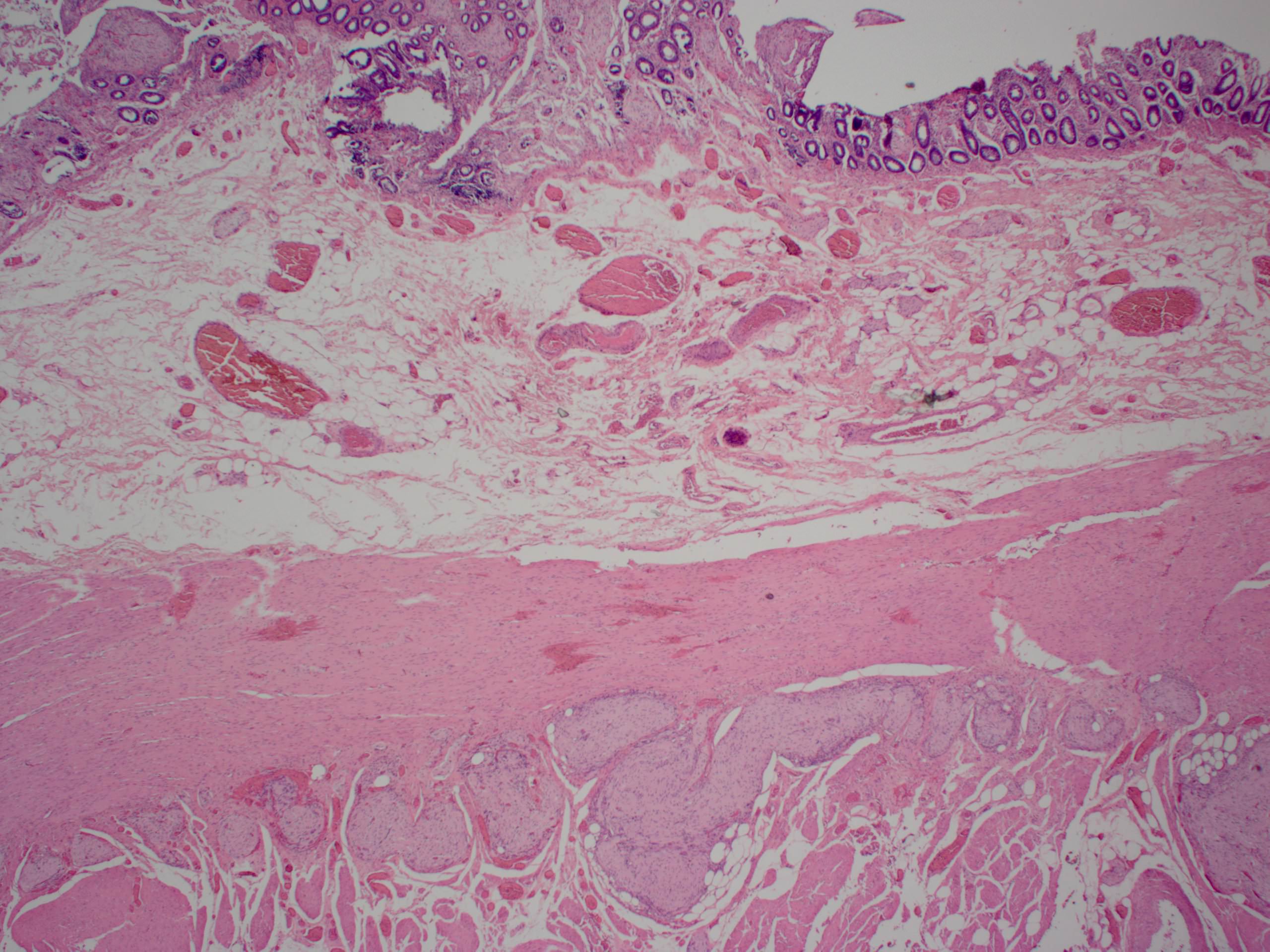
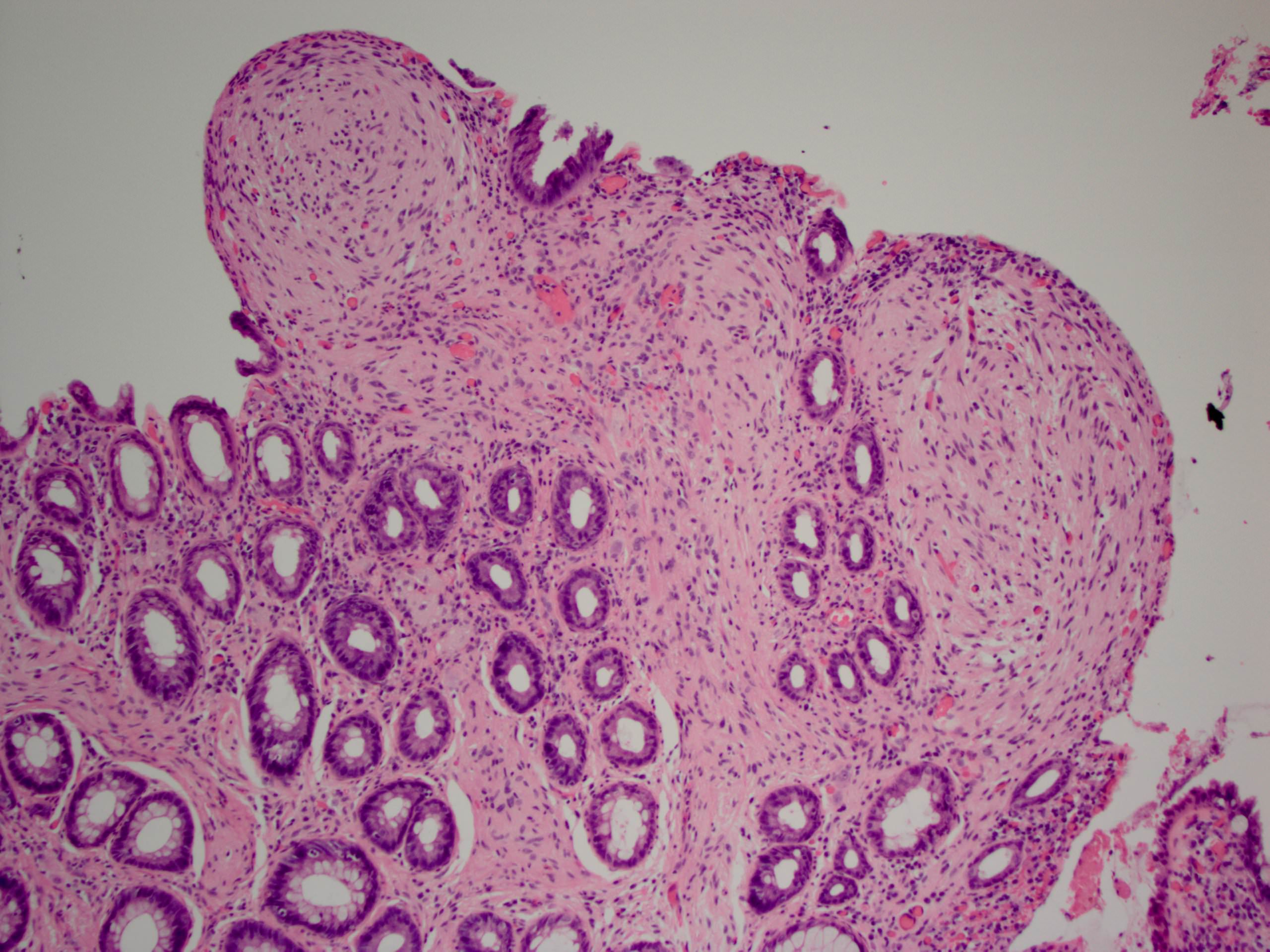
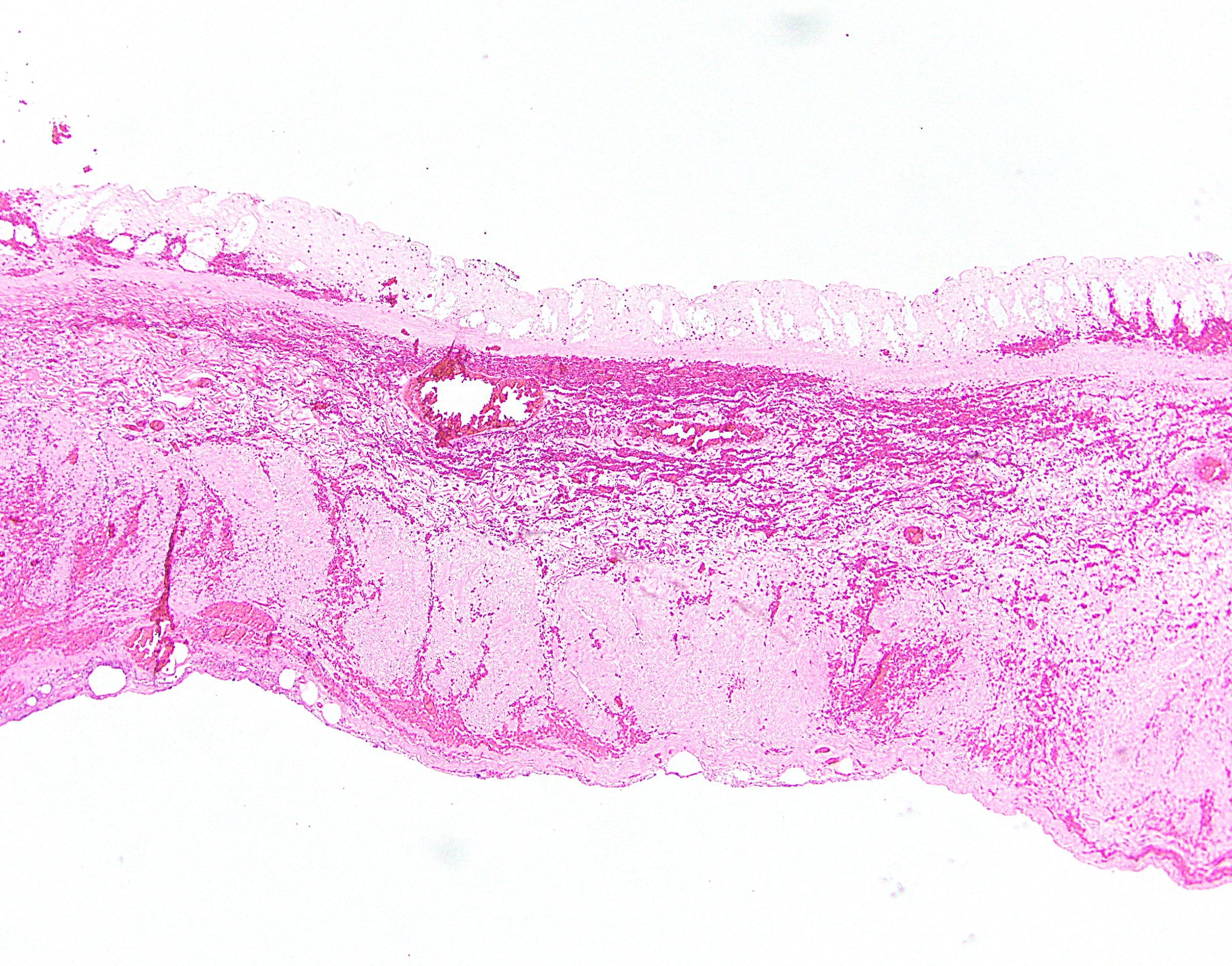
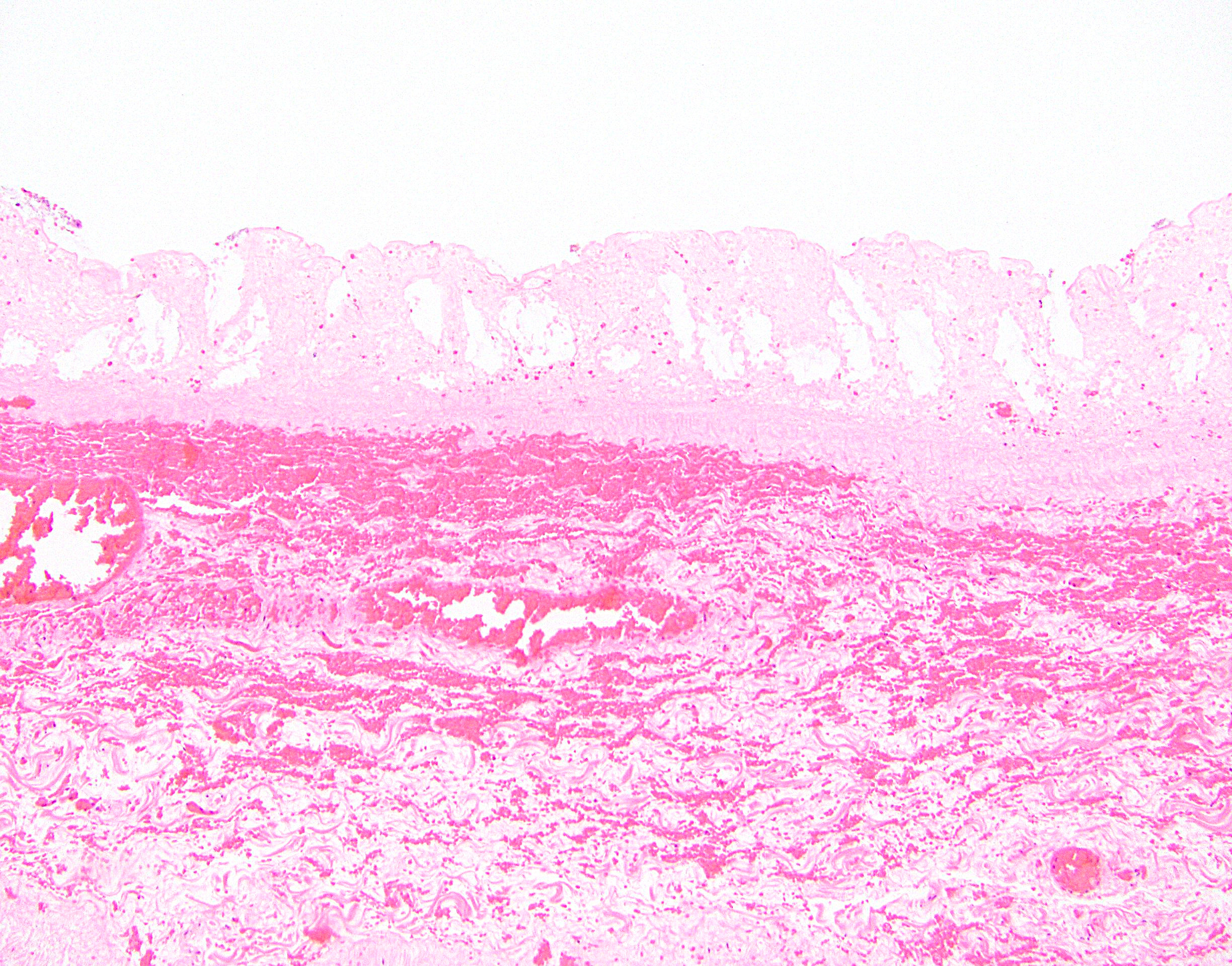
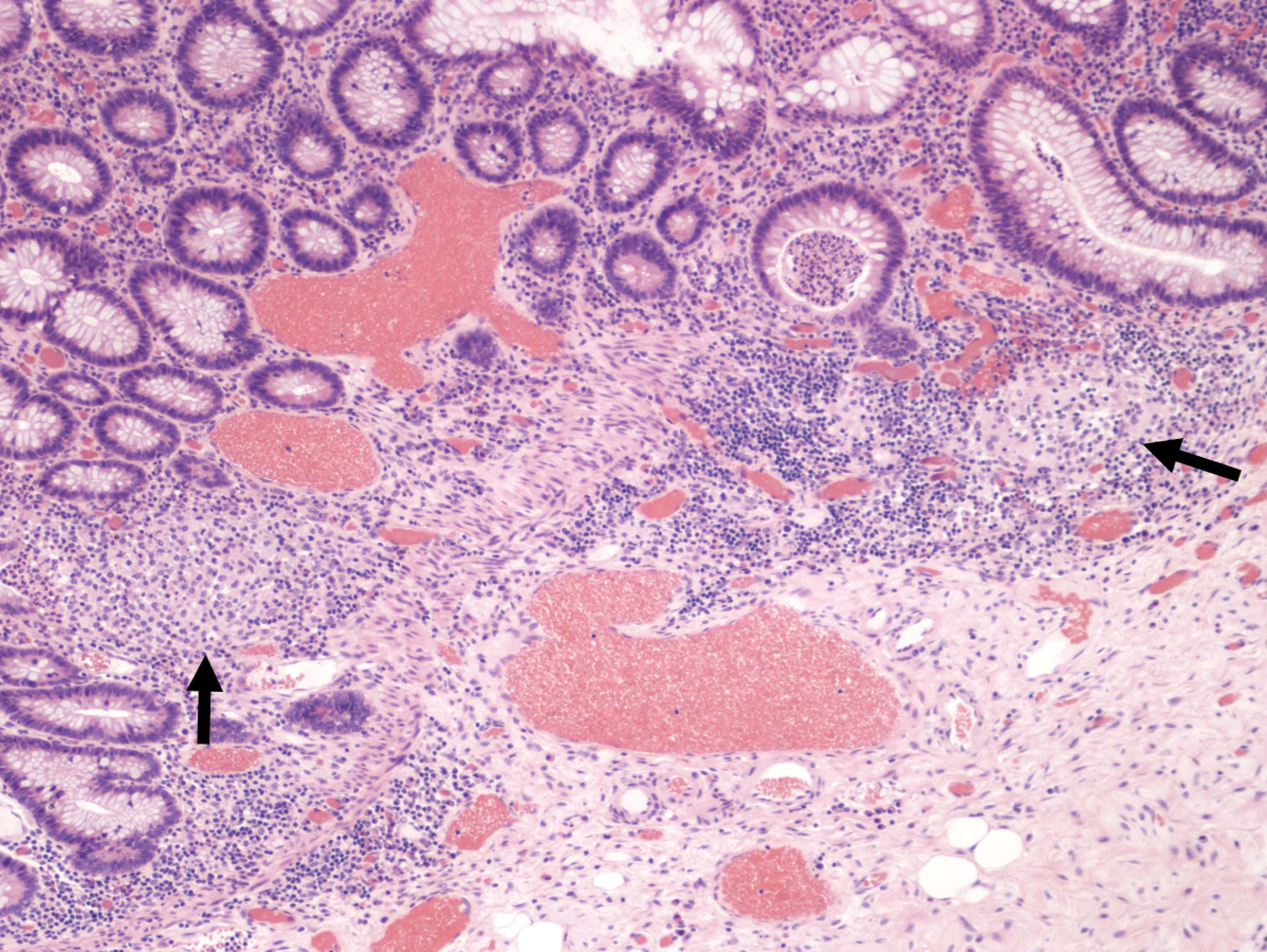
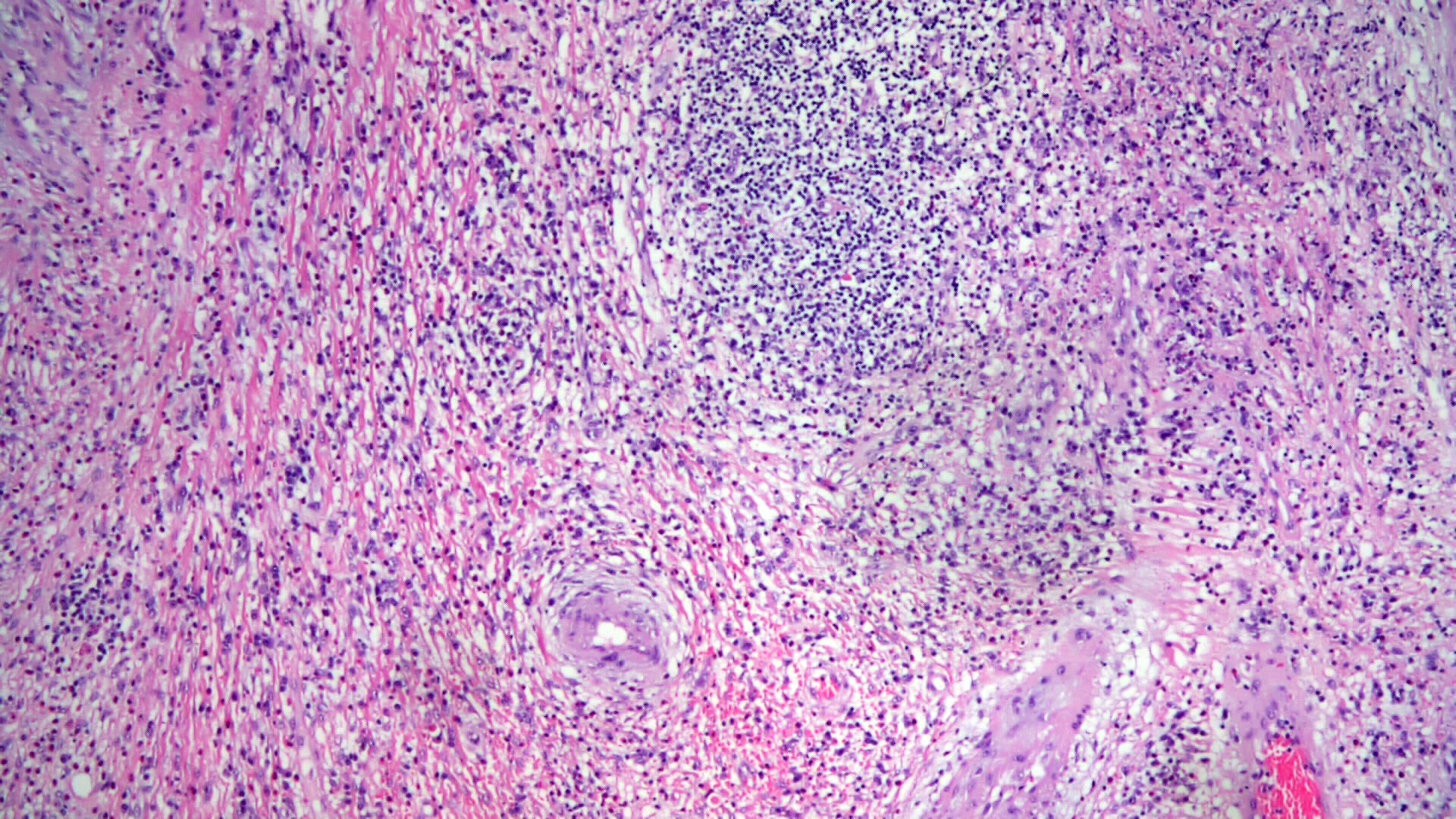
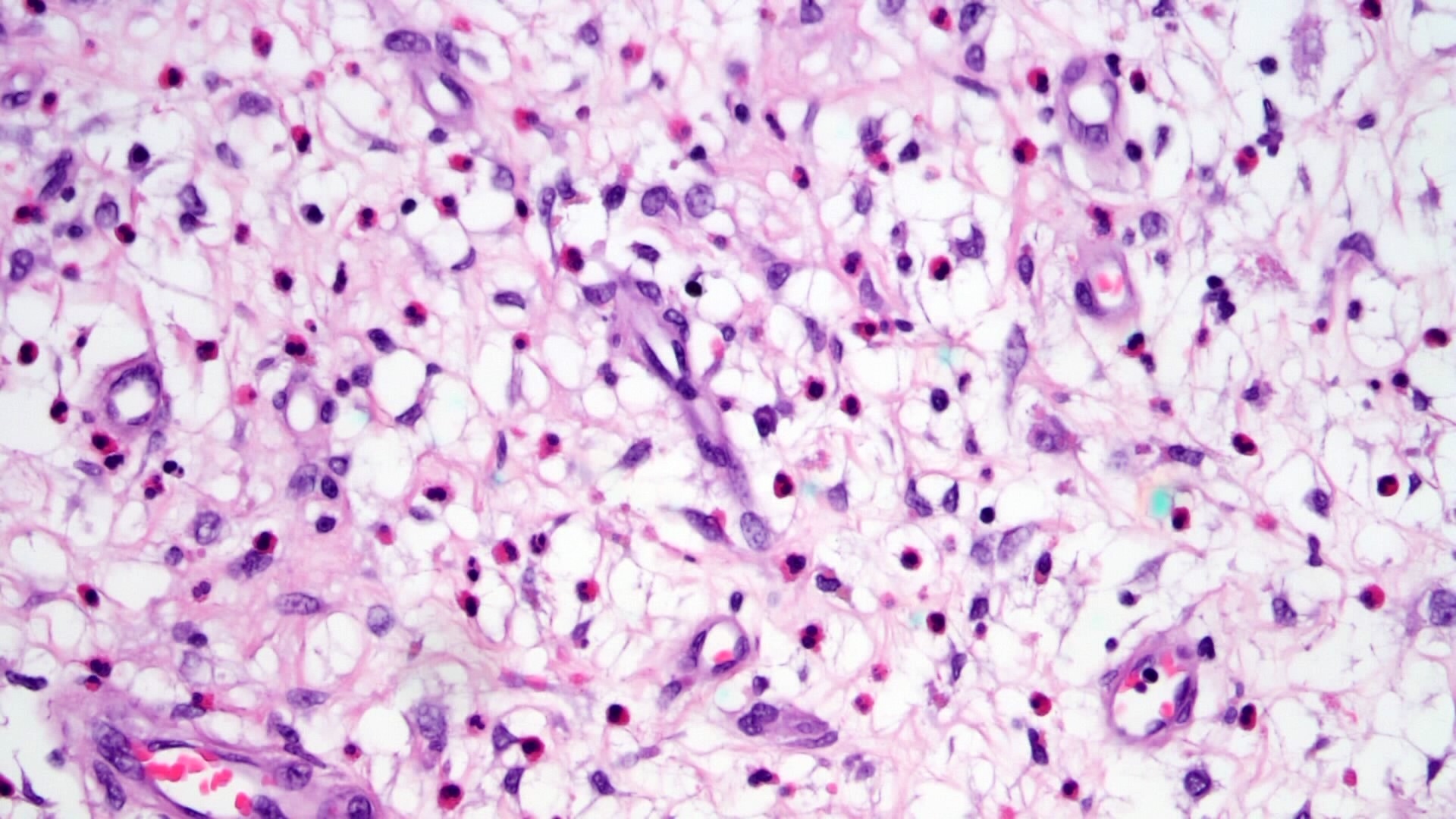
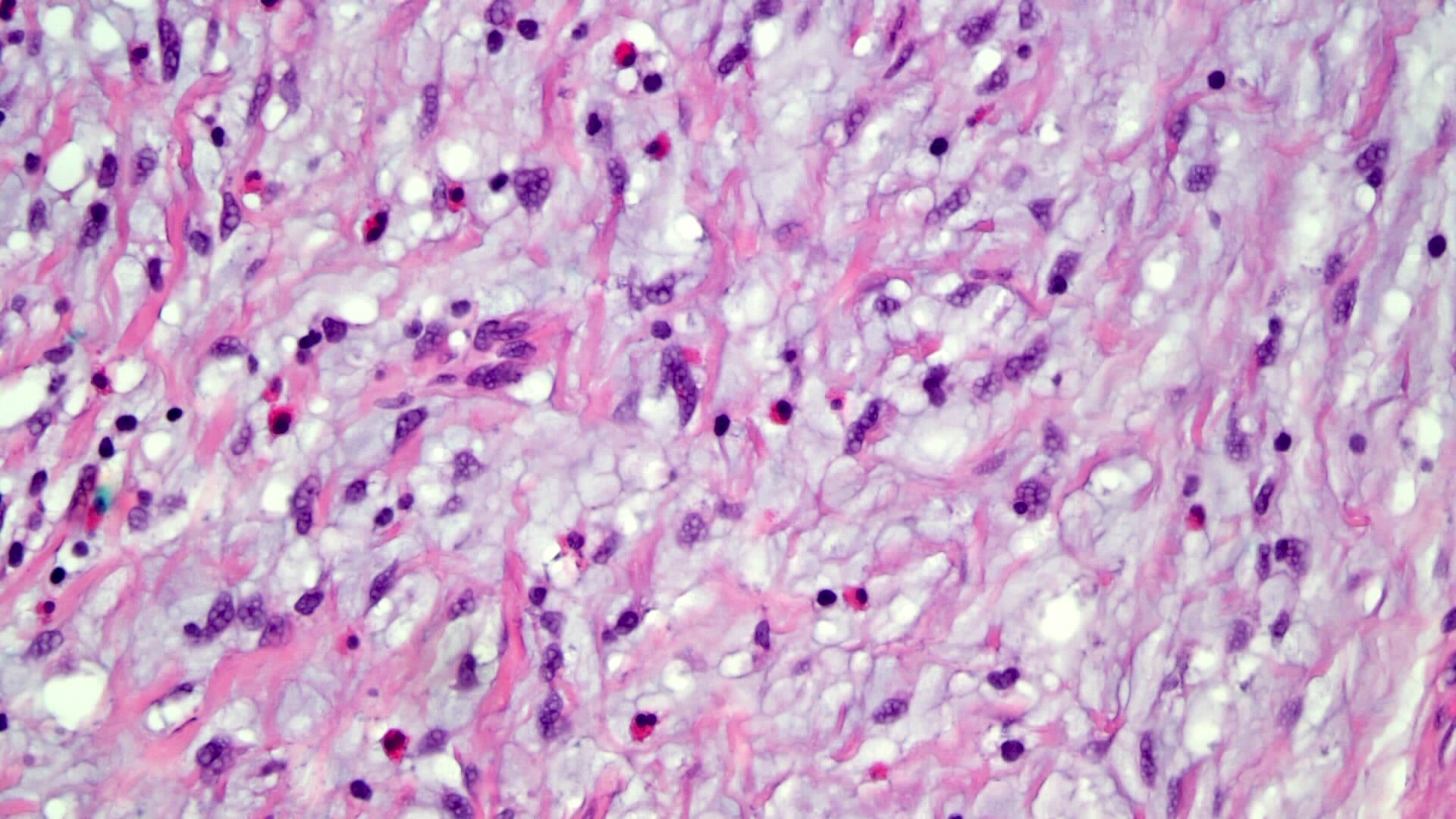
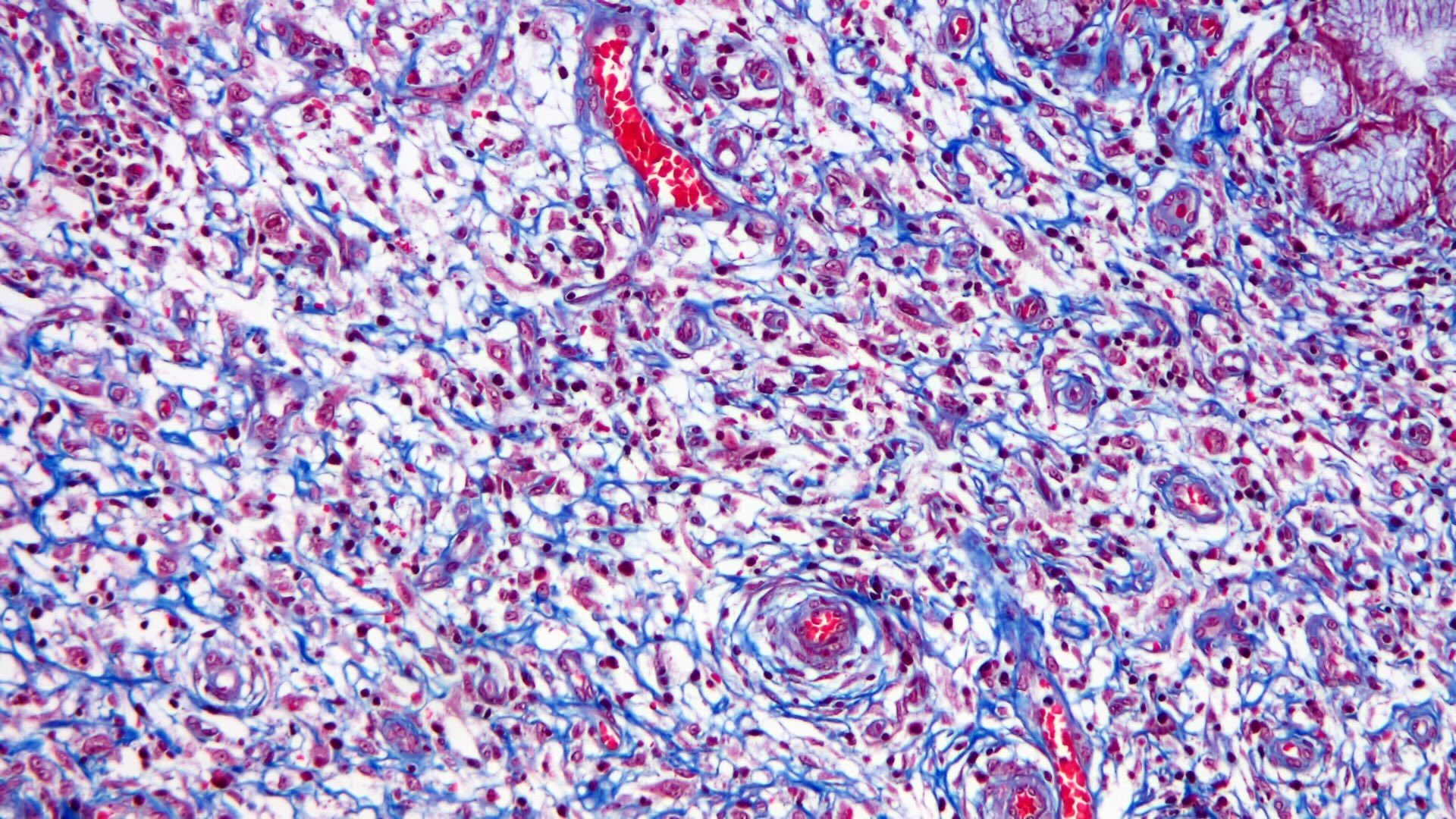
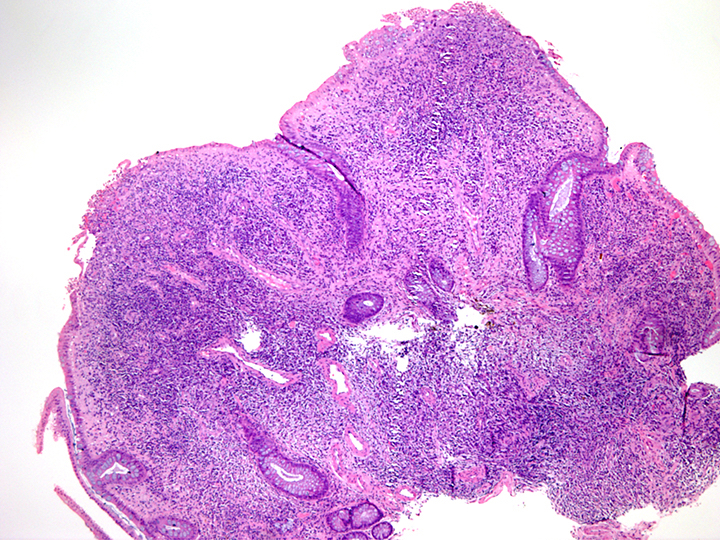
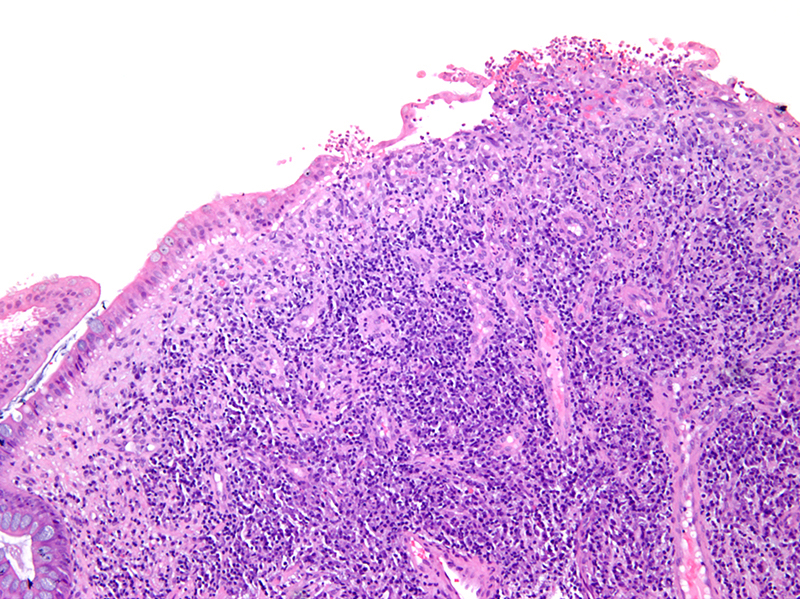
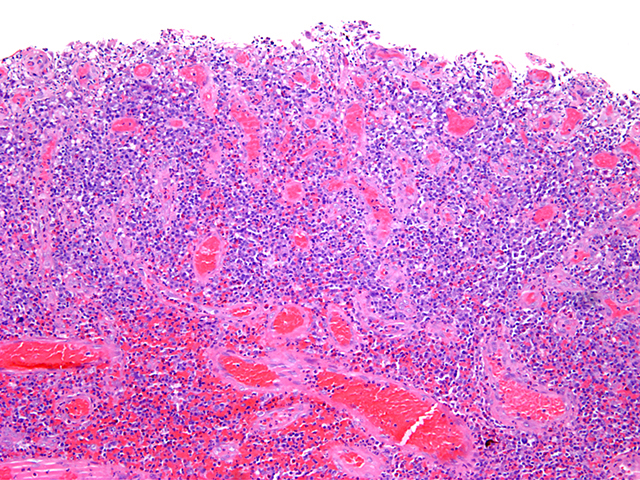
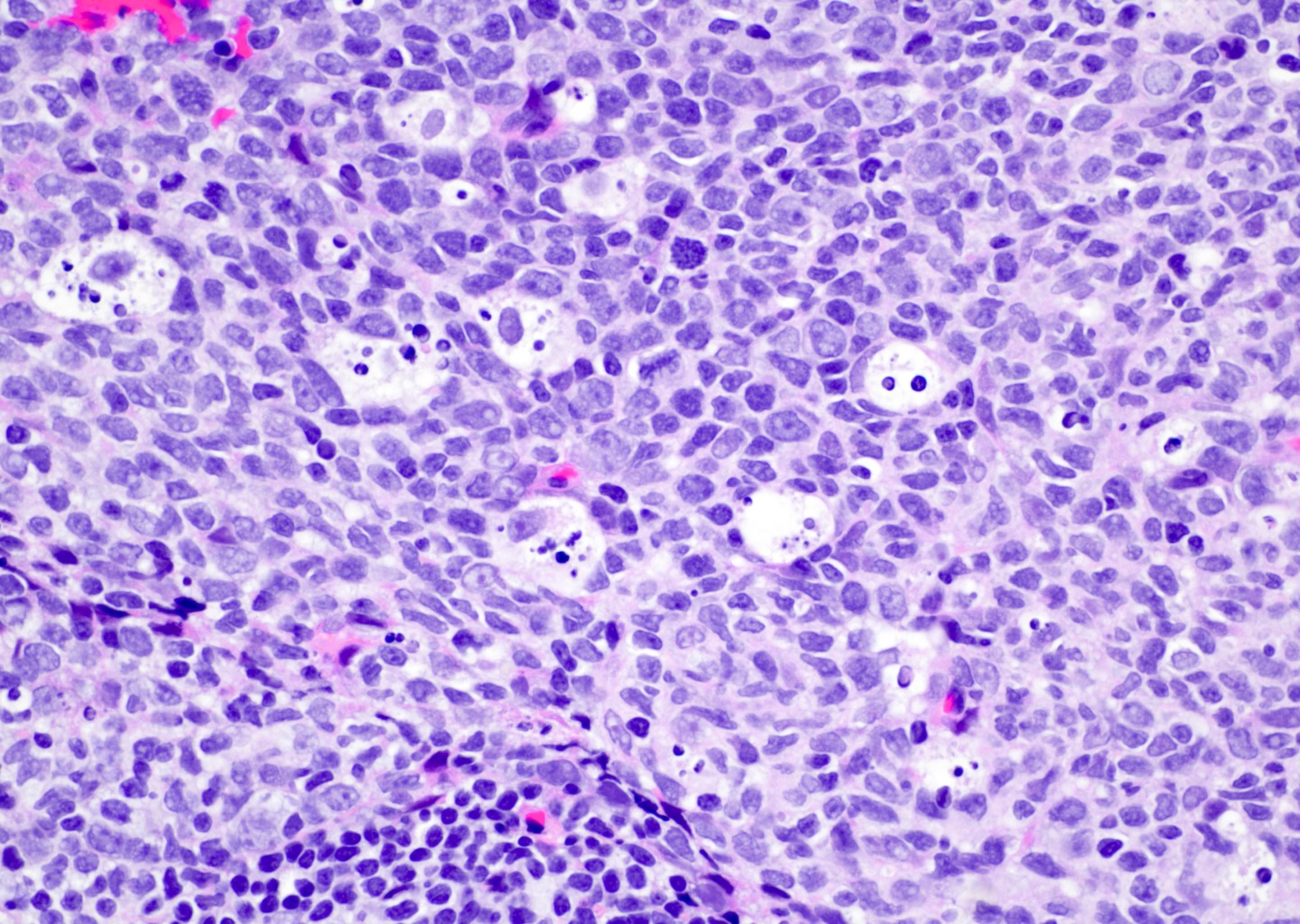
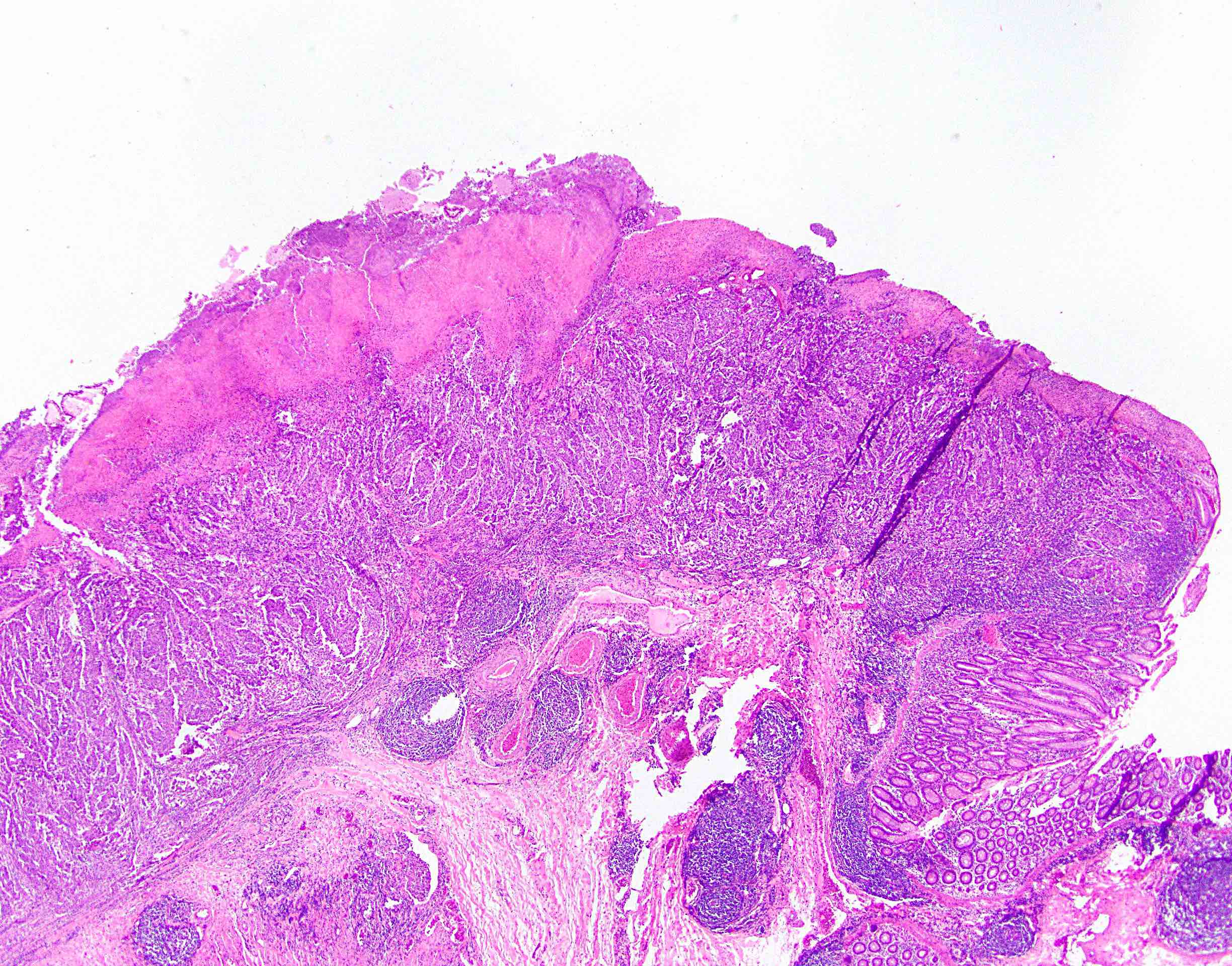
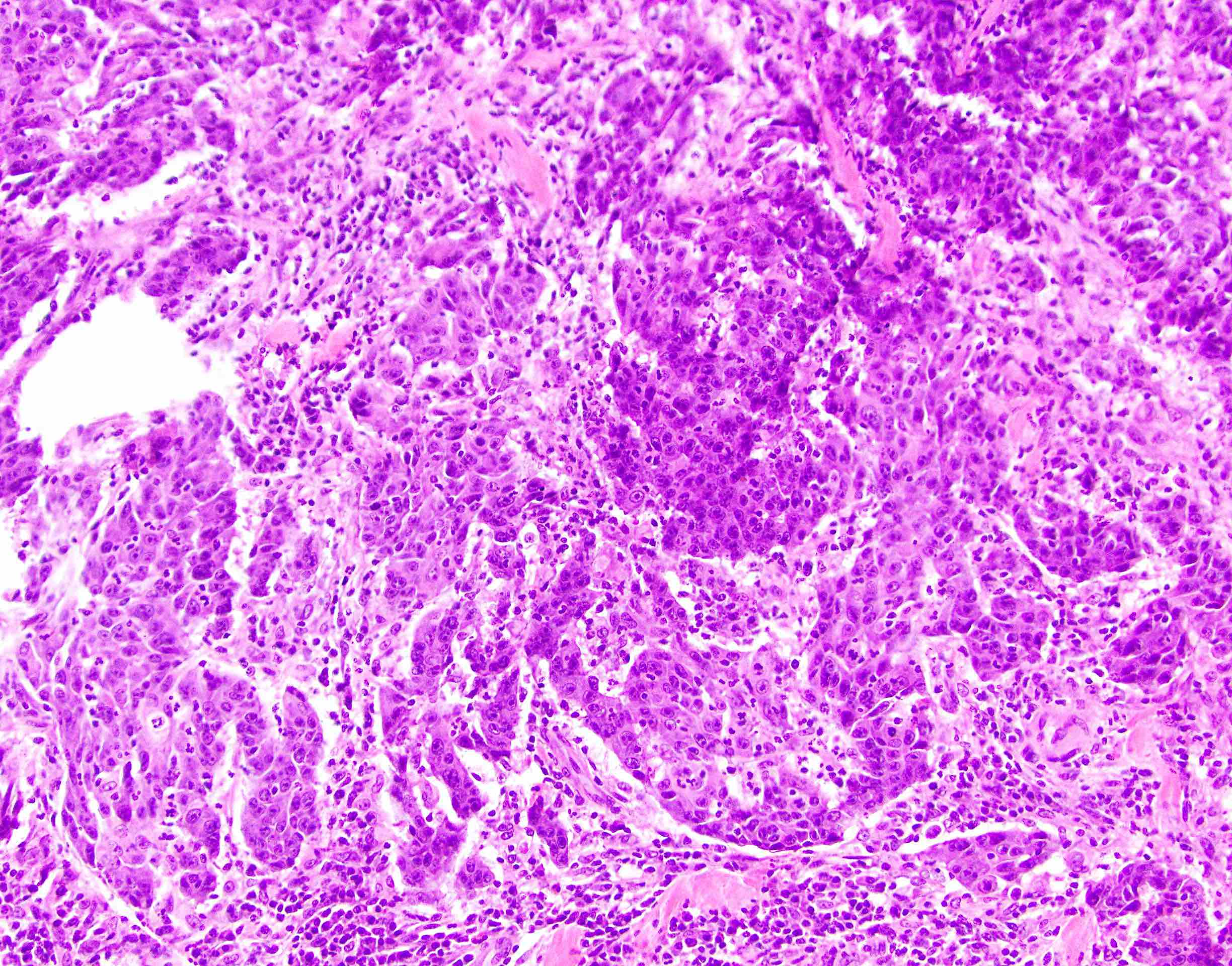
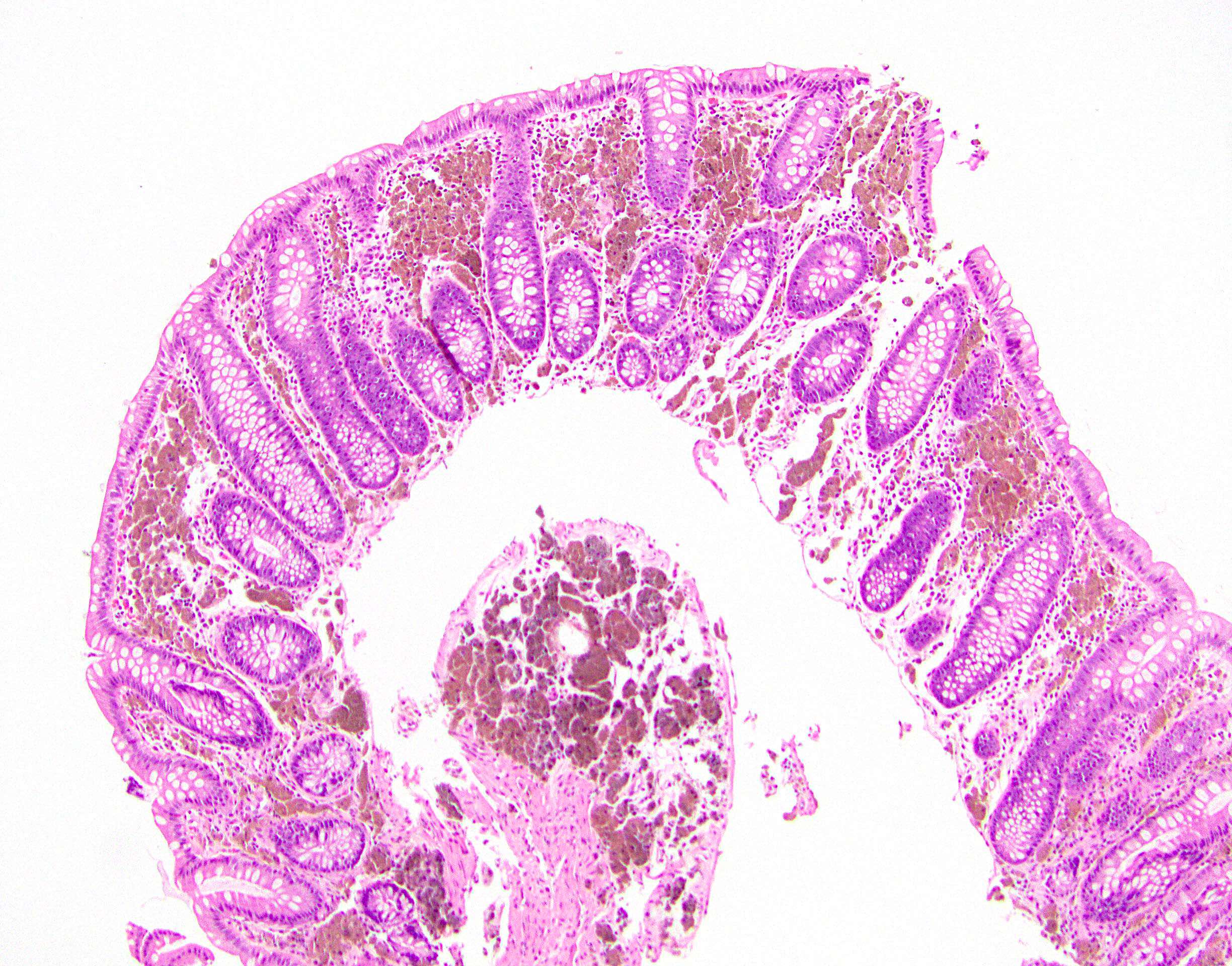
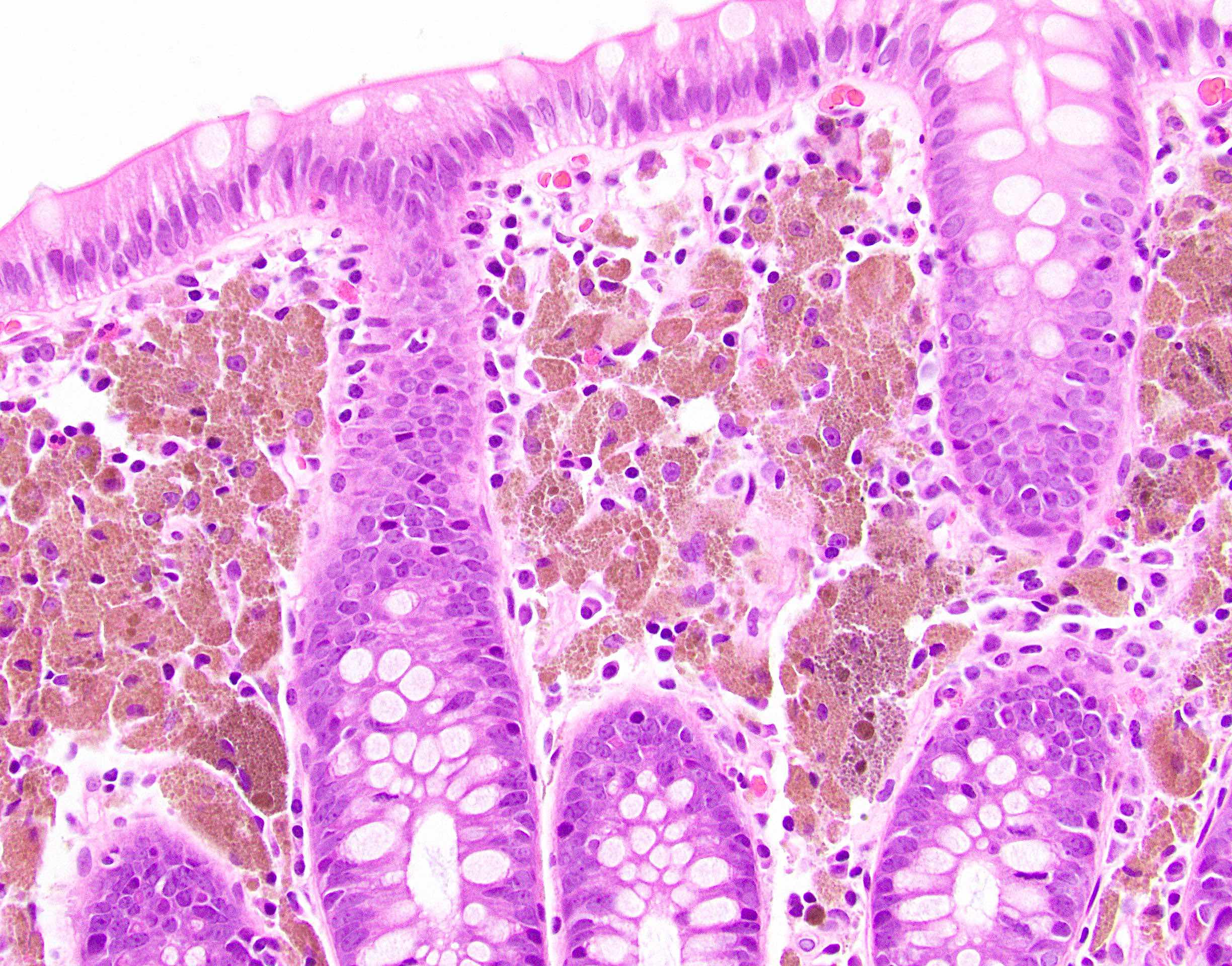
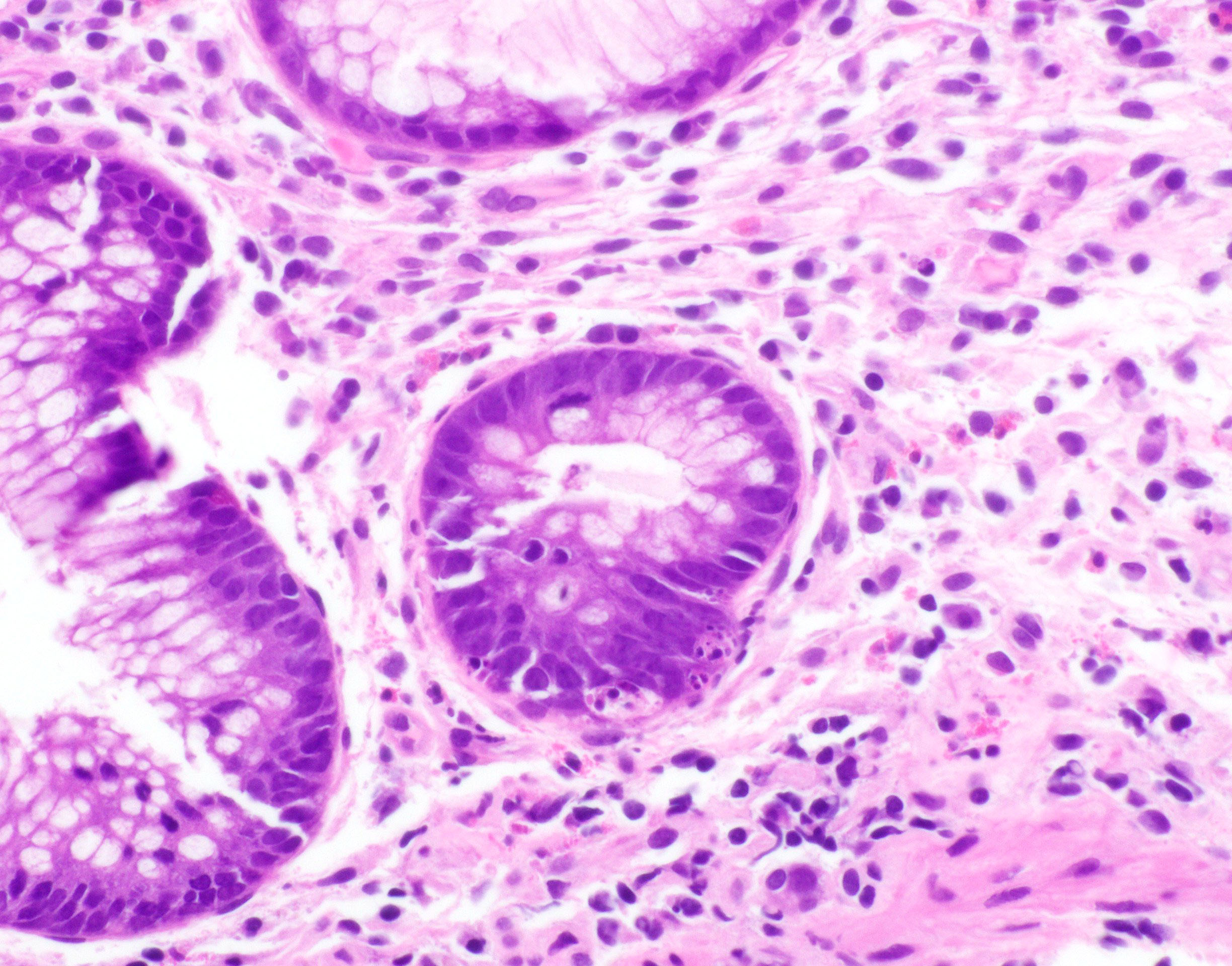
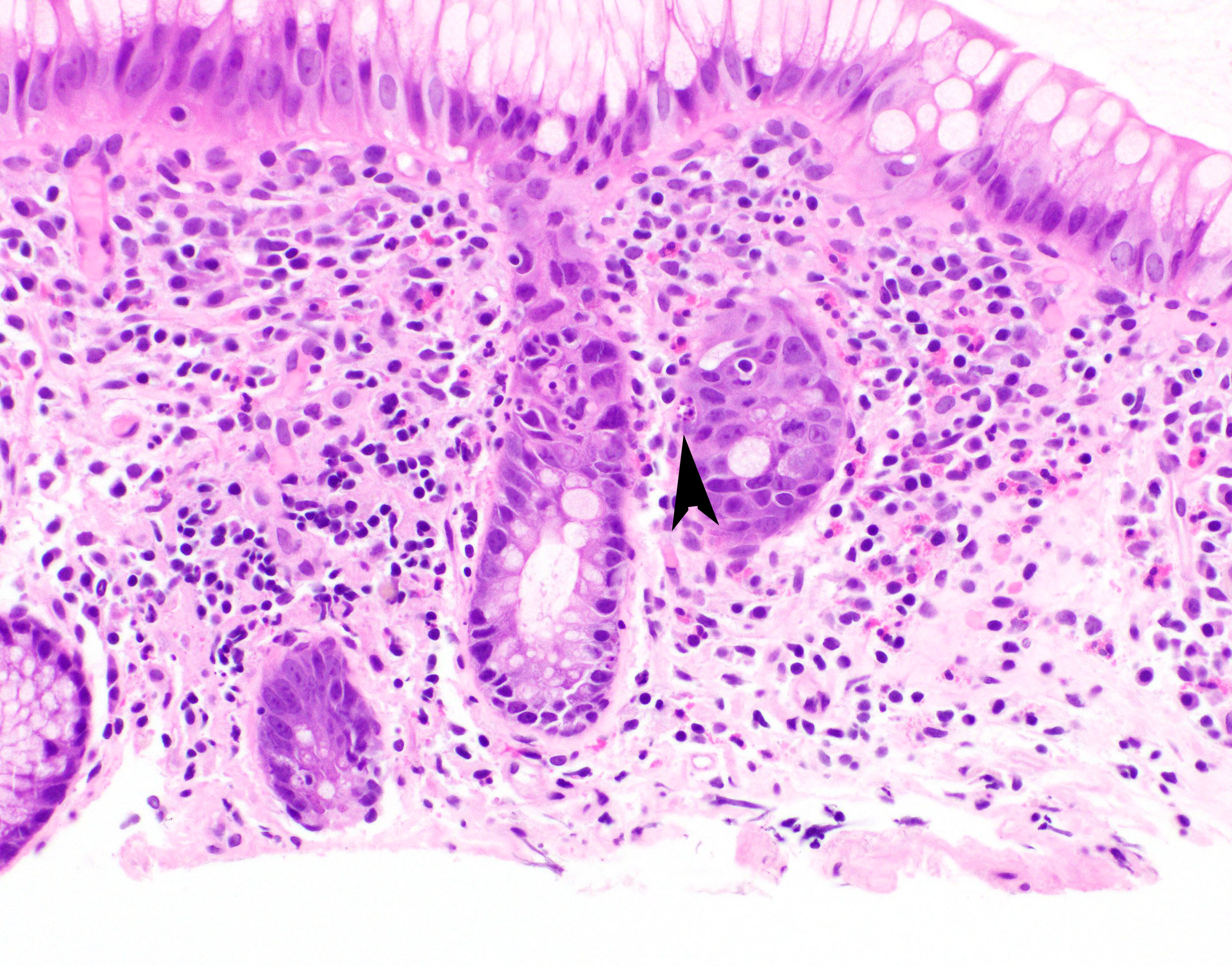
- Lesions begin with superficial mucosal necrosis and progress to erosions and ulcers (AFIP: Topics on the Pathology of Protozoan and Invasive Arthropod Diseases [Accessed 22 April 2022])
- Overlying inflammatory exudates consist of necrotic material, fibrin and inflammatory cells, and often contain E. histolytica trophozoites
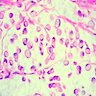
- Chronic cryptitis and crypt architectural distortion may be seen
- Mature ulcers are flask shaped, with a base that is broader than the apex; undermines the overlying mucosa
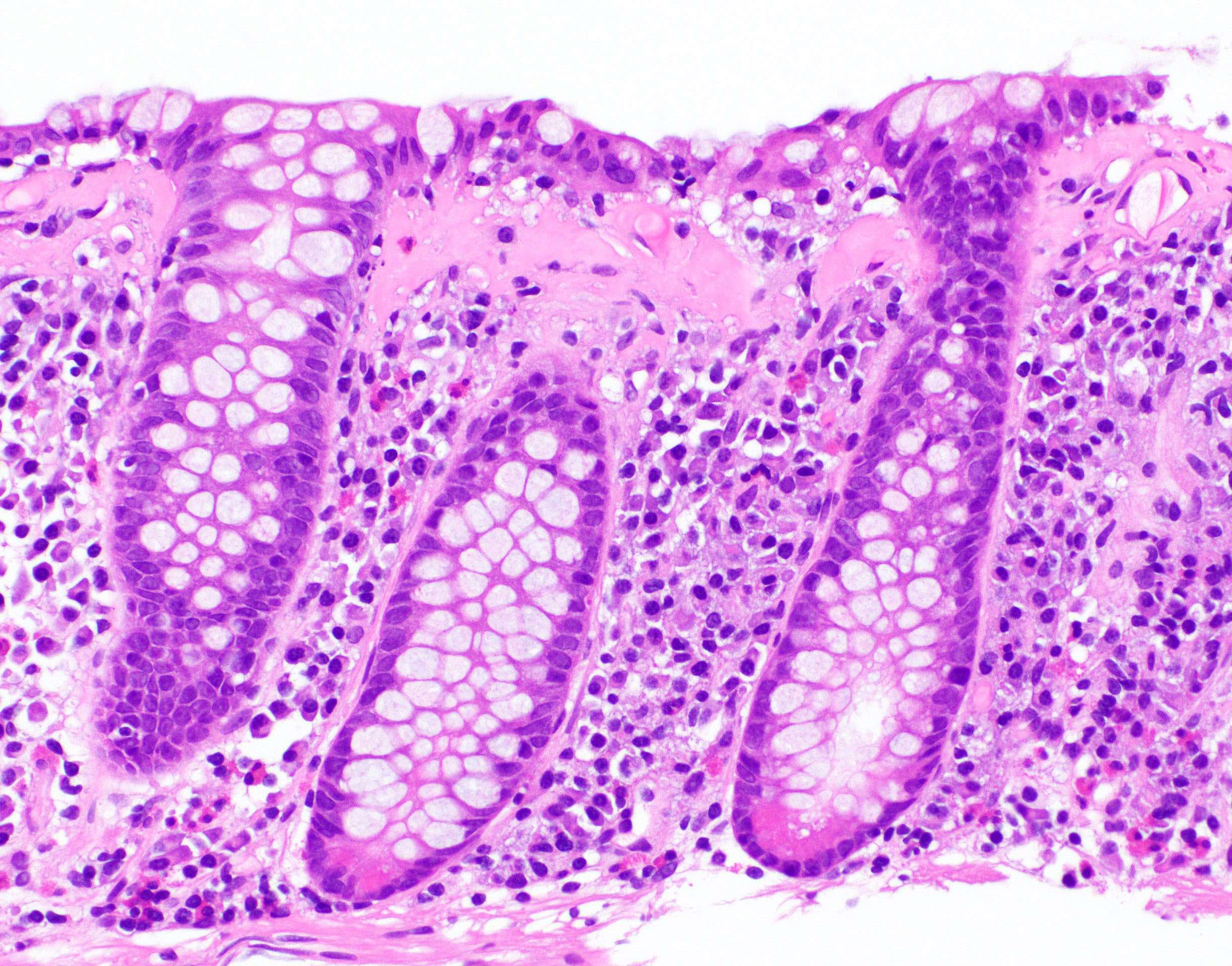
- Ulcer crater is composed of cellular debris, fibrin and trophozoites
- Minimal inflammation initially; later includes neutrophils, histiocytes, lymphocytes, plasma cells and occasionally eosinophils within and around the ulcer
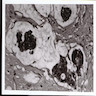
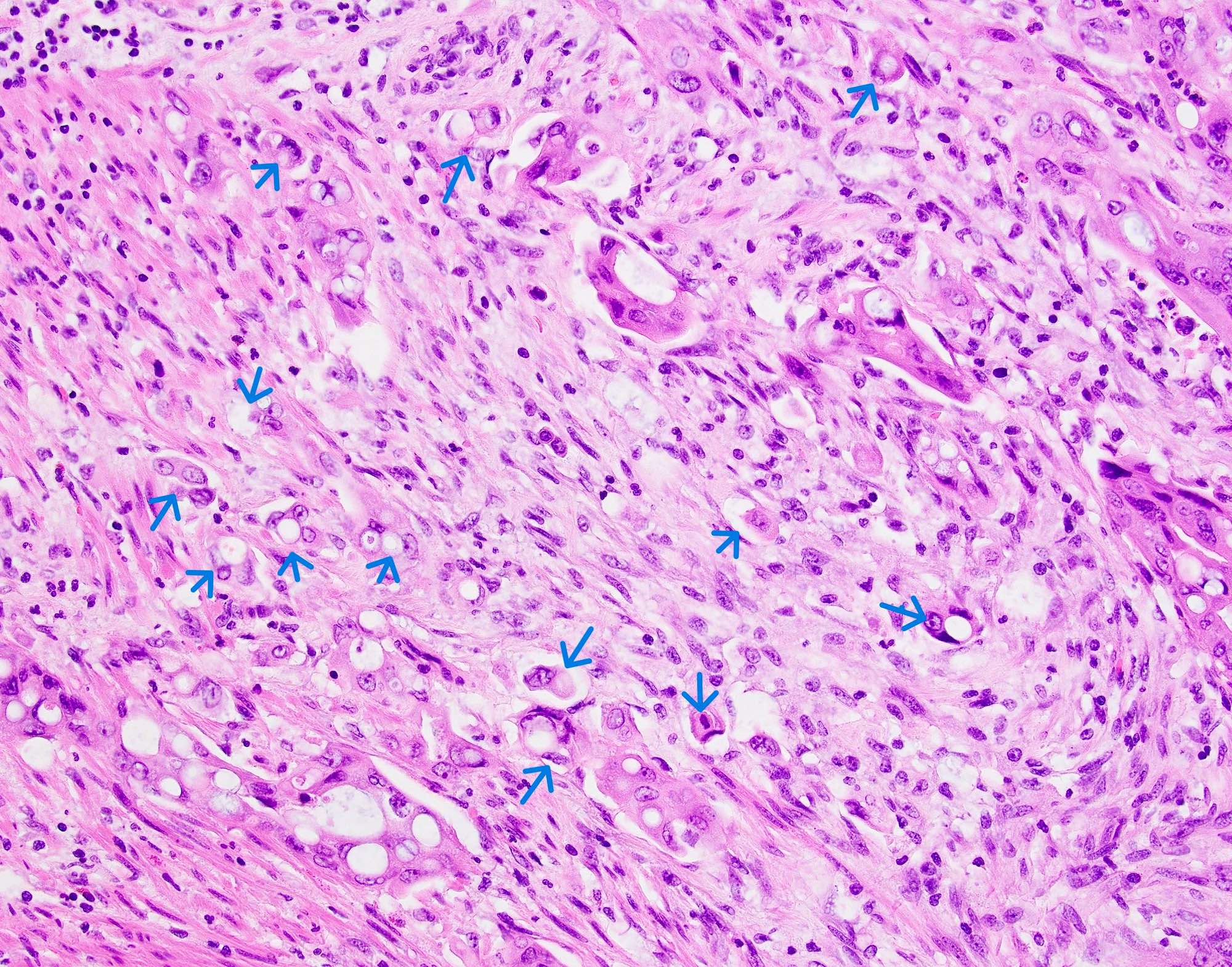
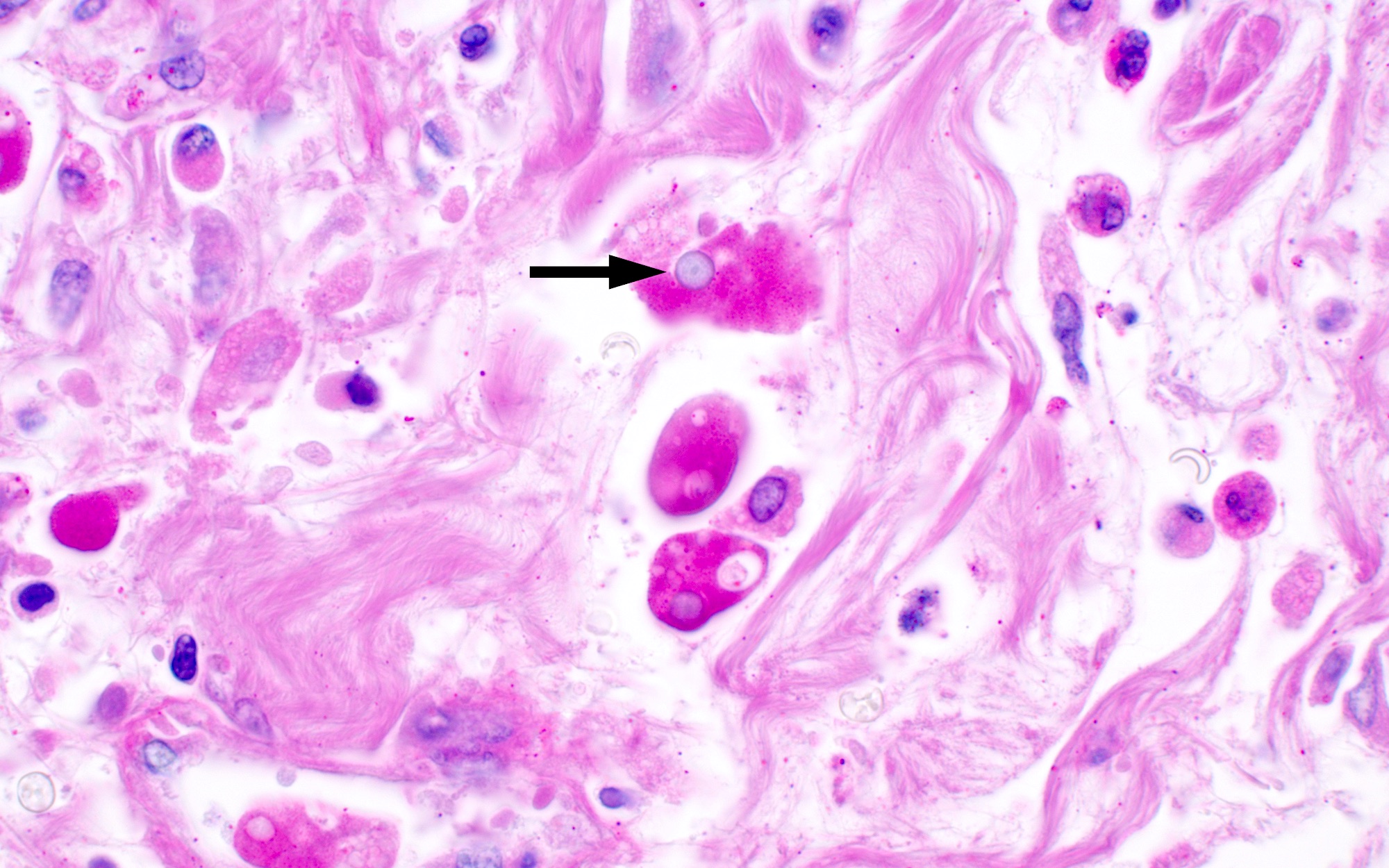
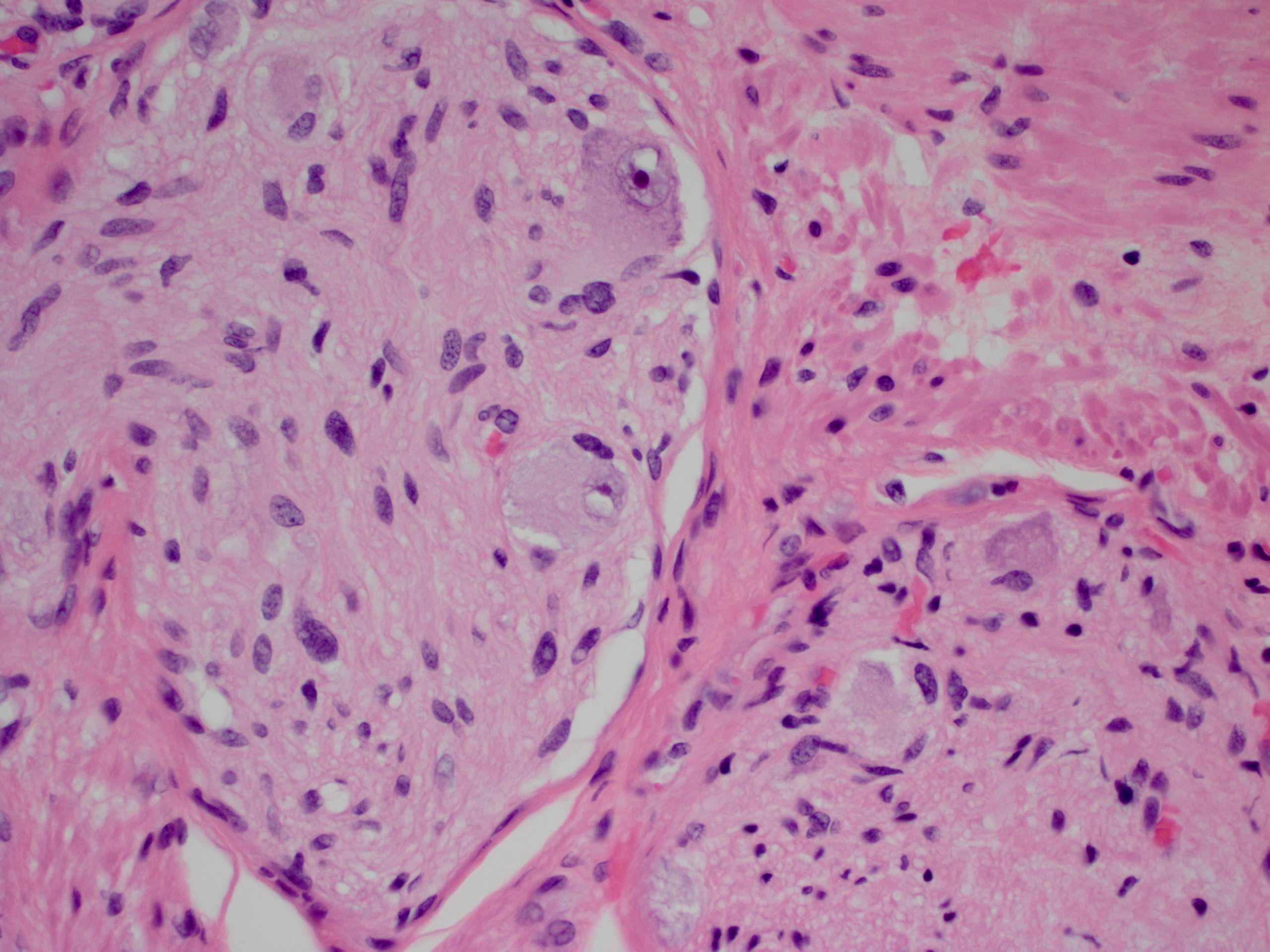
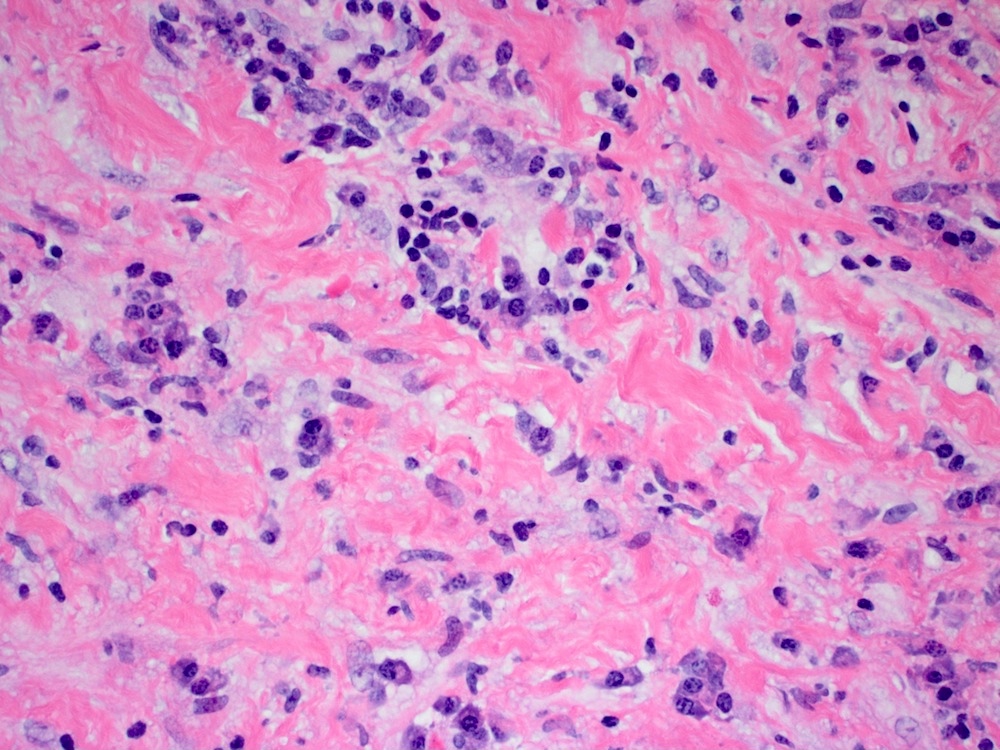
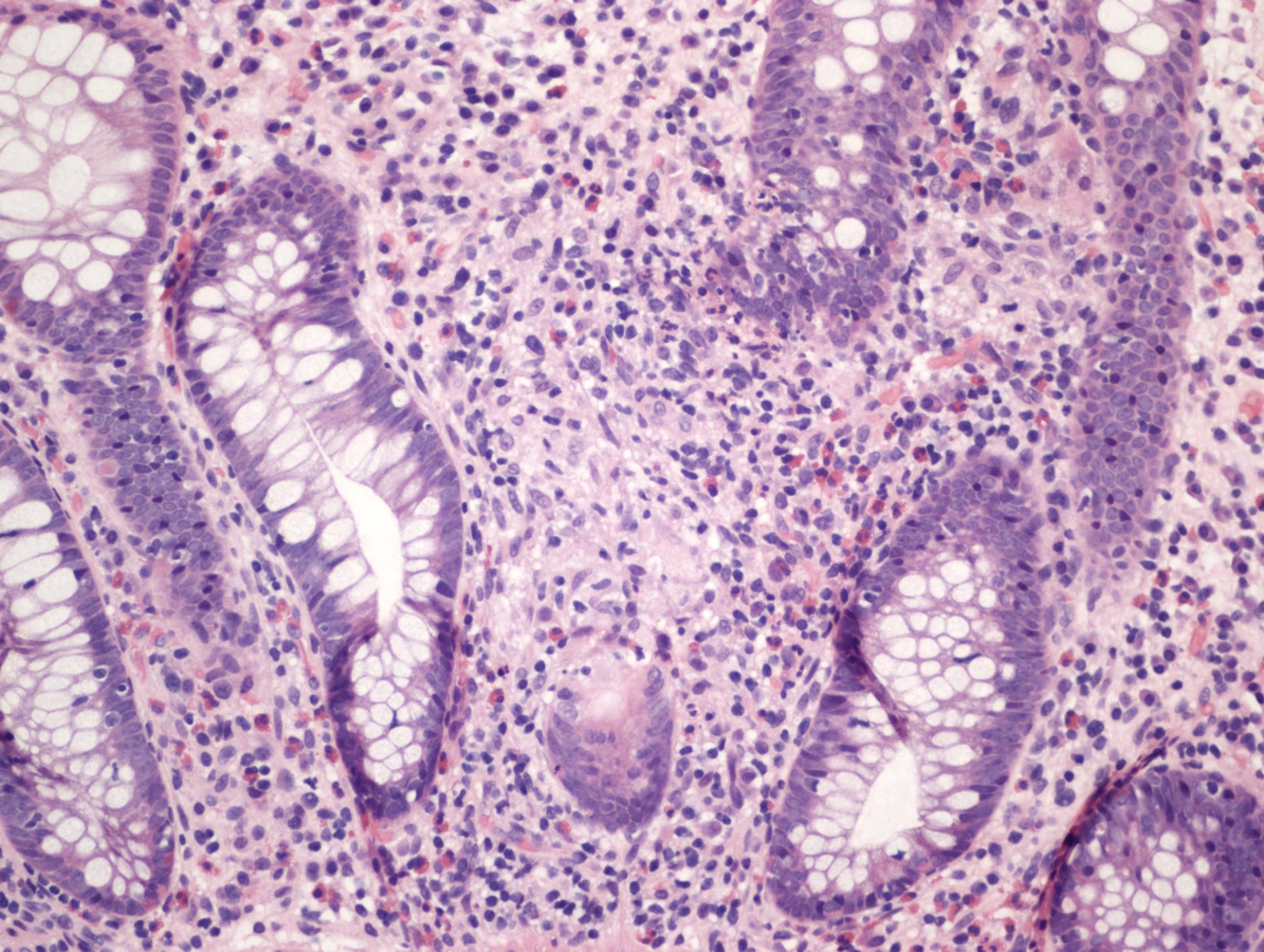
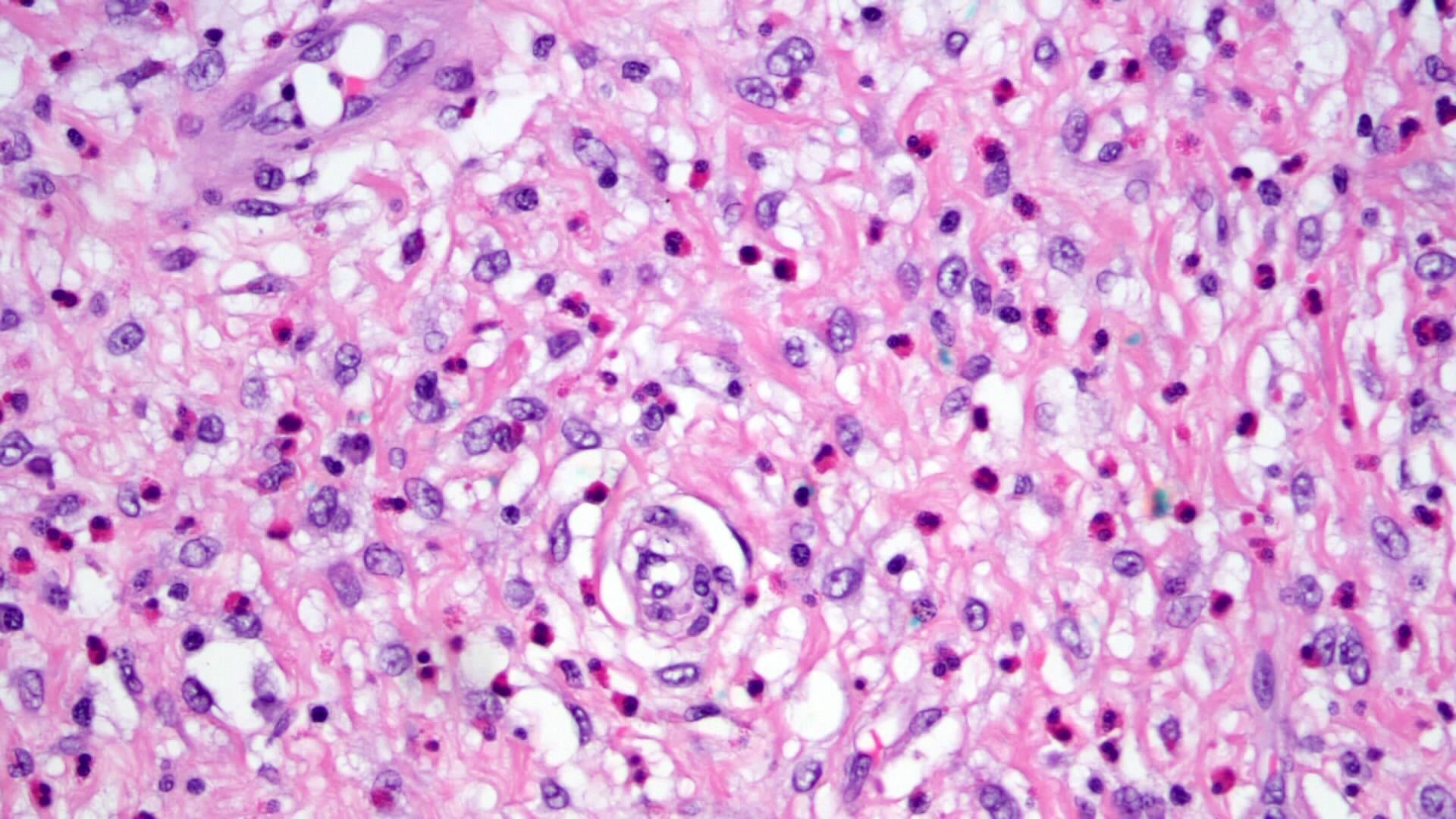
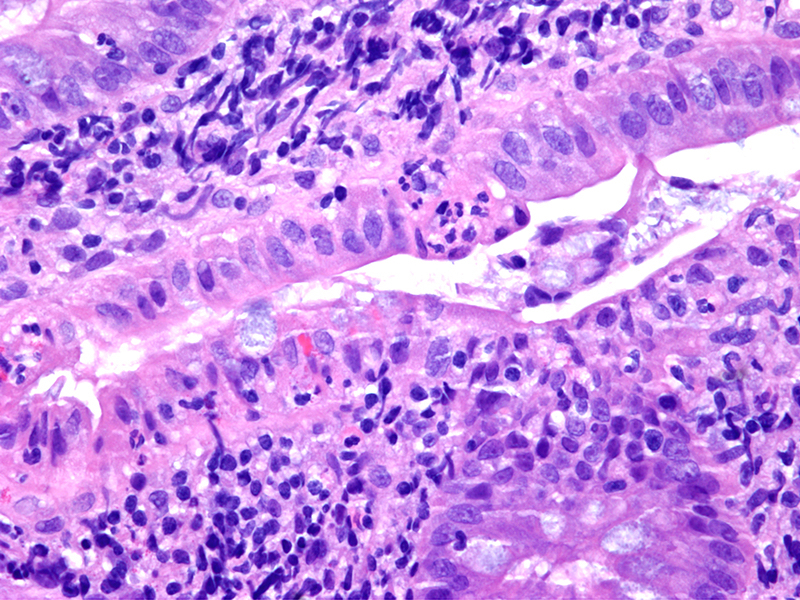
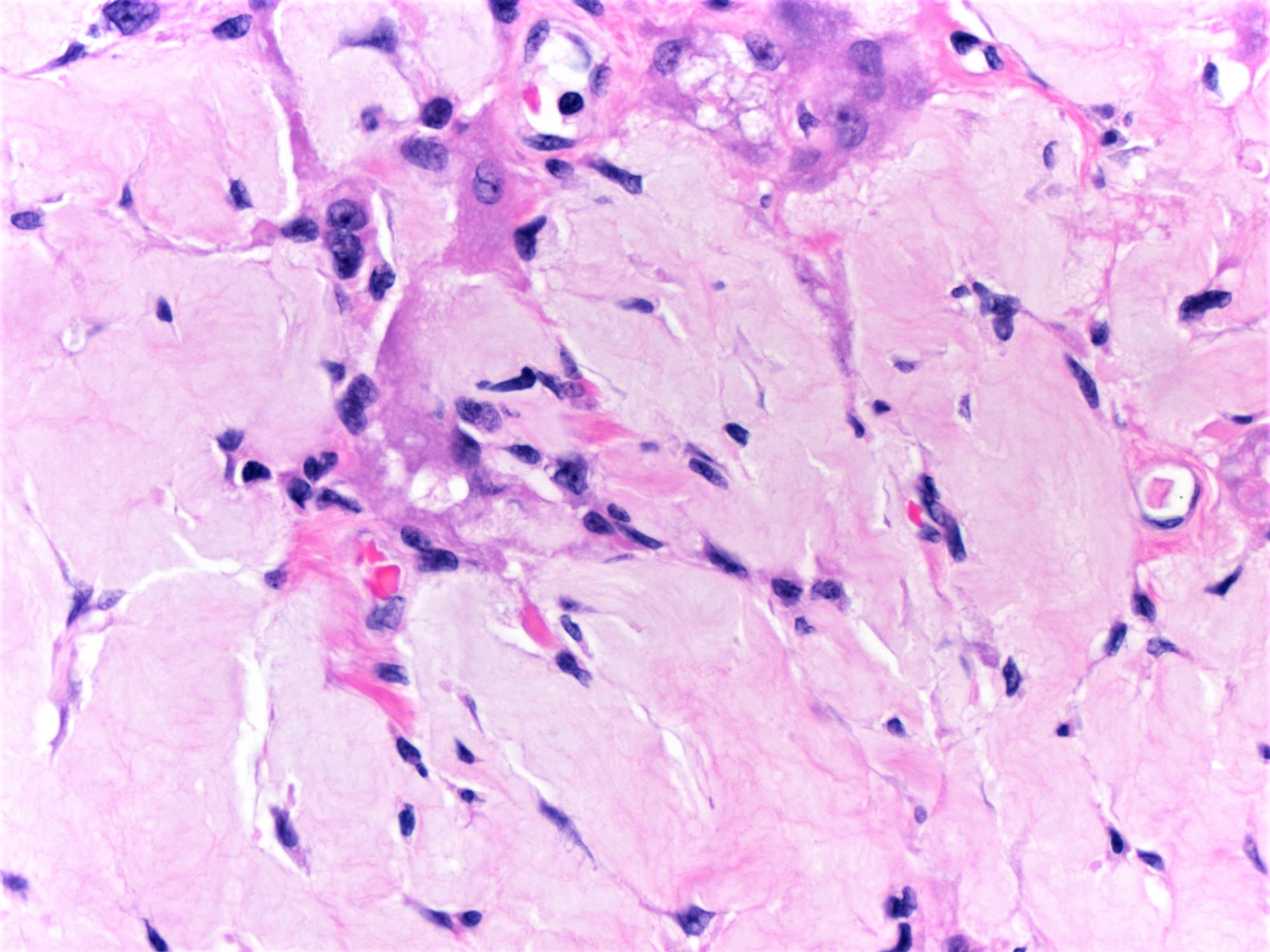
- Amebic trophozoites are best appreciated at the border of viable and necrotic tissue
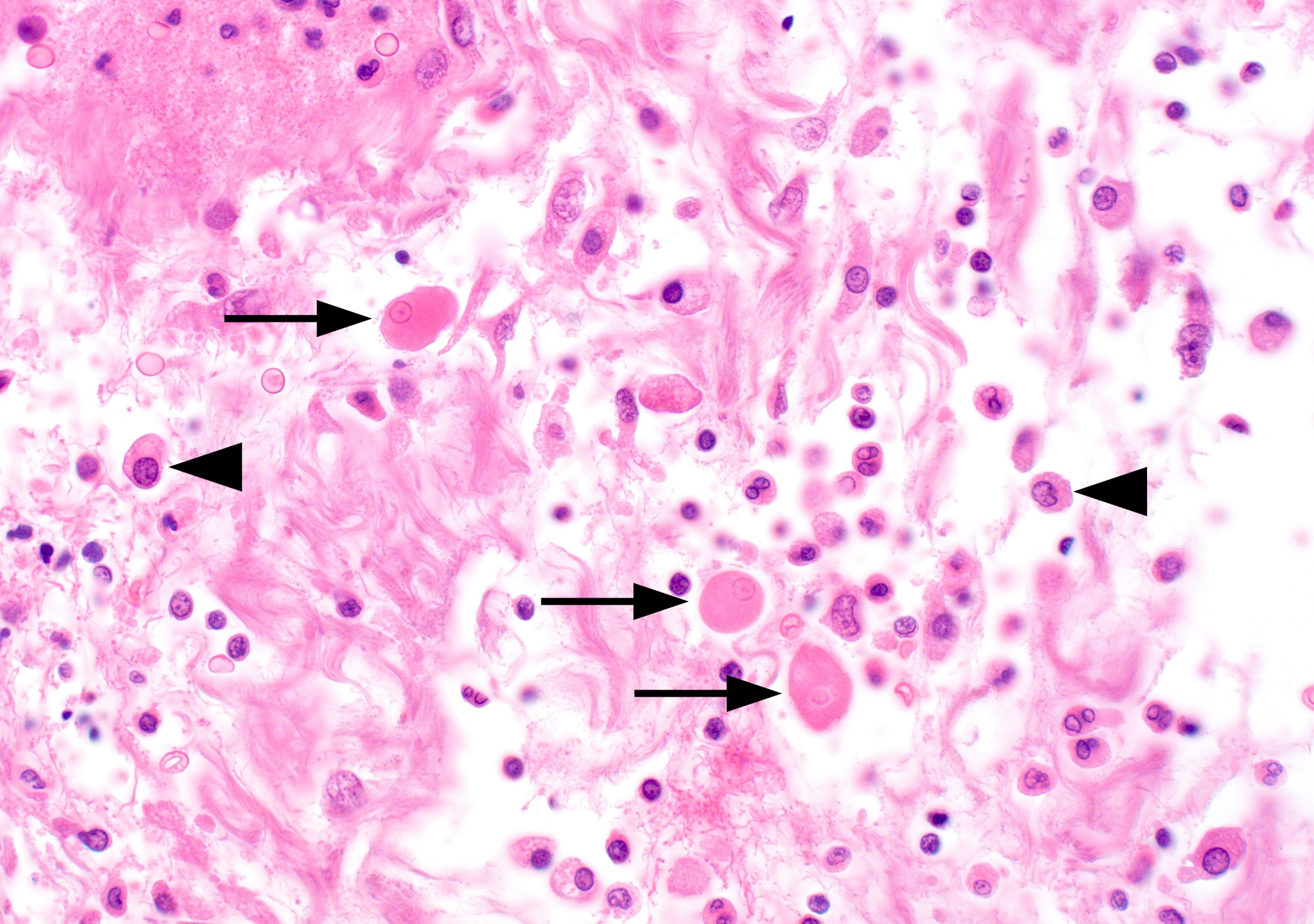
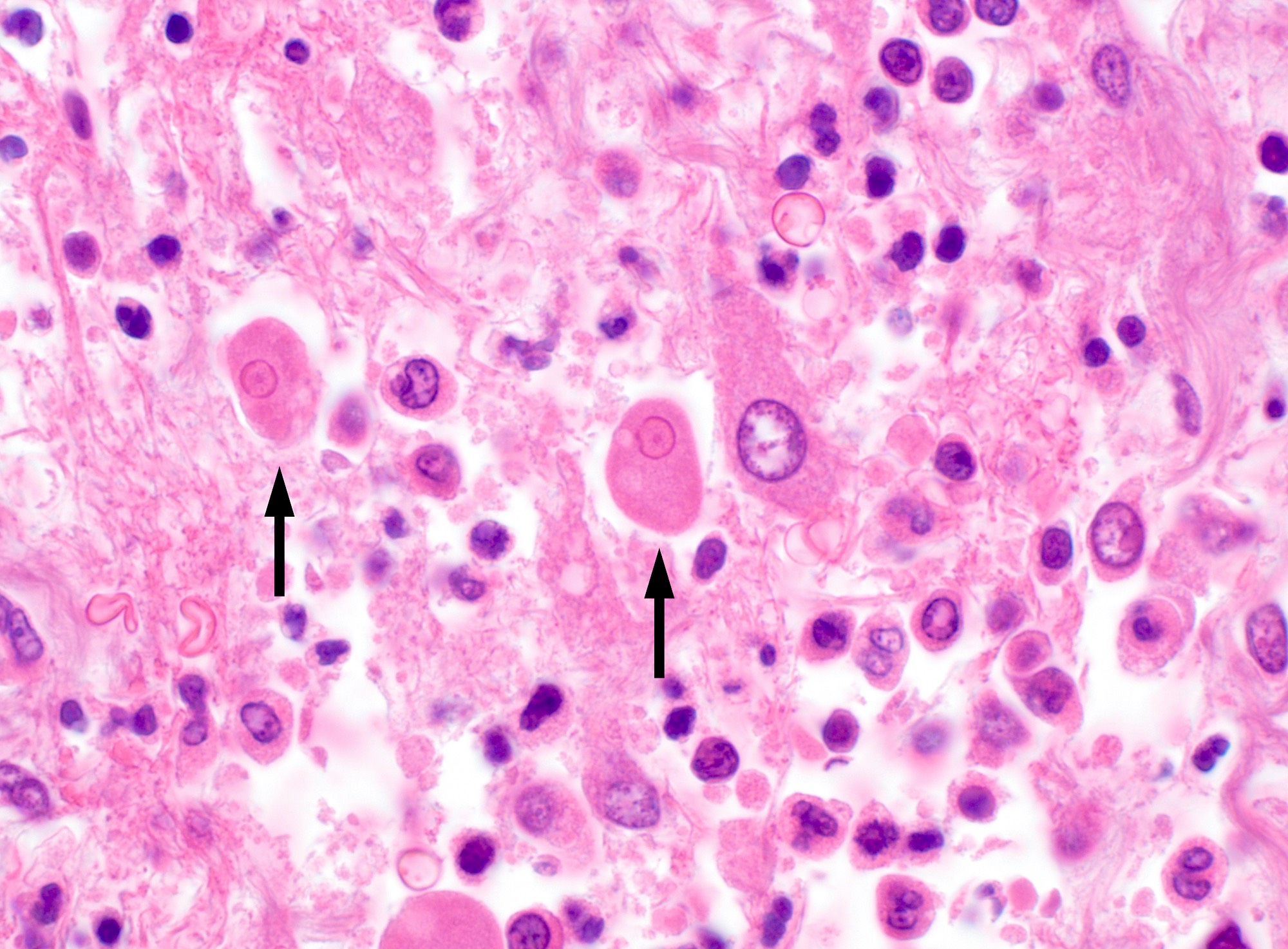
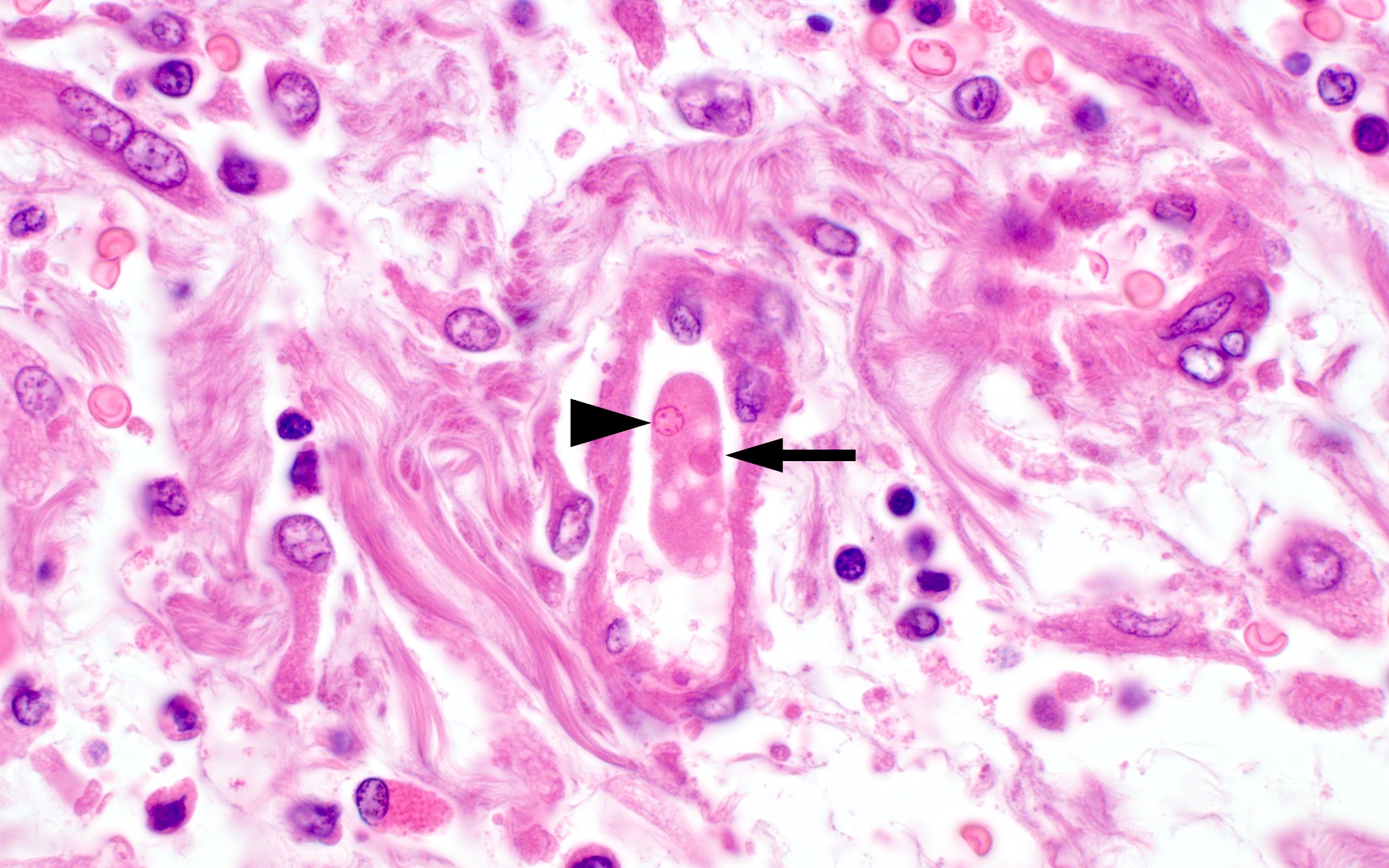
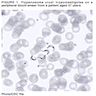
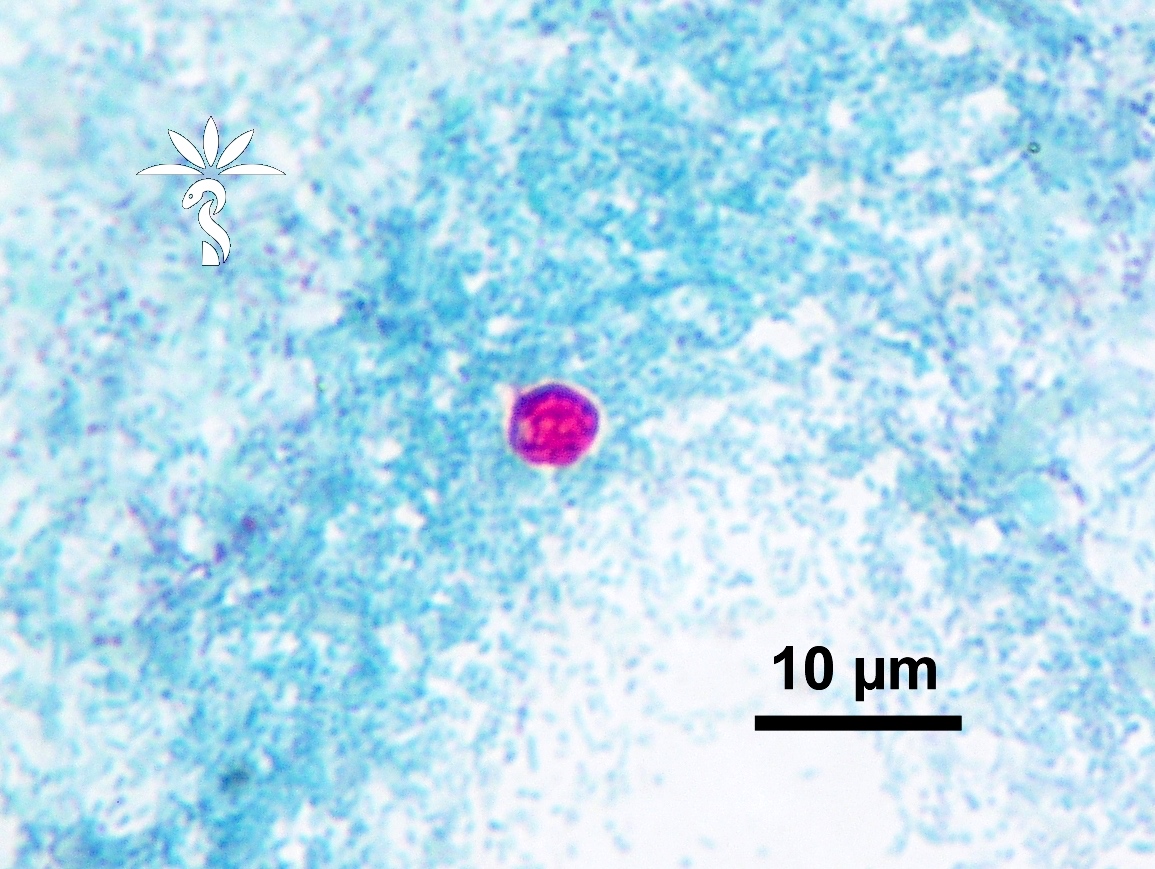
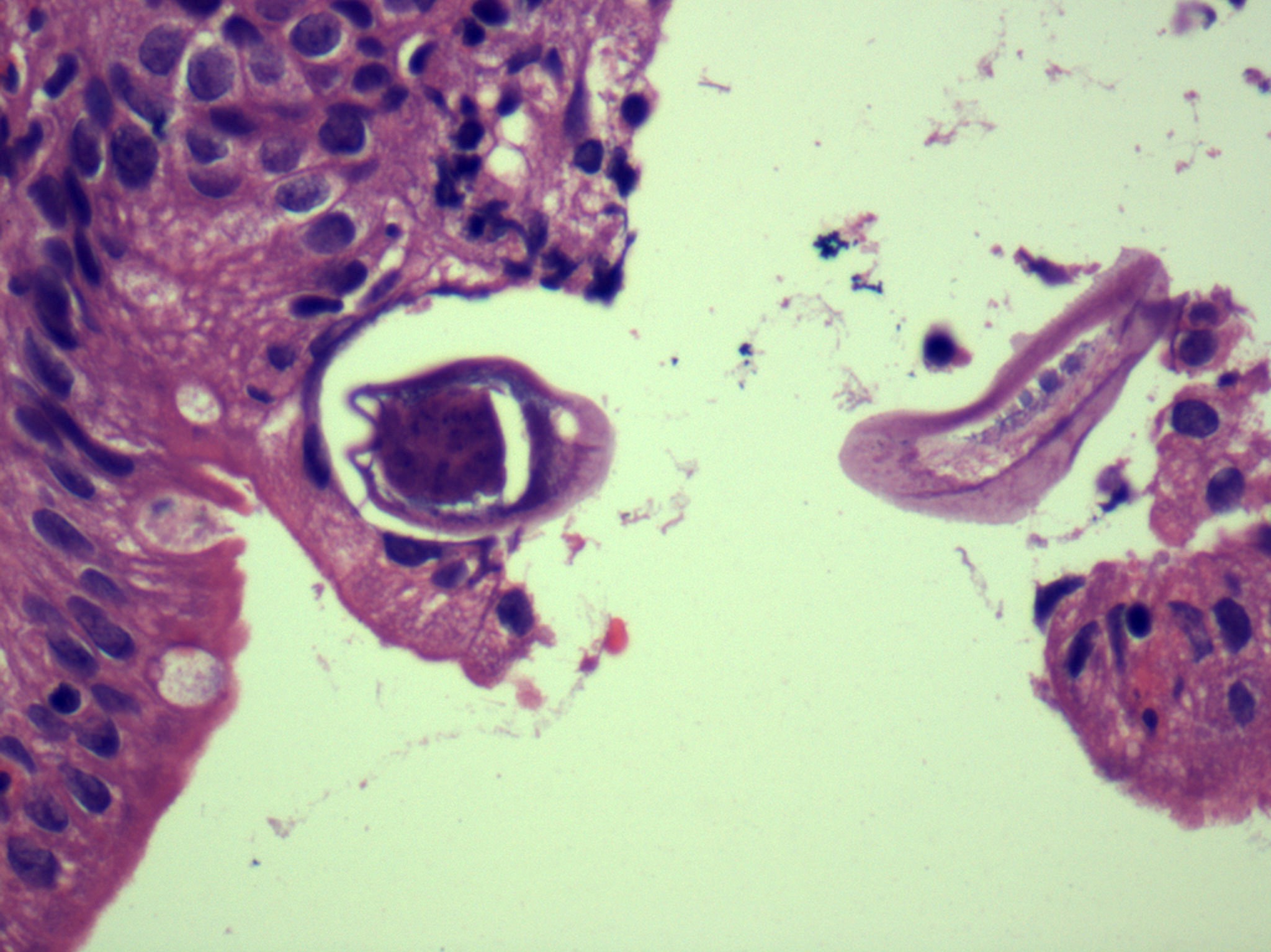
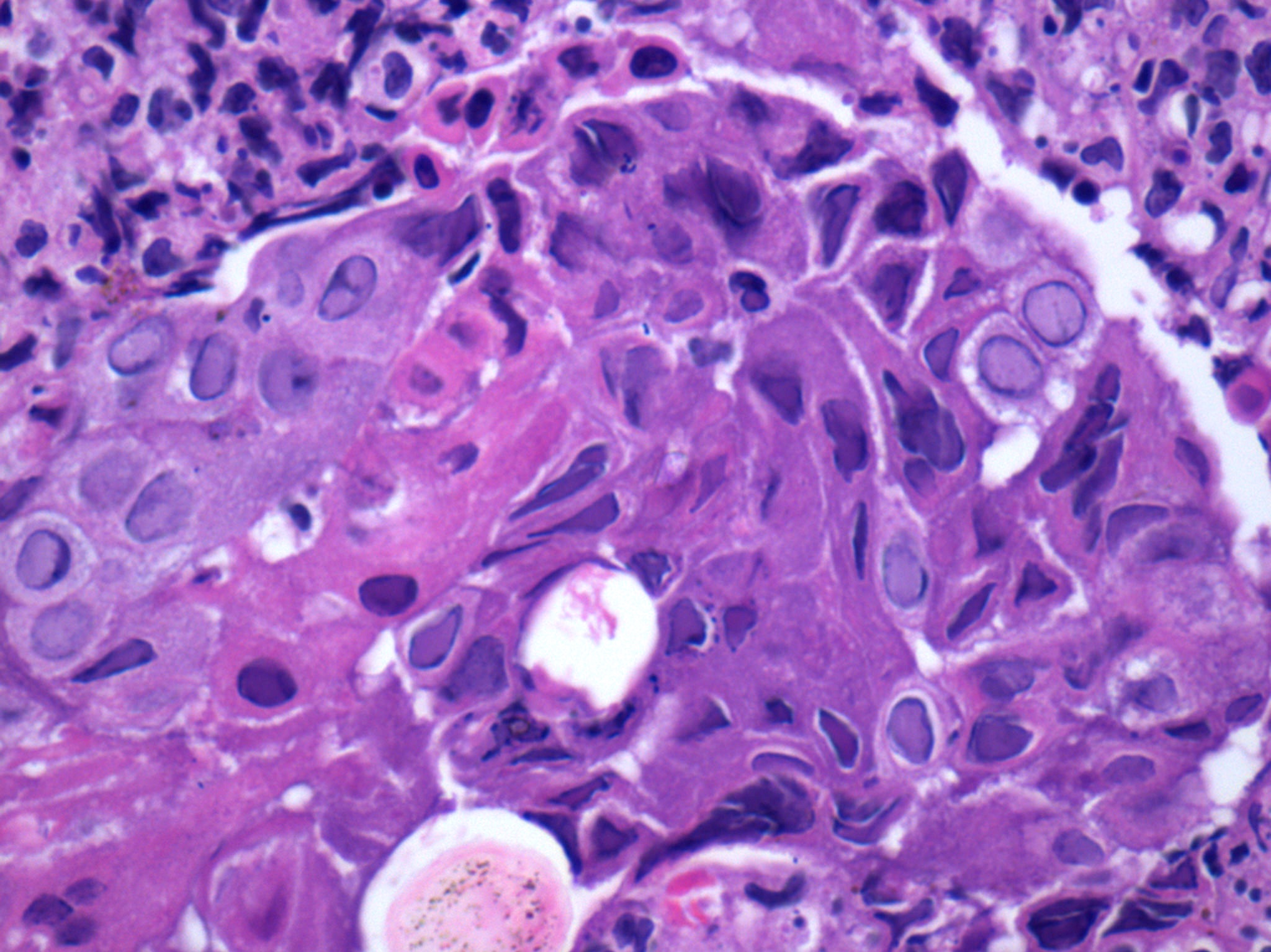
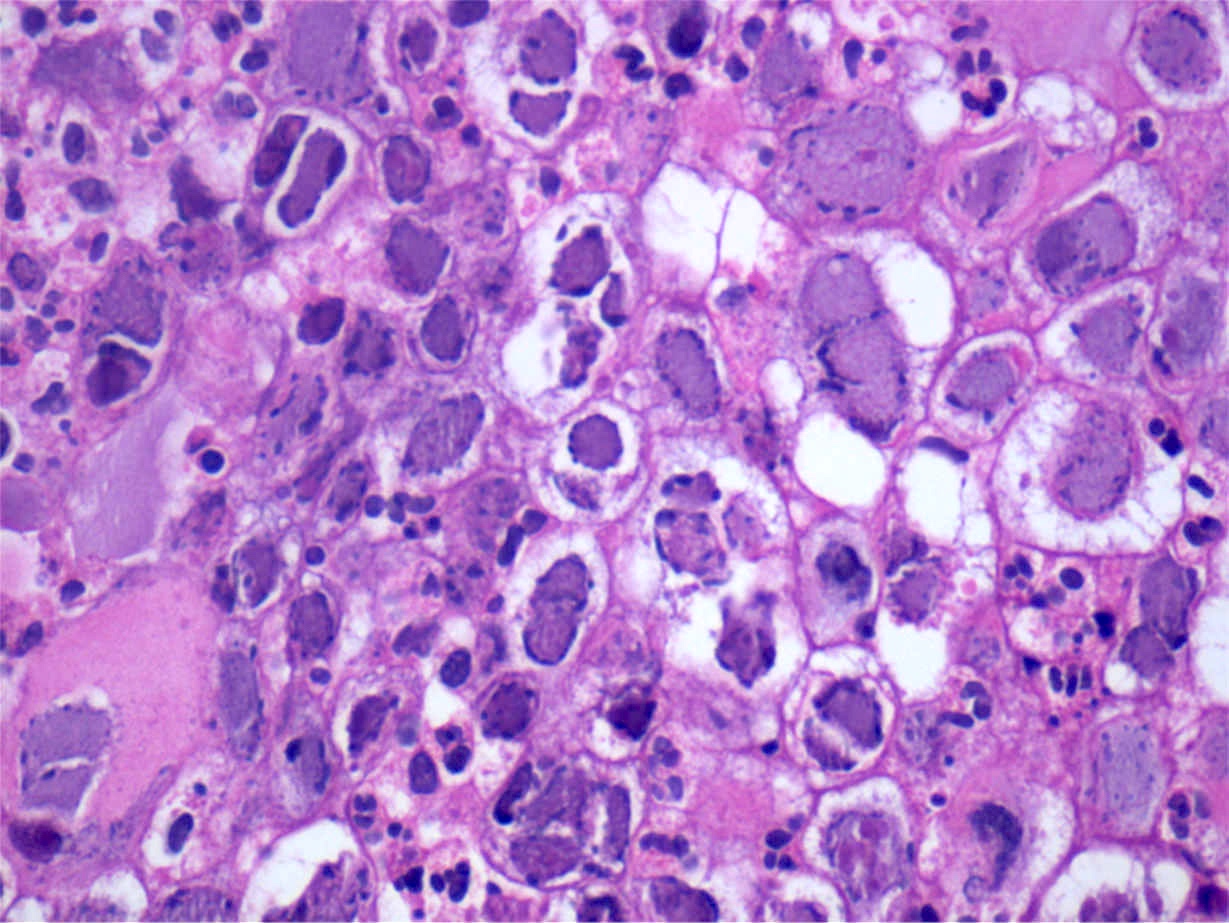
- Trophozoites of Entamoeba histolytica are 10 - 60 micrometers and may resemble macrophages
- Round to oval with pseudopod projections
- Commonly surrounded by a halo in FFPE tissue preparations; caused by retraction during fixation or by parasite toxins
- Cytoplasm is abundant, vacuolated, pink-purple on H&E, and may contain ingested red blood cells
- Nuclei are small and round with a prominent rim of peripheral chromatin and a small, dot-like central karyosome
- Amebomas are comprised of granulation tissue, chronic inflammatory cells and fibrosis
- Clusters of trophozoites are usually seen near submucosa ulcerations
- In trichrome stained stool specimens, peripheral chromatin and discrete karyosome are supportive of E. histolytica / E. dispar (Pritt: Creepy Dreadful Wonderful Parasites Blog - Answer to Case 546 [Accessed 13 October 2023])
Microscopic (histologic) images
Positive stains
- Periodic acid Schiff (PAS) highlights amebic trophozoites by staining them deep pink / magenta
- There are no commercially available IHC stains for E. histolytica
- Reference: AFIP: Topics on the Pathology of Protozoan and Invasive Arthropod Diseases [Accessed 22 April 2022]
Negative stains
- CD68 is negative in amebae, allowing for differentiation from macrophages (AFIP: Topics on the Pathology of Protozoan and Invasive Arthropod Diseases [Accessed 22 April 2022])
Sample pathology report
- Colon, cecum, biopsy:
- Intestinal amebiasis (see comment)
- Comment: The biopsy shows mucosal ulceration with an overlying fibrinous exudate. Within the ulcer bed and exudate are numerous trophozoites of the protozoan pathogen, Entamoeba histolytica, which extend into the submucosa but not into the underlying muscular layer. The trophozoites are readily identified by their abundant dense bubbly cytoplasm and small round nucleus with peripheral rim of condensed chromatin and central dot-like karyosome. Some trophozoites contain ingested erythrocytes. The trophozoites are highlighted with PAS, which stains their cytoplasm deep magenta.
Differential diagnosis
- Appendicitis:
- Acute inflammation
- No infiltrating trophozoites
- Balantidium coli (Balantioides coli):
- Flask shaped ulcers resembling amebiasis
- Large (40 - 200 micrometers) ciliated trophozoites invading the mucosa and submucosa
- Crohn's disease:
- Active chronic colitis
- No infiltrating trophozoites
- Histiocytes (also known as tissue macrophages):
- Present in various inflammatory and infectious conditions
- Similar size to Entamoeba histolytica trophozoites
- Large, often reniform nucleus (versus small round nucleus of E. histolytica)
- CD68+
- Nonpathogenic ameba
- Pseudomembranous colitis:
- Mucopurulent exudate erupts out of crypts to form a mushroom-like layer of karyorrhectic debris and neutrophils that adheres to the epithelial surface
- Crypt necrosis
- Superficial lamina propria with neutrophilic inflammation and some capillary fibrin thrombi
- No infiltrating trophozoites
- Pyogenic abscess (in liver)
- Tuberculosis
- Ulcerative colitis:
- Active chronic colitis
- No infiltrating trophozoites
Additional references
Board review style question #1
A 47 year old man living in Mexico presented with 3 months of gradually increasing abdominal pain and diarrhea. The initial diagnostic workup was unrevealing, so a colonoscopy was performed, which revealed several well demarcated ulcers in the cecum and ascending colon. Biopsies of the ulcers were obtained and above is a representative image of the histopathologic findings. What is the diagnosis?
- Amebiasis
- Balantiosis
- Signet ring carcinoma
- Toxic drug injury
Board review style answer #1
Board review style question #2
Which of the following histochemical stains is useful for highlighting invasive trophozoites of Entamoeba histolytica?
- Alcian blue
- Giemsa
- Masson trichrome
- Mucicarmine
- Periodic acid Schiff
Board review style answer #2
Amyloidosis
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Extracellular deposition of amyloid protein, often around blood vessels
Essential features
- Amyloid deposition in the colon, confirmable with Congo red
- Usually around blood vessels, which can lead to vascular injury
Terminology
- Localized (limited to the colon) or diffuse (present in numerous organs)
Epidemiology
- Can be primary, secondary, hereditary or endocrine related
Pathophysiology
- Overproduction of amyloid protein (AL, AA, ATTR, etc.) due to various causes
- Senile amyloid is often present in GI tract of elderly patients (Pathol Res Pract 1994;190:641)
Etiology
- Can have multiple causes, including malignancy, chronic inflammation, dialysis and endocrine abnormalities; sometimes associated with hemodialysis (Gastroenterology 1989;96:230, Clin Nephrol 2000;53:394, Mod Pathol 1995;8:577)
Clinical features
- Gastrointestinal involvement is seen in most patients with systemic amyloidosis
- May be asymptomatic or cause bleeding, obstruction, perforation or abnormal motility
- Amyloid tumor may clinically resemble carcinoma (AJR Am J Roentgenol 2002;179:536)
- Uncommonly, amyloid is localized to colon and does not require systemic treatment (Amyloid 2003;10:36)
Diagnosis
- Can diagnose with rectal biopsy that includes submucosa (85% sensitivity), though amyloid deposition may be initially discovered in a resection specimen
Radiology description
- Can cause various abnormalities on barium enema (Gastrointest Radiol 1991;16:133)
Case reports
- 65 year old man with amyloid tumor with synchronous adenocarcinoma (J Clin Pathol 1995;48:592)
Treatment
- If systemic, depends on type of amyloid but generally targeted at the cause (myeloma, kidney failure, etc.)
Gross description
- Mucosa may be normal or finely granular
Gross images
Microscopic (histologic) description
- Amyloid present in blood vessel walls and muscularis propria; may be subepithelial; may cause ischemic changes or frank hemorrhage
Microscopic (histologic) images
Positive stains
- Congo red (stains deep pink and demonstrates apple green birefringence, as in other body sites)
Sample pathology report
- Colon, splenic flexure, biopsy:
- Amyloidosis (see comment)
- Comment: The biopsy shows amorphous eosinophilic material present around submucosal blood vessels. On Congo red stain, the material demonstrates apple green birefringence.
Differential diagnosis
- Collagenous colitis:
- Surface epithelial damage, epithelial lymphocytes
- Elastofibromatous change:
- Lacks apple green birefringence on Congo red, elastin stain positive
- Lifting agent granuloma:
- Can contain inflammatory component
- Negative on Congo red
- Pulse granuloma:
- Contains pulse material and inflammatory component
- Negative on Congo red
Additional references
Board review style question #1
Board review style answer #1
B. The material is amyloid. It would stain a salmon pink color with Congo red and demonstrate apple green birefringence.
Comment Here
Reference: Amyloidosis
Comment Here
Reference: Amyloidosis
Anatomy & histology
Table of Contents
Definition / general | Essential features | Terminology | Physiology | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colon: the terminal portion of the gastrointestinal tract situated in the abdomen and the pelvis
- Includes the cecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum
Essential features
- Colonic mucosa has 2 predominant functions:
- Absorption of water and electrolytes from the nutrient poor chyme that passes into the colon from the ileum
- Production of mucus to lubricate the fecal material
- Above functions are accomplished by 2 predominant cell types:
- Absorptive columnar cells absorb water and electrolytes, line the surface epithelial layer and are the major cell type of crypts in the right and transverse colon
- Goblet cells secrete mucus and are the major cell type of the crypts in the left side of the colon
- Inflammatory cells are normally present in the lamina propria and include plasma cells, macrophages, eosinophils and lymphocytes
- Lymphoid aggregates and follicles are also normally present
- Architectural discipline of colon with parallel test tube-like arrangement of crypts separated by a consistent amount of lamina propria in between is important to identify
- Expansion of this lamina propria and dropout of crypts can be signs of chronic injury
Terminology
- Lower gastrointestinal tract, large bowel, large intestine
Physiology
- Colon is lined by mucosa on the luminal surface
- Epithelial layer of the mucosa has columnar cells that absorb water and electrolytes from the nutrient poor chyme received from the small intestine as it passes through the proximal colon (Hall: Guyton and Hall Textbook of Medical Physiology, 14th Edition, 2020)
- Chyme is then lubricated with mucus secreted by the goblet cells primarily in the left side of the colon to eventually form feces that is stored in the distal colon and the rectum (Hall: Guyton and Hall Textbook of Medical Physiology, 14th Edition, 2020)
- Propulsion of fecal material in the colon is achieved by the muscular layer of the colon composed of inner circular layer and outer longitudinal layer (Hall: Guyton and Hall Textbook of Medical Physiology, 14th Edition, 2020)
- Colon plays a critical role in immune defense
- Interactions between innate immune system, adaptive immune system and gut microbiota are important in regulating immune homeostasis
Gross description
- Colon is approximately 1.5 meters long with a diameter of 6 - 7 cm
- From terminal ileum to anal canal, the colon is divided into cecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum
- Segments of the colon that are completely intraperitoneal include the cecum, transverse colon and sigmoid colon (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
- Ascending colon and descending colon and upper third of the rectum are retroperitoneal, with the anterior and lateral surface covered by peritoneum (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
- Middle third of the rectum only has peritoneum on the anterior surface and the lower third is completely nonperitonealized (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
- These correct anatomic landmarks are very important in evaluation of margins and staging of colorectal cancers (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
- Different from small intestine, the colon has epiploic appendages (pedunculated fat on lateral side) and taeniae coli (discontinuous muscular fibers)
Microscopic (histologic) description
- Mucosa is composed of the epithelium, lamina propria and muscularis mucosa
- Epithelium: colonic epithelium is composed of a single layer of the absorptive columnar cells and the goblet cells
- As the epithelium invaginates into the underlying lamina propria, it forms glandular structures called crypts, arranged in a characteristic parallel test tube-like pattern
- Crypt is a functional unit of colon and is primarily lined by the goblet cells
- Crypt also has enteroendocrine cells, Paneth cells and stem cells located at its base
- During the maturation process, the mature epithelial cells migrate toward the surface of the epithelium (luminal migration), while the immature (stem cells) are at the base of the crypts
- On maturation, the Paneth cells migrate to the base of the crypt, instead of the luminal migration
- Enteroendocrine cells also stay in the deeper portion of the crypt and in the middle of the tubule
- Absorptive cells: predominant cells in the right colon
- Columnar cells with eosinophilic cytoplasm, basally located nuclei, small apical mucin vacuoles and apical microvilli
- Primarily line the surface epithelium
- Goblet cells: predominant cells in the left colon
- Large cells with intracytoplasmic mucin and basally located hyperchromatic nuclei
- Mucin composition of goblet cells is different from the mucin in the absorptive cells (Histopathology 2000;37:561)
- Other cell types of the epithelium:
- Enteroendocrine cells:
- Located at the base of the crypts and have eosinophilic secretory granules in the cytoplasm with apically located nuclei
- Apical location of the nuclei helps to differentiate these cells from the Paneth cells
- Paneth cells:
- Paneth cells have a triangular shape with densely eosinophilic cytoplasmic granules
- Nuclei are basally located, unlike enteroendocrine cells described above, a distinction important in the left colon, since the presence of Paneth cells in this part of colon is abnormal and could be a sign of chronic injury
- M cells: typically not identified on routine histology
- Usually associated with lymphoid follicles and seen best with electron microscopy
- Enteroendocrine cells:
- Lamina propria:
- Loose connective tissue rich in capillaries and lymphatics
- Supports the crypts and consists of supportive mesenchymal cells and inflammatory cells
- Mesenchymal cells are divided into 2 types: pericrypt myofibroblasts and subepithelial myofibroblasts
- Inflammatory cells vary in type and number
- Presence of eosinophils, macrophages, lymphocytes and mast cells is normal
- Plasma cells are also normally present and predominantly secrete IgA
- Presence of neutrophils in large number is abnormal
- Muscularis mucosa:
- Muscularis mucosa consists of thin strands of smooth muscle fibers separating the mucosa and submucosa
- Presence of muscularis mucosae in biopsies is critical to evaluate for architectural distortion
- Epithelium: colonic epithelium is composed of a single layer of the absorptive columnar cells and the goblet cells
- Submucosa:
- Consists of loose connective tissue, thin smooth muscle bundles, nerve plexuses (Meissner plexus and Henle deep submucosal plexus) with ganglion cells, stromal cells, adipose tissue and vasculature
- Well circumscribed areas of adipose tissue forming a discrete mass within the submucosa may represent lipomas and may present as a mass on endoscopy
- Mucosal lymphoid aggregates or follicles may extend into submucosa, which may contain the crypts, so called lymphoglandular complex mimicking invasive carcinoma in the submucosa
- Muscularis propria:
- Consists of inner circular layer and outer longitudinal layer and Auerbach nerve plexus in between the 2 muscle layers
- Interstitial cells of Cajal are present within the muscularis propria and play a role in peristalsis
- Subserosa and serosa: subserosa is composed of fibroadipose tissue and is covered by the serosa lined by cuboidal mesothelial cells
- Rectum: a continuation of the sigmoid colon with thicker mucosa and longer crypts primarily lined by the goblet cells; the mucosa gradually transitions from columnar to squamous from the rectum into the anal canal
- References: Westerhoff: Histology for Pathologists, 5th Edition, 2019, Kierszenbaum: Histology and Cell Biology - An Introduction to Pathologists, 5th Edition, 2019
Microscopic (histologic) images
Positive stains
- Enterocytes (absorptive cells): pankeratin AE1 / AE3, CK20, CDX2, SATB2 and villin
- Goblet cells: MUC2, depending on their surface or crypt location in the mucosa; periodic acid Schiff (PAS), PAS diastase (PASD), mucicarmine and Alcian blue also stain the mucin in goblet cells (Histopathology 2000;37:561)
- Enteroendocrine cells: synaptophysin, chromogranin, neuron specific enolase (NSE) and pankeratin AE1 / AE3
- Paneth cells: AE1 / AE3
- Interstitial cells of Cajal: KIT
- Muscularis mucosae and propria: desmin, vimentin, SMA
- Ganglion cells: calretinin
- Enteric glial cells: S100, GFAP, synaptophysin, PDGFRA (R), neuron specific enolase (NSE)
- Inflammatory cells:
- Lamina propria T lymphocytes: CD3+ / CD4+
- Intraepithelial T lymphocytes: surface - CD3+ / CD8+ and TCRαβ+; near lymphoid follicles - CD3+ / CD45RO+ (Chem Immunol 1998;71:1)
- Lamina propria plasma cells: CD138, MUM1 and CD79a
- Macrophages: CD68, CD163, HAM56, MAC387, lysozyme and alpha-1 antitrypsin
- Mast cells: KIT, tryptase
Board review style question #1
Board review style answer #1
D. Paneth cells. They have a triangular shape with densely eosinophilic cytoplasmic granules and basally located nuclei, as opposed to enteroendocrine cells (see image above). Their presence in the left side of the colon can be a sign of inflammatory bowel disease.
Comment Here
Reference: Colon - Anatomy & histology
Comment Here
Reference: Colon - Anatomy & histology
Board review style question #2
Which of the following parts of the colon is entirely intraperitoneal?
- Ascending colon
- Cecum
- Descending colon
- Rectum
Board review style answer #2
B. Cecum. It is entirely intraperitoneal. The ascending and descending colon are retroperitoneal. Upper third of the rectum is covered by the peritoneum on the anterior surface.
Comment Here
Reference: Colon - Anatomy & histology
Comment Here
Reference: Colon - Anatomy & histology
Angiosarcoma (pending)
[Pending]
Anti-PD1 associated colitis
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colitis is an immune related adverse event of anti-PD1 (nivolumab, pembrolizumab) and anti-PDL1 medications (atezolizumab, avelumab and durvalumab)
- PDL1 (programmed death ligand 1) and its receptor PD1 protect host cells from autoreactive T cells; monoclonal antibodies which block this interaction have been approved for treatment of several cancers (see PDL1 topic)
Essential features
- Colitis is an immune related side effect of anti-PD1 and anti-PDL1 therapy
- Often mild (diarrhea); very rarely severe (perforation) (Surg Case Rep 2017;3:94)
- 2 main histologic patterns of injury (Am J Surg Pathol 2017;41:643):
- Active colitis with neutrophilic crypt microabscesses, prominent crypt epithelial cell apoptosis and crypt atrophy / dropout (the most common pattern)
- Lymphocytic colitis-like pattern with surface injury
Epidemiology
- Incidence of 1.3 - 13.6% based on the specific agent, dose and combination of treatment for patients taking anti-PD1 and anti-PDL1 medication (BMJ 2018;360:k793, Front Pharmacol 2017;8:730, Oncoimmunology 2017;6:e1344805)
Sites
- Can affect the entire gastrointestinal tract and liver (Histopathology 2017;70:558, Mod Pathol 2018;31:965)
Pathophysiology
- Caused by immune dysregulation of the gastrointestinal mucosa (Cell 2020;182:655)
Clinical features
- Abdominal pain / cramping, diarrhea, urgency and rectal bleeding in severe form
Diagnosis
- Diagnosis of anti-PD1 colitis should be considered in patients who present with diarrhea or abdominal pain and have a history of treatment with anti-PD1 medication
- Further evaluation with endoscopy and biopsy could confirm the diagnosis
Radiology description
- Segmental / diffuse bowel wall thickening, mucosal hyperenhancement and diffuse or segmental peritoneal fat infiltration with mesenteric vessel engorgement (Eur J Cancer 2018;96:91, Eur Radiol 2021;31:8868)
Case reports
- 51 year old woman with colonic perforation after treatment with pembrolizumab (anti-PD1 inhibitor) for metastatic melanoma (Case Rep Gastrointest Med 2018;2018:3406437)
- 62 year old man with infliximab refractory severe nivolumab induced enterocolitis (Am J Case Rep 2018;19:360)
- 68 year old woman with anti-PD1 induced collagenous colitis (Melanoma Res 2016;26:308)
- 71 year old man with intestinal perforation after nivolumab immunotherapy for a malignant melanoma (Surg Case Rep 2017;3:94)
- 82 year old man with nivolumab associated colitis mimicking ulcerative colitis (Clin Gastroenterol Hepatol 2017;15:A35)
Treatment
- Withholding the immune check point inhibitor
- Most patients respond to corticosteroids
- In steroid refractory cases, tumor necrosis factor α (TNFα) blocking agents such as infliximab and vedolizumab can be used
- Reference: Practice Guideline Gastroenterology 2021;160:1384
Microscopic (histologic) description
- Common patterns of injury (Am J Surg Pathol 2017;41:643, Histopathology 2021;78:532):
- Focal active colitis: neutrophilic crypt microabscesses or cryptitis with prominent crypt epithelial cell apoptosis, focal crypt atrophy / dropout
- Lymphocytic colitis-like pattern of injury
- Other histopathologic patterns include graft versus host disease-like or collagenous colitis patterns of injury (Histol Histopathol 2022;37:699)
- Anti-PD1 associated colitis rarely causes perforation
Microscopic (histologic) images
Negative stains
Sample pathology report
- Colon, biopsy:
- Colonic mucosa with lymphocytic colitis pattern of injury and mild focal active colitis (see comment)
- Comment: Patient’s history of advanced melanoma status post treatment with pembrolizumab is noted. The biopsies show a predominantly lymphocytic colitis pattern of injury with focal mild active colitis. No significant chronicity, no granuloma and no viral cytopathic effects are seen. Overall, these findings are most consistent with anti-PD1 associated colitis.
Differential diagnosis
- Other causes of active colitis with prominent apoptosis, such as infections (CMV colitis), acute graft versus host disease
- Inflammatory bowel disease:
- Basal plasmacytosis, less apoptosis, evidence of chronicity
- Chemotherapy induced colitis:
- Changes depend on the type of medication but may show similar histology; history is probably necessary for distinction
- Ipilimumab associated colitis:
- Lymphoplasmacytic expansion of lamina propria, increased apoptosis and intraepithelial lymphocytes, cryptitis and crypt elongation
- Somewhat similar histology; history is probably necessary for distinction
- Idelalisib associated colitis:
- Somewhat similar histology to anti-PD1 associated colitis; history is probably necessary for distinction (Am J Surg Pathol 2015;39:1661)
Board review style question #1
A 64 year old man with ulcerative colitis recently completed 3 cycles of pembrolizumab therapy for metastatic carcinoma. He underwent a colonoscopy with a biopsy for diarrhea. Which of the following features is key to differentiating anti-PD1 associated colitis from ulcerative colitis?
- Increased crypt epithelial apoptosis, lack of significant chronicity and absence of basal plasmacytosis
- Involvement of the rectum
- Presence of crypt abscesses
- Presence of erosions
Board review style answer #1
A. Increased crypt epithelial apoptosis, lack of significant chronicity and absence of basal plasmacytosis. Increased crypt epithelial apoptosis is most commonly seen in anti-PD1 colitis. On the other hand, presence of significant chronicity and basal plasmacytosis is more in favor of inflammatory bowel disease. Rectum involvement, crypt abscesses and erosions can be seen in both.
Comment Here
Reference: Anti-PD1 associated colitis
Comment Here
Reference: Anti-PD1 associated colitis
Board review style question #2
A 72 year old man with a history of melanoma recently received 3 cycles of nivolumab and developed abdominal pain and diarrhea. A colonoscopy was done which found erythema in the ascending and transverse colon. Histology is shown in the image above. What is the most likely diagnosis?
- Anti-PD1 associated colitis
- Collagenous colitis
- Diverticular associated colitis
- Severe ulcerative colitis
Board review style answer #2
A. Anti-PD1 associated colitis. Based on the history and findings of a mild focal active colitis and mild increase in intraepithelial lymphocytes, the most likely diagnosis is anti-PD1 colitis.
Comment Here
Reference: Anti-PD1 associated colitis
Comment Here
Reference: Anti-PD1 associated colitis
APC gene
Table of Contents
Definition / general | Molecular / cytogenetics description | Molecular / cytogenetics images | Differential diagnosisDefinition / general
- APC is a tumor suppressor "gatekeeper" gene located on 5q that regulates the level of beta-catenin and directs the downstream activity of Wnt / beta catenin pathways (Appl Clin Genet 2015;8:95)
- APC contributes to orderly migration of intestinal cells within the crypt and beta-catenin plays a crucial role in this function; it also plays a role in intercellular adhesion
- Germline mutations in APC have various manifestations, often based on the position of the mutation (among other factors):
- Classic familial adenomatous polyposis (FAP): mutations occur between exon 5 and 5' portion of exon 15; severe polyposis (< 5000 colorectal polyps) associated with mutations between codons 1250 and 1464
- Attenuated FAP: < 100 colorectal adenomas (average 30), later age of onset (< 40 years old); due to mutations at the extreme 5' or 3' ends or in region of exon 9
- Gardner syndrome: variant of FAP with benign osteoid tumors and epidermoid skin cysts
- Hereditary desmoid disease
- Turcot syndrome: multiple colorectal polyps and medulloblastoma
- Congenital hypertrophy of retinal pigment epithelium: benign finding, but can identify individuals at risk for FAP (Acta Ophthalmol Scand 1996;74:338)
- Missense mutation does not lead to classic FAP but increases risk for colorectal carcinoma; found in Ashkenazi Jewish ancestry
Molecular / cytogenetics description
- 90% of cases of classic FAP are caused by germline mutation of APC (Clin Gastroenterol Hepatol 2014;12:1059)
- More than 1500 germline mutations exist, the majority being point mutations (Colon Cancer Gene Variant Databases)
- Exon 15 is the most common target for mutations, at codons 1061 and 1309 (Hum Mol Genet 2001;10:721)
- Mutations are mostly nonsense or frameshift, resulting in a truncated protein
- Biallelic inactivation leads to a loss of protein function (Genet Med 2014;16:101), which causes aberrant transcription of c-myc, cyclin D1 and others
- APC mutations promote the T cell factor-lymphoid enhancer factor (TCF-LEF) pathway and inhibit the cellular adhesion complex, thereby stimulating cell proliferation (Mol Cancer 2003;2:41)
- The gold standard for mutation detection is direct DNA sequencing of all 15 coding exons
- Various other methods are available to detect point mutations, including PCR based methods (Tech Coloproctol 2004;8:s305)
Differential diagnosis
- In FAP-like patients without APC mutation, biallelic mutations of MUTY human homologue (MYH) gene may be found (MUTYH associated polyposis)
- 10% of patients with classic FAP phenotype do not have identifiable mutations in APC or MYH
Apoptotic colopathy
Microscopic (histologic) description
- Reduced intracellular mucin, epithelial apoptosis and sloughing, increase in mitotic figures (Hum Pathol 1998;29:972, Cesk Patol 2011;47:130)
- May have erosion of superficial epithelium, consistent with clinical impression of aphthoid ulcer
- Also increased lymphocytes and neutrophils (Dis Colon Rectum 2006;49:109)
- Patchy mononuclear infiltration in upper part of lamina propria, increased epithelial cell proliferation of individual crypts, basal cryptitis (Cesk Patol 2010;46:37)
- Rarely lamina propria edema or extravasated red blood cells
Differential diagnosis
Atresia
Table of Contents
Definition / general | Treatment | Clinical images | Gross images | Microscopic (histologic) images | Additional referencesDefinition / general
- Imperforate mucosal diaphragm or stringlike segment of bowel
- Very rare (1 per 20,000 live births)
- Associated with other congenital anomalies, including Hirschsprung disease
- May have genetic (J Pediatr Surg 2005;40:390) or vascular cause
- Type 1: bowel and mesentery are intact, but bowel lumen is interrupted by a complete membrane
- Type 2: bowel is discontinuous, connected by a fibrous cord
- Type 3: bowel ends are completely separated and mesentery has a gap
Treatment
- Segmental resection and anastomosis; good prognosis with prompt treatment (J Pediatr Surg 2005;40:1258)
Clinical images
Microscopic (histologic) images
Additional references
Balantidiasis
Table of Contents
Definition / general | Epidemiology | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Disease caused by the ciliate protozoan Balantidium coli
Epidemiology
- B. coli is found worldwide, but disease occurs most commonly in parts of the developing world including Latin America, Southeast Asia, Papua New Guinea and parts of the Middle East
- Estimated prevalence is 1% in Aymara children in northern Bolivia (Am J Trop Med Hyg 1998;59:922);and 0.4% in rural northeast Thailand (Korean J Parasitol 2013;51:727)
- Very low prevalence in industrialized countries
- Humans are usually resistant to infection; disease generally occurs in debilitated or poorly nourished patients
- Pigs are the primary reservoir for human infection and most cases occur in people in close proximity to pigs, although rats and other mammals may also transmit disease
- Human to human transmission is also described
- Infection by ingesting fecally contaminated food or water or from ingesting cysts due to other direct contact with pig or rat excrement
Pathophysiology
- Excystation occurs in the small intestine and trophozoites migrate to the colon
- Invasion into the intestinal wall occurs, where they multiply and cyst formation occurs
Clinical features
- Most infections are asymptomatic
- Symptomatic patients generally suffer disease similar to amebiasis, with diarrhea, dysentery, abdominal pain and weight loss
- Chronic disease is most common, although fulminant colitis may occur, including perforation leading to peritonitis
- Disease in the lung, urinary bladder and bone has been described (see case reports)
- Similarly to E. histolytica, B. coli causes flask shaped ulcers in the large intestine, most commonly in the cecum and rectosigmoid
- Neobalantidium coli is the largest protozoan parasite and only pathogenic ciliate to infect humans (Pritt: Creepy Dreadful Wonderful Parasites Blog - Answer to Case 532 [Accessed 23 August 2019])
Diagnosis
- Diagnosis is usually made by identification of mobile trophozoites in fresh stool or scraped from an ulcer seen during endoscopy
- Rarely the diagnosis is made by examination of urine or bronchioalveolar lavage fluid, or by identification in biopsy or resection specimens
Case reports
- 20 year old man with pulmonary hemorrhage (S Afr Med J 2010;100:534)
- 28 year old man with dysentery (New Microbiol 2013;36:203)
- 47 year old woman with dysentery and non-Hodgkin lymphoma (World J Gastroenterol 2004;10:458)
- 60 year old man with vertebral osteomyelitis and myelopathy (J Neurosurg Spine 2013;18:310)
- 68 year old man with urinary balantidiasis (Trop Parasitol 2014;4:47)
- 72 year old woman with Balantidium coli in urine sediment (J Parasit Dis 2013;37:283)
- Causing severe peritonitis (Eur J Clin Microbiol Infect Dis 2004;23:393)
Treatment
- Tetracycline is the drug of choice
- Alternative treatments include metronidazole, ampicillin, iodoquinol and nitazoxanide
- Longer treatment is necessary if immunosuppressed
Microscopic (histologic) description
- The trophozoite is oval and large, sometimes large enough to be seen without magnification (generally 30-150 μm in length x 20-120 μm in width, rarely up to 200 μm in length), contains circumferential cilia (B. coli is the only ciliated parasite that infects humans)
- It is rapidly mobile
- In tissue sections, the trophozoites are large with kidney-bean shaped nuclei and visible cilia
- Cytostome (oral groove) can be seen on trophozoite (Pritt: Creepy Dreadful Wonderful Parasites Blog - Answer to Case 532 [Accessed 23 August 2019])
- N. coli cysts may be mistaken for helminth eggs (Pritt: Creepy Dreadful Wonderful Parasites Blog - Answer to Case 532 [Accessed 23 August 2019])
Microscopic (histologic) images
Electron microscopy description
- Flattened oval organism covered with cilia with gullet at anterior end
Differential diagnosis
Additional references
Basidiobolomycosis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Poor prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Differential diagnosisDefinition / general
- Basidiobolomycosis is a rare fungal infection caused by environmental saprophyte Basidiobolus ranarum
- It is a member of the order Entomophthorales of the class zygomycetes
- The zygomycetes include two fungal orders:
- Mucorales, involving mainly immunocompromised patients, and
- Entomophthorales, which causes infection in immunocompetent individuals (J Korean Surg 2012;83:325)
- Basidiobolomycosis is endemic in tropical and subtropical regions of Africa, Latin America, Middle East and Asia (Curr Pediatr Res 2013;17:1)
- It mainly involves skin and subcutaneous tissue and rarely infects the gastrointestinal tract
- First case of visceral basidiobolomycosis (GIB) was reported in 1964 (Trans R Soc Trop Med Hyg 1964;58:242)
Essential features
- Basidiobolomycosis is a rare fungal infection caused by Basidiobolus ranarum
- Causes infection in immunocompetent individuals
- Is endemic in tropical and subtropical regions
- Mainly involves skin and subcutaneous tissue, visceral involvement is rare
- Infection is caused by ingestion of contaminated food or use of contaminated toilet paper
- Abdominal pain and fever are most common presenting features
- Histology shows broad septate thin walled fungal hyphae with the Splendore-Hoeppli phenomenon
- Culture is gold standard for diagnosis
- Treatment: combined surgery and medical therapy with antifungals
- Prognosis poor in pediatric patients
ICD coding
- B46.8 - other zygomycoses
Epidemiology
- Ages 18 months to 80 years; male to female ratio of 11:1
- Mean age 37 years (Clin Infect Dis 2012;54:1685)
Sites
- Mostly cause chronic infection in the skin and subcutaneous tissue
- May cause infection in the stomach, small intestine, colon and liver
Pathophysiology
- Infection is acquired by ingestion of infected food (e.g., people with pica) and using contaminated papers for cleaning the skin (e.g., toilet papers)
- Possibly due to ranitidine, which can decrease gastric acidity and allow fungal survival after gastric passing (Iran J Med Sci 2015;40:90)
- Smoking can decrease mucosal WBC function and facilitate fungal infection by B. ranarum (Clin Infect Dis 2012;54:1685)
Etiology
- Basidiobolus ranarum
Clinical features
- Abdominal pain and fever are most common presenting features (J Med Microbiol 2012;61:1770)
- Other features include constipation, diarrhea, gastrointestinal bleeding, mucoid stool and rarely perforated appendicitis (Case Rep Surg 2011;2011:685460)
Diagnosis
- Based on characteristic histopathology findings of fungal hyphae (the Splendore-Hoeppli phenomenon) and culture of B. ranarum from the tissue specimen
- The gold standard for definite diagnosis is culture
- Macroscopically, colonies are yellowish gray in color with a waxy appearance and many radial folds (J Med Microbiol 2012;61:1770)
Laboratory
- Eosinophilia and high ESR (erythrocyte sedimentation rate)
- Elevated levels of cytokines such as IL4, IL10, TNFα (TH2 type cytokines) (J Clin Microbiol 2001;39:2360)
Radiology description
- Colorectal mass is the most common finding, followed by hepatic mass or small bowel mass (Abdom Imaging 2015;40:246)
- Bowel wall thickening was noticed in 25% of the reported cases, whereas bowel perforation or abscess was less commonly seen (Afr J Paediatr Surg 2015;12:193)
Radiology images
Poor prognostic factors
- Pediatric age group at presentation
- Delayed treatment
Case reports
- 4 year old girl with visceral basidiobolomycosis, an unusual fungal infection of viscera (Afr J Paediatr Surg 2015;12:193)
- 34 year old woman with perforated appendicitis with gastrointestinal basidiobolomycosis (Surg Infect (Larchmt) 2014;15:339)
- 36 year old man with diffusely thickened small bowel with edematous change (Medicine (Baltimore) 2015;94;e1430)
Treatment
- Combined surgery and antifungal treatment for a variable period of time (6 months to 2 years) (J Pediatr Surg 2012;47:949)
- Itraconazole, an antifungal agent is the most common drug used
Gross description
- Fungus involves the nonmucosal layers such as submucosa and subserosa of the GI tract, hence endoscopic biopsies, which are superficial and small, usually fail to detect the fungal hyphae (Saudi Med J 2013;34:1068)
Gross images
Microscopic (histologic) description
- Marked infiltration of eosinophils
- Mixed infiltration of PMN leukocytes and granulomatous inflammation
- Thin wall and broad hyphae surrounded by eosinophilic material, which are easily seen by hematoxylin and eosin staining; however periodic acid-Schiff (PAS) and gomori methenamine silver (GMS) can intensify the fungal wall staining
- Zygospores which are very similar to trophozoites of amoeba (Clin Infect Dis 2001;32:1448)
Microscopic (histologic) images
Cytology description
- Broad septate thin walled fungal hyphae along with eosinophils
Differential diagnosis
- Eosinophilic gastroenteritis: no fungal hyphae
- Other granulomatous lesions: no broad septate fungal hyphae
Behcet's syndrome
Table of Contents
Definition / general | Epidemiology | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Multisystem inflammatory disorder of unknown origin, with aphthous stomatitis, genital ulcers, relapsing iritis; also arthritis, cutaneous and vascular disease (eMedicine #1, #2)
- GI involvement in 10% (ileum, cecum); causes pain, diarrhea, melena; rarely perforation (Surg Today 2003;33:383)
Epidemiology
- More common in region from Mediterranean to eastern Asia
- 2 / 3 in males
Case reports
- 16 year old girl with colonic Behcet's disease with pyoderma gangrenosum (World J Gastroenterol 2006;12:979)
- 17 year old monozygotic male twins with concordant colonic Behçet's disease(J Gastroenterol 2005;40:421)
- 17 year old boy with colonic perforation in Behcet's syndrome (World J Gastroenterol 2008;14:6578)
- 20 year old man with Behçet's disease with multiple colon perforations (Turk J Gastroenterol 2010;21:324)
- 21 year old man with multiple perforations along the transverse colon (Clinics (Sao Paulo) 2009;64:1231)
- 29 year old woman with severe colitis (Am J Surg Pathol 1986;10:888)
- 34 year old man with case resembling inflammatory bowel disease (Intern Med 2004;43:243)
- 37 year old woman with case with longitudinal ulcers and granulomas resembling Crohn's (J Gastroenterol Hepatol 2002;17:105)
- 77 year old man with colon cancer arising in an ulcer scar due to intestinal Behçet's disease (Intern Med 2011;50:429)
Treatment
- Steroids
Gross description
- Numerous punched out ulcers of various sizes, shapes and depths in colon
Microscopic (histologic) description
- Ulcers with neutrophils around ulcer bed, lymphocytic vasculitis in submucosal veins
- Note: diagnosis is clinical, not histologic
Differential diagnosis
Brainerd diarrhea
Definition / general
- Chronic watery diarrhea of unknown etiology with acute onset and long duration (Centers for Disease Control, Wikipedia)
- First case in 1983 in Brainerd, Minnesota (USA)
- Similar to epidemic infectious diarrhea, but symptoms usually last 4+ weeks, often years
- Most patients recover within 3 years or less
Case reports
- California restaurant outbreak (Clin Infect Dis 2006;43:62)
- Texas restaurant outbreak (Clin Infect Dis 2006;43:55)
- Cruise ship outbreak (J Infect Dis 1998;177:1041)
Microscopic (histologic) description
- Surface epithelial lymphocytosis (similar to lymphocytic colitis) with no architectural distortion, no degenerative changes, no thickened subepithelial collagen (Am J Surg Pathol 1996;20:1102)
Brown bowel syndrome (pending)
Table of Contents
Definition / generalDefinition / general
[Pending]
Campylobacter jejuni
Table of Contents
Definition / general | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Campylobacteriosis refers to disease caused by Campylobacter species and closely related bacteria
- Campylobacter are comma shaped, gram negative, flagellated, non spore forming, microaerophilic bacteria
- C. jejuni is by far the most common cause of Campylobacteriosis, and it has >90 serotypes
- C. fetus Subsp. fetus infection is not rare
- Other species ("atypical" enteric Campylobacters), including C. coli, C. lari, C. hyointestinalis, Helicobacter cinaedi, C. upsaliensis, Helicobacter fennelliae, and others are rarely isolated and when symptomatic, usually are associated with gastrointestinal illness or periodontal disease
Epidemiology
- C. jejuni:
- Causes 1.3 million (CDC) to >2.0 million (Mandell) infections in US annually
- #1 or #2 most common bacterial cause of diarrheal illness in US
- Commensal in the wild and in domesticated fowl, cattle, sheep, pigs, goats, dogs, cats, rodents
- Has a worldwide distribution
- For human transmission, the most important reservoirs are poultry, sheep, cattle and pigs
- In the developed world disease is generally sporadic and most commonly occurs after ingestion of undercooked poultry or unpasteurized milk
- Infection from eating undercooked beef, raw clams and untreated water also occurs
- Human to human, fecal-oral transmission may occur
- In commercial slaughterhouses, meat, especially poultry, is commonly contaminated by intestinal contents
- Campylobacter die quickly in dry and cold conditions limiting environmental spread
- Pasteurization and chlorine in treated water will kill the organism
- Outbreaks have occurred following malfunctions of municipal water treatment facilities
- Infection may occur from direct contact with companion animals, especially puppies or kittens with diarrhea
- Individuals with occupational exposure to farm animals are at higher risk
- The bacteria is sensitive to gastric acid and patients on acid reducing drugs such as H2 blockers and proton pump inhibitors are at higher risk
- In the developing world, infection is mostly human to human and disease is hyperendemic in children under 2 years old; most children have several infections before the age of 2
- Later in life, most infections are mostly asymptomatic
- C. fetus subsp. fetus:
- Has major reservoir in cattle and sheep
- Reptiles commonly harbor the organism
- Disease usually occurs in elderly / debilitated patients
- Disease also reported in men who have sex with men, and rarely in other healthy patients
Sites
- Disease due to C. jejuni mostly is associated with involvement of the colon; however, the jejunum and ileum may be affected
- Rarely the bladder, gallbladder or pancreas may be infected through direct extension
- C. fetus may cause endocarditis, pericarditis, mycotic aneurysms, pneumonitis or CNS infection
- Rarer enteric Campylobacter species and related organisms usually affect the small and large intestines
Pathophysiology
- Organisms which survive passage through the stomach multiply in bile and colonize upper small intestine, with subsequent involvement of large bowel
- Virulence factors include motility (flagella), adherence, invasion and possibly toxin production
- Adherence and colonization are necessary for invasion
Clinical features
- Disease occurs year round but at a higher incidence in summer and early fall
- Affects people of all ages, but is most common in children under 1 year and adolescents / young adults from 15 to 29 years old
- Disease is more common in males
- It is an important cause of traveler's diarrhea
- C. jejuni:
- Typically causes acute self-limited illness of watery diarrhea, although dysentery may occur
- May have a flu-like prodrome for 1 day of fever, malaise, headache or myalgias
- In addition to diarrhea, patients may experience abdominal pain, tenesmus, nausea or fever
- Symptoms last from one day to a week or longer
- The presentation may mimic acute appendicitis
- Relapse of disease may occur
- Dysentery is more common in children (50%) than adults (15%)
- Ingestion of as few as 500 microorganisms may cause disease, with an incubation period of up to 8 days
- Severity of infection is related to the dose of the inoculate, the virulence of the strain, and the host's immune state
- Ingestion of higher numbers of organisms results in shorter incubation periods
- More severe and prolonged disease occurs in patients with defects in either cellular or humoral immunity
- Complications are uncommon but numerous; includes Guillain-Barré syndrome (GBS) in < 0.1%
- Molecular mimicry between components of C. jejuni and nervous system gangliosides may occur resulting in cross reactivity between antibodies to C. jejuni and nervous system components
- It is estimated that up to half of patients with GBS have had prior C. jejuni infection
- Other potential complications include erythema nodosum, Henoch-Schönlein purpura, myopericarditis, hepatitis, septic abortion, hemolytic uremic syndrome, cellulitis and renal disease including IgA nephropathy and interstitial nephritis
- Toxic megacolon has been reported
- In patients with HLA-B27, reactive arthritis may occur
- Campylobacter has been implicated in immunoproliferative small intestinal disease; may also exacerbate preexisting inflammatory bowel disease
- C. fetus subsp. fetus affects elderly / debilitated patients and causes systemic illness affecting GI tract, heart, blood vessels, lung, nervous system
Diagnosis
- Microscopic evaluation of fresh (<2hr) stool may enable a presumptive diagnosis to be made due to characteristic "darting motility"
- Microscopic stool examination shows leukocytes and red blood cells
- Diagnosis traditionally has been made by microbiologic culture; special techniques are necessary as Campylobacter species grow more slowly than other enteric organisms and will otherwise be overgrown
- C. jejuni grows best at 42°C but will grow at 37°C
- Most laboratories incubate cultures at 42° that may make it impossible to isolate other Campylobacter species besides C. jejuni
- The diagnosis may also be made by PCR of stool or paraffin blocks, but until recently this has been mostly in research settings (Am J Surg Pathol 2006;30:782)
- Recently a PCR based assay for testing stool has been introduced that in addition to Campylobacter group bacteria, also detects Shigella species, Vibrio group, Yersinia enterocolitica, Shiga toxin 1 and 2, Norovirus G1/GII, Rotavirus A and Aeromonas species
- Rarely C. jejuni may be diagnosed by blood culture
- C. fetus subsp fetus is usually detected in blood cultures
- If Campylobacter species infection is suspected, blood cultures should be incubated for two weeks due to its slow growth
- Immunoassays are also available
Prognostic factors
- Usually excellent; worse in immunosuppressed patients
Case reports
- 20 year old pregnant woman with septic abortion caused by Campylobacter jejuni bacteremia (J Chemother 2015 Apr 15 [Epub ahead of print])
- 20 year old man with Campylobacter jejuni as an uncommon cause of splenic abscess (Int J Infect Dis 2014;29:238)
- 65 year old man with mycotic abdominal aortic aneurysm caused by Campylobacter fetus (Ann Vasc Surg 2014;28:1933)
- 80 year old woman with fatal bacteremia caused by Campylobacter gracilis (Emerg Infect Dis 2015;21:1084)
- 91 year old woman with Campylobacter jejuni pancolitis mimicking idiopathic ulcerative colitis (Heart Lung 2005;34:288)
- Campylobacter-associated hemolytic uremic syndrome associated with pulmonary-renal syndrome (J Gen Intern Med 2015 May 23 [Epub ahead of print])
Treatment
- Most cases of C. jejuni do not require antibiotic therapy; if necessary, fluid and electrolyte replacement should be given
- Antibiotic treatment is recommended in the setting of high fever, bloody diarrhea, patients who have more than 8 bowel movements in a day or disease lasting longer than one week
- Erythromycin is generally the drug of choice, although tetracyclines, aminoglycosides, chloramphenicol, quinolones, nitrofuran, and clindamycin are also effective
- Increasing resistance to macrolides (azithromycin, clarithromycin, erythromycin, others) and fluoroquinolones (ciprofloxacin, levofloxacin) is reported
- In patients with hypogammaglobulinemia, fresh frozen plasma in addition to antibiotics may be useful
Microscopic (histologic) description
- Biopsy is rarely performed - the non-specific findings are those of acute self-limited colitis
- Neutrophils are increased within the lamina propria and cryptitis or crypt abscesses often occur
- Crypt architecture is maintained, although neutrophilic infiltrates may make this hard to appreciate
- Basal lymphoplasmacytosis is absent
Microscopic (histologic) images
Differential diagnosis
- Clinical and pathological differential includes:
- Aeromonas
- C. difficile colitis
- Crohn disease
- Shigellosis
- Some Salmonella
- Syphilis
- Ulcerative colitis
Additional references
Candida
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Candida colonies are normal commensals of the gastrointestinal tracts and rarely pathogenic in gut
- Candida infections are usually seen in immunocompromised and debilitated patients due to concurrent chronic diseases, malnutrition, use of immunosuppressive drugs or old age (Int J Contemporary Medical Research 2017;4:76)
- In immunocompetent patients, low pH, regular use of antacids as well as hyperglycemia can cause the yeast to break through the intestinal mucosal barrier and lead to invasive candidiasis and candidemia (Dtsch Arztebl Int 2009;106:837)
Essential features
- Candida colonies are normal commensals of gastrointestinal tracts
- Infections are usually seen in immunocompromised and debilitated patients
- Factors favoring fungal proliferation include: regular antacid use, which lowers pH; use of antibiotics, which diminish useful bacteria; and hyperglycemia, as seen with diabetes
- Candida usually causes mucosal lesions; rarely perforations
- Esophagus is the most commonly affected site in GI
- Gold standard test for diagnosis is culture
- Antifungals are treatment of choice
ICD coding
- B37.8 - Candidiasis of other sites
Epidemiology
- Patients usually middle aged to elderly, with an average age of 65 years
Sites
- Most commonly affected organ is the esophagus, followed by the stomach, the small intestine and the large intestine (Medicine (Baltimore) 1972;51:367, Nihon Shokakibyo Gakkai Zasshi 1986;83:2341)
Pathophysiology
- Under normal conditions, there is homeostasis between C. albicans and the host; however, if the balance is disrupted by low pH, regular use of antacids or hyperglycemia, the yeast can break through the intestinal mucosal barrier and cause invasive candidiasis and candidemia (Dtsch Arztebl Int 2009;106:837)
Etiology
- Candida albicans most commonly; rarely C. tropicalis, C. parapsilosis and C. kefyr (Emerg Med (Los Angel) 2015;5:264)
Diagrams / tables
Clinical features
- Invasive Candida infections are characterized by fever and shock along with low blood pressure, abdominal pain, rigors, an elevated heart rate, respiratory distress and multiorgan failure (Emerg Med (Los Angel) 2015;5:264)
Diagnosis
- Histopathology showing fungal hyphae and spores
- Gold standard is culture for fungi
Laboratory
- Eosinophilia
- Positive fungal culture
- Elevated levels of proinflammatory cytokine IL17 (Curr Opin Microbiol 2011;14:386)
Radiology description
- With intestinal perforation, chest Xray and Xray abdomen in erect view demonstrate gas under right hemidiaphragm
Prognostic factors
- Prognosis of Candida peritonitis is very poor in critically ill surgical patients, with a mortality rate between 52% and 75% (Curr Opin Crit Care 2007;13:195)
- Risk factors associated with increased mortality in Candida peritonitis include: extremes of age, upper GI tract infection as well as patient comorbidities, such as cardiac insufficiency, cirrhosis, diabetes mellitus, renal failure and multiorgan failure (Medicine (Baltimore) 2011:90:69)
Case reports
- 50 year old man diagnosed with acute peritonitis caused by a gastrointestinal perforation (J Clin Diagn Res 2012;6:1564)
- 50 year old man with invasive gastric candidiasis causing perforation and peritonitis (J Krishna Inst Med Sci Univ 2015;4:183)
- 54 and 62 year old women who developed systemic candidiasis (World J Emerg Surg 2013;8:38)
Treatment
- The antifungal agents for Candida peritonitis include polyenes, azoles, echinocandins and flucytosine
- Azoles are fungistatic against most Candida species, whereas the polyenes and echinocandins are fungicidal
- Fluconazole is recommended for patients without prior azole exposure and not at high risk, such as elderly, diabetic and cancer patients (Clin Infect Dis 2009;48:503, Perit Dial Int 2009;29 Suppl 2:S161)
Gross description
- Candida tends to cause mucosal lesions and produce ulcers of varying configuration, mucosal flecks, sloughed mucous membranes, polypoid masses and segmental lesions (J Clin Pathol 1992;45:806)
- With perforation, bowel may be covered by a thick, black, serosal exudate (Acta Paediatr 2003;92:258)
Microscopic (histologic) description
- Ulcer slough with eosinophils and neutrophils
- Classical budding hyphae and spores of Candida admixed with ulcer slough
Microscopic (histologic) images
Cytology description
- Fungal spores and hyphae
Negative stains
Differential diagnosis
- Other fungal infections
- Mucormycosis: broad aseptate hyphae
- Basidiobolomycosis: broad septate; thin walled fungal hyphae
Chagas disease (trypanosomiasis)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Colonic involvement in the chronic phase of infection by the flagellate protozoan Trypanosoma cruzi, causing chagasic megacolon
Essential features
- Infection most commonly involves the heart and GI tract (esophagus is most frequent site, followed by colon)
- Patients with chagasic megacolon suffer from chronic constipation and abdominal pain; in severe cases, there may be several weeks between bowel movements
- Histology shows ganglionitis with neuronal cell depopulation
Terminology
- Also known as American trypanosomiasis
ICD coding
Epidemiology
- Worldwide, an estimated 100 million people are at risk (Am J Trop Med Hyg 2014;90:814); 8 million are chronically infected (CDC - Chagas Disease: Detailed FAQs) with 56,000 new infections a year (Am J Trop Med Hyg 2014;90:814) and 12,000 deaths (PAHO WHO - Chagas disease)
- Chagas disease is endemic in all of the Western hemisphere south of the United States
- Vector borne transmission does not occur in the Caribbean
- Insect vectors are found in the southern United States but vector borne infection is rare there
- It is estimated that 300,000 residents of the United States are infected, the vast majority are immigrants from countries where the disease is endemic (CDC - Chagas Disease: Epidemiology and Risk Factors)
- There are very rare reports of disease in tourists
- Imported cases may occur worldwide
- Insect vector is more common in outdoor settings, including animal burrows, kennels, dog houses, chicken coops, under porches, between rocky structures and wood piles
- It is more commonly found in rural settings
- Deforestation for agriculture has played a role in its spread
- Indoors, it prefers cracks, nooks, crannies and holes typically found in dilapidated housing, including homes with mud walls and thatched roofs, accounting for its association with poverty
- Due to migration, disease is also found in urban settings
- Far less common routes of transmission are congenital, consuming contaminated food or drink, blood transfusion and organ transplants
- 5% of infants born to infected mothers become infected
- Public health efforts, including improving housing and public hygiene, as well as bed nets and screening donated blood, have resulted in declining numbers of T. cruzi infections
- Interestingly, gastrointestinal disease is much more likely to occur in the southern range of endemic Chagas disease
Sites
- Infection most commonly involves the heart and GI tract (esophagus is most frequent site, followed by colon)
- Far less often, other parts of the GI tract, the biliary tract and the central nervous system are affected
Etiology
- Infection is transmitted by blood sucking triatomine bugs, a type of reduviid bug that caries T. cruzi parasites
- They become infected by biting infected mammals including humans
- They multiply in the midgut as epimastigotes, a distinct type of flagellate; in the hindgut they transform into infective metacyclic trypomastigotes
- People are bitten on the face and the insect defecates
- Infection occurs when feces containing trypomastigotes reaches mucous membrane or skin abrasions, often wiped into the wound or conjunctiva by the host's hand
- An inflammatory lesion at the site of infection is known as a chagoma
- Parasites invade several types of host cell, transform into amastigotes and multiply in the cytoplasm
- Amastigotes differentiate into trypomastigotes and subsequently the cell ruptures
- Parasites spread hematogenously or invade tissues especially muscle
- They alternate between intracellular and swimming forms in blood
- Acute infection sometimes is associated with acute illness, usually with mild nonspecific symptoms, although rarely severe or even fatal disease may occur
- Reactivation of infection in the setting of immunosuppression may lead to severe disease
- Chronic Chagas disease occurs in 10 - 30% of infected individuals years or even decades after initial infection, most commonly involving the heart, less likely the gastrointestinal tract
- Cytotoxic T cells invade ganglia, causing reduced numbers of interstitial cells of Cajal, leading to parasympathetic denervation with reduced and eventual failure of relaxation of anal sphincters during defecation
- In some cases the inflammatory response may be contained by enteric glial cells (Hum Pathol 2009;40:244)
Clinical features
- Infection may occur from blood transfusion or organ transplantation and there are rare reports of transmission from contaminated food or laboratory accidents
- In a minority of cases, acute disease occurs that may be lethal due to cardiac or neurologic complications
- Infection is lifelong without treatment
- Most infected subjects remain in an asymptomatic indeterminant phase but 10 - 30% develop cardiac or gastrointestinal tract complications
- Parasite invades the bowel wall and damages the enteric nervous system leading to megacolon
- Patients with chagasic megacolon suffer from chronic constipation and abdominal pain; in severe cases, there may be several weeks between bowel movements
- Chronic disease manifests years or even decades after initial infection
- Fecalith / fecaloma may develop
- Acute obstruction may occur, possibly with volvulus and lead to perforation
- Toxic megacolon may occur
Diagnosis
- Acute disease may be diagnosed by review of a peripheral blood smear; however, other laboratory methods are necessary to diagnosis chronic Chagas disease
Laboratory
- Serologic diagnosis by enzyme linked immunosorbent, immunofluorescence or chemiluminescence methods
- Equivocal serologic tests may be confirmed with PCR based testing but this lacks sensitivity for detecting chronic disease
- False positives may occur; the WHO recommends confirmation with a different format assay
Radiology description
- Megacolon with contraction of anal sphincter
- Lengthening of the distal colon
Prognostic factors
- Immunosuppressed patients may suffer from severe disease
Case reports
- 62 year old man with sigmoid volvulus due to Chagas disease (Indian J Surg 2013;75:162)
Treatment
- Early / mild cases are treated with high fiber diets, laxatives, stool softeners and enemas
- With more severe disease, manual disimpaction may be necessary
- In children, endoscopic emptying may be employed
- Toxic megacolon and volvulus are treated surgically and surgical intervention may be considered for patients with severe chronic disease
- Antiparasitic treatment has a high failure rate and potentially toxic side effects
- Acute infection and children under 18 are usually treated
- Whether to treat indeterminant or chronic symptomatic infection is controversial
- Nifurtimox and benznidazole are available from the CDC
Clinical images
Microscopic (histologic) description
- Ganglionitis with neuronal cell depopulation
- Fibrosis and mast cell infiltrates are also present
Differential diagnosis
- Hirschsprung disease, idiopathic megacolon
Additional references
Board review style question #1
- Which statement is true?
- Chronic Chagas disease most commonly affects the central nervous system
- Patients with acute Chagas disease invariably progress to chronic disease
- The incidence of Chagas disease is increasing
- The majority of individuals infected with T. cruzi spontaneous clear the parasite
- Vector borne transmission of Chagas disease only occurs in the Western hemisphere
Board review style answer #1
E. Vector borne transmission only occurs in the Western hemisphere. Chagas disease is endemic in all of the continental Americas south of the United States but it does not occur in the Caribbean. Vector borne transmission in the southern United States is documented but is rare. Imported cases occur worldwide. Infection is life long but only 10 - 30% of individual who are infected will progress to chronic disease that most commonly affects the heart, followed by the esophagus, then the colon. The infection is transmitted by blood sucking triatomine bugs, a type of reduvid bug that caries T. cruzi parasites. Triatomine bugs are more likely to be found in conditions that occur in dilapidated housing; public health campaigns to improve housing as well as screening of donated blood has resulting in decreasing numbers of new infections over time.
Comment Here
Reference: Chagas disease (trypanosomiasis)
Comment Here
Reference: Chagas disease (trypanosomiasis)
Chemotherapy induced colitis (pending)
[Pending]
Chromosomal instability & MUTYH pathways
Chromosomal instability pathway
- Common pathway in colorectal carcinoma
- Mutations in oncogenes and tumor suppressor genes, including APC, beta catenin, Kras, BRAF, SMAD4, PTEN, p53 and bax
- Have altered total DNA content, cytogenetics, aneuploidy and numerous allelic gains and losses and translocations
- 70% + of colorectal carcinomas (Neoplasia 2008;10:680)
- Etiology
- Alterations in beta catenin pathway appear to be important in ulcerative colitis related and sporadic colon carcinomas (Mod Pathol 2001;14:29)
- RAS signaling pathway (Kras, BRAF, NF1 and RASSF1A) shows dysregulation in at least one gene in 80%
- Activating Kras mutations found in 30 - 40%, associated with poor response to anti-EGFR therapies in primary (Hum Path 2001;32:649Virchows Arch 2008;453:417, N Engl J Med 2008;359:1757) or metastatic disease (J Clin Oncol 2008;26:1626, J Clin Oncol 2008;26:374)
- Clinical features:
- Important in familial adenomatous polyposis and variants (APC gene) and juvenile polyposis (DPC4, PTEN genes)
- High rate of concordance for Kras status between primaries and metastases (Oncologist. 2008;13:1270)
- Patterns of Kras mutation vary based on germline mismatch repair defects and hMLH1 methylation status (Hum Mol Genet 2004;13:2303)
- Updated National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Colon Cancer now recommend that tumors from all patients with stage IV disease be tested for the Kras gene
- Only patients whose tumors have normal (wild type) Kras should receive cetuximab and panitumumab
MUTYH pathway
- See also MUTYH associated polyposis
- MUTYH (also called MYH) gene encodes MUTYH glycosylase, involved in oxidative DNA damage repair (Wikipedia)
- Mutations occur in both copies of MUTYH repair gene
- Genetic pathway is not well understood (Curr Genomics 2008;9:420)
- Biallelic patients (mutations in both genes) have multiple adenomas (Hum Mutat 2006;27:1064) and 93× excess risk of colorectal carcinoma compared to normal controls but account for < 1% of all cases (Am J Hum Genet 2005;77:112)
- Germline mutation of MUTYH gene is a mechanism of development of APC negative familial adenomatous polyposis; these patients may have a family history compatible with recessive inheritance indicating that this may be a different disease; genetic testing may be helpful in screening, diagnosis and management of atypical FAP cases
- Can be detected by high resolution melting analysis
Chronic granulomatous disease (pending)
[Pending]
Chronic intestinal pseudo-obstruction
Table of Contents
Definition / general | Etiology | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Syndrome of intestinal obstruction without mechanical obstruction
- Usually a small bowel disorder but can occur anywhere in GI tract
- Ogilvie syndrome (acute colonic pseudo-obstruction): abrupt onset of abdominal distension (Radiol Med (Torino) 2005;109:370)
Etiology
- Gut motility depends on sympathetic (thoracolumbar) and parasympathetic (vagal) innervation to ganglionated plexi; also enteric nervous system, smooth muscle cells and interstitial cells of Cajal
- Results from autonomic imbalance resulting in sympathetic overactivity affecting some part of colon (Singapore Med J 2009;50:237)
- Usually congenital in children; in adults due to systemic disease (amyloidosis, Chagas disease, dermato / polymyositis, diabetes, muscular dystrophy, myotonic dystrophy, myxedema, scleroderma), drugs (anti-Parkinson, clonidine, ganglionic blockers, phenothiazines, tricyclic antidepressants) or idiopathic (cathartic colon, ceroidosis, Hirschsprung disease, visceral myopathies, visceral neuropathies)
- May be due to loss of interstitial cells of Cajal in small and large bowel (Am J Surg Pathol 2003;27:228)
Prognostic factors
- Often poor long term outcome (Clin Gastroenterol Hepatol 2005;3:449)
- High mortality if perforation or ischemia
Case reports
- 44 year old woman with intestinal amyloidosis with intractable diarrhea (Korean J Gastroenterol 2012;60:172)
- 71 year old woman with pseudo-obstruction with pitch black colon (Ulster Med J 2008;77:54)
- 2 cases of pseudo-obstruction in sickle cell disease (South Med J 2003;96:93)
Treatment
- Diet, octreotide, surgery, transplant
Microscopic (histologic) description
- Visceral myopathy (Am J Surg Pathol 1987;11:846):
- Vacuolar degeneration with swelling and loss of muscle cells, fibrosis of outer longitudinal muscle layer
- Other cases show cytoplasmic vacuoles, marked nuclear enlargement and irregularity and interstitial fibrosis
- May have segmental hypoganglionosis at transitional zone (Am Surg 2011;77:736)
Microscopic (histologic) images
Differential diagnosis
- Ischemic colitis:
- Hemosiderin deposits, fibrous stricture
- Scleroderma:
- Patchy bowel involvement, dense fibrosis affecting inner or all muscle layers, no vacuolar change
- Tuberculosis:
- Stricture, necrotizing granulomas
Additional references
Clostridium botulinum
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Diagrams / tables | Microscopic (histologic) images | Electron microscopy images | Differential diagnosis | Additional referencesDefinition / general
- Botulism is a disease caused by botulinum toxin ("botox"), a potent neurotoxin produced by Clostridium botulinum or closely related microorganisms such as C. baratii or C. butyricum
Epidemiology
- Botulism is uncommon in the developed world
- According to the CDC in the United States, about 145 cases are reported each year, 65% in infants, 20% wound related and 15% foodborne
- Most cases are associated with eating raw or undercooked food containing botulinum toxin or C. botulinum spores
- Clusters of disease associated with eating the same food are common
- Canned (especially home canned alkaline foods), vacuum packed food and fermented or smoked foods are often implicated
- Infant botulism is related to ingestion of spores which colonize and release toxin in the intestine; may be related to honey ingestion (MMWR 2003;52:21)
- Wound botulism is caused by toxin released by C. botulinum that has contaminated a wound; may follow heroin injection (Euro Surveill 2013;18:20630, Anaerobe 2014;30:108)
- Rare cases are associated with inhalation of spores, sometimes in a health care setting
- Recently, iatrogenic botulism has been reported due to botulinum toxin for cosmetic or therapeutic purposes (Clin Neuropharmacol 2012;35:254, Clin Neuropharmacol 2010;33:158, Clin Neuropharmacol 2007;30:310, JAMA 2006;296:2476)
Etiology
- Clostridium are toxin producing, spore forming, anaerobic, gram positive bacilli found in soil and marine sediment
- Botulinum toxin is one of the most dangerous substances known; one millionth of a gram is a lethal dose
- It is a potent neurotoxin that blocks the presynaptic release of acetylcholine across the neuromuscular junction, leading to a toxic neuropathy
- Eight toxins have been identified, A-H; A, B, E, and F have been associated with human disease
- Foodborne botulism is associated with ingesting food containing botulinum toxin while infant botulism is associated with ingesting spores that colonize the intestines and produce toxin; disease in adults via a similar mechanism is rare, but may occur (adult intestinal colonization botulism)
- Wound, inhalation and iatrogenic botulism are discussed above under Epidemiology
Clinical features
- The incubation period for infant botulism is 2 to 4 weeks, with a peak incidence from 2 to 4 months
- Infants usually present with constipation, occasionally hypoventilation, followed by hypotonia, drooling, weak cry, generalized muscular weakness ("floppy baby") and hypoventilation; half have upper airway obstruction and cranial nerve palsies (Pediatr Neurol 2005;32:193)
- Has been associated with sudden infant death syndrome (Lancet 1985;1:237, Lancet 1978;1:1273)
- Patients with foodborne botulism generally present with acute, bilateral, symmetric cranial neuropathies
- Symptoms include lack of coordination of eye muscles, double vision, swallowing difficulties and dizziness
- Subsequently there is descending progressive weakness of the extremities and respiratory muscles
- Patients are generally afebrile, alert, and oriented
- Physical exam demonstrates flaccid muscle weakness of tongue, laryngeal muscles, respiratory muscles and extremities
- Without rapid intervention, patients may die of respiratory paralysis or cardiac arrest
Diagnosis
- A high index of suspicion is generally necessary for diagnosis
- Diagnosis is largely clinical through electromyographic studies
- PCR based assays for the bacteria in stool and gastric aspirates or culture are suggestive
- Detection of toxin in stool, blood, contaminated food or the environment is more definitive
- Testing should be performed in public health laboratories, not Level A Laboratories
- These laboratories or the CDC should be contacted for information on specimen transport
- Public health authorities should be notified as epidemiologic investigations are necessary; foodborne botulism is considered a public health emergency
- Of note, infection does not lead to antibody production, and no serologic testing is available
Prognostic factors
- With good supportive care, the death rate is currently < 5%; previously was > 50%
Case reports
- 27 and 28 year old men with botulism from cocaine inhalation (Clin Infect Dis 2006;43:e51)
- 60 year old woman with abdominal pain, dyspnea and diplopia (N Engl J Med 2015;372:364)
Treatment
- Equine derived heptavalent antitoxin (available from CDC) for food borne and wound botulism
- Debridement with antibiotics for wound botulism
- Human Botulism Immune Globulin Intravenous (BabyBig) is administered for infant botulism; generally antibiotics are not given for infant botulism
- Supportive care, especially respiratory support is critical
Differential diagnosis
- Acute motor axonal neuropathy
- Allergic reaction
- Guillain-Barré syndrome
- Miller Fisher syndrome
- Mosquito borne encephalitis
- Poliomyelitis
- Tick paralysis
Additional references
Colitis cystica profunda
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Radiology description | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Reactive mucosal change characterized by submucosal mucin filled cysts lined by benign epithelium
Essential features
- Mucosal herniation into submucosa secondary to various forms of injury
- Bland histology distinguishes from a true invasive process
Terminology
- Also called colitis cystica polyposa or hamartomatous inverted polyp when presents as polypoid lesion
Epidemiology
- Uncommon; male predilection
Sites
- Can occur anywhere in GI tract, including stomach (gastritis cystica profunda) and small intestine (enteritis cystica profunda)
Pathophysiology
- Secondary to mucosal damage due to ulcerative colitis, Crohn's disease, radiation, diverticulitis or other inflammation / ulceration of bowel; mucus follows granulation tracts to involve large areas of bowel
Clinical features
- Stools contain blood or mucus; patients may have diarrhea or abdominal pain
Radiology description
- Can mimic a malignant process (Abdom Imaging 2007;32:239)
Case reports
- 41 year old woman with recurrence after 20 years (Chir Ital 2005;57:789)
- 42 year old man with diverticulitis (Am J Gastroenterol 2011;106:172)
- After radiation for bladder carcinoma (Arch Pathol Lab Med 1995;119:1170)
Treatment
- Patient education to avoid straining, high fiber diet with bulk laxatives, surgery for rectal prolapse
Gross description
- May appear as a polypoid or submucosal mass
Microscopic (histologic) description
- Either localized or diffuse; similar histology to colorectal polyps in Cowden syndrome although inverted
- Misplaced benign colonic epithelium forming cystic spaces filled with mucin and surrounded by lamina propria; mucin may extravasate into surrounding tissue
Sample pathology report
- Rectum, mass, biopsy:
- Colitis cystica profunda
- Negative for dysplasia or malignancy.
Differential diagnosis
- Mucinous (colloid) carcinoma:
- Infiltrating, epithelium floats in mucin, glandular atypia, tumor desmoplasia, no hemorrhage or hemosiderin, no lamina propria
- Solitary rectal ulcer syndrome:
- May be indistinguishable in late cases of colitis cystica profunda
- Ulcerative colitis:
- No fibromuscular obliteration of lamina propria
Board review style question #1
Board review style answer #1
Collagenous colitis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Form of microscopic colitis clinically associated with chronic watery diarrhea and histologically characterized by colonic intraepithelial lymphocytosis, surface mucosal damage, lamina propria inflammation and a subepithelial collagen band
Essential features
- Subtype of microscopic colitis in which patients present with chronic watery diarrhea and normal or nearly normal endoscopic findings
- Pathophysiology and etiology are unclear but thought to be multifactorial with a possible luminal antigen triggering inflammation and mucosal damage in a genetically predisposed individual
- Histology is characterized by a subepithelial collagen band, intraepithelial lymphocytosis, mixed inflammation in the lamina propria and surface mucosal damage with preserved crypt architecture
ICD coding
- ICD-10: K52.831 - Collagenous colitis
Epidemiology
- Wide age range including children but typically affects adults 50 - 70 years old (Am J Gastroenterol 2017;112:78)
- Strong female predominance (F:M = 9:1) (Am J Gastroenterol 2015;110:265, Am J Gastroenterol 2017;112:78)
- Incidence is 4.14 per 100,000 person years (Am J Gastroenterol 2015;110:265)
- Possible increased prevalence in Caucasians (Am J Gastroenterol 2017;112:78)
Sites
- Colon (right > left / rectum) (Clin Gastroenterol Hepatol 2020;18:2003, Neth J Med 2005;63:137)
Pathophysiology
- Poorly understood
- Presumed mechanism includes a dysregulated immune reaction to an unknown luminal antigen (medication, dietary factor, infectious agent, other) in a genetically predisposed individual (Lancet Gastroenterol Hepatol 2019;4:305)
- Watery diarrhea is a result of inhibited sodium channel upregulation, downregulation of tight junctions and colonocyte aquaporins and bile acid malabsorption (Lancet Gastroenterol Hepatol 2019;4:305)
Etiology
- Strong association with certain medications
- NSAIDs, aspirin, proton pump inhibitors, H2 receptor antagonists, selective serotonin reuptake inhibitors, among others (Am J Gastroenterol 2017;112:78)
- Other specific etiologies are unknown
Clinical features
- Symptoms include chronic or intermittent watery diarrhea, abdominal pain, weight loss, arthralgias, myalgias, fecal urgency, incontinence and fatigue (Am J Gastroenterol 2017;112:78, Frontline Gastroenterol 2019;10:388)
- Colonoscopy is normal or has mild nonspecific findings such as erythema or edema
- Rarely, mucosal tears or perforation can occur during endoscopy, likely as a result of decreased colonic wall compliance (Dig Liver Dis 2017;49:1073)
- Association with other autoimmune diseases (Lancet Gastroenterol Hepatol 2019;4:305, Am J Gastroenterol 2017;112:78, Frontline Gastroenterol 2019;10:388):
- 4.3% of patients with celiac disease also have microscopic colitis
- Also associated with type 1 diabetes, psoriasis and autoimmune thyroiditis
- Smoking is a risk factor (Scand J Gastroenterol 2011;46:1334, Frontline Gastroenterol 2019;10:388)
Diagnosis
- Colonoscopy with colon biopsies are required for diagnosis
- At least 2 biopsies from the ascending, transverse, descending and sigmoid colon are recommended due to patchy involvement of the colon (Am J Gastroenterol 2009;104:774, Am J Gastroenterol 2017;112:78)
Prognostic factors
- Symptomatic remission ranges from 2 - 92% (Am J Gastroenterol 2017;112:78)
- Spontaneous remission may occur in up to 15% of patients (Am J Gastroenterol 1997;92:57)
- If remission is achieved, repeat colonoscopy with biopsy not usually necessary (Frontline Gastroenterol 2019;10:388)
- No increased risk of colorectal adenocarcinoma (J Clin Med 2019;8:1942)
Case reports
- 49 and 61 year old women with collagenous colitis occurring in the setting of inflammatory bowel disease (Gastroenterol Rep (Oxf) 2019;7:218)
- 68 year old woman with melanoma and anti-PD1 induced collagenous colitis (Melanoma Res 2016;26:308)
- 72 year old man with pseudomembranous collagenous colitis (ACG Case Rep J 2016;3:e187)
- 80 year old woman presenting with spontaneous colon perforation in the setting of collagenous colitis (BMC Gastroenterol 2016;16:124)
Treatment
- Discontinuation of any offending medications (Am J Gastroenterol 2017;112:78)
- Antidiarrheals such as loperamide, diphenoxylate or bismuth subsalicylate
- For more severe symptoms, corticosteroids such as budesonide can be used
- Surgical intervention is a last resort
Microscopic (histologic) description
- Thickened subepithelial collagen band, usually with capillary, red blood cell and inflammatory cell entrapment (Histopathology 2015;66:613)
- Collagen band typically > 10 microns
- Occasional cases can have an irregular collagen band that is < 10 microns; some have labeled such cases as "incomplete collagenous colitis"
- Trichrome stain can be useful in equivocal cases
- Surface mucosal damage with loss of mucin, flattening and detachment
- Increased lamina propria inflammation composed of lymphocytes, plasma cells and eosinophils
- Neutrophilic inflammation can be seen but is typically not prominent
- Increased intraepithelial lymphocytes; may be mild (< 20 per 100 epithelial cells)
- Crypt architecture is preserved or minimally distorted
- Paneth cell metaplasia can be seen
- Up to 8% of cases can have crypt atrophy or irregularity (Am J Surg Pathol 2002;26:1414)
- Subepithelial multinucleated giant cells can be seen but have no clinical significance (Pathology 2008;40:671)
- Pseudomembranous collagenous colitis has been described; unclear whether truly a variant of collagenous colitis or due to superimposed infection or medication injury (Dig Dis Sci 2004;49:1763, Pathol Res Pract 2013;209:735)
Microscopic (histologic) images
Positive stains
- Masson trichrome stain highlights the subepithelial collagen band
- CD3 highlights intraepithelial lymphocytes
- Tenascin immunohistochemical stain also highlights the collagen band (Histopathology 2015;66:613)
Negative stains
- Congo red stain
Sample pathology report
- Colon, random, biopsies:
- Colonic mucosa with a thickened subepithelial collagen band, increased intraepithelial lymphocytes and surface epithelial injury, consistent with collagenous colitis
Differential diagnosis
- Lymphocytic colitis:
- Lack of subepithelial collagen band and greater number of intraepithelial lymphocytes
- Inflammatory bowel disease:
- Lack of subepithelial collagen band
- Architectural distortion and prominent neutrophilic inflammation
- Endoscopic evidence of inflammation and clinical history of bloody diarrhea
- Chronic ischemic colitis:
- Diffuse lamina propria hyalinization as opposed to a discrete subepithelial collagen band
- Withered, injured crypts
- Amyloidosis:
- Usually surrounds blood vessels, though rarely may create a subepithelial layer mimicking collagenous colitis
- Congo red stain is positive
- Lack of surface epithelial injury and inflammation
Board review style question #1
A 55 year old female with a 1 year history of chronic watery diarrhea presents for evaluation. Stool ova / parasites and culture are negative for infectious organisms. Colonoscopy is performed and reveals a grossly normal appearing colon. The biopsy histology is shown. Which of the following diagnoses is correct?
- Collagenous colitis
- Crohn's colitis
- Irritable bowel syndrome
- Lymphocytic colitis
Board review style answer #1
Board review style question #2
A 57 year old female was recently diagnosed with collagenous colitis. Which of the following is a characteristic histologic feature of collagenous colitis?
- Architectural distortion
- Cryptitis and crypt abscess
- Granulomas
- Mixed lamina propria inflammation
Board review style answer #2
Colon carcinoma overview
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Etiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Treatment | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Primary epithelial malignancy arising in the colorectum
Essential features
- More than 1 million new cases worldwide per year
- Second or third most common cancer and cause of cancer deaths in men and in women
- 98% of colonic cancers are adenocarcinomas
Epidemiology
- Most common gastrointestinal tumor in U.S.; less common in Africa, Asia and parts of South America
- Affects 5% of the U.S. population during their lifetime
- More common in men than women
- Most common cause of cancer related death among nonsmokers
- Peak age 60 - 79 years
- < 20% of cases occur before age 50
- Rare before age 40 except in patients with a predisposition syndrome
Sites
- May arise anywhere in the colorectum but sigmoid colon and rectum are most common sites
Etiology
- Risk factors: older age, obesity, physical inactivity, alcohol consumption, inflammatory bowel disease, schistosomiasis, family history of colorectal neoplasia
- Polyposis syndromes: familial adenomatous polyposis and variants (APC gene), Lynch syndrome and variants (MLH1, MSH2, MSH6 and PMS2 genes), juvenile polyposis (SMAD4, PTEN genes), Peutz-Jeghers syndrome (STK11 gene)
- Dietary risk factors: low vegetable fiber, high refined carbohydrates, increased beef consumption, decreased vitamins A / C / E
- Low fiber prolongs transit time (toxic oxidative byproducts are in longer contact with colonic mucosa) and alters bacterial flora
- Beef consumption enhances synthesis of bile acids by liver, which may be converted into carcinogens by bile acids
Clinical features
- Screening: colonoscopy, guaiac fecal occult blood test
- Symptoms:
- Right sided carcinomas cause anemia (due to blood loss) and vague abdominal pain
- Left sided carcinomas cause change in bowel habits (diarrhea or constipation) and rectal bleeding
- Some patients may be asymptomatic, and tumors are detected by screening, especially if lesion is early
- Metastases:
- 60% of patients have lymph node or distant metastases at diagnosis
- Most common metastatic sites are regional lymph nodes, liver, peritoneum, lung, ovaries
- Metastases may simulate primary tumors of affected organs
- Prognosis:
- 5 year survival is 40% - 60%
- Most recurrences are within 2 years
Laboratory
- Serum CEA: elevated levels associated with various carcinomas or liver disease
- Useful for monitoring recurrences but not sensitive for early tumors
Radiology description
- Imaging allows for clinical staging (depth of invasion, possibility of metastasis)
Prognostic factors
- Poor prognostic factors: high stage, positive margins (particularly radial margin in rectal carcinoma), poor differentiation, signet ring cells, flat or ulcerative gross configuration, tumor budding, tumor perforation, free tumor cells in peritoneal space, lymphovascular invasion, perineurial invasion (Am J Clin Pathol 2003;119:108)
- Lack of CDX2 expression and reduced claudin 1 expression portend worse survival in stage II tumors (N Engl J Med 2016;374:211, Mod Pathol 2005;18:511)
- HER2 / neu and VEGF are not important prognostic markers (BMC Cancer 2011;11:277)
Treatment
- Resection with endoscopic follow up
- Local excision (endoscopic mucosal resection) for early rectal carcinoma
- Surgical excision of isolated distant metastases
- Radiation therapy or chemotherapy (including neoadjuvant if rectal)
Additional references
Board review style question #1
What is the most common gastrointestinal malignancy in the U.S.?
- Colorectal adenocarcinoma
- Esophageal squamous cell carcinoma
- Gastric adenocarcinoma
- Small bowel adenocarcinoma
Board review style answer #1
Board review style question #2
The following syndromes confer an increased risk of colorectal carcinoma. Which syndrome is properly linked to its causative germline mutation?
- Familial adenomatous polyposis: MLH1 mutation
- Juvenile polyposis: SMAD4 mutation
- Lynch syndrome: STK11 mutation
- Peutz-Jeghers syndrome: APC mutation
Board review style answer #2
Common variable immunodeficiency (CVID)
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Common variable immunodeficiency disorders (CVID) are uncommon conditions of primary B cell dysfunction or T cell impairment leading to hypogammaglobulinemia and IgA or IgM deficiency
- Immunoglobulin deficiency elicits an immunodeficiency state with increased susceptibility to recurrent infections, inflammatory diseases, autoimmune diseases and malignancy (J Allergy Clin Immunol Pract 2016;4:38)
Essential features
- CVID is due to B cell impairment causing hypogammaglobulinemia with either IgA or IgM deficiency
- Infectious, inflammatory, autoimmune and malignant gastrointestinal manifestations can occur in approximately 15% of patients with CVID
- CVID histology in the colon may include one or more of the following: inflammatory bowel disease-like pattern, microscopic colitis pattern, granulomatous inflammation, increased apoptosis, lymphoid aggregates and infections such as cytomegalovirus and herpes simplex virus
- Presence of plasma cells does not rule out CVID because approximately 33% of CVID cases will have plasma cells
Epidemiology
- Second most common immunodeficiency disorder after selective IgA deficiency (Am J Surg Pathol 2007;31:1800)
- True incidence or prevalence is unknown but estimated prevalence is 1:100,000 to 1:10,000 in the general population (J Allergy Clin Immunol Pract 2016;4:38, Br J Haematol 2009;145:709)
- Age of onset varies from childhood to adulthood with a bimodal peak age of onset in the first and third decades of life (J Allergy Clin Immunol Pract 2016;4:38, J Allergy Clin Immunol 2014;134:116, Clin Immunol 1999;92:34)
- Males and females are equally affected (J Allergy Clin Immunol 2014;134:116)
Sites
- All organ systems including the colon can be affected (J Allergy Clin Immunol Pract 2016;4:38)
Pathophysiology
- All patients have B cell loss of function due to an intrinsic defect or lack of extrinsic activation by another immune cell (J Allergy Clin Immunol Pract 2016;4:38)
- T cell loss of function may also contribute to B cell impairment (J Allergy Clin Immunol Pract 2016;4:38)
- B cell impairment leads to reduced or absent IgG, IgA or IgM immunoglobulin levels (J Allergy Clin Immunol Pract 2016;4:38)
- Reduced immunoglobulin levels leads to increased susceptibility to recurrent infections especially of the sinopulmonary and gastrointestinal tract
- Gastrointestinal tract involvement is a manifestation of CVID due to its large surface area and presence of numerous immunoglobulin producing plasma cells; if the plasma cells become dysfunctional, immune dysregulation and infection can result
- Altered immunity can also lead to infection, autoimmune disease, inflammatory disease and malignancy
Etiology
- > 98% of cases do not have an identifiable etiology (J Allergy Clin Immunol Pract 2016;4:38)
- Predominantly sporadic genetic disease with 5 - 25% of cases being familial (J Allergy Clin Immunol Pract 2016;4:38)
- Familial cases are most commonly inherited in an autosomal dominant pattern (J Allergy Clin Immunol Pract 2016;4:38)
- Examples of genes involved in CVID include CD19, CD20, CD21, CD27, CD81, CTLA4, ICOS, IL21 and IL21R (J Allergy Clin Immunol Pract 2016;4:38)
Clinical features
- Infectious, inflammatory, autoimmune and malignant gastrointestinal tract manifestations can occur in approximately 15% of individuals with CVID (Blood 2012;119:1650)
- Symptoms of gastrointestinal tract manifestations in CVID patients include: weight loss, anemia, chronic diarrhea, chronic abdominal discomfort, bloody diarrhea (Dig Dis Sci 2007;52:2977, Am J Gastroenterol 2019;114:648)
- Colonoscopy and biopsy reports from 63 Finnish patients with CVID identified (Am J Gastroenterol 2019;114:648):
- Nonspecific colitis in 14%
- Ulcerative colitis in 8%
- Microscopic colitis in 10%
- Crohn's disease 2%
- Colonic polyps (types not specified) in 30%
- GI malignancies (types not specified) in 3%
- Cytomegalovirus colitis in 2%
- Herpes simplex virus colitis in 2%
Diagnosis
- CVID is diagnosed in the proper clinical situation with the following criteria (J Allergy Clin Immunol Pract 2016;4:38):
- Presentation with immunodeficiency at greater than 2 years of age
- Decreased serum IgG (≥ 2 standard deviations below the mean for age) and decreased serum IgA or IgM
- Absent isohemagglutinins or inability to develop antibodies to vaccines
- Acquired hypogammaglobulinemia is ruled out
- Colonoscopy and biopsy are helpful in the assessment of lower gastrointestinal tract involvement by inflammatory, autoimmune, infectious and malignant sequelae of CVID
Laboratory
- Low serum IgG (≥ 2 standard deviations below the mean for age)
- Low serum IgA or IgM
- Circulating B cells may be normal (54% of cases), increased (19% of cases), decreased (12% of cases) or undetectable (12% of cases) (Blood 2008;112:277)
- Circulating T cells and natural killer cells are usually within normal limits (Clin Immunol 1999;92:34)
- Important to remember: serology may be falsely negative or falsely positive due to the patient's inability to mount a humoral response and due to intravenous immunoglobulin (IVIG) administration, respectively
Case reports
- 31 year old man with nodular lymphoid hyperplasia of stomach, small intestine and large intestine mimicking familial adenomatous polyposis (Indian J Pathol Microbiol 2009;52:530)
- 39 year old man with CVID associated with inflammatory bowel disease and diabetes mellitus type 1 (Clin Med Case Rep 2009;2:67)
- 69 year old woman with CVID and herpes simplex type 1 colitis (Eur J Gastroenterol Hepatol 2006;18:541)
- A case series of 4 patients, ages 19 - 69, with CVID associated with collagenous colitis, gastritis and sprue (Indian J Gastroenterol 2016;35:133)
Treatment
- IVIG may be insufficient to treat inflammatory and autoimmune conditions associated with CVID (J Allergy Clin Immunol Pract 2016;4:38)
- Infections, autoimmune diseases, inflammatory diseases and malignancies need to be treated when they are identified
Microscopic (histologic) description
- Numerous colonic histologic patterns can be seen in CVID including the following (Am J Surg Pathol 2007;31:1800, Am J Gastroenterol 2019;114:648):
- Ulcerative colitis-like pattern and Crohn's disease-like pattern
- Some cases may have active colitis but without features of chronicity such as crypt atrophy / loss and crypt branching
- Lymphocytic colitis pattern and collagenous colitis pattern
- Unclear whether these patterns of injury are mimicking or are true cases of lymphocytic colitis and collagenous colitis
- Granulomatous inflammation
- Etiology may be infectious or inflammatory, fungal and mycobacterial stains are important to rule out infection
- Viral infection (CMV, HSV, etc.)
- Important to thoroughly evaluate for viral inclusions as serology may be negative due to hypoglobulinemia associated with CVID
- Increased apoptosis can occur in colonic epithelium mimicking graft versus host disease, viral infection (CMV) and drug effect (mycophenolate)
- Lymphoid aggregates are a common finding in the colon of CVID patients
- Ulcerative colitis-like pattern and Crohn's disease-like pattern
- Presence of plasma cells does not rule out CVID as reduced plasma cells were identified in 63% of colonic CVID biopsies / resection specimens (Am J Surg Pathol 2007;31:1800)
Microscopic (histologic) images
Contributed by Benjamin J. Van Treeck, M.D and Christopher Hartley, M.D.
Sample pathology report
- Colon, biopsy:
- Colonic mucosa with mild active chronic colitis, increased apoptosis and absence of plasma cells (evaluated with CD138); negative for dysplasia (see comment)
- Comment: These features are consistent with the patient’s history of common variable immunodeficiency. Clinicopathologic correlation is recommended.
Differential diagnosis
- Crohn's colitis:
- Reduced to absent plasma cells is not a feature of Crohn's colitis
- Common variable immunodeficiency disorders (CVID) injury pattern can mimic Crohn's disease to the extent that patients may be diagnosed with Crohn's disease prior to being diagnosed with CVID (Am J Surg Pathol 2007;31:1800)
- Clinical history of CVID is helpful in distinguishing Crohn’s colitis from CVID
- Ulcerative colitis:
- Reduced to absent plasma cells is not a feature of ulcerative colitis
- Clinical history of CVID is helpful in distinguishing ulcerative colitis from CVID
- Lymphocytic colitis:
- Reduced to absent plasma cells is not a feature of lymphocytic colitis
- Clinical history of CVID is helpful in distinguishing lymphocytic colitis from CVID
- Collagenous colitis:
- Reduced to absent plasma cells is not a feature of collagenous colitis
- Clinical history of CVID is helpful in distinguishing collagenous colitis from CVID
- Cytomegalovirus (CMV) colitis:
- Evaluate for CMV viral inclusions
- CMV immunohistochemical stain can be helpful
- CVID can coexist with CMV infection
- Herpes simplex virus (HSV) colitis:
- Evaluate for HSV viral inclusions
- HSV immunohistochemical stain can be helpful
- CVID can coexist with HSV infection
- Acute self limited colitis:
- Reduced to absent plasma cells is not a feature of acute self limited colitis
- Clinical history of CVID and microbiology tests are helpful in distinguishing acute self limited colitis from CVID
- Acute self limited colitis may coexist with CVID
- Lymphoma such as mantle cell lymphoma, follicular lymphoma and others:
- Clonal population of lymphocytes is not a feature of CVID
- CVID may predispose to lymphoma
- Graft versus host disease (GVHD):
- Clinical history of transplant is necessary to diagnosis GVHD
- Reduced to absent plasma cells is not a feature of GVHD
- Sarcoidosis:
- Clinical history may be helpful in differentiating sarcoidosis from CVID
- Sarcoidosis can coexist with CVID (Am J Surg Pathol 2007;31:1800)
Board review style question #1
A patient with chronic diarrhea, recurrent sinopulmonary infections and reduced serum IgG and IgA levels undergoes colonoscopy. The colonic biopsies demonstrate rare lamina propria plasma cells. Which one of the following statements is correct?
- Presence of rare plasma cells does not rule out the possibility of common variable immunodeficiency (CVID) in this patient and should be mentioned in the pathology report
- Presence of rare plasma cells is secondary to chronic diarrhea and is not related to common variable immunodeficiency (CVID) in this patient
- Presence of rare plasma cells rules out the possibility of common variable immunodeficiency (CVID) in this patient
- Presence of rare plasma cells should not be reported and a diagnosis of normal colonic mucosa should be included in the pathology report
Board review style answer #1
A. Presence of rare plasma cells does not rule out the possibility of common variable immunodeficiency (CVID) in this patient and should be mentioned in the pathology report
Comment Here
Reference: Common variable immunodeficiency (CVID)
Comment Here
Reference: Common variable immunodeficiency (CVID)
Board review style question #2
A patient with chronic diarrhea, recurrent sinopulmonary infections and reduced IgG and IgA levels is diagnosed with common variable immunodeficiency (CVID). Which of the following histologic patterns can be seen in colonic biopsies from patients with CVID?
- Inflammatory bowel disease-like pattern, increased apoptosis pattern, colonic mucosal prolapse-like pattern
- Inflammatory bowel disease-like pattern, increased apoptosis pattern, lymphocytic colitis pattern
- Inflammatory bowel disease-like pattern, increased apoptosis pattern, radiation induced colitis-like pattern
- Inflammatory bowel disease-like pattern, ischemic colitis-like pattern, lymphocytic colitis pattern
Board review style answer #2
B. Inflammatory bowel disease-like pattern, increased apoptosis pattern, lymphocytic colitis pattern
Comment Here
Reference: Common variable immunodeficiency (CVID)
Comment Here
Reference: Common variable immunodeficiency (CVID)
Congenital absence of muscularis propria (pending)
Table of Contents
Definition / generalDefinition / general
[Pending]
COVID-19 associated colitis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colonic mucosal damage associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection
Essential features
- Gastrointestinal symptoms seen in up to 50% of coronavirus disease 2019 (COVID-19) patients (Am J Gastroenterol 2020;115:766, Lancet Gastroenterol Hepatol 2020;5:667, BMJ Open Gastroenterol 2021;8:e000578, Gut 2020;69:997)
- SARS-CoV-2 RNA detected in more than 40% of fecal samples in COVID-19 patients (BMJ Open Gastroenterol 2021;8:e000578)
- Potential fecal - oral transmission (Nat Rev Gastroenterol Hepatol 2021;18:269)
- Common clinical presentation: nausea, vomiting, diarrhea, abdominal discomfort and hematochezia (Gastroenterology 2020;159:81, Am J Gastroenterol 2020;115:766, Clin Gastroenterol Hepatol 2020;18:2378, World J Gastroenterol 2020;26:2323)
- Common endoscopic findings: ischemic-like colopathy, ulcer, lower GI bleeding and erythema (BMJ Open Gastroenterol 2021;8:e000578)
- Common histopathologic findings: ischemic colitis with necrosis, submucosal venous microthrombi, withered crypts, erosion / ulceration, focal edema, nonspecific acute inflammation, lamina propria lymphohistiocytic or eosinophilic infiltrate, increased crypt apoptotic bodies, mild architectural distortion and pneumatosis intestinalis; may resemble pseudomembranous colitis (Case Rep Gastroenterol 2021;15:408, BMJ Open Gastroenterol 2021;8:e000578, Arch Pathol Lab Med 2021 May 7 [Epub ahead of print])
ICD coding
Epidemiology
- WHO declared the COVID-19 outbreak, caused by SARS-CoV-2, a global pandemic
- Primarily affecting the respiratory system, COVID-19 can also lead to gastrointestinal symptoms
- Common comorbidities: hypertension, diabetes mellitus, chronic cardiac disease, chronic respiratory disease, chronic renal disease (Clin Gastroenterol Hepatol 2020;18:2378, BMJ Open Gastroenterol 2021;8:e000578)
Pathophysiology
- SARS-CoV-2 binds to the angiotensin converting enzyme 2 (ACE2) receptor through its surface spike protein for host entry
- ACE2 receptor is highly expressed in the intestinal epithelial cells
- Potential mechanisms for COVID-19 associated colitis:
- Altered microbiota homeostasis
- Enterocyte damage
- Release of virulent proteins or toxins
- Hypercoagulable state (Intest Res 2020 Nov 6 [Epub ahead of print], Acta Med Indones 2021;53:96)
Etiology
- SARS-CoV-2, an enveloped positive stranded RNA virus related to the severe acute respiratory syndrome (SARS) virus and the Middle East respiratory syndrome (MERS) virus
Clinical features
- Common clinical presentation:
- Anorexia (26.8%)
- Diarrhea (12.5%)
- Nausea and vomiting (10.2%)
- Abdominal discomfort (9.2%) (Gastroenterology 2020;159:81)
- Gastrointestinal symptoms typically develop within 1 week of onset of COVID-19 infection
- Diffuse abdominal pain / tenderness, metabolic acidosis and hypotension indicate worsening clinical course and potential need for surgical intervention
Diagnosis
- SARS-CoV-2 RNA detection in upper respiratory tract specimens
- SARS-CoV-2 RNA detection in fecal specimens
- Electron microscopy showing SARS-CoV-2 viral particles in colorectal tissue
- Histopathologic evaluation of the gastrointestinal biopsy and resection specimens
- SARS-CoV-2 RNA ISH and immunohistochemical stain on formalin fixed, paraffin embedded (FFPE) sections (Arch Pathol Lab Med 2021 May 7 [Epub ahead of print], Clin Infect Dis 2020 Jul 8 [Epub ahead of print])
Laboratory
- Elevation of prothrombin time and international normalized ratio (INR)
- D dimer > 1,850 ng/mL and presence of at least 1 gastrointestinal symptom were independently associated with major endoscopic abnormalities (BMJ Open Gastroenterol 2021;8:e000578)
- Elevation of liver function test (aspartate aminotransferase [AST], alanine aminotransferase [ALT]) (Am J Gastroenterol 2020;115:766)
Radiology description
- Enhanced CT showed mild dilatation of the colon with diffuse wall thickening and surrounding inflammation (Cureus 2021;13:e13236, Am J Trop Med Hyg 2021;104:1655)
- Computed tomography angiography (CTA) showed pneumatosis intestinalis and mesenteric stranding, highlighting the thromboembolic complications in COVID-19 (Rev Esp Enferm Dig 2020;112:583)
Case reports
- 7 week old boy with COVID-19 and necrotizing enterocolitis-like pneumatosis intestinalis (Pediatr Infect Dis J 2021;40:e85)
- 17 year old boy with trisomy 21 diagnosed with SARS-CoV-2 related ischemic colitis (Pediatr Dev Pathol 2021 May 28 [Epub ahead of print])
- 38, 55 and 74 year old men with cases of COVID-19 disease with colonic manifestations (Am J Gastroenterol 2020;115:948)
- 44 year old man, hospitalized for COVID-19 associated pneumonia, developed pneumatosis intestinalis (BMJ Open Gastroenterol 2020;7:e000434)
- 45 year old man and 68 year old woman with COVID-19 associated ischemic enterocolitis (Am J Trop Med Hyg 2021;104:1655)
- 56, 68 and 76 year old men with COVID-19 pneumonia developed severe colon ischemia (Rev Esp Enferm Dig 2020;112:784)
- 62 year old woman with low rectal cancer treated with low anterior rectal resection, complicated with SARS-CoV-2 infection, late colonic ischemia and colovaginal fistula (Chirurgia (Bucur) 2020;115:677)
- 66 year old man with severe COVID-19 pneumonia and ischemic colitis (Am J Trop Med Hyg 2021;104:63)
- 67 and 68 year old men with severe COVID-19 pneumonia complicated by hematochezia (Case Rep Gastroenterol 2021;15:408)
- 73 year old man presented with COVID-19 disease and ischemic colitis (Am J Emerg Med 2020;38:2758.e1)
- 82 year old woman with COVID-19 infection presenting as acute intestinal ischemia (Korean J Gastroenterol 2020;76:164)
Treatment
- Supportive care, bowel rest and close observation
- Surgical intervention is indicated if there is evidence of perforation, infarction and necrosis (Am J Emerg Med 2020;38:2758.e1)
Microscopic (histologic) description
- Ischemic colitis pattern of injury most common:
- Withered crypts
- Hyalinized stroma
- Erosion / ulceration
- Coagulative necrosis
- Microhemorrhages
- Microthrombi and vascular congestion
- Other nonspecific histopathologic findings:
- Focal edema
- Nonspecific acute inflammation
- Lamina propria lymphohistiocytic or eosinophilic infiltrate
- Increased crypt apoptotic bodies (Arch Pathol Lab Med 2021 May 7 [Epub ahead of print])
- Mild architectural distortion
- Pneumatosis intestinalis
- Pseudomembranous colitis-like presentation
Microscopic (histologic) images
Positive stains
- SARS-CoV-2 immunohistochemical stain (Clin Infect Dis 2020 Jul 8 [Epub ahead of print])
- SARS-CoV-2 in situ hybridization (ISH) (Arch Pathol Lab Med 2021 May 7 [Epub ahead of print])
Electron microscopy description
- Multiple SARS-CoV-2 viral particles in the colonic submucosal venous endothelial cells (Intensive Care Med 2020;46:2081)
Sample pathology report
- Colon, biopsy:
- Ischemic colitis consistent with clinical history of SARS-CoV-2 infection
Differential diagnosis
- Inflammatory bowel disease (IBD):
- Personal or family history of IBD or other autoimmune disorders
- Onset tends to be subacute to chronic
- Marked architectural distortion and prominent acute inflammation
- Ischemic colitis, NOS:
- Noninfectious colitis
- Watershed areas most commonly affected
- Clinical correlation essential
- Medication associated mucosal damage:
- History of medication (such as NASID) use
- May present as chronic mucosal injury pattern
- Acute infectious colitis (other than COVID-19):
- Caused by bacteria or virus
- Acute cryptitis, crypt abscess
- May present with ischemic injury pattern
- Bacterial culture, PCR and sequencing diagnostic
Board review style question #1
A 71 year old man with a medical history of diabetes mellites type 2, coronary artery disease and morbid obesity was hospitalized for COVID-19 pneumonia. On day 3 of hospital stay, he developed left sided abdominal pain and hematochezia. Endoscopy showed mucosal erosions and ulceration at the splenic flexure. A representative photomicrograph of the biopsy is shown. Which is the most likely diagnosis?
- COVID-19 associated colitis
- Diverticulitis
- Inflammatory bowel disease
- Mesenteric ischemia
- Pseudomembranous colitis
Board review style answer #1
A. COVID-19 associated colitis. The biopsy reveals colonic mucosa with focal epithelial mucin depletion, withered crypts, mildly hyalinized stroma, mucosal microthrombi and patchy acute inflammatory infiltrate. In the current clinical setting (patient with COVID-19 pneumonia followed by abdominal pain and hematochezia), this is most compatible with COVID-19 associated ischemic colonic mucosal injury. Clinical correlation is essential.
Comment Here
Reference: COVID-19 associated colitis
Comment Here
Reference: COVID-19 associated colitis
Board review style question #2
What is the most common histologic feature of COVID-19 associated colitis?
- Basal lymphoplasmacytosis and crypt architectural distortion
- Eosinophilic cryptitis and abscess
- Intraepithelial lymphocytosis
- Pseudomembrane material composed of fibrin, mucin, neutrophils and inflammatory debris
- Withered crypts, lamina propria hyalinization and microthrombi
Board review style answer #2
E. Withered crypts, lamina propria hyalinization and microthrombi. The most common histologic feature of COVID-19 associated colitis is ischemic colitis pattern, including withered crypts, lamina propria hyalinization and microthrombi.
Comment Here
Reference: COVID-19 associated colitis
Comment Here
Reference: COVID-19 associated colitis
Cowden syndrome
Table of Contents
Definition / general | Essential features | Sites | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cowden syndrome is one component of the PTEN hamartomatous tumor syndrome, which also includes Bannayan-Riley-Ruvalcaba syndrome, PTEN related Proteus syndrome and Proteus-like syndrome
Essential features
- Syndrome with autosomal dominant inheritance caused by mutations in PTEN and sometimes other genes
- Causes benign hamartomatous overgrowths of skin, GI tract and thyroid
- Also increases risk of malignancy of breast, thyroid, endometrium and colorectum
Sites
- Affected organs include skin, breast, endometrium and thyroid
- Gastrointestinal manifestations:
- Esophagus: glycogenic acanthosis
- Stomach, duodenum and small bowel: multiple hamartomatous polyp, hyperplastic polyp, ganglioneuroma, adenoma and inflammatory polyp
- Possible association with gastric cancer (Am J Gastroenterol 2005;100:476)
- Colon: multiple polyps, usually hamartomas (resemble juvenile polyps); also ganglioneuroma, adenoma, inflammatory polyp, others (J Natl Cancer Inst 2013;105:1607)
- Lifetime colorectal cancer risk ~9 - 16% (Clin Cancer Res 2012;18:400, Gastroenterology 2010;139:1927)
Clinical features
- Gastrointestinal hamartoma found in 35 - 80% of patients; increased risk for colorectal cancer
- Mucocutaneous lesions include trichilemmoma, acral keratosis and oral papilloma
- Macrocephaly; dysplastic gangliocytoma of cerebellum (Lhermitte-Duclos disease)
- Breast fibroadenoma, fibrocysttic disease and carcinoma
- Thyroid goiter, adenoma and carcinoma
- Increased risk for endometrial carcinoma
Diagnosis
Case reports
- 29 year old woman with Cowden syndrome diagnosed from multiple gastric polyposis (World J Gastroenterol 2012;18:861)
- 73 year old man with Cowden syndrome presenting with gastric carcinoma and GI polyposis (Nat Clin Pract Gastroenterol Hepatol 2009;6:184)
Treatment
Microscopic (histologic) description
- Colon polyps: nondysplastic epithelium, dilated glands, expanded stroma; histologically similar to juvenile polyps; may also show intramucosal lipomas (Mod Pathol 2018;31:643)
Microscopic (histologic) images
Molecular / cytogenetics description
- Germline PTEN (phosphate and tensin homologue, 10q23) mutation in 70 - 80% of patients (J Clin Oncol 2004;22:2954)
- Other mutations: SDH (succinate dehydrogenase) B and C mutations, KLLN, PIK3CA, others (OMIM: Cowden Syndrome [Accessed 21 April 2021])
Additional references
Board review style question #1
Cowden syndrome is caused by a germline mutation in what gene?
- BMPR1A
- KRAS
- PTEN
- STK11
Board review style answer #1
Board review style question #2
Board review style answer #2
Crohn's disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Chronic, relapsing, idiopathic inflammatory gastrointestinal disease
- Involvement of the upper and lower gastrointestinal tract in a discontinuous and transmural manner
Essential features
- Segmental, patchy inflammatory bowel disease, often involving the ileum, colon and upper GI tract
- Histologically characterized by chronic active colitis with associated transmural lymphoid aggregates and fissuring ulcers
- Noncaseating granulomas are characteristic but neither sensitive nor specific
- Main differential diagnosis: ulcerative colitis, indeterminate colitis and infectious colitis
Terminology
- Terminal ileitis
- Granulomatous enterocolitis
- Regional enteritis
Epidemiology
- Incidence: 0.003 - 0.02%
- Prevalence: 0.3%
- Slight female predominance in adult onset Crohn's disease
- Onset of disease at 20 - 40 years of age with a second peak described at the fifth to sixth decade of life
- More prevalent in Ashkenazi Jewish populations
- Incidence in Hispanic and Asian populations has increased in recent decades
- Incidence and prevalence are higher in high income countries and in urban areas, compared to low income countries and rural areas
- References: Mayo Clin Proc 2017;92:1088, Lancet 2017;389:1741
Sites
- Ileocolonic involvement: 30 - 40%
- Exclusive colon involvement: 30 - 40%, 50% rectal sparing
- Small bowel involvement: 80%
- Exclusive small bowel involvement: 30 - 40%
- Of patients with colon disease, 20% will develop ileal involvement in the next 10 years
- Of patients with ileal disease, 20% will develop colon involvement in the next 10 years
- 52% have right sided colitis, 40% have left sided colitis and 6% have pancolitis
- Approximately 25% have perianal complications, including fissures and fistulas
- Upper gastrointestinal complications are present in 5 - 30%, 50% in children and adolescents
- < 10% present with isolated perianal, upper gastrointestinal or extraintestinal complications
- Reference: Mod Pathol 2012;25:295
Pathophysiology
- Not fully understood
- Immunobiology: impaired intestinal barrier function and dysregulation of the innate and adaptative immune system responses, with an alteration of gut microbiota
- Deficient mucus biofilm barrier: decreased expression of mucin secretion genes (MUC1, MUC19 and PTGER4) in the terminal ileum in patients with Crohn's disease
- Permeability changes in the intestinal epithelium: altered expression of tight junction proteins (claudin)
- Paneth cell dysfunction
- Impaired autophagy of invasive microbes
- Imbalance of effector T cells and naturally regulatory T cells
- Recruitment and erratic retention of leukocytes
- Dysbiosis:
- Continuous alterations in intestinal microbiota resulting in clustering and reduced diversity in Firmicutes and Bacteroidetes phyla
- Reduction in Faecalibacterium prausnnitzii was associated with an increased recurrence of ileal Crohn's disease in the postoperative setting
- Genetic risk factors:
- Increased risk for individuals who have family history
- Concordance in monozygotic twins is 20 - 50% compared with 10% in dizygotic twins
- Wide genome association studies identified 200 loci associated to Crohn's disease; however, they contribute only a modest relative risk increase
- NOD2, ATG16L1, IL23R gene variants are responsible for some of the heritable risk
- Environmental risk factors:
- Cigarette smoking doubles the risk of developing Crohn's disease
- Reduced fiber dietary intake
- Antibiotic therapy during childhood increases the risk of developing Crohn's disease
- Other medications including nonsteroidal anti-inflammatory drugs, oral contraceptives and aspirin
- Breastfeeding appears to be a protective factor against the development of Crohn's disease
- References: Nat Rev Dis Primers 2020;6:22, Lancet 2012;380:1590
Etiology
- Idiopathic
Clinical features
- Signs and symptoms: abdominal pain, diarrhea, fatigue and weight loss
- Involvement of other GI sites can present as oral painful aphthous ulcers, odynophagia and dysphagia, postprandial vomiting and nausea, and malabsorption (including diarrhea and steatorrhea with associated nutritional deficiencies)
- 3 phenotypic clinical subtypes:
- Inflammatory phenotype: inflammation of the GI tract with no fistula or stenosing process; classic symptoms
- Stricturing phenotype: inflammation can progress to fibrosis and stenosis; symptoms are associated with bowel obstruction (i.e., lack of bowel movements, nausea and vomiting)
- Fistulizing phenotype: continuous transmural inflammation can cause sinus tract formation resulting in fistulas between bowel and other organs (vagina and bladder)
- Extraintestinal manifestations:
- Musculoskeletal: arthritis or arthropathy and bone loss
- Ocular: uveitis, iritis and episcleritis
- Cutaneous: erythema nodosum and pyoderma gangrenosum
- Hepatobiliary: primary sclerosing cholangitis, pyogenic liver abscess
- Renal: secondary amyloidosis leading to renal disease, calcium oxalate and uric acid renal stones
- Pulmonary: bronchiectasis, chronic bronchitis, interstitial lung disease, bronchiolitis obliterans with organizing pneumonia, sarcoidosis
- There are several clinical scoring systems that categorize patients into low and high risk (e.g., Crohn's disease activity index and Harvey-Bradshaw index)
- Endoscopic findings:
- Ileocolonoscopy is the gold standard for diagnosis
- Ulceration: small aphthous ulcers (< 5 mm) and transmural ulcers
- Cobblestone appearance: linear and serpiginous ulcers with intervening nonulcerated mucosa
- Skip lesions: discontinuous lesions surrounded by adjacent normal tissue
- Other (less specific) findings:
- Normal rectal mucosa
- Inflammation of the terminal ileum with no colonic inflammation
- Ileocolonoscopy is the gold standard for diagnosis
- References: Mayo Clin Proc 2017;92:1088, Inflamm Bowel Dis 2011;17:471, Gastroenterol Rep (Oxf) 2018;6:75
Diagnosis
- Diagnosis consists of a combination of clinical findings and complementary diagnostic tests including blood tests, stool tests, upper and lower endoscopic studies, radiologic imaging and histologic evaluation
- The aim is to exclude differential diagnoses, establish a diagnosis and classify the severity of the disease
Laboratory
- C reactive protein: nonspecific marker for acute inflammation; correlates with disease activity
- ASCA (anti-Saccharomyces cerevisiae antibodies): positive in 60 - 70% of patients with Crohn's disease, 10 - 15% of patients with ulcerative colitis and < 5% of patients without inflammatory bowel disease
- pANCA (perinuclear antineutrophil cytoplasmic antibodies): positive in 10 - 15% of patients with Crohn's disease, positive in 60 - 70% of patients with ulcerative colitis
- Patients with Crohn's disease with positive pANCA tend to have an ulcerative colitis resembling phenotype
- Fecal calprotectin: fecal biomarker used to distinguish between inflammatory bowel disease and functional bowel disease; elevated levels of fecal calprotectin indicate the need for further investigation for inflammatory bowel disease
- Reference: Lancet 2017;389:1741
Radiology description
- Frequently used to assess disease in the small bowel: bowel ultrasonography, CT scan and MRI are helpful for the assessment of the extent of the disease and presence of complications
- Small bowel findings:
- Asymmetrical segmental mural hyperenhancement: specific to Crohn's disease; other types of segmental mural hyperenhancement are less specific
- Wall thickening
- Intramural edema: indicative of bowel inflammation
- Strictures: more common with fibrosis and inflammation; proximal bowel dilation can correlate with higher fibrotic burden
- Ulcers: indicative of severe inflammation (Radiology 2018;286:776)
- Pelvic MRI: used to evaluate and define perianal fistula tracts (Radiology 2017;282:628)
Radiology images
Prognostic factors
- Associated with a 1.38 increase in mortality
- Recurrence in approximately 95% of patients after 10 years of diagnosis, most commonly as ileocolonic disease
- Postoperative recurrence is dependent on disease location
- Isolated ileal disease recurrence is proximal to anastomosis
- Ileocolitis disease recurrence frequently occurs both proximal and distal to the anastomosis (Gut 2012;61:1140)
- Increased risk for relapse, surgery or complications:
- Patient features:
- Young age at diagnosis (< 40 years)
- Smoking
- Disease features:
- High disease burden and prolonged duration of disease
- Perianal disease
- Stricturing disease
- Involvement of the upper GI tract
- Requiring corticosteroids during first flare
- Lack of epithelial healing after clinical remission
- Presence of deep ulcers
- Granulomas on biopsy specimen
- Laboratory tests:
- High C reactive protein and ASCA
- High fecal calprotectin levels
- Low serum levels of albumin and hemoglobin
- Patient features:
- Patients with Crohn's disease have an increased risk for:
- Colorectal cancer and death from colorectal cancer (Lancet Gastroenterol Hepatol 2020;5:475)
- Small bowel carcinoma (Ann Oncol 2009;20:574, Dig Liver Dis 2021;53:809)
- Anal squamous cell carcinoma (Cancers (Basel) 2021;13:1445, J Crohns Colitis 2017;11:1011)
- Lymphoproliferative disorders (Ann Oncol 2009;20:574, Dig Liver Dis 2021;53:809)
Case reports
- 3 year old boy with a case of early childhood Crohn's disease (Hosp Pediatr 2016;6:248)
- 12 year old girl with the cobblestone sign (Dtsch Arztebl Int 2017;114:472)
- 20 year old man with type 2 autoimmune pancreatitis with Crohn's disease (Intern Med 2018;57:2957)
- 31 year old man with pulmonary Crohn's disease (Dig Dis Sci 2017;62:64)
- 37 year old woman with unusual presentation of Crohn's disease (BMJ Case Rep 2021;14:e242703)
- 6 cases of colorectal cancer in Crohn's disease (Surg Case Rep 2021;7:152)
Treatment
- Mainstay of treatment is medical therapy with a goal to achieve clinical, endoscopic and histologic remission, demonstrated by complete mucosal healing
- Chosen based on disease stage, severity and location
- Low risk patients: step up therapy; initially less potent medications with better adverse effect profile are used, while more potent medications are reserved for patients who do not respond to the initial approach
- High risk patients: top down therapy; potent therapies, including biologic therapy and immunomodulators, are used early in the disease to prevent complications
- Medical therapies:
- Corticosteroids
- Thiopurines
- Methotrexate
- Anti-TNF agents
- Surgical therapy: approximately 50% of patients with Crohn's disease will have at least 1 surgical procedure due to disease complications
- Stricturing disease: irreversible with medical therapy; if obstructive symptoms are ongoing, surgery may be indicated
- Fistulizing disease: cases of enterovesicular, enterovaginal and enterocutaneous fistulas or sinus tract and abscess formation
- Perianal disease: perianal fistula or abscess
- Reference: Gastroenterology 2014;147:702
Gross description
- Segmental involvement of the bowel
- Creeping fat due to transmural inflammation
- Thickened and fibrotic bowel walls with stricture formation
- Mucosal aphthous ulcers surrounded by hyperemia with eventual formation of a cobblestone appearing mucosa
- Inflammatory pseudopolyps
- Fissures, sinus and fistulous tracts and abscesses in the setting of complicated disease
- Perforations, rarely
- Reference: Odze and Goldblum: Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 3rd Edition, 2015
Gross images
Microscopic (histologic) description
- Similar to ulcerative colitis, untreated cases typically show features of active chronic colitis
- Features of chronicity include:
- Crypt architectural distortion
- Inflammatory expansion of the lamina propria with basal lymphoplasmacytosis
- Paneth cell metaplasia or hyperplasia
- Pyloric gland metaplasia of small bowel and right colon
- Features of activity include neutrophilic inflammation with cryptitis, crypt abscess and ulceration
- Patchy and segmental distribution with skip lesions
- Aphthous ulcers and deep fissuring ulcers
- Granulomas are characteristic but only present in approximately 50% of patients (Colorectal Dis 2011;13:1142)
- Often well formed and sarcoid-like but may be poorly formed or consist of giant cells
- Should be distinguished from crypt rupture granuloma
- Transmural inflammation with lymphoid aggregates in the subserosal adipose tissue
- Sinus tracts and fistula formation
- Biopsies are limited in determining the depth and distribution of inflammation; therefore, diagnosis is often reliant on clinical correlation or examination of the resection specimen (Histopathology 2014;64:317)
- Dysplasia may be present in patients with longstanding disease
- Activity is usually graded similar to ulcerative colitis (Gastroenterology 2007;133:1099):
- Inactive: absence of neutrophils
- Mild: activity involving < 50% of the mucosa
- Moderate: activity involving > 50% of the mucosa; crypt abscesses often seen
- Severe: presence of surface ulceration or erosion
- References: Histopathology 2014;64:317, Best Pract Res Clin Gastroenterol 2019;38:101601
Microscopic (histologic) images
Negative stains
Sample pathology report
- Colon, biopsy:
- Mildly active chronic colitis with multiple nonnecrotizing granulomas (see comment)
- Comment: The histologic findings are suggestive of idiopathic inflammatory bowel disease. Presence of multiple nonnecrotizing granulomas favors Crohn's disease over ulcerative colitis but clinical correlation with distribution of disease is required.
- Colon, resection:
- Severely active chronic colitis with transmural inflammation and fissuring ulcers compatible with Crohn's colitis; no dysplasia or malignancy
Differential diagnosis
- Ulcerative colitis:
- Diffuse involvement of the colon and rectum
- Absence of skip lesions, except in treated cases
- Absence of granulomas, except when associated with ruptured crypts
- Backwash ileitis: in the setting of severe cecal disease, frequently without severe activity or chronic changes
- Indeterminate colitis:
- Diagnosis of exclusion when it is impossible to distinguish between ulcerative colitis and Crohn's disease
- This term is only used when a resection specimen is available
- Infectious colitis:
- Presence of noncaseating granulomas
- Yersinia:
- Central necrosis within granulomas and lack of transmural inflammation
- Salmonella and Campylobacter:
- Poorly circumscribed microgranulomas
- Tuberculosis:
- Florid coalescent granulomatous inflammation, extensive caseous necrosis and nodal granulomas with no intramural granulomas
- Segmental colitis associated with diverticulitis:
- Inflammation limited to segment of colon with diverticulosis
Board review style question #1
Board review style answer #1
Board review style question #2
Board review style answer #2
Cronkhite-Canada syndrome
Table of Contents
Definition / general | Essential features | Sites | Pathophysiology | Clinical features | Case reports | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, nonhereditary polyposis syndrome of unknown etiology
Essential features
- Disorder of middle adulthood, with mean age of diagnosis of 59 years
- Poor prognosis due to associated nutritional complications
- Up to 50% of patients have remissions
- Rarely has a fulminant course (Endoscopy 2010;42:E350)
- Associated carcinomas documented in 15 - 25% of cases (J Clin Pathol 2014;67:891)
Sites
- Polyps affect entire gastrointestinal tract, except the esophagus
- Also associated with ectodermal changes including alopecia, nail atrophy and cutaneous hyperpigmentation
Pathophysiology
- No known genetic link; considered nonhereditary
- May represent an autoimmune disorder (Digestion 2007;75:96)
Clinical features
- Patients present with variable symptoms, including diarrhea, weight loss, nausea, GI bleeding, protein losing enteropathy
Case reports
- 17 year old boy with late onset Cronkhite-Canada syndrome (Eur J Gastroenterol Hepatol 2005;17:1139)
- 54 year old woman with Cronkhite-Canada syndrome associated with myelodysplastic syndrome (World J Gastroenterol 2009;15:5871)
- 59 year old man with Cronkhite-Canada syndrome complicated with huge intramucosal gastric cancer (Gastric Cancer 2009;12:113)
- 63 year old woman with colorectal carcinoma in Cronkhite-Canada syndrome (Z Gastroenterol 2001;39:365)
- 70 year old woman with Cronkhite-Canada syndrome with adenomatous and carcinomatous transformation of colonic polyp (Indian J Gastroenterol 2003;22:189)
- 72 year old man with Cronkhite-Canada syndrome presenting with adenomatous and inflammatory colon polyps (Nat Rev Gastroenterol Hepatol 2010;7:460)
- Acute brain syndrome as a consequence of Cronkhite-Canada syndrome (Psychiatr Danub 2005;17:90)
Microscopic (histologic) description
- Multiple ill defined polyps with cystically dilated glands and crypts associated with an edematous lamina propria containing mononuclear cells and eosinophils, microscopically indistinguishable from juvenile polyps (Am J Surg Pathol 1989;13:940)
- Intervening nonpolypoid mucosa characteristically also has dilated glands, edema, congestion and inflammation (Gastroenterol Res Pract 2009;2009:619378)
Microscopic (histologic) images
Differential diagnosis
- Juvenile polyposis syndrome:
- Intervening nonpolypoid mucosa is histologically unremarkable
Additional references
Board review style question #1
Germline mutation in which of the following genes has been linked to Cronkhite-Canada syndrome?
- BRAF
- KRAS
- None (syndrome is nonhereditary)
- TP53
Board review style answer #1
Board review style question #2
The risk of developing carcinoma in patients with Cronkhite-Canada syndrome is approximately
- 0 - 5%
- 15 - 25%
- 40 - 50%
- 65 - 75%
Board review style answer #2
Cryptosporidium parvum
Table of Contents
Definition / general | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Immunohistochemistry & special stains | Differential diagnosis | Additional referencesDefinition / general
- Disease caused by infection with Cryptosporidium species, a protozoal parasite
- Traditionally considered a coccidian parasite but recent evidence suggests it may be a gregarine parasite (Wikipedia: Gregarinasina [Accessed 13 October 2023])
Epidemiology
- Cryptosporidium has a worldwide distribution (excepting Antarctica)
- Infection is usually person to person through the fecal-oral route, via ingestion of infective oocysts
- In some cases, zoonotic infection from sheep, cows, pigs, rodents, companion animals and other animals may occur
- The oocysts are hardy and are not killed by chlorination of drinking water
- Developing world: primarily affects children under age 5 and in most cases persistent diarrhea occurs that may be compounded by malnutrition; uncommon in adults
- Developed world:
- More common in children but not to the extent of the developing world
- Disease due to:
- Spread of pediatric cases in day care centers
- Travel to developed countries
- Spread in mental institutions
- Contaminated recreational water, including swimming pools, rivers, lakes, fountains
- Cryptosporidium is the most common cause of waterborne disease in recreational water
- Animal handlers
- Food borne spread
- Breakdowns in municipal water purification systems
- United States: 300,000 to 750,000 cases each year, more commonly in summer
- The largest known outbreak occurred in Milwaukee, Wisconsin in 1993, affecting an estimated 400,000 people, although this type of spread is now uncommon in the developed world
- Sexual transmission in men who have sex with men has been reported
- Disease is common in immunosuppressed patients especially AIDS patients with CD4 counts under 100
- There are over 20 species of cryptosporidium:
- C. hominis, the human genotype that primarily infects people and C. parvum, the bovine genotype, are the most important causes of human disease
- C. hominis was formerly known as C. parvum anthroponotic genotype 1
- C. meleagridis, C. Canis, C. felis, C. ubiquitum, C. cuniculus, C. suis, C. muris and other species are known to cause human disease
Sites
- Infection is most common in terminal ileum and proximal colon
- Disease also occurs in proximal small intestine, distal colon, gallbladder, bile ducts and pancreas
- Widespread disease generally occurs with severe immunosuppression and may involve respiratory tract
Pathophysiology
- Ingested oocysts excyst in stomach and small intestine, releasing 4 infective sporozoites that bind to intestinal epithelial cells
- The sporozoite becomes embedded in cell membrane in a parasitophorous vacuole
- Inside the vacuole, the sporozoite undergoes merogony (asexual reproduction) to become trophozoites
- The trophozoites divide to become type I meronts that mature, causing the parasitophorous vacuole to rupture, releasing motile merozoites that bind to epithelial cells and are engulfed
- Merogony is repeated or sexual differentiation occurs and merozoites differentiate into micro and macrogamonts
- The microgamonts release microgametes that penetrate cell walls of cells infected with macrogamonts
- The macrogamont and microgametes fuse and form zygotes
- The zygote undergoes meiosis to form an oocyst containing 4 sporozoites
- There are thin walled and thick walled forms of oocysts
- The thin walled form excysts in the host causing autoinfection while the thick walled oocyst is shed in the environment
- Infection causes enterocolitis or malabsorption
Clinical features
- The incubation period is usually about one week but may be from 1 to 30 days
- In immunocompetent individuals, acute but self-limited profuse watery diarrhea usually occurs
- In children in the developing world, persistent diarrhea occurs but most patients recover
- In immunosuppressed patients, chronic diarrhea occurs that is often debilitating
- In immunosuppressed patients, acalculous cholecystitis, sclerosing cholangitis, pancreatitis, biliary strictures and respiratory disease may occur
Diagnosis
- The diagnosis is usually made by visualization of cysts in stool using immunofluorescence or a modified acid fast stain
- It is important to communicate with the laboratory if there is a suspicion of cryptosporidiosis, as not all laboratories routinely test stool for Cryptosporidiium
- On occasion, the diagnosis is made on biopsy (see Microscopic (histologic) description)
Prognostic factors
- In immunocompetent adults, self limited diarrhea lasting one or two days is the rule
- If the CD4 count is under 100, chronic diarrhea occurs
- In HIV+ patients with CD4 counts under 50 and other immunosuppressed patients with markedly suppressed T cell function, fulminant diarrhea occurs
- In HIV+ patients with CD4 counts greater than 150 - 180, self-limited diarrhea usually occurs
- The ultimate prognosis in chronic and fulminant diarrhea in HIV+ patients is related to the success of therapy to reconstitute the immune system
- In the developing world, children under 5 usually suffer from persistent diarrhea that is often complicated by malnutrition
Case reports
- 2 year old boy with Cryptosporidium infection and celiac disease (J Postgrad Med 2012;58:160)
- 15 year old boy with several day history of profuse watery diarrhea after returning from swimming camp (Pritt: Creepy Dreadful Wonderful Parasites Blog - Case of the Week 543 [Accessed 13 October 2023])
- 43 year old man with cholangiocarcinoma complicating secondary sclerosing cholangitis from cryptosporidiosis (Int Arch Allergy Immunol 2012;159:204)
- 64 year old woman with Cryptosporidiosis causing severe persistent diarrhea (Indian J Med Paediatr Oncol 2014;35:93)
- Pulmonary cryptosporidiosis and immune reconstitution inflammatory syndrome (Int J STD AIDS 2013;24:333)
- Opportunistic parasite infections in patients receiving alemtuzumab (J Clin Pathol 2012;65:92)
Treatment
- Nitazoxanide reduces the severity of disease in immunocompetent patients and has been approved by the FDA for these patients; its effectiveness in immunocompromised patients is unclear although it is used in that setting
- Reconstitution of the immune system through highly active anti-viral therapy in AIDS patients or reduction in immunosuppression in other settings is important
Microscopic (histologic) description
- In tissue biopsies, 2 - 5 μm basophilic round bodies are seen protruding from the apex of enterocytes ("blue beads") within the cell membrane; highlight with Giemsa stain
- Cryptosporidium spp. oocysts measure 4 - 6 micrometers in diameter (Pritt: Creepy Dreadful Wonderful Parasites Blog - Answer to Case 543 [Accessed 13 October 2023])
- Villous atrophy, crypt hyperplasia, cryptitis and increased mixed inflammatory cells within the lamina propria may be seen
Microscopic (histologic) images
Immunohistochemistry & special stains
- Cryptosporidium spp. oocysts are red-pink using a modified acid fast stain (Pritt: Creepy Dreadful Wonderful Parasites Blog - Answer to Case 543 [Accessed 13 October 2023])
- Not all oocysts will reliably stain; some may appear as ghost cells or negative outlines
- Modified safranin stain can also be used and may more reliably stain the oocysts
Differential diagnosis
- Cyclospora:
- Oocysts are 8 μm, not 2 - 5 microns
- Isospora:
- Oocysts are 20 - 30 μm
- Microsporidium:
- An intracellular fungus that is not modified acid fast but may coinfect with Cryptosporidium
Additional references
Cyst of retrorectal space
Table of Contents
Definition / general | Diagrams / tables | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) imagesDefinition / general
- Also called tailgut cyst, retrorectal hamartoma
- Retrorectal space is loose areolar tissue plane between fascia propria of rectum and presacral space
- Rare but most common retrorectal cystic lesions in adults, occurring mostly in middle aged women (Radiographics 2001;21:575)
- Often misdiagnosed clinically (J Am Coll Surg 2003;196:880)
- Arises from remnants of embryonic postanal gut
Types:
- Dermoid cyst: unilocular, lined by squamous epithelium and skin adnexae, no smooth muscle
- Epidermoid cyst: unilocular, lined by squamous epithelium without adnexa
- Rectal duplication cyst: unilocular, lined by colonic, gastric or respiratory epithelium with organized smooth muscle similar to muscularis propria
- Cystic hamartoma: multilocular with squamous, transitional or glandular lining, disorganized smooth muscle, occasionally foreign body granulomatous inflammation
Case reports
- 14 year old boy with prerectal tailgut cyst (World J Gastroenterol 2006;12:5081)
- 36 year old woman with laparoscopy assisted resection of tailgut cyst (Case Rep Gastroenterol 2011;5:22)
- 45 year old woman with tailgut cyst initially misdiagnosed as ovarian tumor (Arch Gynecol Obstet 2005;272:301)
- 46 year old woman with low back pain (Case Rep Med 2012;2012:623142)
- 68 year old man with extension of malignant tailgut cyst to subcutaneous space in buttock (Eur J Dermatol 2010;20:788)
- Causing pseudomyxoma peritonei (Tumori 2009;95:514)
- Two cases with associated adenocarcinoma (Arch Pathol Lab Med 2001;125:1361)
Treatment
- Complete excision recommended to prevent malignant transformation (Am J Clin Pathol 1988;89:139, Arch Pathol Lab Med 2000;124:725)
Gross description
- Multilocular, variable solid areas
Microscopic (histologic) images
Cytomegalovirus (CMV)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cytomegalovirus (CMV) is a double stranded DNA virus and a member of human herpes virus family
- Also known as herpes virus type 5
- 3 patterns of CMV infection:
- Latent infection
- Most common, immunocompetent patients
- Mononucleosis-like syndrome
- Immunocompetent patients
- Tissue invasive disease
- Immunocompromised patients
- Latent infection
- Tissue invasive disease
- Gastrointestinal tract is most commonly involved (30% of tissue invasive cases) (Virol J 2008;5:47)
Essential features
- Double stranded DNA virus and a member of human herpes virus family
- Tissue invasive disease, usually seen in immunocompromised patients
- Most common sites of infection in gastrointestinal tract:
- Colon
- Esophagus
- Symptoms:
- Rectal bleeding (most common)
- Diarrhea
- Abdominal pain
- Fever
- Weight loss
- Microscopy:
- Cytomegalic cells with owl's eye intranuclear viral inclusions
- CMV immunohistochemistry is the gold standard for diagnosis
Terminology
- CMV infection
- CMV antigens or antibodies in blood
- CMV disease
- Clinical symptoms and end organ damage
- CMV colitis
- Identification of characteristic intranuclear / intracytoplasmic inclusions on H&E sections
- Identification of CMV specific antigens by immunohistochemistry (IHC) and clinical symptoms
ICD coding
- ICD-10: B25.8 - other cytomegaloviral diseases
Epidemiology
- Affects 50 - 100% humans worldwide depending on age and race of the population tested
- Infects over half of adults by age 40 (CDC: About Cytomegalovirus (CMV) [Accessed 11 November 2021])
- CMV seroprevalence in the United States is 42 - 93% (Am J Kidney Dis 1991;17:719)
- Immunocompetent patients
- Mean age is 64 - 75 years (Clin Microbiol Infect 2015;21:1121.e1)
- Liver transplant patients
- Cumulative incidence is 4.9% (Transplant Proc 2014;46:832)
- Allogenic hematopoietic stem cell transplant patients
- Incidence is 15 - 25% (Biol Blood Marrow Transplant 2015;21:159)
- Inflammatory bowel disease
- Incidence is 1.5 - 4.5%
- Ulcerative colitis > Crohn’s disease (Eur J Gastroenterol Hepatol 2016;28:1329)
Sites
- Can involve any part of the gastrointestinal tract
- Most common sites:
- Colon
- Esophagus
Pathophysiology
- Spread by saliva, urine, respiratory droplets, sexual contact, breast milk and blood transfusions (Clin Microbiol Rev 1989;2:204, Nihon Rinsho 1998;56:179)
- After initial infection, CMV resides latently in monocytes, fibroblasts, myeloid cells and endothelial cells
- Tissue invasive disease in colon can lead to ulcerative changes, erosion into blood vessels (causing bloody diarrhea), inflammatory polyps, severe inflammation and vasculitis leading to ischemia and transmural necrosis
Etiology
- Cytomegalovirus (CMV)
- Most commonly in immunocompromised patients
- History of AIDS, organ transplantation, hematologic malignancy, cancer therapy and corticosteroid therapy
- Risk factors in immunocompetent patients:
- Renal disease
- Hemodialysis
- Neurological disease
- Rheumatic disease
- Exposure to antibiotics
- Antacids
- Corticosteroid
- Red blood cell transfusion within 1 month of diagnosis of colitis (Clin Infect Dis 2015;60:e20)
- Severe ulcerative colitis (patients treated with high dose corticosteroids)
Clinical features
- Rectal bleeding (most common)
- Diarrhea, abdominal pain, fever, weight loss (StatPearls: Cytomegalovirus Colitis [Accessed 12 November 2021])
Diagnosis
- Surgical resection specimen or biopsy: histopathologic diagnosis
- Clinical symptoms, endoscopic findings, serologic testing, polymerase chain reaction (PCR) and culture
Laboratory
- Histology:
- Gold standard test
- Immunohistochemistry (IHC) for CMV
- Greater sensitivity than hematoxylin & eosin (H&E)
- H&E can detect CMV infected cells
- Cells larger than normal, containing intranuclear or intracytoplasmic inclusions
- CMV infected cells can be confirmed by IHC staining
- Immunohistochemistry (IHC) for CMV
- Gold standard test
- Serology:
- Acute infection
- CMV IgM antibodies
- 4 times increase in titer of CMV IgG specific antibodies 2 - 4 weeks apart
- CMV antigenemia
- Predictor of clinical outcomes
- Less sensitive for diagnosis of CMV colitis
- Real time polymerase chain reaction (PCR) / CMV DNA quantification
- Positive in only 50% of patients with biopsy proven CMV colitis / enteritis
- CMV culture
- High sensitivity and specificity for diagnosis of CMV colitis
- Takes longer to obtain results (1 - 3 weeks)
- May delay diagnosis and timely treatment (J Clin Microbiol 1993;31:2851)
- Acute infection
Radiology description
- Computed tomography:
- Nonspecific findings
- Bowel wall thickening
- Mural edema
- Pericolonic fat stranding
- Free fluid, free air
- Lymphadenopathy (Radiology 1985;155:585)
- Endoscopic findings:
- Easy bleeding, loss of vascular pattern, mucosal edema, erythema, mucinous exudate and wide mucosal defect
- Mucosal defects, punched out ulcers (most common), longitudinal ulcers, irregular ulceration or cobblestone appearance (Emerg Radiol 2020;27:277)
Prognostic factors
- Excellent overall prognosis
- Factors associated with poor prognosis and higher mortality (immunocompetent patients)
- M > F
- Age > 55 years
- Patients requiring surgery
- CMV colitis reactivation with ulcerative colitis, tends to have poorer prognosis
- Timely diagnosis and treatment greatly improves clinical outcomes (StatPearls: Cytomegalovirus Colitis [Accessed 12 November 2021])
Case reports
- 42 year old HIV+ man with progressive abdominal pain, palpable right umbilical mass, fever, asthenia and weight loss (Radiol Case Rep 2018;14:273)
- 48 year old woman with severe abdominal pain and constipation (World J Clin Cases 2021;9:5631)
- 60 year old man with history of sarcoidosis and chronic kidney disease with lower abdominal pain and diarrhea (Indian J Nephrol 2021;31:73)
- 71 year old man with history of posttuberculous aspergilloma admitted to ICU with acute respiratory distress syndrome (Clin Case Rep 2020;9:e03600)
Treatment
- Intravenous (IV) ganciclovir (5 mg/kg twice daily)
- After 3 - 5 days of IV ganciclovir, oral valganciclovir (900 mg, twice daily) (Clin Gastroenterol Hepatol 2015;13:949)
- Foscarnet in ganciclovir resistant or tolerant cases
Clinical images
Gross description
- Nonspecific findings
- Inflamed mucosa, hyperemia, mucosal sloughing
- Punched out ulcers, aphthous ulcers, exudate
- Pseudomembrane formation (Arch Pathol Lab Med 2016;140:854)
Gross images
Microscopic (histologic) description
- Most commonly affected cells:
- Endothelial cells
- Mesenchymal cells
- Macrophages
- Larger (cytomegalic) cells:
- Usually 25 - 35 micrometers
- Typically 2 - 4 times larger than normal
- Owl’s eye:
- Large ovoid or pleomorphic nucleus with basophilic intranuclear inclusions (Cowdry bodies) surrounded by a clear halo (Arch Pathol Lab Med 2016;140:854)
- Thickened nuclear membrane
- Coarse red intracytoplasmic granules (Int Med Case Rep J 2011;4:55)
- Increased apoptotic bodies
- Expansion of lamina propria by mixed inflammatory plasma cell rich infiltrate
- Neutrophilic inflammation
- Should raise suspicion for CMV infection in graft versus host disease and mycophenolate injury (Int J Surg Pathol 2018;26:347, J Clin Pathol 2013;66:8)
- Deep fissuring ulcers, cryptitis, crypt abscess, architectural distortion and pseudopolyp formation
- May mimic inflammatory bowel disease (Am Surg 2007;73:58)
- Submucosal vasculitis and thrombosis of microvessels
Microscopic (histologic) images
Positive stains
- Immunohistochemistry for CMV
Negative stains
- Immunohistochemistry for adenovirus, HSV1, HSV2
Molecular / cytogenetics description
- Real time DNA PCR amplification method:
- Rapid results, high negative predictive value
- Contradictory reports regarding sensitivity
- CMV DNA load > 250 copies/mg tissue may predict resistance to steroid treatment in ulcerative colitis (Am J Gastroenterol 2011;106:2001)
Videos
CMV colitis in ulcerative colitis and immunocompromised states
Sample pathology report
- Colon, random, biopsy:
- CMV colitis
- Immunohistochemical stain for CMV is positive
- Colon, colectomy:
- CMV colitis in the background of severely active ulcerative pancolitis
- Immunohistochemical stain for CMV is positive
- Negative for dysplasia
Differential diagnosis
- Infectious colitis:
- Adenovirus:
- Commonly infects surface epithelial cells
- Homogenous glassy amphophilic nuclear inclusions
- HSV1, HSV2:
- Chromatin margination, multinucleation, nuclear molding
- Adenovirus:
- Graft versus host disease:
- Crypt apoptosis
- Crypt dropout
- Ulceration
- No cytomegalic inclusions
- May coexist with CMV infection
- Inflammatory bowel disease:
- No inclusions
- Ulcerative colitis, Crohn's disease
- Mycophenolate mofetyl induced colitis:
- No inclusions
- Increased apoptosis
- Increased lamina propria eosinophils
Additional references
Board review style question #1
A 71 year old man presents with abdominal pain and diarrhea. Colonoscopy showed diffuse mucosal erythema and irregular ulcerations. The patient undergoes biopsy of the lesion. What is the infected cell and organism causing the histopathologic findings?
- Endothelial cell, adenovirus
- Endothelial cell, cytomegalovirus
- Epithelial cell, adenovirus
- Epithelial cell, cytomegalovirus
Board review style answer #1
B. Endothelial cell, cytomegalovirus. The images show cytomegalic endothelial cells with inclusions. Adenovirus typically affects epithelial cells and shows amphophilic nuclear inclusions.
Comment Here
Reference: Cytomegalovirus (CMV)
Comment Here
Reference: Cytomegalovirus (CMV)
Board review style question #2
What is the gold standard method for diagnosing CMV colitis?
- CMV culture
- CMV DNA polymerase chain reaction (PCR) amplification method
- CMV serology
- Immunohistochemistry for CMV
Board review style answer #2
Diarrhea / dysentery
Table of Contents
Definition / general | Terminology | Epidemiology | Clinical features | Diagrams / tables | Microscopic (histologic) description | Additional referencesDefinition / general
- Normal intestines receive 9 liters of fluid per day (oral intake: 2 L, saliva: 1L, gastric juices: 2L, pancreatic juices: 2L, intestinal juices: 1L); most is reabsorbed in small intestine and colon
Terminology
- Diarrhea: 3 or more loose stools / day; also increase in stool mass, fluidity or frequency of defecation (Wikipedia: Diarrhea [Accessed 22 December 2021], eMedicine: Diarrhea [Accessed 22 December 2021])
- Chronic diarrhea: decrease in stool consistency for more than 4 weeks (Am Fam Physician 2011;84:1119, Best Pract Res Clin Gastroenterol 2012;26:551, Arch Pathol Lab Med 2013;137:83)
- Secretory diarrhea: > 500 mL of fluid stool per day, isotonic with plasma, persists during fasting
- Dysentery: low volume, painful, bloody diarrhea (Wikipedia: Dysentery [Accessed 22 December 2021])
- Exudative disease: purulent bloody stools, persists with fasting - due to bacteria (Salmonella, Shigella, Campylobacter), Entamoeba histolytica, idiopathic inflammatory bowel disease, typhlitis
- Malabsorption: bulky stools, abates with fasting, due to defective intraluminal digestion, primary mucosal cell abnormalities, reduced small bowel surface area, lymphatic obstruction, Giardia lamblia
Epidemiology
- 12,000 deaths/year from dehydration in developing countries - 50% of all deaths before age 5
- Affects 40% of U.S. population - #2 in attack rates in U.S. after common cold
Clinical features
Deranged motility:
- Improper gut neuromuscular function causes decreased transit time
- Due to surgical resection of gut, irritable bowel syndrome (neural dysfunction), hyperthyroidism, diabetic neuropathy, carcinoid syndrome
- Decreased motility due to small bowel diverticula, blind loop, bacterial overgrowth
- Purulent bloody stools, persist with fasting; caused by bacteria (Salmonella, Shigella, Campylobacter), Entamoeba histolytica, idiopathic inflammatory bowel disease, typhlitis (neutropenic colitis in immunosuppressed)
- Bulky stools, abates with fasting; caused by defective intraluminal digestion, primary mucosal cell abnormalities, reduced small intestinal surface area, lymphatic obstruction, Giardia lamblia (MedlinePlus: Malabsorption [Accessed 22 December 2021], Wikipedia: Malabsorption [Accessed 22 December 2021])
- Gut luminal solutes create high stool osmolality, abates with fasting
- Stool osmolality > electrolyte concentration by 50 mOsm
- Associated with lactase deficiency, lactulose therapy, gut lavage, antacids, primary bile acid malabsorption
- > 500 mL of fluid stool per day, isotonic with plasma, persists during fasting
- Infectious (viral damage to epithelium): rotavirus, Norwalk virus, enteric adenoviruses, calicivirus, astrovirus
- Infectious (enterotoxin): Vibrio cholera, E. coli, Bacillus cereus, Clostridium perfringens
- Neoplastic: tumor production of peptides, villous adenoma in distal colon
- Excessive laxatives
Microscopic (histologic) description
- Patchy lesions with variable villus abnormality, rarely severe
- Increased chronic and acute inflammatory infiltrate in epithelium and lamina propria
- Small intestinal bacterial overgrowth: common cause of chronic diarrhea and malabsorption with either villous blunting or normal duodenal biopsy (Arch Pathol Lab Med 2010;134:264)
Additional references
Diversion colitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Inflammatory condition that affects colon distal to an ileostomy or colostomy
- It can also occur in sigmoid segments used in colovaginoplasty procedures
Essential features
- Clinicopathologic condition often arising in colonic segments distal to an ileostomy or colostomy site; it may also occur in colonic pouches, such as those following colovaginoplasty
- Creation of an ostomy site alters the normal flow of fecal material through the bowel and thereby alters the microbiome of the gut resulting in alteration of the nutrients available for colonic enterocytes
- Patients may present with nausea, vomiting, abdominal discomfort and bloody bowel movements
- Histologic features may include lymphoid aggregates with germinal centers, muscularis mucosa hypertrophy, acute inflammation, architectural changes and ulceration
- When it occurs in patients with underlying inflammatory bowel disease, histologic distinction can be challenging
Terminology
- Defunctionalized bowel
ICD coding
Epidemiology
- Generally reported that no significant associations exist between diversion colitis and age of onset, gender, stoma type or mode of surgery (Int J Colorectal Dis 2017;32:1191)
- However, Tominaga et al. reported a slight predominance in men (World J Gastroenterol 2018;24:1734)
- Greatest association with diversion colitis is simply the surgical interruption of the normal flow of fecal material through the bowel
Sites
- Colonic segments distal to colostomy or ileostomy, as well as segments of colon repurposed for use outside of the alimentary canal
Pathophysiology
- Pathophysiology of diversion colitis is thought to be multifactorial
- Disruption in the normal flow of fecal material may lead to changes in the microbiota
- In a normal healthy colon, luminal bacteria produce short chain fatty acids (SCFAs), such as acetate, propionate and butyrate, with butyrate likely being the most important of the 3 (Nutr Rev 1989;47:257)
- These SCFAs have been demonstrated to be important nutrients for colonic enterocytes
- SCFAs may also play a role in vascular smooth muscle relaxation
- Thus, deficiencies in SCFAs may lead to nutritional deficiencies for colonic enterocytes and increased arterial tone (World J Gastroenterol 2018;24:1734)
- It has also been hypothesized that patients with diversion colitis may have an increased population of nitrate reducing bacteria and thus higher levels of nitric oxide (NO); while lower levels of nitric oxide appear to play a protective role for the bowel, it has been hypothesized that an excess of nitric oxide may be toxic to colonic tissue (World J Gastroenterol 2018;24:1734)
Etiology
- Construction of ileostomy or colostomy are common causes for diversion colitis; for instance, this may occur following a Hartmann procedure (proctosigmoidectomy with creation of end colostomy) for management of colorectal cancer, severe diverticulitis or for treatment of refractory fecal incontinence (J Wound Ostomy Continence Nurs 2008;35:231)
- Diversion colitis has also been reported in patients who underwent sigmoid neovaginal surgery for aplasia vaginae secondary to Mayer-Rokitansky-Küster-Hauser syndrome (Müllerian agenesis), as well as those pursuing gender reassignment surgery (Frontline Gastroenterol 2016;7:227)
Clinical features
- Clinical features are highly variable but presentation may include
- Abdominal pain
- Tenesmus
- Anal and rectal pain
- Mucous discharge
- Rectal bleeding
- Some patients may present with endoscopic or histologic findings but may remain asymptomatic
- When evaluating clinical features, it is important to contextualize those signs and symptoms within the appropriate setting; for instance, the above features should raise suspicion for diversion colitis in patients with ileostomies, with some research to suggest that symptoms can arise from months to years after ileostomy construction (World J Gastroenterol 2018;24:1734)
Diagnosis
- Standardized diagnostic criteria have not been codified; thus, diagnosis must be suspected clinically and supported with imaging and histologic evaluation
Radiology description
- Findings are largely nonspecific
- Computed tomography (CT) and magnetic resonance imaging (MRI) scans will demonstrate findings consistent with intestinal discontinuity, such as an ileostomy, colostomy or a colonic pouch
- Affected colonic segments may show fat stranding, wall thickening and fluid distension
- Target or water halo sign may be present, which is suggestive of inflamed colonic mucosa and edematous submucosa
- Reference: Insights Imaging 2019;10:41
Prognostic factors
- Protracted ostomy reliance generally portends a worse prognosis; additionally, inflammatory states, such as infection or underlying bowel disease, are thought to either precipitate, exacerbate or otherwise contribute to diversion colitis (BMJ Case Rep 2021;14:e243284)
Case reports
- 12 year old girl with myelomeningocoele with fecal incontinence managed with colostomy construction and subsequent resection of distal defunctionalized bowel (Histopathology 1994;24:395)
- 19 year old woman with Berdon syndome (megacystis microcolon intestinal hypoperistalsis syndrome [MMIHS]) diagnosed with diversion colitis after ileostomy construction (Dig Dis Sci 2009;54:2338)
- 42 year old woman with androgen insensitivity syndrome with diversion colitis of sigmoid neovagina (Frontline Gastroenterol 2016;7:227)
- 51 year old woman with Crohn's disease and microcarcinoids in association with diversion colitis symptoms presenting more than 17 years after subtotal colectomy and ileostomy (J Crohns Colitis 2008;2:246)
Treatment
- Surgical management with reestablishment of intestinal continuity and stomal closure is generally regarded as the preferred and most successful treatment
- Medical management may be pursued in place of surgical intervention in patients who are not surgical candidates, at risk for anastomotic leakage or those with severe active bowel inflammation (BMJ Case Rep 2021;14:e243284, World J Gastroenterol 2018;24:1734)
- Medical management varies and may include
- Short chain fatty acid (SCFA) enemas
- 5-aminosalicylic acid
- Corticosteroids
- Irrigation with fibers
- Leukocytapheresis
- Autologous fecal transplant
- Dextrose spray
- TNFα inhibitors, such as infliximab
Microscopic (histologic) description
- Lymphoid follicular hyperplasia with germinal centers
- Muscularis mucosa hypertrophy, which is often patchy
- Architectural changes with distortion of crypts, crypt branching and loss of crypts
- Degenerative mucosal surface epithelium with cell exfoliation and pyknotic nuclei
- Loss of goblet cells and mucin
- Mucosal ulceration with granulation tissue
- Varying degrees of inflammation, with features of both activity (neutrophil predominant) and chronicity (lymphoplasmacytic)
- Focal edema
- Pseudopolyps
- Paneth cell metaplasia
- References: Hum Pathol 2022;123:31, World J Gastroenterol 2018;24:1734, Histopathology 1991;19:55
Microscopic (histologic) images
Sample pathology report
- Rectum, stump, biopsy:
- Diversion colitis (see comment)
- Comment: Colonic mucosa with prominent lymphoid aggregates and mild architectural changes compatible with diversion colitis.
Differential diagnosis
- Inflammatory bowel disease (IBD) (ulcerative colitis or Crohn's disease):
- Histologic distinction can be challenging and diversion colitis can co-occur with IBD
- More severe inflammatory changes may indicate superimposed IBD (Hum Pathol 2022;123:31)
- Segmental colitis associated with diverticulosis (SCAD):
- Occurs in segment of colon with diverticula and rectum is typically spared
- Radiation colitis:
- Occurs in patients with history of radiation therapy for malignancy (e.g., of prostate, colon, bladder)
- Acute radiation may demonstrate a number of histologic findings, including nuclear atypia, reduction in mitotic counts, eosinophilic inflammation (including eosinophilic cryptitis and crypt abscess formation), goblet cell derangements and fibroblastic proliferation
- Chronic exposure may result in Paneth cell metaplasia, architectural distortion, fibrosis of lamina propria, chronic inflammation, hyalinized vasculature, vascular ectasia and thrombosis
Board review style question #1
A 5 month old infant is seen in the emergency department with bloody bowel movements. She appears uncomfortable and begins crying with abdominal palpation. She is afebrile and her white blood cell count is within normal limits. Medical history is significant for premature birth complicated by necrotizing enterocolitis with bowel resection and ileostomy construction. A biopsy of the colonic stump is likely to demonstrate which of the following findings?
- Crypt abscesses, Paneth cell metaplasia, marked architectural distortion, basal lymphoplasmacytosis
- Endothelial cells demonstrating owl eye intranuclear inclusions with associated clear halo
- Intraepithelial lymphocytosis
- Prominent lymphoid hyperplasia, muscularis mucosa hypertrophy and crypt abscesses
Board review style answer #1
D. Prominent lymphoid hyperplasia, muscularis mucosa hypertrophy and crypt abscesses. These findings are characteristic of diversion colitis. The patient’s history of bowel resection with ileostomy should raise suspicion for diversion colitis. Answer A is incorrect because these findings are more demonstrative of ulcerative colitis. It can be difficult to separate out the 2 conditions and reports in the literature exist of concomitant ulcerative colitis and diversion colitis. With overlapping symptoms and the potential for finding crypt abscesses in both conditions, it can certainly pose a diagnostic challenge for pathologists trying to differentiate the 2 conditions on biopsy. The patient’s history of ileostomy construction should be a clue that diversion colitis may be favored here. Without a pre-existing diagnosis of inflammatory bowel disease (IBD), this is unlikely. Answer B is incorrect because the characteristic owl eye inclusions are consistent with cytomegalovirus (CMV) colitis. This consideration of this condition may be greater in patients with a history of immunosuppresion; for instance, in patients with human immunodeficiency virus (HIV) or those receiving immunosuppressive therapy following solid organ transplant. Answer C is incorrect because intraepithelial lymphocytosis is characteristic of lymphocytic colitis, which typically presents with watery, nonbloody diarrhea. Microscopic colitis is uncommon in the pediatric population.
Comment Here
Reference: Diversion colitis
Comment Here
Reference: Diversion colitis
Board review style question #2
A 72 year old man with a history of colon cancer undergoes radiation therapy, followed by partial colon resection with construction of an ileostomy. He denies any history of inflammatory bowel disease. The image shown above demonstrates histologic findings of the resected portion of the colon. These findings are most consistent with which clinical presentation?
- Diversion colitis
- Infectious agent
- Radiation colitis
- Ulcerative colitis
Board review style answer #2
A. Diversion colitis. The construction of an ileostomy should always raise suspicion for the possibility of diversion colitis. The prominent lymphoid aggregates are likely the most characteristic finding associated with diversion colitis. Other features appreciated here include patchy hypertrophy of muscularis mucosa, ulceration and loss of colonic crypts. Answer B is incorrect because the clinical vignette does not suggest any fevers or bowel symptoms consistent with an infectious etiology. Infectious colitis typically shows a histologic pattern of acute self limited colitis without architectural distortion. Answer C is incorrect because the presence of lymphoid aggregates should favor diversion colitis over radiation colitis. Radiation colitis is typically pauci-inflammatory with evidence of lamina propria fibrosis and hyalinized ectatic vessels. Answer D is incorrect because while diversion colitis can have overlapping histologic features with inflammatory bowel disease, given that the patient denies any history of underlying inflammatory bowel disease, this is unlikely to be a manifestation of ulcerative colitis.
Comment Here
Reference: Diversion colitis
Comment Here
Reference: Diversion colitis
Diverticular colitis
Table of Contents
Definition / general | Pathophysiology | Treatment | Microscopic (histologic) description | Differential diagnosisDefinition / general
- Colitis related to diverticular disease (diverticulosis) (Pathology 2002;34:568, Colorectal Dis 2002;4:208, Am J Gastroenterol 1992;87:609, Dig Dis 2012;30:29)
- Also called segmental colitis associated with diverticula
- Affects sigmoid colon only with rectal sparing (Am J Surg Pathol 1996;20:94)
- Usually presents with rectal bleeding
- Good prognosis (Endoscopy 2006;38:610); does not appear to evolve to inflammatory bowel disease (Int J Colorectal Dis 2005;20:28)
Pathophysiology
- Due to mucosal prolapse, fecal stasis and relative mucosal ischaemia; also subserosal peridiverticulitis and suppuration causing mass effect (Pathology 2002;34:568)
Treatment
- High fiber diet, antibiotics, aminosalicylates; surgery may be necessary (Colorectal Dis 2001;3:149)
Microscopic (histologic) description
- Transmural inflammation with expansion of lamina propria, cryptitis and crypt abscesses, lymphoid aggregates and distorted crypt architecture
Differential diagnosis
- Inflammatory bowel disease and acute self-limited colitis have different clinical history
Diverticulosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Anatomic change in the colon characterized by outpouchings of mucosa and submucosa through the muscularis (Clin Geriatr Med 2021;37:141)
- Diverticulosis: presence of diverticula, regardless of symptoms
- Diverticular disease: clinically relevant symptomatic diverticulosis
Essential features
- Anatomic change in the colon characterized by outpouchings of mucosa and submucosa through the muscularis
- Diverticulosis can be asymptomatic or symptomatic
- Sigmoid colon / left colon, most common site (90%)
- Mucosa normal to markedly abnormal
- Expansion of lamina propria by lymphoplasmacytic infiltrate, lymphoglandular complexes, mucin depletion and focal Paneth cell metaplasia may be seen
- No rationale for treatment of asymptomatic colonic diverticulosis
Terminology
- Most colonic diverticula are false diverticula
- Mucosa and submucosa herniate through defect or weakness in muscularis layer and is covered by serosa
- True diverticula are uncommon
- Involves the outpouching of all layers of the intestinal wall (e.g., Meckel diverticulum) (Expert Rev Gastroenterol Hepatol 2018;12:683)
- Diverticulosis can be asymptomatic or symptomatic
- Symptomatic diverticulosis:
- Symptomatic uncomplicated diverticular disease (SUDD)
- Diverticulitis (acute, chronic or recurrent)
- Diverticular hemorrhage
- Segmental colitis associated with diverticulosis (SCAD)
- Diverticulitis: uncomplicated or complicated
- Complicated diverticulitis includes abscess, fistula, perforation, obstruction (Clin Geriatr Med 2021;37:141)
ICD coding
- ICD-10: K57.90 - diverticulosis of intestine, part unspecified, without perforation or abscess without bleeding
Epidemiology
- Highest incidence in Western world
- Prevalence increases with age
- > 75% people above 75 years of age
- < 50 years of age: more common in males
- 50 - 70 years of age: slightly more common in females
- > 70 years of age: significantly increased incidence in females (Expert Rev Gastroenterol Hepatol 2018;12:683)
- Left sided diverticula: more common in Western world
- Right sided diverticula: more common in Asia
- Diverticular bleeding 5 - 15%
- Usual source is right sided diverticula
- Possibly due to thinner wall on right side of colon, widened necks and domes of right sided diverticula
- Diverticulitis: in 4 - 15% of patients with diverticula
Sites
- Sigmoid colon / left colon, most common site (90%)
- Right sided colon (5 - 15%)
- Pancolonic involvement (2%) (Int J Colorectal Dis 2018;33:1299)
Pathophysiology
- Occurs in weaker portions of the colonic wall where vasa recta infiltrate the circular muscle layer
- Abnormal colonic motility → exaggerated segmental contractions → increased intraluminal pressure and separation of colonic lumen into chambers (Expert Rev Gastroenterol Hepatol 2018;12:683)
- Sigmoid colon, segment with smallest diameter and largest intraluminal pressure
- In diverticula, vasa recta are separated from intestinal lumen by a layer of mucosa alone
- Exposed to greater amount of injury and can lead to bleeding
Etiology
- Host of complex factors are implicated
- Altered colonic motility
- Visceral hypersensitivity
- Inflammation
- Genetic susceptibility
- Diet, tobacco use
- Medication
- Gut microbiome imbalances (Clin Geriatr Med 2021;37:141)
- Connective tissue disease, Marfan syndrome, Ehlers-Danlos and autosomal dominant polycystic kidney disease
- Can cause structural changes in intestinal walls
Clinical features
- Usually asymptomatic
- Abdominal pain, cramping, altered bowel functions, painless bleeding
- Resembles irritable bowel syndrome (IBS) (Dig Dis 2012;30:64, Curr Opin Gastroenterol 2019;35:27)
Diagnosis
- Frequently is an incidental finding during colonoscopy
- Symptomatic patients, classic presenting symptoms and confirmation with radiology or via colonoscopy (Mayo Clin Proc 2016;91:1094)
- Colonoscopy or Xray following barium enema (StatPearls: Diverticulosis [Accessed 21 April 2022])
- Colonoscopy should be avoided in acute diverticulitis (Mayo Clin Proc 2016;91:1094)
Laboratory
- Fecal calprotectin measurement
- Calprotectin is a calcium and zinc binding protein; can be considered neutrophil specific
- Marker of gastrointestinal inflammation
- Can help in distinguishing IBS from diverticular disease (Expert Rev Gastroenterol Hepatol 2018;12:791)
- Increased white blood cell (WBC), erythrocyte sedimentation rate (ESR), C reactive protein (CRP) in diverticulitis
Radiology description
- Barium enema: barium filled outpouchings; can look similar to polyps
- Ultrasound: gas filled outpouchings
- CT: diverticula outlined by gas
- Intramural diverticula seen as a tiny focus of gas or contrast within the colonic wall (J Clin Gastroenterol 2004;38:S11)
- CT allows grading of severity and detection of complications (Br J Radiol 2020;93:20200670)
Radiology images
Prognostic factors
- Mortality rate in uncomplicated diverticulitis is negligible (StatPearls: Acute Diverticulitis [Accessed 21 April 2022])
- Complicated diverticulitis requiring surgery may lead to death in ~5% of patients
- Perforation of the bowel with resulting peritonitis, risk of death increases to 20%
Case reports
- 15 year old boy with dysmorphic features and learning disabilities presents with acute onset abdominal pain, nausea and vomiting (ACG Case Rep J 2019;6:1)
- 50 year old man with longstanding history of ulcerative colitis and severe diverticulosis on colonoscopy (Rev Esp Enferm Dig 2021;113:550)
- 68 year old man with lower gastrointestinal bleeding and arteriovenous malformations within jejunal diverticulosis (BMC Surg 2019;19:70)
- 83 year old woman with history of chronic abdominal pain is found to have partial midgut volvulus (Am J Case Rep 2021;22:e933180)
- 88 year old man presents with hematochezia and perianal pain (Cureus 2021;13:e14900)
Treatment
- No rationale for treatment of asymptomatic colonic diverticulosis (Pol Przegl Chir 2015;87:203, United European Gastroenterol J 2014;2:413)
- Symptomatic uncomplicated diverticular disease (SUDD)
- Dietary modifications to increase fiber, use of probiotics has been proposed (Neurogastroenterol Motil 2015;27:305)
- Antibiotics in acute complicated diverticulitis (Gastroenterology 2015;149:1944)
Clinical images
Gross description
- Specimen should be fixed and opened longitudinally for easy sampling of diverticula
- Usually, multiple flask shaped invaginations of colonic wall
- Wall thickening may be present (Surg Endosc 2011;25:2586)
- Narrowing of lumen, shortening of tenia and marked thickening of circular muscle layer may be present (J Clin Gastroenterol 2006;40:S108)
- If it extends deeply, perforation and serosal adhesions may be seen
Gross images
Microscopic (histologic) description
- Mucosa may be normal to markedly abnormal
- Expansion of lamina propria by lymphoplasmacytic infiltrate (more prominent in basal half)
- Lymphoglandular complexes, mucin depletion, focal Paneth cell metaplasia (in chronic cases) may be seen (J Clin Gastroenterol 2004;38:S11)
- Muscularis mucosa extends towards surface between elongated crypts
- Shortening of the affected bowel and hypertrophy of circular muscle layer (myochosis) leads to exaggerated mucosal folds (Am J Surg Pathol 1991;15:871)
- Some cases can show lamina propria fibrosis, crypt elongation on the tips of prominent mucosal folds, like mucosal prolapse syndrome in rectum and anus (J Clin Gastroenterol 2008;42:1137)
- Hemosiderin deposition in submucosa may be seen (StatPearls: Diverticulosis [Accessed 21 April 2022])
- Can mimic inflammatory bowel disease
- Hyperplasia of lymphoid aggregates is one of the earliest signs of diverticulitis
- Cryptitis, crypt abscesses, peridiverticular abscess and fistulas may be superimposed on this background in acute diverticulitis
- Tracking abscesses can spread longitudinally or circumferentially and can cause diverticular colitis
- Persistent localized inflammation can lead to phlegmon which is a thickened, firm segment of bowel wall that can lead to strictures and acute or subacute large bowel obstruction (Best Pract Res Clin Gastroenterol 2002;16:543)
Microscopic (histologic) images
Contributed by Bindu Challa, M.D. and Martha M. Yearsley, M.D.
Videos
Histopathology of diverticular disease
Whole slide image of case of diverticulosis with diverticulitis
Sample pathology report
- Colon, sigmoid, segmental resection:
- Diverticulosis
- Two lymph nodes, negative for tumor
- Colon, sigmoid, partial colectomy:
- Segment of colon with diverticulosis, diverticulitis and abscess formation
- Colon, total colectomy:
- Colonic diverticular disease with perforated diverticulum
Differential diagnosis
- Inflammatory bowel disease:
- Diverticular disease associated chronic active colitis can resemble ulcerative colitis
- Wall thickening, transmural lymphoid aggregates and granulomas can mimic Crohn's disease
- Solitary rectal ulcer syndrome and inflammatory cloacogenic polyp:
- Prominent mucosal folds / prolapsed folds adjacent to diverticula can mimic these conditions
- Angiodysplasia:
- Abnormal, tortuous, thin walled blood vessels in mucosa and submucosa
Additional references
Board review style question #1
Board review style answer #1
D. Sigmoid colon. Diverticulosis occurs most commonly in sigmoid colon in 90% of cases.
Comment Here
Reference: Diverticulosis
Comment Here
Reference: Diverticulosis
Board review style question #2
What is the laboratory test that can help distinguish irritable bowel syndrome from diverticular disease?
- Aspartate transaminase
- Fecal calprotectin
- Fecal lactoferrin
- Gamma glutamyl transferase
Board review style answer #2
B. Fecal calprotectin is a marker of gastrointestinal inflammation. Elevated fecal calprotectin favors diverticular disease and helps in distinguishing it from functional gastrointestinal disorders like irritable bowel syndrome.
Comment Here
Reference: Diverticulosis
Comment Here
Reference: Diverticulosis
Drug induced colitis - overview (pending)
Table of Contents
Definition / generalDefinition / general
[Pending]
Duplication
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- GI duplications are uncommon congenital abnormalities, 80% recognised before age 2; colorectal duplications occur in 7% of cases
- Rare in adults, may present with rectal bleeding (J Clin Pathol 2010;63:1080, World J Gastroenterol 2005;11:5072)
- Partial or complete doubling of a variable length of bowel
- Saccular to long, cystic structures
- Often present in mesentery of normal bowel without communication with lumen
- Associated with complex GU abnormalities
Clinical features
- Cystic (> 80%) or tubular; often asymptomatic and undiagnosed
- Symptomatic colonic duplication is rare in adults; present with abdominal pain and intestinal obstruction; also acute abdomen or acute bleeding, necessitating emergency surgery (BMC Surg 2010;10:19)
Case reports
- 16 month old boy with colonic duplication associated with bladder duplication causing diffuse ascites (Diagn Interv Radiol 2009;15:210)
- 10 year old girl with duplication cyst of ascending colon presenting as an ileal volvulus (Afr J Paediatr Surg 2012;9:237)
- 12 year old girl with Y shaped colonic duplication (Chang Gung Med J 2011;34:43)
- 25 year old man with total colorectal and terminal ileal duplication presenting as intussusception and intestinal obstruction (World J Gastroenterol 2012;18:6338)
- 28 year old man with combined duplication of colon and appendix (World J Gastroenterol 2008;14:641)
- 29 year old woman with large tubular colonic duplication (J Korean Surg Soc 2012;82:190)
- 43 year old woman with colonic duplication mimicking a tumor of pancreas (World J Gastroenterol 2008;14:966)
- Mimicking fistulizing Crohn's colitis (Inflamm Bowel Dis 2011;17:E103)
- Two unusual cases of congenital pouch colon and segmental dilatation of colon (J Indian Assoc Pediatr Surg 2011;16:61)
Treatment
- May not require treatment (Yonsei Med J 2005;46:189)
Clinical images
Gross images
Microscopic (histologic) images
Differential diagnosis
- Enteric cysts: less organized smooth muscle, no nerve plexus
Additional references
Dysplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Dysplasia of colonic epithelium identified in setting of colonic inflammatory bowel disease (IBD), usually in colonic biopsies from surveillance colonoscopies
- Precursor of invasive carcinoma
- Can be endoscopically visible or invisible
- Aim of surveillance is to reduce morbidity and mortality from colorectal carcinoma by identifying dysplasia (or early invasive carcinoma)
Essential features
- Precursor of invasive carcinoma in patients with inflammatory bowel disease
- Dysplasia status should be reported for biopsies from surveillance colonoscopies of patients with inflammatory bowel disease:
- Negative for dysplasia
- Indefinite for dysplasia
- Low grade dysplasia
- High grade dysplasia
- Management of dysplasia identified on colonoscopies is dependent on endoscopic appearance (visible [polypoid or nonpolypoid] or invisible) and resectability
Terminology
- Previously used term dysplasia associated lesion or mass (DALM) and related terms (e.g. adenoma-like or nonadenoma-like DALM) should be avoided due to the historical connotation of a high risk of malignancy (Mod Pathol 2018;31:1180)
- Endoscopic appearance based on standardized terminology as described by the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC) is recommended (Gastroenterology 2015;148:639)
ICD coding
- ICD-10:
- D12.0 - benign neoplasm of cecum
- D12.2 - benign neoplasm of ascending colon
- D12.3 - benign neoplasm of transverse colon
- D12.4 - benign neoplasm of descending colon
- D12.5 - benign neoplasm of sigmoid colon
- D12.6 - benign neoplasm of colon, unspecified
- D12.7 - benign neoplasm of rectosigmoid junction
- D12.8 - benign neoplasm of rectum
Sites
- Colon and rectum in areas of colitis
Pathophysiology
- Cytotoxic effect of inflammation leads to repeated cycles of epithelial wounding and repair
- Postulated selective pressure for mutant cells that can survive the inflammatory insult and rapidly repopulate the damaged mucosa
- Epigenetic and genetic changes accumulate in morphologically nondysplastic colonic mucosa long before development of neoplasia
- TP53 mutations and aneuploidy are early events in colitis associated neoplasia and have been identified in nondysplastic colonic mucosa (Carcinogenesis 2018;39:11, Nat Rev Gastroenterol Hepatol 2017;14:218, Gastroenterology 2009;136:542)
- Accumulation of additional mutations, chromosomal abnormalities and epigenetic changes leads to dysplasia and invasive carcinoma
- In comparison, sporadic colorectal neoplasia tends to have APC mutations early and TP53 mutations later in tumorigenesis
Etiology
- Severe, extensive and longstanding chronic inflammation in the setting of ulcerative colitis is associated with an increased risk of colorectal neoplasia (Nat Rev Gastroenterol Hepatol 2017;14:218)
- Extensive colitis related to Crohn's disease is associated with an increased risk of dysplasia and neoplasia (Gastroenterology 2001;120:820)
Clinical features
- Surveillance colonoscopies typically start at 8 years after onset of inflammatory bowel disease (IBD) (Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649, Am J Gastroenterol 2019;114:384)
- Chromoendoscopy, the application of dye to help visualize lesions, can increase the sensitivity in detecting dysplasia
- Has been endorsed for IBD surveillance (Gastroenterology 2015;148:639, Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649)
- Most dysplasia identified in surveillance colonoscopies are endoscopically visible but some dysplasia is endoscopically invisible, i.e. indistinguishable from macroscopically unremarkable colonic mucosa (Gastrointest Endosc 2007;65:998)
- Endoscopic appearance of dysplasia based on descriptors from the Paris classification recommended by the SCENIC panel and other major societies, although other standardized classifications may be used (Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649, Gastroenterology 2015;148:639, Gastrointest Endosc 2003;58:S3, J Crohns Colitis 2021;15:1089):
- Visible dysplasia
- Polypoid: pedunculated or sessile
- Nonpolypoid: superficial elevated, flat or depressed
- Invisible dysplasia
- Visible dysplasia
- Visible lesions are endoscopically resected or biopsied
- Biopsies adjacent to endoscopically resected lesion may also be taken to ensure complete removal
- Random biopsies may be taken to detect endoscopically invisible dysplasia
- Unconventional dysplasia may be more likely than conventional dysplasia to present as flat or invisible lesions (Histopathology 2021;78:814)
Diagnosis
- Detected with colonoscopies, typically as part of surveillance
- Targeted biopsies, endoscopic resections or polypectomies of visible lesions
- Random biopsies may be taken to detect endoscopically invisible dysplasia
Prognostic factors
- Polypoid dysplasia treated by endoscopic polypectomy:
- Low rate of invasive carcinoma on followup (Clin Gastroenterol Hepatol 2004;2:534, Gastroenterology 1999;117:1295, Clin Gastroenterol Hepatol 2014;12:756)
- Increased rate of dysplasia on followup (Clin Gastroenterol Hepatol 2014;12:756)
- Nonpolypoid dysplasia:
- In general, associated with a high rate of metachronous and synchronous carcinoma (J Crohns Colitis 2017;11:649, Am J Gastroenterol 2015;110:1461, Surg Endosc 2021;35:1534)
- Endoscopic resection can be achieved in subset of cases (Inflamm Bowel Dis 2018;24:1196, Gastrointest Endosc 2018;87:1079, Surg Endosc 2021;35:1534, Endoscopy 2017;49:1237, J Gastroenterol Hepatol 2019;34:1581)
- Invisible high grade dysplasia:
- High risk of synchronous and metachronous invasive carcinoma (Am J Gastroenterol 2015;110:1022)
- Invisible low grade dysplasia:
- Prognosis is controversial
- Some studies report a low rate of progression to carcinoma while others suggest a high rate (Clin Gastroenterol Hepatol 2017;15:665, Gastroenterology 2003;125:1311)
- Indefinite for dysplasia:
- Subset will show bonafide dysplasia on followup (Inflamm Bowel Dis 2010;16:1352)
- Unconventional dysplasia may be more likely than conventional dysplasia to be associated with increased risk of high grade dysplasia or carcinoma on followup, particularly hypermucinous, goblet cell deficient and crypt cell dysplasia variants (Histopathology 2021;78:814)
- Unconventional dysplasia may be more likely to present as flat or invisible lesions compared to conventional dysplasia (Histopathology 2021;78:814)
Case reports
- 19 year old woman with 6 year history of ulcerative colitis with invasive carcinoma and high grade dysplasia (BMJ Case Rep 2013;2013:bcr2013200172)
- 42 year old man with polypoid dysplasia in Crohn's colitis (Can J Gastroenterol 2009;23:477)
- 45 year old man with ileorectal anastomosis, low grade dysplasia and invasive adenocarcinoma (SAGE Open Med Case Rep 2017;5:2050313X17692902)
- 76 year old woman with longstanding ulcerative colitis with low and high grade dysplasia (J Gastroenterol Hepatol 2016;31:1797)
- Dysplasia in 5 patients with ulcerative colitis (Intern Med 2016;55:911)
Treatment
- Depends on endoscopic appearance and resectability
- Polypoid dysplasia:
- Endoscopic polypectomy and followup with continued surveillance
- Nonpolypoid dysplasia:
- Endoscopically resectable:
- Endoscopic mucosal resection or submucosal dissection and followup with continued surveillance
- Not amenable to endoscopic resection:
- Cannot exclude underlying invasive carcinoma
- Should be surgically resected
- Endoscopically resectable:
- Invisible dysplasia:
- Refer for chromoendoscopy, if feasible and not already done, as this may help visualize endoscopically the dysplastic lesion (Gastroenterology 2015;148:639)
- Invisible high grade dysplasia:
- Resection, due to high risk of invasive carcinoma
- Invisible low grade dysplasia:
- Continued surveillance or colectomy; multifocal disease may be indication for surgery (Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649, Gastroenterology 2003;125:1311)
- Indefinite for dysplasia:
- Repeat endoscopy posttreatment to reduce inflammatory background (Gastrointest Endosc 2015;81:1101)
Clinical images
Gross description
- Can be macroscopically (endoscopically) unapparent
- Can be polypoid (pedunculated or sessile) or nonpolypoid (superficially elevated, flat or depressed)
Gross images
Microscopic (histologic) description
- Correlate with endoscopic appearance, as this influences management
- Visible dysplasia (polypoid or nonpolypoid)
- Invisible dysplasia
- Low grade dysplasia:
- Preserved nuclear polarity
- Pseudostratified, crowded, elongated and hyperchromatic nuclei
- Lack of surface maturation, i.e. abnormalities persist to surface
- High grade dysplasia:
- May show complex architecture, such as cribriform glands
- Loss of nuclear polarity
- Nuclear pleomorphism, vesicular nuclei and prominent nucleoli
- Indefinite for dysplasia:
- Reserved for cases when distinction between dysplasia and reactive epithelial atypia cannot be made
- Mucosal erosion or ulceration (precluding assessment of surface maturation) or prominent inflammation may be sources of difficulty
- Features distinguishing IBD associated polypoid dysplasia and sporadic adenomas have been described but are not reliable and have no therapeutic implications
- Include lamina propria inflammation, mixture of benign and dysplastic glands at the surface and dysplasia in stalk of polyp (Hum Pathol 1983;14:931, Am J Surg Pathol 1998;22:275)
- Distinction is also not clinically significant as endoscopic polypectomy suffices either way
- More recently, Gui et al. reported features that can be seen in IBD associated nonpolypoid dysplasia but only rarely seen in polypoid dysplasia or sporadic adenomas (Hum Pathol 2020;100:24):
- Dysplastic epithelium associated with a background of inflammatory polyp or granulation tissue, micropapillary or hobnailing surface epithelial cells, nuclear pleomorphism and disarray, and microvesicular or bubbling cytoplasm
- IBD associated nonconventional dysplasia (i.e. not intestinal type) has been described and is relatively common in IBD patients:
- 45% of IBD patients with colorectal cancer and of those, 46% did not have associated conventional dysplasia as reported by Choi et al. (Mod Pathol 2020;33:933)
- 33% of IBD patients with dysplasia as reported by Lee et al. (Histopathology 2021;78:814)
- 38% of carcinoma related lesions and 41% of nonpolypoid dysplasia compared to 7% of polypoid dysplasia as reported by Gui et al. (Hum Pathol 2020;100:24)
- Nonconventional dysplasia subtypes (Histopathology 2021;78:814, Hum Pathol 2020;100:24, Mod Pathol 2020;33:933):
- Hypermucinous (mucinous) dysplasia:
- Tubulovillous or villous architecture with prominent mucinous differentiation
- Usually mild nuclear atypia, especially towards the surface
- Dysplasia with increased Paneth cell differentiation:
- Tubular architecture with elongated, hyperchromatic nuclei
- Foci of increased Paneth cell differentiation, defined by Choi et al. as involving at least 2 contiguous crypts in 2 different foci and increased relative to background mucosa (Mod Pathol 2020;33:933)
- Goblet cell deficient (eosinophilic) dysplasia:
- Goblet cells are nearly or completely absent
- Eosinophilic cytoplasm
- Serrated dysplasia, which includes:
- Traditional serrated adenoma (TSA)-like
- Sessile serrated lesion (SSL)-like
- Serrated lesion not otherwise specified: does not meet criteria for SSL-like or TSA-like dysplasia
- Dysplasia with terminal epithelial differentiation (differentiated dysplasia) (Hum Pathol 2020;100:24, J Clin Pathol 2020;73:391)
- Nuclei are enlarged (can be mildly so), round to oval, slightly irregular, hyperchromatic and mostly nonstratified
- Nucleoli can be present, inconspicuous and occasionally prominent
- Some authors consider at least a subset of differentiated dysplasia as crypt cell dysplasia (see next heading) (Histopathology 2021;78:814, Hum Pathol 2020;100:24, J Clin Pathol 2020;73:391)
- Crypt cell dysplasia:
- Recently proposed to represent a high risk dysplastic lesion but the diagnosis based on histologic features alone is subject to significant interobserver variability and high rates of nondysplastic diagnoses, particularly indefinite for dysplasia (Histopathology 2021;78:814, Histopathology 2019;75:578)
- Atypia is limited to crypt base and does not involve surface epithelium
- Nuclei are round or oval, mildly enlarged, hyperchromatic and show slightly irregular contours
- Hypermucinous (mucinous) dysplasia:
- Dysplasia can show mixed histologic patterns (including mixed nonconventional and conventional dysplasia)
Microscopic (histologic) images
Virtual slides
Positive stains
- Various immunohistochemical stains have been evaluated to help distinguish dysplasia from nondysplastic epithelium but lack of sensitivity and specificity and limited data limit their use
- p53 and Ki67: increased nuclear staining extending to the surface has been suggested to favor dysplasia over regenerative atypia but lack of specificity limits their diagnostic use (Arch Pathol Lab Med 2013;137:338, Semin Diagn Pathol 2015;32:334)
- CK7: positive staining may be a marker of dysplasia but sensitivity and specificity are limited (Mod Pathol 2014;27:303)
- AMACR: strong cytoplasmic staining reported to favor dysplasia (Semin Diagn Pathol 2015;32:334, Hum Pathol 2009;40:166)
Negative stains
- Loss of SATB2 expression described in a subset of IBD associated dysplasia (J Clin Pathol 2020;73:391)
Molecular / cytogenetics description
- TP53 mutations and aneuploidy occur early in IBD associated neoplasia (Nat Rev Gastroenterol Hepatol 2017;14:218)
- Aneuploidy reported to be more common in nonconventional dysplasia (particularly hypermucinous and goblet cell deficient types) than conventional dysplasia (Histopathology 2021;78:814)
- Aneuploidy frequent with invisible and flat dysplasia (J Pathol Transl Med 2021;55:83)
- Case series of crypt cell atypia or dysplasia reported aneuploidy in all 14 cases (Histopathology 2021;78:814, Histopathology 2019;75:578)
- Conventional and nonconventional dysplasia frequently show p53 immunohistochemical mutant pattern expression and other molecular alterations (e.g. KRAS mutations, MLH1 deficiency, etc.) (J Clin Pathol 2020;73:391)
- TSA-like dysplasia in colectomy specimens more likely to show KRAS mutations and less likely to show BRAF mutations than endoscopically resected TSA-like lesions in IBD patients (Hum Pathol 2020;97:19)
Molecular / cytogenetics images
Sample pathology report
- Presence or absence of dysplasia should be reported for biopsies from surveillance colonoscopies as follows (Hum Pathol 1983;14:931, Gut 2000;47:251):
- Negative for dysplasia
- Indefinite for dysplasia
- Low grade dysplasia
- High grade dysplasia
- Polypoid lesion within colitic mucosa, endoscopically resected:
- Colon (sigmoid), polypectomy:
- Polypoid low grade dysplasia
- Colon (sigmoid), polypectomy:
- Nonpolypoid lesion, endoscopically resected piecemeal:
- Colon (ascending), biopsy:
- High grade dysplasia
- Completeness of excision cannot be determined (specimen fragmented)
- Colon (ascending), biopsy:
- Invisible dysplasia identified on random biopsy:
- Rectum, biopsy:
- Low grade dysplasia
- Rectum, biopsy:
- Note: a comment noting that endoscopically resectable lesions that are completely removed can be managed with continued surveillance rather than colectomy may be helpful
Differential diagnosis
- Reactive epithelial atypia:
- May show mild nuclear stratification and crowding, nuclear enlargement and hyperchromasia and nucleoli
- Surface maturation is present
- Nuclear polarity is preserved
- Inflammatory background may be present
- Nonconventional dysplasia, e.g. increased Paneth cell differentiation or goblet cell deficient variant, can mimic reactive, metaplastic changes (Mod Pathol 2020;33:933)
- Features such as lack of surface maturation and cytoarchitectural atypia help establish a diagnosis of dysplasia
- Sporadic adenoma:
- Occurs in colonic segments outside colitic mucosa
- Not related to IBD
- Otherwise similar epithelial changes as those in (conventional) IBD associated dysplasia
Board review style question #1
Which of the following statements regarding surveillance colonoscopies for patients with inflammatory bowel disease is true?
- Complete endoscopic resection of colitis associated polypoid high grade dysplasia can be followed up with continued surveillance
- Most colorectal dysplasia identified in surveillance colonoscopies is endoscopically invisible
- Nonpolypoid dysplasia is never endoscopically resectable and should therefore prompt colectomy
- Polyps with dysplasia present in mucosa outside of mucosa affected by chronic colitis are not considered sporadic adenomas
Board review style answer #1
A. Complete endoscopic resection of colitis associated polypoid high grade dysplasia can be followed up with continued surveillance.
Comment Here
Reference: Dysplasia
Comment Here
Reference: Dysplasia
Board review style question #2
Which of the following statements regarding dysplasia associated with inflammatory bowel disease is true?
- Early genetic changes present in colitis associated neoplasia are similar to those in sporadic neoplasia
- Indefinite for dysplasia may be diagnosed if the distinction between reactive atypia and dysplasia cannot be established
- Loss of nuclear polarity is a feature of low grade dysplasia
- Only patients with ulcerative colitis and not Crohn's disease have an increased risk of colorectal carcinoma
Board review style answer #2
B. Indefinite for dysplasia may be diagnosed if the distinction between reactive atypia and dysplasia cannot be established
Comment Here
Reference: Dysplasia
Comment Here
Reference: Dysplasia
Ehlers Danlos syndrome
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Additional referencesDefinition / general
- Inherited heterogenous group of connective tissue disorders characterized by abnormal collagen synthesis (eMedicine, Wikipedia)
- Six variants of EDS have been described, based on clinical and molecular features (Am J Med Genet 1998;77:31)
Clinical features
- Clinical symptoms vary by clinical variant, and include skin hyperextensibility and fragility / poor healing; joint hyperextensibility with a propensity to dislocation; eye symptoms with corneal rupture and retinal detachment; kyphoscoliosis and rupture of the colon and large arteries
Vascular type (type IV):
- Autosomal dominant; caused by a mutation in COL3A1 gene (J Nippon Med Sch 2008;75:254)
- Three distinct types of mutations, which affect either the rate of synthesis, the secretion of type III procollagen or result in structurally abnormal type III collagen
- Associated with a reduced median survival of only 48 years
- Neonates have increased incidence of clubfoot and hip dislocation, and rarely have subarachnoid hemorrhage (Am J Clin Pathol 1990;93;579)
- In children, inguinal hernia, pneumothorax and recurrent joint dislocation or subluxation are common; skin is translucent with visible veins and is easily bruised; distinctive facial features are often present, including protruding eyes, a thin nose and lips, sunken cheeks and a small chin
- Blood vessels and intestines are typically rich in type III collagen, and adult patients often present with vascular rupture/dissection or gastrointestinal perforation
- Uterine rupture may occur during pregnancy, particularly at delivery
Case reports
- 9 year old boy with type IV disease (Case #163)
- 17 year old boy with recurrent spontaneous pneumothoraces and cavitary lesion on chest X-ray (Intern Med 2009;48:717)
- Neuromuscular manifestations (J Clin Neuromuscul Dis 2009;11:81)
Treatment
- No specific treatment
- Preventative measures are recommended; some patients may improve with high dose Vitamin C
Microscopic (histologic) description
- Often colonic perforation, with mucosal ulceration and thin bowel wall
- May have segmental absence of muscularis propria, with replacement by cellular, reactive fibroblastic proliferation
Additional references
Elastofibromatous change
Table of Contents
Definition / general | Essential features | Terminology | Sites | Clinical features | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Increase in elastin fibers in colonic submucosa
Essential features
- Uncommon collection of amorphous elastotic material in colon
- Seemingly no clinical significance but can mimic amyloid
Terminology
- Also called elastosis
- Called elastofibroma if forms a polyp
Sites
- Occurs throughout gastrointestinal tract
- In colon, favors left side; can present in rectum
Clinical features
- Patients typically older than 45
- No sex predilection
- May cause nonspecific gastrointestinal symptoms or be asymptomatic
Case reports
- 58 year old woman (Am J Surg Pathol 1992;16:793)
- Elastofibromatous polyp of sigmoid colon (Can J Gastroenterol 2003;17:275)
Gross description
- May appear as a polyp or as a linear white lesion
Microscopic (histologic) description
- Eosinophilic to gray amorphous material, sometimes with fibrous tissue, involving submocusa or muscularis mucosae
- May be centered around blood vessels (Am J Clin Pathol 2004;122:232)
Microscopic (histologic) images
Positive stains
- Elastin stain highlights elastic fibers
Negative stains
Sample pathology report
- Descending colon, polypectomy:
- Colon with elastofibromatous change (see comment)
- Comment: The polyp consists of submucosal amorphous material that is negative on a Congo red special stain and positive on an elastin special stain.
Differential diagnosis
- Amyloidosis:
- Shows birefringence with Congo red stain
- Lifting agent granuloma:
- Can contain inflammatory component
- History of polypectomy at site
Board review style question #1
Board review style answer #1
Endometriosis
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Clinical features | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosis | Sample pathology report | Board review style question #1 | Board review style answer #1Definition / general
- Endometriosis arising within colon
Essential features
- Similar to endometriosis elsewhere: can cause clinical symptoms; histology shows endometrial glands, endometrial stroma and hemosiderin
- Malignant transformation is rare
Epidemiology
- Up to 33% with endometriosis have involvement of large or small intestine
Sites
- Usually rectosigmoid or rectovaginal septum (Med Sci Monit 2000;6:787)
- Can involve any portion of colonic wall, from mucosa to serosa
Clinical features
- Typically incidental but can cause pain, obstruction or diarrhea
- Rarely associated with neoplasms or precancerous changes (Am J Surg Pathol 2000;24:513)
Case reports
- 31 year old patient with schistosomiasis (Fertil Steril 2006;85:1060.e1)
- 35 year old woman with obstruction of sigmoid colon (Ugeskr Laeger 2005;167:3604)
- 42 year old woman with mixed germ cell tumor (Am J Clin Pathol 1982;78:555)
- 48 year old woman with endometrial stroma sarcoma (J Korean Med Sci 2002;17:412)
- 65 year old woman with clear cell carcinoma (J Clin Pathol 2001;54:76)
- With endometrioid adenocarcinoma (Am Surg 2005;71:694)
Treatment
- Pain medication, hormonal therapy; may require surgery if causing obstructive symptoms (Clin Colon Rectal Surg 2010;23:72)
Gross description
- Serosal and subserosal nodules < 5 cm; smooth muscle hypertrophy may cause obstruction
- Gray cut surface with minute areas of hemorrhage; can cause adhesions if subserosal
Gross images
Microscopic (histologic) description
- Endometrial glands and stroma plus hemosiderin in deeper layers of colon; usually surrounded by smooth muscle
- Epithelium may have inflammation and ulcers, simulating inflammatory bowel disease or solitary rectal ulcer syndrome
- Often infiltrates along nerves of bowel wall (Hum Reprod 2004;19:996)
- Mucosa usually normal; bowel wall may be fibrotic; cilia help distinguish endometrial glands from colonic glands
Microscopic (histologic) images
Positive stains
Differential diagnosis
Sample pathology report
- Ascending colon, resection:
- Colon with multiple mural and serosal foci of endometriosis
- Negative for malignancy.
- Margins of resection unremarkable.
- Two benign lymph nodes.
Board review style question #1
Board review style answer #1
Eosinophilic gastroenterocolitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Eosinophilic gastrointestinal disorders (EGIDs) consist of eosinophilic esophagitis, eosinophilic gastritis, eosinophilic gastroenteritis, eosinophilic enteritis and eosinophilic colitis (J Allergy Clin Immunol 2004;113:11)
- However, eosinophilic esophagitis differs from eosinophilic gastroenterocolitis (EGEC) in all aspects and should be considered a different entity
- Eosinophilic gastroenterocolitis is an inflammatory disorder characterized by prominent eosinophilic infiltration of the gastrointestinal tract (stomach, duodenum, small intestine or large intestine) with no known causes of tissue eosinophilia
Essential features
- Inflammatory process characterized by the abnormal eosinophilic infiltration of different segments of the gastrointestinal tract in the absence of a known cause (drug reaction, parasitic infection, malignancy, etc.)
- Can occur throughout the gastrointestinal tract; most reported in the stomach, followed by the small intestine and colon
- Its diagnosis requires the presence of:
- Gastrointestinal symptoms
- Demonstration of gastrointestinal eosinophilia by biopsy
- Exclusion of other known causes of tissue eosinophilia
Terminology
- Allergic gastroenteritis
- Eosinophilic gastroenterocolitis
- Eosinophilic gastrointestinal disorder (includes eosinophilic esophagitis)
ICD coding
Epidemiology
- Rare disease
- Overall prevalence of eosinophilic gastroenteritis in the U.S. is 5.1/100,000 (Clin Gastroenterol Hepatol 2017;15:1733)
- Overall prevalence of eosinophilic colitis in the U.S. is 2.1/100,000 (Clin Gastroenterol Hepatol 2017;15:1733)
- In other parts of the world, such as Japan, the prevalence may be up to 5.5 times higher when compared to the U.S. (J Gastroenterol 2013;48:333)
- Disease of both adults and children
- More prevalent in the younger population (18 years or younger) (J Pediatr Gastroenterol Nutr 2016;62:36, Clin Gastroenterol Hepatol 2017;15:1733)
- However, eosinophilic colitis specifically is more prevalent in adults (> 18 years of age) (Clin Gastroenterol Hepatol 2017;15:1733)
- Predominantly affects females (J Pediatr Gastroenterol Nutr 2016;62:36, Dig Dis Sci 2016;61:2585)
- Diagnosis is commonly described in the Caucasian population but reported occurrence in all races and ethnic backgrounds
- Up to 70% of patients have personal or family history of atopic disorders (asthma, eczema or hay fever) (Paediatr Drugs 2002;4:563)
Sites
- Throughout the gastrointestinal tract: most common stomach, followed by small intestine and colon (Scott Med J 1990;35:163)
- 2 or more gastrointestinal sites of involvement are commonly seen
- Rare cases of bile duct involvement have been reported (Gut 1990;31:54)
Pathophysiology
- Although not fully understood, it likely has genetic and environmental factors
- ~10 - 70% of patients with immediate family member history (J Pediatr 2002;141:576)
- Leading key players are mast cells and eosinophils
- Immediate phase of reaction is dependent on the mast cell activation through the high affinity IgE receptor
- Late phase (non-IgE mediated) of reaction involves Th2 lymphocyte and monocytes producing cytokines and chemokines such as eotaxin 1 and interleukin 5, inducing eosinophil accumulation
- Eosinophils, when recruited in the intestinal epithelium, become cytotoxic by producing inflammatory mediators, such as eosinophil peroxidase, eosinophil derived neurotoxin, eosinophil cationic protein and major basic protein, causing destruction of the gastrointestinal epithelium and organ dysfunction (Baillieres Clin Gastroenterol 1996;10:443, N Engl J Med 1990;323:645, Clin Rev Allergy Immunol 2016;50:175)
- Eosinophil's 4 major cationic proteins (Nature 1986;321:613):
- Eosinophil peroxidase (EPO): cytotoxic effect on the epithelium, by the generation of toxic hydrogen peroxide and halide acid leading to tissue damage (J Allergy Clin Immunol 2004;113:11)
- Eosinophil derived neurotoxin (EDN): ribonuclease activity
- Eosinophil cationic protein (ECP): cytotoxic effect on the epithelium, by ion nonselective pore insertion (site of entry to other toxic molecules); also has ribonuclease activity
- Major basic protein (MBP): cytotoxic effect on the epithelium and also plays a role in the degranulation of mast cells and basophils
Etiology
- Cause is unknown
- Some research suggests allergic or immune dysregulation as many patients with eosinophilic gastrointestinal disorders (EGIDs) are also atopic (Adv Immunol 2001;78:291, Pediatr Neonatol 2011;52:272, Paediatr Perinat Epidemiol 2007;21:2)
- EGIDs can be categorized into primary versus secondary (Clin Rev Allergy Immunol 2016;50:175):
- Primary EGIDs are defined as an inflammatory process characterized by the abnormal eosinophilic infiltration of different segments of the gastrointestinal tract in the absence of a known cause (drug reaction, parasitic infection, malignancy, etc.) (J Allergy Clin Immunol 2004;113:11)
- Secondary EGIDs are due to drug reaction, parasitic infection, bacterial infection, hypereosinophilic syndrome, Churg-Strauss syndrome, celiac disease, inflammatory bowel disease, polyarteritis nodosa and other connective disorders
Clinical features
- Nondisease specific gastrointestinal symptoms:
- Abdominal pain
- Vomiting, nausea, diarrhea
- Malnutrition
- Poor growth and weight loss
- Some symptoms may be more frequently observed, depending on the depth of gastrointestinal tract wall involvement (Klein classification) (J Allergy Clin Immunol 2004;113:11, Medicine (Baltimore) 1970;49:299):
- Mucosal pattern: abdominal pain, nausea, vomiting, diarrhea, bleeding, malabsorption, weight loss
- Muscular pattern: pyloric or intestinal obstruction
- Serosal pattern: ascites with eosinophilia
- Often have a prior or concurrent history of atopy (asthma, allergic rhinitis and elevated serum IgE) (Pediatr Neonatol 2011;52:272)
- Some may present with gross or occult hematochezia, which may lead to significant anemia (Pediatr Neonatol 2011;52:272)
- Rare reported cases of EGIDs associated with pancreatitis and duodenal ulcer or perforation (World J Gastroenterol 2009;15:2156, Endoscopy 2011;43:E358)
Diagnosis
- Combination of clinical history, physical exam, endoscopy, radiology and laboratory studies are required as the diagnosis requires the presence of (Gut 1990;31:54):
- Gastrointestinal symptoms
- Demonstration of gastrointestinal eosinophilia by biopsy
- Exclusion of other known causes of tissue eosinophilia
- Endoscopy
- Spectrum of findings from normal appearing to mucosal erythema, edema, friability, granularity, ulceration, thickening of folds, mucosal nodules and whitish specks (Gastrointest Endosc 2002;56:762, Clin Rev Allergy Immunol 2016;50:175)
Laboratory
- No single laboratory test or procedure is diagnostic for EGIDs
- Maintaining a high index of suspicion is essential
- Although not specific, the following laboratory tests / findings may support the diagnosis (Clin Rev Allergy Immunol 2016;50:175):
- Allergy testing to assess allergies that may trigger the onset of symptoms; its utility remains controversial (J Gastroenterol Hepatol 2013;28:1306)
- Peripheral blood eosinophilia (seen in 20 - 80% of cases)
- Elevated erythrocyte sedimentation rate
- Iron deficiency anemia
- Elevated IgE levels
- Low serum albumin
- Fecal levels of eosinophilic cationic protein
- Serum eosinophilic cationic protein and eosinophil derived neurotoxin (Scand J Gastroenterol 2011;46:1074)
- Elevated 2 macroglobulin level (Scand J Gastroenterol 2011;46:1074)
- Stool examination (to rule out parasites)
Radiology description
- Its usefulness is limited
- Ultrasound:
- To detect the presence of bowel wall thickening, ascites or peritoneal nodules
- Reported use in follow up setting and determination of response to treatment (Ultraschall Med 2011;32:E57)
- Computerized tomography:
- May demonstrate nodular, irregular folds and thickening of the stomach and small intestines (J Comput Assist Tomogr 1999;23:417)
- Barium contrast:
- Identification of variable degree of stenosis, mucosal irregularity and thickened mucosal folds (J Clin Pathol 1986;39:1)
- Tc-99m hexamethyl propylene amine oxime (HMPAO) white blood cell scintigraphy:
- Reported uptake in areas of active eosinophilic inflammation
- Useful to determine the extent of disease and assessment of the treatment response (Clin Nucl Med 1997;22:536, Ann Nucl Med 2003;17:601)
- Ultrasound:
Radiology images
Prognostic factors
- Unknown due to the rarity of the disease but observed to be dependent on response to treatment and Klein classification of disease (Clin Gastroenterol Hepatol 2011;9:950)
- Patients have 3 different disease courses (Clin Gastroenterol Hepatol 2011;9:950):
- Single flare: more associated with serosal pattern
- Recurring course: more related to muscular pattern
- Continuous course: more associated with mucosal pattern
Case reports
- 9 year old boy with fever, melena and hematemesis (Turk Pediatri Ars 2020;55:299)
- 14 year old girl with continuous epigastralgia, vomiting and loss of appetite (Allergol Int 2017;66:621)
- 15 year old boy with a duodenal ulcer (Intern Med 2020;59:2249)
- 17 year old boy with nausea and melena for 3 - 4 days (Turk Pediatri Ars 2020;55:299)
- 28 year old man with vague abdominal pain, unintentional weight loss and progressive ascites (Am J Case Rep 2019;20:189)
- 48 year old woman with recurrent eosinophilic peritonitis (Cureus 2020;12:e9422)
- 51 year old woman with eosinophilic gastroenteritis presenting as small bowel obstruction (World J Gastroenterol 2007;13:1758)
- 54 year old man with HIV with recurrent presentations for colitis since initiating emtricitabine / tenofovir (Cureus 2018;10:e3498)
- 66 year old man with a 15 day history of persistent upper abdominal pain and distension (Clin Case Rep 2020;8:2843)
Treatment
- No established standard treatment guidelines are available due to the lack of extensive prospective studies; current treatments are based on case reports and case series
- Treatments are empiric and based on the severity of the clinical manifestation
- Avoidance of allergen or trigger
- Common issue: recurrence of symptoms with the reintroduction of the allergen, such as specific food
- Elemental diet (J Pediatr Gastroenterol Nutr 1996;23:81)
- Anti-inflammatory medication
- Systemic and topical steroids are the mainstay of therapy (Curr Allergy Asthma Rep 2005;5:259)
- Reserved for severe disease presentation or failed dietary restriction
- Serosal EGIDS often have a better response to steroids (Dig Dis Sci 2003;48:1013)
- Other steroid sparing treatment modalities: mast cell stabilizers (cromolyn sodium), mast cell secretion inhibitor (ketotifen), leukotriene receptor antagonist (montelukast), selective Th2, cytokine IL4 and IL5 inhibitors (subplatast tosilate)
- Other specific pathway targeting treatment options: anti-IgE monoclonal antibody (omalizumab), anti-IL5 antibodies (reslizumab and mepolizumab) and anti tumor necrosis factor alpha (infliximab)
- Fecal microbiota transplantation (World J Gastroenterol 2014;20:16368)
- Avoidance of allergen or trigger
Clinical images
Gross description
- Nonspecific findings on the mucosal surface, including thickened mucosa, erythema, erosion, ulceration, white plaques, edema, telangiectasia or submucosal nodules
Microscopic (histologic) description
- Diagnosis requires confirmation by histopathological examination of gastric, small intestinal or large intestinal biopsies (Adv Anat Pathol 2011;18:335)
- Distribution of eosinophils may be patchy (obtaining multiple biopsy samples is helpful)
- Eosinophils are seen in the entire gastrointestinal tract except in the esophagus in a normal physiologic state; they are an essential part of the innate immune system of the digestive tract
- Ranges of normal numbers of eosinophils in different sites of the GI tract and different individuals vary significantly (J Allergy Clin Immunol 2004;113:11, Mod Pathol 1996;9:110)
- Highest concentration of eosinophils found in the cecum and appendix (up to 30/high power field) (Mod Pathol 1996;9:110, J Gastroenterol 2008;43:741)
- Lack of consensus on histological features of EGEC due to the relative rarity of the disease and variation of the numbers of eosinophils but it is characterized by prominent eosinophilic infiltration of the wall (most commonly affected sites: stomach and small intestine) (Case Rep Gastroenterol 2013;7:293)
- No established cutoff for the number of eosinophils/high power field to diagnose EGECs
- Pathologists should comment on the findings if eosinophils are more than expected
- Recently suggested cutoff numbers are shown below but they are not formal diagnostic criteria (Gastroenterol Clin North Am 2014;43:257):
- Stomach: ≥ 30 eosinophils/high power field in 5 high power fields
- Duodenum: > 52 eosinophils/high power field
- Ileum: > 56/high power field in the ileum
- Right colon: > 100/high power field
- Transverse and descending colon: > 84/high power field
- Rectosigmoid colon: > 64/high power field
- Distribution of eosinophils is also an essential clue for EGEC irrespective of the eosinophil counts
- Presence of eosinophils within the crypts (intraepithelial eosinophils and eosinophilic crypt abscesses), clusters of eosinophils in lamina propria and eosinophils in muscularis mucosae are abnormal
- In resection specimens, transmural eosinophilic infiltration may be present
- May accompany other inflammatory cells, especially mast cells
- Other nonspecific changes include edema, mucin depletion, erosion, reactive epithelial changes (foveolar hyperplasia and villous atrophy) and mild architectural distortion
- Areas of increased eosinophilic inflammation may show extracellular deposition of eosinophil granule constituents (Gastroenterology 1992;103:137)
- Klein et al. classified the disease based on the location of the eosinophilic infiltration in the different layers of the intestinal wall (depth of eosinophilic infiltration) into mucosal, muscular and subserosal patterns of disease (Medicine (Baltimore) 1970;49:299)
- Mucosal:
- Most commonly reported; 57 - 100% of cases (Gut 1990;31:54, Clin Gastroenterol Hepatol 2010;8:669)
- Infiltration of the mucosa without the involvement of the muscular or serosal layers
- Muscular:
- Second most commonly reported
- Infiltration of eosinophils predominantly in the muscle layer
- Causes wall thickening, which may lead to gastric outlet obstruction, intestinal obstruction or intussusception without eosinophilic ascites (World J Gastroenterol 2007;13:1758, Am Surg 1997;63:741)
- Subserosal:
- Most unusual form; estimated prevalence of up to 9% in Japan and 13% in the U.S. (Intern Med 1996;35:779)
- Infiltration of eosinophils in the serosa layer
- Hallmark of this type is ascitic fluid with eosinophilia
- Often accompanying peripheral eosinophilia, peritonitis (Medicine (Baltimore) 1970;49:299)
- May present with isolated abdominal ascites or ascites along with symptoms more characteristic of mucosal or muscular EGIDSs
- Chang et al. observed muscular and serosal types of having concomitant mucosal eosinophilic gastroenteritis, suggesting disease progression from mucosal to serosal (Clin Gastroenterol Hepatol 2010;8:669)
- Mucosal:
Microscopic (histologic) images
Contributed by Byoung Uk Park, M.D. and Lizhi Zhang, M.D.
Positive stains
- Major basic protein (MBP) (Allergy 2000;55:985)
Sample pathology report
- Small intestine and stomach, biopsy:
- Marked eosinophilic infiltration identified in both small bowel and gastric mucosa (see comment)
- Comment: The biopsy shows diffuse eosinophilic infiltrate (> 50/high power field) involving the mucosa and submucosa with focal intraepithelial eosinophils. No apparent microorganisms, neutrophils, vasculitis or granulomas are identified. In the appropriate clinical setting, the findings may be indicative of eosinophilic gastroenteritis. Clinical and pathologic correlation and further workup are recommended to rule out secondary causes of the observed eosinophilia.
Differential diagnosis
- Parasitic or bacterial infections:
- Enterobius (pinworms), Ancylostoma (hookworms), Ascaris, Anisakis, Eustoma, Trichuris and Schistosoma
- Toxocara canis can cause eosinophilic ascites
- Should be excluded
- H. pylori infection:
- Biopsy and silver stain to rule out H. pylori infection
- Reported cases of successful treatment of EGIDS with the eradication of H. pylori (Gut 2005;54:1822, J Clin Gastroenterol 2008;42:1063)
- Inflammatory bowel disease (Mayo Clin Proc 1997;72:117):
- May present with peripheral and intestinal eosinophilia
- Lacks the florid eosinophilia
- Hypereosinophilic syndrome:
- Rare and idiopathic condition
- Persistence (> 6 month) of marked peripheral eosinophilia (often exceeding > 1,500 eosinophils/microliter)
- Along with the presence of organ damage or dysfunction related to eosinophilic infiltration and release of mediators
- Most common presenting manifestation: dermatologic and pulmonary (J Allergy Clin Immunol 2009;124:1319)
- Vasculitis disorders (Churg-Strauss syndrome and polyarteritis nodosa):
- Eosinophilic infiltrate of the small vessels
- Peripheral eosinophilia
- Elevated markers of inflammation and autoantibodies
- Connective tissue disorders (scleroderma and dermatomyositis):
- Episodic peripheral eosinophilia and a band-like eosinophil and mast cell infiltrate of the lamina propria
- Drug hypersensitivity:
- Gastrointestinal eosinophilia has been associated with a long list of medications, such as carbamazepine, rifampicin, naproxen, NSAIDs, interferon, azathioprine, enalapril and gold compounds
- Mastocytosis and Langerhans histiocytosis:
- Gastrointestinal eosinophilia is usually seen in GI involvement by mastocytosis and Langerhans histiocytosis
- Besides eosinophils, prominent mononuclear cell infiltration with a nodular or tumefactive growth pattern is present
- Mononuclear cells may show characteristic morphologic features of either mast cells or Langerhans cells which immunohistochemical stains can confirm
Board review style question #1
Board review style answer #1
B. Eosinophils in muscularis mucosae. Prominent eosinophilic infiltration is a hallmark of eosinophilic gastroenterocolitis (EGEC), which is sensitive but not specific for diagnosing EGEC. The cutoff numbers of eosinophilic counts have not been well established. On the other hand, the abnormal distribution of eosinophils, such as muscularis mucosae, in this case, is an essential clue for EGEC.
Comment Here
Reference: Eosinophilic gastroenterocolitis
Comment Here
Reference: Eosinophilic gastroenterocolitis
Board review style question #2
Which of the following statements are true regarding the histologic finding of the above gastric biopsy?
- Although there are increased eosinophils in the lamina propria, the eosinophil count does not meet the diagnostic criteria for eosinophilic gastritis
- Diagnosis of eosinophilic gastritis should be considered but exclusion of other known causes of tissue eosinophilia is needed
- Increased eosinophils in the lamina propria are diagnostic for eosinophilic gastritis
- Observed changes are diagnostic for H. pylori infection
- Similar finding in other sites of the GI tract in this patient is unlikely
Board review style answer #2
B. Diagnosis of eosinophilic gastritis should be considered but exclusion of other known causes of tissue eosinophilia is needed. The picture shows eosinophilia in gastric mucosa, which can be due to various causes, such as eosinophilic gastroenteritis, drug effect, parasites, hypereosinophilic syndrome, etc. The cutoff numbers of eosinophilic counts have not been well established for the diagnosis of eosinophilic gastroenterocolitis. Therefore, it is a diagnosis of exclusion and other known causes of tissue eosinophilia must be ruled out with clinical correlation.
Comment Here
Reference: Eosinophilic gastroenterocolitis
Comment Here
Reference: Eosinophilic gastroenterocolitis
Escherichia coli
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Enteroaggregative E. coli (EAEC) | Shiga toxin producing E. coli (STEC) / enterohemorrhagic E. coli (EHEC) | Enteroinvasive E. coli (EIEC) | Enteropathogenic E. coli (EPEC) | Enterotoxigenic E. coli (ETEC) | Diffusely adherent E. coli (DEAC) | Adherent invasive E. coli (AIEC) | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Escherichia coli (E. coli) is a gram negative, facultative anaerobic, coliform, rod shaped bacterium
- It is a part of the normal flora of the intestine of humans and many warm blooded animals; it provides benefits such as production of vitamin K2 and a stable environment where more beneficial bacteria can prosper (Elife 2015;4:e05826)
- Most cases are transmitted through contaminated food and water
Essential features
- Most cases cause self limiting disease
- Treatment is supportive
Terminology
- Enteroaggregative E. coli (EAEC)
- Shiga toxin producing E. coli (STEC) / enterohemorrhagic E.coli (EHEC)
- Enteroinvasive E. coli (EIEC)
- Enteropathogenic E. coli (EPEC)
- Enterotoxigenic E. coli (ETEC)
- Diffusely adherent E. coli (DEAC)
- Adherent invasive E. coli (AIEC)
ICD coding
- ICD-10:
- B96.20 - unspecified Escherichia coli (E. coli) as the cause of diseases classified elsewhere, Escherichia coli (E. coli), NOS
- B96.21 - Shiga toxin producing Escherichia coli (E. coli) (STEC) O157 as the cause of diseases classified elsewhere
- B96.22 - other specified Shiga toxin producing Escherichia coli (E. coli) (STEC) as the cause of diseases classified elsewhere
- B96.23 - unspecified Shiga toxin producing Escherichia coli (E. coli) (STEC) as the cause of diseases classified elsewhere
- B96.29 - other Escherichia coli (E. coli) as the cause of diseases classified elsewhere, non-Shiga toxin producing E. coli
Enteroaggregative E. coli (EAEC)
- Pattern of adherence characterized by self agglutination, called aggregative adherence, in a stacking pattern
- Affects children (usually < age 2 years) and adults, immunocompromised and HIV+ (in developing and developed countries)
- Also immunocompetent and those without foreign travel
- EAEC adheres to intestinal mucosa and forms a mucoid biofilm
- Common cause of persistent watery diarrhea > 14 days (Clin Infect Dis 2006;43:402, J Infect Dis 1989;159:1061)
- Also fever, abdominal pain
- Laboratory: lactose fermenter, indole positive, citrate negative
- References: J Med Microbiol 2007;56:4, Lancet 1991;337:262, Koneman: Koneman's Color Atlas and Textbook of Diagnostic Microbiology, 6th Edition, 2005
Shiga toxin producing E. coli (STEC) / enterohemorrhagic E. coli (EHEC)
- Major foodborne pathogen (ground beef and salami, unpasteurized milk and juice, sprouts, lettuce)
- Also due to contact with cattle, swimming in contaminated waters
- Most common E. coli strain producing disease in the U.S.
- Serotype O157:H7 is most commonly isolated strain, infectious dose is 100 bacterial cells or less
- Due to verotoxin, Shiga-like toxin in O157:H7 strains
- Symptoms: abrupt onset of abdominal pain, vomiting and bloody diarrhea 3 - 4 days following ingestion with absence of fever
- Complications: up to 10% of patients, especially children under 15 years of age, may develop hemolytic uremic syndrome (HUS), usually 5 - 10 days after onset of diarrhea with up to 5% mortality rate
- Diagnosis: PCR, enzyme immunoassay or culture on sorbitol MacConkey agar (Mol Cell Probes 2006;20:31, J Clin Microbiol 2004;42:1652)
- Laboratory: lactose fermenter, indole positive, citrate negative, colorless growth (sorbitol nonfermenter) on sorbitol MacConkey
- Treatment
- Supportive therapy for HUS (platelets contraindicated), possibly with dialysis or plasmapheresis
- Avoid usage of antiemetic or antiperistaltic agents
- Antibiotics are not beneficial
- Microscopic description
- Necrosis of superficial mucosa, acute inflammation
- Necrotic foci may appear as pseudomembranous inflammation (usually not endoscopically visible)
- Cryptitis and crypt abscesses
- Hemorrhage and edema in lamina propria, capillary thrombosis
- References: Iowa State University: Enterohemorrhagic Escherichia coli and Other E. coli Causing Hemolytic Uremic Syndrome [Accessed 22 February 2023], Infect Immun 2007;75:4199, UpToDate: Shiga Toxin-Producing Escherichia coli - Clinical Manifestations, Diagnosis, and Treatment [Accessed 22 February 2023], Stanford University: Enterohemorrhagic E coli Colitis [Accessed 22 February 2023]
Enteroinvasive E. coli (EIEC)
- Causes dysentery-like syndrome with fever
- Due to contaminated food, water or person to person spread
- Endemic to developing nations with rare outbreaks in industrialized countries
- Reported outbreaks in the U.S. primarily affect children under 5 years of age (Ryan: Sherris Medical Microbiology, 5th Edition, 2010, Ryan: Sherris Medical Microbiology, 4th Edition, 2003)
- Infectious dose for healthy adults is 106
- Similar molecular features to Shigella, due to virulence plasmid that encodes determinants (specific adhesion, outer membrane protein) for entry into epithelial cells and dissemination from cell to cell (Infect Immun 2004;72:5080)
- Invades and destroys colonic epithelium
- Laboratory: lactose fermenter and nonfermenters; lysine decarboxilase negative; nonmotile
- Treatment: generally self limited; fluid replacement as necessary
- Trimethoprim / sulfamethoxazole (TMP SMX) or quinolones may reduce duration of diarrhea
- Micro description: bacterial invasion and destruction of colonic mucosa resulting in inflammation and ulceration
- References: Clin Microbiol Rev 1998;11:142, Am J Trop Med Hyg 2007;76:528, Todar: Todar's Online Textbook of Bacteriology, 2020
Enteropathogenic E. coli (EPEC)
- Some types of EPEC are known as diffusely adherent E. coli (DAEC)
- Major cause of infant diarrhea in developing countries
- Due to contaminated water and meat products
- Virulence mechanism is unrelated to excretion of typical E. coli enterotoxins; diarrhea and symptoms are caused by invasion of host cells (attachment and effacement) rather than by production of toxins
- Has pathogenicity island that encodes proteins which modulate the actin microtubule and intermediate filament networks to allow intimate attachment of bacteria to plasma membrane of infected enterocytes, forming attachment and effacing lesions (J Bacteriol 2006;188:3110)
- Also has large plasmid that contains a cluster of genes encoding bundle forming pili
- Infectious dose is 106 organisms
- Atypical cases lack bundle forming pili and are associated with prolonged diarrhea (Emerg Infect Dis 2006;12:597)
- Clinical features:
- Infantile diarrhea may lead to dehydration, electrolyte imbalance or death
- Watery or bloody diarrhea
- Up to 50% mortality rates in developing countries
- Treatment: self limited; antibiotics may not improve disease and may increase the risk of developing hemolytic uremic syndrome
- Micro description: effacement of brush border microvilli of enterocytes; no bacterial invasion but surface adherent organisms may be identifiable
- References: Food and Drug Administration: Bad Bug Book - Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd Edition, 2012, Todar: Todar's Online Textbook of Bacteriology, 2020, Microbiology (Reading) 2004;150:527, Emerg Infect Dis 2002;8:508
Enterotoxigenic E. coli (ETEC)
- Annually causes 200 million diarrhea episodes and 170,000 to 380,000 deaths, due to contaminated food and water
- Most common cause of traveler's diarrhea (CDC: Enterotoxigenic E. coli (ETEC) [Accessed 15 March 2023])
- Also a leading cause of infant diarrhea in developing countries
- Emerging cause of diarrhea in the U.S. (Rev Environ Health 2008;23:135)
- Due to enterotoxins (heat labile [LT] and heat stable [ST]) produced by noninvasive bacteria that adhere to small intestine and produce cholera-like watery diarrhea
- Strains that can produce both enterotoxins cause more severe diarrhea
- Causes profuse watery diarrhea, abdominal cramping, fever, nausea
- Treatment: prophylaxis with rifaximin, careful food / beverage selection, rehydration
- Drugs vary based on current patterns of antimicrobial resistance; vaccines are currently being tested (Drugs 2006;66:303, Clin Vaccine Immunol 2012;19:1921)
- Micro description: often does not cause inflammation due to its noninvasive nature
- References: Clin Microbiol Rev 2006;19:583, Clin Microbiol Rev 2005;18:465, CDC: Enterotoxigenic E. coli (ETEC) [Accessed 22 February 2023]
Diffusely adherent E. coli (DEAC)
- Extremely heterogeneous group of potentially pathogenic E. coli, which are characterized by their diffuse adherence to epithelial cells
- DAEC isolates are detected not only in humans commonly in children of between 2 and 5 years of age but also in various groups of animals (dogs, calves, cattle, poultry, pigs)
- No diarrheal disease has been reported in adults but the latest data indicates a possible contribution of DAEC strains in the process of the development of colorectal cancer and Crohn's disease (CD) (Clin Microbiol Rev 2014;27:823, Advancements of Microbiology 2019;58:143)
- On the basis of adhesins, DAEC strains have been divided into 2 basic groups: typical DAEC (which binds to the hDAF receptor) and atypical DAEC (which does not bind the hDAF factor)
- DAEC are omitted in the routine diagnosis of infections of the digestive tract due to the huge genotypic diversity of these strains
- Optimum method for diagnosis of DEAC is still under active research; the lack of commonly available diagnostic methods hinders the correct identification of infections caused by DAEC and therefore, the possibility of treatment (BMC Microbiol 2013;13:22, Front Cell Infect Microbiol 2020;10:572951)
Adherent invasive E. coli (AIEC)
- Adherent invasive Escherichia coli (AIEC) pathobiont bacteria have the ability to adhere to and invade intestinal epithelial cells (IECs) as well as to survive and replicate within macrophages
- AIEC bacteria have been linked in the etiology of CD; however, active genetic research is ongoing in order to better establish this link and develop possible treatment modalities to prevent and limit AIEC colonization in CD patients (Int J Mol Sci 2020;21:3734)
Epidemiology
- In the U.S., most outbreaks have been reported in Minnesota, followed by Washington
- Route of transmission is foodborne; mainly by undercooked ground beef in EHEC (Emerg Infect Dis 2005;11:603)
- ETEC organisms are the principal cause of traveler's diarrhea and spread via contaminated food or water
- EIEC / EAEC organisms are bacteriologically similar to Shigella and are transmitted via food, water or by person to person contact
Sites
- Colon and rectum
Pathophysiology
- Depends on the strain of pathogenic E. coli
- Enteroaggregative E. coli
- EAEC adhere to epithelial cells
- EAEC attach to enterocytes via adherence fimbriae and are aided by dispersin, a bacterial surface protein that neutralizes the negative surface charge of lipopolysaccharide
- They also produce enterotoxins similar to Shigella enterotoxin and ETEC ST toxin (Curr Opin Gastroenterol 2005;21:4)
- Enterohemorrhagic E. coli / Shiga toxin producing E. coli (STEC)
- EHEC are classified as E. coli O157:H7 and non-O157:H7 serotypes
- Both serotypes produce Shiga-like toxins
- Hemolytic uremic syndrome (HUS) is caused by Shiga-like toxin which is absorbed by inflamed gastrointestinal mucosa into the circulation, where it alters endothelial cell function leading to platelet activation and aggregation
- Children and the elderly are at highest risk (Med Clin North Am 2013;97:681)
- Enteroinvasive E. coli
- EIEC do not produce toxins, they invade epithelial cells and cause acute self limited colitis (J Vet Sci 2022;23:e28)
- Enterotoxigenic E. coli
- ETEC produce LT toxin and ST toxin
- Both induce chloride and water secretion while inhibiting intestinal fluid absorption
- LT toxin activates adenylate cyclase and stimulates chloride secretion and simultaneously inhibits absorption
- ST toxins bind to guanylate cyclase and stimulate chloride secretion (Gut Microbes 2022;14:2055943)
- Enteroaggregative E. coli
Etiology
- Contaminated food and water
Clinical features
- EAEC organisms cause nonbloody diarrhea that may be prolonged in AIDS patients (Am J Trop Med Hyg 2010;83:158)
- EHEC causes symptoms similar to S. dysenteriae
- Causes large outbreaks of bloody diarrhea and HUS
- HUS includes microangiopathic hemolytic anemia and thrombocytopenia but no neurologic symptoms and no acute renal failure (Med Clin North Am 2013;97:681)
- EIEC is common among young children in developing countries
- Also causes traveler's diarrhea and diarrhea in both children and adults (J Vet Sci 2022;23:e28)
- ETEC causes traveler's diarrhea commonly in underdeveloped regions and children younger than 2 years of age are particularly susceptible
- Secretory, noninflammatory diarrhea, vomiting, dehydration and in severe cases, shock (Gut Microbes 2022;14:2055943)
Diagnosis
- Typically made by clinical findings and history of ingestion of contaminated food like undercooked meat
Laboratory
- Stool culture on sorbitol MacConkey agar (SMAC) or the variant cefixime potassium tellurite sorbitol MacConkey agar (CT SMAC)
- Colonies appear clear on SMAC due to their inability to ferment sorbitol, while sorbitol fermenting colonies of E. coli serotypes appear red
- E. coli DNA extraction method: DNA probes plus PCR techniques for detecting verocytotoxin (MMWR Recomm Rep 2009;58:1, StatPearls: Escherichia Coli [Accessed 1 June 2023])
Radiology description
- CT is usually preferred modality for radiological assessment (AJR Am J Roentgenol 2001;177:619)
- Shows severe diffuse colonic wall thickening, often with a target sign and pericolic stranding
- Involvement of bowel can be segmental or diffuse
Radiology images
Prognostic factors
- Disease is usually self limiting and patients recover on supportive therapy
Case reports
- 32 year old woman with infection due to Shiga toxin producing enterohemorrhagic Escherichia coli (EHEC) presenting as ischemic colitis (IDCases 2019;18:e00629)
- 36 year old woman with severe ulcerative colitis and Coronavirus disease 2019 followed by Escherichia coli 0157:H7 infection (Croat Med J 2021;62:634)
- 75 year old man with pseudomembranous colitis-like lesions associated with hemolytic uremic syndrome and neurological sequelae (BMJ Case Rep 2017;2017:bcr2016218586)
Treatment
- Treatment is mainly supportive
- Antibiotic use with STEC infections is not recommended (Clin Infect Dis 2016;62:1251)
Gross description
- EHEC: colonic mucosa is edematous and erythematous with multiple erosions
- Erosion may show adherent blood clots (Virulence 2013;4:366)
Microscopic (histologic) description
- EAEC, EIEC, ETEC
- No significant microscopic changes
- EHEC
- Foci of epithelial cell damage / necrosis with pyknotic nuclei, vacuolated cytoplasm
- Areas of hemorrhage within lamina propria
Positive stains
- E. coli are gram negative bacilli
Electron microscopy description
- Transmission electron microscopy, in cases of EHEC, shows pyknotic nuclei, damaged membranes and cytoplasmic vacuolization of colonic epithelial cells
Sample pathology report
- Left colon, colectomy:
- Colonic mucosa with focal active colitis, surface erosions and focal areas of hemorrhage (see comment)
- Comment: Focal active colitis pattern is a nonspecific histologic finding that may be seen in association with a variety of conditions. The most common clinical diagnoses rendered upon follow up of patients with a focal active colitis pattern include acute self limited / infectious colitis, irritable bowel syndrome, Crohn's disease, ischemic colitis, antibiotic associated colitis, NSAID related colitis, diverticular disease associated colitis, etc.
Differential diagnosis
- Ischemic colitis:
- Negative culture for E. coli; no significant history
- Other infective colitis:
- Negative culture for E. coli
Additional references
Board review style question #1
Which E. coli strain has been implicated as a possible precursor to the development of colorectal cancer?
- Adherent invasive E. coli (AIEC)
- Diffusely adherent E. coli (DEAC)
- Enteropathogenic E. coli (EPEC)
- Enterotoxigenic E. coli (ETEC)
- Shiga toxin producing E. coli (STEC)
Board review style answer #1
B. Diffusely adherent E. coli (DEAC). Diffusely adherent E. coli (DEAC) found in colons of Crohn's disease and colorectal cancer (CRC) patients have been found to harbor the daaC and afaBC genes. The afa1 positive strains isolated from these patients demonstrate adherence and invasion into various epithelial cells and also induce the increased mRNA expression of VEGF (vascular endothelial growth factor), which promotes angiogenesis in tumors. Multiple other genes and the proinflammatory effect of DEAC adhesions on intestinal epithelium has also been associated with the development of Crohn's disease and CRC according to multiple studies (Gastroenterology 2004;127:80, Advancements of Microbiology 2019;58:143, World J Gastrointest Pathophysiol 2014;5:213). No other strain of E. coli has been associated with the development of CRC.
Comment Here
Reference: Escherichia coli
Comment Here
Reference: Escherichia coli
Board review style question #2
A 45 year old woman with chronic kidney disease status post renal transplant presents to the emergency department with vomiting, bloody diarrhea and dehydration. In light of the recent E. coli O157:H7 outbreak, you suspect E. coli colitis. What is the gold standard for diagnosis?
- Cefixime potassium tellurite sorbitol MacConkey agar (CT SMAC)
- Horse blood agar
- Thayer Martin Agar (VPN agar)
- Thiosulfate citrate bile salts sucrose agar (TCBS)
Board review style answer #2
A. Cefixime potassium tellurite sorbitol MacConkey agar (CT SMAC). Cefixime potassium tellurite sorbitol MacConkey agar (CT SMAC) is a modified form of MacConkey sorbitol agar (SMAC) that contains selective agents cefixime and tellurite. Cefixime inhibits the growth of other enteric organisms, such as Proteus spp. and potassium tellurite inhibits non-O157 Shiga toxin producing E. coli (STEC). The use of CT SMAC has been associated with increased cultural sensitivity for the isolation of E. coli O157:H7. Therefore, it is recommended by the American Society of Microbiology and Centers for Disease Control and Prevention (CDC) for use as a primary isolation medium for E. coli O157:H7 in community acquired diarrhea (Versalovic: Manual of Clinical Microbiology, 10th Edition, 2011). The mechanism includes the production of pink colonies by E. coli strains other than O157:H7 through the fermentation of D sorbitol, whereas the colonies of CT SMAC are colorless after overnight incubation due to the lack of D sorbitol fermentation (ThermoFisher: MacConkey Sorbitol Agar w/ Cefixime and Tellurite [Accessed 15 March 2023).
Comment Here
Reference: Escherichia coli
Comment Here
Reference: Escherichia coli
Familial adenomatous polyposis, attenuated
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Diagnosis | Case reports | Treatment | Gross description | Microscopic (histologic) description | Molecular / cytogenetics description | Videos | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Subtype of familial adenomatous polyposis (FAP) characterized by fewer than 100 adenomatous colorectal polyps
- Colonic adenomatous polyps have high risk for progression to colorectal adenocarcinoma (69% cumulative risk by age 80) (Gastroenterology 2004;127:444)
Essential features
- FAP variant characterized by markedly fewer polyps
- Due to a defect in APC (5q21)
- Patients might not require colectomy
Terminology
- Also called attenuated adenomatous polyposis coli or hereditary flat adenoma syndrome (Dis Colon Rectum 1992;35:411)
Epidemiology
- Phenotypically and genetically heterogeneous (Gut 2006;55:1440)
- Cancers usually develop at age 50 - 55 years, 15 years later than classic FAP
Sites
- Patients may develop gastric fundic gland polyps, gastric or small bowel flat adenomas and gastric / duodenal carcinomas (Cancer 1993;71:2709)
- Extracolonic manifestations of classical FAP are less common in attenuated FAP
Diagnosis
- Classically reserved for patients with fewer than 100 colonic adenomatous polyps but exact diagnostic criteria have not been firmly established
Case reports
- 42 year old woman with presentation of ampullary adenoma (University of Pittsburgh Medical Center: Case 340 Familial Adenomatous Polyposis - A Molecular Diagnostic Approach [Accessed 19 February 2021])
- 42 year old syndromic woman with associated hepatocellular carcinoma (Clin Colorectal Cancer 2012;11:77)
- 60 flat adenomas in proximal colon (Int J Gastrointest Cancer 2003;33:117)
- 2 families with hereditary flat adenoma syndrome (Cancer 1990;66:909)
Treatment
- Patients with fewer than 20 - 30 polyps may not require total colectomy if they have frequent surveillance colonscopies with polypectomies
Gross description
- Polyps are usually more proximal (i.e. right sided) than in classic FAP
- Rectum often spared
- Polyps are flat, slightly raised or plaque-like
- May have minute central depression or umbilication
Microscopic (histologic) description
- Adenomatous polyps are microscopically similar to sporadic type adenomas
Molecular / cytogenetics description
- Associated with pathogenic variants in the 5' and distal 3' end of APC, as well as interstitial deletions of 5q22, which include the APC gene
Videos
Attenuated familial adenomatous polyposis (FAP)
Differential diagnosis
- Familial adenomatous polyposis, classical type:
- Increased numbers of colorectal adenomas and more frequent extraintestinal manifestations
- MUTYH associated polyposis:
- Similar colonic phenotype but mutation lies in MUTYH rather than APC
- Lynch syndrome:
- Patients have few polyps and have germline defects in a mismatch repair protein associated gene (Am J Gastroenterol 2002;97:1822)
Additional references
Board review style question #1
How is attenuated familial adenomatous polyposis different from classic familial adenomatous polyposis?
- Patients develop carcinomas at a younger age
- The adenomas are fewer in number
- The adenomas are smaller in size
- The adenomas occur in the small intestine, not the colon
Board review style answer #1
B. The adenomas are fewer in number
Comment Here
Reference: Familial adenomatous polyposis, attenuated
Comment Here
Reference: Familial adenomatous polyposis, attenuated
Familial adenomatous polyposis, classic
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Autosomal dominant familial polyposis syndrome due to a defect in the APC gene (5q21) which prototypically results in numerous (> 100) colonic adenomatous polyps
Essential features
- Most frequent genetic polyposis syndrome, which is due to a germline mutation in the APC gene (5q21)
- Greater than 100 colorectal adenomatous polyps
- Essentially 100% lifetime risk of colorectal adenocarcinoma
- Variants include Gardner syndrome, Turcot syndrome and attenuated familial adenomatous polyposis (AFAP)
Terminology
- Also known as familial polyposis coli or adenomatous polyposis coli
Epidemiology
- Incidence of 1 per 7 - 30,000 individuals
- Males and females are equally affected
- Colon polyps typically are present by the end of the second decade (mean age: 15.9 years)
- Essentially 100% of patients develop colonic adenocarcinoma without surgical intervention (average age: 39 years)
- 25% of patients will be diagnosed with colorectal carcinoma at time of presentation
Sites
- In addition to colonic adenomatous polyps, patients may develop polyps in the stomach (fundic gland polyps) and small intestine
- May also develop carcinoma of thyroid gland, gallbladder and adrenal gland
- Desmoid tumors are a common extraintestinal manifestation (10 - 25%) - they frequently develop in the abdominal wall following local surgery / trauma
- May develop bone lesions (i.e. osteomas), skin lesions (i.e. epidermal inclusion cysts), dental abnormalities (i.e. supranummary or absent teeth), ocular problems (i.e. congenital hypertrophy of retinal pigment epithelium [CHRPE]) or juvenile nasopharyngeal angiofibromas
- Gardner syndrome encompasses the subset of FAP patients with extraintestinal tumors
- Turcot syndrome encompasses the subset of FAP patients with brain tumors, which are typically medulloblastomas
Pathophysiology
- APC (adenomatous polyposis coli) is a tumor suppressor gene involved in cell cycle control and downregulation of beta catenin through the Wnt signaling pathway
- APC protein is normally involved in apoptosis of colonic epithelial cells
- APC mutations may cause expansion of the crypt base cell population, including crypt stem cells
- Mutant crypt stem cells may clonally expand to form adenomas and carcinomas (Am J Pathol 2004;165:1489)
- When APC is mutated, beta catenin is no longer downregulated and can function to stimulate cell growth
- As explained by the 2 hit hypothesis, patients with a germline APC mutation develop adenomatous polyps when there is inactivation of the remaining normal APC allele
- Colorectal adenocarcinomas in FAP develop in a similar fashion to sporadic type colorectal adenocarcinomas (i.e. mutations in KRAS, TP53)
- Abundance of adenomatous polyps results in the near certain development of colorectal adenocarcinoma
Diagrams / tables
Diagnosis
- Diagnostic criteria:
- 100 or more colorectal adenomatous polyps or
- Germline mutation in APC or
- Family history of FAP with colorectal adenomas (age < 30) or
- Family history of FAP and presence of at least one epidermoid cyst, osteoma or desmoid tumor
- Patients with a history of > 10 colorectal adenomas, a family history of adenomatous polyposis syndromes or a history of adenomas with FAP type extracolonic lesions should undergo assessment for adenomatous polyposis syndrome (Am J Gastroenterol 2015;110:223)
Prognostic factors
- Location of the mutation in APC has an impact on the phenotypic presentation, including number of polyps and presence or absence of desmoid tumors and congenital hypertrophy of retinal pigment epithelium
- Most common causes of noncolorectal carcinoma deaths in FAP patients are duodenal / ampullary carcinoma and complications of desmoid tumor
Case reports
- 11 and 12 year old boys with cancer (Eur J Pediatr 2005;164:306)
- 24 year old woman with FAP and small bowel GIST (Exp Oncol 2015;37:227)
- FAP in 3 generations of a single family (Case Rep Oncol 2014;7:349)
Treatment
- Prophylactic colectomy in late second to early third decade
- Must monitor rectal stump if preserved
- FAP patients who undergo consistent colorectal screening with subsequent surgery have a significantly decreased rate of mortality from colorectal malignancies
- Children at risk for FAP should undergo sigmoidoscopy every 1 - 2 years, beginning at age 10 - 12 years
- Relatives should also be screened
- NSAIDs and aspirin use have been studied to decrease polyp regrowth following subtotal colectomy
Gross description
- Most adenomatous polyps are small (< 0.5 cm) and sessile
- Polyps are more common in the left sided colon, although the entire length of the colon is typically involved
- Polyps may be flat or depressed (Int J SurgPathol 2006;14:133)
Gross images
Microscopic (histologic) images
Contributed by Jennifer Findeis-Hosey, M.D. and @Andrew_Fltv on Twitter
Images hosted on other servers:
Molecular / cytogenetics description
- Hereditary colorectal cancer syndromes are usually due to defect in APC gene at 5q21
- Polyposis patients without APC mutations often have mutations in MYH gene
- Autosomal dominant trait with high degree of penetrance (> 90%)
- 20% represent a new de novo APC mutation without family history
Videos
Full colonoscopy in retroflexed maneuver in a familial adenomatous polyposis coli
Sample pathology report
- Colon, total colectomy:
- Colon with innumerable tubular adenomas, some with focal high grade dysplasia (see comment)
- Negative for malignancy.
- Adenomas focally present at both proximal and distal mucosal resection margins.
- Twelve benign lymph nodes.
- Comment: The findings are consistent with the patient's reported history of familial adenomatous polyposis. Numerous grossly identifiable polyps were submitted for microscopy, including all polyps larger than 1 cm.
Differential diagnosis
- Attenuated FAP:
- Polyposis syndrome also due to an APC mutation but patients have fewer than 100 colorectal polyps
- MYH associated polyposis:
- Polyposis syndrome caused by a mutation in the MYH gene
Additional references
Board review style question #1
Without surgical intervention, patient with familial adenomatous polyposis have lifetime risk of developing colorectal carcinoma of approximately
- 25%
- 50%
- 75%
- 100%
Board review style answer #1
Board review style question #2
Patients with Turcot syndrome develop what tumors in addition to the typical manifestations of familial adenomatous polyposis?
- Desmoid tumors (fibromatosis)
- Gastrointestinal stromal tumors
- Keratoacanthomas
- Medulloblastomas
Board review style answer #2
Focal active colitis
Table of Contents
Definition / general | Essential features | ICD coding | Sites | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Focal active colitis (FAC) is a histologic term that denotes the presence of focal neutrophil infiltration within the colonic crypts or epithelium (Biomedicines 2023;11:2631)
- Nonspecific diagnosis that encompasses infectious (acute self limited) colitis and noninfectious active colitis
Essential features
- Focal active colitis (FAC) is characterized by a single focus or multiple foci of neutrophilic infiltration of the crypts with an otherwise unremarkable colonic mucosa; the crypt architecture is well preserved with no evidence of chronic injury (Cureus 2020;12:e8140)
- FAC can be seen in the setting of acute self limited infectious colitis, evolving inflammatory bowel disease (IBD), ischemic colitis, Clostridium difficile colitis, drug induced or chemical injury, irritable bowel syndrome and bowel preparation artifact (Cureus 2020;12:e8140)
- Incidence of Crohn's disease (CD) in adults presenting with FAC is relatively low and varies between 0 and 13%, whereas the incidence of infectious type colitis has been demonstrated to be nearly 50% (Cureus 2020;12:e8140)
- In pediatric patients, a 24% rate of IBD is seen with FAC; however, when patients with associated terminal ileal inflammation were excluded, the rate dropped to 11% (similar to the rates of adults) (Hum Pathol 2018;74:164)
- FAC remains a diagnostic challenge; one must incorporate the patient's presenting history, medications and findings on endoscopy to help narrow the differential diagnosis
ICD coding
- ICD-11: 1A40.0 - gastroenteritis or colitis without specification of origin
Sites
- Colon
Clinical features
- Diarrhea, nausea, abdominal cramps, increased flatulence (Cureus 2020;12:e8140)
Diagnosis
- Diagnosed on colonoscopic biopsy
Prognostic factors
- Prognosis depends upon the underlying cause whether it is infectious or inflammatory bowel disease
Case reports
- Teenage girl with orbital myositis associated with focal active colitis (Childs Nerv Syst 2012;28:641)
- 82 year old woman presented with complaints of diarrhea, nausea, abdominal cramping, increased flatulence, anorexia and weight loss for the past 3 months (Cureus 2020;12:e8140)
Treatment
- Treatment of underlying etiology, such as inflammatory bowel disease, irritable bowel syndrome or infectious colitis
Microscopic (histologic) description
- Neutrophil mediated surface epithelial injury, neutrophils within epithelial cells (cryptitis) or within crypt lumina (crypt abscess) (Biomedicines 2023;11:2631)
- Expansion of lamina propria with neutrophils and eosinophils
- Well preserved crypt architecture
Microscopic (histologic) images
Sample pathology report
- Colon, biopsy:
- Focal active colitis (see comment)
- Comment: The differential diagnosis for focal active colitis includes infectious process, medication associated injury (e.g., nonsteroidal anti-inflammatory drugs) or emerging inflammatory bowel disease. Histologic features of chronic injury are not seen. Negative for viral cytopathic effect and granulomata.
Differential diagnosis
- Acute self limiting colitis:
- Abundant superficial acute inflammation, cryptitis and crypt abscess without features of chronicity
- Early onset inflammatory bowel disease:
- Especially in pediatric population
- Ileal involvement
- Granulomas may be present
- Drug related colitis:
- Can have various patterns injury
- Increased epithelial apoptosis
- May show increased eosinophils (Br J Clin Pharmacol 2003;56:477)
- Increased intraepithelial lymphocytes
- Ischemic colitis:
- Ulceration and mucosal necrosis
- Lamina propria hyalinization
- Acute inflammatory exudate
- Crypt withering and crypt loss
Additional references
Board review style question #1
A 55 year old man presented with diarrhea, abdominal discomfort and flatulence. Colonoscopy shows patchy erythematous areas in the left colon. Endoscopic biopsy is taken and it shows the histology above. Which of the following statements is the most likely morphological diagnosis?
- Focal active colitis
- Lymphocytic colitis
- Pseudomembranous colitis
- Ulcerative colitis
Board review style answer #1
A. Focal active colitis. Focal active colitis is the correct answer as there is focal cryptitis and crypt abscess formation. The crypt architecture is intact. Answer B is incorrect because there are no increased intraepithelial lymphocytes. Answer C is incorrect because pseudomembranous colitis typically has necroinflammatory exudate on the surface. Answer D is incorrect because crypt distortion is typically seen in ulcerative colitis of patients of this age range.
Comment Here
Reference: Focal active colitis
Comment Here
Reference: Focal active colitis
Board review style question #2
A 17 year old boy presented with bloody diarrhea and elevated fecal calprotectin. He had granulomata on upper GI biopsy and colonoscopic biopsy showed focal active colitis. Which of the following is the most likely underlying diagnosis?
- Crohn's disease
- Lymphocytic colitis
- Pseudomembranous colitis
- Ulcerative colitis
Board review style answer #2
A. Crohn's disease is the correct answer because in the pediatric population, inflammatory bowel disease can just present by focal active colitis, even though the architecture is intact. Answer B is incorrect because lymphocytic colitis has increased intraepithelial lymphocytes. Answer C is incorrect because pseudomembranous colitis shows inflammatory exudate on the mucosal surface. Answer D is incorrect because ulcerative colitis doesn't show granulomas on upper GI biopsies.
Comment Here
Reference: Focal active colitis
Comment Here
Reference: Focal active colitis
Foreign materials / food
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Food histology can mimic drugs and parasites in the gastrointestinal tract and lungs
Essential features
- Foods can be aspirated or found in the gastrointestinal (GI) tract and can mimic bugs or drugs
- It is essential to be aware of food histology in order to avoid misinterpreting a mimicker
- Foreign material found in biopsies, surgical specimens and during autopsies
Terminology
N/A
ICD coding
N/A
Epidemiology
N/A
Sites
- Often found in the GI tract and lungs
- Can be seen during routine screening, within surgical specimens or found during autopsy
Pathophysiology
N/A
Etiology
N/A
Diagrams / tables
N/A
Clinical features
N/A
Diagnosis
- Often foreign material is an incidental finding, which can mimic pathological disease and therefore histology of foreign material is essential
Laboratory
N/A
Radiology description
N/A
Radiology images
N/A
Prognostic factors
N/A
Case reports
- 31 year old woman with bowel endometriosis and schistosomiasis (Fertil Steril 2006;85:1060.e1)
Treatment
N/A
Clinical images
N/A
Gross description
N/A
Gross images
N/A
Frozen section description
N/A
Frozen section images
N/A
Microscopic (histologic) description
N/A
Microscopic (histologic) images
Contributed by Mona Deerwester, M.D., M.Sc.
Virtual slides
N/A
Cytology description
N/A
Cytology images
N/A
Immunofluorescence description
N/A
Immunofluorescence images
N/A
Positive stains
N/A
Negative stains
N/A
Electron microscopy description
N/A
Electron microscopy images
N/A
Molecular / cytogenetics description
N/A
Molecular / cytogenetics images
N/A
Videos
N/A
Sample pathology report
- Skin and soft tissue, right third digit, excision:
- Foreign body reaction (see comment)
- Comment: No evidence of malignancy.
- Mucosal lip, biopsy:
- Foreign body consistent with hyaluronic acid injection (see comment)
- Comment: No evidence of malignancy.
Differential diagnosis
- Epidermal inclusion cyst / epidermoid cyst:
- Smooth, freely movable on palpation often with dilated punctum
- Lined by stratified squamous epithelium
- Lumen contains abundant keratin flakes
- Granulomatous inflammation contains shards of keratin flakes
- Pilomatrixoma:
- Benign tumor arising from hair matrix
- Solid nests of basaloid cells undergoing abrupt trichilemmal type keratinization
- Ghost cells, calcification or ossification with extramedullary hematopoiesis and frequent rupture leading to giant cell reaction
- Sarcoidosis:
- Exclude foreign material and infection (e.g., with microscopic examination with polarized light and stains for microorganisms)
- Rheumatoid nodule:
- Seen on extensor sites, also over the spinous process of vertebrae
- Subcutaneous
- Central fibrinoid necrosis, surrounded by histiocytes and perivascular lymphocytes and neutrophils
- Age of onset: children
- Granuloma annulare:
- Intradermal
- Palisaded or interstitial granulomatous inflammation with mucin
- Minimal epidermal changes
- Gouty tophi:
- Most commonly involving the great toe metatarsophalangeal (MTP) joints
- Tophi are composed of needle shaped aggregates of urate crystals with surrounding foreign body giant cell reaction
- Urate crystals dissolve with routine processing, so fix a smear of crystals in absolute alcohol or nonaqueous fixation
- Under polarized light microscopy, they have a needle-like morphology and strong negative birefringence
- Calcinosis cutis:
- Dystrophic calcification at sites of microtrauma or inflammation
- Lesions are composed of calcium hydroxyapatite
- Multiple subcutaneous papules or nodules that are yellow-white
- Irregular deposits of intensely basophilic acellular material in the dermis and subcutaneous tissue
- Basophilia is so strong that the appearance is of a deep purple
- Deposits are typically well circumscribed with a thin rim of eosinophilic hyalinization and frequently with a host giant cell reaction
- Palisaded neutrophilic granulomatous dermatitis (JAAD Case Rep 2017;3:425):
- Typically associated with underlying disease states, including autoimmune connective tissue disease, lymphoproliferative disorders and infections
- Most common in patients with rheumatoid arthritis
- Can be seen in patients with systemic lupus erythematosus and systemic vasculitis
- Microscopy
- Early lesions present with neutrophilic infiltrates and leukocytoclastic vasculitis
- Fully developed lesions feature palisaded granulomas with collagen trapping and neutrophil remnants
- Necrobiosis lipoidica:
- Atrophic, yellow depressed plaques, usually on legs of diabetic patients
- Layered inflammatory process and alternating zones of necrobiosis involving the full thickness of the dermis
- Changes tend to become more pronounced deeper in the dermis and may extend into the septal panniculus
- Areas of necrobiosis are poorly defined and run into each other with broad foci of inflammatory infiltrate intervening
- Variable histiocytic infiltrate with multinucleated giant cells surrounds these areas
- Accompanying inflammatory infiltrate is predominantly lymphocytic with plasma cells and occasional eosinophils
- Amyloidosis:
- Parasites:
- Infection
- Exclude with tissue cultures and stains for microorganisms (e.g., Fite, GMS, PAS, Gram stain)
- Infection
Additional references
Board review style question #1
Board review style answer #1
B. Foreign body giant cell reaction. Answer A is incorrect because a giant cell reaction surrounding shards of keratin should be seen with an inclusion cyst. Answer C is incorrect because pilomatrixomas have islands of basaloid cells and ghost or shadow cells. Answer D is incorrect because bone is not seen.
Comment Here
Reference: Foreign materials / food
Comment Here
Reference: Foreign materials / food
Board review style question #2
Board review style answer #2
C. Hyaluronic fillers. Answer A is incorrect because cutaneous mucinosis or focal dermal mucinosis is a collection of mucin between dermal collagen bundles. Answer D is incorrect because metastatic mucinous adenocarcinoma should have atypical glands or nests of epithelial cells which appear to be floating in copious mucin. Answer B is incorrect because granuloma annulare should reveal palisading of histiocytes around a focus of necrobiosis and increased mucin deposition.
Comment Here
Reference: Foreign materials / food
Comment Here
Reference: Foreign materials / food
Foreign materials / food (pending)
[Pending]
Ganglioneuroma
Table of Contents
Definition / general | Essential features | Sites | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Benign mesenchymal polyp in the colon
- Unrelated to lesion of the same name in the adrenal gland
Essential features
- Uncommon colonic mesenchymal polyp with prominent ganglion cells
- Associated with Cowden syndrome, especially if multiple polyps
Sites
- Colorectum is most common site in gastrointestinal tract
Clinical features
- Patients with multiple intestinal ganglioneuromas may have Cowden syndrome
- Solitary intestinal ganglioneuromas usually incidental and of no clinical consequence (Am J Surg Pathol 1994;18:250)
- May rarely cause intussusception (J Pediatr Surg 2009;44:e17)
Diagnosis
- Typically found during screening colonoscopy
Case reports
- 48 year old woman with small sessile colon polyp (Diagn Pathol 2008;3:20)
- 65 year old man with solitary ascending colon polyp (Case Rep Gastroenterol 2017;11:434)
Treatment
- Polypectomy generally curative
Gross description
- Usually small (a few millimeters) but can rarely grow to 2 - 3 cm
Microscopic (histologic) description
- Expansion of the lamina propria by bland spindled mesenchymal cells (Schwann cells), lacking atypia or mitotic figures
- Ganglion cells present within the expanded region; may be rare or abundant
Microscopic (histologic) images
Positive stains
- S100 (Schwann cells); synaptophysin, NSE (ganglion cells) (Arch Pathol Lab Med 2011;135:1311)
Sample pathology report
- Transverse colon, polypectomy:
- Ganglioneuroma
Differential diagnosis
- Ganglioneuromatosis:
- Nodular expansile confluence of ganglioneuromatous tissue, often in patients with MEN IIb or neurofibromatosis
- Perineurioma:
- Negative for S100
- Mucosal Schwann cell hamartoma:
- Both lesions S100+ but ganglioneuroma has ganglion cells
Additional references
Board review style question #1
A 60 year old patient undergoes colonoscopy and is found to have a solitary ganglioneuroma. What should be the next step in working up this patient?
- Colectomy for possible ganglioneuromatosis
- Follow up colonoscopy in 1 year
- Genetic screening for Cowden syndrome
- Imaging to search for ganglioneuromas at other sites
- No further workup needed
Board review style answer #1
Ganglioneuromatosis
Table of Contents
Definition / general | Essential features | Terminology | Clinical features | Radiology description | Case reports | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Benign hamartomatous proliferation involving the colonic muscularis propria
- Often associated with MEN IIb (RET mutations) and neurofibromatosis type 1 (NF1 mutations) (Am J Surg Pathol 1994;18:250, Gut 1999;45:143)
Essential features
- Hamartomatous increase of glial and neuronal elements in the muscularis propria
- Has syndromic associations
- Must be distinguished from solitary ganglioneuromas and polypoid ganglioneuromatosis
Terminology
- Also called transmural intestinal ganglioneuromatosis or diffuse intestinal ganglioneuromatosis
Clinical features
- Patients may have diarrhea or intestinal pseudo-obstruction
Radiology description
- Mural thickening and luminal narrowing (AJR Am J Roentgenol. 2004;182:1166)
Case reports
- 45 year old woman with nonsyndromic intestinal ganglioneuromatosis (J Clin Pathol 2007;60:222)
- 63 year old man with prior tubular adenoma and new colon polyp (Case #305)
- 77 year old woman with mucinous adenocarcinoma and hyperparathyroidism (Eur J Gastroenterol Hepatol 1999;11:447)
- Causing chronic diarrhea (Ugeskr Laeger 1998;160:7139)
Gross description
- Poorly demarcated mural thickening, up to 17 cm in length, which can distort surrounding tissue
Microscopic (histologic) description
- Proliferation of neural cells and ganglion cells in muscularis propria and sometimes in other layers
- No evidence of malignancy
Microscopic (histologic) images
Positive stains
- S100 (neural cells and ganglion cells); synaptophysin, NSE (nerve fibers)
Molecular / cytogenetics description
- RET mutations, even without MEN IIb (J Clin Endocrinol Metab 1998;83:4191)
Sample pathology report
- Rectum, resection:
- Segment of large bowel with extensive ganglioneuromatosis, extending to distal resection margin (see comment)
- Proximal resection margin unremarkable.
- Negative for malignancy.
- Three benign lymph nodes.
- Comment: Colorectal ganglioneuromatosis is often seen in the setting of a clinical syndrome, including MEN IIb and neurofibromatosis type 1.
Differential diagnosis
- Crohn's disease:
- May show neural hyperplasia but mucosal and mural inflammation are also present
- Intestinal neuronal dysplasia:
- Controversial entity harboring increased ganglion cells; no mural thickening
- Polypoid ganglioneuroma:
- Small, solitary / few, mucosa based, nonsyndromic (Case #305)
- Ganglioneuromatous polyposis:
- More than 20 polypoid mucosa based lesions, with variability in ganglionic and neural content; associated with Cowden syndrome / PTEN mutations
Board review style question #1
Colonic ganglioneuromatosis is most common in which of the following syndromes?
- Cowden syndrome
- Familial schwannomatosis
- Lynch syndrome
- Neurofibromatosis type 1
Board review style answer #1
Gardner syndrome
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Radiology images | Case reports | Gross images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- A variant of familial adenomatous polyposis (FAP) with prominent extraintestinal manifestations
Essential features
- Features of FAP and extraintestinal lesions, including desmoid tumors, bone osteomas and fibromas
- Due to a mutation in the APC tumor suppressor gene
Terminology
- Rarely also called Plenk-Gardner syndrome
Epidemiology
- Incidence of 1 per million in U.S.
Sites
- Colonic adenomatous polyps plus extraintestinal features, including multiple osteomas (skull, mandible and long bones), epidermal cysts, desmoid fibromatosis (10%, usually arise after intra-abdominal surgery, aggressive and may cause death), fibromas, lipomas, impacted and supernumerary teeth, dental cysts and congenital hypertrophy of retinal pigment epithelium
Radiology images
Case reports
- 11 year old girl presenting with fibromatosis (World J Gastroenterol 2005;11:5408)
Gross images
Additional references
Board review style question #1
Gardner syndrome is caused by a germline mutation in what gene?
- APC
- BRAF
- MLH1
- STK11
Board review style answer #1
Gastric heterotopia
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Clinical features | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Board review style question #1 | Board review style answer #1Definition / general
- Gastric tissue aberrantly present in colon
- See also heterotopia topic
Essential features
- Rare finding that usually consists of oxyntic type gastric tissue
- Helicobacter pylori organisms may be present
Epidemiology
- < 50 cases reported
Sites
- Usually in rectum; can be multifocal (Gut 1988;29:848)
Clinical features
- Mean age 18 years but wide range
- Slight male predominance
- Associated with other heterotopias, with vertebral and digital anomalies
- In rectum, usually presents with rectal bleeding and tenesmus (J Clin Gastroenterol 1994;19:41)
- At colonoscopy, may appear as polyp, ulcer or diverticulum
Case reports
- 34 year old man and 36 year old woman with polypoid lesions in rectum (Arch Pathol Lab Med 1999;123:222)
- 70 year old woman with heterotopia giving rise to adenocarcinoma (Korean J Pathol 2013;47:289)
- Associated with tubulovillous adenoma (N J Med 1995;92:512)
Treatment
- Excision, H2 blockers
Microscopic (histologic) description
- Gastric tissue (typically oxyntic type but may be pyloric type) in colon
- Helicobacter pylori organisms may be present
Microscopic (histologic) images
Sample pathology report
- Rectum, mass, biopsy:
- Gastric heterotopia (see comment)
- Comment: Helicobacter organisms are not identified by immunohistochemistry.
Board review style question #1
Board review style answer #1
Gastrointestinal stromal tumor
Table of Contents
Definition / general | Essential features | Terminology | Sites | Clinical features | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Tumors that differentiate along lines of interstitial cells of Cajal, the gut's pacemaker cells (Mod Pathol 2003;16:366)
Essential features
- Mesenchymal spindle cell neoplasm caused by KIT or PDFRGA mutations
- Risk stratification is based on location, size and mitotic rate
- Very rare in colon; rectal GISTs account for roughly 5% of all GISTs
Terminology
- Leiomyoblastoma and gastrintestinal autonomic nerve tumor (GANT) are old terms no longer used
Sites
- 5% or fewer of GISTs occur in the colorectum (usually the rectum) (Arch Pathol Lab Med 2006;130:1466)
Clinical features
- Median age 67 years (Am J Surg Pathol 2000;24:1339)
- The majority of lesions are aggressive and lead to patient death
- Asymptomatic microscopic "seedling" GISTs are quite rare (Am J Surg Pathol 2008;32:867)
Prognostic factors
- Risk of disease progression for rectal GISTs depends on tumor size and mitotic rate (Semin Diagn Pathol 2006;23:70)
- If mitotic rate is < 5 per 5 square mm, risk of progression is 0% (< 2 cm), 8.5% (> 2 to < 5 cm) or 57% (> 10 cm) (insufficient data for > 5 to < 10 cm)
- If mitotic rate is > 5 per 5 square mm, risk of progression is 54% (< 2 cm), 52% (> 2 to < 5 cm) or 71% (> 10 cm) (insufficient data for > 5 to < 10 cm)
- There is insufficient data for progression estimation in colonic GISTs
Case reports
- Man in mid 40s with GIST of the transverse colon
- 65 year old man with prostatic stromal sarcoma and rectal GIST (Urology 2006;68:672.e11)
- 71 year old man with a large rectal adenoma and interstitial cell of Cajal hyperplasia (Case #428)
Treatment
- Tyrosine kinase inhibitors, including imatinib mesylate (first line) and sunitinib malate
Gross description
- Often large, bulky, intramural masses
- Fish flesh or tannish brown parenchyma with hemorrhage, necrosis and cystic softening
Microscopic (histologic) description
- Mesenchymal tumor usually centered in the muscularis propria
- Spindle cell GISTs: intersecting fascicles of plump spindled cells with eosinophilic cytoplasm within variably hyalinized or edematous stroma
- Epithelioid GISTs: rounded epithelioid cells with pseudo compartmental organization
- Skeinoid fibers (extracellular collagen globules) may be seen
- Muscle infiltration is common but not predictive of behavior
- Rarely has osteoclast-like giant cells (Arch Pathol Lab Med 2004;128:440)
Microscopic (histologic) images
Positive stains
- DOG1 (ANO1), CD117, protein kinase C-theta, CD34 (Am J Surg Pathol 2009;33:1401)
- Alpha smooth muscle actin (30% - 40%), S100 (rare)
- Variable keratin (rare, weak)
Negative stains
Electron microscopy description
- Processes or cell bodies with intermediate filaments showing solitary focal densities
- Attachment plaques with incomplete lamina, rare myofilaments and smooth endoplasmic reticulum (Ultrastruct Pathol 2002;26:269)
Molecular / cytogenetics description
- 80% have mutations in KIT, which encodes CD117; exons affected include 11, 9, 13 and 17 (Nat Rev Cancer 2011;11:865)
- 10% have mutations in PDGFRA; exons affected include 18, 14 and 12; these GISTs are often gastric and epithelioid
- Both mutations have been identified in small incidental GISTs, affirming their importance
- Up to 10% have neither mutation and are associated with syndromes such as neurofibromatosis type 1 and Carney's triad
Sample pathology report
- Ascending colon, resection:
- Gastrointestinal stromal tumor (4.3 cm) (see synoptic report and comment)
- Comment: The tumor is positive for KIT and DOG1 by immunohistochemistry. The mitotic rate is 2 per 5 square mm. Risk stratification for colonic GISTs has not been established but the risk in this case is likely relatively low (an analogous rectal GIST would have a risk of progression of 8.5%).
Differential diagnosis
- Fibromatosis:
- Long, sweeping fascicles and dilated, thin walled blood vessels; nuclear positivity for beta catenin; may be positive for CD117 (Am J Surg Pathol 2000;24:947)
- Inflammatory fibroid polyp:
- Inflammatory myofibroblastic tumor:
- Leiomyoma:
- Bland and brightly eosinophilic; positive for actin and desmin; negative for CD117 (Int J Colorectal Dis 2006;21:84)
- Leiomyosarcoma:
- Marked atypia and necrosis; positive for smooth muscle actin or desmin; negative for CD117; no KIT mutations
Board review style question #1
What is the most common site for gastrointestinal stromal tumors in the large intestine?
- Appendix
- Cecum
- Rectum
- Transverse colon
Board review style answer #1
Board review style question #2
What 2 factors are used to estimate risk of progression in gastrointestinal stromal tumors?
- Ki67 index and necrosis
- Ki67 index and size
- Mitotic rate and necrosis
- Mitotic rate and size
Board review style answer #2
Graft versus host disease
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Complication of allogeneic stem cell transplantation in which T cells from the donor recognize the recipient tissue as foreign, leading to organ damage
- May also occur in some autologous stem cell transplant patients (Mod Pathol 2011;24:117)
Essential features
- Complication of stem cell transplantation in which T cells from the donor recognize the recipient tissue as foreign, leading to organ damage
- Histologically characterized by crypt apoptosis, crypt dropout and ulceration
- Infection (especially cytomegalovirus) and drug induced injury are the most common differential considerations
ICD coding
- ICD-10: D89.813 - graft versus host disease, unspecified
Epidemiology
- Occurs in 30 - 70% of allogeneic stem cell transplant patients (Biol Blood Marrow Transplant 2015;21:389)
- M:F = 1.2:1 (Blood 2012;119:296, Bone Marrow Transplant 2013;48:587)
- Median age: 36 - 43 years (Blood 2012;119:296, Bone Marrow Transplant 2013;48:587)
Sites
- Skin > gastrointestinal tract > liver
- Other sites: mouth, eyes, lungs
Pathophysiology
- Tissue injury occurs following myeloablative or conditioning chemotherapy regimens in preparation for stem cell transplantation and leads to antigen upregulation on host tissues
- Donor T cells recognize host tissue antigens as foreign
- T cell activation and cytokine storm lead to end organ damage (Am J Clin Pathol 2016;145:591)
- Alteration of the microbiome is likely a contributing factor (Biol Blood Marrow Transplant 2018;24:1322)
- In autologous stem cell transplant patients, it is hypothesized to result from failure of self tolerance (Mod Pathol 2011;24:117)
Etiology
- Risk factors: human leukocyte antigen mismatch, age, sex disparity, stem cell source and intensity of the conditioning regimen (N Engl J Med 2017;377:2167)
Clinical features
- Clinically divided into classic acute graft versus host disease (occurring within 100 days of transplant), persistent / recurrent / late onset acute graft versus host disease (occurring > 100 days posttransplant) and chronic graft versus host disease (defined by presence of diagnostic clinical signs and symptoms; no specific features are diagnostic of chronic colonic GVHD)
- Histologic distinction of these groups not possible (Biol Blood Marrow Transplant 2015;21:589)
- Gastrointestinal symptoms: dysphagia, anorexia, nausea, vomiting, diarrhea (watery to mucoid / bloody), weight loss and failure to thrive (Nat Rev Gastroenterol Hepatol 2017;14:711)
- Coexisting skin graft versus host disease is often present, causing diffuse maculopapular rash
- Endoscopic findings range from edema and mild erythema to mucosal erosions, ulceration and denudation (Nat Rev Gastroenterol Hepatol 2017;14:711)
Diagnosis
- Combination of clinical, endoscopic and histologic features
- Biopsy is essential when the clinical presentation is atypical and helps exclude alternative diagnoses
Radiology description
- Bowel wall thickening with mucosal enhancement, mesenteric edema and vascular engorgement (AJR Am J Roentgenol 2017;209:33)
Prognostic factors
- Severity predicts treatment response and mortality (Biol Blood Marrow Transplant 2015;21:389)
- Gastrointestinal tract staging is based on severity of diarrhea and extent of weight loss
Case reports
- 23 year old man with defecation of a colon cast (Ann Saudi Med 2009;29:231)
- 44 year old woman with cytomegalovirus infection (World J Gastroenterol 2013;19:597)
- 56 year old man with idelalisib induced colitis and skin eruption (Clin J Gastroenterol 2017;10:14)
- 63 and 74 year old men with multiple myeloma and hematopoietic stem cell transplantation (Gastroenterology Res 2018;11:52)
- 3 patients with Epstein-Barr virus associated posttransplant lymphoproliferative disorder (Eur J Haematol 2019;103:519)
Treatment
- Increased immunosuppression
Microscopic (histologic) description
- Characteristic histologic features include crypt apoptosis, crypt dropout and ulceration (Biol Blood Marrow Transplant 2015;21:589)
- Inflammation is generally sparse
- Endocrine cell nests may be seen (Am J Surg Pathol 2013;37:1319)
- Fibrosis and crypt architectural changes are markers of longstanding disease but not specific features of chronic graft versus host disease (Am J Clin Pathol 2016;145:591)
- Apoptosis in the colon is not specific to graft versus host disease and the lower diagnostic threshold is debated
- National Institute of Health proposes ≥ 1 apoptotic body per biopsy fragment (Biol Blood Marrow Transplant 2015;21:589)
- Others propose > 6 apoptotic bodies per 10 contiguous crypts for definitive diagnosis of graft versus host disease (Am J Surg Pathol 2013;37:539)
- Cases with ≤ 6 apoptotic bodies per 10 contiguous crypts are considered indeterminate for graft versus host disease (Histopathology 2016;69:802)
- Utility of histologic grading is questionable because of poor correlation with patient outcome
- When grading is performed, the Lerner system is most commonly utilized
- Grade I: crypt apoptosis without crypt dropout
- Grade II: single crypt dropout
- Grade III: contiguous crypt dropout
- Grade IV: diffuse crypt dropout with ulceration
- When grading is performed, the Lerner system is most commonly utilized
- Graft versus host disease in autologous stem cell transplant patients shows more prominent crypt apoptosis but is otherwise histologically identical to allogeneic graft versus host disease (Mod Pathol 2018;31:1619)
- Histologic examination of at least 8 serial sections is recommended to avoid missing rare apoptotic bodies (Biol Blood Marrow Transplant 2015;21:589)
Microscopic (histologic) images
Virtual slides
Negative stains
- Cytomegalovirus IHC, PAS and Giemsa negative for microorganisms (though their presence does not rule out coincident graft versus host disease)
Sample pathology report
- Colon, biopsy:
- Colonic mucosa with increased crypt apoptosis and crypt dropout (see comment)
- Comment: Given the patient's history of allogeneic stem cell transplant, the findings likely represent graft versus host disease.
Differential diagnosis
- Cytomegalovirus colitis:
- Presence of characteristic viral inclusions via immunohistochemistry or on H&E; always helpful to rule out in cases of suspected graft versus host disease
- Chemotherapy or radiation related changes:
- Usually resolve within 20 days
- Epithelial flattening with regenerative atypia, mucin depletion and crypt apoptosis
- Mycophenolate mofetil:
- Increased eosinophils in the lamina propria and fewer neuroendocrine cell nests (Am J Surg Pathol 2013;37:1319)
- Cryptosporidiosis:
- Identification of organisms attached to mucosal surface, Giemsa or PAS stains can highlight organisms; prominent neutrophilic inflammation (Hum Pathol 2010;41:918)
Board review style question #1
A 55 year old man undergoes peripheral blood stem cell transplant for multiple myeloma. 70 days after the transplant, he complains of mucoid to bloody diarrhea 3 - 5 times per day. Colonoscopy shows multiple ulcerations and biopsies are obtained. What is the most likely diagnosis?
- Chemotherapy induced injury
- Crohn's disease
- Graft versus host disease
- Ischemic colitis
- NSAID related injury
Board review style answer #1
Board review style question #2
What is the most characteristic histologic feature of colonic graft versus host disease?
- Architectural distortion
- Crypt apoptosis
- Eosinophilic inflammation
- Lamina propria hyalinization
- Neutrophilic inflammation
Board review style answer #2
Granulomatous colitis
Etiology
- Due to Crohn disease, tuberculosis, Yersinia
Case reports
- 46 year old woman with Propionibacterium acnes, a low virulence skin bacteria (Arch Pathol Lab Med 2001;125:1491)
- 51 year old man with sarcoidosis, a rare cause of granulomatous enterocolitis (J Gastrointestin Liver Dis 2012;21:423)
Differential diagnosis
- Normal germinal centers, tangential sections of blood vessels or pericryptal fibroblastic sheath, inflammatory reaction to extravasated mucin, rarely sarcoidosis (Am J Gastroenterol 1998;93:1949)
Grossing & features to report
Table of Contents
Definition / general | Grossing - polypectomy | Grossing - endoscopic mucosal resection (EMR) or endoscopic submucosal resection (ESD) | Grossing - colectomy | Grossing - colectomy with no tumor | Grossing - colectomy with tumor | Grossing - total mesorectal excision (TME) specimens | Features to report for adenocarcinoma | Colonic biopsy - mandatory to report | Colonic biopsy - recommended but not required to report | Polypectomy - mandatory to report | Polypectomy - recommended but not required to report | Colorectal resection for tumor - mandatory to report | Colorectal resection for tumor - recommended but not required to report | Gross description | Gross images | Sample gross description report | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- This topic describes how to gross specimens obtained from polypectomy and colectomy procedures
- Essential clinical history: clinical diagnosis, procedure performed, prior biopsy or procedure
Grossing - polypectomy
- Describe: size, color, configuration (sessile or pedunculated), single or multiple pieces (piecemeal polypectomy)
- Ink the base or stalk margin if it can be identified
- If stalk present: measure length and diameter of stalk, apply ink to base of stalk
- If stalk not present: look for pale tissue at base of polyp, apply ink to this area
- Submit in toto (Am J Clin Pathol 2001;116:336)
- Small polyp: bisect perpendicular to the stalk or base
- Large polyp: for polyps with wide heads not able to fit in a cassette, trim the sides away from the stalk; take section through surgical margin of stalk, submit in one cassette and put the sides in other cassette(s)
- Sectioning protocol based on polyp size (see table 1)
Grossing - endoscopic mucosal resection (EMR) or endoscopic submucosal resection (ESD)
- Orientation and ink
- If unoriented, ink the deep and peripheral margins with one color
- If oriented, ink the deep margin and peripheral margins with multiple colors (similar to inking a skin eclipse)
- Describe: lesion present or not, size, type, color, shape, borders, distance to all margins
- Identify the closest peripheral margin and section along that axis at 2 mm intervals, submit in toto
Grossing - colectomy
Grossing - colectomy with no tumor
- Identify and record the subsites of colon
- Open the bowel along the antimesenteric border using scissors
- Clean the lumen by gently washing out fecal material
- Fix in formalin overnight (ensure the specimen is immersed in an adequate volume of clean formalin)
- Measure and record the dimensions (length and circumferences), length of mesentery and size of omentum if present
- Describe and record any abnormal findings
- Sections to submit:
- Sections to submit depend on the pathological findings (IBD, ischemia, volvulus, diverticular disease, arteriovenous malformation, etc.)
- Abnormal areas by taking sections perpendicular to mucosal folds (through bowel wall)
- Resection margins
- Representative sections of other organs present (appendix, terminal ileum, etc.)
- At least one representative section from grossly uninvolved colon
- Lymph nodes
- Representative lymph nodes (usually one cassette) suffices
Grossing - colectomy with tumor
- Identify and record the subsites of colon
- Identify the location of the lesion(s) by palpation
- Open the bowel along the uninvolved colonic wall using scissors
- Clean the lumen by gently washing out fecal material
- Ideally, pin the colon on a flat board; immerse in clean formalin, at least overnight
- Measure and record the dimensions of the specimen (length and circumferences), length of mesentery and size of omentum if present
- Measure and describe the lesion(s)
- Measure and record the distance of lesion(s) from margins
- Identify and ink the closest mesenteric margin (see Arch Pathol Lab Med 2009;133:1539 and diagram 3)
- Identify and ink the colonic radial resection margin if present
- Radial (retroperitoneal) margins are present in the proximal ascending colon and the descending colon
- Identify where tumor is close to or extending through a serosal surface and ink any suspicious serosal area with a different color
- Suspicious areas are those areas of serosa that are roughened, granular or appear hypervascular
- Inspect the entire bowel for polyps, other lesions or any abnormality
- All lymph nodes must be found and submitted in their entirety (must record whether nodes are bisected or trisected and submitted in one block or in multiple blocks)
- Best achieved by removing the fat close to the bowel wall and then using inspection and palpation to identify nodes
- Important to examine the fat that remains adhered to the bowel wall, as this is often a location for small nodes
- At least 12 lymph nodes are needed for accurate staging (Am J Surg Pathol 2002;26:179)
- For more tips of lymph node searching, see Grossing & features to report - lymph nodes
Grossing - total mesorectal excision (TME) specimens
- Identify anatomical landmarks and location of tumor by palpation
- Peritoneal reflection is low on the anterior aspect but high on the posterior aspect; the nonperitonealized tissue distal to the reflection is the radial resection margin
- Note that tumors in the upper / proximal rectum will have a serosal covering anteriorly and laterally and a radial margin posteriorly; mid to low / distal rectal tumors have a circumferential radial resection margin beneath the anterior peritoneal reflection
- Photograph in fresh state (useful for correlation with imaging and documentation of completeness of mesorectum)
- Anterior aspect
- Posterior aspect
- Other findings (e.g. significant defects)
- Open the specimen along the anterior aspect from the top and the bottom, leaving the bowel intact at a level just above and just below the tumor
- Place loose gauze (soaked in formalin) into the unopened ends of the bowel
- Fix all rectal cancer specimens for 72 - 96 hours in adequate volume of formalin
- Measure and record the dimensions of the specimen (length and circumferences)
- Evaluation of mesorectum completeness
- Bulk: good, moderate, little
- Surface: smooth or irregular
- Defect (record the depth of defect and extension)
- None > 5 mm
- > 5 mm but no visible muscularis propria
- Down to muscularis propria
- Coning: present or absent
- Completeness of the mesorectum is scored according to the worst area
- Complete (Cancer 2009;115:3400, J Clin Pathol 2007;60:849) (see gross images)
- Intact bulky mesorectum with a smooth surface
- Only minor irregularities of the mesorectal surface
- No surface defects greater than 5 mm in depth
- No coning towards the distal margin of the specimen
- After transverse sectioning, the circumferential margin appears smooth
- Nearly complete (Cancer 2009;115:3400) (see gross images)
- Moderate bulk to the mesorectum
- Irregularity of the mesorectal surface with defects greater than 5 mm but none extending to the muscularis propria
- No areas of visibility of the muscularis propria except at the insertion site of the levator ani muscles
- Incomplete (Cancer 2009;115:3400, J Clin Pathol 2007;60:849) (see gross images)
- Little bulk to the mesorectum
- Defects in the mesorectum down to the muscularis propria
- After transverse sectioning, the circumferential margin appears very irregular
- Complete (Cancer 2009;115:3400, J Clin Pathol 2007;60:849) (see gross images)
- Paint the bare area below the peritoneal reflection with ink
- Describe any other organs / tissues present as appropriate (e.g. vagina, prostate, bladder, etc.)
- Slice through the unopened bowel at 3 - 5 mm intervals
- Photograph (see above):
- All rings laid out sequentially from proximal to distal
- Close up photographs of any ring(s) to show significant findings (e.g. defects, tumor close to radial margin, etc.)
- Inspect slices to record:
- CRM (circumferential radial margin): smooth, regular, moderately irregular, very irregular
- Extent of tumor
- Closest distance of tumor to the CRM and whether it is anterior, posterior or lateral
- Obviously positive nodes and the distance of any positive node to the CRM
- Examine fat away from tumor for lymph nodes
- Inspect the entire bowel for polyps, other lesions or any abnormality
- Sections to submit:
- Tumor (entire tumor if 5 sections or less or 1 section per cm diameter)
- Deepest point of invasion (at least 2 sections)
- Relationship to radial margin (at least 2 sections; may be the same as sections showing deepest invasion)
- Relationship to serosa (at least 2 sections)
- If no obvious tumor following neoadjuvant treatment (Biomed Res Int 2015;2015:574540):
- Take at least 5 blocks from scarred area, including deepest point, relationship to radial margin and relationship to serosa
- If no viable tumour cells are identified microscopically, submit the entire scarred area
- If no viable tumour cells are found after submitting the entire scarred area, cut through at least three levels for each block
- If no viable tumour cells are present after the above steps, report as complete pathologic response or ypT0
- Longitudinal resection margins
- Interface with uninvolved bowel
- Relationship with other organs (if present)
- Uninvolved bowel
- Appendix, terminal ileum, cecum and ileocecal value, if present
- Any other abnormalities
- All the lymph nodes submitted in their entirety (must record whether nodes are bisected or trisected in 1 block or in multiple blocks)
- Tumor (entire tumor if 5 sections or less or 1 section per cm diameter)
Features to report for adenocarcinoma
- Editorial note
- "Mandatory" means for accreditation purposes by the American College of Surgeons Commission on Cancer
- "Recommended" means suggested by the literature
- Protocol may improve quality of pathology report (Colorectal Dis 2011;13:e33)
- Features to report by organization:
Colonic biopsy - mandatory to report
- Biopsy site
- Histologic type
Colonic biopsy - recommended but not required to report
- Tumor size
- Histologic grade
- Depth of invasion (if identifiable)
- Lymphovascular invasion
- Tumor budding
- Type of polyp it arises from (if applicable)
Polypectomy - mandatory to report
- Tumor site
- Specimen integrity (intact or fragmented)
- Polyp size
- Maximum dimensions of intact specimen and intact polyp are mandatory
- Type of polyp
- Histologic type
- Histologic grade
- Maximum size of invasive component
- Tumor thickness - vertical dimension
- Tumor extension
- Distance of invasive carcinoma from margin / polyp base
- Involvement of mucosal / lateral margin by invasive carcinoma or dysplasia
- Lymphovascular invasion
- Small vessel
- Large vessel: intramural or extramural
- Perineural invasion
- Ancillary studies performed (depending on local protocols)
- Microsatellite instability, immunohistochemistry for mismatch repair proteins, mutational analysis (required in some places in patients younger than 70, others in all patients)
Polypectomy - recommended but not required to report
- Aggregated dimensions of fragmented polyp or maximum dimensions of largest piece for fragmented polyp
- Maximum width of invasion
- Tumor budding: low, intermediate or high
- Additional findings
Colorectal resection for tumor - mandatory to report
- Specimen type
- Number of tumors (for multiple primary tumors, each tumor requires a complete report)
- Tumor site(s)
- Tumor location relative to anterior peritoneal reflection for rectal tumors: entirely above, below or straddling
- Tumor size
- Macroscopic tumor perforation
- Macroscopic intactness of mesorectum (required only for rectal tumors)
- Histologic type
- Histologic grade
- Tumor extension
- Margins
- Involved by invasive carcinoma or not
- Involved by low or high grade dysplasia or not
- If all margins are negative, specify closest margin and distance of invasive carcinoma from this margin
- Distance of tumor from CRM (required only for rectal tumors)
- Lymphovascular invasion: present or not identified
- Small vessel
- Large vessel: intramural or extramural
- Perineural invasion
- Treatment effect
- No known presurgical therapy
- Present
- Complete response: no viable cancer cells (can have acellular mucin present)
- Near complete response: single cells or rare small groups of cancer cells
- Partial response: more than single cells or rare small groups of residual cancer with tumor regression
- Poor or no response: extensive cancer with no evident tumor regression
- Tumor deposits (absence / presence and numbers)
- Regional lymph node: number of examined and number of involved
- Ancillary studies performed (depending on local protocols)
- Microsatellite instability, immunohistochemistry for mismatch repair proteins, mutational analysis (required in some places in patients younger than 70, others in all patients)
Colorectal resection for tumor - recommended but not required to report
- Margin:
- Rectum cancer: distance of tumor from negative distal margin
- Tumor budding: number of buds in 1 hotspot field (0.785 mm2)
- Low score: 0 - 4
- Intermediate score: 5 - 9
- High score: 10 or more
- Type of polyp in which invasive carcinoma arose
- Additional pathologic findings (e.g. adenoma, ulcerative colitis, Crohn’s disease, diverticulosis, etc.)
- Checklists: Michigan Cancer Consortium
Gross description
Sample gross description report
Polypectomy:
- The specimen is received in a properly labeled container with the patient's identifiers and accession number, designated as sigmoid polyp
- Specimen: brown polypoid piece(s)
- Number: 1
- Size: 1.2 cm
- Stalk(s): not identified
- Site(s) of attachment: inked
- Section code (entirely submitted):
- (A1) trisected
Colectomy:
Sample 1:
- The specimen is received in a properly labeled container with the patient's identifiers and accession number, designated as right hemicolectomy
- Specimen:
- Terminal ileum: 5.5 x 4.7 cm (length x circumference proximal margin)
- Appendix: 5.5 x 0.6 cm (length x greatest diameter)
- Colon: 13.5 x 8.4 cm (length x circumference distal margin)
- Proximal margin received stapled
- Distal margin received stapled
- Pericolic soft tissue: 8.0 cm in depth
- Number of tumors: 1
- Location(s): within the cecum at the appendiceal orifice
- Configuration: polypoid
- Size: 2.2 x 1.2 x 0.4 cm (length x width x thickness)
- Distance from longitudinal margins: 4.5 cm; 12.5 cm (proximal; distal)
- Extension into pericolic / rectal tissue: absent
- Extension into other organs or tissues: absent
- Tumor perforation: absent
- Serosa underlying tumor:
- Unremarkable
- Distance of tumor to closest serosa: 0.5 cm
- Radial margin:
- Closest distance of direct tumor extension to radial margin: 4.2 cm
- Lesions in nontumoral mucosa: diverticula
- Lymph nodes: within the pericolic soft tissue are multiple possible lymph nodes (0.2 - 0.8 cm)
- Ink code:
- Colon radial margin: black
- Serosal surface: blue
- Proximal and distal resection margins: black
- Section code (representative sections):
- (A1 - A5) entire polypoid lesion with closest serosa
- (A6) closest radial margin
- (A7) proximal margin
- (A8) distal margin
- (A9) appendix
- (A10) diverticulum
- (A11 - A13) multiple intact lymph nodes in each
- (A14 - A16) 1 lymph node in each
Sample 2:
- The specimen is received in a properly labeled container with the patient's identifiers and accession number, designated as rectosigmoid resection
- Specimen:
- Sigmoid colon and rectum 24.5 x 6.0 x 7.5 cm (length x circumference proximal margin x circumference distal margin)
- Proximal margin received stapled
- Distal margin received stapled
- Pericolic and perirectal soft tissue up to 4.5 cm in depth
- Number of tumors: 1
- Location(s): sigmoid colon
- Configuration: annular / circumferential
- Size: 4.1 x 2.2 x 1.5 cm (length x width x thickness)
- Distance from longitudinal margins: 13.0 cm; 6.5 cm (proximal; distal)
- Extension into pericolic / rectal tissue: present
- Extension into other organs or tissues: absent
- Tumor perforation: absent
- Serosa underlying tumor:
- Puckered with fibrous adhesions
- Distance of tumor to closest serosa: 0.0 cm
- Radial margin:
- Closest distance of direct tumor extension to radial margin: 1.2 cm
- Closest distance of involved lymph node or tumor nodule to radial margin: 0.0 cm
- Closest margin is mesenteric (vascular tie)
- Lesions in nontumoral mucosa:
- Polyp(s): 2 sessile polyps (0.3 and 0.6 cm) immediately distal to the mass
- Diverticula
- Lymph nodes: within the pericolic fat are multiple possible lymph nodes (0.2 - 2.8 cm)
- Ink code:
- Colon radial margin: black
- Serosal surface: blue
- Proximal and distal resection margins: black
- Section code (representative sections):
- (A1 - A3) mass and closest radial margin
- (A4 - A6) mass and deepest point of invasion
- (A7 - A8) mass and closest overlying serosa
- (A8) proximal margin
- (A9) distal margin
- (A10) 1 polyp
- (A11 - A12) 1 polyp, bisected
- (A13) lymph node abutting radial margin
- (A14 - A16) multiple intact lymph nodes in each
- (A17 - A20) 1 lymph node in each
- (A20 - A21) 1 lymph node
- (A22 - A25) 1 lymph node
Sample 3:
- The specimen is received in a properly labeled container with the patient's identifiers and accession number, designated as rectosigmoid resection
- Specimen:
- Sigmoid colon and rectum 13.0 x 2.8 x 7.0 cm (length x circumference proximal margin x circumference distal margin)
- Proximal margin received stapled
- Distal margin received stapled
- Pericolic and perirectal soft tissue up to 6.0 cm in depth
- Mesorectum: good bulk, intact with smooth surface
- Defects: none > 5 mm
- Coning: none
- Number of tumors: 1
- Location(s):
- Rectum
- Tumor at anterior peritoneal reflection
- Tumor 6.5 cm distal to posterior peritoneal reflection
- Rectum
- Configuration: annular / circumferential
- Size: 3.5 x 3.5 x 0.7 cm (length x width x thickness)
- Distance from longitudinal margins: 8.0 cm; 1.2 cm (proximal; distal)
- Extension into pericolic / rectal tissue: present
- Extension into other organs or tissues: absent
- Tumor perforation: absent
- Serosa underlying tumor:
- Unremarkable
- Distance of tumor to closest serosa: 0.2 cm
- Radial margin:
- Closest distance of direct tumor extension to radial margin: 0.4 cm
- Closest radial margin is left lateral
- Radial margin on cross section is smooth
- Lesions in nontumoral mucosa: diverticulum
- Lymph nodes: multiple possible tan lymph nodes (0.5 to 2.0 cm) identified
- Ink code:
- Colon radial margin: black
- Rectal posterior radial margin: black
- Rectal anterior radial margin: green
- Serosal surface: blue
- Proximal and distal resection margins: black
- Section code (representative sections):
- (A1) proximal margin
- (A2) distal margin with tumor
- (A3 - A4) tumor, closest to serosa
- (A5) tumor, closest to radial margin
- (A6 - A8) tumor, deepest point of invasion
- (A9) diverticulum
- (A10 - A13) 3 possible lymph nodes in each
- (A14) 2 possible lymph nodes
- (A15 - A17) 1 possible lymph node in each
- (A18 - A20) multiple possible lymph nodes in each
Diagrams / tables
Table 1: sectioning protocol based on polyp size
| | |
| Smaller than 0.4 cm | No sectioning |
| 0.4 - 0.8 cm | Bisect; place in 1 cassette |
| 0.9 - 1.2 cm | Trisect by shaving two sides off central section with stalk; place central section in separate cassette |
| Larger than 1.2 cm | More than 3 sections as appropriate |
Images hosted on other servers:
Additional references
Board review style question #1
You are grossing a subtotal colectomy specimen for a biopsy proven colon adenocarcinoma. You find 50 lymph nodes with 15 of them larger than 1 cm in size. Which of the following will be the most appropriate sections to submit?
- All lymph nodes submitted in their entirety
- Lymph nodes larger than 1 cm submitted in their entirety
- Representative sections of all lymph nodes
- Representative sections of lymph nodes larger than 1 cm and in toto of lymph nodes smaller than 1 cm
Board review style answer #1
A. All lymph nodes submitted in their entirety
Comment Here
Reference: Colon - Grossing & features to report
Comment Here
Reference: Colon - Grossing & features to report
Board review style question #2
You are grossing a TME specimen and found a defect on the mesorectum under the peritoneal reflection down to the muscularis propria. How would you report on the macroscopic evaluation of mesorectum?
- Cannot be determined
- Complete
- Incomplete
- Near complete
Board review style answer #2
Hemangioma
Table of Contents
Definition / general | Essential features | Sites | Clinical features | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Uncommon benign vascular proliferation of the colon
Essential features
- Benign vascular proliferation
- Often related to a syndrome
- Typically a cavernous hemangioma (majority) or capillary hemangioma
Sites
- Most common site in large intestine is rectum
- More common in small intestine than large intestine
Clinical features
- Causes bleeding, melena, anemia, rarely intussusception or obstruction
- Patients are often young and may have a syndrome (blue rubber bleb nevus syndrome, Klippel-Trénaunay syndrome, etc.) (Clin Colon Rectal Surg 2011;24:193)
Case reports
- 1 year and 8 year old boys with vascular lesions of the colon (Korean J Pediatr 2014;57:245)
- 26 year old woman with hemangiomas in rectosigmoid colon and other sites (Turk J Gastroenterol 2006;17:308)
Gross description
- Well circumscribed reddish lesion
Microscopic (histologic) description
- Cavernous hemangioma: localized or diffuse; blood filled sinus-like spaces with scant connective tissue, variable smooth muscle; may be infiltrative but no other concerning features
- Capillary hemangioma: small, closely packed capillaries, rarely multifocal; no features suggestive of malignancy (Dtsch Med Wochenschr 2004;129:1970)
- Other rare variants with distinctive morphology can occur, such as anastomosing hemangioma (Am J Surg Pathol 2013;37:1761)
Microscopic (histologic) images
Sample pathology report
- Sigmoid colon, resection:
- Segment of colon with submucosal cavernous hemangioma (1.4 cm)
- Margins of resection unremarkable.
- Six benign lymph nodes.
Differential diagnosis
- Arteriovenous malformation:
- Abnormal arteries and veins; distinction from hemangiomas may be difficult
- Angiosarcoma:
- Rare; overtly malignant
- Florid vascular proliferation:
- Linked to intussusception or prolapse; spindled lesion with increased cellularity (Mod Pathol 2001;14:1114)
Board review style question #1
What is the most common subtype of hemangioma encountered in the colon?
- Anastomosing hemangioma
- Capillary hemangioma
- Cavernous hemangioma
- Glomeruloid hemangioma
Board review style answer #1
Hepatoid carcinoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Rare, aggressive subtype of adenocarcinoma that morphologically resembles hepatocellular carcinoma and produces alpha fetoprotein
Essential features
- Extremely rare subtype of colorectal carcinoma
- Resembles hepatocellular carcinoma morphologically and sometimes immunohistochemically
- Serum alpha fetoprotein may be elevated
Terminology
- Originally termed alpha fetoprotein producing tumor
Epidemiology
- More common in men
- Fewer than 20 colonic cases reported
Sites
- Hepatoid carcinoma has also been reported in other sites, including stomach, gallbladder and uterus (World J Gastroenterol 2013;19:321)
- Also ampulla, bladder, lung, ovary
Clinical features
- Can occur in patients with ulcerative colitis (World J Gastroenterol 2014;20:12657)
- Serum alpha fetoprotein may be elevated (Hepatobiliary Pancreat Dis Int 2008;7:539)
Case reports
- 42 year old man with rectal hepatoid carcinoma with liver metastases (Hepatobiliary Pancreat Dis Int 2008;7:539)
- 59 year old man with colonic α fetoprotein producing carcinoma (Dis Colon Rectum 2003;46:826)
- 71 year old man with hepatoid carcinoma colliding with liposarcoma (World J Surg Oncol 2007;5:42)
- Rectal tumor arising in ulcerative colitis (Dis Colon Rectum 2000;43:105)
- Sigmoid alpha fetoprotein producing adenocarcinoma (Pathology 1995;27:277)
Treatment
- Surgery, possibly chemotherapy (Pathol Oncol Res 2012;18:93)
Microscopic (histologic) description
- Resembles hepatocellular carcinoma, with trabecular or solid pattern of large polygonal cells with abundant eosinophilic or clear cytoplasm
- Component of traditional adenocarcinoma also often present
- Lymphovascular invasion is common (Histopathology 1997;31:47)
Microscopic (histologic) images
Positive stains
- Alpha fetoprotein, CEA, glypican 3
- Variable Hep Par1, CDX2, CK18, CK19 (Histopathology 2007;51:123)
Sample pathology report
- Sigmoid colon, resection:
- Hepatoid carcinoma of the colon, moderately differentiated (see synoptic report)
Differential diagnosis
- Metastatic hepatocellular carcinoma:
- Unusual in colon; negative for CDX2 and (usually) CK19 (J Gastroenterol Hepatol 2000;15:536)
Board review style question #1
What serum marker is often elevated in patients with hepatoid colon carcinoma?
- Alpha fetoprotein
- CA125
- CA19-9
- HER2
Board review style answer #1
Hirschsprung disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Absent enteric ganglion cells in submucosal and myenteric plexuses in distal rectum and variable length of contiguous intestine, causing functional obstruction and colonic dilation proximal to affected segment (eMedicine: Hirschsprung Disease [Accessed 9 January 2020])
Essential features
- Diagnostic: Absent ganglion cells, submucosal nerve hypertrophy, absent calretinin immunoreactive mucosal innervation and increased cholinergic mucosal innervation (AChE histochemistry or ChT immunohistochemistry)
- Intraoperative:
- Levelling biopsy: identification of ganglion cells
- Frozen section of proximal resection margin: exclude features of transition zone (partial circumferential aganglionosis, myenteric hypoganglionosis, submucosal nerve hypertrophy)
Terminology
- Also called congenital aganglionic megacolon
- Short segment (~80% of patients): aganglionic portion limited to rectosigmoid colon; M:F ≈ 4:1
- Long segment (~20% of patients): aganglionic segment extends proximal to the sigmoid colon, may extend into small bowel; M:F approaches 1:1 as length of aganglionic segment increases; some regard long segment as proximal to splenic flexure and “colonic” as aganglionosis extending to somewhere between sigmoid colon and splenic flexure
- Very (ultra) short segment: aganglionic portion restricted to < 2 cm of the distal most rectum; more common in boys; potentially more difficult to document because this portion of rectum is normally hypoganglionic
- Total colonic aganglionosis (TCA): uncommon; M:F ≈ 1:1; clinical and genetic differences; rectal biopsy may lack submucosal nerve hypertrophy; more difficult to diagnose and manage (Pediatr Surg Int 2016;32:221)
- Pan-intestinal aganglionosis: very rare; aganglionosis of entire small and large intestines
- Skip area (or skip lesion): aganglionic segment is interrupted by a focus or segment of ganglionic bowel; proximal aganglionic portion frequently includes appendix + / - adjacent cecum and distal ileum
- Zonal colonic aganglionosis: interstitial aganglionic segment flanked proximally and distally by ganglionic bowel; distal rectum innervated normally; pathogenesis (likely secondary disruption as opposed to malformation) considered different from classic Hirschsprung disease
- Transition zone: ganglionic but neuroanatomically abnormal, bowel located proximal to aganglionic segment; typically < 5 cm in short segment aganglionosis; may be longer in long segment aganglionosis; may cause persistent obstructive symptoms if not resected with aganglionic segment (Am J Surg Pathol 2016;40:1637)
Epidemiology
- Average: 1 in 5,000 live births (0.75 in Caucasians; 2.8 in Asians) (Clin Genet 2020;97:114)
- Male predominance for short segment aganglionosis (3 - 4.5:1)
- ~80% sporadic
- ~18% have other congenital anomalies; some with specific syndromes
- ~12% have major chromosomal variants; ~10% associated with Down syndrome
- About 5% have neurologic abnormalities that may be serious
- Occasionally associated with intestinal atresia and malrotation, anorectal malformations and other abnormalities of the GI tract
- 5% have a sibling with disease; variation in risk depending on sex, segment length and familiality
- Patients usually present as neonates; 80% present before their first birthday but up to 10% of cases are diagnosed in adulthood
Sites
- Classic Hirschsprung disease invariably includes aganglionosis of the distal rectum and variable length of contiguous bowel
- Short segment, long segment, total colonic and pan-intestinal forms as defined above
Pathophysiology
- Absence of enteric ganglia causes a loss of intrinsic excitatory and inhibitory innervation; net effect is spastic contraction of aganglionic segment, including internal anal sphincter, with impaired peristalsis and defecation
- Bowel proximal to the aganglionic segment undergoes muscular hypertrophy and dilation
- Abnormal extrinsic innervation (enlarged cholinergic nerves) in aganglionic rectum + / - more proximal aganglionic left colon may contribute to spasticity
- Numerous alterations in expression of various receptors, channels, cytoskeletal proteins, neurotrophic factors and other molecules described in ganglionic bowel but functional significance unclear (Pediatr Dev Pathol 2020;23:40)
Etiology
- Proximate cause is failure of neural crest derived neuroglial progenitors to colonize the entire length of the intestinal tract (Dev Biol 2016;417:158)
- Complex genetic basis with at least 24 susceptibility genes; variable penetrance for most genetic alterations; combinatorial effects of low penetrance gene variants (N Engl J Med 2019;380:1421)
- Coding variants and large copy number variants associated with highest risk but less frequently implicated than noncoding variants
- Coding or noncoding mutations RET proto-oncogene appear to play some role in the majority cases
Diagrams / tables
Clinical features
- Most common presentation: failure to pass meconium within 48 hours of birth
- Other findings may include abdominal distension, bilious emesis, explosive defecation in response to manual anal dilatation, bowel perforation and enterocolitis
- Older children may have chronic constipation and failure to thrive
- Down syndrome patients more commonly have associated enterocolitis
- 80% male; usually sporadic (1 per 5,000 live births); 5% have affected sibling
- 10% have Down syndrome; another 5% have other serious neurologic impairment
- Complications: proximal innervated colon may become massively distended (15 cm in diameter) with muscular wall hypertrophy and rupture / perforation, usually near cecum or appendix; also acute intestinal obstruction, enterocolitis with fluid and electrolyte imbalance
- Mortality: currently 5 - 10%
- Eur J Pediatr Surg 2008;18:140
Diagnosis
- Screening tests
- Anorectal manometry - absent rectoanal inhibitory reflex (RAIR, relaxation of internal anal sphincter in response to balloon dilatation of the rectum)
- Contrast enema - ratio of rectal diameter to sigmoid diameter is < 1
- Rectal biopsy, either suction or incisional
- Suction rectal biopsy: most common, does not require sedation in neonate or young infant, samples mucosa and submucosa; no direct visualization of rectum or anal canal; recommend multiple biopsies at different sites (e.g., 2, 3 and 4 cm above the anal verge)
- Incisional biopsy: requires anesthesia, some prefer this for patients > 1 year of age, may sample myenteric plexus in addition to mucosa and submucosa; direct visualization of dentate line
- Pediatr Dev Pathol 2020;23:8
Case reports
- Newborn with volvulus, intestinal malrotation and total colonic aganglionosis (J Neonatal Perinatal Med 2019)
- 2 day old girl with colonic atresia and Hirschsprung disease (Arch Iran Med 2015;18:322)
- 10 day old boy with Hirschsprung disease and a distal skip area (Fetal Pediatr Pathol. 2019;38:437)
- 21 year old man and 22 year old woman who presented with Hirschsprung disease as young adults (Ann Med Surg (Lond) 2019;48:59)
- Biopsy findings in an infant with trisomy 21 and very short segment Hirschsprung disease (Pediatr Dev Pathol 2016;19:87)
Treatment
- Surgical: excision of aganglionic segment and transition zone (ganglionic but neuroanatomically abnormal bowel immediately proximal to the aganglionic segment) with a pullthrough procedure to anastomose ganglionic bowel just superior to the anal canal
- One stage (resection and anastomosis) or two stage (ostomy then later resection and anastomosis)
- Levelling biopsy with intraoperative frozen section should be performed during either procedure to insure ganglionic bowel proximal to aganglionic segment is used for anastomosis
- Biopsy is seromuscular or full thickness
- Identification of myenteric ganglion cells is the key; rebiopsy of more proximal bowel is necessary until ganglion cells are located
- 5 - 10 well oriented sections is usually sufficient
- H&E is satisfactory; some prefer Diff-Quik, Giemsa or toluidine blue
- Use of Giemsa, Diff-Quik or toluidine blue stains in addition to routine H&E has been advocated to improve diagnostic accuracy at frozen section
- Presence of ganglion cells in seromuscular biopsy does not exclude transition zone
- To reduce likelihood of transition zone pullthrough or ostomy, intraoperative frozen section of a full circumference (donut) section from the proximal resection margin (or ostomy site) is recommended (Pediatr Dev Pathol 2020;23:23)
- Histological features of transition zone include:
- Partial circumferential aganglionosis (⅛ or more of circumference devoid of ganglion cells)
- Myenteric hypoganglionosis (⅛ or more of circumference predominantly tiny ganglia with 1 or 2 ganglion cells and minimal neuropil)
- Submucosal nerve hypertrophy (e.g., > 2 submucosal nerves > 40 µm diameter in a 400x field)
- Histological features of transition zone include:
- Surgery successful in most patients
- Treatment failures may be due to transition zone pullthrough, strictures at anastomosis site, twists proximal to the anastomosis, excessive retained aganglionic distal rectum, post-surgical loss of ganglion cells proximal to anastomosis or poorly understood physiological abnormalities in the ganglionic bowel
Gross description
- Aganglionic segment typically narrow with dilated proximal ganglionic bowel and funnel shaped transition near interface
- Resection specimen from Soave pullthrough procedure lacks muscularis propria distally; sleeve of submucosa and mucosa at distal end
- Resection specimen from Swenson or Duhamel pullthrough procedure is full thickness throughout
- Histological sampling of resection should include:
- Full circumference adjacent to proximal margin (confirm normal neuroanatomy)
- Full circumference at distal margin (confirm aganglionosis)
- One or more longitudinal strip of entire resection or closely spaced transverse sections along the length of the resection (establish length of aganglionic segment)
- J Pediatr Surg 2019;54:2017
Gross images
Microscopic (histologic) description
- Aganglionic segment
- Complete absence of submucosal and myenteric ganglion cells
- Submucosal and myenteric nerve hypertrophy in aganglionic rectum; gradually lost in more proximal aganglionic colon; may not be prominent in total colonic aganglionosis; usually assessed subjectively but in neonate nerves > 40 µm in diameter are abnormal; enlarged nerves have extrinsic features including Glut1 positive perineurium but occasional Glut1+ nerves are also present in normal rectum
- Transition zone (any of the following)
- Partial circumferential aganglionosis (⅛ or more of circumference devoid of ganglion cells)
- Myenteric hypoganglionosis (⅛ or more of circumference predominantly tiny ganglia with 1 or 2 ganglion cells and minimal neuropil)
- Submucosal nerve hypertrophy (e.g., > 2 submucosal nerves > 40 µm diameter in a 400x field)
- Ganglia in newborns are smaller than in older children and nucleoli and Nissl substance may be lacking, a situation even more pronounced in premature infants
- Myenteric ganglia in the transition zone may be ectopically situated within the muscularis externa or serosa
- Associated nerve hypertrophy is a helpful clue but is not diagnostic
- Active mucosal inflammation (enterocolitis)
- Stercoral ulcers (sharply demarcated shallow ulcers with mucosal inflammation due to pressure of feces on obstructed colon)
- Fibromuscular dysplasia of submucosal or seromuscular arteries in aganglionic segment or transition zone (Pediatr Dev Pathol 2018;21:363)
Microscopic (histologic) images
Contributed by Raj P. Kapur, M.D., Ph.D.
Positive stains
- Most diagnostically useful are acetylcholinesterase histochemistry (AChE) or choline transporter immunohistochemistry; both demonstrate increased cholinergic innervation in lamina propria and muscularis mucosa
- AChE requires frozen sections and special methodology (Fetal Pediatr Pathol 2016;35:399)
- Choline transporter immunohistochemistry: formalin fixation and paraffin embedding; relatively new and has not been validated in many laboratories; may be more challenging to interpret than AChE (Pediatr Dev Pathol 2017;20:308)
Negative stains
- Calretinin immunohistochemistry
- Most widely used ancillary method
- Run routinely with all rectal biopsies to exclude aganglionosis
- Absence of calretinin staining in muscularis mucosae and lamina propria of aganglionic segment
- Negative result pertains only to mucosa; submucosal nerves retain calretinin positive neurites are retained in submucosal nerves
- Immunoreactive mucosal nerves may be present in rectal biopsy from patient with very short segment aganglionosis due to descending fibers from transition zone (Pediatr Dev Pathol 2014;17:28)
- Many antibodies available to identify neuronal cell bodies (e.g., Hu C/D, Phox2B); seldom used in practice as H&E is sufficient
Electron microscopy description
- Not clinically relevant
- Hypertrophic nerves in aganglionic segment resemble extrinsic peripheral nerves (perineurium, endoneurial collagen, non-myelinating Schwann cells)
Molecular / cytogenetics description
- Complex genetics as described above; see Etiology
- Formal testing generally not indicated but karyotype, genomic array or mutational analysis may be pursued for syndromic cases (e.g., Waardenburg-Shah syndrome, Down syndrome, multiple endocrine neoplasia type 2A)
Sample pathology report
- Rectal biopsy
- Rectum, suction biopsies: findings diagnostic of Hirschsprung disease
- No identifiable ganglion cells
- Submucosal nerve hypertrophy
- Absent calretinin immunoreactive mucosal innervation
- Rectum, suction biopsies: findings diagnostic of Hirschsprung disease
- Pullthrough resection
- Rectosigmoid colon, Soave resection (12.5 cm): Hirschsprung disease with the following features:
- Aganglionosis (distal 4 cm)
- No significant neuromuscular pathology at the proximal surgical margin
- Rectosigmoid colon, Soave resection (12.5 cm): Hirschsprung disease with the following features:
- Resection synoptic report
- Can be used for diagnosis or as comment after diagnosis
- Online resource with dropdown menus for report preparation: https://hematogones.com/surg-path/hirsch
Differential diagnosis
- Clinical
- Chronic idiopathic pseudo-obstruction
- Clinical condition with heterogeneous etiologies, most affect small and large intestine, some primary (e.g., visceral myopathy; myenteric hypoganglionosis), some secondary (e.g., hypothyroidism)
- Megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS)
- Bladder and intestinal dysmotility
- Typically presents pre- or perinatally
- Mutations in genes for smooth muscle contractile proteins
- X-linked intestinal pseudo obstruction
- Meconium ileus
- Especially with perforation, consider cystic fibrosis
- Anatomic intestinal obstruction (e.g., atresia, volvulus, stenosis)
- Anal achalasia
- Spasticity of internal anal sphincter with absent RAIR and reduced nitrergic innervation of sphincter muscle (Pediatr Surg Int 2013;29:855)
- Chronic idiopathic pseudo-obstruction
- Histological (clinical mimics with different diagnostic findings in rectal biopsy)
- Intestinal neuronal dysplasia type B
- Controversial pattern of submucosal hyperganglionosis (Arch Pathol Lab Med 2019;143:235)
- Neuroglial hamartoma syndromes (multiple endocrine neoplasia type 2B, neurofibromatosis, PTEN-hamartoma syndromes)
- Ganglioneuromatous hyperplasia of submucosal and/or myenteric plexuses, + / - mucosal ganglioneuromas
- Investigate clinical history and extra enteric findings
- Confirm with molecular genetic testing
- Primary visceral myopathy
- Changes rarely diagnostic in muscularis propria of rectal biopsy
- Key features include muscle atrophy and fibrosis as well as myocyte vacuolar degeneration
- Mitochondriopathy
- Changes rarely diagnostic in muscularis propria of rectal biopsy
- Key features include muscle atrophy and fibrosis as well as eosinophilic megamitochondria in enteric ganglia
- Intestinal neuronal dysplasia type B
Additional references
- Pathogenesis oriented review (Nat Rev Gastroenterol Hepatol 2018;15:152)
- Normative data for rectal submucosal nerve thickness (J Pediatr Surg 2016;51:1585)
- Puri: Hirschsprung's Disease and Allied Disorders, 4th Edition, 2019
Board review style question #1
A neonate with Hirschsprung disease, diagnosed by suction rectal biopsy, undergoes primary endorectal pullthrough surgery. During the operation, a leveling seromuscular biopsy from the proximal sigmoid colon is sent for frozen section diagnosis. Which of the following diagnoses and recommendations is appropriate, based on the microscopic field shown in the above image?
- No definitive ganglion cell, obtain another biopsy from an upstream location
- Ganglion cells present, recommend pullthrough resection at least 5 cm proximal to site of this biopsy
- Ganglion cells present, insist on rebiopsy of rectum to confirm Hirschsprung disease before resection
- Probably intestinal neuronal dysplasia, recommend additional upstream biopsies to define extent of dysplastic ganglia
- Inadequate sample to assess for ganglion cells, rebiopsy
Board review style answer #1
B. Ganglion cells present, recommend pullthrough resection at least 5 cm proximal to site of this biopsy
Comment Here
Reference: Hirschsprung disease
Comment Here
Reference: Hirschsprung disease
Board review style question #2
A rectal biopsy to exclude Hirschsprung disease may be considered inadequate if it demonstrates
- Submucosal nerve hypertrophy
- Absent calretinin immunoreactive mucosal innervation
- Crowded, coarse, acetylcholinesterase positive mucosal
- Transitional mucosa
- Absent submucosal ganglion cells
Board review style answer #2
Histoplasmosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Histoplasmosis is an invasive mycosis caused by inhalation of the spores of dimorphic fungi Histoplasma capsulatum (Int J Surg Pathol 2017;25:592)
- The most common endemic mycoses in the United States and in certain areas of Mexico, Central America and South America (Infect Dis Clin North Am 2016;30:207)
Essential features
- Invasive mycosis caused by inhalation of the spores of dimorphic fungi Histoplasma capsulatum
- Distal ileum and cecum are most commonly affected, followed by the rectum and descending colon
- Tissue culture and histopathology remain the gold standard for the diagnosis of colonic histoplasmosis
- Small, round to ovoid, narrow based budding yeast cells measuring 2 - 5 micrometers
- Stains positive for Gomori methenamine silver (GMS) stain and periodic acid-Schiff (PAS); negative for mucicarmine and Fontana-Masson silver stain
Terminology
- Also called Cave disease, Darling disease, Ohio Valley disease, reticuloendotheliosis, Spelunker lung and Caver disease (BMJ Case Rep 2016;2016:bcr2016216423)
ICD coding
Epidemiology
- Occurs widely but is highly endemic in Ohio, Mississippi river valleys and Latin America
- Also endemic in Asia, Southeast Asia and India
- Centers for Disease Control records estimate 50,000 infections yearly (Infect Dis Clin North Am 2016;30:207)
- Gastrointestinal (GI) infection by Histoplasma is common and a manifestation of disseminated disease
- Gastrointestinal tract is involved in 50 - 70% of disseminated cases
- Occurs in 0.05% cases in immunocompetent hosts
- Symptoms occur in 3 - 12% of patients (Gastrointest Endosc 2020;91:951, Int J Surg Pathol 2017;25:592)
- At autopsy, about 70% of patients with disseminated histoplasmosis have GI involvement (South Med J 2004;97:172)
Sites
- Distal ileum and cecum are most commonly affected, followed by the rectum and descending colon
- Colon is among the most frequently affected sites in GI (Gastrointest Endosc 2020;91:951)
Pathophysiology
- Dimorphic fungus exists as a mold in the environment
- Once inhaled, it enters the lung and transforms into yeast form at 35 - 37 °C
- Alveolar macrophages phagocytose these yeast forms, which multiply intracellularly until the advent of immune response
- Primary infection is usually asymptomatic and becomes reactivated in immunocompromised states
- Usually manifests as acute or chronic pulmonary disease
- Severe cases: disseminated to the skin, adrenal gland, central nervous system, lymph nodes, spleen, bone marrow and GI tract (Int J Surg Pathol 2017;25:592)
- In most healthy individuals, nonviable organisms persist in granulomas
Etiology
- Risk factors: HIV / AIDS, autoimmune disease, old age, organ transplant, hepatitis C virus infection, hematologic malignancies, corticosteroid use, tumor necrosis factor antagonists, primary immunodeficiency syndromes (ACG Case Rep J 2021;8:e00598, BMC Infect Dis 2013;13:143)
- Most commonly linked to farming, exposure to chicken coops or caves, remodeling or demolition of old buildings and cutting down trees or clearing brush from sites in which blackbirds have roosted (Infect Dis Clin North Am 2016;30:207)
Clinical features
- Symptoms: fever, diarrhea, altered bowel habits, GI bleeding (ACG Case Rep J 2021;8:e00598)
Diagnosis
- Multifaceted approach: history, clinical features, radiographic / endoscopic findings, blood, stool, tissue culture, antigen and antibody testing and molecular testing
- The European Organization for Research and Treatment of Cancer / Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC / MSG) criteria for the diagnosis of invasive fungal infections:
- Proven diagnosis: confirmation by either histopathology or culture
- Probable diagnosis: the presence of an appropriate clinical presentation, a predisposing condition and mycological evidence, such as the presence of antigenuria (J Clin Microbiol 2017;55:1612)
- Tissue culture and histopathology remain the gold standard for the diagnosis of colonic histoplasmosis (Case Rep Med 2018;2018:2723489, J Fungi (Basel) 2020;7:12)
- Slow, takes 4 - 6 weeks
Laboratory
- Culture and microbiology stains:
- Histoplasma capsulatum stains poorly for Gram stain
- Calcuflour white binds to chitin in fungal cell walls and helps in identification
- Colonies show septate hyphae followed by smooth or less commonly spiny microconidia (2 - 5 micrometers in size) and characteristic tuberculate macroconidia (7 - 15 micrometers)
- Small, round to oval, narrow based budding yeast forms at 37 °C
- Antigen detection:
- Galactomannan antigen testing in body fluids: rapid test (Infect Dis Clin North Am 2016;30:207)
- Sensitivity of enzyme immunoassay (EIA) is high in patients with disseminated disease (95%)
- Urine antigen detection: more sensitive than serum
- EIA's quantitative nature also allows for sequential monitoring of antigen clearance
- Crossreactivity can be seen with Blastomyces dermatitidis, Paracoccidioides brasiliensis, T. marneffei and less commonly, Coccidioides immitis and Coccidioides posadasii
- Antibody detection:
- Antibodies detectable after 4 - 8 weeks
- More suitable for diagnosis of subacute or chronic histoplasmosis
- A titer of ≥ 1:32 or 4 fold rise in antibody titers from acute to convalescent phase is strongly suggestive of acute infection
- Antibodies detectable after 4 - 8 weeks
- Molecular methods: polymerase chain reaction (PCR) assays
- Role uncertain
- Variable sensitivities reported in published studies (J Clin Microbiol 2017;55:1612)
Radiology description
- Bowel wall thickening, mass lesions, signs suggesting bowel obstruction, free peritoneal air (J Med Microbiol 2010;59:610)
- Other findings of disseminated disease: hepatosplenomegaly, pulmonary infiltrates, generalized lymphadenopathy
Prognostic factors
- Prognosis is good after early and aggressive treatment; the disease is fatal if remains untreated (Int J Surg Pathol 2017;25:592)
- Improvements in both antiretroviral and antifungal therapies appear to have contributed to a lower prevalence and may favorably impact the prognosis of this disease (Diagn Microbiol Infect Dis 2006;55:195)
Case reports
- 30 year old cachectic woman with a history of HIV and poor compliance with highly active antiretroviral therapy (HAART) presents with diffuse abdominal pain and fever (Cureus 2020;12:e7775)
- 39 year old man with HIV presents with severe pain in the abdomen (Case Rep Med 2018;2018:8923972)
- 57 year old asymptomatic man with a history of orthotopic liver transplant was found to have sessile polyps on elective surveillance colonoscopy (ACG Case Rep J 2021;8:e00598)
- 60 year old woman presents with recurrent diarrhea and abdominal discomfort (Cureus 2018;10:e2951)
- 60 year old man presents with 75 pound weight loss and dysphagia, and was found to have idiopathic Cd4 T lymphocytopenia (Cureus 2021;13:e19748)
Treatment
- According to the Infectious Diseases Society of America, clinical guidelines for the treatment of GI histoplasmosis without CNS involvement: amphotericin B (300 mg/kg daily) for 1 - 2 weeks followed by oral itraconazole (200 mg 3 times daily for 3 days and then 200 mg twice a day for a total of at least 12 months) (Infect Dis Clin North Am 2016;30:207)
Clinical images
Gross description
- Focal / multiple mucosal ulcerations, polypoid lesions, strictures, obstruction due to circumferential thickening (Gig Sanit 1986:39)
Microscopic (histologic) description
- Colonic tissue shows diffuse expansion of lamina propria and submucosa by foamy histiocytes associated with lymphocytes and eosinophils; mucosal ulceration can be seen frequently (Clin Chest Med 2009;30:217)
- Granulomas are less frequently observed
- Organisms are usually intracellular within histiocytes, rarely extracellular mixed with ulcer debris (Am J Clin Pathol 2000;113:64)
- Protoplasm of the organisms retracts during fixation, resulting in a pseudocapsule on routine H&E (Pathologe 2022;43:16)
- GMS and PAS special stains highlight the yeast cell wall and are helpful for characterization of the organisms
- Romanowsky type stains, such as the Wright-Giemsa stain, impart a dark blue hue to the fungal nuclear compartment
- Yeast forms can also pick up Ziehl-Neelsen stain (J Pathol Transl Med 2017;51:482)
- H. capsulatum var. capsulatum: small, round to ovoid, narrow based budding yeast forms measuring 2 - 5 micrometers
- H. capsulatum var. duboisii are larger and measure 6 - 12 micrometers (Infect Dis Clin North Am 2016;30:207)
Microscopic (histologic) images
Virtual slides
Negative stains
Videos
Histoplasmosis colitis
Colonoscopy in histoplasmosis
Sample pathology report
- Colon, cecum, ulcer, biopsy:
- Mucosal and submucosal histiocytic inflammation containing numerous yeasts, morphologically compatible with Histoplasmosis capsulatum (positive GMS and PAS stains, negative mucicarmine and Fontana-Masson stains)
Differential diagnosis
- Infections mimicking histoplasmosis:
- Candida, Pneumocystis, Talaromyces, Cryptococcus, Blastomyces and leishmaniasis:
- Candida glabrata resembles Histoplasma but shows variably sized yeast cells and more frequent budding, lacks halos, is Gram stain positive and is usually extracellular
- Pneumocystis jiroveci and Talaromyces marneffei also resemble Histoplasma but rarely involve colon (Pathologe 2022;43:16)
- Pneumocystis can show GMS and PAS positivity but cysts are larger (5 - 8 micrometers), do not show budding and are predominantly extracellular
- Mucicarmine stains Cryptococcus capsule (3 - 8 micrometers) and produces characteristic halos
- Unencapsulated forms of Cryptococcus: Fontana-Masson stain can be used to stain cryptococcal melanin
- Blastomyces are larger (up to 15 micrometers) and show broad based budding and thick walls and can stain positive for Congo red (J Clin Microbiol 2017;55:1612, J Cutan Pathol 2008;35:27)
- Leishmaniasis shows small (2 - 4 micrometers) round to oval organisms with bar shaped kinetoplast and stains negative on GMS, positive on Giemsa and is usually extracellular (Am J Trop Med Hyg 2010;83:209)
- Candida, Pneumocystis, Talaromyces, Cryptococcus, Blastomyces and leishmaniasis:
- Granulomatous / IBD colitis:
- No fungal organisms
- Malakoplakia:
Additional references
Board review style question #1
A 45 year old man with a history of HIV presents with abdominal pain and diarrhea. Colonoscopy showed erythematous mucosal ulceration in the cecum. Biopsy from the ulcer showed numerous foamy histiocytes. GMS stain highlights numerous intracellular small, round to oval organisms as shown in the above image. Organisms stain positive with PAS and negative with mucicarmine. What is the diagnosis?
- Candidiasis
- Cryptococcosis
- Histoplasmosis
- Leishmaniasis
Board review style answer #1
C. Histoplasmosis. GMS stain highlights numerous intracellular round to oval yeast forms showing narrow based budding, morphologically consistent with histoplasmosis.
Comment Here
Reference: Histoplasmosis
Comment Here
Reference: Histoplasmosis
Board review style question #2
The organisms shown in this image stain positive for GMS and PAS and negative for mucicarmine. What is the gold standard for diagnosis of the tissue invasive disease caused by these organisms?
- Clinical history and imaging findings
- Imaging findings and serology testing
- Serology testing and histopathological examination
- Tissue culture and histopathological examination
Board review style answer #2
D. Tissue culture and histopathological diagnosis. The diagnosis is histoplasmosis. Clinical history and imaging findings can be nonspecific in histoplasmosis, while serology testing can be false negative or show crossreactivity with other organisms. Tissue culture isolates the organisms and histopathological examination with H&E and special stains help in definitive diagnosis.
Comment Here
Reference: Histoplasmosis
Comment Here
Reference: Histoplasmosis
HIV/AIDS associated
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Major causes of gastrointestinal disease in patients who are infected with HIV are opportunistic infections and AIDS associated neoplasms (Radiology 1992:184:761, Arch Pathol Lab Med 2012;136:305)
- Most opportunistic infections happen when the CD4+ T cell count is less than 200/mm3
- CMV infection is the most common opportunistic infection of the bowel (Saudi J Gastroenterol 2009;15:95)
- Other protozoal, viral and bacterial pathogens that can cause opportunistic infections are: cryptosporidium, cyclospora, entamoeba, giardia, herpes, isospora, microsporidia, mycobacteria, parasites, salmonella, shigella, strongyloides
- Kaposi sarcoma and non-Hodgkin lymphoma (NHL) were the most common AIDS defining malignancies (Arch Pathol Lab Med 2012;136:305)
- Primary HIV infection of the gastrointestinal tract has also been described in absence of any known etiological agent
Essential features
- Gastrointestinal tract is a major site for HIV replication; virus localizes in gastric mucosal tissue and depletes CD4+ T cells
Terminology
- AIDS colitis
ICD coding
- AIDS (related complex) B20
Epidemiology
- HIV colitis occurs predominantly in homosexual men; affected individuals range from 26 to 54 years of age (AJR Am J Roentgenol 1997;168:681)
Sites
- Can occur anywhere in gastrointestinal tract
Pathophysiology
- Gastrointestinal tract is a major site of HIV replication
- HIV virus localizes and replicates in gut associated lymphoid tissues (GALT) and specifically targets mucosal CD4+ lymphocytes
- Constant viral replication result in depletion of CD4+ lymphocyte count, leading to secondary opportunistic infections (Immunol Rev 2013;254:54)
- With advanced HIV, there are markedly reduced numbers of CD4+ cells along with a parallel increase in CD8+ T cells (Gastroenterology 1986;91:651)
- There are also significant increases in levels of inflammatory cytokines IL1β and IFN ϒ but a decrease in the levels of IL10 (AIDS 1994;8:461)
Etiology
- HIV virus
Clinical features
- Chronic diarrhea, abdominal pain, rectal bleeding
Diagnosis
- Noninfective, HIV related colitis is easily recognized; this diagnosis may be made once infective pathologies have been excluded and the disease remains refractory to standard therapies for inflammatory bowel disease (Clin Infect Dis 2008;47:133)
Laboratory
- Stool culture, CD4+ T cell count
Radiology description
- Diffuse proctocolitis, consisting of contact bleeding, superficial ulcerations, exudates or loss of vascular pattern
Prognostic factors
- Very low CD4+ T cell count
Case reports
- Chronic colitis associated with HIV infection can be related to intraepithelial infiltration of the colon by CD8+ T lymphocytes (Int J STD AIDS 2008;19:524)
Treatment
- For HIV related diarrhea, maintain adequate hydration and good nutrition
- Antiviral reteroviral therapy ART (HAART) or combination ART
- Thalidomide has been tried for refractory HIV (Clin Infect Dis 2008;47:133)
Clinical images
Gross description
- Superficial ulceration and hemorrhage
Microscopic (histologic) description
- Histologic changes can be minimal or absent in patients with well-controlled HIV
- Mid esophagus commonly effected by HIV
- Causes well circumscribed / linear ulcers with irregular margins and overhanging edematous edges
- Histologically show granulation tissue with mixed acute and chronic inflammatory infiltrate often with eosinophils
AIDS enteropathy
- HIV enteropathy causes villous blunting and atrophy, crypt hypertrophy, increased intraepithelial lymphocytes, variably increased mononuclear cell in lamina propria. Increased mitosis in glandular epithelial cells and increased number of apoptotic enterocytes at the surface
AIDS colonopathy
- Colon show similar changes but presence of apoptotic cells in glandular epithelium more prominent (Ann Intern Med 1991;114:366, J Gastroenterol Hepatol 1994;9:291)
Microscopic (histologic) images
Contributed by Nalini Bansal, M.D.
Images hosted on other servers:
Molecular / cytogenetics description
- HIV nucleic acid identified by in situ hybridization in colonic biopsies
- HIV DNA confirmed by Southern blot analysis
Differential diagnosis
Board review style question #1
What is the most common site of localization and replication of HIV virus?
- Brain
- Gastrointestinal tract
- Liver
- Lungs
Board review style answer #1
Board review style question #2
What is the most common opportunistic infection in AIDS colitis?
- CMV
- Cryptosporidiosis
- Giardia
- Herpes
Board review style answer #2
Board review style question #3
What is the most opportunistic infection occur when CD4+ T cell count is below?
- 200/mm3
- 400/mm3
- 500/mm3
- 700/mm3
Board review style answer #3
HSV
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare in the gastrointestinal tract; more common in the esophagus and in immunocompromised patients
- Painful, discrete ulcers, vesicles or pustular lesions in distal rectum or perianal skin
- Associated with immunocompromise and idiopathic inflammatory bowel disease (IBD)
- Diagnose with viral culture
Essential features
- HSV1 tends to cause predominantly oral and esophageal herpetic lesions
- Commonly acquired during childhood, though it has been associated with proctitis in a minority of cases
- HSV1 is primarily transmitted via oral secretions and has a higher seroprevalence in lower socioeconomic communities
- HSV2 is most often sexually transmitted; it primarily causes genital and rectal infections
Terminology
- Herpetic colitis
- Herpes simplex colitis
ICD coding
Epidemiology
- Estimates suggest that HSV1 has a worldwide seroprevalence of 80% to 90%, whereas HSV2 has a 22% seroprevalence rate in the U.S. (Infect Dis 1998;178:1616)
- Among patients infected with HIV, HSV is the most common viral coinfection and 95% of men who have sex with men (MSM) are seropositive for HSV1, HSV2 or both
Sites
- After primary infection, viral particles are transported retrograde from the peripheral site of infection to dorsal root ganglia (Lancet Neurol 2007;6:1015)
- Virus may become latent for an unpredictable period
- At any time, the virus may be reactivated
- Asymptomatic reactivation may cause viral shedding; symptomatic reactivation frequently causes symptoms that are less severe and shorter in duration than the symptoms of the primary infection but that have a similar distribution (Lancet Neurol 2007;6:1015)
Pathophysiology
- Primary HSV infection causes mild / asymptomatic oral labial (usually HSV1) or genital infections (usually HSV2) in immunocompetent patients
- Latent HSV persists in sensory nerve ganglia
- In immunocompromised patients, HSV infection can lead to systemic infections
- Patients on immunosuppressive agents (i.e., corticosteroids, azathioprine, cyclosporine, tacrolimus or methotrexate) are at increased risk
- Almost 25% of patients have preceding cough or sore throat; 27% have oral lesions and 13% have herpetic skin rashes (Dis Esophagus 2005;18:340)
Etiology
- Most cases of gastrointestinal HSV infection are in immunocompromised hosts
- HSV does not commonly affect the small and large intestine but there are scattered case reports of HSV colitis and jejunitis after bone marrow and solid organ transplantation (Bone Marrow Transplant 1997;20:989)
- Most HSV proctitis cases (70%) are related to HSV2
- Typical endoscopic findings in HSV proctitis include friable mucosa, diffuse distal ulcerations and vesicular lesions (Clin Infect Dis 1999;28:S84)
Diagrams / tables
No information provided
Clinical features
- Symptoms are similar to colitis of other causes and include watery or bloody diarrhea, fever, tenesmus, abdominal pain, nausea, neurological symptoms, fatigue and weight loss
- Symptoms of inflammatory bowel disease may also be present if there is concomitant disease
Diagnosis
- Biopsies and serologic testing
- Immunohistochemical stain with anti-HSV is available for tissue diagnosis
- Immunocytochemistry also has an excellent diagnostic yield, being positive in 87.5% of patients
- Characteristic viral pathology includes multinucleated giant cells and Cowdry type A inclusion
Laboratory
- Serology uses a blood test, which looks for antibodies to the herpes simplex virus (HSV), including HSV1 and HSV2
- Serum HSV IgG and IgM immunoglobulin assays
- Detection of HSV DNA by PCR in colonic biopsies is more reliable due to high seropositivity of HSV worldwide
- Viral cultures have slow turn around time
Radiology description
- Typically normal findings, unless associated with inflammatory bowel disease, which can show toxic megacolon or strictures related to inflammatory bowel disease
Radiology images
No information provided
Prognostic factors
- Overall prognosis for HSV colitis or esophagitis without treatment is poor
- Patients do tend to respond to antiviral medication, including acyclovir (Gut 1989;30:1033)
- Disseminated disease may be fatal
Case reports
- 35 year old woman with ulcerative colitis complicated by herpes simplex colitis (J Crohns Colitis 2007;1:41)
- 43 year old man with ulcerative colitis and concomitant herpes simplex colitis and hepatitis (Medicine (Baltimore) 2016;95:e5082)
- 63 year old woman with colitis due to cytomegalovirus and HSV2 as first presentation of inflammatory bowel disease (GE Port J Gastroenterol 2021;29:56)
Treatment
- No vaccination is available for HSV
- Antiviral therapy with acyclovir
Gross description
- Perianal vesicles and pustules
- Ulceration
- Mucosal friability
Gross images
No information provided
Frozen section description
No information provided
Frozen section images
No information provided
Microscopic (histologic) description
- Exudate with sloughed epithelial cells
- Neutrophilic infiltrate
- Aggregates of macrophages
- Perivascular lymphocyte cuffing
- Characteristic inclusions in sloughed mucosa or mucosa at edge of ulcers
- Inclusions are exclusively nuclear
- Characteristic cytoplasmic inclusions
- Cowdry type A (acidophilic inclusion with surrounding clear halo)
- Some patients, especially HIV+, can have multiple infectious etiologies (Curr Gastroenterol Rep 2008;10:417)
Microscopic (histologic) images
Virtual slides
No information provided
Cytology description
No information provided
Cytology images
No information provided
Immunofluorescence description
No information provided
Immunofluorescence images
No information provided
Positive stains
No information provided
Negative stains
No information provided
Electron microscopy description
No information provided
Electron microscopy images
No information provided
Molecular / cytogenetics description
No information provided
Molecular / cytogenetics images
No information provided
Videos
No information provided
Sample pathology report
- Colon, random, biopsy:
- Herpes simplex virus (HSV) colitis (see comment)
- Comment: Immunohistochemical stain for HSV1 is positive.
- Colon, colectomy:
- Herpes simplex virus (HSV) colitis (see comment)
- Comment: In the background of severely active ulcerative pancolitis. Immunohistochemical stain for HSV1 is positive. Negative for dysplasia.
Differential diagnosis
- Acute self limited / infectious colitis:
- Inflammation of lamina propria (active much more than chronic), edema, hemorrhage
- Intranuclear inclusion, epithelial vacuolization and nuclear disarray can be seen in viral etiologies
- Other viral colitis / CMV colitis:
- Large, ovoid or pleomorphic nucleus with basophilic intranuclear inclusions (owl eyes) surrounded by a clear halo
- Graft versus host disease:
- Crypt apoptosis
- Crypt dropout
- Ulceration
- No microscopic features of margination, multinucleation and molding
- Lack of Cowdry type A and B inclusions
- Inflammatory bowel disease:
- No inclusions
- Microscopic features of chronicity, ulceration, cryptitis or crypt abscess formation
Additional references
Board review style question #1
A 69 year old man presents with abdominal pain and diarrhea. Colonoscopy showed diffuse mucosal erythema. The patient underwent a biopsy of the lesion and histology is shown above. Which organism is causing the histopathologic findings?
- Adenovirus
- Cytomegalovirus
- Hepatitis B virus
- Herpes simplex virus
Board review style answer #1
D. Herpes simplex virus. The image shows Cowdry type A inclusions and the characteristic changes associated with HSV infection, including molding, margination and multinucleation (the 3M phenomenon).
Comment Here
Reference: Colon - HSV
Comment Here
Reference: Colon - HSV
Board review style question #2
What is the gold standard method for diagnosing HSV colitis?
- Histology and immunohistochemistry for HSV
- HSV culture
- HSV DNA polymerase chain reaction (PCR) amplification method
- HSV serology
Board review style answer #2
A. Histology and immunohistochemistry for HSV. The gold standard diagnostic test for tissue invasive HSV disease, including colitis and hepatitis, is histopathologic evidence of herpes simplex virus infection, which can include characteristic eosinophilic intracytoplasmic inclusions as well as positive immunohistochemical staining using labeled HSV1 and HSV2 specific monoclonal antibodies.
Comment Here
Reference: Colon - HSV
Comment Here
Reference: Colon - HSV
HSV
Table of Contents
Definition / general | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Laboratory | Case reports | Treatment | Gross description | Microscopic (histologic) description | Differential diagnosisDefinition / general
- Painful discrete ulcers, vesicles or pustular lesions in distal rectum or perianal skin
- Diagnose with viral culture
Epidemiology
- > 90% of worldwide population is seropositive for HSV by age 30, but HSV colitis is rare (JAMA 2006;296:964)
- Associated with immunocompromise and idiopathic inflammatory bowel disease
Pathophysiology
- Primary HSV infection causes mild/asymptomatic oral labial (usually HSV1) or genital infections (usually HSV2) in immunocompetent patients
- Latent HSV persists in sensory nerve ganglia
- In immunocompromised patients, HSV infection can lead to systemic infections
- Patients on immunosuppressive agents (ie. corticosteroids, azathioprine, cyclosporine, tacrolimus, or methotrexate) are at increased risk
Clinical features
- Symptoms are similar to colitis of other causes and include watery or bloody diarrhea, fever, abdominal pain, nausea, fatigue, and weight loss
- Symptoms of inflammatory bowel disease may also be present if there is concomitant disease
Diagnosis
- Immunohistochemical stain with anti-HSV is available for tissue diagnosis
Laboratory
- Serum HSV IgG and IgM immunoglobulin assays
- Detection of HSV DNA by PCR in colonic biopsies is more reliable due to high seropositivity of HSV worldwide
- Viral cultures have slow turn around time
Case reports
- Newborn with disseminated HSV infection associated with profuse hematochezia and late sigmoid colon perforation (Pediatr Infect Dis J 2002;21:887)
- Child with combined liver and small bowel transplant (Pediatr Transplant 2001;5:374)
- 35 year old woman with HSV colitis, Crohn disease and cirrhosis (Gastroenterol Hepatol (N Y) 2010;6:122)
- 35 year old woman with HSV colitis and ulcerative colitis (J Crohns Colitis 2007;1:41)
- 69 year old woman with HSV1 colitis and common variable immunodeficiency syndrome (Eur J Gastroenterol Hepatol 2006;18:541)
- Woman with fulminant herpes colitis and Crohn disease (J Clin Gastroenterol 1996;22:220)
- HSV colitis and ulcerative colitis, simulating malignancy (Histopathology 2006;49:316)
Treatment
- No vaccination is available for HSV
- Antiviral therapy with acyclovir
Gross description
- Painful discrete ulcers, vesicles or pustular lesions in distal rectum or perianal skin
Microscopic (histologic) description
- Ulceration with neutrophils in lamina propria, cryptitis, crypt abscess, multinucleated giant cells, inclusions in anal transition zone epithelium and perianal skin
Differential diagnosis
Hyperplastic polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Very common type of polyp in the colon and rectum
- Often numerous, particularly within the rectum
- Usually small (5 mm or less)
- Associated with no significant risk of malignant progression
Essential features
- Can occur throughout the large intestine but is most common in the sigmoid colon and rectum
- Associated with no significant risk of malignant progression
- Serrated architecture with a sawtooth appearance, with serrations generally limited to the upper half of the crypts
- Main differential diagnosis is with sessile serrated lesions (SSLs)
- Microvesicular variant not uncommonly contains a BRAF gene mutation
Terminology
- These lesions used to be termed metaplastic polyps
- Microvesicular and goblet cell rich variants exist
- Goblet cell poor variant no longer recognized
ICD coding
- ICD-10: K63.5 - polyp of colon
Epidemiology
- Common lesions and the most common type of serrated colorectal polyp (80%) (Scand J Gastroenterol 2017;52:654)
- Identified most often in adulthood
- M > F (Cancer 1982;49:819)
- Little or no associated risk of colorectal cancer development
Sites
- Large intestine
- Can occur throughout the large intestine but is most common in the sigmoid colon and rectum
- Commonly multiple within the rectum
- Reference: Histopathology 2021;78:780
Pathophysiology
- Unknown
Etiology
- Shares lifestyle risk factors with colorectal adenoma and colorectal cancer; e.g. low dietary fiber content, raised body mass index, increased alcohol consumption and cigarette smoking are all associated with an increased risk of hyperplastic polyp development (Gastroenterology 1997;113:423)
Clinical features
- Almost always asymptomatic
- May be present as part of a spectrum of lesions that also includes sessile serrated lesions, in serrated polyposis (Am J Gastroenterol 2012;107:1315)
Diagnosis
- Diagnosis is made on histological examination
Case reports
- 23 year old woman with juvenile polyposis (Cancer 1979;43:1906)
- 44 year old man with traditional serrated adenoma originating from a goblet cell hyperplastic polyp (Anal Quant Cytopathol Histpathol 2014;36:351)
- 61 year old man with serrated polyposis syndrome (Cureus 2020;12:e9198)
Treatment
- Endoscopic biopsy or removal is often performed to confirm the diagnosis
- No further treatment is required unless the patient is thought to have serrated polyposis, in which case endoscopic surveillance may be warranted
Gross description
- These are small mucosal lesions, typically less than 5 mm in size
- Appear as slightly elevated and pale lesions at endoscopy but lack the mucin cap that is commonly seen with sessile serrated lesions (World J Gastroenterol 2018;24:3250)
Microscopic (histologic) description
- Serrated architecture with a sawtooth appearance, with serrations generally limited to the upper half of the crypts
- Mild nuclear enlargement, stratification and hyperchromasia, limited to the crypt bases
- No dilated, branched or horizontally spreading crypts
- No evidence of conventional dysplasia, e.g. as is seen in colorectal adenomas
- Microvesicular variant is more common and comprises epithelial cells with small mucin vacuoles
- Goblet cell rich variant is less common and contains prominent goblet cells
- Comparison with adjacent nonlesional large intestinal mucosa may facilitate the recognition of goblet cell rich variant and reduce the risk of underdiagnosis, since the serrations are more subtle than in the microvesicular type (Histopathology 2021;78:634)
Microscopic (histologic) images
Positive stains
- Ki67 immunohistochemistry shows proliferation confined to the lower portions of the polyp, mirroring the morphological evidence of maturation towards the surface
Molecular / cytogenetics description
- Microvesicular variant not uncommonly contains a BRAF gene mutation (J Clin Pathol 2009;62:516)
- Goblet cell rich variant may contain a KRAS gene mutation (J Gastroenterol Hepatol 2017;32:358)
Sample pathology report
- Rectum, cold biopsy excision of 4 mm polyp:
- Hyperplastic polyp
Differential diagnosis
- Sessile serrated lesions (SSLs):
- Prominent serration extending into the lower half of the crypts
- Branched, dilated or laterally spreading crypts with little or no intervening lamia propria
- Herniation into the superficial submucosa
- May show conventional dysplasia
- Traditional serrated adenomas:
- Larger size
- Villiform architecture
- Eosinophilic cytoplasm
- Pencillate nuclei
- Ectopic crypts (Histopathology 2021;78:634)
Board review style question #1
Which of the following is characteristic of hyperplastic polyps of the large intestine?
- Are rare lesions
- Different risk factors for development compared with those for adenomas
- Little or no association with an increased risk of colorectal cancer
- Not often multiple within the rectum
- Usually require treatment by bowel resection
Board review style answer #1
C. Hyperplastic polyps that occur outside of the context of polyposis syndrome are not associated with a significant risk of colorectal cancer, in contrast to sessile serrated lesions, which are associated with increased cancer risk.
Comment Here
Reference: Hyperplastic polyp
Comment Here
Reference: Hyperplastic polyp
Board review style question #2
Which of the following histological features is characteristic of a hyperplastic polyp of the large intestine?
- Branched crypts
- Conventional dysplasia
- Dilated glands with little or no intervening lamina propria
- Prominent glandular serration extending down to the crypt bases
- Sawtooth pattern glands within the superficial aspect of the lesion
Board review style answer #2
E. Hyperplastic polyps characteristically contain sawtooth pattern glands within the superficial aspect of the lesion. In contrast to sessile serrated lesions, hyperplastic polyps do not show serration to the crypt bases, branched crypts or dilated crypts.
Comment Here
Reference: Hyperplastic polyp
Comment Here
Reference: Hyperplastic polyp
IBD associated
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colitis associated colorectal cancer (CAC) is a malignant epithelial tumor originating in the large bowel associated with longstanding inflammatory bowel disease (IBD)
Essential features
- Associated with longstanding inflammatory bowel disease
- Other risk factors: disease extent, longstanding anorectal fistula, pseudopolyposis and coexisting primary sclerosing cholangitis (PSC)
- Evenly distributed (left sided and right sided)
- Mucinous adenocarcinoma and signet ring cell adenocarcinoma are common in this setting
ICD coding
- No official coding established
Epidemiology
- Longstanding inflammatory bowel disease patients are at a higher risk of developing colitis associated colorectal cancer compared with the general population; the risks are higher along with longer duration of inflammatory bowel disease
- An inflammatory bowel disease patient with a first degree relative having colorectal adenocarcinoma has an eightfold increase in risk of developing colitis associated colorectal cancer (Clin Gastroenterol Hepatol 2019;17:1807)
- Among inflammatory bowel disease patients, the disease extent (> 50% of the colon), longstanding anorectal fistula, presence of pseudopolyposis and coexisting primary sclerosing cholangitis (PSC) determine higher risk of developing colitis associated colorectal cancer (Clin Colon Rectal Surg 2018;31:168)
Sites
- Colitis associated colorectal cancer is more evenly distributed across the colon, compared with sporadic colorectal adenocarcinoma which is located mainly distal to the splenic flexure (Clin Gastroenterol Hepatol 2006;4:335)
Pathophysiology
- Cytokines such as TNFα, IL1 and IL6 are involved in the promotion of colitis associated colorectal cancer in inflammatory bowel disease patients through chronic inflammation induced accumulation of reactive oxygen species, mutagenic DNA damage and activation of transcription factors such as NFκB and STAT3 (Curr Cancer Drug Targets 2011;11:451, Gastroenterology 2011;140:1807)
Etiology
- Longstanding inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC)
Clinical features
- Similar to sporadic colorectal adenocarcinoma; hematochezia or rectal bleeding may be present
Diagnosis
- Clinical history of longstanding inflammatory bowel disease; endoscopic, histopathologic and radiographic findings
Radiology description
- Two different CT patterns are described:
- Type 1 tumor (47%): clearly visible soft tissue mass
- Type 2 tumor (53%): circumferential thickening of the colorectal wall with stenosis; significant association with the presence of signet ring cells (Abdom Imaging 2013;38:421)
Prognostic factors
- Risk factors: long duration of inflammatory bowel disease, degree of inflammation, disease extent (pancolitis or left sided colitis), longstanding anorectal fistula, pseudopolyposis, coexistenting primary sclerosing cholangitis
- Role of chemoprevention with 5-aminosalicylic acid derivatives (5-ASA) as a protecting factor has been controversial (Dig Dis 2012;30 Suppl 2:55)
Case reports
- 16 year old girl with synchronous colorectal neoplasms in ulcerative pancolitis of 6 years duration (BMJ Case Rep 2013;2013:bcr2013200172)
- 36 year old man with synchronous multifocal colorectal carcinoma after delayed diagnosis of ulcerative pancolitis (Pathol Res Pract 2008;204:905)
- 36 year old man with primary sclerosing cholangitis and inflammatory bowel disease developed two synchronous colitis associated colorectal cancers (World J Gastroenterol 2014;20:12657)
- 41 year old man with colitis associated colorectal cancer developing only 2 years after the first symptoms of ulcerative colitis (J Gastroenterol 2007;42:854)
- 51 year old man with Crohn’s disease developed a mucinous adenocarcinoma with signet ring cell carcinoma 3 years post resection of a sporadic adenocarcinoma (World J Surg Oncol 2013;11:295)
Treatment
- Prophylactic proctocolectomy in patients with primary sclerosing cholangitis and inflammatory bowel disease is still controversial (Gastrointest Cancer Res 2011;4:53)
Microscopic (histologic) description
- Conventional adenocarcinoma most common
- One known histologic cancer variant in this setting is low grade tubuloglandular adenocarcinoma (Mod Pathol 2019;32:884)
- Mucinous carcinomas and signet ring cell adenocarcinomas are much more common in colitis associated colorectal cancer as compared with sporadic cases
- Rarely, other types of carcinoma may be seen, such as neuroendocrine carcinoma, mixed adenocarcinoma / squamous cell carcinoma, undifferentiated carcinoma and pure squamous cell carcinoma (Mod Pathol 1998;11:537, Can J Gastroenterol 2007;21:47)
Microscopic (histologic) images
Positive stains
- CK20, CDX2
- AMACR positive in low grade dysplasia (96%), high grade dysplasia (80%) and adenocarcinoma (71%) (Am J Surg Pathol 2006;30:871)
- p53 (mutation as an early event in colitis associated colorectal cancer but a late event in sporadic colorectal adenocarcinoma) (Int J Mol Sci 2017;18:E1284)
Negative stains
- CK7 (similar to sporadic colorectal adenocarcinoma)
Molecular / cytogenetics description
- Mutation of TP53 occurs at earlier stage in inflammatory bowel disease associated dysplasia and is observed in 50 - 85% of colitis associated colorectal cancers
- KRAS and APC mutations are less frequently seen and occur at later stage in colitis associated colorectal cancer (Oncotarget 2017;8:22175)
- Mutations such as MLH1, RNF43, RPL22 and BRAF have been reported (Pathol Res Pract 2019;215:730)
- IDH1 R132 mutations more common in patients with Crohn’s disease but not ulcerative colitis
- MYC amplification more common in colitis associated colorectal cancer (Gastroenterology 2016;151:278)
Sample pathology report
- Colon, mass, biopsy:
- Adenocarcinoma, well differentiated, arising in a background of chronic active colitis with high grade dysplasia, consistent with colitis associated colorectal adenocarcinoma
Differential diagnosis
- Sporadic colorectal adenocarcinoma:
- Usually affects elderly population, single lesion
- Less likely to have mucinous or signet ring features
- No clinical history of long standing chronic colitis
Board review style question #1
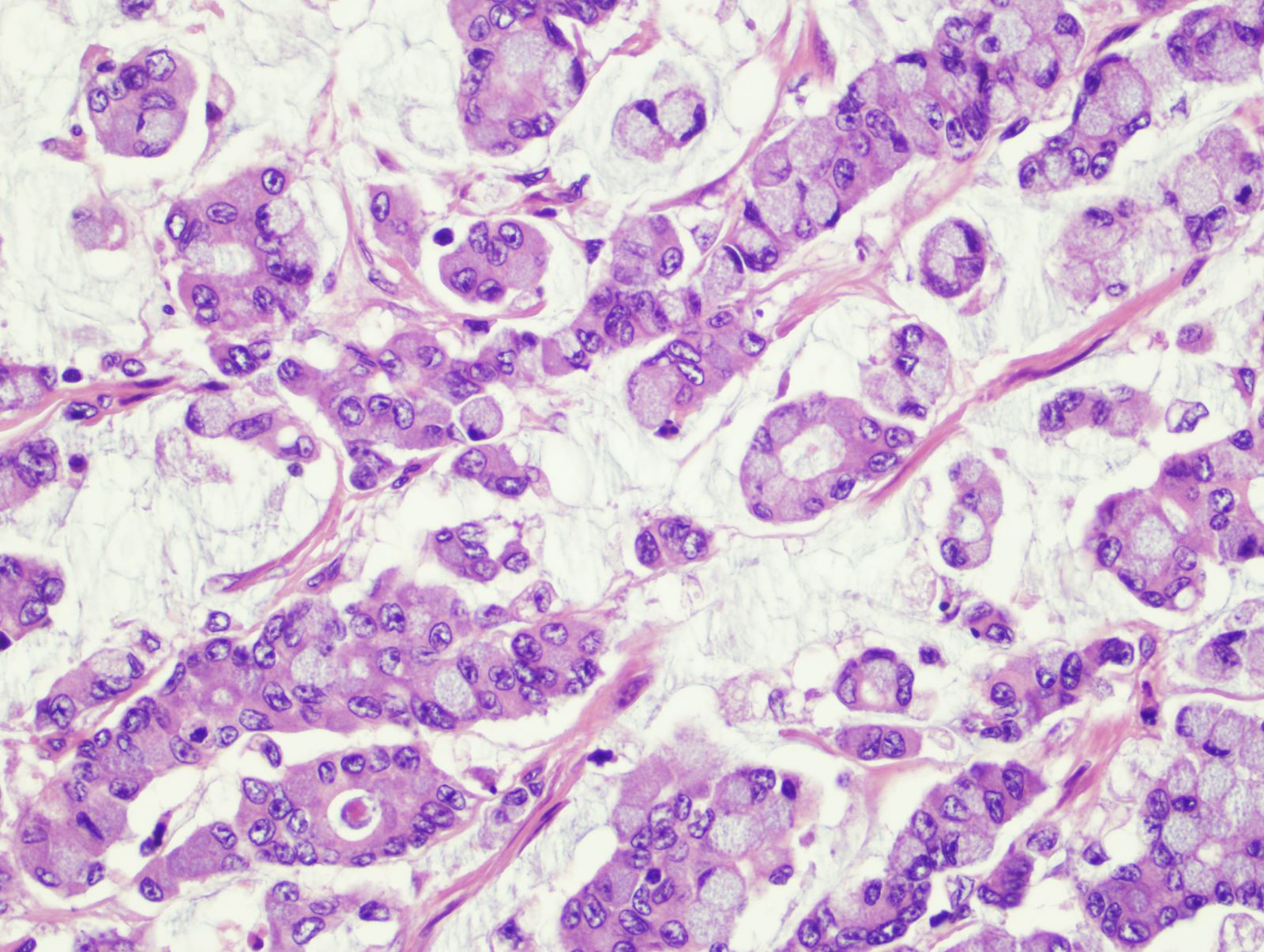
A 32 year old woman with Crohn’s disease presented with abdominal pain and hematochezia. The abdominal CT scan showed a segment of circumferential bowel wall thickening. Colonoscopy showed a stricture in the descending colon. A representative photomicrograph of the biopsy is shown. Which is the most likely diagnosis?
- Colorectal adenocarcinoma, NOS
- Metastatic breast lobular carcinoma
- Metastatic gastric signet ring cell carcinoma
- Signet ring cell carcinoma, colitis associated
Board review style answer #1
D. Signet ring cell carcinoma, colitis associated. The biopsy reveals signet ring cell carcinoma in a young patient with longstanding Crohn’s disease. This is a classic presentation of colitis associated colorectal cancer. Signet ring cell carcinoma and mucinous adenocarcinoma are more common in colitis associated colorectal cancer as compared with sporadic cases.
Comment Here
Reference: IBD associated carcinoma
Comment Here
Reference: IBD associated carcinoma
Board review style question #2
Which mutation often occurs at an early stage of colitis associated colorectal cancer?
- APC
- BRAF
- KRAS
- MLH1
- TP53
Board review style answer #2
E. TP53. TP53 mutation occurs early in colitis associated colorectal cancer but is a late event in sporadic colorectal adenocarcinoma. In contrast, APC mutation occurs early in sporadic colorectal adenocarcinoma but is very rare and usually a late event in colitis associated colorectal cancer.
Comment Here
Reference: IBD associated carcinoma
Comment Here
Reference: IBD associated carcinoma
Idelalisib associated
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colitis caused by the use of idelalisib, a highly potent small molecule phosphoinositide 3 kinase δ (PI3Kδ) inhibitor approved for the treatment of chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL / SLL) and follicular lymphoma
Essential features
- A triad of intraepithelial lymphocytosis, epithelial cell apoptosis and neutrophilic cryptitis in colonic biopsies with moderate to severe diarrheal symptoms along with clinical history of idelalisib therapy (Am J Surg Pathol 2015;39:1661)
- Many patients develop gastrointestinal symptoms during idelalisib therapy
- Diarrhea with enterocolitis is a common adverse effect of long term idelalisib therapy (Am J Surg Pathol 2015;39:1661)
Epidemiology
- Most of the patients with severe gastrointestinal toxicity are older adults (mean age is 65 years)
- M > F
- History of drug intake for an average of 7 months before severe symptom development (Future Oncol 2018;14:2265)
Sites
- Mostly affects the small intestine and colorectum
- Stomach and esophageal involvement can also be seen (Am J Surg Pathol 2015;39:1653)
Pathophysiology
- Diarrhea with enterocolitis is a common adverse effect of long term idelalisib therapy
- Clinically present with loose, watery and nonbloody stools
- Majority of patients who undergo a colonoscopy have endoscopically apparent colitis with erythema, congestion and granularity
- More severe cases can exhibit erosions or ulceration (Am J Surg Pathol 2015;39:1653)
Etiology
- Idelalisib (Zydelig) is a small molecule inhibitor of phosphoinositide 3 kinase δ (PI3Kδ) that was recently approved for treatment of relapsed B cell malignancies, including chronic lymphocytic leukemia and follicular lymphoma
- Median time for onset of severe grade 3 or 4 diarrhea is ~7 months (Am J Surg Pathol 2015;39:1653)
Clinical features
- Diarrhea, nausea, fatigue, cough, pyrexia, chills
- Rarely associated with transaminitis, pneumonitis
Diagnosis
- Physical examination including assessment of patient for fever, dizziness, abdominal pain / cramping and weakness (i.e., rule out risk for sepsis, bowel obstruction, dehydration)
- History of onset and duration of diarrhea
- Travel history to exclude infectious etiologies
- Description of number of stools and stool composition (e.g., watery, bloody, nocturnal)
- Medication profile to identify diarrheagenic agents
- Dietary profile to identify diarrheagenic foods (Leuk Lymphoma 2015;56:2779)
Laboratory
- Stool workup
- Occult blood determination
- Culture for enteric pathogens (Salmonella, Escherichia coli, Campylobacter, Clostridium difficile)
- Complete blood count, electrolytes and blood urea nitrogen / creatine (BUN:Cr) (grade 3: neutropenia, thrombocytopenia, anemia and alanine transaminase [ALT] or aspartate transferase [AST] elevation)
- Consider colonoscopy in selected cases (Leuk Lymphoma 2015;56:2779)
Radiology description
- Majority of patients who undergo a colonoscopy have endoscopically apparent colitis with erythema, congestion and granularity or ulceration
Prognostic factors
- Poor prognostic factors include fatal or severe diarrhea or colitis, hepatotoxicity, pneumonitis and intestinal perforation
- Adverse idelalisib events include pneumonia, pyrexia and febrile neutropenia (Leuk Lymphoma 2015;56:2779)
Case reports
- 40 year old woman with relapsed grade 2 follicular lymphoma presented with 3 days of vomiting, cramping abdominal pain and diarrhea (JAMA Oncol 2016;2:1361)
- 56 year old man with a history of relapsed follicular lymphoma presented with severe diarrhea and a skin eruption mimicking graft versus host disease 6 months after starting idelalisib (Clin J Gastroenterol 2017;10:142)
- 72 year old woman presented with abdominal pain, nonbloody diarrhea for 2 months and 15 lbs of weight loss (Ann Gastroenterol 2016;29:233)
Treatment
- Discontinue idelalisib
- Rule out infection
- Intravenous or oral rehydration if grade 3 diarrhea or colitis
- Budenoside or oral steroids (intravenous if orals are not tolerated)
- Diet modification recommendations such as plenty of fluids, 8 - 12 glasses a day of oral rehydration drinks, 5 - 6 small meals each day and low fat, high protein foods such as lean meat and eggs (Leuk Lymphoma 2015;56:2779)
Microscopic (histologic) description
- Presence of intraepithelial lymphocytosis in both the crypts and surface epithelium
- Apoptotic crypt epithelial cells (sometimes numerous with large exploding apoptotic cells)
- Neutrophilic infiltration of the crypt epithelium with occasional crypt abscesses
- Minority show crypt architectural distortion in those patients that had a prolonged interval between symptom onset and colonoscopy
- Histologic patterns can be divided into 4 distinct morphological categories:
- Predominantly normal
- Apoptotic pattern without intensive inflammatory component
- Infectious colitis-like pattern characterized predominantly by inflammation with varying degrees of attenuated glands (apoptotic cells not a prominent feature)
- Mixed pattern with varying degrees of apoptotic cells, mixed inflammation or attenuated glands, with the most striking features of architectural distortion, mixed inflammation (including intraepithelial lymphocytes and neutrophils and increased eosinophils), crypt abscess / destruction and apoptosis
- References: Am J Surg Pathol 2015;39:1661, Am J Surg Pathol 2015;39:1653
Microscopic (histologic) images
Positive stains
- Intraepithelial lymphocytes are positive for CD8 and TCR alpha / beta (Am J Surg Pathol 2015;39:1653)
Negative stains
- CMV, adenovirus, HHV6 (Am J Surg Pathol 2015;39:1653)
Sample pathology report
- Left colon, biopsy:
- Colonic mucosa with mild acute cryptitis, mucosal eosinophilia, intraepithelial lymphocytes and prominent crypt apoptosis (see comment)
- Comment: The patient's clinical history of refractory follicular lymphoma and treatment with idelalisib are noted. The histologic findings are likely consistent with idelalisib associated colitis.
Differential diagnosis
- Graft versus host disease (GVHD):
- Additional clinical history of a lack of stem cell transplant is perhaps the most helpful in excluding GVHD (Am J Surg Pathol 2015;39:1653)
- Autoimmune enteropathy:
- Predominantly neutrophilic (versus eosinophilic) cryptitis and crypt abscesses, as well as a complete absence of goblet cells (Am J Surg Pathol 2015;39:1653)
- Inflammatory bowel disease (IBD):
- Crypt apoptosis is not typical for inflammatory bowel disease
- Presence of eosinophils within crypt abscesses and the finding of dilated, damaged crypts is more suggestive of a drug related process
- Chronic injury is absent or mild in idelalisib associated colitis
- Mild architectural distortion in the form of few branching crypts may be present but basal plasmacytosis, Paneth cell metaplasia and thickening with splaying of the muscularis mucosae are not observed (Am J Surg Pathol 2015;39:1653)
- Common variable immunodeficiency (CVID):
- May exhibit almost identical histologic features, although cryptitis is not typical for common variable immunodeficiency
- Common variable immunodeficiency demonstrates a paucity or complete absence of plasma cells (Am J Surg Pathol 2015;39:1653)
- Celiac disease:
- Presence of cryptitis and apoptosis are not typical for celiac disease and may help with this distinction (Am J Surg Pathol 2015;39:1653)
- Cytomegalovirus (CMV) and other infectious enterocolitides:
- Relatively mild chronic changes with comparatively prominent features of active injury may suggest an infectious colitis
- Increased eosinophils within the lamina propria and within crypt abscesses suggest a drug related process (Am J Surg Pathol 2015;39:1653)
Additional references
Board review style question #1
A 68 year old man was admitted for evaluation of chronic dehydration, diarrhea, abdominal pain and weight loss for > 1 month (~20 lbs). He has a history of relapsed follicular lymphoma and has been on Zydelig (idelalisib) for ~11 months. Which of the following favors idelalisib associated colitis over inflammatory bowel disease?
- Basal plasmacytosis
- Epithelial cell apoptosis
- Paneth cell metaplasia
- Splaying of muscularis mucosae
Board review style answer #1
B. Epithelial cell apoptosis. Crypt apoptosis is a common feature associated with idelalisib associated colitis and is less commonly seen in patients with inflammatory bowel disease.
Comment Here
Reference: Idelalisib associated colitis
Comment Here
Reference: Idelalisib associated colitis
Board review style question #2
A 56 year old man with a history of relapsed follicular lymphoma status postallogenic bone marrow transplant who developed severe diarrhea with a skin eruption mimicking graft versus host disease (GVHD) 6 months after starting idelalisib. Which of the following histological findings is indicative of idelalisib associated colitis?
- Basal plasmacytosis
- Increased eosinophils within the lamina propria and crypt abscesses
- Lack of apoptosis
- Severe chronic injury
Board review style answer #2
B. Increased eosinophils within the lamina propria and crypt abscesses. Idelalisib associated colitis closely mimics graft versus host disease and can be challenging to distinguish. The presence of prominent neutrophilic cryptitis and crypt abscesses can be helpful to favor idelalisib associated colitis.
Comment Here
Reference: Idelalisib associated colitis
Comment Here
Reference: Idelalisib associated colitis
Idiopathic myointimal hyperplasia of mesenteric veins
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Etiology | Clinical features | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Unusual thickening of mesenteric veins, resulting in gastrointestinal symptoms and ischemic colitis
Essential features
- Rare condition that clinically mimics inflammatory bowel disease in young men
- Thought to result from arteriovenous fistulization
- Histology: hyperplastic veins mimic arteries (distinguish with elastin stain); thick walled vascular proliferation in mucosa
Epidemiology
- Rare; usually occurs in young male adults but can occur in older patients and in women (Gastroenterol Hepatol (N Y) 2012;8:700)
Sites
- Usually (but not always) involves the left colon
Etiology
- Might result from traumatic arteriovenous fistulization (Pathol Res Pract 2006;202:517)
Clinical features
- Diarrhea / constipation, abdominal pain, rectal bleeding
Case reports
- 38 year man with abdominal pain and 40 pound weight loss, clinically diagnosed with inflammatory bowel disease (J Clin Gastroenterol 2005;39:704)
Treatment
- Surgery usually required
Microscopic (histologic) description
- Biopsy findings: ischemic mucosal change accompanied by proliferation of hyperplastic, thick walled, hyalinized blood vessels (Am J Surg Pathol 1996;20:1271)
- Resection findings: mesenteric and mural veins show myointimal hyperplasia, making them somewhat resemble arteries; no vasculitis present; arteries are unaffected (Gastroenterology 1991;101:533)
Microscopic (histologic) images
Positive stains
- Elastin stain confirms that blood vessels with myointimal hyperplasia are veins, not arteries
Sample pathology report
- Descending colon, resection:
- Segment of colon with prominent vascular reactive changes and myointimal hyperplasia of mesenteric veins (see comment)
- Margins of resection unremarkable.
- Seven benign lymph nodes.
- Comment: There is no definitive evidence of chronic colitis. An elastic special stain highlights mesenteric veins with myointimal hyperplasia, distinguishing them from arteries.
Differential diagnosis
- Inflammatory bowel disease:
- Similar clinical picture but histology shows a classic chronic colitis, without vascular abnormalities
- Mesenteric phlebosclerosis:
- Unusual vascular disease seen only in Japanese patients; mesenteric and colonic mucosal vessels are calcified and sclerotic (Dis Colon Rectum 2003;46:209)
Board review style question #1
Which of the following is true about idiopathic myointimal hyperplasia of mesenteric veins?
- An elastin stain can help distinguish arteries from abnormal veins
- Histologic changes are seen in resection but not biopsy specimens
- It classically occurs along the right colon in older female patients
- It is asymptomatic and incidentally observed
Board review style answer #1
A. An elastin stain can help distinguish arteries from abnormal veins
Comment Here
Reference: Idiopathic myointimal hyperplasia of mesenteric veins
Comment Here
Reference: Idiopathic myointimal hyperplasia of mesenteric veins
Idiopathic retroperitoneal fibrosis
Table of Contents
Definition / general | Essential features | Terminology | Sites | Etiology | Clinical features | Laboratory | Radiology images | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Nonneoplastic fibrosis that develops in retroperitoneum and may encroach upon colon
Essential features
- Benign fibrosing process of the retroperitoneum that can rarely involve the gastrointestinal tract secondarily
- Some cases belong to the spectrum of IgG4 related disease
Terminology
- Also called Ormond disease, idiopathic retroperitoneal fibrosis
Sites
- Arises in retroperitoneum, often at aortic bifurcation
- Gastrointestinal tract involvement is rare
Etiology
- IgG4 positive plasma cells implicated as a causative factor (Am J Surg Pathol 2009;33:1833)
- Can also be caused by methysergide or lymphoma or may be idiopathic
Clinical features
- Patients present with lower back or abdominal pain; bowel obstruction is rare
- IgG4 related cases usually seen in males
- Associated with other IgG4 related diseases, such as sclerosing cholangitis, Riedel thyroiditis, inflammatory pseudotumor of orbit (World J Gastroenterol 2013;19:7661)
- Can cause obstructive uropathy
Laboratory
- Increased serum IgG and IgG4 in patients with IgG4 related disease
Case reports
- 39 year old woman with bowel obstruction (Ann Surg 1972;176:199)
Treatment
- Options include steroids, azathioprine, tamoxifen and surgery (Nephrol Dial Transplant 2006;21:2485)
Gross description
- Poorly circumscribed fibrotic mass
Microscopic (histologic) description
- Storiform fibrotic process infiltrated by eosinophils and IgG4 positive plasma cells (which may be sparse)
- May show obliterative phlebitis, fat necrosis
- Similar findings may be observed in lymph nodes (Am J Clin Pathol 1996;105:430)
Microscopic (histologic) images
Positive stains
- IgG4 (in plasma cells)
Sample pathology report
- Transverse colon and adjacent soft tissue mass, excision:
- Prominent bland fibrotic process involving soft tissue and focally extending into colon wall (see comment)
- Negative for malignancy.
- Margins of resection unremarkable.
- Comment: The soft tissue fibrosis shows a storiform pattern and contains abundant chronic inflammation. An immunostain for IgG4 highlights numerous plasma cells. The overall findings are most consistent with idiopathic retroperitoneal fibrosis.
Differential diagnosis
- Sclerosing mesenteritis:
- Involves mesentery rather than retroperitoneum; can also be IgG4 related
- Inflammatory myofibroblastic tumor:
- Spindle cells more prominent; may be positive for ALK1
Additional references
Board review style question #1
Which of the following is true about idiopathic retroperitoneal fibrosis?
- Colon is often secondarily involved
- Most patients present with lower gastrointestinal bleeding
- Numerous IgG4 positive plasma cells are seen histologically
- Obliterative arteritis can be seen
Board review style answer #1
C. Numerous IgG4 positive plasma cells are seen histologically
Comment Here
Reference: Idiopathic retroperitoneal fibrosis
Comment Here
Reference: Idiopathic retroperitoneal fibrosis
Infarct
Table of Contents
Definition / general | Essential features | Clinical features | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Videos | Sample pathology report | Board review style question #1 | Board review style answer #1Definition / general
- Death of colonic tissue due to any marked loss of blood supply to colon, including vascular insufficiency and trauma
- See also ischemic colitis
Essential features
- Necrosis of colon due to loss of blood supply
- Cause may not be evident histologically
- Surgical resection necessary
Clinical features
- Depends on cause but abdominal pain and hematochezia are common
Case reports
- 83 year old woman with intestinal infarct caused by giant cell arteritis (Medicina (B Aires) 1999;59:86)
- Patients with association of rupture abdominal aneurysm and intestinal infarct (Minerva Chir 1996;51:597)
Treatment
- Surgical resection of necrotic colon
Gross description
- Intestine is dusky and necrotic
Microscopic (histologic) description
- Typically transmural colonic necrosis, with nonviable mucosa and edema, hemorrhage and congestion of wall
Videos
Histopathology colon - hemorrhagic infarct
Sample pathology report
- Descending colon, resection:
- Segment of colon with diffuse transmural necrosis and hemorrhage, extending to both resection margins
Board review style question #1
Board review style answer #1
Infarcted epiploic appendages
Table of Contents
Definition / general | Essential features | Terminology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Infarction and subsequent fat necrosis of epiploic appendages (fat containing pouches of colonic peritoneum) that may remain attached or autoamputate and lie loose in the peritoneum
Essential features
- Fat necrosis of epiploic appendage, which may detach and lie loose in the peritoneum
- May cause pain or be discovered as an incidental curiosity
Terminology
- Epiploic appendagitis: inflammation but not infarction of appendages
- Unattached infarcted appendages are known as peritoneal loose bodies or peritoneal mice (J Clin Gastroenterol 2006;40:427)
Sites
- Epiploic appendages are chiefly on transverse and sigmoid colon
- Can occur on appendix (S D Med 2006;59:511)
Etiology
- Due to twisting and torsion of normally thick pedicles of epiploic appendages
- May be associated with obstruction and abscess (J Am Assoc Gynecol Laparosc 1996;3:325)
Clinical features
- Abdominal pain, typically in left lower quadrant
- Rarely causes death (South Med J 1986;79:374)
Diagnosis
- Laparoscopy
Radiology description
- Localized pericolic inflammatory changes (Abdom Imaging 1994;19:449)
Radiology images
Case reports
- 64 year old man with a 7 cm giant peritoneal loose body (J Med Case Rep 2011;5:297)
Gross description
- Firm, gray-white nodules that may resemble metastatic tumor
- Loose bodies can resemble an egg
Gross images
Microscopic (histologic) description
- Central infarcted adipose tissue with peripheral fat necrosis and calcification, surrounded by thick, inflamed fibrotic tissue
Microscopic (histologic) images
Sample pathology report
- Abdominal cavity, loose body, removal:
- Fat necrosis with fibrotic rim, consistent with infarcted epiploic appendage
Differential diagnosis
- Fat necrosis from other cause:
- Not rounded / encapsulated or free floating, may show more inflammation
Additional references
Board review style question #1
Board review style answer #1
Inflammatory bowel disease, indeterminate type
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Sites | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Inflammatory bowel disease (IBD), indeterminate type is a provisional term used to describe cases of IBD in which a definitive diagnosis of ulcerative colitis or Crohn's disease cannot be established on the basis of histopathological and clinical (including radiologic and endoscopic) data
- IBD, indeterminate type is not a specific disease entity and has no established diagnostic criteria (Mod Pathol 2015;28:S30, J Clin Pathol 2004;57:1233)
- Comprises 5 - 15% of IBD cases but most will eventually be declared as 1 of the 2 subtypes (only up to 15% thought to be Crohn's disease)
Essential features
- IBD, indeterminate type is a diagnosis of exclusion and should only be used in resection specimens when it is impossible to distinguish ulcerative colitis from Crohn's disease
- Term should be avoided in the following circumstances:
- Recognized IBD variants: appendiceal or cecal patch in left sided ulcerative colitis, patchy / segmental disease (including rectal sparing) in treated or longstanding ("burned out") ulcerative colitis, fulminant (severe or toxic) ulcerative colitis, ulcerative colitis with backwash ileitis, ulcerative colitis with cryptolytic granulomas, superficial (ulcerative colitis-like) Crohn's disease
- In the presence of convincing criteria for Crohn disease: nonnecrotizing, noninfectious epithelioid granulomas, true segmental colonic or small bowel disease, extensive transmural lymphoid aggregates underlying intact mucosa, definitive perianal or fistulizing disease
- Secondary or superimposed non-IBD colitides, e.g. infectious colitis, ischemic colitis, diverticular disease associated colitis, diversion colitis, microscopic colitis (i.e., lymphocytic or collagenous colitis), pseudomembranous colitis, colitis due to medication related injury (e.g. nonsteroidal anti-inflammatory drugs (NSAIDs), mycophenolate, immune checkpoint inhibitors), etc.
- In the setting of insufficient clinical, pathologic, radiologic or endoscopic data (e.g. on biopsy material in the absence of granulomas): use the term "IBD, unclassified" instead
- Even though up to 15% will ultimately receive a diagnosis of Crohn's disease, patients with IBD, indeterminate type are generally thought to have similar ileal pouch anal anastomosis (IPAA) failure rates as ulcerative colitis; therefore, IPAA surgery is probably warranted in most cases
- Higher rates of IBD, indeterminate type diagnosis are seen in the pediatric population, given the patchy disease distribution often seen in childhood ulcerative colitis
Terminology
- Indeterminate colitis (IC):
- Inexact and confusing term; not limited to IBD per se
- Used in some instances when the etiology of chronic colitis cannot be ascertained from clinicopathologic findings; in these cases, nonspecific colitis or chronic active colitis, type unknown is preferred, accompanied by a note as to the reason for the uncertainty and possible differential diagnoses
- Inflammatory bowel disease, unclassified (IBD-U):
- Primarily a temporary term used during colonoscopy when the pathologist or gastroenterologist favors a diagnosis of IBD but the histologic or endoscopic impression do not permit distinction between ulcerative colitis and Crohn's disease
- In most cases, a diagnosis of ulcerative colitis or Crohn's disease can be yielded upon evaluation of follow up resection specimens or additional clinical data
- Other terms:
- Colitis of uncertain type or etiology (CUTE) (Inflamm Bowel Dis 2008;14:850)
- IBD, NOS (not otherwise specified)
- IBD, unknown etiology
- Uncertain colitis
ICD coding
Sites
- Colon
Diagnosis
- IBD, indeterminate type is a diagnosis of exclusion and requires conflicting histopathological findings in a resection specimen or in the setting of contradictory clinical or radiological data
Laboratory
- Limited clinical utility of serological markers ASCA and pANCA
- ~50% of patients with IBD, indeterminate type are ASCA- / pANCA- (Gastroenterology 2002;122:1242)
- Only in a minority of patients do serologic markers help establish a definitive diagnosis of Crohn's disease (ASCA+ / pANCA-) or ulcerative colitis (ASCA- / pANCA+) (Gastroenterology 2002;122:1242)
Prognostic factors
- Similar ileal pouch anal anastomosis failure rates as ulcerative colitis (J Crohns Colitis 2020;14:1010, Am Surg 2016;82:977, J Gastrointest Surg 2017;21:56, Gastroenterol Hepatol (N Y) 2017;13:466)
- Higher overall ileal pouch anal anastomosis complication rates than ulcerative colitis, including pouch fistula, pelvic sepsis, abscess, perineal complications and ultimate diagnosis of Crohn's disease (J Crohns Colitis 2020;14:1010, J Gastrointest Surg 2017;21:56)
- ~15% of cases ultimately receive a diagnosis of Crohn's disease (Dis Colon Rectum 2000;43:1487)
- Higher rate of the diagnosis seen in the pediatric population, given frequent findings of rectal sparing and patchy disease in childhood ulcerative colitis that generates diagnostic difficulty (Inflamm Bowel Dis 2006;12:258, J Pediatr Gastroenterol Nutr 2007;44:653)
Case reports
- 3 year old boy with bloody diarrhea for 5 months (Am J Gastroenterol 1993;88:105)
- 21 year old woman with longstanding colitis (Semin Gastrointest Dis 2001;12:211)
- 22 year old man with watery diarrhea, fever and nausea (Z Gastroenterol 1997;35:481)
Treatment
- Same as IBD
- Given similar ileal pouch anal anastomosis failure rates as ulcerative colitis, ileal pouch anal anastomosis probably warranted (Gastroenterol Hepatol (N Y) 2017;13:466)
Gross description
- Ulcerative colitis-like colectomy specimen showing predominantly continuous, mucosa based inflammation but with 1 or more Crohn's disease-like features, such as:
- Rectal sparing (except when treated or longstanding colitis)
- Proximal skip lesions (except in pediatric population)
- Ulceration in small bowel (even in the setting of backwash ileitis)
- Deep fissuring ulceration (in the absence of fulminant colitis or history thereof)
- Focal wall thickening
- "Cobblestone" mucosa (deep linear ulcers with intervening normal mucosa)
- Left sided colitis with discontinuous cecal or appendiceal orifice involvement (so called “cecal patch” or “periappendiceal red patch”) is a well recognized variant of ulcerative colitis and should not be designated as IBD, indeterminate type
- References: Mod Pathol 2015;28:S30, J Clin Pathol 2004;57:1233, Histopathology 2007;50:83, J Clin Pathol 2009;62:201
Gross images
Microscopic (histologic) description
- Active mucosal inflammation (same as IBD) (J Clin Pathol 2009;62:201):
- Cryptitis (neutrophilic infiltration of crypt epithelium)
- Crypt abscess (crypt with intraluminal aggregates of neutrophils)
- Erosions
- Ulceration
- Chronic mucosal inflammation (same as IBD) (J Clin Pathol 2009;62:201):
- Increased lymphoplasmacytic inflammation in the lamina propria with loss of gradient (i.e. lack of increased infiltrates near lumen)
- Basal plasmacytosis (so called crypt shortfall)
- Evidence of chronic injury (same as IBD) (J Clin Pathol 2009;62:201):
- Paneth cell metaplasia (Paneth cells are normally absent distal to the mid transverse colon)
- Crypt architectural distortion (branched, dilated and disorganized crypts)
- Microscopic features that are still compatible with ulcerative colitis and do not warrant a diagnosis of Crohn's disease or IBD, indeterminate type (Mod Pathol 2015;28:S30, J Clin Pathol 2004;57:1233):
- Cryptolytic granulomas
- Backwash ileitis: active inflammation in the terminal ileum in the setting of at least similar in intensity active inflammation in the cecum; usually there is minimal or no chronicity in the ileum
- Microscopic features considered major diagnostic criteria for Crohn's disease (Mod Pathol 2015;28:S30, J Clin Pathol 2004;57:1233):
- True segmental disease of the colon (in untreated patients)
- Absence of rectal involvement (in untreated patients)
- Transmural lymphoid aggregates (away from deep mucosal ulcers)
- Penetrating sinus tracts or fistulas (unrelated to other causes, e.g. diverticulitis)
- Nonnecrotizing epithelioid cell granuloma (unrelated to infection or crypt rupture)
- True small intestinal involvement unrelated to backwash ileitis
- Perianal disease unrelated to other causes
- Superficial (ulcerative colitis-like) Crohn's disease: nonnecrotizing epithelioid cell granulomas (unassociated with infection or crypt rupture) in the mucosa or lymph node of a specimen that is otherwise suggestive of ulcerative colitis (i.e. mucosa based chronic active colitis); a diagnosis of IBD, indeterminate type is preferred by some authors in these cases but an explanation as to the reason of the uncertainty should be included
- Dysplasia / carcinoma: same as IBD (Inflamm Bowel Dis 2009;15:1076)
Microscopic (histologic) images
Sample pathology report
- Terminal ileum, cecum, appendix and colon, total abdominal colectomy:
- Severely active chronic colitis with continuous retrograde extension to the hepatic flexure (see comment)
- Comment: The inflammation is generally mucosa based with extensive ulceration and associated transmural disease but diagnostic characteristics of Crohn's disease are lacking. Rectal sparing has been described clinically but should be confirmed histologically, if possible. The ileal segment and appendix are unremarkable.
- The findings are best classified as inflammatory bowel disease, indeterminate type.
Differential diagnosis
- Ulcerative colitis:
- Diffuse mucosa based / superficial involvement of the colorectum
- Well recognized patterns of ulcerative colitis with Crohn's-like features: cecal patch, cryptolytic granulomas, fulminant ulcerative colitis with transmural inflammation in areas of ulceration
- Crohn's disease:
- Transmural lymphoid aggregates in areas of nonulcerated mucosa, nonnecrotizing epithelioid cell granuloma (not associated with ruptured crypts), fissuring ulcers
- Ischemic colitis:
- Involvement of watershed areas (e.g. splenic flexure) mostly
- Surface active inflammation / necrosis
- Hyalinized lamina propria
- Withered / shrunken crypts
- Diversion colitis:
- Prominent mucosa / submucosal follicular lymphoid hyperplasia
- History of fecal stream diversion
- Segmental colitis associated with diverticular disease (SCAD):
- Limited to areas of the colon with known diverticulosis
Board review style question #1
A 26 year old woman with no significant past medical history is found to have severe colitis and undergoes total abdominal colectomy. The gross examination of the resection specimen reveals a predominantly left sided colitis with discontinuous involvement of the cecal and periappendiceal mucosa, as shown in the image above. Microscopic evaluation demonstrates a mucosa based, active chronic colitis without evidence of transmural inflammation. Several nonnecrotizing epithelioid cell granuloma associated with ruptured crypts are identified. Which of the following is true regarding the patient’s pathologic diagnosis?
- A diagnosis of Crohn's disease is warranted due to the presence of discontinuous colitis
- The findings are best classified as inflammatory bowel disease (IBD), type indeterminate because the specimen shows overlapping features of Crohn's disease and ulcerative colitis
- The findings are consistent with a recognized variant of ulcerative colitis
- The presence of nonnecrotizing epithelioid cell granuloma precludes a diagnosis of ulcerative colitis in this case
Board review style answer #1
C. The findings are consistent with a recognized variant of ulcerative colitis
The photograph shows a total abdominal colectomy specimen with a predominantly left sided colitis and discontinuous involvement of the cecal or periappendiceal mucosa. The gross and microscopic findings are convincing for a diagnosis of ulcerative colitis. It is well documented that patients with left sided ulcerative colitis may have discontinuous involvement of the periappendiceal or cecal mucosa, known as a cecal patch. A diagnosis of IBD, indeterminate type should be avoided when dealing with well recognized variants of IBD, such as ulcerative colitis with cecal patch and superficial (ulcerative colitis-like) Crohn's disease. Nonnecrotizing epithelioid cell granulomas associated with ruptured crypts (cryptolytic granulomas) are known to occur in cases of ulcerative colitis and often result in diagnostic difficulties; however, this finding should not preclude a diagnosis of ulcerative colitis.
Comment Here
Reference: Inflammatory bowel disease, indeterminate type
The photograph shows a total abdominal colectomy specimen with a predominantly left sided colitis and discontinuous involvement of the cecal or periappendiceal mucosa. The gross and microscopic findings are convincing for a diagnosis of ulcerative colitis. It is well documented that patients with left sided ulcerative colitis may have discontinuous involvement of the periappendiceal or cecal mucosa, known as a cecal patch. A diagnosis of IBD, indeterminate type should be avoided when dealing with well recognized variants of IBD, such as ulcerative colitis with cecal patch and superficial (ulcerative colitis-like) Crohn's disease. Nonnecrotizing epithelioid cell granulomas associated with ruptured crypts (cryptolytic granulomas) are known to occur in cases of ulcerative colitis and often result in diagnostic difficulties; however, this finding should not preclude a diagnosis of ulcerative colitis.
Comment Here
Reference: Inflammatory bowel disease, indeterminate type
Inflammatory cap polyp
Table of Contents
Definition / general | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- First described in an abstract in 1985 (Br J Surg 1985; 72 (Suppl) S133)
- Rare
- Etiology unknown, but may be associated with mucosal prolapse (Gut 1993;34:562)
- Often presents with mucoid or bloody diarrhea and hypoproteinemia
- Endoscopy: multiple sessile rectosigmoid polyps with adherent white flecks with normal intervening mucosa (Endoscopy 2001;33:262)
Treatment
- Traditionally polypectomy or partial colectomy (Dis Colon Rectum 2004;47:1208)
- Also medical treatment in patients with H. pylori gastritis (Helicobacter 2004;9:651) and using infliximab, an antibody to tumor necrosis alpha (Gastroenterology 2004;126:1868)
Gross description
- Multiple, distinctive, inflammatory rectosigmoid polyps
Microscopic (histologic) description
- Inflamed mucosa with tortuous, elongated crypts attenuated towards the mucosal surface with abundant inflammation in the lamina propria and characteristic "cap" of inflamed and ulcerated granulation tissue on the mucosal surface
Microscopic (histologic) images
Differential diagnosis
- Amebic colitis
- Antibiotic associated colitis
- Inflammatory bowel disease
Inflammatory fibroid polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare mesenchymal polypoid neoplasms that arise anywhere throughout the GI tract but most commonly in the stomach and small intestine
Essential features
- Benign mesenchymal polypoid neoplasm centered in the submucosa
- Rare in the large bowel
- Proliferation of bland spindle cells with a variable myxoid background and varying degree of inflammatory infiltration
- Association with activating PDGFRA gene mutation
Terminology
- Formerly known as Vanek tumor (no longer recommended)
- Terminological confusion in older literature (e.g., granuloblastoma, gastric fibroma with eosinophilic infiltration, eosinophilic granuloma)
- Inflammatory fibroid polyp (IFP) is the recommended term
ICD coding
- ICD-10: K51.419 - inflammatory polyps of colon with unspecified complications
Epidemiology
- Predominance in middle aged adults (median 53 years), with wide variation (4 - 84 years)
- Very rare in children (World J Clin Pediatr 2015;4:160)
- Slight predominance in women (1.3:1) (In Vivo 2021;35:81)
- Increased frequency in menopausal women has led to postulate a potential relationship with androgens (Anticancer Res 2014;34:7203)
Sites
- Most frequent location is the stomach (> 65% in a recent meta analysis of 417 cases), mainly the antrum (In Vivo 2021;35:81)
- < 10% of cases arise in the large bowel
Pathophysiology
- For a long time, it was considered reactive in nature (autoimmune, allergic)
- Discovery of overexpression and mutations in platelet derived growth factor receptor alpha (PDGFRA) has led to considering inflammatory fibroid polyp a neoplastic process
Etiology
- Unknown
- May be familial (Devon polyposis syndrome) (Gut 1992;33:1004)
Clinical features
- Symptoms largely depend on location
- For large bowel lesions, abdominal pain and acute intestinal obstruction are the most frequent presenting symptoms
- Nonmalignant potential
- Some familial cases and occasional association with GI malignancies (Trop Gastroenterol 2014;35:196, Ann Diagn Pathol 2012;16:148)
- Not usually associated with peripheral eosinophilia
- No endoscopic surveillance is required after the histological diagnosis is confirmed
Diagnosis
- Histologic
Prognostic factors
- Good prognosis with no risk of malignization
Case reports
- 23 year old woman with iron deficiency anemia and a 4.5 cm polypoid lesion in the descending colon removed with a needle knife (Yonsei Med J 2008;49:680)
- 50 year old woman with bloating and abdominal pain with a diagnosis of intussusception undergoes an emergency right hemicolectomy (Medicina (Kaunas) 2022;58:310)
- 53 year old man with diabetes and hypercholesterolemia undergoes a routine colonoscopy, which reveals a 4 cm lobular mass in the proximal rectum removed by transanal resection (J Surg Case Rep 2019;2019:rjz164)
- 65 year old woman with mild abdominal pain for 3 years and a large elongated mass in the cecum (Chin Med J (Engl) 2017;130:2130)
Treatment
- Resection is the preferred therapy
- Endoscopic resection is the preferred option, either polypectomy or endoscopic submucosal or radical resection
- Need of surgical resection in some complicated cases
- No further therapy is necessary
Gross description
- Solitary intraluminal, usually sessile polypoid lesion
- Frequently ulcerated
- Can reach large size (giant inflammatory fibroid polyp measuring > 4 cm)
- As opposed to usual gastric inflammatory fibroid polyp, large bowel lesions can infiltrate through the wall, reaching neighboring structures and mimicking malignancy (Kaohsiung J Med Sci 2013;29:460)
Gross images
Microscopic (histologic) description
- Mostly centered in the submucosa but can infiltrate lamina propria
- Well demarcated but encapsulated
- Proliferation of bland mesenchymal cells
- Background with small vessel proliferation, little collagen, inflammatory infiltrates and variable degrees of edema
- Distinctive onion skin arrangements of spindled cells around vessels
- Vessels can be ectatic
- Inflammatory infiltrate rich in eosinophils
- 5 histopathological patterns proposed, probably representing a spectrum of disease over time:
- Classical fibrovascular
- Nodular
- Sclerotic
- Edematous
- Atypical (very rare)
- Scarce mitosis, never atypical
Microscopic (histologic) images
Positive stains
- Spindle cells are usually positive for CD34 (82 - 100%), with a variable intensity
- Variable smooth muscle actin
- PDGFRA frequently positive, even in cases with no PDFGRA mutations by molecular testing (Pathol Int 2018;68:205)
Negative stains
- CD117 (KIT) (although mast cells are CD117+), DOG1 and S100
- Anaplastic lymphoma kinase (ALK)
- Alpha smooth muscle actin can be focally positive
Electron microscopy description
- No recent reports regarding electron microscopy features of this lesion
- Older reports indicate a combination of fibroblastic and histiocytic features in the tumor cells
- Putative origin in primitive submucosal stromal cells
Molecular / cytogenetics description
- Activating mutations in exons 12, 14 and 18 of PDGFRA in > 50% of inflammatory fibroid polyps, with described differences according to location (Mod Pathol 2009;22:1049)
- PDGFRA mutations seem less frequent in large bowel lesions (Pathol Int 2018;68:205)
- Descriptions of germline mutations in PDGFRA leading to familial cases of inflammatory fibroid polyp or gastrointestinal stromal tumor (GIST) (Cancer Genet 2021;256:106)
Sample pathology report
- Large bowel, endoscopic polypectomy
- Inflammatory fibroid polyp (1.3 cm) (see comment)
- Comment: The lesion is centered in the submucosa and is composed of bland proliferation of spindled cells with prominent eosinophils. The lesional cells express CD34 and are negative for CD117 and DOG1.
Differential diagnosis
- Gastrointestinal stromal tumor:
- Inflammatory myofibroblastic tumor:
- Histology can be similar with spindle cell myofibroblasts and fibroblasts on an inflammatory background with lymphocytes and plasma cells
- Eosinophils are less prominent
- Tumor cells express alpha SMA and desmin and are CD34 negative
- ALK expression by IHC with rearrangements of the ALK gene locus 2p23 in up to 70% of cases
- Inflammatory polyp:
- Usually associated with inflammatory bowel disease
- CD34 negative
- Inflammatory myoglandular polyp (BMC Gastroenterol 2010;10:10):
- Rare nonneoplastic polyp of the large bowel
- Pedunculated and more frequent in the sigmoid colon, with a ripe wild strawberry endoscopic aspect
- Inflammatory granulation tissue with engorged capillaries in lamina propria
- Hyperplastic glands with cystic dilatations
- Proliferation of smooth muscle from the muscularis mucosae
- Angiolymphoid hyperplasia with eosinophilia:
- Extremely rare in the large bowel
- Proliferation of immature blood vessels with epithelioid endothelial cells and infiltration by lymphocytes and eosinophils
- Lack of PDGFRA IHC expression
- Perineuroma / fibroblastic polyps:
- Proliferation of spindle cells limited to mucosa and submucosa
- Frequent association with serrated crypts
- IHC: EMA, claudin1 and GLUT1 positive
- Frequent BRAF p.V600E mutations in the epithelial component (Clin Med Insights Pathol 2018;11:1179555718815918)
- Schwannomas:
- Typical pattern of growth with Verocay bodies
- IHC: S100 positive
- Reference: Histopathology 2020;76:342
Board review style question #1
Board review style answer #1
D. It is polypoid and originates in the submucosa. Inflammatory fibroid polyps are usually polypoid and originate in the submucosa, as opposed to other kind of stromal lesions, like gastrointestinal stromal tumors or schwannoma.
Comment Here
Reference: Inflammatory fibroid polyp
Comment Here
Reference: Inflammatory fibroid polyp
Board review style question #2
Which statement is true regarding IHC for PDGFRA in inflammatory fibroid polyps?
- Allows differential diagnosis with GIST
- Always positive
- Always negative
- Confirms reactive nature of the process
- Does not always reflect a mutation in PDGFRA gene
Board review style answer #2
E. Does not always reflect a mutation in PDGFRA gene. IHC for PDFGRA can be positive in cases with no shown mutation of PDGFRA gene.
Comment Here
Reference: Inflammatory fibroid polyp
Comment Here
Reference: Inflammatory fibroid polyp
Inflammatory myofibroblastic tumor
Table of Contents
Definition / general | Essential features | Terminology | Sites | Clinical features | Radiology images | Case reports | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Low grade mesenchymal neoplasm that can arise in the colonic wall or mesentery, usually of children
- See also topic in Soft tissue chapter
Essential features
- Mesenchymal neoplasm, often pediatric, with spindled cells admixed with inflammation
- t(2;5) (TPM3-ALK) translocation
- Rare aggressive variant called epithelioid inflammatory fibroblastic sarcoma
Terminology
- Older terms include called inflammatory pseudotumor, inflammatory fibrosarcoma and plasma cell granuloma
- Epithelioid inflammatory fibroblastic sarcoma refers to a rare, aggressive variant with unique morphologic and molecular features (Am J Surg Pathol 2011;35:135)
Sites
- Can arise in the colonic wall or the mesentery
- Most common site in gastrointestinal tract is stomach
Clinical features
- Patients usually in the pediatric age range (Am J Surg Pathol 2007;31:509)
- Symptoms include anemia, abdominal pain, fever, weight loss and high sedimentation rate (J Pediatr Surg 2001;36:169)
- May recur but metastases rare; these outcomes may be more common in epithelioid inflammatory fibroblastic sarcoma
Case reports
- 11 year old boy with rectal tumor (Arch Iran Med 2006;9:277)
- 35 year old woman with clinical appendicitis, cecal tumor (Scott Med J 2004;49:157)
Gross description
- Circumscribed, often polypoid
Gross images
Microscopic (histologic) description
- Bland spindle cells with abundant amphophilic cytoplasm and variably prominent nucleoli
- Lymphoplasmacytic infiltrate with polyclonal plasma cells
- Background may show myxoid change or laminated / whorled fibrosis
- Epithelioid inflammatory myofibroblastic sarcoma: similar, except cells are more epithelioid, with large nucleoli
Microscopic (histologic) images
Contributed by Raul S. Gonzalez, M.D.
Contributed by Michael Feely, D.O.
Images hosted on other servers:
Positive stains
- Desmin, smooth muscle actin; variable cytoplasmic ALK1 (Mod Pathol 2002;15:931)
- Epithelioid inflammatory myofibroblastic sarcoma: nuclear membrane ALK1
Molecular / cytogenetics description
- Abnormalities of 2p23, usually t(2;5) (TPM3-ALK) translocation, in approximately half of cases; more common in children (Mod Pathol 2001;14:569)
- Epithelioid inflammatory myofibroblastic sarcoma: t(2;2) RANBP2-ALK translocation
Sample pathology report
- Ascending colon, resection:
- Inflammatory myofibroblastic tumor (3.1 cm) (see comment)
- Margins of resection unremarkable.
- Two benign lymph nodes.
- Comment: An immunostain for ALK1 is positive in the tumor.
Differential diagnosis
- Inflammatory fibroid polyp:
- Patients older, tumors smaller with more eosinophils and CD34 positivity (Hum Pathol 2002;33:307)
- Fibromatosis:
- Nuclear positivity for beta catenin
- Schistosomiasis:
- May rarely form inflammatory pseuotumorous reaction (J Natl Med Assoc 2006;98:1365)
Additional references
Board review style question #1
A young male patient presents with abdominal pain. Imaging reveals a large mesenteric mass, which is resected. An image of the tumor’s histology is seen above. Immunohistochemistry shows ALK1 staining of the nuclear membranes. What molecular abnormality is present in this tumor?
- ALK1 deletion
- ALK1 inversion
- t(2;2) RANBP2-ALK translocation
- t(2;5) TPM3-ALK translocation
Board review style answer #1
C. t(2;2) RANBP2-ALK translocation. This is an epithelioid inflammatory myofibroblastic sarcoma.
Comment Here
Reference: Inflammatory myofibroblastic tumor
Comment Here
Reference: Inflammatory myofibroblastic tumor
Inflammatory polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Generic term for nonneoplastic mixture of epithelial and stromal components admixed with inflammatory cells
- Often related to inflammatory bowel disease (Crohn's disease or ulcerative colitis), anastomosis, ischemic colitis or infection
Essential features
- Nonneoplastic colon polyp composed of inflamed mucosa
- Typically shows surface erosion with surrounding granulation tissue and epithelial distortion
Terminology
- Inflammatory polyp as a diagnosis is generally used to describe small foci of nonspecifically inflamed colonic mucosa or inflammatory pseudopolyps
- Inflammatory polyp as a category includes several subtypes, including:
- Inflammatory cap polyp
- Inflammatory fibroid polyp
- Inflammatory myoglandular polyp
- Prolapse associated polyps
ICD coding
- ICD-10: K51.4 - inflammatory polyps of colon
Epidemiology
- Typically second and third decades for inflammatory bowel disease; incidence range of 10 - 20% in ulcerative colitis patients (World J Gastroenterol 2017;23:1541)
- May occur in older patients with peripheral vascular disease
Sites
- Can arise anywhere in the colon, especially at the ileocecal region in Crohn’s disease
- May form at anastomotic sites
Pathophysiology
- Believed to be secondary to repeated bouts of intense inflammation
- Formation of inflammatory polyps may be related to increases in C reactive protein, C4 and procollagen III peptide (World J Gastroenterol 2003;9:619)
Clinical features
- Sporadic inflammatory polyps are usually incidental at colonoscopy
- May present with intussusception or obstructive symptoms
- Presence of pseudopolyps in inflammatory bowel disease may represent recent flare, although lesions are found in active or dormant disease (World J Gastroenterol 2017;23:1541)
- Also may be related to arthropathy or other extracolonic symptoms (Lancet 1969;2:555)
Case reports
- 28 year old woman with pseudosarcomatous changes in inflammatory polyp (Korean J Gastrointest Endosc 2007;35:51)
- 47 year old man with intussusception due to 3 cm inflammatory polyp (Asian J Surg 2005;28:58)
- 62 year old man with inflammatory polyp due to Kirschner wire (Intern Med 2014;53:699)
- 74 year old man with inflammatory polyp with osseous metaplasia (Gastroenterology Res 2012;5:74)
- Patient with inflammatory polyp containing schistosomiasis (bilharzial polyp) (J Clin Gastroenterol 1983;5:169)
Treatment
- Typically treated endoscopically via polypectomy
- Examples related to inflammatory bowel disease may improve with infliximab (J Crohns Colitis 2010;4:707)
- Argon plasma coagulation or ablation for bleeding control
- Surgical resection if profuse bleeding, obstruction or intussusception
Gross description
- Usually sessile and less than 3 cm
- May be pedunculated or filiform
Microscopic (histologic) description
- Often consists of normal colonic mucosa in a polypoid configuration, with increased inflammation (expanded lamina propria and crypt abscesses or cryptitis)
- Epithelium can show various degrees of surface erosion, crypt distortion / dilation or hyperplasia, along with reactive nuclear features within the mucosal epithelial cells
- May consist entirely of granulation tissue (abundant thin walled and dilated vessels surrounded by mixed neutrophilic and lymphoplasmacytic inflammation)
- Reactive stromal cells may be markedly pleomorphic and mimic sarcoma
- Cases associated with inflammatory bowel disease may rarely show epithelial dysplasia
Microscopic (histologic) images
Negative stains
- S100, cytokeratin (reactive stromal cells), CMV
Molecular / cytogenetics description
- Usually no abnormalities
Videos
Inflammatory polyp on colonoscopy
Sample pathology report
- Sigmoid colon, polypectomy:
- Inflammatory polyp
Differential diagnosis
- Juvenile polyp:
- Large cystically dilated glands; wide histologic overlap and the distinction is of little importance in adult patients
- Pyogenic granuloma:
- Lobular arrangement of capillaries within edematous stroma (Ann Diagn Pathol 2005;9:106)
Board review style question #1
Which of the following is not a typical feature of colonic inflammatory polyps?
- Crypt distortion / branching
- Granulation tissue changes
- Microsatellite instability
- Surface mucosal erosion
Board review style answer #1
C. Microsatellite instability. Inflammatory polyps are a benign process with various degrees of mucosal erosion, increased vascular density similar to granulation tissue and architectural changes.
Comment here
Reference: Inflammatory polyp
Comment here
Reference: Inflammatory polyp
Intestinal neuronal dysplasia
Table of Contents
Definition / general | Diagnosis | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stainsDefinition / general
- Also known as neuronal colonic dysplasia, hyperganglionosis, pseudo or variant Hirschsprung disease
- Type A: hypoplastic or aplastic sympathetic innervation
- Type B: hypoplastic or plastic parasympathetic innervation (Virchows Arch A Pathol Anat Histopathol 1992;420:171); has disturbed submucous plexus development (Semin Pediatr Surg 2009;18:206)
- A controversial entity; should diagnose only if no other diagnosis, no obstruction and multiple adequate biopsies (30 sections) of submucosa and muscularis propria available; may be due to delayed maturity of enteric nervous system as 95% have normal gut motility within 1 year
- Associated with neurofibromatosis 1 and MEN2b syndromes
- Associated with hypoganglionosis of myenteric plexus or aganglionosis of rectum
Diagnosis
Diagnostic criteria:
- Biopsies 8 - 10 cm above dentate line, sufficient submucosa, 15 - 20% of ganglia are giant ganglia with more than 8 nerve cells in submucosa of 30 serial sections (Eur J Pediatr Surg 2004;14:384)
- Hyperganglionosis of submucous plexus, giant ganglia and rectal biopsies show either ectopic ganglia, increased acetylcholinesterase activity in lamina propria or increased acetylcholinesterase in nerve fibers around submucosal blood vessels (J Pediatr Surg 2001;36:777, Indian J Pathol Microbiol 2004;47:4)
Case reports
- 2 year old girl with coexisting congenital interstitial cell of Cajal hyperplasia (Am J Surg Pathol 2000;24:1568)
Microscopic (histologic) description
- Resembles Hirschsprung disease with hyperplasia of myenteric nerves and increased acetylcholinesterase staining but with occasional (15 - 20%) submucosal giant ganglia (containing 7 - 10 neurons vs. 3 - 5 normally) and isolated ganglion cells in submucosa
- Note: giant ganglia by themselves are not specific (Virchows Arch 1998;432:103)
Positive stains
- BCL2 to detect immature enteric ganglion cells (Am J Surg Pathol 2005;29:1017)
Intestinal spirochetosis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colonization of the colonic epithelium by the filamentous nontreponemal anaerobic spirochetes, Brachyspira aalborgi or Brachyspira pilosicoli
Essential features
- Colonization of the colonic epithelium by spirochetes of the Brachyspira species
- Most commonly asymptomatic but may present with diarrhea or abdominal pain / cramps
- Diagnosed by histologic examination of colorectal mucosal biopsies that demonstrate a basophilic fringe on the surface epithelium
- May be confirmed with silver stains (Warthin-Starry, Steiner) or Treponema pallidum immunohistochemistry
- Typically treated with metronidazole, if symptomatic; otherwise, may be observed
ICD coding
Epidemiology
- Human intestinal spirochetosis (HIS) was originally recognized in 1967; however, the pathogenicity is still unclear
- HIS has a global distribution and Brachyspira species have been detected on nearly every continent, with the exception of Antarctica
- Prevalence rate is highly variable and is not entirely clear from the current literature; however, there appears to be lower prevalence rates (ranging from 0.2% to 3.2%) among healthy people residing in developed countries and higher prevalence rates (ranging from 10.8% to 64.8%) in developing countries
- In developed nations, the prevalence rates are thought to be highest among men who have sex with men (MSM) and patients with HIV
- More common in men and patients in the fourth or fifth decades of life
- Although rare, it may occur in pediatric patients
- No confirmed seasonality
- References: Br Med J 1967;3:718, J Gastroenterol Hepatol 2022;37:1222, J Bacteriol 2019;201:e00465, Ann Clin Lab Sci 2020;50:386, Annals Diagn Pathol 2015;19:414, Pediatr Gastroenterol Hepatol Nutr 2019;22:193
Sites
- Any portion of the colon may be involved
- A small study noted a predilection for the right colon (Annals Diagn Pathol 2015;19:414)
Pathophysiology
- Pathogenesis, transmission and risk factors remain unclear
- It is debatable whether Brachyspira species represent a commensal or pathogenic organism
- Electron microscopy findings suggest that the spirochetes adhere to the brush border resulting in thickening of the border (creating a so called false brush border)
- Diarrhea is thought to result from the reduction in absorptive surface secondary to loss of microvilli
- References: Ann Clin Lab Sci 2020;50:386, Pediatr Gastroenterol Hepatol Nutr 2019;22:193, J Bacteriol 2019;201:e00465, J Gastroenterol Hepatol 2022;37:1222
Clinical features
- In most children and adults, human intestinal spirochetosis is asymptomatic and often an incidental histologic finding on endoscopic biopsy material
- In symptomatic patients, the 2 most common symptoms are diarrhea (often chronic and watery) (51%) and abdominal cramps or pain (46%)
- Other symptoms may include bloating, weight loss, constipation, nausea, alternating constipation / diarrhea or irritable bowel syndrome-like symptoms and rectal or perirectal bleeding
- Pediatric patients may develop severe symptoms
- Brachyspira pilosicoli has a broader host range including swine, birds, humans and nonhuman primates
- Known cause of diarrhea in pigs and chickens
- Usually asymptomatic in humans but may cause diarrhea, abdominal pain or perirectal bleeding
- Critically ill patients may have spirochetemia
- Brachyspira aalborgi has only been detected in humans, nonhuman primates and opossums
- Typically, not associated with histologic findings or gastrointestinal symptoms
- Some believe this is simply a commensal organism
- Some studies have found an association between human intestinal spirochetosis and juvenile polyps in children
- References: Ann Clin Lab Sci 2020;50:386, J Gastroenterol Hepatol 2022;37:1222, Pediatr Gastroenterol Hepatol Nutr 2019;22:193, J Bacteriol 2019;201:e00465
Diagnosis
- Gold standard for diagnosis is histologic examination of an endoscopic colon or rectal biopsy
- Noninvasive diagnostic methods are not currently routine
- Reference: J Gastroenterol Hepatol 2022;37:1222
Case reports
- 9 year old boy with recurrent abdominal pain (Arch Pathol Lab Med 2004;128:823)
- 38 year old man with worsening fatigue and dyspnea (Am J Med 2019;132:e654)
- 49 year old man with positive fecal occult blood test result (Clin Gastroenterol Hepatol 2022;20:e1226)
- 60 year old man with hepatitis C cirrhosis and progressive weight loss (Int J Surg Pathol 2014;22:709)
Treatment
- Metronidazole is the most commonly utilized antibiotic for symptomatic patients
- Symptoms will resolve in the majority of patients (81%); in the minority (5%) they will not
- Asymptomatic patients do not usually require treatment
- References: J Gastroenterol Hepatol 2022;37:1222, Ann Clin Lab Sci 2020;50:386
Gross description
- The majority of cases appear grossly normal on endoscopy; rarely, nonspecific erythematous lesions may be seen (Pediatr Gastroenterol Hepatol Nutr 2019;22:193, Ann Clin Lab Sci 2020;50:386)
Microscopic (histologic) description
- The characteristic histologic feature of human intestinal spirochetosis is a blue fringe or fuzzy appearance of the surface colonic epithelium on H&E staining, which may be referred to as a so called false brush border, confluent forest or carpeting
- Rarely, there may be nonspecific lymphocytic, neutrophilic or eosinophilic inflammation in the lamina propria or epithelium; lymphoid aggregates or follicles may also be seen
- Organisms may be highlighted by silver stains (Steiner, Warthin-Starry) or immunohistochemical stains for Treponema pallidum, which crossreacts with Brachyspira species
- Reference: Virchows Arch 2020;477:57, J Bacteriol 2019;201:e00465, J Gastroenterol Hepatol 2022;37:1222, Pediatr Gastroenterol Hepatol Nutr 2019;22:193, BMC Infect Dis 2021;21:721
Microscopic (histologic) images
Positive stains
- Organisms may be highlighted by silver stains (Steiner, Warthin-Starry) or immunohistochemical stains for Treponema pallidum, which crossreacts with Brachyspira species
Electron microscopy description
- Nontreponemal spirochetes are attached perpendicularly to the luminal border of enterocytes
- The head of the spirochetes anchors between microvilli, while the tail is directed towards the colonic lumen
- Creates a confluent forest of spirochetes on the surface epithelium, while the microvilli are reduced
- References: J Gastroenterol Hepatol 2022;37:1222, J Bacteriol 2019;201:e00465
Electron microscopy images
Sample pathology report
- Colon, random, biopsy:
- Intestinal spirochetosis (see comment)
- Comment: H&E stained sections demonstrate a basophilic fringe on the surface of the colonic epithelium that is highlighted by a Warthin-Starry stain for spirochetes. The findings are compatible with intestinal spirochetosis.
Differential diagnosis
- Prominent glycocalyx:
- Careful pathologic examination of the surface epithelium and use of special stains (silver or T. pallidum immunohistochemistry) (J Gastroenterol Hepatol 2022;37:1222)
- Enteroadherent Escherichia coli:
- Gram negative on tissue Gram stain
- Normal colon biopsy:
- Findings are subtle and could be missed; always look at the surface of the epithelium in random colon biopsies for the basophilic fringe
Additional references
Board review style question #1
A 50 year old man with chronic watery diarrhea had random colon biopsies taken during a colonoscopy, which demonstrated macroscopically normal mucosa throughout the colon. A representative H&E image of his colon biopsies is shown above, as well as a Warthin-Starry stain performed to confirm the morphologic impression. What is the most likely diagnosis?
- Enteroadherent Escherichia coli
- Intestinal spirochetosis
- Normal colonic mucosa
- Normal colonic mucosa with prominent glycocalyx
Board review style answer #1
B. Intestinal spirochetosis. The H&E image demonstrates the classic basophilic fringe on the surface epithelium, which is characteristic of intestinal spirochetosis. This is confirmed with the Warthin-Starry stain that highlights the organisms. The findings of intestinal spirochetosis may be subtle and appear like normal colonic mucosa. It is important to evaluate the surface epithelium for the presence of the spirochetes in random colon biopsies performed for diarrhea. Enteroadherent E. coli would be strongly gram negative, if performed.
Comment Here
Reference: Intestinal spirochetosis
Comment Here
Reference: Intestinal spirochetosis
Board review style question #2
Which of the following statements is true regarding human intestinal spirochetosis (HIS)?
- All patients diagnosed histologically with human intestinal spirochetosis should be treated with antibiotics
- A majority of patients with human intestinal spirochetosis are symptomatic
- The most common symptoms in symptomatic patients are diarrhea and abdominal pain / cramps
- The most commonly utilized treatment for symptomatic patients is clarithromycin
- The pathogenesis, transmission and risk factors for human intestinal spirochetosis are clearly understood and delineated
Board review style answer #2
C. The most common symptoms in symptomatic patients are diarrhea (which is commonly chronic and watery) and abdominal pain / cramps. Most patients with human intestinal spirochetosis diagnosed histologically are asymptomatic and do not require treatment but may be followed or observed. Metronidazole is the most commonly used antibiotic therapy in symptomatic patients. The pathogenesis, transmission and risk factors for human intestinal spirochetosis are still unclear.
Comment Here
Reference: Intestinal spirochetosis
Comment Here
Reference: Intestinal spirochetosis
Intramucosal carcinoma
Table of Contents
Definition / general | Essential features | Terminology | Clinical features | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Malignant change confined to mucosa (considered pTis in AJCC staging)
- Proper cutoff between high grade dysplasia and intramucosal carcinoma is not entirely clear
Essential features
- Adenocarcinoma solely within the mucosal layer of the colon
- Usually within a polyp but may occur in flat mucosa in inflammatory bowel disease
- Term typically not used by Western pathologists, who prefer high grade dysplasia
Terminology
- Controversial term that reflects differing thresholds for malignancy between the West (higher threshold) and Japan (lower threshold), as Japanese pathologists often diagnose as malignant what Western pathologists might call high grade dysplasia
- United States Preventive Services Task Force specifically recommends against using the term intramucosal carcinoma for lesions confined to the mucosa (Gastrointest Endosc 2020;92:997)
Clinical features
- Up to 2% of cases may metastasize to lymph nodes (J Clin Oncol 2010;28:e469)
- Earlier theory that colonic mucosa has no lymphatic channels may be erroneous (Histopathology 2015;67:167)
- Intramucosal carcinoma on biopsy may portend unsampled invasive disease (Ann Surg Oncol 2009;16:3267)
Case reports
- 51 year old man with sporadic juvenile polyp of the colon harboring an intramucosal adenocarcinoma (Int J Surg Pathol 2015;23:571)
- 71 year old man with TisN0M1 sigmoid colon cancer (Ann Coloproctol 2014;30:141)
Treatment
- Polypectomy should be sufficient for intramucosal carcinoma confined to a polyp, even with a poorly differentiated component (Am J Surg Pathol 2007;31:1882)
- Endoscopic mucosal resection may be curative (J Gastroenterol Hepatol 2011;26:1619)
Gross description
- May appear as a flat / ulcerated lesion or arise in a polyp
Microscopic (histologic) description
- Glandular proliferation showing high grade nuclei and architectural complexity, extending no deeper than the muscularis mucosae
- Necrosis, desmoplasia and single malignant cells may suggest intramucosal carcinoma over high grade dysplasia but this remains subjective
- Often arises in an adenoma but may occur in flat colonic mucosa (such as in patients with inflammatory bowel disease)
- May rarely show signet ring cells (Virchows Arch 2009;454:115)
Microscopic (histologic) images
Sample pathology report
- Rectum, mass, biopsy:
- Tubulovillous adenoma with extensive high grade dysplasia (see comment)
- Comment: The findings are compatible with an interpretation as intramucosal carcinoma, though this concept is rarely applied to colorectal lesions. The sample is relatively superficial and an unsampled invasive process cannot be excluded.
Differential diagnosis
- High grade dysplasia:
- Cutoff unclear; see above
Board review style question #1
Which of the following is true about intramucosal carcinoma of the colorectum?
- It only arises within traditional serrated adenomas
- Roughly 10% of cases metastasize to lymph nodes despite early stage
- There are well defined histologic criteria to distinguish it from high grade dysplasia
- Usage of the term differs between Eastern and Western pathologists
Board review style answer #1
D. Usage of the term differs between Eastern and Western pathologists
Comment Here
Reference: Intramucosal carcinoma
Comment Here
Reference: Intramucosal carcinoma
Ipilimumab associated colitis
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Clinical features | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Colitis caused by use of the anti-CTLA4 medication, ipilimumab
Essential features
- Colitis is a somewhat common side effect of ipilimumab
- Can be mild (diarrhea) or severe (perforation, death)
- Histology: increased intraepithelial lymphocytes, increased apoptosis, cryptitis
Epidemiology
- Occurs in up to 29% of patients taking ipilimumab, an immunologic agent used for melanoma and other malignancies (Melanoma Res 2015;25:321)
Sites
- Can affect entire gastrointestinal tract, even liver (Am J Surg Pathol 2015;39:1075)
Pathophysiology
- Causes dysregulation of gastrointestinal mucosal immunity
Clinical features
- Diarrhea, colitis
Radiology description
- Mesenteric vessel engorgement and bowel wall thickening (Am J Roentgenol 2013;200:W468)
Prognostic factors
- Side effects are dose dependent, schedule related and cumulative (Scientifica (Cairo) 2013;2013:857519)
Case reports
- 52 year old man with perforating ipilimumab associated colitis (J Surg Case Rep 2014;2014:rju010)
Treatment
- Steroids are usually effective but anti-TNF agents or surgery may be required for severe cases (Dig Dis Sci 2016;61:2132)
Microscopic (histologic) description
- Lymphoplasmacytic expansion of lamina propria; increased apoptosis and intraepithelial lymphocytes; cryptitis and crypt elongation (Am J Surg Pathol 2008;32:1130)
- Severe cases may show ulceration/perforation, pseudopolyp formation and crypt dilation, without lymphocytosis or apoptosis (J Clin Gastroenterol 2013;47:781)
Microscopic (histologic) images
Sample pathology report
- Sigmoid colon, biopsy:
- Colonic mucosa with mild acute inflammation, expanded lamina propria, increased intraepithelial lymphocytes and focally increased apoptosis (see comment)
- Comment: The patient's use of ipilimumab is noted. The findings are most consistent with ipilimumab associated colitis.
Differential diagnosis
- Inflammatory bowel disease:
- Less apoptosis; signs of chronicity
- Idelalisib associated colitis:
- Also caused by immune modulation (PI3K inhibitor); similar histology; history probably necessary for distinction (Am J Surg Pathol 2015;39:1661)
- Anti-PD1 associated colitis:
- Also caused by immune modulation; similar histology; history probably necessary for distinction (Am J Surg Pathol 2017;41:643)
Board review style question #1
Which of the following is true about gastrointestinal tract injury secondary to ipilimumab use?
- It can cause ulceration and perforation
- It is an extremely rare side effect
- It only occurs in the colon
- The main histologic findings are Paneth cell metaplasia and a thickened basement membrane
Board review style answer #1
Board review style question #2
A 55 year old man with a history of metastatic melanoma presents with diarrhea and undergoes colonoscopy, which finds musical erythema. A biopsy is taken, with histology shown above. What is the most likely diagnosis?
- Ipilimumab induced colitis
- Lymphocytic colitis
- Segmental colitis associated with diverticulosis
- Ulcerative colitis
Board review style answer #2
Ischemic colitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Ischemic colitis is a leading cause of acute abdomen in elderly patients
- Gross and microscopic findings depend on the severity and duration of ischemia
- Classic findings include superficial mucosa necrosis / regeneration, withered crypts and lamina propria hyalinization
Essential features
- Gross and microscopic spectrum of ischemic colitis varies by the duration and extent of ischemia
- Classic ischemic type mucosal injury pattern is described as superficial mucosa necrosis / regeneration, withered crypts and lamina propria hyalinization; this histology is not specific for ischemia and may be seen with infections (C. difficile, E. coli O157:H7), medications and inflammatory bowel disease
- In cases with severe sudden ischemia, the affected segment of bowel is infarcted with red, congested or grayish dusky discoloration with transmural necrosis
Terminology
- Ischemic colitis
- Noninfectious colitis
ICD coding
- ICD-10: K55.9 - Vascular disorder of intestine, unspecified
Epidemiology
- Incidence: 4.5 - 44 per 100,000 (WMJ 2016;115:196)
- Most common in older patients and mainly in debilitated elderly women (Front Surg 2017;4:47)
- Younger patients can be affected; this is on the rise (WMJ 2016;115:196)
Sites
- Watershed areas most commonly affected (splenic flexure and rectosigmoid colon); the right colon is involved in 20 - 25% of cases (WMJ 2016;115:196)
Pathophysiology
- 2 categories: vascular factors and bowel factors, both leading to inadequate blood flow to the colonic wall and causing ischemic injury (WMJ 2016;115:196)
- Vascular factors:
- Transient hypoperfusion is the most common cause
- Vasospasm of the colonic vessels is another mechanism, either due to systemic hypoperfusion which shunts blood away from intestine or due to vasoconstrictive effects of certain drugs and substances
- Hypercoagulable states, such as coagulation factor deficiencies
- Vasculitis, such as systemic lupus erythematous and antiphospholipid syndrome
- Bowel factors:
- Constipation, irritable bowel syndrome (IBS), fecal impaction, colonic obstruction and any other condition that increases the intraluminal pressure, which may compromise the blood flow the colonic wall, leading to ischemic injury
- Extent of ischemic changes in the intestines depends on the severity, rate of onset and duration of ischemia
- Transient, reversible vascular obstruction often leads to mild superficial changes, whereas sudden, complete obstruction results in complete infarction of the affected segment with transmural necrosis (see colon infarct)
Etiology
- Most cases represent nonocclusive ischemia caused by a sudden decrease in blood flow in the small arterioles of the colon, resulting from a low volume state (Front Surg 2017;4:47)
- Risk factors include atherosclerotic disease, aortic surgery, coronary artery bypass graft, atrial fibrillation, oral contraceptives, hereditary coagulopathies, cocaine abuse, strenuous physical activity, bacterial pathogens (cytomegalovirus and E. coli), hypertension, diabetes mellitus and hypoalbuminemia
- Constipation, hyperuricemia, smoking may contribute to disease development in young adults
- Other risk factors in younger patients: collagen vascular diseases, amyloidosis or cocaine use
- Idiopathic myointimal hyperplasia of mesenteric veins (Am J Surg Pathol 2017;41:1657)
Clinical features
- Classic symptoms in elderly are abdominal pain, hematochezia and leukocytosis
- Left sided ischemic colitis presents with acute abdominal pain and lower gastrointestinal bleeding
- Right sided ischemic colitis presents with severe abdominal pain but rarely bloody diarrhea
- Full thickness involvement of the colonic wall can lead to perforation with peritonitis and sepsis
- Reference: Front Surg 2017;4:47
Diagnosis
- CT scan is the investigation of choice (BMJ 2016;355:i6600)
- CT angiogram finds abnormalities in the vasculature (Front Surg 2017;4:47)
- Xrays can help in detecting perforation by demonstrating gas under the diaphragm
- Colonoscopy should be performed within 48 hours to visualize mucosa; it can show edematous and fragile mucosa, segmental erythema, erosions, hemorrhages and ulceration
- An endoscopic finding, the so called colon single stripe sign represents a single line of erythema with erosion or ulceration placed along the longitudinal axis of the large bowel; this sign is highly suggestive of ischemic injury and indicates a milder disease
- Pseudomembranes can be seen, characterized by elevated yellow-white nodules or plaques on the mucosal surfaces of the colon biopsy (Dis Mon 2015;61:181)
Laboratory
- Complete blood count, metabolic panel and liver function tests are required to assess the physiologic status of the patient (Front Surg 2017;4:47)
- C reactive protein and neutrophil count are likely to be raised
- Serum lactate levels may be raised as a result of systemic dysfunction and hypoperfusion (BMJ 2016;355:i6600)
Radiology description
- CT may appear normal in mild disease (Front Surg 2017;4:47)
- In more advanced but nongangrenous forms of the disease, CT will often show colonic wall thickening, thumbprinting, colonic dilatation and pericolic fat stranding, with or without the presence of ascitic fluid
- Double halo or target sign may be present
- Emboli or thrombus occluding the inferior mesenteric artery is occasionally seen while the corresponding colonic wall appears thin due to the lack of reperfusion
- Air within the mesenteric or the portal venous system or pneumatosis coli is a serious finding indicating bowel infarction
Prognostic factors
- If the ischemia is limited to the mucosa, the disease will be transient and complete recovery is expected
- Ischemia involving the muscularis propria can lead to scarring and stricture formation
- If ischemia involves full thickness of the bowel wall, it may lead to gangrene and perforation of the colonic wall with peritonitis and sepsis
- Reference: Front Surg 2017;4:47
Case reports
- 20 year old man with ischemic colitis associated with rhabdomyolysis and heat stroke after intense exercise (Korean J Gastroenterol 2019;74:115)
- 35 year old woman with end stage renal disease on hemodialysis who developed ischemic colitis associated with sevelamer crystals (Indian J Nephrol 2019;29:191)
- 38 year old woman with schizoaffective disorder presenting with olanzapine induced ischemic colitis (Rev Esp Enferm Dig 2016;108:507)
- 55 year old woman with mass forming ischemic colitis (Case Rep Pathol 2019;2019:8927872)
- 59 year old man with ischemic colitis caused by polycythemia vera (Exp Ther Med 2018;16:3663)
Treatment
- Transient ischemia is managed conservatively and has a relatively good prognosis (Front Surg 2017;4:47)
- Conservative therapy includes fluid resuscitation, optimizing cardiac output, bowel rest and discontinuing the etiologic agent
- Antibiotic therapy can be started
- For severe disease, urgent laparotomy and removal of the necrotic part of the colon is recommended
Gross description
- Ulcers, pseudopolyps
- Frank blood or dark mucus in lumen
- Segmental thinning in areas of full thickness infarction or gangrene
- Tan-brown dusky areas of the bowel mucosa
- Pseudomembranous appearance in exudative cases
- Perforation can be identified in severe cases
- Fibrosis and stricture formation in late cases
- When grossing specimens, carefully dissect blood vessels and submit numerous sections to detect vascular lesions
- Rarely, ischemic colitis can produce mass forming lesions (Am J Surg Pathol 2015;39:1275)
- References: Dig Dis Sci 2009;54:2009, World J Gastroenterol 2008;14:7302
Gross images
Microscopic (histologic) description
- Early transient ischemia may result in mild changes, such as mucosal edema, denudation and congestion
- With more advanced ischemia, superficial mucosal necrosis ensues, which results in crypt damage (withering) or loss and hyalinization of the lamina propria
- A reduced number and size of crypts and fibrosis in lamina propria suggests a more severe injury
- Necrosis, ulceration and granulation tissue extend into submucosa and surrounding smooth muscle fibers of muscularis mucosae in more severe cases
- Acute cases may exhibit acute inflammatory exudate (pseudomembranes) on the mucosa, even when noninfectious
- Atypical reactive or degenerative changes may be seen, mimicking dysplasia or malignancy (e.g. pseudosignet ring cells)
- Hemosiderin / hemorrhage and edema in lamina propria
- May see crypt abscesses (especially in cases with reperfusion); however, fissures, lymphoid follicles and granulomas are absent
- In patients with chronic ischemia, histologic findings may represent a mixture of ischemic features described above, along with foci of chronic mucosal injury, such as crypt architectural distortion, Paneth cell metaplasia, basal lymphoplasmcytosis and active inflammation, mimicking inflammatory bowel disease
- Vessels may show necrotizing phlebitis, hyaline thrombi or capillary microthrombi, cholesterol crystals (atheroembolism) or myointimal hyperplasia
- Cholesterol crystals, when present in vascular lumens, support a diagnosis of atheroembolism
- In some cases, underlying etiology can be identified
- References: Goldblum: Rosai and Ackerman's Surgical Pathology, 11th Edition, 2017, Arch Pathol Lab Med 2001;125:224, Am J Surg Pathol 1997;21:706
Microscopic (histologic) images
Sample pathology report
- Colon, biopsy:
- Colonic mucosa with changes consistent with ischemic type mucosal injury / colitis (see comment)
- Comment: Possible etiologies for this ischemic type mucosal injury include true ischemic colitis, infection (such as E. coli 0157:H7) and a drug reaction (including oral contraceptives, NSAIDs, digoxin and ergotamine derivatives). Clinical and endoscopic correlation is recommended.
- Colon, colectomy:
- Ischemic colitis with transmural necrosis / hemorrhage / perforation
- The resection margins are viable.
Differential diagnosis
- Drug / medication related injury:
- Ischemic pattern of injury in young adults and involvement of nonwatershed areas should raise the possibility of drug induced injury
- Drugs / medication related ischemic type injury (Arch Pathol Lab Med 2016;140:748)
- Characterized by eosinophilic, lymphocytic and granulocytic infiltration (World J Gastroenterol 2019;25:967)
- Increased intraepithelial apoptosis (mycophenolate mofetil)
- Fragments of resins in cases associated with kayexalate or bile acid squestrants
- Common drugs: oral contraceptives, cocaine, ergotamine derivatives, sumatriptan, kayexalate, cholestyramine
- Crohn's disease:
- Often younger patients
- Transmural inflammation, lymphoid aggregates
- No necrosis
- Granulomas
- Infectious colitis:
- Occurs in epidemiological clusters
- Younger patients
- Children, elderly and immunocompromised are susceptible
- Bacterial colitis is the most common cause
- Pseudomembranous colitis is caused by Clostridium difficile (Am J Surg Pathol 1997;21:706):
- Typically, history of use of antibiotics such as vancomycin is present and useful in raising a clinical suspicion of C. difficile colitis; stool antigen testing for presence of C. difficile toxin is confirmatory
- Presence of pseudomembranes is usually more widespread in the colon in infectious etiologies and more segmental in ischemia
- Hyalinization of lamina propria and specific vascular pathology are usually absent in C. difficile colitis
- Crypt atrophy, lamina propria hemorrhage and full thickness mucosal atrophy favor a diagnosis of ischemia over C. difficile (Am J Surg Pathol 1997;21:706)
- Ulcerative colitis:
- Often younger patients
- Cryptitis and crypt abscesses
- Basal plasmacytosis
- No fibrosis of lamina propria
- No hemosiderin deposition
Board review style question #1
A 62 year old woman, status post coronary artery bypass graft, complains of abdominal pain and bloody stools. A CT scan is performed, which shows significant thickening of the colon wall. Biopsy is performed, which shows changes consistent with ischemic colitis. What is the most common site of ischemic colitis?
- Ascending colon
- Cecum
- Hepatic flexure
- Rectum
- Splenic flexure
Board review style answer #1
Board review style question #2
A 73 year old woman presents with severe abdominal pain and a recent history of passing bloody stools. The pain began about 3 days ago and is more concentrated at the left side of the abdomen. Physical examination shows decreased to absent bowel sounds, abdominal distention and diffuse tenderness to palpation over her abdomen. An Xray is performed, which shows thumbprinting of the colon. What is the most likely histologic change seen in biopsy of the colon in patients with ischemic colitis?
- Crypt abscess with granulomas
- Increased lamina propria eosinophils
- Necrotizing vasculitis, fibrin thrombi and lamina propria hyalinization
- Pseudomembrane formation
Board review style answer #2
C. Necrotizing vasculitis, fibrin thrombi and lamina propria hyalinization
Comment Here
Reference: Ischemic colitis
Comment Here
Reference: Ischemic colitis
Juvenile (retention) polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Most common type of pediatric intestinal polyp, with prominent, cystically dilated glands and inflammatory stroma (> 90% of childhood colon polyps) (Clin Colon Rectal Surg 2008;21:280)
- Juvenile refers to the type of polyp (hamartomatous), not the age of presentation
- Majority are solitary (Gastroenterol Hepatol (N Y) 2010;6:185); solitary juvenile polyps have minimal malignant potential (Clin Pediatr (Phila) 2011;50:910); multiple juvenile polyps may indicate a premalignant condition (juvenile polyposis coli or juvenile polyposis syndrome [JPS]) (Clin Gastroenterol Hepatol 2010;8:795)
Essential features
- Most common type of pediatric intestinal polyp, with prominent, cystically dilated glands and inflammatory stroma
- Solitary juvenile polyps have minimal malignant potential; multiple juvenile polyps may indicate a premalignant condition (juvenile polyposis coli or juvenile polyposis syndrome [JPS])
- Juvenile polyposis syndrome is autosomal dominant and caused by a germline defect in SMAD4 or BMPR1A
Terminology
- Retention polyps and juvenile hamartomatous polyps (Can J Gastroenterol 2007;21:233)
- Juvenile polyposis syndrome (WHO 5th edition):
- > 3 - 5 juvenile polyps in the colorectum or
- Juvenile polyps throughout the gastrointestinal tract or
- Any number of juvenile polyps with a positive family history of juvenile polyposis (World J Gastroenterol 2011;17:4839)
- Other syndromes involving hamartomatous gastrointestinal polyps should be ruled out clinically or by pathological examination
Epidemiology
- Age: 3 to 10 years; sporadic polyps are uncommon before 2 years of age and rare in the first year of life (Clin Colon Rectal Surg 2008;21:280)
- M:F = 1.4:1 (Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1995;36:197)
Sites
- Majority in distal colon / rectosigmoid
- Less frequently seen in stomach and small bowel
Pathophysiology
- Earliest morphologic lesions are focal areas of mucosal hyperplasia, which seem to evolve into minute hyperplastic polyps
- These become inflamed, frequently with ulceration and scarring of their surface, sealing over some of the crypts, with subsequent cystic dilatation of the crypts, accumulation of mucus within the cysts, enlargement of the structure to form a typical juvenile polyp and further inflammation and scarring
- In some of the larger polyps, focal epithelial atypia develops
- Second hypothesis: landscape defect, in which an abnormal stromal environment leads to neoplastic transformation of adjacent epithelium (World J Gastroenterol 2011;17:4839)
- Juvenile polyposis syndrome is caused by a germline defect in SMAD4 or BMPR1A (Am J Surg Pathol 2011;35:530)
Etiology
- Juvenile polyposis syndrome is autosomal dominant
Clinical features
- Most frequent presentation: painless hematochezia
- Others: hematochezia with pain, abdominal pain, chronic iron deficiency anemia, intussusceptions and prolapsing rectal mass (J Pediatr Gastroenterol Nutr 2019;69:668, J Pediatr Gastroenterol Nutr 2020;71:491, Case Rep Surg 2014;2014:856765, Cureus 2020;12:e11222)
- Sessile or pedunculated polyp on endoscopy
Diagnosis
- Colonoscopy is the modality of choice (J Pediatr Gastroenterol Nutr 2019;69:668)
- Capsule endoscopy (Clin Gastroenterol Hepatol 2020;18:e73)
- Genetic testing and screening colonoscopies if suspicion of juvenile polyposis syndrome (J Pediatr Gastroenterol Nutr 2020;71:491)
Radiology description
- Filling defect on barium enema
- Rounded hypoechoic nodule within the lumen of gastrointestinal tract with a peripheral hyperechoic layer (Ital J Pediatr 2020;46:66)
Prognostic factors
- Extremely low risk of malignant transformation unless syndromic (J Pediatr Gastroenterol Nutr 2019;69:668)
- Recurrence is common in juvenile polyps; occurs with both multiple and solitary polyps (Clin Gastroenterol Hepatol 2010;8:795)
Case reports
- 3 year old girl with juvenile polyp in small intestine presenting with chronic iron deficiency anemia (J Pediatr Gastroenterol Nutr 2020;71:491)
- 10 year old boy with rectal prolapse and lower abdominal pain (Cureus 2020;12:e11222)
- 14 year old boy with osseous metaplasia in a juvenile polyp presenting with bleeding per rectum (J Clin Diagn Res 2013;7:2004)
- 37 year old man with a solitary colorectal juvenile polyp identified during followup of ulcerative colitis (World J Gastroenterol 2020;26:877)
- 46 year old man presented with a 6 week history of loose stools and hematochezia (BMJ Case Rep 2018;2018:bcr2018224770)
Treatment
- Many of these polyps outgrow their blood supply, become ischemic and autoamputate
- For solitary juvenile polyp:
- Colonoscopy / sigmoidoscopy and polypectomy
- No further evaluation or followup is needed after polypectomy
- Adenomatous changes in a juvenile polyp: followup and screening endoscopy is recommended (Clin Colon Rectal Surg 2008;21:280)
- For juvenile polyposis syndrome:
- High suspicion of JPS: endoscopic screening of the colon and upper gastrointestinal tract at age 15 or at the time of first symptoms
- Followup: recommended every 2 to 3 years in patients with juvenile polyposis syndrome
- Surgery: considered in patients with colorectal polyposis unmanageable by endoscopy (> 50 - 100 polyps), those with severe gastrointestinal bleeding or diarrhea, juvenile polyps with dysplasia and patients with a strong family history of colorectal cancer (World J Gastroenterol 2011;17:4839)
Gross description
- Sessile or pedunculated hamartomatous polyps
- 5 mm to 50 mm, typically spherical, lobulated and pedunculated with surface erosion
- Reference: World J Gastroenterol 2011;17:4839
Gross images
Microscopic (histologic) description
- Characterized by an abundance of edematous lamina propria with inflammatory cells and cystically dilated glands lined by cuboidal to columnar epithelium with reactive changes (nonneoplastic, hamartomatous, epithelial retentions)
- Dilated glands filled with mucus and inspissated inflammatory debris
- Juvenile polyposis syndrome:
- Polyps appear similar to sporadic solitary juvenile polyps, although syndromic polyps often have a frond-like growth pattern with less stroma, fewer dilated glands and more proliferative smaller glands
- JPS polyps frequently show neoplastic changes to the epithelium not common in sporadic solitary juvenile polyps
- Reference: World J Gastroenterol 2011;17:4839
Microscopic (histologic) images
Virtual slides
Positive stains
- TP53 mutations (p53 mutated pattern staining) can be seen with high grade dysplasia in polyps of juvenile polyposis syndrome
Negative stains
- p53 shows wild type pattern staining in benign solitary juvenile polyp, as there is no dysplastic change
Molecular / cytogenetics description
- Germline mutation in the SMAD4 (18q21.1) or BMPR1A (10q23.2) gene is found in 40 - 60% of patients with juvenile polyposis syndrome
- JPS patients with SMAD4 mutations are associated with more aggressive gastric polyps and appear to have a higher risk for gastric cancer (J Pediatr Gastroenterol Nutr 2019;68:453)
Videos
Histopathology of juvenile polyps
Endoscopy of juvenile polyps
Sample pathology report
- Colon, polypectomy:
- Solitary juvenile polyp (see comment)
- Comment: Negative for dysplasia or malignancy
Differential diagnosis
- Cronkhite-Canada syndrome:
- Changes in the surrounding mucosa, clinical findings (nail atrophy, skin pigment, alopecia)
- Hyperplastic polyp:
- Sawtoothed appearance of glands, cystic dilatation not prominent
- Inflammatory polyp:
- Common in inflammatory bowel disease and diverticulitis; irregularly dilated glands, no prominent cystic dilationn
- Peutz-Jeghers polyp:
- Prominent arborizing smooth muscle bundles
- Tubular adenoma:
- Cytologic dysplasia, cystic dilation usually not prominent, inflamed stroma usually not prominent
Additional references
Board review style question #1
Board review style answer #1
B. Hamartomatous polyp. The picture shows a juvenile polyp with cystically dilated glands in an edematous and inflammatory stroma with ulceration. Juvenile polyp is a hamartomatous polyp.
Comment Here
Reference: Juvenile (retention) polyp
Comment Here
Reference: Juvenile (retention) polyp
Board review style question #2
Which of the following is true regarding juvenile polyp?
- Most common clinical presentation is intussusception
- Mostly seen in proximal stomach
- Solitary juvenile polyp has minimal risk of malignant transformation
- Uncommon type of pediatric polyp
Board review style answer #2
C. Solitary juvenile polyp has minimal risk of malignant transformation. Juvenile polyp is the most common type of pediatric intestinal polyp, mostly seen in distal colon. Painless rectal bleed is the most common clinical presentation. Solitary juvenile polyps have minimal malignant potential; however, multiple juvenile polyps may indicate a premalignant condition (juvenile polyposis coli or juvenile polyposis syndrome [JPS]).
Comment Here
Reference: Juvenile (retention) polyp
Comment Here
Reference: Juvenile (retention) polyp
Juvenile polyposis syndrome
Table of Contents
Definition / general | Essential features | Sites | Pathophysiology | Clinical features | Case reports | Treatment | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Autosomal dominant syndrome leading to growth of numerous juvenile polyps, predominantly in the colon
Essential features
- Syndrome that encompasses 3 particular phenotypic groups, including juvenile polyposis of infancy, juvenile polyposis coli (colonic involvement only) and generalized juvenile polyposis
- Diagnostic criteria:
- 3 - 10 juvenile polyps, depending on criterion used
- Juvenile polyps throughout entire GI tract
- Any number of polyps in a patient with a family history of juvenile polyposis (Histopathology 1988;13:619)
- Associated risk of upper or lower GI malignancies is approximately 55% (Ann Surg Oncol 1998;5:751)
- Juvenile polyposis of infancy is a rare form that usually manifests before age 2, is not hereditable and is associated with a poor prognosis
Sites
- Polyps found throughout the GI tract, with the exception of the juvenile polyposis coli form, which is restricted to the colon
Pathophysiology
- SMAD4 mutations account for up to 20% of familial cases, while BMPR1A mutations are found in approximately 25% of cases (Am J Med Genet 2004;129C:44)
- Syndrome has incomplete penetrance and a large proportion of affected individuals have no family history (OMIM: Juvenile Polyposis Syndrome [Accessed 4 March 2021])
Clinical features
- If symptomatic, children typically present with painless rectal bleeding or prolapsing of polyps through rectum
Case reports
- 51 year old man with juvenile polyposis syndrome affecting the stomach (J Med Case Rep 2008;2:314)
Treatment
- Annual upper and lower endoscopic examinations with polypectomies
- Surgery reserved for diffuse polyposis not amenable to polypectomy or for associated malignancies
- Screening of family members recommended
Microscopic (histologic) description
- Numerous cystic and dilated crypts or glands with inspissated mucin and occasional intraluminal neutrophils
- Lamina propria typically edematous with associated lymphocytes, plasma cells and occasional eosinophils and neutrophils
- Filiform, multilobated forms with increased glandular to stroma ratio may be encountered and have been variably termed nonclassic or atypical polyps
- May contain areas of conventional dysplasia (Arch Pathol Lab Med 1996;120:1032)
Microscopic (histologic) images
Videos
Colonoscopy of juvenile polyposis
Sample pathology report
- Colon, total colectomy:
- Colon with numerous juvenile polyps, some with focal low grade dysplasia (see comment)
- Negative for high grade dysplasia or malignancy.
- Eight benign lymph nodes.
- Comment: The findings are consistent with the patient’s reported history of juvenile polyposis. Numerous grossly identifiable polyps were submitted for microscopy, including the largest.
Differential diagnosis
- Similar polyps:
- Can also occur sporadically in nonsyndromic children and adults
- Multiple juvenile type polyps:
- Have also been associated with Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome (PTEN mutations), as well as hereditary hemorrhagic telangiectasia (SMAD4 / DPC4, ENG or ACVRL1 mutations)
- Polyps of Cronkhite-Canada syndrome:
- Histologically similar but intervening mucosa is also involved
Board review style question #1
Which of the following is a criterion for diagnosis of juvenile polyposis syndrome?
- 1 juvenile polyp measuring > 5 cm
- At least 5 juvenile polyps in a patient with a family history of juvenile polyposis
- Juvenile polyps throughout entire GI tract
- More than 25 juvenile polyps
Board review style answer #1
Kaposi sarcoma
Table of Contents
Definition / general | Essential features | Epidemiology | Etiology | Clinical features | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Vascular malignancy caused by human herpes virus 8 (see topic in Soft tissue chapter)
Essential features
- Vascular malignancy most often seen in AIDS patients with HHV8 infection
- Rare in gastrointestinal tract
Epidemiology
- Most common gastrointestinal malignancy attributable to AIDS (Am J Gastroenterol 1991;86:715)
- Currently rare; incidence decreased from use of HAART for AIDS treatment
Etiology
- HHV8 infection
Clinical features
- Usually no clinical sequelae (Gastroenterology 1985;89:102)
- Rarely causes diarrhea, protein losing enteropathy or abdominal pain
Prognostic factors
- Degree of immunosuppression influences prognosis (Scand J Gastroenterol 1991;26:1007)
Case reports
- 77 year old woman with extensive colonic disease that resolved after chemotherapy (J Cancer Res Ther 2012;8:112)
- 87 year old man with coexisting colon cancer (Acta Dermatovenerol Alp Panonica Adriat 2011;20:83)
Treatment
- Chemotherapy and radiation preferred over surgery
Gross description
- Reddish brown to purple macules or polyps, typically 0.5 - 1.5 cm
Microscopic (histologic) description
- Lamina propria based lesion composed of spindle cells with mild to moderate atypia, arranged in vague fascicles and separated by slit-like vessels
- Extravasated red blood cells common
- PAS positive, diastase resistant hyaline globules sometimes seen
Microscopic (histologic) images
Negative stains
Sample pathology report
- Rectum, mass, biopsy:
- Vascular proliferation most consistent with Kaposi sarcoma (see comment)
- Comment: An immunostain for HHV8 is positive in the lesional cells.
Differential diagnosis
- Angiosarcoma:
- Negative for HHV8; often epithelioid in gastrointestinal tract
- Gastrointestinal stromal tumor:
- Melanoma:
- Positive for S100
Board review style question #1
Board review style answer #1
Leiomyoma
Table of Contents
Definition / general | Essential features | Terminology | Sites | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Benign smooth muscle proliferation of colon
Essential features
- Benign incidental tumor usually arising in muscularis mucosae
Terminology
- Symplastic leiomyoma refers to benign tumor with bizarre nuclear atypia and possibly increased mitotic activity
Sites
- Most gastrointestinal tract leiomyomas arise in colon
- Majority arise in rectosigmoid (Mod Pathol 2001;14:950)
Clinical features
- Benign
- More common in men
- Median age is 62 years
Diagnosis
- Generally discovered incidentally during colonoscopy
Case reports
- 10 month old girl with leiomyoma of the rectum (Pediatr Surg Int 2003;19:104)
- 54 year old woman with giant retroperitoneal mass (Obstet Gynecol 2003;101:1132)
Treatment
- Total excision is adequate treatment
Clinical images
Gross description
- Firm, white, well delineated nodule or polyp, generally < 1 cm
Microscopic (histologic) description
- Well differentiated smooth muscle cells with red fibrillar cytoplasm, arranged in fascicles and arising from muscularis mucosae (Arch Pathol Lab Med 2011;135:1311)
- Usually no mitotic activity or necrosis
- Symplastic leiomyomas demonstrate significant atypia and possibly increased mitotic activity
Microscopic (histologic) images
Positive stains
Sample pathology report
- Sigmoid colon, polypectomy:
- Leiomyoma
Differential diagnosis
- Gastrointestinal stromal tumor:
- Positive for CD117, arises in muscularis propria (Mod Pathol 2015;28:S47)
- Leiomyosarcoma:
- Larger, more aggressive / destructive, abundant mitoses, arises in muscularis propria
Additional references
Board review style question #1
What is the most common location of origin for leiomyomas of the colon?
- Muscularis mucosae
- Muscularis propria
- Submucosal blood vessels
- Subserosal blood vessels
Board review style answer #1
Leiomyosarcoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Etiology | Clinical features | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Malignant smooth muscle tumor of colon
Essential features
- Rare, with poor prognosis
- Arises in muscularis propria (Mod Pathol 2015;28 Suppl 1:S47)
Terminology
- Most older reports of colonic leiomyosarcomas were probably GIST
Epidemiology
- Rare in colon; less common than GIST (Am J Surg Pathol 2000;24:1339)
Sites
- Small intestine and colon most common GI sites for leiomyosarcoma
Etiology
- May be a rare late effect of pelvic radiation (Hepatogastroenterology 2003;50:1933, Am Surg 1999;65:6)
Clinical features
- Adult patients often die of disease
- Tumors in infants may have more favorable prognosis (J Pediatr Surg 2004;39:1257)
Prognostic factors
- Tumor size > 5 cm suggests poor prognosis (Histopathology 2013;63:194)
Treatment
- Surgical resection, including of metastases (J Gastrointest Oncol 2015;6:E70)
Gross description
- Intraluminal, bulging / polypoid mass measuring up to 25 cm
Microscopic (histologic) description
- Resembles leiomyosarcoma at other sites: malignant smooth muscle cells with prominent nuclear atypia, coagulative necrosis and at least 1 mitosis per 10 high power fields
- Rare examples have osteoclast-like giant cells (Arch Pathol Lab Med 2004;128:440)
Microscopic (histologic) images
Positive stains
Molecular / cytogenetics description
- Negative for KIT mutations
Sample pathology report
- Transverse colon, resection:
- Leiomyosarcoma (4.7 cm) (see comment)
- Margins of resection unremarkable.
- Three benign lymph nodes.
- Comment: The tumor shows prominent nuclear atypia, numerous mitotic figures and regions of necrosis. Immunohistochemical stains for SMA and desmin are positive in the tumor. Leiomyosarcomas are rare in the colon and risk factors for progression in this location remain poorly defined.
Differential diagnosis
- Gastrointestinal stromal tumor:
- Metastatic leiomyosarcoma:
- May require clinical correlation (South Med J 1997;90:1238)
Board review style question #1
Board review style answer #1
Lifting agent granuloma
Table of Contents
Definition / general | Essential features | ICD coding | Sites | Pathophysiology | Diagnosis | Prognostic factors | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Submucosal granulomatous reactive process due to the use of lifting agents during colonoscopy
Essential features
- Reactive process in the submucosa arising from reaction to lifting agents injected during colonoscopy
- Appearance changes over time
- Often forms prominent, hard, hyalinized, homogenously eosinophilic amorphous ribbons and globules with foreign body giant cells
- Should be differentiated from mimickers such as amyloid and pulse granuloma
ICD coding
- ICD-10: M60.28 - foreign body granuloma of soft tissue, not elsewhere classified, other site
Sites
- Luminal gastrointestinal tract
Pathophysiology
- Lifting agents such as Eleview (Cosmo Technologies, 2015, also known as SIC-8000) and ORISE (Boston Scientific, 2018) are injected in the submucosa underneath a lesion to create a fluid cushion between muscularis mucosae and muscularis propria in order to visualize that lesion for endoscopic resection
- Immediately after injection, the material has an acellular mucin-like quality (basophilic, bubble-like, amorphous)
- Over time (~ 2 - 3 months) it becomes prominent, hard, hyalinized, homogenously eosinophilic, forming amorphous ribbons and globules with foreign body giant cells, scattered eosinophils and fibrosis
- Median time interval for an Eleview or ORISE lifting agent granuloma to form is 16 weeks between injection and resection; however, the material can be seen as long as 31 weeks post injection (Am J Surg Pathol 2020;44:793)
Diagnosis
- Histology
Prognostic factors
- Outcomes of lifting agent granulomas are unknown as long term case studies are unavailable
- Portion of the gel not resected during polypectomy likely remains in the submucosa
- Hyalinized ribbons represent degrading agent or tissue reaction to the foreign agent (Am J Surg Pathol 2020;44:793)
Case reports
- 54 year old man had a partial colonic resection for an endoscopically unresectable tubular adenoma of the right colon (Case of the Month #507)
- 55 year old woman with a 35 mm tubular adenoma with high grade dysplasia in the rectum (Am J Clin Pathol 2020;153:630)
- 64 year old man with biopsy proven 15 mm, moderately differentiated adenocarcinoma in the gastric cardia (Am J Clin Pathol 2020;153:630)
- 73 year old woman with a 20 mm mucinous adenocarcinoma in the sigmoid (Am J Clin Pathol 2020;153:630)
- 76 year old man with a 70 mm tubulovillous adenoma, without high grade dysplasia, located in the ascending colon (Am J Clin Pathol 2020;153:630)
Gross description
- Often forms a well circumscribed submucosal nodule
Microscopic (histologic) description
- Morphology of the lifting agents changes with time (Am J Surg Pathol 2020;44:793, Am J Clin Pathol 2020;153:630, Mod Pathol 2020;33:1581):
- Immediately after injection: lifting agents demonstrate acellular mucin-like quality with basophilic, amorphous and bubbly extracellular material
- 1 day after injection: infiltrating neutrophils and a more solid and less bubbly texture
- 2 - 3 months after injection: prominent hard, hyalinized, homogenously eosinophilic, amorphous ribbons and globules with foreign body giant cells, scattered eosinophils and fibrosis
- ORISE and Eleview are neither refractile nor polarizable (Am J Surg Pathol 2020;44:793)
Microscopic (histologic) images
Contributed by Maryam Kherad Pezhouh, M.D., M.Sc.
Contributed by Catherine E. Hagen, M.D. (Case #507)
Positive stains
- Mucicarmine is positive in fresh state and negative after ~3 months (Mod Pathol 2020;33:1581, Am J Surg Pathol 2020;44:793)
- Studies have reported variable (positive, faint, negative) staining for PAS and PASD (Mod Pathol 2020;33:1581, Am J Surg Pathol 2020;44:793, Am J Clin Pathol 2020;153:630)
Negative stains
- Generally the lifting agent material is negative for Congo red and Alcian blue and is not evident on trichrome stain (Am J Surg Pathol 2020;44:793, Am J Clin Pathol 2020;153:630)
Sample pathology report
- Colon, left, polyp, polypectomy:
- Tubulovillous adenoma (see comment)
- Comment: The submucosa adjacent to tubular adenoma contains prominent hyalinized, homogenously eosinophilic, amorphous ribbons and globules with foreign body giant cells, scattered eosinophils and fibrosis consistent with lifting agent granuloma. Congo red stain is negative for amyloid. (Am J Surg Pathol 2020;44:793)
Differential diagnosis
- Amyloid:
- Prominent intramucosal and perivascular distribution
- Positive for Congo red (negative in lifting agent granuloma or pulse granuloma)
- Pulse granuloma:
- Benign entity occurring within the gastrointestinal tract
- Foreign body reaction to legume material embedded in the bowel wall or peritoneum due to ulceration or perforation (Arch Pathol Lab Med 2001;125:822)
- Round in shape (well circumscribed), contains ribbons of glassy material and calcifications
- In contrast, lifting agent granulomas do not contain entrapped calcifications but show irregular, infiltrative pattern (border) that occurs secondary to forceful injection of gel into tissue (Mod Pathol 2020;33:1581)
Additional references
Board review style question #1
Which stain is most helpful to differentiate between lifting agent granuloma and amyloidosis?
- Congo red because amyloid stains positive with Congo red
- Congo red because lifting agent stains positive with Congo red
- Congo red because lifting agent granulomas are birefringent as they have two different refractile indices
- Thioflavin T stain because lifting agent granulomas are nonpolarizable
Board review style answer #1
A. Congo red because amyloid stains positive with Congo red
Comment Here
Reference: Lifting agent granuloma
Comment Here
Reference: Lifting agent granuloma
Board review style question #2
Histologically, pulse granuloma can be differentiated from lifting agent granuloma as they
- Are round in shape (well circumscribed), contain ribbons of glassy material and calcifications and are more limited in size
- Demonstrate acellular mucin-like quality with basophilic, amorphous and bubbly extracellular material with prominent hemorrhage
- Demonstrate prominent intramucosal and perivascular distributions
- Do not contain entrapped calcifications but show irregular, infiltrative pattern (border)
Board review style answer #2
A. Are round in shape (well circumscribed), contain ribbons of glassy material and calcifications and are more limited in size
Comment Here
Reference: Lifting agent granuloma
Comment Here
Reference: Lifting agent granuloma
Board review style question #3
Which of the following features is characteristic of submucosal lifting agents (Eleview or ORISE)?
- Association with ruptured diverticula
- Involvement of vascular walls
- Positive for Congo red stain
- Surrounding giant cell reaction
Board review style answer #3
D. Surrounding giant cell reaction. Submucosal lifting agents frequently show a surrounding giant cell reaction. Amyloid will stain positive for Congo red and frequently is seen within vessels walls. Pulse granulomas are frequently seen in association with ruptured diverticula.
Comment Here
Reference: Lifting agent granuloma
Comment Here
Reference: Lifting agent granuloma
Lipoma
Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Clinical features | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Benign colonic neoplasm composed of mature adipose tissue
Essential features
- Most common submucosal mesenchymal lesion of colon
- Typically small and benign but can cause symptoms if large
Epidemiology
- Incidence may be as high as 4.4% but is more likely less than 1% (Gastroenterol Hepatol (N Y) 2011;7:490)
Sites
- Usually submucosal in right colon
Clinical features
- More common in elderly patients
- Larger lesions can cause symptoms, such as abdominal pain and intussusception (Am Surg 2006;72:83)
- Typically solitary but rare patients may have multiple or diffuse lesions (Histopathology 2001;38:81)
- Patients may have concurrent malignancy (J Surg Oncol 1991;47:170)
Case reports
- 83 year old woman with ascending colon lipoma (World J Gastroenterol 2005;11:3167)
Treatment
- Removal by colonoscopy or surgery (Eur J Surg 1991;157:51)
Gross description
- Soft, yellow cut surface
- Size can vary; large lesions may have overlying ulceration
Gross images
Microscopic (histologic) description
- Benign, well circumscribed (but not encapsulated) adipose tissue, usually in the submucosa
- Some cases may show prominent fibrosis or vasculature (Virchows Arch 2019;474:309)
- Overlying mucosa usually normal but may show an adenoma or a serrated polyp (World J Clin Cases 2013;1:124)
- May have overlying mucosal ulceration, atypical stromal cells and florid vascular proliferation due to repeated intussusception (Pathol Int 2005;55:160)
- Intramucosal lipomas may occur sporadically or in Cowden syndrome (Mod Pathol 2018;31:643)
Microscopic (histologic) images
Sample pathology report
- Ascending colon, polypectomy:
- Colonic mucosa with focus of benign submucosal fat, compatible with lipoma
- Descending colon, polypectomy:
- Intramucosal lipoma (see comment)
- Comment: Intramucosal lipomas have been associated with Cowden syndrome in some patients. Additional clinical evaluation may be warranted.
Differential diagnosis
- Lipomatous hypertrophy of ileocecal valve:
- Also benign; not well circumscribed
- Pseudolipomatosis:
- Artifact caused by air insufflation or disinfectant (Gastrointest Endosc 1989;35:93)
- Should not form a mass
- Numerous small rounded empty spaces without nuclei; S100 negative (Ulus Cerrahi Derg 2015;32:90)
Board review style question #1
Benign fatty lesions of the colon usually arise in what layer of the wall?
- Lamina propria
- Muscularis mucosae
- Muscularis propria
- Submucosa
- Subserosa
Board review style answer #1
Low grade tubuloglandular adenocarcinoma
Table of Contents
Definition / general | Essential features | Epidemiology | Clinical features | Prognostic factors | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Board review style question #1 | Board review style answer #1Definition / general
- Uncommon type of colorectal adenocarcinoma seen in patients with inflammatory bowel disease (Am J Surg Pathol 2006;30:1022)
Essential features
- Arises in patients with ulcerative colitis or Crohn's disease
- Low grade histology may be difficult to distinguish from benign glands
- Can arise directly from low grade dysplasia
Epidemiology
- ~10% of inflammatory bowel disease associated colon carcinomas are low grade tubuloglandular subtype
Clinical features
- Median age 41.5 years
- More common in ulcerative colitis than Crohn's disease
- Patients may have synchronous conventional colorectal adenocarcinoma (see IBD associated carcinoma)
Prognostic factors
- Patients generally do well unless a synchronous conventional colorectal carcinoma is also present
Gross description
- May not be identifiable grossly; may be only identified on random sections
Microscopic (histologic) description
- Small or medium sized glands with round or tubular shape
- Malignant glands are extremely well differentiated and might only be identifiable as malignant based on location deep within the bowel wall
- Desmoplasia is rare or absent
- Overlying mucosa may show only low grade dysplasia
- Areas of conventional adenocarcinoma may be visible
Microscopic (histologic) images
Negative stains
- MUC6 (0%)
Molecular / cytogenetics description
- Often harbors IDH1 mutation (Am J Surg Pathol 2014;38:1147)
- 55% of cases lack MLH1 expression by immunohistochemistry
Sample pathology report
- Ascending colon, resection:
- Low grade tubuloglandular adenocarcinoma (see synoptic report)
- Background severely active chronic colitis, consistent with patient’s reported history of ulcerative colitis.
Board review style question #1
Board review style answer #1
Lymphocytic colitis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Chronic nonulcerating colitis; subtype of microscopic colitis
- Common cause of chronic nonbloody diarrhea in older adults with normal or near normal colonoscopy and increased intraepithelial lymphocytes as the histologic hallmark
Essential features
- Cause of chronic watery diarrhea, often in older females
- Normal, edematous or mildly erythematous mucosa on endoscopy
- Colonic intraepithelial lymphocytosis (> 20 per 100 enterocytes) with diffuse increase in lamina propria inflammatory cells
ICD coding
- ICD-10: K52.832 - lymphocytic colitis
Epidemiology
- Overall incidence 4.85 per 100,000 person years (Am J Gastroenterol 2015;110:265)
- Older adults (50 - 70 years) but wide age range
- F > M
- F:M = 1.62 (Am J Gastroenterol 2015;110:265)
- No ethnic predilection
- 9 - 16% of patients undergoing colonoscopy for watery diarrhea (Eur J Gastroenterol Hepatol 2015;27:963)
Sites
- Worse in right than left colon (Neth J Med 2005;63:137)
- Rectal sparing in 8%
Pathophysiology
- Multifactorial (Inflamm Bowel Dis 2016;22:450)
- Impaired epithelial barrier
- Reaction to luminal antigens
- Genetic predisposition
- Smoking (Eur J Gastroenterol Hepatol 2017;29:587)
- Hormonal influence
- Abnormalities in fluid homeostasis
- Autoimmunity
- HLA-DQ2 and DQ1.3, HLD-DR3DQ2 haplotypes
- Bile acid malabsorption (Hepatogastroenterology 2002;49:432)
- Infection, viral or bacterial
- Medication induced (Am J Gastroenterol 2017;112:78)
- Acarbose, aspirin, beta blockers, carbamazepine, H2 receptor antagonists, NSAIDs, proton pump inhibitor, selective serotonin reuptake inhibitors (SSRI), statins, ticlopidine
Etiology
- Not clearly determined yet
Clinical features
- Classic symptom is chronic nonbloody watery diarrhea
- Other symptoms include urgency, fecal incontinence, abdominal pain, weight loss
- Some asymptomatic
- Associated autoimmune disorders (Gut 2004;53:536)
- Thyroiditis
- Celiac disease
- Diabetes mellitus
- Psoriasis
- Rheumatoid arthritis
Diagnosis
- Normal colonoscopy or mild nonspecific erythema or edema
- Biopsies from all segments of the colon, proximal to rectosigmoid
- Proximal to rectosigmoid
- Rectal biopsy alone cannot rule it out
- Proximal to rectosigmoid
Laboratory
- Mild anemia, elevated erythrocyte sedimentation
- Autoantibodies (ANA, ANCA, antithyroid peroxidase antibodies)
- Fecal leukocytes may be present
- Elevated fecal calprotectin
- Fecal eosinophil derived proteins
- Negative stool cultures, ova and parasites
- Negative lactose malabsorption test (Am J Gastroenterol 2017;112:78)
Radiology description
- Normal barium enema
Prognostic factors
- Benign clinical course, chronic intermittent or continuous (Clin Transl Gastroenterol 2019;10:e00071)
- Reduced risk of colorectal neoplasia including decreased prevalence of polyps (Dig Dis Sci 2012;57:161, Am J Gastroenterol 2015;110:1056)
Case reports
- 54 year old man with nasopharyngeal squamous cell carcinoma on pembrolizumab (Am J Gastroenterol 2018;113:629)
- 59 year old man with intractable diarrhea, weight loss and hypertension (Am J Case Rep 2019;20:111)
- 68 year old man with lymphocytic colitis diagnosis 40 years after ulcerative colitis (ACG Case Rep J 2018;5:e82)
Treatment
- Decrease risk factors:
- Smoking cessation
- Discontinuation of drugs
- Mild cases:
- Loperamide, cholestyramine, mesalazine, bismuth (Am J Gastroenterol 2017;112:78)
- Moderate to severe cases:
- Budesonide (Gastroenterology 2009;136:2092)
- Nonresponsive cases:
- Immunomodulators (azathioprine, 6-mercaptopurine), anti-TNF (J Crohns Colitis 2011;5:612)
- Surgical diversion (Dis Colon Rectum 2002;45:123)
Gross description
- Normal, edematous or mildly erythematous mucosa on endoscopy
Microscopic (histologic) description
- Increased intraepithelial lymphocytes (Hum Pathol 1989;20:18)
- > 20 IELs per 100 epithelial cells, away from lymphoid aggregates
- Increased lamina propria inflammatory cells
- Lymphocytes, plasma cells, eosinophils, occasional neutrophils
- Predominantly upper half of the mucosa
- Less prominent in left colon
- Preserved / intact crypt architecture (Hum Pathol 1989;20:18)
- Surface epithelial damage (Hum Pathol 1989;20:18)
- Flattening, mucin depletion, vacuolization, nuclear irregularities
- Unremarkable subepithelial collagen (< 10 μm)
- Rare acute cryptitis, crypt abscess (30 - 38% of cases) (Am J Surg Pathol 2002;26:1414)
- Focal and mild, not predominant
- Paneth cell metaplasia, rarely seen
- Less frequent findings:
- Subepithelial giant cells
- Ruptured crypt granulomas
Microscopic (histologic) images
Positive stains
- CD3
- Not needed routinely
- May be helpful in borderline cases (Hum Pathol 2016;48:25)
Negative stains
- Negative for thickening of subepithelial collagen band by:
- Trichrome
- van Gieson
- Tenascin (Virchows Arch 2001;438:435)
Videos
Microscopic colitis
Sample pathology report
- Colon, random biopsies:
- Colonic mucosa with increased intraepithelial lymphocytes, consistent with lymphocytic colitis
Differential diagnosis
- Collagenous colitis:
- Thickening and qualitative abnormalities of subepithelial collagen band with mild increase in intraepithelial lymphocytes
- Inflammatory bowel disease (IBD):
- Architectural distortion
- Basal lymphoplasmacytosis
- Cryptitis, crypt abscesses
- Paneth cell metaplasia
- Acute infectious colitis:
- Edema
- Luminal, lamina propria, surface neutrophils and cryptitis
- No mononuclear inflammation or increased intraepithelial lymphocytes
- Checkpoint inhibitor induced colitis (Cancer 2019;125:1768):
- Scattered crypt apoptosis
- Basal lymphoplasmacytosis
- May otherwise mimic lymphocytic colitis; clinical history essential
- Autoimmune enteropathy:
- Loss of Paneth and goblet cells
- Basal crypt intraepithelial lymphocytes and apoptosis
- Crypt distortion and crypt abscesses
- No surface intraepithelial lymphocytosis
Additional references
Board review style question #1
Which of the following histologic findings best describes colonic mucosa in lymphocytic colitis?
- Crypt architectural distortion, basal lymphoplasmacytosis and crypt abscesses
- Depleted lamina propria, increased crypt apoptosis and crypt distortion
- Intraepithelial lymphocytes, surface epithelial damage, intact crypt architecture and lamina propria expansion
- Intraepithelial lymphocytes, thick subepithelial collagen band with entrapped inflammatory cells
- Lamina propria hyalinization, hemorrhage and withering crypts
Board review style answer #1
C. Intraepithelial lymphocytes, surface epithelial damage, intact crypt architecture and lamina propria expansion. Answer A best fits inflammatory bowel disease. Answer B is an apoptotic colopathy pattern of injury that can be seen in graft versus host disease and mycophenolate induced injury, for example. Answer D describes collagenous colitis and answer E corresponds to ischemic colitis.
Comment Here
Reference: Lymphocytic colitis
Comment Here
Reference: Lymphocytic colitis
Board review style question #2
A 53 year old woman presents with a history of intermittent diarrhea of unexplained origin. The colonoscopy shows mild erythema throughout the colon. Based on representative images of random colon biopsies, what is the most likely diagnosis?
- Idiopathic inflammatory bowel disease
- Infectious colitis
- Ischemic colitis
- Lymphocytic colitis
- Normal histology
Board review style answer #2
E. Normal histology. The left image shows normal architecture. The intraepithelial lymphocytes on the right image are overlying a lymphoid aggregate. Intraepithelial lymphocytes should be evaluated away from the mucosal lymphoid aggregates.
Comment Here
Reference: Lymphocytic colitis
Comment Here
Reference: Lymphocytic colitis
Lymphoid polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign lesion that may endoscopically resemble a mucosal polyp
- Predominantly asymptomatic and incidental
Essential features
- Endoscopically, these lesions may appear as small nodules (Case Rep Gastrointest Med 2018;2018:5758689)
- Frequently sampled as a polyp biopsy or polypectomy
- Consideration of a neoplastic hematolymphoid process is suggested when the lymphoid aggregate appears monotonous and without an obvious germinal center; very small nonneoplastic lymphoid follicles may not develop germinal centers
Terminology
- Also called nodular lymphoid hyperplasia, lymphoid aggregate, rectal tonsil
- The term pseudolymphoma is not recommended
- Various terminologies may be used to report the findings of lymphoid polyp on a pathology report
- May be reported as reactive lymphoid aggregate if there is a prominent germinal center with polymorphous population of cells and tingible body macrophages
- Other reporting terminology may be colonic mucosa with prominent lymphoid aggregate; otherwise with no significant histopathological changes
- The latter may be used when there is no other associated lesion in addition to the colonic lymphoid polyp
- Note: there are instances when certain lesions, such as hyperplastic polyp or sessile serrated lesions / polyp, may occur alongside lymphoid polyp (see Microscopic (histologic) images)
Epidemiology
- More frequent in younger population and in children
- Nodular lymphoid hyperplasia, normally between 2 and 10 mm, may occur as a normal physiology (World J Gastrointest Endosc 2014;6:534)
- Certain immunodeficiency disorders and infections are associated with prominent lymphoid aggregates and may appear polypoid on endoscopic examination
- Examples include common variable immunodeficiency (CVID), selective IgA deficiency, Giardia infection, human immunodeficiency virus infection (uncommon), celiac disease and Helicobacter pylori infection
Sites
- Widely distributed throughout the stomach, small intestine and large bowel
- Polypoid endoscopic findings are more frequent in the colon and particularly in the ileocecal region because of prominent Peyer patches
Pathophysiology
- Lymphoid follicles may occur as the normal component of intestinal or gut associated mucosal lymphoid tissue (Ann N Y Acad Sci 2010;1207:E86)
- Gut associated lymphoid tissue (GALT) contains a significant percentage of immune cells (up to 70%)
- Mucosa overlying the lymphoid follicles is specialized follicle associated epithelium that is rich in macrophages and specialized antigen presenting cells (Biomedicines 2022;10:226)
- Average diameter of lymphoid aggregates ranges from 0.1 to 0.7 mm
- Reactive hyperplasia of GALT due to various infections can occur and lead to the appearance of polypoid or granular mucosa
- Associated with adenovirus infection, immunodeficiency (low IgA and IgM)
- EBV associated with immunosuppression may cause progression of the benign lymphoid follicle to lymphoma (Am J Surg Pathol 2019;43:1253)
Etiology
- No specific etiology has been associated and such lesions are frequently seen
- Certain specific conditions have an association; before considering a strong association be reported on pathology diagnosis, it is recommended to have more clinical information
- ~20% of adults with CVID may have nodular lymphoid hyperplasia or have more frequent lymphoid polyps; diffuse nodular hyperplasia may occur more frequently in the proximal small bowel (Indian J Pathol Microbiol 2009;52:530)
Clinical features
- Usually localized but may show diffuse involvement of mucosa when the portion of colon is diverted from the fecal stream (see Colon-Diversion colitis)
- Rectal lymphoid polyps are usually asymptomatic but colonic lymphoid polyps may present with gastrointestinal bleeding
- Children: multiple lesions; larger lymphoid polyps may cause intestinal obstruction and intussusception
- Adults: usually an isolated lesion
- Subtypes: idiopathic (most common), reactive, hypogammaglobulinemia associated
- Awareness of associated conditions such as CVID or IgA deficiency can be helpful to correlate clinical findings with histology
- Such systemic associations are more common with lymphoid aggregates in the small bowel and with increased numbers of lymphoid aggregates at an unusual mucosal site
- Frequent physiological lymphoid aggregates normally occur as Peyer patches in the ileocecal region and should generally not raise concern
- Nodular lymphoid hyperplasia is associated with some other conditions, such as common variable immunodeficiency, selective IgA deficiency, Giardia infection, human immunodeficiency virus infection, celiac disease and Helicobacter pylori infection, etc. (Arch Pathol Lab Med 2013;137:83)
- Associated conditions are due to the antigenic stimulation of lymphoglandular complexes
- A study done on lymphoid aggregates suggests prediction of chronic diarrhea in a subset of patients
- References: Case Rep Surg 2019;2019:9017863, Int J Colorectal Dis 1991;6:165, Indian J Pathol Microbiol 2009;52:530, Endoscopy 2013;45:320, Toxicol Pathol 2006;34:599
Diagnosis
- Diagnosis is made on light microscopy on routine H&E stain
- Special stains are usually not required except when the lymphoid aggregate appears neoplastic on the H&E stain
Prognostic factors
- Overall favorable outcome with no follow up required for benign lymphoid aggregates
Case reports
- 47 year old man with a history of left colon cancer, left colon resection for 12 years and rectal bleeding (Case Rep Pathol 2015;2015:646270)
- 64 year old woman with change in bowel habit and anemia (Case Rep Gastrointest Med 2018;2018:5758689)
- 11 cases of GALT associated pseudoinvasion / epithelial misplacement (J Pathol Transl Med 2020;54:135)
Treatment
- Polypectomy
Gross description
- Small polypoid or raised lesions, which generally are sessile or may have broad stalk
- Surface erosions with acute inflammation over the lymphoid polyp / follicle clinically and endoscopically may appear as an aphthoid ulceration, which may indicate possibility of Crohn's colitis but is not diagnostic
Microscopic (histologic) description
- Intestinal mucosal associated lymphoid tissue (MALT) is distributed throughout the large intestinal tract as lymphoglandular complexes (World J Gastrointest Oncol 2019;11:59)
- They may be located within the lamina propria or extend to the deeper layers within muscularis mucosae or submucosa
- Antigenic stimulation of lymphoglandular complex leads to prominent lymphoid aggregates, thereby forming lymphoid polyp
- Benign lymphoid follicles covered by columnar (colonic mucosa) or transitional epithelium (anorectal mucosa)
- Surface epithelium is usually flat but may have reactive epithelial changes in the form of surface hyperplasia in the distal rectum
- Germinal centers may be seen in larger lymphoid polyps
- Reactive lymphoid follicles may also be present in the lamina propria (more commonly) or submucosa
- Intraepithelial lymphocytes are commonly present over a lymphoid polyp and should not be considered pathologic
- Intraepithelial neutrophils over a lymphoid follicle is a pathologic finding and should be reported in the final diagnosis as focal acute inflammation
- It is discouraged to use the term colitis if the acute inflammation is identified in focal areas; this is to prevent the patient from falsely being clinically labeled with chronic inflammatory bowel disease
- Presence or absence of dysplasia in the surface epithelium of the lymphoid polyp is not dependent on the nature of underlying lymphoid follicle
- Herniation of normal colonic epithelium or dysplastic epithelium (tubular adenoma) may occur and could pose a diagnostic challenge to evaluate for invasion
- This could be further challenging if the herniated epithelium has high grade dysplasia (Am J Surg Pathol 2018;42:1083)
Positive stains
- Admixture of CD20 positive B cells and CD3 positive T cells
- Kappa and lambda stains show polyclonal cells
- Ki67 proliferation index is low
- Reference: Toxicol Pathol 2006;34:599
Negative stains
- BCL2 is negative in germinal centers (only scattered T cells in germinal centers stain positive)
Sample pathology report
- Colon, polyp, polypectomy:
- Colonic mucosa with lymphoid aggregate; otherwise, with no significant histopathologic findings (see comment)
- Comment: The mucosal lymphoid tissue shows polymorphous population of lymphoid tissue. There is an admixture of CD20 positive B cells and CD3 positive T cells. CD45 stain shows no aberrant expression in the regions stained by CD20.
- Note: Immunohistochemical stains are not required for the majority of cases. Comment may be added for cases where there are immunohistochemical stains performed based on morphologic (H&E) concern. In routine cases without immunohistochemistry, comment is not required.
Differential diagnosis
- MALT lymphoma and follicular lymphoma:
- More uniform appearance of small to medium sized type B lymphocytes with histological overlap with MALT lymphoma
- Translocation t(14;18)(q32;q21) is a characteristic finding for follicular lymphoma
- Lymphoma immunohistochemical panel workup is essential for the subclassification of neoplastic hematolymphoid process
- Inverted lymphoglandular polyp (Case Rep Pathol 2015:2015:646270):
- In some instances, the dysplastic colonic epithelium may herniate or extend into the prominent mucosal or submucosal lymphoid aggregates
- Although the location of dysplastic epithelium may raise a concern for an invasive malignancy, the herniated tissue still retains the mucosal morphology with intact lamina propria and without any desmoplastic reaction
- Smooth muscle markers such as desmin immunohistochemistry may be misleading in cases where herniation of dysplastic epithelium extends into submucosal lymphoid aggregates
- On the contrary, invasive adenocarcinoma extending to the lymphoid aggregate will lack lamina propria and may have angulated pattern of arrangement of malignant glands
- Frequently, fibrous stroma or desmoplastic reaction will accompany invasive malignancy extending to the lymphoid aggregates
Board review style question #1
A 14 year old boy presented with small sessile polypoid areas in the terminal ileum. A wide mucosal biopsy was performed with concern for inflammatory bowel disease. There was no clinical history of failure to thrive or frequent infections. Which of the following is the most likely diagnosis?
- IgA deficiency
- MALT lymphoma
- Mucosa associated lymphoid tissue (Peyer patches)
- Small bowel follicular lymphoma
Board review style answer #1
C. Mucosa associated lymphoid tissue (Peyer patches). Mucosa associated lymphoid tissue is the most likely diagnosis because of the age of the patient and presence of reactive germinal centers and lymphoid follicles, which do not show a fused pattern. Answer A is incorrect because IgA deficiency disease will present with numerous lymphoid follicles in the upper gastrointestinal tract and higher propensity to infections. Answers B and D are incorrect because lymphomas will appear to have monotonous small to medium sized lymphocytes and generally lack lymphoid aggregate.
Comment Here
Reference: Lymphoid polyp
Comment Here
Reference: Lymphoid polyp
Board review style question #2
Board review style answer #2
C. Polymorphous population of lymphocytes in the germinal centers. Nonneoplastic germinal center will have admixture of cells of variable sizes. Answer B is incorrect because a neoplastic hematolymphoid process will have a monotonous appearance of small to medium sized lymphocytes with high Ki67 proliferation index. Answer A is incorrect because benign follicles frequently show germinal center and presence of tingible body macrophages. Answer D is incorrect because BCL2 should be negative in germinal centers of benign mucosal lymphoid aggregate.
Comment Here
Reference: Lymphoid polyp
Comment Here
Reference: Lymphoid polyp
Lynch syndrome
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology / etiology | Etiology | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Negative stains | Molecular / cytogenetics description | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Autosomal dominant hereditary disorder due to mutation in a mismatch repair (MMR) gene
Essential features
- Most common hereditary colorectal carcinoma syndrome (accounts for 2 - 5% of all colorectal carcinomas)
- 80% of patients develop colorectal carcinoma; also increased risk of endometrial carcinoma (33%), ovarian carcinoma (5%) and cancers of small bowel, stomach, upper urinary tract and brain (Fam Cancer 2005;4:245)
- Muir-Torre syndrome is a clinical variant
- See also MSI testing for Lynch syndrome, MLH1, MSH2
Terminology
- Initially known as hereditary nonpolyposis colon cancer (HNPCC) but this terminology has been criticized (World J Gastroenterol 2006;12:4943)
Epidemiology
- Patients with Lynch syndrome tend to develop carcinomas at an earlier age than the general population (average age: 44 years old)
Sites
- Colon cancers more likely to occur in the proximal colon
Pathophysiology / etiology
- Mutation in MMR gene results in defective repair of DNA sequence mismatches, which most frequently occur in long, repetitive DNA sequences (such as seen in microsatellite regions, hence the term microsatellite instability [MSI])
- Accumulation of DNA mismatches lead to increased risk of developing malignant neoplasms
Etiology
- Typically due to mutation in an MMR gene: most commonly due to a germline mutation in MSH2 or MLH1; less frequently due to germline mutations in MSH6 or PMS2
- Mutations in EPCAM (epithelial cellular adhesion molecule) / TACSTD1 (tumor associated calcium signal transducer 1) gene may result in Lynch syndrome: EPCAM gene is adjacent to MSH2 and mutations in EPCAM may result in MSH2 promoter hypermethylation and subsequent inactivation of MSH2
- Rarely due to inactivation through germline promoter hypermethylation of MLH1; differs from the somatic MLH1 hypermethylation, which may be seen in sporadic colon cancers
- Rarely the result of inactivation of CHEK2 (cell cycle checkpoint kinase 2)
Diagnosis
- Amsterdam I screening criteria (all 4 must be met):
- 3 or more family members with a confirmed diagnosis of colorectal cancer, one of whom is a first degree (parent, child, sibling) relative of the other two
- 2 successive affected generations
- 1 or more colon cancers diagnosed before age 50
- Familial adenomatous polyposis has been excluded (Dis Colon Rectum 1991;34:424)
- These criteria are relatively sensitive but not specific for Lynch syndrome, since these patients may lack MMR gene mutations
- Amsterdam II criteria (all 4 must be met):
- 3 or more family members with HNPCC related cancers, one of whom is a first degree relative of the other two
- 2 successive affected generations
- 1 or more HNPCC related cancers diagnosed before age 50
- Familial adenomatous polyposis has been excluded (Gastroenterology 1999;116:1453)
- Revised Bethesda criteria (any criteria must be met; these are guidelines for when tumors should be tested for MSI):
- Colorectal cancer diagnosed before age 50 years
- Presence of synchronous or metachronous colorectal cancers or other HNPCC associated tumors, regardless of age
- Colorectal cancer with MSI-H histology (see below) diagnosed before age 60 years
- Colorectal cancer diagnosed in one or more first degree relatives with an HNPCC related tumor, with one of the cancers being diagnosed before age 50 years
- Colorectal cancer diagnosed in 2 or more first or second degree relatives with HNPCC related tumors, regardless of age (J Natl Cancer Inst 2004;96:261)
- Recommended screening for patients with Lynch syndrome:
- Full colonoscopy every 1 - 2 years beginning at age 20 - 25 years
- Annual screening for endometrial cancer beginning at age 25 - 35
- Annual urinalysis and cytologic examination beginning at age 25
- Annual skin surveillance and upper GI endoscopy beginning at age 35 when gastric cancer is part of the family spectrum (JAMA 2006;296:1507)
- At colonscopic screening, patients have similar rate of adenomas as nonsyndrome patients but much higher incidence of carcinoma (Gastroenterology 2006;130:1995)
Laboratory
- Germline testing for mutations can be performed
- Microsatellite Instability testing of tumor specimens via PCR is widely utilized; this typically consists of a panel of 5 mono / dinucleotide repeats which are analyzed, and a shift in PCR product size of tumor versus normal indicates instability; designation of MSI-H requires instability in at least 30% of examined loci
- Immunohistochemical testing panel for 4 MMR proteins (MLH1, MSH2, PMS2, MSH6) is widely utilized
- BRAF V600E mutation analysis may be performed on cases with loss of MLH1 and PMS2 IHC staining: if mutation is present, then Lynch syndrome is virtually excluded
- MLH1 gene promoter hypermethylation may be utilized to determine sporadic versus Lynch syndrome related colon cancers
Prognostic factors
- Syndromic cancers have better survival than nonsyndromic
Case reports
- 53 year old man with duodenal follicular lymphoma and hereditary nonpolyposis colorectal cancer (Mod Pathol 2000;13:586)
- 64 year old woman with hereditary nonpolyposis colorectal cancer syndrome (Arch Pathol Lab Med 2003;127:E60)
- History and molecular genetics of Lynch syndrome in family (JAMA 2005;294:2195)
Treatment
- 5-FU chemotherapy does not appear to be helpful (Isr Med Assoc J 2005;7:520, Eur J Cancer 2009;45:1890)
- Risk of endometrial carcinoma and ovarian carcinoma is reduced by prophylactic surgery (N Engl J Med 2006;354:261)
- Unresectable or metastatic MMR deficient carcinomas can be treated with PDL1 inhibitors, regardless of PDL1 status by immunohistochemistry (Clin Cancer Res 2019;25:3753)
Gross description
- Usually proximal colon; 18% multiple and 40% metachronous (multiple separate occurrences)
Microscopic (histologic) description
- Tumoral features suggestive of MSI-H etiology include: tumor infiltrating lymphocytes and peritumoral lymphocytes, Crohn's-like lymphoid reaction, mucinous features, medullary features, tumoral heterogeneity and absence of dirty necrosis
Negative stains
- Defective mismatch repair genes can be reliably detected (negative staining) by immunohistochemistry (Am J Clin Pathol 2004;122:389, N Engl J Med 2005;352:1851)
- Loss of only PMS2 staining is suggestive of PMS2 mutation
- Loss of only MSH6 staining is suggestive of MSH6 mutation
- Loss of MLH1 and PMS2 staining may be seen in MLH1 mutation (although sporadic colon cancers may also have this profile; if this profile is observed, follow up with BRAF V600E mutation analysis or MLH1 hypermethylation studies is warranted)
- Loss of MSH2 and MSH6 staining may be seen in MSH2 mutation
Molecular / cytogenetics description
- Defect in mismatch repair genes (caretaker genes that proofread DNA replication): typically MLH1 or MSH2, while MSH6 and PMS2 are less common (Gastroenterology 2006;130:312)
- Rarely due to EPCAM mutation, germline promoter hypermethylation of MLH1, inactivation of CHEK2 (cell cycle checkpoint kinase 2)
- Associated with microsatellite instability (MSI; microsatellites are dinucleotide repeat sequences, such as (CA)n, normally present in human genome), although MSI is not specific (World J Gastroenterol 2006;12:4745)
Additional references
Board review style question #1
What is the most common hereditary colorectal cancer syndrome?
- Familial adenomatous polyposis
- Juvenile polyposis
- Lynch syndrome
- MUTYH associated polyposis
Board review style answer #1
Board review style question #2
Lynch syndrome usually arises from a germline mutation in a gene coding for a mismatch repair protein. A germline mutation in which of the following genes could also cause Lynch syndrome?
- BRAF
- CDH1
- EPCAM
- MUTYH
Board review style answer #2
Malakoplakia
Table of Contents
Definition / general | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slidesDefinition / general
- Marked histiocytic infiltrate, more common in bladder, that may diffusely involve colon
- Usually adults, associated with immunosuppression
- Due to defective inflammatory response to gram negative bacterial infection
- Rarely associated with colorectal carcinoma in elderly men (Pathology 2002;34:332)
Case reports
- 2 cases of pediatric malakoplakia of colon (Pediatr Surg Int 2010;26:323)
- 4 year old boy with coexisting celiac disease and chronic granulomatous disease (Indian J Gastroenterol 2006;25:163)
- 54 year old man with liver transplant (Arch Pathol Lab Med 2004;128:e133)
- 58 year old woman with liver transplant (Can J Gastroenterol 2007;21:753)
- 64 year old man with colonic adenocarcinoma (World J Gastroenterol 2007;13:6109)
- 74 year old man with malakoplakia adjacent to adenocarcinoma (J Clin Pathol 1993;46:959)
Gross description
- Diffuse mucosal involvement and submucosal thickening; may cause mass
Microscopic (histologic) description
- Marked histiocytic infiltrate containing Michaelis-Gutmann bodies (calcospherites) that may resemble signet ring cells at frozen section
Microscopic (histologic) images
Mantle cell lymphoma
Table of Contents
Definition / general | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Uncommon in colon
- Often presents as multiple lymphomatoid polyposis
- Often present in benign appearing mucosa (Curr Opin Gastroenterol 2005;21:80)
- 88% males, mean age 61 years
- Also involves stomach and small intestine
- Mean survival historically less than 3 years; may be improving with newer treatments
- Rarely coexists as incidental finding with adenocarcinoma (Arch Pathol Lab Med 2003;127:E64, Mod Pathol 2001;14:811)
Case reports
- 60 year old man with rectal tumor (Hepatogastroenterology. 2001;48:675)
- 61 year old man with diffuse involvement of GI tract without polyposis (J Gastroenterol 2004;39:995)
- 71 year old woman with ulcerative colitis (Am J Surg Pathol 1996;20:1024)
- Woman in 80s (University of Pittsburgh Case #441)
Gross description
- Nodular, sessile or polypoid lesions, widely spaced and confluent studded or cobblestone appearance
- Each polyp 2 mm to 2 cm
- May be dominant tumor mass in ileocecum
- Endoscopically normal mucosa may have small tumor infiltrates also
Microscopic (histologic) description
- Mantle cells (small lymphocytes with pale cytoplasm and cleaved nuclei), often invasion of submucosa, proliferation around germinal centers and sparing of mucosa
- Late epithelial invasion and ulceration
- Note: may also be minute lymphoid infiltrates in known mantle cell patients
Positive stains
- CD20, CD5 and cyclin D1 / bcl1
- Immunohistochemistry for cyclin D1 may be more sensitive than FISH for t(11;14) or PCR for IgH (Am J Surg Pathol 2006;30:1274)
Differential diagnosis
- Inflammatory bowel disease: may need immunostains to differentiate (Dig Dis Sci 1992;37:934)
- MALT lymphoma: may present as multiple lymphomatoid polyposis but has lymphoepithelial lesions and is negative for CD5 and cyclin D1
- Multiple lymphoid polyps: benign germinal centers in children, patients with Gardner's syndrome
- Nodular lymphoid hyperplasia: benign, associated with common variable immunodeficiency syndrome
Mast cell disorders
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare condition, described in 2006, occurring in cases of chronic intractable diarrhea (Arch Pathol Lab Med 2006;130:362)
- Not intended as a specific diagnosis (Ann Allergy Asthma Immunol 2008;101:645)
- Helps select patients amenable to specific mast cell targeted therapies
- No uniform or standardized criteria for diagnosis
- Must be distinguished from GI involvement by systemic mastocytosis, a clonal proliferation of mast cells with specific WHO diagnostic criteria
Essential features
- Chronic intractable diarrhea
- Normal endoscopy
- Increase in the number of mast cells in the gastrointestinal biopsies with sheet and nest formation
Terminology
- Also called mastocytic colitis
- Allergic mastocytic gastroenteritis and colitis (Gastroenterol Res Pract 2012;2012:950582)
ICD coding
- ICD-10: K52.9 - noninfective gastroenteritis and colitis, unspecified
Epidemiology
- Chronic diarrhea is a frequent health problem, affecting 5 - 10% of the adult population (Dig Dis 2018;36:409)
- Despite normal colonoscopy, biopsy can reveal abnormalities in almost 30% of cases (20% corresponding to microscopic colitis)
- No gender differences
- Any age
Sites
- Mast cells can be increased anywhere along the gastrointestinal tract
- The most important differences from controls and other disorders are found in the left colon
Pathophysiology
- Mast cells are immunological cells derived from a CD34+ progenitor in the bone marrow
- They are granulated and measure ~20 microns
- They can be activated through IgE dependent (allergic) and IgE independent pathways
- 2 types, according to granule contents: those containing only tryptase and those that also contain chymase and carboxypeptidase
- In the normal mucosa, the tryptase containing mast cells are located in the mucosa (reactive), while the chymase containing ones are mainly submucosal (constitutive)
- They act on the regulation of intestinal permeability, secretion, peristalsis, immunity and angiogenesis
- Upon activation, mast cells are involved in allergies, anaphylaxis, gastrointestinal disorders, malignancies and cardiovascular diseases (Cancers (Basel) 2021;13:3316)
Etiology
- Unclear
- Some patients have a history of allergies
Clinical features
- Chronic intractable diarrhea with normal endoscopy
- Can also be associated with abdominal pain or bloating
Diagnosis
- Based on the clinical history and confirmed by histopathology
Case reports
- 21 year old healthy woman with a 6 month history of diarrhea, abdominal pain and weight loss (Gastroenterol Hepatol 2017;40:467)
- 39 year old man with a longstanding history of diarrhea alternating with constipation, suggestive of irritable bowel disease (J Gastrointestin Liver Dis 2018;27:327)
- 70 year old man with a 3 month history of diarrhea (Intest Res 2016;14:280)
Treatment
- Antihistamines (H1 and H2)
- Mast cell stabilizers, including cromolyn
- Steroids (e.g., prednisone, budesonide) (J Gastrointestin Liver Dis 2018;27:327)
- Inhibitors of mast cell mediators, inhibitors of tryptase and chymase
- Leukotriene antagonists (e.g., montelukast, zileuton)
- Kinase inhibitor (e.g., imatinib, nilotinib, dasatinib)
Gross description
- Normal mucosa on endoscopy
Microscopic (histologic) description
- Normal mucosal architecture
- Increased number of mast cells in the lamina propria
- Might be associated with an increase in eosinophils
- Scattered mast cells but also nests and sheets
- Sometimes presence of a subepithelial band of mast cells
- Hard to recognize in routine H&E stained slides
- Controversy regarding the cutoff value for mast cells
- Most reports recommend more than 20 mast cells/high power field (HPF), although cutoff can be different for the left and right colon and other gastrointestinal locations
- Lack of standardization for counting
- Routine mast cell counting is not recommended at this time
- Significant overlap exists between mast cell counts in asymptomatic control patients and patients with suspected mastocytic enterocolitis, arguing against the clinical utility of routinely counting mast cells in colonic biopsies (Am J Surg Pathol 2014;38:832)
- Patients with gastrointestinal disorders, other than mastocytic enterocolitis showing increases in mast cells, can also benefit from mast cell stabilizing therapies
Microscopic (histologic) images
Positive stains
- Immunohistochemistry is necessary for reliable mast cell counting
- Mast cells are positive for KIT (CD117) (Cells 2021;10:444)
- Also express tryptase (Immunol Allergy Clin North Am 2014;34:315)
Negative stains
Sample pathology report
- Large bowel, endoscopic biopsy:
- Large bowel mucosa with slight increase in lamina propria mast cells (see comment)
- Comment: Large bowel mucosa with a slight expansion of the lamina propria related to an increase in the number of mast cells in the immunohistochemical stains for KIT. The mast cell count is 36 per high power field.
Differential diagnosis
- Systemic mastocytosis (SM):
- Gastrointestinal tract can be involved in 60 - 80% of SM patients (Cancers (Basel) 2021;13:3316)
- Major criterion to diagnose SM is the presence of multifocal clusters of spindled mast cells in the bone marrow, absent in mastocytic colitis (Am J Hematol 2021;96:508)
- WHO recognizes several subtypes:
- Indolent / smoldering SM
- Advanced
- Aggressive SM
- SM with associated hematological neoplasms
- Mast cell leukemia
- Irritable bowel syndrome (IBS):
- Mast cells can be significantly increased in IBS population
- Overlap in the number of mast cells between groups is too large to be useful in clinical practice (Dig Dis 2018;36:409)
- Inflammatory bowel disease (IBD):
- Increase and degranulation of mast cells can release inflammatory mediators that initiate and maintain neutrophilic infiltration
- Typical clinical, endoscopic and histopathological data of Crohn's disease or ulcerative colitis
- Mast cells can be increased in varying grades in many other gastrointestinal pathologies but history, endoscopy and other histopathological features should allow differential:
- Collagenous colitis:
- Typical thickening of the basement membrane
- Motility disorders (Chagas disease, Hirschsprung disease):
- Absence of ganglion cells in the nerves
- Radiation associated enteritis / colitis:
- History
- Diverticular disease:
- History and endoscopy
- Collagenous colitis:
Board review style question #1
The picture shows KIT immunostaining in a large bowel biopsy of a patient with chronic diarrhea. Which of the following statements regarding KIT immunostaining is true?
- Mast cell counts under 20/HPF should never be reported
- Should be performed in all large bowel biopsies with normal endoscopy
- The presence of a subepithelial band of mast cells is diagnostic of mastocytic enterocolitis
- There are clear differences between normal population and patients with different diseases in the number of mast cells
- There are no uniform criteria for the diagnosis of mastocytic enterocolitis
Board review style answer #1
E. There are no uniform criteria for the diagnosis of mastocytic enterocolitis. To date, the criteria for the diagnosis of mastocytic enterocolitis are not standardized and although most authors recommend 20/HPF, the most recent guidelines and meta analysis emphasize the large overlap in the mast cell numbers between controls and patients with diarrhea and other gastrointestinal pathologies. On the other hand, patients with lower mast cell counts can benefit from therapy, so it is recommended to report the absolute number of mast cells per HPF.
Comment Here
Reference: Mastocytic enterocolitis
Comment Here
Reference: Mastocytic enterocolitis
Board review style question #2
Mastocytic enterocolitis is a(n)
- Clearly defined clinicopathological disease
- Diagnosis that can only be made on imaging
- Disease of young allergic people with a clear clinical phenotype
- Orientative diagnosis to help select patients that are candidates for targeted therapies
- Outdated entity that no longer exists
Board review style answer #2
D. Mastocytic enterocolitis is an orientative diagnosis to help select patients that are candidates for targeted therapies. This diagnosis is almost never certain on pure histopathological criteria and is only useful to select candidates to mast cell stabilizing therapies, as a significant number can respond to them.
Comment Here
Reference: Mastocytic enterocolitis
Comment Here
Reference: Mastocytic enterocolitis
Medullary carcinoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- WHO recognized subtype of colon carcinoma with solid growth pattern (Hum Pathol 1999;30:843)
- Relatively rare; usually quoted as < 1% of colorectal neoplasms (Int J Oncol 2010;37:901)
Essential features
- Strongly associated with high degree of microsatellite instability (MSI), indicative of loss of normal DNA repair gene function
- Often better clinical outcome independent of stage than microsatellite stable tumors or tumors with low levels of microsatellite instability
- Usually no / few nodal metastases
Terminology
- Older names: undifferentiated carcinoma, solid type poorly differentiated carcinoma, large cell minimally differentiated carcinoma
Epidemiology
- Often elderly women (average age ~70 years)
Sites
- Usually right sided
Case reports
- 79 year old woman with black, ulcerated cecal tumor (Arch Pathol Lab Med 2005;129:113)
Treatment
- Surgery and chemotherapy (although 5-FU not effective, as with other MSI high tumors)
Gross description
- Large, ulcerating colon mass
Microscopic (histologic) description
- Malignant, well circumscribed neoplasm with solid growth pattern (no gland formation) and pushing border
- Can form nested, organoid or trabecular patterns
- Cells are uniform, polygonal to round and medium sized with amphophilic cytoplasm, vesicular nuclei with prominent nucleoli and frequent mitotic activity
- Mucin production absent or very focal
- Prominent lymphocytic infiltration within and around tumor
Positive stains
Negative stains
- MLH1 and MSH2; neuroendocrine markers (Mod Pathol 2002;15:741)
- CDX2 (positive in 19%), CK7 (positive in 13%), CK20 (positive in 44%) (Hum Pathol 2009;40:398)
Molecular / cytogenetics description
- Associated with microsatellite instability; therefore may be sporadic or arise in Lynch syndrome
- BRAF mutations common (Ann Surg Oncol 2015;22:2988)
- KRAS mutations uncommon (Am J Pathol 2001;159:2239)
Sample pathology report
- Cecum, biopsy:
- Poorly differentiated carcinoma, consistent with medullary carcinoma (see comment)
- Comment: Immunohistochemical stains for MLH1 and PMS2 show aberrant loss of nuclear expression in the tumor. Staining for MSH2 and MSH6 is unremarkable (i.e. retained).
Differential diagnosis
- Lymphoepithelioma-like carcinoma:
- Very rare in colon
- EBER+
- Neuroendocrine carcinoma:
- Positive for neuroendocrine markers (Am J Clin Pathol 2005;123:56)
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
Board review style answer #2
Melanosis coli
Table of Contents
Definition / general | Essential features | Sites | Etiology | Clinical features | Diagnosis | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Deposition of dark melanin-like pigment in colonic macrophages
Essential features
- Pigment deposition in colon with striking gross and macroscopic features but minimal direct clinical consequences
- Linked to use of anthraquinone laxatives
- Common endoscopic finding
Sites
- Can involve all parts of colon and rectum but typically spares mucosal regions with lymphoid nodules, polyps or carcinomas
- Should biopsy nonpigmented regions in these patients
Etiology
- Linked to use of anthraquinone laxatives but may be secondary to any increase in colonic epithelium apoptosis (Histopathology 1997;30:160)
Clinical features
- Not associated with malignant changes, although it allows small polyps to be more easily identified (Z Gastroenterol 1997;35:313)
Diagnosis
- Colonoscopy
Case reports
- 40 year old woman with colonic lymphoid hyperplasia in melanosis coli (Arch Pathol Lab Med 2001;125:1110)
- 50 year old woman taking laxatives (N Engl J Med 2013;368:2303)
- 83 year old man with melanosis coli involving pericolonic lymph nodes associated with the herbal laxative Swiss Kriss (Arch Pathol Lab Med 2004;128:565)
Gross description
- Diffuse brown-black discoloration of colonic mucosa
Microscopic (histologic) description
- Diffuse deposition of melanized ceroid in macrophages of lamina propria (Gastrointest Endosc 1997;46:131)
- Silver stains may show abnormalities of myenteric plexus
Microscopic (histologic) images
Positive stains
Negative stains
Videos
Melanosis coli
Sample pathology report
- Random colon, biopsy:
- Colonic mucosa with melanosis coli, otherwise within normal limits
Differential diagnosis
- Blue / green / red bowel:
- Coloration different
- No histologic changes
- Brown bowel / ceroidosis:
- Pigment in smooth muscle cells
- Hemochromatosis:
- Pigment in epithelial cells
- Pseudomelanosis:
- Black pigment in macrophages of duodenum
Board review style question #1
Board review style answer #1
Metastases
Table of Contents
Definition / general | Essential features | Clinical features | Clinical images | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Secondary involvement of colon by a neoplasm of extracolonic origin
- Uncommon (for example, occurs in 2% of breast and lung carcinomas)
Essential features
- Metastases more common in small intestine than in colon
- Primary lesion is often but not always identifiable
- Most common primaries are stomach, lung, prostate, breast and ovarian carcinomas and melanoma
Clinical features
- Endometrioid adenocarcinoma: if associated with colonic endometriosis, then represents an enigmatic primary rather than a metastasis
- Leukemia: usually involves right colon and terminal ileum, as polypoid or transmural infiltrates
- Melanoma: may have long interval between primary lesion and metastases (Dis Colon Rectum 2003;46:441)
- Prostate: more common to be direct spread / invasion than a metastasis
- Resections for rectal cancer may rarely contain lymph nodes with metastatic prostate carcinoma
Clinical images
Case reports
- 41 year old man with gastric metastasis to colon (Kaohsiung J Med Sci 2004;20:552)
- 55 and 57 year old women with breast metastases to colon (Tech Coloproctol 2004;8:s135)
- 60 year old man with bladder metastasis to colon (Dig Liver Dis 2006;38:609)
- 64 year old man with lung metastasis to colon (World J Gastroenterol 2014;20:5930)
Gross description
- Central ulceration extending toward mucosa but often sparing it
Microscopic (histologic) description
- Disease usually resembles primary tumor microscopically
- Metastases may be present along serosal surface or within the wall but mucosal involvement is uncommon
- Adjacent / overlying colonic epithelium is not dysplastic
Microscopic (histologic) images
Sample pathology report
- Descending colon, resection:
- Segment of colon with multiple serosal foci of metastatic high grade serous carcinoma, consistent with known ovarian primary
- Margins of resection unremarkable.
- Eight lymph nodes, negative for malignancy (0/8).
Differential diagnosis
- Gastrointestinal stromal tumor:
- Primary colorectal carcinoma:
- Precursor lesion may be present; colonic adenocarcinomas are usually CK20+, CDX2+, CK7-
Board review style question #1
While metastases to the colon are rare, some diseases metastasize there more often than others. Which of the following malignancies metastasizes to the colon relatively frequently?
- Melanoma
- Salivary gland carcinoma
- Synovial sarcoma
- Urothelial carcinoma
Board review style answer #1
Micropapillary carcinoma
Table of Contents
Definition / general | Essential features | Epidemiology | Pathophysiology | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Uncommon subtype of colonic adenocarcinoma with distinctive behavior
- Officially recognized in WHO classification
Essential features
- Histology: small clusters of malignant cells with abundant eosinophilic cytoplasm and pleomorphic nuclei
- High rate of lymph node metastasis (even when early stage) and poor prognosis
Epidemiology
- Micropapillary features may be present in 9% to 19% of colorectal carcinomas (Hum Pathol 2006;37:809, Mod Pathol 2007;20:729)
Pathophysiology
- Lymph node metastasis are very common, even in pT1 / pT2 tumors (Am J Surg Pathol 2009;33:1287)
- Prognosis worse than for conventional colorectal carcinoma (Colorectal Dis 2012;14:e567)
Case reports
- 72 year old woman with invasive micropapillary carcinoma of sigmoid colon (Int J Clin Exp Pathol 2008;1:457)
Microscopic (histologic) description
- Small clusters of malignant cells with abundant eosinophilic cytoplasm and pleomorphic nuclei
- Micropapillae inhabit lacunar-like spaces and demonstrate a reverse polarity configuration, with apical surfaces facing the periphery rather than the center
- Lymphovascular invasion is commonly present
- Morphology is similar to micropapillary carcinomas of other organs (Adv Anat Pathol 2004;11:297)
- Coexisting regions of conventional adenocarcinoma also usually present
Positive stains
- MUC1 (inside out staining pattern) (Histopathology 2005;47:479)
- SOX2, NOTCH3 (stem cell markers) (Mod Pathol 2013;26:1123)
Molecular / cytogenetics description
- Increased rate of TP53 alterations and lower rate of microsatellite instability (Mod Pathol 2011;24:729)
Sample pathology report
- Ascending colon, mass, biopsy:
- Carcinoma with prominent micropapillary features (see comment)
- Comment: Micropapillary carcinoma of the colon has a reported propensity to metastasize to lymph nodes even when early stage.
Differential diagnosis
- Tumor budding:
- Does not show reverse polarity (Histopathology 2002;40:127)
Board review style question #1
Board review style answer #1
Microsatellite instability pathway
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Microsatellites (short tandem repeats) are repetitive DNA sequences composed of repeating motifs of 2 - 10 nucleotides
- When there are defects in mismatch repair (MMR) genes (MLH1, PMS2, MSH2, MSH6), mutations introduced into microsatellite regions during DNA synthesis are not repaired, which results in microsatellite instability (MSI)
- Microsatellite instability (MSI) is one of the pathways implicated in colorectal adenocarcinoma (CRC) carcinogenesis; colorectal cancer with MSI is referred to as MSI / microsatellite unstable or MMR deficient (dMMR) colorectal cancer
Essential features
- Microsatellite instability (MSI) results from abnormal function of one or more mismatch repair genes (MLH1, PMS2, MSH2, MSH6)
- 10 - 15% of colorectal adenocarcinomas (CRC) are MSI / MMR deficient (dMMR)
- dMMR colorectal cancer arises due to sporadic hypermethylation of the MLH1 promoter or due to germline mutations in MMR genes (Lynch syndrome)
- In pathology, colorectal cancer is initially screened for MSI by assessing the presence of MMR proteins using immunohistochemistry
- Loss of one or more MMR proteins is followed by either MLH1 hypermethylation testing or BRAF mutation testing; the absence of MLH1 hypermethylation or a BRAF mutation prompts germline mutation to assess for Lynch syndrome
- Identifying dMMR colorectal cancer is significant as these tumors have a better stage adjusted survival compared to microsatellite stable (MSS) tumors
Terminology
- Lynch syndrome was formerly known as hereditary nonpolyposis colorectal cancer (HNPCC) syndrome
- Biallelic germline mutation in an MMR related gene is termed constitutional mismatch repair deficiency
Epidemiology
- 10 - 15% colorectal cancer is dMMR (Clin Cancer Res 2012;18:1506)
- 80% of dMMR colorectal cancer is sporadic
- Hypermethylation of MLH1 promoter
- ≥ 60 years old
- F > M
- 20% of dMMR colorectal cancer is familial
- Autosomal dominant, germline mutation (Lynch syndrome)
- ~ 40 - 50 years old
- M = F
- 80% of dMMR colorectal cancer is sporadic
Sites
- dMMR colorectal cancer has predilection for the right colon (Arch Surg 1977;112:170)
- Lynch syndrome related colorectal cancer arises from tubular adenomas (Mod Pathol 2017;30:1144)
- Sporadic dMMR colorectal cancer arises from sessile serrated adenomas / polyps (SSA / P) (Histopathology 2007;50:113)
Pathophysiology
- MMR pathway (see diagram 1) (J Gastroenterol 2020;55:15)
- Corrects DNA base substitution mismatch, insertions, deletions or slippage made during DNA replication
- Errors are corrected through the action of two protein complexes:
- MutSα: heterodimer of mutS homologue 2 (MSH2) and mutS homologue 6 (MSH6) proteins
- MutSβ: heterodimer of MSH2 and mutS homologue 3 (MSH3) proteins
- MutSα or MutSβ bind to the area with the defect and recruit MutLα (heterodimer of mutL homologue 1 [MLH1] and postmeiotic segregation increased 2 [PMS2] proteins)
- PMS2 endonuclease makes a nick at 5’ to the mismatch (upstream)
- Exonuclease 1 is recruited and activated by MSH2 or MLH1
- Catalyzes excision of the nascent DNA strand up to and slightly beyond the mismatch
- DNA excision gap is resynthesized by polymerase δ and nick is sealed by DNA ligase I
- MLH1 is required for the function of PMS2 (when MLH1 is lost, PMS2 must also be lost)
- MSH2 is required for the function of MSH6 (when MSH2 is lost, MSH6 must also be lost)
Etiology
- Also see pathophysiology
- Colorectal cancer is MSS / MMR proficient (pMMR) or MSI / MMR deficient (dMMR) (see diagram 2)
- pMMR colorectal cancer: intact MMR genes
- dMMR colorectal cancer: abnormality affecting one or more MMR genes
- Sporadic
- MLH1 promoter hypermethylation
- 50% BRAF V600E mutation
- ARID1A may have a role in carcinogenesis (Hum Pathol 2014;45:2430)
- Familial - Lynch syndrome
- Germline mutation followed by somatic mutation in wild type allele
- Mutations in MLH1, PMS2, MSH2, MSH6, EPCAM (adjacent to MSH2)
- Other:
- Rare cases with isolated loss of PMS2
- Patients present at an older age and have a lower risk for carcinoma at other sites (J Clin Oncol 2015;33:319)
- dMMR tumors lacking germline mutations and MLH1 hypermethylation
- 70%: double somatic mutations in MMR gene (J Pathol 2014;234:548, Gastroenterology 2014;147:1308)
- Rare cases with isolated loss of PMS2
- Sporadic
Diagrams / tables
Clinical features
- Lynch syndrome is associated with increased risk of carcinoma at other sites
- Gastrointestinal: colon, small intestine, stomach, hepatobiliary, pancreas
- Genitourinary: kidney, bladder, prostate
- Gynecological: endometrium, ovary
Diagnosis
- Clinical assessment
- Patients with dMMR colorectal cancer may be asymptomatic and have an incidentally discovered mass during surveillance colonoscopy, while others may present with signs of MSS colorectal cancer (anemia, fatigue, weight loss, change in bowel habits or hematochezia) - prompting colonoscopy
- If there is clinical suspicion for Lynch syndrome, screening may be done using the Amsterdam Criteria or Bethesda System:
- Amsterdam criteria (Dis Colon Rectum 1991;34:424)
- At least 3 relatives with colorectal cancer (1 is a first degree relative of the other 2)
- At least 2 consecutive generations involved
- At least 1 person with colorectal cancer diagnosed < 50 years old
- Familial adenomatous polyposis (FAP) excluded
- Bethesda system (revised) (J Natl Cancer Inst 1997;89:1758)
- Colorectal cancer diagnosed < 50 years old
- Presence of other colorectal cancer or other Lynch syndrome associated tumors
- Colorectal cancer with MSI-H phenotype diagnosed < 60 years old (MSI-H versus MSI-L discussed under Molecular / cytogenetics section)
- Patient with colorectal cancer with 2+ first or second degree relatives with a Lynch syndrome associated tumor
- Amsterdam criteria (Dis Colon Rectum 1991;34:424)
- Pathologic assessment (see diagram 3)
- Most institutions test all colorectal cancer cases for MMR deficiency
- Immunohistochemistry (IHC): IHC markers for MLH1, PMS2, MSH2, MSH6
- Loss of one or more markers prompts additional testing
- BRAF mutation status (IHC or PCR)
- MLH1 methylation status
- Absence of methylation or BRAF mutation prompts germline testing
- Next generation sequencing
- Loss of one or more markers prompts additional testing
- Specific staining patterns
- MLH1 / PMS2 loss: favor sporadic
- MSH2 / MSH6 loss: favor familial
- Isolated PMS2 loss: favor familial
- Isolated MSH6 loss: favor familial
- Immunohistochemistry (IHC): IHC markers for MLH1, PMS2, MSH2, MSH6
- Most institutions test all colorectal cancer cases for MMR deficiency
Laboratory
- Similar to findings as MSS colorectal cancer: anemia with or without elevated CEA
Radiology description
- Similar to findings as MSS colorectal cancer (Radiographics 2000;20:419)
- Luminal obstruction with or without soft tissue mass
- Fat stranding
- Lymphadenopathy
Prognostic factors
- dMMR colorectal cancer
- Better stage adjusted survival compared to MSS tumors (Cancer Epidemiol Biomarkers Prev 2001;10:917)
- Prognosis for colorectal cancer associated with Lynch syndrome and MLH1 promoter hypermethylation is the same (Genet Med 2016;18:863)
Case reports
- 57 year old white woman with a history of colon cancer status post hemicolectomy and diagnosis of Lynch syndrome (BMJ Case Rep 2018;2018)
- 61 year old man had a colonoscopy that showed metachronous colorectal carcinoma with massive submucosal invasion (World J Surg Oncol 2017;15:140)
- 62 year old man with mismatch repair deficient metastatic colorectal adenocarcinoma, urothelial carcinoma and a history of sebaceous carcinomas (Cancer Biol Ther 2017;18:651)
Treatment
- Surgery
- pMMR colorectal cancer
- Node negative disease = surgical excision
- Higher stage (T3, T4) or LN positive = consider chemotherapy
- dMMR colorectal cancer
- Surgical excision
- Lynch: with or without prophylactic hysterectomy
- pMMR colorectal cancer
- Immunotherapy
- dMMR solid tumor: pembrolizumab (Science 2017;357:409)
- Metastatic dMMR colorectal cancer: nivolumab (Lancet Oncol 2017;18:1182)
- Other
- BRAF mutation = limited response to EGFR targeted therapies (N Engl J Med 2015;372:2509)
Gross description
- Larger masses with a polypoid / exophytic appearance
- Classic colorectal cancer appearance: ulcerated lesion with heaped up / rolled edges
- Reference: Dis Colon Rectum 1977;20:661
Gross images
Microscopic (histologic) description
- dMMR colorectal cancer histologic characteristics (Cancer 2001;91:2417, Anticancer Res 1994;14:1631):
- Mucinous, poorly differentiated, signet ring or medullary subtypes
- Increased intratumoral / peritumoral lymphocytes
- Crohn-like reaction
- Relative lack of intraluminal (dirty) necrosis
- Tumoral heterogeneity
- Decreased tumor budding (Hum Pathol 2011;42:1833)
Microscopic (histologic) images
Negative stains
- MSI / dMMR colorectal cancer: 1 or more MMR proteins absent (MLH1, PMS2, MSH2, MSH6)
- Note: Several pitfalls exist when interpreting MMR (Mod Pathol 2019;32:1)
Molecular / cytogenetics description
- BRAF somatic mutation testing
- DNA change C.1799T>A
- Amino acid change: p.V600E (Val600Glu)
- BRAF mutation absent: send for somatic tests to evaluate tumor for Lynch syndrome
- Germline mutation testing
- PCR amplification of seven markers including five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) and two pentanucleotide repeat markers (Penta C and Penta D) followed by separation of the PCR products by capillary electrophoresis
- ≥ 2 unstable loci = MSI-H, < 1 unstable loci = MSI-L, 0 unstable loci = MSS
- PCR amplification of seven markers including five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) and two pentanucleotide repeat markers (Penta C and Penta D) followed by separation of the PCR products by capillary electrophoresis
- Reference: Cancer Res 1998;58:5248
Molecular / cytogenetics images
Sample pathology report
- Interpretations for MSI immunohistochemistry, BRAF mutation or hypermethylation status should be included in a colorectal adenocarcinoma pathology report. Examples are as follows:
- MSI (by IHC) interpretation:
- pMMR colorectal cancer:
- Immunohistochemical stains for the mismatch repair proteins MLH1, MSH2, MSH6 and PMS2 were performed. The tumor cells demonstrate retained nuclear staining for all four proteins, indicating the tumor is MMR proficient (pMMR) and likely microsatellite stable.
- dMMR colorectal cancer:
- Immunohistochemical stains demonstrate the tumor is mismatch repair protein deficient (dMMR) with loss of expression of MLH1 and PMS2, while nuclear expression of MSH2 and MSH6 is retained. BRAF mutational analysis (or methylation status) is being performed to determine if this represents a sporadic type carcinoma.
- pMMR colorectal cancer:
- BRAF mutation interpretation:
- Absence of mutation:
- BRAF mutational analysis was performed and the V600E mutation was not identified. These are somatic tests being performed to evaluate the tumor phenotype. dMMR tumors may be sporadic or associated with Lynch syndrome / hereditary nonpolyposis colorectal cancer. Referral to a hereditary cancer screening program is recommended.
- Presence of mutation:
- Molecular testing demonstrated the presence of BRAF V600E mutation, which correlates with sporadic hypermethylation of the MLH1 promoter. However, if there is a strong family history, caution should be exercised in excluding patients from germline screening on the basis of BRAF V600E mutations.
- Absence of mutation:
- MSI (by IHC) interpretation:
Differential diagnosis
- Rectal carcinoma status post neoadjuvant therapy:
- Can lose expression of MSH6 by IHC despite being MSS (Am J Surg Pathol 2010;34:1798)
Additional references
Board review style question #1
A 64 year old woman presents for routine colonoscopy and a mass is identified in the ascending colon. She undergoes a partial hemicolectomy. Representative histologic sections of the mass are shown. Which of the following features is associated with MSI-H colorectal carcinoma?
- Extensive intraluminal (dirty) necrosis
- Lack of a Crohn-like reaction
- Micropapillary subtype
- Mucinous features
Board review style answer #1
D. Mucinous features. Histologically, traditional MSS colorectal adenocarcinomas are seen as invasive irregular glands surrounded by a desmoplastic stroma. The cells are composed of elongated hyerchromatic nuclei, surrounding luminal (dirty) necrosis. In contrast, MSI / dMMR colorectal carcinomas are associated with mucinous / poorly differentiated / medullary / signet ring cell subtypes, a Crohn-like reaction (dense lymphoid aggregates), increased intratumor / peritumoral lymphocytes, relative lack of intraluminal necrosis and tumoral heterogeneity. Although many institutions regularly test colorectal carcinoma cases for MSI, these features in particular should signal one to consider an MSI / dMMR tumor.
Comment Here
Reference: Microsatellite instability pathway
Comment Here
Reference: Microsatellite instability pathway
Board review style question #2
Sporadic MMR deficient colorectal carcinoma is due to promoter hypermethylation of which of the following genes?
- EPCAM
- MLH1
- MSH2
- MSH6
- PMS2
Board review style answer #2
B. Hypermethylation of the MLH1 (heterodimer of mutL homologue 1) promoter is the most common cause of sporadic microsatellite unstable (MSI) CRC. MLH1 is part of a heterodimer, which also includes post-meiotic segregation increased 2 (PMS). MLH1/PMS2 are members of the DNA mismatch repair (MMR) pathway, which fixes mutations in microsatellite regions made during DNA replication. Defects in any of the MMR genes (including MLH1) prevent these mutations from being repaired, resulting in MSI/MMR deficient (dMMR) CRC. In contrast to promoter hypermethylation that leads to sporadic MMR deficient colorectal carcinoma, Lynch syndrome is due to germline mutations in any of the MMR genes.
Comment Here
Reference: Microsatellite instability pathway
Comment Here
Reference: Microsatellite instability pathway
Mixed neuroendocrine nonneuroendocrine neoplasm
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Extremely rare and aggressive with a high grade neuroendocrine component (in the majority of cases)
- Composed of neuroendocrine cells, nonneuroendocrine cells or amphicrine cells that show both neuroendocrine and nonneuroendocrine cell differentiation (Int J Surg Case Rep 2020;76:125)
Essential features
- Mix of neuroendocrine and nonneuroendocrine components, each constituting at least 30% of the tumor; both components should be histologically and immunohistochemically proven
- Biological behavior is predominantly driven by the endocrine component, which is mostly poorly differentiated (World J Gastroenterol 2020;26:5181)
- Prognosis of mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN) is intermediate between that of pure adenocarcinoma and pure poorly differentiated neuroendocrine carcinoma (Neuroendocrinology 2017;105:412)
- When considering treatment, the more aggressive component of MiNEN should be considered first; MiNEN containing a well differentiated neuroendocrine tumor (NET) (grades 1 or 2) component and an adenocarcinoma component should be treated as adenocarcinoma (J Chin Med Assoc 2015;78:454)
Terminology
- Mixed adenoneuroendocrine carcinomas, glandular neuroendocrine mixed tumor, mixed epithelial endocrine neoplasms, mixed adenoneuroendocrine tumors (J Clin Med 2020;9:273, World J Gastroenterol 2020;26:5181)
ICD coding
Epidemiology
- Age range of 32 - 96 years (Mol Clin Oncol 2018;9:219)
- M > F
- < 2% of all colorectal malignancies (Mol Clin Oncol 2018;9:219)
Sites
- Colonic MiNEN represents 11.2% of all gastroenteropancreatic tract MiNEN (World J Gastroenterol 2019;25:5991)
- In the colon, the rectosigmoid / sigmoid colon is more frequent than the ascending / transverse colon, cecum or ileocecal valve (Case Rep Otolaryngol 2020;2020:5927610)
Pathophysiology
- 3 main hypotheses:
- First hypothesis: 2 components (endocrine and nonendocrine) arise independently from distinct precursor cells and unite
- Second hypothesis: 2 components originate from a common pluripotent stem cell that undergoes biphenotypic differentiation during tumorigenesis
- Third hypothesis: 2 components arise from a common precursor but postulates that the neuroendocrine component develops from an originally nonneuroendocrine cell, via progressive accumulation of genetic aberrations (World J Gastroenterol 2019;25:5991)
Etiology
- Association with longstanding intestinal inflammatory disease (BMC Cancer 2005;5:157, J Clin Gastroenterol 1998;26:353)
Clinical features
- Mass lesion, gastrointestinal bleeding, bowel obstruction, intussusception or paraneoplastic syndromes, rarely carcinoid syndrome
Diagnosis
- Discovered during colonoscopy, some are diagnosed on evaluation of distant metastasis
- Imaging features are nonspecific and necessitate histopathologic confirmation
- CT is helpful in identifying and staging the disease
Laboratory
Radiology description
- Contrast enhanced CT may reveal intraluminal heterogeneous enhancing mass, irregular wall thickening and mesenteric haziness (J Clin Imaging Sci 2013;3:10)
- CT may additionally show pneumoperitoneum, free intraperitoneal fluid, particularly around the cecum and in the pelvis (Chirurgia (Bucur) 2017;112:152)
- PET / CT using 68Ga-Dotatate may provide localization of neuroendocrine components (AJR Am J Roentgenol 2018;211:267)
Radiology images
Prognostic factors
- High tumor grade
- Increased number of genetic / chromosomal alterations (J Clin Med 2020;9:273)
- Prognosis of MiNEN is intermediate between that of pure adenocarcinoma and pure poorly differentiated neuroendocrine carcinoma (Neuroendocrinology 2017;105:412)
- Disease stage and tumor type
- Vascular invasion and CD117 expression (worse prognosis) (Am J Surg Pathol 2012;36:601)
- Large cell type, peritumoral lymphoid reaction tumor and microsatellite instability (better prognosis) (Am J Surg Pathol 2012;36:601)
- Higher volume of high grade neuroendocrine component (> 50%) has worse survival rate (J Chin Med Assoc 2015;78:454)
- Low grade MiNEN composed of adenoma with well differentiated low grade neuroendocrine tumors; behaves as an indolent disease (Am J Surg Pathol 2018;42:1503)
Case reports
- 36 year old woman with ascending colonic polyposis (Int Cancer Conf J 2017;6:175)
- 45 year old man with colon tumor demonstrating thyroid and liver metastasis (Case Rep Otolaryngol 2020;2020:5927610)
- 64 year old man with chronic anemia and right colonic intussusception due to a giant pedunculated lesion (Case Rep Surg 2016;2016:7684364)
- 66 year old man with abdominal pain, loss of weight, anemia, irregular circumferential mass in the left half of transverse colon (Ann Med Surg (Lond) 2015;4:399)
- 68 year old woman with abdominal pain, nausea and vomiting with rising carcinoembryonic antigen (CEA) and alpha fetoprotein (AFP) (Int J Clin Exp Pathol 2014;7:6395)
- 85 year old woman with anemia and laterally spreading tumor in the transverse colon (Int J Surg Case Rep 2020;76:125)
Treatment
- First consideration is the more aggressive component of MiNEN
- MiNEN containing a well differentiated NET (grades 1 or 2) component and an adenocarcinoma component, should be treated as adenocarcinoma (J Chin Med Assoc 2015;78:454)
- Surgery preferred for nearly all potentially curable cases
- Chemotherapy and radiotherapy considered on case by case basis (World J Gastroenterol 2020;26:5181)
- Antiprogrammed death 1 receptor monoclonal antibody (pembrolizumab) therapy is effective where 5 - 10% of tumor cells express the programmed cell death receptor ligand 1 (J Immunother 2019;42:274)
Gross description
- Polypoid or ulcerated tumor with raised edges or large fungating mass (World J Gastroenterol 2020;26:5181)
- Cut surface of the tumor often shows white tan, poorly circumscribed lesions with an infiltrating border
- Focal necrosis and foci of hemorrhage may be seen
Microscopic (histologic) description
- 2 components include epithelial / nonneuroendocrine component (mostly adenocarcinoma; less commonly, squamous cell carcinoma, acinar cell carcinoma, adenoma and other tumors) and neuroendocrine component, each constituting at least 30% of the tumor
- This cut off value only applies to resected specimens after histological examination of the entire neoplasm within the specimen
- Previously, these mixed neoplasms were classified under the category of mixed adenoneuroendocrine carcinoma; however, it is now recognized that the nonneuroendocrine component is not necessarily adenocarcinoma and either one or both components may not be carcinomas
- Both components should be histologically and immunohistochemically proven
- Per WHO, evaluate and grade each histologic component separately
- All neuroendocrine components are classified according to the WHO criteria based on mitotic and Ki67 index, into well differentiated neuroendocrine tumors (G1, G2 and G3) and poorly differentiated neuroendocrine carcinomas (small or large cell neuroendocrine tumors)
- Neuroendocrine component is mostly high grade and demonstrate a high mitotic index
- Small cell type: nested or diffuse proliferation of tumor cells with round to oval nuclei, finely dispersed chromatin, inconspicuous nucleoli and scant cytoplasm
- Large cell type: cells with abundant cytoplasm, vesicular nuclei and prominent nucleoli (Endocr Pathol 2016;27:284)
- Prominent oncocytic differentiation represented by large cells with marked eosinophilia, has also been reported in a mixed tumor of the transverse colon (Endocr Pathol 2013;24:54)
- Amphicrine tumors are distinguished by a divergent immunophenotype in which both exocrine and neuroendocrine traits are expressed in the same cell (Cancer Cell Int 2019;19:310)
- About 5% of colorectal MiNEN show low grade neoplasms, combining adenomas with G1 / G2 NET (Neuroendocrinology 2017;105:412)
- Because of possible response to platinum based treatments, the presence of even a minor component of small cell neuroendocrine carcinoma (SCNEC) should be mentioned in the diagnosis
- Presence of a focal (< 30%) neuroendocrine component does not change the diagnostic categorization but it may be mentioned in the report
Microscopic (histologic) images
Contributed by Lewis A. Hassell, M.D. and Catherine E. Hagen, M.D.
Positive stains
- Adenocarcinoma component: Alcian blue, pancytokeratin AE1 / AE3, cytokeratins and CDX2
- Acinar cell carcinoma component: BCL10, trypsin
- Neuroendocrine component: commonly used immunohistochemical neuroendocrine markers (chromogranin A, synaptophysin, CD56 and neuron specific enolase)
- Synaptophysin and chromogranin A are reported as the most reliable neuroendocrine markers
- Neuroendocrine cells may be positive for peptide hormones (somatostatin, ACTH adrenocorticotropic hormone, VIP vasoactive peptide)
- Both epithelial and endocrine components may show cyclin D1, p53 and beta catenin positivity (Am J Surg Pathol 2011;35:413)
- E-cadherin, CEA, villin and chromogranin A (Int J Clin Exp Pathol 2014;7:6395)
- CD133, a marker of cancer stem cells (reported in 64% of MiNEN of digestive tract), has been associated with tumor aggressiveness (Tohoku J Exp Med 2013;229:301)
Molecular / cytogenetics description
- Mutations in TP53, RB1, PTEN, adenomatous polyposis coli (APC), P13KCA, BRAF, KRAS, MYC, BCL9 and FOXP1, SMARC4 gene (Anticancer Res 2014;34:5517, World J Gastroenterol 2020;26:5181)
- Abnormalities of chromosome 5q, 11q, 17q and 18q have also been identified (World J Gastroenterol 2020;26:5181)
- MSI instability (Endocr Relat Cancer 2018;25:583)
Videos
Mixed neuroendocrine and nonneuroendocrine carcinoma of the esophagus
Mixed neuroendocrine nonneuroendocrine neoplasms
Sample pathology report
- Sigmoid colon, resection:
- Mixed neuroendocrine nonneuroendocrine neoplasm with well differentiated adenocarcinoma (G1, 30%) and poorly differentiated small cell neuroendocrine carcinoma (70%)
- See synoptic report for more details
Brief synoptic reportTumor site Sigmoid colon Histologic type Mixed neuroendocrine nonneuroendocrine carcinoma Size 4.5 x 2.0 x 1.2 cm Depth of invasion Subserosal adipose tissue Lymphovascular invasion Present Perineural invasion Not identified Margins Uninvolved by invasive carcinoma, intramucosal
adenocarcinoma, high grade dysplasia and adenomaLymph nodes with metastatic tumor 2 Lymph nodes examined 15 Tumor deposits Not identified Distant metastasis Not applicable AJCC staging pT3 N1
Differential diagnosis
- Neuroendocrine carcinoma:
- No epithelial nonneuroendocrine component is identified
- Adenocarcinoma with scattered neuroendocrine cells:
- Adenocarcinoma with a minor neuroendocrine component that is < 30% of the entire tumor
Additional references
Board review style question #1
An 8 mm polypoid mass located in the sigmoid colon was resected. The microscopic examination revealed a biphasic tumor that was positive for epithelial and neuroendocrine markers, each component accounting for > 30% of the tumor (see images above: chromogranin, trypsin). Which statement is true regarding this lesion?
- It is a common colorectal neoplasm
- It is mostly an aggressive tumor
- More common in females
- Recognized as part of the multiple endocrine neoplasia type 1 group of tumors
- Treatment is determined by the predominant (volume wise) component
Board review style answer #1
B. It is mostly an aggressive tumor
Comment Here
Reference: Mixed neuroendocrine nonneuroendocrine neoplasm
Comment Here
Reference: Mixed neuroendocrine nonneuroendocrine neoplasm
Mucinous adenocarcinoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Prognostic factors | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- WHO recognized subtype of colorectal carcinoma with mucin lakes comprising at least 50% of tumor mass, though utility of 50% cutoff is unclear (Histopathology 2019;74:406)
Essential features
- Most common subtype of colorectal carcinoma
- Mucin production easily observed on histology
- Some cases are microsatellite instability (MSI) high
- Unclear whether worse prognosis than conventional colorectal carcinoma
Terminology
- Variably called colloid carcinoma
Epidemiology
- Relatively common (10% of all colorectal carcinomas) (Cancer 1976;37:1891)
- Incidence is stable (Dis Colon Rectum 2005;48:1161)
Sites
- Usually arises in the right colon (J Clin Pathol 2012;65:381)
Pathophysiology
- Associated with microsatellite instability, though most mucinous carcinomas are not MSI high (Cancer 2005;103:2023, Am J Pathol 2001;158:527)
Clinical features
- Typically presents at more advanced stage and with greater nodal involvement than standard colorectal carcinoma (Dis Colon Rectum 1993;36:49, Gastroenterol Hepatol 2002;25:534)
- May recur locally following excision (World J Surg Oncol 2012;10:109)
Prognostic factors
- May have same or worse prognosis compared with standard colorectal carcinoma (Ann Oncol 2012;23:135, J Surg Oncol 2000;73:70)
Case reports
- 11 year old girl with colloid carcinoma of rectum (J Postgrad Med 1993;39:218)
- 77 year old man with large mucinous adenocarcinoma (Dig Surg 2003;20:69)
Gross description
- Exophytic, gelatinous mass
Gross images
Microscopic (histologic) description
- Adenocarcinoma with strips of tumor cells floating in large extracellular mucin lakes comprising at least half of tumor mass
- Signet ring cells (with intracellular mucin) may also be present but should comprise less than half of tumor
Microscopic (histologic) images
Positive stains
- CDX2 (100%)
- MUC2 (100%)
- CK20 (98%)
- Beta catenin (nuclear) (64%)
- CK7 (19%, usually rectal)
- MUC6 (5%)
- Reference: Am J Surg Pathol 2011;35:1830
Molecular / cytogenetics description
- KRAS mutations more common and TP53 mutations less common than in standard colorectal carcinomas (Mod Pathol 2006;19:59)
Sample pathology report
- Ascending colon, resection:
- Mucinous adenocarcinoma, well differentiated (see synoptic report)
Differential diagnosis
- Colitis cystica profunda:
- May form a mass and demonstrate copious extracellular mucin but epithelium is clearly benign
- Signet ring cell carcinoma:
- More than half of tumor composed of signet ring cells
Additional references
Board review style question #1
Which of the following is true about mucinous adenocarcinoma of the colorectum?
- It is the most common subtype of colorectal carcinoma
- It unequivocally has a better prognosis than standard colorectal carcinoma
- It usually affects pediatric patients
- Signet ring cells are always at least a minor component
Board review style answer #1
A. It is the most common subtype of colorectal carcinoma
Comment Here
Reference: Mucinous adenocarcinoma
Comment Here
Reference: Mucinous adenocarcinoma
Mucosal prolapse polyp (pending)
Table of Contents
Definition / generalDefinition / general
[Pending]
Mucosal Schwann cell hamartoma
Table of Contents
Definition / general | Essential features | Terminology | Sites | Clinical features | Diagnosis | Case reports | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Benign mucosal proliferation composed of Schwann cells
Essential features
- Benign, incidental spindle cell lesion
- Not associated with any clinical syndrome
- Positive for S100, negative for EMA
Terminology
- Has been called neuroma or neurofibroma in past
Sites
- Can arise anywhere in the colorectum but more common distally
Clinical features
- Average patient age is 62 years, with a female predominance (Am J Surg Pathol 2009;33:781)
- Not associated with any particular syndrome
- Lesions do not recur
Diagnosis
- Discovered incidentally during colonoscopy
Case reports
- 20 year old man with multiple colonic lesions (BMC Gastroenterol 2015;15:128)
- 59 year old man with ulcerative colitis (Gastroenterol Hepatol (N Y) 2013;9:183)
Gross description
- Small (6 mm or less) sessile polyp
Microscopic (histologic) description
- Poorly circumscribed mucosal proliferation of spindle cells, with no whorling, no palisading, no fasciculation
- Nuclei are generally small, bland and elongated
Microscopic (histologic) images
Positive stains
- S100 (lesional cells)
- Neurofilament / NFP (rare intralesional axons)
Negative stains
Sample pathology report
- Transverse colon, polypectomy:
- Mucosal Schwann cell hamartoma (see comment)
- Comment: An immunohistochemical stain for S100 is positive.
Differential diagnosis
- Perineurioma:
- Ganglioneuroma:
- Ganglion cells present
- Neurofibroma:
- Unlikely to involve mucosa
- Benign epithelioid peripheral nerve sheath tumor:
- Cells are epithelioid; lesion extends into superficial submucosa (Am J Surg Pathol 2005;29:1310)
- Tactile corpuscle-like body:
- Usually small, incidental finding in mucosal biopsy (Am J Surg Pathol 2015;39:1668)
Additional references
Board review style question #1
Board review style answer #1
Muir-Torre syndrome
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Clinical variant of Lynch syndrome with cutaneous sebaceous neoplasms (including sebaceous adenoma, sebaceoma and sebaceous carcinoma) or keratoacanthomas in addition to visceral malignancies of Lynch syndrome
- See also Lynch syndrome, sebaceous adenoma: skin, sebaceous carcinoma: skin, breast
Essential features
- Variant of Lynch syndrome with skin manifestations
- More common in men
Terminology
- Also called Torre-Muir syndrome
Epidemiology
- Sebaceous neoplasms present at mean age of 53 years
- M:F = 3:2
Sites
- Sebaceous tumors are most common below the neck
- Colorectal tumors are more commonly located in proximal colon
Etiology
- Typically due to germline mutations in a DNA mismatch repair gene: most commonly MSH2 (60 - 90%); may be due to mutation in MLH1 or MSH6
- May also be due to inactivation through hypermethylation of a DNA mismatch repair gene
Clinical features
- Skin neoplasm may be presenting lesion 22% of time (J Am Acad Dermatol 1999;41:681)
- Colorectal adenocarcinoma is prototypical tumor of Lynch syndrome (typically proximal colon); however, patients may develop other low grade carcinomas, including endometrial carcinomas (15%) and genitourinary carcinomas
Diagnosis
- Clinical algorithms include Amsterdam criteria and revised Bethesda criteria
- Mayo Muir-Torre syndrome risk scoring system assesses whether patients with sebaceous neoplasms are in need of further evaluation for Lynch Syndrome (Genet Med 2014;16:711)
- Can screen using immunohistochemical testing for loss of mismatch repair protein expression (MLH1, MSH2, MSH6, PMS2) or microsatellite instability testing via PCR
- Germline genetic testing
Prognostic factors
- Muir-Torre associated neoplasms tend to be less aggressive than the same tumors in nonsyndromic patients
Case reports
- 58 year old man with retroperitoneal undifferentiated pleomorphic sarcoma associated with Muir-Torre syndrome (J Cutan Pathol 2013;40:730)
- Unusual case of hepatocellular carcinoma in noncirrhotic background in Muir-Torre patient (Fam Cancer 2012;11:7)
- Sebaceous adenoma arising within an ovarian mature cystic teratoma in Muir-Torre syndrome (Ann Diagn Pathol 2012;16:485)
Microscopic (histologic) description
- Cutaneous tumors demonstrate sebaceous origin or differentiation, including coarsely vacuolated cytoplasm and starry nuclei (mulberry cells) (Arch Pathol Lab Med 2014;138:1685)
- Muir-Torre syndrome associated sebaceous tumors are often nodulocystic
- Cystic sebaceous neoplasms have only been reported in patients with Muir-Torre syndrome
- Colorectal adenocarcinomas tend to have mucinous features, with tumor infiltrating lymphocytes and Crohn-like reaction
Microscopic (histologic) images
Positive stains
- EMA in sebaceous neoplasms
Negative stains
- Loss of MSH2 and MSH6 staining in colonic polyps (Arch Dermatol 2006;142:1039)
- CK15 and MSH2 in sebaceous neoplasms (J Cutan Pathol 2006;33:634, Dermatol Online J 2006;12:4, Am J Clin Dermatol 2007;8:315)
Molecular / cytogenetics description
- Autosomal dominant inheritance
- Mutations most commonly in MLH1 or MSH2 mismatch repair genes (3p22.2 and 2p21, respectively)
- Microsatellite instability is present in approximately 70% of Muir-Torre syndrome associated tumors
- Germline testing confirms the diagnosis
Differential diagnosis
- Lynch syndrome:
- Similar presentation and genetics, except no sebaceous neoplasms
Additional references
Board review style question #1
Muir-Torre syndrome is considered a clinical variant of what syndrome?
- Cowden syndrome
- Juvenile polyposis syndrome
- Li-Fraumeni syndrome
- Lynch syndrome
Board review style answer #1
Board review style question #2
Which of the following is a typical manifestation of Muir-Torre syndrome?
- Cylindroma
- Desmoid fibromatosis
- Sebaceous adenoma
- Trichilemmoma
Board review style answer #2
MUTYH associated polyposis
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Autosomal recessive polyposis syndrome characterized by development of numerous colon adenomas but usually fewer than in classic familial adenomatous polyposis
Essential features
- Attenuated polyposis syndrome with biallelic MUTYH mutations and no FAP mutation
- Increased risk for colorectal carcinoma
Terminology
- Outdated terms include:
- MYH associated polyposis
- Familial adenomatous polyposis 2
- Autosomal recessive adenomatous polyposis
Epidemiology
- Accounts for < 1% of colorectal carcinoma (Gastroenterology 2009;136:1251)
Sites
- Adenomatous polyps develop in the colon and sometimes in the duodenum
- Colorectal carcinomas are often proximal (Gastroenterology 2010;138:2044)
Clinical features
- Mean age of diagnosis is about 50 years
- Patients often have between 10 and 100 polyps (Int J Cancer 2004;109:680)
- Biallelic mutations confer a 50 fold risk of colorectal carcinoma and monoallelic mutation a 3 fold risk (Cancer Epidemiol Biomarkers Prev 2006;15:312)
- Patients can also develop ovarian, bladder and breast carcinoma, as well as sebaceous gland tumors
Diagnosis
- Molecular testing to confirm biallelic MUTYH mutations
Case reports
- 2 patients with duodenal carcinoma (J Clin Pathol 2006;59:1212)
Microscopic (histologic) description
- Patients most commonly develop colonic tubular adenomas that resemble sporadic adenomas
- Sessile serrated polyps can also occur
Microscopic (histologic) images
Molecular / cytogenetics description
- MUTYH is located at 1p34 and repairs oxidative DNA damage that otherwise can lead to carcinoma (Mod Pathol 2013;26:1371)
- Most common mutations are Y165C and G382D
Differential diagnosis
- Familial adenomatous polyposis and attenuated familial adenomatous polyposis:
- Presentations can overlap but these patients have an APC mutation
Additional references
Board review style question #1
How does MUTYH associated polyposis differ from familial adenomatous polyposis?
- It is autosomal recessive
- Molecular testing cannot confirm the diagnosis
- Patients never develop upper gastrointestinal tract adenomas
- The polyps are all serrated
Board review style answer #1
Mycobacterium avium-intercellulare (pending)
[Pending]
Mycophenolate mofetil associated colitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Gastrointestinal tract toxicity related to mycophenolate therapy
- Mycophenolate mofetil (CellCept) is a therapeutic immunosuppressant agent that inhibits the proliferation of B and T cells through noncompetitive, reversible inhibition of inosine monophosphate dehydrogenase and is used for (Pharmacotherapy 1997;17:1178):
- Prevention of acute rejection of solid organ transplants
- Treatment of various inflammatory and autoimmune disorders
- Prophylaxis of acute graft versus host disease (GVHD) and treatment of chronic graft versus host disease
Essential features
- Gastrointestinal tract toxicity commonly seen in transplant patients taking mycophenolate, particularly renal transplant recipients
- Symptoms resolve with medication withdrawal or dose reduction
- Histologically characterized by crypt apoptosis, crypt injury, lamina propria eosinophils and architectural distortion
- Lack of neuroendocrine cell aggregates, presence of eosinophils and paucity of apoptotic microabscesses can help distinguish from graft versus host disease
Terminology
- Also called CellCept colitis
ICD coding
- ICD-10: Z79.899 - other long term (current) drug therapy
Epidemiology
- Mean age about 35 - 50 (World J Gastrointest Endosc 2017;9:405, Clin Transplant 2014;28:1244)
- M:F = 1:1
- More common among solid organ transplant patients, especially kidney transplant recipients (World J Gastrointest Endosc 2017;9:405, Ann Gastroenterol 2015;28:366)
- Reported incidence among patients taking mycophenolate is approximately 30% (Clin Transplant 2014;28:1244)
Sites
- Anywhere in gastrointestinal tract can be involved:
- Colon > upper gastrointestinal tract
- Right colon > left colon / rectum (Ann Gastroenterol 2015;28:366)
Pathophysiology
- Not well understood
- Possible mechanisms include direct toxic effect of drug, antimetabolite effect on enterocytes leading to reduced crypt proliferation or immune dysregulation (Histopathology 2013;63:649, Am J Surg Pathol 2008;32:1367)
Etiology
- Some correlation with mycophenolate dosage (Transplantation 1998;65:1450)
Clinical features
- Watery diarrhea, nausea, vomiting
- Severe cases can result in malabsorption and weight loss
- Onset varies widely from within 6 months of starting mycophenolate to > 10 years (Case Rep Gastrointest Med 2016;2016:3058407)
- Endoscopically characterized by ulceration, erythema, hyperemia and coarse appearing mucosa but endoscopic appearance may also be normal (World J Gastrointest Endosc 2017;9:405, Ann Gastroenterol 2015;28:366)
Diagnosis
- Endoscopy with tissue biopsy
- Symptom resolution following drug withdrawal / dose reduction
- Exclusion of other etiologies (e.g. infection, other drugs, etc.)
Prognostic factors
- Patients universally do well following withdrawal or dose reduction
Case reports
- 31 year old woman with mycophenolate induced colitis after 10 years of use (Case Rep Gastrointest Med 2016;2016:3058407)
- 33 year old man with late onset mycophenolate mofetil related colitis (Am J Health Syst Pharm 2014;71:1858)
- 44 year old woman with systemic sclerosis and mycophenolate mofetil induced colitis (BMJ Case Rep 2018;2018:bcr-2018-224829)
- 55 year old man, a heart transplant recipient, with mycophenolate mofetil induced colitis (BMJ Case Rep 2018;2018:bcr-2017-224035)
- 64 year old man with mycophenolate mofetil induced colitis mimicking ischemic colitis (Case Rep Gastroenterol 2014;8:95)
Treatment
- Dose reduction or discontinuation of mycophenolate
Microscopic (histologic) description
- Crypt apoptosis with no or few apoptotic microabscesses
- Increased eosinophils (> 15/10 high powered fields) present in lamina propria
- Neutrophilic inflammation may also be present in lamina propria; cryptitis and crypt abscess may be present but usually focal (J Clin Pathol 2013;66:8)
- Lack of lamina propria endocrine cell aggregates
- Architectural distortion
- Pseudopyloric gland metaplasia can be seen (Int J Surg Pathol 2003;11:295)
- Injured dilated eosinophilic crypts may be present but typically to a lesser degree than graft versus host disease
- Can have Crohn's-like appearance with patchy inflammation and lymphoid hyperplasia (Int J Surg Pathol 2003;11:295)
- Cases are not routinely graded but if necessary the Lerner system can be used (Am J Surg Pathol 2013;37:1319)
Microscopic (histologic) images
Negative stains
- CMV immunostain
Sample pathology report
- Colon, biopsy:
- Colonic mucosa with increased crypt apoptosis and mild architectural distortion (see comment)
- Comment: Given the patient’s reported use of mycophenolate, the histologic features are suggestive of mycophenolate colitis (or give differential diagnosis as appropriate).
Differential diagnosis
- Cytomegalovirus (CMV) colitis:
- Presence of viral inclusions on H&E or immunohistochemistry
- Graft versus host disease (GVHD):
- Fewer lamina propria eosinophils
- Presence of apoptotic microabscesses
- Presence of endocrine cell aggregates in lamina propria (Am J Surg Pathol 2013;37:1319)
- Exceedingly rare in solid organ transplant patients
- Inflammatory bowel disease:
- Prominent neutrophilic cryptitis and crypt abscess with architectural distortion
- Limited crypt apoptosis
- Clinical history is important (bloody diarrhea, lack of mycophenolate use)
Board review style question #1
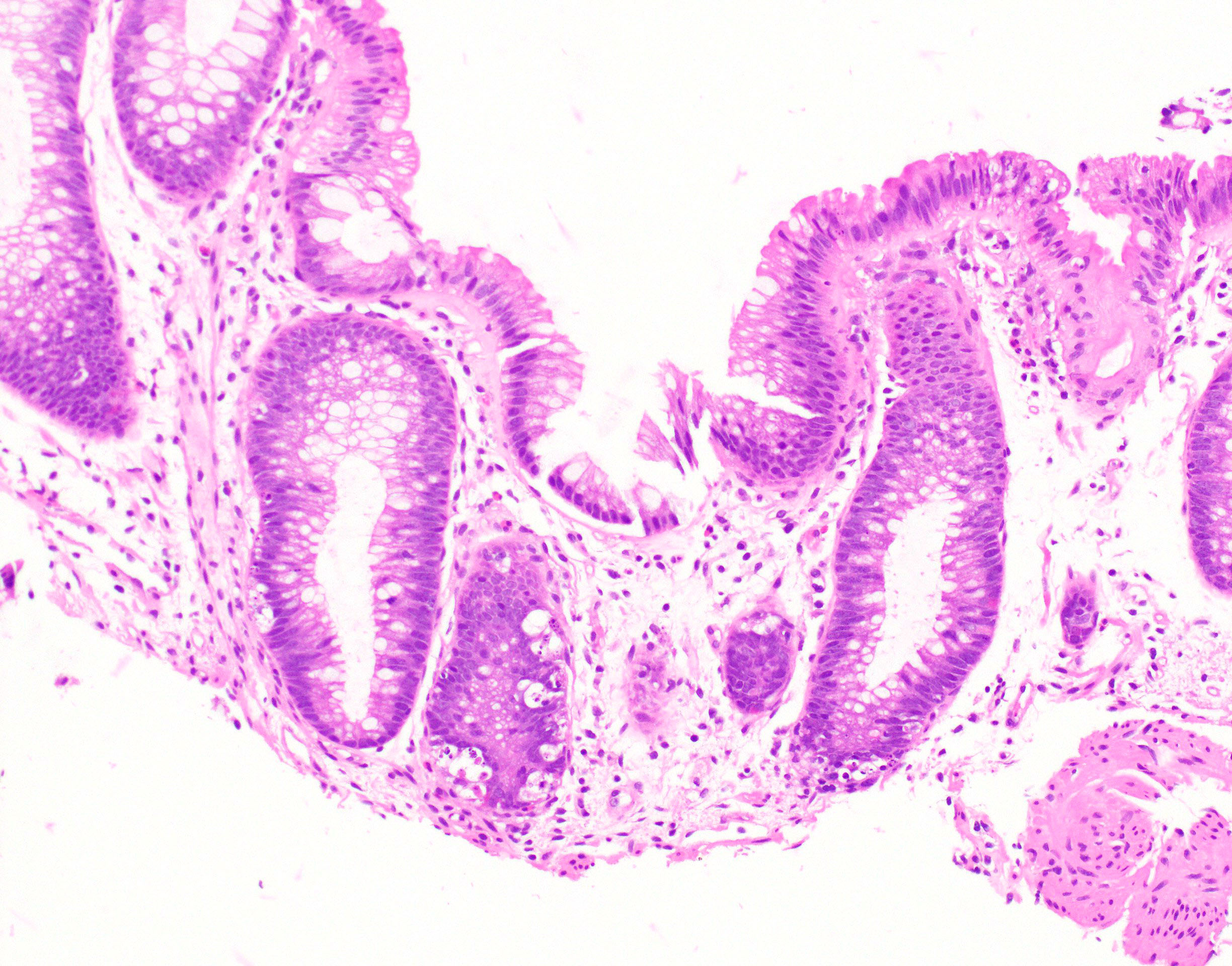
A 45 year old man who underwent a kidney transplant one year ago presents complaining of watery diarrhea. A colonoscopy and biopsy are performed. CMV immunohistochemistry is negative. Based on the histologic findings, which of the following is the most likely diagnosis?
- Graft versus host disease
- Infectious colitis
- Mycophenolate toxicity
- Ulcerative colitis
Board review style answer #1
Board review style question #2
Which of the following histologic features is not helpful in distinguishing mycophenolate toxicity from graft versus host disease?
- Apoptotic microabscesses
- Architectural distortion
- Endocrine cell aggregates
- Eosinophilic inflammation
Board review style answer #2
Necrotizing enterocolitis
Table of Contents
Definition / general | Etiology | Clinical features | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Acute, necrotizing inflammation of terminal ileum or ascending colon, common at day 2-4 in premature or low birth weight neonates (eMedicine #1, #2, J Clin Pathol 1979;32:1090)
- May be associated with Hirschsprung disease, thrombosis of abdominal aorta, H2 blockers (Pediatrics 2006;117:e137), chemotherapy for hematopoietic malignancies (Int J Hematol 2005;82:319)
- Mortality rate of 15-30% (Semin Fetal Neonatal Med 2011;16:145); poorer survival in preterm infants of HIV+ mothers (Pediatr Dev Pathol 2012;15:293)
Etiology
- Oral feeding of neonates with immature gut immune system causes release of proinflammatory cytokines; bacteria in food produce more cytokines which injure mucosa; intestinal blood flow may be disturbed
- IL10 may prevent by attenuating intestinal inflammation (Am J Surg 2012;203:428)
Clinical features
- Symptoms: abdominal distention, loss of bowel sounds, blood-stained stool
- Complications: bowel perforation, short bowel syndrome, malabsorption (due to ileal resection), strictures, recurrence
Treatment
- Fluids and surgery if gangrene/perforation
Gross description
- Necrotic mucosa, submucosal gas-filled cysts
Gross images
Microscopic (histologic) description
- Early: mucosal edema, hemorrhage, necrosis, pneumatosis cystoides intestinalis
- Late: hemorrhagic and gangrenous bowel wall with strictures
Microscopic (histologic) images
Differential diagnosis
- Candidiasis: see Fetal Pediatr Pathol 2010;29:172
Neuroendocrine carcinoma
Table of Contents
Definition / general | Essential features | Terminology | Sites | Epidemiology | Clinical features | Prognostic factors | Case reports | Radiology images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Poorly differentiated neuroendocrine malignancy, including large cell and small cell neuroendocrine carcinoma (