Superpage
Superpage Topics
Adrenal insufficiency-diagnosis
Aldosterone
Allergy testing (pending)
Anion gap (pending)
Anticardiolipin antibodies
Aspartate aminotransferase
Assay interferences
CK-MB (creatine kinase isoenzyme MB)
Calcitonin
Cancer biomarkers (pending)
Captopril suppression test
Cardiac troponins
Chemistry analyzer selection (pending)
Creatine kinase
Drugs of abuse
Electrolytes
Fludrocortisone suppression test
Folate (pending)
HIV testing (pending)
Hemoglobin A1C
Hemoglobin electrophoresis (pending)
Hepatitis B testing
Hepatitis C testing (pending)
High sensitivity CRP
Hyperaldosteronism
Hypercortisolism
Hyperlipidemia
Hyperthyroidism-lab diagnosis
Hypothyroidism-lab diagnosis
Immunofluorescence testing (pending)
Important wavelengths for various tests (pending)
Interference in protein electrophoresis
LD1-Lactate dehydrogenase isoenzyme 1
Lipid panel
Liver function test panel
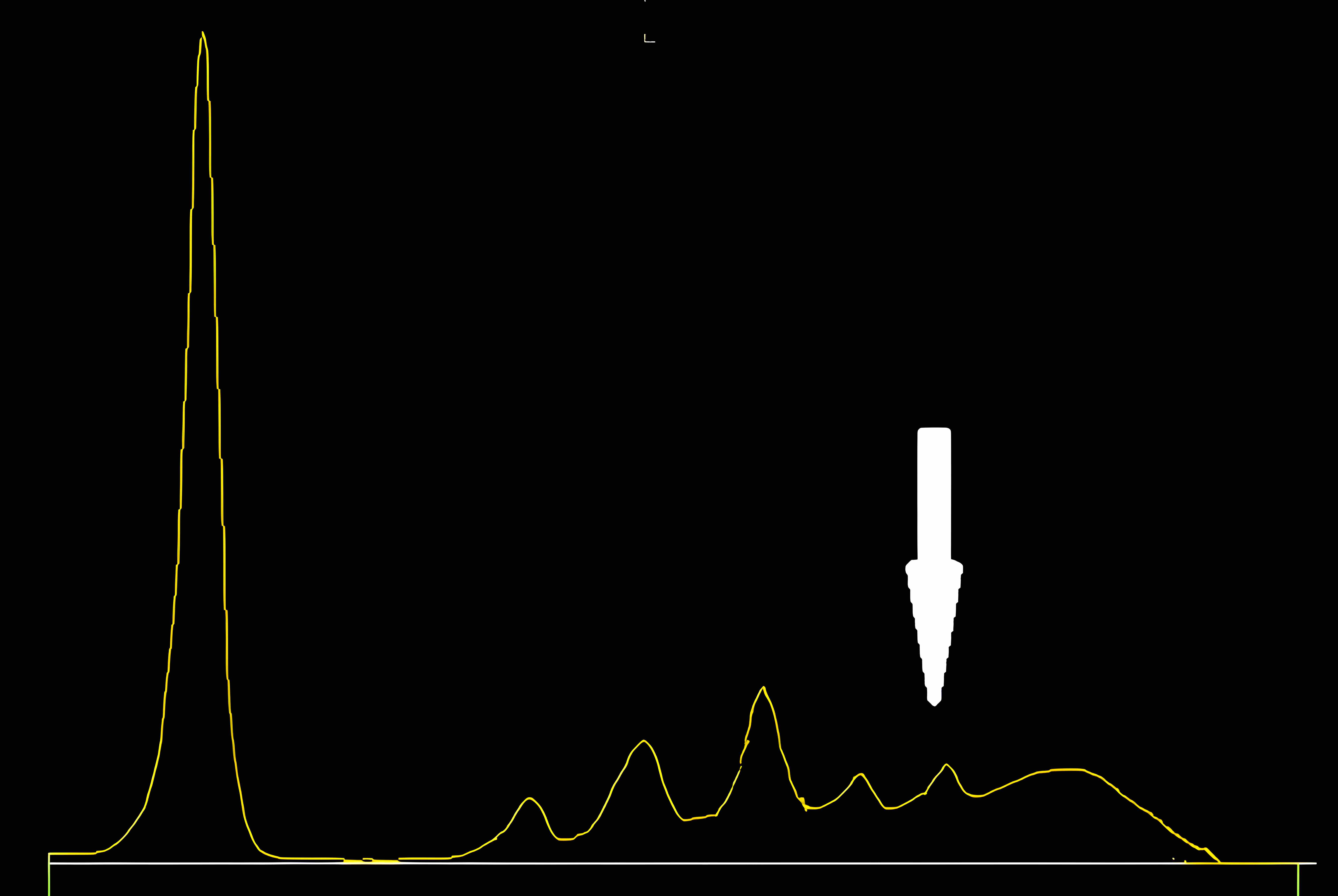
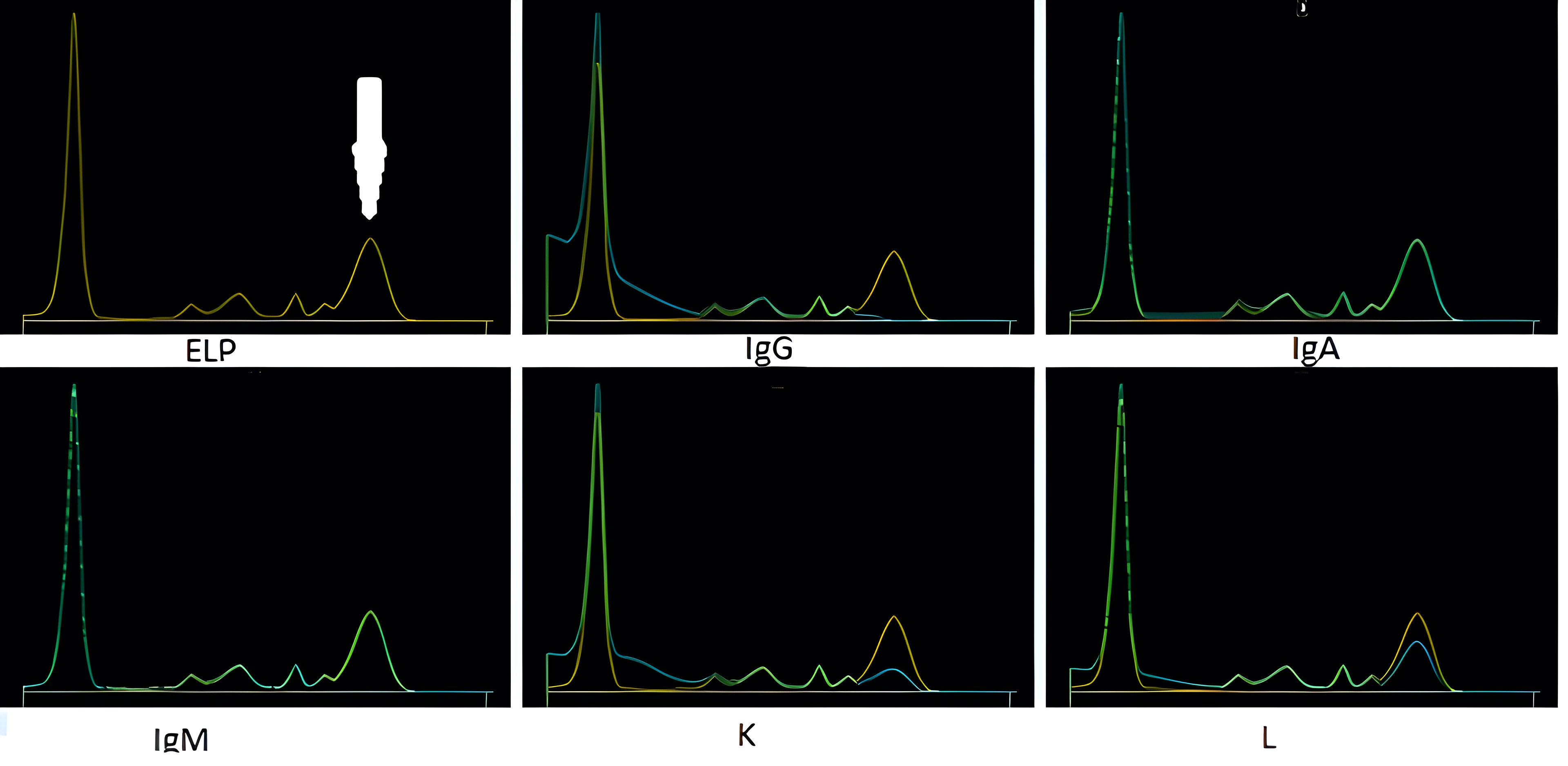
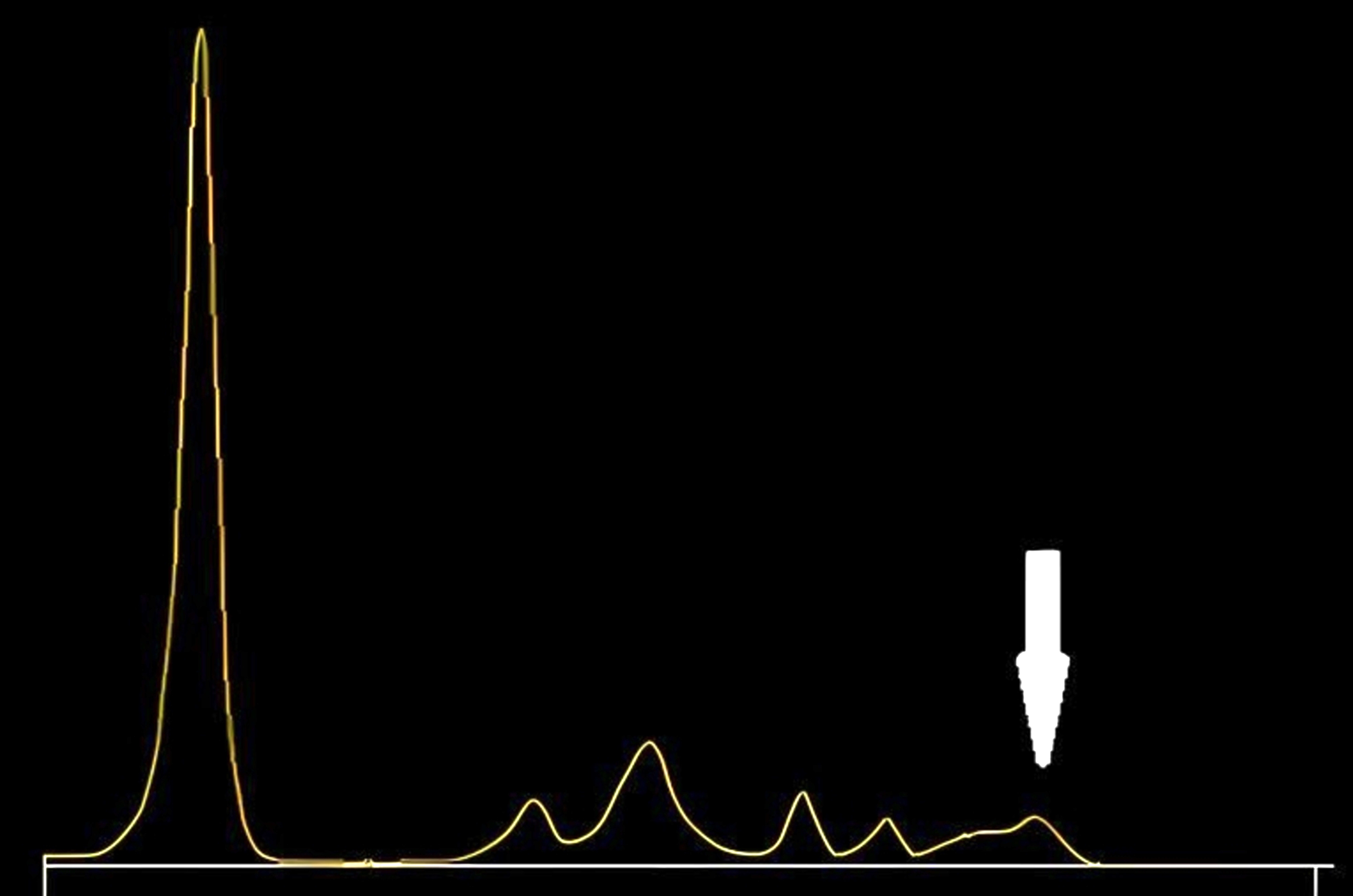
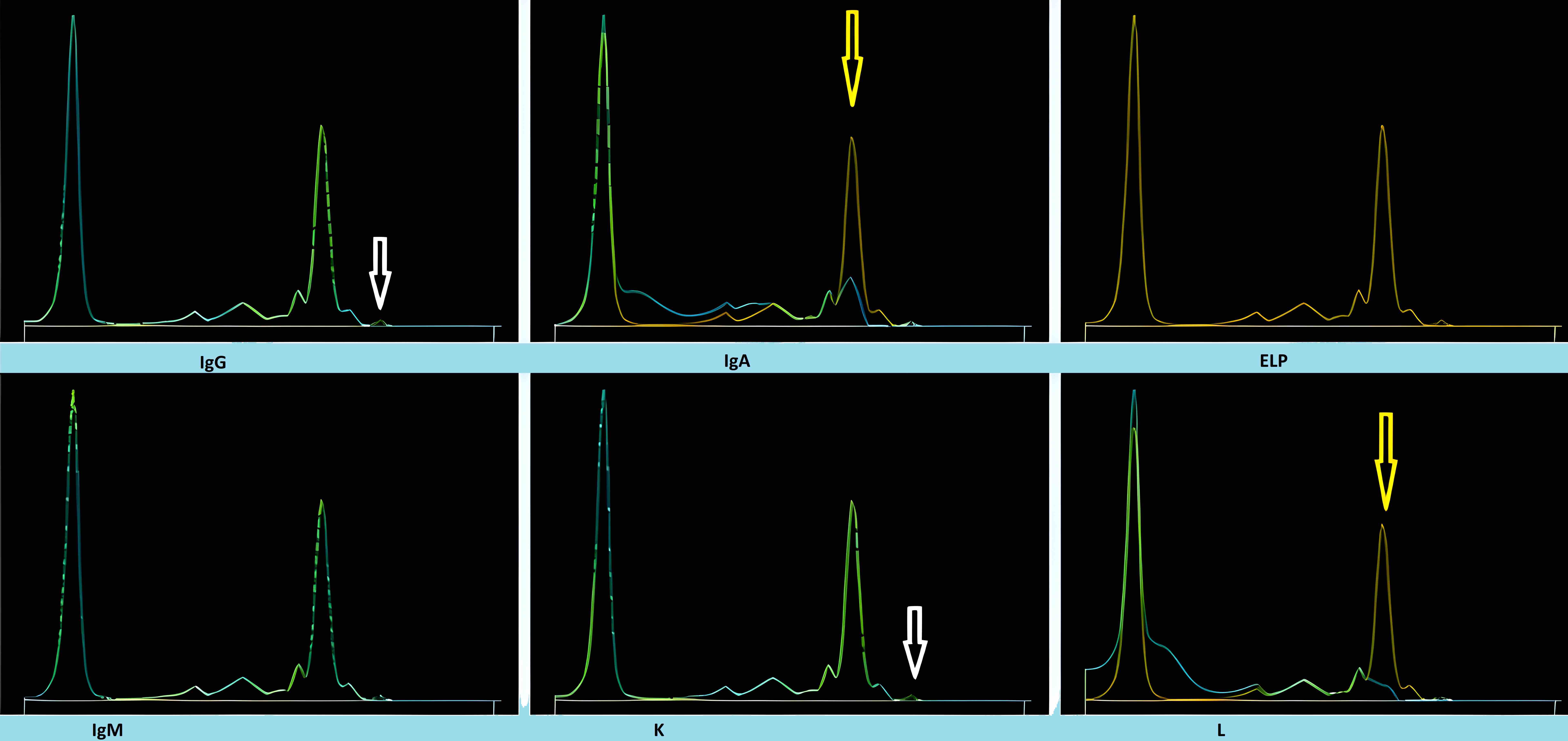
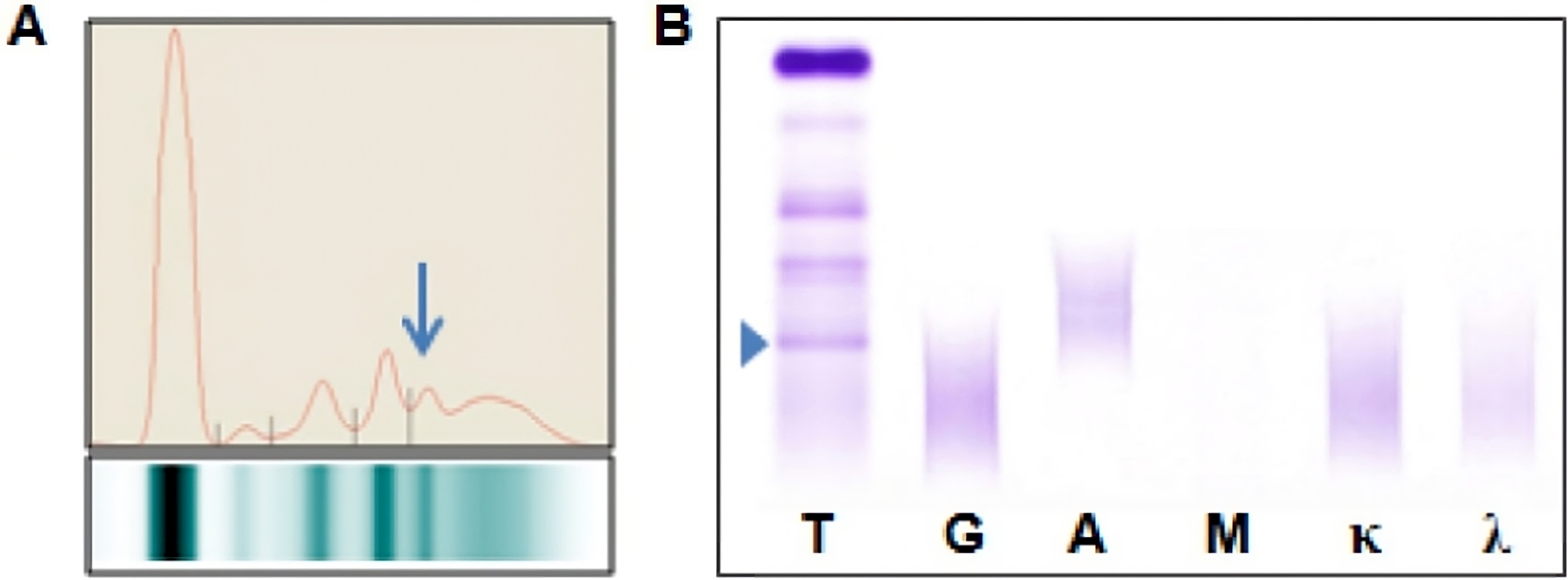
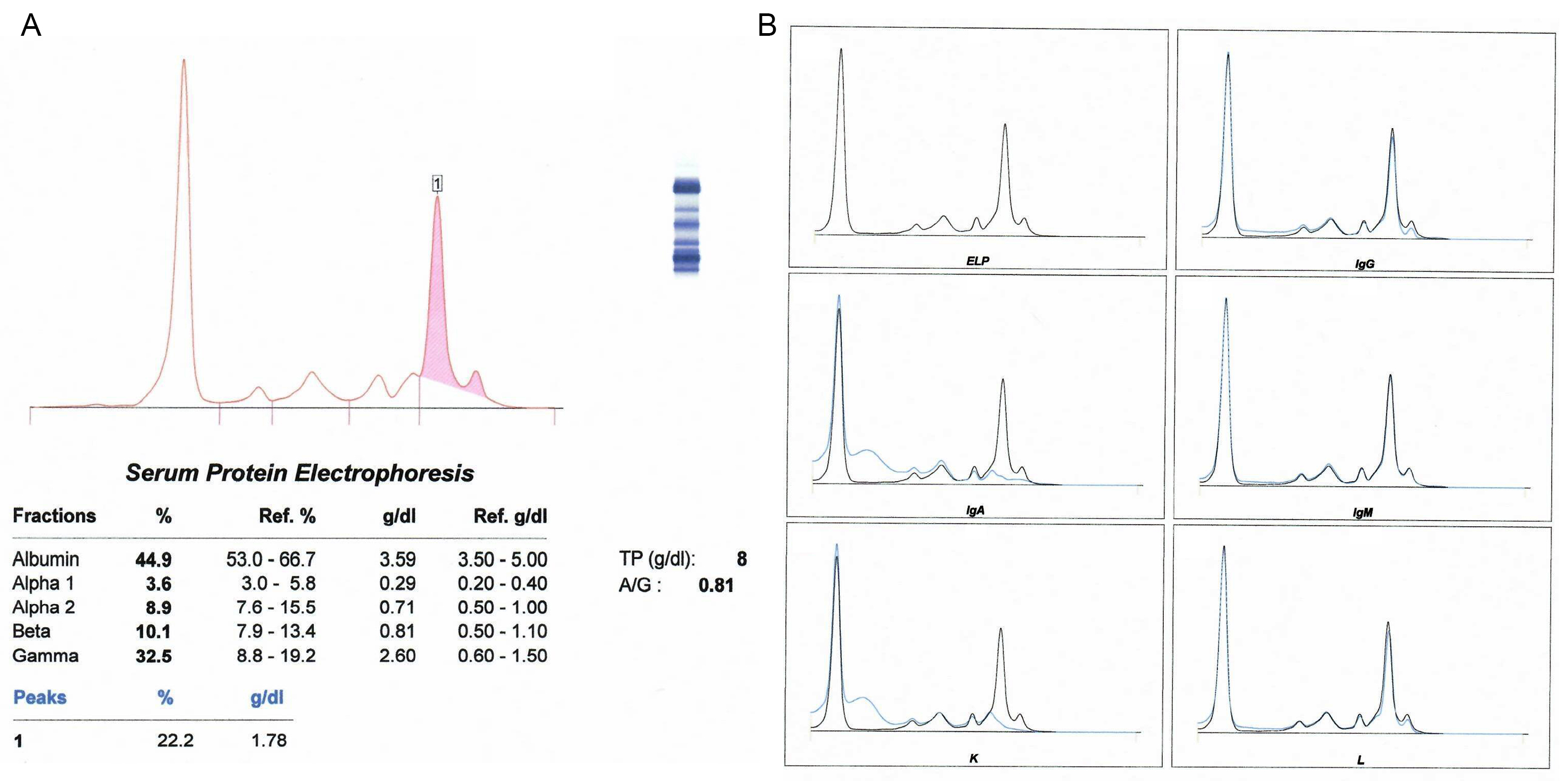
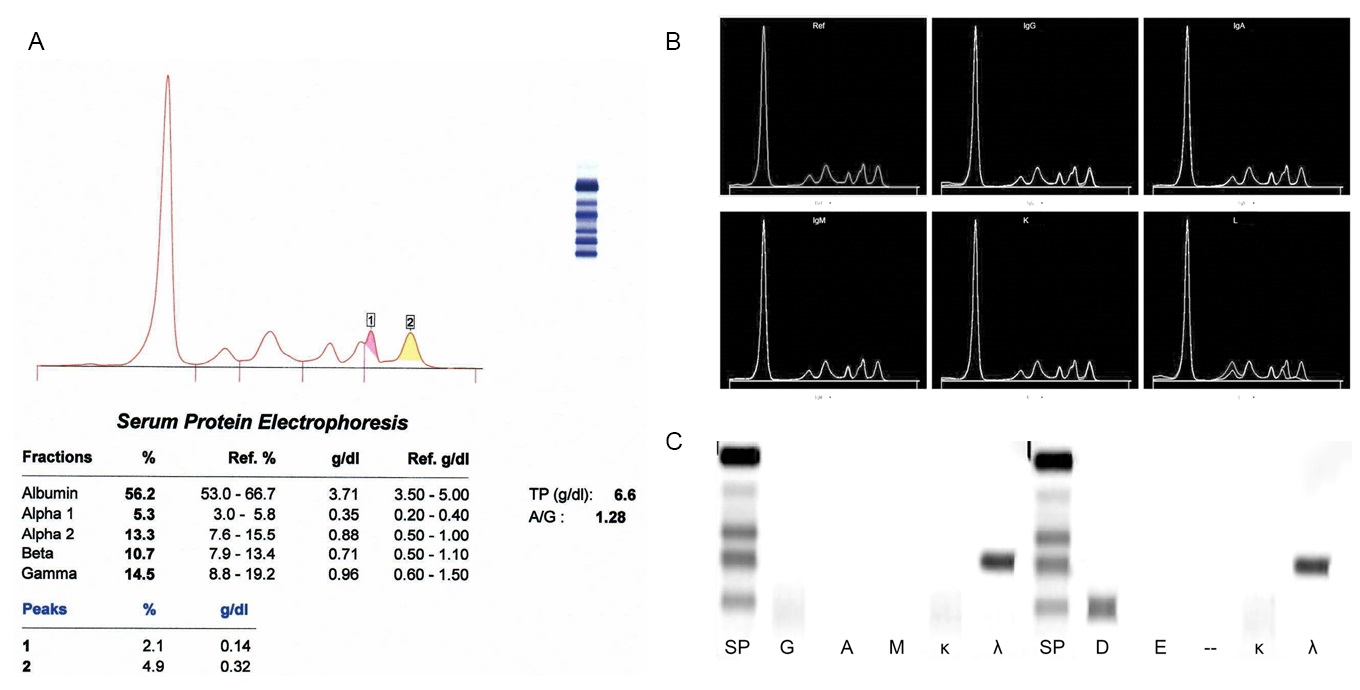
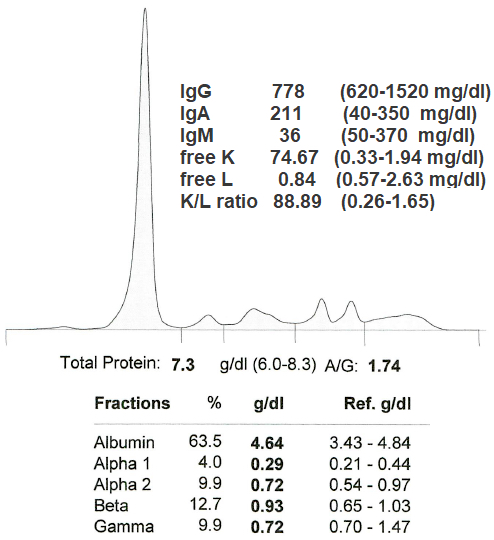
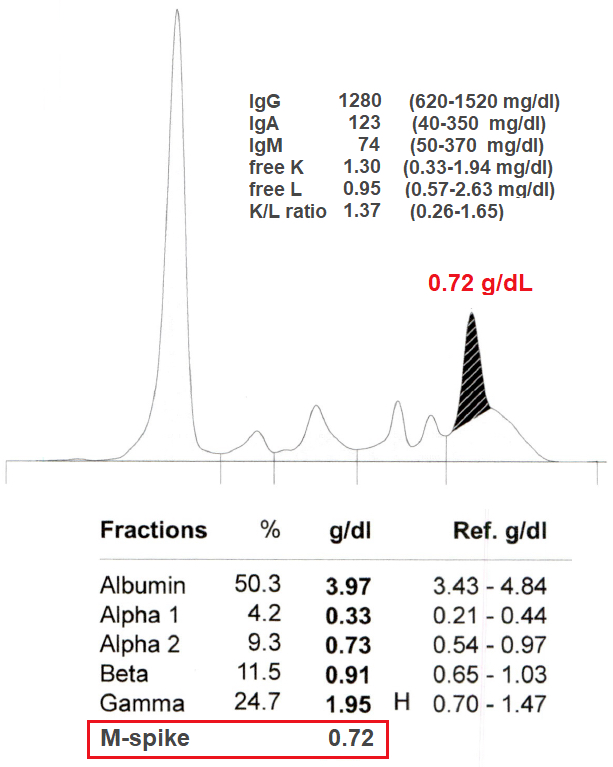
Monoclonal gammopathy testing
Natriuretic peptides (BNP and Amino-terminal proBNP)
Newborn screening
Parathyroid hormone
Pregnancy / menopause hormone levels (pending)
Renal function test panel (pending)
Rheumatoid arthritis (pending)
Saline suppression test
Screening thyroid disorders
Sensitivity and specificity (pending)
Sepsis biomarkers (pending)
Serum free light chain test
Syphilis (pending)
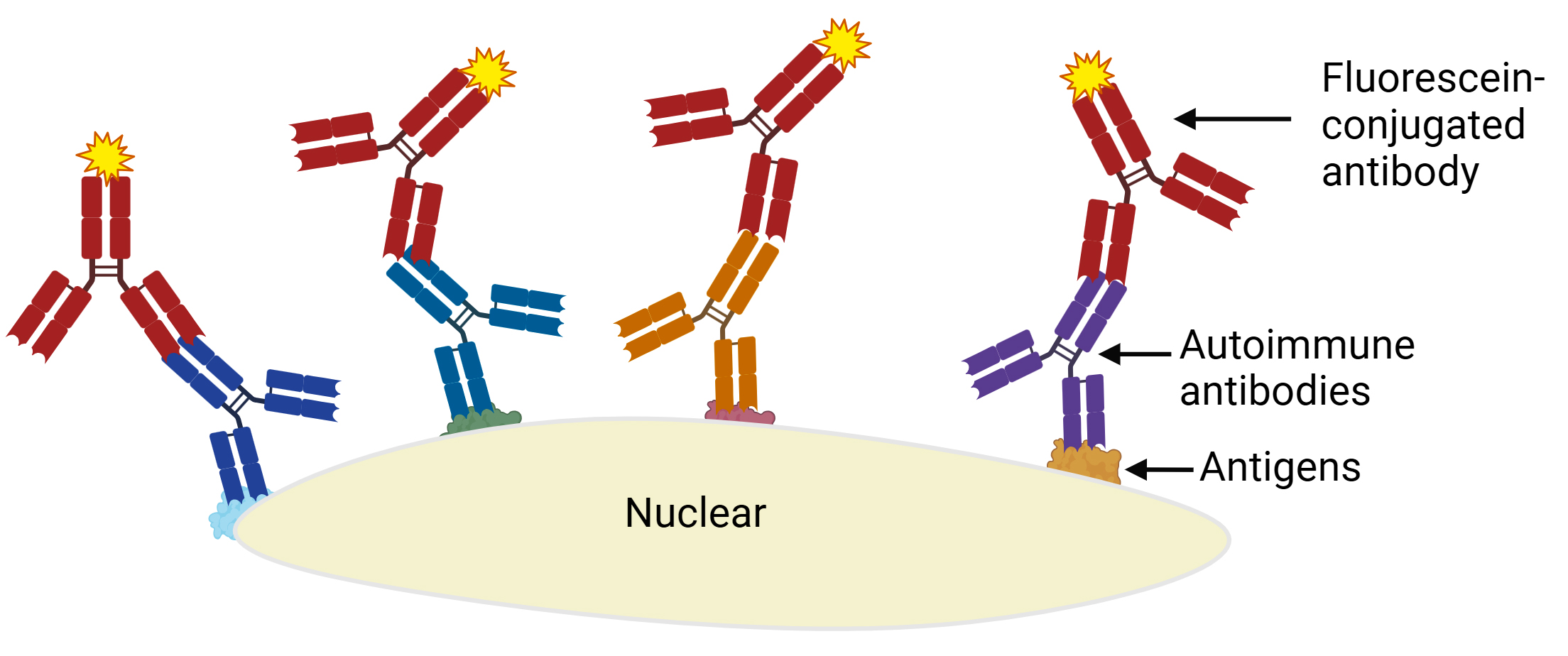
Systemic lupus erythematosus (SLE)
Therapeutic drug monitoring (pending)
Thyroglobulin
Thyroid autoantibodies
Thyroid function panel
Toxicology-general
Toxicology-general-part 2 (pending)
Transferrin
Tumor markers (pending)
Urinalysis (pending)
Urinalysis selection (pending)
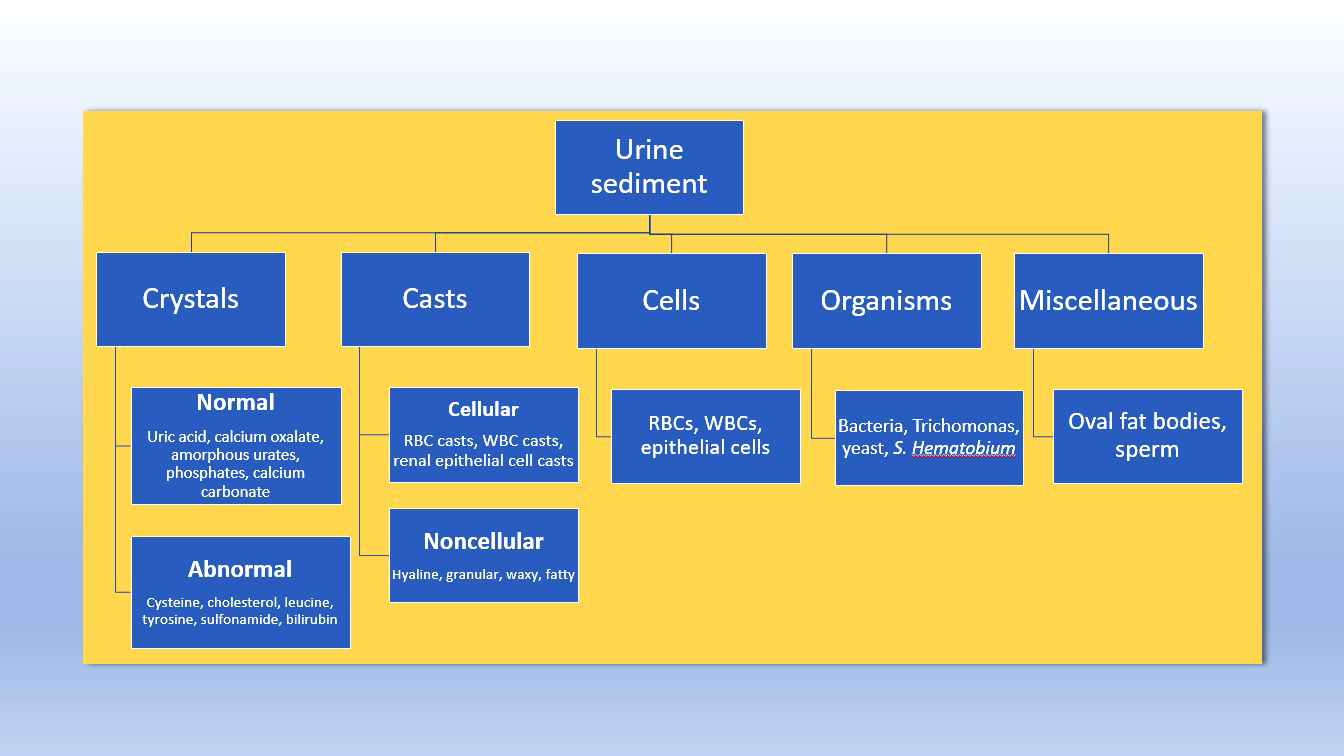
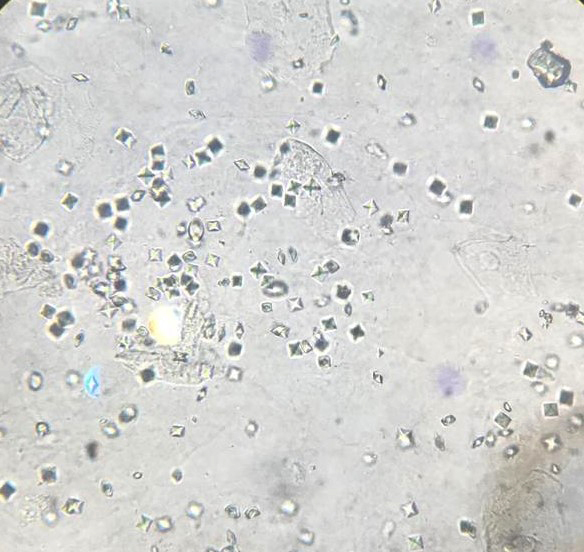
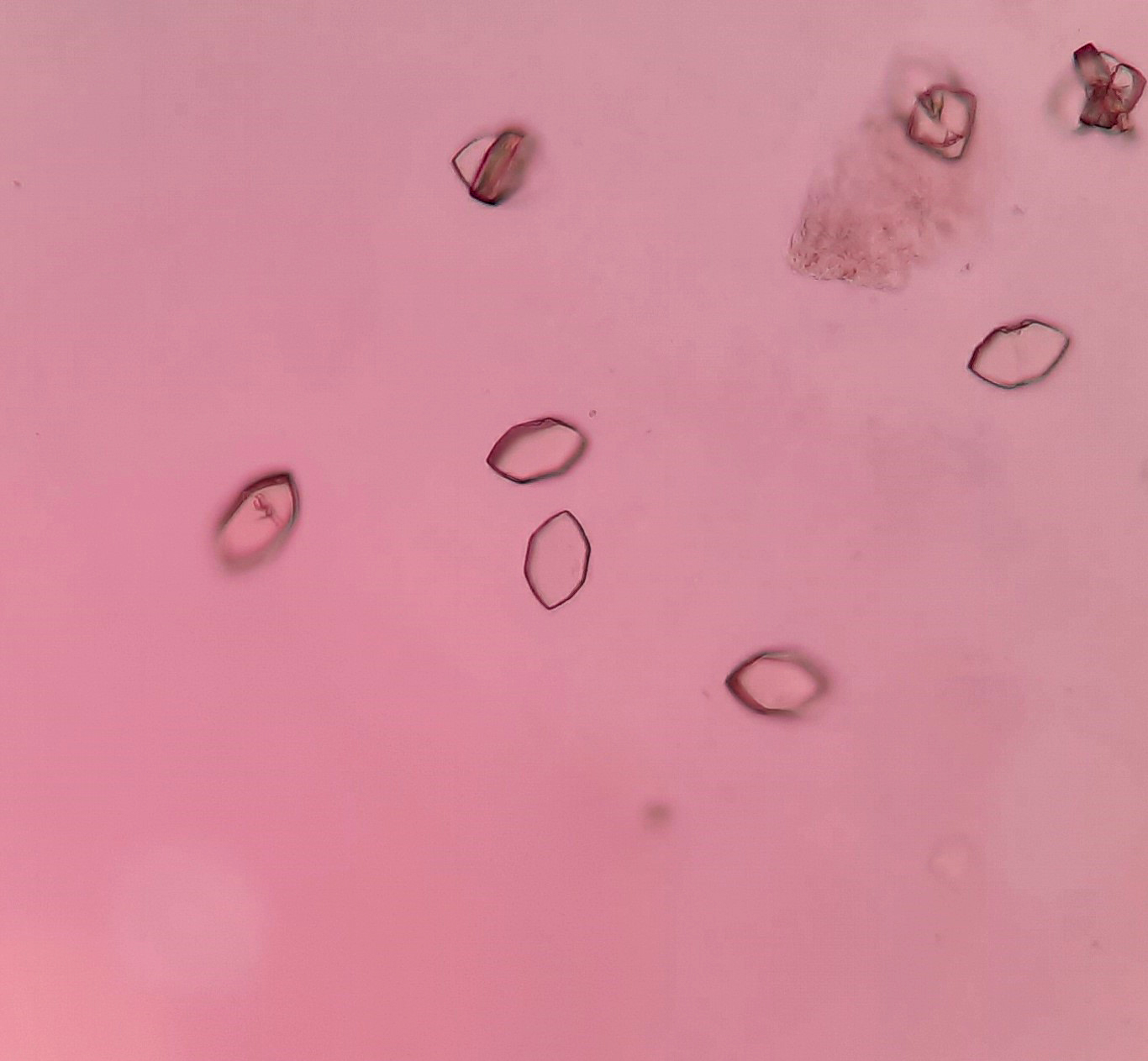
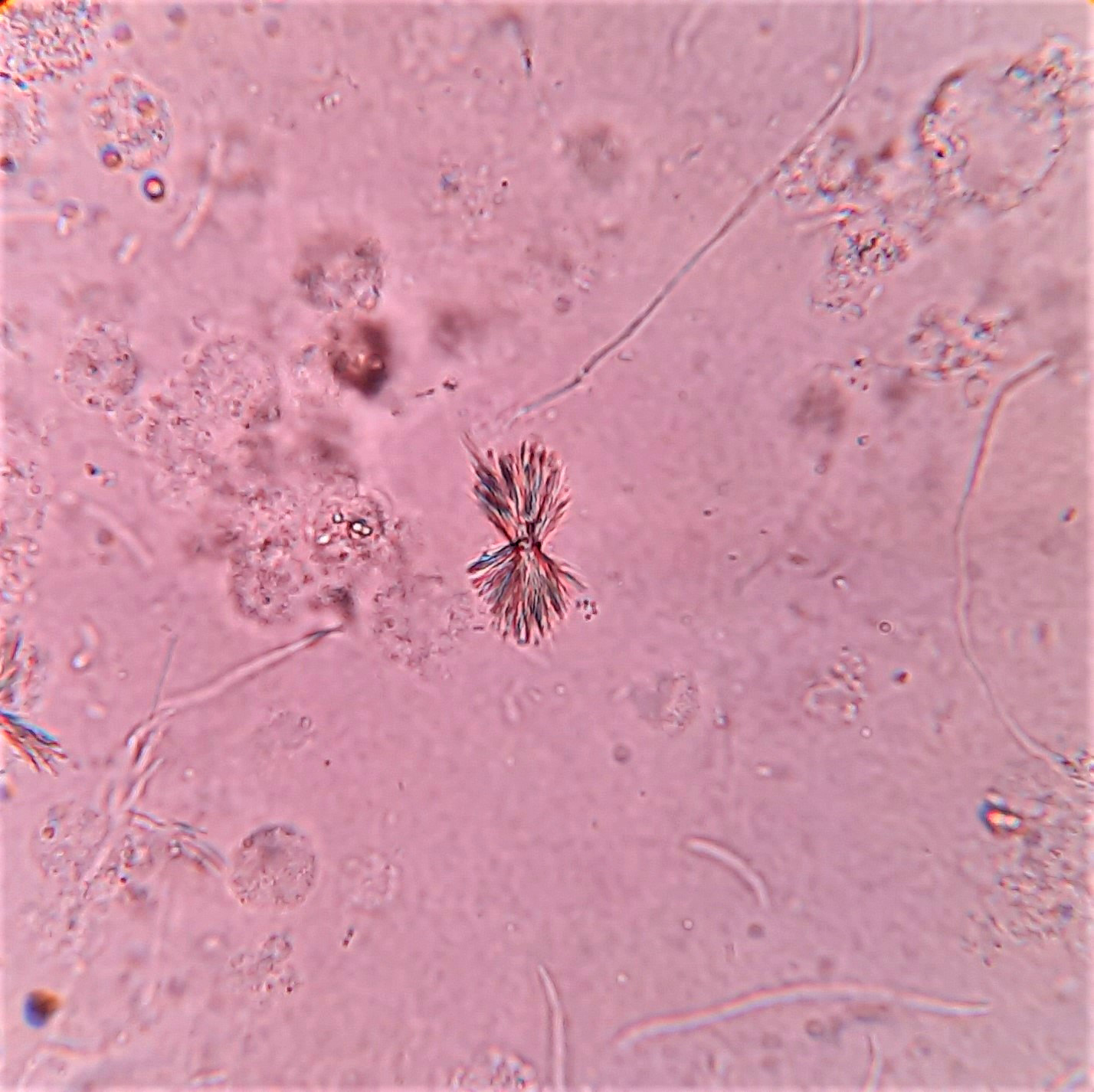
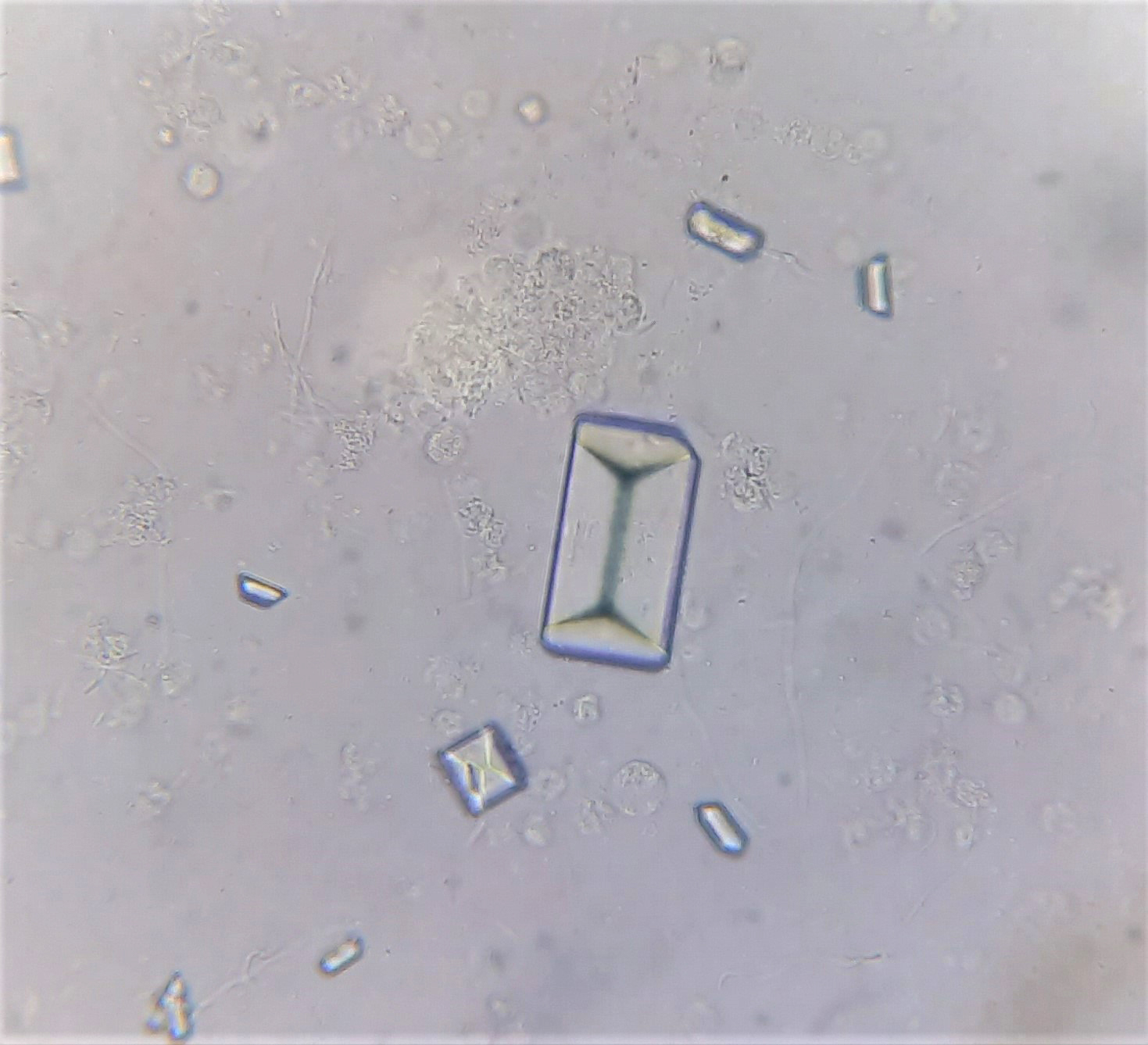
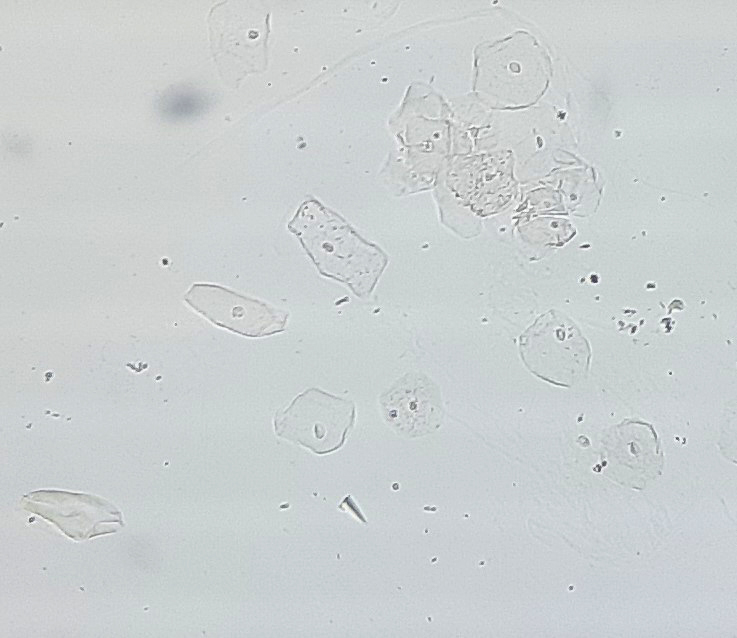
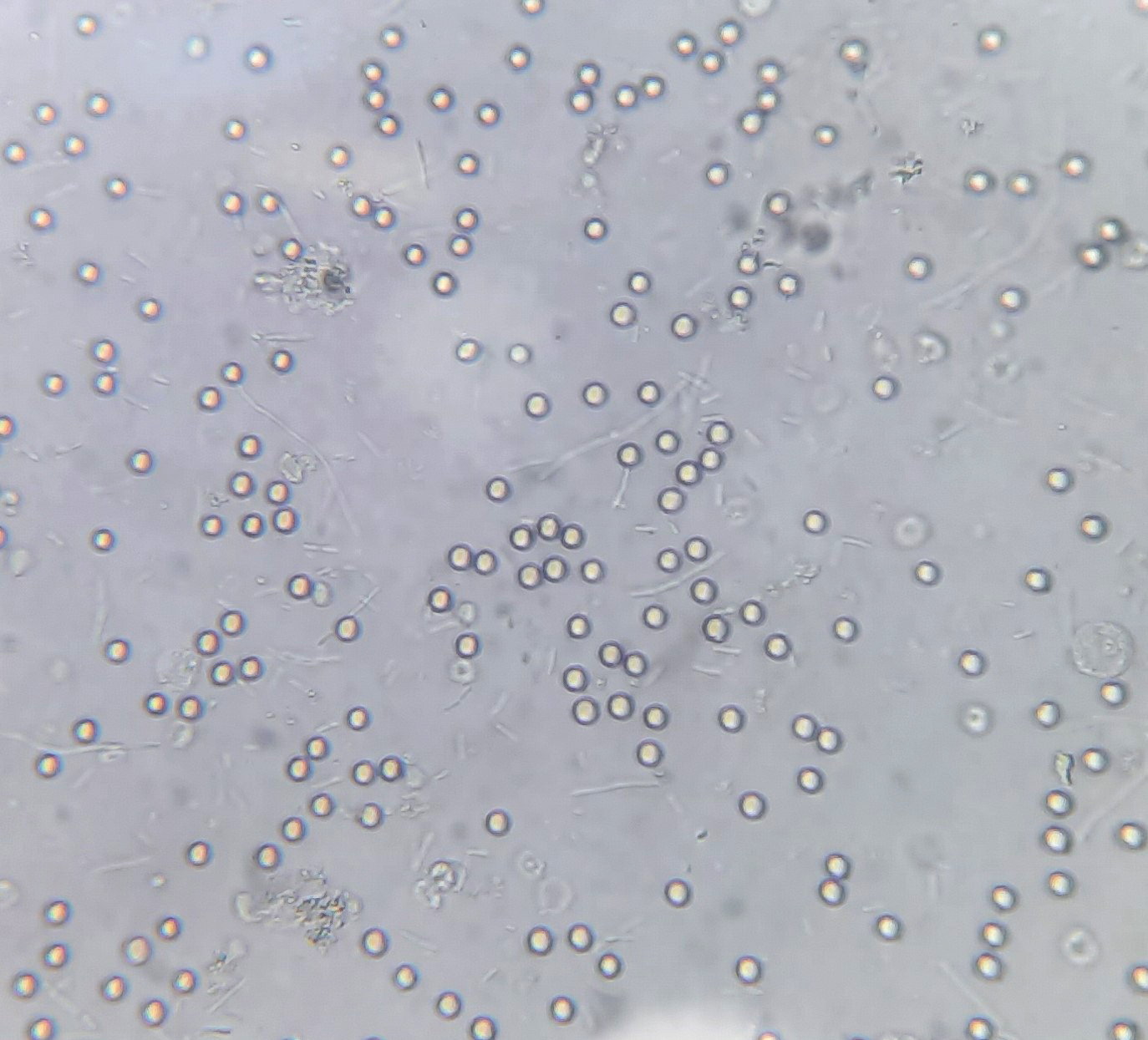
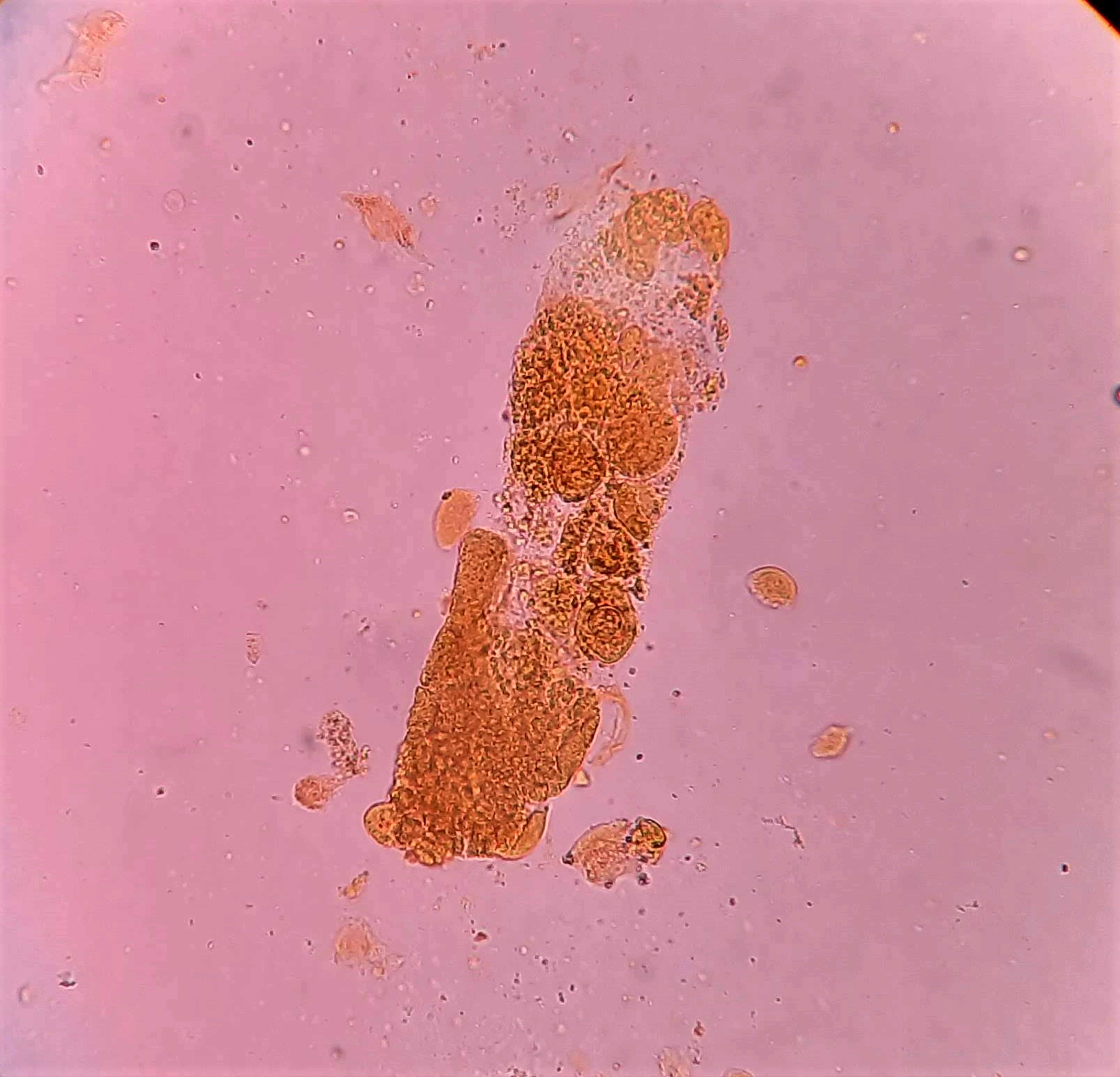
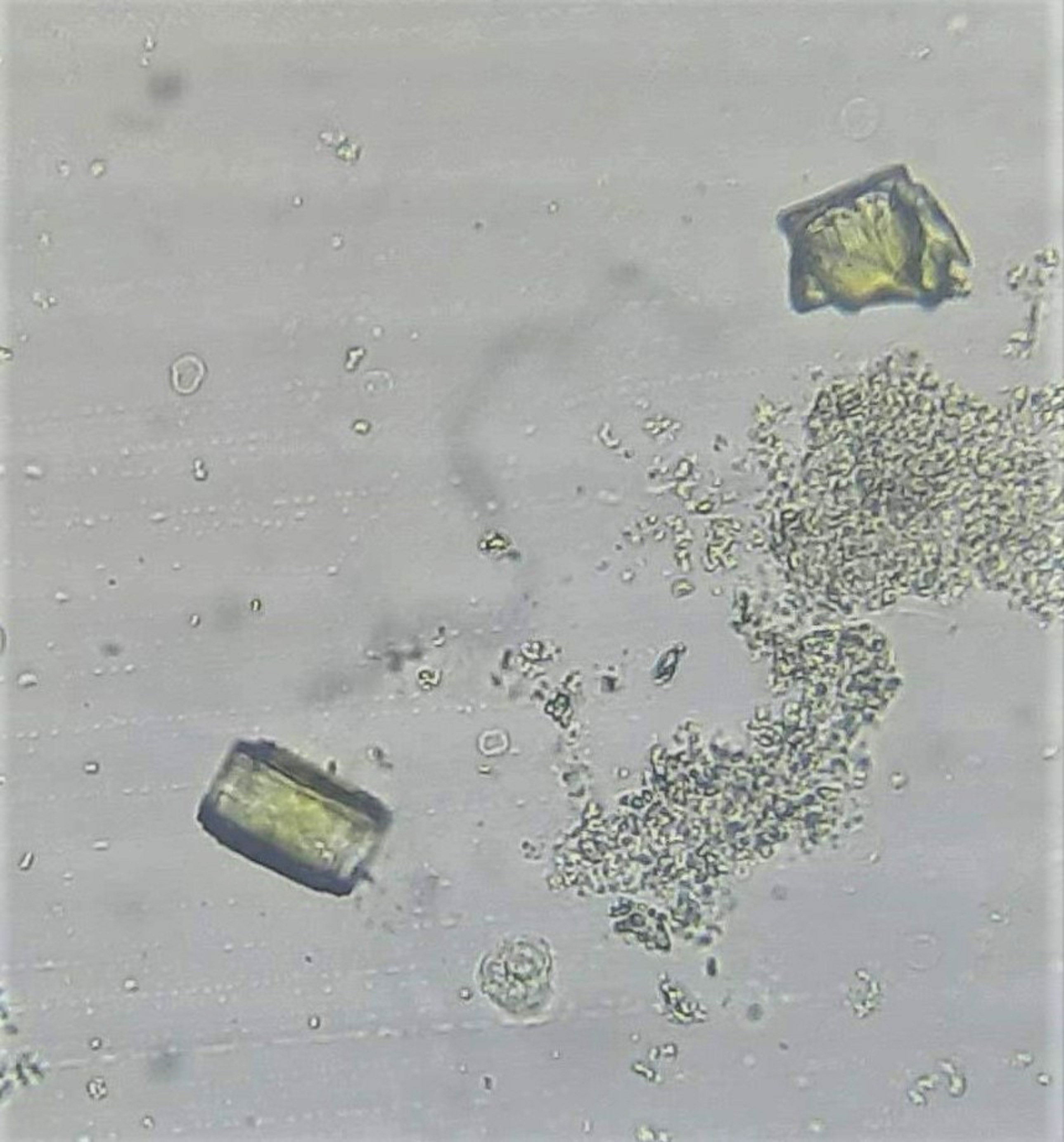
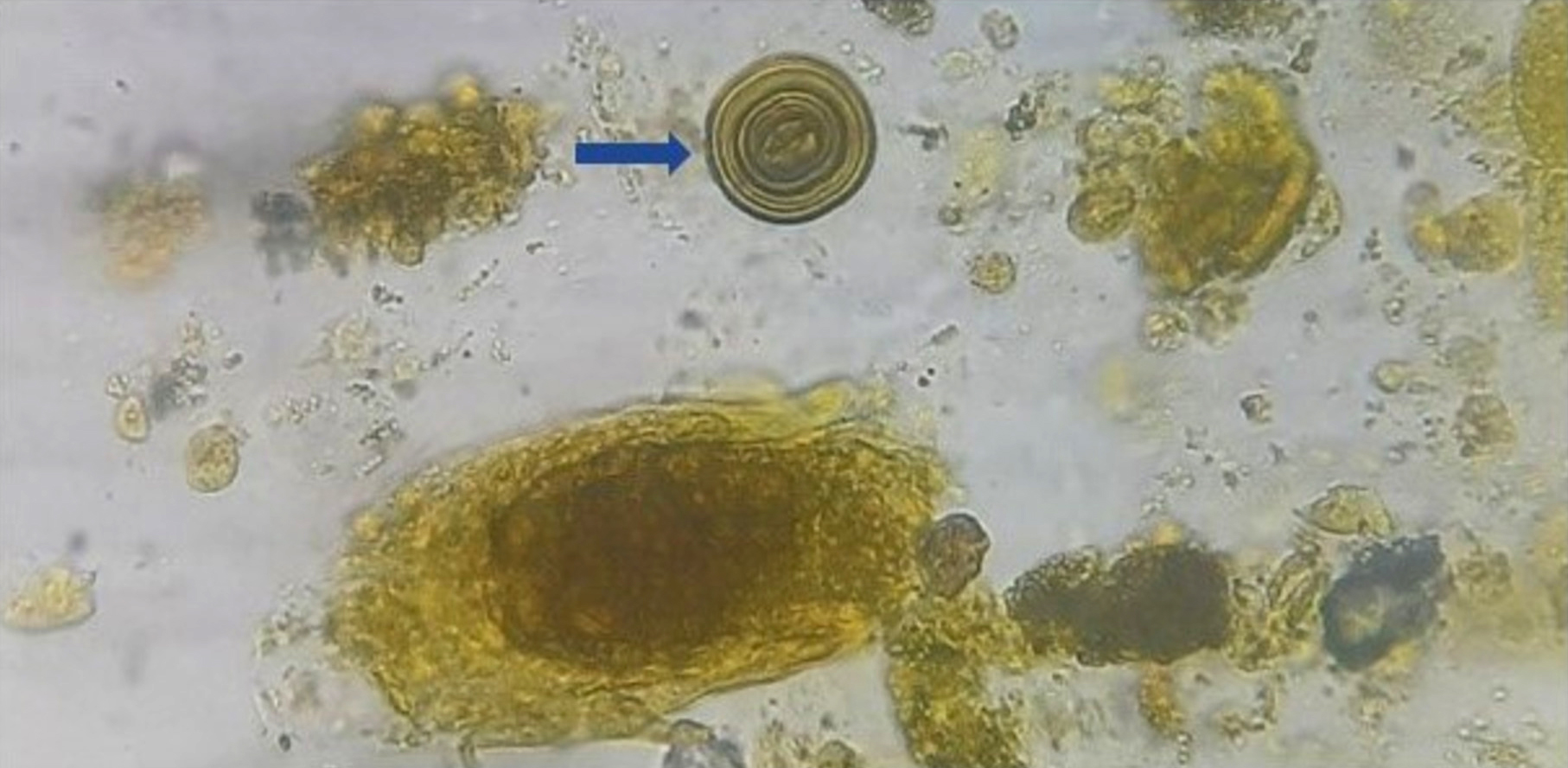
Urine crystals & microscopy
Utilization management strategies
Utilization management strategies (pending)
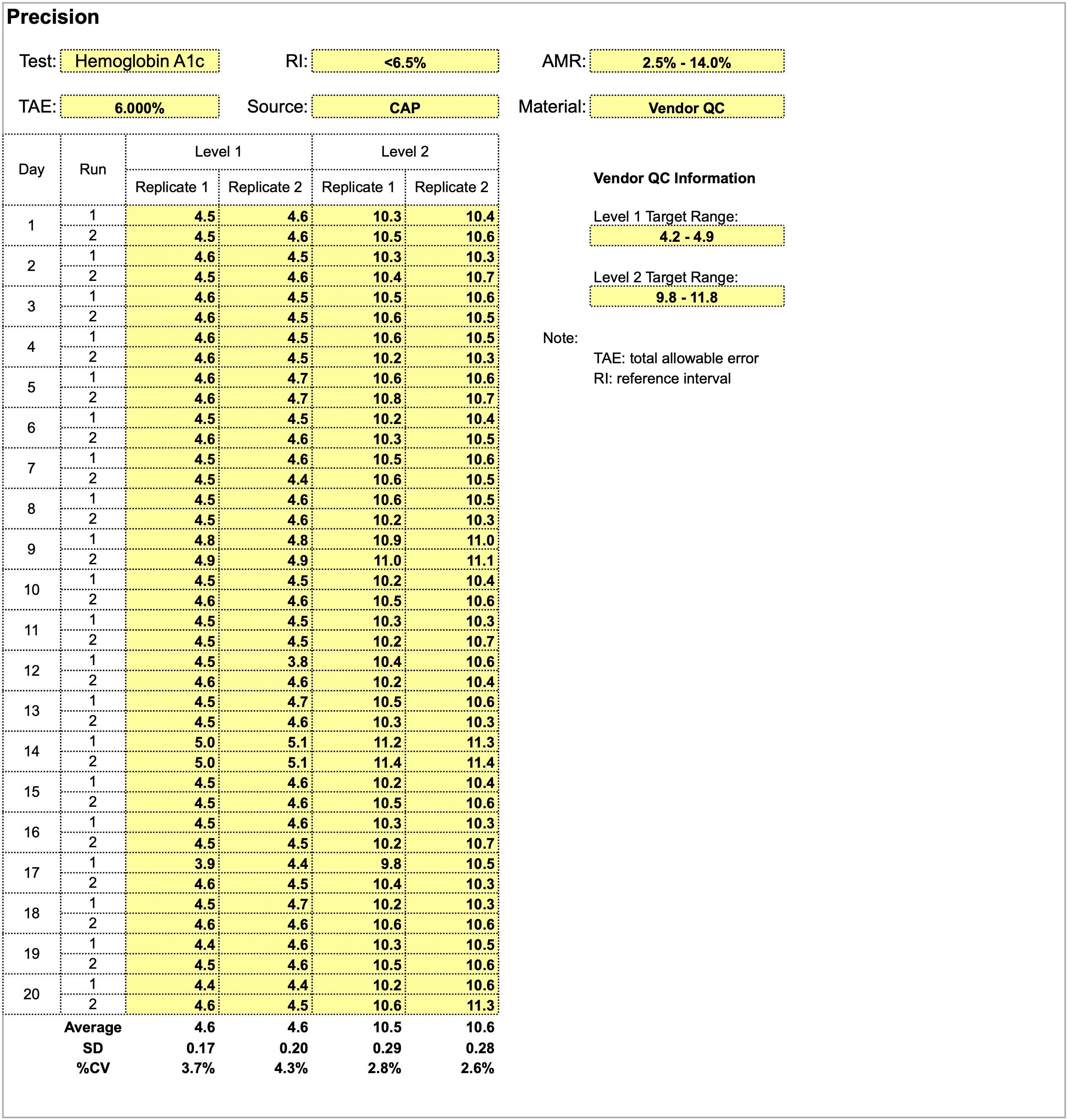
Validating a new quantitative assay
Vitamin B12 (pending)
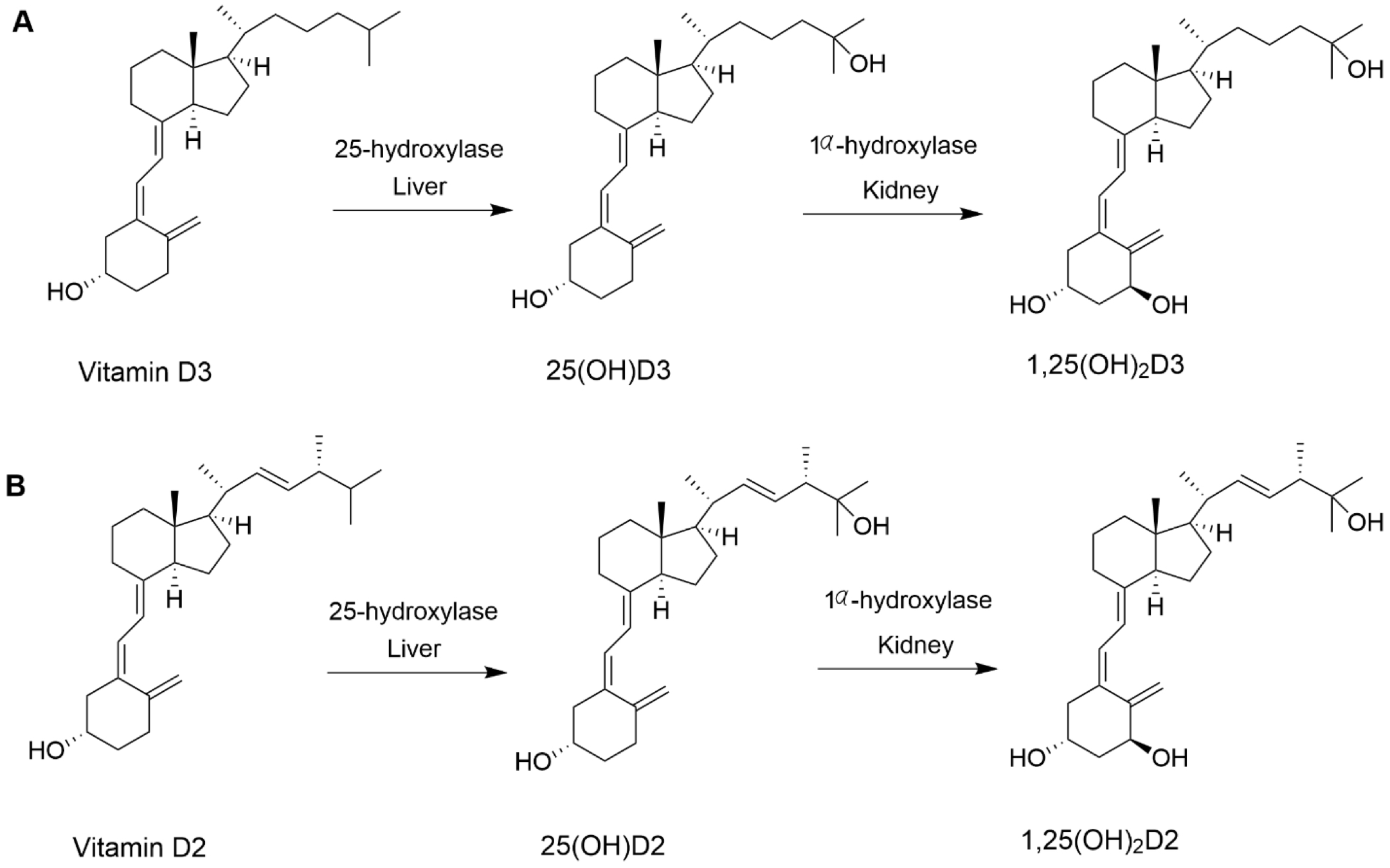
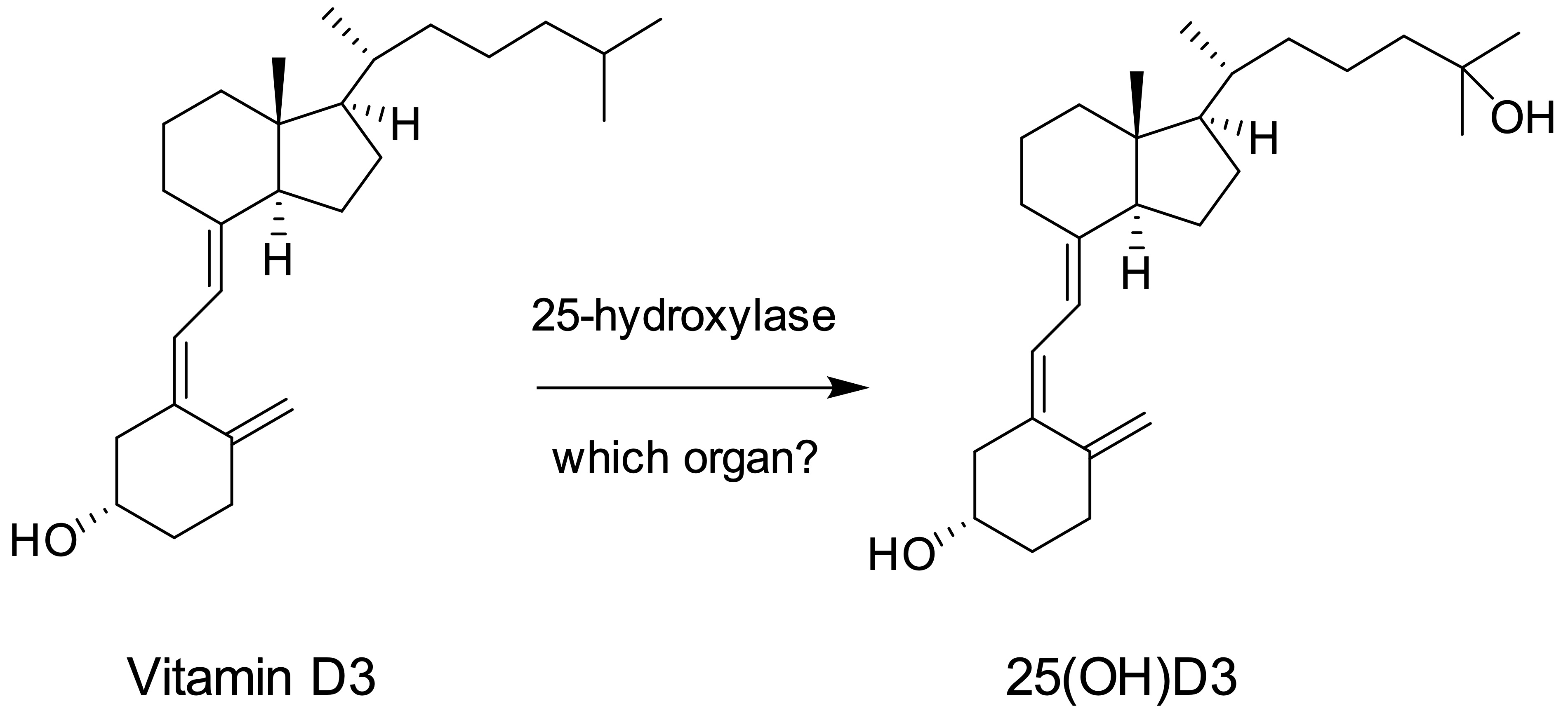
Vitamin D
lactate dehydrogenaseAdrenal insufficiency-diagnosis
Table of Contents
Definition / general | Essential features | Diagrams / tables | Clinical features | Laboratory diagnosis | Additional testing for primary adrenal insufficiency | Factors that can impact test results | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Deficiency in the production of glucocorticoids (e.g., cortisol) due to a disorder of the adrenal gland (primary adrenal insufficiency), inadequate pituitary adrenocorticotrophin hormone (ACTH; secondary adrenal insufficiency) or suppression of ACTH due to decreased corticotrophin releasing hormone (CRH; tertiary adrenal insufficiency)
Essential features
- Clinical presentation of adrenal insufficiency is nonspecific; therefore, diagnosis is confirmed via laboratory testing
- Low basal cortisol levels are indicative of insufficiency; abnormal response on the ACTH stimulation test is confirmatory
- Concurrent high or low pituitary ACTH levels can differentiate between primary and secondary / tertiary adrenal insufficiency, respectively
- Additional laboratory work up is needed to confirm the etiology of primary adrenal insufficiency; the most common causes are autoimmune adrenalitis in adults and congenital adrenal hyperplasia in children
Diagrams / tables
Clinical features
- Clinical symptoms are often vague and nonspecific
- Weight loss, anorexia, nausea and vomiting, postural dizziness, headaches, weakness, fatigue, hyponatremia, hypoglycemia, muscle cramps and abdominal pain
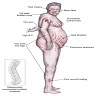
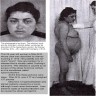
- Primary adrenal insufficiency only: skin hyperpigmentation, salt craving, hyperkalemia, postural hypotension and volume depletion
- Adrenal crisis: life threatening medical emergency mainly seen in primary adrenal insufficiency patients or in secondary / tertiary adrenal insufficiency under conditions of severe physiologic stress
- Features: severe hypotension and shock, often accompanied by loss of consciousness
- Reference: Lancet 2021;397:613
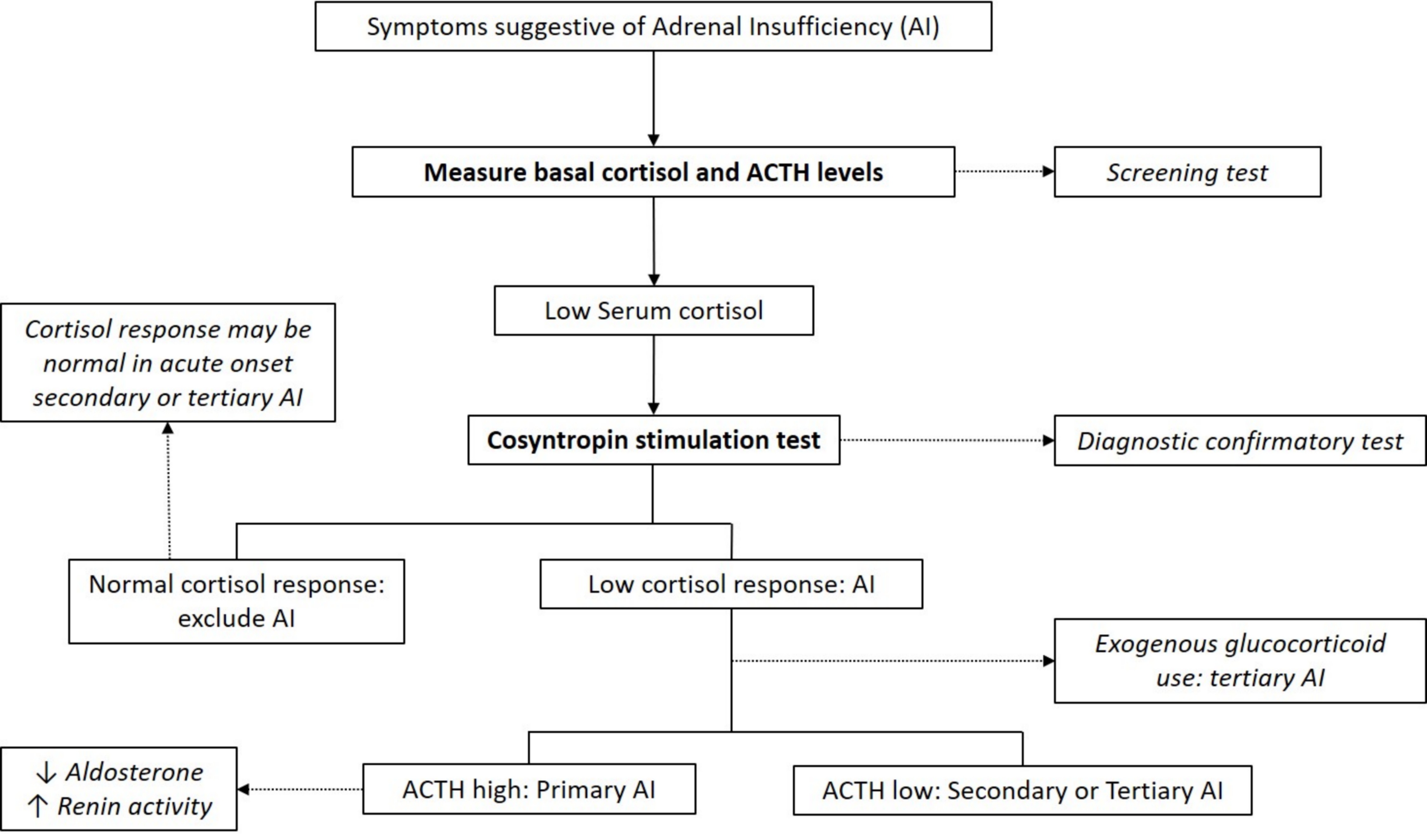
Laboratory diagnosis
- Cortisol typically peaks in the morning
- Early morning (8:00 AM) plasma or serum cortisol < 5 μg/dL (< 140 nmol/L) highly suggestive of adrenal insufficiency (J Clin Endocrinol Metab 2016;101:364)
- Basal cortisol > 14 μg/dl (> 400 nmol/L) on commonly used automated immunoassays excludes adrenal insufficiency (Clin Endocrinol (Oxf) 2017;86:177)
- ACTH levels in conjunction with low morning cortisol:
- High ACTH > 2 fold the upper reference limit → primary adrenal insufficiency (absence of feedback loop to pituitary gland)
- Low ACTH → secondary or tertiary adrenal insufficiency
- Note: serum / plasma cortisol measures total cortisol (i.e., cortisol bound to cortisol binding globulin [CBG] and albumin)
- Free cortisol can be measured in saliva and urine
- Salivary free cortisol can be utilized for screening
- Recommended for the diagnosis of critical illness related corticosteroid insufficiency (CIRCI) in critically ill patients (Crit Care Med 2017;45:2078)
- Urinary free cortisol commonly used for the diagnosis of Cushing syndrome (hypercorticolism); not recommended for adrenal insufficiency testing
- ACTH stimulation test (cosyntropin stimulation test)
- Diagnostic gold standard test for primary adrenal insufficiency
- Cosyntropin: synthetic form of the biologically active region of ACTH
- Standard dose: 250 μg for adults and children ≥ 2 years of age, 125 μg for children < 2 years of age and 15 μg/kg for infants
- Cortisol levels measured at baseline and 30 and 60 minutes after cosyntropin administration
- Peak cortisol levels < 18 μg/dL (500 nmol/L) at 30 or 60 minutes confirms adrenal insufficiency diagnosis (J Clin Endocrinol Metab 2016;101:364)
- Low dose cosyntropin stimulation test (1 μg) not recommended
- Note:
- Test cannot differentiate between primary and secondary adrenal insufficiency
- Stimulated cortisol levels may appear normal in cases of acute onset secondary or tertiary adrenal insufficiency (e.g., post recent pituitary / brain surgery or trauma)
- Diagnostic gold standard test for primary adrenal insufficiency
- Multiday ACTH stimulation test
- Can differentiate between primary and secondary (or tertiary) adrenal insufficiency
- Rationale:
- Chronic ACTH deficiency in secondary / tertiary adrenal insufficiency leads to adrenocortical atrophy and decreased responsiveness to stimulation
- This results in an absent or low cortisol response 30 - 60 minutes after cosyntropin / Synacthen administration
- Can be overcome by sustained ACTH stimulation, from 8 hours to 5 days
- Dosage: single intramuscular or intravenous dose, 250 μg/day
- Insulin tolerance test (ITT)
- Can differentiate primary from secondary adrenal insufficiency
- Can also identify acute onset central adrenal insufficiency not detected by the ACTH stimulation test
- Rationale: hypoglycemia is a potent stressor for activation of the hypothalamus pituitary adrenocortical axis
- IV insulin (0.1 U/kg body weight) administered after overnight fasting, with samples collected at baseline and variable intervals for up to 120 minutes
- Hypoglycemia confirmed by > 50% reduction in glucose concentration below baseline or at concentration < 45 mg/dL
- Low cortisol (post ITT induced hypoglycemia) confirms adrenal insufficiency
- Concurrent high ACTH → primary adrenal insufficiency; low ACTH → secondary or tertiary adrenal insufficiency
- Note: high risk test due to the sequelae of severe hypoglycemia
- Metyrapone test
- Metyrapone: blocks the conversion of 11-deoxycortisol to cortisol, causing decreased cortisol while increasing ACTH and 11-deoxycortisol
- No increase in ACTH and 11-deoxycortisol confirms adrenal insufficiency
- Dosage: 30 mg/kg body weight
- Administered at midnight and levels of cortisol, ACTH and 11-deoxycortisol measured next morning at 8:00 AM
- Note: can precipitate an adrenal crisis due to aggravated hypocortisolemia
- CRH stimulation test
- Can differentiate between secondary and tertiary adrenal insufficiency
- Not recommended in routine practice
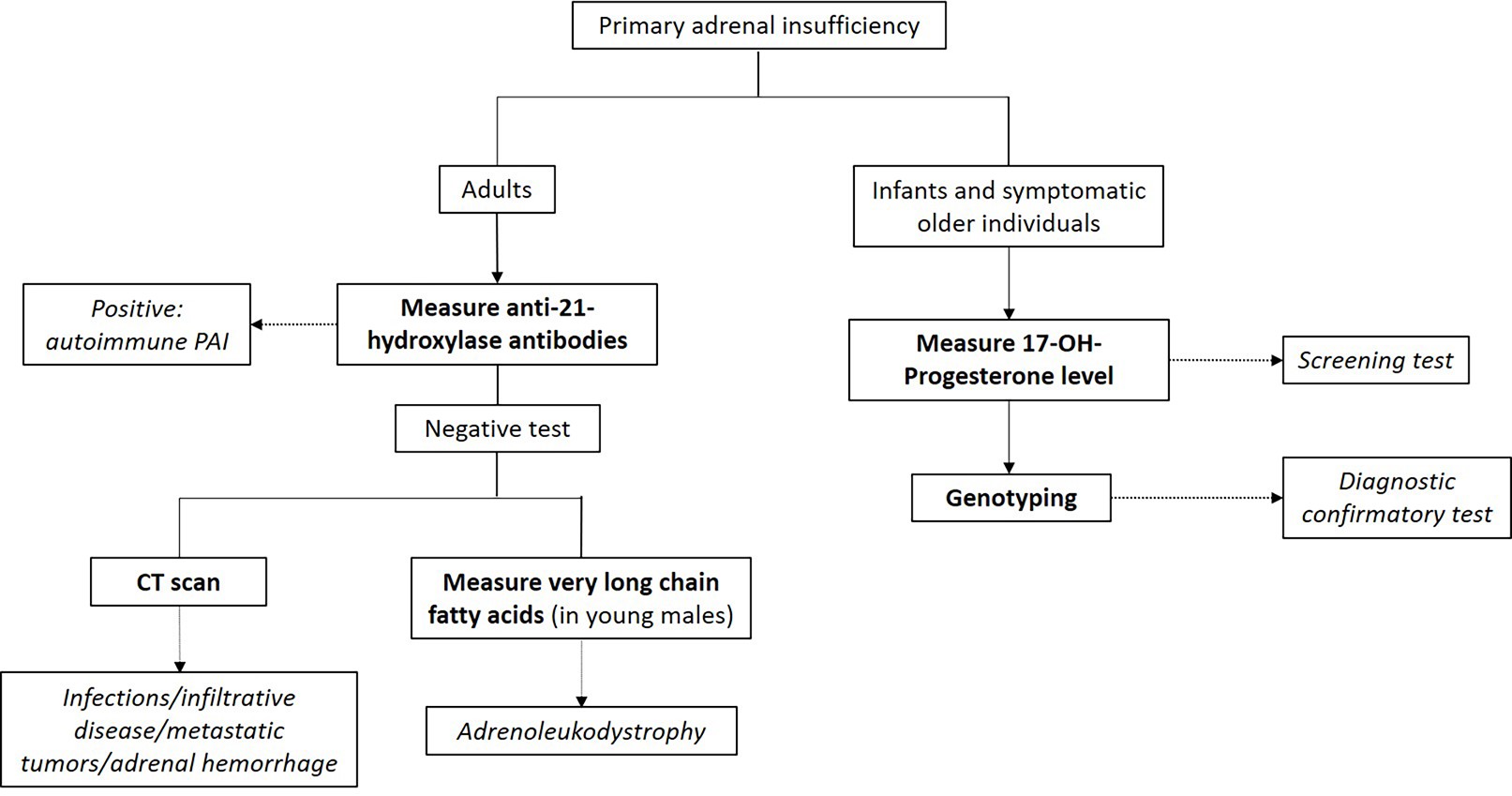
Additional testing for primary adrenal insufficiency
- Mineralocorticoid (body salt balance) function evaluation
- Tests for serum electrolytes, aldosterone levels and plasma renin concentration / activity
- Hyponatremia, hyperkalemia, decreased aldosterone and increased renin concentration (or activity) indicates primary adrenal insufficiency diagnosis
- Autoantibodies against 21-hydroxylase
- Autoimmune primary adrenal insufficiency: most common cause of primary adrenal insufficiency in the western world
- Congenital adrenal hyperplasia (CAH) testing
- CAH: autosomal recessive disorder of defective steroidogenesis
- Most cases are due to 21-hydroxylase deficiency (95%), with 11-hydroxylase deficiency accounting for the majority of the remaining cases
- Screening: elevated 17-hydroxyprogesterone
- Second tier screening: 17-hydroxyprogesterone measured by liquid chromatography-mass spectrometry (LC-MS); if not available, cosyntropin stimulation test should be performed (J Clin Endocrinol Metab 2018;103:4043)
- Basal or cosyntropin stimulated 17-hydroxyprogesterone > 1,000 ng/dL is diagnostic
- Usually > 5,000 ng/dL in classic CAH
- 21-deoxycortisol:
- Due to 21-hydroxylase deficiency (in CAH), accumulated 17-hydroxyprogesterone is converted to 21-deoxycortisol by 11-hydroxylase
- Can be used for CAH screening
- More specific than 17-hydroxyprogesterone as a marker for 21-hydroxylase deficiency CAH (J Pediatr 2021 Mar;230:161)
- Virtually absent in normal patients and in CAH cases caused by 11-hydroxylase deficiency
- Confirmatory test: genotyping
Factors that can impact test results
- Levels of CBG: total cortisol can appear to be falsely elevated or decreased due to a corresponding change in CBG levels (J Pediatr Endocrinol Metab 2018;31:107)
- Albumin: cortisol binds to albumin and therefore measured total cortisol can be affected by albumin concentration, albeit to a lesser extent than CBG
- Pregnancy: cortisol and CBG increase in pregnancy and may cause normal appearing morning cortisol levels; higher diagnostic thresholds for the cosyntropin stimulation test are indicated (Curr Opin Endocrinol Diabetes Obes 2017;24:184)
Additional references
Board review style question #1
A patient presents to the clinic with low basal cortisol, high ACTH, hypokalemia, hyperpigmentation and anti 21-hydroxylase antibodies. What is the patient's likely diagnosis?
- Adrenoleukodystrophy
- Autoimmune primary adrenal insufficiency
- Congenital adrenal hyperplasia
- Secondary adrenal insufficiency
Board review style answer #1
B. Autoimmune primary adrenal insufficiency. Low basal cortisol, high ACTH, hypokalemia and hyperpigmentation are all features of primary adrenal insufficiency. Autoantibodies against 21-hydroxylase are detected in 90% of patients with autoimmune adrenalitis.
Comment Here
Reference: Adrenal insufficiency-diagnosis
Comment Here
Reference: Adrenal insufficiency-diagnosis
Aldosterone
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Diagrams / tables | Pathophysiology | Clinical features | Test indications | Laboratory | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
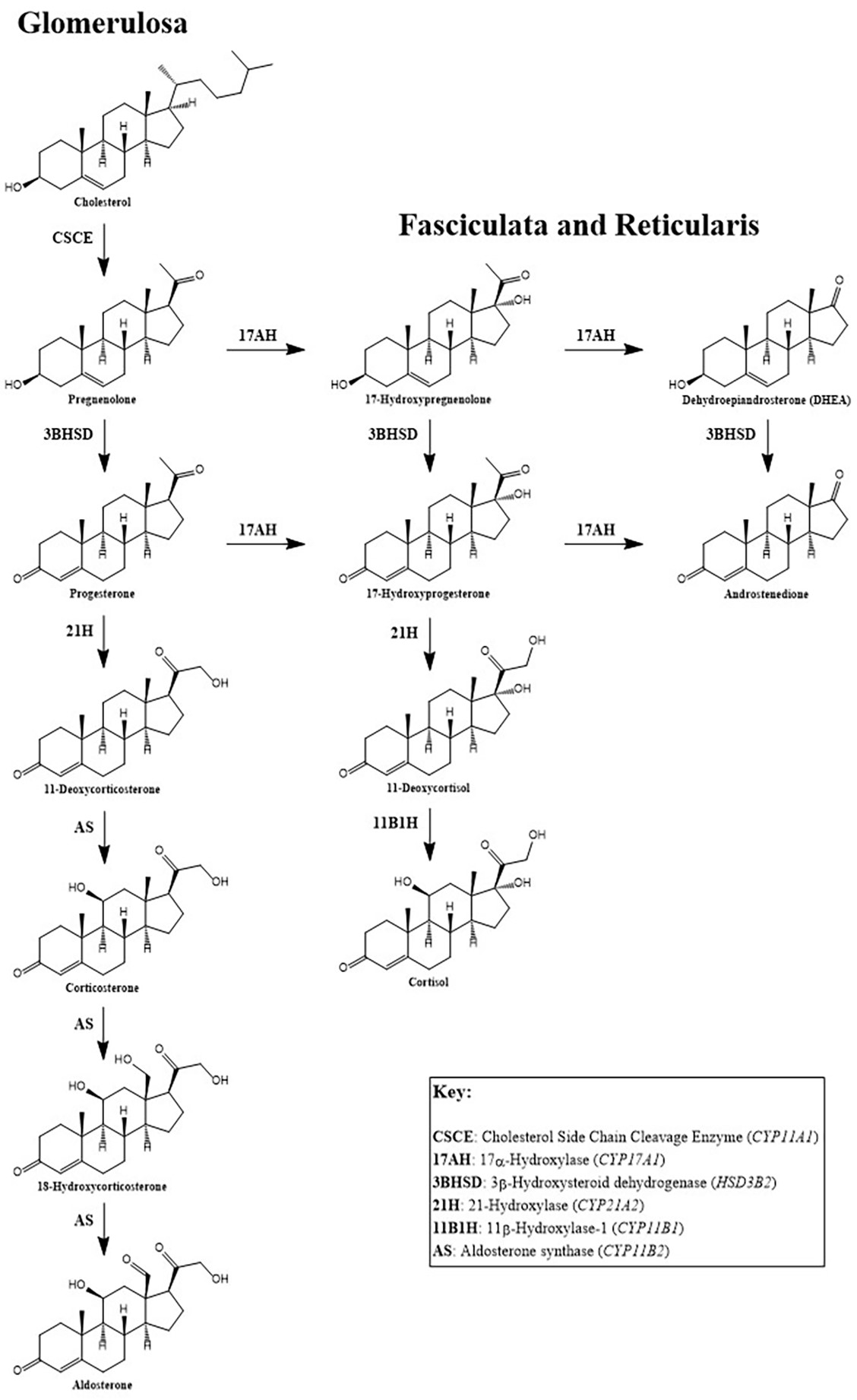
- Aldosterone is a mineralocorticoid hormone derived from cholesterol in the zona glomerulosa (the outer layer) of the adrenal cortex
Essential features
- Aldosterone helps control water and salt balance; production is controlled by the renin angiotensin aldosterone system (RAAS), adrenocorticotropic hormone (ACTH) and extracellular potassium concentration
- Most patients with hyperaldosteronism present with normokalemic hypertension; hypoaldosteronism should be considered in any patient with persistent hyperkalemia
- Adrenal adenoma and bilateral adrenal hyperplasia are the most common causes of hyperaldosteronism; causes of hypoaldosteronism include disorders that reduce aldosterone synthesis and cause aldosterone resistance
- Screening test is a concurrent measurement of plasma renin, aldosterone and calculation of aldosterone to renin ratio (ARR); confirmatory testing and subtype classification should be followed with positive screening results
- Many variables in preanalytical, analytical and postanalytical steps can affect plasma aldosterone and renin results; these factors should be considered when interpreting test results
Terminology
- Steroid hormone
- Mineralocorticoid hormone
ICD coding
Pathophysiology
- Aldosterone production is controlled by the RAAS in response to decreased renal perfusion and reduced tubular sodium content as well as increased ACTH and extracellular potassium concentration (Heart Fail Rev 2005;10:7)
- RAAS involves multiple organ systems, including the kidney, liver, lung, vasculature, adrenal cortex and brain
- Activation of juxtaglomerular (JG) cells causes the intracellular cleavage of prorenin to renin
- Mature renin is stored in the granules of the JG cells and is released into circulation upon stimulation (Annu Rev Physiol 2011;73:377)
- Circulating renin cleaves angiotensin, primarily synthesized and secreted by the liver, to form inactive angiotensin I
- Inactive angiotensin I is further cleaved to form active angiotensin II mediated by the angiotensin converting enzyme (ACE) expressed on plasma membranes of vascular endothelial cells in the lung (J Clin Pathol 1983;36:938)
- Binding of angiotensin II to the angiotensin II type I receptor (AT1R) causes the aldosterone mediated effects of vasoconstriction and sodium and water reabsorption (Blood Press 2003;12:70)
- Primary physiological function of aldosterone is to control the balance of water and salts in the kidney by sodium retention and potassium excretion
- Second action of aldosterone is to promote the excretion of hydrogen ions while retaining bicarbonate
Clinical features
Causes of hyperaldosteronism (Jameson: Harrison's Endocrinology, 3rd Edition, 2013, Eur J Endocrinol 2019;180:R45)
| Primary hyperaldosteronism (isolated excess production of aldosterone) | |
| Bilateral (micronodular) adrenal hyperplasia | 60% |
| Adrenal (Conn) adenoma | 40% |
| Glucocorticoid remediable aldosteronism (ACTH driven) | 1% |
| Secondary hyperaldosteronism (excessive activation of the RAAS) | |
| Renin producing tumor | |
| Renal artery stenosis | |
| Edematous disorders (e.g., heart failure, cirrhosis with ascites, nephrotic syndrome) | |
| Other rare causes (pseudoprimary aldosteronism due to exogenous aldosterone or enhanced mineralocorticoid activity) | |
| Syndrome of apparent mineralocorticoid excess | |
| Cushing syndrome | |
| Glucocorticoid resistance | |
| Adrenocortical carcinoma | |
| Congenital adrenal hyperplasia | |
| Progesterone induced hypertension | |
| Liddle syndrome | |
- Clinical manifestations of hyperaldosteronism
- Primary and secondary hyperaldosteronism present similarly but can be differentiated by laboratory tests and diagnostic studies
- Most patients present with normokalemic hypertension; hypokalemia can be only seen in the more severe cases (J Clin Endocrinol Metab 2016;101:1889)
Causes of hypoaldosteronism (adapted from UpToDate: Etiology, Diagnosis, and Treatment of Hypoaldosteronism [Accessed 8 August 2023], StatPearls: Hypoaldosteronism [Accessed 8 August 2023])
| Reduced aldosterone production | ||
| Hyporeninemic hypoaldosteronism | Kidney disease, most often diabetic nephropathy | Most common acquired causes |
| Nonsteroidal anti-inflammatory drugs | ||
| Calcineurin inhibitors | ||
| Angiotensin inhibitors (ACE inhibitors, angiotensin II receptor blockers and direct renin inhibitors) | ||
| Chronic heparin therapy | ||
| Primary adrenal insufficiency | Infrequent causes | |
| Severe illness | ||
| Inherited disorders | Congenital hypoaldosteronism (21 hydroxylase deficiency and isolated hypoaldosteronism) | |
| Pseudohypoaldosteronism type 2 (Gordon syndrome) | ||
| Aldosterone resistance | ||
| Inhibition of the epithelial sodium channel | Potassium sparing diuretics | |
| Antibiotics (trimethoprim and pentamidine) | ||
| Voltage defects | Markedly reduced distal sodium delivery | |
| Acquired or congenital defects in sodium reabsorption by the distal tubule principal cells (obstructive uropathy), systemic lupus erythematosus (SLE) and sickle cell disease | ||
| Inherited disorders | Pseudohypoaldosteronism type 1 | |
- Clinical manifestations of hypoaldosteronism
- Persistent hyperkalemia with no obvious cause
- Mild metabolic acidosis with a normal anion gap
Test indications
- Hyperaldosteronism (J Clin Endocrinol Metab 2016;101:1889)
- Resistant hypertension; blood pressure remains above 140/90 mm Hg on concurrent use of 3 antihypertensive agents (1 agent should be a diuretic) of different classes taken at maximally tolerated doses
- Controlled BP (< 140/90 mm Hg) on 4 or more antihypertensive drugs
- Hypertension and spontaneous or diuretic induced hypokalemia
- Hypertension and adrenal incidentaloma
- Hypertension and sleep apnea
- Hypertension and a family history of early onset hypertension or cerebrovascular accident at a young age (< 40 years)
- Hypertensive first degree relatives of patients with primary aldosteronism
- Hypoaldosteronism (UpToDate: Etiology, Diagnosis, and Treatment of Hypoaldosteronism [Accessed 8 August 2023], StatPearls: Hypoaldosteronism [Accessed 8 August 2023])
- Patients on medications that can impair aldosterone release (see Clinical features)
- Critically ill patients, patients with diabetes or nephropathies from various causes, patients with sickle cell disease or HIV infection
- Patients who undergo adrenalectomy for Conn syndrome (Surgery 2018;163:183)
Laboratory
- Hyperaldosteronism (J Clin Endocrinol Metab 2016;101:1889)
- Screening test of hyperaldosteronism is concurrent measurement of plasma renin activity (PRA) or direct renin concentration (DRC) and aldosterone concentration (PAC) and calculation of aldosterone to renin ratio (ARR)
- Confirmatory testing with a positive ARR screening result includes saline infusion, oral sodium loading, fludrocortisone suppression and captopril challenge
- For oral sodium loading, aldosterone is measured in the 24 hour urine collection
- Adrenal CT and adrenal vein sampling (AVS) are used for subtype classification of confirmed hyperaldosteronism
- AVS should be performed to lateralize adrenal mass when surgical treatment is feasible, with or without cosyntropin stimulation
- Cortisol corrected aldosterone ratio of high side to low side > 4:1 indicates unilateral aldosterone production
- Ratio of < 3:1 suggests bilateral aldosterone production (Surgery 2004;136:1227)
- Hypoaldosteronism
- PRA or PAC, PAC and serum cortisol can be used to differentiate different causes of hypoaldosteronism
Differential diagnosis of hyper or hypoaldosteronism based on laboratory tests (Endotext: Hyperaldosteronism [Accessed 8 August 2023], UpToDate: Etiology, Diagnosis, and Treatment of Hypoaldosteronism [Accessed 8 August 2023])
| Disease | Laboratory test | |||
| PAC | PRA or PRC | ARR | Serum cortisol | |
| Primary hyperaldosteronism | ↑ | ↓ | ↑ | x |
| Pseudoprimary hyperaldosteronism | ↓ | ↓ | / | x |
| Secondary hyperaldosteronism | ↑ | ↑ | ↑ | x |
| Hyporeninemic hypoaldosteronism | ↓ | ↓ | / | → |
| Primary adrenal insufficiency | ↓ | ↑ | / | ↓ |
| Congenital adrenal hyperplasia | ↓ | ↑ | / | → |
- Preanalytical requirements (J Clin Endocrinol Metab 2016;101:1889)
- Normalize serum potassium
- Unrestricted dietary salt intake
- Withdraw interfering medications that markedly or moderately affect the ARR if possible or commence medications that minimally affect the ARR
- Collect blood samples midmorning after the patient has been up (sitting, standing or walking) for a minimum of 2 hours and then seated for 5 - 15 min prior to collection
- For either renin mass or activity, it is recommended that samples are collected and processed at room temperature (not chilled), followed by rapid freezing of separated plasma at -20 °C to prevent cryoactivation
- Cryoactivation may result in a false elevation of renin mass or activity
| Medications that markedly affect the ARR (withdraw for at least 4 weeks) | Spironolactone, eplerenone, amiloride and triamterene |
| Potassium wasting diuretics | |
| Products are derived from licorice root | |
| Medications that moderately affect the ARR (withdraw for at least 2 weeks) | Beta adrenergic blockers, central alpha 2 agonists (e.g., clonidine, alpha methyldopa) and nonsteroidal anti-inflammatory drugs |
| Angiotensin converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors and dihydropyridine calcium channel antagonists | |
| Medications that minimally affect ARR | Verapamil slow release, hydralazine, prazosin, doxazosin, terazosin |
- Analytical limitations (Clin Biochem 2015;48:377)
- DRC assay crossreactivity with prorenin is most significant in the low renin state
- Lack of assay standardization is a common problem with both CLIA and LC MS / MS assay for aldosterone and renin measurement
- Postanalytical considerations
- PRA: reporting units of ng/mL/h is most commonly used
- Plasma aldosterone concentration (PAC): reporting patient posture and providing posture specific (supine and upright) reference intervals
- ARR cutoff values depend on assay and reporting units; numbers with asterisks indicate the most commonly adopted cutoff values (J Clin Endocrinol Metab 2016;101:1889)
| PRA, ng/mL/h | PRA, pmol/L/min | DRC, mU/L | DRC, ng/L | |
| PAC (as ng/dL) | 20 | 1.6 | 2.4 | 3.8 |
| 30* | 2.5 | 3.7 | 5.7 | |
| 40 | 3.1 | 4.9 | 7.7 | |
| PAC (as pmol/L) | 750* | 60 | 91 | 144 |
| 1000 | 80 | 122 | 192 |
- Comparison studies of platforms or methodologies
- Methodology to measure aldosterone and renin assay
- Assays have evolved from radioimmunoassay (RIA) and chemiluminescent immunoassay (CLIA) to liquid chromatography tandem mass spectrometry (LC MS / MS) assay
- CLIA is commonly used on automated instruments; the results from RIA and CLIA are shown to be comparable (J Hypertens 2016;34:920, Prague Med Rep. 2021;122:80)
- LC MS / MS method is available in large reference laboratories
- Aldosterone was substantially lower by LC MS / MS than immunoassay, presumably due to antibody crossreactivity with structurally similar metabolites in immunoassay but other contributing factors may also play a role (J Endocr Soc 2022;6:bvac049)
- Clinicians should be aware of assay characteristics
- As the current diagnostic cutoffs are from previous studies based on RIA and CLIA, there is a need to update clinical guidelines to reflect the differences between CLIA and modern LC MS / MS assays
- Methodology to measure aldosterone and renin assay
- Different renin assays (PRA versus DRC)
- PRA assay measures the concentration of angiotensin I produced
- DRC assay directly measures renin mass (concentration)
- For most patients, both assays give comparable information; however, renin activity and mass assays may give conflicting results in patients taking direct renin inhibitors and in patients with significantly high renin activity, high estrogen states (e.g., pregnancy) or congestive heart failure (Pract Lab Med 2021;25:e00229)
Board review style question #1
Which of the following is commonly associated with hyperaldosteronism?
- Adrenal nodules
- Easily managed hypertension
- High plasma potassium
- Hypertension after 50 years of age
- Increased plasma sodium
Board review style answer #1
A. Adrenal nodules. Bilateral (micronodular) adrenal hyperplasia is the most common cause of hyperaldosteronism. Answer B is incorrect because resistant hypertension is presented in hyperaldosteronism. Answer D is incorrect because early onset hypertension before 40 years old is a clinical indication of hyperaldosteronism. Answer C is incorrect because most patients with hyperaldosteronism present with normokalemic hypertension. Answer E is incorrect because plasma sodium tends to be normal due to the concurrent fluid retention.
Comment Here
Reference: Aldosterone
Comment Here
Reference: Aldosterone
Board review style question #2
Which of the following factors can cause misinterpretation of aldosterone, renin or the aldosterone to renin ratio (ARR)?
- Interferences with the testing methods
- Patients without restriction on dietary salt intake
- Sample collected and processed at room temperature, followed by rapid freezing
- Upright position during blood collection
- Use of selective serotonin reuptake inhibitors (SSRIs) prior to testing
Board review style answer #2
A. Interferences with the testing methods. Assay specific interferences can cause erroneous results. Answer E is incorrect because SSRIs can affect metanephrines but are not known to affect aldosterone or renin. Answer B is incorrect because the endocrinology practice guideline does not recommend dietary salt restriction. Sodium restriction can significantly raise plasma renin activity (PRA), normalize the ARR and could cause falsely negative screening results (J Clin Endocrinol Metab 2016;101:3989). Answer D is incorrect because samples should be collected after the patient has been up (sitting, standing or walking) for a minimum of 2 hours. Answer C is incorrect because the sample should be collected and processed at room temperature and separated plasma should be rapidly frozen to prevent cryoactivation and false elevation of renin.
Comment Here
Reference: Aldosterone
Comment Here
Reference: Aldosterone
Allergy testing (pending)
[Pending]
Anion gap (pending)
[Pending]
Anticardiolipin antibodies
Aspartate aminotransferase
Terminology
- Previously known as Serum Glutamic Oxaloacetic Transaminase (SGOT)
- Transaminase classification EC 2.6 (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1)
- Distinct from ALanine aminoTransferase (ALT), another hepatic aminotransferase previously known as serum glutamic pyruvic transaminase (SGPT)
Pathophysiology
- Ubiquitously distributed in tissues
- Has different specific activity (rate of NADH oxidation per gram of protein) in red cells, heart, liver, muscle, brain, kidneys and placenta
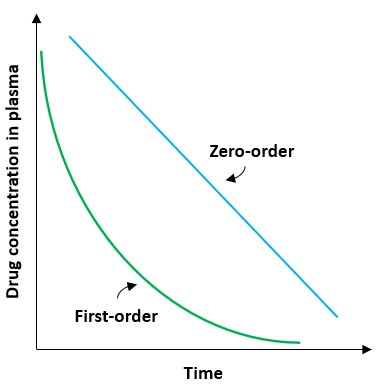
- After myocardial infarction, is released into the circulation and becomes elevated at 6 to 10 hours, peaks at 24-36 hours (at serum levels of 2 to 10 times upper limit of reference range)
- Levels remain high for 3 - 5 days (Circulation 1955;11:711, Clin Chem 1988;34:225)
Laboratory
Function
Methodology
- Catalyzes the transfer of an amino group to the keto acid in the conversion of conversion of aspartate and alpha-ketoglutarate to oxaloacetate and glutamate, with pyridoxal phosphate (Vitamin B6) as a cofactor
- Found in hepatocyte cytoplasm and mitochondria as two isoenzymes, but this has no clinical significance
Methodology
- May be assayed spectrophotometrically in a coupled reaction with malate dehydrogenase in the presence of NADH (Karmen 1955 J Clin Invest 1955;34:126, Amador and Wacker 1962 Clin Chem 1962;8:343)
- One unit oxidizes one micromole of NADH per minute at 25°C and pH 7.4 under the specified conditions
- Laboratory methods for aminotransferases should be supplemented with pyridoxal phosphate, to avoid falsely decreased activities in samples obtained from malnourished individuals with low endogenous vitamin B6 concentrations
Interpretation
Indications for testing (serum)
Limitations
Reference ranges
- For acute myocardial infarction, use has been superseded by cardiac troponins
- Screening test for liver disease, although ALT is more specific
- Increases in AST and ALT are higher when hepatocytes are damaged by viruses or toxic substances than in biliary obstruction
- In elderly, AST elevation is associated with obesity and consuming >3 alcoholic drinks / day (Aliment Pharmacol Ther 2009;30:1137)
- To assess prognosis after liver transplantation (Transplant Proc 2009;41:1727)
- To assess prognosis in autoimmune hepatitis (Clin Gastroenterol Hepatol 2008;6:1389)
- To assess effectiveness of treatment for liver disease (decline in levels may be due to disease resolution, or severe disease with minimal enzyme left for release)
- AST/ALT ratio is useful:
- Value > 2:1 is suggestive of alcoholic liver disease (General Practice Notebook)
- Can predict hepatic fibrosis and outcome in primary biliary cirrhosis (J Clin Gastroenterol 2009;43:876)
- Note that ratio is generally higher in women than men (Dig Dis Sci 2008;53:799)
- AST/platelet ratio index is useful:
- Can predict liver fibrosis in chronic hepatitis B (Dig Liver Dis 2008;40:267) or HIV / Hepatitis C co-infection (Liver Int 2008;28:486)
- Levels in vaginal washing fluid may predict preterm premature rupture of membranes (Fetal Diagn Ther 2008;24:425)
- A more sensitive marker of muscle damage than ALT, but less sensitive than CK, aldolase and myoglobin (Clinical Chemistry 2009;55:1573)
Limitations
- For acute myocardial infarction, AST elevation is nonspecific in the absence of crushing chest pain and ECG changes of ST elevation, ST depression or T-wave inversion
- High AST levels are associated with acute pancreatitis, celiac disease, exercise, hemolysis, hypothyroidism, liver disease (see above), muscle disease (see above), post-delivery, post-intramuscular injection, post-surgery, premature rupture of membranes, renal infract, sepsis (General Practice Notebook)
- In leukemia patients, falsely elevated levels may be due to pneumatic transport of specimen (Ann Clin Biochem 2010;47:94)
- Children may have isolated elevated levels, often due to macroenzyme form of AST (Am J Gastroenter 2005; 100;243), but phenomenon appears to be benign (J Pediatr 2009;154:744)
Reference ranges
- Female: 6 - 34 IU/L
- Male: 8 - 40 IU/L (may vary between laboratories)
- High value: needs to be interpreted in the context of chest pain and ECG findings
Assay interferences
Table of Contents
Definition / general | Essential features | Terminology | Types of interferences | Mechanisms of interference | Serum indices | Other common interferents | Strategies to handle interferents | Board review style question #1 | Board review style answer #1Definition / general
- Interference is defined as a cause of clinically significant difference in the assay result, due to another component or property of the sample
- Most interferences are missed by quality control processes and can lead to undetected discrepant test results, which can lead to patient harm
Essential features
- HIL (hemolysis, icterus, lipemia) interference can be detected on most automated analyzers
- Other types of interference are often missed and require awareness by both laboratorians and clinicians
- Use Clinical and Laboratory Standards Institute (CLSI) EP07 as a resource for interference testing (CLSI: EP07 - Interference Testing in Clinical Chemistry [Accessed 16 April 2021])
Terminology
- Analyte: component represented in the name of a measurable quantity
- Matrix: all components of a material system, except the analyte
- Measurand: particular quantity subject to measurement
Types of interferences
- Interferents can originate from both exogenous and endogenous sources (Clin Chem Lab Med 2020;58:350)
- Endogenous:
- Metabolites that arise from pathological conditions (free hemoglobin, bilirubin, lipidemia)
- Macrocomplexes (macroprolactin, macroenzymes)
- Antianalyte antibodies (antithyroglobulin)
- Paraproteins
- Specimen matrix itself
- Exogenous:
- Compounds given to patient for treatment (e.g. drugs, anticoagulants, intravenous saline or dextrose solutions)
- Ingested substances (biotin, a type of vitamin B)
- Environmental contaminants (powder from gloves, atmospheric air)
- Sample additives from phlebotomy processes (anticoagulants, preservatives)
- Identifying the presence of most interferents depends on clinicians recognizing discordant test results and communicating with laboratorians
Mechanisms of interference
- Broad categories of interference include:
- Chemical interference: interferent disrupts assay reaction
- Spectral interference: interferent has similar spectral properties to the measurand
- Physical interference: interferent alters physical properties of the sample or measurand
- Enzymatic interference: interferent alters activity of enzyme(s) used in the assay reaction
- Nonselectivity: interferent mimics measurand in the assay reaction
- Additive interference: interferent or additional measurand introduced into sample
- The same interferent can interfere differently, depending on the assay
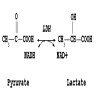
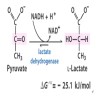
- In vitro hemolysis can cause chemical interference (hemoglobin inhibits certain reactions), spectral interference (hemoglobin has characteristic red color) and additive interference (red blood cells have high intracellular concentrations of certain analytes, e.g. potassium, lactate dehydrogenase, magnesium, phosphorus and AST)
- Critical to assess interferent in context of assay in question
- Reference: Rifai: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th Edition, 2017
Serum indices
- Most high volume automated analyzers can measure serum HIL (hemolysis, icterus, lipemia) indices on patient samples
- Assays susceptible to HIL interference can be set to flag patient results, if indices are present above established threshold
- Manufacturers will typically provide HIL interference data in assay information but may still require laboratory verification / validation (refer to CLSI guidelines) (CLSI: C56 - Hemolysis, Icterus, and Lipemia/Turbidity Indices as Indicators of Interference in Clinical Laboratory Analysis [Accessed 16 April 2021])
- Serum indices are measured photometrically based on characteristic absorbance (Ann Clin Biochem 2016;53:527):
- Hemolysis absorbs light between 340 - 440 nm and 540 - 580 nm
- Bilirubin (icterus) absorbs light between 400 - 500 nm
- Lipemia (caused by lipid particles) can absorb light between 300 - 700 nm
Common tests affected by HIL interference:
| | | |
| Hemolysis |
|
|
Icterus |
| |
| Lipemia |
| |
|
||
Other common interferents
- Biotin (vitamin B7) will interfere with immunoassays that use the streptavidin - biotin system
- Interference can be negative or positive, depending on assay format (i.e. competitive or noncompetitive)
- Heterophilic antibodies can interfere with any type of immunoassay
- Defined as antibodies that can react nonspecifically with different molecules
- Typically causes false positive sandwich immunoassay result but may also cause false negative results
- Often increased in patients with autoimmune or inflammatory conditions
- Human antianimal antibodies can interfere with immunoassay, if it uses antibodies produced by the animal in question
- Most frequent cause is human antimouse antibodies
- Macrocomplexes (endogenous analytes that self polymerize or complex with immunoglobulins) will cause discrepantly high results that are not indicative of patient status
- Most commonly affected tests are aspartate aminotransferase, creatine kinase, amylase and prolactin
- Reference: Rifai: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th Edition, 2017
Strategies to handle interferents
- HIL interferences should be thoroughly assessed and validated prior to implementation of an assay
- Use manufacturer provided information and CLSI guidelines (C56-A and EP07) as reference (CLSI: C56 - Hemolysis, Icterus, and Lipemia/Turbidity Indices as Indicators of Interference in Clinical Laboratory Analysis [Accessed 16 April 2021], CLSI: EP07 - Interference Testing in Clinical Chemistry [Accessed 16 April 2021])
- Rare or unexpected interferents can be handled with alternative solutions (though none are guaranteed):
- Dilute the interferent out with appropriate diluent
- Remove the interferent with treatment (i.e. polyethylene glycol [PEG] precipitation for macrocomplexes)
- Estimate the degree of discrepancy caused by interferent
Board review style question #1
Which of the following statements about assay interferences is true?
- An interferent typically exhibits the same interference properties across different assays
- Interferents exhibit similar interference properties across different assays
- The recognition of assay interference by biotin and heterophilic antibodies often depends on awareness by clinicians
- Most automated analyzers are unable to detect HIL (hemolysis, icterus, lipemia) interferences
Board review style answer #1
C. The recognition of assay interference by biotin and heterophilic antibodies often depends on awareness by clinicians
Comment Here
Reference: Assay interferences
Comment Here
Reference: Assay interferences
CK-MB (creatine kinase isoenzyme MB)
Table of Contents
Definition / general | Pathophysiology | Clinical features | Laboratory | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Sensitive and specific test for myocardial infarction, now widely replaced by troponin
Pathophysiology
- Catalyzes the conversion of creatine to phosphocreatine, consuming adenosine triphosphate (ATP) and generating adenosine diphosphate (ADP)
Clinical features
- CK isoenzyme MB rises some 4 - 6 hours after the onset of chest pain, peaks within 12 - 24 hours, and returns to baseline levels within 24 - 48 hours
- CK-MB is usually ordered, along with total CK in persons with chest pain to determine whether the pain is due to myocardial infarction
- May also be ordered in a person with a high CK to determine whether damage is in the heart
Laboratory
Test methodology
Test indications
Test limitations
Reference ranges
- Electrophoresis:
- Serum creatine kinase (CK) is separated into 3 isoenzymes by electrophoretic separation on agarose gel
- Colorimetric results allow for improved workflow management as the gels do not have to be scanned immediately
- The permanent patterns combined with a clear gel background means scanning and quantitation are easy
- Immunoassays are also commonly used
Test indications
- Troponin has largely replaced CK-MB in many hospitals, although some centers still rely on CK-MB (Wikipedia - CPK-MB test)
Test limitations
- Some patients have a variant of CK-BB called "Macro CK", which complexes to IgG or IgA antibody
- It migrates between MM and BB on the gel, and may falsely increase CK-MB values
- CK-MB can be elevated with massive rhabdomyolysis, even though the concentration is low in skeletal muscle
- Electrophoresis of CK with values of total CK under 100 U/L may cause false positive CK-MB values
Reference ranges
- If the value of CK-MB is elevated and the ratio of CK–MB to total CK (relative index) is more than 2.5 - 3, it is likely that the heart was damaged
- A high CK with a relative index below 2.5 - 3.0 suggests that skeletal muscle and not cardiac muscle was damaged
Additional references
Board review style question #1
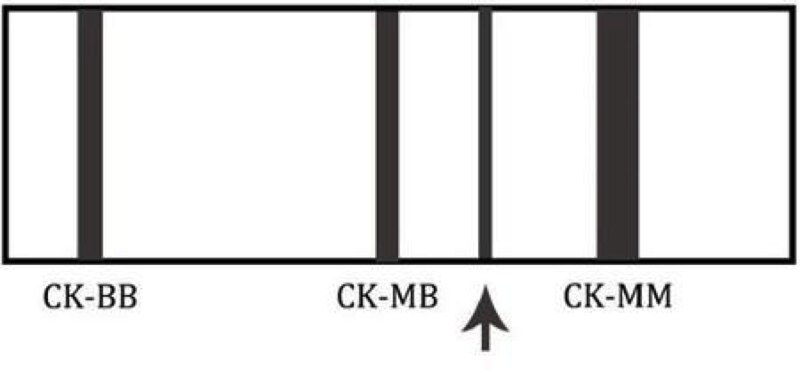
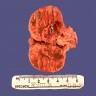
This is an image of a creatinine kinase electrophoresis. Which of the following patients would likely have this electrophoretic pattern?
- 32 year old healthy male who just finished a marathon
- 45 year old female with widely metastatic breast cancer
- 55 year old male with acute onset chest pain
- 78 year old asymptomatic female
Board review style answer #1
D. A 78 year old asymptomatic female - this is the CK electrophoretic pattern seen in macro CK, a benign finding in healthy, elderly females. Macro CK is a result of a CK antibody conjugate and typically appears between CK-MM and CK-MB.
Choices 'a' and 'b' would not have a macro CK band. A 45 year old female (c) may have an additional band that migrates to the right of CK-MM. This band is mitochondrial CK and is seen in patients with widely metastatic disease.
Comment Here
Reference: CK-MB (creatine kinase isoenzyme MB)
Choices 'a' and 'b' would not have a macro CK band. A 45 year old female (c) may have an additional band that migrates to the right of CK-MM. This band is mitochondrial CK and is seen in patients with widely metastatic disease.
Comment Here
Reference: CK-MB (creatine kinase isoenzyme MB)
Calcitonin
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Laboratory | Calcitonin stimulation test | Videos | Board review style question #1 | Board review style answer #1Definition / general
- Polypeptide hormone produced by parafollicular cells (C cells) of the thyroid gland
- Controls serum calcium level; specifically, reduces blood calcium, opposing the effects of parathyroid hormone
Essential features
- Calcitonin is a polypeptide hormone produced by parafollicular cells (C cells) of the thyroid gland to control the calcium level
- Calcitonin is a sensitive and specific tumor marker for diagnosis and follow up of C cell disorder, including C cell hyperplasia (CCH) and medullary thyroid carcinoma (MTC)
- Serum calcitonin can be falsely elevated in several conditions
- Calcitonin stimulation tests may be used when the basal calcitonin level is indeterminate
Terminology
- Also known as thyrocalcitonin
Pathophysiology
- Calcitonin (32 amino acids) is a polypeptide produced almost exclusively by parafollicular C cells of the thyroid gland
- Calcitonin results from cleavage and posttranslational processing of procalcitonin (116 amino acids, see Diagram 1), a precursor peptide derived from preprocalcitonin (141 amino acids)
- Secretion of calcitonin is stimulated by an increase in serum calcium and gastrin / pentagastrin
- Function
- Calcitonin reduces calcium level in the blood
- Major effect: inhibits osteoclasts from resorbing bone
- Minor effect: inhibits renal tubular cell and intestine from reabsorption of calcium
- Procalcitonin is a marker of systemic inflammation and can be used as a diagnostic marker of sepsis and antibiotic therapy (J Intensive Care 2017;5:51)
- See Diagram 2
- Calcitonin reduces calcium level in the blood
Diagrams / tables
Laboratory
- Immunochemiluminometric, 2 site, 2 steps assay that is highly sensitive and specific for monomeric calcitonin
Indications
- A sensitive and specific tumor marker for diagnosis and follow up of C cell disorders, including medullary thyroid carcinoma (MTC) and C cell hyperplasia (CCH)
- Basal serum calcitonin correlates well with tumor size and extent of metastasis of MTC (Thyroid 2015:25:567)
- To detect the presence of residual disease, a calcitonin level should be checked 3 to 6 months after the initial operation (total thyroidectomy)
- Calcitonin doubling time can be used as a prognostic factor (Clin Endocrinol 2010;72:534)
- If the doubling time is longer than 24 months, the 5 and 10 year survival rates are 100% and 100%, respectively
- When the doubling time is less than 6 months, the 5 and 10 year survival rates are 23% and 15%, respectively
- Calcitonin doubling time calculator
- Calcitonin doubling time can be used as a prognostic factor (Clin Endocrinol 2010;72:534)
- Calcitonin measurement in fine needle aspirate (FNA) washouts of thyroid nodule or suspicious neck lymph node is an additional tool if FNA biopsy findings are inconclusive (Clin Endocrinol 2014;80:135)
Limitations
- Calcitonin negative MTC (nonsecretory MTC) is rare and mostly occurs in sporadic MTC (J Cancer Res Clin Oncol 2016;142:2023)
- Despite the low or undetectable calcitonin serum level, many of these cases show tissue expression of calcitonin or its precursor by immunostaining or ISH (BMC Endocr Disord 2019;19:45)
- Serum calcitonin can be falsely elevated in several conditions, including chronic renal failure, autoimmune thyroiditis, large cell lung cancers, prostate cancer, mastocytosis, gastrointestinal and pulmonary neuroendocrine tumors, hyperparathyroidism and on proton pump inhibitor drugs treatment (Endocrinol Metab Clin North Am 2017;46:631)
- Heterophile antibodies can cause falsely elevated serum calcitonin levels (Clin Chem 2005;51:208)
- Owing to variability in calcitonin measurements among different commercial assays, individual patient samples should be evaluated using the same assay whenever possible
- Different assays may use antisera that recognize different epitopes of the calcitonin molecule
Reference ranges
- Gender differences and age related changes in normal calcitonin level exist but no significant ethnic differences observed
- Adults
- Males: < 19 ng/L
- Females: < 14 ng/L
- Children: (Clin Chem 2004;50:1828)
- < 6 months: < 40 ng/L
- 6 months to 3 years: < 15 ng/L
- Older children same as adults
Conversion factor
- Multiply by 0.293 to convert from ng/L to pmol/L
Interpretation
- Screening or diagnosis of MTC
- < 10 ng/L: normal
- 10 - 100 ng/L: indeterminate (need calcitonin stimulation test)
- > 100 ng/L: suspected MTC (see Diagram 3)
- Follow up monitoring and prognostic assessment
- < 10 ng/L: no residual tumor tissue
- 10 - 150 ng/L: possible local disease (i.e. neck)
- > 150 ng/l: possible distant metastases (see Diagram 4)
- Reference: Nat Clin Pract Endocrinol Metab 2009;5:35
Calcitonin stimulation test
Indications
Methodology
Interpretation
Adverse effects
- Early diagnosis of neoplastic CCH or micro MTC in RET mutation carriers
- Differentiation MTC from CCH, the preoperative recognition of which should avoid unnecessary thyroidectomies
- Identifying the possible coexistence of nonthyroidal neuroendocrine tumors of the foregut, pancreas, prostate and lung that can be distinguished from a C cell disease by the absence of response to the stimulation test
- Reference: J Clin Endocrinol Metab 2014;99:1656
Methodology
- 2 ways to stimulate calcitonin secretion for diagnostic testing:
- Calcium stimulation test: give 2.5 mg of elemental calcium/kg bodyweight of 10% calcium gluconate (1 ml = 9 elemental calcium) IV at rate of 5 ml/min for a minimum of 3 minutes
- Pentagastrin stimulation test: give 0.5 μg/kg body weight of pentagastrin IV for 10 seconds (pentagastrin is now unavailable in many countries)
- Blood is taken at time 0, 2, 5 and 10 or 15 minutes after administration of stimulants to determine calcitonin levels (see Diagram 5)
Interpretation
- Calcium stimulation test and pentagastrin stimulation test show similar diagnostic value in MTC after thyroidectomy (Neuro Endocrinol Lett 2016;37:485)
- Peak stimulated calcitonin (Nat Clin Pract Endocrinol Metab 2009;5:35)
- < 10 ng/L: absence of C cell disease
- 10 - 100 ng/L: indeterminate (probable false positive result)
- 101 - 500 ng/L: probable CCH
- 501 - 1,000 ng/L: probable MTC
- > 1,000 ng/L: MTC
Adverse effects
- Nausea, vomiting, abdominal cramping, urgency to micturate, warm feeling, altered gustatory sensation, extremity or facial paresthesia, tachycardia, bradycardia, substernal discomfort and dizziness (Endocrine 2014;46:549)
- Testing is contraindicated in patients older than 60 years old and those with hypertension or coronary artery disease
- Calcium stimulation test is better tolerated than pentagastrin stimulation test
Videos
Regulation of blood calcium via PTH and calcitonin
Customizing imaging based on calcitonin levels in medullary thyroid cancer
Board review style question #1
A 30 year old male without a history of underlying disease or medication presented with right thyroid nodule, 3 cm. Fine needle aspiration cytology showed atypical cells suspicious for medullary thyroid carcinoma. His calcitonin level was 30 ng/L (normal < 10 ng/L). What is the most appropriate management of this patient?
- Check CEA level
- Consult surgeon for thyroidectomy
- CT chest / abdomen screening for metastasis
- Perform calcium stimulation test
- Repeat fasting calcitonin
Board review style answer #1
D. Perform calcium stimulation test. Calcitonin level is indeterminate (10 - 100 ng/L). After excluding other causes of falsely high calcitonin, calcium or pentagastrin test should be performed in this patient.
Comment Here
Reference: Calcitonin
Comment Here
Reference: Calcitonin
Cancer biomarkers (pending)
[Pending]
Captopril suppression test
Definition / general
Indication:
Rationale:
Methodology:
Normal range:
Adverse effects:
- Confirms the diagnosis of primary (hyper) aldosteronism
- One of four tests recommended for screening or confirmation: also oral sodium loading, saline infusion and fludrocortisone suppression (Horm Metab Res 2010;42:406)
- As effective as sodium loading in confirming the diagnosis of primary aldosteronism (Hypertension 2001; 37:1440, Curr Hypertens Rep 2002;4:245)
- Note: Captopril challenge test is used to diagnose renal artery stenosis, differs in that plasma renin (not aldosterone) is measured (Wikipedia - Captopril challenge test)
Rationale:
- Captopril inhibits conversion of angiotensin I to angiotensin II, thereby decreasing aldosterone production in normal individuals
- In patients with primary hyperaldosteronism, the plasma aldosterone levels are NOT suppressed by captopril
Methodology:
- Measure plasma aldosterone levels at baseline and 2 hours after patient takes 25mg of captopril orally
Normal range:
- Plasma aldosterone concentration (PAC) <15 ng/dl (416pmol.L) and PAC to plasma renin activity (PRA) ratio less than 50
Adverse effects:
- Marked hypotension may occur due to captopril intake
- Blood pressure should be monitored regularly
Additional references
Cardiac troponins
Table of Contents
Definition / general | Essential features | ICD coding | Laboratory | Interpretation | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Cardiac troponins are the preferred biomarkers for the evaluation, detection and diagnosis of acute chest pain or myocardial injury
- Myocardial injury is defined as blood levels of cardiac troponin (cTn) that are elevated above the 99th percentile upper reference limit (URL) (Glob Heart 2018;13:305)
Essential features
- High sensitivity troponin (hs-cTn) by definition is the assay that detects cTn concentrations with a coefficient of variation (CV) < 10% at or below the 99th percentile upper reference limits and measurable in > 50% of normal healthy individuals (J Am Heart Assoc 2014;3:e000403)
- The definition of myocardial injury was defined by the Fourth Universal Definition of Myocardial Infarction (2018) as cTn levels that are elevated above the 99th percentile URL (Glob Heart 2018;13:305)
- No myocardial injury: cTn values ≤ 99th percentile URL or not detectable (Glob Heart 2018;13:305)
- Myocardial injury: cTn values > 99th percentile URL without symptoms or signs of myocardial ischemia
- All nonischemic myocardial injury is classified as acute if there is a rise or fall of cTn values, unless a change of ≤ 20% was observed on serial testing (Circulation 2020;141:161)
- Myocardial infarction: clinical evidence of myocardial ischemia and a rise or fall of cTn values > 99th percentile URL (Glob Heart 2018;13:305)
- A single positive hs-cTn value > 99th percentile upper reference limit is sensitive for but not specific for myocardial infarction, for which diagnosis may require serial testing
- Pattern change in hs-cTn values (delta) is also assay specific
- Interpretation of the delta needs to account for other clinical data such as the history (notably the onset of symptoms), electrocardiography changes and imaging
- In patients with chronically elevated hs-cTn, the absence of significant change defined as < 20% delta is indicative of chronic myocardial injury (Circulation 2022;146:569)
- hs-cTn is recommended as an important biomarker for the evaluation and diagnosis of acute chest pain by the 2021 American Heart Association (AHA) / American College of Cardiology (ACC) / American Society of Echocardiography / American College of Chest Physicians / Society for Academic Emergency Medicine / Society of Cardiovascular Computed Tomography and International Federation of Clinical Chemistry and Laboratory Medicine Task Force on Clinical Applications of Bio-Markers (IFCC TF-CB) (Circulation 2021;144:e368)
Laboratory
- hs-cTn assays should measure cTn above the limit of detection in ≤ 50% of healthy subjects (J Am Heart Assoc 2014;3:e000403)
- Analytical requirement for hs-cTn is the assay that has a CV of ≤ 10% at the 99th percentile (Clin Biochem 2015;48:201, J Am Heart Assoc 2014;3:e000403)
- High precision of the assay allows the determination of small differences in cTn over time
- 99th percentile may be different for serum, plasma and whole blood (Clin Biochem 2015;48:201)
- 99th percentile must be determined individually for each assay, as assays are not standardized (Clin Biochem 2015;48:201)
- Assay specific 99th percentile URL should be derived from a sample size of at least 400 male and 400 female healthy subjects
- Healthy subjects should be screened with questionnaires to exclude those with cardiovascular comorbidities and those on cardiovascular medications
- NT-proBNP (N terminal pro-B type natriuretic peptide), hemoglobin A1C and estimated glomerular filtration rate (eGFR) are used to exclude subclinical disease, recommended by the most recent (2022) IFCC and American Association of Clinical Chemistry (AACC) guidelines (Clin Chem 2022;68:1022)
- There have been multiple hs-cTn assays approved by the U.S. Food and Drug Administration (FDA) for clinical use in the United States since 2017 (Clin Chem 2021;67:70)
- Women have lower 99th percentiles than men and all the FDA approved hs-cTn assays report gender specific 99th percentile URL
- 2021 AHA / ACC guidelines recognize gender specific hs-cTn URLs but do not encourage their use (Circulation 2021;144:e368)
- Below are the recommendations of hs-cTn assays by the American Association for Clinical Chemistry Academy and International Federation of Clinical Chemistry and Laboratory Medicine Task Force on Clinical Applications of Bio-Markers (IFCC TF-CB) (Clin Chem 2018;64:645)
|
|
|
|
|
|
|
|
|
|
Interpretation
- Development of rapid risk stratification protocols with evidence based studies with hs-cTn assays is required and important for the assessment of patients with acute chest pain
- Sample collection and acceptable turnaround times are critical to establish the rapid rule in and rule out algorithms
- Laboratory analytical quality is essential for the accuracy of hs-cTn results and should be reliable for decision making
- Assessment of patients with the suspected acute coronary syndrome (ACS) should integrate a multidisciplinary team effort that includes laboratory medicine, emergency medicine, internal medicine, family medicine and cardiology
- Below is a summary of the latest guidelines for implementing the hs-cTns assay in clinical practice (Glob Heart 2018;13:305)
- Criteria for cardiac procedural myocardial injury: arbitrarily defined by increases of cTn values (> 99th percentile URL) in patients with normal baseline values (≤ 99th percentile URL) or a rise of cTn values > 20% of the baseline value when it is above the 99th percentile URL but it is stable or falling
2018 Fourth Universal Definition of Myocardial Infarction criteria
| Myocardial infarction (MI) | Criteria |
| Type 1 | Detection of a rise or fall of cTn values with at least 1 value above the 99th percentile URL and with at least 1 of the following:
|
| Type 2 | Detection of a rise or fall of cTn values with at least 1 value above the 99th percentile URL and evidence of an imbalance between myocardial oxygen supply and demand unrelated to acute coronary atherothrombosis, requiring at least 1 of the following:
|
| Type 3 | Patients who suffer cardiac death, with symptoms suggestive of myocardial ischemia accompanied by presumed new ischemic electrocardiogram changes or ventricular fibrillation but die before blood samples for biomarkers can be obtained or before increases in cardiac biomarkers can be identified or myocardial infarction is detected by autopsy examination |
| Type 4a | Coronary intervention related myocardial infarction is arbitrarily defined by an elevation of cTn values > 5 times the 99th percentile URL in patients with normal baseline values; in patients with elevated preprocedure cTn in whom the cTn level is stable (≤ 20% variation) or falling, the postprocedure cTn must rise by > 20%; however, the absolute postprocedural value must still be at least 5 times the 99th percentile URL and in addition, 1 of the following elements is required:
|
| Type 4b | A subcategory of percutaneous coronary intervention (PCI) related myocardial infarction is stent / scaffold thrombosis, as documented by angiography or autopsy using the same criteria for type 1 myocardial infarction |
| Type 4c | Defined as focal or diffuse restenosis or a complex lesion associated with a rise or fall of cTn values above the 99th percentile URL applying, the same criteria utilized for type 1 myocardial infarction |
| Type 5 | Coronary artery bypass grafting (CABG) related myocardial infarction is arbitrarily defined as an elevation of cTn values > 10 times the 99th percentile URL in patients with normal baseline cTn value; in patients with elevated preprocedure cTn in whom cTn levels are stable (≤ 20% variation) or falling, the postprocedure cTn must rise by > 20%; however, the absolute postprocedural value still must be > 10 times the 99th percentile URL and in addition, 1 of the following elements is required:
|
European Society of Cardiology (ESC) guidelines
| Years | Recommendations | References | |||
| 2011 |
| Eur Heart J 2011;32:2999 | |||
| 2015 |
|
Board review style question #1
For the high sensitivity cardiac troponin (hs-cTn) assay, which of the following criteria are correct?
- CV of ≤ 10% at the 99th percentile and detected in at least 50% of acute myocardial infarction patients
- CV of ≤ 10% at the 99th percentile and detected in at least 50% of healthy subjects
- CV of ≤ 10% at the 99th percentile and detected in at least 99% of healthy subjects
- CV of ≤ 20% at the 99th percentile and detected in at least 50% of acute myocardial infarction patients
Board review style answer #1
B. CV of ≤ 10% at the 99th percentile and detected in at least 50% of healthy subjects. High sensitivity troponin (hs-cTn) by definition is the assay that detects cTn concentrations with a coefficient of variation (CV) ≤ 10% at or below the 99th percentile upper reference limits and measurable in > 50% of normal healthy individuals (J Am Heart Assoc 2014;3:e000403).
Comment Here
Reference: Cardiac troponins
Comment Here
Reference: Cardiac troponins
Board review style question #2
Which of the following is the correct recommendation for the 99th percentile of high sensitivity cardiac troponin (hs-cTn)?
- The 99th percentile may be different for serum, plasma and whole blood
- The analytical requirement for hs-cTn is the assay has a % CV at the 99th percentile of ≤ 20%
- The assay specific 99th percentile URL should be derived from a sample size of 120 male and 120 female healthy subjects
- The hs-cTn assays are standardized and the 99th percentile does not need to be determined individually for each assay
Board review style answer #2
A. The 99th percentile may be different for serum, plasma and whole blood (Clin Biochem 2015;48:201). Analytical requirement for hs-cTn is the assay that has a CV of ≤ 10% at the 99th percentile (Clin Biochem 2015;48:201, J Am Heart Assoc 2014;3:e000403). Assay specific 99th percentile URL should be derived from a sample size of at least 400 male and 400 female healthy subjects. 99th percentile must be determined individually for each assay, as assays are not standardized (Clin Biochem 2015;48:201).
Comment Here
Reference: Cardiac troponins
Comment Here
Reference: Cardiac troponins
Chemistry analyzer selection (pending)
[Pending]
Creatine kinase
Table of Contents
Definition / general | Terminology | Pathophysiology | Diagrams / tables | Interpretation | Laboratory | Additional referencesDefinition / general
- Muscle related enzyme released into blood after muscle cell death
- Serum levels are used to diagnosis acute myocardial infarction, rhabdomyolysis, muscular dystrophy and acute renal failure
Terminology
- Also known as CK, creatine phosphokinase (CPK), phospho-creative kinase, EC 2.7.3.2
- "Creatinine kinase" is an incorrect term
- Creatinine is a break-down product of creatine phosphate in muscle produced at a fairly constant rate, and used to calculate creatinine clearance and glomerular filtration rate
Pathophysiology
- Present in heart, brain, skeletal and intestinal smooth muscle (acts as energy reservoir for rapid rebuffering and regeneration of ATP), but in different concentrations and with different ratios of the M (muscle) and B (brain) dimeric units
- CK from brain almost never crosses the blood-brain barrier
- There are three different isoenzymes: CK-MM, CK-BB and CK-MB
- Skeletal muscle expresses CK-MM (98%) and low levels of CK-MB (1% in type 1 fibers, 2 - 6% in type 2 fibers, higher amounts during skeletal muscle regeneration)
- Myocardium expresses CK-MM (70%) and CK-MB (25 - 30%, higher in right heart than left heart)
- Creatine kinase catalyses the conversion of creatine to phosphocreatine, consuming adenosine triphosphate (ATP) and generating adenosine diphosphate (ADP)
Interpretation
Acute myocardial infarction
- Markers are ordered as a panel, because different markers have different time frames for detection
- American College of Cardiology / American Heart Association recommend results within 30 - 60 minutes of admission, which precludes prolonged serial measures of serum levels of markers
- Suggested point of care multimarker algorithm to detect acute MI:
- Troponin I >= 0.4 ng/mL (0.4 μg/L) in any specimen
- Doubling of myoglobin between 2 sequential specimens with any detectable TnI at least by the second of the 2 specimens, or
- Myoglobin (doubling) and CK-MB concentrations increasing by 50% or more in 2 or 3 specimens (Am J Clin Pathol 2008;129:788)
- Algorithm for CK-MB testing:
- If total CK < 80 IU/L, don’t do CK-MB
- Do CK-MB (reference range is 0 - 4.9 ng/mL) if total CK is between 80 - 500 IU/L
- Do CK-MB (no reference range) and CK-MB% (reference range 0.0 - 1.0%) if total CK > 500 IU/L
Laboratory
Test methodology
CK-MB Mass Assay
Test indications
Test limitations
Reference ranges
- Continuous Spectrophotometric Rate Determination
- Temperature: 30 degrees C, pH: 7.4
- Wavelength: A340nm
- Light path: 1 cm
CK-MB Mass Assay
- An immunometric assay using a monoclonal antibody, in which CK-MB is considered an antigen
- Test can be reported in < 1 hour using various automatic platforms
- Qualitative level is usually reported with relative index / relative percent (CK-MB / total CK)
- Values suggestive of acute MI are 5 ng/ml or greater and relative index of 2% or greater (Abbott Point of Care: Creatine [Accessed 20 December 2021], BeckmanCoulter)
Test indications
- CK levels were historically used in emergency room patients to test for myocardial infarction (now often replaced by troponin), rhabdomyolysis, muscular dystrophy, myositis, myocarditis, malignant hyperthermia, neuroleptic malignant syndrome
- Levels are determined specifically in patients with chest pain and cardinal features of chest pain
- Activity is estimated in the course of acute ischemic heart disease to diagnose acute myocardial infarction (AMI) and to estimate infarct size
- CK-MB activity is normal in 25 - 50% of patients with MI at time of admission (Emerg Med Clin North Am 2001;19:321), rises some 4 - 6 hours after the onset of chest pain, peaks within 12 - 24 hours, and returns to baseline levels within 36 - 48 hours
- These times may be shorted considerably by thrombolytic therapy
Test limitations
- High serum levels indicate injury to muscle, including rhabdomyolysis, myocardial infarction, muscular dystrophy, myositis, myocarditis, malignant hyperthermia and neuroleptic malignant syndrome
- Also seen in hypothyroidism
- The use of statin medications, commonly used to decrease serum cholesterol levels, may be associated with elevation of the CPK level in 1% of the patients taking these medications, and with actual muscle damage in a much smaller proportion
- CK-MB and CK-BB are quite labile
- Specimens should be frozen if the assay cannot be performed within 24 hours
Reference ranges
- Normal values are usually between 25 and 200 U/L, may be lower in women
- High values for CK-MB: need to be interpreted in the context of chest pain and ECG findings or other muscle damages
- Patients whose CK does not decline 50% or more within 48 hours of peak have increased risk of reinfarction or death (Clin Chem 1989;35:414)
Additional references
Drugs of abuse
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Detection methodologies | Common drugs of abuse | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Drug abuse is a global major public health problem
- Includes prescription and nonprescription drugs that are either overdosed or illicitly abused for pleasure
- Testing is performed for patient compliance in pain management and for addiction management
Essential features
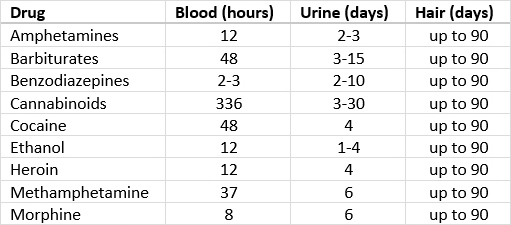
- Drugs of abuse are commonly analyzed in urine because of the longer window period for detection; less commonly analyzed in serum or plasma
- Drugs are excreted in urine, either in their native form or as their metabolites
- Absence of drugs in the urine indicates inappropriate specimen collection, diversion (i.e. not taking the prescribed dose), diluted urine or adulterated urine
- Dilution of urine samples can be confirmed by measuring the urine creatinine levels (normal 24 hour urine creatinine: 500 - 2000 mg/day; random urine creatinine: 20 - 300 mg/dL)
- Identification of drugs of abuse in pregnant women / neonates during antenatal testing can have serious consequences
- For neonates, meconium can be used to detect drug abuse by the pregnant mother for up to 4 - 5 months before delivery; results are reported as ng/g (Clin Chem 2018;64:1671)
- Cutoff levels for urine testing of drugs of abuse are established in ng/ml for both the screen and confirmation
Terminology
- Meconium
- Kinetic interaction of microparticles in solution (KIMS)
- Liquid chromatography tandem mass spectrometry (LC-MS / MS)
- Gas chromatography mass spectrometry (GC-MS)
ICD coding
- ICD-10: F01-F99 - mental, behavioral and neurodevelopmental disorders
Detection methodologies
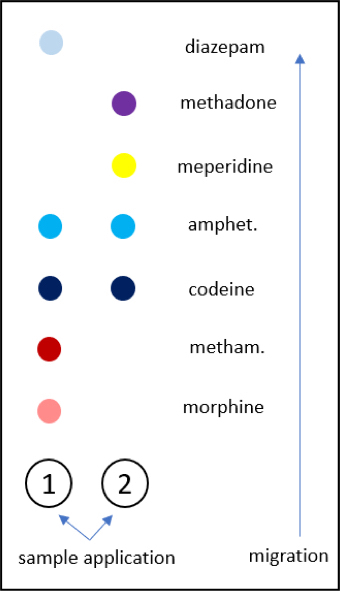
- Immunoassay screening for drugs of abuse is performed mostly by the kinetic interaction of microparticles in solution (KIMS) method
- Other immunoassay methods, such as enzyme multiplied immunoassay technique (EMIT) and cloned enzyme donor immunoassay (CEDIA), are also performed for drugs of abuse testing
- Results are provided as positive or negative based on the cutoff concentration detection limits
- Immunoassays recognize or detect only 1 or certain drugs in a class and cannot differentiate between the main drug and their metabolites
- Confirmation of drugs of abuse is carried out by quantitative measurement by liquid chromatography tandem mass spectrometry (LC-MS / MS) or gas chromatography mass spectrometry (GC-MS) methods
- These methods are highly sensitive and specific to the drugs of interest
- Can also detect the parent drug and its metabolite
- Current trend for toxicology laboratories to convert from GC-MS to LC-MS / MS due to the higher throughput and improved cost savings of the latter (AACC: Liquid Chromatography Tandem Mass Spectrometry [Accessed 22 February 2021])
- Point of care testing (POCT) methods / assays are available for some drugs of abuse testing
- However, they should be used only for emergency purposes and the results should be confirmed by more specific and sensitive methods (Ann Clin Biochem 2021 Feb 1 [Epub ahead of print])
Common drugs of abuse
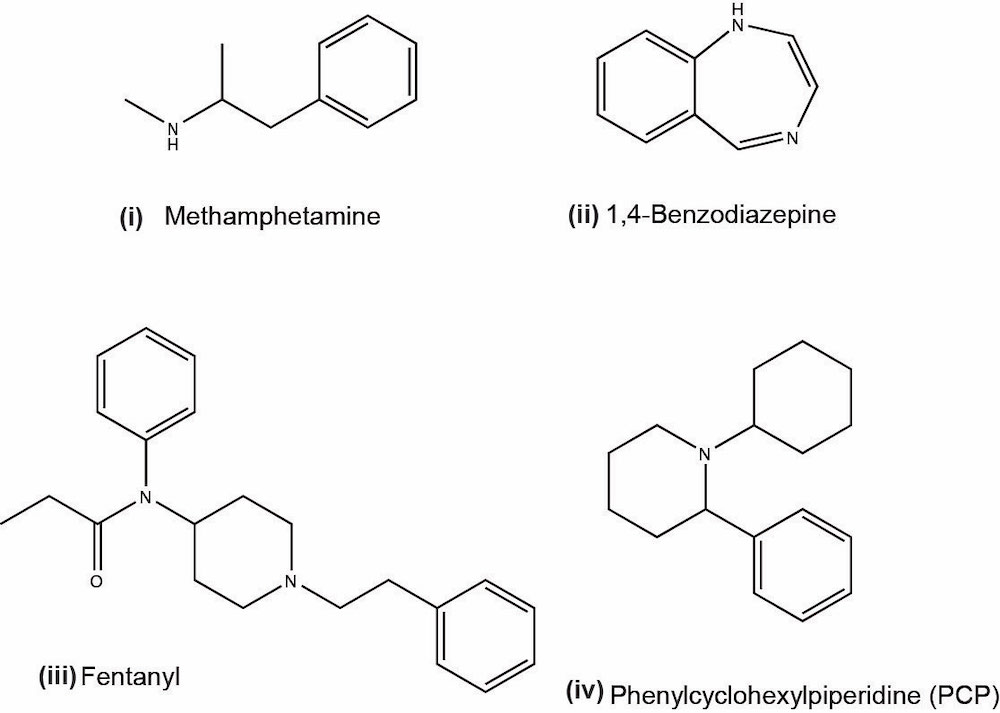
- Amphetamines:
- Belong to phenylethylamine class of drugs and stimulate central nervous system (CNS)
- Pharmacologically used for the treatment of narcolepsy, obesity and ADHD
- High doses and frequent heavy use can create an amphetamine induced psychosis
- Forms of amphetamines used in abuse include crystal methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA), also referred to as ecstasy
- Amphetamines are metabolized by liver and eliminated in urine with an average detection window of 3 days
- Quantitative LC-MS / MS based detection cutoff concentrations for amphetamine is 50 ng/ml and for all others, such as methamphetamine and MDMA (ecstasy), is 200 ng/ml
- Barbiturates:
- Class of sedative drugs
- CNS depressants used in treatment of insomnia and seizures
- Short acting sedative barbiturates, such as pentobarbital, secobarbital and amobarbital, are more subjected to abuse compared to long acting phenobarbital that is rarely abused
- Withdrawal symptoms include agitation, anxiety, insomnia, nausea and vomiting
- Detection window for barbiturates ranges from 24 hours to 4 days (for long acting phenobarbital)
- Quantitative analysis by GC-MS can detect individual barbitals with positive cutoff of 50 ng/ml
- Benzodiazepines:
- Include a wide range of compounds that consist of a benzene ring, phenyl ring and a diazepine ring, with a common molecular structure
- LC-MS / MS quantitative detection positive cutoff for most benzodiazepines is 20 ng/ml
- LC-MS / MS can also identify individual derivatives, including lorazepam, oxazepam and flurazepam
- Cocaine:
- Cocaine or coke is a strong, addictive CNS stimulant, chemically an alkaloid made from the leaves of the coca plant
- Cocaine salt form is most commonly abused through snorting (intranasal delivery) or by intravenous injection
- Base form, also known as crack, is consumed as smoke after heating
- Cocaine is metabolized to benzoylecgonine, norcocaine or ecgonine methyl ester and excreted in urine, mostly as benzoylecgonine that can be detectable for 1 - 3 days
- Both oral fluid and urine samples can be tested for detection of cocaine or its metabolite benzoylecgonine; however, because of the short half life of cocaine, benzoylecgonine is the most common analyte measured for cocaine (J Appl Lab Med 2020;5:935)
- Side effects of cocaine abuse cause heart attack, stroke, headache and seizures
- Quantitative GC-MS detection in urine can identify cocaine and its metabolite benzoylecgonine with a positive cutoff of 50 ng/ml
- Cannabinoids:
- Cannabinoids are the products of the plant Cannabis sativa or indica and the most highly consumed drugs of abuse
- Commonly known or available as marijuana, pot and weed; consumed as smoke
- Consumption of cannabinoids results in euphoria, relaxation and mood changes, including psychosis and panic attacks
- Marijuana withdrawal symptoms include anxiety, insomnia and anorexia
- Major psychoactive component of cannabinoids is delta-9-tetrahydrocannabinol (THC), which gets deposited in the adipose tissue after consumption
- THC is mainly metabolized to 11-hydroxy-delta-9-THC by the liver CYP450 enzymes
- THC has a longer detection window ranging from 3 to 90 days, depending on the usage
- Quantitative LC-MS / MS detects THC metabolite, 11-Nor-9-carboxy-THC, with cutoff concentration of 15 ng/ml
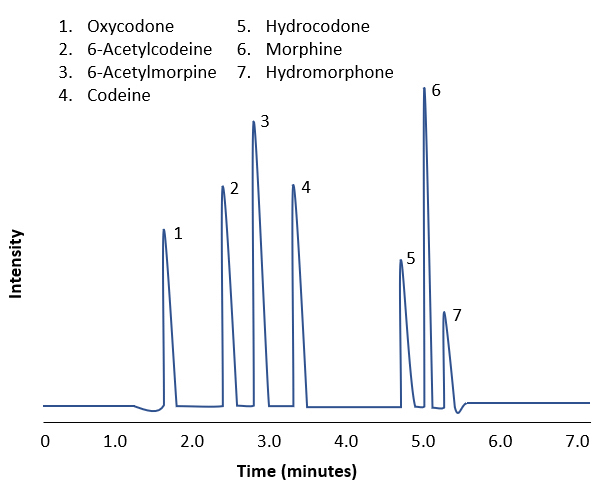
- Opioids:
- Opioids are a group of compounds that bind to the opioid receptors
- Natural alkaloids opioids, also called opiates, are morphine and codeine and are derived from the opium plant
- Other opioids include semisynthetic heroin, hydrocodone, oxycodone and synthetic opioids such as fentanyl, methadone and tramadol
- Opioids are used as analgesics and are highly addictive, causing nausea, sedation, physical dependence and breathlessness
- Opioid withdrawal symptoms include vomiting, diarrhea, muscle and joint pain, restlessness
- Morphine is metabolized majorly to morphine-3-glucuronide and minorly to hydromorphone when used longterm
- Immunoassay screening for opiates can detect morphine and codeine but not the semisynthetic and synthetic opioids
- Quantitative LC-MS / MS detects hydrocodone, oxycodone and their metabolites, including morphine and codeine, with cutoff concentration of 20 ng/ml
- Fentanyl:
- Synthetic opioid that is 50 - 100 times more potent than morphine
- Prescription drug for management of severe pain or postsurgery
- Illicitly synthesized and sold as China Girl, China White and Dance Fever
- Severe respiratory distress is a common display of fentanyl overdose
- Half life of fentanyl in blood is 3 - 10 hours and in urine is 2 - 3 days
- In a suspected case of fentanyl abuse or overdose, the urine opioid screening results will be negative and a targeted analysis of fentanyl testing is necessary (MMWR Morb Mortal Wkly Rep 2019;68:687)
- Metabolized by CYP450 enzyme in a CYP3A4 mediated N-dealkylation to norfentanyl (major) and less than 1% to hydroxyfentanyl and hydroxynorfentanyl
- Quantitative LC-MS / MS detects hydrocodone, oxycodone and their metabolites, including morphine and codeine, with cutoff concentration of 20 ng/ml
- Phencyclidine (PCP):
- Phencyclidine or phenylcyclohexyl piperidine (PCP) is a synthetic drug that was originally developed as an anesthetic and now is a Schedule II controlled substance
- Mind altering drug; belongs to the class of hallucinogens and its recreational use has been increasing in recent years (J Med Toxicol 2015;11:321)
- PCP is most commonly taken by smoking; street name is angel dust
- Serious effects of PCP abuse include agitation, anxiety, coma and death (with high doses of more than 20 mg)
- Quantitative LC-MS / MS detection cutoff for PCP is 10 ng/ml
Diagrams / tables
Contributed by Vishnu Amaram Samara, Ph.D.
Images hosted on other servers:
General cutoff limit concentrations of common drug classes by immunoassay screening and LC-MS / MS quantitative confirmation
| | typical cutoff limits (ng/mL) | limits using LC-MS / MS (ng/mL) |
| Amphetamine | ||
| Barbiturates | ||
| Benzodiazepines | ||
| Cannabinoids | ||
| Cocaine | ||
| Opiates | ||
| Phencyclidine |
Additional references
Board review style question #1
In highly suspected cases of drug abuse where the urine results are negative, the dilution of urine samples can be confirmed by measuring which of the following?
- Serum creatinine
- Serum electrolytes
- Urine creatinine
- Urine electrolytes
- Urine volume
Board review style answer #1
C. Urine creatinine. A random urine average creatinine concentration is 20 - 300 mg/dL and if the creatinine concentration is less than 20 mg/dL, the urine is diluted leading to negative drug results.
Comment Here
Reference: Drugs of abuse
Comment Here
Reference: Drugs of abuse
Electrolytes
Table of Contents
Definition / general | Essential features | Sodium | Potassium | Chloride | Bicarbonate | Calcium | Phosphate | Magnesium | Laboratory investigations for electrolytes | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sodium is the principal extracellular cation, while potassium is the principal intracellular cation
- Principal extracellular anions are chloride and bicarbonate, while principal intracellular anions are proteins and phosphate
Essential features
- Hyponatremia is dependent on plasma osmolality and volume status of the patient
- Kidneys play an important role in electrolyte balance
- Chloride concentrations in plasma usually parallels those of sodium
- Most common method of analysis of electrolytes is by ion selective electrodes
Sodium
- Sodium plays a central role in water balance and maintenance of extracellular fluid (ECF) osmotic pressure
- Sodium balance is controlled by renal blood flow and aldosterone
- Sodium and its associated anions (mainly chloride) contribute 90% or more to the measured plasma osmolality
- Serum osmolality can be measured directly or calculated from serum sodium, urea and glucose
- Urine sodium excretion is dependent on diet; urine sodium exhibits diurnal variation with lower sodium excretion occurring at night
- Preanalytical factors affecting sodium measurements
- Exercise
- Prolonged tourniquet application
- Sample collection: order of collection, needle size, mixing techniques, collection tubes
- Posture
- Postprandial
- Stress
- Medications
- Weather / seasons
- Transportation of samples
- Reference: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018
Hyponatremia
- Defined as plasma serum sodium concentration of < 135 mmol/L
- Affects ~5% of adults and 35% of hospitalized patients (JAMA 2022;328:280)
- Clinical features include weakness, nausea, headache, vomiting, somnolence, seizures, cardiorespiratory distress
- Acute severe hyponatremia can be associated with brain herniation, respiratory arrest, permanent brain damage and death (Nephrol Dial Transplant 2014;29:i1)
- Cause: depends on the measurement of plasma osmolality
- Hypo-osmotic hyponatremia: most common form; due to excess water loss or increased extracellular fluid volume and is also dependent on volume status
- Hypervolemia: renal failure, congestive heart failure, liver cirrhosis with ascites, nephrotic syndrome
- Hypovolemia
- With increased renal loss (> 20 mmol/L urine sodium): diuretics, renal diseases, metabolic alkalosis, mineralocorticoid deficiency, carbonic anhydrase inhibitors
- With decreased renal loss (< 10 mmol/L urine sodium): vomiting, diarrhea, burns
- Euvolemia: diuretics, syndrome of inappropriate antidiuretic hormone secretion (SIADH), hypothyroidism, secondary hypoadrenalism
- Hyperosmotic hyponatremia
- Severe hyperglycemia
- Uremia
- Mannitol diuresis
- Iso-osmotic hyponatremia: plasma osmolality is normal with decreased sodium concentration
- Pseudohyponatremia: electrolyte exclusion effect due to hyperlipidemia or hyperproteinemia
- Proteins and lipids decrease water content and can alter ion concentrations measured
- Usually occurs in patients with hyperlipidemia or hyperproteinemia
- Occurs in indirect ion selective electrode (ISE) measurements; most automated analyzers use the indirect ion selective electrode method
- Results in underestimation of sodium
- Pseudohyponatremia: electrolyte exclusion effect due to hyperlipidemia or hyperproteinemia
- References: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018, Nephrol Dial Transplant 2014;29:i1, JAMA 2022;328:280
Hypernatremia
- Plasma sodium concentration > 145 mmol/L
- Always hyperosmolar in nature
- Can present as acute or chronic hypernatremia; clinical features include weakness, malaise, delirium, tremors, irritability, confusion, coma, ataxia (Diagnosis (Berl) 2022;9:403)
- Cause depends on volume status (Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018)
- Hypovolemic hypernatremia: decreased extracellular fluid caused by renal (osmotic diuretics) or extrarenal (diarrhea, fever, burns, excessive sweating and respiratory losses, poor water intake) loss of hypo-osmotic fluid leading to dehydration
- Hypervolemic hypernatremia: net gain of water and sodium with a higher sodium gain compared to water; occurs in hospitalized patients on hypertonic saline or bicarbonate infusion, Conn syndrome or Cushing syndrome with excess mineralocorticoid, accidental salt ingestion
- Normovolemic / euvolemic hypernatremia: normal extracellular fluid volume; can be renal (glycosuria, increased urea load, diabetes insipidus) or extrarenal (hyperventilation)
- Artifactual or pseudohypernatremia: can occur following the use of collection tubes with sodium heparin as anticoagulant
Potassium
- Major intracellular cation
- 2% present in extracellular fluid; ~90% of the daily K+ intake is excreted in the urine and ~10% is excreted by the gastrointestinal tract
- Intracellular potassium is maintained by energy dependent sodium - potassium ATPase pump
- Extracellular H+ ions affect the entry of potassium into all cells
- Kidney is the major organ responsible for K+ homeostasis (major site is the distal convoluted tubule) (Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018)
- Preanalytical factors affecting potassium measurements
- Hemolysis falsely elevates K+ value (intracellular release)
- Serum K+ is higher than plasma potassium due to release during clot formation
- Thrombocytosis / leukocytosis
- Refrigeration of whole blood specimens
- In unspun specimens at 37 °C
- Prolonged tourniquet application
- Exercise
Hypokalemia
- Plasma potassium < 3.5 mmol/L
- Clinical features include muscle weakness, paralysis, irritability, tachycardia, cardiac conduction defects, cardiac arrest
- Redistribution
- Alkalosis
- Insulin response
- Hypothermia
- Familial hypokalemic periodic paralysis
- Pseudohypokalemia in thrombocytosis or leukocytosis
- Beta adrenergic excess
- True potassium deficit
- Renal (if renal potassium loss is > 30 mmol/day)
- Renal tubular acidosis
- Fanconi syndrome (disorder of renal proximal tubules leading to excessive excretion of certain electrolytes and amino acids)
- Diuretic phase of acute tubular necrosis (ATN)
- Medications: diuretics, penicillins, amphotericin B toxicity
- Glucocorticoid excess
- Mineralocorticoid excess
- Nonrenal (renal potassium loss is < 30 mmol/day)
- Diarrhea, vomiting, GI fistula
- Starvation
- Excessive sweating
- Renal (if renal potassium loss is > 30 mmol/day)
- Reference: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018
Hyperkalemia
- Plasma potassium > 5.0 mmol/L
- Clinical features include mental confusion, weakness, tingling, flaccid paralysis of extremities, bradycardia and respiratory muscle weakness
- Severe hyperkalemia (> 7 mmol/L) can lead to cardiac arrest
- Causes
- Redistribution
- Acidemia transfer of intracellular K to extracellular fluid
- Tissue hypoxia
- Diabetic ketoacidosis
- Severe burns
- Massive intravascular hemolysis
- Status epilepticus (sustained muscular activity)
- Tumor lysis syndrome
- Rhabdomyolysis
- Potassium retention
- Renal failure
- Addison disease (adrenal insufficiency, Na depletion also occurs)
- Medications: ACE inhibitors, NSAIDs and angiotensin II receptor blockers, potassium sparing diuretics (Crook: Clinical Biochemistry and Metabolic Medicine, 8th Edition, 2012)
- Pseudohyperkalemia
- Hemolysis
- Thrombocytosis
- Leukocytosis
- Redistribution
Chloride
- Chloride concentrations in plasma usually follow those of sodium
- Useful for calculating anion gap
- Disturbances usually reflect acid base disturbances
- Elevated chloride in sweat remains a mainstay diagnostic indicator for cystic fibrosis
- Reference: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018
Hypochloremia
- Usually parallels causes of hyponatremia
- Prolonged vomiting, however, can lead to a significant loss of chloride (hypochloremia alkalosis)
- Respiratory acidosis is a common cause of decreased chloride with normal sodium levels
- Reference: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018
Hyperchloremia
- Hyperchloremia usually accompanies hypernatremia (diabetes insipidus, dehydration, excess normal saline solution treatment)
- Can also be seen in respiratory alkalosis
- Reference: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018
Bicarbonate
- Bicarbonate makes up the second largest fraction (behind Cl-) of plasma anions
- Includes (1) plasma bicarbonate ion (HCO3-), (2) carbonate ion (CO32-) and (3) CO2 bound in plasma carbamino compounds (RCNHCOOH)
- Actual bicarbonate ion concentration is not measured in clinical laboratories
- Analyte usually measured in plasma is total CO2, which includes bicarbonate and dissolved CO2 (dCO2) but is often referred to as serum bicarb
- The most important buffer of plasma is the bicarbonate / carbonic acid pair
- Because HCO3- and CO2 are the major buffers of the body, pH is typically expressed as a function of their ratio
- Disorders of CO2 are usually referred to as respiratory disorders and disorders of HCO3- are referred to as metabolic disorders
- Kidneys have the predominant role of regulating the systemic HCO3- concentration
- Methods for total CO2 measurement with today’s automated instruments may be electrode based or enzymatic
- Reference: Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018
Calcium
- Calcium has a major structural role in the formation of bone and is important in blood coagulation, neurological and neuromuscular function and intracellular signaling
- Majority of calcium in the body is localized to bone (~99%)
- ~1% of calcium that is not associated with bone makes up the slowly exchangeable pool and the rapidly exchangeable pool
- Slowly exchangeable pool encompasses calcium in dystrophic sites (such as atheromas and damaged cartilage) and in subcellular organelles (such as the mitochondria and the endoplasmic reticulum)
- Rapidly exchangeable pool includes calcium in systemic circulation, interstitial fluid and cellular cytoplasm as well as calcium that has recently been deposited on bone surfaces
- Plasma total calcium exists as free calcium (40 - 50%); protein bound calcium to mostly albumin (~40%) or complexed (~10%) to anions such as citrate, lactate and phosphate
- Causes of hypercalcemia include hyperparathyroidism, malignancy, endocrine disorders, granulomatous diseases, drugs, immobilization and other miscellaneous causes
- Causes of hypocalcemia include decreased parathyroid hormone action, deficient vitamin D action, extreme dietary calcium deficiency, healing phase of various forms of bone disease and calcium saponification (e.g., acute pancreatitis)
- References: McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 22nd Edition, 2011, Crook: Clinical Biochemistry and Metabolic Medicine, 8th Edition, 2012, Clarke: Contemporary Practice in Clinical Chemistry, 4th Edition, 2020
Phosphate
- Phosphate is involved in many critically important biochemical processes, including energy metabolism (e.g., adenosine triphosphate [ATP]), nucleic acid metabolism, cell signaling, bone formation and maintenance of acid / base balance (especially related to urinary phosphate buffering)
- The majority of total body phosphate resides as organic phosphate complexed with proteins, lipids and carbohydrates
- In blood, organic phosphate is located primarily in erythrocytes, with the plasma containing mostly inorganic phosphate
- Inorganic phosphate (noncomplexed phosphate) in plasma is a very small proportion of total body phosphate
- ~10% of the serum phosphate is bound to proteins; 35% is complexed with sodium, calcium and magnesium; the remaining 55% is free
- Only inorganic phosphorus is measured in routine clinical settings
- Causes of hyperphosphatemia include hypoparathyroidism, pseudohypoparathyroidism, renal failure, hypervitaminosis D, cell lysis (tumor lysis, burns, shock, etc.), increased bone turnover or release from bone (thyrotoxicosis, prolonged immobilization, glucocorticoid withdrawal or deficiency)
- Causes of hypophosphatemia include inadequate intake (e.g., inadequate dietary intake or malabsorption of intestinal phosphate), phosphate redistribution into cells (e.g., acute respiratory alkalosis, insulin administration), excessive loss (e.g., renal phosphate wasting, rickets with secondary hyperparathyroidism, heavy metal poisoning, drug induced renal phosphate wasting)
- References: McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 22nd Edition, 2011, Crook: Clinical Biochemistry and Metabolic Medicine, 8th Edition, 2012, Clarke: Contemporary Practice in Clinical Chemistry, 4th Edition, 2020
Magnesium
- Magnesium is the fourth most abundant cation in the body (following sodium, potassium and calcium)
- Total body magnesium is ~50 - 60% in bone and 40 - 50% in soft tissue
- It is the second most abundant intracellular cation (following potassium)
- Only 1% of the total body magnesium (TBMg) is in extracellular fluid
- No primary hormone controls circulating magnesium concentrations
- In serum, ~55% of magnesium is ionized or free magnesium (Mg++), 30% is associated with proteins (primarily albumin) and 15% is complexed with phosphate, citrate and other anions
- Hypermagnesemia is mostly iatrogenic (e.g., infusion of magnesium sulfate in the treatment of preeclampsia or eclampsia in pregnant women)
- Other causes of hypermagnesemia include excessive oral intake, increased gastrointestinal tract absorption (e.g., hypomotility and milk alkali syndrome), cellular release (e.g., tumor lysis syndrome and rhabdomyolysis) and contraction of the circulating blood volume
- Causes of hypomagnesemia include malabsorption, malnutrition and renal magnesium wasting from drugs (e.g., loop or thiazide diuretics, cisplatin, cyclosporine or tacrolimus), endocrine disorders (e.g., primary hyperaldosteronism, hypoparathyroidism and hyperthyroidism) or osmotic diuresis
- References: McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 22nd Edition, 2011, Crook: Clinical Biochemistry and Metabolic Medicine, 8th Edition, 2012, Clarke: Contemporary Practice in Clinical Chemistry, 4th Edition, 2020
Laboratory investigations for electrolytes
- Specimen is usually serum, plasma or urine; others include capillary blood, heparinized whole blood (for blood gas and pH determinations), body fluid aspirates, feces and sweat (chloride)
- Anion gap
- ([Na+] + [K+]) − ([Cl-] + [HCO-3])
- Potassium is commonly omitted from the calculation
- Elevated anion gap (> 12 mEq/L) = metabolic acidosis
- Reduced anion gap (< 3 mEq/L) may be due to hypoalbuminemia as albumin is the primary unmeasured anion
- Laboratory methods used to determine electrolyte concentrations include (Rifai: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th Edition, 2018)
- Atomic absorption spectrophotometry
- Flame emission spectrophotometry
- Ion selective electrodes (direct using undiluted sample or indirect using diluted sample)
- Enzymatic spectrophotometry
- Coulometric - amperometric titration (sweat chloride)
- Ion selective electrodes is the most common method used
- Typical reference intervals
- Serum sodium: 136 - 145 mmol/L
- Serum potassium: 3.5 - 5.1 mmol/L
- Serum chloride: 98 - 107 mmol/L
Board review style question #1
A patient is found to have plasma potassium of 6.9 mmol/L during his annual health check. The analysis was done a few hours after collection. He does not have any known health issues and is not on any medications. His complete blood count was normal. His renal functions are normal and his last potassium check was normal a few months ago. What is the most likely cause of the high potassium result?
- Diabetic ketoacidosis
- Diarrhea
- Hemolysis
- Thrombocytosis
- Tumor lysis syndrome
Board review style answer #1
C. Hemolysis. Hyperkalemia in a healthy person is most likely due to a preanalytic issue, the most common being hemolysis. Hemolysis falsely elevates potassium in plasma and can occur due to delayed processing of whole blood specimens or refrigeration of whole blood. A repeat assay should be requested. Answer D is incorrect because the complete blood count was normal and therefore rules out thrombocytosis as a cause. Answer A is incorrect because a patient with diabetic ketoacidosis will be critically ill. Answer E is incorrect because tumor lysis syndrome can cause hyperkalemia but in this patient, the patient had no history of malignancy or therapy. Answer B is incorrect because diarrhea would cause hypokalemia and not hyperkalemia.
Comment Here
Reference: Electrolytes
Comment Here
Reference: Electrolytes
Board review style question #2
What is pseudohyponatremia caused by?
- Hypoproteinemia
- Hyperlipidemia
- Liver cirrhosis
- Nephrotic syndrome
- Renal failure
Board review style answer #2
B. Hyperlipidemia. Pseudohyponatremia occurs as a result of how the blood sample is processed for serum sodium measurement. Using automated indirect ion selective electrode methods, proteins and lipids decrease the water content, which alters the ion concentrations measured and gives a falsely low sodium result. It is also called the electrolyte exclusion effect. Answer A is incorrect because hyperproteinemia (and not hypoproteinemia) gives the same electrolyte exclusion effect as hyperlipidemia. Answers C, D and E are incorrect because these are all causes of hypoproteinemia and not hyperproteinemia.
Comment Here
Reference: Electrolytes
Comment Here
Reference: Electrolytes
Fludrocortisone suppression test
Table of Contents
Definition / generalDefinition / general
Indication:
Rationale:
Procedure:
Normal reference:
Adverse effects:
Clinical, other:
- Confirms the diagnosis of primary (hyper) aldosteronism
- One of four tests recommended for screening or confirmation
- Also:
- Oral sodium loading
- Aaline infusion and captopril suppression (Horm Metab Res 2010;42:406)
Rationale:
- Fludrocortisone suppresses aldosterone production in normal subjects (molecule is structurally similar to cortisone, Wikipedia - Fludrocortisone), but not in patients with primary aldosteronism
Procedure:
- Correct hypokalemia if patient is hypokalemic before administering this test
- Give fludrocortisone 0.2 mg twice daily with 500 mg NaCl supplementation for 3 days (Scand J Clin Lab Invest 2009;69:234)
- Measure urine aldosterone excretion on the third day
Normal reference:
- Levels should be < 10 g/d (28 nmol/d) in normal subjects
- Normal suppression excludes primary hyperaldosteronism
Adverse effects:
- May precipitate severe hypokalemia
- Serum potassium should be monitored regularly during the test
- Due to sodium loading, this test should not be used in the elderly and in patients with severe hypertension
- Test should be used with care and is not a first line screening test
Clinical, other:
- Intravenous saline load test is a reasonably good alternative to this more expensive and complex test to diagnose primary aldosteronism after a positive screening test (J Clin Endocrinol Metab 2006;91:2618)
Folate (pending)
[Pending]
HIV testing (pending)
Hemoglobin A1C
Table of Contents
Definition / general | Essential features | History | Terminology | ICD coding | Chemical aspects | Indications for testing | Laboratory | Laboratory analysis | Frequency of testing | Reference values | Variations | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Gold standard laboratory test used to evaluate long term glucose control
Essential features
- Indicator of an average of body blood sugar levels over the preceding 90 days
- A standard of care (SOC) test for diagnosis and management of diabetes
- Elevated levels of HbA1c have been identified as a significant risk factor for cardiovascular diseases and stroke in subjects who may have diabetes
- HbA1c exhibits positive correlations with cholesterol, triglycerides and low density lipoprotein cholesterol, as well as an inverse correlation with high density lipoprotein cholesterol
History
- First isolated by Huisman et al. in 1958
- Characterized as a glycoprotein by Bookchin and Gallop in 1968
- Elevated levels of HbA1c were first reported in diabetic patients by Rahbar et al. in 1969 (Indian J Endocrinol Metab 2012;16:528)
- Chemical pathway leading to the formation of HbA1c was identified by Bunn et al. in 1975
- First proposed to be used as a biomarker for monitoring levels of glucose among diabetics by Koenig et al. in 1967 (Biomark Insights 2016;11:95)
Terminology
- Synonyms: hemoglobin A1C, glycated hemoglobin, glycosylated hemoglobin, HbA1c or A1C (StatPearls: Hemoglobin A1C [Accessed 28 September 2022])
ICD coding
- ICD-10: R73.09 - other abnormal glucose
Chemical aspects
- HbA1c is a minor portion of adult hemoglobin (HbA)
- Formed by the nonenzymatic condensation of the carbonyl group of glucose and the amino group at the N terminus of the beta chain of hemoglobin A in red blood cells (RBCs), resulting in a labile aldimine or Schiff base (reversible reaction)
- As RBCs circulate, some of the aldimine undergoes a slow, irreversible conversion (Amadori rearrangement) to a stable ketoamine and forms HbA1c (Sensors (Basel) 2017;17:1798)
- Increase in glycated hemoglobin is proportional to both the level of blood glucose and lifespan of the red cell (Biomark Insights 2016;11:95)
- This specific characteristic of the hemoglobin biomarker is utilized for estimating the average blood glucose levels over the previous 2 - 3 months
Indications for testing
- Diagnostic criterion of diabetes: A1c ≥ 6.5% (American Diabetic Association) (J Gen Intern Med 2014;29:388)
- Monitor long term glycemic control
- Adjust therapy in diabetic patients
- Assess quality of diabetic care
- Predict risk for development of complications related to diabetes (Biomark Insights 2016;11:95)
Laboratory
- Specimen for testing:
- Preferred dipotassium ethylenediaminetetraacetic acid (K2 EDTA); venous whole blood specimen collected in ethylenediaminetetraacetic acid (EDTA) is required
- Tubes containing heparin, potassium oxalate or sodium fluoride are acceptable
- Studies have reported no difference when collected in tripotassium ethylenediaminetetraacetic acid (K3 EDTA), sodium citrate, sodium heparin or sodium fluoride / ethylenediaminetetracetic acid disodium salt (Na2 EDTA) blood tubes (J Lab Physicians 2013;5:143)
- HemaSpot devices:
- Can be used as an alternative to current blood collection methods if calibrated as per standards
- Use dry blood spots, which are tested within 3 days of preparation (Diabet Med 2020;37:1463)
- Advantages:
- Provide convenient sample collection service for those living in remote or rural locations
- Provide sample collection service for those who are in urban areas but might be working or housebound
- Specimen integrity:
- Whole blood specimens in K2 or K3 EDTA are stable for up to 7 days stored at 2 - 8 °C, 3 days at room temperature (15 - 30 °C) or 12 months at -70 °C
- Timing of test:
- Convenience; not a timed testing or bound by fasting state
- Can be collected at any time
Laboratory analysis
- Methods based on charge difference:
- Ion exchange chromatography
- Capillary electrophoresis
- Methods based on structural difference:
- Immunoassay
- Enzymatic assay
- Affinity chromatography (Am J Clin Pathol 2014;141:5)
- Point of care testing:
- Based on latex immunoagglutination inhibition method (J Diabetes Res 2020;2020:2037565)
Frequency of testing
- At least 2 times a year in patients who are meeting treatment goals and have stable glycemic control
- At least 4 times a year to assess glycemic status and as needed in patients whose therapy has recently changed or who are not meeting glycemic goals (Clin Diabetes 2022;40:10)
Reference values
| Glycosylated hemoglobin (HbA1c) | mmol/L | Status |
| < 5.7% | 39 | Normal |
| 5.7 - 6.4% | 39 - 47 | Prediabetes |
| 6.5% or higher | 48 | Diabetes |
| National Glycohemoglobin Standardization Program (NGSP) HbA1c (%) | International Federation of Clinical Chemistry Working Group (IFCC) HbA1c (mmol/mol) | Estimated average glucose (eAG) (mg/dL) | Estimated average glucose (eAG) (mmol/L) |
| 5.0 | 31 | 97 | 5.4 |
| 6.0 | 42 | 126 | 7.0 |
| 7.0 | 53 | 154 | 8.6 |
| 8.0 | 64 | 183 | 10.2 |
| 9.0 | 75 | 212 | 11.8 |
| 10.0 | 86 | 240 | 13.3 |
| 11.0 | 97 | 269 | 14.9 |
| 12.0 | 108 | 298 | 16.6 |
- Officially, there is worldwide consensus that HbA1c should be reported in both NGSP (%) and IFCC (mmol/mol) units along with eAG (in either mmol/L or mg/dL) (World J Methodol 2016;6:133)
- The formula (NGSP = [0.09148 * IFCC] + 2.152) describes the relationship between NGSP (column 1) and IFCC (column 2) (World J Methodol 2016;6:133)
Variations
- Any condition that prolongs the life of RBCs or is associated with decreased red cell turnover exposes the cell to glucose for a longer period of time and results in falsely elevated A1C levels (J Gen Intern Med 2014;29:388):
- Asplenia
- Iron deficiency anemia
- Vitamin B12 or folate deficiency
- Severe hypertriglyceridemia (concentrations > 1,750 mg/dL)
- Severe hyperbilirubinemia (concentrations > 20 mg/dL)
- Uremia
- Chronic alcohol consumption
- Chronic salicylate ingestion
- Conditions that shorten the life of RBCs or are associated with increased red cell turnover shortens the exposure of the cell to glucose, resulting in falsely low A1C levels:
- Anemia from acute or chronic blood loss (hemolytic anemia, such as sickle cell anemia, spherocytosis)
- Splenomegaly
- End stage renal disease
- Pregnancy: can cause falsely low A1c through second trimester and high results in third trimester; hence, not useful for diagnosing gestational diabetes (J Gen Intern Med 2014;29:388)
- A1C results in a recently transfused patient should be considered uninterpretable
- Vitamin C supplementation can either increase or decrease the HbA1c level depending on the method used for its measurement (World Health Organization: Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus [Accessed 29 September 2022])
- Not a suitable index for glycemic control for patients with short term fluctuations of plasma glucose
- When accurate HbA1c measurement is not possible, as in the above conditions, alternative measures of chronic glycemia such as fructosamine or glycated serum albumin should be used
- These measure the glycation of serum proteins rather than hemoglobin and reflect glycemia over the preceding 2 - 4 weeks (J Diabetes Sci Technol 2020;14:883)
Additional references
Board review style question #1
HbA1c is usually a reliable indicator of diabetic control in
- Adjusting therapy in diabetic patients
- Known cases of hemoglobinopathies
- Monitoring immediate term glycemic control
- Predicting risk for development of neural complications in diabetics
Board review style answer #1
Board review style question #2
Falsely low HbA1c laboratory values are seen in which one of the following conditions?
- B12 deficiency anemia
- Chronic alcoholism
- Hyperbilirubinemia
- Sickle cell anemia
Board review style answer #2
D. Sickle cell anemia. Increased RBC turnover shortens the exposure of the cells to glucose, causing the A1c level to be falsely reduced.
Comment Here
Reference: Hemoglobin A1C
Comment Here
Reference: Hemoglobin A1C
Hemoglobin electrophoresis (pending)
[Pending]
Hepatitis B testing
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Diagrams / tables | Pathophysiology | Clinical features | Test indications | Hepatitis B virus screening and testing recommendations (CDC, 2023) | Laboratory | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hepatitis B testing is used for screening, diagnosis and monitoring of hepatitis B virus (HBV) infections, which involves measurement of HBV specific antigens, antibodies and HBV DNA
- Test results of individual HBV markers or a combination of markers can help determine whether a patient has acute or chronic HBV infection, is immune to HBV due to past infection or vaccination or is susceptible to HBV infection as well as provide information on the level of viral replication and infectivity
Essential features
- HBV is considered noncytopathic: both liver damage and viral control are mostly immune mediated, where the clinical outcome of HBV infection depends on the convoluted interplay between virus replication and host immune response
- Chronic hepatitis B is defined as an HBV infection lasting > 6 months and is associated with a higher risk of liver related complications including cirrhosis, hepatic failure and hepatocellular carcinoma (HCC)
- Diagnosis of HBV infection requires serology tests, including HBsAg, anti-HBs, total anti-HBc and IgM anti-HBc, which are used for screening and testing patients with increased risk for HBV infection
- Presence of HBsAg indicates acute or chronic infection, with the exception of transient positivity due to recent vaccination; positive specimens are required to be retested by a neutralization assay to confirm the presence of HBsAg
- After identifying a patient with HBV infection, testing for HBeAg, anti-HBe and HBV DNA can provide information on the level of viral replication, infectivity and immunity to guide clinical management
Terminology
- Hepatitis B virus (HBV)
- Acute hepatitis B
- Chronic hepatitis B
- Hepatitis B surface antigen (HBsAg)
- Hepatitis B surface antibody (anti-HBs)
- Total antibody to hepatitis B core antigen / hepatitis B core antibody, total (total anti-HBc)
- IgM antibody to hepatitis B core antigen / hepatitis B core antibody, IgM (IgM anti-HBc)
- Hepatitis B e antigen (HBeAg)
- Hepatitis B e antibody (anti-HBe)
- Hepatitis B DNA viral load
ICD coding
Pathophysiology
- HBV, part of the Hepadnaviridae family, is a DNA virus that enters hepatocytes through a receptor sodium taurocholate cotransporting polypeptide (J Hepatol 2016;64:S32)
- Relaxed circular DNA (rcDNA) in the viral capsid is converted to covalently closed circular DNA (cccDNA) in the hepatocyte nucleus, where cccDNA is transcribed into the pregenomic RNA (pgRNA, reverse transcribed into HBV DNA) and mRNAs (translated into viral proteins) (Lancet 2023;401:1039)
- Persistence of cccDNA in hepatocytes is the main reason for the difficulty in HBV eradication and for reactivation of HBV replication (J Hepatol 2021;75:706)
- HBV DNA can integrate into the host genomic DNA and contributes to hepatocarcinogenesis (Microorganisms 2021;9:1787)
- HBV is considered noncytopathic: both liver damage and viral control are mostly immune mediated; the clinical outcome of HBV infection depends on the convoluted interplay between virus replication and host immune response
- Resolution of acute HBV infection is mainly mediated through the adaptive immune response and high level anti-HBs can persist decades after recovery (Nat Rev Immunol 2022;22:19)
- Patients chronically infected with HBV have impaired innate and adaptive immune response to HBV, in which the impairment in HBV specific T cell responses is believed to be mostly due to immune exhaustion (J Hepatol 2020;73:52)
Clinical features
- Overview
- HBV is transmitted through contact with infected blood or body fluids, such as during pregnancy or delivery, through sex or by injection drug use, with the greatest risk for chronic infection occurring during perinatal infection
- Coinfection with other viruses is seen with HBV infection, such as hepatitis D virus, hepatitis C virus and human immunodeficiency virus (HIV)
- Clinical manifestation of acute HBV infection varies from asymptomatic (~70%) to symptomatic (~30%) hepatitis presenting with nonspecific symptoms (i.e., fatigue, anorexia, nausea, right upper quadrant discomfort and jaundice) and uncommonly, fulminant hepatitis (encephalopathy and coagulopathy, < 1% in immunocompetent adults) (Lancet 2023;401:1039)
- Acute viral hepatitis infections are usually associated with significant elevation of aminotransferases (10 - 100 times the upper limit of normal range), such as alanine transaminase (ALT) and aspartate transaminase (AST), with AST/ALT ratio typically < 1 (Clarke: Contemporary Practice in Clinical Chemistry, 4th Edition, 2020)
- Patients with chronic HBV infection have an increased risk for liver related complications, including cirrhosis, liver failure and HCC
- Acute HBV infection
- Incubation period of HBV can range from 1 to 6 months; HBV DNA may be the only marker detectable in the first 2 weeks, whereas HBsAg appears in the serum 2 - 10 weeks after exposure, before symptom onset and elevated aminotransferases (see Figure 1)
- In the early phase of an acute infection, HBsAg, IgM anti-HBc and HBeAg are all positive with HBV DNA detectable in high levels
- During recovery, HBsAg disappears and seroconverts to anti-HBs
- In resolved hepatitis B, total anti-HBc will remain positive and anti-HBs will develop to protect against future infection; HBV DNA is undetectable in serum but can persist in the liver
- Chronic HBV infection
- Chronic hepatitis B is defined as an HBV infection lasting > 6 months, typically with HBsAg and total anti-HBc present but not IgM anti-HBc (Hepatology 2018;67:1560)
- HBeAg and anti-HBe are variably present and HBV DNA levels also vary (see Figure 1)
- Patients with chronic HBV infection may go through 5 different clinical phases, though not all patients go through all phases: immune tolerant, immune active, inactive carrier, HBeAg negative immune active and resolved or occult HBV infection
- Some patients with chronic HBV infection eventually become HBsAg negative
- Chronic hepatitis B is defined as an HBV infection lasting > 6 months, typically with HBsAg and total anti-HBc present but not IgM anti-HBc (Hepatology 2018;67:1560)
- Isolated total anti-HBc positive is used to describe the serological pattern with positive total anti-HBc but negative HBsAg and anti-HBs
- Occult hepatitis B refers to a small fraction of isolated total anti-HBc positive individuals who present with low level chronic viremia, in whom HBsAg is absent in the serum but HBV DNA is detectable
Clinical phases of chronic HBV infection (adapted from Lancet 2023;401:1039)
| Clinical phases | Alanine transaminase | HBeAg | Anti-HBe | HBsAg | Anti-HBs | HBV DNA |
| Immune tolerant | Normal | + | - | + | - | ++++ |
| Immune active | Elevated | + | - | + | - | ++++ |
| Inactive carrier | Normal | - | + | + | - | ++ |
| HBeAg negative Immune active | Elevated | - | + / - | + | - | ++ |
| Resolved or occult HBV infection | Normal | - | + | - | + / - | + / - |
Test indications
- In general, assessment of liver disease activity and staging is mainly based on aminotransferases, platelet count and elastography
- Diagnosis of HBV infection requires serology tests, including HBsAg, anti-HBs, total anti-HBc and IgM anti-HBc, for screening and testing patients with increased risk or suspicion of HBV infection
- After identifying a patient with HBV infection, testing for HBeAg, anti-HBe and HBV DNA can provide information on the level of viral replication and infectivity to help guide clinical management
- HBeAg status, aminotransferase levels and HBV DNA viral load are used to monitor and guide treatment decisions for patients with chronic HBV infection (Lancet 2023;401:1039)
- HBV screening and testing recommendations by the Centers for Disease Control and Prevention (CDC) in 2023 involve using the triple panel, HBsAg, anti-HBsAg and total anti-HBc, for universal HBV screening
Hepatitis B virus screening and testing recommendations (CDC, 2023)
- Universal HBV screening
- HBV screening at least once during a lifetime for adults aged ≥ 18 years using the triple panel (new recommendation)
- Screening pregnant persons
- HBV screening for all pregnant persons during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing
- Risk based testing
- Testing for all persons with a history of increased risk for HBV infection, regardless of age, if they might have been susceptible during the period of increased risk
- Susceptible persons include those who have never been infected with HBV and either did not complete a hepatitis B vaccine series or are known to be vaccine nonresponders
- Periodic testing for susceptible persons, regardless of age, with ongoing risk for exposures, while risk for exposures persists
- Persons who have an increased risk for acquiring HBV infection, including the following
- Infants born to HBsAg positive pregnant women
- Persons born in regions with HBV infection prevalence of ≥ 2%
- U.S. born persons not vaccinated as infants whose parents were born in regions with HBV infection prevalence of ≥ 8%
- Persons who are injecting drug users or have a history of injecting drug use (IDU)
- Persons incarcerated or formerly incarcerated in a jail, prison or other detention setting (new recommendation)
- Persons with HIV infection
- Persons with hepatitis C virus (HCV) infection or a past HCV infection (new recommendation)
- Men who have sex with men
- Persons with sexually transmitted infections (STIs), past STIs or multiple sex partners (new recommendation)
- Household contacts or former household contacts of persons with known HBV infection
- Needle sharing or sexual contacts of persons with known HBV infection
- Persons on maintenance dialysis, including in center or home hemodialysis and peritoneal dialysis (Kidney Med 2019;1:347)
- Persons with elevated alanine transaminase or aspartate transaminase levels of unknown origin
- Anyone who requests HBV testing regardless of disclosure of risk
- Testing for all persons with a history of increased risk for HBV infection, regardless of age, if they might have been susceptible during the period of increased risk
- Reference: CDC: Viral Hepatitis Surveillance and Case Management - Guidance for State, Territorial, and Local Health Departments [Accessed 23 February 2024]
Laboratory
- Methodology
- Chemiluminescent immunoassay (CLIA) is the most common platform used for hepatitis B serology testing
- HBsAg
- Protein on the surface of HBV that can be detected in either acute or chronic infections
- Presence of HBsAg indicates infection, with exception of transient positivity within 30 days after a dose of hepatitis B vaccine (CDC: Viral Hepatitis - Interpretation of Hepatitis B Serologic Test Results [Accessed 19 February 2024])
- Positive HBsAg specimens are required to be retested by a neutralization assay, which uses the principle of specific antibody neutralization to confirm the presence of HBsAg
- Some HBsAg mutants are not detectable by older assays
- HBsAg mutant strains can be detected by some HBsAg assays that first became available in the United States in 2015, including Abbott ARCHITECT instrument, ETI-MAK-2 PLUS and Siemens ADVIA Centaur XP or XPT instrument (Kidney Med 2019;1:347)
- Alternative testing options include quantitative HBV DNA or total anti-HBc with follow up HBV DNA if positive
- Anti-HBs
- Antibodies develop as part of the normal immune response to HBV infection or hepatitis B vaccination
- Appearance of anti-HBs and decline of HBsAg indicates recovery from HBV infection and development of immunity
- Anti-HBs concentration > 10 mIU/mL after completion of a vaccine series indicates immunity
- Testing for anti-HBs within 6 months of hepatitis B immune globulin administration can lead to inaccurate assessment of immune status (Dev Biol Stand 1983;54:347)
- Total anti-HBc
- Antibodies develop in all HBV infections (resolved or current) and typically persist for life
- Immunity gained through vaccination does not develop anti-HBc
- Total anti-HBc detects both IgG and IgM antibodies to HBcAg
- No commercially available test for IgG anti-HBc alone
- IgM anti-HBc
- Antibodies develop after HBsAg during the early phase of HBV infection and typically wane around 1 year postexposure in both acute and chronic HBV infections
- During acute HBV infection, there can be a period of 1 - 6 months between the loss of HBsAg and the appearance of anti-HBs, when diagnosis has to rely on IgM anti-HBc
- IgM anti-HBc should only be tested when acute HBV infection is a concern
- Other HBV markers
- HBeAg is a marker for viral replication and high infectivity; HBeAg is positive during acute HBV infection and remains positive in the early phases of chronic HBV infection
- Anti-HBe can be used to monitor response to treatment and progression of chronic HBV infection; positive anti-HBe with negative HBeAg are seen in patients with resolved HBV infection or in the later phases of chronic HBV infection
- HBV DNA test is a measure of viral load, which indicates HBV replication status to guide treatment decisions and to assess response; HBV DNA levels can also predict the risk of cirrhosis and HCC
- Emerging tests may provide additional information on patients with chronic HBV infection, such as quantification of HBsAg, HBV RNA and hepatitis B core related antigen (HBcrAg); HBV RNA and HBcrAg are considered more reliable serum markers of cccDNA transcription than HBV DNA or HBsAg level, which can be derived from both cccDNA and integrated HBV DNA (Gastroenterology 2019;156:355)
Hepatitis B testing results interpretation (adapted from MMWR Recomm Rep 2018;67:1)
| HBsAg | Total anti- HBc | IgM anti- HBc | Anti- HBs | HBV DNA | Possible interpretation |
| - | - | - | - | - | Never infected; susceptible if never vaccinated or vaccine failure |
| + | - | - | - | + or - | Early acute infection (if HBV DNA is positive); transiently positive for HBsAg after vaccination (if HBV DNA is negative) |
| + | + | + | - | + | Acute infection |
| - | + | + | + or - | + or - | Acute resolving infection: window period if anti-HBs is negative |
| - | + | - | + | - | Recovered from past infection and immune |
| + | + | - | - | + | Chronic HBV infection |
| - | - | - | + | - | Immune from vaccination; passive anti-HBs transfer after hepatitis B immune globulin administration |
| - | + | - | - | + or - | Isolated total anti-HBc positive* |
| - | + or - | - | + or - | + | Occult HBV infection |
| + or - | + | + or - | + or - | + | Possible HBsAg mutant infection |
- *Isolated total anti-HBc positive could result from
- Loss of anti-HBs after past resolved infection; HBV DNA is negative
- False positive total anti-HBc (i.e., susceptible); HBV DNA is negative
- To resolve the ambiguity of a false positive total anti-HBc result, test a follow up sample 4 - 8 weeks later
- If found positive, interpret as a resolved infection
- If negative, interpret as false positive
- Passive maternal transfer of total anti-HBc to infant born to a HBsAg positive gestational parent for up to 24 months; HBV DNA is negative
- Occult HBV infection: HBV DNA is positive, typically at low levels; anti-HBs may be positive or negative
- HBsAg mutant infection: HBV DNA is positive, typically at high levels; anti-HBs may be positive or negative
Board review style question #1
Which of the following is the most appropriate test for the diagnosis of acute hepatitis B virus (HBV) infection?
- Hepatitis B DNA testing
- Hepatitis B e antigen (HBeAg)
- Hepatitis B surface antibody (anti-HBsAg)
- Hepatitis B surface antigen (HBsAg)
- Total antibody to hepatitis B core antigen (total anti-HBc)
Board review style answer #1
D. Hepatitis B surface antigen (HBsAg). The presence of HBsAg indicates HBV infection, either acute or chronic. Answers A, B, C and E are incorrect because these tests are useful for differentiating infection phases and assessing infectivity or immunity but cannot be used for the diagnosis of acute HBV infection.
Comment Here
Reference: Hepatitis B testing
Comment Here
Reference: Hepatitis B testing
Board review style question #2
| HBsAg | Negative |
| Total anti-HBc | Positive |
| IgM anti-HBc | Positive |
| Anti-HBsAg | Negative |
| HBeAg | Negative |
| HBV DNA | Negative |
What is the possible interpretation of the hepatitis B testing results shown above?
- Active chronic HBV infection
- Early acute HBV infection
- Occult HBV infection
- Recovered from past HBV infection and immune
- Resolving acute HBV infection (window period)
Board review style answer #2
E. Resolving acute infection (window period). During acute HBV infection, there can be a period of 1 - 6 months between the loss of HBsAg and the appearance of anti-HBs, when diagnosis has to rely on IgM anti-HBc. Answer B is incorrect because HBsAg, HBeAg and HBV DNA are negative. Answer D is incorrect because anti-HBsAg is negative, indicating a lack of immunity. Answers A and C are incorrect because IgM anti-HBc is positive.
Comment Here
Reference: Hepatitis B testing
Comment Here
Reference: Hepatitis B testing
Hepatitis C testing (pending)
[Pending]
High sensitivity CRP
Definition / general
- hsCRP is an enhanced sensitivity C-reactive protein (CRP) immunoassay with a lowered measurement cutoff
Laboratory
Methodology
Indications
Limitations
Reference ranges
- Laser nephelometry
Indications
- In the JUPITER trial of apparently healthy persons without hyperlipidemia but with elevated high-sensitivity C-reactive protein levels, rosuvastatin significantly reduced the incidence of major cardiovascular events (N Engl J Med 2008;359:2195)
- This effect is thought to be due to the effect of statins on inflammation, which is detected by hsCRP
- hsCRP assessment for cardiovascular disease in asymptomatic individuals seems to be most useful for those classified as intermediate risk on the basis of traditional risk factors (e.g. an NCEP-ATP III global risk score between 5% and 20%), and who do not already warrant chronic treatment with aspirin and a statin
Limitations
- Most useful for patients with intermediate risk for cardiovascular disease (Circ Cardiovasc Qual Outcomes 2008;1:92, Ann Intern Med 2009;151:483)
- For low risk patients, if their risk increases 3x (e.g. from 1% to 3%), their absolute cardiovascular risk is still low, so the hsCRP test has no practical value
- High risk patients are candidates for chronic aspirin and lipid-lowering therapy regardless of their hsCRP test results
- However, a recent study concludes that risk based statin treatment without hs-CRP testing is more cost-effective than hs-CRP screening, assuming that statins have good long-term safety and provide benefits among low-risk people with normal hs-CRP (Circulation 2010;122:1478)
Reference ranges
- Low risk: under 1 mg/L
- Intermediate risk: 1-3 mg/L
- High risk: > 3 mg/L
Additional references
Hyperaldosteronism
Table of Contents
Definition / general | Terminology | Diagrams / tables | Etiology | Clinical features | Laboratory | Diagnosis | Case reports | Treatment | Additional referencesDefinition / general
- Disorder caused by excess secretion of aldosterone
- See also Adrenal gland - Hyperaldosteronism
Terminology
- Primary hyperaldosteronism: cause is in the adrenal gland
- Secondary hyperaldosteronism: cause is extra-adrenal
- Tertiary hyperaldosteronism: cause is renal juxtaglomerular cells
Diagrams / tables
Etiology
- Primary hyperaldosteronism causes:
- Idiopathic adrenal hyperplasia: most common cause
- Conn syndrome: aldosterone producing adrenal adenoma, rarely adrenal carcinoma
- Secondary hyperaldosteronism causes:
- Increased levels of plasma renin from non-adrenal pathology
- Includes:
- Congestive heart failure
- Pregnancy (due to estrogen)
- Decreased renal perfusion (renal arterial stenosis, nephrosclerosis)
- Gypoalbuminemia
- Ovarian tumor
- Hyperthyroidism
- Tertiary hyperaldosteronism (Bartter syndrome):
- Hypertrophy and hyperplasia of renal juxtaglomerular cells, causing elevated plasma renin, angiotensin II and aldosterone, hypokalemic alkalosis but no hypertension
- Some cases are autosomal recessive
- Infants or adults (eMedicine - Bartter Syndrome, Wikipedia - Bartter syndrome)
- Glucocorticoid suppressible hyperaldosteronism:
- Also called familial hyperaldosteronism type I
- Rare, familial
- Due to mutation which causes developmental derangement of cortical zonation, with hybrid cells between glomerulosa and fasciculata that are under the influence of ACTH, but can be suppressed by dexamethasone
- Familial cases:
- Early onset hypertension and severe target organ damage
Clinical features
- Causes urinary loss of potassium and hypokalemia, sodium retention and hypertension
- May cause up to 14% of cases of refractory hypertension (Arq Bras Cardiol 2009;92:39)
- Hypokalemia (present in 63%, Dtsch Arztebl Int 2009;106:305) causes weakness, paresthesias, visual disturbances, tetany
- Sodium retention causes volume overload which suppresses the renin-angiotensin system and reduces plasma renin activity
- Volume overload causes polyuria, polydipsia, nocturia, hypertension, alkalosis, hypernatremia
Laboratory
- High serum sodium, low serum potassium, metabolic alkalosis
Diagnosis
Tests for primary hyperaldosteronism
- Nonsuppressible aldosterone excretion with normal cortisol excretion, low plasma renin
- Screening tests:
- Preferred screening test is Ratio of plasma aldosterone concentration (PAC, in ng/dl) to plasma renin activity (PRA, in ng/ml/hr)
- Ratio >30 is strongly suggestive of primary hyperaldosteronism
- Confirmatory test:
- Serum aldosterone level, urine aldosterone levels, saline suppression test
Case reports
- 28 year old woman with adrenal adenoma also causing hypocalcemia (J Korean Med Sci 2009;24:1220)
Treatment
- Surgery for adenoma
- Patients with bilateral adrenal hyperplasia need spironolactone or other antihypertensive drugs
Additional references
Hypercortisolism
Table of Contents
Definition / general | Terminology | Epidemiology | Clinical features | Diagnosis | Laboratory | Clinical images | Gross images | Additional referencesDefinition / general
- Disorder of chronic exposure to high levels of cortisol in the blood, either endogenous or exogenous
Terminology
- Also called Cushing syndrome in Adrenal Chapter
- Cushing disease refers to a pituitary adenoma that produces excess ACTH
- Subclinical hypercortisolism:
- Due to incidental adrenal masses that may secrete cortisol autonomously, with no signs or symptoms of hypercortisolism (Eur J Endocrinol 2009;160:87)
- Associated with high prevalence of hypertension, diabetes mellitus (Eur J Endocrinol 2005;153:837), obesity, dyslipidemia and osteoporosis
Epidemiology
- Adrenal adenoma (eMedicine - Adrenal Incidentaloma) or hyperplasia
- Adrenal carcinoma
- ACTH secreting pituitary adenoma (Cushing disease)
- Other tumors with ectopic ACTH production
- Exogenous glucocorticoids
Clinical features
- Obesity (buffalo hump is characteristic), hypertension, glucose intolerance, moon facies, easy bruisability, striae, proximal muscle weakness, bone loss, osteonecrosis of femur head, menstrual irregularities
- With androgen excess, females show virilization
- Some patients may present with neuropsychological changes
Diagnosis
- A single test cannot be used to diagnose hypercortisolism
- Primary hypercortisolism: high serum cortisol, low plasma ACTH
- Secondary hypercortisolism: high serum cortisol, high plasma ACTH
Laboratory
- Increase in daily urinary cortisol excretion
- High midnight salivary cortisol levels
- Increase in late evening serum cortisol levels
Low dose dexamethasone suppression test:
- Absence of suppression supports a diagnosis of Cushing syndrome (J Clin Endocrinol Metab 2006;91:2582), but suppression also occurs in some patients with Cushing syndrome (J Clin Endocrinol Metab 2004;89:1222)
- This test is not very helpful in patients with abnormal levels of cortisol binding globulin
High dose dexamethasone suppression test:
- This test is useful in differentiating primary hypercortisolism from ACTH secreting pituitary adenoma
- No suppression is noted in patients with ectopic ACTH secretion or adrenal adenoma
CRH after dexamethasone test:
- Makes the dexamethasone test more sensitive
- Urinary and salivary cortisol should be measured twice (N Engl J Med 1986;314:1329)
CRH stimulation test:
- Usually done with equivocal plasma ACTH levels
- Indicated to differentiate Cushing disease from Cushing syndrome
- Pituitary tumor will show increase in ACTH and cortisol levels
- Adrenal tumor and ectopic ACTH production will not show any increase
Additional references
Hyperlipidemia
Table of Contents
Definition / general | Pathophysiology | Diagrams / tables | Types of hyperlipidemia | Management | Clinical images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Term used to denote excess of lipids, mainly cholesterol and triglycerides (TG), in the blood
- Strong relationship between high plasma lipids and coronary heart disease
- Lowering LDL and raising HDL decrease the progression of coronary atherosclerosis, the process responsible for majority of cardiovascular diseases (CVD)
Pathophysiology
-
Lipid metabolism
- Cholesterol and triglycerides are not water soluble and circulate bound to lipoproteins
- These consist of nonpolar core of TG and cholesteryl esters surrounded by a layer of phospholipids, cholesterol and proteins known as apolipoproteins (see Diagrams / tables below)
-
Classification of lipoproteins
- Chylomicrons: large particles that carry dietary fat (mainly TG) from the intestine to the liver
- Very low density lipoprotein (VLDL): carries endogenous TG synthesized in the liver to the tissues
- Low density lipoprotein (LDL): is formed from intermediate density lipoprotein (IDL) by hepatic lipase
- It carries cholesterol from liver to tissues
- High density lipoprotein (HDL): carries cholesterol from tissues to liver
Types of hyperlipidemia
-
Primary hyperlipidemia:
- There are many types of familial dyslipidemia; common forms include:
- Familial hyeprcholestrolemia (FH):
- Autosomal dominant
- LDL receptor defects
- In heterozygotes, cholesterol levels are in the range of 6 - 12 mmol/L, while in homozygotes, levels can be as high as 20 mmol/L
- Clinical features: tendon xanthoma, corneal arcus and xanthelasma (see Clinical images)
- Polygenic hypercholesterolemia:
- Most common form of familial hyperlipidemia
- Plasma cholesterol is not as high as FH and is influenced by environmental factors such as diet
- Familial combined hyperlipidemia:
- Results in elevated cholesterol and TG
- Associated with diabetes, obesity, cutaneous manifestations of hyperlipidemia and premature ischemic heart disease (IHD)
- Familial chylomicronemia:
- Failure to metabolise the chylomicrons due to deficiency of lipoprotein lipase or apoC-II
- Presents with pancreatitis, hepatosplenomegaly and eruptive xanthomata
- Familial hypertriglyceridemia:
- Autosomal dominant
- Associated with eruptive xanthomas, diabetes and pancreatitis
Secondary hyperlipidemia:
- Alcoholism
- Diabetes
- Cushing syndrome
- Renal failure
- Cholestasis
- Nephrotic syndrome
- Hypothyroidism
- Drugs: β blockers, thiazides, steroids and antiretrovirals
Management
- Exclude secondary causes of dyslipidemia
- Lifestyle modifications (Am Fam Physician 2010;81:1097):
- Diet
- Reduce total fat intake to 30% or less of total energy and intake of dietary cholesterol to less than 300 mg/day
- Eat at least 5 portions of fruits and vegetables per day and ≥ 2 portions of fish per week
- Reduce alcohol consumption to 2 drinks per day for men and 1 drink per day for women
- Smoking cessation
- Encourage physical activity
- Diet
- Assess the following before starting treatment:
- Blood pressure
- Body mass index (BMI)
- Total cholesterol, non HDL cholesterol, HDL cholesterol and triglycerides
- HbA1c
- Renal function and eGFR
- Transaminase levels
- Thyroid stimulating hormone
- Primary prevention (treatment of those with no evidence of disease):
- Treat with statins if lipids remain high despite nondrug therapy and 10 year CVD risk is ≥ 10%
- Secondary prevention (treatment of those who have proven CVD):
- Start treatment immediately, irrespective of initial cholesterol levels
- Patients with diabetes are considered as secondary prevention
- Hypercholesterolemia
- Statins are the drugs of choice for the treatment of hypercholesterolemia
- They inhibit HMG CoA reductase required for cholesterol synthesis in the liver
- Possible side effects: myalgia, myositis, abdominal pain and abnormal liver function tests
- Statins are contraindicated in pregnancy, breastfeeding and active liver disease
- Bile acid sequestrants: they act by binding bile acids, preventing their reabsorption, which promotes hepatic conversion of cholesterol into bile acids
- Gastrointestinal side effects are common
- Ezetimibe (Zetia or Ezetrol) inhibits the intestinal absorption of cholesterol
- Fibrates act mainly by decreasing serum triglycerides
- They tend to be less effective than statins at lowering cholesterol
- They also increase HDL concentration
- Statins are the drugs of choice for the treatment of hypercholesterolemia
- Hypertriglyceridemia:
- Responds best to fibrates, nicotinic acid or fish oil
Additional references
Board review style question #1
- When do you start treatment with statins?
- 10 year CVD risk is ≥ 10%
- 10 year CVD risk is ≥ 15%
- 10 year CVD risk is ≥ 20%
- 10 year CVD risk is ≥ 30%
- 10 year CVD risk is ≥ 50%
Board review style answer #1
Hyperthyroidism-lab diagnosis
Table of Contents
Definition / general | Etiology | Diagnosis | Laboratory | Treatment | Additional referencesDefinition / general
- See also Hyperthyroidism in Thyroid chapter
- Hyperfunction of gland (usually elevated levels of free T3 / T4, but see subclinical hyperthyroidism below)
- Thyrotoxicosis refers to clinical effects of free thyroid hormone
- Source may not be thyroid gland
- May be masked by nonthyroidal illness syndrome (J R Soc Med 2003;96:185)
Etiology
- Graves disease (85%-circulating autoantibodies to TSH receptor)
- Overdose of exogenous thyroid hormone, hyperfunctioning multinodular goiter or thyroid adenoma, thyroiditis, struma ovarii, iodide ingestion, choriocarcinoma/hydatidiform mole, maternal Graves disease, pituitary adenoma
Diagnosis
- High free T4
- TSH may be low (primary hyperthyroidism) or high (secondary hyperthyroidism)
- In subclinical hyperthyroidism, T3 and T4 are normal but TSH is low
Laboratory
Primary hyperthyroidism
Secondary hyperthyroidism
Subclinical hyperthyroidism
T3 hyperthyroidism
T4 hyperthyroidism
- Intrinsic thyroid gland abnormality producing excess T4
- High free T4, low TSH, normal TRH stimulation test
Secondary hyperthyroidism
- Abnormal TRH stimulation test (problem is at level of pituitary, including TSH producing tumors), high TSH, high free T4
Subclinical hyperthyroidism
- Low TSH (< 0.1 μIU/ml), normal T3 and T4 (Eur J Endocrinol 2005;152:1), no clinical hyperthyroidism
- May be due to exogenous thyroid (Hormones (Athens) 2006;5:119)
- Patients have increased risk of atrial fibrillation, cardiac death and osteoporosis
T3 hyperthyroidism
- 1 - 4% of hyperthyroid patients
- Low TSH, high free T3, normal free T4
- Associated with early treatment of hyperthyroidism with antithyroid drugs
T4 hyperthyroidism
- High T4, normal T3
- Due to primary hyperthyroidism causes, also iodine, amiodarone (Arch Pathol Lab Med 2003;127:e275), pregnancy (2%)
Treatment
- Beta blockers for symptoms, thionamide-type drugs to block new hormone synthesis, iodine to block release of T4/T3
Additional references
Hypothyroidism-lab diagnosis
Table of Contents
Definition / general | Laboratory | Thyrotropin releasing hormone | Thyroid binding globulin | Additional referencesDefinition / general
- See also Hypothyroidism in Thyroid chapter
- May initially have transient hyperthyroidism
- May cause macrocytic, nonmegaloblastic anemia with normal RDW
Laboratory
Primary hypothyroidism
Secondary hypothyroidism
Tertiary disease
Subclinical hypothyroidism
Cretinism
Myxedema
- Due to:
- Destruction or ablation of thyroid gland (surgery, radiation, Hashimoto thyroiditis, developmental)
- Interference with thyroid hormone synthesis (idiopathic, genetic J Med Genet 2005;42:379)
- Drugs (lithium, iodide, methimazole, PTU)
- Iodine ingestion or iodine deficiency (Intern Med 2007;46:391)
- T4 is low causing high TSH
- Other causes:
- Chronic renal failure has normal TSH, low T3 and T4 (Nucl Recept 2005;3:1)
- Bromide intoxication (Am J Clin Pathol 1988;89:802)
Secondary hypothyroidism
- Pituitary disorder causes reduced TSH secretion
Tertiary disease
- Hypothalamic lesion causes reduced TRF secretion
Subclinical hypothyroidism
- High TSH, normal T3 and T4
- No clinical symptoms of hypothyroidism (Am Fam Physician 2005;71:1763)
Cretinism
- Hypothyroidism developing during infancy/childhood
- May be due to maternal hypothyroidism (maternal T3/T4 crosses placenta and is critical to fetal brain development before fetal thyroid gland develops)
- Now rare due to newborn testing and iodine supplementation (Pediatrics 2006;117:2290)
Myxedema
- Chronic hypothyroidism in older child or adult
Thyrotropin releasing hormone
Definition / general
Laboratory
Interpretation
- Also called thyrotropin releasing factor
- Produced by hypothalamus
- Enhances TSH synthesis and stimulates its secretion
- Also affects prolactin and other pituitary hormones to a lesser extent
- Also produced in pancreatic beta cell, where it colocalizes with insulin, highest levels in neonates (Acta Biomed 2007;78:216)
- Feedback inhibition due to circulating T3 and T4
- Regulated also by leptin, which mediates food appetite
- Resistance occurs rarely (OMIM: TRHR [Accessed 8 October 2021])
Laboratory
- Methodology:
- Can measure by RIA but specimen must be frozen
- TRH stimulation test:
- Measure baseline TSH
- Give IV bolus of TRH
- Measure TSH (should rise within 5 minutes, peak in 20 minutes)
- Fold increase is a better measure of response than absolute increase
Interpretation
- Exaggerated: primary hypothyroidism
- Suppressed: hyperthyroid patients (including Graves disease), also multinodular goiter, renal failure, Cushing syndrome, depression, drugs
- No response: pituitary disorder (may not be necessary for diagnosis) (J Clin Endocrinol Metab 2003;88:5696)
Thyroid binding globulin
Definition / general
Pathophysiology
Laboratory
Indications:
- Deficiency does not cause thyroid disease by itself
- Increases / decreases cause increase / decrease in total T3 and T4
- However, free T3 and T4 are unchanged
Pathophysiology
- Produced in liver
- Major carrier protein for T3 and T4
- Has highest affinity but lowest concentration of the carrier proteins
- Other thyroid carrier proteins are transthyretin and albumin, which have lower affinity but higher concentration
Laboratory
- Interpreting T3 and T4 levels that do not match clinical findings or other laboratory results
- For example, total T3 and T4 levels are very low, TSH is normal, free T3 and T4 are normal, patient is clinically euthyroid
- Methodology:
- Chemiluminescence immunoassay
- Reference range:
- 13 - 39 μg/dL (150 - 360 nmol/L)
- High values:
- Inherited abnormalities, drugs (clofibrate, estrogens, 5-FU, heroin / methadone), hepatitis, pregnancy, idiopathic)
- Low values:
- Inherited abnormalities, drugs (androgens, glucocorticoids), liver failure, malnutrition, nephrotic syndrome, idiopathic)
Additional references
Immunofluorescence testing (pending)
[Pending]
Important wavelengths for various tests (pending)
[Pending]
Interference in protein electrophoresis
Table of Contents
Definition / general | Essential features | Factors interfering with electrophoresis | Types of interferences | Fibrinogen | Hemolysis | Human antianimal antibodies (HAAAs) | IgG4 related disease (IgG4 RD) | Contrast dyes | Antifungals and antibiotics | Gelatin based plasma substitutes | Hydroxocobalamin | Monoclonal therapies | Monoclonal immunoglobulin rapid accurate molecular mass (miRAMM) assay | Diagrams / tables | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Interference causes a clinically significant difference in the assay result due to another component or property of the sample
- For serum electrophoresis, it can be exogenous or endogenous
Essential features
- Interference in serum electrophoresis can cause misinterpretation, leading to improper patient management
- Immunofixation, immunosubtraction and clinical assessment can mitigate most of the interferences
Factors interfering with electrophoresis
- Analytical interferences
- Important causes of laboratory error
- Clinically significant consequences, including over or undertreatment and misdiagnosis
Types of interferences
| Endogenous | Exogenous |
|
|
- Reference: Clin Biochem 2018;51:72
Fibrinogen
- Fibrinogen is not normally present in serum specimens if adequate preanalysis is performed
- Fibrinogen may be present in serum
- Disorders of coagulation
- Anticoagulation therapy
- When a plasma sample is erroneously provided instead of serum
- When serum protein electrophoresis (SPEP) is performed on these samples, fibrinogen migrates to the β / γ region and it may be misinterpreted as a monoclonal immunoglobulin
- Following immunofixation electrophoresis (IFE) the apparent monoclonal protein is absent, this combined with the characteristic localization of the band in the β / γ region should establish the identity of this band as fibrinogen
- Although not routinely performed in diagnostic practice, IFE with antifibrinogen antibodies provides solid proof that the band is indeed fibrinogen
- Commercial sources of antiserum used for IFE may contain antibodies with specificity towards proteins that are typically absent from serum, such as fibrinogen, which can produce clinically misleading results
- For proper M protein analysis of these rare cases, either another blood sample should be obtained or the fibrinogen should be selectively eliminated prior to SPEP (Clin Chim Acta 2006;368:192)
- See image 1
- Reference: Clin Biochem 2018;51:72
Hemolysis
- The 2 main interference mechanisms are spectral interference from high concentrations of hemoglobin and direct release of analytes from red blood cells
- Hemolysis can be broadly divided into either prephlebotomy (in vivo hemolysis) or during phlebotomy (in vitro) causes
- In SPEP, hemoglobin and hemoglobin complexes that show up as discrete bands in the α2 and β regions may be misinterpreted as monoclonal proteins
- This is easily avoided by identifying hemolyzed specimens prior to interpretation or by reflexing to IFE to confirm the presence of any abnormal bands
- Reference: Clin Biochem 2018;51:72
Human antianimal antibodies (HAAAs)
- HAAAs are typically formed after exposure to animal proteins (either through occupational exposure or medical therapy)
- The most common HAAAs encountered are human mouse antibodies (HAMAs)
- Disorders affected by HAMAs include cases of organ transplantation, cancer, inflammation, infection and kidney disease, etc. (Clin Chem 1999;45:942)
- HAMAs interfere with serum IFE and may falsely appear as monoclonal proteins
- When SPEP / IFE in isolation are not sufficient to drive decision making, clinical awareness of this issue is the best course of action
- Reference: Clin Biochem 2018;51:72
Contrast dyes
- Contrasts dyes, used in imaging testing, interfere with capillary zone electrophoresis (CZE)
- Migrate to α2 globulin fraction or less frequently the β2 globulin fraction
- Reflex testing to IFE or immunotyping can confirm absence of a monoclonal component
- Clinicians should be aware that imaging using contrast dyes should be done after blood collection
- Reference: Clin Biochem 2018;51:72
Antifungals and antibiotics
| Drugs | Peak zones on CZE |
| 5 fluorocytosine (5FC) | Cathodal region of γ globulin fraction |
| Ceftriaxone | Anodal region of the prealbumin fraction |
| Sulfamethoxazole | Albumin peak |
| Piperacillin with tazobactam | α2 β1 globulin region |
- These interferences are more likely to occur when blood samples are collected closer to the time of perfusion, especially in the context of renal failure and drug accumulation
- Reflex testing to IFE or immunotyping can confirm the absence of a monoclonal component
- See image 3
- Reference: Clin Biochem 2018;51:72
Gelatin based plasma substitutes
- Gelatin based plasma substitutes can increase the γ globulin fraction, with a polyclonal pattern shifted to the β2 globulin fraction, simulating a βγ block in CZE
- Reflex testing to IFE or immunotyping can confirm absence of monoclonal component
- SPEP is rarely performed in an emergency context of infusion of gelatin based substitutes; together with their short half life (~2.5 hours), the risk of this type of interference is uncommon
- Reference: Clin Biochem 2018;51:72
Hydroxocobalamin
- Interference with SPEP concerning the α1 globulin fraction
- Hydroxocobalamin is the standard therapy for cyanide poisoning
- Interference will be much easier to detect on SPEP as hypoproteinemia is very frequently associated with severe burns
- Reflex testing to IFE or immunotyping can confirm absence of a monoclonal component
- Reference: Clin Biochem 2018;51:72
Monoclonal therapies
- Many of the available monoclonal therapeutics can appear as monoclonal IgG kappa by IFE
- Of the monoclonal drugs used for non-plasma cell dyscrasia based diseases, only rituximab and bevacizumab in therapeutic doses produced a visible M protein on SPEP / IFE
- Drugs like siltuximab, daratumumab and elotuzumab (all IgG kappa monoclonal antibodies) can appear as a small M protein, most often in concentrations of no more than 1 g/L or 0.1 g/dL, when used in therapeutic doses (Clin Chem 2010;56:1897)
- The main concern with the presence of these therapies is misclassification of disease response as having a very good partial response (VGPR) as opposed to a complete response
- Practically applicable mitigation response to monoclonal therapy varies with subtype of clonal immunoglobulin produced by plasma cell neoplasm
| IgG plasma cell neoplasm | Plasma cell neoplasm other than IgG |
|
|
- Daratumumab specific immunofixation electrophoresis reflex assay (DIRA) using an antibody targeting daratumumab to alter the migration of daratumumab during electrophoresis should be utilized
- Reference: Clin Biochem 2018;51:72
Monoclonal immunoglobulin rapid accurate molecular mass (miRAMM) assay
- miRAMM assay uses affinity purification followed by microflow LC-ESI-Q-TOF MS to determine the exact mass of monoclonal immunoglobulin chains
- This method is effectively immune to interferences including those from a monoclonal therapy because it will resolve each immunoglobulin peak by its unique mass and allow for it to be identified and differentiated (Methods 2015;81:56)
- Reference: Clin Biochem 2018;51:72
Diagrams / tables
Board review style question #1
Which of the following statements regarding electrophoreses after initiation of monoclonal therapy is true?
- Electrophoresis studies should be done before administration of monoclonal antibodies
- Immunofixation electrophoresis (IFE) will show a peak in the anodal part of the γ region with daratumumab
- Short time interval between serum protein electrophoresis (SPEP) and drug administration should decrease chances of interference
- SPEP showing a peak of 300 mg/dL will always mean a very good partial response (VGPR)
Board review style answer #1
A. Electrophoresis studies should be done before administration of monoclonal antibodies. Before each dose, the concentration of therapeutic monoclonal will be lowest and thus give less interference.
Answer C is incorrect because with longer intervals between the dose and testing, interference will be less.
Answer B is incorrect because daratumumab usually migrates to the cathodal part of the γ region.
Answer D is incorrect because a value of < 1 g/dL can be due to therapeutic monoclonal therapy.
Comment Here
Reference: Interference in protein electrophoresis
Comment Here
Reference: Interference in protein electrophoresis
Board review style question #2
Serum electrophoresis of a 60 year old man identifies a peak. Immunofixation electrophoresis (IFE) is performed. Which most the following is most consistent with the findings?
- Patient has multiple myeloma
- Plasma sample of patient with macrocytic anemia
- Serum sample obtained immediately after monoclonal therapy
- Serum sample of patient with IgG related disease
Board review style answer #2
B. Plasma sample of patient with macrocytic anemia. Plasma contains fibrinogen; plasma sample was erogenously run instead of serum.
Answer C is incorrect because IFE shows no monoclonal antibody.
Answer D is incorrect because the band is in β / γ region.
Answer A is incorrect because IFE shows no monoclonal antibody.
Comment Here
Reference: Interference in protein electrophoresis
Comment Here
Reference: Interference in protein electrophoresis
LD1-Lactate dehydrogenase isoenzyme 1
Definition / general
- LDH measures the amount of serum lactate dehydrogenase (LDH), which is released into the circulation with an acute myocardial infarction (AMI) due to tissue damage
- LDH is elevated on the second day after chest pain and remains elevated for up to 4 days
- LD1 is the heart specific form of the enzyme present in AMI between 12 and 24 hours after onset of chest pain
Pathophysiology
- LDH is an enzyme (EC 1.1.1.27) ubiquitous in tissue
- It has five isoenzymes, each with a different composition of M-type and H-type subunits in a tetrameric structure
- LD1 (HHHH) is present in cardiac muscle and erythrocytes
Laboratory
Methodology
Indications
Limitations
Reference ranges
- LD2 is usually the predominant form in serum
- In acute myocardial infarction, the serum levels of LD1 are greater than LD2 (a "flipped pattern")
- However, the use of LD1 to diagnose AMI has been largely superseded by Troponin I or T
- The isoenzyme 1 of LD is measured by immunoprecitation of LD 2-5 and measuring the residual activity
- Also by electrophoresis and staining of agarose media
Indications
- Patients presenting 12+ hours after the onset of chest pain or other symptoms suggestive of an acute myocardial infarction
Limitations
- The LD1 isoenzyme typically is elevated in acute renal failure and with hemolytic anemia
Reference ranges
- Must interpret values in context of clinical findings
Lipid panel
Table of Contents
Definition / general | Essential features | Terminology | Indications for testing | Laboratory | Lipid types | Reference ranges | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Set of chemistry tests to measure the level of various fats in a person's blood
Essential features
- Typical lipid profile includes testing for total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides (TG), very low density lipoprotein cholesterol (VLDL-C) and non-HDL cholesterol
- Maintaining desirable concentrations of each lipid lowers the risk of heart attack and stroke
Terminology
- Lipid profile
- Complete cholesterol test
- Lipoproteins
Indications for testing
- Known risk factors for developing heart disease (e.g., cigarette smoking, history of heart disease or atherosclerosis, history of stroke or heart attack)
- Family history of heart disease
- High blood pressure
- Obesity (Mayo Clin Proc Innov Qual Outcomes 2019;3:251)
- As a part of routine health check up, a fasting or nonfasting lipoprotein profile should be obtained at least every 5 years in all adults (≥ 20 years of age)
- Current guidelines from the National Heart, Lung, and Blood Institute recommend that children aged 9 - 11 years of age and young adults 17 - 21 years of age be screened for high cholesterol, regardless of risk factors
Laboratory
- Specimen for testing: serum (blood sample)
- Preferred: serum gel barrier tube (gold top)
- Alternative: plasma separator tube (green top containing sodium heparin or lithium heparin)
- Collection instructions:
- Serum gel or plasma separator tube must be centrifuged within 2 hours of draw time
- Red top tube must be centrifuged and aliquoted within 2 hours of draw time
- Rejection criteria:
- Sample showing gross hemolysis
- Underfilled or overfilled tube
- Anticoagulants other than heparin
- Possible interferences:
- Lipemic and icteric samples
- Plasma mixed with anticoagulants, such as oxalates, citrates and fluoride
- Methodology:
- Spectrophotometric (colorimetric)
- Variations:
- Change from an upright to a supine position due to dilutional effect can reduce the cholesterol levels by 10% and triglycerides by 12%
- Prolonged tourniquet application (2 - 5 minutes) can increase cholesterol by 5 - 15%
- Cholesterol is slightly higher in winter than in summer and the opposite is true for triglycerides
- Timing of test:
- Sample drawn after overnight fasting of 12 hours
- Though a fasting sample is preferred, recent guidelines recommend measurement of nonfasting lipids may be an equivalent or better predictor of cardiovascular conditions as nonfasting lipid profiles can reflect real status of circulating lipids (Ann Transl Med 2021;9:386)
- Principle:
- Cholesterol and triglycerides measured using enzymatically coupled reactions
- HDL: measured directly
- LDL: calculated / measured directly
- VLDL: calculated
Lipid types
- Total cholesterol:
- Lipids are synthesized by the body and transported by 5 classes of lipoproteins (HDL, LDL, VLDL, chylomicrons and IDL)
- Cholesterol is insoluble and requires transporters (e.g., lipoproteins)
- HDL and LDL play contradictory roles in cholesterol metabolism
- Total cholesterol by itself has a low, predictive value with regard to coronary artery disease (CAD); it provides only a baseline value to decide if further investigation of lipid metabolism must be carried out
- Since lipids cannot dissolve in water, they need to be broken down for ease of transportation in blood; proteins that aid in this process are lipoproteins (HDL and LDL)
- Triglycerides:
- Fatty acids complexed with glycerol, which represent the storage form of fat that can be used later as an energy source
- Ideal levels are < 150 mg/dL
- Independent risk factor for peripheral vascular disease and ischemic heart disease (IHD)
- Elevated in patients with acute and chronic pancreatitis, diabetes mellitus, diseases of lipid metabolism, endocrine and chronic inflammatory diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus) (NCBI Bookshelf: Endotext [Accessed 23 February 2022])
- Levels can help to classify various lipoprotein disorders and in the assessment of risk factors for atherosclerosis and coronary artery disease (CAD)
- HDL cholesterol:
- Also called good cholesterol or atheroprotective cholesterol
- Preferred levels > 40 mg/dL in men and > 50 mg/dL in women
- Type of lipoprotein that absorbs cholesterol and carries it back to the liver, for elimination from the body (Sports Med 2014;44:211)
- Levels are inversely associated with incidence of CAD
- Levels are used for early recognition of atherosclerosis risk and for monitoring patients when on lipid lowering medications
- LDL cholesterol:
- Also called bad cholesterol as it transports cholesterol from liver to peripheral tissues
- Preferred levels < 130 mg/dL
- High levels of LDL cholesterol increase risk for heart disease and stroke by accumulating on the walls of blood vessels (J Clin Lipidol 2014;8:473)
- Oxidized LDL is found in higher levels in smokers, diabetics and patients with insulin resistance
- Lipoprotein a (Lp[a]):
- Similar to LDL but contains an additional protein, apolipoprotein A (apo[a])
- Not routinely tested for as a part of lipid panel
- Interferes with plasminogen activation and inhibits fibrinolysis, leading to unopposed intravenous thrombosis and possible myocardial infarction
- Levels assessed in patients with personal history of (or first degree relative with) premature atherosclerotic vascular disease (particularly if otherwise considered low risk) and in patients with severe hypercholesterolemia (LDL-C ≥ 190 mg/dL) (Curr Atheroscler Rep 2021;23:51)
- VLDL cholesterol:
- Type of LDL cholesterol that is synthesized by liver and is mainly a transporter of triglycerides
- High levels of VLDL cholesterol have been associated with the development of plaque deposits on artery walls, which narrow the passage and restrict blood flow
- Non-HDL cholesterol:
- Calculated by subtracting HDL-C value from total cholesterol value
- Non-HDL-C is used to assess risk of heart disease or to decide on treatment targets for cholesterol lowering drug therapy (Am J Prev Cardiol 2021;6:100174)
Reference ranges
- Total cholesterol:
- < 18 years: < 170 mg/dL
- Adult: see below
- HDL cholesterol: males ≥ 40 mg/dL; females ≥ 50 mg/dL
- VLDL cholesterol: 5 - 40 mg/dL
- Lp(a): < 30 mg/dL
- Non-HDL cholesterol:
- < 100 mg/dL target level for high risk cardiovascular disease
- < 130 mg/dL optimal for general population
Total cholesterol
| Desirable | Borderline high | High |
| < 200 mg/dL | 200 - 239 mg/dL | ≥ 240 mg/dL |
Triglycerides
| Normal | Borderline high | High | Very high |
| < 150 mg/dL | 150 - 199 mg/dL | 200 - 499 mg/dL | ≥ 500 mg/dL |
LDL cholesterol
| Desirable | Above desirable | Borderline high | High | Very high |
| < 100 mg/dL | 100 - 129 mg/dL | 130 - 159 mg/dL | 160 - 189 mg/dL | ≥ 190 mg/dL |
- Variations:
- Results for each component of a lipid profile must be interpreted in context with other risk factors, including age, sex, smoking status, family and personal history of heart disease
- References: Circulation 2019;139:e1046, Circulation 2019;140:e596
Additional references
Board review style question #1
HDL cholesterol is called the good cholesterol because it
- Contains enzymes to break down cholesterol
- Is a negative regulator of cholesterol synthesis
- Transports cholesterol from liver to tissues for further metabolism
- Transports cholesterol from tissues to liver for excretion
Board review style answer #1
Board review style question #2
The level of which of the following is inversely related to the risk of cardiovascular disease?
- High density lipoprotein (HDL)
- Intermediate density lipoprotein (IDL)
- Low density lipoprotein (LDL)
- Very low density lipoprotein (VLDL)
Board review style answer #2
Liver function test panel
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Utility | CPT code | Proficiency testing | Instrumentation | Individual analytes | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Used for diagnosing liver disease in patients with history or clinical symptoms of liver dysfunction
- Aberrant test results may indicate the need for additional testing in order to assess the etiology
- Results within the panel are evaluated together to look for disease pattern
- Effectiveness of treatment for liver disease can be monitored by interval testing
- May reflect abnormalities in other organs as well, such as skeletal muscles, pancreas or heart (J Infus Nurs 2005;28:108, Ulster Med J 2012;81:30)
Essential features
- Alanine aminotransferase (ALT) is more liver specific than aspartate aminotransferase (AST), even though both are elevated in liver disease
- ALT elevation persists longer than AST
- Serum alkaline phosphatase (ALP) levels are increased in hepatobiliary disease and bone disease with increased osteoblastic activity
- Hepatic versus bone elevations can be distinguished by isoenzyme electrophoresis
- Hypoproteinemia, including hypoalbuminemia, can be caused by
- Primary factors, e.g. impaired synthesis from liver disease or
- Secondary factors, e.g. diminished protein intake, increased breakdown, malabsorption and increased renal excretion
- Hyperproteinemia may be seen in dehydration or as a result of increased production of proteins in inflammatory or neoplastic states
- Direct and indirect bilirubin are both increased in hepatic diseases, e.g. hepatitis and tumors and in obstructive lesions, e.g. carcinomas of the pancreas, common bile duct and ampulla of Vater
Terminology
- Liver function tests (LFTs)
- Hepatic function panel
- Liver profile
Pathophysiology
- Liver performs many functions, including the uptake, metabolism, conjugation and excretion of endogenous and exogenous compounds
- Immunological function: phagocytosis of microorganisms and removal of endotoxins from portal blood by the hepatic reticuloendothelial system
- Synthesis of vitamin K dependent coagulation proteins (factors II, VII, IX, X, protein C, protein S and protein Z) and factor V, XIII, fibrinogen, antithrombin, α2-plasmin inhibitor and plasminogen, as well as albumin and proteins
- Orchestrate secondary hemostasis and fibrinolysis
- Their plasma concentrations are used as indirect indicators of liver synthesis function
- Von Willebrand factor and factor VIII not synthesized by the liver and not affected by hepatic insufficiency
- Liver disease leads to decrease in the synthesis of albumin and coagulation factors, which results in increase in prothrombin time (PT) and international normalized ratio (INR)
- Hepatobiliary system metabolizes bilirubin, a process that involves transport of bilirubin into the hepatocyte, its conjugation to glucuronic acid and its secretion into bile canaliculi and the enterohepatic system
- Hepatitis leads to focal cellular injury due to destruction of hepatocytes by viruses, chemicals or trauma
- Can result in jaundice, which is caused by the blocking of bilirubin conjugation and excretion
- Causes elevation in both direct and indirect bilirubin levels
- Chronic passive congestion of the liver from congestive heart failure causes back pressure from the right heart to be transmitted to hepatic sinusoids from the inferior vena cava and hepatic veins
- Causes sinusoidal dilation and leads to physical damage of hepatocytes
- Results in mild increase in aminotransferases and occasionally mild hyperbilirubinemia
- Reference: Ann Surg 2013;257:27
Utility
- Tests in the panel include
- Liver enzymes, measured in international units per liter of blood
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- Alkaline phosphatase (ALP)
- Proteins, measured in g/dL or mg/dL
- Albumin
- Total protein (TP)
- Bilirubin - total bilirubin and direct bilirubin
- Liver enzymes, measured in international units per liter of blood
- Indications for testing (J Infus Nurs 2005;28:108)
- Symptoms of liver disease, e.g. jaundice and edema
- Hepatotoxic medications, e.g. chemotherapy
- Hematologic and coagulation disorders, e.g. bleeding and cytopenias
- Hepatotoxicity from exposure to environmental toxins
- Suspicion for cancer of the hepatobiliary system
- Potential exposure to hepatitis viruses
CPT code
- 80076: hepatic function panel
Proficiency testing
- Graded proficiency testing (PT) provides an external check on a laboratory’s performance of the assays
- PT program by the College of American Pathologists (CAP) is commonly used by laboratories in the U.S. for LFTs
- C-A 2020 General Chemistry / Therapeutic Drugs
- Laboratory test results are evaluated based on a range of acceptability around the peer group mean
- Must have at least 10 laboratories in the peer group
- Other PT programs approved by CLIA (Clinical Laboratory Improvement Amendments) include
(CMS: CLIA Approved Proficiency Testing Programs - 2020 [Accessed 1 December 2020])
- Accutest, Inc.
- American Academy of Family Physicians (AAFP) Proficiency Testing
- American Association of Bioanalysts (AAB)
- American Proficiency Institute (API)
- Medical Laboratory Evaluation (MLE) Program
- Puerto Rico Proficiency Testing Service
- Wisconsin State Laboratory of Hygiene Proficiency Testing (WSLH PT)
- PT program by the College of American Pathologists (CAP) is commonly used by laboratories in the U.S. for LFTs
Instrumentation
- Common instrument platforms include
- Abbott
- Beckman Coulter
- Ortho Clinical Diagnostics
- Roche
- Siemens
Individual analytes
- Alanine aminotransferase (ALT)
- Synonym
- Serum glutamic pyruvic transaminase (SGPT)
- Indications
- Diagnosis and monitoring of liver disease associated with hepatic necrosis
- Test methodology
- Photometric rate, L-alanine with pyridoxal-5-phosphate
- Example of reference ranges:
- Males
- ≥ 1 year: 7 - 55 U/L
- Not established for patients < 12 months of age
- Females
- ≥ 1 year: 7 - 45 U/L
- Not established for patients < 12 months of age
- Males
- Interpretation
- Present primarily in liver cells and elevated even prior to clinical signs and symptoms appearing in viral hepatitis and other liver diseases with hepatic necrosis
- A more liver specific enzyme than aspartate aminotransferase (AST), even though both are elevated in liver disease; however, the elevation of ALT activity persists longer than AST
- Elevated values seen in parenchymal liver diseases with destruction of hepatocytes; 20 - 50 times elevations are mostly seen and may reach as high as 100 times the upper limit
- ALT is as high as or higher than AST in infections and inflammatory conditions of the liver; the ALT:AST ratio, normally less than 1, becomes greater than 1
- Elevated ALT levels persist longer than AST
- Preanalytic considerations
- Pyridoxal phosphate is a cofactor in the reaction and is required for enzyme activity
- Synonym
- Aspartate aminotransferase (AST)
- Synonym
- Serum glutamic oxaloacetic transaminase (SGOT)
- Indications
- Diagnosing and monitoring of liver disease that results in destruction of hepatocytes
- Test methodology
- Photometric rate, L-aspartate with pyridoxyl-5-phosphate
- Example of reference ranges:
- Males
- 0 - 11 months: not established
- 1 - 13 years: 8 - 60 U/L
- ≥ 14 years: 8 - 48 U/L
- Females
- 0 - 11 months: not established
- 1 - 13 years: 8 - 50 U/L
- ≥ 14 years: 8 - 43 U/L
- Males
- Interpretation
- Elevated in alcoholism, cirrhosis, hepatitis, drug therapy and biliary disease
- Depressed in uremia, B6 deficiency and drug therapy
- Typically lower than ALT in infections and inflammatory conditions of the liver; the ALT:AST ratio, normally less than 1, becomes greater than 1
- Elevated before clinical signs and symptoms of disease appear
- In primary or metastatic carcinoma of the liver, 5 - 10 times elevations of both AST and ALT occur, with AST being higher than ALT but levels are usually normal in early malignancy
- Elevations more short lived than ALT
- May also be elevated in disorders of the heart, skeletal muscle and kidney
- Preanalytic considerations
- Pyridoxal phosphate is a cofactor in the reaction and is required for enzyme activity
- Synonym
- Alkaline phosphatase (ALP)
- Indications
- Diagnosing and monitoring treatment of liver, bone, intestinal and parathyroid diseases
- Test methodology
- Colorimetric
- Example of reference ranges:
- Males
- 0 - 14 days: 83 - 248 U/L
- 15 days - < 1 year: 122 - 469 U/L
- 1 - < 10 years: 142 - 335 U/L
- 10 - < 13 years: 129 - 417 U/L
- 13 - < 15 years: 116 - 468 U/L
- 15 - < 17 years: 82 - 331 U/L
- 17 - < 19 years: 55 - 149 U/L
- ≥ 19 years: 40 - 129 U/L
- Females
- 0 - 14 days: 83 - 248 U/L
- 15 days - < 1 year: 122 - 469 U/L
- 1 - < 10 years: 142 - 335 U/L
- 10 - < 13 years: 129 - 417 U/L
- 13 - < 15 years: 57 - 254 U/L
- 15 - < 17 years: 50 - 117 U/L
- ≥ 17 years: 35 - 104 U/L
- Males
- Interpretation
- Increase in ALP greater than 4 times the upper reference value is seen in extrahepatic obstruction, for example by stones or cancer of the head of the pancreas
- Greater than 4 times the upper reference value increase is seen in advanced primary liver cancer or widespread hepatic metastases
- Greater than 2 times the upper reference value can predict transplant free survival rates of patients with primary biliary cholangitis
- Normal to moderately increased (less than 3 times) in hepatic parenchymal diseases, e.g. infectious hepatitis
- Reaction to drug therapy may also lead to elevated ALP levels; an ALT / ALP based criterion can be used to distinguish the type of liver injury in drug induced hepatic toxicity
- Liver cirrhosis also causes elevations in intestinal ALP isoenzyme as it is an asialoglycoprotein that is cleared by the hepatic asialoglycoprotein receptors
- Hepatic versus bone elevations can be distinguished by isoenzyme electrophoresis
- Postanalytic considerations
- Age specific reference values should be used as values are higher in children and adolescents with bone growth
- Indications
- Albumin
- Indications
- Diagnosis and monitoring of nutritional diseases, liver function and renal function
- Test methodology
- Photometric, bromocresol green
- Example of reference ranges:
- ≥ 12 months: 3.5 - 5.0 g/dL
- Not established for patients < 12 months of age
- Interpretation
- Hyperalbuminemia occurs in dehydration
- Hypoalbuminemia (below 2.0 g/dL) occurs in edema
- Decreased serum albumin levels can be seen with reduced intake in malnutrition; increased turnover associated with glucocorticoid use and excessive loss in protein losing enteropathy, nephrotic syndrome or burn injury
- Postanalytic considerations
- Albumin values measured by the bromocresol green method may be different from those measured by electrophoresis
- Indications
- Total protein (TP)
- Indications
- Diagnosis of diseases involving the liver, kidneys, bone marrow and other metabolic or nutritional disorders
- Test methodology
- Colorimetric, biuret
- Example of reference range:
- ≥ 1 year: 6.3 - 7.9 g/dL
- Not established for patients < 12 months of age
- Interpretation
- Inflammation, infection and late stage liver disease cause increase in acute phase reactants and polyclonal immunoglobulins, leading to mild hyperproteinemia
- Multiple myeloma and other malignant paraproteinemias often cause moderate to marked hyperproteinemia
- Hypogammaglobulinemia, nephrotic syndrome and protein losing enteropathy lead to hypoproteinemia
- Preanalytic considerations
- If the patient is in the recumbent position at the time of collection, total protein concentration is characteristically 0.4 - 0.8 mg/dL lower
- Indications
- Total bilirubin
- Synonym
- Neonatal bilirubin
- Indications
- Diagnosis of liver, hemolytic, hematologic and metabolic disorders, including hepatitis and gallbladder obstructive disease
- Monitoring the efficacy of neonatal phototherapy
- Test methodology
- Photometric, diazonium salt (DPD)
- Example of reference ranges:
- 0 - 6 days: refer to BiliTool for information on age specific (postnatal hour of life) serum bilirubin values
- 7 - 14 days: < 15.0 mg/dL
- 15 days - 17 years: ≤ 1.0 mg/dL
- ≥ 18 years: ≤ 1.2 mg/ dL
- Critical values above 16.0 mg/dL only for patients under the age of 1
- Interpretation
- Sum of direct bilirubin (associated with liver) and indirect bilirubin (bound to albumin in circulation)
- Less than 20 mg/dL of total serum bilirubin (TSB) typically poses no risk of kernicterus (central nervous system damage caused by hyperbilirubinemia); however, premature infants may be affected at lower levels
- TSB guides decision to start phototherapy
- Phototherapy is stopped when TSB reaches 14 - 15 mg/dL
- Preanalytic considerations
- Ship specimen in amber vial or wrapped in aluminum foil to protect from light which causes photoisomerization of bilirubin in samples and falsely increases measured direct bilirubin
- Hemolyzed specimens should be rejected as hemoglobin interferes with the diazo reaction, resulting in falsely low levels
- Compounds, e.g. penicillin, sulfisoxazole and acetylsalicylic acid, compete for binding sites on serum albumin and lead to lower serum bilirubin levels
- Synonym
- Direct bilirubin
- Synonym
- Bilirubin, conjugated
- Indications
- Used in the diagnosis and treatment of liver, hemolytic, hematologic and metabolic disorders, including hepatitis and gallbladder obstruction
- Test methodology
- Photometric, diazotized sulfanilic acid
- Example of reference ranges:
- < 31 days: 0 - 0.6 mg/dL
- ≥ 31 days: 0 - 0.3 mg/dL
- Interpretation
- Both direct and indirect bilirubin are increased in hepatic diseases, such as hepatitis and tumors or obstructive lesions, such as carcinomas of the pancreas, common bile duct and ampulla of Vater
- Should be assessed in conjunction with total and indirect levels and the clinical setting
- Preanalytic considerations
- Ship specimen in amber vial or wrapped in aluminum foil to protect from light which can cause falsely elevated values (see discussion above for total bilirubin)
- Hemolyzed specimens should be rejected as hemoglobin interferes with the diazo reaction, resulting in falsely low levels
- Synonym
- Reference: Med Clin North Am 2014;98:1
Additional references
Board review style question #1
Which of the following is true about AST and ALT?
- ALT levels help to guide neonatal phototherapy
- ALT:AST ratio is normally greater than 1
- AST levels may also be elevated in disorders of the heart, skeletal muscle and kidney
- AST levels persist longer than ALT levels
Board review style answer #1
C. AST levels may also be elevated in disorders of the heart, skeletal muscle and kidney. Since AST is widely expressed in tissues other than the liver, it is less specific for liver disease.
Comment Here
Reference: Liver function test panel
Comment Here
Reference: Liver function test panel
Board review style question #2
Which of the following is true regarding serum alkaline phosphatase (ALP) levels?
- Markedly increased in hepatic parenchymal diseases, such as infectious hepatitis
- Measured using the photometric, diazonium salt method
- Measured using the same reference values for children, adolescents and adults
- Used in the diagnosis of liver, bone, intestinal and parathyroid diseases
Board review style answer #2
D. Used in the diagnosis of liver, bone, intestinal and parathyroid diseases. ALP can be normal to moderately increased (less than 3 times) in hepatic parenchymal diseases, such as infectious hepatitis. Serum ALP level is measured using the colorimetric method. There should be separate reference values for children and adolescents than adults due to bone growth in the pediatric population.
Comment Here
Reference: Liver function test panel
Comment Here
Reference: Liver function test panel
Monoclonal gammopathy testing
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Laboratory | Interpretation | Test limitations | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Monoclonal gammopathies are plasma cell disorders, ranging from benign monoclonal gammopathy of undetermined significance (MGUS) to malignant diseases of multiple myeloma (MM) and amyloid light chain (AL) amyloidosis, with characteristic excretion of a monoclonal immunoglobulin (M protein)
- Monoclonal protein (M protein) usually demonstrates a single restricted peak / dense band in the gamma, beta or alpha 2 region
- Immunofixation or immunosubtraction is used to identify the heavy chain and light chain types of the M spike
Essential features
- Interpretation of serum electrophoresis and immunoglobulin profiles forms an important part of the investigation of immune function
- Current available methods to detect M proteins include gel and capillary serum and urine protein electrophoresis (SPEP and UPEP), serum and urine immunofixation (sIFE and uIFE), immunosubtraction (ISUB) and mass spectrometry (MS)
- International Myeloma Working Group in 2014 and the College of American Pathologists in 2021 published a consensus and guidelines on diagnosis of multiple myeloma
- There are still challenges in different methodologies, although they have advantages and disadvantages, especially the potential interferences caused by therapeutic monoclonal antibodies
Terminology
- Monoclonal protein / spike (M protein / M spike)
- Immunoparesis (suppression of uninvolved immunoglobulin isotypes in myeloma)
- Serum / urine protein electrophoresis (SPEP / UPEP)
- Immunofixation electrophoresis (IFE)
- Immunosubtraction (ISUB)
Laboratory
- Protein electrophoresis can be performed on agarose gels, capillary electrophoresis (CE) and mass spectrometry; gel and capillary electrophoresis are FDA approved, while mass spectrometry (MALDI-TOF) electrophoresis is a laboratory developed test (LDT) (Blood Cancer J 2021;11:24)
- Immunosubtraction (ISUB), an alternative to immunofixation (IFE) on agarose gels, runs on CE
- Serum protein electrophoresis is performed either on gel or by capillary based technology in order to discover / monitor paraproteins
- Urine protein electrophoresis is performed either on gel or by capillary based technology in order to discover / monitor free light chains (Bence Jones protein) in light chain myeloma or intact monoclonal immunoglobulin, particularly in poor renal function
- Cerebrospinal fluid (CSF) protein electrophoresis is performed mostly using gel based technique for the diagnosis of tumors in the central nervous system or neurological illness; a serum protein electrophoresis should be performed simultaneously to rule out potential elevated CSF proteins from systemic production
- IFE / ISUB is performed to identify / distinguish monoclonal proteins in serum / CSF or Bence Jones proteins in urine
- Nephelometric immunoassay techniques are performed to detect either an intact immunoglobulin M protein or a monoclonal free light chain
IFE (Sebia) ISUB (Sebia) MALDI-TOF MS (Mass Fix) Gel Yes No No Band / peak Present Absent Present Automated No Yes Yes Technically demanding More Less More Labor intensive Very much Much less (automated) In between FDA approved Yes Yes No IgD and IgE Yes No No
Interpretation
- IgG is the major immunoglobulin in the γ region
- Elevations of IgG occur with infections, liver disease, autoimmune disease and multiple myeloma (accounting for 52%)
- Reduction of IgG may indicate genetic immunodeficiency, nonsecretory myeloma or amyloid AL
- IgA is the other major isotype of dimer immunoglobulin
- Elevations of IgA occur with cirrhosis, mucosal infections and malignancy (multiple myeloma accounting for 21%)
- Elevations of IgA usually cause increase in the β and β-γ regions, which is termed β-γ bridging
- IgA deficiency is most commonly seen in congenital immunodeficiencies
- IgM is a pentamer immunoglobulin
- Elevations of IgM may occur with infections or malignancy (multiple myeloma accounting for 0.5%)
- Majority of lymphoplasmacytic lymphoma (Waldenström macroglobulinemia)
- IgM deficiency rarely happens
- IgD is one of the major isotypes of immunoglobulin in lymphocytes
- Elevations of IgD may occur with hyper IgD syndrome or malignancy
- IgD multiple myeloma accounts for around 2%
- IgE is another isotype of immunoglobulin
- IgE multiple myeloma is rarely seen (< 0.1%) (Adv Lab Med 2022;3:79)
- Serum free light chains (sFLC) (free light chain only monoclonal proteins accounting for 20%) are important biomarkers for the diagnosis and management of plasma cell disorders such as multiple myeloma (Arch Pathol Lab Med 2022;146:575)
- Diagnosis of multiple myeloma:
- 2014 International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma (Lancet Oncol 2014;15:e538):
- Clonal bone marrow plasma cells ≥ 10% or biopsy proven bony or extramedullary plasmacytoma
- Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder (CRAB - hypercalcemia, renal failure, anemia and bone lesions)
- FLC κ/λ ≥ 100
- > 1 focal lesion (≥ 5 mm) on MRI studies
- 2014 International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma (Lancet Oncol 2014;15:e538):
2021 College of American Pathologists laboratory detection and initial diagnosis of monoclonal gammopathies: guideline from the College of American Pathologists in collaboration with the American Association for Clinical Chemistry and the American Society for Clinical Pathology (Arch Pathol Lab Med 2022;146:575)
| Guideline statement | Strength of recommendation |
| 1. Clinical care providers should order both serum protein electrophoresis (SPEP) and serum free light chains (sFLC) for the initial detection of monoclonal immunoglobulin protein (M protein) in all patients with suspected monoclonal gammopathies | Strong recommendation |
| 2. Laboratorians should confirm a SPEP abnormality suspicious for an M protein with additional testing by serum immunofixation electrophoresis (sIFE) or alternative method with similar sensitivity | Strong recommendation |
| 3. Laboratorians or clinical care providers should follow up an abnormal sFLC ratio for the presence of an M protein with a serum IFE or alternative method with similar sensitivity | Conditional recommendation |
| 4. Clinical care providers should order SPEP, sFLC, serum IFE and urine IFE for the initial detection of M protein in all patients with suspected amyloid light chain (AL) amyloidosis | Strong recommendation |
| 5. Clinical care providers should not order heavy / light chain isotype assay (hlc) for initial detection of M protein in patients with suspected monoclonal gammopathies | Strong recommendation |
| 6. Clinical care providers should not use total / intact light chains for the quantitation of M proteins in patients with suspected myeloma | Strong recommendation |
| 7. In patients with intact M proteins outside the gamma region by SPEP, laboratories should use total immunoglobulin (IgA, IgG or IgM) for the quantitation of the M proteins; quantitation of a band in the beta region by SPEP can be performed if the M protein is distinguished from background normal protein bands | Conditional recommendation |
| 8. Laboratorians should report both quantitative levels of free kappa and free lambda and the rFLC when the sFLC assay is performed | Strong recommendation |
| 9. Clinical care providers may use rFLC, IgM isotype, M protein > 1.5 g/dL and immunoparesis as risk factors for progression to MM or a B cell lymphoproliferative disorder | Conditional recommendation |
Test limitations
- Despite major advances over the last decade, there are still challenges in routine practice of monoclonal gammopathy measurements
- Hidden light chains in monoclonal proteins have been recognized on gel based immunofixation, as may be seen as a separate large clonal heavy chain without a comigrating light chain band
- Reason for hidden light chains may be the inaccessible light chain epitopes in the folded tertiary or quaternary structure of some monoclonal proteins; one of the possible approaches to revealing the hidden light chain band is to use the chemical reduction agent 2-mercaptoethanol or dithiothreitol in serum samples by breaking up disulfide bonds
- There have been quite a few cases reporting the detection of therapeutic antibodies at their high dose, including daratumumab, siltuximab, ofatumumab, elotuzumab, pembrolizumab and belantamab mafodotin; as a result, the likelihood of encountering interferences from therapeutic antibodies in the clinical laboratory has been increasing
- With current technologies used in most clinical laboratories for the detection, identification and isotyping of M proteins, there is limited ability to recognize the presence of monoclonal antibody therapeutics at the analytical phase of testing
- Given the limited analytical solutions to the problem of monoclonal antibody interferences, newer approaches, such as robust mass spectrometry, are needed to overcome the challenges
- Reference: Keren: Protein Electrophoresis in Clinical Diagnosis, 1st Edition, 2012
Board review style question #1
A 74 year old man was admitted to the hospital due to complications from chronic heart failure disease. Skeletal Xrays revealed diffuse areas of patchy demineralization that could represent osteoporosis. Serum protein electrophoresis (A) was performed with capillary electrophoresis results shown above. For the serum protein electrophoresis (SPEP), measured protein was 8 g/dL (reference interval: 6.4 - 8.3 g/dL) and the shaded area has a density estimate of 1.78 g/dL, indicating possible abnormalities in the gamma region. The sample was reflexed to immunosubtraction (B). What is the most probable explanation for these findings?
- Monoclonal protein free kappa is present
- Monoclonal protein IgA kappa is present
- Oligoclonal protein is present
- Polyclonal protein IgA kappa is present
Board review style answer #1
B. Monoclonal protein IgA kappa is present. The serum protein electropherogram result suggests 2 monoclonal gammopathies in the γ globulin region. Immunofixation electrophoresis (IFE) identified these as IgA kappa. The Xrays suggest the possibility of lytic lesions. The chronic heart failure is unrelated. The patient has multiple myeloma with IgA kappa monoclonal proteins.
Comment Here
Reference: Monoclonal gammopathy testing
Comment Here
Reference: Monoclonal gammopathy testing
Board review style question #2
An 84 year old woman was seen in the emergency department after experiencing the sudden onset of severe pain in the left femoral region. She declined history of falling or trauma. She also denied back pain. Skeletal Xrays revealed a fracture of the left femoral region and a compression fracture of a lower lumbar vertebra. Skeletal Xrays also showed multiple punched out lesions. Serum protein electrophoresis (A) was performed with capillary electrophoresis results shown above. For the serum protein electrophoresis (SPEP), measured protein was 6.6 g/dL (reference interval: 6.4 - 8.3 g/dL) and the shaded area has a density estimate of 0.14 g/dL and 0.32 g/dL in the beta 2 and gamma region, respectively, indicating possible abnormalities in the beta 2 and gamma regions. The sample was reflexed to immunosubtraction (B) to identify the subtype of monoclonal proteins. The immunosubtraction results showed subtraction for λ light chain only in both peaks, suggesting 2 monoclonal λ light chains but no corresponding heavy chains subtracted. Due to lack of anti-IgD and E antisera for immunosubtraction, immunofixation (C) was performed to identify the subtype of monoclonal proteins. Immunofixation against D and E antisera confirmed the presence of an IgD monoclonal protein in the gamma region. The D and λ bands migrate separately, D is in gamma region while λ is in beta region, which is discrepant with the findings of 2 monoclonal λ light chains in immunosubtraction. For the discrepancy between immunofixation and immunosubtraction results, what else can be done using immunofixation to further evaluate this specimen?
- No need to repeat immunofixation because it is monoclonal IgD only + free λ
- Repeat immunofixation after bringing specimen to 37 °C
- Repeat immunofixation after chemical reduction of serum specimen with 2-mercaptoethanol
- Repeat immunofixation with higher dilution of serum to get the concentration lower
Board review style answer #2
C. Repeat immunofixation after chemical reduction of serum specimen with 2-mercaptoethanol. The medical laboratories should be aware of the described scenario. The failure to detect light chains of certain intact M proteins is most likely due to the structural inaccessibility of light chains. It is recommended that treatment with reduction agents or use of an alternative methodology or immunosubtraction might be helpful for investigating suspected heavy chain only cases, due to the limitation of conventional methodology.
Comment Here
Reference: Monoclonal gammopathy testing
Comment Here
Reference: Monoclonal gammopathy testing
Natriuretic peptides (BNP and Amino-terminal proBNP)
Definition / general
- Brain natriuretic peptide (BNP), now known as B-type natriuretic peptide (also BNP), is a 32 amino acid polypeptide secreted by the cardiac ventricles in response to excessive stretching of cardiomyocytes (Wikipedia - Brain natriuretic peptide)
- BNP was originally identified in extracts of porcine brain, although in humans it is produced mainly in the cardiac ventricles
- BNP is co-secreted with a 76 amino acid N-terminal fragment (NT-proBNP), which is biologically inactive
Clinical features
- Reduces misdiagnosis of congestive heart failure, which occurs 50% to 75% of the time
- NT-proBNP is superior to BNP for predicting mortality and morbidity for heart failure (Clin Chem 2006;52:1528), and coexisting renal disease and heart failure (Clin Chem 2007;53:1511)
Laboratory
Indications
Reference ranges
Limitations
- Evaluation of dyspneic patient with suspected congestive heart failure, regardless of renal function (J Am Coll Cardiol 2006;47:91)
- B-type natriuretic peptide levels are higher in patients with congestive heart failure than in dyspnea from other causes (J Am Coll Cardiol 2002;39:202, N Engl J Med 2004;350:647)
- NT-proBNP measurement is a valuable addition to standard clinical assessment for the identification and exclusion of acute CHF in the emergency department setting (Am J Cardiol 2005;95:9480)
Reference ranges
- BNP levels below 100 pg/mL indicate no heart failure
Limitations
- Determination of endogenous BNP with the AxSYM assay using frozen plasma samples may not be valid after 1 day, but NT-proBNP as measured by the Elecsys assay may be stored at -20 degrees C for at least four months without a relevant loss of the immunoreactive analyte (Clin Chem Lab Med 2004;42:942)
Additional references
Newborn screening
Table of Contents
Definition / general | Essential features | Newborn screening (NBS) | History | Current status in the U.S. | Disorders included | Addition of disorders to the NBS panel | Regulation | Monitoring | Methodologies | Potential confounding factors | Limitations | Management | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Newborn screening (NBS) is one of the most successful preventive health programs in the U.S. intended to identify clinically occult but potentially serious disorders that require immediate intervention
- The NBS program screens for some genetic, endocrine and metabolic disorders as well as hearing loss and critical congenital heart defects (CCHDs)
Essential features
- The goal of NBS is to detect the presence of treatable disorders early enough to provide an intervention and improve long term outcome
- NBS is performed in all states in the U.S.
- NBS consists of dried blood spot (DBS) screening, hearing loss screening and congenital heart disease screening
Newborn screening (NBS)
- Refers to a group of tests performed on newborns to identify early presymptomatic, treatable disorders, for which timely intervention is critical to improve long term outcome
- Intended to identify clinically occult but potentially serious disorders that require immediate intervention
- Considered a great public health achievement
- It is a state mandated public health program that begins with a heel poke for every baby before hospital discharge
- Parents have a right to opt out of NBS testing; each state has a procedure for the opt out process
- NBS is not a diagnostic test; it identifies babies at increased risk for certain genetic, metabolic or functional diseases
- The 6 pillars for a successful NBS program include education, testing, follow up, diagnosis, intervention or management, and evaluation (Mol Genet Metab 2001;74:64)
- The NBS program in the United States includes screening for
- Hearing loss
- Critical congenital heart defects (CCHD)
- Dried blood spot screening
- Testing for metabolic, hematologic, endocrine and other inheritable disorders
- Disorders included in the NBS panel are ones that can cause significant morbidity, mortality or intellectual disability without intervention
- Reference: Birth Defects Res 2020;112:350
History
- In 1963, Dr. Robert Guthrie developed a simple screening assay for the detection of phenylketonuria (PKU)
- NBS testing is performed from a dried blood spot
- DBS enables population screening due to ease of collection and the ability to mail the samples to off site, state centralized laboratories
- In the U.S., ~3,400 infants/year receive early intervention for diseases identified via NBS
- Reference: Annu Rev Nurs Res 2011;29:113
Current status in the U.S.
- In the U.S., ~4 million infants undergo NBS annually
- NBS is performed in all states and each state screens for at least 31 out of the 38 core or primary disorders
- Some states do not test for any of the secondary disorders while others test for up to 25 out of the 26 secondary disorders
- References: Pediatr Clin North Am 2019;66:369, NewSTEPs: Newborn Screening Status for All Disorders [Accessed 14 February 2024]
Disorders included
- Newborn screening primarily includes disorders listed on the recommended universal screening panel (RUSP)
- The RUSP is a list of disorders that the Secretary of the Department of Health and Human Services (HHS) recommends states include on their NBS panel
- 38 core conditions and 26 secondary conditions are included on the RUSP
- Disorders are categorized as
- Metabolic disorders
- Organic acid condition
- Fatty acid oxidation disorders
- Amino acid disorders
- Endocrine disorders
- Hemoglobin disorders
- Other disorders including lysosomal storage disorders, severe combined immunodeficiency (SCID), spinal muscular atrophy (SMA)
- Metabolic disorders
- Screening for hearing loss and CCHD is included in NBS and is performed in the hospital
- Most states screen for the majority of disorders on the RUSP and may also screen for additional disorders
- Full list of all disorders screened: HRSA: Recommended Uniform Screening Panel [Accessed 14 February 2024]
Addition of disorders to the NBS panel
- Criteria for inclusion of disorders
- To be included as a primary target condition in an NBS program, a condition should meet the following minimal criteria
- It can be identified at a time (24 - 48 hours after birth) at which it would not ordinarily be detected clinically
- A test with appropriate sensitivity and specificity is available for it
- There should be available treatment of the condition (Genet Med 2006;8:1S)
- To be included as a primary target condition in an NBS program, a condition should meet the following minimal criteria
- Each state is responsible for adding disorders to their NBS panel
- Most states have a public or expert advisory committee to support the process
- Advocacy groups may directly approach individual legislators to add specific disorders by statute
- Most states use the RUSP as a guide for what disorders should be included in the NBS panel
- Disorders included in the RUSP were chosen based on
- Evidence that supports the potential net benefit of screening
- Ability of states to screen for the disorder
- Availability of effective treatments
- New disorders are added to the RUSP following a formal process, requiring support from the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) and recommended by the HHS
- Process for adding a new disorder to the RUSP includes
- Submission of a nomination package to the committee
- Committee reviews the nomination package
- Committee votes on whether or not to send a nomination to evidence based review
- External group conducts an evidence based review
- Committee considers the evidence
- Committee votes on whether or not to recommend addition of the disorder to the RUSP
- Committee makes a recommendation
- HHS Secretary responds
- Reference: HRSA: Advisory Committee on Heritable Disorders in Newborns and Children [Accessed 14 February 2024]
Regulation
- NBS Saves Lives Act
- In 2008, U.S. Congress passed the original Newborn Screening Saves Lives Act (public law 110-204), which established national NBS guidelines and helped facilitate comprehensive NBS in every state; this act has been reauthorized since then, the latest being in 2021 (through FY2026) (Congress.gov: H.R.482 - Newborn Screening Saves Lives Reauthorization Act of 2021 [Accessed 23 February 2024])
- In the U.S., NBS is a state mandated public health program
- NBS programs are regulated and administered by the state, allowing each program to meet the state's specific needs
- Federal government also plays a role in administering and adding to the RUSP
Monitoring
- 8 quality indicators are used to evaluate the success of the NBS program (NewSTEPs: Quality Indicators [Accessed 14 February 2024])
- Percent or number of unsatisfactory specimens due to improper collection or transport
- Percent of DBS with at least 1 missing data field on the form
- Percent of newborns not receiving the NBS
- Percent of newborns that are lost to follow up
- Percent of infants with out of range results that require further testing
- Percent of missed cases
- Percent of confirmed positive cases
- Timeliness of the NBS activities
Methodologies
| Laboratory method | Disorders |
| Immunoassays | Congenital hypothyroidism Congenital adrenal hyperplasia Cystic fibrosis |
| Electrophoresis | Hemoglobinopathies |
| High performance liquid chromatography | Hemoglobinopathies |
| Tandem mass spectrometry (MS/MS) | Amino acidemias Organic acidemias Fatty acid oxidation Urea cycle disorders Pompe disease Mucopolysaccharidosis, type I X linked adrenoleukodystrophy |
| DNA based (RT-PCR, next generation sequencing) | Severe combined immunodeficiencies Spinal muscular atrophy Cystic fibrosis (second tier) |
| Fluorimetry or colorimetry (digital microfluidics platform or plate based enzyme assay) | Pompe disease Mucopolysaccharidosis, type I |
Potential confounding factors
- Timing of sample collection: 24 - 48 hours; this is enough time for babies to feed, which is important for the detection of some metabolic disorders (e.g., phenylketonuria)
- DBS should arrive at the lab ideally within 24 hours of collection with a goal to have results by 5 days of life for critical disorders and 7 days for the remaining disorders (HRSA: Newborn Screening Timeliness Goals [Accessed 14 February 2024])
- Some states require a second screening within 7 - 14 days after the first sample collection to improve the sensitivity and specificity of screening
- Delay in receipt of the DBS by the measuring laboratory may lead to erroneous results
- Some metabolites are greatly affected by a variety of prenatal factors, including gestational age, birth weight and nutritional status, underlying illness, total parenteral nutrition and use of antibiotics (Pediatr Clin North Am 2019;66:369)
- Premature and sick infants are more likely to have a false positive / negative result on their initial screen due to immaturity of enzymes involved in metabolic pathways or clinical interventions received
- Blood transfusions: can give false results in hemoglobinopathy screening or enzyme screening; NBS should be carried out before transfusions or when the blood products will no longer interfere with testing
- Temperature and humidity of test cards, for example, can falsely lower biotinidase activity (HRSA: Newborn Screening Timeliness Goals [Accessed 14 February 2024])
- Maternal disorders, such as previously diagnosed metabolic disorders, may cause false positive results (B12 deficiency, hypothyroidism / hyperthyroidism)
- Medication use in pregnancy such as steroids or propylthiouracil may affect screening for congenital adrenal hyperplasia or hypothyroidism (Pediatr Clin North Am 2019;66:369)
Limitations
- Trade off to maximizing assay sensitivity is a large number of false positive results
- Depending on the disorder, it is estimated that > 90% of abnormal NBS results are false positives
- Increasing the number of disorders included in the NBS panel will increase the burden of false positive results
- Support for mandatory NBS could deteriorate if false positive results become excessive
- Reference: Pediatr Clin North Am 2019;66:369
Management
- A positive newborn screen sets off the management, which includes contacting the ordering facility and primary care physician
- State follow up team ensures that the family and specialists are notified
- Diagnostic testing is ordered by the specialist in a clinical laboratory followed by treatment if condition is confirmed
- Management includes emergency management and supportive / maintenance therapy; the urgency of follow up depends on the disorder
- Some disorders require dietary management to decrease the substrate in cases of enzyme deficiencies or to provide deficient metabolites; other modes of management include enzyme replacement, liver / stem cell transplant, gene therapy, toxin removal (hemodialysis / peritoneal dialysis)
- Management requires a multidisciplinary approach that often involves parents, nutritionists, geneticists, social workers, medical laboratory, school, community, therapists and psychologists
- The ACMG (American College of Medical Genetics and Genomics) and the National Coordinating Center for the Regional Genetics Networks have developed action (ACT) sheets as resources for healthcare providers on information regarding clinical decisions and next steps in diagnosis
- In addition, there are diagnostic algorithms for each disorder that list the confirmatory tests recommended to confirm the diagnosis (ACMG: ACT Sheets and Algorithms [Accessed 14 February 2024])
- NBS continues to expand; newer technologies for screening and confirmation of disorders and newer novel treatment options continue to push the expansion of NBS disorders
- Other parts of the world, like Europe, China, Latin America, Asia Pacific and the Middle East, have NBS programs in place with varying numbers of disorders and babies screened from country to country (International Society for Neonatal Screening (ISNS) [Accessed 14 February 2024])
- Reference: Indian J Pediatr 2021;88:679
Board review style question #1
Which of the following statements is true about the newborn screening program?
- The best time to collect heel prick blood spots from babies is immediately after birth
- Detects all inborn errors of metabolism
- It includes dried blood spot screening, hearing loss screening and congenital heart disease screening
- It is a federally mandated program in the United States
- It is estimated that ~20% of initial newborn screen results are false positives
Board review style answer #1
C. It includes dried blood spot screening, hearing loss screening and congenital heart disease screening. Answer D is incorrect because newborn screening is a state mandated program in the United States. Answer B is incorrect because it does not detect all inborn errors of metabolism. Answer A is incorrect because blood spots should be collected between 24 - 48 hours of birth after feeding has been established. Answer E is incorrect because false positives account for ~90% of cases; each positive result must be confirmed as soon as possible.
Comment Here
Reference: Newborn screening
Comment Here
Reference: Newborn screening
Board review style question #2
Which of the following is necessary for addition of a new disorder to the recommended universal screening panel (RUSP) for newborn screening?
- Disorders added to RUSP must be supported by patient advocacy groups
- Availability of a screening test with appropriate sensitivity and specificity
- The disorder has to be clinically present in the newborn period
- There must be a federal law passed mandating the addition of the disorder to the RUSP
- The screening test must be approved by the United States Food and Drug Administration (FDA)
Board review style answer #2
B. Availability of a screening test with appropriate sensitivity and specificity. This is one of the criteria for adding a disorder to the recommended universal screening panel (RUSP). Answer D is incorrect because no federal law is required for addition of disorders; the RUSP is a guide to states for newborn screening (NBS). Answer C is incorrect because the disorder does not have to present clinically in the newborn period. Answer A is incorrect because there is no requirement that disorders added to RUSP must be supported by patient advocacy groups. Answer E is incorrect because the screening test does not have to be FDA approved.
Comment Here
Reference: Newborn screening
Comment Here
Reference: Newborn screening
Parathyroid hormone
Table of Contents
Definition / general | Essential features | Pathophysiology | Diagrams / tables | Clinical features | Laboratory | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hormone secreted by parathyroid glands for maintaining blood calcium homeostasis
- Has reciprocal effect on phosphate metabolism
- Its release controlled by ionized calcium level with negative feedback system
- 84 amino acids derived from cleavage of prepro parathyroid hormone (PTH); biologic activity due to 34 amino acids at amino terminus; other portions are inert but may give false positives in detection systems
- Binding of PTH to its receptor stimulates cAMP and phosphatidylinositol diphosphate
- Note: PTH related protein is rarely produced by benign lesions (Am J Clin Pathol 1996;105:487)
Essential features
- Maintains serum calcium within a tight range of around 9 - 10 mg/dL
- Mobilizes calcium from bone, increases calcium reabsorption from the kidney and stimulates calcium absorption from the gut (Compr Physiol 2016;6:561)
- Primary hyperparathyroidism leads to elevated PTH, elevated blood calcium and reduced blood phosphate; managed most commonly by parathyroidectomy
- Intraoperative rapid PTH assays are useful as an indicator for successful surgical removal of the PTH hypersecreting parathyroid tissue
- Both second and third generation PTH assays measure 1-84 full length intact PTH protein
- Third generation assays are more specific and do not target truncated protein fragments
- While second and third generation assays tend to correlate well (correlation coefficient close to 1.0), third generation assays may generate significantly lower absolute PTH values (Metabolism 2013;62:1416)
Pathophysiology
- PTH maintains serum calcium homeostasis by exerting the following biological effects:
- Activates and increases the number of osteoclasts, mobilizing calcium from bone
- Increases renal tubular reabsorption of calcium
- Activates 1-alpha-hydroxylase, which increases conversion of inactive vitamin D to the active dihydroxy form in kidneys
- Active form of vitamin D promotes GI calcium absorption
- Increases urinary phosphate excretion, which reduces calcium loss
- Reference: Front Horm Res 2018;50:1
Diagrams / tables
Clinical features
- Primary hyperparathyroidism
- Relatively common endocrine disorder (up to 80 per 100,000) caused by overactive parathyroid glands
- 80% single adenoma, 10 - 15% hyperplasia, 5% multiple adenomas (Nat Rev Endocrinol 2018;14:115)
- Presents with elevated circulating PTH, hypercalcemia and hypophosphatemia
- Can lead to osteoporosis, bone fractures, hypercalciuria and nephrolithiasis (Best Pract Res Clin Rheumatol 2020;101514)
- Managed with parathyroidectomy that leads to rapid decline in PTH level
- Intraoperative rapid PTH assay: certain assays enable results in less than 20 minutes (Endocr Pract 2011;17:2)
- Short half life of PTH (2 - 5 minutes) provides sensitive indication of successful removal of hypersecreting parathyroid gland(s)
- Serial intraoperative PTH levels for trending are a common practice
- Secondary hyperparathyroidism
- Common complication of chronic renal disease that can lead to reduced vitamin D production
- Low serum calcium or elevated phosphate leads to parathyroid hyperplasia and elevated circulating PTH (Ther Apher Dial 2019;23:309)
- Elevated PTH can result in abnormal bone turnover (renal osteodystrophy)
- Tertiary hyperparathyroidism
- Most common in patients with chronic secondary hyperparathyroidism who have been on dialysis for years
- Observed in 1 - 3% of patients with renal failure (Am J Otolaryngol 2017;38:630)
- Hypertrophied parathyroid glands oversecrete PTH in an autonomic fashion despite resolution of the underlying condition (e.g. renal transplant)
- Resulting hypercalcemia and hyperphosphatemia can lead to diffuse calcinosis
- Hypoparathyroidism
- Relatively rare heterogeneous condition characterized by insufficient circulating PTH (Expert Opin Drug Saf 2017;16:617)
- Leads to hypocalcemia and hyperphosphatemia
- Treated by vitamin D (inactive and active) and oral calcium tablets
- Hypercalcemia of malignancy (breast, lung, kidney, myeloma) due to:
- Release of PTH related protein, usually in advanced disease (squamous cell carcinoma, lung cancer, hepatoma) or
- Osteolytic metastases with local release of cytokines (IL1, TNF alpha) (multiple myeloma, breast cancer, renal cell carcinoma)
Laboratory
- Second generation (intact PTH) assays widely used today
- However, the intact assay also detects N truncated fragments (e.g. 7-84 PTH) that may exert effects opposite to those of full length 1-84 PTH
- Different assays measure varying types and amounts of these circulating fragments, which can lead to inconsistent results among platforms (National Kidney Foundation: Parathyroid Hormone and Secondary Hyperparathyroidism in Chronic Kidney Disease [Accessed 15 July 2020])
- Third generation PTH assay specifically targets 1-84 PTH without detecting 7-84 PTH and other fragments (Clin Chem Lab Med 2018;56:1476)
- May potentially enable better standardization of PTH values across platforms than second generation assays
- Values tends to be numerically lower than those from second generation assays
- Close communication with clinicians warranted when changing assays between generations
- Primary hyperparathyroidism
- Type of PTH assay used will not affect diagnosis or management because the precise concentration of PTH is less relevant (Endocr Rev 2019;40:1468)
- Secondary hyperparathyroidism due to chronic kidney disease
- Clinical guideline recommends maintaining PTH level at twofold to ninefold above the upper limit of normal, corresponding to about 130 - 600 pg/mL (depending on assay platform used) (National Kidney Foundation: Parathyroid Hormone and Secondary Hyperparathyroidism in Chronic Kidney Disease [Accessed 15 July 2020])
- Intraoperative rapid PTH assay
- Third generation assays can provide a faster indication of treatment success than second generation assays (Surgery 2020 [Epub ahead of print])
Board review style question #1
Which of the following sets of laboratory values is characteristic of primary hyperparathyroidism?
- Elevated blood PTH, elevated calcium, elevated phosphate
- Elevated blood PTH, reduced calcium, elevated phosphate
- Elevated blood PTH, elevated calcium, reduced phosphate
- Elevated blood PTH, reduced calcium, reduced phosphate
Board review style answer #1
C. In primary hyperparathyroidism, the elevated blood PTH physiologically increases serum calcium and reciprocally reduces serum phosphate.
Comment Here
Reference: Parathyroid hormone
Comment Here
Reference: Parathyroid hormone
Board review style question #2
Which of the following describes the difference between second generation and third generation PTH assays?
- Second generation PTH assays detect the 1-84 intact PTH protein in addition to the N truncated 7-84 fragment, while the third generation assays detect only the 1-84 intact PTH
- PTH values are numerically interchangeable between second generation and third generation assays
- Second generation PTH assays provide faster results as compared to third generation assays for intraoperative PTH evaluation
- Third generation PTH assays are more comprehensive than second generation assays by detecting more circulating PTH fragments
Board review style answer #2
A. Third generation PTH assays do not detect 7-84 PTH and other fragments, which second generation assays typically do.
Comment Here
Reference: Parathyroid hormone
Comment Here
Reference: Parathyroid hormone
Pregnancy / menopause hormone levels (pending)
[Pending]
Renal function test panel (pending)
[Pending]
Rheumatoid arthritis (pending)
[Pending]
Saline suppression test
Definition / general
Indication:
Methodology:
- To confirm the diagnosis of primary aldosteronism (J Clin Endocrinol Metab 2006;91:2618)
- Other tests recommended for screening or confirmation are captopril suppression test and fludrocortisone suppression (Horm Metab Res 2010;42:406)
Methodology:
- Infuse 2L of 0.9% saline over 4 hours ("saline infusion test") OR give oral NaCl tablets 10 - 12 gm daily for 3 days ("oral sodium loading test")
- Blood levels: plasma aldosterone greater than 5 ng/dl (140 pmol/L) confirms the diagnosis of primary hyperaldosteronism
- Urine levels: collect 24 hour urine for aldosterone, creatinine (to assess the adequacy of urine collection), and sodium (to ensure adequate intake) on the last day of sodium chloride administration
- Aldosterone levels > 10 μg/24 hours (28 nmol/24 hours) confirms the diagnosis
Clinical features
- More difficult to perform than captopril suppression test, with comparable accuracy in patients with high sodium intake (Hypertension 2007;50:424)
Screening thyroid disorders
Table of Contents
Definition / general | Terminology | Epidemiology | Pathophysiology / etiology | Screening adult thyroid disorders | Screening pregnancy related thyroid disorders | Diagrams / tables | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Molecular / cytogenetics description | Videos | Additional referencesDefinition / general
- Congenital hypothyroidism is the most common congenital endocrine / metabolic disorder, with an estimated prevalence of 1 per 1,600 to 3,500 newborn infants (for TSH cutoffs of 10 and 20 mU/L, respectively)
- Screening for neonatal hypothyroidism was established on a worldwide basis in the 1970s, now it is acclaimed as the most successful pediatric screening test, which approaches a cost to benefit ratio of 10:1 (J Clin Endocrinol Metab 1999;84:4332)
- Screening for neonatal hypothyroidism is a part of a newborn screening blood test panel for congenital disorders including phenylketonuria, congenital adrenal hyperplasia, cystic fibrosis and others (from 2 to 40 conditions, depending on country / state)
- Congenital hypothyroidism screening is most efficient in countries without iodine deficiency
- Screening is not widely adopted in countries with moderate to severe iodine deficiency, where efforts should be made to supply sufficient iodine to the population
- Only 25% of babies are born in countries with newborn screening programs in place; for the other 75%, diagnosis is made when development of clinical features prompts thyroid function testing (Orphanet J Rare Dis 2010;5:17)
- The major effect of screening for congenital hypothyroidism is that it allows treatment to be started much earlier than in infants diagnosed on the basis of clinical findings, preventing severe intellectual disability (Braverman, Cooper: Werner & Ingbar's The Thyroid, Tenth Edition, 2012)
- Clinical guidelines on screening were issued in the USA (Pediatrics 2006;117:2290) and Europe (J Clin Endocrinol Metab 2014;99:363)
Terminology
- Congenital hypothyroidism = neonatal or fetal hypothyroidism
- Congenital hypothyroidism is permanent if the absent, ectopic or dyshormonogenetic thyroid gland is confirmed by thyroid imaging and ancillary studies, or if at any time during the first year of life serum TSH rises above 20 mU/L presumably due to insufficient T4 replacement (Pediatrics 2006;117:2290, Orphanet J Rare Dis 2010;5:17)
Epidemiology
- Birth prevalence of congenital hypothyroidism has almost tripled, from about 1 in 6,700 newborns by clinical diagnosis to about 1 in 2,500 in the screening era, which is due to population based screening (Braverman, Cooper: Werner & Ingbar's The Thyroid, Tenth Edition, 2012)
- Incidence of congenital hypothyroidism is currently increasing, which became evident after adjustment for screening effect (Nat Rev Endocrinol 2011;8:104)
- F : M = 2:1, Asians > Hispanics > Whites >> Blacks
- Risk factors:
- Maternal hypothyroidism due to iodine deficiency and other causes (Pediatr Int 2007;49:76)
- Multiple pregnancies (J Clin Endocrinol Metab 2007;92:3141, Eur J Endocrinol 2005;153:765)
- Older mother
- Preterm birth
- Down syndrome in infants
Pathophysiology / etiology
- Thyroid dysgenesis and dyshormonogenesis (prevalence ratio 5:1) are the two main causes of congenital hypothyroidism
- See details on mechanism in aplasia and dyshormonogenetic goiter topics
- Less frequent causes, usually of transient hypothyroidism, are transplacental passage of maternal antithyroid drugs, maternal blocking antibodies, iodine deficiency or excess and germline mutations in the TSH receptor
- Central congenital hypothyroidism (pituitary or hypothalamic) is much rarer, incidence is 1:25,000 to 1:100,000 births
Screening adult thyroid disorders
- The US Preventive Services Task Force concludes that evidence is insufficient to recommend for or against routine screening for thyroid disease in adults (Am Fam Physician 2004;69:2415, Ann Intern Med 2004;140:128)
- American Thyroid Association recommends screening every 5 years beginning at age 35, particularly in women (Arch Intern Med 2000;160:1573)
- High incidence of unrecognized thyroid disease in elderly (Recenti Prog Med 2004;95:308)
- Recommended screening test is TSH
- Abnormal values should be confirmed with a fresh specimen
Diagrams / tables
Clinical features
If no laboratory screening:
- Signs of clinically evident congenital hypothyroidism: jaundice, lethargy, hypothermia, constipation, macroglossia, poor feeding, hoarse cry, wide posterior fontanel
- However, in > 95% of infants, signs are often subtle and not present at birth, because newborn continues to be protected by maternal hormones for 3 - 4 weeks
- Growth and developmental delay are usually apparent by 4 - 6 months
- Extrathyroidal congenital abnormalities (heart malformations and facial dysmorphism) can be a part of syndromic congenital hypothyroididsm
Laboratory
Methodology
Algorithm
Actions if TSH is high
- Routinely performed on all newborns
- Every infant should be tested before discharge from the nursery, optimally by 2 to 4 days of age
- Specimens collected in the first 24 to 48 hours of life may lead to false positive TSH elevations when using any screening test approach
- Use dry blood spots to measure both T4 and TSH
- TSH alone screening is common in Europe and Japan due to higher cost effectiveness
- Cord blood is rarely used because of mixing with maternal blood and logistics (J Clin Endocrinol Metab 2002;87:4072)
- If measure only TSH (checking for values of 10 mU/L or higher), have false positives from premature or severely stressed infants and miss infants with hypothalamic or pituitary disease (J Clin Res Pediatr Endocrinol 2013;5:8)
- If measure only T4 (checking for low values), have high false positive rate due to prematurity or congenital absence of thyroid binding globulin, and miss patients with elevated TSH but normal T4
- It is recommended that screening should be conducted in centralized laboratories under external quality control programs, covering 50,000 - 100,000 newborns per year (Paediatric Thyroidology Endocr Dev 2014;26:44)
Algorithm
- TSH less than 10 mU/L: no further action for newborns
- TSH 10 - 20 mU/L: retest at 2 - 6 weeks
- TSH > 20 mU/L: endocrine workup needed
- Preterm and low birth weight infants (J Med Screen 2005;12:166), twins (An Pediatr (Barc) 2006;65:129), and all neonates admitted to an intensive care unit should be retested at 2 weeks
Actions if TSH is high
- Immediate recall for further workup, usually via family doctor or direct contact with further referral to pediatric endocrinology center
- Many screening programs achieve recall in 8 to 14 days, which allows median treatment age of 14 days
- Rapid TSH and free T4 measurement in a new venous blood sample
- Serum free T4 is used to stratify hypothyroidism as biochemically severe, moderate and mild (< 5, 5 to < 10, and 10 to 15 pmol/l, respectively)
- Immediate hormone replacement therapy
- Thyroid imaging studies including scintiscan, sonography and knee Xray
- After initiation of therapy, the usual recommendation is to monitor TSH and T4 levels at weekly intervals for 1 month, at monthly intervals for 3 months, followed by a continuous followup every 3 months during the first 2 years of life and later every 6 months (Nat Rev Endocrinol 2011;8:104)
- Genetic workup is optional
Radiology description
- Thyroid scintigraphy with sodium pertechnetate (99mTc) within 24 hours of a positive screening result (Braverman, Cooper: Werner & Ingbar's The Thyroid, Tenth Edition, 2012)
- Ultrasonography to evaluate the gland location
Prognostic factors
- Early diagnosis and treatment (not later than 3 months of age, optimally within 2 weeks) of hypothyroidism are critical to prevent intellectual disability
- Mean IQ of children with congenital hypothyroidism before neonatal screening was 86, which has been improved to 95 - 105 (control IQ = 110) in the current screening era (Arch Dis Child 2011;96:374)
Case reports
- Newborn girl with illustrative case of congenital hypothyroidism (J Clin Endocrinol Metab 2011;96:2959)
Treatment
- T4 treatment should be started immediately if the results of screening are frankly abnormal (TSH > 30 - 40 mU/L), and any delay in obtaining results of the confirmatory serum or scintigraphy tests should not delay treatment (Braverman, Cooper: Werner & Ingbar's The Thyroid, Tenth Edition, 2012)
- Use single, daily dose (10 to 15 μg / kg) of oral levothyroxine; avoid mixing with soy (Arch Dis Child 2004;89:37, Endocr Pract 2001;7:193) or iron (South Med J 1997;90:637)
- The goal of therapy is to normalize T4 within 2 weeks and TSH within 1 month
- May be discontinued after 3 years of age if hypothyroidism was proven to be transient
- If no permanent cause of hypothyroidism was found by scan (aplasia / ectopia) or there was no TSH increase after the newborn period, then T4 administration should be discontinued for 30 days at some point after the child is 3 years of age
- If the FT4 and TSH concentrations remain in the reference range, euthyroidism is assumed and a diagnosis of transient hypothyroidism recorded
Molecular / cytogenetics description
- See aplasia and dyshormonogenetic goiter topics
Additional references
Sensitivity and specificity (pending)
[Pending]
Sepsis biomarkers (pending)
[Pending]
Serum free light chain test
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Pathophysiology | Clinical features & test indications of conditions where serum free light chain testing is useful | Laboratory | Common commercial platforms and methodologies | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Serum free light chain testing is required for detecting and monitoring patients with monoclonal gammopathies (Arch Pathol Lab Med 2022;146:575)
Essential features
- Measures the concentration of serum free kappa and lambda immunoglobulin light chains
- Recommended to be used in connection with serum protein electrophoresis (SPEP)
- Abnormal ratio of serum free kappa/serum free lambda (rFLC) supports diagnosis of a monoclonal gammopathy
- Elevated rFLC may also be found in patients with chronic kidney disease or chronic inflammation
- Used to calculate risk of progression
Terminology
- Bence Jones protein
- Monoclonal protein (M protein)
- Paraprotein
ICD coding
Pathophysiology
- Plasma cells produce immunoglobulin light chains in excess of immunoglobulin heavy chains
- Most free kappa light chains circulate as monomers (~22.5 kDa) while most free lambda light chains circulate as dimers (~44 kDa)
- Due to their small size, their half life ranges from 2 to 6 hours (Br J Haematol 2004;126:348)
- When the monoclonal free light chains (MFLC) pass into the renal tubules, they may bind to uromodulin (also known as Tamm-Horsfall protein) leading to cast formation that obstructs the distal tubules and can cause pyelonephritis (J Clin Invest 1997;99:732)
- MFLC may form the beta pleated sheets of AL (light chain type) amyloid that deposit in and damage a wide variety of organs: cardiac, renal, neural, hepatic and gastrointestinal
- MFLC that do not form beta pleated sheets also may deposit in glomeruli or cardiac tissues as monoclonal immunoglobulin (or light chain deposition) disease (Clin J Am Soc Nephrol 2012;7:231)
Clinical features & test indications of conditions where serum free light chain testing is useful
- Non-IgM monoclonal gammopathy of undetermined significance (non-IgM MGUS): no clinical features
- Serum monoclonal protein (non-IgM type) < 3 g/dL
- Bone marrow clonal plasma cells < 10%
- In low risk patients (IgG isotype, M protein < 1.5 g/dL, normal ratio of free kappa/free lambda), bone marrow biopsy can be deferred
- No CRAB (increased calcium, renal disease [elevated serum creatinine ≥ 2 mg/dL], anemia or bone lesions) symptoms of end organ damage due to the monoclonal process (Lancet Oncol 2014;15:e538)
- Abnormal free light chain ratio (< 0.26 or > 1.65) is associated with higher risk for progression when using the Freelite assay (manufactured by The Binding Site, Thermo Fisher Scientific) on a BNII nephelometer (manufactured by Siemens) (N Engl J Med 2018;378:241)
- Caution: this range has not been validated for this purpose on contemporary instruments or other methods to measure FLC (see Laboratory below)
- Smoldering multiple myeloma (SMM): no clinical features
- IgG or IgA monoclonal protein present, with measurement of the M protein (≥ 3 g/dL) or
- Bone marrow clonal plasma cells 10 - 60%
- No CRAB signs of end organ damage, anemia or bone lesions due to the monoclonal process
- Ratio of the involved serum free light chain isotype (iFLC) to the uninvolved free light chain isotype (uFLC) of > 20 (iFLC/uFLC > 20) is 1 of 3 risk factors for progression; the other risk factors are bone marrow plasma cells percentage > 20% and the M protein > 2 g/dL (Blood Cancer J 2018;8:59)
- Multiple myeloma (MM), also known as plasma cell myeloma (PCM)
- Bone marrow clonal plasma cells ≥ 10% or extramedullary plasmacytoma and 1 or more of the following myeloma defining events (solitary plasmacytoma with ≥ 10% clonal bone marrow plasma cells is considered as multiple myeloma)
- CRAB symptom(s) attributed to the plasma cell proliferative process (any 1)
- Increased serum calcium > 1 mg/dL (> 0.25 mmol/L) higher than the upper limit of normal or > 11 mg/dL (> 2.75 mmol/L)
- Renal insufficiency: serum creatinine > 2 mg/dL (> 177 μmol/L) or creatinine clearance < 50 mL/min
- Anemia: hemoglobin value of < 10 g/dL or a value of > 2 g/dL below the lower limit of normal
- Bone lesions: 1 or more osteolytic lesions on skeletal radiography, computed tomography (CT or positron emission tomography CT [PET CT])
- Bone marrow clonal plasma cells ≥ 60%
- Involved / uninvolved serum free light chain ratio ≥ 100 (involved FLC level must be ≥ 100 mg/L)
- Caution: this level was established using the Freelite method on a BNII nephelometer
- This level has not been verified at this time on other methods for free light chain measurement or on the Freelite method on other instruments (see Laboratory below)
- > 1 focal lesion on magnetic resonance imaging (MRI) studies (at least 5 mm in size)
- CRAB symptom(s) attributed to the plasma cell proliferative process (any 1)
- Bone marrow clonal plasma cells ≥ 10% or extramedullary plasmacytoma and 1 or more of the following myeloma defining events (solitary plasmacytoma with ≥ 10% clonal bone marrow plasma cells is considered as multiple myeloma)
- IgM monoclonal gammopathy of undetermined significance (IgM MGUS): no clinical features
- Serum IgM monoclonal protein < 3 g/dL
- Bone marrow clonal plasma cells < 10%
- No evidence of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy or hepatosplenomegaly attributed to the underlying lymphoproliferative disorder
- Abnormal free light chain ratio (< 0.26 or > 1.65) is associated with higher risk for progression when using the Freelite system on a BNII nephelometer (Am J Hematol 2023;98:348)
- Caution: this range has not been validated for this purpose on contemporary instruments or other methods to measure FLC (see Laboratory below)
- Waldenström macroglobulinemia
- IgM M monoclonal protein (any size) and
- ≥ 10% Bone marrow infiltration by small lymphoplasmacytic cells with specific phenotype excluding other lymphoproliferative disorders
- Anemia, constitutional symptoms, hyperviscosity, lymphadenopathy or hepatosplenomegaly due to the lymphoproliferative disorder
- Serum FLC has been recommended to follow response to therapy and progression of disease (Leuk Lymphoma 2008;49:864)
- Light chain monoclonal gammopathy of undetermined significance (light chain MGUS): no clinical features
- No M protein on SPEP or immunofixation
- No underlying lymphoproliferative disease
- Abnormal FLC ratio, determined with Freelite assay on Optilite Analyzer (manufactured by The Binding Site, Thermo Fisher Scientific)
- eGFR ≥ 60 mL/min/1.73 m²
- Age < 70 years: FLC ratio < 0.44 or > 2.16
- Age ≥ 70 years: FLC ratio < 0.46 or > 2.59
- Elevated iFLC
- Age < 70 years: kappa > 39.0 or lambda > 36.7 mg/L
- Age ≥ 70 years: kappa > 55.8 or lambda > 48.0 mg/L
- eGFR < 60 mL/min/1.73 m²
- eGFR 45 - 49: FLC ratio < 0.46 or > 2.62
- eGFR 30 - 44: FLC ratio < 0.48 or > 3.38
- eGFR < 30: FLC ratio < 0.54 or > 3.30
- Elevated FLC
- eGFR 45 - 59: kappa > 83.6 or lambda > 65.1 mg/L
- eGFR 30 - 44: kappa > 103.3 or lambda > 73.2 mg/L
- eGFR < 30: kappa > 265.1 or lambda > 150.9 mg/L
- Further, this range has not been verified at this time on other methods or on the Freelite method on other instruments (Blood 2023;142:535, J Appl Lab Med 2023;8:742, Clin Chem Lab Med 2023;61:e229)
- eGFR ≥ 60 mL/min/1.73 m²
- Increased level of iFLC
- No immunoglobulin heavy chain by immunofixation, immunosubtraction or MASS-FIX
- Bone marrow clonal plasma cells < 10%
- No CRAB signs of end organ damage, anemia or bone lesions due to the monoclonal process
- Urine monoclonal protein < 500 mg/24 h
- Monoclonal gammopathy of renal significance (MGRS): evidence of renal disease due to damage from monoclonal immunoglobulin free light chains or heavy chains
- Demonstration of monoclonal light chain deposition in the kidney usually accompanied by circulating monoclonal FLC (Neth J Med 2019;77:243)
- AL amyloidosis
- Clinical symptoms of broad organ involvement: cardiac, renal, hepatic or gastrointestinal involvement
- Monoclonal FLC indicated by abnormal serum free light chain ratio and increase of involved isotype, monoclonal free light chain band by urine or serum immunofixation or biopsy demonstration of amyloid by Congo red technique or ultrastructure analysis and demonstration of monoclonal free light chain (Blood 2020;136:2620)
- Reference: Mayo Clin Proc 2016;91:101
Laboratory
- Diagnostic range of 0.26 - 1.65 established by using the entire range of individuals' serum as controls on the Freelite assay performed on the Dade-Behring BNII nephelometer in 2002 has not been confirmed as correct with most contemporary methods and instruments
- < 3% of laboratories performing serum FLC assays currently use the BNII instrument with the Freelite assays (CAP Survey 2023)
- Recent studies report that the range differs depending on which method and which instrument the laboratory uses (Clin Biochem 2018;58:100)
- Current studies suggest using a more statistically reproducible 99% range for the specific method and instrument used (Blood Cancer J 2022;12:133, Clin Biochem 2023;118:110604)
- iStopMM study, which uses the Freelite method on the Optilite, reports the 99% range as (Blood 2023;142:535)
Kappa FLC (mg/L) Lambda FLC (mg/L) FLC ratio Age < 70 yr 6.3 - 39.0 5.9 - 36.7 0.44 - 2.16 Age ≥ 70 yr 7.0 - 55.8 6.4 - 48.0 0.46 - 2.59
- Several commercial methods are available for measuring serum free light chains
Common commercial platforms and methodologies
| Company | Methodology | Instrument(s)* |
| The Binding Site (Thermo Fisher Scientific) | Polyclonal anti-light chain reagents Immunonephelometric | Binding Site Optilite Binding Site SPAplus Siemens Nephelometer Roche Cobas c500 |
| Siemens | Monoclonal anti-light chain reagents Immunonephelometric | Siemens Nephelometer |
| Diazyme Reagent | Polyclonal anti-light chain reagents Immunoturbidimetric | Roche Cobas c500 |
| Sebia | Polyclonal anti-light chain reagents Enzyme linked immunosorbent assay (ELISA) | AP22 ELITE analyzer |
Additional references
Board review style question #1
This serum is from a 58 year old woman with back pain and hemoglobin of 7.4 g/dL. Her bone marrow clonal plasma cells were > 60%. Which of the following is the most likely diagnosis?
- IgA kappa multiple myeloma
- IgG kappa multiple myeloma
- IgM kappa Waldenström macroglobulinemia
- Kappa light chain multiple myeloma
Board review style answer #1
D. Kappa light chain multiple myeloma is the most likely diagnosis because she meets the IMWG criteria for multiple myeloma; she is anemic with a hemoglobin of 7.4 and her bone marrow clonal plasma cells are > 60%. Kappa light chain myeloma is the most likely isotype because her free K is 35x the upper limit of normal with a free K/L ratio 40x the upper limit of normal. Answers A, B and C are incorrect because it is not likely to be an IgG, IgAK or IgMK process given the absence of an M spike and absence of elevated IgG, IgA and IgM levels.
Comment Here
Reference: Free light chains
Comment Here
Reference: Free light chains
Board review style question #2
This serum is from a clinically well 76 year old woman with a normal CBC, no evidence of renal disease and normal calcium. The immunofixation identified the M spike as IgGK. Which of the following is the most likely diagnosis?
- Monoclonal gammopathy of undetermined significance (MGUS)
- Multiple myeloma (MM)
- Smoldering multiple myeloma (SMM)
- Waldenström macroglobulinemia (WM)
Board review style answer #2
A. Monoclonal gammopathy of undetermined significance (MGUS) is the most likely diagnosis because she is well, not anemic and her M spike measures only 0.72 g/dL. Answer B is incorrect because the patient is clinically well with no evidence of renal disease and has a normal CBC. Answer C is incorrect because an M spike of ≥ 3g/dL or a bone marrow clonal plasmacytosis of ≥ 10% would be needed to upgrade her condition to SMM. In this case she has a normal free K/L, a normal CBC and no myeloma defining events. Bone marrow biopsy can be deferred in patients categorized as low risk MGUS (IgG isotype, M protein < 1.5 g/dL, normal FLC ratio) and who lack features suspicious for MM. Answer D is incorrect because she is clinically well with a normal CBC and the M protein is not IgM.
Comment Here
Reference: Free light chains
Comment Here
Reference: Free light chains
Syphilis (pending)
Systemic lupus erythematosus (SLE)
Table of Contents
Definition / general | Essential features | ICD coding | Diagrams / tables | Pathophysiology | Clinical features | Test indications | Laboratory | Immunofluorescence description | Immunofluorescence images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Systemic lupus erythematosus (SLE) is a chronic autoimmune disease marked by autoantibodies or immune complex deposits causing inflammation and damage in organs like the kidneys, heart, blood vessels, central nervous system (CNS), skin, lungs, muscles and joints; its severity varies from mild to life threatening (Ann Rheum Dis 2010;69:1603)
Essential features
- Antinuclear antibodies (ANAs) are present in systemic and organ specific diseases; ANA testing is important for diagnosing specific autoimmune conditions but not suitable for general screening due to poor sensitivity
- SLE patients typically have a strongly positive ANA test (≥ 1:80 titer); an ANA screening immunoassay or HEp-2 immunofluorescence with comparable performance is recommended
- Points are assigned based on 7 clinical categories and 3 immunologic test categories once the ANA criterion is met
- Exclusions apply if another cause is more likely than SLE and criteria need not occur simultaneously
- Score of ≥ 10 is indicative of SLE
ICD coding
- ICD-10: M32.9 - systemic lupus erythematosus, unspecified
Diagrams / tables
Pathophysiology
- In SLE, multiple organs are affected due to autoimmune related inflammation
- This damage results from immune system characteristics (specifically, the loss of nucleic acid tolerance and interferon system activation)
- SLE hallmarks are autoantibodies against nucleic acids (notably targeting dsDNA and RNA / RNA binding proteins) and an interferon stimulated gene (ISG) signature in blood and affected tissues
- Recent findings show that nucleic acids bound to SLE related autoantibodies trigger interferon production in plasmacytoid dendritic cells (pDCs) upon endocytosis
- This link highlights the role of endosomal Toll-like receptor (TLR) in SLE
- Additionally, interferon's diverse impact on dendritic cell (DC) and B cell differentiation has been reported (Ann Rheum Dis 2023;82:999)
- See Diagrams / tables for an overview of SLE pathogenesis
Clinical features
- SLE symptoms include fatigue, fevers, skin rashes and joint pain / swelling
- Flares and remissions occur variably
- Additional symptoms involve sun sensitivity, oral ulcers, arthritis, lung / heart / kidney problems, seizures, psychosis and blood / immune abnormalities
- See Diagrams / tables for a summary of clinical features
- Reference: Arthritis Rheumatol 2019;71:1400
Test indications
- Early SLE signs and symptoms lack specificity
- Rheumatologists need to use specific criteria for final diagnosis
- Women, especially ages 15 - 44, are at the highest risk for SLE (Arthritis Rheum 2012;64:2319)
- Black, Hispanic / Latino, Asian and American Indian people / Alaska Natives are more affected than White people (Imboden: Current Diagnosis & Treatment in Rheumatology, 3rd Edition, 2013)
- New 2019 European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) classification criteria allow earlier and more accurate SLE classification; these criteria are detailed in Diagrams / tables (Arthritis Rheumatol 2019;71:1400)
- ANA test positivity (≥ 1:80) is an entry criterion; ANA screening immunoassay or HEp-2 (human epidermoid carcinoma cells) immunofluorescence is recommended
- Points are assigned based on 7 clinical and 3 immunologic categories once the ANA criterion is met
- Exclusion happens if another cause seems more likely than SLE; criteria do not need to occur simultaneously
- Score ≥ 10 indicates SLE
Laboratory
- Common hematologic manifestations include anemia of chronic disease, autoimmune hemolytic anemia, leukopenia and thrombocytopenia
- Low complement C3 or C4 levels
- Antiphospholipid antibodies can be detected
- Strongly positive ANA is associated with SLE but it lacks specificity
- Can be seen in some healthy individuals (especially older female) as well as those with other autoimmune conditions, certain cancers and medications
- ANA detection relies on indirect immunofluorescence assay (IFA) using HEp-2 cells and solid phase assays
- Both methods have limitations that reduce antibody detection sensitivity; clinicians should understand these assays' strengths and weaknesses used in their laboratory
- In 2009, due to concerns regarding solid phase assay sensitivity, the ACR recommended initial ANA testing using IFA with HEp-2 cells (Ann Rheum Dis 2010;69:1420, Clin Chem Lab Med 2023;61:1167)
- 2019 EULAR / ACR classification criteria strongly advocated IFA ANA testing as an entry criterion
- IFA and solid phase immunoassays for ANA testing involves the following steps
- IFA utilizes a HEp-2 cell line characterized by large nuclei, aiding in the visualization of staining patterns; these cells incorporate nearly all clinically relevant autoantigens, making them suitable for detecting corresponding autoantibodies
- Process involves fixing cells on glass slides, incubating them with patient serum, washing off nonadherent proteins and introducing fluorescein conjugated antibodies that bind to human antibodies interacting with HEp-2 cell antigens (see Diagrams / tables)
- ANA staining patterns in the nucleus, like homogeneous, speckled, nucleolar, dot and nuclear envelope patterns, are observed using an ultraviolet microscope (see Immunofluorescence images)
- Upon initial fluorescence detection at 1:80 dilution, serum undergoes serial dilution until fluorescence is detectable in less than half of the cells
- Advantages include broad autoantibody detection, aiding SLE diagnosis
- However, interpreting results requires skilled technicians; automated analyzers simplify the process but ANA patterns lack specificity, often requiring further solid phase testing
- Some autoantigens may not be detectable in HEp-2 cells, leading to potential false negatives
- Efforts to standardize ANA pattern nomenclature are ongoing through the International Consensus on Autoantibody Patterns (ICAP)
- Solid phase assays include enzyme linked immunosorbent assay (ELISA), fluorescent microsphere and immunoline assays, utilizing fixed autoantigens on surfaces to detect serum autoantibodies categorized as positive, indeterminate or negative
- These assays involve fixing autoantigens to a solid surface, incubating patient serum and using a labeled secondary antibody to detect bound autoantibodies
- They are efficient for high throughput ANA tests, identifying specific autoantibodies rather than patterns
- However, they might lack sensitivity compared to IFA, using fewer antigens and potentially reducing SLE diagnostic sensitivity (Arthritis Rheumatol 2019;71:1400)
- IFA utilizes a HEp-2 cell line characterized by large nuclei, aiding in the visualization of staining patterns; these cells incorporate nearly all clinically relevant autoantigens, making them suitable for detecting corresponding autoantibodies
- SLE specific antibodies
- Anti-double stranded (dsDNA) antibodies
- Discovered in 1957
- Have long been established as diagnostic markers for systemic lupus erythematosus (SLE)
- Serve as reliable indicators of disease activity in SLE
- Current methodologies for detecting anti-dsDNA antibodies include Farr radioimmunoassay, Crithidia luciliae indirect immunofluorescence test (CLIFT), enzyme linked immunosorbent assay (ELISA), fluoroenzyme immunoassay (FEIA) and chemiluminescent immunoassay (CIA)
- Anti-Smith antibodies
- First identified in 1966
- Exhibit high specificity for SLE and are often detectable prior to diagnosis
- Several techniques are available for detecting anti-Sm antibodies
- While immunoprecipitation using a radioactive immunoassay (RIA) is considered the gold standard, in routine practice, alternative methods such as indirect immunofluorescence tests (IIFT), enzyme linked immunosorbent assays (ELISA), addressable laser bead immunoassays (ALBIA), line immunoassays (LIA), chemiluminescent immunoassays (CLIA) and fluorescent enzyme immunoassays (FEIA) are more commonly used
- Anti-double stranded (dsDNA) antibodies
Immunofluorescence description
- See Laboratory
Board review style question #1
A 52 year old South American woman presented with fever (102 °F) and fatigue. She also has arthralgias and Raynaud phenomenon. Her primary care provider ordered an antinuclear antibody (ANA) screening test; the pattern on HEp-2 cells with serum diluted to 1:80 is shown in the figure. What is the ANA pattern?
- Centromere
- Homogeneous
- Negative
- Nucleolar
- Speckled
Board review style answer #1
B. Homogeneous. This is a homogeneous pattern, demonstrating homogenous and regular fluorescence across all nucleoplasms. The nucleoli may be stained or not stained depending on cell substrate. Mitotic cells (metaphase, anaphase and telophase) have the chromatin mass intensely stained in a homogeneous hyaline fashion (ICAP: ANA Patterns [Accessed 25 January 2024]). Answers A, C, D and E are incorrect because the pattern in the image does not match these choices.
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Board review style question #2
The IFA ANA result (> 1:80) was interpreted as homogeneous pattern in a 52 year old South American woman who presented with fever (102 °F) and fatigue, with arthralgias and Raynaud phenomenon. The provider ordered routine extractable nuclear antigen (ENA) profile test (Ro [SSA], La [SSB], Sm [Smith], RNP, Scl-70 and Jo1). Both Sm and RNP were found to be positive. What condition or diagnosis would you suspect for this patient?
- Rheumatoid arthritis
- Scleroderma
- Sjögren syndrome
- Systemic lupus erythematosus (SLE)
Board review style answer #2
D. Systemic lupus erythematosus (SLE). According to the 2019 EUALR / ACR new classification criteria, a positive ANA test with a titer of ≥ 1:80 and a score of ≥ 10 is indicative of SLE.
Answer A is incorrect because it remains uncertain how this information could contribute to the clinical management of rheumatoid arthritis. Currently, ANA positivity is not utilized as a screening tool for diagnosing rheumatoid arthritis. There has been no extensive population based cohort study investigating ANA positivity specifically in rheumatoid arthritis patients.
Answer B is incorrect because there is no clear evidence supporting a diagnosis of systemic sclerosis, based on this patient's testing results and clinical presentation. When scleroderma affects both the skin and internal organs, it is referred to as systemic sclerosis. Antinuclear antibodies (ANAs) are present in ~95% of systemic sclerosis patients, making ANA testing a crucial initial step in disease assessment. The observed ANA patterns in systemic sclerosis can vary widely, including homogeneous, centromere, nucleolar, speckled nuclear patterns or reticular / ANA cytoplasmic pattern; however, the results of an ANA test alone are not sufficient for a scleroderma diagnosis. According to the European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) criteria for the classification of systemic sclerosis, positivity for anticentromere, antitopoisomerase I or anti-Sd 70 antibodies is typical.
Answer C is incorrect because there is no clear evidence supporting a diagnosis of Sjögren syndrome, based on this patient's testing results and clinical presentation. For individuals presenting with persistent symptoms of dry eyes or mouth, an unexplained increase in dental caries, parotid gland enlargement or abnormal results of specific serologic tests (e.g., anti-Ro / SSA antibodies with or without anti-La / SB antibodies, rheumatoid factor and hyperglobulinemia), Sjögren syndrome should be considered.
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Answer A is incorrect because it remains uncertain how this information could contribute to the clinical management of rheumatoid arthritis. Currently, ANA positivity is not utilized as a screening tool for diagnosing rheumatoid arthritis. There has been no extensive population based cohort study investigating ANA positivity specifically in rheumatoid arthritis patients.
Answer B is incorrect because there is no clear evidence supporting a diagnosis of systemic sclerosis, based on this patient's testing results and clinical presentation. When scleroderma affects both the skin and internal organs, it is referred to as systemic sclerosis. Antinuclear antibodies (ANAs) are present in ~95% of systemic sclerosis patients, making ANA testing a crucial initial step in disease assessment. The observed ANA patterns in systemic sclerosis can vary widely, including homogeneous, centromere, nucleolar, speckled nuclear patterns or reticular / ANA cytoplasmic pattern; however, the results of an ANA test alone are not sufficient for a scleroderma diagnosis. According to the European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) criteria for the classification of systemic sclerosis, positivity for anticentromere, antitopoisomerase I or anti-Sd 70 antibodies is typical.
Answer C is incorrect because there is no clear evidence supporting a diagnosis of Sjögren syndrome, based on this patient's testing results and clinical presentation. For individuals presenting with persistent symptoms of dry eyes or mouth, an unexplained increase in dental caries, parotid gland enlargement or abnormal results of specific serologic tests (e.g., anti-Ro / SSA antibodies with or without anti-La / SB antibodies, rheumatoid factor and hyperglobulinemia), Sjögren syndrome should be considered.
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Therapeutic drug monitoring (pending)
[Pending]
Thyroglobulin
Definition / general
- Prohormone of T3 and T4 synthesized by thyroid follicular cells and cosecreted into serum with T3 and T4
- Serum level is affected by thyroid mass, thyroid injury and TSH receptor stimulation
- Tyrosine residues of thyroglobulin are iodinated with thyroid peroxidase to produce mono- and diiodotyrosyls, which combine to form T3 (mono+di) and T4 (di + di)
- Thyroglobulin releases T3 and T4 into circulation via lysosomal degradation
Laboratory
Indications
Methodology
Monitoring recurrence of well differentiated thyroid carcinoma
Limitations
Reference range
High values
Low levels
- Used primarily to monitor recurrence of well differentiated thyroid cancer after total thyroidectomy or I-131 treatment (Endocr Regul 2006;40:53)
- Also to diagnose thyroid dysgenesis in congenital hypothyroidism (J Med Genet 2005;42:379) or to distinguish subacute thyroiditis (elevated levels) from factitious thyrotoxicosis (undetectable levels despite elevated T3 and T4 levels)
Methodology
- Immunoassay
Monitoring recurrence of well differentiated thyroid carcinoma
- Give patient recombinant human TSH, and measure thyroglobulin 72 hours after last dose
- Rise of serum thyroglobulin above 2 ng/ml (if had remnant ablation and receiving hormone suppressive therapy) correlates with presence of recurrent disease
- Can confirm recurrent disease by whole body iodine scan
- Well differentiated tumors typically have 10x rise in thyroglobuln
- Poorly differentiated tumors may not concentrate iodide, may have blunted response to TSH stimulation
- Can also monitor change of thyroglobulin level when receiving T4 treatment post-thyroidectomy (J Clin Endocrinol Metab 2005;90:5047)
Limitations
- 20% with thyroid cancer have antithyroglobulin antibodies which interfere with assay (falsely lower serum concentration by binding to thyroglobulin and making it "invisible" to the assay)
- Should determine presence of antibodies when measure thyroglobulin level for tumor monitoring
- If antibodies are present, PCR may be useful to measure thyroglobulin
- Also interference by heterophile antibodies (J Clin Endocrinol Metab 2003;88:3069)
Reference range
- < 30 ng/ml (45 pmol/L)
High values
- Graves disease, thyroiditis, nodular goiter, well differentiated thyroid carcinoma
Low levels
- Factitious thyrotoxicosis (surreptitious use of thyroid hormone-levels are undetectable although T3 or T4 are elevated)
Thyroid autoantibodies
Table of Contents
General | TSH receptor antibodies | Thyroperoxidase antibodies | Thyroglobulin antibodies | Board review style question #1 | Board review style answer #1General
- Antibody testing for evaluation of autoimmune thyroid disorders:
- TSH receptor antibodies (including thyroid stimulating immunoglobulin and thyrotropin binding inhibiting immunoglobulin)
- Thyroperoxidase antibody
- Thyroglobulin antibody
TSH receptor antibodies
Definition / general
Clinical features
Laboratory
Diagrams
Images hosted on other servers:
- TSH (thyroid stimulating hormone) receptor antibodies (TRAbs) may have different effects on thyroid function
- Stimulating antibodies (TSAbs) cause hyperthyroidism
- Blocking antibodies (TBAbs) cause hypothyroidism
- Neutral TRAbs have no effect
Clinical features
- Clinical utility (J Clin Endocrinol Metab 2013;98:2247):
- Confirm diagnosis of Graves disease (not routine use)
- Diagnosis of euthyroid Graves orbitopathy
- Predict remission before withdrawing antithyroid drug
- Pregnant women with previous Graves disease: determine risk of fetal / neonatal hyperthyroidism or hypothyroidism (depends on functional type of TRAbs)
Laboratory
- Methodology:
- 2 methods for measuring TRAbs (Endocr Pract 2020;26:97) (see diagram):
- Conventional immunoassay, binding assay or thyrotropin binding inhibiting immunoglobulins (TBII) assay:
- Advantage: international standardization, commercial automated immunoassays
- Disadvantage: does not differentiate the functional type of the antibodies
- Bioassay:
- Advantage: discriminates the functional type (TSAbs or TBAbs), preferred use in prediction of fetal / neonatal risk for hypothyroidism in pregnant women with active or treated autoimmune thyroid disease
- Disadvantage: more time consuming, requires experienced laboratory technician and available only in some centers
- Conventional immunoassay, binding assay or thyrotropin binding inhibiting immunoglobulins (TBII) assay:
- 2 methods for measuring TRAbs (Endocr Pract 2020;26:97) (see diagram):
- Third generation TBII assays have a sensitivity of over 97.2% and a specificity of over 98.3% for the diagnosis of Graves disease versus other causes of hyperthyroidism (Clin Endocrinol (Oxf) 2011;75:127)
- Reference range of TBII assay:
- Elecsys (Roche Diagnostics, Mannheim, Germany): normal cutoff < 1.75 IU/L
Diagrams
Images hosted on other servers:
Thyroperoxidase antibodies
Definition / general
Clinical features
Laboratory
- Thyroperoxidase antibodies (TPOAbs) were formerly known as microsomal antibodies, which is still used in literature
- Almost all Hashimoto thyroiditis patients and nearly 75% of Graves disease patients have detectable TPOAbs (Horm Metab Res 2019;51:765)
- Moderately increased levels of TPOAbs may be found in 5 - 20% of the normal population and patients with nonthyroid autoimmune diseases, such as pernicious anemia, type I diabetes or other autoimmune disease (Front Immunol 2017;8:521)
Clinical features
- Clinical utility:
- Diagnosis of autoimmune thyroid disease
- Predict the likelihood of progression to permanent overt hypothyroidism in patients with subclinical hypothyroidism (N Engl J Med 2017;376:2556)
- Predict risk of pregnancy loss in high risk patients and determine the need for thyroxine replacement therapy during pregnancy (Thyroid 2017;27:315)
Laboratory
- Methodology:
- Electrochemiluminescence immunoassay (ECLIA), chemiluminescence enzyme immunoassay (CLEIA) (Endocr J 2017;64:955)
- Reference range:
- Varies between different immunoassays due to TPO antigen preparations used to coat solid phase (Endocr J 2017;64:955)
- IMMULITE 2000 (Siemens Healthcare Diagnostics, NY, USA): normal cutoff < 35 IU/mL
Thyroglobulin antibodies
Definition / general
Clinical features
Laboratory
- Thyroglobulin antibodies (TgAbs) were formerly known as colloid antigen
- Marker of autoimmune thyroid disease (along with TPOAbs)
- TgAbs are detectable in 10% of the general population and 20% of thyroid cancer patients (J Clin Endocrinol Metab 2005;90:5566, J Clin Endocrinol Metab 1998;83:1121)
- TgAbs are also detectable in nonthyroid autoimmune diseases, such as pernicious anemia, type I diabetes or other autoimmune diseases (Front Immunol 2017;8:521)
- TgAbs interfere with Tg measurement causing falsely low or undetectable Tg immunometric assay (IMA) values (Best Pract Res Clin Endocrinol Metab 2013;27:701)
Clinical features
- Clinical utility:
- Diagnosis of autoimmune thyroid disease (together with TPOAbs)
- Surrogate marker of differentiated thyroid cancer useful in postoperative follow up protocols (routine use with serum Tg levels) (Thyroid 2016;26:1)
Laboratory
- Methodology:
- Electrochemiluminescence immunoassay, chemiluminescence enzyme immunoassay (Endocr J 2017;64:955)
- Reference range:
- Varies between different immunoassays due to Tg antigen preparations used to coat solid phase (Endocr J 2017;64:955)
- IMMULITE 2000 (Siemens Healthcare Diagnostics, NY, USA): normal cutoff < 40 IU/mL
Board review style question #1
A 30 year old woman presented with bilateral proptosis. She denied a history of palpitation, perspiration or weight loss, clinically consistent with euthyroidism. Physical examination revealed mild exophthalmos and diffuse thyroid enlargement. Thyroid function test was normal. What is the best choice for the next laboratory test in this patient?
- IGF1 receptor antibodies
- Microsomal antibodies
- Thyroglobulin antibodies
- Thyroperoxidase antibodies
- TSH receptor antibodies
Board review style answer #1
E. TSH receptor antibodies. The patient presented with symptoms of euthyroid Graves orbitopathy, therefore TSH receptor antibodies should be performed to definitively diagnose this condition.
Comment Here
Reference: Thyroglobulin, TSH receptor and thyroperoxidase antibodies
Comment Here
Reference: Thyroglobulin, TSH receptor and thyroperoxidase antibodies
Thyroid function panel
Table of Contents
Thyroid function test interpretation | Thyroid stimulating hormone (TSH) | Thyroid hormone (T4, T3 and rT3) | Total thyroid hormone measurement | Free thyroid hormone measurement | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Thyroid function test interpretation
- Thyroid function test (TFT) algorithm (Best Pract Res Clin Endocrinol Metab 2013;27:745)
- Initial screening with serum thyroid stimulating hormone (TSH) level is the most common approach
- If TSH level is abnormal, free thyroxine (T4) should be evaluated
- A commonly used screening panel is TSH with reflex to free T4
- Free triiodothyronine (T3) can be added for the evaluation of hyperthyroidism when free T4 is not elevated
- Potential confounding factors for TFT interpretation
- Age: age specific reference ranges are important in newborn and elderly
- Pregnancy
- Low TSH (first trimester; secondary to increased human chorionic gonadotropin [hCG])
- High total T4 and T3 (from first trimester; secondary to increased thyroxine binding globulin [TBG])
- Changes in free T4 and T3
- Trimester specific reference ranges should be used wherever possible
- Levothyroxine (LT4) therapy: mildly elevated free T4 (but normal free T3), intermittent hormone ingestion may result in normal or elevated free T4 but fails to normalize TSH
- Confounding medications / supplements: alter thyroid physiology (e.g. amiodarone) or cause artifactual laboratory results (e.g. heparin or biotin) (N Engl J Med 2019;381:749)
- Nonthyroidal illness (NTI): different TFT results depend on severity of illness (eMedicine: Euthyroid Sick Syndrome [Accessed 25 June 2020])
- Mild illness: low free T3, normal free T4, normal or low TSH (also known as low T3 syndrome)
- Severe illness: very low free T3, low free T4, normal or low TSH
- Recovery phase: low free T3, low free T4, high TSH (usually < 20 μU/mL)
- TFT patterns and associated clinical conditions (Best Pract Res Clin Endocrinol Metab 2013;27:745)
- Low free T3/T4 and high TSH: primary hypothyroidism
- Normal free T3, low free T4 and high TSH: early primary hypothyroidism
- High free T3/T4 and low TSH: primary hyperthyroidism (toxic adenoma, multinodular goiter or Graves disease)
- Normal free T3, high free T4 and low TSH (T4 toxicosis): iodine induced hyperthyroidism, primary hyperthyroidism with NTI
- High free T3, normal free T4 and low TSH (T3 toxicosis): primary hyperthyroidism in iodine deficiency area, earliest stages of primary hyperthyroidism
- Low free T3/T4 and normal or low TSH: central hypothyroidism, NTI
- High free T3/T4 and normal or high TSH: interference, familial dysalbuminemic hyperthyroxinemia (FDH), TSH secreting pituitary adenoma, resistance to thyroid hormone, acute psychiatric disorder, drug (amiodarone, heparin)
- Normal free T3/T4 and high TSH: subclinical hypothyroid, poor compliance of LT4, NTI recovery phase, TSH resistance, assay interference (heterophile antibodies, macroTSH)
- Normal free T3/T4 and low TSH: subclinical hyperthyroidism, recent treatment of thyrotoxicosis, assay interference, drug (steroid, dopamine)
Thyroid stimulating hormone (TSH)
-
General
- Also called thyrotropin
- Hypothalamic-pituitary-thyroid (HPT) axis regulates TSH release
- Hypothalamus secretes the thyroid releasing hormone (TRH), which stimulates thyrotrophs in the anterior pituitary to secrete TSH which consists of alpha subunit (same sequence as luteinizing hormone, follicle stimulating hormone, human chorionic gonadotropin [hCG]) and beta unit (differs from other hormones)
- Binding TSH to TSH receptor on thyroid follicular cell promotes increased uptake of iodide into thyroid follicular cells by active transport via sodium iodide symporter, synthesis of thyroglobulin, formation of T3 and T4, release of T3 and T4 into circulation (Ento Key: Thyroid Physiology and Thyroid Function Testing [Accessed 25 June 2020])
- T4 and T3 can exert negative feedback on TSH levels, with high levels of T3/T4 decreasing TSH (negative feedback) and low levels of T3/T4 increasing TSH levels from the anterior pituitary (positive feedback)
- There is a log linear relationship between T3/T4 and TSH with minor changes in TH resulting in major changes in TSH (Ento Key: Thyroid Physiology and Thyroid Function Testing [Accessed 25 June 2020])
- Most TSH assays use a "sandwich" format with 2 antibodies - capture and labeled detection - directed against different epitopes on TSH
- Presence of human anti-animal antibodies in a patient's serum can interfere with TSH measurement if directed against the same species as the assay antibodies, leading to falsely high TSH (Endocr Rev 2018;39:830)
- Depends on different platforms and methods: average 0.4 - 4 μU/mL
- Initial screening test for thyroid dysfunction unless the conditions below are suspected; in such cases, add free T4 and possibly also free T3 (PLoS One 2018;13:e0196631, Best Pract Res Clin Endocrinol Metab 2013;27:745):
- Confounding intercurrent illness or medication use
- Recent treatment for hyper or hypothyroidism
- Erratic or poor adherence to levothyroxine therapy
- Genetic or acquired disorders of the HPT axis
- Interference in thyroid function panel
- Annual screening for patients receiving thyroid hormone replacement therapy (target is 1 - 4 μU/mL) (wait 6 weeks to retest due to altered dose)
- Monitor target of thyroid hormone suppressive therapy after thyroidectomy in patients with differentiated thyroid carcinoma (target is 0.5 - 2.0 μU/mL if low risk, 0.1 - 0.5 μU/mL if moderate risk and < 0.1 μU/mL if high risk) (Thyroid 2016;26:1)
TSH measurement
TSH reference value
Indications for TSH testing
Thyroid hormone (T4, T3 and rT3)
General
- The 2 principal thyroid hormones are thyroxine (also known as T4 or L-3,5,3',5'-tetraiodothyronine) and triiodothyronine (T3 or L-3,5,3'-triiodothyronine) (Ento Key: Thyroid Physiology and Thyroid Function Testing [Accessed 25 June 2020])
- Thyroxine (T4)
- Usual daily production is 110 nmol/day; half life is 5 - 7 days
- 100% produced in thyroid
- 50% of T4 is 5' deiodinated to form T3, 40% is 5 deiodinated to form reverse T3 via 5-deiodinase (Ento Key: Thyroid Physiology and Thyroid Function Testing [Accessed 25 June 2020])
- Triiodothyronine (T3)
- Usual daily production is 50 nmol/day; half life is 1 day
- 80% from deiodination of T4 in nonthyroid tissue, 20% arises in thyroid gland
- Most of the TH circulating in the blood is bound to TH binding proteins: thyroxine binding globulin (TBG), transthyretin (TTR) and albumin
- Only a very small fraction of unbound TH (0.03% of T4 and 0.3% of T3) is biologically active and signal all tissues by binding TH nuclear receptor (THR)
- TH is metabolized by deiodination, conjugation with glucuronide and sulfate, ether link cleavage and side chain modification (oxidative deamination and decarboxylation) (Endotext: Metabolism of Thyroid Hormone [Accessed 25 June 2020])
- Reverse T3 (rT3)
- Also known as 3,3',5'-triiodothyronine
- Biologically inactive metabolite of T4 formed by selective deiodination
- Not routinely measured
- Serum rT3 levels are increased in systemic nonthyroidal illnesses except chronic renal failure and in patients taking drugs such as amiodarone, propylthiouracil, dexamethasone, propranolol and ipodate
Total thyroid hormone measurement
- Easier to measure than free TH
- Many inherited, acquired conditions and drugs can alter TBG level
- Pregnancy, contraceptive pills, estrogen receptor modulators, 5-fluorouracil, liver diseases, methadone, heroin, acute intermittent porphyria, hypothyroidism and inherited TBG excess (TBG-E) increase serum TBG resulting in high total TH but normal free TH
- Androgens, corticosteroids, hyperthyroidism, terminal illnesses and inherited TBG deficiency (TBG-D) decrease serum TBG resulting in low total TH but normal free TH
- Serum total TH is usually measured by immunoassay (automated competitive binding chemiluminescent assays)
- Serum total TH is also measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) which results in more specific and accurate TH value than immunoassay but this technique needs expensive equipment and clinical expertise (Anal Bioanal Chem 2010;397:1831)
Free thyroid hormone measurement
- Free T4 measurement is preferred to total T4 whereas total T3 is preferred to free T3 (Best Pract Res Clin Endocrinol Metab 2013;27:689)
- Presence of factors in serum that affect free TH measurement include:
- Interferences (T4/T3 antibodies, human anti-animal antibodies) (Endocr Rev 2018;39:830)
- Variant thyroid hormone binding proteins: dominantly inherited genetic variants of albumin (familial dysalbuminemic hyperthyroxinemia [FDH] or transthyretin [transthyretin associated hyperthyroxinemia (TTR-AH)])
- Drugs (e.g. heparin and aspirin) (N Engl J Med 2019;381:749)
Methods of free TH measurement
- Free T4 index
- Free T4 index is calculated by dividing the total T4 by the T3 uptake ratio (percentage)
- T3 uptake test is performed by incubating the patient's serum with radiolabeled T3 tracer and subsequently adding resin that traps the remaining unbound radiolabeled T3; the value reported is the percent of tracer bound to the resin, which varies inversely with the number of available free binding sites for T3
- T3 uptake was designed to distinguish TBG excess and deficiency from hyperthyroidism and hypothyroidism:
- Hyperthyroidism: high serum total T4, high T3 uptake, high free T4 index
- TBG excess: high serum total T4, low T3 uptake, normal free T4 index
- Hypothyroidism: low serum total T4, low T3 uptake, low free T4 index
- TBG deficiency: low serum total T4, high T3 uptake, normal free T4 index
- Free TH ligand assays (indirect method) (Biochemia Medica 2016;26:436)
- Competition immunoassay is used
- Serum TH compete with labeled TH analog for a fixed number of TH antibody binding sites
- Serum TH compete with an immobilized TH analog for a labeled TH antibody
- Free hormone concentration is inversely proportional to the signal generated with fluorescence or chemiluminescence
- Different immunoassay platforms are available
- 1 step labeled T4 analog (Immulite, Siemens)
- 1 step analog 2 step incubation (Cobas, Roche Diagnostics)
- 2 step labeled T4 analog method (DXi, Beckman Coulter, Architect, Abbott)
- 1 step labeled antibody method (Vitros, Ortho Clinical Diagnostics)
- 2 step method was less susceptible to T4/3 autoantibodies than the 1 step method
- Competition immunoassay is used
- Ultrafiltration (UF) and equilibrium dialysis (ED) (direct method) (Best Pract Res Clin Endocrinol Metab 2013;27:689)
- Methods with physical separation against ultrafiltrate / dialysate membrane and direct measurement of free T4 in protein depleted serum by immunoassay or LC-MS/MS
- Not affected by changes in binding protein concentration, T4/T3 autoantibodies or nonthyroidal illness
- Free thyroid hormone concentrations measured by LC-MS/MS correlate to a greater degree with log TSH values compared to concentrations measured by immunoassay (Clin Chem 2009;55:1380)
- Time consuming, expensive and requires rigorous control of the pH, temperature, membrane type / cutoff, minimum dilution and dialysis buffer
- Methods with physical separation against ultrafiltrate / dialysate membrane and direct measurement of free T4 in protein depleted serum by immunoassay or LC-MS/MS
Reference value
- Depends on different platforms and methods
- Cobas, Roche Diagnostics
- FT4 12 - 22 pmol/L (0.93 - 1.7 ng/dL)
- FT3 3.1 - 6.8 pmol/L (2 - 4.4 pg/mL)
Conversion factor
- FT4: multiply by 12.87 to convert from ng/dL to pmol/L
- FT3: multiply by 1.54 to convert from pg/dL to pmol/L
Board review style question #1
A 65 year old man with no history of thyroid disease is admitted to the medical intensive care unit for septic shock. Thyroid function test is performed due to hyponatremia: TSH 5 μU/mL (0.3 - 4.1), free T4 0.9 ng/dL (0.8 - 1.8), free T3 1.2 pg/mL (1.6 - 4).
What is the most likely cause of abnormal thyroid function test in this patient?
What is the most likely cause of abnormal thyroid function test in this patient?
- Heterophile antibodies
- Nonthyroidal illness
- Primary hypothyroidism
- Subclinical hypothyroidism
- TSH producing pituitary adenoma
Board review style answer #1
B. Nonthyroidal illness. TFT showed normal free T4, low T3 and slightly elevated TSH. Nonthyroidal illness is the likely background condition. In the early course of illness, free T3 will firstly decrease due to decrease of peripheral conversion while free T4 is still maintained; TSH level is variable (0.1 - 20 μU/mL). Heterophile antibodies is incorrect because normally heterophile antibodies will interfere with TSH measurement, causing falsely high TSH but normal free T4 and T3 level. Primary hypothyroidism is incorrect because free T4 level is normal and TSH level is too low for primary hypothyroidism. Subclinical hypothyroidism is incorrect because free T3 level is low. TSH producing pituitary adenoma is incorrect because free T3 and T4 level is not high. TFT pattern in TSH producing pituitary adenoma is high free T3/T4 and normal or high TSH.
Comment Here
Reference: Thyroid function panel
Comment Here
Reference: Thyroid function panel
Board review style question #2
A 55 year old woman with weight gain and fatigue was found to have an elevated serum TSH and was started on levothyroxine (LT4). She was referred for assistance in managing thyroid replacement therapy. Physical examination showed a pulse rate of 80 beats per minute, no goiter or thyroidectomy scar and normal deep tendon reflexes.
Which of the following is the most likely explanation for these findings?
| Date | TSH (0.3 - 4.1 μU/mL) | FT4 (0.8 - 1.8 ng/dL) | Levothyroxine dose (mg/day) |
| June 2019 | 12 | 1.3 | None |
| September 2019 | 11 | 1.7 | 0.075 |
| December 2019 | 10.5 | 2.1 | 0.100 |
Which of the following is the most likely explanation for these findings?
- Free T4 autoantibodies
- Heterophile antibodies
- Poor absorption of levothyroxine
- Resistance to thyroid hormone
- TSH producing pituitary adenoma
Board review style answer #2
B. Heterophile antibodies. Normal free T4 and high TSH on 06/2019 were compatible with subclinical hypothyroidism. Differential diagnosis is NTI recovery phase, TSH resistance, assay interference (heterophile antibodies, macroTSH), poor absorption of levothyroxine. After trial of LT4 replacement TFT, there were high free T4 and high TSH (similar level as the initial TSH) levels. The most likely explanation is that the patient had heterophile antibodies that caused falsely elevated TSH. When the patients takes LT4, free T4 level is elevated but TSH still high at the same level because of heterophile antibodies.
Free T4 autoantibodies is incorrect since free T4 level was normal at initial evaluation. Poor absorption of levothyroxine is incorrect since free T4 level increased after LT4 treatment. Resistance to thyroid hormone is incorrect since free T4 level was normal at presentation. TSH producing pituitary adenoma is incorrect since the patient did not have clinical thyrotoxicosis and free T4 level was normal at initial evaluation.
Comment Here
Reference: Thyroid function panel
Comment Here
Reference: Thyroid function panel
Toxicology-general
Table of Contents
Definition / general | Essential features | Terminology | Drug detection techniques in serum and urine | General principles / pharmacokinetics / pharmacodynamics | Biological samples amenable for testing | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Multidisciplinary field focusing on the diagnosis, management and prevention of chemical agent toxicities in living systems, including
- Acute or chronic poisoning, drug overdoses, employment drug screening, industrial accidents, environmental exposures, envenomations, adverse drug reactions and therapeutic drug monitoring
- Due to a wide range of potential chemical entities faced in practice, several analytical techniques may be required to detect the toxin of interest
Essential features
- Numerous analytical techniques are used to identify the drug / toxin of interest across multiple biological samples
- Immunochemical assays are typically used as initial screening
- Thin layer chromatography, high performance liquid chromatography and gas chromatography / mass spectrometry are used for confirmation
- Wide variety of tissue samples can be tested (urine, serum, saliva, hair)
- Large window of detection period depending on sample type and drug of interest
- Drug half life affects the ability to detect the compound in body fluids; drug metabolites may be tested in certain circumstances
Terminology
- Thin layer chromatography (TLC)
- High performance liquid chromatography (HPLC)
- Gas chromatography / mass spectrometry (GC / MS)
Drug detection techniques in serum and urine
- Immunochemical assays (immunoassays)
- Rapid and relatively inexpensive analysis with minimal sample preparation
- Commonly used as initial screening for drugs of abuse (J Anal Toxicol 2005;29:359)
- Cannot differentiate between drug classes (e.g. opiates); however, some cross reactivity can occur (Drug Test Anal 2014;6:492)
- Positive results should be confirmed with independent, more specific method (e.g. gas chromatography / mass spectrometry, high performance liquid chromatography / mass spectrometry) (Clin Chem 1988;34:471)
- Examples include enzyme mediated immunologic technique (EMIT) and fluorescence polarization immunoassay (FPIA) (Forensic Sci Int 1990;48:27)
- Chromatographic techniques
- Thin layer chromatography
- Qualitative assessment for drugs of abuse and their metabolites (Ann Clin Biochem 1993;30:163)
- Several compounds separated based on their polarity relative to solid stationary phase (silica gel)
- Urine (preferred), serum, gastric contents
- Disadvantage: skilled experience needed to interpret results
- See Diagrams / tables, figure 1
- Gas chromatography / mass spectrometry
- Wide range of drugs can be detected (Ther Drug Monit 2002;24:247)
- Gold standard method for volatile (nonpolar) compounds within several body fluids and hair
- Qualitative and quantitative
- Highly sensitive and specific
- High performance liquid chromatography / mass spectrometry
- Similar features as with gas chromatography / mass spectrometry but with the advantage that it can be used for nonvolatile (polar) compounds such as opiates (J Pain Palliat Care Pharmacother 2013;27:322)
- Less sample preparation and increased turnaround times than gas chromatography / mass spectrometry (Clin Toxicol (Phila) 2012;50:733)
- See Diagrams / tables, figure 2
- Thin layer chromatography
General principles / pharmacokinetics / pharmacodynamics
- Most drugs are excreted in urine (J Nucl Med Technol 2018;46:221)
- Either unaffected or converted to metabolites of the parent drug
- Some drugs enter enterohepatic circulation and are excreted in stool
- Most metabolism occurs in liver (cytochrome P450 system) (Hepat Mon 2016;16:e32636)
- Useful parameters include bioavailability, half life, therapeutic range, toxic levels, transport, metabolism, elimination, steady state and mechanism of action
Drug elimination kinetics
- Zero order (linear): constant quantity of drug is eliminated per unit time (J Nucl Med Technol 2018;46:221)
- Examples: phenytoin, ethanol, warfarin, heparin, salicylates and theophylline
- First order (nonlinear): constant fraction of drug is eliminated per unit time (J Nucl Med Technol 2018;46:221)
- Most (> 95%) drugs used at therapeutic levels are eliminated this way
- See Diagrams / tables, figure 3
Drug half life
- Time taken for half of the drug that was initially present in serum to be excreted
- Many drugs have a half life independent of their concentrations because they are eliminated via first order kinetics
Steady state
- Represents overall absorption of drug that is in dynamic equilibrium with its elimination (J Nucl Med Technol 2018;46:81)
- Reached after about 4 - 5 half lives with continuous drug administration
Bioavailability
- Fraction of unchanged drug reaching systemic circulation (J Nucl Med Technol 2018;46:221)
- Fraction of drug binds albumin in blood; remaining drug is free
- Some small molecules compete for albumin binding, affecting total drug concentration
- Affected by physical drug properties, drug formulations, route of administration, fed versus fasted state, drug-drug interactions, plasma protein binding, disease state of individual, P450 enzyme induction / inhibition and metabolic differences
Volume of distribution (Vd)
- Total amount of drug in body/drug concentration in plasma (J Nucl Med Technol 2018;46:221)
- A theoretical volume; uniform distribution of drug between plasma and rest of body
- Lipophilic drugs have high volume of distribution (molecules sequestered in adipose tissue); hydrophilic drugs have low volume of distribution
Lethal dose (LD50)
- Amount of an ingested substance that kills 50% of test subjects; expressed in mg/kg (Regul Toxicol Pharmacol 2016;80:274)
General considerations
- Anion gap, osmolal gap and oxygen saturation gap should be determined when an overdose is suspected
- Anion gap (mmol/L) = [Na+] - [Cl- + HCO3-] (J Emerg Nurs 2005;31:109)
- Assessment for metabolic acidosis
- Normal value: 8 - 16 mmol/L; metabolic acidosis likely with values > 30 mmol/L
- Increased anion gap in methanol, acetaminophen, carbon monoxide, epinephrine, nitroprusside, formaldehyde, ethylene glycol, salicylates and propylene glycol (Clin Toxicol (Phila) 2015;53:589)
- Lowered anion gap in hypoalbuminemia
- Assessment for metabolic acidosis
- Osmolal gap (mOsm/kg) = (2 x Na) + (BUN/2.8) + (glucose/18) (Postgrad Med 2017;129:456)
- Difference between serum osmolarity and calculated osmolality
- Increased in acute alcohol poisoning (ethanol, methanol, etc.)
- Oxygen saturation gap (J Anaesthesiol Clin Pharmacol 2014;30:86)
- Difference between oxygen saturation given by pulse oximetry and arterial blood gas
- > 5% suggests hemoglobin-oxygen abnormality
- Increased in agents affecting hemoglobin binding
- Carbon monoxide, cyanide, hydrogen sulfide, methemoglobin
Biological samples amenable for testing
- Urine: used for initial screening (Arch Intern Med 1988;148:2407)
- Advantages: relatively stable, longer window of detection and high concentration of drug / metabolites
- Disadvantages: sample subject to adulteration
- Check for tampering: specimen color, odor, temperature, specific gravity, creatinine and nitrite
- Serum: collected in red top tube (no additives)
- Detection windows shorter than urine
- Saliva: easy to perform, rugged, amenable to point of collection testing; window period comparable to serum (Forensic Sci Int 2005;150:165, Clin Chem 2009;55:1910)
- Sweat: convenient, less invasive measure than blood or urine; wearable patch applied to patient (J Chromatogr B Biomed Sci Appl 1999;733:247)
- Hair: longest detection window period (up to 90 days); immunoassay and gas chromatography / mass spectrometry (Mass Spectrom Rev 2013;32:312)
- Initial screening: immunoassays or thin layer chromatography
- Confirmation (independent method): gas chromatography / mass spectrometry or high performance liquid chromatography / mass spectrometry
- Window of detection depends on drug and specimen type and drug of interest (blood, urine, hair) (Acta Clin Belg 2000;55:323, see Diagrams / tables, figure 4)
Diagrams / tables
Additional references
- Rifai: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th Edition, 2018, McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2016, Kumar: Robbins & Cotran Pathologic Basis of Disease, 9th Edition, 2014, Mais: Quick Compendium of Clinical Pathology, 4th Edition, 2018
Board review style question #1
A 32 year old man was found dead at home. The medical examiner was called to the decedent's home to investigate the cause of death. At the decedent's home, there were multiple empty prescription pill bottles scattered around and an in situ needle was found within the vein of the man's antecubital fossa. Foaming around the nose and mouth was also noted. Which of the following techniques should be used to definitively identify all the potential opiates the deceased may have ingested?
- Enzyme mediated immunologic technique
- Fluorescence polarization immunoassay
- Gas chromatography / mass spectrometry
- High pressure liquid chromatography / mass spectrometry
- Ion absorption spectroscopy
Board review style answer #1
D. High pressure liquid chromatography should be used to definitely characterize all the opiates ingested by the decedent. Enzyme mediated immunologic technique and fluorescence polarization immunoassay are immunochemical assays used as screening tests but are not definitive. Gas chromatography / mass spectrometry is possible but sample preparation (i.e. derivatization of the compounds) would add additional steps and make the sample preparation more labor intensive and time consuming. Gas chromatography / mass spectrometry is used for mostly nonpolar analytes of interest.
Comment Here
Reference: Toxicology-general
Comment Here
Reference: Toxicology-general
Board review style question #2
Which of the following drugs is eliminated from the body following a nonlinear (exponential) kinetic elimination process?
- Aspirin
- Ethanol
- Heparin
- Phenytoin
- Propranolol
- Warfarin
Board review style answer #2
E. Propranolol (and > 95% of available drugs) follow first order elimination kinetics (i.e. constant fraction per unit time). The remaining < 5% of drugs follow zero order elimination kinetics (i.e. constant amount per unit time).
Comment Here
Reference: Toxicology-general
Comment Here
Reference: Toxicology-general
Toxicology-general-part 2 (pending)
[Pending]
Transferrin
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Pathophysiology | Test indications | Laboratory | Interpretation | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Glycoprotein responsible for storage and transport of iron in serum
Essential features
- Delivers iron to marrow for heme synthesis and transfers iron to all tissues for absorption, utilization and storage
- Used to calculate total iron binding capacity (TIBC) and iron saturation; in patients with iron deficiency, the degree of transferrin saturation is an extremely sensitive indicator of functional iron depletion
- Scavenger of free toxic iron; in screening for hereditary hemochromatosis, transferrin saturation provides a better indication of the homozygous genotype than does ferritin
- Negative acute phase reactant (Int J Infect Dis 2022;116:74)
- Can sequester iron to prevent acquisition by pathogenic bacteria (Coord Chem Rev 2021;449:214186)
Terminology
- Iron binding component
- Serotransferrin
- Siderophilin
- β1 metal binding globulin
- β2 transferrin: specific to cerebrospinal fluid and perilymph fluid of the inner ear; useful for diagnosing cerebrospinal fluid (CSF) leak (Int Forum Allergy Rhinol 2016;6:8)
ICD coding
- Not applicable unless the diagnosis relates to iron deficiency / disorders
Pathophysiology
- Transferrin (Tf) is a 687 amino acid glycoprotein, which is the major β globulin in serum; it is synthesized in the liver in response to acute decreases in the concentration of circulating iron
- Unbound form of the molecule apotransferrin has 2 binding sites for free iron (Fe3+), which binds either monoferrically or diferrically (when iron is abundant)
- Receptor for transferrin (TfR) is found on most cells and endocytoses in the transferrin / iron complex (Medicines (Basel) 2019;6:85)
- Transferrin binds and transports ferric iron stored by ferritin from intracellular and mucosal sources for delivery to bone marrow for heme synthesis and transfers iron to all tissues for absorption, utilization and storage
- Proportional to TIBC
- In iron deficient anemia, transferrin is expected to be elevated
- Liver disease, inflammatory states, thalassemia and conditions that cause iron overload usually result in a decrease in transferrin
- Physiologically normal decrease in iron concentration with a concurrent elevation of transferrin should be expected during pregnancy
- Chronic saturation of transferrin occurs in hemochromatosis (hereditary and idiopathic) and transfusional hemosiderosis; chronic saturation of transferrin results in reduced iron binding capacity, hence iron is not excreted normally and deposits in tissue (Trends Pharmacol Sci 2021;42:640)
- Transferrin also plays a role in regulation of other metals in human serum, such as Ti(IV), Co(III), Ga(III), Cr(III) and Zn(II) (Coord Chem Rev 2021;449:214186)
Test indications
- As a transport protein of iron in the blood, transferrin is a measure of iron binding capacity
- Useful in the diagnosis of disorders of iron metabolism as a component of an iron panel, including iron saturation, serum ferritin and TIBC
Laboratory
- Assays
- Nephelometry
- Turbidimetry
- Preanalytical factors
- Multiple situations may alter the transferrin level and total iron binding capacity
- Confounding factors, such as medications (chloramphenicol reduces transferrin receptor expression), iron supplements and iron fortified multivitamins, recent transfusion or recent ingestion of a high iron meal (J Cell Physiol 1999;180:334)
- Iron contamination due to oral ingestion or other administration should be avoided
- Transferrin is usually ordered as part of an iron study; therefore, the specimen requirements are similar
- Fasting serum in the morning is preferred, utilizing a serum or heparinized plasma tube (red or green top)
- Serum separator tube, such as gold topped tube, containing clot activator and gel
- Containers with EDTA are not acceptable as EDTA binds metal ions with high affinity; similarly, oxalate and citrate have lower pH and will bind ferric iron
- Typical reference range: 2.0 - 3.6 g/L
Interpretation
- Causes of high transferrin
- Iron deficiency
- Causes of low transferrin
- Liver diseases
- Anemia of chronic disease
- Kidney insult or injury
- Infection and acute phase reaction
- Malignancy
- Atransferrinemia due to transferrin gene mutation
- Iron overload such as hemochromatosis (Trends Pharmacol Sci 2021;42:640)
Additional references
Board review style question #1
A 17 year old unrestrained male passenger of a motor vehicle accident arrived at the emergency department with multiple fractures of the bilateral upper and lower extremities, hematomas and contusions. The patient exhibited signs of confusion and lethargy. The presence of nonpurulent serous drainage from the nose was concerning as it could indicate a cerebrospinal fluid leak from a suspected cranial trauma. What is the most specific test to determine the source of the rhinorrhea?
- β2 transferrin
- β trace protein
- Glucose
- Radionuclide cisternography
- Ring sign
Board review style answer #1
A. β2 transferrin. β2 transferrin is a transferrin variant found in cerebrospinal fluid (CSF), perilymph of the ear and vitreous humor of the eye. It is a highly specific marker for CSF. Answer D is incorrect because radionucleotide cisternography is very specific for identifying a CSF leak; however, it cannot distinguish between a rhinologic versus an otologic source. Answer B is incorrect because β trace protein, although shown in some studies to rival β2 transferrin in specificity and sensitivity, is also present in blood. In certain patient groups, β trace protein is decreased and should not be used. Answer E is incorrect because ring sign is a visible halo sometimes found at the bedside of patients with bloodstains mixed with CSF. It has poor specificity for CSF detection. Answer C is incorrect because although the use of glucose testing strips on rhinorrhea specimens was once thought to be a positive indication of the presence of CSF, studies have shown that glucose can be detected in otherwise healthy patients. The detection of glucose in rhinorrhea is not considered sensitive or specific for CSF (Int Forum Allergy Rhinol 2016;6:8).
Comment Here
Reference: Transferrin
Comment Here
Reference: Transferrin
Board review style question #2
An 18 year old woman with past medical history of sickle cell trait, iron deficiency and neutropenia presents to the office for follow up. She states that she has been having right lower quadrant pain for the past week. The pain can become so severe that she rates it at 7/10. It causes nausea and abdominal fullness. She denies fever, colicky pain or flank pain. The patient serum transferrin concentration is 390 mg/dL (normal: 200 mg/dL to 360 mg/dL). Which of the following is most likely observed in this patient?
- Ferritin concentration of 10 ng/mL
- Ferritin concentration of 200 ng/mL
- Iron concentration of 100 μg/dL
- Iron saturation 80%
- Total iron binding capacity of 200 μg/dL
Board review style answer #2
A. Ferritin concentration of 10 ng/mL. Understanding the normal ranges for transferrin, iron saturation, iron concentration and TIBC will demonstrate that normal ferritin concentration in women is 24 to 307 ng/mL. The patient has a history of anemia and presents with an elevated transferrin; therefore, the ferritin concentration should be decreased. Answers B, C, D and E are incorrect because these responses are consistent with normal iron levels.
Comment Here
Reference: Transferrin
Comment Here
Reference: Transferrin
Board review style question #3
Hypertransferrinemia can be seen in patients with
- Acute myocardial infarction
- Cirrhosis
- Hypoalbuminemia
- Iron deficiency
- Nephrotic syndrome
Board review style answer #3
D. Iron deficiency. Iron deficiency is the only option that is compatible with an elevated transferrin level. Answer C is incorrect because hypotransferrinemia usually accompanies hypoalbuminemia and is also an indication of protein malnutrition. Answer E is incorrect because nephrotic syndrome causes loss of proteins such as transferrin. Answer B is incorrect because cirrhosis results in decreased production of all proteins synthesized in the liver. Answer A is incorrect because hypotransferrinemia is associated with acute myocardial infarction rather than normal or excess transferrin. Transferrin is also a negative acute phase reactant and would be decreased in the hours and days following an acute event such as an infarction.
Comment Here
Reference: Transferrin
Comment Here
Reference: Transferrin
Tumor markers (pending)
[Pending]
Urinalysis (pending)
[Pending]
Urinalysis selection (pending)
[Pending]
Urine crystals & microscopy
Table of Contents
Definition / general | Essential features | Diagrams / tables | Indications for testing | Laboratory | Types of elements in urine | Automated urine analyzers | Urine reagent strips | Microscopic (histologic) images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Standard component of complete urinalysis
- One of the oldest laboratory tests in medicine is to look for abnormal microscopic constituents in urine (JAMA Netw Open 2020;3:e2013959)
Essential features
- Urine analysis is the third major diagnostic screening test in the clinical laboratory, only preceded by serum / plasma chemistry profiles and complete blood count analysis
- As sample collection is done by the patient, it is more likely to be susceptible to preanalytical issues
- Routine urine microscopic examination includes looking for cellular, formed elements and organisms
Diagrams / tables
Indications for testing
- Helps diagnose:
- Kidney disease
- Urinary tract infection
- Cancers of urinary tract
- Reactions to medicines
- Prostate infection
- Liver disease
- Yeast, viral, parasitic infections
Laboratory
- Specimen for testing:
- First void urine preferred in routine practice
- Midstream urine is the most appropriate sample, since contaminating elements, such as bacteria, analytes and formed particles, are minimized
- Collection instructions:
- Clean catch urine or midstream portions of first morning urine
- Samples should be collected in a wide mouthed, sterile container made of nonabsorbing materials, with no influence on any analyte
- Due to influence on the viability of bacteria, the use of soap or antiseptics while washing the genitalia is not recommended (Ann Biol Clin (Paris) 2018;76:609)
- Catheter urine sample for bacteriological study or culture in bed ridden patients or patients with obstruction of urinary tract
- For infants, a clean plastic bag can be attached around genitalia and left in place for some time for sample to be collected
- Volume required:
- 12 mL is the recommended urine volume for adult patients
- Range of 5 - 12 mL of urine is acceptable to examine the formed elements in urine
- Test protocol and acceptance of smaller urine specimens is determined by the individual laboratory
- Procedure:
- Centrifuge urine at 1,500 rpm for 10 minutes
- Resuspend sediment in 1 drop of urine an observe under microscope
- Examine first using low power and then high power
- Lower the condenser and minimize the light to view elements
- Possible interferences:
- Dietary intake, exercise
- Medications
- Increased time lag between sampling and analysis
- For urinalysis that cannot be performed within 2 hours of collection
- Lack of temperature control and lack of addition of a preservative to samples will lower the quality of urinary test results
- If testing by strip method, test can be performed within 24 hours if urine is refrigerated without preservatives (Biochem Med (Zagreb) 2014;24:89)
- For urinalysis that cannot be performed within 2 hours of collection
Types of elements in urine
- Urinary crystals:
- Crystals are formed in urine when it is supersaturated with certain minerals, which may be due to
- Increased excretion of these constituents
- Reduced urine volume, an alteration in urine pH
- A combination of the above factors
- Crystals can be seen in both healthy and disease states
- Crystals are formed in urine when it is supersaturated with certain minerals, which may be due to
| Normal crystals | |||
| Crystals | Ph of urine | Identification | Remarks |
| Uric acid | Acidic |
| Urate nephrolithiasis or acute urate nephropathy, gout and leukemia |
| Calcium oxalate | Acidic or alkaline |
| Dietary oxalate ingestion (spinach, tomato, cabbage and asparagus) and nephrolithiasis |
| Calcium phosphate | Alkaline |
| In rare cases, could be caused by hypoparathyroidism |
| Triple phosphate | Alkaline |
| In patients with struvite stones and in urinary tract infection with Proteus |
| Calcium carbonate | Alkaline |
| Normal |
| Ammonium biurate | Alkaline |
| Old or longstanding urine sample |
| Amorphous | Phosphates - seen in alkaline urine and soluble in acetic acid |
| Can be confused with degenerating cells |
| Abnormal crystals | |||
| Crystals | Ph of urine | Identification | Remarks |
| Bilirubin | Acidic |
| Associated with increased conjugated bilirubin, severe liver disorders of obstructive cause |
| Cholesterol | Acidic |
| Nephrotic syndrome |
| Cysteine | Acidic |
| Genetic disease - cysteinuria (Nephrol Ther 2015;11:174) |
| Leucine | Acidic / neutral |
| Liver disorders with impaired amino acid metabolism |
| Tyrosine | Acidic / neutral |
| Liver disorders with impaired amino acid metabolism |
| Sulfonamide | Acidic / neutral |
| Drug related |
- Urinary casts:
- Casts are cylindrical, cigar shaped microscopic structures composed mainly of mucoprotein (Tamm-Horsfall mucoprotein, also known as uromodulin) that is secreted by epithelial cells lining the loops of Henle, the distal tubules and the collecting ducts
- As they are formed in the distal and collecting tubules, their presence in urine suggests renal parenchymal pathology
- Named depending on the predominant constituent
- Reported as number seen per low power field (10x objective)
| Noncellular casts | ||
| Type of cast | Appearance | Remarks |
| Hyaline |
| Seen normally (not associated with disease states); seen after strenuous exercise and with nonrenal diseases, such as dehydration (EJIFCC 2021;32:410) |
| Granular |
| Seen in acute glomerulonephritis and pyelonephritis; appears muddy brown in acute tubular necrosis |
| Waxy |
| Final stage of cast degeneration: indicates tubular injury of a more chronic nature than granular or cellular casts and is likely associated with low urine flow; always of pathologic significance (associated with end stage renal failure) |
| Fatty |
| Seen in nephrotic syndrome; may represent tubular degeneration |
| Cellular casts | ||||||||
| Type of cast | Appearance | Remarks | ||||||
| White blood cell (WBC) |
| |||||||
- Cells:
- Reported as the number of each type found per average high power field (HPF)
| Cell type | Description | Remarks | |||||||||
| Renal tubular epithelial |
| Tubular damage (like acute tubular necrosis) pyelonephritis, viral infection of kidney, allograft rejection and salicylate or heavy metal poisoning (Clin J Am Soc Nephrol 2010;5:402) | |||||||||
| Transitional epithelial |
| Seen after catheterization and in transitional cell carcinoma | |||||||||
| Squamous epithelial |
|
- Organisms:
| Organism | Description | Remarks | |||
| Bacteria |
| In a wet preparation, presence of bacteria should be reported only when urine is fresh; presence of only bacteria without pus cells indicates contamination with vaginal or skin flora | |||
| Yeast cells |
| Seen in immunocompromised state, vaginal candidiasis or diabetes mellitus | |||
| Trichomonas vaginalis |
| Causes vaginitis in females and are thus contaminants in urine
Note: wet preparation of genital secretions or wet mount is the diagnostic method of choice (Sci Rep 2019;9:11074) | |||
| Schistosoma haematobium |
|
- Miscellaneous:
| Element | Description | Remarks |
| Sperm |
| Significant in infertility cases and sexual abuse
May be seen in males after a nocturnal emission or ejaculation Presence in the urine is of significance in the case of retrograde ejaculation |
Automated urine analyzers
- Automated urine analyzers enhance productivity and efficiency in the laboratories by increasing the reproducibility and throughput and reducing potential human errors
- Reduces the time and labor required to process urine samples
- Automated analyzers help standardize analysis so that laboratories can accurately recognize pathological elements in urine (Clin Chem Lab Med 2015;53:s1509)
- One study comparing 3 automated urine analyzers (Roche's Cobas 6500, Sysmex's UN3000-111b and Iris's iRICELL 3000) showed fairly good correlation among the 3 platforms and with manual microscopy (Pract Lab Med 2021;24:e00203)
- However, microscopic confirmation by experienced technologists is still necessary to avoid errors and definitively classify urine sediments, especially in pathologic specimen samples and images (particularly dysmorphic cells, bacteria, yeasts, casts and crystals)
Urine reagent strips
- Urine strips are firm, plastic strips onto which several separate reagent areas are affixed
- Analytes detected can be ascorbic acid, glucose, bilirubin, ketone (acetoacetic acid), specific gravity, blood, pH, protein, urobilinogen, nitrite and leukocytes
- Reagent strips are packaged along with a drying agent in a plastic bottle with a twist off cap
- Each strip is stable and ready to use upon removal from the bottle
- Entire reagent strip is disposable
- Results are obtained by direct comparison of the test strip with the color blocks printed on the bottle label
- Procedure:
- Completely immerse reagent areas of the strip in fresh, well mixed urine
- While removing, touch the side of the strip against the rim of the urine container to remove excess urine
- Blot the lengthwise edge of the strip on an absorbent paper towel to further remove excess urine and avoid running over
- Compare each reagent area to its corresponding color blocks on the color chart and read at the times specified
- Proper read time is critical for optimal results
- Interpretation of microscopic findings can be correlated with strip findings for better interpretation
Microscopic (histologic) images
Board review style question #1
A urine sample from a 38 year old man with alcohol induced cirrhosis, ascites and jaundice is shown above. The appearance of the crystal is golden brown, with specific gravity = 1.015 and pH = 6.0. Identify the sediment finding (arrow)
- Cholesterol crystals
- Cysteine crystals
- Leucine crystals
- Tyrosine crystals
Board review style answer #1
C. Leucine crystals are seen in acidic urine. They appear as concentric circles with radial striations and are yellow-brown in color. They resemble a cross section of a tree trunk and are associated with liver disorder.
Comment Here
Reference: Urine crystals & microscopy
Comment Here
Reference: Urine crystals & microscopy
Board review style question #2
Which of the following types of urinary cast has serrated edges and is an indicator of end stage renal disease?
- Fatty cast
- Granular cast
- Waxy cast
- White blood cell (WBC) cast
Board review style answer #2
C. Waxy cast is seen in the setting of prolonged low urine flow.
Comment Here
Reference: Urine crystals & microscopy
Comment Here
Reference: Urine crystals & microscopy
Utilization management strategies
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Diagrams / tables | Overview | Plan - establishing target(s) | Do - intervention(s) and planning | Study - monitoring metrics | Act - common pitfalls | Laboratory formularies | Resources | Videos | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Clinical laboratory has an essential role to maintain, audit and revise laboratory test ordering practices to ensure appropriate utilization
- Laboratory testing stewardship is a common target for utilization initiatives and can be achieved through various approaches
Essential features
- Laboratory utilization initiatives commonly target tests that are ordered inappropriately or offer minimal value to patient management
- It is essential to apply quality / process improvement framework(s) to utilization initiatives for implementation and monitoring of success or failure
- Approaches to overutilization issues can vary from hard interventions, such as removing tests from the ordering system to soft interventions such as dissemination of educational materials
Terminology
- Laboratory utilization: a framework of strategies to ensure appropriate use of laboratory testing to provide quality and cost effective patient care
- Quality improvement: a process of improving the quality and safety of healthcare delivery using a systematic approach that is guided by data
- Plan Do Study Act: an iterative problem solving model used for improving a process or implementing a change
- Quality improvement intervention: an effort to change / improve the healthcare structure, process or outcomes of care by means of a systems level change
- Outcome measures: indicator of the success of the quality improvement initiative in relation to the goal(s)
- Process measures: indicator of compliance with actions implemented to achieve the goal(s) of a quality improvement initiative
- Balancing measures: indicator of consequences, whether intended or not, of implementing a quality improvement initiative
ICD coding
No information provided
Diagrams / tables
None
Overview
- Laboratory testing stewardship is not a novel concept and is inherently addressed within typical quality management systems / processes
- Examples include repatriating referred out testing or assessing financial feasibility of low volume testing
- Advent of Choosing Wisely has made laboratory tests a priority area for utilization initiatives
- Using a quality / process improvement framework in designing, implementing and monitoring a utilization initiative is recommended
- Iterative Plan, Do, Study, Act (PDSA) cycles is an example of an appropriate framework
Plan - establishing target(s)
- First phase involves generating ideas for targets or auditing data to identify opportunities (Health Quality Ontario: Measurement for Quality Improvement [Accessed 29 February 2024])
- Internal approaches include
- Reviewing test menu / formulary
- Reviewing laboratory ordering mechanisms (paper requisitions, computerized order entry builds)
- Reviewing laboratory data (inappropriately co-ordered tests, excessive repeat testing)
- Reviewing referred out testing
- External approaches include (see Resources below)
- Reviewing medical specialty guidelines that dictate appropriate test utilization
- Reviewing repeat testing guidelines
- Reviewing Choosing Wisely guidelines that focus on medical resource stewardship
- Other considerations or approaches
- Benchmarking utilization performance against other laboratories / institutions if data is available
- Cost effectiveness of test(s)
- Diagnostic yield of test(s)
Do - intervention(s) and planning
- Design of suitable intervention(s) occurs once utilization target(s) have been chosen
- Interventions can vary in their effectiveness to produce and sustain test utilization targets (Biochem Med (Zagreb) 2014;24:223)
- Examples of strong or hard interventions include
- Blocking repeating test orders
- Removing test(s) from menu / formulary
- Requiring approval or explicit privileges for specific test orders
- Implementing reflexive testing algorithms
- Examples of weaker or soft interventions include
- Providing audit and feedback reports to clinicians
- Implementing automatic reminders (best practice advisory popups) within computerized test order system
- Disseminating educational material
- Examples of strong or hard interventions include
- All types of interventions have inherent weaknesses and it is best to combine different intervention modalities for utilization initiatives
- Other factors that must be considered in planning to implement a utilization based intervention
- Stakeholder management: have all the necessary and appropriate stakeholders been consulted and provided feedback?
- Feasibility: is it feasible to successfully implement the intervention with the provided resources?
- Potential impact: have all possible positive and negative impacts from this intervention been discussed / accounted for?
- Sustainability: have all possible barriers to sustainability of the initiative been discussed / accounted for?
- Scenario validation: depending on the proposed intervention(s), does there need to be simulation and validation of anticipated scenarios?
Study - monitoring metrics
- Common quality improvement frameworks recommend measuring multiple metrics (Health Quality Ontario: Measurement for Quality Improvement [Accessed 29 February 2024])
- Outcome measures represent measurable data related to the target(s) of the utilization initiative
- E.g., to decrease the number of free thyroxine orders by 20% within 1 year of the intervention implementation date
- Process measures represent measurable data related to the processes required to achieve the utilization outcome measure
- E.g., to audit and review 100% of the existing computerized order sets that contain free thyroxine
- Balancing measures represent measurable data related to unintended consequences borne from the utilization initiative
- E.g., to monitor the number of patient safety reports related to the inability to explicitly order a free thyroxine test for 1 year after implementation of the intervention
- Outcome measures represent measurable data related to the target(s) of the utilization initiative
- Common outcome measures for laboratory test utilization initiatives (Clin Chim Acta 2014:427:127)
- Number of tests per patient
- Number of inpatient tests per discharge
- Number of inpatient tests per hospital day
- Number of tests per requisition
- Benchmarking data, especially for outcome measures, is important to assess and monitor success of utilization initiatives after implementation of intervention(s)
- External benchmarking services are available but peer group may not be ideal or appropriate and could require additional financial resources
- Internal benchmarking is a more common approach but data extraction / analysis can be challenging to handle internally and lack of peer group data may result in misleading interpretation of own performance
Act - common pitfalls
- After each PDSA cycle, there should be refinement of the initiative based on lessons learned from the current cycle (i.e., metrics / measures data) (Health Quality Ontario: Measurement for Quality Improvement [Accessed 29 February 2024])
- Common pitfalls that hinder successful utilization initiatives include
- Clinicians finding alternative means to ordering tests that were overlooked during planning phase
- Excluding medical trainees from the initial stakeholder discussions
- Clinician fatigue from automated reminders or ongoing review of educational materials
- Overestimation of financial savings as most routine clinical laboratory tests are relatively inexpensive
Laboratory formularies
Resources
- Choosing Wisely initiative has now expanded to over a dozen countries, each with recommendations from professional medical societies
- Additional countries include Canada, Australia, United Kingdom and Japan
- American Society of Clinical Pathology has published Choosing Wisely recommendations focused exclusively on laboratory testing (ASCP: Thirty Five Things Physicians and Patients Should Question [Accessed 5 January 2024])
- Royal College of Pathologists publishes an extensive guideline on minimum retesting intervals in laboratory medicine (RCPath: National Minimum Retesting Intervals in Laboratory Medicine [Accessed 5 January 2024])
- Clinical Laboratory Sciences Institute has a published guideline (CLSI: CLSI GP48 - Developing and Managing a Medical Laboratory (Test) Utilization Management Program [Accessed 5 January 2024])
Videos
None
Additional references
None
Board review style question #1
Which of the following statements is the most correct concerning common laboratory test utilization targets?
- Erythrocyte sedimentation rate (ESR) should be prioritized above C reactive protein (CRP) as an inflammatory marker for patients with undiagnosed conditions
- H. pylori serology testing represents a viable alternative to stool antigen or breath tests when determining presence / absence of H. pylori infection
- Lipase should be prioritized above amylase in cases of suspected acute pancreatitis
- Red blood cell folate still represents an essential blood test as it is a common cause of macrocytic anemia
- Uncoupling routine liver enzyme testing often results in significant financial savings
Board review style answer #1
C. Lipase should be prioritized above amylase in cases of suspected acute pancreatitis. There is much evidence demonstrating that lipase is a more sensitive and specific marker of acute pancreatitis than amylase.
Answer E is incorrect because liver enzymes are commonly ordered routine biochemistry tests that are offered on large scale automated platforms. Decreasing utilization of tests run on such platforms often does not result in significant financial savings.
Answer D is incorrect because red blood cell folate represents a test with minimal clinical value; treatment with folate supplementation is a more cost effective approach than testing for folate levels when there is suspected deficiency.
Answer A is incorrect because ESR is a nonspecific inflammation marker that is influenced by various factors, whereas CRP is sensitive and specific to the acute phase of inflammation.
Answer B is incorrect because serology testing has minimal value given the advent of alternative noninvasive testing methods with higher sensitivity and specificity (such as breath testing or stool antigen testing).
Comment Here
Reference: Utilization management strategies
Comment Here
Reference: Utilization management strategies
Utilization management strategies (pending)
[Pending]
Validating a new quantitative assay
Table of Contents
Definition / general | Essential features | Terminology | Overview | Precision | Bias and accuracy | Linearity | Reference interval | Other studies | Analytical validation tools | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Proper analytical validation of a new assay is essential prior to patient testing
- Using Clinical Laboratory Standards Institute (CLSI) guidelines as a framework for designing new assay validations is highly recommended
Essential features
- Minimum requirements for analytical validation of a quantitative assay include studies for precision, bias, accuracy, linearity and reference interval
- Required validation studies each have an associated CLSI standard which outlines how to design, carry out and evaluate the specific analytical aspect (CLSI: Method Evaluation Standards [Accessed 1 December 2022])
- Statistical software, such as EP Evaluator and Analyse-it, are useful for analyzing the validation data
Terminology
- Total allowable error (TAE): the amount of error that is allowable without invalidating the interpretation of a test result
- Precision: closeness of agreement between values obtained by replicate measurements
- Bias: estimate of a systematic measurement error
- Linearity: ability to provide results that are directly proportional to the concentration / amount of analyte in the test sample within a given range
Overview
- Precision, bias, accuracy, linearity and reference interval studies are typically the minimum requirements
- Establish performance limits for validation studies a priori using appropriate total allowable error goals
- Laboratory medicine associations have defined total allowable error goals for clinical assays: EFLM Biological Variation, CLIA, CAP, RCPA, IQMH
- Factors to consider for each validation study:
- Sample: what type of samples to use?
- Target values: what concentrations are relevant?
- N (number): how many samples / replicates are needed?
- Limits: what are the performance limits?
Precision
- Precision studies establish variance within replicate measurements and the likelihood / magnitude of random errors
- Refer to CLSI EP05-A3 for designing precision studies:
- Sample: quality control (QC) material, optimally from a third party vendor
- Target values: at least 2 levels of QC near medical decision limit(s)
- N: 20 x 2 x 2 format consisting of 2 runs of duplicate measurements daily for 20 days for each level of QC
- Limits: total observed variance should not typically exceed 33% of the total allowable error
- For the 20 x 2 x 2 format, CLSI recommends calculating the coefficient of variation (%CV) via 2 way nested analysis of variance (ANOVA) (see Analytical validation tools below)
- Reference: CLSI: EP05-A3 - Evaluation of Precision of Quantitative Measurement Procedures [Accessed 1 December 2022]
Bias and accuracy
- Bias studies establish agreement to a predicate assay and the magnitude of systematic error
- Refer to CLSI EP09-A3 for designing bias studies:
- Sample: patient samples with known values as reference standard for comparison
- Target values: preferably an even distribution across the analytical measuring range (AMR)
- N: minimum of 40 patient sample singleton measurements, preferably tested over several days
- Limits: mean bias (absolute or relative) should not typically exceed half of the total allowable error
- Additional analyses to perform for bias studies:
- Regression analyses are typically performed to assess absolute or proportional bias in the form of a regression line
- Passing-Bablock or Deming are the recommended regression methods (see Analytical validation tools below)
- Differences (absolute or relative) are typically plotted on Bland-Altman plots to assess bias trends across the analytical measuring range
- Consider inclusion of higher metrological order samples such as proficiency testing materials or certified reference material to establish bias against a reference method
- Regression analyses are typically performed to assess absolute or proportional bias in the form of a regression line
- Reference: CLSI: EP09-A3 - Measurement Procedure Comparison and Bias Estimation Using Patient Samples [Accessed 1 December 2022]
Linearity
- Linearity studies verify vendor quoted analytical measuring range and reportable limits to be implemented with the assay
- Refer to CLSI EP06 for designing linearity studies:
- Sample: patient derived sample pools preferable (see below for preparation steps)
- Target values: preferably equally spaced concentrations across the analytical measuring range bounded by sample pools at or near the low and high limits
- N: minimum of 5 sample pools, each tested in duplicate
- Limits: various schema are used to evaluate linearity, though CLSI recommends bias limits be used for linearity
- Linearity samples are ideally matrix matched, patient derived sample pools
- Typical preparation involves serial dilutions of a high sample pool with a low sample pool
- If patient derived sample pools are not attainable, consider alternatives:
- Vendor provided material (linearity, calibrator, quality control, etc.)
- High sample pool diluted with vendor diluent
- Reference: CLSI: EP06 - Evaluation of Linearity of Quantitative Measurement Procedures [Accessed 1 December 2022]
Reference interval
- Reference interval (RI) studies verify vendor quoted reference intervals, which represent the normal range for the assay
- Refer to CLSI EP28 for designing reference interval studies:
- Sample: samples from apparently healthy volunteers
- Target values: not applicable
- N: minimum of 20 samples per appropriate stratification (i.e., an assay with reference intervals for males and females would require 40 samples)
- Limits: ≥ 95% of samples fall within vendor quoted reference interval(s)
- Reference: CLSI: EP28 - Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory [Accessed 1 December 2022]
Other studies
- Additional studies (stability, interferences, carryover, limit of quantification / detection, dilutional recovery) should be considered if appropriate for the assay in question
- Stability studies to establish the viability of samples for testing across time and storage conditions (i.e., temperature) after collection, as well as any specific sample processing or transportation needs for labile analytes
- Consider adopting CLSI based continuous study design (Clin Chem Lab Med 2020;58:188)
- Interference studies to evaluate interfering substances that can cause a clinically significant difference in assay results; assays on automated analyzers are typically evaluated for interference effects from hemolysis, icterus and lipemia (CLSI: EP07 - Interference Testing in Clinical Chemistry [Accessed 1 December 2022])
- Carryover studies to ensure that instrumentation prevents samples being analyzed from contaminating subsequent samples; especially relevant for assays with a wide expected range of values (i.e., beta hCG, serum free light chains) (CLSI: EP10A3AMD - Preliminary Evaluation of Quantitative Clinical Laboratory Measurement Procedures [Accessed 1 December 2022])
- Limit of quantification / detection (analytical sensitivity) studies to establish assay performance at the lower limit of the analytical measuring range; especially relevant for assays with laboratory or clinical requirements near the lower limit of measurement (i.e., thyroglobulin, high sensitivity cardiac troponins) (CLSI: EP17 - Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures [Accessed 1 December 2022])
- Dilutional recovery studies to establish appropriate dilution protocol (i.e., dilution factor, diluent) for high concentration samples beyond the upper limit of the analytical measuring range for undiluted sample analysis (CLSI: EP34 - Establishing and Verifying an Extended Measuring Interval Through Specimen Dilution and Spiking [Accessed 1 December 2022])
Analytical validation tools
- Statistical programs / software are useful tools to aid the analyses and calculations required for analytical validation studies based on CLSI standards
- EP Evaluator (Data Innovations LLC) is a standalone software
- Analyse-it (Analyse-it Software, Ltd.) is an Excel add on software
Board review style question #1
Using the provided dataset for precision studies for a hemoglobin A1c assay, which of the following statements is true?
- A positive shift in both levels is observed on day 14
- Level 1 quality control shows more consistency and stable performance compared to level 2
- Quality control material is inappropriate for precision studies
- The total allowable error of 6% is too stringent and should be widened
Board review style answer #1
A. A positive shift in both levels is observed on day 14. Overall, precision studies show adequate performance for both levels of quality control material in the 20 x 2 x 2 format and are acceptable. Level 2 quality control shows a smaller CV and more consistency than level 1. A total allowable error of 6% is too wide as a precision goal for hemoglobin a1c, which has relatively small absolute values.
Comment Here
Reference: Validating a new quantitative assay
Comment Here
Reference: Validating a new quantitative assay
Board review style question #2
Using the provided dataset for method comparison studies for a hemoglobin A1c assay, which of the following statements is true?
- The data shows acceptable linearity
- The result suggests analytical errors due to carryover
- There is an increasing negative bias of the new method with increasing HbA1c concentrations
- There is an insufficient number of data points for the analysis to be conclusive
Board review style answer #2
C. While the average bias of -1.5% is within the total allowable error, it is clear that there is an increasing negative bias of the new method with increasing HbA1c concentrations (current method is negatively biased against new method). The bias hovers around 6% starting around 6.0% A1c units and greater, which encompasses the medical decision limit of 6.5% A1c units. This bias renders the method comparison result not acceptable for patient testing.
Comment Here
Reference: Validating a new quantitative assay
Comment Here
Reference: Validating a new quantitative assay
Vitamin B12 (pending)
[Pending]
Vitamin D
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Diagrams / tables | Pathophysiology | Clinical features | Laboratory | Test indications | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Vitamin D plays an important role in the maintenance of calcium and phosphate homeostasis by regulating calcium absorption and osteoclastic / osteoblastic activity
- Plasma concentrations of vitamin D should be interpreted in context with other measures of calcium and phosphate homeostasis, such as parathyroid hormone, calcium, phosphate and alkaline phosphatase
Essential features
- Vitamin D3 is endogenously synthesized in skin exposed to sunlight, whereas vitamin D2 is the dietary form found in plants and fungi
- Vitamin D2 and D3 are converted in the liver to 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3, respectively
- 25-hydroxyvitamin D [25(OH)D] has a long biological half life (2 - 3 weeks) and is the primary form of vitamin D in the body; therefore, it is the best biomarker to assess vitamin D deficiency
- 1,25-dihydroxyvitamin D [1,25(OH)2D] is the biologically active form of the vitamin but has a short half life (~4 hours) and is present in significantly lower concentrations than 25-hydroxyvitamin D
- In the United States, ergocalciferol or vitamin D2 supplement is provided as a high dose oral capsule (50,000 IU); various low dosage vitamin D3 capsule options exist
Terminology
- Vitamin D3: cholecalciferol
- 25-hydroxyvitamin D3: calcidiol, calcifediol, 25-hydroxycholecalciferol, 25(OH)D3
- Vitamin D2: ergocalciferol
- 25-hydroxyvitamin D2: 25-hydroxyergocalciferol, 25(OH)D2
- 1,25-dihydroxyvitamin D: calcitriol, 1,25(OH)2D
ICD coding
- ICD-10: E55 - vitamin D deficiency
Pathophysiology
- Vitamin D3 is synthesized from 7-dehydrocholesterol in the skin exposed to UVB radiation
- Vitamin D2 originates from dietary sources and is found in plants and fungi
- Vitamin D2 and D3 have similar functions; laboratory testing measures the total value of D2 and D3 in the blood
- In the liver, vitamin D is converted to 25(OH)D by 25-hydroxylase
- 25(OH)D is a prohormone that represents the main reservoir and transport form of vitamin D and is bound by a transport protein while in circulation (Am J Clin Nutr 2012;95:1357)
- In renal tubular cells, 25(OH)D is converted to the active form, 1,25(OH)2D, by 1-α-hydroxylase, which is tightly regulated by calcium, phosphate and parathyroid hormone (PTH) concentrations in the body (Nat Rev Endocrinol 2020;16:234)
- Overall effect of vitamin D in the body is to increase calcium and phosphate levels in the blood
- 1,25(OH)2D is transported in the bloodstream to the intestine via binding to vitamin D binding protein (DBP) and promotes absorption of calcium and phosphate in the small intestine
- 1,25(OH)2D also increases osteoclastic activity in bones
- In the kidney, 1,25(OH)2D increases calcium reabsorption by the distal renal tubules and phosphate reabsorption by the proximal tubules through its synergy with PTH (McPherson: Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2017)
- 24,25-dihydroxyvitamin D is an inactive vitamin D metabolite produced in the kidney by 24-α-hydroxylase
Clinical features
- Vitamin D deficiency risk factors
- Exclusively breast fed infants
- More common in dark skin than light skin individuals
- Intestinal diseases preventing absorption (e.g. Celiac disease, Crohn’s disease)
- Renal and liver diseases leading to insufficient enzymes for metabolism
- Medications (e.g. phenobarbital and rifampin) can activate hepatic p450 enzymes that lead to vitamin D degradation
- Consequences of vitamin D deficiency
- Hypocalcemia
- Hypophosphatemia
- Rickets (softening of bones in children)
- Osteomalacia and osteoporosis
- Unregulated production of 1,25-dihydroxyvitamin D in sarcoidosis or granulomatous diseases
- Uncommon cause of hypercalcemia
- Elevated calcium with low PTH level
Laboratory
- Methodology to measure levels
- Because of its relatively stable plasma concentration and longer half life, 25(OH)D is the best measure of overall vitamin D status
- Chemiluminescent immunoassay (CLIA) is commonly used on automated instruments for high throughput vitamin D assays in clinical settings
- Vitamin D binding protein concentration and binding kinetics may affect the accuracy of 25-hydroxyvitamin D measured by immunoassay
- Antibodies used in many immunoassays often have lower crossreactivity with 25-hydroxyvitamin D2 and therefore may underestimate total vitamin D activity (Hormones (Athens) 2020;19:97)
- Liquid chromatography tandem mass spectrometry (LC-MS / MS) method is the reference method for the quantification of 25(OH)D and 1,25(OH)2D and can accurately quantitate both vitamin D2 and D3 (Mass Spectrom Rev 2015;34:2)
- Under uncommon circumstances (e.g., chronic renal disease or hypercalcemia), 1,25(OH)2D testing may be indicated
- Diminished levels may be due to reduced 1α-hydroxylase enzyme activity in renal disease, leading to secondary hyperparathyroidism (parathyroid gland hyperplasia)
- Because of its relatively stable plasma concentration and longer half life, 25(OH)D is the best measure of overall vitamin D status
- Test limitations
- Antibodies used in immunoassays for vitamin D have variable reactivity toward 25-hydroxyvitamin D2 and D3, which may result in a bias between different vitamin D immunoassays (Rifai: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th Edition, 2018)
- Presence of inactive vitamin D metabolites may introduce a positive bias for both immunoassays and LC-MS / MS methods (Mass Spectrom Rev 2015;34:2)
- Reference ranges
- Plasma vitamin D concentration is considered insufficient if < 20 ng/mL and deficient if < 10 ng/mL
- Reference range of 25(OH)D is 10 - 65 ng/mL in serum or plasma
- Vitamin D toxicity may occur at concentrations > 100 ng/mL (Rifai: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th Edition, 2018)
- Reference range of circulating 1,25(OH)2D is 15 - 60 pg/mL (McPherson: Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2017)
- Plasma vitamin D concentration is considered insufficient if < 20 ng/mL and deficient if < 10 ng/mL
Test indications
- Vitamin D deficiency results from inadequate dietary intake, intestinal malabsorption, decreased synthesis or defective vitamin D receptors, causing rickets in children and osteomalacia in adults (McPherson: Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2017)
- Vitamin D deficiency may cause hypocalcemia, which is followed by increased secretion of PTH (secondary hyperparathyroidism)
- 1-α-hydroxylase deficiency is caused by a mutation in CYP27B1 (chromosome 12q14.1), leading to vitamin D dependent rickets type I
- Hypercalcemia is commonly associated with primary hyperparathyroidism
- Elevated PTH increases the conversion of 25(OH)D to the active form 1,25(OH)2D in the kidney
- Increased 1,25(OH)2D and hypercalcemia may be associated with granulomatous diseases such as sarcoidosis, tuberculosis and granulomas (McPherson: Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2017)
Additional references
Board review style question #1
Board review style answer #1
C. Liver. Vitamin D is metabolized to 25(OH)D by 25-hydroxylase in the liver. 25(OH)D is converted to the active form 1,25(OH)2D by 1-α-hydroxylase in renal tubular cells.
Comment Here
Reference: Vitamin D
Comment Here
Reference: Vitamin D
Board review style question #2
- Which of the following is true about the major function of vitamin D?
- Increases the absorption of calcium and phosphate from the gastrointestinal tract
- Maintenance of normal bone formation, turnover and strength
- Raises the circulating calcium concentration while lowering phosphate concentration
- Responsible for the regulation of total body phosphate
- Stimulates parathyroid hormone synthesis and secretion
Board review style answer #2
A. Increases the absorption of calcium and phosphate from the gastrointestinal tract. The active form of vitamin D, 1,25(OH)2D, is transported to the small intestine and promotes absorption of calcium and phosphate from the diet.
Comment Here
Reference: Vitamin D
Comment Here
Reference: Vitamin D
lactate dehydrogenase
Table of Contents
Definition / general | Pathophysiology | Diagrams / tables | Laboratory | Additional referencesDefinition / general
- LDH measures the amount of serum lactate dehydrogenase (LDH), which is released into the circulation after tissue damage
- Causes of tissue damage include acute myocardial infarction (AMI), hepatitis and acute renal failure
- LDH is elevated on the second day after AMI, and remains elevated for up to 4 days
- LD1 is usually measured
Pathophysiology
- LDH is an enzyme (EC 1.1.1.27) ubiquitous in tissue (Wikipedia - Lactate dehydrogenase)
- It has five isoenzymes, each with a different composition of M-type and H-type subunits in a tetrameric structure
- The M-type isoenzyme is predominant in liver and skeletal muscle
- The H-type isoenzyme is predominant in heart and renal cortex
- LDH catalyzes the conversion of pyruvate to lactate in the glycolytic pathway
Laboratory
Methodology
Indications
Limitations
Reference ranges
- The measurement of the enzyme uses the reduction of the coenzyme NAD to NADH with increasing optical density and oxidation of lactic acid to pyruvate
- Alternatively, one can follow the reverse reaction of pyruvate to lactate and NADH to NAD at 340 nm
Indications
- Patients presenting 12+ hours after the onset of chest pain
- High levels are suggestive of acute myocardial infarction
- Staging (S classification) patients with nonseminomatous testicular cancer
- Evaluating patients with metastatic cancer
- Assessing nature of pleural / pericardial fluids: ratio of fluid LDH to upper limit of normal serum LDH of more than 0.6 suggests an inflammatory process (exudate)
Limitations
- Elevated in hemolyzed specimens, since enzyme is present in erythrocytes
Reference ranges
- A typical range is 105 - 333 IU/L
- Must interpret values in context of patient clinical findings
Additional references
Recent Chemistry, toxicology & urinalysis Pathology books
Find related Pathology books: lab medicine, microbiology, body fluid/urinalysis, management